Cell Applications of Optiprep
OptiPrep™ Application Sheet C01
Preparation of density gradient solutions
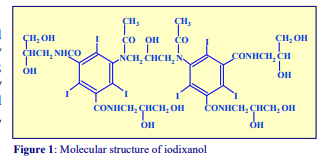
1. OptiPrep™ OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml. Iodixanol is a nonionic molecule with a molecular mass of 1550 (see Figure 1).
2. Handling OptiPrep™ Exposure (several months) of iodixanol solutions to direct sunlight will cause a slow release of iodine (solution turns yellow); OptiPrep™ should therefore be stored away from strong sunlight. On standing, iodixanol may „settle out“ of concentrated solutions, which should be well mixed before use.
3. Osmolality The observed osmolality of OptiPrep™ depends on the mode of measurement (vapour pressure or freezing point); moreover the situation is complicated by the tendency of the iodixanol molecules to associate non-covalently in a concentrated aqueous solution. Measured values for its osmolality are thus lower than might be expected. Importantly however, when OptiPrep™ is diluted with a buffered isoosmotic solution, the iodixanol oligomers dissociate and all dilutions are isoosmotic. Under normal operating conditions therefore OptiPrep™ behaves as if it had an osmolality of approx 290 mOsm.

4. Preparation of density solutions
The following methodology is based on use of 0.85% (w/v) NaCl, 10 mM Tricine-NaOH, pH 7.4 as a cell suspension medium. To keep the bufferconcentration constant throughout any gradient a40% (w/v) iodixanol working solution (WS) is first prepared by mixing 4 vol. of OptiPrep with 2 vol. of Solution A that contains 3x the required buffer concentration (see Box 1). Gradient solutions of theappropriate density are then produced from the WS by further dilution with Solution B. Densities of solutions produced in this manner are given in Table 1.
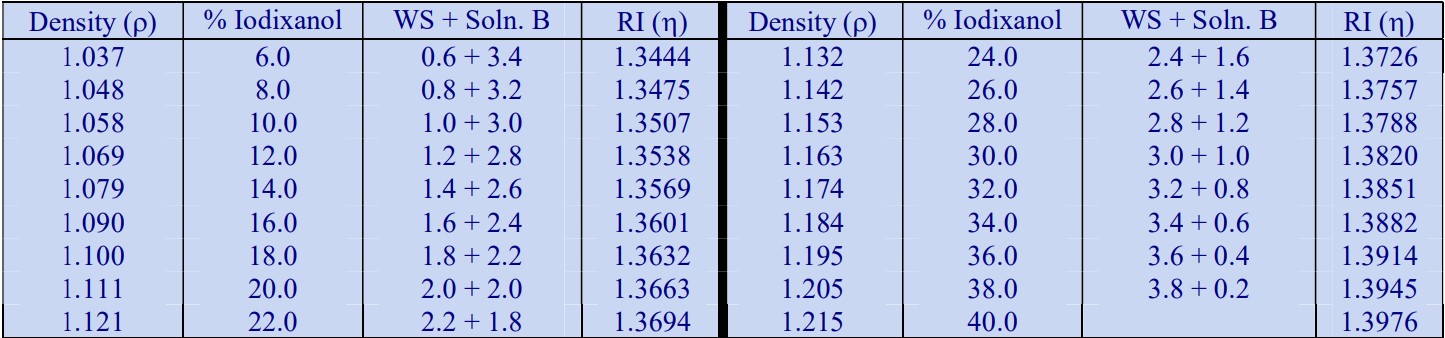
Table 1.
Any other organic buffer (e.g. Tricine) may be substituted for HEPES. Low concentrations (1-2mM) of other additives such as Mg2+ or Ca2+ in Solution B can also be maintained constant in the gradient by inclusion in Solution A at 3-6 mM. The density and refractive index of such solutions will only be marginally altered from the figures in the table. The osmolality of all these gradient solutions will be in the range 285-305 mOsm. If maintenance of a constant buffer and/or divalent cation concentration is deemed unnecessary the OptiPrep may simply be diluted directly with Solution B (with or without low concentrations of divalent cations). There will only be marginal differences in the density and osmolality of the gradient solutions (compared to those in Table 1).
5. Use of balanced salt and culture media
Working solutions and gradient solutions may also be prepared by diluting OptiPrep™ directly with any cell suspension media such as Hanks Balanced Salt Solution (HBSS) or serum-free culture medium (e.g. RPMI or DMEM) which have the same density as Solution B (approx. 1.006 g/ml). The density of the gradient solutions may be marginally higher (up by approx. 0.001 g/ml) than those given in Table 1. Refractive indices may also be slightly higher (by 0.0001-0.0004). The os molality of all solutions will again be in the range 285-305 mOsm.
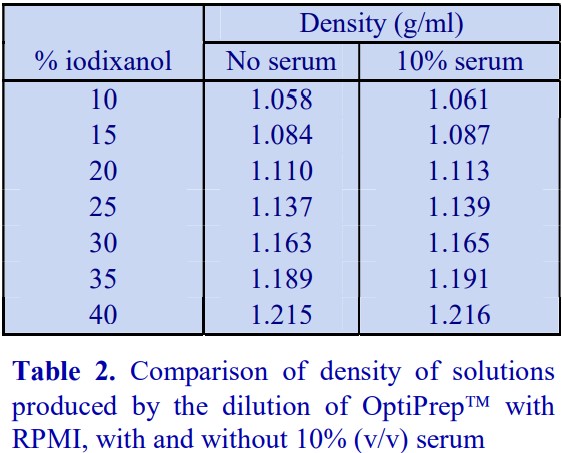
6. Use of serum-containing media
A standard cell culture medium containing 10% (v/v) serum may also be used to dilute OptiPrep™ directly; because the density of the diluent (approx 1.009 g/ml) is higher than that of any balanced salt medium, the density of the gradient solutions will be correspondingly higher. Table 2 compares the densities of selected gradient solutions produced by RPMI with and without the serum.
5. Calculation of density
As long as the density of the diluent is known then Equation 1 can be used to calculate the density of any solution produced from the diluent and a working or stock solution of iodixanol.
Equation 1: 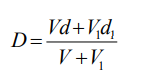
D = density of mixture; V = volume of iodixanol stock solution; d = density iodixanol stock solution;
V1 = volume of diluent; d1 = density of diluent
OptiPrepTM Application Sheet C01; 8th edition, January 2020
OptiPrep™ Application Sheet C02
Preparation of gradients for cells
1 Discontinuous gradients
1a Overlayering technique
The most widely used method for producing discontinuous gradients is to start with the densest solution and layer solutions of successively lower densities on top using some form of pipette or
syringe. Tilt the centrifuge tube (approx. 45°); place the tip of the pipette or syringe against the wall of the tube, about 1 cm above the meniscus of the denser solution, and gently deliver a slow and steady stream of liquid. This allows the liquid to spread over the tube surface and minimizes any mixing due to a sudden increase in liquid flow. Once a steady flow is established keep the tip of the pipette or syringe just above the meniscus of the liquid and against the wall of the tube.
From a pipette
Use a rubber two- or three-valve pipette filler to aliquot and dispense the gradient solutions. Check that the release valve when pressed gently, allows the delivery of a slow and steady flow of liquid. Do not use a pipette filler that uses positive pressure to deliver the liquid, as a slow even flow is often difficult to attain. Always take up more of the gradient medium than is required as it is easier and more accurate to empty the pipette to a graduation mark than to try to empty it completely.
From an automatic pipette
For small volume gradients an automatic pipette may be used. Always cut off the end of the plastic pipette tip to reduce the flow velocity of the liquid.
From a Pasteur pipette
Plastic Pasteur pipettes can be used conveniently for larger volume gradients, particularly those in calibrated centrifuge tubes. It requires some practise however to maintain a steady liquid flow by depressing the bulb of the pipette.
From a syringe
A syringe with a wide-bore stainless-steel filling cannula (i.d. approx 0.8 mm) is suitable for most gradient volumes, but make sure that the barrel can move easily and smoothly when a small pressure is applied. Placing the index finger around the bottom of the plunger, rather than around the barrel, restricts the movement of the plunger when it is depressed and thus achieves a more controlled liquid flow. Always take up more of the gradient medium than is required for the step as it is more accurate
to empty the syringe to a graduation mark than to try to empty it completely.
- Metal filling cannulas can be purchased from most surgical instrument suppliers.
1b Underlayering technique
Although the overlayering technique is probably the most widely used, the easier method is to underlayer successively denser solutions beneath the lighter solutions. The only important
requirement is that no air bubbles are introduced which may disturb the lower density layers above; for this reason a syringe with a metal filling cannula is the best tool for this procedure. Generally the existing steps are disturbed less as the outflowing liquid spreads upwards through the conical section of the bottom of the tube.
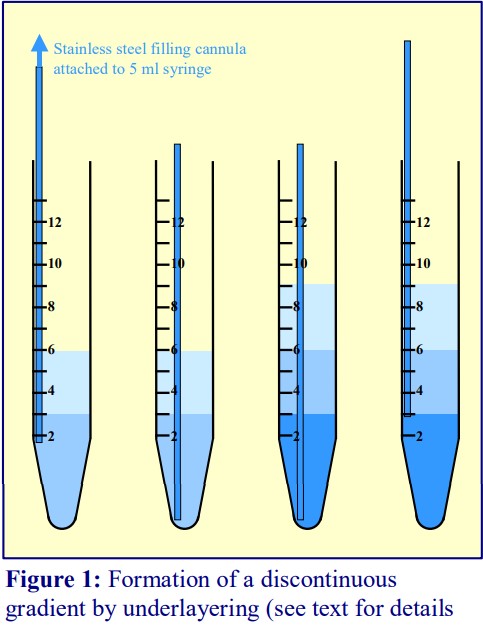
- To underlayer 3 ml of liquid, take up 4 ml into the syringe and expel to the 3.5 ml mark to ensure that the cannula is full of liquid.
- Dry the outside of the cannula.
- Move the tip of the cannula to the bottom of the tube, sliding it slowly down the wall of the tube (Figure 1).
- Depress the plunger to the 0.5 ml mark.
- After a few seconds (to allow all of the liquid to be delivered into the tube) slowly withdraw the cannula, again against the wall of the tube.
- Repeat the procedure with successively denser solutions.
2 Continuous gradients
Continuous gradients may be made by allowing discontinuous gradients to diffuse or by using a gradient maker specifically designed for this purpose.
2a By diffusion of discontinuous gradients
Y diffusion of discontinuous gradients Once a discontinuous gradient is formed, the sharp boundaries between the layers, which are observed as a sudden change in refractive index, start to disappear as the solute molecules diffuse down the concentration gradient from each denser layer to each lighter layer. Thus the density discontinuities between each layer will slowly even out and the gradient will eventually become linear (Figure 2), and given sufficient time the density will become completely uniform.
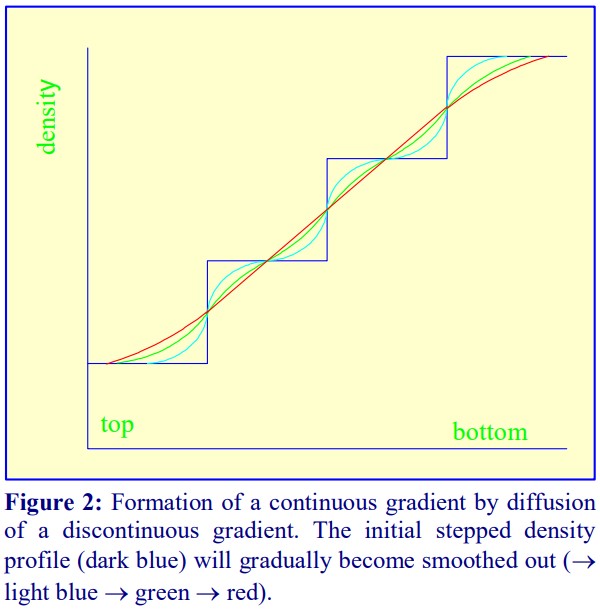
For a particular medium, the rate of diffusion across an interface is dependent on temperature, the cross-sectional area of the interface and the viscosity of the solution. In addition the rate at which the gradient becomes linear will also be a function of the distance between the interfaces. Thus a linear gradient will form more rapidly at room temperature than at 4°C and if the distance between interfaces is minimized.
The precise timing for the formation of a continuous linear gradient will depend on the dimensions of the tube, the number of layers, the concentrations of iodixanol and the temperature. If the gradients are prepared the day before the experiment and left in the refrigerator overnight then this can be a convenient approach. At room temperature the time may be reduced to approx. 4h. A series of trial experiments should be carried out in which the time is varied and the density profile of the formed gradient checked by fractionation and refractive index measurement to establish the correct conditions. In the absence of refractometer, density profiles can also be determined by absorbance measurements.
Because the continuous gradient is formed by a physical process, so long as the temperature and time are well controlled, the shape of the gradient is highly reproducible. The sample may be applied to the gradient after diffusion or it may be incorporated into one or more of the layers before diffusion. The latter strategy eliminates any interface between the sample and the gradient and may improve resolution. It is only useful however if the gradients can be rapidly prepared at room temperature.
2b Using a two-chamber gradient maker
Using a two-chamber gradient maker The traditional way of constructing a continuous gradient is to use a standard two-chamber gradient maker (Figure 3). It consists of two identical chambers connected close to their bases by a tapped channel (T). One of the chambers (the mixing chamber – B in Figure 3) has an outlet directly opposite the inlet from the tapped channel.
- Set up the device with the mixing chamber (B) resting on a magnetic stirrer (M) and the outlet tube leading via a peristaltic pump (P) to the bottom of the centrifuge tube.
- Place the chosen high-density solution in the non-mixing chamber (A) and then momentarily open the tap (T) to allow dense liquid to fill the connecting tube.
- Pour an equal volume of the low-density solution in the mixing chamber (B).
- Place two identical stirring bars (SB) in the two chambers (this ensures that the height of the two solutions is the same.
- In rapid sequence, switch on the pump (P) and the magnetic stirrer (M) and then open the connecting tap (T). As the levels in the two chambers fall synchronously, reduce the speed of the stirrer to avoid generating air bubbles that may enter the gradient and disturb it.
- Make sure that the pump is turned off before any air bubbles reach the bottom of the delivery tube at the end of the operation.
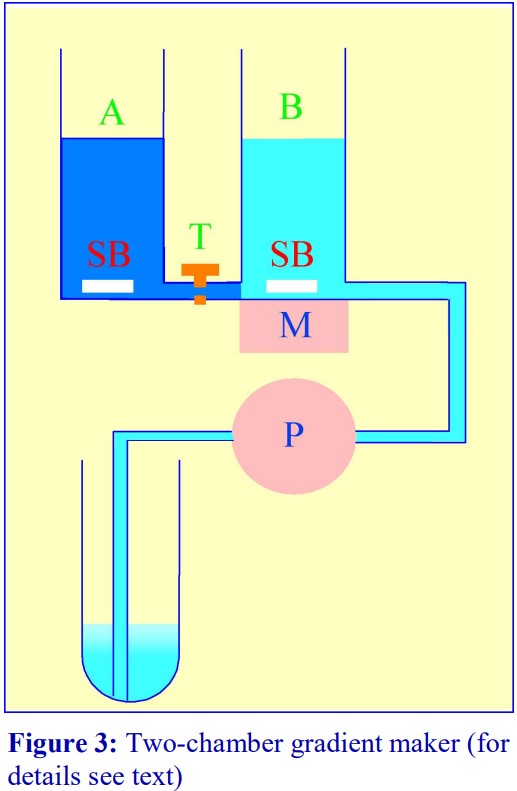
- The larger the density difference between the two gradient solutions the more vigorous must be the stirring to ensure good mixing. If the stirring bar in chamber B is too close to the inlet from the connecting tube, it is possible in the initial stages for the low-density medium to back flow into the high-density medium.
- The correct pumping speed depends on the volume of the gradient and the quality of the pump (ideally the outflow from the pump should not pulsate), but for a standard 10- 30% (w/v) or iodixanol gradient (of 12-15 ml total volume) a flow rate of approx 2 ml/min is satisfactory. Pumps that impart little or no pulsation to the liquid flow are commonly available from many sources.
- The gradient can alternatively be produced high density end-first, in which case the location of the two solutions needs to be reversed and the delivery tube to the centrifuge tube must be placed against the wall of the centrifuge tube near to its top, so the gradient flows down the tube smoothly. This is can pose some problems of mixing in the centrifuge tube if the flow down the tube wall is in the form of large drops rather than a continuous stream (this may be minimized by tilting the tube), on the other hand the tendency of the low density medium to float to the surface of the high density medium in the mixing chamber (B) aids mixing. The Labconco Auto Densi-Flow gradient unloader can be used to deposit a gradient high-density end first. Although this device is no longer commercially available, it will be found in many laboratories and often appears in laboratory equipment websites.
- To guard against air bubbles entering the delivery tube, a bubble trap could be included between mixer and pump. Although air bubbles are a major problem if they reach the bottom of the centrifuge tube (low density first delivery), they are no less a problem for high-density first delivery as they interfere with the smooth flow of liquid down the tube wall.
- It is possible to produce up to three gradients at a time; some gradient mixers have a three-outlet manifold. However such a device requires three tubes to pass through the peristaltic pump. It is the only reliable configuration of the delivery tube; simply splitting the liquid flow from a single tube through the pump cannot guarantee precisely equal delivery to all three tubes.
2c Gradient Master

An alternative device for the generation of continuous density gradients – the Gradient Master – produces the gradient by controlled mixing of the low and high-density solutions layered in the centrifuge tube. The tubes are rotated at a pre-set angle – usually 80° – to increase the cross-sectional area of the interface – and speed (usually 20 rpm) for about 2 min (Figure 4). The
density profile of the gradient generally becomes more shallow with time. The simplicity of the
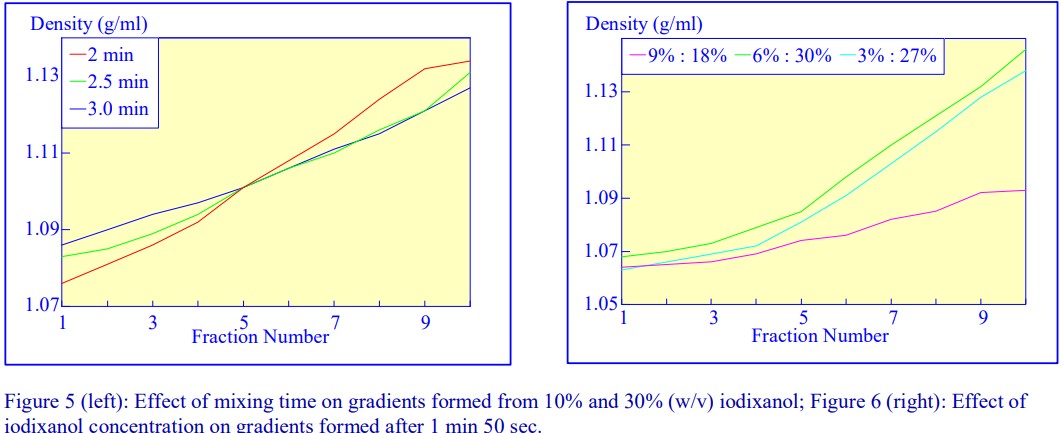
technique and the highly reproducible nature of the gradients make this a very attractive method; up to 6 gradients (17 ml tubes) can be formed at once. Some examples with iodixanol solutions are given in Figures 5 and 6.
- A very important advantage of this technique over the use of a two-chamber gradient mixer is that if it is necessary to make the sample part of the gradient, any potentially hazardous biological sample is contained within the centrifuge tube and does not contaminate the gradient forming device.
- For more information on the Gradient Master™ and other similar instruments contact the manufacturers at www.biocompinstruments.com
2d Freeze-thawing
The final manner in which continuous gradients can be produced is by freezing a solution of uniform density for at least 30 min at -20C and then thawing at room temperature for 30-60 min. These times are for tubes of approximately 5 ml volume. The freeze-thaw cycles can then be repeated; this modulates the density profile of the gradient. Generally as the number of freeze-thaw cycles increases, the gradient becomes markedly less dense at the top. The method can produce gradients that are more or less linear. Because the shape of the gradient depends on the rate of freezing and thawing, as well as the number of freeze-thaw cycles (and the volume of the tube), the precise conditions required need to be worked out for a particular laboratory. Under well-controlled conditions however, the profiles are highly reproducible. An example of the procedure with an iodixanol solution is given in Figure 7 (data kindly supplied by Dr C A Borneque, CNRS, Centre de Génétique Moléculaire, 91198 Gif sur Yvette, France).

2e Non-linear gradients
It is not always desirable to use a linear gradient and either convex, concave, S-shaped or more complex gradient density profiles may be required to effect a particular resolution of
particles. Convex gradients are sometimes particularly useful for the resolution of a sample containing a high concentration of particles of a wide range of densities. The steep density profile at the top of the gradient provides stable conditions for high capacity and the shallower high-density region provides high resolution.
From discontinuous gradients by diffusion
If each of the layers of the initially discontinuous gradient is of the same volume then diffusion will produce a linear gradient. The diffusion process however is also a very convenient way of producing a gradient that is not linear with volume. Convex or concave gradients or gradients containing a shallow median section can be produced by increasing the volume of the denser, lighter or median density layers respectively. The shape of the gradient may also be altered by changing the density interval between adjacent layers. Clearly reducing the density interval will make the gradient more shallow. It is important to test the density profile that is formed from such discontinuous gradients, but once satisfactory conditions are established the profile will be highly reproducible.
Using a gradient mixer
Convex and concave gradients cannot be produced with the standard two-chamber gradient mixer (see Figure 3). However if the non-mixing chamber is made twice the diameter of the mixing chamber, then with low-density solution in the mixing chamber a convex gradient is produced; if the locations of the low density and high-density solutions are reversed, a concave gradient is produced.
Using a Gradient Master
(see Section 2c) By using non-equal volumes of the two density solutions the gradient shape may also be changed.
OptiPrep Application Sheet C03
Mononuclear cells, lymphocytes, monocytes and polymorphonuclear leukocytes from blood: a methodological review
- This Application Sheet summarizes the development of methodologies for purifying these cells using iodinated density gradient media.
- RC01 is a reference list reporting the use of iodixanol for purifying these cells, according to species, cell type and research topic
1. Iodinated density gradient media
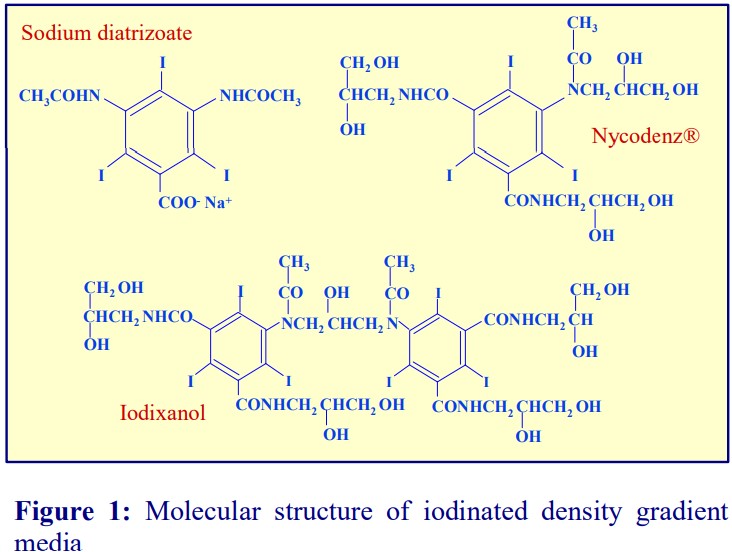
In the early nineteen-sixties Arne Bøyum, who was working in Oslo on the fractionation of blood leukocytes, recognized that the derivatives of triiodobenzoic acid that were being synthesized as X-ray imaging agents (for human intravenous injection) would also make ideal density gradient media for mammalian cell fractionation. The modern version of the medium that he devised for the purification of human peripheral blood mononuclear cells (PBMCs), which is marketed under the trade-name Lymphoprep™, is almost identical to that described in Boyum’s seminal paper published in 1968 [1]. It contains the ionic compound sodium diatrizoate (also known as Hypaque™); its molecular structure is shown in Figure 1. Later non-ionic derivatives, which are better tolerated by cells, were produced as X-ray imaging agents. These included iohexol (known under the commercial name NycodenzⓇ) in the early nineteen-eighties and about ten years later iodixanol, which is more or less a dimer of NycodenzⓇ (see Figure 1). Iodixanol is available commercially as a sterile 60% (w/v) solution called OptiPrep™. The density gradient media are produced in facilities that operate under strict EU cGMP compliance and to the European Pharmacological Standard of <1.0 endotoxin unit/ml. The actual measured levels of endotoxin are regularly <0.13 units/ml. This information, together with density and osmolality data, is available on the Certificate of Analysis that accompanies each batch of medium.
- Because of their use as X-ray imaging agents, these compounds have been clinically tested; no other density gradient media conform to this high standard. Functions of the biological particles are well retained.
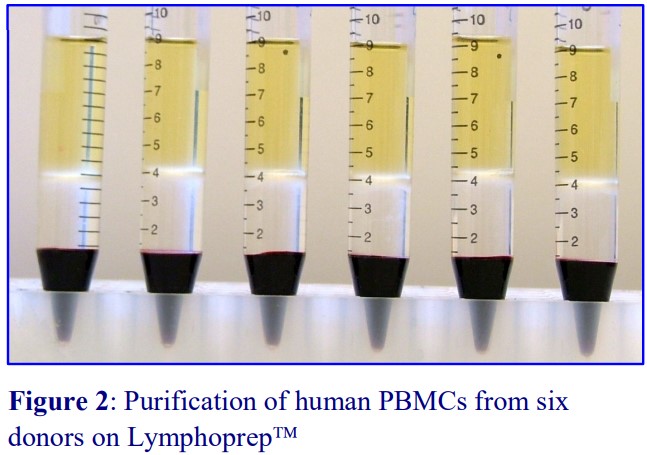
2. Density barrier isolation of human PBMCs
2a. Lymphoprep
The isolation of human PBMCs is undoubtedly the most frequently performed of any density gradient technique.
- The composition of Lymphoprep™ is: 9.1% (w/v) sodium diatrizoate and 5.7% (w/v) polysaccharide; density = 1.077 ± 0.001 g/ml, osmolality = 290 ± 15 mOsm (<0.13 endotoxin units/ml).
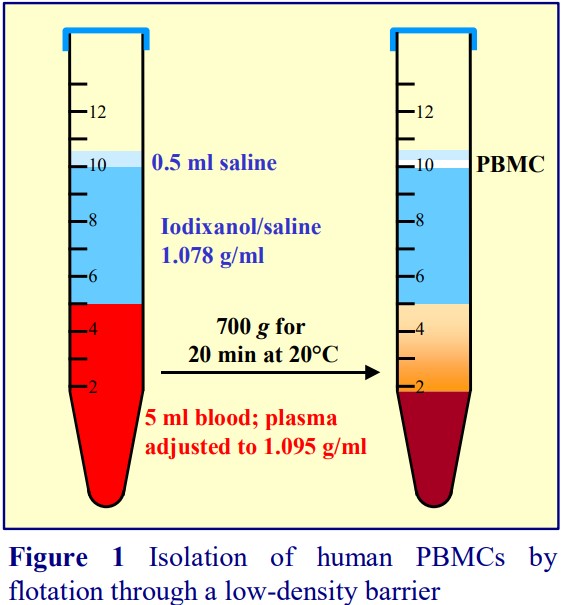
The polysaccharide, which contributes to the overall density of the medium, also aggregates the erythrocytes to enhance their rate of sedimentation. The standard protocol is to dilute blood with an equal volume of saline; layer 6 ml over 3 ml of Lymphoprep™ and centrifuge at 800 g for 20 min. Typical results are shown in Figure 2.

For frequent processing of large numbers of blood samples the Lymphoprep Tube offers a time-saving option. Tubes are pre-filled with Lymphoprep™, contained below a porous plastic frit, thus permitting the diluted blood to be poured onto the frit. During centrifugation the erythrocytes pellet through the frit; displacing the medium upwards, allowing the PBMCs to band at the plasma/medium interface above the frit. The PBMCs may be recovered simply by pouring off the liquid from the tube. The procedure is illustrated in Figure 3. Lymphoprep™ Tubes containing 2 ml or 10 ml of Lymphoprep™ are available.
2b. Polysaccharide-free media
There is evidence that the polysaccharide in any of the commercial PBMC isolation media can be adsorbed on to the surface of lymphocytes and affect their mitogenic stimulation [2]. The polysaccharide-free medium (NycoprepⓇ 1.077) containing 14.1% (w/v) NycodenzⓇ, 0.44% (w/v) NaCl, 5 mM Tricine-NaOH, pH 7.0 is however no longer commercially available. A solution of identical density and osmolality can be easily prepared from OptiPrepTM (see Section 2c).
2c. From OptiPrep
The 1.077 g/ml solution for human PBMC isolation may also be prepared by dilution of 5 vol. of OptiPrep™ with 17 vol. of any suitable isoosmotic medium. The methodology is described in OptiPrep™ Application Sheet C04 (see Section 8)
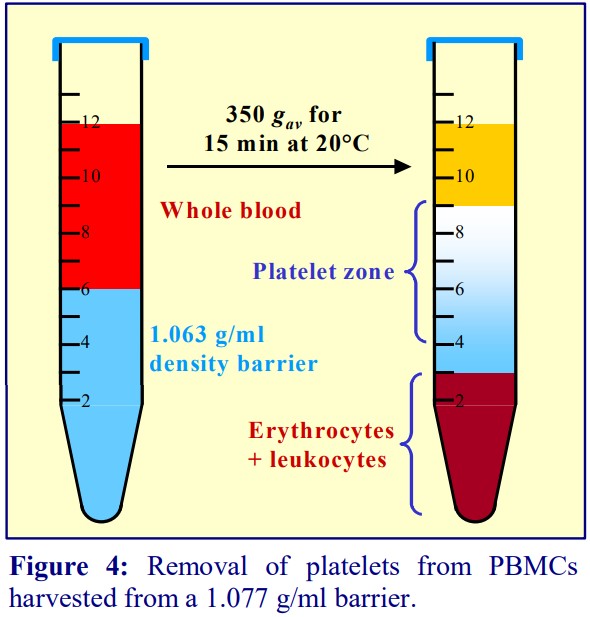
2d. Removal of platelets from PBMCs isolated on a density barrier
A drawback of any sedimentation on to a density barrier is that the platelets co-band with the PBMCs. The routine procedure to remove platelets is to dilute the interface harvest with saline and centrifuge at a speed (approx. 300 g) and time (approx. 5 min) that will loosely pellet the PBMCs but leave most of the platelets in the supernatant. After very careful removal of the majority of the supernatant, the dilution with saline and centrifugation is repeated twice. The procedure is tedious and inefficient. A simple sedimentation velocity separation was developed to prepare platelets from whole blood for functional studies (see Figure 4). It is equally efficacious for the removal of platelets from a PBMC preparation. The PBMC harvest from above the 1.077 g/ml barrier is diluted with saline and layered over a 1.063 g/ml solution prepared from OptiPrep™ and centrifuged as described in Figure 4. All of the PBMCs sediment to the bottom of the tube, while the platelets form a broad band just beneath the interface. The method was originally worked out using Nycodenz [3].
- The methodology is described in OptiPrep™ Application Sheet C13 (see Section 8)
3. Flotation isolation of human PBMCs
3a. Mixer strategy
In 1990 Ford and Rickwood [4] published a method in which the plasma itself became the density barrier. A 19% (w/v) NycodenzⓇ solution (ρ = 1.100 g/ml) was added to an equal volume of whole blood to raise the density of the plasma to 1.077 g/ml. During centrifugation at 1500 g for 30 min at 20°C the erythrocytes and polymorphonuclear leukocytes (PMNs) sediment while the PBMCs float to the top and are recovered from the meniscus and the medium below it. In the modern version OptiPrep™ is simply mixed with the blood. An advantage of the method is that if the blood is mixed with the OptiPrep™ upon collection, the centrifugation may be carried out up to 24 h later. A small disadvantage is that the final density of the plasma depends on the haematocrit of the blood.
- The methodology is described in OptiPrep™Application Sheet C05
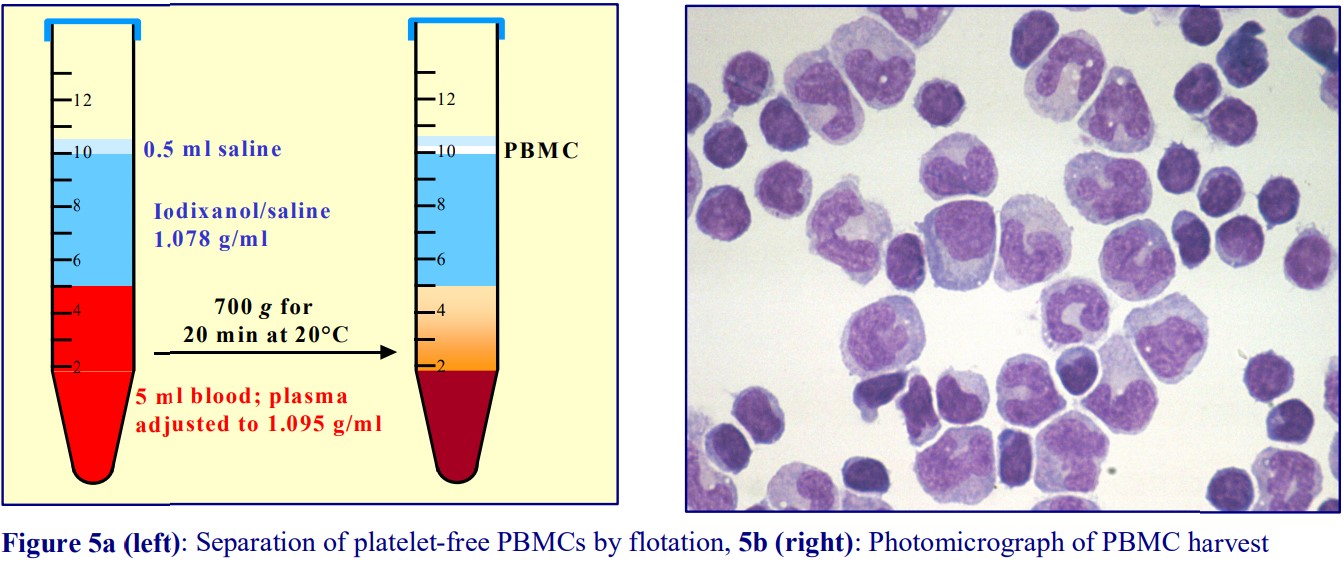
3b. Platelet-free PBMCs
Platelet contamination can be avoided entirely in a barrier flotation strategy. The plasma in the blood is adjusted to 1.095 g/ml (by addition of a 40% iodixanol solution); a solution of 1.077 g/ml (OptiPrep™ diluted with buffered saline) and a small volume of saline are layered on top. The PBMCs float to the top interface; all of the other cells and platelets remain at the bottom of the tube (see Figures 5a and 5b).
- The methodology is described in OptiPrep™ Application Sheet C06 (see Section 8)
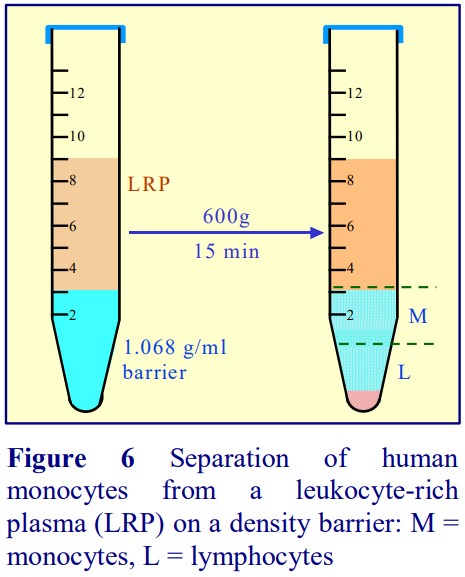
4. Purification of monocytes from human blood
All monocyte purification methods use a leukocyte-rich plasma (LRP) rather than whole blood. The LRP may be prepared as a buffy coat by low speed centrifugation (400 g for 10-15 min) of whole blood or by allowing the erythrocytes to aggregate and sediment at 1 g in the presence of 0.6% (w/v) polysucrose.
4a. Sedimentation on to a density barrier
Boyum [5,6] introduced a NycodenzⓇ density barrier (ρ = 1.068 g/ml) for resolving monocytes and lymphocytes from a leukocyte-rich plasma (LRP). It had a slightly raised osmolality (335 mOsm) to enhance the density difference between the monocytes and the osmoticallysensitive lymphocytes (whose density is increased preferentially). The method is very effective and the purity of the monocytes is greater than 90% but the monocytes do not form a distinct band; they are concentrated in the upper half of a broad turbid zone within the density barrier (see Figure 6). In the modern version of this method the density barrier is prepared by dilution of OptiPrep™ with a hyperosmotic buffered saline of 1.05% (w/v) NaCl, 10 mM Tricine-NaOH, pH 7.0.
- The methodology is described in OptiPrep™ Application Sheet C46 (see Section 8)
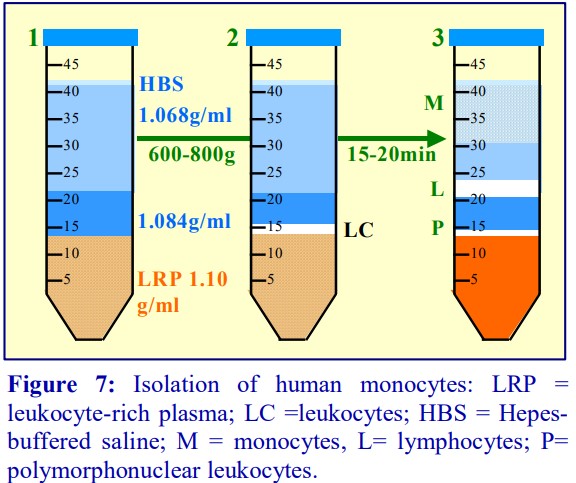
4b. Flotation through a discontinuous gradient
In the alternative strategy developed by GrazianiBowering et al [9], OptiPrep™ is added to the LRP to raise its density to approx 1.1 g/ml. The leukocytes will rapidly float to the top of this dense plasma (Figure 7:1-3) when this suspension is centrifuged. In this way the mononuclear cells initially form a narrow band at the interface between the sample and a 1.084 g/ml solution layered on top (2). The monocytes, because of their size and density, migrate upwards through this layer and through a second lowdensity barrier (ρ=1.068 g/ml). The smaller and denser lymphocytes tend to float more slowly, and in this way a separation between the two types of cells is effected Figure 7:2-3). Polymorphonuclear leukocytes (granulocytes) from the LRP tend to remain at the top interface of the sample zone.
- Flow cytometry analysis of the monocyte-rich band showed that only 3.4% of cells were CD3+ (i.e. Tcells); 1.6% of cells were CD14+
/CD4 , 6.9% were CD14+ /CD4+ and 84.1% were CD14+ /CD4+ , i.e. a total of 92.6% were identified as monocytes [7]. - The methodology is described in OptiPrep™ Application Sheet C10 (see Section 8)
- It has also been adapted to the use of whole blood in OptiPrep™ Application Sheet C11 (see Section 8)
5. Purification of human polymorphonuclear leukocytes (PMNs)
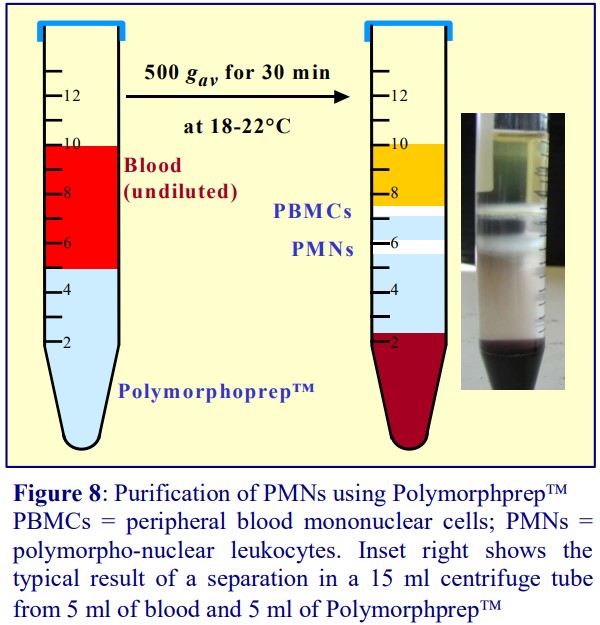
5a. From whole blood [8]
Polymorphprep™ contains 13.8% (w/v) sodium diatrizoate and 8% (w/v) Dextran 500; it has a density of 1.113 g/ml, a raised osmolality of 445 mOsm. It is the only medium capable of separating PBMCs and PMNs in one step from whole blood. The use of whole blood is essential: water in the dextran-aggregated erythrocytes, which sediment ahead of the leukocytes, passes into the Polymorphprep™ under the influence of the osmotic pressure gradient, effectively diluting the medium. As a consequence the osmotic pressure inside the erythrocytes increases; thus as they continue to sediment through the medium the osmotic pressure gradient between the cell and the medium and the loss of water from the cells progressively decline. The end result is the creation of a continuous density gradient in the medium. It is in this continuous gradient that the PBMCs and PMNs are resolved (see Figure 8). The efficacy of the method relies on the use of fresh blood from healthy donors.
- The methodology is described in the Polymorphprep™ Application Sheet (see Section 8).
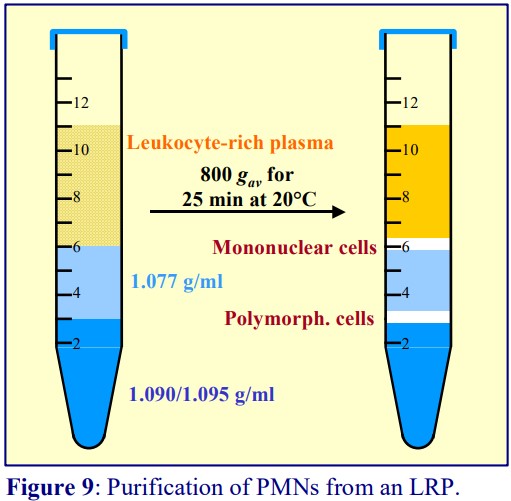
5b. From a leukocyte-rich plasma (LRP)
The LRP is best prepared from whole blood by allowing the erythrocytes to aggregate and sediment at 1 g in the presence of 0.6% (w/v) polysucrose. If this is then layered over a solution of density 1.077 g/ml (for example Lymphoprep™) and centrifuged at 600-700 g for 20 min, then the PBMCs will band at the interface and the PMNs will pellet. This is quite a common approach. However, the pelleting and consequent aggregation of PMNs at the bottom of the tube disturbs the functional integrity of the cells. Pelleting can be avoided by including a high-density cushion beneath the 1.077 g/ml layer. The easiest strategy is to prepare both layers by dilution of OptiPrep™ with a buffered saline (see Figure 9). The method is more robust than the Polymorphprep method; it is less dependent on the time from drawing the blood.
- The methodology is described in OptiPrep™ Application Sheet C12 (see Section 8).
6. Mononuclear cells (MCs) and neutrophils from experimental animals
6a. Using a 1.077 g/ml density barrier
Although commercial media designed for isolation of human blood PBMCs (see Section 2a) such as Lymphoprep™ or Histopaque™ 1.077 have been used for rodent and rabbit blood, the yields are lower because lymphocytes from these species have a higher median density than those of human blood. Consequently there are some commercial media (e.g. Histopaque™ 1.083), which address this problem simply by raising the density of the medium from 1.077 g/ml to 1.083 g/ml. This effectively improves the yield of MCs but significantly increases the contamination from neutrophils. Bøyum et al [9] overcame this serious problem by using a 1.077 g/ml of slightly reduced osmotic pressure (265 mOsm). Lymphocytes are osmotically-sensitive, neutrophils are not; thus reduction of the osmotic pressure effectively reduces the density of lymphocytes but has no effect at all on the density of the neutrophils. A 1.077 g/ml, 265 mOsm density barrier is thus the only means of obtaining rodent and rabbit MCs in high yield without neutrophil contamination.
- The methodology has also been used for MCs from canine, porcine and bovine blood.
- The reduced osmolality barrier is no longer available commercially as NycoprepⓇ 1.077A; it is however prepared very easily from OptiPrep™; the methodology is described in Application Sheet C43 (see Section 8).
- The reduced osmolality barrier is also used for the purification of MCs from a variety of animal tissues.
If a leukocyte-rich plasma (LRP) is used instead of whole blood the same reduced osmolality 1.077 g/ml barrier may be used for the simultaneous isolation of neutrophils, which will pellet. The pellet will also contain erythrocytes not aggregated by the polysucrose during the preparation of the LRP. After removal of the MCs and all of the liquid above the neutrophil pellet, the latter is suspended in isotonic ammonium chloride to lyse the erythrocytes selectively. Finally the neutrophils are pelleted and resuspended in saline.
- The methodology is described in OptiPrep™ Application Sheet C44.
6b Using a mixer flotation strategy
The method described in Section 3a has also been adapted to rat, mouse and bovine blood described in
OptiPrep Application Sheets C07, C08 and C09 respectively).
7. Clinical trials
There are now several papers from groups that have cultured the PBMCs purified in iodixanol gradients for administration to groups of patients with cancer [10-14].
8. Density Gradient Media technical literature
The OptiPrep™ Application Sheets described in the above text may all be accessed from the Index of this “cell-app” file. Other relevant OptiPrep™ Application Sheets that address gradient preparation may also be accessed from the Index. The Polymorphprep Application Sheet may be accessed from “Products”, on the www.Optiprep.com website.
9. References
1. Boyum, A. (1968) Isolation of mononuclear cells and granulocytes from human blood: Isolation of mononuclear cells by one centrifugation and of granulocytes by combining centrifugation and sedimentation at 1g Scand. J. Clin. Lab. Invest., 21 (Suppl. 97), 77-89
2. Feucht, H.E., Hadam, M.R., Frank, F. and Reithmuller, G. (1980) Efficient separation of human T lymphocytes from venous blood using PVP-coated colloidal silica particles (Percoll) J. Immunol. Meth., 38, 43-51
3. Ford, T.C., Graham, J. and Rickwood, D. (1990) A new, rapid, one-step method for the isolation of platelets from human blood Clin. Chim. Acta, 192, 115-120
4. Ford, T. C. and Rickwood, D. (1990) A new one-step method for the isolation of human mononuclear cells
J. Immunol. Meth., 134, 237-241
5. Bøyum, A., Berg, T. and Blomhoff, R. (1983) Fractionation of mammalian cells In: Iodinated density gradient media – a practical approach (ed D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 147-171
6. Bøyum, A., Lovhaug, D., Tresland, L. and Nordlie, E.M. (1983) Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality Scand. J. Immunol., 34, 697-712
7. Graziani-Bowering, G.M., Graham, J. and Filion, L.G. (1997) A quick, easy and inexpensive method for the isolation of human peripheral blood monocytes J. Immunol. Meth., 207, 157-168
8. Ferrante, A. and Thong, Y.H. (1980) Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method J. Immunol. Meth., 36, 109-117
9. Bøyum, A., Løvhaug, D., Tresland, I. and Nordlie, E.M. (1991) Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality Scand. J. Immunol., 34, 697-712
10. Kurosaki, M., Horiguchi, S., Yamasaki, K., Uchida, Y., Motohashi, S., Nakayama, T., Sugimoto, A. and
Okamoto, Y. (2011) Migration and immunological reaction after the administration of GalCer-pulsed antigen-presenting cells into the submucosa of patients with head and neck cancer Cancer Immunol. Immunother., 60, 207–215
11. Motohashi, S., Nagato, K., Kunii, N., Yamamoto, H., Yamasaki, K., Okita, K., Hanaoka, H., Shimizu, N.,
Suzuki, M., Yoshino, I., Taniguchi, M., Fujisawa, T. and Nakayama, T. (2009) A phase I-II study of - 6 galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer J. Immunol., 182, 2492–2501
12. Ishikawa, A., Motohashi, S., Ishikawa, E., Fuchida, H., Higashino, K., Otsuji, M., Iizasa, T., Nakayama,
T., Taniguchi, M. and Fujisawa, T. (2005) A phase I study of -galactosylceramide (KRN7000) – pulsed dendritic cells in patients with advanced and recurrent non – small cell lung cancer Clin. Cancer Res., 11, 1910-1917
13. Motohashi, S., Ishikawa, A., Ishikawa, E., Otsuji, M., Iizasa, T., Hanaoka, H., Shimizu, N., Horiguchi, S.,
Okamoto, Y., Fujii, S-i., Taniguchi, M., Fujisawa, T. and Nakayama, T. (2006) A phase 1 study of in vitro expanded natural T killer cells in patients with advanced and recurrent non-small cell lung cancer Clin. Cancer Res., 12, 6079-6085
14. Uchida, T., Horioguchi, S., Tanaka, Y., Yamamoto, H., Kunii, N., Motohashi, S., Taniguchi, M.,
Nakayama, T. and Okamoto, Y. (2008) Phase I study of α-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer Cancer Immunol. Immunother., 57, 337-345
OptiPrep™ Application Sheet C03; 8th edition, February 2020
OptiPrep™ Application Sheet C04
Isolation of mononuclear cells from human blood by sedimentation on to a density barrier
- OptiPrep™ is a sterile 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes – a methodological review” compares all of the currently available methodologies
- OptiPrep™ Reference List RC01 “Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes” provides a comprehensive list of all the published papers reporting
the use of OptiPrep™ - To access RC01 return to the initial list of Folders and select “Reference Lists”
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
A simple, effective method for the isolation of peripheral blood mononuclear cells (PBMCs) from human blood was first reported by Boyum in the mid-sixties [1]. Since then, the commercial medium known as Lymphoprep™, which contains sodium diatrizoate (9.6% w/v) and a polysaccharide (5.6% w/v), has been widely used for isolating the PBMCs. This simple isoosmotic density barrier (1.077 g/ml), separates the mononuclear cells from the denser polymorphonuclear leukocytes and erythrocytes. The polysaccharide aggregates the erythrocytes to increase their rate of sedimentation. It is however well established that the polysaccharide may interact with the surface of lymphocytes. Moreover, the presence of an impermeant ion (diatrizoate) in the medium may also affect the GibbsDonnan equilibrium of ions across the membrane. A non-ionic derivative of diatrizoate (NycodenzⓇ), was therefore developed. An iso-osmotic solution of 14.1% (w/v) NycodenzⓇ, 0.44% NaCl and 5 mM Tricine-NaOH, pH 7.0 [2,3], with the same density as Lymphoprep™ separates the PBMCs in exactly the same manner. Omission of a polysaccharide requires a slightly longer centrifugation time to achieve satisfactory pelleting of the erythrocytes. Identical separations can be obtained by replacing NycodenzⓇ with iodixanol. Because iodixanol is available as a 60% (w/v) solution in water (ρ = 1.32 g/ml) with no additives (OptiPrep™), the 1.077- 1.078 g/ml density barrier can be made up in the operator’s own choice of buffer and additives. The routine OptiPrep™ diluent for cells is usually 0.85% (w/v) NaCl containing 10 mM of a suitable buffer; this is normally either Hepes-NaOH or Tricine-NaOH. In an alternative strategy for the isolation of human PBMCs, the plasma itself is adjusted to a density of 1.077 g/ml cells; consequently during the centrifugation the PBMCs float to the surface of the plasma. The efficacy of this technique appears to be less species-sensitive than the density barrier strategy. This technique is described in Application Sheet C05. A modification of this flotation strategy allows the isolation of human PBMCs that are contaminated neither by platelets nor plasma. This is described in Application Sheet C06.
- This Application Sheet describes the preparation of the 1.077 g/ml barrier from OptiPrep™.
2. Choice of anticoagulant
EDTA (final concentration 1.5-2.0 mM) is the anticoagulant of choice. Both citrate and heparin are acceptable but, for reasons that are unclear, heparin is more likely to cause less than optimal separations with some blood samples. Excellence in Separations OptiPrep™ Application Sheet C04

3. Solution preparation
A. OptiPrep™ (60%, w/v iodixanol)
B. Saline solution: 0.85% (w/v) NaCl, 10 mM Hepes (or Tricine) at pH 7.0-7.6 (see Note 1) Shake the OptiPrep™ bottle gently before use and
make up the density barrier using 5 vol. of OptiPrep™ + 17 vol. of Solution B (see Note 2).
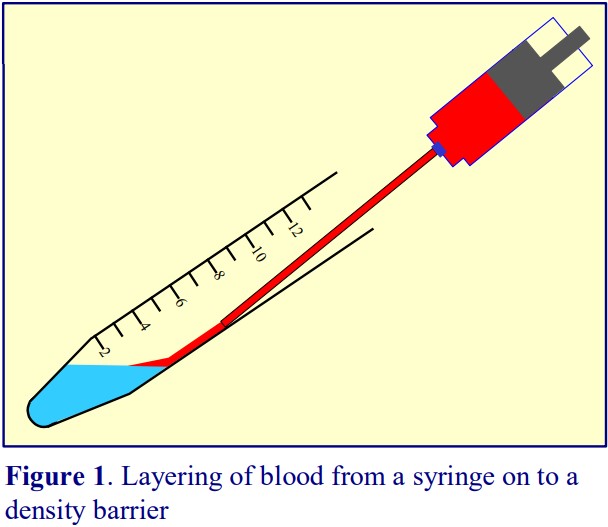
4. Protocol
1. Collect human blood by venepuncture into a suitable anticoagulant; e.g. mix 10 ml of blood gently with 150l of 100 mM di-potassium EDTA. Then dilute with an equal volume of Solution B (see
Note 3).
2. Deliver 3 ml of the barrier solution into a 15 ml conical tube; then layer 6 ml of the diluted blood on top. To achieve a sharp interface, tilt the tube and deliver the blood from a 10 ml plastic syringe attached to a metal filling cannula (see Notes 4 and 5 and Figure 1).
3. Centrifuge at 700 g for 20 min at 20°C (see Notes 6-8).
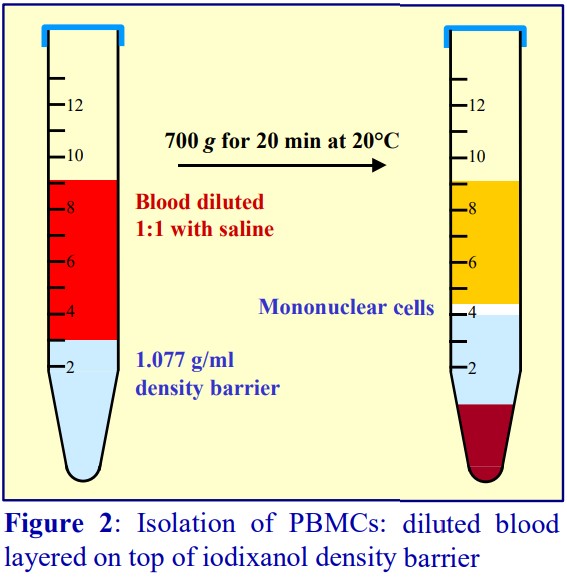
4. Harvest the PBMCs from the interface (see Figure 2 and Notes 9 and 10)
5. Notes
1. Any balanced salt solution or culture medium may be used as Solution B.
2. OptiPrep™ is quite viscous; when withdrawing an aliquot into an automatic pipette do this slowly and likewise, expel it slowly into the mixing vessel.
3. High yields (>95%) of PBMCs are only obtained if the whole blood is diluted with saline. With undiluted blood yields are reduced to <85% because the interface between the sample and the
medium is less stable and there is a tendency for the blood cells to „stream“ through the medium, carrying erythrocytes and mononuclear cells into the pellet.
4. Wide-bore stainless-steel filling cannulas (i.d. approx 0.8 mm) are readily available from surgical equipment supplies companies. By tilting the tube and positioning the tip of the cannula 1-2 cm above the density barrier, a more or less continuous stream of blood can be maintained, thus producing a sharp interface (see Figure 1).
5. Larger volumes of diluted blood (e.g. 8-9 ml) are permissible, but it may be necessary to increase the centrifugation time by approx. 5 min; the cells at the top of the sample will be exposed to a lower g-force than in the 3+6 ml format. In a 50 ml tube, use 10 ml of barrier and 20 ml of diluted blood.
6. It is recommended that Lymphoprep™ separations be carried out at 800 g for 20 min; with this iodixanol barrier 700 g is sufficient; the presence of a polysaccharide in Lymphoprep makes the solution more viscous, hence the higher recommended g-force.
7. The separation may be carried out equally effectively at 4°C, but it may be necessary to increase the centrifugation time by 5 min to overcome the slightly raised viscosity of the density barrier at the lower temperature.
8. Do not use the brake to decelerate the rotor. Rapid changes in the rpm create a vortex in the liquid and “swirling” of the pellet and banded cells. Keep Hepes (free acid) or Tricine as a 100 mM stock solution at 4°C; Hepes (2.38g) or Tricine (1.79g) per 100 ml water. Solution B: Dissolve 0.85g NaCl in 50 ml water; add 10 ml of Hepes or Tricine stock solution; adjust to pH 7.4 with 1 M NaOH and make up to 100 ml.
9. The cells will be contaminated with platelets from the plasma above the cells. Partial removal of platelets from human PBMCs can be carried out by pelleting the cells preferentially at a low RCF, 250-300 g for 10 min (no brake). The cells can be resuspended in saline and the washing process repeated. At these low g-forces the pellet is very loosely-packed and great care must be
taken during aspiration of the supernatant to avoid losing cells. Moreover pelleting and resuspending any cells is potentially damaging to the cells and should be avoided if possible..
10. If complete removal of platelets is important, the PBMCs harvested from the barrier interface should be diluted with an equal volume of Solution B (or the plasma) and the platelets separated on a 1.063 g/ml density barrier (see Application Sheet C13).
6. References
1. Boyum, A. (1968) Isolation of mononuclear cells and granulocytes from human blood: Isolation of mononuclear cells by one centrifugation and of granulocytes by combining centrifugation and sedimentation at 1g Scand. J. Clin. Lab. Invest., 21 (Suppl. 97), 77-89
2. Ford, T. C. and Rickwood, D. (1982) Formation of isotonic Nycodenz gradients for cell separations Anal. Biochem. 124, 293-298
3. Bøyum, A., Berg, T. and Blomhoff, R. (1983) Fractionation of mammalian cells In: Iodinated density gradient media – a practical approach (ed. D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 147-171
OptiPrep™ Application Sheet C04: 8th edition, February, 2020
OptiPrep™ Application Sheet C05
Isolation of human peripheral blood mononuclear cells by flotation (iodixanol mixer technique)
- OptiPrep™ is a sterile 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes – a methodological review” compares all of the currently available
methodologies - OptiPrep™ Reference List RC01 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes” provides a comprehensive list of all the published papers reporting
the use of OptiPrep™ - To access RC01 return to the initial list of Folders and select “Reference Lists”
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box
1. Background
The most commonly used technique for the isolation of peripheral blood mononuclear cells (PBMCs) from human blood is to centrifuge whole blood (diluted 1:1 with saline) over an isoosmotic 1.077 g/ml density barrier. For more information see “Mononuclear cells” – Application Sheet C04 in index. An alternative strategy devised by Ford and Rickwood [1] simplifies the procedure. A 19% (w/v) NycodenzⓇ solution (ρ = 1.100 g/ml), produced commercially as NycoPrep™ Mixer, was added to an equal volume of whole blood to raise the density of the plasma to 1.077 g/ml. During centrifugation at 1500 g for 30 min at 20°C the erythrocytes and polymorphonuclear leukocytes (PMNs) sediment while the PBMCs float to the top and are recovered from the meniscus and the medium below it. NycoPrep™ Mixer is no longer available but the technique has been adapted very successfully to the use of OptiPrep™ and is described below.
- OptiPrep™ can be mixed with whole blood directly or if preferred a buffered Working Solution containing 37% (w/v) iodixanol (ρ = 1.199 g/ml) can be added. Strategies for preparing Working Solutions for cells are described in Application Sheet C01.
2. Solutions required
A. OptiPrep™ (shake gently before use)
B. Diluent: 0.85% (w/v) NaCl, 30 mM Tricine-NaOH, pH 7.4 (for Working Solution only)
C. Tricine-buffered saline (TBS): 0.85% NaCl, 10 mM Tricine-NaOH, pH 7.4
3. Protocol
1. If using a 37% (w/v) iodixanol Working Solution: mix 3.7 vol of OptiPrep™ with 2.3 vol of Solution B.
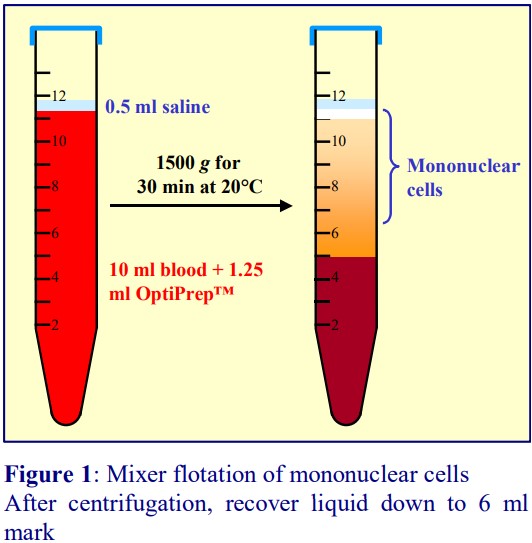
2. Mix whole blood gently but thoroughly (by repeated inversion) with OptiPrep™ or the Working Solution (WS) according to Table 1 (see Notes 1-3) in a suitable capped centrifuge tube (e.g. 15 ml tubes for 5-12 ml samples).
3. Layer approx 0.5 ml of Solution C on top and centrifuge at 1500 g for 30 min at 20°C (see Figure 1 and Note 4).
4. Collect the PBMCs from the meniscus downwards to about 1 cm from the cell pellet (see Figure 1).
5. Dilute the collected material with two volumes of buffered-saline and pellet the cells at 250-500 g for 5-10 min (see Notes 5-8).
4. Notes
1. The mixer based on Nycodenz was formulated so that equal volumes of blood and medium were mixed together to produce the required density. By using solutions of higher density (either OptiPrep™ or the Working Solution prepared from it) blood sample volumes are increased by only 12.5% or 25% (respectively) after mixing, thus the environment of the cells is closer to that of the original plasma and larger blood volumes are easier to handle (see Note 9).
2. The actual increase in density of the plasma will depend of the haematocrit of the blood and the density of the plasma. The volumes given in Table 1 assume that the hematocrit is approx. 46% (adult male average) and the plasma density is approx 1.022 g/ml. The hematocrit of normal adult female blood tends to be lower, approx 43%. If contamination of the PBMCs by PMNs is routinely unacceptable, the amount of OptiPrep™or WS added should be reduced.
3. If the aim is to isolate monocytes from the mononuclear cells, and if addition of OptiPrep to the blood is chosen, rather than the 37% iodixanol working solution, it is beneficial to spike the OptiPrep™ with 8.5% NaCl, 10 mM Hepes-NaOH, pH 7.4 (volume ratio of 1:0.01).
4. The layer of TBS on top of the blood is not critical to the separation, but it facilitates the harvesting of the PBMCs from the meniscus.
5. Table 2 shows the numbers of PBMCs recovered from 5 ml or 20 ml of blood from eight healthy donors. They represent recoveries of 92-98% from the original blood sample. Recoveries are volume independent, approx four times the number of cells being recovered from four times the blood volume. No granulocytes were observed in any of the PBMC harvests and the erythrocyte contamination was 1-3% of total cells.
6. Recoveries and purity of PBMCs isolated by flotation in iodixanol are almost identical to those obtained with Lymphoprep™. The ease of operation however makes the mixer-flotation technique the method of choice especially when handling large numbers of potentially pathogenic samples. The results are in line with those of Kaden et al [2] who compared Lymphoprep™ with a mixer based on NycodenzⓇ; these workers found that the PBMC harvests were essentially identical by both techniques.
7. The cells will be contaminated with platelets from the plasma. Partial removal of platelets from human PBMCs can be carried out by pelleting the cells preferentially at a low RCF (250 g for 10 min). The cells can be resuspended in saline and the washing process repeated. However pelleting and resuspending any cells is potentially damaging to the cells and should be avoided.
8. If complete removal of platelets is important, the PBMCs should be diluted with an equal volume of Solution C; layered over an equal volume of iodixanol, ρ = 1.063 g/ml, (5 vol OptiPrep™ + 22 vol Solution C) and centrifuged at 350 g for 15 min at 20°C. The platelets form a wide band just below the interface; the entire liquid is aspirated and the PBMC pellet resuspended in a suitable medium. For more details see “Platelets (human)” Application Sheet C13 in index.
9. If the blood has to be stored before fractionation then it is useful to note that if the density of the blood is raised by addition of the dense medium immediately after drawing, then the loss of recovery and purity of the PBMCs that is observed with density barrier techniques, is much less marked. This is probably related to the fact that once the density of the plasma has been raised, the PBMCs do not settle out upon standing [3].
5. References
1. Ford, T. C. and Rickwood, D. (1990) A new one-step method for the isolation of human mononuclear cells J. Immunol.
Meth., 134, 237-241
2. Kaden, J., Schönemann, C., Leverenz, S. and Koch, B. (1994) Optimized lymphocyte isolation. One-step procedure for
isolation of human lymphocytes by means of NycoPrep Mixer Recovery and purity in comparison with other separation
media Allergologie Jahrgang, 17, 429-433
3. Ford, T. C. and Rickwood, D. (1992) Improved isolation of mononuclear cells from stored blood Clin. Chim. Acta, 206,
249-252
OptiPrep™ Application Sheet C05: 8th edition, February 2020
OptiPrep™ Application Sheet C06
Isolation of human peripheral blood mononuclear cells by flotation (low density iodixanol density barrier)
- OptiPrep™ is a sterile 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes – a methodological review” compares all of the currently available
methodologies - OptiPrep™ Reference List RC01 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes” provides a comprehensive list of all the published papers reporting
the use of OptiPrep™ - To access RC01 return to the initial list of Folders and select “Reference Lists”
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box
1. Background
The isolation of human peripheral blood mononuclear cells (PBMCs) presented in OptiPrep™ Application Sheets C03 and C04 represent two approaches to PBMC purification in a non-ionic
medium without polysaccharide. Application Sheet C04 describes a “traditional” approach of layering the blood over a ρ = 1.077 g/ml density barrier. Application Sheet C05 describes a simpler “mixer” approach in which the blood is adjusted to = 1.077-1.078 g/ml and the PBMCs allowed to float to the surface. This Application Sheet presents a third option in which the blood is adjusted to a density considerably higher than that of the PBMCs (ρ = 1.095 g/ml) and layered beneath a ρ = 1.078 g/ml density barrier. As with the mixer technique, the PBMCs float to the surface, but this is the only system in which the cells do not band adjacent to the plasma-containing sample layer. The low-density barrier acts as a „buffer-zone“ which „washes” the PBMCs free of soluble plasma proteins and particulate contaminants such as platelets at the same time as they are purified from other blood cells.
2. Solutions required (see Note 1)

A. OptiPrep™ (shake gently before use)
B. Diluent: 0.85% (w/v) NaCl, 30 mM Tricine-NaOH, pH 7.4 (for Working Solution only)
C. Tricine-buffered saline (TBS): 0.85% NaCl, 10 mM
Tricine-NaOH, pH 7.4
3. Protocol
1. Make a Working Solution of 40% (w/v) iodixanol: mix 4 ml of OptiPrep™ and 2 ml of Solution B.
2. Adjust the plasma of whole blood to approx ρ = 1.095 g/ml by adding 2.7 ml of the Working Solution to 10 ml of whole undiluted blood (see Notes 2 and 3).
3. Prepare the ρ = 1.078 g/ml density barrier solution by diluting 5 ml of Working Solution with 9.6 ml of Solution C.
4. Using a syringe and metal cannula underlayer 5 ml of the density barrier with 5 ml of blood in a 15 ml centrifuge tube (see Note 4).
5. Layer approx 0.5 ml of Solution C on top (see Note 5) and centrifuge at 700 gav for 20 min at 20°C.
6. The PBMCs band on the top of the 1.078 g/ml barrier (see Figure 1). Remove the band with a pipette or syringe and metal cannula. Excellence in Separations OptiPrep™ Application Sheet C06
Keep Tricine as 100 mM stock solution at 4°C; 1.79g per 100 ml water. Dissolve 0.85 g NaCl in 50 ml water; add 30 ml or 10 ml Tricine stock (for Solution B or C respectively); adjust to pH 7.4 with 1 M NaOH and make up to 100 ml
7. To pellet the cells, dilute the suspension with an equal volume of Solution C and centrifuge at 400 g for 10 min (see Notes 6 and 7).
4. Notes
1. The composition of the diluents can be tailored to suit the operator’s own requirements so long as the density remains approx 1.006 g/ml. Tricine-NaOH buffers are used in the protocol but any suitable buffer may be substituted. Strategies for preparing Working Solutions for cells are described in Application Sheet C01.
2. OptiPrep™ can be mixed with whole blood directly, but a buffered Working Solution containing 40% (w/v) iodixanol (ρ = 1.216 g/ml) is the recommended option.
3. A minor modification to this method has been investigated [1] in which the blood plasma was adjusted to 1.1 g/ml rather than 1.095 g/ml. This seemed beneficial to the recovery of PBMCs, but only from those samples whose erythrocytes sedimented at this higher density. If most of the erythrocytes floated up to the bottom of the 1.078 g/ml layer, then the recovery of PBMCs was marginally worse.
4. For more information on layering of gradient solutions see Application Sheet C02.
5. It is recommended that a small volume of saline be layered on top of the 1.078g/ml layer: this facilitates harvesting of the PBMCs and avoids their banding at a water/air interface. It is not however critical in any way to the separation.
6. In an in-depth survey of PBMC isolation methods for proteomic analysis Roos et al [2] reported that the yield of PBMCs by this method was as good as the standard density-barrier sedimentation and the contamination by platelets the lowest.
7. If contamination from lymphocytes is not a problem, this flotation method is sometimes used in studies of monocyte function (e.g. see ref 3)
5. References
1. Ahmed, Y., Walton, L. J. and Graham, J. M. (2004) An improved method for isolation of mononuclear cells from peripheral blood 12th Int. Congr. Immunol., Abstr. 1758
2. De Roos, B., Duthie, S.J., Polley, A.C.J., Mulholland, F., Bouwman, F.G., Heim, C., Rucklidge, G.J., Johnson, I.T., Mariman, E.C., Daniel, H. and Elliott, R.M. (2008) Proteomic methodological recommendations for studies involving human plasma, platelets and peripheral blood mononuclear cells J. Proteome Res., 7, 2280-2290
3. Hartrick, C.T. (2002) Increased production of nitric oxide stimulated by interferon- from peripheral blood monocytes in patients with complex regional pain syndrome Neurosci. Lett., 323, 75-77.
OptiPrep™ Application Sheet C06: 7th edition, February 2020
OptiPrep™ Application Sheet C07
Isolation of rat blood mononuclear cells by flotation (iodixanol mixer technique)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes – a methodological review” compares all of the currently available
methodologies - OptiPrep™ Reference List RC01 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes” provides a comprehensive list of all the published papers reporting
the use of OptiPrep™ - To access RC01 return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box
1. Background
Standard human peripheral blood mononuclear cell (PBMC) isolation media such as Lymphoprep are less effective for the isolation of these cells from the blood of certain experimental
animals. The density of the PBMCs from mice, rats and rabbits is apparently slightly higher than that from humans. Some commercial media simply address this problem by having a correspondingly raised density. This simple solution however fails to address the simultaneous problem that the density of the polymorphonuclear leukocytes (PMNs) is the same. Thus although recoveries of PBMCs are satisfactory, contamination from PMNs can be significant. The alternative strategy solves this problem by maintaining the density at 1.077 g/ml, while reducing the osmolality of the medium from 295 mOsm to 265 mOsm. The density of the osmotically-sensitive PBMCs is thus reduced to a value less than 1.077 g/ml, while the density of the other cells is unaffected. In this manner, the difference in density between the PBMCs and the PMNs is enhanced and the cells behave essentially the same as those from human blood [1].
Human PBMCs may also be isolated by flotation: the method involves adjustment of the density of the plasma of whole blood to approx 1.078 g/ml by addition of a dense solution, which allows cells with a density lower than 1.078 g/ml to float to the surface during the centrifugation [2]. Initially this method was carried out using NycodenzⓇ but was subsequently adapted to the use of OptiPrep™. This flotation strategy, for reasons that are not clear, allows satisfactory separation of PBMCs and PMNs from other species without modulation of the osmolality. It seems not to be species-sensitive and has now been successfully applied to rat blood using OptiPrep™.
- OptiPrep™ can either be mixed with whole blood directly or if preferred a buffered Working
Solution containing 37% (w/v) iodixanol (ρ = 1.199 g/ml) can be added. - Tricine-NaOH buffer is used in the protocol but any suitable buffer may be substituted. Strategies
for preparing Working Solutions for cells are described in Application Sheet C01.

2. Solutions required
A. OptiPrep™ (shake gently before use)
B. Diluent: 0.85% (w/v) NaCl, 30 mM Tricine-NaOH, pH 7.4 (for Working Solution only)
C. Tricine-buffered saline (TBS): 0.85% NaCl, 10 mM Tricine-NaOH, pH 7.4
D. Working Solution of 37% iodixanol: mix 3.7 vol. of OptiPrep™ with 2.3 vol. of Solution B (optional).
3. Protocol
1. Anaesthetize the animal with CO2 and collect the blood (approx 10 ml) by cardiac puncture into a 10 ml syringe containing 1 ml of 3.8% (w/v) citrate as anticoagulant.
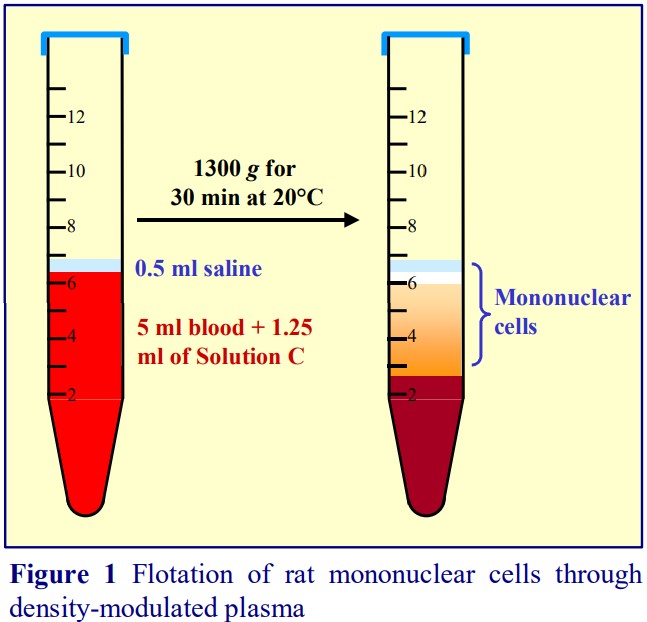
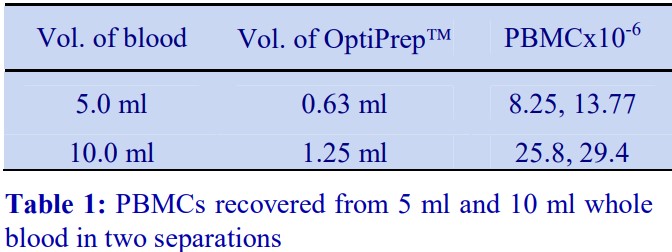
2. Mix 5 ml of whole rat blood gently but thoroughly (by repeated inversion) with 0.625-0.63 ml of OptiPrep™ or 1.25-1.26 ml of Solution D, in a suitable capped centrifuge tube (see Note 1).
3. Layer a small volume (0.5 ml) of Solution C on top (see Figure 1 and Note 2).
4. Centrifuge at 1300 g for 30 min at 20°C. Collect the PBMCs from the meniscus downwards to about 0.5 cm from the cell pellet (see Figure 1).
5. Dilute the collected material with two volumes of buffered-saline and pellet the cells at 250-500 g for 5-10 min (see Notes 3 and 4).
4. Notes
1 Larger volumes of blood can be processed if the ratio of blood to added OptiPrep is kept constant.
2. The small volume of saline on top of the sample is not required for the fractionation, but it facilitates harvesting the PBMCs, from the top of the plasma. It also prevents the cells from collecting at, and adhering to, the walls of the tube at the meniscus.
3 Recoveries of PBMCs from two experiments at two different blood volumes are given in Table 1.
4 As with the purification of human PBMCs (see Application Sheet C04) the cells will be contaminated with platelets in the plasma. Partial removal of platelets from human PBMCs can be carried out by pelleting the cells preferentially at a low RCF (250-300 g for 10 min). A more efficient method for removing platelets from human PBMCs is described in “Platelets (human)” Application Sheet C13 in index. The method has been successfully used with rodent cells.
5. References
1. Boyum, A., Lovhaug, D., Tresland, L. and Nordlie, E. M. (1991) Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality Scand. J. Immunol., 34, 697-712
2. Ford, T. C. and Rickwood, D. (1990) A new one-step method for the isolation of human mononuclear cells J. Immunol. Meth., 134, 237-241
OptiPrep™ Application Sheet C07: 9th edition, January 2020
OptiPrep™ Application Sheet C08
Isolation of mononuclear cells from mouse blood by flotation (iodixanol mixer technique)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density of 1.32 g/ml
- OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes – a methodological review” compares all of the currently available
methodologies - OptiPrep™ Reference List RC01 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes” provides a comprehensive list of all the published papers reporting
the use of OptiPrep™ - To access RC01 return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box
1. Background
Standard human peripheral blood mononuclear cell (PBMC) isolation media such as Lymphoprep are less effective for the isolation of these cells from the blood of certain experimental
animals because of the slightly higher density of the PBMCs from mice, rats and rabbits. Some commercial media address this problem by having a correspondingly raised density. This simple
solution however fails to address the simultaneous problem that the density of the polymorphonuclear leukocytes (PMNs) is the same. Thus although recoveries of PBMCs are satisfactory, contamination from PMNs can be significant. The alternative strategy solves this problem by maintaining the density at 1.077 g/ml, while reducing the osmolality of the medium from 295 mOsm to 265 mOsm. The density of the osmotically-sensitive PBMCs is thus reduced to a value less than 1.077 g/ml, while the density of the other cells is unaffected. In this manner, the difference in density between the PBMCs and the PMNs is enhanced and the cells behave essentially the same as those from human blood [1]. For more details see Application Sheet C43.
Human PBMCs may also be isolated by flotation: the method involves adjustment of the density of the plasma of whole blood to approx 1.078 g/ml by addition of a dense solution, which allows cells with a density lower than 1.078 g/ml to float to the surface during the centrifugation [2]. Initially this method was carried out using NycodenzⓇ but was subsequently adapted to the use of OptiPrep™. This flotation strategy, for reasons that are not clear, allows satisfactory separation of PBMCs and PMNs from other species without modulation of the osmolality; it seems not to be species-sensitive.

2. Solutions required
A. OptiPrep™ (shake gently before use)
B. Tricine-buffered saline (TBS): 0.85% NaCl, 10 mM Tricine-NaOH, pH 7.4 (see Note 1)
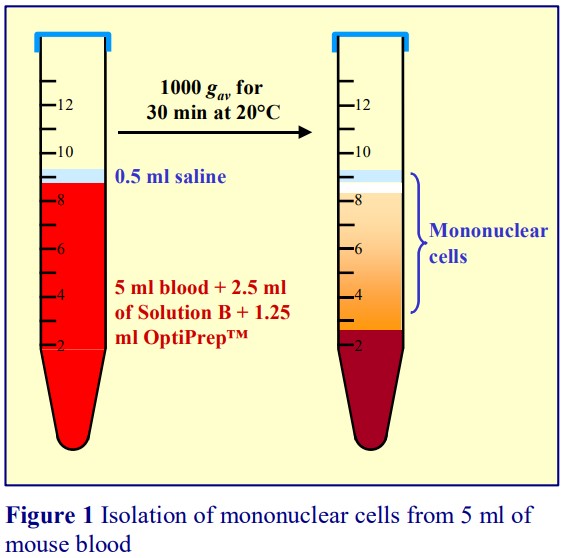
1. Anaesthetize the animal with CO2 and collect the blood (0.5-1.0 ml) by cardiac puncture into a 2 ml syringe containing 0.1 ml of 3.8% (w/v) citrate as anticoagulant.
2. For 0.25-0.5 ml of blood: To 5.0 ml of Solution B, add 1.5 ml of OptiPrep™, and mix well. Then add 5.0 ml of this medium to the mouse blood by gentle and repeated inversion.
3. For 5 ml of blood: Dilute with 2.5 ml of Solution B and then mix with 1.25 ml of OptiPrep™
4. Transfer the blood to a suitable capped tube; layer0.5 ml Solution B on top (see Figure 1) andcentrifuge at 1000 gav for 30 min at 20°C (see Note 2).
5. Collect the PBMCs from the meniscus downwardsto about 0.5 cm from the cell pellet (Figure 1).
6. Dilute the suspension with two volumes of SolutionB and pellet the cells at 300-400 g for 5-10 min (seeNotes 3 -5).
4. Notes
1 Any suitable buffer may be used, but Tricine is the buffer of choice for many cell types.
2 The small volume of saline on top of the sample is not required for the fractionation, but it facilitates harvesting the PBMCs, from the top of the plasma. It also prevents the cells from collecting at, and adhering to, the walls of the tube at the meniscus.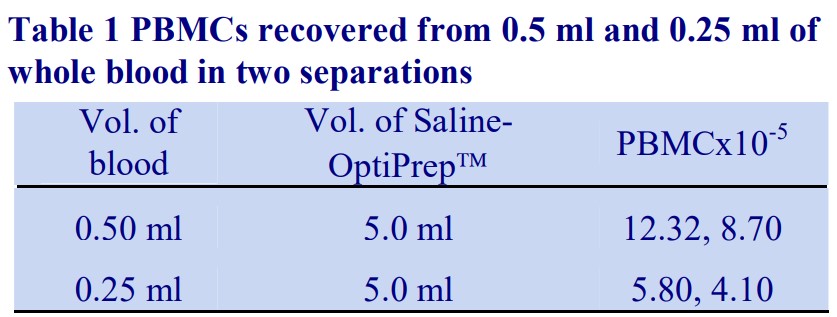
3 Total recoveries of PBMCs from two experiments at two different blood volumes (from single animals) are given in Table 1.
