Subcellular References
OptiPrep Reference List RS01
Purification of nuclei from tissues and cells
- This Reference List provides a complete list of publications reporting the use of OptiPrep™ for the isolation of nuclei: the references are sorted alphabetically into sections according cell type or tissue source. Within each section references are listed alphabetically according to first author.
- Key words in the article titles are highlighted in light blue
- Application Sheet S10a provides a practical review of the current OptiPrep™-based methodologies
Annelids
Tweetena, K.A. and Morris, S.J. (2016) Flow cytometry analysis of DNA ploidy levels and protein profiles distinguish between populations of Lumbriculus (Annelida: Clitellata) Invert. Biol., 135, 385–399
BHK cells
Ilina, P., Hyvonen, Z., Saura, M., Sandvig, K., Yliperttula, M. and Ruponen, M. (2012) Genetic blockage of endocytic pathways reveals differences in the intracellular processing of non-viral gene delivery systems J. Control. Release, 163, 385–395
Brain cells (human), healthy and disease tissue (see also “Human tissues”)
Aiello, G., Ballabio, C., Ruggeri, R., Fagnocchi, L., Anderle, M., Morassut, I., Caron, D., Garilli, F., Gianno, F. et al (2019) Truncated BRPF1 cooperates with smoothened to promote adult Shh medullo-blastoma Cell Rep., 29, 4036–4052
Castelijns, B., Baak, M.L., Timpanaro, I.S., Wiggers, C.R.M., Vermunt, M.W., Shang, P., Kondova, I., Geeven, G., Bianchi, V. et al (2020) Hominin-specific regulatory elements selectively emerged in oligodendrocytes and are disrupted in autism patients Nat. Comm., 11: 301
Corces, M.R., Trevino, A.E., Hamilton, E.G., Greenside, P.G., Sinnott-Armstrong, N.A., Vesuna, S., Satpathy, A.T., Rubin, A.J., Montine, K.S. et al (2017) An improved ATAC -seq protocol reduces background and enables interrogation of frozen tissues Nat. Meth., 14, 959-962
Del-Aguila, J.L., Li, Z., Dube, U., Mihindukulasuriya, K.A., Budde, J.P., Fernandez, M.V., Ibanez, L., Bradley, J., Wang, F. et al (2019) A single-nuclei RNA sequencing study of Mendelian and sporadic AD in the human brain Alzheimer’s Res. Ther., 11: 71
Erwin, J.A., Paquola, A.C.M., Singer, T., Gallina, I., Novotny, M., Quayle, C., Bedrosian, T.A., Alves, F.I.A., Butcher, C.R. et al (2016) L1-associated genomic regions are deleted in somatic cells of the healthy human brain Nat. Neurosci, 19, 1583-1591
Frigero, C.S., Fiers, M., Voet, T. and De Strooper, B. (2017) Identification of low allele frequency mosaic mutations in Alzheimer disease In Genomic Mosaicism in Neurons and Other Cell Types: Neuromethods, 131, (ed. Frade, J.M. and Gage, F.H.) Springer Science+Business Media, LLC, pp 361-378
Garcia-Esparcia, P., Hernández-Ortega, K., Koneti, A., Gil, L., Delgado-Morales, R., Castaño, E., Carmona, M. and Ferrer, I. (2015) Altered machinery of protein synthesis is region- and stage-dependent and isassociated with α-synuclein oligomers in Parkinson’s disease Acta Neuropathol. Comm., 3: 76
Hoffner, G., Island, M-L. and Djian, P. (2005) Purification of neuronal inclusions of patients with Huntington’s disease reveals a broad range of N-terminal fragments of expanded huntingtin and insoluble polymers J. Neurochem., 95, 125-136
Iuchi, S., Hoffner, G., Verbeke, P., Djian, P. and Green, H. (2003) Oligomeric and polymeric aggregates formed by proteins containing expanded polyglutamine Proc. Natl. Acad. Sci. USA, 100, 2409-2414
Jessa, S., Blanchet-Cohen, A., Krug, B., Vladoiu, M., Coutelier, M., Faury, D., Poreau, B., De Jay, N., Hébert, S., Monlong, J. et al (2019) Stalled developmental programs at the root of pediatric brain tumors Nat. Genet., 51, 1702–1713
Kaeser, G.E. and Chun, J. (2017) Flow cytometric and sorting analyses for nuclear DNA content, nucleotide sequencing, and interphase FISH In Genomic Mosaicism in Neurons and Other Cell Types: Neuromethods, 131, (ed. Frade, J.M. and Gage, F.H.) Springer Science+Business Media, LLC, pp 43-55
Krishnaswami, S.R., Grindberg, R.V., Novotny, M., Venepally, P., Lacar, B., Bhutani, K., Linker, S.B. et al (2016) Using single nuclei for RNA-seq to capture the transcriptome of postmortem neurons Nat. Protoc., 11, 499-524
Mathys, H., Davila-Velderrain, J., Peng, Z., Gao, F., Mohammadi, S., Young, J.Z., Menon, M., He, L., Abdurrob, F. et al (2019) Single-cell transcriptomic analysis of Alzheimer’s disease Nature, 570, 332-337
Nagaraja, S., Quezada, M.A., Gillespie, S.M., Arzt, M., Lennon, J.J., Woo, P.J., Hovestadt, V., Kambhampati, M., Filbin, M.G. et al (2019) Histone variant and cell context determine H3K27M reprogramming of the enhancer landscape and oncogenic state Mol. Cell, 76, 965–980
Nagy, C., Maitra, M., Theroux, J-F., Djambazian, H. and Turecki, G. (2018) Single-cell transcriptome of the depressed and suicidal brain Biol. Psychiatry, 83, Abstr 118 Perez-Rodriguez, D., Kalyva, M., Leija-Salazar, M., Lashley, T., Tarabichi, M., Chelban, V., Gentleman, S., Schottlaender, L., Franklin, H. (2019) Investigation of somatic CNVs in brains of synucleinopathy cases using targeted SNCA analysis and single cell sequencing Acta Neuropathol. Comm., 7: 219
Pinho, R., Paiva, I., Gotovac Jerčić, C., Fonseca-Ornelas, L., Gerhardt, E., Fahlbusch, C., Garcia-Esparcia, P., Kerimoglu, C., Pavlou, M.A.S. et al (2019) Nuclear localization and phosphorylation modulate pathological effects of alpha-synuclein Hum. Mol. Genet., 28, 31-50
Reed, P.J., Wang, M., Erwin, J.A., Paquola, A.C.M. and Gage, F.H. (2017) Single-cell whole genome amplification and sequencing to study neuronal mosaicism and diversity In Genomic Mosaicism in Neurons and Other Cell Types: Neuromethods, 131, (ed. Frade, J.M. and Gage, F.H.) Springer Science+Business Media, LLC, pp 253-268
Renthal, W., Boxer, L.D., Hrvatin, S., Li, E., Silberfeld, A., Nagy, M.A., Griffith, E.C., Vierbuchen, T. and Greenberg, M.E. (2018) Characterization of human mosaic Rett syndrome brain tissue by single-nucleus RNA sequencing Nat. Neurosci., 21, 1670–1679
Sousa, A.M.M., Zhu, Y., Raghanti, M.A., Kitchen, R.R., Onorati, M., Tebbenkamp, A.T.N., Stutz, B. (2017) Molecular and cellular reorganization of neural circuits in the human lineage Science, 358, 1027-1032
Wierman, M.B., Burbulis, I.E., Chronister, W.D., Bekiranov, S. and McConnell, M.J. (2017) Single-cell CNV detection in human neuronal nuclei In Genomic Mosaicism in Neurons and Other Cell Types: Neuromethods, 131, (ed. Frade, J.M. and Gage, F.H.) Springer Science+Business Media, LLC, pp 109-131
Brain tissue/spinal cord/neural cells (rodents), healthy and disease sources
Caro, P., Gómez, J., Arduini, A., González-Sánchez, M., González-García, M., Borrás, C., Viña, J., Puertas, M.J., Sastre, J. and Barja, G. (2010) Mitochondrial DNA sequences are present inside nuclear DNA in rat tissues and increase with age Mitochondrion 10, 479–486
Cheadle, L., Tzeng, C.P., Kalish, B.T., Harmin, D.A., Rivera, S., Ling, E., Nagy, M.A., Hrvatin, S. et al (2018) Visual experience-dependent expression of Fn14 is required for retinogeniculate refinement Neuron 99, 525–539
Clemen, C.S., Herr, C., Hövelmeyer, N. and Noegel, A.A. (2003) The lack of annexin A7 affects functions of primary astrocytes Exp. Cell Res., 291, 406-414
Clemens, A.W., Wu, D.Y., Moore, R., Christian, D.L., Zhao, G. and Gabel, H.W. (2020) MeCP2 represses enhancers through chromosome topology-associated DNA methylation Mol. Cell. 77, 279–293
Fernandez-Albert, J., Lipinski, M., Lopez-Cascales, M.T., Rowley, M.J., Martin-Gonzalez, A.M., del Blanco, B., Corces, V.G. and Barco, A. (2019) Immediate and deferred epigenomic signatures of in vivo neuronal activation in mouse hippocampus Nat. Neurosci., 22, 1718-1730
Foran, E., Bogush, A., Goffredo, M., Roncaglia, P., Gustincich, S., Pasinelli, P., and Trotti, D. (2011) Motor neuron impairment mediated by a sumoylated fragment of the glial glutamate transporter EAAT2 Glia, 59, 1719–1731
Gao, Z., Lee, P., Stafford, J.M., von Schimmelmann, M., Schaefer, A. and Reinberg, D. (2014) An AUTS2– Polycomb complex activates gene expression in the CNS Nature, 516, 349-354
German, D.C., Ng, M.C., Liang, C.L., McMahon, A and Iacopino, A.M. (1997) Calbindin-D28k in nerve cell nuclei Neuroscience, 81, 735-743
