Virus Application of Optiprep
OptiPrep™ Application Sheet V01
Preparation of density gradient solutions
1. OptiPrep™
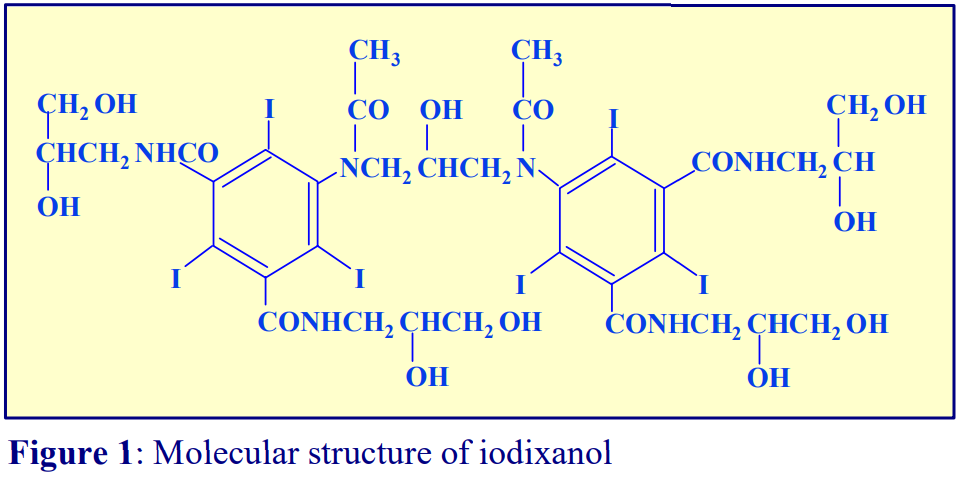
OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml. Iodixanol is a nonionic molecule with a molecular mass of 1550 (see Figure 1).
 2. Handling OptiPrep™
2. Handling OptiPrep™
Exposure (several months) of iodixanol solutions to direct sunlight will cause a slow release of iodine (solution turns yellow); OptiPrep™ should therefore be stored away from strong sunlight. On standing, iodixanol may „settle out“ of concentrated solutions, which should be well mixed before use.
3. Osmolality
The observed osmolality of OptiPrep™ depends on the mode of measurement (vapour pressure or freezing point); moreover the situation is complicated by the tendency of the iodixanol molecules to associate non-covalently in a concentrated aqueous solution. Measured values for its osmolality are thus lower than might be expected. Importantly however, when OptiPrep™ is diluted with a buffered isoosmotic solution, the iodixanol oligomers dissociate and all dilutions are isoosmotic. Under normal operating conditions therefore OptiPrep™ behaves as if it had an osmolality of approx 290 mOsm.
4. Preparation of density solutions
Traditionally viruses have been purified in gradients containing high concentrations of sucrose, glycerol or CsCl. The particles have therefore been isolated in grossly hyperosmotic conditions. OptiPrep™ offers the opportunity to isolate them under isoosmotic conditions. In many instances the density of a virus in iodixanol will be considerably lower than that in CsCl and slightly lower than that in sucrose or glycerol. Commonly the solutions used to suspend viruses are phosphate-, Tris- or HEPES-buffered buffered saline (or 0.25 M sucrose). The solutions may contain low concentrations of additives such as EDTA (1 mM), KCl (2.5 mM) or MgCl2 (1 mM).
If it is important to maintain the concentration of the buffer and additives constant throughout the gradient, then the general strategy is to start by making a dense working solution (WS). For example make a 50% (w/v) iodixanol working solution by diluting 5 vol. of OptiPrep™ with a 1 vol. of a diluent containing 6x the required concentrations of buffer and additives. Note that the concentration of the osmotic balancer (NaCl or sucrose) is not similarly increased six-fold; if it were then the solution would be hyperosmotic. The WS will then contain the correct concentration of buffer and additives and be approximately isoosmotic; this can then be further diluted with the normal medium to provide solutions of lower density. The WS can also be added directly to a sample to adjust its density. Iodixanol solutions produced in this manner will be in the range 285-305 mOsm. The use of alternative organic buffers at similar concentrations will have no significant effect on the density and osmolality of the solutions.
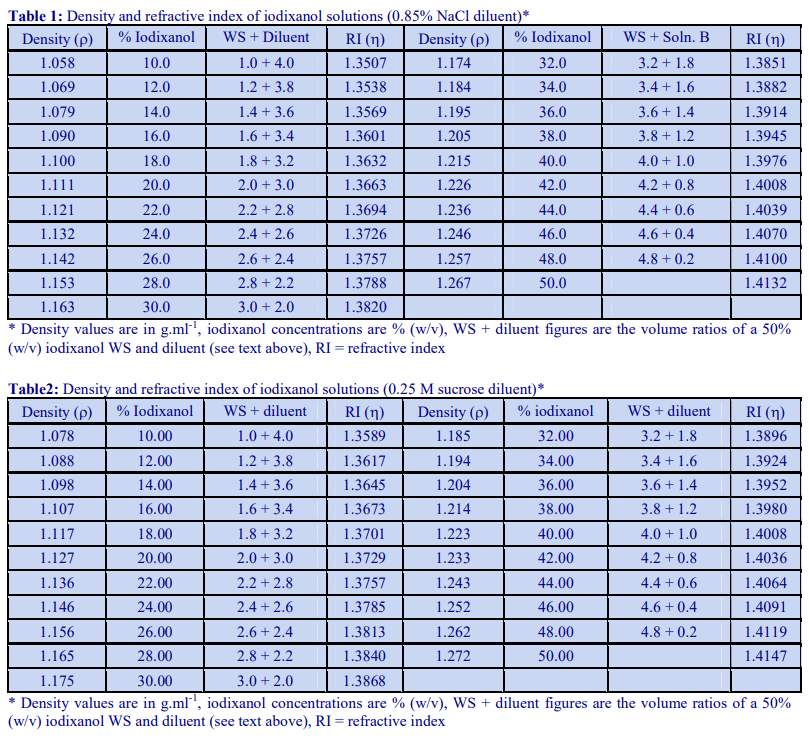
Tables 1 and 2 give the density of solutions produced by dilution of a 50% (w/v) iodixanol WS with either 0.85% NaCl, 10 mM Tris-HCl, pH 7.4 (Table 1) or 0.25 M sucrose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.4 (Table 2). To maintain a constant buffer concentration in the solutions (Table 1), the 50% iodixanol WS was produced by diluting 5 vol. of OptiPrep™ with 1 vol. of 0.85% NaCl, 60 mM Tris-HCl, pH 7.4. Similarly the 50% iodixanol WS in Table 2 was produced by diluting 5 vol. of OptiPrep™ with 1 vol. of 0.25 M sucrose, 6 mM EDTA, 60 mM Tris-HCl, pH 7.4.
5 Calculation of density
As long as the density of the diluent is known then Equation 1 can be used to calculate the density of any solution produced from the diluent and a working or stock solution of iodixanol.
Equation 1:
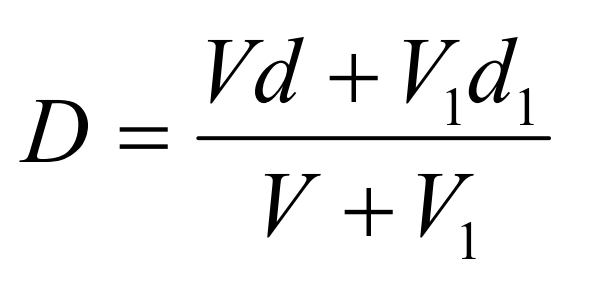
D = density of mixture; V = volume of iodixanol stock solution; d = density iodixanol stock solution;
V1 = volume of diluent; d1 = density of diluent
OptiPrep™ Application Sheet V01; 8th edition, March 2020
OptiPrep™ Application Sheet V02
Preparation of discontinuous and continuous gradients
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V-number.
1. Discontinuous gradients
1a Overlayering technique
The most widely used method for producing discontinuous gradients is to start with the densest solution and layer solutions of successively lower densities on top using some form of pipette or syringe. Tilt the centrifuge tube (approx. 45°); place the tip of the pipette or syringe against the wall of the tube, about 1 cm above the meniscus of the denser solution, and gently deliver a slow and steady stream of liquid. This allows the liquid to spread over the tube surface and minimizes any mixing due to a sudden increase in liquid flow. Once a steady flow is established keep the tip of the pipette or syringe just above the meniscus of the liquid and against the wall of the tube.
From a pipette
Use a rubber two- or three-valve pipette filler to aliquot and dispense the gradient solutions. Check that the release valve when pressed gently, allows the delivery of a slow and steady flow of liquid. Do not use a pipette filler that uses positive pressure to deliver the liquid, as a slow even flow is often difficult to attain. Always take up more of the gradient medium than is required as it is easier and more accurate to empty the pipette to a graduation mark than to try to empty it completely.
From an automatic pipette
For small volume gradients an automatic pipette may be used. Always cut off the end of the plastic pipette tip to reduce the flow velocity of the liquid.
From a Pasteur pipette
Plastic Pasteur pipettes can be used conveniently for larger volume gradients, particularly those in calibrated centrifuge tubes. It requires some practise however to maintain a steady liquid flow by depressing the bulb of the pipette.
From a syringe
A syringe with a wide-bore metal filling cannula (i.d. approx 1 mm) is suitable for most gradient volumes, but make sure that the barrel can move easily and smoothly when a small pressure is applied. Placing the index finger around the bottom of the plunger, rather than around the barrel, restricts the movement of the plunger when it is depressed and thus achieves a more controlled liquid flow. Always take up more of the gradient medium than is required for the step as it is more accurate to empty the syringe to a graduation mark than to try to empty it completely.
- Metal filling cannulas can be purchased from most surgical instrument suppliers.
1b Underlayering technique
Although the overlayering technique is probably the most widely used, the easier method is to underlayer successively denser solutions beneath the lighter solutions. The only important requirement is that no air bubbles are introduced which may disturb the lower density layers above; for this reason a syringe with a metal filling cannula is the best tool for this procedure. Generally the existing steps are disturbed less as the outflowing liquid spreads upwards through the hemispherical section of the bottom of the tube.
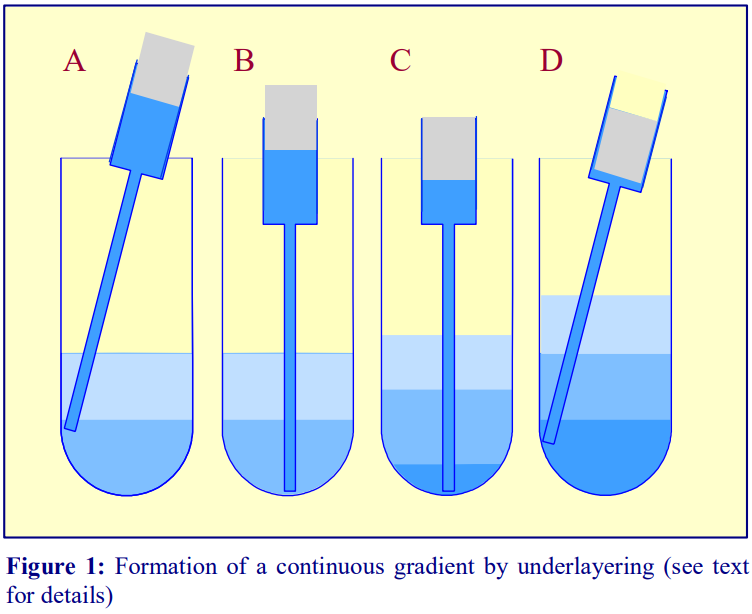 1. To underlayer 4 ml of liquid, take up 5 ml into the syringe and expel to the 4.5 ml mark to ensure that the cannula is full of liquid.
1. To underlayer 4 ml of liquid, take up 5 ml into the syringe and expel to the 4.5 ml mark to ensure that the cannula is full of liquid.
2. Dry the outside of the cannula.
3. Move the tip of the cannula to the bottom of the tube, sliding it slowly down the wall of the tube (Figure 1A-B)
4. Depress the plunger to the 0.5 ml mark (Figure 1C).
5. After a few seconds (to allow all of the liquid to be delivered into the tube) slowly withdraw the cannula, again against the wall of the tube (Figure 1D).
6. Dry the outside of the cannula and repeat the procedure with successively denser solutions.
2. Continuous gradients
Continuous gradients may be made by allowing discontinuous gradients to diffuse or by using a gradient maker specifically designed for this purpose.
 2a By diffusion of discontinuous gradients
2a By diffusion of discontinuous gradients
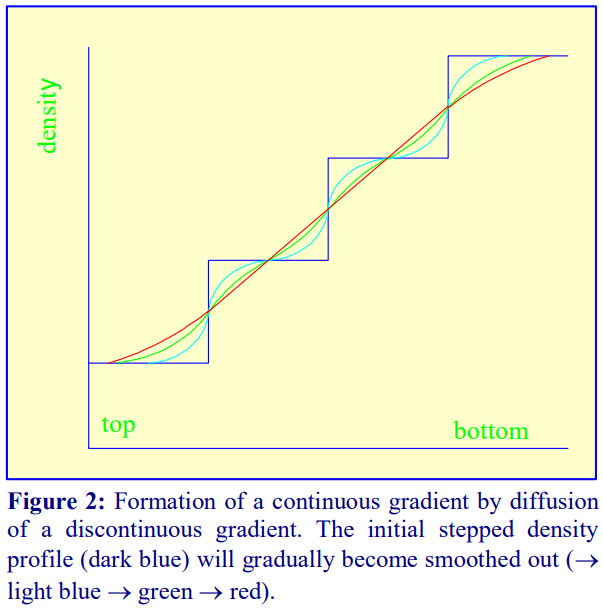
Once a discontinuous gradient is formed, the sharp boundaries between the layers, which are observed as a sudden change in refractive index, start to disappear as the solute molecules diffuse down the concentration gradient from each denser layer to each lighter layer. Thus the density
discontinuities between each layer will slowly even out and the gradient will eventually become linear (Figure 2), and given sufficient time the density will become completely uniform.
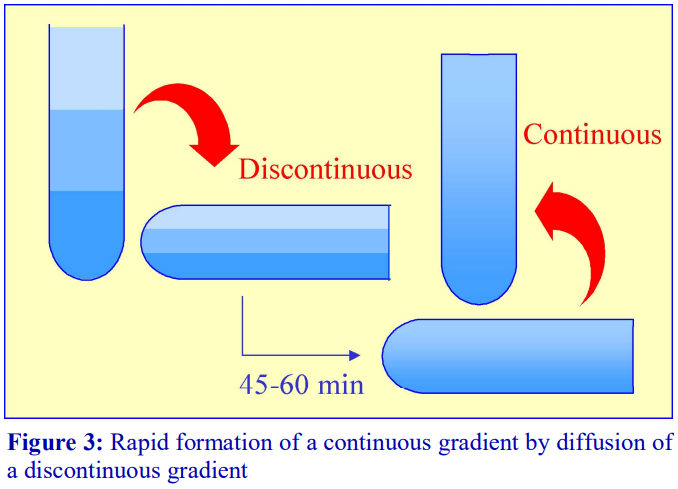
For a particular medium, the rate of diffusion across an interface is dependent on temperature and the cross-sectional area of the interface. In addition the rate at which the gradient becomes linear will also be a function of the distance between the interfaces. Thus a linear gradient will form more rapidly at room temperature than at 4°C and if the distance between interfaces is reduced and the cross-sectional area increased. This can be achieved as follows (Figure 3).
1. Produce a discontinuous gradient by the underlayering (Section 1b) or overlayering (Section1a) method. Unless the gradient is to be very shallow use 3 or 4 layers that increase in steps of about 5-10% (w/v) iodixanol.
2. Seal the tube well with Parafilm or a plastic stopper and carefully rotate the tube to a horizontal position and leave for 45-60 min.
 3. Return the tube to the vertical, cool to 4°C if required and apply the sample to the gradient (either over- or underlayered).
3. Return the tube to the vertical, cool to 4°C if required and apply the sample to the gradient (either over- or underlayered).
The precise timing for the formation of a continuous linear gradient will depend on the dimensions of the tube, the number of layers and the concentrations of iodixanol. A series of trial experiments should be carried out in which the time is varied and the density profile of the formed gradient checked by fractionation and refractive index measurement.
Because the continuous gradient is formed by a physical process, so long as the temperature and time are well controlled, the shape of the gradient is highly reproducible. If the diffusion is allowed to occur in a vertically maintained tube the process will take longer and at 4°C it may take more than 10 h. If however the gradients can be prepared the day before the separation and left in the refrigerator overnight then this can be a convenient approach. With gradients prepared rapidly at room temperature the virus suspension may be incorporated into one or more of the layers if the time at 18- 22°C is not considered deleterious and the tube is reliably sealed; elimination of interfaces can improve resolution. Although most virus purifications are carried at 4°C, a few are executed at room temperature.
 2b Using a two-chamber gradient maker
2b Using a two-chamber gradient maker
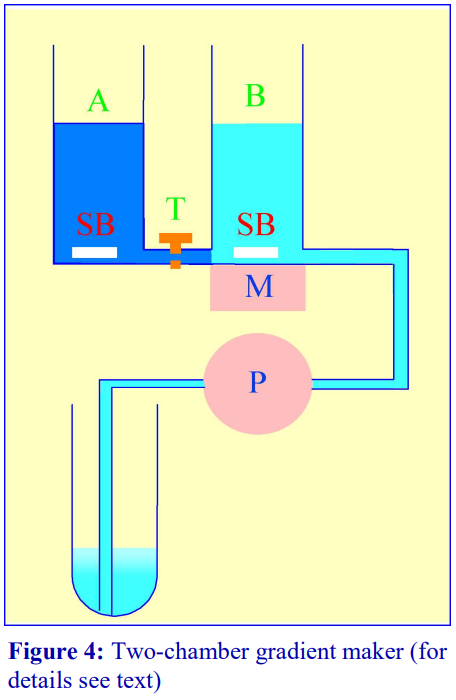
The traditional way of constructing a continuous gradient is to use a standard two-chamber gradient maker (Figure 4). It consists of two identical chambers connected close to their bases by a tapped channel (T). One of the chambers (the mixing chamber – B in Figure 4)) has an outlet directly opposite the inlet from the tapped channel.
1. Set up the device as shown in Figure 4 with the mixing chamber (B) resting on a magnetic stirrer (M) and the outlet tube leading via a peristaltic pump (P) to the bottom of the centrifuge tube.
2. Place the chosen high-density solution in the non-mixing chamber (A) and then momentarily open the tap (T) to allow dense liquid to fill the connecting tube.
3. Pour an equal volume of the low-density solution in the mixing chamber (B).
4. Place two identical stirring bars (SB) in the two chambers (this ensures that the height of the two solutions is the same.
5. In rapid sequence, switch on the pump (P) and the magnetic stirrer (M) and then open the connecting tap (T). As the levels in the two chambers fall synchronously, reduce the speed of the stirrer to avoid generating air bubbles that may enter the gradient and disturb it.
6. Make sure that the pump is turned off before any air bubbles reach the bottom of the delivery tube at the end of the operation.
- The larger the density difference between the two gradient solutions the more vigorous must be the stirring to ensure good mixing. If the stirring bar is too close to the inlet from the connecting tube, it is possible in the initial stages for the low-density medium to back flow into the highdensity medium.
- The correct pumping speed depends on the volume of the gradient and the quality of the pump (ideally the outflow from the pump should not pulsate), but for a standard 10-30% (w/v) iodixanol gradient (of 12-15 ml total volume) a flow rate of approx 2 ml/min is satisfactory. Pumps that impart little or no pulsation to the liquid flow are commonly available from many sources.
- The gradient can alternatively be produced high density end-first, in which case the location of the two solutions needs to be reversed and the delivery tube to the centrifuge tube must be placed against the wall of the centrifuge tube near to its top, so the gradient flows down the tube smoothly. This is can pose some problems of mixing in the centrifuge tube if the flow down the tube wall is in the form of large drops rather than a continuous stream (this may be minimized by tilting the tube), on the other hand the tendency of the low density medium to float to the surface of the high density medium in the mixing chamber aids mixing. The Auto Densi-Flow gradient unloader can be used to deposit a gradient high-density end first with no disturbance. Although this device is no longer commercially available, it will be found in many laboratories. For details of this device see Section 4e of Application Sheet V04.
- To guard against air bubbles entering the delivery tube, a bubble trap could be included between mixer and pump. Although air bubbles are a major problem if they reach the bottom of the centrifuge tube (low density first delivery) they are no less a problem for high-density first delivery as they interfere with the smooth flow of liquid down the tube wall.
- It is possible to produce up to three gradients at a time; some gradient mixers have a three-outlet manifold. However such a device requires three tubes to pass through the peristaltic pump. It is the only reliable configuration of the delivery tube; simply splitting the liquid flow from a single tube through the pump cannot guarantee precisely equal delivery to all three tubes.
 2c Gradient Master™
2c Gradient Master™
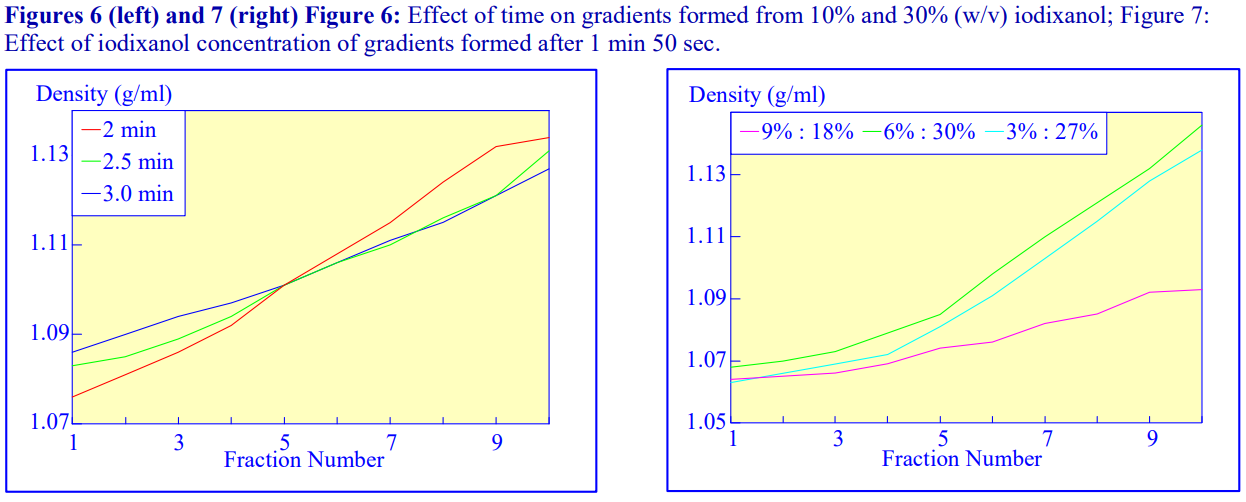
An alternative device for the generation of continuous density gradients – the Gradient Maste™ – produces the gradient by controlled mixing of the low and high-density solutions layered in the centrifuge tube. The tubes are rotated at a pre-set angle – usually 80° – to increase the cross-sectional area of the interface – and speed (usually 20 rpm) for about 2 min (Figure 5). The density profile of the gradient generally becomes more shallow with time. The simplicity of the technique and the highly reproducible nature of the gradients make this a very attractive method; up to 6 gradients (17 ml tubes) can be formed at once. Some examples with iodixanol solutions are given in Figures 6 and 7.
- A very important advantage of this technique over the use of a two-chamber gradient mixer is that if it is necessary to make the sample part of the gradient, any potentially hazardous biological sample is contained within the centrifuge tube and does not contaminate the gradient forming device and ancillary tubing.
- For more information on the Gradient Master™ and other similar instruments contact the manufacturers at www.biocompinstruments.com
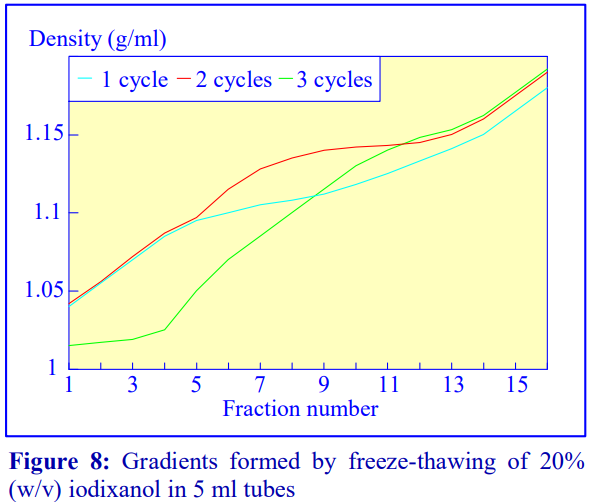 2d Freeze-thawing
2d Freeze-thawing
The final manner in which continuous gradients can be produced is by freezing a solution of uniform density for at least 30 min at -20°C and then thawing at room temperature for 30-60 min. These times are for tubes of approximately 5 ml volume. The freeze-thaw cycles can then be repeated; this modulates the density profile of the gradient. Generally as the number of freeze-thaw cycles increases, the gradient becomes markedly less dense at the top. The method can produce gradients that are more or less linear. Because the shape of the gradient depends on the rate of freezing and thawing, as well as the number of freeze-thaw cycles (and the volume of the tube), the precise conditions required need to be worked out for a particular laboratory. Under well-controlled conditions however, the profiles are highly reproducible. An example of the procedure with an iodixanol solution is given in Figure 8 (data kindly supplied by Dr C A Borneque, CNRS, Centre de Génétique Moléculaire, 91198 Gif sur Yvette, France).
2e Non-linear gradients
It is not always desirable to use a linear gradient and either convex, concave, S-shaped or more complex gradient density profiles may be required to effect a particular resolution of particles. Convex gradients are sometimes particularly useful for the resolution of a sample containing a high concentration of particles of a wide range of densities. The steep density profile at the top of the gradient provides stable conditions for high capacity and the shallower high-density region provides high resolution.
From discontinuous gradients by diffusion
If each of the layers of the initially discontinuous gradient is of the same volume then diffusion will produce a linear gradient. The diffusion process however is also a very convenient way of producing a gradient that is not linear with volume. Convex or concave gradients or gradients containing a shallow median section can be produced by increasing the volume of the denser, lighter or median density layers respectively. The shape of the gradient may also be altered by changing the density interval between adjacent layers. Clearly reducing the density interval will make the gradient more shallow. It is important to test the density profile that is formed from such discontinuous gradients, but once satisfactory conditions are established the profile will be highly reproducible. Fraction Number
Using a gradient mixer
Convex and concave gradients cannot be produced with the standard two-chamber gradient mixer (see Figure 4). However if the non-mixing chamber is made twice the diameter of the mixing chamber, then with low-density solution in the mixing chamber a convex gradient is produced; if the locations of the low density and high-density solutions are reversed, a concave gradient is produced.
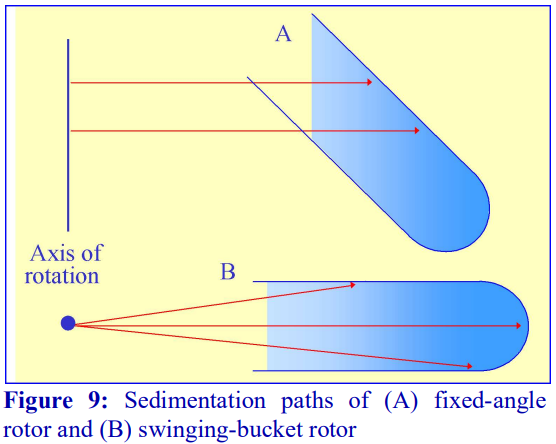 3. Types of rotor used with preformed gradients
3. Types of rotor used with preformed gradients
Traditionally, preformed gradients of sucrose or glycerol are run in a swinging-bucket rotor and today this remains the most popular choice of rotor for any density gradient centrifugation. Sedimentation path lengths tend to be long, but because of the relatively low viscosity of iodixanol solutions, centrifugation times need not be correspondingly long. However, because iodixanol is able to form its own gradient by self-generation in the centrifugal field, it is not good practice to carry out buoyant density banding of smaller particles such as viruses through pre-formed gradients at RCFs in excess of 250,000 gav for more than 3-4 h. Under these conditions iodixanol molecules towards the bottom of the tube may start to form a self-generated gradient and thus may deform the pre-formed density profile in the high-density region. At lower RCFs (e.g. 100,000 g) there will essentially be no density profile modulation.
Fixed-angle rotors are generally less frequently used for pre-formed gradients. Because of the angle at which the tube is held, particles tend to sediment to the wall of the tube due to the radial centrifugal field (Figure 9A); this does not occur in a swinging-bucket rotor, although even in this type of rotor, only those particles in the middle of the sample move in a plane parallel to the walls of the tube (Figure 9B). Swinging-bucket rotors were also often perceived as having an advantage over fixed-angle rotors for gradient work since the gradient always maintains the same orientation with respect to the long axis of the tube. However, so long as the particles do not adhere to the wall of the tube, a fixed-angle rotor can provide a useful alternative and there are many successful examples, particularly now that slow acceleration and deceleration facilities are now widely available on centrifuges to permit smooth reorientations of the gradient.
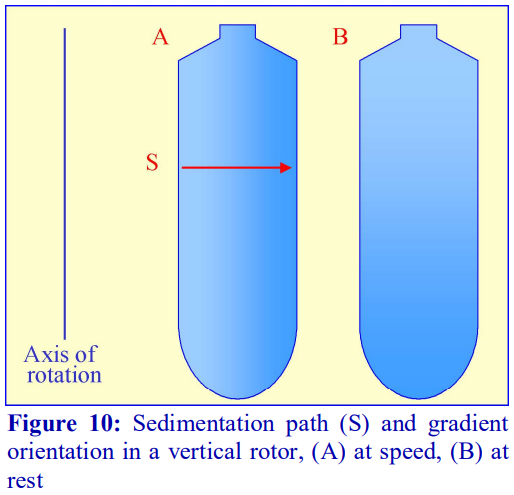 If a fixed-angle rotor is satisfactory for a particular gradient separation, then the shorter sedimentation path length of such a rotor compared to that of a swinging-bucket rotor of the same capacity permits a shorter centrifugation time. This situation is taken to its logical conclusion with a vertical rotor which will have the shortest path length, i.e. the width of the tube and, like the swinging-bucket rotor, the sedimentation or flotation of particles is relatively unaffected by the tube wall (Figure 10). In these rotors therefore, centrifugation times are reduced to a minimum. In a rotor such as the Beckman VTi65.1 or VTi50 the long axis of the tubes is much larger than their diameter (Figure 10), so the steep gradient formed during centrifugation reorients to a relatively shallow one at rest. Therefore, so long as there is no mixing of the tube contents during deceleration, small volume fractionation of the gradient will provide very high resolution.
If a fixed-angle rotor is satisfactory for a particular gradient separation, then the shorter sedimentation path length of such a rotor compared to that of a swinging-bucket rotor of the same capacity permits a shorter centrifugation time. This situation is taken to its logical conclusion with a vertical rotor which will have the shortest path length, i.e. the width of the tube and, like the swinging-bucket rotor, the sedimentation or flotation of particles is relatively unaffected by the tube wall (Figure 10). In these rotors therefore, centrifugation times are reduced to a minimum. In a rotor such as the Beckman VTi65.1 or VTi50 the long axis of the tubes is much larger than their diameter (Figure 10), so the steep gradient formed during centrifugation reorients to a relatively shallow one at rest. Therefore, so long as there is no mixing of the tube contents during deceleration, small volume fractionation of the gradient will provide very high resolution.
- It is important that the gradient is so designed to prevent particles from sedimenting to the wall of the tube, as these will tend to fall back into the medium during reorientation and unloading and thus contaminate the rest of the gradient.
- Near-vertical rotors, which hold the tube at approx. 8 to the vertical, overcome this pellet problem.
- Vertical and near-vertical rotors can provide the most efficient form of centrifugation in gradients. They can be particularly effective for rate-zonal separations, since any sample placed on top of a gradient achieves a very small radial thickness after reorientation.
- Because the surface area of any banded material is much higher in a vertical or near-vertical rotor than in a swinging-bucket rotor during centrifugation, particles that have a significant rate of diffusion (Mr<5×105 ) may exhibit band broadening due to this diffusion.
The use of large volume zonal rotors for gradient centrifugation is beyond the scope of this text; for information the reader is referred to relevant review articles [1,2].
4. Types of tube for gradient centrifugation
Choice of tube material (polyallomer, polycarbonate etc) is usually governed by considerations of optical transparency, resistance to chemicals or sterilizing (autoclaving) procedures (see manufacturers specifications for more information); generally speaking there is no specific advantage or disadvantage of using one particular type of tube material for gradient centrifugation from a fractionation point of view. The tube material may also restrict the maximum permitted RCF.
Choice of tube type (open-topped, screw-capped, sealed etc) is dictated by the selection of rotor type, the RCF that is required (many tube types cannot be run at the maximum speed of the rotor); the degree of containment that is required and a consideration of the type of gradient harvesting that is to be carried out. For details on gradient harvesting see Section 4i of Application Sheet V04.
Gradient centrifugation in low-speed and high-speed centrifuges is not generally carried out in special tubes, unless special containment is required; the standard thick walled polycarbonate, polyallomer, polypropylene or polystyrene tubes employed for all low- and high-speed centrifugation are satisfactory.
In ultracentrifugation a wide range of tube styles are available and the reader is directed to the appropriate technical manuals published by the centrifuge companies. Principally polyallomer and polycarbonate or Ultra-Clear™ (Beckman trade-name) are used. For simplicity and convenience only those tubes manufactured by Beckman Instruments will be described although other companies supply an essentially similar range of tubes although there may be some differences in the mode of sealing.
- Swinging-bucket rotors Tubes for swinging bucket rotors are traditionally open topped (the seal being provided the screw-cap on the bucket), thin-walled and made from polyallomer or UltraClear™. Occasionally thick-walled polycarbonate is available. See also “Vertical rotors” below.
- Fixed-angle rotors The types of material used for tubes for fixed-angle rotors are broadly similar to those for swinging-bucket rotors. Some of the thick walled tubes are open-topped and do not require caps, others have a variety of capping devices. Thin-walled tubes always require caps. The thick walled variety with a simple screw cap is not ideally suited to some forms of gradient harvesting. For details on gradient harvesting see Section 4i of Application Sheet V04. See also “Vertical rotors”, below.
- Vertical rotors The only types of tube recommended for vertical rotors are thin-walled sealed tubes made either from polyallomer or Ultraclear™; these are also available for many swingingbucket and fixed-angle rotors. In swinging-bucket rotors however there is usually a variable reduction in tube volume compared to the standard thin-walled open-topped tubes. Beckman manufacture two types:
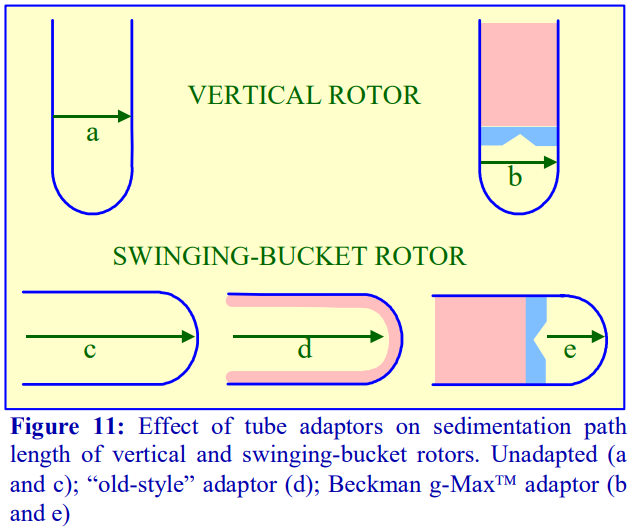 Quick-Seal™ and Optiseal™, the former are sealed by a heat and the latter by a central plastic plug.
Quick-Seal™ and Optiseal™, the former are sealed by a heat and the latter by a central plastic plug. - Through the use of adaptors and spacers most rotors accommodate a range of tubes of a volume considerably smaller than that of the rotor tube pocket, many of which may have a restricted maximum RCF (compared to the standard thin walled tube). Traditionally the smaller volume tubes for swinging-bucket and fixed-angle had a much-reduced diameter but the length was only slightly less than that of the fullvolume tube (Figure 11).
- g-Max™ tubes: Because of the advantage of a short sedimentation path length, some of the swinging-bucket and fixed-angle rotors have been adapted to take shorter sealed tubes so that the path length is reduced. They are also available for vertical rotors but in these rotors the sedimentation path length is unchanged, only the volume is altered (Figure 11).
5. References
1. Graham, J. M. (1984) Separations in zonal rotors In: Centrifugation – a practical approach (ed D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 219-249
2. Graham, J. M. (1992) Separations in zonal rotors In: Preparative centrifugation – a practical approach (ed D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 315-350
OptiPrep™ Application Sheet V02; 8th edition, January 2020
OptiPrep™ Application Sheet V03
Preparation of self-generating gradients
1. Background
Iodixanol, like solutions of heavy metal salts (e.g. CsCl) can form a gradient from a solution of uniform density under the influence of the centrifugal field. Once the solute begins to sediment through the solvent a concentration gradient is formed which is opposed by back-diffusion of the solute. With a sufficiently high RCF, at equilibrium, the sedimentation of the solute is exactly balanced by the diffusion and the gradient is stable. It is possible to calculate the time for a selfgenerating gradient to reach equilibrium and it is described by the following equation:
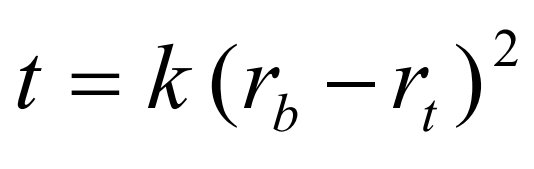
t is the time in hours; rb and rt the distance from the centre of rotation to the bottom and top of the gradient respectively and k is a constant, which depends on the diffusion coefficient and viscosity of the solute and on temperature [1]. The slope of the gradient is given by the equation:
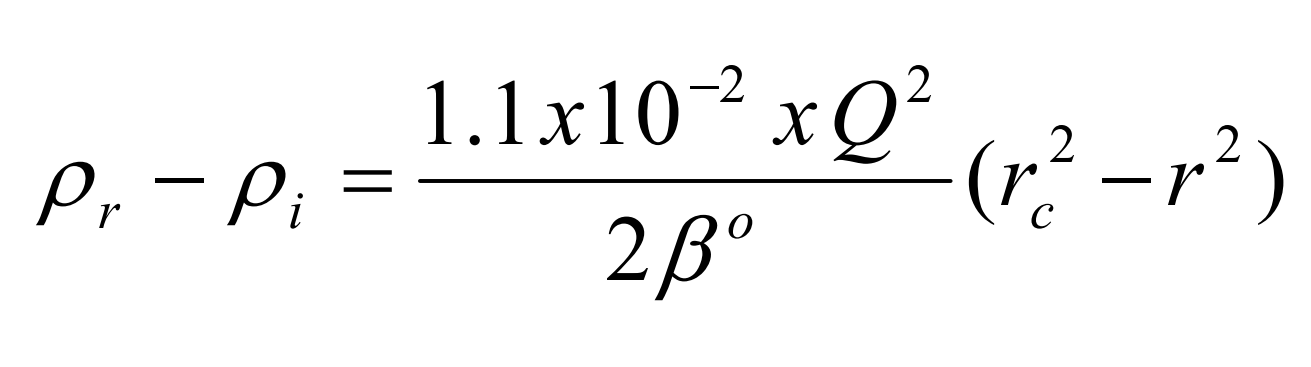
where ρ r is the density at a point r cm from the axis of rotation, ρ i is the starting density of the homogeneous solution, rc is the distance in cm from the axis of rotation where the density of the gradient = ρ i , Q is the rotor speed in rpm and ρ o is a constant depending on the solute [1].
The shape of the gradient that is formed for a particular solute thus depends on the following factors:
- sedimentation path length of the rotor
- time of centrifugation
- speed of centrifugation
- temperature
The big advantages of the use of any self-generated gradient are the ease of sample handling (the sample is simply adjusted to the required starting concentration of iodixanol) and the great reproducibility of the gradient density profile under a particular set of centrifugation parameters.
 2. Self-generated gradient formation
2. Self-generated gradient formation
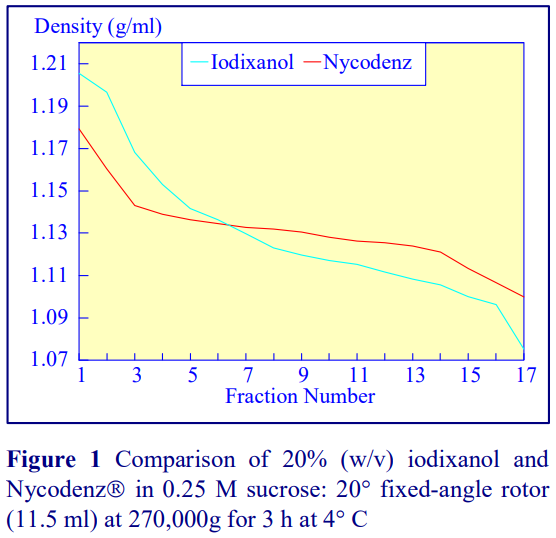
Iodixanol is able to form useful self-generating gradients in 1-4 h depending on the centrifugation speed and the rotor [2]. Figure 1 compares the gradient density profile generated from 20% (w/v) iodixanol and 20% (w/v) NycodenzⓇ in 0.25 M sucrose in a 20° fixed-angle rotor at 270,000gav for 3 h at 4°C. Clearly a steeper gradient is formed from the iodixanol and this is a function of the higher molecular mass of iodixanol (approx. twice that of NycodenzⓇ): it therefore sediments rather more rapidly and diffuses more slowly.
2a Types of rotor
Swinging-bucket rotors, which have rather long sedimentation path lengths, are little used for the formation of self-generating gradients. The shorter sedimentation path length rotors are much better suited to this task. Vertical and near-vertical rotors are particularly useful, although some fixed-angle rotors (preferably those with shallow angles of 20-24°) may be used.
Gradients generated in the Beckman TLN100 near-vertical rotor (for the TLX120 table-top ultracentrifuge) which accommodates tubes of 3.5-4.0 ml, the Beckman VTi65.1 vertical rotor (for an appropriate floor-standing ultracentrifuge) which accommodates tubes of approx 11.0 ml (but which can be adapted down to smaller volumes) and the Beckman NVT65 (a near vertical rotor of similar tube capacity to that of the VTi65.1) are particularly useful for iodixanol self-generated gradients. The TLN100 and VTi65.1 rotors have approximately the same sedimentation path length (about 17 mm), that of the NVT65 is marginally longer (approx 25 mm); consequently under the same centrifugation conditions, they generate rather similar gradient profiles.
2b Time of centrifugation
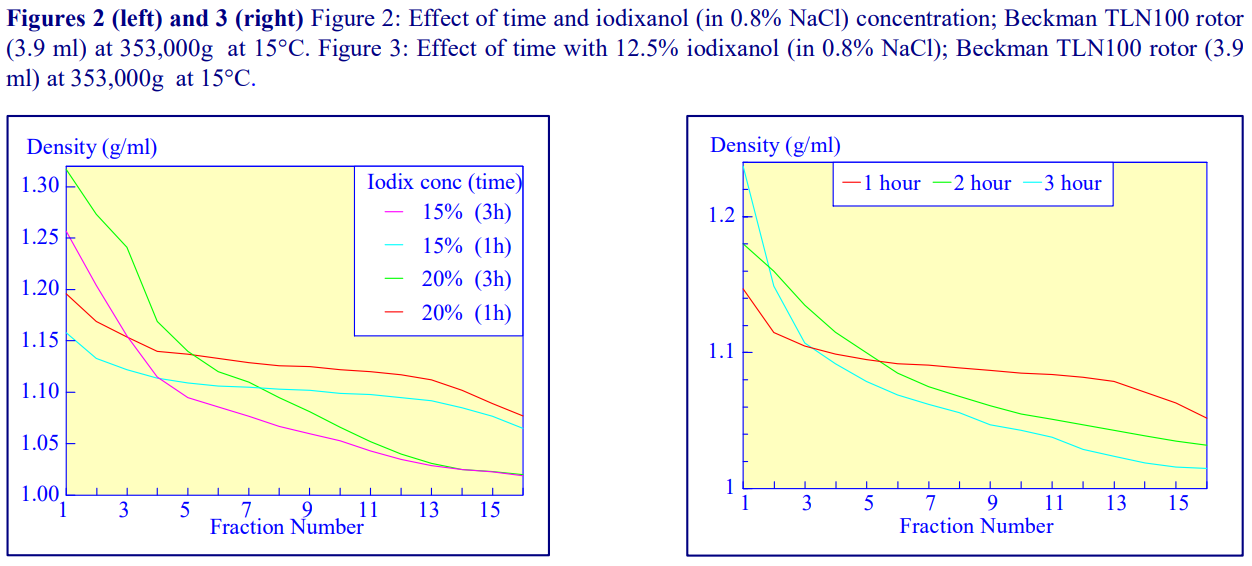
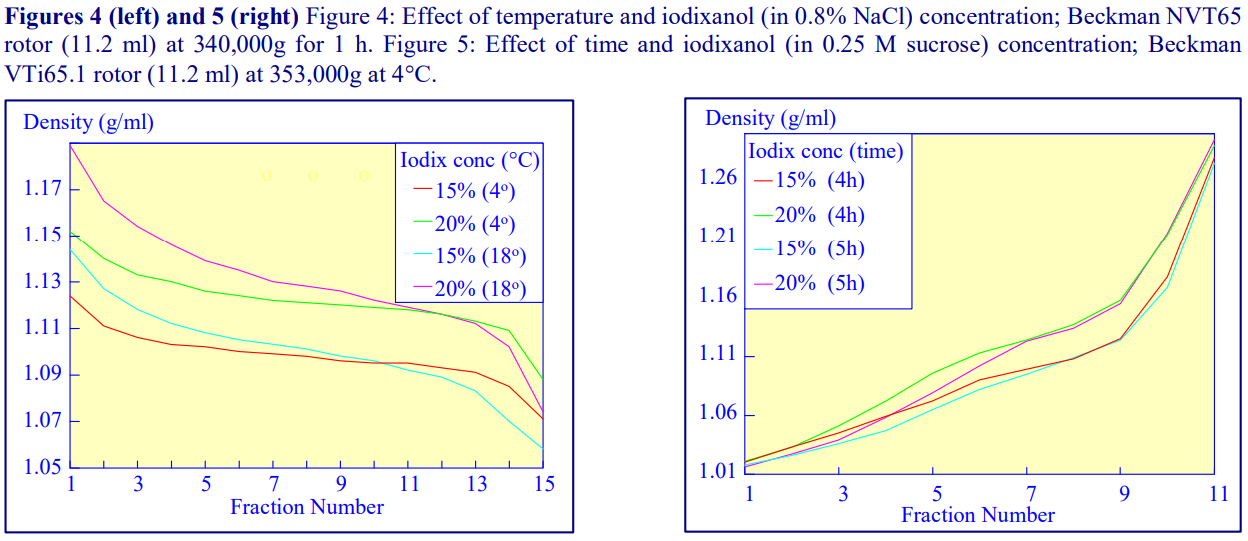
After 1 h at 15-18oC, centrifugation at approx 350,000gav, gradients generated in the TLN100 are Sshaped (i.e. they contain a relatively shallow region in the middle) and span a relatively narrow density range, while after 3 h, gradients are considerably steeper and cover a much wider density range. Figure 2 compares two starting concentrations of iodixanol at these two times, while Figure 3 compares three times (1, 2 and 3 h) using a 12.5% (w/v) iodixanol starting concentration with the same rotor. The exponential nature of the gradient becomes more apparent with time but times greater than 3 h result in little further change in the shape of the gradient at 350,000g, indicating that an equilibrium point has been reached.
2c Temperature
Higher temperatures tend to promote the formation of steeper gradients, although this effect is more apparent at shorter times of 1 h than at longer times of centrifugation. Figure 4 compares the formation of gradients at 4°C and 18°C in the NVT65 rotor at two iodixanol concentrations after centrifugation at approx 340,000gav for 1 h. At 4°C, using 0.25 M sucrose as osmotic balancer, the gradients approach equilibrium more slowly: the excellent gradient profiles produced in the VTi65.1 with 15% or 20% (w/v) iodixanol at 4 h are very similar to those at 5 h (Figure 5), compare with Figure 2 (using NaCl as osmotic balancer at 15°C).
2d Iodixanol concentration
Other than changing the density range covered by the gradient (Figures 2 and 4-8) the starting concentration of iodixanol has rather little effect on the rate of gradient formation or shape of gradient profile. The shape of the gradient can be made more linear at lower RCFs by using two layers of iodixanol (e.g. 10% and 30%, w/v) rather than a single uniform concentration (20%, w/v).
2e RCF
As the RCF decreases, the gradient becomes more shallow in the middle of the tube; the minimum RCF that produces a useful gradient will vary with the time and the rotor type. In the VTi65.1 vertical rotor, even at 170,000gav, a useful shallow gradient is produced within 3 h (Figure 7). In very high performance rotors that can run at up to 150,000 rpm (and also have very short sedimentation path lengths – see next section), self-generated gradients can form in as little as 15 min (Figure 8)
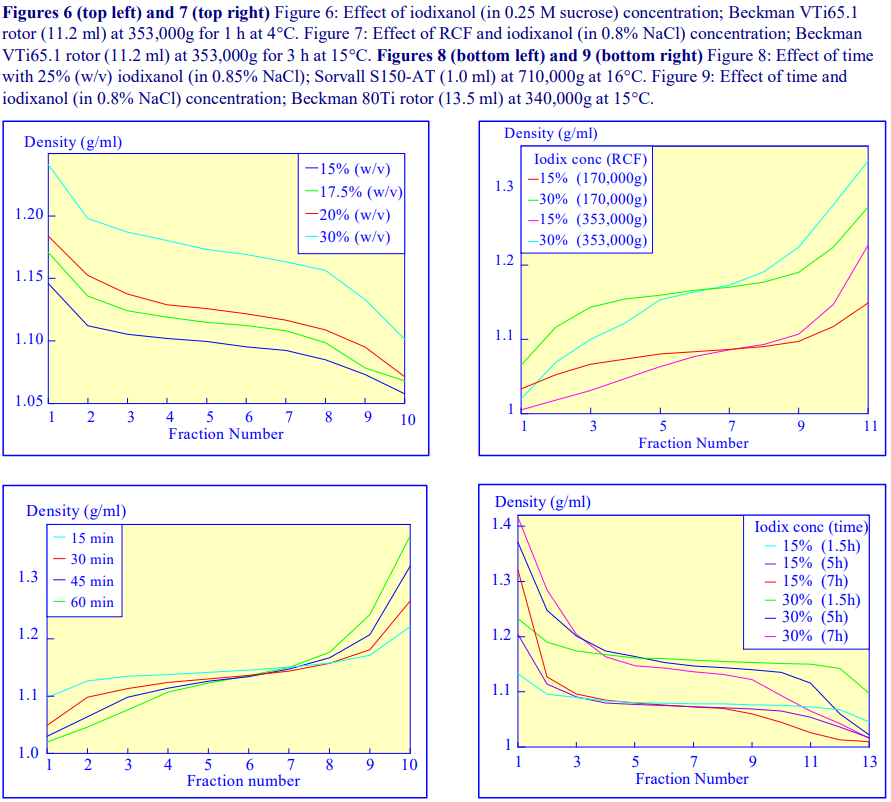
2f Sedimentation path length
The longer the sedimentation path length of the rotor, the greater the tendency to form S-shaped gradients. Figure 9 compares the gradient formed from 15% or 30% (w/v) iodixanol using the 80Ti fixed-angle rotor with a sedimentation path length of 43 mm (13.5 ml tube volume) at a series of times. At 70,000 rpm, (equivalent to 345,000gav) approx 5 h is required to produce a useful gradient (compare with Figures 2-7).
3. References
1. Dobrota, M. and Hinton, R. (1992) Conditions for density gradient separations In Preparative centrifugation – a practical approach (ed D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 77-142.
2. Ford, T., Graham, J. and Rickwood, D. (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of self generated gradients Anal. Biochem., 220, 360-366.
OptiPrep™ Application Sheet V03; 7th edition, January 2020
OptiPrep™ Application Sheet V04
Harvesting gradients
1. Introduction
The mode of harvesting depends very much on the type of tube used for the gradient, the distribution of particles in the gradient and the aim of the fractionation. Thick-walled tubes cannot be unloaded by any of the methods that involve piercing the tube wall with a needle and tubes with a narrow neck, such as some sealed tubes, make access with the tip of an automatic pipette impossible.
2. Tube handling prior to band or gradient recovery
The traditional open topped flexible-walled tubes for swinging-bucket rotors pose few, if any, problems for any mode of sample recovery. Heat-sealed or crimp-sealed tubes pose the biggest problems and for some modes of harvesting it may be necessary to slice off the top, to convert it to an open-topped tube.
- Do not use a scalpel blade
- Use a special tube cutter (Seton Scientific, Los Gatos, CA; sales@setonscientific.com) – the Beckman tube slicer (Section 4f) is a possible alternative
3. Recovery of individual bands of material
If the position of the particles of interest has been clearly established and, if there is more than one band in the gradient, the linear separation of those bands is 1 cm, then the band(s) may be removed individually by aspiration.
3a. Using a Pasteur pipette or syringe (applicable to any open-topped tube)
If a syringe is used, attach it to a flat-tipped metal cannula (i.d. 0.8-1.0 mm) not to a syringe needle. Metal filling cannulas may be obtained from any surgical equipment supplies company.
- Place the tip of the pipette or cannula at the top of the band of interest and aspirate the liquid very slowly, moving it across the diameter of the tube.
- To minimise the aspiration of any liquid from below the band, the tip of a glass Pasteur pipette may be fashioned into an L-shape.
- If the band of interest is below other material in the gradient then remove the latter first.
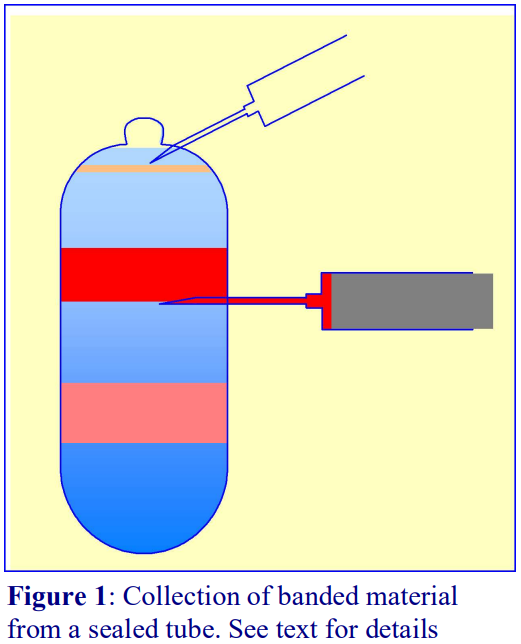 3b. Using a syringe (flexible-walled tubes only)
3b. Using a syringe (flexible-walled tubes only)
It is also possible to collect a specific band within the gradient by puncturing the tube wall with a needle attached to a syringe.
- To allow easy piercing of the tube wall; the centrifuge tube is best restricted by some sort of tube clamp.
- Insert the needle just below the band and with the inlet to the needle (bevel uppermost); aspirate the band into the syringe (Figure 1).
- If a sealed tube is used, air must be allowed to displace the falling column of liquid in the tube (see Figure 1) by puncturing the tube close to its top with another syringe needle.
- Once the band has been aspirated, the syringe needle is withdrawn and the hole in the tube sealed with silicone grease.
- The procedure may be repeated to harvest a denser band.
4. Harvesting the entire gradient into a series of equal volume fractions
The volume of each fraction collected from a gradient is determined as much by the operator’s requirements as by the resolving power of the gradient. As a general rule however, the volume of each fraction should be approx 5% of the gradient volume, but this may be decreased or increased for higher or lower resolution respectively.
4a. Using a Pasteur pipette, automatic pipette or syringe (applicable to any open-topped tube)
Most Pasteur pipettes are calibrated on the stem so if the tip of the pipette or cannula (attached to a 1 or 2 ml syringe) is placed at the meniscus, the total gradient may be collected in suitably sized fractions. If an automatic pipette is used, trim the end of the tip to make the orifice diameter 0.8-1.0 mm. The method is however tedious, prone to error and difficult to obtain equal volume fractions because of the need to keep the tip of the cannula or pipette at the meniscus without occasionally aspirating some air or removing some of the gradient from below the meniscus. For a crude fractionation however into four or five gradient cuts it is quite satisfactory.
4b. Aspiration form the bottom using a peristaltic pump
Ideally the harvesting system should be devised so that the effluent from the tube should not have to pass through a pump, but as long as the dead space volume of the tubing is small compared to the volume of the gradient it is permissible to insert a narrow rigid tube to the bottom of the centrifuge tube and to aspirate the contents (dense-end first). Theoretically, mixing will occur in the vertical section of the collection tubing as the decreasingly dense medium enters the bottom of the tube. In practice however this seems not to be a serious problem, again as long as the enclosed volume of the collecting tube is small compared to that of the gradient.
- If there is a pellet, make sure that the tip of collecting tube is maintained above it.
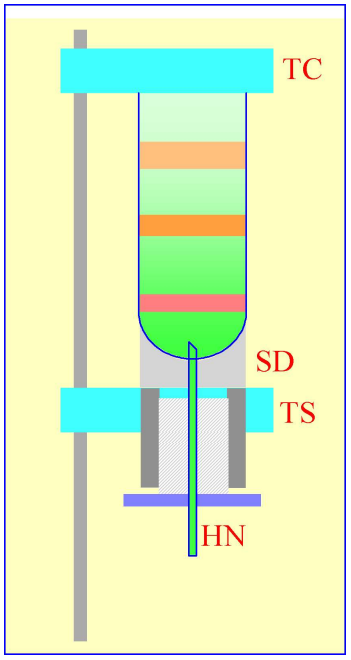
Figure 2: Gradient collection (dense-end first) by tube puncture. The tube is clamped between the sealing disc (SD) and the tube support (TS). A hollow needle (HN) is advanced through the bottom of the tube, sometimes by a screw-device (shown by the hatched area) or, more commonly by a pivoted lever
4c. Tube puncture
Practically this is best achieved by securing the tube vertically in some form of clamping device and to advance the needle through a rubber seal into the bottom of the tube by a screw or lever mechanism (Figure 2). The Beckman-Coulter Fraction Recovery System incorporates such a device. If sealed tubes are used, then either the central plug should be removed (Optiseal™) or the top punctured with a syringe needle (Quick-Seal™) to allow air to displace the liquid, which exits the tube under gravity. The system is simple and the dead space of the collecting tube is very small and the gradient is collected almost ideally, the hemispherical section of the bottom of the tube directing banded material into the collecting needle.
Because of the viscosity of the dense end of some gradients, gravitational flow will be slow at first and then speed up as the viscosity of the liquid decreases. To overcome this, the effluent from the hollow needle can be passed through a small volume peristaltic pump. So long as the dead space of the silicone tubing is small compared to the volume of the gradient, resolution is not seriously sacrificed.
- Thick-walled tubes cannot be used and it may not be a useful method if there is a large pellet, which may obstruct the hollow needle.
- Collecting equal volume fractions by this method or that described in Section 4b is not easy. The low-tech answer is to use calibrated collection tubes, although this requires continual attention from the operator to move on the delivery tube at the appropriate time. This problem is discussed further in Section 4h.
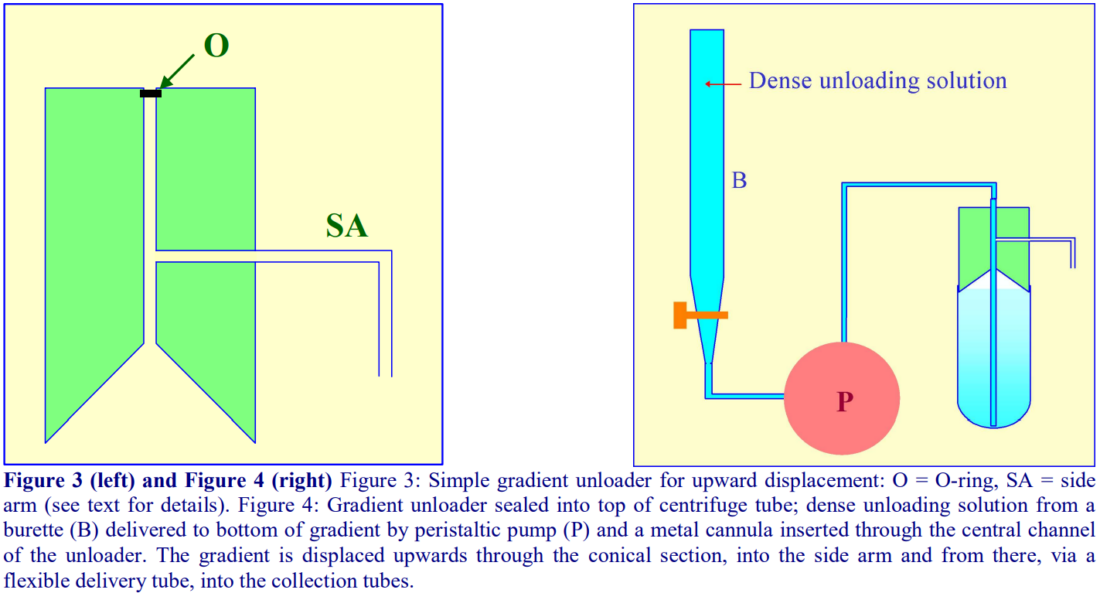
4d. Upward displacement
A dense liquid introduced to the bottom of the tube can displace the entire gradient upwards and with a suitable device attached to the top of the tube, the gradient can be delivered into the collection tubes. The use of a burette to contain the unloading solution does allow the collection of equal volume fractions.
4d-1. Delivery of dense liquid through a central tube inserted into the gradient
A simple device fashioned from a cylindrical block of Perspex (Lucite or acrylic) shown in Figure 3 can be produced by any laboratory workshop. To fit flexible-walled tubes the cylinder should be slightly tapered towards the bottom (not shown in figure). The block contains a central channel, which leads to a hollowed-out cone, and a side-arm, which connects with the central channel. The dense unloading solution is introduced to the bottom via a long metal cannula inserted down the central channel and through the gradient (Figure 4). The gradient is displaced upwards by the incoming dense liquid into the cone and an O-ring around the cannula diverts the flow into the collection tubes via the side-arm.
- For rigid-walled open-topped tubes the collecting device requires sealing on to the tube with a gasket, under pressure. Such a device can indeed be used for any type of tube and one is incorporated as one of the options in the Beckman-Coulter Fraction Recovery System.
- By placing the dense unloading solution in a burette and delivering it to the bottom of the centrifuge tube via a peristaltic pump (Figure 4), the unloading process can be executed at a uniform flow rate
- By using the graduations on the burette to signal the manual advancement of the delivery tube to the next collection tube, it is the only method that guarantees equal volume fractions.
- The gradient could alternatively be collected using an automated fraction collector (see Sections 4g and 4h).
- The best unloading medium is a low viscosity, dense, non-water-miscible, fluorocarbon such as perfluorodecalin (ρ = 1.9 g/ml). This was previously commercially available from Axis-Shield and its distributors as Maxidens. Perfluorodecalin can currently be purchased from F2 Chemicals Ltd, Lea Lane, Lea Town, Preston PR4 0RZ, UK (tel: +44 (0)1772 775802, fax +44 (0)1772 775808); contact name Gerry May (gerry.may@fluoros.co.uk). Also available from the same company is a similar fluorocarbon containing a blue dye (Flutec-blue), which makes visual assessment of the progress of gradient unloading very easy.
- The rate of gradient unloading should be 1-2 ml/min for 10-20 ml gradients and 0.5-1.0 ml/min for smaller volume gradients.
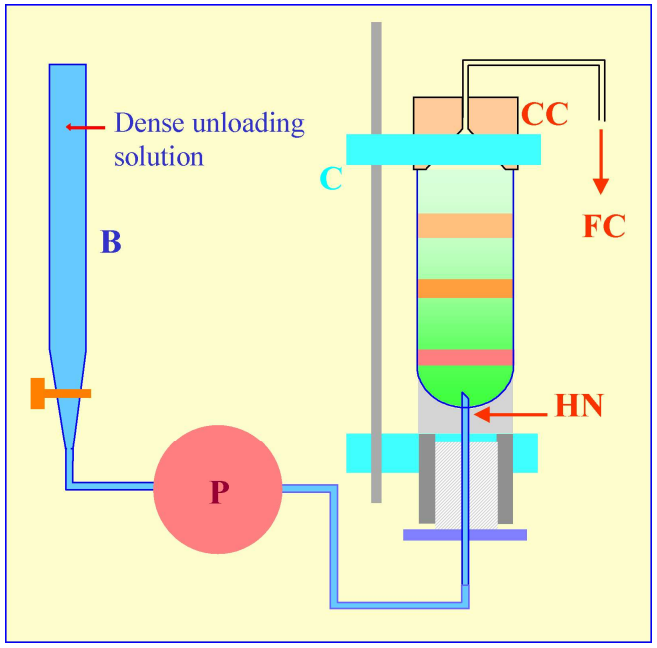 Figure 5: Gradient harvesting by upward displacement with a dense medium delivered by tube puncture. The hollow needle (HN) is completely filled with the dense unloading solution from the burette (B) using the peristaltic pump (P) before the tube is located within the clamping device of a Beckman-Coulter Fraction Recovery System. The conical collection head (CC) is located sealed on to the tube, and the tube held vertically, by the clamp (C). When the pump (P) is reactivated after puncturing the tube, the dense unloading solution displaces the gradient upwards through the conical collection head (CC) and into the fraction collection tubes (FC).
Figure 5: Gradient harvesting by upward displacement with a dense medium delivered by tube puncture. The hollow needle (HN) is completely filled with the dense unloading solution from the burette (B) using the peristaltic pump (P) before the tube is located within the clamping device of a Beckman-Coulter Fraction Recovery System. The conical collection head (CC) is located sealed on to the tube, and the tube held vertically, by the clamp (C). When the pump (P) is reactivated after puncturing the tube, the dense unloading solution displaces the gradient upwards through the conical collection head (CC) and into the fraction collection tubes (FC).
4d-2. Delivery of dense liquid by tube puncture
An alternative mode of delivering the dense unloading solution to the bottom of the tube is by tube puncture. In this case the burette is attached via the pump to the lower end of the hollow needle (Figure 5), which must be primed with the dense solution, prior to tube puncture. The hollow needle (HN) of the Beckman-Coulter Fraction Recovery System has an important design feature – the exit port is on the vertical side of the needle, thus its sharp point is solid. This not only facilitates tube puncture, fragments of tube material removed by the puncturing process or any pellet in the tube, are much less likely to impede the flow of the dense unloading solution than if the exit port was tip-located, as in a standard syringe needle.