4 As with the purification of human PBMCs by this method the cells will be contaminated with platelets in the plasma. Partial removal of platelets from human PBMCs can be carried out by pelleting the cells preferentially at a low RCF (250-300 g for 10 min). The cells can be resuspended in saline and the washing process repeated. Whether this is a satisfactory method for mouse PBMCs is not clear.
5 Complete removal of platelets from human PBMCs can be achieved by dilution with an equal volume of Solution B; layering over an equal volume of iodixanol, ρ = 1.063 g/ml, (5 vol. OptiPrep™ + 22 vol. Solution B) and centrifugation at 350 g for 15 min at 20°C. The platelets form a wide band just below the interface; the entire liquid is aspirated and the PBMC pellet resuspended in a suitable medium. For more details see Application Sheet C12. The method has been successfully used with rodent cells.
5. References
1 Boyum, A., Lovhaug, D., Tresland, L. and Nordlie, E. M. (1991) Separation of leucocytes: improved cell purity by fine
adjustments of gradient medium density and osmolality Scand. J. Immunol., 34, 697-712
2 Ford, T. C. and Rickwood, D. (1990) A new one-step method for the isolation of human mononuclear cells J. Immunol.
Meth., 134, 237-241
OptiPrep™ Application Sheet C08: 8th edition, January 2020
OptiPrep™ Application Sheet C09
Isolation of ruminant and equine peripheral blood mononuclear cells in iodixanol gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes – a methodological review” compares all of the currently available
methodologies - OptiPrep™ Reference List RC01 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes” provides a comprehensive list of all the published papers reporting
the use of OptiPrep - To access RC01 return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box
1. Background
For the isolation of human peripheral blood mononuclear cells (PBMCs) either Lymphoprep or a solution of 14.1% (w/v) NycodenzⓇ, 0.44% (w/v) NaCl and 5 mM Tricine-NaOH, pH 7.0 have been successfully used. Lymphoprep™ contains the ionic diatrizoate and a polysaccharide, while NycodenzⓇ is non-ionic and the solution contains no polysaccharide. Both density gradient media have been used for isolating PBMCs from ruminant blood and it seems that the density of PBMCs from ruminants is closer to that of human PBMCs, compared to those from experimental animals such as rodents. This OptiPrep™ Application Sheet describes two methods for the isolation of PBMCs from ruminants. Protocol A describes the familiar strategy of sedimentation on to a density barrier, while Protocol B presents a strategy in which the density of whole blood is adjusted to a value just greater
than that of the PBMCs, which allows them to float to the surface [1]. Although this flotation technique was developed for human blood, it also seems to be rather broadly applicable to the blood of many species. Protocol B was devised for bovine blood, but it is almost certainly applicable to the blood of other ruminants and horses.

2. Solutions required (see Note 1)
A. OptiPrep™ (shake gently before use)
B. Diluent: 0.85% (w/v) NaCl, 30 mM Tricine-NaOH, pH 7.4 (Protocol B only)
C. Tricine-buffered saline (TBS): 0.85% NaCl, 20 mM Tricine-NaOH, pH 7.4
3a. Protocol A
1. Collect blood using heparin, citrate or EDTA as anticoagulant and dilute with an equal volume of Solution C (see Note 2).
2. Prepare a 1.078 g/ml solution by diluting 1.4 vol. of Solution A with 4.6 vol. of Solution C.
3. In a suitable centrifuge tube layer 2 vol. of diluted blood over 1 vol. of 1.078 g/ml solution.
4. Centrifuge at 800 g for 30 min.
5. Allow the rotor to decelerate without the brake and then collect the PBMCs from the interface.
6. Dilute the collected material with two volumes of Solution C and pellet the cells at 500 g for 15 min (see Notes 3-5).
3b. Protocol B
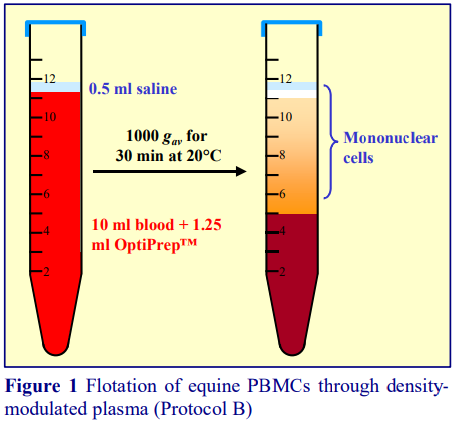
1. Collect blood using heparin, citrate or EDTA as anticoagulant (see Note 2)
2. To prepare a 37% (w/v) iodixanol Working Solution: mix 3.7 vol. of OptiPrep™ with 2.3 vol. of Solution B (see Note 6).
3. In a suitable capped centrifuge tube mix 10 ml of whole blood with 1.25 ml of OptiPrep™ or 2.5 ml of Working Solution by repeated inversion and then layer 0.5 ml of Solution C on top (see Note 7).
4. Centrifuge at 1000 gav for 30 min at 20C (see Note 8).
5. Allow the rotor to decelerate without the brake and then collect the PBMCs from the meniscus downwards to about 0.5 cm from the cell pellet as shown in Figure 1.
6. Dilute the collected material with two volumes of buffered-saline and pellet the cells at 300-400 g for 15 min (see Notes 3-5 and 9).
- In the Reference List (Section 5) refs 2-6 describe the use of ruminant blood; refs 7 and 8 equine blood
4. Notes
1. Tricine-NaOH buffer is used in the protocol but any suitable buffer may be substituted. Strategies for preparing Working Solutions for cells are described in Application Sheet C01.
2. Choice of the optimal anticoagulant is best determined empirically.
3. In the case of human blood, harvesting PBMCs from the medium is often carried out at 250-300 g for 10 min. This is insufficient to pellet all the bovine PBMCs – 300-400 g for 15 min recovers all the cells.
4. As with the purification of human PBMCs the cells will be contaminated with platelets in the plasma. Partial removal of platelets from human PBMCs can be carried out by pelleting the cells preferentially at a low RCF (250-300 g for 10 min). The cells are then resuspended in saline and the washing process repeated. Whether this is a satisfactory method for bovine PBMCs has not been rigorously tested.
5. Complete removal of platelets from human PBMCs can be achieved by dilution with an equal volume of Solution C; layering over an equal volume of iodixanol, ρ = 1.063 g/ml, (5 vol. OptiPrep™ + 22 vol. Solution C) and centrifugation at 350 g for 15 min at 20C. The platelets form a wide band just below the interface; the entire liquid is aspirated and the PBMC pellet resuspended in a suitable medium. Whether this is a satisfactory method for bovine PBMCs has not been tested. For more details see “Platelets (human)” Application Sheet C13 in index.
6. If addition of unbuffered OptiPrep™ to the blood in Step 3 of Protocol B is regarded as undesirable then use the buffered Working Solution containing 37% (w/v) iodixanol (ρ = 1.199 g/ml).
7. The small volume of saline on top of the sample is not required for the fractionation, but it facilitates harvesting the PBMCs, from the top of the plasma. It also prevents the cells from collecting at, and adhering to, the walls of the tube at the meniscus.
8. Olsen and Storset [2] used a 35 min rather than a 30 min centrifugation for calf blood.
9. Total recoveries of PBMCs from two flotation experiments with 10 ml of bovine blood were 10.85 x 10^6 and 12.65 x 10^6
5. References
1 Ford, T. C. and Rickwood, D. (1990) A new one-step method for the isolation of human mononuclear cells J.Immunol. Meth., 134, 237-241
2 Olsen, I. and Storset, A.K. (2001) Innate IFN- production in cattle in response to MPP14, a secreted protein from Mycobacterium avium subsp. paratuberculosis Scand. J. Immunol., 54, 305-3130
3 Nagaoka, K., Sakai, A., Nojima, H., Suda, Y., Yokomizo, Y., Imakawa, K., Sakai, S. and Christenson, R.K. (2003) A chemokine, interferon (IFN)--inducible protein 10 kDa, is stimulated by IFN- and recruits immune cells in the ovine endometrium Biol. Reprod., 68, 1413-1421
4 Imakawa, K., Nagaoka, K., Nojima, H., Hara, Y. and Christensen, R.K. (2005) Changes in immune cell distribution and IL-10 production are regulated through endometrial IP-10 expression in the goat uterus Am. J. Reprod. Immunol., 53, 54-64
5 Wang, J., Zhou, X., Pana, B., Yang, L., Yin, X., Xu, B. and Zhao, D. (2013) Investigation of the effect of Mycobacterium bovis infection on bovine neutrophils functions Tuberculosis, 93, 675-687
6 Lin, J., Zhao, Da,1, Wang, J., Wang, Y., Li, H., Yin, X., Yang, L. and Zhou, X. (2015) Transcriptome changes upon in vitro challenge with Mycobacterium bovis in monocyte-derived macrophages from bovine tuberculosis-infected and healthy cows Vet. Immunol. Immunopathol., 163, 146–156
7 Ellison, S.P., Greiner, E., Brown, K.W. and Kennedy, T. (2004) Experimental infection of horses with culturederived Sarcocystis neurona merozoites as a model for equine protozoal myeloencephalitis Int. J. Appl. Res. Vet. Med., 2, 79-89
8 Pronost, S., Legrand, L., Pitel, P-H., Wegge, B., Lissens, J., Freymuth, F., Richard, E. and Fortier, G. (2012) Outbreak of equine herpesvirus myeloencephalopathy in France: a clinical and molecular investigation Transbound. Emerg. Dis., 59, 256–263
OptiPrep™ Application Sheet C09; 8th edition, January 2020
OptiPrep™ Application Sheet C10
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes – a methodological review” compares all of the currently available
methodologies - OptiPrep™ Reference List RC01 “Purification of mononuclear cells, monocytes and
polymorphonuclear leukocytes” provides a comprehensive list of all the published papers reporting
the use of OptiPrep™. To access return to the initial list of Folders and select “Reference Lists”. - To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box - An alternative flotation method from whole blood is described in Application Sheet C11 (see
index). A sedimentation method (from a leukocyte-rich plasma) is also available for the isolation
of a monocyte-rich fraction (see Application Sheet C46)
1. Background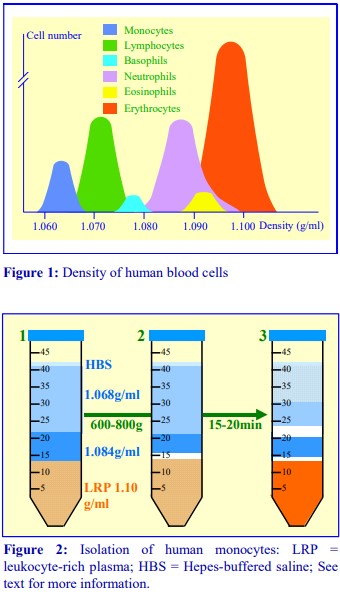
The monocytes in human peripheral blood, account for, on average, about 8% of the leukocyte population. They tend to be larger (15-20 µm) than lymphocytes (6-20 µm) and they also have a slightly lower density (Figure 1). These properties allow some scope for their separation by centrifugation. Boyum [1] introduced a NycodenzⓇ density barrier (ρ = 1.068 g/ml) for resolving
monocytes and lymphocytes from a leukocyte-rich plasma. This is commercially available as NycoprepⓇ 1.068. It has a slightly raised osmolality (335 mOsm); this enhances the density difference
between the monocytes and the osmotically-sensitive lymphocytes, whose density is increased. The method is very effective and the purity of the monocytes is greater than 90% but the monocytes do not form a distinct band; they are concentrated in the upper half of a broad turbid zone within the NycoprepⓇ 1.068. In the alternative strategy developed by Graziani-Bowering et al [2], which is the subject of this Application Sheet, OptiPrep™ is added to a leukocyte-rich plasma (LRP) to raise its density to approx 1.1 g/ml and overlaid by two lower density layers of 1.084 and 1.068 g/ml (Figure 2-1). The leukocytes rapidly float to the top of the plasma layer when the tube is centrifuged and initially form a narrow band at the interface between the sample and the 1.084 g/ml solution layer (Figure 2-2). The monocytes (because of their size and low density) migrate upwards through this layer and into the 1.068 g/ml layer. The smaller and denser lymphocytes tend to float more slowly, and in this way a separation between the two types of cells is effected (Figure 2-3). Because of the heterogeneity of the monocyte population the monocyte band is diffuse and may occupy at least a 10 ml zone below the HBS. Polymorphonuclear leukocytes (granulocytes) from the LRP tend to remain at the top interface of the sample zone.
2. Solutions required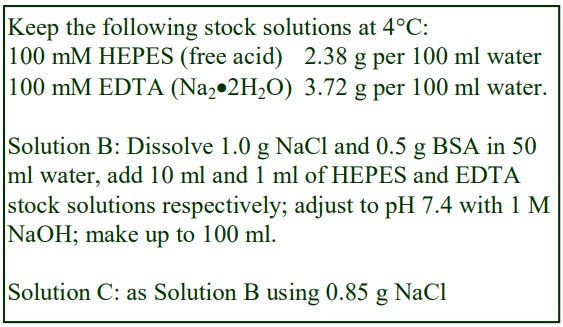
A. OptiPrep™ (shake gently before use)
B. Diluent: 1.0% (w/v) NaCl, 1 mM EDTA, 10 mM HEPES-NaOH, pH 7.4, containing 0.5% (w/v) bovine serum albumin (make up fresh)
C. HEPES-buffered saline: 0.85% (w/v) NaCl, 1 mM EDTA, 10 mM HEPES-NaOH, pH 7.4, containing 0.5% (w/v) bovine serum albumin (make up fresh)
1. Prepare two solutions of 1.068 g/ml and 1.084 g/ml by mixing OptiPrep™ and solution B using the following volume ratios: 1 vol. + 4 vol. and 1 vol. + 3 vol. respectively (see Note 1).
2. Centrifuge freshly drawn, whole blood (anti-coagulant 1.5 mM EDTA final concentration) at 400 gav in a swinging-bucket rotor, at about 20°C, for 10-15 min.
3. Harvest the buffy coat in approx 10 ml of the plasma supernatant (LRP). Some erythrocytes will also be collected but try to keep them to a minimum. Over 80% of the leukocytes are recovered in this manner.
4. Mix the LRP with OptiPrep™ (10 ml + 4 ml respectively) and in a 50 ml centrifuge tube overlayer with 7.5 ml of the 1.084 g/ml solution and 20.0 ml of the 1.068 g/ml solution and then layer a small volume of Solution C (approx. 0.5 ml) on top (see Notes 3-5).
5. Centrifuge at 600-800 gav in a swinging-bucket rotor for 20-25 min at 20°C. Do not use the brake during deceleration (see Notes 6 and 7).
6. Collect the monocytes that float into the 1.068 g/ml layer (see Figure 2 and Notes 8 and 9).
4. Notes
1. As this method separates the monocytes and lymphocytes on the basis of density and size, small differences in run conditions from laboratory to laboratory may influence its success. Improved
recoveries of monocytes may be obtained by adjusting the density of the 1.084 g/ml layer within the range 1.079-1.089 g/ml. There is evidence that it may be preferable to prepare the two density
gradient solutions by diluting the OptiPrep™ Stock with a culture medium (RPMI or DMEM) containing 10% serum. The small increase in the density of two solutions (an increase of approx 0.002 g/ml) may also be beneficial. For density tables see Application Sheet C01.
2. The method only works satisfactorily on fresh blood (used within 2 h of drawing) from healthy individuals.
3. For smaller amounts of LRP use a 15 ml tube and scale down all volumes to maintain the geometry of the interfaces observed in a 50 ml tube. For more information about preparing discontinuous gradients for cell fractionation see Application Sheet C02.
4. The topmost layer of HEPES-buffered saline is important – cells reaching the top of the 1.068 g/ml in the absence of the saline tend to adhere to the wall of the tube.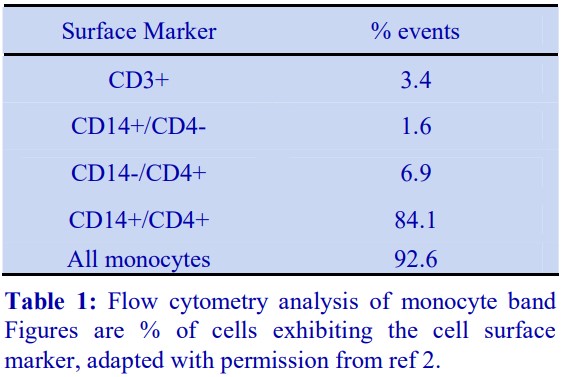
5. In the preparation of the density solutions, a 1% NaCl solution is used rather than a 0.85% NaCl solution because of the sensitivity of monocytes to reductions in ionic strength. It may therefore be useful dilute the LRP with a medium other than OptiPrep™ itself. Dilution of OptiPrep™ (4.5 vol.) with 0.5 vol. of 8% NaCl, 10 mM Hepes-NaOH, pH 7.4 will produce a solution containing 54% iodixanol and 0.8% NaCl, with a density of 1.293 g/ml. Dilution of the LRP (10 ml) with 4.5 ml of this solution will raise the density of the LRP in the same way as adding 4 ml of OptiPrep™
but the ionic strength will be raised. This modification has not been investigated but it may improve monocyte viability.
6. Increasing the time of centrifugation will increase yields but decrease purity. If lymphocyte contamination is unacceptable, try reducing the centrifugation time or harvest less of the 1.069
g/ml layer or make the middle density barrier 1.079 g/ml rather than 1.084 g/ml.
7. Note that in Application Sheet C11, the centrifugation is carried out at 4°C; this may improve monocyte viability, but the lower temperature has not been investigated with the method
described in this Application Sheet.
8. Because of small variations in tube sizes and centrifugation conditions the precise position of the monocyte band may vary. The monocyte band isolated by this technique has been analyzed by flow cytometry and contains at least 90-95% monocytes with only 3-5% contamination from Tcells. Results from a typical experiment are given in Table 1.
9. A comparison of the production of cytokines from monocytes isolated from the blood of pregnant women by iodixanol flotation and elutriation showed no statistically significant difference between the two methods [3]. Although the method was developed primarily for the isolation of monocytes, it has been used for the simultaneous recovery of lymphocytes [4] and both lymphocytes and PMNs [5].
- Ovine monocytes have also been isolated by this method [6]
5. References
1. Bøyum, A. (1983) Isolation of human blood monocytes with Nycodenz, a new non-ionic iodinated gradient medium Scand. J. Immunol., 17, 429-436
2. Graziani-Bowering, G.M., Graham, J. and Filion, L.G. (1997) A quick, easy and inexpensive method for the isolation of human peripheral blood monocytes J. Immunol. Meth., 207, 157-168
3. Nutt, J.C., Willis, C.C., Morris, J.M. and Gallery, E.D.M. (2004) Isolating pure populations of monocytes from the blood
of pregnant women: comparison of flotation in iodixanol with elutriation J. Immunol. Meth., 293, 215-218
4. Chehadeh, W., Bouzidi, A., Alm, G., Wattré, P.and Hober, D. (2001) Human antibodies isolated from plasma by affinity chromatography increase the coxsackievirus B4-induced synthesis of interferon- by human peripheral blood mononuclear cells in vitro J. Gen. Virol., 82, 1899-1907
5. Dumont, L.J., Luka, J., van den Broeke, T., Whitley, P., Ambruso, D.R. and Elfath, M.D. (2001) The effect of leukocytereduction method on the amount of human cytomegalovirus in blood products: a comparison of apheresis and filtration methods Blood, 97, 3640-3647
6. Berger, S.T. and Griffin, F.T. (2006) A comparison of ovine monocyte-derived macrophage function following infection with Mycobacterium avium ssp. avium and Mycobacterium avium ssp. paratuberculosis Immunol. Cell Biol., 84, 349- 356
OptiPrep™ Application Sheet C10; 7th edition, January 2020
OptiPrep™ Application Sheet C11
Isolation of a monocyte-rich fraction from whole human blood by iodixanol barrier flotation
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes – a methodological review” compares all of the currently available
methodologies - OptiPrep™ Reference List RC01 “Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes” provides a comprehensive list of all the published papers reporting
the use of OptiPrep™ - To access RC01 return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background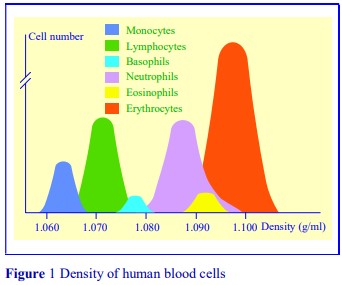
Monocytes in human peripheral blood, account for, on average, about 8% of the leukocyte population. They tend to be larger (15-20 µm) than lymphocytes (6-20 µm) and they also have a slightly
lower density (Figure 1). A method developed by Graziani-Bowering et al [1] permits the separation of monocytes from lymphocytes on the basis of rate of flotation from a leukocyte-rich plasma (LRP). The method provides highly purified and viable monocytes [1]; see Application Sheet C10: “Separation of monocytes from a leukocyte-rich plasma by flotation through a discontinuous iodixanol gradient”. However because of the requirement to prepare a buffy-coat fraction from whole blood, the yield of monocytes is always compromised by the inevitable loss of leukocytes that occurs during the preparation of such a fraction. This step also adds to the overall time of preparation. Moreover the platelets in the blood also sediment into the buffy coat fraction and the close juxtaposition of platelets and monocytes may lead to activation of the latter. The method originally devised by GrazianiBowering et al [1] has therefore been modified in an attempt to reduce any activation of the monocytes (by platelets or by the isolation procedure itself) to a minimum. The method described in this Application Sheet relies on the same principle of separation as that described in Application Sheet C10 (i.e. the more rapid rate of flotation of monocytes compared to lymphocytes) and in both cases the vast majority of the platelets remains in the high-density sample zone and do not co-band with the monocytes. The method described in this OptiPrep Application Sheet is carried out at 4°C; this tends to reduce activation and minimizes the vesiculation within the cytoplasm of the monocytes that is sometimes observed when the separations are carried out at room temperature. When this vesiculation occurs yields are very low.
- The monocytes produced by the methods described in this OptiPrep™ Application Sheet have been quantified by esterase and Sudan black staining. Purity of monocytes isolated by this method is not as high as that of cells isolated by the C10 method; recoveries however are more reproducible.
- In accordance with the observations of Filion et al [2], the OptiPrep™ used to increase the density of the blood sample is “spiked” with NaCl in order to avoid the decrease of ionic strength that occurs if neat OptiPrep is used. This improves the recovery and function of the monocytes.
- See Section 5 for a new mixer strategy for the isolation of monocytes from whole blood.
2. Solutions required (see Note 1)
A. OptiPrep™
B. 8.5% (w/v) NaCl
C. Diluent: Routine culture medium (e.g. RPMI or DMEM) containing 10% serum.
3. Protocol
- Use polypropylene tubes for all operations. Take care to mix the OptiPrep™ gently before removing an aliquot.
1. Collect 10 ml of blood using EDTA (2 mM final concentration as anticoagulant).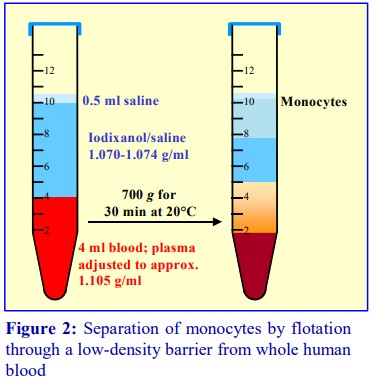
2. Prepare a 1.070 g/ml OR 1.072 g/ml OR 1.074 g/ml density barrier solution by mixing Solution A with Solution C at one of the following volume ratios: 11.8 + 48.2, 12.2 + 47.8 or 12.6 + 47.4 respectively (see Notes 2-4).
3. Mix 5.4 vol. of Solution A with 0.6 vol. of Solution B to make a 54% (w/v) iodixanol solution (see Note 5).
4. Mix 2 ml of the 54% iodixanol with 10 ml of whole blood by several very gentle inversions.
5. In a 15 ml centrifuge tube, layer 6 ml of the chosen density barrier solution over 4 ml of blood, and then layer approx 0.5 ml of Solution C on top (see Notes 6 and 7).
6. Centrifuge at 700 g in a swinging-bucket rotor for 30 min at 20°C. Do not use the brake during deceleration (see Note 8).
7. Collect the monocytes that float to the top of the 1.072 or 1.074 g/ml layer (Figure 2). The band may be quite diffuse and occupy 2-3 ml.
8. Dilute the collected cells with 2 vol of Solution C and harvest by centrifugation and resuspend the pellet gently in any medium as required (see Note 9).
4. Notes
1. It has currently not been ascertained if improved yields or purity might be achieved if the ionic strength of Solution C was increased in line with that of the OptiPrep (see Step 3 of the
protocol)
2. The choice of density for the low-density barrier depends to some extent on the operator’s requirements. Use of a 1.072 g/ml barrier will give a monocyte preparation that is approx. 85-90% pure (as estimated by esterase staining) but the yields are only approx. 30%. A 1.074 g/ml density barrier will permit the recovery of more monocytes (approx. 60% of the total) but the contamination by lymphocytes is proportionately greater (approx. 80-85% pure). The 1.070 g/ml barrier has not been investigated.
3. For rat blood, a density for the upper layer of 1.076 g/ml is suggested.
4. The protocol describes the use of a single low-density layer prepared by dilution of OptiPrep with a routine culture medium containing 10% fetal calf serum, but a two-layer format of approx
1.084 g/ml and 1.068 g/ml described in Application Sheet C10 may give better monocyte purity.
5. The method only works satisfactorily on fresh blood (used within 2 h of drawing) from healthy individuals and with EDTA as anticoagulant.
6. The uppermost layer of medium is not required for the separation process, but it avoids the banding of cells at a liquid/air interface and also prevents the cells from adhering to the walls of
the tube at the meniscus.
7. There are a number of possible variants to this method that might be investigated. For example: (1) using a 50 ml centrifuge tube so that the radial distance occupied by the sample is less, which might be accompanied by increasing the volume of the low-density barrier, or (2) using a two-layer gradient format as in Application Sheet C10.
8. Increasing the centrifugation time to 40 min may improve the yield of monocytes with the lower density barriers.
9. The purity of the monocytes is not as high as that obtained by using the method described in Application Sheet C10. Nevertheless this might be regarded as a very useful preliminary step
prior to the more economical use of antibody-coated beads to remove residual lymphocytes by negative selection.
5. Mixer strategy for whole blood monocyte preparations
Yin et al [3] have reported a simple mixer technology for the isolation of monocytes from whole human blood. It resembles the methodology described in Application Sheet C05 for the isolation of
PBMCs. Yin et al [3] mixed whole blood and OptiPrep™ in the ratio of 8:1; layered a small volume of buffered saline and top and centrifuged at 1500 g for 30 min. The cells recovered from the interface between the saline and the plasma contained >90% monocytes.
6. References
1. Graziani-Bowering, G.M., Graham, J. and Filion, L.G. (1997) A quick, easy and inexpensive method for the isolation of human peripheral blood monocytes J. Immunol. Meth., 207, 157-168
2. Filion, L.G., Graziani-Bowering, G., Matusevicius, D. and Freedman, M.S. (2003) Monocyte-derived cytokines in multiple sclerosis Clin. Exp. Immunol., 131, 324-334
3. Yin, K., Deng, X., Mo, Z-C., Zhao, G-J., Jiang, J., Cui, L-B., Tan, C-Z., Wen, G-B., Fu, Y. and Tang, C-K. (2011) Tristetraprolin-dependent post-transcriptional regulation of inflammatory cytokine mRNA expression by apolipoprotein A-I Role of ATP-binding membrane cassette transporter A1 and signal transducer and activator of transcription 3 J. Biol. Chem., 286, 13834–13845
OptiPrep™ Application Sheet C11; 7th edition, January 2020
OptiPrep™ Application Sheet C12
Isolation of human polymorphonuclear leukocytes (granulocytes) from a leukocyte rich plasma in a discontinuous iodixanol gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes – a methodological review” compares all of the currently available methodologies. For isolation of PMNs from experimental animals see Application Sheet C44.
- OptiPrep™ Reference List RC01 “Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes” provides a comprehensive list of all the published papers reporting the use of OptiPrep
- To access RC01 return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box 1
1. Background
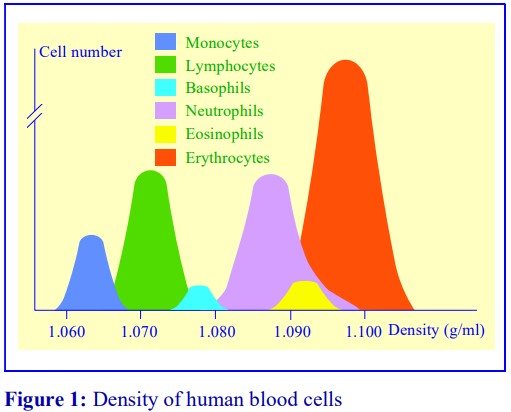
With the exception of basophils, the polymorphonuclear leukocytes (PMNs) or granulocytes from human peripheral blood have densities predominantly above 1.080 g/ml, while mononuclear
cells (MCs) have densities below 1.077 g/ml (see Figure 1). Since the density of erythrocytes significantly overlaps that of the denser neutrophils there is only one means by which PMNs may be isolated from whole human blood using a single step method and that is to use the density gradient medium called Polymorphprep™. It contains diatrizoate and a polysaccharide [1] and has a high
density (1.113 g/ml) and osmolality (445 mOsm). The high osmolality causes loss of water from the erythrocytes; creating a continuous gradient in which the MCs and PMNs band according to their buoyant densities. It is a very reliable method but the donor must be healthy; not presenting with any mild anemia and the separation must be carried out as soon as possible after the collection. There are two alternative strategies that involve the use of a simple density barrier (ρ = 1.077 g/ml) solution, rather than a two-layer density gradient. (1) Whole blood is layered over the density barrier; after centrifugation the MCs band at the interface, while the erythrocytes and PMNs sediment through the barrier to form a pellet. The latter is then recovered and the erythrocytes selectively lysed in isotonic NH4Cl or ice-cold water to leave a pure PMN fraction. Neither of these alternatives may be regarded as ideal. (2) In the other alternative a leukocyte-rich plasma (LRP), prepared from whole blood by polysucrose-aggregation of the erythrocytes (as described in this Application Sheet), is layered over the density barrier which separates the MCs and PMNs. The latter form a pellet at the bottom of the tube. These two alternatives may be considered less suitable because the formation of a pellet of the cells of interest, may lead to some aggregation of the PMNs with consequent impairment of function. Because of the possible undesirable effects of pelleting the PMNs, it is strongly recommended that an LRP is used; a dense cushion (1.090-1.095 g/ml) may then be placed beneath the 1.077 g/ml layer in order to band the PMNs rather than pellet them. The two density gradient solutions can be easily prepared from OptiPrep and it is this method that is described in the Application Sheet.
- Preparation of the leukocyte-rich plasma is achieved by adjusting the blood to 0.6% (w/v) polysucrose and allowing the aggregated erythrocytes to sediment at 1 g. There are however several popular alternatives that are described in Notes 1 and 2.
2. Solutions required
A. OptiPrep™ (shake gently before use).
B. Diluent: 0.85% (w/v) NaCl, 1 mM EDTA, 20 mM HEPES-NaOH, pH 7.4
C. Polysucrose: 6% (w/v) polysucrose (Mr = 400-500 x 103 ) in 0.85% (w/v) NaCl
D. Lysis buffer: 0.83% (w/v) NH4Cl, 10 mM HEPESNaOH, pH 7.0
E. 1.8 (w/v) NaCl, 20 mM Hepes-NaOH, pH 7.4
- Strategies for preparing density solutions for
mammalian cells are described in Application
Sheet C01.
3. Protocol
1. To 9 vol. of freshly drawn blood (containing 2 mM EDTA as anticoagulant) add 1 vol. of Solution C (see Notes 1 and 2).
2. Allow the aggregated erythrocytes to settle to the bottom (20-40 min at room temperature); then aspirate the entire supernatant.
3. Prepare the following density solutions from OptiPrep™ and Solution B (respectively): 1.077 g/ml, 5 vol. + 17 vol. and EITHER 1.090 g/ml, 8 vol. + 22 vol.; OR 1.095 g/ml, 17 vol. + 43 vol. (see Notes 3 and 4).
4. Underlayer 5 ml of LRP with 2.5-3.0 ml of 1.077 g/ml solution and the same volume of EITHER 1.090 g/ml OR 1.095 g/ml (see Fig. 2 and Note 5).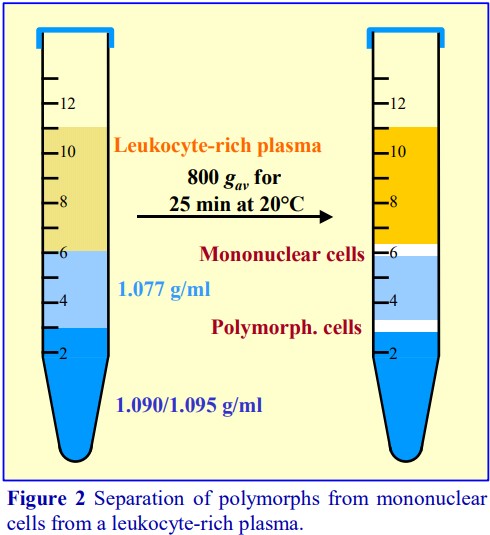
5. Centrifuge at 18-22°C for 25 min at 800 g.
6. Harvest the PMNs from the lower interface and the mononuclear cells from the upper interface (see Fig. 2).
7. Dilute the PMN suspension with an equal volume of Solution B and collect the PMNs by centrifugation at 250-350 g for 10 min.
8. Resuspend the pellet in a suitable medium for analysis.
9. To remove residual erythrocyte contamination of the PMNs, resuspend the cell pellet in 3 ml of Solution D and incubate at 37C for 7 min OR resuspend the PMNs in 3 ml ice-cold distilled water, then after 30 sec add an equal volume of Solution E.
10. Harvest the PMNs by centrifugation and resuspend in a suitable medium (see Note 6).
4. Notes
1. If exposure of the cells to polysucrose is deemed undesirable (there is evidence that this macromolecule can adsorb to the surface of leukocytes) then centrifuge the blood at 200 gav for 15-
20 min at 18-22°C and harvest the buffy coat in the plasma supernatant from the top of the packed erythrocytes and use this in Step 4.
2. Methylcellulose can be added to the blood is an alternative to polysucrose for erythrocyte aggregation. A variant of the method for removing the erythrocytes, adapted from Boyum [2], is to layer the blood over 12% (w/v) iodixanol in 130 mM NaCl, containing 1.66% methylcellulose. After standing at 1g the aggregated erythrocytes sediment to the bottom of the tube [3,4].
3. If the density of this cushion is 1.090 g/ml a small percentage of the neutrophils and most of the eosinophils will sediment through this layer. If a density of 1.095 g/ml is chosen, virtually all of the PMNs will be retained by the high-density barrier. On the other hand, fewer of the residual erythrocytes in the LRP will contaminate the PMN band using the lower density cushion. See
section 5 for some examples.
4. Occasionally the low-density 1.077 g/ml iodixanol layer is replaced with a routine commercial peripheral blood mononuclear (PBMC) isolation medium such as Lymphoprep™. There may be
some merit in the use of this solution as one of its components, 5.7% (w/v) polysucrose, may cause the residual erythrocytes to aggregate further and thus assist their sedimentation into a pellet. On the other hand this concentration of polysucrose is almost ten times higher than that to which the blood is adjusted in Step 2 of the protocol. See Section 5 for some examples.
5. For more information about preparing discontinuous gradients see Application Sheet C02.
6. In a recent study of twelve healthy males [5], the two-layer iodixanol gradient consistently gave a yield of 95%.
5. Methodological review
Some of the gradient and centrifugation conditions described in published papers reporting the use of the method described in this Application Sheet to purify PMNs from primate peripheral blood are summarized in Table 1.
The methodology has been used, with minor variations, for the isolation of PMNs from bovine, guinea pig, mouse, rabbit and rat sources. For references see Reference List RC01.
6. References
1. Ferrante, A. and Thong, Y.H. (1980) Optimal conditions for simultaneous purification of mononuclear and polmorphonuclear leucocytes from human blood by the Hypaque-Ficoll method J. Immunol. Meth., 36, 109-117
2. Boyum, A. (1968) Isolation of mononuclear cells and granulocytes from human blood: Isolation of mononuclear cells by one centrifugation and of granulocytes by combining centrifugation and sedimentation at 1g Scand. J. Clin. Lab. Invest., 21, 1-89
3. Niggli, V. (2003) Microtubule-disruption-induced and chemotactic-peptide-induced migration of human neutrophils: implications for differential sets of signaling pathways J. Cell Sci., 116, 813-822
4. Dehghani Zadeh, A., Seveau, S., Halbwachs-Mecarelli, L. and Keller, H.U. (2003) Chemotactically-induced redistribution of CD43 as related to polarity and locomotion of human polymorphonuclear leucocytes Biol. Cell, 95, 265-273
5. Radom-Aizik, S., Zalvidar, Jr., F., Leu, S-Y., Galasetti, P. and Cooper, D.M. (2008) Effects of 30 min of aerobic exercise on gene expression in human neutrophils J. Appl. Physiol., 104, 236-243
6. Yuan, Z-N, Tolo, K., Schenck, K. and Helgeland, K. (1999) Increased levels of soluble Fc receptor III in gingival fluid periodontal lesions Oral Microbiol. Immunol., 14, 172-175
7. Hudgens, J., Langkamp-Henken, B., Stechmiller, J.K., Herrlinger-Garica, K.A. and Nieves, C. (2005) Immune function is impaired with a mini nutritional assessment score indicative of malnutrition in nursing home elders with pressure ulcers J. Parenter. Enteral Nutr. 28, 416-422
8. Chiu, H-C., Liang, J-S., Wang, J-S. and Lu, J-F. (2006) Mutational analyses of Taiwanese kindred with X-linked adrenaleukodystrophy Pediatr. Neurol., 35, 250-256
9. Channon, J.Y., Seguin, R.M. and Kasper, L. (2000) Differential infectivity and division of Toxoplasma gondii in human peripheral blood leukocytes Infect. Immun., 68, 4822-4826
10. Channon, J.Y., Miselis, K.A:, Minns, L.A, Dutta, C. and Kasper, L.H. (2002) Toxoplasma gondii induces granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor secretion by human fibroblasts: implications for neutrophil apoptosis Infect. Immun., 70, 6048-6057
11. Shen, L., Fahey, J.V., Hussey, S.B., Asin, S.N., Wira, C.R. and Fanger, M.W. (2004) Synergy between IL-8 and GMCSF in reproductive tract epithelial cell secretions promotes enhanced neutrophil chemotaxis Cell. Immunol., 230, 23- 32
12. Shen, L., Smith, J.M., Shen, Z., Hussey, S.B., Wira, C.R. and Fanger, M.W. (2006) Differential regulation of neutrophil chemotaxis to IL-8 and fMLP by GM-CSF: lack of direct effect of oestradiol Immunology, 117, 205-212
13. Smith, J.M., Wira, C.R., Fanger, M.W. and Shen, L. (2006) Human fallopian tube neutrophils – a distinct phenotype from blood neutrophils Am. J. Reprod. Immunol., 56, 218-229
14. Shen, L., Smith, J.M., Shen, Z., Eriksson, M., Sentman, C. and Wira, C.R. (2007) Inhibition of human neutrophil degranulation by transforming growth factor-1 Clin. Exp.
Immunol.,149, 155-161
15. Smith, J.M., Shen, Z., Wira, C.R., Fanger, M.W. and Shen, L. (2007) Effects of menstrual cycle status and gender on human neutrophil phenotype Am. J. Reprod. Immunol., 58, 111-119
16. Pioli, P.A., Jensen, A.L., Weaver, L.K., Amiel, E., Shen, Z., Shen, L., Wira, C.R. and Guyre, P.M. (2007) Estradiol attenuates lipopolysaccharide-induced CXC ligand 8 production by human peripheral blood monocytes J. Immunol., 179, 6284-6290
17. Lau, M., Vayntrub, T., Grumet, F.C., Lowsky, R., Strober, S., Hoppe, R., Larson, M., Holm, B., Reitz, B. and Borie, D (2004) Short tandem repeat analysis to monitor chimerism in Macaca Fasicularis Am. J. Transplant., 4, 1543-1548
OptiPrep™ Application Sheet C12; 8th edition, January 2020
OptiPrep™ Application Sheet C13
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RC02) “The purification of platelets from whole blood and
their removal from blood leukocyte preparations” provides a comprehensive list of all the relevant
published papers: to access return to the initial list of Folders and select “Reference Lists” - To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box
1. Background
Although platelet-rich plasma (PRP) is relatively easy to produce by centrifugation of whole blood, yields of platelets may be variable, because many of them are trapped within the erythrocyte layer. Although they can be recovered by washing these cells with isotonic saline, it is a general rule that to avoid activation of the platelets, the number of centrifugations and resuspensions should be kept to a minimum. Another problem is that aspiration of the PRP must be performed carefully to avoid contamination from leucocytes in the buffy coat which lies atop the erythrocytes. To provide a highly purified platelet fraction from human blood Ford et al [1] layered whole blood over a density barrier of NycodenzⓇ (ρ = 1.063 g/ml) that allowed the erythrocytes and leucocytes to pellet during centrifugation at 350g. The platelets, because of their small size, sediment much more slowly; they form a broad band extending into the density barrier from just above the interface. The platelets recovered from this density barrier method have been used directly in aggregation studies; the NycodenzⓇ did not interfere with this process [1]. An iodixanol barrier of the same density can be substituted for the NycodenzⓇ; this has no effect on the separation or yield of platelets.
- Recently the purity of the iodixanol-isolated platelets has been validated by flow cytometry and their functional integrity confirmed [2,3].
2. Solutions required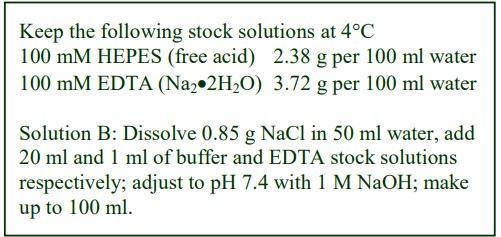
A. OptiPrep™ (shake the bottle gently before use)
B. Diluent: 0.85% (w/v) NaCl, 20 mM HEPESNaOH, pH 7.4, 1 mM EDTA
3. Protocol
1. Collect blood by venepuncture into a suitable anti-coagulant (EDTA or citrate).
2. Produce the ρ = 1.063 g/ml density barrier by mixing 5 vol OptiPrep™ with 22 vol of Solution B (see Note 1).
3. In a centrifuge tube layer 1 vol of blood over 1 vol of density barrier (see Figure 1 and Notes 2 and 3) and centrifuge at 350 g for 15 min at 20°C in a swinging-bucket rotor and allow the rotor to decelerate without the brake.
4. Harvest the autologous plasma and the platelet-containing band as shown in Figure 1.
5. Towards the bottom (lowest 2-3 mm) of the platelet band there will be a slight increase in the contamination by leucocytes and erythrocytes (up to 3-5%) while the contamination in the bulk of the platelet band is <1%.
4. Notes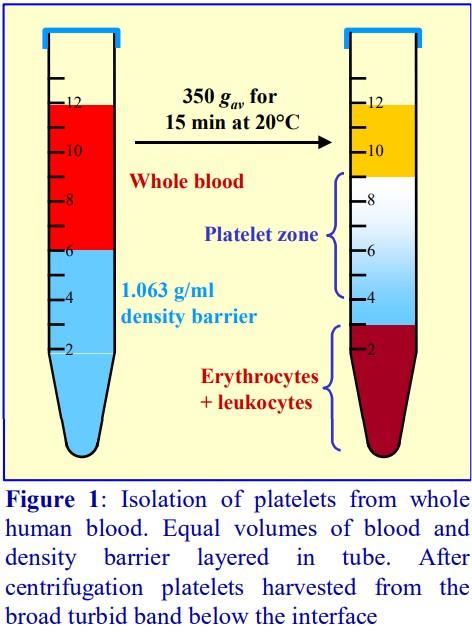
1 For more information on preparing density solutions see Application Sheet C01.
2 The separation of the platelets is based on their slow rate of sedimentation, so it is very important that the centrifugation speed and time is carefully adhered to. Higher speeds and longer times will result in the platelet band moving closer to the cell pellet.
3 To permit an adequate linear separation of the platelets from the pellet the density barrier column needs to be approx 5 cm, thus in a 15 ml centrifuge tube there should be a minimum of 5
ml of density barrier. For small volumes (1-5 ml of blood) use 5 ml of barrier; for larger volumes of blood, use an equal volume of barrier. The column height of the density barrier in larger volume tubes should be maintained.
- The method can be used both to prepare platelets for analysis and to remove them from other cell types such as peripheral blood mononuclear cell suspensions.
5. Methodological variation
A three-layer iodixanol gradient has been used for the very successful production of highlypurified functional platelets by Birschmann et al [4,5] from platelet concentrates (ex blood bank). The
washed platelets were layered on top of 14, 14 and 15 ml respectively of 6%, 7.8% and 10.2% (w/v) iodixanol (equivalent to 10, 13 and 17% (v/v) OptiPrep) and centrifuged at 300 g for 20 min. After discarding the top 7.5 ml, the highly-purified platelets were recovered in the next 12.5 ml.
6. References
1. Ford, T.C., Graham, J. and Rickwood, D. (1990) A new, rapid, one-step method for the isolation of platelets from human blood Clin. Chim. Acta, 192, 115-120
2. Bagamery, K., Kvell, K., Barnet, M., Landau, R. and Graham, J. (2005) Are platelets activated after a rapid, one-step density gradient centrifugation? Evidence from flow cytometric analysis Clin. Lab. Haem., 27, 75-77
3. Bagamery, K., Kvell, K., Landau, R. and Graham, J. (2005) Flow cytometric analysis of CD41-labeled platelets isolated by the rapid, one-step OptiPrep method from human blood Cytometry Part A, 65A, 84-87
4. Birschmann, I., Mietner, S., Dittrich, M., Pfrang, J., Dandekar, T. and Walter, U. (2008) Use of functional highly purified human platelets for the identification of new proteins of the IPP signaling pathway Thromb. Res., 122, 59–68
5. Gambaryan, S., Kobsar, A., Hartmann, S., Birschmann, I., Kuhlencordt, P.J., Müller-Esterl, W., Lohmann, S.M. and Walter, U. (2008) NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity J. Thromb. Haemost., 6, 1376-1384
OptiPrep™ Application Sheet C13; 8th edition, January 2020
OptiPrep™ Application Sheet C14
Removal of non-viable cells from a cell suspension
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List RC03 “Viable/non-viable cell separation” provides a list of all the published papers reporting the use of OptiPrep™: to access return to the initial list of Folders and select “Reference Lists”
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
Isolation of cells from a lavage of a body cavity or from the mechanical or enzymic dissociation of a tissue will inevitably render a number of cells non-viable, which must be removed prior to further processing. Other important scenarios are the removal of non-viable cells after electroporation and the retrieval of viable cells from a valuable line of cultured cells after an incubator failure. Non-viable cells may also release intracellular components, for example hydrolytic enzymes and DNA, into the suspension; these also need to be removed from the aqueous environment of any recovered viable cells.
Non-viable cells, which no longer enclose an osmotic space, are significantly denser than viable cells and thus should be easily separated from them across a density barrier. The actual density of the non-viable cells will be partly related to that of their viable counterparts.
Strategy 1
A simple method developed with NycodenzⓇ, and subsequently extended to OptiPrep™, is to centrifuge the cell suspension over a solution whose density is higher than that of the viable cells; the non-viable cells pellet and the viable ones are recovered from the interface (Figure 1). Once the cells have lost their osmotic competence and become leaky to the gradient solute, their density should increase significantly and theoretically their density should exceed approx 1.15 g/ml. With this strategy however there is no need to use such a high density for the barrier as long as it is of a sufficient density to retain the viable cells. Consequently the density of barriers used to effect this separation varies from approx 1.060 to 1.15 g/ml, although in the majority of cases the density is at least 1.080 g/ml.
Strategy 2
A drawback of Strategy 1 is that the viable cells band adjacent to the sample zone and hence will remain in contact with released intracellular macromolecules (Figure 1). In Strategy 2, the cell suspension is adjusted to a density of 1.15-1.16 g/ml, layered beneath a solution whose density is greater than that of the viable cells, thus allowing these viable cells to float away from the sample zone (Figure 2) and any released macromolecules. Suspending the cells in a high concentration of the gradient solute will also be a more efficient way for the solute to enter the intracellular space of the non-viable cells. A density of 1.12 g/ml has been chosen for the upper layer, as this is likely to be high enough to allow the flotation of any viable mammalian or non-mammalian cell. It might however be replaced by a solution of any density higher than that of the cell of interest.
- Strategy 2 has been used very successfully for recovering a small number of viable cells from a vast preponderance of non-viable cultured cells.
- The following protocols use OptiPrep™ rather than NycodenzⓇ because of the easier solution preparation with the former.
2. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. OptiPrep™ Diluent: culture medium (RPMI or DMEM) + 10% serum (see Note 1).
 3. Protocol
3. Protocol
3a. Strategy 1
1. Make a suitable density barrier solution by diluting OptiPrep™ with Solution B. See Notes 2-4 for detailed information on choice of the correct density solution.
2. Put a small volume of the density barrier in 15 ml (approx. 2 ml) or 50 ml (approx 5 ml), conical plastic centrifuge tubes and carefully layer the cell suspension on top.
3. Centrifuge in a swinging bucket rotor at approx 800 g for 15-20 min (see Note 5).
4. Non-viable cells will pellet and any macromolecular material, and residual enzymes from any enzymicallydigested tissue, will mostly remain in the supernatant, above the interfacial band of viable cells (see Figure 1).
5. Remove as much of the supernatant as possible: harvest the cell band; dilute with two volumes of Solution B (or any balanced salt solution); pellet the cells at 200-400 g for 10 min and resuspend them in a suitable medium.
 3b. Strategy 2
3b. Strategy 2
1. Prepare a 40% (w/v) iodixanol working solution (WS) by diluting 2 vol. of OptiPrep™ with 1 vol. of Solution B. Dilute the WS further with Solution B to give a 22% (w/v) iodixanol solution (approx ρ = 1.12 g/ml). See Notes 2-4 for more information on density solution preparation.
2. Carefully mix 1 vol. of WS with 0.45 vol. of cell suspension by gentle repeated inversion and transfer to a centrifuge tube (up to 6.0 ml in a 15 ml centrifuge tube).
3. Overlay this with 3.0 ml of the 22% (w/v) iodixanol (see Note 6) and 0.5 ml of culture medium or Solution B (scale up or down as appropriate).
4. Centrifuge at 800 g for 20-25 min (see Note 6).
5. Collect the viable cells from the top interface (see Figure 2); dilute with 2 vol. of culture medium or Solution B and harvest the cells at 200-400 g for 10 min.
4. Notes
1. Solution B may be any suitable balanced salt solution, or a Tricine or HEPES-buffered saline (with or without serum) may be substituted for the culture medium. Low concentrations of Mg2+ and/or Ca2+ and glucose may be included in the saline solution.
2. To recover all the viable cells from a mixed cell population containing cells with a broad range of densities, a density barrier of at least 1.11 g/ml should be chosen. If the requirement is to recover a single cell type of known density, then the density of the barrier might be reduced. Viable cells that have a relatively low density (e.g. lymphocytes, thymocytes, some bone marrow progenitor cells) can be separated from non-viable cells on density barrier of approx 1.09 g/ml.
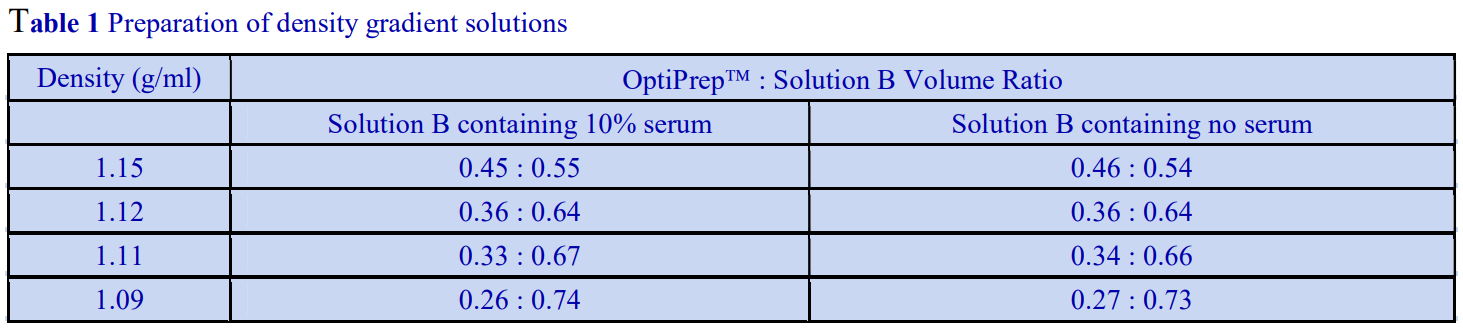
3. The precise amounts of OptiPrep™ and Solution B will vary with the density of the latter but the values given in Table 1 will cover most cases. If the barrier is ineffective in removing the nonviable cells its density must be decreased; if viable cells are lost into the pellet its density must be increased. For more information about preparing iodixanol density gradient solutions and more extensive density tables see Application Sheet C01.
4. Table 2 lists some of the cell types processed using OptiPrep™, together with the centrifugation conditions (if given) in the text.
5. It may be necessary to increase either the g-force or the time of centrifugation to achieve a satisfactory result.
6. It is permissible to omit the layer of ρ = 1.12 g/ml but this provides a useful “clean zone” to separate the viable cells from all of the non-viable cells plus any released cytosolic components plus any residual digestive enzymes (if these were used for tissue disaggregation). All of these remain in the load zone.
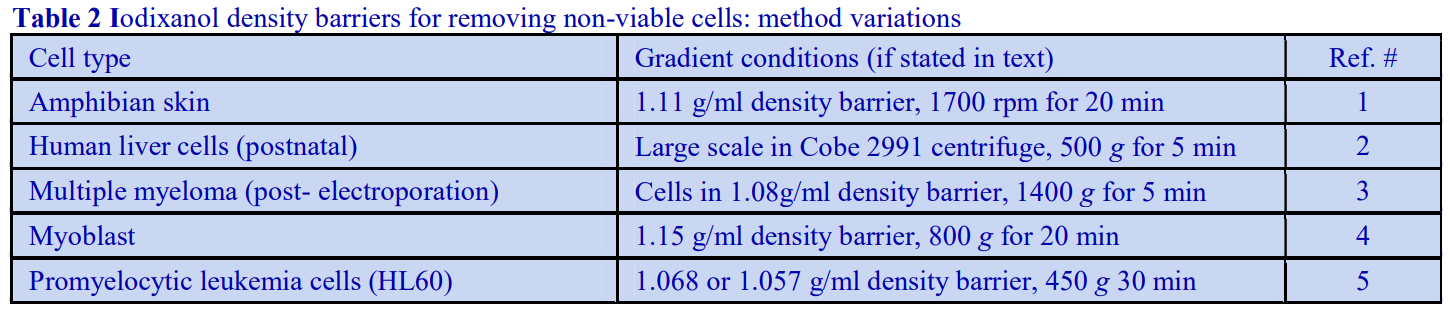
5. References
1. Cox, T.C. (1999) Calcium and ATP regulation of ion transport in larval frog skin J. Comp. Physiol. B, 169, 344-350
2. Schmelzer, E., Zhang, L., Bruce, A., Wauthier, E., Ludlow, J., Yao, H-l., Moss, N., Melhem, A., McClelland, R., Turner, W., Kulik, M., Sherwood, S., Tallheden, T., Cheng, N., Furth, M.E. and. Reid, L.M. (2007) Human hepatic stem cells from fetal and postnatal donors J. Exp. Med., 204, 1973-1987
3. Chatterjee, M., Stuhmer, T., Herrmann, P., Bommert, K., Dorken, B. and Bargou, R.C. (2002) Combined disruption of both the MEK/ERK and the IL-6R/STAT3 pathways is required to induce apoptosis of multiple myeloma cells in the presence of bone marrow stromal cells Blood, 104, 3712-3721
4. Benabdallah, B.F., Bouchentouf, M. and Tremblay, J.P. (2005) Improved success of myoblast transplantation in mdx mice by blocking the myostatin signal Transplantation 79, 1696-1702
5. Hauert, A.B., Martinelli, S., Marone, C. and Niggli, V. (2002) Differentiated HL-60 cells are a valid model system for the analysis of human neutrophil migration and chemotaxis Int. J. Biochem. Cell Biol., 34, 838-854
OptiPrep™ Application Sheet C14; 8th edition, January 2020
OptiPrep™ Application Sheet C15
Fractionation of a mixed population of cells
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box
1. Background
If neither an OptiPrep™ nor a Nycodenz based method is presently available for the purification or enrichment of a particular cell type from a body fluid or lavage or from a mechanically or
enzymically-dissociated tissues, this Application Sheet contains some simple suggestions for the design of a new gradient system. Although there may be a published method for the isolation of a particular cell type using one of the polysaccharide or colloidal silica (PercollⓇ) media, the use of OptiPrep™ will provide several important advantages. The very low endotoxin levels of OptiPrep™ and the lack of interaction of iodixanol with cells are important properties not exhibited by these other media. This Application Sheet will confine its recommendations to the use of OptiPrep™ because of the ease of preparation of gradient solutions (simple dilution of OptiPrep™ with saline); NycodenzⓇgradient solutions must be prepared from NycodenzⓇ powder. Although positive selection with antibody-bound magnetic beads may provide highly purified cells very easily, the interaction of beads with the cell surface may lead to unpredictable functional changes. Even if an OptiPrep™ based gradient cannot provide the purity of positive selection, a preliminary enrichment of particular cell type can make the subsequent use of negative selection with antibodybound beads a more economical and very attractive alternative strategy. Although the “traditional” means of applying a cell suspension to pre-formed gradient is simply to layer it on top, there alternative strategy of layering it in a dense solution under the gradient is an increasingly used alternative that can often provide improved resolution. This is particularly the case if the aim is to isolate a minor population of low-density cells from a mixture of predominant denser cells.
- Any non-viable cells should be removed from the suspension during the preliminary stages of any gradient fractionation; see “Removal of non-viable cells from a cell suspension” – Application Sheet C14 in index.
2. Solutions and reagents required (see Note 1)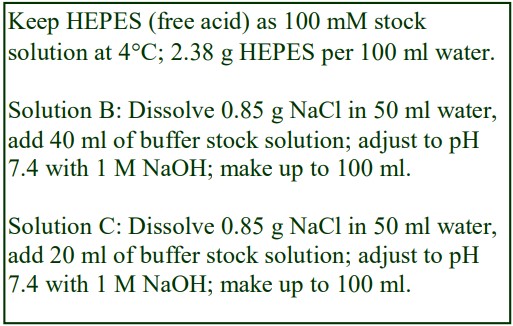
A. OptiPrep™
B. OptiPrep™ Diluent: 0.85% (w/v) NaCl, 40 mM HEPES-NaOH, pH 7.4
C. Working Solution (WS) Diluent: 0.85% (w/v) NaCl, 20 mM HEPES-NaOH, pH 7.4
3. Protocols
3a. Iodixanol working solution preparation
1. Shake the bottle of OptiPrep™ gently before use.
2. Make a 30% (w/v) iodixanol working solution (approx = 1.16 g/ml) by mixing equal volumes of OptiPrep and Solution B. Dilute further with Solution C to produce solutions of lower density.
3b. Preparation of cell suspension for gradient loading
3b-1. Non-viable cells removed using Strategy 1 of Application Sheet C13
If in the subsequent fractionation, the cells are to be layered on top of the gradient: harvest the cell band (taking as little of the density barrier as possible). Dilute the suspension with 2 vol. of Solution C and pellet the cells at approx 250-400g for 10-20 min. Resuspend the pellet in Solution C. If in the subsequent fractionation, the cells are to be layered beneath the gradient: discard as much of the top layer as possible and harvest the cell band, together with most of the density barrier, but avoiding the pellet. Then mix gently to resuspend the cells: the density of the suspension should be approx 1.12 g/ml.
3b-2. Non-viable cells removed using Strategy 2 of Application Sheet C13
If in the subsequent fractionation, the cells are to be layered on top of the gradient: harvest the cell band in the top layer of culture medium (taking as little of the 1.12 g/ml layer as possible) and dilute with 3 volumes of Solution C or culture medium. If the density of the cell suspension is not less than that of the top of the subsequent gradient, the cells will have to be pelleted and resuspended (see above). If in the subsequent fractionation, the cells are to be layered beneath the gradient: discard as much of the top layer of culture medium and harvest the cell band in 4-5 ml of the 1.12 g/ml layer. The density of this suspension may need to be increased by mixing with a small volume of the 1.16 g/ml iodixanol Working Solution.
3c. Fractionation by buoyant density
1. Prepare a preformed, continuous gradient, with a density range of 1.03 to 1.10 g/ml (this is approximately equivalent to 5-20% (w/v) iodixanol. Alternatively construct a discontinuous gradient with several layers covering the same density range (see Notes 4 and 5).
2. Layer the recovered cell sample (see Note 4) either under or on top of the gradient and centrifuge at 800-1000 g for 20-30 min at 20C (or 4C) in a swinging bucket rotor (see Note 6).
3. Identify the cell types in each band, which, during centrifugation, will band at respective their buoyant densities (see Notes 7-9).
- With a little patience and experimentation, a suitable separation method can be developed. Once
the banding characteristics have been determined, it may be possible to devise a simplified density
barrier or two-step discontinuous gradient.
3d. Fractionation on the basis of cell size
1. If two types of cell, with the same buoyant density, but of different sizes are present, the larger will sediment (or float up) more quickly than the smaller until they reach the point in the gradient
equivalent to their buoyant density. With continuing centrifugation, the smaller cells will reach the same point. So time of centrifugation is important in separations by size.
2. Suspend the cells in Solution C and layer them (see Note 10) on top of a preformed continuous gradient (approx 1.03-1.09 g/ml).
3. Centrifuge at 600 gav for 10 min and examine to determine if a separation has been achieved. If not try other centrifugation times (8, 12, 15 min etc).
4. Notes
1. All gradient solutions prepared as described in Section 2 will have an osmolality in the range 290- 305 mOsm. Solution B has double the buffer concentration of that of Solution C so that the buffer concentration in the gradient solutions (and hence in the gradient) is constant. The same principle could be applied to any other low concentration additive that might be deemed an important component of the gradient for maintenance of cell viability, e.g. MgCl2 and/or CaCl2 at 1-2 mM. If this is unimportant then Solutions B and C may be identical and be any isotonic buffered saline, balanced salt solution or a routine culture medium; none of these substitutions will have a significant effect on the final density or osmolality of the gradient solutions. If 10% serum is included then this will slightly increase the density of all the solutions. For more information see Application Sheet C01.
2. There are some instances in which separation may benefit from a small increase in ionic strength of the gradient solutions, see for example Application Sheet C46 – “Isolation of monocytes from a human leukocyte-rich plasma on a density barrier” in index; or the use of a significantly hyperosmotic medium maybe beneficial to the separation of a particular cell type, see for example Application Sheet C16 – “Pancreatic islets” in index.
3. For information on the preparation of discontinuous and continuous gradients for cell separations see Application Sheet C02.
4. If the step to remove non-viable cells is omitted the cells can be suspended directly in a highdensity solution (approx 22.5% (w/v) iodixanol or Nycodenz) for bottom-loading or in Solution C for top-loading.
5. It may be beneficial to carry out the centrifugation at 4C in some cases, in which case it may be necessary to increase the time of centrifugation.
6. The resolving power of a discontinuous gradient depends on the density interval of adjacent layers. Cells that band at any interface will have a range of densities between those of the two layers.
7. If the cells of interest co-band with other types, then the gradient may be too steep to resolve them, in which case a shallower gradient (or layers covering a smaller density interval) may be required. Alternatively different cell types may have the same density, and only be separable on the basis of size.
8. If all of the cells band either towards the top or bottom of the gradient, its density range should be modulated to avoid this.
9. Resolution on the basis of sedimentation (or flotation) rate is inversely proportional to the depth of the sample; sample volume must therefore be kept to a minimum.
OptiPrep™ Application Sheet C15; 8th edition, December 2019
OptiPrep™ Application Sheet C16
Purification of Islets of Langerhans from porcine, primate and rodent pancreas in a discontinuous iodixanol gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RC04) “Purification of pancreatic islets” provides a protocol
review and list of all published papers: to access return to the initial list of Folders and select
“Reference Lists” - To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box
1. Background
This protocol is based upon an islet isolation method using the University of Wisconsin solution (UWS) as the medium for both collagenase digestion of the tissue at 37°C and
for all post-digestion operations (mechanical dispersion, filtration etc) carried out at 0-4°C [1- 3]. Some workers may prefer to restrict the use of UWS to the “cold” steps (it may be slightly cytotoxic at 37°C, or it may inhibit digestion in other species); in which case the digestion should be carried out in Hanks Balanced Salt Solution (HBSS) or in a tissue culture medium such as
RPMI (see Note 1). If such a medium is also used for the preparation of the density gradient solutions, modifications will need to be made to the volumes of OptiPrep™ and medium because these culture media have a lower density than that of UWS (see Notes 2 and 3). The protocol uses a Working Solution containing 30% (w/v) iodixanol produced by mixing OptiPrep™ with an equal volume of double strength UWS (2x). The crude islet suspension is adjusted to ρ = 1.10 g/ml (osmolality approx 380 mOsm) by mixing with the Working Solution and gradient solutions are subsequently prepared by diluting the Working Solution with standard (1x) UWS (see Note 2). The protocol is described as a flow diagram in Figure 1.
- Optimal recoveries may vary with the species and pre-gradient procedures and may require minor adjustments to the gradient. Section 5 contains some information on rat islet isolation.
2. Solutions required
A. OptiPrep™ (shake gently before use)
B. OptiPrep™ diluent: UWS(x2).
C. Diluent for gradient solutions: UWS (see Note 7).
D. Working Solution (WS, ρ = 1.206 g/ml): mix equal volumes of Solutions A and B and transfer 10ml aliquots to 50 ml conical centrifuge tubes. Keep these at 4C.
E. Low-density barrier solution (ρ = 1.090 g/ml): mix 10 ml WS with 26.36 ml of UWS and keep at 4oC (see Notes 8 and 9).
3. Protocol
1. Digest the pancreatic tissue with collagenase in UWS (or other chosen medium) at 37C, then carry out all subsequent operations (mechanical dispersion, filtering etc) in UWS at 0-4°C.
2. Centrifuge the digest for 2 min at 200 g at 4°C and gently resuspend the pellet in UWS and make up to volume (a multiple of 20 ml) with this medium (e.g. 10-12 ml of packed tissue pellet in
80 ml).
3. Transfer 20 ml of digest suspension into each of the prepared centrifuge tubes containing 10 ml of WS and mix rapidly but gently by repeated inversion or pouring repeatedly between two centrifuge tubes.
4. Layer 8 ml of the low-density barrier solution over the suspension and top up the tube with 10 ml of (1x) UWS.
5. Centrifuge at 500 g for 5 min at 4°C (see Note 10). Islets band at the top interface; acinar tissue remains in the load zone (see Figure 1 and Note 11).
6. Harvest the islets using a syringe and wide-bore metal cannula; dilute with an equal volume of (1x) UWS and pellet at 200 g for 4 min.
4. Notes
1. If a medium such as HBSS or RPMI is used for the cold isolation steps, the tissue should be preincubated in cold UWS for 60 min before addition of the Working Solution. The gradient however
may require significant adjustment of density and perhaps osmolality [2].
2. UWS(x2) has a density of 1.092 g/ml. Double strength HBSS or RPMI have a lower density (approx 1.012 g/ml), consequently the amount of single-strength medium required to produce solutions of the appropriate density will require modifying (see Notes 3 and 8).
3. For more information about preparing density gradient solutions for mammalian cells see Application Sheet C01.
4. Neutralization of the lactobionic acid should be carried out slowly and carefully.
5. Allopurinol is kept at the same concentration as in UWS (1x) as higher concentrations are difficult to dissolve.
6. For sources of pentastarch (hydroxyethylstarch) powder contact Fresenius Kabi AG, Germany (www.fresenius-kabi.com) or B. Braun, USA (www.bbraunusa.com).
7. UWS may be purchased commercially or it can be prepared using half the concentration of the reagents in Solution B (except allopurinol which should be at the same concentration). Alternatively it may be prepared by diluting Solution B with an equal volume of water (check pH is still 7.4), but note that the allopurinol concentration will be half that normally in UWS (1x).
8. It may be necessary to modulate the density of this layer [2] according to the isolation method that is used or if islets are purified from other species. Table 1 gives the volumes of UWS and Working Solution required to produce solutions of different densities.
9. It may be an advantage to produce the barrier solution in RPMI; this can act as a preliminary means of washing the islets free from UWS, as they float to the upper interface. Good results have been obtained with barrier solutions prepared by diluting OptiPrep™ with RPMI or RPMI containing 10% serum: 3.2 ml of OptiPrep™ and 8.8 ml of RPMI gives a solution of ρ = 1.090 g/ml; if RPMI containing 10% serum is used the density is approx 1.092 g/ml.
10. Recently it has been suggested that the recovery, purity, resistance to fragmentation and insulin response to glucose are all improved by reducing the RCF to 100 g [4]. Longer centrifugation times may consequently be required. 11. Unacceptable levels of acinar tissue contamination in the islet layer normally imply that the density of the barrier layer is too high and should be reduced.
5. Rat islets
Some rat islet preparation methods incorporate a useful technical operation that is worth noting briefly here. Panza et al [5] first concentrated the cells at an interface by centrifuging the tissue digest over 5 ml of OptiPrep™ (250 g for 15 min). The supernatant was removed (except for 2-3 ml) and the residual material in the tube was mixed, then iodixanol solutions of 1.135, 1.120, 1.096 g/ml and saline layered on top. After centrifugation at 800 g for 30 min the islets banded at the 1.096 g/ml – saline interface. This is a convenient way of keep the gradient volume to a relatively small volume without pelleting the cells first. Buchanan et al [6] used a similar strategy but after mixing the residual material a 10-25% v/v OptiPrep™ gradient was layered on top and centrifuged at 800 g for 25 min.
6. References
1. Van der Burg, M. P. M., Basir, I. and Bouwman, E. (1998) No porcine islet loss during density gradient purification in a novel iodixanol in University of Wisconsin solution Transplant. Proc., 30, 362-363
2. Van der Burg, M. P. M., Basir, I., Zwaan, R.P. and Bouwman, E. (1998) Porcine islet preservation during isolation in University of Wisconsin solution Transplant. Proc., 30, 360-361
3. Van der Burg, M. P. M., Zwaan, R. P. and Bouwman, E. (1998) Markedly improved outcome of adult porcine islet isolation, purification, and culture using Liberase-P1 versus Collagenase-P, and a novel gradient of OptiPrep in University of Wisconsin solution Horm. Metab. Res., 30 A23
4. Shibata, S., Sageshima, J., Hiraoka, K., Zhang, H., Koyama, K., Sutherland, D. E. R. and Hering, B .J. (2001) Low-speed isopycnic islet separation is effeictive and yields islets with superior quantity and quality Abstr. Int. Pancreas Islets Transplant. Assoc., Innsbruck, p5
5. Panza, J.L., Wagner, W.R., Rilo, H.L.R., Rao, R.H., Beckman, E.J. and Russell, A.J. (2000) Treatment of rat pancreatic islets with reactive PEG Biomaterials, 21, 1155-1164
6. Buchanan, C.M., Phillips, A.R.J. and Cooper, G.J.S. (2001) Preptin derived from proinsulin growth factor II (preIGFII) is secreted from pancreatic islet -cells and enhances insulin secretion Biochem. J., 360, 431-439
7. Acknowledgements
We thank Dr M.P.M. van der Burg, Department of Surgery, University Hospital, Leiden, NL
2300RC, Netherlands for his help and comments in the preparation of this Application Sheet.
OptiPrep™Application Sheet C16; 9th edition, January 2020
OptiPrep™ Application Sheet C17
Purification and processing of animal (non-human) and avian spermatozoa
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- For human spermatozoa see Application Sheet C18
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box - Section 2 describes the optimal method for the enrichment of viable spermatozoa from bovine
semen using OptiPrep™. - Sections 3-5 summarize some of the other published methods for sperm from rodents (3), equine
sperm (4), and Xenopus laevis sperm and spermatids (5). - Section 6 describes the use of OptiPrep for cryopreservation of sperm from a number of species.
1. Introduction
In ejaculates, viable spermatozoa of normal morphology are sometimes a very low percentage of the total cell population. This Application Sheet presents a detailed protocol for the recovery of a
highly viable fraction of bovine spermatozoa for use in fertilization. The recommended strategy involves, in the first instance, adjustment of the density of a semen sample to approx. 1.17 g/ml.
Ideally, two lower density solutions are then layered on top, so that the viable semen of normal morphology band at the interface between theses two layers. These cells are thus completely separated from both the non-viable cells (and any soluble material released from partially broken cells), which all remain in the load zone, and any morphologically abnormal cells that band at the top of the least dense layer. This result is depicted in Figure 1. Iodixanol is the gradient solute of choice: firstly, all of the solutions are easily prepared by dilution of OptiPrep™ with any buffered saline or a special diluent formulated for the maintenance of sperm viability, while NycodenzⓇ solutions must be prepared from NycodenzⓇ powder. Secondly, to raise the density of the ejaculate to approx 1.170 g/ml it is necessary to mix it with a high-density medium (usually >1.26 g/ml). NycodenzⓇ solutions are hyperosmotic above ρ = 1.16 g/ml, thus the seminal fluid would also become hyperosmotic. Consequently with Nycodenz the semen has to be loaded at a lower density in the middle, or top of the gradient. This is not the case with iodixanol; OptiPrep™ or a
dense solution prepared from OptiPrep™ and the chosen diluent, can be added to a raw ejaculate without increasing its osmolality (see Notes 1 and 2).
2. Purification of bovine spermatozoa
2a. Solutions required
A. OptiPrep™ (shake gently before use)
B. Diluent: Hanks Buffered Salt Solution (HBSS) or other suitable ambient temperature diluent such as Ruthin Diluent (RD); see also Note 2.
2b. Protocol
1. Assess a freshly taken ejaculate for viability and then mix with an equal volume of Solution A to raise its density to approx. 1.170 g/ml.
2. Prepare the two gradient solutions: 1.119 g/ml (4 vol. OptiPrep™ + 7 vol. HBSS) and 1.154 g/ml (9 vol. OptiPrep™ + 10 vol. HBSS). Using RD mix respectively 4 vol. + 8 vol. and 9 vol. + 11 vol.
(see Notes 2 and 3).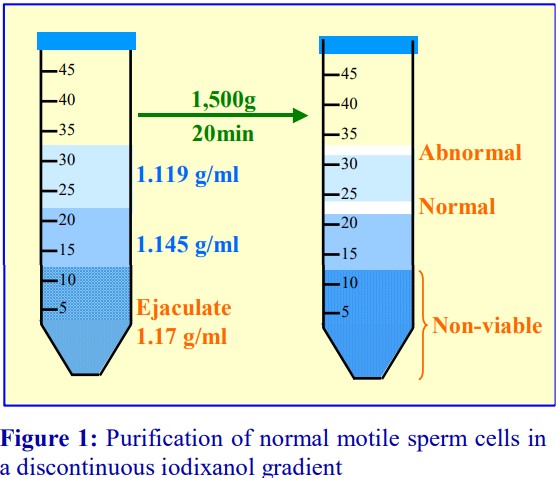
3. In a suitable tube (50 ml) layer 10 ml of each of the two gradient solutions and underlayer these with 10-15 ml of the sample-OptiPrep™ mixture (density approximately 1.17 g/ml), to form a
three-step gradient (see Figure 1 and Notes 4 and 5).
4. Centrifuge the gradient in a swinging-bucket rotor at 1500 gav for 20 min at approx. 20C.
5. After centrifugation, deformed sperm, cytoplasmic droplets, detached heads and tails band at the top of the gradient (A). Motile cells of normal morphology band at the 1.119/1.154 g/ml interface (B) while in the loading area a pellet (D) and some particulate material in suspension (C) contain immotile sperm (see Figure 1). The sperm cells from interface B can then be checked for viability and fertility and stored (see Notes 6-9).
2c. Notes
1. Iodixanol is essentially a dimer of NycodenzⓇ, it therefore has approximately twice the molecular mass and solutions have half the osmolality of NycodenzⓇ
2. The diluent solution may be any solution thought appropriate by individual workers. The final density of the diluted fractions will depend upon the density of the diluent. Common physiological salt solutions, such as phosphate-buffered saline, Hanks Buffered Salt Solution (HBSS) or a more complex medium such as RPMI, have densities close to 1.005 – 1.006 g/ml. It may however be
preferable to use a medium designed to preserve the viability and motility of sperm cells at room temperature. These so-called ambient temperature diluents (for example Ruthin Diluent)
frequently contain polyhydric alcohols such as sorbitol and consequently have a slightly higher density (ρ = 1.018 g/ml). Details of the Ruthin Diluent can be obtained from Dr Stuart Revell,
Genus Freezing Unit, Llanrhydd, Ruthin, Denbighshire, LL15 2UP, UK.
3. The volume of OptiPrep™ and medium required to prepare the density solutions will vary with the density of the medium. For more information about preparing density gradient solutions for
mammalian cells see Application Sheet C01.
4. For more information on preparation of discontinuous gradients see Application Sheet C02.
5. More recently Garrett et al [1] simplified the gradient: the semen (diluted first with Eqcellsire™) was mixed with an equal volume of OptiPrep™; 8 ml of this suspension was then overlaid with 1
ml of a 1.15 g/ml solution and centrifuged at 1000 g for 15 min. The viable sperm banded at the top of the gradient.
6. The quality of the semen has been assessed by using membrane integrity as an indicator of general cell function and viability. The Osmotic Resistance Test (ORT) described by Revell and Mrode [2] and the fluorescent analysis method described by Harrison and Vickers [3] have been used to check membrane integrity. The motile band from the 1.119/1.154 g/ml interface shows over 95% viability by these tests, while the pelleted material and particulate material remaining in the loading layer are found to be 99% non-viable cells by these methods.
7. Routinely, the viable, motile spermatozoa are diluted in skimmed milk, glucose and glycerol, to provide 1.5×107 per A.I. straw and deep-frozen. When the straws are subsequently thawed and subjected to ORT, 74% of the sperm are still viable as judged by membrane integrity and activity.
8. Ejaculates from other species have shown similar but not identical banding characteristics: small changes to the precise densities of the layers may be required. The solutions and protocol in this Application Sheet will serve as a useful starting point from which adjustments to the final densities of the gradient layers can be made, to optimize the fractionation of material from other species.
9. This methodology has also been reported in ref 4.
3. Rodent sperm
Separation of viable and non-viable sperm cells was achieved on double layer iodixanol gradient of 15% and 24% (w/v) iodixanol; after centrifugation at 400 g for 20 min the viable sperm banded at the interface of the two iodixanol solutions [5-8]
4. Equine sperm
Stuhtmann et al [9] and Heutelbeck et al [10,11] used similar two-layer gradients for stallion sperm of 1.090 and 1.165 g/ml or 1.090 and 1.170 g/ml and commented that these gradients promoted better retention of morphology and progressive motility and that the gradient purification was more effective if carried out just after collection rather then immediately before cryopreservation.
5. Xenopus laevis sperm and spermatids
A discontinuous iodixanol gradient of 12%, 20% and 30% (w/v) iodixanol was used to separate the lighter spermatids from the denser mature semen [12].
6. Concentration of semen on to a dense cushion, prior to freezing
Compared with simple pelleting, sedimentation on to a dense cushion of iodixanol prior to cooling and freezing considerably improves the recovery and motility of viable sperm. The cushion is usually OptiPrep™ itself and the centrifugation conditions vary from 800 g for 10 min to 1000 g for 20 min. To harvest the band of spermatozoa, a narrow metal cannula is used to remove the liquids above and below the cells. The spermatozoa band is often described as a “pellet”; a “sharp band” is the more accurate description. The method has been widely used for equine sperm [13-33]; this has also been observed for gazelle semen [34], elephant semen [35-38], boar semen [39,40], donkey semen [41], buffalo semen [42], porcine semen [43], ram semen [44] and rodent semen [45,46]. Some more recent references reporting the use of OptiPrepTM are listed in Section 7: refs. # 47-53 Important technical note: It is not known if any of the published methods using the iodinated density gradient medium Nycodenz® can be translated direrctly to the use of OptiPrepTM. Although solutions of the same % (w/v) will have the same density, the osmolality of iodixanol solutions will be approx. half those of Nycodenz®. Unlike those of Nycodenz®, iodixanol solutions can be made approx. isoosmotic with plasma at all densities.
Some more recent references reporting the use of OptiPrepTM are listed in Section 7: refs. #
47-53
Important technical note: It is not known if any of the published methods using the iodinated density gradient medium Nycodenz® can be translated direrctly to the use of OptiPrepTM. Although solutions of the same % (w/v) will have the same density, the osmolality of iodixanol solutions will be approx. half those of Nycodenz®. Unlike those of Nycodenz®, iodixanol solutions can be made approx. isoosmotic with plasma at all densities.
