Cell References
OptiPrep™ Reference List RC01
Mononuclear cells, monocytes and polymorphonuclear leukocytes
- This Reference List divides the published papers into cell type and (where necessary) method type and/or source, species and research topic: within each group references are listed alphabetically according to first author.
- A companion Application Sheet (C03) is a methodological review of iodixanol gradient technology for purifying all leukocyte types from blood.
1 Monocytes
1a From a leukocyte-rich plasma (discontinuous flotation gradient)
Note that monocytes are also prepared from mononuclear cell preparations (see Section 2) by
antibody-bead negative selection
1a-1 Human
Adherence (to endothelial cells)
AbdAlla, S., Lother, H., Langer, A., el Faramawy, Y. and Quitterer, U. (2004) Factor XIIIA transglutaminase crosslinks AT1 receptor dimers of monocytes at the onset of atherosclerosis Cell, 119, 343-354
Aspinall, A.I., Curbishley, S.M., Lalor, P.F., Weston, C.J., Blahova, M., Liaskou, E., Adams, R.M., Holt, A.P. and Adams, D.H. (2010) CX3CR1 and vascular adhesion protein-1-dependent recruitment of CD161 monocytes across human liver sinusoidal endothelium Hepatology, 51, 2030-2039
Belcher, J.D., Marker, P.H., Weber, J.P., Hebbel, R.P. and Vercellotti, G.M. (2000) Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion Blood, 96, 2451- 2459
Blomqvist, H.M. and Olsson, A.G. (2003) Monocyte chemoattractant protein-1 and CC-chemokine receptor-2 in severe hypercholesterolaemia Scand. J. Clin. Lab. Invest., 63, 513-520
Brevig, T., Holst, B., Ademovic, Z., Rozlosnik, N., Rohrmann, J.H., Larsen, N.B., Hansen, O.C. and Kingshott, P. (2005) The recognition of adsorbed and denatured proteins of different topographics by β2 integrins and effects on leukocyte adhesion and activation Biomaterials, 26, 3039-3053
Crosley, L.K., Bashir, S., Nicol, F., Arthur, J.R., Hesketh, J.E. and Sneddon, A.A. (2013) The single-nucleotide polymorphism (GPX4c718t) in the glutathione peroxidase 4 gene influences endothelial cell function: Interaction with selenium and fatty acids Mol. Nutr. Food Res., 57, 2185–2194
Del Conde, I., Nabi, F., Tonda, R., Thiagarajan, P., Lopez, J.A. and Kleiman, N.S. (2005) Effect of P-selectin on phosphatidylserine exposure and surface-dependent thrombin generation on monocytes Arterioscler. Thromb. Vasc. Biol., 25, 1065-1070
Ferreira, A.M., Isaacs, H., Hayflick, J.S., Rogers, K.A. and Sandig, M. (2006) The p110δ isoform of PI3K differentially regulates β1 and β2 integrin-mediated monocyte adhesion and spreading and modulates diapedesis Microcirculation, 13, 439-456
Galettis, A., Campbell, S., Morris, J.M., Jackson, C.J., Twigg, S.M. and Gallery, E.D.M. (2004) Monocyte adhesion to decidual endothelial cells is increased in pregnancies complicated by type 1 diabetes but not by gestational diabetes Diabetes Care, 27, 2514-2515
Humphries, J., Gossage, J.A., Modarai, B., Burnand, K.G., Sisson, T.H., Murdoch, C. and Smith, A. (2009) Monocyte urokinase-type plasminogen activator up-regulation reduces thrombus size in a model of venous thrombosis J. Vasc. Surg., 50, 1127-1134
Ohlsson, S., Hellmark, T., Pieters, K., Sturfelt, G., Wieslander, J. and Segelmark, M. (2005) Increased monocyte transciption of the proteinase 3 gene in small vessel vasculitis Clin. Exp. Immunol., 141, 174-182
Ronald, J.A., Ionescu, C.V., Rogers, K.A. and Sandig, M. (2001) Differential regulation of transendothelial migration of THP-1 cells by ICAM-1/LFA-1 and VCAM-1/VLA-4 J. Leukoc. Biol., 70, 601-609
Schwartz, B.R., Karsan, A., Bombeli, T. and Harlan, J.M. (1999) A novel 1 integrin-dependent mechanism of leukocyte adherence to apoptotic cells J. Immunol., 162, 4842-4848
Sneddon, A.A., McLeod, E., Wahle, K.W.J. and Arthur, J.R. (2006) Cytokine-induced monocyte adhesion to endothelial cells involves platelet-activating factor: Suppression by conjugated linoleic acid Biochim. Biophys. Acta, 1761, 793-801
Ward, J.R., Francis, S.E., Marsden, L., Suddason, T., Lord, G.M., Dower, S.K., Crossman, D.C. and Sabroe, I. (2009) A central role for monocytes in Toll-like receptor-mediated activation of the vasculature Immunology, 128, 58–68
Zimmermann, H., Weston, C.J., Curbishley, S.M. and Adams, D.H.(2012) The role of vascular-adhesion-protein 1 (vap-1) in mediating monocyte migration across inflamed hepatic sinusoidal endothelium Gut, 61, A124
Angiogenic/immune responses
Agostini, L., Martinon, F., Burns, K., McDermott, M.F., Hawkins, P.N. and Tschopp, J. (2004) NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder Immunity, 20, 319-325
Aittomaki, S., Pesu, M., Groner, B., Janne, O.A., Palvimo, J.J. and Silvennoinen, O. (2000) Cooperation among Stat1, glucocorticoid receptor, and PU.1 in transcriptional activation of the high-affinity Fcγ receptor I in monocytes J. Immunol., 164, 5689-5697
Ammons, M.C.B., Siemsen, D.W., Nelson-Overton, L.K., Quinn, M.T. and Gauss, K.A. (2007) Binding of pleomorphic adenoma gene-like 2 to the tumor necrosis factor (TNF)--responsive region of the NCF2 promoter regulates p67phox expression and NADPH oxidase activity J. Biol. Chem., 282, 17941-17952
Cousins, S.W., Espinosa-Heidelmann, D.G. and Csaky, K.G. (2004) Monocyte activation in patients with agerelated macular degeneration Arch. Ophthalmol., 122, 1013 1018
Filion, L.G., Matusevicius, D., Graziani-Bowering, G.M., Kumar, A. and Freedman, M. (2003) Monocytederived IL12, CD86 (B7-2) and CD40L expression in relapsing and progressive multiple sclerosis Clin. Immunol., 106 127-138
Filion, L.G., Graziani-Bowering, G., Matusevicius, D. and Freedman, M.S. (2003) Monocyte-derived cytokines in multiple sclerosis Clin. Exp. Immunol., 131, 324-334
Hong, G., Davis, B., Khatoon, N., Baker, S.F. and Brown J. (2003) PPARγ-dependent anti-flammatory action of rosiglitazone in human monocytes: suppression of TNFα secretion is not mediated by PTEN regulation Biochem. Biophys. Res. Commun., 303, 782-787
Li, C-Y., Chou, T-C., Lee, C-H., Tsai, C-S., Loh, S-H. and Wong, C-S. (2003) Adrenaline inhibits lipopolysaccharide-induced macrophage inflammatory protein-1α in human monocytes: the role of β-receptors Anesth. Analg., 96, 518-523
Lommatzsch, M., Schloetcke, K., Klotz, J., Schuhbaeck, K., Zingler, D., Zingler, C., Schulte-Herbruggen, O., Gill, H., Schuff-Werner, P. and Virchow, J.C. (2005) Brain-derived neurotrophic factor in platelets and airflow limitation in asthma Am. J. Respir. Crit. Care Med., 171, 115-120
Martin, T., Möglich, A., Felix, I., Förtsch, C., Rittlinger, A., Palmer, A., Denk, S., Schneider, J., Notbohm, L. et al (2018) Rho‑inhibiting C2IN‑C3 fusion toxin inhibits chemotactic recruitment of human monocytes ex vivo and in mice in vivo Arch. Toxicol., 92, 323–336
Miller, L.A., Li, C and Hyde, D.M. (2000) Expression of the HML-1 epitope on human monocytes is independent of αE integrin mRNA Inflammation, 24, 195-205
Nau, G.J., Horzempa, J., O’Dee, D., Brown, M.J., Russo, B.C., Hernandez, A., Dillon, S.T., Cheng, J., Kane, L.P., Sanker, S. and Hukrie, N.A. (2019) A predicted Francisella tularensis DXD-motif glycosyltransferase blocks immune activation Virulence 10, 643–656
Ohlsson, S., Wieslander, J. and Segelmark, M. (2004) Circulating cytokine profile in anti-neutrophilic cytoplasmatic autoantibody-associated vasculitis: prediction of outcome Mediators Inflamm., 13, 275-283
Scott, C., Bonner, J., Min, D., Boughton, P., Stokes, R., Cha, K.M., Walters, S.N., Maslowski, K., Sierro, F., Grey, S.T., Twigg, S., McLennan, S. and Gunton, J.E. (2014) Reduction of ARNT in myeloid cells causes immune suppression and delayed wound healing Am. J. Physiol. Cell. Physiol., 307, C349–C357
Xue, M., March, L., Sambrook, P.N., Fukudome, K. and Jackson, C.J. (2007) Endothelial protein C receptor is overexpressed in rheumatoid arthritis (RA) synovium and mediates the anti-inflammatory effects if activated protein C in RA monocytes Ann. Rheum. Dis., 66, 1574-1580
Atherogenesis
Cheng, H-P., Gong, D., Zhao, Z-W., He, P-P., Yu, X-H., Ye, Q., Huang, C., Zhang, X., et al (2018) MicroRNA-182 promotes lipoprotein lipase expression and atherogenesis by targeting histone deacetylase 9 in apolipoprotein E-knockout mice Circul. J., 82, 28-38
Atherosclerosis
Risko, P., Plateník, J., Buchal, R., Potockova, J. and Kraml, P.J. (2018) Long-term donors versus non-donor men: Iron metabolism and the atherosclerotic process Atherosclerosis 272, 14-20
Bacterial interactions
Brown, M.J., Russo, B.C., O’Dee, D.M., Schmitt, D.M. and Nau, G.J. (2014) The contribution of the glycine cleavage system to the pathogenesis of Francisella tularensis Microb. Infect., 16, 300-309
Horzempa, J., Tarwacki, D.M., Carlson Jr., P.E., Robinson, C.M. and Nau, G.J. (2008) Characterization and application of a glucose-repressible promoter in Francisella tularensis Appl. Envir. Microbiol., 74, 2161-2170
Horzempa, J., Carlson Jr, P.E., O’Dee, D.M., Shanks, R.M.Q. and Naum G.J. (2008) Global transcriptional response to mammalian temperature provides new insight into Francisella tularensis pathogenesis BMC Microbiol., 8: 172
Jung, J-Y., Gleave Parson, M., Kraft, J.D., Lyda, L., Kobe, B., Davis, C., Robinson, J., Pena, M.O.M. and Robinson, C.M. (2016) Elevated interleukin-27 levels in human neonatal macrophages regulate indoleamine dioxygenase in a STAT-1 and STAT-3-dependent manner Immunology, 149, 35–47
Mancilla-Herrera I., Alvarado-Moreno, J.A., Cérbulo-Vázquez, A., Prieto-Chávez, J.L., Ferat-Osorio, E., López-Macías, C., Estrada-Parra, S., Isibasi, A. and Arriaga-Pizano, L. (2015) Activated endothelial cells limit inflammatory response, but increase chemoattractant potential and bacterial clearance by human monocytes Cell Biol. Int., 39, 721–732
Martins, R., Maier, J., Gorki, A-D., Huber, K.V.M., Sharif, O., Starkl, P., Saluzzo, S., Quattrone, F., Gawish, R., Lakovits, K., Aichinger, M.C. et al (2016) Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions Nat. Immunol., 17, 1361-1372
Roberts, L.L. and Robinson, C.M. (2014) Mycobacterium tuberculosis infection of human dendritic cells decreases integrin expression, adhesion and migration to chemokines Immunology, 141, 39–51
Robinson, C.M., Jung, J-Y. and Nau, G.J. (2012) Interferon-γ, tumor necrosis factor, and interleukin-18 cooperate to control growth of Mycobacterium tuberculosis in human macrophages Cytokine, 60, 233–241
Chemotaxis
Li, Y., Nishiura, H., Tokita, K., Kouike, Y., Taniguchi, C., Iwahara, M., Nishino, N., Hamad, Y., Asakawa, M. and Yamamoto, T. (2009) Elastin peptide receptor-directed monocyte chemotactic polysaccharides derived from seaweed sporophyll and from infectious fungus Microb. Pathog. 45, 423–434
Magazine, H.I., Chang, J., Goumon, Y. and Stefano, G.B. (2000) Rebound from nitric oxide inhibition triggers enhanced monocyte activation and chemotaxis J. Immunol., 165, 102-107
Mancilla-Herrera I., Alvarado-Moreno, J.A., Cérbulo-Vázquez, A., Prieto-Chávez, J.L., Ferat-Osorio, E., López-Macías, C., Estrada-Parra, S., Isibasi, A. and Arriaga-Pizano, L. (2015) Activated endothelial cells limit inflammatory response, but increase chemoattractant potential and bacterial clearance by human monocytes Cell Biol. Int., 39, 721–732
Martin, T., Möglich, A., Felix, I., Förtsch, C., Rittlinger, A., Palmer, A., Denk, S., Schneider, J., Notbohm, L. et al (2018) Rho‑inhibiting C2IN‑C3 fusion toxin inhibits chemotactic recruitment of human monocytes ex vivo and in mice in vivo Arch. Toxicol., 92, 323–336
Papaspyridonos, M., McNeill, E., de Bono, J.P., Smith, A., Burnand, K.G., Channon, K.M. and Greaves, D.R. (2008) Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction Arterioscler. Thromb. Vasc. Biol., 28, 433-440
Ritter, U. and Moll, H. (2000) Monocyte chemotactic protein-1 stimulates the killing of Leishmania major by human monocytes, acts synergistically with IFN- and is antagonized by IL-4 Eur. J. Immunol., 30, 3111-3120
Cord blood
Kraft, J.D., Horzempa, J., Davis, C., Jung, J-Y., Pena, M.M.O. and Robinson, C.M. (2013) Neonatal macrophages express elevated levels of interleukin-27 that oppose immune responses Immunology, 139, 484– 493
Dendritic cell, derived
Alvarez, Y., Municio, C., Alonso, S., San Román, J.A., Sánchez Crespo, M. and Fernández, N. (2009) Cyclooxygenase-2 iduced by zymosan in human monocyte-derived dendritic cells shows high stability, and its expression is enhanced by atorvastatin J. Pharmacol. Exp. Ther., 329, 987-994
Jung, J-Y., Roberts, L.L. and Robinson, C.M. (2015) The presence of interleukin-27 during monocyte-derived dendritic cell differentiation promotes improved antigen processing and stimulation of T cells Immunology, 144, 649–660
Roberts, L.L. and Robinson, C.M. (2014) Mycobacterium tuberculosis infection of human dendritic cells decreases integrin expression, adhesion and migration to chemokines Immunology, 141, 39–51
Valera, I., Fernández, N., García Trinidad, A., Alonso, S., Brown, G.D., Alonso, A. and Sánchez Crespo, M. (2008) Costimulation of dectin-1 and DC-SIGN triggers the arachidonic acid cascade in human monocytederived dendritic cells J. Immunol., 180, 5727-5736
Drug delivery (liposomes)
Qin, J., Chen, D.W., Hu, H.Y., Cui, Q., Qiao, M.X. and Chen, B.Y. (2007) Surface modification of RGDliposomes for selective drug delivery to monocytes/neutrophils in brain Chem. Pharm. Bull., 55, 1192-1197
Qin, J., Chen, D.W., Hu, H.Y., Qiao, M.X., Zhao, X.L. and Chen, B.Y. (2007) Body distribution of RGDmediated liposomes in brain-targeting drug delivery Yakugaku Zasshi, 127, 1497-1501
Exercise effects
Périard, J.D., Ruell, P.A., Thompson, M.W. and Caillaud, C. (2015) Moderate- and high-intensity exhaustive exercise in the heat induce a similar increase in monocyte Hsp72 Cell Stress Chaperones, 20, 1037–1042
Wang, D., Cai, Ge. J. and Yin, L. (2015) Brief exercises affect gene expression in circulating monocytes Scand. J. Immunol., 82, 429–435
Heat-shock protein see Exercise effects
Immune responses see Angiogenic/immune responses
Inflammatory responses
Chaudhuri, N., Paiva, C., Donaldson, K., Duffin, R., Parker, L.C., Sabroe, I. (2010) Diesel exhaust particles override natural injury-limiting pathways in the lung Am. J. Physiol. Lung. Cell. Mol. Physiol. 299, L263–L271
Chaudhuri, N., Jary, H., Lea, S., Khan, N., Piddock, K.C., Dockrell, D.H., Donaldson, K., Duffin, R., Singh, D., Parker, L.C. and Sabroe, I. (2012) Diesel exhaust particle exposure in vitro alters monocyte differentiation and function PloS One, 7: e51107
Chen, S.S.H., Jenkins, A.J. and Majewski, H. (2009) Elevated plasma prostaglandins and acetylated histone in monocytes in Type 1 diabetes patients Diabet. Med., 26, 182–186
Digby, J.E., Martinez, F., Jefferson, A., Ruparelia, N., Chai, J., Wamil, M., Greaves, D,R, and Choudhury, R.P. (2012) Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms Arterioscler. Thromb. Vasc. Biol., 32, 669-676
Mancilla-Herrera I., Alvarado-Moreno, J.A., Cérbulo-Vázquez, A., Prieto-Chávez, J.L., Ferat-Osorio, E., López-Macías, C., Estrada-Parra, S., Isibasi, A. and Arriaga-Pizano, L. (2015) Activated endothelial cells limit inflammatory response, but increase chemoattractant potential and bacterial clearance by human monocytes Cell Biol. Int., 39, 721–732
Menu, P., Mayor, A., Zhou, R., Tardivel, A., Ichijo, H., Mori, K. and Tschopp, J. (2012) ER stress activates the NLRP3 inflammasome via an UPR-independent pathway Cell Death Dis., 3: e261
Oo, Y.H., Weston, C.J., Lalor, P.F., Curbishley, S.M., Withers, D.R., Reynolds, G.M., Shetty, S. et al (2010) Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver J. Immunol., 184, 2886–2898
Papaspyridonos, M., McNeill, E., de Bono, J.P., Smith, A., Burnand, K.G., Channon, K.M. and Greaves, D.R. (2008) Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction Arterioscler. Thromb. Vasc. Biol., 28, 433-440
Xue, M., March, L., Sambrook, P.N. and Jackson, C.J. (2007) Differential regulation of matrix metalloproteinase 2 and matrix metalloproteinase 9 by activated protein C: Relevance to inflammation in rheumatoid arthritis Arthritis Rheumatism, 56, 2864-2874
Zimmermann, H., Weston, C.J., Curbishley, S.M. and Adams, D.H.(2012) The role of vascular-adhesion-protein 1 (vap-1) in mediating monocyte migration across inflamed hepatic sinusoidal endothelium Gut, 61, A124
Leishmania
Ritter, U. and Moll, H. (2000) Monocyte chemotactic protein-1 stimulates the killing of Leishmania major by human monocytes, acts synergistically with IFN- and is antagonized by IL-4 Eur. J. Immunol., 30, 3111-3120
Leukapheresis samples, from
Akiyama, Y., Oshita, C., Kume, A., Iizuka, A., Miyata, H., Komiyama, M., Ashizawa, T., Yagoto, M. et al (2012) α-Type-1 polarized dendritic cell-based vaccination in recurrent high-grade glioma: a phase I clinical trial BMC Cancer, 12: 623
Lipoprotein lipase
Cheng, H-P., Gong, D., Zhao, Z-W., He, P-P., Yu, X-H., Ye, Q., Huang, C., Zhang, X., et al MicroRNA-182 promotes lipoprotein lipase expression and atherogenesis by targeting histone deacetylase 9 in apolipoprotein E-knockout mice Circul. J., 82, 28-38
Liver/liver tumous
Aspinall, A.I., Curbishley, S.M., Lalor, P.F., Weston, C.J., Blahova, M., Liaskou, E., Adams, R.M., Holt, A.P. and Adams, D.H. (2010) CX3CR1 and vascular adhesion protein-1-dependent recruitment of CD161 monocytes across human liver sinusoidal endothelium Hepatology, 51, 2030-2039
Wu, Q., Zhou, W., Yin, S., Zhou, Y., Chen, T., Qian, J., Su, R., Hong, L. et al (2019) Blocking triggering receptor expressed on myeloid cells-1-positive tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-programmed cell death ligand 1 resistance in liver cancer Hepatology, 70, 198- 214
LPS induced responses
Creery, D., Angel, J.B., Aucoin, S., Weiss, W., Cameron, W.D., Diaz-Mitoma, F. and Kumar, A. (2002) Nef protein of human immunodeficiency virus and lipopolysaccharide induce expression of CD14 on human monocytes through differential utilization of interleukin-10 Clin. Diagnost. Lab. Immunol., 9, 1212-1221
Widing, L., Bechensteen, A.G., Mirlashari, M.R., Vetlesen, A. and Kjeldsen-Kragh, J. (2007) Evaluation of nonleukoreduced red blood cell transfusion units collected at delivery from the placenta Transfusion 47, 1481- 1487
Macrophage differentiation/function
Alvarez, Y., Municio, C., Alonso, S., Sánchez Crespo, M. and Fernández, N. (2009) The induction of IL-10 by zymosan in dendritic cells depends on CREB activation by the coactivators CREB-binding protein and TORC2 and autocrine PGE2 J. Immunol., 183, 1471–1479
Bordet, E., Maisonnasse, P., Renson, P., Bouguyon, E., Crisci, E., Tiret, M., Descamps, D., Bernelin-Cottet, C. et al (2018) Porcine alveolar macrophage-like cells are pro-inflammatory pulmonary intravascular macrophages that produce large titers of porcine reproductive and respiratory syndrome virus Sci. Rep., 8: 10172
Carlson, P.E., Carroll, J.A., O’Dee, D.M. and Nau, G.J. (2007) Modulation of virulence factors in Francisella tularensis determines human macrophage responses Microb. Pathogen. 42, 204-214
Chaudhuri, N., Jary, H., Lea, S., Khan, N., Piddock, K.C., Dockrell, D.H., Donaldson, K., Duffin, R. et al (2012) Diesel exhaust particle exposure in vitro alters monocyte differentiation and function PloS One, 7: e51107
Cousins, S.W., Espinosa-Heidelmann, D.G. and Csaky, K.G. (2004) Monocyte activation in patients with agerelated macular degeneration Arch. Ophthalmol., 122, 1013 1018
Goto-Koshino, Y., Ohno, K., Nakajima, M., Mochizuki, H., Kanemoto, H. and Tsujimoto, H. (2011) A rapid and simple method to obtain canine peripheral blood-derived macrophages J. Vet. Med. Sci., 73, 773–778
Inoue, M., Niki, M., Ozeki, Y., Nagi, S., Chadeka, E.A., Yamaguchi, T., Osada-Oka, M., Ono, K., Oda, T. et al (2018) High-density lipoprotein suppresses tumor necrosis factor alpha production by mycobacteria infected human macrophages Sci. Rep., 8: 6736
Jung, J-Y., Madan-Lala, R., Georgieva, M., Rengarajan, J., Sohaskey, C.D., Bange, F-C. and Robinson, C.M. (2013) The intracellular environment of human macrophages that produce nitric oxide promotes growth of mycobacteria Infect. Immun., 81, 3198–3209
Jung, J-Y. and Robinson, C.M. (2014) IL-12 and IL-27 regulate the phagolysosomal pathway in mycobacteriainfected human macrophages Cell Commun. Signal., 12: 16
Kraft, J.D., Horzempa, J., Davis, C., Jung, J-Y., Pena, M.M.O. and Robinson, C.M. (2013) Neonatal macrophages express elevated levels of interleukin-27 that oppose immune responses Immunology, 139, 484–493
Lejal, N., Truchet, S., Bechor, E., Bouguyon, E., Khedkar, V., Bertho, N., Vidic, J., Adenot, P., Soliere, S., Pick, E. and Slama-Schwok, A. (2018) Turning off NADPH oxidase-2 by impeding p67phox activation in infected mouse macrophages reduced viral entry and inflammation BBA – Gen. Subjects, 1862, 1263–1275
Liang, C-P., Han, S., Okamoto, H., Carnemolia, R., Tabas, I., Accili, D. and Tali, A.R. (2004) Increased CD36 protein as a response to defective insulin signaling in macrophages J. Clin. Invest., 113, 764-773
Li, C-Y., Chou, T-C., Lee, C-H., Tsai, C-S., Loh, S-H. and Wong, C-S. (2003) Adrenaline inhibits lipopolysaccharide-induced macrophage inflammatory protein-1 in human monocytes: the role of α-receptors Anesth. Analg., 96, 518-523
Quesniaux, V., Erard, F. and Ryffel, B. (2010) Adjuvant activity on murine and human macrophages In Vaccine Adjuvants (ed. Davies, G.) Methods Mol. Biol., 626, 117-130, Humana Press, Totowa, NJ, USA Robinson, C.M., O’Dee, D., Hamilton, T. and Nau, G.J. (2010) Cytokines involved in interferon-γ production by human macrophages J. Innate Immun., 2, 56–65
Robinson, C.M., Jung, J-Y. and Nau, G.J. (2012) Interferon-γ, tumor necrosis factor, and interleukin-18 cooperate to control growth of Mycobacterium tuberculosis in human macrophages Cytokine, 60, 233–241
Tyner, J.W., Uchida, O., Kajiwara, N., Kim, E.Y., Patel, A.C., O’Sullivan, M.P., Walter, M.J. et al (2005) CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival viral infection Nat. Med., 11, 1180-1187
Vosper, H., Patel, L., Graham, T.L., Khoudoli, G.A., Hill, A., Macphee, C.H., Pinto, I., Smith, S.A. et al (2001) The peroxisome proliferator-activated receptor delta promotes lipid accumulation in human macrophages J. Biol. Chem. 276, 44258-4426
Metalloproteinases
Bao, W., Min, D., Twigg, S.M., Shackel, N.A., Warner, F.J., Yue D.K., McLennan, S.V. (2010) Monocyte CD147 is induced by advanced glycation end products and high glucose concentration: possible role in diabetic complications Am. J. Physiol. Cell Physiol., 299, C1212–C121
Ludwig, A., Berkhout, T., Moores, K., Groot, P. and Chapman G. (2002) Fractalkine is expressed by smooth muscle cells in response to IFN-γ and TFN-α and is modulated by metalloproteinase activity J. Immunol., 168, 604-612
Methodology
Graziani-Bowering, G.M., Graham, J. and Filion, L.G. (1997) A quick, easy and inexpensive method for the isolation of human peripheral blood monocytes J. Immunol. Methods, 207, 157-168
Nutt, J.C., Willis, C.C., Morris, J.M. and Gallery, E.D.M. (2004) Isolating pure populations of monocytes from the blood of pregnant women: comparison of flotation in iodixanol with elutriation J. Immunol. Methods, 293, 215-218
MicroRNA-182
Cheng, H-P., Gong, D., Zhao, Z-W., He, P-P., Yu, X-H., Ye, Q., Huang, C., Zhang, X., et al MicroRNA-182 promotes lipoprotein lipase expression and atherogenesis by targeting histone deacetylase 9 in apolipoprotein E-knockout mice Circul. J., 82, 28-38
Transcription factors
Scott, C., Bonner, J., Min, D., Boughton, P., Stokes, R., Cha, K.M., Walters, S.N., Maslowski, K. et al (2014) Reduction of ARNT in myeloid cells causes immune suppression and delayed wound healing Am. J. Physiol. Cell. Physiol., 307, C349–C357
Oncohaemotology patients
Herrero-Sánchez, M.C., Angomás, E.B., de Ramón, C., Tellería, J.J., Corchete, L.A., Alonso, S., del Carmen Ramos, M., Peñarrubia, M.J. et al (2018) Polymorphisms in receptors involved in opsonic and nonopsonic phagocytosis, and correlation with risk of infection in oncohematology patients Infect. Immun., 86: 00709-18
Oxidation
Gauss, K.A., Bunger, P.L., Larson, T.C., Young, C.J., Nelson-Overton, L.K., Siemsen, D.W. and Quinn, M.T. (2005) Identification of a novel tumor necrosis factor α-responsive region in the NCF2 promoter J. Leukoc. Biol., 77, 267-278
Gauss, K.A., Bunger, P.L., Crawford, M.A., McDermott, B.E., Swearingen, R., Nelson-Overton, L.K., Siemsen, D.W., Kobayashi, S.D., DeLeo, F.R. and Quinn, M.T. (2006) Variants of the 5’-untranslated region of human NCF2: expression and translational efficiency Gene, 366, 169-179
VEGF transfection
Magazine, H.I., Chang, J., Goumon, Y. and Stefano, G.B. (2000) Rebound from nitric oxide inhibition triggers enhanced monocyte activation and chemotaxis J. Immunol., 165, 102-107
Modarai, B., Humphries, J., Gossage, J.A., Waltham, M., Burnand, K.G., Kanaganayagam, G.S., Afuwape, A., Paleolog, E., Smith, A., Wadoodi, A. (2008) Adenovirus-mediated VEGF gene therapy enhances venous thrombus recanalization and resolution Arterioscler. Thromb. Vasc. Biol., 28, 1752-1759
Phagocytosis
Herrero-Sánchez, M.C., Angomás, E.B., de Ramón, C., Tellería, J.J., Corchete, L.A., Alonso, S., del Carmen Ramos, M., Peñarrubia, M.J. et al (2018) Polymorphisms in receptors involved in opsonic and nonopsonic phagocytosis, and correlation with risk of infection in oncohematology patients Infect. Immun., 86: 00709-18
Pluripotent stem cells
Isogai, S., Yamamoto, N., Hiramatsu, N., Goto, Y., Hayashi, M., Kondo, M. and Imaizumi, K. (2018) Preparation of induced pluripotent stem cells using human peripheral blood monocytes Cell. Reprogram. 20, 247-355
Somatic stem cells
Eve, D.J., Sanberg, P.R., Buzanska, L., Sarnowska, A. and Domanska-Janik, K. (2018) Human somatic stem cell neural differentiation potential In Human Neural Stem Cells, Results and Problems in Cell
Thiosemicarbazones
Moreno-Rodríguez, A., Salazar-Schettino, P.M., Bautista, J.L., Hernández-Luis, F., Torrens, H., GuevaraGómez, Y., Pina-Canseco, S. et al (2014) In vitro antiparasitic activity of new thiosemicarbazones in strains of Trypanosoma cruzi Eur. J. Medic. Chem., 87, 23-29
Virus interactions
Chehadeh, W., Bouzidi, A., Alm, G., Wattré, P.and Hober, D. (2001) Human antibodies isolated from plasma by affinity chromatography increase the coxsackievirus B4-induced synthesis of interferon-α by human peripheral blood mononuclear cells in vitro J. Gen. Virol., 82, 1899-1907
Creery, D., Angel, J.B., Aucoin, S., Weiss, W., Cameron, W.D., Diaz-Mitoma, F. and Kumar, A. (2002) Nef protein of human immunodeficiency virus and lipopolysaccharide induce expression of CD14 on human monocytes through differential utilization of interleukin-10 Clin. Diagnost. Lab. Immunol., 9, 1212-1221
Dumont, L.J., Luka, J., van den Broeke, T., Whitley, P., Ambruso, D.R. and Elfath, M.D. (2001) The effect of leukocyte-reduction method on the amount of human cytomegalovirus in blood products: a comparison of apheresis and filtration methods Blood, 97, 3640-3647
Mackewicz, C.E., Yuan, J., Tran, P., Diaz, L., Mack, E., Selsted, M.E. and Levy, J.A. (2003) -Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors AIDS, 17, F23-F32
Wound healing
Scott, C., Bonner, J., Min, D., Boughton, P., Stokes, R., Cha, K.M., Walters, S.N., Maslowski, K., Sierro, F., Grey, S.T., Twigg, S., McLennan, S. and Gunton, J.E. (2014) Reduction of ARNT in myeloid cells causes immune suppression and delayed wound healing Am. J. Physiol. Cell. Physiol., 307, C349–C357
1a-2 Macaque
Tongaonkar, P., Tran, P., Roberts, K., Schaal, J., Osapay, G., Tran, D., Ouellette, A.J. and Selsted, M.E. (2011) Rhesus macaque θ-defensin isoforms: expression, antimicrobial activities and demonstration of a prominent role in neutrophil granule microbicidal activities J. Leukoc. Biol., 89, 283–290
1a-3 Murine
Abd Alla, J., Langer, A., Elzahwy, S.S., Arman-Kalcek, G., Streichert, T. and Quitterer, U. (2010) Angiotensinconverting enzyme inhibition down-regulates the pro atherogenic chemokine receptor 9 (CCR9)-chemokine ligand 25 (CCL25) axis J. Biol. Chem., 285, 23496-23505
Wu, Q., Zhou, W., Yin, S., Zhou, Y., Chen, T., Qian, J., Su, R., Hong, L. et al (2019) Blocking triggering receptor expressed on myeloid cells-1-positive tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-programmed cell death ligand 1 resistance in liver cancer Hepatology, 70, 198- 214
1a-4 Ovine
Berger, S.T. and Griffin, F.T. (2006) A comparison of ovine monocyte-derived macrophage function following infection with Mycobacterium avium ssp. avium and Mycobacterium avium ssp. paratuberculosis Immunol. Cell Biol., 84, 349-356
1b From whole blood (discontinuous flotation gradient)
Anwar, K., Voloshyna, I., Littlefield, M.J., Carsons, S.E., Wirkowski, P.A., Jaber, N.L., Sohn, A., Eapen, S. and Reiss, A.B. (2011) COX-2 Inhibition and inhibition of cytosolic phospholipase A2 increase CD36 expression and foam cell formation in THP-1 cells Lipids 46, 131–142
Burdo, T.H., Wood, M.R. and Fox, H.S. (2007) Osteopontin prevents monocyte recirculation and apoptosis J. Leukoc. Biol. 81, 1504-1511
Goodman, R.S., Kirton, C.M., Oostingh, G.J., Schon, M., Clark, M.R., Bradley, J.A. and Taylor, C.J. (2008) PECAM-1 polymorphism affects monocyte adhesion to endothelial cells Transplantation, 85, 71-477
Ivanov, I.I., Apta, B.H.R., Bonna, A.M. and Harper, M.T. (2019) Platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes Sci. Rep., 9: 13397
2 Mononuclear cells (barrier sedimentation gradient)
2a-1 Blood (canine)
Goto-Koshino, Y., Tomiyasu, H., Suzuki, H., Tamamoto, T., Mizutani, N., Fujino, Y., Ohno, K. and Tsujimoto, H. (2014) Differential expression of CD45 isoforms in canine leukocytes Vet. Immunol. Immunopathol., 160, 118–122
2a-2 Blood (chicken)
Gan, L., Tian, Y., Zhao, Y., Shan, X-q., Zhou, W., Xia, B-B., Chen, J., Wang. M-L. and Zhao, J. (2019) Enhancing immunogenicity and protective efficacy of inactivated avian influenza H9N2vaccine with recombinant chicken IFN-α in chicken Vet. Microbiol., 234, 77–82
Xu, S., Xue, C., Li, J., Bi, Y. and Cao, Y. (2011) Marek’s disease virus type 1 microRNA miR-M3 suppresses cisplatin-induced apoptosis by targeting SMAD2 of the transforming growth factor beta signal pathway J. Virol., 85, 276-285
2a-3 Blood (equine)
Ellison, S.P., Greiner, E., Brown, K.W. and Kennedy, T. (2004) Experimental infection of horses with culturederived Sarcocystis neurona merozoites as a model for equine protozoal myeloencephalitis Int. J. Appl. Res. Vet. Med., 2, 79-89
Pronost, S., Legrand, L., Pitel, P-H., Wegge, B., Lissens, J., Freymuth, F., Richard, E. and Fortier, G. (2012) Outbreak of equine herpesvirus myeloencephalopathy in France: a clinical and molecular investigation Transbound. Emerg. Dis., 59, 256–263
2a-4 Blood (fish)
Li, J., Das, S., Herrin, B.R., Hirano, M. and Cooper, M.D. (2013) Definition of a third VLR gene in hagfish Proc. Natl. Acad. Sci., 110, 15013–15018
Godahewa G.I., Perera, N.C.N., Umasuthan, N., Wan, Q., Whang, I. and Lee, J. (2016) Molecular characterization and expression analysis of B cell activating factor from rock bream (Oplegnathus fasciatus) Dev. Comp. Immunol., 55, 1-11
Oh, M., Bathige, S.D.N.K., Kim, Y., Lee, S., Yang, H., Kim, M-J. and Lee, J. (2017) A CXCL ortholog from Hippocampus abdominalis: Molecular features and functional delineation as a pro-inflammatory chemokine Fish Shellfish Immunol., 67, 218-227
Thulasitha, W.S., Umasuthan, N., Whang, I., Lim, B-S., Jung, H-B., Noh, J.K. and Lee, J. (2015) A CXC chemokine gene, CXCL12, from rock bream, Oplegnathus fasciatus: Molecular characterization and transcriptional profile Fish Shellfish Immunol., 45, 560-566
Umasuthan, N., Wan, Q., Revathy, K.S., Whang, I., Noh, J.K., Kim, S., Park, M-A. and Lee, J. (2014) Molecular aspects, genomic arrangement and immune responsive mRNA expression profiles of two CXC chemokine receptor homologs (CXCR1 and CXCR2) from rock bream, Oplegnathus fasciatus Fish, Shellfish Immunol., 40, 304-318
2a-5 Blood (human)
Adrenoleukodystrophy
Hung, K-L., Wang, J-S., Keng, W.T., Chen, H-J., Liang, J-S., Ngu, L.H. and Lu, J-F.(2013) Mutational analyses on X-linked adrenoleukodystrophy reveal a novel cryptic splicing and three missense mutations in the ABCD1 gene Pediatr. Neurol., 49, 185-190
Bacterial infections
Télleza, G.A., Zapata, J.A, Johan, Toro, L.J., Henao, D.C., Bedoya, J.P., Rivera, J.D., Trujillo, J.V. et al (2018) Identification, characterization, immunolocalization, and biological activity of Lucilin peptide Acta Tropica 185, 318–326
Cancer/virus studies
Ishikawa, A., Motohashi, S., Ishikawa, E., Fuchida, H., Higashino, K., Otsuji, M., Iizasa, T., Nakayama, T., Taniguchi, M. and Fujisawa, T. (2005) A phase I study of α-galactosylceramide (KRN7000) – pulsed dendritic cells in patients with advanced and recurrent non – small cell lung cancer Clin. Cancer Res., 11, 1910-1917
Kurosaki, M., Horiguchi, S., Yamasaki, K., Uchida, Y., Motohashi, S., Nakayama, T., Sugimoto, A. and Okamoto, Y. (2011) Migration and immunological reaction after the administration of α GalCer-pulsed antigen-presenting cells into the submucosa of patients with head and neck cancer Cancer Immunol. Immunother., 60, 207–215
Motohashi, S., Ishikawa, A., Ishikawa, E., Otsuji, M., Iizasa, T., Hanaoka, H., Shimizu, N., Horiguchi, S. et al (2006) A phase 1 study of in vitro expanded natural T killer cells in patients with advanced and recurrent nonsmall cell lung cancer Clin. Cancer Res., 12, 6079-6085
Motohashi, S., Nagato, K., Kunii, N., Yamamoto, H., Yamasaki, K., Okita, K., Hanaoka, H., Shimizu, N. et al (2009) A phase I-II study of α-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer J. Immunol., 182, 2492– 2501
Petersen, L., Petersen, C.C., Møller-Larsen, A. and Hokland, M.E. (2010) Short-term exposure to human cytomegalovirus–infected fibroblasts induces a proportional increase of active CD94/NKG2A+ natural killer cells Hum. Immunol., 71, 29–35
Petersen, C.C., Nederby, L., Roug, A.S., Skovbo, A., Peterslund, N.A., Hokland, P., Nielsen, B. and Hokland, M. (2011) Increased expression of CD69 on T cells as an early immune marker for human cytomegalovirus reactivation in chronic lymphocytic leukemia patients Viral Immunol., 24, 165–169
Stokes, C.A., Ismail, S., Dick, E.P., Bennett, J.A., Johnston, S.L., Edwards, M.R., Sabroe, I. and Parker, L.C. (2011) Role of interleukin-1 and MyD88-dependent signaling in rhinovirus infection J. Virol., 85, 7912-7921
Sun, C., Feng, L., Zhang, Y., Xiao, L., Pan, W., Li, C., Zhang, L. and Chen, L. (2012) Circumventing antivector immunity by using adenovirus-infected blood cells for repeated application of adenovirus-vectored vaccines: proof of ccncept in rhesus macaques J. Virol., 86, 11031-11042
Uchida, T., Horioguchi, S., Tanaka, Y., Yamamoto, H., Kunii, N., Motohashi, S., Taniguchi, M., Nakayama, T. and Okamoto, Y. (2008) Phase I study of α-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer Cancer Immunol. Immunother., 57, 337-345
Yang, Z., Tang, T., Wei, X., Yang, S. and Tian, Z. (2015) Type 1 innate lymphoid cells contribute to the pathogenesis of chronic hepatitis B Innate Immun., 21, 665–673
Basophil isolation
Youssef, L.A., Pharm, B., Wilson, B.S. and Oliver, J.M. (2002) Proteasome-dependent regulation of Syk tyrosine kinase levels in human basophils J. Allergy Clin. Immunol., 110, 366-373
DNA repair pathways
Healing, E., Charlier, C.F., Meira, L.B. and Elliott, R.M. (2019) A panel of colorimetric assays to measure enzymatic activity in the base excision DNA repair pathway Nucleic Acids Res., 47: e61
Cell proliferation
Mukherjee, S., Giamberardino, C., Thomas, J., Evans, K., Goto, H., Ledford, J.G., Hsia, B., Pastva, A.M. and Wright, J.R. (2012) Surfactant protein A integrates activation signal strength to differentially modulate T cell proliferation J. Immunol., 188, 957–967
Parmar, S., Thompson, A.A.R., Higgins, K.R., Sabroe, I., Parker, L.C., Lawrie, A., Arnold, J., Walker, S. et al (2012) Elucidating the mechanism by which monocytes can inhibit hypoxic Pa-Smc proliferation Am. J. Respir. Crit. Care Med., 185, A5660
Schmitt, D.M., O’Dee, D.M., Horzempa, J., Carlson Jr., P.E., Russo, B.C., Bales, J.M., Brown, M.J. and Nau, G.J. (2012) A Francisella tularensis live vaccine strain that improves stimulation of antigen-presenting cells does not enhance vaccine efficacy PLoS One, 7: e31172
Stechmiller, J.K., Langkamo-Henken, B., Childress, B., Herrlinger-Garcia, K.A., Hugens, J., Tian, L., Percival, S.S. and Steele, R. (2005) Arginine supplementation does not enhance serum nitric oxide levels in elderly nursing home residents with pressure ulcers Biol. Res. Nurs., 6, 289-299
Corticosteroids
Bodor, N., Zubovics, Z., Kurucz, I., Solyom, S. and Bodor, E. (2017) Potent analogues of etiprednol dicloacetate, a second generation of soft corticosteroids J. Pharmacy Pharmacol., 69, 1745–1753
Endocannabinoid system
Chiang, K.P., Gerber, A.L., Sipe, J.C. and Cravatt, B.F. (2004) Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use Hum. Mol. Genet., 13, 2113-2119
Exercise/fasting effects
Elliott, R.M., de Roos, B., Duthie, S.J., Bouwman, F.G., Rubio-Aliaga, I., Crosley, L.K., Mayer, C., Polley, A.C. et al (2014) Transcriptome analysis of peripheral blood mononuclear cells in human subjects following a 36 h fast provides evidence of effects on genes regulating inflammation, apoptosis and energy metabolism Genes Nutr., 9: 432
Radom-Aizik, S., Zaldivar, Jr. F., Leu, S-Y., Adams, G.R., Oliver, S. and Cooper, D.M. (2012) Effects of exercise on microRNA expression in young males peripheral blood mononuclear cells Clin.Trans. Sci., 5, 32–38
Radom-Aizik, S., Zaldivar, F., Leu, S-Y. and Cooper, D.M. (2009) Brief bout of exercise alters gene expression in peripheral blood mononuclear cells of early- and late-pubertal males Pediatr. Res., 65, 447–452
Radom-Aizik, S., Zaldivar, F., Leu, S. and Cooper, D.M. (2009) A brief bout of exercise alters gene expression and distinct gene pathways in peripheral blood mononuclear cells of early- and late-pubertal females J. Appl. Physiol., 107, 168–175
Immunogenicity
Liu, P., Chen, S., Li, X., Qin, L., Huang, K., Wang, L., Huang, W., Li, S., Jia, B., Zhong, M., Pan, G., Cai, J. and Pei, D. (2013) Low immunogenicity of neural progenitor cells differentiated from induced pluri-potent stem cells derived from less immunogenic somatic cells PLoS One, 8: e69617
Inflammatory processes
Das, N., Dewan, V., Grace, P.M., Gunn, R.J., Tamura, R., Tzarum, N., Watkins, L.R., Wilson, I.A. and Yin, H. (2016) HMGB1 activates proinflammatory signaling via TLR5 leading to allodynia Cell Rep., 17, 1128–1140
Grundtner, R., Dornmair,K., Dahm, R., Flügel,A., Kawakami,N., Zeitelhofer, M., Schoderboeck, L., Nosov, M. et al (2007) Transition from enhanced T cell infiltration to inflammation in the myelin-degenerative central nervous system Neuobiol. Dis., 28, 261-275
Radom-Aizik, S., Zaldivar, Jr. F., Leu, S-Y., Adams, G.R., Oliver, S. and Cooper, D.M. (2012) Effects of exercise on micro-RNA expression in young males peripheral blood mononuclear cells Clin.Trans. Sci., 5, 32– 38
Saurer, L., Rihs, S., Birrer, M., Saxer-Seculic, N., Radsak, M. and Mueller, C. (2012) Elevated levels of serumsoluble triggering receptor expressed on myeloid cells-1 in patients with IBD do not correlate with intestinal TREM-1 mRNA expression and endoscopic disease activity J. Crohn’s Colitis, 6, 913–923
Macrophage/monocyte differentiation
Chaudhuri, N., Jary, H., Lea, S., Khan, N., Piddock, K.C., Dockrell, D.H., Donaldson, K., Duffin, R., Singh, D., Parker, L.C. and Sabroe, I. (2012) Diesel exhaust particle exposure in vitro alters monocyte differentiation and function PloS One, 7: e51107
Inoue, M., Niki, M., Ozeki, Y., Nagi, S., Chadeka, E.A., Yamaguchi, T., Osada-Oka, M., Ono, K., Oda, T. et al (2018) High-density lipoprotein suppresses tumor necrosis factor alpha production by mycobacteria infected human macrophages Sci. Rep., 8: 6736
Voloshyna, I., Hai, O., Littlefield, M.J., Carsons, S. and Reiss, A.B. (2013) Resveratrol mediates antiatherogenic effects on cholesterol flux in human macrophages and endothelium via PPAR and adenosine Eur. J. Pharmacol., 698, 299–309
Sample processing
De Roos, B., Duthie, S.J., Polley, A.C.J., Mulholland, F., Bouwman, F.G., Heim, C., Rucklidge, G.J., Johnson, I.T. et al (2008) Proteomic methodological recommendations for studies involving human plasma, platelets and peripheral blood mononuclear cells J. Proteome Res., 7, 2280-2290
Holland, N.T., Smith, M.T., Eskenazi, B. and Bastaki, M. (2003) Biological sample collection and processing for molecular epidemiological studies Mutat. Res., 543, 217-234
Holland, N.T., Pfleger, L., Berger, E., Ho, A. and Bastaki, M. (2005) Molecular epidemiology biomarkers – sample collection and processing considerations Tox. Appl. Pharmacol., 206, 261-268
α-Synuclein
Barbour, R., Kling, K., Anderson, J.P., Banducci, K., Cole, T., Diep, L., Fox, M., Goldstein, J.M., Soriano, F., Seubert, P. and Chilcote, T.J. (2008) Red blood cells are the major source of alpha-synuclein in blood Neurodegener. Dis., 5, 55-59
T-cell apoptosis
Oh, J., Kim, S-H., Ahn, S. and Lee, C-E. (2012) Suppressors of cytokine signaling promote Fas-induced apoptosis through downregulation of NF-B and mitochondrial Bfl-1 in leukemic T cells J. Immunol., 189, 5561–5571
VEGF
Kusumanto, Y.H., Dam, W.A., Hospers, G.A.P., Meijer, C. and Mulder, N.H. (2003) Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor Angiogenesis, 6, 283-287
Webb, N.J.A., Watson, C.J., Roberts, I.S.D., Bottomley, M.J., Jones, C.A., Lewis, M.A., Postlethwaite, R.J. and Benchley, P.E.C. (1999) Circulating vascular endothelial growth factor is not increased during relapses of steroid-sensitive nephrotic syndrome Kidney Int., 55, 1063-1071
Vitamin B12 uptake
Obeid, R., Kuhlmann, M., Kirshc, C-M. and Herrmann, W. (2005) Cellular uptake of vitamin B12 in patients
with chronic renal failure Nephron Clin. Pract., 99, c42-c48
2a-6 Blood (non-human primate)
Meng, W., Pan, W., Zhang, A.J.X., Li, Z., Wei, G., Feng, L., Dong, Z., Li, C. et al (2013) Rapid generation of human-like neutralizing monoclonal antibodies in urgent preparedness for influenza pandemics and virulent infectious diseases PLoS One, 8: e66276
Stittelaar, K., Wyatt, L.S., de Swart, R.L., Vos, H.W., Groen, J., van Amerongen, G., van Binnendijk, R.S., Rozenblatt, S., Moss, B. and Osterhaus, A.D.M.E. (2000) Protective immunity in macaques vaccinated with a modified vaccinia virus Ankara-based measles virus vaccine in the presence of passively acquired antibodies J. Virol., 74, 4236-4243
Stittelaar, K.J., Kuiken, T., de Swart, R.L., van Amerongen, G., Vos, H.W., Niesters, H.G.M., van Schalkwijk, P., van der Kwast, T., Wyatt, L.S., Moss, B. and Osterhaus, A.D.M.E. (2001) Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques Vaccine, 19, 3700-3709
Sun, C., Feng, L., Zhang, Y., Xiao, L., Pan, W., Li, C., Zhang, L. and Chen, L. (2012) Circumventing antivector immunity by using adenovirus-infected blood cells for repeated application of adenovirus-vectored vaccines: proof of ccncept in rhesus macaques J. Virol., 86, 11031-11042
Van der Kruyl, A.C., van den Burg, R., Hoyer, M.J. Gruters, R.A., Osterhaus, A.D.M.E. and Berhout, B. (2004) SIVdrl detection in captive mandrills: are mandrill infected with a third strain of simian immunodeficiency virus? Retrovirology, 1: 36
2a-7 Blood (porcine)
Kim, S.J., Han, Y.W., Rahman, Md.M., Kim, S.B., Uyanga, E., Lee, B.M., Kim, J.H., Roh, Y.S. et al (2010) Live attenuated Salmonella enterica serovar Typhimurium expressing swine interferon-α has antiviral activity and alleviates clinical signs induced by infection with transmissible gastroenteritis virus in piglets Vaccine 28, 5031–5037
Kim, S.J., Kim, S.B., Han, Y.W., Uyangaa, E., Kim, J.H., Choi, J.Y., Kim, K. and Eo, S.K. (2012) Coadministration of live attenuated Salmonella enterica serovar Typhimurium expressing swine interleukin-18 and interferon-α provides enhanced Th1-biased protective immunity against inactivated vaccine of pseudorabies virus Microbiol. Immunol., 56, 529–540
Kim, S.B., Kim, S.J., Lee, B.M., Han, Y.W., Rahman, M., Uyangaa, E., Kim, J.H., Choi, J.Y. et al (2012) Oral administration of Salmonella enterica serovar Typhimurium expressing swine interleukin-18 induces Th1- biased protective immunity against inactivated vaccine of pseudorabies virus Vet. Microbiol., 155, 172–182
Lannes, N., Python, S. and Summerfield, A. (2012) Interplay of foot-and-mouth disease virus, antibodies and plasmacytoid dendritic cells: virus opsonization under non-neutralizing conditions results in enhanced interferon-alpha responses Vet. Res., 43: 64
Lee, B.M., Han, Y.W., Kim, S.B., Rahman, M.M., Uyangaa, E., Kim, J.Y., Roh, Y.S. et al (2011) Enhanced protection against infection with transmissible gastroenteritis virus in piglets by oral co-administration of live attenuated Salmonella enterica serovar Typhimurium expressing swine interferon-α and interleukin-18 Comp. Immunol. Microbiol. Infect. Dis., 34, 369– 380
2a-8 Blood (rodent)
Aleksandrov, A.P., Belij-Rammerstorfer, S., Mirkov, I., Subota, V., Kulas, J., Kataranovski, D. and Kataranovski, M. (2018) Oral warfarin affects some aspects of systemic immunomodulation with topical dinitrochlorobenzene (DNCB) in rats Cutan. Ocul. Toxicol., 47, 29–35
Cifre, M., Palou, A. and Oliver, P. (2018) Cognitive impairment in metabolically obese, normal-weight rats: identification of early biomarkers in peripheral blood mononuclear cells Mol. Neurodegen., 13:14
Horibe, T., Kawamoto, M., Kohno, M. and Kawakami, K. (2012) Cytotoxic activity to acute myeloid leukemia cells by Antp-TPR hybrid peptide targeting Hsp90 J. Biosci. Bioeng., 114, 96-103
Islama, M.A., Hooiveld, G.J.E.J., van den Berg, J.H.J., Boekschoten, M.V., van der Velpen, V., Murk, A.J., Rietjens, I.M.C.M. and van Leeuwen, F.X.R. (2015) Plasma bioavailability and changes in PBMC gene expressionafter treatment of ovariectomized rats with a commercial soysupplement Toxicol. Rep., 2, 308–321
King, A., Houlihan, D.D., Kavanagh, D., Haldar, D., Luu, N., Owen, A., Suresh, S., Than, N.N. et al (2017) Sphingosine-1-phosphate prevents egress of hematopoietic stem cells from liver to reduce fibrosis Gastroenterology, 153, 233–248
Kloth, C., Gruben, N., Ochs, M., Knudsen, L., and Lopez-Rodriguez, E (2019) Flow cytometric analysis of the leukocyte landscape during bleomycin induced lung injury and fibrosis in the rat Am. J. Physiol. Lung. Cell. Mol. Physiol., 317, L109–L126
Konieczna, J., Sánchez, J., Palou, M., Picó, C. and Palou, A. (2015) Blood cell transcriptomic-based early biomarkers of adverse programming effects of gestational calorie restriction and their reversibility by leptin supplementation Sci. Rep., 5: 9088
Kuan, W-L., Poole, E., Fletcher, M., Karniely, S., Tyers, P., Wills, M., Barker, R.A. and Sinclair, J.J. (2012) A novel neuroprotective therapy for Parkinson’s disease using a viral noncoding RNA that protects mitochondrial Complex I activity J. Exp. Med., 209, 1-10
Lühder, F., Kebir, H., Odoardi, F., Litke, T., Sonneck, M., Alvarez, J.I., Winchenbach, J., Eckert, N. et al (2017) Laquinimod enhances central nervous system barrier functions Neurobiol. Dis., 102, 60–69
Masuo, Y., Ohba, Y., Yamada, K., Al-Shammari, A.H., Seba, N., Nakamichi, N., Ogihara, T., Kunishima, M. and Kato, Y. (2018) Combination metabolomics approach for identifying endogenous substrates of carnitine/organic cation transporter OCTN1 Pharm. Res., 35: 224
Mendez-David, I., David, D.J., Guilloux, J-P., Hen, R. and Gardier, A.M. (2015) 5-HT 4 Receptor subtype, βarrestin level, and rapid-onset effects of antidepressant drugs In Neuromethods., 95, Serotonin Receptor Technologies: (ed. Blenau, W. and Baumann, A.) Springer Science+Business Media, New York, pp 101-121
Miller, B.C., Sen, D.R., Abosy, R.A., Bi, K., Virkud, Y.V., LaFleur, M.W., Yates, K.B., Lako, A., Felt, K. (2019) Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade Nat. Immunol., 326, 326–336
Patel, K., Trivedi, R.N., Durgampudi, C., Noel, P., Cline, R.A., DeLany, J.P., Navina, S. and Singh, V.P. (2015) Lipolysis of visceral adipocyte triglyceride by pancreatic lipases converts mild acute pancreatitis to severe pancreatitis independent of necrosis and inflammation Am. J. Pathol., 185, 808-819
Petrov, P.D., Bonet, M.L., Reynés, B., Oliver, P., Palou, A. and Ribot, J. (2016) Whole blood RNA as a source of transcript-based nutrition- and metabolic health-related biomarkers PLoS One, 11: e0155361
Qin, J., Yang, X., Zhang, R-X., Luo, Y-X., Li, J-L., Hou, J., Zhang, C. et al (2015) Monocyte mediated brain targeting delivery of macromolecular drug for the therapy of depression Nanomed: Nanotechnol. Biol. Med., 11, 391–400
Ruiz, E., Oliver, P. and Palou, A. (2015) Gene expression of peripheral blood mononuclear cells is affected by cold exposure Reynés, B., García- Am. J. Physiol. Regul. Integr. Comp. Physiol., 309, R824–R834
Subota, V., Mirkov, I., Demenesku, J., Aleksandrov, A.P., Ninkov, M., Mileusnic, D., Kataranovski, D. and Kataranovski, M., (2016) Transdermal toxicity of topically applied anticoagulant rodenticidewarfarin in rats Environ. Toxicol. Pharmacol., 41, 232–240
Tang, X., Wang, X., Zhao, Y.Y., Curtis, J.M. and Brindley, D.N. (2017) Doxycycline attenuates breast cancer related inflammation by decreasing plasma lysophosphatidate concentrations and inhibiting NF-κB activation Mol. Cancer, 16: 36
2a-9 Blood (ruminant)
Imakawa, K., Nagaoka, K., Nojima, H., Hara, Y. and Christensen, R.K. (2005) Changes in immune cell distribution and IL-10 production are regulated through endometrial IP-10 expression in the goat uterus Am. J. Reprod. Immunol., 53, 54-64
Lin, J., Zhao, Da,1, Wang, J., Wang, Y., Li, H., Yin, X., Yang, L. and Zhou, X. (2015) Transcriptome changes upon in vitro challenge with Mycobacterium bovis in monocyte-derived macrophages from bovine tuberculosisinfected and healthy cows Vet. Immunol. Immunopathol., 163, 146–156
Nagaoka, K., Sakai, A., Nojima, H., Suda, Y., Yokomizo, Y., Imakawa, K., Sakai, S. and Christenson, R.K. (2003) A chemokine, interferon (IFN)--inducible protein 10 kDa, is stimulated by IFN-γ and recruits immune cells in the ovine endometrium Biol. Reprod., 68, 1413-1421
Wang, J., Zhou, X., Pana, B., Yang, L., Yin, X., Xu, B. and Zhao, D. (2013) Investigation of the effect of Mycobacterium bovis infection on bovine neutrophils functions Tuberculosis, 93, 675-687
2b Semen (human)
Semen (human)
Byrn, R.A. and Kiessling, A.A. (1998) Analysis of human immunodeficiency virus in semen: indications of a genetically distinct virus reservoir J. Reprod. Immunol., 41, 161-176
Eyre, R.C., Zheng, G. and Kiessling, A.A. (2000) Multiple drug resistance mutations in human immunodeficiency virus in semen but not blood of a man on antiretroviral therapy Urology, 55, 591xvii-591xx
2c Tissues
Bone marrow
Aliotta, J.M., Pereira, M., Johnson, K.W., de Paza, N., Dooner, M.S., Puente, N., Ayala, C., Brilliant, K. et al (2010) Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription Exp. Hematol., 38, 233–245
Aliotta, J.M., Lee, D., Puente, N., Faradyan, S., Sears, E.H., Amara, A., Goldberg, L., Dooner, M.S., Pereira, M. and Quesenberry, P.J. (2012) Progenitor/stem cell fate determination: interactive dynamics of cell cycle and microvesicles Stem Cells Dev., 21, 1627-1638
Aliotta, J.M., Pereira, M., Amaral, A., Sorokina, A., Igbinoba, Z., Hasslinger, A., El-Bizri, R., Rounds, S.I., Quesenberry, P.J. and Klinger, J.R. (2013) Induction of pulmonary hypertensive changes by extracellular vesicles frommonocrotaline-treated mice Cardiovasc. Res., 100, 354–362
Aliotta, J.M., Pereira, M., Sears, E.H., Dooner, M.S., Wen, S., Goldberg, L.R. and Quesenberry, P.J. (2012) (2015) Lung-derived exosome uptake into and epigenetic odulation of marrow progenitor/stem and ifferentiated cells J. Extracell. Vesicles, 4:26166
Evans, C.A., Tonge, R., Blinco, D., Pierce, A., Shaw, J., Lu, Y., Hanzah, H.G., Gray, A. et al (2004) Comparative proteomics of primitive hematopoietic cell populations reveals differences in expression of proteins regulating motility Blood, 103, 3751-3759
Liu, L., Papa, E.F., Dooner, M.S., Machan, J.T., Johnson, K.W., Goldberg, L.R., Quesenberry, P.J. and Colvin, G.A. (2012) Homing and long-term engraftment of long- and short-term renewal hematopoietic stem cells PLoS One, 7: e31300
Mukai, M., Suruga, N., Saeki, N. and Ogawa, K. (2017) EphA receptors and ephrin-A ligands are upregulated by monocytic differentiation/maturation and promote cell adhesion and protrusion formation in HL60 monocytes BMC Cell Biol., 18: 28
Unwin, R.D., Smith, D.L., Blinco, D., Wilson, C.L., Miller, C.J., Evans, C.A., Jaworska, E., Baldwin, S.A. et al (2006) Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells Blood, 107, 4687-4694
Whetton, A.D., Lu, Y., Pierce, A., Carney, L. and Spooncer, E. (2003) Lysophospholipids synergistically promote primitive hematopoietic cell chemotaxis via a mechanism involving Vav1 Blood, 102, 2798-2802
Brain
Kim, J.H., Choi, J.Y., Kim, S.B., Uyangaa, E., Patil, A.M., Han, Y.W., Park, S-Y., Lee, J.H., Kim, K. and Eo, S.K. (2015) CD11chi dendritic cells regulate Ly-6Chi monocyte differentiation to preserve immune-privileged CNS in lethal neuroinflammation Sci. Rep., 5: 17548
Kim, S.B., Choi, J.Y., Kim, J.H., Uyangaa, E., Patil, A.M., Park, S-Y., Lee, J.H. et al (2015) Amelioration of Japanese encephalitis by blockage of 4-1BB signaling is coupled to divergent enhancement of type I/II IFN responses and Ly-6Chi monocyte differentiation J. Neuroinflamm., 12: 216
Kim, S.B., Choi, J.Y., Uyangaa, E., Patil, A.M., Hossain, F.M.A., Hur, J., Park, S-Y. et al (2016) Blockage of indoleamine 2,3-dioxygenaseregulates Japanese encephalitis via enhancement of type I/II IFN innate and adaptive T-cell responses J. Neuroinflam. 13: 79
Heart
Dobaczewski, M., Xia, Y., Bujak, M., Gonzalez-Quesada, C. and Frangogiannis, N.G. (2010) CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells Am. J. Pathol., 176, 2177–2187
Intestine
Goodyear, A.W., Kumar, A., Dowa, S. and Ryan, E.P. (2014) Optimization of murine small intestine leukocyte isolation for global immune phenotype analysis J. Immunol. Methods, 405, 97–108
Henderson, A.J., Kumar, A., Barnett, B., Dow, S.W. and Ryan, E.P. (2012) Consumption of rice bran increases mucosal immunoglobulin A concentrations and numbers of intestinal Lactobacillus spp. J. Med. Food, 15, 469–475
Lee, J-A., Kim, Y-M., Kim, T-H., Lee, S-H., Lee, C-A., Cho, C-W., Jeon, J-w., Park, J-k. et al (2016) Nasal delivery of chitosan-coated poly(lactide-co-glycolide)-encapsulated honeybee (Apis mellifera) venom promotes Th 1-specific systemic and local intestinal immune responses in weaned pigs Vet. Immunol. Immunopath., 178, 99–106
Okamoto, T., Uemoto, S. and Tabata, Y. (2012) Prevention of trinitrobenzene sulfonic acid-induced experimental colitis by oral administration of a poly(lactic-coglycolic acid) microsphere containing prostaglandin E2 receptor subtype 4 agonist J. Pharmacol. Exp. Ther., 341, 340–349