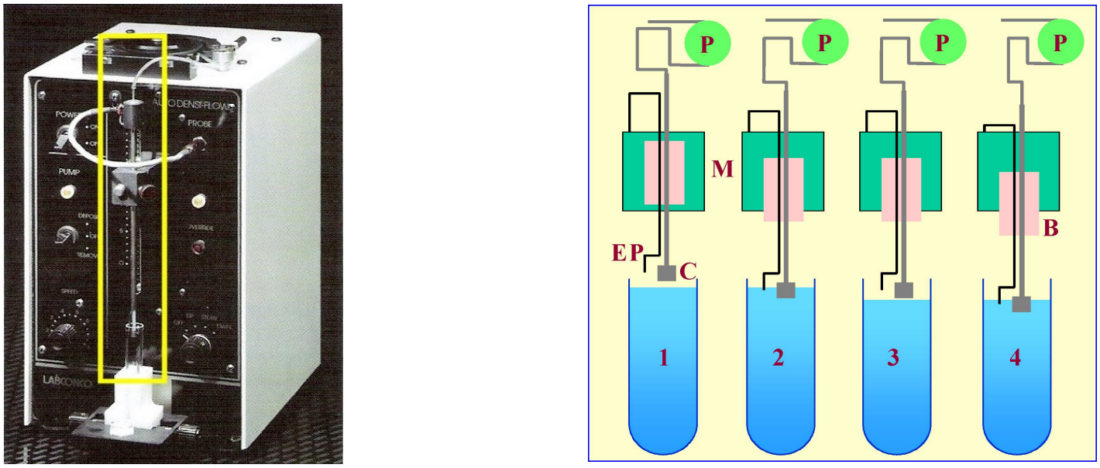
4e. Automatic aspiration from the meniscus
The Auto Densi-Flow™, produced by the Labconco Corporation comprises a hollow metal tube that terminates in a small collection head (Figures 6 and 7); the upper end of the tube is connected to a peristaltic pump, which aspirates the gradient. The motor, which is activated when the electronic probe (mounted at the side of the collection head) is in a non-conductive medium (air), advances the collection head towards the gradient until the tip of the probe reaches the meniscus of the gradient (Figure 7, 1 and 2). Now the tip of the probe is in an aqueous conductive medium, the motor stops and the gradient starts to be aspirated by the pump and the meniscus falls (Figure 7, 3). The motor is consequently re-activated as the meniscus recedes from the probe and the collection head advances further downwards until again the probe reaches the meniscus (Figure 7,4) and so on. For clarity, the procedure has been described and shown in Figure 7 in an exaggerated step-like manner. In reality, the aspiration of the gradient and the steady advance of the collection head occur almost simultaneously. In this way the entire gradient is collected in a smooth and continuous fashion.
- Note that the collection head of this device also provides an excellent means of depositing a continuous gradient, dense end first, from a two-chamber gradient maker. In this mode the motor moves the collection head upwards; the sequential activation and deactivation of the motor by the rising meniscus being the reverse of the collection mode.
- IMPORTANT NOTE: although this device is no longer produced by Labconco, many remain available in laboratories and second hand machines are available from instrument “recycling” companies.
4f. Biocomp Instruments piston fractionator
Rather different to the other types of fractionator, the Biocomp piston fractionator comprises a piston containing a central channel, which at its lower end expands outwards in the form of a curved conical section. As the piston advances down the tube, the gradient is displaced upwards into the central channel. The progressively decreasing volume of the conical section experienced by the displaced liquid effectively increases the linear separation of particles in the gradient and so maximises resolution. The device is available in conjunction with a detection system (and the Biocomp Gradient Master™ gradient former – see Application Sheet V02 Section 2c). The device is only suitable for open-topped tubes and tubes of different diameters require their own piston.
4g. Integrated automatic gradient harvesting process
In the system illustrated in Figure 8, the Labconco Auto Densi-flow gradient unloader is being used to harvest the gradient (from the meniscus) from a standard tube for a swinging-bucket rotor. The effluent from the peristaltic pump on top of the Auto Densi-flow is directed to the collection head of a Gilson FC205 fraction collector for dispensing into a 96-well polypropylene “MasterBlock DeepWell” plate (Greiner Bio-One Inc). These MasterBlocks can easily accommodate volumes of up to 2.0 ml. The multi-well plate format for gradient collection allows simple gradient analysis if the gradient fractions are subsequently sampled using a multiple channel automatic pipette (see below); it also provides an easy means of storage. A standard 96-well plate can replace the large-volume MasterBlock for the collection of smaller gradient volumes.
 Any gradient unloader that incorporates a peristaltic pump to maintain a reasonably consistent flow rate can be linked up to fraction collector, but note that as the density of the liquid progressively changes so do other physical parameters such as viscosity and surface tension. Drop size will thus vary during the collection process and fraction volumes will change progressively during a drop-counting collection process. So whether the fraction advance is signaled by drop number or time, there will be a progressive change in fraction volume whose severity depends on the density-range of the gradient. Nevertheless if this change is acceptable, it will at least be reproducible from gradient to gradient. This fraction collection system has been used very successfully for analysis of lipoprotein banding in self-generated iodixanol gradients [1,2].
Any gradient unloader that incorporates a peristaltic pump to maintain a reasonably consistent flow rate can be linked up to fraction collector, but note that as the density of the liquid progressively changes so do other physical parameters such as viscosity and surface tension. Drop size will thus vary during the collection process and fraction volumes will change progressively during a drop-counting collection process. So whether the fraction advance is signaled by drop number or time, there will be a progressive change in fraction volume whose severity depends on the density-range of the gradient. Nevertheless if this change is acceptable, it will at least be reproducible from gradient to gradient. This fraction collection system has been used very successfully for analysis of lipoprotein banding in self-generated iodixanol gradients [1,2].
4h. Influence of tube type on harvesting strategy
- Open topped thin walled tubes (polyallomer, polycarbonate or Beckman Ultraclear™) for swinging-bucket or fixed-angle rotors can be unloaded by any of the above methods.
- Thick-walled open-top tubes can be unloaded by any of the methods except tube puncture (Sections 4c and 4d-1b).
- Thick-walled tubes (screw-capped) with a wide shoulder are best unloaded from the meniscus (Section 4e) using the Labconco Auto Densi-flow or aspiration from the bottom (Section 4b). Upward displacement (Section 4d-1) may be satisfactory if the shoulder is narrow, sloped or rounded, otherwise material may get trapped at the shoulder.
- Heat-sealed or crimp-sealed tubes cannot be unloaded directly by any of the methods except tube puncture (Section 4c). Any other method of unloading requires the tube to be cut horizontally just below the shoulder (see Section 2). This is might cause disturbance to the gradient unless carried out very carefully.
- Sealed tubes that are sealed by a central plastic plug (e.g. Beckman Optiseal™ tubes) can be unloaded by any of the methods. Note however that upward displacement is best carried out using the Section 4d-2 option with a length of Teflon tubing secured to the neck of the tube by a silicone rubber collar to carry the gradient effluent to the collection tubes. Note also that the neck of some of the smaller volume sealed tubes may be too narrow to accept the collection head of the Labconco Auto Densi-flow machine (Section 4e).
OptiPrep™ Application Sheet V04; 8th edition, January 2020
OptiPrep™ Application Sheet V05
Analysis of gradients
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V-number.
1. Density determination
Once gradients have been fractionated, it is often important that the density of each fraction is measured accurately. The most direct method is to weigh accurately known volumes of liquid using a pycnometer; however, this is very time consuming and it is more convenient to determine the density of a fraction by measuring the refractive index, which has the added advantage of requiring as little as 20-50 µl of sample. The simple linear relationship between refractive index (η) and the density (ρ) is ρ = Aη – B. The refractive index of gradient solutions is increased by the presence of other solutes (e.g. sucrose and NaCl), thus the values of the two constants A and B vary with the presence and concentration of the solute.
For extensive tables relating % (w/v) concentration of iodixanol, density and refractive index of solutions used for the fractionation of viruses see Application Sheet V01.
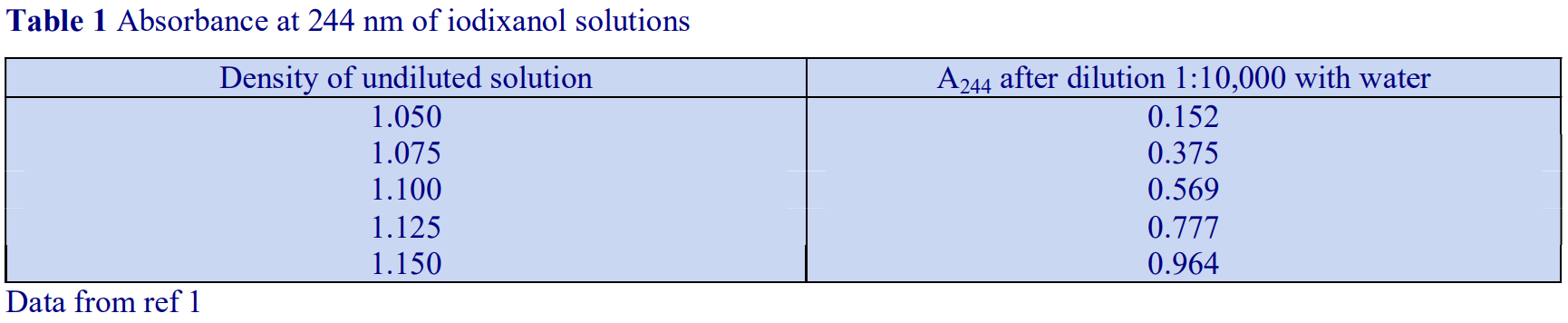
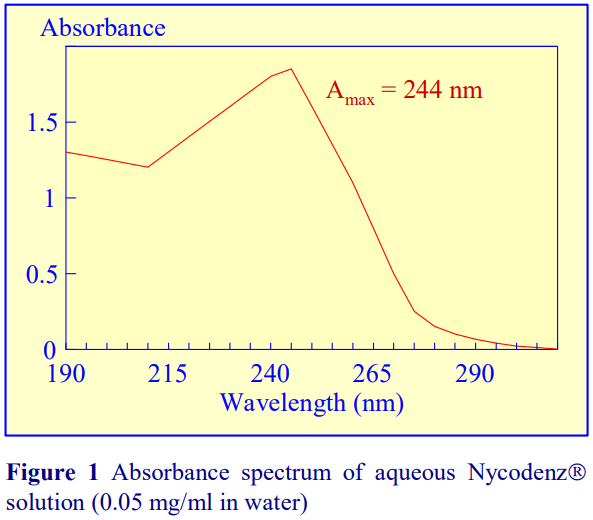 If a refractometer is not available then an alternative method of determining the density of gradient fractions is to measure the absorbance (optical density) of the fractions. All iodinated density gradient media absorb strongly in the UV (see Figure 1). If the absorbance is measured at approx 244 nm (the absorbance maximum for NycodenzⓇ and iodixanol) the gradient samples will need to be diluted 1:10,000 with water to get an absorbance value that can be measured accurately. Table 1 gives a few values for iodixanol solutions, measured in a standard 1 cm path length quartz cell in a single beam spectrophotometer. The need to dilute the solution also means that any other potentially interfering material will be diluted out at the same time.
If a refractometer is not available then an alternative method of determining the density of gradient fractions is to measure the absorbance (optical density) of the fractions. All iodinated density gradient media absorb strongly in the UV (see Figure 1). If the absorbance is measured at approx 244 nm (the absorbance maximum for NycodenzⓇ and iodixanol) the gradient samples will need to be diluted 1:10,000 with water to get an absorbance value that can be measured accurately. Table 1 gives a few values for iodixanol solutions, measured in a standard 1 cm path length quartz cell in a single beam spectrophotometer. The need to dilute the solution also means that any other potentially interfering material will be diluted out at the same time.
Alternatively, if the absorbance is measured at a higher wavelength, dilution is not required. Table 2 gives a few absorbance values for NycodenzⓇ solutions at 350 nm and 360 nm. Care must be taken to use the correct blank to ensure that other components in the gradient fractions that absorb at, or near these wavelengths do not interfere with the measurement of the gradient medium.
1a Absorbance measurements using a Multi-well Plate Reader
The wide availability of Multi-well Plate Readers which routinely have the facility for measurement of absorbance at 340 nm considerably simplify the measurement of absorbance on gradient fractions, particularly if the gradient has already been collected in a multi-well plate. Multiple-channel automatic pipettes also facilitate the transfer of liquid aliquots between plates.
1. Transfer 100 μl of each of the fractions into 100 μl of water in the wells of a second plate.
2. Complete the transfer and mixing by three repeated aspirations into and expulsions from the pipette tips.
3. Measure the absorbance of the solutions in each well in a standard plate reader using a 340 nm filter, against a suitable blank.
For iodixanol concentration above 35% (w/v), it may be necessary to make a second dilution of the solutions (again 100 μl into 100 μl of water) to avoid absorbance values above 1.2.
Six different types of multi-well plate have been tested for their suitability. A flat-bottomed 96- well polystyrene plate, which has the lowest background absorbance of any plate tested (approx 0.130 at 340 nm), is available from Greiner BioOne Inc (Cat. # 655101). The inter-well variability of the absorbance was also one of the lowest of all those tested (± 0.007).
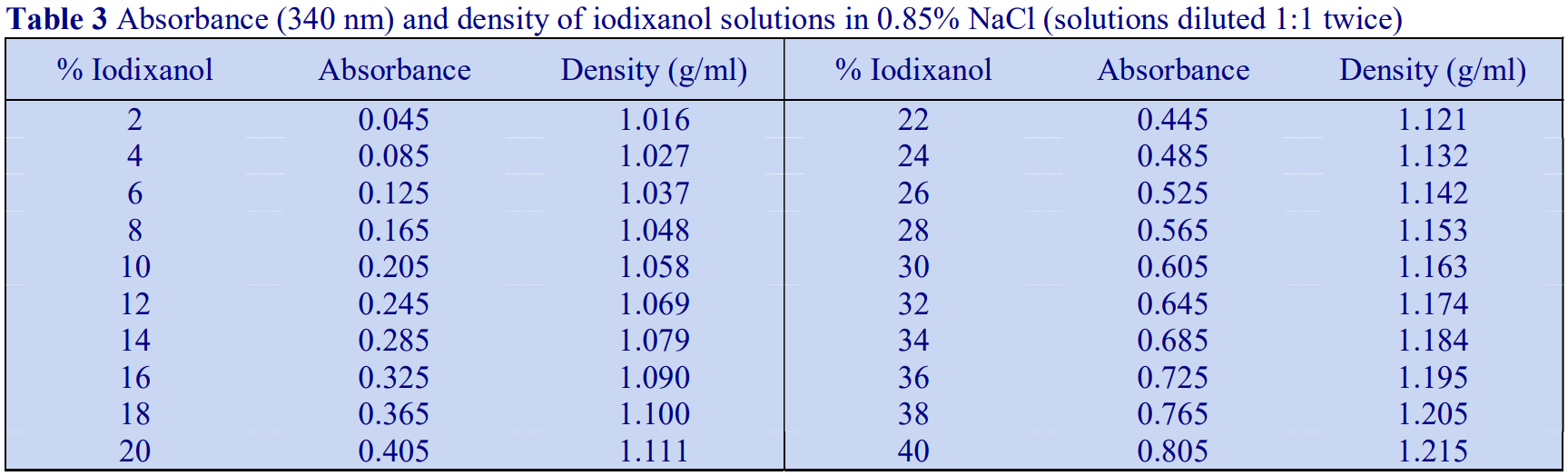
Absorbance values of a range of iodixanol solutions produce by dilution of OptiPrep™ with either saline or 0.25 M sucrose are given in Tables 3 and 4 respectively. The absorbance measurements were made against saline and 0.25 M sucrose blanks, which accounts for the slight distortion of the measured values of samples diluted with sucrose.
2. Particle detection
Although the quantitative distribution of cells through a gradient can be determined by using a haemocytometer or an electronic particle counter, turbidometric analysis is a more general method used for all types of light-scattering particles. Particulate matter can be detected and semi-quantified by light-scattering measurements at 500-600 nm, while particles containing macromolecules bearing porphyrin prosthetic groups (e.g. haem groups) can be monitored by Soret band absorbance at 400- 420 nm.
3. Nucleic acids, proteins and polysaccharides
Although solutions of iodinated media absorb strongly in the ultraviolet region of the spectrum, as their absorbance maximum is different to that of proteins and nucleic acids, it may be possible in some cases, through use of the correct blank (i.e. from a blank gradient unloaded in exactly the same manner as the test gradient) to determine their distribution spectrophotometrically. Normally however, nucleic acids, proteins and polysaccharides are assayed spectrophotometrically by chemical methods (Table 5 and ref 2). Unlike metrizamide, neither NycodenzⓇ nor iodixanol contain a sugar residue, therefore they do not interfere with the orcinol or diphenylamine reactions for the estimation of the ribose and deoxyribose of RNA and DNA respectively [3]; polysaccharides and sugars can be determined using the phenol/H2SO4 assay [4]. Sensitive dye binding assays for protein [5,6] and DNA [7] are also unaffected by the presence of the gradient media. Protein assays based on Coomassie blue give the most reliable data. The Folin Ciocalteu reagent [8] however cannot be carried out unless the concentration of NycodenzⓇ or iodixanol is less than 5% (w/v): this situation however can often be attained if the final assay volume is 1-2 ml and the volume of gradient fraction used is 50 µl. Even at higher concentrations of gradient medium, an appropriate correction can be made to produce a linear relationship between protein concentration and absorbance (Table 6 gives an example). In addition to these spectrophotometric methods, fluorimetric assays of nucleic acids [9,10] and proteins [11] can also be carried out in the presence of NycodenzⓇ or iodixanol. Many of these protocols are listed in ref 12.
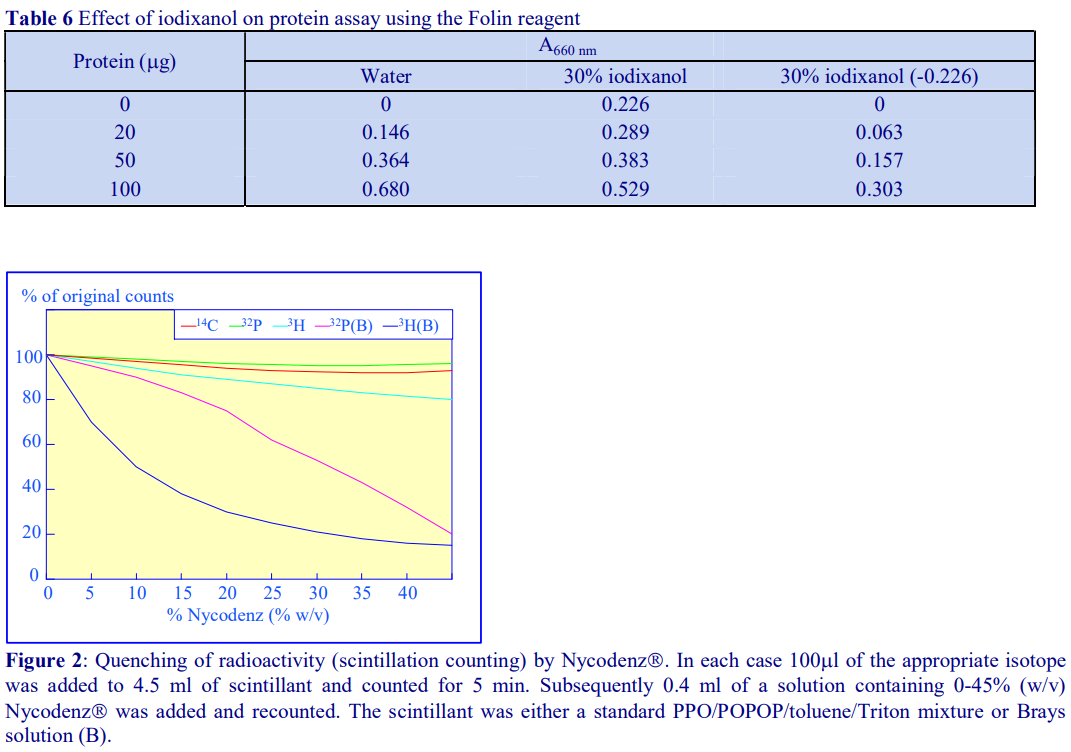
4. Radioactivity assays
Analysis of gradients material may either involve the radiolabeling of the material prior to fractionation or the use of radiolabeled reagents in functional assays. NycodenzⓇ and iodixanol quench 3H, 32P and 14C to an extent that is dependent on the energy of the emission, although, as shown in Figure 2, the degree of quenching is also dependent upon the scintillant used. Toluene-based scintillant, containing 4.0 g 2,5-diphenyloxazole (PPO) and 0.05 g 1.4-bis – 2(5-phenyloxazolyl) benzene, (POPOP) per litre and mixed with one half its volume of Triton X-100 is quite resistant to quenching, while Brays scintillant is much less suitable. The extent of quenching may be minimized by diluting the samples prior to counting, or it can be eliminated completely by acid precipitating the material in the gradient fractions and counting each precipitate after collection on filters and drying.
5. Electrophoresis
SDS-polyacrylamide and agarose gel electrophoresis can be carried out directly on gradient samples, as long as the concentration of protein or nucleic acid in the gradient fractions is sufficiently high for analysis. If the protein for example requires concentration, neither NycodenzⓇ nor iodixanol interfere with TCA precipitation.
6. Removal of gradient medium and concentration of particles
It may be necessary to remove either NycodenzⓇ or iodixanol from the gradient fractions either to concentrate the particles or if the medium does interfere with some add-on process. Viruses can be pelleted from fractions after dilution with 1-2 volumes of a low-density buffer such as a buffered salt or sucrose solution. Particles should be sedimented at either a slightly higher RCF and/or longer centrifugation time than that used to pellet the particles from the low-density solution itself, to take account of the slightly raised density and viscosity caused by the presence of the gradient medium. RCFs in excess of 150,000g should be avoided for iodixanol-containing solutions; otherwise there may be some sedimentation of the solute molecule itself.
Removal of iodixanol and NycodenzⓇ from gradient samples containing virus is best achieved by filtration through microcentrifuge ultrafiltration cones such as those manufactured by Whatman
(VectaspinⓇ) or Millipore (AmiconⓇ Ultra 4). Successful use of two other membrane devices has been reported in the literature – Vivaspin membranes from Sartorius and Centricon Plus 70 centrifugal filters from Millipore, or a PBHK Centrifugal Plus-20 filter unit with an Ultracel PL membrane (100 kDa cut off). An alternative is dialysis in large-pore size tubing or in a GeBAflex dialysis tube (Gene Bio Applications (GeBA) Ltd.). The latter are certainly more convenient than dialysis tubing for small
volumes, the tubes are available with 0.25, 0.8 and 3.0 ml capacities and MWt cut-offs up to 14,000. Tangential flow filtration is also an effective alternative. Passage down a column of Sephadex G25 is another possibility.
7. References
1. Schroeder, M., Schafer, R. and Friedl, P. (1997) Spectrophotometric determination of iodixanol in subcellular fractions of mammalian cells Anal. Biochem., 244, 174-176
2. Rickwood, D., Ford, T. and Graham, J. (1982) Nycodenz: A new nonionic iodinated gradient medium Anal. Biochem., 123, 23-31
3. Schneider, W.C. (1957) Determination of nucleic acids in tissues by pentose analysis Meth. Enzymol., 3, 680-684
4. Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.E. and Smith, F. (1956) Colorimetric method for determination of sugars and related substances Anal. Chem., 28, 350-356
5. Schaffner, W. and Weissman, C. (1973) A rapid, sensitive, and specific method for the determination of protein in dilute solution Anal. Biochem., 56, 502-510
6. Bradford, M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding Anal. Biochem., 72, 248-254
7. Peters, D.L. and Dahmus, M.E. (1979) A method of DNA quantitation for localization of DNA in metrizamide gradients Anal. Biochem., 93, 306-311
8. Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with the Folin phenol reagent J. Biol. Chem., 193, 265-275
9. Fong, J., Schaffer, F.L. and Kirk, P.K. (1953) The ultramicrodetermination of glycogen in liver. A comparison of the anthrone and reducing-sugar methods Arch. Biochem. Biophys., 45, 319-326
10. Karsten, U. and Wollenberger, A. (1977) Improvements in the ethidium bromide method for direct fluorometric estimation of DNA and RNA in cell and tissue homogenates Anal. Biochem., 77, 464-469
11. Bohlen, P., Stein, S., Dairman, W. and Udenfriend, S. (1973) Fluorometric assay of proteins in the nanogram range Arch. Biochem. Biophys., 155, 213-220
12. Ford, T. and Graham, J.M. (1983) Enzymatic and chemical assays compatible with iodinated density gradient media In: Iodinated Density Gradient Media – a practical approach (ed D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 195-216
OptiPrep™ Application Sheet V05; 5th edition, January 2020
OptiPrep™ Application Sheet V06
Purification of viruses and viral vectors using OptiPrep™
- This OptiPrep™ Application Sheet summarizes the range of methods, using OptiPrep™, for the purification of viruses and the principal viral vectors involved in studies on the transduction of cells and tissues
1. Comparison with other density gradient media
Compared to CsCl and sucrose there are procedural advantages to the use of OptiPrep™:
- OptiPrep™ is a sterile solution of 60% (w/v) iodixanol; it is simply diluted with saline to prepare sterile gradient solutions. It is the only gradient medium manufactured under strict FDA and EU cGMP compliance.
- CsCl and sucrose are both toxic to cells.
- Iodixanol is non-toxic to cells; it has very low endotoxin levels (<1 EU/ml); measured levels on each batch are usually <0.13 EU/ml.
- CsCl must be removed prior to HPLC or gel electrophoresis; iodixanol rarely needs removing prior to further processing, except maybe for some electron microscopy studies.
- CsCl gradients lead to major reductions in viral infectivity. Virus from iodixanol gradients shows a higher % recovery of infectivity and much lower average particle/infectivity ratios compared to that from CsCl gradients.
- Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless have serious effects on viral structure; in particular the loss of surface glycoproteins from enveloped viruses [1].
2. Practical considerations in the selection of a gradient technology
2a. Pre-gradient procedures
2a-1. Pelleting
The choice of a gradient method must take into consideration the often large volumes of culture fluid that require processing. This is particularly the case of large-scale viral vector production. After clarifying the suspension (removal of cells and large cellular fragments) using low speed centrifugation (2,000-4,000 g for 15- 20 min) or passage through a suitable filter (pore size 0.2-0.45 μm), the simple practice of pelleting the virus at 50,000-150,000 g for 1-4 h, can often lead to a serious loss of infectivity. Nevertheless, this remains a common approach using either sucrose or CsCl gradients, prior to loading the resuspended virus pellet on top of the gradient. Further loss of infectivity may be caused by the liquid shearing forces required to resuspend the virus pellet; this can however be reduced by allowing the pellet to disperse in a suitable medium overnight.
If the virus-containing suspension is a cell lysate containing subcellular organelles, the initial clarification step will remove large debris and nuclei. A 0.2 μm filter will remove many of the larger organelles (mitochondria, lysosomes, peroxisomes etc). The subsequent virus pelleting conditions will co-sediment most of the microsomal membrane vesicles, except perhaps at lower g-forces and times (e.g. 50,000 g for 1.5 h), which may be insufficient to pellet the smallest vesicles.
It is not entirely clear whether the loss of infectivity that occurs during pelleting is due to the aggregation of the viral particles, the high hydrostatic pressure at the bottom of the tube or the shearing forces that are necessary to disperse the virus pellet after the centrifugation, or a combination of all three effects.
- In order to overcome the shearing force problems some workers have allowed pelleted Semliki Forest virus to disperse itself in a buffered saline solution at 4°C overnight.
2a-2. Low-density barriers
A commonly used alternative to direct pelleting from the virus-containing fluid is the use of a low-density barrier through which the virus is pelleted. A variety of types of barrier have been used, which traditionally were 15-20% (w/v) sucrose or, occasionally, 30%. More recently, these have been replaced with 5-15% (w/v) iodixanol barriers and since an iodixanol gradient is used in the subsequent purification, then it makes for good practice to expose the virus to just one type of gradient solute. The g-forces and centrifugation times are similar to those used in direct pelleting, i.e. generally 50,000-160,000 g for 1-2 h. This barrier technique will allow some preliminary purification from soluble proteins and from small low-density vesicles. The virus pellet can be resuspended in a solution of any density for further purification.
2a-3. High-density barriers
Sedimentation of the virus on to a small volume of a dense solution (cushion) considerably reduces this loss of activity but requires careful recovery of the virus if it is to be layered on top of a subsequent gradient. These problems are abrogated if the virus is subsequently bottom-loaded rather than top-loaded. Commonly the cushion is either 50% (w/v) iodixanol or pure OptiPrep™. With sucrose and CsCl gradients: because of their high osmolality; the banding density of the virus will depend on the mode of loading.
If the virus is subsequently to be loaded on top of a pre-formed continuous or discontinuous gradient then there are some limitations to the cushion-banding technique that need to be considered, if for example the iodixanol concentration at the top of the subsequent gradient is, for example 6% (w/v). Even with just 0.5 ml of a 50% (w/v) iodixanol cushion, mixing the residual contents of the tube after removal of the majority of the supernatant would require at least 5 ml of supernatant to reduce the iodixanol concentration to <5% iodixanol. In this situation therefore it is important to remove as much as the cushion as possible before harvesting the virus band.
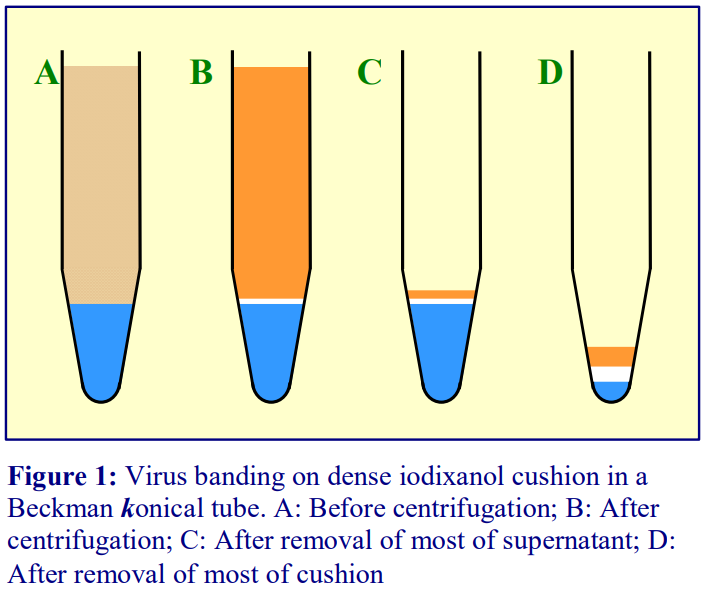 Conical tubes facilitate this process, and Beckman manufacture konical tubes for all their swinging-bucket rotors. Small volumes of cushion occupy a greater linear height in a conical tube than in the traditional round-bottomed ultracentrifuge tube and most of this cushion can also be more easily removed prior to harvesting the virus band in a small volume of supernatant. A thin metal cannula or length of Teflon tubing, attached to a syringe may be used to remove as much of the cushion as possible after centrifugation.
Conical tubes facilitate this process, and Beckman manufacture konical tubes for all their swinging-bucket rotors. Small volumes of cushion occupy a greater linear height in a conical tube than in the traditional round-bottomed ultracentrifuge tube and most of this cushion can also be more easily removed prior to harvesting the virus band in a small volume of supernatant. A thin metal cannula or length of Teflon tubing, attached to a syringe may be used to remove as much of the cushion as possible after centrifugation.
Coleman et al [2] only used 0.22 ml of cushion and removed all of the supernatant (except for the last 0.22 ml) and then harvested all of the remaining liquid (including the cushion) and diluted the suspension 2.5x with buffer before centrifuging it at 6000 g for 24 h at 4°C to pellet HIV-1. Since the final volume of suspension was very small, it was possible to pellet the virus efficiently at this very gentle g-force. This would not have been feasible with a large volume of virus suspension, because of the longer sedimentation path length and low gforce at the top of the sample.
If the concentrated virus is to be loaded in a dense solution beneath a pre-formed gradient for a separation on the basis of its buoyant density then the need to eliminate as much of the cushion as possible does not apply.
Because iodixanol gradients can be made more or less isoosmotic over the entire density range (unless the ionic strength of the gradient, or part of the gradient is deliberately raised) the banding density is considerably less dependent on the mode of loading. Bottom loading of the virus sample has one particular advantage: any soluble proteins remaining in the virus suspension will remain in the load zone at the bottom of the tube rather than sediment in the same direction as the virus in the top-loaded version
3. Modern gradient loading alternatives
Some of the modern gradient loading alternatives with OptiPrep™ are shown in Figures 2-4. Figure 2 (see next page) describes a method devised by Adair et al [3] for the purification of hepatitis C virus in which a suspension of virus in saline was layered over virus in 40% (w/v) iodixanol (A). After allowing the iodixanol to diffuse for 12 h at 4°C; the end-result was a 0-40% (w/v) linear gradient of iodixanol in which the virus was dispersed (B); this was then centrifuged for 12 h to band the virus isopynically (C). The lack of any interfaces and the initial low concentration of the virus minimises any potential interaction between the virus particles and contaminants.
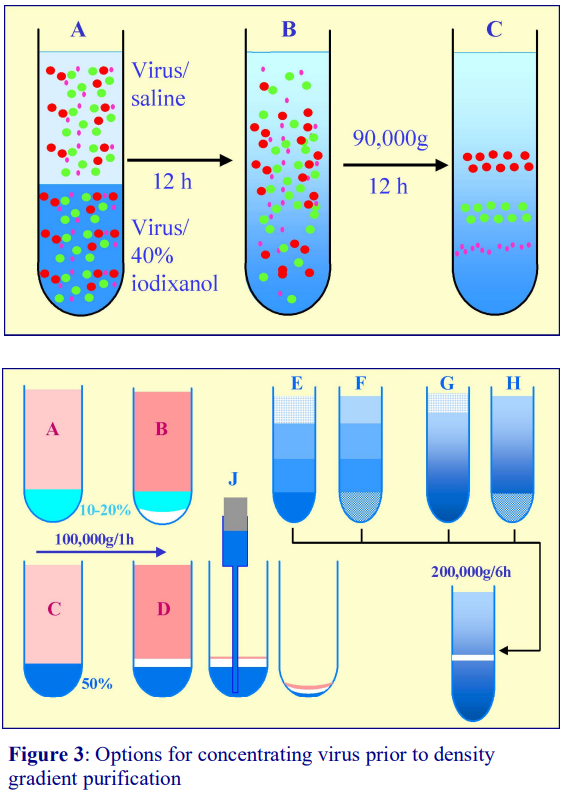 Figure 2: Purification of hepatitis C virus in a linear iodixanol gradient (Adair et al: see ref 3).
Figure 2: Purification of hepatitis C virus in a linear iodixanol gradient (Adair et al: see ref 3).
Figure 3 summarizes some of the options for concentration of a virus prior to using either a continuous or discontinuous iodixanol gradient for purification. In the initial centrifugation, the virus is layered over a small volume of iodixanol solution which is either less (A) or more (C) dense than the virus. After centrifugation the virus will sediment to form a pellet (B) below the less dense barrier or band at the interface of the denser barrier (D). In the first option the virus will be largely separated from soluble proteins and slowly sedimenting material, but may lose infectivity due to the pelleting. In the second option the virus will retain higher infectivity and bottom-loading of the virus under a subsequent discontinuous or continuous gradient (F,H) is the obvious method of choice for the second stage purification. Pelleting the virus (B) allows either top-loading or botttom-loading of the subsequent gradient, since the entire liquid phase above the pellet can be aspirated. To top-load the subsequent gradient (E,G) after concentrating the virus on a dense cushion (D) as much of the dense liquid as possible must be removed. A flat-tipped metal filling cannula attached to a syringe (J) is best suited to this task. The use of Beckman “konical” tubes are also an advantage.
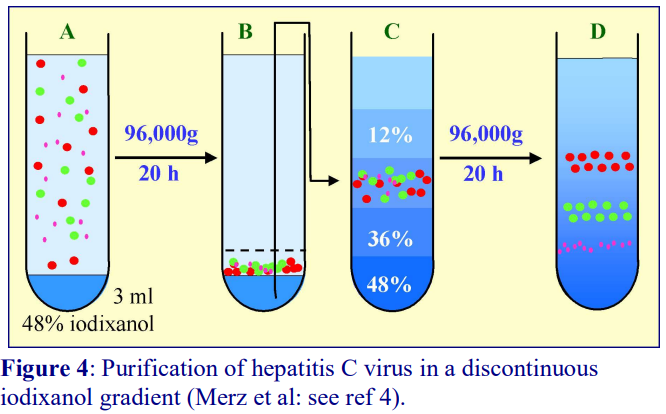 Figure 4 describes a novel way of overcoming the problems of handling virus that has been concentrated on to a dense cushion of iodixanol. It was devised by Merz et al [4] for hepatitis C virus, which is firstly sedimented from a clarified culture medium on to 3 ml of a 48% (w/v) solution of iodixanol (density 1.257 g/ml). The virus suspension was first layered over a 3 ml cushion of 48% iodixanol, then after centrifugation at 96,000 g overnight. The use of a relatively low g-force for a long time period will minimize any tendency for the virus to aggregate, but 2-3 h at 200,000 g would be an alternative. Then 6 ml was aspirated from the bottom of the tube (i.e. the virus was now suspended in 24% (w/v) iodixanol and this was made part of a discontinuous gradient and recentrifuged for a further 20 h to band the virus according to its density. Again the use of relatively low g-forces minimizes the effect on virus infectivity.
Figure 4 describes a novel way of overcoming the problems of handling virus that has been concentrated on to a dense cushion of iodixanol. It was devised by Merz et al [4] for hepatitis C virus, which is firstly sedimented from a clarified culture medium on to 3 ml of a 48% (w/v) solution of iodixanol (density 1.257 g/ml). The virus suspension was first layered over a 3 ml cushion of 48% iodixanol, then after centrifugation at 96,000 g overnight. The use of a relatively low g-force for a long time period will minimize any tendency for the virus to aggregate, but 2-3 h at 200,000 g would be an alternative. Then 6 ml was aspirated from the bottom of the tube (i.e. the virus was now suspended in 24% (w/v) iodixanol and this was made part of a discontinuous gradient and recentrifuged for a further 20 h to band the virus according to its density. Again the use of relatively low g-forces minimizes the effect on virus infectivity.
3. Purification of rAAV and parvovirus vectors
There are two methods (see Figure 5) for the purification of rAAV vectors and each has its own advantages. The continuous gradient (Figure 5a) was designed for a near-vertical rotor and it has the great merit of ease of setting up, but the rAAV may be less well resolved from low MWt soluble proteins. The lack of any interfaces will however minimise any particulate aggregation. Hermens et al [5] compared iodixanol and CsCl gradients for rAAV purification and found that both % recovery and infectivity were considerably better with an iodixanol gradient. The more widely-used discontinuous gradient (Figure 4b) developed by Zolotukhin et al [6] was designed for a 39 ml fixed-angle rotor. Large-volume swinging-bucket rotors are not normally capable of achieving 350,000 g, with such a rotor the centrifugation time would need to be increased. Any soluble proteins band well away from the rAAV [6]. The NaCl in the 15% (w/v) iodixanol minimises any association between the rAAV and these proteins. Zolotukhin et al [6] observed that OptiPrep™ routinely produced more than 50% recovery of rAAV, which was more than 99% pure. Moreover the rAAV product had particle-to-infectivity ratios of less than 100 – significantly better than conventional methods. Both methods also permit complete purification of rAAV in 1 working day.
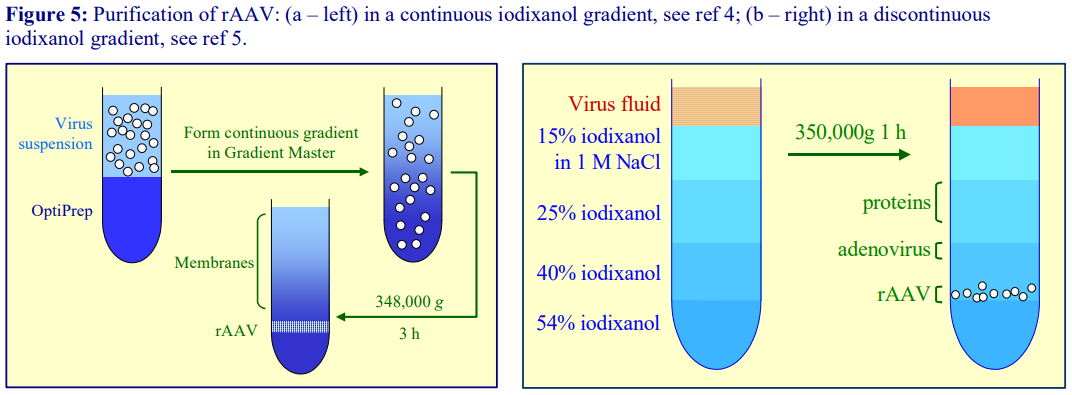
- Parvovirus vectors have also generally been purified using the discontinuous iodixanol gradient method.
4. Purification of adenovirus vectors
In 2005 the discontinuous iodixanol gradient described in Figure 5b was first reported for the purification of adenovirus vectors by Manninen et al [7]. The gradients are centrifuged in an approx. 12 ml swinging-bucket rotor at 100,000 g for 6 h. Later Arpiainen et al [8] used a modified gradient of 15%, 30% and 40% (w/v) iodixanol with centrifugation at 100,000 g for 14-16 h.
5. Self-generated iodixanol gradients
The ability of iodixanol to form self-generated gradients (see OptiPrep™ Application Sheet V03 for more details) considerably simplifies the process of purifying viruses. Herpes simplex virus (HSV) was the first virus to be purified in self-generated gradients of iodixanol and it is now a very widely used method for HSV vectors and other viruses. Self-generated gradients make sample handling very easy; the virus is first sedimented on to a 50% (w/v) iodixanol cushion (1-2 in Fig. 6). The gforce will depend on the virus. All of the supernatant is discarded (3) except for a volume equal to that of the cushion. After mixing the remaining liquids – the virus suspension (now in 25% iodixanol) is transferred to a tube for a vertical or near-vertical rotor and centrifuged at approx. 350,000 g for 1.5-2.5 h (4-5). During this time the gradient forms and the virus moves to its banding density. As with the continuous gradient method for rAAV (see Section 3, above) there are no interfaces to produce particulate aggregation. The shape of the density profile changes gradually from 1 to approx. 4.5 h, after which the profile is more or less stable. Figure 7 compares infectivity and density profiles after centrifugation of a suspension of HSV in 25% (w/v) iodixanol in a Beckman VTi65.1 vertical rotor after centrifugation at 350,000 g for 1.5 and 2.5 h. The shallower S-shaped gradient formed after 1.5 h allows a better separation of the lighter immature virus.
- Some short path-length small-volume fixed-angle rotors can replace the vertical or near-vertical rotors that are usually used for the construction of self-generated gradients.
6. Sedimentation velocity gradients
The final type of iodixanol gradient that was developed primarily for HIV by Dettenhoffer and Yu [9] comprises an essentially linear gradient, usually covering the range 6-18% (w/v) iodixanol, in which the sample is top-loaded. It is a sedimentation velocity gradient, which removes the more slowly sedimenting Vif gene,
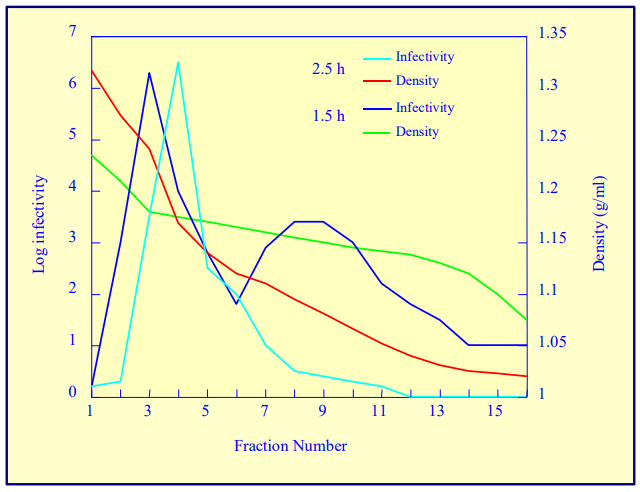 Figure 7. Purification of Herpes simplex virus in a self-generated iodixanol gradient. Effect of time of centrifugation at 350,000g in a vertical rotor; iodixanol starting concentration = 25% (w/v).
Figure 7. Purification of Herpes simplex virus in a self-generated iodixanol gradient. Effect of time of centrifugation at 350,000g in a vertical rotor; iodixanol starting concentration = 25% (w/v).
soluble proteins and microvesicles. Importantly the latter includes the exosomes that are often essential to resolve from the intact virus. Its use has been extended to several other viruses of similar structure.
7. Comments from the lierature
Lock et al [10] noted that yields are routinely greater than 1×1014 genome copies per run, with capsid protein purity exceeding 90%. Improved transduction both in vitro and in vivo was observed when compared with small-scale, CsCl gradient-purified vectors. Moreover the OptiPrep™ method effectively separates infectious particles from empty capsids.
Segura et al [11] observed that the low viscosity and isoosmotic nature of iodixanol solutions helps preserve virus particle integrity and functionality. The authors stated that the low toxicity compared to CsCl allows for direct in vitro or in vivo experimentation directly without the need to remove the iodixanol. Buclez et al [12], by modifying the standard discontinuous iodixanol gradient for the purification of rAAV, noted that by combining this with an earlier tangential flow filtration, “it was possible to purify several litres of crude lysate produced by baculovirus expression vector system in only one working day”.
Ammersbach, M. and Bienzle, D [13] noted that the iodixanol sedimentation velocity gradient, first introduced by Detenhoffer and Yu [9] for HIV purification, had the advantages of being both isoosmotic and low viscosity. It was less damaging to viruses and cells, required shorter centrifugation times and subsequent electrophoresis or HPLC could be executed without dialysis. Kol et al [14] also noted that the morphological and mechanical properties are far better preserved in iodixanol than in sucrose gradients.
8. OptiPrep™ Application Sheets/Reference Lists
To access other Application Sheets return to the 2020Virapp file and select the appropriate Vnumber. Comprehensive lists of references describing the use of OptiPrep™ methods are listed under “Reference Lists”.
9. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Coleman J.E., Huentelman, M.J., Kasparov, S., Metcalfe, B.L., Paton, J.F.R., Katovich, M.J., SempleRowland, S.L. and Raizada, M.K. (2003) Efficient large scale production and concentration of HIV-1- based lentiviral vectors for use in vivo Physiol. Genomics, 12, 221-228
3. Adair, R., Patel, A.H., Corless, L., Griffin, S., Rowlands, D.J. and McCormick, C.J. (2009) Expression of hepatitis C virus (HCV) structural proteins in trans facilitates encapsidation and transmission of HCV subgenomic RNA J. Gen. Virol., 90, 833–842
4. Merz, A., Long, G., Hiet, M-S., Brügger, B., Chlanda, P., Andre, P., Wieland, F., Krijnse-Locker, J. and Bartenschlager, R. (2011) Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome J. Biol. Chem., 286, 3018-3032
5. Hermens, W.T.J.M.C., Ter Brake, O., Dijkhuizen, P.A., Sonnemans, M.A.F., Grimm, D., Kleinschmidt, J.A. and Verhaagen, J. (1999) Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous Human Gene Ther., 10, 1885-1891
6. Zolotukhin, S., Byrne, B.J., Mason, E., Zolotukhin, I., Potter, M., Chesnut, K., Summerford, C., Samulski, R.J. and Muzyczka, N. (1999) Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield Gene Ther., 6, 973-985
7. Manninen, A., Verkade, P., Le Jay, S., Torkko, J., Kasper, M., Fullerkrug, J. and Simons, K. (2005) Caveolin-1 is not essential for biosynthetic apical membrane transport Mol. Cell Biol., 25, 10087-10096
8. Arpiainen, S., Järvenpää, S-M., Manninen, A., Viitala, P., Lang, M.A., Pelkonen, O. and Hakkola, J. (2008) Coactivator PGC-1α regulates the fasting inducible xenobiotic-metabolizing enzyme CYP2A5 in mouse primary hepatocytes Toxicol. Appl. Pharmacol., 232, 135-141
9. Dettenhofer, M. and Yu, X.F. (1999) Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif virions J. Virol., 73, 1460-1467
10. Lock, M., Alvira, M., Vandenberghe, L.H., Samanta, A., Toelen, J., Debyser, Z. and Wilson, J.M. (2010) Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale Hum. Gene Ther., 21, 1259–1271
11. Segura, M.M., Kamen, A.A. and Garnier, A. (2011) Overview of current scalable methods for purificationn of viral vectors In, Viral Vectors for Gene Therapy: Methods and Protocols, Methods in Molecular Biology, 737 (eds. Merten O.W. and Al-Rubeai, M.) Springer Science+Business Media, pp 89-116
12. Buclez, P-O., Florencio, G.D., Relizani, K., Beley, C., Garcia, L. and Benchaouir, R. (2016) Rapid, scalable, and low-cost purification of recombinant adeno-associated virus produced by baculovirus expression vector system Mol. Ther. Meth. Clin. Dev., 3: 16035
13. Ammersbach, M. and Bienzle, D. (2011) Methods for assessing feline immunodeficiency virus infection, infectivity and purification Vet. Immunol. Immunopathol.,143, 202– 214
14. Kol, N., Tsvitov, M., Hevroni, L., Wolf, S.G., Pang, H-B., Kay, M.S. and Rousso, I. (2010) The effect of purification method on the completeness of the immature HIV-1 Gag shell J. Virol. Methods 169, 244–247
OptiPrep™ Application Sheet V06: 6th edition, January 2020
OptiPrep™ Application Sheet V07
Purification of Group I (ds)DNA viruses: adenovirus and removal of helper virus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- Whether this method can be applied to other Adenoviridae members with similar morphology, macromolecular composition and size can only be determined experimentally
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
- See Section 6 for details of separation of helper-dependent adenoviral vector from the helper virus.
- A short bibliographical review of the literature is given in Sections 7 and 8.
- See Section 9 for publications from 2018
1. Background
Adenovirus is important on two accounts, firstly most children by the age of 15 have been infected with at least one type of adenovirus and it is responsible for a wide variety of upper respiratory tract infections. Secondly it is a popular choice as a viral vector for potential use in gene therapy. The latter in particular would clearly benefit from isolation methods which are both effective and cause little or no damage to the viral particles. Density gradient centrifugation has always played an important part in the concentration and purification of virus particles but the gradient media that have been used most prominently, sucrose and CsCl, pose a number of problems. Both media are highly hyperosmotic at the densities used to band viruses (sucrose solutions are also very viscous) and generally have to be removed either by pelleting the virus or by dialysis, prior to further processing or analysis. CsCl also leads to poor recoveries and low infectivity of adenovirus isolates.
Because of the very low water activity of CsCl solutions, viruses tend to have significantly higher density in this medium compared to media such as sucrose or any of the iodinated density gradient media, although the magnitude of this difference varies from virus to virus. Many viruses in CsCl have a density of approx 1.34 g/ml, in iodixanol the density range is generally 1.16-1.22 g/ml, although some viruses may be as low as 1.14 g/ml or as high as 1.24 g/ml.
OptiPrep is widely regarded as the gradient medium of choice for the purification of other vectors such as rAAV and there is huge bibliography of papers reporting the use of OptiPrep. Now the method has been extended to adenovirus [1], again with major increases (five- to tenfold) in infectivity titer over CsCl [2]. Infectivity measurements and many add-on techniques can be carried out without the need to dialyze the medium. The following methodology is adapted from ref 2.
- See Note 1 for details of a modified gradient used by Arpiainen et al [3]
 2. Solutions required
2. Solutions required
A. OptiPrep™
B. 10xPhosphate-buffered saline containing 10 mM MgCl2 and 25 mM KCl (10xPBS-MK)
C. Phosphate-buffered saline containing 1 mM MgCl2 and 2.5 mM KCl (PBS-MK)
D. 2 M NaCl in PBS-MK
E. Working solution of 54% (w/v) iodixanol in PBS-MK: mix 9 vol of OptiPrep with 1 vol of Solution B.
3. Ultracentrifuge rotor requirements
Swinging-bucket rotor with approx 14 ml tubes, e.g. Beckman SW41Ti or SW40Ti
4. Protocol
1. Prepare the following gradient solutions: 15% (w/v) iodixanol containing 1 M NaCl in PBS-MK: 1.5 vol. of Solution E + 2.7 vol. of Solution D + 1.2 vol of Solution C. 25% (w/v) iodixanol in PBS-MK: 2.5 vol. of Solution E + 2.9 vol. of Solution C 40% (w/v) iodixanol in PBS-MK: 4.0 vol. of Solution E + 1.4 vol. of Solution C.
2. Disrupt the virus-containing cells by 4 cycles of freeze-thawing: snap-freeze the cells in liquid N2 or dry ice-methanol; thaw at room temperature, then vortex the suspension twice for 30 sec, with a 15 sec rest on ice between each vortexing.
3. Clarify the cell lysate clarified by centrifugation at 2000 g for 10 min.
4. Underlayer 4 ml of clarified lysate with 1 ml of 15% iodixanol; 3 ml of 25% iodixanol, 3 ml of 40% iodixanol and 1 ml of 54% iodixanol working solution (see Figure 1 and Notes 2 and 3).
5. Fill the tubes with a small volume of low-density mineral oil on the top and centrifuge at 100,000 gav for 6-16 h at 4°C (see Notes 4-7).
6. Either collect the whole gradient (see Figure 1) in 1 ml fractions dense end first or use a syringe (attached to a 22 gauge needle) inserted just beneath the adenovirus band to remove the band directly in 0.5-1.0 ml (see Notes 8 and 9).
5. Notes
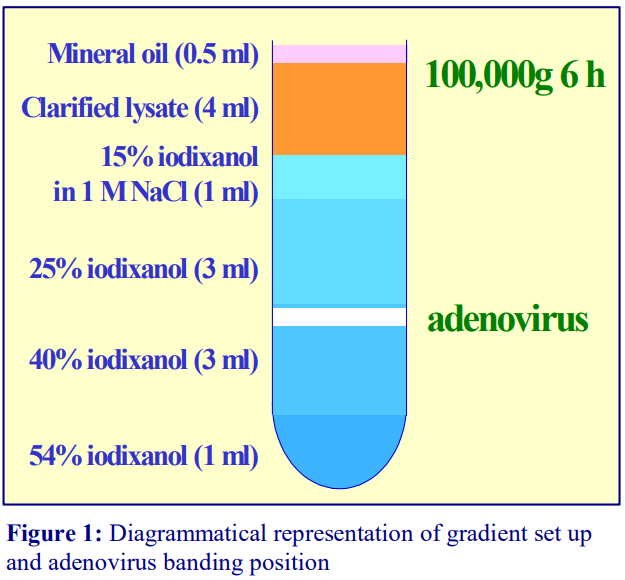 1. Arpiainen et al [3] used a modified gradient of 15%, 30% and 40% (w/v) iodixanol with centrifugation at 100,000 g at 4°C for 14-16 h.
1. Arpiainen et al [3] used a modified gradient of 15%, 30% and 40% (w/v) iodixanol with centrifugation at 100,000 g at 4°C for 14-16 h.
2. For larger or smaller volume tubes scale up or down the volume of the layers and sample proportionally. Peng et al [4] used 6.5 ml of the clarified lysate, 2 ml each of the 15%, 25% and 40% iodixanol solutions and 0.5 ml of 54% iodixanol.
3. Peng et al [4] created the gradient by underlayering the sample, starting with the most dense solution. This is the reverse of the more usual underlayering strategy (i.e. starting with the least dense solution). For information on preparing discontinuous gradients see Application Sheet V02.
4. Six hours is the minimum requirement at 100,000g. Although many swinging-bucket rotors are capable of higher g-forces, this relatively low g-force may minimize aggregation of the virus particles at the first interface.
5. Soluble proteins band broadly in the 25% iodixanol layer and their encroachment of the adenovirus band might be reduced by centrifuging for 6 h rather than 16 h.
6. The adenovirus bands at the top of the 40% iodixanol layer, sometimes as a doublet. All of the contaminating proteins in the lysate band broadly in the 25% iodixanol and may reach the 25%/40% iodixanol interface.
7. Peng et al [4] used approx. 200,000 g for only 1 h. Under these conditions the adenovirus bands at the 25%/40% interface [4].
8. For more information on the collection of gradients and the recovery of banded material see Application Sheet V04.
9. In many cases it is unnecessary to remove the iodixanol before processing the sample further. If it is however a requirement of any downstream procedures, size exclusion chromatography is often the method of choice [4]. For more information see Application Sheet V05.
6. Separation of helper-dependent adenoviral vector from the helper virus
An excellent example of use the very shallow iodixanol gradients that can be formed by self generation in a vertical rotor was devised by Dormond et al [5] for the separation of helper-dependent adenoviral vectors (HDV) from the helper virus (HV). Very often the difference in density between these two types of particle is very small and only a self-generated gradient is able to offer an easy solution to the problem of their discrimination. In this case the difference in banding position differed by little more than 0.01 g/ml, yet the peak separation of the sharply banding particles was three fractions. The authors reported that by using two rounds of gradient centrifugation the helper virus contamination was reduced from 2.57% to 0.03%. The absence of any interface where particles could aggregate is clearly a big benefit in the use of self-generated gradients. In the method, the virus suspension was adjusted to 38.7% (w/v) iodixanol and centrifuged (in approx 13 ml tubes) at 180,000 g
for 3 h at 4°C in a vertical rotor. The HV banded at 40.3-41.6% iodixanol, while the HDV banded at 37.6-39.4% [6]. Iodixanol was removed by size exclusion chromatography [6]. For more information on self-generated gradients see Application Sheet V03.
7. Bibliographical review
1. Manninen et al [1] used this adenovirus purification method in their studies on the role of caveolin-1 in apical membrane transport by generating caveolin-1-deficient Madin-Darby canine kidney (MDCK) cells using virus-mediated RNA interference.
2. Elevation of the peroxisome proliferators-activated receptor γ co-activator PGC-1 by adenovirus mediated gene transfer, which increased transcription of the xenobiotic-metabolizing enzyme CYP2A5 transcription [3].
3. Using an iodixanol discontinuous gradient Peng et al [4] reported a fourfold improvement in yield of an adenovector with RGD-modified fibre proteins, compared to the CsCl method.
4. Targeting of caveolin to lipid bodies in adipocytes that express high levels of caveolins [7].
5. The role of LN5 in the spreading, proliferation, wound-edge migration, and apical–basal polarization of MDCK cells [8].
6. To test whether synapsin plays a role in Glut4 traffic, by expression of a site 1 phosphorylation mutant (S10A synapsin) in 3T3-L1 adipocytes [9].
7. Deletion of transcription domain CR3 of adenoviral E1A13S protein and anti-tumour activity in drug-resistant cells [10].
8. Study of the use of cell-derived isolates for the production of seasonal influenza vaccine [11].
9. Iodixanol gradient purification used in adenovirus targeting to prostate-specific membrane antigen.[12].
10. El-Andaloussi et al [13] have described the oncolytic potential of iodixanol-purified adenovirusparvovirus chimeras.
11. Several authors have commented on the advantages of using a biologically-friendly density gradient medium (OptiPrep™), rather than a toxic heavy metal salt such as CsCl: for direct in vitro and in vivo experimentation and potential clinical applications; the improved function:particle ratio; the rapid execution of the methodology and the avoidance of desalting techniques [14-16].
12. Papers have described new transfection methods [17], the advantage of adenovirus-parvovirus chimeras in oncolysis [18] and rAAV co-infection [19].
13. More recently iodixanol gradient-purified recombinant adenovirus vectors have been used in gene transfer studies in cardiomyocytes and rat heart. Koivisto et al [20] studied the activation transcription factor 3 (ATF3), a stress-activated immediate early gene that may have both a detrimental and a cardioprotective role in the heart, using cardiomyocytes. Moilanenet al [21] investigated the function of the WD-repeat domain 12 (WDR12) in early-onset myocardial infarction (MI), using adult rat heart. Papers covering treatment of lung carcinoma [22], osteoclast function [23] and oxidant induction in prostate carcinoma treatment [24].
8. References
1. Manninen, A., Verkade, P., Le Jay, S., Torkko, J., Kasper, M., Fullerkrug, J. and Simons, K. (2005) Caveolin-1 is not essential for biosynthetic apical membrane transport Mol. Cell Biol., 25, 10087-10096
2. Manninen, A. (2006) Personal communication
3. Arpiainen, S., Järvenpää, S-M., Manninen, A., Viitala, P., Lang, M.A., Pelkonen, O. and Hakkola, J. (2008) Coactivator PGC-1α regulates the fasting inducible xenobiotic-metabolizing enzyme CYP2A5 in mouse primary hepatocytes Toxicol. Appl. Pharmacol., 232, 135-141
4. Peng, H.H., Wu, S., Davis, J.J., Wang, L., Roth, J.A., Marini III, F.C. and Fang, B. (2006) A rapid and efficient method for purification of recombinant adenovirus with arginine–glycine–aspartic acid-modified fibers Anal. Biochem., 354, 140-147
5. Dormond, E., Chahal, P., Bernier, A., Tran, R., Perrier, M. and Kamen, A. (2010) An efficient process for the purification of helper-dependent adenoviral vector and removal of helper virus by iodixanol ultracentrifugation J. Virol. Meth., 165, 83-89
6. Dormond, E. and Kamen, A.A. (2011) Manufacturing of adenovirus vectors: production and purification of helper dependent adenovirus In.Viral Vectors for Gene Therapy: Methods and Protocols (eds Merten, O-W. and Al-Rubeai, M.), Methods Mol. Biol., 737, Springer Science+Business Media, pp 136-156
7. Le Lay, S., Hajduch, E., Lindsay, M.R., Le Lièpvre, X., Thiele, C., Ferré, P., Parton, R.G., Kurzchalia, T., Simons, K. and Dugail, I. (2006) Cholesterol-induced caveolin targeting to lipid droplets in adipocytes: a role for caveolar endocytosis Traffic, 7, 549-561
8. Mak, G.Z., Kavanaugh, G.M., Buschmann, M.M., Stickley, S.M., Koch, M., Goss, K.H., Waechter, H., Zuk, A. and Matlin, K.S. (2006) Regulated synthesis and functions of laminin 5 in polarized Madin-Darby canine kidney epithelial cells Mol. Biol. Cell, 17, 3664-3677
9. Muretta, J.M., Romenskaia, I., Cassiday, P.A. and Mastick, C.C. (2007) Expression of a synapsin Iib site phosphorylation mutant in 3T3-L1 adipocytes inhibits basal intracellular retention of Glut4 J. Cell Sci., 120, 1168-1177
10. Rognoni, E., Widmaier, M., , C., Mantwill, K., Holzmüller, R., Gansbacher, B., Kolk, A., Schuster, T., Schmid, R.M., Saur, D., Kaszubiak, A., Lage, H. and Holm, P.S. (2009) Adenovirus-based virotherapy enabled by cellular YB-1 expression in vitro and in vivo Cancer Gene Ther., 16, 753–763
11. Murata, H., Macauley, J., Lewis Jr., A.M. and Peden, K. (2011) Plaque purification as a method to mitigate the risk of adventitious-agent contamination in influenza vaccine virus seeds Vaccine 29, 3155–3161
12. Wu, P., Kudrolli, T.A., Chowdhury, W.H., Liu, M.M., Rodriguez, R. and Lupold, S.E. (2010) Adenovirus targeting to prostate-specific membrane antigen through virus-displayed, semirandom peptide library screening Cancer Res; 70, 9549–9553
13. El-Andaloussi, N., Bonifati, S., Kaufmann, J.K., Mailly, L., Daeffler, L., Deryckère, F., Nettelbeck, D.M., Rommelaere, J. and Marchini, A. (2012) Generation of an adenovirus-parvovirus chimera with enhanced oncolytic potential J. Virol., 86, 10418-10431
14. Segura, M.M., Kamen, A.A. and Garnier, A. (2011) Overview of current scalable methods for purification of viral vectors In, Viral Vectors for Gene Therapy: Methods and Protocols, Methods in Molecular Biology, 737 (eds. Merten O.W. and Al-Rubeai, M.) Springer Science+Business Media, pp 89-116
15. Giménez-Alejandre, M., Gros, A. and Alemany, R. (2011) Construction of capsid-modified adenoviruses by recombination in yeast and purification by iodixanol-gradient In Methods Mol. Biol., 797, Oncolytic Viruses: Methods and Protocols, (ed. Kirn, D.H. et al.), Springer Science+Business Media, pp 21-34
16. Giacca, M. and Zacchigna, S. (2012) Virus-mediated gene delivery for human gene therapy J. Control. Release, 161, 377–388
17. Okada, H., Iizuka, T., Mochizuki, H., Nihira, T., Kamiya, K., Inoshita, A., Kasagi, H., Kasai, M. and Ikeda, K. (2012) Gene transfer targeting mouse vestibule using adenovirus and adeno-associated virus vectors Otol. Neurotol., 33, 655-659
18. El-Andaloussi, N., Bonifati, S., Kaufmann, J.K., Mailly, L., Daeffler, L., Deryckère, F., Nettelbeck, D.M., Rommelaere, J. and Marchini, A. (2012) Generation of an adenovirus-parvovirus chimera with enhanced oncolytic potential J. Virol., 86, 10418-10431
19. Laborda, E., Puig-Saus, C., Cascalló, M., Chillón, M. and Alemany, R. (2013) Adeno-associated virus enhances wild-type and oncolytic adenovirus spread Hum. Gene Ther. Methods 24, 372–380
20. Koivisto, E., Acosta, A.J., Moilanen, A-M., Tokola, H., Aro, J., Pennanen, H., Sakkinen, H., Käikkonen, L., Ruskoaho, H. and Rysä, J. (2014) Characterization of the regulatory mechanisms of activating transcription factor 3 by hypertrophic stimuli in rat cardiomyocytes PLoS One, 9: e105168
21. Moilanen, A-M., Rysä, J., Kaikkonen, L., Karvonen, T., Mustonen, E., Serpi, R., Szabó, Z., Tenhunen, O., Bagyura, Z., et al (2015) WDR12, a member of nucleolar PeBoW complex, is up-regulated in failing hearts and causes deterioration of cardiac function PLoS One, 10: e0124907
22. Catani, J.P.P., Medrano, R.F.V., Hunger, A., Del Valle, P., Adjemian, S., Bertolini Zanatta, D., Kroemer, G., Costanzi-Strauss, E. and Strauss, B.E. (2016) Intratumoral immunization by p19Arf and interferon-β gene transfer in a heterotopic mouse model of lung carcinoma Translat. Oncol., 9, 565–574
23. Sztacho, M., Segeletz, S., Sanchez-Fernandez, M.A., Czupalla, C., Niehage, C. and Hoflack, B. (2016) BAR proteins PSTPIP112 regulate podosome dynamics and the resorption activity of osteoclasts PLoS One, 11, e0164829
24. Tamura, R.E., Hunger, A., Fernandes, D.C., Laurindo, F.R., Costanzi-Strauss, E. and Strauss, B.E. (2017) Induction of oxidants distinguishes susceptibility of prostate carcinoma cell lines to p53 gene transfer mediated by an improved adenoviral vector Hum. Gene Ther., 28, 639-653
9. Publications reporting the use of OptiPrepTM (from 2018)
1. Sharon, D. and Kamen, A. (2018) Advancements in the design and scalable production of viral gene transfer vectors Biotech. Bioeng., 115, 25–40
2. Zou, X-H., Bib, Z-X., Guo, X-J., Zhang, Z., Zhao, Y., Wang, M., Zhu, Y-L., Jie, H-Y. et al (2018) DNA assembly technique simplifies the construction of infectious clone of fowl adenovirus J. Virol. Meth., 257, 85–92
3. Parras-Moltó, m., Rodríguez-Galet, a., Suárez-Rodríguez, P. and López-Bueno, A. (2018) Evaluation of bias induced by viral enrichment and random amplification protocols in metagenomic surveys of saliva DNA viruses Microbiome, 6: 119
4. Houldcroft, C.J., Roy, S., Morfopoulou, S., Margetts, B.K., Depledge, D.P., Cudini, J., Shah, D., Brown, J.R. et al (2018) Use of whole-genome sequencing of adenovirus in immunocompromised pediatric patients to identify nosocomial transmission and mixed-genotype infection J. Infect. Dis., 218, 1261-1271
5. De Luna Vieira, I., Tamura, R.E., Hunger, A. and Strauss, B.E. (2019) Distinct roles of direct transduction versus exposure to the tumor secretome on murine endothelial cells after melanoma gene therapy with interferon-β and p19Arf J. Interferon Cytokine Res., 39, 246-258
11. Acknowledgements
We are extremely grateful to Professor Kai Simons and Dr Aki Manninen, Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, for invaluable information in the preparation of this Application Sheet.