7. References
1. Garrett, L.J.A., Revell, S.G. and Leese, H.J. (2008) Adenosine triphosphate production by bovine spermatozoa and its relationship to semen fertilizing ability J. Androl., 29, 449-458
2. Revell, S.G. and Mrode, R.A. (1994) An osmotic resistance test for bovine semen Animal Reprod. Sci., 36, 77-86
3. Harrison, R.A.P. and Vickers, S.E. (1990) Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa J. Reprod. Fert., 88, 343-352
4. Beer-Ljubić, B., Aladrović, J., Marenjak, T.S., Majić-Balić, I., Laškaj, R. and Milinković-Tur, S. (2012) Biochemical properties of bull spermatozoa separated in iodixanol density solution Res. Vet. Sci., 92, 292- 294
5. Stein, K.K., Go, J.C., Primakoff, P. and Myles, D.G. (2005) Defects in secretory pathway trafficking during sperm development in Adam2 knockout mice Biol. Reprod., 73, 1032-1038
6. Stein, K.K., Go, J.C., Lane, W.S., Primakoff, P. and Myles, D.G. (2006) Proteomic analysis of sperm regions that mediate sperm-egg interactions Proteomics, 6, 3533-3543
7. Nishimura, H., Myles, D.G. and Primakoff, P. (2007) Identification of an ADAM2-ADAM3 complex on the surface of mouse testicular germ cells and cauda epididymal sperm J. Biol. Chem., 282, 17900-17907
8. Nishimura, H., Gupta, S., Myles, D.G. and Primakoff, P. (2011) Characterization of mouse sperm TMEM190, a small transmembrane protein with the trefoil domain: evidence for co-localization with IZUMO1 and complex formation with other sperm proteins Reproduction, 141, 437–451
9. Stuhtmann, G., Oldenhof, H., Peters, P., Klewitz, J., Martinsson, G. and Sieme, H. (2012) Iodixanol density gradient centrifugation for selecting stallion sperm for cold storage and cryopreservation Anim. Reprod. Sci., 133, 184– 190
10. Heutelbeck, A., Oldenhof, H., Henke, S., Martinsson, G. and Sieme, H. (2012) Delayed cryopreservation of stallion sperm: effect of iodixanol density gradient centrifugation J. Equine Vet. Sci., 32, 488-489
11. Heutelbeck, A., Oldenhof, H., Rohn, K., Martinsson, G., Morrell, J.M. and Sieme, H. (2015) Use of density centrifugation for delayed cryopreservation of stallion sperm: perform sperm selection directly after collection or after storage? Reprod. Dom. Anim., 50, 76–83
12. Teperek, M., Simeone, A., Gaggioli, V., Miyamoto, K., Allen, G.E., Erkek, S., Kwon, T., Marcotte, E.M., Zegerman, P. et al (2016) Sperm is epigenetically programmed to regulate gene transcription in embryos Genome Res., 26, 1034-1046
13. Revell, S.G., Pettit, M.T. and Ford, T.C. (1997) Use of centrifugation over iodixanol to reduce damage when processing stallion sperm for freezing. Proc. Joint Meeting, Society for the Study of Fertility, Abstr. Series No. 92, 38 (abstr.)
14. Sieme, H., Knop, K. and Rath, D. (2006) Effects of cushioned centrifugation on sperm quality in stallion semen stored cooled at 5°C for 24h, and stored cooled for 2h or 24h and then frozen Animal Reprod. Sci., 94, 99-103
15. Loomis, P.R. (2006) Advanced methods for handling and preparation of stallion semen Vet. Clin. Equine 22, 663-676
16. Saragusty, J., Gacitua, H., Pettit, M.T. and Arav, A. (2007) Directional freezing of equine semen in large volumes Reprod. Dom. Anim., 42, 610-615
17. Waite, J.A., Love, C.C., Brinsko, S.P., Teague, S.R., Salazar Jr. J.L., Mancill, S.S. and Varner, D.D. (2008) Factors impacting equine sperm recovery rate and quality following cushioned centrifugation Theriogenology, 70, 704-714
18. Varner, D.D., Love, C.C., Brinsko, S.P., Blanchard, T.L., Hartman, D.L., Bliss, S.B., Carroll, B.S. and Eslick, M.C. DVMd (2008) Semen processing for the subfertile stallion J. Equine Vet. Sci., 28, 677-685
19. Webb, G.W. Dean, M.M. (2009) Effect of centrifugation technique on post-storage characteristics of stallion spermatozoa J. Equine Vet. Sci., 29, 675-680
20. Mari, G., Morganti, C.M., Rizzato, G., Mislei, B., Iacono, E. and Merlo, B. (2010) Comparison of density gradient and simple centrifugation of equine spermatozoa: effect on fertility of an oligospermic-subfertile stallion Animal Reprod. Sci., 121S, S153–S154
21. Salazar Jr, J.L., Teague, S.R., Love, C.C., Brinsko, S.P., Blanchard, T.L. and Varner, D.D. (2011) Effect of cryopreservation protocol on postthaw characteristics of stallion sperm Theriogenology, 76, 409–418
22. Hoogewijs, M., Morrell, J., Van Soom, A., Govaere, J., Johannisson, A., Piepers, S., De Schauwer, C., De Kruif, A. and S. De Vliegher (2011) Sperm selection using single layer centrifugation prior to cryopreservation can increase thawed sperm quality in stallions Equine Vet. J., 43 (Suppl. 40), 35-41
23. Blanchard, T.L., Brinsko, S.P., Love, C.C., Vest, D.D., Berezowski, C.B., Wendt, K.M., Stich, K. and Varner, D.D. (2012) Case study of processing and insemination techniques: attempts to improve fertility of an aged stallion with dilute semen of poor quality J. Equine Vet. Sci., 32, 5-11
24. Edmond, A.J., Brinsko, S.P., Love, C.C., Blanchard, T.L., Teague, S.R. and Varner, D.D. (2012) Effect of centrifugal fractionation protocols on quality and recovery rate of equine sperm Theriogenology, 77, 959– 966
25. Bliss, S.B., Voge, J.L., Hayden, S.S., Teague, S.R., Brinsko, S.P., Love, C.C., Blanchard, T.L. and Varner, D.D. (2012) The impact of cushioned centrifugation protocols on semen quality of stallions Theriogenology 77, 1232–1239
26. Love, C.C., Blanchard, T.L., Varner, D.D., Brinsko, S.P., Voge, J., Bliss, S., Sudderth, K., Teague, S. and LaCaze, K. (2012) Effect of daily semen centrifugation and resuspension on the longevity of equine sperm quality following cooled storage Theriogenology, 77, 1911–1917
27. Len, J.A., Beehan, D.P., Lyle, S.K. and Eilts, B.E. (2013) Cushioned versus noncushioned centrifugation: Sperm recovery rate and integrity Theriogenology, 80, 648–653
28. Ponthier, J., Franck, T., Parrilla-Hernandez, S., Niesten, A., de la Rebiere, G., Serteyn, D. and Deleuze, S. (2014) Concentration, activity and biochemical characterization of myeloperoxidase in fresh and post-thaw equine semen and their implication on freezability Reprod. Dom. Anim., 49, 285–291
29. Bradecamp, E.A. (2014) Centrifugation of semen: cushion technique In Equine Reproductive Procedures, (ed. Dascanio, J. and McCue, P.) JohnWiley & Sons, Inc., pp 429-432
30. Heutelbeck, A., Oldenhof, H., Rohn, K., Martinsson, G., Morrell, J.M. and Sieme, H. (2015) Use of density centrifugation for delayed cryopreservation of stallion sperm: perform sperm selection directly after collection or after storage? Reprod. Dom. Anim., 50, 76–83
31. Stawicki, R.J., McDonnell, S.M., Giguère, S. and Turner, R.M. (2016) Pregnancy outcomes using stallion epididymal sperm stored at 5C for 24 or 48 hours before harvest Theriogenology, 85, 698–702
32. Blommaert, D., Franck, T., Donnay, I., Lejeune, J-P., Detilleux, J. and Serteyn, D. (2016) Substitution of egg yolk by a cyclodextrin-cholesterol complex allows a reduction of the glycerol concentration into the freezing medium of equine sperm Cryobiology, 72, 27-32
33. Voge, J., Varner, D.D., Blanchard, T.L., Meschini, M., Turner, C., Teague, S.R., Brinsko, S.P. and Love, C.C. (2106) The effects of urine concentration, and cushion centrifugation to remove urine, on the quality of coolstored stallion sperm Theriogenology 86, 1294–1298
34. Saragusty, J., Gacitua, H., King, R. and Arav, A. (2006) Post-mortem semen cryopreservation and characterization in two different endangered gazelle species (Gazella gazella and Gazella dorcas) and one subspecies (Gazella gazelle acaiae) Theriogenology, 66, 775-784
35. Hermesa, R., Behr, B., Hildebrandt, T.B., Blottner, S., Sieg, B., Frenzel, A., Knieriem, A., Saragusty, J. and Rath, D. (2009) Sperm sex-sorting in the Asian elephant (Elephas maximus) Animal Reprod. Sci., 112, 390– 396
36. Saragusty, J., Hildebrandt, T.B., Behr, B., Knieriem, A., Kruse, J. and Hermes, R. (2009) Successful cryopreservation of Asian elephant (Elephas maximus) spermatozoa Anim. Reprod. Sci., 115, 255–266
37. Hildebrandt, T.B., Hermes, R., Saragusty, J., Potier, R., Schwammer, H.M., Balfanz, F., Vielgrader, H., Baker, B., Bartels, P. and Göritza, F. (2012) Enriching the captive elephant population genetic pool through artificial insemination with frozen-thawed semen collected in the wild Theriogenology, 78, 1398–1404
38. Hermes, R., Saragusty, J., Goritz, F., Bartels, P., Potier, R., Baker, B., Streich, W.J. and Hildebrandt, T.B. (2013) Freezing African elephant semen as a new population management tool PLoS One 8: e57616
39. Matás, C., Decuadro, G., Martínez-Miró, S. and Gadea, J. (2007) Evaluation of a cushioned method for centrifugation and processing for freezing boar semen Theriogenology 67, 1087-1091
40. Zhang, W., Yi, K., Chen, C., Hou, X. and Zhou, X. (2012) Application of antioxidants and centrifugation for cryopreservation of boar spermatozoa Anim. Reprod. Sci., 132, 123– 128
41. Saragusty, J., Lemma, A., Hildebrandt, T.B. and Goèritz, F. (2017) Follicular size predicts success in artificial insemination with frozen-thawed sperm in donkeys PloS One, 12: e0175637
42. Swami, D.S., Kumar, P., Malik, R.K., Saini, M., Kumar, D. and Jan, M.H. (2017) The cryoprotective effect of iodixanol in buffalo semen cryopreservation Anim. Reprod. Sci., 179, 20–26
43. Romar, R., Funahashi, H. and Coy, P. (2016) In vitro fertilization in pigs: New molecules and protocols to consider in the forthcoming years Theriogenology, 85, 125–134
44. Cirit, U., Bagıs, H., Demir, K., Agca, C., Pabuccuoglu, S., Varısli, O., Clifford-Rathert, C., Agcac, Y. (2013) Comparison of cryoprotective effects of iodixanol, trehalose and cysteamine on ram semen Anim. Reprod. Sci., 139, 38– 44
45. Kim, S., Agca, C. and Agca, Y. (2013) Effects of iodixanol during rat epididymal sperm cryopreservation Cryobiology, 67, 398–442
46. Kim, S., Hooper, S., Agca, C. and Agca, Y. (2016) Post-thaw ATP supplementation enhances cryoprotective effect of iodixanol in rat spermatozoa Reprod. Biol. Endocrinol., 14: 5
47. Chuawongboon, P., Sirisathien, S., Jatuporn Pongpeng, J., Sakhong, D., Nagai, T. and Vongpralub, T. (2017) Effects of supplementation of iodixanol to semen extender on quality and fertilization ability of frozen– thawed Thai native bull sperm Anim. Sci. J., 88, 1311–1320
48. Ali, A., Ahmad, E., Ijaz, N., Ahmad, W. and Ulhassan, F. (2018) Quality of cryopreserved buffalo spermatozoa improved from poor quality ejaculates Cryobiology, 80, 167
49. Agca, C., Timonin, M., Kim, S., Epperson, K. and Agca, Y. (2018) Cryosurvival of Mus musculus and peromyscus spermatozoa in the presence of iodixanol Cryobiology, 80, 185
50. Brom-de-Luna, J.G., Siqueira Canesin, H., Wright, G. and Hinrichs, K. (2018) Culture of somatic cells isolated from frozen-thawed equine semen using fluorescence-assisted cell sorting Anim. Reprod. Sci., 190, 10–17
51. Marqui, F.N., Martins Jr., A., da Cruz, T.E., Berton, T.I.U., de Paula Freitas-Dell’Aqua, C., Dell’Aqua Júnior, J.A., Oba, E. (2018) Addition of iodixanol in bull freezing extender improves the sperm membranes integrity Anim. Reprod. Sci. (Abstr), 194, e1–e27
52. Hermes, R., Hildebrandt, T.B. and Göritz, F. (2018) Cryopreservation in rhinoceros – setting a new benchmark for sperm cryosurvival PLoS One, 13: e0200154
53. Palazzese L., Gosalvez, J., Anzalone, D.A., Loi, P. and Saragusty, J. (2018) DNA fragmentation in epididymal freeze-dried ram spermatozoa impairs embryo development J. Reprod. Devel., 64, 393-400
OptiPrep™ Application Sheet C17; 8th edition, January 2020
OptiPrep™ Application Sheet C18
Purification of viable human spermatozoa in iodixanol or NycodenzⓇ gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box
1. Background
Human ejaculates contain variable proportions of viable spermatozoa of normal morphology, sometimes a very low percentage of the total cell population but Mortimer [1] stressed the need to avoid direct pelleting of sperm cells in any fertilization studies. The use of various density gradient techniques for the enrichment of motile sperm media has therefore been investigated and this application sheet presents some of the recommended methods. Although gradients of PercollⓇ became popular in the early nineteen-eighties for the enrichment of motile spermatozoa from human semen, several groups of workers recognized that the use of an iodinated density gradient medium that was already approved for human injection as an X-ray contrast agent offered a much more attractive solution for this procedure. Earlier papers reported the use of NycodenzⓇ (Section 2), but more recently OptiPrep™ (Section 3) has been used for this purpose.
- Gellert-Mortimer et al [2] showed that 60% of the sperm isolated from a four-step NycodenzⓇ gradient retained their motility after 21 h, while this figure was only 5% with a PercollⓇ gradient. The method is summarized in Section 2. Mortimer [3] also stressed that, particularly in the case of the semen from asthenozoospermic individuals, a NycodenzⓇ gradient gave superior results.
2. NycodenzⓇ
2a. Solutions and reagents required
A. NycodenzⓇ powder
B. Diluent: 6 mM KCl, 10 mM Tricine (or HEPES) – NaOH, pH 7.4
C. Any buffered saline balanced salt solution or culture medium
2b. Solution preparation
Published papers report the use of NycoprepⓇ 1.15, a solution of 27.6% (w/v) Nycodenz containing 3mM KCl, 0.3 mM CaNa2-EDTA, 5 mM Tris-HCl, pH 7.4. This isoosmotic solution is no
longer commercially available, but a similar stock solution can be made up easily from Nycodenz powder. In this Application Sheet the Tris is replaced by either Tricine of HEPES (see box), which are much more cell-friendly, and the EDTA omitted. To make 100 ml of the 1.15 g/ml stock solution place approx. 50 ml of Solution B in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 27.6 g of Nycodenz in small amounts until dissolved. Allow the solution to cool to room temperature and then make up to 100 ml with water. Filter sterilize if required (see Note 1).
2c. Protocol
Gellert-Mortimer et al [2] diluted the 27.6% NycodenzⓇ stock solution with a Ham’s F10 medium supplemented with 1mM calcium lactate, 20 mM NaHCO3, 5 mM KHCO3 and 0.5 mM MgSO4, to
produce the gradient solutions. Sbracia et al [4] used Human Tubule Fluid (HTF) containing 0.5% bovine serum albumin (BSA).
1. Measure out aliquots of 4.85 ml, 6.9 ml and 9.0 ml of the 27.6% NycodenzⓇ stock solution and dilute each with the supplemented Ham’s F10 medium to 13.8 ml. This produces solutions of 9.7%, 13.8% and 18% (w/v) Nycodenz (see Note 2).
2. Form discontinuous gradients from 1.6 ml, 1.6 ml, 3.2 ml and 1.6 ml of the 9.7%, 13.8%, 18% and 27.6% (w/v) NycodenzⓇ solutions (see Notes 2 and 3).
3. Dilute the liquefied semen with an equal volume of the supplemented Ham’s F10 medium and layer it on top of the gradient (see Note 2).
4. Centrifuge at 350 g for 12 min (see Note 2).
5. Collect the motile semen that band at the bottom of the 18% NycodenzⓇ layer (see Notes 2 and 4)
2d. Notes on the NycodenzⓇ methodology
1. There is no obvious reason why iodixanol should not be substituted for Nycodenz in these methods; it would certainly be an easier option. To produce a stock solution of density 1.15 g/ml dilute 27.6 ml of OptiPrep™ to 60 ml with the chosen diluent (see Section 2c).
2. Sbracia et al [4] used a simplified two-layer gradient from 2 ml each of 9.7% and 18% (w/v) NycodenzⓇ. The semen was diluted in the HTF+BSA medium prior to loading and the gradient was centrifuged at 400 g for 20 min. In this format the semen form a pellet.
3. For the construction of discontinuous gradients see Application Sheet C02.
4. Sbracia et al [4] reported that the long-term retention of sperm motility (after 24 h) was substantially improved in Nycodenz compared to PercollⓇ: 35% versus 26% with 60% versus 40% of the initial motility respectively. NycodenzⓇ samples also exhibited a higher retention of total motile sperm. The sperm motility index, a multiple of velocity and motility in the sample (a measure of the efficiency of the sperm population in sperm-oocyte interaction, was 75% higher in the NycodenzⓇ. Refs 5 and 6 review some of the current technology.
3. OptiPrep™
3a. Purification by flotation
The optimal protocol for the isolation of viable sperm cells of normal morphology from bovine sperm is to adjust the density of the raw ejaculate to approx 1.170 g/ml and place this beneath a two layer gradient (1.154 and 1.119 g/ml). OptiPrep™ can be added to a raw ejaculate without increasing its osmolality. In this respect OptiPrep™ offers an advantage over NycodenzⓇ as solutions of this solute are hyperosmotic above ρ = 1.16 g/ml. Viable cells float upwards to their buoyant banding density [7]. Those of normal morphology band at the 1.154 and 1.119 g/ml interface and non-viable cells either pellet or remain in the load zone, see Application Sheet C17. This methodology was very successfully adapted by Smith et al [8,9] to human semen; the two upper layers of iodixanol were adjusted to 1.05 and 1.15 g/ml interface. The method described in below is adapted from refs 8 and 9.
3a-1. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. Modified Human Tubal Fluid (mHTF)
3a-2. Protocol
1. Dilute OptiPrep™ with mHTF to obtain solutions of ρ = 1.15 and 1.05 g/ml – approx 27.5% and 8%, w/v iodixanol respectively (see Notes 1 and 2 in Section 3c).
2. Mix the liquefied semen with OptiPrep™ (4 vol. + 6.5 vol. respectively) to raise its density to approx ρ = 1.17 g/ml.
3. Prepare the discontinuous gradients from 3 ml each of the ρ = 1.15 and 1.05 g/ml solutions in a 15 ml tube (see Note 3 in Section 3c).
4. Layer the dense semen suspension below the gradient and centrifuge at 1500 g for 40 min.
5. Collect the viable sperm cells of normal morphology from the 1.05/1.15 g/ml interface.
6. Dilute the 5 vol. of mHTF and harvest the cells by centrifugation at 500 g for 15 min (see Note 4 Section 3c).
3b. Purification by sedimentation
The protocol described below takes account of the fact that the customary procedure for purifying human sperm cells involves layering the semen on top of the gradient rather than below it in a dense solution. It should be regarded as a trial procedure rather than a definitive procedure and may require modification to suit the particular medium used and/or in the light of experience. The density of the top layer has been increased to allow for possible variation in the density of the applied sample. It is adapted from ref. 10.
3b-1. Protocol
1. Dilute OptiPrep™ with mHTF (see Note 2) to obtain solutions of = 1.09 and 1.132 g/ml (approx 16% and 24%, w/v iodixanol respectively). See Notes 5-9 in Section 3c for more information on
density selection and quality of the purified semen.
2. Layer the liquefied ejaculate on top of equal volumes (1 ml was recommended in ref 8) of the two density barriers.
3. Centrifuge at 400 g for 20 min at room temperature.
4. Remove and discard the upper layers containing abnormal cells and harvest the motile normal cells from the lower interface.
5. Harvest the cells after dilution as in Section Protocol 3a-2.
4. Notes
1. The osmolality of these solutions is approx 280 mOsm.
2. The volume of OptiPrep™ and medium required to prepare the density solutions will vary with the density of the medium. If the density of the diluent is significantly different to that of mHTF it may be necessary to adjust the volumes of OptiPrep and diluent required to produce a particular density. To determine the amounts of OptiPrep™ and medium to mix together use the equation
described in Application Sheet C01.
3. For information on the preparation of discontinuous and continuous gradients for cell separations see Application Sheet C02.
4. Smith et al [8] reported that 78% of the motile and 99% of the morphologically normal sperm cells were recovered in the interfacial band and they, and other workers [10-14] have concluded that the method was a suitable nontoxic alternative to PercollⓇ.
5. Harrison [10] reported that these two densities produced best recovery of viable sperm (30-34%) but layers of 18% and 27% (w/v) iodixanol, equivalent to densities of 1.100 and 1.148 g/ml were almost as effective. A lower layer with a density as high as approx ρ = 1.16 g/ml should still allow non-viable cells to pellet and this higher density may improve the recovery of viable cells. The recommended density of the upper layer may also require modulation to suit the operator’s requirements. The aim of this layer is to separate the viable sperm cells, which sediment through it
and band at 1.09/1.132 g/ml interface, from abnormal cells which band at the sample/1.09 g/ml interface or within the 1.09 g/ml layer.
6. Van den Bergh et al [11] used a three-layer gradient of 7.5%, 15% and 30% (w/v) iodixanol.
7. Kaftani et al [12] compared a 10.5% and 21% (w/v) iodixanol gradient (approx. equivalent to 1.062 and 1.117 g/ml) with the standard Percoll gradient and found no significant difference between the two gradient media in terms of recovery of % motile sperm or morphology.
8. There are other published methods in which the sedimentation strategy (Protocol B) has been variously modified to take account of particular laboratory or clinical requirements, some of which
use polysucrose as an additive to the iodixanol solution [13-15]. A number of publications have compared some or all of the available methods, for example ref. 16.
9. More recently Araki et al [17] used a two layer gradient of 16% and 24% (w/v) iodixanol to purify human sperm.
5. Other OptiPrep™ applications
The use of OptiPrep™ as a cushion on to which the sperm may be concentrated prior to cryopreservation, which has been widely used for non-human sperm applications (see OptiPrep™
Application Sheet C17), has more recently been extended to human samples [18]. Jallouk et al [19] used nanoparticles to reduce sperm and vaginal epithelium cytotoxicity and subsequently concentrated the modified sperm cells on to an OptiPrep™ cushion, which also allowed the unbound nanoparticles
to sediment. Iodixanol solutions have also been used to separate sperm cells from leukocyte in studies on individuals with HIV infection [20,21] and more recently used in cryopreservation studies [22]. A recent publication has reviewed the technologies used for purifying and cryopreservation of human sperm [23]
6. References
1. Mortimer, D. (1991) Sperm preparation techniques and iatrogenic failures of in-vitro fertilization Hum. Reprod., 6, 173-176
2. Gellert-Mortimer, S.T., Clarke, G.N., Baker, H.W.G., Hyne, R.V. and Johnston, W.I. (1988) Evaluation of Nycodenz and Percoll density gradients for the selection of motile human spermatozoa Fertil. Steril., 49, 335-341
3. Mortimer, D. (1994) Sperm recovery techniques to maximize fertilizing capacity Reprod. Fertil. Dev., 6, 25-31
4. Sbracia, M., Sayme, N., Grasso, J., Vigue, L. and Huszar, G. (1996) Sperm function and choice of preparation media: comparison of Percoll and Accudenz discontinuous density gradients J. Androl., 17, 61-67
5. Lee, C-H. (1996) Review: in vitro spermicidal tests Contraception, 54, 131-147
6. Henkel, R.R. and Schill, W-B. (2003) Sperm preparation for ART Reprod. Biol. Endocrinol., 1:108, 1-22
7. Revell, S.G., Ford, T.C., Pettit, M.T., Green, D. and Graham, J. (1997) Selection of motile spermatozoa of normal morphology from bovine ejaculates by centrifugation in an iodixanol gradient Liverpool John Moores University, “Control of Human Fertility”, Seminar Report, pp. 84-88
8. Smith, T. T., Byers, M., Kaftani, D. and Whitford, W. (1997) The use of iodixanol as a density gradient material for separating human sperm from semen Arch. Androl., 38, 223-230
9. Smith, T. T., Turner, D. and Whitford, W. (1996) Use of iodixanol as a density gradient material for the isolation of motile, morphologically normal human sperm from semen J. Androl., 21st Annual Meeting Suppl., Abstr. 043
10. Harrison, K. (1997) Iodixanol as a density gradient medium for the isolation of motile spermatozoa J. Assisted Reprod. Genet., 14, 385-387
11. Van den Bergh, M., Emiliani, S., Biramane, J., Vannin, A-S. and Englert, Y. (1999) Autocontrolled, randomized comparison between a tri-layer density gradient (OptiPrep) and the migration-sedimentation-gravity method Human Reprod. Suppl., 14, 211
12. Kaftani, D., Byers, M. and Smith, T.T. (1997) The use of OptiPrep to prepare human sperm for the assisted reproductive technologies Am. Soc. Reprod. Med., Abstr. 264
13. Andersen, C. Y. and Grinsted, J. (1997) A new method for the purification of human motile spermatozoa applying density-gradient centrifugation; Polysucrose media compared to Percoll media J. Assisted Reprod. Genet., 14, 624-628
14. Makkar, G., Ng, H-Y., Yeung, S-B. and Ho, P-C. (1999) Comparison of two colloidal silica-based sperm separation media with a non-silica-based medium Fertil. Steril. 72, 796-802
15. Ding, D-C., Huang, Y-C., Liu, J-Y. and Wu, G-J. (2002) Comparison of nitric oxide production and motion characteristics after 3-layer Percoll and IxaPrep preparation methods of human sperm Arch. Gynecol. Obstet., 266, 210-213
16. Tucker, K.E. and Jansen, C.A.M. (2002) Sperm separation techniques: comparison and evaluation of gradient products In: Proceedings 2nd International workshop for Embryologists: Troubleshooting activities in the ART lab. (Ed. R. Basuray and D. Mortimer)
17. Araki, Y., Yao, T., Asayama, Y., Matsuhisa, A. and Araki, Y. (2015) Single human sperm cryopreservation method using hollow-core agarose capsules Fertil. Steril., 104, 1004–1009
18. Sieme, H. and Oldenhof, H. (2015) Sperm cleanup and centrifugation processing for cryopreservation In Methods in Molecular Biology, 1257, Cryopreservation and Freeze-Drying Protocols (ed. Wolkers, W.F. and Oldenhof, H.) Springer Science+Business Media New York, pp 343-352
19. Jallouk, A.P., Moley, K.H., Omurtag, K., Hu, G., Lanza, G.M., Wickline, S.A. and Hood, (2014) Nanoparticle incorporation of melittin reduces sperm and vaginal epithelium cytotoxicity PLoS One, 9: e95411
20. Byrn, R.A. and Kiessling, A.A. (1998) Analysis of human immunodeficiency virus in semen: indications of a genetically distinct virus reservoir J. Reprod. Immunol., 41, 161-176
21. Eyre, R.C., Zheng, G. and Kiessling, A.A. (2000) Multiple drug resistance mutations in human immunodeficiency virus in semen but not blood of a man on antiretroviral therapy Urology, 55, 591xvii-591xx
22. Arav, A. and Saragusty, J. (2018) Preservation of gametes and embryos Animal Biotech., 1, (Niemann, H. and Wrenzycki, C. ed.) Springer International Publishing AG, Springer Nature, pp. 235-267
23. Arav, A. and Saragusty, J. (2018) Preservation of gametes and embryos Animal Biotech., 1, (Niemann, H. and Wrenzycki, C. ed.) Springer International Publishing AG, Springer Nature, pp. 235-267
OptiPrep™ Application Sheet C18; 8th edition, January 2020
OptiPrep™ Application Sheet C19
Purification of intact plant protoplasts by density gradient flotation
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box
1. Background
Once the cellulose walls are removed from plant cells, they become sensitive to osmotic changes in the environment, shrinking or swelling in response to the environment, with consequent changes to their buoyant densities. In the preparation of plant protoplasts the osmolality of the medium used to digest the plant walls is therefore important: use of a digest mixture whose osmolality is 1.8 x that of the living plant tissue is a widely used guideline [1]. Similar attention must be given to devising a suitable density gradient for the purification of the intact protoplasts away from the debris of the digested walls, broken protoplasts and the digestion medium itself. NycodenzⓇ or iodixanol solutions can be adjusted to an appropriate osmolality by dissolution of a suitable salt or by addition of a sorbitol or mannitol solution of known osmolality (see Note 1). Important osmoticum components can be added while still maintaining an appropriate physiological osmolality. The osmolality of the gradient solutions should be the same as that of the digesting solutions and will depend on the source material.
- In this Application Sheet a detailed method for the isolation of barley or wheat leaf protoplasts using a simple discontinuous iodixanol gradient is given (Section 2)
- Section 3 summarizes some of the NycodenzⓇ methods
2. Isolation of protoplasts for leaf tissue using OptiPrep™
2a. Solutions required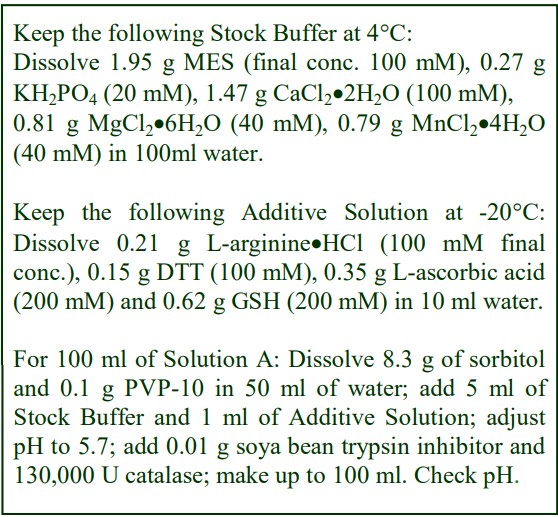
A. Plasmolysing solution: 5 mM MES, 1 mM KH2PO4, 0.44 M D-sorbitol, 5 mM CaCl2, 2 mM MgCl2, 2 mM MnCl2, 1 mM L-arginine, 1 mM dithiothreitol (DTT), 0.1% (w/v) polyvinylpyrrolidone (PVP-10), 2 mM glutathione (GSH), 2 mM L-ascorbic acid, 0.01% (w/v) soybean trypsin inhibitor, 1300 U /ml catalase, adjusted to pH 5.7.
B. Digest solution: 2%(w/v) CellulysinTM and 0.5%(w/v) macerase in Solution A.
C. Isolation buffer: as Solution A without the catalase or trypsin inhibitor.
D. OptiPrep™(shake the bottle gently before use)
E. Working Stock: dissolve 0.6 g KCl in 100 ml Solution D (500 mOsm).
- If a sterile preparation is required, all solutions should be filter sterilized.
2b. Protocol
2b-1. Determination of leaf osmolality
1. Pulverize leaf tissue (3 g is convenient) after freezing in liquid N2.
2. After thawing in a sealed tube, to exclude condensation, centrifuge the mixture at 30,000g for 20 min at 4oC. Measure the osmolality of the supernatant by depression of freezing point.
3. The osmolality of the digest and gradient solutions is then set at 1.8x that of the tissue.
2b-2. Sterilization
If required, before processing, the leaf blades should be surface sterilized by sequential washing in 1% sodium hypochlorite containing 0.01% (v/v) Tween-80 (5 min); sterile distilled water (x3), 70% (v/v) ethanol (2 min) and finally sterile distilled water again (x3). This procedure not only surface sterilizes the tissue but also weakens the cuticle thus aiding protoplast release [1].
2b-3. Protoplast preparation (adapted from ref 1)
1. Place the leaf tissue in Solution A (50 ml /g of tissue) for 30 min at 20C.
2. Remove the leaves from the solution and cut in to 0.5-1 mm pieces and place in 9 cm Petri dishes (2 g of tissue per dish) containing Solution B (10 ml /g of tissue).
3. Digest the tissue at 20°C for 3 h, with shaking at 40 rpm for the first and last 30 min.
4. After digestion filter the contents of each culture plate through Nylon mesh (pore size 100 µm). Wash off the tissue retained by the mesh in isolation buffer; mash lightly to release more protoplasts, and filter again.
5. Wash the mesh through with the buffer and make up the volume of filtrate from each plate to 30 ml in 50 ml Sterilin centrifuge tubes.
2b-4. Purification of protoplasts
1. Add 7.5 ml of Solution E to each 30 ml of digest to make a final density of close to 1.07 g/ml (see Note 2).
2. Dilute 2 ml of Solution E with 20 ml of Solution C (approx 1.03 g/ml). Overlayer the digest mixture with 10 ml of this solution.
3. Finally, layer 2-3 ml of the Solution C on top.
4. Centrifuge the tubes at 200 g for 4 min in a swinging-bucket rotor at 4C (see Note 3).
5. After centrifugation, a band of material is found at the top of the medium and in the overlying buffer. The medium from this band down to the 1.03/1.07 g/ml interface is clear of material. The 1.07 g/ml layer contains particulate material and there is also a pellet.
6. Harvest the band at the top using a plastic Pasteur pipette with the tip cut off to increase the size of the orifice and thus reduce damage to the delicate protoplasts.
7. The top band contains over 95% intact protoplasts, with the remainder just showing signs of lysing and releasing chloroplasts. The number of intact protoplasts remaining in the 1.07 g/ml layer is insignificant (see Notes 4-8).
2c. Notes
1. For more information on the preparation of working solutions and gradient solutions of the appropriate density and osmolality see Application Sheet S02 (Subcellular membranes index).
2. Plant material other than wheat and barley leaves may require small modifications to the densities of the two gradient layers. When calculating the volumes of Solutions C and E required to produce a particular density, the densities of both solutions must be taken into account (see Note 1).
3. Centrifugation is actually not necessary for this preparation: the size of the protoplasts means that they will float to the top of the density barrier in about 30 min at 1 g.
4. Recovery of protoplasts with barley is approx 4×106 per gram of tissue; using wheat, the yield is significantly lower.
5. The harvested protoplasts suspension contains a small amount of 1.03 g/ml layer (about 2% iodixanol) which has no effect whatsoever on the protoplasts.
6. The protoplasts are in high concentration and free of any residual enzyme activity, thus eliminating the need for washing. Washing any cell (but particularly delicate plant protoplasts) by pelleting
and resuspending them several times is very damaging. This protocol eliminates this requirement.
This protocol was developed for wheat or barley leaves. It has also been used to purify protoplasts from the grass Glyceria fluitans [2]. The flotation method was also used for Gracilaria gracilis
(Gracilariales, Rhodophyta) although the gradient was modified [3]: the crude fraction was suspended in 34.8 % (w/v) iodixanol (approx 1,189 g/ml) and solutions of 19.2 % iodixanol (approx. 1.105 g/ml) and 0% iodixanol layered on top (all solutions contained artificial sea water). After centrifugation at 160 g for 10 min the protoplasts banded at the 0%/19.2% iodixanol interface. For more details regarding the preparation of the protoplasts see ref. 3.
3. Use of NycodenzⓇ gradients
3a. Protoplasts from barley grain aleurone
In the method developed by Bethke et al [4] Gamborg B5 medium (minimal organics) was used in the preparation of the protoplasts, but for the gradient isolation this was supplemented with a variety of additives. Three gradient solutions of densities 1.26 g/ml (5 g NycodenzⓇ + 10 g of medium), 1.18 g/ml (3 g NycodenzⓇ + 10 g of medium) and 1.08 g/ml (sorbitol in medium) were produced with an osmotic pressure of 1150-1200 mmol.kg-1. Protoplasts were very gently suspended in the densest medium and the two lower density solutions layered on top. Because of the small volume of each step (350 μl) the protoplasts gather at the top interface after only 10 min (see also refs 5-9 for other papers reporting use of this method). A similar three-layered gradient uses slightly higher concentrations of NycodenzⓇ of 70% and 50% (w/v) [10-12].
3b. Protoplasts from barley endosperm
A pellet of crude protoplast, obtained at 40-50 g for 2-4 min, was suspended in 2 ml of 40% (w/v) NycodenzⓇ (in 50 mM CaCl2, 25 mM MES, pH 5.5), overlaid with 1 ml each of 26.6%, 13.3% and
6.6% NycodenzⓇ (produced by dilution of the 40% Nycodenz solution with the protoplast medium). After centrifugation at 40-50 g for 4min, two bands of protoplasts were obtained at the top of the gradient [13].
3c. Protoplasts from Amaranthus
A NycodenzⓇ-sorbitol gradient has been used for the isolation of Amaranthus cotyledon protoplasts [14]. The gradient contained three layers of (1) 20.5% (w/v) NycodenzⓇ, 0.25 M sorbitol,
(2) 8.2% NycodenzⓇ, 0.4 M sorbitol and (3) 4.1% NycodenzⓇ, 0.45 M sorbitol. All solutions contained 10 mM CaCl2, 1% BSA (w/v) and 25 mM MES, pH 6.0. In this case the crude protoplast suspension was layered on top and after centrifugation at 200 g for 4 min, the protoplasts were recovered from the lowest interface.
3d. Protoplasts from Scots pine bud callus
Flotation through a layer of 6% (w/v) NycodenzⓇ was used [15].
4. References
1. Sarhan, F. and Cesar, D. (1988) High yield isolation of mesophyll protoplasts from wheat, barley and rye Physiologia Plantarum, 72, 337-342
2. Matthews, D. J., Moran, B. M., McCabe, P. F. and Otte, M. L. (2004) Zinc tolerance, uptake, accumulation and distribution in plants and protoplasts of five European populations of the wetland grass Glyceria fluitans Aquatic Bot., 80, 39-52
3. Huddy, S.M., Meyers, A.E. and Coyne, V.E. (2015) Regeneration of whole plants from protoplasts of Gracilaria gracilis (Gracilariales, Rhodophyta) J Appl. Phycol. 27, 427–435
4. Bethke, P.C., Hillmer, S. and Jones, R.L. (1996) Isolation of intact protein storage vacuoles from barley aleurone: identification of aspartic and cysteine proteases Plant Physiol., 110, 521-529
5. Swanson, S.J., Bethke, P.C. and Jones, R.L. (1998) Barley aleurone cells contain two types of vacuoles: characterization of lytic organelles by use of fluorescent probes Plant Cell, 10, 685-698
6. Bethke, P.C., Lonsdale, J.E., Fath, A. and Jones, R.L. (1999) Hormonally regulated programmed cell death in barley aleurone cells Plant Cell, 11, 1033-1046
7. Fath, A., Bethke, P.C. and Jones, R.L. (1999) Barley aleurone cell death is not apoptotic: characterization of nuclease activities and DNA degradation Plant J., 20, 305-315
8. Fath, A., Bethke, P.C., Belligni, M.V., Spiegel, Y.N. and Jones, R.L. (2001) Signalling in the cereal aleurone: hormones, reactive oxygen and cell death New Phytologist, 151, 99-107
9. Fath, A., Bethke, P.C., Belligni, M.V., Spiegel, Y.N. and Jones, R.L. (2001) Signalling in the cereal aleurone: hormones, reactive oxygen and cell death New Phytologist, 151, 99–107
10. Zorec, R. and Tester, M. (1992) Cytoplasmic calcium stimulates exocytosis in a plant secretory cell Biophys. J., 63, 864-867
11. Zorec, R. and Tester, M. (1993) Rapid pressure driven exocytosis-endocytosis cycle in a single plant cell FEBS Lett., 333, 283-286
12. Homann, U. and Tester, M. (1997) Ca2+ -independent and Ca2+ /GTP-binding protein-controlled exocytosis in a plant cell Proc. Natl. Acad. Sci. USA, 94, 6565-6570
13. Lee, B.T., Murdoch, K., Topping, J., Jones, M.G.K. and Kreis, M. (1991) Transient expression of foreign genes introduced into barley endosperm protoplasts by PEG-mediated transfer or into intact endosperm tissue by microprojectile bombardment Plant Sci., 78, 237-246
14. Elliott, D.C. and Yuguang, Y (1989) Cytokinin and fusicoccin effects on calcium transport in Amaranthus protoplasts Plant Science, 65), 243-252
15. Hohtola, A. and Kvist, A-P. (1991) Preparation of protoplasts from callus derived from buds of mature scots pine and subsequent induction of cell proliferation Tree Physiol., 8, 423-428
OptiPrep™ Application Sheet C19; 7th edition, January 2020
OptiPrep™ Application Sheet C20
Purification of leukocyte fractions from rodent/rabbit peritoneal exudates
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
Hattori et al [1] harvested the cells from the peritoneal fluid; lysed the residual erythrocytes in 0.2% NaCl and restored isotonicity by adding an equal volume of 1.6% NaCl. The MCs and PMNs were then separated on NycoprepⓇ 1.077A. This medium, which was primarily produced for the separation of MCs and PMNs from rodent blood, is no longer commercially available but may be
prepared from NycodenzⓇ powder (14.1% (w/v) NycodenzⓇ, 0.30% (w/v) NaCl, 5 mM TricineNaOH, pH 7.2). A solution of the same density and osmolality (ρ = 1.077 ρ 0.001 g/ml; osmolality 265 mOsm) may more easily be prepared from OptiPrep™.
Frevert et al [2] also used a method that was worked out for the separation of MCs and PMNs from rodent blood. In this method, developed by Freeman et al [3], an isoosmotic solution (NycoPrepⓇ 1.15) was used, which is no longer commercially available. This solution containing 27.6% (w/v) NycodenzⓇ in 3 mM KCl, 0.3 mM CaNa2-EDTA 5 mM Tris-HCl, pH 7.5 (density = 1.15 g/ml) was subsequently diluted with the same KCl, EDTA, Tris solution containing 0.75 g NaCl to produce solutions of 18.4% and 13.8% NycodenzⓇ (ρ = 1.098 and 1.075 respectively). The peritoneal cell suspension (2-6 ml) was layered on top of 2.5 ml of each of the density solutions and centrifuged at 400 g for 30 min at 26°C. The PMNs banded around the lower interface.
Fisker et al [4] developed a single NycodenzⓇ barrier to separate MCs and PMNs; the density and osmolality of which was modulated according to the type of peritoneal exudates (from a thioglycolatestimulated or an unstimulated animal). For cells from stimulated rats the optimum density and osmolality of the barrier was 1.106 g/ml and 400 mOsm respectively, for non-stimulated rats 1.091 g/ml and 325 mOsm. Sawant and McMurray [5] used the same strategy for guinea pig peritoneal exudates. The density barrier solutions were again prepared from NycoPrepⓇ 1.15. The MCs banded at the interface and the PMNs formed a pellet.
- An identical solution to NycoPrep 1.15 may be prepared from NycodenzⓇpowder or from OptiPrep. Both options are given (see Note 1).
2. Reagents and solutions required (see Note 2)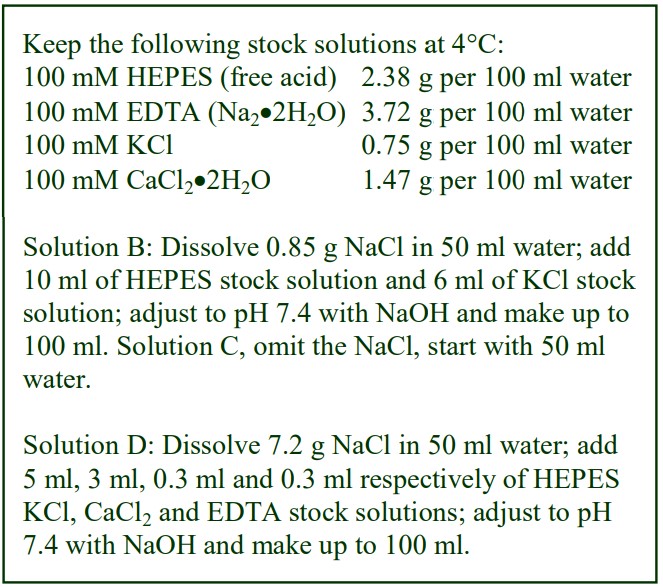
A. OptiPrep™(shake the bottle gently before use) OR NycodenzⓇ powder
B. Diluent (OptiPrep™): 0.85% (w/v) NaCl, 6 mM KCl, 10 mM HEPES-NaOH, pH 7.4 OR
C. Diluent (NycodenzⓇ): 6 mM KCl, 10 mM HEPES-NaOH, pH 7.4
D. Hyperosmotic diluent: 3 mM KCl, 0.3 mM Na2- EDTA, 0.3 mM CaCl2, 1.245 M NaCl, 5 mM HEPES-NaOH, pH 7.4.
E. Lavage solution: Krebs Ringer salt solution buffered with 25 mM HEPES to pH 7.4 containing 1% (w/v) bovine serum albumin.
3. Protocol
1. OptiPrep™: Dilute 4.6 ml of solution A with 5.4 ml of solution B to produce a Working Solution of approx 1.15 g/ml. OR
2. NycodenzⓇ To make 100 ml of the 1.15 g/ml Working Solution place approx. 50 ml of Solution C in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 27.6 g of NycodenzⓇ in small amounts until dissolved. Allow the solution to cool to room temperature and then make up to 100 ml with water. Filter sterilize if required.
3. To make up a 1.106 g/ml and 400 mOsm density barrier, mix the chosen Working Solution with Solution D and water in the volume ratio of 0.7:0.08:0.22 respectively; to make up a 1.091 g/ml and 325 mOsm density barrier use ratios of 0.6:0.06:0.34.
4. Collect the peritoneal cells in the Solution E and centrifuge at 300 g for 10 min at 20°C; then wash the cells in Solution E once and resuspend the cells in this medium to about 107 cells/ml.
5. Underlayer 7 ml of the cell suspension with 3 ml of density barrier and centrifuge at 700 g for 20 min at 20°C. The mononuclear cells and PMNs separate across the density barrier
(see Note 3).
4. Notes
1. Making up the 1.15 g/ml working solution from OptiPrep™ is easier than it is from NycodenzⓇpowder and the two solutions will have the same physical properties. It is very unlikely that the separation will differ but it should be stressed that the use of OptiPrep™ has not been validated.
2. NycoprepⓇ 1.15 contained 3 mM KCl, 5 mM Tris-HCl, pH 7.5 and 0.3 mM CaNa2EDTA. The latter was included to improve the long-term stability of the solution. It has been omitted from the
working solutions. If the concentration of EDTA is important to the separation however, 0.6 mM CaNa2EDTA or Na2EDTA may be included in Solutions B or C. For more information about preparing density gradient solutions for mammalian cells see Application Sheet C01.
3. The authors reported ca. 95% purity of the mononuclear cells (top band) and PMNs (bottom band).
4. More recently it has been shown, using iodixanol gradients to purify rabbit macrophages, that these cells can be induced by human recombinant GM-CSF and M-CSF [6]. Radovanovic et al [7]
showed the effect of silicon-rich water intake on the systemic inflammation and functional characteristics of rodent peritoneal macrophages that were chronically exposed to dietary aluminum.
5. References
1. Hattori, H., Imai, H., Hanamoto, A., Furuhama, K. and Nakagawa, Y. (2005) Up-regulation of phospholipid hydroperoxide gluthatione peroxidase in rat casein-induced polymorphonuclear neutrophils Biochem. J., 389, 279-287
2. Frevert, C.W., Huang, S., Danace, H., Paulauskis, J.D. and Kobzik, L. (1995) Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation J. Immunol., 154, 335-344
3. Freeman, G.E., Dalton, C.A. and Brooks, P.M. (1991) A Nycodenz gradient method for the purification of neutrophils from the peripheral blood of rats J. Immunol. Meth., 139, 241-249
4. Fisker S., Kudahl, K. and Sonne, O. (1990) Isolation of rat peritoneal mononuclear and polymorphonuclear leucocytes on discontinuous gradients of Nycodenz J. Immunol. Meth., 133, 31-38
5. Sawant, K.V. and McMurray, D.N. (2007) Guinea pig neutrophils infected with Mycobacterium tuberculosis produce cytokines which acitivate alveolar macrophages in non-contact cultures Infect. Immun., 75, 1870-1877
6. Yamane, K. and Leung, K-P. (2016) Rabbit M1 and M2 macrophages can be induced by human recombinant GM-CSF and M-CSF FEBS Open Bio, 6, 945–953
7. Radovanovic, Z., Djindjic, B., Dzopalic, T., Veljkovic, A., Dunjic, M., Krstic, D., Djindjic, N. and Nedeljkovic, B. (2018) Effect of silicon-rich water intake on the systemic and peritoneal inflammation of rats with chronic low levels of aluminum ingestion J. Trace Elements Med. Biol., 46, 96–102
OptiPrep™ Application Sheet C20; 6th edition, January 2020
OptiPrep™ Application Sheet C21
Dendritic cells from tissues by flotation through a low-density barrier
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List RC05 “Dendritic cells from blood and tissues” compares all of
the current methodologies and provides a full list of all the published papers reporting the use of
OptiPrep™: to access return to the initial list of Folders and select “Reference Lists” - To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and
type the C-Number in the Find Box
1. Background
Since dendritic cells (DC) were recognized as playing an important role in the induction of cellmediated responses [1], there has been a rapid growth in research into the function of these cells and
methods for their purification. Gradients of either albumin or metrizamide, although providing an effective enrichment of DC, tended to cause some functional alteration of the cells (see ref 2 for details). However, because cells are much more tolerant of NycodenzⓇ, this iodinated density gradient medium rapidly became established as the medium of choice for DC cell purification from peripheral blood and from lymphoid tissues. More recently methods have been developed for isolation of DC from tissues in iodixanol gradients because of the even higher tolerance of this solute by cells. Compared to other cell types in tissues such as spleen, thymus, lymph nodes etc., the DCs have a low density and one of the most commonly Nycodenz-based techniques is simply to isolate lowdensity cells by layering the disaggregated cell suspension over a barrier of density 1.077-1.080 g/ml (e.g. refs 3-5). This sedimentation strategy has also been used with OptiPrep™ and it is described in “Dendritic cells” Application Sheet C41 in index. Another common approach is to suspend the crude cells in a solution of NycodenzⓇ of density 1.077 g/ml and then allow the DCs to float to the top during centrifugation (e.g. refs 6-9). This approach too has been adapted to OptiPrep™, but to improve the purity of the DCs there has been a tendency to reduce the density of the suspending solution (as low as 1.061 g/ml). It is described in “Dendritic cells” Application Sheet C22 in index.  The protocol described in this Application Sheet was first described for the isolation of DC from mouse Peyer’s patches [10], but it has since been extended to their isolation from blood, lymph nodes, spleen and thymus and the isolation of Langerhans cells from skin. Like that in Application Sheet C22 it involves flotation but the crude suspension is adjusted to 1.085 g/ml and the separating low-density barrier is layered on top (see Figure 1). The major advantage of this approach is that the DCs are separated from the original dense cell suspension by the low density barrier, which is free of other cells, any cells partially disrupted by the earlier disaggregation process or any residual enzymes used in this process. This is not the case with any other strategy. The 1.065 g/ml barrier almost acts as a “washing” solution. The following protocol is adapted from ref 10.
The protocol described in this Application Sheet was first described for the isolation of DC from mouse Peyer’s patches [10], but it has since been extended to their isolation from blood, lymph nodes, spleen and thymus and the isolation of Langerhans cells from skin. Like that in Application Sheet C22 it involves flotation but the crude suspension is adjusted to 1.085 g/ml and the separating low-density barrier is layered on top (see Figure 1). The major advantage of this approach is that the DCs are separated from the original dense cell suspension by the low density barrier, which is free of other cells, any cells partially disrupted by the earlier disaggregation process or any residual enzymes used in this process. This is not the case with any other strategy. The 1.065 g/ml barrier almost acts as a “washing” solution. The following protocol is adapted from ref 10.
- Note that in many cases the density barrier enrichment of DCs is followed by further purification using antibody-bound beads
2. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. Suspension solution: Hank’s Balanced Salt Solution (without Ca2+ and Mg2+)
C. Diluent: 0.88% (w/v) NaCl, 1 mM EDTA, 0.5% (w/v) bovine serum albumin (BSA), 10 mM HEPES-NaOH, pH 7.4.
D. Digest solution: RPMI (DMEM or IMDM) containing 5% fetal calf serum (FCS), 10 U/ml collagenase (see Note 1).
3. Protocol
3a. Dissociation of the tissue (see Note 2)
1. Preparation of a single cell suspension by dissociation of the chosen tissue will only be described in general terms in this Application Sheet. Detailed protocols may vary from laboratory to
laboratory.
2. Digest the finely chopped tissue twice in the Solution D at 37°C for 30 min.
3. Pass the digest through a stainless steel sieve.
4. Harvest the cells by centrifugation and wash them as required in a balanced salt medium (see Note 3).
3b. Gradient separation (see Note 4)
1. Make up an 11.5% (w/v) iodixanol solution (ρ = 1.065 g/ml) from Solutions A and C (1:4.2 v/v).
2. Suspend the washed cell pellet in Solution B and mix gently but thoroughly with OptiPrep (3:1 v/v) to give a 15% (w/v) iodixanol solution (ρ = 1.085 g/ml). Overlayer 4 ml of this suspension with 5 ml of the 11.5% iodixanol solution (step 1) and 3 ml of Solution B.
3. Centrifuge at 600 gav for 15 min at room temperature (approx 20°C).
4. Allow the rotor to decelerate without the brake and harvest the DC from the top of the 11.5% iodixanol layer (see Figure 1 and Note 5).
4. Notes
1. The composition of the digest medium is only given as a basic recipe, other components such as antibiotics; glutamine may be included as required by the operator.
2. The operator should use whatever digest protocol is effective for the chosen tissue. It is important however that the handling of the cells after digestion should be carried out as gently as possible to avoid potential damage to the cells. Sometimes DNase I and/or EDTA are included in the final cell suspension medium to reduce any cell aggregation.
3. This medium should not contain Ca2+ or Mg2+ but inclusion of FCS may be permissible.
4. Some publications report the use of an upper layer of density higher than 1.065 g/ml (up to 1.071 g/ml) and media of a composition different to that of Solution C for diluting the OptiPrep™. More
information on gradient solution preparation is in Application Sheet C01.
5. Ruedl et al [10] reported that from Peyer’s patch material 3-5% of the total cells in the gradient were recovered at the upper interface, and the enrichment of DC over the starting material should be 30-60x (Ruedl, C. personal communication).
5. References
1. Barfoot, R., Denham, S., Gyure, L. A., Hall, J. G., Hobbs, Jackson, L. E. and Robertson, D. (1989) Some properties of dendritic macrophages from peripheral lymph
Immunology, 68, 233-239
2. McLellan, A. D., Starling, G. C. and Hart, D. N. J. (1995) Isolation of human blood dendritic cells by discontinuous Nycodenz gradient centrifugation J. Immunol. Meth., 184, 81-89
3. Leenen, P.J.M. Radošević, K. Voerman, J.S. Salomon, B., van Rooijen, N., Klatzmann, D. van Ewijk, W. (1998) Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover J. Immunol., 160, 2166-2173
4. Drake, D.R., Shawver, M.L., Hadley, A., Butz, E., Maliszewski, C. and Lukacher, A.E. (2001) Induction of polyomavirus-specific CD8+ T lymphocytes by distinct dendritic cell subpopulations J. Virol., 75, 544-547
5. Voisine, C., Hubert, F-X., Trinite, B., Heslan, M. and Josien, R. (2002) Two phenotypically distinct subsets of spleen dendritic cells in rats exhibit different cytokine production and T cell stimulatory activity J. Immunol., 169, 2284-2291
6. Kronin, V., Winkel, K., Suss, G. Kelso, A., Heath, W., Kirberg, J., von Boehmer, H. and Shortman. K. (1996) A subclass of dendritic cells regulates the response of naïve CD8 T cells by limiting their IL-2 production J. Immunol., 157, 3819- 3627
7. Vremec, D. and Shortman, K. (1997) Dendritic cell suptype in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes J. Immunol., 159, 565-573
8. Vremec, D., Pooley, J., Hochrein, H., Wu, L. and Shortman, K. (2000) CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen J. Immunol., 164, 2978-2986
9. Kamath, A.T. Pooley, J., O’Keeffe, M.A. Vremec, D., Zhan, Y. Lew, A.M., D’Amico, A., Wu, L., Tough, D.F. and Shortman, K. (2000) The development, maturation, and turnover rate of mouse spleen dendritic cell populations J. Immunol., 165, 6762-6770
10. Ruedl, C., Rieser, C., Bock, G., Wick, G. and Wolf, H. (1996) Phenotypic and functional characterization of CD11c+ dendritic cell population in mouse Peyer’s patches Eur. J. Immunol., 26, 1801-1806
6. Acknowledgements
We would like to thank Dr Christiane Ruedl for her help in preparation of this Application Sheet.
OptiPrep™ Application Sheet C21; 8th edition, January 2020
OptiPrep™ Application Sheet C22
Isolation of dendritic cells from tissues by a mixer technique
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List RC05 “Dendritic cells from blood and tissues” compares all of the current methodologies and provides a full bibliography of all the published papers reporting the use of OptiPrep™: to access return to the initial list of Folders and select “Reference Lists”
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
Since dendritic cells (DC) were recognized as playing an important role in the induction of cellmediated responses [1], there has been a rapid growth in research into the function of these cells and
methods for their purification. Gradients of either albumin or metrizamide, although providing an effective enrichment of DC, tended to cause some functional alteration of the cells (see ref 2 for details). However, because cells are much more tolerant of Nycodenz, this iodinated density gradient medium rapidly became established as the medium of choice for DC cell purification from peripheral blood and from lymphoid tissues. More recently methods have been developed for isolation of DC from tissues in iodixanol gradients because of the even higher tolerance of this solute by cells. Compared to other cell types in tissues such as spleen, thymus, lymph nodes etc., the DCs have a low density and one of the most commonly NycodenzⓇ-based techniques is simply to isolate lowdensity cells by layering the disaggregated cell suspension over a barrier of density 1.077-1.080 g/ml (e.g. refs 3-5). This sedimentation strategy has also been used with OptiPrep™ and it is described in “Dendritic cells” Application Sheet C41, see index. Another common approach is to suspend the crude cells in a solution of NycodenzⓇ of density 1.077 g/ml to allow the DCs to float to the top during centrifugation, while all of the denser cells pellet (e.g. refs 6-9). This strategy too has been adapted to OptiPrep™, but to improve the purity of the DCs there has been a tendency to reduce the density of the suspending solution (approx 1.061 g/ml). This separation protocol was first described for the isolation of DC from mouse Peyer’s patches, lymph nodes and spleen and for the isolation of Langerhans cells from skin [10-12]. The method is described in this Application Sheet and it is the easiest of all the methods to execute. A third option, also a flotation technique is as follows: the cell suspension is adjusted to a density of 1.085 g/ml with OptiPrep™and the DC allowed to float up through an iodixanol solution of density 1.065 g/ml layered on top. This is described in “Dendritic cells” Application Sheet C21, see index.
2. Solutions required (see Note 1 for the NycodenzⓇ
A. OptiPrep™ (shake the bottle gently before use)
B. Digest solution: RPMI containing 5% heatinactivated fetal calf serum (FCS), 10 U/ml collagenase and 5 g/ml DNase I, pH 7.4 (see Note 2).
C. Phosphate buffered saline (without Ca2+ and Mg2+) containing 5% fetal calf serum (FCS) 5 mM EDTA and 5 μg/ml DNase I.
D. Diluent: 0.8% (w/v) NaCl, 5 mM EDTA, 10 mM Tricine-NaOH, pH 7.4
E. 30% (w/v) Iodixanol working solution: mix equal vols. of OptiPrep™ and Solution D.
3. Protocol (adapted from refs 3 and 4)
3a. Dissociation of the tissue (see Note 3)
Preparation of a single cell suspension by dissociation of the chosen tissue will only be described in general terms in this Application Sheet. Detailed protocols may vary from laboratory to laboratory.
1. Digest the finely chopped tissue in Solution B at 37°C for 10 min.
2. Pass the digest through a stainless steel sieve.
3b. Gradient separation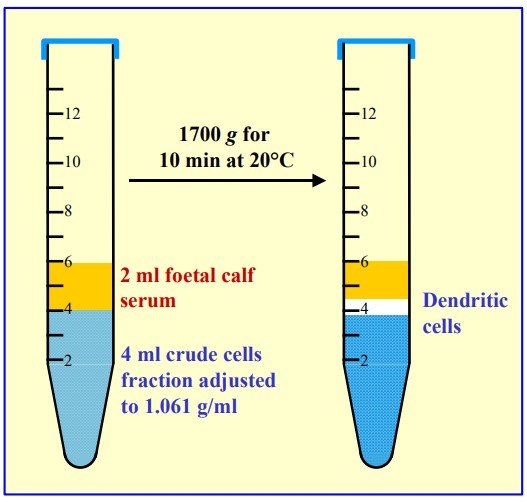
Carry out all operations at 4°C, making sure that all solutions and equipment are pre-cooled.
1. Harvest the cells by centrifugation at 540 g for 5 min and wash them twice in Solution C.
2. Prepare an isoosmotic solution of 10.5% (w/v) iodixanol (ρ = 1.061 g/ml) by diluting 10.5 vol. of Solution E with 19.5 vol. of Solution D (see Notes 4- 6).
3. Suspend the washed cell pellet in this solution at approx. 1.5×108 cells/ml.
4. Transfer 3-4 ml to a centrifuge tube and overlay with 2 ml of FCS (see Note 7).
5. Centrifuge at 1700 g for 10 min using a slow acceleration mode (see Note 8)
6. Allow the rotor to decelerate without the brake and harvest the DC from the FCS/sample interface (see Figure 1 and Note 9).
4. Notes
1. The most widely used NycodenzⓇ option was published in 1996 [6]. Prepare a stock solution of 1.16 g/ml as follows: place 50 ml of water or the chosen buffer in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 30.5 g of NycodenzⓇ powder in small amounts until dissolved. Allow the solution to cool to room temperature and then make up to 100 ml with water or the buffer. Filter sterilize if required. To make a 1.077 g/ml solution dilute the stock to 14% (w/v) NycodenzⓇ with 0.154 M NaCl, 4 mM KCl, 5 mM EDTA, 5 mM HEPES-NaOH, pH 7.2 containing 5% FCS (adjusted to 10 mM EDTA).
2. The composition of the digest medium is only given in outline; other components such as antibiotics or glutamine may be included as required by the operator.
3. The operator should use whatever digest protocol is effective for the chosen tissue (see refs. 1 and 2 for more information). It is important however that the handling of the cells after digestion should be carried out as gently as possible to avoid potential damage to the cells. DNase I and/or EDTA are included in the cell medium to reduce any cell aggregation to a minimum.
4. The composition of the isoosmotic solutions used in the gradient separation may be adjusted to
suit the requirements of the operator. EDTA prevents any aggregation.
5. For the NycodenzⓇ option, the cells should be suspended in the 14% (w/v) NycodenzⓇ solution (see Note 1). The density of the solution used to suspend the cells should be adjusted to suit the operator’s requirements. Iodixanol or NycodenzⓇ solutions up to 14.5% (w/v) may be used.
6. The density of the DC may depend on the pre-gradient treatment and also the material source. It may be necessary to modulate the density of the cell suspension medium in the light of experience.
7. It is important to put a low-density layer on top of the cell suspension, to avoid banding the DC at an air/water interface. This low-density layer however could alternatively be Solution D (± FCS).
8. Centrifugation conditions vary quite widely; times as long as 20 min at 1,700 g [13] or g-forces as low as 600 g for 25 min [14,15].
9. The harvested DC may be further purified using the appropriate MAb-coated magnetic beads. This initial gradient purification step allows the bead purification to be performed more efficiently.
5. References
1. Barfoot, R., Denham, S., Gyure, L. A., Hall, J. G., Hobbs, Jackson, L. E. and Robertson, D. (1989) Some properties of dendritic macrophages from peripheral lymph Immunology, 68, 233-239
2. McLellan, A. D., Starling, G. C. and Hart, D. N. J. (1995) Isolation of human blood dendritic cells by discontinuous Nycodenz gradient centrifugation J. Immunol. Meth., 184, 81-89
3. Leenen, P.J.M. Radošević, K. Voerman, J.S. Salomon, B., van Rooijen, N., Klatzmann, D. van Ewijk, W. (1998) Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover J. Immunol., 160, 2166-2173
4. Drake, D.R., Shawver, M.L., Hadley, A., Butz, E., Maliszewski, C. and Lukacher, A.E. (2001) Induction of polyomavirus-specific CD8+ T lymphocytes by distinct dendritic cell subpopulations J. Virol., 75, 544-547
5. Voisine, C., Hubert, F-X., Trinite, B., Heslan, M. and Josien, R. (2002) Two phenotypically distinct subsets of spleen dendritic cells in rats exhibit different cytokine production and T cell stimulatory activity J. Immunol., 169, 2284-2291
6. Kronin, V., Winkel, K., Suss, G. Kelso, A., Heath, W., Kirberg, J., von Boehmer, H. and Shortman. K. (1996) A subclass of dendritic cells regulates the response of naïve CD8 T cells by limiting their IL-2 production J. Immunol., 157, 3819- 3627
7. Vremec, D. and Shortman, K. (1997) Dendritic cell suptype in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes J. Immunol., 159, 565-573
8. Vremec, D., Pooley, J., Hochrein, H., Wu, L. and Shortman, K. (2000) CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen J. Immunol., 164, 2978-2986
9. Kamath, A.T. Pooley, J., O’Keeffe, M.A. Vremec, D., Zhan, Y. Lew, A.M., D’Amico, A., Wu, L., Tough, D.F. and Shortman, K. (2000) The development, maturation, and turnover rate of mouse spleen dendritic cell populations J. Immunol., 165, 6762-6770
10. Anjuere, F., Martin, P., Ferrero, I., Fraga, M. L., del Hoyo, G. M., Wright, N. and Ardavin, C. (1999) Definition of dendritic cell subpopulations present in the spleen, Peyer’s patches, lymph nodes, and skin of the mouse Blood, 93, 590-598
11. Ardavin, C., Martinez del Hoyo, G., Martin, P., Anjuere, F., Arias, C. F., Marin, A. R., Ruiz, S., Parrillas, V. and Hernandez, H. (2001) Origin and differentiation of dendritic cells Trends Immunol., 22, 691-700
12. Martin, P., Martinez del Hoyo, G., Anjuère, F., Fernandez Arias, C., Hernandez Vargas, H., Fernandez-L, A., Parrillas, V. and Ardavin, C. (2002) Characterization of a new subpopulation of mouse CD8+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential Blood, 100, 383-390
13. Mattei, F., Schiavoni, G., Belardelli, F. and Tough, D.F. (2001) IL-15 is expressed by dendritic cells in response to type I IFN, souble-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation J. Immunol., 167, 1179-1187
14. McLellan, A.D., Kapp, M., Eggert, A., Linden, C., Bommhardt, U., Bröcker, E-B., Kämmerer, U. and Kämpgen, E. (2002) Anatomic location and T-cell stimulatory functions of mouse dendritic cell subsets defined by CD4 and CD8 expression Blood, 99, 2084-2093
15. Douillard, P., Stoitzner, P., Tripp, C.H., Clair-Moninot, V., Aït-Yahia, S., McLellan, A.D., Eggert, A., Romani, N. and Saeland, S. (2005) Mouse lymphoid tissue contains distinct subsets of langerin/CD207+ dendritic cells, only one of which represents epidermal-derived Langerhans cells J. Invest. Dermatol., 125, 983-994
6. Acknowledgements
We wish to thank Dr Carlos Ardavin, Dept. of Cell Biology, Faculty of Biology, Complutense University, 28040 Madrid, Spain for providing information regarding the methodology described in this Application Sheet.
OptiPrep™ Application Sheet C22; 9th edition, January 2020
OptiPrep™ Application Sheet C23
Isolation of a mouse motoneuron enriched fraction from spinal cord on a density barrier
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List RC06 “Neural cells from brain and spinal cord – reference list” provides a comprehensive bibliography of all the published papers reporting the use of OptiPrep™: to access return to the initial list of Folders and select “Reference Lists”
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
The growth and differentiation of spinal motoneurons are dependent on various genetic and epigenetic factors, which influence both functional and morphological characteristics [1]. Involvement
of a number of trophic molecules is known to be an important part of these processes [1]. Consequently there is a considerable amount of research carried out on cultured motoneurons, which can be derived from spinal cord. The general procedure can be summarized as follows: Spinal cords are dissected from embryos and following a combined enzymic and mechanical disruption of the tissue, debris is removed by pelleting the cells through a cushion of bovine serum albumin. A motoneuron-rich fraction is then isolated from the cell pellet prior to cell culture. Bataille et al [1] layered the crude cell pellet from rat embryo spinal cord over two layers of NycodenzⓇ of density, ρ = 1.047 and 1.065 g/ml and analyzed the cells that banded at the top of each NycodenzⓇ layer. The majority of the cells banded at the lower interface and the diameter of these cells (approx 4.3 μm) was much lower than the minor population of cells (motoneurons) around the upper interface (approx 6.7 m). These large low-density cells contained very high levels of acetylcholine, which was virtually absent from the smaller denser cells at the lower interface. It seems
clear from this earlier method that the motoneurons, as a result of their larger size are less dense than the other cells. Martinou [2] reported that the denser layer contained cholinergic cells other than motoneurons. In some cases the density of the two layers was changed, e.g. 1.035 and 1.092 g/ml [3] and for mouse embryo spinal cord 1.042 and 1.065 g/ml [4]. The gradient format was later simplified to a single low-density NycodenzⓇ cushion usually of density 1.055 g/ml [5,6], although densities as low as 1.035 g/ml have also been used [7]. Duong et al. [8] were first to report the use of OptiPrep™; they used a single layer of approx 10.5% (w/v) iodixanol (ρ = 1.06 g/ml) to purify mouse motoneurons, which remained on top of the density barrier after centrifugation. This simple barrier system has been described in all subsequent papers but, as with NycodenzⓇ methodology, the barrier density has often been reduced. For rodent motoneurons the iodixanol concentration may be 5.5-6.5% (w/v), equivalent to ρ = 1.035-1.040 g/ml [9-13] but for chick motoneurons [14,15] this as low as 5% (w/v) iodixanol (ρ = 1.035 = 1.032 g/ml). Since the NycodenzⓇ and OptiPrep™ procedures are so similar, only the latter is given in this Application Sheet. It is based on ref 8.
2. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. Hank’s Balanced Salt Solution (without Ca2+ and Mg2+) C. 3.5% (w/v) Bovine serum albumin in Solution B.
D. 0.025% trypsin in isotonic solution
3. Protocol
3a. Isolation of a total cellular fraction
1. Carry out all operations at room temperature
2. After dissection of the mouse embryo spinal columns, incubate them in Solution D for 20 min.
3. Dissociate the tissue by repeated passage through a syringe needle (21 gauge).
4. Layer the suspension over Solution C and centrifuge at 120 g for 10 min to remove cell debris.
5. Discard the supernatant and resuspend the pellet in Solution B.
3b. Isolation of a neuron-rich fraction
1. Dilute OptiPrep™ with Solution B to give a 1.06 g/ml solution, equivalent to 10.4% (w/v) iodixanol (see Notes 1 and 2).
2. Layer the resuspended pellet over the 1.06 g/ml solution.
3. Centrifuge at 400 g for 25 min (see Note 3).
4. Collect the banded cells in the upper layer, dilute with Solution B and centrifuge at 700 g for 10 min to pellet the motoneuron fraction.
5. Wash the cell pellet as required. See refs 1 and 7 for information on motoneuron culture (see Note 4).
4. Notes
1. For motoneurons from other species it may be necessary to modulate the density of the lower layer. For more information on gradient solution preparation see Application Sheet C01.
2. Modulating the density of the barrier will change the yield and purity of the motoneurons. Payne et al [16] isolated the largest cells from trypsinized mouse embryo spinal cords by sequential
centrifugation over cushions of 5.2% iodixanol (15 min at 800 g) and 4% bovine serum albumin (10 min at 470 g). A similar strategy was used for chick embryo neurons [17].
3. The centrifugation conditions are quite varied, 800-900 g for approx 15 min is quite common [11,12,14,15], but g-forces as low as 100 g [13] have been used.
4. Misgeld et al [18] noted that from 1-2 mouse spinal cords, 10-25,000 motoneurons could be purified by this method.
5. References
1. Bataille, S., Portalier, P., Coulon, P. and Ternaux, J-P. (1998) Influence of acetylcholinesterase on embryonic spinal rat motoneurones growth in culture: a quantitative morphometric study Eur. J. Neurosci., 10, 560-572
2. Martinou, J.C., Le Van Thai, A., Cassar, G., Roubinet, F. and Weber, M.J. (1989) Characterization of two factors enhancing choline acetyltransferase activity in cultures of purified rat motoneurons J. Neurosci., 9, 3645-3658
3. Guigoni, C. and Coulon, P. (2002) Rabies virus is not cytolytic for rat spinal motoneurons in vitro J. Neurovirol., 8, 306-317
4. Demierre, B., Martinou, J.C. and Kato, A.C. (1990) Embryonic motoneurons grafted into the adult CNS can differentiate and migrate Brain. Res., 510, 355-359
5. Martinou, J-C., Bierer, F., Van Thai, A.L. and Weber, M.J. (1989) Influence of the culture substratum on the expression of choline acetyltransferase activity in purified motoneurons from rat embryos Develop. Brain Res., 47, 251-262
6. Martinou, J-C., Martinou, I. and Kato, A.C. (1992) Cholinergic differentiation factor (CDF/LIF) promotes survival of isolated rat embryonic motoneurons in vitro Neuron, 8, 737-744
7. Copray, J.C.V.M. and Liem, R.S.B. (1993) Survival and neurite formation of mesencephalic trigeminal neurons of the rat in vitro Arch. Oral Biol., 38, 547-557
8. Duong, F. H. T., Warter, J. M., Poindron, P. and Passilly, P. (1999) Effect of the nonpeptide neurotrophic compound SR57746A on the phenotypic survival of purified mouse motoneurons Br. J. Pharmacol., 128, 1385-1392
9. Andries, M., Van Damme, P., Robberecht, W. and van den Bosch, L. (2007) Ivermectin inhibits AMPA receptormediated excitotoxicity in cultured motir neurons and extends the life span of a transgenic mouse model of amytrophic lateral sclerosis Neurobiol. Dis., 25, 8-16
10. Grosskreutz, J., Haastert, K., Dewil, M., Van Damme, P., Callewaert, G., Robberecht, W., Dengler, R. and Van den Bosch, L. (2007) Role of mitochondria in kinate-induced fast Ca2+ transients in cultured spinal motor neurons Cell Calcium, 42, 59-69
11. De Paola, M., Diana, V., Bigini, P. and Mennini, T. (2008) Morphological features and responses to AMPA receptormediated excitotoxicity of mouse motor neurons: comparison in purified, mixed anterior horn or motor neuron/glia cocultures J. Neurosci. Meth., 170, 85-95
12. Van Damme, P., Van Hoecke, A., Lambrechts, D., Vanacker, P., Bogaert, E., van Swieten, J., Carmeleit, P., Van Den Bosch, L. and Robberecht, W. (2008) Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival J. Cell Biol., 181, 37-41
13. Corey, J.M., Gertz, C.C., Wang, B-S., Birrell, L.K., Johnson, S.L., Martin, D.C. and Feldman, E.L. (2008) The design of electrospun PLLA nanofiber scaffolds compatible with serum-free growth of primary motor neuron and sensory neurons Acta Biomat., 4, 863-875
14. Taylor, A.R., Robinson, M.B. and Milligan, C.E. (2007) In vitro methods to prepare astrocyte and motoneuron cultures for the investigation of potential in vivo interactions Nat. Protoc., 2, 1499-1507
15. Macosko, J.C., Newbern, J.M., Rockford, J., Chisena, E.N., Brown, C.M., Holzwarth, G.M. and Milligan, C.E. (2008) Fewer active motors per vesicle may explain slowed vesicle transport in chick motoneurons after three days in vitro Brain Res., 1211, 6-12
16. Payne, A.M., Zheng, Z., Messi, M.L., Milligan, C.E., Gonzalez, E. and Delbono, O. (2006) Motor neurone targeting of IGF-1 prevents specific force decline in ageing mouse muscle J. Physiol., 570, 283-294
17. Taylor, A.R., Gifondorwa, D.J., Newbern, J.M., Robinson, M.B., Strupe, J.L., Prevette, D., Oppenhiem, R.W. and Milligan, C.E. (2007) Astrocyte and muscle-derived secreted factors differentially regulate motoneuron survival J. Neurosci., 27, 634-644
18. Misgeld, T., Kummer, T.T., Lichtman, J.W. and Sanes, J.R. (2005) Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter Proc. Natl. Acad. Sci. USA, 102, 11088-11093
OptiPrep™ Application Sheet C23; 8th edition, January 2020
OptiPrep™ Application Sheet C24
Isolation of a progenitor cell-enriched fraction
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
- Section 1 describes the more traditional sedimentation from a cell suspension on to a density barrier; Section 2 describes the use of the sample itself as the density barrier; Section 3 briefly describes a strategy for neural tissue progenitors
1. Sedimentation on to a density barrier
1a Introduction
The study of the proliferation and maturation of stem cells from bone marrow is a major area of cell and molecular biology research. Usually the resolving power of simple density barriers is insufficient to allow progenitor cells to be isolated in a sufficiently pure form for further analysis and culture. Nevertheless, the low-density cell fraction harvested from the barrier interface can provide a progenitor cell-enriched population that allows a more effective and economical use of immunomagnetic beads to remove lineage cells (using a cocktail of lineage-specific monoclonal antibodies).
Although metrizamide was used for density barriers prior to 1984, the lower cell toxicity of NycodenzⓇ, made this the medium of choice. Mayanagi et al [1] also emphasized the lack of toxicity of NycodenzⓇ (compared to PercollⓇ) and the avoidance of positive selection with antibody-beads was important in recovering viable progenitor cells. Bertoncello et al [2,3] reported the use of a 1.085 g/ml NycodenzⓇ barrier to isolate low-density cells from a suspension of nucleated bone marrow cells; 3 ml containing 108 cells were layered over 5 ml of the density barrier and centrifuged at 100 g for 30 min, at 4°C and the progenitor cell-enriched fraction harvested from the interface. A barrier of the same density was used by Erlich et al [4] and Sitnicka et al [5]. Lower density barriers, 1.080 g/ml [6], 1.077 g/ml [7-10] and 1.068 g/ml [11] have also been used and frequently the centrifugation is carried out at room temperature rather than at 4°C. In some cases, a shorter centrifugation time (20 min) has been employed at 1000 g [12]; in others a g-force of 400 g was used [5,6]. The 1.077 g/ml density barrier has been widely used since ready-made solutions of this density were previously available as NycoPrep™ 1.077 and NycoPrep™ 1.077A, the latter being the more popular for murine samples (see also Section 3). The density barrier approach has since been adapted to OptiPrep™; several publications report the use of a ρ = 1.08 g/ml iodixanol barrier [13-17]. As with the NycodenzⓇ methodology however, there are a number of variations for the density of the iodixanol barrier: 1.077 g/ml [18-21] and 1.074 g/ml [22].
Progenitor-enriched fractions have also been isolated from human blood [23] and cord blood [24] using NycoPrep™ 1.077; those from murine blood [25,26] and fetal liver [27-30] on NycoPrep™ 1.077A. Again the g-force is rather variable – 400-1000 g. Using OptiPrep™, progenitor cells have been enriched from blood (1.072 g/ml) [31] and thymus (1.05 g/ml) [32-34].
Important note: As many NycodenzⓇ gradient solutions were routinely used as NycoPrep™ 1.077A or diluted from NycoPrep™ 1.15, neither of which are now commercially available, the following methodology is based on the use of OptiPrep™ (a sterile solution of 60% w/v iodixanol) to avoid making up solutions from Nycodenz™ powder.
Modified density barriers, in which the density of the sample is raised before layering on the barrier, or the use of discontinuous gradients, may provide higher enrichments of progenitor cells than a simple density barrier. For more information on the advantages of using such techniques see “Hepatic Kupfer cells”, Application Sheet C28 in index.
 1b. Solutions required (see Note 1)
1b. Solutions required (see Note 1)
A. OptiPrep™ (shake the bottle gently before use)
B. Iscove’s modified Dulbecco’s medium Hank’s (IMDM)
C. Fetal calf serum (FCS)
D. Phosphate buffered saline (PBS) OR
E. Tricine buffered saline
1c. Protocol
Carry out all operations at room temperature (see Note 2)
1. Harvest bone marrow material in Solution B containing 2% FCS.
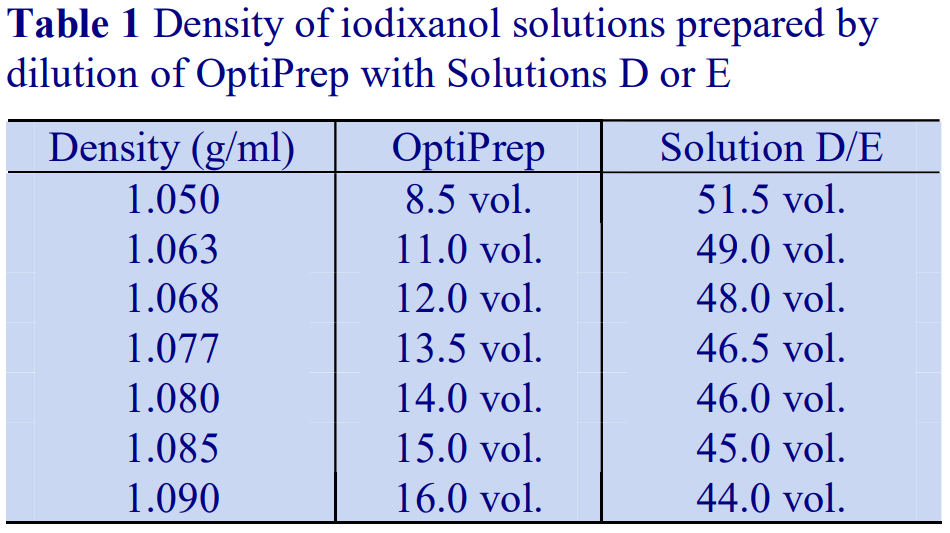 2. Dissociate the bone marrow cells by passing three times through a 25-gauge needle.
2. Dissociate the bone marrow cells by passing three times through a 25-gauge needle.
3. Filter the suspension through 200 μm nylon mesh.
4. To prepare a density barrier solution of approx 395 mOsm, dilute OptiPrep™ directly with Solution D or E (with or without 5% FCS) to give a solution of the chosen density (see Table 1 and Notes 3 and 4).
5. To prepare a density barrier solution of 1.077 g/ml and osmolality approx 265 mOsm (equivalent to Nycoprep 1.077A) start by diluting Solution E with water (volume ratio 2.5:0.5 respectively); this solution has an osmolality of approx 242 mOsm. Dilute OptiPrep™ with the 242 mOsm solution using a volume ratio of 2.7:9.3 respectively.