Wang, X., O’Gorman, M.R.G., Bu, H-F., Koti, V., Zuo, X-L. and Tan, X-D. (2009) Probiotic preparation VSL#3 alters the distribution and phenotypes of dendritic cells within the intestinal mucosa in 57BL/10J mice J. Nutr. 139, 1595–1602
Zellweger, R.M., Prestwood, T.R. and Shresta, S. (2010) Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease Cell Host Microbe 7, 128–139
Liver
Dai, K., Huang, L., Sun, X., Yang, L. and Gong, Z. (2015) Hepatic CD206-positive macrophages express amphiregulin to promote the immunosuppressive activity of regulatory T cells in HBV infection J. Leukoc. Biol., 98, 1071–1080
Henning, J.R., Graffeo, C.S., Rehman, A., Fallon, N.C., Zambirinis, C.P., Ochi, A., Barilla, R., Jamal, M. et al (2013) Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice Hepatology, 58, 589-602
Juchem, K.W., Sacirbegovic, F., Zhang, C., Sharpe, A.H., Russell, K., McNiff, J.M., Demetris, A.J., Shlomchik, M.J. and Shlomchik, W.D. (2018) PD-L1 prevents the development of autoimmune heart disease in graft-versus-host disease J. Immunol., 200, 834–846
Lian Z-X., Okada, T., He, X-S., Kita, H., Liu, Y-J., Ansari, A.A., Kikuchi, K., Ikehara, S. and Gershwin, M.E. (2003) Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations J. Immunol., 170, 2323-2330
Mehal, W., Sheikh, S.Z., Gorelik, L. and Flavell, R.A. (2005) TGF-β signaling regulates CD8+ T cell responses to high- and low-affinity TCR interactions Int. Immunol., 17, 531-538
Mouralidarane, A., Soeda, J., Visconti-Pugmire, C., Samuelsson, A-M., Pombo, J., Maragkoudaki, X., Butt, A., Saraswati, R. et al (2013) Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice Hepatology, 58, 128-138
Nasr, I.W., Reel, M., Oberbarnscheidt, M.H., Mounzer, R.H., Baddoura, F.K., Ruddle, N.H. and Makkis, F.G. (2007) Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection Am. J. Transplant., 7, 1071-1079
Obhrai, J.S., Oberbarnscheidt, M.H., Hand, T.W., Diggs, L., Chalasani, G. and Lakkis, F.G. (2006) Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs J. Immunol., 176, 4051- 4058
Raftery, M.J., Wolter, E., Fillatreau, S., Meisel, H., Kaufmann, S.H.E. and Schönrich, G. (2014) NKT cells determine titer and subtype profile of virus-specific IgG antibodies during herpes simplex virus infection J. Immunol., 192, 4294–4302
Ravichandran, G., Neumann, K., Berkhout, L.K., Weidemann, S., Langeneckert, A.E., Schwinge, D., Poch, T., Huber, S., Schiller, B. et al (2019) Interferon-γ-dependent immune responses contribute to the pathogenesis of sclerosing cholangitis in mice J. Hepatol., 71, 773–782
Salzberger, W., Martrus, G., Bachmann, K., Goebels, H., Heû, L., Koch, M. Langeneckert, A., Lunemann, S. et al (2018) Tissue-resident NK cells differ in their expression profile of the nutrient transporters Glut1, CD98 and CD71 PLoS One, 13: e0201170
Seshadri, S., Allan, D.S.J., Carlyle, J.R. and Zenewicz, L.A. (2017) Bacillus anthracis lethal toxin negatively modulates ILC3 function through perturbation of IL-23-mediated MAPK signalling PLoS Pathog., 13: e1006690
Tzeng, H-T., Tsai, H-F., Liao, H-J., Lin, Y-J., Chen, L., Chen, P-J. and Hsu, P-N. (2012) PD-1 blockage reverses immune dysfunction and hepatitis B viral oersistence in a mouse animal model PLoS One, 7: e39179
Lung
Koyama, S., Akbay, E.A., Li, Y.Y., Herter-Sprie, G.S., Buczkowski, K.A., Richards, W.G., Gandhi, L., Redig, A.J., Rodig, S.J. et al (2016) Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints Nat. Comm., 7: 10501
Licona-Limón, P., Henao-Mejia, J., Temann, A.U., Gagliani, N., Licona-Limón, I., Ishigame, H., Hao, L., Herbert, D.R. and Flavell, R.A. (2013) Th9 cells drive host immunity against gastrointestinal worm infection Immunity, 39, 744–757
Sayes, F., Blanc, C., Ates, L.S., Deboosere, N., Orgeur, M., Le Chevalier, F., Gröschel, M.I., Frigui, W., Song, O-y., et al (2018) Multiplexed quantitation of intraphagocyte Mycobacterium tuberculosis secreted protein effectors Cell Rep., 23, 1072–1084
Pancreatic lymph nodes
Mirlekar, B., Michaud, D., Searcy, R., Greene, K. and Pylayeva-Gupta, Y. (2018) IL35 Hinders endogenous antitumor T-cell immunity and responsiveness to immunotherapy in pancreatic cancer Cancer Immunol. Res., 6; 1014–1024
Spleen
Horibe, T., Kawamoto, M., Kohno, M. and Kawakami, K. (2012) Cytotoxic activity to acute myeloid leukemia cells by Antp-TPR hybrid peptide targeting Hsp90 J. Biosci. Bioeng., 114, 96-103
Kitazawa, Y., Ueta, H., Hünig, T., Sawanobori, Y. and Matsuno, K. (2015) A novel multicolor immunostaining method using ethynyldeoxyuridine for analysis of in situ immuno-proliferative response Histochem. Cell Biol., 144, 195–208
Kivi, G., Teesalu, K., Parik, J., Kontkar, E., Ustav Jr, M., Noodla, L., Ustav, M. and Männik, A. (2016) HybriFree: a robust and rapid method for the development of monoclonal antibodies from different host species BMC Biotechnol., 16: 2
Lee, J-A., Kim, Y-M., Kim, T-H., Lee, S-H., Lee, C-A., Cho, C-W., Jeon, J-w., Park, J-k. et al (2016) Nasal delivery of chitosan-coated poly(lactide-co-glycolide)-encapsulated honeybee (Apis mellifera) venom promotes Th 1-specific systemic and local intestinal immune responses in weaned pigs Vet. Immunol. Immunopath., 178, 99–106
Mirlekar, B., Michaud, D., Searcy, R., Greene, K. and Pylayeva-Gupta, Y. (2018) IL35 Hinders endogenous antitumor T-cell immunity and responsiveness to immunotherapy in pancreatic cancer Cancer Immunol. Res., 6; 1014–1024
Tumour tissue
Buchan, S.L., Dou, L., Remer, M., Booth, S.G., Dunn, S.N., Lai, C., Semmrich, M., Teige, I., Martensson, L., Penfold, C.A. et al (2018) Antibodies to costimulatory receptor 4-1BB enhance anti-tumor immunity via T regulatory cell depletion and promotion of CD8 T cell effector function Immunity, 49, 958–970
Crawford, G., Hayes, M.D., Seoane, R.C., Ward, S., Dalessandri, T., Lai, C., Healy, E., Kipling, D. et al (2018) Epithelial damage and tissue γδ T cells promote a unique tumor-protective IgE response Nat. Immunol., 19, 859–870
Mirlekar, B., Michaud, D., Searcy, R., Greene, K. and Pylayeva-Gupta, Y. (2018) IL35 Hinders endogenous antitumor T-cell immunity and responsiveness to immunotherapy in pancreatic cancer Cancer Immunol. Res., 6; 1014–1024
3 Mononuclear cells (mixer flotation)
3a-1 Blood (human and non-human primates)
Bouwens, M., Afman, L.A. and Müller, M. (2007) Fasting induces changes in peripheral blood mono-nuclear cell gene expression profiles related to increases in fatty acid β-oxidation: functional role of peroxisome proliferator–activated receptor α in human peripheral blood mononuclear cells Am. J. Clin. Nutr., 86, 1515- 1523
Guo, H., Zhang, H., Lu, L., Ezzelarab, M.B. and Thomson, A.W. (2015) Generation, cryopreservation, function and in vivo persistence of ex vivo expanded cynomolgus monkey regulatory T cells Cell. Immunol., 295, 19–28
Huang, K., Liu, PF., Li, X., Chen, SB., Wang, LH., Qin, L., Su, ZH. Et al (2014) Neural progenitor cells from human induced pluripotent stem cells generated less autogenous immune response Sci. China Life Sci., 57, 62– 170
Hutchinson, M.R., La Vincente, S.F. and Somogyi, A.A. (2004) In vitro opioid induced proliferation of peripheral blood immune cells correlates with in vivo cold pressor pain tolerance in humans: a biological marker of pain tolerance Pain, 110, 751-755
Kang, K.B., van der Zypp, A., Iannazzo, L. and Majewski, H. (2006) Age-related changes in monocyte and platelet cyclooxygenase expression in healthy male humans and rats Translat. Res., 148, 289-294
Kwok, Y.H., Hutchinson, M.R., Gentgall, M.G. and Rolan, P.E. (2012) Increased responsiveness of peripheral blood mononuclear cells to in vitro TLR 2, 4 and 7 ligand stimulation in chronic pain patients PLoS One, 7: e44232
Müller, T.H., Döscher, A., Schunter, F. and Scott, C.S. (1997) Manual and automated methods for the determination of leukocyte counts at extreme low levels: comparative evaluation of the Nageotte chamber Transfus. Sci., 18, 505-515
Nievergelt, A., Marazzi, J., Schoop, R., Altmann, K-H. and Gertsch, J. (2011) Ginger phenylpropanoids inhibit IL-1b and prostanoid secretion and disrupt arachidonate-phospholipid remodeling by targeting phospholipases A2 J. Immunol., 187, 4140–4150
Zhang, Y., Li, S-K. and Tsui, S.K-W. (2015) Genome-wide analysis of DNA methylation associated with HIV infection based on a pair of monozygotic twins Genomics Data 6, 12–15
3a-2 Blood (rodent)
DiJoseph, J.F., Dougher, M.M., Kalyandrug, L.B., Armellino, D.C., Boghaert, E.R., Hamann, P.R., Moran, J.K. and Damle, N.K. (2006) Antitumor efficacy of a combination of CMC-544 (inotuzumab ozogamicin), a CD22-targeted cytotoxic immunoconjugate of calicheamicin, and rituximab against non-Hodgkin’s B-cell lymphoma Clin. Cancer Res., 12, 242-249
Grace, P.M., Fabisiak, T.J., Green-Fulgham, S.M., Anderson, N.D., Strand, K.A., Kwilasz, A.J. Galer, E.L., Walker, F.R., Greenwood, B.N. et al (2016) Prior voluntary wheel running attenuates neuropathic pain Pain, 157, 2012–2023
Houghton, J., Macera-Bloch, L.S., Harrison, L., Kim, K.H. and Korah, R.M. (2000) Tumor necrosis factor alpha and interleukin 1 up-regulate gastric mucosal Fas antigen expression in Helicobacter pylori infection Infect. Immun., 68, 1189-1195
Kang, K.B., van der Zypp, A., Iannazzo, L. and Majewski, H. (2006) Age-related changes in monocyte and platelet cyclooxygenase expression in healthy male humans and rats Translat. Res., 148, 289-294
Kwok, Y.H., Tuke, J., Nicotra, L.L., Grace, P.M., Rolan, P.E., and Hutchinson, M.R. (2013) TLR 2 and 4 responsiveness from isolated peripheral blood mononuclear cells from rats and humans as potential chronic pain biomarkers PLoS One, 8: e77799
Mendez-David, I., El-Ali, Z., Hen, R., Falissard, B., Corruble, E., Gardier, A.M., Kerdine-Römer, S. and David, D.J. (2013) A method for biomarker measurements in peripheral blood mononuclear cells isolated from anxious and depressed mice: β-arrestin 1 protein levels in depression and treatment Front. Pharmacol, 4: 124 Mishra, R.S., Carnevale, K.A. and Cathcart, M.K. (2008) iPLA2β: front and center in human monocyte chemotaxis to MCP-1 J. Exp. Med., 205, 347-359
Montminy Paquette, S., Dawit, H., Hickey, M.B., Merisko-Liversidge, E., Almarsson, O. and Deaver, D.R. (2014) Long-acting atypical antipsychotics: characterization of the local tissue response Pharm. Res., 31, 2065–2077
Nosov, M., Wilk, M., Morcos, M., Cregg, M., O’Flynn, L., Treacy, O. and Ritter, T. (2012) Role of lentivirusmediated overexpression of programmed death-ligand 1 on corneal allograft survival Am. J. Transplant., 12, 1313–1322
Shahrara, S., Proudfoot, A.E.I., Woods, J.M., Ruth, J.H., Amin, M.A., Park, C.C., Haas, C.S., Pope, R.M., Haines, G.K., Zha, Y.Y. and Koch, A.E. (2005) Amelioration of rat adjuvant-induced arthritis by Met-RANTES Arthritis Rheumatism, 52, 1907-1919
Shao, X., Rivera, J., Niang, R., Casadevall, A. and Goldman, D.L. (2005) A dual role for TGF-1 in the control and persistence of fungal pneumonia J. Immunol., 175, 6757-6763
Wada, Y., Lu, R., Zhou, D., Chu, J., Przewloka, T., Zhang, S., Li, L., Wu, Y., Qin, J., Balasubramanyam, V., Barsoum, J. and Ono, M. (2007) Selective abrogation of Th1 response by STA-5326, a potent IL-12/IL-23 inhibitor Blood, 109, 1156-1164
3a-3 Blood (ruminant)
Graham-Brown, J., Hartley, C., Clough, H., Kadioglu, A., Baylis, M. and Williams, D.J.L. (2018) Dairy heifers naturally exposed to Fasciola hepatica develop a type 2 immune response and concomitant suppression of leukocyte proliferation Infect. Immun., 86: e00607-17
Olsen, I. and Storset, A.K. (2001) Innate IFN- production in cattle in response to MPP14, a secreted protein from Mycobacterium avium subsp. paratuberculosis Scand. J. Immunol., 54, 305-313
Wan, Y., Tan, J., Asghar, W., Kim, Y-t., Liu, Y. and Iqbal, S.M. (2011) Velocity effect on aptamer-based circulating tumor cell isolation in microfluidic devices J. Phys. Chem. B 115, 13891–13896
Wang, Y., Zhou, X., Lin, J., Yin, F., Xu, L., Huang, Y., Ding, T. and Zhao, D. (2011) Effects of Mycobacterium bovis on monocyte-derived macrophages from bovine tuberculosis infection and healthy cattle FEMS Microbiol. Lett., 321, 30–36
3a-4 Cord blood
Elias, M., Choudhury, N. and Smit Sibinga, CTh. (2003) Cord blood from collection to expansion: Feasibility in a regional blood bank Indian J. Padiatr., 70, 327-336
3a-5 Tissues
Bone marrow
Liu, L., Papa, E.F., Dooner, M.S., Machan, J.T., Johnson, K.W., Goldberg, L.R., Quesenberry, P.J. and Colvin, G.A. (2012) Homing and long-term engraftment of long- and short-term renewal hematopoietic stem cells PLoS One, 7: e31300
Liver
Au-Yeung, B.B. and Fowell, D.J. (2007) A key role for Itk in both IFNγ and IL-4 production by NKT cells J. Immunol., 179, 111-119
Cabrera, M., Pewe, L.L., Harty, J.T. and Frevert, U. (2013) In vivo CD8+ T cell dynamics in the liver of Plasmodium yoelii immunized and infected mice PloS One, 8: e70842
Ginnandrea, M., Pierce, R.H. and Crispe, I.N. (2009) Indirect action of tumor necrosis factor-alpha in liver injury during the CD8+ T cell response to an adeno-associated virus vector in mice Hepatology, 49, 2010-2020
Ishigame, H., Mosaheb, M.M., Sanjabi, S. and Flavell, R.A. (2013) Truncated form of TGF-βRII, but not its absence, induces memory CD8+ T cell expansion and lymphoproliferative disorder in mice J. Immunol., 190, 6340–6350
John, B. and Crispe, I.N. (2005) LR-4 regulates CD8+ T cell trapping in the liver J. Immunol., 175, 1643-1650
John, B., Klein, I. and Crispe, I.N. (2007) Immune role of hepatic TLR-4 revealed by orthotopic mouse liver transplantation Hepatology, 45, 178-186
Klein, I. and Crispe, I.N. (2006) Complete differentiation of CD8+ T cells activated locally within the transplanted liver J. Exp. Med., 203, 437-447
Polakos, N.K., Klein, I., Richter, M.V., Zaiss, D.M., Giannandrea, M., Crispe, I.N. and Topham, D.J. (2007) Early intrahepatic accumulation of CD8+ T cells provides a source of effectors for nonhepatic immune responses J. Immunol., 179, 201-210
Sanjabi, S., Mosaheb, M.M. and Flavell, R.A. (2009) Opposing effects of TGF- and IL-15 cytokines control the number of short-lived effector CD8+ T cells Immunity 31, 131–144
Spahn, J., Pierce, R.H. and Crispe, I.N. (2011) Ineffective CD8+ T-cell immunity to adeno-associated virus can result in prolonged liver injury and fibrogenesis Am. J. Pathol., 179, 2370–2381
Wuensch, S.A., Pierce, R.H. and Crispe, I.N. (2006) Local intrahepatic CD8+ T cell activation by a non-selfantigen results in full functional differentiation J. Immunol., 177, 1689-1697
Zenewicz, L.A., Yancopoulos, G.D., Valenzuela, D.M., Murphy, A.J., Karow, M. and Flavell, R.A. (2007) Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation Immunity, 27, 647-659
Lung
Ishigame, H., Mosaheb, M.M., Sanjabi, S. and Flavell, R.A. (2013) Truncated form of TGF-βRII, but not its absence, induces memory CD8+
T cell expansion and lymphoproliferative disorder in mice J. Immunol., 190, 6340–6350
Licona-Limón, P., Henao-Mejia, J., Temann, A.U., Gagliani, N., Licona-Limón, I., Ishigame, H., Hao, L., Herbert, D.R. and Flavell, R.A. (2013) Th9 cells drive host immunity against gastrointestinal worm infection Immunity, 39, 744–757
Sanjabi, S., Mosaheb, M.M. and Flavell, R.A. (2009) Opposing effects of TGF- and IL-15 cytokines control the number of short-lived effector CD8+ T cells Immunity 31, 131–144
Spleen
DiJoseph, J.F., Dougher, M.M., Kalyandrug, L.B., Armellino, D.C., Boghaert, E.R., Hamann, P.R., Moran, J.K. and Damle, N.K. (2006) Antitumor efficacy of a combination of CMC-544 (inotuzumab ozogamicin), a CD22-targeted cytotoxic immunoconjugate of calicheamicin, and rituximab against non-Hodgkin’s B-cell lymphoma Clin. Cancer Res., 12, 242-249
Lee, I-C., Ko, J-W., Park, S-H., Shin, N-R., Shin, I-S., Moon, C., Kim, S-H., Yun, W-K. et al (2018) Copper nanoparticles induce early fibrotic changes in the liver via TGF-β/Smad signaling and cause immunosuppressive effects in rats Nanotoxicol., 12, 637–651
Liang, Y., Song, D-Z., Liang, S., Zhang, Z-F., Gao, L-X. and Fan, X-H. (2017) The hemagglutininneuramidinase protein of Newcastle disease virus upregulates expression of the TRAIL gene in murine natural killer cells through the activation of Syk and NF-κB PLoS One, 12: e0178746
Shehata, H.M., Khan, S., Chen, E., Fields, P.E., Flavell, R.A. and Sanjabia, S. (2018) Lack of Sprouty 1 and 2 enhances survival of effector CD8+ T cells and yields more protective memory cells Proc. Natal. Acad. Sci. USA, 115, E8939–E8947
Ueta, H., Kitazawa, Y., Sawanobori, Y., Ueno, T., Ueha, S., Matsushima, K. and Matsuno, K. (2018) Single blood transfusion induces the production of donor-specific alloantibodies and regulatory T cellsmainly in the spleen Int. Immunol., 30, 53–67
4 Mononuclear cells (barrier flotation)
4a Human blood
Ahmed, Y., Walton, L.J. and Graham, J. (2004) An improved method for isolation of mononuclear cells from peripheral blood 12th Int. Congr. Immunol., Abstr. 1758
De Roos, B., Duthie, S.J., Polley, A.C.J., Mulholland, F., Bouwman, F.G., Heim, C., Rucklidge, G.J., Johnson, I.T., Mariman, E.C., Daniel, H. and Elliott, R.M. (2008) Proteomic methodological recommendations for studies involving human plasma, platelets and peripheral blood mononuclear cells J. Proteome Res., 7, 2280- 2290
Hartrick, C.T. (2002) Increased production of nitric oxide stimulated by interferon-γ from peripheral blood monocytes in patients with complex regional pain syndrome Neurosci. Lett., 323, 75-77
Wong, J., McLennan, S.V., Molyneaux, L., Min, D., Twigg, S.M. and Yue, D.K. (2009) Mitochondrial DNA content in peripheral blood monocytes: relationship with age of diabetes onset and diabetic complications Diabetologia, 52, 1953–1961
4b Tissues (mouse)
Pinkaew, D., Le, R.J., Chen, Y., Eltorky, M., Teng, B-B. and Fujise, K. (2013) Fortilin reduces apoptosis in macrophages and promotes atherosclerosis Am. J. Physiol. Heart Circ. Physiol., 305, H1519–H1529
5 Polymorphonuclear leukocytes (granulocytes)
5a Bovine peripheral blood
Wang, J., Zhou, X., Pan, B., Wang, H., Shi, F., Gan, W., Yang, L. et al (2013) Expression pattern of interferoninducible transcriptional genes in neutrophils during bovine tuberculosis infection DNA Cell Biol., 32, 480-486
Wang, J., Zhou, X., Pana, B., Yang, L., Yin, X., Xu, B. and Zhao, D. (2013) Investigation of the effect of Mycobacterium bovis infection on bovine neutrophils functions Tuberculosis, 93, 675-687
5b Guinea pig peripheral blood
Takahashi, M., Jeevan, A., Sawant, K., Mc Murray, D.N. and Yoshimura, T. (2007) Cloning and characterization of guinea pig CXCR1 Mol. Immunol., 44, 878-888
5c Human peripheral blood
Barbour, T.D., Ling, G.S., Ruseva, M.M., Fossati-Jimack, L., Cook, H.T., Botto, M. and Pickering, M.C. (2016) Complement receptor 3 mediates renal protection in experimental C3 glomerulopathy Kidney Int., 89, 823–832
Channon, J.Y., Seguin, R.M. and Kasper, L. (2000) Differential infectivity and division of Toxoplasma gondii in human peripheral blood leukocytes Infect. Immun., 68, 4822-4826
Channon, J.Y., Miselis, K.A:, Minns, L.A, Dutta, C. and Kasper, L.H. (2002) Toxoplasma gondii induces granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor secretion by human fibroblasts: implications for neutrophil apoptosis Infect. Immun., 70, 6048-6057
Chiu, H-C., Liang, J-S., Wang, J-S. and Lu, J-F. (2006) Mutational analyses of Taiwanese kindred with Xlinked adrenaleukodystrophy Pediatr. Neurol., 35, 250-256 Fossati-Jimack, L., Ling, G.S., Cortini, A. Szajna, M., Malik, T.H., McDonald, J.U., Pickering, M.C. et al (2013) Phagocytosis is the main CR3-mediated function affected by the lupus-associated variant of CD11b in human myeloid cells PLoS One, 8: e57082
Hudgens, J., Langkamp-Henken, B., Stechmiller, J.K., Herrlinger-Garica, K.A. and Nieves, C. (2004) Immune function is impaired with a mini nutritional assessment score indicative of malnutrition in nursing home elders with pressure ulcers J. Parenter. Enteral Nutr., 28, 416-422
Hung, K-L., Wang, J-S., Keng, W.T., Chen, H-J., Liang, J-S., Ngu, L.H. and Lu, J-F.(2013) Mutational analyses on X-linked adrenoleukodystrophy reveal a novel cryptic splicing and three missense mutations in the ABCD1 gene Pediatr. Neurol., 49, 185-190
Kusumanto, Y.H., Dam, W.A., Hospers, G.A.P., Meijer, C. and Mulder, N.H. (2003) Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor Angiogenesis, 6, 283-287
Martins, R., Maier, J., Gorki, A-D., Huber, K.V.M., Sharif, O., Starkl, P., Saluzzo, S. et al (2016) Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions Nat. Immunol., 17, 1361- 1372
Mikami, J., Kurokawa, Y., Takahashi, T., Miyazaki, Y., Yamasaki, M., Miyata, H., Nakajima, K. et al (2016) Antitumor effect of antiplatelet agents in gastric cancer cells: an in vivo and in vitro study Gastric Cancer, 19, 817–826
Pioli, P.A., Jensen, A.L., Weaver, L.K., Amiel, E., Shen, Z., Shen, L., Wira, C.R. and Guyre, P.M. (2007)
Estradiol attenuates lipopolysaccharide-induced CXC ligand 8 production by human peripheral blood monocytes J. Immunol., 179, 6284-6290
Ponath, V. and Kaina, B. (2017) Death of monocytes through oxidative burst of macrophages and neutrophils: killing in trans PLoS One: e0170347
Ponath, V., Heylmann, D., Haak, T., Woods, K., Becker, H. and Kaina, B. (2019) Compromised DNA repair and signalling in human granulocytes J. Innate Immun., 11, 74–85
Qin, J., Chen, D.W., Hu, H.Y., Cui, Q., Qiao, M.X. and Chen, B.Y. (2007) Surface modification of RGDliposomes for selective drug delivery to monocytes/neutrophils in brain Chem. Pharm. Bull., 55, 1192-1197 Qin, J., Chen, D.W., Hu, H.Y., Qiao, M.X., Zhao, X.L. and Chen, B.Y. (2007) Body distribution of RGDmediated liposomes in brain-targeting drug delivery Yakugaku Zasshi, 127, 1497-1501
Radom-Aizik, S., Zalvidar, Jr., F., Leu, S-Y., Galasetti, P. and Cooper, D.M. (2008) Effects of 30 min of aerobic exercise on gene expression in human neutrophils J. Appl. Physiol., 104, 236-243
Radom-Aizik, S., Zaldivar, F., Oliver, S., Galassetti, P. and Cooper, D.M. (2010) Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes J. Appl. Physiol., 109, 252–261
Rossy, J., Schlicht, D., Engelhardt, B., Niggli, V. (2009) Flotillins interact with PSGL-1 in neutrophils and upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod PLoS One 4: e5403
Salzberg, A.C., Harris-Becker, A., Popova, E.Y., Keasey, N., Loughran, T.P., Claxton, D.F. and Grigoryev, S.A. (2017) Genome-wide mapping of histone H3K9me2 in acute myeloid leukemia reveals large chromosomal domains associated with massive gene silencing and sites of genome instability PLoS One, 12: e0173723
Shen, L., Fahey, J.V., Hussey, S.B., Asin, S.N., Wira, C.R. and Fanger, M.W. (2004) Synergy between IL-8 and GM-CSF in reproductive tract epithelial cell secretions promotes enhanced neutrophil chemotaxis Cell. Immunol., 230, 23-32
Shen, L., Smith, J.M., Shen, Z., Hussey, S.B., Wira, C.R. and Fanger, M.W. (2006) Differential regulation of neutrophil chemotaxis to IL-8 and fMLP by GM-CSF: lack of direct effect of oestradiol Immunology, 117, 205-212
Shen, L., Smith, J.M., Shen, Z., Eriksson, M., Sentman, C. and Wira, C.R. (2007) Inhibition of human neutrophil degranulation by transforming growth factor-1 Clin. Exp. Immunol., 149, 155-161
Smith, J.M., Wira, C.R., Fanger, M.W. and Shen, L. (2006) Human fallopian tube neutrophils – a distinct phenotype from blood neutrophils Am. J. Reprod. Immunol., 56, 218-229
Smith, J.M., Shen, Z., Wira, C.R., Fanger, M.W. and Shen, L. (2007) Effects of menstrual cycle status and gender on human neutrophil phenotype Am. J. Reprod. Immunol., 58, 111-119
Ward, J.R., Heath, P.R., Catto, J.W., Whyte, M.K.B., Milo, M. and Renshaw, S.A. (2011) Regulation of neutrophil senescence by microRNAs PloS One 6: e15810
Wardle, D.J., Burgon, J., Sabroe, I., Bingle, C.D., Whyte, M.K.B. and Renshaw, S.A. (2011) Effective caspase inhibition blocks neutrophil apoptosis and reveals Mcl-1 as both a regulator and a target of neutrophil caspase activation PloS One 6: e15768
Yuan, Z-N., Tolo, K., Schenck, K. and Helgeland, K. (1999) Increased levels of soluble Fc receptor III in gingival fluid from periodontal lesions Oral Microbiol. Immunol., 14, 172-175
5d Mouse
Sellami, M., Meghraoui-Kheddar, A., Terryn, C., Fichel, C., Bouland, N., Diebold, M., Guenounou, M., HéryHuynh, S., and Le Naour, R. (2016) Induction and regulation of murine emphysema by elastin peptides Am. J. Physiol. Lung Cell. Mol. Physiol. 310: L8–L23, 2016
5e Primate (non-human) blood
Lau, M., Vayntrub, T., Grumet, F.C., Lowsky, R., Strober, S., Hoppe, R., Larson, M., Holm, B., Reitz, B. and Borie, D (2004) Short tandem repeat analysis to monitor chimerism in Macaca Fasicularis Am. J. Transplant., 4, 1543-1548 (2004)
5f Rabbit blood
Frevert, C.W., Goodman, R.B., Kinsella, M.G., Kajikawa, O., Ballman, K., Clark-Lewis, I., Proudfoot, A.E.I., Wells, T.N.C. and Martin, T.R. (2002) Tissue-specific mechanisms control the retention of IL-8 in lungs and skin J. Immunol., 168, 3550-3556
Matute-Bello, G., Frevert, C.W., Kajikawa, O., Skerrett, S.J., Goodman, R.B., Park, D.R. and Martin, T.R. (2001) Septic shock and acute lung injury in rabbits with peritonitis Am. J. Respir. Crit. Care Med., 163, 234-243
5g Rat blood
Aleksandrov, A.P., Belij-Rammerstorfer, S., Mirkov, I., Subota, V., Kulas, J., Kataranovski, D. and Kataranovski, M. (2018) Oral warfarin affects some aspects of systemic immunomodulation with topical dinitrochlorobenzene (DNCB) in rats Cutan. Ocul. Toxicol., 47, 29–35
Belij, S., Miljković, D., Popov, A., Subota, V., Timotijević, G., Slavić, M., Mirkov, I., Kataranovski, D. and Kataranovski, M. (2012) Effects of subacute oral warfarin administration on peripheral blood granulocytes in rats Food Chem. Toxicol., 50, 1499–1507
Djokic, J., Ninkov, M., Mirkov, I., Aleksandrov, A.P., Zolotarevski, L., Kataranovski, D. and Kataranovski, M. (2014) Differential effects of cadmium administration onperipheral, blood granulocytes in rats Environ. Toxicol. Pharmacol., 37, 210-219
Mishra, A., Guo, Y., Zhang, L., More, S., Weng, T., Chintagari, N.R., Huang, C., Liang, Y., Pushparaj, S. et al (2016) A critical role for P2X7 receptor–induced VCAM-1 shedding and neutrophil infiltration during acute lung injury J. Immunol., 197, 2828–2837
Piubelli, C., Galvani, M., Hamdan, M., Domenici, E. and Righetti, P.G. (2002) Proteome analysis of rat polymorphonuclear leukocytes: A two-dimensional electrophoresis/ mass spectrometry approach Electrophoresis, 23, 298-310
Subota, V., Mirkov, I., Demenesku, J., Aleksandrov, A.P., Ninkov, M., Mileusnic, D., Kataranovski, D. and Kataranovski, M., (2016) Transdermal toxicity of topically applied anticoagulant rodenticide warfarin in rats Environ. Toxicol. Pharmacol., 41, 232–240
OptiPrep™ Reference List RC01; 5th edition, January 2020
OptiPrep™ Reference List RC02
Purification of platelets from whole blood/removal from blood leukocyte preparations
- OptiPrep™ is a sterile 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- This Reference List principally provides (in Section 2) a list of all papers reporting the use of
- OptiPrep™ in the purification of platelets from blood taken humans and from experimental animals and for their removal from leukocyte preparations. Section 1 briefly summarizes the advantages of using the OptiPrep™ methodology.
1. The OptiPrep™ technology
The routine method for the purification of platelets from whole blood is to centrifuge the blood at a g-force that will sediment the erythrocytes and leukocytes, while leaving the smaller platelets suspended in the plasma supernatant. This is simple concept is technically very difficult; if the g-force is high enough to sediment all of
the erythrocytes and leukocytes then many of the platelets will also be in the cell pellet; if it is sufficiently low to prevent the majority of platelets from pelleting then many of less dense leukocytes will also remain in the plasma supernatant. As a result the procedure needs to be repeated several times to recover more of the platelets from the pellet and to remove more leukocytes from the supernatant. It becomes a very tedious process.
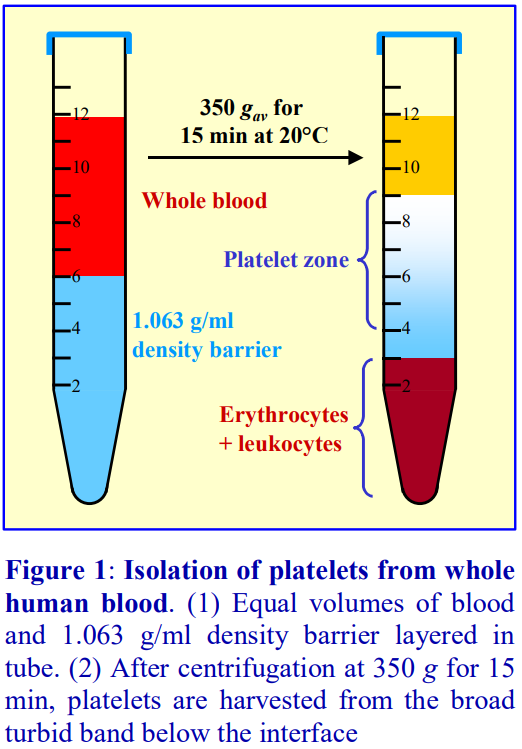 In the one-step OptiPrep™ method, OptiPrep™ is diluted with a buffered saline to produce a solution of density 1.063 g/ml (lower
In the one-step OptiPrep™ method, OptiPrep™ is diluted with a buffered saline to produce a solution of density 1.063 g/ml (lower
than all of the leukocytes); an equal volume of whole blood is layered over it and centrifuged at 350 g for 15 min. The procedure is summarized in Figure 1. Typically 5 ml each of blood and the density barrier are used in a standard 15 ml tube. The separation is based on the much lower sedimentation rate of the platelets. The yields are approx. 90-92%. The method is equally effective for removing platelets from previously purified leukocyte fractions,
particularly human peripheral blood mononuclear cells (PBMCs) produced by centrifugation of blood over a density barrier of approx. 1.077 g/ml (e.g. Lymphoprep™). A common method of removing the platelets is to dilute the cell harvest with saline and to use the lowest feasible g-force to pellet the cells selectively (usually about 300-350 g for 5-10 min). This is then repeated at least twice more – like the preparation of platelets by differential centrifugation (see above) this is inefficient, tedious and detrimental to the cells. Instead the platelets can be removed in a single step by layering the diluted harvest over the 1.063 g/ml barrier.
The method is described in OptiPrep™ Application Sheet (C13); this may be accessed from the Index of the “Mammalian and non-mammalian cells” file on the following website: www.OptiPrep.com (click on “Methodology”).
2. Reference List
- The references are listed according to source of platelets and research topic. Within each section references are listed alphabetically by first author.
Canine platelets
Isolation and purification
Bonnefont-Rebeix, C., de Carvalho, C.M., Bernaud, J., Chabanne, L., Marchal, T. and Rigal, D. (2006) CD86 molecule is a specific marker for canine monocyte-derived dendritic cells Vet. Immunol. Immunopathol., 109, 167-176
Hennink, I., van Leeuwen, M.W., Penning, L.C. and Piek, C.J. (2018) Increased number of tissue factor protein expressing thrombocytes in canine idiopathic immune mediated hemolytic anemia Vet. Immunol. Immunopathol., 196, 22–29
Trichler, S.A., Bulla, S.C., Thomason, J., Lunsford, K.V. and Bulla, C. (2013) Ultra-pure platelet isolation from canine whole blood BMC Vet. Res., 9:144
Human platelets
Acetylcholinesterase expression
Camp, S., De Jaco, A., Zhang, L., Marquez, M., De La Torre, B. and Taylor, P. (2008) Acetylcholinesterase expression in muscle is specifically controlled by a promoter-selective enhancesome in the first intron J. Neurosci., 28, 2459-2470
Activation
Bagamery, K. and Landau, R. (2005) Different platelet activation levels in non-pregnant, normotensive pregnant, pregnancy-induced hypertensive and pre-eclamptic women. A pilot study of flow cytometric analysis Eur. J. Obstet. Gynecol. Reprod. Biol., 121, 117-123
Calzi, S.L., Purich, D., Chang, K., Afzal, A., Phillips, J., Agarwal, A., Segal, M. and Grant, M.B. (2007) Cytoskeletal rearrangements via vasodilator-stimulated phosphoprotein (VASP) phosphorylation and redistribution in human retinal endothelial cells (HREC), endothelial precursor cells (EPC) and platelets:
implications for retinal vessel repair Invest. Ophthalmol. Vis. Sci., 48, E-Abstr 4096 Natal, C., Restituto, P., Inigo, C., Colina, I., Diez, J. and Varo, N. (2008) The proinflammatory mediator CD40 ligand is increased in the metabolic syndrome and modulated by adiponectin J. Clin. Endocrinol. Metab., 93, 2319-2327
α2-Adrenoreceptors
Piletz, J., Baker, R. and Halaris, A. (2008) Platelet imidazoline receptors as state markers of depressive symptomatology J. Psychiatr. Res., 42, 41-49
Aggregation
Onselaer, M-B., Eeckhoudt, S., de Meester, C., Hue, L., Vanoverschelde, J-L., Bertrand, L., Oury, C., Beauloye, C., Horman, S. (2010) Calcium and calmodulin-dependent kinase kinase beta signalling controls human platelet aggregation Circulation, 122, Abstract 17868
Calzi, S.L., Purich, D., Chang, K., Afzal, A., Phillips, J., Agarwal, A., Segal, M. and Grant, M.B. (2007) Cytoskeletal rearrangements via vasodilator-stimulated phosphoprotein (VASP) phosphorylation and redistribution in human retinal endothelial cells (HREC), endothelial precursor cells (EPC) and platelets: implications for retinal vessel repair Invest. Ophthalmol. Vis. Sci., 48, E-Abstr 4096
Calzi, S.L., Purich, D.L., Chang, K.H., Afzal, A., Nakagawa, T., Busik, J.V., Agarwal, A., Segal, M.S. and Grant, M.B. (2008) Carbon monoxide and nitric oxide mediate cytoskeletal reorganization in microvascular cells via vasodilator-stimulated phosphoprotein phosphorylation evidence for blunted responsiveness in diabetes Diabetes, 57, 2488-2494
AMP-activated protein kinase
Onselaer, M-B., Oury, C., Hunter, R.W., Erckhoudt, S., Barile, N., Lecut, C., Morel, N. et al (2014) The Ca2+/calmodulin-dependent kinase kinase β-AMP-activated protein kinase-α1 pathway regulates phosphorylation of cytoskeletal targets in thrombin-stimulated human platelets J. Thromb. Haemost., 12, 973– 986
Amyotrophic lateral sclerosis (sporadic)
Hishizawa, M., Yamashita, H., Akizuki, M., Urushitani, M. and Takahashi, R. (2019) TDP-43 levels are higher in platelets from patients with sporadic amyotrophic lateral sclerosis than in healthy controls Neurochem. Internat., 124, 41–45
Antioxidative protection
Poniedziałek, B., Mleczek, M., Niedzielski, P., Siwulsk, M., Gasecka, M., Kozak, L., Komosa, A. and Rzymski, P. (2017) Bio‑enriched Pleurotus mushrooms for deficiency control and improved antioxidative protection of human platelets? Eur. Food Res. Technol., 243, 2187–2198
Atherogenesis
Heger, L.A., Hortmann, M., Albrecht, M., Colberg, C., Peter, K., Witsch, T., Stallmann, D., Zirlik, A., Bode, C., Duerschmied, D. and Ahrens, I. (2019) Inflammation in acute coronary syndrome: Expression of TLR2 mRNA is increased in platelets of patients with ACS PLoS One, 14: e0224181
Bacterial infections/interactions
Arbesu, I., Bucsaiova, M., Fischer, M.B. and Mannhalter, C. (2016) Platelet-borne complement proteins and their role in platelet–bacteria interactions J. Thromb. Haemost. 14, 2241–2252
Fejes, A.V., Best, M.G., van der Heijden, W.A., Vancura, A., Verschueren, H., de Mast, Q., Wurdinger, T. and Mannhalter, C. (2018) Impact of Escherichia coli K12 and O18:K1 on human platelets: Differential effects on platelet activation, RNAs and proteins Sci. Rep., 8: 16145
Calumenin – thrombospondin-1 binding
Hansen, G.A.W., Vorum, H., Jacobsen, C. and Honore, B. (2009) Calumenin but not reticulocalbin forms a Ca2+-dependent complex with thrombospondin-1. A potential role in haemostasis and thrombosis Mol. Cell Biochem., 320, 25–33
Cancer cells, interactions with
Best, M.G., Sol, N., In ‘t Veld, S.G.J.G., Vancura, A., Muller, M., Niemeijer, A-L. N., Fejes, A.V., Fat, LA.T.K., In ‘t Veld, A.E.H. Leurs, C. et al (2017) Swarm intelligence enhanced detection of non-small-cell lung cancer using tumor-educated platelets Cancer Cell, 32, 238–252
Bulla, S.C., Badial, P.R., Silva, R.C., Lunsford, K. and Bulla (2017) Platelets inhibit migration of canine osteosarcoma cells J. Comp. Path., 156, 3-13
Da Costa Barros, T.A., de Oliveira Batista, D., de Carvalho, A.T., da Costa Faria, N.R., Barreto-Vieirae, D.F., Jácome, F.C., Barth, O.M., Ribeiro Nogueira, R.M. et al (2019) Different aspects of platelet evaluation in dengue: Measurement of circulating mediators, ability to interact with the virus, the degree of activation and quantification of intraplatelet protein content Virus Res., 260, 163–172
Hayashi, Y., Jia, W., Kidoya, H., Muramatsu, F., Tsukada, Y. and Takakura, N. (2019) Galectin-3 inhibits cancer metastasis by negatively regulating integrin β3 expression Am. J. Pathol., 189, 900-910
Hickish, T., Mohanty, P., Michael, S., Shivaswamy, S., Sunley, K., Varshney, A., Martin, R. and Simard, J. (2017) Modulation of platelet levels by an anti-IL-1α antibody (MABp1) in advanced colorectal cancer patients Eur. J. Cancer, 72, Suppl.1, S70
Zhang, X., Wang, J., Chen, Z., Hu, Q., Wang, C., Yan, J., Dotti, G., Huang, P, and Gu, Z. (2018) Engineering PD-1-presenting platelets for cancer immunotherapy Nano Lett., 5716−5725
Carbon monoxide
Calzi, S.L., Purich, D.L., Chang, K.H., Afzal, A., Nakagawa, T., Busik, J.V., Agarwal, A., Segal, M.S. and Grant, M.B. (2008) Carbon monoxide and nitric oxide mediate cytoskeletal reorganization in microvascular cells via vasodilator-stimulated phosphoprotein phosphorylation evidence for blunted responsiveness in diabetes Diabetes, 57, 2488-2494
Coagulation factor
Dashty, M., Akbarkhanzadeh, V., Zeebregts, C.J., Spek, C.A., Sijbrands, E.J., Peppelenbosch, M.P. and Rezaee, F. (2012) Characterization of coagulation factor synthesis in nine human primary cell types Nat. Sci. Rep., 2: 787
Community-acquired pneumonia
Laškaj, R., Salvica, D., Čepelak, I. and Kuzman, I. (2007) Gamma-glutamyltransferase activity and total antioxidant status in serum and platelets of patients with community-acquired pneumonia Arch. Med. Res., 38, 424-431
Laškaj, R., Dodig, S., Čepelak, S. and Kuzman, I. (2009) Superoxide dismutase, copper and zinc concentrations in platelet-rich plasma of pneumonia patients Ann. Clin. Biochem., 46, 123–128
Dengue virus haemorrhagic fever
Noisakran, S., Chokephaibulkit, K., Songprakhon, P., Onlamoon, N., Hsiao, H-M., Villinger, F., Ansari, A. and Perng, G.C. (2009) A re-evaluation of the mechanisms leading to Dengue hemorrhagic fever Ann. N.Y. Acad. Sci., 1171, E24-E35
Tsai, J-J., Chang, J-S., Chang, K., Chen, P-C., Liu, L-T., Ho, T-C., Tan, S.S., Chien, Y-W., Lo, Y-C., Pern, GC. (2017) Transient monocytosis subjugates low platelet count in adult Dengue patients Biomed Hub 2: 457785
Diabetic patients
Calzi, S.L., Purich, D.L., Chang, K.H., Afzal, A., Nakagawa, T., Busik, J.V., Agarwal, A., Segal, M.S. and Grant, M.B. (2008) Carbon monoxide and nitric oxide mediate cytoskeletal reorganization in microvascular cells via vasodilator-stimulated phosphoprotein phosphorylation evidence for blunted responsiveness in diabetes Diabetes, 57, 2488-2494
Exosomes
Aatonen, M., Valkonen, S., Böing, A., Yuana, Y., Nieuwland, R. and Siljander, P. (2017) Isolation of plateletderived extracellular vesicles In Methods Mol. Biol., 1545, Exosomes and Microvesicles: Methods and Protocols (ed. Hill, A.F.) Springer Science+Business Media, LLC pp 177-188
Chiang, C-y. and Chen, C. (2018) An increased level of CD41-positive extracellular vesicles recovered by 100,000 ×g centrifugation from stimulated platelets J. Extracell. Ves., 7, Suppl. 1, Abstr. # PF05.07
Factor VIII
Gilbert, G.E., Novakovic, V.A., Shi, J., Rasmussen, J. and Pipe, S.W. (2015) Platelet binding sites for factor VIII in relation to fibrin and phosphatidylserine Blood, 126, 1237-1244
Meems, H., Meijer, A.B., Cullinan, D.B., Mertens, K. and Gilbert, G.E. (2009) Factor VIII C1 domain residues Lys 2092 and Phe 2093 contribute to membrane binding and cofactor activity Blood 114, 3938-3946
Phillips, J.E., Lord, S.T. and Gilbert, G.E. (2004) Fibrin stimulates platelets to increase factor VIIIa binding site expression J. Thromb. Haemost., 2, 1806-1815
Guanyl cyclase/cGMP signaling
Gambaryan, S., Kobsar, A., Hartmann, S., Birschmann, I., Kuhlencordt, P.J., Müller-Esterl, W., Lohmann, S.M. and Walter, U. (2008) NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity J. Thromb. Haemost., 6, 1376-1384
HPA-1a antigen
Ahlen, M.T., Husebekk, A., Killie, M.K., Skogen, B. and Stuge, T.B. (2009) T-cell responses associated with neonatal alloimmune thrombocytopenia: isolation of HPA-1a–specific, HLA-DRB3*0101–restricted CD4+ T cells Blood, 113, 3838-3844
ILK-PINCH-Parvin complex signalling
Birschmann, I., Mietner, S., Dittrich, M., Pfrang, J., Dandekar, T. and Walter, U. (2008) Use of functional highly purified human platelets for the identification of new proteins of the IPP signaling pathway Thromb. Res., 122, 59–68
Imidazoline receptors
Piletz, J., Baker, R. and Halaris, A. (2008) Platelet imidazoline receptors as state markers of depressive symptomatology J. Psychiatr. Res., 42, 41-49
Metabolic syndrome
Natal, C., Restituto, P., Inigo, C., Colina, I., Diez, J. and Varo, N. (2008) The proinflammatory mediator CD40 ligand is increased in the metabolic syndrome and modulated by adiponectin J. Clin. Endocrinol. Metab., 93, 2319-2327
Methodology
Bagamery, K., Kvell, K., Barnet, M., Landau, R. and Graham, J. (2005) Are platelets activated after a rapid, one-step density gradient centrifugation? Evidence from flow cytometric analysis Clin. Lab. Haem., 27, 75-77
Bagamery, K., Kvell, K., Landau, R. and Graham, J. (2005) Flow cytometric analysis of CD41-labeled platelets isolated by the rapid, one-step OptiPrep method from human blood Cytometry Part A, 65A, 84-87
Wrzyszcz, A., Urbaniak, J., Sapa, A. and Woźniak, M. (2017) An efficient method for isolation of representative and contamination-free population of blood platelets for proteomic studies Platelets, 28, 43-53
Nitric oxide
Calzi, S.L., Purich, D.L., Chang, K.H., Afzal, A., Nakagawa, T., Busik, J.V., Agarwal, A., Segal, M.S. and Grant, M.B. (2008) Carbon monoxide and nitric oxide mediate cytoskeletal reorganization in microvascular cells via vasodilator-stimulated phosphoprotein phosphorylation evidence for blunted responsiveness in diabetes Diabetes, 57, 2488-2494
Gambaryan, S., Kobsar, A., Hartmann, S., Birschmann, I., Kuhlencordt, P.J., Müller-Esterl, W., Lohmann, S.M. and Walter, U. (2008) NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity J. Thromb. Haemost., 6, 1376-1384
Prostanoid receptors
Coleman, R.A., Woodrooffe, A.J., Clark, K.L., Toris, C.B., Fan, S., Wang, J.W. and Woodward, D.F. (2019) The affinity, intrinsic activity and selectivity of a structurally novel EP2 receptor agonist at human prostanoid receptors Br. J. Pharmacol., 176, 687–698
Proteomics
Rieckmann, J.C., Geiger, R., Hornburg, D., Wolf, T., Kveler, K., Jarrossay, D., Sallusto, F. et al (2017) Social network architecture of human immune cells unveiled by quantitative proteomics Nat. Immunol., 18, 583-593
Tsai, J-J., Chang, J-S., Chang, K., Chen, P-C., Liu, L-T., Ho, T-C., Tan, S.S., Chien, Y-W., Lo, Y-C., Pern, GC. (2017) Transient monocytosis subjugates low platelet count in adult Dengue patients Biomed Hub 2: 457785
Removal from cell preparations
Astley, S.B., Elliott, R.M., Archer, D.B. and Southon, S. (2004) Evidence that dietary supplementation with carotenoids and carotenoid-rich foods modulates the DNA damage: repair balance in human lymphocytes Br. J. Nutr., 91, 63-72
Beppu, S., Nakajima, Y., Shibasaki, M., Kageyama, K., Mizobe, T., Shime, N. and Matsuda, N. (2009) Phosphodiesterase 3 inhibition reduces platelet activation and monocyte tissue factor expression in knee arthroplasty patients Anesthesiology; 111,1227–1237
Brown, A.J., Watts, G.F., Burnett, J.R., Dean, R.T. and Jessup, W. (2000) Sterol 27-hydroxylase acts on 7- ketocholesterol in human atherosclerotic lesions and macrophages in culture J. Biol. Chem., 275, 27627-27633
Ivetic, N., Nazi, I., Karim, N., Clare, R., Smith, J.W., Moore, J.C., Hope, K.J., Kelton, J.G. and Arnold, D.M. (2016) Producing megakaryocytes from a human peripheral blood source Transfusion 56, 1066–1074
Jain, A. and Munn, L.L. (2009) Determinants of leukocyte margination in rectangular microchannels PLoS One 4: e7104
Kockx, M., Rye, K-A., Gaus, K., Quinn, C. M., Wright, J., Sloane, T., Sviridov, D., Fu, Y. et al (2004) Apolipoprotein A-I stimulated apolipoprotein E secretion from human macrophages is independent of cholesterol efflux J. Biol. Chem., 279, 25966-25977
Mohan, S., Mohan, N., Valente, A.J. and Sprague, E.A. (1999) Regulation of low shear flow-induced HAEC VCAM-1 expression and monocyte adhesion Am. J. Physiol. Cell Physiol., 276, C1100-C1107
Mohan, S., Hamuro, M., Sorrescu, G.P., Koyoma, K., Sprague, E.A., Jo, H., Valente, A.J., Prihoda, T.J. and Natarajan, M. (2003) IκBα-dependent regulation of low-shear flow-induced NF-κB activity: role of nitric oxide Am. J. Physiol. Cell Physiol., 284, C1039-C1047
Pecher, G., Schirrmann, T., Kaiser, L. and Schenk, J.A. Efficient cryopreservation of dendritic cells transfected with cDNA of a tumour antigen for clinical application Biotechnol. Appl. Biochem., 34, 161-166
Pecher, G., Häring, A., Kaiser, L. and Thiel, E. (2002) Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trial Cancer Immunol. Immunother., 51, 669-673
Retinal vascular repair
Calzi, S.L., Purich, D., Chang, K., Afzal, A., Phillips, J., Agarwal, A., Segal, M. and Grant, M.B. (2007) Cytoskeletal rearrangements via vasodilator-stimulated phosphoprotein (VASP) phosphorylation and redistribution in human retinal endothelial cells (HREC), endothelial precursor cells (EPC) and platelets: implications for retinal vessel repair Invest. Ophthalmol. Vis. Sci., 48, E-Abstr 4096
Serine residue, differential phosphorylation of
Calzi, S.L., Purich, D.L., Chang, K.H., Afzal, A., Nakagawa, T., Busik, J.V., Agarwal, A., Segal, M.S. and Grant, M.B. (2008) Carbon monoxide and nitric oxide mediate cytoskeletal reorganization in microvascular cells via vasodilator-stimulated phosphoprotein phosphorylation evidence for blunted responsiveness in diabetes Diabetes, 57, 2488-2494
α-Synuclein
Barbour, R., Kling, K., Anderson, J.P., Banducci, K., Cole, T., Diep, L., Fox, M., Goldstein, J.M., Soriano, F., Seubert, P. and Chilcote, T.J. (2008) Red blood cells are the major source of alpha-synuclein in blood Neurodegener. Dis., 5, 55-59
Thrombin stimulation
Hansen, G.A.W., Vorum, H., Jacobsen, C. and Honore, B. (2009) Calumenin but not reticulocalbin forms a Ca2+-dependent complex with thrombospondin-1. A potential role in haemostasis and thrombosis Mol. Cell Biochem., 320, 25–33
Thrombocytopenia
Ahlen, M.T., Husebekk, A., Killie, M.K., Skogen, B. and Stuge, T.B. (2009) T-cell responses associated with neonatal alloimmune thrombocytopenia: isolation of HPA-1a–specific, HLA-DRB3*0101–restricted CD4+
cells Blood, 113, 3838-3844
Noisakran, S., Chokephaibulkit, K., Songprakhon, P., Onlamoon, N., Hsiao, H-M., Villinger, F., Ansari, A. and Perng, G.C. (2009) A re-evaluation of the mechanisms leading to Dengue hemorrhagic fever Ann. N.Y. Acad. Sci., 1171, E24-E35
Tissue factor pathway
Girard, T.J., Grunz, K., Lasky, N.M., Malone, J.P. and Broze Jr, G.J. (2018) Re-evaluation of mouse tissue factor pathway inhibitor and comparison of mouse and human tissue factor pathway inhibitor physiology J. Thromb. Haemost., 16, 2246–2257
Ventricular tachycardia
Josephs, K., Patel, K., Janson, C.M., Montagna, C. and McDonald, T.V. (2017) Compound heterozygous CASQ2 mutations and long-term course of catecholaminergic polymorphic ventricular tachycardia Mol. Genet. Genom. Med. 5, 788–794
Primate bone marrow platelets
Dengue virus
Noisakran, S., Onlamoona, N., Hsiao, H-M., Clark, K.B,., Villingera, F., Ansaria, A.A. and Perng, G.C. (2012) Infection of bone marrow cells by dengue virus in vivo Exp. Hematol., 40, 250–259
Rodent platelets
Aggregation
Camp, S., Zhang, L., Krejci, E., Dobbertin, A., Bernard, V., Girard, E., Duysen, E.G., Lockridge, O., De Jaco, A. and Taylor, P. (2010) Contributions of selective knockout studies to understanding cholinesterase disposition and function Chem-Biol. Interact., 187, 72–77
Chen, B., Guo, L., Fan, C., Bolisetty, S., Joseph, R., Wright, M.M., Agarwal, A. and George, J.F. (2009) Carbon monoxide rescues heme oxygenase-1-deficient mice from arterial thrombosis in allogeneic aortic transplantation Am. J. Pathol., 175, 422–429
Cunningham, T.J., Souayah, N., Jameson, B., Mitchell, J. and Yao, L. (2004) Systemic treatment of cerebral cortex lesions in rats with a new secreted phospholipase A2 inhibitor J. Neurotrauma, 21, 1683-1691
Platelet depletion
Hu, M., Li, S., Menon, S., Liu, B., Hu, M.S., Longaker, M.T. and Lorenza, H.P. (2016) Expansion and hepatic differentiation of adult blood-derived CD34+ progenitor cells and promotion of liver regeneration after acute injury Stem Cells Translat. Med., 5, 723-732
Platelet destruction in vivo
Bakchoul, T., Kubiak, S., Krautwurst, A., Roderfeld, M., Siebert, H.C., Bein, G., Sachs, U.J. and Santoso, S. (2011) Low-avidity anti-HPA-1a alloantibodies are capable of antigen-positive platelet destruction in the NOD/SCID mouse model of alloimmune thrombocytopenia Transfusion, 51, 2455-2461
Platelet survival
Fuhrmann, J., Jouni, R., Alex, J., Zöllner, H., Wesche, J., Greinacher, A. and Bakchoul, T. (2016) Assessment of human platelet survival in the NOD/SCID mouse model: technical considerations Transfusion, 56, 1370-1376
Thrombocytopenia model
Bakchoul, T., Fuhrmann, J., Chong, B.H., Bougie, D. and Aster, R. (2015) Recommendations for the use of the non-obese diabetic/severe combined immunodeficiency mouse model in autoimmune and drug-induced thrombocytopenia J, Thromb.Haemost., 13, 1–4
Tissue factor pathway
Girard, T.J., Grunz, K., Lasky, N.M., Malone, J.P. and Broze Jr, G.J. (2018) Re-evaluation of mouse tissue factor pathway inhibitor and comparison of mouse and human tissue factor pathway inhibitor physiology J. Thromb. Haemost., 16, 2246–2257
OptiPrep™ Reference List RC02; 5th edition, February 2020
OptiPrep™ Reference List RC03
Viable/non-viable cell separation
This Reference List includes, in Section 2, a short summary of the methodology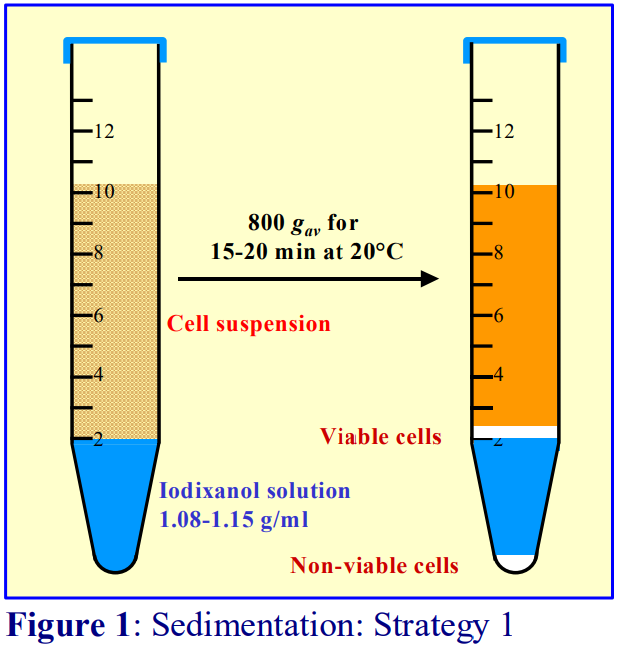
1. Introduction
Studies of cells from tissues (mammalian and non-mammalian) involve both mechanical and enzymic disaggregation of for example, liver or spleen or plant leaves, to release single cells. Prior to fractionation of different cell types it is often necessary to remove partially disrupted cells, released organelles and cytoplasm from damaged cells, other often fibrous debris from the tissue and any residual enzymes used in the disaggregation process.
2. Methodology
2a. Sedimentation mode
In iodixanol solutions, non-viable cells are denser than viable cells and there are two basic strategies for separating out 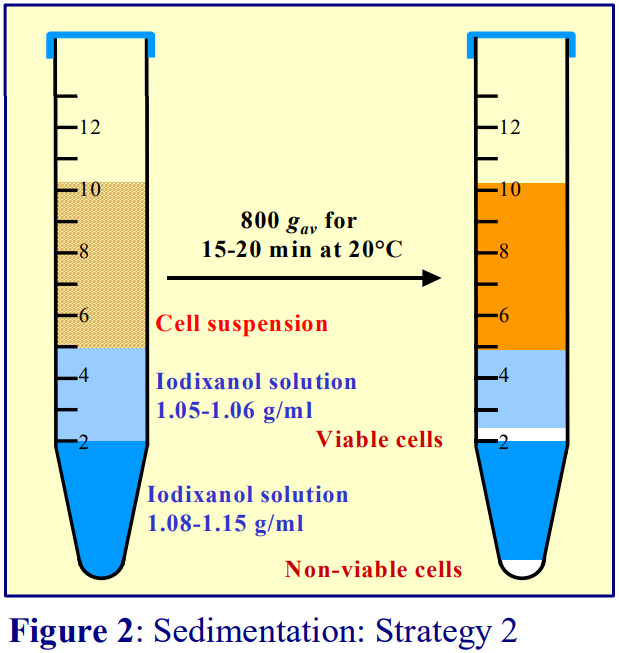 the less dense viable cells, sedimentation or flotation. The often-used Sedimentation Strategy 1, is a simple density barrier (Figure 1). The density of the barrier is chosen to be slightly higher than that of the cells of interest. At the end of the centrifugation the viable cells band and non-viable cells separate acrosss the barrier. The problems with this simple system are: (1) soluble proteins in the sample layer diffuse into the barrier; (2) while most nuclei will probably pellet, most subcellular particles released from disrupted cells will remain in sample layer zone; (3) partially disrupted cells will band across the viable cell layer. In Sedimentation Strategy 2 (Figure 2) a lower density layer (1.05- 1.06 g/ml) is interposed between the resolving layer and the sample. The viable and non-viable cells are again separated across the denser layer but the diffusing soluble proteins will not reach the viable cell layer and there will be less contamination by smaller subcellular particles.
the less dense viable cells, sedimentation or flotation. The often-used Sedimentation Strategy 1, is a simple density barrier (Figure 1). The density of the barrier is chosen to be slightly higher than that of the cells of interest. At the end of the centrifugation the viable cells band and non-viable cells separate acrosss the barrier. The problems with this simple system are: (1) soluble proteins in the sample layer diffuse into the barrier; (2) while most nuclei will probably pellet, most subcellular particles released from disrupted cells will remain in sample layer zone; (3) partially disrupted cells will band across the viable cell layer. In Sedimentation Strategy 2 (Figure 2) a lower density layer (1.05- 1.06 g/ml) is interposed between the resolving layer and the sample. The viable and non-viable cells are again separated across the denser layer but the diffusing soluble proteins will not reach the viable cell layer and there will be less contamination by smaller subcellular particles.
In both top-loading strategies all of the particles (cells, non-viable cells, partially broken cells, organelles) will be moving in the same direction and the problems described above will be compounded by the tendency for particles to aggregate at the interfaces. Hence it is usually necessary to wash the recovered viable cells several times to remove contamination by smaller particles and partially-disrupted cells.
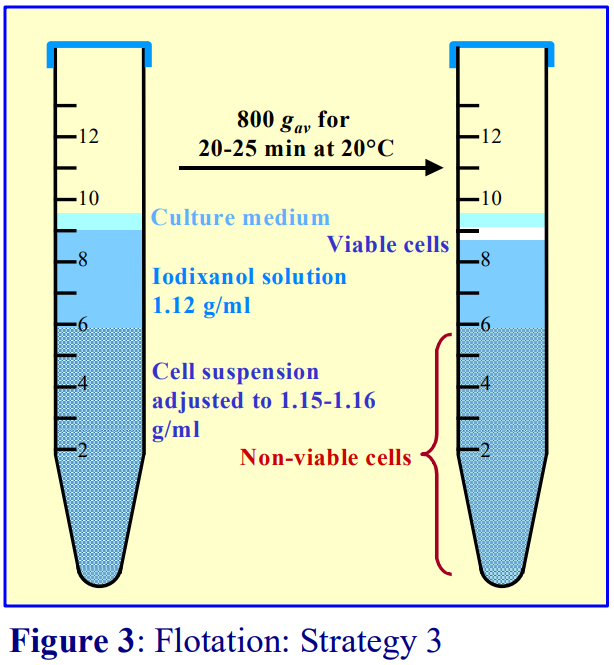 2b. Flotation mode (Strategy 3)
2b. Flotation mode (Strategy 3)
In the flotation mode the sample is adjusted to a density greater than that of the viable cells (Figure 3); this will allow the layering a lower density solution whose density is still greater than that of the viable cells. A small layer of saline is layered on top. During the centrifugation the viable cells float to the top interface; non-viable, partially broken cells and nuclei sediment slowly towards the bottom of the tube. The topmost layer (culture medium or saline) is not critical to the separation but prevents the viable cells from banding at an air/liquid interface. The big advantage of the method is that the viable cells move in the opposite direction to that of the non-viable and partially broken cells and nuclei. Aggregation at interfaces is therefore minimized and there should be layer of clean liquid that is essentially devoid of any contaminants.
2c. Gradient solution preparation
All of the gradient solutions are very easily prepared from OptiPrep, as sterile 60% (w/v) solution, which makes the preparation of gradient solutions very easy. The methodology using iodixanol gradients for removal of non-viable cells is given in Application Sheet C14. This is accessible from the following website: www.OptiPrep.com
Note that although we recommend the OptiPrep™-based flotation strategy (outlined above and described in detail in Application Sheet C14), which should serve as a broadly applicable methodology, there are examples in the literature of the use of technical variations that may have a particular advantage, either for sample handling or for any subsequent technology. For example sedimentation on to a 1.10 g/ml barrier was used for melanoma cells [1,2]; the cell suspension was mixed with an equal volume of 24% (w/v) iodixanol (OptiPrep™ diluted with growth medium) and centrifuged at 800 g for 15 min to float viable epithelial cells [3]; HL60 cells were layered on top of three iodixanol solutions of density 1.057, 1.068 and 1.083 g/ml and centrifuged at 450 g for 30 min; the viable cells banded at the 1.057/1.068 g/ml interface [4]; a two-step iodixanol gradient (400 g for 15 min) was used for progenitor cells [5] and a plasma cell suspension was mixed 3.3:1 with OptiPrep™ (14% w/v iodixanol final concentration) , centrifuged at 1400 g for 5 min to float the viable cells [6].