Jordi, E., Heiman, M., Marion-Poll, L., Guermonprez, P., Cheng, S.K., Nairn, A.C. Greengard, P. and Giraulta, J-A. (2013) Differential effects of cocaine on histone posttranslational modifications in identified populations of striatal neurons Proc. Natl. Acad. Sci. USA, 110, 9511-9516
Korb, E., Herre, M., Zucker-Scharff, I., Gresack, J., Allis, C.D. and Darnell, R.B. (2017) Excess translation of epigenetic regulators contributes to fragile X syndrome and is alleviated by Brd4 inhibition Cell, 170, 1209–1223
Kraus, K., Kleene, R., Henis, M., Braren, I., Kataria, H., Sharaf, A., Loers, G., Schachner, M. and Lutz, D. (2018) A fragment of adhesion molecule L1 binds to nuclear receptors to regulate synaptic plasticity and motor coordination Mol. Neurobiol., 55, 7164–7178
Kumar, V., Jong, Y-J.I. and O’Malley, K.L. (2008) Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ release J. Biol. Chem., 283, 14072-14083
Lutz, D., Wolters-Eisfeld, G., Joshi, G., Djogo, N., Jakovcevski, I., Schachner, M. and Kleene, R. (2012) Generation and nuclear translocation of sumoylated transmembrane fragment of cell adhesion molecule L1 J. Biol. Chem., 287, 17161–17175
Ma, S., Hsieh, Y-P., Ma, J. and Lu, C. (2018) Low-input and multiplexed microfluidic assay reveals epigenomic variation across cerebellum and pre-frontal cortex Sci. Adv. 4: eaar8187
Ma, S., de la Fuente Revenga, M., Sun, Z., Sun, C., 1, Murphy, T.W., Xie, H., González-Maeso, J. and Lu, C. (2018) Cell-type-specific brain methylomes profiled via ultralow-input microfluidics Nat. Biomed. Engineer., 2, 183–194
Marcora, E. and Kennedy, M.B. (2010) The Huntington’s disease mutation impairs Huntingtin’s role in the transport of NF-kB from the synapse to the nucleus Hum. Mol. Genet., 19, 4373–4384
Marion-Poll, L., Montalban, E., Munier, A., Hervé, D. and Girault, J-A. (2014) Fluorescence-activated sorting of fixed nuclei: a general method for studying nuclei from specific cell populations that preserves posttranslational modifications Eur. J. Neurosci., 39, 1234–1244
Mellén, M., Ayata, P., Dewell, S., Kriaucionis, S. and Heintz, N. (2012) MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system Cell 151, 1417–1430
Mellén, M., Ayata, P. and Heintz, N. (2017) 5-hydroxymethylcytosine accumulation in postmitotic neurons results in functional demethylation of expressed genes Proc. Natl. Acad. Sci. USA, 114, E7812–E7821
Merritt, S.E., Mata, M., Nihalani, D., Zhu, C., Hu, X. and Holzman, L.B. (1999) The mixed lineage kinase DLK utilizes MKK7 and not MKK4 as substrate J. Biol. Chem., 274, 10195-10202
Palmowski, P., Rogowska-Wrzesinska, A., Williamson, J., Beck, H.C., Mikkelsen, J.D., Hansen, H.H. and Jensen, O.N. (2014) Acute phencyclidine treatment induces extensive and distinct protein phosphorylation in rat frontal cortex J. Proteome Res., 13,1578-1592
Sharma, N., Pollina, E.A, Nagy, M.A., Yap, E-L., DiBiase, F.A., Hrvatin, S., Hu, L., Lin, C. and Greenberg, M.E. (2019) ARNT2 tunes activity-dependent gene expression through NCoR2-mediated repression and NPAS4- mediated activation Neuron 102, 390–406
Tillotson, R., Selfridge, J., Koerner, M.V., Gadalla, K.K.E., Guy, J., De Sousa, D., Hector, R.D., Cobb, S.R. and Bird, A. (2017) Radically truncated MeCP2 rescues Rett syndrome-like neurological defects Nature 550, 398-401
Tuesta, L.M., Djekidel, M.N., Chen, R., Lu, F., Wang, W., Sabatini, B.L. and Zhang, Y. (2019) In vivo nuclear capture and molecular profiling identifies Gmeb1 as a transcriptional regulator essential for dopamine neuron function Nat. Comm., 10: 2508
Von Schimmelmann, M., Feinberg, P.A., Sullivan, J.M., Ku, S.M., Badimon, A., Duff, M.K., Wang, Z., Lachmann, A., Dewell, S. et al (2016) Polycomb repressive complex 2 (PRC2) silences genes responsible for neurodegeneration Nat. Neurosci., 19, 1321-1330
Yamamoto, Y., Jones, K.A., Mak, B.C., Muehlenbachs, A. and Yeung, R.S. (2002) Multicompartmental distribution of tuberous sclerosis gene products, hamartin and tuberin Arch. Biochem. Biophys., 404, 210-217
Zetsche, B., Heidenreich, M., Mohanraju, P., Fedorova, I., Kneppers, J., DeGennaro, E.M., Winblad, N., Choudhury, S.R. et al (2017) Multiplex gene editing by CRIS PR–Cpf1 using a single crRNA array Nat. Biotechnol., 35 31-34
Zhu, B., Hsieh, Y-P., Murphy, T.W., Zhang, Q., Naler, L.B. and Lu, C. (2019) MOWChIP-seq for low-input and multiplexed profiling of genome-wide histone modifications Nature Protoc., 14, 3366–3394
Caco-2 cells
Barta, C.A., Sachs-Barrable, K., Feng, F. and Wasan, K.M. (2008) Effects of monoglycerides on Pglycoprotein: modulation of the activity and expression in Caco-2 cell monolayers Mol. Pharmaceut., 5, 863-875
Caenorhabditis elegans
Steiner, F.A., Talbert, P.B., Kasinathan, S., Deal, R.B. and Henikoff, S. (2012) Cell-type-specific nuclei purification from whole animals for genome-wide expression and chromatin profiling Genome Res., 22:766–777
Steiner, F.A. and Henikoff, S. (2015) Cell type-specific afinity purification of nuclei for chromatin profiling in whole animals In The Nucleus, Methods in Mol. Biol. 1228 (ed. Hancock, R.) Springer Science+Business Media New York, pp 3-14
Carcinoma cells: see also “Hepatoma cells” and “Human tissues (frozen)”
Cohen, R.N., van der Aa, M.A.E.M., Macaraeg, N., Lee, A.P. and Szoka, F.C. (2009) Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection J. Control. Release 135 (2009) 166–174
Corsi, L., Geminiani, E., Avallone, R. and Baraldi, M. (2005) Nuclear location-dependent role of peripheral benzodiapine receptor (PBR) in hepatic tumoral cell lines proliferation Life Sci., 76, 2523-2533
Dubash, A.D., Guilluy, C., Srougi, M.C., Boulter, E., Burridge, K. and García-Mata, R. (2011) The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals PLoS One, 6: e17380
Dumas, N.A., He, D., Frost, A.R. and Falany, C.N. (2008) Sulfotransferase 2B1b in human breast: Differences in subcellular localization in African American and Caucasian women J. Steroid Biochem Mol. Biol., 111, 171-177
Duxin J.P., Dao, B., Martinsson, P., Rajala, N., Guittat, L., Campbell, J.L., Spelbrink, J.N. and Stewart, S.A. (2009) Human Dna2 is a nuclear and mitochondrial DNA maintenance protein Mol. Cell. Biol., 29, 4274-4282
Falany, C.N., He, D., Dumas, N., Frost, A.R. and Falany, J.L. (2006) Human cytosolic sulfotransferase 2B1: Isoform expression, tissue specificity and subcellular localization J. Steroid Biochem. Mol. Biol., 102, 214-221
He, D. and Falany, C.N. (2006) Characterization of proline-serine-rich carboxyl terminus in human sulfotransferase 2B1b: immunogenicity, subcellular localization, kinetic properties, and phosphorylation Drug. Metab. Dispos., 34, 1749-1755
Henaff, D., Rémillard-Labrosse, G., Loret, S. and Lippé, R. (2013) Analysis of the early steps of herpes simplex virus 1 capsid tegumentation J. Virol., 87, 4895–4906
Hensen, F., Moretton, A., van Esveld, S., Farge, G. and Spelbrink, J.N. (2018) The mitochondrial outer membrane location of the EXD2 exonuclease contradicts its direct role in nuclear DNA repair Sci. Rep., 8: 5368
Huff, L.P., DeCristo, M.J., Trembath, D., Kuan, P.F., Yim, M., Liu, J., Cook, D.R., Miller, R., Der, C.J. and Cox, A.D. (2013) The role of Ect2 nuclear RhoGEF activity in ovarian cancer cell transformation Genes Cancer, 4, 460-475
Ilina, P., Hyvonen, Z., Saura, M., Sandvig, K., Yliperttula, M. and Ruponen, M. (2012) Genetic blockage of endocytic pathways reveals differences in the intracellular processing of non-viral gene delivery systems J. Control. Release, 163, 385–395
Iordanskiy, S., Berro, R., Altieri, M., Kashanchi, F. and Bukrinsky, M. (2006) Intracytoplasmic maturation of the human immunodeficiency virus type I reverse transcription complexes determines their capacity to integrate into chromatin Retrovirology, 3, 1-12
Iordanskiy, S.N. and Bukrinsky, M.I. (2009) Analysis of viral and cellular proteins in HIV-1 reverse transcription complexes by co-immunoprecipitation In: HIV Protocols 2nd edition, Methods Mol. Biol. 485 (eds. Prasad, V.R. and Kalpana, G.V.), Humana Press, Totowa, NJ pp 121-134