OptiPrep™ Application Sheet V07; 9th edition, January 2020
OptiPrep™ Application Sheet V08
Purification of Group I (ds)DNA viruses: Herpesviridae and Baculoviridae in a self-generated gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RV01) provides a full bibliography of papers reporting the use of iodixanol gradients for purification and analysis of Group I viruses; to access return to the initial list of Folders and select “Reference List”.
- The self-generated gradient strategy described in this Application Sheet was developed for Herpes simplex 1; it can certainly be extended to other viruses of the Herpesviridae family but centrifugation time or iodixanol starting concentration require modulation to optimize the process. Some comments about this and the purification of other herpesviruses are given in Sections 6-9.
- Use of alternative pre-formed gradients is described in Application Sheet V09.
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
In the following protocol, the viral particles are first concentrated on top of a dense cushion of iodixanol, in place of pelleting the virus. Subsequently, after removal of most of the supernatant, the contents of the tube are simply mixed so that the virus is suspended in 25% (w/v) iodixanol. This suspension is then centrifuged in a tube for a vertical or near-vertical rotor. The self-generated gradient that is formed is designed to band virus particles towards the bottom of gradient while allowing any contaminating membrane material to band at lower densities. The process is simple and requires many fewer manipulations than the routine techniques with sucrose and CsCl gradients. Self-generated gradients also have the merit of high reproducibility.
In all comparative studies between CsCl and iodixanol, it has been shown that the recovery of virus infectivity is much higher and the particle: infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which is much higher than iodixanol. Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast, many add-on techniques can be performed and cells infected with virus, without dialysis of iodixanol.
 2. Solutions required
2. Solutions required
A. OptiPrep™
B. Diluent: 0.85% (w/v) NaCl, 60 mM Hepes-NaOH, pH 7.4
C. Working solution of 50% iodixanol (ρ = 1.272 g/ml): mix 5 vol of solution A with 1 vol of solution B (see Section 5, Note 1).
D. HEPES buffered saline: 0.85% NaCl (w/v), 10 mM HEPES-NaOH, pH 7.4.
3. Ultracentrifuge rotor requirements For concentration of the virus: a swinging-bucket rotor of suitable volume to accommodate the volume of crude virus suspension and capable of 100,00-200,000gav, such as the Beckman SW28 or Beckman SW28.1 or equivalent rotors.
For gradient purification: any vertical or near-vertical rotor with tube capacity of approx 12 ml and capable of approx 350,000g. The sedimentation path length of the rotor should be 17-25 mm. Separations described in this Application Sheet were obtained with a Beckman VTi65.1 vertical rotor, NVT65 near-vertical rotor or NVT65.2 near-vertical rotor. High performance fixed-angle rotors may only be used for the rapid formation of self-generated gradients if the tube volume is relatively small (<6 ml). For a summary of the range of density profiles achievable with OptiPrep™ see Application Sheet V03.
4. Protocol
1. Clarify the virus suspension by centrifugation at 1000 g for 10 min.
2. Transfer a known volume of the supernatant to suitable tubes for a swinging-bucket rotor and underlay with a small volume (2-4 ml) of Solution C (see Section 5, Note 2).
3. Centrifuge at 160,000 gav for 1 h to band the virus sharply at the working solution interface.
4. Remove all of the supernatant except for a volume equal to the volume of cushion and mix the residual contents of the tubes. This will produce a concentrated virus suspension in 25% (w/v) iodixanol.
5. Transfer the suspension to tubes suitable for a vertical or near-vertical rotor to band the virus in a self-generated gradient of iodixanol.
6. Any tubes that are not filled should be topped up and mixed with 25% (w/v) iodixanol (mix equal volumes of Solutions C and D).
7. Centrifuge at 350,000 gav for 2.5h and at the end of the centrifugation use either a controlled deceleration programme or turn off the brake below 2000 rpm.
8. Either harvest the virus band with a syringe and metal cannula or unload the entire gradient by tube puncture, or other suitable method. Under the centrifugation conditions described the Herpes virus will band in the bottom third of the tube.
- See Section 6 for more information on use of other rotors and centrifugation conditions.
5. Notes
1. Strategies for preparing working solutions are given in Application Sheet V01.
2. The actual volumes will depend on the total volume of virus fluid and the volume of the tubes used. For example: for approx. 15 ml supernatant per tube use 1-2 ml cushion solution, for approx. 35 ml use 2-4 ml.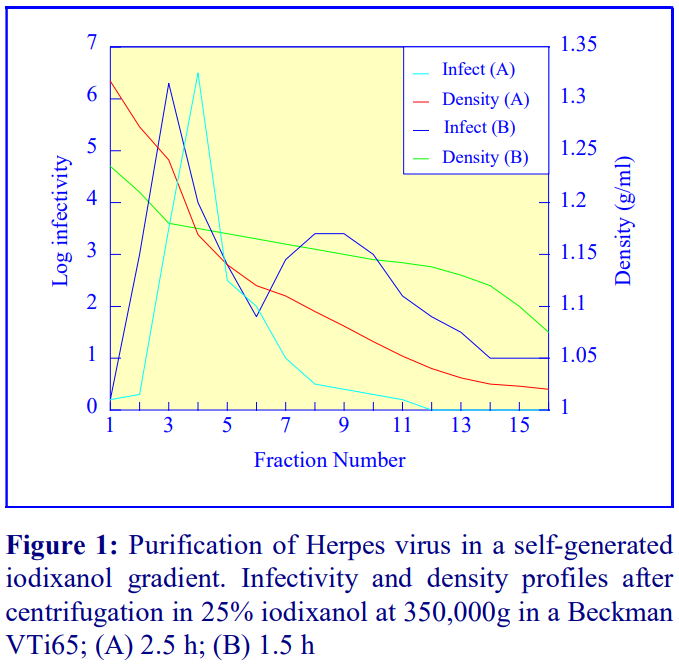 3. Since the virus bands close to the bottom of the gradient and contaminating membranes are lighter, collection from the bottom is the method of choice. For more information on harvesting gradients see Application Sheet V04.
3. Since the virus bands close to the bottom of the gradient and contaminating membranes are lighter, collection from the bottom is the method of choice. For more information on harvesting gradients see Application Sheet V04.
4. The separations shown in Figure 1 were obtained with a Beckman VTi65.1 vertical rotor. After approx 2.5-3.5 h the gradient that is generated is relative shallow towards the top and becomes progressively steeper in the denser regions. It is very effective for banding the virus sharply near the bottom of the tube (see Figure 1A) while any membranous contamination bands at lower densities. At shorter times (e.g. 1.5 h) the gradient is more S-shaped and shallower in the virus-banding area; this permits possible subfractionation of the virus (Figure 1B). In Figure 1A the shoulder seen in the infectivity profile becomes more clearly resolved at the shorter time (Figure 1B). The significance of this subfractionation has not been investigated.
5. Herpes virus (and astrovirus) harvested from iodixanol gradients have been used to re-infect cells without removal of the medium. Preliminary analysis shows that recoveries of more than 90% are achieved.
6. The method can be scaled up for the use of larger vertical rotors such as the Beckman VTi50, but the longer sedimentation path length and lower maximum RCF means that longer centrifugation times will be necessary.
6. Brief review of other published methods reporting the use of self-generated gradients
The methodology as described above has been extensively used and reported by a group working on, amongst other topic areas, CD4+ and CD8+ T cell and dendritic cell interactions and inflammatory reactions in Herpes stromal keratitis, at the University of Pittsburgh School of Medicine [2-7]. For a more extensive listing of publications from this group see Reference List RV03.
Published papers also a reveal some variations in the centrifugation conditions used for the selfgenerated iodixanol gradient banding of the virus; some are relatively trivial; some are significant. Most g-forces are in the range 330,000-402,000 g [8-12] and centrifugation times in the range 3-4.5 h [8-12] in Beckman vertical or near-vertical rotors (tube volumes approx. 5-11 ml). Some notable exceptions to this are 58,000 g for 3.5 h [13] and 70,000 g for 3.5 h [14]. Gradient formation at these lower g-forces has not been investigated in any detail; the gradients are more likely to be shallow in the middle and steep in the lower and higher density sections. It is not known if this has any beneficial effects regarding the purification of the virus. Small changes to the iodixanol starting concentration have also been made: 22% [9] and 20% [11]. In a very detailed account of the methodology [15] an 11 ml tube was charged with 9.2 ml of the virus in 24% (w/v) iodixanol, overlaid with 1.5 ml of 22 % iodixanol. The gradient was generated at approx 300,000 g and the time varied from 4-15 h; during this time period the density profile of the gradient will change and approach equilibrium: longer times may permit the resolution of subpopulations of the virus.
7. Epstein-Barr virus
The same strategy of virus concentration on a 50% (w/v) iodixanol barrier, followed by a selfgenerated gradient formed from 25% iodixanol (350,000 g for 2.4h) has been used by a group at the University of Birmingham (UK) for the purification and analysis of Epstein-Barr virus [16-24]. Although vertical or near-vertical rotors are the recommended rotors for self-generated gradients, small-volume, high-performance swinging-bucket rotors are a possible alternative, especially if they are used in conjunction with small volume tubes with so-called g-Max adaptors (Beckman), which reduce the path length of the tube rather than its diameter. Even some high-performance swinging bucket rotors, similarly adapted, may be an option.
Using a flotation separation, Ruiss et al commented that the banding of Epstein-Barr virus at 1.03- 1.08 g/ml in iodixanol gradients was much lower than that in hyperosmotic sucrose gradients (1.13- 1.18 g/ml) [25].
8. Cytomegalovirus
Murine cytomegalovirus has been purified under similar conditions to those described in Sections 2-4 [26]. The gradient has also been self-generated using rather lower g-forces and longer centrifugation times – 144,000 g for 16 h [27].
9. Baculovirus
After centrifuging the virus on to a 50% (w/v) iodixanol cushion (80,000 g for 1 h, the fluid above the virus band was removed and the virus harvested in the residual cushion and the iodixanol concentration adjusted to 25% (w/v) and the virus banded in self-generated gradient in a Beckman NVT65 (near-vertical rotor) at 350,000 g for 3 h [28].
Nucleocapsids from a 1% NP-40 (in a buffered saline containing 2 mM EDTA) treated baculovirus suspension (6 ml) can be separated on a two-layer iodixanol gradient (3 ml each of 25% and 50% w/v) centrifuged at 16,000 g for 3 h. The capsids were concentrated just above the barrier of the two iodixanol solutions [29].
- Segura et al [30] commented in a review of gene therapy viral vectors that iodixanol’s celland virus-friendly properties made it much more useful in gradient ´purification than CsCl
8. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Xu, M., Lepisto, A.J. and Hendricks, R.L. (2004) CD154 signaling regulates the Th1 response to herpes simplex virus-1 and inflammation in infected corneas J. Immunol., 173, 1232-1239
3. Sheridan, B.S., Khanna, K.M., Frank, G.M. and Hendricks, R.L. (2006) Latent virus influences the generation and maintenance of CD8+ T cell memory J. Immunol., 177, 8356-8364
4. Divito, S.J. and Hendricks, R.L. (2008) Activated inflammatory infiltrate in HSV-1-infected corneas without Herpes stromal keratitis Invest. Ophthalmol. Vis. Sci., 49, 1488-1495
5. Sheridan, B.S., Cherpes, T.L., Urban, J., Kalinski, P. and Hendricks, R.L. (2009) Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes J. Virol., 83, 2237-2245
6. St. Leger, A.J., Peters, B., Sidney, J., Sette, A. and Hendricks, R.L. (2011) Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice J. Immunol., 186, 3927–3933
7. Frank, G.M., Buela, K-A.G., Maker, D.M., Harvey, S.A.K. and Hendricks, R.L. (2012) Early responding dendritic cells direct the local NK response to control herpes simplex virus 1 infection within the cornea J. Immunol., 188, 1350–1359
8. Argnani, R., Boccafogli, L., Marconi, P.C. and Manservigi, R. (2004) Specific targeted binding of herpes simplex virus type 1 to hepatocytes via the human hepatitis B virus preS1 peptide Gene Ther., 11, 1087-1098
9. Goins, W.F., Krisky, D.M., Wolfe, D.P., Fink, D.J. and Glorioso, J.C. (2002) Development of replication-defective herpes simplex virus vectors Methods Mol. Med., 69, 481-507
10. Burton, E.A., Huang, S., Goins, W.F. and Glorioso, J.C. (2003) Use of the herpes simplex viral genome to construct gene therapy vectors Methods Mol. Med., 76, 1-31
11. Goss, J.R., Natsume, A., Wolfe, D., mata, M., Glorioso, J.C. and Fink, D. (2004) Delivery of herpes simplex virus-based vectors to the nervous system Methods Mol. Biol., 246, 309-322 (2004)
12. Caselli, E., Galavan, M., Cassai, E., Caruso, A., Sighinolfi, L. and Di Luca, D. (2005) Human herpesvirus 8 enhances human immunodeficiency virus replication in acutely infected cells and induces reactivation in latently infected cells Blood, 106, 2790-2797
13. Shah, A.C., Price, K.H., Parker, J.N., Samuel, S.L., Meleth, S., Cassady, K.A., Gillespie, G.Y., Whitley, R.J. and Markert, J.M. (2006) Serial passage through human glioma xenografts selects for a 134.5 herpes simplex virus type 1 mutant that exhibits decreased neurotoxicity and prolongs survival of mice with experimental brain tumors J. Virol., 80, 7308-7315
14. Caselli, E., Fiorentini, S., Amici, C., Di Luca, D., Caruso, A. and Santoro, M.G. (2007) Human herpesvirus 8 acute infection of endothelial cells induces monocyte chemoattractant protein 1-dependent capillary-like structure formation: role of the IKK/NF-B pathway Blood, 109, 2718-2726
15. Fraefel, C., Marconi, P. and Epstein, A.L. (2011) Herpes simplex virus type 1-derived recombinant and amplicon vectors In.Viral Vectors for Gene Therapy: Methods and Protocols (eds Merten, O-W. and Al-Rubeai, M.), Methods Mol. Biol., 737, Springer Science+Business Media, pp 303-343
16. Shannon-Lowe, C., Adland, E., Bell, A.I., Delecluse, H-J., Rickinson, A.B. and Rowe, M. (2009) Features distinguishing Epstein-Barr virus infections of epithelial cells and B cells: viral genome expression, genome maintenance, and genome amplification J. Virol., 83, 7749-7760
17. Shannon-Lowe, C. and Rowe, M. (2011) Epstein-Barr virus infection of polarized epithelial cells via the basolateral surface by memory B cell-mediated transfer infection Plos Pathog., 5: e1001338
18. Long, H.M., Leese, A.M., Chagoury, O.L., Connerty, S.R., Quarcoopome, J., Quinn, L.L., Shannon-Lowe, C. and Rickinson, A.B. (2011) Cytotoxic CD4+ T cell responses to EBV contrast with CD8 responses in breadth of lytic cycle antigen choice and in lytic cycle recognition J. Immunol., 187, 92–101
19. Tierney, R.J., Kao, K-Y., Nagra, J.K. and Rickinson, A.B. (2011) Epstein-Barr virus BamHI W repeat number limits EBNA2/EBNA-LP coexpression in newly infected B cells and the efficiency of B-cell transformation: a rationale for the multiple W repeats in wild-type virus strains J. Virol., 85, 12362–12375
20. Hernando, H., 1, Islam, A.B.M.M.K., Rodríguez-Ubreva, J., Forné, I., Ciudad, L., Imhof, A., Shannon-Lowe, C. and Ballestar, E. (2014) Epstein–Barr virus-mediated transformation of B cells induces global chromatin changes independent to the acquisition of proliferation Nucleic Acids Res., 42, 249–263
21. Rowe, M., Raithatha, S. and Shannon-Lowe, C. (2014) Counteracting effects of cellular notch and Epstein-Barr virus EBNA2: implications for stromal effects on virus-host interactions J. Virol., 88, 12065–12076
22. Campion, E.M., Hakimjavadi, R., Loughran, S.T., Phelan, S., Smith, S.M., D’Souza, B.N., Tierney, R.J., Bell, A.I., Cahill, P.A. and Walls, D. (2014) Repression of the proapoptotic cellular BIK/NBK bene by Epstein-Barr virus antagonizes transforming growth factor 1-induced B cell apoptosis J. Virol., 88, 5001–5013
23. Rowe, M., Raithatha, S. and Shannon-Lowe, C. (2014) Counteracting effects of cellular notch and Epstein-Barr virus EBNA2: implications for stromal effects on virus-host interactions J. Virol., 88, 12065–12076
24. Fitzsimmons, L., Bell, A. and Rowe, M., Tierney, R.J., Shannon-Lowe, C.D., (2015) Unexpected patterns of Epstein– Barr virus transcription revealed by a High throughput PCR array for absolute quantification of viral mRNA Virology 474, 117–130
25. Ruiss, R., Jochum, S., Wanner, G., Reisbach, G., Hammerschmidt, W. and Zeidler, R. (2011) A virus-like particle-based Epstein-Barr virus vaccine J. Virol., 85, 13105–13113
26. Rupp, B., Ruzsics, Z., Sacher, T. and Koszinowski, U.H. (2005) Conditional cytomegalovirus replication in vitro and in vivo J. Virol., 79, 486-494
27. LaRocca, T.J., Jeong, D., Kohlbrenner, E., Lee, A., Chen, JQ., Hajjar, R.J. and Tarzami, S.T. (2012) CXCR4 gene transfer prevents pressure overload induced heart failure J. Mol. Cell. Cardiol., 53, 223–232
28. Strauss, R., Hüser, A., Ni, S., Tuve, S., Kiviat, N., Sow, P.S., Hofmann, C. and Lieber, A. (2007) Baculovirus-based vaccination vectors allow for efficient induction of immune responses against Plasmodium falciparum circumsporozoite protein Mol. Ther., 15, 193-202
29. Wang, Q., Bosch, B-J., Vlak, J.M., van Oers, M.M., Rottier, P.J. and van Lent, J.W.M. (2016) Budded baculovirus particle structure revisited J. Invertebr. Pathol., 134, 15–22
30. Segura, M.M., Kamen, A.A. and Garnier, A. (2011) Overview of current scalable methods for purification of viral vectors In, Viral Vectors for Gene Therapy: Methods and Protocols, Methods in Molecular Biology, 737 (eds. Merten O.W. and Al-Rubeai, M.) Springer Science+Business Media, pp 89-116
OptiPrep™ Application Sheet V08; 10th edition, January 2020
OptiPrep™ Application Sheet V09
Purification of Group I (ds)DNA viruses: Herpesviridae and Asfaviridae in a pre-formed gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RV01) provides a full bibliography of papers reporting the use of iodixanol gradients for purification and analysis of Group I viruses; to access return to the initial list of Folders and select “Reference Lists”.
- Use of a self-generated gradient strategy for Herpes virus is described in Application Sheet V08.
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number
1. Background
There are presently eight members of the Herpesviridae family of viruses (HHV1-8), including human herpes virus 1 and 2 (HHV-1 and HHV-2), the Epstein-Barr virus (HHV-4), human cytomegalovirus and Karposi’s sarcoma associated herpes virus (KSHV). They enveloped viruses and share a common structure. These and others have been purified in pre-formed continuous or discontinuous NycodenzⓇ or iodixanol gradients. The use of sucrose gradients is also common, but the use of this gradient solute for any enveloped virus has serious disadvantages:
- Palker [1] was one of the first to point out that the movement of virus particles through a sucrose gradient caused loss of surface glycoproteins from enveloped viruses
- Zhu and Yuan [2] noted that most of the glycoprotein gB was stripped from the surface of KSHV in sucrose gradients (even if the severity of any surface hydrodynamic shear was reduced by using low g-forces), while this was not observed in NycodenzⓇ gradients
- Use of iodixanol gradients overcame the problem of the cellular vesicle contamination of HHV-6A that was observed in sucrose gradients [3]
2. Clarification of virus suspension
Virus-containing suspensions are usually clarified (separated from cellular debris) by low-speed centrifugation, often followed by filtration through a 0.45 μm filter. Centrifugation speeds are usually carried out at approx. 4000 g for 30 min (e.g. refs 2 and 4), sometimes 8000 g for 15 min [5], which may be in addition to the 4000 g step [2].
3. Concentration of virus
Concentration of virus particles by rapid sedimentation from a saline solution can cause serious loss of infectivity and there are a number of strategies that minimize this problem. Xiao et al [6] pelleted Epstein-Barr virus at 22,000 g overnight rather than a higher g-force for a shorter time. There are many instances of the use of a low-density cushion through which the virus sediments: 5 ml of 5% sucrose at approx. 100,000 g for 1 h [2], 20% sorbitol, at 64,000 g for 1 h [7]; 10% NycodenzⓇ at 35,000 g [4] or 20% iodixanol at 141,000 g for 1 h [8].
To avoid entirely the pelleting of the virus, banding on to a dense cushion is often a strategy used for a number of viruses. Garrigues et al [9] first concentrated KSHV on a 50% (w/v) iodixanol cushion. This has also been used for Rhadinovirus [10]. More information on methods for virus concentration is provided in Application Sheet V06.
4. Gradient solutions
It is highly likely that any method describing the use of a NycodenzⓇ gradient can be transposed directly to an iodixanol gradient of the same % (w/v) concentration range. Certainly, it is much more simple to prepare sterile gradient solutions from OptiPrep™ than from powdered NycodenzⓇ. The preparation of solutions from both sources is described below.
4a. Buffer preparation
The concentrated virus is commonly suspended in PBS [2] or a Tris-HCl buffered 100 mM NaCl containing EDTA at pH 7.2-7.4; the Tris concentration varies from 10 mM [6] to 50 mM [7] and that of the EDTA from 1 mM [6] to 10 mM [7]. The chosen gradient medium (Nycodenz or iodixanol) is usually made up in the same medium or sometimes in 1 mM potassium phosphate, pH 7.4 [11,12].
4b. NycodenzⓇ
To make up a stock solution of 50% (w/v) NycodenzⓇ place 50 ml of buffer in a 150 ml beaker on a heated magnetic stirrer set at approx. 50C and add 50 g of NycodenzⓇ powder in small amounts until dissolved. Allow the solution to cool to room temperature and make up to 100 ml with buffer. Filter sterilize if required.
Nycodenz solutions in water are hyperosmotic above approx 30% (w/v) and 50% (w/v) NycodenzⓇ has an osmolality of approx. 485 mOsm, thus if the stock solution is made up in a buffer containing 100 mM NaCl, the resultant solution will be > 700 mOsm. To avoid such high osmolalities the NaCl should be omitted and the stock solution of 50% (w/v) NycodenzⓇ made up in, for example, 10 mM EDTA, 50 mM Tris-HCl, pH 7.4. Subsequent dilutions of the stock are made with the complete buffer. A 35% (w/v) NycodenzⓇ solution in 0.5 mM phosphate was diluted with 0.25 M sucrose, 0.5 mM phosphate [13]; all these solutions will be approx. isoosmotic.
4c. Iodixanol
As dilution of OptiPrep™ (60% w/v iodixanol) with any volume of an isoosmotic salt solution will produce a solution that is also isoosmotic, it can be diluted directly with the chosen buffer, but if it is important to keep the concentrations of EDTA and Tris constant then a 50% (w/v) iodixanol stock should be prepared form 5 vol. of OptiPrep™ and 1 vol. of a 6x EDTA/Tris buffer. Iodixanol solutions are certainly easier to prepare than NycodenzⓇ solutions and they can probably be used as a substitute in NycodenzⓇ methods, but no direct comparative study has been made.
5. Ultracentrifuge rotor requirements
The routine requirement is for a swinging-bucket rotor capable of 100,00-140,000 gav, the Beckman SW28, SW28.1, SW41Ti or equivalent rotors are commonly used.
6. Gradient preparation (all Nycodenz and iodixanol concentrations are %, w/v)
Discontinuous gradients of the following formats have been used: 20% and 35% NycodenzⓇ [2] and 20% and 40% NycodenzⓇ [6]. More discriminating multi-step iodixanol gradients were introduced by Garrigues et al [9] for KSHV and subsequently used by Hahn et al [14]; these comprised 20,25, 30 and 40% (w/v) iodixanol. For Herpes simplex a broader range of 20,30,40 and 50% (w/v) iodixanol was used [8].
Continuous gradients are more widely used; a commonly used one is 1 ml or 2 ml each of 24%, 26%, 28%, 30%, 32%, 34%, 36%, 38%, 40% and 42% NycodenzⓇ [10,11,15,16] which is allowed to diffuse overnight at 4°C. It is probably easier to construct such a gradient from equal volumes of 24% and 42% NycodenzⓇ using a two-chamber gradient maker or Gradient Master™. Other continuous gradients are: 20-35% [2], 10-50% [7,17], 10-40% [18], 15-35% [13]. Iodixanol gradients of 5-25% (w/v) have been reported [3].
- For more information about the preparation of both discontinuous and continuous gradients
7. Gradient centrifugation
Commonly 0.5-2.0 ml of virus suspension is layered on top of the gradient and centrifuged at approx 70-120,000 g for 2-4, usually at 4°C. Use a slow acceleration and deceleration program if they are available – if not, turn off the brake during deceleration from 3000 rpm.
8. Gradient analysis
With discontinuous gradients the virus may be aspirated from the interface between the two gradient solutions. Unload continuous gradients in small equal volume fractions either low- or highdensity end first (commonly 20-25 fractions are collected). The precise banding position of the gradient may depend on the virus type or any pre-gradient treatments of the virus. The density of HHV is normally in the range 1.13-1.15 g/ml (equivalent to approx. 24-29% NycodenzⓇ, but being an enveloped virus its precise density may also depend on the osmolality of the gradient (see Sections 4b and 4c). In the case of HHV-5, the gradient was able to resolve virus particles from dense bodies [7] and recombinant HHV-4 was find to contain a denser fraction (1.18-1.20 g/ml) consistent with deenveloped particles or partially damaged virus that was not observed with the wild-type virus [10]. For more information about unloading methods see Application Sheet V04.
- Iodixanol gradients have also been used for the purification of African swine fever virus; no detailed methodology was provided [19,20] but it is highly likely that one of the pre-formed gradients described in this Application Sheet would be effective.
9. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Zhu, F.X. and Yuan, Y. (2003) The ORF45 protein of Kaposi’s sarcoma-associated herpesvirus is associated with purified virions J. Virol., 77, 4221-4230
3. Hammarstedt, M., Ahlqvist, J., Jacobson, S., Garoff, H. and Fogdell-Hahn, A. (2007) Purification of infectious human herpesvirus 6A virions and association of host cell proteins Virol J. 4:101
4. Lee, J.I., Gant Luxton, G.W. and Smith, G.A. (2006) Identification of an essential domain in the herpesvirus VP1/2 tegument protein: the carboxy terminus directs incorporation into capsid assemblons J. Virol., 80, 12086-12094
5. Xiao, J., Palesky, J.M., Herrera, R. And Tugizov, S.M. (2007) Characterization of the Epstein–Barr virus glycoprotein BMRF-2 Virology, 359, 382-396
6. Farina, A., Santarelli, R., Gonnella, R., Bei, R., Muraro, R., Cardinali, G., Uccini, S., Ragona, G., Frati, L., Faggioni, A. and Angeloni, A. (2000) The BFRF1 gene of Epstein-Barr virus encodes a novel protein J. Virol., 74, 3225-3244
7. Varnum, S.M., Streblow, D.N., Monroe, M.E., Smith, P., Auberry, K.J., Pasa-Tolic, L., Wang, D., Camp, D.G., Rodland, K., Wiley, S., Britt, W., Shenk, T., Smith, R.D. and Nelson, J.A. (2004) Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome J. Virol., 78, 10960-10966
8. Jambunathan, N., Chowdhury, S., Subramanian, R., Chouljenko, V.N., Walker, J.D. and Kousoulas, K.G. (2011) Site-specific proteolytic cleavage of the amino terminus of herpes simplex virus glycoprotein K on virion particles inhibits virus entry J. Virol., 85, 12910–12918
9. Garrigues, H.J., Rubinchikova, Y.E., DiPersio, C. and Rose, T.M. (2008) Integrin Vβ3 binds to the RGD motif of glycoprotein B of Kaposi’s sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor J. Virol., 82, 1570-1580
10. Bruce, A.G., Bakke, A.M., Gravett, C.A., DeMaster, L.K., Bielefeldt-Ohmann, H., Burnside, K.L. and Rose, T.M. (2009) The ORF59 DNA polymerase processivity factor homologs of Old World primate RV2 rhadinoviruses are highly conserved nuclear antigens expressed in differentiated epithelium in infected macaques Virol. J., 6:205
11. Lake, C.M. and Hutt-Fletcher, L.M. (2000) Epstein-Barr virus that lacks glycoprotein gN impaired in assembly and infection J. Virol., 74, 11162-11172
12. Akula, S.M., Wang, F-Z., Vieira, J. and Chandran, B. (2001) Human herpesvirus 8 interaction with target cells involves heparin sulfate Virology, 282, 245-255
13. Fowler, E., Raab-Traub, N. and Hester, S. (1985) Purification of biologically active Epstein-Barr virus by affinity chromatography and non-ionic density gradient centrifugation J. Virol. Meth., 11, 59-74
14. Hahn, A.S., Kaufmann, J.K., Wies, E., Naschberger, E., Panteleev-Ivlev, J., Schmidt, K., Holzer, A., Schmidt, M., Chen, J., König, S., Ensser, A., Myoung, J., Brockmeyer, N.H., Stürzl, M., Fleckenstein, B. and Neipel, F. (2012) The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi’s sarcoma–
associated herpesvirus Nat. Med., 18, 961-966
15. Naranatt, P.P., Krishnan, H.H., Svojanovsky, S.R., Bloomer, C., Mathur, S. and Chandran, B. (2004) Host gene induction and transcriptional reprogramming in Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells Cancer Res., 64, 72-84
16. Grange, P.A., Marcelin, A-G., Calvez, V., Chauvel, C., Escande, J-P. and Dupin, N. (2005) Salivary lactoferrin is recognized by the human herpesvirus-8 J. Invest. Dermatol., 124, 1249-1258
17. Bechtel, J.T., Winant, R. and Ganem, D. (2005) Host and viral proteins in the virion of Kaposi’s sarcomaassociated herpesvirus J. Virol., 79, 4952-4964
18. Hoffmann, D. and Wildner, O. (2007) Comparison of herpes simplex virus- and conditionally replicative adenovirus-based vectors for glioblastoma treatment Cancer Gene Ther., 14, 627-639
19. Zhang, F., Hopwood, P., Abrams, C.C., Downing, A., Murray, F., Talbot, R., Archibald, A., Lowden, S. and Dixon, L.K. (2006) Macrophage transcriptional responses following in vitro infection with a highly virulent African swine fever isolate J. Virol., 80, 10514-10521
20. Lithgow, P., Takamatsu, H., Werling, D., Dixon, L. and Chapman, D. (2014) Correlation of cell surface marker expression with African swine fever virus infection Vet. Microbiol., 168, 413-419
OptiPrep™ Application Sheet V09; 6th edition, January 2020
OptiPrep™ Application Sheet V10
Purification of Group I (ds)DNA viruses: human papillomavirus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RV01) “Group I Viruses” provides a bibliography of all published papers reporting the use of iodixanol gradients for the purification of papillomavirus. To access return to the initial list of Folders and select “Reference Lists”.
- Human papillomavirus is a small non-enveloped DNA virus and the method described in this OptiPrep™ Application Sheet may be applicable to other similar papillomaviruses.
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
There are now many published papers that report the use of iodixanol gradients not only to purify viruses but also to investigate their assembly. In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which, in solutions of the same density, is much higher than that of iodixanol.
Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast both infectivity measurements using cultured cells and many add-on techniques can be performed without dialysis of iodixanol. Combined with the availability of OptiPrep as a sterile solution, this makes the use of OptiPrep™ for virus purification much more convenient than the use of either CsCl or sucrose.
The protocol described in this OptiPrep™ Application Sheet for papillomavirus vector purification, which is adapted from refs 2-4, has also been used for the purification of pseudovirus carrying a secreted alkaline phosphatase (SEAP) reporter gene [5]. The iodixanol solutions are prepared in PBS supplemented with additional NaCl, KCl and divalent cations. Note that ref. 4 contains an excellent detailed account of all the methodology associated with culture and transfection of 293TT cells, harvesting and maturation of virus, virus purification and assay.
2. Solutions required (see Note 1, in Section 6)
A. OptiPrep™ B. 10xPBS
B. 10xPBS
C. OptiPrep™ diluent: 3.125 M NaCl, 4.5 mM CaCl2, 2.5 mM MgCl2, 10.5 mM KCl
D. 39% (w/v) Iodixanol Working Solution (WS): mix 3.9 vol. Solution A, 0.6 vol. of Solution B, 1.2 vol. of Solution C and 0.3 vol. of water (see Note 2)
E. WS Diluent: mix 0.6 vol. of Solution B, 1.2 vol. of Solution C and 4.2 vol. of water
3. Pre-gradient protocols
This Application Sheet is concerned primarily with the density gradient purification of papillomavirus, but it is important to point out that there are a number of pre-gradient protocols that contribute significantly to the success of the purification procedure. These may vary from laboratory to laboratory (see Note 3) and their detailed consideration is outside the scope of this text. It is worth noting however that Buck et al [4] considered that most of the viral capsids were too fragile to allow processing immediately after release from the host cells by a detergent lysis buffer and consequently devised a maturation regimen in which the cells, suspended in PBS (supplemented with 9.5 mM MgCl2), containing 0.35% Brij 58, 0.1% Benzonase and 0.1% Plasmid Safe, are incubated at 37°C for at least 16 h. After this, the lysate is clarified by salt extraction prior to density gradient centrifugation. For more details of the maturation protocol see refs 3 and 4. However Buck and Thomson [6] now consider that Triton X-100 is the detergent of choice. This detergent is used at a concentration of 0.4%. Although the cell lysis capacity of Triton X-100 and Brij 58 are similar, solutions of Triton X-100 are considerably more stable than those of Brij 58, which have to be re-made every few weeks. See ref 6 for more details.
4. Rotor requirements
Swinging-bucket rotor with approx 5 ml tubes (e.g. Beckman SW50.1 or SW55Ti or Sorvall AH650) for 5 ml gradients (see Note 4)
5. Protocol
1. Dilute Solution D with Solution E to produce a 27% (w/v) iodixanol solution and in tubes for the 5 ml swinging-bucket rotor prepare 4.2 ml continuous gradients from equal volumes of Solution D and the 27% iodixanol solution using a two-chamber gradient maker or a Gradient Master™ (see Note 5). For more information about the preparation of continuous gradients see Application Sheet V02.
2. OR make up two iodixanol solutions of 27% and 33% (w/v) and prepare, by underlayering, a discontinuous gradient from 1.4 ml each of these two solutions and Solution D. Allow the gradients to diffuse at room temperature for 3-4 h (see Notes 5 and 6). For more information on the preparation of continuous from discontinuous gradients see Application Sheet V02.
3. Bring the cell lysate to 4°C; adjust the salt concentration to 0.85 M by addition of 0.17 vol. of 5 M NaCl and incubate for 10-20 min (see Note 7).
4. Clarify the suspension by centrifugation at 5000 g for 10 min in a microfuge (see Note 8).
5. Aspirate and keep the virus-containing supernatant.
6. Resuspend the pellet in approx 0.25 ml of Solution E and repeat step 4.
7. Aspirate the supernatant; combine with the first supernatant and repeat step 4 (see Note 9).
8. Layer the clarified virus suspension on top of the continuous iodixanol gradients and centrifuge at 234,000 g for 3.5 h at 16°C. Use a slow acceleration and deceleration program up to and down from 2000 rpm. If such a facility is not available, turn off the brake during deceleration below 2000 rpm (see Note 10).
9. Collect the gradient by tube puncture or, if the band is sufficiently well defined, retrieve the banded virus (about half to two thirds of the way down the tube) using a syringe (see Notes 11 and 12).
6. Notes
1. The mode of preparing the solutions ensures that the concentrations of buffer and ions are constant throughout the gradient. Any suitable buffer can be used for suspending the virus and for making the gradient solutions and its composition may vary from laboratory to laboratory. As long as the buffer has a low density (approx 1.006 g/ml) the density of the gradients will not be compromised. It might for example be a cell culture medium (e.g. DMEM or RPMI) rather than a balanced salt solution. Application Sheet V01 gives more details on the making up of gradient solutions.
2. In the original method [2-4], a 46% (w/v) iodixanol solution was used as the stock solution from which three gradient solutions of 39%, 33% and 27% (w/v) iodixanol were prepared. In this adaptation the 39% (w/v) stock solution is used as densest gradient solution and the source of the lower density solutions.
3. Cell lysis may also be achieved by freeze-thawing: Pejawar-Gaddy et al [7] for example used this technique with the cells suspended in 150 mM NaCl, 2 mM MgCl2, 1 mM CaCl2, 50 mM TrisHCl, pH 7.0; clarified the suspension at 8000 g for 20 min and delipidated the supernatant before pelleting the virus particles through a 40% sucrose cushion.
4. Larger volume tubes are permissible (e.g. in the Beckman SW41) but the time will need increasing to compensate for the longer sedimentation path and lower RCF (see Note 7) If a vertical rotor is substituted for the swinging-bucket rotor (e.g. Beckman VTi90 or VTi65.1), the shorter sedimentation path length will permit shorter centrifugation times.
5. For larger rotor tubes scale up all volumes proportionately.
6. Sometimes the sample is layered on a discontinuous gradient, without prior diffusion [7,8]. There are also small variations in the concentrations of iodixanol used for the discontinuous iodixanol gradient; for example 26%, 32% and 38% iodixanol [7]. Pejawar-Gaddy et al [7] reported that the capsomeres banded at the 26%/32% interface and the capsids at 32%/38% interface.
7. The volumes used for the cell lysis should be small enough to allow for loading on to the density gradients. Buck et al [2,4] used 0.65 ml per 108 cells. Although the efficacy of the density gradient is considered to be due, at least partly, to sedimentation velocity, the volume of sample can be as much as 2/3 of the gradients volume. This may reflect a very rapid sedimentation of the viral particles to the gradient interface.
8. Higher centrifugation speeds have been used for the clarification step, e.g. 16,300 g [9].
9. If the volume of virus suspension is too large, sediment it on to a small cushion (approx. 0.5 ml) of Solution D; a conical-bottomed Beckman konical tube is ideal for this. When aspirating the banded virus make sure that the final iodixanol concentration is no more than approx. 15% (w/v), to facilitate layering on to the gradient. For more information on concentrating virus suspensions see Application Sheet V06.
10. There is some variation in the g-force used for the gradient separation, occasionally higher values are used, e.g. 300,000 g [7]. Larger volume rotors can be used at lower RCFs for longer times, e.g. the SW41 at 200,000 g for 4.75 h [4].
11. For more information on harvesting gradients see Application Sheet V04.
12. In spite of the toxicity of CsCl gradients (see Section 1) there are many examples in the published literature of purification schedules that use sequential iodixanol and CsCl gradients.
7. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Buck, C.B., Pastrana, D.V., Lowy, D.R. and Schiller, J.T. (2004) Efficient intracellular assembly of papillomaviral vectors J. Virol., 78, 751-757
3. Schiller, J.T. (2006) Natl. Cancer Inst. Lab. Tech. File
4. Buck, C.B., Pastrana, D.V., Lowy, D.R. and Schiller, J.T. (2005) Generation of HPV pseudovirions using transfection and their use in neutralization assays Meth. Mol. Med., 119, 445-462
5. Pastrana, D.V., Buck, C.B., Pang, Y-Y. S., Thompson, C.D., Castle, P.E., Fitzgerald, P.C., Kjaer, S.K., Lowy, D.R. and Schiller, J.T. (2004) Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18 Virology, 321, 205-216
6. Buck, C. and Thompson, C. (2008) Alternative protocol: removal of capsids containing cellular DNA fragments http://home.ccr.cancer.gov/LCO/
7. Pejawar-Gaddy, S., Rajawat, Y., Hilioti, Z., Xue, J., Gaddy, D.F., Finn. O.J., Viscidi, R.P. and Bossis, I. (2010) Generation of a tumor vaccine candidate based on conjugation of a MUC1 peptide to polyionic papillomavirus viruslike particles Cancer Immunol. Immunother. 59, 1685–1696
8. Karanam, B., Peng, S., Li, T., Buck, C., Day, P.M. and Roden, R.B.S. (2010) Papillomavirus infection requires γ secretase J. Virol., 84, 10661–10670
9. Fraillery, D., Baud, D., Pang, S.Y-Y., Schiller, J., Bobst, M., Zosso, N., Ponci, F. and Nardelli-Haefliger, D. (2007) Salmonella enterica serovar typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever Clin. Vaccine Immunol., 14, 1285-1295
8. Acknowledgements
We are indebted to Dr Chris Buck, Laboratory of Cellular Oncology, N.C.I., Bethesda, MD 20892- 4263 for his kind help in the preparation of this OptiPrep Application Sheet.
OptiPrep™ Application Sheet V10; 9th edition, January 2020
OptiPrep™ Application Sheet V11
Purification of Group I (ds)DNA viruses: Polyomaviridae
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
- See OptiPrep™ Reference List (RV01) “Group I Viruses” for a full list of published papers. To access return to the initial list of Folders and select “Reference Lists”.
1. Background
There are now many published papers that report the use of iodixanol gradients not only to purify viruses but also to investigate their assembly. In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which, in solutions of the same density, is much higher than that of iodixanol. Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast both infectivity measurements using cultured cells and many add-on techniques can be performed without dialysis of iodixanol. Combined with the availability of OptiPrep™ as a sterile solution, this makes the use of OptiPrep™ for virus purification much more convenient than the use of either CsCl or sucrose.
The protocol described in this OptiPrep™ Application Sheet (Section 2) was first developed in 2004 for papillomavirus vector purification [2-4]; in 2009 however, Pastrana et al [5] applied the methodology to the purification of the polyomavirus associated with Merkel cell carcinoma. Although there is an earlier report by Nakanishi et al [6] in which the 39-33-27% (w/v) iodixanol gradient was used for other types of polyomavirus (SV-40, JC virus, BK virus and B-lymphotropic papovavirus). The iodixanol solutions are prepared in PBS supplemented with additional NaCl, KCl and divalent cations. Section 2 describes the principal steps involved in the gradient procedure.
- Section 3 summarizes other gradient strategies and includes a few details from many of the more recent published papers.
- Section 4 contains some brief comments regarding the purification of SV40
- Section 5a is the reference list; Section 5b contains more recent references
2. Three-step discontinuous gradient method
This method has been adapted from refs 4, 5 and 7; other gradients are described in Section 3.
2a. Solutions required (see Note 1 in Section 2e)
 A. OptiPrep™
A. OptiPrep™
B. 10xPBS
C. OptiPrep™ diluent: 3.125 M NaCl, 4.5 mM CaCl2, 2.5 mM MgCl2, 10.5 mM KCl
D. 39% (w/v) Iodixanol Working Solution (WS): mix 3.9 vol. Solution A, 0.6 vol. of Solution B, 1.2 vol. of Solution C and 0.3 vol. of water (see Note 2)
E. WS Diluent: mix 0.6 vol. of Solution B, 1.2 vol. of Solution C and 4.2 vol. of water
2b. Pre-gradient protocols
This Application Sheet is concerned primarily with the density gradient purification of polyomavirus. The pre-gradient protocols may vary from laboratory to laboratory and are outside the scope of this text. It is worth noting however that Pastrana et al [5] considered that most polyoma viral capsids are too fragile to allow processing immediately after release from the host cells by a detergent lysis buffer. These workers therefore devised a maturation regimen in which the cells, suspended in PBS (supplemented with 9.5 mM MgCl2), containing 0.4% Triton X100, a 0.1% RNase A/T1 cocktail (and an antibiotic), are incubated at 37°C overnight. For more details of the maturation protocol see ref 5. Nakanishi et al [6] used a medium containing 10 mM Tris–HCl pH 7.5, 2 mM MgCl2, 0.25% Brij 58 and 5 U/ml of Benzonase incubated for only 30 min at 37 °C.
These pre-gradient protocols [2-4] were originally developed for human papillomavirus and also used Brij 58 as the detergent. However Buck and Thomson [7] now consider that Triton X-100 is the detergent of choice. Although the cell lysis capacity of Triton X-100 and Brij 58 are similar, solutions of Triton X-100 are considerably more stable than those of Brij 58, which have to be re-made every few weeks. Moreover Buck and Thomson [7] noted that Triton X-100 is superior to Brij 58 for polyoma vectors. Sunyaev et al [8] released JC virus from the cells using a mechanical homogenization device.
2c. Rotor requirements
Swinging-bucket rotor with approx 5 ml tubes (e.g. Beckman SW50.1 or SW55Ti or Sorvall AH650) for 5 ml gradients (see Note 3)
2d. Protocol
1. Dilute Solution D with Solution E to produce a 27% (w/v) iodixanol solution and in tubes for the 5 ml swinging-bucket rotor prepare 4.2 ml continuous gradients from equal volumes of Solution D and the 27% iodixanol solution using a two-chamber gradient maker or a Gradient Master™ (see Note 4). For more information about the preparation of continuous gradients see Application Sheet V02.
2. OR make up two iodixanol solutions of 27% and 33% (w/v) and prepare, by underlayering, a discontinuous gradient from 1.4 ml each of these two solutions and Solution D. Allow the gradients to diffuse at room temperature for 3-4 h (see Note 4). For more information on the preparation of continuous from discontinuous gradients see Application Sheet V02.
3. Bring the cell lysate to 4°C; adjust the salt concentration to 0.85 M by addition of 0.17 vol. of 5 M NaCl and incubate for 10-20 min (see Note 5).
4. Clarify the suspension by centrifugation at 5000 g for 10 min in a microfuge (see Note 6).
5. Aspirate and keep the virus-containing supernatant.
6. Resuspend the pellet in approx 0.25 ml of Solution E and repeat step 4.
7. Aspirate the supernatant; combine with the first supernatant and repeat step 4 (see Note 7).
8. Layer the clarified virus suspension on top of the continuous iodixanol gradients and centrifuge at 234,000 g for 3.5 h at 16°C. Use a slow acceleration and deceleration program up to and down from 2000 rpm. If such a facility is not available, turn off the brake during deceleration below 2000 rpm (see Note 8).
9. Collect the gradient by tube puncture or, if the band is sufficiently well defined, retrieve the banded virus (about half to two thirds of the way down the tube) using a syringe (see Note 9).
2e. Notes
1. The mode of preparing the solutions ensures that the concentrations of buffer and ions are constant throughout the gradient. Any suitable buffer can be used for suspending the virus and for making the gradient solutions and its composition may vary from laboratory to laboratory. As long as the buffer has a low density (approx 1.006 g/ml) the density of the gradients will not be compromised. It might for example be a cell culture medium (e.g. DMEM or RPMI) rather than a balanced salt solution. Application Sheet V01 gives more details on the making up of gradient solutions.
2. In the original papillomavirus method [2-4], a 46% (w/v) iodixanol solution was used as the stock solution from which three gradient solutions of 39%, 33% and 27% (w/v) iodixanol were prepared. In this adaptation the 39% (w/v) stock solution is used as densest gradient solution and the source of the lower density solutions.
3. Larger volume tubes are permissible (e.g. in the Beckman SW41) but the time will need increasing to compensate for the longer sedimentation path and lower RCF (see Note 7) If a vertical rotor is substituted for the swinging-bucket rotor (e.g. Beckman VTi90 or VTi65.1), the shorter sedimentation path length will permit shorter centrifugation times.
4. For larger rotor tubes scale up all volumes proportionately.
5. The volumes used for the cell lysis should be small enough to allow for loading on to the density gradients. Buck et al [2,4] used 0.65 ml per 108 cells in their original papillomavirus methodology. Although the efficacy of the density gradient is considered to be due, at least partly, to sedimentation velocity, the volume of sample can be as much as 2/3 of the gradients volume. This may reflect a very rapid sedimentation of the viral particles to the gradient interface.
6. Higher and lower centrifugation speeds have been used for the clarification step, e.g. 8,000 g [8] and 1,500 g [9] for 15 min. In the latter example filtration through a
7. If the volume of virus suspension is too large, sediment it on to a small cushion (approx. 0.5 ml) of Solution D; a conical-bottomed Beckman konical tube is ideal for this. When aspirating the banded virus make sure that the final iodixanol concentration is no more than approx. 15% (w/v), to facilitate layering on to the gradient. For more information on concentrating virus suspensions see Application Sheet V24. Hamilton et al [9] centrifuged the clarified supernatant at 105,000 g for 90 min to concentrate JC and BK virus as a pellet before resuspending it in buffer. Sunyaev et al [8] pelleted JC virus through a 40% sucrose cushion at 100,000 g for 5 h, followed by treatment with 0.25% deoxycholate.
8. Larger volume rotors can be used at lower RCFs for longer times, e.g. the SW41 at 200,000 g for 4.75 h [4].
9. For more information on harvesting gradients see Application Sheet V04.
3. Comments and recently published papers
Sunyaev et al [8] also used a discontinuous gradient, covering a similar density range (25-40% iodixanol), in which the viral particles were loaded in one of the median layers, centrifuged at 180,000 g for 17 h in a fixed-angle rotor (Beckman 50.2Ti rotor). Median sample loading has been used for high resolution of certain cell organelles, but it has been used relatively rarely for viruses. Median loading allows lighter and denser particles to move in opposite directions during the centrifugation, thus minimizing aggregation of different particles. A simple two layer gradient (26% and 32% w/v iodixanol) centrifuged at 165,000 g for 4 h was used to band Merkel carcinoma polyomavirus at the interface between the two layers (the virus was released from cells by freeze-thawing) [9]. Hamilton et al [10], like Sunyaev et al [8], used a much longer centrifugation time (160,000 g for 16 h) with a 15- 36% (w/v) continuous iodixanol.
Using the purification methodology described in this Application Sheet Schowalter et al [11] described the identification of two previously unknown skin-tropic polyoma viruses and Feng et al [12] reported the banding of fully-encapsidated Merkel cell carcinoma virions at a density of 1.24 g/ml, while those of JC virus banded at approx. 1.20 g/ml. The values were significantly lower than those observed in CsCl and sucrose gradients because of the much lower osmolality of iodixanol gradients.
The authors emphasized the superiority of iodixanol gradients over those of CsCl or sucrose for studies on these viruses.
- Other published papers reporting the use of similar OptiPrep-based methods for polyomavirus, pseudovirions and virus-like particles are given in refs 13-31.
- Notably the method isolated encapsidated virions from the plasma of transplant patients [32].
- Polyoma-like particles have been isolated from Nicotiana benthamiana leaves in a 20%, 30%, 40%, 50% (w/v) iodixanol gradient at 140,000g, for 3 h [33]
- In a recent large study of polyoma contamination of ground beef samples Peretti et al [34] concentrated detergent-soluble capsids on to a 39% (w/v) iodixanol cushion (1.5 ml) by centrifugation at 110,000 g for 2 h, then carefully removed all the supernatant except for the bottom 1.5 ml. The viral material now in 3 ml of 19.5% (w/v) iodixanol was then loaded on to the regular 27%-33%-39% (w/v) iodixanol density gradient and centrifuged for 5 h at 234,000g. The authors concluded that this strategy was applicable to “any DNA virus with a detergent-soluble capsid that is impermeant to nucleases and capable of migrating down an OptiPrep ultracentrifuge gradient”.
- Hurdis et al [35] investigated the structure of the genome and minor capsid proteins using cryoelectron microscopy
- Liu et al [36] studied the mechanisms of Merkel Cell polyoma infection.
- Nguyen et al [37] investigated the association of polyomavirus with pruritic and dyskeratotic dermatoses.
4. Summary of methods for purification of Simian Virus 40 (SV40)
Nakanishi et al [6] used an identical gradient to that described in Section 2, using a slightly lower g- force of 190,000 g. In the most frequently used method however [38] the cells were first lysed in a buffered saline containing 0.5% Brij58, which was then clarified at 16,000 g for 10 min. The supernatant was loaded on to a 20%, 40% discontinuous iodixanol gradient and centrifuged at approx. 240,000 g for 2 g. The virus banded at the interface. The same gradient has been reported in several published papers [39-43], or 20% and 50% [44, 45] or occasionally 20% and 55% (w/v) iodixanol [46, 47]. The centrifugation conditions have generally been 220-235,000 g for 2 h, although in refs 46 and 47 the g-force was lower – 160,000 g).
- Other OptiPrep Application Sheets that may be useful are:
- Concentration of virus particles: see Application Sheet V06
- Harvesting gradients: see Application Sheet V04
- Analysis of gradients: see Application Sheet V05
5a References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Buck, C.B., Pastrana, D.V., Lowy, D.R. and Schiller, J.T. (2004) Efficient intracellular assembly of papillomaviral vectors J. Virol., 78, 751-757
3. Schiller, J.T. (2006) Natl. Cancer Inst. Lab. Tech. file
4. Buck, C.B., Pastrana, D.V., Lowy, D.R. and Schiller, J.T. (2005) Generation of HPV pseudovirions using transfection and their use in neutralization assays Meth. Mol. Med., 119, 445-462
5. Pastrana, D.V., Tolstov, Y.L., Becker, J.C., Moore, P.S., Chang, Y. and Buck, C.B. (2009) Quantitation of human seroresponsiveness to Merkel cell polyomavirus PLoS Pathog., 5: e1000578
6. Nakanishi, A., Chapellier, B., Maekawa, N., Hiramoto, M., Kuge, T., Takahashi, R-u., Handa, H. and Imai, T. (2008) SV40 vectors carrying minimal sequence of viral origin with exchangeable capsids Virology, 379, 110-117
7. Buck, C. and Thompson, C. (2008) Alternative protocol: removal of capsids containing cellular DNA fragments http://home.ccr.cancer.gov/LCO/
8. Sunyaev, S.R., Lugovskoy, A., Simon, K. and Gorelik, L. (2009) Adaptive mutations in the JC virus protein capsid are associated with progressive multifocal leukoencephalopathy (PML) PLoS Genetics 5:e1000368
9. Viscidi, R.P., Rollison, D.E., Sondak, V.K., Silver, B., Messina, J.L., Giuliano, A.R., Fulp, W., Ajidahun, A. and Rivanera, D. (2011) Age-specific seroprevalence of Merkel cell polyomavirus, BK virus, and JC virus Clin. Vaccine Immunol., 18, 1737–1743
10. Hamilton, R.S., Gravell, M. and Major, E.O. (1999) Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus J. Clin. Microbiol., 38, 105- 109
11. Schowalter, R.M., Pastrana, D.V., Pumphrey, K.A., Moyer, A.L. and Buck, C.B. (2010) Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin Cell Host Microbe, 7, 509–515
12. Feng, H., Kwun, H.J., Liu, X., Gjoerup, O., Stolz, D.B., Chang, Y. and Moore, P.S. (2011) Cellular and viral factors regulating Merkel cell polyomavirus replication PLoS One, 6: e22468
13. Tolstov, Y., Pastrana, D.V., Feng, H., Becker, J.C., Jenkins, F.J., Moschos, S., Chang, Y., Bick, C.B. and Moore, P.S. (2009) Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays Int. J. Cancer, 125, 1250-1256
14. Jilek, S., Jaquiéry, E., Hirsch, H.H., Lysandropoulos, A., Canales, M., Guignard, L., Schluep, M., Pantaleo, G., Du Pasquier, R.A. (2010) Immune responses to JC virus in patients with multiple sclerosis treated with natalizumab: a cross-sectional and longitudinal study Lancet Neurol., 9, 264–72
15. Pastrana, D.V., Pumphrey, K.A., Çuburu, N., Schowalter, R.M., Buck, C.B. (2010) Characterization of monoclonal antibodies specific for the Merkel cell polyomavirus capsid Virology 405 (2010) 20–25
16. Schowalter, R.M., Pastrana, D.V. and Buck, C.B. (2011) Glycosaminoglycans and sialylated glycans sequentially facilitate Merkel cell polyomavirus infectious entry PLoS Pathog., 7: e1002161
17. Neumann, F., Borchert, S., Schmidt, C., Reimer, R., Hohenberg, H., Fischer, N., and Grundhoff, A. (2011) Replication, gene expression and particle production by a consensus Merkel cell polyomavirus (MCPyV) genome PLoS One 6: e29112
18. Pastrana, D.V., Wieland, U., Silling, S., Buck, C.B. and Pfister, H. (2012) Positive correlation between Merkel cell polyomavirus viral load and capsid-specific antibody titer Med. Microbiol. Immunol., 201, 17–23
19. Pastrana, D.V., Brennan, D.C., Cuburu, N., Storch, G.A., Viscidi, R.P., Randhawa, P.S. and Buck, C.B. (2012) Neutralization serotyping of BK polyomavirus infection in kidney transplant recipients PLoS Pathog., 8: e1002650
20. Neu, U., Henge, H., Blaum, B.S., Schowalter, R.M., Macejak, D., Gilbert, M., Wakarchuk, W.W., Imamura, A., Ando, H., Kiso, M., Arnberg, N., Garcea, R.L., Peters, T., Buck, C.B. and Stehle, T. (2012) Structures of Merkel cell polyomavirus VP1 complexes define a sialic acid binding site required for infection PLoS Pathog., 8: e1002738
21. Schowalter, R.M., Reinhold, W.C. and Buck, C.B. (2012) Entry tropism of BK and Merkel cell polyomaviruses in cell culture PLoS One, 7: e42181
22. Buck, C.B., Phan, G.Q., Raiji, M.T., Murphy, P.M., McDermott, D.H. and McBride, A.A. (2012) Complete genome sequence of a tenth human polyomavirus J. Virol., 86, 10887
23. Maginnis, M.S., Ströh, L.J., Gee, G.V., O’Hara, B.A., Derdowski, A., Stehle, T. and Atwood, W.J. (2013) Progressive multifocal leukoencephalopathy-associated mutations in the JC polyomavirus capsid disrupt lactoseries tetrasaccharide c binding mBio, 4: e00247-13
24. Li, J., Wang, X., Diaz, J., Tsang, S.H., Buck, C.B. and You, J. (2013) Merkel cell polyomavirus large T antigen disrupts host genomic integrity and inhibits cellular proliferation J. Virol., 87, 9173–9188
25. Pastrana, D.V., Ray, U., Magaldi, T.G., Schowalter, R.M., Çuburu, N. and Buck, C.B. (2013) BK polyomavirus genotypes represent distinct serotypes with distinct entry tropism J. Virol., 87, 10105–10113
26. Schowalter, R.M. and Buck, C.B. (2013) The Merkel cell polyomavirus minor capsid protein PLoS Pathog., 9: e1003558
27. Tsang, S.H., Wang, X., Li, J., Buck, C.B. and You, J. (2014) Host DNA damage response factors localize to Merkel cell polyomavirus DNA replication sites to support efficient viral DNA replication J. Virol., 88, 3285–3297
28. Yatawara, A., Gaidos, G., Rupasinghe, C.N., O’Hara, B.A., Pellegrini, M., Atwood, W.J. and Mierke, D.F. (2015) Small-molecule inhibitors of JC polyomavirus infection J. Pept. Sci., 21, 236–242
29. Randhawa, P., Pastrana, D.V., Zeng, G., Huang, Y., Shapiro, R., Sood, P., Puttarajappa, C., Berger, M., Hariharan, S. and Buck, C.B. (2015) Commercially available immunoglobulins contain virus neutralizing antibodies against all major genotypes of polyomavirus BK Am. J. Transplant., 15, 1014–1020
30. Ray, U., Cinque, P., Gerevini, S., Longo, V., Lazzarin, A., Schippling, S., Martin, R., Buck, C.B. and Pastrana, D.V. (2015) JC polyomavirus mutants escape antibody-mediated neutralization Sci. Transl. Med. 7: 306ra151
31. Hurdiss, D.L., Morgan, E.L., Thompson, R.F., Prescott, E.L., Panou, M.M., Macdonald, A. and Ranson, N.A. (2016) New structural insights into the genome and minor capsid proteins of BK polyomavirus using cryoelectron microscopy Structure, 24, 528–536
32. Ho, J., Jedrych, J.J., Feng, H., Natalie, A.A., Grandinetti, L., Mirvish, E., Crespo, M.M., Yadav, D., Fasanella, K.E., et al (2015) Human polyomavirus 7–associated pruritic rash and viremia in transplant recipients J. Infect. Dis., 211, 1560–1565
33. Catrice, E.V.B. and Sainsbury, F. (2015) Assembly and purification of polyomavirus-like particles from plants Mol. Biotechnol., 57, 904–913
34. Peretti, A., FitzGerald, P.C., Bliskovsky, V., Buck, C.B. and Pastrana, D.V. (2015) Hamburger polyomaviruses J. Gen. Virol., 96, 833–839
35. Hurdiss, D.L., Morgan, E.L., Thompson, R.F., Prescott, E.L., Panou, M.M., Macdonald, A. and Ranson, N.A. (2016) New structural insights into the genome and minor capsid proteins of BK polyomavirus using cryoelectron microscopy Structure, 24, 528–536
36. Liu, W., Yang, R., Payne, A.S.,Schowalter, R.M., Spurgeon, M.E., Lambert, P.F., Xu, X., Buck, C.B. and You, J. (2016) Identifying the target cells and mechanisms of Merkel Cell polyomavirus infection Cell Host Microbe, 19, 775–787
37. Nguyen, K.D., Lee, E.E., Yue, Y., Stork, J., Pock, L., North, J.P., Vandergriff, T., Cockerell, C., Hosler, G.A. et al (2017) Human polyomavirus 6 and 7 are associated with pruritic and dyskeratotic dermatoses J. Am. Acad. Dermatol., 76, 932-940
38. Inoue, T. and Tsai, B. (2011) A large and intact viral particle penetrates the endoplasmic reticulum membrane to reach the cytosol PLoS Pathog., 7: e1002037
39. Ravindran, M.S., Bagchi, P., Inoue, T. and Tsai, B. (2015) A non-enveloped virus hijacks host disaggregation machinery to translocate across the endoplasmic reticulum membrane PLoS Pathog., 11: e1005086
40. Ravindran, M.S., Engelke, M.F., Verhey, K.J. and Tsai, B. (2017) Exploiting the kinesin-1 molecular motor to generate a virus membrane penetration site Nat. Comm., 8: 15496
41. Dupzyk, A., Williams, J.M., Bagchi, P., Inoue, T. and Tsai, B. (2017) SGTA-dependent regulation of Hsc70 promotes cytosol entry of simian virus 40 from the endoplasmic reticulum J. Virol., 91: e00232-17
42. Walczak, C.P., Ravindran, M.S., Inoue, T. and Tsai, B. (2014) A cytosolic chaperone complexes with dynamic membrane J-proteins and mobilizes a non-enveloped virus out of the endoplasmic reticulum PLoS Pathog., 10: e1004007
43. Bagchi, P., Walczak, C.P. and Tsaia, B. (2015) The endoplasmic reticulum membrane J protein C18 executes a distinct role in promoting simian virus 40 membrane penetration J. Virol., 89, 4058-4068
44. Enomoto, T., Kukimoto, I., Kawano, M-a., Yamaguchi, Y., Berk, A.J. and Handa, H. (2011) In vitro reconstitution of SV40 particles that are composed of VP1/2/3 capsid proteins and nucleosomal DNA and direct efficient gene transfer Virology, 420, 1–9
45. Kitai, Y., Fukuda, H., Enomoto, T., Asakawa, Y., Suzuki, T., Inouye, S. and Handa, H. (2011) Cell selective targeting of a simian virus 40 virus-like particle conjugated to epidermal growth factor J. Biotechnol., 155, 251– 256
46. Murata, H., Teferedegne, B., Lewis, A.M. and Peden, K. (2009) A quantitative PCR assay for SV40 neutralization adaptable for high-throughput applications J. Virol. Methods 162, 236–244
47. Murata, H., Macauley, J., Lewis Jr., A.M. and Peden, K. (2011) Plaque purification as a method to mitigate the risk of adventitious-agent contamination in influenza vaccine virus seeds Vaccine 29, 3155–3161
48. Bagchi, P., Walczak, C.P. and Tsaia, B. (2015) The endoplasmic reticulum membrane J protein C18 executes a distinct role in promoting simian virus 40 membrane penetration J. Virol., 89, 4058-4068
5b References
Becker, M., Dominguez, M., Greune, L., Soria-Martinez, L., Pfleiderer, M.M., Schowalter, R., Buck, C.B., Blaum, B.S., Schmidt, M.A. and Schelhaasa, M. (2019) Infectious entry of Merkel cell polyomavirus J. Virol., 93: e02004-18
Peretti, A., Geoghegan, E.M., Pastrana, D.V., Smola, S., Feld, P., Sauter, M., Lohse, S., Ramesh, M., Lim, E.S. et al (2018) Characterization of BK polyomaviruses from kidney transplant recipients suggests a role for APOBEC3 in driving in-host virus evolution Cell Host Microbe 23, 628–635
6. Acknowledgements
We are indebted to Dr Chris Buck, Laboratory of Cellular Oncology, N.C.I., Bethesda, MD 20892- 4263 for his kind help in the preparation of this OptiPrep Application Sheet.
OptiPrep™ Application Sheet V11; 6th edition, March 2020
OptiPrep™ Application Sheet V12
Purification of Group I (ds)DNA viruses: Poxviridae
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- This Application Sheet primarily covers the purification and analysis of modified Vaccinia virus (Ankara), but Section 6 also briefly covers Molluscipoxvirus
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
There are now many published papers that report the use of iodixanol gradients not only to purify viruses but also to investigate their assembly. In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which, in solutions of the same density, is much higher than iodixanol.
Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast both infectivity measurements using cultured cells and many add-on techniques can be performed without dialysis of iodixanol. Combined with the availability of OptiPrep™ as a sterile solution, this makes the use of OptiPrep™ for virus purification and assembly analysis much more convenient than the use of either CsCl or sucrose.
Section 4a in this OptiPrep™ Application Sheet [2] describes the purification of modified vaccinia virus Anakara (MVA), released from infected cells by sonication, by banding at an interface between two solutions of iodixanol (22% and 32%).