1. Quintana E., Shackleton, M., Sabel, M.S., Fullen, D.R., Johnson, T.M. and Morrison, S.J. (2008) Efficient tumour formation by single human melanoma cells Nature 456, 593-5991
2. Quintana, E., Shackleton, M., Foster, H.R., Fullen, D.R., Sabel, M.S., Johnson, T.M. and Morrison, S.J. (2010) Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized Cancer Cell, 18, 510–523
3. Bruce, A.T., Sangha, N., Richmond, A., Johnson, K., Jones, S., Spencer, T. and Ludlow, J.W. (2010) Use of iodixanol self-generated density gradients to enrich for viable urothelial cells from non-neurogenic and neurogenic bladder tissue Tissue Eng., Part C Methods 16, 33-40
4. Hauert, A.B., Martinelli, S., Marone, C. and Niggli, V. (2002) Differentiated HL-60 cells are a valid model system for the analysis of human neutrophil migration and chemotaxis Int. J. Biochem. Cell Biol., 34, 838-8541282
5. Howell, O.W., Scharfman, H.E., Herzog, H., Sundstrom, L.E., Beck-Sickinger, A and Gray, W.P. (2003) Neuropeptide Y is neuroproliferative for hippocampal presursor cells J. Neurochem., 86, 646-659
6. Chatterjee, M., Stuhmer, T., Herrmann, P., Bommert, K., Dorken, B. and Bargou, R.C. (2004) Combined disruption of both the MEK/ERK and the IL-6R/STAT3 pathways is required to induce apoptosis of multiple myeloma cells in the presence of bone marrow stromal cells Blood, 104, 3712-3721
3. Reference list of papers reporting the use of OptiPrep™
Amphibian cells
Cox, T.C. (1999) Calcium and ATP regulation of ion transport in larval frog skin J. Comp. Physiol. B 169, 344-350
Bacteria
Dehusa, O., Pfitzenmaier, M., Stuebs, G., Fischer, N., Schwaeble, W., Morath, S., Hartung, T., Geyer, A. and Hermann, C. (2011) Growth temperature-dependent expression of structural variants of Listeria monocytogenes lipoteichoic acid Immunobiology, 216, 24–31
B cells
Methot, S. P., Litzler, L.C., Subramani, P.G., Eranki, A.K., Fifield, H., Patenaude, A-M., Gilmore, J.C., Santiago, G.E., Bagci, H., Côté, J-F. et al (2018) A licensing step links AID to transcription elongation for mutagenesis in B cells Nat. Comm., 9: 1248
Bone marrow cells
Khoramian Tusi, B., Wolock, S.L., Weinre, C., Hwang, Y., Hidalgo, D., Zilionis, R., Waisman, A., Huh, J.R., Klein, A.M. and Socolovsky, M. (2018) Population snapshots predict early haematopoietic and erythroid hierarchies Nature, 555, 54-60
Bronchoalveolar lavage cells
Kotzin, J.J., Spencer, S.P., McCright, S.J., Uthaya Kumar, D.B., Collet, M.A., Mowel, W.K., Elliott, E.N., Uyar, A. et al (2016) The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan Nature, 537, 239-243
Breast tumour/cancer cells
Chopra, S.S., Jenney, A., Palmer, A., Niepel, M., Chung, M., Mills, C., Sivakumaren, S.C., Liu, Q., Chen, J-Y. (2020) Torin2 exploits replication and checkpoint vulnerabilities to cause death of PI3K-activated triplenegative breast cancer cells Cell Systems 10, 66–81
Green, J.L., La, J., Yum, K.W., Desai, P., Rodewald, L-W., Zhang, X., Leblanc, M., Nusse, R., Lewis, M.T. and Wahl, G.M. (2013) Paracrine Wnt signaling both promotes and inhibits human breast tumor growth Proc. Natl. Acad. Sci. USA, 110, 6991–6996
Cancerous tissue
Zierhut, C., Yamaguchi, N., Paredes, M., Luo, J-D., Carroll, T. and Funabiki, H. (2019) The cytoplasmic DNA sensor cGAS promotes mitotic cell death Cell 178, 302–315
Cardiomyocytes
Shaikh, S.R., Dumaual, A.C., Castillo, A., LoCasio, D., Siddiqui, R.A., Stillwell, W. and Wassall, S.R. (2004) Oleic and docosahexaenoic acid differentially phase separate from lipid raft molecules: a comparative NMR, DSC, AFM, and detergent extraction study Biophys. J., 87, 1752-1766
Siddiqui, R.A., Shaikh, S.R., Kovacs, R., Stillwell, W. and Zaloga, G. (2004) Inhibition of phenylephrineinduced cardiac hypertrophy by docosahexaenoic acid J. Cell. Biochem., 92, 1141-1159
Wennicke, K., Debierre-Grockiego, F., Wichmann, D., Brattig, N.W., Pankuweit, S., Maisch, B., Schwarz, R.T. and Ruppert, V. (2008) Glycosylphosphatidylinositol-induced cardiac myocyte death might contribute to the fatal outcome of Plasmodium falciparum malaria Apoptosis, 13, 857-866
Wichmann, D., Schwarz, R.T., Ruppert, V., Ehrhardt, S., Cramer, J.P., Burchard, G.D., Maisch, B. and Debierre-Grickiego, F. (2007) Plasmodium falciparum glycosylphosphatidylinositol induces limited apoptosis in liver and spleen mouse tissue Apoptosis, 12, 1037-1041
Colonic macrophages
Filardy, A.A., He, J., Bennink, J., Yewdell, J. and Kelsall, B.L.(2016) Posttranscriptional control of NLRP3 inflammasome activation in colonic macrophages Mucosal Immunol., 9, 850-858
Dendritic cells
Kapadia, C.H., Tian, S., Perry, J.L., Sailer, D., Luft, C.J., DeSimone, J.M. (2018) Extending antigen release from particulate vaccines results in enhanced antitumor immune response J. Control. Release, 269, 393–404
Electroporation-treated cells
Steinbrunn, T., Chatterjee, M., Bargou, R.C. and Stühmer, T. (2014) Efficient transient transfection of human
multiple myeloma cclls by electroporation – an appraisal PLoS One, 9: e97443
Epithelial cells
Bruce, A.T., Sangha, N., Richmond, A., Johnson, K., Jones, S., Spencer, T. and Ludlow, J.W. (2010) Use of iodixanol self-generated density gradients to enrich for viable urothelial cells from non-neurogenic and neurogenic bladder tissue Tissue Eng., Part C Methods 16, 33-40
Hepatocytes (see also “Human foetal liver cells”)
Schmelzer, E., Wauther, E. and Reid, L.M. (2006) The phenotypes of pluripotent human hepatic progenitors Stem Cell.,24, 1852-1858
Sicklick, J.K., Li, Y-X., Melhem, A., Schmelzer, E., Zdanowicz, M., Huang, J., Caballero, M., Fair, J.H., Ludlow, J.W., McLelland, R.E., Reid, L.M. and Diehl, A.M. (2006) Hedgehog signaling maintains resident hepatic progenitors throughout life Am. J. Physiol. Gastrointest. Liver Physiol., 290, G859-G870
HL-60 cells
Hauert, A.B., Martinelli, S., Marone, C. and Niggli, V. (2002) Differentiated HL-60 cells are a valid model system for the analysis of human neutrophil migration and chemotaxis Int. J. Biochem. Cell Biol., 34, 838- 8541282
Human foetal liver cells
Khoramian Tusi, B., Wolock, S.L., Weinre, C., Hwang, Y., Hidalgo, D., Zilionis, R., Waisman, A., Huh, J.R., Klein, A.M. and Socolovsky, M. (2018) Population snapshots predict early haematopoietic and erythroid hierarchies Nature, 555, 54-60
Schmelzer, E., Zhang, L., Bruce, A., Wauthier, E., Ludlow, J., Yao, H-l., Moss, N., Melhem, A., McClelland, R., Turner, W., et al (2007) Human hepatic stem cells from fetal and postnatal donors J. Exp. Med., 204, 1973- 1987
Schmelzer, E. and Reid, L.M. (2009) Human telomerase activity, telomerase and telomeric template expression in hepatic stem cells and in livers from fetal and postnatal donors Eur. J. Gastroenterol. Hepatol., 21, 1191– 1198
Lipid droplets
Pan, C.W., Horvath, D.G., Braza, S., Moore, T., Lynch, A., Feit, C. and Abbyad, P. (2019) Sorting by interfacial tension (SIFT): label-free selection of live cells based on single-cell metabolism Lab Chip, 19, 1344- 1351
Melanoma cells
Quintana E., Shackleton, M., Sabel, M.S., Fullen, D.R., Johnson, T.M. and Morrison, S.J. (2008) Efficient tumour formation by single human melanoma cells Nature 456, 593-5991
Quintana, E., Shackleton, M., Foster, H.R., Fullen, D.R., Sabel, M.S., Johnson, T.M. and Morrison, S.J. (2010) Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized Cancer Cell, 18, 510–523
Quintana, E., Piskounova, E., Shackleton, M., Weinberg, D., Eskiocak, U., Fullen, D.R., Johnson, T.M. and Morrison, S.J. (2012) Human melanoma metastasis in NSG mice correlates with clinical outcome in patients Sci. Transl. Med., 4: 159ra149
Myeloma cells
Chatterjee, M., Stuhmer, T., Herrmann, P., Bommert, K., Dorken, B. and Bargou, R.C. (2004) Combined disruption of both the MEK/ERK and the IL-6R/STAT3 pathways is required to induce apoptosis of multiple myeloma cells in the presence of bone marrow stromal cells Blood, 104, 3712-3721
Olsen, O.E., Sankar, M., Elsaadi, S., Hella, H., Buene, G., Darvekar, S.R., Misund, K., Katagiri, T., Knaus, P. and Holien, T. (2018) BMPR2 inhibits activin and BMP signaling via wild-type ALK2 J. Cell Sci., 131: jcs213512
Stühmer, T., Chatterjee, M., Hildebrandt, M., Herrmann, P., Gollasch, H., Gerecke, C., Theurich, S., Cigliano, L., Manz, R.A. et al (2005) Nongenotoxic activation of the p53 pathway as a therapeutic strategy for multiple myeloma Blood, 106, 3609-3617
Våtsveen, T,K., Børset, M., Dikic, A., Tian, E., Micci, F., Lid, A.H.B., Meza-Zepeda, L.A., Coward, E. et al (2016) VOLIN and KJON-Two novel hyperdiploid myeloma cell lines Genes Chromosomes Cancer, 55, 890– 901
Zöllinger, A., Stühmer, T., Chatterjee, M., Gattenlöhner, S., Haralambieva, E., Müller-Hermelink, H-K., Andrulis, M., Greiner, A., Wesemeier, C., Rath, J.C., Einsele, H. and Bargou, R.C. (2008) Combined functional and molecular analysis of tumor cell signaling defines 2 distinct myeloma subgroups: Akt-dependent and Aktindependent multiple myeloma Blood, 112, 3403-3411
Myoblasts/myocytes
Benabdallah, B.F., Bouchentouf, M. and Tremblay, J.P. (2005) Improved success of myoblast transplantation in mdx mice by blocking the myostatin signal Transplantation, 79, 1696-1702
Liu, S.J. (2013) Characterization of functional capacity of adult ventricular myocytes in long-term culture Int. J. Cardiol., 168, 1923–1936
Pancreatic acinar cells
Mankad, P., James, A., Siriwardena, A.K., Elliott, A.C. and Bruce, J.I.E. (2012) Insulin protects pancreatic acinar cells from cytosolic calcium overload and inhibition of plasma membrane calcium pump J. Biol. Chem., 287, 1823–1836
Progenitor cells
Howell, O.W., Scharfman, H.E., Herzog, H., Sundstrom, L.E., Beck-Sickinger, A and Gray, W.P. (2003) Neuropeptide Y is neuroproliferative for hippocampal presursor cells J. Neurochem., 86, 646-659
Stühmer, T., Chatterjee, M., Hildebrandt, M., Herrmann, P., Gollasch, H., Gerecke, C., Theurich, S., Cigliano, L., Manz, R.A. et al (2005) Nongenotoxic activation of the p53 pathway as a therapeutic strategy for multiple myeloma Blood, 106, 3609-3617
Spinal cells
Nguyen, H.X., Galvan, M.D. and Anderson, A.J. (2008) Characterization of early and terminal complement proteins associated with polymorphonuclear leukocytes in vitro and in vivo after spinal injury J. Neuroinflamm., 5:26
Spleen cells
Deloizy, C., Fossum, E., Barnier-Quer, C., Urien, C., Chrun, T., Duval, A., Codjovi, M., Bouguyon, E., Maisonnasse, P. et al (2017) The anti-influenza M2e antibody response is promoted by XCR1 targeting in pig skin Sci. Rep., 7: 7639
Westhorpe, C.L.V., Norman, M.U., Hall, P., Snelgrove, S.L., Finsterbusch, M., Li, A., Lo, C., Tan, Z.H. et al (2018) Effector CD4+ T cells recognize intravascular antigen presented by patrolling monocytes Nat. Comm., 9: 747
T-cells
Westhorpe, C.L.V., Norman, M.U., Hall, P., Snelgrove, S.L., Finsterbusch, M., Li, A., Lo, C., Tan, Z.H. et al (2018) Effector CD4+ T cells recognize intravascular antigen presented by patrolling monocytes Nat. Comm., 9: 747
Urological cancer cells
Oates, J.E., Grey, B.R., Addla, S.K., Samuel, J.D., Hart, C.A., Ramani, V.A.C., Brown, M.D. and Clarke, N.W. (2009) Hoechst 33342 side population identification is a conserved and unified mechanism in urological cancers Stem Cell Devel., 18, 1515-1521
OptiPrep™Reference List RC03; 5th edition, March 2020
OptiPrep™ Reference List RC04
Purification of pancreatic islets
References are listed according to source: canine p1; human pp1-8, murine pp8-10, porcine pp10-16, primate (non-human) p16, rat pp16-18
Each of these sections is divided further into research topic groups. References are listed alphabetically within each section according to first author and in cases of multiple entries, are listed chronologically. A list of review articles is provided on pp18-19.
For the original methodology using a discontinuous iodixanol gradient see Application Sheet C16.
Canine
Harrington, S., Williams, S.J., Otte, V., Barchman, S., Jones, C., Ramachandran, K. and Stehno-Bittel, L. (2017) Improved yield of canine islet isolation from deceased donors BMC Vet. Res., 13: 264
Harrington, S., Williams, J., Rawal, S., Ramachandran, K. and Stehno-Bittel, L. (2017) Hyaluronic acid/collagen hydrogel as an alternative to alginate for long-term immunoprotected islet transplantation Tissue Eng. Part A, 23, 1088-1099
Human
Autoimmune response
Rutman, A.K., Negi, S., Gasparrini, M., Hasilo, C.P., Tchervenkov, J., and Paraskevas, S. (2018) Immune response to extracellular vesicles from human islets of Langerhans in patients with type 1 diabetes Endocrinology, 159, 3834–3847
Chronic pancreatitis
Bellin, M.D., Freeman, M.L., Schwarzenberg, S.J., Dunn, T.B., Beilman, G.J., Vickers, S.M., Chinnakotla, S., Balamurugan, A.N. et al (2011) Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis Clin. Gastroenterol. Hepatol., 9, 793–799
Matsumoto, S., Takita, M., Shimoda, M., Sugimoto, K., Itoh, T., Chujo, D., SoRelle, J.A., Tamura, Y. et al (2012) Impact of tissue volume and purification on clinical autologous islet transplantation for the treatment of chronic pancreatitis Cell Transplant., 21, 625–632
Balamurugan, A., Loganathan, G., Tweed, B., Tucker, W., Mokshagundam, S., Williams, S. and Hughes, M. (2016) Isolating high islet mass even from alcoholic pancreatitis pancreases intended for clinical islet autotransplantation: improved strategies to human islet isolation technique Am. J. Transplant., 16(S3), abstr 81 Continuous gradients in bottles (large scale) Shimoda, M., Itoh, T., Iwahashi, S., Takita, M., Sugimoto, K., Kanak, M.A., Chujo, D., Naziruddin, B., Levy, M.F., Grayburn, P.A. and Matsumoto, S. (2012) An effective purification method using large bottles for human pancreatic islet isolation Islets 4: 6
Encapsulation
Baron, M., Veres, A., Wolock, S.L., Faust, A.L., Gaujoux, R., Vetere, A., Ryu, J.H., Wagner, B.K. et al (2016) A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure Cell Systems 3, 346–360
Enck, K., McQuilling, J.P., Orlando, G., Tamburrini, R., Sivanandane, S. and Opara, E.C. (2017) Selective osmotic shock (SOS)-based islet isolation for microencapsulation In Methods Mol. Biol., 1479, Cell Microencapsulation: Methods and Protocols (ed. Opara, E.C.) Springer Science+Business Media, LLC pp 191-198
Weaver, J.D., Headen, D.M., Coronel, M.M., Hunckler, M.D., Shirwan, H. and García, A.J. (2019) Synthetic poly(ethylene glycol)‐based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site Am J Transplant. 2019, 19, 1315–1327
Exosome production
Hasilo, C.P., Negi, S., Allaeys, I., Cloutier, N., Rutman, A.K., Gasparrini, M., Bonneil, E., Thibault, P., Boilard, E. and Paraskevas, S. (2017) Presence of diabetes autoantigens in extracellular vesicles derived from human islets Sci. Rep., 7, 5000
Rutman, A.K., Negi, S., Gasparrini, M., Hasilo, C.P., Tchervenkov, J., and Paraskevas, S. (2018) Immune response to extracellular vesicles from human islets of Langerhans in patients with type 1 diabetes Endocrinology, 159, 3834–3847
Extracellular matrix attachment
Loganathan, G., Subhashree, V., Narayanan, S., Tweed, B., Goedde, M.A., Gunaratnam, B., Tucker, W.W., Goli, P. et al (2019) Improved recovery of human islets from young donor pancreases utilizing increased protease dose to collagenase for digesting peri‐islet extracellular matrix Am. J. Transplant., 19, 831–843
Peloso, A., Urbani, L., Cravedi, P., Katari, R., Maghsoudlou, P., Alvarez Fallas, M.E., Sordi, V., Citro, A. et al (2016) The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas Ann. Surg., 264, 169–179
Gene delivery
Cheng, K., Fraga, D., Zhang, C., Kotb, M., Gaber, A.O., Guntaka, R.V. and Mahato, R.I. (2004) Adenovirusbased vascular endothelial growth factor gene delivery to human pancreatic islets Gene Ther., 11, 1105-1116
Mahato, R.I., Henry, J., Narang, A.S., Sabek, O., Fraga, D., Kotb, M. and Gaber, A.O. (2003) Cationic lipid and polymer based gene delivery to human pancreatic cells Mol. Ther., 7, 89-100
Narang, A.S., Cheng, K., Henry, J., Zhang, C., Sabek, O., Fraga, D., Kotb, M., Gaber, O. and Mahato, R.I. (2004) Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets Pharmaceut. Res., 21, 15-25
Large scale multi-site production
Ricordi, C., Goldstein, J.S., . Balamurugan, A.M., Szot, G.L., Kin, T., Liu, C., Czarniecki, C.W., Barbaro, B., Bridges, N.D. et al (2016) National Institutes of Health–sponsored clinical islet transplantation consortium phase 3 trial: manufacture of a complex cellular product at eight processing facilities Diabetes, 65, 3418–3428
N-glycan profiles
Miyagawa, S., Maeda, A., Kawamura, T., Ueno, T., Usui, N., Kondo, S., Matsumoto, S., Okitsu, T., Goto, M. and Nagashima, H. (2014) A comparison of the main structures of N-glycans of porcine islets with those from humans Glycobiology, 24, 125–138
Pancreatectomy (complications)
Shahbazov, R., Naziruddin, B., Salam, O., Saracino, G., Levy, M.F., Beecherl, E. and Onaca, N. (2020) The impact of surgical complications on the outcome of total pancreatectomy with islet auto-transplantation Am. J. Surgery, 219, 99-105
Progenitor cells
Lee, S., Lee, C.M. and Kim, S.C. (2016) Adult human pancreas-derived cells expressing stage-specific embryonic antigen 4 differentiate into Sox9-expressing and Ngn3-expressing pancreatic ducts in vivo Stem Cell Res. Ther., 7: 162
Transcription regulation
Lawrence, M.C., McGlynn, K., Shao, C., Duan, L., Naziruddin, B., Levy, M.F. and Cobb, M.H. (2008) Chromatin-bound mitogen-activated protein kinases transmit dynamic signals in transcription complexes in β- cells Proc. Natl. Acad. Sci. USA., 105, 13315-13320
Transplantation
Allotransplant and autotransplant recipients
Bellin, M.D., Sutherland, D.E.R., Beilman, G.J., Hong-McAtee, I., Balamurugan, A.N., Hering, B.J. and Moran, A. (2011) Similar islet function in islet allotransplant and autotransplant recipients, despite lower islet mass in autotransplants Transplantation, 91, 367–372
Autotransplantation
Saravanan, P.B., Kanak, M.A., Chang, C.A., Darden, C., Yoshimatsu G., Lawrence, M.C. and Naziruddin, B. (2018) Islet damage during isolation as assessed by miRNAs and the correlation of miRNA levels with posttransplantation outcome in islet autotransplantation Am. J. Transplant., 18, 982-989
Shahbazov, R., Naziruddin, B., Yadav, K., Saracino, G., Yoshimatsu, G., Kanak, M.A., Beecher, E., Kim, P.T. and Levy, M.F. (2018) Risk factors for early readmission after total pancreatectomy and islet auto transplantation HPB, 20, 166–174
Anti-inflammatory strategy
Mita, A., Ricordi, C., Messinger, S., Miki, A., Misawa, R., Barker, S., Molano, R.D., Haertter, R. et al (2010) Antipro-inflammatory effects of iodixanol (OptiPrep)-based density gradient purification on human islet preparations Cell Transplant., 19, 1537–1546
Takita, M., Matsumoto, S., Shimoda, M., Chujo, D., Itoh, T., SoRelle, J.A., Purcell, K., Onaca, N., Naziruddin, B. and Levy. M.F. (2012) Safety and tolerability of the T-cell depletion protocol coupled with anakinra and etanercept for clinical islet cell transplantation Clin. Transplant., 26, E471–E484
Clinical problems
Naziruddin, B., Iwahashi, S., Kanak, M.A., Takita, M., Itoh, T. and Levy, M.F. (2014) Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation Am. J. Transplant., 14, 428–Takita, M., Matsumoto, S., Noguchi, H., Shimoda, M., Ikemoto, T., Chujo, D., Tamura, Y., Olsen, G.S. et al (2012) Adverse events in clinical islet transplantation: one institutional experience Cell Transplant., 21, 547– 551
Wang, L-j., Young, S., Misawa, R., Azzam, R., Wang, X., Gołąb, K., Cochet, O., Savari, O., Tibudan, M. et al (2014) Chronic pancreatitis and primary sclerosing cholangitis—first report of intrahepatic autologous islet tansplantation J. Gastrointest. Surg., 18, 845–850
Collagenase effects
Balamurugan, A.N., Breite, A.G., Anazawa, T., Loganathan, G., Wilhelm, J.J., Papas, K.K., Dwulet, F.E., McCarthy, R.C. and Hering, B.J. (2010) Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay Transplantation, 89, 954–961
Culture effects and islet function
Gaber, A. O., Fraga, D.W., Callicutt, C.S., Gerling, I.C., Sabek, O.M. and Kotb, M.Y. (2001) Improved in vivo pancreatic iselt function after prolonged in vitro islet culture Transplantation, 72, 1730-1736
Hering, B.J., Kandaswamy, R., Harmon, J.V., Ansite, J.D., Clemmings, S.M., Sakai, T., Paraskevas, S., Eckman, P.M. et al (2004) Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody Am. J. Transplant., 4, 390-401
Noguchi, H., Naziruddin, B., Jackson, A., Shimoda, M., Ikemoto, T., Fujita, Y., Chujo, D., Takita, M. et al (2010) Low-temperature preservation of isolated islets is superior to conventional islet culture before islet transplantation Transplantation, 89, 47-54
Noguchi, H., Naziruddin, B., Jackson, A., Shimoda, M., Ikemoto, T., Fujita, Y., Chujo, D., Takita, M. et al (2012) Fresh islets are more effective for islet transplantation than cultured islets Cell Transplant., 21, 517–523
Rush, B.T., Fraga, D.W., Kotb, M.Y., Sabek, O.M., Lo, A., Gaber, L.W., Halim, A-B. and Gaber, A.A. (2004) Preservation of human pancreatic islet in vivo function after 6-month culture in serum-free media Transplantation, 77, 1147-1154
Damage, miRNA assessment
Saravanan, P.B., Kanak, M.A., Chang, C.A., Darden, C., Yoshimatsu G., Lawrence, M.C. and Naziruddin, B. (2018) Islet damage during isolation as assessed by miRNAs and the correlation of miRNA levels with posttransplantation outcome in islet autotransplantation Am. J. Transplant., 18, 982-989
Donor pancreas optimization
Matsumoto, S., Noguchi, H., Takita, M., Shimoda, M., Tamura, Y., Olsen, G., Naziruddin, B., Onaca, N. and Levy, M.F. (2010) ET-Kyoto ductal injection and density-adjusted purification combined with potent antiinflammatory strategy facilitated single-donor islet transplantation: case reports Transplant. Proc., 42, 2159– 2161
Noguchi, H. and Matsumoto, S. (2008) Islet transplantation at the Diabetes Research Institute Japan J. Hepatobiliary Pancreat. Surg., 15, 278-283
Noguchi, H., Naziruddin, B., Jackson, A., Shimoda, M., Ikemoto, T., Fujita, Y., Chujo, D., Takita, M. et al (2010) Low-temperature preservation of isolated islets is superior to conventional islet culture before islet transplantation Transplantation, 89, 47-54
Donor selection (living)
Noguchi, H. and Matsumoto, S. (2008) Islet transplantation at the Diabetes Research Institute Japan J. Hepatobiliary Pancreat. Surg., 15, 278-283
Donor selection (marginal cadaver)
Nagata, H., Matsumoto, S., Okitsu, T., Iwanaga, Y., Noguchi, H., Yonekawa, Y., Kinukawa, T., Shimizu, T. et al (2006) Procurement of the human pancreas for pancreatic islet transplantation from marginal cadaver donors Transplantation, 82, 327-331
Donor selection (non-heart beating)
Matsumoto, S. and Tanaka, K. (2005) Pancreatic islet transplantation using non-heart-beating donors (NHBDs) J. Hepatobiliary Pancreat. Surg., 12, 227-230
Noguchi, H., Iwanaga, Y., Okitsu, T., Nagata, H., Yonekawa, Y. and Matsumoto, S. (2006) Evaluation of islet transplantation from non-heart beating donors Am. J. Transplant., 6, 2476-2482
Noguchi, H. and Matsumoto, S. (2008) Islet transplantation at the Diabetes Research Institute Japan J. Hepatobiliary Pancreat. Surg., 15, 278-283
Noguchi, H., Yamada, Y., Okitsu, T., Iwanaga, Y., Nagata, H., Kobayashi, N., Hayashi, S. and Matsumoto, S. (2008) Secretory unit of islet in transplantation (SUIT) and engrafted islet rate (EIR) indexes are useful for evaluating single islet transplantation Cell Transplant., 17, 121-128
Okitsu, T., Matsumoto, S., Iwanaga, Y., Noguchi, H., Nagata, H., Yonekawa, Y., Maekawa, T. and Tanaka, K. (2005) Kyoto islet isolation method: the optimized one for non-heart-beating donors with highly efficient islet retrieval Transplant. Proc., 37, 3391-3392
Saito, T., Gotoh, M., Satomi, S., Uemoto, S., Kenmochi, T., Itoh, T., Kuroda, Y., Yasunami, Y., Matsumoto, S. and Teraoka, S. (2010) Islet transplantation using donors after cardiac death: report of the Japan Islet Transplantation Registry Transplantation, 90, 740–747
Donor selection (paediatric patients)
Bellin, M.D., Blondet, J.J., Beilman, G.J., Dunn, T.B., Balamurugan, A.N., Thomas, W., Sutherland D.E.R., Moran, A. (2010) Predicting islet yield in pediatric patients undergoing pancreatectomy and autoislet transplantation for chronic pancreatitis Pediatr. Diabetes, 11, 227–234
Donor selection (review)
Kin, T. (2010) Islet isolation for clinical transplantation In Adv. Exp. Med. Biol., 654, The Islets of Langerhans (ed. Islam, M.S.) Springer Science + Business Media pp. 683-710
Donor selection (single donor)
Hering, B.J., Kandaswamy, R., Ansite, J.D., Eckman, P.M., Nakano, M., Sawada, T., Matsumoto, I., Ihm, S-H. et al (2005) Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes J. Am. Med. Assoc. 830-835
Matsumoto, S., Noguchi, H., Takita, M., Shimoda, M., Tamura, Y., Olsen, G., Naziruddin, B., Onaca, N. and Levy, M.F. (2010) ET-Kyoto ductal injection and density adjusted purification combined with potent antiinflammatory strategy facilitated single-donor islet transplantation: case reports Transplant. Proc., 42, 2159–2161
Immunosuppression of diabetic patient
Bellin, M.D., Kandaswamy, R., Parkey, J., Zhang, H-J., Liu, B., Ihm, S.H., Ansite, J.D., Witson, J. et al (2008) Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes Am. J. Transplant., 8, 2463-2470
Hering, B.J., Kandaswamy, R., Ansite, J.D., Fckman, P.M., Nakano, M., Sawada, T., Matsumoto, I., Ihm, S-H., Zhang, H-J., Hunter, D.W. and Sutherland, D.E.R. (2003) Successful single donor islet transplantation in type 1 diabetes Int. Pancreas Islet Transpl. Assoc., Abstr. 012
Hering, B.J., Kandaswamy, R., Harmon, J.V., Ansite, J.D., Clemmings, S.M., Sakai, T., Paraskevas, S., Eckman, P.M. et al (2004) Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody Am. J. Transplant., 4, 390-401
Takita, M., Matsumoto, S., Shimoda, M., Chujo, D., Itoh, T., SoRelle, J.A., Purcell, K., Onaca, N., Naziruddin, B. and Levy. M.F. (2012) Safety and tolerability of the T-cell depletion protocol coupled with anakinra and etanercept for clinical islet cell transplantation Clin. Transplant., 26, E471–E484
OptiPrep – Ficoll comparison
Mita, A., Ricordi, C., Messinger, S., Miki, A., Misawa, R., Barker, S., Molano, R.D., Haertter, R. et al (2010) Antipro-inflammatory effects of iodixanol (OptiPrep)-based density gradient purification on human islet preparations Cell Transplant., 19, 1537–1546
Supplemental islet transplantation
Matsumoto, S., Takita, M., Shimoda, M., Chujo, D., Itoh, T., Iwahashi, S., SoRelle, J.A., Tamura, Y. et al (2011) Insulin independence by supplemental islet transplantation 5 years after initial islet transplantation J. Diabetes, 3, 353–355
Warm ischaemia
Matsumoto, S. and Tanaka, K. (2005) Pancreatic islet transplantation using non-heart-beating donors (NHBDs) J. Hepatobiliary Pancreat. Surg., 12, 227-230
Nagata, H., Matsumoto, S., Okitsu, T., Iwanaga, Y., Noguchi, H., Yonekawa, Y., Kinukawa, T., Shimizu, T. et al (2006) Procurement of the human pancreas for pancreatic islet transplantation from marginal cadaver donors Transplantation, 82, 327-331
Yield/viability/function
Allogenic blood transfusion
Yoshimatsu, G., Shahbazov, R., Saracino, G., Lawrence, M.C., Kim, P.T., Onaca, N., Beecher, E.E., Naziruddin, B. and Levy, M.F. (2017) The impact of allogenic blood transfusion on the outcomes of total pancreatectomy with islet autotransplantation Am. J. Surg., 214, 849-855
β-cell proliferation
Purwana, I., Zheng, J., Li, X., Deurloo, M., Son, D.O., Zhang, Z. et al (2014) GABA promotes human -cell proliferation and modulates glucose homeostasis Diabetes, 63, 4197–4205
Chemokine/Cytokine production
Mita, A., Ricordi, C., Miki, A., Barker, S., Khan, A., Alvarez, A., Hashikura, Y., Miyagawa, S., and Ichii, H. (2008) The purification method using iodixanol (OptiPrep)-based density gradient significantly reduce cytokine/chemokine production from human islet preparations, leading to prolonged cell survival during culture Transplantation 86 (Suppl. 2) 570
Mita, A., Ricordi, C., Miki, A., Barker, S., Khan, A., Alverez, A., Hashikura, Y., Miyagawa, S. and Ichii, H. (2009) Purification method using iodixanol (OptiPrep)-based density gradient significantly reduces cytokine chemokine production from human islet preparations, leading to prolonged β-cell survival during pretransplantation culture Transplant. Proc., 41, 314-315
Sabek, O.M., Fraga, D.W., Henry, J., Gaber, L.W., Kotb, M. and Gaber, A.O. (2007) Expression of transforming growth factor-β by human islets: impact in islet viability and function Cell Transplant., 16, 775-785
Collagenase
Balamurugan, A.N., Green, M.L., Breite, A.G., Loganathan, G., Wilhelm, J.J., Tweed, B., Vargova, L. Lockridge, A., Kuriti, M. et al (2016) Identifying effective enzyme activity targets for recombinant class I and class II collagenase for successful human islet isolation Transplant. Dir., 2, e54
Loganathan, G., Subhashree, V., Breite, A.G., Tucker, W.W., Narayanan, S., Dhanasekaran, M., Mokshagundam, S., Green, M.L., Hughes, M.G. et al (2018) Beneficial effect of recombinant rC1rC2 collagenases on human islet function: Efficacy of low-dose enzymes on pancreas digestion and yield Am. J. Transplant., 18, 478–485
Culture (short-term) and other treatments of isolated islets
Ihm, S-H., Matsumoto, I., Zhang, H.J., Ansite, J.D. and Hering, B.J. (2009) Effect of short-term culture on functional and stress-related parameters in isolated human islets Transplant Int., 22, 207-216
Noguchi, H., Naziruddin, B., Jackson, A., Shimoda, M., Ikemoto, T., Fujita, Y., Chujo, D., Takita, M. et al (2010) Low-temperature preservation of isolated islets is superior to conventional islet culture before islet transplantation Transplantation, 89, 47-54
Noguchi, H., Naziruddin, B., Jackson, A., Shimoda, M., Ikemoto, T., Fujita, Y., Chujo, D., Takita, M. et al (2012) Fresh islets are more effective for islet transplantation than cultured islets Cell Transplant., 21, 517–523
Donor type and age, effect of
Anazawa, T., Balamurugan, A.N., Bellin, M., Zhang, H.J., Matsumoto, S., Yonekawa, Y., Tanaka, T., Loganathan, G. et al (2009) Human islet isolation for autologous transplantation: comparison of yield and function using SERVA/Nordmark versus Roche enzymes Am. J. Transplant., 9, 2383–2391
Avila, J.G., Agarwal, A., Turgeon, N., Cano, J.A., Turner, A., Russell, M.C., Kirk, A.D., Pearson, T., C.P. Larsen (2009) Identification of critical factors leading to successful islet isolations and transplantation Am. J. Transplant., 9, Supp 2, 406
Bellin, M.D., Blondet, J.J., Beilman, G.J., Dunn, T.B., Balamurugan, A.N., Thomas, W., Sutherland D.E.R., Moran, A. (2010) Predicting islet yield in pediatric patients undergoing pancreatectomy and autoislet transplantation for chronic pancreatitis Pediatr. Diabetes, 11, 227–234
Ihm, S-H., Matsumoto, I., Sawada, T., Nakano, M., Zhang, H.J., Ansite, J.D., Sutherland, D.E.R. and Hering, B.J. (2006) Effect of donor age on function of isolated human islets Diabetes, 55, 1361-1368
Matsumoto, I., Sawada, T., Nakano, M., Sakai, T., Liu, B., Ansite, J.D., Zhang, H-J., Kandaswamy, R., Sutherland, D.E.R. and Hering, B.J. (2004) Improvement in islet yield from obese donors for human islet transplants Transplantation, 78, 880-885
Matsumoto, S., Okitsu, T., Iwanaga, Y., Noguchi, H., Nagata, H., Yonekawa, Y., Yamada, Y., Fukuda, K. et al (2006) Successful islet transplantation from nonheartbeating donor pancreata using modified Ricordi islet isolation method Transplantation 82, 460-465
Ductal perfusion
Matsumoto, S., Noguchi, H., Takita, M., Shimoda, M., Tamura, Y., Olsen, G., Naziruddin, B., Onaca, N. and Levy, M.F. (2010) ET-Kyoto ductal injection and density-adjusted purification combined with potent antiinflammatory strategy facilitated single-donor islet transplantation: case reports Transplant. Proc., 42, 2159– 2161
Matsumoto, S., Noguichi, H., Shimoda, M., Ikemoto, T., Naziruddin, B., Jackson, A., Tamura, Y., Olson, G. et al (2010) Seven consecutive successful clinical islet isolations with pancreatic ductal injection Cell Transplant., 19, 291–297
Takita, M., Itoh, T., Shimoda, M., Kanak, M.A., Shahbazov, R., Kunnathodi, F., Lawrence, M.C., Naziruddin, B. and Levy, M.F. (2014) Pancreatic ductal perfusion at organ procurement enhances islet yield in human islet isolation Pancreas, 43, 1249–1255
Endotoxin levels in reagents
Linetsky, E., Inverardi, L., Kenyon, N.S., Alejandro, R. and Ricordi, C. (1998) Endotoxin contamination of reagents used during isolation and purification of human pancreatic islets Transplant. Proc., 30, 345-346
Enzyme digestion
Balamurugan, A.N., Loganathan, G., Bellin, M.D., Wilhelm, J.J., Harmon, J., Anazawa, T., Soltani, S.M., Radosevich, D.M. et al (2012) A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products Transplantation, 93, 693–702
Functional properties of gradient isolates
Sweet, I.R., Cook, D.L., Wiseman, R.W., Greenbaum, C.J., Lernmark, A., Matsumoto, S., Teague, J.C. and Krohn, K.A. (2002) Dynamic perifusion to maintain and assess isolated pancreatic islets Diabetes Techn. Therapeut., 4, 67-76
GABA, effects of
Prud’homme, G.J., Glinka, Y., Hasilo, C., Paraskevas, S., Li, X. and Wang, Q. (2013) GABA protects human islet cells against the deleterious effects of immunosuppressive drugs and exerts immunoinhibitory effects alone Transplantation, 96, 616-623
Purwana, I., Zheng, J., Li, X., Deurloo, M., Son, D.O., Zhang, Z. et al (2014) GABA promotes human β-cell proliferation and modulates glucose homeostasis Diabetes, 63, 4197–4205
Gradient methodology
Anazawa, T., Matsumoto, S., Yonekawa, Y., Loganathan, G., Wilhelm, J.J., Soltani, S.M., Papas, K.K., Sutherland, D.E.R., Hering, B.J. and Balamurugan, A.N. (2011) Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation /allotransplantation Transplantation, 91, 508–51
Mita, A., Ricordi, C., Messinger, S., Miki, A., Misawa, R., Barker, S., Molano, R.D., Haertter, R. et al (2009) Superiority of iodixanol (OptiPrep) over Ficoll in human islet purification Am. J. Transplant., 9 Supp. 2, 406
Noguchi, H., Ikemoto, T., Naziruddin, B., Jackson, A., Shimoda, M., Fujita, Y., Chujo, D., Takita, M. et al (2009) Iodixanol-controlled density gradient during islet purification improves recovery rate in human islet isolation Transplantation, 87, 1629–1635
Noguchi, H., Naziruddin, B., Shimoda, M., Fujita, Y., Chujo, D., Takita, M., Peng, H., Sugimoto, K. et al (2012) Evaluation of osmolality of density gradient for human islet purification Cell Transplant., 21, 493–500
Van der Burg, M.P.M., Ranuncoli, A., Molano, R., Kirlew, T., Ringers, J., Bouwman, E. and Ricordi, C. (1998) Efficacy of the novel iodixanol – UWS density gradient for human islet purification Acta Diabetol., 35, 247
Van der Burg, M.P.M., Ranuncoli, A., Molano, R., Kirlew, T., Ringers, J., Bouwman, E., Terpstra, O.T. and Ricordi, C. (1999) OptiPrep for human islet purification Cell Transplant., 8, abstr. 57
Yonekawa, Y., Balamurugan, A.N., Matsumoto, S., Tanaka, T., Gilmore, T.R., Ansite, J.D., Zhang, H., Sutherland, D.E.R. and Hering, B.J. (2007) The use of test gradients to determine the bottom layer density for subsequent continuous isopycnic purification of human islets on a COBE 2991 cell processor CTS-IPITA-IXA, Minneapolis 2007 Joint Conference Abstracts, p 480
HMGB1 release levels
Itoh, T., Takita, M., SoRelle, J.A., Shimoda, M., Sugimoto, K., Chujo, D., Qin, H., Naziruddin, B., Levy, M.F. and Matsumoto, S. (2012) Correlation of released HMGB1 levels with the degree of islet damage in mice and humans and with the outcomes of islet transplantation in mice Cell Transplant., 21, 1371–1381
IL-1β and TNF-α blockage
Matsumoto, S., Takita, M., Chaussabel, D., Noguchi, H., Shimoda, M., Sugimoto, K., Itoh, T., Chujo, D. et al (2011) Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1β and TNF-α Cell Transplant., 20, 1641–1647
Metabolic assessment
Lundberg, R., Beilman, G.J., Dunn, T.B., Pruett, T.L., Chinnakotla, S.C., Radosevich, D.M., Robertson, R.P., Ptacek, P. et al (2013) Metabolic assessment prior to total pancreatectomy and islet autotransplant: utility, limitations and potential Am. J. Transplant., 13, 2664–2671
Multi-centre analysis
Kaddis, J.S., Danobeitia, J.S., Niland, J.C., Stiller, T. and Fernandez, L.A. (2010) Multicenter analysis of novel and established variables associated with successful human islet isolation outcomes Am. J. Transplant., 10, 646–656
Non-heart-beating cadavers
Liu, X., Matsumoto, S., Okitsu, T., Iwanaga, Y., Noguchi, H., Yonekawa, Y., Nagata, H., Kamiya, H. et al (2008) Analysis of donor- and isolation-related variables from non-heart-beating donors (NHBDs) using the Kyoto islet isolation method Cell Transplant., 17, 649-656
Matsumoto, S., Okitsu, T., Iwanaga, Y., Noguchi, H., Nagata, H., Yonekawa, Y., Yamada, Y., Fukuda, K. et al (2006) Successful islet transplantation from nonheartbeating donor pancreata using modified Ricordi islet isolation method
Pancreas treatment and preservation prior to gradient purification
Avila, J.G., Agarwal, A., Turgeon, N., Cano, J.A., Turner, A., Russell, M.C., Kirk, A.D., Pearson, T., C.P. Larsen (2009) Identification of critical factors leading to successful islet isolations and transplantation Am. J. Transplant., 9, Supp 2, 406
Choi, S.J., Kim, F., Schwartz, M.W. and Wisse, B.E. (2010) Cultured hypothalamic neurons are resistant to inflammation and insulin resistance induced by saturated fatty acids Am. J. Physiol. Endocrinol. Metab., 298, E1122–E1130
Matsumoto, S., Rigley, T.H., Qualley, S.A., Kuroda, Y., Reems, J.A. and Stevens, R.B. (2002) Efficacy of the oxygen-charged static two-layer method for short-term pancreas preservation and islet isolation from nonhuman primate and human pancreata Cell Transplant. 11, 769-777
Sabek, O.M., Cowan, P., Fraga, D.W., Gaber, A.O. (2008) The effect of isolation methods and the use of different enzymes on islet yield and in vivo function Cell Transplant., 17, 785-792
Shimoda, M., Noguchi, H., Naziruddin, B., Fujita, Y., Chujo, D., Takita, M., Peng, H., Tamura, Y. et al (2010) Assessment of human islet isolation with four different collagenases Transplant. Proc., 42, 2049–2051
Shimoda, M., Itoh, T., Sugimoto, K., Takita, M., Chujo, D., Iwahashi, S., SoRelle, J.A., Naziruddin, B., Levy, M.F., Grayburn, P.A. and Matsumoto, S. (2011) An effective method to release human islets from surrounding acinar cells with agitation in high osmolality solution Transplant. Proc., 43, 3161–3166
Szot, G.L., Lee, M.R., Tavakol, M.M., Lang, J., Dekovic, F., Kerlan, R.K., Stock, P.G. and Posselt, A.M. (2009) Successful clinical islet isolation using a GMP manufactured collagenase and neutral protease Transplantation; 88, 753–756
Portal vein infusion
Shahbazov, R., Yoshimatsu, G., Haque, W.Z., Khan, O.S., Saracino, G., Lawrence, M.C., Kim, P. T. et al (2107) Clinical effectiveness of a pylorus-preserving procedure on total pancreatectomy with islet autotransplantation Am. J. Surgery, 213, 1065-1071
Thymoglobulin induction
Matsumoto, S., Takita, M., Chaussabel, D., Noguchi, H., Shimoda, M., Sugimoto, K., Itoh, T., Chujo, D., SoRelle, J., Onaca, N., Naziruddin, B. and Levy, M.F. (2011) Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1β and TNF-α Cell Transplant., 20, 1641–1647
Two-layer pancreas preservation prior to gradient
Matsumoto, S., Rigley, T.H., Qualley, S.A., Kuroda, Y., Reems, J.A. and Stevens, R.B. (2002) Efficacy of the oxygen-charged static two-layer method for short-term pancreas preservation and islet isolation from nonhuman primate and human pancreata Cell Transplant. 11, 769-777
Matsumoto, S., Rigley, T.H., Reems, J.A., Kuroda, Y. and Stevens, R.B. (2003) Improved islet yields from Macaca Nemestrina and marginal human pancreata after two-layer method preservation and endogenous trypsin inhibition Am. J. Transplant., 3, 53-63
Murine
Amyloid polypeptide
Rodriguez Camargo, D.C., Tripsianes, K., Buday, K., Franko, A., Göbl, C., Hartlmüller, C., Sarkar, R., Aichler, M., Mettenleiter, G. et al (2017) The redox environment triggers conformational changes and aggregation of hIAPP in Type II Diabetes Sci. Rep., 7: 44041
Apoptotic ER signals
Ladiges, W.C., Knoblaugh, S.E., Morton, J.F., Korth, M.J., Sopher, B.L., Baskin, C.R., MacAuley, A.,
Goodman, A.G., LeBoeuf, R.C. and Katze, M.G. (2005) Pancreatic -cell failure and diabetes in mice with a
delition mutation of the endoplastic reticulum molecular chaperone gene P58IPK Diabetes, 54, 1074-1081
β cell function
Bollyky. P.L., Bice, J.B., Sweet, I.R., Falk, B.A., Gebe, J.A., Clark, A.E., Gersuk, V.H., Aderem, A., Hawn, T.R. and Nepom, G.T. (2009) The Toll-like receptor signaling molecule Myd88 contributes to pancreatic Betacell homeostasis in response to injury PLoS One, 4:e5063
Ladiges, W.C., Knoblaugh, S.E., Morton, J.F., Korth, M.J., Sopher, B.L., Baskin, C.R., MacAuley, A., Goodman, A.G., LeBoeuf, R.C. and Katze, M.G. (2005) Pancreatic β-cell failure and diabetes in mice with a delition mutation of the endoplastic reticulum molecular chaperone gene P58IPK Diabetes, 54, 1074-1081
Pechhold, K., Koczwara, K., Zhu, X., Harrison, V.S., Walker, G., Lee, J. and D.M. Harlan (2009) Blood glucose levels regulate pancreatic -cell proliferation during experimentally-induced and spontaneous autoimmune diabetes in mice PLoS One 4:e4827
Bone marrow spheroidsl
Oh, B.J., Jin, S-M., Hwang, Y., Choi, J.M., Lee, H-S., Kim, G., Kim, G., Park, H.J. et al (2018) Highly angiogenic, nonthrombogenic bone marrow mononuclear cell–derived spheroids in intraportal islet transplantation Diabetes, 67, 473–485
Cilia, function of
Schmitz, F., Burtscher, I., Stauber, M., Gossler, A. and Lickert, H. (2017) A novel Cre-inducible knock-in ARL13B-tRFP fusion cilium reporter Genesis, 55: e23073
Encapsulation
Baron, M., Veres, A., Wolock, S.L., Faust, A.L., Gaujoux, R., Vetere, A., Ryu, J.H., Wagner, B.K. et al (2016) A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure Cell Systems 3, 346–360
Weaver, J.D., Headen, D.M., Coronel, M.M., Hunckler, M.D., Shirwan, H. and García, A.J. (2019) Synthetic poly(ethylene glycol)‐based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site Am J Transplant. 2019, 19, 1315–1327
Endocrine cell proliferation and function
Pechhold, K., Zhu, X., Harrison, V.S., Lee, J., Chakrabarty, S., Koczwara, K., Gavrilova, O. and Harlan, D.M. (2009) Dynamic changes in pancreatic endocrine cell abundance, distribution, and function in antigen-induced and spontaneous autoimmune diabetes Diabetes 58, 1175-1184
Pechhold, S., Stouffer, M., Walker, G., Martel, R., Seligmann, B., Hang, Y., Stein, R., Harlan, D.M. and Pechhold, K. (2009) Transcriptional analysis of intracytoplasmically stained, FACS-purified cells by highthroughput, quantitative nuclease protection Nat. Biotech., 27, 1038-1042
Gene regulation
Org, T., Rebane, A., Kisand, K., Laan, M., Haljasorg, U., Andreson, R. and Peterson, P. (2009) AIRE activated tissue specific genes have histone modifications associated with inactive chromatin Hum. Mol. Genet., 18, 4699–4710
Graft rejection, inhibition
Guo, M., Han, S., Liu, Y., Guo, W., Zhao, Y., Liu, F., Shi, X., Ding, G. and Wang, Q. (2019) Inhibition of allogeneic islet graft rejection by VISTA-conjugated liposome Biochem. Biophys. Res. Comm., 516: 914-920
Insulin secretion
Suwandhi, L., Hausmann, S., Braun, A., Gruber, T., Heinzmann, S.S., Gálvez, E.J.C., Buck, A., Legutko, B., Israel, A., Feuchtinger, A. et al (2018) Chronic D-serine supplementation impairs insulin secretion Mol. Metab., 16, 191-202
Islet hormonal release
Amisten, S., Meidute-Abaraviciene, S., Tan, C., Olde, B., Lundquist, I., Salehi, A. and Erlinge, D. (2010) ADP mediates inhibition of insulin secretion by activation of P2Y13 receptors in mice Diabetologia, 3, 1927–1934
Parandeh, F., Abaraviciene, S.M., Amisten, S., Erlinge, D. and Salehi, A. (2008) Uridine diphosphate (UDP) stimulates insulin secretion by activation of P2Y6 receptors Biochem. Biophys. Res. Commun., 370, 499-503
Islet neogenesis associated protein
Taylor-Fishwick, D.A., Bowman, A., Hamblet, N., Bernard, P., Harlan, D.M. and Vinik, A.I. (2006) Islet neoegenesis associated protein transgenic mice are resistant to hyperglycemia induced by streptozotocin J.
Endocrinol., 190, 729-737
Taylor-Fishwick, D.A., Bowman, A., Korngiebel-Rosique. M.C. and Vinik, A.I. (2008) Pancreatic islet immunoreactivity to the Reg protein INGAP J. Histochem. Cytochem., 56, 183-191
Maturation
Bastidas-Ponce, A., Roscioni, S.S., Burtscher, I., Bader, E., Sterr, M., Bakhti, M. and Lickert, H. (2017) Foxa2 and Pdx1 cooperatively regulate postnatal maturation of pancreatic β-cells Mol. Metab., 6, 524-534
Pancreatic development
Banga, A., Akinci, E., Greder, L.V., Dutton, J.R. and Slack, J.M.W. (2012) In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts Proc. Natl. Acad. Sci. USA, 109, 15336–15341
Yang, Y., Akinci, E., Dutton, J,R., Banga, A. and Slack, J.M.W. (2013) Stage specific reprogramming of mouse embryo liver cells to a beta cell-like phenotype Mech. Dev., 130, 602–612
Von Hippel-Landau syndrome
Shen, H-C.J., Adem, A., Ylaya, K., Wilson, A., He, M., Lorang, D., Hewitt, S.M., Pechhold, K. et al (2009) Deciphering von Hippel-Lindau (VHL/Vhl)-associated pancreatic manifestations by inactivating Vhl in specific pancreatic cell populations PLoS One, 4:e4897
Yield, viability and function
Bader, E., Migliorini, A., Gegg, M., Moruzzi, N. Gerdes, J., Roscioni, S.S., Bakhti, M., Brand, E., Irmler, M., Beckers, J., Aichler, M. et al (2016) Identification of proliferative and mature β-cells in the islets of Langerhans Nature, 535, 430-434
Machida, T., Tanemura, M., Ohmura, Y., Tanida, T., Wada, H., Kobayashi, S., Marubashi, S., Eguchi, H. et al (2013) Significant improvement in islet yield and survival with modified ET-Kyoto solution: ETKyoto/neutrophil elastase inhibitor Cell Transplant., 22, 159–173
Ohmura, Y., Tanemura, M., Kawaguchi, N., Machida, T., Tanida, T., Deguchi, T., Wada, H., Kobayashi, S. et al (2010) Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice Transplantation, 90, 1366–1373
Tanemura, M., Machida, T., Nagano, H., Wada, H., Kobayashi, S., Marubashi, S., Eguchi, H., Ito, T., Mori, M. and Doki, Y. (2011) The inhibition of neutrophil elastase ameliorates islet yield and islet graft survival Int. J. Transplant, 11, 249
Wu, C., Zhang, Y., Jiang, Y., Wang, Q., Long, Y., Wang, C., Cao, X. and Chen, G. (2013) Apoptotic cell administration enhances pancreatic islet engraftment by induction of regulatory T cells and tolerogenic dendritic cells Cell. Mol. Immunol., 10, 393–402
Porcine
Adhesion characteristics
Nakashima, Y., Miyagi-Shiohira, C., Kobayashi, N., Saitoh, I., Watanabe. M. and Noguchi, H. (2018) Adhesion characteristics of porcine pancreatic islets and exocrine tissue to coating materials Islets, 10:3 e1460294
Antioxidant effects
Chung, S.S., Kim, M., Lee, J.S., Ahn, B.Y., Jung, H.S., Lee, H.M. and Park, K.S. (2011) Mechanism for antioxidative effects of thiazolidinediones in pancreatic-cells Am. J. Physiol. Endocrinol. Metab., 301, E912– E921
Culture of
Weegman, B.P., Taylor, M.J., Baicu, S.C., Mueller, K., O’Brien, T.D., Wilson, J. and Papas, K.K. (2016) Plasticity and aggregation of juvenile porcine islets in modified culture: preliminary observations Cell Transplant., 25, 1763–1775
Donor-age effects
Smith, K.E., Purvis, W.G., Davis, M.A., Min, C.G., Cooksey, A.M., Weber, C.S., Jandova, J., Price, N.D. et al (2018) In vitro characterization of neonatal, juvenile, and adult porcine islet oxygen demand, β-cell function, and transcriptomes Xenotransplantation 25: e12432
Glycans
Kim, Y-G., Harvey, D.J., Yang, Y-H., Park, C-G. and Kim, B-G. (2009) Mass spectrometric analysis of the glycosphingolipid-derived glycans from miniature pig endothelial cells and islets: identification of NeuGc epitope in pig islets J. Mass. Spectrom., 44, 1489-1499
Kim, Y-G., Gil, G-C., Jang, K-S., Lee, S., Kim, H-i., Kim, J-S., Chung, J., Park, C-G., Harvey, D.J. and Kim, B-G. (2009) Qualitative and quantitative comparison of N-glycans between pig endothelial and islet cells by high-performance liquid chromatography and mass spectrometry-based strategy J. Mass. Spectrom., 44, 1087- 1104
Miyagawa, S., Maeda, A., Kawamura, T., Ueno, T., Usui, N., Kondo, S., Matsumoto, S., Okitsu, T., Goto, M. and Nagashima, H. (2014) A comparison of the main structures of N-glycans of porcine islets with those from humans Glycobiology, 24, 125–138
Hyperglycemia
Jiang X.-F., Qian, T-L., Chen, D., Lu, H-W., Xue, P., Yang, X-W., Zhang, L-H., Hu, Y-Z. and Zhang, D-W. (2018) Correction of hyperglycemia in diabetic rats with the use of microencapsulated young market pig islets Transplant. Proc., 50, 3895-3899
Immune reactions
Haque, M.R., Jeong, J-H. and Byun, Y. (2016) Combination strategy of multi-layered surface camouflage using hyperbranched polyethylene glycol and immunosuppressive drugs for the prevention of immune reactions against transplanted porcine islets Biomaterials, 84, 144-156
Jung, K.C., Park, C-G., Jeon, Y.K., Park, H.J., Ban, Y.L., Min, H.S, Kim, E.J., Kim, J.Y. et al (2011) In situ induction of dendritic cell–based T cell tolerance in humanized mice and nonhuman primates J. Exp. Med., 208, 2477-2488
Kawamoto, K., Tanemura, M., Saga, A., Komoda, H., Fumimoto, Y., Deguchi, T., Machida, T., Sawa, Y., Nishida, T. and Ito, T. (2008) Adenoviral-mediated overexpression of either membrane-bound human FasL or human decoy Fas can prolong pig islet xenograft survival in a rat transplant model Transplant. Proc., 40, 477-479
Lalain, S., Chaillous, L., Gouin, E. and Saï, P. (1999) Intensity and mechanisms of in vitro xeno-recognition of adult pig pancreatic islet cells by CD4 + and CD8 + lymphocytes from type I diabetic or healthy subjects Diabetologia, 42, 330-335
Lalain, S., Gianello, P., Gouin, E. and Sai, P. (2001) In vitro recognition and impairment of pig islet cells by baboon immune cells Transplantation, 72, 1541-1548
Ock, S.A., Lee, J., Oh, K.B., Hwang, S., Yun, I.J., Ahn, C., Chee, Hk., Kim, H. et al (2016) Molecular immunology profiles of monkeys following xenografting with the islets and heart of -1,3-galactosyltransferase knockout pigs Xenotransplantation, 23, 357–369
Rijkelijkhuizen, J.K., Bouwman, E., van der Burg, M.P., Ringers, J., Ossevoort, M.A., Kuhn, E.M., Frost, P. and Jonker, M. (2000) Successful suppression of the early rejection of pig islets in monkeys Cell Transplant., 9, 909-912
Rijkelijkhuizen, J.K.R.A., Haanstra, K.G., Wubben, J., Töns, A., Roos, A., van Gijlswijk-Janssen, D.J., Ringers, J., Bouwman, E. and Jonker, M. (2003) T-cell specific immunosuppresion results in more than 53 days survival of porcine islets of Langerhans in the monkey Transplantation, 76, 1359-1368
You, S., Gouin, E. and Saï, P. (1998) Spleen cells of non-obese diabetic mice fed with pig splenocytes display modified proliferation and reduced aggressiveness in vitro against pig islet cells Diabetologia, 41, 955-962
You, S., Gouin, E. and Sai, P. (2002) Feeding NOD mice with pig splenocytes induces transferable mechanisms that modulate cellular and humoral xenogeneic reactions aginst pig spleen or islet cells Clin. Exp. Immunol., 127, 412-422
Insulin release
Lalain, S., Clémenceau, B., Gouin, E. and Sai, P. (2001) In vitro co-incubation of pig islet cells with xenogeneic human blood mononuclear cells causes loss of insulin release during perifusion: involvement of non-T-cell- and T-cell-mediated mechanism Hum. Immunol., 62, 607-614
Renner, S., Fehlings, C., Herbach, N., Hofmann, A., von Waldthausen, D.C., Kessler, B., Ulrichs, K., Chodnevskaja, I. et al (2010) Glucose intolerance and reduced proliferation of pancreatic β-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function Diabetes 59, 1228–1238
You, S., Rivereau, A-S., Gouin, E. and Sai, P. (2001) Co-incubation of pig-islet cells with spleen cells from nonobese mice causes decreased insulin release by non-T cell- and T-cell-mediated mechanisms Clin. Exp. Immunol., 125, 25-31
Nano-encapsulation of islets
Haque, M.R., Jeong, J-H., Lee, K-W., Shin, D.Y., Kim, G-S., Kim, S.J. and Byun, Y. (2018) Effects of transplanted islets nano-encapsulated with hyperbranched polyethylene glycol and heparin on microenvironment reconstruction and glucose control Bioconjugate Chem., 29, 2945−2953
Preservation (MK solution)
Kuwae, K., Miyagi-Shiohira, C., Hamada, E., Tamaki, Y., Nishime, K., Sakai, M., Yonaha, T., Makishi, E. et al (2019) Excellent islet yields after 18-h porcine pancreas preservation by ductal injection, pancreas preservation with MK solution, bottle purification, and Iilet purification using idixanol with UW solution and iodixanol with MK solution J. Clin. Med. 2019, 8: 1561
Proteome analysis
Nakashima, Y., Miyagi-Shiohira, C., Kobayashi, N., Saitoh, I., Watanabe, M. and Noguchi, H. (2017) A proteome analysis of pig pancreatic islets and exocrine tissue by liquid chromatography with tandem mass spectrometry Islets, 9, 159-176
Surface modification
SoRelle, J.A., Kanak, M.A., Itoh, T.,. Horton, J.M., Naziruddin, B. and Kane, R.R. (2015) Comparison of surface modification chemistries in mouse, porcine and human islets J. Biomed. Mater. Res. Part A, 103A, 869– 877
Transplantation
Encapsulation of islets
Darrabie, M.D., Kendall, W.F. and Opara, E.C. (2005) Characteristics of poly-L-ornithine-coated alginate microcapsules Biomaterials, 26, 6846-6852
Pakhomov, O., Honiger, J., Gouin, E., Cariolet, R., Reach, G. and Darquy, S (2002) Insulin treatment of mice recipients preserves β-cell function in porcine islet transplantation Cell Transplant., 11, 721-728
Park, H-S., Kim, J-W., Lee, S-H., Yang, H.K., Ham, D-S., Sun, C-L., Hong, T.H., Khang, G. et al (2017) Antifibrotic effect of rapamycin containing polyethylene glycol-coated alginate microcapsule in islet xenotransplantation J. Tissue Eng. Regen. Med., 11, 1274–1284
Yang, H.K., Ham, D-S., Park, H-S., Rhee, M., You, Y.H., Kim, M.J., Shin, J. et al (2016) Long-term efficacy and biocompatibility of encapsulated islet transplantation with chitosan-coated alginate capsules in mice and canine models of diabetes Transplantation, 100, 334–343
Zhu, H., Yu, L., He, Y., Lyu, Y. and Wang, B. (2015) Microencapsulated pig islet xenotransplantation as an alternative treatment of diabetes Tissue Eng., Part B, 21, 474-489
Gradient influence of diabetes reversal
Matsumoto, S., Shibata, S., Kirchoff, N., Hiraoka, K., Sageshima, J., Zhang, X.W., Gilmore, T., Ansite, J., Zhang, H.J., Suthreland, D.E.R. and Hering, B.J. (1999) Immediate reversal of diabetes in primates following intraportal transplantation of porcine islets on a new histidine-lactobionate-iodixanol gradient Transplantation, 67, S220
Min, T., Yi, L., Chao, Z., Haitao, Z., Wei, W., Liang, Y. and Bo, W. (2010) Superiority of Visipaque (iodixanol)-controlled density gradient over Ficoll-400 in adult porcine islet purification Transplant. Proc., 42, 1825–1829
Non-human primates, into
Lee, J-I., Kim, J., Choi, Y-J., Park, H-J., Park, H-J., Wi, H.J., Yoon, S., Shin, J-S., Park, J.K. et al (2018) The effect of epitope-based ligation of ICAM-1 on survival and retransplantation of pig islets in nonhuman primates Xenotransplantation.25: e12362
Virus transmission
Brewer, L., LaRue, R., Hering, B., Brown, C. and Njenga, M.K. (2004) Transplanting encephalomyocarditis virus-infected porcine islet cells reverses diabetes in recipient mice but also transmits the virus Xenotransplantation, 11, 160-170
Clemenceau, B., Jegou, D., Martignat, L. and Sai, P. (2001) Long-term follow-up failed to detect in vitro transmission of full-length porcine endogenous retroviruses from specific pathogen-free pig islets to human cells Diabetologia, 44, 2044-2055
Clemenceau, B., Jegou, D., Martignat, L, and Saï, P. (2002) Microchimerism and transmission of porcine endogenous retrovirus from a pig cell line or specific pathogen-free pig islets to mouse tissues and human cells during xenografts in nude mice Diabetologia, 45, 914-923
Myers, S. E., Brewer, L., Shaw, D.P., Greene, W.H., Love, B.C., Hering, B.J., Brad Spiller, O., Kariuki Njenga, M. (2004) Prevalent human coxsaxkie B-5 virus infects porcine islet cells primarily using the coxsackieadenovirus receptor Xenotransplantation, 11, 536-546
Xenotransplantation
ATP levels
Kim, J.H., Park, S.G., Lee, H.N., Lee, Y.Y., Park, H.S., Kim, H-I., Yu, J.E., Kim, S.H., Park, C-G. et al (2009) ATP measurement predicts porcine islet transplantation outcome in nude mice Transplantation, 87, 166-169
Endothelial cell co-transplantation
Kang, S., Park, H.S., Jo, A., Hong, S.H., Lee, H.N., Lee, Y.Y., Park, J.S., Jung, H.S., Chung, S.S. and Park, K.S. (2012) Endothelial progenitor cell cotransplantation enhances islet engraftment by rapid revascularization Diabetes, 61, 866–876
Fas expression, effect of
Kawamoto, K., Tanemura, M., Saga, A., Komoda, H., Fumimoto, Y., Deguchi, T., Machida, T., Sawa, Y., Nishida, T. and Ito, T. (2008) Adenoviral-mediated overexpression of either membrane-bound human FasL or human decoy Fas can prolong pig islet xenograft survival in a rat transplant model Transplant. Proc., 40, 477-479
Human mesenchymal stem cells
Lee, H-S., Song, S., Shin, D.Y., Kim, G-S., Lee, J-H., Cho, C-W., Lee, K.W., Park, H., Ahn, C. et al (2018) Enhanced effect of human mesenchymal stem cells expressing human TNF-αR-Fc and HO-1 gene on porcine islet xenotransplantation in humanized mice Xenotransplantation, 25: e12342
Immune suppression status of recipients
Kirchhof, N., Shibata, S., Wijkstrom, M., Salerno, C.T., Clemmings, S.M., Heremans, Y., Galili, U., Sutherland, D.E.R., Dalmasso, A.P. and Hering B.J. (2004) Reversal of diabetes in non-immunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection Xenotransplantation, 11, 396-407
Pakhomov, O., Honiger, J., Gouin, E., Cariolet, R., Reach, G. and Darquy, S (2002) Insulin treatment of mice recipients preserves β-cell function in porcine islet transplantation Cell Transplant., 11, 721-728