Kung, C-P., and Raab-Traub, N. (2008) Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor through effects on Bcl-3 and STAT3 J. Virol., 82, 5486-5493
Kung, C-P. and Raab-Traub, N. (2010) Epstein-Barr virus latent membrane protein 1 modulates distinctive NF-B pathways through C-terminus-activating region 1 to regulate epidermal growth factor receptor expression J. Virol., 84, 6605-6614
Liffers, S-T., Maghnouj, A., Munding, J.B., Jackstadt, R., Herbrand, U., Schulenborg, T., Marcus, K., KleinScory, S., Schmiegel, W., Schwarte-Waldhoff, I., Meyer, H.E., Stühler, K. and Hahn, S.A. (2011) Keratin 23, a novel DPC4/Smad4 target gene which binds 14-3-3ε BMC Cancer. 11: 137
Lu, Z., Ghosh, S., Wang, Z. and Hunter, T. (2003) Down-regulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion Cancer Cell, 4, 499-515
Martin, T.M., Wysocki, B.J., Beyersdorf, J.P., Wysocki, T.A. and Pannier, A.K. (2014) Integrating mitosis, toxicity, and transgene expression in a telecommunications packet-switched network model of lipoplex-mediated gene delivery Biotechnol. Bioeng., 111, 1659–1671
Morrison, J.A., Gulley, M.L., Pathmanathan, R. and Raab-Traub, N. (2004) Differential signaling pathways are activated in the Epstein-Barr virus-activated malignancies nasopharyngeal carcinoma and Hodgkin lymphoma Cancer. Res.,64, 5251-5260
Morrison, T.E. and Kenney, S.C. (2004) BZLF1, an Epstein-Barr virus immediate-early protein, induces p65 nuclear translocation while inhibiting p65 transcriptional function Virology, 328, 219-232
Parelkar, S.S., Letteri, R., Chan-Seng, D., Zolochevska, O., Ellis, J., Figueiredo, M. and Emrick, T. (2014) Polymer-peptide delivery platforms: effect of oligopeptide orientation on polymer-based DNA delivery Biomacromolecules, 15, 1328-1336
Rémillard-Labrosse, G., Guay, G. and Lippe, R. (2006) Reconstitution of herpes simplex virus type 1 nuclear capsid egress in vitro J. Virol., 80, 9741-9753
Rémillard-Labrosse, G. and Lippé, R. (2011) In vitro nuclear egress of herpes simplex virus type 1 capsids Methods 55, 153–159
Salman, E.D., He, D., Runge-Morris, M., b, Kocarek, T.A. and Falany, C.N. (2011) Site-directed mutagenesis of human cytosolic sulfotransferase (SULT) 2B1b tophospho-mimetic Ser348Asp results in an isoform with increased catalytic activity J. Steroid Biochem. Mol. Biol., 127, 315– 323
Thornburg, N.J., Pathmanathan, R. and Raab-Traub, N. (2003) Activation of nuclear factor-kB p50 homodimer/Bcl-3 complexes in nasapharyngeal carcinoma Cancer. Res., 63, 8293-8301
Thornburg, N.J. and Raab-Traub, N. (2007) Induction of epidermal growth factor receptor expression by Epstein-Barr virus latent membrane protein 1 C-terminal-activating region 1 is mediated by NF-kB p50 homodimer/Bcl-3 complexes J. Virol., 81, 12954-12961
Van Gaal, E.V.B., Oosting, R.S., van Eijk, R., Bakowska, M., Feyen, D., Kok, R.J., Hennink, W.E., Crommelin, D.J.A. and Mastrobattista, E. (2011) DNA nuclear targeting sequences for non-viral gene delivery Pharm. Res., 28, 1707–1722
Zippin, J.H., Farrell, J., Huron, D., Kamenetsky, M., Hess, K.C., Fischman, D.A., Levin, L.R. and Buck, J. (2004) Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear camp microdomain J. Cell Biol., 164, 527-534
CHO cells
Macaraeg, N.F., Reilly, D.E. and Wong, A.W. (2013) Use of an anti-apoptotic CHO cell line for transient gene expression Biotechnol. Prog., 29, 1050–1058
Nomani, A., Hyvönen, Z., Pulkkinen, E., Hiekkala, M., Ruponen, M.(2014) Intracellular gene delivery is dependent on the type of non-viral carrier and defined by the cell surface glycosaminoglycans J. Control. Release, 187, 59–65
Valenzuela, S.M., Martin, D.K., Por, S.B., Robbins, J.M., Warton, K., Bootcov, M.R., Schofield, P.R., Campbell, T.J. and Breit, S.N. (1997) Molecular cloning and expression of a chloride ion channel of cell nuclei J. Biol. Chem., 272, 12575-12582
Ziraksaz, Z., Nomani, A., Ruponen, M., Soleimani, M., Tabbakhian, M. and Haririan, I. (2013) Cell-surface glycosaminoglycans inhibit intranuclear uptake but promote post-nuclear processes of polyamidoamine dendrimer–pDNA transfection Eur. J. Pharmaceut. Sci., 48, 55–63
Drosophila melanogaster
Groen, C.M., Jayo, A., Parsons, M. and Tootle, T.L. (2015) Prostaglandins regulate nuclear localization of Fascin and its function in nucleolar architecture Mol. Biol. Cell, 26, 1901-1917
Shmueli, A., Shalit, T., Okun, E. and Shohat‑Ophir, G, (2018) The Toll pathway in the central nervous system of flies and mammals Neuromol. Med., 20, 419–436
Steiner, F.A., Talbert, P.B., Kasinathan, S., Deal, R.B. and Henikoff, S. (2012) Cell-type-specific nuclei purification from whole animals for genome-wide expression and chromatin profiling Genome Res., 22:766–777
Ye, Y., Gu, L., Chen, X., Shi, J., Zhang, X. and Jiang, C. (2016) Chromatin remodeling during the in vivo glial differentiation in early Drosophila embryos Sci. Rep., 6: 33422
Ye, Y., Li, M., Gu, L., Chen, X., Shi, J., Zhang, X. and Jiang, C. (2017) Chromatin remodeling during in vivo neural stem cells differentiating to neurons in early Drosophila embryos Cell Death Different., 24, 409–420
Endothelial cells
Hahn, A.S. and Desrosiers, R.C. (2013) Rhesus monkey rhadinovirus uses Eph family receptors for entry into B cells and endothelial cells but not fibroblasts PLoS Pathog., 9: e1003360
Lucero, H.A., Kintsurashvili, E., Marketou, M.E. and Gavras, H. (2010) Cell signaling, internalization, and nuclear localization of the angiotensin converting enzyme in smooth muscle and endothelial cells J. Biol. Chem., 285, 5555-5568
Epithelial cells
Garcia-Mata, R., Dubash, A.D., Sharek, L., Carr, H.S., Frost, J.A. and Burridge, K. (2007) The nuclear RhoA exchange factor Net1 interacts with proteins of the Dlg family, affects their localization and influences their tumor suppressor activity Mol. Cell. Biol., 27, 8683-8697
Hyvönen, Z., Hämäläinen, V., Ruponen, M., Lucas, B., Rejman, J., Vercauteren, D., Demeester, J., De Smedt, S. and Braeckmans, K. (2012) Elucidating the pre- and post-nuclear intracellular processing of 1,4- dihydropyridine based gene delivery carriers J. Control. Release, 162, 167–175
Fibroblasts
Botting, C., Lu, X. and Triezenberg, S.J. (2016) H2AX phosphorylation and DNA damage kinase activity are dispensable for herpes simplex virus replication Virol. J., 13: 15
Brock, I., Krüger, M., Mertens, T. and von Einem, J. (2013) Nuclear targeting of human cytomegalovirus large tegument protein pUL48 is essential for viral growth J. Virol., 87, 6005–6019
Burke, C.W., Gardner, C.L., Steffan, J.J., Ryman, K.D. and Klimstra, W.B. (2009) Characteristics of alpha/beta interferon induction after infection of murine fibroblasts with wild-type and mutant alphaviruses Virology 395, 121–132
Duxin J.P., Dao, B., Martinsson, P., Rajala, N., Guittat, L., Campbell, J.L., Spelbrink, J.N. and Stewart, S.A. (2009) Human Dna2 is a nuclear and mitochondrial DNA maintenance protein Mol. Cell. Biol., 29, 4274-4282
Gaboreanu, A-M., Hrstka, R., Xu, W., Shy, M., Kamholz, J., Lilien, J. and Balsamo, J. (2007) Myelin protein zero/P0 phosphorylation and function require an adaptor protein linking it to RACK1 and PKC J. Cell. Biol., 177, 707-716
Hahn, A.S. and Desrosiers, R.C. (2013) Rhesus monkey rhadinovirus uses Eph family receptors for entry into B cells and endothelial cells but not fibroblasts PLoS Pathog., 9: e1003360
Hurwitz, S.N., Nkosi, D., Conlon, M.M., York, S.B., Liu, X., Tremblay, D.C. and Meckes, D.G. (2017) CD63 regulates Epstein-Barr virus LMP1 exosomal packaging, enhancement of vesicle production, and noncanonical NF-B signaling J. Virol., 91: e02251-16
Kegel, K., Meloni, A.R., Yi, Y., Kim, Y.J., Doyle, E., Cuiffo, B.G., Sapp, E., Wang, Y., Qin, Z-H., Chen, J.D., Nevins, J.R., Aronin, N. and DiFgilia, M. (2002) Huntingtin is present in the nucleus, interacts with the transcriptional corepressor C-terminal binding protein, and represses transcription J. Biol. Chem., 277, 7466-7476
Krishnan, H.H., Sharma-Walia, N., Streblow, D.N., Naranatt, P.P. and Chandran, B. (2006) Focal adhesion kinase is critical for entry of Kaposi’s sarcoma-associated herpesvirus into target cells J. Virol., 80, 1167-1180