Section 4b in this OptiPrep Application Sheet describes a slightly more sophisticated technique in which the intracellular mature virus (IMV) was processed from a post-nuclear supernatant of homogenized infected cells [3]. The iodixanol gradient used to purify the IMV was continuous rather than discontinuous [4]. This same gradient was also used to purify the extracellular enveloped virus (EEV) form of the virus (from the culture medium) that is formed as the IMV is exocytosed at the cell surface [4]. More recently this continuous gradient has been used to separate IMV and EEV particles from MVA preparations [5] and the purification of a recombinant vaccinia virus (VV-Osp-A) [6].
- The notes referred to in the following methods can be found in Section 5
 2. Solutions required (see Note 1)
2. Solutions required (see Note 1)
A. OptiPrep™
B. OptiPrep™ diluent: 60 mM Tris-HCl, pH 9.0 (see Note 1)
C. 50% (w/v) Iodixanol Working Solution: mix 5 vol. Solution A with 1 vol. of Solution B
D. Diluent: 10 mM Tris-HCl, pH 9.0
E. Phosphate-buffered saline or cell culture medium
F. Barrier (for Protocol B only): 36% (w/v) sucrose in 10 mM Tris-HCl, pH 9.0 (see Step 6 of Protocol B)
3. Rotor requirements
Protocol 4a: Swinging-bucket rotors for 39 ml or 17 ml gradients, such as the Beckman SW28, Beckman SW28.1 or Sorvall AH629; for 5 ml gradients, rotors such as the Beckman SW55Ti, MLS50 or Sorvall AH650 Protocol 4b: Swinging-bucket rotor for 17 ml gradients, such as the Beckman SW28, Beckman SW28.1 or Sorvall AH629 4A. Protocol (adapted from ref 2) Carry out all operations at 4°C
1. Wash the infected cells in Solution E; resuspend in the same medium and freeze-thaw the cells.
2. Release the virus from the cells by mild sonication.
3. Clarify the suspension by low speed centrifugation (approx 1000 g for 15 min) to remove cellular debris.
4. To concentrate the virus transfer the suspension to 17 ml or 39 ml tubes for the swinging-bucket rotor and underlay with a cushion of 2-5 ml of Solution C using a syringe and metal cannula (see Note 2)
5. Centrifuge at 80,000 gav for 3 h
6. Using a syringe and metal cannula remove as much of the cushion as possible (below the virus band) then recover the virus band from each gradient in 2-3 ml of the supernatant (see Notes 3 and 4).
7. If necessary these concentration steps can be repeated in tubes for the 5 ml swinging-bucket rotor using just 1 ml of 50% iodixanol cushion and centrifuging at 128,000 g for 2 h (see Note 5).
8. During the final concentration centrifugation, mix Solution C with Solution D to make solutions of 22% and 32% (w/v) iodixanol (see Note 6).
9. Layer the concentrated virus suspension over equal volumes of the two iodixanol solutions and centrifuge at 50,000 gav for 3 h. In a 5 ml tube use 0.5-1.0 ml of virus suspension over approx. 2 ml of each iodixanol solution; in a 17 ml tube use 2-3 ml of virus over approx. 7 ml of each iodixanol solution (see Notes 7 and 8).
10. Harvest the purified virus from the interface between the two iodixanol solutions.
4B. Protocol (adapted from refs 3 and 4)
Carry out all operations at 4°C
For pre-gradient processing of IMV follow Steps 1-7
For pre-gradient processing of EEV follow Steps 8-9
For purifying IMV and EEV follow Steps 10-13
1. Scrape the infected cells from the dish and pellet them at approx. 600g for 10 min.
2. Suspend the cells in Solution D.
3. To release the IMV homogenize the cells using 10-12 strokes of a tightly fitting pestle (Wheaton Type A) of a Dounce homogenizer (see Note 9).
4. Centrifuge the homogenate at approx 1000 g for 5 min to pellet the nuclei and carefully aspirate the supernatant.
5. Repeat Step 4 using the post-nuclear supernatant in order to maximize the removal of the nuclei.
6. Concentrate and partially purify the virus by centrifuging it in 17 ml tubes through Solution F at 70,000 gav for 30 min (see Note 10).
7. Remove all the supernatant and suspend the viral pellet in 2 ml of Solution D.
8. Harvest the culture medium from the cells and clarify it at 2000 g for 10 min.
9. Pellet the virus at 75,000 gav for 30 min and resuspend in 2 ml of Solution D.
10. Sonicate the viral suspension (water bath sonicator) for 1 min.
11. Mix Solution C with Solution D to make solutions of 22% and 32% (w/v) iodixanol and use equal volumes (7.5 ml) of the two iodixanol solutions in a two-chamber gradient maker or a Gradient Master™ to make a continuous gradient in a tube for the 17 ml swinging-bucket rotor (see Note 11).
12. Layer the viral suspension over the iodixanol gradient and centrifuge at 75,000 gav for 45 min (see Note 12).
13. Collect the banded IMV (higher density) and/or EEV (lower density). Each band may be collected separately using a syringe or the whole gradient may be unloaded by upward displacement, aspiration from the meniscus or by tube puncture in 0.5-1.0 ml fractions (see Notes 13 and 14).
5. Notes
1. Any low-density solution compatible with the virus and/or with the subsequent analysis can be used to dilute the OptiPrep™ or a Working Solution produced from OptiPrep™. More details on the making up of gradient solutions may be found in Application Sheet V01.
2. Underlayering the small volume of dense cushion is certainly much easier than overlayering the large volume of virus-containing fluid.
3. It is important to avoid contaminating the recovered concentrated virus with too much of the dense cushion since the virus suspension will subsequently be layered on top of a 22% iodixanol solution. For more information on the concentration of virus see Application Sheet V06.
4. The use of an interface to concentrate the virus, rather than pelleting, may make the production of a non-aggregated suspension of the virus easier and also avoids the loss of infectivity that often accompanies pellet formation.
5. A second round of concentration may be even more useful when larger volumes of virus suspension are used in the first concentration step.
6. Sandgren et al [7] used a discontinuous gradient of 16/32% (w/v) iodixanol (140,000 g for 1h) and then, after dilution of the virus collected from the interface, it was concentrated on to a 50% iodixanol cushion at 24,000 g for 45 min in a 14 ml tube of a swinging-bucket rotor.
7. Make sure that the virus suspension has a sufficiently low density to permit layering on the 22% iodixanol.
8. It may be permissible to use larger volumes of virus and smaller volumes of the gradient solutions; this will need to be confirmed by recovery and purity data.
9. There are a variety of methods for homogenizing cells. The hypoosmotic Solution D would facilitate the breakage by causing cell swelling, but this may not be optimal for the preservation of nuclear integrity. Since the nuclei are subsequently removed from the homogenate by centrifugation (Step 4) this may be a point worth investigating further. For more information on cell disruption see ref 8 and Application Sheet S06 (see Subcellular membrane index).
10. Since Sancho et al [5] recognized that the final iodixanol gradient was more efficient than the original sucrose gradient [3] in the recovery of infective EEVs, replacement of the sucrose barrier by an iodixanol one might merit consideration. It is difficult to predict what might be a suitable solution because of the big difference between the osmolality of sucrose and iodixanol solutions, but a 20% (w/v) solution might be a good starting point. This will need to be confirmed by recovery and purity data.
11. A continuous gradient can alternatively be constructed by allowing a discontinuous gradient (22%, 25%, 28% and 32% iodixanol layers) to diffuse. For more information on making gradients see Application Sheet V02.
12. The sample loaded on to the 22-32% (w/v) iodixanol gradient could also be an MVA suspension [5].
13. For more information on harvesting gradients see Application Sheet V04.
14. The denser band is essentially pure IMVs, while the lighter EEV band contains some IMV contamination.
6. Molluscipoxvirus
Bugert [9] purified this virus from skin lesions using iodixanol gradients and obtained excellent structural analysis data from the isolate, but did not provide a detailed methodology.
7. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Sandbulte, M. R., Platt, R. and Roth, J. A. (2004) T cells from a high proportion of apparently naive cattle can be activated by modified vaccinia virus Ankara (MVA) Viral Immunol., 17, 39-49
3. Jensen, O.N., Houthaeve, T., Shevchenko, A., Cudmore, S., Ashford, T., Mann, M., Griffiths, G. and KrijnseLocker, J. (1996) Identification of the major membrane and core proteins of vaccinia virus by twodimensional electrophoresis J. Virol., 70, 7485-7497
4. Krijnse-Locker, J., Kuehn, A., Schleich, S., Rutter, G., Hohenberg, H., Wepf, R. and Griffiths, G. (2000) Entry of the two infectious forms of vaccinia virus at the plasma membrane is signaling-dependent for the IMV but not the EEV Mol. Biol. Cell, 11, 2497-2511
5. Sancho, M. C., Schleich, S., Griffiths, G. and Krijnse-Locker, J. (2002) The block in assembly of modified vaccinia virus Ankara in HeLa cells reveals new insights into vaccinia virus morphogenesis J. Virol., 76, 8318-8334
6. Scheckelhoff, M.R., Telford, S.R. and Hu, L.T. (2006) Protective efficacy of an oral vaccine to reduce carriage of Borrelia burgdorferi (strain N40) on mouse and tick reservoirs Vaccine, 24, 1949-1957
7. Sandgren, K.J., Wilkinson, J., Miranda-Saksena, M., McInerney, G.M., Byth-Wilson, K., Robinson, P.J. and Cunningham, A.L. (2010) A differential role for macropinocytosis in mediating entry of the two forms of vaccinia virus into dendritic cells PLoS Pathogens, 6: e1000866
8. Bugert, J.J. (2007) Genus molluscipoxvirus In Poxviruses (ed. Mercer, A., Schmidt, A. and Weber, O.) Birkhäuser Verlag, Basel, Switzerland, pp 89-112
7. Acknowledgements
We would like to thank Jacomine Krijnse-Locker, European Molecular Biology Laboratory, Heidelberg for valuable information in the preparation of this Application Sheet.
OptiPrep™ Application Sheet V12; 8th edition, January 2020
OptiPrep™ Application Sheet V13
Purification of Group I (ds)DNA viruses (non-mammalian sources): Iridoviridae
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
- Note that the purification of viruses that grow in other non-mammalian cells (e.g. algae, protozoa, marine arthropods, plant cells, etc) is summarized in Application Sheet V37).
1. Background
This Application Sheet describes the purification of a Group I (ds)DNA iridovirus from the Singapore grouper.
In all comparative studies between CsCl and iodixanol, it has been shown that the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from enveloped viruses has been noted [2]. This may be related to its viscosity, which is much higher than that of iodixanol. Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast, many add-on techniques can be performed and cells infected with virus, without dialysis of iodixanol.
 2. Iridovirus purification
2. Iridovirus purification
The following protocol is adapted from ref 1. The virus was grown in grouper embryonic cells and the cells were lysed in a buffered NaCl solution, but the gradient solutions were prepared by dilution of OptiPrep™ with isoosmotic buffered sucrose.
2a. Solutions required
A. OptiPrep™
B. Cell lysis buffer: 0.1 M NaCl, 5 mM EDTA, 50 mM
Tris-HCl, pH 7.4
C. OptiPrep™ dilution buffer: 7.4% (w/v) sucrose, 4 mM EDTA, 40 mM Tris-HCl, pH 7.4 (see Section 2d Note 1)
D. Dilution buffer for gradient stock solution: 7.4% (w/v) sucrose, 2 mM EDTA, 20 mM Tris-HCl, pH 7.4
2b. Ultracentrifuge rotor requirements
Swinging-bucket rotor (e.g. Beckman SW28 or SW41Ti) and a vertical or near-vertical rotor (e.g. Beckman Vti65.1 or NVT65) – see Section 2d Note 2.
2c. Protocol
1. Homogenize the cells using Dounce homogenizer or similar device to release the virus and clarify and centrifuge at 1500 g for 20 min to remove cellular debris. The supernatant may be clarified by passage through a 0.22 μm filter.
2. Dilute the OptiPrep™ with an equal volume of Solution C to produce an isoosmotic 30% (w/v) iodixanol solution containing 2 mM EDTA and 20 mM Tris-HCl, pH 7.4; then diluted this stock solution with Solution D to produce 5%, 10% and 20% (w/v) iodixanol solutions.
3. In the chosen swinging-bucket rotor layer equal volumes of the, 5%, 10%, 20% and 30% (w/v) iodixanol and layer the crude virus suspension on top. See Application Sheet V02 for more information about making these gradients.
4. Centrifuge at 55,000 g for 1 h. The virus should band at the 20%/30% iodixanol interface.
5. Remove the solutions from above the virus band, then aspirate the virus using a syringe and metal cannula, taking as little of the 30% (w/v) solution as possible (see Application Sheet V04 for information about harvesting material from gradients)
6. Dilute the virus suspension with 25% (w/v) iodixanol (gradient stock diluted with Solution D) and transfer to tubes for the chosen vertical or near-vertical rotor and centrifuge at approx 350,000 g for 2-3 h (see Section 2d Note 2).
7. Collect the visible band of virus.
2d. Notes
1. The production of a stock solution of 30% (w/v) iodixanol that contains 2mM EDTA and 20 mM Tris-HCl, pH 7.4 in order to make the gradient solutions ensures not only that all the gradient solutions are approx. isoosmotic, but also they all contain 2 mM EDTA and 20 mM Tris. If this is regarded as unnecessary then simply dilute the OptiPrep™ with Solution D. For more information on the preparation of density gradient solutions see Application Sheet V01.
2. Wu et al [2] used a Beckman SW41 rotor for creating a self-generated gradient and centrifuged the tubes at 87,000 g overnight. Most commonly (see Application Sheet V03) a vertical or nearvertical rotor is used at a much higher g-force for much shorter time (as recommended). The type of gradient that forms at 87,000 g overnight in a swinging-bucket rotor has not been investigated. The operator must choose between the two alternatives.
3. References
1. Wu, J., Chan, R., Wenk, M.R. and Hew, C-L. (2010) Lipidomic study of intracellular Singapore grouper iridovirus Virology 399, 248–256
2. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445Dettenhoffer, M. and Yu, XF. (1999) J. Virol., 73, 1460-1467
OptiPrep™Application Sheet V13; 3rd edition, January 2020
OptiPrep™ Application Sheet V14
Purification of Group II (ss)DNA viruses: Parvoviridae: recombinant adenoassociated virus (rAAV) and avian AAV
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- This Application Sheet describes two methods for purifying rAAV with pre-formed gradients.
- Zolotukhin et al [1] devised a discontinuous gradient (Section 2); this method has also been used for avian AAV [2]. Hermens et al [3] devised a continuous gradient (Section 3).
- The Zolotukhin et al method [1] has also been used for the purification of viruses of the Parvovirinae subfamily [4], see Application Sheet V16 for more details.
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
- There are three important related files:
1. Application Sheet V06 “Purification of viruses and viral vectors using OptiPrep™”: a summary of the basic methodologies for all viruses
2. Reference Lists RV02-1 (Part A) and RV02-2 list over 1200 references related to rAAV research : to access return to the initial list of Folders and select “Reference Lists”
1. Background
Viral vectors that are of potential use in gene therapy would clearly benefit from isolation methods which are both effective and cause little or no damage to the viral particles. Density gradient centrifugation has always played an important part in the concentration and purification of virus particles but the gradient media that have been used most prominently, sucrose and CsCl, pose a number of problems. Both media are highly hyperosmotic at the densities used to band viruses (sucrose solutions are also very viscous) and generally have to be removed either by pelleting the virus or by dialysis, prior to further processing or analysis. CsCl also leads to poor recoveries and low infectivity of rAAV isolates.
Because of the very low water activity of CsCl solutions, viruses tend to have significantly higher density in this medium compared to media such as sucrose or any of the iodinated density gradient media, although the magnitude of this difference varies from virus to virus. Many viruses in CsCl have a density of approx 1.34 g/ml, in iodixanol the density range is generally 1.16-1.22 g/ml, although some viruses may be as low as 1.14 g/ml or as high as 1.24 g/ml. rAAV falls into the latter category.
OptiPrep™ is widely regarded as the gradient medium of choice for rAAV purification. Compared to CsCl gradients:
- Recovery of virus from the gradient is at least ten times greater
- Particle:infectivity titer is up to 100x lower
- Infectivity measurements and many add-on techniques can be carried out without the need to dialyze the medium.
2. Discontinuous gradient
The method is adapted from Zolotukhin et al [1].
 2a. Solutions required
2a. Solutions required
A. OptiPrep™
B. 10xPhosphate-buffered saline containing 10 mM MgCl2 and 25 mM KCl (10xPBS-MK)
C. Phosphate-buffered saline containing 1 mM MgCl2 and 2.5 mM KCl (PBS-MK)
D. 2 M NaCl in PBS-MK
E. Working solution: 54% (w/v) iodixanol in PBS-MK: mix 9 vol of OptiPrep with 1 vol Solution B.
2b. Ultracentrifuge rotor requirements
Fixed-angle rotor with approx 39 ml sealed tubes capable of approx 350,000 g (e.g. Beckman 70Ti or Sorvall T865; see Section 4, Note 1).
2c. Protocol
1. Prepare the following gradient solutions (see Section 4, Notes 2 and 3): 15% (w/v) iodixanol containing 1 M NaCl in PBS-MK: 1.5 vol. of Solution E + 2.7 vol. of Solution D + 1.2 vol of Solution C.
25% (w/v) iodixanol in PBS-MK: 2.5 vol. of Solution E + 2.9 vol. of Solution C
40% (w/v) iodixanol in PBS-MK: 4.0 vol. of Solution E + 1.4 vol. of Solution C.
2. Clarify the cell lysate clarified by centrifugation at 4000 g for 20 min.
3. Underlayer 10-15 ml of clarified lysate with 9 ml of 15% iodixanol; 6 ml of 25% iodixanol, 5 ml of 40% iodixanol and 5 ml of the 54% iodixanol working solution (see Note 3). Use a long metal cannula (0.8 mm i.d.) attached to a syringe or via tubing to a peristaltic pump to load the tube (see Section 4, Note 4).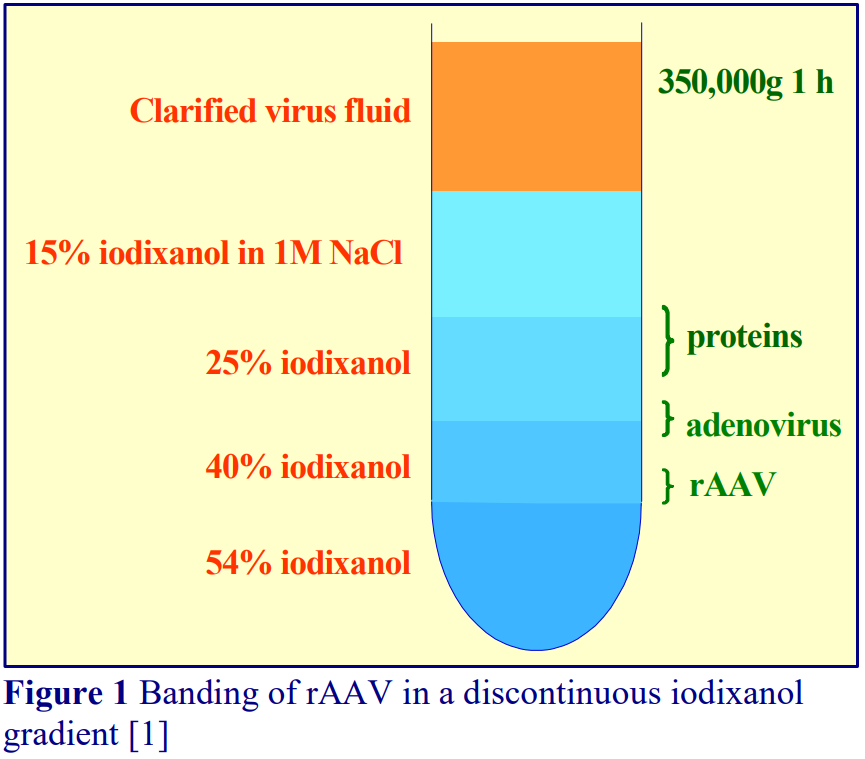 4. Centrifuge at 350,000 gav for 1 h at 18°C. Use a slow acceleration and deceleration programme (up to and below 2000 rpm) if this facility is
4. Centrifuge at 350,000 gav for 1 h at 18°C. Use a slow acceleration and deceleration programme (up to and below 2000 rpm) if this facility is
available on the centrifuge, or turn off the brake below 2000 rpm during deceleration.
5. Either collect the whole gradient (Figure 1) in 1- 2 ml fractions dense end first or use a syringe inserted just below the 40%/54% interface to aspirate no more than 2-3 ml of the 40% layer (see Section 4, Notes 5-8).
3. Continuous gradient
The method is adapted from Hermens et al [3] who used a preformed continuous gradient in a nearvertical rotor. After release of rAAV from cultured cells by freeze/thawing, the virus particles from the clarified fluid were first concentrated either by ammonium sulfate precipitation or by cellufine sulfate column chromatography [3].
3a. Solutions required
A. OptiPrep™
B. Phosphate-buffered saline
3b. Ultracentrifuge rotor requirements
Near-vertical rotor with approx 5 ml tubes, capable of approx 360,000g (see Section 4, Note 9)
3c. Protocol
1. After concentration by ammonium sulfate precipitation or chromatography, suspend rAAV in phosphate-buffered saline, pH 7.4.
2. Transfer 2.7 ml of rAAV-containing fluid to a suitable tube and underlayer with OptiPrep™ to fill the tube.
3. After sealing the tube, form the gradient in a Gradient Master by rotating at 20 rpm at 80° for 12 min (see Section 4, Note 10)
4. Centrifuge at 71,000 rpm (348,000 gav) for 3 h at 16C (see Section 4, Note 10).
5. Collect the gradient from the bottom by tube puncture, the rAAV bands close to the bottom of the gradient (see Section 4, Note 11).
4. Notes
1. For smaller volume tubes scale down all volumes proportionally. It may be necessary to increase the centrifugation time proportionally if the rotor cannot achieve 350,000 gav.
2. Phenol red (0.01 µg/ml) may be included in the alternate gradient layers to enhance visual identification of the layers. At 350,000 g, the iodixanol itself will sediment and may make the interfaces less obvious.
3. Aggregation of rAAV with proteins in the cell lysate can pose a serious problem to its isolation as the aggregates are heterogeneous and consequently exhibit a broad range of densities. Inclusion of 1 M NaCl in the 15% iodixanol prevents this aggregation and allows the rAAV to be isolated as a single band in the 40% iodixanol layer.
4. Because of the large volumes used in this gradient, the use of a peristaltic pump to introduce the iodixanol solutions makes this task easier. For more information on preparing discontinuous gradients see Application Sheet V02.
5. All of the contaminating proteins in the lysate band within the 25% iodixanol layer and more than 99% of any adenovirus contaminant bands at a lower density (<1.22 g/ml.) than the rAAV.
6. Great care needs to be exercised in removing the rAAV band to avoid contamination not only from any adenovirus but also empty capsids which are also band at a lower density.
7. Further purification by ion exchange or heparin affinity chromatography can be carried out directly on the iodixanol-containing fractions.
8. For many applications such as electrophoresis, infection of cultured cells, administration to experimental animals removal of the iodixanol is not a requirement. If it is an absolute requirement to remove (or at least reduce the concentration of) the iodixanol then some form of ultrafiltration is widely regarded as the most effective method: Vivaspin membranes from Sartorius and Centricon Plus 70 centrifugal filters from Millipore, or a PBHK Centrifugal Plus-20 filter unit with an Ultracel PL membrane (100 kDa cut off). Tangential flow filtration is also effective. For more information on this subject see Application Sheet V06
9. In ref 2 a NVT90 was used; other vertical and near vertical rotors with similar sedimentation path lengths may be suitable.
10. Because of the high density of rAAV, it is necessary to ensure that the bottom of the gradient is sufficiently dense to avoid the rAAV from reaching the wall of the tube during centrifugation. The gradient that is generated by the Gradient Master will tend to increase sharply in density towards the bottom and this will be enhanced during the subsequent centrifugation.
11. For more information about harvesting gradients see Application Sheet V04.
- Lock et al [5] compared the use of iodixanol and CsCl methods for the purification of rAAV and noted the considerable improved transduction (both in vitro and importantly in vivo) of the iodixanol-purified material. Moreover only the iodixanol gradient separated infectious particles from empty capsids, which the authors deemed “a desirable property for reducing toxicity and unwanted immune responses during preclinical studies”.
- There are many reviews of the rAAV technology; some provide very detailed protocols of the entire procedure, some are concerned with comparisons of the available methodology; these are all listed in Reference List RV02. Just two of the latest publications are given: refs. 6 and 7. The former provides very detailed methodology for the entire purification procedure the latter compares handling of two different serotypes rAAV2/5 and rAAV2/9 during the continuous collection of rAAV from producer cell medium.
5. References
1. Zolotukhin, S., Byrne, B.J., Mason, E., Zolotukhin, I., Potter, M., Chesnut, K., Summerford, C., Samulski, R.J. and Muzyczka, N. (1999) Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield Gene Ther., 6, 973-985
2. Matsui, R., Tanabe, Y. and Watanabe, D. (2012) Avian adeno-associated virus vector efficiently transduces neurons in the embryonic and post-embryonic chicken brain PLoS One 7: e48730
3. Hermens, W.T.J.M.C., Ter Brake, O., Dijkhuizen, P.A., Sonnemans, M.A.F., Grimm, D., Kleinschmidt, J.A. and Verhaagen, J. (1999) Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous Human Gene Ther., 10, 1885-1891
4. Bloom, M.E., Best, S.M., Hayes, S.F., Wells, R.D., Wolfinbarger, J.B., McKenna, R. and AgbandjeMcKenna, M. (2001) Identification of Aleutian mink disease parvovirus capsid sequences mediating antibody-dependent enhancement of infection, virus neutralization, and immune complex formation J. Virol., 75, 11116-11127
5. Lock, M., Alvira, M., Vandenberghe, L.H., Samanta, A., Toelen, J., Debyser, Z. and Wilson, J.M. (2010) Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale Hum. Gene Ther., 21, 1259–1271
6. Burger, C. and Nash, K.R. (2016) Small-scale recombinant adeno-associated virus purification In Gene Therapy for Neurological Disorders: Methods and Protocols: Methods in Molecular Biology, vol. 1382 (ed. Manfredsson, F.P.) Springer Science+Business Media New York, pp 95-106
7. Benskey, M.J., Sandoval, I.M. and Manfredsson, F.P. (2016) Continuous collection of adeno-associated virus from producer cell medium significantly increases total viral yield Hum. Gene Ther. Meth., 27, 32-45
OptiPrep™ Application Sheet V14; 11th edition, January 2020
OptiPrep™ Application Sheet V15
Purification of Group II (ss)DNA viruses: Protoparvovirus – murine minute virus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
- The OptiPrep™ Reference List (RV02-1), Part B contains a list of papers describing the use of iodixanol gradients for all Group II viruses (except rAAV); to access return to the initial list of Folders and select “Reference Lists”.
1. Background
Density gradient centrifugation has always played an important part in the concentration and purification of virus particles but the gradient media that have been used most prominently, sucrose and CsCl, pose a number of problems. Both media are highly hyperosmotic at the densities used to band viruses (sucrose solutions are also very viscous) and generally have to be removed either by pelleting the virus or by dialysis, prior to further processing or analysis. CsCl also leads to poor recoveries and low infectivity of virus isolates.
Because of the very low water activity of CsCl solutions, viruses tend to have significantly higher density in this medium compared to media such as sucrose or any of the iodinated density gradient media, although the magnitude of this difference varies from virus to virus. Many viruses in CsCl have a density of approx 1.34 g/ml, in iodixanol the density range is generally 1.16-1.22 g/ml, although some viruses may be as low as 1.14 g/ml or as high as 1.26 g/ml. MMV is one of the denser ones banding at approx 1.26 g/ml.
In studies where the iodixanol and CsCl gradients have been compared:
- Recovery of virus from the gradient is at least ten times greater
- Particle:infectivity titer is up to 100x lower
- Infectivity measurements and many add-on techniques can be carried out without the need to dialyze the medium.
2. Protocols
In the method devised by Cotmore and Tattershall [1], the virus was released from infected cells by three cycles of freeze-thawing in 50 mM Tris-HCl, 0.5 mM EDTA, pH 8.7. Virus was purified in a discontinuous gradient of iodixanol.
 The gradient is able to discriminate mature virions from viral DNA, which is much lighter – the low density of DNA banding is a characteristic only of iodixanol gradients
The gradient is able to discriminate mature virions from viral DNA, which is much lighter – the low density of DNA banding is a characteristic only of iodixanol gradients
2a. Solutions required (see Section 2d Note 1)
A. OptiPrep™
B. 10xPhosphate-buffered saline containing 10 mM MgCl2 and 25 mM KCl, pH 7.2
C. 5 mM EDTA, 500 mM Tris-HCl, pH 7.8
D. 0.5 mM EDTA, 50 mM Tris-HCl, pH 7.8
2b. Ultracentrifuge rotor requirements
Swinging-bucket rotor with approx. 13 ml tubes, such as Beckman SW41Ti
2c. Protocol
1. Prepare the following iodixanol gradient solutions in EDTA-Tris: 15% (w/v) iodixanol, mix 15 vol. OptiPrep™ with 6 vol. of Solution C and 39 vol. of water; 35% (w/v) iodixanol, mix 35 vol. OptiPrep™ with 6 vol. of Solution C and 19 vol. of water (see Section 2d Note 2).
2. Prepare the following iodixanol gradient solutions in PBS-Mg-K: 45% (w/v) iodixanol, mix 45 vol. OptiPrep™ with 5 vol. of Solution B and 10 vol. of water; 55% (w/v) iodixanol, mix 55 vol. OptiPrep™ with 5 vol. of Solution B (see Section 2d Note 2).
3. Release the virus from the cells in Solution D by three cycles of freeze-thawing.
4. Clarify the suspension by centrifugation at 15,000 g for 30 min at 4°C.
5. Prepare discontinuous gradients from 1 ml of 55%, 2 ml of 45%, 2 ml of 35% and 1.5 ml of 15% iodixanol (see Section 2d Notes 3 and 4). For more information on the construction of discontinuous gradients see Application Sheet V02.
6. Layer approx. 6 ml of the clarified virus suspension on top of the gradient, to fill the tube according to the manufacturer’s specifications.
7. Centrifuge at approx. 150,000 gav for 18 h at 18°C. Allow the rotor to decelerate to zero using a controlled deceleration program, or turn off the brake below 2000 rpm.
8. Harvest the gradient in 0.5-1 ml fractions dense-end first. For more information about harvesting gradients see Application Sheet V04.
2d. Notes
1. In the original methodology [1], all of the gradient solutions were prepared in PBS. Later [2] improved resolution was obtained by making up the denser two solutions in PBS, 1 mM MgCl2, 2 mM KCl, pH 7.2 and the lighter two solutions in 50 mM Tris-HCl (pH 8.7), 0.5 mM EDTA. This strategy was also reported in subsequent publications [3-11]. Farr et al [12] used two types of gradient, one at pH 7.5 in which gradient solutions contained PBS, 1 mM MgCl2, 2 mM KCl, pH 7.2 pH 7.5 or 50 mM MES (pH 5.5) 120 mM NaCl, 1 mM MgCl2, 2 mM KCl. The authors compared the tryptic digestion of virions at pH 5.5 and 7.5. Wild-type virions after trypsin treatment banded close the 45%/55% iodixanol boundary at both pHs. However a threoninesubstituted variant shifted the banding in the pH 7.5 gradient to a lower density. Plevka et al [13] also studied similar gradients (see Note 3).
2. Smaller volume modified gradients of 0.5 ml 55% iodixanol and 1 ml each of 45%, 35%, 25% iodixanol and 0.5 ml of 15% iodixanol in 5 ml tubes (e.g. Beckman SW50.1 or SW55Ti) are centrifuged at 140,000 gav for 20 h.
3. Cotmore and Tattershall [14] used similar iodixanol gradients of 55% (0.75 ml), 45% (1.5 ml), 35% (1 ml) and 15% (0.75 ml) in PBS containing 5 mM KCl and 1 mM MgCl2 (in 5 ml tubes) in an in vitro analysis of genome uncoating. The gradients resolve fully infectious virions from less dense empty particles. Studies showed that mutant forms displayed a distinctive shift towards less dense profiles.
3. References
1. Cotmore, S.F. and Tattersall, P. (2005) Encapsidation of minute virus of mice DNA: Aspects of the translocation mechanism revelaed by the structure of partially packaged genomes Virology, 336, 100-112
2. D’Abramo Jr., A.M., Ali, A.A., Wang, F., Cotmore, S.F. and Tattersall, P. (2005) Host range mutants of minute virus of mice with a single VP2 amino acid change require additional silent mutations that regulate NS2 accumulation Virology, 340, 143-154
3. Paglino, J., Burnett, E. and Tattershall, P. (2007) Exploring the contribution of disgtal P4 promoter elements to the oncoselectivity of minute virus of mice Virology, 361, 174-184
4. Cotmore, S.F., Hafenstein, S. and Tattersall, P. (2010) Depletion of virion-associated divalent cations induces parvovirus minute virus of mice to eject its genome in a 3’-to-5’ direction from an otherwise intact viral particle J. Virol., 84, 1945-1956
5. Ruiz, Z., Mihaylov, I.S., Cotmore, S.F. and Tattersall, P. (2011) Recruitment of DNA replication and damage response proteins to viral replication centers during infection with NS2 mutants of Minute Virus of mice (MVM) Virology 410, 375–384
6. Li, L., Cotmore, S.F. and Tattersall, P. (2012) Maintenance of the flip sequence orientation of the ears in the parvoviral left-end hairpin is a nonessential consequence of the critical asymmetry in the hairpin stem J. Virol., 86, 12187-12197
7. Li, L., Cotmore, S.F. and Tattersall, P. (2013) Parvoviral left-end hairpin ears are essential during infection for establishing a functional intranuclear transcription template and for efficient progeny genome encapsidation J. Virol., 87, 10501–10514
8. Halder, S., Cotmore, S., Heimburg-Molinaro, J., Smith, D.F., Cummings, R.D., Chen, X., Trollope, A.J., North, S.J., Haslam, S.M., Dell, A., Tattersall, P., McKenna, R. and Agbandje-McKenna, M. (2014) Profiling of glycan receptors for minute virus of mice in permissive cell lines towards understanding the mechanism of cell recognition PLoS One, 9: e86909
9. Rostovsky, I. and Davis, C. (2015) Induction of an embryonic mouse innate immune response following inoculation in utero with minute virus of mice J. Virol., 89, 2182-2191
10. Mihaylov, I.S., Cotmore, S.F. and Tattersall, P. (2014) Complementation for an essential ancillary nonstructural protein function across parvovirus genera Virology, 468-470, 226–237
11. Rostovsky, I. and Davis, C. (2015) Induction of an embryonic mouse innate immune response following inoculation in utero with minute virus of mice J. Virol., 89, 2182-2191
12. Farr, G.A., Cotmore, S.F. and Tattersall, P. (2006) VP2 cleavage and the leucine ring at the base of the fivefold cylinder control pH-dependent externalization of both the VP1 N terminus and the genome of minute virus of mice J. Virol., 80, 161-171
13. Plevka, P., Hafenstein, S., Li, L., D’Abramo, A., Cotmore, S.F., Rossmann, M.G. and Tattersall, P. (2011) Structure of a packaging-defective mutant of minute virus of mice indicates that the genome is packaged via a pore at a 5-Fold axis J. Virol., 85, 4822–4827
14. Cotmore, S.F. and Tattersall, P. (2012) Mutations at the base of the icosahedral five-fold cylinders of minute virus of mice induce 3’-to-5’ genome uncoating and critically impair entry functions J. Virol., 86, 69-80
15. Lang, S.I., Boelz, S., Stroh-Dege, A.Y., Rommelaere, J., Dinsart, C. and Cornelis, J.J. (2005) The infectivity and lytic activity of minute virus of mice wild-type and derived vector particles are strikingly different J. Virol., 79, 289-298
16. Grekova, S., Zawatzky, R., Hörlein, R., Cziepluch, C., Mincberg, M., Davis, C., Rommelaere, J. and Daeffler, L. (2010) Activation of an antiviral response in normal but not transformed mouse cells: a new determinant of minute virus of mice oncotropism J. Virol., 84, 516-531
17. Grekova, S.P., Raykov, Z., Zawatzky, R., Rommelaere, J. and Koch, U. (2012) Activation of a gliomaspecific immune response by oncolytic parvovirus Minute Virus of Mice infection Cancer Gene Ther., 19, 468-475
OptiPrep™ Application Sheet V15; 6th edition, January 2020
OptiPrep™ Application Sheet V16
Purification of Group II (ss)DNA viruses: Parvovirinae – parvovirus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
- This Application Sheet describes a gradient that was first developed by Zolotukhin et al [1] for the purification of rAAV and it has been widely applied also to parvovirus. Section 4 describes some of the variations to the methodology.
- In the OptiPrep™ Reference List (RV02-1), Part B contains a list of papers describing the use of iodixanol gradients for all Group II viruses (except rAAV); to access return to the initial list of Folders and select “Reference Lists”.
1. Background
Viral vectors that are of potential use in gene therapy would clearly benefit from isolation methods which are both effective and cause little or no damage to the viral particles. Density gradient centrifugation has always played an important part in the concentration and purification of virus particles but the main gradient media that have been used (sucrose and CsCl), pose a number of problems. Both are highly hyperosmotic at the densities used to band viruses (sucrose solutions are also very viscous). Both have to be removed either by pelleting the virus or by dialysis, prior to further processing or analysis. CsCl also leads to poor recoveries and low infectivity of parvovirus and rAAV isolates.
Because of the very low water activity of CsCl solutions, viruses tend to have significantly higher density in this medium compared to media such as sucrose or any of the iodinated density gradient media, although the magnitude of this difference varies from virus to virus. Many viruses in CsCl have a density of approx 1.34 g/ml, in iodixanol the density range is generally 1.16-1.22 g/ml, although some viruses may be as low as 1.14 g/ml or as high as 1.24 g/ml.
- OptiPrep™ is widely regarded as the gradient medium of choice for purification of viruses and viural vectors. Compared to CsCl gradients recovery of virus from the gradient is at least ten times greater and the particle:infectivity titer is usually at least 100x lower. Infectivity measurements and many add-on techniques can be carried out without the need to dialyze the medium
- Lock et al [1] compared the use of iodixanol and CsCl methods for the purification of rAAV and noted the considerable improved transduction (both in vitro and importantly in vivo) of the iodixanol-purified material. Moreover only the iodixanol gradient separated infectious particles from empty capsids, which the authors deemed “a desirable property for reducing toxicity and unwanted immune responses during preclinical studies”.
- Brandenburger et al [2] who used the method described below for parvovirus also commented that the recovery of the virus is more efficient from iodixanol gradients that from CsCl gradients; that there is no loss of infectivity and that iodixanol prevents aggregation of virus particles. The authors also noted that no dialysis of the isolated virus was required prior to downstream processing.
- In 2001 Bloom et al [3] reported the use of the Zolotukhin et al [4] rAAV purification method, for the purification of Aleutian mink disease parvovirus and since that time many other papers have referenced essentially the same method for other parvoviruses, often with some modifications [5- 16].
- The centrifugation conditions of the gradient were modified by Farr and Tattersall [17] to the use of a swinging-bucket rotor (Beckman SW41Ti) at approx 150,000 gav for 18 h (see also ref. 18). Paglino et al [19] described these gradient conditions as effective for the separation of full and empty capsids. Other modifications of the centrifugation conditions include the use of 110,000 g for 6.5 h at 25C [20].
2. Discontinuous gradient method
The method is adapted from that devised by Zolotukhin et al [4] for r-AAV and described by Bloom et al for parvovirus [4]
2a. Solutions required (see box)
A. OptiPrep™ B. 10xPhosphate-buffered saline containing 10 mM MgCl2 and 25 mM KCl (10xPBS-MK)
B. 10xPhosphate-buffered saline containing 10 mM MgCl2 and 25 mM KCl (10xPBS-MK)
C. Phosphate-buffered saline containing 1 mM MgCl2 and 2.5 mM KCl (PBS-MK)
D. 2 M NaCl in PBS-MK
E. Working solution of 54% (w/v) iodixanol in PBS-MK: mix 9 vol of OptiPrep™ with 1 vol of Solution B.
2b. Ultracentrifuge rotor requirements
Fixed-angle rotor with approx 39 ml sealed tubes capable of approx 350,000 g (e.g. Beckman 70Ti or Sorvall T865; (see Section 3, Note 1).
2c. Protocol
1. Prepare the following gradient solutions (see Section 3 Notes 2 and 3): 15% (w/v) iodixanol containing 1 M NaCl in PBS-MK: 1.5 vol. of Solution E + 2.7 vol. of Solution D + 1.2 vol of Solution C.
25% (w/v) iodixanol in PBS-MK: 2.5 vol. of Solution E + 2.9 vol. of Solution C
40% (w/v) iodixanol in PBS-MK: 4.0 vol. of Solution E + 1.4 vol. of Solution C.
2. Clarify the cell lysate clarified by centrifugation at 4000 g for 20 min.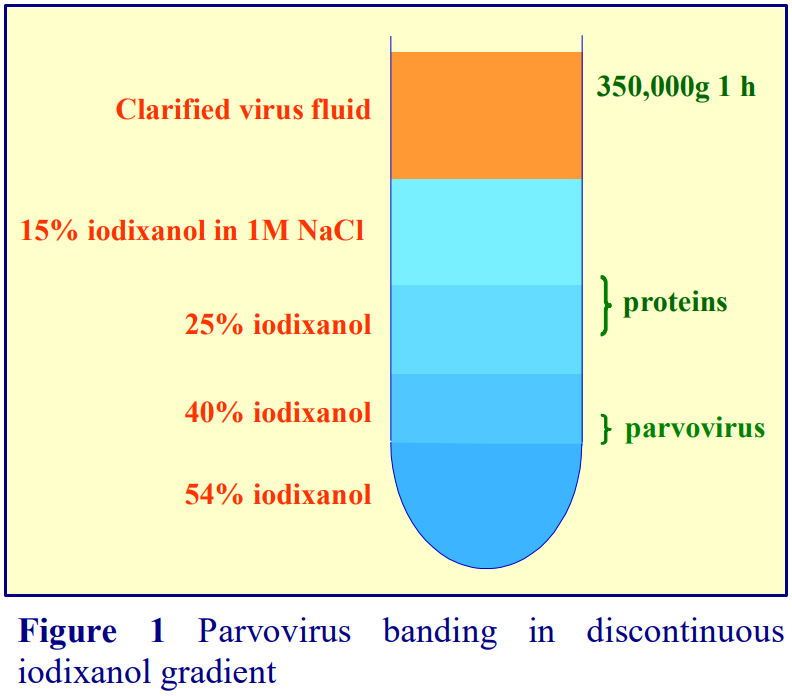 3. Underlayer 10-15 ml of clarified lysate with 9 ml of 15% iodixanol; 6 ml of 25% iodixanol, 5 ml of 40% iodixanol and 5 ml of the 54% iodixanol working solution (see Section 3, Note 1). Use a long metal cannula (0.8 mm i.d.) attached to a syringe or via tubing to a peristaltic pump to load the tubes (see Section 3, Notes 4 and 5).
3. Underlayer 10-15 ml of clarified lysate with 9 ml of 15% iodixanol; 6 ml of 25% iodixanol, 5 ml of 40% iodixanol and 5 ml of the 54% iodixanol working solution (see Section 3, Note 1). Use a long metal cannula (0.8 mm i.d.) attached to a syringe or via tubing to a peristaltic pump to load the tubes (see Section 3, Notes 4 and 5).
4. Centrifuge at 350,000 gav for 1 h at 18C. Use a slow acceleration and deceleration programme (up to and below 2000 rpm) if this facility is available on the centrifuge, or turn off the brake below 2000 rpm during deceleration (see Section 3, Note 5).
5. Either collect the whole gradient (Figure 1) in 1-2 ml fractions dense end first or use a syringe inserted at the 40%/54% interface to aspirate 4 ml of the 40% layer (see Section 3, Notes 6-8).
3. Notes
1. Smaller capacity rotors may be substituted. For smaller volume tubes scale down all volumes proportionately.
2. Aggregation of parvovirus with proteins in the cell lysate can pose a serious problem to its isolation as the aggregates are heterogeneous and consequently exhibit a broad range of densities. Inclusion of 1 M NaCl in the 15% iodixanol prevents this aggregation and allows the virus to be isolated as a single band above the 40%/54% iodixanol interface (see Figure 1).
3. Phenol red (0.01 µg/ml) may be included in the alternate gradient layers to aid the layering process.
4. If large volumes of solutions are used in creation of this gradient, the use of a peristaltic pump to introduce the iodixanol solutions makes this task easier. For more information on preparing discontinuous gradients see Application Sheet V02.
5. The gradients described in refs 17 and 18 were reduced to a total volume of 6 ml with a 5 ml sample layer. The lower g-force of 150,000 g (for a longer time) may reduce any interfacial aggregation of particles and proteins.
6. All of the contaminating proteins in the lysate band at the 25%/40% iodixanol interface.
7. For more information about harvesting gradients see Application Sheet V04.
8. Ion exchange, affinity chromatography, incubations with cells or SDS-PAGE can be carried out directly on the iodixanol-containing fractions. Only electron microscopy may require removal of the iodixanol. For more information about gradient analysis see Application Sheet V05
4. Self-generated iodixanol gradients Hendrie et al [21] used a self-generated iodixanol gradient to purify parvovirus. Cells were lysed in a routine Tris-buffered saline by freeze/thawing and after removal of cell debris at 4100 g for 30 min the supernatant was adjusted to 1 M NaCl. It was then mixed with OptiPrep™ to a final iodixanol concentration of 36% (w/v) and centrifuged at 290,000 g for 3 h. All operations were carried out at 4°C. The virus banded sharply at approx 1.29 g/ml.
- For more information about self-generated gradients see Application Sheet V03
5. References
1. Lock, M., Alvira, M., Vandenberghe, L.H., Samanta, A., Toelen, J., Debyser, Z. and Wilson, J.M. (2010) Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale Hum. Gene Ther., 21, 1259–1271
2. Brandenburger, A. and Velu, T. (2004) Autonomous parvovirus vectors: preventing the generation of wildtype or replication-competent virus J. Gene Med., 6, S203 S211
3. Zolotukhin, S., Byrne, B.J., Mason, E., Zolotukhin, I., Potter, M., Chesnut, K., Summerford, C., Samulski, R.J. and Muzyczka, N. (1999) Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield Gene Ther., 6, 973-985
4. Bloom, M.E., Best, S.M., Hayes, S.F., Wells, R.D., Wolfinbarger, J.B., McKenna, R. and AgbandjeMcKenna, M. (2001) Identification of Aleutian mink disease parvovirus capsid sequences mediating antibody-dependent enhancement of infection, virus neutralization, and immune complex formation J. Virol., 75, 11116-11127
5. Hendrie, P.C. and Russell, D.W. (2001) Homologous gene targeting using an autonomous parvovirus vector Am. Soc. Gene Ther., 4th Annu. Meeting Abstr. 515
6. Maxwell, I.H., Terrell, K.L. and Maxwell, F. (2002) Autonomous parvovirus vectors Methods, 28, 168-181
7. Brown, C.S., DiSumma, F.M., Rommelaere, J., Dege, A.Y., Cornelis, J.J., Dinsart, C. and Spaan, W.J.M. (2002) Production of recombinant H1 parvovirus stocks devoid of replication-competent viruses Hum. Gene Ther., 13, 2135-2145
8. Abschuetz, A., Kehl, T., Geibig, R., Leuchs, B., Rommelacre, J. and Régnier-Vigouroux, A. (2006) Oncolytic murine autonomous parvovirus, a candidate vector for glioma gene therapy, is innocuous to normal and immunocompetent mouse glial cells Cell Tissue Res., 325, 423-436
9. Krüger, L., Eskerski, H., Dinsart, C., Cornelis, J., Rommelaere, J., Haberkorn, H. and Kleinschmidt, J.A. (2008) Augmented transgene expression in transformed cells using a parvoviral hybrid vector Cancer Gene Ther., 15, 252-267
10. Dempe, S., Stroh-Dege, A.Y., Schwarz, E., Rommelaere, J. and Dinsart, C. (2010) SMAD4: a predictive marker of PDAC cell permissiveness for oncolytic infection with parvovirus H-1PV Int. J. Cancer, 126, 2914–2927
11. Bhat, R., Dempe, S., Dinsart, C. and Rommelaere, J. (2011) Enhancement of NK cell antitumor responses using an oncolytic parvovirus Int. J. Cancer, 128, 908–919
12. Alkassar, M., Gärtner, B., Roemer, K., Graesser, F., Rommelaere, J., Kaestner, L., Haeckel, I. and Graf, N. (2011) The combined effects of oncolytic reovirus plus Newcastle disease virus and reovirus plus parvovirus on U87 and U373 cells in vitro and in vivo J Neurooncol., 104, 715–727
13. Moralès, O., Richard, A., Martin, N., Mrizak, D., Sénéchal, M., Miroux, C., Pancré, V., Rommelaere, J., Caillet-Fauquet, P., de Launoit, Y. and Delhem, N. (2012) Activation of a helper and not regulatory human CD4+ T cell response by oncolytic H-1 parvovirus PLoS One, 7: e32197
14. Allaume, X., El-Andaloussi, N., Leuchs, B., Bonifati, S., Kulkarni, A., Marttila, T., Kaufmann, J.K., Nettelbeck, D.M., Kleinschmidt, J., Rommelaere, J. and Marchinia, A. (2012) Retargeting of rat parvovirus H-1PV to cancer cells through genetic engineering of the viral capsid J. Virol., 86, 3452–3465
15. Weiss, N., Stroh-Dege, A., Rommelaere, J., Dinsart, C. and Salomé, N. (2012) Infectivity of progeny virions H-1PV efficiently stimulates export and protein-coding sequence of parvovirus J. Virol., 86, 7554-7564
16. Raykov, Z., Grekova, S.P., Hörlein, R., Leuchs, B., Giese, T., Giese, N.A., Rommelaere, J., Zawatzky, R. and Daeffler, L. (2013) TLR-9 contributes to the antiviral innate immune sensing of rodent parvoviruses MVMp and H-1PV by normal human immune cells PLoS One, 8: e55086
17. Farr, G.A. and Tattersall, P.A (2004) A conserved leucine that constricts the pore through the capsid fivefold cylinder plays a central role in parvoviral infection Virology, 323, 243-256
18. Farr, G.A., Zhang, L-G. and Tattersall, P. (2005) Parvoviral virions deploy a capsid-tethered lipolytic enzyme to breach the endosomal membrane during cell entry Proc. Natl. Acad. Sci. USA, 102, 17148-17153
19. Paglino, J.C., Ozduman, K. and van den Pol, A.N. (2012) LuIII parvovirus selectively and efficiently targets, replicates in, and kills human glioma cells J. Virol. 2012, 86(13):7280-7291
20. Nelson, C.D.S., Minkkinen, E., Bergkvist, M., Hoelzer, K., Fisher, M., Bothner, B. and Parrish, C.R. (2008) Detecting small changes and additional peptides in the canine parvovirus capsid structure J. Virol., 82, 10397-10407
21. Hendrie, P.C., Hirata, R.K. and Russell, D.W. (2003) Chromsomal integration and homologous gene targeting by replication-incompetent vectors based on the autonomous parvovirus minute virus mice J. Virol., 77, 13136-13145
OptiPrep™ Application Sheet V16; 4th edition, January 2020
OptiPrep™ Application Sheet V17
Purification of Group III (ds)RNA viruses: Reoviridae
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
Density gradient centrifugation has always played an important part in the concentration and purification of virus particles but the gradient media that have been used most prominently, sucrose and CsCl, pose a number of problems. Both media are highly hyperosmotic at the densities used to band viruses (sucrose solutions are also very viscous) and generally have to be removed either by pelleting the virus or by dialysis, prior to further processing or analysis. CsCl also leads to poor recoveries and low infectivity of parvovirus and rAAV isolates.
Because of the very low water activity of CsCl solutions, viruses tend to have significantly higher density in this medium compared to media such as sucrose or any of the iodinated density gradient media, although the magnitude of this difference varies from virus to virus. Many viruses in CsCl have a density of approx 1.34 g/ml, in iodixanol the density range is generally 1.16-1.22 g/ml, although some viruses may be as low as 1.14 g/ml or as high as 1.24 g/ml.
OptiPrep™ is widely regarded as the gradient medium of choice for purification. Compared to
CsCl gradients:
- Recovery of virus from the gradient is at least ten times greater
- Particle:infectivity titer is up to 100x lower
- Infectivity measurements and many add-on techniques can be carried out without the need to dialyze the medium.
2. Methodology
There are rather few published papers reporting the use of iodixanol gradients for the purification and analysis of Class III viruses. The first by Jaafar et al [1] was concerned with study of a Seadornavirus (Banna virus) cores; the authors reported a multi-step purification, which incorporated a discontinuous 10, 20, 30, 40 and 55% (w/v) iodixanol (in 100 mM Tris-HCl, pH 7.5) and centrifuged at 210,000 g for 2 h. It was followed by a CsCl gradient. The same group (ref 2) extended the method to a new member of the group (Dinovernavirus) using the same iodixanol gradient but omitting the CsCl gradient. Banding at the 40-55% (w/v) iodixanol interface was observed in both cases. Later Attoui et al [3] used an OptiPrep™ cushion to concentrate Liao ning virus (another Seadornavirus).
Rotavirus was investigated using a continuous iodixanol gradient [4] centrifuged at 80,000 g for 16 h. Gradient solutions were prepared by dilution of OptiPrep™ with buffered 0.25 M sucrose. The majority of the dsRNA banded in the ρ = 1.11-1.15 g/ml region (approx. 16.5-25% w/v iodixanol). The authors considered that this was consistent with the nascent sub-viral particles residing in neoplasms and that they were associated with lipid droplets. Gradients were similar to those used by other workers to show that hepatitis C virus isolated from liver samples, for example, were also associated with lipid [5]. For more information on the use for such gradients see Application Sheet V19
A Birnavirus (Espirito Santo virus) has been purified in a 12-35% (w/v) iodixanol gradient centrifuged for 16 h at 76,000 g and then re-centrifuged through a second gradient of 20-35% (w/v) iodixanol for 3 h at 90,000 g [6].
An interesting aspect of the replication of rotaviruses is that this process occurs in cytoplasmic inclusion bodies termed viroplasms. It is known that these viroplasms interact (both physically and functionally) with lipid droplets (LDs). Iodixanol gradients have been used to purify these particles, which have a density of 1.11-1.15 g/ml [7-9].
3. References
1. Jaafar, F.M., Attoui, H., Mertens, P.P.C., de Micco, P. and de Lamballerie, X. (2005) Structural organization of an encephalitic human isolate of Banna virus (genus Seadornavirus, family Reoviridae) J. Gen. Virol., 86, 1147-1157
2. Attoui, H., Jaafar, F.M., Belhouchet, M., Biagini, P., Cantaloube, J-F., de Micco, P. and de Lamballerie, X. (2005) Expansion of family reoviridae to include nine-segmented dsRNA viruses: isolation and characterization of a new virus designated aedes pseudoscutellaris reovirus assigned to a proposed genes (Dinovernavirus) Virology, 343, 212-223
3. Attoui, H., Jaafar, F.M., Belhouchet, M., Tao, S., Chen, B., Liang, G., Tesh, R.B., de Micco, P. and de Lamballerie, X. (2006) Liao ning, a new Chinese seadornavirus that replicates in transformed and embryonic mammalian cells J. Gen. Virol., 87, 199-208
4. Cheung, W., Gill, M., Esposito, A., Kaminski, C.F., Courousse, N., Chwetzoff, S., Trugnan, G. Keshavan, N., Lever, A. and Desselberger, U. (2010) Rotaviruses associate with cellular lipid droplet components to replicate in viroplasms, and compounds disrupting or blocking lipid droplets inhibit viroplasm formation and viral replication J. Virol., 84, 6782-6798
5. Nielsen, S.U., Bassendine, M.F., Martin, C., Lowther, D., Purcell, P.J., King, B.J., Neely, D., Toms, G.L. (2008) Characterization of hepatitis C RNA-containing particles from human liver by density and size J. Gen. Virol., 89, 2507-2517
6. Vancini, R., Paredes, A., Ribeiro, M., Blackburn, K., Ferreira, D., Kononchik, Jr. J.P., Hernandez, R. and Brown, D. (2012) Espirito Santo virus: a new Birnavirus that replicates in insect cells J. Virol., 86, 2390- 2399
7. Lever, A. and Desselberger, U. (2016) Rotavirus replication and the role of cellular lipid droplets: New therapeutic targets? J. Formosan Med. Assoc., 115, 389-394
8. Cheung, W., Gaunt, E., Lever, A. and Desselberger, U. (2016) Rotavirus replication: the role of lipid droplets in viral gastroenteritis, Elsevier Inc pp 175-187
9. Trejo-Cerro, O., Eichwald, C., Schraner, E.M., Silva-Ayala, D., López, S., Ariasa, C.F. (2018) Actindependent nonlytic rotavirus exit and infectious virus morphogenetic pathway in nonpolarized cells J. Virol., 92: e02076-17
OptiPrepρ™ Application Sheet V17; 5th edition, January 2020
OptiPrep™ Application Sheet V18
Purification of Group III (ds)RNA viruses: Reoviridae
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- This Application Sheet covers the purification of two genera of this family: Norovirus (Norwalk virus) and Lagovirus (rabbit haemorrhagic disease virus). Whether the methods described in this Application Sheet can be applied to other viruses of the same family can only be determined experimentally
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. General background to use of iodixanol
There are now many published papers that report the use of iodixanol gradients not only to purify viruses but also to investigate their assembly. In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function. Both CsCl and sucrose must be dialyzed before infectivity can be measured. In contrast both infectivity measurements using cultured cells and many add-on techniques can be performed without dialysis of iodixanol. Combined with the availability of OptiPrep™ as a sterile solution, this makes the use of OptiPrep™ for virus purification much more convenient than the use of either CsCl or sucrose.
2. Norwalk virus
2a. Introduction
Using recombinant bacuolvirus-infected insect cells Bertolotti-Ciarlet et al [1] have recovered fulllength and mutant Norwalk virus capsid proteins from the cell supernatant. After clarification of the cell supernatant the Norwalk virus-like particles were concentrated by sedimentation and then purified in a self-generated iodixanol gradient.
2b. Solutions required
A. OptiPrep™
B. Diluent: Phosphate-buffered saline (see Section 2e: Note 1)
2c. Ultracentrifuge rotor requirements
For small volume gradients an NVT65.2 (5 ml) near-vertical rotor is recommended. However the appropriate density profile can be generated by almost any vertical or near-vertical rotor with a tube capacity of 3-12 ml and a maximum RCF of approx 350,000gav. The sedimentation path length of the rotor should be no more than approx. 25 mm, thus the Beckman VTi65.1 vertical rotor, NVT65 nearvertical rotor (both 12 ml) or the TLN100 near-vertical rotor (3 ml) are acceptable alternatives (see Note 1). High performance fixed-angle rotors may only be used for the rapid formation of selfgenerated gradients if the tube volume is relatively small (less than 6 ml).
- For examples of self-generated density gradient profiles with these rotors see Application Sheet V03.
The following protocol is adapted from ref 1.
2d. Protocol
1. Harvest the culture medium from the cell monolayer.
2. Clarify the medium by centrifugation at 700 g for 15 min.
3. Complete the clarification by centrifugation at 20,000 g for 30 min.
4. Pellet the particles at 120,000 g for 2 h (see Section 2e: Note 2)
5. Suspend the pellet in approx 2.5 ml of Solution B and mix with OptiPrep™ to produce a final iodixanol concentration of 30% (w/v) (see Section 2e: Note 1)
6. Transfer the suspension to tubes for the NVT65.2 rotor or other suitable vertical or near-vertical rotor and centrifuge at approx. 350,000 gav for 3 h at 4°C (see ™).
7. At the end of the centrifugation use either a controlled deceleration programme or turn off the brake below 2000 rpm.
8. Unload the gradient by tube puncture, upward displacement of aspiration from the meniscus in a series of equal volume fractions (15-20 fractions irrespective of the gradient volume) and analyze the fractions as required. The virus-like particles band in the top third of the gradient (see Section 2e: Note 4)
2e: Note
1. In step 5 of the protocol the suspension containing the virus-like particles is simply mixed with an equal volume of OptiPrep™, which will mean that the buffer concentration will be reduced by 50%. If this is deemed unacceptable then first produce a Working Solution of 54% (w/v) iodixanol by mixing 5.4 vol. of OptiPrep™ with 0.6 vol. of 10xPBS. Other strategies for preparing working solutions are given in Application Sheet V01.
2. The ideal way of concentrating the virus is sedimentation on to a dense cushion of iodixanol, rather than pelleting, which can lead to loss of infectivity. The method is very easy to use when the subsequent purification is in a self-generated gradient. In a swinging-bucket rotor underlayer the clarified virus-containing solution with 2-3 ml of OptiPrep™ and centrifuge at 120,000 g for 2 h. Remove all the supernatant except for a volume equal to that of the OptiPrep™ cushion and mix well with the latter before transferring to a tube for the near-vertical or vertical rotor. For more information on concentration of virus prior to gradient purification see Application Sheet V06.
3. If using larger volumes it may be necessary to increase the centrifugation time.
4. For more information on harvesting gradients see Application Sheet V04.
3. Rabbit haemorrhagic disease virus
3a. Introduction
Previous methods for the purification of this virus from rabbit liver homogenates have been very lengthy and recoveries of high titre virus not easy to achieve. This new method, first published by Teixeira et al [2], involving a single discontinuous iodixanol gradient was developed from an earlier method for purifying rAAV by Zolotukhin et al [3]. This methodology is described in Application Sheet V14. This protocol is adapted from ref 2.
 3b. Solutions required
3b. Solutions required
A. OptiPrep™
B. 10xPhosphate-buffered saline containing 10 mM MgCl2 and 25 mM KCl (10xPBS-MK)
C. Phosphate-buffered saline containing 1 mM MgCl2 and 2.5 mM KCl (PBS-MK)
D. 2 M NaCl in PBS-MK
E. Working solution of 54% (w/v) iodixanol in PBS-MK: mix 9 vol. of OptiPrep™ with 1 vol. of Solution B.
3c. Ultracentrifuge rotor requirements
The rotor used by the Teixeira et al [2], a Beckman 75Ti fixed angle rotor is no longercommercially available; either a 70Ti or 80Ti fixed-angle rotor is the closest substitute. Using a fixedangle rotor for running a density gradient at high g-forces may not be the best choice; a vertical or near vertical-rotor is more suited to this task (see Section 3 of Application Sheet V02 for details). In this case either the Beckman VTi65.1 or the NVT65 would the most obvious substitutes. Although no medium-volume swinging-bucket rotor can achieve the necessary g-force, the Beckman SW41 (maximum g-force 200,000 gav) run for a proportionately longer time might be a good choice. Alternatively, if the total gradient volume is scaled down, rotors such as the SW55Ti can approach the required g-force. All these alternatives would need to be properly evaluated before using in the method described in this Application Sheet.
3d. Protocol
1. Prepare the following gradient solutions (see Section 3e, Notes 1 and 2): 15% (w/v) iodixanol containing 1 M NaCl in PBS-MK: 1.5 vol. of Solution E + 2.7 vol. of Solution D + 1.2 vol. of Solution C.
25% (w/v) iodixanol in PBS-MK: 2.5 vol. of Solution E + 2.9 vol. of Solution C
40% (w/v) iodixanol in PBS-MK: 4.0 vol. of Solution E + 1.4 vol. of Solution C.5% (w/v) iodixanol in PBS-MK:
2. Produce a liver homogenate in PBS using a Dounce homogenizer and centrifuge at 900 g for 10 min (see Section 3e Note 3)
3. Centrifuge the post-nuclear supernatant at 4000 g for 20 min and then clarify by passing through a 0.2 μm filter (see Section 3e Note 4)
4. Transfer the clarified supernatant (2.5 ml) to tubes for the chosen rotor and underlayer sequentially (using a syringe and metal cannula) with 2.25 ml, 1.5 ml, 1.25 ml and 1.25 ml respectively of the 15%, 25%, 40% and 54% iodixanol solutions (see Section 3e Note 5).
5. Centrifuge at 350,000 gav for 1h 20 min, using slow acceleration to and deceleration from, 2000 rpm (see Section 3e Note 6).
6. The virus bands either within the 40% iodixanol layer or at its lower interface (see Section 3e Note 7).
3e. Notes
1. For smaller or larger volume tubes scale down or up all volumes proportionally.
2. Phenol red (0.01 µg/ml) may be included in the alternate gradient layers to enhance visual identification of the layers. Diffusion of the iodixanol and, during centrifugation at 350,000 g, some sedimentation of the iodixanol itself, will make the interfaces less obvious.
3. Teixeira et al [2] froze the material after this step.
4. A 15% (w/v) iodixanol cushion has also been used for concentrating this virus [4].
5. For more information about the construction of discontinuous gradients see Application Sheet V02.
6. It may be necessary to increase the centrifugation time proportionally if the rotor cannot achieve 350,000 gav.
7. Teixeira et al [2] concluded that the single short iodixanol gradient centrifugation was the method of choice compared to earlier procedures involving the use of either sucrose or CsCl gradients. It is not only a rapid procedure but also the infectivity and purity of the rabbit haemorrhagic disease virus was superior to other methods. – see also Section 1 for more information on the advantages of using iodixanol. See refs 2 and 5 for more information about the properties of the iodixanolpurified virus.
4. References
1. Bertolotti-Ciarlet, A., White, L.J., Chen, R., Prasad, B.V.V. and Estes, M.K. (2002) Structural requirements for the assembly of Norwalk virus-like particles J. Virol., 76, 4044-4055
2. Teixeira, L., Marques, R.M., Aguas, A.P. and Ferreira, P.G. (2011) A simple and rapid method for isolation of caliciviruses from liver of infected rabbits Res. Vet. Sci., 91, 164–166
3. Zolotukhin, S., Byrne, B.J., Mason, E., Zolotukhin, I., Potter, M., Chesnut, K., Summerford, C., Samulski, R.J. and Muzyczka, N. (1999) Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield Gene Ther., 6, 973-985
4. Crisci, E., Fraile, L., Moreno, N., Blanco, E., Cabezónd, R., Costa, C., Mussá, T., Baratelli, M., MartinezOrellana, P., Ganges, L., Martínez, J., Bárcenac, J. and Montoya, M. (2012) Chimeric calicivirus-like particles elicit specific immune responses in pigs Vaccine 30, 2427–2439
5. Teixeira, L., Marques, R.M., Águas, A.P. and Ferreira, P.G. (2012) Regulatory T cells are decreased in acute RHDV lethal infection of adult rabbits Vet. Immunol. Immunopathol., 148, 343– 347
OptiPrep™Application Sheet V18; 9th edition, January 2020
OptiPrep™ Application Sheet V19
Purification of Group IV ((+)ss) RNA viruses: Flaviviridae family and hepatitis E in pre-formed gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- This Optiprep™ Application Sheet is concerned principally with the following:
Hepacivirus: hepatitis C virus and hepatitis C virus-like particles (Sections 2-7)
Flavivirus: Dengue virus, West Nile virus and yellow fever virus (Section 8b)
Pestivirus: bovine viral diarrhea virus (Section 8a)
Hepevirus: hepatitis E (Section 8c)
Picornaviridae: hepatitis A (Section 8d) - The OptiPrep™ Reference List (RV04) provides a full bibliography of all published papers reporting the use of iodixanol gradients for the purification of Group IV viruses; to access return to the initial list of Folders and select “Reference Lists”. The viruses are listed according to the Baltimore scheme of “Family, Genus and Species”.