6. Layer 5 ml of the bone marrow cell suspension (107 cells/ml) over 3 ml of the chosen density barrier (see Note 5).
7. Centrifuge at 450 g for 20 min; allow the rotor to decelerate without the brake (see Notes 6 and 7).
8. Collect the cells from the interface; dilute with an equal volume of Solution D or E (+ 5% FCS); wash twice and resuspend in the same medium prior to lineage committed cell depletion.
1d. Notes
1. To dilute the OptiPrep™ use any suitable medium, two examples are given as Solutions D or E; culture medium may also be used. As long as the solution is isoosmotic, then the dilutions will also be isoosmotic.
2. Some papers report the use of centrifugation temperatures of 4°C.
3. Step 5 describes the preparation of an alternative reduced osmolality OptiPrep™ density solution.
4. Dilutions of OptiPrep™ with any isotonic medium (including culture medium) will give the same densities as those shown in Table 1. These diluents all have a density of approx 1.006 g/ml. Inclusion of FCS will however modulate the density slightly upwards. FCS has a density of approx 1.032 g/ml, so any balanced salt solution containing 10% FCS for example will have a density of 1.009 g/ml. The density of the OptiPrep™/saline mixtures given in Table 1 will be increased by 0.002-0.003 g/ml. If 5% FCS is used the density difference is proportionately less. For more information on preparing gradient solutions see Application Sheet C01.
5. Since the megakaryocytic progenitor cells are less dense than the majority of other cell types, it may be worth considering using a flotation strategy in which the bone marrow cell suspension is adjusted to the barrier density (e.g. 1.085 g/ml) and the progenitor cells harvested from the top of the solution. This approach has been used for fetal liver cells [35].
6. The published g-force, time and temperature values are rather variable. What effect lower or higher values might have on the separation can only be determined by experimentation.
7. Using the brake may create a vortex in the liquid and cause loss of definition of the interfacial band and even contamination from denser cells.
2. Progenitor cells from blood using a mixer strategy
This was first introduced for the isolation of mononuclear cells from human blood and later rat and mouse blood (see “Mononuclear cells” Application Sheets C05, C07 and C08 respectively in index). It has also been applied to the isolation of progenitor cells. The crude cell suspension is PBS + 5% FCS (30 ml) was mixed with 10.1 ml of OptiPrep™ and 4 ml of water. This adjusts the cell suspension medium to approx 1.077 g/ml. A small volume of buffered saline is placed on top to prevent the low-density cells from banding at an air-liquid interface during centrifugation [36-41]. The strategy avoids the build up and possible aggregation of cells at the interface between the sample and the density barrier in the standard barrier sedimentation strategy (see Section 1). It allows the denser cells to sediment while the low-density cells float. The 4 ml of water used in the preparation of the sample could certainly be replaced with saline if required, without affecting the separation.
- In a symposium abstract Hu et al [42] also reported a new OptiPrep™-based method for the purification of blood-derived mesenchymal stem cells.
3. Discontinuous gradients
A novel method [43,44] used gradient solutions produced by mixing OptiPrep™ with NycoPrep 1.077A. The latter is now commercially unavailable but a solution of the same density and reduced osmolality can be produced from OptiPrep™ by dilution with a reduced osmolality saline solution see “Mononuclear cells” Application Sheet C43 in index for more information) Solutions can be prepared as follows: use 0.7% (w/v) NaCl, 10 mM Tricine, pH 7.0 to dilute OptiPrep™ to produce solutions of 1.050, 1.080, 1.090 g/ml (approx. iodixanol concentrations if 8.4, 14 and 16% w/v). The gradient is centrifuged at 400 g for 15 min to isolate a low density fraction.
The iodixanol density gradient devised by Brewer et al [45] for the isolation of hippocampal motoneurons is also used as a gradient for purifying neural progenitor cells [46-50]. The method is described in detail in “Brain motoneurons” Application Sheet C36 in index. Abbosh et al [50] reported a two-step gradient of 1.058 and 1.11 g/ml for postnatal hippocampal precursors; He and Shen [52] and Nunan et al [53] also used a discontinuous iodixanol gradient for the isolation of glial progenitor cells, but gave no details.
The oval cells of liver, which are considered to be the equivalent of progenitor cells in the liver, may potentially be propagated in vitro and used restoratively in some liver diseases. Three layer gradients of 13%, 16 and 18% (w/v) iodixanol (OptiPrep™ diluted with HBSS, supplemented with 0.2% BSA (10 ml, 10 ml and 5 ml respectively) are overlaid with the non-parenchymal cells (see Application Sheet C24) in 11% iodixanol and centrifuged at 6,500 g for 30 min [54]. The oval cells are enriched at the 11/13% interface.
4. References
1. Mayanagi, T., Kurosawa, R., Ohnuma, K., Ueyama, A., Ito, K. and Takahashi, J. (2003) Purification of mouse primordial germ cells by Nycodenz Reproduction, 125, 667-675
2. Bertoncello, I., Bartelmez, S.H., Bradley, T.R. and Hodgson, G.S. (1987) Increased Qa-m7 antigen expression is characteristics of primitive hemopoietic progenitors in regenerating marrow J. Immunol., 139, 1096-1103
3. Bertoncello, I., Bradley, T.R., Hodgson, G.S. and Dunlop, J.M. (1991) The resolution, enrichment, and organization of normal bone marrow high proliferative potential colony-forming cell subsets on the basis of rhodamine-123 fluorescence Exp. Hematol., 19, 174-178
4. Erlich, S., Miranda, S.R.P., Visser, J.W.M., Dagan, A., Gatt, S. and Schuchman, E.H. (1999) Fluorescence-based selection of gene-corrected hematopoietic stem and progenitor cells from acid sphingomyelinase-deficient mice: implications for Niemann-Pick disease gene therapy and the development of improved stem cell gene transfer procedures Blood, 93, 80-86
5. Sitnicka, E., Ruscetti, F.W., Priestley, G.V., Wolf, N.S. and Bartelmez, S.H. (1996) The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells Blood, 88, 82-88
6. Hodohara, K., Fujii, N., Yamamoto, N. and Kaushansky, K. (2000) Stromal cell-derived factor-1 (SD-1) acts together with thrombopoietin to enhance the development of megakaryocytic progenitor cells (CFU-MK) Blood, 95, 769-775
7. Morgan, B., Sun, L., Avitahl, N., Andrikopoulos, K., Ikeda, T., Gonzales, E., Wu, P., Neben, S. and Georgopoulos, K. (1997) Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation EMBO J., 16, 2004-2013
8. Nibbs, R.J.B., Wylie, S.M., Pragnell, I.B. and Graham, G.J. (1997) Cloning and characterization of a novel murine chemokine receptor, D6 J. Biol. Chem., 272, 12495-12504
9. Tsai, S., Fero, J. and Bartelmez, S. (2000) Mouse jagged2 is differentially expressed in hematopoietic progenitors and endothelial cells and promotes the survival and proliferation of hematopoietic progenitors by direct cell-to-cell contact Blood, 96, 95-957
10. Weich, N.S., Fitzgerald, M., Wang, A., Calvetti, J., Yetz-Aldape, J., Neben, S. and Turner, K.J. (2000) Recombinant human interleukin-11 synergizes with steel factor and interleukin-3 to promote directly the early stages of murine megakaryocyte development in vitro Blood, 95, 503-509
11. Berkovic, D., Bensch, M., Bertram, J., Wille, T., Haase, D., Binder, C. and Fleer. E.A.M. (2001) Effects of hexadecylphosphocholine on thrombocytopoiesis Eur. J. Cancer, 37, 503-511
12. Nichogiannopoulou, A., Trevisan, M., Neben, S., Friedrich, C. and Georgopoulos, K. (1999) Defects in hemopoietic stem cell activity in Ikaros mutant mice J. Exp. Med., 190, 1201-1213
13. Rojnuckarin, P., Drachman, J.G. and Kaushansky, K. (1999) Thrombopoietin-induced activation of the mitogenactivated protein kinase (MAPK) pathway in normal megakaryocytes: role in endomitosis Blood, 94, 1273-1282
14. Hodohara, K., Fujii, N., Yamamoto, N. and Kaushansky, K. (2000) Stromal cell-derived factor-1 (SDF-1) acts together with thrombopoietin to enhance the development of megakaryocytic progenitor cells (CFU-MK) Blood, 95, 769-775
15. Geddis, A.E., Fox, N.E. and Kaushansky, K. (2001) Phosphatidylinositol 3-kinase is necessary but not sufficient for thrombopoietin- induced proliferation in engineered Mpl-bearing cell lines as well as in primary megakaryocytic progenitors J. Biol. Chem. 276, 34473-34479
16. Wang, Q., Miyakawa, Y., Fox, N. and Kaushansky, K. (2000) Interferon- directly represses megakaryopoiesis by inhibiting thrombopoietin- induced signaling through induction of SOCS-1 Blood, 96, 2093-2099
17. Carow, C.E., Fox, N.E. and Kaushansky, K. (2001) Kinetics of endomitosis in primary murine megakaryocytes J. Cell. Physiol., 188, 291-303
18. Whetton, A.D., Lu, Y., Pierce, A., Carney, L. and Spooncer, E. (2003) Lysophospholipids synergistically promote primitive hematopoietic cell chemotaxis via a mechanism involving Vav1 Blood, 102, 2798-2802
19. Evans, C.A., Tonge, R., Blinco, D., Pierce, A., Shaw, J., Lu, Y., Hanzah, H.G., Gray, A., Downes, C.P., Gaskell, S.J., Spooncer, E. and Whetton, A.D. (2004) Comparative proteomics of primitive hematopoietic cell populations reveals differences in expression of proteins regulating motility Blood, 103, 3751-3759
20. Unwin, R.D., Smith, D.L., Blinco, D., Wilson, C.L., Miller, C.J., Evans, C.A., Jaworska, E., Baldwin, S.A., Barnes, K., Pierce, A., Spooncer, E. and Whetton, A.D. (2006) Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells Blood, 107, 4687-4694
21. Dooner, M.S., Aliotta, J.M., Pimental, J., Dooner, G.J., Abedi, M., Colvin, G., Liu, Q., Weier, H-U., Johnson, K. and Quesenberry, P.J. (2008) Conversion potential of marrow cells into lung cells fluctuates with cytokine-induced cell cycle Stem Cells Devel., 17, 207-219
22. Broudy, V.C. and Lin, N.L. (2004) AMG531 stimulates megakaryopoiesis in vitro by binding to Mpl Cytokine, 25, 52-
23. Hepburn, M.D., Nagesh, K., Heppleston, A.D., Cachia, P.G. and Pippard, M.J. (2001) Timing of the appearance of multipotential and committed haemopoietic progenitors in peripheral blood after mobilization in patients with lymphoma Clin. Lab. Hematol., 23, 119-124
24. Zeng, F., Chen, M-J., Huang, W-Y., Yan, J-B., Xiao, Y-P., Gong, Z-J., Ren, Z-R. and Huang, S-Z. (2005) In utero transplantation of human hematopoetic stem cells into fetal goats under B-type ultrasonographic scan: an experimental model for the study of potential prenatal therapy Eur. J. Obstet. Gynecol. Reprod. Biol., 118, 170-173
25. Meister, B., Maurer, H., Simma, B., Kern, H., Ulmer, H., Hittmair, A. and Fink, F-M. (1997) The effect of recombinant human erythrpoietin on circulating hematopoietic progenitor cells in anemic premature infants Stem Cells, 15, 359-363
26. Yamamoto, Y., Yasumizu, R., Amou, Y., Watanabe, N., Nishio, N., Toki, J., Fukuhara, S. and Ikehara, S. (1996) Characterization of peripheral blood stem cells in mice Blood, 88, 445-454
27. Kurata, H., Mancini, G.C., Alespeiti, G., Migliaccio, A.R. and Migliaccio, G. (1998) Stem cell factor induces proliferation and differentiation of fetal progenitor cells in the mouse Br. J. Hematol., 101, 676-687
28. Barcena, A., Muench, M.O., Song, K.S., Ohkuba, T. and Harrison, M.R. (1999) Role of CD95/Fas and its ligand in the regulation of the growth of human CD34++CD38- fetal liver cells Exp. Hematol., 27, 1428-1439
29. Huie, M.A., Cheung, M-C., Muench, M.O., Becerril, B., Kan, Y.W. and Marks, J.D. (2001) Antibodies to human fetal erythroid cells from a non-immune phage antibody library Proc. Natl. Acad. Sci. USA, 98, 2682-2687
30. Shao, J., Stapleton, P.L., Lin, Y.S. and Gallagher, E.P. (2007) Cytochrome P450 and glutathione S-transferase mRNA expression in human fetal liver hematopoietic stem cells Drug Metab. Dispos., 35, 168-175
31. Porat, Y., Porozov, S., Belkin, D., Shimoni, D., Fisher, Y., Belleli, A., Czeiger, D., Silverman, W.F., Belkin, M., Battler, A., Fulga, V. and Savion, N. (2006) Isolation of an adult blood-derived progenitor cell population capable of differentiation into angiogenic, myocardial and neural lineages Br. J. Haematol., 135, 703-714
32. Porritt, H.E., Rumfelt, L.L., Tabrizifard, S., Schmitt, T.M., Zuniga-Pflucker, J.C. and Petrie, H.T. (2004) Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages Immunity, 20, 735-745
33. Tabrizifard, S., Olaru, A., Plotkin, J., Fallahi-Sichani, M., Livak, F. and Petrie, H.T. (2004) Analysis of transcription factor expression during discrete stages of postnatal thymocyte differentiation J. Immunol., 173, 1094-1102
34. Umland, O., Mwangi, W.N., Anderson, B.M., Walker, J.C. and Petrie, H.T. (2007) The blood contains multiple distinct progenitor populations with clonogenic B and T lineage potential J. Immunol., 178, 4147-4152
35. Muench, M., Cupp, J., Polakoff, J. and Roncarolo, M.G. (1994) Expression of CD33, CD38, and HLA-DR on CD34+ human fetal liver progenitors with a high proliferative potential Blood, 83, 3170-3181
36. Moore, B.E., Colvin, G.A., Dooner, M.S. and Quesenberry, P.J. (2005) Lineage-negative bone marrow cells travel bidirectionally in the olfactory migratory stream but maintain hematopoietic phenotype J. Cell. Physiol., 202, 147-152
37. Colvin, G.A., Dooner, M.S., Dooner, G.J., Sanchez-Guijo, F.M., Demers, D.A., Abedi, M., Ramanathan, M., Chung, S., Pascual, S. and Quesenberry, P.J. (2007) Stem cell continuum: Directed differentiation hotspots Expt. Hematol., 35, 96-107
38. Dooner, M.S., Aliotta, J.M., Pimental, J., Dooner, G.J., Abedi, M., Colvin, G., Liu, Q., Weier, H-U., Johnson, K. and Quesenberry, P.J. (2008) Conversion potential of marrow cells into lung cells fluctuates with cytokine-induced cell cycle Stem Cells Devel., 17, 207-219
39. Dooner, G.J., Colvin, G.A., Dooner, M.S., Johnson, K.W. and Quesenberry, P.J. (2008) Gene expression fluctuations in murine hematopoietic stem cells with cell cycle progression J. Cell. Physiol., 214, 786-795
40. Aliotta, J.M., Pereira, M., Johnson, K.W., de Paza, N., Dooner, M.S., Puente, N., Ayala, C., Brilliant, K., Berza, D., Lee, D., Ramratnam, B., McMillan, P.N., Hixson, D.C., Josic, D. and Quesenberry, P.J. (2010) Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription Exp. Hematol., 38, 233–245
41. Quesenberry, P.J., Dooner, G.J., Del Tatto, M., Colvin, G.A., Johnson, K. and Dooner, M.S. (2010) Expression of cell cycle–related genes with cytokine-induced cell cycle progression of primitive hematopoietic stem cells Stem Cells Devel., 19, 453-460
42. Hu, M.S., Huang, K-J., Li, S., Wu, J-C., Lo, D.D., Hyun, J.S., Chung, M.T., Hu, M., Longaker, M.T. and Lorenz, P. (2014) Blood-derived mesenchymal stem cells heal calvarial defects and promote wound healing J. Am. Coll. Surg., 219, S85-S86
43. Del Carmen Rodriguez, M., Bernad, A. and Aracil, M. (2004) Interleukin-6 deficiency affects bone marrow stromal precursors, resulting in defective hematopoietic support Blood, 103, 3349-3354
44. Franco, S., van de Vrugt, H.J., Fernandez, P., Aracil, M., Arwert, F. and Blasco, M.A. (2004) Telomere dynamics in Fancg-deficient mouse and human cells Blood, 104, 3927-3935
45. Brewer, G. J., Espinosa, J., McIlhaney, M. P., Pencek, T. P., Kesslak, J. P., Cotman, C., Viel, J. and McManus, D. C. (2001) Culture and regeneration of human neurons after brain surgery J. Neurosci. Meth., 107, 15-23
46. Tatebayashi, Y., Iqbal, K. and Grundke-Iqbal, I. (1999) Dynamic regulation of expression and phosphorylation of Tau by fibroblast growth factor-2 in neural progenitor cells from adult rat hippocampus J. Neurosci., 19, 5245-5254
47. Tatebayashi, Y., Haque, N., Tung, Y-C., Iqbal, K. and Grundke-Iqbal, I. (2004) Role of tau phosphorylation by glycogen synthase kinase-3 in the regulation of organelle transport J. Cell Sci., 117, 1653-1663
48. Chen, H., Tung, Y-C., Li, B., Iqbal, K. and Grundke-Iqbal, I. (2007) Trophic factors counteract elevated FGF-2- induced inhibition of adult neurogenesis Neurobiol. Aging, 28, 1148-1162
49. Staquicini, F.I., Dias-Neto, E., Li, J., Snyder, E.Y., Sidmanb, R.L., Pasqualini, R. and Arap, W. (2009) Discovery of a functional protein complex of netrin-4, laminin 1 chain, and integrin 61 in mouse neural stem cells Proc. Natl. Acad. Sci. USA, 106, 2903–2908
50. Chohan, M.O., Li, B., Blanchard, J., Tung, Y-C., Heaney, A.T., Rabe, A., Iqbal, K. and Grundke-Iqbal, I. (2011) Enhancement ofdentate gyrus neurogenesis, dendritic and synaptic plasticity and memory by aneurotrophic peptide Neurobiol. Aging, 32, 1420-1434
51. Abbosh, C., Lawkowski, A., Zaben, M. and Gray, W. (2011) GalR2/3 mediates proliferative and trophic effects of galanin on postnatal hippocampal precursors J. Neurochem. (2011) 117, 425–436
52. He. P. and Shen, Y. (2009) Interruption of -catenin signaling reduces neurogenesis in Alzheimer’s disease J. Neurosci., 29, 6545– 6557
53. Nunan, R., Sivasathiaseelan, H., Khan, D., Zaben, M. and Gray, W. (2014) Microglial VPAC1R mediates a novel mechanism of neuroimmune-modulation of hippocampal precursor cells via IL-4 release Glia, 62, 1313–1327
54. Yovchev, M.I., Dabeva, M.D. and Oertel, M. (2013) Isolation, characterization, and transplantation of adult liver progenitor cells In Methods Mol. Biol., 976, Stem Cells and Aging: Methods and Protocols, (ed. Tursen, K.) Springer Science+Business Media, LLC pp 37-51
OptiPrep™Application Sheet C24; 8th edition, January 2020
OptiPrep™ Application Sheet C25
Hepatic non-parenchymal (stellate, Kupffer and endothelial) cells – a short methodological survey
- This Application Sheet provides a review of the density gradient methods for purification of the three main types of non-parenchymal cells (stellate, Kupffer and sinusoidal endothelial).
- The companion Reference Lists RC07 and RC08 provide complete lists of publications that report the use of OptiPrep™. RC07 is devoted entirely to stellate cells, while RC08 provides similar information for both Kupffer and sinusoidal endothelial cells. RC08 also covers, nonparenchymal epithelial cells, NK cells, oval cells and progenitor cells.
1. Total non-parenchymal cell fraction
An essential preliminary procedure in the isolation of the total non-parenchymal cells (NPC) from liver is the disaggregation of the liver tissue by enzymic perfusion. The aim of collagenase digestion (Method 1) of the liver is to release both NPC and parenchymal cells (PC) as intact cells. In a modified perfusion strategy, the liver is perfused with a mixture of collagenase and Pronase or Clostridium perfringens enterotoxin (Method 2), which destroys the PC selectively [1,2].
In Method 1 the bulk of the more rapidly sedimenting PC may then separated from the NPC by repeated differential pelleting at 50 g for 1-4 min. Although this method is simple, NPC yield is usually low. It is both more common and more effective to carry out the 50 g centrifugation once; to harvest all the cells from the supernatant by centrifugation at a higher g-force and then use a density barrier (prepared from OptiPrep™) to resolve the two types of cell. A common approach is to adjust the density of the cell suspension to approx 1.071 g/ml; this allows the NPC to float to the top and the PC and residual erythrocytes to pellet during the centrifugation [3,4]. In Method 2, the digest is often adjusted to a higher density (approx. 1.096 g/ml) to allow the NPC to float to the top. A recent detailed procedure described the use of equal volumes (20 ml) of 8.2% and 17.6% (w/v) iodixanol [5]. The NPCs are suspended in the denser layer. After centrifugation at 1400 g for 30 min, the top 15ml was discarded and the NPCs recovered in from the interface in the remaining low density solution.
- Detailed methods to purify a total NPC fraction are described in OptiPrep™ Application Sheet C26
- A total NPC fraction is often a starting point for purification of Kupffer cells and/or endothelial cells by non-density gradient methods (adherence to a substratum or centrifugal elutriation).
- Stellate cells possess a distinctively low density and are successfully purified simply by modifying the format of the NPC flotation gradient (see Section 5).
2. Kupffer cells
The discontinuous gradient used to prepare the initial NPC fraction varies considerably; for example flotation through a 1.080-1.090 g/ml layer [6] or sedimentation on a two-layer gradient of 1.066 and 1.097 g/ml [7,8]. Further purification is then achieved by suspension of the interfacial cells in DMEM supplemented with 10% fetal bovine serum and 1% L-glutamine (plus the usual antibiotics) and adherence to culture dishes coated with coated with 2.5% glutaraldehyde-fixed BSA [9] or to non-collagen coated plates [10] or by elutriation [7]. The adherence method can provide a greater than 90% purity for Kupffer cells [11].
- More information about the density gradient systems may be obtained from OptiPrep™ Application Sheet C15. The latter is an Application Sheet that provides suggested gradient methods that might be applied to a variety of mixed cell types.
- A recent paper [12] reported the use of a more sophisticated gradient that avoided the need for the subsequent elutriation. In a five layer iodixanol gradient of 24%, 17%, 11.5%, 8.4% and 0% (w/v) iodixanol (total NPCs in the 24% layer) the Kupffer cells banded at the 8.4%/11.5% interface.
3. Sinusoidal endothelial cells
Generally the gradients used for the NPC isolation are similar to those described in Sections 1 and 2, Further purification is then achieved by suspension of the cells in DMEM supplemented with 10% fetal bovine serum and 1% L-glutamine (plus the usual antibiotics) and adherence to culture dishes coated with human fibronectin (1 mg/cm2) [9], by elutriation [3,4], using magnetic beads [13] or by flow cytometry [14].
4 Progenitor cells
Grozdanov et al [15] harvested the NPC from the interface between a two-layer 1.063 and 1.079 g/ml gradient and progenitor cells were subsequently identified by flow cytometry.
- More information about the density gradient systems for the isolation of progenitor cells from a variety of tissues may be obtained from OptiPrep™ Application Sheet C24; this can be accessed via the website www.Optiprep.com. Click on “Methodology” then “Mammalian and non-mammalian cells” and follow the links from the Index.
 5. Stellate cells
5. Stellate cells
Stellate cells are perhaps the most widely studied type of hepatic cell, particularly with regard to their differentiation into myofibroblasts that occurs in liver fibrosis. Being the least dense of the NPC, these cells are regularly isolated by flotation from a dense medium. The method described by Borouwer et al [2] initially used NycodenzⓇ, but it was subsequently adapted to iodixanol. Of the two methods described in Figure 1, the format described in the lower panel has the advantage of banding the stellate cells at an interface separated from any unpelleted residual material originally present in the sample layer.
- For more information about the methods for the isolation of stellate cells from liver and pancreas may be obtained from OptiPrep™ Application Sheet C27; this can be accessed via the following website www.Optiprep.com Click om “Methodology” then “Mammalian and nonmammalian cells” and follow the links from the Index.
Figure 1 Separation of stellate cells by flotation Solution A is the solution used to suspend the stellate cells (SC). See text for more information
6 References
1. Boyum, A., Berg, T. and Blomhoff, R. (1983) Fractionation of mammalian cells In Iodinated density gradient media – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 147-171
2. Brouwer, A., Hendricks, H. F. J., Ford, T. and Knook, D. L. (1991) Centrifugation separations of mammalian cells In Preparative centrifugation – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 271-314
3. Nedredal, G. I., Elvevold, K. H., Ytrebo, L. M., Olsen, R., Revhaug, A. and Smedsrod, B. (2003) Liver sinusoidal endothelial cells represent an important blood clearance system in pigs Comp. Hepatol., 2:1.
4. Elvevold, K.H., Nedredal, G.I., Revhaug, A. and Smedsrød, B. (2004) Scavenger properties of cultivated pig liver endothelial cells Comp. Hepatol., 3, 1-11
5. Meyer, J., Lacotte, S., Morel, P., Gonelle-Gispert, C. and Bühler, L. (2016) An optimized method for mouse liver sinusoidal endothelial cell isolation Exp. Cell Res., 349, 291–301
6. Zelnickova, P., Matiasovic, J., Pavlova, B., Kudlackove, H., Kovaru, F. and Faldyna, M. (2008) Quantitative nitric oxide production by rat, bovine and porcine macrophages Nitric Oxide, 19, 36-41
7. Valatas, V., Xidakis, C., Roumpaki, H., Kolios, G. and Kouroumalis, E.A. (2003) Isolation of rat Kupffer cells: a combined methodology for highly purified primary cultures Cell Biol Int., 27, 67-73
8. Kolios, G., Valatas, V., Manousou, P., Xidakis, C., Notas, G. and Kouroumalis, E. (2008) Nitric oxide and MCP-1 regulation in LPS activated rat Kupffer cells Mol. Cell. Biochem., 319, 91-98
9. Malerød, L., Juvet, L.K., Gjøen, T. and Berg, T. (2002) The expression of scavenger receptor class B, type I (SR-BI) and caveolin-1 in parenchymal and nonparenchymal liver cells Cell Tissue Res., 307, 173-180
10. Hu, S., Yin, S., Jiang, X., Huang, D. and Shen, G. (2009) Melatonin protects against alcoholic liver injury by attenuating oxidative stress, inflammatory response, and apoptosis Eur. J. Pharmacol., 616, 287–292
11. Banerjee, A., Abdelmegeed, M.A., Jang, S. and Song, B-J. (2015) Increased sensitivity to binge alcoholinduced gut leakiness and inflammatory liver disease in HIV transgenic rats PLoS One, 10: e0140498
12. Schreiber, R., Taschler, U., Wolinski, H., Seper, A., Tamegger, S.N., Graf, M., Kohlwein, S.D., Haemmerle, G., Zimmermann, R., Zechner, R. and Lass, A. (2009) Esterase 22 and beta-glucuronidase hydrolyze retinoids in mouse liver J. Lipid Res., 50, 2514–2523
13. Connolly, M.K., Bedrosian, A.S., Malhotra, A., Henning, J.R., Ibrahim, J., Vera, V., Cieza-Rubio, N.E., Hassan, B.U., Pachter, H.L., Cohen, S., Frey, A.B. and Miller, G. (2010) In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity J. Immunol., 185, 2200–2208
14. Connolly, M.K., Mallen-St. Clair, J., Bedrosian, A.S., Malhotra, A., Vera, V., Ibrahim, J., Henning, J., Pachter, H.L., Bar-Sagi, D., Frey, A.B. and Miller, G. (2010) Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and pre-invasive intra-abdominal tumor J. Leukoc. Biol., 87, 713–725
15. Grozdanov, P.N., Yovchev, M.I. and Dabeva, M.D. (2006) The oncofetal protein glypican-3 is a novel marker of hepatic progenitor/oval cells Lab. Invest., 86, 1272-1284
OptiPrep™ Application Sheet C25; 5th edition, February 2020
OptiPrep™ Application Sheet C26
Purification of hepatic non-parenchymal cells on a density barrier
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Application Sheet C25 is a short introductory survey that compares some of the established methodologies for hepatic cells.
- There are also two related Application Sheets: C27 “Preparation of stellate cells from liver and pancreas” and C28 – “Enrichment of hepatic Kupffer cells in a discontinuous gradient” see index.
- To access other Application Sheets referred to in the text, return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box.
1. Background
Parenchymal and non-parenchymal cells (PC and NPC) may be prepared by collagenase digestion of the liver using a tissue perfusion system. The PC are then separated from the NPC by differential pelleting at 50 g for 1-4 min. It is however necessary to repeat this centrifugation (maybe twice more) to remove PC from the supernatant; moreover the NPC yield is usually low. It is both more common and more effective to carry out the 50 g centrifugation once; to harvest all the cells from the supernatant by centrifugation at a higher g-force and then use a density barrier prepared from one of the of iodinated density gradient media to resolve the two types of cell. Many workers prefer a modified perfusion strategy, which involves a mixture of collagenase and Pronase or Clostridium perfringens enterotoxin to destroy the PC selectively [1,2].
The purification of NPC is a starting point for the isolation of Kupffer and endothelial cells; the NPC are then processed further by centrifugal elutriation to obtain reasonably pure populations of these important cells, although there are instances of the reverse situation in which the elutriation is the first step (e.g. ref. 3). Although metrizamide was widely used for the density barrier step prior to 1984, NycodenzⓇ subsequently became more popular (over 200 papers) because of its lower toxicity to cells. More recently iodixanol gradients have become a popular choice and because of the ease of gradient solution preparation from OptiPrep™, the  following methodology is based solely on use of this medium.
following methodology is based solely on use of this medium.
- Note that two-layer discontinuous gradients are able to improve the purity of Kupffer cells, see Application Sheet C28.
2. Solution selection and preparation
2a. Buffer salt solution
A crude NPC preparation may be suspended in a routine balanced salt solution such as Hank’s Balances Salt Solution (HBSS) or more often this is an NPC customized medium such as Gey’s balanced salt solution and this may be prepared as described in the box. The chosen solution is also used in the preparation of the gradient solutions. Unless the solution contains 10% serum, the density of any balanced salt solution (or culture medium) is likely to be very similar, if not identical, to GBSS or HBSS (i.e. approx 1.006 g/ml). A medium containing 10% serum has a density of approx 1.009 g/ml and the amount of iodixanol should be adjusted to account for this.
- For more information on the preparation of gradient solutions see Application Sheet C01.
2b. Gradient solution preparation
The rationale regarding the preparation of iodixanol solutions is given in Application Sheet C01. A major advantage of the use of this medium over NycodenzⓇ is the ease of solution preparation; for NPC enrichment OptiPrep™ is simply diluted with either HBSS or GBSS to give the appropriate density and all the solutions of any density will be isoosmotic. NycodenzⓇ and iodixanol solutions of the same % (w/v) have almost identical densities. It is highly likely that iodixanol can substitute directly for NycodenzⓇ in any protocol if the latter solutions are isoosmotic, but as far as we know, direct comparisons between the efficacies of the two solutes have not been made. Therefore, for completeness, some NycodenzⓇ variants are described.
3. Protocols
3a. Density barrier format
The crude cell suspension may be layered over a barrier of the chosen density or the suspension may be adjusted to the chosen density and a small volume of GBSS or HBSS layered on top (this is often termed a “Mixer Format”). In the former all the cells will sediment to or through the barrier; in the latter cells will float to the interface; remain in the barrier or sediment to form a pellet. The layer of salt solution in the second format prevents the floating cells from banding at an air/liquid interface and aids recovery of the cell layer. Most centrifugations are carried out at 4°C.
3b. Removal of erythrocytes and cell debris from a crude NPC fraction Protocol 3b-1 (all iodixanol concentrations are %, w/v)
1. Layer the NPC suspension over a barrier of ρ = 1.14-1.16 g/ml; published methods report the use of 28% [4] or 24% [5] iodixanol. Higher NycodenzⓇ concentrations (approx 29%) have also been used [6-8].
2. Centrifuge to band the NPC at the interface; a wide range of conditions have been used – 5 min at 310 g [4], 15 min at 500 g [5] and 15 min at 1500 g [6].
Protocol 3b-2
1. Mix the NPC suspension with OptiPrep™ (volume ratio of 3.4:2.6 respectively).
2. Layer 0.5-1.0 ml of GBSS on top; centrifuge at 400 g for 15 min [9] and collect the cells at the interface.
3c. Purification of NPC
Because of the simplicity of using OptiPrep™ in a Mixer Format, the following protocols describe the use of this strategy. Some protocol variations and comments, together with a brief summary of the major NycodenzⓇ based methods are given in the Section 3c-3.
3c-1. Collagenase-dispersed cells (adapted from refs 10 and 11)
1. Prepare a 12.6% iodixanol solution: mix OptiPrep™ with any balanced salt solution or culture medium (12.6 vol. + 47.4 vol. respectively).
2. Centrifuge a suspension of the collagenase-dispersed cells at 50 g for 3 min.
3. Recover the supernatant and centrifuge it again at 50 g.
4. Recover the supernatant and centrifuge it at 850 g for 10 min.
5. Remove and discard all of the supernatant and resuspend the pellet in the 12.6% iodixanol.
6. Layer 1-2 ml of balanced salt solution or culture medium on top and centrifuge at 3300 g for 30 min to pellet the residual PC cells and erythrocytes.
7. Allow the rotor to decelerate without the brake.
8. Harvest the non-PC cells from top of the barrier.
3c-2. Collagenase/Pronase-dispersed cells (adapted from refs 1 and 2)
1. Make a solution of 40% iodixanol: mix 4 vol. of OptiPrep™ and 2 vol. of GBSS.
2. Suspend the crude non-parenchymal cells in GBSS.
3. Mix 40% iodixanol with the cell suspension, thoroughly (but gently) so that the final concentration
of iodixanol is 17% (w/v) iodixanol solution (ρ = 1.096 g/ml).
4. Layer 1-2 ml of GBSS on top and centrifuge at 400 g for 15 min at 20°C.
5. Allow the rotor to decelerate without the brake.
6. Collect the non-PC cells, which band at the interface between GBSS and the 17% iodixanol layer.
3c-3. Protocol variations
OptiPrep™
Note that the two protocols (3c-1 and 3c-2) describe two ways of processing the sample. In 3c-1 the cells are sedimented as a pellet and then resuspended in the solution of the required density, while in 3c-2 this pelleting step is omitted and the suspension mixed with a high-density solution. In some instances the cell suspension is simply mixed with OptiPrep™ rather than a 40% iodixanol working solution. The final concentrations of iodixanol are variable, e.g. 11.5% [12] 13.2%, [13,14] and 16.8% [15]. A wide variation in centrifugation conditions has been reported, from 200 g for 20 min to 1500 g for 25 min.
A rather more sophisticated gradient was recommended by Yovchev et al [16]. OptiPrep is diluted with a HEPES- buffered saline, pH 7.4 containing 0.2% bovine serum albumin to produce solutions of 11%, 13%, 16% and 18% (w/v) iodixanol. The NPC pellets were suspended in 11% iodixanol and each 10 ml of suspension was underlayered with 10 ml each of the 13% and 16% iodixanol solutions and 5 ml of 18% iodixanol. The gradients were centrifuged at 6,500 g for 30 min and were allowed to decelerate without the brake. NPC’s banded at the 11-13% and 13%-16% interfaces. The lower density interface was particularly enriched in stellate and oval cells. For full details see ref 16.
NycodenzⓇ
The NycodenzⓇ literature reveals use of both the more traditional layering of the crude fraction over a density barrier and also the Mixer Format. NycodenzⓇ density barriers of approx 15.7% [17,18], 16% [19] and 16.75% [20-22] are commonly used; centrifugation conditions vary from 600- 1700 g for 15-20 min. In the Mixer Format 28-30% NycodenzⓇ has been added to the cell suspension to adjust it to 15.8% [23-24], 16.7% [25-28] or 17.5% [29,30]; the centrifugation conditions are generally 1500-1700 g for 15-20 min.
4. References
1. Boyum, A., Berg, T. and Blomhoff, R. (1983) Fractionation of mammalian cells In Iodinated density gradient media – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 147-171
2. Brouwer, A., Hendricks, H. F. J., Ford, T. and Knook, D. L. (1991) Centrifugation separations of mammalian cells In Preparative centrifugation – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 271-314
3. Gaustad, R., Berg, T. and Fonnum, F. (1992) Heterogeneity of carboxylesterases in rat liver cells Biochem. Pharmacol., 44, 827-829
4. Malerød, L., Juvet, L.K., Gjøen, T. and Berg, T. (2002) The expression of scavenger receptor class B, type I (SR-BI) and caveolin-1 in parenchymal and nonparenchymal liver cells Cell Tissue Res., 307, 173-180
5. Shao, B., Lu, M., Katz, S.C., Varley, A.W., Hardwick, J., Rogers, T.E., Ojogun, N., Rockey, D.C., DeMatteo, R.P. and Munford, R.S. (2007) A host lipase detoxifies bacterial lipopolysaccharides in the liver and spleen J. Biol. Chem., 282, 13726-13735
6. Hong, G., Bazin-Redureau, M., Gires, P. and Scherrmann, J.M. (1998) Hepatic disposition and toxicity of cationized goat immunoglobulin G and Fab fragments in isolated perfused rat liver Drug. Metab. Dispos., 26, 661-669
7. Milosevic, N., Schawalder, H. and Maier, P. (1999) Kupffer cell-mediated differential down-regulation of cytochrome P450 metabolism in rat hepatocytes Eur. J. Pharmacol., 368, 75-87
8. Upadhya, G.A. and Strasberg, S.M. (1999) Evidence that actin disassembly is a requirement of matrix metalloproteinase secretion by sinuosoidal endothelial cells during cold preservation in the rat Hepatology, 30, 169-176
9. Katz, S.C., Pillarisetty, V.G., Bleier, J.I., Shah, A.B. and DeMatteo, R.P. (2004) Liver sinusoidal endothelial cells are insufficient to activate T cells J. Immunol., 173, 230-235
10. Nedredal, G. I., Elvevold, K. H., Ytrebo, L. M., Olsen, R., Revhaug, A. and Smedsrod, B. (2003) Liver sinusoidal endothelial cells represent an important blood clearance system in pigs Comp. Hepatol., 2:1.
11. Elvevold, K.H., Nedredal, G.I., Revhaug, A. and Smedsrød, B. (2004) Scavenger properties of cultivated pig liver endothelial cells Comp. Hepatol., 3, 1-11
12. Zhu, J., Huang, X. and Yang, Y. (2007) Innate immune response to adenoviral vectors is mediated by both toll-like receptor-dependent and -independent pathways J. Virol., 81, 3170-3180
13. Kuniyasu, Y., Qamar, A., Sheikh, S.Z., Jhandier, M.N., Hakim, W. and Mehal, W.Z. (2005) Blocking intrahepatic deletion of activated CD8+ T cells by an altered peptide ligand Cell. Immunol., 238, 31-37
14. Klein, I., Cornejo, J.C., Polakos, N.K., Beena, J., Wuensch, S.A., Topham, D.J., Pierce, R.H. and Crispe, N.J. (2007) Kupffer cell heterogeneity: functional properties of bone marrow-derived and sessile hepatic macrophages Blood, 110, 4077-4085
15. Zelnickova, P., Matiasovic, J., Pavlova, B., Kudlackove, H., Kovaru, F. and Faldyna, M. (2008) Quantitative nitric oxide production by rat, bovine and porcine macrophages Nitric Oxide, 19, 36-41
16. Yovchev, M.I., Dabeva, M.D. and Oertel, M. (2013) Isolation, characterization, and transplantation of adult liver progenitor cells In Methods Mol. Biol., 976, Stem Cells and Aging: Methods and Protocols, (ed. Tursen, K.) Springer Science+Business Media, LLC pp 37-51
17. Knolle, P., Schlaak, J., Uhrig, A. Kempf, P. Meyer zum Büschenfelde, K.H. and Gerken, G. (1995) Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge J. Hepatol., 22, 226-229
18. Schmitz, F., Bresciani, R., Hartmann, H. and Braulke, T. (1995) Effect of insulin-like growth factor II on uptake of arylsulfatase A by cultured rat hepatocytes and Kupffer cells J. Hepatol., 22, 356-363
19. Yoshioka, M., Nakajima, Y., Ito, T. Mikarni, O. Tanaka, S., Miyazaki, S. and Motoi, Y. (1997) Primary culture and expression of cytokine mRNAs by lipopolysaccharide in bovine Kupffer cells Vet. Immunol. Immunopathol., 58, 155-163
20. Petermann, H., Heymann, S., Vogl, S. and Dargel, R. (1966) Phagocytic function and metabolite production in thioacetamide-induced liver cirrhosis: a comparative study in perfused livers and cultured Kupffer cells J. Hepatol., 24, 468-477
21. Petermann, H., Vogl, S., Schulze, E. And Dargel, R. (1999) Chronic liver injury alters basal and stimulated nitric oxide production and 3H-thymidine incorporation in cultured sinusoidal endlthelial cells from rats J. Hepatol., 31, 284-292
22. Novosyadlyy, R., Dargel, R. and Scharf, J-G. (2005) Expression of insulin-like growth factor-I ans insulinlike growth factor binding proteins during thioacetamide induced liver cirrhosis in rats Growth Horm. IGF Res., 15, 313-323
23. Ten Hagen, T.L.M., Van Vianen, V. and Bakker-Woudenberg, I.A.J.M. (1996) Isolation and characterization of murine Kupffer cells and splenic macrophages J. Immunol. Meth., 193, 81-91
24. Luedde, T., Assmus, U., Wüstefeld, T., zu Vilsendorf, A.M., Roskams, T., Schmidt-Supprian, M., Rajewsky, K., Brenner, D.A., Manns, M.P., Pasparakis, M. and Trautwein, C. (2005) Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis but protects from ischemia/reperfusion injury J. Clin. Invest., 115, 849-859
25. Juillerat, M., Marceau, N., Coeytaux, S., Sierra, F., Kolodziejczyk, E. and Guigoz, Y. (1997) Expression of organ-specific structures and functions in long-term cultures of aggregates from adult rat liver cells Toxicol. in Vitro, 11, 57-69
26. Pestel, S., Jungermann, K., Götze, O. and Schieferdecker, L. (2002) Inhibition by prostaglandin E2 of anaphylatoxin C5a-but not zymosan-induced prostanoid release from rat Kupffer cells Lab. Invest., 82, 463- 471
27. Pestel, S., Schlaf, G., Götze, O., Jungermann, K. and Schieferdecker, H.L. (2003) Differences in the involvement of prostanoids from Kupffer cells in the meditation of anaphylylatoxin C5a-, zymosan-, and lipopolysaccharide-dependent hepatic glucose output and flow reduction Lab. Invest., 83, 1733-1741
28. Pestel, S., Jungermannm, K. and Schieferdecker, H.L. (2005) Re-evaluation of thin layer chromatography as an alternative method for the quantification of prostaglandins from rat Kupffer cells Prostaglandins Other Lipid Mediat., 75, 123-139
29. De Rijke, Y.B., Jurgens, G., Hessels, e.M., Hermann, A. and van Berkel, T.J. (1992) In vivo fate and scavenger receptor recognition of oxidized lipoprotein (a) isoforms in rats J. Lipid Res., 33, 1315-1325
30. Ling, W., Lougheed, M., Suzuki, H., Buchan, A., Kodama, T., Steinbrecher, U.P. (1997) Oxidized or acetylated low density lipoproteins are rapidly cleared by the liver in mice with disruption of the scavenger receptor class A type I/II gene J. Clin. Invest., 100, 244-252
OptiPrep™ Application Sheet C26; 8th edition, January 2020
OptiPrep™ Application Sheet C27
Preparation of stellate cells from liver and pancreas
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml Optiprep™ Application Sheet C26 “Purification of hepatic non-parenchymal cells on a density barrier” compares some of the methodologies for purifying these cells.
- Optiprep™ Reference List RC07 “Hepatic and pancreatic stellate cells” provides a comprehensive bibliography of all the published papers reporting the use of
- OptiPrep™ for the isolation of these cells
- To access the Reference List return to the initial list of Folders and select “Reference Lists”
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
Hepatic stellate cells represent up to 15% of the total liver cells and their ability to transdifferentiate into myofibroblast-like cells is regarded as a key event in hepatic fibrosis. There is therefore considerable clinical research interest in the isolation of these cells; after blood leukocytes and dendritic cells they are perhaps the most widely studied of any mammalian cell. Stellate (sometimes called fat-storing or Ito) cells are the least dense of the non-parenchymal cells (NPC) and unlike the other NPC (Kupffer cells and endothelial cells), they can be effectively purified to 90-95% purity using a simple density barrier or two-step discontinuous gradient. The latter is widely used in studies requiring purification of both stellate and Kupffer cells and is described in Application Sheet C26 – see index. Pancreatic stellate cells also mediate fibrosis in chronic pancreatitis and are also the least dense cell in the tissue; very similar gradient strategies are therefore used in their isolation.
2. Preparation of the cell suspension
The detailed methodology in this Application Sheet is confined to the density barrier separation; methods for preparing the tissue cell digests, which are probably well established in the laboratory, are merely summarized below.
2a. Liver digestion
Parenchymal cells are routinely prepared by collagenase digestion of the liver using a tissue perfusion system. These cells are then separated from the non-parenchymal cells by differential pelleting at 50 g for 1-4min. Although the non-parenchymal cells can be isolated from the 50 g supernatant, the yields are usually low. The most widely used procedure is to perfuse the liver with a mixture of collagenase and Pronase or enterotoxin to destroy the parenchymal cells selectively [1,2].
2b. Pancreas digestion
The preparation of cell suspensions from pancreas follows more traditional lines of digesting the dissected and finely minced tissue with Pronase and collagenase at 37°C for 30 min before filtering through a nylon on stainless steel mesh [3].
2c. Cell suspension
The cells are normally pelleted from the crude suspension and maybe washed once or twice prior to density barrier separation in order to remove any residual enzymes and/or endotoxin. Deoxyribonuclease I may also be added to degrade any DNA released from damaged cells, which would otherwise cause aggregation of the cells. Cells are suspended in an isoosmotic salt solution; either a general-purpose medium such as Hanks Balanced Salt Solution (HBSS), which may be supplemented with Ca2+ if required, or a customized medium: Gey’s Balanced Salt Solution (GBSS) is routinely used for hepatic cells.
3. Gradient selection
NycodenzⓇ
Since 1986 NycodenzⓇ has been widely used for the purification of hepatic stellate cells. One of the simplest methods, which was first described in 1987 by Schäfer et al [4] involved the addition of an isoosmotic solution of 28.7% (w/v) NycodenzⓇ to a suspension of NPC in order to raise its density to approx 1.072 g/ml (13.2% NycodenzⓇ). A layer of balanced salt solution is layered on top and after the centrifugation stellate cells are recovered from just above the interface. Subsequently Gressner and Zerbe [5] reduced the density to approx 1.049 g/ml (8.2% NycodenzⓇ) to improve the purity and the latter has been widely used (e.g. refs 6-9). The final concentration of Nycodenz in the cell suspension may also be as high as 14.35% [10]. In some cases the NPC suspension is layered over a NycodenzⓇ cushion, which may be 13% [11], 9% [12] or 8.2% [13]. In few instances the crude cell suspension is layered on a discontinuous gradient of 8.2% and 15.6 % (w/v) NycodenzⓇ [14,15] but this gradient format is normally reserved for the purification of stellate cells and Kupffer cells.
The purification of pancreatic stellate cells using NycodenzⓇ is almost exclusively carried out according to the methods for hepatic cells [4,5] and was first described by Apte et al [16]. Generally the concentration to which the crude cell suspension is adjusted is approx. 11.4% (w/v) [e.g. refs 16- 19] but there are variations: 12% [20], 13.2% [21] and 15.1% [22].
 OptiPrep™
OptiPrep™
Since 1998 OptiPrep™ has also been used for the isolation of stellate cells from both liver and pancreas; Bachem et al [23] published the first paper in which the cell suspension was layered over a density barrier and Peterson and Rowden [24] used a multi-step discontinuous gradient of 5%, 10%, 20% and 25% iodixanol. The strategy of adjusting the density of the NPC suspension to a density just higher than that of the stellate cells (as with NycodenzⓇ) has also become popular. Also as with NycodenzⓇ, the final density of the liquid has tended to be reduced, examples as low as 1.045 g/ml (equivalent to an iodixanol concentration of 7.2% w/v) have been reported [3].
4. Methodological options
Because of the ease of use of OptiPrep™ the following methodology is based on the use of this medium. The
preparation of a suitable NycodenzⓇ stock solution and its use are however given in Section 7. Only the flotation strategy is given, since this is well established as the method of choice in the isolation of the least dense particle from a mixture of predominantly denser particles. A variant of the regular flotation method (Strategy A) is given in Strategy B, in which the low-density resolving solution is layered upon the NPC suspension adjusted to 1.084 g/m/l (see Figures 1 and 2).
Strategy 2 is the preferred one since the stellate cells are separated from the sample by a “clean” resolving layer; they are thus completely divorced from the denser cells and any residual soluble components such as the digesting enzymes and any cytoplasmic material released from broken cells. This strategy is widely used in the purification of pancreatic islets and of dendritic cells from mouse spleen, thymus and lymph nodes.
 5. Solutions required
5. Solutions required
A. Gey’s balanced salt solution (GBSS) or Hank’s Balanced Salt Solution, with or without Ca2+/Mg2+ (see Note 1).
B. OptiPrep™ (shake the bottle gently before use)
C. Iodixanol (40% (w/v)) working solution: mix 4 vol. of Solution B and 2 vol. of Solution A (see Note 2).
6. Protocol
Carry out all operations at 4°C.
6b. Strategy A
1. Mix Solution C with Solution A so that the final concentration of iodixanol is 8.0-11.5% (w/v) iodixanol solution (ρ = 1.050-1.065 g/ml) and use this to suspend the final washed cell pellet. Alternatively suspend the cells in Solution A and mix with Solution C to produce an 8.0-11.5% iodixanol suspension (see Note 3).
2. Transfer 10-20 ml to a centrifuge tube and layer 8-10 ml of Solution A on top (see Note 4).
3. Centrifuge at 1400 g for 15-20 min; allow the rotor to decelerate without the brake (see Note 5).
4. Collect the cells, which band at the interface between Solution A and the sample (see Figure 1).
6c. Strategy B
1. Mix Solution C with Solution A so that the final concentration of iodixanol is 15% (w/v) iodixanol solution (ρ = 1.084 g/ml) and use this to suspend the final washed cell pellet (see Notes 6 and 7).
2. Dilute Solution C with Solution A to produce a solution containing 8.0-11.5% (w/v) iodixanol ( = 1.050-1.065 g/ml) (see Note 3).
3. Layer 5-10 ml of this solution over the same volume of cell suspension (in 15% iodixanol); then layer approx 5 ml of Solution A on top (see Note 8).
4. Centrifuge at 1400 g for 15-20 min; allow the rotor to decelerate without the brake (see Note 5).
5. Collect the cells, which band at the interface between Solution A and the low-density barrier (see Figure 2).
7. Notes
1. Any medium, compatible with the cells, may be used. For more information about the preparation of density solutions for cells see Application Sheet C01.
2. NycodenzⓇ stock solution option: Make up Solution A without the NaCl. Place 50 ml of this in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 28.7 g of NycodenzⓇ powder in small amounts until dissolved. Allow the solution to cool to room temperature and then make up to 100 ml with Solution A (minus NaCl). Filter sterilize if required. Dilute it with Solution A (complete) to produce solutions of the same concentration as the iodixanol solutions described in either Strategy A or B.
3. Use whichever concentration of iodixanol (or NycodenzⓇ) that provides the optimal recovery and purity of stellate cells.
4. Alternatively layer the cell suspension under Solution A using a syringe and metal cannula.
5. Use of the brake causes vortex formation in the liquid and mixing of the contents.
6. The actual density of this cell layer is not particularly critical, so long as it is dense enough to support the low-density solution.
7. In the two-layer method described by Brouwer et al [2] and others, the cell suspension was placed in the low-density layer.
8. Alternatively layer the cell suspension under the lower density solution using a syringe and metal cannula.
8. References
1. Boyum, A., Berg, T. and Blomhoff, R. (1983) Fractionation of mammalian cells In Iodinated density gradient media – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 147-171
2. Brouwer, A., Hendricks, H. F. J., Ford, T. and Knook, D. L. (1991) Centrifugation separations of mammalian cells In Preparative centrifugation – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 271-314
3. Shek, F. W-T., Benyon, R. C., Walker, F. M., McCrudden, P. R., Pender, S. L. F., Williams, E. J., Johnson, P. A., Johnson, C. D., Bateman, A. C., Fine, D. R. and Iredale, J. P. (2002) Expression of transforming growth factor-1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis Am. J. Pathol., 160, 1787-1798
4. Schafer, S., Zerbe, O. and Gressner, A.M. (1987) The synthesis of proteoglycans in fat-storing cells of rat liver Hepatology, 7, 680-687
5. Gressner A.M. and Zerbe, O. (1987) Kupffer cell-mediated induction of synthesis and secretion of proteoglycans by rat liver fat-storing cells in culture J. Hepatol., 5, 299-310
6. Zhang, L.P., Takahara, T., Yata, Y., Furui, K., Jin, B., Kawada, N. and Watanabe, A. (1999) Increased expression of plasminogen activator and plasminogen activator inhibitor during liver fibrogenesis of rats: role of stellate cells J. Hepatol., 31, 703-711
7. Arias, M., Lahme, B., Van de Leur, E., Gressner, A.M. and Weiskirchen, R. (2002) Adenoviral delivery of an antisense RNA complementary to the 3’ coding sequence of transforming growth factor-1 inhibits fibrogenic activities of hepatic stellate cells Cell Growth Differ., 13, 265-273
8. Kurikawa, N., Suga, M., Kuroda, S., Yamada, K. and Ishikawa, H. (2003) An angiotensin II type 1 receptor antagonist, olmesartan medoxomil, improves experimental liver fibrosis by suppression of proliferation and collagen synthesis in activated hepatic stellate cells Br. J. Pharmacol., 139, 1085-1094
9. Yoshiji, H., Kuriyama, S., Noguchi, R., Yoshii, J., Ikenaka, Y., Yanase, K., Namisaki, T., Kitade, M., Yamazaki, M., Tsujinoue, H. and Fukui, H. (2005) Combination of interferon-β and angiotensin-converting enzyme inhibitor, perindopril, attenuates the murine liver fibrosis development Liver Int., 25, 153-161
10. Paradis, V. Scoazec, J.W., Kollinger, M., Holstege, A., Moreau, A., Feldmann, G. and Bedossa, P. 1996) Cellular and subcellular localization of acetaldehyde-protein adducts in liver biopsies from alcoholic patients J. Histochem. Cytochem., 44, 1051-1057
11. Nakamura, T., Arii, S., Monden, Z., Furutani, M., Takeda, Y., Imamura, M., Tominaga, M. and Okada, Y. (1998) Expression of the Na+ /Ca2+ exchanger emerges in hepatic stellate cells after activation in association with liver fibrosis Proc. Natl. Acad. Sci. USA, 95, 5389-5394
12. Bataller, R., Sancho-Bru, P., Ginès, P., Lora, J.M., Al-Garawi, A., Solé, M., Colmenero, J., Nicolás, J.M., Jiménez, W., Weich, N., Gutiérrez–Ramos, J-C., Arroyo, V. and Rodés, J. (2003) Activated human hepatic stellate cells express the rennin-angiotensin system and synthesize angiotensin II Gastroenterology, 125, 117-125
13. Horani, A. Muhanna, N., Pappo, O., Melhem, A., Alvarez, C.E., Doron, S., Wehbi, W., Dimitrios, K., Friedman, S.L. and Safadi, R. (2007) Beneficial effect of glatiramer acetate (Copaxone) on immune modulation of experimental hepatic fibrosis Am. J. Physiol. Gastrointest. Liver Physiol., 292, G628-G638
14. Shafiei, M.S. and Rockey, D.C. (2006) The role of integrin-linked kinase in liver wound healing J. Biol. Chem., 281, 24863-24872
15. Novosyadlyy, R., Dudas, J., Pannem, R., Ramadori, G. and Scharf, J-G. (2006) Crosstalk between PDGF and IGF-I receptors in rat liver myofibroblasts: implication for liver fibrogenesis Lab. Invest., 86, 710-723
16. Apte, M.V., Haber, P.S., Applegate, T.L., Norton, I.D., McCaughan, G.W., Korsten, M.A., Pirola, R.C. and Wilson, J.S. (1998) Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture Gut, 43, 128-133
17. Masamune, A., Kikuta, K., Satoh, M., Satoh, A. and Shimosegawa, T. (2002) Alcohol activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells J. Pharmacol. Exp. Ther., 302, 36-42
18. Phillips, P.A., McCarroll, J.A., Park, S., Wu, M-J., Pirola, R., Korsten, M., Wilson, J.S. and Apte, M.V. (2003) Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover Gut, 52, 275- 282
19. Shimizu, K., Shiratori, K., Kobayashi, M. and Kawamata, H. (2004) Troglitazone inhibits the progression of chronic pancreatitis and the profibrogenic activity of pancreatic stellate cells via a PPAR-independent mechanism Pancreas, 29, 67-74
20. Jaster, R., Sparmann, G., Emmrich, J. and Liebe, S. (2002) Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells Gut, 51, 579-5844.1027
21. Ohnishi, N., Miyata, T., Ohnishi, H., Yasuda, H., Tamada, K., Ueda, N., Mashima, H. and Sugano, K. (2003) Activin A is an autocrine activator of rat pancreatic stellate cells: potential therapeutic role of follistatin for pancreatic fibrosis Gut, 52, 1487-1493
22. Tanioka, H., Mizushima, T., Shirahige, A., Matsushita, K., Ochi, K., Ichimura, M., Matsumura, N., Shinji, T., Tanimoto, M. and Koide, N. (2006) Xanthine oxidase-derived free radicals directly activate rat pancreatic stellate cells J. Gastroenterol. Hepatol., 21, 537-544
23. Bachem, M.G., Schneider, E., Gro, H., Weidenbach, H., Schmid, R.M., Menke, A., Siech, M., Beger, H., Grunert, A. and Adler, G. (1998) Identification, culture, and characterization of pancreatic stellate cells in rat and humans Gastroenterology, 115, 421-432
24. Peterson, T.C. and Rowden, G. (1998) Drug-metabolizing enzymes in rat liver myofibroblasts Biochem. Pharmacol., 55, 703-708
OptiPrep™Application Sheet C27; 9th edition, January 2020
OptiPrep™ Application Sheet C28
Enrichment of hepatic Kupffer cells in a discontinuous gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- Application Sheet C25 “Hepatic non-parenchymal cells (stellate, Kupffer and endothelial) cells – a short methodological survey” compares some of the methodologies for these cells.
- OptiPrep™ Reference List RC08 “Hepatic non-parenchymal, Kupffer and sinusoidal endothelial cells (and other liver cell types)” provides a comprehensive list of all the published papers reporting the use of OptiPrep™ for the isolation of these cells. To access return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
Parenchymal and non-parenchymal cells (PC and NPC) may be prepared by collagenase digestion of the liver using a tissue perfusion system. The PCs are then separated from the NPC by differential pelleting at 50 g for 1-4 min. It is however necessary to repeat this centrifugation (maybe twice more) to remove PC from the supernatant; moreover the NPC yield is usually low. It is both more common and more effective to carry out the 50 g centrifugation once; to harvest all the cells from the supernatant by centrifugation at a higher g-force and then use a density barrier prepared from one of the of iodinated density gradient media to resolve the two types of cell. Many workers prefer a modified perfusion strategy; it uses a mixture of collagenase and Pronase or Clostridium perfringens enterotoxin to destroy the PC selectively [1,2]. Hendriks et al [3] preferred Pronase because of the uncertain commercial availability of the enterotoxin and the latter’s possible cause of cell blebs.
One- or two layer density gradient centrifugation alone may not be sufficiently discriminating to provide a pure preparation of Kupffer cells, but this technique can provide an important initial enrichment for these cells prior to the use of centrifugal elutriation, adherence of the Kupffer cells to a plastic surface; sometimes both elutriation and surface adherence are used. Antibody-bound magnetic beads have also been used as a final purification step. See Section 5 for more information about additional procedures. The methods in this Application Sheet may simply provide a pure preparation of total NPC or of a NPC fraction also impoverished in the lighter stellate cells (see Section 4). However multiple-layer flotation gradients may be able achieve an improved resolution of Kupffer cells from other NPC types – see Section 4c.
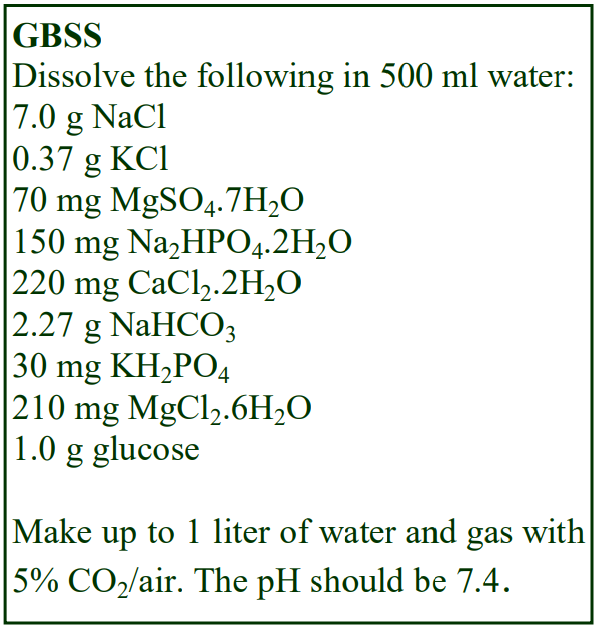 2. Solution selection and preparation
2. Solution selection and preparation
The solution used to suspend the crude NPC suspension and to dilute the OptiPrep™ may be a routine buffered saline such as PBS [4,5], which may be supplemented with 1% BSA [6] or a balanced salt solution such as Hank’s Balanced Salt Solution (HBSS) [7-11] or an NPC customized medium such as Gey’s balanced salt solution [12-15] and this may be prepared as described in the box. In a few instances a commercial culture medium is used, such as F12 [16] or RPMI, which may be supplemented with 1% BSA [17] or 10% FCS [18,19]. Only when the solution contains 10% serum is the density of a culture medium likely to be significantly different to PBS, HBSS or GBSS (i.e. approx 1.006 g/ml). A medium containing 10% serum has a density of approx 1.009 g/ml.
- For more information on the preparation of gradient solutions see Application Sheet C01.
3. Species source
The species source for the liver cells may very well influence the detailed methods used in the pregradient stages such as perfusion of the liver, enzymic and physical disaggregation of the tissue and washing of the released cells. There are some significant differences in the density gradient methodology used in preparing the Kupffer cell-enriched fraction, but whether any of this is species related is not known. Most papers report the use of either rat [6-12, 20-23] or mouse liver [5, 13-16, 18, 24, 25], but pig [4, 17, 26, 27] and human [28] are also used as sources.
4. Protocols
Note that in many published methods the gradients are described in terms of % OptiPrep™; often this is % (v/v) OptiPrep™. Sometimes however it is actually % (w/v) iodixanol; i.e. iodixanol and OptiPrep™ are used synonymously, which is incorrect (OptiPrep™ is the commercial name for a solution of 60% (w/v) iodixanol). In the following text all gradient solutions are given as % (w/v) iodixanol.
4a. Flotation from a density-adjusted cell suspension
This is the simplest strategy in which the crude NPC suspension is mixed with OptiPrep™ to a certain concentration of iodixanol; a small volume of saline or balanced salt solution (2-3 ml) is layered on top and centrifuged. The NPC float to the interface with the saline and any PC, residual erythrocytes, non-viable cells or cell fragments either pellet or remain suspended in the load zone. Some examples are given in Table 1. Although some workers omit the upper layer, its presence is recommended since it prevents the cells banding at an air/liquid interface. Most centrifugations are at 4°C
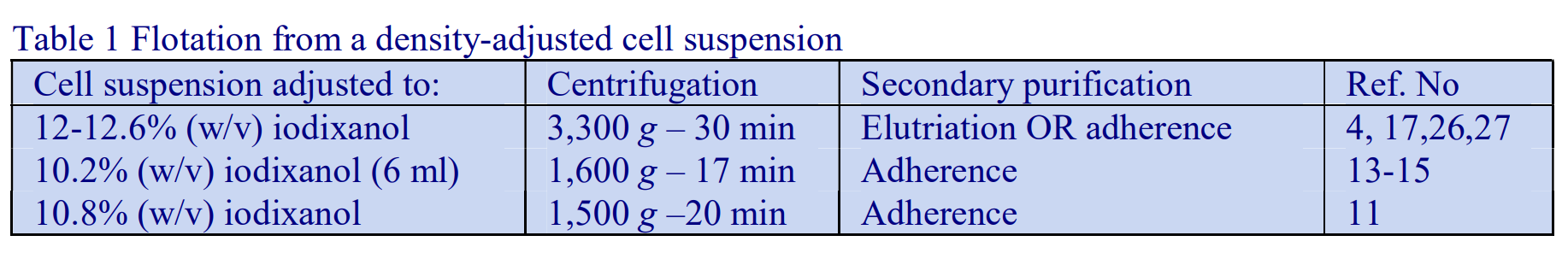
4b. Two-density layer sedimentation
The crude NPC preparation in HBSS is adjusted to 11.7% (w/v) iodixanol (approx ρ = 1.066 g/ml); layered over a solution of 17.6% (w/v) iodixanol (approx ρ = 1.097 g/ml) and overlaid by HBSS. After centrifugation at 1400 g for 17 min at 4°C NPC banded at the top and bottom of the 11.7% iodixanol layer; both layers were further processed by elutriation or adherence [7]. This configuration was also used by Yang et al [29]. In a slight modification of this two-layer gradient, the cell suspension was layered over the two iodixanol solutions rather being adjusted to the lower density [8-10, 20-22, 25]. Park et al [16] used a similar strategy layering the crude cell suspension over 8.2% and 15.6% (w/v) iodixanol; while der Flier et al [30] increased the density of the lower layer to 17.6%. More recently Hyun et al [31] separated the stellate and Kupffer + endothelial cells using 11.5% and 20% (w/v) iodixanol.
4c. Multiple layer gradients – flotation
Schreiber et al [32] diluted OptiPrep™ with Krebs-Henseleit buffer containing 1.25 mM CaCl2 and 1.2 mM Na2SO4 to produce solutions of 17%, 11.5% and 8.4% (w/v) iodixanol. The crude NPCs were suspended in 24% iodixanol; this was overlaid by the lower density solutions and finally the buffer. After centrifugation at 1,400 g for 20 min at 4°C, the stellate and Kupffer cells banded predominantly at the interface of the buffer/8.4% and 8.4/11.5% interfaces respectively.
4d. Removal of debris
Sometimes a quite dense solution of 24% (w/v) iodixanol is used as a barrier merely to remove debris and non-viable cells, which tend to be denser than the Kupffer cells [5, 18, 33]. This can also be achieved simply by adjusting the cell suspension to approx 26% (w/v) iodixanol and after layering a small volume of HBSS on top, centrifuging at 400 g for 15 min and collecting the cells from the interface [19].
5. Add-on procedures
To include elutriation schedules is beyond the scope of this Application Sheet, but some of the commonly used adherence methods can be briefly summarized: either dishes coated with glutaraldehyde-fixed bovine serum albumin [4, 6, 26] or collagen have been used [13, 14, 16]. Antibody-bound bead techniques were reported in refs 5, 18, 19, 24, 33 and 34.
6. References
1. Boyum, A., Berg, T. and Blomhoff, R. (1983) Fractionation of mammalian cells In Iodinated density gradient media – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 147-171
2. Brouwer, A., Hendricks, H. F. J., Ford, T. and Knook, D. L. (1991) Centrifugation separations of mammalian cells In Preparative centrifugation – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 271-314
3. Hendriks, H.F.J., Brouwer, A. and Knook, D.L. (1990) Isolation, purification, and characterization of liver cell types Methods Enzymol., 190, 49-58
4. Elvevold, K., Nedredal, G.I., Revhaug, A., Bertheussen, K. And Smedsrød, B. (2005) Long-term preservation of high endocytic activity in primary cultures of pig liver sinusoidal endothelial cells Eur. J. Cell Biol., 84, 749-764
5. Shao, B., Lu, M., Katz, S.C., Varley, A.W., Hardwick, J., Rogers, T.E., Ojogun, N., Rockey, D.C., DeMatteo, R.P. and Munford, R.S. (2007) A host lipase detoxifies bacterial lipopolysaccharides in the liver and spleen J. Biol. Chem., 282, 13726-13735
6. Malerød, L., Juvet, L.K., Gjøen, T. and Berg, T. (2002) The expression of scavenger receptor class B, type I (SR-BI) and caveolin-1 in parenchymal and nonparenchymal liver cells Cell Tissue Res., 307, 173-180
7. Valatas, V., Xidakis, C., Roumpaki, H., Kolios, G. and Kouroumalis, E.A. (2003) Isolation of rat Kupffer cells: a combined methodology for highly purified primary cultures Cell Biol Int., 27, 67-73
8. Xidakis, C., Ljumovic, D., Manousou, P., Notas, G., Valatas, V., Kolios, G., and Kouroumalis, E. (2005) Production of pro- and anti-fibrotic agents by rat kupffer cells; the effect of octreotide Digest. Dis. Sci., 50, 935-941
9. Charalampopoulos, I., Androulidaki, A., Minas, V., Chatzaki, E., Tsatsanis, C., Nota, G., Xidakis, C., Kolios, G., Kouroumalis, E., Margioris, A.N. and Gravanis, A. (2006) Neuropeptide urocortin and its receptors are expressed in rat Kupffer cells Neuroendocrinology, 84, 49-57
10. Xidakis, C., Mastrodimou, N., Notas, G., Renieri, E., Kolios, G., Kouroumalis, E. and Thermos, K. (2007) RT-PCR and immunocytochemistry studies support the presence of somatostatin, cortistatin and somatostatin receptor subtypes in rat Kupffer cells Regul. Pept., 143, 76-82
11. Baranova, I.N., Bocharov, A.V., Vishnyakova, T.G., Kurlander, R., Chen, Z., Fu, D., Arias, I.M., Csako, G., Patterson, A.P. and Eggerman, T.L. (2010) CD36 is a novel serum amyloid A (SAA) receptor mediating SAA binding and SAAinduced signaling in human and rodent cells J. Biol. Chem., 285, 8492–8506
12. DeLeve, L.D., Wang, X., McCuskey, M.K. and McCuskey, R.S. (2006) Rat liver endothelial cells isolated by anti-CD31 immunomagnetic separation lack fenestrae and sieve plates Am. J. Physiol. Gastrointest. Liver Physiol., 291, G1187- G1189
13. Hu, S., Yin, S., Jiang, X., Huang, D. and Shen, G. (2009) Melatonin protects against alcoholic liver injury by attenuating oxidative stress, inflammatory response, and apoptosis Eur. J. Pharmacol., 616, 287–292
14. Hu, S., Shen, G., Zhao, W., Wang, F., Jiang, X. and Huang, D. (2010) Paeonol, the main active principles of Paeonia moutan, ameliorates alcoholic steatohepatitis in mice J. Ethnopharmacol., 128, 100–106
15. Lv, X., Chen, Z., Li, J., Zhang, L., Liu, H., Huang, C. and Zhu, P. (2010) Caffeine protects against alcoholic liver injury by attenuating inflammatory response and oxidative stress Inflamm. Res., 59, 635–645
16. Park, J.K., Cho, K., Johnson, J. and Perez, R.V. (2004) Induction of MIP-1 in Kupffer cell by portal venous transfusion Transplant Immunol., 13, 33-38
17. Nedredal, G.I., Elvevold, K.H., Ytrebø, L.M., Olsen, R., Revhaug, A. and Smedsrød, B. (2003) Liver sinusoidal endothelial cells represent an important blood clearance system in pigs Comp. Hepatol., 2:1
18. Burt, B.M., Plitas, G., Stableford, J.A., Nguyen, H.M., Bamboat, Z.M., Pillarisetty, V.G. and DeMatteo, R.P. (2008) CD11c identifies a subset of murine liver natural killer cells that responds to adenoviral hepatitis J. Leukoc. Biol., 84, 1039-1046
19. Connolly, M.K., Bedrosian, A.S., Malhotra, A., Henning, J.R., Ibrahim, J., Vera, V., Cieza-Rubio, N.E., Hassan, B.U., Pachter, H.L., Cohen, S., Frey, A.B. and Miller, G. (2010) In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity J. Immunol., 185, 2200–2208
20. Valatas, V., Kolios, G., Manousou, P., Notas, G., Xidakis, C., Diamantis, I. and Kouroumalis, E. (2004) Octreotide regulates CC but not CXC LPS-induced chemokine secretion in rat Kupffer cells Br. J. Pharm., 141, 477-487
21. Valatas, V., Kolios, G., Manousou, P., Xidakis, C., Notas, G., Ljumovic, D. and Kouroumalis, E.A. (2004) Secretion of inflammatory mediators by isolated rat Kupffer cells; the effect of octreotide Regul. Pept., 120, 215-225
22. Kolios, G., Valatas, V., Manousou, P., Xidakis, C., Notas, G. and Kouroumalis, E. (2008) Nitric oxide and MCP-1 regulation in LPS activated rat Kupffer cells Mol. Cell. Biochem., 319, 91-98
23. Xie, G., Wang, L., Wang, X., Wang, L. and DeLeve, L.D (2010) Isolation of periportal, midlobular, and centrilobular rat liver sinusoidal endothelial cells enables study of zonated drug toxicity Am. J. Physiol. Gastrointest. Liver Physiol., 299, G1204–G1210
24. Zhu, J., Huang, X. and Yang, Y. (2007) Innate immune response to adenoviral vectors is mediated by both toll-like receptor-dependent and -independent pathways J. Virol., 81, 3170-3180
25. Meng, Z., Fu, X., Chen, X., Zeng, S., Tian, Y., Jove, R., Xu, R. and Huang, W. (2010) miR-194 is a marker of hepatic epithelial cells and suppresses metastasis of liver cancer cells in mice Hepatology, 52, 2148-2157
26. Elvevold, K.H., Nedredal, G.I., Revhaug, A. and Smedsrød, B. (2004) Scavenger properties of cultivated pig liver endothelial cells Comp. Hepatol., 3:1
27. Nedredal, G.I., Elvevold, K., Ytrebø, L.M., Fuskevåg, O-M., Pettersen, I., McCourt, P.A., Bertheussen, K., Smedsrød, b. and Revhaug, A. (2009) Porcine liver sinusoidal endothelial cells contribute significantly to intrahepatic ammonia metabolism Hepatology, 50, 900-908
28. Wallace, K., Cowie, D.E., Konstantinou, D.K., Hill, S.J., Tjelle, T.E., Axon, A., Koruth, M., White, S.A., Carlsen, H., Mann, D.A. and Wright, M.C. (2010) The PXR is a drug target for chronic inflammatory liver disease J. Steroid Biochem. Mol, Biol., 120, 137–148
29. Yang, H., Tong, C., Fu, C., Xu, Y., Liu, X., Chen, Q., Zhang, Y., Lü, S., Li, N. and Long, M. (2016) Analyses of movement and contact of two nucleated cells using a gas-driven micropipette aspiration technique J. Immunol. Meth., 428, 20–29
30. Van der Flier, A., Liu, Z., Tan, S., Chen, K., Drager, D., Liu, T., Patarroyo-White, S., Jiang, H. and Light, D.R. (2015) FcRn rescues recombinant factor VIII Fc fusion protein from a VWF independent FVIII clearance pathway in mouse hepatocytes PLoS One, 10: e0124930
31. Hyun, J., Wang, S., Kim, J., Rao, K.M., Park, S.Y., 2, Chung, I., Ha, C-S., Kim, S-W., Yun, Y.H. and Jung, Y. (2016) MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression Nat. Comm., 7: 10993
32. Schreiber, R., Taschler, U., Wolinski, H., Seper, A., Tamegger, S.N., Graf, M., Kohlwein, S.D., Haemmerle, G., Zimmermann, R., Zechner, R. and Lass, A. (2009) Esterase 22 and beta-glucuronidase hydrolyze retinoids in mouse liver J. Lipid Res., 50, 2514–2523
33. Connolly, M.K., Mallen-St. Clair, J., Bedrosian, A.S., Malhotra, A., Vera, V., Ibrahim, J., Henning, J., Pachter, H.L., Bar-Sagi, D., Frey, A.B. and Miller, G. (2010) Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and pre-invasive intra-abdominal tumor J. Leukoc. Biol., 87, 713–725
34. Katz, S.C., Pillarisetty, V.G., Bleier, J.I., Shah, A.B. and DeMatteo, R.P. (2004) Liver sinusoidal endothelial cells are insufficient to activate T cells J. Immunol., 173, 230-235
OptiPrep™ Application Sheet C28; 4th edition, January 2020
OptiPrep™ Application Sheet C29
Isolation of cells from pulmonary tissue Endothelial from epithelial cells, type I epithelial cells and macrophagest
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
- For other pulmonary cells methods see the companion Application Sheet C30
- See Reference List RC09 for a list of published papers on all pulmonary cells
1. Background
NycodenzⓇ gradients have been used very successfully for the isolation of Type II pneumocytes [1,2] and Viscardi et al [2] pointed out the advantages of using this medium and the potential problems of using the colloidal silica medium PercollⓇ for this task. Alveolar macrophages have also been purified using NycodenzⓇ gradients [3].
OptiPrep™ has been employed, as a continuous gradient, in the separation of endothelial and epithelial cells [4] and also of alveolar macrophages [5] and as a discontinuous gradient in the isolation of Type I epithelial cells [6]. Pu et al [4] studied the regulation of calcitonin secretion in pulmonary
neuroendocrine cells, which banded with the epithelial cells. Borok et al [6] were able to confirm the involvement of Type I cells in transalveolar Na+ transport. Starr et al [5] studied the effects of bronchofibrinous pneumonia in cattle. All three separations are described in this OptiPrep™ Application Sheet. See Section 6 for other published methods.
2. Separation of endothelial and epithelial cells (adapted from ref 4)
2a. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. Culture medium, e.g. RPMI-1640 or DMEM (see Note 1)
C. 1% (w/v) collagenase II in Solution B
D. Solution B + 10% fetal bovine serum
2b. Protocol
1. Finely mince the dissected lung tissue, using scissors, into 1 mm3 pieces
2. Incubate in 10 ml Solution C at 37 for 2-3 h (see Notes 2 and 3).
3. Add an equal volume of Solution D and centrifuge the cell suspension at 600 g for 20 min.
4. Resuspend the cell pellet in 5 ml of Solution D.
5. Mix 0.5 ml of OptiPrep with 4.5 ml of the cell suspension to make a final density of 1.04 g/ml (see Note 4).
6. Make a solution of density 1.15 g/ml by mixing 3 ml of Solution D and 2.5 ml of OptiPrep (see Note 4).
7. Prepare a continuous gradient from 5 ml each of the cell suspension (1.04 g/ml) and the 1.15 g/ml solutions using a Gradient Master (see Note 5).
8. After centrifuging at 600 g for 20 min, harvest the banded material from the gradient using a syringe and metal cannula (see Note 6).
3. Isolation of alveolar epithelial type I cells (adapted from ref 6)
3a. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. Culture medium, e.g. RPMI-1640 or DMEM (see Note 1)
3b. Protocol
- For details of lavage procedure and tissue disaggregation with elastase see ref 6.
1. Suspend the crude single cell suspension in Solution B.
2. Produce four solution of density 1.012, 1.033, 1.047 and 1.068 g/ml by diluting OptiPrep™ with Solution B using, respectively, the following volume ratios: 2 + 58, 5 + 55, 8 + 52 and 12 + 48.
3. Layer 2 ml of each gradient solution, followed by the crude cell suspension in a 15 ml centrifuge tube and centrifuge at 600 g for 15 min.
4. Harvest the type I cells that band in the 1.033 g/ml layer.
4. Isolation of alveolar macrophages (adapted from ref 5)
4a. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. Lavage medium: phosphate-buffered saline (PBS)
C. Cell suspension medium: Ca2+/Mg2+ – free Hank’s Balanced Salt Solution (see Note 1)
4b. Protocol
1. Prepare two iodixanol solutions of 5% (w/v) and 30% (w/v) iodixanol by diluting OptiPrep™ with Solution C using, respectively, the following volumes ratios: 5 + 55 and 1 + 1.
2. Lavage the lungs using Solution B (see Note 7)
3. Pass the lavage through a 22 μm pore-size nylon membrane filter.
4. Collect the cells by centrifugation at 500 g for 15 min and resuspend in Solution C.
5. During the centrifugation produce a linear gradient in a 15 ml tube from 5 ml each of the 5% and 30% iodixanol solutions using a two-chamber gradient maker or a Gradient Master™ (see Note 8).
6. Layer the cell suspension on top and centrifuge at 800 g for 20 min.
7. Collect the macrophages that band at approx 15% iodixanol.
5. Notes
1. Any suitable isoosmotic culture medium or balanced salt solution may be used.
2. The time required for tissue disaggregation may need modification depending on the animal species being used; Syrian golden hamsters were used in the study by Pu et al [4].
3. Alternative more elaborate techniques for tissue disaggregation involving lavage of the lungs prior to use of elastase rather than collagenase might be used (see ref 6).
4. The ratio of OptiPrep™ to diluent will depend on whether fetal bovine serum (FBS) is included; with FBS (as in this case) the diluent will have a density of approx 1.009 g/ml, without FBS the density will be approx 1.006 g/ml. For more information on the preparation of density solutions see Application Sheet C01.
5. If a Gradient Master™ is unavailable then a two-chamber gradient maker might be used, but to avoid vigorous stirring of the cell suspension. It might be more suitable to make the 1.04 g/ml solution from Solution A and Solution D (i.e. a cell-free solution) and then to layer the crude cell suspension (in Solution D) on top. Alternatively the continuous gradient might be produced by diffusion from a discontinuous gradient using 2.5 ml each of 1.040 g/ml (containing cells), 1.075g/ml, 1.110 g/ml and 1.145 g/ml (equivalent to approx 6%, 13%, 20% and 27% (w/v) iodixanol). For information on the preparation of continuous gradients for cell separations see Application Sheet C02.