Rijkelijkhuizen, J.K.R.A., Töns, Terpstra, O.T. and Bouwman, E. (2010) Transplantation of long-term cultured porcine islets in the rat: prolonged graft survival and recipient growth on reduced immunosuppression Cell Transplant., 19, 387-398
Tian, M., Lv, Y., Zhai, C., Zhu, H., Yu, L. and Wang, B. (2013) Alternative immunomodulatory strategies for xenotransplantation: CD80/CD86-CTLA4 pathway-modified immature dendritic cells promote xenograft srvival PLoS One, 8: e69640
Pancreas pre-gradient treatments
Anazawa, T., Balamurugan, A.N., Papas, K.K., Avgoustiniatos, E.S., Ferrer, J., Matsumoto, S., Sutherland, E.D.R. and Hering, B.J. (2010) Improved method of porcine pancreas procurement with arterial flush and ductal injection enhances islet isolation outcome Transplant. Proc., 42, 2032–2035
Jin, S-M., Shin, J.S., Kim, K.S., Gong, C-H., Park, S.K., Kim, J-S., Yeom, S-C., Hwang, E.S. et al (2011) Islet isolation from adult designated pathogen-free pigs: use of the newer bovine nervous tissue–free enzymes and a revised donor selection strategy would improve the islet graft function Xenotransplantation 18, 369-379
Preservation of β-cell function
Pakhomov, O., Honiger, J., Gouin, E., Cariolet, R., Reach, G. and Darquy, S (2002) Insulin treatment of mice recipients preserves β-cell function in porcine islet transplantation Cell Transplant., 11, 721-728
T cell effects
Jung, K.C., Park, C-G., Jeon, Y.K., Park, H.J., Ban, Y.L., Min, H.S, Kim, E.J., Kim, J.Y. et al (2011) In situ induction of dendritic cell–based T cell tolerance in humanized mice and nonhuman primates J. Exp. Med., 208, 2477-2488
Zhai, C., Yu, L., Zhu, H., Tian, M., Xiaogang, Z., Bo, W. (2011) Porcine CTLA4-Ig prolong islet xenografts in rats by downregulating the direct pathway of T-cell activation Xenotransplantation 18, 40–45
VCAM-1, expression of
Lee, S., Ha, I.S., Kim, J.Y., Park, K.S., Han, K.H., Kim, S-H., Chae, Y.C., Kim, S.H., Kim, Y.H. et al (2008) Hydrogen peroxide-induced VCAM-1 expression in pancreatic islets and β-Cells through extracellular Ca2+ influx Transplantation, 86, 1257-1266
Yield/viability/function
Allograft functions
Krickhahn, M., Meyer, T., Buchler, C., Thiede, A. and Ulrichs, K. (2001) Highly efficient isolation of porcine islets of Langerhans for xenotransplantation: numbers, purity, yield and in vitro function Ann. Transplant., 6, 48-54
Matsumoto, S, Zhang, H.J., Gilmore, T., van der Burg, M.P., Sutherland, D.E.R. and Hering, B.J. (1998) Large scale isopycnic islet purification utilizing non-toxic, endotoxin-free media facilitates immediate single-donor pig islet allograft function Transplantation, 66, S30
Collagenase
Green, M., Beechler, C., Breite, D., Dwulet, F. and McCarthy, R. (2013) Optimization of a porcine islet isolation and purification procedure that utilizes recombinant collagenase Xenotransplantation, 20, 333
Green, M.L., Breite, A,G., Beechler, C.A., Dwulet, F.E. and McCarthy, R.C. (2017) Effectiveness of different molecular forms of C. histolyticum class I collagenase to recover islets Islets, 9, 177-181
Culture effects on transplantation
Rijkelijkhuizen, J.K.R.A., Bouwman, E. and van der Burg, M.P.M. (1999) Viability of fresh vs. cultured pig islets for transplant Cell Transplant., 8, abstr. 6
Rijkelijkhuizen, J.K.R.A., van der Burg, M.P.M., Töns, A., Terpstra, O.T. and Bouwman, E. (2006) Pretransplant culture selects for high-quality porcine islets Transplantation, 32, 403-407
Van der Burg, M.P.M., Zwaan, R.P. and Bouwman, E. (1998) Markedly improved outcome of adult porcine islet isolation, purification, and culture using Liberase-P1 versus Collagenase-P, and a novel gradient of OptiPrep in University of Wisconsin solution Horm. Metab. Res., 30, A23
Gradient methodology, yield and purity
Ebi, N., Miyagi-Shiohira, C., Hamada, E., Tamaki, Y., Masamoto, M., Makishi, E., Nakashima, Y., Kobayashi, N. et al (2018) Evaluation of islet purification methods for making a continuous density gradient and loading tissue Cell Med., 10, 1-7
Krickhahn, M., Meyer, T., Buchler, C., Thiede, A. and Ulrichs, K. (2001) Highly efficient isolation of porcine islets of Langerhans for xenotransplantation: numbers, purity, yield and in vitro function Ann. Transplant., 6, 48-54
Min, T., Yi, L., Chao, Z., Haitao, Z., Wei, W., Liang, Y. and Bo, W. (2010) Superiority of Visipaque (iodixanol)-controlled density gradient over Ficoll-400 in adult porcine islet purification Transplant. Proc., 42, 1825–1829
Miyagi-Shiohira, C., Kobayashi, N., Saitoh, I., Watanabe, M., Noguchi, Y., Matsushita, M. and Noguchi, H. (2017) The evaluation of islet purification methods that use large bottles to create a continuous density gradient Cell Med., 9, 45–51
Miyagi-Shiohira, C., Nakashima, Y., Ebi, N., Hamada, E., Tamaki, Y., Kuwae, K., Kobayashi, N., Saitoh, I. et al (2018) Tissue loading before and after the creation of a continuous density gradient in porcine islet purification Cell Med., 10, 1-7
Nakashima, Y., Miyagi-Shiohira, C, Ebi, N., Hamada, E., Tamaki, Y., Kuwae, K., Kobayashi, N. et al (2018) A Comparison of pancreatic islet purification using iodixanol with University of Wisconsin solution and with NaLactobionate and histidine solution Cell Med., 10, 1-7 Okitsu, T. (2013) Manual adult porcine islet isolation technique and optimal condition for adult pig islets Xenotransplantation, 20, 349
Sack, F.D., Schwuchow, J.M., Wagner, T. and Kern, V. (2001) Gravity sensing in moss protonemata Adv. Space Res., 27, 871-876
Van der Burg, M.P.M., Basir, I. and Bouwman, E. (1998) No porcine islet loss during density gradient purification in a novel iodixanol in University of Wisconsin solution Transplant. Proc., 30, 362-363
Van der Burg, M.P.M., Zwaan, R.P. and Bouwman, E. (1998) Markedly improved outcome of adult porcine islet isolation, purification, and culture using Liberase-P1 versus Collagenase-P, and a novel gradient of OptiPrep in University of Wisconsin solution Horm. Metab. Res., 30, A23
Van der Burg, M.P.M., Rijkelijkhuizen, J.K.R.A., Zwaan, R.P. and Bouwman, E. (1999) Adult pig islet recovery during Liberase isolation, OptiPrep purification and culture for transplantation in nude mice Cell Transplant., 8, abstr. 58
Van der Burg, M.P.M. and Graham, J.M. (2003) Iodixanol density gradient preparation in University of Wisconsin solution for porcine islet purification Sci. World J., 3, 1154-1159
Gradient yield, prediction of
Anazawa, T., Balamurugan, A.N., Matsumoto, S., LaFreniere, S.A., O’Brien, T.D., Sutherland, D.E.T. and Hering, B.J. (2010) Rapid quantitative assessment of the pig pancreas biopsy predicts islet yield Transplant. Proc., 42, 2036–2039
Jin, S-M., Kim, K.S., Lee, S-Y., Gong, C-H., Park, S.K., Yu, J.E., Yeom, S-C.,Yoon, T.W., Ha, J., Park, C-G. and Kim, S-J. (2010) Enhanced prediction of porcine islet yield and posttransplant outcome using a combination of quantitative histomorphometric parameters and flow cytometry Cell Transplant., 19, 299–311
Islet function enhancement
Lee, Y.Y., Hong, S.E., Lee, Y.J., Chung, S.S., Jung, H.S., Park, S.G. and Park, K.S. (2010) Tauroursodeoxycholate (TUDCA), chemical chaperone, enhances function of islets by reducing ER stress Biochem. Biophys. Res. Comm., 397, 735–739
Paraskevas, S., Aikin, R., Maysinger, D., Lakey, J.R.T., Cavanagh, T.J., Hering, B., Wang, R. and Rosenberg, L. (1999) Activation and expression of ERK, JNK, and p38 MAP-kinases in isolated islets of Langerhans: implications for cultured islet survival FEBS Lett., 455, 203-208
Large scale sterile separations
Klaffschenkel, R.A., Biesemeier, A., Waidmann, M., Northoff, H., Steurer, W., Königsrainer, A. and Lembert, N. (2007) A closed system for islet isolation and purification using the COBE2991 cell processor may reduce the need of clean room facilities Cell Transplant., 16, 587-594
Lembert, N., Biesemeier, A., Klaffschenkel, R. and Königsrainer, A. (2006) A closed system for the preparation of islets of Langerhans using the COBE2991 cell processor Cytotherapy, 8, Suppl. 2, 30
Matsumoto, S, Zhang, H.J., Gilmore, T., van der Burg, M.P., Sutherland, D.E.R. and Hering, B.J. (1998) Large scale isopycnic islet purification utilizing non-toxic, endotoxin-free media facilitates immediate single-donor pig islet allograft function Transplantation, 66, S30
Shimoda, M., Noguchi, H., Fujita, Y., Takita, M., Ikemoto, T., Chujo, D., Naziruddin, B., Levy, M.F., Kobayashi, N., Grayburn, P.A. and Matsumoto, S. (2012) Islet purification method using large bottles effectively achieves high islet yield from pig pancreas Cell Transplant., 21, 501–508
Method optimization
Shibata, S., Sageshima, J., Hiraoka, K., Zhang, H., Koyama, K., Sutherland, D.E.R. and Hering, B.J. (2001) Low-speed isopycnic islet separation is effeictive and yields islets with superior quantity and quality Int. Pancreas Islet Transplant. Assoc. Abstr. p. 5
Morphology, islet
Krickhahn, M., Bühler, C., Meyer, T., Thiede, A. and Ulrichs, K. (2002) The morphology of islets within the porcine donor pancreas determines the isolation result: Successful isolation of pancreatic islets can now be achieved from young market pigs Cell Transplant., 11, 827-838
Jin, S-M., Lee, H-S., Oh, S-H., Park, H.J., Park, J.B., Kim, J.H. and Kim, S.J. (2014) Adult porcine islet isolation using a ductal preservation method and purification with a density gradient composed of histidinetryptophan-ketoglutarate solution and iodixanol Transplant. Proc., 46, 1628-1632
Osmolality effects
Miyagi-Shiohira, C., Kobayashi, N., Saitoh, I., Watanabe, M., Noguchi, Y., Matsushita, M. and Noguchi, H. (2017) Comparison of purification solutions with different osmolality for porcine islet purification (2016) Cell Med. 9, 53–59
Pre-gradient treatments
Anazawa, T., Balamurugan, A.N., Papas, K.K., Avgoustiniatos, E.S., Ferrer, J., Matsumoto, S., Sutherland, E.D.R. and Hering, B.J. (2010) Improved method of porcine pancreas procurement with arterial flush and ductal injection enhances islet isolation outcome Transplant. Proc., 42, 2032–2035
Loganathan, G., Graham, M.L., Spizzo, T., Tiwari, M., Lockridge, A.D., Soltani, S., Wilhelm, J.J., Balamurugan, A.N, and Hering, B.J. (2014) Pretreatment of donor pigs with a diet rich in soybean oil increases the yield of isolated islets Transplant. Proc., 46, 1945-1949
Matsumoto, S., Okitsu, T., Iwanaga, Y., Noguchi, H., Nagata, H., Yonekawa, Y., Yamada, Y., Fukuda, K. et al (2006) Successful islet transplantation from nonheartbeating donor pancreata using modified Ricordi islet isolation method Transplantation 82, 460-465
Matsumoto, S., Noguchi, H., Hatanaka, N., Shimoda, M., Kobayashi, N., Jackson, A., Onaca, N., Naziruddin, B. and Levy, M.F. (2009) Estimation of donor usability for islet transplantation in the United States with the Kyoto islet isolation method Cell Transplant., 18, 549–556
Noguchi, H., Ueda, M., Hayashi, S., Kobayashi, N., Okitsu, T., Iwanaga, Y., Nagata, H., Nakai, Y. and Mastsumoto, S. (2008) Ductal injection of preservation solution increases islet yields in islet isolation and improves islet graft function Cell Transplant., 17, 69-81
Van der Burg, M.P.M., Basir, I., Zwaan, R.P. and Bouwman, E. (1998) Porcine islet preservation during isolation in University of Wisconsin solution Transplant. Proc., 30, 360-361
Van der Burg, M.P.M., Zwaan, R.P. and Bouwman, E. (1998) Markedly improved outcome of adult porcine islet isolation, purification, and culture using Liberase-P1 versus Collagenase-P, and a novel gradient of OptiPrep in University of Wisconsin solution Horm. Metab. Res., 30, A23
Van der Burg, M.P.M., Rijkelijkhuizen, J.K.R.A., Zwaan, R.P. and Bouwman, E. (1999) Adult pig islet recovery during Liberase isolation, OptiPrep purification and culture for transplantation in nude mice Cell Transplant., 8, abstr. 58
Wee, Y.M., Kim, S.C., Koo, S.K., Kim, Y.H., Jung, E.J., Choi, M.Y., Park, Y.H., Park, K.T., Lim, D.G. and Han, D.J. (2008) Improved islet yields after purification following the novel endogenous trypsin inhibitor and histidine-tryptophan-ketoglutarate treatment in pigs Transplant. Proc., 40, 2585-2587
Special pathogen-free (SPF) pigs
Kim, J.H., Kim, H-I., Lee, K-W., Yu, J.E., Kim, S.H., Park, H.S., Ihm, S-H., Ha, J. et al (2007) Influence of strain and age differences on the yields of porcine islet isolation: extremely high islet yields from SPF CMS miniature pigs Xenotransplantation, 14, 60-66
Kim, H-I., Lee, S-Y., Jin, S.M., Kim, K.S., YU, J.E., Yeom, S-C., Yoon, T.W., Kim, J.H., Ha, J., Park, C-G. and Kim, S-J. (2009) Parameters for successful pig islet isolation as determined using 68 specific-pathogen-free miniature pigs Xenotransplant., 16, 11-18
Trypsin inhibition
Noguchi, H., Naziruddin, B., Jackson, A., Shimoda, M., Fujita, Y., Chujo, D., Takita, M., Peng, H. et al (2012) Comparison of ulinastatin, gabexate mesilate, and nafamostat mesilate in preservation solution for islet isolation Cell Transplant., 21, 509–516
Shimoda, M., Noguchi, H., Fujita, Y., Takita, M., Ikemoto, T., Chujo, D., Naziruddin, B., Levy, M.F., Kobayashi, N., Grayburn, P.A. and Matsumoto, S. (2012) Improvement of porcine islet isolation by inhibition of trypsin activity during pancreas preservation and digestion using α1-antitrypsin Cell Transplant., 21, 465–471
Primates (non-human)
Abouaish, J., Graham, M., Bansal-Pakala, P., Loganathan, G., Soltani, S.M., Tiwari, M., Yuasa, T., Papas, K.K. et al (2011) Successful isolation and transplantation of nonhuman primate islets using a novel purified enzyme blend Transplantation, 92, e41-e42
Haque, M.R., Kim, J., Park, H., Lee, H.S., Lee, K.W., Al-Hilal, T.A., Jeong, J-H., Ahn, C-H. et al (2017) Xenotransplantation of layer-by-layer encapsulated non-human primate islets with a specified immunosuppressive drug protocol J. Control. Release, 258, 10–21
Jin, S-M., Shim, W., Oh, B.J., Oh, S-H., Yu, S.J., Choi, J.M., Park, H.J., Park, J.B. and Kim, J.H. (2017) Anakinra protects against serum deprivation-induced inflammation and functional derangement in islets isolated from nonhuman primates Am. J. Transpl., 17, 365–376
Lei, J., Kim, J.I., Shi, S., Zhang, X., Machaidze, Z., Lee, S., Schuetz, C., Martins, P.N., Oura, T., et al (2015) Pilot study evaluating regulatory T cell–promoting immunosuppression and nonimmunogenic donor antigen delivery in a nonhuman primate islet allotransplantation model Am. J. Transplant., 15, 2739–2749
Matsumoto, S., Rigley, T.H., Qualley, S.A., Kuroda, Y., Reems, J.A. and Stevens, R.B. (2002) Efficacy of the oxygen-charged static two-layer method for short-term pancreas preservation and islet isolation from nonhuman primate and human pancreata Cell Transplant. 11, 769-777
Matsumoto, S., Rigley, T.H., Reems, J.A., Kuroda, Y. and Stevens, R.B. (2003) Improved islet yields from Macaca Nemestrina and marginal human pancreata after two-layer method preservation and endogenous trypsin inhibition Am. J. Transplant., 3, 53-63
Park, H., Park, J.B., Kim, J.H., Lee, K.W., Lee, H.S., Kim, G-S., Shin, D-Y., et al (2017) Simultaneous subtotal pancreatectomy and streptozotocin injection for diabetes modeling in cynomolgus monkeys Transplant. Proc., 49, 1142-1149
Sasikala, M., Rao, G.V., Vijayalakshmi, V., Pradeep, R., Pothani, S., Kumar, P.P., Gaddipati, R., Sirisha, G. et al (2013) Long-term functions of encapsulated islets grafted in nonhuman primates without immunosuppression Transplantation, 96, 624-632
Rat
Allografts
Dellê, H. and Noronha, I.L. (2010) Induction of indoleamine 2,3-dioxygenase by gene delivery in allogeneic islets prolongs allograft survival Am. J. Transplant. 10, 1918-1924
Amino acid transporter
Chessler, S.D., Simonson, W.T., Sweet, I.R. and Hammerle, L.P. (2002) Expression of the vesicular inhibitory amino acid transporter in pancreatic islet cells Diabetes, 51, 1763-1771
β cell imaging
Sweet, I.R., Cook, D.L., Lernmark, A., Greenbaum, C.J., Wallen, A.R., Marcum, E.S., Stekhova, S.A. and Krohn, K.A. (2004) Systematic screening of potential -cell imaging agents Biochem. Biophys. Res. Commun., 314, 976-983
Ca2+ metabolism
Jung, S-R., Reed, B.J. and Sweet, I.R. (2009) A highly energetic process couples calcium influx through L-type calcium channels to insulin secretion in pancreatic -cells Am. J. Physiol. Endocrinol. Metab., 297, E717–E727
Moustafa, A. and Habara, Y. (2016) Reciprocal interaction among gasotransmitters in isolated pancreatic β- cells Free Radical Biol. Med. 90, 47–58
Rountree, A.M., Neal, A.S., Lisowski, M., Rizzo, N., Radtke, J., White, S., Luciani, D.S., Kim, F., Hampe, C.S. and Sweet, I.R. (2014) Control of insulin secretion by cytochrome c and calcium signaling in islets with impaired metabolism J. Biol. Chem., 289, 19110–19119
Encapsulation
Baron, M., Veres, A., Wolock, S.L., Faust, A.L., Gaujoux, R., Vetere, A., Ryu, J.H., Wagner, B.K. et al (2016) A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure Cell Systems 3, 346–360
Donor nutrition
Mishima, T., Kuroki, T., Tajima, Y., Adachi, T., Hirabaru, M., Tanaka, T., Kitasato, A., Takatsuki, M. and Eguchi, S. (2014) Dietary zinc supplementation to the donor improves insulin secretion after islet transplantation in chemically induced diabetic rats Pancreas, 43, 236-239
Pareta, R., McQuilling, J.P., Sittadjody, S., Jenkins, R., Bowden, S., Orlando, G., Farney, A.C., Brey, E.M. and Opara, E.C. (2014) Long-term function of islets encapsulated in a redesigned alginate microcapsule construct in omentum pouches of immune-competent diabetic rats Pancreas, 43, 605-613
GAD-GABA system
Suckow, A.T., Sweet, I.R., Van Yserloo, B., Rutledge, E.A., Hall, T.R., Waldrop, M. and Chessler, S.D. Identification and characterization of a novel isoform of the vesicular γ-aminobutyric acid transporter with glucose-regulated expression in rat islets J. Mol. Endocrinol., 36, 187-199
Glucose transport
Shu, S., Liu, H., Wang, M., Su, D., Yao, L. and Wang, G. (2014) Subchronic olanzapine treatment decreases the expression of pancreatic glucose transporter 2 in rat pancreatic β cells J. Endocrinol. Invest., 37, 667–673
Hyperglycemia
Jiang X.-F., Qian, T-L., Chen, D., Lu, H-W., Xue, P., Yang, X-W., Zhang, L-H., Hu, Y-Z. and Zhang, D-W. (2018) Correction of hyperglycemia in diabetic rats with the use of microencapsulated young market pig islets Transplant. Proc., 50, 3895-3899
Insulin secretion
Buchanan, C.M., Phillips, A.R.J. and Cooper, G.J.S. (2001) Preptin derived from proinsulin growth factor II (preIGF-II) is secreted from pancreatic islet β-cells and enhances insulin secretion Biochem. J., 360, 431-439
Cao, D-S., Zhong, L., Hsieh, T-h., Abooj, M., Bishnoi, M., Hughes, L. and Premkumar, L.S. (2012) Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells PLoS One, 7: e38005
Suckow, A.T., Comoletti, D., Waldrop, M., Mosedale, M., Egodage, S., Taylor, P. and Chessler, S.D. (2008) Expression of neurexin, neuroligin, and their cytoplasmic binding partners in the pancreatic β-cells and the involvement of neuroligin in insulin secretion Endocrinology, 149, 6006-6017
Sweet, I.R., Khalil, G., Wallen, A.R., Steedman, M., Schenkman, K.A., Reems, J.A., Kahn, S.E. and Callis, J.B. (2002) Continuous measurement of oxygen consumption by pancreatic islets Diabetes Techn. Therapeut., 4, 661-672
Zang, X-L., Yang, J-K., Yu, M. and Xue, G-F. (2009) Improved, low-cost methods for pancreatic islet purification in rats Transplant. Proc., 41, 4297-4301
Ischaemic preconditioning
Delaune, V., Lacotte, S., Gex, Q., Slits, F., Kahler-Quesada, A., Lavallard, V., Peloso, A., Orci, L.A., Berney, T. and Toso, C. (2019) Effects of remote ischaemic preconditioning on intraportal islet trans-plantation in a rat model Transpl. Inter., 32: 323–333
Mitochondrial function
Sweet, I.R., Cook, D.L., DeJulio, E., Wallen, A.R., Khalil, G., Callis, J. and Reems, J-A. (2004) Regulation of ATP/ADP in pancreatic islets Diabetes, 53, 401-409
Sweet, I.R., Gilbert, M., Jensen, R., Sabek, O., Fraga, D.W., Gaber, A.O. and Reems, J. (2005) Glucose stimulation of cytochrome C reduction and oxygen consumption as assessment of human islet quality Transplantation, 80, 1003-1011
Sweet, I.R. and Gilbert, M. (2006) Contribution of calcium influx in mediating glucose-stimulated oxygen consumption in pancreatic islets Diabetes, 55, 3509-3519Particulate O2 generation
Particulate oxygen-generation
McQuilling, J.P., Sittadjody, S., Pendergraft, S., Farney, A.C. Opara, E.C. (2017) Applications of particulate oxygen-generating substances (POGS) in the bioartificial pancreas Biomater. Sci., 2017, 5, 2437–2447
PEG interaction
Panza, J.L., Wagner, W.R., Rilo, H.L.R., Rao, R.H., Beckman, E.J. and Russell, A.J. (2000) Treatment of rat pancreatic islets with reactive PEG Biomaterials, 21, 1155-1164
Purification
Dellê, H., Saito, M.H., Yoshimoto, P.M. and Noronha , I.L. (2007) The use of iodixanol for the purification of rat pancreatic islets Transplant. Proc., 39, 467-469
Sawada, T., Matsumoto, I., Nakano, M., Kirchhof, N., Sutherland, D.E.R. and Hering, B.J. (2003) Improved islet yield and function with ductal injection of university of Wisconsin solution before pancreas preservation Transplantation, 75, 1965-1969
RNA
Derr, A., Yang, C., Zilionis, R., Sergushichev, A., Blodgett, D.M., Redick, S., Bortell, R., Luban, J., Harlan, D.M., Kadener, S. et al (2016) End Sequence Analysis Toolkit (ESAT) expands the extractable information from single-cell RNA-seq data Genome Res., 26, 1397-1410
Kiba, T., Tanemura, M. and Yagyu, K. (2013) High-quality RNA extraction from rat pancreatic islet Cell Biol. Int. Rep., 9999, 1–4
Review articles
Bellin, M.D. and Sutherland, D.E.R. (2010) Pediatric islet autotransplantation: indication, technique and outcome Curr. Diab. Rep., 10, 326–331
Chhabra, P. et al (2014) Overcoming barriers in clinical islet transplantation: Current limitations and future prospects Curr. Probl. Surg., 51, 49–86
Gaglia, J.L., Shapiro, A.M.J. and Weir, G.C. (2005) Islet transplantation: progress and challenge Arch. Med. Res., 36, 273-280
Hawthorne, W.J., Williams, L. and Chew, Y.V. (2016) Clinical islet isolation In Pancreatic Islet Isolation, Advances in Experimental Medicine and Biology (ed © Ramírez-Domínguez, M.) Springer International Publishing Switzerland, pp 89-122
Ikemoto, T., Noguchi, H., Shimoda, M., Naziruddin, B., Jackson, A., Tamura, Y., Fujita, Y., Onaca, N., Levy, M.F. and Matsumoto, S. (2009) Islet cell transplantation for the treatment of type 1 diabetes in the USA J Hepatobiliary Pancreat. Surg., 16, 118-123
Kandeel, F., Smith, C.V., Todorov, I. and Mullen, Y (2003) Advances in islet cell biology. From stem cell differentiation to clinical transplantation: conference report Pancreas, 27, e63-e78
Langer, R.M. (2010) Islet transplantation: lessons learned since the Edmonton breakthrough Transplant. Proc., 42, 1421–1424
Linetsky, E. and Ricordi, C. (2020) Islet isolation for autotransplantation, following total or near total pancreatectomy In Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas, 2, Elsevier Inc. Chapter 4 pp. 67-87
Liu, E.H. and Harlan, D.M. (2008) Islet cell transplantation. How effective is it? In Contemporary Endocrinology: Controversies in Treating Diabetes: Clinical and Research Aspects (ed. LeRoith, D. and Vinik, A.I.), Humana Press, Totowa, NJ, pp. 11-32
McCall, M. and Shapiro, A.M.J. (2014) Islet cell transplantation Semin. Pediatr. Surg., 23, 83–90
Matsumoto, S. (2010) Islet cell transplantation for Type 1 diabetes J. Diabetes 2 (2010) 16–22
Matsumoto, S. (2011) Autologous islet cell transplantation to prevent surgical diabetes J. Diabetes, 3, 328–336
Onaca, N., Naziruddin, B., Matsumoto, S., Noguchi, H., Klintmalm, G.B. and Levy, M.F. (2007) Pancreatic islet cell transplantation: update and new developments Nutr. Clin. Pract., 22, 485-493
Rafati, S., Le, C., Rajotte, R.V. and Rayat, G.R. (2012) Cell separation, perfusion from tissue, organelle fractionation: A comparison of the methods used for porcine islet isolation for transplantation as a treatment for type 1 diabetes mellitus In Comprehensive Sampling and Sample Preparation, Vol. 3, Extraction Techniques
and Applications: Biological/Medical and Environmental/Forensics, Elsevier Inc., pp 33-51
Soria, B., Hmadcha, A., Bedoya, F.J. and Tejedo, J.R. (2007) Generation of islets from stem cells Principles of Tissue Engineering, 3rd. edition (ed. Lanza, R., Langer, R. and Vacanti, P.) Elsevier, Inc., pp605-618
Shapiro, A. M. J. (2003) Islet transplants and impact on secondary diabetic complications: does C-peptide protect the kidney J. Am. Soc. Nephrol., 14, 2214-2216
Shapiro, A.M.J., Nanij, S. and Lakey, J.R.T. (2003) Clinical islet transplant: current and future directions towards tolerance Immunol. Rev., 96, 219-236
Shapiro, A.M.J. and Ricordi, C. (2004) Unraveling the secrets of single donor success in islet transplantation Am. J. Transplant., 4, 295-298
Stevens, R.B., Matsumoto, S. and Marsh, C. (2001) Is islet transplantation a realistic therapy for the treatment of type 1 diabetes in the near future? Clin. Diabetes, 19, 51-60
Ulrichs, K., Eber, S., Schneiker, B., Gahn, S., Strauß, A., Moskalenko, V. and Chodnevskaja, I. (2012) Isolation of porcine pancreatic isletsfor xenotransplantation In Xenotransplantation: Methods and Protocols, Methods Mol. Biol., 885 (ed. Costa, C. and Máñez, R.), Springer Science+Business Media, LLC, pp 213-232
White, S.A., James, R.F.L., Swift, S.M., Kimber, R.M. and Nicholson, M.L. (2001) Human islet cell transplantation – future prospects Diabet. Med., 18, 78-103
OptiPrep™ Reference List RC04; 5th edition, February 2020
OptiPrep™ Reference List RC05
Dendritic cells from blood and tissues
Section 2 provides a reference list of publications that report the use of OptiPrep™ for the purification of dendritic cells (DC) from blood and tissues. There is also a brief review of the methodological options for the gradient separations (Section 1). All Application Sheets described in Section 1 can be accessed on the following website: www.Optiprep.com. Click on “Methodology” and then “Mammalian and non-mammalian cells” and follow the links from the Index.
1. Methodological review
1a. Sedimentation on to a barrier
Prior to the introduction of NycodenzⓇ, gradients of either albumin or metrizamide were commonly used in DC purification. These media however tended to cause functional alteration of the cells [1]. Because cells are more tolerant of NycodenzⓇ, after 1994 this iodinated density gradient medium rapidly became established as the medium of choice for DC purification from peripheral blood and from lymphoid tissues. Density barriers of ρ = 1.076 g/ml to 1.088 g/ml [2-7], the majority being approx 1.077 g/ml, have been used in the “traditional” format in which the crude cell fraction is layered on top. DCs band at the interface and further enrichment is often carried out by negative selection with antibody-bound beads. McLellan et al [8,9] introduced a lower density of 1.068 g/ml for DC isolation from human peripheral blood mononuclear cells, depleted in Tlymphocytes by rosetting with neuraminidase-treated sheep erythrocytes. Use of this medium has also been extended to tissues [10,11]. McLellan et al [8] noted that the 1.068 g/ml barrier was also effective for removing denser T-lymphocytes. This strategy of layering the cell suspension on top of a barrier has been extended to the use of OptiPrep™ for isolation of DCs from various tissues (12-17). The barrier sedimentation method is described in Application Sheet C41.
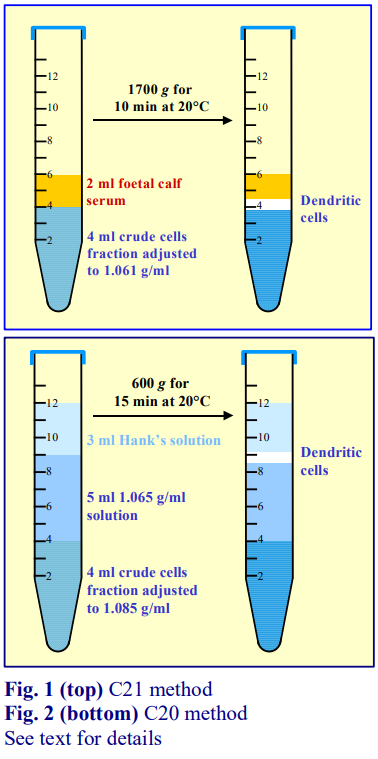 1b. Flotation methods
1b. Flotation methods
Generally speaking isolation of the least dense particle from a mixture of predominantly denser particles (and this is essentially what is happening in the isolation of DCs), is better accomplished by flotation rather than sedimentation. If the cells are suspended in a solution whose density is slightly higher than DCs, the latter will float to the top while the denser cells will either remain suspended in, or sediment slowly to the bottom, of the suspending medium. An important facet of this strategy is that avoids the rapid accumulation of cells, and consequent aggregation, that occurs during sedimentation on to a density barrier. This strategy was first introduced by Shortman’s group in 1996 [18-21], in which the cells from spleen, thymus, lymph nodes etc were suspended in a solution of NycodenzⓇ (ρ = 1.077 g/ml). This approach was adapted by Anjuere et al [22] to OptiPrep™ in 1999 and there has been tendency towards the use of lower densities for the suspending solution (e.g. 1.061 g/ml) to improve the purity of the DC harvest. This method (see Fig. 1) is described in Application Sheet C22.
A modification to this flotation format is to suspend the cells in a medium of higher density, which is placed below the separating solution of ρ = 1.065 g/ml [23]. This is a commonly used format for the selective isolation of a low density cell type; its advantage is that the band of cells is divorced from the crude fraction layer, which is likely to contain residual tissue dispersing enzymes and components from partially disrupted cells. This method (see Fig. 2) is described in Application Sheet C21.
1c. References (to Section 1)
1. McLellan, A. D., Starling, G. C. and Hart, D. N. J. (1995) Isolation of human blood dendritic cells by discontinuous Nycodenz gradient centrifugation J. Immunol. Meth., 184, 81-89
2. Durant, S., Alves, V., Coulaud, J. and Homo-Delarche, F. (2002) Nonobese diabetic (NOD) mouse dendritic cells stimulate insulin secretion by prediabetic islets Autoimmunity, 35, 449-4551059
3. Jang, M.H., Sougawa, N., Tanaka, T., Hirata, T., Hiroi, T., Tohya, K., Guo, Z., Umemoto, E., Ebisuno, Y., Yang, B-G., Seoh, J-Y., Lipp, M., Kiyono, H. and Miyasaka, M. (2006) CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes J. Immunol., 176, 803-810
4. De Creus, A., Lau, A.H., Hackstein, H., Raimondi, G. and Thomson, A.W. (2005) Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin J. Immunol., 174, 2037-2045
5. Leenen, P.J.M, Radosevic, K., Voerman, J.S.A., Salomon, B., van Rooijen, N., Klatzmann, D. and van Ewijk, W. (1998) Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover J. Immunol., 160, 2166-2173
6. Den Haan, J.M.M., Lehar, S.M. and Bevan, M.J. (2000) CD8+ but not CD8- cells cross-prime cytotoxic T cells in vivo J. Exp. Med., 192, 1685-1695
7. Trinite, B., Chauvin, C., Pêche, H., Voisine, C., Heslan, M. and Josien, R. (2005) Immature CD4-CD103+ rat dendritic cells induce rapid caspase-independnet apoptosis-like cell death in various tumor and nontumor cells and phagacytose their victims J. Immunol., 175, 2408-2417
8. McLellan, A.D., Starling, G.C. and Hart, D.N.J. (1995) Isolation of human blood dendritic cells by discontinous Nycodenz gradient centrifugation J. Immunol. Meth., 184, 81-89
9. McLellan, A.M., Heiser, A., Sorg, R.V., Fearnley, D.B. and Hart, D.N. (1998) Dermal dendritic cells associated with T lymphocytes in normal human skin display an activated phenotype J. Invest. Dermatol., 111, 841-849
10. Moghaddami, M., Swart, B., Reynolds, P., Diener, K. and Brown, M.P. (2002) Flt3 ligand expands dendritic cell numbers in normal and malignant murine prostate Immunol. Cell Biol., 80, 370-381
11. Chen, L., Arora, M., Yarlagadda, M., Oriss, T.B., Krishnamoorthy, N., Ray, A. and Ray, P. (2006) Distinct responses of lung and spleen dendritic cells to the TLR9 agonist CpG oligodeoxynucleotide J. Immunol., 177, 2373-2383
12. Bonneau, M., Epardaud, M., Payot, F., Niborski, V., Thoulouze, M-I., Bernex, F., Charley, B., Riffault, S., Guilloteau, L.A. and Schwartz-Cornil, I. (2006) Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node J. Leukoc. Biol., 79, 268-276
13. Lee, J-E., Kang, C-S., Guan, X-Y., Kim, B-T., Kim, S-H., Lee, Y-M., Moon, W-S. and Kim, D-K. (2007) Discoidin domain receptor 2 is involved in the activation of bone marrow-derived dendritic cells caused by type I collagen Biochem. Biophys. Res. Comm., 352, 244-250
14. Eaton, K.A., Benson, L.H., Haeger, J. and Gray, B.M. (2006) Role of transcription factor T-bet expression by CD4+ cells in gastritis due to Helicobacter pylori in mice Infect. Immun., 74, 4673-4684
15. Cervantes-Barragan, L., Züst, R., Weber, F., Spiegel, M., Lang, K.S., Akira, S., Thiel, V. and Ludewig, B. (2007) Control of coronavirus infection through plasmacytoid dendritic cell-derived type I interferon Blood, 109, 1131-1137
16. Luckashenak, N.A., Ryszkiewicz, R.L., Ramsey, K.D. and Clements, J.L. (2006) The Src homology 2 domaincontaining leukocyte protein of 76-kDa adaptor links integrin ligation with p44/42 MAPK phosphorylation and podosome distribution in murine dendritic cells J. Immunol., 177, 5177-5185
17. Hansen, S., Lo, B., Evans, K., Neophytou, P., Holmskov, U. and Wright, J.R. (2007) Surfactant protein D augments bacterial association but attenuates major histocompatibility complex class II presentation of bacterial antigens Am. J. Respir. Cell. Mol. Biol., 36, 94-102
18. Kronin, V., Winkel, K., Suss, G. Kelso, A., Heath, W., Kirberg, J., von Boehmer, H. and Shortman. K. (1996) A subclass of dendritic cells regulates the response of naïve CD8 T cells by limiting their IL-2 production J. Immunol., 157, 3819-3627
19. Vremec, D. and Shortman, K. (1997) Dendritic cell suptype in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes J. Immunol., 159, 565-573
20. Vremec, D., Pooley, J., Hochrein, H., Wu, L. and Shortman, K. (2000) CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen J. Immunol., 164, 2978-2986
21. Kamath, A.T. Pooley, J., O’Keeffe, M.A. Vremec, D., Zhan, Y. Lew, A.M., D’Amico, A., Wu, L., Tough, D.F. and Shortman, K. (2000) The development, maturation, and turnover rate of mouse spleen dendritic cell populations J. Immunol., 165, 6762-6770
22. Anjuere, F., Martin, P., Ferrero, I., Fraga, M. L., del Hoyo, G. M., Wright, N. and Ardavin, C. (1999) Definition of dendritic cell subpopulations present in the spleen, Peyer’s patches, lymph nodes, and skin of the mouse Blood, 93, 590-598
23. Ruedl, C., Rieser, C., Bock, G., Wick, G. and Wolf, H. (1996) Phenotypic and functional characterization of CD11c+ dendritic cell population in mouse Peyer’s patches Eur. J. Immunol., 26, 1801-1806
2. OptiPrep™ Reference list
References are divided alphabetically into sections according to tissue or cell source and, where the number of references demands, sorted further into research topic sub-sections. Within each section references are listed alphabetically according to first author. This bibliography was last updated in September 2019.
Blood
Monocyte-derived
Akiyama, Y., Tanosaki, R., Inoue, N., Shimada, M., Hotate, Y., Yamamoto, A., Yamazaki, N., Kawashima, I. et al (2005) Clinical response in Japanese metastatic melanoma patients treated with peptide cocktail-pulsed dendritic cells J. Transplant. Med., 3, 1-10
Alvarez, Y., Municio, C., Alonso, S., San Román, J.A., Sánchez Crespo, M. and Fernández, N. (2009) Cyclooxygenase-2 induced by zymosan in human monocyte-derived dendritic cells shows high stability, and its expression is enhanced by atorvastatin J. Pharmacol. Exp. Ther., 329, 987-994
Oo, Y.H., Weston, C.J., Lalor, P.F., Curbishley, S.M., Withers, D.R., Reynolds, G.M., Shetty, S., Harki, J. et al (2010) Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver J. Immunol., 184, 2886–2898
Pecher, G., Schirrmann, T., Kaiser, L. and Schenk, J.A. (2001) Efficient cryopreservation of dendritic cells transfected with cDNA of a tumour antigen for clinical application Biotechnol. Appl. Biochem., 34, 161-166
Pinchuk, L.M., Boyd, B.L., Kruger, E.F., Roditi, I. and Furger, A. (2003) Bovine dendritic cells generated from monocytes and bone marrow progenitors regulate immunoglobulin production in peripheral blood B cells Comp. Immun. Microbiol. Infect. Dis., 26 233-249
Suehiro, Y., Hasegawa, A., Iino, T., Sasada, A., Watanabe, N., Matsuoka, M., Takamori, A., Tanosaki, R., Utsunomiya, A. et al (2015) Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study Br. J. Haematol., 169, 356–367
Valera, I., Fernández, N., García Trinidad, A., Alonso, S., Brown, G.D., Alonso, A. and Sánchez Crespo, M. (2008) Costimulation of dectin-1 and DC-SIGN triggers the arachidonic acid cascade in human monocytederived dendritic cells J. Immunol., 180, 5727-5736
PBMC-derived
Deloizy, C., Bouguyon, E., Fossum, E., Sebo, P., Osicka, R., Bole, A., Pierres, M. Biacchesi, S. et al (2016) Expanding the tools for identifying mononuclear phagocyte subsets in swine: Reagents to porcine CD11c and XCR1 Dev. Comp. Immunol., 65, 31-40
Fiebach, A.R., Guzylack-Piriou, L., Python, S., Summerfield, A. and Ruggli, N. (2011) Classical swine fever virus Npro limits type I interferon induction in plasmacytoid dendritic cells by interacting with interferon regulatory factor 7 J. Virol., 85, 8002-8011
Nagato, K., Motohashi, S., Ishibashi, F., Okita, K., Yamasaki, K., Moriya, Y., Hoshino, H., Yoshida, S. et al (2012) Accumulation of activated invariant natural killer T cells in the tumor microenvironment after αgalactosylceramide-pulsed antigen presenting cells J. Clin. Immunol., 32, 1071–1081
Reid, E., Juleff, N., Gubbins, S., Prentice, H., Seago, J. and Charleston, B. (2011) Bovine plasmacytoid dendritic cells are the major source of type I interferon in response to foot-and-mouth disease virus in vitro and in vivo J. Virol., 85, 4297–4308
Ruscanu, S., Jouneau, L., Urien, C., Bourge, M., Lecardonnel, J., Moroldo, M., Loup, B., Dalod, M., ElhmouziYounes, J. et al (2013) Dendritic cell subtypes from lymph nodes and blood show contrasted gene expression programs upon bluetongue virus infection J. Virol., 87, 9333–9343
Strowig, T., Brilot, F., Arrey, F., Bougras, G., Thomas, D., Muller, W.A. and Münz, C. (2008) Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-γ PLoS Pathog., 4:e27
Peripheral blood
Zamora-Pineda, J., Kumar, A., Suh, J.H., Zhang, M. and Saba, J.D. (2016) Dendritic cell sphingosine-1- phosphate lyase regulates thymic egress J. Exp. Med., 213, 2773–2791
Bone marrow
Anti-tumour immune response
Kapadia, C.H., Tian, S., Perry, J.L., Sailer, D., Luft, C.J., DeSimone, J.M. (2018) Extending antigen release from particulate vaccines results in enhanced antitumor immune response J. Control. Release, 269, 393–404
Apoptosis
Yamazaki, T., Akiba, H., Iwai, H., Matsuda, H., Aoki, M., Tanno, Y., Shin, T., Tsichiya, H., Pardoll, D.M., Okumura, K., Azuma, M. and Yagita, H. (2002) Expression of programmed death 1 ligands by murine T cells and APC J. Immunol., 169, 5538-5545
Derivation, development and maturation
Figueiredo, R.T., Fernandez, P.L., Mourao-Sa, D.S., Porto, B.N., Dutra, F.F., Alves, L.S., Oliviera, M.F., Oliviera, P.L., Graca-Souza, A.V. and Bozza, M.T. (2007) Characterization of heme as activator of toll-like receptor 4 J. Biol. Chem., 282, 20221-20229 Lee, J-E., Kang, C-S., Guan, X-Y., Kim, B-T., Kim, S-H., Lee, Y-M., Moon, W-S. and Kim, D-K. (2007)
Discoidin domain receptor 2 is involved in the activation of bone marrow-derived dendritic cells caused by type I collagen Biochem. Biophys. Res. Commun., 352, 244-250
Li, J-C., Park, J-H., Foss, D. and Goldschneider, I. (2009) Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus J. Exp. Med., 206, 607-622
Immune responses
Functional control
Williams, M.A., Bauer, S., Lu, W., Guo, J., Walter, S., Bushnell, T.P., Lillehoj, E.P. and Georas, S.N. (2010) Deletion of the mucin-like molecule Muc1 enhances dendritic cell activation in response to toll-like receptor ligands J. Innate Immun., 2, 123–143
Infection (Helicobacter)
Eaton, K.A., Benson, L.H., Haeger, J. and Gray, B.M. (2006) Role of transcription factor T-bet expression by CD4+ cells in gastritis due to Helicobacter pylori in mice Infect. Immun., 74, 4673-4684
Kao, J.Y., Pierzchala, A., Rathinavelu, S., Zavros, Y., Tessier, A. and Merchant, J.L. (2006) Somatostatin inhibits dendritic cell responsiveness to Helicobacter pylori Regul. Pept., 134, 23-29
Kao, J.Y., Rathinavelu, S., Eaton, K.A., Bai, L., Zavross, Y., Takami, M., Pierzchala, A. and Merchant, J.L. (2006) Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense Am. J. Physiol Gastrointest. Liver Physiol., 291, G73-G81
Luther, J., Owyang, S.Y., Takeuchi, T., Cole, T.S., Zhang, M., Liu, M., Erb-Downward, J., Rubenstein, J.H. et al (2011) Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis Gut 60, 1479-1486
Rathinavelu, S., Kao, J.Y., Zavros, Y. and Merchant, J.L. (2005) Helicobacter pylori outer membrane protein 18 (Hp1125) induces dendritic cell maturation and function Helicobacter, 10, 424-432
Sun, X., Zhang, M., El-Zataari, M., Owyang, S.Y., Eaton, K.A., Liu, M., Chang, Y-M., Zou, W. and Kao, J.Y. (2013) TLR2 mediates Helicobacter pylori-induced tolerogenic immune response in mice PLoS One, 9: e74595
Sun, X., Zhang, M., El-Zaatari, M., Huffnagle, G.B. and Kao, J.Y. (2017) CCR2 mediates Helicobacter pyloriinduced immune tolerance and contributes to mucosal homeostasis Helicobacter 22: e12366
Takabayashi, H., Shinohara, M., Mao, M., Phaosawasdi, P., El–Zaatari, M., Zhang, M., Ji, T., Eaton, K.A., Dang, D., Kao, J. and Todisco, A. (2014) Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice Gastroenterology, 147, 396–406
Tanaka, H., Yoshida, M., Nishiumi, S., Ohnishi, N., Kobayashi, K., Yamamoto, K., Fujita, T., Hatakeyama, M. and Azuma, T. (2010) The CagA protein of Helicobacter pylori suppresses the functions of dendritic cell in mice Arch. Biochem., Biophys., 498, 35–42
Zhang, M., Berndt, B.E., Eaton, K.A., Rathinavelu, S., Pierzchala, A. and Kao, J.Y. (2008) Helicobacter pyloripulsed dendritic cells induce H. pylori-specific immunity in mice Helicobacter, 13, 200-208
Infections (other than Helicobacter)
Awasthi, S. and Cropper, J. (2006) Immunophenotype and functions of fetal baboon bone-marrow derived dendritic cells Cell. Immunol., 240, 31-40
Awasthi, S., Vilekar, P., Conkleton, A. and Rahman, N. (2019) Dendritic cell-based immunization induces Coccidioides Ag2/PRA-specific immune response Vaccine, 37, 1685–1691
Benjamin, C.F., Lundy, S.K., Lukacs, N.W., Hogaboam, C.M. and Kunkel, S.L. (2005) Reversal of long-term sepsis-induced immunosuppression by dendritic cells Blood, 105, 3588-3595
Bittencourt, V.C.B., Figueiredo, R.T., da Silva, R.B., Mourão-Sá, D.S., Fernandez, P.L., Sassaki, G.L., Mulloy, B., Bozza, M.T. and Barreto-Bergter, E. (2006) An -glucan of Pseudallescheria boydii is involved in fungal phagocytosis and toll-like receptor activation J. Biol. Chem., 281, 22614-22623
Martin, P., Martinez del Hoyo, G., Anjuère, F., Fernandez Arias, C., Hernandez Vargas, H., Fernandez-L, A., Parrillas, V. and Ardavin, C. (2002) Characterization of a new subpopulation of mouse CD8+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential Blood, 100, 383-390
Schindler, D., Gutierrez, M.G., Beineke, A., Rauter, Y., Rohde, M., Foster, S., Goldmann, O. and Medina, E. (2012) Dendritic cells are central coordinators of the host immune response to Staphylococcus aureus bloodstream infection Am. J. Pathol., 181, 1327–1337
Shetron-Rama, L.M., Herring-Palmer, A.C., Huffnagle, G.B. and Hanna, P. (2010) Transport of Bacillus anthracis from the lungs to the draining lymph nodes is a rapid process facilitated by CD11c+ cells Microb. Pathog., 49, 38-46
Vilekar, P., Awasthi, V., Lagisetty, P., King, C., Shankar, N., Awasthi, S. (2010) In vivo trafficking and immunostimulatory potential of an intranasally-administered primary dendritic cell-based vaccine BMC Immunol., 11, 60
Inflammatory response
Berndt, B.E., Zhang, M., Chen, G-H., Huffnagle, G.B. and Kao, J.Y. (2007) The role of dendritic cells in the development of acute dextran sulfate sodium colitis J. Immunol., 179, 6255-6262
Takabayashi, H., Shinohara, M., Mao, M., Phaosawasdi, P., El–Zaatari, M., Zhang, M., Ji, T. et al (2014) Antiinflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice Gastroenterology, 147, 396–406
Vilekar, P., Awasthi, S., Natarajan, A., Anant, S. and Awasthi, V. (2012) EF24 suppresses maturation and inflammatory response in dendritic cells Int. Immunol., 24, 455–464
Tumour interactions
Ghansah, T., Vohra, N., Kinney, K., Weber, A., Kodumudi, K., Springett, G., Sarnaik, A.A. and Pilon-Thomas, S. (2013) Dendritic cell immunotherapy combined with gemcitabine chemotherapy enhances survival in a murine model of pancreatic carcinoma Cancer Immunol. Immunother., 62, 1083–1091
Komine, H., Kuhn, L., Matsushita, N., Mulé, J.J. and Pilon-Thomas, S. (2013) Examination of MARCO activity on dendritic cell phenotype and function using a gene knockout mouse PLoS One, 8: e67795
Matsushita, N., Pilon-Thomas, S.A., Martin, L.M. and Riker, A.I. (2008) Comparative methodologies of regulatory T cell depletion in a murine melanoma model J. Immunol. Methods, 333, 167-179
Perez-Shibayama, C., Gil-Cruz, C., Nussbacher, M., Allgäuer, E., Cervantes-Barragan, L., Züst, R. and Ludewig, R. (2103) Dendritic cell-specific delivery of Flt3L by coronavirus vectors secures induction of therapeutic antitumor immunity PLoS One, 8: e81442
Pilon-Thomas, S., Mackay, A., Vohra, N. and Mulé. J.J. (2010) Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma J. Immunol., 184, 3442– 3449
Viruses
Acosta-Ramirez, E., Pérez-Flores, R., Majeau, N., Pastelin-Palacios, R., Gil-Cruz, C., Ramírez-Saldaña, M., Manjarrez-Orduño, N., Cervantes-Barragán, L. et al (2008) Translating innate response into long-lasting antibody response by the intrinsic antigen-adjuvant properties of papaya mosaic virus Immunology, 124, 186-197
Cervantes-Barragán, L., Kalinke, U., Züst, R., König, M., Reizis, B., López-Macías, C., Thiel, V. and Ludewig, B. (2009) Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection J. Immunol., 182, 1099-1106
Interleukin production
Berndt, B.E., Zhang, M., Owyang, S.Y., Cole, T.S., Wang, T.W., Luther, J., Veniaminova, N.A., Merchant, J.L. et al (2012) Butyrate increases IL-23 production by stimulated dendritic cells Am. J. Physiol. Gastrointest. Liver Physiol., 303, G1384–G1392
Lipopolysaccharide stimulation
Berndt, B.E., Zhang, M., Owyang, S.Y., Cole, T.S., Wang, T.W., Luther, J., Veniaminova, N.A. et al (2012) Butyrate increases IL-23 production by stimulated dendritic cells Am. J. Physiol. Gastrointest. Liver Physiol., 303, G1384–G1392
MHC class II expression
Goth, S.R., Chu, R.A. and Pessah, I.N. (2006) Oxygen tension regulates the in vitro maturation of GM-CSF expanded murine bone marrow dendritic cells by modulating class II MHC expression J. Immunol. Methods, 308, 179-191
LeibundGut-Landemann, S., Waldburger, J-M., Reis e Sousa, C. Acha-Orbea, H. and Reith, W. (2004) MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells Nat. Immunol., 5, 899-908
T-cell function
Kodumudi, K.N., Weber, A., Sarnaik, A.A. and Pilon-Thomas, S. (2012) Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma J. Immunol., 189, 5147–5154
Wang, S.H., Chen, G-H., Fan, Y., Van Antwerp, M. and Baer, J.R. (2009) Tumor necrosis factor-related apoptosis-inducing ligand inhibits experimental autoimmune thyroiditis by the expansion of CD4+CD25+ regulatory T cells Endocrinology, 150, 2000–2007
Vaccine production in cancer
Gatza, E. and Okada, C.Y. (2006) Adjuvant IL-15 does not enhance the efficacy of tumor cell lysate-pulsed dendritic cell vaccines for active immunotherapy of T cell lymphoma Cancer Immunol. Immunother., 55, 420- 432
Hosoi, A., Takeda, Y., Sakuta, K., Ueha, S., Kurachi, M., Kimura, K., Maekawa, R. and Kakimi, K. (2008) Dendritic cell vaccine with mRNA targeted to the proteasome by polyubiquitination Biochem. Biophys. Res. Commun., 371, 242-246
Kao, J.Y., Zhang, M., Chen, C-M. and Chen, J-J. (2005) Superior efficacy of dendritic cell-tumor fusion vaccine compared with tumor lysate-pulsed dendritic cell vaccine in colon cancer Immunol. Lett., 101, 154-159
Kao, J.Y., Zhang, M., Chen, C-M., Pierzchala, A. and Chen, J-J. (2006) Aberrant T helper cell response in tumor-bearing mice limits the efficacy of dendritic cell vaccine Immunol. Lett., 105, 16-25
Koike, N., Pilon-Thomas, S. and Mule, J.J. (2008) Nonmyeloablative chemotherapy followed by T-cell adoptive transfer and dendritic cell-based vaccination results in rejection of established melanoma J. Immunother., 31, 402-412
Zhang, M., Berndt, B.E., Chen, J-J. and and Kao, J.Y. (2008) Expression of a soluble TGF-β receptor by tumor cells enhances dendritic cell/tumor fusion vaccine efficacy J. Immunol., 181, 3690-3697
Brain meninges
Flach, A.C., Litke, T., Strauss, J., Haberl, M., Gómez, C.C., Reindl, M., Saiz, A., Fehlingd, H-J., Wienands, J. et al (2016) Autoantibody-boosted T-cell reactivation in the target organ triggers manifestation of autoimmune CNS disease Proc. Natl. Acad. Sci. USA, 113, 3323-3328
Inflammatory cells
Čolić, M., Gazivoda, D., Vučević, D., Vasilijić, S., Rudolf, R. and Lukić, A. (2009) Proinflammatory and immunoregulatory mechanisms in periapical lesions Mol. Immunol., 47, 101–113
Vasilijić, S., Savić, Vasilev, S., Vučević, D., Gašić, S., Majstorović, I., Janković, S. and Čolić, M. (2005) Dendritic cells acquire tolerogenic properties at the site of sterile granulomatous inflammation Cell. Immunol., 233, 148-157
Intestinal tract
Arques, J.L., Hautefort, I., Ivory, K., Bertelli, E., Regoli, M., Clare, S., Hinton, J.C.D. and Nicoletti, C. (2009) Salmonella induces flagellin- and MyD88-dependent migration of bacteria-capturing dendritic cells into the gut lumen Gastroenterology 137, 579-587
Chang, S-Y., Song, J-H., Guleng, B., Cotoner, C.A., Arihiro, S., Zhao, Y., Chiang, H-S., O’Keeffe, M. et al (2013) Circulatory antigen processing by mucosal dendritic cells controls CD8+ T cell activation Immunity 38, 153–165
Eksteen, B., Mora, J.R., Haughton, E.L., Henderson, N.C., Lee–Turner, L., Villablanca E.J., Curbishley, S.M. et al (2009) Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells Gastroenterology, 137, 320–329
Hu, Q., Ren, H., Li, G., Wang, D., Zhou, Q., Wu, J., Zheng, J., Huang, J. et al (2019) STING-mediated intestinal barrier dysfunction contributes to lethal sepsis EBioMedicine 41, 497–508 Kim, J-I., Song, J-Y., Ko, H-J., Kweon, M-N., Kang, C-Y., Reinecker, H-C and Chang, S-Y. (2018) CX3CR1+ macrophages and CD8+ T cells control intestinal IgA production J. Immunol., 201, 1287–1294
Lee, M-R., Seo, G-Y., Kim, Y-M., Kim, P-H. (2011) iNOS potentiates mouse Ig isotype switching through AID expression Biochem. Biophys. Res. Comm., 410, 602–607
Padassi, H., Acharya, M., Zhang, A., Mukhopadhyay, S., Kwon, M., Chow, C., Stuart, L.M., Savill, J. and Lacy–Hulbert, A. (2011) Preferential expression of integrin v8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells Gastroenterology, 141, 1813–1820
Sidhu, M., Cotoner, C.A., Guleng, B., Arihiro, S., Chang, S., Duncan, K.W., Ajami, A.M., Chau, M. and Reinecker, H-C. (2011) Small molecule tyrosine kinase inhibitors for the treatment of intestinal inflammation Inflamm. Bowel Dis., 17, 2416–2426
Sundberg, T.B., Choi, H.G., Song, J-H., Russell, C.N., Hussain, M.M., Graham, D.B., Khor, B., Gagnon, J. et al (2014) Small-molecule screening identifies inhibition of salt-inducible kinases as a therapeutic strategy toenhance immunoregulatory functions of dendritic cells Proc. Natl. Acad. Sci. USA, 111, 12468–12473
Lamina propria
Park, S-M., Omatsu, T., Zhao, Y., Yoshida, N., Shah, P., Zagani, R. and Reinecker, H-C. (2019) T cell fate following Salmonella infection is determined by a STING-IRF1 signaling axis in mice Commun. Biol., 2: 464
Langerhans cells
Anjuère, F., Martin, P., Ferrero, I., Lopez Fraga, M., Martinez del Hoyo, G., Wright, N. and Ardavin, C. (1999) Definition of dendritic cell subpopulations present in the spleen, Peyer’s patches, lymph nodes, and skin of the mouse Blood, 93, 590-598
Anjuère, F., Martinez del Hoyo, G., Martin, P. and Ardavin, C. (2000) Langerhans cells develop from a lymphoid-committed precursor Blood, 96, 1633-1637
Contreras, V., Urien, C., Guiton, R., Alexandre, Y., Vu Manh, T-P., Andrieu, T., Crozat, K. et al (2010) Existence of CD8α-like dendritic cells with a conserved functional specialization and a common molecular signature in distant mammalian species J. Immunol., 185, 3313–3325
Dimitroff, C.J., Kupper, T.S. and Sackstein, R. (2003) Prevention of leukocyte migration to inflamed skin with a novel fluorosugar modifier of cutaneous lymphocyte-associated antigen J. Clin. Invest., 112, 1008-1018
Goddard, S., Youster, J., Morgan, E. and Adams, D.H. (2004) Interleukin-10 secretion differentiates dendritic cells from human liver and skin Am. J. Pathol., 164, 511-519
Jux, B., Kadow, S. and Esser, C. (2009) Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice J. Immunol., 182, 6709–6717
Kuipers, H., Schnorfeil, F.M., Fehling, H-J., Bartels, H. and Brocker, T. (2010) Dicer-dependent microRNAs control maturation, function and maintenance of Langerhans cells in vivo J. Immunol., 185, 400–409
Peña-Cruz, V., McDonough, S.M., Diaz-Griffero, F., Crum, C.P., Carrasco, R.D. and Freeman, G.J. (2010) PD-1 on immature and PD-1 ligands on migratory human Langerhans cells regulate antigen-presenting cell activity J. Invest. Dermatol., 130, 2222–2230
Polak, M.E., Newell, L., Taraban, V.Y., Pickard, C., Healy, E., Friedmann, P.S., Al-Shamkhani, A. and ArdernJones, M.R. (2012) CD70–CD27 interaction augments CD8+ T-cell activation by human epidermal Langerhans clls J. Invest. Dermatol., 132, 1636–1644
Ruedl, C., Koebel, P. and Karjalainen, K. (2001) In vivo-matured Langerhans cells continue to take up and process native proteins unlike in vitro-matured counterparts J. Immunol., 166, 7178-7182
Stutte, S., Jux, B., Esser, C. and Förster, I. (2008) CD24a expression levels discriminate Langerhans cells from dermal dendritic cells in murine skin and lymph nodes J. Invest. Dermatol., 128, 1470-1475
Sirvent, S., Vallejo, A.F., Davies, J., Clayton, K., Wu, Z., Woo, J., Riddell, J., Chaudhri, V.K., Stumpf, P. et al (2020) Genomic programming of IRF4-expressing human Langerhans cells Nat. Comm., 11: 313
Liver
Cavassani, K.A., Moreira, A.P., Habiel, D., Ito, T., Coelho, A.L., Allen, R.M., Hu, B. et al (2013) Toll like receptor 3 plays a critical role in the progression and severity of acetaminophen-induced hepatotoxicity PLoS One, 8: e65899
Connolly, M.K., Ayo, D., Malhotra, A., Hackman, M., Bedrosian, A.S., Ibrahim, J., Cieza-Rubio, N.E. et al (2011) Dendritic cell depletion exacerbates acetaminophen hepatotoxicity Hepatology, 54, 959-968
Cremer, I., Dieu-Nosjean, M-C., Maréchal, S., Dezutter-Dambuyant, C., Goddard, S., Adams, D., Winter, N., Menetrier-Caux, C. et al (2002) Long-lived immature dendritic cells mediated by TRANCE-RANK interaction Blood, 100, 3646-3655
Eksteen, B., Mora, J.R., Haughton, E.L., Henderson, N.C., Lee–Turner, L., Villablanca E.J., Curbishley, S.M. et al (2009) Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells Gastroenterology, 137, 320–329
Goddard, S., Youster, J., Morgan, E. and Adams, D.H. (2004) Interleukin-10 secretion differentiates dendritic cells from human liver and skin Am. J. Pathol., 164, 511-519
Hansen, S., Lo, B., Evans, K., Neophytou, P., Holmskov, U. and Wright, J.R. (2007) Surfactant protein D augments bacterial association but attenuates major histocompatibility complex class II presentation of bacterial antigens Am. J. Respir. Cell. Mol. Biol., 36, 94-102
Oo, Y.H., Weston, C.J., Lalor, P.F., Curbishley, S.M., Withers, D.R., Reynolds, G.M., Shetty, S. et al (2010) Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver J. Immunol., 184, 2886–2898
Wiegard, C., Wolint, P., Frenzel, C., Cheruti, U., Schmitt, E., Oxenius, A., Lohse, A.W. and Herkel, J. (2007) Defective T helper response of hepatocyte-stimulated CD4 T cells impairs antiviral CD8 response and viral clearance Gastroenterology 133, 2010-2018
Lung
Ainsua-Enrich, E., Hatipoglu, I., Kadel, S., Turner, S., Paul, J., Singh, S., Bagavant, H. and Kovats, S. (2019) IRF4-dependent dendritic cells regulate CD8+ T-cell differentiation and memory responses in influenza infection Mucosal Immunol., 12, 1025–1037
Awasthi, S., Wolf, R. and White, G. (2009) Ontogeny and phagocytic function of baboon lung dendritic cells Immunol. Cell Biol., 87, 419–427
Awasthi, S., Madhusoodhanan, R. and Wolf, R. (2011) Surfactant protein-A and toll-like receptor-4 modulate immune functions of preterm baboon lung dendritic cell precursor cells Cell. Immunol., 268, 87–96
Bordet, E., Maisonnasse, P., Renson, P., Bouguyon, E., Crisci, E., Tiret, M., Descamps, D., Bernelin-Cottet, C. et al (2018) Porcine alveolar macrophage-like cells are pro-inflammatory pulmonary intravascular macrophages that produce large titers of porcine reproductive and respiratory syndrome virus Sci. Rep., 8: 10172
Bosteels, C., Lambrecht, B.N. and Hammad, H. (2018) Isolation of conventional murine lung dendritic cell subsets Current Protoc. Immunol., 120, 3.7B.1–3.7B.16
Hansen, S., Lo, B., Evans, K., Neophytou, P., Holmskov, U. and Wright, J.R. (2007) Surfactant protein D augments bacterial association but attenuates major histocompatibility complex class II presentation of bacterial antigens Am. J. Respir. Cell. Mol. Biol., 36, 94-102
Hayashi, T., Beck, L., Rossetto, C., Gong, X., Takikawa, O., Takabayashi, K., Broide, D.H. and Raz, E. (2004) Inhibition of experimental asthma by indoleamine 2,3-dioxygenase J. Clin. Invest., 114, 270-279
Ho, A.W.S., Prabhu, N., Betts, R.J., Ge, M.Q., Dai, X., Hutchinson, P.E., Lew, F.C. et al (2011) Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells J. Immunol., 187, 6011–6021
Maisonnasse, P., Bouguyon, E., Piton, G., Ezquerra, A., Urien, C., Deloizy, C., Bourge, M., Leplat, J-J., Simon, G. et al (2016) The respiratory DC/macrophage network at steady-state and upon influenza infection in the swine biomedical model Mucosal Immunol., 9, 835-849
Kannan, Y., Li, Y., Coomes, S.M., Okoye, I.S., Pelly, V.S. Sriskantharajah, S., Geuckel, E., Webb, L., Czieso, S., Nikolov, N. et al (2017) Tumor progression locus 2 reduces severe allergic airway inflammation by inhibiting Ccl24 production in dendritic cells J. Allergy Clin. Immunol., 139, 655-66