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
- Sections 2 and 5b address the problems associated with larger volumes of virus suspension.
1. Background
In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which, in solutions of the same density, is much higher than that of iodixanol. Most iodixanol gradients can also be made isoosmotic over the entire density range. Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast both infectivity measurements using cultured cells and many add-on techniques can be performed without dialysis of iodixanol. Combined with the availability of OptiPrep™ as a sterile solution, this makes the use of OptiPrep™ for virus purification and assembly analysis much more convenient than the use of either CsCl or sucrose. Thus iodixanol is being increasingly used for hepatitis C virus particle purification from lysed cultured cells, conditioned culture medium or plasma samples from patients.
2. Concentration of virus particles
After clarification of the virus-containing fluid by centrifugation at 3000-4000 g for 10 min and/or filtration through a 0.2 or 0.45 m filter it is often necessary to concentrate the virus before applying to a gradient. Direct pelleting from culture the suspension, e.g. at 125,000 g for 4 h, may lead to loss of infectivity. One useful alternative is to use a centrifugal ultrafiltration, for example the Centricon PBHK Centrifugal Plus-20 filter unit with an Ultracel PL membrane (100 kDa cut off) as described by Yi et al [2,3]. Bartolomé et al [4] centrifuged viral particles from sera over 10% sucrose at 100,000 gav for 17 h. Virus can also be precipitated from culture fluid by incubating it with 0.25 vol. of 40% (w/v) polyethylene glycol 8000 in PBS at 4°C overnight [5,6]. After collection of the virus by centrifugation it was suspended in 0.85% (w/v) NaCl, 0.02% bovine serum albumin, 10 mM HEPES-NaOH, pH 7.55; and pelleted through a 20% (w/v) iodixanol cushion by centrifugation for 6 h at 190,000 gav.
Use of an iodixanol cushion to concentrate the virus was also used by Elmowalid et al [7], but in this case the iodixanol concentration was 40% (w/v). Banding of the virus at an interface is probably the gentlest method of concentration and is ideal if the virus is subsequently used to bottom load the gradient [7]. If the gradient is to be top-loaded however, virus concentration on to a dense cushion may be problematical. A top-loading strategy means that the virus suspension must contain <10% iodixanol and great care must be exercised in recovering as little of the cushion as possible when the virus is harvested. In Beckman konical tubes the volume of the dense cushion can be very small (e.g. in the Beckman SW41Ti, approx 0.5-1.0 ml) and if most of this cushion is aspirated (using fine bore Teflon tubing attached to a syringe) before the virus band is harvested, it is possible to achieve a suitably low iodixanol concentration in the harvest.
 For more information on density cushions see Section 5b. For more information on handling large volumes of virus see Application Sheet V06 and Section 5b of this Application Sheet.
For more information on density cushions see Section 5b. For more information on handling large volumes of virus see Application Sheet V06 and Section 5b of this Application Sheet.
3. Gradient solution preparation
3a. NaCl as osmotic balancer (see Box 1, from ref 8)
A. OptiPrep™
B. Buffer: 120 mM HEPES-NaOH, pH 7.6, 0.12% (w/v) bovine serum albumin (BSA)
C. Salt solution: 1 M NaCl
- Prepare 10% (w/v) iodixanol by diluting 1 vol. of OptiPrep™ with 1 vol. of Solution B, 0.75 vol. of Solution C and 3.25 vol. of water
- Prepare 40% (w/v) iodixanol by diluting 4 vol. of OptiPrep™ with 1 vol. of Solution B, 0.3 vol. of Solution C and 0.7 vol. of water.
 3b. Sucrose as osmotic balancer (see Box 2, from ref 9)
3b. Sucrose as osmotic balancer (see Box 2, from ref 9)
A. OptiPrep™
B. Diluent 1: 0.25 M sucrose, 12 mM EDTA, 60 mM Tris-HCl 5 pH 8.0 C. Diluent 2: 0.25 M sucrose, 2 mM EDTA, 10 mM
Tris-HCl 5 pH 8.0
- Prepare 50% (w/v) iodixanol by mixing 5 vol. of OptiPrep™ with 1 vol. of Solution A.
- Dilute the 50% iodixanol with Solution C to give lower concentrations of iodixanol.
3c. Notes
The two solutions described in Section 3a provide a positive gradient of iodixanol (10-40%) and a negative gradient of NaCl (0.125-0.05M) and are designed to maintain isoosmotic conditions. However when OptiPrep™ is simply diluted with any buffered isoosmotic solution, the resulting solution should also be approx. isoosmotic, see Application Sheet V01. Thus it may be sufficient simply to dilute the OptiPrep™ with 0.85% (w/v) NaCl, 0.02% bovine serum albumin, 20 (or 10) mM HEPES-NaOH, pH 7.6. The recipe in Section 3b maintains constant concentrations of EDTA and Tris in all solutions, with sucrose as the osmotic balancer [9]. A similar strategy may be used with NaCl as osmotic balancer [10]. Other methods use Hank’s balanced salt solution [2,3] to dilute the OptiPrep™ or the cell lysis medium containing 1 mM MgCl2, 1 mM CaCl2, 1 mM PMSF, 10 mM Tris-HCl, pH 7.4 [7].
4. Sedimentation in pre-formed gradients (adapted from ref 8)
4a. Protocol
1. Using a two-chamber gradient maker or a Gradient Master™ prepare an approx. 12 ml gradient in tubes (for a swinging-bucket rotor with approx 14 ml tubes, e.g. Beckman SW41Ti) from equal volumes of the two iodixanol solutions described in Section 3a (see Section 4b, Notes 1-3).
2. Layer 0.5-1.0 ml of the crude virus suspension on top and centrifuge at 197,000 gav for at least 6 h at 4°C (see Section 4b, Notes 1-3). Box 1
3. Collect the gradient in 0.5-1.0 ml fractions either by tube puncture or aspiration from the meniscus or if the band of virus is sufficiently distinct retrieve it using a syringe. For more information on harvesting gradients see Application Sheet V04.
4b. Notes
1. Lindenbach et al [8] was the first group to use this 10-40% iodixanol gradient and this technique is probably the most widely used. Although 10-40% iodixanol is a common range for the gradient, others are equally successful: 10-50% [10], 6-56% [9] and 20-50% for purifying hepatitis C viruslike particles released from insect cells infected by recombinant baculoviruses [6].
2. If a mechanical device for creating a continuous gradient is not available, then make up a discontinuous gradient from equal volumes of 10%, 20%, 30% and 40%. Because gradients are centrifuged for at least 6 h these discontinuous gradients will become more or less linear (depending on the volume of the gradient steps) by diffusion during centrifugation. For more information on preparing continuous gradients see Application Sheet V02.
3. Occasionally discontinuous gradients from 10-50% (or 10-40%) iodixanol are produced using only a 5% step interval. Often they may be allowed to diffuse at 4°C for 4-24 h, prior to loading the sample or the sample may be loaded without any diffusion time. Centrifugation at g-forcesbetween 90,000 g and 250,000 g has been reported. Moreover the centrifugation forces and times do not necessarily relate to the size of the gradient: examples are 230,000 gav for 5 h (4 ml gradient) to 90,000 gav for 24 h or 190,000 gav for 6-16 h (12-13 ml gradient). Just a few of the variations in gradient construction and g-force are given in Table 1.
5. Flotation in pre-formed gradients (adapted from ref 17)
5a. Protocol
1. Prepare 10%, 20%, 30% and 40% iodixanol solutions using any of the buffers described in Section 3; a Tris-buffered 0.85% NaCl solutions was described in ref 17 (see Section 5b).
2. In approx 14 ml tubes for a swinging-bucket rotor, use a syringe attached to a thin metal cannula to prepare gradients by underlayering from approx 3.0 ml of each of the iodixanol solutions and the buffered saline. For more information about the preparation of discontinuous gradients see Application Sheet V02.
3. Adjust the virus suspension to approx. 45% iodixanol by mixing with OptiPrep™ and underlayer each gradient with approx. 1.0 ml of the suspension (see Section 5b).
4. Centrifuge at 154,000 g for 16 h at 4°C (see Section 5b).
5. Collect the gradient by tube puncture or, if the band is sufficiently well defined, retrieve it using a syringe. For more information on harvesting gradients see Application Sheet V04.
5b. Notes
The method can be scaled down to smaller volume rotors by reducing all volumes proportionately. Smaller gradients will become more or less continuous due to diffusion during centrifugation. A preformed continuous gradient may also be bottom-loaded for a flotation separation, e.g. an 8-30% iodixanol gradient underlayered by the hepatitis C virus in 40% iodixanol [7]. In this case the virus had previously and very conveniently been concentrated on a 40% iodixanol cushion [7]. There are other examples of the use of flotation gradients, a selection is provided in Table 2.
A few interesting variants of these strategies, which facilitate the use of larger volumes of virus suspension are worth mentioning:
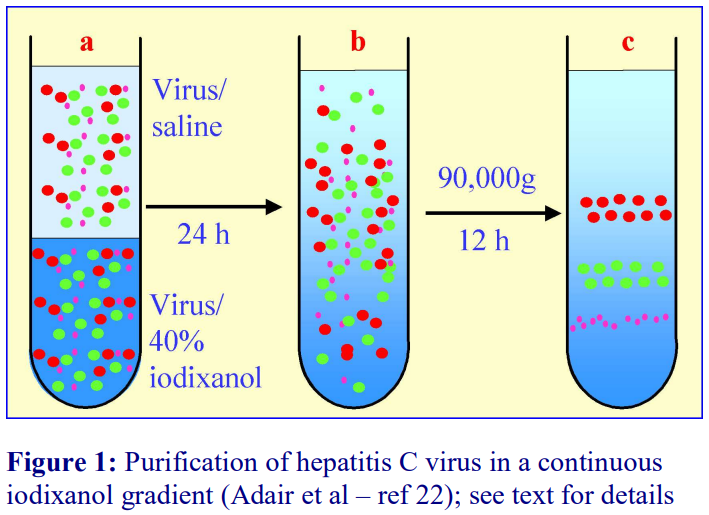 Ref 22 described the following method: as with the routine bottom-loading, the crude virus suspension was adjusted to 40% (w/v) iodixanol, but this was overlaid by an equal volume of the suspension, to fill the tube (Fig 1a). After sealing the tube, it was gently reoriented to a horizontal position. After 24 h at 4°C, the tube was returned to the vertical. During this time, diffusion of the iodixanol will have created a linear gradient (Fig 1b) and the particles will be randomly distributed through it. This procedure is described in Application Sheet V02. After removing 0.5 ml from the top of the tube it was centrifuged at 90,000 g for 24 h. (Fig 1c) to band the particles isopynically. The big advantage of this procedure is that, rather like a self-generated gradient (see Application Sheet V20), there are no interfaces that may cause the build-up and aggregation of particles, in this case the gradient was able to resolve replicon RNA from HCV RNA.
Ref 22 described the following method: as with the routine bottom-loading, the crude virus suspension was adjusted to 40% (w/v) iodixanol, but this was overlaid by an equal volume of the suspension, to fill the tube (Fig 1a). After sealing the tube, it was gently reoriented to a horizontal position. After 24 h at 4°C, the tube was returned to the vertical. During this time, diffusion of the iodixanol will have created a linear gradient (Fig 1b) and the particles will be randomly distributed through it. This procedure is described in Application Sheet V02. After removing 0.5 ml from the top of the tube it was centrifuged at 90,000 g for 24 h. (Fig 1c) to band the particles isopynically. The big advantage of this procedure is that, rather like a self-generated gradient (see Application Sheet V20), there are no interfaces that may cause the build-up and aggregation of particles, in this case the gradient was able to resolve replicon RNA from HCV RNA.
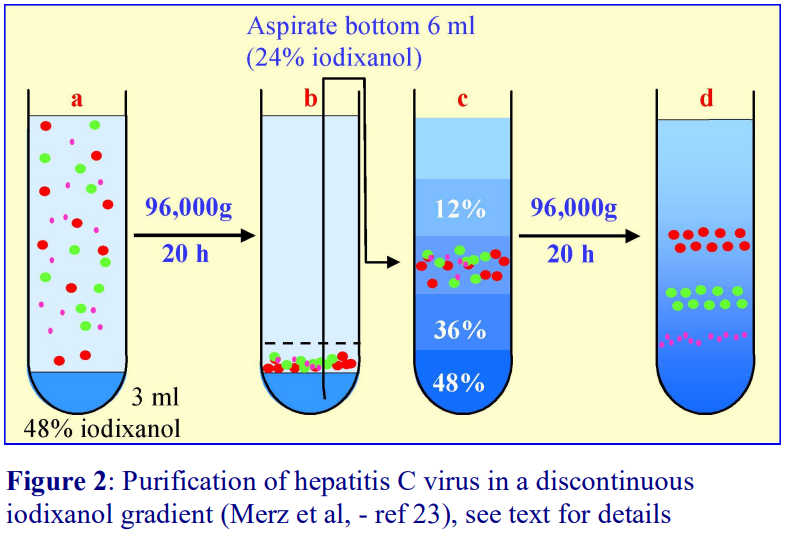 In a similar protocol Merz et al [23] used a 3 ml 48% (w/v) iodixanol cushion initially to concentrate the HCV at 96,000 g for 20 h (Fig 2a-b). From the bottom of the tube 6 ml was removed (i.e. the cushion, banded virus and 3 ml of sample buffer) – so the concentrated virus was now in 24% iodixanol. This was used to construct a discontinuous gradient (Fig 2b-c) of 48%, 36%, 24%, 12% and 0% (saline), which was centrifuged at 96,000 g for 20 h (Fig 2d). The gradient displayed distinct banding of core protein, E2 protein and ApoE [23].
In a similar protocol Merz et al [23] used a 3 ml 48% (w/v) iodixanol cushion initially to concentrate the HCV at 96,000 g for 20 h (Fig 2a-b). From the bottom of the tube 6 ml was removed (i.e. the cushion, banded virus and 3 ml of sample buffer) – so the concentrated virus was now in 24% iodixanol. This was used to construct a discontinuous gradient (Fig 2b-c) of 48%, 36%, 24%, 12% and 0% (saline), which was centrifuged at 96,000 g for 20 h (Fig 2d). The gradient displayed distinct banding of core protein, E2 protein and ApoE [23].
6. Buoyant density of virus particles
Generally virus particles band around 1.09-1.12 g/ml [12] but the density of infectious virions was <1.093 g/ml according to ref 24. Often at least two peaks of RNA are identified in the gradient. Lindenbach et al [16] for example noted that virus from cultured cells banded principally around 1.14 g/ml, while that from animal serum was <1.10 g/ml. Yi et al [2] reported that RNA from virus containing the H77-S genotype was biphasically distributed at the same two densities; the JFH-1 strain contained mainly the denser form, while in the H77-S/△E1p7 mutant this form was virtually absent. Intracellular virus was identified at 1.09-1.12 g/ml while extracellular virus banded broadly from 1.03- 1.12 g/ml [25]. The bimodal distribution of particles (1.086 and 1.152-1.155 g/ml) is confirmed in studies by Farquhar et al [26]. Nielsen et al [27] showed that while membrane-encapsulated particles from human liver had a density of approx 1.08 g/ml, the free nucleocapsid banded at approx 1.2 g/ml. Analysis of particles from the plasma of infected patients identified particles <1.08 g/ml that were associated with VLDL [9].
7. Sedimentation velocity gradients
Nielsen et al [9] have also used iodixanol gradients for determining the size of virus particles isolated from human plasma. The 4-24% (w/v) continuous iodixanol gradients were centrifuged at 90,000 gav for 2 or 4 h. The authors compared the sedimentation coefficient (s20,w) before and after detergent-treatment; the values were approx 215S and 180S respectively.
8. Other Flaviviridae viruses
8a. Pestivirus (Bovine diarrhea virus)
The virus was purified by flotation through a discontinuous iodixanol gradient [28]. Medium from a cell culture was clarified at 4000 g for 30 min and the supernatant centrifuged at 125,000 g for 4h to concentrate the virus as a pellet. The latter was resuspended in 0.35 ml of PBS and mixed with an equal volume of OptiPrep™ (final concentration of iodixanol = 30% w/v). Layers of 25%, 20%, 15%, 10% and 5% (w/v) iodixanol (OptiPrep™ diluted with PBS) were layered on top in tubes for a Beckman SW60Ti swinging-bucket rotor (4 ml tubes). After centrifugation at 168,000 g for 4 h the gradient was probably close to being a linear one; virus banded at approx 16.5% (w/v) iodixanol. Since the virus is bottom-loaded in the gradient, this is a method that would allow the first concentration step to be carried out, not by pelleting, but by banding on to a small cushion of OptiPrep™. More recently, a top loaded discontinuous gradient comprising 2 ml each of 10%, 22%, 24% and 26% (w/v) iodixanol at 150,000 g for 2 h was used; the virus banded at the 22%/24% interface [29-30]. More information on concentration of virus prior to gradient purification can be found in Application Sheet V06.
8b. Flavivirus
8b-1 Dengue virus
Virus from a cell culture supernatant was concentrated by pelleting through 20% (w/v) sucrose at 72,000 g for 5 h. The resuspended pellet was layered on a 10-40% (w/v) iodixanol gradient and centrifuged at 164,000 g for 2 h. Virus was recovered from gradient fractions (after dilution with buffer) by pelleting at 72,000 g for 5 h [31-33]. Other workers have used the same concentration technique, with a marginally shallower gradient (9-36% iodixanol) and much milder centrifugation conditions – 30,000 g for 2.5 h [34,35]. In a simple two-layer 20/55% (w/v) iodixanol discontinuous gradient (210,000 g for 2 h) the virus bands at the interface [36,37].
Smith et al [38] and Vancini et al [39] used two rounds of centrifugation, also in a top-loaded twolayer gradient of 12% and 35% (w/v) iodixanol; the first centrifugation was executed at approx. 105,000 g for 8-16 h and the second at approx. 150,000 g for 3 h. An important aim of the centrifugation was to concentrate the virus and to separate the virus from the bovine serum albumin in the virus preparation. The authors [38,39] stressed the importance of using a gradient solution that was less viscous than the traditional sucrose and importantly non-toxic to cells so that vaccine strains could be administered to African green monkeys in the gradient harvest containing 33% (w/v) iodixanol. It was also observed that other gradient purification and concentration reagents such as PEG may induce fusion events between viral particles [39]. Essentially the same two-layer gradient has also been used in a single overnight centrifugation [40] and for a shorter time (2.75 h) [41].
Multi-layer discontinuous gradients have also been used. A post-nuclear supernatant from infected cultured cells was fractionated on a discontinuous gradient of 6%, 18%, 30% and 48% (w/v) iodixanol centrifuged overnight at approx. 100,000 gav in a study of Dengue virus replication complexes; the gradient showed that the increased fatty acid synthesis that occurs in infected cells is associated with a membrane compartment that also contains most of the Dengue RNA [42]. Zaitseva et al [43] used a 15%, 20%, 25%, 40% (w/v) iodixanol gradient, centrifuged at approx. 340,000 g for 1.5 h to separate a Dengue virus fraction loaded with a lipophilic fluorescent protein (which banded at the 20-25% interface) from any unincorporated protein. Virus in 5% (w/v) iodixanol has been layered over 10%, 20%, 25% and 35% (w/v) iodixanol centrifuged at 240,000 g for 1.5 h (the virus banding at the 20-25% interface [44]. A similar gradient is reported in ref 45.
8b-2 West Nile virus
The virus was concentrated by pelleting at 48,000 g, for 15 h and then the pellet resuspended in buffered saline (or culture medium) and layered on a 15-55% (w/v) iodixanol gradient and centrifuged at 100,000 g for 18 h [46,47]. Whether the relatively low g-forces used reflect the particular sensitivity of this virus to centrifugal forces is not clear. Vancini et al [39] also used their 12%/35% (w/v) iodixanol gradient (see Section 8b-1) for West Nile virus purification.
8b-3. Yellow fever virus
Yellow fever virus was purified and analyzed on a 5-40% (w/v) iodixanol gradient centrifuged at approx. 175,000 gav for 7 h. The gradient was described as “rate-zonal” but under these centrifugation conditions, it is more likely to be a buoyant density separation. Whatever the nature of the separation, a very interesting observation made by Patkar et al [48] was that some amino acid deletion sequences made to the capsid protein caused a significant shift in the banding position compared to that of the wild-type. The gradient is capable of very high discriminatory powers.
8c. Hepatitis E virus
Continuous iodixanol gradients of 8-40% (w/v) [49], 6-56% (w/v) [50] and 0-40% (w/v) [51] have been used to compare the properties of virus that is released into the culture medium from infected cell monolayers versus that from cell lysates [49] or that which is present in blood serum or in fecal matter from infected animals [50,51]. The iodixanol gradients clearly showed that the virus in plasma orculture medium was enveloped and had a density in the range 1.08-1.11 g/ml while that from cell lysates and fecal matter was much higher (1.20-1.25 g/ml), characteristic of a non-enveloped form.
8d. Hepatitis A virus (Picornaviridae)
Feng et al [52] were the first group to devise a gradient (8-40% w/v iodixanol, centrifuged at 141,000 g for 48 h) that distinguished two populations of hepatitis A virus: a low density one (1.06- 1.10 g/ml) and a high density one (1.22-1.28 g/ml). The low density one was enveloped and the high density one was non-enveloped; Hofer [53] identified the low-density population as “exosome-like” and noted that unlike the dense population it contained no viral capsids. Feng and Lemon [54] commented that the envelope may be gained from the host by the virus to evade host antibodies. This dichotomy of virus macromolecules was further studied in ref. 55 using the established 8-40% iodixanol gradient as described in ref 52. The same gradient has been reported in other publications but the centrifugation time was reduced to 24 h and the speed increased slightly from 141,000g to 165,500 g [56, 57]. The only gradient variation was introduced by Kapsch et al [58]: a 15-50% iodixanol step gradient centrifuged at 141,000 g for 48 h.
9. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Yi, M., Villanueva, R.A., Thomas, D.L., Wakita, T. and Lemon, S.M. (2006) Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells Proc. Natl. Acad. Sci. USA, 103, 2310-2315
3. Yi, M., Ma, Y., Yates, J. and Lemon, S.M. (2007) Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus J. Virol., 81, 629-638
4. Bartolomé, J., López-Alcorocho, J.M., Castillo, I., Rodriguez-Iñigo, E., Quiroga, J.A., Palacios, R. and Carreño, V. (2007) Ultracentrifugation of serum samples allows detection of hepatitis C virus RNA in patients with occult hepatitis C. J. Virol., 81, 7710-7715
5. Kato, T., Matsumura, T., Heller, T., Saito, S., Sapp, R.K., Murthy, K., Wakita, T. and Liang, T.J. (2007) Production of infectious hepatitis C virus of various genotypes in cell cultures J. Virol., 81, 4405-4411
6. Yu, X., Qiao, M., Atanasov, I., Hu, Z., Kato, T., Liang, T.J. and Zhou, Z.H. (2007) Cryo-electron microscopy and threedimensional reconstructions of hepatitis C virus particles Virology, 126, 126-134
7. Elmowalid, G.A., Qiao, M., Jeong, S-H., Borg, B.B., Baumert, T.F., Sapp, R.K., Hu, Z., Murthy, K. and Liang, T.J. (2007) Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees Proc. Natl. Acad. Sci. USA, 104, 8427-8432
8. Lindenbach, B.D., Evans, M.J., Syder, A.J., Wolk, B., Tellinghuisen, T.L., Liu, C.C., Maruyama, T., Hynes, R.O., Burton, D.R., McKeating, J.A. and Rice, C.M. (2005) Complete replication of hepatitis C virus in cell culture Science, 309, 623-626
9. Nielsen, S.U., Bassendine, M.F., Burt, A.D., Martin, C., Pumeechockchai, W. and Toms, G.L. (2006) Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients J. Virol., 80, 2418-2428
10. Gastaminza, P., Kapadia, S.B. and Chisari, F. (2006) Differential biophysical properties of infectious intracellular and secreted hepatitis C virus particles J. Virol., 80, 11074-11081
11. Wahid, A., Helle, F., Descamps, V., Duverlie, G., Penin, F. and Dubuisson, J. (2013) Disulfide bonds in hepatitis C virus glycoprotein E1 control the assembly and entry functions of E2 glycoprotein J. Virol., 87, 1605-1617
12. Prentoe, J., Jensen, T.B., Meuleman, P., Serre, S.B.N., Scheel, T.K.H., Leroux-Roels, G., Gottwein, J.M. and Bukh, J. (2011) Hypervariable region 1 differentially impacts viability of hepatitis C virus strains of genotypes 1 to 6 and impairs virus neutralization J. Virol., 85, 2224-2234
13. Carlsen, T.H.R., Scheel, T,K,H, Ramirez, S., Foung, S.K.H. and Bukha, J. (2013) Characterization of hepatitis C virus recombinants with chimeric E1/E2 envelope proteins and identification of single amino acids in the E2 stem region important for entry J. Virol., 87, 1385-1399
14. Angus, A.G.N., Loquet, A., Stack, S.J., Dalrymple, D., Gatherer, D., Penin, F. and Patela, A.H. (2012) Conserved glycine 33 residue in flexible domain I of hepatitis C virus core protein is critical for virus infectivity J. Virol., 86, 679-690
15. Maillard, P., Walic, M., Meuleman, P., Roohvand, F., Huby, T., Le Goff, W., Leroux-Roels, G., Pécheur, E.I. and Budkowska, A. (2011) Lipoprotein lipase inhibits hepatitis C virus (HCV) infection by blocking virus cell entry PLoS One, 6: e26637
16. Lindenbach, B.D., Meuleman, P., Ploss, A., Vanwolleghem, T., Syder, A.L., McKeating, J.A., Lanford, R.E., Feinstone, S.M., Major, M.E., Leroux-Roels, G. and Rice, C.M. (2006) Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro Proc. Natl. Acad. Sci. USA, 103, 3805-3809
17. Steinmann, E., Brohm, C., Kallis, S., Bartenschlager, R. and Pietschmann, T. (2008) Efficient trans-encapsidation of hepatitis C virus RNAs into infectious virus-like particles J. Virol., 82, 7034-7046
18. Haid, S., Pietschmann, T. and Pécheur, E.I. (2009) Low pH-dependent hepatitis C virus membrane fusion depends on E2 integrity, target lipid composition, and density of virus particles J. Biol. Chem., 284, 17657–17667
19. Bitzegeio, J., Bankwitz, D., Hueging, K., Haid, S., Brohm, C., Zeisel, M.B., Herrmann, E., Iken, M.. Ott, M., Baumert, T.F. and Pietschmann, T. (2010) Adaptation of hepatitis C virus to mouse CD81 permits infection of mouse cells in the absence of human entry factors PLoS Pathogens, 6, e:1000978
20. Haid, S., Windisch, M.P., Bartenschlager, R. and Pietschmann, T. (2010) Mouse-specific residues of claudin-1 limit hepatitis C virus genotype 2a infection in a human hepatocyte cell line J. Virol., 84, 964-975
21. Pietschmann, T., Zayas, M., Meuleman, P., Long, G., Appel, N., Koutsoudakis, G., Kallis, S., Leroux-Roels, G., Lohmann, V. and Bartenschlager, R. (2009) Production of infectious genotype 1b virus particles in cell culture and impairment by replication enhancing mutations PLoS Pathog., 5:e1000475
22. Adair, R., Patel, A.H., Corless, L., Griffin, S., Rowlands, D.J. and McCormick, C.J. (2009) Expression of hepatitis C virus (HCV) structural proteins in trans facilitates encapsidation and transmission of HCV subgenomic RNA J. Gen. Virol., 90, 833–842
23. Merz, A., Long, G., Hiet, M-S., Brügger, B., Chlanda, P., Andre, P., Wieland, F., Krijnse-Locker, J. and Bartenschlager, R. (2011) Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome J. Biol. Chem., 286, 3018-3032
24. Buck, M. (2008) Direct infection and replication of naturally occurring hepatitis C virus genotypes 1, 2, 3 and 4 in normal human hepatocyte cultures PloS One, 3:e2660
25. Ma, Y., Yates, J., Liang, Y., Lemon, S.M. and Yi, MK. (2008) NS3 helicase domains involved in infectious intracellular hepatitis C virus particle assembly J. Virol., 82, 7624-7639
26. Farquhar, M.J., Harris, H.J., Diskar, M., Jones, S., Mee,, C.J., Nielsen, S.U., Brimacombe C.L., Molina, S., Toms, G.L., Maurel, P., Howl, J., Herberg, F.W., van IJzendoorn, S.C.D., Balfe, P. and McKeating J.A. (2008) Protein kinase Adependent step(s) in hepatitis C virus entry and infectivity J. Virol., 82, 8797-8811
27. Nielsen, S.U., Bassendine, M.F., Martin, C., Lowther, D., Purcell, P.J., King, B.J., Neely, D., Toms, G.L. (2008) Characterization of hepatitis C RNA-containing particles from human liver by density and size J. Gen. Virol., 89, 2507- 2517
28. Maurer, K., Krey, T., Moennig, V., Thiel, H-J. and Rümenapf, T. (2004) CD46 is a cellular receptor for bovine viral diarrhea virus J. Virol., 78, 1792-1799
29. Fredericksen, F., Delgado, F., Cabrera, C., Yáñez, A., Gonzalo, C., Villalba, M. and Olavarría, V.H. (2015) The effects of reference genes in qRT-PCR assays for determining the immune response of bovine cells (MDBK) infected with the Bovine Viral Diarrhea Virus 1 (BVDV-1) Gene, 569, 95–103
30. Fredericksen, F., Carrasco, G., Villalba, M. and Olavarría, V.H. (2015) Cytopathic BVDV-1 strain induces immune marker production in bovine cells through the NF-κB signaling pathway Mol. Immunol., 68, 213–222
31. White, L.J.. Parsons, M.M., Whitmore, A.C., Williams, B.M., de Silva, A. and Johnston, R.E. (2007) An immunogenic and protective alphavirus replicon particle-based Dengue vaccine overcomes maternal antibody interference in weanling mice J. Virol., 81, 10329-10339
32. Wahala, W.M.P.B., Kraus, A.A., Haymore, L.B., Accavitti-Loper, M.A. and de Silva, A.M. (2009) Dengue virus neutralization by human immune sera: Role of envelope protein domain III-reactive antibody Virology 392, 103–113
33. Wahala, W.M.P.B., Donaldson, E.F., de Alwis, R., Accavitti-Loper, M.A., Baric, R.S. and de Silva, A.M. (2010) Natural strain variation and antibody neutralization of dengue serotype 3 viruses PLoS Pathogens, 6: e1000821
34. Hacker, K., White, L. and de Silva, A.M. (2009) N-Linked glycans on dengue viruses grown in mammalian and insect cells J. Gen. Virol., 90, 2097–2106
35. Raheel, U., Jamal, M. and Zaidi, N.U.S.S. (2015) A molecular approach designed to limit the replication of mature DENV2 in host cells Viral Immunol., 28, 378–384
36. Zybert, I.A., van der Ende-Metselaar, H., Wilschut, J. and Smit, J.M. (2008) Functional importance of dengue virus maturation: infectious properties of immature virions J. Gen. Virol., 89, 3047–3051
37. Rodenhuis-Zybert, I.A., van der Schaar, H.M., da Silva Voorham, J.M., van der Ende-Metselaar, H., Lei, H-Y., Jan Wilschut, J. and Smit, J.M. (2010) Immature dengue virus: a veiled pathogen? PLoS Pathogens, 6:e1000718
38. Smith, K.M., Nanda, K., McCarl, V., Spears, C.J., Piper, A., Ribeiro, M., Quiles, M., Briggs, C.M., Thomas, G.S., Thomas, M.E., Brown, D.T. and Hernandez, R. (2012) Testing of novel dengue virus 2 vaccines in African green monkeys: safety, immunogenicity, and efficacy Am. J. Trop. Med. Hyg., 87, 743–753
39. Vancini, R., Kramer, L.D., Ribeiro, M., Hernandez, R. and Brown, D. (2013) Flavivirus infection from mosquitoes in vitro reveals cell entry at the plasma membrane Virology 435, 406–414
40. Briggs, C.M., Smith, K.M., Piper, A., Huitt, E., Spears, C.J., Quiles, M., Ribeiro, M., Thomas, M.E., Brown, D.T. and Hernandez, R. (2014) Live attenuated tetravalent dengue virus host range vaccine is immunogenic in African green monkeys following a single vaccination J. Virol., 88, 6729–6742
41. Hadjilaou, A., Green, A.M., Coloma, J. and Harris, E. (2015) Single-cell analysis of B cell/antibody cross-reactivity using a novel multicolor FluoroSpot assay J. Immunol., 195, 3490–3496
42. Heaton, N.S., Perera, R., Berger, K.L., Khadka, S., LaCount, D.J., Kuhn, R.J. and Randall, G. (2010) Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis Proc. Natl. Acad. Sci. USA, 107, 17345–17350
43. Zaitseva, E., Yang, S-T., Melikov, K., Pourmal, S., Chernomordik, L.V. (2010) Dengue virus ensures its fusion in late endosomes using compartment-specific lipids PloS Pathogens, 6: e1001131
44. Zicari, S., Arakelyan, A., Fitzgerald, W., Zaitseva, E., Chernomordik, L.V., Margolis, L. and Grivel, J-C. (2016) Evaluation of the maturation of individual Dengue virions with flow virometry Virology, 488, 20–27
45. Alayli, F. and Scholle, F. (2016) Dengue virus NS1enhances viral replication and pro-inflammatory cytokine production in human dendritic cells Virology, 496, 227–236
46. Thompson, B.S., Moesker, B., Smit, J.M., Wilschut, J., Diamond, M.S. and Fremont, D.H. (2009) A therapeutic antibody against West Nile virus neutralizes infection by blocking fusion within endosomes PLoS Pathog., 5:e1000453
47. Vogt, M.R., Moesker, B., Goudsmit, J., Jongeneelen, M., Austin, K., Oliphant, T., Nelson, S., Pierson, T.C., Wilschut, J., Throsby, M. and Diamond, M.S. (2009) Human monoclonal antibodies against West Nile Virus induced by natural infection neutralize at a post-attachment step J. Virol., 83, 6494–6507
48. Patkar, C.G., Jones, C.T., Chang, Y-h., Warrier, R. and Kuhn, R.J. (2007) Functional requirements of the yellow fever virus capsid protein J. Virol., 81, 6471-6481
49. Yin, X., Ambardekar, C., Lu, Y. and Feng, Z. (2016) Distinct entry mechanisms for nonenveloped and quasi-enveloped hepatitis E viruses J. Virol., 90, 4232-4242
50. Allweiss, L., Gass, S., Giersch, K., Groth, A., Kah, J., Volz, T., Rapp, G., Schöbel, A. et al (2016) Human liver chimeric mice as a new model of chronic hepatitis E virus infection and preclinical drug evaluation J. Hepatol., 64, 1033–1040
51. Behrendt, P., Bremer, B., Todt, D., Brown, R.J.P., Heim, A., Manns, M.P., Steinmann, E. and Wedemeyer, H. (2016) Hepatitis E virus (HEV) ORF2 antigen levels differentiate between acute and chronic HEV infection J. Infect. Dis., 214, 361–368
52. Feng, Z., Hensley, L., McKnight, K.L., Hu, F., Madden, V., Ping, L-F., Jeong, S-H., Walker, C., Lanford, R.E. and Lemon, S.M. (2013) A pathogenic picornavirus acquires an envelope by hijacking cellular membranes Nature 496, 367- 371
53. Hofer, U. (2013) Cloak and dagger Nat. Rev. Microbiol., 11, 3026
54. Feng, Z. and Lemon, S.M. (2014) Peek-a-boo: membrane hijacking and the pathogenesis of viral hepatitis Trends Microbiol., 22, 59-64
55. McKnight, K.L., Xiec, L., González-Lópeza, O., Rivera-Serrano, E.E., Chen, X. and Lemon, S.M. (2017) Protein composition of the hepatitis A virus quasi-envelope Proc. Natl. Acad. Sci. USA, 114, 6587–6592
56. Hirai-Yuki, A., Hensley, L., Whitmire, J.K. and Lemon, S.M. (2016) Biliary secretion of quasi-enveloped human hepatitis A virus mBio, 7: e01998-16
57. Das, A., Hirai-Yuki, A., González-López, O., Rhein, B., Moller-Tank, S., Brouillette, R., Hensley, L., Misumi, I. et al (2017) TIM1 (HAVCR1) is not essential for cellular entry of either quasi-enveloped or naked hepatitis A virions mBIO, 8: e00969-17
58. Kapsch, A-M., Farcet, M.R., Antoine, G. and Kreil, T.R. (2017) A nonenveloped virus with a lipid envelope: hepatitis A virus as used in virus-reduction studies Transfusion 57, 1433–1439
OptiPrep™ Application Sheet V19; 8th edition, January 2020
OptiPrep™ Application Sheet V20
Purification of Group IV ((+)ss) RNA viruses: Flaviviridae family: hepatitis C particles in a self-generated gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- In OptiPrep™ Reference List (RV04) Section 4c covers publications on the purification and analysis of hepatitis C virus”; it provides a full bibliography of all published papers reporting the use of iodixanol gradients; to access return to the initial list of Folders and select “Reference Lists”.
- For the purification of the virus from cultured cells in pre-formed gradients by buoyant density or sedimentation velocity see Application Sheet V19.
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Introduction
The methodology described in this Application Sheet was primarily developed by the groups based in the Institutes of Cellular Medicine and of Cell and Molecular Biosciences at the University of Newcastle (UK) for the recovery of hepatitis C virus particles from macerates of human infected liver, removed during transplantation and processed directly or from frozen material. The macerated samples have been analyzed in a self-generated iodixanol gradient [1,2]. Although the use of a self-generated gradient is not a common approach for hepatitis C virus, the method is used widely for many retroviruses and has the major advantage of simplicity and safety of sample handling, especially when human samples are involved.
- See Section 6 for use of plasma samples and also for studies on the virus from cultured cells.
 2. Solutions required
2. Solutions required
A. OptiPrep™
B. Buffer: 0.25 M sucrose, 15 mM EDTA 30 mM TrisHCl, pH 8.0
C. Tisssue lysis buffer: 150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 0.2% bovine serum albumin (BSA) Add protease inhibitors to Solution C as required.
3. Rotor requirements For gradient purification: fixed-angle rotor with 10 ml sealed tubes (e.g. Beckman 50Ti) or a vertical or nearvertical rotor (e.g. Beckman VTi65.1 or NVT65) with similar tubes (see Section 5)
4. Protocol
1. Homogenize 400-500 mg liver in 10 ml of Solution C using up to 60 strokes of the pestle of a tight-fitting Dounce homogenizer. This must be carried out in an isolation cabinet to avoid exposure to aerosols.
2. Centrifuge at 8000 g for 5 min at 4°C, then pass the supernatant through a 0.45 m filter.
3. In tubes for the chosen rotor mix 4.2 ml of OptiPrep™, 3.3 ml of Solution B and 2.5 ml of the supernatant (see Section 5, Notes 1 and 2).
4. In a 10 ml fixed-angle tube centrifuge at 165,000 gav for 24 h at 4°C (see Section 5, Note 2).
5. Allow the rotor to decelerate using a slow-deceleration program below 2000 rpm, or turn off the brake below 2000 rpm, then collect the gradient in 0.5-0.7 ml fractions, low density end first. For more information on harvesting gradients see Application Sheet V04.
5. Notes
1. Note that this recipe for the self-generated gradient is a slightly simplified version of that in ref. 1. In that example 2.2 ml of the macerate was mixed with 7.8 ml of a solution containing 32% (w/v) iodixanol, 0.1 M sucrose, 13 mM Tris-HCl, pH 8.0 and 6.5 mM EDTA [1]. In a later example [2] 4.2 ml of the macerate was mixed with 6.24 ml of a solution containing 40% (w/v) iodixanol, 60 mM sucrose, 16 mM Tris-HCl, pH 8.0 and 27 mM EDTA [1]. The final concentration of iodixanol in both cases was however the same at approx. 25% (w/v).
2. Nielsen et al [1,2] used a Beckman 50Ti fixed-angle rotor with approx. 10 ml tubes. Self-generated gradients are more usually prepared in high-performance short sedimentation path length vertical and near-vertical rotors. If these rotors are used at 265-365,000 g self-generated gradients can be formed in 1-3 h see for example the isolation of Herpes virus in Application Sheet V08. It should be noted however that retention of viral infectivity might be enhanced at lower g-forces.
6. Other sample applications
More recently the self-generated gradient approach has been extended to the analysis of human plasma samples. In these samples the particles of interest are primarily low density, hence the reduced iodixanol concentration. Felmlee et al [3] mixed 2 ml of plasma with 8 ml of a solution containing 16% (w/v) iodixanol, 2.5 mM EDTA, 12.5 mM Tris-HCl, pH 8.0, 176 mM sucrose and 6.25 mM of both MgSO4 and MgCl2. A higher performance Beckman 70Ti fixed-angle rotor was used at approx. 266,000 gav for 16 h. A smaller plasma volume (0.5 ml) was used by Bridge et al [4], adjusted to very similar concentrations of iodixanol, buffer etc. In the lower performance 50Ti rotor the time required at 165,000 gav was 24 h. Sheridan et al [5] used this latter method for plasma. Virus from cultured cells has also been analyzed in self-generated iodixanol gradients [6].
- For more information on the creation of self-generated gradients see Application Sheet V03
7. References
1. Nielsen, S.U., Bassendine, F., Burt, A.D., Bevitt, D.J. and Toms, G.L. (2004) Characterization of the genome and structural proteins of hepatitis C virus resolved from infected human liver J. Gen. Virol., 85, 1497-1507
2. Nielsen, S.U., Bassendine, M.F., Martin, C., Lowther, D., Purcell, P.J., King, B.J., Neely, D., Toms, G.L. (2008) Characterization of hepatitis C RNA-containing particles from human liver by density and size J. Gen. Virol., 89, 2507- 2517
3. Felmlee, D.J., Sheridan, D.A., Bridge, S.H., Nielsen, S.U., Milne, R.W., Packard, C.J., Caslake, M.J., McLauchlan, J., Toms, G.L., Neely, R.D.G. and Bassendine, M.F. (2010) Intravascular transfer contributes to postprandial increase in numbers of very-low-density hepatitis C virus particles Gastroenterology 139, 1774–1783
4. Bridge, S.H., Sheridan, D.A., Felmlee, D.J., Nielsen, S.U., Thomas, H.C., Taylor-Robinson, S.D., Neely, R.D.G., Toms, G.L. and Bassendine, M.F. (2011) Insulin resistance and low-density apolipoprotein B-associated lipoviral particles in hepatitis C virus genotype 1 infection Gut, 60, 680-687
5. Sheridan, D.A., Bridge, S.H., Felmlee, D.J., Crossey, M.M.E., Thomas, H.C., Taylor-Robinson, S.D., Toms, G.L., Neely, R.D.G. and Bassendine, M.F. (2012) Apolipoprotein-E and hepatitis C lipoviral particles in genotype 1 infection: Evidence for an association with interferon sensitivity J. Hepatol., 57, 32–38
6. Vassilaki, N., Friebe, P., Meuleman, P., Kallis, S., Kaul, A., Paranhos-Baccalà, G., Leroux-Roels, G., Mavromara, P. and Bartenschlager, R. (2008) Role of the hepatitis C virus core+1 open reading frame and core cis-acting RNA elements in viral RNA translation and replication J. Virol., 82, 11503-11515
OptiPrep™ Application Sheet V20; 5th edition, January 2020
OptiPrep™ Application Sheet V21
Purification of Group IV ((+)ss) RNA viruses: Nidovirales: Coronaviridae and Arteriviridae
- OptiPrep is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- Whether the protocols described in this Application Sheet can be applied to other members of the Nidovirales order, such as those viruses of the Roniviridae can only be determined by experimentation.
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
There are now many published papers that report the use of iodixanol gradients not only to purify viruses but also to investigate their assembly. In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which, in solutions of the same density, is much higher than that of iodixanol.
Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast both infectivity measurements using cultured cells and many add-on techniques can be performed without dialysis of iodixanol. Combined with the availability of OptiPrep™ as a sterile solution, this makes the use of OptiPrep™ for virus purification and assembly analysis much more convenient than the use of either CsCl or sucrose.
Clarified suspensions of murine coronavirus [2] and later human coronavirus [3] were first concentrated in PEG 8000 (containing 0.5 M NaCl) and subsequently purified in NycodenzⓇ gradients. Initially the virus was concentrated at the boundary of a 10% and 50% (w/v) Nycodenz discontinuous gradient (83,000 g for 3.5 h) and then it was further purified in a continuous 10-50% NycodenzⓇ gradient (83,000 g for 16 h). The NycodenzⓇ solutions were made up in 0.1 M NaCl, 1 mM EDTA, 100 mM Tris-maleate, pH 6.2.
However, because of the relative ease of using OptiPrep™, this Application sheet will focus on the use of this medium for the purification of the severe acute respiratory syndrome coronavirus (SARS-CoV) (Coronaviridae) and the porcine reproductive and respiratory syndrome virus (PRRSV), which is an Arteriviridae virus. SARS-CoV has been purified principally on the basis of buoyant density in either a self-generated gradient [4-8] or a pre-formed continuous gradient [9,10], while for PRRSV both selfgenerated [11-13] and sedimentation velocity gradients have been used [14]. All three options are described in this Application Sheet in Sections 2-4 respectively.
- Note that methods from some of the more recent papers are briefly discussed in Section 5.
2. Self-generated gradient (adapted from refs 4-8)
2a. Solutions required
A. OptiPrep™
B. Phosphate buffered saline (PBS) (see Section 2d, Note 1)
2b. Rotor requirements
For virus concentration: swinging-bucket rotors with approx 17 or 38 ml tubes (e.g. Beckman SW28 or SW28.1)
For self-generated gradient: near-vertical rotor with tube capacity of 5-13 ml (e.g. Beckman NVT65, NVT65.2, NVT90 or similar rotor.
2c. Protocol
1. Freeze-thaw the cells three times in Solution B.
2. Clarify the cell lysate by centrifugation at 3,000 g for 20 min at 4°C.
3. Prepare a 50% (w/v) iodixanol solution by diluting 5 vol. of OptiPrep™ with 1 vol. of PBS (see Section 2d, Note 2).
4. Concentrate the virus by sedimentation on to a small cushion of the 50% iodixanol by centrifugation at 50,000 g for 1.5 h in the chosen swinging-bucket rotor. In 38 ml tubes use 3-4 ml of cushion, in 17 ml tubes use 1-2 ml. Make sure that the volume of virus-containing liquid in each tube is known, this is important for Step 5 (see Section 2d, Note 3).
5. Carefully aspirate all of the supernatant except for a small volume equivalent to that of the cushion (see Section 2d, Notes 4 and 5).
6. Mix the contents of the tubes very well and then transfer them to sealed tubes for the chosen nearvertical rotor (see Section 2d, Note 6).
7. Centrifuge at 350-400,000 g for approx. 3.5 h at 4°C and allow the rotor to decelerate using a slow deceleration program below 4000 rpm or turn off the brake below 4000 rpm (see Section 2d, Note 6).
8. Collect the gradient by aspiration from the meniscus, upward displacement with a dense medium or tube puncture and analyze the fractions. For more information on harvesting gradients see Application Sheet V04. The banding density of coronavirus is approx 1.2 g/ml according to Huang et al [6].
2d. Notes
1. The PBS may be replaced by any buffered saline solution.
2. When OptiPrep™ is diluted with PBS, the final solution will be approximately isoosmotic, but if it is considered that the ionic strength of the final solution is not sufficiently high, then mix the OptiPrep™ instead with 0.6 vol. of 10xPBS and 0.4 vol. of water.
3. The use of Beckman “konical” tubes permits the volume of cushion to be reduced; this might be convenient with large volumes of virus-containing fluid. For more information on concentrating virus see Application Sheet V06.
4. Note that Berry et al [4] removed a volume of supernatant equivalent to 1.5x that of the cushion, so that when the residual material was mixed, the final concentration of iodixanol was 20%; while Huang et al [6] removed a volume equivalent to two thirds of that of the cushion, so that when the residual material was mixed, the final concentration of iodixanol was 30%. In the mid-course approach adopted here, mixing the cushion with the same volume of supernatant will reduce the iodixanol concentration to 25% (w/v). All three approaches probably work equally well, but because density profile of the self-generated gradient will be different in each case, the position of the virus in the tube will also be different.
5. In their purification strategy for PRRSV, Li and Murtaugh [11-13] collected the pelleted virus from two rounds of 0.5 M sucrose barrier sedimentation and then suspended the virus in 20% (w/v) iodixanol and used a self-generated gradient created at a lower centrifugation speed of 250,000 g for a longer time – 9 h.
6. Vertical rotors of the same capacity are permissible and the gradient that is generated will be more or less identical, but a small cushion of 0.5 ml of 40% iodixanol should be included to stop any dense material from reaching the tube wall.
3. Pre-formed gradients (adapted from ref 9; but see also Section 3d Notes)
3a. Solutions required
A. OptiPrep™
B. Phosphate buffered saline (PBS)
3b. Rotor requirements
For virus concentration: swinging-bucket rotor with approx 13 ml tubes (e.g. Beckman SW41Ti)
3c. Protocol
1. Concentrate and partially purify the virus by sedimentation through a 15% (w/v) iodixanol cushion (dilute 1.5 vol. of OptiPrep with 4.5 vol. of Solution B) at 67,000 g for 2.5 h (see Section 3d).
2. Resuspend the virus in 1-2 ml of PBS.
3. Prepare solutions of 12% and 30% (w/v) iodixanol by diluting 1.2 vol. and 1 vol. of OptiPrep™ with 4.8 vol. and 1 vol. of PBS respectively (see Section 3d).
4. In tubes for the swinging-bucket rotor prepare a linear gradient from equal volumes (5.5-6.0 ml) of the 12% and 30% iodixanol solutions using a two chamber gradient maker or a Gradient Master™ Alternatively make a discontinuous gradient from 12%, 18%, 24% and 30% (w/v) iodixanol and allow the gradient to diffuse. For more information on making continuous gradients see Application Sheet V02.
5. Layer 1-2 ml of the virus suspension on top of the gradient and centrifuge at 260,000 g for 2.5 h. Allow the rotor to decelerate using a slow deceleration program or turn off the brake at 2000 rpm.
6. Collect the gradient in 0.7 ml fractions low-density end first and analyze them for the virus; for more information on gradient unloading see Application Sheet V04.
3d. Notes
Virus concentration: Beniac et al [9] concentrated the virus by pelleting at approx 140,000 g for 90 min. The recommended method of pelleting through a cushion at a lower g-force for a longer time is rather more gentle, but the best method is to sediment the virus on to a dense cushion. The latter does however pose a problem for subsequent layering of the virus on top of a gradient starting at 12% iodixanol – for a more complete discussion of the problems and possible solutions see Application Sheet V06.
An alternative top-loaded 10-40% (w/v) iodixanol gradient was used by Tseng et al [10] for SARScoronavirus; it was centrifuged in 5 ml tubes at approx. 175,000 g for 16 h. The gradient solutions were again made up by dilution of OptiPrep with PBS. The gradient was initially formed from 1.25 ml each of 10, 20, 20 and 40% (w/v) iodixanol, but during the centrifugation it will become continuous and more or less linear. Tseng et al [10] compared the particles expressed from cultured cells transfected with either the M protein or M plus the N nucleocapsid proteins. Interestingly the viral-like particles expressed from the cells with M protein banded at a slightly lower density (1.13 g/ml) than those with M+N proteins (1.14 g/ml). The gradient thus exhibits a high resolving power. Human coronavirus has also been isolated on a shallow 10-20% iodixanol gradient, centrifuged at 175,000 g for 18 h [15,16].
4. Sedimentation-velocity gradient (adapted from ref 14)
4a. Solutions required
A. OptiPrep™
B. Buffered saline: 150 mM NaCl, 1 mM EDTA, 50 mM Tris-HCl, pH 7.4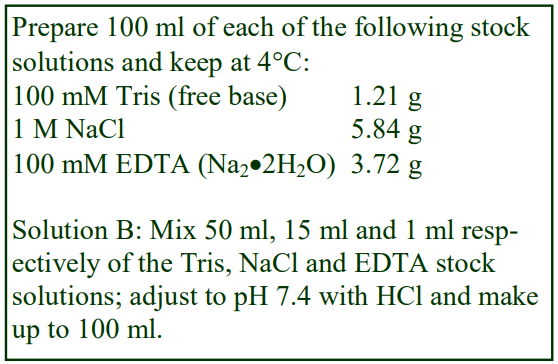 C. Gradient solutions: 10%, 12.5%, 15%, 17.5% and 20% (w/v) iodixanol, dilute OptiPrep™ with Solution B at the following volume ratios respectively, 1:5, 1.25:4.75, 1.5:4.5, 1.75:4.25 and 2:4
C. Gradient solutions: 10%, 12.5%, 15%, 17.5% and 20% (w/v) iodixanol, dilute OptiPrep™ with Solution B at the following volume ratios respectively, 1:5, 1.25:4.75, 1.5:4.5, 1.75:4.25 and 2:4
4b. Rotor requirements
For virus concentration: swinging-bucket rotors with approx 17 or 38 ml tubes (e.g. Beckman SW28 or SW28.1) For sedimentation-velocity gradient: swinging-bucket rotor with tube capacity of approx.13 ml (e.g. Beckman 41Ti or similar rotor)
4c. Protocol
1. Concentrate and partially purify the virus by sedimentation through a 15% (w/v) iodixanol cushion (dilute 1.5 vol. of OptiPrep™ with 4.5 vol. of Solution B) at 67,000 g for 2.5 h (see Section 4d).
2. Towards the end of step 1 make a discontinuous gradient from 2.4 ml each of 10%, 12.5%, 15%, 17.5% and 20% (w/v) iodixanol by underlayering or overlayering. For more information on making gradients see Application Sheet V02.
3. Resuspend the viral pellet from Step 1 in Solution B and layer 1 ml on top of each gradient.
4. Centrifuge at 41,000 rpm (200,000 gav) for 2 h at 4°C.
5. Collect the gradient by aspiration from the meniscus, upward displacement with a dense medium or tube puncture and analyze the fractions for virus. For more information on gradient unloading see Application Sheet V04.
4d. Notes
Use whichever rotor is more suitable for the volume of virus fluid. Only 1-2 ml of cushion is required in a 17 ml tube or 3-4 ml in a 38 ml tube. The ideal way of concentrating the virus is sedimentation on to a dense cushion of iodixanol, rather than pelleting. This however may be less convenient when, as in this case, the concentration of iodixanol in the viral suspension needs to be <10% (w/v) to permit loading on the gradient. When recovering the band of virus as little as possible of the cushion must be aspirated. For more information on concentration of virus see Application Sheet V06.
5. Recent publications
De Wit et al [17] have studied Middle East respiratory syndrome coronavirus (MERS-CoV), pelleting the virus through a simple 15% (w/v) iodixanol cushion OptiPrep™. Tseng et al [18] investigated the conditions that promote the formation of virus-like SARS-CoV particles, in which the virus was purified in a 5 ml 10-40% (w/v) iodixanol gradient at approv. 150,000 g for 16 h. SARS-Corona virions have also been purified in shallow 20-30 % (w/v) iodixanol gradients at 111,000 g for 18 h [19] Giles et al [20,21] used a self-generated gradient method very similar to that described in Section 2 to purify the nidovirus associated with wobbly possum disease; the concentrated virus was adjusted to 25% (w/v) iodixanol and centrifuges in a small volume (approx 4 ml tubes) vertical rotor at 200,000 g for 6.5 h.
6. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Talbot, P.J., Lapierre, J., Daniel, C., Dugre, R. and Trepanier, P. (1989) Growth of a murine coronavirus in a microcarrier cell culture system J. Virol. Meth., 25, 63-70
3. Lachance, C., Arbour, N., Cashman, N.R. and Talbot, P.J. (1998) Involvement of aminopeptidase N (CD13) in infection of human neural cells by human coronavirus 229E J. Virol., 72, 6511-6519
4. Berry, J.D., Jones, S., Drebot, M.A., Andonov, A., Sabara, M., Yuan, X.Y., Weingartl, H., Fernando, L., Marszal, P., Gren, J., Nicolas, B., Andonova, M., Ranada, F., Gubbins, M.J., Ball, T.B., Kitching, P., Li, Y., Kabani, A. and Plummer, F. (2004) Development and characterization of neutralizing monoclonal antibody to the SARs-coronavirus J. Virol. Meth., 120, 87-96
5. Gubbins, M. J., Plummer, F.A., Yuan, X.Y., Johnstone, D., Drebot, M., Andonova, M., Andonov, A. and Berry, J.D. (2004) Molecular characterization of a panel of murine monoclonal antibodies specific for the SARS-coronavirus Mol. Immunol., 42, 125-136
6. Huang, Y., Yang, Z-y., Kong, W-p. and Nabel, G.J. (2004) Generation of synthetic severe acute respiratory syndrome coronavirus pseudoparticles: implications for assembly and vaccine production J. Virol., 78, 12557-12565
7. Yang, Z-Y., Huang, Y., Ganesh, L., Leung, K., Kong, W-P., Schwartz, O., Subbarao, K. and Nabel, G.J. (2004) pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-sign J. Virol., 78, 5642-5680
8. Hatakeyama, S., Matsuoka, Y., Ueshiba, H., Komatsu, N., Itoh, K., Shichijo, S., Kanai, T., Fukushi, M., Ishida, I., Kirikae, T., Sasazuki, T. and Miyoshi-Akiyama, T. (2008) Dissection and identification of regions required to form pseudoparticles by the interaction between the nucleocapsid (N) and membrane (M) proteins of SARS coronavirus Virology, 380, 99-108
9. Beniac, D.R., deVarennes, S.L., Andonov, A., He, R. and Booth, T.F. (2007) Conformational reorganization of the SARS Coronavirus spike following receptor binding: implications for membrane fusion PloS ONE, 10:e1082
10. Tseng, Y-T., Wang, S-M., Huang, K-J., Lee, A.I-R., Chiang, C-C. and Wang, C-T. (2010) Self-assembly of severe acute respiratory syndrome coronavirus membrane protein J. Biol. Chem., 285, 12862–12872
11. Li, J. and Murtaugh, M.P. (2012) Dissociation of porcine reproductive and respiratory syndrome virus neutralization from antibodies specific to major envelope protein surface epitopes Virology, 433, 367–376
12. Li, J. amd Murtaugh, M.P. (2015) Functional analysis of porcine reproductive and respiratory syndrome virus N-glycans in infection of permissive cells Virology, 477, 82–88
13. Li, J., Tao, S., Orlando, R. and Murtaugh, M.P. (2015) N-glycosylation profiling of porcine reproductive and respiratory syndrome virus envelope glycoprotein 5 Virology 478, 86–98 14. Delputte, P.L., Meerts, P., Costers, S. and Nauwynck, H.J. (2004) Effect of virus-specific antibodies on
attachment, internalization and infection of porcine reproductive and respiratory syndrome virus in primary macrophages Vet. Immunol. Immunopathol., 102, 179-188
15. Milewska, A., Zarebski, M., Nowak, P., Stozek, K., Potempa, J. and Pyrca, K. (2014) Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells J. Virol., 88, 13221–13230
16. Milewska, A., Kaminski, K., Ciejka, J., Kosowicz, K., Zeglen, S., Wojarski, J., Nowakowska, M., Szczubiałka, K. and Pyrc, K. (2016) HTCC: broad range inhibitor of coronavirus entry PLoS One, 11: e0156552
17. De Wit, E., Prescott, J., Baseler, L., Bushmaker, T., Thomas, T., Lackemeyer, M.G., Martellaro, C., MilnePrice, S., Haddock, E., Haagmans, B.L., Feldmann, H. and Munster, V.J. (2013) The Middle East respiratory syndrome coronavirus (MERS-CoV) does not replicate in Syrian hamsters PLoS One, 8: e69127
18. Tseng, Y-T., Wang, S-M., Huang, K-J. and Wang, C-T. (2014) SARS-CoV envelope protein palmitoylation or nucleocapid association is not required for promoting virus-like particle production J. Biomed. Sci., 21:
19. Kuo, L., Hurst-Hess, K.R., Koetzner, C.A. and Masters, P.S. (2016) Analyses of coronavirus assembly interactions with interspecies membrane and nucleocapsid protein chimeras J. Virol., 90, 4357-4368
20. Giles, J.C., Perrott, M.R. and Dunowska, M. (2015) Primary possum macrophage cultures support the growth of anidovirus associated with wobbly possum disease J. Virol. Methods, 222, 66–71
21. Giles, J., Perrott, M., Roe, W. and Dunowska, M. (2016) The aetiologyof wobbly possum disease: Reproduction of the disease with purified nidovirus Virology, 491, 20–26
OptiPrep™ Application Sheet V21; 9th edition, February 2020
OptiPrep™ Application Sheet V22
Purification of Group IV ((+)ss) RNA viruses: Togaviridae: Alphavirus and Rubivirus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- Whether any of the methods described in this Application Sheet can be applied to any Togaviridae alphavirus with similar morphology, macromolecular composition and size, other than those described, can only be determined experimentally.
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Introduction
Regarding Togaviridae alphaviruses, the majority of publications reporting the use of iodixanol gradients are concerned with Semliki Forest virus, for which detailed protocols are given in Section 2. Other Togaviridae alphaviruses have also been prepared and analyzed in iodixanol gradients and subsequent sections briefly present methodologies for Sindbis virus (Section 3), Venezuelan equine encephalitis virus (Section 4), Chikungunya virus (Section 5) and Rubella (Section 6)
In all comparative studies between CsCl and iodixanol, it has been shown that the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which is much higher than that of iodixanol. Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast, many add-on techniques can be performed and cells infected with virus, without dialysis of iodixanol.
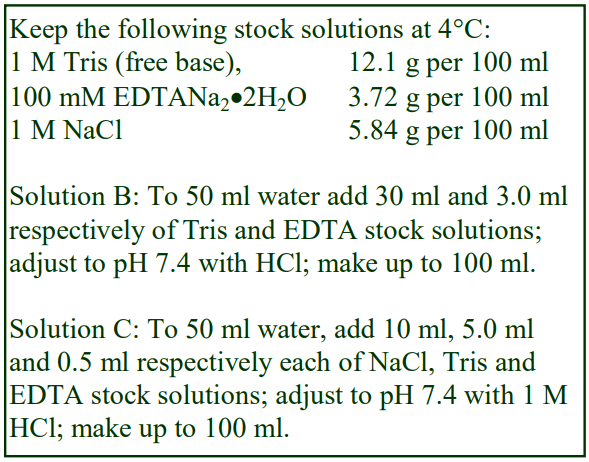 2. Semliki forest virus
2. Semliki forest virus
The protocol below is adapted from refs 2 and 3 (see also Section 2e).
2a. Solutions required
A. OptiPrep™
B. OptiPrep™ diluent: 3.0 mM EDTA, 0.3 M Tris-HCl, pH 7.4
C. Suspension medium: 0.1 M NaCl, 0.5 mM EDTA, 50 mM Tris-HCl, pH 7.4
D. Iodixanol working solution: 50% iodixanol, 0.5 mM EDTA, 50 mM Tris-HCl, pH 7.4; mix 5 vol. of OptiPrep™ with 1 vol. of Solution B (see Note 1)
2b. Ultracentrifuge rotor requirements
Swinging-bucket rotor with 13-14 ml tubes (e.g. Beckman SW41Ti or Sorvall TH641).
2c. Protocol
1. Produce solutions of 5% and 30% (w/v) iodixanol by diluting Solution D with Solution C (see Notes 2 and 3).
2. Harvest the virus-containing supernatant from the cells.
3. Clarify the suspension by centrifugation at 1500 g for 20 min.
4. Concentrate the virus suspension by pelleting it through a 5% iodixanol barrier at 160,000 gav for 1 h (see Note 4).
5. Allow the pellet to disperse itself overnight at 4°C in 1-2 ml of Solution C (see Notes 4 and 5).
6. Using a two-chamber gradient maker or a Gradient Master™ prepare a continuous gradient from approx 6 ml each of the 5% and 30% iodixanol solutions (see Notes 6-8).
7. Layer the crude virus suspension (1.0-1.5 ml) on top of the gradient and centrifuge at 160,000 gav for 1.5 h at 4°C.
8. Collect the gradient by upward displacement, low-density end first in approx 0.8-1.0 ml fractions (see Note 9). The virus bands sharply towards the bottom of the gradient.
2d. Notes
1. The production of a working solution as described ensures that the buffer and EDTA concentration is constant throughout the gradient. If Solution B also contains 0.6 M NaCl, the NaCl concentration will also be constant but the dense part of the gradient will be hyperosmotic. For more information on the preparation of density gradient solutions see Application Sheet V01.
2. If a gradient making device is unavailable, then make up solutions of 5%, 11%, 17%, 24% and 30% (w/v) iodixanol (see Note 6).
3. For the isolation of Gag particles Hammarstedt et al [1] used a 5-20% (w/v) iodixanol gradient.
4. The ideal way of concentrating the virus is sedimentation on to a dense cushion of iodixanol, rather than pelleting. This however may be less convenient when, as in this case, the concentration of iodixanol in the viral suspension needs to be <4% (w/v) to permit loading on to the gradient. When recovering the band of virus as little as possible of the cushion must be aspirated. It may however be permissible to raise the density of the top of the gradient to 7.5% (w/v) iodixanol without compromising the purification. In which case the stringent requirement for a virus suspension of <4% iodixanol need not apply. For more information on concentration of virus prior to gradient purification see Application Sheet V06.
5. The overnight dispersal of the pellet avoids the often harsh shear conditions required to resuspend virus pellets and consequently may give improved retention of infectivity.
6. Alternatively make a discontinuous gradient from equal volumes of the 5%, 11%, 17%, 24% and 30% iodixanol solutions (see Note 2) and allow the formation of a continuous gradient by diffusion (approx. 5 h at room temperature, or overnight at 4°C). For more information on making gradients see Application Sheet V02.
7. If forming a gradient by diffusion confirm that it is continuous by checking the density of a blank gradient. For more information about density measurement see Application Sheet V05.
8. If larger volumes of crude virus are to be purified then proportionately larger volume gradients must be used.
9. Collection of the gradient by tube puncture may be a useful alternative. For more information on harvesting gradients see Application Sheet V04.