6. Endothelial cells + macrophages make up the least dense band (approx 1.049 g/ml), epithelial cells band at 1.059-1/062 g/ml, fibroblasts and erythrocytes at 1.069-1.096 g/ml and the densest band (1.113 g/ml) contained fibroblasts + non-viable cells + cell debris.
7. For more information see ref 5.
8. Alternatively the continuous gradient might be produced by diffusion from a discontinuous gradient using 2.5 ml each of 5%, 10%, 15%, 20% and 30% (w/v) iodixanol. For information on the preparation of continuous gradients for cell separations see Application Sheet C02.
6. Other published papers Mossel et al [7] and Yu et al [8] employed two-layer iodixanol gradient (1.04 and 1.08 g/ml) for part of the isolation process in studies on alveolar type I and type II cells.
7. References
1. Schultz, C.J., Torres, E., Londos, C. and Torday, J. S. (2002) Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells Am. J. Physiol. Lung Cell Mol. Physiol., 283, L288-L296
2. Viscardi, R.M., Ullsperger, S. and Resau, J.H. (1992) Reproducible isolation of type II pneumocytes from fetal and adult rat lung using Nycodenz density gradients Exp. Lung Res., 18, 225-245
3. Killingsworth, C.R., Shore, S.A., Alessandrini, F., Dey, R.D. and Paulauskis, J.D. (1997) Rat alveolar macrophages express preprotachykinin gene-I mRNA-wncoding tachykinins Am. J. Physiol. Lung Cell Mol. Physiol., 273, L1073- L1081
4. Pu, F.R., Manning, F.C.R., Brannigan, A.E. and Crosby, S. R. (2001) Differential regulation of calcitonin secretion in normal and neoplastic pulmonary neuroendocrine cells in vitro Exptl. Lung Res., 27, 689-703
5. Starr, A.E., Dan, T., Minhas, K., Shewen, P.E. and Coomber, B.L. (2004) Potential involvement of gelatinases and their inhibitors in Mannheimia haemolytica pneumonia in cattle Infect. Immun., 72, 4393-4400
6. Borok, Z., Liebler, J.M., Lubman, R.L., Foster, M.J., Zhou, B., Li, X., Zabski, S. M., Kim, K-J. and Crandall, E.D. (2002) Alveolar epithelial ion and fluid transport Na transport proteins are expressed by rat alveolar epithelial type I cells Am. J. Physiol. Lung Cell Mol. Physiol., 282, L599-L608
7. Mossel, E.C., Wang, J., Jeffers, S., Edeen, K.E., Wang, S., Cosgrove, G.P., Funk, C.J., Manzer, R., Miura, T.A., Pearson, L.D., Holmes, K.V. and Mason, R.J. (2008) SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells Virology, 372, 127-135
8. Yu, W.C.L., Chan, R.W.Y., Wang, J., Travanty, E.A., Nicholls, J.M., Peiris, J.S.M., Mason, R.J. and Chan, M.C.W. (2011) Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses J. Virol., 85, 6844-6855
OptiPrep™Application Sheet C29; 7th edition, February 2020
OptiPrep™ Application Sheet C30
Isolation of rat alveolar type II pneumocytes, lymphoid and myeloid cells
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
- For other pulmonary cell methods see Application Sheet C29
- See Reference List RC09 for a list of published papers on all pulmonary cells
1. Background
Alveolar (pneumocyte) type II pneumocytes are widely studied because they synthesize and secrete the phospholipid-rich lung surfactant, which lines the air-alveolar interface and prevents alveolar collapse by lowering surface tension at low lung volumes. Isolation of these cells from both adult and foetal lung is an important prerequisite for the culture and study. Viscardi et al [1-6], who successfully developed a NycodenzⓇ gradient technique, emphasised the importance of the non-toxic, non-invasive properties of this gradient medium and pointed out that although PercollⓇ gradients had been used previously for purifying these cells, the potentially toxic nature of a polyvinyl-pyrollidone-coated silica colloid was of considerable concern in studies of their function.
Viscardi et al [1] reported that that the purity of type II pneumocytes from adult lung on NycodenzⓇ gradients was over 80%; very similar figures were obtained from foetal lung tissue. The recovery of these cells from adult lung was over 70%, while for foetal lung this figure was rather lower at 45-50%. Both the purity and the viability of the cells (approx. 97%) from NycodenzⓇ gradients were considerably higher than that obtained from cells purified by IgG panning. The cells from NycodenzⓇ gradients moreover had almost four times the plating efficiency. Other workers [7-9] have reported an even higher purity of >90% for the cells from foetal lung tissue. The same gradient system has also been used by Driscoll et al [10,11] and Johnston et al [12].
- Recently discontinuous iodixanol gradients have been used to purify these cells [13-19]
2. Preparation of cell suspension
The treatment of the lung tissue prior to gradient centrifugation is a complex operation, the detail of which varies from laboratory to laboratory and is outside the scope of this Application Sheet. For detailed information see refs 1 and 7-13. Briefly the preparation of the cell suspension involves five steps:
1. Rats are anaesthetized and heparin injected as an anticoagulant.
2. After tracheostomy the vasculature is perfused with a buffered saline medium such as 140 mM NaCl, 5 mM KCl, 2.5 mM Na2HPO4, 10 mM HEPES, 2.0 mM CaCl2, 1,3 mM MgSO4, pH 7.4 to remove blood cells [1]. Sometimes this is supplemented with glucose, nystatin and an antibioticantimycotic [12].
3. To remove macrophages, the lungs are lavaged several times with 140 mM NaCl, 5 mM KCl, 2.5 mM Na2HPO4, 10 mM HEPES, 6 mM glucose, 0.2 mM EGTA, pH 7.4 followed by lavage with the Step 2 buffer [1]. Sometimes the lavage procedure uses the Step 2 buffer (minus divalent cations) followed by Step 2 buffer [12].
4. Partial disaggregation of the cells by lavage with either elastase [1] or pronase [12]
5. After removal of the trachea, the lung tissue is minced in a solution containing DNase I (in Step 2 buffer) and foetal bovine serum and then incubated at 37C for 5 min in a trypsinizing flask [1].
 3. Cell fractionation in NycodenzⓇ
3. Cell fractionation in NycodenzⓇ
The following protocol is adapted from ref 1.
3a. Reagents and solutions required (see Note 1)
1. NycodenzⓇ powder
2. NycodenzⓇ solvent: 3 mM KCl, 0.3 mM EDTA, 5 mM HEPES-NaOH, pH 7.5 (Solution 1)
3. Working Solution Diluent: 0.75% (w/v) NaCl, 3 mM KCl, 0.3 mM EDTA, 5 mM HEPES-NaOH, pH 7.5 (Solution 2)
3b. Protocol
1. Prepare a 27.6% (w/v) NycodenzⓇ stock solution in Solution 1. Place 50 ml of Solution 1 in a 150
ml beaker on a magnetic stirrer set at approx. 50°C and add the NycodenzⓇ in small amounts until dissolved. Allow the solution to cool to room temperature and then make up to 100 ml with Solution 1. Filter sterilize if required.
2. Dilute the Nycodenz stock solution with Solution 2 to produce 4.6% (w/v) NycodenzⓇ solution for a continuous gradient or three solutions of 20.7%, 13.8% and 4.6% NycodenzⓇ for a discontinuous gradient (see Note 2).
3. Filter the lung tissue (in approx 25 ml buffer) sequentially through 2- and 4-ply cotton gauze,
followed by 100-, 37- and 15-ρm nylon mesh.
4. Dilute with cell suspension buffer to 50 ml and centrifuge at 130 g for 10 min (see Note 3).
5. Suspend the cell pellets in DMEM containing 2% foetal bovine serum.
6. Produce a continuous NycodenzⓇ gradient of 1.03-1.15 g/ml using a two-chamber gradient maker or a Gradient Master™ using 4 ml each of the 27.6% and 4.6% NycodenzⓇ solutions.
7. Alternatively produce a discontinuous gradient from 2 ml each the 27.6%, 20.7%, 13.8% and 4.6% NycodenzⓇ solutions (see Step 2) by underlayering from a syringe and metal cannula and allow a continuous gradient to form by diffusion (see Notes 4 and 5).
8. Layer 3 ml of the cell suspension (approx 1 x 107 cells/ml) on top of the gradient.
9. Centrifuge at 1500 g for 20 min at 15°C. 10. Harvest the band of cells (at approx. 1.056 g/ml) just below the sample/gradient interface (see Note 6).
4. Cell fractionations using OptiPrep™
4a Separation of Alveolar Type II cells
In recent publications [13-28] a simple two layer gradient of iodixanol of densities 1.040 and 1.080 g/ml was described for the partial purification of Type II alveolar cells from human lung tissue prior to further purification by negative selection with magnetic beads. As iodixanol is available as a sterile solution of density 1.32 g/ml (OptiPrep™) it considerably simplifies gradient solution preparation. If an ordinary balanced salt or buffered saline solution is used to dilute the OptiPrep™, then these two densities are equivalent to approx 6.5% and 14% (w/v) iodixanol, i.e. OptiPrep™:diluent ratios of 6.5:53.5 and 14:46.
4b Separation of mononuclear/lymphoid/haematopoietic type cells
The separation of myeloid and lymphoid cells has also been achieved using a two-layer iodixanol gradient of 4% and 14.5% (w/v), centrifuged at 600 g for 20 min [29]. This gradient was also used to separate haematopoietic cells [30]. Leukocytes (mononuclear cells) from lung tissue have been separated on a 1.079 g/ml barrier prepared from OptiPrep™ (diluted with RPMI containing 10% FCS) [31,32].
5. Notes
1. As all of the solutions used are isoosmotic, it is highly likely that iodixanol can be substituted for Nycodenz in the methods in Section 3; certainly its availability as a sterile 60% (w/v) solution (OptiPrep™) would make solution preparation much easier. OptiPrep™ would simply be diluted with a suitable buffered saline to the same % (w/v) iodixanol as the NycodenzⓇ % (w/v). See Application Sheet C01 for more information.
2. See Steps 6 and 7 for information about choice of gradient solution preparation.
3. This pelleting step is sometimes carried out at higher g-forces, e.g. 500 g [12]
4. Metal “filling” cannulas can be obtained from any hospital supplies company.
5. If the tube is capped and carefully rotated to a horizontal position, diffusion to form a linear gradient will occur in about 1 h at room temperature. If the tube is maintained in a vertical position this process will take at least 6 h. It is always advisable to check that the density gradient is linear by unloading the gradient in a series of equal volume fractions and determining their density by measuring the refractive index or absorbance. For more information about the preparation gradients see ref 29 and Application Sheet C02.
6. The broad band at approx. 1.086 g/ml contains fibroblasts, endothelial cells and macrophages, while any erythrocytes band close to the bottom of the gradient.
6. References
1. Viscardi, R. M., Ullsperger, S. and Resau, J. H. (1992) Reproducible isolation of Type II pneumocytes from fetal and adult rat lung using Nycodenz gradients Exp. Lung Res., 18, 225-245
2. Viscardi, R.M. and McKenna, M.C. (1993) Developmental changes in cholinephosphate cytidylyl-transferase activity and microsomal phospholipid fatty acid composition in alveolar type II cells Life Sci., 54, 1411-1421
3. Viscardi, R.M. and Strauss, K. (1996) Phospholipid transfer activities are enriched in cytosolic fractions of freshly isolated type II cells (TII) compared with whole lung Pediatr. Res., 39, Suppl. 2:354
4. Viscardi, R.M. and Strauss, K (1996) Role of phosphatidylinositol transfer protein in type II pneumocytes: regulation of choline phosphate cytidylyltransferase Pediatr. Res., 39, Suppl. 2:355
5. Viscardi, R.M., Strauss, K. and Hasday, J.D. (1997) Oleic acid stimulates rapid translocation of cholinephosphate cytidylyltransferase in type II cells Biochim. Biophys. Acta, 1349, 157-170
6. Viscardi, R.M. and Strauss, K.A. (1999) Developmental changes in phosphatidylinositol transfer protein concentration and phospholipid transfer activities in rat type II cells Exp. Lung Res., 25, 561-576
7. Torday, J. S., Sun, H. and Qin, J. (1998) Prostaglandin E2 integrates the effects of fluid distension and glucocorticoid on lung maturation Am. J. Physiol. Lung Cell. Mol. Physiol., 274, L106-L111
8. Schultz, C. J., Torres, E., Londos, C. and Torday, J. S. (2002) Role of adipocyte differentiation-related protein in surfactant phospholipids synthesis Am. J. Physiol. Lung Cell. Mol. Physiol., 283, L288-L296
9. Torday, J. S. (2003) Parathyroid hormone-related protein is a gravisensor in lung and bone cell biology Adv. Space Res,, 32, 1569-1576
10. Driscoll, K. E., Deyo, L. C., Howard, B. W., Poynter, J. and Carter, J. M. (1995) Characterizing mutagenesis in the hprt gene of rat alveolar epithelial cells Exp. Lung Res., 21, 941-956
11. Driscoll, K. E., Deyo, L. C., Carter, J. M., Howard, B. W., Hassenbein, D. G. and Bertram, T. A. (1997) Effects of particle exposure and particle-elicited inflammatory cells on mutation in rat alveolar epithelial cells Carcinogenesis, 18, 423-430
12. Johnston, C. J., Driscoll, K. E., Finkelstein, J. N., Baggs, R., O’Reilly, M. A., Carter, J., Gelein, R. and Oberdorster, G. (2000) Pulmonary chemokine and mutagenic responses in rats after subchronic inhalation of amorphous and crystalline silica Toxicol. Sci., 56, 405-413
13. Manzer, R., Wang, J., Nishina, K., McConville, G. and Mason, R.J. (2006) Alveolar epithelial cells secrete chemokines in response to IL-1 and lipopolysaccharide but not to ozone Am. J. Respir. Cell Mol., 34, 158-166
14. Wang, J., Edeen, K., Manzer, R., Chang, Y., Wang, S., Chen, X., Funk, C.J., Cosgrove, G.P., Fang, X. an Mason, R.J. (2007) Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro Am. J. Respir. Cell Mol. Biol., 36, 661-668
15. Mossel, E.C., Wang, J., Jeffers, S., Edeen, K.E., Wang, S., Cosgrove, G.P., Funk, C.J., Manzer, R., Miura, T.A., Pearson, L.D., Holmes, K.V. and Mason, R.J. (2008) SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells Virology, 372, 127-135
16. Miura, T.A., Wang, J., Holmes, K.V. and Mason, R.J. (2007) Rat coronaviruses infect rat alveolar type I epithelial cells and induce expression of CXC chemokines Virology, 369, 288-298
17. Wang, J., Oberley-Deegan, R., Wang, S., Nikrad, M., Funk, C.J., Hartshorn, K.L. and Mason, R.J. (2009) Differentiated human aveolar type II cells secrete antiviral IL-29 (IFN-1) in response to influenza A infection J. Immunol., 182,1296–1304
18. Kosmider, B., Loader, J.E., Murphy, R.C. and Mason, R.J. (2010) Apoptosis induced by ozone and oxysterols in human alveolar epithelial cells Free Radical Biol. Med., 48, 1513–1524
19. Ito, Y. and Mason, R.J. (2010) The effect of interleukin-13 (IL-13) and interferon- (IFN-) on expression of surfactant proteins in adult human alveolar type II cells in vitro Respir. Res., 11, 157
20. Yu, W.C.L., Chan, R.W.Y., Wang, J., Travanty, E.A., Nicholls, J.M., Peiris, J.S.M., Mason, R.J. and Chan, M.C.W. (2011) Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses J. Virol., 85, 6844-6855
21. Kosmider, B., Messier, E.M., Chu, H.W. and Mason, R.J. (2011) Human alveolar epithelial cell injury induced by cigarette smoke PLoS One 6: e26059
22. Messier, E.M., Bahmed, K., Tuder, R.M., Chu, H.W., Bowler, R.P. and Kosmider, B. (2013) Trolox contributes to Nrf2- mediated protection of human and murine primary alveolar type II cells from injury by cigarette smoke Cell Death Dis., 4: e573
23. Goetzman, E.S., Alcorn, J.F., Bharathi, S.S., Uppala, R., McHugh, K.J., Kosmider, B., Chen, R., Zuo, Y.Y., Beck, M.E., McKinney, R.W., Skilling. H., Suhrie, K.R., Karunanidhi, A., Yeasted, R., Otsubo, C., Ellis, B., Tyurina, Y.Y., Kagan, V.E., Mallampalli, R.K. and Vockley, J. (2014) Long-chain acyl-CoA dehydrogenase deficiency as a cause of pulmonary surfactant dysfunction J. Biol. Chem., 289, 10668–10679
24. Ito, Y., Correll, K., Schiel, J.A., Finigan, J.H., Prekeris, R., and Mason, R.J. (2014) Lung fibroblasts accelerate wound closure in human alveolar epithelial cells through hepatocyte growth factor/c-Met signaling. Am. J. Physiol. Lung Cell. Mol. Physiol., 307, L94–L105, 2014
25. Ito, Y., Correll, K., Zemans, R.L., Leslie, C.C., Murphy, R.C., Mason, R.J. (2015) Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-/EGFR signaling Am. J. Physiol. Lung Cell Mol. Physiol. 308: L1178–L1188
26. Epa, A.P., Thatcher, T.H., Pollock, S.J., Wahl, L.A., Lyda, E., Kottmann, R.M., Phipps, R.P. and Sime, P.J. (2015) Normal human lung epithelial cells inhibit transforming growth factor-β induced myofibroblast differentiation via prostaglandin E2 PLoS One, 10: e0135266
27. Xie, W., Wang, H., Liu, Q., Li, Y., Wang, J., Yao, S. and Wu, Q. (2016) ResolvinD1 reduces apoptosis and inflammation in primary human alveolar epithelial type 2 cells Lab. Invest., 96, 526–536
28. Zemski Berry, K. A., Murphy, R.C., Kosmider, B. and Mason, R.J. (2017) Lipidomic characterization and localization of phospholipids in the human lung J. Lipid Res., 58, 926–933
29. Ledford, J.G., Goto, H., Potts, E.N., Degan, S., Chu, H.W., Voelker, D.R., Sunday, M.E., Cianciolo, G.J., Foster, W.M. Kraft, M. and Wright, J.R. (2009) SP-A preserves airway homeostasis during mycoplasma pneumoniae infection in mice J. Immunol., 182, 7818–7827
30. Holmer, S.M., Evans, K.S., Asfaw, Y.G., Saini, D., Schell, W.A., Ledford, J.G., Frothingham, R., Wright, J.R., Sempowski, G.D. and Perfect, J.R. (2014) Impact of surfactant protein D, interleukin-5, and eosinophilia on Cryptococcosis Infect. Immun., 82, 83–693
31. Bolles, M., Deming, D., Long, K., Agnihothram, S., Whitmore, A., Ferris, M., Funkhouser, W., Gralinski, L., Totura, A., Heise, M. and Baric, R.S. (2011) A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge J. Virol., 85, 12201–12215
32. Akbay, E.A, Koyama, S., Carretero, J., Altabef, A., Tchaicha, J.H., Christensen, C.L., Mikse, O.R., Cherniack, A.D., Beauchamp, E.M., Pugh, T.J. et al. (2013) Activation of the PD-1 pathway contributes to immune escape in EGFRdriven lung tumors Cancer Discov., 3, 1355–1363
OptiPrep™ Application Sheet C30; 7th edition, February 2020
OptiPrep™ Application Sheet C31
Purification of protozoa
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
- There are three Application Sheets devoted to protozoa: C32 – Plasmodium, C33 – Toxoplasma,
while this Application Sheet C31 includes the following sections:
(1) A simple density barrier for recovering protozoa (Cyclospora) from foodstuffs
(2) A discontinuous gradient for purification of Cryptosporidium oocysts
(3) A discontinuous gradient for separation of morphologically distinct merozoites (Sarcocystis)
(4) Comments on other pathogens
1. Isolation of protozoa from foodstuffs
1a. Background
A simple density barrier prepared from OptiPrep™, which permitted the identification of Cyclospora in frozen raspberries [1], should be more generally applicable.
 1b. Solutions required
1b. Solutions required
A. OptiPrep™ (shake gently before use)
B. Salt buffer: 150 mM NaCl, 10 mM EDTA, 100 mM Tris-HCl. pH 8.0
1c. Protocol (from ref 1)
1. Carry out any pre-treatment of the sample that is necessary, then wash the crude cells twice in Solution B and resuspend in the same solution.
2. Mix equal volumes of OptiPrep™ and Solution B and transfer 4 ml to tube for a low speed centrifuge and overlay with 4 ml of the crude suspension.
3. Centrifuge at 250 g for 15 min at 15°C and harvest the parasites by aspirating the top layer plus the top 1.5 ml of the density barrier.
2. Purification of Cryptosporidium oocysts
2a. Background
Cryptosporidium parvum and Cryptosporidium meleagridis oocysts have been purified from fecal matter, usually from bovine or porcine sources, generally in simple two-layer NycodenzⓇ gradients [2- 9], although for the partial separation of Type 1 and Type 2 oocysts linear gradients of the same solute have been used [10]. Chesnot and Schwartzbrod [11] noted that NycodenzⓇ gradients were superior to those of PercollⓇ for oocyst purification, when considering both “recovery and particulate load”. The NycodenzⓇ solutions have been made up either in phosphate-buffered saline [2] or water [6]; there will be a small difference in density between the two types of solvent, but otherwise it is unlikely that the solvent will have any significant effect on the separation.
2b. Sample preparation
Widmer et al [2] first removed coarse debris from the fecal matter by filtration through gauze or low speed centrifugation. The suspension was then mixed with 2 vol. of saturated NaCl and centrifuge at 1000 g for 15 min. After diluting the supernatant with 3 vol. of water, the crude oocysts were pelleted at 4000 g for 15 min before resuspension in a small volume of water.
Akiyoshi et al [7] concentrated the fecal material by centrifugation at 4000 g for 10-15 min. The pellet was resuspended in 0.5% Tween 80 and filtered through gauze to remove debris. After vortexing with an equal volume of diethyl ether the suspension was recentrifuged at 4000 g and all of the ether and aqueous layers aspirated. The crude oocysts were again finally resuspended in small volume of water.
2c. Gradient purification
Widmer et al [2] used a two-layered gradient of 15% and 30% (w/v) NycodenzⓇ with centrifugation at 100,000 g for 1 h; this method was also reported in ref 3-6. Akiyoshi et al [7] and Chappell et al [8] used layers of 10% and 25% and much milder centrifugation conditions of 4000 g for 30 min. Zuckerman et al [9] used the same gradient but a higher g-force – 20,000 g. To make 100 ml of a stock solution of either 25% or 30% (w/v) NycodenzⓇ place approx. 50 ml of water or phosphate-buffered saline (PBS) in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 25 g or 30 g of NycodenzⓇ in small amounts until dissolved. Allow the solution to cool to room temperature and then make up to 100 ml with the chosen solvent. Filter sterilize if required. Dilute the stock solution with water (or PBS) to make 10% or 15% (w/v) solutions, then layer 2.5 ml of each of the NycodenzⓇ solutions in tubes for a swinging-bucket rotor, followed by the sample and centrifuge as required. The oocysts band at the interface between the two NycodenzⓇ solutions.
To separate Type 1 and Type 2 oocysts use either a linear 15-30% or 15-25% (w/v) NycodenzⓇ continuous gradient and centrifuged at 50,000 g for 1 h [10]. Type 2 oocysts are recovered from the top half of the gradient, while although Type 1 oocysts are mainly in the bottom half, there are significant numbers of Type 2 here also.
- As far as we know OptiPrep™ has not been investigated for any of these separations
3. Isolation of morphologically distinct forms of Sarcocystis neurona
3a. Background
To be able to study the cell and molecular biological characteristics of the parasites that cause debilitating diseases in a variety of livestock requires an effective system for their growth in, and release from, cultured cells. Subsequent density gradient centrifugation is often an important add-on technique for the separation of the parasites from cell debris and for resolving morphologically distinct forms of the parasite.
Sarcocystis neurona grows relatively slowly in host cells and the merozoites are also released from the cells rather slowly. Ellison et al [12,13] have described a procedure that uses the calcium ionophore A23187 to cause rapid and synchronous release of the merozoites. A discontinuous iodixanol gradient was also developed by Ellison et al [12] to resolve the different morphological forms of the parasite. Although the merozoite release and density gradient are customized to Sarcocystis neurona, the density gradient strategy may have a wider application to the fractionation of merozoites from any parasite-infected cell monolayers. This Application Sheet is therefore concerned solely with these procedures rather than the cell culture itself.
 3b. Solutions required
3b. Solutions required
A. OptiPrep™ (shake gently before use)
B. Any isoosmotic solution: RPMI, DMEM, Hank’s balanced salt solution, phosphate-buffered saline, etc.
C. A23187 in Solution B (1 μM).
 3c. Protocol (adapted from ref 12)
3c. Protocol (adapted from ref 12)
1. After washing the infected cell monolayer three times in Solution B, incubate in Solution C for 40 min at 37° C in 5% CO2/95% air (see Note 1).
3. Prepare three density gradient solutions of 1.03, 1.04 and 1.06 g/ml (equivalent to 5.4%, 6.4% and 10.3%, w/v iodixanol) by diluting Optiprep™ with Solution B (see Note 2).
4. Transfer 4 ml of the 10.3% iodixanol solution to a 15 ml centrifuge tube and overlayer with the same volume of the two other gradient solutions.
5. Centrifuge the merozoite-containing solution at 300-500 g for 10 min and resuspend the pellet gently in Solution B.
6. Layer 1 ml of the suspension on top of the gradient and centrifuge at 1000 g for 25 min at 20°C (see Note 3).
7. Harvest the banded material and any pellet from the gradient and process as required. To pellet the recovered material dilute the sample with two volumes of Solution B and centrifuge at 300-500g for 10 min (see Figure 1 and Notes 4 and 5).
3d. Notes
1. Any suitable strategy for the efficient release of merozoites should be used.
2. The density of the various gradient solutions may require modulation for the fractionation of parasites from other sources. This can only determined in the light of experience.
3. If the host cell debris persistently contaminates the merozoites then it may be beneficial to layer the suspension in a dense solution (e.g. 1.08 g/ml) beneath the gradient and allow the merozoites to band by flotation. For more information on the preparation of density gradient solutions see Application Sheet C01.
4. The morphology and the nucleus:cytoplasm ratio of the three forms of merozoites that banded in the gradient were distinctively different (see Figure 1). The parasites that banded at interface A were tear-shaped to oblong, while those at interface B were more rounded with a lower nucleus:cytoplasm ratio. The early merozoites banded at interface C along with host cells. For more detailed information about the identity and properties of the banded material see ref 12.
4. Other pathogens
A final continuous 10-50% (w/v) iodixanol gradient was added to an earlier Percoll barrier and continuous sucrose gradient [14-16] in order to improve the purification of spores of the protozoan Enterocytozoon bieneusi (a parasite found in the faeces of primates causing major gastrointestinal symptoms, particularly in patients with AIDS) . Moreover this additional purification step did not cause any loss of recovery (a problem associated with earlier density gradient steps). The gradient was a 10- 50% (w/v) iodixanol (OptiPrep™ diluted with 0.25 M sucrose/1 mM EDTA/10 mM Tris-HCl, pH 7.4) centrifuged at 30,000g for 60 min. The densest spores from a previous gradient [14] were further resolved in the iodixanol gradient into two distinct populations of median density 1.15 and 1.16 g/ml. As with the Sarcocystis neurona separations described above, iodixanol gradients demonstrate an ability to resolve different cell types that is lacking in other density gradient media. Gradients produced from OptiPrep™ have also been used to purify Mattesia orzaephili oocysts [17].
5. Separation of protozoa from bacteria, organic matter and engineered nanomaterials
The new technology of using carbon nanotubes in agriculture and consumer product processing requires effective methods for separating these synthetic particles from biological particles such as protozoa, bacteria and residual fecal matter. Mortimer et al [18] have developed iodixanol gradients that can achieve these types of separation. After an initial centrifugation (5 min @ 600 g) most of the bacteria and free nanotubes (supernatant) remained in the supernatant. To remove the residual free nanotubes from the pelleted material, the latter was resuspended; layered over 10% (w/v) iodixanol and centrifuged at approx. 1800 g for 5min. Re-centrifugation (same g-force and time) of the pelleted material over 20% iodixanol, resolved the protozoa (interface) from the pelleted bacteria and fecal matter. The fate of large number of organic and inorganic compounds in the environment is a major global problem: the uptake of graphene by Tetrahymena thermophila has been studied using iodixanol gradients [19] and iodixanol gradients have successfully been used in the detection of Toxoplasma gondii oocysts in soil samples[20].
6. References
1. Ho, A.Y., Lopez, A.S., Eberhart, M.G., Levenson, R., Finkel, B.S., da Silva, A.J., Roberts, J.M., Orlandi, P.A., Johnson, C.C. and Herwaldt, B.L. (2002) Outbreak of cyclosporiasis associated with imported raspberries, Philadelphia, Pennsylvania, 2000 Emerging Infect. Dis., 8, 783-788
2. Widmer, G., Tchack, L., Chappell, C.L. and Tzipori, S. (1998) Sequence polymorphism in the -tubulin gene reveals heterogeneous and variable population structures in Cryptosporidium parvum Appl. Environ. Microbiol., 64, 4477-4481
3. Widmer, G., Orbacz, E.A. and Tzipori, S. (1999) -tubulin mRNA as a marker of Cryptosporidium parvum oocyst viability Appl. Envir. Microbiol., 65, 1584-1588
4. Widmer, G., Akiyoshi, D., Buckholt, M.A., Feng, X., Rich, S.M., Deary, K.M., Bowman, C.A, Xu, P., Wang, Y., Wang, X., Buck, C.A. and Tzipori, S. (2000) Animal propagation and genomic survey of a genotype 1 isolate of Crystosporidium parvum Mol. Biochem. Parasitol., 108, 187-197
5. Feng, X., Rich, S.M., Akiyoshi, D., Tumwine, J.K., Kekitiinwa, A., Nabukeera, N., Tzipori, S. and Widmer, G. (2000) Extensive polymorphism in Cryptosporidium parvum identified by multilocus microsatellite analysis Appl. Envir. Microbiol., 66, 3344-3349
6. Feng, X., Rich, S.M., Tzipori, S. and Widmer, G. (2002) Experimental evidence for genetic recombination in the opportunistic pathogen Cryptosporidium parvum Mol. Biochem. Parasitol., 119, 55-62
7. Akiyoshi, D.E., Dilo, J., Pearson, C., Chapman, S., Tumwine, J. and Tzipori, S.et al (2003) Characterization of Cryptosporidium meleagridis of human origin passaged through different host species Infect. Immun., 71, 1828-1832
8. Chappell, C.L., Okhuysen, P.C., Langer-Curry, R., Widmer, G., Akiyoshi, D.E., Tanriverdi, S. and Tzipori, S. (2006) Cryptosporidum hominis: experimental challenge of healthy adults Am. J. Trop. Med., 75, 851-857
9. Zuckerman, U., Armon, R., Tzipori, S. and Gold, D. (1999) Evaluation of a portable differential continuous flow centrifuge for concentration of Cryptosporidium oocysts and Giardia cysts from water J. Appl. Microbiol., 86, 955-961
10. Tanriverdi, S., Özkan Arslam, M., Akiyoshi, D.E., Tzipori, S. and Widmer, G. (2003) Identification of genotypically mixed Cryptosporidium parvum populations in humans and calves Mol. Biochem. Parasitol., 130, 13-22
11. Chesnot, T. and Schwartzbrod, J. (2004) Quantitative and qualitative comparison of density-based purification methods for detection of Cryptosporidium oocysts in turbid environmental matrices J. Microbiol. Meth., 58, 375-386
12. Ellison, S.P., Greiner, E. and Dame, J.B. (2001) In vitro culture and synchronous release of Sarcocystis neurona merozoites from host cells Vet. Parasitol., 95, 251-261
13. Ellison, S.P., Greiner, E., Brown, K.W. and Kennedy, T. (2004) Experimental infection of horses with culture-derived Sarcocystis neurona merozoites as a model for equine protozoal myeloencephalitis Intern. J. Appl. Res. Vet. Med., 2, 79-89
14. Zhang, Q., Singh, I., Sheoran, A., Feng, X., Nunnari, J., Carville, A. and Tzipori, S. (2005) Production and characterization of monoclonal antibodies against Enterocytozoon bieneusi purified from rhesus macaques Infect. Immun., 73, 5166-5172
15. Akiyoshi, D.E., Morrison, H.G., Lei, S., Feng, X., Zhang, Q., Corradi, N., Mayanja, H., Tumwine, J.K., Keeling, P.J., Weiss, L.M. and Tzipori, S. (2009) Genomic survey of the non-cultivatable opportunistic human pathogen, Enterocytozoon bieneusi PLoS Pathog., 5: e1000261
16. Zhang, Q., Feng, X., Nie, W., Golenbock, D.T., Mayanja-Kizza, H., Tzipori, S. and Feng, H. (2011) MyD88-dependent pathway is essential for the innate immunity to Enterocytozoon bieneusi Parasite Immunol., 33, 217–225
17. Lord, J.C. (2007) Detection of Mattesia oryzaephili (Neogregarinorida: Lipotrophidae) in grain beetle laboratory colonies with an enzyme-linked immunoadsorbent assay J. Invertebr. Pathol., 94, 74-76
18. Mortimer, M., Petersen, E.J., Buchholz, B.A. and Holden, P.A. (2016) Separation of bacteria, protozoa and carbon nanotubes by density gradient centrifugation Nanomaterials 6: 181
19. Dong, S., Xia, T., Yang, Y., Lin, S. and Mao, L. (2018) Bioaccumulation of 14C‑labeled graphene in an aquatic food chain through direct uptake or trophic transfer Environ. Sci. Technol., 52, 541−549
20. Escotte-Binet, S., Da Silva, A.M., Cancès, B., Aubert, D., Dubey, J., La Carbona, S., Villena, I. and Poulle, M-L. (2019) A rapid and sensitive method to detect Toxoplasma gondii oocysts in soil samples Vet. Parasitol., 274: 108904
OptiPrep™ Application Sheet C31; 8th edition, February 2020
OptiPrep™ Application Sheet C32
Purification of malarial parasites (Plasmodium falciparum, Plasmodium berghei, Plasmodium vivax, and Plasmodium yoelii)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
- See Section 4 regarding use of OptiPrep™ for these separations
1. Background
The use of a 12.5% (w/v) NycodenzⓇ density barrier to enrich for either gametocytes or ookinetes from cultures of Plasmodium falciparum was first described by Carter et al [1] who reported that viability of the parasites purified in this manner is greater than those purified in PercollⓇ. Later, a 10% (w/v) NycodenzⓇ cushion was used to harvest macrogametes and zygotes from Plasmodium berghei while if 12% (w/v) NycodenzⓇ was used the material contained, in addition, ookinetes [2]. In a threelayer gradient of 6%, 11% and 16% (w/v) NycodenzⓇ, macrogametes and zygotes from Plasmodium falciparum banded at the 6%/11% interface [3,4]. This three-layer gradient is a widely used approach for separating various forms of the organism [5-7].
Mons et al [8], who used either a 16% or 16.5% (w/v) NycodenzⓇ cushion to concentrate the parasites from Plasmodium vivax cultures, reported that although the interfacial material contained mainly parasitized erythrocytes, some leukocytes, large erythrocytes and erythrocyte ghosts were observed. The enrichment on the 16% NycodenzⓇ was noticeably higher (400-4200x) than on the 15% NycodenzⓇ (10-200x). The 16.5% NycodenzⓇ barrier was also found to provide an approx. five-fold enrichment of reticulocytes. A barrier of 12-16% (w/v) is widely used to purify a variety of parasitized erythrocytes.
Important Note: in many instances the concentration of NycodenzⓇ is reported as 50% or 60%; these figures are actually the volume percentage of NycoprepⓇ 1.15, which is no longer available commercially. Nycoprep 1.15 was an isoosmotic solution containing 27.6% (w/v) NycodenzⓇ, thus a 50% (v/v) solution is equivalent to 13.8% (w/v) NycodenzⓇ and a 60% solution is equivalent to approx 16.5% (w/v) NycodenzⓇ. Only this concentration format is given in this Application Sheet.
2. Solution preparation
NycodenzⓇ solutions must now be prepared by dissolution of NycodenzⓇ powder in a suitable medium. We suggest making a 30% (w/v) NycodenzⓇ stock solution. To 50 ml of water (stirred gently at 60°C) slowly add 30 g of NycodenzⓇ until completely dissolved. Allow the solution to cool to room temperature; add 10 ml of 100 mM Tris, HEPES or Tricine; adjust the pH to 7.0-7.5 and make up to 100 ml with water. This stock solution may be filter-sterilized if required for storage. When diluted with a balanced salt solution, buffered saline solution or culture medium, to produce solutions of lower density, theses solutions will be approx. isoosmotic with mammalian plasma.
An exception to this preparation of NycodenzⓇ density gradient solutions from powdered NycodenzⓇ, is the purification of Plasmodium falciparum gametocytes by banding at the interface of a NycoprepⓇ 1.077 barrier. NycoprepⓇ 1.077, which is normally used for the purification of human peripheral blood mononuclear cells, is still available commercially (see Table 1)
3. NycodenzⓇ density gradient fractionation
There are such a variety of published pre-gradient operations, density gradient conditions and gradient separation characteristics that selection of one methodology would not be useful. Instead some of the centrifugation protocols and their fractionation characteristics are summarized in Table 1.

4. Use of OptiPrep™
It is very likely that iodixanol can be substituted for NycodenzⓇ in these applications. Certainly the availability of iodixanol as a 60% (w/v) solution (OptiPrep™) makes gradient solution preparation much easier than is the case with NycodenzⓇ. Iodixanol and NycodenzⓇ solutions of the same % (w/v) concentration have almost identical densities, but solutions of NycodenzⓇ are hyperosmotic above 1.15 g/ml, in contrast to those of iodixanol which can be made isoosmotic at all densities. Whether the osmolality of NycodenzⓇ solutions plays an important role in achieving the separations described in this Application Sheet is not known. Comparisons can only be made empirically. For the preparation of iodixanol gradient solutions see Application Sheet C01.
Janse et al [31] described detailed protocols for these organisms. The standard stock solution of NycodenzⓇ was a 1.15 g/ml solution containing 5 mM Tris-HCl, 0.3 mM Ca/Na2 EDTA and 3 mM KCl, which was diluted 1:1 with PBS. 30 ml of schizont-containing blood was underlaid with 10 ml of this medium and centrifuged at 450 g for 20 min to band the schizonts at the interface. The authors also indicated that NycodenzⓇ was considerable less toxic to schizonts than PercollⓇ. A later paper [32] also reported that NycoprepⓇ 1.077A (no longer commercially available) or OptiPrep™ might be used for the isolation of mature schizonts from cultures. The method for the preparation of an iodixanol solution of similar density and osmolality to NycoprepⓇ 1.077A is described in OptiPrep™ Application Sheet C43. The solutions described in Table 1 of Application Sheet C01 are isoosmotic.
More recently Plasmodium falciparum [33,34] and Plasmodium berghei have been purified in iodixanol gradients [33,35]. Cha et al [35] described the use of a two-layer gradient of 15.4 and 10.2% iodixanol centrifuged at 16,500 g for 10 min. The organisms banded at the top of the lower density layer.
5. References
1. Carter, E.H., Suhrbier, A., Beckers, P.J.A. and Sinden, R.E. (1987) The in vitro cultivation of P. falciparum ookinetes, and their enrichment on Nycodenz gradients Parasitology, 95, 25-30
2. Dearsly, A.L., Nicholas, J. and Sinden, R.E. (1987) Sexual development in Plasmodium berghei: the use of mitomycin C to separate infective gametocytes in vivo and ookinetes in vitro Int. J. Parasitol., 17, 1307-1312
3. Quakyi, I.A., Carter, R., Rener, J., Kumar, N., Good, M.F. and Miller, L.H. (1987) The 230 kCa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies J. Immunol., 139, 4213-4217
4. Vermeulen, A.N., Ponnudurai, T., Beckers, P.J.A., Verhave, J-P., Smits, M.A. and Meuwissen, J.H.E.T. (1985) Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito J. Exp. Med., 162, 1460-1476
5. Contreras, C.E., Ploton, I.N., Siliciano, R.F., Karp, C.L., Viscidi, R. and Kumar, N. (1998) Mapping of specific and promiscuous HLA-DR-restricted T-cell epitopes on the Plasmodium falciparum 27-kidalton sexual stage-specific antigen Infect. Immun., 66, 3579-3590
6. Dechering, K.J., Thompson, J., Dodemont, H.J., Eling, W. and Konings, R.N.H. (1997) Developmentally regulated expression of pfs 16, a marker for sexual differentiation of the human malaria parasite Plasmodium falciparum Mol. Biochem. Parasitol., 89, 235-244
7. Healer, J., Graszynski, A. and Riey, E. (1999) Phagocytosis does not play a major role in naturally axquired transmission-blocking immunity to Plasmodium falciparum malaria Infect. Immun., 67, 2334-2339
8. Mons, B., Croon, J.J.A.B., van der Star, W. and van der Kaay, H.J. (1988) Erythrocytic shizogony and invasion of Plasmodium vivax in vitro Int. J. Parasitol., 18, 307-311
9. Al-Layan, E.M., Williams, G.T. and Hurd, H. (2002) Apoptosis in the malaria protozoan, Plasmodium berghei: a possible mechanism for limiting intensity of infection in the mosquito Int. J. Parasitol., 32, 1133-1143
10. Arrighi, R.B.G. and Hurd, H. (2002) The role of Plasmodium berghei ookinete proteins in binding to basal lamina components and transformation into oocysts Int. J. Parasitol., 32, 91-98
11. Blanco, A.R.A., Paez, A., Gerold, P., Dearsly, A.L., Margos, G., Schwarz, R.T., Barker, G., Rodriguez, M.C. and Sinden, R.E. (1999) The biosynthesis and post-translational modification of Pbs21 an ookinete-surface protein of Plasmodium berghei Mol. Biochem. Parasitol., 98, 163-173
12. Carter, V., Cable, H.C., Underhill, B.A., Williams, J. and Hurd, H. (2003) Isolation of Plasmodium berghei ookinetes in culture using Nycodenz density gradient columns and magnetic isolation Malaria J., 2:35
13. Dessens, J.T., Beetsma, A.L., Dimopoulos, G., Wengelnik, K., Crisanti, A., Kafatos, F.C. and Sinden, R.E. (1999) CTRP is essential for mosquito infection by malaria ookinetes EMBO J., 18, 6221-6227
14. Janse, C.J. and Waters, A.P. (1995) Plasmodium berghei: the application of cultivation and purification techniques to molecular studies of malaria parasites Parasitol. Today, 11, 138-143
15. Kocken, C.H.M., van der Wel, A. ., Dubbeld, M.A., Narum, D.L., van de Rijke, F.M., van Gemert, G-J., van der Linde, X., Bannister, L.H., Janse, C., Waters, A.P., and Thomas, A.W. (1998) Precise timing of expression of a Plasmodium falciparum-derived transgene in Plasmodium berghei is a critical determinant of subsequent subcellular localization J. Biol. Chem., 273, 15119-15124
16. Kocken, C.H.M., Narum, D.L., Massougbodji, A., Ayivi, B., Dubbeld, M.A., van der Wel, A., Conway, D.J., Sanni, A. and Thomas, A.W. (2000) Molecular charaterization of Plasmodium reichenowi apical membrane antigen-1 (AMA-1), comparison with P. falciparum AMA-1, and antibody-mediated inhibition of red cell invasion Mol. Biochem. Parasitol., 109, 147-156
17. Kongkasuriyachai, D., Fujioka H. and Kumar, N. (2004) Functional analysis of Plasmodium falciparum parasitophorous vacuole membrane protein (pfs16) during gametocytogenesis and gametogenesis by targeted gene disruption Mol. Biochem. Parasitol., 133, 275-285
18. Kumar, N. (1997) Protein phsophorylation during sexual differentiation in the malaria parasite Plasmodium falciparum Mol. Biochem. Parasitol., 87, 205-210
19. Lobo, C.A., Dhar, R. and Kumar, N. (1999) Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies Infect. Immun. 67, 1688-1693
20. Margos, G., Navarette, S., Butcher, G., Davies, A., Willers, C., Sinden, R.E. and Lachmann, P.J. (2001) Interaction between host complement and mosquito-midgut-stage Plasmodium berghei Infect. Immun., 69, 5064-6071
21. Mota, M.M., Thathy, V., Nussenzweig, R.S. and Nussenzweig, V. (2001) Gene targeting in the rodent malaria parasite Plasmodium yoelii Mol. Biochem. Parasitol., 113, 271-278
22. Narum, D.L. and Thomas, A.W. (1994) Differential localization of full-length and processed forms of PF83/AMA-1 an apical membrane antigen of Plasmodium falciparum merozoites Mol. Biochem. Parasitol., 67, 59-68
23. Narum, D.L., Ogun, S.A., Thomas, A.W. and Holder, A.A. (2000) Immunization with parasite-derived apical membrane antigen 1 or passive immunization with a specific monoclonal antibody protects BALB/c mice against lethal Plasmodium yoelii YM blood-stage infection Infect. Immun., 68, 2899-2906
24. Rodriguez, M.C., Margos, G., Compton, H., Ku, M., Lanz, H., Rodriguez, M.H. and Sinden, R.E. (2002) Plasmodium berghei: routine production of pure gametocytes, extracellular gemetes, zygotes, and ookinetes Exp. Parasitol., 101, 73- 76
25. Fivelman, Q.L., McRobert, L., Sharp, S., Taylor, C.J., Saeed, M., Swales, C.A., Sutherland, C.J. and Baker, D.A. (2007) Improved synchronous production of Plasmodium falciparum gametocytes in vitro Mol. Biochem. Parasitol., 154, 119- 123
26. Leber, W., Skippen, A., Fivelman, Q., Bowyer, P.W., Cockroft, S. and Baker, D.A. (2009) A unique phosphatidylinositol 4-phosphate 5-kinase is activated by ADP-ribosylation factor in Plasmodium falciparum Int. J. Parasitol., 39, 645-653
27. Schnick, C., Polley, S.D., Fivelman, Q.L., Ranford-cartwright, L.C., Wilkinson, S.R., Branningham, J.A., Wilkinson, A.J. and Baker, D.A. (2009) Structure and non-essential function of glycerol kinase in Plasmodium falciparum blood stages Mol. Microbiol., 71, 533-545
28. Fonager, J., Cunningham, D., Jarra, W., Koernig, S., Henneman, A.A., Langhorn, J. and Preiser, P. (2007) Transcription and alternative splicing in the yir multigene family of the malaria parasite Plasmodium y. yoelii: Identification of motifs suggesting epigenetic and post-transcriptional control of RNA expression Mol. Biochem. Parasitol., 156, 1-11
29. Ramalingam, J.K., Hunke, C., Gao, X., Grüber, G. and Preiser, P.R. (2008) ATP/ADP Binding to a Novel Nucleotide Binding Domain of the Reticulocyte-binding Protein Py235 of Plasmodium yoelii J. Biol. Chem., 283, 36386-36396
30. Cunningham, D., Fonager, J., Jarra, W., Carret, C., Preiser, P and Langhorne, J. (2009) Rapid changes in transcription profiles of the Plasmodium yoelii yir multigene family in clonal populations: lack of epigenetic memory? PloS One, 4, e4285 (2009)
31. Janse, C.J., Ramesar, J. and Waters, A.P. (2006) High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei Nat. Protoc., 1, 346–356
32. Wykes, M.N., Kay, J.G., Manderson, A., Liu, X.Q., Brown, D.L., Richard, D.J., Wipasa, J., Jiang, S.H., Jones, M.K., Janse, C.J., Waters, A.P., Pierce, S.K., Miller, L.H., Stow, J.L. and Good, M.F. (2011) Rodent blood-stage Plasmodium survive in dendritic cells that infect naive mice Proc. Natl. Acad. Sci. USA, 108, 11205–11210
33. Ma, J., Trop, S., Baer, S., Rakhmanaliev, E., Arany, Z., Dumoulin, P., Zhang, H., Romano, J., Coppens, I., Levitsky, V. and Levitskaya, J. (2013) Dynamics of the major histocompatibility complex class I processing and presentation pathway in the course of malaria parasite development in human hepatocytes: implications for vaccine development PLoS One, 8: e75321
34. Hain, A.U.P., Bartee, D., Sanders, N.G., Miller, A.S., Sullivan, D.J., Levitskaya, J., Freel Meyers, C. and Bosch, J. (2014) Identification of an Atg8-Atg3 protein-protein interaction inhibitor from the Medicines for Malaria Venture Malaria Box active in blood and liver stage Plasmodium falciparum parasites J. Med. Chem. 2014, 57, 4521−4531
35. Cha, S-J., Park, K., Srinivasan, P., Schindler, C.W., van Rooijen, N., Stins, M. and Jacobs-Lorena, M. (2015) CD68 acts as a major gateway for malaria sporozoite liver infection J. Exp. Med., 212, 1391-1403
OptiPrep™ Application Sheet C32; 8th edition, January 2020
OptiPrep™ Application Sheet C33
Purification of Toxoplasma gondii from cell cultures (Part A) Separation of sporocysts and oocyst walls (Part B)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
Part A
1. Background
Toxoplasma gondii can be maintained in cell culture using Vero, Chinese hamster ovary (CHO) cells or human foreskin fibroblasts (HFF). Coppens et al [1] developed a simple continuous 10-30% NycodenzⓇ gradient to purify the parasite cells away from the host cell material; it has been used in many later studies [2-6]. Although iodixanol gradients have been used separating oocyst walls and sporocysts, they have not been used in this particular Toxoplasma application. Since NycodenzⓇ is only available as a powder, while iodixanol solutions are prepared by simple dilution of OptiPrep™, this alternative is given in the methodology below. Although it is highly likely that this modification would be effective in the purification of the organism, it has not been validated. The following methodology is adapted from ref 1.
2. Solution preparation
A. NycodenzⓇ powder OR
B. OptiPrep™ (shake gently before use)
C. Phosphate-buffered saline (PBS)
D. For NycodenzⓇ solutions only: Phosphate buffer: 100 vol. of 1.78% (w/v) Na2HPO4.2H2O + 25 vol. 1.38% (w/v) NaH2PO4.H2O
To make up a 30% (w/v) NycodenzⓇ stock solution place 50 ml of water in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 30 g of NycodenzⓇ powder in small amounts until dissolved. Allow the solution to cool to room temperature; add 5 ml of Solution D and then make up to 100 ml with water. Filter sterilize if required (see Note 1). For the continuous gradient prepare also a solution of 10% (w/v) NycodenzⓇ by diluting the 30% stock solution with Solution C. For a discontinuous gradient alternative also make up similarly a 20% NycodenzⓇ solution (see Section 3, Step 2).
For the iodixanol option simply dilute OptiPrep™ (60% w/v iodixanol) with Solution C to make a 10% and 30% OR 10%, 20% and 30% (w/v) iodixanol solutions (see Note 1 and Section 3, Step 2).
3. Protocol
1. Culture the Toxoplasma in Vero cells, CHO cells or HFF (see ref 1).
2. Preparation prepare continuous gradients (total volume approx 8 ml in a 15 ml tube or approx 30 ml in a 50 ml tube) of NycodenzⓇ or iodixanol from equal volumes of 10% and 30% NycodenzⓇ or iodixanol using a two-chamber gradient maker or Gradient Master™. If neither of these devices is available prepare discontinuous gradients from equal volumes of 10%, 20% and 30% NycodenzⓇ or iodixanol; carefully rotate the tubes to a horizontal position and allow the gradient to form by diffusion (see Note 2).
3. During gradient production harvest parasites from the cell culture supernatants and pass the suspensions twice through a 27-gauge syringe needle to disrupt any contaminating cells.
4. Wash the Toxoplasma three times in Solution C; centrifuging the suspension each time at 1000 g for 10 min.
5. Finally suspend the pellet in 10% NycodenzⓇ or iodixanol and layer on top of the continuous gradient (see Note 3).
6. Centrifuge at 2000 g for approx. 30 min (see Note 4).
7. Harvest the Toxoplasma that bands around 1.09-1.11 g/ml (just above half way down the gradient).
4. Notes
1. The 30% (w/v) NycodenzⓇ solution will be slightly hyperosmotic (approx. 315 mOsm); all the iodixanol solutions will be isoosmotic with mammalian cells.
2. Diffusion of the discontinuous gradient should take no more than about 1 h at room temperature. If the tubes are kept vertical, the process will take several hours. For more information see Application Sheet C02. Because the Toxoplasma bands at a density just below that of 20% (w/v) NycodenzⓇ or iodixanol, a discontinuous gradient may be effective in the purification process but this has not been tested (as far as we know).
3. The residual buffer on and in the pellet will dilute the gradient medium to allow layering on the 10-30% gradient. If difficulty is encountered in layering the sample, dilute it with about 0.2 ml of Solution C.
4. Do not use the brake for deceleration of the rotor.
5. References
1. Coppens, I., Sinai, A.P. and Joiner, K.A. (2000) Toxoplasma gondii exploits low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition J. Cell Biol., 149, 167-180E
2. Coppens, I. and Joiner, K.A. (2003) Host but not parasite cholesterol controls Toxoplasma cell entry by modulating organelle discharge Mol. Biol. Cell, 14, 3804-3820
3. Quittnat, F., Nishikawa, Y., Stedman, T.T., Voelker, D.R., Choi, J-Y., Zahn, M.M., Murphy, R.C., Barkley, R.M., Pypaert, M., Joiner, K.A. and Coppens, I. (2004) On the biogenesis of lipid bodies in ancient eukaryotes: synthesis of triacylglycerols by a Toxoplasma DGAT1-related enzyme Mol. Biochem. Parasitol., 138, 107-122
4. Nishikawa, Y., Quittnat, F., Stedman, T.T., Voelker, D.R., Choi, J-Y., Zahn, M.., Yang, M., Pypaert, M., Joiner, K.A. and Coppens, I.et al (2005) Host cell lipids control cholesteryl ester synthesis and storage in intracellular Toxoplasma Cell. Microbiol., 7, 849-867
5. Massimine, K.M., Doan, L.T., Atreya, C.A., Stedman, T.T., Anderson, K.S., Joiner, K.A. and Coppens, I et al (2005) Toxoplasma gondii is capable of exogenous folate transport a likely expansion of the BT1 family of transmembrane proteins Mol. Biochem. Parasitol., 144, 44-54
6. Sehgal, A., Bettiol, S., Pypaert, M., Wenk, M.R., Kaasch, A., Blader, I.J., Joiner, K.A. and Coppens, I. (2005) Peculiarities of host cholesterol transport to the unique intracellular vacuole containing Toxoplasma Traffic, 6, 1125- 1141
Part B
1. Background
The resistance of Toxoplasma gondii is thought to be related to the oocyst wall that surrounds the sporocysts [1]. To investigate the nature and functional properties of the oocyst wall, divorced from the sporocysts could be an important step in understanding the infectious properties of this organism.
Although PercollⓇ gradients were able to provide a purified sporocyst fraction, because these particles do not all band in a discrete manner in such gradients, they were unable to provide a simultaneous isolation of a pure oocyst wall fraction [1]. Gradients formed from OptiPrep™ on the other hand are able to provide purified sporocysts and oocyst walls in the same gradient.
The following protocol is adapted from ref 1. Everson et al investigated top-loaded and bottomloaded discontinuous iodixanol gradients and both alternatives are presented in this Application Sheet. It describes only the gradient separation and not the method for mechanical fragmentation of the oocysts –see ref 1 for this information. See ref 2 for a review of Toxoplasma methodology.
 2. Solutions required
2. Solutions required
E. OptiPrep™ (shake gently before use)
F. Diluent: 0.25 M sucrose, 15 mM Tris-HCl, pH 7.5
3. Protocol
1. Fragment the oocysts using glass beads and vortexing according to ref 1.
2. Prepare the following density gradient solutions by diluting OptiPrep™ with Solution B: 2.5%, 5%, 10%, 15%, 20%, 25% and 30% (v/v) OptiPrep™. For bottom loading omit the 30% OptiPrep™ (see Note 1 and Important Note at the end of Section 4).
3. For bottom loading only: mix 7 vol. of the fragmented oocyst suspension with 3 vol. of OptiPrep™ (i.e. adjust the suspension to 30% v/v OptiPrep™) and use this in place of the 30% OptiPrep™ (step 1).
4. Prepare a discontinuous gradient from equal volumes (1-2 ml) of each of the iodixanol solutions; Underlayering is probably the easiest way of creating the gradient (see Notes 2-4).
5. For top-loading only, load the gradient with the fragmented oocysts in 2.5% v/v OptiPrep™ (see Note 5).
6. Centrifuge at 1000 g for approx. 1 h (see Note 6). Do not use the brake for deceleration. 7. Harvest the intact sporocysts which band between 5% and 15% iodixanol and the oocyst walls which band at the 25%/30% interface (see Figure 1 and Notes 7 and 8).
7. Harvest the intact sporocysts which band between 5% and 15% iodixanol and the oocyst walls which band at the 25%/30% interface (see Figure 1 and Notes 7 and 8).
4. Notes
1. Everson et al [1] investigated gradients with step intervals of both 2.5% and 5% OptiPrep™.
2. Underlayering using a metal cannula attached to a 1-2 ml syringe is the best way of creating multiple step gradients. Alternatively a small volume (“low-pulse”) peristaltic pump might be used to introduce each layer, dense end first. Use the pump to take up the aliquot of solution and then reverse the flow to expel into the centrifuge tube. For more information see Application Sheet C02.
3. The gradient will become more or less continuous (particularly if 2.5% steps are used) due to the mixing that is bound to occur and diffusion during the setting up and centrifugation.
4. Dumètre and Dardè [3] used bottom loading with slightly different discontinuous iodixanol gradient of 30%, 25%, 20%, 15% and 5% (v/v). This was also used by Fritz et al [4].
5. Top-loaded gradients tend to give a lower yield of oocyst walls than do bottom-loaded gradients. Although the yield of sporocysts was greater with top-loaded gradients, contamination by some non-sporulated oocysts was greater.
6. Everson et al [1] investigated centrifugation times from 20-100 min. One hour is probably optimal, although if flotation is used some of the non-sporulated oocysts, which are found below the 15% iodixanol, have probably not had time to reach their banding density.
7. Either aspirate observable bands of material or unload the entire gradient by careful aspiration from the meniscus; use a flat-tipped metal cannula (0.8 mm i.d.) attached to a 1-2 ml syringe. Most gradient unloaders are designed for use with flexible thin-walled tubes and not the screw-cap thick-walled tubes routinely used for cells. For more information regarding the harvesting of gradients see Application Sheet S08, accessed from the “Subcellular Membranes” index.
8. Other groups have also reported the use of these OptiPrep™ techniques for the studies of oocysts and sporocysts (e.g. see ref 5 and 6).
IMPORTANT NOTE: published papers below often describe gradient solutions as “% v/v iodixanol”. Since OptiPrep™ is the commercial name for a 60% (w/v) iodixanol solution it has been assumed that the solutions are actually % v/v OptiPrep™.
5. References
1. Everson, W. V., Ware, M. W., Dubey, J. P. and Lindquist, H. D. L. (2002) Isolation of purified oocyst walls and sporocysts from Toxoplasma gondii J. Eukaryot. Microbiol., 49, 344-349
2. Dumètre, A. and Dardé, M-L. (2003) How to detect Toxoplasma gondii oocysts in environmental samples FEMS Microbiol. Rev., 27, 651-661
3. Dumètre, A. and Dardé, M-L. (2005) Immunomagnetic separation of Toxoplasma gondii oocysts using a monoclonal antibody directed against the oocyst wall J. Microbiol. Meth., 61, 209-217
4. Fritz, H.M., Bowyer, P.W., Bogyo, M., Conrad, P.A. and Boothroyd, J.C. (2012) Proteomic analysis of fractionated toxoplasma oocysts reveals clues to their environmental resistance PLoS One 7: e29955
5. Gondim, L.F.P., Wolf, A., Vrhovec, M.G., Pantchev, N., Bauer, C., Langenmayer, M.C., Bohne, W. Teifke, J. P. et al (2016) Characterization of an IgG monoclonal antibody targeted to both tissue cyst and sporocyst walls of Toxoplasma gondii Exp. Parasitol., 163, 46-56
6. Escotte-Binet, S., Da Silva, A.M., Cancès, B., Aubert, D., Dubey, J., La Carbona, S., Villena, I. and Poulle, M-L. (2019) A rapid and sensitive method to detect Toxoplasma gondii oocysts in soil samples Vet. Parasitol., 274: 108904
OptiPrep™ Application Sheet C33; 6th edition January 2020
OptiPrep™ Application Sheet C35
Isolation of neuroglia, inflammatory and glial cells from neural tissue
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List RC06 “Isolation of neural cells from brain and spinal cord” provides a comprehensive bibliography of all the published papers reporting the use of
- OptiPrep™: to access return to the initial list of Folders and select “Reference Lists” To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
In neural tissue there are numerous types of “support cells” which are grouped under the term “Neuroglia” and this Application Sheet is concerned with just two of them. Iodixanol gradients have been used to purify neuroglial cells and also to remove myelin from disaggregated CNS tissue prior to the use of antibody-bound beads to purify oligodendrocytes.
Microglial cells have been isolated both from mouse brain, using the standard methodology of tissue mincing followed by papain [1] or trypsin digestion [2] to disaggregate the cells. They have also been isolated from culture after a six-stage in vitro differentiation of mouse embryonic stem cells [2]. The latter involves expansion of undifferentiated stem cells, followed by generation of embryoid bodies, selection and expansion of nestin-positive cells, differentiation into neurons and expansion of microglia. Details of this methodology are beyond the scope of this Application Sheet and the reader is referred to ref 2 for details.
2. Purification of glial cells from mouse brain
For the gradient purification Bettinger et al [1] used four-step gradient which was overlaid by the sample, while Tsuchiya et al [2] used a two layer gradient flotation strategy; both are given in the protocol. More recently O’Mahony et al [3] also used a four-step gradient to resolve oligodendrocytes, neurons + glial accessory cells and microglia.
2a. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. Hank’s Balanced Salt Solution (see Note 1)
2b. Protocol
1. Prepare the following iodixanol solutions by diluting OptiPrep™ with Solution B: for the four-step gradient [1] 4%, 5.5%, 7% and 10% (w/v) iodixanol OR for the two-step gradient [2] 9.6% and 21.6% (w/v) iodixanol (see Note 2).
2. Mince the brain tissue and pass through a 70 μm mesh sieve.
3. Suspend the mince in Solution B and dissociate the tissue by one of the following methods: (a) 500 μg papain per brain for 20 min at room temperature followed by DNase I (1 mg/ml) at 37°C for 5 min [1] OR (b) 0.25% trypsin and 0.1 mg/ml DNase I for 20 min at 37°C [2].
4. Dilute the suspension with Solution B and harvest the cells by centrifugation at 1,500 g for 10 min (see Note 3).
5. Resuspend the cell pellet in Solution B (for the four-step gradient) OR the 21.6% (w/v) iodixanol solution (for the two-step gradient).
6. For the four-step gradient, layer 1 ml of each of 4%, 5.5%, 7% and 10% (w/v) iodixanol and layer the cell suspension on top OR for the two step gradient layer an equal volume of 9.6% (w/v) iodixanol on top of the cell suspension in 21.6% (w/v) iodixanol (see Notes 4 and 5).
7. Centrifuge at 3000 g for 20 min (four-step gradient) OR 670 g for 20 min (two-step gradient) (see Notes 6 and 7).
8. In the four-step gradient the microglia band in the lowermost layer; in the two-step gradient, they band at the interface (see Notes 7-9).
2c. Notes
1. Any suitable medium – a buffered saline solution or a culture medium can be used in place of the Hank’s Balanced Salt Solution.
2. The gradient used by O’Mahony et al [3] comprised 9%, 12%, 15% and 21% (w/v) iodixanol prepared by diluting OptiPrep™ with Hibernate A/B27 medium. While more recently Song et al [4] and Hong et al [5] pelleted the microglia through a 7.5%, 10.0%, 13.5% and 17% gradient (800 g for 15 min).
3. Tsuchiya et al [2] used a 5 min centrifugation for this step.
4. Larger gradient volumes may provide improved resolving power [3].
5. Discontinuous gradients are normally most easily prepared by underlayering (i.e. low density first) using a syringe (2 ml) and a long metal cannula; overlayering solutions, particularly those which differ in density by only a small amount, is more difficult. For more information about preparing gradients see Application Sheet C02.
6. Tsuchiya et al [2] specified a temperature of 24°C for this step.
7. The gradient system described by O’Mahony et al [3] was centrifuged at 800 g for 15 min. After centrifugation the top 6 ml of the gradient was discarded as debris-containing, then in increasing density bands containing (1) oligodendrocytes, (2) neurons + glial accessory cells, (3) neurons and (4) microglia, were observed.
8. A 6% or 6.2% (w/v) iodixanol barrier has also been used in the separation of neurons and glial cells [6-10].
9. A barrier flotation method was employed by Tucsek et al [11]; cells were suspended in approx. 21% (w/v) iodixanol (OptiPrep™ diluted with Hank’s balanced salt solution) and overlaid with 9.6% iodixanol. After 20 min at 670 g the microglia were harvested from the interface.
3. Removal of myelin for purification of cell fractions from rat brain
The trituration and incubation processes involved in the production of a suitable cell suspension for the isolation of oligodendrocytes from the mid-brain and cerebellar tissue from rat brain are very complex and may vary from laboratory to laboratory. For information on these methods see Section 4. This brief comment is concerned only with the simple one-step gradient strategy for removal of the myelin: Mix the dissociated cell suspension with OptiPrep™ so that the final iodixanol concentration is 9% (w/v) and centrifuge at 800 g for 20 min. Discard the myelin in the supernatant and harvest the cellcontaining fraction in an appropriate medium (see refs 12-16).
4. Other neuronal cell purifications
Oligodendrocytes have been isolated on a four step-gradient prepared from OptiPrep™ with a purity of >90% [3,17-19]. The gradients are similar to those described in “Isolation of brain motoneurons” Application Sheet C36 in index; the oligodendrocytes tend to be relatively low density and are recovered from the top of the gradient.
Astrocytes have also bee purified in a four-step iodixanol gradient [20]. Firstly a working solution (WS) of approx 29.7% (w/v) iodixanol (ρ = 1.161 g/ml) by diluting OptiPrep™ with a 10 mM MOPS – 137 mM NaCl. This was further diluted with DMEM containing 10% foetal bovine serum (FBS). Complete culture medium (containing FBS) has a density of approx 1.009 g/ml. This was then used to dilute the iodixanol WS to produce the four solutions described in Table 1.
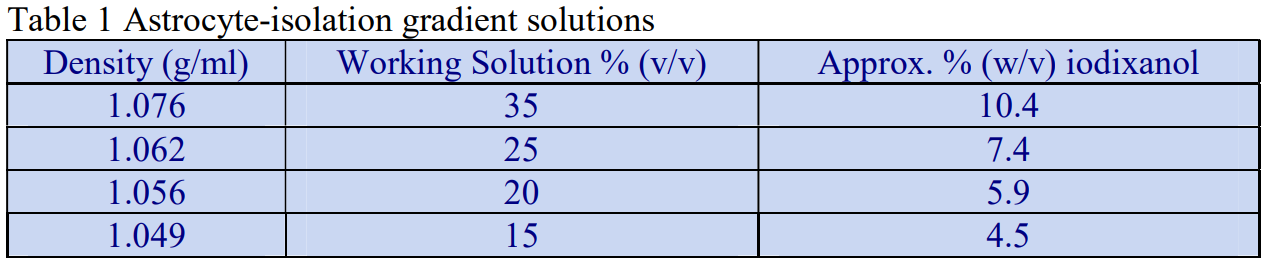
A discontinuous gradient comprising 1 ml of each the gradient solutions was overlayered by 6 ml of the crude cell fraction and centrifuged at approx. 800 g for 15 min [20]. The astrocytes banded across the 1.062/1.056 g/ml interface. These cells have also been purified using the four-step iodixanol gradient described in OptiPrep Application Sheet C36 (see above) [21].
Iodixanol gradients are also a valuable aid to studies on the inflammatory response after traumatic spinal injury. By layering dissociated spinal cord cells over a four-step discontinuous gradient of 4.5%, 6%, 7.5% and 10.5% (w/v) iodixanol (OptiPrep™ initially diluted to 30% iodixanol with 0.15 M NaCl, 10 mM MOPS, pH 7.4 and then further diluted with Hanks Balanced Salt Solution) and centrifuging at 1900 rpm for 15 min., debris remained at the top interface, neurons banded at the three lower interfaces and inflammatory cells and glial cells pelleted [22,23]. Beck et al [24] compared OptiPrep™ with other methods including Percoll™ gradients and concluded that only OptiPrep™ gradients permitted a correct quantitative assessment of the presence of PMNs in spinal injury tissue.
- A methodological review of a number of OptiPrep™-based techniques is given in ref 25.
5. References
1. Bettinger, I., Thanos, S. and Paulus, W. (2002) Microglia promote glioma migration Acta Neuropathol., 103, 351-355
2. Tsuchiya, T., Park, K.C., Toyonaga, S., Yamada, S.M., Nakabayashi, H., Nakai, E., Ikawa, N., Furuya, M., Tominaga, A. and Shimizu, K. (2005) Characterization of microglia induced from mouse embryonic stem cells and their migration into brain parenchyma J. Neuroimmunol., 160, 210-218
3. O’Mahony, A., Raber, J., Montano, M., Foehr, E., Han, V., Lu, S-m., Kwon, H., LeFevour, A., Chakraborty-Sett, S. and Greene, W.C. (2006) NF-B/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity Mol. Biol. Cell., 26, 7283-7298
4. Song, D-Y., Yu, H-N., Park, C-R., Lee, J-S., Lee, J-Y., Park, B-G., Woo, R-S., Han, J-T., Cho, B-P. and Baik, T-K. (2013) Down-regulation of microglial activity attenuates axotomized nigral dopaminergic neuronal cell loss BMC Neurosci., 14: 112
5. Hong, H-B., Krause, H.J., Sohn, S-W., Baik, T-K., Park, J.H., Shin, S-W., Park, C-H. and Song, D-Y. (2014) In situ measurement of superoxide and hydroxyl radicals by frequency mixing detection technique Anal. Biochem., 447, 141-145
6. Goethals, S., Ydens, E., Timmerman, V. and Janssens, S. (2010) Toll-like receptor expression in the peripheral nerve Glia, 58, 1701–1709
7. Papa, S., Rossi, F., Ferrari, R., Mariani, A., De Paola, M., Caron, I., Fiordaliso, F., Bisighini, C., Sammali, E., Colombo, C., Gobbi, M., Canovi, M., Lucchetti, J., Peviani, M., Morbidelli, M., Forloni, G., Perale, G., Moscatelli, D. and Veglianese, P. (2013) Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury ACS Nano, 7, 9881–9895
8. Papa, S., Ferrari, R., De Paola, M., Rossi, F., Mariani, A., Caron, I., Sammali, E., Peviani, M., Dell’Oro, V., Colombo, C., Morbidelli, M., Forloni, G., Perale, G., Moscatelli, D. and Veglianese, P. (2014) Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury J. Control. Release, 174, 15-26
9. Mariani, A., Fanelli, R., Re Depaolini, A. and De Paola, M. (2015) Decabrominated diphenyl ether and methylmercury impair fetal nervous system development in mice at documented human exposure levels. Develop. Neurobiol., 75, 23–38
10. Papa, S., Caron, I., Erba, E., Panini, N., De Paola, M., Mariani, A., Colombo, C., Ferrari, R., Pozzer, D. et al (2016) Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury Biomaterials, 75, 13-24
11. Tucsek, Z., Toth, P., Sosnowska, D., Gautam, T., Mitschelen, M., Koller, A., Szalai, G., Sonntag, W.E., Ungvari, Z. and Csiszar, A. (2014) Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease J. Gerontol. A Biol. Sci. Med..Sci., 69, 1212–1226
12. Chari, D.M., Phil, A., Crang, A.J. and Blakemore, W.F. (2003) Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age J. Neuropathol. Exp. Neurol., 62, 908-816
13. Li, G., Crang, A.J., Rundle, J.L. and Blakemore, W.F. (2002) Oligodendrocyte progenitor cells in the adult rat CNS express myelin oligodendrocyte glycoprotein (MOG) Brain Pathol., 12, 463-471
14. Luyt, K., Varadi, A., Halfpenny, C.A., Scolding, N.J. and Molnar, E. (2004) Metabotropic glutamate receptors are expressed in adult human glial progenitor cells Biochem. Biophys. Res. Comm., 319, 120-129
15. Crang, A.J., Gilson, J.M., Li, W-W. and Blakemore, W.F. (2004) The remyelinating potential and in vitro differentiation of MOG-expressing oligodendrocyte precursors isolated from the adult rat CNS Eur. J. Neurosci., 20, 1445-1460
16. Janes, K., Wahlman, C., Little, J.W., Doyle, T., Tosh, D.K., Jacobson, K.A. and Salvemini, D. (2015) Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in amodel of oxaliplatin-induced peripheral neuropathy Brain, Behav. Immun., 44 91–99
17. Donati D, Akhyani N, Fogdell-Hahn A, Cermelli C, Cassiani-Ingoni R, Vortmeyer A, Heiss JD, Cogen P, Gaillard WD, Sato S, Theodore WH, Jacobson S. (2003) Detection of human herpesvirus-6 in mesial temporal lobe epilepsy surgical brain resections Neurology 61,1405–1411
18. Cassiani-Ingoni, R., Greenstone, H.L., Donati, D., Fogdell-Hahn, A., Martinell, E., Refai, D., Martin, R., Berger, E.A. and Jacobson, S. (2005) CD46 on glial cells can function as a receptor for viral glycoprotein-mediated cell–cell fusion Glia, 52, 252-258
19. Sotnikov, I., Veremeyko, T., Starossom, S.C., Barteneva, N., Weiner, H.L. and Ponomarev, E.D. (2013) Platelets recognize brain-specific glycolipid structures, respond to neurovascular damage and promote neuroinflammation PLoS One, 8: e58979
20. Kerstetter, A.E. and Miller, R.H. (2012) Isolation and culture of spinal cord astrocytes In Methods Mol. Biol., 814, Astrocytes: Methods and Protocols (ed. Milner, R.) Springer Science+Business Media, LLC pp 93-104
21. Freeman, K.A., Fullerton, D.A., Foley, L.S., Bell, M.T., Cleveland, Jr, J.C., Weyant, M.J. Mares, J. et al (2015) Spinal cord protection via alpha-2 agonist-mediated increase in glial cell-line–derived neurotrophic factor J. Thorac. Cardiovasc. Surg., 149, 578-86
22. Galvan, M.D., Luchetti, S., Burgos, A.M., Nguyen, H.X., Hooshmand, M.J., Hamers, F.P.T. and Anderson, A.J. (2008) Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury J. Neurosci., 28, 13876 –13888
23. Nguyen, H.X., Galvan, M.D. and Anderson, A.J. (2008) Characterization of early and terminal complement proteins associated with polymorphonuclear leukocytes in vitro and in vivo after spinal injury J. Neuroinflamm., 5:26
24. Beck, K.D., Nguyen, H.X., Galvan, M.D., Salazar, D.L., Woodruff, T.M. and Anderson, A.J. (2010) Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment Brain 133, 433–447
25. Vincent, A.M. and Feldman, E.L. (2010) Primary sensory and motor neuron cultures In, Protocols for Neural Cell Culture, Springer Protocols Handbooks, (ed. Doering, L.C.), Humana Press (Springer Science+Business Media), Totowa, NJ. pp 161-173
OptiPrep™ Application Sheet C35; 8th edition, February 2020
OptiPrep™ Application Sheet C36
Isolation of brain motoneurons
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Reference List RC06 “Isolation of neural cells from brain and spinal cord” provides a comprehensive bibliography of all the published papers reporting the use of
- OptiPrep™: to access return to the initial list of Folders and select “Reference Lists”
To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box - For the isolation of neuroglial cells see OptiPrep™ Application Sheet C35
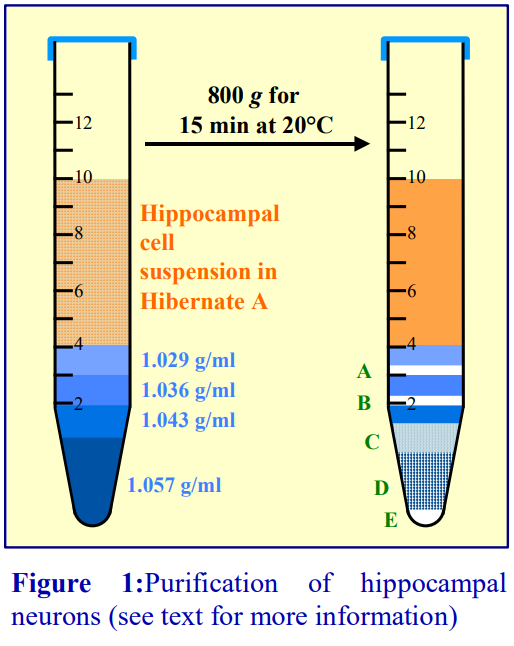 1. Background
1. Background
One of the first NycodenzⓇ-based methods for the isolation of hippocampal neurons from rat was published by Brewer [1]. It used a general-purpose commercial medium, NycoPrepⓇ 1.15 diluted with Hibernate A; the gradient comprised four layers of 15, 20, 25 and 35% (v/v) NycoPrepⓇ 1.15, equivalent to densities of approx 1.029, 1.036, 1.043 and 1.057 g/ml. The crude neuronal cell suspension (6 ml) was placed on top of 1 ml each of the gradient solutions. After centrifugation at 800 g for 15 min the disposition of cells was as shown diagrammatically in Figure 1. The sample zone contained mainly cellular debris; band A contained mainly oligodendroglia; neurons banded across the 1.043 g/ml layer and also within the 1.057 g/ml layer (B, C and D) and the pellet (E) contained mainly microglia [1]. The method was also applied to human post-mortem brain slices [2].
There are a couple of variants of the NycodenzⓇ gradient, both of which reduced the number of layers; one used three layers of 20, 35% and 100% (v/v) NycoPrep 1.15 [3] and the other used just 20% and 60% (v/v) NycoPrepⓇ 1.15 [4]. The ref 4 method also increased the g-force to 2000 g for 20 min. Note that NycoPrepⓇ 1.15 is no longer commercially available.
In 2001 Brewer et al [5] adapted NycodenzⓇ technology to OptiPrep™ and extended the method to human cortical tissue obtained at surgery; the density range of the gradient was again approx 1.029- 1.057 g/ml. As with the NycodenzⓇ gradient (Figure 1) material is observed at each interface and in a pellet. Although neurons are present throughout the gradient, they are most highly enriched in fractions C and D [6]. Sometimes only the densest layer is harvested [5]. Marks et al [7] observed that the plating out of neurons directly from rat hippocampi after enzymic disaggregation and mechanical triturition became notably less efficient as the age of the animals increased from 8 to 35 days. However poor plating efficiency could be overcome by increasing the cell concentration in the suspension applied to substratum. This was achieved by banding the neurons in a four-step discontinuous iodixanol gradient covering the density range 1.026-1.055 g/ml. In this case the neurons were recovered from the lower layer after + the pellet after discarding the upper layers [7]. Liu et al [8] used a similar strategy, although the gradient density range was slightly different.
- The following protocol (adapted from refs 5 and 6) describes preparation of gradient solutions and the gradient centrifugation step, prior techniques for disaggregating the tissue are not included. An excellent detailed account of all the methodology associated with the isolation and culturing of neurons has been produced by Brewer and Torricelli [6].
 2. Solutions required
2. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. OptiPrep™ diluent e.g. 0.85% NaCl, 10 mM MOPS-NaOH, pH 7.4 (see Note 1)
C. Working solution (30%, w/v) iodixanol): mix equal volumes of Solutions A and B.
D. Suspension medium (SM): see Note 2.
3. Protocol
1. Prepare a single cell suspension from the tissue by enyzmic disaggregation and triturition [8].
2. Suspend the cells in the medium of choice, this is normally Hibernate A (see Note 3) which can be supplemented as required [1-3].
3. Prepare gradient solutions of density 1.057, 1.043, 1.036 and 1.029 g/ml: mix Solution C with Solution D at these volume ratios: 0.33:0.67, 0.23:0.77, 0.18:0.82 and 0.13:0.87 (see Notes 4 and 5).
4. In a 15 ml centrifuge tube layer 1 ml of each of the four density solutions and then 6 ml of the cell suspension on top.
5. Centrifuge at 1900 rpm for 15 min at room temperature. Turn the brake off during the deceleration.
6. The distribution of material in the gradient is shown in Figure 1. Brewer et al [5] collected the fraction marked D, while Marks et al [7] included the pelleted material in the neuron harvest (see Note 6).
7. Dilute the fraction with 1-2 volumes of Solution D and harvest the cells by centrifugation.
4. Notes
1. The choice of OptiPrep™ diluent may vary with operational requirements. Brewer et al [3] observed that saline (0.8-0.9% NaCl) buffered with 10 mM MOPS-NaOH, pH 7.4 gave superior results. This diluent may however be any buffered isoosmotic solution; it may be, for example, Hibernate A (see Note 3). For more information about gradient solution preparation see Application Sheet C01.
2. Solution D is usually Hibernate A (see Note 3) but can be any suitable medium that is compatible with neurons. Ehrenreich et al [9] for example used Hanks Balanced Salt Solution; Vasko et al [10] used L-15 medium.
3. For sources of Hibernate A contact Dr Gregory Brewer, Dept of Molecular Biology, Microbiology and Biochemistry, South Illinois University School of Medicine, Springfield, IL 62794-9626 (gbrewer@siumed.edu).
4. The optimal density of the four layers may require some experimentation. For neurons from other species in particular, it may be necessary to modulate the density of the layers. Precise densities should be adjusted in the light of experience. More information on preparation of solutions of different densities can be obtained from Application Sheet C01.
5. The inclusion of a denser layer might be considered (e.g. 1.07 g/ml) if some denser contaminants are present. Whether improved resolution could be obtained by loading the sample in a dense solution below the gradient is one operational variant that might be considered.