Montgomery, S.A., Berglund, P., Beard, C.W. and Johnston, R.E. (2006) Ribosomal protein S6 associates with alphavirus nonstructural protein 2 and mediates expression from alphavirus messages J. Virol., 80, 7729-7739
Montgomery, S.A. and Johnston, R.E. (2007) Nuclear import and export of Venezuelan equine encephalitis virus nonstructural protein 2 J. Virol., 81, 10268-10279
Naranatt, P.P., Krishnan, H.H., Smith, M.S. and Chandran, B. (2005) Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus J. Virol., 79, 1191-1206
Pandey, S., Talukdar, I., Jain, B.P. and Goswami, S.K. (2017) GSK3β and ERK regulate the expression of 78 kDa SG2NA and ectopic modulation of its level affects phases of cell cycle Sci. Rep., 7: 7555
Qin, Z-H., Wang, Y., Kikly, K.K., Sapp, E., Kegel, K.B., Aronin, N. and DiFiglia, M. (2001) Pro-caspase-8 is pre-dominantly localized in mitochondria and released into cytoplasm upon apoptotic stimulation J. Biol. Chem., 276, 8079-8086
Sinzger, C., Kahl, M., Laib, K., Klingel, K., Rieger, P., Plachter, B. and Jahn, G. (2000) Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus J. Gen. Virol., 81, 3021-3035
Xia, Y., Wang, J., Liu, T-J., Yung, W.K.A., Hunter, T., Lu, Z. (2007) c-Jun down-regulation by HDAC3- dependent transcriptional repression promotes osmotic stress-induced cell apoptosis Mol. Cell, 25, 219-232
Glioblastoma see Neuroblastoma
Granulosa (ovarian) cells
Chen, H., Guo, J.H., Lu, Y.C., Ding, G.L., Yu, M.K., Tsang, L.L., Fok, K.L. et al (2012) Impaired CFTRdependent amplification of FSH-stimulated estrogen production in cystic fibrosis and PCOS J. Clin. Endocrinol. Metab., 97, 923–932
Heart tissue
Bhattacharyya, S., Sathe, A.A., Bhakta, M., Xing, C. and Munshi, N.V. (2019) PAN-INTACT enables direct isolation of lineage-specific nuclei from fibrous tissues PLoS One. 14: e0214677
Monroe, T.O., Hill, M.C., Morikawa, Y., Leach, J.P., Heallen, T., Cao, S., Krijger, P.H.L., de Laat, W., Wehrens, X.H.T., Rodney, G.G. and Martin, J.F. (2019) YAP partially reprograms chromatin accessibility to directly induce adult cardiogenesis in vivo Devel. Cell, 48, 765–779
HEK cells
Choi, Y.B., Sandford, G. and Nicholas, J. (2012) Human herpesvirus 8 interferon regulatory factor-mediated BH3-only protein inhibition via Bid BH3-B mimicry PLoS Pathog., 8: e1002748
Cooper, H.M. and. Spelbrink, J.N. (2008) The human SIRT3 protein deacetylase is exclusively mitochondrial Biochem. J., 411, 279-285
Dubash, A.D., Guilluy, C., Srougi, M.C., Boulter, E., Burridge, K. and García-Mata, R. (2011) The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals PLoS One, 6: e17380
Duxin J.P., Dao, B., Martinsson, P., Rajala, N., Guittat, L., Campbell, J.L., Spelbrink, J.N. and Stewart, S.A. (2009) Human Dna2 is a nuclear and mitochondrial DNA maintenance protein Mol. Cell. Biol., 29, 4274-4282
Guilluy, C., Dubash, A.D. and García-Mata, R. (2011) Analysis of RhoA and Rho GEF activity in whole cells and the cell nucleus Nat. Protocols 6, 2050-2060
Hurwitz, S.N., Nkosi, D., Conlon, M.M., York, S.B., Liu, X., Tremblay, D.C. and Meckes, D.G. (2017) CD63 regulates Epstein-Barr virus LMP1 exosomal packaging, enhancement of vesicle production, and noncanonical NF-B signaling J. Virol., 91: e02251-16
Joyal, J-S., Nim, S., Zhu, T., Sitaras, N. et al (2014) Subcellular localization of coagulation factor II receptorlike 1 in neurons governs angiogenesis Nature Med., 20, 1165-1173
Lang, W-H., Calloni, G. and Vabulas, R.M. (2018) Polylysine is a proteostasis network-engaging structural determinant J. Proteome Res., 17, 1967−1977
Liffers, S-T., Maghnouj, A., Munding, J.B., Jackstadt, R., Herbrand, U., Schulenborg, T., Marcus, K. et al (2011) Keratin 23, a novel DPC4/Smad4 target gene which binds 14-3-3ε BMC Cancer. 11: 137
Sergin, I., Jong, Y-J.I., Harmon, S.K., Kumar, V. and O’Malley, K.L. (2017) Sequences within the C terminus of the metabotropic glutamate receptor 5 (mGluR5) are responsible for inner nuclear membrane localization J. Biol. Chem., 292, 3637–3655
HeLa cells
Du, M. and Chen, Z.J. (2018) DNA-induced liquid phase condensation of cGAS activates innate immune signaling Science 361, 704–709
Hepatoma cells
Barthelson, R.A., Lambert, G.M., Vanier, C., Lynch, R.M. and Galbraith, D.W. (2007) Comparison of the contributions of the nuclear and cytoplasmic compartments to global gene expression in human cells BMC Genom., 8:340
Wua, M-J., Ke, P-Y., and Horng, J-T. (2014) RacGTPase-activating protein 1 interacts with hepatitis C virus polymerase NS5B to regulate viral replication Biochem. Biophy. Res. Comm., 454, 19–24
Human tissues (frozen)
Corces, M.R., Trevino, A.E., Hamilton, E.G., Greenside, P.G., Sinnott-Armstrong, N.A., Vesuna, S., Satpathy, A.T., Rubin, A.J., Montine, K.S. et al (2017) An improved ATAC -seq protocol reduces background and enables interrogation of frozen tissues Nat. Meth., 14, 959-962
Mathys, H., Davila-Velderrain, J., Peng, Z., Gao, F., Mohammadi, S., Young, J.Z., Menon, M., He, L., Abdurrob, F. et al (2019) Single-cell transcriptomic analysis of Alzheimer’s disease Nature, 570, 332-337
Keratinocytes
Morrison, J.A., Klingelhutz, A.J. and Raab-Traub, N. (2003) Epstein-Barr virus latent membrane protein 2A activates β-catenin signaling in epithelial cells J. Virol., 77, 12276-12284
Morrison, J.A. and Raab-Traub, N. (2005) Roles of the ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of -catenin signaling J. Virol., 79, 2375-2382
Siler, C.A. and Raab-Traub, N. (2008) Rhesus lymphocryptovirus latent membrane protein 2A activates βcatenin signaling and inhibits differentiation in epithelial cells Virology, 377, 273-279
Kidney
Gwathmey, T.M., Pendergrass, K.D., Pirro, N.T., Shaltout, H.A., Reid, S.D., Rose, J.C. and Chappell, M.C. (2009) Nuclear AT2 receptors mediate angiotensin II-dependent generation of nitric oxide FASEB J., 23, Abstr. 606.9
Gwathmey, T-Y.M., Shaltout, H.A., Pendergrass, K.D., Pirro, N.T., Figueroa, J.P., Rose, J.C., Diz, D.I. and Chappell, M.C. (2009) Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production Am. J. Physiol. Renal Physiol., 296, F1484–F1493
Gwathmey, T.M., Pendergrass, K.D., Reid, S.D., Rose, J.C., Diz, D.I. and Chappell, M.C. (2010) Angiotensin- (1-7)–angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus Hypertension, 55, 166-171
Gwathmey, T.M., Westwood, B.M., Pirro, N.T., Tang, L., Rose, J.C., Diz, D.I. and Chappell, M.C. (2010) Nuclear angiotensin-(1–7) receptor is functionally coupled to the formation of nitric oxide Am. J. Physiol. Renal Physiol., 299, F983–F990
Gwathmey, TY.M., Shaltout, H.A., Rose, J.C., Diz, D.I. and Chappell, M.C. (2011) Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney Hypertension, 57, 620-626
Gwathmey, T.M., Alzayadneh, E.M., Pendergrass, K.D. and Chappell, M.C. (2012) Novel roles of nuclear angiotensin receptors and signaling mechanisms Am. J. Physiol. Regul. Integr. Comp. Physiol., 302, R518–R530
Pendergrass, K.D., Averill, D.B., Ferrario, C.M., Diz, D.I. and Chappell, M.C. (2006) Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2.Lewis rat Am. J. Physiol. Renal Physiol., 290, F1497-F1506
Leishmania
Jardim, A., Hardie, D.B., Boitz, J. and Borchers, C.H. (2018) Proteomic profiling of Leishmania donovani promastigote subcellular organelles J. Proteome Res., 17, 1194−1215
Liver
Anderson, D.D. and Stover, P.J. (2009) SHMT1 and SHMT2 are functionally redundant in nuclear de novo thymidylate biosynthesis PLoS One, 4:e5839
Caro, P., Gómez, J., Arduini, A., González-Sánchez, M., González-García, M., Borrás, C., Viña, J., Puertas, M.J., Sastre, J. and Barja, G. (2010) Mitochondrial DNA sequences are present inside nuclear DNA in rat tissues and increase with age Mitochondrion 10, 479–486
Diaz, M.B., Lange, M., Heldmaier, G. and Klingenspor, M. (2004) Depression of transcription and translation during daily torpor in the Djungarian hamster (Phodopus sungorus) J. Comp. Physiol. B, 174, 495-502
Field, M.S., Kamynina, E., Agunloye, O.C., Liebenthal, R.P., Lamarre, S.G., Brosnan, M.E., Brosnan, J.T. and Stover, P.J. (2014) Nuclear enrichment of folate cofactors and methylenetetrahydrofolate dehydrogenase 1 (MTHFD1) protect de novo thymidylate biosynthesis during folate deficiency J. Biol. Chem., 289, 29642–29650