2e. SFV sedimentation velocity separation
The relatively short centrifugation time employed in the method described above may be because it is based, at least in part, on sedimentation velocity. More recently SFV has been purified by a method that more clearly relies on sedimentation velocity for its efficacy [4]. The virus was first concentrated by pelleting through a sucrose cushion (see Application Sheet V06 for other methods of concentration). A 7.5%-27.5% (w/v) iodixanol gradient was generated by diffusion overnight at 4°C from a multiple step discontinuous gradient (0.65 ml each of 7.5% and 10% iodixanol and 0.5 ml each of 11.25%, 12.5%, 13.75%, 15%………27.5% iodixanol). No more than 0.25 ml of concentrated virus was placed on top and centrifuged in 14 ml tubes at 85,000 g for 30 min. It is highly likely that an approx. 13 ml continuous gradient generated from equal volumes of 7.5% and 27.5% (w/v) iodixanol would substitute for the diffused multi-step gradient (see Application Sheet V02 for pre-formed continuous gradient methodology).
3. Sindbis virus
Sindbis virus nucleocapsids have been analyzed in a continuous iodixanol gradient. After cell lysis in a hypoosmotic buffer containing 4% Triton X100, a post-nuclear supernatant was loaded onto a continuous isoosmotic 0-30% (w/v) iodixanol gradient containing 0.1% Triton X100, 100 mM NaCl, 1 mM EDTA and 50 mM Tris-HCl, pH 7.4 and centrifuged at approx. 120,000 g for 2.5 h (approx 14 ml tubes). The nucleocapsids peaked in fractions approximately two-thirds of the distance from the top of the gradient [5-8]. Iodixanol gradients are also able to resolve two types of virus, the denser of which is more infectious [9].
Snyder et al [10] also reported the use of a 0 to 30% (w/v) iodixanol gradient in a standard buffered saline containing 1 mM EDTA); the gradient was top-loaded with a crude virus suspension and centrifuged at approx 125,000 g for 2 h. Both Sindbis and Chikungunya virus (see Section 5) were purified using this gradient.
4. Venezuelan equine encephalitis virus
Prior to the isolation of pre-viral nucleocapsids the virus was concentrated by pelleting through a 25% (w/v) iodixanol cushion at 115,000 g for 3 h (see Application Sheet V06 for methods of concentration). The pre-viral nucleocapsids were then purified in a 10-60% (w/v) iodixanol gradient under the same centrifugation conditions; all procedures were carried out at 4°C [11].
Because of the safety problems regarding the development of attenuated HIV as a vaccine, Jurgens et al [12] investigated the use of the genome from the Venezuelan equine encephalitis virus, modified to express SHIV89.6P genes encoding the Gag and Env proteins of HIV. Essentially the same methodology of an iodixanol cushion and subsequent gradient was used as described in ref 11; the only significant difference being the slightly narrower density range of the gradient (10-50% w/v iodixanol).
Ref 13 also reports the use of iodixanol gradients for the final stage purification of this virus.
5. Chikungunya virus
The regulation of Chikungunya virus-like particle production by the E2 proteins has been studied by Akahata and Nabel [14] using a self-generated iodixanol gradient that was first introduced to study HIV tropism. A crude suspension of particles was adjusted to 30% (w/v) iodixanol and centrifuged at 160,000 gav (45,000 rpm) for 6 h in a Beckman VTi50 vertical rotor, to generate a gradient that spanned the range 1.01-1.22 g/ml. These studies on the Chikungunya virus revealed that the capsid protein banded at significantly higher density than the virus-like particles. This method was also used by Urakami et al [15]. For more information on self-generated iodixanol gradients see OptiPrep™ Application Sheet V03.
6. Rubella virus
PEG-pelleted Rubella virus has been purified on 0-54% (w/v) iodixanol gradients (containing 20 mM Tris-HCl, pH 8.0, 120 mM NaCl, 1 mM EDTA) centrifuged at 175,000 g for 2 h [16]; the authors demonstrated the significant difference in the organization of Rubella structural proteins compared to those of other viruses in the Togaviridae alphavirus group.
7. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Hammarstedt, M, Wallengren, K., Pedersen, K.W., Roos, N. and Garoff, H. (2000) Minimal exclusion of plasma membrane proteins during retrovirus envelope formation Proc. Natl. Acad. Sci. USA, 97, 7527-7532
3. Sjøberg, M. and Garoff, H (2003) Interactions between the transmembrane segments of the alphavirus E1 and E2 proteins play a role in virus budding and fusion J. Virol., 77, 3441-3450
4. Kalvodova, L., Sampaio, J.L., Cordo, S., Ejsing, C.S., Shevchenko, A. and Simons, K. (2009) The lipidomes of vesicular stomatitis virus, Semliki Forest virus and the host plasma membrane analyzed by quantitative shotgun mass spectrometry J. Virol., 83, 7996-8003
5. Snyder, J.E., Azizgolshani, O., Wu, B., He, Y., Lee, A.C., Jose, J., Suter, D.M., Knobler, C.M., Gelbart, W.M. and Kuhn, R.J. (2011) Rescue of infectious particles from preassembled alphavirus nucleocapsid cores J. Virol., 85, 5773–5781
6. Tang, J., Jose, J., Chipman, P., Zhang, W., Kuhn, R.J. and Baker, T.S. (2011) Molecular links between the E2 envelope glycoprotein and nucleocapsid core in sindbis virus J. Mol. Biol., 414, 442–459
7. Jose, J., Przybyla, L., Edwards, T.J., Perera, R., Burgner II, J.W. and Kuhn, R.J. (2012) Interactions of the cytoplasmic domain of Sindbis virus E2 with nucleocapsid cores promote alphavirus budding J. Virol., 86, 2585-2599
8. Snyder, J.E., Berrios, C.J., Edwards, T.J., Jose, J., Perera, R. and Kuhn, R.J. (2012) Probing the early temporal and spatial interaction of the Sindbis virus capsid and E2 proteins with reverse genetics J. Virol., 86, 12372-12383
9. Sokoloski, K.J., Snyder, A.J., Liu, N.H., Hayes, C.A., Mukhopadhyay, S. and Hardy, R.W. (2013) Encapsidation of host-derived factors correlates with enhanced infectivity of Sindbis virus J. Virol., 87, 12216–12226
10. Snyder, J.E., Kulcsar, K.A., Schultz, K.L.W., Riley, C.P., Neary, J.T., Marr, S., Jose, J., Griffin, D.E. and Kuhn, R.J. (2013) Functional characterization of the alphavirus TF protein J. Virol., 87, 8511–8523
11. Lamb, K., Lokesh, G.L., Sherman, M. and Watowich, S. (2010) Structure of a Venezuelan equine encephalitis virus assembly intermediate isolated from infected cells Virology 406, 261–269
12. Jurgens, C.K., Young, K.R., Madden, V.J., Johnson, P.R. and Johnston, R.E. (2012) A novel self-replicating chimeric lentivirus-like particle J. Virol., 86, 246-261
13. Porta, J., Jose, J., Roehrig, J.T., Blair, C.D., Kuhn, R.J. and Rossmann, M.G. (2014) Locking and blocking the viral landscape of an alphavirus with neutralizing antibodies J. Virol., 88, 9616–9623
14. Akahata, W. and Nabel, G. J. (2012) A specific domain of the Chikungunya virus E2 protein regulates particle formation in human cells: implications for alphavirus vaccine design J. Virol., 86, 8879-8883
15. Urakami, A., Sakurai, A., Ishikawa, M., Yap, M.L., Flores-Garcia, Y., Haseda, Y., Aoshi, T., Zavala, F.P. et al (2017) Development of a novel virus-like particle vaccine platform that mimics the immature form of alphavirus Clin. Vacc. Immunol., 24: e00090-17
16. Battisti, A.J., Yoder, J.D., Plevka, P., Winkler, D.C., Prasad, V.M., Kuhn, R.J., Frey, T.K., Steven, A.C. and Rossmann, M.G. (2012) Cryo-electron tomography of rubella virus J. Virol., 86, 11078-11085
OptiPrep™ Application Sheet V22; 9th edition, February 2020
OptiPrep™ Application Sheet V23
Purification of Group V ((-)ss) RNA viruses: Arenaviruses: Lassa virus, Tacaribe virus, Junin virus, Lymphocytic choriomeningitic virus (LCV)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
- This OptiPrep™ Application Sheet primarily describes the purification of Lassa virus (the methodology can probably be applied to other arenaviruses), with comments regarding Junin virus (Section 6) and LCV (Section 7)
- Section 8 describes the gradient analysis of Tacaribe virus nucleoproteins.
1. Background
Dettenhoffer and Yu [1] were the first to report the use of discontinuous 6-18% (w/v) iodixanol gradient in a sedimentation velocity mode to purify HIV-1 virions without affecting the infectivity of the virus. This is described in Application Sheet V34. The technique was subsequently extended to the purification of Lassa virus by Lenz et al [2] and Strecker et al [3]. These workers have used the technique to study the processing of Lassa virus glycoproteins grown in Vero-E6 cells. Purification has also been carried out on an 8%/30% iodixanol discontinuous gradient [4].
All comparative studies between CsCl and iodixanol show that recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [5]. This may be related to its viscosity, which is much higher than that of iodixanol. Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast, many add-on techniques can be performed and cells infected with virus, without dialysis of iodixanol.
The following protocol is adapted from ref 2.
2. Solutions required
A. OptiPrep™
B. Phosphate-buffered saline
C. Gradient solutions: dilute OptiPrep™ with Solution B to give density solutions of 7.2 and 18% (w/v) iodixanol (see Notes 1 and 2)
3. Ultracentrifuge rotor requirements
Swinging-bucket rotor with 13-14 ml tubes (e.g. Beckman SW41Ti or Sorvall TH641).
4. Protocol
1. Concentrate the virus suspension by pelleting it, from a clarified cell culture supernatant, through a density barrier (see Note 3) and resuspend it in a small volume of Solution B.
2. Using a two-chamber gradient maker or a Gradient Master™ prepare a continuous gradient from approx 6 ml each of the two iodixanol solutions (see Notes 4-6).
3. Layer the crude virus suspension (1.0-1.5 ml) on top of the gradient and centrifuge at 200,000 gav for 1.5 h at 4°C (see Notes 7 and 8).
4. Collect the gradient by upward displacement, low-density end first in approx 0.8-1.0 ml fractions (see Note 9). The virus bands sharply at approx 14% (w/v) iodixanol.
5. Notes
1. For more information on the preparation of density gradient solutions see Application Sheet V01.
2. If a gradient making device is unavailable, then make up solutions of 7.0%, 10%, 13%, 16% and 19% (w/v) iodixanol.
3. Lenz et al [3] pelleted Lassa virus through a 20% sucrose cushion (2 h at 48,000 gav); to maintain an isoosmotic environment for the virus, the 20% sucrose might be replaced by 15% (w/v) iodixanol. The ideal way of concentrating the virus is sedimentation on to a dense cushion of iodixanol, rather than pelleting. This however may be less convenient when, as in this case, the concentration of iodixanol in the viral suspension needs to be <5% (w/v) to permit loading on to the gradient. When recovering the band of virus as little as possible of the cushion must be aspirated. For more information on concentration of virus prior to gradient purification see Application Sheet V06.
4. Alternatively make a discontinuous gradient from equal volumes of 7.0%, 10%, 13%, 16% and 19% (w/v) iodixanol and allow the formation of a continuous gradient by diffusion (approx. 5 h at room temperature, or overnight at 4°C). For more information on gradient formation see Application Sheet V02.
5. Confirm that the gradient is continuous by checking the density of a blank gradient. For more information about density measurement see Application Sheet V05.
6. Dettenhoffer and Yu [1], who introduced the sedimentation velocity strategy for HIV-1, prepared gradients that were “essentially continuous” by layering solutions with a 1.2% iodixanol concentration interval. It takes considerable practice to be able to form discontinuous gradients from numerous small volume steps, irrespective of whether a pipette or a syringe is used and whether an overlayering or underlayering technique is used; see Application Sheet V02.
7. If larger volumes of crude virus are to be purified then larger volume gradients must be used. As this is a rate-zonal separation the volume of crude virus suspension should not exceed 10-15% of the gradient volume.
8. If the separation is to be carried out at higher temperatures then it may be necessary to reduce the centrifugation time to take account of the reduced viscosity of the gradient.
9. Collection of the gradient by tube puncture may be a useful alternative. For more information on harvesting gradients see Application Sheet V04.
6. Pelleting through a density cushion
A simple cushion of 10% (w/v) iodixanol (150,000 g for 2 h) has been used to separate fluorescent dye-labelled Junin virus from the dye solution in flow cytometry studies [6-8].
7. Lymphocytic choriomeningitis virus (LCV)
LCV was purified in a continuous iodixanol gradient generated by diffusion from 7%, 10%, 13%, 16% and 19% (w/v) iodixanol, centrifuged at 122,000 g for 12 h [9]
8. Analysis of Tacaribe virus nucleoproteins
In a study of RNA replication in Tacaribe virus Baird et al [10] developed a very useful iodixanol gradient for the analysis of replication transcription complexes (RTCs). The virus-containing cells are lysed in a medium containing K-aspartate, K-glutamate and K-gluconate (all 38 mM), !0 mM KHCO3, 2 mM MgCl2 (or 5 mM EDTA), 2mM DTT, 10μM ZnCl2 and 20 mM MOPS pH 7.1 (plus protease inhibitors), either by Dounce homogenization or addition of a detergent (2% NP40). A 15-48% (w/v) iodixanol gradient (containing the same reagents as the lysis medium) is formed in 5 ml tubes for a swinging-bucket rotor; either using a gradient former or by allowing a discontinuous gradient (equal volumes of 15%, 26%, 37% and 48% iodixanol) to diffuse. After adjusting the sample to 50% (w/v) iodixanol it is layered beneath the continuous gradient and centrifuged at 100,000 g for 20 h. For more information on gradient formation and the underlayering of samples see Application Sheet V02.
As with all flotation gradients, soluble proteins remain in the load zone, allowing the other macromolecules and macromolecular complexes to float into the gradient. Most of the Tacaribe virus nucleoprotein banded at a density that confirmed its association with the virus membrane [6]. Importantly the gradient was also able to distinguish the full length RNAs (which co-banded with the nucleoprotein) and denser mRNA nucleoprotein. Baird et al [10] also observed that the gradient was able to resolve other novel RNA species. For more information on the analysis see ref 6.
9. References
1. Dettenhoffer, M. and Yu, X-F. (1999) Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif virions J. Virol., 73, 1460-1467
2. Lenz, O., ter Meulen, J., Feldmann, H., Klenk, H-D. and Garten, W. (2000) Identification of a novel consensus sequence at the cleavage site of the Lassa virus glycoprotein J. Virol., 74, 11418-11421
3. Strecker, T., Eichler, R., ter Meulen, J., Weissenhorn, W., Klenk, H.D., Garten, W. and Lenz, O. (2003) Lassa virus Z protein is a matrix protein sufficient for the release of virus-like particles J. Virol., 77, 10700- 10705
4. Eichler, R., Lenz, O., Strecker, T. and Garten, W. (2003) Signal peptide of Lassa virus glycoprotein GP-C exhibits an unusual length FEBS Lett., 538, 203-206
5. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
6. Gaudin, R. and Barteneva, N.S. (2015) Sorting of small infectious virus particles by flow virometry reveals distinct infectivity profiles Nat. Commun, 6: 6022
7. Gaudin, R. and Kirchhausen, T. (2015) Superinfection exclusion is absent during acute Junin virus infection of Vero and A549 cells Sci. Rep., 5: 15990
8. Chou, Y-y., Cuevas, C., Carocci, M., Stubbs, S.H., Ma, M., Cureton, D.K., Luke Chao, L., Evesson, F. et al (2016) Identification and characterization of a novel broad-spectrum virus entry inhibitor J. Virol., 90, 4494-4510
9. Ziegler, C.M., Eisenhauer, P., Bruce, E.A., Weir, M.E., King, B.R., Klaus, J.P., Krementsov, D.N. et al (2016) The lymphocytic choriomeningitis virus matrix protein PPXY late domain drives the production of defective interfering particles PLoS Pathog., 12: e1005501
10. Baird, N.L., York, J. and Nunberg, J.H. (2012) Arenavirus infection induces discrete cytosolic structures for RNA replication J. Virol., 86, 11301-11310
OptiPrep™ Application Sheet V23; 8th edition, January 2020
OptiPrep™ Application Sheet V24
Purification of Group V ((-)ss) RNA viruses: Bunyaviridae – Hantavirus, Orthobunyavirus and Phlebovirus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- This Application Sheet covers Bunyamwera virus (Section 2), Hantavirus (Section 3), Rift Valley fever virus (Section 2), Hazara virus (Section 4).
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
- For purification and analysis of other Group V ((–)ss) RNA viruses see OptiPrep™ Application Sheets V23 and V25-V28.
1. Background
There are now many published papers that report the use of iodixanol gradients not only to purify viruses but also to investigate their assembly. In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which, in solutions of the same density, is much higher than that of iodixanol.
Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast both infectivity measurements using cultured cells and many add-on techniques can be performed without dialysis of iodixanol. Combined with the availability of OptiPrep™ as a sterile solution, this makes the use of OptiPrep™ for virus purification and assembly analysis much more convenient than the use of either CsCl or sucrose.
Bunyamwera virus and Rift Valley fever virus (Section 2) have been purified in continuous gradients of iodixanol; Sin Nombre hantavirus (Section 3) in self-generated and pre-formed discontinuous iodixanol gradients. Whether a particular method can be applied to any –ve sense RNA virus can only be determined experimentally.
2. Analysis of Bunyamwera virus
The protocol is adapted from refs 2 and 3. See Section 2d, Notes 1 and 6 re Rift Valley virus.
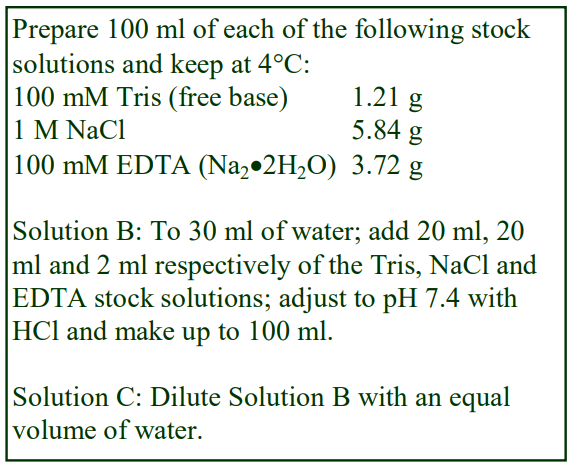 2a. Solutions required (see box ⇒)
2a. Solutions required (see box ⇒)
A. OptiPrep™
B. OptiPrep™ Diluent: 0.2 M NaCl, 2 mM EDTA, 0.02 M Tris-HCl, pH 7.4
C. Working solution (30% w/v iodixanol): Mix equal volumes of OptiPrep™ and Solution B.
D. Buffer: 0.1 M NaCl, 1 mM EDTA, 0.01 M Tris-HCl, pH 7.4
Include protease inhibitors in Solutions B and D as required.
2b. Rotor requirements
Swinging-bucket rotors with approx 13 ml tubes (e.g. Beckman SW41Ti) and approx 38 ml (e.g.
Beckman SW28)
2c. Protocol
1. Clarify the culture medium or the cell lysate by centrifugation at 3,700 g for 20 min at 4°C (see Section 2d, Note 2).
2. Using a swinging-bucket rotor concentrate and partially purify the virus by sedimentation through a 20% (w/v) iodixanol cushion (dilute Solution C with Solution D) at 67,000 g for 2.5 h (see Section 2d, Note 3).
3. During step 2 prepare two iodixanol solutions of 13% and 22% (w/v) by diluting Solution C with Solution D (see Section 2d, Note 4).
4. In tubes for the 13 ml swinging-bucket rotor use either a two-chamber gradient maker or a Gradient Master™to prepare approx 12 ml continuous gradients from equal volumes the 13% and 22% iodixanol solutions (see Section 2d, Note 4).
5. Resuspend the virus pellet from step 2 in Solution D and apply approx 0.5 ml to each gradient.
6. Centrifuge the gradients at 250,000 g for 1.5 h. Use a slow deceleration program or turn off the brake during deceleration from 3000 rpm.
7. Collect the gradient by aspiration from the meniscus, upward displacement with a dense medium or tube puncture (for more information on harvesting gradients see Application Sheet V04) and analyze the fractions (see Section 2d, Note 5).
2d. Notes
1. Rift Valley virus was concentrated by ultrafiltration using a centrifugal filter device, rather than by sedimentation on to a dense cushion of iodixanol (see Step 2 in Protocol 2c). The virus was then purified on a 10-30% (w/v) iodixanol gradient [4,5]; the 10% and 30% iodixanol solutions should be prepared from OptiPrep™ using the same dilution procedure, with the same NaCl/EDTA/Tris solutions as described above. After forming the gradient in 14 ml tubes from equal volumes of the 10 and 30% iodixanol solutions, the gradient was top-loaded with the concentrated virus and the centrifugation was carried out at 210,000 g for 1.5 h at 4°C. The virus banded approx. half-way down the gradient.
2. Intracellular forms of the virus can be released by three rounds of freeze-thawing [2].
3. Novoa et al [2] pelleted the virus through a 30% (w/v) sucrose cushion; to maintain an isoosmotic environment for the virus, the 30% sucrose has been replaced by 20% (w/v) iodixanol. The ideal way of concentrating the virus is sedimentation on to a dense cushion of iodixanol, rather than pelleting. This however may be less convenient when, as in this case, the concentration of iodixanol in the viral suspension needs to be <13% (w/v) to permit loading on to the gradient. When recovering the band of virus as little as possible of the cushion must be aspirated. For more information on concentration of virus prior to purification see Application Sheet V06.
4. Novoa et al [2] prepared nine solutions between 13% and 22% iodixanol (in 1% steps) and layered equal volumes (dense end first) in a centrifuge tube, snap-freezing each layer in dry ice before applying the next. The tubes were kept frozen until required and continuous gradients were formed by allowing the tubes to thaw at room temperature overnight. For more information on preparing continuous gradients see Application Sheet V02.
5. Virus particles of increasing density can be harvested from the gradient: Type I has an annular structure (immature precursors); Type II is an intermediate dense form (these are both intracellular forms) and finally a dense extracellular form, distinguished morphologically from the Type II form [2].
6. Ariza et al [6] studied the nucleocapsid protein structure from Bunyamwera virus using the iodixanol gradient method of Novoa et al [2]. Working with Rift Valley fever virus Weingart et al [7] first concentrated the virus by pelleting through a 20% (w/v) iodixanol cushion (115,500 g for 1 h); then suspended the virus in a buffered saline (containing 1 mM EDTA) and then banded the virus in a discontinuous gradient of 10, 15, 20, 25 and 30% (w/v) iodixanol (210,000 g 1.5 hr).
3. Analysis of Hantavirus (self-generated gradient)
The protocol is adapted from refs 8-11 (see also Section 3d, Notes 1 and 2).
3a. Solutions required
A. OptiPrep™
B. OptiPrep™ Diluent: Any suitable iso-osmotic balanced salt solution
3b. Rotor requirements
Swinging-bucket rotor with approx. 13 ml tubes (e.g. Beckman SW41Ti) Near-vertical rotor with approx. 5 ml tubes (e.g. Beckman NVT65.2)
3c. Protocol
1. Dilute 5 vol. of OptiPrep™ with 1 vol. of Solution B to produce a 50% (w/v) iodixanol solution.
2. In tubes for the swinging-bucket rotor underlayer approx. 10 ml of the crude virus suspension with
2 ml of the 50% iodixanol solution using a syringe and metal cannula (see Section 3d, Note 3).
3. Centrifuge at approx 190,000 g for 3 h at 4°C.
4. Using a syringe and metal cannula collect 4 ml of liquid from the bottom of the tube i.e. the cushion + the banded virus + 2 ml of the balanced salt solution).
5. Mix the suspension with an extra 1 ml of the 50% iodixanol and transfer to an Optiseal™ for the near-vertical rotor (see Section 3d, Notes 3-5).
6. Centrifuge at approx 350,000 gav for 5 h and allow the rotor to decelerate from 2000 to 0 rpm using a controlled deceleration program (over at least 5 min) or turn off the brake at 2000 rpm.
7. Collect the gradient by tube puncture or any other method; the virus will band in the bottom half of the gradient. For more information on harvesting gradients see Application Sheet V04.
3d. Notes
1. An alternative method in which the concentrated virus was layered over pre-formed continuous gradient formed by diffusion overnight at 4°C from 1.5 ml of 50%, 42%, 35%, 28%, 21%, 14% and 7% (w/v) iodixanol (OptiPrep™ diluted with HEPES-buffered 135 mM NaCl) centrifuged at 25,000 g for 15 h was devised by Huiskonen et al [12]. Although it may be acceptable to use a higher g-force for a shorter time, there is little doubt that the use of relatively low g-forces for longer times will provide the best resolution of any biological particles; moreover during the 15 h centrifugation the gradient will certainly become continuous and at the low speed of 25,000 g there will be little or no sedimentation of the iodixanol molecules themselves. A similar gradient was used by Li et al [13] at approx/ 110,000 g for 3 h.
2. More recently a 5-25% (w/v) iodixanol gradient was generated from a discontinuous one (5% step interval); after layering each step the gradient was frozen and after the final freezing the gradient was top-loaded with sample and centrifuged at 28,000 g for 1.5 h [14].
3. For larger volumes of virus use for example a Beckman SW28 for the virus concentration step and an NVT65 for the self-generated gradient and scale up all the volumes proportionately to fill the tubes. Although the larger volume swinging-bucket rotor cannot be run at 190,000 g, it is probably not necessary to increase the centrifugation time in step 3 to band the virus (although this should be checked out).
4. Vertical rotors of the same capacity are permissible and the gradient that is generated will be more or less identical, but a small cushion of 0.5 ml of OptiPrep™ should be included to stop any dense material from reaching the tube wall.
5. The final concentration of iodixanol is 30% (w/v).
4. Hazara virus/Crimean-Congo hemorrhagic fever virus
Hazara virus and Crimean-Congo hemorrhagic fever virus are members of the Nairovirus genus; Surtees et al [15] purified Hazara virus after precipitation in polyethylene glycol at 4000 g for 30 min. The pellet was resuspended in a buffered saline and transferred to the top of a 5-25% (w/v) iodixanol gradient and the virus was banded sharply in the gradient after centrifugation at 250,000 g for 2.5 h. Crimean-Congo hemorrhagic fever virus was purified by Wang et al [16] using a modification of the methodology used by Ariza et al [6] for orthobunyaviruses. The gradient covered the same density range as described above for Hazara virus, the gradient centrifugation conditions were however modified to 28,000 g for 1.5 h.
5. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Novoa, R.R., Calderita, G., Cabezas, P., Elliott, R.M. and Risco, C. (2005) Key Golgi factors for structural and functional maturation of bunyamwera virus J. Virol., 79, 10852-10863
3. Cabezas, P. and Risco, C. (2006) Studying cellular architecture in three dimensions with improved resolution: Ta replicas revisited Cell Biol. Int., 30, 747-754
4. Freiberg, A.N., Sherman, M.B., Morais, M.C., Holbrook, M.R. and Watowich, S.J. (2008) Threedimensional organization of Rift Valley fever virus revealed by cryoelectron tomography J. Virol., 82, 10341-10348
5. Wolf, M.C., Freiberg, A.N., Zhang, T., Akyol-Ataman, Z., Grock, A., Hong, P.W., Li, J., Watson, N.F. et al (2010) A broad-spectrum antiviral targeting entry of enveloped viruses Proc. Natl. Acad. Sci. USA, 107, 3157–3162
6. Ariza, A., Tanner, S.J., Walter, C.T., Dent, K.C., Shepherd, D.A., Wu, W., Matthews, S.V., Hiscox, J.A., Green, T.J., Luo, M., Elliott, R.M., Fooks, A.R., Ashcroft, A.E., Stonehouse, N.J., Ranson, N.A., Barr, J.N. and Edwards, T.A. (2013) Nucleocapsid protein structures from orthobunyaviruses reveal insight into ribonucleoprotein architecture and RNA polymerization Nucleic Acids Res., 41, 5912–5926
7. Weingart, H.M., Zhang, S., Marszal, P., McGreevy, A., Burton, L. and Wilson, W.C. (2014) Rift valley fever virus incorporates the 78 kDa glycoprotein into virions matured in mosquito C6/36 cells PLoS One, 9: e87385.
8. Prescott, J.B., Hall, P.R., Bondu-Hawkins, V.S., Ye, C. and Hjelle, B. (2007) Early innate immune responses to Sin Nombre Hantavirus occur independently of IFN regulatory factor 3, characterized pattern recognition receptors and viral entry J. Immunol., 179, 1796-1802
9. Bisoffi, M., Hjelle, B., Brown, D.C., Branch, D.W., Edwards, T.L., Brozik, S.M., Bondu-Hawkins, V.S. and Larson, R.S. (2008) Detection of viral bioagents using a shear horizontal surface acoustic wave biosensor Biosens. Bioelectron., 23, 1397-1403
10. Hall, P.R., Hjelle, B., Brown, D.C., Ye, C., Bondu-Hawkins, V., Kilpatrick, K.A. and Larson, R.S. (2008) Multivalent presentation of antihantavirus peptides on nanoparticles enhances infection blockade Antimicrob. Agents Chemother., 52, 2079-2088
11. Buranda, T., Wu, Y., Perez, D., Jett, S.D., BonduHawkins, V., Ye, C., Edwards, B., Hall, P., Larson, R.S., Lopez, G.P., Sklar, L.A. and Hjelle, B. (2010) Recognition of decay accelerating factor and avb3 by inactivated hantaviruses: Toward the development of high-throughput screening flow cytometry assays Anal. Biochem., 402, 151–160
12. Huiskonen, J.T., Hepojoki, J., Laurinmäki, P., Vaheri, A., Lankinen, H., Butcher, S.J. and Grünewald, K. (2010) Electron cryotomography of Tula hantavirus suggests a unique assembly paradigm for enveloped viruses J. Virol., 84, 4889–4897
13. Li, S., Rissanen, I., Zeltina, A., Hepojoki, J., Raghwani, J., Harlos, K., Pybus, O.G., Huiskonen, J.T. and Bowden, T.A. (2016) A molecular-level account of the antigenic hantaviral surface Cell Rep., 15, 959–967
14. Guo, Y., Wang, W., Sun, Y., Ma, C., Wang, X., Wang, X., Liu, P., Shen, S. et al (2016) Crystal structure of the core region of hantavirus nucleocapsid protein reveals the mechanism for ribonucleoprotein complex formation J. Virol., 90, 1048-1061
15. Surtees, R., Dowall, S.D., Shaw, A., Armstrong, S., Hewson, R., Carroll, M.W., Mankouri, J., Edwards, T.A., Hiscox, J.A. and Barr. J.N. (2016) Heat shock protein 70 family members interact with Crimean-Congo hemorrhagic fever virus and Hazara virus nucleocapsid proteins and perform a functional role in the nairovirus replication cycle J. Virol., 90, 9305-9326
16. Wang, X., Li, B., Guo, Y., Shen, S., Zhao, L., Zhang, P., Sun, Y., Sui, S-F., Deng, F. and Lou, Z. (2016) Molecular basis for the formation of ribonucleoprotein complex of Crimean-Congo hemorrhagic fever virus J. Struct. Biol., 196, 455–465
OptiPrep™ Application Sheet V24; 9th edition, March 2020
OptiPrep™ Application Sheet V25
Purification of Group V ((-)ss) RNA viruses: Ebola virus capsid assembly analysis and purification of Ebola pseudovirus in self-generated gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
Huang et al [1] have used the OptiPrep™ self-generated gradient method to study the assembly of Ebola virus; in particular they have used it to analyze the influence of nucleoprotein and various virionassociated proteins on the assembly of capsids in transfected 293T cells.
The procedure is particularly noteworthy for the use of larger volume tubes than is normal and this is one of the first examples of the use of the Beckman VTi50 vertical rotor (tube volume 39 ml) for creating self-generated iodixanol gradients. The tube volumes are much larger than the ones normally used for these self-generated gradients (<13 ml) and the maximum RCF of the rotor is approx 190,000gav against the more routine value of 350,000gav. In spite of these less favourable rotor parameters, very acceptable gradients that demonstrate only a slight flattening in the middle can be obtained after 6 h.
The method may be applicable for the intact virion. Being an enveloped virus, it would probably have a lower density than the capsid, although this has not been validated experimentally. A selfgenerated gradient has however been used for the purification of an Ebola pseudovirus, specifically a luciferase-expressing lentiviral vector pseudotyped with envelopes from Ebola virus [2].
The following protocol is adapted from ref 1. See Section 6 for a brief note on the self-generated gradient used for purification of the Ebola pseudovirus.
2. Solutions required
A. OptiPrep™
B. Lysis medium: 0.05% Tween 20 in phosphate-buffered saline (see Note 1)
3. Ultracentrifuge rotor requirements
The Beckman VTi50 vertical rotor used by Huang et al [1] accommodates 39 ml tubes. If the volume of lysate is manageable in a smaller volume rotor, then any vertical or near-vertical rotor with tube capacity of approx 12 ml and capable of approx 350,000g would be suitable, such as the Beckman VTi65.1 vertical rotor or NVT65 near-vertical rotor. These rotors have a sedimentation path length <25 mm and are more efficient than the VTi50 for gradient self-generation.
- For more information on self-generated gradients in the VTi65.1 and NVT65 rotors consult Application Sheet V03
4. Protocol
1. Suspend the cells in the Solution B and lyse them by freeze-thawing (three cycles).
2. Clarify the virus suspension by centrifugation at 1500 g for 10 min.
3. Mix the clarified lysate with an equal volume of OptiPrep™ to produce a final iodixanol concentration of 30% (w/v).
4. Transfer the suspension to tubes suitable for a vertical or near-vertical rotor. In the Beckman VTi50 centrifuge at 160,000 gav (45,000 rpm) for 6 h. In the Beckman VTi65.1 vertical rotor or NVT65 near-vertical rotor use either the same RCF and centrifugation time or double the RCF and halve the centrifugation time.
5. At the end of the centrifugation use either a controlled deceleration programme or turn off the brake below 2000 rpm.
6. Unload the gradient by tube puncture, upward displacement of aspiration from the meniscus in a series of equal volume fractions (20-25 fractions irrespective of the gradient volume) and analyze the fractions as required. The capsid bands at approx 1.17 g/ml (see Note 2).
5. Notes
1. In step 3 of the protocol the lysate is simply mixed with an equal volume of OptiPrep, which will mean that the buffer and Tween 20 concentrations will be reduced by 50%. If this is deemed unacceptable then first produce a Working Solution of 54% (w/v) iodixanol by mixing 5.4 vol. of OptiPrep™ with 0.6 vol. of 10xPBS containing 0.5% Tween 20.
2. For more information on harvesting gradients see Application Sheet V04.
6. Ebola pseudovirus
Kim et al [2] mixed the suspension with an equal volume of OptiPrep™ (i.e. the final iodixanol concentration was 30%, w/v) and centrifuged it at 421,000 g for 3 .5 h in a Beckman NVT100 rotor. The pseudovirus displayed a median density of approx 1.10 g/ml.
7. References
1. Huang, Y., Xu, L., Sun, Y. and Nabel, G.J. (2002) The assembly of Ebola virus nucleocapsid requires virionassociated proteins 35 and 24 and posttranslational modification of nucleoprotein Mol. Cell, 10, 307-316
2. Kim, J-O., Chakrabarti, B.K., Guha-Niyogi, A., Louder, M.K., Mascola, J.R., Ganesh, L. and Nabel, G.J. (2007) Lysis of human immunodeficiency virus type 1 by a specific secreted human phospholipase A2 J. Virol., 81, 1441-1450
OptiPrep™ Application Sheet V25; 8th edition, January 2020
OptiPrep™ Application Sheet V26
Purification of Group V ((-)ss) RNA viruses: Orthomyxoviridae
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- This Application Sheet covers the purification of influenza A virus
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
- Section 6 is devoted to the purification of virus-like particles
1. Background
The Orthomyxoviridae family comprises influenza virus A, B and C, Isavirus and Thogotovirus. All of the published papers reporting the use of iodixanol gradients for the purification and analysis of Orthomyxoviridae viruses are concerned with influenza virus A. It is highly likely that, because of the basic overall similarity in structure, the methods described below would be suitable for any members of this family.
There are now many published papers that report the use of iodixanol gradients not only to purify viruses but also to investigate their assembly. In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which, in solutions of the same density, is much higher than that of iodixanol.
Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast both infectivity measurements using cultured cells and many add-on techniques can be performed without dialysis of iodixanol. Combined with the availability of OptiPrep™ as a sterile solution, this makes the use of OptiPrep™ for virus purification and assembly analysis much more convenient than the use of either CsCl or sucrose.
2. Solutions required
A. OptiPrep™ (60% w/v iodixanol)
B. OptiPrep™ diluent: any suitable buffered saline such as PBS is suitable or a buffer such as 100 mM NaCl, 1 mM EDTA, 10 mM Tris-HCl, pH 7.4 [2,3]. As long as the solution is isoosmotic, then all iodixanol solutions produced from OptiPrep™ will also be isoosmotic. For more information on the production of gradient solutions see OptiPrep™ Application Sheet V01.
3. Rotor requirements
A routine swinging-bucket rotor capable of approx. 100,000 g (e.g. Beckman SW28 or SW41Ti)
4. Protocol (carry out all operations at 4°C)
4a. Clarification and concentration of virus fluid
The virus is normally grown in a cell culture (often MDCK cells) although Chou et al [3] used the allantoic fluid of chicken eggs. An initial clarification of the virus-containing isolate to remove cells and larger debris is carried out usually between 400 g and 800 g for 5-15 min [4-6] although much higher speeds are sometimes used such as 2600 g for 5 min [2] or in the case of allantoic fluid 3000 g for 30 min [3].
The virus is usually, but not always [6] concentrated prior to gradient purification. Sometimes the virus is pelleted through a 20% (w/v) sucrose cushion at approx. 100,000 g for 2h [2,3] or a 14% (w/v) iodixanol cushion for 1 h at 55,000 g [4] or 88,000 g [5]. The higher g-force and time necessary for the sucrose cushion reflect the higher viscosity of this medium. Since the virus is finally purified in an iodixanol gradient it may be less stressful for the virus to use the same medium for the cushion.
4b. Gradient purification
Continuous iodixanol gradients have often been used for the final purification step. The following conditions have been used: 10-30% (w/v) iodixanol at approx 100-120,000 g for 3 h [2,3], 14-26% (w/v) at 55,000 g for 45 min [5] and two sequential gradient centrifugations through 10-26% (w/v) iodixanol at 55,000 g for 45 min [6,7]. The short time and low g-forces used in the latter two examples may suggest that the higher g-forces and longer times more frequently reported for this and other viruses may be unnecessary. More recently Hutchinson et al [8] used a 10-40% (w/v) iodixanol gradient following concentration through a 10% iodixanol cushion. In an interesting adaptation by Thompson et al [9] the sample was adjusted to 20% (w/v) iodixanol before fractionation on 25.5-40% (w/v) iodixanol gradient at 350,000 g for 6h. The raised density of the sample layer would minimize any aggregation at the gradient interface. This study also investigated the resolution from baculovirus.
Discontinuous gradients of 10%, 15%, 20%, 25% and 30% (w/v) iodixanol at, 100, 000 g for 3 h have also been used [10]; the virus banded at the 15/20 boundary was the most pure, although virus was present at the two adjacent boundaries.
A detailed comparison of the various iodixanol methodologies has not been carried out. It is worth pointing out however that because of the generally lower density of viruses in iodixanol gradients compared to sucrose; the consequent lower viscosity of iodixanol gradients may allow the use of more mild centrifugation conditions.
5. Comments
Shaw et al [2] compared the banding of influenza virus in sucrose and iodixanol gradients. Firstly to band the virus it was necessary to use a 30-60% sucrose gradient, which covers a density range of 1.12 to 1.28 g/ml; while with the range of a 10-30% iodixanol gradient was 1.058-1.16 g/ml. This reflects the lower density of the virus in an isoosmotic iodixanol gradient compared that of a hyperosmotic sucrose gradient. In the latter the virus has a median density of approx 1.20 g/ml, while in iodixanol it was approx 1.14 g/ml. Another serious consequence of the hyperosmotic nature of sucrose gradients is that all osmotically active particles, such as membrane vesicles, also lose water and approach a limiting density as they move through the gradient. So the sucrose gradients were unable to resolve the CD9 protein of the virus from the MHC-1 marker for exosomes; while the density of the latter in the iodixanol gradient (approx 1.10 g/ml) was much lower than that of the virus. The gradients described by Latham and Galarza for virus-like particles (see Section 6) have also been used by LeBouder et al [11] for influenza virus (2h at 80,000 g). A self-generated iodixanol gradient (starting concentration 18% (w/v) iodixanol) centrifuged in a vertical rotor at 350,000 g for 6 h has also been used [12].
6. Virus-like particles (VLPs)
The purification of VLPs in iodixanol gradients was first described by Latham and Galarza [13]. After pelleting from a clarified culture medium the VLPs were pelleted at 200,000 g and loaded on to a 14-60% (w/v) iodixanol gradient centrifuged at 200,000 3 g for 3.5 h. Similar centrifugation conditions were reported in refs 14-16, but Sulli et al [17] used a shallower gradient of 10-30% (w/v) iodixanol. An identical gradient was used by Chlanda et al [18] centrifuged at 200,000 g for 2 h after an initial concentration by pelleting through a 30% sucrose cushion. The purification and use of VLPs has recently been reviewed [19].
- For information on preparation of continuous gradients see OptiPrep™ Application Sheet V02
- For information on harvesting of gradients see OptiPrep™ Application Sheet V04
- For information on gradient analysis see OptiPrep™ Application Sheet V05
- For more information on virus concentration see OptiPrep™ Application Sheet V06
7. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Shaw, M.L., Stone, K.L., Colangelo, C.M., Gilcicek, E.E. and Palese, P. (2008) Cellular proteins in influenza virus particles PLoS Pathog., 4:e1000085
3. Chou, Y-Y., Vafabakhsh, R., Doganay, S., Gao, Q., Ha, T. and Palese (2012) One influenza virus particle packages eight unique viral RNAs as shown by FISH analysis Proc. Natl. Acad. Sci. USA, 109, 9101–9106
4. Speshock, J.L., Doyon-Reale, N., Rabah, R., Neely, M.N. and Roberts, P.C. (2007) Filamentous influenza virus A infection predisposes mice to fatal septicemia following superinfection with Streptococcus pneumoniae Serotype 3 Infect. Immun., 75, 3102-3111
5. Yang, Y., Leggat, D., Herbert, A., Roberts, P.C. and Sundick, R.S. (2009) A novel method to incorporate bioactive cytokines as adjuvants on the surface of virus particles J. Interferon Cytokine Res., 29, 9-23
6. Herbert, A.S., Heffron, L., Sundick, R. and Roberts, P.C. (2009) Incorporation of membrane-bound, mammalian-derived immunomodulatory proteins into influenza whole virus vaccines boosts immunogenicity and protection against lethal challenge Virol. J., 6:42
7. Khan, T., Heffron, C.L., High, K.P. and Roberts, P.C. (2014) Tailored vaccines targeting the elderly using whole inactivated influenza vaccines bearing cytokine immunomodulators J. Interferon Cytokine Res., 34,129-139
8. Hutchinson, E.C., Charles, P.D., Hester, S.S., Thomas, B., Trudgian, D., Martınez-Alonso, M. and Fodor, E. (2014) Conserved and host-specific features of influenza virion architecture Nat. Commun., 5: 4816
9. Thompson, C.M., Petiot, E., Mullick, A., Aucoin, M.G., Henry, O. and Kamen, A.A. (2015) Critical assessment of influenza VLP production in Sf9 and HEK293 expression systems BMC Biotechnol., 15: 31
10. Yang, X., Steukers, L., Forier, K., Xiong, R., Braeckmans, K., Van Reeth, K. and Nauwynck, H. (2014) A beneficiary role for neuraminidase in influenza virus penetration through the respiratory mucus PLoS One, 9: e110026
11. LeBouder, F., Morello, E., Rimmelzwaan, G.F., Bosse, F., Pechoux, C., Delmas, B. and Riteau, B. (2008) Annexin II incorporated into influenza virus particles supports virus replication by converting plasminogen into plasmin J. Virol., 82, 6820-6828
12. Le Rua, A., Jacob, D., Transfiguracion, J., Ansorge, S., Henry, O. and Kamena, A.A. (2010) Scalable production of influenza virus in HEK-293 cells for efficient vaccine manufacturing Vaccine, 28, 3661–3671
13. Latham, T. and Galarza, J.M. (2001) Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins J. Virol., 75, 6154-6165
14. Galarza, J.M., Latham, T. and Cupo, A. (2005) Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge Viral Immunol., 18, 244-251
15. Galarza, J.M., Latham, T. and Cupo, A. (2005) Virus like particle vaccine conferred complete protection against a lethal influenza virus challenge Viral Immunol., 18, 365-372
16. Matassov, D., Cupo, A. and Galarza, J.M. (2007) A novel intranasal virus-like particle (VLP) vaccine designed to protect against the pandemic 1918 influenza A virus (H1N1) Viral Immunol., 20, 441-452
17. Sulli, C., Banik, S.S.R., Schilling, J., Moser, A., Xiang, X., Payne, R., Wanless, A., Willis, S.H., Paes, C., Rucker, J.B. and Doranz, B.J. (2013) Detection of proton movement directly across viral membranes to identify novel influenza virus M2 inhibitors J. Virol., 87, 10679-10686
18. Chlanda, P., Mekhedov, E., Waters, H., Sodt, A., Schwartz, C., Nair, V., Blank, P.S. and Zimmerberg, J. (2017) Palmitoylation contributes to membrane curvature in influenza A virus assembly and hemagglutininmediated membrane fusion J. Virol. 91, e00947-17
19. Thompson, C.M., Petiot, E., Lennaertz, A., Henry, O. and Kamen, A.A. (2013) Analytical technologies for influenza virus-like particle candidate vaccines: challenges and emerging approaches Virol. J. 10: 141
OptiPrep™ Application Sheet V26; 4th edition, January 2020
OptiPrep™ Application Sheet V27
Purification of Group V ((-)ss) RNA viruses: Rhabdoviridae: Rabies virus (Lyssavirus) and vesicular stomatitis virus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- Whether the method described in this Application Sheet can be applied to other members of the Rhabdoviridae can only be determined experimentally
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
In all comparative studies between CsCl and iodixanol, it has been shown that recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which is much higher than that of iodixanol. Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast, many add-on techniques can be performed and cells infected with virus, without dialysis of iodixanol.
2. Rabies virus (Lyssavirus)
Rabies virus has been purified in 20-40% iodixanol gradients [2], following concentration by sedimentation through a low-density barrier on to a high-density cushion. Although in the original method this double barrier comprised 20% and 60% sucrose, this has been replaced by a 12%/50% iodixanol double barrier in this OptiPrep™ Application Sheet. In an earlier publication Finke and Conzelmann [3] cited the use of a 10-40% iodixanol gradient, which was also described in ref 4. Klingen et al [5] described the use of a 10-35% iodixanol gradient. Use of density barrier concentration steps introduces the problem of subsequent layering of the collected virus on top of the iodixanol gradient. The density of the recovered liquid must obviously be below that of the top of the iodixanol gradient. If one of the newer alternative iodixanol gradients (10-40% or 10-35%) is chosen then the two-layer gradient described for concentrating the virus may have to be eliminated as in ref 4. Klingen et al [5] used a size exclusion column to concentrate the virus.
 2a. Solutions required
2a. Solutions required
A. OptiPrep™
B. OptiPrep™ diluent: 0.15 M NaCl, 6.0 mM EDTA, 0.3 M Tris-HCl, pH 7.4
C. Suspension medium: 0.15 M NaCl, 1.0 mM EDTA, 50 mM Tris-HCl, pH 7.4
D. Iodixanol (50% w/v) working solution: mix 5 vol. of OptiPrep™ with 1 vol. of Solution B (see Section 2d, Note 1)
2b. Ultracentrifuge rotor requirements
Virus concentration: Swinging-bucket rotor e.g. Beckman SW28 or SW28.1 (see Section 2d, Note 2)
Virus purification: Swinging-bucket rotor e.g. Beckman SW28.1 (see Section 2d, Note 3)
2c. Protocol (adapted from refs 2 and 4)
1. Prepare a 12% (w/v) iodixanol solution by diluting Solution D with Solution C (1.2:3.8 volume ratio).
2. Clarify the cell supernatant by centrifugation at 1500 g for 20 min.
3. Transfer clarified cell supernatants to tubes for the chosen swinging-bucket rotor. Underlayer 8 vol. of supernatant with approx 3.5 vol. of 12% (w/v) iodixanol and 1 vol. of 50% (w/v) iodixanol (see Section 2d, Notes 4-6).
4. Centrifuge at 120,000 g for 2 h; allow the rotor to decelerate from 2000 rpm without the brake.
5. Towards the end of this centrifugation prepare two further solutions of iodixanol of 20% and 40% (w/v) by diluting Solution D with Solution C (2:3 and 4:1 volume ratios respectively) and prepare a 13-14 ml continuous gradient from equal volumes of the two iodixanol solutions using a twochamber gradient maker or Gradient Master in 17 ml tubes for the swinging-bucket rotor (see Section 2d, Notes 7 and 8).
6. Carefully aspirate the liquid above the rabies virus band above the dense iodixanol layer, leaving approx 1 ml of the upper layer.
7. Using a thin metal cannula or a length of narrow-bore Teflon tubing attached to a 2 ml syringe remove as much as possible of the 50% iodixanol (see Section 2d, Note 9).
8. Harvest the rabies virus in the remaining 12% iodixanol, taking as little as possible of any residual 50% iodixanol (see Section 2d, Note 10).
9. Dilute the harvested virus with 1-2 vol. of Solution C (if necessary) and layer on top of the 20-40% (w/v) iodixanol gradient to fill the tube and centrifuge at 27,000 rpm (approx 90,000 gav) for 18 h.
10. Collect the gradient by aspiration from the meniscus, upward displacement with a dense medium or tube puncture (see Section 5, Note 11) and analyze the fractions. The virus bands in the top third of the gradient. When the 10-40% iodixanol gradient is used in a Beckman SW28 rotor, the first 8 ml of the gradient can be discarded; the virus bands maximally in the 3-6 ml cut of the following gradient [4].
- See Note 12 for a brief summary of more recently published methods
5. Notes
1. The production of a working solution from OptiPrep™ and Solution B, as described, ensures that the buffer and EDTA concentration is constant throughout the gradient. If Solution B also contains six times the NaCl concentration of Solution C, the NaCl concentration will also be constant but the dense part of the gradient will be very hyperosmotic. For more information on the preparation of density gradient solutions see Application Sheet V01.
2. Use whatever rotor is suitable to the volume of clarified cell supernatant (see Step 3).
3. The method can be scaled down to smaller volume tubes as required, or it can be scaled up to the Beckman SW28, as in ref 4.
4. Conical tubes facilitate this process, and Beckman manufacture so-called konical™ tubes for all their swinging-bucket rotors. This two-barrier format achieves both a partial purification and a concentration of the virus. For more information on concentrating virus see Application Sheet V06.
5. For more information on setting up discontinuous gradients see Application Sheet V02.
6. If the 10-35% iodixanol gradient is chosen for the virus purification, the 12% iodixanol layer might be omitted from the concentration step and the 50% iodixanol layer reduced to 40% iodixanol (see Note 10).
7. For the 10-35% gradient use volume ratios of 1:4 and 3.5:1.5 respectively. For a 10-40% gradient use 1:4 and 4:1 respectively.
8. If a gradient making device is unavailable, then make a discontinuous gradient (5-10% iodixanol steps) and allow the formation of a continuous gradient by diffusion. For more information about making continuous gradients see Application Sheet V02.
9. Removing most of the dense cushion is facilitated by the use of Beckman konical tubes.
10. The iodixanol concentration of the harvested virus suspension needs to be <20% (w/v) to permit layering on top of the next gradient or <10% if the 10-35% or 10-40% iodixanol gradient is chosen. But note that the harvested virus may be diluted with a small volume of buffer in Step 9. If a suitably low iodixanol concentration cannot be attained then an alternative means of concentration will be required such as the size exclusion column used by Klingen et al [5] or centrifugal ultrafiltration using for example the Centricon PBHK Centrifugal Plus-20 filter unit with an Ultracel PL membrane (100 kDa cut off) as described by Yi et al [6] for the removal of iodixanol from hepatitis C virus preparations. For more information on concentrating virus see Application Sheet V06.
11. Once the banding position of the virus has been well established it may be permissible to harvest the virus band with a syringe. See Application Sheet V04 for gradient harvesting methods.
12. More recently gradients have been constructed from 1 ml each of 20%, 30%, 40% and 50% (w/v) iodixanol (112,000g for 18h) for banding virus-like particles [7], although in a later publication the time was reduced to 6 h [8].
3. Vesicular stomatitis virus (VSV)
3a. Density separations
VSV (Vesiculovirus) has been purified in an iodixanol gradient, using a broadly similar approach to that described above for rabies virus, although the centrifugation conditions were rather different [9, 10]. Solutions for the continuous gradient were prepared in a similar manner except that the solution used for suspending the virus contained 100 mM NaCl, 0.5 mM EDTA and 50 mM Tris-HCl, pH 7.4. Gradient solutions also contained these components at the same concentrations. An approx. 10 ml continuous gradient (15-35% (w/v) iodixanol) was prepared in tubes for a Beckman SW41 rotor and the crude virus suspension layered on top. There were two major differences in the protocol; the gradient was centrifuged at 160,000 g for 1.5 h and the initial concentration of the virus was achieved simply by pelleting at 28,000 g for 90 min. Otherwise the protocol was in essence, very similar to that described above and the virus banded in the middle of the gradient. This methodology was also used by Kim et al [11] and Beug et al [12] in their studies on Smac-mimetics.
A 5-50% (w/v) iodixanol gradient, centrifuged at 160,000 g for 1.5 h was described by Cuevas et al [13] for purification of VSV-mCherry, VSV-GFP and VSV-A3853C. A similar gradient was described in refs 14 and 15 but no other details were provided.
3b. Sedimentation velocity separation
After concentration by sedimentation through a 20% (w/v) sucrose barrier, the pellet was resuspended in HEPES-buffered saline overnight and layered on top of a continuous 7.5-27.5% (w/v) iodixanol gradient [16-18]. The gradients were centrifuged at approx. 85,000 gav for 25-30 min. The position of the virus band under these conditions was not given. There is no reason why the sucrose cushion would not be replaced with an iodixanol solution of the same density (approx. 12% iodixanol).
- A simple centrifugation through a 10% (w/v) iodixanol cushion has been used to concentrate and partially purify the VSV [19, 20]. For more information on concentrating virus see Application Sheet V06
4. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Finke, S., Brzozka, K. and Conzelmann, K-K. (2004) Tracking fluorescence-labeled rabies virus: enhanced green fluorescent protein-tagged phosphoprotein P supports virus gene expression and formation of infectious particles J. Virol., 78, 12333-12343
3. Finke, S. and Conzelmann, K-K. (2003) Dissociation of rabies virus matrix protein functions in regulation of viral RNA synthesis and virus assembly J. Virol., 77, 12704-12082
4. Marschalek A., Drechsel, L. amd Conzelmann, K-K. (2012) The importance of being short: The role of rabies virus phosphoprotein isoforms assessed by differential IRES translation initiation Eur. J. Cell Biol., 91, 17– 23
5. Klingen, Y., Conzelmann, K-K. and Finke, S. (2008) Double-labeled rabies virus: live tracking of enveloped virus transport J. Virol., 82, 237-245
6. Yi, M., Villanueva, R.A., Thomas, D.L., Wakita, T. and Lemon, S.M. (2006) Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells Proc. Natl. Acad. Sci. USA, 103, 2310-2315
7. Fontana, D., Kratje, R., Etcheverrigaray, M. and Prieto, C. (2015) Immunogenic virus-like particles continuously expressed inmammalian cells as a veterinary rabies vaccine candidate Vaccine, 33, 4238–4246
8. Fontana, D., Etcheverrigaray, M., Kratje, R. and Prieto, C. (2016) Development of rabies virus-like particles for vaccine applications: production, characterization, and protection studies In Vaccine Design: Methods and Protocols, Vol. 1: Vaccines for Human Diseases, Methods in Molecular Biology, vol. 1403 (ed. Thomas, S.) Springer Science+Business Media New York pp 155-166
9. Diallo, J-S., Vähä-Koskela, M., Le Boeuf, F. and Bell, J. (2011) Propagation, purification, and in vivo testing of oncolytic vesicular stomatitis virus strains In Methods Mol. Biol., 797, Oncolytic Viruses: Methods and Protocols, (ed. Kirn, D.H. et al.), Springer Science+Business Media, pp 127-140
10. Arulanandam, R., Batenchuk, C., Varette, O., Zakaria, C., Garcia, V., Forbes, N.E., Davis, C. Krishnan, R. et al (2015) Microtubule disruption synergizes with oncolytic virotherapy by inhibiting interferon translation and potentiating bystander killing Nat. Commun., 6: 6410
11. Kim, D-S., Dastidar, H., Zhang, C., Zemp, F.J., Lau, K., Ernst, M., Rakic, A., Sikdar, S., Rajwani, J. et al (2017) Smac mimetics and oncolytic viruses synergize in driving anticancer T-cell responses through complementary mechanisms Nat. Comm., 8: 344
12. Beug, S.T., Beauregard, C.E., Healy, C., Sanda, T., St-Jean, M., Chabot, J., Walker, D.E., Mohan, A., Earl, N. et al (2017) Smac mimetics synergize with immune checkpoint inhibitors to promote tumour immunity against glioblastoma Nat. Comm., 8: 14278
13. Cuevas, J.M., Durán-Moreno, M. and Sanjuán, R. (2017) Multi-virion infectious units arise from free viral particles in an enveloped virus Nat. Microbiol., 2: 17078
14. Diallo, J-S., Le Boeuf, F., Lai, F., Cox, J., Vaha-Koskela, M., Abdelbary, H., MacTavish, H., Waite, K., Falls, T., Wang, J., Brown, R., Blanchard, J.E., Brown, E.D., Kirn, D.H., Hiscott, J., Atkins, H. Lichty, B.D. and Bell, J.C. (2010) A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers Mol. Ther., 18, 1123-1129
15. Garijo, R., Hernández-Alonso, P., Rivas, C., Diallo, J-S. and Sanjuán, R. (2014) Experimental evolution of an oncolytic vesicular stomatitis virus with increased selectivity for p53-deficient cells PLoS One, 9: e102365
16. Kalvodova, L., Sampaio, J.L., Cordo, S., Ejsing, C.S., Shevchenko, A. and Simons, K. (2009) The lipidomes of vesicular stomatitis virus, Semliki Forest virus and the host plasma membrane analyzed by quantitative shotgun mass spectrometry J. Virol., 83, 7996-8003
17. Hastie, E., Besmer, D.M., Shah, N.R., Murphy, A.M., Moerdyk-Schauwecker, M., Molestina, C., Das Roy, L., Curry, J.M., Mukherjee, P. and Grdzelishvili, V.Z. (2013) Oncolytic vesicular stomatitis virus in an immunocompetent model of MUC1-positive or MUC1-null pancreatic ductal adenocarcinoma J. Virol., 87, 10283–10294.
18. Moerdyk-Schauwecker, M,. Hwang, S-I., Grdzelishvili, V.Z. (2014) Cellular proteins associated with the interior and exterior of vesicular stomatitis virus virions. PLoS One, 9: e104688
19. Betancourt, D., Ramos, J.C. and Barber, G.N. (2015) Retargeting oncolytic vesicular stomatitis virus to human T-cell lymphotropic virus Type 1-associated adult T-cell leukemia J. Virol., 89, 11786-11800
20. Betancourt, D., de Queiroz, N.M.G.P., Xia, T., Ahn, J. and Barber, G.N. (2017) Cutting edge: innate immune augmenting vesicular stomatitis virus expressing Zika virus proteins confers protective immunity J. Immunol., 198, 3023–3028
OptiPrep™ Application Sheet V27; 8th edition, February 2020
OptiPrep™ Application Sheet V28
Purification of Group V ((-)ss) RNA viruses: Paramyxoviridae – Paramyxovirinae and Pneumovirinae
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- This Application Sheet covers the purification of human respiratory syncytial virus (Section 2), measles virus (Section 3), Newcastle disease virus (Section 4), swine paramyxovirus (Section 5) and henipavirus (Section 6).
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
The Paramyxoviridae family is divided into two subfamilies: Paramyxovirinae and Pneumovirinae. The Paramyxovirinae comprises a large number of genuses including Avulavirus (Newcastle disease virus) and Morbillivirus, which includes measles virus but also other important forms such as Rinderpest virus and canine distemper virus. A new genus Respirovirus has recently been added, in which has been placed newly isolated swine paramyxoviruses. The Pneumovirinae comprises both human and bovine respiratory syncytial virus and the avian pneumovirus. This Application Sheet covers the isolation of such viruses. Although different methodologies have been developed for specific viruses, it is very likely that, since all paramyxoviruses have a similar structure then it is likely that the individual methods are more widely applicable; it must be stressed however that this has not been tested.
There are now many published papers that report the use of iodixanol gradients not only to purify viruses but also to investigate their assembly. In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which, in solutions of the same density, is much higher than that of iodixanol.
Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast both infectivity measurements using cultured cells and many add-on techniques can be performed without dialysis of iodixanol. Combined with the availability of OptiPrep™ as a sterile solution, this makes the use of OptiPrep™ for virus purification and assembly analysis much more convenient than the use of either CsCl or sucrose.
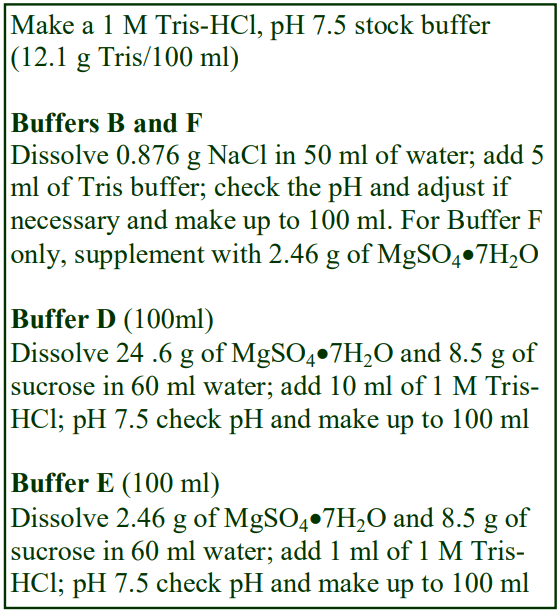 2. Analysis of respiratory syncytial virus (adapted from ref 2)
2. Analysis of respiratory syncytial virus (adapted from ref 2)
2a. Solutions required (see box ⇒ and Section 2d, Note 1)
A. OptiPrep™
B. 150 mM NaCl, 50 mM Tris-HCl, pH 7.5
C. 50% (w/v) PEG6000 in Solution B
D. 1 M MgSO4, 0.25 M sucrose, 100 mM Tris-HCl, pH 7.5
E. 100 mM MgSO4, 0.25 M sucrose, 10 mM Tris-HCl, pH 7.5
F. 100 mM MgSO4, 150 mM NaCl, 50 mM Tris-HCl, pH 7.5
3b. Rotor requirements
Swinging-bucket rotor with approx 13 ml tubes (e.g. Beckman SW41Ti)
2c. Protocol (carry out all operations at 4°C)
1. Prepare a 52% (w/v) iodixanol solution by mixing 5.2 vol. of OptiPrep™ with 0.6 vol. of Solution D and 0.2 vol. of water. Dilute some of the 52% iodixanol solution with Solution E to produce solutions of 36% and 20% (w/v) iodixanol (see Section 2d, Note 2).
2. Clarify the virus-containing culture fluid by centrifugation at 3000 g for 20 min.
3. Concentrate the virus by adding Solution C to the 3000 g supernatant (1 vol. + 4 vol. respectively) and stir gently at 4°C for 90 min.
4. Centrifuge the virus at 3250 g for 20 min.
5. Remove the supernatant and re-centrifuge the pellet to remove the remaining fluid; suspend the pellet in 1.0 ml of Solution F.
6. In tubes for the swinging-bucket rotor prepare a discontinuous gradient from approx 4.0 ml each of the three iodixanol solutions (52%, 36% and 20%). For more information on preparing discontinuous gradients see Application Sheet V02.
7. Layer the virus on top of the gradient and centrifuge at approx. 150,000 g for 90 min. Use a slow deceleration program or allow the rotor to decelerate from 3000 rpm without the brake.
8. During the centrifugation produce continuous gradients from equal volumes (approx 5.5 ml) of 20% and 52% iodixanol using either a two-chamber gradient maker or a Gradient Master™. For more information on preparing continuous gradients see Application Sheet V02.
9. Recover the band of virus from 20-30% interface of the discontinuous gradient and dilute with 2 vol. of Solution F (see Section 2d, Note 3).
10. Layer the virus suspension on top of the prepared continuous gradient and centrifuge at 150,000 g for 18 h. Use a slow deceleration program or allow the rotor to decelerate from 3000 rpm without the brake.
11. Collect the gradient by aspiration from the meniscus, upward displacement with a dense medium or tube puncture (for more information on harvesting gradients see Application Sheet V04) and analyze the fractions (see Section 2d, Note 4).
2d. Note
1. The sucrose solutions were treated with 0.1% diethylpyrocarbonate (DEPC) at 37ЁC for 24 h to inactivate any RNases and then heated to 60C for 3 days to remove the DEPC. Check pH and volume of solutions after this treatment and readjust if required [3].
2. Sufficient volumes of the three iodixanol solutions must be made for at least four gradients; two discontinuous gradients (Step 6) and two continuous gradients (Step 8). If neither of the devices for preparing continuous gradients are available (Step 8), then make up discontinuous gradients as described in Step 6 and allow them to diffuse overnight at 4°C.
3. This virus harvest is to be reloaded on to the second gradient in Step 10 so its density must be sufficiently low to allow this. When recovering the band sample as little of the 36% iodixanol layer as possible.
4. The method has also been used by Murawski et al [4]. Whether any of the methods used for other paramyxoviruses, described briefly in the following sections, are also applicable to respiratory syncytial virus can only be determined by experimental validation.
3. Measles virus
3a. Methods
Hallek et al [5] purified measles virus, grown in Vero cells; after lysis of the cells the viruscontaining fluid was clarified at 1,500 g for 10 min and the supernatant applied to a continuous 6-36% (w/v) iodixanol gradient, centrifuged at approx 200,000 g for 2h.
Brindley and Plemper [6] also cultivated the virus in Vero cells and after lysing the cells in 100 mM NaCl, 1 mM EDTA, 10 mM Tris-HCl, pH 7.8, the lysate was clarified at 5,000 g, for 20 min at 4°C to pellet nuclei and cell debris. Two solutions of 10% and 30% (w/v) iodixanol were prepared in the lysis buffer (see Application Sheet V01 for details on gradient solution preparation) and used for preparing a discontinuous gradient. After loading the lysate on top, the tubes were centrifuged at 100,000 g for 90 min. The concentrated virus was harvested from the interface in the minimum volume of liquid and, followed by loading on a continuous 10%-30% (w/v) iodixanol gradient and recentrifuged at 100,000 g for 14 h. All operations were carried out at 4°C. The gradient was collected in equivolume fractions and analyzed (see Section 3b Notes 1 and 2).