6. The optimal harvesting procedure should be worked out once the composition of the zones of banded material (A-D in Figure 1) and the pellet has been verified.
5. References
1. Brewer, G.J. (1997) Isolation and culture of adult rat hippocampal neurons J. Neurosci. Meth., 71, 143-155
2. McManus, D.Q. and Brewer, G.J. (1997) Culture of neurons from postmortem rat brain Neurosci. Lett., 224, 193-196
3. Perez Velazquez, J.L., Kokarovtseva, L., Weisspapir, M. and Frantseva, M.V. (2003) Anti-porin antibodies prevent excitotoxic and ischemic damage to brain tissue J. Neurotrouma, 20, 633-647
4. Coulon, P., Ternaux, J-P., Flamand, A. and Tufffereau, C. (1998) An avirulent mutant of rabies virus is unable to infect motoneurons in vivo and in vitro J. Virol., 72, 273-278
5. Brewer, G. J., Espinosa, J., McIlhaney, M. P., Pencek, T. P., Kesslak, J. P., Cotman, C., Viel, J. and McManus, D. C. (2001) Culture and regeneration of human neurons after brain surgery J. Neurosci. Meth., 107, 15-23
6. Brewer, G.J. and Torricelli, J.R. (2007) Isolation and culture of adult neurons and neurospheres Nat. Protoc., 2, 1490- 1498
7. Marks, J. D., Bindokas, V. P. and Zhang, X-M. (2001) Maturation of vulnerability to excitotoxicity: intracellular mechanisms in cultured postnatal hippocampal neurons Develop. Brain Res., 124, 101-116
8. Liu, Y., Ford, B., Mann, M. A. and Fischbach, G. D. (2001) Neuregulins increase 7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus J. Neurosci., 21, 5660-5669
9. Ehrenreich, H., Degner, D., Meller, J., Brinew, M., Behe, M., Hasselblatt, M., Woldt, H., Falkai, P., Knerlich, F., Jacob, S., von Ahsen, N., Maier, W., Bruck, W., Ruther, E., Cerami, A., Becker, W. and Siren, A-L. (2004) Erythropoietin: a candidate compound for neuroprotection in schizophrenia Mol. Psychiatry, 9, 42-54
10. Vasko, M.R., Guo, C. and Kelley, M.R. (2005) The multifunctional DNA repair/redox enzyme Apel/Ref-1 promotes survival of neurons after oxidative stress DNA Repair, 4, 367-379
6. Acknowledgements
We would like to thank Dr Gregory Brewer, Dept of Molecular Biology, Microbiology and Biochemistry, South Illinois University School of Medicine, Springfield, IL 62794-9626, for his comments in the preparation of this application sheet.
OptiPrep™Application Sheet C36; 8th edition, February 2020
OptiPrep™ Application Sheet C37
Isolation of renal cells: (1) Proximal tubule cells and (2) Interstitial cells and thin loop of Henlé cells
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
- NycodenzⓇ gradients have been used routinely for the fractionation of renal cells (see Sections 1 and 2); it is only very recently that papers reporting the use of OptiPrep™ have been published (see Section 4).
1. Proximal tubule cells
1a. Background
Proximal tubule cells are used as an in vitro model for studies on nephrotoxicity and the method developed by Boogaard et al [1,2] yields 90-95% of proximal tubule cells with a high viability (97%). The perfusion technique is an important part of the methodology and is provided in the protocol, but for information about the preliminary ligation of blood vessels, the operator should refer to ref 1. As with all isolations of cells from tissues, the release of viable cells as a single cell suspension by disaggregation of the tissue with collagenase is a critical part of the procedure.
The following protocol is adapted from ref 1 (see Section 3, Notes 1 and 2).
 1b. Solutions required (see Note 3)
1b. Solutions required (see Note 3)
A. Perfusion medium 1: Calcium-free Hank’s Balanced Salt Solution containing 0.5 mM EGTA and 25 mM HEPES
B. Perfusion Medium 2: As solution A without EGTA.
C. Perfusion Medium 3: As Solution B containing 4 mM CaCl2 and 0.12% collagenase
D. Perfusion Medium 4: As Solution B containing 2.5% (w/v) bovine serum albumin (BSA)
E. NycodenzⓇ stock: 40% (w/v) in water (see Note 4)
F. NycodenzⓇ diluent: 67 mM KCl, 12.2 mM CaCl2, 100 mM HEPES-NaOH, pH 7.4
G. NycodenzⓇ (34%, w/v) solution: Mix 3.4 vol. of Solution E with 0.6 vol. of Solution F and 2 vol. of water
H. Low density barrier solution: mix 1 vol. of solution G with 4 vol. of Solution D (see Note 5).
1c. Protocol
Carry out Steps 1-3 at 37°C and step 5 onwards at 0-4°C. Keep Solutions D, G and H at 0-4°C
1. Perfuse the kidneys with 150 ml of Solution A at 37°C at 10 ml/min; once all the blood has been washed out reduce the flow rate to 7.5 ml/min.
2. After removing kidneys, continue perfusion with 25 ml of Solution B.
3. Perfuse in a recirculating system with Solution C for 18 min.
4. Wash out Solution C with 10 ml Solution D.
5. Remove the capsule and disperse the tissue in Solution D.
6. Filter the cell suspension through two layers of nylon gauze (80 mesh).
7. Centrifuge the cells at 80 g for 3 min and wash the pellet three times in Solution D (see Note 6).
8. Resuspend the cells in 4 ml of Solution D and mix with 2 ml of Solution G.
9. Overlayer 3 ml of the cell suspension with 1 ml of Solution H and 0.5 ml of Solution D (see Note 7).
10. Centrifuge at 2300 g for 3 min (see Note 8).
11. Harvest the proximal tubule cells from the lower interface.
2. Interstitial cells and thin loop of Henlé cells
2a. Background
Interstitial cells and thin limb of Henlé cells from the disaggregated inner medulla have been purified in two steps. Firstly, they are separated from inner medullary collecting duct cells using magnetic beads coated with Dolichos biflorus Agglutinin, which binds almost exclusively to the collecting duct cells. Subsequently purification of the non-collecting duct cells is achieved in a continuous Nycodenz gradient with a density range of 1.052-1.093 g/ml; this is approximately equivalent to 9.4%-17% NycodenzⓇ [3-6]. In the following truncated protocol only the gradient centrifugation is described; for information regarding the preparation of the crude medullary cell suspension see ref 5. The method is taken from ref 5.
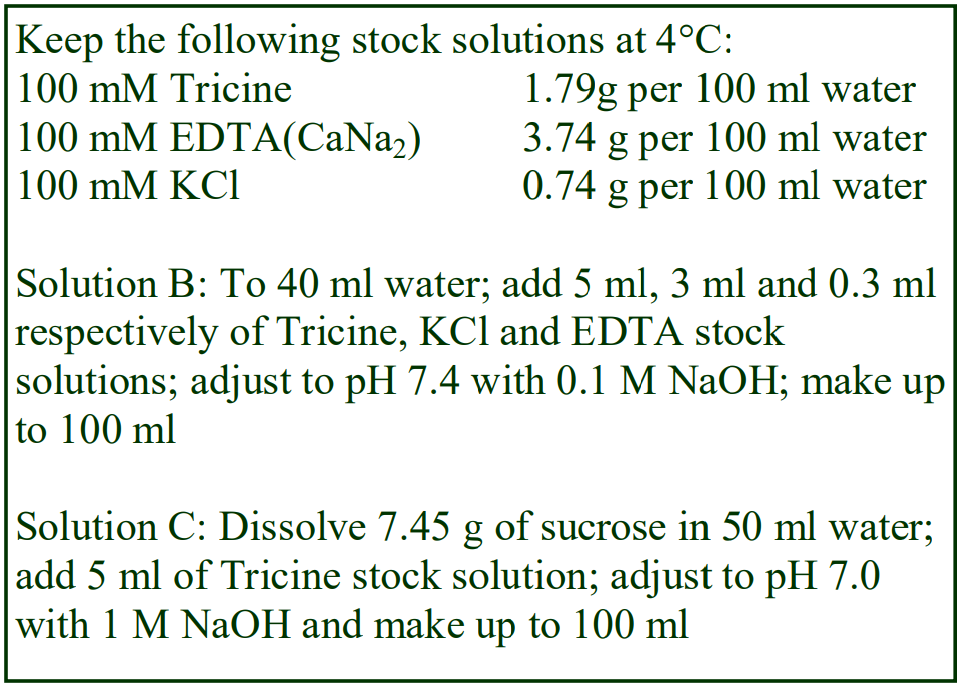 2b. Solutions required (see Notes 3 and 9)
2b. Solutions required (see Notes 3 and 9)
A. NycodenzⓇ Stock Solution, 28% (w/v), ρ = 1.15 g/ml (see step 1 of Protocol)
B. Diluent: 3mM KCl, 0.3 mM CaNa2-EDTA, 5 mM Tricine-NaOH, pH 7.4
C. 7.45% (w/v) sucrose in 5 mM Tricine-NaOH, pH 7.4
2c. Protocol (density gradient only)
1. To make Stock Solution place approx. 50 ml of Solution B in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 28 g of NycodenzⓇ in small amounts until dissolved. Allow the solution to cool to room temperature and then make up to 100 ml with Solution B. Filter sterilize if required.
2. Dilute the 28% NycodenzⓇ solution with Solution C to produce solutions of 8% and 20% (w/v) NycodenzⓇ.
3. In a 15 ml centrifuge tube layer 2 ml of the 8% NycodenzⓇ over 3 ml of the 20% NycodenzⓇ solution and allow a continuous gradient to form by diffusion (see Note 10).
4. Layer the crude cell suspension on top and centrifuge at 1,500 g for 45 min.
5. Allow the rotor to decelerate without the brake.
6. The interstitial cells band at 1.081-1.093 g/ml, approx. equivalent to 15-17% NycodenzⓇ [3,4]. In this same gradient, the thin limb of Henlé cells band predominantly at 1.052-1.069 g/ml, approx equivalent to 9.4%-12.5% NycodenzⓇ [5,6].
7. Recover the appropriate layers using a syringe and metal cannula or unload the gradient in a series of equal volume fractions (see Note 11)
3. Notes
1. A number of other published papers have also reported the use of this methodology for the isolation of rat renal proximal tubule cells [7-18], more or less as described by Boogaard et al [1,2]. There have however, been some small modifications to both the buffers and the gradient. Schaaf et al [19] used a Hank’s Balanced Salt Solution (HBSS) containing PIPES rather than HEPES; these workers also suspended the cells in 14% (w/v) NycodenzⓇ rather than 11.3% NycodenzⓇ and 9% NycodenzⓇ was layered on top rather than 6.8% NycodenzⓇ.
2. Kruidering et al [20] obtained the cells from pigs and used a perfusion medium (Eurocollins, pH 7.4) comprising 177 mM glucose, 10 mM NaHCO3, 15 mM KCl, 42 mM K2HPO4, 15 mM KH2PO4 and 2 mM glycine. In this case, the minced cortex was washed with Ca2+/Mg2+-free HBSS containing 25 mM HEPES and 2 mM glycine. Disaggregation of the minced tissue was achieved in 0.07% collagenase in the same HBSS solution supplemented with 4 mM CaCl2 and 1 mM deferoxamine. The density gradient was rather different, being composed of 8.5% (5 ml), 11.3% (10 ml) and 17% (w/v) NycodenzⓇ (5 ml); the cells being in the 11.3% NycodenzⓇ layer. The centrifugation time was 6 min and the proximal tubule cells were recovered from the upper interface.
3. It is not known if iodixanol can be substituted for NycodenzⓇ in these separations; certainly its availability as a sterile 60% (w/v) solution (OptiPrep™) would make solution preparation more easy – see Application Sheet C01.
4. Keeping NycodenzⓇ as a sterile stock solution of 40% (w/v) in water is a convenient source for preparation of a broad range of gradients. To make the stock solution place approx. 50 ml of water in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 40 g of Nycodenz in small amounts until dissolved. Allow the solution to cool to room temperature and then make up to 100 ml with water. Filter sterilize if required.
5. This 6.8% NycodenzⓇ solution has a density of approx 1.037 g/ml.
6. Carry out the resuspension of the cells after each centrifugation very gently, with the minimum of shearing forces.
7. For more information about making gradients see Application Sheet C02.
8. Do not use the brake to decelerate the rotor.
9. Although in ref 5 Tris was used as the buffer, the more “cell-friendly” Tricine has been substituted in this Application Sheet. The approx. isoosmotic solution of sucrose (Solution C) used as the diluent for the stock NycodenzⓇ solution has a density of approx 1.28 g/ml. In view of the known toxicity of sucrose to mammalian cells, a variant might be to use an isoosmotic 4.5% (w/v) solution of NycodenzⓇ, which has the same density. This can be produced by dilution of Solution A with any normal buffered saline solution.
10. Diffusion was allowed to occur after the tube was capped and rotated to a horizontal position; for more details on the formation of continuous gradients see Application Sheet C02.
11. A gradient unloading device such as the Beckman Fraction Recovery System may also be used. Information for calculating density from refractive index and absorbance measurements can be found in Application Sheet S09 (Subcellular membranes index).
4. OptiPrep™
More recently there has been considerable interest in the use OptiPrep™ in the selection of renal cells for transplantation, the well-established innocuous nature of the gradient medium being cited as being of prime importance.
The first reports of the use of OptiPrep™ for rodent kidney cells involved studies on chronic kidney disease using cultured kidney cells [21]. First of all a 15% (w/v) iodixanol barrier (ρ = 1.085 g/ml) was used to remove erythrocytes from the initial cell suspension of UNFX cells. After an initial period of culture the cells were fractionated on a four-step discontinuous iodixanol gradient of 7%, 11%, 13% and 16% (w/v) iodixanol (approx. ρ = 1.042, 1.064, 1.074 and 1.090 g/ml). After centrifugation at 800 g for 20 min at room temperature, the cells were resolved into four major subfractions. Epithelial cells of the tubular and collecting ducts were enriched in the second band across the 7%/11% iodixanol interface (designated 1.045-1.063 g/ml), while non-tubular cells were enriched in the fourth band across the 11%/16% iodixanol interface (designated 1.073-1.091 g/ml). The technique was later extended and optimized to kidney cells of other species [22]: for canine kidney the four iodixanol concentrations were 7%, 10%, 11% and 16% (w/v) and for human 7%, 9%, 11% and 16% (w/v).
Centrifugation of a suspension of urothelial cells (neurogenic and non-neurogenic tissue) from patient bladder biopsy material considerably facilitated the recovery of viable cells for culture [23]. More recently Genheimer et al [24] used a similar gradient system of 16%, 13%, 11% and 7% (w/v) iodixanol; OptiPrep™ was diluted with un-supplemented keratinocyte serum-free medium (KSFM), and the same centrifugation conditions, for rat kidney cells. The authors employed a mixture of cells from the second and fourth band (at a 97:3 ratio) as a source of injectable bioactive renal tubular cells. For the use of viable tubule cells in a regenerative medicine approach to “neurogenic bladder disease that is refractory to medical treatment” Presnell et al [22] and Bruce et al [23] have stressed the importance of the use a density gradient medium (iodixanol) that is manufactured according to cGMP and which, as an X-ray imaging agent, is approved for clinical use. These publications [21-24] have highlighted the potential use of renal tubular epithelial cells for transplantation into patients with chronic kidney disease.
Detailed methodologies have been published by Bruce et al [25] and Halberstadt et al [26]. Bruce et al [25] used a 30% (w/v) iodixanol stock from equal volumes of OptiPrep™ and KSFM, which was mixed 1:1 with the crude cell suspension and a small volume of PBS layered on top. Cells were collected from the interface and pellet after centrifugation at 800 g for 15 min. After further culture of the cells they were resolved by sedimentation through 7% and 16% (w/v) iodixanol (800 g for 20 min. The cells at the sample/7% iodixanol layer were enriched in tubular epithelial cells and collecting duct cells, while proximal tubular cells were resolved at the lower interface. Halberstadt et al [26] used a second gradient of 7%, 11%, 13% and 16% (w/v) iodixanol at 800 g for 20 min.
5. References
1. Boogaard, P.J., Mulder, G.J. and Nagelkerke, J.F. (1989) Isolated proximal tubule cells from rat kidney as an in vitro model for studies on nephrotoxicity: I An improved method fir preparation of proximal tubular cells and their functional characterization by α-methylglucose uptake Toxicol. Appl. Pharmacol., 101, 135-143
2. Boogaard, P.J., Slikkerveer, A., Nagelkerke, J.F. and Mulder, G.J. (1991) The role of metallothionein in the reduction of cisplastin-induced nephrotoxicity by Bi3+ -pretreatment in the rat in vivo and in vitro Biochem. Pharmacol., 41, 369-375
3. Theilig, F., Bostanjoglo, M., Pavenstadt, H., Grupp, C., Holland, G., Slosarek, I., Gressner, A.M., Russwurm, M., Koesling, D. and Bachmann, S. (2001) Cellular distribution and function of soluble guanylyl cyclase in rat kidney and liver J. Am. Soc. Nephrol., 12, 2209-2220
4. Steffgen, J., Kampfer, K., Grupp, C., Langenberg, C., Muller, G. A. and Grunewald, R. W. (2003) Osmoregulation of aldose reductase and sorbitol dehydrogenase in cultivated interstitial cells of rat renal inner medulla Nephrol. Dial. Transplant., 18, 2255-2261
5. Grupp, C., Begher, M., Cohen, D., Raghunath, M., Franz, H-E. and Muller, G.A. (1998) Isolation and characterization of the lower portion of the thin limb of Henle in primary culture Am. J. Physiol., 274, F775-F782
6. Boisvert, C., Pare, C., Veyrat-Durebex, C., Robert, A., Dubuisson, S., Morel, G. and Gaudreau, P. (2002) Localization and regulation of a functional GHRH receptor in the rat renal medulla Endocrinology, 143, 1475-1484
7. Haenen, H.E.M.G., Spenkelink, A., Teunissen, C., Temmink, J.H.M., Koemana, J.H. and van Bladeren, P.J. (1996) Transport and metabolism of glutathione conjugates of menadione and ethacrynic acid in confluent monolayers of rat proximal tubular cells Toxicology 112, 117-130
8. Haenen, H.E.M.G., Bleijlevens, E., Elzerman, H., Temmink, J.H.M., Koeman, J.H. and van Bladeren, P.J. (1996) Cytotoxicity of 2-tert-butyl hydroquninone glutathione conjugates after apical and basolateral exposure of rat renal proximal tubular cell monolayers Toxicol. in Vitro, 10, 141-148
9. Terlouw, S.A., Masereeuw, R., van den Broek, P.H.H., Notenboom, S. and Russel, F.G.M. (2001) Role of multidrug resistance protein 2 (MRP2) in glutathione-bimane efflux from Caco-2 and rat proximal tubule cells Br. J. Pharmacol., 134, 931-938
10. Van de Water, B., Zoeteweij, J.P., de Bont, H.J.G.M., Mulder, G.J. and Nagelkerke, J.F. (1994) Role of mitochondrial Ca2+ in the oxidative stress-induced dissipation of the mitochondrial membrane potential: Studies in isolated proximal tubular cells using the nephrotoxin 1,2-dichlorovinyl-L-cysteine J. Biol. Chem., 269, 14546-14552
11. Van de Water, B., Nagelkerke, J.F. and Stevens, J.L. (1999) Dephosphorylation of ofcal adhesion kinase (FAK) and loss of focal contacts precedes caspase-mediated cleavage of FAK during apoptosis in renal epithelial cells J. Biol. Chem., 274, 13328-13337
12. Van de Water, B., Tijdens, I.B., Verbrugge, A., Huigsloot, M., Dihal, A.A., Stevens, J.L., Jaken, S. and Mulder, G.J. (2000) Cleavage of the actin-capping protein -adducin at Asp-Asp-Ser-Asp633-Ala by caspase-3 is preceded by its phosphorylation on serine 726 in cisplantin-induced apoptosis of renal epithelial cells J. Biol. Chem., 275, 25805-25813
13. Boogaard, P.J., Commandeur, J.N.M., Mulder, G.J., Vermeulen, N.P.E. and Nagelkerke, J.F. (1989) Toxicity of the cysteine-S-conjugates and mercapturic acids of dour structurally related difluoroethylenes in isolated proximal tubular cells from rat kidney uptake of the conhugates and activation to toxic metbolites Biochem. Pharmacol., 38, 3731-3741
14. Boogaard, P.J., Zoeteweij, J.P., Van Berkel, T.J.C., Van’t Noordende, J.M., Mulder, G.J. and Nagelkerke, J.F. (1989) Primary culture of proximal tubular cells from normal rat kidney as an in vitro model to study mechanisms of nephrotoxicity Biochem. Pharmacol., 39, 1335-1345
15. Boogaard, P.J., Nagelkerke, J.F. and Mulder, G.J. (1990) Renal proximal tubular cells in suspension or in primary culture as in vitro models to study nephrotoxicity Chem.-biol. Interactions, 76, 251-292
16. Viver, M.M.I., Nagelkerke, J.F. and Mulder, G.J. (1992) Stereospecific glutathione conjugation of (R)- and (S)-2- bromoisovalerylurea in freshly isolated rat kidney tubular cells Biochem. Pharmacol., 43, 902-904
17. Haenen, H.E.M.G., Rogmans, P., Temmink, J.H.M., and van Bladeren, P.J. (1994) Differential detoxification of two thioether conjugates of menadione in confluent monolayers of rat renal proximal tubular cells Toxic. in Vitro, 8, 207-214
18. De Graauw, M., Le Dévédec, S., Tijdens, I., Smeets, M.J., Deelder, A.M. van de Water, B. (2007) Proteomic analysis of alternative protein tyrosine phosphorylation in 1,2-dichlorovinyl-cysteine-induced cytotoxicity in primary cultured rat renal proximal tubular cells J. Pharmacol. Exp. Ther., 322, 89-100
19. Schaaf, G.J., de Groene, E.M., Maas, R.F., Commandeur, J.N.M. and Fink-Gremmels, J. (2001) Characterization of biotransformation enzyme activities in primary rat proximal tubular cells Chemico-Biol., Interact., 134, 167-190
20. Kruidering, M., van de Water, B., de Heer, E., Mulder, G.J. and Nagelkerke, J.F. (1997) Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: Mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain J. Pharmacol. Exp. Therapeut., 280, 638-649
21. Kelley, R., Werdin, E.S., Bruce, A.T., Choudhury, S., Wallace, S.M., Ilagan, R.M., Cox, B.R., Tatsumi-Ficht, P., Rivera, E.A., Spencer, T., Rapoport, H.S., Wagner, B.J., Guthrie, K., Jayo, M.J., Bertram, T.A., Presnell, S.C. (2010) Tubular cell-enriched subpopulation of primary renal cells improves survival and augments kidney function in rodent model of chronic kidney disease Am. J. Physiol. Renal Physiol., 299, F1026–F1039
22. Presnell, S.C., Bruce, A.T., Wallace, S.M., Choudhury, S., Genheimer, C.W., Cox, B., Guthrie, K., Werdin, E.S., Tatsumi-Ficht, P., Ilagan, R.M., Kelley, R.W., Rivera, E.A., Ludlow, J.W., Wagner, B.J., Jayo, M.J. and Bertram, T.A. (2011) Isolation, characterization, and expansion methods for defined primary renal cell populations from rodent, canine, and human normal and diseased kidneys Tissue Eng., Part C, 17, 261-273
23. Bruce, A.T., Sangha, N., Richmond, A., Johnson, K., Jones, S., Spencer, T. and Ludlow, J.W. (2010) Use of iodixanol self-generated density gradients to enrich for viable urothelial cells from non-neurogenic and neurogenic bladder tissue Tissue Eng., Part C Methods 16, 33-40
24. Genheimer, C.W., Ilagan, R.M., Spencer, T., Kelley, R.W., Werdin, E., Choudhury, S., Jain, D., Ludlow, J.W. and Basu, J. (2012) Molecular characterization of the regenerative response induced by intrarenal transplantation of selected renal cells in a rodent model of chronic kidney disease Cells Tissues Organs, 196, 374–384
25. Bruce, A.T., Guthrie, K.I. and Kelley, R. (2013) Ex vivo culture and separation of functional renal cells In Methods Mol. Biol., 1001, Organ Regeneration: Methods and Protocols (eds. Basu, J. and Ludlow, J.W.) Springer Science+Business Media, LLC pp 53-64
26. Halberstadt, C., Robbins, N., McCoy, D.W., Guthrie, K.I., Bruce, A.T., Knight, T.A. and Payne, R.G. (2013) Formulation of selected renal cells for implantation into a kidney In Methods Mol. Biol., 1001, Organ Regeneration: Methods and Protocols (eds. Basu, J. and Ludlow, J.W.) Springer Science+Business Media, LLC pp 279-287
OptiPrep™ Application Sheet C37; 7th edition, January 2020
OptiPrep™ Application Sheet C38
(I) Maintenance of cells in suspension for chemical/physical measurements
(II) Microfluidic cell encapsulation/cell sorting
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
PART I: Maintenance of cells in suspension for chemical/physical measurements
1. Background
This is a non-centrifugal application, in which OptiPrep™ is mixed with a suspension of cells in order to prevent their sedimentation during an extended physical measurement or separation. The first published paper to report this novel application was in 2001, when Farinas, Chow and Wada [1] commented in the abstract to their paper: “Recent developments in microfluidics have enabled the design of a lab-on-a-chip system capable of measuring cellular membrane potential. The chip accesses liquid samples sequentially by sipping from a micro-plate through a capillary, mixes the samples with cells flowing through a microchannel, contacts the cells with potential-sensitive dyes, and reads out cellular responses using fluorescence detection”. Later Culbertson [2] noted that the use of OptiPrep™ enabled the rate of cell entry into the main chip channel of a microfluidic device to remain constant over the course of an entire experiment. The technique permits the identification of single cells with unique compositional characteristics that may presage the later development of a disease state. In its simplest form, a medium with the same density as the cell, which in no way interacts with the cell, maintains the cell in a state of continuous suspension.
Section 2 briefly describes solution preparation and the physical parameters of some the solutions that have been used in these studies. Section 3 summarizes the cell types that have been studied and types of physico-chemical measurements that were carried out. Section 4 lists some more recent publications. Section 6 is the reference list.
2. Solution preparation
Mix OptiPrep™ (shake the bottle gently before use) with saline or a balanced salt solution to give a solution of suitable density, according to the figures in Table 1. Table 2 summarizes a few of the conditions reported in the literature. For more information on making up solutions see Application Sheet C01.
- The actual density required will depend on the cell type; it can be chosen by suspending the cells in a series of density solutions and observing the disposition of the cells after centrifugation. Because of the heterogeneity of any cell population however it is impossible to select a density that will allow all the cells to remain in suspension, some will float and some will sediment. If the cell suspension contains a variety of cell types the problem will be exaggerated. For example human peripheral blood mononuclear cells have a density range of 1.058-1.078 g/ml.
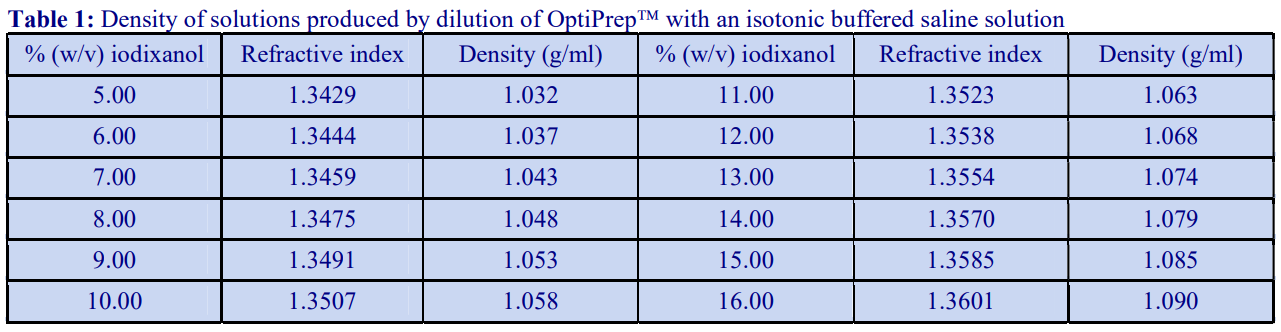
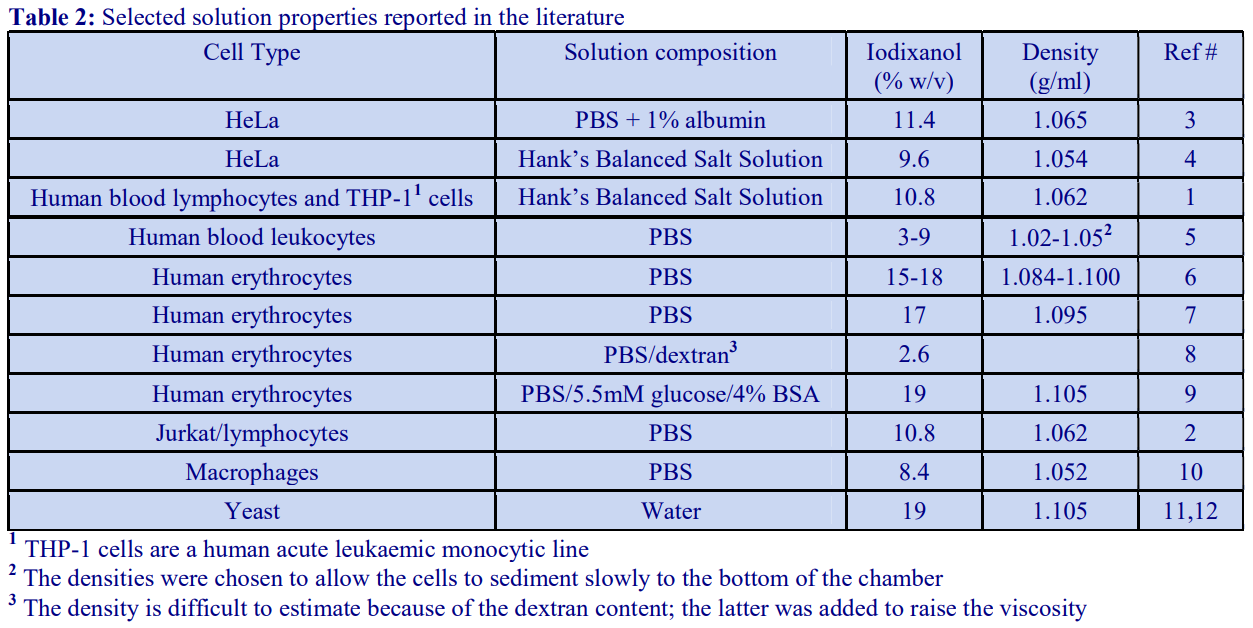
3. Cell types and study topic
Breast carcinoma cells
Iso-acoustic focusing for phenotyping [29]
Radiometric assays [50]
Viability of cells in alginate particles [13]
Bronchial epithelial cells
Confocal light absorption and scattering spectroscopic (CLASS) microscopy [14]
Cancer cell lines, distinction of
SERS-microfluidic droplet platform [58]
CHO cells
Microfluidic cytometry [15, 48]
Embryonic spinal cord cells
Ribosome reduction [57]
Embryonic stem cells
Gene expression in single cells [16]
Erythrocytes
Acoustic cell manipulation [38]
Blood viscosity changes in response to exercise [17]
Channel flow profiling [9]
Cholesterol [53]
Density matching [39]
Highly mono-dispersed microdroplet production [35]
Lysis kinetics [7]
Nitric oxide scavenging by haemoglobin [6, 8]
Phase separation in micro-channel networks [31]
Plasma membrane cholesterol [52]
Shape and mechanical properties of erythrocytes [18]
Escherishia coli
Genetically encoded synthesis of nanomaterials [32]
Fibroblasts
Hydrogel encapsulation [12]
Francisella tularensis
Cell sorting [10]
HeLa cells
Cell heterogeneity in metal ion responses [4]
Fluorescence-activated microfluidic devices for cell identification and sorting [3, 19, 20, 21]
Microfluidic cytometry [45, 49]
Hepatocarcinoma cells
Microfluidic cytometry [15]
Hepatocytes
Microfluidic cytometry [42]
HL60 cells
Differential detection photothermal spectroscopy: label-free detection [37]
Human stem cells
Pancreatic β-cell transformation [52]
Industrial effluent analysis
Membrane bioreactors [22]
Jurkat cells
Microfluidic cytometry [15, 43, 46]
Microfluidic devices for cell identification and sorting [2]
Leukocytes (human)
Acoustic cell manipulation [38]
Continuous concentration of cells [23]
Density matching [39]
Microfluidic devices for cell identification and sorting; orientation-dependent elastic light scattering [5]
Lung adenocarcinoma epithelial cells
Microfluidic cytometry [41]
Lymphocytic cells (incl. lymphoblasts and other similar cells)
Cell membrane potential measurements using potential-sensitive dyes [1]
Cell size and density changes; response to growth factor deprivation [24]
Hydrogel encapsulation [12]
Iso-acoustic focusing for phenotyping [29]
Microfluidic cytometry [15, 43]
PCR-based sorting [30]
Viscoelastic carrier fluids [51]
Mesenchymal stem cells
Microfluidic cytometry [47]
Mouse bone marrow stromal cells
Microfluidic cytometry [44]
Mouse hybridoma cells
Analysis and sorting of single cells [25]
Mouse pro-B lymphoid cells
Cell size, density and deformability [26]
Mouse insulinoma cells
Enzymatic crosslinking of tyramine-functionalized polymer droplets [34]
Neural cells
Density matching [40]
Single cell barcoding – droplet microfluidics [33]
Prostate cancer cells
Printed droplet microfluidics: dispensing of picoliter droplets and cells [36]
Rat liver lysosomes (stained with red tracker dye)
Confocal light absorption and scattering spectroscopic (CLASS) microscopy [14]
Review articles
Extracellular matrix [56]
Recent advances in research involving chemical and biological reactions in microdroplets [27]
Staphylococcus aureus
Microchannels [54]
Tumor-infiltrating myeloid cells
Tumour growth regulation [55]
Yeast cells
Acoustic cell manipulation [38]
Biogenic magnetization [28]
Hydrogel encapsulation [12]
Polyglycerol microgel particles for the micro-encapsulation of yeast cells [11]
4. Physical measurements (recent publications)
Density matching: see refs 59 and 60
Droplet sorting/merging: see ref 61
Microfluidic cytometry: see refs 62-66
Single cell analysis: see ref 67
5. References
1. Farinas, J., Chow, A.W. and Wada, H.G. (2001) A microfluidic device for measuring cellular membrane potential Anal. Biochem., 295, 138-142
2. Culbertson, C. (2006) Single cell analysis on microfluidic devices Methods Mol. Biol., 339, 203-216
3. Wang, M.M., Tu, E., Raymond, D.E., Yang, J.M., Zhang, H., Hagen, N., Dees, B., Mercer, E.M. et al (2005) Microfluid sorting of mammalian cells by optical force switching Nature Biotech., 23, 83-87
4. Ma, H., Gibson, E.A., Dittmer, P.J., Jimenez, R. and Palmer, A.E. (2012) High-throughput examination of fluorescence resonance energy transfer-detected metal-ion response in mammalian cells J. Am. Chem.Soc., 134, 2488−2491
5. Watson, D., Hagen, N., Diver, J., Marchand, P. and Chachisvilis, M. (2004) Elastic light scattering from single cells: orientational dynamics in optical trap Biophys. J., 87, 1298-1306
6. Azarov, I., Huang, K.T., Basu, S., Gladwin, M.T., Hogg, N. and Kim-Shapiro, D.B. (2005) Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation J. Biol. Chem., 280, 39024-39032
7. SooHoo, J.R., Herr, J.K., Ramsey, J.M. and Walker, G.M. (2012) Microfluidic cytometer for the characterization of cell lysis Anal. Chem., 84, 2195−2201
8. Azarov, I., Liu, C., Reynolds, H., Tsekouras, Z., Lee, J.S., Gladwin, M.T. and Kim-Shapiro, D.B. (2011) Mechanisms of slower nitric oxide uptake by red blood cells and other hemoglobin-containing vsicles J. Biol. Chem., 286, 33567–33579
9. Roman, S., Lorthois, S., Duru, P. and Risso, F. (2012) Velocimetry of red blood cells in microvessels by the dual-slit method: Effect of velocity gradients Microvasc. Res., 84, 249–261
10. Perroud, T.D., Kaiser, J.N., Sy, J.C., Lane, T.W., Branda, C.S. Singh, A.K. and Patel, K.D. (2008) Microfluidic-based cell sorting of Francisella tularensis infected macrophages using optical forces Anal. Chem., 80, 6385-6372
11. Steinhilber, D., Seiffert, S., Heyman, J.A., Paulus, F., Weitz, D.A. and Haag, R. (2011) Hyperbranched polyglycerols on the nanometer and micrometer scale Biomaterials, 32, 1311-1316
12. Rossow, T., Heyman, J.A., Ehrlicher, A.J., Langhoff, A., Weitz, D.A., Haag, R. and Seiffert, S. (2012) Controlled synthesis of cell-laden microgels by radical-free gelation in droplet microfluidics J. Am. Chem. Soc., 134, 4983−4989
13. Akbar, S. and Pirbodaghi, T. (2014) Microfluidic encapsulation of cells in alginate particles via an improved internal gelation approach Microfluid Nanofluidics, 16, 773–777
14. Itzkan, I., Qiu, L., Fang, H., Zaman, M.M., Vitkin, E., Ghiran, I.C., Salahuddin, S., Modell, M. et al (2007) Confocal light absorption and scattering spectroscopic microscopy monitors organelles in live cells with no exogenous labels Proc. Natl. Acad. Sci. USA, 104, 17255-17260
15. Cheung, M.C., McKenna, B., Wang, S.S., Wolf, D. and Ehrlich, D.J. (2015) Image-based Ccll-resolved screening assays in flow Cytometry Part A, 87A, 541-548
16. Klein, A.M., Mazutis, L., Akartuna, I., Tallapragada, N., Veres, A., Li, V., Peshkin, L., Weitz, D.A. and Kirschner, M.W. (2015) Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells Cell, 161, 1187–1201
17. Buono, M.J., Krippes, T., Kolkhorst, F.W., Williams, A.T. and Cabrales, P. (2016) Increases in core temperature counterbalance effects of haemoconcentration on blood viscosity during prolonged exercise in the heat Exp. Physiol., 101.2, 332–342
18. Shen, Z., Coupier, G., Kaoui, B., Polack, B., Harting, J., Misbah, C. and Podgorski, T. (2016) Inversion of hematocrit partition at microfluidic bifurcations Microvasc. Res., 105, 40–46
19. Eastburn, D.J., Sciambi, A. and Abate, A.R. (2014) Identification and genetic analysis of cancer cells with PCR-activated cell sorting Nucleic Acids Res., 42: e128
20. Rajauria, S., Axline, C., Gottstein, C. and Cleland, A.N. (2015) High-speed discrimination and sorting of submicron particles using a microfluidic device Nano Lett.,15, 469− 475
21. Pallaoro, A., Hoonejani, M.R., Braun, G.B., Meinhart, C.D. and Moskovits, M. (2015) Rapid identification by surface-enhanced Raman spectroscopy of cancer cells at low concentrations flowing in a microfluidic channel ASC Nano, 9, 4328–4336
22. Blanco, L., Hermosilla, D., Blanco, A., Swinnen, N., Prieto, D. and Negro, C. (2015) Assessment of the performance of membrane bioreactors applied to the treatment of industrial effluents containing poly(vinyl alcohol) Ind. Eng. Chem. Res., 54, 5442−5449
23. Martel, J.M., Smith, K.C., Dlamini, M., Pletcher, K., Yang, J., Karabacak, M., Haber, D.A., Kapur, R. and Toner, M. (2015) Continuous flow microfluidic bioparticle concentrator Sci. Rep., 5: 11300
24. Hecht, V.C., Sullivan, L.B., Kimmerling, R.J., Kim, D-H., Hosios, A.M., Stockslager, M.A., Stevens, M.M., Kang, J.H. et al (2016) Biophysical changes reduce energetic demand in growth factor-deprived lymphocytes J. Cell Biol., 212, 439-447
25. Mazutis, L., Gilbert, J., Ung, W.L., Weitz, D.A., Griffiths, A.D. and Heyman, J.A. (2013) Single-cell analysis and sorting using droplet-based microfluidics Nat. Protoc., 8, 870-891
26. Byun, S., Hecht, V.C. and Manalis, S.R. (2015) Characterizing cellular biophysical responses to stress by relating density, deformability, and size Biophys. J., 109, 1565–1573
27. Devenish, S.R.A., Kaltenbach, M., Fischlechner, M. and Hollfelder, F. (2013) Droplets as reaction compartments for protein nanotechnology In Methods Mol. Biol., 996, Protein Nanotechnology: Protocols, Instrumentation, and Applications (ed. Gerrard, J.A.) Springer Science+Business Media, LLC pp 269-286
28. Nishida, K. and Silver, P.A. (2012) Induction of biogenic magnetization and redox control by a component of the target of rapamycin complex 1 signaling pathway PLoS Biol., 10: e1001269
29. Augustsson, P., Karlsen, J.T., Su, H-W., Bruus, H. and Voldman, J. (2016) Iso-acoustic focusing of cells for size-insensitive acousto-mechanical phenotyping Nat. Comm., 7: 11556
30. Pellegrino, M., Sciambi, A., Yates, J.L., Mast, J.D., Silver, C. and Eastburn, D.J. (2016) RNA-Seq following PCR-based sorting reveals rare cell transcriptional signatures BMC Genom., 17: 361
31. Clavica, F., Homsy, A., Jeandupeux, L. and Obrist, D. (2016) Red blood cell phase separation in symmetric and asymmetric microchannel networks: effect of capillary dilation and inflow velocity Sci. Rep., 6: 36763
32. Liu, X., Lopez, P.A., Giessen, T.W., Giles, M., Way, J.C. and Silver, P.A. (2016) Engineering geneticallyencoded mineralization and magnetism via directed evolution Sci. Rep., 6: 38019
33. Zilionis, R., Nainys, J., Veres, A., Savova, V., Zemmour, D, Klein, A.M. and Mazutis, L. (2017) Single-cell barcoding and sequencing using droplet microfluidics Nat. Protoc., 12, 44-73
34. Kamperman, T., Henke, S., Zoetebier, B., Ruiterkamp, N., Wang, R., Pouran, B., Weinans, H., Karperien, M. and Leijten, J. (2017) Nanoemulsion-induced enzymatic crosslinking of tyramine-functionalized polymer droplets J. Mater. Chem. B, 2017, 5, 4835-4844
35. Crawford, D.F., Smith, C.A. and Whyte, G. (2017) Image-based closed-loop feedback for highly monodispersed microdroplet production Sci. Rep., 7: 10545 6
36. Cole, R.H., Tang, S-Y., Siltanen, C.A., Shahi, P., Zhang, J.Q., Poust, S., Gartner, Z.J. and Abate, A.R. (2017) Printed droplet microfluidics for on demand dispensing of picoliter droplets and cells Proc. Natl. Acad. Sci. USA, 114, 8728–8733
37. Maceiczyk, R.M., Hess, D., Chiu, F.W.Y., Stavrakis, S. and deMello, A.J. (2017) Differential detection photothermal spectroscopy: towards ultra-fast and sensitive label-free detection in picoliter & femtoliter droplets Lab Chip, 17, 3654–3663
38. Lenshof, A., Johannesson, C., Evander, M., Nilsson, J. and Laurel, T. (2016) Acoustic cell manipulation In Microtechnology for Cell Manipulation and Sorting; Microsystems and Nanosystems (eds Lee, W. et al.), Springer International Publishing Switzerland, pp 129-173
39. Mutlu, B.R., Smith, K.C., Edd, J.F., Nadar, P., Dlamini, M., Kapur, R. and Toner, M. (2017) Nonequilibrium inertial separation array for high-throughput, large volume blood fractionation Sci. Rep., 7: 9915
40. Allen, W.E., DeNardo, L.A., Chen, M.Z., Liu, C.D., Loh, K.M., Fenno, L.E., Ramakrishnan, C., Deisseroth, K. and Luo, L. (2017) Thirst-associated preoptic neurons encode an aversive motivational drive Science 357, 1149–1155
41. Xia, B., Krutkramelis, K. and Oakey, J. (2106) Oxygen-purged microfluidic device to enhance cell viability in photopolymerized PEG hydrogel microparticles Biomacromolecules 17, 2459−2465
42. Siltanen, C., Diakataou, M., Lowen, J., Haque, A., Rahimian, A., Stybayeva, G. and Revzin, A. (2017) One step fabrication of hydrogel microcapsules with hollow core for assembly and cultivation of hepatocyte spheroids Acta Biomaterialia, 50, 428–436
43. Shahi, P., Kim, S.C., Haliburton, J.R., Gartner, Z.J. Abate, A. R. (2017) Abseq: Ultrahigh-throughput single cell protein profiling with droplet microfluidic barcoding Sci. Rep., 7: 44447
44. Mao, A.S., Shin, J-W., Utech, S., Wang, H., Uzun, O., Li, W., Cooper, M., Hu, Y. Zhang, L., Weitz, D.A. and Mooney, D.J. (2017) Deterministic encapsulation of single cells in thin, tunable microgels for niche modelling and therapeutic delivery Nat Mater., 16, 236-243
45. Cheng, Z., Wu, X.,, Cheng, J. and Liu, P. (2017) Microfluidic fluorescence-activated cell sorting (μFACS) chip with integrated piezoelectric actuators for low-cost mammalian cell enrichment Microfl. Nanofl., 21: 9
46. Yan, Z., Clark, I.C. and Abate, A.R. (2017) Rapid encapsulation of cell and polymer solutions with bubbletriggered droplet generation Macromol. Chem. Phys., 218: 1600297
47. Lienemann, P.S., Rossow, T., Mao, A.S., Vallmajo-Martin, Q., Ehrbar, M. and Mooney, D.J. (2017) Single cell-laden protease-sensitive microniches for long-term culture in 3D Lab Chip, 17, 727-437
48. Rajeswari, P.K.P., Joensson, H.N., Andersson-Svahn, H. (2017) Droplet size influences division of mammalian cell factories in droplet microfluidic cultivation Electrophoresis, 38, 305–310
49. Fiedler, B.L., Van Buskirk, S., Carter, K.P., Qin, Y., Carpenter, M.C., Palmer, A.E. and Jimenez, R. (2017) Droplet microfluidic flow cytometer for sorting on transient cellular responses of genetically-encoded sensors Anal. Chem., 89, 711−719
50. Gallina, M.E., Kim, T.J., Shelor, M., Vasquez, J., Mongersun, A., Kim, M., Tang, S.K.Y., Abbyad, P. and Pratx, G. (2017) Toward a droplet-based single-cell radiometric assay Anal. Chem., 89, 6472−6481
51. Holzner, G., Stavrakis, S. and deMello, A. (2017) Elasto-inertial focusing of mammalian cells and bacteria using low molecular, low viscosity PEO solutions Anal. Chem., 89, 11653−11663
52. Veres, A., Faust, A.L., Bushnell, H.L., Engquist, E.N., Kenty, J.H-R., Harb, G., Poh, Y-C., Sintov, E., Gürtler, M. et al (2019) Charting cellular identity during human in vitro β-cell differentiation Nature 569, 368-373
53. Ayuyan, A.G. and Cohen, F.S. (2018) The chemical potential of plasma membrane cholesterol: implications for cell biology Biophy. J., 114, 904–918
54. Mutlu, B.R., Edd, J.F. and Toner, M. (2018) Oscillatory inertial focusing in infinite microchannels Proc. Natl. Acad. Sci. USA. 115, 7682-7687
55. Zilionis, R., Engblom, C., Pfirschke, C., Savova, V., Zemmour, D., Saatcioglu, H.D., Krishnan, I., Maroni, G., Meyerovitz, C.V. et al (2019) Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species Immunity 50, 1317–1334
56. Matellan, C. and del Rı́o Hernández, A.E. (2019) Engineering the cellular mechanical microenvironment – from bulk mechanics to the nanoscale J. Cell Sci., 132, jcs229013
57. Niepel, M., Hafner, M., Mills, C.E., Subramanian, K., Williams, E.H., Chung, M., Gaudio, B., Barrette, A.M. et al (2019) A multi-center study on the reproducibility of drug-response assays in mammalian cell lines Cell Sys., 9, 35–48
58. Cong, L., Liang, L., Cao, F., Sun, D., Yue, J., Xu, W., Liang, C. and Xu, S. (2019) Distinguishing cancer cell lines at a single living cell level via detection of sialic acid by dual-channel plasmonic imaging and by using a SERS-microfluidic droplet platform Microchim. Acta 186: 367
59. Yang, X., Zhou, T., Zwang, T.J., Hong, G., Zhao, Y., Viveros, R.D., Fu, T-M., Gao, T. and Lieber, C.M. (2019) Bioinspired neuron-like electronics Nat. Mater., 510, 510–517
60. Li, Q., Tang, F., Huo, X., Huang, X., Zhang, Y., Wang, X. and Zhang, X. (2019) Native state single-cell printing system and analysis for matrix effects Anal. Chem. 2019, 91, 8115−8122
61. Chung, M.T., Kurabayashi, K. and Cai, D. (2019) Single-cell RT-LAMP mRNA detection by integrated droplet sorting and merging Lab Chip, 19, 2425-2434
62. Kim, S.C., Clark, I.C., Shahi, P. and Abate, A.R. (2018) Single-cell RT-PCR in microfluidic droplets with integrated chemical lysis Anal. Chem., 90, 1273−1279
63. Losserand, S., Coupier, G. and Podgorski, T. (2019) Migration velocity of red blood cells in microchannels Microvasc. Res., 124, 30–36
64. Lee, J., Mena, S.E. and Burns, M.A. (2019) Micro-particle operations using asymmetric traps Sci. Rep. 9: 1278
65. Rahimian, A., Siltanen, C., Feyzizarnagh, H., Escalante, P. and Revzin, A. (2019) Microencapsulated immunoassays for detection of cytokines in human blood ACS Sens., 4, 578−585
66. Li, L., Wu, P., Luo, Z., Wang, L., Ding, W., Wu, T., Chen, J., He, J., He, Y. et al (2019) Dean flow assisted single cell and bead encapsulation for high performance single cell expression profiling ACS Sens. 4, 1299−1305
67. Matuła, K., Rivello, F. and Huck, W.T.S. (2020) Single-cell analysis using droplet microfluidics Adv. Biosys., 4: 1900188
(II) Microfluidic cell encapsulation/cell sorting
This new application of OptiPrepTM has only very recently featured regularly in the literature; publications are listed alphabetically according to cell type, or “methodology” or “reviews”.
Adipose tissue stem cells
Morandi, E.M., Verstappen, R., Zwierzina, M.E., Geley, S., Pierer, G. and Ploner, C. (2016) ITGAV and ITGA5 diversely regulate proliferation and adipogenic differentiation of human adipose derived stem cells Sci. Rep., 6: 28889
Bacteria
Jusková, P., Schmid, Y.R.F., Stucki, A., Schmitt, S., Held, M. and Dittrich, P.S. (2019) “Basicles”: microbial growth and production monitoring in giant lipid vesicles ACS Appl. Mater. Interfaces, 11, 34698−34706
Carcinoma cells
Eastburn, D.J., Sciambi, A. and Abate, A.R. (2013) Ultrahigh-throughput mammalian single-cell reversetranscriptase polymerase chain reaction in microfluidic drops Anal. Chem., 85, 8016-8021
Jiang, Z., Xia, B., McBride, R. and Oakey, J. (2017) A microfluidic-based cell encapsulation platform to achieve high long-term cell viability in photopolymerized PEGNB hydrogel microspheres J. Mater. Chem. B, 5, 173-180
Pei, H., Li, L., Wang, Y., Sheng, R., Wang, Y., Xie, S., Shui, L., Si, H. and Tang, B. (2019) Single-cell phenotypic profiling of CTCs in whole blood using an integrated microfluidic device Anal. Chem., 91, 11078−11084
Sun, D., Cao, F., Cong, L., Xu, W., Chen, Q., Shic, W. and Xu, S. (2019) Cellular heterogeneity identified by single-cell alkaline phosphatase (ALP) via a SERRSmicrofluidic droplet platform Lab Chip, 19, 335-342
B-lymphocytes
Eastburn, D.J., Sciambi, A. and Abate, A.R. (2013) Ultrahigh-throughput mammalian single-cell reversetranscriptase polymerase chain reaction in microfluidic drops Anal. Chem., 85, 8016-8021
Holzner, G., Du, Y., Cao, X., Choo, J., deMello, A.J. and Stavrakis, S. (2018) An optofluidic system with integrated microlens arrays for parallel imaging flow cytometry Lab Chip, 18, 3631–3637
Bone marrow cells
Asensio, M.A., Lim, Y.W., Wayham, N., Stadtmiller, K., Edgar, R.C., Leong, J., Leong, R., Mizrahi, R.A.
Adams, M.S. et al (2019) Antibody repertoire analysis of mouse immunization protocols using microfluidics and molecular genomics mAbs 11, 870–883
Petukhov, V., Guo, J., Baryawno, N., Severe, N., Scadden, D.T., Samsonova, M.G. and Kharchenko, P.V. (2018) dropEst: pipeline for accurate estimation of molecular counts in droplet-based single-cell RNA-seq experiments Genome Biol. 19: 78
Breast cancer cells
Hinohara, K., Wu, H-J., Vigneau, S., McDonald, T.O., Igarashi, K.J., Yamamoto, K.N., Madsen, T., Fassl, A and Egri, S.B. et al (2018) KDM5 histone demethylase activity links cellular transcriptomic heterogeneity to therapeutic resistance Cancer Cell 34, 939–953
Hsu, M.N., Wei, S-C., Guo, S., Phan, D-T., Zhang, Y. and Chen, C-H. (2018) Smart hydrogel microfluidics for single-cell multiplexed secretomic analysis with high sensitivity Small, 14: 1802918
Erythrocytes
Crawford, D.F., Smith, C.A. and Whyte, G. (2017) Image-based closed-loop feedback for highly mono-dispersed microdroplet production Sci. Rep., 7: 10545
Fenech, M., Girod, V., Claveria, V., Meance, S., Abkarian, M. and Charlot, B. (2019) Microfluidic blood vasculature replicas using backside lithography Lab Chip, 19, 2096-2106
Heart cells/tissue
Guerzoni, L.P.B., Tsukamoto, Y., Gehlen, D.B., Rommel, D., Haraszti, T., Akashi, M. and De Laporte, L. (2019) A layer-by-layer single-cell coating technique to produce injectable beating mini heart tissues via microfluidics Biomacromolecules, 20, 3746−3754
HEK cells
Kolb, L., Allazetta, S., Karlsson, M., Girgin, M., Weber, W. and Lutolf, M.P. (2019) High-throughput stem cellbased phenotypic screening through microniches Biomater. Sci., 7, 3471-3479
Jurkat cells
Clark, I.C. and Abate, A.R. (2018) Microfluidic bead encapsulation above 20 kHz with triggered drop formation Lab Chip, 18, 3598–3605
Xu, Y., Lee, J-H., Li, Z., Wang, L., Ordog, T. and Bailey, R.C. (2018) A droplet microfluidic platform for efficient enzymatic chromatin digestion enables robust determination of nucleosome positioning Lab Chip, 18, 2583-2592
Lymph node cells
Asensio, M.A., Lim, Y.W., Wayham, N., Stadtmiller, K., Edgar, R.C., Leong, J., Leong, R., Mizrahi, R.A. Adams, M.S. et al (2019) Antibody repertoire analysis of mouse immunization protocols using microfluidics and molecular genomics mAbs 11, 870–883
Lymphoblasts
Mutafopulos, K., Spink, P., Lofstrom, C.D., Lu, P.J., Lu, H., Sharpe, J.C., Franke, T. and Weitz. D.A. (2019) Traveling surface acoustic wave (TSAW) microfluidic fluorescence activated cell sorter (μFACS) Lab Chip, 19, 2435-2443
Methodology
Doonan, S.R., Lin, M. and Bailey, R.C. (2019) Droplet CAR-Wash: continuous picoliter-scale immunocapture and washing Lab Chip, 19, 1589-1598
Park, J., Destgeer, G., Kim, H., Cho, Y. and Sung, H.J. (2018) In-droplet microparticle washing and enrichment using surface acoustic wave-driven acoustic radiation force Lab Chip, 18, 2936–2945
Sahore, V., Doonan, S.R. and Bailey, R.C. (2018) Droplet microfluidics in thermoplastics: device fabrication, droplet generation, and content manipulation using integrated electric and magnetic fields Anal. Methods, 10, 4264–4274
Microglia
Gunner, G., Cheadle, L., Johnson, K.M., Ayata, P., Badimon, A., Mondo, E., Nagy, M.A., Liu, L., Bemiller, S.M. et al (2019) Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signalling Nat. Neurosc., 22, 1075–1088
Mononuclear cells
Karthick, S., Pradeep, P.N., Kanchana, P. and Sen, A.K. (2018) Acoustic impedance-based size-independent isolation of circulating tumour cells from blood using acoustophoresis Lab Chip, 18, 3802–3813
Mouse lung cancer cells
Petukhov, V., Guo, J., Baryawno, N., Severe, N., Scadden, D.T., Samsonova, M.G. and Kharchenko, P.V. (2018) dropEst: pipeline for accurate estimation of molecular counts in droplet-based single-cell RNA-seq experiments Genome Biol. 19: 78
Myeloid leukaemia tumour
Pellegrino, M., Sciambi, A., Treusch, S., Durruthy-Durruthy, R., Gokhale, K., Jacob, J., Chen, T.X., Geis, J.A. et al (2018) High-throughput single-cell DNA sequencing of acute myeloid leukemia tumors with droplet microfluidics Genome Res.,28, 1345–1352
Neurons
Luo, B., Tian, L., Chen, N., Ramakrishna, S., Thakor, N. and Yang, I.H. (2018) Electrospun nanofibers facilitate better alignment, differentiation, and long-term culture in an in vitro model of the neuromuscular junction (NMJ) Biomater. Sci., 6, 3262–3272
Pancreatic islets
Weaver, J.D., Headen, D.M., Coronel, M.M., Hunckler, M.D., Shirwan, H. and García, A.J. (2019) Synthetic poly(ethylene glycol)‐based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site Am J Transplant. 2019, 19, 1315–1327
Promyelocytic leukaemia cells
Hsu, M.N., Wei, S-C., Guo, S., Phan, D-T., Zhang, Y. and Chen, C-H. (2018) Smart hydrogel microfluidics for single-cell multiplexed secretomic analysis with high sensitivity Small, 14: 1802918
Reviews
Ding, Y., Choo, J. and deMello, A.J. (2017) From single‑molecule detection to next‑generation sequencing: microfluidic droplets for high‑throughput nucleic acid analysis Microfluid Nanofluid, 21: 58
Matellan, C. and del Rı́o Hernández, A.E. (2019) Engineering the cellular mechanical microenvironment – from bulk mechanics to the nanoscale J. Cell Sci., 132, jcs229013
Spleen cells
Asensio, M.A., Lim, Y.W., Wayham, N., Stadtmiller, K., Edgar, R.C., Leong, J., Leong, R., Mizrahi, R.A. Adams, M.S. et al (2019) Antibody repertoire analysis of mouse immunization protocols using microfluidics and molecular genomics mAbs 11, 870–883
Mutafopulos, K., Spink, P., Lofstrom, C.D., Lu, P.J., Lu, H., Sharpe, J.C., Franke, T. and Weitz. D.A. (2019) Traveling surface acoustic wave (TSAW) microfluidic fluorescence activated cell sorter (μFACS) Lab Chip, 19, 2435-2443
Stem cells
Siltanen, C., Yaghoobi, M., Haque, A., You, J., Lowen, J., Soleimani, M. and Revzin, A. (2016) Microfluidic fabrication of bioactive microgels for rapid formation and enhanced differentiation of stem cell spheroids Acta Biomater., 34, 125–132
T-cells
Segaliny, A.I., Li, G., Kong, L., Ren, C., Chen, X., Wang, J.K., Baltimore, D., Wu, G. and Zhao, W. (2018) Functional TCR T cell screening using single-cell droplet microfluidics Lab Chip, 18, 3733–3749
Yeast cells
Manna, P., Hung, S-T., Mukherjee, S., Friis, P., Simpson, D.M., Lo, M.N., Palmer, A.E. and Jimenez, R. (2018) Directed evolution of excited state lifetime and brightness in FusionRed using a microfluidic sorter Integr. Biol., 10, 516-526
Zebrafish cells
Tang, Q., Iyer, S., Lobbardi, R., Moore, J.C., Chen, H., Lareau, C., Hebert, C., Shaw, M.L. et al (2017) Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing J. Exp. Med., 214, 2875–2887
OptiPrep™ Application Sheet C38; 1st edition, February 2020
OptiPrep™ Application Sheet C39
Purification of bacteria from soil, stream-water, clinical specimens and food
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box.
- Section 7 briefly addresses the isolation of bacteria from host tissues/organisms
- Note that purification of obligate intracellular bacteria is addressed in Application Sheet C49
1. Background
The use of a NycodenzⓇ density barrier of approx 1.3 g/ml density to isolate bacteria from soil and surface water samples was first reported in 1995 by Lindahl and Bakken [1]; this paper also contains a review of the methods used for soil dispersion. The preferred method for the detachment of the bacteria from the soil samples is the use of a rotating blades blender, normally a Waring blender. Other techniques have been investigated including sonication, reciprocal shaking and a rotating pestle, but the blender was preferred over these other techniques as the most reliable [1]. The sterile medium used to suspend the soil particles is quite variable; in some cases water is used; other examples are a 0.05 M phosphate buffer, 0.15% NaCl, a phosphate-buffered saline solution, organic buffer such as 10-50 mM Tris-HCl, pH 7.5-8.0, which may be supplemented with either 0.2M NaCl or 1 mM EDTA.
The published literature widely reports the use of this NycodenzⓇ 1.3 g/ml density barrier. In terms of the recovery, purity and functional integrity of the bacteria, NycodenzⓇ has been shown to be superior both to inorganic salts such as CsCl and Na2WO4 and other non-ionic gradient media such as metrizamide and PercollⓇ [2,3]. Lindahl [4] noted that the NycodenzⓇ barrier method could effect an almost complete separation of bacteria from the soil particles and percentage recoveries can be as high as 80% [5]. There are a few examples of the use of a slightly lower density for the barrier, e.g. 1.26 g/ml [6].
The same NycodenzⓇ density barrier strategies have been used for the isolation of bacteria from fecal matter [7,8] and from food [9]. Hazebrouck et al [8] used a 1.26 g/ml barrier.
Now the methodology for the isolation of Salmonella enterica from soil has been adapted to iodixanol [10] and the latter’s availability as a sterile solution of density 1.32 g/ml (OptiPrep™) makes it very easy to use in such an application. Neat OptiPrep™ is used directly as the density barrier or diluted with a buffered isoosmotic solution, i.e. there is no need to for the lengthy preparation of a dense solution from NycodenzⓇ powder nor is sterilization of the solution required. Because of the ease of solution preparation the method given in Sections 3-5 is based solely on the use of OptiPrep™.
In a recent paper by Pascaud et al [11], a three-layered gradient of 1.10, 1.15 and 1.30 g/ml (2 ml of each) was produced by dilution of OptiPrep ™ with 0.25 M sucrose, 6 mM EDTA, 60 mM Tris-HCl, pH 7.4 and the soil suspension (5 ml) layered on top. Nousiainen et al [12] have also adapted the standard NycodenzⓇ to OptiPrep™.
2. Centrifugation strategies
In the original methodology [1], 2 ml of the suspension was layered upon 7 ml of the NycodenzⓇ barrier and centrifuged at 10,000 g for 20 min. The volume ratio of sample:barrier is normally 2-3:1. Much larger scale separations are also possible e.g. 25 ml over 11.6 ml [13] 90 ml of sample over 30 ml of density barrier [14]. The most frequently used centrifugation conditions are 10,000 g for 20-30 min; occasionally higher g-forces are used, e.g. 25,000 g for 1 h [15] occasionally much lower, e.g. 3000 g for 20 min with a sample:barrier volume ratio of 10 [16].
The methodology has also been adapted for multiple analyses to the use of approx 2 ml microcentrifuge tubes with 800 μl layered over 700 μl of density barrier [e.g. ref 6]. Ref. 15 describes the use of a 1.34 g/ml barrier.
3. Reagents required
A. OptiPrep™ (shake the bottle gently before use)
B. Saline (sterile) or other suitable buffer (see Section 1)
4. Centrifuge requirement
High-speed centrifuge or ultracentrifuge with swinging-bucket rotor of appropriate tube volume, or microcentrifuge
5. Protocol
1. Sieve the soil through a 2 mm mesh and suspend in saline (10 g soil per 100 ml).
2. Macerate in a Waring blender (or other rotating blades device) at full speed for a total time of 3-5 min at 4°C, using 1 min “bursts” and 1 min “rests” to dissipate any heat (see Note 1).
3. Density barrier method: Either use pure OptiPrep ™ for the barrier or mix 10 vol. of OptiPrep with 0.1 vol. of a 100x buffer (see Notes 2). Then layer the macerated sample over the chosen density barrier using a volume ratio of 4:2.5 (see Notes 3 and 4).
4. Discontinuous gradient method: Dilute OptiPrep ™ with 0.25 M sucrose, 6 mM EDTA, 60 mM Tris-HCl, pH 7.4 to produce 14%, 25% and 55% (w/v) iodixanol. Layer 2 ml of each in a tube and layer the soil suspension (5 ml) layered on top (see Note 5). For more information on production of gradient solutions using a sucrose solution see Application Sheet S01 (Subcellular membranes index).
5. Density barrier method: Centrifuge at 10,000-25,000 g at 4C for 20-40 min (see Note 6) and then harvest all of the material above the soil pellet (see Note 7).
6. Discontinuous gradient method: Centrifuge at 2600 g for 1 h; then aspirate the liquid above the 55% (w/v) iodixanol layer.
7. Dilute the bacterial suspension with 3 vol. of sterile saline; pellet the bacteria at 10,000-20,000 g for 20-60 min and resuspend in a suitable medium (see Note 8).
- Isolation of bacteria from stream water is described in ref 17.
6. Notes
1. The precise conditions for dispersion are quite variable. Some are much more gentle, e.g. 3×1 min at low speed, in others the rest periods of much longer duration are used, e.g. 5 min. If a speed is stipulated it is often approx. 20,000 rpm.
2. If the presence of a buffer and/or saline is required in the density barrier, this dilution step will only cause a small change in the density of the 60% (w/v) iodixanol.
3. The relative volumes of sample and barrier are also variable, but usually the sample volume exceeds that of the barrier and ratio given is one that is commonly used. Rapp et al [18] underlayered 15 ml of the processed soil suspension with 9 ml of OptiPrep.
4. In a recent paper by Pascaud et al [19] the density of the barrier was reduced to 1.20 g/ml, i.e. approx. 36% (w/v) iodixanol (OptiPrep diluted with a buffered solution of 0.25 M sucrose containing 6 mM EDTA).
5. Pascaud et al [19] used a three-layer gradient of 1.10, 1.15 and 1.30 g/ml; this is equivalent to approx. 14.5, 25 and 55% (w/v) iodixanol when OptiPrep is diluted with a buffered solution of 0.25 M sucrose. After centrifugation at 2,600 g for 1 h, the bacteria banded on top of the 1.15 g/ml layer.
6. The time for the centrifugation will vary with the total volume of sample + density barrier; for larger volumes the time may need to be increased.
7. Often the bacteria are harvested from the interfacial material and only the top half of the cushion. Because of the high density of the barrier the harvest requires considerable dilution (maybe as much as 10x) with buffer in order to reduce the density of the collected liquid.
8. The centrifugation conditions used to pellet the bacteria will depend on the sedimentation properties of the bacteria, the amount of cushion in the harvest and the total volume of the bacterial suspension after dilution; they vary from 16,000 g for 60 min to 100-200,000 g for 10-20 min.
7. Isolation of bacteria from a host tissue or organism
Because of the great variability in host tissue or organism, only a brief outline of a possible strategy for the study of the bacteria consortium. Woyke et al [20] studied the bacterial populations in annelids by loading an extract in PBS on to a 5 ml 1.083-1.146 g/ml NycodenzⓇ (equivalent to 15- 27.5% w/v) gradient. It was centrifuged at 10,000 g for 1 h and collected in approx 0.25 ml fractions. Bacteria were harvested from the densest fractions and the metagenomic high MWt DNA analyzed. As far as is known, no similar separations have been carried out using OptiPrep™, although it is highly likely that gradients covering a similar density range would be effective. Using an iodixanol gradient, the isolation has been reported of the following organism: Chromulinavorax destructans, a bacterium which infects the aquatic Spumella elongata (21).
8. Recent publications reporting the use of iodixanol gradients
Frampton et al [22] have reported on the draft genome sequence of “Candidatus Liberibacter europaeus” ASNZ1, assembled from broom psyllids in New Zealand. A 30% OptiPrep column centrifuged at 100,000g for 2 h to analyze the extracted material.
Gionchetta et al [23] used an iodixanol gradient to study the effect of investigated the resistance of streambed bacteria to drought conditions.
Burz et al [24] investigated the storage conditions of transplantable stool samples in relation to their bacterial content.
Petersen et al [25] assessed the risk of biological contamination of engineered nanomaterials with respect to their various contacts with the human population.
9. References
1. Lindahl, V. and Bakken, L.R. (1995) Evaluation of methods for extraction of bacteria from soil FEMS Microbiol. Ecol., 16, 135-142
2. Rockne, K.J., Liang, W., Young, L.Y. and Taghon, G.L. (2003) Toxicity of density separation media to Esherichia coli and Myobacterium strain PC01: implications for density-separation of soils and sediments FEMS Microbiol. Ecol., 43, 185-189
3. Robe, P., Nalin, R., Capellano, C., Vogel, T.M. and Simonet, P. (2003) Extraction of DNA from soil Eur. J. Soil Biol., 39, 183-190
4. Lindahl, V. (1996) Improved soil dispersion procedures for total bacterial counts, extraction of indigenous bacteria and cell survival J. Microbiol. Meth., 25, 279-286
5. Musovic, S., Oregaard, G., Kroer, N. and Sørensen, S.J. (2006) Cultivation-independent examination of horizontal transfer and host range of an IncP-1 plasmid among Gram-positive and Gram-negative bacteria indigenous to the barley rhizosphere Appl. Envir. Microbiol., 72, 6687-6692
6. Backman, A. and Jansson, J.K. (2004) Degradation of 4-chlorophenol at low temperature and during extreme temperature fluctations by Arthrobacter chlorophenolicus A6 Microbiol. Ecol., 48, 246-253
7. Manichanh, C., Rigottier-Gois, L., Bonnaud, E., Gloux, K., Pelletier, E., Frangeul, L., Nalin, R., Jarrin, C., Chardon, P., Marteau, P., Roca, J. and Dore, J. (2006) Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach Gut, 55, 205-211
8. Hazebrouck, S., Oozeer, R., Adel-Patient, K., Langella, P., Rabot, S., Wal, J-M. and Corthier, G. (2006) Constitutive delivery of bovine ß-lactoglobulin to the digestive tracts of gnotobiotic mice by engineered Lactobacillus casei Appl. Envir. Microbiol., 72, 7460-7467
9. Stevens, K.A. and Jaykus, L-A. (2004) Bacterial separation and concentration from complex sample matrices. A review Crit. Rev. Microbiol., 30, 7-24
10. Klerks, M.M., van Bruggen, A.H.C., Zijlstra, C. and Donnikov, M. (2006) Comparison of methods of extracting Salmonella enterica serovar Enteritidis DNA from environmental substrates and quantification of organisms by using a general internal procedural control Appl. Envir. Microbiol., 72, 3879-3886
11. Pascaud, A., Amellal, S., Soulas, M-L. and Soulas, G. (2009) A fluorescence-based assay for measuring the viable cell concentration of mixed microbial communities in soil J. Microbiol. Methods, 76, 81–87
12. Nousiainen, A.O., Björklöf, K., Sagarkar, S., Nielsen, J.L., Kapley, A. and Jørgensen, K.SD. (2015) Bioremediation strategies for removal of residual atrazine in the boreal groundwater zone Appl. Microbiol. Biotechnol., 99, 10249–10259
13. Teyssier-Cuvelle, S., Mougel, C. and Nesme, X. (1999) Direct conjugal transfers of Ti plasmid to soil microflora Mol. Ecol., 8, 1273-1284
14. Lindahl, V., Frostegård, Å., Bakken, L. and Baath, E. (1997) Phospholipid fatty acid composition of size fractionated indigenous soil bacteria Soil Biol. Biochem., 29, 1565-1569
15. Amaral. J.A. and Knowles, R. (1997) Inhibition of methane comsumption in forest soils and pure cultures of methanotrophs by aqueous forest soil extracts Soil Biol. Biochem., 29, 1713-1720
16. Muirhead, R.W., Collins, R.P. and Bremer, P.J. (2005) Erosion and subsequent transport state of Escherichia coli from cowpats Appl. Environ, Microbiol., 71, 2875-2879
17. Freixa, A., Acuna, V., Casellas, M., Pecheva, S. and Roman, A.M. (2017) Warmer night-time temperature promotes microbial heterotrophic activity and modifies stream sediment community Glob. Change Biol., 23, 3825–3837
18. Rapp, D., Richaume, A., Jame, P., Rigou, P., Rezaei, H., Alcouffe, P., Chapel, J-P., Quiquampoix, H. and Potier, P. (2011) Evidence for proteolysis of a recombinant prion protein in a lamb brain-amended loamy soil Eur. J. Soil Sci., 62, 607–616
19. Pascaud, A., Soulas, M-L., Amellal, S. and Soulas, G. (2012) An integrated analytical approach for assessing the biological status of the soil microbial community Eur. J. Soil Biol., 49, 98-106
20. Woyke, T., Teeling, H., Ivanova, N.N., Huntemann, M., Richter, M., Gloeckner, F.O., Boffelli, D., Anderson, I.J., et al, (2006) Symbiosis insights through metagenomic analysis of a microbial consortium Nature, 443, 950-955
21. Deeg, C.M., Zimmer, M.M., George, E.E., Husnik, F., Keeling, P.J. and Suttle, C.A. (2019) Chromulinavorax destructans, a pathogen of microzooplankton that provides a window into the enigmatic candidate phylum Dependentiae PLoS Pathog. 15: e1007801
22. Frampton, R.A., Thompson, S.M., Kalamorz, F., David, C.,, Addison, S.M. and Smith, G.R. (2018) Draft Genome Sequence of a “Candidatus Liberibacter europaeus” Strain Assembled from Broom Psyllids (Arytainilla spartiophila) from New Zealand Prokaryotes, 6: e00430-18
23. Gionchetta, G., Oliva, F., Menéndez, M., Laseras, P.L. and Romaní, A.M. (2019) Key role of streambed moisture and flash storms for microbial resistance and resilience to long-term drought Freshwater Biol., 64, 306–322
24. Burz, S.D., Abraham, A-L., Fonseca, F., David, O., Chapron, A., Béguet-Crespe, F., Cénard, S., Le Roux, K., Patrascu, O., Levenez, F. et al (2019) A guide for ex vivo handling and storage of stool samples intended for fecal microbiota Transpl. Sci. Rep., 9: 8897
25. Petersen, E.J., Mortimer, M., Burgess, R.M., Handy, R., Hanna, S., Ho, K.T., Johnson, M., Loureiro, S. et al (2019) Strategies for robust and accurate experimental approaches to quantify nanomaterial bioaccumulation across a broad range of organisms Environ. Sci.: Nano, 6, 1619-1656
OptiPrep™Application Sheet C39; 8th edition, February 2020
OptiPrep™ Application Sheet C40
Isolation of mononuclear cells from tissues
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes – a methodological review” compares all of the currently available methodologies
- OptiPrep™ Reference List RC01 “Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes – a bibliographical review” provides a comprehensive bibliography of all the published papers reporting the use of OptiPrep™
- To access RC01 return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text, return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
Mononuclear cells (MCs) from a variety of tissues, predominantly liver, spleen, intestine and bone marrow, have been purified by using a strategy similar to that used for the isolation of these cells from blood, namely sedimentation onto a density barrier. This density barrier has commonly been a NycodenzⓇ solution, often in the form of one of the ready-made NycoprepⓇ solutions; NycoprepⓇ 1.077 [1,2] for human blood, NycoprepⓇ1.077A [3-8] for rodent blood: the former was formulated for isolation of MC from human blood, the latter from rodent blood. Neither of these ready-made solutions is now commercially available, However solutions of the same density and osmolality may very easily, be prepared from OptiPrep™ (see Section 3).
More recently MCs from liver have been have been purified on an iodixanol density barrier of approx ρ = 1.084 g/ml [9,10] or banded between a two-layer gradient of 1.051 and 1.077 g/ml [11]. Other tissues from which MCs have been prepared using sedimentation onto an iodixanol density barrier are rat spleen [12], spinal cord [12] and bone marrow [13-15]. As far as is known, these barrier sedimentation methods have not been executed using a low-osmolality density barrier. The iodixanol barrier solutions have been produced by dilution of OptiPrep™ with regular saline or culture medium.
In the alternative “mixer” strategy the sample is simply adjusted to a density just higher than that of the MCs so that the latter float to the top during the centrifugation, introduced for human blood by Ford and Rickwood [16] using NycodenzⓇ. This was later adapted to the use of OptiPrep™ and extended to both mouse and rat blood. It has now been used successfully for the isolation of MCs from liver [17-22] and spleen [23,24]. This technology should be applicable to any mouse or rat tissue.
The most commonly used tissues for the isolation of lymphocytes are bone marrow, spleen, intestine and liver. Since this Application Sheet was first prepared however, the use of iodixanol gradients has additionally been reported for their isolation from brain, heart and lung tissue. Although the general gradient strategies have not changed, there is considerable variation in some of the details, some of which will be indicated in Section 6.
- This Application Sheet presents the density gradient methods for resolving the lymphocytes and not the methods that are used to disaggregate the tissues. Methods for preparing a total nonparenchymal cell fraction are described in Application Sheet C26
- Section 2 describes the options for sedimentation on to a density barrier
- Section 3 describes the two-layer gradient
- Section 4 describes the flotation strategy
- Section 5 contains important notes to Sections 2, 3 and 4
- Section 6 briefly describes some of the methodological variations reported in recent papers
 2. Sedimentation on to a density barrier
2. Sedimentation on to a density barrier
2a. Solution preparation
A. OptiPrep™ (60%, w/v iodixanol) – shake the bottle gently before use
B. Buffered saline
2a-1 Isoosmotic barrier solution
Dilute Solution A with Solution B to obtain isoosmotic solutions of lower density. The density of this barrier solution may be modulated to improve either the purity or yield of mononuclear cells. For more information on the density of iodixanol solutions see Application Sheet C01. The published methods quote % (w/v) iodixanol concentrations from 12.6% (see ref 17), which has a density of approx. 1.072 g/ml, to 15% (e.g. see refs 9, 10, 25) with a density of approx. 1.084 g/ml and also includes the standard 1.077 g/ml barrier [26] and for intestinal cells a much lower density barrier (1.055 g/ml) was employed at 1700 g for 10 min [27]. Centrifugation conditions are normally in the range 600-1000 g for 10-20 min.
2a-2 Hypoosmotic 1.077 g/ml barrier solution
Dilute Solution B with water (2.5 vol. + 0.5 vol.) and then mix 2.7 vol. of Solution A with 9.3 vol. of the diluted saline solution (see Note 1).
2b. Protocol (adapted from refs 9 and 10)
1. Layer 2 vol. of cell suspension (see Section 2) on top of 1 vol. of the density barrier (see Note 2).
2. Centrifuge at 750 g for 20 min at room temperature (see Note 3).
3. Allow the rotor to decelerate without the brake and harvest the MCs from the interface.
3. Sedimentation in a two-layer gradient
3a. Solutions required
A. OptiPrep™ (60%, w/v iodixanol)
B. Culture medium (RPMI 1640)
3b. Protocol (adapted from ref 11)
1. Shake the OptiPrep™ gently before use.
2. Make up two solutions of 1.051 and 1.078 g/ml by mixing Solutions A and B in the following volume ratios (1:5.8) and (1:3.5) respectively (see Note 4)
3. Suspend the cells in the 1.051 g/ml solution and layer over an equal volume of the 1.078 g/ml solution (see Note 2).
4. Centrifuge at 750 g for 20 min at room temperature.
5. Allow the rotor to decelerate without the brake and harvest the MCs from the interface (see Note 5).
4. Flotation strategy
4a. Solutions required
A. OptiPrep™ (60%, w/v iodixanol)
B. Culture medium (RPMI 1640) Keep Hepes (free acid) or Tricine as a 100 mM stock solution at 4°C; Hepes (2.38 g) or Tricine (1.79 g) per 100 ml water. Solution B: Dissolve 0.85 g of NaCl in 50 ml water; add 10 ml of Hepes or Tricine stock solution; adjust to pH 7.2-7.4 with 1 M NaOH and make up to 100 ml.
4b. Protocol (adapted from ref 20)
1. Shake the OptiPrep™ gently before use and mix 4 vol. of Solution A with 2 vol. of Solution B to produce a solution of density 1.215 g/ml (see Note 7).
2. Suspend the cells in 3.9 ml of Solution B and mix gently but thoroughly with 2.1 ml of the 1.215 g/ml solution (see Note 8 and 9).
3. Layer 1 ml of Solution B on top and centrifuge at 1500 g for 20 min at 4°C (see Notes 5 and 10).
4. Allow the rotor to decelerate without the brake and harvest the MCs from the interface.
5. Notes
1. This solution is equivalent to Nycoprep 1.077A and has a density of 1.077 g/ml and an osmolality of approx 265 mOsm.
2. The relative volumes of sample and density barrier are probably not critical but the given ratio is widely used.
3. The separation on an isoosmotic barrier should not be temperature dependent but lower temperatures may require a further 5 min of centrifugation because of the increased viscosity at lower temperatures. Use of the hypoosmotic medium should be carried out at room temperature because the movement of water across an osmotic gradient is reduced at low temperatures.
4. The density of the 1.078 g/ml solution might be modulated upwards if too many MCs are lost to the pellet. For intestine and liver mononuclear cells Zellweger et al used densities of 1.052 and 1.076 g/ml [28]. This was also very similar to the method of Dai et al [29]
5. The separation should not be temperature dependent but lower temperatures may require a further
5 min of centrifugation because of the increased viscosity at lower temperatures.