Garcia, D., Hellberg, K., Chaix, A., Wallace, M., Herzig, S., Badur, M.G., Lin, T., Shokhirev, M.N., Pinto, A.F.M., Ross, D.S., et al (2019) Genetic liver-specific AMPK activation protects against diet-induced obesity and NAFLD Cell Rep., 26, 192–208
Graham, J., Ford, T. and Rickwood, D (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
Hofer, T. and Moller, L. (2002) Optimization of the workup procedure for the analysis of 8-oxo-7, 8-dihydro2’-deoxyguanosine with electrochemical detection Chem. Res. Toxicol., 15, 426-432
Linder, B., Grozhik, A.V., Olarerin-George, A.O., Meydan, C., Mason, C.E. and Jaffrey, S.R. (2015) Singlenucleotide-resolution mapping of m6A and m6Am throughout the transcriptome Nat. Methods, 12, 767-772
MacFarlane, A.J., Anderson, D.D., Flodby, P., Perry, C.A., Allen, R.H., Stabler, S.P. and Stover, P.J. (2011) Nuclear localization of de novo thymidylate biosynthesis pathway is required to prevent uracil accumulation in DNA J. Biol. Chem., 286, 44015–44022
Pan, P., Treat, M.D. and van Breukelen, F. (2014) A systems-level approach to understanding transcriptional regulation by p53 during mammalian hibernation J. Exp. Biol., 217, 2489-2498
Pyhtila, B., Zheng, T., Lager, P.J., Keene, J.D., Reedy, M.C. and Nicchita, C.V. (2008) Signal sequence- and translation-independent mRNA localization to the endoplasmic reticulum RNA, 14, 445-453
Provost, J.J., Fudge, J., Israelit, S., Siddiqi, A.R. and Exton, J.H. (1996) Tissue-specific distribution and subcellular distribution of phospholipase D in rat: evidence for distinct RhoA- and ADP-ribosylation factor (ARF)-regulated isoenzymes Biochem. J., 319, 285-291
Robertson, A.B., Robertson, J., Fusser, M. and Klungland, A. (2014) Endonuclease G preferentially cleaves 5- hydroxymethylcytosine-modified DNA creating a substrate for recombination Nucleic Acids Res., 42, 13280–13293
Sucajtys-Szulc, E., Szolkiewicz, M., Swierczynski, J. and Rutkowski, B. (2016) Up-regulation of Hnf1a gene expression in the liver of rats with experimentally induced chronic renal failure – A possible link between circulating PCSK9 and triacylglycerol concentrations Atherosclerosis 248, 17-26
Van Breukelen, F. and Martin, S.L. (2002) Reversible depression of transcription during hibernation J. Comp. Physiol. B., 172, 355-361
Yamamoto, Y., Jones, K.A., Mak, B.C., Muehlenbachs, A. and Yeung, R.S. (2002) Multicompartmental distribution of tuberous sclerosis gene products, hamartin and tuberin Arch. Biochem. Biophys., 404, 210-217
Zhou, W., Zhang, Y., Hosch, M.S., Lang, A., Zwacka, R.M. and Engelhardt, J.F. (2001) Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion Hepatology, 33, 902-914
Lymphoid/monocytic cells
Bowick, G.C., Fennewald, S.M., Scott, E.P., Zhang, L-H., Elsom, B.L., Aronosn, J.F., Spratt, H.M., Luxon, B.A., Gorenstein, D.G. and Herzog, N.K. (2007) Identification of differentially activated cell-signaling networks associated with Pichinde virus pathogenesis by using systems kinomics J. Virol., 81, 1923-1933
Engelke, R., Riede, J., Hegermann, J., Wuerch, A., Eimer, S., Dengjel, J. and Mittler, G. (2014) The quantitative nuclear matrix proteome as a biochemical snapshot of nuclear organization J. Proteome Res., 13, 3940−3956
Seres, T., Knickelbein, R.G., Warshaw, J.B. and Johnston, Jr, R.B. (2000) The phagacytosis-associated respiratory burst in human monocytes is associated with increased uptake of glutathione J. Immunol., 165, 3333-3340
Thornburg, N.J., Kusano, S. and Raab-Traub, N. (2005) Identification of Epstein-Barr virus RK-BARF0- interacting proteins and characterization of expression pattern J. Virol., 78, 12848-12856 (2004) Tomlinson, C.C. and Damania, B. (2004) The K1 protein of Kaposi’s sarcoma-associated herpesvirus activates the Akt signaling pathway J. Virol., 78, 1918-1927
Weichhart, T., Haidinger, M., Katholnig, K., Kopecky, C., Poglitsch, M., Lassnig, C., Rosner, M., Zlabinger, G.J. et al (2011) Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells Blood, 117, 4273-4283
Macrophages
Andreyev, A.Y., Shen, Z., Guan, Z., Ryan, A., Fahy, E., Subramaniam, S., Raetz, C.R.H., Briggs, S. and Dennis, E.A. (2010) Application of proteomic marker ensembles to subcellular organelle identification Mol. Cell. Proteomics, 9, 388–402
Andreyev, A.Y., Fahy, E., Guan, Z., Kelly, S., Li, X., McDonald, J.G., Milne, S., Myers, D., Park, H., et al (2010) Subcellular organelle lipidomics in TLR-4-activated macrophages J. Lipid Res., 51, 2785–2797
Bowick, G.C., Fennewald, S.M., Scott, E.P., Zhang, L-H., Elsom, B.L., Aronosn, J.F., Spratt, H.M., Luxon, B.A., Gorenstein, D.G. and Herzog, N.K. (2007) Identification of differentially activated cell-signaling networks associated with Pichinde virus pathogenesis by using systems kinomics J. Virol., 81, 1923-1933
Bowick, G.C., Spratt, H.M., Hogg, A.E., Endsley, J.J., Wiktorowicz, J.E., Kurosky, A., Luxon, B.A., Gorenstein, D.G. and Herzog, N.K. (2009) Analysis of the differential host cell nuclear proteome induced by attenuated and virulent hemorrhagic arenavirus infection J. Virol., 83, 687-700
Kassas, N., Tanguy, E., Thahouly, T., Fouillen, L., Heintz, D., Chasserot-Golaz, S., Bader, M-F., Grant, N.J. and Vitale, N. (2017) Comparative characterization of phosphatidic acid sensors and their localization during frustrated phagocytosis J. Biol. Chem., 292, 4266–4279
Satyanarayanan, S.K., El Kebir, D., Soboh, S., Butenko, S., Sekheri, M., Saadi, J., Peled, N., Assi, S., Othman, A. et al (2019) IFN-β is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation Nat. Comm., 10: 3471
Mammary tissue
Dumas, N.A., He, D., Frost, A.R. and Falany, C.N. (2008) Sulfotransferase 2B1b in human breast: Differences in subcellular localization in African American and Caucasian women J. Steroid Biochem Mol. Biol., 111, 171-177
Mesenchymal stem cells
Kelly, A.M., Plautz, S.A., Zempleni, J. and Pannier, A.K. (2016) Glucocorticoid cell priming enhances transfection outcomes in adult human mesenchymal stem cells Mol. Ther., 24, 331–341
Microglial cells: see Neural/neural progenitor/microglial cells
Mouse embryo cells (10T1/2)
Staus, D.P., Weise-Cross, L., Mangum, K.D., Medlin, M.D., Mangiante, L., Taylor, J.M. and Mack, C.P. (2014) Nuclear RhoA signaling regulates MRTF-dependent SMC-specific transcription Am. J. Physiol. Heart Circ. Physiol., 307, H379–H390
Mouse mammary tissue
Fujiwara, S., Baek, S., Varticovski, L., Kim, S. and Hager, G.L. (2019) High quality ATAC-Seq data recovered from cryopreserved breast cell lines and tissue Sci. Rep., 9: 516
Muscle and myoblasts
Franko, A., von Kleist-Retzow, J.C., Böse, M., Sanchez-Lasheras, C., Brodesser, S., Krut, O., Kunz, W.S., Wiedermann, D. et al (2012) Complete failure of insulin-transmitted signaling, but not obesity-induced insulin resistance, impairs respiratory chain function in muscle J. Mol. Med., 90, 1145–1160
Hao, Y. and Gu, X.H. (2014) Effects of heat shock protein 90 expression on pectoralis major oxidation in broilers exposed to acute heat stress Poultry Sci., 93, 2709–2717
Parelkar, S.S., Chan-Seng, D. and Emrick, T. (2011) Reconfiguring polylysine architectures for controlling polyplex binding and non-viral transfection Biomaterials, 32, 2432-2444
Neural/neural progenitor/microglial cells
Ayata, P., Badimon. A., Strasburger, H.J., Duff, M.K., Montgomery, S.E., Loh, Y-H.E., Ebert, A., Pimenova, A.A, et al (2018) Epigenetic regulation of brain region-specific microglia clearance activity Nat. Neurosci., 21, 1049–1060
Fortin Ensign, S.P., Roos, A., Mathews, I.T., Dhruv, H.D., Tuncali, S., Sarkaria, J.N., Symons, M.H. Loftus, J.C. et al (2016) SGEF is regulated via TWEAK/Fn14/NF-B signaling and promotes survival by modulation of the DNA repair response to temozolomide Mol. Cancer Res.; 14, 302–12
Grindberg, R.V., Yee-Greenbaum, J.L., McConnell, M.J., Novotny, M., O’Shaughnessy, A.L., Lambert, G.M., Araúzo-Bravo, M.J. et al (2013) RNA-sequencing from single nuclei Proc. Natl. Acad. Sci. USA, 110, 19802–19807