Liljeroos et al [7] also used a discontinuous gradient to concentrate the virus. Iodixanol solutions were prepared using a Tris buffered 180 mM NaCl (without EDTA) and the virus sedimented on to a 2 ml 54% (w/v) iodixanol cushion through an 8 ml layer of 20% (w/v) iodixanol by centrifugation at 134,000 g for 4 h. The harvested band was diluted with the buffered saline and concentrated in an Amicon spin column before being loaded on a second discontinuous gradient of 15%, 25%, 35% and 54% (w/v) iodixanol and centrifuged at the same speed for approx. 15 h (see Section 3b Notes 1 and 2).
- For information on preparation of gradients see Application Sheet V02
3b. Notes
1. When a virus is banded at an interface between two layers of iodixanol and it is subsequently overlaid on to a second similar gradient great care has to be taken in harvesting the banded material so that removal of too much of the denser layer is avoided. This can be facilitated by removal of the bulk of the higher density layer first, before removing the bulk of the low density layer. For more information on harvesting of gradients see Application Sheet V04.
2. Although the harvest can be diluted and reduced in volume by ultrafiltration to permit reloading in a low-density medium on top of a second gradient, a useful alternative would be to perform the second gradient by flotation. The sample is adjusted to a higher density and loaded at the bottom of a second discontinuous or continuous gradient.
4. Newcastle disease virus (NDV)
Biswas et al [8] investigated the interaction between NDV and human serum using a continuous 10-26% (w/v) iodixanol gradient, centrifuged at approx. 80,000 gav for 2 h. The separation in the gradient probably encompassed some degree resolution according to sedimentation rate. The fusion and matrix proteins banded towards the middle of the gradient and human serum caused a small high density shift of the proteins and the appearance of a significant amount of aggregated virus towards the bottom of the gradient. Complement C3 fractions from the serum had a broader distribution, also in the middle of the gradient, overlapping the fusion and matrix proteins, but did not appear in the very dense region. In the absence of virus these complement proteins banded only in a low density region.
5. Swine paramyxoviruses
Two separate swine paramyxoviruses have been purified in 14-26 (w/v) iodixanol gradients prepared by dilution of OptiPrep™ with PBS [9]. The cell lysates were layered one top of the gradient and centrifuged for at 250,000 g for 1.5 h in a swinging-bucket rotor. The banding density of the viruses was not stated.
6. Henipavirus
Henipavirus [10] has been purified by the same velocity iodixanol gradient as that described for HIV (see Application Sheet V34)
- For information on gradient analysis see Application Sheet V05
6. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Gias, E., Nielsen, S.U., Morgan, L.A.F. and Toms, G.L. (2008) Purification of human respiratory syncytial virus by ultracentrifugation in iodixanol density gradient J. Virol. Meth., 147, 328-332
3. Nielsen, S.U. (2008) Personal communication
4. Murawski, M.R., Bowen, G.N., Cerny, A.M., Anderson, L.J., Haynes, L.M., Tripp, R.A., Kurt-Jones, E.A. and Finberg, R.W. (2009) Respiratory syncytial virus activates innate immunity through Toll-Like receptor 2 J. Virol., 83, 1492-1500
5. Hallak, L.K., Merchan, J.R., Storgard, C.M., Loftus, J.C. and Russell, S.J. (2005) Targeted measles virus vector displaying echistatin infects endothelial cells via v3 and leads to tumor regression Cancer Res., 65, 5292-5300
6. Brindley, M.A. and Plemper, R.K. (2010) Blue native PAGE and biomolecular complementation reveal a tetrameric or higher-order oligomer organization of the physiological measles virus attachment protein H J. Virol., 84, 12174-12184
7. Liljeroos, L., Huiskonen, J.T., Ora, A., Susi, P. and Butcher, S.J. (2011) Electron cryotomography of measles virus reveals how matrix protein coats the ribonucleocapsid within intact virions Proc. Natl. Acad. Sci. USA, 108, 18085–18090
8. Biswas, M., Johnson, J.B., Kumar, S.R.P., Parks, G.D. and Subbiaha, E. (2012) Incorporation of host complement regulatory proteins into Newcastle disease virus enhances complement evasion J. Virol., 86, 12708-12716
9. Qiao, D., Janke, B.H. and Elankumaran, S. (2009) Molecular characterization of glycoprotein genes and phylogenetic analysis of two swine paramyxoviruses isolated from United States Virus Genes, 39, 53–65
10. Akiyama, H., Miller, C., Patel, H.V., Hatch, S.C., Archer, J., Ramirez, N-G.P. and Gummuluru, S. (2014) Virus particle release from glycosphingolipid-enriched microdomains is essential for dendritic cellmediated capture and transfer of HIV-1 and Henipavirus J. Virol., 88, 8813–8825
OptiPrep™ Application Sheet V28; 4th edition, January 2020
OptiPrep™ Application Sheet V29
Purification and analysis of Group VI (ss)RNA-RT viruses: Retroviridae: Alpharetrovirus: Rous sarcoma virus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RV06) lists all of the published papers that have reported the use of iodixanol gradients for the purification of viruses within Group VI (Baltimore classification scheme). To access the Reference List return to the initial list of Folders and select “Reference Lists”.
- This Application Sheet describes the use of continuous buoyant density pre-formed gradient for purification of Rous sarcoma virus, which belongs to the Alpharetrovirus genus of retroviruses.
- The retrovirus group is extremely diverse; whether the methods described in this Application Sheet can be applied to another retrovirus, of the same or different genus can only be determined experimentally. For other retroviral isolation methods see the Virus Index
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
There are now many published papers that report the use of iodixanol gradients not only to purify viruses but also to investigate their assembly. In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which, in solutions of the same density, is much higher than iodixanol.
Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast both infectivity measurements using cultured cells and many add-on techniques can be performed without dialysis of iodixanol. Combined with the availability of OptiPrep as a sterile solution, this makes the use of OptiPrep™ for virus purification and analysis more convenient than the use of either CsCl or sucrose.
In the protocol described in this OptiPrep™ Application Sheet, RSV is banded according to its buoyant density and it is adapted from ref 2. The pre-gradient strategy is adapted from ref 3.
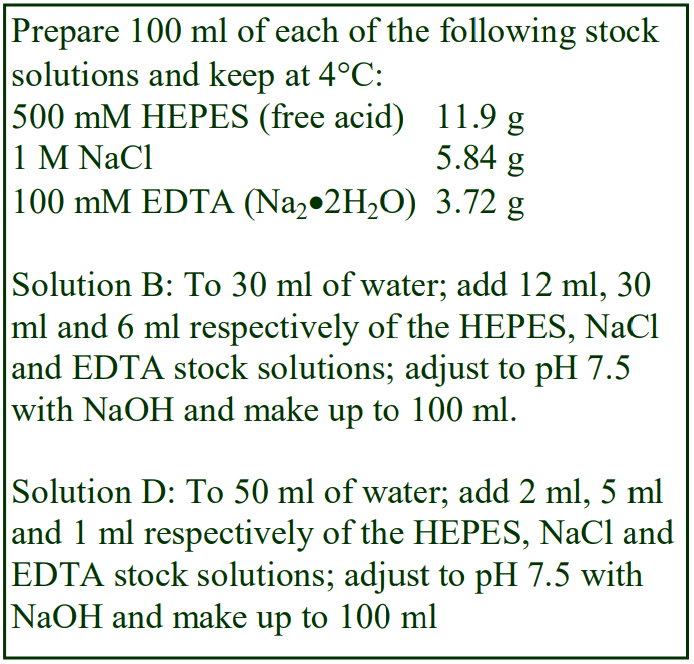 2. Solutions required (see Note 1)
2. Solutions required (see Note 1)
A. OptiPrep™
B. OptiPrep™ diluent:, 300 mM NaCl, 6 mM EDTA, 60 mM HEPES-NaOH (pH 7.5)
C. Working Solution (50%, w/v iodixanol): Mix 5 vol. of Solution A with 1 vol. of Solution B (see Note 1)
D. Working solution diluent: 50 mM NaCl, 1 mM EDTA, 10 mM HEPES-NaOH (pH 7.5)
3. Ultracentrifuge rotor requirements
For concentrating the virus from large volumes of culture fluid: swinging-bucket rotor with 36-39 ml tubes (e.g. Beckman SW28 or Sorvall AH629)
For the iodixanol gradient: swinging-bucket rotor with 13-14 ml tubes (e.g. Beckman SW41Ti or Sorvall TH641) (see Note 2).
4. Protocol
1. Once the virus has been released from the cells clarify the suspension by low speed centrifugation (approx. 5000 g for 15 min) to remove cellular debris.
2. If required filter the supernatant through a 0.45 μm filter.
3. Prepare a 12% (w/v) iodixanol solution (1.2 vol. of Solution C with 3.8 vol. of Solution D).
4. To concentrate the virus, use the tubes for the 36-39 ml swinging-bucket rotor and underlay the suspension with 5 ml of the 12% iodixanol using a syringe and metal cannula (see Notes 3 and 4).
5. Centrifuge at 80,000 gav for 2.5 h.
6. During the centrifugation make solutions of 10% and 40% (w/v) iodixanol (mix Solution C and Solution D 1:4 and 4:1 respectively). Then use equal volumes (5-6 ml) of the two iodixanol solutions in a two-chamber gradient maker or a Gradient Master™ to make a continuous gradient in a tube for the 14 ml swinging-bucket rotor (see Note 5).
7. Resupend the virus pellet in 1-2 ml of 5% (w/v) iodixanol (1 vol. of Solution C + 9 vol. of Solution D) and layer on top of the 10-40% iodixanol gradient.
8. Centrifuge at 160,000 gav for 4 h at 4°C.
9. Unload the gradient either by upward displacement, aspiration from the meniscus or by tube puncture in 0.5-1.0 ml fractions. If the RSV forms a visible band (about 2/3rds down the gradient), it can alternatively be recovered using a syringe and metal cannula (see Notes 6 and 7).
5. Notes
1. The mode of preparing the solutions ensures that the concentrations of NaCl, buffer and EDTA are constant throughout the gradient. If this is not considered important, the OptiPrep™ may simply be diluted with the virus suspension solution. Any suitable buffer can be used for suspending the virus and for making the gradient solutions. It may be customized to the operator’s own requirements, as long as the buffer has a low density (approx 1.006 g/ml) the density of the gradients will not be compromised. It might be a routine phosphate buffered saline [4] or a cell culture medium (e.g. DMEM or RPMI) supplemented with any additives as required. For more details on the making up of gradient solutions see Application Sheet V01.
2. Larger volume gradients are permissible (e.g. in the Beckman SW28) but the time will need increasing to compensate for the lower RCF. If a vertical rotor is substituted for the swingingbucket rotor (e.g. Beckman VTi50 or VTi65.1), the shorter sedimentation path length will permit shorter centrifugation times.
3. Vogt and Simon [3] sedimented the RSV through a 15% sucrose layer, this has been substituted with the more virus-friendly iodixanol in this protocol.
4. Virus concentration by pelleting, either directly or through a low-density layer may be undesirable. This procedure can result in some loss of infectivity either because of the physical aggregation of particles, high hydrostatic pressure at the bottom of the tube or the dispersal forces used to resuspend the pellet (or a combination of all of these problems). A useful alternative is to sediment the virus on to a small cushion (2-3 ml) of 45% w/v iodixanol or even pure OptiPrep™. However, unless the virus band is harvested with the minimum amount of cushion, it may have to be diluted to an unacceptable volume for loading on top of the subsequent density gradient. In this case, the iodixanol concentration must be <10% (w/v). The use of Beckman “konical” tubes overcomes this problem to some extent. Moreover, as long as the purification is based on buoyant density and sedimentation velocity then the sample volume is not really important. For buoyant density banding the sample may alternatively be layered beneath the gradient, in which case the contamination from the cushion is irrelevant. For more information on concentration of virus prior to gradient purification see Application Sheet V06.
5. A continuous gradient can alternatively be constructed by allowing a discontinuous gradient (10%, 20%, 30% and 40% iodixanol layers) to diffuse. For more information on making gradients see Application Sheet V02.
6. For more information on harvesting gradients see Application Sheet V04.
7. The median density of wild-type RSV is approx 1.14 g/ml [2]. The banding density of a capsid (CA)-deleted Gag mutant was 1.16 g/ml, while that of a matrix (MA)-deleted mutant was considerably lower – 1.09 g/ml [2], indicating that the MA domain is required for proper HSV assembly while the major homology region (included in CA) is not. Iodixanol gradients were also used to purify Rous sarcoma virus in a study of monoclonal antibody binding to the receptorbinding site [4].
6. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Lee, E-G., Yeo, A., Kraemer, B., Wickens. M. and Linial, M. (1999) The Gag domains required for avian retroviral RNA encapsidation determined by using two independent assays J. Virol., 73, 6282-6292
3. Vogt, V.M. and Simon, M.N. (1999) Mass determination of Rous sarcoma virus virions by scanning transmission electron microscopy J. Virol., 73, 7050-7055
4. Ochsenbauer-Jambor, C., Delos, S. E., Accavitti, M. A., White, J. M. and Hunter, E. (2002) Novel monoclonal antibody directed at the receptor binding site on the avian sarcoma and leukosis virus env complex J. Virol., 76, 7518-7527
OptiPrep™ Application Sheet V29; 8th edition, January 2020
OptiPrep™ Application Sheet V30
Purification and analysis of Group VI (ss)RNA-RT viruses: Retroviridae: Betaretrovirus: Mason-Pfizer Monkey virus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiprepTM Reference List (RV06) provides a comprehensive list of all published papers reporting the isolation of Group VI viruses on iodixanol gradients. To access RV06 return to the initial list of Folders and select “Reference Lists”. This Application Sheet summarizes the published methods for purification of viruses of the Retroviridae family.
- This Application Sheet describes the use of continuous sedimentation velocity or buoyant density pre-formed gradients for purification of the Mason-Pfizer monkey virus, which belongs to the Betaretrovirus genus of retroviruses.
- For other retroviral isolation methods see the Virus Index
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
Dettenhoffer and Yu [1] were the first to report the use of discontinuous 6-18% (w/v) iodixanol gradient in a sedimentation velocity mode to purify HIV-1 virions without affecting the infectivity of the virus (see Application Sheet V34). The technique was subsequently extended to the purification of Mason-Pfizer monkey virus by Gottwein et al [2] and the protocol is described in Section 2; an alternative buoyant density method [3,4] is described in Section 3.
In all comparative studies between CsCl and iodixanol, it has been shown that the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [5]. This may be related to its viscosity, which is much higher than that of iodixanol. Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast, many add-on techniques can be performed and cells infected with virus, without dialysis of iodixanol.
2. Sedimentation velocity method (adapted from ref. 2)
2a. Solutions required
A. OptiPrep™
B. Phosphate-buffered saline
C. Gradient solutions: dilute OptiPrep™ with Solution B to give a series of density solutions from 6 to 18% (w/v) iodixanol in 1.2% steps (i.e. 11 solutions, see Notes 1 and 2 in Section 2d)
2b. Rotor requirements
Virus concentration: Swinging-bucket rotor with approx 30-38 ml tubes (e.g. Beckman SW28) or 13-14 ml tubes (e.g. Beckman SW41Ti), see Note 3 (Section 2d) Gradient purification: Swinging-bucket rotor with 13-14 ml tubes (e.g. Beckman SW41Ti)
2c-1. Virus concentration
1. Harvest the cell supernatants and filter through a 0.45 μm filter.
2. Concentrate the virus by pelleting it through a density barrier (see Notes 3 and 4, Section 2d).
3. Resuspend it in a small volume of Solution B (see Step 2 of the next section).
2c-2. Gradient purification
1. Prepare a discontinuous gradient from approx 1 ml of each density solution. This is best accomplished by overlayering using a peristaltic pump first to draw each 1 ml into a plastic tube and then reversing the flow to expel it gently on top of the denser layer. Alternatively prepare a continuous gradient (approx 12 ml total) from equal volumes of the 6% and 18% iodixanol solutions using a two-chamber gradient maker or a Gradient Master™ (see Note 5, Section 2d).
2. Layer the virus suspension (approx 1.0 ml) on top of the gradient and centrifuge at 164,000 gav for 30 min at 4°C (see Notes 6-9, Section 2d).
2d. Notes
1. One of the practical alternatives, which might be considered, is the use of a continuous 6-18%
iodixanol gradient rather than a multi-step discontinuous gradient (see Note 5).
2. For more information on the preparation of density gradient solutions see Application Sheet V01.
3. Use a rotor convenient to the virus suspension volume. The tube volume and the relative amount of virus-containing solution and cushion are not critical and can vary with the amount of material.
4. Gottwein et al [2] pelleted the virus through a 20% sucrose cushion (1 h at 53,000g); to maintain an isoosmotic environment for the virus, the 20% sucrose might be replaced by 15% (w/v) iodixanol. The ideal way of concentrating the virus, to avoid loss of infectivity, is sedimentation on to a dense cushion of iodixanol, rather than pelleting. This however may be less convenient for this sedimentation velocity separation as the concentration of iodixanol in the viral suspension needs to be <5% (w/v) to permit loading on to the gradient. For more information on concentration of virus prior to gradient purification see Application Sheet V06.
5. A third option is to make an easier discontinuous gradient from equal volumes of 6.0%, 9.0%, 12.0, 15.0% and 18.0% (w/v) iodixanol and allow the formation of a continuous gradient by diffusion (approx. 5 h at room temperature, or overnight at 4°C). For more information on making gradients see Application Sheet V02.
6. If larger volumes of virus suspension are to be purified then larger volume gradients must be used. As this is a sedimentation-velocity separation the volume of crude virus suspension should not exceed 10-15% of the gradient volume.
7. Allow the rotor to decelerate from 2000 rpm without the brake.
8. For more information on harvesting gradients see Application Sheet V04.
9. In an alternative 10-55% (w/v) iodixanol gradient centrifuged at 215,000 g for 40 min was used to separate unassembled proteins (top of gradient) from denser assembled virus-like particles [6].
3. Buoyant density method (adapted from ref. 3)
3a. Solutions required
A. OptiPrep™
B. Phosphate-buffered saline or any similar medium
C. Gradient solutions: dilute OptiPrep™ with Solution B to give solutions of 10% and 40% (w/v) iodixanol
3b. Rotor requirements
Virus concentration: Swinging-bucket rotor with approx 30-38 ml tubes (e.g. Beckman SW28) or 13-14 ml tubes (e.g. Beckman SW41Ti) (see Note 1) Gradient purification: Swinging-bucket rotor with 13-14 ml tubes (e.g. Beckman SW41Ti)
3c. Protocols
3c-1. Virus concentration
1. Harvest the cell supernatants; filter through a 0.45 μm filter; then sediment the virus from the filtered crude suspension on to a 1 ml (Beckman SW41Ti) or 2.5 ml (Beckman SW28) cushion of OptiPrep™ and centrifuge at 35,000 g for 1 h. (see Note 2).
2. Recover the virus particles from just above the cushion, being very careful to retrieve as little of the cushion as possible (see Note 2).
3c-2. Virus purification
1. Prepare approx. 12 ml gradients in tubes for the Beckman SW41Ti rotor from equal volumes of the two iodixanol gradient solutions using a two-chamber gradient maker or a Gradient Master™ or allow a discontinuous gradient of equal volumes of 10%, 20%, 30% and 40% (w/v) iodixanol to diffuse. For more information on making gradients see Application Sheet V02.
2. Make sure that the harvested virus suspension from the iodixanol cushion is of a sufficiently low density to allow it to be layered on 10% iodixanol. Dilute with Solution B if necessary.
3. Layer the virus suspension on the gradient and centrifuge at 35,000 g for 16 h (see Note 3).
4. Collect the gradient in a series of equal volume fractions or harvest the viral particles by aspiration into a syringe and metal cannula (see Note 4).
3d. Notes
1. Use a rotor convenient to the virus suspension volume.
2. For more information on concentration of virus on to a density barrier see ™
3. There is no doubt that relatively low g-forces, as used in this protocol, are beneficial to any viral particle, but banding times could be reduced to 3-4 h by increasing the g-force to say 120,000 g, what affect this might have on the infectivity of the virus particles is not known.
4. The wild-type virus particles band at approx 1.125 g/ml (equivalent to 23% (w/v) iodixanol); interestingly Gag protein processing mutants banded at a slightly higher density [3]. For more information on harvesting gradients see ™.
4. References
1. Dettenhoffer, M. and Yu, X-F. (1999) Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif virions J. Virol., 73, 1460-1467
2. Gottwein, E., Bodem, J., Müller, B., Schmechel, A., Zentgraf, H. and Kräusslich, H-G. (2003) The MasonPfizer monkey virus PPPY and PSAP motifs both contribute to virus release J. Virol., 77, 9474-9485
3. Wildová, M., Hadravová, R. Štokrová, J., Křížová, I., Ruml, T., Hunter, E., Pichová, I. and Rumlová, M. (2008) The effect of point mutations within the N-terminal domain of Mason-Pfizer monkey virus capsid protein on virus core assembly and infectivity Virology, 380, 157-163
4. Voráčková, I., Ulbrich, P., Diehl, W.E. and Ruml, T. (2014) Engineered retroviral virus-like particles for receptor targeting Arch.Virol., 159, 677–688
5. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
6. Fuzik, T., Pichalova, R., Schur, F.K.M., Strohalmova, K., Križova, I., Hadravova, R., Rumlova, M., Briggs, J.A.G., Ulbrich, P. and Ruml, T. (2016) Nucleic acid binding by Mason-Pfizer monkey virus CA promotes virus assembly and genome packaging J. Virol., 90, 4593-4603
OptiPrep™Application Sheet V30; 8th edition, January 2020
OptiPrep™ Application Sheet V31
Purification of Group VI (ss)RNA-RT viruses: Retroviridae: Deltaretrovirus, human T-cell lymphotropic virus (HTLV-1) and human endogenous retrovirus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RV06) provides a full bibliography of all published papers reporting the use of iodixanol gradients for Group VI viruses; to access return to the initial list of Folders and select “Reference Lists”.
- This Application Sheet principally describes the use of a self-generated gradient for purification of members of the Deltaretrovirus genus. Section 2 briefly describes use of pre-formed gradients.
- The retrovirus group is extremely diverse; whether the methods described in this Application Sheet can be applied to another retrovirus can only be determined experimentally.
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Self-generated gradients
1-1. Background
In the following protocol [1,2], the viral particles are first concentrated on top of a dense cushion of iodixanol, instead of pelleting the virus. Subsequently, after removal of most of the supernatant, the contents of the tube are simply mixed so that the virus is suspended in 20% (w/v) iodixanol. This suspension is then centrifuged in a tube for a vertical or near-vertical rotor. The self-generated gradient that is formed is designed to band virus particles towards the bottom of gradient while allowing any contaminating membrane material to band at lower densities. Self-generated gradients have the merit of high reproducibility and ease of execution. This strategy was first worked out for Herpes virus; see Application Sheet V08.
In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [3]. This may be related to its viscosity, which is much higher than iodixanol. Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast many add-on techniques can be performed and cells infected with virus, without dialysis of iodixanol. The following protocol is adapted from ref 2.
 1-2. Solutions required
1-2. Solutions required
A. OptiPrep™
B. Diluent: 0.85% (w/v) NaCl, 60 mM Hepes-NaOH, pH 7.4
C. Working solution of 50% iodixanol (ρ = 1.272 g/ml): mix 5 vol of solution A with 1 vol of solution B (see Section 1-5, Note 1).
D. HEPES buffered saline: 0.85% NaCl (w/v), 10 mM HEPES-NaOH, pH 7.4. 1-3. Ultracentrifuge rotor requirements
For concentration of the virus (if required): a swinging-bucket rotor of suitable volume to accommodate the volume of crude virus suspension and capable of 100,00-200,000 gav (e.g. Beckman SW28 or Beckman SW28.1 or equivalent rotors).
For gradient purification: any vertical or near-vertical rotor with tube capacity of approx 12 ml and capable of approx 350,000g. The sedimentation path length of the rotor should be 17-25 mm. Separations described in this Application Sheet were obtained with a Beckman VTi65.1 vertical rotor, NVT65 near-vertical rotor or NVT65.2 near-vertical rotor (see Section 1-5, Note 2). High performance fixed-angle rotors may only be used for the rapid formation of self-generated gradients if the tube volume is relatively small (less than 6 ml). For more information on self-generated gradients see Application Sheet V03.
1-4. Protocol
1. Clarify the virus suspension by centrifugation at 1000 g for 10 min.
2. Transfer a known volume of the supernatant to suitable tubes for a swinging-bucket rotor and underlay with a small volume (2-4 ml) of Solution C (see Section 1-5, Note 3).
3. Centrifuge at 160,000 gav for 1 h to band the virus sharply at the working solution interface.
4. Remove all of the supernatant except for an amount equal to the 1.5x the volume of cushion.
5. Mix the residual contents of the tubes. This will produce a concentrated virus suspension in 20% (w/v) iodixanol.
6. Transfer the suspension to tubes suitable for a vertical, near-vertical or low-angle fixed-angle rotor to band the virus in a self-generated gradient of iodixanol.
7. Any tubes that are not filled should be topped up and mixed with 20% (w/v) iodixanol (mix Solutions C and D in the volume ratio 1:1.5).
8. Centrifuge at 350,000 gav for 3.5h and at the end of the centrifugation use either a controlled deceleration programme or turn off the brake below 2000 rpm (see Section 1-5, Note 2).
9. Either harvest the virus band with a syringe and metal cannula or unload the entire gradient by tube puncture, or other suitable method. Under the centrifugation conditions described the retrovirus will band close to the bottom of the tube (see Section 1-5, Notes 4-6).
1-5. Notes
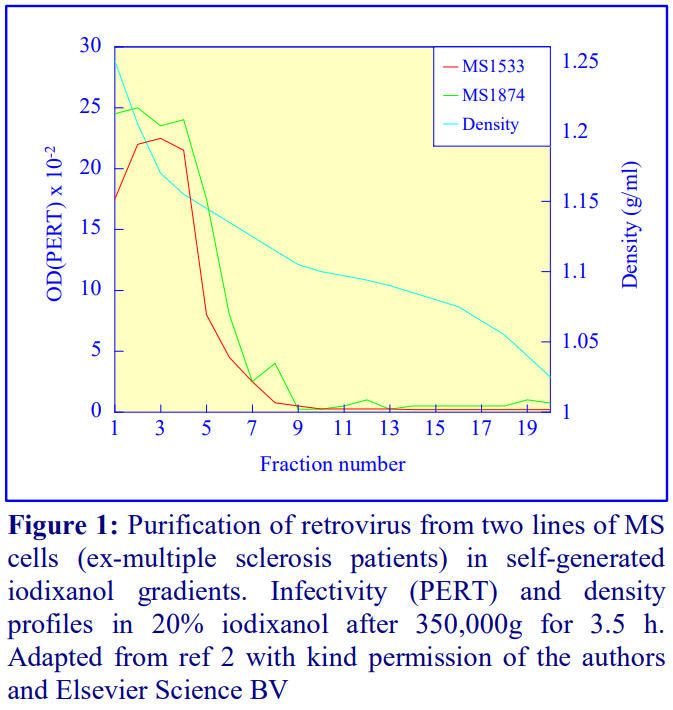 1. Strategies for preparing working solutions are given in Application Sheet V01.
1. Strategies for preparing working solutions are given in Application Sheet V01.
2. The method can be scaled up for the use of larger vertical rotors such as the Beckman VTi50, but the longer sedimentation path length and lower maximum RCF means that longer centrifugation times will be necessary. Work with Ebola virus suggests that 6 h at 45,000 rpm is satisfactory. See Application Sheet V25 for more details.
3. The actual volumes will depend on the totalvolume of virus fluid and the volume of the tubes used. For example: for approx. 15 ml supernatant per tube use 1-2 ml cushion solution, for approx. 35 ml use 2-4 ml.
4. Since the virus bands close to the bottom of the gradient and contaminating membranes are lighter, collection from the bottom is the method of choice. For more information on harvesting gradients see Application Sheet V04.
5. The separations shown in Figure 1 were obtained with a Beckman NVT65 near-vertical rotor. After approx 3.5 h the gradient that is generated is more or less linear, but does become progressively steeper in the densest regions. It is very effective for banding the virus sharply near the bottom of the tube (see Figure 1) while any membranous contamination bands at lower densities. If it is deemed advantageous to band the virus further from the bottom of the tube, increase the starting iodixanol concentration to 25% (w/v).
6. Møller-Larsen and Christensen [1] observed that iodixanol retrovirus particles isolated from iodixanol gradients showed much less damage than those isolated from other gradient media. These authors also noted that the method could be used to purify HTLV-1. The method has been subsequently used in investigations into the relation of retrovirus to multiple sclerosis [4-7]. Retroviral particles (HERV-H) have also been isolated from the supernatants from human melanoma cells on a continuous iodixanol gradient in a swinging-bucket rotor centrifuged at 287,000 g for 18 h [8]. The virus particles, as measured by reverse transcriptase assay, had a low density of approx 1.10 g/ml.
2. Other gradient systems
Hirschl et al [9] showed that melanoma cells produced viral particles that were homologous to human endogenous retrovirus. In their short communication there was no detailed description of the continuous iodixanol gradient, which was centrifuged at 287,000 g for 18 h.. However the endogenous retrovirus clearly banded within the density range of 1.06-1.11 g/ml (equivalent to approx. 10.5-20% (w/v) iodixanol. Contreras-Galindo et al [10] analyzed human endogenous retrovirus K from patients with lymphoma and breast cancer. The HERV-K was first concentrated by pelleting at 45,000 g for 2 h through a 20% (w/v) iodixanol cushion., prior to gradient centrifugation. In a later publication the same group [11] studied the endogenous retrovirus particles from patients infected with HIV-1. The authors used the same 20% iodixanol cushion to concentrate the virus and then loaded the virus suspension on a 10-50% (w/v) iodixanol gradient , centrifuged at 350,000 g for 6 h. The particles had a density of approx 1.16 g/ml [11].
HTLV-1 has been isolated using a velocity gradient similar to that that is widely used for HIV (see Application Sheet V34); the top-loaded 6-20% (w/v) iodixanol centrifuged at 80,000 g for 3h [12]. More recently Cao ey al [13] first pelleted HTLV-1 through an 8% (w/v) iodixanol cushion and then purified the virus through a 10-50% iodixanol gradient – no other details were given.
7. References
1. Møller-Larsen, A. and Christensen, T. (1998) Isolation of a retrovirus from multiple sclerosis patients in selfgenerated iodixanol gradients J. Virol. Meth., 73, 151-161
2. Christensen, T., Dissing Sørenson, P., Riemann, H., Hansen, H.J., Munch, M., Haahr, S. and Møller-Larsen, A. (2000) Molecular characterization of HERV-H variants associated with multiple sclerosis Acta Neurol. Scand., 101, 229-238
3. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
4. Christensen, T., Sorenson, P. D., Hansen, H. J. and Møller-Larsen, A. (2003) Antibodies against a human endogenous retrovirus and the preponderance of env splice variants in multiple sclerosis patients Multiple Sclerosis, 9, 6-15
5. Brudek, T., Christensen, T., Hansen, H.J., Bobecka, J. and Møller-Larsen, A. (2004) Simultaneous presence of endogenous retrovirus and herpes virus antigens has profound effect on cell-mediated immune responses: implications for multiple sclerosis AIDS AIDS Res. Hum. Retrovir., 20, 415-423
6. Christensen, T. (2005) Association of human endogenous retroviruses with multiple sclerosis and possible interactions with herpes virus Rev. Med. Oncol., 15, 179-211
7. Muster, T., Waltenberger, A., Grassauer, A., Hirschl, S., Caueig, P., Romirer, I., Fodinger, D., Seppele, H., Schanab, O., Magin-Lachmann, C., Lower, R., Jansen, B., Pehamberger, H. and Wolff, K. (2003) An endogenous retrovirus derived from human melanoma cells Cancer Res., 63, 8735-8741
8. Hirschl, S., Schanab, O., Seppele, H., Waltenberger, A., Humer, J., Wolff, K., Pehamberger, H. and Muster, T. (2007) Sequence variability of retroviral particles derived from human melanoma cells: Melanomaassociated retrovirus Virus Res., 123, 211-215
9. Hirschl, S., Schanab, O., Seppele, H., Waltenberger, A., Humer, J., Wolff, K., Pehamberger, H. and Muster, T. (2007) Sequence variability of retroviral particles derived from human melanoma cells: Melanomaassociated retrovirus Virus Res., 123, 211-215
10. Contreras-Galindo, R., Kaplan, M.H., Leissner, P., Verjat, T., Ferlenghi, I., Bagnoli, F., Giusti, F., Dosik, M.H., Hayes, D.F., Gitlin, S.D. and Markovitz, D.M. (2008) Human endogenous retrovirus K (HML-2) elements in the plasma of people with lymphoma and breast cancer J. Virol., 82, 9329-9336
11. Contreras-Galindo, R., Kaplan, M.H., Contreras-Galindo, A.C., Gonzalez-Hernandez, M.J., Ferlenghi, I., Giusti, F., Lorenzo, E., Gitlin, S.D., Dosik, M.H., Yamamura, Y. and Markovitza, D.M. (2012) Characterization of human endogenous retroviral elements in the blood of HIV-1-infected individuals J.
Virol., 86, 262–276
12. Hémonnot, B., Molle, D., Bardy, M., Gay, B., Laune, D., Devaux, C. and Briant, L. (2006) Phosphorylation of the HTLV-1 matrix L-domain-containing protein by virus-associated ERK-2 kinase Virology, 349, 430- 439
13. Cao, S., Maldonado, J.O., Grigsby, I.F., Mansky, L.M. and Zhang, W. (2015) Analysis of human T-cell leukemia virus type 1 particles by using cryo-electron tomography J. Virol., 89, 2182-2191
8. Acknowledgements
We wish to thank Dr A Møller-Larsen, Institute of Medical Microbiology, University of Aarhus, DK-8000 Aarhus C, Denmark for his cooperation in the preparation of this Application Sheet.
OptiPrep™ Application Sheet V31; 8th edition, January 2020
OptiPrep™ Application Sheet V32
Purification and analysis of Group VI (ss)RNA-RT viruses: Retroviridae:Gammaretrovirus: murine oncornavirus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number
- Application Sheet V06 provides a summary of the OptiPrep™ virus purification methodology.
- The OptiPrep™ Reference List (RV06) provides a full bibliography of all published papers reporting the use of iodixanol gradients for the purification of Group VI viruses; to access return to the initial list of Folders and select “Reference Lists”.
1. Background
Dettenhoffer and Yu [1] were the first to report the use of discontinuous 6-18% (w/v) iodixanol gradient in a sedimentation velocity mode to purify HIV-1 virions without affecting the infectivity of the virus. This is described in Application Sheet V34. The technique was subsequently extended to the purification of murine oncornavirus by Fujisawa et al [2].
In all comparative studies between CsCl and iodixanol, it has been shown that the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from enveloped viruses has been noted [3]. This may be related to its viscosity, which is much higher than that of iodixanol. Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast, many add-on techniques can be performed and cells infected with virus, without dialysis of iodixanol.
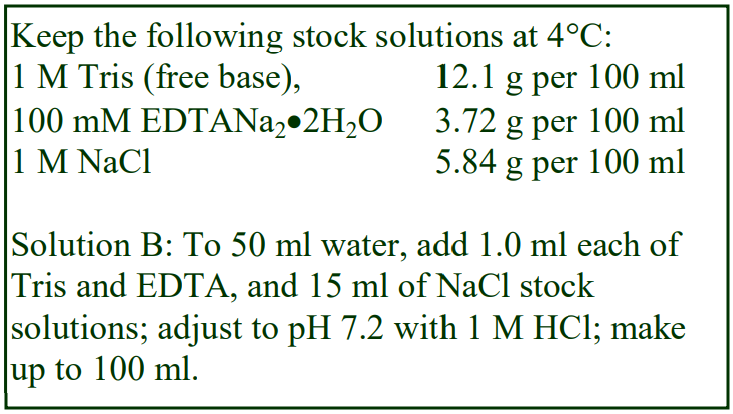 The following protocol is adapted from ref 2.
The following protocol is adapted from ref 2.
2. Solutions required
A. OptiPrep™
B. Suspension Buffer: 0.15 M NaCl, 1 mM EDTA, 10 mM Tris-HCl, pH 7.2
C. Gradient solutions: dilute OptiPrep with Solution B to give two solutions of 6 and 18% (w/v) iodixanol (see Notes 1 and 2)
3. Ultracentrifuge rotor requirements Swinging-bucket rotor with 13-14 ml tubes (e.g. Beckman SW41Ti or Sorvall TH641).
4. Protocol
1. Clarify the supernatant from infected cells by centrifugation at 1500 g for 20 min and pass through a 0.22 μm filter.
2. Concentrate the virus suspension by pelleting it through a density barrier at 100,000 g for 2 h (see Note 3) and resuspend it in a small volume of Solution B.
3. Using a two-chamber gradient maker or a Gradient Master™ prepare a continuous gradient from approx 6 ml each of the two iodixanol solutions in the 13-14 ml tubes (see Notes 4 and 5).
4. Layer the crude virus suspension (1.0-1.5 ml) on top of the gradient and centrifuge at 187,000 gav for 1 h 20 min at 4°C (see Notes 6 and 7).
5. Collect the gradient by upward displacement, low-density end first in approx 0.8-1.0 ml fractions
(see Note 8). The virus bands sharply, 1-2 ml from the bottom of the gradient.
5. Notes
1. For more information on the preparation of density gradient solutions see Application Sheet V01.
2. If a gradient making device is unavailable, then make up solutions of 6.0%, 9.0%, 12.0, 15.0% and 18.0% (w/v) iodixanol.
3. Fujisawa et al [2] pelleted the virus through a 20% sucrose cushion; to maintain an isoosmotic environment for the virus, the 20% sucrose might be replaced by 15% (w/v) iodixanol. The ideal way of concentrating the virus is sedimentation on to a dense cushion of iodixanol, rather than pelleting. This however may be less convenient when, as in this case, the concentration of iodixanol in the viral suspension needs to be <5% (w/v) to permit loading on to the gradient. When recovering the band of virus as little as possible of the cushion must be aspirated. For more information on concentration of virus prior to gradient purification see Application Sheet V06.
4. Alternatively make a discontinuous gradient from equal volumes of 6.0%, 9.0%, 12.0, 15.0% and 18.0% (w/v) iodixanol and allow the formation of a continuous gradient by diffusion (approx. 5 h at room temperature, or overnight at 4°C). For more information on making gradients see Application Sheet V02.
5. Dettenhoffer and Yu [1], who introduced the sedimentation velocity strategy for HIV-1, prepared gradients that were “essentially continuous” by layering solutions with a 1.2% iodixanol concentration interval. It takes considerable practice to be able to form discontinuous gradients from numerous small volume steps, irrespective of whether a pipette or a syringe is used and whether an overlayering or underlayering technique is used; see Application Sheet V02.
6. If larger volumes of crude virus are to be purified then larger volume gradients must be used. As this is a sedimentation-velocity separation the volume of crude virus suspension should not exceed 10-15% of the gradient volume.
7. If the separation is to be carried out at higher temperatures then it may be necessary to reduce the centrifugation time to take account of the reduced viscosity of the gradient.
8. Collection of the gradient by tube puncture may be a useful alternative. For more information on harvesting gradients see Application Sheet V04.
6. References
1. Dettenhoffer, M. and Yu, X-F. (1999) Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif virions J. Virol., 73, 1460-1467
2. Fujisawa, R., McAtee, F.J., Favara, C., Hayes, S.F. and Portis, J.L. (2001) N-terminal cleavage fragment of glycosylated Gag is incorporated into murine oncornavirus particles J. Virol., 75, 11239-11243
3. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445Dettenhoffer, M. and Yu, XF. (1999) J. Virol., 73, 1460-1467
OptiPrep™ Application Sheet V32; 8th edition, January 2020
OptiPrep™ Application Sheet V33
Purification and analysis of Group VI (ss)RNA-RT viruses: Retroviridae: Gammaretrovirus: Moloney murine leukemia virus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- Application Sheet V06 provides a summary of the OptiPrepTM virus purification methodology.
- The OptiPrep™ Reference List (RV06) provides a full bibliography of all published papers reporting the use of iodixanol gradients for the purification of Group VI viruses; to access return to the initial list of Folders and select “Reference Lists”.
- This Application Sheet describes the use of continuous sedimentation velocity or buoyant density pre-formed gradients for purification of members of the Gammaretrovirus genus.
- The retrovirus group is extremely diverse; whether the methods described in this Application Sheet can be applied to another retrovirus, of the same or different genus can only be determined experimentally. For other retroviral isolation methods see the Virus Index
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
Dettenhoffer and Yu [1] developed a sedimentation velocity iodixanol gradient to purify HIV-1 virions without affecting the infectivity of the virus. Furthermore the iodixanol gradient was shown to provide better resolution from vesicle and macromolecular contaminants than a buoyant density sucrose gradient. Another important point about sucrose gradients is that although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [2]. This may be related to its viscosity, which is much higher than iodixanol. This iodixanol gradient strategy has now been extended to the Moloney murine leukemia virus (MLV) by Onafuwa-Nuga et al [3] and Leblanc et al [4].
Prior to loading on to the gradient Onafuwa-Nuga et al [3] pelleted the virions before resuspension in DMEM containing 10% calf serum. High-speed pelleting and resuspension of the pellet by shearing forces, although effective and simple, can however lead to a significant loss of infectivity. Better recovery of viral infectivity can be obtained by sedimentation on to a dense iodixanol cushion. A small volume of dense cushion [5,6] can be used for banding retroviruses (50,000 g for 1.5-3 h) from a very large volume of virus-containing fluid. However, the top of the sedimentation-velocity gradient used for purifying the virus has a low density (6% iodixanol), so when recovering the virus band from the dense cushion it is necessary to ensure that the iodixanol concentration in the virus suspension is <5%. Coleman et al [6] overcame this problem by diluting the harvest with approx 4 vol. of buffer (total volume approx 4 ml); this permits the virus to be efficiently pelleted by very gentle centrifugation for 24 h [6]. Low-speed centrifugation of the original large volume of culture fluid, on the other hand, would lead to a very poor recovery.
The virus has also been purified on the basis of its buoyant density using OptiPrep™ [7].
The strategies of high-density cushion concentration (Section 2) and purification in a sedimentation velocity gradient (Section 3) and buoyant density gradient (Section 4) will be described in this Application Sheet.
2. Virus concentration (adapted from refs 5 and 6)
- The notes referred to in the following methods can be found in Section 5
2a. Solutions required
A. OptiPrep™
B. Buffered saline solution (Hepes or phosphate-buffered)
2b. Rotor requirements
Swinging-bucket rotor (e.g. Beckman SW28) with approx 30-38 ml tubes (see Note 1)
2c. Protocol
1. Harvest the cell supernatants and filter through a 0.45 μm filter.
2. Transfer approx 32-33 ml of the supernatant to tubes for the swinging-bucket rotor and underlayer with 5 ml of OptiPrep™. With konical tubes, the volume of supernatant can be 28-29 ml with 1-2 ml of OptiPrep™ (see Notes 2 and 3).
3. Centrifuge at 50,000 g for 1.5 h at 4° C (see Notes 4 and 5).
4. Carefully aspirate all but 2-3 ml of the supernatant; in konical tubes this can be reduced to 1-2 ml (see Note 6).
5. Collect the banded virus in the residual supernatant, removing as little as possible of the cushion.
6. If the density of the virus suspension is too high to be loaded on the subsequent gradient dilute it with 1 volume of Solution B and pellet the virus at 6000 g for 24 h at 4°C (see Note 7).
3. Purification in sedimentation velocity gradients (adapted from ref 3)
3a. Solutions required
A. OptiPrep™
B. Phosphate-buffered saline
C. Gradient solutions: dilute OptiPrep™ with Solution B to give a series of density solutions from 6 to 18% (w/v) iodixanol in 1.2% steps (i.e. 11 solutions, see Notes 8 and 9)
3b. Rotor requirements
Swinging-bucket rotor with 13-14 ml tubes (e.g. Beckman SW41Ti or Sorvall TH641; see Note 10)
3c. Protocol
1. Prepare a discontinuous gradient from approx 1 ml of each density solution. This is probably best accomplished by overlayering using a peristaltic pump first to draw each 1 ml of liquid into a plastic tube and then reversing the flow to expel it gently on top of the denser layer (see Note 11).
2. Layer the virus suspension (< 2.0 ml) on top of the gradient and centrifuge at 100,000 gav for 1 h at 4°C (see Notes 12 and 13). Use slow acceleration and deceleration programs if they are available or turn off the brake during deceleration from 3000 rpm.
3. Collect the gradient by upward displacement, low-density end first in approx 0.8-1.0 ml fractions (see Note 14). The virus bands just below the middle of the gradient.
4. Purification in buoyant density gradients (adapted from ref 7)
4a. Solutions required
A. OptiPrep™
B. Phosphate-buffered saline
C. Gradient solutions: dilute OptiPrep™ with Solution B to give solutions of 10% and 30% (w/v) iodixanol (20 ml of each).
4b. Rotor requirements
Swinging-bucket rotor with 38-39 ml tubes, e.g. Beckman SW28 (see Note 15)
4c. Protocol
1. Using a two-chamber gradient maker or a Gradient Master™ prepare a gradient in tubes for the swinging-bucket rotor using 17 ml each of the 10% and 30% iodixanol solutions (see Note 15). For more information on making continuous gradients see Application Sheet V02.
2. Layer 3-4 ml of the concentrated virus on top of the gradient and centrifuge at 100,000 g for 4 h; use a slow-deceleration program or turn off the brake during deceleration from 3000 rpm (see Note 15).
3. Collect the gradient by tube puncture or aspiration from the meniscus in approx 5.0 ml fractions (see Note 15). The virus bands in the middle third of the gradient.
5. Notes
1. The best rotors for concentrating virus are certainly swinging-bucket ones and the best tubes are the conical-bottomed “konical” tubes of Beckman. The small cross-sectional area of the tube close to its bottom means that a smaller volume of cushion can be used, and recovery of the banded virus without simultaneous aspiration of the cushion itself, is facilitated.
2. The alternative to banding is simply to pellet the virus at 50,000 g for 1.5 h.
3. Underlayering the virus-containing fluid with the cushion, using a syringe attached to a long metal cannula is certainly the preferred method. Overlayering such a small volume of cushion with a large volume of supernatant is bound to lead to mixing problems.
4. Coleman et al [6] used 2.5 h.
5. Allow the rotor to decelerate using a slow-deceleration program or turn off the brake below 2000 rpm to avoid “Coriolus” mixing of the banded virus.
6. It may be more convenient to use a syringe + long metal cannula to remove the cushion first.
7. Coleman et al [6] only used 0.22 ml of cushion and removed all of the supernatant (except for the last 0.22 ml) and then harvested all of the remaining liquid (including the cushion) and diluted the suspension 2.5x with buffer before the 24 h centrifugation. With the more convenient larger volume of cushion, the method described in step 4 of the protocol should allow easy harvest of the banded virus from a konical tube without removing more than 0.2 ml of cushion.
8. One of the practical alternatives, which might be considered, is the use of a continuous gradient rather than a multi-step discontinuous gradient (see Note 5). If this option is used then prepare just 6 and 18% iodixanol.
9. For more information on the preparation of density gradient solutions for viruses see Application Sheet V01.
10. If larger volumes of crude virus are to be purified then larger volume gradients must be used. As this is a rate-zonal separation the volume of crude virus suspension should not exceed approx 15% of the gradient volume.
11. Using the more normal pipette or a syringe, considerable practice is required to be able to form discontinuous gradients of numerous small volume steps, irrespective of whether an overlayering or underlayering technique is used. The recommended use of a pump may facilitate the process. Since however diffusion of iodixanol will occur during the centrifugation, it may be even easier to make a continuous gradient from equal volumes of the densest and lightest solutions. For more information see Application Sheet V02.
12. If it is necessary to concentrate the virus before layering on the gradient make sure that the density of the virus suspension is low enough to permit layering on the gradient. For more information see Application Sheet V06.
13. More recently Eckwahl et al [7] used a gradient of 8-22% (w/v) iodixanol centrifuged at 88,000 g for 1 h: the virus banded very sharply at approx. 17-18% iodixanol.
14. Collection of the gradient by tube puncture may be a useful alternative. For more information on harvesting gradients see Application Sheet V04.
15. Smaller volume rotors (e.g. Beckman SW41Ti, tube volume approx. 13 ml) can almost certainly be used; scale down the volumes of gradient solutions and sample proportionately.
16. Ref 8 reports the collection of just three fractions of 12.5 ml, 10 ml and 14.5 ml (high-density end first) – the virus banding in the middle fraction.
6. References
1. Dettenhoffer, M. and Yu, X-F. (1999) Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif virions J. Virol., 73, 1460-1467
2. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
3. Onafuwa-Nuga, A.A., King, S.R. and Telesnitsky, A. (2005) Nonrandom packaging of host RNAs in moloney murine leukemia virus J. Virol., 79, 13528-13537
4. Leblanc, P., Alais, S., Porto-Carriero, I., Lehmann, S., Grassi, J., Raposo, G. and Darlix, J.L. (2006) Retrovirus infection strongly enhances scrapie infectivity release in cell culture EMBO J., 25, 2674-2685
5. Ganesh, L., Leung, K., Loré, K., Levin, R., Panet, A., Schwartz, O., Koup, R.A. and Nabel, G.J. (2004) Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cellmediated transmission of virus resistant to broadly neutralizing antibodies J. Virol., 78, 11980-11987
6. Coleman J.E., Huentelman, M.J., Kasparov, S., Metcalfe, B.L., Paton, J.F.R., Katovich, M.J., SempleRowland, S.L. and Raizada, M.K. (2003) Efficient large scale production and concentration of HIV-1-based lentiviral vectors for use in vivo Physiol. Genomics, 12, 221-228
7. Eckwahl, M.J., Sim, S.,. Smith, D., Telesnitsky, A. and Wolin, S.L. (2015) A retrovirus packages nascent host noncoding RNAs from a novel surveillance pathway Genes Devel., 29, 646–657
8. Segura, M.M., Garnier, A., Di Falco, M.R., Whissell, G., Meneses-Acosta, A., Arcand, N. and Kamen, A. (2008) Identification of host proteins associated with retroviral vector particles by proteomic analysis of highly purified vector preparations J. Virol., 82 1107-1117
OptiPrep™ Application Sheet V33; 9th edition, January 2020
OptiPrep™ Application Sheet V34
Purification and analysis of Group VI (ss)RNA-RT viruses: Retroviridae: Lentivirus: Human immunodeficiency virus – 1 (HIV-1) and lentivirus vectors
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RV06) provides a full bibliography of all published papers reporting the use of iodixanol gradients for Group VI viruses; to access return to the initial list of Folders and select “Reference Lists”.
- Application Sheet V06 provides a summary of the OptiPrep™ virus purification methodology which has been developed over the last 20 years.
- This Application Sheet covers continuous sedimentation velocity, buoyant density pre-formed gradients and self-generated gradients, for the purification of members of the Lentivirus genus.
- The retrovirus group is extremely diverse; whether the methods described in this Application Sheet can be applied to another retrovirus, of the same or different genus can only be determined experimentally. For other retroviral isolation methods see the Virus Index
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Background
1a. Virus concentration (see Section 2)
A simple iodixanol density cushion can be used to concentrate the virus. Depending on the density of the cushion, such a technique may also partially purify the virus. A small volume of very dense cushion [1,2] was used for banding viral particles from a very large volume of virus-containing fluid. This stage can be carried out at 50,000 g for a short time (1.5-3 h). Should it be necessary to remove the iodixanol after recovery of the banded material, the virus can be efficiently pelleted from a small volume by gentle centrifugation for a long period [2]. Low-speed centrifugation of the original large volume on the other hand would lead to very poor recovery. These procedures lead to improved infectivity, compared to simple high-speed pelleting. Low-density cushions of 8.4% [3] and 6% [4] (w/v) iodixanol have been used to pellet HIV-1 and HIV-1 cores respectively and to separate them from more slowly-sedimenting contaminating particles and soluble proteins.
1b. Purification and analysis in a pre-formed sedimentation velocity gradient (see Section 3)
Dettenhoffer and Yu [5] developed a sedimentation velocity iodixanol gradient to purify HIV-1 virions without affecting the infectivity of the virus. In buoyant density sucrose gradients the extracellular Vif gene always co-purifies with the virus and the latter is also contaminated with cellderived microvesicles. In rate-zonal iodixanol gradients on the other hand the HIV-1 was effectively separated both from Vif and from the microvesicles. Another important point about sucrose gradients is that although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless have serious effects on viral structure; in particular the loss of surface glycoproteins from retroviruses has been noted [6]. This may be related to its viscosity, which is much higher than that of iodixanol. This sedimentation velocity method is certainly the most widely used of the three gradient options.
1c. Purification and analysis in a self-generated gradient (see Section 4)
Self-generated gradients are highly reproducible and the ease of sample handling makes them very attractive. This strategy was first described using Nycodenz; the clarified virus suspension was adjusted to 25% NycodenzⓇ and centrifuged in a vertical rotor at 200,000 g for 24 h [7,8]. The strategy has been extended to the use of iodixanol, which forms these gradients more readily [9-11].
1d. Purification and analysis in a pre-formed buoyant density gradient (see Section 5)
Warrilow et al [12] formed a 3.6 ml 20-60% (w/v) iodixanol gradient by diffusion of a multi-step discontinuous gradient; banding of HIV-1 was carried out at 150,000 g for 20 h.
2. Virus concentration on to a dense cushion (adapted from ref 2; see also Note 1)
- The notes referred to in this and the following methods can be found in Section 6
2a. Solutions required
A. OptiPrep™
B. Buffered saline solution (Hepes or phosphate-buffered)
2b. Rotor requirements
Swinging-bucket rotor (e.g. Beckman SW28) with approx 30-38 ml tubes (see Note 2)
2c. Protocol
1. Harvest the cell supernatants and filter through a 0.45 μm filter.
2. Transfer approx 32-33 ml of the supernatant to tubes for the swinging-bucket rotor and underlayer with 5 ml of OptiPrep™. With konical tubes, the volume of supernatant can be 28-29 ml with 1-2 ml of OptiPrep™ (see Note 3).
3. Centrifuge at 50,000 g for 1.5 h at 4° C (see Notes 4 and 5).
4. Carefully aspirate all but 3-4 ml of the supernatant; in konical tubes this can be reduced to 1-2 ml.
5. Collect the banded virus in the residual supernatant, removing as little as possible of the cushion (see Note 6).
6. If the density of the virus suspension is too high to be loaded on a subsequent gradient dilute it with 1 volume of Solution B and pellet the virus at 6000 g for 24 h at 4°C (see Note 7).
3. HIV-1 purification in sedimentation velocity gradients (adapted from ref 5, see Note 8)
3a. Solutions required
A. OptiPrep™
B. Phosphate-buffered saline
C. Gradient solutions: dilute OptiPrep™ with Solution B to give a series of density solutions from 6 to 18% (w/v) iodixanol in 1.2% steps (i.e. 11 solutions, see Notes 9-11)
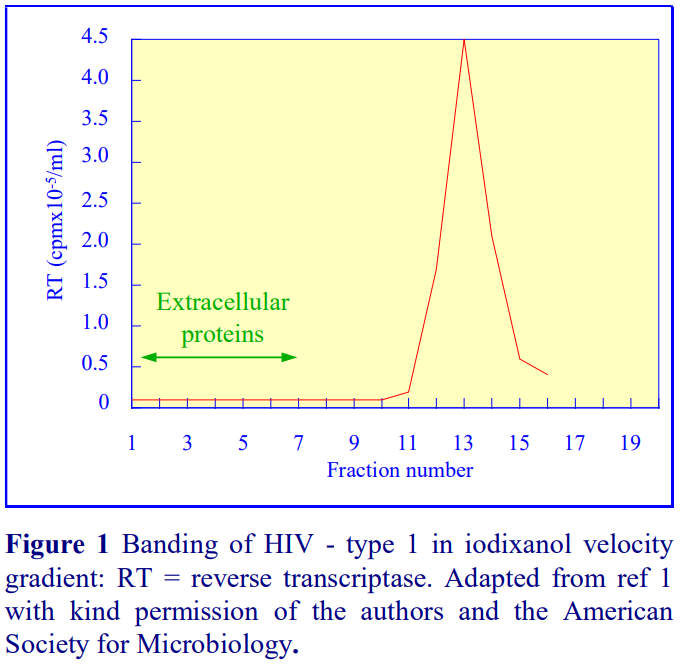 3b. Rotor requirements
3b. Rotor requirements
Swinging-bucket rotor with 13-14 ml tubes (e.g. Beckman SW41Ti or Sorvall TH641; see Note 122
3c. Protocol
1. Prepare a discontinuous gradient from approx. 1 ml of each density solution. This is probably best
accomplished by overlayering using a peristaltic pump first to draw each 1 ml of liquid into a plastic tube and then reversing the flow to expel it gently on top of the denser layer (see Note 13).
2. Layer the concentrated virus suspension (approx 1.0 ml) on top of the gradient and centrifuge at 200,000 gav for 1.5 h (see Notes 5, 14 and 15).
3. Collect the gradient by upward displacement, low-density end first in approx 0.8-1.0 ml fractions (see Note 16). The virus bands sharply in the bottom third of the gradient (Figure 1).
4. HIV-1 purification in a self-generated gradient (adapted from ref 9, see Note 8)
4a. Rotor requirements
Vertical or near vertical rotor: e.g. Beckman VTi65.1 or NVT65 (both approx 13 ml tubes)
4b. Protocol
1. Mix equal volumes of the clarified cell supernatants and OptiPrep™ and centrifuge in the chosen vertical or near vertical rotor. Use approx 350,000 gav for 3-3.5 h (see Note 17).
2. Use a slow-deceleration program or turn off the brake below 2000 rpm.
3. Collect the gradient dense end first by tube puncture or, if the tube type permits it, low density end first, by upward displacement with a dense medium or aspiration from the meniscus (see Notes 16 and 18).
5. HIV-1 purification in a pre-formed continuous gradient (adapted from ref 12, see Note 8)
5a. Solutions required
A. OptiPrep™
B. Stock 100x buffer: 2 M NaCl, 100 mM MgCl2, 50 mM β-mercaptoethanol, 500 mM Tris-HCl, pH 7.4
C. Stock buffer: 20 mM NaCl, 1 mM MgCl2, 0.5 mM β-mercaptoethanol, 5 mM Tris-HCl, pH 7.4
5b. Rotor requirements
Swinging-bucket rotor for approx. 4 ml tubes (e.g. Beckman SW60Ti)
5c. Protocol
1. Prepare an approx. 55% (w/v) iodixanol solution from 11 ml of OptiPrep™, 1.0 ml of water and 120 μl of Solution B. Dilute this further with Solution C to make solutions of 50%, 45%, 40%, 35%, 30%, 25% and 20% (w/v) iodixanol (see Note 19).
2. In 4 ml tubes for the swinging bucket rotor layer 0.45 ml of each of the eight iodixanol solutions, dense end first and allow the gradient to diffuse at room temperature for 4 h (see Note 19).
3. Bring the gradient to 4°C and layer approx. 0.4 ml of the virus solution on top of the gradient to fill the tube.
4. Centrifuge at 150,000 g for 20 h (see Note 20) using slow acceleration and deceleration (to and from approx 3000 rpm) programs, if available; if not available turn of the brake during deceleration from 3000 rpm.
5. Unload the gradient in approx 0.4 ml fractions low-density end first. HIV-1 bands in the top quarter of the gradient (see Note 16).
6. Notes
1. For more information on concentrating virus see Application Sheet V06
2. The best rotors for concentrating virus on to a cushion are swinging-bucket ones and the best tubes are the conical-bottomed “konical” tubes of Beckman. The small cross-sectional area of the tube close to its bottom means that a smaller volume of cushion can be used, and recovery of the banded virus without simultaneous aspiration of the cushion itself, is facilitated.
3. Underlayering the virus-containing fluid with the cushion, using a syringe attached to a long metal cannula is certainly the preferred method. Overlayering such a small volume of cushion with a large volume of supernatant is bound to lead to mixing problems.
4. Coleman et al [2] used 2.5 h.
5. Allow the rotor to decelerate using a slow-deceleration program or turn off the brake below 2000 rpm to avoid “Coriolus” mixing of the banded virus.
6. It may be more convenient to use a syringe + long metal cannula to remove the cushion first.
7. Coleman et al [2] only used 0.22 ml of cushion and removed all of the supernatant (except for the last 0.22 ml) and then harvested all of the remaining liquid (including the cushion) and diluted the suspension 2.5x with buffer before the 24 h centrifugation. With the more convenient larger volume of cushion, the method described in Steps 4 and 5 of the protocol should allow easy harvest of the banded virus from a konical tube without removing more than 0.2 ml of cushion.
8. It is not known how widely applicable any of these methods is to the purification of other members of the Retroviridae, but it is highly likely that each may be used for other virus types, even though optimization of either the density range of the gradient and/or the centrifugation conditions may be needed for purity maximization.
9. Sometimes gradients of 0-18% iodixanol have been used for purifying HIV-1 (e.g. refs 13 and 14).
10. One of the practical alternatives, which might be considered, is the use of a continuous gradient. If this option is used then prepare just 6 and 18% iodixanol.
11. For more information on the preparation of density gradient solutions see Application Sheet V01.
12. If larger volumes of crude virus are to be purified then larger volume gradients must be used. As this is a rate-zonal separation the volume of crude virus suspension should not exceed approx 10- 15% of the gradient volume. 13. Using the more normal pipette or a syringe, considerable practice is required to be able to form discontinuous gradients of numerous small volume steps, irrespective of whether an overlayering or underlayering technique is used. Since however diffusion of iodixanol will occur during the centrifugation, it may be easier to make a continuous gradient from 6 and 18% iodixanol. For more information see Application Sheet V02.
14. If it is necessary to concentrate the virus before layering on the gradient make sure that the density of the virus suspension is low enough to permit layering on the gradient. Generally RCFs of either 100,000 g [14-16] or 250,000 g [5,17,18] have been used, occasionally 183,000 g [13] or 200,000 g [19]. Centrifugation times are usually 1.5 h [5,13,14,17-21], sometimes 3.0 h [15,16].
15. A vertical or near-vertical rotor would actually improve the resolution further because of the large surface area of the liquid in the tube during the centrifugation and the short path length would mean a much shorter centrifugation time.
16. Collection of the gradient by tube puncture may be a useful alternative. For more information on harvesting gradients see Application Sheet V04.
17. This was first carried out in a 39 ml tube vertical rotor (Beckman VTi50) at 240,000 g for 6 h [9], later in the 5 ml tube near-vertical NVT100 at 420,000 g for 3.5 h [11].
18. The method is a high resolution one – small shifts in density of V3 loop mutants compared to wild-type have been reported [9] and released Gag bands in denser regions of the gradient [11].
19. Since the virus bands at a relatively low density omission of the 55% solution may be permissible. A 50% iodixanol solution may be prepared from 10 ml of OptiPrep™, 2.0 ml of water and 120 μl of Solution B. It is also likely that the number of steps might be reduced (10% iodixanol increments); formation of a continuous gradient by diffusion will take about 6 h in a 4 ml tube. Larger volume tubes will require longer diffusion times. Continuous gradients can also be made from equal volumes of the densest and lightest solutions using a two-chamber gradient maker or Gradient Master™. For more information see Application Sheet V02.
20. Shorter times at higher g-forces may be permissible but have not been validated.
7. References
1. Ganesh, L., Leung, K., Loré, K., Levin, R., Panet, A., Schwartz, O., Koup, R.A. and Nabel, G.J. (2004) Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies J. Virol., 78, 11980-11987
2. Coleman J.E., Huentelman, M.J., Kasparov, S., Metcalfe, B.L., Paton, J.F.R., Katovich, M.J., Semple-Rowland, S.L. and Raizada, M.K. (2003) Efficient large scale production and concentration of HIV-1-based lentiviral vectors for use in vivo Physiol. Genomics, 12, 221-228
3. Klingen, Y., Conzelmann, K-K. and Finke, S. (2008) Double-labeled rabies virus: live tracking of enveloped virus transport J. Virol., 82, 237-245
4. Cavrois, M., Neidleman, J., Yonemoto, W., Fenard, D and Greene, W.C. (2004) HIV-1 virion fusion assay: uncoating not required and no effect of Nef on fusion Virology, 328, 36-44
5. Dettenhoffer, M. and Yu, X-F. (1999) Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif virions J. Virol., 73, 1460-1467
6. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
7. Nielsen, C.M., Kvinesdal, B. and Vestergaard, B.F. (1989) Antigen-antibody reaction in solution in capture competition immunoassay for human immunodeficiency virus antibodies J. Clin. Microbiol., 27, 1609-1612
8. Pedersen, M., Nielsen, C.M. and Permin, H. (1991) HIV antigen-induced release of histamine from basophils from HIV infected patients. Mechanism and relation to disease progression and immunodeficiency Allergy, 46, 206-212
9. Yang, Z-Y., Chakrabati, B.K., Xu, L., Welcher, B., Kong, W-p., Leung, K., Panet, A., Mascola, J.R. and Nabel, G.J. (2004) Selective modification of variable loops alters tropism and enhances immunogenicity of human immunodeficiency virus type 1 envelope J. Virol., 78, 4029-4036
10. Akahata, W., Yang, Z-Y. and Nabel, G.J. (2005) Comparative immunogenicity of human immunodeficiency virus particles and corresponding polypeptides in a DNA vaccine J. Virol., 79, 626-631
11. Kim, J-O., Chakrabarti, B.K., Guha-Niyogi, A., Louder, M.K., Mascola, J.R., Ganesh, L. and Nabel, G.J. (2007) Lysis of human immunodeficiency virus type 1 by a specific secreted human phospholipase A2 J. Virol., 81, 1441-1450
12. Warrilow, D., Meredith, L., Davis, A., Burrell, C., Li, P. and Harrich, D. (2008) Cell factors stimulate human immunodeficiency virus type 1 reverse transcription in vitro J. Virol., 82, 1425-1437
13. Mouland, A. J., Mercier, J., Luo, M., Bernier, L., DesGroseillers, L. and Cohen, E. A. (2000) The double-stranded RNAbinding protein Staufen is incorporated in human Immunodeficiency virus type 1: evidence for a role in genomic encapsidation J. Virol., 74, 5441-5451
14. Chen, S., Khorchid, A., Javanbakht, H., Gabor, J., Stello, T., Shiba, K., Musier-Forsyth, K. and Kleiman, L. (2001) Incorporation of lysyl-tRNA synthetase into human immunodeficiency virus type 1 J. Virol., 75, 5043-5048
15. Tritel, M. and Resh, M. (2000) Kinetic analysis of human immunodeficiency virus type 1 assembly reveals the presence of sequential intermediates J. Virol., 74, 5845-5855
16. Tritel, M. and Resh, M. (2001) The late stage of human immunodeficiency virus type 1 assembly is an energy-dependent process J. Virol., 75, 5473-5481
17. Henriksson, P., Pfeiffer, T., Zentgraf, H., Alke, A. and Bosch, V. (1999) Incorporation of wild-type and C-terminally truncated human epidermal growth factor receptor into human immunodeficiency virus-like particles: insight into the processes governing glycoproteins incorporation into retroviral particles J. Virol., 73, 9294-9302
18. Gurer, C., Cimarelli, A. and Luban, J. (2002) Specific incorporation of heat shock protein 70 family members into primate lentiviral virions J. Virol., 76, 4666-4670
19. Sova, P., Volsky, D. J., Wang, L. and Chao, W. (2001) Vif is largely absent from human immunodeficiency virus type 1 mature virions and associates with viral particles containing unprocessed J. Virol., 75, 5504-5517
20. Müller, B., Tessmer, U., Schubert, U. and Krausslich, H-G. (2000) Human immunodeficiency virus type 1 Vpr protein is incorporated into the virion in significantly smaller amounts than Gag and is phosphorylated in infected cells J. Virol., 74, 9727-9731
21. Müller, B., Patschinsky, T. and Krausslich, H-G. (2002) The late-domain-containing protein p6 is the predominant phosphoprotein of human immunodeficiency virus type 1 particles J. Virol., 76, 1015-1024
8. Acknowledgements
We wish to thank Dr Xiang-Fang Yu, Department of Molecular Microbiology and Immunology, Johns Hopkins University school of Medicine, Baltimore, MD 21205, USA for his cooperation in the preparation of this Application Sheet.