6. The ratio of Solution A:Solution B may be modulated in the light of data on the recovery of MCs. In step 3 it is possible to mix the sample directly with OptiPrep rather than with the ρ = 1.215 g/ml medium; the latter is the chosen method because complete mixing of OptiPrep with the sample requires rather more vigorous agitation, which may be deleterious to the cells.
7. The final v/v ratio of OptiPrep™ in the sample is 22%, variations include 21% [17,18], 21.5% [21,22] and 24% [23]. So the final concentration of iodixanol varies from 12.6% (w/v) to 14.4% (w/v), equivalent to densities of approx. 1.072-1.082 g/ml.
8. The small layer of culture medium on top of the sample does not influence the separation, but it prevents the MCs from banding at an air/liquid interface, which causes aggregation problems.
9. For the isolation of mononuclear cells from mouse bone marrow, Mukai et al [30] floated the mononuclear cells from a dense sample layer (1.090 g/ml), through a 1.08 g/ml layer using very mild centrifugation conditions of 100 g for 20 min.
10. For bone marrow cells, 30 ml of cell suspension was mixed with 10.1 ml of OptiPrep™ and 4 ml
of water; then overlaid with 5 ml of PBS and centrifuged at 1000 g for 30 min [31] – note that the final iodixanol concentration in the sample was 13.7% (w/v).
6. Recent methods and variations in methodology
- Lung lymphocytes have been purified on both the normal density barrier [32] and a slightly denser barrier of approx 16.5% (w/v) iodixanol (described in the paper as 27.5% (v/v) OptiPrep™) [33] similar to that used for intestinal cells [34].
- Two-layer gradients of 15% (w/v) and 11% (w/v) iodixanol (equivalent to 1.085 and 1.063 g/ml density) were applied to resolve the lymphocytes in the top zone of the 15% layer and macrophages + dendritic cells just above the interface [35]
- Brain lymphocytes have only relatively recently been isolated using OptiPrep™ using a three-layer gradient of 5%, 10% and 18% (w/v) iodixanol at 800 g for 30 min; the leukocytes collected at the 10-18% interface [36-38]
- Recently published papers describe the use of iodixanol gradients for the isolation of mononuclear
cells from bone marrow [39,40], liver [41] and spleen [42].
7. References
1. Zhang, Z., Kaptanoglu, L., Haddad, W., Ivancic, D., Alnadjim, Z., Hurst, S., Tishler, D., Luster, A.D., Barrett, T.A. and Fryer, J. (2002) Donor T cell activation initiates small bowel allograft rejection through an IFN-γ-inducible protein-10- dependent mechanism J. Immunol., 168, 3205-3212
2. Fukutome, K., Watarai, S., Mukamoto, M. and Kodama, H. (2001) Intestinal mucosal immune response in chickens following intraocular immunization with liposome-associated Salmonella enterica serovar enteritidis antigen Devel. Comp. Immunol., 25, 475-484
3. Medana, I., Li, Z., Flügel, A., Tschopp, J., Wekerle, H. and Neumann, H. (2001) Fas ligand (CD95L) protects neurons against perforin-mediated T lymphocyte cytotoxicity J. Immunol., 167, 674-681
4. Villey, I., Caillol, D., Selz, F., Ferrier, P. and de Villartay, J-P. (1996) Defect in rearrangement of the most 5’TCR-Jα following targeted deletion of T earlyα (TEA): implications for TCRα locus accessibility Immunity, 5, 331-342
5. Wurbel, M-A., Malissen, M., Guy-Grand, D., Meffre, E., Nussenzweig, M.C., Richelme, M., Carrier, A. and Malissen, B. (2001) Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor γδ gut intraepithelial lymphocytes Blood, 98, 2626-2632
6. Ćupić, V., Čolić, M., Pavičić, L., Vučević, D. and Varagić, V.M. (2001) Immunomodulatory effect of xylazine, an 2 adrenergic agonist, on rat spleen cells in culture J. Neuroimmunol., 113, 19-29
7. Becker, J.C., Varki, N., Gillies, S.D., Furukawa, K. and Reisfeld, R.A. (1996) Long-lived and transferable tumor immunity in mice after targeted interleukin-2 therapy J. Clin. Invest., 98, 2801-2804
8. Arstila, T., Arstila, T.P., Calbo, S., Selz, F., Malassis-Seris, M., Vassalli, P., Kourlisky, P. and Guy-Grand, D. (2000) Identical T cell clones are located within the mouse gut epithelium and lamina propria and circulate in the thoracic lymph duct J. Exp. Med., 191, 823-834
9. Obhari, J.S., Oberbarnscheidt, M.H., Hand, T.W., Diggs, L., Chalasani, G. and Lakkis, F.G. (2006) Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs J. Immunol., 176, 4051-4058
10. Nasr, I.W., Reel, M., Oberbarnscheidt, M.H., Mounzer, R.H., Baddoura, F.K., Ruddle, N.H. and Makkis, F.G. (2007) Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection Am. J. Transplant., 7, 1071-1079
11. Lian Z-X., Okada, T., He, X-S., Kita, H., Liu, Y-J., Ansari, A.A., Kikuchi, K., Ikehara, S. and Gershwin, M.E. (2003) J. Immunol., 170, 2323-2330
12. Flügel, A., Berkowicz, T., Ritter, T., Labeur, M., Jenne, D.E., Li, Z., Ellwart, J.W., Willem, M., Lassmann, H. and Wekerle, H. (2001) Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis Immunity, 14, 547-560
13. Whetton, A.D., Lu, Y., Pierce, A., Carney, L. and Spooncer, E. (2003) Lysophospholipids synergistically promote primitive hematopoietic cell chemotaxis via a mechanism involving Vav1 Blood, 102, 2798-2802
14. Evans, C.A., Tonge, R., Blinco, D., Pierce, A., Shaw, J., Lu, Y., Hanzah, H.G., Gray, A., Downes, C.P., Gaskell, S.J., Spooncer, E. and Whetton, A.D. (2004) Comparative proteomics of primitive hematopoietic cell populations reveals differences in expression of proteins regulating motility Blood, 103, 3751-3759
15. Unwin, R.D., Smith, D.L., Blinco, D., Wilson, C.L., Miller, C.J., Evans, C.A., Jaworska, E., Baldwin, S.A., Barnes, K., Pierce, A., Spooncer, E. and Whetton, A.D. (2006) Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells Blood, 107, 4687-4694
16. Ford, T. C. and Rickwood, D. (1990) A new one-step method for the isolation of human mononuclear cells J. Immunol. Meth., 134, 237-241
17. Mehal, W., Sheikh, S.Z., Gorelik, L. and Flavell, R.A. (2005) TGF- signaling regulates CD8+ T cell responses to highand low-affinity TCR interactions Int. Immunol., 17, 531-538
18. John, B. and Crispe, I.N. (2005) LR-4 regulates CD8+ T cell trapping in the liver J. Immunol., 175, 1643-1650
19. Klein, I. and Crispe, I.N. (2006) Complete differentiation of CD8+ T cells activated locally within the transplanted liver J. Exp. Med., 203, 437-447
20. Wuensch, S.A., Pierce, R.H. and Crispe, I.N. (2006) Local intrahepatic CD8+ T cell activation by a non-self- antigen results in full functional differentiation J. Immunol., 177, 1689-1697
21. Au-Yeung, B.B. and Fowell, D.J. (2007) A key role for Itk in both IFN and IL-4 production by NKT cells J. Immunol., 179, 111-119
22. Polakos, N.K., Klein, I., Richter, M.V., Zaiss, D.M., Giannandrea, M., Crispe, I.N. and Topham, D.J. (2007) Early intrahepatic accumulation of CD8+
T cells provides a source of effectors for nonhepatic immune responses J. Immunol., 179, 201-210
23. DiJoseph, J.F., Dougher, M.M., Kalyandrug, L.B., Armellino, D.C., Boghaert, E.R., Hamann, P.R., Moran, J.K. and Damle, N.K. (2006) Antitumor efficiacy of a combination of CMC-544 (inotuzumab ozogamicin), a CD22-targeted cytotoxic immunoconjugate of calicheamicin, and rituximab against non-Hodgkin’s B-cell lymphoma Clin. Cancer Res., 12, 242-249
24. Kivi, G., Teesalu, K., Parik, J., Kontkar, E., Ustav Jr, M., Noodla, L., Ustav, M. and Männik, A. (2016) HybriFree: a robust and rapid method for the development of monoclonal antibodies from different host species BMC Biotechnol., 16:
25. Dobaczewski, M., Xia, Y., Bujak, M., Gonzalez-Quesada, C. and Frangogiannis, N.G. (2010) CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells Am. J. Pathol., 176, 2177–2187
26. Aliotta, J.M., Pereira, M., Johnson, K.W., de Paza, N., Dooner, M.S., Puente, N., Ayala, C., Brilliant, K., Berza, D., Lee, D., Ramratnam, B., McMillan, P.N., Hixson, D.C., Josic, D. and Quesenberry, P.J. (2010) Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription Exp. Hematol., 38, 233–245
27. Wang, X., O’Gorman, M.R.G., Bu, H-F., Koti, V., Zuo, X-L. and Tan, X-D. (2009) Probiotic preparation VSL#3 alters the distribution and phenotypes of dendritic cells within the intestinal mucosa in 57BL/10J mice J. Nutr. 139, 1595–1602
28. Zellweger, R.M., Prestwood, T.R. and Shresta, S. (2010) Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease Cell Host Microbe 7, 128–139
29. Dai, K., Huang, L., Sun, X., Yang, L. and Gong, Z. (2015) Hepatic CD206-positive macrophages express amphiregulin to promote the immunosuppressive activity of regulatory T cells in HBV infection J. Leukoc. Biol., 98, 1071–1080
30. Mukai, M., Suruga, N., Saeki, N. and Ogawa, K. (2017) EphA receptors and ephrin-A ligands are upregulated by monocytic differentiation/maturation and promote cell adhesion and protrusion formation in HL60 monocytes BMC Cell Biol., 18: 28
31. Liu, L., Papa, E.F., Dooner, M.S., Machan, J.T., Johnson, K.W., Goldberg, L.R., Quesenberry, P.J. and Colvin, G.A. (2012) Homing and long-term engraftment of long- and short-term renewal hematopoietic stem cells PLoS One, 7: e31300
32. Licona-Limón, P., Henao-Mejia, J., Temann, A.U., Gagliani, N., Licona-Limón, I., Ishigame, H., Hao, L., Herbert, D.R. and Flavell, R.A. (2013) Th9 cells drive host immunity against gastrointestinal worm infection Immunity, 39, 744–757
33. Koyama, S., Akbay, E.A., Li, Y.Y., Herter-Sprie, G.S., Buczkowski, K.A., Richards, W.G., Gandhi, L., Redig, A.J., Rodig, S.J. et al (2016) Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints Nat. Comm., 7: 10501
34. Goodyear, A.W., Kumar, A., Dowa, S. and Ryan, E.P. (2014) Optimization of murine small intestine leukocyte isolation for global immune phenotype analysis J. Immunol. Methods, 405, 97–108
35. Kitazawa, Y., Ueta, H., Hünig, T., Sawanobori, Y. and Matsuno, K. (2015) A novel multicolor immunostaining method using ethynyldeoxyuridine for analysis of in situ immuno-proliferative response Histochem. Cell Biol., 144, 195–208
36. Kim, J.H., Choi, J.Y., Kim, S.B., Uyangaa, E., Patil, A.M., Han, Y.W., Park, S-Y., Lee, J.H., Kim, K. and Eo, S.K. (2015) CD11chi dendritic cells regulate Ly-6Chi monocyte differentiation to preserve immune-privileged CNS in lethal neuroinflammation Sci. Rep., 5: 17548
37. Kim, S.B., Choi, J.Y., Kim, J.H., Uyangaa, E., Patil, A.M., Park, S-Y., Lee, J.H., Kim, K., Han, Y.W. and Eo, S.K. (2015) Amelioration of Japanese encephalitis by blockage of 4-1BB signaling is coupled to divergent enhancement of type I/II IFN responses and Ly-6Chi monocyte differentiation J. Neuroinflamm., 12: 216
38. Kim, S.B., Choi, J.Y., Uyangaa, E., Patil, A.M., Hossain, F.M.A., Hur, J., Park, S-Y. et al (2016) Blockage of indoleamine 2,3-dioxygenaseregulates Japanese encephalitis via enhancement of type I/II IFN innate and adaptive Tcell responses J. Neuroinflam. 13: 79
39. Aliotta, J.M., Pereira, M., Sears, E.H., Dooner, M.S., Wen, S., Goldberg, L.R. and Quesenberry, P.J. (2015) Lungderived exosome uptake into and epigenetic odulation of marrow progenitor/stem and ifferentiated cells J. Extracell. Vesicles, 4:26166
40. Wen, S., Dooner, M., Cheng, Y., Papa, E., Del Tatto, M., Pereira, M., Deng, Y., Goldberg, L., Aliotta1, J., Chatterjee, D. et al (2016) Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells Leukemia, 30, 2221–2231
41. King, A., Houlihan, D.D., Kavanagh, D., Haldar, D., Luu, N., Owen, A., Suresh, S., Than, N.N. et al (2017) Sphingosine-1-phosphate prevents egress of hematopoietic stem cells from liver to reduce fibrosis Gastroenterology, 153, 233–248
42. Liang, Y., Song, D-Z., Liang, S., Zhang, Z-F., Gao, L-X. and Fan, X-H. (2017) The hemagglutinin-neuramidinase protein of Newcastle disease virus upregulates expression of the TRAIL gene in murine natural killer cells through the activation of Syk and NF-κB PLoS One, 12: e0178746
8. Acknowledgements
We thank Beena John of the David H. Smith Center for Vaccine Biology, University of Rochester Medical Center, Rochester, NY 14642 for valuable help in preparation of this Application Sheet.
OptiPrep™Application Sheet C40; 10th edition, January 2020
OptiPrep™ Application Sheet C41
Isolation of dendritic cells from tissues by sedimentation on to a density barrier
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List “Dendritic cells from blood and tissues” (RC05) compares all of the currently available methodologies and provides a comprehensive bibliography of all the published papers reporting the use of OptiPrep™ listed according to the research topic: to access return to the initial list of Folders and select “Reference Lists”
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
Since dendritic cells (DC) were recognized as playing an important role in the induction of cellmediated responses [1], there has been a rapid growth in research into the function of these cells and methods for their purification. Gradients of either albumin or metrizamide, although providing an effective enrichment of DC, tended to cause some functional alteration of the cells (see ref 2 for details). However, because cells are more tolerant of NycodenzⓇ, this iodinated density gradient medium rapidly became established as the medium of choice for DC cell purification from peripheral blood and from lymphoid tissues.
NycodenzⓇ density barriers of ρ = 1.076 g/ml [3] to 1.084 g/ml [4], the majority being approx 1.077 g/ml (e.g. refs. 5-9), have been used in the “traditional” format in which the crude cell fraction is layered on top. There are also instances of the use of the customized medium Nycoprep 1.068 [10,11], which was designed for the purification of monocytes from a human leukocyte-rich plasma. This strategy of layering the cell suspension on top of a barrier has been extended to the use of OptiPrep™ for isolation of DCs from lymph [12], bone marrow [13], bone marrow cell cultures [14], spleen [15], thymus [16] and lung [17].
- For alternative flotation protocols see Application Sheets C21 and C22
2. Preparation of cells
For the standard preparation of DCs from spleen and thymus by collagenase digestion and filtration see Application Sheet C21. Lung DCs are prepared by perfusion of the tissue via the pulmonary artery with phosphate-buffered saline containing heparin prior to digestion with collagenase (see ref. 15 for more details). For the preparation of DCs from bone marrow cell cultures, the cells flushed from murine femurs and tibias are cultured in the standard RPMI/10% FCS in the presence of 10 ng/ml IL-4 and granulocyte-macrophage colony-stimulating factor for 6 days prior to the harvesting of loosely adherent and non-adherent cells (see ref. 14 or more details of this cell preparation protocol).
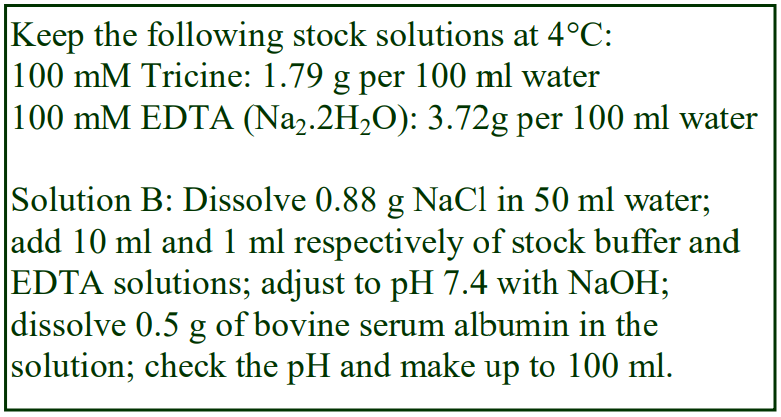 3. Purification of dendritic cells (adapted from ref 14)
3. Purification of dendritic cells (adapted from ref 14)
3a. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. Diluent: 0.88% (w/v) NaCl, 1 mM EDTA, 0.5% bovine serum albumin (BSA), 10 mM Tricine-NaOH, pH 7.4 (see Note 1)
C. Density barrier: 2.3 vol. of Solution A + 9.7 vol. of Solution B (see Note 2)
D. Hank’s Balanced Salt Solution (see Note 3) Excellence in Separations OptiPrep™ Application Sheet C41
3b. Protocol
Carry out all operations at 4°C, making sure that all solutions and equipment are pre-cooled.
1. Harvest the cells by centrifugation at 540 g for 5 min and wash them twice in Solution D.
2. Suspend the washed cell pellet in this solution (6 ml).
3. Transfer 6 ml of Solution C or other chosen density barrier to a centrifuge tube and overlay with cell suspension (see Note 4).
4. Centrifuge at 600-700 g for 5-10 min; use a slow acceleration if available (see Note 5).
5. Allow the rotor to decelerate without the brake and harvest the DCs from the interface (see Note 6).
4. Notes
1. In some cases the OptiPrep diluent is a culture medium supplemented with FCS and EDTA. Any approximately isoosmotic solution compatible with the cells may be used.
2. The density of this barrier is approx. 1.067 g/ml and equivalent to 11.6% (w/v) iodixanol; others have used 12% [15]. For lung DCs Hansen et al [17] employed a two layer gradient of 4% and 16% (w/v) iodixanol.
3. The solution used to suspend the cells prior to layering on the density barrier and the solution used to dilute the OptiPrep™ may be any compatible isoosmotic medium. Hansen et al [17] used Hank’s Balanced Salt Solution (HBSS) containing 5% FCS and 2 mM EDTA for both, while Cervantes-Barragan et al [15] replaced the HBSS with PBS and Luckashenak et al [16] used PBS for suspending the cells and HBSS for diluting the OptiPrep™ (both containing 0.2% BSA and 2 mM EDTA.
4. The relative volumes of sample and density barrier are probably not very critical; 2ml over 2 ml is a quite common format with NycodenzⓇ barriers.
5. The time may be increased to 20 min. Most centrifugations are carried out at 4°C but higher temperatures may be used. There is a broad range of centrifugation conditions with NycodenzⓇ barriers; approx. 800 g for 20 min is common but 530 g for 20 min to 2800 g for 15 min have been described.
6. In the case of the two-layer gradient, the DCs band at the lower interface. Eaton et al [14] reported that the purity of the DCs from bone marrow cultures, as judged by flow cytometry, was approx. 85%. The harvested DC may be further purified by negative selection using the appropriate MAbcoated magnetic beads. Carrying out this initial gradient purification step allows the bead purification by negative selection to be performed more efficiently. Ref. 19 reviews DC methodology and function.
5. References
1. Barfoot, R., Denham, S., Gyure, L. A., Hall, J. G., Hobbs, Jackson, L. E. and Robertson, D. (1989) Some properties of dendritic macrophages from peripheral lymph Immunology, 68, 233-239
2. McLellan, A. D., Starling, G. C. and Hart, D. N. J. (1995) Isolation of human blood dendritic cells by discontinuous Nycodenz gradient centrifugation J. Immunol. Meth., 184, 81-89
3. Durant, S., Alves, V., Coulaud, J. and Homo-Delarche, F. (2002) Nonobese diabetic (NOD) mouse dendritic cells stimulate insulin secretion by prediabetic islets Autoimmunity, 35, 449-4551059
4. Jang, M.H., Sougawa, N., Tanaka, T., Hirata, T., Hiroi, T., Tohya, K., Guo, Z., Umemoto, E., Ebisuno, Y., Yang, B-G., Seoh, J-Y., Lipp, M., Kiyono, H. and Miyasaka, M. (2006) CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes J. Immunol., 176, 803-810
5. Simons, P.J., Delemarre, F.G.A. and Drexhage, H.A. (1998) Antigen-presenting dendritic cells are regulators of the growth of thyrocytes: a role of interleukin-1β and interleukin-6 Endocrinology, 139, 3148-3156
6. Leenen, P.J.M, Radosevic, K., Voerman, J.S.A., Salomon, B., van Rooijen, N., Klatzmann, D. and van Ewijk, W. (1998) Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover J. Immunol., 160, 2166-2173
7. Den Haan, J.M.M., Lehar, S.M. and Bevan, M.J. (2000) CD8+ but not CD8- cells cross-prime cytotoxic T cells in vivo J. Exp. Med., 192, 1685-1695
8. Roberts, J.M., Yang, J. and Ronchese, F. (2004) IL-4 deficiency does not impair the ability of dendritic cells to initiate CD4+ and CD8+ T cell responses in vivo Int. Immunol., 16, 1451-1458
9. Trinite, B., Chauvin, C., Pêche, H., Voisine, C., Heslan, M. and Josien, R. (2005) Immature CD4-CD103+ rat dendritic cells induce rapid caspase-independnet apoptosis-like cell death in various tumor and nontumor cells and phagacytose their victims J. Immunol., 175, 2408-2417
10. Moghaddami, M., Swart, B., Reynolds, P., Diener, K. and Brown, M.P. (2002) Flt3 ligand expands dendritic cell numbers in normal and malignant murine prostate Immunol. Cell Biol., 80, 370-381
11. Chen, L., Arora, M., Yarlagadda, M., Oriss, T.B., Krishnamoorthy, N., Ray, A. and Ray, P. (2006) Distinct responses of lung and spleen dendritic cells to the TLR9 agonist CpG oligodeoxynucleotide J. Immunol., 177, 2373-2383
12. Bonneau, M., Epardaud, M., Payot, F., Niborski, V., Thoulouze, M-I., Bernex, F., Charley, B., Riffault, S., Guilloteau, L.A. and Schwartz-Cornil, I. (2006) Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node J. Leukoc. Biol., 79, 268-276
13. Lee, J-E., Kang, C-S., Guan, X-Y., Kim, B-T., Kim, S-H., Lee, Y-M., Moon, W-S. and Kim, D-K. (2007) Discoidin domain receptor 2 is involved in the activation of bone marrow-derived dendritic cells caused by type I collagen Biochem. Biophys. Res. Comm., 352, 244-250
14. Eaton, K.A., Benson, L.H., Haeger, J. and Gray, B.M. (2006) Role of transcription factor T-bet expression by CD4+ cells in gastritis due to Helicobacter pylori in mice Infect. Immun., 74, 4673-4684
15. Cervantes-Barragan, L., Züst, R., Weber, F., Spiegel, M., Lang, K.S., Akira, S., Thiel, V. and Ludewig, B. (2007) Control of coronavirus infection through plasmacytoid dendritic cell-derived type I interferon Blood, 109, 1131-1137
16. Luckashenak, N.A., Ryszkiewicz, R.L., Ramsey, K.D. and Clements, J.L. (2006) The Src homology 2 domain-containing leukocyte protein of 76-kDa adaptor links integrin ligation with p44/42 MAPK phosphorylation and podosome distribution in murine dendritic cells J. Immunol., 177, 5177-5185
17. Hansen, S., Lo, B., Evans, K., Neophytou, P., Holmskov, U. and Wright, J.R. (2007) Surfactant protein D augments bacterial association but attenuates major histocompatibility complex class II presentation of bacterial antigens Am. J. Respir. Cell. Mol. Biol., 36, 94-102
18. Kronin, V., Winkel, K., Suss, G. Kelso, A., Heath, W., Kirberg, J., von Boehmer, H. and Shortman. K. (1996) A subclass of dendritic cells regulates the response of naïve CD8 T cells by limiting their IL-2 production J. Immunol., 157, 3819- 3627
19. Ardavin, C., Martinez del Hoyo, G., Martin, P., Anjuere, F., Arias, C. F., Marin, A. R., Ruiz, S., Parrillas, V. and Hernandez, H. (2001) Origin and differentiation of dendritic cells Trends Immunol., 22, 691-700
OptiPrep™Application Sheet C41; 6th edition, January 2020
OptiPrep™ Application Sheet C42
Purification of macrophages and foam cells
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Introduction
This Application Sheet is concerned primarily with the purification of macrophages from either alveolar lavages or peritoneal fluid (Section 2); however these cells have also been isolated from a variety of other sources such as lung tissue, intestinal lamina propria and spinal cord tissue (see Section 3). Section 4 is concerned with the foam cells found in atherosclerotic lesions. Section 5 describes more recent applications using OptiPrep™.
It is also recognized that macrophages are related to dendritic cells from a variety of tissue sources and to blood monocytes. For the purification of these cells please refer to the following Application Sheets:
- Dendritic cells – barrier flotation: Application Sheet C21
- Dendritic cells – barrier sedimentation: Application Sheet C41
- Dendritic cells – mixer flotation: Application Sheet C22
- Monocytes – leukocyte-rich plasma – flotation: Application Sheet C10
- Monocytes – whole blood – flotation: Application Sheet C11
2. Isolation from peritoneal fluid and alveolar lavages
There is no single methodology that has been widely adopted for the purification of macrophages, so this Application Sheet provides a selection of density gradients, taken from the literature. Many of the published papers report the use of NycoPrepⓇ 1.15, an isoosmotic solution of 27.6% (w/v) NycodenzⓇ in 3 mM KCl, 0.3 mM CaNa2-EDTA 5 mM Tris-HCl, pH 7.5. This is no longer commercially available, so we suggest that solutions of the same density and osmolality should be prepared from OptiPrep™. The alternative in many cases is to prepare the solutions from NycodenzⓇ powder; this is time consuming and the solutions will require sterilization. See Application Sheet C01 for more information on preparing gradient solutions from OptiPrep™.
2a. Sedimentation on to a density barrier
2a-1. Background
Rat peritoneal macrophages require separation from lymphocytes, neutrophils and erythrocytes; Fisker et al [1] carried out a detailed study of the gradient requirements for unstimulated rats and for rats receiving thioglycollate. The optimal properties of the density barrier that produced the highest yields and purity of macrophages were different for the two types of animal. For the normal animal the optimal density and osmolality were 1.091 g/ml and 325 mOsm; for the stimulated rat the figures were 1.106 g/ml and 400 mOsm. Data in subsequent papers confirmed this [2,3]. Fisker et al [1] used NycoPrep 1.15; since this solution is isoosmotic it is simple to mimic this solution with OptiPrep™. The following method is adapted from ref 1.
2a-2. Solutions required (see Box on next page for more details about solution preparation)
A. OptiPrep™ (shake the bottle gently before use)
B. OptiPrep™ diluent: 0.85% (w/v) NaCl, 6 mM KCl, 0.6 mM EDTA, 0.6 mM CaCl2, 10 mM HEPES-NaOH, pH 7.0
C. Hyperosmotic mixer: 1.245 M NaCl, 3 mM KCl, 0.3 mM EDTA, 0.3 mM CaCl2, 5 mM HEPESNaOH, pH 7.0
 2a-3. Protocol
2a-3. Protocol
1. Prepare the 27.6% (w/v) iodixanol stock solution by mixing 27.5 vol. of Solution A with 32.5 vol. of Solution B.
2. Select the appropriate density barrier (see Section 2a-1); for the 1.091 g/ml/325 mOsm barrier, mix 0.6 vol. of the 27.5% (w/v) iodixanol stock solution with 0.34 vol. of water and 0.06 vol. of Solution C; 1.106 g/ml and 400 mOsm barrier, mix 0.7 vol. of the 27.5% (w/v) iodixanol stock solution with 0.22 vol. of water and 0.08 vol. of Solution C (see Note 1). Check the osmotic pressure with an osmometer if possible.
3. Layer 7 ml of the cell suspension over 3 ml of the chosen density barrier and centrifuge at 700 g for 20 min at room temperature.
4. Collect the macrophages from the interface.
2a-4. Notes
1. The only other example of a density barrier was very different to that described above. Cells from a peritoneal cavity lavage in RPMI were layered on NycoPrep 1.068™ (600 g for 10 min). This commercial medium is also no longer available and its primary purpose was to isolate human monocytes from a leukocyte-rich plasma. The macrophages are described as banding in a median zone within the NycoPrep 1.068™ [4]. Like the density barriers described above it does have a raised osmolality. A medium of identical density and osmolality may again be readily prepared from OptiPrep™ and this is described in Application Sheet C46.
2b. Two-layer discontinuous gradients
2b-1. Flotation
In the first example of a two-layer gradient for peritoneal macrophages [5], a stock solution of approx. 25% (w/v) NycodenzⓇ, 10 mM EDTA was diluted with Hank’s Balanced Salt Solution (HBBS) (containing 10 mM EDTA) to produce two solutions of approx. 19% and 14.5% NycodenzⓇ. The cells from the peritoneal lavage were suspended in the 19% NycodenzⓇ (12ml); the 14.5% (16 ml) NycodenzⓇ and a layer of HBBS (12 ml) were laid on top. After 30 min at 400 g the macrophages banded above the 14.5% layer and the neutrophils below it. The density solutions can be made up from OptiPrep™: dilute with an equal volume of HBSS containing 20 mM EDTA to produce a 30% (w/v) iodixanol solution; then dilute further to 14.5% and 19% (w/v) iodixanol with HBSS-10 mM EDTA.
2b-2. Sedimentation
The method developed by Freeman et al [6] for the separation of neutrophils and mononuclear cells from blood has also been used for the isolation of rat alveolar macrophages [7]. This methodology is described in Section 3 of Application Sheet C44.
2c. Multi-step gradients
Separation of alveolar and peritoneal macrophages from lymphocytes and neutrophils has occasionally been executed in four-step discontinuous gradients, the density solutions being prepared by dilution of NycoPrep™ 1.15 with PBS containing 4 mM EDTA [8]. The gradient of approx. 12%, 14.5%, 17.5% and 20% (w/v) NycodenzⓇ was centrifuged at 500 g for 45 min and macrophages banded at the interface of the top two layers. To produce these density solutions from OptiPrep™ and to mimic the EDTA profile of the NycodenzⓇ gradient, first dilute OptiPrep™ with an equal volume of
PBS to produce a 30% (w/v) iodixanol solution and then dilute further with PBS containing 4 mM EDTA to make solutions of 12%, 14.5%, 17.5% and 20% (w/v) iodixanol.
3. Isolation from spinal tissue and lamina propria Beck et al [9] described a four-layer discontinuous gradient of density 1.029, 1.037, 1.056 and
1.061 g/ml. The solutions were produced by dilution of OptiPrep™ with 0.15 M NaCl, 10 mM MOPS, pH 7.4. 1 ml of each of the solutions was overlaid by 6 ml of the crude spinal cord cells and centrifuged at approx 700-800 g for 15 min at room temperature. The glial and inflammatory cells were recovered from the pellet. Iodixanol gradients have also been used for spinal cord tissue by Galvan et al [10] and Mavrikis Cox [11].
Lamina propria macrophages were suspended in approx. 5 ml of 5.5% (w/v) NycodenzⓇ and layered over 3 ml 11% (w/v) NycodenzⓇ. After centrifugation at 650 g for 20 min the macrophages banded at the interface [12]. The solutions were prepared by dilution of NycoPrepⓇ 1.15 with PBS; solutions of approx the same density (5.5% and 11% w/v iodixanol) may be prepared by simple dilution of OptiPrep™ with PBS.
4. Foam cells
Foam cells, being lipid-laden macrophages from atherosclerotic lesions are likely to be less dense than other macrophages. After collagen treatment of the lesion-containing material and sieving, the washed cells were layered over a five-layer discontinuous NycodenzⓇ gradient of 0.5, 1.0, 5.0, 10 and 30% (w/v) NycodenzⓇ and centrifuged at 1200 g for 15 min at 10°C [13,14]. The foam cells banded at the 5%-10% interface. A gradient of exactly the same density profile can be generated by diluting OptiPrep™ with buffered saline, balanced salt solution or culture medium to give the same % (w/v) iodixanol solutions.
5. Publications reporting the use of OptiPrep™
Macrophages and hepatic stellate cells have been separated from liver by successive centrifugations on 8.2% and 17.6% (w/v) iodixanol barriers [15]. Sometimes the crude liver cell fraction is adjusted to 17% (w/v) iodixanol, overlaid with a buffered salt solution; after centrifugation the macrophages have floated to the interface [16]. Hepatic macrophages have also been recovered on a much lower density barrier of 8.2% (w/v) iodixanol [17]. Other papers also report the use of iodixanol gradients tissues for liver macrophages (e.g. refs 18 and 19).
Macrophages from other sources that have been isolated in iodixanol gradients are: human tumour tissue [20], lung tissue [21,22], mouse small intestine [23] and rabbit peripheral blood [24]. Macrophages have also been recovered from subcutaneous surgical sponge implants in mice by flotation on a 10% (w/v) iodixanol barrier [25]. More recently macrophages were isolated from a human buffy coat fraction after sequential removal of PMNs and lymphocytes using iodixanol density barriers and platelets by differential centrifugation [26].
This type of OptiPrepTM-based methodology is becoming increasingly popular in modern macrophage studies (see for example refs 27-31).
6. References
1. Fisker, S., Kudahl, K. and Sonne, O. (1990) Isolation of rat peritoneal mononuclear and polymorphonuclear leucocytes on discontinuous gradients of Nycodenz J. Immunol. Meth., 133, 31-38
2. Kudahl, K., Fisker, S. and Sonne, O. (1991) A thrombin receptor in resident rat peritoneal macrophages Exp. Cell Res., 193, 45-53
3. Fisker, S., Kudahl, K. and Sonne, O. (1992) In vivo inflammatory stimulation induces a transient change in the binding of thrombin to rat peritoneal macrophages Exp. Cell Res., 201, 145-153
4. Palacios-Corona, R., Ortiz-Navarrete, V.F., Said-Fernandez, S., Rodriguez-Padilla, C. and Gonzales-Garza, T. (1999) Detection of a factor released by L5178Y lymphoblasts that inhibits mouse macrophage-activation induced by lipopolysaccharides Arch. Med. Res., 30, 298-302
5. Antonissen, A.C.J.M., Lemmens, P.J.M.R., van den Bosch, J.F. and van Boven, C.P.A (1986) Transfer of enhanced resistance against Listeria monocytogenes induced with ribosomal RNA and the adjuvant dimethyldioctadecylammonium bromide Immunol. Lett., 14, 21-28
6. Freeman, G.E., Dalton, C.A. and Brooks, P.M. (1991) A Nycodenz gradient for the purification of neutrophils from the peripheral blood of rats J. Immunol. Meth., 139, 241-249
7. Killingsworth, C.R., Shore, S.A., Alessandrini, F., Dey, R.D. and Paulauskis, J.D. (1997) Rat alveolar macrophages express preprotachykinin gene-I mRNA-wncoding tachykinins Am. J. Physiol. Lung Cell Mol. Physiol., 273, L1073-L1081
8. Ganz, T., Rayner, J.R., Valore, E.V., Tumolo, A., Talmadge, K. and Fuller, F. (1989) The structure of the rabbit macrophage defensin genes and their organ- specific expression J. Immunol., 143, 1358-1365
9. Beck, K.D., Nguyen, H.X., Galvan, M.D., Salazar, D.L., Woodruff, T.M. and Anderson, A.J. (2010) Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment Brain 133, 433–447
10. Galvan, M.D., Luchetti, S., Burgos, A.M., Nguyen, H.X., Hooshmand, M.J., Hamers, F.P.T. and Anderson, A.J. (2008) Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury J. Neurosci., 28, 13876 –13888
11. Mavrikis Cox, G., Kithcart, A.P., Pitt, D., Guan, Z., Alexander, J., Williams, J.L., Shawler, T., Dagia, N.M. et al (2013) Macrophage migration inhibitory factor potentiates autoimmune-mediated neuroinflammation J. Immunol., 191, 1043–1054
12. Kambarage, D.M., Bland, P.W., Strokes, C.R., Brown, P. and Skuse, A.M. (1995) Ultrastructural, histochemical and immunohistochemical features of porcine intestinal lamina propria macrophages, peripheral blood monocytes and splenic adherent cells J. Comp. Path., 112, 63-77
13. De Vries, H.E., Buchner, B., van Berkel, T.J.C. and Kuiper, J. (1999) Specific interaction of oxidized low-density lipoprotein with macrophage-derived foam cells isolated from rabbit atherosclerotic lesions Arterioscler. Thromb. Vasc. Biol., 19, 638-645
14. Cipollone, F., Fazia, M.L., Iezzi, A., Cuccurullo, C., De Cesare, D., Ucchino, S., Spigonardo, F., Marchetti, A. et al (2005) Association between prostaglandin E receptor subtype EP4 overexpression and unstable phenotype in atherosclerotic plaques in human Arterioscler. Thromb. Vasc. Biol., 25, 1925-1931
15. He, F., Guo, F-C., Li, Z., Yu, H-C., Ma, P-F., Zhao, J-L., Feng, L., Li, W-N. et al (2015) Myeloid-specific disruption of recombination signal binding protein Jj ameliorates hepatic fibrosis by attenuating inflammation through cylindromatosis in mice Hepatology, 61, 303-314
16. Kannan, Y., Perez-Lloret, J., Li, Y., Entwistle, L.J., Khoury, H., Papoutsopoulou, S., Mahmood, R., Mansour, N.R. et al (2016) TPL-2 regulates macrophage lipid metabolism and M2 differentiation to control TH2-mediated immunopathology PLoS Pathog., 2: e1005783
17. Yu, H-C., Bai, L., Yang, Z-X., Qin, H-Y., Tao, K-S., Han, H. and Dou, K-F. (2016) Blocking Notch signal in myeloid cells alleviates hepatic ischemia reperfusion injury by repressing the activation of NF-κB through CYLD Sci. Rep., 6: 32226
18. Eguchi, A., Lazaro, R.G., Wang, J., Kim, J., Povero, D., Willliams, B., Ho, S.B., Stearkel, P., Schnabl, B. et al (2017) Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a microRNA barcode that can be detected in blood Hepatology, 65, 475-490
19. Reid, D.T., McDonald, B., Khalidc, T., Vo, T., Schenck, L.P., Surette, M.G., Beck, P.L., Reimer, R.A. et al (2016) Unique microbial-derived volatile organic compounds in portal venous circulation in murine nonalcoholic fatty liver disease Biochim. Biophys. Acta, 1862, 1337–1344
20. Sprinzl, M.F., Reisinger, F., Puschnik, A., Ringelhan, M., Ackermann, K., Hartmann, D., Schiemann, M., Weinmann, A., Galle, P.R., Schuchmann, M., Friess, H., Otto, G., Heikenwalder, M. and Protzer, U. (2013) Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells Hepatology, 57, 2358-2368
21. Kim, E.Y., Battaile, J.T., Patel, A.C., You, Y., Agapov, E., Grayson, M.H., Benoit, L.A., Byers, D.E. et al (2008) Persistent activation of an innate response translates respiratory viral infection into chronic lung disease Nat. Med., 14, 633-640
22. Johnson, C., Jahid, S., Voelker, D.R. and Fan, H. (2011) Enhanced proliferation of primary rat type II pneumocytes by Jaagsiekte sheep retrovirus envelope protein Virology, 412, 349–356
23. Zhong, Z., Zhai, Y., Bu, P., Shah, S. and Qiao, L. (2017) Papilloma-pseudovirus eradicates intestinal tumours and triples the lifespan of ApcMin/+ mice Nat. Comm., 8: 15004
24. Yamane, K. and Leung, K-P. (2016) Rabbit M1 and M2 macrophages can be induced by human recombinant GM-CSF and M-CSF FEBS Open Bio, 6, 945–953
25. Thomas, A.C., Eijgelaar, W.J., Daemen, M.J.A.P. and Newby, A.C. (2015) The pro-fibrotic and antiinflammatory foam cell macrophage paradox Genomics Data 6, 136–138
26. Yuko Goto-Koshino, Ohno, K., Nakajima, M., Mochizuki, H., Kanemoto, H. and Tsujimoto, H. (2011) A rapid and simple method to obtain canine peripheral blood-derived macrophages J. Vet. Med. Sci., 73, 773–778
27. Radovanovic, Z., Djindjic, B., Dzopalic, T., Veljkovic, A., Dunjic, M., Krstic, D., Djindjic, N. and Nedeljkovic, B. (2018) Effect of silicon-rich water intake on the systemic and peritoneal inflammation of rats with chronic low levels of aluminum ingestion J. Trace Elements Med. Biol., 46, 96–102
28. Lejal, N., Truchet, S., Bechor, E., Bouguyon, E., Khedkar, V., Bertho, N., Vidic, J., Adenot, P., Soliere, S., Pick, E. and Slama-Schwok, A. (2018) Turning off NADPH oxidase-2 by impeding p67phox activation in infected mouse macrophages reduced viral entry and inflammation BBA – Gen. Subjects, 1862, 1263–1275
29. Inoue, M., Niki, M., Ozeki, Y., Nagi, S., Chadeka, E.A., Yamaguchi, T., Osada-Oka, M., Ono, K., Oda, T. et al (2018) High-density lipoprotein suppresses tumor necrosis factor alpha production by mycobacteria infected human macrophages Sci. Rep., 8: 6736
30. Bordet, E., Maisonnasse, P., Renson, P., Bouguyon, E., Crisci, E., Tiret, M., Descamps, D., Bernelin-Cottet, C. et al (2018) Porcine alveolar macrophage-like cells are pro-inflammatory pulmonary intravascular macrophages that produce large titers of porcine reproductive and respiratory syndrome virus Sci. Rep., 8: 10172
31. Zheng, X-L., Wu, J-P., Gong, Y., Hong, J-B., Xiao, H-Y., Zhong, J-W., Xie, B., Li, B-M. et al (2019) IL-25 protects against high-fat diet-induced hepatic steatosis in mice by inducing IL-25 and M2a macrophage production Immunol. Cell Biol., 97, 165–177
32. Buechler, M.B., Kim, K-W., Onufer, E.J., Williams, J.W., Little, C.C., Dominguez, C.X., Li, Q., Sandoval, W., Cooper, J.E. et al (2019) A stromal niche defined by expression of the transcription factor WT1 mediates programming and homeostasis of cavity-resident macrophages Immunity 51, 119–130
OptiPrep™ Application Sheet C42; 5th edition January 2020
OptiPrep™ Application Sheet C43
Isolation of mononuclear cells from rat, mouse and rabbit blood on a density barrier
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density of 1.32 g/ml
- OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes – a methodological review” compares all of the currently available methodologies
- OptiPrep™ Reference List RC01 “Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes – a bibliographical review” provides a comprehensive bibliography of all the published papers reporting the use of OptiPrep™
- To access C03 and RC01 return to the initial list of Folders and select “Application Sheets” or “Reference Lists”. To access other Application Sheets referred to in the text, return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
In 1982 Bøyum [1] published a new method for the isolation of human PBMCs that employed a medium of the same density as Lymphoprep™ containing no polysaccharide. This medium containing 14.1% (w/v) NycodenzⓇ, 0.44% NaCl, 5 mM Tricine-NaOH, pH 7.0 (ρ = 1.077 ± 0.001 g/ml; osmolality = 295 mOsm) is no longer commercially produced. This solution was subsequently modified [2] to provide a simple method for the preparation of mononuclear cells (MCs) from rodent and rabbit blood, which have a higher density than those from human blood. The solution contained 14.1% (w/v) NycodenzⓇ, 0.30% (w/v) NaCl, 5 mM Tricine-NaOH, pH 7.2 ρ = 1.077 ± 0.001 g/ml; osmolality 265 mOsm). Reducing the osmolality of the solution causes the MCs, but not the polymorphonuclear leukocytes (PMNs), to gain water, thus their density decreases. This strategy gives better resolution of the MCs from PMNs than using a barrier of raised density.
This medium, which was commercially produced by Axis-Shield as NycoprepⓇ 1.077A, is no longer available. However a medium of identical density and osmolality can be easily produced from OptiPrep™.
 2. Solution preparation (see Note 1)
2. Solution preparation (see Note 1)
A. OptiPrep™ (60%, w/v iodixanol) – shake the bottle gently before use
B. Buffered saline (isoosmotic): 0.85% (w/v) NaCl, 10 mM Tricine, pH 7.0
3. Protocol
1. Make up the density barrier: Dilute Solution B with water (volume ratio 2.5:0.5 respectively); this solution has an osmolality of approx 242 mOsm. Dilute OptiPrep™ with this solution using a volume ratio 2.7: 9.3 respectively (see Note 2).
2. Collect the blood by cardiac puncture into a syringe containing anticoagulant; EDTA, citrate, ACD or heparin is usually satisfactory (see Note 3).
3. Dilute the blood with an equal volume of Solution B.
4. In a 15 ml centrifuge tube carefully layer 6 ml of diluted blood over 3 ml of the density barrier (avoid mixing at the interface). Alternatively the blood may be underlaid with the density barrier using a syringe and metal cannula (see Notes 4 and 5).
5. Centrifuge at 700 g for 20 min at approx. 20°C in a swinging-bucket rotor.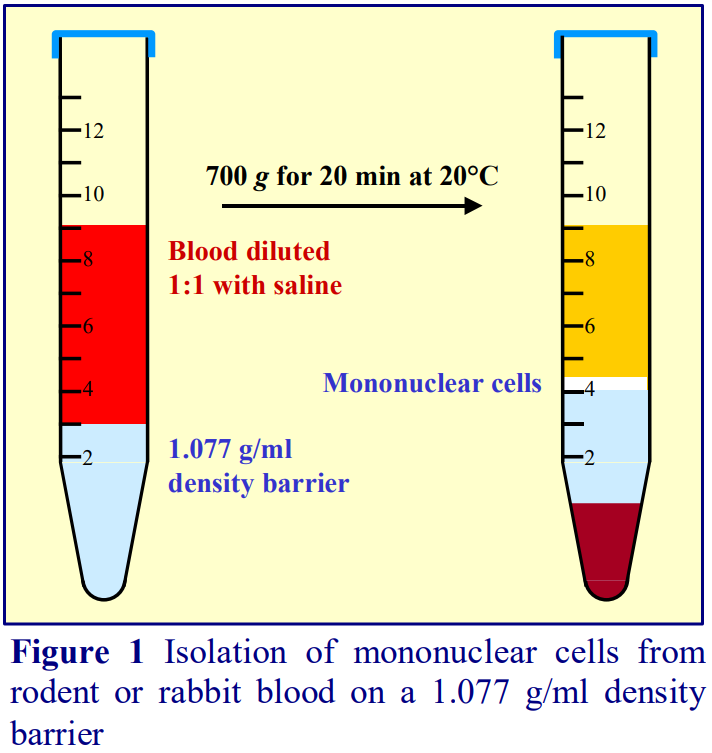 6. After centrifugation the MCs form a sharp band at the interface (see Figure 1).
6. After centrifugation the MCs form a sharp band at the interface (see Figure 1).
7. Remove the plasma layer to just above MC band (Figure 1) and then recover the band of MCs. This is best achieved using a syringe attached to a metal cannula (i.d. 0.8 mm).
8. Dilute the cell harvest with 2 vol. of solution B to reduce the density of the solution; pellet the cells by centrifugation at 400 g for 10 min
9. Resuspend the MC pellet in Solution B and process as required.
4. Notes
1. The Tricine in the saline solutions may be replaced by any suitable organic buffer (e.g. HEPES)
2. If an osmometer is available check the osmolality of these solutions; it should be 265 mOsm (± 10 mOsm). The dilutions should be prepared as accurately as possible. If there is significant loss of mononuclear cells to the pellet, try increasing the density of the barrier slightly (2.8 vol. of OptiPrep™ and 9.2 vol. of the diluted saline).
3. Rat blood in particular is prone to coagulation and higher concentrations of anticoagulant than those used for human blood may be required. We recommend the use of EDTA and have found that the final concentration of EDTA should be 3-4 mM.
4. For other blood volumes keep to a ratio of diluted blood to density barrier of 2:1. For small volumes of mouse blood use a smaller volume (narrower) tube.
5. Flat-tipped metal cannulas can be purchased from many surgical instrument companies.
- MCs from rat and mouse blood may also be isolated by flotation using OptiPrep™, see respectively Applications Sheets C07 and C08
5. References
1. Bøyum, A., Berg, T. and Blomhoff, R. (1982) Fractionation of mammalian cells In: Iodinated density gradient media – a practical approach (ed D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 147-171
2. Bøyum, A., Lovhaug, D., Tresland, L. and Nordlie, E.M. (1991) Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality Scand. J. Immunol., 34, 697-712
OptiPrep™ Application Sheet C43; 7th edition, January 2020
OptiPrep™ Application Sheet C44
Isolation of polymorphonuclear leukocytes (granulocytes) from rat, mouse, guinea pig and rabbit blood
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
A common approach to the problem of isolating rodent and rabbit polymorphonuclear leukocytes (PMNs) was to use a density barrier of NycoprepⓇ 1.077A (ρ = 1.077 g/ml, osmolality = 265 mOsm). The method was first published by Bøyum et al [1]. This customized medium separated the mononuclear cells (MCs), which band at the interface, from the PMNs and the erythrocytes, which sediment through the barrier to form a pellet. To retrieve the PMNs, the erythrocytes may be selectively lysed in isotonic ammonium chloride solution or ice-cold distilled water. An alternative approach is to remove the erythrocytes from whole blood first by aggregation with dextran [2-4], methylcellulose [5,6] hetastarch [7] or Plasmagel [8,9] and then layer the resulting leukocyte-rich plasma (LRP) over the NycoprepⓇ 1.077A, so that the PMN pellet contains only a very small percentage of residual erythrocytes. NycoprepⓇ 1.077A is no longer commercially available but an iodixanol solution of the same density and osmolality can be easily prepared from OptiPrep and it is this method that is described in Section 2.
There are also a few examples of the use of other density barriers and two-layer discontinuous gradients, prepared from both NycodenzⓇ and OptiPrep™. A few examples are given in Section 3. Polymorphprep™, a medium normally restricted to the use of human blood, has also been used in a few cases (see Section 4).
 2. Reduced osmolality density barrier
2. Reduced osmolality density barrier
2a. Solution preparation (see Box)
A. OptiPrep™ (60%, w/v iodixanol) – shake the bottle gently before use
B. Buffered saline (isoosmotic): 0.85% (w/v) NaCl, 10 mM Tricine-NaOH, pH 7.0
C. Buffered saline containing EDTA
D. 20% (w/v) polysucrose (dextran), MWt 450,000 in Solution B (see Section 2c, Note 1)
E. Erythrocyte lysis solution
2b. Protocol
1. Start by preparing the hypoosmotic 1.077 g/ml density barrier: dilute Solution B with water (2.5 vol. + 0.5 vol.) and then mix 2.7 vol. of OptiPrep™ with 9.3 vol. of the diluted saline solution (see Section 2c, Note 2).
2. Collect the blood by cardiac puncture into a syringe containing a volume of Solution C equal to that of the expected blood volume (see Section 2c, Note 3).
3. Mix 9.25 vol. of blood with 0.75 vol. of Solution D (see Section 2c, Notes 4-6).
4. Allow the blood to stand at room temperature for 30 min then remove the leukocyte-rich plasma (LRP) from above the aggregated erythrocyte pellet.
5. Layer 1 vol. of the leukocyte-rich plasma over 0.5 vol. of the 1.077 g/ml density barrier prepared in step 1 (see Section 2c, Note 7).
6. Centrifuge at 700 g for 20 min at room temperature and remove all of the liquid above the pellet by aspiration (see Section 2c, Note 8).
7. Suspend the pellet in 5 vol. of Solution E and lyse the erythrocytes by incubation at 37°C for 7 min (see Section 2c, Note 9).
8. Sediment the PMNs at 250-300 g for 15 min and resuspend in Solution B or process as required (see Section 2c Note 10).
2c. Notes
1. Polysucrose is best dissolved by slowly adding the liquid to the solid, stirring with a glass rod after addition of each 1-2 ml aliquot.
2. If an osmometer is available check the osmolality of these solutions; the osmolality of the barrier solution should be 26510 mOsm. The dilutions should be prepared as accurately as possible. OptiPrep™ is quite viscous and when dispensing volumes with an automatic pipette the liquid should be aspirated into, and ejected from, the pipette tip slowly. If there is significant loss of mononuclear cells to the pellet, try increasing the density of the barrier slightly (2.8 vol. of OptiPrep™ and 9.2 vol. of the diluted saline).
3. Compared to human blood collected by venepuncture, both mouse and rat blood (collected by cardiac puncture) is very prone to clotting. Even if anticoagulant is present in the syringe, coagulation can still be problematical. This is minimized if the blood is also diluted directly by pre-charging the syringe with a volume of saline containing EDTA (4 mM) at twice the required final concentration (Solution C). If this approach is not used, then dilute the blood with an equal volume of Solution B before proceeding to Step 3. We have found that EDTA is the most reliable and use it at 2 mM final concentration; other workers use concentrations as high as 4 or 5 mM or other anticoagulants such as citrate or heparin. See Section 1 above for more information on preparing the LRP.
4. It is also possible to use whole blood rather than an LRP in this separation and to remove the erythrocytes from the pellet (see step 6) by aggregation in polysucrose. Although this approach is rather uncommon, it does reduce the time before the gradient separation of the PMNs from the mononuclear cells and platelets (see ref 10).
5. If the blood is not diluted during cardiac puncture (see Note 3) it is common to add an equal volume of 6% (w/v) polysucrose [2,3], although half this concentration is often effective. On the other hand if the blood has been diluted during cardiac puncture, the use of a small volume of a high concentration polysucrose solution (20%) minimizes any further dilution of the blood; make sure that the viscous polysucrose is mixed well with the blood by repeated gentle inversion. If the aggregation is unsatisfactory, double the volume of 20% polysucrose.
6. Other erythrocyte aggregating agents may be used: 2% (w/v) methylcellulose is added at a volume ratio to whole blood of 1:10 [6]; 2-3 ml of Plasmagel was added to 5 ml of heparinized whole blood by Song et al [8]; equal volumes of whole blood and 6% (w/v) hetastarch in 0.9% NaCl is a third option [7].
7. Because of the functional problems that can arise from the pelleting of PMNs, some workers include a small volume of a dense cushion to prevent this; a cushion of density 1.11 g/ml can be simply produced by diluting 1 vol. of OptiPrep™ with 2 vol. of the saline solution prepared in Step 1. A typical separation in a 15 ml conical tube therefore might be 5 ml of LRP/saline, 2.5 ml of the 1.077 g/ml density barrier and 1.0 ml of cushion. For small volumes of mouse blood use a smaller volume (narrower) tube.
8. If a cushion is used remove all of the liquid above the PMN band and, using a syringe attached to a length of Teflon tubing, remove as much of the cushion as possible.
9. Lysis using ice-cold distilled water for 30 sec before adding an equal volume of double strength saline is an alternative.
10. For a selection of papers reporting the use of NycoPrep 1.077A for rabbit PMNs and rodent PMNs see refs 11-13 and 2,4,6,14-19 respectively. Rat PMNs have been prepared using this OptiPrep™- based method [20]
- For more recent papers reporting the use of the OptiPrep™ methodology see refs 21-25
3. Other density barriers and two-layer discontinuous gradient
Rodent PMNs have also been separated from mononuclear cells by pelleting through a 1.09 g/ml barrier at 600 g for 15 min [26]. This was prepared from NycodenzⓇ but could easily be formed by dilution of OptiPrep™ with a regular buffered saline solution and is equivalent to an iodixanol concentration of 16% (w/v). From a functional point of view, it would be better to include a small dense cushion of about 1.11 g/ml (20% iodixanol) to prevent the pelleting of the PMNs.
The use of a two layer discontinuous gradient was developed by Freeman et al [27] who started with an isoosmotic solution of 27.6% (w/v) NycodenzⓇ in 3 mM KCl, 0.3 mM CaNa2-EDTA 5 mM Tris-HCl, pH 7.5 (this isoosmotic solution, which was available as NycoPrep 1.15 is no longer produced commercially). It was diluted with the same KCl, EDTA, Tris solution containing 0.75 g NaCl/100 ml to produce solutions of 18.4% and 13.8% NycodenzⓇ (ρ = 1.098 and 1.075 respectively). A rat leukocyte-rich plasma (2-6 ml) was layered on top of 2.5 ml of each of the density solutions and centrifuged at 400 g for 30 min at 26°C. The PMNs banded around the lower interface. The diluent was slightly hypoosmotic (see Section 2). Iodixanol solutions of the same % (w/v) concentration and very similar osmolality could be produced by a two-step dilution of OptiPrep™:
1. Dilute OptiPrep™ with an equal volume of normal buffered saline (Solution B in Section 2a) to produce an isoosmotic 30% (w/v) iodixanol solution
2. Dilute this further to produce 18.4% and 13.8% (w/v) iodixanol solutions with a 0.75% (w/v) NaCl solution in 10 mM Tricine-NaOH, pH 7.0 Rabbit PMNs have also been isolated from an LRP using a two-layer iodixanol gradient (density 1.085 and 1.086 g/ml) with centrifugation at 500 g for 35 min [28,29]
4. Polymorphprep™
There are a few reported cases of the use of Polymorphprep™ for rat [30-33], mouse [34-36] and rabbit [37-40] PMNs. Although the rationale behind the use of this medium for human PMNs is that the use of whole blood is essential to the separation, see Polymorphprep™ Application Sheet (on home page, select “Products”) for more details, Shibata et al [39] used a PMN-rich suspension from which the erythrocytes had been removed. McCartney-Francis et al [31] diluted the Polymorphprep™ 5:1 for rat PMNs and the use of a high concentration of EDTA (7.7 mM) may reflect the aggregation problems sometimes encountered with rat blood [32]. Wen et al [35] used this medium for the removal of PMNs from mouse leukocytes prior to isolation of macrophages and monocytes. In a variant of the two-layer discontinuous gradient the dense cushion layer was replaced by Polymorphprep™, the high osmolality of this layer presumably allowing the majority of the erythrocytes to sediment through it [41].
5. Other species
PMNs have been isolated from bovine [42,43] and guinea pig blood [44] in iodixanol gradients
6 References
1. Bøyum, A., Løvhaug, D., Tresland, I. And Nordlie, E.M. (1991) Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality Scand. J. Immunol., 34, 697-712
2. Connor, T.J., Kelly, J.P. and Leonard, B.E. (1997) Forced swim test-induced neurochemical, endocrine and immune changes in the rat Pharmacol. Biochem. Behav., 58, 961-967
3. Connor, T.J., Kelly, J.P. and Leonard, B.E. (1997) Forced swim test-induced endocrine and immune changes in the rat: effect of subacute desipramine treatment Pharmacol. Biochem. Behav., 59, 171-177
4. Kataranovski, M., Vlaški, M., Kataranovski, D., Tošić, N., Mandić-Radić, S. and Todorović, V. (2003) Immunotoxicity of epicutanously applied anticoagulant rodenticide warfarin: evaluation by contact hypersensitivity to DNCB in rats Toxicology, 188, 83-100
5. Bautista, A.P. (1995) Chronic alcohol intoxication enhances the expression of CD18 adhesion molecules on rat neutrophils and release of a chemotactic factor Kupffer cells Alcohol. Clin. Exp. Res., 19, 285-290
6. Rossoni, G., Berti, F., Trenot, F., Cattaneo, F., Porta, R., Pescador, R. and Ferro, L. (1999) Chronic oral defibrotide counteracts hypercholesterolemia noxious effects on cardiovascular function in the rabbit Thromb. Res., 94, 327-338
7. Silliman, C.C., Voelkel, N.F., Allard, J.D., Elzi, D.J., Tuder, R.M., Johnson, J.L. and Ambruso, D.R. (1998) Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model J. Clin. Invest., 101, 1458-1467
8. Song, C., Earley, B. and Leonard, B.E. (1996) Behavioural and immunological effects of the antihistamine terfanadine in olfactory bulbectomized rats Eur. Neuropsychopharmacol., 6, 157-162
9. Song, C. and Leonard, B.E. (1998) Comparison between the effects of receptor ligand JO1784 and neuropeptide Y on immune functions Eur. J. Pharmacol., 345, 79-87
10. Tassiopoulos, A.K., Hakim, T.S., Finck, C.M., Pedoto, A., Hodell, M.G., Landas, S.J. and McGraw, D.J. (1998) Neutrophil sequestration in the lung following acute aortic occlusion starts during ischaemia and can be attenuated by tumour necrosis factor and nitric oxide blockade Eur. J. Endovasc. Surg., 16, 36-42
11. Rossoni, G., Berti, F., Trento, F., Cattaneo, F., Porta, R., Pescador, R. and Ferro, L. (1999) Chronic oral Defibrotide counteracts hypercholesterolemia noxious effects on cardiovascular function in the rabbit Thromb. Res., 94 327–338
12. Gratz, S., Rennen, H.J.J.M., Boerman, O.C., Oyen, W.J.G., Burma, P. and Corstens, F.H.M. (2001) 99mTcinterleukin-8 for imaging acute osteomyelitis J. Nucl. Med., 42, 1257–1264
13. Gratz, S., Rennen, H.J.J.M., Boerman, O.C., Oyen, W.J.G., and Corstens, F.H.M. (2001) Rapid imaging of experimental colitis with 99mTc-interleukin-8 in rabbits J. Nucl. Med., 42, 917–923
14. Wuyts, A., Haelens, A., Proost, P., Lenaerts, J-P., Conings, R., Opdenakker, C. and Damme, J.V. (1996) Identification of mouse granulocyte chemotactic protein-2 from fibroblasts and epithelial cells functional comparison with natural KC and macrophage inflammatory protein-2 J. Immunol., 157, 1736-1 743
15. Lou, Y., Xia, D., Han, W., Wang, Y., Li, X., Li, Y., Rui, M., Ding, P., Song, Q., Zhang, Y. and Ma, D. (2003) Molecular cloning and characterization of rat chemokine-like factor 1 and 2 Gene 307, 125–132
16. Morgan, B.P., Griffiths, M., Khanom, H., Taylor, S.M. and Neal, J.W. (2004) Blockade of the C5a receptor fails to protect against experimental autoimmune encephalomyelitis in rats Clin. Exp. Immunol., 138, 430– 438
17. Knudsen, E., Benestad, H.B., Seierstad, T. and Iversen, P.O. (2004) Macrophages in spleen and liver direct the migration pattern of rat neutrophils during inflammation Eur. J. Haematol., 73, 109–122
18. Shimazawa, M., Kondo, K., Hara, H., Nakashima, M. and Umemura, K. (2005) Sulfatides, L- and P-selectin ligands, exacerbate the intimal hyperplasia occurring after endothelial injury Eur. J. Pharmacol., 520, 118 – 126
19. Laragione, T., Yarlett, N.C., Brenner, M., Mello, A., Sherry, B., Miller, E..J., Metz, C.N. and Gulko, P.S. (2007) The arthritis severity quantitative trait loci Cia4 and Cia6 regulate neutrophil migration into inflammatory sites and levels of TNF- and nitric oxide J. Immunol., 178, 2344–2351
20. Belij, S., Miljković, D., Popov, A., Subota, V., Timotijević, G., Slavić, M., Mirkov, I., Kataranovski, D. and Kataranovski, M. (2012) Effects of subacute oral warfarin administration on peripheral blood granulocytes in rats Food Chem. Toxicol., 50, 1499–1507
21. Djokic, J., Ninkov, M., Mirkov, I., Aleksandrov, A.P., Zolotarevski, L., Kataranovski, D. and Kataranovski, M. (2014) Differential effects of cadmium administration onperipheral, blood granulocytes in rats Environ. Toxicol. Pharmacol., 37, 210-219
22. Subota, V., Mirkov, I., Demenesku, J., Aleksandrov, A.P., Ninkov, M., Mileusnic, D., Kataranovski, D. and Kataranovski, M., (2016) Transdermal toxicity of topically applied anticoagulant rodenticidewarfarin in rats Environ. Toxicol. Pharmacol., 41, 232–240
23. Aleksandrov, A.P., Belij-Rammerstorfer, S., Mirkov, I., Subota, V., Kulas, J., Kataranovski, D. and Kataranovski, M. (2018) Oral warfarin affects some aspects of systemic immunomodulation with topical dinitrochlorobenzene (DNCB) in rats Cutan. Ocul. Toxicol., 47, 29–35
24. Sellami, M., Meghraoui-Kheddar, A., Terryn, C., Fichel, C., Bouland, N., Diebold, M., Guenounou, M., Héry-Huynh, S., and Le Naour, R. (2016) Induction and regulation of murine emphysema by elastin peptides Am. J. Physiol. Lung Cell. Mol. Physiol. 310: L8–L23, 2016
25. Mishra, A., Guo, Y., Zhang, L., More, S., Weng, T., Chintagari, N.R., Huang, C., Liang, Y., Pushparaj, S. et al (2016) A critical role for P2X7 receptor–induced VCAM-1 shedding and neutrophil infiltration during acute lung injury J. Immunol., 197, 2828–2837
26. Johnsen, H., Odden, E., Johnsen, B.A. and Fonnum, F. (1988) Metabolism of T-2 toxin by blood cell carboxylesterases Biochem. Pharmacol., 37, 3193-3197
27. Freeman, G.E., Dalton, C.A. and Brooks, P.M. (1991) A Nycodenz gradient for the purification of neutrophils from the peripheral blood of rats J. Immunol. Meth., 139, 241-249
28. Matute-Bello, G., Frevert, C.W., Kajikawa, O., Skerrett, S.J., Goodman, R.B., Park, D.R. and Martin, T.R. (2001) Septic shock and acute lung injury in rabbits with peritonitis Am. J. Respir. Crit. Care Med., 163, 234-243
29. Frevert, C.W., Goodman, R.B., Kinsella, M.G., Kajikawa, O., Ballman, K., Clark-Lewis, I., Proudfoot, A.E.I., Wells, T.N.C. and Martin, T.R. (2002) Tissue-specific mechanisms control the retention of IL-8 in lungs and skin J. Immunol., 168, 3550-3556
30. Mizuno, M., Nishikawa, K., Okada, N., Matsuo, S., Ito, K. and Okada, H. (1999) Inhibition of a membrane complement regulatory protein by a monoclonal antibody induces acute lethal shock in rats primed with lipopolysaccharide J. Immunol., 162, 5477–5482
31. McCartney-Francis, N.L., Song, X-y., Mizel, D.E. and Wahl, S.M. (2001) Selective inhibition of inducible nitric oxide synthase exacerbates erosive joint disease J. Immunol., 166, 2734–2740
32. Fujisawa, H., Nakagawa, S., Ohkubo, Y., Matsui, M., Yamaguchi, S., Kawamura, M., Hatanaka, K., Kawakubo, Y., Hiramoto, Y., Kobayashi, H., Harada, Y. (2005) Local and systemic expression of inducible nitric oxide synthase in comparison with that of cyclooxygenase-2 in rat carrageenin-induced pleurisy Nitric Oxide 12, 80–88
33. Lipton, B.P., Bautista, A.P., Delcarpio, J.B. and McDonough, K.H. (2001) Effects of endotoxin on neutrophil-mediated I/R injury in isolated perfused rat hearts Am. J. Physiol. Heart Circ. Physiol., 280, H802-H811
34. Yamauchi, A., Kim, C., Li, S., Marchal, C.C., Towe, J., Atkinson, S.J. and Dinauer, M.C. (2004) Rac2- deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles J. Immunol., 173, 5971-5979
35. Wen, R., Jou, S-T., Chen, Y., Hoffmeyer, A. and Wang, D. (2002) Phospholipase Cγ2 is essential for specific functions of FcεR and FcγR J. Immunol., 169, 6743–6752
36. Carlyon, J.A., Akkoyunlu, M., Xia, L., Yago, T., Wang, T., Cummings, R.D., McEver, R.P. and Fikrig, E. (2003) Murine neutrophils require α1,3-fucosylation but not PSGL-1 for productive infection with Anaplasma phagocytophilum Blood, 102, 3387-3395
37. Shibata, K., Kitayama, S., Morita, K., Shirakawa, M., Okamoto, H. and Dohi, T. (1994) Regulation by protein kinase C of platelet-activating factor- and thapsigargin-induced calcium entry in rabbit neutrophils Jpn. J. Pharmacol., 66, 273-276
38. Shibata, K., Morita, K., Kitayama, S., Okamoto, H. and Dohi, T. (1996) Ca2+ entry induced by calcium influx factor and its regulation by protein kinase C in rabbit neutrophils Biochem. Pharmacol., 52, 167-171
39. Shibata, K., Yoshino, H., Mizuno, N., Shinohara, H., Morita, K., Kitayama, S., Kurihara, H. and Dohi, T. (2000) Mediation by platelet-activating factor of 12 hydroxyeicosatetraenoic acid-induced cytosolic free calcium concentration elevation in neutrophils Prostaglandin Other Lipid Mediat., 62, 385-394
40. Okamoto, K., Kanoe, M., Yaguchi, Y., Inoue, T. and Watanabe, T. (2006) Effects of a collagenolytic cell wall component from Fusobacterium necrophorum subsp. necrophorum on rabbit tissue-culture cells Vet. J. 171, 380-382
41. Ishida-Okawara, A., Ito-Ihara, T., Muso, E., Ono, T., Saiga, K., Nemoto, K. and Suzuki, K. (2004) Neutrophil contribution to the cresecentic glomerulonephritis in SCG/Kj mice Nephrol. Dial. Transplant., 19, 1708-1715
42. Wang, J., Zhou, X., Pan, B., Wang, H., Shi, F., Gan, W., Yang, L., Yin, X., Xu, B. and Zhao, D. (2013) Expression pattern of interferon-inducible transcriptional genes in neutrophils during bovine tuberculosis infection DNA Cell Biol., 32, 480-486
43. Wang, J., Zhou, X., Pana, B., Yang, L., Yin, X., Xu, B. and Zhao, D. (2013) Investigation of the effect of Mycobacterium bovis infection on bovine neutrophils functions Tuberculosis, 93, 675-687
44. Takahashi, M., Jeevan, A., Sawant, K., Mc Murray, D.N. and Yoshimura, T. (2007) Cloning and characterization of guinea pig CXCR1 Mol. Immunol., 44, 878-888
OptiPrep™Application Sheet C44; 10th edition, February 2020
OptiPrep™ Application Sheet C45
Isolation of peripheral blood mononuclear cells from non-human primates
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
For the isolation of human peripheral blood mononuclear cells (PBMCs) from whole blood, sedimentation onto a barrier of density 1.077 g/ml is probably the most widely used technique. There are two commercially available media from Axis-Shield PoC AS; Lymphoprep™ (the most widely used medium) contains diatrizoate and a polysaccharide and NycoprepⓇ 1.077, which contains a buffered solution of NycodenzⓇ. This density barrier method for human PBMCs has also been adapted to the use of OptiPrep™ (see Application Sheet C04). Published papers report the use of both Lymphoprep™ and NycoprepⓇ 1.077 for the isolation of PBMCs from many types of higher primate but there have been no detailed comparative studies between different primates. The method described in this Application Sheet (Section 2), devised by Stittelaar et al [1,2] was designed specifically for macaque PBMCs (see also ref 3), but it has now been extended to mandrills [4].
An alternative “mixer” technique was devised for human PBMCs in 1990 [5], which has also been adapted to the use of OptiPrep™ technology (see Application Sheet C05); this has now been extended to cynomolgus macaques and is described in Section 3.
2. Density barrier method
2a. Solutions required
A. OptiPrep™ (shake gently before use)
B. Ten-times concentrated phosphate buffered saline (10xPBS).
C. Polysaccharide solution: 6% (w/v) polysucrose 400 (MWt 400,000) in water.
 2b. Protocol (adapted from ref 1, see Section 2c)
2b. Protocol (adapted from ref 1, see Section 2c)
1. Prepare the density barrier by mixing Solutions A, B and C in the following volume ratio: 16.7:8.3: 75.
2. In a centrifuge tube layer 2 volumes of heparinized blood over 1 volume of the density barrier (see Figure 1 and Section 2c).
3. Centrifuge at 600 g for 20 min; allow the rotor to decelerate without the brake.
4. Remove the PBMC that band at the interface (see Figure 1).
5. Dilute the collected material with two volumes of buffered-saline and pellet the cells at 250-500 g for 5-10 min.
2c. Notes
Sittelar et al [1,2] specified the use of heparinized blood and used the blood undiluted. Human blood is normally diluted with an equal volume of saline before applying to the density barrier. It is not entirely clear from other publications which strategy was adopted [3,4,6]. Use whichever method provides the optimal results.
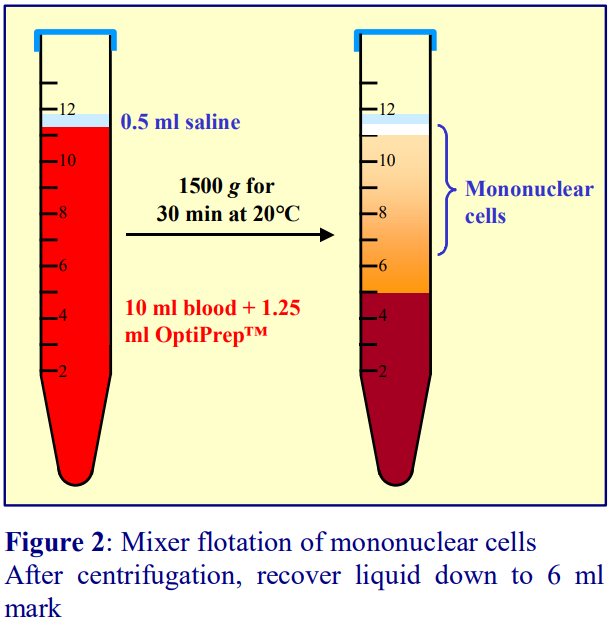 3. Mixer strategy
3. Mixer strategy
3a. Solutions required
OptiPrep™ (shake gently before use) Buffered saline
3b. Protocol
Mix 10 ml of blood with 1.25 ml of OptiPrep™ by several GENTLE inversions and proceed as described in Figure 2.
3c. Notes
Guo et al [7] who investigated this method concluded that the mixer strategy described here gave consistent yields, which are described here, that were higher than any of the established alternative procedures.
4. References
1. Stittelaar, K. J., Wyatt, L. S., de Swart, R. L., Vos, H. W., Groen, J., van Amerongen, G., van Binnendijk, R. S., Rozenblatt, S., Moss, B. and Osterhaus, A. D. M. E. (2000) Protective immunity in macaques vaccinated with a modified vaccinia virus Ankara-based measles virus vaccine in the presence of passively acquired antibodies J. Virol., 74, 4236- 4243
2. Stittelaar, K.J., Kuiken, T., de Swart, R.L., van Amerongen, G., Vos, H.W., Niesters, H.G.M., van Schalkwijk, P., van der Kwast, T., Wyatt, L.S., Moss, B. and Osterhaus, A.D.M.E. (2001) Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques Vaccine, 19, 3700-3709
3. Sun, C., Feng, L., Zhang, Y., Xiao, L., Pan, W., Li, C., Zhang, L. and Chen, L. (2012) Circumventing antivector immunity by using adenovirus-infected blood cells for repeated application of adenovirus-vectored vaccines: proof of ccncept in rhesus macaques J. Virol., 86, 11031-11042
4. Van der Kuyl, A.C., van den Burg, R., Hoyer, M.J., Gruters, R.A., Osterhaus, A.D.M.E. and Berkhout, B. (2004) SIVdrl detection in captive mandrills: are mandrill infected with a third strain of simian immunodeficiency virus? Retrovirology, 1:36
5. Ford, T. C. and Rickwood, D. (1990) A new one-step method for the isolation of human mononuclear cells J. Immunol. Meth., 134, 237-241
6. Meng, W., Pan, W., Zhang, A.J.X., Li, Z., Wei, G., Feng, L., Dong, Z., Li, C., Hu, X., Sun, C., Luo, Q., Yuen, K-Y., Zhong, N., Chen, L. (2013) Rapid generation of human-like neutralizing monoclonal antibodies in urgent preparedness for influenza pandemics and virulent infectious diseases PLoS One, 8: e66276
7. Guo, H., Zhang, H., Lu, L., Ezzelarab, M.B. and Thomson, A.W. (2015) Generation, cryopreservation, function and in vivo persistence of ex vivo expanded cynomolgus monkey regulatory T cells Cell. Immunol., 295, 19–28
OptiPrep™Application Sheet C45; 7th edition, January 2020
OptiPrep™ Application Sheet C46
Isolation of monocytes from a human leukocyte-rich plasma on a density barrier
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density of 1.32 g/ml
- The OptiPrep™ Application Sheet C03 “Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes: a methodological review” compares all of the currently available methodologies for human leukocytes. See Applications Sheets C10 and C11 for other monocyte methods.
- A Reference List (RC01) of all the published papers reporting the use of OptiPrep™ is also available: to access return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
The monocytes in human peripheral blood, account for, on average, about 8% of the leukocyte population. They tend to be larger (15-20 µm) than lymphocytes (6-20 µm) and they also have a slightly lower density. These properties allow some scope for their separation by centrifugation. Boyum et al [1,2] introduced a NycodenzⓇ density barrier (ρ = 1.068 g/ml) for resolving monocytes and lymphocytes from a leukocyte-rich plasma (LRP). It has a slightly raised osmolality (335 mOsm); this enhances the density difference between the monocytes and the osmotically-sensitive lymphocytes, whose density is increased. The method is very effective and the purity of the monocytes is greater than 90% but the monocytes do not form a distinct band; they are concentrated in the upper half of a broad turbid zone within the density barrier (see Figure 1 on page 2).
This medium, which was commercially produced by Axis-Shield as NycoprepⓇ 1.068, is no longer available. However a medium of identical density and osmolality can be easily produced from OptiPrep™.
 2. Solution preparation (see Section 4, Note 1)
2. Solution preparation (see Section 4, Note 1)
A. OptiPrep™ (60%, w/v iodixanol) – shake the bottle gently before use
B. Buffered saline (isoosmotic): 0.85% (w/v) NaCl, 10 mM Tricine-NaOH, pH 7.0
C. Buffered saline: 1.05% (w/v) NaCl, 10 mM Tricine-NaOH, pH 7.0
D. Polysucrose: 6% (w/v) polysucrose (Mr = 400-500 x 103) in Solution B (optional, see Step 2 of the Protocol)
3. Protocol
3a. Make up the density barrier
Mix 0.12 vol. of OptiPrep™ 0.48 vol. of Solution D. If an osmometer is available check the osmolality of the density barrier solutions; it should be 335 ± 10 mOsm and the density 1.068 ± 0.001 g/ml. Volumes must be dispensed as accurately as possible; any deviation from the recommended osmolality and/or density will affect the separation. Particular attention must be made to dispensing the OptiPrep™, because of its viscosity.
3b. Prepare the LRP
Always use freshly drawn, whole blood (anti-coagulant 1.5-2.0 mM EDTA). The leukocyte-rich plasma may be prepared as a buffy coat fraction or by polysucrose sedimentation of the erythrocytes.
Buffy coat: Centrifuge the blood at 400 g in a swinging-bucket rotor, at about 20°C, for 10-15 min. Harvest the buffy coat layer, on the top of the erythrocytes, in the plasma supernatant (LRP). Some erythrocytes will also be collected but try to keep them to a minimum. Over 80% of the leukocytes can be recovered in this manner.
Erythrocyte aggregation: Mix 9 vol. of blood with 1 vol. of Solution F by gentle inversions and allow the aggregated erythrocytes to settle to the bottom (20-40 min at room temperature); then aspirate the entire supernatant.
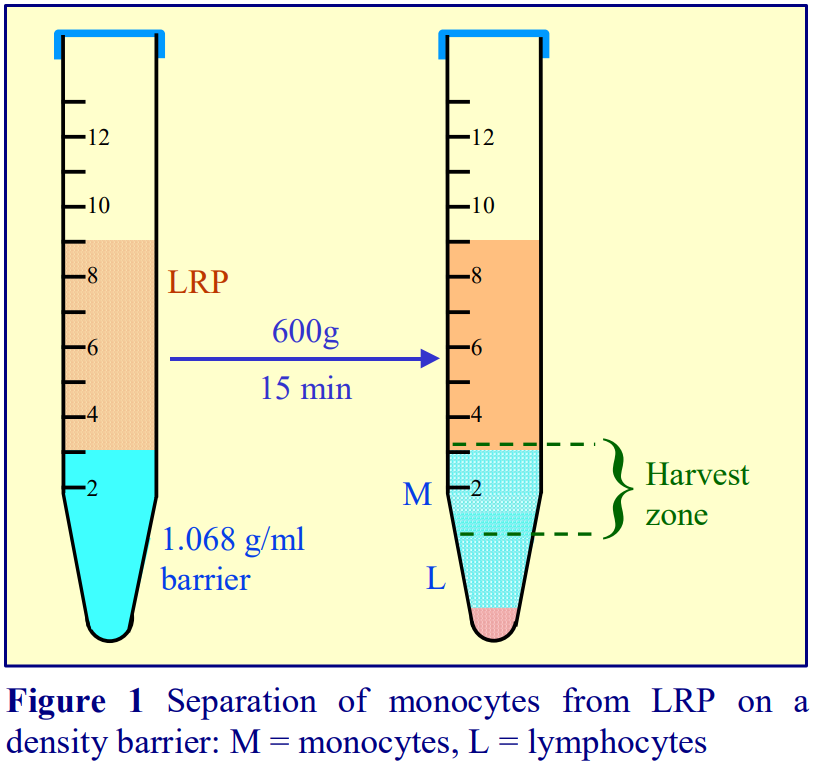 3c. Monocyte separation
3c. Monocyte separation
In a 15 ml centrifuge tube carefully layer 6 ml of LRP over 3 ml of the density barrier (avoid mixing at the interface). Alternatively the LRP may be underlaid with the density barrier using a syringe and metal cannula (see Section 4, Note 2) and centrifuge at 600 g for 15 min at approx. 20°C in a swinging-bucket rotor. Allow the rotor to decelerate without the brake (see Section 4, Note 3).