Ito-Ishida, A., Yamalanchili, H.K., Shao, Y., Baker, S.A., Heckman, L.D., Lavery, L.A., Kim, J-y., Lombardi, L.M. et al (2018) Genome-wide distribution of linker histone H1.0 is independent of MeCP2 Nat. Neurosci., 21, 794–798
Kegel, K.B., Kim, M., Sapp, E., McIntyre, C., Castano, J.G., Aronin, N. and DiFiglia, M. (2000) Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation and autophagy J. Neurosci., 20, 7268-7278
Merritt, S.E., Mata, M., Nihalani, D., Zhu, C., Hu, X. and Holzman, L.B. (1999) The mixed lineage kinase DLK utilizes MKK7 and not MKK4 as substrate J. Biol. Chem., 274, 10195-10202
Platt, R.J., Chen, S., Zhou, Y., Yim, M.J. et al (2014) CRISPR-Cas9 knockin mice for genome editing and cancer modeling Cell, 159, 440–455
Yang, C.Z., Li, H.L., Zhou, Y., Chai, R.C., Zhao, R., Dong, Y., Xu, Z.Y., Lau, L.T., Yingge, Z., Teng, J., Chen, J. and Yu, A.C.H. (2011) A new specialization in astrocytes: glutamate- and ammonia-induced nuclear size changes J, Neurosci. Res., 89, 2041–2051
Neuroblastoma/glioblastoma cells
Burbulis, I.E., Wierman, M.B., Wolpert, M., Haakenson, M., Lopes, M-B., Schiff, D., Hicks, J., Loe, J. et al (2018) Improved molecular karyotyping in glioblastoma Mutat. Res. Fund. Mol. Mech. Mutagen., 811, 16–26
Kleene, R., Mzoughi, M., Joshi, G., Kalus, I., Bormann, U., Schulze, C., Xiao, M-F., Dityatev, A. and Schachner, M. (2010) NCAM-induced neurite outgrowth depends on binding of calmodulin to NCAM and on nuclear import of NCAM and fak fragments J. Neurosci., 30, 10784 –10798
Lutz, D., Wolters-Eisfeld, G., Joshi, G., Djogo, N., Jakovcevski, I., Schachner, M. and Kleene, R. (2012) Generation and nuclear translocation of sumoylated transmembrane fragment of cell adhesion molecule L1 J. Biol. Chem., 287, 17161–17175
Placenta
He, D., Meloche, C.A., Dumas, N.A., Frost, A.R. and Falany, C.N. (2004) Different subcellular localization of sulphotransferase 2B1b in human placenta and prostate Biochem. J., 379, 533-540
Plant tissues and cells
Bedell, J.A., Budiman, M.A., Nunberg, A., Citek, R.W., Robbins, D., Jones, J., Flick, E., Rohlfing, T., Fries, J., Bradford, K. et al (2005) Sorghum genome sequencing by methylation filtration PLoS Biol 3: e13
Dahan, J., Pichereaux, C., Rossignol, M., Blanc, S., Wendehenne, D., Pugin, A. and Bourque, S. (2009) Activation of a nuclear-localized SIPK in tobacco cells challenged by cryptogein, an elicitor of plant defence reactions Biochem. J., 418, 191–200
Ford, T.C., Baldwin, J.P. and Lambert, S.J. (1998) Rapid enzyme-free preparation of starch-free nuclei from plants facilitates studies of chromatin structure. Plant proteins in abiotic stress responses Plant Protein Club, 1998 Annual Symposium, University of York, p24
Lannoo, N., Peumans, W.J., Van Pamel, E., Alvarez, R., Xiong, T-C., Hause, G., Mazars, C. and Van Damme, E.J.M. (2006) Localization and in vitro binding studies suggest that the cytoplasmic/nuclear tobacco lectin can interact in situ with high-mannose and complex N-glyc FEBS Lett., 580, 6329-6337
Liu, Z., Zhu, Y., Gao, J., Yu, F., Dong, A. and Shen, W-H. (2009) Molecular and reverse genetic characterization of nucleosome assembly protein1 (NAP1) genes unravels their function in transcription and nucleotide excision repair in Arabidopsis thaliana Plant J., 59, 27–38
Mazars, C., Bourque, S., Mithöfer, A., Pugin, A. and Ranjeva, R. (2009) Calcium homeostasis in plant cell nuclei New Phytologist, 181, 261-274
Regulski, M., Lu, Z., Kendall, J., Donoghue, M.T.A., Reinders, J., Llaca, V., Deschamps, S., Smith, A., Levy, D., McCombie, W.R. et al (2013) The maize methylome influences mRNA splice sites and reveals widespread paramutation-like switches guided by small RNA Genome Res., 23, 1651-1662
Schouppe, D., Ghesquière, B., Menschaert, G., De Vos, W.H., Bourque, S., Trooskens, G., Proost, P., Gevaert, K. and Van Damme, E.J.M. (2011) Interaction of the tobacco lectin with histone proteins Plant Physiol., 155, 1091–1102
Testard, A., Da Silva, D., Ormancey, M., Pichereaux, C., Pouzet, C., Jauneau, A., Grat, S. et al (2016) Calcium- and nitric oxide-dependent nuclear accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase in response to long chain bases in tobacco BY-2 cells Plant Cell. Physiol., 57, 2221–2231
Timko, M.P., Rushton, P.J., Laudeman, T.W., Bokowiec, M.T., Chipumuro, E., Cheung, F., Town, C.D. and Chen, X. (2008) Sequencing and analysis of the gene-rich space of cowpea BMC Genomics, 9:103
Xiong, T.C., Jauneau, A., Ranjeva, R. and Mazars, C. (2004) Isolated plant nuclei as mechanical and thermal sensors involved in calcium signaling Plant J., 40, 12-21
Rat1 cells
Hurwitz, S.N., Nkosi, D., Conlon, M.M., York, S.B., Liu, X., Tremblay, D.C. and Meckes, Jr. D.G. (2017) CD63 regulates Epstein-Barr virus LMP1 exosomal packaging, enhancement of vesicle production, and noncanonical NF-κB signaling J. Virol., 91: e02251-16
Retinal ganglion cells
Joyal, J-S., Nim, S., Zhu, T., Sitaras, N. et al (2014) Subcellular localization of coagulation factor II receptorlike 1 in neurons governs angiogenesis Nature Med., 20, 1165-1173
Retinal pigment epithelial cells
Hyvönen, Z., Hämäläinen, V., Ruponen, M., Lucas, B., Rejman, J., Vercauteren, D., Demeester, J., De Smedt, S. and Braeckmans, K. (2012) Elucidating the pre- and post-nuclear intracellular processing of 1,4- dihydropyridine based gene delivery carriers J. Control. Release, 162, 167–175
Shark tissues
Roa, J.N. and Tresguerres, M. (2017) Bicarbonate-sensing soluble adenylyl cyclase is present in the cell
cytoplasm and nucleus of multiple shark tissues Physiol. Rep., 5: e13090
Smooth muscle cells
Lucero, H.A., Kintsurashvili, E., Marketou, M.E. and Gavras, H. (2010) Cell signaling, internalization, and nuclear localization of the angiotensin converting enzyme in smooth muscle and endothelial cells J. Biol. Chem., 285, 5555-5568
Spleen
Chen, K.G., Valencia, J.C., Lai, B., Zhang, G., Paterson, J.K., Rouzard, F., Berens, W., Wincovitch, S.M., et al (2006) Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas Proc. Natl. Acad. Sci. USA, 103, 9903-9907
Yamamoto, Y., Jones, K.A., Mak, B.C., Muehlenbachs, A. and Yeung, R.S. (2002) Multicompartmental distribution of tuberous sclerosis gene products, hamartin and tuberin Arch. Biochem. Biophys., 404, 210-217
Testis
Ookawara, T., Kizaki, T., Takayama, E., Imazeki, N., Matsubara, O., Ikeda, Y., Suzuki, K., Ji, L.L., Tadakuma, T., Taniguchi, N. and Ohno, H. (2002) Nuclear translocation of extracellular superoxide dismutase Biochem. Biophys. Res. Commun., 296, 54-61
Yamamoto, Y., Jones, K.A., Mak, B.C., Muehlenbachs, A. and Yeung, R.S. (2002) Multicompartmental distribution of tuberous sclerosis gene products, hamartin and tuberin Arch. Biochem. Biophys., 404, 210-217
Thymus
Ookawara, T., Kizaki, T., Takayama, E., Imazeki, N., Matsubara, O., Ikeda, Y., Suzuki, K., Ji, L.L., Tadakuma, T., Taniguchi, N. and Ohno, H. (2002) Nuclear translocation of extracellular superoxide dismutase Biochem. Biophys. Res. Commun., 296, 54-61
Thyroid cancer tissue
Corces, M.R., Trevino, A.E., Hamilton, E.G., Greenside, P.G., Sinnott-Armstrong, N.A., Vesuna, S., Satpathy, A.T., Rubin, A.J., Montine, K.S. et al (2017) An improved ATAC -seq protocol reduces background and enables interrogation of frozen tissues Nat. Meth., 14, 959-962
Vero cells
Botting, C., Lu, X. and Triezenberg, S.J. (2016) H2AX phosphorylation and DNA damage kinase activity are dispensable for herpes simplex virus replication Virol. J., 13: 15
Xenopus
Amin, N.M., Greco, T.M., Kuchenbrod, L.M., Rigney, M.M., Chung, M-I., Wallingford, J.B., Cristea, I.M. and Conlon, F.L. (2014) Proteomic profiling of cardiac tissue by isolation of nuclei tagged in specific cell types (INTACT) Development, 141, 962-973
OptiPrepTM Reference List RS01 8th edition, January 2020
OptiPrep Reference List RS02
Purification and analysis of peroxisomes
There are several OptiPrep™ Application Sheets that are relevant to the isolation and analysis of peroxisomes using iodixanol gradients and three types of gradient have been used:
Continuous iodixanol gradients: principally for the purification of peroxisomes from mammalian liver, but they have also been used for organelles from fungi, mainly yeast and also from plants.