OptiPrep™ Application Sheet V34; 9th edition, January 2020
OptiPrep™ Application Sheet V35
Purification and analysis of Group VI (ss)RNA-RT viruses: Retroviridae: Spumaretrovirinae: Foamy viruses
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RV06) provides a full bibliography of all published papers reporting the use of iodixanol gradients for Group VI viruses; to access return to the initial list of Folders and select “Reference Lists”.
- Application Sheet V06 provides a summary of the OptiPrep™ virus purification methodology which has been developed over the last 20 years.
- This Application Sheet describes the use of continuous and discontinuous buoyant density gradients for purification of human and feline foamy virus (Spumaretrovirinae).
- The retrovirus group is extremely diverse; whether the methods described in this Application Sheet can be applied to another retrovirus, of the same or different genus can only be determined experimentally. For other retroviral isolation methods see the Virus Index
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number
1. Background
There are now many published papers that report the use of iodixanol gradients not only to purify viruses but also to investigate their assembly. In all comparative studies between CsCl and iodixanol, the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from retroviruses has been noted [1]. This may be related to its viscosity, which, in solutions of the same density, is much higher than iodixanol.
Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast both infectivity measurements using cultured cells and many add-on techniques can be performed without dialysis of iodixanol. Combined with the availability of OptiPrep™ as a sterile solution, this makes the use of OptiPrep™ for virus purification and analysis much more convenient than that of CsCl or sucrose.
Foamy virus can be banded according to its buoyant density in either a discontinuous or continuous gradient; both types of gradient will be described, see Step 6 or Step 7 respectively. Some of the variations in methodology are presented in Table 1 at the end of this Application Sheet. The protocols are adapted principally from refs 2 and 3.
2. Solutions required (see Section 5, Note 1)
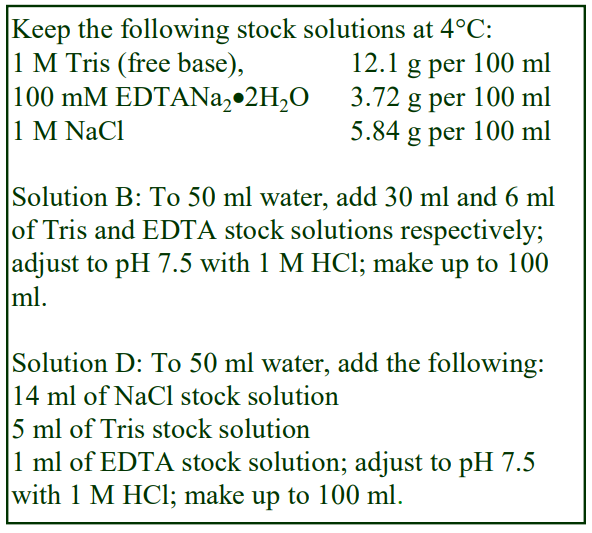 A. OptiPrep™
A. OptiPrep™
B. OptiPrep™ diluent: 6 mM EDTA, 300 mM Tris-HCl, pH 7.5
C. 50% (w/v) Iodixanol Working Solution: mix 5 vol. Solution A with 1 vol. of Solution B
D. Diluent: 0.14 M NaCl, 1 mM EDTA, 50 mM Tris-HCl, pH 7.5
3. Rotor requirements
For concentrating the virus from large volumes of culture fluid: swinging-bucket rotor with 36-39 ml tubes (e.g. Beckman SW28 or Sorvall AH629) Excellence in Separations OptiPrep™ Application Sheet V35
For the iodixanol gradient: swinging-bucket rotor with either 5 ml (e.g. Beckman SW55Ti or Sorvall AH650) 14 ml tubes (e.g. Beckman SW41Ti) or 17 ml tubes (e.g. Beckman SW28.1 or Sorvall AH629). See Section 5, Note 2 for more information.
4. Protocol
1. Once the virus has been released from the cells, clarify the suspension by low speed centrifugation (approx 2000 g for 15 min) to remove cellular debris.
2. If required filter the supernatant through a 0.45 μm filter.
3. Prepare a 12% (w/v) iodixanol solution (1.2 vol. of Solution C with 3.8 vol. of Solution D).
4. To concentrate the virus, use the tubes for the 36-39 ml swinging-bucket rotor and underlay the suspension with 5 ml of the 12% iodixanol using a syringe and metal cannula (see Section 5, Notes 3 and 4).
5. Centrifuge at 80,000 gav for 2.5 h
6. For a discontinuous gradient: During Step 5 prepare from Solutions C and D: 10%, 20%, 30% and 40% (w/v) iodixanol, and layer 1 ml or 2.5 ml of each in 5 or 14 ml tubes, respectively (see Section 5, Notes 5 and 6).
7. For a continuous gradient: During Step 5 prepare from Solutions C and D two solutions of 10% and 32% (w/v) iodixanol and using a two-chamber gradient or a Gradient Master™ make a 4 ml or 10 ml gradient (5 ml or 15 ml tubes respectively) from equal volumes of the two solutions (see Section 5, Note 5).
8. Resuspend the virus pellet in 5% (w/v) iodixanol (1 vol. of Solution C + 9 vol. of Solution D) and layer over the chosen gradient (see Section 5, Note 4).
9. Centrifuge at 122,000 gav for 4-16 h at 4°C
10. Unload the gradient either by upward displacement, aspiration from the meniscus or by tube puncture in 0.2-0.5 ml fractions. Once the position of the foamy virus in the tube has been established, it can alternatively be recovered using a syringe (see Section 5, Note 7).
- For a brief summary of some of the variations in gradient and centrifugation conditions see Section 5, Notes 8-11)
5. Notes
1. The mode of preparing the solutions described in this OptiPrep™ Application Sheet ensures that the concentrations of buffer and EDTA are constant throughout the gradient. If this is not considered important, the OptiPrep™ may simply be diluted with the virus suspension solution. Any suitable buffer can be used for suspending the virus and for making the gradient solutions. It may be customized to the operator’s own requirements, as long as the buffer has a low density (approx 1.006 g/ml) the density of the gradients will not be compromised. Some of the variations are given in Table 1. More details on the making up of gradient solutions are given in Application Sheet V01.
2. Larger volume gradients are permissible (e.g. in the Beckman SW41) but the time will need increasing to compensate for the lower RCF. If a vertical rotor is substituted for the swingingbucket rotor (e.g. Beckman VTi90 or VTi65.1), the shorter sedimentation path length will permit shorter centrifugation times.
3. Baldwin and Linial [2] sedimented the foamy virus through a 20% sucrose layer, this has been substituted with the more virus-friendly iodixanol in this protocol.
4. Virus concentration by pelleting, either directly or through a low-density layer may be undesirable. This procedure can result in some loss of infectivity either because of the physical aggregation of particles, high hydrostatic pressure at the bottom of the tube or the dispersal forces used to resuspend the pellet (or a combination of all of these problems). A useful alternative is to sediment the virus on to a small cushion (2-3 ml) of 45% w/v iodixanol or even pure OptiPrep™. However, unless the virus band is harvested with the minimum amount of cushion, it may have to be diluted to an unacceptable volume for loading on top of the subsequent density gradient. The use of Beckman “konical” tubes overcomes this problem to some extent. Moreover, as long as the purification is based on buoyant density rather than sedimentation velocity then the sample volume is not really important. For buoyant density banding, the mode of banding in this protocol, the sample may alternatively be layered beneath the gradient, in which case the contamination from the cushion is irrelevant. For more information on concentrating virus see Application Sheet V06. Occasionally the iodixanol is only used as a cushion for the concentration step [4].
5. A number of gradient variations are summarized in Table 1. Most methods use either 14 ml or 5 ml tubes, but smaller volume tubes have been used. For more information on making continuous and discontinuous gradients see Application Sheet V02.
6. More recently the range of the discontinuous gradient has been changed to 20-55% iodixanol, this may allow loading of the sample on top of the gradient more easy [10,11].
7. For more information on harvesting gradients see Application Sheet V04.
9. The banding density of foamy virus is in the range 1.12-1.15 g/ml [2], but the precise value may vary with centrifugation conditions. Some of the variations in centrifugation conditions are given in Table 1. Recently Spannaus and Bodem [12] used a 6-35% (w/v) iodixanol gradient centrifuged at approx. 172,000 g for 1.5 h, within which the virus banded in the 19-25% (w/v) iodixanol zone.
10. In the 20-55% (w/v) iodixanol reported in ref 11, the peak density may be slightly higher at approx. 1.17 g/ml. This particular paper reported the use of a 5 ml swinging-bucket rotor centrifuged at approx 100,000 g for 18 h; it was used to show the similar banding of wt, △GR1 and △GR1Ala mutants. The 15-40% (w/v) iodixanol gradient, centrifuged at 197,000 g for 3 h in the short sedimentation path-length Beckman TLS55 rotor used by Swiersy et al [13], was described as a sedimentation velocity gradient. It was used to analyze Pol processing products: p85PR-RT and p40IN coincided with the Gag and env proteins; the resolving power of the gradient allowed the authors to identify a shift to higher densities of the p127Pol protein. In a recent paper by Lee et al [14], the 20-55% (w/v) iodixanol gradient was changed to 20-50%. It clearly demonstrated that the wt virus-like particles (VLPs) peaked at approx 1.12-1.14 g/ml, while VLPs bearing Gag-Pol fusions banded at a slightly lower density. Hamann et al [15] used a small-volume gradient (15- 40% w/v iodixanol) constructed from nine equivolume steps of 0.22 ml, centrifuged at 197,000 g for 3 h to investigate particle morphology of mutant and wild-type particles. The gradient was able to distinguish wild-type and mutant forms indicating a variation of morphology induced Gagnucleic acid interactions.
11. Spannaus et al [16] have recently published a paper documenting the methodology for purification of foamy viruses.
6. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
2. Baldwin, D. N. and Linial, M.L. (1999) Proteolytic activity, the carboxy terminus of Gag, and the primer binding site are not required for Pol incorporation into foamy virus particles J. Virol., 73, 6387-6393
3. Wilk, T., Geiselhart, V., Frech, M., Fuller, S.D., Flugel, R.M. and Lochelt, M. (2001) Specific interaction of a novel foamy virus env leader protein with the N-terminal Gag domain J. Virol., 75, 7995-8007
4. Rua, R., Lepelley, A., Gessain, A. and Schwartz, O. (2012) Innate sensing of foamy viruses by human hematopoietic cells J. Virol., 86, 909-918
5. Lindemann, D., Pietschmann, T., Picard-Maurreau, M., Berg, A., Heinkelein, M., Thurow, J., Knaus, P., Zentgraf, H. and Rethwilm, A. (2001) A particle-associated glycoprotein signal peptide essential for virus maturation and infectivity J. Virol., 75, 5762-5771
6. Geiselhart, V., Schwantes, A., Bastone, P., Frech, M. and Lochelt, M. (2003) Features of the Env leader protein and the N-terminal Gag domain of feline foamy virus important for virus morphogenesis Virology, 310, 235-244
7. Geiselhart, V., Bastone, P., Kempf, T., Schnolzer, M. and Lochelt, M. (2004) Furin-mediated cleavage of the feline foamy virus Env leader protein J. Virol., 78, 13573-13581
8. Shaw, K.L., Lindemann, D., Mulligan, M.J. and Goepfert, P.A. (2003) Foamy virus envelope glycoprotein is sufficient for particle budding and release J. Virol., 77, 2338-2348
9. Cartellieri, M., Rudolph, W., Herchenröder, O., Lindemann, D. and Rethwilm, A. (2005) Determination of the relative amounts of Gag and Pol proteins in foamy virus particles Retrovirology, 2:44
10. Life, R.B., Lee, E-G., Eastman, S.W. and Linial, M.L. (2008) Mutations in the amino terminus of foamy virus Gag disrupt morphology and infectivity but do not target cell assembly J. Virol., 82, 6109-6119
11. Lee, E-G. and Linial, M.L. (2008) The C terminus of foamy retrovirus Gag contains determinants for encapsidation of Pol protein into virions J. Virol., 82, 10803-10810
12. Spannaus, R. and Bodem, J. (2014) Determination of the protease cleavage site repertoire-the RNase H but not the RT domain is essential for foamy viral protease activity Virology, 454-455, 145–156
13. Swiersy, A., Wiek, C., Reh, J., Zentgraf, H. and Lindemann, D. (2011) Orthoretroviral-like prototype foamy virus gag-00pol expression is compatible with viral replication Retrovirology, 8: 66
14. Lee, E-G., Sinicrope, A., Jackson, D.L., Yu, S.F. and Linial, M.L. (2012) Foamy virus Pol protein expressed as a Gag-Pol fusion retains enzymatic activities, allowing for infectious virus production J. Virol., 86, 5992– 6001
15. Hamann, M.V., Müllers, E., Reh, J., Stanke, N., Effantin, G., Weissenhorn, W. Lindemann, D. (2014) The cooperative function of arginine residues in the prototype foamy virus Gag C-terminus mediates viral and cellular RNA encapsidation Retrovirology, 11: 87
16. Spannaus, R., Miller, C., Lindemann, D. and Bodem, J. (2017) Purification of foamy viral particles Virology 506, 28–33
OptiPrep™ Application Sheet V35; 9th edition, January 2020
OptiPrep™ Application Sheet V36
Purification and analysis of Group VII (ds RNA-RT): Hepadnaviridae: Orthohepadnavirus: hepatitis B virus
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number
- For recent reports of the use of iodixanol gradients see Section 3
1. Background
The two genera of the Hepadnaviridae family are Orthohepadnavirus and Avihepadnavirus which are represented by hepatitis B virus and duck hepatitis B virus respectively, both of which have been purified and analyzed using iodixanol gradients.
In all comparative studies between CsCl and iodixanol, it has been shown that the recovery of virus infectivity is much higher and the particle:infectivity ratio much lower when viruses are purified in iodixanol. Although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless also have serious effects on certain important aspects of viral function; in particular the loss of surface glycoproteins from enveloped viruses has been noted [1]. This may be related to its viscosity, which is much higher than that of iodixanol. Like CsCl, sucrose must be dialyzed before infectivity can be measured. In contrast, many add-on techniques can be performed and cells infected with virus, without dialysis of iodixanol.
2. Methodology and results
Prior to the development of an OptiPrep based method, Kock et al [2] had used a 10-50% (w/v) Nycodenz gradient (the solid Nycodenz was dissolved in an isoosmotic solution of 140 mM NaCl, 1.5 mM MgCl2, 50 mM Tris-HCl, pH 8.0 containing 0.5% NP40). Centrifugation was carried out at approx. 250,000 gmax for 40 min at 20°C (in a Beckman TLS55 swinging-bucket rotor). The gradient showed that the viral DNA sedimented with the core protein of duck hepatitis B virus. The method was adapted to a discontinuous gradient of iodixanol (10% increments) using the same centrifugation conditions and applied to human hepatitis B virus grown in mammalian cultured cells [3,4]. The density range would have been almost identical in the two cases, but, whilst the denser regions of the Nycodenz gradient would have been hyperosmotic, the entire iodixanol gradient would have been more or less isoosmotic. Whether this has any influence on the banding density of the virus is not clear.
The 10-50% (w/v) iodixanol gradient effectively demonstrated that an HBV Pol-interacting host factor (DDX3) was incorporated into the nucleocapsid and that this was dependent on HBV-Pol. The core proteins demonstrated a peak banding around three-quarters of the way down the gradient. This was equivalent to approx. 35% (w/v) iodixanol or approx. 1.19 g/ml. The same group also showed the incorporation of Pol into nucleocapsid was proteinase K-resistant.
Bardens et al [5] used a very different gradient format, the cells were Dounce homogenized in a hypotonic medium containing 1 mM MgCl2, 10 mM Tris-HCl, pH 7.5 (no detergent) and after an initial low speed centrifugation to remove cell debris the supernatant was adjusted to 40% (w/v) iodixanol and overlaid by a solution of 28% (w/v) iodixanol and centrifuged at 100,000 g for 3h. The virus particles presumably banded around the interface between the two iodixanol solutions.
The only paper reporting the analysis of duck HBV was concerned with the inhibition of HBV replication [6]. The cells were homogenized in a routine medium often used in membrane fractionation studies (0.25 M sucrose, 1 mM EDTA, 60 mM HEPES-NaOH, pH 7.4). Any un-homogenized cells and partially disrupted cells were sedimented at 100 g for 4 min; the pellets were re-homogenized in the same medium after being washed twice; all of the supernatants were bulked together. A post-nuclear + heavy mitochondria supernatant was prepared at 2,500 g for 10 min (the pellet was washed and the supernatants combined). This supernatant was loaded on to a discontinuous gradient of 5, 10, 15, 20, 25 and 30% (w/v) iodixanol. These solutions were prepared from dilutions of OptiPrep™ with the homogenization buffer (see Application Sheet V01 for more information about the preparation of gradient solutions). The gradients were centrifuged at 288,000 g for 2 h (Beckman SW41Ti rotor). They were used to investigate the effects of treatment of cells with a cationic peptide Deca-(arg)8, which has been shown have significant anti-viral activity. Abdul et al [6] demonstrated that the DNA, nucleocapsid and preS/S proteins banded quite broadly towards the bottom of the gradient (ρ = 1.14/1.22 g/ml), although some capsids and preS/S banded at a slightly lower density. Deca-(arg)8 treatment caused a marked shift of the DNA and core protein to a lower and more sharply-defined density while the preS/S proteins shifted to a similarly well-defined higher density. The gradient thus seems to offer a high-resolution analysis of these macromolecules.
3. Recent published papers
Komatsu et al [7] used a discontinuous iodixanol gradient in a small volume fixed-angle rotor (6%, 10%, 20%, 30%, 40%, 50% (w/v), i.e covering a similar density range to that described in refs 3 and 4. The authors used a small-volume high performance rotor (probably the Beckman TLA-110 or similar) for 4 h at approx 400,000 gav to purify the virus from patient specimens. The gradient would undoubtedly become continuous (due to diffusion and self-generation) during this time, but whether these centrifugation conditions would allow a discrimination of enveloped and non-enveloped virions is not clear. Verrier et al [8] also investigated the virus isolated from patient specimens in a 10-45% (w/v) iodixanol gradient but did not provide any further experimental detail. Virus was also isolated both from HepG2215 cells and patient plasma [9]. Li et al [10] refer to a method first used by Feng et al [11] for hepatitis A virus which involves the use of an 8-40% (w/v) iodixanol gradient, centrifuged at 141,000 g for 48 h. It is not clear if the virus banding was similar or different. In the studies by Lam et al [12] the hepatitis B virus that was expressed into the culture fluid from HepaRG cells was analyzed on an 18-50% iodixanol gradient centrifuged at 134,000 g for 2 h.
4. References
1. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445Dettenhoffer, M. and Yu, XF. (1999) J. Virol., 73, 1460-1467
2. Kock, J., Kann, M., Pütz, G., Blum, H.E. and von Weizsäcker, F. (2003) Central role of a serine phosphorylation site with duck hepatitis B virus core protein for capsid trafficking and genome release . Biol. Chem., 278, 28123-28129
3. Wang, H., Kim, S. and Ryu, W-S. (2009) DDX3 DEAD-Box RNA helicase inhibits hepatitis B virus reverse transcription by incorporation into nucleocapsids J. Virol., 83, 5815–5824
4. Kim, S., Lee, J. and Ryu, W-S. (2009) Four conserved cysteine residues of the hepatitis B virus polymerase are critical for RNA pregenome encapsidation J. Virol., 83, 8032-8040
5. Bardens, A., Döring, T., Stieler, J. and Prange, R. (2011) Alix regulates egress of hepatitis B virus naked capsid particles in an ESCRT-independent manner Cell. Microbiol., 13, 602–619
6. Abdul, F., Ndeboko, B., Buronfosse, T., Zoulim, F., Kann, M., Nielsen, P.E. and Cova, L. (2012) Potent inhibition of late stages of hepadnavirus replication by a modified cell penetrating peptide PLoS One, 7: e48721
7. Komatsu, H., Inui, A., Murano, T., Tsunoda, T., Sogo, T. 2 and Fujisawa, T. (2015) Lack of infectivity of HBV in feces from patients with chronic hepatitis B virus infection, and infection using chimeric mice BMC Res. Notes, 8: 366
8. Verrier, E.R., Colpitts, C.C., Bach, C., Heydmann, L., Weiss, A., Renaud, M., Durand, S.C., Habersetzer, F., Durante, D. et al (2016) A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses Hepatology, 36, 35-48
9. Zannetti, C., Roblot, G., Charrier, E., Ainouze, M., Tout, I., Briat, F., Isorce, N., Faure-Dupuy, S., Michelet, M. et al (2016) Characterization of the inflammasome in human Kupffer cells in response to synthetic agonists and pathogens J. Immunol., 197, 356-367
10. Li, F., Cheng, L., Murphy, C.M., Reszka-Blanco, N.J., Wu, Y., Chi, L., Hu, J. and Su, L. (2016) Minicircle HBV cccDNA with a Gaussia luciferase reporter for investigating HBV cccDNA biology and developing cccDNA-targeting drugs Sci. Rep., 6: 36483
11. Feng, Z., Hensley, L., McKnight, K.L., Hu, F., Madden, V., Ping, L-F., Jeong, S-H., Walker, C., Lanford, R.E. and Lemon, S.M. (2013) A pathogenic picornavirus acquires an envelope by hijacking cellular membranes Nature 496, 367-371
12. Lam, A.M., Ren, S., Espiritu, C., Kelly, M., Lau, V., Zheng, L., Hartman, G.D., Flores, O.A. and Klumpp, K. (2017) Hepatitis B virus capsid assembly modulators, but not nucleoside analogs, inhibit the production of extracellular pregenomic RNA and spliced RNA variants Antimicrob. Agents Chemother., 61: e00680-17
OptiPrep™ Application Sheet V36; 4th edition, January 2020
OptiPrep™ Application Sheet V37
Purification of viruses from non-mammalian cells and tissues
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number - This Application Sheet describes principally the isolation of viruses (Phycodnaviridae) from algal cells (Section 1), but also viruses from protozoa (Section 2), nematodes (Section 3), marine arthropods (Section 4), plant cells (Section 5) and yeast (Section 6).
- Note that Application Sheet V13 describes the purification of the Group I iridovirus isolated from an aquatic species (Singapore grouper fish)
- Note that Application Sheet V38 describes the purification of bacteriophages
1. Isolation of viruses from algal sources (Phycodnaviridae)
Lawrence and Steward [1], recommended making all iodixanol gradient solutions by dilution of OptiPrep™ with the regular culture medium. This will maintain the normal osmolality of the solutions. This approach has been adopted by many authors.
Moniruzamann et al [2] purified the Megaviridae virus Aureococcus anophagefferns (trivial name “brown-tide virus”) using a discontinuous gradient of 2.6 ml each of 25%, 30%, 35% and 40% (w/v) iodixanol, on to which 1.5 ml of sample was layered and centrifuged at 185,000 g for 14 h. Certainly during this time the gradient would have become more or less continuous. Lawrence and Steward [1] recommended the use of a 4 ml gradient (total volume) with 1 ml of sample at approx. for 200,000 g, for 4.25 h, or in a scaled-up version (13 ml tubes in a Beckman SW41 rotor) at approx. the same g-force for 7.25 h. The authors also noted that for most downstream techniques (except electron microscopy) it was not necessary to remove the iodixanol and Malitsky et al [3] observed that the iodixanol gradient maintained the virus morphology and infectivity.
Essentially the same gradient and centrifugation conditions were used by Rosenwasser et al [4] and Schatz et al [5] for the purification of Emiliania huxley virus.
Paramecium bursaria Chlorella virus 1 (a “Giant” virus) was treated with 1% (v/v) NP-40 before sedimenting and resuspension in 50 mM Tris-HCl, pH 7.8 before initial loading on to a 10-40% (w/v) sucrose gradient and centrifugation for 20 min at 48,000 gav. The recovered banded virus was treated with proteinase K to remove contaminating proteins before being centrifuged into a 20-40% (w/v) iodixanol gradient at 48,000 gav, for 4h [6]. Dunigan et al [7] reported the same methodology and that the banding density of the virus was 1.178 g/ml (equivalent to approx. 33% (w/v) iodixanol. Working with the Acanthocytosis turfacea Chlorella virus, Petro et al [8] replaced the sucrose gradient with an iodixanol gradient and after treating with proteinase K to remove extraneous protein, treated the virus to a third round of iodixanol gradient centrifugation.
2. Isolation from protozoa (amoeba)
Faustovirus (Vermamoeba vermiformis) is an unusually dense virus, probably because it has two protein shells and the iodixanol gradient used to purify it is correspondingly dense [9]: the top-loaded 12 ml 40-60% (w/v) iodixanol gradient was centrifuged at 100,000 g for 24 h. The gradient effectively separated the intact virus from empty virions.
3. Orsay virus
Orsay virus infects nematodes (Caenorhabditis elegans or Caenorhabditis briggsae): it has unusually been purified in three stages [10]. In the first stage the virus in a buffered saline medium containing 2-mercaptoethanol and Triton X-100 was sedimented through a four step discontinuous iodixanol gradient, comprising 15%, 25%, 32.5% and 40% (w/v) iodixanol at 150,000 g for 3 h. The iodixanol solutions contained a routine buffered saline containing EDTA and 2-mercaptoethanol; the 15% layer however contained 1 M NaCl. In this respect the method is very similar to that used for the purification of recombinant adeno-associated virus (rAAV) devised by Zolotukhin et al [11]; the high salt is present to minimize aggregation of the virus with soluble proteins at the first two interfaces. While the gradients are very similar, the centrifugation conditions are rather different: 150,000 g for 3 h for the Orsay virus and 350,000 g for 1 h for the rAAV. The former conditions used by Jiang et al [10] are likely to reduce any aggregation to a minimum.
The iodixanol in the harvested virus was then removed by ultrafiltration; the suspension treated with 1.0% Triton X100 and applied to a second gradient (25-45% w/v iodixanol), which also contained EDTA and 2-mercaptoethanol, and centrifuged at 150,000 gav for 16 h. After harvesting the virus, it was adjusted to 40% (w/v) iodixanol and re-centrifuged in a near-vertical rotor (Beckman NVT90) at 250,000 g for 1.5 h. This step uses the ability of iodixanol to form self-generated gradients. It is important to use either a near-vertical or a vertical rotor to allow the formation of gradient with a useful density profile. Some small volume fixed-angle rotors can be used in place of the near-vertical rotor.
- For more information on the formation of self-generated gradients, using iodixanol, see Application Sheet V03.
- The authors [10] commented that iodixanol gradients permitted the recovery of virus of much higher purity, compared to that from CsCl-purified gradients. Moreover it permitted the detection of particles containing a minor post-translational modified variant of the fusion protein
4. From marine arthropods
White spot syndrome virus (WSSV) is a major pathogen for marine crustaceans; it has been isolated from Penaeus vannamei by Dantas-Lima et al [12]. After maceration of the shrimps in a buffered saline, large contaminants were removed from the suspension (3000 g for 20 min) the suspension (60 ml) was underlaid with a 10 ml cushion of 50% (w/v) iodixanol and centrifuged at 60,000 g for 2 h to concentrate the virus at the interface. After removal of the bottom 7.5 ml of cushion (this is best achieved by using a syringe attached to a long flat-tipped metal filling cannula, i.d. approx 1mm – see Application Sheet V02 for more details). The bottom 5 ml of the remaining material is then recovered (i.e 2.5 ml of 50% idixanol + virus band + 2.5 ml of maceration medium) and layered under a discontinuous iodixanol gradient of 5%, 10%, 15% and 20% (w/v) iodixanol and centrifuged at 80,000 g for 3 h. The virus banding pattern depended on whether the original harvest was from tissues or haemolymph. For more information on this see ref 12.
- The authors commented on the very high purity and infectivity of the virus recovered from iodixanol gradients compared to that from CsCl gradients
- 5. From plant cells
- Because of the relative paucity of information on plant viruses this section summarizes information from papers reporting the use of both NycodenzⓇ and OptiPrep™. Section 5-1 is concerned with the isolation of plant viruses using NycodenzⓇ gradients. As far as we know only red clover necrotic mosaic virus has been purified in iodixanol gradients (Section 5-2-2). The latter has been used more widely however for isolation of animal viruses grown in plant cells (these methods are summarized in Section 5-2-1).
- 5-1 Isolation of plant viruses in NycodenzⓇ
One of the earliest papers that reported the use of Nycodenz® was that by Gugerli [13] who studied the banding of a number of plant viruses: various luteoviruses, tymoviruses, nepoviruses, tobamoviruses (including tobacco mosaic virus) and hodeiviruses. Gradients in the range 30-60% (w/v) Nycodenz® were used and the viruses generally had densities in the range 1.23-1.28 g/ml and significant advantages over the use of CsCl were noted (1.23 g/ml is approx equivalent to 42% Nycodenz®). Habili et al [14] reported the use the same gradients for a luteovirus (barley yellow dwarf virus). Cowpea mosaic virus consists of two separately encapsidated RNA molecules which, along with empty capsids, can also be fractionated on these 30-60% Nycodenz® gradients at approx 160,000 g for 15 h at 15°C.. The empty capsids band at a low density, while the other two particles are usually termed “middle” and “bottom” [15-18], reflecting their position in the gradient.
5-2 OptiPrep™-based methods
5-2-1 Virus-like particles propagated in plant cells
Blue-tongue virus-like particles (BTVLP) have been propagated in Nicotiania benthamiana using a plant expression system. The harvesting procedure involved the use of a 20-50% (w/v) iodixanolgradient generated from 3 ml each of four solutions (10% increment), which was overlaid by 24 ml of leaf extract and centrifuged at 85,000 gmax for 3h. The virus banded in the 30-40% iodixanol zone [19]. Brillault et al [20] used a very similar gradient. Van Zyl et al [21] first concentrated the BTVLP on to a 40% iodixanol cushion (79,000 g for 2 h) then analyzed it on a discontinuous iodixanol gradient (20- 60%) under the same centrifugation conditions. Similar gradients were used for purifying polyoma VLPs, also propagated in Nicotiania benthamiana leaves [22].
HIV virus-like particles (VLPs), also grown in Nicotiania benthamiana, were layered on top of a 10%, 20%, 30%, 40%, 50%, 60% (w/v) iodixanol gradient and centrifuged 210,000 g for 4 h. The VLPs banded around the original position of the 10%-20% interface [23, 24]. Human papillomavirus pseudovirions have also been grown in Nicotiania benthamiana. Larger particles from the plant extract were first removed by low speed centrifugation (10,000 g for 15 min) and concentrated in a two layer (30% – 50%) sucrose gradient at approx. 110,000 g for 75 min. After recovery of the VLPs and dialysis to remove the sucrose, they were layered atop a 20%, 33%, 40%, 50% (w/v) iodixanol gradient and centrifuged at approx. 110,000 g for 4h. The VLPs banded around 33% iodixanol [25].
- A review of plant-grown viruses and VLPs has recently been published [26].
- 5-2-2 Red clover necrotic mosaic virus (RCNMV)
Lockney et al [27, 28] studied the potential for RCNMV capsid to be used in specific cell targeting in the delivery of therapeutic reagents. The capsid was labeled with a fluorescent peptide but the authors gave rather little detailed information about the purification procedure. A generic 0-54% (w/v) iodixanol gradient (containing 20 mM Tris-HCl, pH 8.0, 120 mM NaCl, 1 mM EDTA) centrifuged at 175,000 g for 2 h may be a good starting point. The fluorescent-marked particles banded about twothirds the way down the gradient. It may be necessary to modulate the centrifugation time (or speed).
6 Yeast retrotransposons
Tf1 retrotransposons are being studied as important models for the activity of retroviruses. They have similar structures and propagation mechanisms. Kim et al [29] used the discontinuous iodixanol gradient method that is widely used for the purification of rAAV (see Application Sheet V14). Various Gag species were identified in the denser regions of the gradient that were assembled into virus-like particles (VLPs); these were well separated from the lighter ones that did not assembles into such particles [29]. Ty1 retrotransposons in VLPs were resolved in a discontinuous 5-50% (w/v) iodixanol gradient centrifuged at approx 200,000 g for 3h [30]
7 References
1. Lawrence, J.E. and Steward, G.F. (2010) In Manual of Aquatic Viral Ecology Chapter 17 (Eds. Wilhelm, S.W., Weinbauer, M.G. and Suttle, C.A. American Society of Limnology and Oceanography, Inc. pp 166– 181
2. Moniruzzaman, M., LeCleir, G.R., Brown, C.M., Gobler, C.J., Bidle, K.D., Wilson, W.H. and Wilhelm, S.W. (2014) Genome of brown tide virus (AaV), the little giant of the Megaviridae, elucidates NCLDV genome expansion and host–virus coevolution Virology, 466-467, 60–70
3. Malitsky, S., Ziv, C., Rosenwasser, S., Zheng, S., Schatz, D., Porat, Z., Ben-Dor, S., Aharoni, A. and Vardi, A. (2016) Viral infection of the marine alga Emiliania huxleyi triggers lipidome remodeling and induces the production of highly saturated triacylglycerol New Phytol., 210, 88–96
4. Rosenwasser, S., Mausz, M.A., Schatz, D., Sheyn, U., Malitsky, S., Aharoni, A., Weinstock, E., Tzfadia, O.,Ben-Dor, S., Feldmesser, E. et al (2014) Rewiring host lipid metabolism by large viruses determines the fate of Emiliania huxleyi, a bloom-forming alga in the ocean Plant Cell, 26, 2689–2707
5. Schatz, D., Shemi, A., Rosenwasser, S., Sabanay, H., Wolf, S.G., Ben-Dor, S. and Vardi, A. (2014) Hijacking of an autophagy-like process is critical for the life cycle of a DNA virus infecting oceanic algal blooms New Phytologist, 204, 854–863
6. Wulfmeyer, T., Polzer, C., Hiepler, G., Hamacher, K., Shoeman, R., Dunigan, D.D., Van Etten, J.L., Lolicato, M., Moroni, A., Thiel, G. and Meckel, T. (2012) Structural organization of DNA in chlorella viruses PLoS One, 7: e30133
7. Dunigan, D.D., Cerny, R.L., Bauman, A.T., Roach, J.C., Lane, L.C., Agarkova, I.V., Wulser, K., YanaiBalser, G.M., Gurnon, J.R., Vitek, J.C., Kronschnabel, B.J., Jeanniard, A., Blanc, G., Upton, C., Duncan, G.A., McClung, O.W., Ma, F. and Van Ettena, J.L. (2012) Paramecium bursaria chlorella virus 1 proteome reveals novel architectural and regulatory features of a giant virus J. Virol., 86, 8821-8834
8. Petro, T.M., Agarkova, I.V., Zhou, Y., Yolken, R.H., Van Etten, J.L. and Dunigana, D.D. (2015) Response of mammalian macrophages to challenge with the chlorovirus Acanthocystis turfacea chlorella virus 1 J. Virol., 89, 12096-12107
9. Klose, T., Reteno, D.G., Benamar, S., Hollerbach, A., Colson, P., La Scola, B. and Rossmann, M.G. (2016) Structure of faustovirus, a large dsDNA virus Proc. Natl. Acad. Sci. USA, 113, 6206-6211
10. Jiang, H., Franz, C.J., Wu, G., Renshaw, H., Zhao, G., Firth, A.E. and Wang, D. (2014) Orsay virus utilizes ribosomal frameshifting to express a novel protein that is incorporated into virions Virology 450-451, 213– 221
11. Zolotukhin, S., Byrne, B.J., Mason, E., Zolotukhin, I., Potter, M., Chesnut, K., Summerford, C., Samulski, R.J. and Muzyczka, N. (1999) Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield Gene Ther., 6, 973-985
12. Dantas-Lima, J.J., Corteel, M., Cornelissen, M., Bossier, P., Sorgeloos, P. and Nauwynck, H.J. (2013) Purification of white spot syndrome virus by iodixanol density gradient centrifugation J. Fish Dis., 36, 841– 851
13. Gugerli, P. (1984) Isopycnic centrifugation of plant viruses in Nycodenz density gradients J. Virol. Meth., 9, 249-258
14. Habili, N., McInnes, J.L. and Symons, R.H. (1987) Nonradioactive, photobiotin-labelled DNA probes for the routine diagnosis of barley yellow dwarf virus J. Virol. Meth., 16, 225-237
15. Holness, C.L., Lomonossoff, G.P, Evans, D. and Maule, A.J. (1989) Identification of the initiation codons for translation of cowpea mosaic virus middle component RNA using site-directed mutagenesis of an infectious cDNA clone Virology, 172, 311-320 15
16. Dessens, J.T. and Lomonossoff, G.P. (1991) Mutational analysis of the putative catalytic triad of the cowpea mosaic virus 24K protease Virology, 184, 738-746 16
17. Taylor, K.M., Spall, V.E., Butler, J.G. and Lomonossoff, G.P. (1999) The cleavable carboxyl-terminus of the small coat protein of cowpea mosaic virus is involved in RNA encapsidation Virology, 255, 129-137
18. King, D.P., Montague, N., Ebert, K., Reid, S.M., Dukes, J.P., Schädlich, L., Belsham, G.J. and Lomonossoff, G.P. (2007) Development of a novel recombinant encapsidated RNA particle: Evaluation as an internal control for diagnostic RT-PCR J. Virol. Meth., 146, 218-225
19. Thuenemann, E.C., Meyers, A.E., Verwey, J., Rybicki, E.P. and Lomonossoff, G.P. (2013) A method for rapid production of heteromultimeric protein complexes in plants: assembly of protective bluetongue viruslike particles Plant Biotechnol. J. 11, 839–846 20. Van Zyl, A.R., Meyers, A.E. and Rybicki, E.P. (2016) Transient Bluetongue virus serotype 8 capsid protein expression in Nicotiana benthamiana Biotech. Rep., 9, 15–24
21. Brillault, L., Jutras, P.V., Dashti, N., Thuenemann, E.C., Morgan, G., Lomonossoff, G.P., Landsberg, M.J.
and Sainsbury, F. (2017) Engineering recombinant virus-like nanoparticles from plants for cellular delivery ACS Nano,11, 3476−3484
22. Catrice, E.V.B. and Sainsbury, F. (2015) Assembly and purification of polyomavirus-like particles from plants Mol. Biotechnol., 57, 904–913
23. Kessans, S.A., Linhart, M.D., Matoba, N. and Mor, T. (2013) Biological and biochemical characterization of HIV-1 Gag/dgp41 virus-like particles expressed in Nicotiana benthamiana Plant Biotech. J., 11, 681–690
24. Meador, L.R., Kessans, S.A., Kilbourne, J., Kibler, K.V., Pantaleo, G., Esteban Roderiguez, M., Blattman, J.N., Jacobs, B.L. and Mor, T.S. (2017) A heterologous prime-boosting strategy with replicating Vaccinia virus vectors and plant-produced HIV-1 Gag/dgp41 virus-like particles Virology, 507, 242–256
25. Lamprecht, R.L., Kennedy, P., Huddy, S.M., Bethke, S., Hendrikse, M., Hitzeroth, I.I. and Rybicki, E.P. (2016) Production of human papillomavirus pseudovirions in plants and their use in pseudovirion-based neutralization assays in mammalian cells Sci. Rep., 6: 20431
26. Van Zyl, A.R. and Hitzeroth, I.I. (2016) Purification of virus-like particles (VLPs) from plants In VaccineDesign: Methods and Protocols, Vol. 2: Vaccines for Veterinary Diseases, Methods in Molecular Biology, vol. 1404 (ed. Thomas, S.) Springer Science+Business Media New York pp 569-579
27. Lockney, D.M., Guenther, R.N., Loo, L., Overton, W., Antonelli, R., Clark, J., Hu, M., Luft, C., Lommel,S.A. and Franzen, S. (2011) The Red clover necrotic mosaic virus capsid as a multifunctional cell targetingplant viral nanoparticle Bioconjugate Chem. 22, 67–73
28. Lockney, D., Franzen, S. and Lommel, S. (2011) Viruses as nanomaterials for drug delivery In Biomedical Nanotechnology: Methods and Protocols, Methods Mol. Biol., 726 (ed. Hurst, S.J.), Springer Science+Business Media, pp 207-2217
29. Kim, M-K., Claiborn, K.C. and Levin, H.L. (2005) The long terminal repeat-containing retrotransposon Tf1 possesses amino acids in gag that regulate nuclear localization and particle formation J. Virol., 79, 9540- 9555
30. Moore, S.P. and Garfinkel, D.J. (2009) Functional analysis of N-terminal residues of Ty1 integrase J. Virol., 83, 9502-9511
OptiPrep™ Application Sheet V37; 7th edition March 2020
OptiPrep™ Application Sheet V38
Purification and analysis of bacteriophages
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- This Application Sheet summarizes the use of iodixanol gradients for the purification of:
1. A lipid-containing marine bacteriophage PM2
2. Bacteriophages DSSE3Φ2 and EE36Φ1 and virus-like particles from marine roseobacters
3. Proheads from bacteriophage Φ29
4. Bacteriophage KPP12
5. Podoviridae phage C1
6. Pyrococcus abyssi and Thermococus prieurii
7. Bacteriophage S13’
8. Bacteriophage CP-51
9. Cyanophage SEIV-1
10. Bacteriophage ΦM9
11. Thermotagales-infecting virus
12. Virus-like particles (VLPs) LPs from E. coli (Acinetobacterphage)
13. Purification of the ‘phage T4 base-plate complex
14. MS-2 phage
15. Qβ phages
- Whether any of the methods can be applied to other bacteriophages with similar morphology, macromolecular composition and size can only be determined experimentally.
- To access other Application Sheets referred to in the text: return to the 2020Virapp file and select the appropriate V number.
1. Lipid-containing bacteriophage PM2
1a. Introduction
This method for purifying PM2 was devised by Kivela et al [1,2]. Other lipid-containing bacteriophages are known to exist, such as PRD1 and Φ6, which resemble adenoviruses (see ref 1 for details) and the iodixanol gradient described in this Application Sheet may also be applicable to these particles. The methodology may be broadly applicable to any bacteriophage, but those not containing any lipid would probably have higher densities, so the density range of the gradient for non-lipid-containing bacteriophages may require modulation for such particles.
The PM2 bacteriophage is particularly sensitive to the type of medium, which is used to purify the particles, and loss of infectivity is a major problem with the more traditional media such as CsCl and sucrose. In CsCl the PM2 bacteriophage has a banding density of approx 1.28 g/ml and in sucrose the value is approx 1.26 g/ml [1]. In 55% (w/v) sucrose (1.26 g/ml) there is a rapid loss of infectivity, the specific infectivity of the bacteriophage was reduced by as much as 98%, while the effect of CsCl was to drastically reduce the overall yield of virus recovered from the gradient rather than directly inhibit infectivity.
Because of the problems of CsCl at all concentrations and of sucrose at the high concentrations required to band the virus according to buoyant density, Kivela et al [1,2] have retained an initial 5- 20% rate zonal sucrose gradient but then replaced the subsequent sucrose (or CsCl) buoyant density gradient with an iodixanol gradient. It should be noted that Dettenhoffer and Yu [3] used a rate-zonal iodixanol gradient to purify HIV1 (for more information see Application Sheet V34) and it may be feasible to use this also for the preliminary purification (see next page) of PM2. The following protocol is adapted from refs 1 and 2.
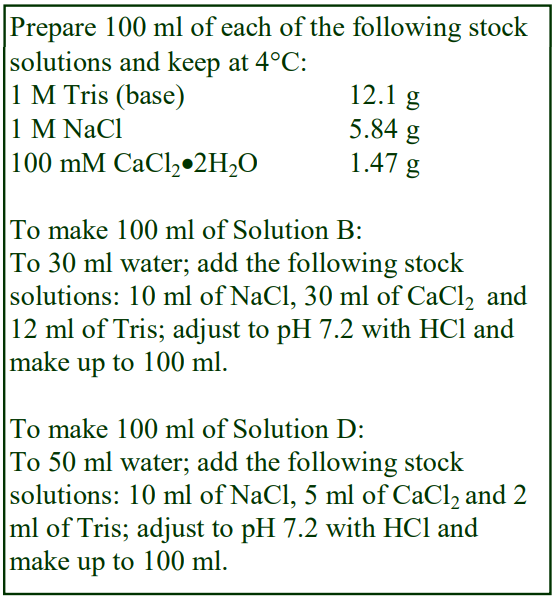 1b. Solutions required
1b. Solutions required
A. OptiPrep™ (60%, w/v iodixanol)
B. OptiPrep™ diluent: 100 mM NaCl, 30 mM CaCl2, 120 mM Tris-HCl, pH 7.2
C. OptiPrep™ Working Solution (50% iodixanol): mix 5 vol of Solution A with 1 vol of Solution B.
D. PM2 buffer: 100 mM NaCl, 5 mM CaCl2, 20 mM TrisHCl, pH 7.2.
1c. Ultracentrifuge rotor requirements
Swinging-bucket rotor with 4-5 ml tubes Beckman SW 50Ti, Sorvall TH660 or equivalent).
1d. Protocol
1d-1. Preliminary purification (see Note 1)
1. Precipitate the virus from the clarified fluid in 8% (w/v) polyethylene glycol (PEG 6000) at 4°C.
2. Suspend the pellet in 1 M NaCl, 10 mM CaCl2 20 mM Tris-HCl, pH 7.2 and load on to linear sucrose gradients (5-20%, w/v) in the same buffer (see Note 2).
3. Centrifuge at 75,000gav for 1h 10 min at 15°C and collect the banded virus.
4. Pellet the virus at 75,000gav for 3.5h min at 5°C and then resuspend the virus in Solution D (700 μl/liter of lysate).
1d-2. OptiPrep™ purification
1. Dilute Solution C with Solution D to prepare 5% and 40% (w/v) iodixanol solutions
2. Make linear 5-40% iodixanol gradients using a two-chamber gradient maker or Gradient Master™ in tubes (4-5 ml) for a swinging-bucket rotor and load the virus suspension on top (see Note 3).
3. Centrifuge at 200,000g for 16 h at 10°C.
4. PM2 bacteriophage bands at 1.16 g/ml in iodixanol. Collect the virus bands and process as required (see Note 4).
1e. Notes
1. The preliminary purification steps described briefly here are described in detail by Kivela et al [1,2]. Other strategies may be used prior to the purification in the subsequent overnight iodixanol gradient.
2. To avoid the exposure of the virus to different types of gradient media it may be possible to substitute a rate-zonal iodixanol gradient for the sucrose one. Dettenhoffer and Yu [3] used a 6- 18% iodixanol gradient with centrifugation at 250,000g for 1.5 h for purifying HIV-1; see Application Sheet V34.
3. If neither of these devices is available create a continuous gradient by allowing a discontinuous gradient (5%, 17%, 28%, 40% iodixanol) to diffuse; for more information on making continuous gradients see Application Sheet V02.
4. It should be noted that any add-on purification steps such as HPLC, re-infection of microorganisms and many analytical techniques could be carried out directly on gradient fractions without the need to remove the gradient medium since iodixanol is non-ionic and very “particle-friendly”.
2. Bacteriophages DSSE3Φ2 and EE36Φ1 and virus-like particles from marine roseobacters
Zhao et al [4,5] concentrated the bacteriophages from clarified lysates of cultured roseobacter cells by polyethylene glycol precipitation; after resuspension in culture medium they were loaded on to a 10- 50% (w/v) iodixanol gradient. The gradients for the purification of the DSSE3Φ2 and EE36Φ1 bacteriophages were centrifuged at 200,000 g for 2 h [4]; the gradients for the detection of induced virus-like particles were centrifuged for half the time [5]. Roseobacter phages were also purified in iodixanol gradients by Zhan et al [6] for DNA analysis and genome sequencing [7].
3. Proheads from bacteriophage Φ29
Iodixanol gradients were used in the analysis of the structure of bacteriophage Φ29, the details of which are outside the scope of this Application Sheet; for information see refs 8 and 9.
4. Bacteriophage KPP12
This method was developed by Fukuda et al [10]. The phage was isolated from Pseudomonas aeruginosa cells and after lysis of the latter, the phage was PEG precipitated. OptiPrep™ was diluted with saline to produce gradient solutions of 30%, 35% and 40% (w/v) iodixanol and the crude phage suspension layered on top of a discontinuous gradient formed from the three solutions. The phage banded in the gradient after centrifugation at 200,000 g for 2 h. The density range of the isoosmotic gradient was approx. 1.16–1.22 g/ml; by comparison a CsCl gradient in the range 1.3-1.7 g/ml was required for similar banding. This highlights the big advantages of using iodixanol over CsCl; solutions of the latter are not only toxic, they are also hugely hyperosmotic and dialysis is essential before further analysis. Fukuda et al [10] found that the material isolated in iodixanol gradients was extremely stable.
5. Podoviridae phage C1 [11]
The phage, grown in Streptococcus was suspended in 0.2 M NaCl, 10 mM MgSO4, 20 mM TrisHCl, pH 7.4. After concentration on to a 50% (w/v) iodixanol cushion and then purified in a 15-35% (w/v) iodixanol gradient (in the same buffer). Centrifugation was carried out at 200,000 g for 2 h. For convenience the gradient purification can be executed by flotation; the virus can collected along with part or all of the 50% iodixanol cushion (from the concentration step) and then diluted as required to 35% iodixanol. More information on handling viruses from concentration steps can be found in Application Sheet V06.
6. Archeaviruses [12]
Gorlas, A. and Geslin, C. [12] chose iodixanol as the preferred gradient for the purification and analysis of Pyrococcus abyssi and Thermococcus prieurii over the commonly-used CsCl because of the significant improvement in the recovery of infectivity. The ‘phages were purified in 30-45% iodixanol gradients that were centrifuged at 180,000 g for 6 h.
7. Bacteriophage S13’ [13]
The ability of this ‘phage to infect Staphylococcus aureus is of great potential therapeutic value as a possible treatment for respiratory diseases caused by this bacterium. The ‘phage was purified in a discontinuous iodixanol gradient (30%, 35%, 40% w/v iodixanol) centrifuged for 2h at 200,000 g [13] and then subjected to a second gradient of just two layers (30% and 40%) to band the virus sharply at the interface [14].
8. Bacteriophage CP-51 [15]
Klumpp et al [14] also stressed the possible use of iodixanol-purified phage-based diagnostics/ therapeutics for the Bacillus ACT group of pathogens
9. Cyanophage S-EIV-1 [16]
After filtration of the bacterial lysate through a 0.45 μm filter, the ‘phage was concentrated by ultrafiltration loaded onto a discontinuous 20, 30, 40, 50% (w/v) iodixanol gradient and centrifuged at approx 85,000 g for 8 h to band the ‘phage.
10. Bacteriophage ΦM9 [17]
In a similar approach to that outlined in 7 (above), the ‘phage was purified first in a continuous iodixanol gradient 10-50% (w/v) iodixanol at 200,000 g for 2 h. Following harvesting the ‘phage was suspension was adjusted to approx. 20% (w/v) iodixanol to allow application on to a discontinuous gradient of 35% and 50% iodixanol and centrifuged at 200,000 g for 3 h to allow concentration of the ‘phage at the interface.
11. Thermotagales-infecting virus
Viruses that occur in the bacteria of deep-sea hydrothermal ecosystems have been investigated by Lossouarn et al [18]. The tailed hexagonal bacterium was purified in an 30-45% (w/v) iodixanol gradient (OptiPrep™ was diluted with a Tris-buffered 100 mM NaCl, 5 mM CaCl2, 20 mM MgCl2) at 40,000 g for 5 h. The position of the well-defined virus band was not stated in the paper.
12. Virus-like particles (VLPs) LPs from E. coli [19]
VLPs bearing the Acinetobacter phage AP205 capsid protein were grown in E. coli) and purified in an iodixanol gradient adapted from that described in Application Sheet V10 for papillomavirus. The top-loaded discontinuous gradient of 23%, 29% and 35% (w/v) iodixanol (OptiPrep diluted with phosphate-buffered saline) was centrifuged at approx. 300,000 g for 3 h (at 16 C). Acinetobacter phage was also purified in studies on VLP vaccines [20].
13. Purification of the ‘phage T4 base-plate complex [21]
The base-plate complex was purified in a 10-40% (w/v) iodixanol gradient (plus a 50% cushion), centrifuged at 35,000 g for 24 h.
14. MS-2 phage
The phage was adjusted to 20% (w/v) iodixanol; underlaid by 40% iodixanol and centrifuged at 160,000 g for 7 h in a swinging-bucket rotor [22]. Dai et al [23] used a top-loaded 10, 20, 30, 40, 50% (w/v) iodixanol gradient centrifuged at 100,000 g over night.
15. Qβ-phages
The crude phage suspension was adjusted to 20% (w/v) iodixanol; layered over an equal volume of 40% iodixanol and centrifuged at 36,000 rpm for 18 h at 15°C to band the phage [24].
16. Bacteriophage ΦM5
The crude phage suspension was purified by layering on top of a 10, 20, 30, 40, 50% (w/v) iodixanol gradient, centrifuged at 100,000 g overnight [25]
15. References
1. Kivela, H. M., Mannisto, R. H., Kalkkinen, N. and Bamford, D. H. (1999) Purification and protein composition of PM2, the first lipid-containing bacterial virus to be isolated Virology, 262, 364-374
2. Kivela, H. M., Kalkkinen, N. and Bamford, D. H. (2003) Bacteriophage PM2 has a protein capsid surrounding a spherical proteinaceous lipid core J. Virol., 76, 8169-8178
3. Dettenhoffer, M. and Yu, X-F. (1999) Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif virions J. Virol., 73, 1460-1467
4. Zhao, Y., Wang, K., Jiao, N. and Chen, F. (2009) Genome sequences of two novel phages infecting marine roseobacters Environ. Microbiol., 11, 2055–2064
5. Zhao, Y., Wang, K., Ackermann, H-W., Halden, R.U., Jiao, N. and Chen, F. (2010) Searching for a “hidden” prophage in a marine bacterium Appl. Environ. Microbiol., 76, 589-595
6. Zhan, Y., Huang, S., Voget, S., Simon, M. and Chen, F. (2016) A novel roseobacter phage possesses features of podoviruses, siphoviruses, prophages and gene transfer agents Sci. Rep., 6: 30372
7. Li, B., Zhang, S., Long, L. and Huang, S. (2016) Characterization and complete genome sequences of three N4-like Roseobacter phages isolated from the South China sea Curr. Microbiol., 73, 409–418
8. Tao, Y., Olson, N. H., Xu, W., Anderson, D. L., Rossmann, M. G. and Baker, T.S. (1998) Assembly of a tailed bacterial virus and its genome release studied in three dimensions Cell, 95, 431-437
9. Sun, J., Cai, Y., Moll, W-D. and Guo, P. (2006) Controlling bacteriophage phi29 DNA-packaging motor by addition or discharge of a peptide at N-terminus of connector protein that interacts with pRNA Nucleic Acids Res., 34, 5482-5490
10. Fukuda, K., Ishida, W., Uchiyama, J., Rashe, M., Kato, S-i., Morita, T., Muraoka, A., Sumi, T., Matsuzaki, S., Daibata, M. and Fukushima, A. (2012) Pseudomonas aeruginosa keratitis in mice: effects of topical bacteriophage KPP12 administration PLoS One, 7: e47742
11. Aksyuk, A.A., Bowman, V.D., Kaufmann, B., Fields, C., Klose, T., Holdaway, H.A., Fischetti, V.A. and Rossmann, M.G. (2012) Structural investigations of a Podoviridae streptococcus phage C1, implications for the mechanism of viral entry Proc. Natl. Acad. Sci. USA, 109, 14001-14006
12. Gorlas, A. and Geslin, C. (2013) A simple procedure to determine the infectivity and host range of viruses infecting anaerobic and hyperthermophilic microorganisms Extremophiles 17, 349–355
13. Takemura-Uchiyama, I., Uchiyama, J., Osanai, M., Morimoto, N., Asagiri, T., Ujihara, T., Daibata, M., Sugiura, T. and Matsuzaki, S. (2014) Experimental phage therapy against lethal lung-derived septicemia caused by Staphylococcus aureus in mice Microbes Infect., 16, 512-517
14. Takemura-Uchiyama, I., Uchiyama, J., Kato, S-i., Inoue, T., Ujihara, T., Ohara, N., Daibata, M. and Matsuzaki, S. (2013) Evaluating efficacy of bacteriophage therapy against Staphylococcus aureus infections using a silkworm larval infection model FEMS Microbiol. Lett., 347, 52–60
15. Klumpp, J., Schmuki, M., Sozhamannan, S., Beyer, W., Fouts, D.E., Bernbach, V., Calendar, R. and Loessner, M.J. (2014) The odd one out: Bacillus ACT bacteriophage CP-51 exhibits unusual properties compared to related Spounavirinae W.Ph.and Bastille Virology, 462-463, 299–308
16. Chénard, C., Chan, A.M., Vincent, W.F. and Suttle, C.A. (2015) Polar freshwater cyanophage S-EIV1 represents a new widespread evolutionary lineage of phages ISME J., 9, 2046-2058
17. Johnson, M.C., Tatum, K.B., Lynn, J.S., Brewer, T.E., Lu, S., Washburn, B.K., Stroupe, M.E. and Jones, K.M. (2015) Sinorhizobium meliloti phage M9 defines a new group of T4 superfamily phages with unusual genomic features but a common T=16 capsid J. Virol., 89, 10945-10958
18. Lossouarn, J., Nesbø, C.L., Mercier, C., Zhaxybayeva, O., Johnson, M.S., Charchuck, R., Farasin, J., Bienvenu, L. et al (2015) ‘Ménage à trois’: a selfish genetic element uses a virus to propagate within Thermotogales Environ. Microbiol., 17, 3278–3288
19. Thrane, S., Janitzek, C.M., Matondo, S., Resende, M., Gustavsson, T., de Jongh, W.A., Clemmensen, S., Roeffen, W., van de Vegte-Bolmer, M. et al (2016) Bacterial superglue enables easy development of efficient virus-like particle based vaccines J. Nanobiotech., 14: 30
20. Janitzek, C.M., Matondo, S., Thrane, S., Nielsen, M.A., Kavishe, R., Mwakalinga, S.B., Theander, T.G., Salanti, A. and Sander, A.F. (2016) Bacterial superglue generates a Full-length circumsporozoite protein virus-like particle vaccine capable of inducing high and durable antibody responses Malar. J., 15: 545
21. Taylor, N.M.I., Prokhorov, N.S., Guerrero-Ferreira, R.C., Shneider, M.M., Browning, C., Goldie, K.N., Stahlberg, H. and Leiman, P.G. (2016) Structure of the T4 baseplate and its function in triggering sheath contraction. Nature, 533, 346-352
22. Brié, A. Bertrand, I., Meo, M., Boudaud, N. and Gantzer, C. (2016) The effect of heat on the physicochemical properties of bacteriophage MS2 Food Environ. Virol., 8, 251–261
23. Dai, X., Li, Z., Lai, M., Shu, S., Du, Y., Zhou, H. and Sun, R. (2017) In situ structures of the genome and genomedelivery apparatus in a single-stranded RNA virus Nature, 541, 112-116
24. Loison, P., Majou, D., Gelhaye, E., Boudaud, N. and Gantzer, C. (2016) Impact of reducing and oxidizing agents on the infectivity of Qβ phage and the overall structure of its capsid FEMS Microbiol. Ecol., 92: fiw153
25. Johnson, M.C., Sena-Veleza, M., Washburn, B.K., Platt, G.N., Lu, S., Brewer, T.E., Lynn, J.S., Stroupe, M.E. and Jones, K.M. (2017) Structure, proteome and genome of Sinorhizobium meliloti phage ΦM5: A virus with LUZ24-like morphology and a highly mosaic genome J. Struct. Biol., 200, 343–359
OptiPrep™ Application Sheet V38; 8th edition, January 2020
OPTIPREP™ VIRUS APPLICATION SHEET INDEX
- All of the general information on gradient production and analysis plus pre-gradient virus concentration are contained in Application Sheets V01-V05.
- V06 summarizes the range of virus purification strategies
- All the virus gradient protocols (V07-V36) have been listed according to the seven groups of the Baltimore Virus Classification scheme.
- Within each group, Families (sometimes preceded by the Order name), and where appropriate Subfamilies, are listed alphabetically in italics. Within each Family or Subfamily individual viruses are also listed alphabetically.
- In some cases more than one Application Sheet for a specific virus type may be provided, if significantly different practical strategies are available.
- Protocols for one particular virus in a Group may be applicable to other viruses in the same group with a similar overall structure and composition, but this can only be determined experimentally.
- Yeast retrotransposons, although not part of the Baltimore Virus Classification system, are considered similar in structure to retroviruses. They are included in Group VI listings.
- Plant Virus (V37) and Bacteriophage (V38) Application Sheets can accessed from this index after the entry for Group VII viruses.
- To access the Application Sheet(s) referred to in the list: return to the 2020Virapp file and select the appropriate V number.
1. Gradient preparation, virus concentration and gradient analysis
Preparation of density gradient solutions [Application Sheet V01]
Preparation of discontinuous and continuous gradients [Application Sheet V02]
Preparation of self-generated gradients [Application Sheet V03]
Harvesting gradients [Application Sheet V04]
Analysis of gradients [Application Sheet V05]
2. Virus purification strategies (a review) [Application Sheet V06]
3. Virus purification
Group I (ds)DNA viruses
Adenoviridae
Adenovirus [Application Sheet V07]
Helper-dependent virus, separation from [Application Sheet V07]
Asfaviridae
African swine fever virus [Application Sheet V09]
Baculoviridae
Baculovirus vectors [Application Sheet V08]
Herpesviridae
Cytomegalovirus
Self-generated gradient [Application Sheet V08]
Pre-formed gradient [Application Sheet V09]
Epstein-Barr virus
Self-generated gradient [Application Sheet V08]
Pre-formed gradient [Application Sheet V09]
Herpesviridae (contd)
Herpes simplex virus
Self-generated gradient [Application Sheet V08]
Pre-formed gradient [Application Sheet V09]
Herpes virus vectors [Application Sheet V08]
Rhadinovirus [Application Sheet V09]
Iridoviridae
Iridovirus [Application Sheet V13]
Papillomaviridae
Human papillomavirus [Application Sheet V10]
Phycodnaviridae
Chlorovirus [Application Sheet V13]
Polyomaviridae
BK virus [Application Sheet V11]
JC virus [Application Sheet V11]
Merkel cell carcinoma virus [Application Sheet V11]
Simian virus 40 (SV40) [Application Sheet V11]
Poxviridae
Molluscipoxvirus [Application Sheet V12]
Vaccinia virus [Application Sheet V12]
Group II (ss)DNA viruses
Anelloviridae
Torque teno virus [Application Sheet V15]
Parvoviridae
Densovirinae
Dependovirus
Avian adeno-associated virus [Application Sheet V14]
Recombinant adeno-associated virus [Application Sheet V14]
Parvovirinae
Minute virus of mice [Application Sheet V15]
Parvovirus [Application Sheet V16]
Group III (ds)RNA viruses
Reoviridae
Birnavirus
Espirito Santo virus [Application Sheet V17]
Sedoreovirinae
Rotavirus [Application Sheet V17]
Seadornavirus [Application Sheet V17]
Spinareovirinae
Dinovernavirus [Application Sheet V17]
Group IV ((+)ss)RNA viruses
Caliciviridae
Lagovirus
Rabbit haemorrhagic fever virus [Application Sheet V18]
Norovirus
Norwalk virus [Application Sheet V18]
Flaviviridae
Flavivirus
Dengue virus [Application Sheet V19]
West Nile virus [Application Sheet V19]
Yellow fever virus [Application Sheet V19]
Hepacivirus
Hepatitis C virus
Pre-formed gradient [Application Sheet V19]
Self-generated [Application Sheet V20]
Hepevirus
Hepatitis E virus [Application Sheet V19]
Pestivirus
Bovine diarrhea virus [Application Sheet V19]
Nidovirales – Arteriviridae
Porcine reproductive & respiratory syndrome virus [Application Sheet V21]
Nidovirales – Coronaviridae
SARS coronavirus [Application Sheet V21]
Togaviridae
Alphavirus
Chikungunya virus [Application Sheet V22]
Semliki Forest virus [Application Sheet V22]
Sindbis virus [Application Sheet V22]
Venezuelan equine encephalitis virus [Application Sheet V22]
Rubivirus
Rubella virus [Application SheetV22]
Tombusviridae
Dianthovirus
Red clover mosaic virus [Application Sheet V22]
Group V ((-)ss) RNA viruses
Arenaviridae
Arenavirus
Lassa virus [Application Sheet V23]
Tacaribe virus [Application Sheet V23]
Bunyaviridae
Hantavirus
Hantavirus [Application Sheet V24]
Orthobunyavirus
Bunyamweravirus [Application Sheet V24]
Phlebovirus
Rift valley fever virus [Application Sheet V24]
Filoviridae
Ebola virus [Application Sheet V25]
Orthomyxoviridae
Influenza virus [Application Sheet V26]
Rhabdoviridae
Lyssavirus
Rabies virus [Application Sheet V27]
Vesiculovirus
Vesicular stomatitis virus [Application Sheet V27]
Paramyxoviridae
Paramyxovirinae
Avulavirus
Newcastle disease virus [Application Sheet V28]
Morbillivirus
Measles virus [Application Sheet V28]
Respirovirus
Swine paramyxovirus [Application Sheet V28]
Pneumovirinae
Human respiratory syncytial virus [Application Sheet V28]
Group VI (ss)RNA-RT viruses
Retroviridae
Alpharetrovirus
Rous sarcoma virus [Application Sheet V29]
Betaretrovirus
Mason-Pfizer monkey virus (Simian retrovirus) [Application Sheet V30]
Deltaretrovirus
Human T cell lymphotropic virus [Application Sheet V31]
Gammaretrovirus
Moloney murine leukaemia virus [Application Sheet V33]
Murine oncornavirus [Application Sheet V32]
Lentivirus
Feline immunodeficiency virus [Application Sheet V34]
Human immunodeficiency virus [Application Sheet V34]
Spumaretroviridae
Spumaviridae
Foamy virus [Application Sheet V35]
Yeast retrotransposons [Application Sheet V37]
Group VII (ds)RNA-RT
Hepadnaviridae
Orthohepadnavirus
Hepatitis B [Application Sheet V36]
Plant viruses [Application Sheet V37]
Bacteriophages [Application Sheet V38]
OptiPrep™ Virus Application Sheet Index February 2020