Majlessi, L., Brodin, P., Brosch, R., Rojas, M-J., Khun, H., Huerre, M., Cole, S.T. and Leclerc, C. (2005) Influence of ESAT-6 secretion system 1 (RD1) of Mycobacterium tuberculosis on the interaction between mycobacteria and the host immune system J. Immunol., 174, 3570-3579
Okunishi, K., Dohi, M., Nakagome, K., Tanaka, R., Mizuno, S., Matsumoto. K., Miyazaki, J-i., Nakamura, T. and Yamamoto, K. (2005) A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function J. Immunol., 175, 4745-4753
Simeone, R., Sayes, F., Song, O., Gröschel, M.I., Brodin, P., Brosch, R. and Majlessi, L. (2015) Cytosolic access of mycobacterium tuberculosis: critical impact of phagosomal acidification control and demonstration of occurrence in vivo PLoS Pathog., 10: e1004650
Tang, Y., Guan, S.P., Chua, B.Y.L., Zhou, Q., Ho, A.W.S., Wong, K.H.S., Wong, K.L., Wong, W.S.F. and Kemeny, D.M. (2012) Antigen-specific effector CD8 T cells regulate allergic responses via IFN-γ and dendritic cell function J. Allergy Clin. Immunol., 129, 1611-20
Van de Laar, L., Guilliams, M. and Tavernier, S. (2016) Isolation of conventional dendritic cells from mouse lungs In Methods Mol. Biol., 1423, Dendritic Cell Protocols (eds. Segura, E. and Onai, N.) Springer Science+Business Media LLC, pp 139-152
Zhou, Q., Ho, A.W.S., Schlitzer, A., Tang, Y., Wong, K.H.S., Wong, F.H.S., Chua, Y.L. et al (2014) GM-CSF– licensed CD11b+ lung dendritic cells orchestrate Th2 immunity to Blomia tropicalis J. Immunol., 193, 496–509
Lymph
Bertho, N., Marquet, F., Pascale, F., Kang, C., Bonneau, M. and Schwartz-Cornila, I. (2011) Steady state pig dendritic cells migrating in skin draining pseudo-afferent lymph are semi-mature Vet. Immunol., Immunopathol., 144, 430– 436
Bonneau, M., Epardaud, M., Payot, F., Niborski, V., Thoulouze, M-I., Bernex, F., Charley, B., Riffault, S., Guilloteau, L.A. and Schwartz-Cornil, I. (2006) Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node J. Leukoc. Biol., 79, 268-276
Chan, S.S.M., Mastroeni, P., McConnell, I. and Blacklaws, B.A. (2008) Salmonella infection of afferent lymph dendritic cells J. Leukoc. Biol., 88, 272-279
Contreras, V., Urien, C., Jouneau, L., Bourge, M., Bouet-Cararo, C., Bonneau, M., Zientara, S., Klonjkowski, B. and Schwartz-Cornil I. (2012) Canine recombinant adenovirus vector induces an immunogenicity-related gene expression profile in skin-migrated CD11b+ -type DCs PLoS One, 7: e52513
Hemati, B., Contreras, V., Urien, C., Bonneau, M., Takamatsu, H-H., Mertens, P.P.C., Bréard, E., Sailleau, C., Zientara, S. and Schwartz-Cornil, I. (2009) Bluetongue virus targets conventional dendritic cells in skin lymph J. Virol., 83, 8789-8799
Marquet, F., Bonneau, M., Pascale, F., Urien, C., Kang, C., Schwartz-Cornil, I. and Bertho, N. (2011) Characterization of dendritic cells subpopulations in skin and afferent lymph in the swine model PloS One, 6: e16320
Matthews, K., Bailey, S.L., Gossner, A.G., Watkins, C., Dalziel, R.G. and Hopkins, J. (2007) Gene gundelivered pGM-CSF adjuvant induces enhanced emigration of two dendritic cell subsets from the skin Scand. J. Immunol., 65, 221-229
Muixı, L., Contreras, V., Collado, J.A., Alexandre, Y., Ballingall, K., Bonneau, M., Jaraquemada, D. and Schwartz-Cornil, I. (2012) Unraveling features of the natural MHC class II peptidome of skin-migrated dendritic cells Int. Immunol., 24, 59–69
Pascale, F., Contreras, V., Bonneau, M., Courbet, A., Chilmonczyk, S., Bevilacqua, C., Eparaud, M. et al (2008) Plasmacytoid dendritic cells migrate in afferent skin lymph J. Immunol., 180, 5963-5972
Reid, E., Juleff, N., Gubbins, S., Prentice, H., Seago, J. and Charleston, B. (2011) Bovine plasmacytoid dendritic cells are the major source of type I interferon in response to foot-and-mouth disease virus in vitro and in vivo J. Virol., 85, 4297–4308
Ruscanu, S., Pascale, F., Bourge, M., Hemati, B., Elhmouzi-Younes, J., Urien, C., Bonneau, M., Takamatsu, H. et al (2012) The double-stranded RNA bluetongue virus induces type I interferon in plasmacytoid dendritic cells via a MYD88-dependent TLR7/8-independent signaling pathway J. Virol., 86, 5817–5828
Schwartz-Cornil, I., Epardaud, M. and Bonneau, M. (2006) Cervical duct cannulation in sheep for collection of afferent lymph dendritic cells from head tissues Nat. Protoc., 1, 874-879
Sigmundsdottir, H., Pan, J., Debes, G.F., Alt, C., Habtezion, A., Soler, D. and Butcher, E.C. (20070 1,2DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27 Nat. Immunol., 8, 285-293
Lymph node
Derivation, development and maturation
Bailey, S., Schreiner, B., McMahon, E.J. and Miller, S. (2007) CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+
TH-17 cells in relapsing EAE Nat. Immunol., 8, 172-180
Li, J-C., Park, J-H., Foss, D. and Goldschneider, I. (2009) Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus J. Exp. Med., 206, 607-622
Pascual, D.W., Wang, X., Kochetkova, I., Callis, G. and Riccardi, C. (2008) The absence of lymphoid CD8+ dendritic cell maturation in L-selectin-/- respiratory compartment attenuates antiviral immunity J. Immunol., 181, 1345–1356
Ruedl, C., Koebel, P., Bachmann, M., Hess, M. and Karjalainen, K. (2000) Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes J. Immunol., 165, 4910-4916
Yang, G-X., Lian, Z-X., Kikuchi, K., Liu, Y-J., Ansari, A.A, Ikhara, S. and Gershwin, M.E. (2005) CD4- plasmacytoid dendritic cells (pDCs) migrate in lymph nodes by CpG inoculation and represent a potent functional subset of pDCs J. Immunol., 174, 3197-3203
Endotoxin
Wang, X., Wang, Q., Zhang, X., Li, Y., Wang, J., Hou, C., Chen, J. Shen, B., Shi, Y. and Zhang, J. (2015) Endotoxic shock-expanded murine CD11clowCD45RB+ regulatory dendritic cells modulate inflammatory T cell responses through multiple mechanisms Sci. Rep., 5: 10653
Immune responses
Autoimmunity and autoimmune diseases
Poliani, P.L., Kisand, K., Marrella, V., Ravanini, M., Notarangelo, L.D., Villa, A., Peterson, P. and Facchetti, F. (2010) Human peripheral lymphoid tissues contain autoimmune regulator-expressing dendritic cells Am. J. Pathol., 176, 1104-1112
Dietary influences
DePaolo, R.W., Abadie, V., Tang, F., Fehlner-Peach, H., Hall, J.A., Wang, W., Marietta, E.V., Kasarda, D.D. et al (2011) Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens Nature, 471, 220-224
Devkota, S., Wang, Y., Musch, M.W., Leone, V., Fehlner-Peach, H., Nadimpalli, A., Antonopoulos, D.A., Jabri, B. and Chang, E.B. (2012) Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il102/2 mice Nature 487, 104-108
Functional control
Basu, S. and Srivastava, P. (2005) Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells Proc. Natl. Acad. Sci. USA, 102, 5120-5125
Hess, E., Duheron, V., Decossas, M., Lezot, F., Berdal, A., Chea, S., Golub, R., Bosisio, M.R., Bridal, S.L., Choi, Y., Yagita, H. and Mueller, C.G. (2012) RANKL induces organized lymph node growth by stromal cell proliferation J. Immunol., 188, 1245–1254
Le, T.T.T., Gardner, J., Hoang-Le, D., Schmidt, C.W., MacDonald, K.P., Lambley, E., Schroder, W.A., Ogbourne, S.M. and Suhrbier, A. (2009) Immunostimulatory cancer chemotherapy using local ingenol-3- angelate and synergy with immunotherapies Vaccine, 27, 3053–3062
Westwood, J.A., Haynes, N.M., Sharkey, J., McLaughlin, N., Pegram, H.J., Schwendener, R.A., Smyth, M.J., Darcy, P.K. and Kershaw, M.H. (2009) Toll-like receptor triggering and T-cell co-stimulation induce potent antitumor immunity in mice Clin. Cancer Res., 15, 7624–33
Immunotherapy
Anjuère, F., George-Chandy, A., Audant, F., Rousseau, D., Homlgren, J. and Czerlinsky, C. (2003) Transcutaneous immunization with cholera toxin B subunit adjuvant suppresses IgE antibody responses via selective induction of Th1 immune responses J. Immunol., 170, 1586-1592
Anjuère, F., Luci, C., Lebens, M., Rousseau, D., Hervouet, C., Milon, G., Holmgren, J., Ardavin, C. and Czerlinsky, C. (2004) In vivo adjuvant-induced mobilization and maturation of gut dendritic cells after oral administration of cholera toxin J. Immunol., 173, 5103-5111
Langlet, C., Tamoutounour, S., Henri, S., Luche, H., Ardouin, L., Grégoire, C., Malissen, B. and Guilliams, M. (2012) CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization J. Immunol., 188, 1751–1760
Malm, M., Krohn, K. and Blazevic, V. (2011) Immunization with dendritic cells transfected in vivo with HIV-1 plasmid DNA induces HIV-1-specific immune responses Arch. Virol., 156, 1607–1610
Tan, L.K., Huang, C-H., Kuo, I-C., Liew, L.M. and Chua, K.Y. (2006) Intramuscular immunization with DNA construct containing Der p 2 and signal peptide sequences primed strong IgE production Vaccine, 24, 5762- 5771
Infection
Bonneau, M., Epardaud, M., Payot, F., Niborski, V., Thoulouze, M-I., Bernex, F., Charley, B., Riffault, S., Guilloteau, L.A. and Schwartz-Cornil, I. (2006) Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node J. Leukoc. Biol., 79, 268-276
Chen, C-C., Chiu, C-H., Lin, Y-Y., Shi, H.N. and Walker, W.A. (2009) Effect of probiotics Lactobacillus acidophilus on Citrobacter rodentium colitis: the role of dendritic cells Pediatr. Res. 65, 169–175
Martin, P., Martinez del Hoyo, G., Anjuère, F., Fernandez Arias, C., Hernandez Vargas, H., Fernandez-L, A., Parrillas, V. and Ardavin, C. (2002) Characterization of a new subpopulation of mouse CD8+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential Blood, 100, 383-390
Padassi, H., Acharya, M., Zhang, A., Mukhopadhyay, S., Kwon, M., Chow, C., Stuart, L.M., Savill, J. and Lacy-Hulbert, A. (2011) Preferential expression of integrin v8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells Gastroenterology, 141, 1813–1820
Integrin expression
Padassi, H., Acharya, M., Zhang, A., Mukhopadhyay, S., Kwon, M., Chow, C., Stuart, L.M., Savill, J. and Lacy-Hulbert, A. (2011) Preferential expression of integrin v8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells Gastroenterology, 141, 1813–1820
Interferons
Martin, P., Martinez del Hoyo, G., Anjuère, F., Fernandez Arias, C., Hernandez Vargas, H., Fernandez-L, A., Parrillas, V. and Ardavin, C. (2002) Characterization of a new subpopulation of mouse CD8+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential Blood, 100, 383-390
Watt, S.V., Andrews, D.M., Takeda, K., Smyth, M.J. and Hayakawa, Y. (2008) IFN-γ-dependent recruitment of mature CD27high NK cells to lymph nodes primed by dendritic cells J. Immunol., 181, 5323-5330
Irradiation effects
Gorman, S., Tan, J.W-Y., Thomas, J.A., Townley, S.L., Stumbles, P.A., Finlay-Jones, J.J. and Hart, P.H. (2005) Primary defect in UVB-induced systemic immunomodulation does not relate to immature or functionally impaired APCs in regional lymph nodes J. Immunol., 174, 6677-6685
McLoone, P., Woods, G.M. and Norval, M. (2005) Decrease in Langerhans cells and increase in lymph node dendritic cells following chronic exposure of mice to suberythemal doses of solar simulated radiation Photochem. Photobiol., 81, 1168-1173
Migration
Lo, T-H., Silveira, P.A., Fromm, P.D., Verma, N.D., Vu, P.A., Kupresanin, F., Adam, R., Kato, M., Cogger, V.C., Clark, G.J., and Hart, D.N.J. (2016) Characterization of the expression and function of the C-type lectin receptor CD302 in mice and humans reveals a role in dendritic cell migration J. Immunol., 197, 885–898
Psoriasis
Terhorst, D., Chelbi, R., Wohn, C., Malosse, C., Tamoutounour, S., Jorquera, A., Bajenoff, M., Dalod, M., Malissen, B. and Henri, S. (2015) Dynamics and transcriptomics of skin dendritic cells and macrophages in an imiquimod-induced, biphasic mouse model of psoriasis J. Immunol., 195, 4953–4961
Viruses
Brackenbury, L.S., Carr, B.V., Stamataki, Z., Prentice, H., Lefevre, E.A., Howard, C.J. and Charleston, B. (2005) Identification of a cell population that produces alpha/beta interferon in vitro and in vivo in response to noncytopathic bovine viral diarrhea virus J.Virol., 79, 7738-7744
Malm, M., Krohn, K. and Blazevic, V. (2011) Immunization with dendritic cells transfected in vivo with HIV-1 plasmid DNA induces HIV-1-specific immune responses Arch. Virol., 156, 1607–1610
Martin, P., Ruiz, S.R., Martinez del Hoyo, G., Anjuère, F., Hernandez Vargos, H., Lopez-Bravo, M. and Ardavin, C. (2002) Dramatic increase in lymph node dendritic cell number during infection by the mouse mammary tumor virus occurs by a CD62L-dependent blood-borne DC recruitment Blood, 99, 1282-1288
Martin, P., Martinez del Hoyo, G., Anjuère, F., Fernandez Arias, C., Hernandez Vargas, H., Fernandez-L, A., Parrillas, V. and Ardavin, C. (2002) Characterization of a new subpopulation of mouse CD8+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential Blood, 100, 383-390
Ruscanu, S., Jouneau, L., Urien, C., Bourge, M., Lecardonnel, J., Moroldo, M., Loup, B., Dalod, M. et al (2013) Dendritic cell subtypes from lymph nodes and blood show contrasted gene expression programs upon bluetongue virus infection J. Virol., 87, 9333–9343
MHC class I/class II expression
Stutte, S., Jux, B., Esser, C. and Förster, I. (2008) CD24a expression levels discriminate Langerhans cells from dermal dendritic cells in murine skin and lymph nodes J. Invest. Dermatol., 128, 1470-1475
Wang, P., Dong, S., Zhao, P., He, X. and Chen, M. (2018) Direct loading of CTL epitopes onto MHC class I complexes on dendritic cell surface in vivo Biomaterials 182, 92–103
Salmonella infection
Park, S-M., Omatsu, T., Zhao, Y., Yoshida, N., Shah, P., Zagani, R. and Reinecker, H-C. (2019) T cell fate following Salmonella infection is determined by a STING-IRF1 signaling axis in mice Commun. Biol., 2: 464
T cells
Development and differentiation
Jaensson, E., Uronen-Hansson, H., Pabst, O., Eksteen, B., Tian , J., Coombes, J.L., Berg, P-L., Davidsson, T., Powrie, F., Johansson-Lindbom, B., Agace, W.W. (2008) Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans J. Exp Med., 205, 2139-2149
Function
Bachy, V., Hervouet, C., Becker, P.D., Chorro, L., Carlin, L.M., Herath, S., Papagatsias, T., Barbaroux, J-B. et al (2013) Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays Proc. Natl. Acad. Sci. USA, 110, 3041-3046
Castiglioni, P., Lu, C., Lo, D., Croft, M., Langlade-Demoyen, P., Zanetti, M. and Gerloni, M. (2003) CD4 T cell priming in dendritic cell-deficient mice Int. Immunol., 15, 127-136
Hervouet, C., Luci, C., Rol, N., Rousseau, D., Kissenpfennig, A., Malissen, B., Czerkinsky, C. and Anjuère, F. (2010) Langerhans cells prime IL-17–producing T cells and dampen genital cytotoxic responses following mucosal immunization J. Immunol., 184, 4842–4851
Nguyen, D.D., Wurbel, M-C., Goettel, J.A., Eston, M.A., Ahmed, O.S., Marin, R., Boden, E.K., Villablanca, E.J. et al (2012) Wiskott–Aldrich syndrome protein deficiency in innate immune cells leads to mucosal immune dysregulation and colitis in mice Gastroenterology, 143, 719–729
Sakuraba, A., Sato, T., Kamada, N., Kitazume, M., Sugita, A. and Hibi, T.(2009) Th1/Th17 Immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease Gastroenterology, 137, 1736–1745
Wang, X., Wang, Q., Zhang, X., Li, Y., Wang, J., Hou, C., Chen, J. Shen, B., Shi, Y. and Zhang, J. (2015) Endotoxic shock-expanded murine CD11clowCD45RB+ regulatory dendritic cells modulate inflammatory T cell responses through multiple mechanisms Sci. Rep., 5: 10653
Zhan, X-X., Liu, Y., Yang, J-F., Wang, G-Y., Mu, L., Zhang, T-S., Xie, X-L., Wang, J-H. et al (2013) Alltrans-retinoic acid ameliorates experimental allergic encephalomyelitis by affecting dendritic cell and monocyte development Immunology, 138, 333–345
Migration
Dimitroff, C.J., Kupper, T.S. and Sackstein, R. (2003) Prevention of leukocyte migration to inflamed skin with a novel fluorosugar modifier of cutaneous lymphocyte-associated antigen J. Clin. Invest., 112, 1008-1018
Eksteen, B., Mora, J.R., Haughton, E.L., Henderson, N.C., Lee–Turner, L., Villablanca E.J., Curbishley, S.M., Aspinall, A.I., Von Andrian, U.H. and Adams, D.H. (2009) Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells Gastroenterology, 137, 320–329
Proliferation
Shi, H.N., Liu, Y. and Nagler-Anderson, C. (2000) Enteric infection acts as an adjuvant for the response to a model food antigen J. Immunol., 165, 6174-6182
Virus responses
Ruedl, C., Schwarz, K., Jegerlehner, A., Storni, T., Manolova, V. and Bachmann, M.F. (2005) Virus-like particles as carriers for T-cell epitopes: limited inhibition of T-cell priming by carrier-specific antibodies J. Virol., 79, 717-724
Vaccines
Contreras, V., Urien, C., Guiton, R., Alexandre, Y., Vu Manh, T-P., Andrieu, T., Crozat, K., Jouneau, L. et al (2010) Existence of CD8-like dendritic cells with a conserved functional specialization and a common mol Zlotkowska, D., Maddaloni, M., Riccardi C., Walters, N., Holderness, K., Callis, G., Rynda-Apple, A. and Pascual, D.W. (2012) Loss of sialic acid binding domain redirects protein 1 to enhance M cell-directed vaccination PLoS One 7: e36182ecular signature in distant mammalian species J. Immunol., 185, 3313–3325
Periapical lesions (dental)
Colic, M., Gazivoda, D., Vasilijic, S., Vucevic, D. and Lukic, A. (2010) Production of IL-10 and IL-12 by antigen-presenting cells in periapical lesions J. Oral Pathol. Med., 39, 690–696
Peyer’s patch
Anjuère, F., Martin, P., Ferrero, I., Lopez Fraga, M., Martinez del Hoyo, G., Wright, N. and Ardavin, C. (1999) Definition of dendritic cell subpopulations present in the spleen, Peyer’s patches, lymph nodes, and skin of the mouse Blood, 93, 590-598
Lopez-Guerrero, D.V., Meza-Perez, S., Ramirez-Pliego, O., Santana-Calderon, M.A., Espino-Solis, P., Gutierrez-Xicotencatl, L., Flores-Romo, L. and Esquivel-Guadarrama, F.R. (2010) Rotavirus infection activates dendritic cells from Peyer’s Patches in adult mice J. Virol., 84, 1856-1866
Park, S-M., Omatsu, T., Zhao, Y., Yoshida, N., Shah, P., Zagani, R. and Reinecker, H-C. (2019) T cell fate following Salmonella infection is determined by a STING-IRF1 signaling axis in mice Commun. Biol., 2: 464 Ruedl, C., Rieser, C., Bock, G., Wick, G. and Wolf, H. (1996) Phenotypic and functional characterization of CD11c+ dendritic cell population in mouse Peyer’s patches Eur. J. Immunol., 26; 1801-1806
Ruedl, C., Koebel, P., Bachmann, M., Hess, M. and Karjalainen, K. (2000) Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes J. Immunol., 165, 4910-4916
Salazar-Gonzales, R.M., Niess, J.H., Zammit, D.J., Ravindran, R., Srinivasan, A., Maxwell, J.R., Stoklasek, T. et al (2006) CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches Immunity, 24, 623-632
Shi, H.N., Liu, Y. and Nagler-Anderson, C. (2000) Enteric infection acts as an adjuvant for the response to a model food antigen J. Immunol., 165, 6174-6182
Rickettsia infections
Bechelli, J., Smalley, C., Zhao, X., Judy, B., Valdes, P., Walker, D.H. and Fang, R. (2016) MyD88 mediates instructive signaling in dendritic cells and protective inflammatory response during rickettsial ifection Infect. Immun. 84, 883-893
Skin
Hervé, P-L., Descamps, D., Deloizy, C., Dhelft, V., Laubreton, D., Bouguyon, E., Boukadiri, A., Dubuquoy, C. et al (2016) Non-invasive epicutaneous vaccine against Respiratory Syncytial Virus: Preclinical proof of concept J. Control. Release, 243, 146–159
Polak, M.E., Ung, C.Y., Masapust, J., Freeman, T.C. and Ardern-Jones, M.R. (2017) Petri net computational modelling of Langerhans cell interferon regulatory factor network predicts their role in T cell activation Sci. Rep., 7: 668
Spinal cord – see Brain meninges
Spleen
Apoptosis
Gossner, A., Roupaka, S., Foster, J., Hunter, N. and Hopkins, J. (2011) Transcriptional profiling of peripheral lymphoid tissue reveals genes and networks linked to SSBP/1 scrapie pathology in sheep Vet. Microbiol., 153, 218–228
Nakayama, M., Akiba, H., Takeda, K., Kojima, Y., Hashiguchi, M., Azuma, M., Yagita, H.and Okumura, K. (2009) Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation Blood, 113, 3821-3830
Yamazaki, T., Akiba, H., Iwai, H., Matsuda, H., Aoki, M., Tanno, Y., Shin, T., Tsichiya, H., Pardoll, D.M., Okumura, K., Azuma, M. and Yagita, H. (2002) Expression of programmed death 1 ligands by murine T cells and APC J. Immunol., 169, 5538-5545
Derivation, development and maturation
B cell memory
Kang, SA., Keener, A.B., Jones, S.Z., Benschop, R.J., Caro-Maldonado, A., Rathmell, J.C., Clarke, S.H., Matsushima, G.K., Whitmire, J.K. and Vilen, B.J. (2016) IgG-immune complexes promote B cell memory by inducing BAFF J. Immunol., 196, 196–206
CD4 subsets
Stojić-Vukanić, Z., Bufan, B., Pilipović, I., Vujnović, I., Nacka-Aleksić, M., Petrović, R., Arsenović-Ranin, N. and Leposavić, G. (2016) Estradiol enhances capacity of TLR-matured splenic dendritic cells to polarize CD4+ lymphocytes into IL-17/GM-CSF-producing cells in vitro Int. Immunopharmacol., 40, 244–253
Yang, G-X., Lian, Z-X., Kikuchi, K., Liu, Y-J., Ansari, A.A, Ikhara, S. and Gershwin, M.E. (2005) CD4- plasmacytoid dendritic cells (pDCs) migrate in lymph nodes by CpG inoculation and represent a potent functional subset of pDCs J. Immunol., 174, 3197-3203
CD8 subsets
Anjuère, F., Martin, P., Ferrero, I., Lopez Fraga, M., Martinez del Hoyo, G., Wright, N. and Ardavin, C. (1999) Definition of dendritic cell subpopulations present in the spleen, Peyer’s patches, lymph nodes, and skin of the mouse Blood, 93, 590-598
Kamachi, F., Harada, N., Usui, Y., Sakanishi, T., Ishii, N., Okumura, K., Miyake, S. and Akiba, H. (2014) OX40 ligand regulates splenic CD8
dendritic cell-induced Th2 responses in vivo Biochem.Biophys. Res. Comm., 444, 235–240
Martin, P., Martinez del Hoyo,G., Anjuère, F., Riuz, S.R., Arias, C.F., Marin A.R. and Ardavin, C. (2000) Concept of lymphoid versus myeloid dendritic cell lineages revisited: both CD8α – and CD8α + dendritic cells are generated from CD4low lymphoid-committed precursors Blood, 96, 2511-2519
Martinez Del Hoyo, G.M., Martin, P., Arias, F.C., Marin, A.R. and Ardavin, C. (2002) CD8α + dendritic cells originate from CD8α – dendritic cell subset by a maturation process involving CD8α, DEC-205, and CD24 upregulation Blood, 99, 999-1004
Ruedl, C., Koebel, P., Bachmann, M., Hess, M. and Karjalainen, K. (2000) Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes J. Immunol., 165, 4910-4916
CD11 subsets
Ferrero, I., Held, W., Wilson, A., Tacchini-Cottier, F., Radtke, F. and MacDonald, H.R. (2002) Mouse CD11c+ B220+ Gr1+ plasmacytoid dendritic cells develop independently of the T-cell lineage Blood, 100, 2852-2857
Li, J-C., Park, J-H., Foss, D. and Goldschneider, I. (2009) Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus J. Exp. Med., 206, 607-622
Ruedl, C., Koebel, P., Bachmann, M., Hess, M. and Karjalainen, K. (2000) Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes J. Immunol., 165, 4910-4916
Chemokines
Van Rijn, A., Paulis, L., te Riet, J., Vasaturo, A., Reinieren-Beeren, I., van der Schaaf, A., Kuipers, A.J., Schulte, L.P. et al (2016) Semaphorin 7A promotes chemokine-driven dendritic cell migration J. Immunol., 196, 459–468
Estradiol
Stojić-Vukanić, Z., Nacka-Aleksić, M., Bufan, B., Pilipović I., Arsenović-Ranin, N., Djikić, J., Kosec, D. and Leposavić, G. (2015) 17β-Estradiol influences in vitro response of aged rat splenic conventional dendritic cells to TLR4 and TLR7/8 agonists in an agonist specific manner Int. Immunopharmacol., 24 (2015) 24–35
Functional analysis
Tetlak, P. and Ruedl, C. (2016) Analysis of dendritic cell function using Clec9A-DTR transgenic mice In Methods Mol. Biol., 1423, Dendritic Cell Protocols (eds. Segura, E. and Onai, N.) Springer Science+Business Media LLC, pp 275-289
Growth factors
Waskow, C., Liu, K., Darrasse-Jèze, G., Guermonprez, P., Ginhoux, F., Merad, M., Shengelia, T., Yao, K. and Nussenzweig, M. (2008) The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues Nat. Immunol., 9, 676-683
IFN production
Kreutz, M., Bakdash, G., Dolen, Y., Sköld, A.E., van Hout-Kuijer, M.A., de Vries, J.M. and Figdor, C.G. (2015) Type I IFN-mediated synergistic activation of mouse and human DC subsets by TLR agonists Eur. J. Immunol., 45, 2798–2809
Niedzielska, M., Raffi, F.A.M., Tel, J., Muench, S., Jozefowski, K., Alati N., Lahl, K., Mages, J., Billmeier, U. et al (2015) Selective expression of the MAPK phosphatase Dusp9/MKP-4 in mouse plasmacytoid dendritic cells and regulation of IFN- production J. Immunol., 195, 1753–1762
MHCII expression
Chen, X., Reed-Loisel, L.M., Karlsson, L. and Jensen, P.E. (2006) H2-O expression in primary dendritic cells J. Immunol., 176, 3548-3556
Landmann, S., Mühlethaler-Mottet, A., Bernasconi, L., Suter, T., Waldburger, J-M., Masternak, K., Arrighi, JF., Hauser, C., Fontana, A. and Reith, W. (2001) Maturation of dendritic cells is accompanied by rapid transcriptional silencing of class II transactivator (CIITA) expression J. Exp. Med., 194, 379-391
Li, J-C., Park, J-H., Foss, D. and Goldschneider, I. (2009) Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus J. Exp. Med., 206, 607-622
Notch-1
Radtke, F., Ferrero, I., Wilson, A., Lees, R., Aguet, M. and MacDonald, H.R. (2000) Notch1 deficiency dissociates the intrathymic development of dendritic cells and T cells J. Exp. Med., 191, 1085-1093
T cell helper cells
Ruedl, C., Kopf, M. and Bachmann, M.F. (1999) CD8+ T cells mediate CD40-independent maturation of dendritic cells in vivo J. Exp. Med., 189, 1875-1883
Transplantation
Banovic, T., Markey, K.A., Kuns, R.D., Olver, S.D., Raffelt, N.C., Don, A.L., Degli-Esposti, M.A., Engwerda, C.R., MacDonald, K.P.A. and Hill, G.R. (2009) Graft-versus-host disease prevents the maturation of plasmacytoid dendritic cells J. Immunol., 182, 912–920
Detection in vitro
Zarnini, A.H., Moazzeni, S-M., Shokri, F., Salehnia, M., Dokouhaki, P., Shojaeian, J. and Jeddi-Tehrani, M. (2006) The efficient isolation of murine splenic dendritic cells and their cytochemical features Histochem. Cell Biol., 126, 275-282
Immune responses
Autoimmunity and autoimmune diseases
Chua, Y.L., Liong, K.H., Huang, C-H., Wong, H.S., Zhou, Q., Ler, S.S., Tang, Y., Low, C.P., Koh, H.Y. et al (2016) Blomia tropicalis–specific TCR transgenic Th2 cells induce inducible BALT and severe asthma in mice by an IL-4/IL-13–dependent mechanism J. Immunol., 197, 3771–3781
Ding, Y., Seow, S.V., Huang, C.H., Liew, L.M., Lim, Y.C., Kuo, C. and Chua, K.Y. (2009) Coadministration of the fungal immunomodulatory protein FIP-Fve and a tumour-associated antigen enhanced antitumour immunity Immunology, 128, e881–e894
Gelderman, K.A., Hultqvist, M., Holmberg, J., Olafsson, P. and Holmdahl, R. (2006) T cell surface redox levels determine T cell reactivity and arthritis susceptibility Proc. Natl. Acad. Sci., USA, 103, 12831-12836
Hayashi, T., Beck, L., Rossetto, C., Gong, X., Takikawa, O., Takabayashi, K., Broide, D.H. and Raz, E. (2004) Inhibition of experimental asthma by indoleamine 2,3-dioxygenase J. Clin. Invest., 114, 270-279
Homann, D., Jahrels, A., Wolfe, T., Hughes, A., Coon, B., van Stipdonk, M.J.B., Prillman, K.R., Schoenberger, S.P. and von Herrath, M.G. (2002) CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells Immunity, 16, 403-415
Kim, Y.S., Yang, S.H., Kang, H.G., Seong, E.Y., Lee, S,H., Gao, W., Kenny, J., Zheng, X.X. and Strom, T.B. (2006) Distinctive role of donor strain immature dendritic cells in the creation of allograft tolerance Int. Immunol., 18, 1771-1777
Motegi, S-I., Okazawa, H., Murata, Y., Kanazawa, Y., Saito, Y., Kobayashi, H., Ohnishi, H., Oldenborg, P-A., Ishikawa, O., and Matozaki, T. (2008) Essential roles of SHPS-1 in induction of contact hyper-sensitivity of skin Immunol. Lett., 121, 52-60
Okada, T., Lian, Z-X., Hsu, T., Naiki, M., Ansari, A.A., Robinson, D., Kung, H-J., Boyd, R. and Gershwin, E. (2002) Elevated C-met in thymic dendritic cells of New Zealand black mice Dev. Immunol., 9, 29-34
Okunishi, K., Dohi, M., Nakagome, K., Tanaka, R., Mizuno, S., Matsumoto. K., Miyazaki, J-i., Nakamura, T. and Yamamoto, K. (2005) A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function J. Immunol., 175, 4745-4753
Sonderegger, I., Iezzi, G., Maier, R., Schmitz, N., Kurrer, M. and Kopf, M. (2008) GM-CSF mediates autoimmunity by enhancing IL-6–dependent Th17 cell development and survival J. Exp. Med., 205, 2281-2294
Tomizawa, T., Kaneko, Y., Kaneko, Y., Saito, Y., Ohnishi, H., Okajo, J., Okuzawa, C., Ishikawa-Sekigami, T. et al (2007) Resistance to experimental autoimmune encephalomyelitis and impaired T cell priming by dendritic cells in Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 mutant mice J. Immunol., 179, 869-877
Dietary responses
Devkota, S., Wang, Y., Musch, M.W., Leone, V., Fehlner-Peach, H., Nadimpalli, A., Antonopoulos, D.A., Jabri, B. and Chang, E.B. (2012) Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il102/2 mice Nature 487, 104-108
Functional control
Luckashenak, N.A., Ryszkiewicz, R.L., Ramsey, K.D. and Clements, J.L. (2006) The Src homology 2 domaincontaining leukocyte protein of 76-kDa adaptor links integrin ligation with p44/42 MAPK phosphorylation and podosome distribution in murine dendritic cells J. Immunol., 177, 5177-5185
Stojić-Vukanić , Z., Bufan, B., Arsenović-Ranin, N., Kosec, D., Pilipović I., Perišić , Nanut, M.P. and Leposavić, G. (2013) Aging affects AO rat splenic conventional dendritic cell subset composition, cytokine synthesis and T-helper polarizing capacity Biogerontology, 14, 443–459
Vucevic, D., Melliou, E., Vasilijic, S., Gasic, S., Ivanovski, P., Chinou, I. and Colic, M. (2007) Fatty acids from royal jelly modulate dendritic cell-mediated immune response in vitro Int. Immunopharmacol., 7, 1211-1220
Zarnani, A.H., Moazzeni, S.M., Shokri, F., Salehnia, M., Dokouhaki, P., Ghods, R., Mahmoodi, A.R. and Jeddi-Tehrani, M. (2008) Microenvironment of the feto–maternal interface protects the semiallogenic fetus through its immunomodulatory activity on dendritic cells Fertil. Steril., 90, 781-788
Immunotherapy
Poulin, L.F., Salio, M., Griessinger, E., Anjos-Afonso, F., Craciun, L., Chen, J-L., Keller, A.M., Joffre, O., Zelenay, S., Nye, E., Le Moine, A., Faure, F., Donckier, V., Sancho, D., Cerundolo, V., Bonnet, D. and Reis e Sousa, C. (2010) Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells J. Exp. Med., 207, 1261-1271
Infection
Bayat, A.A., Akhondi, M.M., Ostadkarampour, M., Rezania, S. and Zarnani, A.H. (2009) Mutual helper effect in co-pulsing of dendritic cells with 2 antigens: a novel approach for improvement of dendritic-based vaccine efficacy against tumors and infectious diseases simultaneously J. Immunother., 32, 325–332
Chen, C-C., Louie, S., McCormick, B.A., Walker, W.A. and Shi, H.N. (2006) Helminth-primed dendritic cells: alter the host response to enteric bacterial infection J. Immunol., 176, 472-483
Chen, C-C., Chiu, C-H., Lin, Y-Y., Shi, H.N. and Walker, W.A. (2009) Effect of probiotics Lactobacillus acidophilus on Citrobacter rodentium colitis: the role of dendritic cells Pediatr. Res. 65, 169–175
Didierlaurent, A., Ferrero, I., Otten, L.A., Dubois, B., Reinhardt, M., Carlsen, H., Blomhoff, R., Akira, S., Kraehenbuhl, J-P. and Sirard, J-C. (2004) Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response J. Immunol., 172, 6922-6930
Martin, P., Martinez del Hoyo, G., Anjuère, F., Fernandez Arias, C., Hernandez Vargas, H., Fernandez-L, A., Parrillas, V. and Ardavin, C. (2002) Characterization of a new subpopulation of mouse CD8+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential Blood, 100, 383-390
Miura, T., Kudo, T., Matsuki, A., Sekikawa, K., Tagawa, Y-C., Iwakura, Y. and Nakane, A. (2001) Effect of 6- hydroxydopamine on host resistance against Listeria monocytogenes infection Infect. Immun., 69, 7234-7241
Sashinami, H., Nakane, A., Iwakura, Y. And Sasaki, M. (2003) Effective induction of acquired resistance to Listeria monocytogenes by immunizing mice with in vivo-infected dendritic cells Infect. Immun.,71, 117-125
Shi, H.N., Liu, Y. and Nagler-Anderson, C. (2000) Enteric infection acts as an adjuvant for the response to a model food antigen J. Immunol., 165, 6174-6182
Shojaeian, J., Jeddi-Tehrani, M., Dokouhaki, P., Mahmoudi, A.R., Ghods, R., Bozorgmehr, M., Nikoo, S. et al (2009) Non-hematopoietic cells control the outcome of infection with Listeria monocytogenes in a nucleotide oligomerization domain 1-dependent manner Infect. Immun., 77, 2908-2918
Simeone, R., Sayes, F., Song, O., Gröschel, M.I., Brodin, P., Brosch, R. and Majlessi, L. (2015) Cytosolic access of mycobacterium tuberculosis: critical impact of phagosomal acidification control and demonstration of occurrence in vivo PLoS Pathog., 10: e1004650
Inflammatory responses
Abe, K., Nguyen, K.P., Fine, S.D., Mo, J-H., Shen, C., Shenouda, S., Corr, M., Jung, S., Lee, J., Eckmann, L. and Raz, E. (2007) Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells Proc. Natl. Acad. Sci. USA, 104, 17022-17027
DePaolo, R.W., Abadie, V., Tang, F., Fehlner-Peach, H., Hall, J.A., Wang, W., Marietta, E.V., Kasarda, D.D. et al (2011) Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens Nature, 471, 220-224
Schroder, W.A., Le, T.T.T., Major, L., Street, S., Gardner, J., Lambley, E., Markey, K., MacDonald, K.P., Fish, R.J., Thomas, R. and Suhrbier, A. (2010) A physiological function of inflammation-associated SerpinB2 is regulation of adaptive immunity J. Immunol., 184, 2663–2670
Soruri, A., Kim, S., Kiafard, Z. and Zwirner, J. (2003) Characterization of C5aR expression on murine myeloid and lymphoid cells by the use of a novel monoclonal antibody Immunol. Lett., 88, 47-52
Wang, X., Wang, Q., Zhang, X., Li, Y., Wang, J., Hou, C., Chen, J. Shen, B., Shi, Y. and Zhang, J. (2015) Endotoxic shock-expanded murine CD11clowCD45RB+
regulatory dendritic cells modulate inflammatory T cell responses through multiple mechanisms Sci. Rep., 5: 10653
Interferons
Suto, A., Nakajima, H., Tokumasa, N., Takatori, H., Kagami, S-I., Suzuki, K. and Iwamoto, I. (2005) Murine plasmacytoid dendritic cells produce IFN-γ upon IL-4 stimulation J. Immunol., 175, 5681-5689
Listeria
Arnold-Schrauf, C., Dudek, M., Dielmann, A., Pace, L., Swallow, M., Kruse, F., Kühl, A.A., Holzmann, B., Berod, L. and Sparwasser, T. (2014) Dendritic cells coordinate innate immunity via MyD88 signaling to control
Listeria monocytogenes infection Cell Rep., 6, 698–708 Martinez del Hoyo, G., Ramírez-Huesca, M., Levy, S., Boucheix, C., Rubinstein, E., Minguito de la Escalera,
M., González-Cintado, L., Ardavín, C. et al (2015) CD81 controls immunity to Listeria infection through Racdependent inhibition of proinflammatory mediator release and activation of cytotoxic T cells J. Immunol., 194, 6090–6101
Prion proteins
Gossner, A.G., Bennet, N., Hunter, N. and Hopkins, J. (2009) Differential expression of Prnp and Sprn in scrapie infected sheep also reveals Prnp genotype specific differences Biochem. Biophys. Res. Commun., 378, 862–866
Gossner, A., Roupaka, S., Foster, J., Hunter, N. and Hopkins, J. (2011) Transcriptional profiling of peripheral lymphoid tissue reveals genes and networks linked to SSBP/1 scrapie pathology in sheep Vet. Microbiol., 153, 218–228
Martinez del Hoyo, G., Lopez-Bravo, M., Metharom, P., Ardavin, C. and Aucouturier, P. (2006) Prion protein expression by mouse dendritic cells is restricted to the nonplasmacytoid subsets and correlates with the maturation state J. Immunol., 177, 6137-6142
Tumour interactions
Futagawa, T., Akiba, H., Kodama, T., Takeda, K., Hosoda, Y., Yagita, H. and Okumura, K. (2002) Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells Int. Immunol., 14, 275-286
Nierkens, S., den Brok, M.H., Garcia, Z., Togher, S., Wagenaars, J., Wassink, M., Boon, L., Ruers, T.J. et al (2011) Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells Cancer Res., 71, 6428–37
Okajo, J., Kaneko, Y., Murata, Y., Tomizawa, T., Okuzawa, C., Saito, Y., Kaneko, Y., Ishikawa-Sekigami, T. et al (2007) Regulation by Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 of α- galactosylceramide-induced antimetastatic activity and Th1 and Th2 responses of NKT cells J. Immunol., 178, 6164-6172
Perez-Shibayama, C., Gil-Cruz, C., Nussbacher, M., Allgäuer, E., Cervantes-Barragan, L., Züst, R. and Ludewig, R. (2103) Dendritic cell-specific delivery of Flt3L by coronavirus vectors secures induction of therapeutic antitumor immunity PLoS One, 8: e81442
Shojaeian, J., Jeddi-Tehrani, M., Dokouhaki, P., Mahmoudi, A.R., Ghods, R., Bozorgmehr, M., Nikoo, S. et al (2009) Mutual helper effect in co-pulsing of dendritic cells with 2 antigens: a novel approach for improvement of dendritic-based vaccine efficacy against tumors and infectious diseases simultaneously J. Immunother., 32, 325–332
Vaccination
Zlotkowska, D., Maddaloni, M., Riccardi C., Walters, N., Holderness, K., Callis, G., Rynda-Apple, A. and Pascual, D.W. (2012) Loss of sialic acid binding domain redirects protein σ1 to enhance M cell-directed vaccination PLoS One 7: e36182
Viruses
Andrews, D.M., Estcourt, M.J., Andoniou, C.E., Wikstrom, M.E., Khong, A., Voigt, V., Fleming, P., Tabarias, H., Hill, G.R., van der Most, R.G., Scalzo, A.A., Smyth, M.J. and Degli-Esposti, M.A. (2010) Innate immunity defines the capacity of antiviral T cells to limit persistent infection J. Exp. Med., 207, 1333-1343
Cervantes-Barragan, L., Züst, R., Weber, F., Spiegel, M., Lang, K.S., Akira, S., Thiel, V. and Ludewig, B. (2007) Control of coronavirus infection through plasmacytoid dendritic cell-derived type I interferon Blood, 109, 1131-1137
Fossum, E., Grødeland, G., Terhorst, D., Tveita, A.A., Vikse, E., Mjaaland, S., Henri, S., Malissen, B. and Bogen, B. (2015) Vaccine molecules targeting Xcr1 on cross-presenting DCs induce protective CD8+ T-cell responses against influenza virus Eur. J. Immunol., 45, 624–635
Ruedl, C., Schwarz, K., Jegerlehner, A., Storni, T., Manolova, V. and Bachmann, M.F. (2005) Virus-like particles as carriers for T-cell epitopes: limited inhibition of T-cell priming by carrier-specific antibodies J. Virol., 79, 717-724
Wiegard, C., Wolint, P., Frenzel, C., Cheruti, U., Schmitt, E., Oxenius, A., Lohse, A.W. and Herkel, J. (2007) Defective T helper response of hepatocyte-stimulated CD4 T cells impairs antiviral CD8 response and viral clearance Gastroenterology 133, 2010-2018
Wikstrom, M.E., Fleming, P., Kuns, R.D., Schuster, I.S., Voigt, V., Miller, G., Clouston, A.D., Tey, S-K., Andoniou, C.E. et al (2015) Acute GVHD results in a severe DC defect that prevents T-cell priming and leads to fulminant cytomegalovirus disease in mice Blood, 126, 1503-1514
Williams, M.A., Tan, J.T., Adams, A.B., Durham, M.M., Shirasugi, N., Whitmire, J.K., Harrington, L.E., Ahmed, R., Pearson, T.C. and Larsen, C.P. (2001) Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance J. Immunol., 167, 4987-4995
Züst, R., Cervantes-Barragán, L., Kuri, T., Blakqori, G., Weber, F., Ludewig, B. and Thiel, V. (2007) Coronavirus non-structural protein 1 is a major pathogenicity factor: implications for the rational design of coronavirus vaccines PLoS Pathog., 3, 1062-1072
MHC class II expression/interactions
Bonasio, R., Scimone, M.L., Schaerli, P., Grabie, N., Lichtman, A.H. and von Adrian, U.H. (2006) Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus Nat. Immunol., 7, 1092-1100
Brocker, T. (1997) Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expression dendritic cells J. Exp. Med., 186, 1223-1232
Clausen, B.E., Waldburger, J-M., Schwenk, F., Barras, E., Mach, B., Rajewsky, K., Forster, I. and Reith, W. (1998) Residual MHC class II expression on mature dendritic cells and activated B cells in RFX5-deficient mice Immunity, 8, 143-155
Culina, S., Mauvais, F-X., Hsu, H-T., Burgevin, A., Guénette, S., Moser, A., and van Endert, P. (2014) No major role for insulin-degrading enzyme in antigen presentation by MHC molecules PLoS One, 9: e88365
Keller, A.M., Groothuis, T.A., Veraar, E.A.M., Marsman, M., Maillette de Buy Wenniger, L. Janssen, H., Neefjes, J. and Borst, J. (2007) Costimulatory ligand CD70 is delivered to the immunological synapse by shared intracellular trafficking with MHC class II molecules Proc. Natl. Acad. Sci. USA, 104, 5989-5994
Koyama, M., Kuns, R.D., Olver, S.D., Raffelt, N.C., Wilson, Y.A., Don, A.L.J., Lineburg, K.E., Cheong, M. et al (2012) Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versushost disease Nat. Med., 18, 135-142
Kroger, C.J., Spidale, N.A., Wang, B. and Tisch, R. (2017) Thymic dendritic cell subsets display distinct efficiencies and mechanisms of intercellular MHC transfer J. Immunol., 198, 249–256
LeibundGut-Landemann, S., Waldburger, J-M., Reis e Sousa, C. Acha-Orbea, H. and Reith, W. (2004) MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells Nat. Immunol., 5, 899-908
Vucevic, D., Melliou, E., Vasilijic, S., Gasic, S., Ivanovski, P., Chinou, I. and Colic, M. (2007) Fatty acids from royal jelly modulate dendritic cell-mediated immune response in vitro Int. Immunopharmacol., 7, 1211-1220
Waldburger, J-M., Suter, T., Fontana, A., Acha-Orbea, H. and Reith, W. (2001) Selective abrogation of major histocompatibility complex class II expression on extrahematopoietic cells in mice lacking promoter IV of the class II transactivator gene J. Exp. Med., 194, 393-406
Migration
Bonasio, R., Scimone, M.L., Schaerli, P., Grabie, N., Lichtman, A.H. and von Adrian, U.H. (2006) Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus Nat. Immunol., 7, 1092-1100
Lo, T-H., Silveira, P.A., Fromm, P.D., Verma, N.D., Vu, P.A., Kupresanin, F., Adam, R. et al (2016) Characterization of the expression and function of the C-type lectin receptor CD302 in mice and humans reveals a role in dendritic cell migration J. Immunol., 197, 885–898
van Rijn, A., Paulis, L., te Riet, J., Vasaturo, A., Reinieren-Beeren, I., van der Schaaf, A., Kuipers, A.J., Schulte, L.P. et al (2016) Semaphorin 7A promotes chemokine-driven dendritic cell migration J. Immunol., 196, 459–468
Polarizing capacity
Stojic-Vukanić, Z., Pilipović, I., Bufan, B., Stojanović, M. and Leposavić, G. (2020) Age and sex determine CD4+ T cell stimulatory and polarizing capacity of rat splenic dendritic cells Biogerontology, 21, 83–107
Salmonella infection
Park, S-M., Omatsu, T., Zhao, Y., Yoshida, N., Shah, P., Zagani, R. and Reinecker, H-C. (2019) T cell fate following Salmonella infection is determined by a STING-IRF1 signaling axis in mice Commun. Biol., 2: 464
Stimulating capacity
Stojic-Vukanić, Z., Pilipović, I., Bufan, B., Stojanović, M. and Leposavić, G. (2020) Age and sex determine CD4+ T cell stimulatory and polarizing capacity of rat splenic dendritic cells Biogerontology, 21, 83–107
T cells
Development and differentiation
Bailey, S., Schreiner, B., McMahon, E.J. and Miller, S. (2007) CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+
TH-17 cells in relapsing EAE Nat. Immunol., 8, 172-180 Brocker, T. (1997) Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex
class II-expression dendritic cells J. Exp. Med., 186, 1223-1232
Hegazy, A.N. and Klein, C. (2008) Ex vivo priming of CD4 T cells converts immunological tolerance into effective antitumor immunity in a murine model of acute lymphoblastic leukemia Leukemia, 22, 2070
Tokumasa, N., Suto, A., Kagami, S-i., Furuta, S., Hirose, K., Watanabe, N., Saito, Y., Shimoda, K., Iwamoto, I. and Nakajima, H. (2007) Expression of Tyk2 in dendritic cells is required for IL-12, IL-23 and IFN-γ production and the induction of Th1 cell differentiation Blood, 110, 553-560
Yamaguchi, E., Chiba, S., Kumano, K., Kunisato, A., Takahashi, T., Takahashi, T. and Hirai, H. (2002) Expression of Notch ligands, Jagged1, and Delta1 in antigen presenting cells in mice Immunol. Lett., 81, 59-64
Function
Cavanagh, L.L., Bonasio, R., Mazo, I.B., Halin, C., Cheng, G., van der Velden, A.W.M., Cariappa, C. et al (2005) Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells Nat. Immunol., 6, 1029-1037
Dresch, C., Ackermann, M., Vogt, B., de Andrade Pereira, B., Shortman, K., and Fraefel, C. (2011)1 Thymic but not splenic CD81 DCs can efficiently cross-prime T cells in the absence of licensing factors Eur. J. Immunol., 41, 2544–2555
Eksteen, B., Mora, J.R., Haughton, E.L., Henderson, N.C., Lee–Turner, L., Villablanca E.J., Curbishley, S.M. et al (2009) Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells Gastroenterology, 137, 320–329
Fossum, E., Grødeland, G., Terhorst, D., Tveita, A.A., Vikse, E., Mjaaland, S., Henri, S., Malissen, B. and Bogen, B. (2015) Vaccine molecules targeting Xcr1 on cross-presenting DCs induce protective CD8+ T-cell responses against influenza virus Eur. J. Immunol., 45, 624–635
Hegazy, A.N. and Klein, C. (2008) Ex vivo priming of CD4 T cells converts immunological tolerance into effective antitumor immunity in a murine model of acute lymphoblastic leukemia Leukemia, 22, 2070
Kawashima, S., Hirose, K., Iwata, A., Takahashi, K., Ohkubo, A., Tamachi, T., Ikeda, K., Kagami, S-i. and Nakajima, H. (2012) -Glucan curdlan induces IL-10–producing CD4+ T cells and inhibits allergic airway inflammation J. Immunol., 89, 5713–5721
MacDonald, K.P.A., Kuns, R.D., Rowe, V., Morris, E.S., Banovic, T., Bofinger, H., O’Sullivan, B. et al (2007) Effector and regulatory T-cell function is differentially regulated by RelB within antigen-presenting cells during GVHD Blood, 109, 5049-5057
Markey, K.A., Gartlan, K.H., Kuns, R.D., MacDonald, K.P.A. and Hill, G.R. (2015) Imaging the immunological synapse between dendritic cells and T cells J. Immunol. Meth., 423, 40–44
Mayer, C.T., Huntenburg, J., Nandan, A., Schmitt, E., Czeloth, N. and Sparwasser, T. (2013) CD4 blockade directly inhibits mouse and human CD4+ T cell functions independent of Foxp3+ Tregs J. Autoimmun., 47, 73-82
Mazo, I.B., Honczarenko, M., Leung, H., Cavanagh, L.L., Bonasio, R., Weninger, W., Enegelke, K., Xia, L. et al (2005) Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells Immunity, 22, 259-270
Motegi, S-I., Okazawa, H., Murata, Y., Kanazawa, Y., Saito, Y., Kobayashi, H., Ohnishi, H., Oldenborg, P-A., Ishikawa, O., and Matozaki, T. (2008) Essential roles of SHPS-1 in induction of contact hyper-sensitivity of skin Immunol. Lett., 121, 52-60
Nguyen, D.D., Wurbel, M-C., Goettel, J.A., Eston, M.A., Ahmed, O.S., Marin, R., Boden, E.K., Villablanca, E.J. et al (2012) Wiskott–Aldrich syndrome protein deficiency in innate immune cells leads to mucosal immune dysregulation and colitis in mice Gastroenterology, 143, 719–729
Nguyen, V., Pearson, K., Kim, J-H., Kamdar, K. and DePaolo, R.W. (2015) Retinoic acid can exacerbate T cell intrinsic TLR2 activation to promote tolerance PLoS One, 10: e0118875
Padassi, H., Acharya, M., Zhang, A., Mukhopadhyay, S., Kwon, M., Chow, C., Stuart, L.M., Savill, J. and Lacy–Hulbert, A. (2011) Preferential expression of integrin αvβ8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells Gastroenterology, 141, 1813–1820
Poulin, L.F., Salio, M., Griessinger, E., Anjos-Afonso, F., Craciun, L., Chen, J-L., Keller, A.M., Joffre, O. et al (2010) Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells J. Exp. Med., 207, 1261-1271
Rabanal, N.R., Franch, A., Noé, V., Pelegrí, C., Ciudad, C.J., Castellote, C. and Castell, M. (2003) CD4 expression decrease by antisense oligonucleotides: inhibition of rat CD4+ cell reactivity Oligonucleotides, 13 217-228
Tay, Q.W., Ho, L.C., Low, P.Y. and Kemeny, D.M. (2012) CD8 T cell-dendritic cell crosstalk up-regulates CD40L expression and IL-12p70 production Immunology, 137 (Suppl. 1), Abstr. P0874
Ulges, A., Klein, M., Reuter, S., Gerlitzki, B., Hoffmann, M., Grebe, N. Staudt, V. et al (2015) Protein kinase CK2 enables regulatory T cells to suppress excessive TH2 responses in vivo Nat. Immunol., 16, 267-275
Wong, K.L., Tang, L.F.M., Lew, F.C.L., Wong H.S.K., Chua, Y.L., MacAry, P.A. and Kemeny, D.M. (2009) CD44high memory CD8 T cells synergize with CpG DNA to activate dendritic cell IL-12p70 production J. Immunol., 183, 41–50
Zhan, X-X., Liu, Y., Yang, J-F., Wang, G-Y., Mu, L., Zhang, T-S., Xie, X-L., Wang, J-H., Liu, J-M., Kong, QF., Li, H-L. and Sun, B. (2013) All-trans-retinoic acid ameliorates experimental allergic encephalomyelitis by affecting dendritic cell and monocyte development Immunology, 138, 333–345
Proliferation
Bailey, S., Schreiner, B., McMahon, E.J. and Miller, S. (2007) CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ TH-17 cells in relapsing EAE Nat. Immunol., 8, 172-180
Fan, J., Edsen-Moore, M.R., Turner, L.E., Cook, R.T., Legge, K.L., Waldschmidt, T.J. and Schlueter, A.J. (2011) Mechanisms by which chronic ethanol feeding limits the ability of dendritic cells to stimulate T-cell proliferation Alcohol Clin. Exp. Res., 35, 47–59
Hegazy, A.N. and Klein, C. (2008) Ex vivo priming of CD4 T cells converts immunological tolerance into effective antitumor immunity in a murine model of acute lymphoblastic leukemia Leukemia, 22, 2070
Nakayama, M., Takeda, K., Kawano, M., Takai, T., Ishii, N. and Ogasawara, K. (2011) Natural killer (NK)– dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells Proc. Natl. Acad. Sci. USA, 108, 18360–18365
Yamazaki, T., Akiba, H., Koyanagi, A., Azuma, M., Yagita, H. and Okumura, K. (2005) Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-γ-induced nitric oxide production J. Immunol., 175, 1586-1592
Virus responses
Andrews, D.M., Estcourt, M.J., Andoniou, C.E., Wikstrom, M.E., Khong, A., Voigt, V., Fleming, P. et al (2010) Innate immunity defines the capacity of antiviral T cells to limit persistent infection J. Exp. Med., 207, 1333-1343
Cormier, S.A., Shrestha,, B., Saravia, J., Lee, G.I., Shen, L., DeVincenzo, J.P., Kim, Y-i. and You, D. (2014) Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection J. Virol., 88, 9350–9360
Puttur, F., Arnold-Schrauf, C., Lahl, K., Solmaz, G. Lindenberg, M., Mayer, C.T., Gohmert, M., Swallow, M. et al (2013) Absence of Siglec-H in MCMV infection elevates interferon alpha production but does not enhance viral clearance PLoS Pathog., 9: e1003648
Ruscanu, S., Jouneau, L., Urien, C., Bourge, M., Lecardonnel, J., Moroldo, M., Loup, B., Dalod, M. et al (2013) Dendritic cell subtypes from lymph nodes and blood show contrasted gene expression programs upon bluetongue virus infection J. Virol., 87, 9333–9343
Toll-like receptors
Babdor, J., Descamps, D., Adiko, A.C., Tohmé, M., Maschalidi, S., Evnouchidou, I., Vasconcellos, L.R., De Luca, M. et al (2017) IRAP+ endosomes restrict TLR9 activation and signalling Nat. Immunol., 18, 509-518
Thymus
Apoptosis
Vasilijić, S., Cilić, M. and Vucević, D. (2003) Granulocyte-macrophage colony stimulating factor is an antiapoptotic cytokine for thymic dendritic cells and a significant modelator of their accessory function Immunol. Lett., 86, 99-112
Derivation, development and maturation
Anjuère, F., Martin, P., Ferrero, I., Lopez Fraga, M., Martinez del Hoyo, G., Wright, N. and Ardavin, C. (1999) Definition of dendritic cell subpopulations present in the spleen, Peyer’s patches, lymph nodes, and skin of the mouse Blood, 93, 590-598
Ferrero, I., Held, W., Wilson, A., Tacchini-Cottier, F., Radtke, F. and MacDonald, H.R. (2002) Mouse CD11c+ B220+ Gr1+ plasmacytoid dendritic cells develop independently of the T-cell lineage Blood, 100, 2852-2857
Li, J-C., Park, J-H., Foss, D. and Goldschneider, I. (2009) Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus J. Exp. Med., 206, 607-622
Luche, H., Ardouin, L., Teo, P., See, P., Henri, S., Merad, M., Ginhoux, F. and Malissen, B. (2011) The earliest intrathymic precursors of CD8 + thymic dendritic cells correspond to myeloid-type double-negative 1c cells Eur. J. Immunol., 41, 2165–2175
Radtke, F., Ferrero, I., Wilson, A., Lees, R., Aguet, M. and MacDonald, H.R. (2000) Notch1 deficiency dissociates the intrathymic development of dendritic cells and T cells J. Exp. Med., 191, 1085-1093
Ruedl, C., Koebel, P., Bachmann, M., Hess, M. and Karjalainen, K. (2000) Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes J. Immunol., 165, 4910-4916
Spidale, N.A., Wang, B. and Tisch, R. (2014) Cutting edge: antigen-specific thymocyte feedback regulates homeostatic thymic conventional dendritic cell maturation J. Immunol., 193, 21–25
Waskow, C., Liu, K., Darrasse-Jèze, G., Guermonprez, P., Ginhoux, F., Merad, M., Shengelia, T., Yao, K. and Nussenzweig, M. (2008) The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues Nat. Immunol., 9, 676-683
Homeostasis regulation
Saito, Y., Iwamura, H., Kaneko, T., Ohnishi, H., Murata, Y., Okazawa, H., Kanazawa, Y., Sato-Hashimoto, M. et al (2010) Regulation by SIRP of dendritic cell homeostasis in lymphoid tissues Blood, 116, 3517-3525
Immune responses
Luckashenak, N.A., Ryszkiewicz, R.L., Ramsey, K.D. and Clements, J.L. (2006) The Src homology 2 domaincontaining leukocyte protein of 76-kDa adaptor links integrin ligation with p44/42 MAPK phosphorylation and podosome distribution in murine dendritic cells J. Immunol., 177, 5177-5185
Martin, P., Martinez del Hoyo, G., Anjuère, F., Fernandez Arias, C., Hernandez Vargas, H., Fernandez-L, A., Parrillas, V. and Ardavin, C. (2002) Characterization of a new subpopulation of mouse CD8+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential Blood, 100, 383-390
Martinez del Hoyo, G., Lopez-Bravo, M., Metharom, P., Ardavin, C. and Aucouturier, P. (2006) Prion protein expression by mouse dendritic cells is restricted to the nonplasmacytoid subsets and correlates with the maturation state J. Immunol., 177, 6137-6142
Okada, T., Lian, Z-X., Hsu, T., Naiki, M., Ansari, A.A., Robinson, D., Kung, H-J., Boyd, R. and Gershwin, E. (2002) Elevated C-met in thymic dendritic cells of New Zealand black mice Dev. Immunol., 9, 29-34
Okada, T., Inaba, M., Naiki, M., Lian, Z.X., Gershwin, M.E. and Ikehara, S. (2007) Comparative immunobiology of thymic DC mRNA in autoimmune-prone mice J. Autoimmun., 28, 41-45
MHC class II expression
Brocker, T., Riedinger, M. and Karjalainen, K. (1997) Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo J. Exp. Med., 185, 541-550
Kroger, C.J., Spidale, N.A., Wang, B. and Tisch, R. (2017) Thymic dendritic cell subsets display distinct efficiencies and mechanisms of intercellular MHC transfer J. Immunol., 198, 249–256
LeibundGut-Landemann, S., Waldburger, J-M., Reis e Sousa, C. Acha-Orbea, H. and Reith, W. (2004) MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells Nat. Immunol., 5, 899-908
Li, J-C., Park, J-H., Foss, D. and Goldschneider, I. (2009) Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus J. Exp. Med., 206, 607-622
Waldburger, J-M., Suter, T., Fontana, A., Acha-Orbea, H. and Reith, W. (2001) Selective abrogation of major histocompatibility complex class II expression on extrahematopoietic cells in mice lacking promoter IV of the class II transactivator gene J. Exp. Med., 194, 393-406
T cells
Dresch, C., Ackermann, M., Vogt, B., de Andrade Pereira, B., Shortman, K., and Fraefel, C. (2011)1 Thymic but not splenic CD81 DCs can efficiently cross-prime T cells in the absence of licensing factors Eur. J. Immunol., 41, 2544–2555
Schäumann, J., Pittoni, P., Tonti, E., MacDonald, H.R., Dellabona, P. and Casorati, G. (2005) Targeted expression of human CD1d in transgenic mice reveals independent roles for thymocytes and thymic APCs in positive and negative selection of Vα14i NKT cells J. Immunol., 175, 7303-7310
Yamaguchi, E., Chiba, S., Kumano, K., Kunisato, A., Takahashi, T., Takahashi, T. and Hirai, H. (2002) Expression of Notch ligands, Jagged1, and Delta1 in antigen presenting cells in mice Immunol. Lett., 81, 59-64
Tonsil
Del Cacho, E., Gallego, M., Lopez-Bernard, D.F., Sanchez-Acedo, C. and Liillehoj, H.S. (2008) Isolation of chicken follicular dendritic cells J. Immunol. Methods, 334, 59-69
Hallissey, C.M., Heyderman, R.S. and Williams, N.A. (2014) Human tonsil-derived dendritic cells are poor inducers of T cell immunity to mucosally encountered pathogens J,. Infect. Dis., 209, 1847-1856
Polak, M.E., Borthwick, N.J., Gabriel, F.G., Jager, M.J. and Cree, I.A. (2008) Activation of tonsil dendritic cells with immuno-adjuvants BMC Immunol., 9:10
Puebla-Clark, L., Parra-Sánchez, H., Reséndiz, M., Valenzuela, O. and Hernández, J. (2019) Tonsil conventional dendritic cells are not infected by porcine reproductive and respiratory syndrome virus Virology, 529, 65–72
Tumour tissue
Polak, M.E. (2011) Isolation of inflammatory cells from human tumours In Cancer Cell Culture: Methods and Protocols, 2nd Edition (ed. Cree. I.A.) Methods Mol. Biol., 731, Springer Science+Business Media pp 201-208
Vaginal
De Bernardis, F., Lucciarini, R., Boccanera, M., Amantini, C., Arancia, S., Morrone, S., Mosca, M., Cassone, A. and Santoni, G. (2006) Phenotypic and functional characterization of vaginal dendritic cells in a rat model of Candida albicans vaginitis Infect. Immun., 74, 4282-4294
OptiPrep™ Reference List RC05; 6th edition, January 2020
OptiPrep™ Reference List RC06
Isolation of cells from brain and spinal cord
The methodology for the isolation various types of neural cell (motoneurons and neuroglial cells) from brain and spinal cord using OptiPrep is well-established and presented in detail in the OptiPrep Application Sheets that can be accessed via the following website: www.Optiprep.com Click on “Methodology then “Mammalian and non-mammalian cells” and follow the links from the Index). Application Sheet C23: Motoneurons from spinal cords; Application Sheet C36: Motoneurons from brain and Application Sheet C35: Microglial cells
This Reference List brings together all of the known published papers reporting the use of OptiPrep for neural cells. The references are sorted into the following sections:
1. Methodology, 2. Motoneurons (sub-divided alphabetically according cell/tissue source (e.g. “Mouse brain cortex (adult)” or “Mouse brain mesencephalon (post-natal)”) 3. Myelin removal from cell preparations and 4. Neuroglial and other cells. The references may be further divided into research topic areas. Within each section references are listed alphabetically according to first author.
Important information: for convenience, papers published since mid-2019 are listed in Section 5; these will be incorporated into the main index at a later date.