Discontinuous iodixanol gradients: for mammalian liver and kidney, some marine organisms and plants.
Self-generated iodixanol gradients: for mammalian liver and cultured cells
- Application Sheet S11 describes the use of a pre-formed continuous iodixanol gradient
- Application Sheet S12 describes the use of a discontinuous iodixanol gradient
- Application Sheet S13 describes the use of a self-generated iodixanol gradient
- Application Sheet S57 describes the use of a continuous iodixanol gradient for yeast peroxisomes
In addition there are two Application Sheets that are devoted to the analysis of the light mitochondrial fraction (LMF) and although these are not devoted specifically to peroxisomes, the latter are analyzed as part of a more general analysis of the LMF organelles, which include
mitochondria, lysosomes and sometimes Golgi in addition to peroxisomes.
- Application Sheet S15 describes the use of pre-formed continuous gradient
- Application Sheet S16 describes the use of self-generated gradient
The bibliography below is divided into gradient type, then tissue or cell source. References are listed alphabetically according to first author and then, if required, chronologically. To aid identification of research topics, these are highlighted in blue.
1. Continuous gradients
1a. Brain (rodent)
Nawrotzki, R., Islinger, M., Vogel, I., Völkl, A. and Kirsch, J. (2012) Expression and subcellular distribution of gephyrin in non-neuronal tissues and cells Histochem. Cell. Biol., 137, 471–482
1b. Fat pad (mammary)
Vapola, M.H., Rokka, A., Sormunen, R.T., Alhonen, L., Schmitz W., Conzelmann, E., Wärri, A., Grunau, S., Antonenkov, V.D. and Hiltunen, J.K. (2014) Peroxisomal membrane channel Pxmp2 in the mammary fat pad is essential for stromal lipid homeostasis and for development of mammary gland epithelium in mice Dev. Biol., 391, 66–80
1c. Fibroblasts
Wiesinger, C., Kunze, M., Regelsberger, G., Forss-Petter, S. and Berger, J. (2013) Impaired very long-chain Acyl-CoA β-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dyfunction J. Biol. Chem., 288, 19269-19279
1d. Fungi
1d-1 Paracoccidioides brasiliensis
Brito, W.deA., Rezende, T.C.V., Parente, A.F., Ricart, C.A.O., de Sousa, M.V., Báo, N. and Soares, C.M.deA. (2011) Identification, characterization and regulation studies of the aconitase of Paracoccidioides brasiliensis Fungal Biol., 115, 697-707
1d-2 Yeast
Antonenkov, V.D., Mindthoff, S., Grunau, S., Erdmann, R. and Hiltunen, J.K. (2009) An involvement of yeast peroxisomal channels in transmembrane transfer of glyoxylate cycle intermediates Int., J. Biochem. Cell Biol., 41, 2546–2554
Cramer, J., Effelsberg, D., Girzalsky, W. and Erdmann, R. (2015) Isolation of peroxisomes from yeast Cold Spring Harb. Protoc; doi:10.1101/pdb.top074500
Cramer, J., Effelsberg, D., Girzalsky, W. and Erdmann, R. (2015) Small-scale purification of peroxisomes for analytical applications Cold Spring Harb. Protoc; doi:10.1101/pdb.prot083717
Debelyy, M.O., Platta, H.W., Saffian, D., Hensel, A., Thoms, S., Meyer, H.E., Warscheid, B., Girzalsky, W. and Erdmann, R. (2011) Ubp15p, a ubiquitin hydrolase associated with the peroxisomal export machinery J. Biol. Chem., 286, 28223–28234
Effelsberg, D., Cruz-Zaragoza, L.D, Tonillo, J., Schliebs, W. and Erdmann, R. (2015) Role of Pex21p for piggyback import of Gpd1p and Pnc1p into peroxisomes of Saccharomyces cerevisiae J. Biol. Chem., 290, 25333–25342
Effelsberg, D., Cruz-Zaragoza, L.D., Schliebs, W. and Erdmann, R. (2016) Pex9p is a new yeast peroxisomal import receptor for PTS1-containing proteins J. Cell Sci., 129, 4057-4066
Einwachter, H., Sowinski, S., Kunau, W-H. and Schliebs, W. (2001) Yarrowia lipolytica Pex20p, Saccharomyces cerevisiae Pex18p/Pex 21p and mammalian Pex5pL fulfil a common function in the early steps of the peroxisomal PTS2 import pathway EMBO Rep., 2, 1035-1039
Grunau, S., Mindthoff, S., Rottensteiner, H., Sormunen, R.T., Hiltunen, J.K., Erdmann, R. and Antonenkov, V.D. (2009) Channel-forming activities of peroxisomal membrane proteins from the yeast Saccharomyces cerevisiae FEBS J., 276, 1698–1708
Grunau, S., Lay, D., Mindthoff, S., Platta, H.W., Girzalsky, W., Just, W.W. and Erdmann, R. (2011) The phosphoinositide 3-kinase Vps34p is required for pexophagy in Saccharomyces cerevisiae Biochem. J. 434, 161–170
Kerssen, D., Hambruch, E., Klaas, W., Platta, H.W., de Kruijff, B., Erdmann, R., Kunau, W-H. and Schleibs, W. Membrane association of the cycling peroxisome import receptor Pex5p J. Biol. Chem., 281, 27003-27015
Mindthoff, S., Grunau, S., Steinfort, L.L., Girzalsky, W., Hiltunen, J.K., Erdmann, R. and Antonenkov, V.D. (2016) Peroxisomal Pex11 is a pore-forming protein homologous to TRPM channels Biochim. Biophys. Acta, 1863, 271–283
Oeljeklaus, S., Reinartz, B.S., Wolf, J., Wiese, S., Tonillo, J., Podwojski, K., Kuhlmann, K., Stephan, C. et al (2012) Identification of core components and transient interactors of the peroxisomal importomer by dual-track stable isotope labeling with amino acids in cell culture analysis J. Proteome Res. 2012, 11, 2567−2580
Platta, H.W., Grunau, S., Rosenkrantz, K., Girzalsky, W. and Erdmann, R. (2005) Functional role of the AAA peroxins in dislocation of the cycling PTS1 receptor back to the cytosol Nat. Cell Biol., 7, 817-822
Schäfer, A., Kerssen, D., Veenhuis, M., Kunau, W-H. and Schliebs, W. (2004) Functional similarity between the peroxisomal PTS2 receptor binding protein Pex18p and the N-terminal half of the PTS1 receptor Pex5p Mol. Cell Biol., 24, 8895-8906
Thoms, S., Debelyy, M.O., Nau, K., Meyer, H.E. and Erdmann, R. (2008) Lpx1p is a peroxisomal lipase required for normal peroxisome morphology FEBS J., 275, 504-514
Welker, S., Rudolph, B., Frenzel, E., Hagn, F., Liebisch, G., Schmitz, G., Scheuring, J., Kerth, A., Blume, A. et al (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function Mol. Cell, 39, 507–520
1e HEK cells
Okumoto, K., Ono, T., Toyama, R., Shimomura, A., Nagata, A. and Fujiki, Y. (2018) New splicing variants of mitochondrial Rho GTPase-1 (Miro1) transport peroxisomes J. Cell Biol., 217, 619–633
1f HeLa cells
Abe, S., Nagai, T., Masukawa, M., Okumoto, K., Homma, Y., Fujiki, Y. and Mizuno, K. (2017) Localization of protein kinase NDR2 to peroxisomes and its role in ciliogenesis J. Biol. Chem., 292, 4089–4098
Hosoi, K-i., Miyata, N., Mukai, S., Furuki, S., Okumoto, K., Cheng, E.H. and Fujiki, Y. (2017) The VDAC2–BAK axis regulates peroxisomal membrane permeability J. Cell Biol., 216, 709–721
Riccio, V., Demers, N., Hua, R., Vissa, M., Cheng, D.T., Strilchuk, AW., Wang, Y., McQuibban, G.A. and Kim, P.K. (2019) Deubiquitinating enzyme USP30 maintains basal peroxisome abundance by regulating pexophagy J. Cell Biol., 218, 798–807
1g. Liver (rodent)
Antonenkov, V.D., Sormunen, R.T. and Hiltunen, J.K. (2004) The behavior of peroxisomes in vitro: mammalian peroxisomes are osmotically sensitive particles Am. J. Physiol., 287, C1623-C16350
Antonenkov, V.D., Rokka, A., Sormunen, R.T., Benz, R. and Hiltunen, J.K. (2005) Solute traffic across mammalian peroxisomal membrane – single-channel conductance monitoring reveals pore-forming activities in peroxisomes Cell. Mol. Life Sci., 62, 2886-2895