After centrifugation the monocytes are contained in the top half of the turbid layer beneath the interface (Figure 1). Aspirate the plasma supernatant to about 3 mm above the barrier interface (Figure 1). Then very slowly aspirate the remaining plasma and the upper half of the turbid layer (“Harvest zone in Figure 1). This is best achieved using a syringe attached to a metal cannula (i.d. 0.8 mm) as the aspiration must be carried out very slowly to avoid drawing up the lymphocytes from the bottom half of the turbid layer (see Section 4, Note 4). The pellet will contain lymphocytes, polymorphonuclear leukocytes and residual erythrocytes.
Dilute the cell harvest with 2 vol. of solution C to reduce the density of the solution; pellet the cells by centrifugation at 400 g for 10 min and resuspend the monocyte pellet in Solution B or as required.
4. Notes
1. The Tricine in the saline solutions may be replaced by any suitable organic buffer (e.g. HEPES).
2. For other LRP volumes keep to a ratio of LRP to density barrier of 2:1.
3. To retain the resolution of the monocytes and lymphocytes it is essential that the density barrier layer is disturbed as little as possible after the centrifugation.
4. Flat-tipped metal cannulas can be purchased from many surgical instrument companies.
5. References
1. Bøyum, A., Berg, T. and Blomhoff, R. (1983) Fractionation of mammalian cells In: Iodinated density gradient media – a practical approach (ed D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 147-171
2. Bøyum, A., Lovhaug, D., Tresland, L. and Nordlie, E.M. (1983) Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality Scand. J. Immunol., 34, 697-712
OptiPrep™Application Sheet C46; 4th edition, February 2020
OptiPrep™ Application Sheet C47
Purification of enterochromaffin-like (ECL) cells from gastric mucosa
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
- Purification of parietal and chief cells is described in Application Sheet C48
1. Background
ECL cells have been purified mainly from rat or mastomys gastric mucosa, although occasionally ileal mucosa is used. Following dispersal of the mucosal cells by enzymic digestion (usually with Pronase) the first stage in the purification procedure is the isolation of a small cell fraction by centrifugal elutriation. Subsequently the ECL cells are purified by density gradient centrifugation either on a two-layer discontinuous gradient or a simple density barrier. Both NycodenzⓇ and iodixanol gradients have been used for the gradient step but the density and ionic composition of the solutions are very similar. The current availability of NycodenzⓇ only as a powder and of iodixanol as a sterile isoosmotic 60% (w/v) solution (OptiPrep™) makes the preparation of the gradient solutions much easier with the latter. Consequently it is the use of OptiPrep™ that is described in the following protocol. Section 5 summarizes some of the NycodenzⓇ technology. The following protocol is adapted from refs 1-3.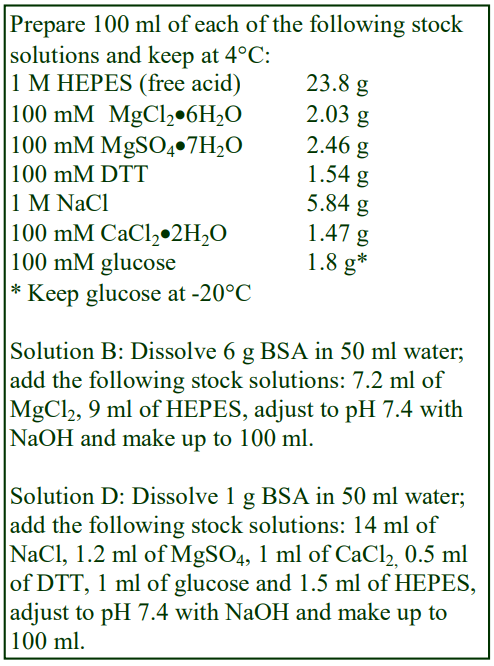
2. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. OptiPrep™ diluent: 60 mg/ml BSA, 7.2 mM MgCl2, 90 mM HEPES-NaOH pH 7.4.
C. OptiPrep™ Working Solution (WS) of 50% (w/v) iodixanol: mix 5 vol. of OptiPrep™ with 1 vol. of Solution B.
D. WS diluent: 10 mg/ml BSA, 140 mM NaCl, 1.2 mM MgSO4, 1 mM CaCl2, 0.5 mM DTT, 1 mM glucose, 15 mM HEPES-NaOH, pH 7.4
3. Protocol
1. Disperse the gastric mucosal cells by Pronase digestion and partially purify the ECL cells by centrifugal elutriation (see ref. 1 for more details).
2. Dilute Solution C with solution D to produce a solution of 10.8% (w/v) iodixanol (approx equivalent to ρ = 1.061 g/ml). For the alternative two-layer gradient make up in addition a 15% iodixanol solution.
3. Layer 1 ml of the ECL cell containing suspension (2×106 cells) over 10 ml of the 10.8% iodixanol solution in a 15 ml centrifuge tube, or use 5 ml each of the 10.8% and 15% iodixanol (see Note 1).
4. Centrifuge at 1000 rpm for 5 min (at speed), using slow acceleration (400 rpm.min-1) and no brake during deceleration. The ECL cells band just above the 10.8% iodixanol layer.
4. Notes
1. As this method isolates the least dense cell from a mixed population containing denser cells, it may be worthwhile considering an alternative flotation strategy. The cells should be suspended in a 15% (w/v) iodixanol solution and layered beneath the 10.8% iodixanol solution. This flotation strategy has been used very successfully for the isolation of other low-density cells from tissue digests. As an example see “Dendritic cells” Application Sheet C21 in index.
5. NycodenzⓇ technology
NycodenzⓇ gradients [4-8] contain essentially the same salts, buffer, BSA and DTT composition as the iodixanol solutions. To make up an approximately isoosmotic stock solution of density approx. 50 ml of water in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 27.6 g of NycodenzⓇ in small amounts until dissolved. Allow the solution to cool to room temperature; add 16.6 ml of Solution B and then make up to 100 ml with water. Filter sterilize if required. As with iodixanol solutions, make further dilutions with Solution D.
The approach with NycodenzⓇ has been rather different: most methods use a two-layer gradient in which the ECL band at the interface between these two solutions rather than at the interface below the sample. With solutions of approx. 13.8% and 10.4% (w/v) NycodenzⓇ [9,10] the ECL cells banded at the interface between the two NycodenzⓇ solutions. The concentration of the lighter solution is often reduced further: approx 9.2% [4-8] or 7% (w/v) NycodenzⓇ [11]. There is no obvious reason why this approach could not be extrapolated to the use of OptiPrep™. The density of the 10.4% NycodenzⓇ solution, through which the ECL cells sediment, is approx 1.058 g/ml, only slightly less dense than the 10.8% iodixanol solution, on which the cells float (see above). It may be necessary to investigate fine changes to the density of any iodixanol solution that may be used when substituting this medium for NycodenzⓇ in order to optimize the purification.
6. References
1. Lindström, E., Lerner, U. H. and Håkanson, R. (2001) Isolated rat stomach ECL cells generate prostaglandin E2 in response to interleukin-1β, tumor necrosis factor-α and bradykinin Eur. J. Pharmacol., 416, 255-263
2. Lindström, E. and Håkanson, R. (2001) Neurohormonal regulation of secretion from isolated rat stomach ECL cells: a critical reappraisal Regul. Pept., 97, 169-180
3. Lindström, E., Eliasson, L., Björkqvist, and Häkanson, R. (2001) Gastrin and the neuropeptide PACAP evoke secretion from rat stomach histamine-containing (ECL) cells by stimulating influx of Ca2+ through different Ca2+ channels J. Physiol., 535, 663-677
4. Tang, L.H., Luque, E.A., Efstathiou, J.A., Bortecen, K.H., Kidd, M., Tarasova, N.I. and Modlin, I.M. (1997) Gastrin receptor expression and function during rapid transformation of the enterochromaffin-like cells in an African rodent Regul. Pept., 72, 9-18
5. Bufler, J., Choi, G.C., Franke, C., Schepp, W. and Prinz, C. (1998) Voltage-gated Ca2+ currents in rat gastric enterochromaffin-like cells Am. J. Physiol. Cell Physiol., 274, C424-C429
6. Kidd, M., Tang, L.H., Schmid, S.W., Miu, K. and Modlin, I.M. (1998) A polyamine pathway-mediated mitogenic mechanism in enterochromaffin-like cells of Mastomys Am. J. Physiol. Gastrointest. Liver Physiol., 275, G370-G376
7. Mahr, S., Neumayer, N., Kolb, H.J., Schepp, W., Classen, M. and Prinz, C. (1998) Growth factor effects on apoptosis of rat gastric enterochromaffin-like cells Endocrinology, 139, 4380-4390
8. Kinoshita, Y., Nakata, H., Kishi, K., Kawanami, C., Sawada, M. and Chiba, T. (1998) Comparison of the signal transduction pathways activated by gastrin in enterochromaffin-like and parietal cells Gastroenterology, 115, 93-100
9. Modlin, I.M., Tang, L.H., Lawton, G.P., Darr, U.M., Zhu, Z-H. and Soroka, C.J. (1994) Enterochromaffin-like cell pathobiology of Mastomys Ann. N. Y. Acad, Sci., 733, 365-379
10. Lawton, G.P., Tang, L., Kidd, M., Chinery, R., Miu, K. and Modlin, I.M. (1996) Regulation of mastomys ECL cell function by transforming growth factor alpha J. Surg. Res., 60, 293-302
11. Lambretcht, N.W.G., Yakubov, I., Zer, C. and Sachs, G. (2006) Transcriptomes of purified gastric ECL and parietal cells: identification of a novel pathway regulating acid secretion Physiol. Genomics, 25, 153-165
OptiPrep™Application Sheet C47; 6th edition, January 2020
OptiPrep™ Application Sheet C48
Fractionation of parietal and chief cells from the gastric mucosa
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
- Purification of gastric ECL cells is described in Application Sheet C47
1. Background
The acid-secreting parietal cell from rabbit gastric mucosa provides a very useful model for studying both the regulation of ion-transport and intracellular signaling pathways. Since 1986 the most widely used strategy is to purify parietal cells from Pronase/ collagenase-disaggregated gastric mucosa on either continuous or discontinuous NycodenzⓇ gradients. The purity of the parietal cells is variously reported as 80-95%; sometimes additional purity is achieved by centrifugal elutriation of the NycodenzⓇ-purified fraction; sometimes the elutriation is executed before the NycodenzⓇ gradient.
 1a. Continuous NycodenzⓇ gradients
1a. Continuous NycodenzⓇ gradients
One of the first descriptions of the method was by Chew and Brown [1]; NycoPrep™ 1.15 (an isoosmotic solution of 27.6% NycodenzⓇ, ρ = 1.15 g/ml) was used to prepare the gradient solutions. The NycoPrep™ 1.15 was supplemented with bovine serum albumin (BSA), DTT, KCl, MgSO4 and buffer, which reduced the density of the solution to 1.139 g/ml. Further dilutions of this NycodenzⓇ Working Solution were made with an isoosmotic diluent containing NaCl, BSA, DTT, MgSO4 and buffer, to produce solutions of 1.095, 1.073 and 1.049 g/ml. Gradients were produced from equal volumes (2 ml) of each of the gradient solutions, which were allowed to diffuse to form a continuous gradient. The gastric mucosal cell suspension (2 ml) was layered on top and centrifuged at 1000 g for 8 min [2]. The distribution of material on a continuous gradient is shown in Figure 1. The gradient also resolves the chief cells, which band towards the bottom of the gradient. If chief cells are not required Chew [2] observed that the densest layer may be omitted, permitting the cells to be applied in double the
volume; this reduction in cell concentration reduced clumping. Note that NycoPrep 1.15 is no longer commercially available.
Benn et al [3] used continuous gradients covering exactly the same density range but added NycodenzⓇ to the cell suspension to raise its density to just below that of the top of the gradient and used 600 g rather than 1000 g. Cell recoveries of >95% were obtained. The authors also noted that use of Percoll™ gave much inferior recoveries and purity of parietal cells and also stimulated acid and cAMP secretion by gastric cells. If elutriation is carried out prior to a continuous gradient, the latter is often 1.04-1.08 g/ml [4-6]
1b. Discontinuous NycodenzⓇ gradients
Chew [2] used the same solutions of 1.139, 1.095, 1.073 and 1.049 g/ml as a discontinuous gradient and achieved similar results to the continuous gradient. In a discontinuous gradient the parietal cells band at the 1.049/1.073 g/ml interface. Berglindh [7] also used a four-step gradient of 1.10, 1.075, 1.05 and 1.0375 g/ml, the latter containing the cell suspension. There are several options in which just two layers are used as the gradient: 8 ml of 1.050 and 5 ml of 1.075 g/ml, overlaid with 2 ml of the cell suspension is a common format [8-10]. Like Berglindh [7], Malinowska [11] suspended the cells in the low-density solution (1.0375 g/ml), layered over a 1.078 g/ml NycodenzⓇ solution and a 40% (w/w) sucrose solution. In all these two-layer NycodenzⓇ gradients the parietal cells band at the interface between the NycodenzⓇ solutions.
1c. Centrifugation conditions
There is a quite diverse range of reported centrifugation conditions: 1000 g for 8 min [1,2], 800 g for 10 min [7], 600 g for 8 min [3] and 200 g for 10 min [8,9]. The higher g-forces, associated with gradients with a maximum density 𝘨1.10 g/ml, may be required for the banding of chief cells; it should be pointed out however that studies with other secreting cells suggest that lower g-forces promote better retention of viability and function.
1d. OptiPrep™ gradients
Chew et al [12-14] have replaced NycodenzⓇ with iodixanol as the density gradient medium but otherwise kept the continuous gradient density range and other aspects of the centrifugation the same. The authors also reported that iodixanol gradients gave improved purities of parietal cells.
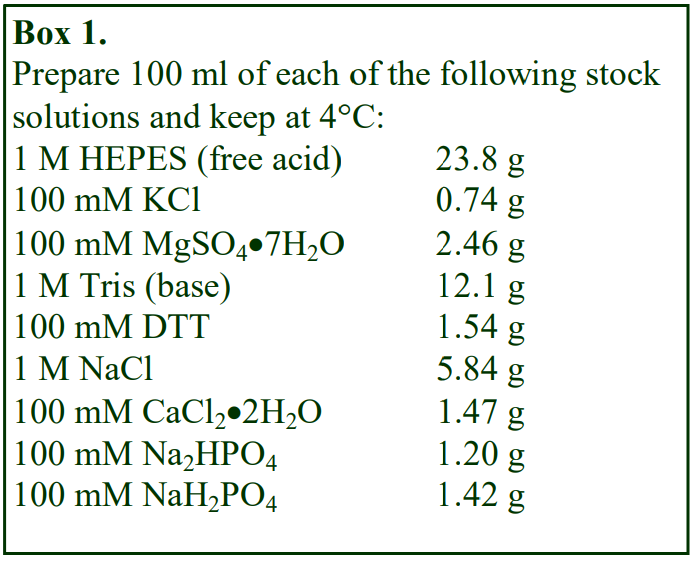 2. Solution selection and gradient preparation
2. Solution selection and gradient preparation
2a. Cell suspension solution
The cell suspension medium is usually a regular balanced salt solution, e.g. 132.4 mM NaCl, 5.4 mM KCl, 5 mM Na2HPO4, 1 mM NaH2PO4, 1.2 mM MgSO4 and 1 mM CaCl2, commonly supplemented with various additions that are added immediately before use: 2 mg/ml BSA, 10 mM glucose, 0.5 or 1.0 mM DTT, 1 mM pyruvate (11 g per 100 ml), 10 mM HEPES, 0.01 mg/ml phenol red and buffered to pH 7.4 with NaOH. Concentrated stocks of these reagents, except the pyruvate and BSA can be kept for several weeks in the refrigerator (see Box 1).
2b. NycodenzⓇ solutions and gradients
Many of the reported density solutions were prepared from NycoprepⓇ 1.15 (an isoosmotic solution of 27.6% NycodenzⓇ containing 3mM KCl, 0.3 mM CaNa2-EDTA, 5 mM Tris-HCl, pH 7.4, ρ = 1.15 g/ml). This is no longer commercially available so all solutions must be made from NycodenzⓇ powder. The CaNa2-EDTA was present only to increase solution stability during autoclaving and may be omitted and the buffer and inorganic salt content may changed to suit the operator’s requirements. Berglindh [7] prepared a solution containing 5.4 mM KCl, 1.2 mM MgSO4, 15 mM Tris-HEPES, pH 7.4 and 10 mg/ml BSA.
To make 100 ml of this ρ = 1.15 g/ml stock solution place approx. 50 ml of water in a 150 ml beaker on a heated magnetic stirrer set at approx. 50C and add 27.6 g of NycodenzⓇ in small amounts until dissolved. Allow the solution to cool to room temperature; add 0.5 g of BSA followed by the appropriate solutions from Box 1: 5.4 ml of KCl, 1.2 ml of MgSO4 and 1.5 ml of HEPES, adjust to pH 7.4 with the Tris solution and finally make up to 100 ml with water. Filter sterilize if required.
Further dilutions of the ρ = 1.15 g/ml NycodenzⓇ solution are then made by dilution with a simple isoosmotic diluent such as 132 mM NaCl, 5.4 mM MgSO4, 0.5 mM DTT, 10 mg/ml BSA, 15 mM HEPES-Tris, pH 7.4 [2], or 128 mM NaCl, 5 mM KCl, 4 mg/ml BSA, 0.3 mM Ca/Na-EDTA, 5 mM Tris-HCl, pH 7.4 [8] or sometimes the cell suspension medium is used [7,11]. Select a gradient from Sections 1a and 1b and prepare the appropriate solutions from the 1.15 g/ml NycodenzⓇ stock solution and an isoosmotic diluent using the data in Table 1.
 Table 1: Density of solutions produced by mixing Nycodenz solution (ρ = 1.15 g/ml) with an isoosmotic diluent
Table 1: Density of solutions produced by mixing Nycodenz solution (ρ = 1.15 g/ml) with an isoosmotic diluent
Discontinuous gradients: The four-layer format of 1.139, 1.095, 1.073 and 1.049 g/ml [1,2] has been executed with 2 ml of each of the solutions in a 15 ml tube, while the 1.10, 1.075, 1.05 and 1.0375 g/ml gradient described in ref 7 comprised 10ml, 20 ml, 10 ml and 7 ml respectively in a 50 ml tube. In the two-layer format the volume of the low-density solution is usually, but not always, larger than the high-density solution e.g. 8ml and 5 ml in a 50 ml tube [9]. For information on the preparation of discontinuous gradients see Application Sheet C02.
Continuous gradients: In the case of parietal cells these are routinely produced by allowing a discontinuous to diffuse; this is normally accomplished by letting the tube stand vertically in a refrigerator overnight or by capping the tube; smoothly rotating it to a horizontal position and leaving at room temperature for approx. 3 h. The continuous 1.049-1.139 g/ml gradients [1,2] however were generated from layers of 1.139, 1.095, 1.073 and 1.049 g/ml with the tube in a horizontal position overnight at 6-8^°C. Continuous gradients may also be produced rather more rapidly using a twochamber gradient maker or a Gradient Master, both of these methods require the use only of the least dense and the most dense solution. For information on the preparation of continuous gradients see Application Sheet C02.
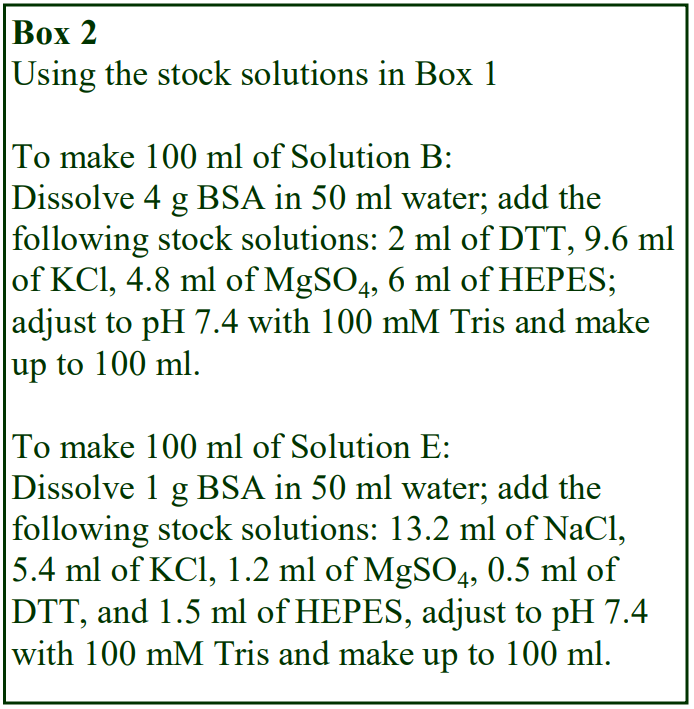 2c. Iodixanol solutions (Box 2: based on ref 12)
2c. Iodixanol solutions (Box 2: based on ref 12)
A. OptiPrep™ (shake gently before use)
B. Additive solution: 40 mg/ml bovine serum albumin (BSA), 2.0 mM DTT, 9.6 mM KCl, 4.8 mM MgSO4, 60 mM TrisHEPES, pH 7.4.
C. OptiPrep™ diluent: Mix 10 ml of 10x Hank’s Buffered Salt Solution (containing Ca and Mg) with 50 ml of Solution B; adjust to pH 7.4 with 100 mM Tris if necessary and make up to 100 ml with water.
D. OptiPrep™ Working Solution (WS) of 24.4% (w/v) iodixanol (ρ = 1.134 g/ml): mix 9 vol. of OptiPrep™ with 11 vol. of Solution C and 2 vol. of water (see Note 1).
E. WS diluent: 10 mg/ml BSA, 132 mM NaCl, 5.4 mM KCl, 1.2 mM MgSO4, 0.5 mM DTT, 15 mM Tris-HEPES, pH 7.4
Iodixanol solutions have been used for the four-step continuous gradient (or discontinuous) gradient format developed by Chew et al [12-14]. Table 2 describes the preparation of the 1.048, 1.070 and 1.091 g/ml solutions from the 24.4% (w/v) iodixanol working solution (1.134 g/ml) for the preparation of the continuous gradient described in ref 12. It also gives the recipes for other density solutions that might be used as a NycodenzⓇ substitute. See above for the preparation of both discontinuous and continuous gradients. A discontinuous gradient was also used by Nishi et al [15]; the density of the solutions (1,049, 1.073, 1.095 and 1.139 g/ml) was similar but not identical to that used by Chew et al [12-14]. Separation of the parietal and chief cells using the gradient described has also been 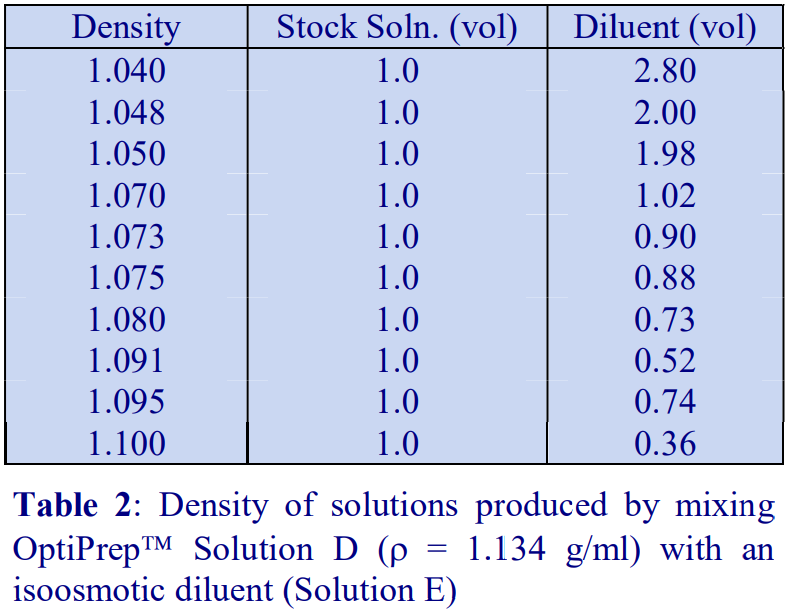 used by Noreldin et al [16]:
used by Noreldin et al [16]:
- Zhang et al [17] have also used the discontinuous iodixanol gradient strategy
- Use of a continuous iodixanol gradient was also reported by Singh et al [18] and by Sashidara et al [19].
3. Processing the cell suspension
Pellet the cells from the enzyme digest at 200 g for 8 min and aspirate the supernatant. Resuspend the cell pellet gently in the cell suspension medium (see Section 2a) or in the lowest density gradient solution (see Section 1b) at 0.1-0.15 ml of packed cells per ml. In 12-15 ml tube layer 2 ml of the cells on top of the gradient. In 50 ml tubes use a proportionately larger volume. Reducing the cell concentration will minimize any cell clumping that may occur.
- For details on preparation of gastric mucosa and its enzymic digestion see refs 1, 2, 12, 20 and 21.
4. Centrifugation
Centrifuge the four-layers gradients at 600-1000 g for 8 min or the two-layer gradients at 200 g for 10 min. See Section 1c for comments about choice of conditions. Use a slow acceleration program if available and allow the rotor to decelerate without the brake. Sudden changes in rpm create vortices in the gradients and the consequent mixing can seriously disturb the gradient. Temperatures vary from approx 10°C to room temperature. Harvest the parietal cells that band at the top of a continuous gradient (see Figure 1) or at the interface below the least dense layer of a discontinuous gradient. Dilute the harvest with at least 2 volumes of Solution E (Section 2c) or the cell suspension medium and harvest by centrifugation at 200 g for 10 min and resuspend the pellet as required.
5. Technical Note
Parietal cells are the least dense of a mixed population of mainly denser cells. In this respect they are not unlike monocytes and dendritic cells. Flotation methods for isolating the least dense cell type have been more successful than sedimentation methods in purifying such cells. Flotation of parietal cells may also provide a purer isolate than sedimentation and avoid subsequent elutriation.
6. References
1. Chew, C. S. and Brown, M.R. (1986) Release of intracellular Ca2+ and elevation of inositol triphosphate by secretagogues in parietal and chief cells isolated from rabbit gastric mucosa Biochim. Biophys. Acta, 888, 116-125
2. Chew, C. S. (1990) cAMP technologies, functional correlates in gastric parietal cells Meth. Enzymol., 191, 640-661
3. Benn, S.E., Canfield, S.P. and Curwain, B.P. (1987) Preparation of purified parietal cells from rat using Nycodenz density gradients J. Physiol., 391, 9P
4. Roche, S., Gusdinar, T., Bali, J-P-. and Magous, R. (1991) Biphasic kinetics of inositol 1,4,5-trisphosphate accumulation in gastrin-stimulated parietal cells FEBS Lett., 282, 147-151
5. Gros, L., Hollande, F., Thorens, B., Kervran, A. and Bataille, D. (1995) Comparative effects of GLP-1-(7-36) amide, oxytomodulin and glucagons on rabbit gastric parietal cell function Eur. J. Pharmacol., 288, 319-327
6. Nagano, M., Chastre, E., Choquet, A., Bara, J., Gespach, C. and Kelly, P.A. et al (1995) Expression of prolactin and growth hormone receptor genes and their isoforms in the gastrointestinal tract Am. J. Physiol. Gastrointest. Liver Physiol., 268, G431-442
7. Berglindh, T. (1990) Gastric glands and cells: Preparation and in vitro methods Meth. Enzymol., 192, 93-107
8. Lewis, J.L., Goldenring, J.R., Asher, V.A. and Modlin, I.M. (1989) Pancreastatin: a novel peptide inhibitor of parietal cell signal transduction Biochem. Biophys. Res. Comm., 163, 667-673
9. Lewis, J.J., Goldenring, J.R., Asher, V.A. and Modlin, U.M. (1990) Effects of epidermal growth factor on signal transduction in rabbit parietal cells Am. J. Physiol. Gastrointest. Liver Physiol., 258, G476-G483
10. Tsunoda, Y., Modlin, I.M. and Goldenring, J.R. (1993) Tyrosine kinase activities in the modulation of stimulated parietal cell acid secretion Am. J. Physiol. Gastrointest. Liver Physiol., 264, G351-G356
11. Malinowska, D.H. (1990) Cl- channel blockers inhibit acid secretion in rabbit parietal cells Am. J. Physiol. Gastrointest. Liver Physiol., 259, G536-G543
12. Chew, C. S., Parente, J. A., Chen, X., Chaponnier, C. and Cameron R. S. (2000) The LIM and SH3 domain-containing protein, lasp-1, may link the cAMP signaling pathway with dynamic membrane restructuring activities in ion transporting epithelia J. Cell Sci., 113, 2035-2045
13. Chew, C.S., Okamoto, C.T., Chen, X. and Qin, H.Y. (2005) IQGAPs are differentially expressed and regulated in polarized gastric epithelial cells Am. J. Physiol. Gastrointest. Liver Physiol., 288, G376-G387
14. Chew, C.S., Okamoto, C.T., Chen, X. and Thomas, R. (2005) Debrin E2 is differentially expressed and phosphorylated in parietal cells in the gastric mucosa Am. J. Physiol. Gastrointest. Liver Physiol., 289, G320-G331
15. Nishi, M., Aoyama, F., Kisa, F., Zhu, H., Sun, M., Lin, P., Ohta, H., Van, B., Yamamoto, S., Kakizawa, S., Sakai, H., Ma, J., Sawaguchi, A. and Takeshima, H. (2012) TRIM50 protein regulates vesicular trafficking for acid secretion in gastric parietal cells J. Biol. Chem., 287, 33523–33532
16. Noreldin, A.E., Sogabe, M., Yamano, Y., Uehara, M., Mahdy, M.A.A., Elnasharty, M.A., Sayed-Ahmed, A., Warita, K. and Hosaka, Y.Z. (2016) Spatial distribution of osteoblast activating peptide in the rat stomach Acta Histochem., 118, 109–117
17. Zhang, G., Ducatelle, R., Mihi, B., Smet, A., Flahou, B. and Haesebrouck, F. (2016) Helicobacter suis affects the health and function of porcine gastric parietal cells Vet. Res., 47: 101
18. Singh, N., Singh, P., Shrivastva, S., Mishra, S.K., Lakshmi, V., Sharma, R. and Palit, G. (2012) Gastroprotective effect of anti-cancer compound rohitukine: possible role of gastrin antagonism and H+ K+ – ATPase inhibition Naunyn-Schmiedeberg’s Arch Pharmacol (2012) 385:277–286
19. Sashidhara, K.V., Avula, S.R., Mishra, V., Palnati, G.R., Singh, L.R., Singh, N., Chhonker, Y.S. et al (2015) Identification of quinoline-chalcone hybrids as potential antiulcer agents Eur. J. Medicin. Chem., 89, 638-653
20. Lindström, E., Lerner, U. H. and Håkanson, R. (2001) Isolated rat stomach ECL cells generate prostaglandin E2 in response to interleukin-1β, tumor necrosis factor-α and bradykinin Eur. J. Pharmacol., 416, 255-263
21. Le Goascogne, C., Sananes, N., Eychenne, B., Gouezou, M., Baulieu. E-M. and Robel, P. (1995) Androgen biosynthesis in the stomach: expression of cytochrome P450 17α-hydroxylase/17,20-lyase messenger ribonucleic acid and protein, and metabolism of pregnenolone and progesterone by parietal cells of the rat gastric mucosa Endocrinology, 136, 1744- 1752
7. Acknowledgements
We would like to thank Professor Catherine Chew, Cellular Biology and Anatomy, Medical College of Georgia, Augusta, GA 30912 for her assistance in the preparation of this Application Sheet.
OptiPrep™Application Sheet C48; 9th edition, February 2020
OptiPrep™ Application Sheet C49
Purification of bacteria and bacterial minicells from cultured cells
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
- Note that purification of bacteria from soil, clinical specimens, host species/tissues and food is described in Application Sheet C39.
1. Introduction
Although NycodenzⓇ has been widely used in the purification of a broad range of bacteria from soil samples (and other biological matrices), see Application Sheet C39, there are in addition some other specific examples of the purification of bacteria, which have been primarily grown in either mammalian or non-mammalian cells (or organisms), using both NycodenzⓇ and OptiPrep™. Some of these methods are summarized in this Application Sheet. The majority of the published methodology is concerned with obligate intracellular bacteria and two of these, Chlamydophila abortus and Piscirickettsia salmonis are considered in Sections 2 and 3. Sections 4-10 summarize some of the other important applications of OptiPrep™ for a broad range of other bacteria. The interesting development of the possible clinical uses of bacterial minicells is described in Section 10.
2. Elementary bodies of Chlamydophila abortus
2a. Introduction
To purify the elementary bodies of Chlamydophila abortus Everson et al [1] used a density barrier of 18% (w/v) NycodenzⓇ containing 0.13 M NaCl, 3 mM KCl, 0.3 mM CaNa2EDTA, 5 mM Tris-HCl, pH 7.2. It was prepared from NycoprepⓇ 1.15 (an isoosmotic solution of 27.6% NycodenzⓇ containing 3mM KCl, 0.3 mM CaNa2-EDTA, 5 mM Tris-HCl, pH 7.4, ρ = 1.15 g/ml). This is no longer commercially available so all solutions must be made from NycodenzⓇ powder as described below. The CaNa2-EDTA in the NycoprepⓇ 1.15 was present only to increase solution stability during autoclaving and may not be necessary for the separation. It may either be omitted or replaced by Na2- EDTA. The Tris, in the example below, has been replaced by the more cell-friendly buffer Tricine. It is highly probable that NycodenzⓇ can be replaced by iodixanol, but this has not been verified experimentally.
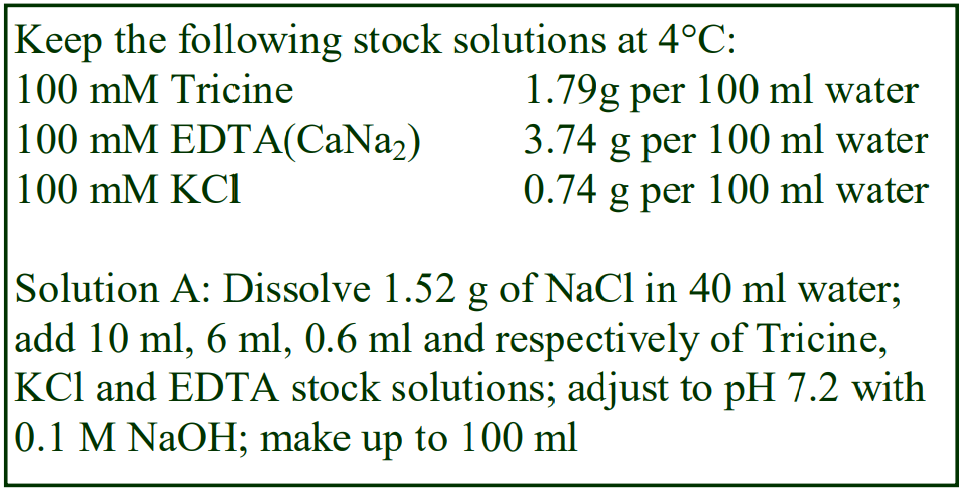 2b. Density barrier solution preparation
2b. Density barrier solution preparation
2b-1 NycodenzⓇ
To make up the density barrier solution place 50 ml of Solution A, which contains 0.26 M NaCl, 6 mM KCl, 0.6 mM CaNa2EDTA, 10 mM Tricine-HCl, pH 7.2. (see box), in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 18 g of NycodenzⓇ in small amounts until dissolved. Allow the solution to cool to room temperature and then make up to 100 ml with water. Filter sterilize if required.
2b-2 Iodixanol
If the iodixanol option is chosen dilute 1.8 vol. of OptiPrep™ with 2.1 vol. of Solution A and 2.1 vol. of water (see Section 2e, Note 1).
2. Centrifuge requirements
Ultracentrifuge with a swinging-bucket rotor, e.g. Beckman SW28; a similar volume rotor in a high-speed centrifuge, e.g. Sorvall HB4, can be substituted but the centrifugation time may have to be extended to make up for the slightly lower g-force capability (see Section 2e, Note 2).
2d. Protocol (adapted from ref 1)
1. Detach the cultured cell monolayer by the normal exposure to trypsin-EDTA in DMEM.
2. Harvest the cells by centrifugation and wash twice in medium containing 10% FCS.
3. Suspend the cells in a 10x dilution of phosphate-buffered saline and disrupted the swollen cells by homogenization in a Dounce homogenizer.
4. Remove cell debris at 250 g for 5 min, then raise the osmolality of the homogenate by addition of an equal volume of phosphate buffer containing 0.4 M sucrose.
5. Load the suspension on to a solution of 18% (w/v) NycodenzⓇ containing 0.13 M NaCl, 3 mM KCl, 0.3 mM CaNa2EDTA, 5 mM Tris-HCl, pH 7.2 and centrifuged at 35,000 g for 40 min (or 27,000 g for 1 h) in a swinging-bucket rotor for an ultracentrifuge or high-speed centrifuge respectively.
6. Harvest the elementary bodies from the interface and wash in phosphate-buffered saline.
2e. Notes
1. It is likely that one of the main determinants of the density of bacteria is the osmolality of the medium; thus the NaCl strength in the OptiPrep™ diluent may require modulation to optimize separations.
2. Most modern high-speed rotors with tube capacities of < 50 ml are capable of approx 27,000 g
3. Piscirickettsia salmonis
3a. Introduction
This is an obligate intracellular bacterium that requires fish host cells to replicate. Both diatrizoate (methylglucamine salt) and Percoll™ have been used for the purification [2] but yields are poor and contamination by host cell components remains a problem [2]. Iodixanol gradients give better yields and improved resolution from 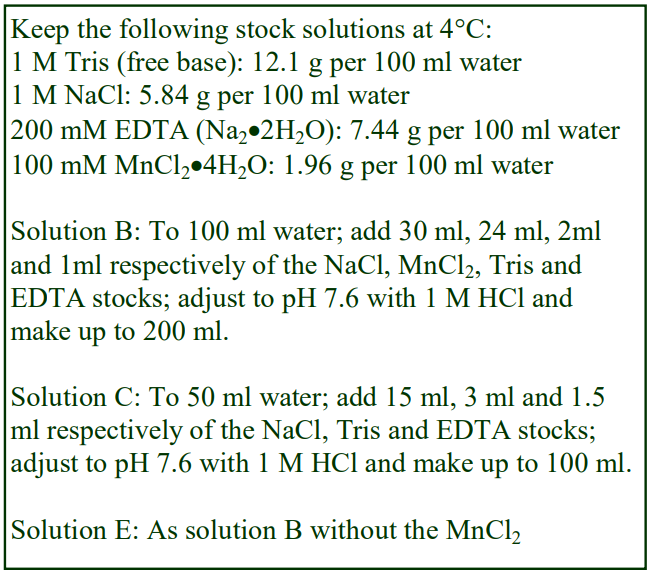 host cell components. The methodology, although developed for Piscirickettsia may be more widely applicable for other obligate intracellular organisms. After growth of the organism in cultured cells, the bacteria are harvested from the cell culture fluid. If sufficient numbers of bacteria are not expressed into the culture fluid, it may be necessary to disrupt the cells.
host cell components. The methodology, although developed for Piscirickettsia may be more widely applicable for other obligate intracellular organisms. After growth of the organism in cultured cells, the bacteria are harvested from the cell culture fluid. If sufficient numbers of bacteria are not expressed into the culture fluid, it may be necessary to disrupt the cells.
3b. Solutions required
A. OptiPrep™ (shake gently before use)
B. Salt buffer: 150 mM NaCl, 12 mM MnCl2, 1 mM EDTA, 10 mM Tris-HCl pH 7.6
C. OptiPrep™ diluent: 150 mM NaCl, 3 mM EDTA, 30 mM Tris-HCl pH 7.6
D. Working Solution (WS) of 40% (w/v) iodixanol: mix 4 vol. of OptiPrep™ with 2 vol. of Solution C.
E. WS diluent: 150 mM NaCl, 1 mM EDTA, 10 mM Tris-HCl pH 7.6
3c. Ultracentrifuge requirements
Swinging-bucket rotor with 17 ml tubes (e.g. Beckman SW28 or 28.1 or Sorvall AH629 (see Section 3e, Note 1)
3d. Protocol (adapted from refs 2 and 3)
Carry out all operations (except step 5) at 4°C
1. Make up three gradient solutions of 22%, 24% and 26% (w/v) iodixanol by diluting Solution D with Solution E and in 17 ml tubes make up discontinuous gradients from 4.5 ml of each solution (see Section 3e, Note 2).
2. Allow the gradients to become linear by diffusion for 1 h at room temperature then bring them to 4°C (see Section 3e, Note 2).
3. Collect the bacteria-containing fluid and centrifuge at 200 g for 10 min to remove host cell debris.
4. Concentrate the bacteria by centrifugation of the 200 g supernatant at 10,000 g for 45 min.
5. Suspend the pellet in 6 ml of Solution B and incubate with 20 U of DNase I at 30°C for 1 h.
6. Stop the reaction by adding 0.6 ml of the 200 mM EDTA stock solution.
7. Layer 3 ml on top of each gradient and centrifuge at 25,000 g for 3 h. Then harvest the sharp band of bacteria from the top third of the gradient (see Section 3e, Note 3).
3e. Notes
1. If an ultracentrifuge is not available, the g-force required is sufficiently low that a swinging bucket rotor for a high-speed centrifuge may be acceptable (e.g. Beckman JS24.15).
2. Alternatively a continuous gradient might be made using a two-chamber gradient maker or a Gradient Master™. For more information about preparing both discontinuous and continuous gradients see Application Sheet C02.
3. Piscirickettsia salmonis bands at a density of approx. 1.13 g/ml; other bacteria may have different banding densities and it may be necessary to modify the gradient density range in some cases. Contaminant organelles from the host cell band at higher densities.
4. Isla et al [4] used the same gradient strategy.
4. Listeria monocytogenes [5]
This bacteria are grown intracellularly in monocyte culture and released from the cells by distilled water lysis. The bacteria are then purified away from cell debris and cytosol by centrifugation through a discontinuous gradient of 60, 30 and 20% (w/v) iodixanol at 45,000 g for 1 h [5].
5. Anaplasma phagocytophilum [6]
Anaplasma phagocytophilum is grown in the human promyelocytic cell line (HL-60). To purify the bacteria released by homogenization of the cells the crude fraction is layered atop a discontinuous iodixanol gradient of 30% (6 ml), 25%, (5 ml), 20% and then 3.5 ml each of 17.5%, 15%, 12.5% (w/v) iodixanol (in an isoosmotic buffer). The gradient is centrifuged at 87,000 g for 74 min at 4°C. The bacteria band at the lowermost interface.
6. Rickettsia
Rickettsia typhi was grown in L929 mouse fibroblasts; cell lysates were first centrifuged at 1000 g for 5 min to remove cell nuclei and partially broken cells and then at 14,000 g for 10 min to pellet the bacteria [7]. After suspension of the pellet in a phosphate-buffered 218 mM sucrose (containing 4.9 mM potassium glutamate) the bacteria are pelleted through a 20% (w/v) iodixanol cushion (prepared by dilution of OptiPrep with the same buffered sucrose) at 14,000 g for 10 min. Pelc et al [8] used a similar strategy. Smalley et al [9] used a discontinuous gradient of 20%, 26% and 32% OptiPrep™ for purification of Rickettsia australis but did not give any more detailed information. A similar gradient was used by Bechelli et al [10].
7. Chlamydia trachomatis (different morphological forms); see refs 11-13
The persistent form, reticulate body and elementary body from Chalmydia trachomatis have been separated in a gradient of 54%, 44%, 40%, 34% and 24% (v/v) OptiPrepTM, formed by dilution of OptiPrep™ with the widely used phosphate-buffered 218 mM sucrose (containing 4.9 mM potassium glutamate). The top loaded sample was centrifuged at 100,000 g for 1 h at 4°C: the persistent form banded above the 24-34% interface, the reticulate body above the 34-40% and the elementary body in the 44% layer. Allbritton et al [13] used the same methodology as that described in ref 11.
8. Cyanobacteria
Fujishiro et al [14] have published a paper on the establishment of a pure culture of the cyanobacterium Aphanothece sacrum that required the isolation of the organism in a very pure form. A complex medium of inorganic salts was used to suspend the organism and aggregates were filtered; sonication was used to remove the dense exopolysaccharide matrix.
After sonication, the crude cell suspension was centrifuged at 14,000 g for 10 min; the cell pellet was resuspended in complex medium (see ref 8 for details of the composition of the medium). The suspension was loaded on to a 40-45% (w/v) iodixanol gradient and the cyanobacterium banded sharply in the middle of the gradient after centrifugation at 112,700 g for 2 h [14]. Thus the banding density of the cyanobacterium was approx. 1.22 g/ml.
9. Density determination of bacteria and bacterial protoplasts
NycodenzⓇ gradients (1.10-1.46 g/ml), or sequential centrifugation in NycodenzⓇ solutions of increasing density, are used to determine the density of bacterial spore protoplasts (e.g. refs 15 and 16). Lewis et al [17] stressed the importance of understanding how bacteria such as Escherichia coli O157:H7 and Listeria innocua survive and proliferate. The authors investigated the buoyant masses of live and dead cells using a suspended microchannel resonator (SMR). Preparation of solutions from OptiPrep™ that were either more or less dense than the cells and which minimized any osmolarity changes were integral to the measurements.
- A review of some of the methodology for purifying a variety of pathogenic bacteria is given in refs 18 and 19.
10. Bacterial minicells
Minicells are the products of an aberrant division of the bacterial cell that contains RNA and protein, but no chromosomal DNA. They are “capable of delivering heterologous antigens to the class I antigen presentation pathway stimulating immune responses both in vitro and in vivo”. Carleton et al [20]. Briefly, bacteria were removed from the culture at 2000 g for 10 min and the minicells sedimented from the supernatant at 10,000 g for 30 min; the pellet was resuspended in medium and applied to a 5–20% (w/v) iodixanol gradient, centrifuged at 20,000 g for 20 min. The minicells that were recovered from the gradient contained <0.001% bacteria. Similar conditions were used by MacDiarmid et al [21].
11. References
1. Everson, J. S., Garner, S. A., Fane, B., Liu, B-L., Lambden, P. R. and Clarke, I. N. (2002) Biological properties and cell tropism of Chp2, a bacteriophage of the obligate intracellular bacterium Chlamydophila abortus J. Bacteriol., 184, 2748-2754
2. Henriquez, V., Rojas, M.V., and Marshall, S.H. (2003) An alternative efficient procedure for purification of the obligate intracellular fish bacterial pathogen Piscirickettsia salmonis Appl. Environ. Microbiol., 69, 6268-6271
3. Marshall, S.H., Conejeros, P., Zahr, M., Olivares, J., Gómez, F., Cataldo, P. and Henríquez, V. (2007) Immunological characterization of a bacterial protein isolated from salmonid fish naturally infected with Piscirickettsia salmonis Vaccine, 25, 2095-2102
4. Isla, A., Haussmann, D., Vera, T., Kausel, G. and Figueroa, J. (2014) Identification of the clpB and bipA genes and an evaluation of their expression as related to intracellular survival for the bacterial pathogen Piscirickettsia salmonis Vet. Microbiol., 173, 390–394
5. Dehusa, O., Pfitzenmaier, M., Stuebs, G., Fischer, N., Schwaeble, W., Morath, S., Hartung, T., Geyer, A. and Hermann, C. (2011) Growth temperature-dependent expression of structural variants of Listeria monocytogenes lipoteichoic acid Immunobiology, 216, 24–31
6. Troese, M.J., Kahlon, A., Ragland, S.A., Ottens, A.K., Ojogun, N., Nelson, K.T., Walker, N.J., Borjesson, D.L. and Carlyon, J.A. (2011) Proteomic analysis of Anaplasma phagocytophilum during infection of human myeloid cells identifies a protein that is pronouncedly upregulated on the infectious dense-cored cell Infect. Immun., 79, 4696-4707
7. Sears, K.T., Ceraul, S.M., Gillespie, J.J., Allen Jr., E.D., Popov, V.L., Ammerman, N.C., Rahman, M.S. and Azad, A.F. (2012) Surface proteome analysis and characterization of surface cell antigen (Sca) or autotransporter family of Rickettsia typhi PLoS Pathog., 8: e1002856
8. Pelc, R.S., McClure, J.C., Kaur, S.J., Sears, K.T., Rahman, M.S, and Ceraul, S.M. (2015) Disrupting protein expression with peptide nucleic acids reduces infection by obligate intracellular Rickettsia PloS One, 10: e0119283
9. Smalley, C., Bechelli, J., Rockx-Brouwer, D., Saito, T., Azar, S.R., Ismail, N., Walker, D.H. and Fang, R. (2016) Rickettsia australis activates inflammasome in human and murine macrophages PLoS One, 11: e0157231
10. Bechelli, J., Smalley, C., Zhao, X., Judy, B., Valdes, P., Walker, D.H. and Fang, R. (2016) MyD88 mediates instructive signaling in dendritic cells and protective inflammatory response during rickettsial infection Infect. Immun. 84, 883-893
11. Frohlich, K., Hua, Z., Wang, J. and Shen, L. (2012) Isolation of Chlamydia trachomatis and membrane vesicles derived from host and bacteria J. Microbiol. Meth., 91, 222–230
12. Hua, Z., Frohlich, K.M., Zhang, Y., Feng, X., Zhang, J. and Shen, Li. (2015) Andrographolide inhibits intracellular Chlamydia trachomatis multiplication and reduces secretion of proinflammatory mediators produced by human epithelial cells FEMS Pathog. Dis., 73, 1–11
13. Albritton, H., Kozlowski, P.A., Lillis, R.A., McGowin, C.L., Siren, J.D., Taylor, S.N., Ibana, J.A., Buckner, L.R., Shen, L. and Quayle, A.J. (2017) A novel whole-bacterial enzyme linkedimmunosorbant assay to quantify Chlamydia trachomatis specific antibodies reveals distinct differences between systemic and genital compartments PLoS One, 12: e0183101
14. Fujishiro, H., Ogawa, T., Matsuoka, M., Nagahama, K., Takeshima, Y. and Hagiwara, H. (2004) Establishment of a pure culture of the hitherto uncultured unicellular cyanobacterium Aphanothece sacrum, and phylogenetic position of the organism Appl. Environ. Microbiol., 70, 3338-3345
15. Popham, D.L., Gilmore, M.E. and Setlow, P. (1999) Roles of low-molecular-weight penicillin-binding proteins in Bacillus subtilis spore peptidoglycan synthesis and spore properties J. Bacteriol., 181, 126-132
16. Young, S.B. and Setlow, P. (2004) Mechanisms of killing of Bacillus subtilis spores by Decon and OxoneTM, two general decontaminants for biological agents J. Appl. Microbiol., 96, 289-301
17. Lewis, C.L., Craig, C.C. and Senecal, A.G. (2014) Mass and density measurements of live and dead Gramnegative and Gram-positive bacterial populations Appl. Environ. Microbiol., 80, 3622–3631
18. Austin, B. and Austin, D.A. (2012) Miscellaneous pathogens In, Bacterial Fish Pathogens: Disease of Farmed and Wild Fish Springer Science+Business Media Dordrecht, pp 413-441
19. Austin, B. and Austin, D.A. (2016) Miscellaneous pathogens In Bacterial Fish Pathogens, Springer International Publishing Switzerland, pp. 603-642
20. Carleton, H.A., Lara-Tejero, M., Liu, X. and Galan, J.E. (2013) Engineering the type III secretion system in non-replicating bacterial minicells for antigen delivery Nat. Commun., 4: 1590
21. MacDiarmid, J.A., Mugridge, N.B. Weiss, J.C., Phillips, L. Burn, A.L., Paulin, R.P., Haasdyk, J.E., Dickson, K-A., Brahmbhatt, et al (2007) Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics Cancer Cell, 11, 431-445
OptiPrep™ Application Sheet C49; 6th edition, January 2020
OptiPrep™ Application Sheet C50
Fractionation of coelomocyte cell populations from sea urchins
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Background
The coelomocytes of echinoderms, such as the purple sea urchin Stronglyocentrotus purpuratus, which are free-floating cells in the coelomic fluid, have been divided into at least four types, amoeboid phagocytes, red spherule cells, colourless spherule cells and vibratile cells [1,2]. A variety of discontinuous gradients have been used to fractionate these cells, including sucrose [3] sodium metrizoate [4] and Percoll [5]. All of these suffer from serious disadvantages:
- Sucrose gradients pose the problem of high osmolality
- The silica particles of PercollⓇ may be engulfed by the potentially phagocytic cells [1]
- Both polysucrose and silica particles have a tendency to adhere to cell surfaces
- The extreme sensitivity of coelomocytes to lipopolysaccharide [1] makes PercollⓇ less than ideal, in view of the significant levels of endotoxin in this medium
OptiPrep™ offers some important advantages over these other gradient media for the fractionation of coelomocytes; it is a true solute and not a colloid, it has the lowest endotoxin levels (< 1 EU/ml) of any commercial density gradient medium and, as the solution contains only iodixanol and no other solutes, it is easy to prepare solutions which are compatible with non-mammalian cells. The following protocol is adapted from ref 1. Iodixanol gradients have also been used by Johnston et al [6] for cell fractionation of the sea anemone Aiptasia pallida.
2. Solutions required
A. OptiPrep™ (shake the bottle gently before use)
B. Ca2+/Mg2+-free sea water containing 70 mM EDTA, 50 mM imidazole, pH 7.5
3. Protocol
1. Prepare the following solutions by diluting OptiPrep™ with Solution B: 3%, 6%, 12%, 18% and 42% (w/v) iodixanol and keep at 4°C (see Notes 1-4).
2. Introduce (through the peristomeum) into the coelomic cavity of the sea urchin, a 23-gauge needle attached to a syringe charged with chilled Solution B. Use a volume equal to that of the recoverable coelomic fluid (see next step).
3. Withdraw the coelomic fluid into the syringe.
4. Prepare a discontinuous gradient from 5 ml each of the five iodixanol solutions (see Notes 5-8).
5. Layer 5 ml of the coelomocyte-containing fluid on top of the gradient and centrifuge at 1500 g for 30 min at 4°C (see Note 9).
4. Recover the banded material (see Figure 1) using a syringe attached to a metal cannula and if required dilute with 2-3 volumes of Solution B before pelleting and resuspending in Solution B (see Notes 10-13).
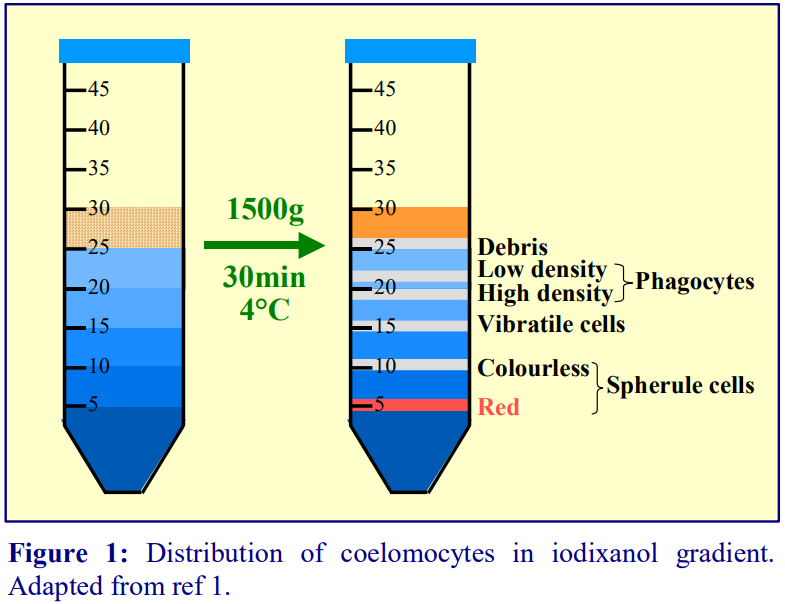 4. Notes
4. Notes
1. The methodology described has also been used by Liao et al [7] for Stronglyocentrotus purpuratus
2. Li et al [8] used a similar density gradient in which the densest 42% (w/v) iodixanol layer was replaced by a 36% (w/v) iodixanol layer, was used for the fractionation of cells from the green sea urchin Strongylocentrotus droebachiensis. The same centrifugation conditions were used as described in Section 3.
3. A gradient for fractionating Paracentrotus lividus coelomocytes comprised three layers of 6%, 12% and 18% (w/v) iodixanol in 0.5 M NaCl, 10 mM EDTA [9].
4. More recently a four-layer gradient has been described for fractionating Paracentrous lividus: OptiPrepTM was diluted with 0.5M NaCl, 10 mM EDTA to produce four solutions of 10%, 20% 30% and 70% (v/v) OptiPrep (equivalent to 6%, 12%, 18% and 42% w/v iodixanol). The toploaded gradient, centrifuged at 800 g for 30 min, resolved amoebocytes, vibratile cells, colourless sphaerulocytes and red cells [10].
5. For more information about preparing gradients see Application Sheet C02.
6. The volumes described are for a standard 50 ml centrifuge tube. The method can be scaled up or down proportionately – for example in a 15 ml tube use 2 ml each of the coelomic fluid and iodixanol solutions.
7. If the primary interest is the low and high-density phagocytes, a revised gradient of 3%, 5%, 7.5%, 10% (w/v) iodixanol might be investigated.
8. With other types of echinoderm, it may be necessary to modulate the density of one or more of the layers.
9. Arizza et al [9] used 800 g for 30 min at 7°C.
10. To avoid disturbance to the gradient layers use a programmed slow-acceleration if available, and to avoid disturbance to the banded cells during deceleration, turn off the brake.
11. Visible clumping of cells at one or more of the interfaces will lead to poor resolution; this is usually due to the use of too high a cell concentration. This problem may be exaggerated if other species of sea urchin or sea anemone are used. If this problem persists (in spite of reducing the cell concentration), it might be alleviated by adjusting the sample to 2.5% (w/v) iodixanol, so that the cells and, in particular the debris, sediment to the first interface more slowly.
12. Either aspirate observable bands of material or unload the entire gradient by careful aspiration from the meniscus; use a flat-tipped metal cannula (0.8 mm i.d.) attached to a 1-2 ml syringe. Most gradient unloaders are designed for use with flexible thin-walled tubes and not the screw-cap thick-walled tubes routinely used for cells. For more information regarding the harvesting of gradients see Application Sheet S08, accessed from “General Methods” of the Subcellular Membranes index.
13. Figure 1 shows the expected distribution of cell types in the gradient. The three interfaces of the Arizza et al [9] gradient, in order of increasing density, contained 90% amoebocytes, 84% vibratile cells, 90% uncoloured spherulocytes and 91% red cells (pellet).
14. Reference 11 provides a comprehensive methodology regarding the collection, handling and analysis of sea urchin coelomocytes.
5. References
1. Gross, P.S., Clow, L.A., Smith, L.C. (2000) SpC3, the complement homologue from the purple sea urchin, Strongylocentrotus purpuratus, is expressed in two subpopulations of the phagocytic coelomocytes Immunogenetics, 51, 1034-1044
2. Terwilliger, D.P., Clow, L.A., Gross, P.S. and Smith, L.C. (2004) Constitutive expression and alternative splicing of the exons encoding SCRs in Sp152, the sea urchin homologue of complement factor B. Implications on the evolution of the Bf/C2 gene family Immunogenetics, 56, 531-543
3. Edds, K.T. (1993) Cell Biology of Echinoid Coelomocytes I. Diversity and Characterization of Cell Types J. Invert. Pathol., 61, 173-178
4. Gerardi, P., Lassegues, M. and Canicatti, C. (1990) Cellular distribution of sea urchin antibacterial activity Biol. Cell, 70, 153-157.
5. Smith, L.C. and Davidson, E.H. (1992) The echinoid immune system and the phylogenetic occurrence of immune mechanisms in deuterostomes Immunol. Today, 13, 356-361
6. Johnston, C., Larkin, K., Woodley, C. and Morris, P.J. (2002) Marine invertebrate cell lines in the study of coral physiology and pathology Marine Biomed. Environ. Sci. Ann. Res. Open House, Medical University of South Carolina, Abstr.
7. Liao, W-Y. and Fugmann, S.D. (2017) Lectins identify distinct populations of coelomocytes in Strongylocentrotus purpuratus PLoS One, 12: e0187987
8. Li, C., Blencke, H-M., Haug, T., Jørgensen, Ø. and Stensvåg, K. (2014) Expression of antimicrobial peptides in coelomocytes and embryos of the green sea urchin (Strongylocentrotus droebachiensis) Dev. Comp. Immunol., 43, 106-113
9. Arizza, V., Giaramita, F.T., Parrinello, D., Cammarata, M. and Parrinello, N. (2007) Cell cooperation in coelomocyte cytotoxic activity of Paracentrotus lividus coelomocytes Comp. Biochem. Physiol. Part A, 147, 389-394
10. Barca, A., Vacca, F., Vizioli, J., Drago, F., Vetrugno, C., Verri, T. and Pagliara, P. (2017) Molecular and expression analysis of the Allograft inflammatory factor 1 (AIF-1) in the coelomocytes of the common sea urchin Paracentrotus lividus Fish Shellfish Immunol., 71, 136-143
11. Smith, L.C., Hawley, T.S., Henson, J.H., Majeske, A.J., Oren, M. and Rosentalk, B. (2019) Methods for collection, handling, and analysis of sea urchin coelomocytes Meth. Cell Biol., 150, 357-389
6. Acknowledgements
We are indebted to Dr L. Courtney Smith for her help in the preparation of this OptiPrep™ Application Sheet.
OptiPrep™ Application Sheet C50; 8th edition, February 2020
OptiPrep™ Application Sheet C51
Analysis of gradients
- To access other Application Sheets referred to in the text return to the Cell Index; key Ctrl “F” and type the C-Number in the Find Box
1. Density determination
Once samples have been collected from a gradient, it may be important to determine their density by collecting fractions from a blank gradient run in parallel. The most direct method is to weigh accurately known volumes of liquid using a pycnometer; however, this is very time consuming. It is more convenient to determine the density of a fraction by measuring the refractive index (RI), which has the added advantage of requiring as little as 20-50 µl of sample. For extensive tables relating % (w/v) concentration of iodixanol, density and RI of iodixanol solutions produced by the dilution of OptiPrep™ with routine buffered saline solutions see Application Sheet C01. Because the RI of gradient solutions is increased by the presence of other solutes (e.g. salts, buffer etc), the precise value of the RI will vary with the presence and concentration of these solutes. Thus it might be wise to construct a simple graph of RI against iodixanol concentration, from measurements made on solutions of iodixanol prepared by mixing OptiPrep™ with the specific cell medium used in the study
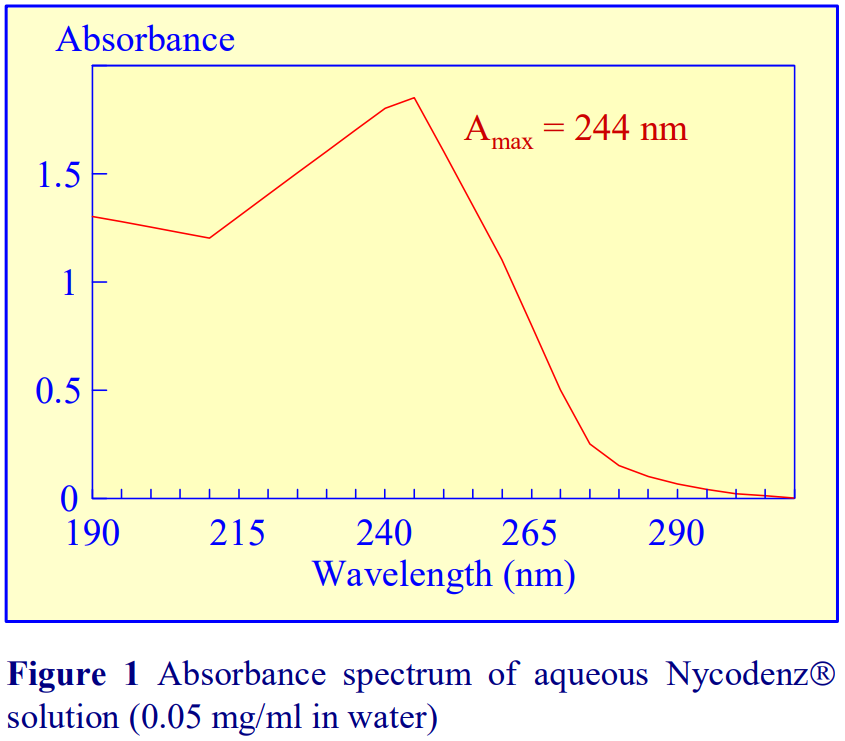 If a refractometer is not available then an alternative method of determining the density of gradient fractions is to measure the absorbance (optical density) of the fractions. All iodinated density gradient media absorb strongly in the UV (see Figure 1). If the absorbance is measured at approx 244 nm (the absorbance maximum for NycodenzⓇ and iodixanol) the gradient samples will need to be diluted 1:10,000 with water to get an absorbance value that can be measured accurately. Table 1 gives a few values for iodixanol solutions, measured in a standard 1 cm path length quartz cell in a single beam spectrophotometer. The need to dilute the solution also means that any other potentially interfering material will be diluted out at the same time.
If a refractometer is not available then an alternative method of determining the density of gradient fractions is to measure the absorbance (optical density) of the fractions. All iodinated density gradient media absorb strongly in the UV (see Figure 1). If the absorbance is measured at approx 244 nm (the absorbance maximum for NycodenzⓇ and iodixanol) the gradient samples will need to be diluted 1:10,000 with water to get an absorbance value that can be measured accurately. Table 1 gives a few values for iodixanol solutions, measured in a standard 1 cm path length quartz cell in a single beam spectrophotometer. The need to dilute the solution also means that any other potentially interfering material will be diluted out at the same time.
Alternatively, if the absorbance is measured at a higher wavelength, dilution is not required. Table 2 gives a few absorbance values for NycodenzⓇ solutions at 350 nm and 360 nm. Care must be taken to use the correct blank to ensure that other components in the gradient fractions that absorb at, or near these wavelengths do not interfere with the measurement of the gradient medium.
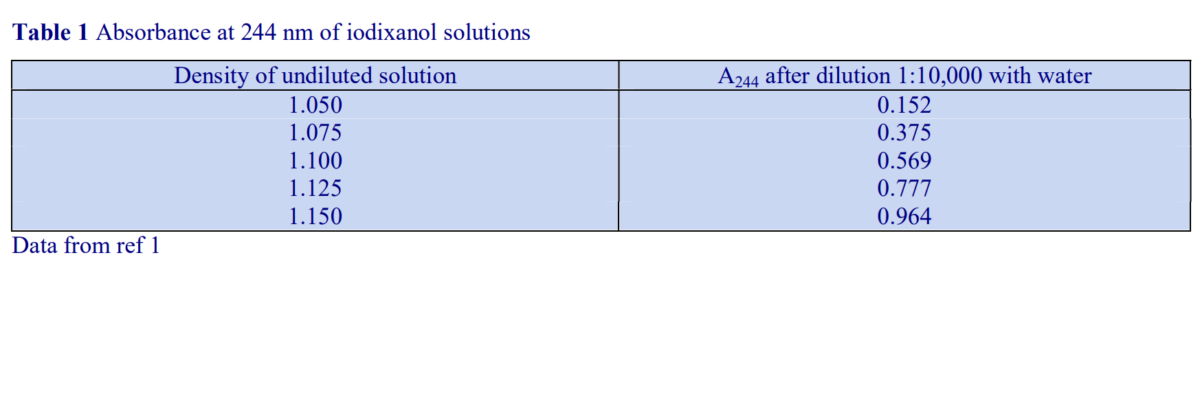

Absorbance measurements using a Multi-well Plate Reader
The wide availability of Multi-well Plate Readers which routinely have the facility for measurement of absorbance at 340 nm, considerably simplify the measurement of absorbance on blank gradient fractions. Multiple-channel automatic pipettes also facilitate the transfer of liquid aliquots between plates.
1. Transfer 100 μl of each of the fractions into 100 μl of water in the wells of a plate.
2. Complete the transfer and mixing by three repeated aspirations into and expulsions from the pipette tips.
3. Measure the absorbance of the solutions in each well in a standard plate reader using a 340 nm filter, against a suitable blank.
- Six different types of multi-well plate have been tested for their suitability. A flat-bottomed 96- well polystyrene plate, which has the lowest background absorbance of any plate tested (approx 0.130 at 340 nm), is available from Greiner BioOne Inc (Cat. # 655101). The inter-well variability of the absorbance was also one of the lowest of all those tested (± 0.007)
Absorbance values of a range of iodixanol solutions produce by dilution of OptiPrep™ with saline are given in Table 3. The absorbance measurements were made against saline blanks.

2. Nucleic acids, proteins and polysaccharides
Nucleic acids, proteins and polysaccharides in isolated gradient fractions are often assayed spectrophotometrically by chemical methods (Table 4 and ref 2). Unlike metrizamide, neither NycodenzⓇ nor iodixanol contain a sugar residue, therefore they do not interfere with the orcinol or diphenylamine reactions for the estimation of the ribose and deoxyribose of RNA and DNA respectively [3]; polysaccharides and sugars can be determined using the phenol/H2SO4 assay [4]. Sensitive dye binding assays for protein [5,6] and DNA [7] are also unaffected by the presence of the gradient media. Protein assays based on Coomassie blue give the most reliable data. The Folin Ciocalteu reagent [8] however cannot be carried out unless the concentration of NycodenzⓇ or iodixanol is less than 5% (w/v): this situation however can often be attained if the final assay volume is 1-2 ml and the volume of gradient fraction used is 50 µl. Even at higher concentrations of gradient medium, an appropriate correction can be made to produce a linear relationship between protein concentration and absorbance (Table 5 gives an example). In addition to these spectrophotometric methods, fluorimetric assays of nucleic acids [9,10] and proteins [11] can also be carried out in the presence of NycodenzⓇ or iodixanol. Many of these protocols are listed in ref 12.
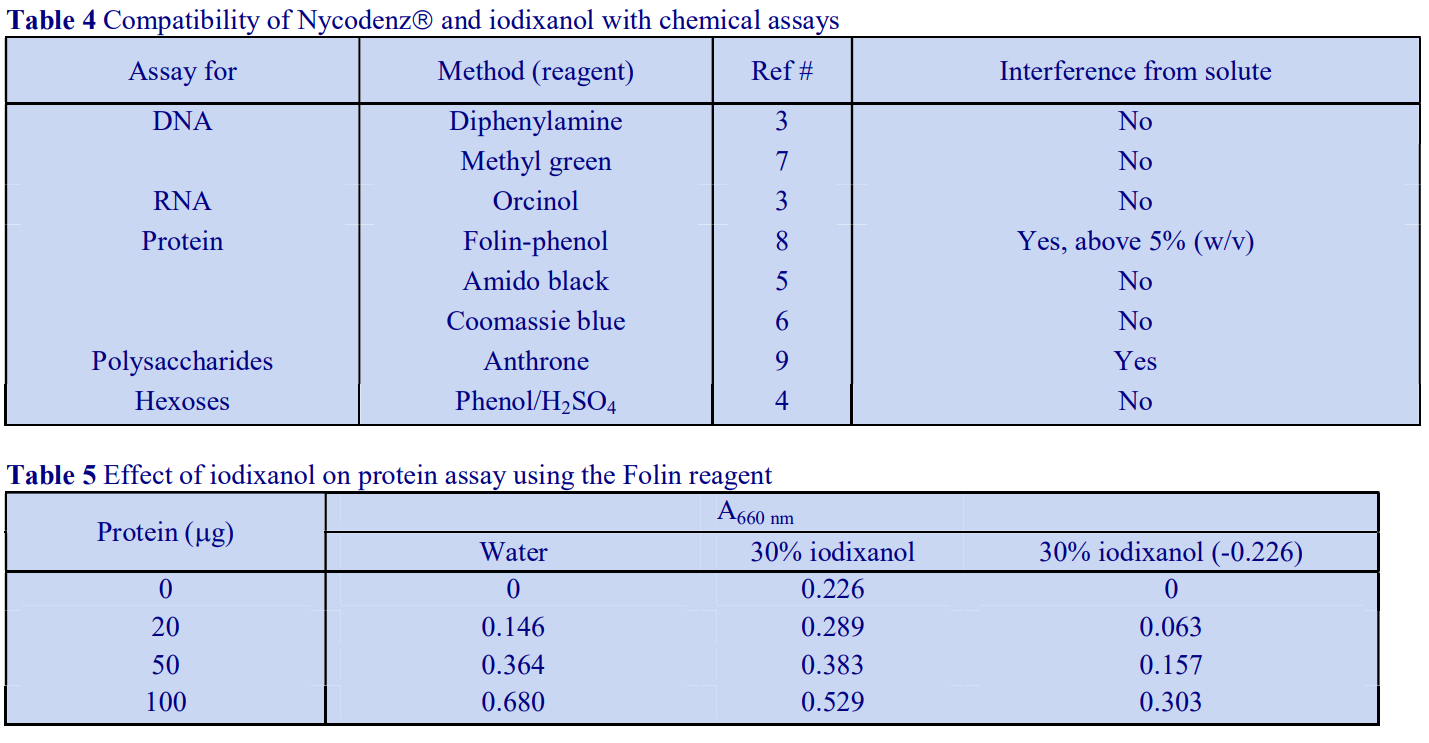
3. Electrophoresis
SDS-polyacrylamide and agarose gel electrophoresis can be carried out directly on gradient samples, as long as the concentration of protein or nucleic acid in the gradient fractions is sufficiently high for analysis. This contrasts with PercollⓇ whose colloidal silica particles interfere with the smooth migration of macromolecules into the gel; this gradient medium must therefore be removed prior to analysis. If the protein for example requires concentration, neither Nycodenz nor iodixanol interfere with TCA precipitation.
4. Removal of gradient medium and concentration of particles
It may be necessary to remove either NycodenzⓇ or iodixanol from the gradient fractions either to concentrate the particles or if the medium does interfere with some add-on process. Cells may be pelleted from fractions after dilution with 1-2 volumes of a low-density buffer such as a buffered salt solution.
5. References
1. Schroeder, M., Schafer, R. and Friedl, P. (1997) Spectrophotometric determination of iodixanol in subcellular fractions of mammalian cells Anal. Biochem., 244, 174 176
2. Rickwood, D., Ford, T. and Graham, J. (1982) Nycodenz: A new nonionic iodinated gradient medium Anal. Biochem., 123, 23-31
3. Schneider, W.C. (1957) Determination of nucleic acids in tissues by pentose analysis Meth. Enzymol., 3, 680-684
4. Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.E. and Smith, F. (1956) Colorimetric method for determination of sugars and related substances Anal. Chem., 28, 350-356
5. Schaffner, W. and Weissman, C. (1973) A rapid, sensitive, and specific method for the determination of protein in dilute solution Anal. Biochem., 56, 502-510
6. Bradford, M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding Anal. Biochem., 72, 248-254
7. Peters, D.L. and Dahmus, M.E. (1979) A method of DNA quantitation for localization of DNA in metrizamide gradients Anal. Biochem., 93, 306-311
8. Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with the Folin phenol reagent J. Biol. Chem., 193, 265-275
9. Fong, J., Schaffer, F.L. and Kirk, P.K. (1953) The ultramicrodetermination of glycogen in liver. A comparison of the anthrone and reducing-sugar methods Arch. Biochem. Biophys., 45, 319-326
10. Karsten, U. and Wollenberger, A. (1977) Improvements in the ethidium bromide method for direct fluorometric estimation of DNA and RNA in cell and tissue homogenates Anal. Biochem., 77, 464-469
11. Bohlen, P., Stein, S., Dairman, W. and Udenfriend, S. (1973) Fluorometric assay of proteins in the nanogram range Arch. Biochem. Biophys., 155, 213-220
12. Ford, T. and Graham, J.M. (1983) Enzymatic and chemical assays compatible with iodinated density gradient media In: Iodinated Density Gradient Media – a practical approach (ed D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 195-216
OptiPrep™ Application Sheet C51; 5th edition, November 2019
OPTIPREP™ APPLICATION SHEET INDEX
EUKARYOTIC AND PROKARYOTIC CELLS
- The Index is divided into two sections:
A. General methods for preparing and analysing gradients
B. An alphabetical cell type index. Commonly isolated cells such as mononuclear cells are often further categorized into species or tissue source. If a cell type is not in this index, it may be necessary to develop a customized method: in Section A see “Fractionation of a mixed population of cells” for some guidance.
- Click on the relevant [Application Sheet] for a detailed protocol. In some cases more than one Application Sheet for a specific cell type may be provided if different practical strategies are available.
A. GENERAL METHODOLOGY
Preparation of gradient solutions [Application Sheet C01]
Preparation of gradients [Application Sheet C02]
Fractionation of a mixed population of cells [Application Sheet C15]
Analysis of gradients [Application Sheet C51]
B. ALPHABETICAL CELL INDEX
Alveolar cells (see “Pulmonary cells”)
Bacteria
Anaplasma phagocytophilum [Application Sheet C49]
Chlamydophila abortus [Application Sheet C49]
Cyanobacteria [Application Sheet C49]
Listeria monocytogenes [Application Sheet C49]
Obligate intracellular bacteria [Application Sheet C49]
Piscirickettsia [Application Sheet C49]
Rickettsia typhi [Application Sheet C49]
Spore protoplast density determination [Application Sheet C49]
Soil, stream-water, clinical specimens and food [Application Sheet C39]
Cells in suspension, maintenance of [Application Sheet C38]
Erythrocytes [Application Sheet C34]
Cryptosporidium (see “Protozoa”)
Cyclospora (see “Protozoa”)
Dendritic cells
Barrier flotation [Application Sheet C21]
Barrier sedimentation [Application Sheet C41]
Mixer flotation [Application Sheet C22]
Enterocyozoon bieneusi (see “Protozoa”)
Erythrocytes (normal and sickle cells) [Application Sheet C34]
Erythrocytes, removal from blood and bone marrow [Application Sheet C34]
Foam cells [Application Sheet C42]
Gastric cells
Parietal cells [Application Sheet C48]
ECL cells [Application Sheet C47]
Granulocytes (see “Polymorphonuclear leukocytes”)
Hepatic cells
Non-parenchymal cells – a short methodological survey [Application Sheet C25]
Kupffer cells [Application Sheet C28]
Non-parenchymal cells [Application Sheet C26]
Stellate cells [Application Sheet C27]
Kidney cells (see “Renal”)
Langerhans cells (see “Dendritic cells”)
Lymphocytes
Blood and tissues, from (see “Mononuclear cells”)
Macrophages [Application Sheet C42]
Mattesia orzaephili (see “Protozoa”)
Microfluidic cell encapsulation/cell sorting [Application Sheet C38]
Monocytes (human)
Leukocyte-rich plasma
Barrier sedimentation [Application Sheet C46]
Flotation [Application Sheet C10]
Methodological review [Application Sheet C03]
Whole blood, from [Application Sheet C11]
Mononuclear cells
Bone marrow [Application Sheet C40]
Equine peripheral blood [Application Sheet C09]
Human peripheral blood
Barrier flotation [Application Sheet C06]
Barrier sedimentation [Application Sheet C04]
Mixer flotation [Application Sheet C05]
Intestine [Application Sheet C40]
Liver [Application Sheet C40]
Methodological review [Application Sheet C03]
Mouse blood
Barrier sedimentation [Application Sheet C43]
Mixer flotation [Application Sheet C08]
Non-human primate peripheral blood [Application Sheet C45]
Peritoneal exudates [Application Sheet C20]
Rabbit blood [Application Sheet C43]
Rat blood
Barrier sedimentation [Application Sheet C43]
Mixer flotation [Application Sheet C07]
Ruminant peripheral blood [Application Sheet C09]
Spinal cord [Application Sheet C40]
Spleen [Application Sheet C40]
Neural cells
Inflammatory cells (spinal cord injury) [Application Sheet C35]
Microglial cells [Application Sheet C35]
Motoneurons (brain, various sites) [Application Sheet C36]
Motoneurons (spinal cord) [Application Sheet C23]
Oligodendrocytes (see “Microglial cells”)
Neutrophils
see “Polymorphonuclear leukocytes”
Pancreatic islets [Application Sheet C16]
Pancreatic stellate cells [Application Sheet C27]
Plant protoplasts [Application Sheet C19]
Platelets (human) [Application Sheet C13]
Polymorphonuclear leukocytes
Human peripheral blood [Application Sheet C12]
Mouse blood [Application Sheet C44]
Non-human primate peripheral blood (see “Human
peripheral blood”)
Peritoneal exudates [Application Sheet C20]
Rat blood (see “ Mouse blood”)
Rabbit blood (see “ Mouse blood”)
Spinal injury, quantitative assessment in [Application Sheet C35]
Progenitor cells (bone marrow and other tissues)
Barrier sedimentation [Application Sheet C24]
Protozoa
Cryptosporidium [Application Sheet C31]
Cyclospora [Application Sheet C31]
Enterocytozoon bieneusi [Application Sheet C31]
Mattesia orzaephili [Application Sheet C31]
Plasmodium [Application Sheet C32]
Sarcocystis neurona [Application Sheet C31]
Tetrahymena thermophila [Application Sheet C31]
Toxoplasma
Purification from cell cultures [Application Sheet C33]
From soil samples [Application Sheet C33]
Separation of sporocysts and oocyst walls [Application Sheet C33]
Pulmonary cells
Endothelial cells [Application Sheet C29]
Epithelial cells (Type I) [Application Sheet C29]
Epithelial cells (Type II) [Application Sheet C30]
Lymphoid cells [Application Sheet C30]
Macrophages [Application Sheet C29]
Myeloid cells [Application Sheet C30]
Renal cells
Interstitial and thin loop of Henlé cells [Application Sheet C37]
Proximal tubule [Application Sheet C37]
Reticulocytes [Application Sheet C34]
Sarcocystis neurona (see “Protozoa”)
Sea urchin coelomocytes [Application Sheet C50]
Sickle cells (see “Erythrocytes”)
Sperm cells
Bovine [Application Sheet C17]
Equine [Application Sheet C17]
Elephant [Application Sheet C17]
Gazelle [Application Sheet C17]
Human [Application Sheet C18]
Mouse [Application Sheet C17]
Porcine [Application Sheet C17]
Turkey/rooster [Application Sheet C17]
Spleen
Dendritic cells (see “Dendritic cells”)
Mononuclear cells (see “Mononuclear cells”)
Splenocytes (see “Mononuclear cells”)
Stem cells (see “Progenitor cells”)
Thrombocytes (see “Platelets”)
Thymus
Dendritic cells (see “Dendritic cells”)
Viable/non-viable cells [Application Sheet C14]
OptiPrep™Application Sheets – Cell Index March 2020