1. Methodology
Brinn, M., O’Neill, K., Musgrave, I., Freeman, B.J.C., Henneberg, M. and Kumaratilake, J. (2016) An optimized method for obtaining adult rat spinal cord motorneurons to be used for tissue culture J. Neurosci., Meth., 273, 128–137
Brewer, G.J. and Torricelli, J.R. (2007) Isolation and culture of adult neurons and neurospheres Nat. Protoc., 2, 1490-1498
Graber, D.J. and Harris, B.T. (2013) Purification and culture of spinal motor neurons Cold Spring Harb. Protoc., prot074161, pp 319-326
Faron-Górecka, A., Szlachta, M., Kolasa, M., Solich, J., Górecki, A., Kuśmider, M., Zurawek, D. and Dziedzicka-Wasylewska, M. (2019) Understanding GPCR dimerization in Methods in Cell Biology, 149, 155-178
Hazen, J.L., Duran, M.A., Smith, R.P., Rodriguez, A.R., Martin, G.S., Kupriyanov, S., Hall, I.M. and Baldwin, K.K. (2017) Using cloning to amplify neuronal genomes for whole-genome sequencing and comprehensive mutation detection and validation In Genomic Mosaicism in Neurons and Other Cell Types, Neuromethods,
131, (ed. Frade, J.M. and Gage, F.H.) Springer Science+Business Media, LLC, pp 163-185 Katzenell, S., Cabrera, J.R., North, B.J. and Leib, D.A. (2017) Isolation, purification, and culture of primary murine sensory neurons In Innate Antiviral Immunity: Methods and Protocols, Methods in Molecular Biology, 1656, (ed. Mossman, K.) Springer Science+Business Media LLC pp. 229-51
Price, P.J. and Brewer, G.J. (2001) G Serum-free media for neural cell cultures, adult and embryonic In Protocols for Neural Cell CultureJ. Physiol., 535, 663-677 (Ed. Federoff, S. and Richardson, A.) Humana Press, Southam, K.A., King, A.E., Blizzard, C.A., McCormack, G.H. and Dickson, T.C. (2015) A novel in vitro primary culture model of the lower motor neuron–neuromuscular junction circuit In Neuromethods, 103, Microfluidic and Compartmentalized Platforms for Neurobiological Research (ed. Biffi, E.) Springer Science+Business Media New York, pp 181-193, Totowa, N.J., USA pp 255-264
2. Motoneurons
Chicken embryo spinal cord
Macosko, J.C., Newbern, J.M., Rockford, J., Chisena, E.N., Brown, C.M., Holzwarth, G.M. and Milligan, C.E. (2008) Fewer active motors per vesicle may explain slowed vesicle transport in chick motoneurons after three days in vitro Brain Res., 1211, 6-12
Newbern, J., Taylor, A., Robinson, M., Li, L. and Milligan, C.E. (2005) Decreases in phosphoinositide-3- kinase/Akt and extracellular signal-regulated kinase 1/2 signaling activate components of spinal motoneuron death J. Neurochem., 94, 1652-1665
Newbern, J., Taylor, A., Robinson, M., Lively, M.O. and Milligan, C.E. (2007) c-Jun N-terminal kinase signaling regulates events associated with both health and degeneration in motoneurons Neuroscience, 147, 68- 692
Robinson, M.B., Taylor, A.R., Gifondorwa, D.J., Tytell, M. and Milligan, C.E. (2008) Exogenous Hsc70, but not thermal preconditioning, confers protection to motoneurons subjected to oxidative stress Develop. Neurobiol., 68, 1-17
Taylor, A.R., Gifondorwa, D.J., Newbern, J.M., Robinson, M.B., Strupe, J.L., Prevette, D., Oppenhiem, R.W. and Milligan, C.E. (2007) Astrocyte and muscle-derived secreted factors differentially regulate motoneuron survival J. Neurosci., 27, 634-644
Taylor, A.R., Robinson, M.B. and Milligan, C.E. (2007) In vitro methods to prepare astrocyte and motoneuron cultures for the investigation of potential in vivo interactions Nat. Protoc., 2, 1499-1507
Taylor, A.R., Gifondorwa, D.J., Robinson, M.B., Strupe, J.L., Prevette, D., Johnson, J.E., Hempstead, B., Oppenheim, R.W. and Milligan, C.E. (2012) Motoneuron programmed cell death in response to ProBDNF Develop. Neurobiol., 72, 699–712
Fish
Da Silva, C.A., de Morais, E.C.P., Costa, M.D.M, Ribas, J.L.C., Guiloski, I.C., Ramsdorf, W.A., Zanata, S.M. et al (2014) Saxitoxins induce cytotoxicity, genotoxicity and oxidative stress in teleost neurons in vitro Toxicon, 86, 8–15
Souto, S., Olveira, J.G., Vazquez‑Salgado, L., Dopazo, C.P. and Bandín, I. (2018) Betanodavirus infection in primary neuron cultures from sole Vet. Res., 49: 86
Freshwater turtle
Cocilova, C.C. and Milton, S.L. (2016) Characterization of brevetoxin (PbTx-3) exposure in neurons of theanoxia-tolerant freshwater turtle (Trachemys scripta) Aquat. Toxicol., 180, 115–122
Hamster brain cortex
Hollister, J.R., Lee, K.S., Dorward, D.W. and Baron, G.S. (2015) Efficient uptake and dissemination of scrapie prion protein by astrocytes and fibroblasts from adult hamster brain PLoS One, 10: e0115351Magalhaes, A.C., Baron, G.S., Lee, K.S., Steele-Mortimer, O., Dorward, D., Prado, M.A.M. and Caughey, B. (2005) Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells J. Neurosci., 25, 5207- 5216
Human brain cortex (at autopsy)
Konishi, Y., Lindhilm, K., Yang, L-B., Li, R. and Shen, Y. (2002) Isolation of living neurons from human elderly brains using the immunomagnetic sorting DNA-linker system Am. J. Pathol., 161, 1567-1576
Human brain cortex (ex-surgery)
Brewer, G.J., Espinosa, J., McIlhaney, M.P., Pencek, T.P., Kesslak, J.P., Cotman, C., Viel, J. and McManus, D.C. (2001) Culture and regeneration of human neurons after brain surgery J. Neurosci Meth., 107, 15-23
Gibbons, H.M. and Dragunow, M. (2010) Adult human brain cell culture for neuroscience research Int. J. Biochem. Cell Biol., 42, 844–856
Human embryonic spinal cord
Sundaramoorthy, V., Walker, A.K., Tan, V., Fifita, J.A., Mccann, E.P., Williams, K.L., Blair, I.P., Guillemin, G.J. et al (2015) Defects in optineurin- and myosin VI-mediated cellular trafficking in amyotrophic lateral sclerosis Hum. Mol. Genet., 24, 3830–3846
Human fetal brain
Ataman, B., Boulting, G.L., Harmin, D.A., Yang, M.G., Baker-Salisbury, M., Yap, E-L., Malik, A.N., Mei, K., Rubin, A.A. et al (2016) Evolution of Osteocrin as an activityregulated factor in the primate brain Nature, 539, 242-247
Human spinal cord
Colón, A., Guo, X., Akanda, N., Cai, Y. and Hickman J.J. (2017) Functional analysis of human intrafusal fiber innervation by human γ-motoneurons Sci. Rep., 7: 17202
Santhanam, N., Kumanchik, L., Guo, X., Sommerhage, F., Cai, Y., Jackson, M., Martin, C., Saad, G. et al (2018) Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics Biomaterials, 166, 64-78
Mouse brain amygdala
Mou, L., Dias, B.G. Gosnell, H. and Ressler, K.J. (2013) Gephryin plays a key role in BDNF-dependent regulation of amygdala surface GABAARS Neuroscience 255, 33–44
Mouse brain cerebellar granule
Benson, M.D., Romero, M.I., Lush, M.E., Lu, R., Henkemeyer, M. and Parada, L.F. (2005) Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth Proc. Natl. Acad. Sci. USA, 102, 10694-10699
Davis, T.H., Chen, C. and Isom, L.L. (2004) Sodium channels β1 subunits promote neurite outgrowth in cerebellar granule neurons J. Biol. Chem., 279, 51424-51432
Sharkey, L.M., Cheng, X., Drews, V., Buchner, D.A., Jones, J.M., Justice, M.J., Waxman, S.G., Dib-Hajj, S.D. Meisler, M.H. (2009) The ataxia3 mutation in the N-terminal cytoplasmic domain of sodium channel Nav1.6 disrupts intracellular trafficking J. Neurosci., 29, 2733–2741
Mouse brain cortex (adult)
Barsukova, A., Komarov, A., Hajnoczky, G., Bernardi, P., Bourdette, D. and Forte, M. (2011) Activation of the mitochondrial permeability transition pore modulates Ca2+ responses to physiological stimuli in adult neurons Eur. J, Neurosci., 33, 831–842
Barsukova, A.G., Bourdette, D. and Forte, M. (2011) Mitochondrial calcium and its regulation in neurodegeneration induced by oxidative stress Eur. J. Neurosci., 34, 437–447
Barsukova, A.G., Forte, M. and Bourdette, D. (2012) Focal increases of axoplasmic Ca2+, aggregation of sodium–calcium exchanger, N-type Ca2+ channel, and actin define the sites of spheroids in axons undergoing oxidative stress J. Neurosci., 32, 12028 –12037
Benson, M.D., Romero, M.I., Lush, M.E., Lu, R., Henkemeyer, M. and Parada, L.F. (2005) Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth Proc. Natl. Acad. Sci. USA, 102, 10694-10699
Cao, L., Pu, J., Scott, R.H., Ching, J. and McCaig, C.D. (2015) Physiological electrical signals promote chain migration of neuroblasts by up-regulating P2Y1 purinergic receptors and enhancing cell adhesion Stem Cell Rev. Rep., 11, 75–86
Capó-Vélez, C.M., Morales-Vargas, B., García-González, A., Grajales-Reyes, J.G., Delgado-Vélez, M., Madera, B., Báez-Pagán, C.A., Quesada, O. and Lasalde-Dominicci, J.A. (2018) The alpha7-nicotinic receptor contributes to gp120-induced neurotoxicity: implications in HIV-associated neurocognitive disorders Sci. Rep., 8: 1829
Dong, Y., Digman, M.A. and Brewer, G.J. (2019) Age- and AD-related redox state of NADH in subcellular compartments by fluorescence lifetime imaging microscopy GeroScience 41, 51–67
Feng, X., Bader, B.M., Yang, F., Segura, M., Schultz, L.,Schröder, O, H-U., Rolfs, A. and Luo, J. (2018) Improvement of impaired electrical activity in NPC1 mutant cortical neurons upon DHPG stimulation detected by micro-electrode array Brain Res., 1694, 87–93
Ghosh, D., LeVault, K.R., Barnett, A.J. and Brewer, G.J. (2012) A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons J. Neurosci., 32, 5821–5832
Ghosh, D., LeVault, K.R. and Brewer, G.J. (2014) Dual-energy precursor and nuclear erythroiderelated factor 2 activator treatment additively improve redox glutathione levels and neuron survival in aging and Alzheimer mouse neurons upstream of reactive oxygen species Neurobiol. Aging, 35, 179-190
Kolasa, M., Solich, J., Faron-Górecka, A., Zurawek, D., Pabian, P., Ukasiewicz, S., Kuśmider, M., SzafranPilch, K. et al (2018) Paroxetine and low-dose risperidone induce serotonin 5-HT1A and dopamine D2 receptor heteromerization in the mouse prefrontal cortex Neuroscience 377, 184–196
Leon, J., Sakumi, K., Castillo, E., Sheng, Z., Oka, S. and Nakabeppu, Y. (2016) 8-Oxoguanine accumulation in mitochondrial DNA causes mitochondrial dysfunction and impairs neuritogenesis in cultured adult mouse cortical neurons under oxidative conditions Sci. Rep., 6: 22086
Li, S., Nie, E.H., Yin, Y., Benowitz, L.I., Tung, S., Vinters, H.V., Bahjat, F.R., Stenzel-Poore, M.P. et al (2015) GDF10 is a signal for axonal sprouting and functional recovery after stroke Nat. Neurosci., 18, 1737-1745
Lopez, J.R., Lyckman, A., Oddo, S., LaFerla, F.M., Querfurth, H.W., Shtifman, A. (2008) Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice J. Neurochem., 105, 262-271
Magalhaes, A.C., Baron, G.S., Lee, K.S., Steele-Mortimer, O., Dorward, D., Prado, M.A.M. and Caughey, B. (2005) Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells J. Neurosci., 25, 5207-5216
Nathan, B.P., Jiang, Y., Wong, G.K., Shen, F., Brewer, G.J. and Struble, R.G. (2002) Apolipoprotein E4 inhibits, and apolipoprotein E3 promotes neurite outgrowth in cultured adult mouse cortical neurons through the low-density lipoprotein receptor-related protein Brain Res., 928, 96-105
Nathan, B.P., Barsukova, A.G., Shen, F., McAsey, M. and Struble, R.G. (2004) Estrogen facilitates neurite extension via apolipoprotein E in cultured adult mouse cortical neurons Endocrinology, 145, 3065-3073
Nicoll, M.P., Hann, W., Shivkumar, M., Harman, L.E.R., Connor, V., Coleman, H.M., Proença, J.T. and Efstathiou, S. (2016) The HSV-1 latency-associated transcript functions to repress latent phase lytic gene expression and suppress virus reactivation from latently infected neurons PLoS Pathog., 12: e1005539
Tiwari, M., Lopez-Cruzan, M., Morgan, W.W. and Herman, B. (2011) Loss of caspase-2-dependent apoptosis induces autophagy after mitochondrial oxidative stress in primary cultures of young adult cortical neurons J. Biol. Chem., 286, 8493–8506
Wils, H., Kleinberger, G., Janssens, J., Pereson, S., Joris, G., Cuijt, I., Smits, V., Ceuterick-de Groote, C. et al (2010) TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration Proc. Natl. Acad. Sci. USA, 107, 3858–3863
Xu, Y-h., Xu, K., Sun, Y., Liou, B., Quinn, B., Li, R-h., Xue, L., Zhang, W., Setchel, K.D.R., Witte, D. and Grabowski, G.A. (2014) Multiple pathogenic proteins implicated in neuronopathic Gaucher disease mice Hum. Mol. Genet., 23, 3943–3957
Mouse brain cortex (juvenile)
Osada, K., Tamamaki, N., Song, S-Y., Kakazu, N., Yamazaki, Y., Makino, H., Sasaki, A., Hirayama, T. et al (2005) Developmental pluripotency of the nuclei of neurons in the cerebral cortex of juvenile mice J. Neurosci., 25, 8368-8374
Wang, Y-J., Wang, X., Lu, J-J., Li, Q-X.,Gao, C-Y., Liu, X-H., Sun, Y., Yang, M. et al (2011) p75NTR regulates Aβ deposition by increasing Aβ production but inhibiting A aggregation with its extracellular domain J. Neurosci., 31, 2292–2304
Mouse brain cortex (neo-natal)
Tang, Z., Arjunan, P., Lee, C., Li, Y., Kumar, A., Hou, X., Wang, B., Wardega, P., Zhang, F. et al (2010) Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3β phosphorylation J. Exp. Med., 207, 867-880
Mouse brain cortex (post-natal)
Ahmed, A.I., Shtaya, A.B., Zaben, M.J., Owens, E.V., Kiecker, C. and Gray, W.P. (2012) Endogenous GFAPpositive neural stem/progenitor cells in the postnatal mouse cortex are activated following traumatic brain injury J. Neurotrauma, 29, 828–842
Agarwal, A.K., Tunison, K., Dalal, J.S., Nagamma, S.S., Hamra, F.K., Sankella, S., Shao, X., Auchus, R.J. and Garg, A. (2017) Metabolic, reproductive, and neurologic abnormalities in Agpat1-null mice Endocrinology, 158, 3954–3973
Barsukova, A.G., Forte, M. and Bourdette, D. (2012) Focal increases of axoplasmic Ca2+, aggregation of sodium–calcium exchanger, N-type Ca2+ channel, and actin define the sites of spheroids in axons undergoing oxidative stress J. Neurosci., 32, 12028 –12037
Berretta, A., Gowing, E.K., Jasoni, C.L. and Clarkson, A.N. (2016) Sonic hedgehog stimulates neurite outgrowth in a mechanical stretch model of reactive-astrogliosis Sci. Rep., 6: 21896 Caracciolo, L., Marosi, M., Mazzitelli, J., Latifi, S., Sano, Y., Galvan, L., Kawaguchi, R., Holley, S., Levine,
M.S. et al (2018) CREB controls cortical circuit plasticity and functional recovery after stroke Nat. Comm., 9: 2250
Chuang, J-H., Tung, L-C., Yin, Y. and Lin, Y. (2013) Differentiation of glutamatergic neurons from mouse embryonic stem cells requires raptor S6K signaling Stem Cell Res., 11, 1117-1128
Finelli, M.J., Sanchez-Pulido, L., Liu, K.X., Davies, K.E. and Oliver, P.L. (2016) The evolutionarily conserved Tre2/Bub2/Cdc16 (TBC), lysin motif (LysM), domain catalytic (TLDc) domain is neuroprotective against oxidative stress J. Biol. Chem., 291, 2751–2763
Finelli, M.J., Paramo, T., Pires, E., Ryan, B.J., Wade-Martins, R., Biggin, P.C., McCullagh, J. and Oliver1, P.L. (2019) Oxidation resistance 1 modulates glycolytic pathways in the cerebellum via an interaction with glucose6-phosphate isomerase Mol. Neurobiol., 56, 1558–1577
Fujita, T., Chen, M.J., Li, B., Smith, N.A., Peng, W., Sun, W., Toner, M.J., Kress, B.T. et al (2014) Neuronal transgene expression in dominant-negative SNARE mice J. Neurosci., 34,16594 –16604
Goto-Silva, L., McShane, M.P., Salinas, S., Kalaidzidis, Y., Schiavo, G. and Zerial, M. (2019) Retrograde transport of Akt by a neuronal Rab5-APPL1 endosome Sci. Rep., 9: 2433
Ingram, N.T., Khankan, R.R. and Phelps, P.E. (2016) Olfactory ensheathing cells express a7 integrin to mediate their migration on laminin PloS One, 11: e0153394
Kruger, L.C., O’Malley, H.A., Hull, J.M., Kleeman, A., Patino, G.A. and Isom, L.L. (2016) β1-C121W is down but not out: epilepsy-associated Scn1b-C121W results in a deleterious gain-of-function J. Neurosci., 36, 6213–6224
Kuo, L-C., Song, Y-Q., Yao, C-A., Cheng, I,H., Chien, C-T., Lee, G-C., Yang, W-C. and Lin, Y. (2019) Ginkgolide a prevents the amyloid-β-induced depolarization of cortical neurons J. Agric. Food Chem., 67, 81−89
Parker, K., Berretta, A., Saenger, S., Sivaramakrishnan, M., Shirley, S.A., Metzger, F. and Clarkson, A.N. (2017) PEGylated insulin-like growth factor-I affords protection and facilitates recovery of lost functions postfocal ischemia Sci. Rep., 7: 241
Mouse brain hippocampus (adult)
Agarwal, A.K., Tunison, K., Dalal, J.S., Nagamma, S.S., Hamra, F.K., Sankella, S., Shao, X., Auchus, R.J. and Garg, A. (2017) Metabolic, reproductive, and neurologic abnormalities in Agpat1-null mice Endocrinology, 158, 3954–3973
Arneson, D., Zhang, G., Ying, Z., Zhuang, Y., Byun, H.R., Ahn, I.S., Gomez-Pinilla, F. and Yang, X. (2018) Single cell molecular alterations reveal target cells and pathways of concussive brain injury Nat. Comm., 9: 3894
Ghosh, D., LeVault, K.R., Barnett, A.J. and Brewer, G.J. (2012) A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons J. Neurosci., 32, 5821–5832
Ghosh, D., LeVault, K.R. and Brewer, G.J. (2014) Dual-energy precursor and nuclear erythroiderelated factor 2 activator treatment additively improve redox glutathione levels and neuron survival in aging and Alzheimer mouse neurons upstream of reactive oxygen species Neurobiol. Aging, 35, 179-190
Varghese, K., Das, M., Bhargava, N., Stancescu, M., Molnar, P., Kindy, M.S. and Hickman, J.J. (2009) Regeneration and characterization of adult mouse hippocampal neurons in a defined in vitro system J. Neurosci. Meth., 177, 51–59
Mouse brain hippocampus (neo-natal)
Mou, L., Heldt, S.A. and Ressler, K.J. (2011) Rapid brain-derived neurotrophic factor-dependent sequestration of amygdala and hippocampal GABAA receptors vis different tyrosine receptor kinase B-mediated phosphorylation pathways Neuroscience, 176, 72–85
O’Mahony, A., Raber, J., Montano, M., Foehr, E., Han, V., Lu, S-m., Kwon, H., LeFevour, A., ChakrabortySett, S. and Greene, W.C. (2006) NF-κB/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity Mol. Biol. Cell., 26, 7283-7298
Wang, X.Q., Deriy, L.V., Foss, S., Huang, P., Lamb, F.S., Kaetzel, M.A., Bindokas, V., Marks, J.D. and Nelson, D.J. (2006) CLC-3 channels modulate excitatory synaptic transmission in hippocampal neurons Neuron, 52, 321-333
Mouse brain hippocampus (post-natal)
Chen, M., Geoffroy, C.G., Wong, H.N., Tress, O., Nguyen, M.T., Holzman, L.B., Jin, Y. and Zheng, B. (2106) Leucine Zipper-bearing Kinase promotes axon growth in mammalian central nervous system neurons Sci. Rep., 6: 31482
Jinadasa, T., Szabó, E.Z., Numata, M. and Orlowski, J. (2014) Activation of AMP-activated protein kinase regulates hippocampal neuronal pH by recruiting Na+ /H+ exchanger NHE5 to the cell surface J. Biol. Chem., 289, 20879–20897
Mahan, A.L., Mou, L., Shah, N., Hu, J-H., Worley, P.F. and Ressler, K.J. (2012) Epigenetic modulation of Homer1a transcription regulation in amygdala and hippocampus with Pavlovian fear conditioning J. Neurosci., 32, 4651– 4659
Mouse brain mesencephalon (post-natal)
Tiwari, M., Herman, B. and Morgan, W.W. (2011) A knockout of the caspase 2 gene produces increased resistance of the nigrostriatal dopaminergic pathway to MPTP-induced toxicity Exp. Neurol., 229, 421–428
Mouse brain olfactory bulb (post-natal)
Pathania, M., Torres-Reveron, J., Yan, L., Kimura, T., Lin, T.V., Gordon, V., Teng, Z-Q., Zhao, X. et al (2012) miR-132 enhances dendritic morphogenesis, spine density, synaptic integration and survival of newborn olfactory bulb neurons PLoS One, 7: e38174
Mouse brain striatum (adult)
Ena, S.L., De Backer, J.F., Schiffmann, S.N. and de Kerchove d’Exaerde, A. (2013) FACS array profiling identifies ecto-5’ nucleotidase as a striatopallidal neuron-specific gene involved in striatal-dependent learning J. Neurosci., 33, 8794–8809
Lambot, L., Rodriguez, E.C., Houtteman, D., Li, Y., Schiffmann, S.N., Gall, D., and de Kerchove d’Exaerde, A. (2016) Striatopallidal neuron NMDA receptors control synaptic connectivity, locomotor, and goal-directed behaviors J. Neurosci., 36, 4976–4992
Szafran-Pilch, K., Faron-Górecka, A., Kolasa, M., Żurawek, D., Szlachta, M., Solich, J., Kuśmider, M., Dziedzicka-Wasylewska, M. (2017) Antidepressants promote formation of heterocomplexes of dopamine D2 and somatostatin subtype 5 receptors in the mouse striatum Brain Res. Bull., 135, 92–97
Mouse brain trigeminal ganglia
Arneson, D., Zhang, G., Ying, Z., Zhuang, Y., Byun, H.R., Ahn, I.S., Gomez-Pinilla, F. and Yang, X. (2018) Single cell molecular alterations reveal target cells and pathways of concussive brain injury Nat. Comm., 9: 3894
Bertke, A.S., Swanson, S.M., Chen, J., Imai, Y., Kinchington, P.R. and Margolis, T.P. (2011) A5-Positive primary sensory neurons are nonpermissive for productive infection with herpes simplex virus 1 in vitro J. Virol., 85, 6669–6677
Bertke, A.S., Apakupakul, K., Ma. AA., Imai, Y., Gussow. A.M., Wang, K., Cohen, J.I., Bloom, D.C., Margolis, T.P. (2012) LAT region factors mediating differential neuronal tropism of HSV-1 and HSV-2 do not act in trans PLoS One, 7: e53281
Cohen, C., Corpet, A., Roubille, S., Maroui, M.A., Poccardi, N., Rousseau, A., Kleijwegt, C., Binda, O. et al (2018) Promyelocytic leukemia (PML) nuclear bodies (NBs) induce latent/quiescent HSV-1 genomes chromatinization through a PML NB/Histone H3.3/H3.3 chaperone axis PLOS Pathog., 14, e1007313
Ives, A.M. and Bertke, A.S. (2017) Stress hormones epinephrine and corticosterone selectively modulate herpes simplex virus 1 (HSV-1) and HSV-2 productive infections in adult sympathetic, but not sensory neurons (2017) J. Virol., 91: e00582-17
Maroui, M.A., Callé, A., Cohen, C., Streichenberger, N., Texier, P., Takissian, J., Rousseau, A., Poccardi, N., Welsch, J. et al (2016) Latency entry of herpes simplex virus 1 is determined by the interaction of its genome with the nuclear environment PLoS Pathog., 12: e1005834
Katzenell, S. and Leib, D.A. (2016) Herpes simplex virus and interferon signaling induce novel autophagic clusters in sensory neurons J. Virol., 90, 4706-4719
Messer, H.G.P., Jacobs, D., Dhummakupt, A. and Bloom, D.C. (2015) Inhibition of H3K27me3-specific histone demethylases JMJD3 and UTX blocks reactivation of herpes simplex virus 1 in trigeminal ganglion neurons J. Virol., 89, 3417-3420
Rosato, P.C. and Leib, D.A. (2014) Intrinsic innate immunity fails to control herpes simplex virus and vesicular stomatitis virus replication in sensory neurons and fibroblasts J. Virol., 88, 9991-10001
Zhang, Y., Dai, J., Tang, J., Zhou, L. and Zhou, M. (2017) MicroRNA-649 promotes HSV-1 replication by directly targeting MALT1 J. Med. Virol., 89, 1069–1079
Mouse embryo
Barber, S.C., Higginbottom, A., Mead, R.J., Barber, S. and Shawa, P.J. (2009) An in vitro screening cascade to identify neuroprotective antioxidants in ALS Free Radical Biol. Med. 46 1127–1138
Guo, W., Pang, K., Chen, Y., Wang, S., Li, H., Xu, Y., Han, F., Yao, H. et al (2019) TrkB agonistic antibodies superior to BDNF: utility in treating motoneuron degeneration Neurobiol. Dis., 132: 104590
Mouse embryo ventral midbrain
Miller, N., Shi, H., Zelikovich, A.S. and Ma, Y-C. (2016) Motor neuron mitochondrial dysfunction in spinal muscular atrophy Hum.Mol. Genet., 25, 3395–3406
Mouse spinal cord (adult)
Benoy, V., Vanden Berghe, P., Jarpe, M., Van Damme, P., Robberecht, W. and Van Den Bosch, L. (2017) Development of improved HDAC6 inhibitors as pharmacological therapy for axonal Charcot–Marie–tooth disease Neurotherapeutics, 14, 417–428
Benoy, V., Van Helleputte, L., Prior, R., d’Ydewalle, C., Haeck, W., Geens, N., Scheveneels, W., Schevenels, B., Cader, M.Z. (2018) HDAC6 is a therapeutic target in mutant GARS-induced Charcot-Marie-Tooth disease Brian, 141, 673–687
Galvan, M.D., Luchetti, S., Burgos, A.M., Nguyen, H.X., Hooshmand, M.J., Hamers, F.P.T. and Anderson, A.J. (2008) Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury J. Neurosci., 28, 13876 –13888
Foley, L.S., Fullerton, D.A., Bennett, D.T., Freeman, K.A., Mares, J., Bell, M.T., Cleveland, Jr, J.C., Weyant, M.J. et al (2015) Spinal cord ischemia-reperfusion injury induces erythropoietin receptor expression Ann. Thorac. Surg., 100, 41–46
Foran, E., Bogrush, A., Goffredo, M., Roncaglia, P., Gustincich, S., Pasinelli, P., Trotti, D. (2011) Motor neuron impairment mediated by a sumoylated fragment of the glial glutamate transporter EAAT2 Glia 59, 1719–1731
Khayrullina, G., Bermudez, S. and Byrnes, K.R. (2015) Inhibition of NOX2 reduces locomotor impairment, inflammation, and oxidative stress after spinal cord injury J. Neuroinflammation, 12: 172
Montoya-Gacharna, J.V., Sutachan, J.J., Chan, W.S., Sideris, A., Blanck, T.J.J. and Recio-Pinto, E. (2012) Preparation of adult spinal cord motor neuron cultures under serum-free conditions In Neurotrophic Factors: Methods and Protocols, Methods in Molecular Biology, vol. 846, (Ed. Skaper, S.D.) Springer Science+Business Media, pp 103-116
Rotem, N., Magen, I., Ionescu, A., Gershoni-Emek, N., Altman, T., Costa, C.J., Gradus, T., Pasmanik-Chor, M. et al (2017) ALS along the axons – expression of coding and noncoding RNA differs in axons of ALS models Sci. Rep., 7: 44500
Shen, S., Benoy, V., Bergman, J.A., Kalin, J.H., Frojuello, M., Vistoli, G., Haeck, W., Van Den Bosch, L. and Kozikowski, A.P. (2016) Bicyclic-capped histone deacetylase 6 inhibitors with improved activity in a model of axonal Charcot-Marie-Tooth disease ACS Chem. Neurosci., 7, 240−258
Wang, P-Y., Koishi, K., McGeachie, A.B., Kimber, M., MacLaughlin, D.T., Donahoe, P.K. and McLennan, I.S. (2005) Mullerian inhibiting substance acts as a motor neuron survival factor in vitro Proc. Natl. Acad. Sci. USA, 102, 16421-16425
Xie, Y., Zhou, B., Lin, M-Y., Wang, S., Foust, K.D. and Sheng, Z-H. (2015) Endolysosomal deficits augment mitochondria pathology in spinal motor neurons of asymptomatic fALS mice Neuron 87, 355–370
Zeisel, A., Hochgerner, H., Lönnerberg, P., Johnsson, A., Memic, F., van der Zwan, J., Häring, M., Braun, E.,
Borm, L.E. et al (2018) Molecular architecture of the mouse nervous system Cell 174, 999–1014
Mouse spinal cord (embryo)
Adherence
Vodouhe, C., Schmittbuhl, M., Boulmedais, F., Bagnard, D., Vautier, D., Schaaf, P., Egles, C., Voegel, J-C. and Ogier, J. (2004) Effect of functionalization of multilayered polyelectrolyte films on motoneuron growth Biomaterials, 26, 545-554
Amyotrophic lateral sclerosis
Bernard-Marissal, N., Moumen, A., Sunyach, C., Pellegrino, C., Dudley, K., Henderson, C.E., Raoul, C. and Pettmann, B. (2012) Reduced calreticulin levels link endoplasmic reticulum stress and Fas-triggered cell death in motoneurons vulnerable to ALS J. Neurosci., 32, 4901– 4912
Blizzard, C.A., Southam, K.A., Dawkins, E., Lewis, K.E., King, A.E., Clark, J.A. and Dickson, T.C. (2015) Identifying the primary site of pathogenesis in amyotrophic lateral sclerosis – vulnerability of lower motor neurons to proximal excitotoxicity Dis. Model. Mech., 8, 215-224
Bowerman, M., Salsac, C., Coque, E., Eiselt, E., Deschaumes, R.G., Brodovitch, A., Burkly, L.C., Scamps, F. and Raoul, C. (2015) Tweak regulates astrogliosis, microgliosis and skeletal muscle atrophy in a mouse model of amyotrophic lateral sclerosis Hum. Mol. Genet., 24, 3440–3456
Camu, W., Tremblier, B., Plassot, C., Alphandery, S., Salsac, C., Pageot, N., Juntas-Morales, R., Scamps, F., Daures, J-P. and Raoul, C. (2014) Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis Neurobiol. Aging, 35, 1198-1205
De Paola, M., Sestito, S.E., Mariani, A., Memo, C., Fanelli, R., Freschi, M., Bendotti, C., Calabrese, V. and Peri, F. (2016) Synthetic and natural small molecule TLR4 antagonists inhibit motoneuron death in cultures from ALS mouse model Pharmacol. Res., 103, 180–187
Ferraiuolo, L., Higginbottom, A., Heath, P.R., Barber, S., Greenald, D., Kirby, J. and Shaw, P.J. (2011) Dysregulation of astrocyte–motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis Brain, 134, 2627–2641
Gershoni-Emek, N., Mazza, A., Chein, M., Gradus-Pery, T., Xiang, X., Wan Li, K., Sharan, R. and Perlson, E. (2016) Proteomic analysis of dynein-interacting proteins in amyotrophic lateral sclerosis synaptosomes reveals alterations in the RNA-binding protein Staufen1 Mol. Cell. Proteom., 15, 506–522
Hogg, M.C., Mitchem, M.R., König, H-G. and Prehn, J.H.M. (2016) Caspase 6 has a protective role in SOD1G93A transgenic mice Biochim. Biophys. Acta, 1862, 1063–1073
Hounoum, B.M., Mavel, S., Coque, E., Patin, F., Vourc’h, P., Marouillat, S., Nadal-Desbarats, L., Emond, P., Corcia, P. et al (2017) Wildtype motoneurons, ALS-linked SOD1 mutation and glutamate profoundly modify astrocyte metabolism and lactate shuttling Glia, 65, 592–605
Hoye, M.L., Koval, E.D., Wegener, A.J., Hyman, T.S., Yang, C., O’Brien, D.R., Miller, R.L., Cole, T., Schoch, K.M., Shen, T., Kunikata, T. et al (2017) MicroRNA profiling reveals marker of motor neuron disease in ALS models J. Neurosci., 37, 5574 –5586
Ionescu, A., Gradus, T., Altman, T., Maimon, R., Avraham, N.S., Geva, M., Hayden, M. and Perlson, E. (2019) Targeting the sigma-1 receptor via pridopidine ameliorates central features of ALS pathology in a SOD1G93A model Cell Death Dis., 10: 210
Lautenschläger, J., Prell, T., Ruhmer, J., Weidemann, L., Witte, O.W., Grosskreutz, J. (2013) Overexpression of human mutated G93A SOD1 changes dynamics of the ER mitochondria calcium cycle specifically in mouse embryonic motor neurons Exp. Neurol., 247, 91–100
Lee, J.K., Shin, J.H., Hwang, S.G., Gwag, B.J., McKee, A.C., Lee, J, Kowall, N.W., Ryu, H., Lim, D-S. and Choi, E-J. (2013) MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS Proc. Natl. Acad. Sci. USA, 110, 12066–12071
Maimon, R., Ionescu, A., Bonnie, A., Sweetat, S., Wald-Altman, S., Inbar, S., Gradus, T., Trotti, D., Weil, M., Behar, O. and Perlson, E. (2018) miR126-5p Downregulation facilitates axon degeneration and NMJ disruption via a non–cell-autonomous mechanism in ALS J. Neurosci., 38, 5478 –5494
Martínez-Palma, L., Miquel, E., Lagos-Rodríguez, V., Barbeito, L., Cassina, A. and Cassina, P., (2019) Mitochondrial modulation by dichloroacetate reduces toxicity of aberrant glial cells and gliosis in the SOD1G93A rat model of amyotrophic lateral sclerosis Neurotherapeutics, 16, 203–215
Miller, N., Shi, H., Zelikovich, A.S. and Ma, Y-C. (2016) Motor neuron mitochondrial dysfunction in spinal muscular atrophy Hum.Mol. Genet., 25, 3395–3406
Nardo, G., Trolese, M.C., Verderio, M., Mariani, A., de Paola, M., Riva, N., Dina, G., Panini, N. et al (2018) Counteracting roles of MHCI and CD8+ T cells in the peripheral and central nervous system of ALS SOD1G93A mice Mol. Neurodegen., 13: 42
Otsmane, B., Aebischer, J., Moumen, A. and Raoul, C., (2014) Cerebrospinal fluid-targeted delivery of neutralizing anti-IFNγ antibody delays motor decline in an ALS mouse model NeuroReport, 25, 49–54
Penndorf, D., Tadić, V., Witte, O.W., Grosskreutz, J. and Kretz, A. (2017) DNA strand breaks and TDP-43 mislocation are absent in the murine hSOD1G93A model of amyotrophic lateral sclerosis in vivo and in vitro PLoS ONE 12(8): e0183684.
Prell, T., Lautenschlager, J., Witte, O.W., Carri, M.T. and Grosskreutz, J. (2012) The unfolded protein response in models of human mutant G93A amyotrophic lateral sclerosis Eur. J. Neurosci., 35, 652-660
Prell, T., Lautenschläger, J., Weidemann, L., Ruhmer, J., Witte, O.W. and Grosskreutz, J. (2014) Endoplasmic reticulum stress is accompanied by activation of NF-κB in amyotrophic lateral sclerosis J. Neuroimmunol., 270, 29–36
Sengupta, S., Le, T.T., Adam, A., Tadić, V., Stubendorff, B., Keiner, S., Kloss, L., Prell, T., Witte, O.W. and Grosskreutz, J. (2019) Interferon-γ receptor 1 and GluR1 upregulated in motor neurons of symptomatic hSOD1G93A mice Eur. J. Neurosci., 49, 62–78
Sunyach, C., Michaud, M., Arnoux, T., Bernard-Marissal, N., Aebischer, J., Latyszenok, V., Gouarné, C.
Raoul, C., Pruss, R.M., Bordet, T. and Pettmann, B. (2012) Olesoxime delays muscle denervation, astrogliosis, microglial activation and motoneuron death in an ALS mouse model Neuropharmacology, 62, 2345-2352
Szelechowski, M., Amoedo, N., Obre, E., Léger, C., Allard, L., Bonneu, M., Claverol, S., Lacombe, D. et al (2018) Metabolic reprogramming in amyotrophic lateral sclerosis Sci. Rep., 8: 3953
Tadi V., Adam, A., Goldhammer, N., Lautenschlaeger, J., Oberstadt, M., Malci, A., Le, T.T., Sengupta, S., Stubendorff, B. et al (2019) Investigation of mitochondrial calcium uniporter role in embryonic and adult motor neurons from G93AhSOD1 mice Neurobiol. Aging, 75, 210- 222
Tadić, V., Malsi, A., Goldhammer, N., Stubendorff, B., Sengupts, S., Prell, T., Keiner, S. et al (2017) Sigma 1 receptor activityion modifies intracellular calcium exchange in the G93AHSOD1 ALS model Neuroscience, 359, 105–118
Van Damme, P., Braeken, D., Callewaert, G., Robberecht, W. and Van Den Bosch, L. (2005) GluR2 deficiency accelerates motor neuron degeneration in a mouse model of amyotropic lateral sclerosis J. Neuropathol. Exp. Neurol., 64, 605-612
Vargas, M.R., Johnson, D.A. and Johnson, J.A. (2011) Decreased glutathione accelerates neurological deficit and mitochondrial pathology in familial ALS-linked hSOD1G93A mice model Neurobiol. Dis., 43, 543–551
Andermann syndrome
Bowerman, M., Salsac, C., Bernard, V., Soulard, C., Dionne, A., Coque, E., Benlefki, S., Hince, P. and Dion, P.A. (2017) KCC3 loss-of-function contributes to Andermann syndrome by inducing activity-dependent neuromuscular junction defects Neurobiol. Dis., 106, 35–48
Antioxidants
Bahia, P.K., Pugh, V., Hoyland, K., Hensley, V., Rattray, M. and Williams, R.J. (2012) Neuroprotective effects of phenolic antioxidant tBHQ associate with inhibition of Fox03a nuclear translocation and activity J. Neurochem., 123, 182–191
Apoptosis
Palandri, A., Salvador, V.R., Wojnacki, J., Vivinetto, A.L., Schnaar, R.L. and Lopez, P.H.H. (2015) Myelinassociated glycoprotein modulates apoptosis of motoneurons during early postnatal development via NgR/p75NTR receptor-mediated activation of RhoA signaling pathways Cell Death Dis., 6: e1876
Raoul, C., Barthelemy, C., Couzinet, A., Hancock, D., Pettmann, B. and Hueber, A-O. (2005) Expression of a dominant negative form of Daxx in vivo rescues motoneurons from Fas (CD95)-induced cell death J. Neurobiol., 62, 178-188
Segura, M.F., Sole, C., Pascual, M., Moubarak, R.S., Perez-Garcia, M.J., Gozzelino, R., Iglesias, V., Badiola, N. et al (2007) The long form of Fas apoptotic inhibitory molecule is expressed specifically in neurons and protects them against death receptor-triggered apoptosis J. Neurosci., 27, 11228-11241
Ugolini, G., Raoul, C., Ferri, A., Haenggeli, C., Yamamoto, Y., Salaun, D., Henderson, C.E., Kato, A.C., Pettmann, B. and Hueber, A-O. (2003) Fas/tumor necrosis factor receptor death signaling is required for axotomy-induced death of motoneurons in vivo Neuroscience, 23, 8526-8531
Astrocyte effects
Diana, V., Ottolina, A., Botti, F., Fumagalli, E., Calcagno, E., De Paola, M., Cagnotto, A., Invernici, G., Parati, E., Curti, D. and Mennini, T. (2010) Neural precursor-derived astrocytes of wobbler mice induce apoptotic death of motor neurons through reduced glutamate uptake Exp. Neurol., 225, 163–172
Vargas, M.R., Johnson, D.A., Sirkis, D.W., Messing, A. and Johnson, J.A. (2008) Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis J. Neurosci., 28, 13574 –13581
Axonal degeneration
Benzina, O., Cloitre, T., Martin, M., Raoul, C., Gergely, C. and Scamps, F. (2014) Morphology and intrinsic excitability of regenerating sensory and motor neurons grown on a line micropattern PLoS One, 9: e110687
Ca2+ regulation
Barneo-Muñoz, M., Juárez1, P., Civera-Tregón, A., Yndriago, L., Pla-Martin, D., Zenker, J., Cuevas-Martín, C., Estela, A. et al (2015) Lack of GDAP1 induces neuronal calcium and mitochondrial defects in a knockout mouse model of Charcot-Marie-Tooth neuropathy PLoS Genet., 11: e1005115
Gou-Fabregas, M., Garcera, A., Mincheva, S., Perez-Garcia, M.J., Comella, J.X. and Soler, R.M. (2009) Specific vulnerability of mouse spinal cord motoneurons to membrane depolarization J. Neurochem., 110, 1842- 1854
Langou, K., Moumen, A., Pellegrino, C., Aebischer, J., Medina, I., Aebischer, P. and Raoul, C. (2010) AAVmediated expression of wild-type and ALS-linked mutant VAPB selectively triggers death of motoneurons through a Ca2+-dependent pathway J. Neurochem., 114, 795–809
Rainey-Smith, S.R., Andersson, D.A., Williams, R.J. and Rattray, M. (2010) Tumour necrosis factor alpha induces rapid reduction in AMPA receptor-mediated calcium entry in motor neurons by increasing cell surface expression of the GluR2 subunit: relevance to neurodegeneration J. Neurochem., 113, 692–703
Rodriguez-Garcia, A., Rojo-Ruiz, J., Navas-Navarro, P., Aulestia, F.J., Gallego-Sandin, S., Garcia-Sancho, J. and Alonso, M.T. (2014) GAP, an aequorin-based fluorescent indicator for imaging Ca2+ in organelles Proc. Natl. Acad. Sci. USA, 111, 2584–2589
Rousset, M., Cens, T., Menard, C., Bowerman, M., Bellis, M., Brusés, J., Raoul, C., Scamps, F. and Charnet, P. (2015) Regulation of neuronal high-voltage activated CaV2 Ca2+ channels by the small GTPase RhoA Neuropharmacology 97 (2015) 201e209
Charcot-Marie-Tooth (see also “Mouse spinal cord (adult)”) Barneo-Muñoz, M., Juárez1, P., Civera-Tregón, A., Yndriago, L., Pla-Martin, D., Zenker, J., Cuevas-Martín,
C., Estela, A. et al (2015) Lack of GDAP1 induces neuronal calcium and mitochondrial defects in a knockout mouse model of Charcot-Marie-Tooth neuropathy PLoS Genet., 11: e1005115
Jacquier, A., Delorme, C., Belotti, E., Juntas-Morales, R., Solé, G., Dubourg, O., Giroux, M., Maurage, C-A., Castellani, V. et al (2017) Cryptic amyloidogenic elements in mutant NEFH causing Charcot-Marie-Tooth 2 trigger aggresome formation and neuronal death Acta Neuropathol. Comm., 5: 55
Cytokines
De Paola, M., Visconti, L., Vianello, E., Mattana, F., Banfi, G., Corsi, M.M., Beghi, E. and Mennini, T. (2011) Circulating cytokines and growth factors in professional soccer players: correlation with in vitro-induced motor neuron death Eur. J. Neurol., 18, 85–92
Mir, M., Asesnio, V.J., Tolosa, L., Gou-Fabregas, M., Soler, R.M., Lladó, J. and Olmos, G (2009) Tumor necrosis factor alpha and interferon gamma cooperatively induce oxidative stress and motoneuron death in rat spinal cord embryonic explants Neuroscience 162, 959–971
Degeneration/ER stress
Bernard-Marissal, N., Médard, J-J., Azzedine, H. and Chrast, R. (2015) Dysfunction in endoplasmic reticulum mitochondria crosstalk underlies SIGMAR1 loss of function mediated motor neuron degeneration Brian, 138, 875–890
Montague, K., Malik, B., Gray, A.L., La Spada, A.R., Hanna, M.G., Szabadkai, G. and Greensmith, L. (2014) Endoplasmic reticulum stress in spinal and bulbar muscular atrophy: a potential target for therapy Brain, 137, 1894–1906
Drug delivery
Mauri, E., Veglianese, P., Papa, S., Mariani, A., De Paola, M., Rigamonti, R., Chincarini, G.M.F., Vismara, I. et al (2017) Double conjugated nanogels for selective intracellular drug delivery RSC Adv., 7, 30345-30356
Facial nerve injury
Greene, J.J., McClendon, M.T., Stephanopoulos, N.S., Álvarez, Z., Stupp, S.I., Richter, C-P. (2018) Electrophysiological assessment of a peptide amphiphile nanofiber nerve graft for facial nerve repair J. Tissue Eng. Regen. Med., 12, 1389–1401
Glutamate, effects of
Hounoum, B.M., Blasco, H., Coque, E., Vourc’h, P., Emond, P., Corcia, P., Andres, C.R., Raoul, C. and Mavel, S. (2018) The metabolic disturbances of motoneurons exposed to glutamate Mol. Neurobiol., 55, 7669– 7676
Growth factors
De Paola, M., Visconti, L., Vianello, E., Mattana, F., Banfi, G., Corsi, M.M., Beghi, E. and Mennini, T. (2011) Circulating cytokines and growth factors in professional soccer players: correlation with in vitro-induced motor neuron death Eur. J. Neurol., 18, 85–92
Payne, A.M., Zheng, Z., Messi, M.L., Milligan, C.E., Gonzalez, E. and Delbono, O. (2006) Motor neurone targeting of IGF-1 prevents specific force decline in ageing mouse muscle J. Physiol., 570, 283-294
Inflammasomes
Ydens, E., Demon, D., Lornet, G., De Winter, V., Timmerman, V., Lamkanfi, M. and Janssens, S. (2015) Nlrp6 promotes recovery after peripheral nerve injury independently of inflammasomes J. Neuroinflam., 12: 143
Methodology
De Paola, M., Diana, V., Bigini, P. and Mennini, T. (2008) Morphological features and responses to AMPA receptor-mediated excitotoxicity of mouse motor neurons: comparison in purified, mixed anterior horn or motor neuron/glia cocultures J. Neurosci. Meth., 170, 85-95
Fallini, C., Bassell, G.J. and Rossoll, W. (2010) High-efficiency transfection of cultured primary motor neurons to study protein localization, trafficking, and function Mol. Neurodegener., 5:17
Faron-Górecka, A., Szlachta, M., Kolasa, M., Solich, J., Górecki, A., Kuśmider, M., Zurawek, D. and Dziedzicka-Wasylewska, M. (2019) Understanding GPCR dimerization in Methods in Cell Biology, 149, 155- 178
Guettier-Sigrist, S., Appert-Collin, A., Duong, F., Azzouz, M., Coupin, G., Gies, J-P. and Poindron, P. (2005) Improved methods for culturing rat and mouse motor neurons Bio Valley Monogr. Basel Karger, 1, 96-108
Hoque, R., Farooq, A., Ghani, A., Gorelick, F. and Mehal, W.Z. (2014) Lactate reduces liver and pancreatic injury in Toll-like receptor-and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity Gastroenterology, 146, 1763–1774
Wang, W., Qi, B., Lv, H., Wu, F., Liu, L., Wang, W., Wang, Q., Hu, L., Hao, Y. and Wang, Y. (2017) A new method of isolating spinal motor neurons from fetal mouse J. Neurosci. Meth., 288, 57–61
Micro-RNA expression
Yardeni, T. and Hornstein, E. (2017) Protocol for high-content screening for the impact of overexpressed microRNAs on primary motor neurons Neuromethods (2017) 128: 11–19
Yardeni, T., Fine, R., Joshi, Y., Gradus-Pery, T., Kozer, N., Reichenstein, I., Yanowski, E., Nevo, S., WeissTishler, H. et al (2018) High content image analysis reveals function of miR-124 upstream of Vimentin in regulating motor neuron mitochondria Sci. Rep., 8: 59
Muscular atrophy
Malik, B., Devine, H., Patani, R., La Spada, A.R., Hanna, M.G. and Greensmith, L. (2019) Gene expression analysis reveals early dysregulation of disease pathways and links Chmp7 to pathogenesis of spinal and bulbar muscular atrophy Sci. Rep., 9: 3539
Neuronal culture
Ito, K., Ohkawara, B., Yagi, H., Nakashima, H., Tsushima, M., Ota, K., Konishi, H., Masuda, A., Imagama, S., Kiyama, H., Ishiguro, N. and Ohno, K. (2018) Lack of Fgf18 causes abnormal clustering of motor nerve terminals at the neuromuscular junction with reduced acetylcholine receptor clusters Sci. Rep., 8: 434
Neurotrophic factors
Appert-Collin, A., Hugel, B., Levy, R., Niederhoffer, N., Coupin, G., Lombard, Y., André, P., Poindron, P. and Gies, J.P. (2006) Cyclin dependent kinase inhibitors prevent apoptosis of postmitotic mouse motoneurons Life Sci., 79, 484-490
Duong, F.H.T., Warter, J.M., Poindron, P. and Passilly, P. (1999) Effect of the nonpeptide neurotrophic compound SR57746A on the phenotypic survival of purified mouse motoneurons Br. J. Pharmacol., 128, 1385- 1392
Mincheva, S., Garcera, A., Gou-Fabregas, M., Encinas, M., Dolcet, X. and Soler, R.M. (2011) The canonical nuclear factor-B pathway regulates cell survival in a developmental model of spinal cord motoneurons J. Neurosci., 31, 6493– 6503
Nitric oxide
Bishop, A., Gooch, R., Eguchi, A., Jeffrey, S., Smallwood, L., Anderson, J. and Estevez, A.G. (2009) Mitigation of peroxynitrite-mediated nitric oxide (NO) toxicity as a mechanism of induced adaptive NO resistance in the CNS J. Neurochem., 109, 74-84
Bishop, A., Green Hobbs, K., Eguchi, A., Jeffrey, S., Smallwood, L., Pennie, C., Anderson, J. and Estevez, A.G. (2009) Differential sensitivity of oligodendrocytes and motor neurons to reactive nitrogen species: implications for multiple sclerosis J. Neurochem., 109, 93-104
Lee, J., Ryu, H. and Kowall, N.W. (2009) Differential regulation of neuronal and inducible nitric oxide synthase (NOS) in the spinal cord of mutant SOD1 (G93A) ALS mice Biochem. Biophys. Res. Commun., 387, 202–206
Programmed cell death
Aebischer, J., Sturny, R., Andrieu, D., Rieusset, A., Schaller, F., Geib, S., Raoul, C. and Muscatelli, F. (2011) Necdin protects embryonic motoneurons from programmed cell death PloS One 6: e23764
Receptors
Barak, R., Yom-Tov, G., Guez-Haddad, J., Gasri-Plotnitsky, L., Maimon, R., Cohen-Berkman, M., McCarthy, A.A., Perlson, E., Henis-Korenblit, S., Isupov, M.N. and Opatowsky, Y. (2019) Structural principles in Robo activation and auto-inhibition Cell, 177, 272–285
Spinal muscular atrophy (see also “Zebrafish”)
Arumugam, S., Mincheva-Tasheva, S., Periyakaruppiah, A., de la Fuente, S., Soler, R.M. and Garcera, A. (2018) Regulation of survival motor neuron protein by the nuclear factor-Kappa B pathway in mouse spinal cord motoneurons Mol. Neurobiol., 55, 5019–5030
Chen, Y-C., Chang, J-G., Jong, Y-J., Liu, T-Y. and You, C-Y. (2015) High expression level of Tra2-β1 is responsible for increased SMN2 exon 7 inclusion in the testis of SMA mice PLoS One 10: e0120721
Martin, J.E., Nguyen, TK.T., Grunseich, C., Nofziger, J.H., Lee, P.R., Fields, D., Fischbeck, K.H. and Foran. E. (2017) Decreased motor neuron support by SMA astrocytes due to diminished MCP1 secretion J. Neurosci., 37, 5309-5318
Miller, N., Feng, Z., Edens, B.M., Yang, B., Shi, H., Sze, C.C., Hong, B.T. Su, S.C. et al (2015) Nonaggregating tau phosphorylation by cyclin-dependent kinase 5 contributes to motor neuron degeneration in spinal muscular atrophy J. Neurosci., 35, 6038–6050
Miller, N., Shi, H., Zelikovich, A.S. and Ma, Y-C. (2016) Motor neuron mitochondrial dysfunction in spinal muscular atrophy Hum.Mol. Genet., 25, 3395–3406
Montague, K., Malik, B., Gray, A.L., La Spada, A.R., Hanna, M.G., Szabadkai, G. and Greensmith, L. (2014) Endoplasmic reticulum stress in spinal and bulbar muscular atrophy: a potential target for therapy Brain, 137, 1894–1906
Superoxide dismutase
Aebischer, J., Cassina, p., Otsmane, b., Moumen, A., Seilhean, D., Meininger, V., Barbeito, L., Pettmann, B. and Raoul, C. (2011) IFNγ triggers a LIGHT-dependent selective death of motoneurons contributing to the noncell-autonomous effects of mutant SOD1 Cell Death Differ. 18, 754–768
Bernard-Marissal, N., Moumen, A., Sunyach, C., Pellegrino, C., Dudley, K., Henderson, C.E., Raoul, C. and Pettmann, B. (2012) Reduced calreticulin levels link endoplasmic reticulum stress and Fas-triggered cell death in motoneurons vulnerable to ALS J. Neurosci., 32, 4901– 4912
Fischer, L.R., Igoudjil, A., Magrane, J., Li, Y., Hansen, J.M., Manfredi, G. and Glass, J.D. (2011) SOD1 targeted to the mitochondrial intermembrane space prevents motor neuropathy in the Sod1 knockout mouse Brain, 134, 196–209
Lautenschläger, J., Prell, T., Ruhmer, J., Weidemann, L., Witte, O.W., Grosskreutz, J. (2013) Overexpression of human mutated G93A SOD1 changes dynamics of the ER mitochondria calcium cycle specifically in mouse embryonic motor neurons Exp. Neurol., 247, 91–100
Lee, J., Ryu, H. and Kowall, N.W. (2009) Motor neuronal protection by L-arginine prolongs survival of mutant SOD1(G93A) ALS mice Biochem. Biophys. Res. Comm., 384, 524–529
Lee, J.K., Shin, J.H., Hwang, S.G., Gwag, B.J., McKee, A.C., Lee, J, Kowall, N.W., Ryu, H., Lim, D-S. and Choi, E-J. (2013) MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS Proc. Natl. Acad. Sci. USA, 110, 12066–12071
Lunn, J.S., Sakowski, S.A., Kim, B., Rosenberg, A.A. and Feldman, E.L. (2009) Vascular endothelial growth factor prevents G93A-SOD1-induced motor neuron degeneration Develop. Neurobiol., 69, 871-884
Prell, T., Lautenschlager, J., Witte, O.W., Carri, M.T. and Grosskreutz, J. (2012) The unfolded protein response in models of humabn mutant G93A amyotrophic lateral sclerosis Eur. J. Neurosci., 35, 652-660
Raoul, C., Estévez, A.G., Nishimune, H., Cleveland, D.W., de Lapeyrière. O., Henderson, C.E., Haase, G. and Pettmann, B. (2002) Motoneuron death triggered by a specific pathway downstream of Fas: Potentation by ALS-linked SOD1 mutations Neuron, 35, 1067-1083
Sunyach, C., Michaud, M., Arnoux, T., Bernard-Marissal, N., Aebischer, J., Latyszenok, V., Gouarné, C. Raoul, C., Pruss, R.M., Bordet, T. and Pettmann, B. (2012) Olesoxime delays muscle denervation, astrogliosis, microglial activation and motoneuron death in an ALS mouse model Neuropharmacology, 62, 2345-2352
Vargas, M.R., Johnson, D.A. and Johnson, J.A. (2011) Decreased glutathione accelerates neurological deficit and mitochondrial pathology in familial ALS-linked hSOD1G93A mice model Neurobiol. Dis., 43, 543–551
Survival
Garcera, A., Mincheva, S., Gou-Fabregas, M., Caraballo-Miralles, V., Lladó, J., Comella, J.X. and Soler, R.M. (2011) A new model to study spinal muscular atrophy: Neurite degeneration and cell death is counteracted by BCL-XL overexpression in motoneurons Neurobiol. Dis., 42, 415–426
Wang, P-Y., Koishi, K. and McLennan, I.S. (2007) BMP6 is axonally transported by motoneurons and supports their survival in vitro Mol. Cell Neurosci., 34, 653-661
Zhang, H., Xing, L., Rossoll, W., Wichterle, H., Singer, R.H. and Bassell, G.J. (2006) Multiprotein complexes of the survival of motor neuron protein SMN with gemins traffic to neuronal processes and growth cones of motor neurons J. Neurosci., 26, 8622-8632
Synaptic differentiation
Misgeld, T., Kummer, T.T., Lichtman, J.W. and Sanes, J.R. (2005) Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter Proc. Natl. Acad. Sci. USA, 102, 11088-11093
Toll-like receptors
Goethals, S., Ydens, E., Timmerman, V. and Janssens, S. (2010) Toll-like receptor expression in the peripheral nerve Glia, 58, 1701–1709
Tyrosine kinase B
Zahavi, E.E., Steinberg, N., Altman, T., Chein, M., Joshi, Y., Gradus-Pery, T. and Perlson, E. (2018) The receptor tyrosine kinase TrkB signals without dimerization at the plasma membrane Sci. Signal. 11: eaao4006
Virus receptor transport
Zussy, C. and Salinas, S. (2014) Study of adenovirus and CAR axonal transport in primary neurons In Methods Mol. Biol., 1089, Adenovirus: Methods and Protocol (ed. Chillón, M. and Bosch, A.) Springer Science+Business Media, LLC pp 71-78
Mouse spinal cord (neo-natal)
Pavelko, K., Howe, C.L., Drescher, K.M., Gamez, J.D., Johnson, A.J., Wei, T., Ransohoff, R.M. and Roriguez, M. (2003) Interleukin-6 protects anterior horn neurons from lethal virus-induced injury J. Neurosci., 23, 481- 492
Mouse spinal cord (post-natal)
Benedusi, V., Martorana, F., Brambilla, L., Maggi, A. and la Rossi, D. (2012) The peroxisome proliferatoractivated receptor (PPAR) controls natural protective mechanisms against lipid peroxidation in amyotrophic lateral sclerosis J. Biol. Chem., 287, 35899–35911
Foley, L.S., Fullerton, D.A., Mares, J., Sungelo, M., Weyant, M.J., Cleveland, Jr, J.C. and Reece, T.B. (2017) Erythropoietin’s beta common receptor mediates neuroprotection in spinal cord neurons Ann. Thorac. Surg. 104, 1909–1914
Freeman, K.A., Puskas, F., Bell, M.T., Mares, J.M., Foley, L.S., Weyant, M.J., Cleveland, J.C. et al (2015) Alpha-2 agonist attenuates ischemic injury in spinal cord neurons J. Surg. Res., 195, 21-28
Häring, M., Zeisel, A., Hochgerner, H., Rinwa, P., Jakobsson, J.E.T., Lönnerberg, P., La Manno, G. Sharma, N. et al (2018) Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types Nat. Neurosci., 21, 869–880
Kempf, A., Montani, L., Petrinovic, M.M., Schroeter, A., Weinmann, O., Patrignani, A. and Schwab, M.E. (2013) Upregulation of axon guidance molecules in the adult central nervous system of Nogo-A knockout mice restricts neuronal growth and regeneration Eur. J. Neurosci., 38, 3567–3579
Lee, S., Ives, A.M. and Bertke, A.S. (2015) Herpes simplex virus 1 reactivates from autonomic ciliary ganglia independently from sensory trigeminal ganglia to cause recurrent ocular disease J. Virol., 89, 8383-8391
Milligan, C. and Gifondorwa, D. (2011) Isolation and culture of postnatal spinal motoneurons In Methods Mol. Biol., 793, Neurodegeneration: Methods and Protocols (ed. Manfredi, G. and Kawamata, H.) Springer Science+Business Media, LLC Xu, J., Huang, G., Zhang, K., Sun, J., Xu, T., Li, R., Tao, H. and Xu, W. (2014) Nrf2 activation in astrocytes contributes to spinal cord ischemic tolerance induced by hyperbaric oxygen preconditioning J. Neuotrauma, 31, 1343–1353
Mouse thoracic ganglia
Furlan, A., La Manno, G., Lübke, M., Häring, M., Abdo, H., Hochgerner, H., Kupari, J., Usoskin, D. et al (2016) Visceral motor neuron diversity delineates a cellular basis for nipple- and pilo-erection muscle control Nature Neurosci., 19, 1331-1340
Rat brain amygdala
Jasnow, A.M., Ressler, K.J., Hammack, S.E., Chhatwal, J.P. and Rainnie, D.G. (2009) Distinct subtypes of cholecystokinin (CCK)-containing interneurons of the basolateral amygdala identified using a CCK promoterspecific lentivirus J. Neurophysiol., 101, 1494–1506
Rat brain cerebellar
Martinez, J., Stessin, A.M., Campana, A., Hou, J., Nikulina, E., Buck, J., Levin, L.R. and Filbin, M.T. (2014) Soluble adenylyl cyclase is necessary and sufficient to overcome the block of axonal growth by myelinassociated factors J. Neurosci., 34, 9281–9289
Rat brain cortex (adult)
Jones, T.T. and Brewer, G.J. (2009) Critical age-related loss of cofactors of neuron cytochrome C oxidase reversed by estrogen Exp. Neurol., 215, 212–219
Jones, T.T. and Brewer, G.J. (2010) Age-related deficiencies in complex I endogenous substrate availability and reserve capacity of complex IV in cortical neuron electron transport Biochim. Biophys. Acta, 1797, 167– 176
Martin-Montañez , E., Pavia, J., Santin, L.J., Boraldi, F., Estivill-Torrus, G., Aguirre, J.A. and GarciaFernandez, M. (2014) Involvement of IGF-II receptors in the antioxidant and neuroprotective effects of IGF-II on adult cortical neuronal cultures Biochim. Biophys. Acta, 1842, 1041–1051
Martín-Montañez, E., Millon, C., Boraldi, F., Garcia-Guirado, F., Pedraza, C., Lara, E., Santin, L.J., Pavia, J. and Garcia-Fernandez, M. (2017) IGF-II promotes neuroprotection and neuroplasticity recovery in a longlasting model of oxidative damage induced by glucocorticoids Redox Biol., 13, 69–81
McCormick, A.M., Maddipatla, M.V.S.N., Shi, S., Chamsaz, E.A, Yokoyama, H., Joy, A. and Leipzig, N.D. (2014) Micropatterned coumarin polyester thin films direct neurite orientation ACS Appl. Mater. Interfaces, 6, 19655−19667
Patel, J.R. and Brewer, G. J. (2008) Age-related differences in NFκB translocation and Bcl-2/Bax ratio caused by TNFα and Abeta42 promote survival in middle-age neurons and death in old neurons Exp. Neurol., 213, 93- 100
Peterson, S.L., Nguyen, H.X., Mendez, O.A. and Anderson, A.J. (2017) Complement protein C3 suppresses axon growth and promotes neuron loss Sci. Rep., 7: 12904
Singh, A.K., Jiang, Y., Gupta, S., Younus, M. and Ramzan, M. (2013) Anti-inflammatory potency of nanoformulated puerarin and curcumin in rats subjected to the lipopolysaccharide-induced inflammation J. Med. Food, 16, 899–911
Viel, J.J., McManus, D.Q., Brewer, G.J. (2004) Postmortem effect of pentobarbital anesthetic on survival of adult cortical neurons in primary culture Brain Res., 1009, 219-222
Yip, P.K., Wong, L-F., Pattinson, D., Battaglia, A., Grist, J., Bradbury, E.J., Maden, M., McMahon, S.B. and Mazarakis, N.D. (2006) Lentiviral vector expressing retinoic acid receptor β2 promotes of function after corticospinal tract injury in the adult rat spinal cord Hum. Mol. Genet., 15, 3107-3118
Yip, P.K., Wong, L-F., Sears, T.A., Yáñez-Muñoz, R.J. and McMahon, S.B. (2010) Cortical overexpression of neuronal calcium sensor-1 induces functional plasticity in spinal cord following unilateral pyramidal tract injury in rat PLoS Biol., 8: e1000399
Rat brain cortex (embryo)
Cheng, Y.C., Chen, T-A., Chen, C-Y., Liang, C.M. and Liang, S-M. (2012) 3′Poly-G-tailed ODNs inhibit Fspondin to induce cell death and neurite retraction in rat embryonic neurons Mol. Neurobiol., 45, 536–549
Rat brain cortex (neo-natal)
De Vellis, J., Ghiani, C.A., Wanner, I.B. and Cole, R. (2010) Preparation of normal and reactive astrocyte cultures In, Protocols for Neural Cell Culture, Springer Protocols Handbooks, (ed. Doering, L.C.), Humana Press (Springer Science+Business Media), Totowa, NJ. pp 193-215
Peterson, S.L., Nguyen, H.X., Mendez, O.A. and Anderson, A.J. (2017) Complement protein C3 suppresses axon growth and promotes neuron loss Sci. Rep., 7: 12904
Rat brain cortex (post-natal)
Foret, M.K., Do Carmo, S., Lincoln, R., Greene, L.E., Zhang, W., Cuello, A.C. and Cosa, G. (2019) Effect of antioxidant supplements on lipid peroxidation levels in primary cortical neuron cultures Free Radical Biol. Med., 130, 471–477
Hannila, S.S., Siddiq, M.M., Carmel, J.B., Hou, J., Chaudhry, N., Bradley, P.M.J., Hilaire, M. Richman, E.L., Hart, R.P. and Filbin, M.T. (2013) Secretory leukocyte protease inhibitor reverses inhibition by CNS myelin, promotes regeneration in the optic nerve, and suppresses expression of the transforming growth factor-β
signaling protein Smad2 J. Neurosci., 33, 5138 –5151
He, H., Deng, K., Siddiq, M.M., Pyie, A., Mellado, W., Hannila, S.S. and Filbin, M.T. (2016) Cyclic AMP and polyamines overcome inhibition by myelin-associated glycoprotein through eIF5A-mediated increases in p35 expression and activation of Cdk5 J. Neurosci., 36, 3079 –3091
Khankan, R.R., Wanner, I.B. and Phelps, P.E. (2015) Olfactory ensheathing cell–neurite alignment enhances neurite outgrowth in scar-like cultures Exp. Neurol., 269, 93–101
Khankan. R.R., Griffis, K.G., Haggerty-Skeans, J.R., Zhong, H., Roy, R.R., Edgerton, V.R. and Phelps, P. (2016) Olfactory ensheathing cell transplantation after a complete spinal sord transection mediates neuroprotective and immunomodulatory mechanisms to facilitate regeneration J. Neurosci., 36, 6269-6286 Piroli, G.G., Manuel, A.M., Patel, T., Walla, M.D., Shi, L., Lanci, S.A., Wang, J., Galloway, A. et al (2019)
Identification of novel protein targets of dimethyl fumarate modification in neurons and astrocytes reveals actions independent of Nrf2 stabilization Mol. Cell. Proteom., 18, 504–519
Siddiq, M.M., Hannila, S.S., Carmel, J.B., Bryson, J.B., Hou, J., Nikulina, E., Willis, M.R., Mellado, W. et al (2015) Metallothionein-I/II promotes axonal regeneration in the central nervous system J. Biol. Chem., 290, 16343–16356
Rat brain stem
Evans, J., Sumners, C., Moore, J., Huentelmann, M.J., Deng, J., Gelband, C.H. and Shaw, G. (2002) Characterization of mitotic neurons derived from adult rat hypothalamus and brain stem J. Neurophysiol., 87, 1076-1085
Hernandez-Morato, I., Pitman, M.J. and Sharma, S. (2016) Muscle specific nucleus ambiguus neurons isolation and culturing J. Neurosci. Meth., 273, 33–39
Rat brain dentate gyrus
Howell, O.W., Doyle, K., Goodman, J,.H., Scharfman, H.E., Herzog, H., Pringle, A., Beck-Sickinger, A. G. and Gray, W.P. (2005) Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus J. Neurochem., 93, 560-570
Rat brain hippocampus (adult)
Cady, C., Evans, M.S. and Brewer, G.J. (2001) Age-related differences in NMDA responses in cultured rat hippocampal neurons Brain Res., 921, 1-11
Edwards, D., Das, M., Molnar, P. and Hickman, J.J. (2010) Addition of glutamate to serum-free culture promotes recovery of electrical activity in adult hippocampal neurons in vitro J. Neurosci. Methods, 190, 155–163
Gamerdinger, M., Hajieva1, P., Kaya1, A.M., Wolfrum, U., Hartl, F.U. and Behl, C. (2009) Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3 EMBO J., 28, 889–901
Hajieva, P., Kuhlmann, C., Luhmann, H.K. and Behl, C. (2009) Impaired calcium homeostasis in aged hippocampal neurons Neurosci. Lett., 451, 119–123
Jones, T.T. and Brewer, G.J. (2009) Critical age-related loss of cofactors of neuron cytochrome C oxidase reversed by estrogen Exp. Neurol., 215, 212–219
Jones, T.T. and Brewer, G.J. (2010) Age-related deficiencies in complex I endogenous substrate availability and reserve capacity of complex IV in cortical neuron electron transport Biochim. Biophys. Acta, 1797, 167–176
Kour, S. and Rath, P.C. (2016) All-trans retinoic acid induces expression of a novel intergenic long noncoding RNA in adult rat primary hippocampal neurons J. Mol. Neurosci., 58, 266-276
Kour, S. and Rath, P.C. (2017) Age-related expression of a repeat-rich intergenic long noncoding RNA in the rat brain Mol. Neurobiol., 54, 639–660
Majd, S., Rastegar, K., Zarifkar, A. and Takhshid, M.A. (2007) Fibrillar beta-amyloid (Aβ) elevates extracellular Aβ in cultured hippocampal neurons of adult rats Brain Res., 1185, 321-327
Majd, S., Zarifkar, A., Rastegar, K. and Takhshid, M.A. (2008) Culturing adult rat hippocampal neurons with long-interval changing media Iran. Biomed. J., 12, 101-107
Majd, S., Zarifkar, A., Rastegar, K. and Takhshid, M.A. (2008) Different fibrillar Aβ 1-42 concentrations induce adult hippocampal neurons to reenter various phases of the cell cycle Brain Res., 1218, 224-229
Majd, S., Chegini, F., Chataway, T., Zhou, X-F. and Gai, W, (2013) Reciprocal induction between α-synuclein and β-amyloid in adult rat neurons Neurotox. Res., 23, 69–78
Marks, J.D., Boriboun, C. and Wang, J. (2005) Mitochondrial nitric oxide mediates decreased vulnerability of hippocampal neurons from immature animals to NMDA J. Neurosci., 25, 6561-6575
Ray, B., Bailey, J.A. Sarkar, S. and Lahiri, D.K. (2009) Molecular and immunocytochemical characterization of primary neuronal cultures from adult rat brain: Differential expression of neuronal and glial protein markers J. Neurosci. Methods, 184, 294–302
Vasko, M.R., Guo, C. and Kelley, M.R. (2005) The multifunctional DNA repair/redox enzyme Apel/Ref-1 promotes survival of neurons after oxidative stress DNA Repair, 4, 367-379
Vasko, M.R., Guo, C., Thompson, E.L. and Kelley, M.R. (2011) The repair function of the multifunctional DNA repair/redox protein APE1 is neuroprotective after ionizing radiation DNA Repair, 10, 942– 952
Wang, S., Huang, G., Wang, Y., Huang, T., Lin, S. and Gu, J. (2013) Up-regulation of immunoglobulin G gene expression in the hippocampus of rats subjected to acute immobilization stress J. Neuroimmunol., 258, 1–9
Zurawek, D., Gruca, P., Antkiewicz-Michaluk, L. and Dziedzicka-Wasylewska, M. (2019) Resilient phenotype in chronic mild stress paradigm is associated with altered expression levels of miR-18a-5p and serotonin 5- HT1a receptor in dorsal part of the hippocampus Mol. Neurobiol., 56, 7680–7693
Rat brain hippocampus (embryo)
Cheng, Y.C., Chen, T-A., Chen, C-Y., Liang, C.M. and Liang, S-M. (2012) 3′Poly-G-tailed ODNs inhibit Fspondin to induce cell death and neurite retraction in rat embryonic neurons Mol. Neurobiol., 45, 536–549
Henriquez, B., Bustos, F.J., Aguilar, R., Becerra, A., Simon, F., Montecino, M. and van Zundert, B. (2013) Ezh1 and Ezh2 differentially regulate PSD-95 gene transcription in developing hippocampal neurons Mol. Cell. Neurosci., 57, 130–143
Rat brain hippocampus (juvenile)
Gamerdinger, M., Hajieva1, P., Kaya1, A.M., Wolfrum, U., Hartl, F.U. and Behl, C. (2009) Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3 EMBO J., 28, 889–901
Rat brain hippocampus (neo-natal)
Choi, S.J., Kim, F., Schwartz, M.W. and Wisse, B.E. (2010) Cultured hypothalamic neurons are resistant to inflammation and insulin resistance induced by saturated fatty acids Am. J. Physiol. Endocrinol. Metab., 298, E1122–E1130
Ehrenreich, H., Degner, D., Meller, J., Brines, M., Béhe, M., Hasselblatt, M., Woldt, H., Falkai, P. et al (2004) Erythropoietin: a candidate compound for neuroprotection in schizophrenia Mol. Psychiatry., 9, 42-54
Fester, L., Zhou, L., Bütow, A., Huber, C., von Lossow, R., Prange-Kiel, J., Jarry, H. and Rune, G.M. (2009) Cholesterol-promoted synaptogenesis requires the conversion of chelosterol to estradiol in the hippocampus Hippocampus, 19, 692-705
Fordyce, C.B., Jagasia, R., Zhu, X. and Schlichter, L.C. (2005) Microglia Kv1.3 channels contribute to their ability to kill neurons J. Neurosci., 25, 7139-7149
Howell, O.W., Scharfman, H.E., Herzog, H., Sundstrom, L.E., Beck-Sickinger, A and Gray, W.P. (2003) Neuropeptide Y is neuroproliferative for hippocampal precursor cells J. Neurochem., 86, 646-659
Howell, O.W., Doyle, K., Goodman, J,.H., Scharfman, H.E., Herzog, H., Pringle, A., Beck-Sickinger, A. G. and Gray, W.P. (2005) Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus J. Neurochem., 93, 560-570
Kretz, O., Fester, L., Wehrenberg, U., Zhou, L., Brauckmann, S., Zhao, S., Prange-Kiel, J., Naumann, T., Jarry, H., Frotscher, M. and Rune, G. M. (2004) Hippocampal synapses depend on hippocampal estrogen synthesis J. Neurosci., 24, 5913-5921
Liu, Y., Ford, B., Mann, M.A. and Fischbach, G.D. (2001) Neuregulins increase α7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus J. Neurosci., 21, 5660-5669
Li, Q., Lau, A., Morris, T.J., Guo, L., Fordyce, C.B. and Stanley, E.F. (2004) A syntaxin 1, Go, and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization J. Neurosci., 24, 4070-4081
Marks, J.D., Bindokas, V.P. and Zhang, X-M. (2001) Maturation of vulnerability to excitotoxicity: intracellular mechanisms in cultured postnatal hippocampal neurons Dev. Brain Res., 124, 101-116
Marks, J.D., Boriboun, C. and Wang, J. (2005) Mitochondrial nitric oxide mediates decreased vulnerability of hippocampal neurons from immature animals to NMDA J. Neurosci., 25, 6561-6575
Nuñez-Villena, F., Becerra, A., Echeverría, C., Briceño, N., Porras, O., Armisén, R., Varela, D., Montorfano, I, Sarmiento, D. and Simon, F. (2011) Increased expression of the transient receptor potential melastatin 7 channel is critically involved in lipopolysaccharide-induced reactive oxygen species-mediated neuronal death Antioxid. Redox Signal., 15, 2425–2438
Qin, J., Berdyshev. E., Goya, J., Natarajan, V. and Dawson, G. (2010) Neurons and oligodendrocytes recycle sphingosine 1-phosphate to ceramide: significance for apoptosis and multiple sclerosis J. Biol. Chem., 285, 14134-14143
Von Schassen, C., Fester, L., Prange-Kiel, J., Lohse, C., Huber, C., Böttner, M. and Rune, G.M. (2006) Oestrogen synthesis in the hippocampus: role in axon outgrowth J. Neuroendocrinol., 18, 847-856
Wang, X.Q., Deriy, L.V., Foss, S., Hunag, P., Lamb, F.S., Kaetzel, M.A., Bindokas, V., Marks, J.D. and Nelson, D.J. (2006) CLC-3 channels modulate excitatory synaptic transmission in hippocampal neurons Neuron, 52, 321-333
Wong, W. and Schlichter, L.C. (2004) Differential recruitment of Kv1.4 and Kv4.2 to lipid rafts by PSD-95 J. Biol. Chem., 279, 444-452
Zhou, L., Lehan, N., Wehrenberg, U., Disteldorf, E., von Lossow, R., Mares, U., Jarry, H. and Rune, G.M. (2007) Neuroprotection by estradiol: A role of aromatase against spine synapse loss after blockade of GABAA receptors Exp. Neurol., 203, 72-81
Rat brain hippocampus (post-natal)
Bonnas, C., Wüstefeld, L., Winkler, D., Kronstein-Wiedemann, R., Dere, E. Specht, K., Boxberg, M. Tonn, T. et al (2017) EV-3, an endogenous human erythropoietin isoform with distinct functional relevance Sci. Rep., 7: 3684
Fester, L., Zhou, L., Ossig, C., Labitzke, J., Blaute, C., Bader, M., Vollmre, G., Jarry, H. and Rune, G.M. (2017) Synaptodin is regulated br aromatase actvity J. Neurochem., 140, 126–139
Hannila, S.S., Siddiq, M.M., Carmel, J.B., Hou, J., Chaudhry, N., Bradley, P.M.J., Hilaire, M. Richman, E.L., Hart, R.P. and Filbin, M.T. (2013) Secretory leukocyte protease inhibitor reverses inhibition by CNS myelin, promotes regeneration in the optic nerve, and suppresses expression of the transforming growth factor- signaling protein Smad2 J. Neurosci., 33, 5138 –5151
Khan, D., Khan, M., Runesson, J., Zaben, M. and Gray, W.P. (2017) GalR3 mediates galanin proliferative effects on postnatal hippocampal precursors Neuropeptides 63, 14–17
Siddiq, M.M., Hannila, S.S., Carmel, J.B., Bryson, J.B., Hou, J., Nikulina, E., Willis, M.R., Mellado, W. et al (2015) Metallothionein-I/II promotes axonal regeneration in the central nervous system J. Biol. Chem., 290, 16343–16356
Rat brain hypothalamus
Choi, S.J., Kim, F., Schwartz, M.W. and Wisse, B.E. (2010) Cultured hypothalamic neurons are resistant to inflammation and insulin resistance induced by saturated fatty acids Am. J. Physiol. Endocrinol. Metab., 298, E1122–E1130
Evans, J., Sumners, C., Moore, J., Huentelmann, M.J., Deng, J., Gelband, C.H. and Shaw, G. (2002) Characterization of mitotic neurons derived from adult rat hypothalamus and brain stem J. Neurophysiol., 87, 1076-1085
Malikov, V., and Madeira, M.D. (2013) Regulation of ER protein expression by 17-estradiol in cultured neurons of hypothalamic ventromedial nucleus Neurochem. Res., 38, 82–89
Rat brain mesencephalic area
Majd, S., Smardencas, A., Parish, C.L. and Drago, J. (2011) Development of an in vitro model to evaluate the regenerative capacity of adult brain-derived tyrosine hydroxylase-expressing dopaminergic neurons Neurochem. Res., 36, 967–977
Rat brain striatal
Rivera-Ramírez, N., Montejo-Lopez, W., Lopez-Mendez, M.C., Guerrero-Hernandez, A., Molina-Hernandez, A., García-Hernandez, U. and Arias-Montano, J.A. (2016) Histamine H3 receptor activation stimulates calcium mobilization in a subpopulation of rat striatal neurons in primary culture, but not in synaptosomes Neurochem. Int., 101, 38-47
Rat brain, ventral brain stem
Halum, S.L., McRae, B., Bijangi-Vishehsaraei, K. and Hiatt, K. (2012) Neurotrophic factor-secreting autologous muscle stem cell therapy for the treatment of laryngeal denervation injury Laryngoscope, 122, 2482–2496
Rat brain trigeminal ganglia
Pita-Thomas,W., Barroso-Garcia, G., Moral, V., Hackett, A.R., Cavalli, V. and Nieto-Diaz, M. (2017) Identification of axon growth promoters in the secretome of the deer antler velvet Neuroscience, 340, 333-344
Rat dorsal root ganglion cells
Smith, T.P., Smith, S.N. and Sweitzer, S.M. (2014) Endothelin-1 induced desensitization in primary afferent neurons Neurosci. Lett., 582, 59–64
Van Helleputte, L., Kater, M., Cook, D.P., Eykens, C., Rossaert, E., Haeck, W., Jaspers, T., Geens, N., Vanden Bergh, P., Gysemans, C. et al (2018) Inhibition of histone deacetylase 6 (HDAC6) protects against vincristine
induced peripheral neuropathies and inhibits tumor growth Neurobiol. Dis., 111, 59-69
Zeisel, A., Hochgerner, H., Lönnerberg, P., Johnsson, A., Memic, F., van der Zwan, J., Häring, M., Braun, E., Borm, L.E. et al (2018) Molecular architecture of the mouse nervous system Cell 174, 999–1014
Rat enterorhinal cortex
Olsen, L.C., O’Reilly, K.C., Liabakk, N.B., Witter, M.P. and Sætrom, P. (2017) MicroRNAs contribute to postnatal development of laminar differences and neuronal subtypes in the rat medial entorhinal cortex Brain Struct. Funct., 222, 3107–3126
Rat spinal cord (adult)
Alizadeh, A., Santhosh, K.T., Kataria, H., Gounni, A.S. and Karimi-Abdolrezaee, S. (2018) Neuregulin-1 elicits a regulatory immune response following traumatic spinal cord injury J. Neuroinflam., 15: 53
Brinn, M., Zhao, S., Kumaratilake, J., Lu, T-F., Freeman, B., Al-Sarawi, S. and Henneberg, M. (2015) Axon stretch growth of adult primary motor neurons J. Neurochem, 134 (Suppl. 1), 333
Chen, J., Cui, Z., Yang, S., Wu, C., Li, W., Bao, G., Xu, G., Sun, Y., Wang, L. and Zhang, J. (2017) The upregulation of annexin A2 after spinal cord injury in rats may have implication for astrocyte proliferation Neuropeptides 61, 67–76
Dey, S., Bose, S., Kumar, S., Rathore, R., Mathur, R. and Jain, S. (2017) Extremely low frequency magnetic field protects injured spinal cord from the microglia- and iron-induced tissue damage Electromagn. Biol. Med., 36, 330–340
Doan, L.V., Eydlin, O., Piskoun, B., Kline, R.P., Recio-Pinto, E., Rosenberg, A.D., Blanck, T.J.J. and Xu, F.
(2014) Despite differences in cytosolic calcium regulation, lidocaine toxicity is similar in adult and neonatal rat dorsal root ganglia in vitro Anesthesiology, 120, 50-61
He, S-Q., Yang, F., Perez, F.M., Xu, Q., Shechter, R., Cheong, Y-K., Carteret, A.F., Dong, X., Sweitzer, S.M., Raja, S.N. and Guan, Y. (2013) Tolerance develops to the antiallodynic effects of the peripherally acting opioid loperamide hydrochloride in nerve-injured rats Pain, 154, 2477–2486
Huang, S., Liu, X., Zhang, J., Bao, G., Xu, G., Sun, Y., Shen, Q., Lian, M., Huang, Y. and Cui, Z. (2015) Expression of peroxiredoxin 1 after traumatic spinal cord injury in rats Cell. Mol. Neurobiol., 35, 1217–1226
Nguyen, H.X., Beck, K.D. and Anderson, A.J. (2011) Quantitative assessment of immune cells in the injured spinal cord tissue by flow cytometry: a novel use for a cell purification method J. Visualized Exp., 50: 1-5
Rat spinal cord (embryo)
Amyotrophic lateral sclerosis
Andries, M., Van Damme, P., Robberecht, W. and van den Bosch, L. (2007) Ivermectin inhibits AMPA receptor-mediated excitotoxicity in cultured motor neurons and extends the life span of a transgenic mouse model of amytrophic lateral sclerosis Neurobiol. Dis., 25, 8-16
Kia, A., McAvoy, K., Krishnamurthy, K., Trotti, D. and Pasinelli, P. (2018) Astrocytes expressing ALS-linked mutant FUS induce motor neuron death through release of tumor necrosis factor-alpha Glia, 66, 1016–1033
Song, W., Song, Y., Kincaid, B., Bossy, B. and Bossy-Wetzel, E. (2013) Mutant SOD1G93A triggers mitochondrial fragmentation in spinal cord motor neurons: Neuroprotection by SIRT3 and PGC-1α Neurobiol. Dis., 51, 72–81
Wang, W., Li, L., Lin, W-L., Dickson, D.W., Petrucelli, L., Zhang, T. and Wang, X. (2013) The ALS diseaseassociated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons Hum. Mol. Genet., 22, 4706–4719
Apoptosis
Carlton, E., Teng, Q., Federici, T., Yang, J., Riley, J. and Boulis, N.M. (2008) Fusion of the tetanus toxin C fragment binding domain and Bcl-xL for protection of peripheral nerve neurons Neurosurgery, 63, 1175-1184
Gandelman, M., Levy, M., Cassina, P., Barbeito. L. and Beckman, J.S. (2013) P2X7 receptor-induced death of motor neurons by a peroxynitrite/FAS-dependent pathway J. Neurochem., 126, 382-388
Arginase 1
Estévez, A.G., Sahawneh, M.A., Lange, P.S., Bae, N., Egea, M. and Ratan, R.R. (2006) Arginase 1 regulation of nitric oxide production is key to survival of trophic factor-deprived motor neurons J. Neurosci., 26, 8512- 8516
Ma, T.C., Campana, A., Lange, P.S., Lee, H-H., Banerjee, K., Bryson, J.B., Mahishi L., Alam, S. et al (2010) A large-scale chemical screen for regulators of the arginase 1 promoter identifies the soy isoflavone daidzein as a clinically approved small molecule that can promote neuronal protection or regeneration via a cAMPindependent pathway J. Neurosci., 30, 739-748
Astrocyte interactions
Domeniconi, M., Hempstead, B.L. and Chao, M.V. (2007) Pro-NGF secreted by astrocytes promotes motor neuron cell death Mol. Cell. Neurosci., 34, 271-279
Miquel, E., Cassina, A., Martínez-Palma, L., Bolatto, C., Trías, E., Gandelman, M., Radi, R., Barbeito, L. and Cassina, P. (2012) Modulation of astrocytic mitochondrial function by dichloroacetate improves survival and motor performance in inherited amyotrophic lateral sclerosis PLoS One, 7: e34776
Ragancokova, D., Jahn, K., Kotsiari, A., Schlesinger, F., Haastert, K., Stangel, M., Petri, S. and Krampfl, K. (2009) Analysis of neuroprotective effects of valproic acid on primary motor neurons in monoculture or cocultures with astrocytes or Schwann cells Cell. Mol. Neurobiol., 29, 1037–1043
Wanner, I.B., Deik, A., Torres, M., Rosendahl, A., Neary, J.T., Lemmon, V.P. and Bixby, J.L. (2008) A new in vitro model of the glial scar inhibits axon growth Glia, 56, 1691-1709
Axon regeneration
Liu, Y., Grumbles, R.M. and Thomas, C.K. (2013) Electrical stimulation of embryonic neurons for 1 hour improves axon regeneration and the number of reinnervated muscles that function J. Neuropathol. Exp. Neurol., 72, 697-707
Ca2+ regulation
Castillo, C., Norcini, M., Baquero-Buitrago, J., Levacic, D., Medina, R., Montoya-Gacharna, J.V., Blanck, T.J.J., Dubois, M. and Recio-Pintoa, E. (2011) The N-methyl-D-aspartate-evoked cytoplasmic calcium increase in adult rat dorsal root ganglion neuronal somata was potentiated by substance P pertreatment in a protein kinase C-dependent manner Neuroscience, 177, 308–320
Grosskreutz, J., Haastert, K., Dewil, M., Van Damme, P., Callewaert, G., Robberecht, W., Dengler, R. and Van den Bosch, L. (2007) Role of mitochondria in kainate-induced fast Ca2+ transients in cultured spinal motor neurons Cell Calcium, 42, 59-69
Haastert, K., Grosskreutz, J., Jaeckel, M., Laderer, C., Bufler, J., Grothe, C. and Claus, P. (2005) Rat embryonic motoneurons in long-term co-culture with Schwann cells – a system to investigate motoneuron diseases on a cellular level in vitro J. Neurosci. Meth., 142, 275-284
Jahn, K., Grosskreutz, J., Haastert, K., Ziegler, E., Schlesinger, F., Grothe, C., Dengler, R. and Bufler, J. (2006) Temporospatial coupling of networked synaptic activation of AMPA-type glutamate receptor channels and calcium transients in cultured motoneurons Neuroscience, 142, 1019-1029
Culture of
Guettier-Sigrist, S., Appert-Collin, A., Duong, F., Azzouz, M., Coupin, G., Gies, J-P. and Poindron, P. (2005) Improved methods for culturing rat and mouse motor neurons Bio Valley Monogr. Basel Karger, 1, 96-108
Sepehr, A., Ruud, J. and Mohseni, S. (2009) Neuron survival in vitro is more influenced by the developmental age of the cells than by glucose condition Cytotechnology, 61, 73–79
Vincent, A.M. and Feldman, E.L. (2010) Primary sensory and motor neuron cultures In, Protocols for Neural Cell Culture, Springer Protocols Handbooks, (ed. Doering, L.C.), Humana Press (Springer Science+Business Media), Totowa, NJ. pp 161-173
Culture, nanofibres
Corey, J.M., Gertz, C.C., Wang, B-S., Birrell, L.K., Johnson, S.L., Martin, D.C. and Feldman, E.L. (2008) The design of electrospun PLLA nanofiber scaffolds compatible with serum-free growth of primary motor neuron and sensory neurons Acta Biomat., 4, 863-875
Gertz, C.C., Leach, M.K., Birrell, L.K., Martin, D.C., Feldman, E.L. and Corey, J.M. (2010) Accelerated neuritogenesis and maturation of primary spinal motor neurons in response to nanofibers Dev. Neurobiol., 70, 589–603
Leach, M.K., Feng, Z-Q., Gertz, C.C., Tuck, S.J., Regan, T.M., Naim, Y., Vincent, A.M. and Corey, J.M. (2011) The culture of primary motor and sensory neurons in defined media on electrospun poly-L-lactide nanofiber scaffolds J. Visualized Exp., 48: 1-5
Martin, D.C., Wu, J., Shaw, C.M., King, Z., Spanninga, S.A., Richardson-Burns, S., Hendricks, J. and Yang, J. (2010) The morphology of Poly(3,4-Ethylenedioxythiophene) Polymer Rev., 50, 340–384
Gene expression
Rossi, S.L., Lumpkin, C.J., Harris, A.W., Holbrook, J., Gentillon, C., McCahan, S.M., Wang, W. and Butchbach, M.E.R. (2016) Identification of early gene expression changes in primary cultured neurons treated with topoisomerase I poisons Biochem. Biophys. Res. Comm., 479, 319-324
Growth factors
De Muynck, L., Herdewyna, S., Beel, S., Scheveneels, W., Van Den Bosch, L., Robberecht , W. and Van Damme, P. (2013) The neurotrophic properties of progranulin depend on the granulin E domain but do not require sortilin binding Neurobiol. Aging, 34, 2541-2547
Poesen, K., Lambrechts, D., Van Damme, P., Dhondt, J., Bender, F., Frank, N., Bogaert, E., Claes, B., Heylen, L. et al (2008) Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration J. Neurosci., 15, 10451-10459
Wang, J., Van Damme, P., Cruchaga, C., Gitcho, M.A., Vidal, J.M., Seijo-Martinez, M., Wang, L., Wu, J.Y., Robberecht, W. and Goate, A. (2010) Pathogenic cysteine mutations affect progranulin function and production of mature granulins J. Neurochem. (2010) 112, 1305–1315
Inflammation
Beck, K.D., Nguyen, H.X., Galvan, M.D., Salazar, D.L., Woodruff, T.M. and Anderson, A.J. (2010) Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment Brain 133, 433–447
Methodology
Faron-Górecka, A., Szlachta, M., Kolasa, M., Solich, J., Górecki, A., Kuśmider, M., Zurawek, D. and Dziedzicka-Wasylewska, M. (2019) Understanding GPCR dimerization in Methods in Cell Biology, 149, 155- 178
Graber, D.J. and Harris, B.T. (2013) Purification and culture of spinal motor neurons Cold Spring Harb. Protoc., prot074161, pp 319-326
Southam, K.A., King, A.E., Blizzard, C.A., McCormack, G.H. and Dickson, T.C. (2015) A novel in vitro primary culture model of the lower motor neuron–neuromuscular junction circuit In Neuromethods, 103,
Microfluidic and Compartmentalized Platforms for Neurobiological Research (ed. Biffi, E.) Springer Science+Business Media New York, pp 181-193
Muscular atrophy/re-innervation
Liu, Y., Grumbles, R.M. and Thomas, C.K. (2014) Electrical stimulation of transplanted motoneurons improves motor unit formation J. Neurophysiol., 112, 660–670
Na+ -K+ -2Cl- co-transporter
Chabwine, J.N., Talavera, K., Verbert, L., Eggermont, J., Vanderwinden, J-M., De Smedt, H., Van Den Bosch, L., Robberecht, W., Callewaert, G. (2009) Differential contribution of the Na+ -K+ -2Cl⁻ cotransporter NKCC1 to chloride handling in rat embryonic dorsal root ganglion neurons and motor neurons FASEB J., 23, 1168– 1176
Nerve repair/growth
Feng, Z-Q., Franz, E.W., Leach, M.K., Winterroth, F., White, C.M., Rastogi, A., Gu, Z-Z. and Corey, J.M. (2016) Mechanical tension applied to substrate films specifies location of neuritogenesis and promotes major neurite growth at the expense of minor neurite development J. Biomed Mater. Res. Part A, 104A, 966–974
Irobi, J., Almeida-Souza, L., Asselbergh, B., De Winter, V., Goethals, S., Dierick, I., Krishnan, J. et al (2010) Mutant HSPB8 causes motor neuron-specific neurite degeneration Hum. Mol. Genet., 19, 3254–3265
Montoya-Gachana, J.V., Sutachan, J.J., Chan, W.S., Sideris, A., Blanck, T.J.J. and Recio-Pinto, E. (2009) Muscle-conditioned media and cAMP promote survival and neurite outgrowth of adult spinal cord motor neurons Exp. Neurol., 220 303–315
Neuromuscular junction/signalling
Das, M., Rumsey, J.W., Bhargava, N., Stancescu, M. and Hickman, J.J. (2010) A defined long-term in vitro tissue engineered model of neuromuscular junctions Biomaterials 31, 4880-4888
Southam, K.A., King, A.E., Blizzard, C.A., McCormack, G.H. and Dickson, T.C. (2015) A novel in vitro primary culture model of the lower motor neuron–neuromuscular junction circuit In Neuromethods, 103,
Microfluidic and Compartmentalized Platforms for Neurobiological Research (ed. Biffi, E.) Springer Science+Business Media New York, pp 181-193
Neurotrophic factors
Deinhardt, K., Reversi, A., Berninghausen, O., Hopkins, C.R., and Schiavo, G. (2007) Neurotrophins redirect p75NTR from a clathrin-independent to a clathrin-dependent pathway coupled to axonal transport Traffic, 8, 1736-1749
Niu, C. and Yip, H.K. (2011) Neuroprotective signaling mechanisms of telomerase are regulated by brainderived neurotrophic factor in rat spinal cord motor neurons J. Neuropathol. Exp. Neurol., 70, 634-652
Van Damme, P., Van Hoecke, A., Lambrechts, D., Vanacker, P., Bogaert, E., van Swieten, J., Carmeleit, P., Van Den Bosch, L. and Robberecht, W. (2008) Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival J. Cell Biol., 181, 37-41
NFE2-related factor 2
Sporn, M., Beal, M.F. and Kiaei, M. (2011) Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis Free Radic. Biol. Med., 51, 88–96
Nitric oxide
Estévez, A.G., Sahawneh, M.A., Lange, P.S., Bae, N., Egea, M. and Ratan, R.R. (2006) Arginase 1 regulation of nitric oxide production is key to survival of trophic factor-deprived motor neurons J. Neurosci., 26, 8512- 8516
Non-NMDA receptors
King, A.E., Dickson, T.C., Blizzard, C.A., Foster, S.S., Chung, R.S., West, A.K., Chuah, M.I. and Vickers, J.C. (2007) Excitotoxicity mediated by non-NMDA receptors causes axonopathy in long-term cultured spinal motor neurons Eur. J. Neurosci., 26, 215102159
Schwann cell interactions
Haastert, K., Grosskreutz, J., Jaeckel, M., Laderer, C., Bufler, J., Grothe, C. and Claus, P. (2005) Rat embryonic motoneurons in long-term co-culture with Schwann cells – a system to investigate motoneuron diseases on a cellular level in vitro J. Neurosci. Meth., 142, 275-284
Ragancokova, D., Jahn, K., Kotsiari, A., Schlesinger, F., Haastert, K., Stangel, M., Petri, S. and Krampfl, K. (2009) Analysis of neuroprotective effects of valproic acid on primary motor neurons in monoculture or cocultures with astrocytes or Schwann cells Cell. Mol. Neurobiol., 29, 1037–1043
Rumsey, J.W., Das, M., Stancescu. M., Bott, M., Fernandez-Valle, C. and Hickman, J.J. (2009) Node of Ranvier formation on motoneurons in vitro Biomaterials 30, 3567–3572
SOD-1
Lobsiger, C.S., Boillée, S. and Cleveland, D.W. (2007) Toxicity from different SOD1 mutants dysregulates the complement system and the neuronal regenerative response in ALS motor neurons Proc. Natl. Acad. Sci. USA, 104, 7319-7326
Magrané, J., Sahawneh, M.A., Przedborski, S., Estévez, A.G. and Manfredi, G. (2012) Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant SOD1 motor neurons J. Neurosci., 32, 229 –242
Marchetto, M.C.N., Muotri, A.R., Mu, Y., Smith, A.M., Cezar, G.G. and Gage, F.H. (2008) Non-cellautonomous effect of human SOD1G37R astrocytes on motor neurons derived from human embryonic stem cells Cell Stem Cell. 3, 649-657
Sahawneh, M.A., Ricart, K.C., Roberts, B.R., Bomben, V.C., Basso, M., Ye, Y., Sahawneh, J., Franco, M.C., Beckman, J.S. and Estévez, A.G. (2010) Cu,Zn-superoxide dismutase increases toxicity of mutant and zincdeficient superoxide dismutase by enhancing protein stability J. Biol. Chem., 285, 33885-33897
Song, W., Song, Y., Kincaid, B., Bossy, B. and Bossy-Wetzel, E. (2013) Mutant SOD1G93A triggers mitochondrial fragmentation in spinal cord motor neurons: Neuroprotection by SIRT3 and PGC-1α Neurobiol. Dis., 51, 72–81
Trumbull, K.A., McAllister, D., Gandelman, M.M., Fung, W.Y., Lew, T., Brennan, L., Lopez, N., Morré, J., Kalyanaraman, B., Beckman, J.S. (2012) Diapocynin and apocynin administration fails to significantly extend survival in G93A SOD1 ALS mice Neurobiol. Dis., 45, 137–144
Spinal motor control
Guo, X., Ayala, J.E., Gonzalez, M., Stancescu, M., Lambert, S. and Hickman, J.J. (2012) Tissue engineering the monosynaptic circuit of the stretch reflex arc with co-culture of embryonic motoneurons and proprioceptive sensory neurons Biomaterials, 33, 5723-5731
Topoisomerase poisons
Rossi, S.L., Lumpkin, C.J., Harris, A.W., Holbrook, J., Gentillon, C., McCahan, S.M., Wang, W. and Butchbach, M.E.R. (2016) Identification of early gene expression changes in primary cultured neurons treated with topoisomerase I poisons Biochem. Biophys. Res. Comm., 479, 319-324
Ubiquitin ligase
Lassot, I., Robbins, I., Kristiansen, M., Rahmeh, R., Jaudon, F., Magiera, M.M., Mora, S., Vanhille, L., Lipkin, A., Pettmann, B., Ham, J. and Desagher, S. (2010) Trim17, a novel E3 ubiquitin-ligase, initiates neuronal apoptosis Cell Death Differ. 17, 1928–1941
Valproic acid
Ragancokova, D., Song, Y., Nau, H., Dengler, R., Krampfl, K. and Petri, S. (2010) Modulation of synaptic transmission and analysis of neuroprotective effects of valproic acid and derivates in rat embryonic motoneurons Cell. Mol. Neurobiol., 30, 891–900
Viral transduction
Eleftheriadou, I., Trabalza, A., Ellison, S.M., Gharun, I.K. and Mazarakis, N.D. (2014) Specific retrograde transduction of spinal motor neurons using lentiviral vectors targeted to presynaptic NMJ receptors Mol. Ther., 22, 1285–1298
Hislop, J.N., Islam, T.A., Eleftheriadou, I., Carpentier, D.C.J., Trabalza, A., Parkinson, M., Schiavo, G. and Mazarakis, N.D. (2014) Rabies virus envelope glycoprotein targets lentiviral vectors to the axonal retrograde pathway in motor neurons J. Biol. Chem., 289, 16148-16163
Peluffo, H., Foster, E., Ahmed, S.G., Lago, N., Hutson, T.H., Moon, L., Yip, P., Wanisch, K., CaraballoMiralles, V., Olmos, G., Llado, J., McMahon, S.B. and Yáñez Muñoz, R.J. (2013) Efficient gene expression from integration-deficient lentiviral vectors in the spinal cord Gene Ther., 20, 645–657
Trabalza, A., Georgiadis, C., Eleftheriadou, I., Hislop, J.N., Ellison, S.M., Karavassilis, M.E. and Mazarakis, N.D. (2013) Venezuelan equine encephalitis virus glycoprotein pseudotyping confers neurotropism to lentiviral vectors Gene Ther., 20, 723–732
Rat spinal cord (post-natal)
Southam, K.A., King, A.E., Blizzard, C.A., McCormack, G.H. and Dickson, T.C. (2013) Microfluidic primary culture model of the lower motor neuron–neuromuscular junction circuit J. Neurosci. Methods, 218, 164– 169
Rat ventral mesencephalic neurons
Lautenschläger, J., Mosharov, E.V., Kanter, E., Sulzer, D. and Kaminski Schierle, G.S. (2018) An easy-toimplement protocol for preparing postnatal ventral mesencephalic cultures Front. Cell. Neurosci., 12: 44
Turtle cerebral hemisphere
Milton, S.L., Nayak, G., Kesaraju, S., Kara, L. and Prentice, H.M. (2007) Suppression of reactive oxygen species production enhances neuronal survival in vitro and in vivo in the anoxia-tolerant turtle Trachemys scripta J. Neurochem., 101, 993-1001
Nayak, G., Prentice, H.M. and Milton, S.L. (2009) Role of neuroglobin in regulating reactive oxygen species in the brain of the anoxia-tolerant turtle Trachemys scripta J. Neurochem., 110, 603–612
Nayak, G.H., Prentice, H.M. and Milton, S.L. (2011) Neuroprotective signaling pathways are modulated by adenosine in the anoxia tolerant turtle J.Cereb. Blood Flow Metab., 31, 467–475
Zebrafish (spinal muscular atrophy)
Boyd, P.J., Tu, W-Y., Shorrock, H.K., Groen, E.J.N., Carter, R.N., Powis, R.A., Thomson, D., Graham, L.C. (2017) Bioenegergetic status modulates motor neuron vulnerability and pathogenesis in a zebrafish model of spinal muscular atrophy PLoS Genet., 13: e1007644
Gassman, A., Hao, L.T., Bhoite, L., Bradford, C.L., Chien, C-B., Beattie, C.E. and Manfredi, J.P. (2013) Small molecule suppressors of Drosophila kinesin deficiency rescue motor axon development in a zebrafish model of spinal muscular atrophy PLoS One, 8: e74325
3. Myelin removal from cell preparations
Crang, A.J., Gilson, J.M., Li, W.-W. and Blakemore, W.F. (2004) The remyelinating potential and in vitro differentiation of MOG-expressing oligodendrocyte precursors isolated from the adult rat CNS Eur. J. Neurosci., 20, 1445-1460
Janes, K., Wahlman, C., Little, J.W., Doyle, T., Tosh, D.K., Jacobson, K.A. and Salvemini, D. (2015) Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy Brain, Behav. Immun., 44 91–99
Luyt, K., Varadi, A., Halfpenny, C.A., Scolding, N.J. and Molnar, E. (2004) Metabotropic glutamate receptors are expressed in adult human glial progenitor cells Biochem. Biophys. Res. Commun., 319, 120-129
Margul, D.J., Park, J., Boehler, R.M., Smith, D.R., Johnson, M.A., McCreedy, D.A., He, T., Ataliwala, A., Kukushliev, T.V., Liang, J., Sohrabi, A. et al (2016) Reducing neuroinflammation by delivery of IL-10 encoding lentivirus from multiple-channel bridges Bioeng. Translat. Med., 1, 136-148
Mavrikis Cox, G., Kithcart, A.P., Pitt, D., Guan, Z., Alexander, J., Williams, J.L., Shawler, T., Dagia, N.M., Popovich, P.G., Satoskar, A.R. and Whitacre, C.C. (2013) Macrophage migration inhibitory factor potentiates autoimmune-mediated neuroinflammation J. Immunol., 191, 1043–1054.
4. Neuroglial and other cells
Astrocytes
Eijkelkamp, N., Steen-Louws, C., Hartgring, S.A.Y., Willemen, H.L.D.M., Prado, J., Lafeber, F.P.J.G., Heijnen, C.J., Hack, C.E., van Roon, J.A.G. and Kavelaars, A. (2016) IL4-10 Fusion protein is a novel drug to treat persistent inflammatory pain J. Neurosci., 36, 7353–7363
Eelen, G., Dubois, C., Cantelmo, A.R., Goveia, J., Brüning, U., DeRan, M., Jarugumilli, G., van Rijssel, J., Saladino, G., Comitani, F. and Zecchin, A. (2018) Role of glutamine synthetase in angiogenesis beyond glutamine synthesis Nature, 561, 63-69
Freeman, K.A., Fullerton, D.A., Foley, L.S., Bell, M.T., Cleveland, Jr, J.C., Weyant, M.J. Mares, J. et al (2015) Spinal cord protection via alpha-2 agonist-mediated increase in glial cell-line–derived neurotrophic factor J. Thorac. Cardiovasc. Surg., 149, 578-86
Kerstetter, A.E. and Miller, R.H. (2012) Isolation and culture of spinal cord astrocytes In Methods Mol. Biol., 814, Astrocytes: Methods and Protocols (ed. Milner, R.) Springer Science+Business Media, LLC pp 93-104
Inflammatory cells
Mavrikis Cox, G., Kithcart, A.P., Pitt, D., Guan, Z., Alexander, J., Williams, J.L., Shawler, T., Dagia, N.M., Popovich, P.G., Satoskar, A.R. and Whitacre, C.C. (2013) Macrophage migration inhibitory factor potentiates autoimmune-mediated neuroinflammation J. Immunol., 191, 1043–1054
Macrophages
Turtzo, L.C., Lescher, J., Janes, L., Dean, D.D., Budde, M.D. and Frank, J.A. (2014) Macrophagic and microglial responses after focal traumatic brain injury in the female rat J. Neuroinflam., 11: 82
Microglial cells
Beck, K.D., Nguyen, H.X., Galvan, M.D., Salazar, D.L., Woodruff, T.M. and Anderson, A.J. (2010) Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment Brain 133, 433–447
Bell, M.T., Puskas, F., Agoston, V.A., Cleveland, Jr, J.C., Freeman, K.A., Gamboni, F., Herson, P.S., Meng, X. and Smith, P.D. (2013) Toll-like receptor 4–dependent microglial activation mediates spinal cord ischemia– reperfusion injury Circulation, 128 [suppl 1], S152-S156
Bettinger, I., Thanos, S. and Paulus, W. (2002) Microglia promote glioma migration Acta Neuropathol., 103, 351-355
Chen, J., Cui, Z., Yang, S., Wu, C., Li, W., Bao, G., Xu, G., Sun, Y., Wang, L. and Zhang, J. (2017) The upregulation of annexin A2 after spinal cord injury in rats may have implication for astrocyte proliferation
Neuropeptides 61, 67–76
Dyck, S., Kataria, H., Alizadeh, A., Santhosh, K.T., Lang, B., Silver, J. and Karimi-Abdolrezaee, S. (2018) Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPσ receptors promotes a bene-ficial inflammatory response following spinal cord injury J. Neuroinflam., 15: 90
Fumagalli, S., Fiordaliso, F., Perego, C., Corbelli, A., Mariani, A., De Paola, M. and De Simoni, M-G. (2019) The phagocytic state of brain myeloid cells after ischemia revealed by superresolution structured illumination microscopy J. Neuroinflamm., 16: 9
Galvan, M.D., Luchetti, S., Burgos, A.M., Nguyen, H.X., Hooshmand, M.J., Hamers, F.P.T. and Anderson, A.J. (2008) Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury J. Neurosci., 28, 13876 –13888
Hong, H-B., Krause, H.J., Sohn, S-W., Baik, T-K., Park, J.H., Shin, S-W., Park, C-H. and Song, D-Y. (2014) In situ measurement of superoxide and hydroxyl radicals by frequency mixing detection technique Anal. Biochem., 447, 141-145
Mariani, A., Fanelli, R., Re Depaolini, A. and De Paola, M. (2015) Decabrominated diphenyl ether and methylmercury impair fetal nervous system development in mice at documented human exposure levels. Develop. Neurobiol., 75, 23–38
Mauri, E., Veglianese, P., Papa, S., Mariani, A., De Paola, M., Rigamonti, R., Chincarini, G.M.F., Vismara, I. et al (2017) Double conjugated nanogels for selective intracellular drug delivery RSC Adv., 7, 30345-30356
Mauri, E., Veglianese, P., Papa, S., Mariani, A., De Paola, M., Rigamonti, R., Chincarini, G.M.F., Rimondo, S., Sacchetti, A. and Rossi, F. (2017) Chemoselective functionalization of nanogels for microglia treatment Eur. Polymer J., 94, 143–151
Mavrikis Cox, G., Kithcart, A.P., Pitt, D., Guan, Z., Alexander, J., Williams, J.L., Shawler, T. et al (2013) Macrophage migration inhibitory factor potentiates autoimmune-mediated neuroinflammation J. Immunol., 191, 1043–1054
O’Mahony, A., Raber, J., Montano, M., Foehr, E., Han, V., Lu, S-m., Goethals, S., Ydens, E., Timmerman, V. and Janssens, S. (2010) Toll-like receptor expression in the peripheral nerve Glia, 58, 1701–1709
Kwon, H., LeFevour, A., Chakraborty-Sett, S. and Greene, W.C. (2006) NF-B/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity Mol. Biol. Cell., 26, 7283-7298
Papa, S., Rossi, F., Ferrari, R., Mariani, A., De Paola, M., Caron, I., Fiordaliso, F., Bisighini, C. et al (2013) Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury ACS Nano, 7, 9881–9895
Papa, S., Ferrari, R., De Paola, M., Rossi, F., Mariani, A., Caron, I., Sammali, E., Peviani, M. et al (2014) Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury J. Control. Release, 174, 15-26
Papa, S., Caron, I., Erba, E., Panini, N., De Paola, M., Mariani, A., Colombo, C., Ferrari, R., Pozzer, D. et al (2016) Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury Biomaterials, 75, 13-24
Sarkar, D., Chastain, L., Cabrera and Shrivastava, P. (2016) Ethanol exposures during the developmental period increase microglia sensitivity to a stress challenge during adulthood: a possible cause for the stress hyperresponse in fetal alcohol exposed offspring Neuropsychopharmacology 41, S136-137
Shrivastava, P., Cabrera, M.A., Chastain, L.G., Boyadjieva, N.I., Jabbar, S., Franklin, T. and Sarkar, D.K. (2017) Mu-opioid receptor and delta-opioid receptor differentially regulate microglial inflammatory response to control proopiomelanocortin neuronal apoptosis in the hypothalamus: effects of neonatal alcohol J. Neuroinflamm., 14: 83
Song, D-Y., Yu, H-N., Park, C-R., Lee, J-S., Lee, J-Y., Park, B-G., Woo, R-S., Han, J-T. et al (2013) Downregulation of microglial activity attenuates axotomized nigral dopaminergic neuronal cell loss BMC Neurosci., 14: 112
Tsuchiya, T., Park, K.C., Toyonaga, S., Yamada, S.M., Nakabayashi, H., Nakai, E., Ikawa, N. et al (2005) Characterization of microglia induced from mouse embryonic stem cells and their migration into brain parenchyma J. Neuroimmunol., 160, 210-218
Tucsek, Z., Toth, P., Sosnowska, D., Gautam, T., Mitschelen, M., Koller, A., Szalai, G., Sonntag, W.E., Ungvari, Z. and Csiszar, A. (2014) Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease J. Gerontol. A Biol. Sci. Med..Sci., 69, 1212–1226
Turtzo, L.C., Lescher, J., Janes, L., Dean, D.D., Budde, M.D. and Frank, J.A. (2014) Macrophagic and microglial responses after focal traumatic brain injury in the female rat J. Neuroinflam., 11: 82
Vincent, A.M. and Feldman, E.L. (2010) Primary sensory and motor neuron cultures In, Protocols for Neural Cell Culture, Springer Protocols Handbooks, (ed. Doering, L.C.), Humana Press (Springer Science+Business Media), Totowa, NJ. pp 161-173
Yang, B., Parsha, K., Migliati, E. and Savitz, S. (2014) Bone marrow mononuclear cells may enhance recovery after stroke by modulating the microglial response Stroke, 45, Abstr. A192
Zhuravleva, M., Rizvanov, A. and Mukhamedshina, Y. (2016) Effect of GDNF on morphology, proliferation, and phagocytic activity of rat neonatal cortex isolated microglia BioNanoSci., 6, 379–383
Neural progenitor cells
Pathania, M., De Jay, N., Maestro, N., Harutyunyan, A.S., Nitarska, J., Pahlavan, P., Henderson, S., Mikael, L.G., Richard-Londt, A, et al (2017) H3.3K27M Cooperates with Trp53 loss and PDGFRA gain in mouse embryonic neural progenitor cells to induce invasive high-grade gliomas Cancer Cell, 32, 684–700
Oligodendrocytes
Cassiani-Ingoni, R., Greenstone, H.L., Donati, D., Fogdell-Hahn, A., Martinell, E., Refai, D., Martin, R., Berger, E.A. and Jacobson, S. (2005) CD46 on glial cells can function as a receptor for viral glycoproteinmediated cell–cell fusion Glia, 52, 252-258
Chari, D.M., Phil, A., Crang, A.J. and Blakemore, W.F. (2003) Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age J. Neuropathol. Exp. Neurol., 62, 908-816
Li, G., Crang, A.J., Rundle, J.L. and Blakemore, W.F. (2002) Oligodendrocyte progenitor cells in the adult rat CNS express myelin oligodendrocyte glycoprotein (MOG) Brain Pathol., 12, 463-471
O’Mahony, A., Raber, J., Montano, M., Foehr, E., Han, V., Lu, S-m., Kwon, H. et al (2006) NF-B/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity Mol. Biol. Cell., 26, 7283-7298
Sotnikov, I., Veremeyko, T., Starossom, S.C., Barteneva, N., Weiner, H.L. and Ponomarev, E.D. (2013) Platelets recognize brain-specific glycolipid structures, respond to neurovascular damage and promote neuroinflammation PLoS One, 8: e58979
5. Recently published papers
Amyotrophic lateral scelerosis; mouse embryo; primary motor neurons
Tischbein, M., Baron, D.M., Lin, Y-C., Gall, K.V., Landers, J.E., Fallini, C. and Bosco, D.A. (2019) The RNAbinding protein FUS/TLS undergoes calcium mediated nuclear egress during excitotoxic stress and is required for GRIA2 mRNA processing J. Biol. Chem., 294, 10194–10210
Astrocyte purification (mouse hindbrain)
Jin, J., Smith, M.D., Kersbergen, C.J., Kam, T-I., Viswanathan, M., Martin, K., Dawson, T.M., Dawson, V.L., Zack, D.J. et al (2019) Glial pathology and retinal neurotoxicity in the anterior visual pathway in experimental autoimmune encephalomyelitis Acta Neuropathol. Comm., 7: 125
Charcot-Marie-Tooth disease; mouse ventral spinal cord cells; Fernandez-Lizarbe, S., Civera-Tregon, A., Cantarero, L., Herrer, I., Juarez, P., Hoenick, J. and Palau, F.
(2019) Neuroinflammation in the pathogenesis of axonal Charcot-Marie-Tooth disease caused by lack of GDAP1 Exp. Neurol., 320, 113004
Cortical neurons (mouse foetus)
Park, D-J., Kang, J-B., Shah, F-A., Jin, Y-B. and Koh, P-O. (2020) Quercetin attenuates decrease of thioredoxin expression following focal cerebral ischemia and glutamate-induced neuronal cell damage Neuroscience, 428, 38–49
Embryonic spinal cord (mouse embryo); amyotrophic lateral sclerosis
Giampetruzzi, A., Danielson, E.W., Gumina, V., Jeon, M., Boopathy, S., Brown, R.H., Ratti, A., Landers, J.E. and Fallini, C. (2019) Modulation of actin polymerization affects nucleocytoplasmic transport in multiple forms of Nat. Comm., 10: 3827
Hippocampal neurons (various ages); Alzheimer’s disease
Dong, Y., Sameni, S., Digman, A. and Brewer. G.J. (2019) Reversibility of age-related oxidized free NADH redox states in Alzheimer’s disease neurons by imposed external Cys/CySS redox shifts Sci. Rep., 9: 11274
Hippocampal neurons:
Memory storage
Zhu, Y., Huang, M., Bushong, E., Phan, S., Uytiepo, M., Beutter, E., Boemer, D., Tsui. K., Ellisman, M. and Maximov, A. (2019) Class IIa HDACs regulate learning and memory through dynamic experience-dependent repression of transcription Nat. Comm., 10: 3469
Pyramidal cell (epilepsy)
Yang, F., Yang, L., Wataya-Kaneda, M., Teng, L. and Katayama, I. (2020) Epilepsy in a melanocyte-lineage mTOR hyperactivation mouse model: A novel epilepsy model PLoS One, 15: e0228204
Hypothalamus
Lipoprotein receptors
Lee, S.D., Priest, C., Bjursell, M., Gao, J., Arneson, D.V., Ahn, I.S., Diamante, G., van Veen, J.E., Massa, M.G., Calkin, A.C., et al (2019) IDOL regulates systemic energy balance through control of neuronal VLDLR expression Nature Metab., 1090, 1089–1100
Microglial cells
Chastain, L.G., Franklin, T., Gangisetty, O., Cabrera, M.A., Mukherjee, S., Shrivastava, P., Jabbar, S. and Sarkar, D.K. (2019) Early life alcohol exposure primes hypothalamic microglia to later-life hyper-sensitivity to immune stress: possible epigenetic mechanism Neuropsychopharmacol., 44, 1579–1588
Mauri, E., Veglianese, P., Papa, S., Rossetti, A., De Paola, M., Mariani, A., Posel, Z., Posocco, P., Sacchetti, A. and Rossi, F. (2020) Effects of primary amine-based coatings on microglia internalization of Nanogels Colloids Surf. B: Biointerfaces, 185: 110574
Motor axon growth
Katiyar, K.S., Struzyna, L.A., Das, S. and Cullen, D.K. (2019) Stretch growth of motor axons in custom mechanobioreactors to generate long‐projecting axonal constructs J. Tissue Eng. Regen. Med., 13, 2040–2054
Mouse adult brain cortex; neuronal projection Chen, X., Sun, Y-C., Zhan, H., Kebschull, J.M., Fischer, S., Matho, K., Huang, Z.J., Gillis, J. and Zador, A.M. (2019) High-throughput mapping of long-range neuronal projection using in situ sequencing Cell, 179, 772– 786
Mouse embryo spinal cord; neuron degeneration
García-Morales, V., Rodríguez-Bey, G., Gómez-Pérez, L., Domínguez-Vías, G., González-Forero, D., Portillo, F., Campos-Caro, A., Gento-Caro, A. et al (2019) Sp1-regulated expression of p11 contributes to motor neuron degeneration by membrane insertion of TASK1 Nat. Comm., 10: 3784
Mouse post-natal; neurite outgrowth
Ossingera,A., Bajica, A., Pana, S., Andersson, B., Ranefall, P., Hailer, N.P. and Schizasa, N. (2020) A rapid and accurate method to quantify neurite outgrowth from cell and tissue cultures: Two image analytic approaches using adaptive thresholds or machine learning J. Neurosci. Meth., 331: 108522
Niemann-Pick disease
Park, M.H., Choi, B.J., Jeong, M.S., Lee, J.Y., Jung, I.K., Park, K.H., Lee, H.W., Yamaguchi, T. et al (2019) Characterization of the subventricular-thalamo-cortical circuit in the NP-C mouse brain, and new insights regarding treatment Mol. Ther., 27, 1507-1526
Prefontal cortex (mouse brain); neuropsychiatric disease
Bhattacherjee, A., Djekidel, M.N., Chen, R., Chen, W., Tuesta, L.M. and Zhang, Y. (2019) Cell type-specific transcriptional programs in mouse prefrontal cortex during adolescence and addiction Nat. Comm., 10: 4169
Rat embryonic spinal cord; motor axon growth
Casci, I., Krishnamurthy, K., Kour, S., Tripathy, V., Ramesh, N., Anderson, E.N., Marrone, L., Grant, R.A. et al (2019) Muscleblind acts as a modifier of FUS toxicity by modulating stress granule dynamics and SMN localization Nat. Comm., 10: 5583
Rat embryonic spinal cord; synaptogenesis; ribosome reduction
Costa, R.O., Martins, H., Martins, L.F., Cwetsch, A.W., Mele, M., Pedro, J.R., Tome, D., Jeon, N.L. et al (2019) Synaptogenesis stimulates a proteasome-mediated ribosome reduction in axons Cell Rep., 28, 864–876
OptiPrep™Reference List RC06; 7th edition, February 2020
OptiPrep™ Reference List RC07
Hepatic and pancreatic stellate cells
- This Reference List RC07 provides a complete list of publications that report the use of OptiPrep™ for the purification of hepatic and pancreatic stellate cells. It complements Application Sheet C25 which provides a brief overview of the separation technology and Reference List RC08: a complete list of published papers primarily reporting the analysis of hepatic Kupffer and sinusoidal endothelial cells; it also lists papers on non-parenchymal epithelial cells, NK cells, oval cells and progenitor cells.
- References are divided into following sections based on cell source:
- Human liver – p1
- Human pancreas – p3
- Mouse liver – p3
- Mouse pancreas – p11
- Rat liver – p11
- Rat pancreas – p 17
- Sections on human, mouse and rat liver are further sorted into sub-sections alphabetically according research topic.
- Within each section or sub-section references are listed alphabetically according to first author (multiple examples are listed chronologically).
Important note: the number of published papers on rodent liver stellate cells that refer to fibrosis, fibrogenesis and liver injury is so huge that they are listed only under the analytical study.
Human liver
Activation
Hong, Y., Li, S., Wang, J. and Li, Y. (2018) In vitro inhibition of hepatic stellate cell activation by the autophagy-related lipid droplet protein ATG2A Sci. Rep., 8: 9232
Longato, L., Andreola, F., Davies, S.S., Roberts, J.L., Fusai, G., Pinzani, M., Moore, K., Rombouts, K. (2017) Reactive gamma-ketoaldehydes as novel activators of hepatic stellate cells in vitro Radic. Biol. Med., 102, 162-
Apoptosis
Singh, H.D., Otano, I., Rombouts, K., Singh, K.P., Peppa, D., Gill, U.S., Böttcher, K., Kennedy, P.T.F., Oben, J. et al (2017) TRAIL regulatory receptors constrain human hepatic stellate cell apoptosis Sci. Rep., 7: 5514
Cirrhosis
Casas‐Grajales, S., Alvarez‐Suarez, D., Ramos‐Tovar, E., Buendía‐Montaño, L.D., Reyes‐Gordillo, K., Camacho, J., Tsutsumi, V., Lakshman, M.R. and Muriel, P. (2019) Stevioside inhibits experimental fibrosis by down‐regulating profibrotic Smad pathways and blocking hepatic stellate cell activation Basic Clin. Pharmacol. Toxicol. 2019, 124, 670–6800
De Mesquita, F.C., Guixé-Muntet, S., Fernández-Iglesias, A., Maeso-Díaz, R., Vila, S., Hide, D., OrtegaRibera, M., Rosa, J.L. et al (2017) Liraglutide improves liver microvascular dysfunction in cirrhosis: Evidence from translational studies Sci. Rep., 7: 3255
Cryopreservation
Nakamura, A., Ueno, T., Yagi, Y., Okuda, K., Ogata, T., Nakamura, T., Torimura, T., Iwamoto, H., Ramadoss, S., Sata, M., Tsutsumi, V., et al (2010) Human primary cultured hepatic stellate cells can be cryopreserved Med. Mol. Morphol., 43, 107–115
Fibrosis
Chen, J.Y., Newcomb, B., Zhou, C., Pondick, J.V., Ghoshal, S., York, S.R., Motola, D.L., Coant, N., Yi, J.K., Mao, C. et al (2017) Tricyclic antidepressants promote ceramide accumulation to regulate collagen production in human hepatic stellate cells Sci. Rep., 7: 44867
Casas‐Grajales, S., Alvarez‐Suarez, D., Ramos‐Tovar, E., Buendía‐Montaño, L.D., Reyes‐Gordillo, K., Camacho, J., Tsutsumi, V., Lakshman, M.R. and Muriel, P. (2019) Stevioside inhibits experimental fibrosis by down‐regulating profibrotic Smad pathways and blocking hepatic stellate cell activation Basic Clin. Pharmacol. Toxicol. 2019, 124, 670–6800
Gene transfer
Perugorria, M.J., Wilson, C.L., Zeybel, M., Walsh, M., Amin, S., Robinson, S., White, S.A., Burt, A.D., Oakley, F., Tsukamoto, H., Mann, D.A. and Mann, J. (2012) Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast tansdifferentiation Hepatology, 56, 1129-1139
Growth factors and growth factor receptors/signalling
Barnaeva, E., Nadezhda, A., Hannappel, E., Sjogren, M.H. and Rojkind, M. (2007) Thymosin β4 upregulates the expression of hepatocyte growth factor and downregulates the expression of PDGF-β receptor in human hepatic stellate cells Ann. N.Y. Acad. Sci., 1112, 154-160
Reyes-Gordillo, K., Shah, R., Popratiloff, A., Fu, S., Hindle, A., Brody, F. and Rojkind, M. (2011) Thymosin- β4 (Tβ4) blunts PDGF-dependent phosphorylation and binding of AKT to actin in hepatic stellate cells Am. J. Pathol., 178, 2100–2108
Hepatitis B
Pallett, L.J., Gill, U.S., Quaglia, A., Sinclair, L.V., Jover-Cobos, M., Schurich, A., Singh, K.P., Thomas, N. et al. (2015) Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells Nat. Med., 21, 591-600
Hepatitis (T-cell mediated)
Chen, L., Gu, J., Qian, Y., Li, M., Qian, Y., Xu, M., Li, J., Wen, Y., Xia, L. et al (2019) Deletion of C-C motif chemokine ligand 5 worsens invariant natural killer T–cell mediated hepatitis via compensatory up-regulation of CXCR2–related chemokine activity Cell. Mol. Gastroenterol. Hepatol., 7, 623–639
Hypertension
Jalan, R., De Chiara, F., Balasubramaniyan, V., Andreola, F., Khetan, V., Malago, M., Pinzani, M., Mookerjee, R.P. and Rombouts, K. (2016) Ammonia produces pathological changes in human hepatic stellate cells and is a target for therapy of portal hypertension J. Hepatol., 64, 823–833
Memory T-cells
Swadling, L., Pallett, L.J., Diniz, M.O., Baker, J.M., Amin, O.E., Stegmann, K.A., Burton, A.R., Schmidt, N.M., Jeffery-Smith, A. et al (2020) Human liver memory CD8+ T cells use autophagy for tissue residence Cell Rep., 30, 687–698
Phosphoinositides
Rombouts, K. and Carloni, V. (2016) Determination and characterization of tetraspanin-associated phosphoinositide-4 kinases in primary and neoplastic liver cells In Methods Mol. Biol., 1376, Astrocytes: Methods and Protocols (ed. Waugh, M.G.) Springer Science+Business Media, LLC pp 203-212
RNA
Zhou, C., York, S.R., Chen, J.Y., Pondick, J.V., Motola, D.L., Chung, R.T. and Mullen, A.C. (2016) Long noncoding RNAs expressed in human hepatic stellate cells form networks with extracellular matrix proteins Genome Med., 8: 31
Zhou, C., York, S.R., Chen, J.Y., Pondick, J.V., Motola, D.L., Chung, R.T. and Mullen, A.C. (2016) Long noncoding RNAs expressed in human hepatic stellate cells form networks with extracellular matrix proteins Genome Med., 8: 31
Transcription factors
E-box DNA
Vincent, K.J., Jones, E., Arthur, M.J.P., Smart, D.E., Trim, J., Wright, M.C. and Mann, D.A. (2001) Regulation of E-box DNA binding during in vivo and in vitro activation of rat and human hepatic stellate cells Gut, 49, 713-719
Metalloproteinases
Bertrand-Philippe, M., Ruddell, R.G., Arthur, M.J.P., Thomas, J., Mungalsingh, N. and Mann, D.A. (2004) Regulation of tissue inhibitor of metalloproteinase 1 gene transcription by RUNX1 and RUNX2 J. Biol. Chem., 279, 24530-24539
Perugorria, M.J., Wilson, C.L., Zeybel, M., Walsh, M., Amin, S., Robinson, S., White, S.A. et al (2012) Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast tansdifferentiation Hepatology, 56, 1129-1139
Methylation
Perugorria, M.J., Wilson, C.L., Zeybel, M., Walsh, M., Amin, S., Robinson, S., White, S.A. et al (2012) Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast tansdifferentiation Hepatology, 56, 1129-1139
Vitamin D
Beilfuss, A., Sowa, J-P., Sydor, S., Beste, M., Bechmann, L.P., Schlattjan, M., Syn, W-K., Wedemeyer, I. et al (2015) Vitamin D counteracts fibrogenic TGF-β signalling in human hepatic stellate cells both receptordependently and independently Gut, 64, 791–799
Human pancreas
Armstrong, T., Packham, G., Murphy, L.B., Bateman, A.C., Conti, J.A., Fine, D.R., Johnson, C.D., Benyon, R.C. and Iredale, J.P. (2004) Type 1 collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma Clin. Cancer Res., 10, 7427-7437
José, A., Rovira-Rigau, M., Luna, J., Giménez-Alejandre, M., Vaquero, E., García de la Torre, B., Andreu, D., Alemany, R. and Fillat, C. (2014) A genetic fiber modification to achievematrix-metalloprotease-activated infectivity of oncolytic adenovirus Journal of Control. Release, 192, 148–156
Mouse liver
Anthocyanins
Jiang, X., Shen, T., Tang, X., Yang, W., Guo, H. and Ling, W. (2017) Cyanidin-3-O-β-glucoside combined with its metabolite protocatechuic acid attenuated the activation of mice hepatic stellate cells Food Funct., 2017, 8, 2945–2957
Antigenic targeting
Wu, F., Wuensch, S.A., Azadniv, M., Ebrahimkhani, M.R. and Crispe, I.N. (2009) Galactosylated LDL nanoparticles: a novel targeting delivery system to deliver antigen to macrophages and enhance antigen specific T cell responses Mol. Pharmaceut., 6, 1506-1517
Apoptosis
Duan, Y., Gu, X., Zhu, D., Sun, W., Chen, J., Feng, J., Song, K., Xu, F., He, X. and He, X. (2014) Schistosoma japonicum soluble egg antigens induce apoptosis and inhibit activation of hepatic stellate cells: a possible molecular mechanism Int. J. Parasitol., 44, 217–224
Tao, Y-y., Yan, X-c., Zhou, T., Shen, L., Liu, Z-l. and Liu, C-h., (2014) Fuzheng Huayu recipe alleviates hepatic fibrosis via inhibiting TNF-α induced hepatocyte apoptosis BMC Complement. Altern. Med., 14: 449
Autoimmune hepatitis
Murthy, A., Shao, Y.W., Defamie, V., Wedeles, C., Smookler, D. and Khokha, R. (2012) Stromal TIMP3 regulates liver lymphocyte populations and provides protection against Th1 T cell-driven autoimmune hepatitis J. Immunol., 188, 2876–2883
Autophagy
Chen, M., Liu, J., Yang, W. and Ling, W, (2017) Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signalling Autophagy, 13, 813–1827
B cell activity
Thapa, M., Chinnadurai, R., Velazquez, V.M., Tedesco, D., Elrod, E., Han, J-H., Sharma, P., et al (2015) Liver fibrosis occurs through dysregulation of MyD88-dependent innate B-cell activity Hepatology, 61, 2067-2079
Bile duct ligation
Cui, W., Matsuno, K., Iwata, K., Ibi, M., Matsumoto, M., Zhang, J., Zhu, K., Katsuyama, M., Torok, N.J. and Yabe-Nishimura, C. (2011) NOX1/Nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation Hepatology, 54, 949-958
Carcinogenesis
Seifert, L., Deutsch, M., Alothman, S., Alqunaibit, D., Werba, G., Pansari, M., Pergamo, M., Ochi, A. (2015) Dectin-1 regulates hepatic fibrosis and hepatocarcinogenesis by suppressing TLR4 signalling pathways Cell Rep., 13, 1–13
Wright, J.H., Johnson, M.M., Shimizu-Albergine, M., Bauer, R.L., Hayes, B.J., Surapisitchat, J., Hudkins, K.L., Riehle, K.J., Johnson, S.C., et al (2014) Paracrine activation of hepatic stellate cells in platelet-derived growth factor C transgenic mice: Evidence for stromal induction of hepatocellular carcinoma Int. J. Cancer, 134, 778–788
Wright, J.H., Johnson, M.M., Shimizu-Albergine, M., Bauer, R.L., Hayes, B.J., Surapisitchat, J., Hudkins et al (2014) Paracrine activation of hepatic stellate cells in platelet-derived growth factor C transgenic mice: Evidence for stromal induction of hepatocellular carcinoma Int. J. Cancer, 134, 778–788
Cell-cell communication
Xiong, X., Kuang, H., Ansari, S., Liu, T., Gong, J., Wang, S., Zhao, X-Y., Ji, Y., Li, C., Guo, L. et al (2019) Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis Mol. Cell, 75, 644–660
Chemokine receptor
Lee, Y-S., Eun, H.S., Kim, S.Y., Jeong, J-M., Seo, W., Byun, J-S., Jeong, W-I. and Yi, H-S. (2106) Hepatic immunophenotyping for streptozotocin-induced hyperglycemia in mice Sci. Rep., 6: 30656
Chronic liver jnjury
Kim, J-W., Yang, D., Jeong, H., Park, S., Lee, M-H., Lim, C.W. and Kim, B. (2019) Dietary zerumbone, a sesquiterpene, ameliorates hepatotoxin‐mediated acute and chronic liver injury in mice Phytother. Res., 33, 1538–1550
Connective tissue/collagen
Huang, G. and Brigstock, D.R. (2011) Integrin expression and function in the response of primary culture hepatic stellate cells to connective tissue growth factor (CCN2) J. Cell. Mol. Med., 15, 1087-1095
Oben, J.A., Yang, S., Lin, H., Ono, M. and Diehl. A.M. (2003) Acetylcholine promotes the proliferation and collagen gene expression of myofibroblastic hepatic stellate cells Biochem. Biophys. Res. Commun., 300, 172- 177
Oben, J.A., Yang, S., Lin, H., Ono, M. and Diehl, A.M. (2003) Norepinephrine and neuropeptide Y promote proliferation and collagen gene expression of hepatic myofibroblastic stellate cells Biochem. Biophys. Res. Commun., 302, 685-690
Cytokines
Kandhi. R., Bobbala, D., Yeganeh. M., Mayhue, M., Menendez, A. and Ilangumaran, S. (2016) Negative regulation of the hepatic fibrogenic response by suppressor of cytokine signalling 1 Cytokine, 82, 58–69
Li, P., Li, Y., Zhu, L., Yang, Z., He, J., Wang, L., Shang, Q., Pan, H., Wang, H., Ma, X. et al (2018) Targeting secreted cytokine BMP9 gates the attenuation of hepatic fibrosis BBA – Mol. Basis Dis., 1864, 709–720
Ogiso, H., Ito, H., Ando, T., Arioka, Y., Kanbe, A., Ando, K., Ishikawa, T. et al (2016) The deficiency of indoleamine 2,3-dioxygenase aggravates the CCl4-induced liver fibrosis in mice PLoS One, 11: e0162183
Drug effects
Liang, Y-J., Luo, J., Yuan, Q., Zheng, D., Liu, Y-P., Shi, L., Zhou, Y., Chen, A-L. et al (2011) New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum PLoS One 6: e20247
Dystroglycan
Kastanis, G.J., Hernandez-Nazara, Z., Nieto, N,, Rincón-Sanchez, A.R., Popratiloff, A., Dominguez-Rosales, J.A., Lechuga, C.G., Rojkind, M. (2011) The role of dystroglycan in PDGF-BB-dependent migration of activated hepatic stellate cells/myofibroblasts Am. J. Physiol. Gastrointest. Liver Physiol., 301, G464–G474
EphB2 receptor tyrosine kinase
Mimche, P.N., Lee, C.M., Mimche, S.M., Thapa, M., Grakoui, A., Henkemeyer, M. and Lamb, T.J. (2018) EphB2 receptor tyrosine kinase promotes hepatic fibrogenesis in mice via activation of hepatic stellate cells Sci. Rep., 8: 2532
Epigenetic therapy
Zeybel, M., Luli, S., Sabater, L., Hardy, T., Oakley, F., Leslie, J., Page, A., Salvador, E.M., Sharkey, V., Tsukamoto, H., Chu, D.C.K. et al (2017) A proof-of-concept for epigenetic therapy of tissue fibrosis: inhibition of liver fibrosis progression by 3-deazaneplanocin A Mol. Ther., 25, 218-231
Fatty liver disease
Zhong, L., Huang, L., Xue, Q., Liu, C., Xu, K., Shen, W. and Deng, L. (2019) Cell‐specific elevation of Runx2 promotes hepatic infiltration of macrophages by upregulating MCP‐1 in high‐fat diet‐induced mice NAFLD J. Cell. Biochem. 120, 11761-11774
Fibrosis
Ben-Shoshan, S.O., Kagan, P., Sultan, M., Barabash, Z., Dor, C., Jacob-Hirsch, J., Harmelin, A., Pappo, O. et al (2017) ADAR1 deletion induces NFκB and interferon signaling dependent liver inflammation and fibrosis RNA Biol., 14, 587–602
Casas‐Grajales, S., Alvarez‐Suarez, D., Ramos‐Tovar, E., Buendía‐Montaño, L.D., Reyes‐Gordillo, K., Camacho, J., Tsutsumi, V., Lakshman, M.R. and Muriel, P. (2019) Stevioside inhibits experimental fibrosis by down‐regulating profibrotic Smad pathways and blocking hepatic stellate cell activation Basic Clin. Pharmacol. Toxicol. 2019, 124, 670–6800
Chen, L., Li, J., Zhang, J., Dai, C., Liu, X., Wang, J. et al (2015) S100A4 promotes liver fibrosis via activation of hepatic stellate cells J. Hepatol., 62, 156-164
Chen, L. and Brigstock, D.R. (2017) Cellular or exosomal microRNAs associated with CCN gene expression in liver fibrosis In CCN Proteins: Methods and Protocols, Methods Mol. Biol., 1489, (ed. Takigawa, M.) Springer Science+Business Media, LLC, pp 465-480
Chen, L. and Brigstock, D.R. (2017) Analysis of pathological activities of CCN proteins in fibrotic diseases: liver fibrosis In CCN Proteins: Methods and Protocols, Methods Mol. Biol., 1489, (ed. Takigawa, M.) Springer Science+Business Media, LLC, pp 445-463
Chen, W., Wu, X., Yan, Z., Xu, A., Yang, A. and You, H. (2019) Multitranscriptome analyses reveal prioritized genes specifically associated with liver fibrosis progression independent of etiology Am. J. Physiol. Gastrointest. Liver Physiol., 316, G744–G754
Chen, X., Li, X-F., Chen, Y., Zhu, S., Li, H-D., Chen, S-Y., Wang, J-N., Pan, X-Y., Bu, F-T., Huang, C. and Li, J. (2019) Hesperetin derivative attenuates CCl4-induced hepatic fibrosis and inflammation by Gli-1-dependent mechanisms Int. Immunopharm., 76: 105838
Jiang, X., Shen, T., Tang, X., Yang, W., Guo, H. and Ling, W. (2017) Cyanidin-3-O-β-glucoside combined with its metabolite protocatechuic acid attenuated the activation of mice hepatic stellate cells Food Funct., 2017, 8, 2945–2957
Jiang, Y., Zhao, Y., He, F. and Wang, H. (2019) Artificial microRNA-mediated Tgfbr2 and Pdgfrb co-silencing ameliorates carbon tetrachloride–induced hepatic fibrosis in mice Hum. Gene Ther., 30, 179-196
Kagan, P., Sultan, M., Tachlytski, I., Safran, M. and Ben-Ari, Z. (2017) Both MAPK and STAT3 signal transduction pathways are necessary for IL-6-dependent hepatic stellate cells activation PLoS One, 12: e0176173
Kim, J., Hyun, J., Wang, S., Lee, C., Lee, J-W., Moon, E-Y., Cha, H., Diehl, A.M. and Jung, Y. (2017) Thymosin beta-4 regulates activation of hepatic stellate cells via hedgehog signalling Sci. Rep., 7: 3815
Kong, De-L., Kong, F-Y., Liu, X-Y., Yan, C., Cui, J., Tang, R-X. and Zheng, K-Y. (2019) Soluble egg antigen of Schistosoma japonicum induces pyroptosis in hepatic stellate cells by modulating ROS production Parasites Vectors, 12: 475
Lao, Y., Li, Y., Zhang, P., Shao, Q., Lin, W., Qiu, B., Lv, Y., Tang, L., Su, S et al (2018) Targeting endothelial Erk1/2-Akt axis as a regeneration strategy to bypass fibrosis during chronic liver injury in mice Mol. Ther., 26, 2779-2797
Li, P., Li, Y., Zhu, L., Yang, Z., He, J., Wang, L., Shang, Q., Pan, H., Wang, H., Ma, X. et al (2018) Targeting secreted cytokine BMP9 gates the attenuation of hepatic fibrosis BBA – Mol. Basis Dis., 1864, 709–720
Li, Y., Pu, S., Liu, Q., Li, R., Zhang, J., Wu, T., Chen, L., Li, H. et al (2019) An integrin-based nanoparticle that targets activated hepatic stellate cells and alleviates liver fibrosis J. Control. Release, 303, 77–90
Mimche, P.N., Lee, C.M., Mimche, S.M., Thapa, M., Grakoui, A., Henkemeyer, M. and Lamb, T.J. (2018) EphB2 receptor tyrosine kinase promotes hepatic fibrogenesis in mice via activation of hepatic stellate cells Sci. Rep., 8: 2532
Strowitzki, M.J., Kirchberg, J., Tuffs, C., Schiedeck, M., Ritter, A.S., Biller, M., Harnoss, J.M., Lasitschka F. et al (2018) Loss of prolyl-hydroxylase 1 protects against biliary fibrosis via attenuated activation of hepatic stellate cells Am. J. Pathol., 188, 2826-2838
You, K., Li, S-Y., Gong, J., Fang, J-H., Zhang, C., Zhang, M., Yuan, Y., Yang, J. and Zhuang, S-M. (2018) Micro RNA-125b promotes hepatic stellate cell activation and liver fibrosis by activating RhoA signaling Mol. Ther. Nucl. Acids, 12, 57-66
Yu, F., Dong, B., Dong, P., He, Y., Zheng, J. and Xu, P. (2020) Hypoxia induces the activation of hepatic stellate cells through the PVT1‑miR‑152‑ATG14 signaling pathway Mol. Cell. Biochem., 465, 115–123
Zhan, T., Ma, H., Jiang, S., Zhong, Z., Wang, X., Li, C., Yu, D., Liu, L., Xu, J. and Xia, C. (2019) Interleukin-9 blockage reduces early hepatic granuloma formation and fibrosis during Schistosoma japonicum infection in mice Immunology, 158, 296–303
Zhao, X-K., Yu, L., Cheng, M-L., Che, P., Lu, Y-Y., Zhang, Q., Mu, M., Li, H. et al (2017) Focal adhesion kinase regulates hepatic stellate cell activation and liver fibrosis Sci. Rep., 7: 4032
Zhu, J., Luo, Z., Pan, Y., Zheng, W., Li, W., Zhang, Z., Xiong, P., Xu, D. et al (2019) H19/miR‐148a/USP4 axis facilitates liver fibrosis by enhancing TGF‐β signaling in both hepatic stellate cells and hepatocytes J. Cell. Physiol. 234, 9698–9710
Zou, X., Ramachandran, P., Kendall, T.J., Pellicoro, A., Dora, E., Aucott, R.L., Manwani, K., Man, T.Y. et al (2018) 11Beta-hydroxysteroid dehydrogenase-1 deficiency or inhibition enhances hepatic myofibroblast activation in murine liver fibrosis Hepatology, 67, 2167-2181
Gene transfer
Perugorria, M.J., Wilson, C.L., Zeybel, M., Walsh, M., Amin, S., Robinson, S., White, S.A., Burt, A.D., Oakley, F., Tsukamoto, H., Mann, D.A. and Mann, J. (2012) Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast tansdifferentiation Hepatology, 56, 1129-1139
Growth factors and growth factor receptors/signalling
Bahrami, A.J., Gunaje, J.J., Hayes, J., Riehle, K.J., Kenerson, H.L., Yeung, R.S., Stempien-Otero, A.S., Campbell, J.S. and Mahoney Jr, W.M. (2014) Regulator of G-protein signalling-5 is a marker of hepatic stellate cells and expression mediates response to liver injury PLoS One, 9: e108505
Huang, G., Besner, G.E. and Brigstock, D.R. (2012) Heparin-binding epidermal growth factor-like growth factor suppresses experimental liver fibrosis in mice Lab. Invest., 92, 703–712
Kastanis, G.J., Hernandez-Nazara, Z., Nieto, N,, Rincón-Sanchez, A.R., Popratiloff, A., Dominguez-Rosales, J.A., Lechuga, C.G., Rojkind, M. (2011) The role of dystroglycan in PDGF-BB-dependent migration of activated hepatic stellate cells/myofibroblasts Am. J. Physiol. Gastrointest. Liver Physiol., 301, G464–G474
Tsai, S-M. and Wang, W-P. (2011) Expression and function of fibroblast growth factor (FGF) 7 during liver regeneration Cell. Physiol. Biochem., 27, 641-652
Gut microbiota
Bigorgne, A.E., John, B., Ebrahimkhani, M.R., Shimizu-Albergine, M., Campbell, J.S. and Crispe, I.N. (2016) TLR4-dependent secretion by hepatic stellate cells of the neutrophil-chemoattractant CXCL1 mediates liver response to gut microbiota PLoS One, 11: e0151063
Hedgehog signalling
Hyun, J., Wang, S., Kim, J., Rao, K.M., Park, S.Y., 2, Chung, I., Ha, C-S. et al (2016) MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression Nat. Comm., 7: 10993
Kim, J., Hyun, J., Wang, S., Lee, C., Lee, J-W., Moon, E-Y., Cha, H., Diehl, A.M. and Jung, Y. (2017) Thymosin beta-4 regulates activation of hepatic stellate cells via hedgehog signalling Sci. Rep., 7: 3815
Hepatitis (T-cell mediated)
Chen, L., Gu, J., Qian, Y., Li, M., Qian, Y., Xu, M., Li, J., Wen, Y., Xia, L. et al (2019) Deletion of C-C motif chemokine ligand 5 worsens invariant natural killer T–cell mediated hepatitis via compensatory up-regulation of CXCR2–related chemokine activity Cell. Mol. Gastroenterol. Hepatol., 7, 623–639
Hyperglycaemia
Lee, Y-S., Eun, H.S., Kim, S.Y., Jeong, J-M., Seo, W., Byun, J-S., Jeong, W-I. and Yi, H-S. (2106) Hepatic immunophenotyping for streptozotocin-induced hyperglycemia in mice Sci. Rep., 6: 30656
Hypoxia
Yu, F., Dong, B., Dong, P., He, Y., Zheng, J. and Xu, P. (2020) Hypoxia induces the activation of hepatic stellate cells through the PVT1‑miR‑152‑ATG14 signaling pathway Mol. Cell. Biochem., 465, 115–123
Indoleamine 2,3-dioxygenase
Ogiso, H., Ito, H., Ando, T., Arioka, Y., Kanbe, A., Ando, K., Ishikawa, T., Saito, K., Hara, A., Moriwaki, H., Shimizu, M. and Seishima, M. (2016) The deficiency of indoleamine 2,3-dioxygenase aggravates the CCl4- induced liver fibrosis in mice PLoS One, 11: e0162183
Integrin expression
Huang, G. and Brigstock, D.R. (2011) Integrin expression and function in the response of primary culture hepatic stellate cells to connective tissue growth factor (CCN2) J. Cell. Mol. Med., 15, 1087-1095
Martin, K., Pritchett, J., Llewellyn, J., Mullan, A.F., Athwal, V.S., Dobie, R., Harvey, E., Zeef, L. et al (2016) PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis Nat. Comm., 7: 12502
Interferon signalling
Ben-Shoshan, S.O., Kagan, P., Sultan, M., Barabash, Z., Dor, C., Jacob-Hirsch, J., Harmelin, A., Pappo, O. et al (2017) ADAR1 deletion induces NFκB and interferon signalling dependent liver inflammation and fibrosis RNA Biol., 14, 587–602
Interleukin expression
Kagan, P., Sultan, M., Tachlytski, I., Safran, M. and Ben-Ari, Z. (2017) Both MAPK and STAT3 signal transduction pathways are necessary for IL-6-dependent hepatic stellate cells activation PLoS One, 12: e0176173
Mchedlidze, T., Waldner, M., Zopf, S., Walker, J., Rankin, A.L., Schuchmann, M., Voehringer, D. et al (2013) Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis Immunity, 39, 357–371
Seo, W., Eun, H.S., Kim, S.Y., Yi, H-S., Lee, Y-S., Park, S-H., Jang, M-J., Jo, E., Kim, S.C. et al (2016) Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis Hepatology 64, 616-631
Tan, Z., Qian, X., Jiang, R., Liu, Q., Wang, Y., Chen, C., Wang, X., Ryffe, B. and Sun, B. (2013) IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation J. Immunol., 191, 1835–1844
Intrahepatic signaling
Xiong, X., Kuang, H., Ansari, S., Liu, T., Gong, J., Wang, S., Zhao, X-Y., Ji, Y., Li, C., Guo, L. et al (2019) Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis Mol. Cell, 75, 644–660
Ischemia/reperfusion injury
Akateh, C., Beal, E.W., Kim, J-L., Reader, B.F., Maynard, K., Zweier, J.L., Whitson, B.A. and Black, S.M. (2019) Intrahepatic delivery of pegylated catalase is protective in a rat ischemia/reperfusion injury model J. Surgic. Res., 238, 152-163
Leishmania
Khadem, F., Gao, X., Mou, Z., Jia, P., Movassagh, H., Onyilagha, C., Gounni, A.S., Wright, M.C. and Uzonna, J.E. (2016) Hepatic stellate cells regulate liver immunity to visceral Leishmaniasis through P110δ-dependent induction and expansion of regulatory T cells in mice Hepatology, 63, 620-632
Lipid metabolism
Jeong, W-I., Osei-Hyiaman, D., Park, O., Liu, J., Batkai, S., Mukhopadhyay, P., Horiguchi, N., Harvey-White, J. et al (2008) Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver Cell Metab., 7, 227-235
Lipopolysaccharide
Chen, M., Liu, J., Yang, W. and Ling, W, (2017) Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signalling Autophagy, 13, 813–1827
Macrophage-mediated liver injury
Lee, Y-S., Kim, M-H., Yi, H-S., Kim, S.Y., Kim, H-H., Kim, J.H., Yeon, J.E., Byun, K.S. et al (2018) CX3CR1 differentiates F4/80low monocytes into pro-inflammatory F4/80high macrophages in the liver Sci. Rep., 8:15076
Zhong, L., Huang, L., Xue, Q., Liu, C., Xu, K., Shen, W. and Deng, L. (2019) Cell‐specific elevation of Runx2 promotes hepatic infiltration of macrophages by upregulating MCP‐1 in high‐fat diet‐induced mice NAFLD J. Cell. Biochem. 120, 11761-11774.
Mesenchymal/mesothelial cells
Li, Y., Wang, J. and Asahina, K. (2013) Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial–mesenchymal transition in liver injury Proc. Natl. Acad. Sci. USA, 110, 2324-2329
Li, Y., Lua, I., French, S.W. and Asahina, K. (2016) Role of TGF- signalling in differentiation of mesothelial cells to vitamin A-poor hepatic stellate cells in liver fibrosis Am. J. Physiol. Gastrointest. Liver Physiol., 310, G262–G272
Lua, I., James, D., Wang, J., Wang, K.S. and Asahina, K. (2014) Mesodermal mesenchymal cells give rise to myofibroblasts, but not epithelial cells, in mouse liver injury Hepatology, 60, 311-322
Sicklick, J.K., Choi, S.S., Bustamente, M., McCall, S.J., Hernández-Pérez, E., Huang, J., Li, Y-X., Rojkind, M. and Diehl, A.M. (2006) Evidence for epithelial-mesenchymal transitions in adult liver cells Am. J. Physiol. Liver Physiol., 291, G575-G583
Methodology
Liu, W., Hou, Y., Chen, H., Wei, H., Lin, W., Li, J., Zhang, M., He, F. and Jiang, Y. (2011) Sample preparation method for isolation of single-cell types from mouse liver for proteomic studies Proteomics 11, 3556–3564
Chang, W., Yang, M., Song, L., Shen, K., Wang, H., Gao, X., Li, M., Niu, W. and Qin, X. (2014) Isolation and culture of hepatic stellate cells from mouse liverActa Biochim. Biophys. Sin., 46, 291–298
Weiskirchen, S., Tag, C.G., Sauer-Lehnen, S., Tacke, F. and Weiskirchen, R. (2017) Isolation and culture of primary murine hepatic stellate cells In Fibrosis: Methods and Protocols, Methods in Molecular Biology, 1627, Rittié, L. (ed.), Springer Science+Business Media LLC pp165-191
MHC
Zhou, C-L., Kong, D-L., Liu, J-F., Lu, Z-K., Guo, H-F., Wang, W., Qiu, J-F., Liu, X-J. and Wang, Y. (2017) MHC II− , but not MHC II+ , hepatic stellate cells contribute to liver fibrosis of mice in infection with Shistosoma japonicum BBA – Mol. Basis Disease, 1863, 1848–1857
Microfluidic chip mimicking
Du, Y., Li, N., Yang, H., Luo, C., Gong, Y., Tong, C., Gao, Y., Lü, S. and Long, M. (2017) Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip Lab. Chip, 17, 782-794
NADPH oxidase – NOX1 isoform
Cui, W., Matsuno, K., Iwata, K., Ibi, M., Matsumoto, M., Zhang, J., Zhu, K., Katsuyama, M., Torok, N.J. and Yabe-Nishimura, C. (2011) NOX1/Nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation Hepatology, 54, 949-958
NFκB signalling
Ben-Shoshan, S.O., Kagan, P., Sultan, M., Barabash, Z., Dor, C., Jacob-Hirsch, J., Harmelin, A., Pappo, O. et al (2017) ADAR1 deletion induces NFκB and interferon signalling dependent liver inflammation and fibrosis RNA Biol., 14, 587–602
He, F., Guo, F-C., Li, Z., Yu, H-C., Ma, P-F., Zhao, J-L., Feng, L., Li, W-N. et al (2015) Myeloid-specific disruption of recombination signal binding protein Jj ameliorates hepatic fibrosis by attenuating inflammation through cylindromatosis in mice Hepatology, 61, 303-314
He, X., Pu, G., Tang, R., Zhang, D. and Pan, W. (2014) Activation of nuclear factor kappa B in the hepatic stellate cells of mice with Schistosomiasis japonica PloS One 9: e104323
NK cell killing
Jeong, W-I., Park, O. and Gao, B. (2008) Abrogation of the antifibrotic effect of natural killer cells/interferon- contributes to alcohol acceleration of liver fibrosis Gastroenterology 134, 248-258
Radaeva, S., Sun, R., Jaruga, B., Nguyen, V.T., Tian, Z. and Gao, B. (2006) Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners Gastroenterology, 130, 434-452
Radaeva. S., Wang, L., Radaeva, S., Jeong, W-I., Park, O. and Gao, B. (2007) Retinoic acid signalling sensitizes hepatic stellate cells to NK cell killing via upregulation of NK cell activating ligand RAE1 Am. J. Physiol. Gastrointest. Liver Physiol., 293, G809-G816
Notch signalling
He, F., Guo, F-C., Li, Z., Yu, H-C., Ma, P-F., Zhao, J-L., Feng, L., Li, W-N. et al (2015) Myeloid-specific disruption of recombination signal binding protein Jj ameliorates hepatic fibrosis by attenuating inflammation through cylindromatosis in mice Hepatology, 61, 303-314
Paracrine stimulation
Corbett, L., Mann, J. and Mann, D.A. (2015) Non-canonical Wnt predominates in activated rat hepatic stellate cells, influencing HSC survival and paracrine stimulation of Kupffer cells PLoS One, 10: e0142794
Wright, J.H., Johnson, M.M., Shimizu-Albergine, M., Bauer, R.L., Hayes, B.J., Surapisitchat, J., Hudkins et al (2014) Paracrine activation of hepatic stellate cells in platelet-derived growth factor C transgenic mice: Evidence for stromal induction of hepatocellular carcinoma Int. J. Cancer, 134, 778–788
PDGF signalling
Wright, J.H., Johnson, M.M., Shimizu-Albergine, M., Bauer, R.L., Hayes, B.J., Surapisitchat, J., Hudkins, K.L., Riehle, K.J., Johnson, S.C., et al (2014) Paracrine activation of hepatic stellate cells in platelet-derived growth factor C transgenic mice: Evidence for stromal induction of hepatocellular carcinoma Int. J. Cancer, 134, 778–788
Wright, J.H., Johnson, M.M., Shimizu-Albergine, M., Bauer, R.L., Hayes, B.J., Surapisitchat, J., Hudkins, K.L. et al (2014) Paracrine activation of hepatic stellate cells in platelet-derived growth factor C transgenic mice: Evidence for stromal induction of hepatocellular carcinoma Int. J. Cancer, 134, 778–788
Zhang, X., Tan, Z., Wang, Y., Tang, J., Jiang, R., Hou, J., Zhuo, H., Wang, X., Ji, J., Qin, X. and Sun, B. (2015) PTPRO-associated hepatic stellate cell activation plays a critical role in liver fibrosis Cell. Physiol. Biochem., 35, 885-898
Proteoglycans
Bukong, T.N., Maurice, S.B., Chahal, B., Schaeffer, D.F. and Winwood, P.J. (2016) Versican: a novel modulator of hepatic fibrosis Lab. Invest., 96, 361–374
Proteomics
Liu, W., Hou, Y., Chen, H., Wei, H., Lin, W., Li, J., Zhang, M., He, F. and Jiang, Y. (2011) Sample preparation method for isolation of single-cell types from mouse liver for proteomic studies Proteomics 11, 3556–3564
Retinoic acid/ester
Chen, M., Liu, J., Yang, W. and Ling, W, (2017) Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signalling Autophagy, 13, 813–1827
Dunham, R.M., Thapa, M., Velazquez, V.M., Elrod, E.J., Denning, T.L., Pulendran, B. and Grakoui, A. (2013) Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid J. Immunol., 190, 2009–2016
Jie, Z., Liang, Y., Yi, P., Tang, H., Soong, L., Cong, Y., Zhang, K. and Sun, J. (2017) Retinoic acid regulates immune responses by promoting IL-22 and modulating S100 proteins in viral hepatitis J. Immunol., 198, 3448– 3460
Radaeva, S., Sun, R., Jaruga, B., Nguyen, V.T., Tian, Z. and Gao, B. (2006) Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners Gastroenterology, 130, 434-452
Radaeva, S., Wang, L., Radaeva, S., Jeong, W-I., Park, O. and Gao, B. (2007) Retinoic acid signalling sensitizes hepatic stellate cells to NK cell killing via upregulation of NK cell activating ligand RAE1 Am. J. Physiol. Gastrointest. Liver Physiol., 293, G809-G816
Schreiber, R., Taschler, U., Wolinski, H., Seper, A., Tamegger, S.N., Graf, M., Kohlwein, S.D. et al (2009) Esterase 22 and beta-glucuronidase hydrolyze retinoids in mouse liver J. Lipid Res., 50, 2514–2523
Taschler, U., Schreiber, R., Chitraju, C., Grabner, G.F., Romauch, M., Wolinski, H., Haemmerle, G. et al (2015) Adipose triglyceride lipase is involved in the mobilization of triglyceride and retinoid stores of hepatic stellate cells Biochim. Biophys. Acta, 1851, 937–945
RNA (various types)
Hyun, J., Wang, S., Kim, J., Rao, K.M., Park, S.Y., 2, Chung, I., Ha, C-S., Kim, S-W., Yun, Y.H. and Jung, Y. (2016) MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression Nat. Comm., 7: 10993
Leask, A., Chen, S., Pala, D., Brigstock, D.R. (2008) Regulation of CCN2 mRNA expression and promoter activity in activated hepatic stellate cells J. Cell. Commun. Signal., 2, 49-56
You, K., Li, S-Y., Gong, J., Fang, J-H., Zhang, C., Zhang, M., Yuan, Y., Yang, J. and Zhuang, S-M. (2018) Micro RNA-125b promotes hepatic stellate cell activation and liver fibrosis by activating RhoA signaling Mol. Ther. Nucl. Acids, 12, 57-66
Yu, F., Zheng, J., Mao, Y., Dong, P., Lu, Z., Li, G., Guo, C., Liu, Z. and Fan, X. (2015) Long non-coding RNA growth arrest-specific transcript 5 (GAS5) inhibits liver fibrogenesis through a mechanism of competing endogenous RNA J. Biol. Chem., 290, 28286–28298
Zheng, J., Dong, P., Mao, Y., Chen, S., Wu, X., Li, G., Lu, Z. and Yu, F. (2015) lincRNA-p21 inhibits hepatic stellate cell activation and liver fibrogenesis via p21 FEBS J., 282, 4810–4821
Schistosome infection
Duan, Y., Gu, X., Zhu, D., Sun, W., Chen, J., Feng, J., Song, K., Xu, F., He, X. and He, X. (2014) Schistosoma japonicum soluble egg antigens induce apoptosis and inhibit activation of hepatic stellate cells: a possible molecular mechanism Int. J. Parasitol., 44, 217–224
He, X., Pu, G., Tang, R., Zhang, D. and Pan, W. (2014) Activation of nuclear factor kappa B in the hepatic stellate cells of mice with Schistosomiasis japonica PloS One 9: e104323
Kong, De-L., Kong, F-Y., Liu, X-Y., Yan, C., Cui, J., Tang, R-X. and Zheng, K-Y. (2019) Soluble egg antigen of Schistosoma japonicum induces pyroptosis in hepatic stellate cells by modulating ROS production Parasites Vectors, 12: 475
Liang, Y-J., Luo, J., Lu, Q., Zhou, Y., Wu, H-W., Zheng, D., Ren, Y-Y., Sun, K-Y., Wang, Y. and Zhang, Z-S. (2012) Gene profile of chemokines on hepatic stellate cells of schistosome-infected mice and antifibrotic roles of CXCL9/10 on liver non-parenchymal cells PLoS One, 7: e42490
Luo, X., Zhang, D., Xie, J., Su, Q., He, X., Bai, R., Gao, G. and Pan, W. (2018) MicroRNA-96 promotes schistosomiasis hepatic fibrosis in mice by suppressing Smad7 Mol. Ther. Meth. Clin. Devel., 11, 73-82
Wang, M., Abais, J.M., Meng, N., Zhang, Y., Ritter, J.K., Li, P-L. and Tang, W-X. (2014) Upregulation of cannabinoid receptor-1 and fibrotic activation of mouse hepatic stellate cells during Schistosoma J. infection: Role of NADPH oxidase Free Radic. Biol. Med., 71, 109–120
Wang, Y., Lin, C., Cao, Y., Duan, Z., Guan, Z., Xu, J., Zhu, X-Q. and Xia, C. (2017) Up-regulation of Interleukin-21 contributes to liver pathology of schistosomiasis by driving GC immune responses and activating HSCs in mice Sci. Rep., 7: 16682
Zhan, T., Ma, H., Jiang, S., Zhong, Z., Wang, X., Li, C., Yu, D., Liu, L., Xu, J. and Xia, C. (2019) Interleukin-9 blockage reduces early hepatic granuloma formation and fibrosis during Schistosoma japonicum infection in mice Immunology, 158, 296–303
Zhou, C-L., Kong, D-L., Liu, J-F., Lu, Z-K., Guo, H-F., Wang, W., Qiu, J-F., Liu, X-J. and Wang, Y. (2017) MHC II− , but not MHC II+ , hepatic stellate cells contribute to liver fibrosis of mice in infection with Shistosoma japonicum BBA – Mol. Basis Disease, 1863, 1848–1857
Steatohepatitis
Asakawa, M., Itoh, M., Suganami, T., Sakai, T., Kanai, S., Shirakawa, I., Yuan, X., Hatayama, T., Shimada, S. et al (2019) Upregulation of cancer-associated gene expression in activated fibroblasts in a mouse model of non-alcoholic steatohepatitis Sci. Rep., 9: 19601
Kim, K.H., Kim, S.H., Han, D.H., Jo, J.S., Lee, Y-h. and Lee, M-S, (2018) Growth differentiation factor 15 ameliorates non-alcoholic steatohepatitis and related metabolic disorders in mice Sci. Rep., 8: 6789
McCommis, K.S., Hodges, W.T., Brunt, E.M., Nalbantoglu, I., McDonald, W.G., Holley, C., Fujiwara, H. et al (2017) Targeting the mitochondrial pyruvate carrier attenuates fibrosis in a mouse model of nonalcoholic steatohepatitis Hepatology, 65, 1543-1556
Pulli, B., Ali, M., Iwamoto, Y., Zeller, M.W.G., Schob, S., Linnoila, J.J. and Chen, J.W. (2015) Myeloperoxidase–hepatocyte–stellate cell cross talk promotes hepatocyte injury and fibrosis in experimental nonalcoholic steatohepatitis Antioxid. Redox Signal., 23, 1255–1269
T-cells
Chinnadurai, R. and Grakoui, A. (2010) B7-H4 mediates inhibition of T cell responses by activated murine hepatic stellate cells Hepatology, 52, 2177-2185
Dunham, R.M., Thapa, M., Velazquez, V.M., Elrod, E.J., Denning, T.L., Pulendran, B. and Grakoui, A. (2013) Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid J. Immunol., 190, 2009–2016
Feng, M., Wang, Q., Jiang, Z., Ding, J., Wang, H., Wang, M., Lu, L. and Guan, W. (2016) Adoptive transferred hepatic stellate cells attenuated drug-induced liver injury by modulating the rate of regulatory T cells/T helper 17 cells Clin. Immunol., 165, 12–18
Feng, M., Wang, Q., Jiang, Z., Ding, J., Wang, H., Wang, M., Lu, L. and Guan, W. (2016) Adoptive transferred hepatic stellate cells attenuated drug-induced liver injury by modulating the rate of regulatory T cells/T helper 17 cells Clin. Immunol., 165, 12–18
Ichikawa, S., Mucida, D., Tyznik, A.J., Kronenberg, M. and Cheroutre, H. (2011) Hepatic stellate cells function as regulatory bystanders J. Immunol., 186, 5549-5555
Khadem, F., Gao, X., Mou, Z., Jia, P., Movassagh, H., Onyilagha, C., Gounni, A.S., Wright, M.C. and Uzonna, J.E. (2016) Hepatic stellate cells regulate liver immunity to visceral Leishmaniasis through P110δ-dependent induction and expansion of regulatory T cells in mice Hepatology, 63, 620-632
TGF-β signalling
Li, Y., Lua, I., French, S.W. and Asahina, K. (2016) Role of TGF-β signalling in differentiation of mesothelial cells to vitamin A-poor hepatic stellate cells in liver fibrosis Am. J. Physiol. Gastrointest. Liver Physiol., 310, G262–G272
Toll-like receptor signalling
Seifert, L., Deutsch, M., Alothman, S., Alqunaibit, D., Werba, G., Pansari, M., Pergamo, M., Ochi, A. (2015) Dectin-1 regulates hepatic fibrosis and hepatocarcinogenesis by suppressing TLR4 signalling pathways Cell Rep., 13, 1–13
Seo, W., Eun, H.S., Kim, S.Y., Yi, H-S., Lee, Y-S., Park, S-H., Jang, M-J., Jo, E., Kim, S.C. et al (2016) Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis Hepatology 64, 616-631
Transcription
Inflammatory response
Elsharkawy, A.M., Oakley, F., Lin, F., Packham, G., Mann, D.A, and Mann, J. (2010) The NF-κB p50:p50:HDAC-1 repressor complex orchestrates transcriptional inhibition of multiple pro-inflammatory genes J. Hepatol., 53, 519-527
Oakley, F., Mann, J., Nailard, S., Smart, D.E., Mungalasingh, N., Constandinou, C., Ali, S., Wilson, S.J. et al (2005) Nuclear factor-B1 (p50) limits the inflammatory and fibrogenic responses to chronic injury Am. J. Pathol., 166, 695-708
Metalloproteinases
Murthy, A., Shao, Y.W., Defamie, V., Wedeles, C., Smookler, D. and Khokha, R. (2012) Stromal TIMP3 regulates liver lymphocyte populations and provides protection against Th1 T cell-driven autoimmune hepatitis J. Immunol., 188, 2876–2883
Perugorria, M.J., Wilson, C.L., Zeybel, M., Walsh, M., Amin, S., Robinson, S., White, S.A., Burt, A.D., Oakley, F., Tsukamoto, H., Mann, D.A. and Mann, J. (2012) Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast tansdifferentiation Hepatology, 56, 1129-1139
Methylation
Perugorria, M.J., Wilson, C.L., Zeybel, M., Walsh, M., Amin, S., Robinson, S., White, S.A. et al (2012) Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast tansdifferentiation Hepatology, 56, 1129-1139
Viral hepatitis
Jie, Z., Liang, Y., Yi, P., Tang, H., Soong, L., Cong, Y., Zhang, K. and Sun, J. (2017) Retinoic acid regulates immune responses by promoting IL-22 and modulating S100 proteins in viral hepatitis J. Immunol., 198, 3448– 3460
Wnt system
Corbett, L., Mann, J. and Mann, D.A. (2015) Non-canonical Wnt predominates in activated rat hepatic stellate cells, influencing HSC survival and paracrine stimulation of Kupffer cells PLoS One, 10: e0142794
Mouse pancreas
Erkan, M., Adler, G., Apte, M.V., Bachem, M.G., Buchholz, M., Detlefsen, S., Esposito, I., Friess, H. et al (2012) StellaTUM: current consensus and discussion on pancreatic stellate cell research Gut, 61, 172-178
Charrier, A., Chen, R., Chen, L., Kemper, S., Hattori, T., Takigawa, M. and Brigstock, D.R. (2014) Connective tissue growth factor (CCN2) and microRNA-21 are components of a positive feedback loop in pancreatic stellate cells (PSC) during chronic pancreatitis and are exported in PSC-derived exosomes J. Cell Commun. Signal., 8, 147–156
Lawrencia, C., Charrier, A., Huang, G. and Brigstock, D.R. (2009) Ethanol-mediated expression of connective tissue growth factor (CCN2) in mouse pancreatic stellate cells Growth Factors, 27, 91–99
Liu, J., Gao, M., Nipper, M., Deng, J., Sharkey, F.E., Johnson, R.L., Crawford, H.C., Chen, Y. and Wang, P. (2019) Activation of the intrinsic fibroinflammatory program in adult pancreatic acinar cells triggered by Hippo signaling disruption PLoS Biol., 17: e3000418
Ulmasov, B., Xu, Z., Tetri, L.H., Inagami, T. and Neuschwander-Tetri, B.A. (2009) Protective role of angiotensin II type 2 receptor signaling in a mouse model of pancreatic fibrosis Am. J. Physiol. Gastrointest. Liver Physiol., 296, G284–G294
Rat liver (majority of papers relate to fibrosis)
Adipogenesis
Jiang, Y., Wang, S., Zhao, Y., Lin, C., Zhong, F., Jin, L., He, F. and Wang, H. (2015) Histone H3K9 demethylase JMJD1A modulates hepatic stellate cells activation and liver fibrosis by epigenetically regulating peroxisome proliferator-activated receptor γ FASEB J. 29, 1830–1841
Alcoholic liver injury
Byun, J-S., Suh, Y-G., Yi, H-S., Lee, Y-S. and Jeong, W-I. (2013) Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice J, Hepatol., 58, 342–349
Apoptosis
Habens, F., Srinivasan, N., Oakley, F., Mann, D.A., Ganesan, A. and Packham, G. (2005) Novel sulfasalazine analogues with enhanced NF-kB inhibitory and apoptosis promoting activity Apoptosis, 10, 481-491
Oakley, F., Meso, M., Iredale, J.P., Green, K., Marek, C.J., Zhou, X., May, M.J. et al (2005) Inhibition of inhibitor of κB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis Gastroenterology, 128, 108-120
Atorvastatin effects
Klein, S., Klösel, J., Schierwagen, R., Körner, C., Granzow, M., Huss, S., Reza Mazar, I.G. et al (2012) Atorvastatin inhibits proliferation and apoptosis, but induces senescence in hepatic myofibroblasts and thereby attenuates hepatic fibrosis in rats Lab. Invest., 92, 1440–1450
Autophagic cell death
Shaker, M.E., Ghani, A., Shiha, G.E., Ibrahim, T.M., Mehal, W.Z.(2013) Nilotinib induces apoptosis and autophagic cell death of activated hepatic stellate cells via inhibition of histone deacetylases Biochim. Biophys. Acta, 1833, 1992–2003
Bile duct ligation
Kageyama, Y., Ikeda, H., Watanabe, N., Nagamine, M., Kusumoto, Y., Yashiro, M., Satoh, Y., Shimosawa, T., Shinozaki, K., et al (2012) Antagonism of sphingosine 1-phosphate receptor 2 causes a selective reduction of portal vein pressure in bile duct-ligated rodents Hepatology, 56, 1427-1438
Chemotaxis
Gong, J., Han, J., He, J., Liu, J., Han, P., Wang, Y., Li, M., Li, D., Ding, X. et al (2017) Paired related homeobox protein 1 regulates PDGF-induced chemotaxis of hepatic stellate cells in liver fibrosis Lab. Invest., 97, 1020–1032
Cirrhosis
De Mesquita, F.C., Guixé-Muntet, S., Fernández-Iglesias, A., Maeso-Díaz, R., Vila, S., Hide, D., OrtegaRibera, M., Rosa, J.L. et al (2017) Liraglutide improves liver microvascular dysfunction in cirrhosis: Evidence from translational studies Sci. Rep., 7: 3255
Tang, W-P., Akahoshi, T., Piao, J-S., Narahara, S., Murata, M., Kawano, T., Hamano, N. et al (2015) Basic fibroblast growth factor-treated adipose tissue-derived mesenchymal stem cell infusion to ameliorate liver cirrhosis via paracrine hepatocyte growth factor J. Gastroenterol. Hepatol., 20, 1065–1074
Tang, W-P., Akahoshi, T., Piao, J-S., Narahara, S., Murata, M., Kawano, T., Hamano, N., Ikeda, T. and Hashizume, M. (2016) Splenectomy enhances the therapeutic effect of adipose tissue-derived mesenchymal stem cell infusion on cirrhosis rats Liver Int.,36, 1151–1159
Connective tissue/collagen
Jiroutova, A., Slavkovsky, R., Cermakova, M., Majdiakova, L., Hanovcova, I., Bolehovska, R., Hadjzlerova, M., Radilova, H., Ruszova, E. and Kanta, J. (2007) Expression of mRNAs related to connective tissue metabolism in rat hepatic stellate cells and myofibroblasts Exp. Toxicol. Pathol., 58, 263-273
Mousavi, S.A., Fønhus, M.S. and Berg, T. (2009) Up-regulation of uPARAP/Endo180 during culture activation of rat hepatic stellate cells and its presence in hepatic stellate cell lines from different species BMC Cell Biol., 10:39
Contractile properties
Laleman W., Van Landeghem L, Severi T, Vander Elst I, Zeegers M, Bisschops R, Van Pelt J, Roskams T, Cassiman D, Fevery J, Nevens F. (2007) Both Ca2+-dependent and -independent pathways are involved in rat hepatic stellate cell contraction and intrahepatic hyperresponsiveness to methoxamine Am. J. Physiol. Gastrointest. Liver Physiol., 292, G556–G564
Laleman, W., van Landeghem, L., van der Elst, I., Zeegers, M., Fevery, J. and Nevens, F. (2007) Nitroflurbiprofen, a nitric oxide-releasing cyclooxygenase inhibitor, improves cirrhotic portal hypertension in rats Gastroenterology, 132, 709-719
3D-Assembly
Weng, Y-S., Chang, S-F., Shih, M-C., Tseng, S-H. and Lai, C-H. (2017) Scaffold-free liver-on-a-chip with multiscale organotypic cultures Adv. Mater., 29: 1701545
DNA methylation
Page, A., Paoli, P., Salvador, E.M., White, S., French, J. and Mann, J. (2016) Hepatic stellate cell transdifferentiation involves genome-wide remodeling of the DNA methylation landscape J. Hepatol., 64, 661–673
Zheng, J., Wu, c., Lin, Z., Guo, Y., Shi, L., Dong, P., Lu, Z., Gao, S., Liao, Y., Chen, B. and Yu, F. (2014) Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNAmediated control of DNA methylation – a novel mechanism suppressing liver fibrosis FEBS J., 281, 88–103
Drug effects
Habens, F., Srinivasan, N., Oakley, F., Mann, D.A., Ganesan, A. and Packham, G. (2005) Novel sulfasalazine analogues with enhanced NF-kB inhibitory and apoptosis promoting activity Apoptosis, 10, 481-491
Khan, F., Peltekian, K.M. and Peterson, T.C. (2008) Effect of interferon-alpha, Ribavirin, pentoxifylline, and interleukin-18 antibody on hepatitis C sera-stimulated hepatic stellate cell proliferation J. Interferon Cytokine Res., 28, 643-652
Laleman W., Van Landeghem L, Severi T, Vander Elst I, Zeegers M, Bisschops R, Van Pelt J, Roskams T, Cassiman D, Fevery J, Nevens F. (2007) Both Ca2+-dependent and -independent pathways are involved in rat hepatic stellate cell contraction and intrahepatic hyperresponsiveness to methoxamine Am. J. Physiol. Gastrointest. Liver Physiol., 292, G556–G564 Laleman, W., van Landeghem, L., van der Elst, I., Zeegers, M., Fevery, J. and Nevens, F. (2007)
Nitroflurbiprofen, a nitric oxide-releasing cyclooxygenase inhibitor, improves cirrhotic portal hypertension in rats Gastroenterology, 132, 709-719
Oakley, F., Meso, M., Iredale, J.P., Green, K., Marek, C.J., Zhou, X., May, M.J., Millward-Sadler, H., Wright, M.C. and Mann, D.A. (2005) Inhibition of inhibitor of B kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis Gastroenterology, 128, 108-120
Peterson, T.C. and Rowden, G. (1998) Drug-metabolizing enzymes in rat liver myofibroblasts Biochem. Pharmacol., 55, 703-708
Epigallocatechin
Fu, Y. and Chen, A. (2006) The phyto-chemical (−)-epigallocatechin gallate suppresses gene expression of epidermal growth factor receptor in rat hepatic stellate cells in vitro by reducing the activity of Egr-1 Biochem. Pharmacol., 72, 227-238
Fu, Y., Zheng, S., Lu, S.C. and Chen, A. (2008) Epigallocatechin-3-gallate inhibits growth of activated hepatic stellate cells by enhancing the capacity of glutathione synthesis Mol. Pharmacol., 73, 1465-1473
Yumei, F., Zhou, Y., Zheng, S. and Chen, A. (2006) The antifibrogenic effect of (-)-epigallocatechin gallate results from the induction of de novo synthesis of glutathione in passaged rat hepatic stellate cells Lab. Invest., 86, 697-709
Fatty liver disease
Li, B-H., He, F-P., Yang, X, Chen, Y-W. and Fan, J-G. (2017) Steatosis induced CCL5 contributes to earlystage liver fibrosis in nonalcoholic fatty liver disease progress Translat. Res., 180, 103–117
Gene transfer
Gao, R., McCormick, C.J., Arthur, M.J.P., Ruddell, R., Oakley, F., Smart, D.E., Murphy, F.R., Garris, M.P.G. and Mann, D.A. (2002) High efficiency gene transfer into cultured primary rat and human hepatic stellate cells using baculovirus vectors Liver 22, 15-22
Smith, P.G., Oakley, F., Fernandez, M., Mann, D.A., Lemoine, N.R. and Whitehouse, A. (2005) Herpesvirus saimiri-based vector biodistribution using noninvasive optical imaging Gene Ther., 12, 1465-1476
Growth factors and growth factor receptors/signalling
Cassiman, D., Denef, C., Desmet, V.J. and Roskams, T. (2001) Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors Hepatology, 33, 148-158
De Leve, L.D., Wang, X. and Wang, L. (2016) VEGF-sdf1 recruitment of CXCR7+ bone marrow progenitors of liver sinusoidal endothelial cells promotes rat liver regeneration Am. J. Physiol. Gastrointest Liver Physiol., 310, G739–G746
Fu, Y. and Chen, A. (2006) The phyto-chemical (−)-epigallocatechin gallate suppresses gene expression of epidermal growth factor receptor in rat hepatic stellate cells in vitro by reducing the activity of Egr-1 Biochem. Pharmacol., 72, 227-238
Jiroutova, A., Slavkovsky, R., Cermakova, M., Majdiakova, L., Hanovcova, I., Bolehovska, R., Hadjzlerova, M. et al (2007) Expression of mRNAs related to connective tissue metabolism in rat hepatic stellate cells and myofibroblasts Exp. Toxicol. Pathol., 58, 263-273
Lin, J. and Chen, A. (2008) Activation of peroxisome proliferator-activated receptor-g by curcumin blocks the signalling pathways for PDGF and EGF in hepatic stellate cells Lab. Invest., 88, 529-540
Tao, Y-Y., Wang, Q-L., Shen, L., Fu, W-W, and Liu, C-H. (2013) Salvianolic acid B inhibits hepatic stellate cell activation through transforming growth factor beta-1 signal transduction pathway in vivo and in vitro Exp. Biol. Med., 238, 1284–1296
Verma-Gandhu, M., Peterson, M.R. and Peterson, T.C. (2007) Effect of fetuin, a TGF antagonist and pentoxifylline, a cyrokine antagonist on hepatic stellate cell function and fibrotic parameters in fibrosis Eur. J. Pharmacol., 572, 220-227
Yumei, F., Zhou, Y., Zheng, S. and Chen, A. (2006) The antifibrogenic effect of (-)-epigallocatechin gallate results from the induction of de novo synthesis of glutathione in passaged rat hepatic stellate cells Lab. Invest., 86, 697-709
Zhang, Z., Zha, Y., Hu, W., Huang, Z., Gao, Z., Zang, Y., Chen, J., Dong, L. and Zhang, J. (2013) The Autoregulatory feedback loopof microRNA-21/programmed cell death protein 4/activation protein-1 (MiR21/PDCD4/AP-1) as a driving force for hepatic fibrosis development J. Biol. Chem., 288, 37082-37093
Hedgehog signalling
Hyun, J., Wang, S., Kim, J., Kim, G.J. and Jung, Y. (2015) MicroRNA125b-mediated Hedgehog signalling influences liver regeneration by chorionic plate-derived mesenchymal stem cells Sci. Rep., 5: 14135
Interleukin expression
Byun, J-S., Suh, Y-G., Yi, H-S., Lee, Y-S. and Jeong, W-I. (2013) Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice J, Hepatol., 58, 342–349
Mesenchymal/mesothelial cells
Hyun, J., Wang, S., Kim, J., Kim, G.J. and Jung, Y. (2015) MicroRNA125b-mediated Hedgehog signalling influences liver regeneration by chorionic plate-derived mesenchymal stem cells Sci. Rep., 5: 14135
Tang, W-P., Akahoshi, T., Piao, J-S., Narahara, S., Murata, M., Kawano, T., Hamano, N. et al (2015) Basic fibroblast growth factor-treated adipose tissue-derived mesenchymal stem cell infusion to ameliorate liver cirrhosis via paracrine hepatocyte growth factor J. Gastroenterol. Hepatol., 20, 1065–1074
Tang, W-P., Akahoshi, T., Piao, J-S., Narahara, S., Murata, M., Kawano, T., Hamano, N., Ikeda, T. and Hashizume, M. (2016) Splenectomy enhances the therapeutic effect of adipose tissue-derived mesenchymal stem cell infusion on cirrhosis rats Liver Int.,36, 1151–1159
Myofibroblast (proliferation/migration/senescence)
Klein, S., Klösel, J., Schierwagen, R., Körner, C., Granzow, M., Huss, S., Reza Mazar, I.G., Weber, S., van den Ven, P.F.M., Pieper-Fürst, U., et al (2012) Atorvastatin inhibits proliferation and apoptosis, but induces
senescence in hepatic myofibroblasts and thereby attenuates hepatic fibrosis in rats Lab. Invest., 92, 1440–1450 Myofibroblast transdifferentiation – see “Transcription factors – Methylation”
Neurotransmitters
Cassiman, D., van Pelt, J., de Vos, R., van Lommel, F., Desmet, V., Yap, S-H. and Roskams, T. (1999) Synaptophysin: a novel marker for human and rat hepatic stellate cells Am. J. Pathol., 155, 1831-1839
Neutrophil interactions
Zhou, Z., Xu, M-J., Cai, Y., Wang, W., Jiang, J.X., Varga, Z.V., Feng, D., Pacher, P. et al (2108) Neutrophil– hepatic stellate cell interactions promote fibrosis in experimental steatohepatitis Cell. Mol. Gastroenterol. Hepatol., 5, 399–413
Opioid system
Ebrahimkhani, M.R., Kiani, S., Oakley, F., Kendall, T., Shariftabrizi, A., Tavangar, S.M., Moezi, L. et al (2006) Naltrexone, an opioid receptor antagonist, attentuates liver fibrosis in bile duct ligated rats Gut, 55, 1606-1616
Oxidases/oxygen species
Ping, J., Li, J-T., Liao, Z-X., Shang, L. and Wang, H. (2011) Indole-3-carbinol inhibits hepatic stellate cells proliferation by blocking NADPH oxidase/reactive oxygen species/p38 MAPK pathway Eur. J. Pharmacol., 650, 656–662
Peroxisome proliferators-activated receptor-γ
Jiang, Y., Wang, S., Zhao, Y., Lin, C., Zhong, F., Jin, L., He, F. and Wang, H. (2015) Histone H3K9 demethylase JMJD1A modulates hepatic stellate cells activation and liver fibrosis by epigenetically regulating peroxisome proliferator-activated receptor γ FASEB J. 29, 1830–1841
Lin, J. and Chen, A. (2008) Activation of peroxisome proliferator-activated receptor-g by curcumin blocks the signalling pathways for PDGF and EGF in hepatic stellate cells Lab. Invest., 88, 529-540
Mann, J., Chu, D.C.K., Maxwell, A., Oakley, F., Zhu, N.L., Tsukamoto, H. and Mann, D.A. (2010) MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis Gastroenterology, 138, 705–714
Portal hypertension
Bockx, I., Verdrengh, K., Vander Elst, I., van Pelt, J., Nevens, F., Laleman, W. and Cassiman, D. (2012) Highfrequency vagus nerve stimulation improves portal hypertension in cirrhotic rats Gut, 61, 604-612
Kageyama, Y., Ikeda, H., Watanabe, N., Nagamine, M., Kusumoto, Y., Yashiro, M., Satoh, Y., Shimosawa, T., Shinozaki, K., Tomiya, T., et al (2012) Antagonism of sphingosine 1-phosphate receptor 2 causes a selective reduction of portal vein pressure in bile duct-ligated rodents Hepatology, 56, 1427-1438
RNA (various types)
Jiroutova, A., Slavkovsky, R., Cermakova, M., Majdiakova, L., Hanovcova, I., Bolehovska, R., Hadjzlerova, M., Radilova, H., Ruszova, E. and Kanta, J. (2007) Expression of mRNAs related to connective tissue metabolism in rat hepatic stellate cells and myofibroblasts Exp. Toxicol. Pathol., 58, 263-273
Zhang, Z., Gao, Z., Hu, W., Yin, S., Wang, C., Zang, Y., Chen, J., Zhang, J. and Dong, L. (2013) 3,3’- Diindolylmethane ameliorates experimental hepatic fibrosis via inhibiting miR-21 expression Br. J. Pharmacol., 170, 649–660
Zhang, Z., Zha, Y., Hu, W., Huang, Z., Gao, Z., Zang, Y., Chen, J., Dong, L. and Zhang, J. (2013) The Autoregulatory feedback loopof microRNA-21/programmed cell death protein 4/activation protein-1 (MiR21/PDCD4/AP-1) as a driving force for hepatic fibrosis development J. Biol. Chem., 288, 37082-37093
Zheng, J., Wu, c., Lin, Z., Guo, Y., Shi, L., Dong, P., Lu, Z., Gao, S. et al (2014) Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation – a novel mechanism suppressing liver fibrosis FEBS J., 281, 88–103
Sirtuin-1 activation (carnosol-mediated)
Zhao, H., Wang, Z., Tang, F., Zhao, Y., Feng, D. Li, Y., Hu, Y., Wang, C. et al (2018) Carnosol-mediated Sirtuin 1 activation inhibits Enhancer of Zeste Homolog 2 to attenuate liver fibrosis Pharmacol. Res., 128, 327– 337
Steatohepatitis
Bravo, M., Raurell, I., Hide, D., Fernández-Iglesias, A., Gil, M., Barberá, A., Salcedo, M.T., Augustin, S., Genescà, J. and Martell, M. (2019) Restoration of liver sinusoidal cell phenotypes by statins improves portal hypertension and histology in rats with NASH Scientific Reports | (2019) 9:20183
Zhou, Z., Xu, M-J., Cai, Y., Wang, W., Jiang, J.X., Varga, Z.V., Feng, D., Pacher, P. et al (2108) Neutrophil– hepatic stellate cell interactions promote fibrosis in experimental steatohepatitis Cell. Mol. Gastroenterol. Hepatol., 5, 399–413
TGFβ signalling
Xu, A., Li, Y., Zhao, W., Hou, F., Li, X., Sun, L., Chen, W., Yang, A. et al (2018) PHP14 regulates hepatic stellate cells migration in liver fibrosis via mediating TGF-β1 signaling to PI3Kγ/AKT/Rac1 pathway J. Mol. Med., 96, 119–133
Toll-like receptor signalling
Byun, J-S., Suh, Y-G., Yi, H-S., Lee, Y-S. and Jeong, W-I. (2013) Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice J, Hepatol., 58, 342–349
Wilson, C.L., Mann, J., Walsh, M., Perugoria, M.J., Oakley, F., Wright, M.C., Brignole, C., Di Paolo, D., Perri, P., Ponzoni, M., Karin, M. and Mann, D.A. (2014) Quiescent hepatic stellate cells functionally contribute to the hepatic innate immune response via TLR3 PLoS One, 9: e83391
Transcription factors
E-box DNA
Vincent, K.J., Jones, E., Arthur, M.J.P., Smart, D.E., Trim, J., Wright, M.C. and Mann, D.A. (2001) Regulation of E-box DNA binding during in vivo and in vitro activation of rat and human hepatic stellate cells Gut, 49, 713-719
Metalloproteinases
Bertrand-Philippe, M., Ruddell, R.G., Arthur, M.J.P., Thomas, J., Mungalsingh, N. and Mann, D.A. (2004) Regulation of tissue inhibitor of metalloproteinase 1 gene transcription by RUNX1 and RUNX2 J. Biol. Chem., 279, 24530-24539
Fowell, A.J., Collins, J.E., Duncombe, D.R., Pickering, J.A., Rosenberg, W.M.C. and Benyon, R.C. (2011) Silencing tissue inhibitors of metalloproteinases (TIMPs) with short interfering RNA reveals a role for TIMP-1 in hepatic stellate cell proliferation Biochem. Biophys. Res. Comm., 407, 277–282
Jiroutova, A., Slavkovsky, R., Cermakova, M., Majdiakova, L., Hanovcova, I., Bolehovska, R., Hadjzlerova, M. et al (2007) Expression of mRNAs related to connective tissue metabolism in rat hepatic stellate cells and myofibroblasts Exp. Toxicol. Pathol., 58, 263-273
Smart, D.E., Vincent, K.J., Arthur, M.J.P., Eickelberg, O., Castellazzi, M., Mann, J. and Mann, D.A. (2001) JunD regulates transcription of the tissue inhibitor of metalloproteinases-1 and interleukin-6 genes in activated hepatic stellate cells J. Biol. Chem., 276, 24414-24421
Trim, J.E., Samra, S.K., Arthur, M.J.P., Wright, M.C., McAulay, M., Beri, R. and Mann, D.A. (2000) Upstream tissue inhibitor of metalloproteinases-1 (TIMP-1) element-1, a novel and essential regulatory DNA motif in the human TIMP-1 gene promoter, directly interacts with a 30-kDa nuclear protein J. Biol. Chem., 275, 6657-6663
Methylation
Mann, J., Oakley, F., Akiboye, F., Elsharkawy, A., Thorne, A.W. and Mann, D.A. (2007) Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: Implications for wound healing and fibrogenesis Cell Death Different., 14, 275-285
Mann, J., Chu, D.C.K., Maxwell, A., Oakley, F., Zhu, N.L., Tsukamoto, H. and Mann, D.A. (2010) MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis Gastroenterology, 138, 705–714
Zheng, J., Wu, c., Lin, Z., Guo, Y., Shi, L., Dong, P., Lu, Z., Gao, S., Liao, Y., Chen, B. and Yu, F. (2014) Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNAmediated control of DNA methylation – a novel mechanism suppressing liver fibrosis FEBS J., 281, 88–103
NF-κB
Ben-Shoshan, S.O., Kagan, P., Sultan, M., Barabash, Z., Dor, C., Jacob-Hirsch, J., Harmelin, A., Pappo, O. et al (2017) ADAR1 deletion induces NFκB and interferon signalling dependent liver inflammation and fibrosis RNA Biol., 14, 587–602
Elsharkawy, A.M., Wright, M.C., Hay, R.T., Arthur, M.J.P., Hughes, T., Bahr, M.J., Degitz, K. and Mann, D.A. (1999) Persistent activation of nuclear factor – κB in cultured rat hepatic stellate cells involves the induction of potentially novel Rel-like factors and prolonged changes in the expression of IκB family proteins Hepatology, 30, 761-769
Habens, F., Srinivasan, N., Oakley, F., Mann, D.A., Ganesan, A. and Packham, G. (2005) Novel sulfasalazine analogues with enhanced NF-kB inhibitory and apoptosis promoting activity Apoptosis, 10, 481-491
Oakley F., Mann, J., Ruddell, R.G., Pickford, J., Weinmaster, G. and Mann, D.A. (2003) Basal expression of IκBα is controlled by the mammalian transcriptional repressor RBP-J (CBF1) and its activator Notch1 J. Biol. Chem., 278, 24359-24370
Transdifferentiation
Page, A., Paoli, P.P., Hill, S.J., Howarth, R., Wu, R., Kweon, S-M., French, J., White, S. et al (2015) Alcohol directly stimulates epigenetic modifications in hepatic stellate cells J. Hepatol., 62, 388–397
Ubiquitin
Wilson, C.L., Murphy, L.B., Leslie, J., Kendrick, S., French, J., Fox, C.R., Sheerin, N.S., Fisher, A. Robinson, J.H., Tiniakos, D.G., Gray, D.A., Oakley, F. and Mann, D.A. (2015) Ubiquitin C-terminal hydrolase 1: A novel functional marker for liver myofibroblasts and a therapeutic target in chronic liver disease J. Hepatol., 63, 1421–1428
Vagus nerve stimulation
Bockx, I., Vander Elst, I., Roskams, T. and Cassiman, D. (2010) The hepatic vagus nerve stimulates hepatic stellate cell proliferation in rat acute hepatitis via muscarinic receptor type 2 Liver Int., 30, 693-702
Bockx, I., Verdrengh, K., Vander Elst, I., van Pelt, J., Nevens, F., Laleman, W. and Cassiman, D. (2012) Highfrequency vagus nerve stimulation improves portal hypertension in cirrhotic rats Gut, 61, 604-612
Wnt/β-catenin
He, L., Gubbins, J., Peng, Z., Medina, V., Fei, F., Asahina, K., Wang, J., Kahn, M., Rountree, C.B. and Stiles, B.L. (2016) Activation of hepatic stellate cell in Pten null liver injury model Fibrogenesis Tissue Repair 9: 8
Rat pancreas
Bachem, M.G., Schneider, E., Groß, H., Weidenbach, H., Schmid, R.M., Menke, A., Siech, M., Beger, H., Grunert, A. and Adler, G. (1998) Identification, culture, and characterization of pancreatic stellate cells in rat and humans Gastroenterology, 115, 421-432
Ben-Harosh, Y., Anosov, M., Salem, H., Yatchenko, Y, and Birk, R. (2017) Pancreatic stellate cell activation is regulated by fatty acids and ER stress Exp. Cell Res., 359, 76-85
Gao, R. and Brigstock, D.R. (2005) Connective tissue growth factor (CCN2) in rat pancreatic stellate cell function: integrin α5β1 as a novel CCN2 receptor Gastroenterology, 129, 1019-1030
González, A.M., Garcia, T., Samper, E., Rickmann, M., Vaquero, E.C. and Molero, X. (2011) Assessment of the protective effects of oral tocotrienols in arginine chronic-like pancreatitis Am. J. Physiol. Gastrointest. Liver Physiol., 301, G846–G855
Kaku, T., Oono, T., Zhao, H., Gibo, J., Kawabe, K., Ito, T. and Takayanagi, R. (2007) IS-741 attenuates local migration of monocytes and subsequent pancreatic fibrosis in experimental chronic pancreatitis induced by dibutyltin dichloride in rats Pancreas, 34, 299-309
Rickmann, M., Vaquero, E.C., Malagelada, J.R. and Molero, X. (2007) Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway Gastroenterology, 132, 2518-2532
Schmid-Kotsas, A., Gross, H-J., Menke, A., Weidenbach, H., Adler, G., Siech, M., Beger, H., Grunert, A. and Bachem, M.G. (1999) Lipopolysaccharide-activated macrophages stimulate the synthesis of collagen type 1 and C-fibronectin in cultured pancreatic stellate cells Am. J. Pathol., 155, 1749-1758
Shek, F.W-T., Benyon, R.C., Walker, F.M., McCrudden, P.R., Pender, S.L.F., Williams, E.J., Johnson, P.A., Johnson, C.D., Bateman, A.C., Fine, D.R. and Iredale, J.P. (2002) Expression of transforming growth factor-β1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis Am. J. Pathol., 160,1787-1798
Shek, F.W., Walker, F.M.J., Bateman, A.C., Benyon, R.C., Iredale, J.P. and Fine, D.R. (2003) A reliable technique for rodent pancreatic stellate cells isolation Pancreatology, 3, 209-269
Siech, M., Zhou, Z., Zhou, S., Bair, B., Alt, A., Hamm, S., Gross, H.,. Mayer, J., Beger, H.G., Tian, X., Kornmann, M. and Bachem, M.G. (2009) Stimulation of stellate cells by injured acinar cells: a model of acute pancreatitis induced by alcohol and fat (VLDL) Am. J. Physiol. Gastrointest. Liver Physiol., 297, G1163– G1171
Yu, F-X., Su, L-F., Dai, C-L., Wang, Y., Teng Y-Y., Fu, J-H., Zhang, Q-Y. and Tang, Y-H. (2015) Inhibition of pancreatic stellate cell activity by adipose-derived stem cells Hepatobiliary Pancreat. Dis. Int., 14, 215-221
OptiPrep™ Reference List RC07; 4th edition, February 2020
OptiPrep™ Reference List RC09
Pulmonary cells
- This Reference List provides a list of all the published papers relating to the purification of pulmonary cells, listed by cell type
- Within each cell type papers are listed alphabetically by first author
- Multiple entries from the same first author are listed chronologically.
- For a detailed methodology of the purification of these cells see OptiPrepTM Application Sheets C29 and C30.
- To aid identification of relevant publications key words in the titles are highlighted in light blue
Alveolar epithelial
Kosmider, B., Loader, J.E., Murphy, R.C. and Mason, R.J. (2010) Apoptosis induced by ozone and oxysterols in human alveolar epithelial cells Free Radical Biol. Med., 48, 1513–1524
Alveolar Type I
Borok, Z., Liebler, J.M., Lubman, R.L., Foster, M.J., Zhou, B., Li, X., Zabski, S.M., Kim, K-J. and Crandall, E.D. (2002) Alveolar epithelial ion and fluid transport Na transport proteins are expressed by rat alveolar epithelial type I cells Am. J. Physiol. Lung Mol. Physiol., 282, L599-608
Mossel, E.C., Wang, J., Jeffers, S., Edeen, K.E., Wang, S., Cosgrove, G.P., Funk, C.J., Manzer, R., Miura, T.A., Pearson, L.D., Holmes, K.V. and Mason, R.J. (2008) SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells Virology, 372, 127-135
Yu, W.C.L., Chan, R.W.Y., Wang, J., Travanty, E.A., Nicholls, J.M., Peiris, J.S.M., Mason, R.J. and Chan, M.C.W. (2011) Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses J. Virol., 85, 6844-6855
Alveolar Type II
Epa, A.P., Thatcher, T.H., Pollock, S.J., Wahl, L.A., Lyda, E., Kottmann, R.M., Phipps, R.P. and Sime, P.J. (2015) Normal human lung epithelial cells inhibit transforming growth factor-β induced myofibroblast differentiation via prostaglandin E2 PLoS One, 10: e0135266
Goetzman, E.S., Alcorn, J.F., Bharathi, S.S., Uppala, R., McHugh, K.J., Kosmider, B., Chen, R., Zuo, Y.Y., Beck, M.E., McKinney, R.W. et al (2014) Long-chain acyl-CoA dehydrogenase deficiency as a cause of pulmonary surfactant dysfunction J. Biol. Chem., 289, 10668–10679
Ito, Y. and Mason, R.J. (2010) The effect of interleukin-13 (IL-13) and interferon-γ (IFN-γ) on expression of surfactant proteins in adult human alveolar type II cells in vitro Respir. Res., 11, 157
Ito, Y., Correll, K., Schiel, J.A., Finigan, J.H., Prekeris, R., and Mason, R.J. (2014) Lung fibroblasts accelerate wound closure in human alveolar epithelial cells through hepatocyte growth factor/c-Met signaling. Am. J. Physiol. Lung Cell. Mol. Physiol., 307, L94–L105,
Ito, Y., Correll, K., Zemans, R.L., Leslie, C.C., Murphy, R.C., Mason, R.J. (2015) Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-α/EGFR signaling Am. J. Physiol. Lung Cell Mol. Physiol. 308: L1178–L1188
Kosmider, B., Messier, E.M., Chu, H.W. and Mason, R.J. (2011) Human alveolar epithelial cell injury induced by cigarette smoke PLoS One 6: e26059
Lambot, L., Rodriguez, E.C., Houtteman, D., Li, Y., Schiffmann, S.N., Gall, D., and de Kerchove d’Exaerde, A. (2016) Striatopallidal neuron NMDA receptors control synaptic connectivity, locomotor, and goal-directed behaviors J. Neurosci., 36, 4976–4992
Lin, C.R., Bahmed, K., Tomar, D., Marchetti, N., Criner, G.J., Bolla, S., Wilson, M.A., Madesh, M. and Kosmider, B. (2019) The relationship between DJ-1 and S100A8 in human primary alveolar type II cells in emphysema. Am. J. Physiol. Lung. Cell. Mol. Physiol., 317, L791–L804
Manzer, R., Wang, J., Nishina, K., McConville, G. and Mason, R.J. (2006) Alveolar epithelial cells secrete chemokines in response to IL-1β and lipopolysaccharide but not to ozone Am. J. Respir. Cell Mol., 34, 158-166
Messier, E.M., Bahmed, K., Tuder, R.M., Chu, H.W., Bowler, R.P. and Kosmider, B. (2013) Trolox contributes to Nrf2-mediated protection of human and murine primary alveolar type II cells from injury by cigarette smoke Cell Death Dis., 4: e573
Miura, T.A., Wang, J., Holmes, K.V. and Mason, R.J. (2007) Rat coronaviruses infect rat alveolar type I epithelial cells and induce expression of CXC chemokines Virology, 369, 288-298
Mossel, E.C., Wang, J., Jeffers, S., Edeen, K.E., Wang, S., Cosgrove, G.P., Funk, C.J., Manzer, R., Miura, T.A., Pearson, L.D., Holmes, K.V. and Mason, R.J. (2008) SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells Virology, 372, 127-135
Tan, L.H., Bahmed, K., Lin, C-R., Marchetti, N., Bolla, S., Criner, G.J., Kelsen, S., Madesh, M. and Kosmider, B. (2018) The cytoprotective role of DJ-1 and p45 NFE2 against human primary alveolar type II cell injury and emphysema Sci. Rep., 8: 3555
Wang, J., Edeen, K., Manzer, R., Chang, Y., Wang, S., Chen, X., Funk, C.J., Cosgrove, G.P., Fang, X. and Mason, R.J. (2007) Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro Am. J. Respir. Cell Mol. Biol., 36, 661-668
Wang, J., Oberley-Deegan, R., Wang, S., Nikrad, M., Funk, C.J., Hartshorn, K.L. and Mason, R.J. (2009) Differentiated human aveolar type II cells secrete antiviral IL-29 (IFN-1) in response to influenza A infection J. Immunol., 182, 1296–1304
Yu, W.C.L., Chan, R.W.Y., Wang, J., Travanty, E.A., Nicholls, J.M., Peiris, J.S.M., Mason, R.J. and Chan, M.C.W. (2011) Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses J. Virol., 85, 6844-6855
Zemski Berry, K. A., Murphy, R.C., Kosmider, B. and Mason, R.J. (2017) Lipidomic characterization and localization of phospholipids in the human lung J. Lipid Res., 58, 926–933
Human
Bahmed, K., Boukhenouna, S., Karim, L., Andrews, T., Lin, J., Powers, R., Wilson, M.A., Lin, C-R., Messier,E. et al (2019) The effect of cysteine oxidation on DJ-1 cytoprotective function in human alveolar type II cells Cell Death Dis., 10: 638
Bahmed, K., Lin, C.R., Simborio H., Karim L., Aksoy M., Kelsen S., Tomar D., Madesh M., Elrod J., et al (2019) The role of DJ-1 in human primary alveolar type II cell injury induced by e-cigarette aerosol. Am J Physiol Lung Cell Mol Physiol 317, L475–L485
Correll. K.A., Edeen, K.E., Zemans, R.L., Redente, E.F., Serban, K.A., Curran-Everett, D., Edelman, B.L., Mikels-Vigdal, A. and Mason, R.J. (2019) Transitional human alveolar type II epithelial cells suppress extracellular matrix and growth factor gene expression in lung fibroblasts Am. J. Physiol. Lung Cell. Mol. Physiol., 317, L283–L294
Kosmider, B., Mason, R.J. and Bahmed, K. (2018) Isolation and characterization of human alveolar type II cells In Lung Innate Immunity and Inflammation: Methods and Protocols, Methods in Mol. Biol., 1809, (ed. Alper, S., and Janssen, W.J.) Springer Science+Business Media, LLC, pp 83-90
Lin, C.R., Bahmed, K., Tomar, D., Marchetti, N., Criner, G.J., Bolla, S., Wilson, M.A., Madesh, M. and Kosmider, B. (2019) The relationship between DJ-1 and S100A8 in human primary alveolar type II cells in emphysema. Am. J. Physiol. Lung. Cell. Mol. Physiol., 317, L791–L804
Rodent
Cottage, C.T., Peterson, N., Kearley, J., Berlin, A., Xiong, X., Huntley, A., Zhao, W., Brown, C. et al (2019) Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice Commun. Biol., 2: 307
Jansing, N.L., McClendon, J., Kage, H., Sunohara, M., Alvarez, J.R., Borok, Z. and Zemans, R.L. (2018) Isolation of rat and mouse alveolar type II epithelial cells In Lung Innate Immunity and Inflammation: Methods and Protocols, Methods in Mol. Biol., 1809, (ed. Alper, S, and Janssen, W.J.) Springer Science+Business Media, LLC, pp 69-82
Lin, C.R., Bahmed, K., Tomar, D., Marchetti, N., Criner, G.J., Bolla, S., Wilson, M.A., Madesh, M. and Kosmider, B. (2019) The relationship between DJ-1 and S100A8 in human primary alveolar type II cells in emphysema. Am. J. Physiol. Lung. Cell. Mol. Physiol., 317, L791–L804
Emphysema
Kosmider, B., Lin, C-R., Karim, L., Tomar, D., Vlasenko, L., Marchetti, N., Bolla, S., Madesh, M., Criner, G.J. and Bahmed, K. (2019) Mitochondrial dysfunction in human primary alveolar type II cells in emphysema EBioMed., 46, 305–316
Endothelial and epithelial cells
Pu, F.R., Manning, F.C.R., Brannigan, A.E. and Crosby, S.R. (2001) Differential regulation of calcitonin secretion in normal and neoplastic pulmonary neuroendocrine cells in vitro Exp. Lung Res., 27, 689-703
Fibroblasts
Waise, S., Parker, R., Rose-Zerilli, M.J.J., Layfield, D.M., Wood, O., West, J., Ottensmeier, C.H., Thomas, G.J. and Hanley, C.J. (2019) An optimised tissue disaggregation and data processing pipeline for characterising fibroblast phenotypes using single-cell RNA sequencing Sci. Rep., 9: 9580
Haematopoietic cells
Holmer, S.M., Evans, K.S., Asfaw, Y.G., Saini, D., Schell, W.A., Ledford, J.G., Frothingham, R., Wright, J.R., Sempowski, G.D. and Perfect, J.R. (2014) Impact of surfactant protein D, interleukin-5, and eosinophilia on Cryptococcosis Infect. Immun., 82, 83–693
Human lung/lymph node cells
Gibbings, S.L. and Jakubzick, C.V. (2018) A consistent method to identify and isolate mononuclear phagocytes from human lung and lymph nodes In Type 2 Immunity: Methods and Protocols, Methods in Mol. Biol., 1799, (ed. Reinhardt, R.L.), Springer Science+Business Media, LLC, pp 381-395
Leukocytes
Bolles, M., Deming, D., Long, K., Agnihothram, S., Whitmore, A., Ferris, M., Funkhouser, W., Gralinski, L., Totura, A., Heise, M. and Baric, R.S. (2011) A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge J. Virol., 85, 12201–12215
Low density cells
Majlessi, L., Sayes, F., Bureau, J-F., Pawlik, A., Michel, V., Jouvion, G., Huerre, M., Severgnini, M. et al (2017) Colonization with Helicobacter is concomitant with modified gut microbiota and drastic failure of the immune control of Mycobacterium tuberculosis Mucosal Immunol., 10, 1178-1189
Low density cells
Akbay, E.A, Koyama, S., Carretero, J., Altabef, A., Tchaicha, J.H., Christensen, C.L., Mikse, O.R., Cherniack, A.D., Beauchamp, E.M., Pugh, T.J. et al. (2013) Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors Cancer Discov., 3, 1355–1363
Ledford, J.G., Goto, H., Potts, E.N., Degan, S., Chu, H.W., Voelker, D.R., Sunday, M.E., Cianciolo, G.J., Foster, W.M. Kraft, M. and Wright, J.R. (2009) SP-A preserves airway homeostasis during mycoplasma pneumoniae infection in mice J. Immunol., 182, 7818–7827
Macrophages
Bordet, E., Maisonnasse, P., Renson, P., Bouguyon, E., Crisci, E., Tiret, M., Descamps, D., Bernelin-Cottet, C. et al (2018) Porcine alveolar macrophage-like cells are pro-inflammatory pulmonary intravascular macrophages that produce large titers of porcine reproductive and respiratory syndrome virus Sci. Rep., 8: 10172
Starr, A.E., Dan, T., Minhas, K., Shewen, P.E. and Coomber, B.L. (2004) Potential involvement of gelatinases and their inhibitors in Mannheimia haemolytica pneumonia in cattle Infect. Immun., 72, 4393-4400
Wang, J., Oberley-Deegan, R., Wang, S., Nikrad, M., Funk, C.J., Hartshorn, K.L. and Mason, R.J. (2009) Differentiated human aveolar type II cells secrete antiviral IL-29 (IFN-λ1) in response to influenza A infection J. Immunol., 182, 1296–1304
Yu, W.C.L., Chan, R.W.Y., Wang, J., Travanty, E.A., Nicholls, J.M., Peiris, J.S.M., Mason, R.J. and Chan, M.C.W. (2011) Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses J. Virol., 85, 6844-6855
Mononuclear phagocytes
Gibbings, S.L. and Jakubzick, C.V. (2018) A consistent method to identify and isolate mononuclear phagocytes from human lung and lymph nodes In Type 2 Immunity: Methods and Protocols, Methods in Mol. Biol., 1799, (ed. Reinhardt, R.L.), Springer Science+Business Media, LLC, pp 381-395
Mycobacterium infection
Majlessi, L., Sayes, F., Bureau, J-F., Pawlik, A., Michel, V., Jouvion, G., Huerre, M., Severgnini, M. et al (2017) Colonization with Helicobacter is concomitant with modified gut microbiota and drastic failure of the immune control of Mycobacterium tuberculosis Mucosal Immunol., 10, 1178-1189
Myeloid cells
Ledford, J.G., Goto, H., Potts, E.N., Degan, S., Chu, H.W., Voelker, D.R., Sunday, M.E., Cianciolo, G.J., Foster, W.M. Kraft, M. and Wright, J.R. (2009) SP-A preserves airway homeostasis during mycoplasma pneumoniae infection in mice J. Immunol., 182, 7818–7827
Pleural effusion cells
Schweppe, R.E., Pozdeyev, N., Pike, L.A., Korch, C., Zhou, Q., Sams, S.B., Sharma, V., Pugazhenthi, U. Raeburn, C. et al (2019) Establishment and characterization of four novel thyroid cancer cell lines and PDX models expressing the RET/PTC1 rearrangement, BRAFV600E, or RASQ61R as drivers Mol, Cancer Res., 17, 1036-1048
OptiPrep™ Reference List RC09: 1st edition, January 2020