Antonenkov, V.D., Sormunen, R.T., Ohlmeier, S., Amery, L., Fransen, M., Mannaerts, G.P. and Hiltunen, J.K. (2006) Localization of a portion of the liver isoform of fatty-acid-binding protein (L-FABP) to peroxisomes Biochem. J., 394, 475-484
Antonenkov, V.D., Ohlmeier, S., Sormunen, R.T. and Hiltunen, J.K. (2007) UK114, a YjgF/Yer057p/UK114 family protein highly conserved from bacteria to mammals, is localized in rat liver peroxisomes Biochem. Biophys. Res. Commun., 357, 252-257
Costello, J.L., Castro, I.G., Camões, F., Schrader, T.A., McNeall, D., Yang, J., Giannopoulou, E-A., Gomes, S., Pogenberg, V. et al (2017) Predicting the targeting of tail-anchored proteins to subcellular compartments in mammalian cells J. Cell Sci., 130, 1675-1687
Gijsbers, S., Van der Hoeven, G. and Van Veldhoven, P.P. (2001) Subcellular study of sphingoid base phosphorylation in rat tissues: evidence for multiple sphingosine kinases Biochim. Biophys. Acta, 1532, 37-50
Graham, J., Ford, T. and Rickwood, D (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
Islinger, M., Lüers, G.H., Zischka, H., Ueffing, M. and Völkl, A. (2006) Insights into the membrane proteome of rat liver peroxisomes: Microsomal glutathione-S-transferase is shared by both subcellular compartments Proteomics, 6, 804-816
Islinger, M. and Weber, G. (2008) Free flow isoelectric focusing: a method for the separation of both hydrophilic and hydrophobic proteins of rat liver peroxisomes In Methods Mol. Biol., 432, Organelle Proteomics (ed. Pflieger, D, and Rossier, J.) Humana Press, Totowa, NJ, pp 199-215
Islinger, M., Li, K.W., Seitz, J., Völkl, A. and Lüers, G.H. (2009) Hitchhiking of Cu/Zn superoxide dismutase to peroxisomes – evidence for a natural piggyback import mechanism in mammals Traffic, 10, 1711–1721
Islinger, M., Li, K.W., Loos, M., Liebler, S., Angermüller, S., Eckerskorn, C., Weber, G., Abdolzade, A. and Völkl, A. (2010) Peroxisomes from the heavy mitochondrial fraction: isolation by zonal free flow electrophoresis and quantitative mass spectrometrical characterization J. Proteome Res., 9, 113–124
Islinger, M., Abdolzade-Bavil, A., Liebler, S., Weber, G. and Völkl, A. (2012) Assessing heterogeneity of peroxisomes: isolation of two subpopulations from rat liver In Liver Proteomics: Methods and Protocols, Methods Mol. Biol., 909 (eds Josic, D. and Hixson, D.C.) Springer Science+Business Media, New York 2012
Joly, E., Bendyan, M., Roduit, R., Saha, A.K., Ruderman, N.B. and Prentki, M. (2005) Malonyl-CoA decarboxylase is present in the cytosolic, mitochondrial and peroxisomal compartments of rat hepatocytes FEBS Lett., 579, 6581-6586
Kerr, E.W., Shumar, S.A. and Leonardi, R. (2019) Nudt8 is a novel CoA diphosphohydrolase that resides in the mitochondria FEBS Lett., 593, 1133–1143
Koch, A., Thiemann, M., Grabenbauer, M., Yoon, Y., McNiven, M.A. and Schrader, M. (2003) Dynamin-like protein 1 is involved in peroxisomal fission J. Biol. Chem., 278, 8597-8605
Koch, A., Yoon, Y., Bonekamp, N.A., McNiven, M.A. and Schrader, M. (2005) A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells Mol. Biol. Cell, 16, 5077-5086
Lewin T.M., Van Horn, C.G., Krisans, S.K. and Coelman, R.A. (2002) Rat liver acyl-CoA synthetase 4 is a peripheral-membrane protein located in two distinct subcellular organelles, peroxisomes and mitochondrialassociated membranes Arch. Biochem. Biophys., 404, 263-270
Manner, A. and Islinger, M. (2017) Isolation of peroxisomes from rat liver and cultured hepatoma cells by density gradient centrifugation In Peroxisomes: Methods and Protocols: Methods Mol. Biol., 1595 (ed. Schrader, M.), Springer Science+Business Media LLC, pp 1-11
Mustacich, D.J., Leonard, S.W., Patel N.K. and Traber, M.G. (2010) α-Tocopherol β-oxidation localized to rat liver mitochondria Free Radic. Biol. Med., 48, 73–81
Nawrotzki, R., Islinger, M., Vogel, I., Völkl, A. and Kirsch, J. (2012) Expression and subcellular distribution of gephyrin in non-neuronal tissues and cells Histochem. Cell. Biol., 137, 471–482
Priore, P., Giudetti, A.M., Natali, F., Gnoni, G.V. and Geelen, M.J.H. (2007) Metabolism and short-term metabolic effects of conjugated linoleic acids in rat hepatocytes Biochim. Biophys. Acta, 1771, 1299-1307
Van Veldhoven, P.P., Baumgart, E. and Mannaerts, G.P. (1996) Iodixanol (OptiPrep), an improved density gradient medium for the iso-osmotic isolation of rat liver peroxisomes Anal. Biochem. 237, 17-23
Villalobos-García, D. and Hernández-Muñoza, R. (2019) Lactate-stimulated ethanol oxidation: Revisiting an old hypothesis Biochem. Pharmacol., 164, 283–288
Westin, M.A.K., Hunt, M.C. and Alexson, S.E.H. (2007) Peroxisomes contain a specific phytanoylCoA/Pristanoyl-CoA thioesterase acting as a novel auxiliary enzyme in α- and β-oxidation of methyl-branched fatty acids in mouse J. Biol. Chem., 282, 26707-26716
Zhang, D., Yu, W., Geisbrecht, B.V., Gould, S.J., Sprecher, H. and Schulz, H. (2002) Functional characterization of ∆3, ∆2-enoyl-CoA isomerase from rat liver J. Biol. Chem., 277, 9127-9132
1h. Mammary fat pad
Vapola, M.H., Rokka, A., Sormunen, R.T., Alhonen, L., Schmitz W., Conzelmann, E., Wärri, A., Grunau, S., Antonenkov, V.D. and Hiltunen, J.K. (2014) Peroxisomal membrane channel Pxmp2 in the mammary fat pad is essential for stromal lipid homeostasis and for development of mammary gland epithelium in mice Dev. Biol., 391, 66–80
1i. Plant tissues
Arai, Y., Hayashi, M. and Nishimura, M. (2008) Proteomic analysis of highly purified peroxisomes from etiolated Soybean cotyledons Plant Cell Physiol., 49, 526-539
Hossain, Z. and Komatsu, S. (2014) Soybean proteomics In Plant Proteomics: Methods Mol. Biol., 1072 (ed. Jorrin-Novo, J.V. et al), Springer Science+Business Media, LLC, pp 315-331
Komatsu, S. and Ahsan, N. (2009) Soybean proteomics and its application to functional analysis J. Proteom., 72, 325-336
Palma, J.M., Corpas, F.J. and del Rio, L.A. (2009) Proteome of plant peroxisomes: new perspectives on the role of these organelles in cell biology Proteomics, 9, 2301-2312
Reumann, S. (2011) Toward a definition of the complete proteome of plant peroxisomes: Where experimental proteomics must be complemented by bioinformatics Proteomics 11, 1764–1779
1j. Retinal pigment cells
Abe, S., Nagai, T., Masukawa, M., Okumoto, K., Homma, Y., Fujiki, Y. and Mizuno, K. (2017) Localization of protein kinase NDR2 to peroxisomes and its role in ciliogenesis J. Biol. Chem., 292, 4089–4098
1k. Review
Antonenkov, V.D. and Hiltunen, J.K. (2006) Peroxisomal membrane permeability and solute transfer Biochim. Biophys. Acta, Mol. Cell Res., 1763, 1697-1706
2. Discontinuous gradients
2a. HEK cells
Ge, L., Melville, D., Zhang, M. and Schekman, R. (2013) The ER–Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis eLife, 2: e00947
Zhang, J., Kim, J., Alexander, A., Cai, S., Tripathi, D.N., Dere, R., Tee, A.R., Tait-Mulder, J., Di Nardo, A., Han, J.M., Kwiatkowski, E., Dunlop, E.A., Dodd, K.M., Folkerth, R.D., Faust, P.L., Kastan, M.B., Sahin, M. and Walker, C.L. (2013) A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS Nat. Cell Biol., 15, 1186-1196
2b. HeLa cells
Luo, J., Liao, Y-C., Xiao, J. and Song, B-L. (2017) Measurement of cholesterol transfer from lysosome to peroxisome using an in vitro reconstitution assay In Cholesterol Homeostasis; Methods and Protocols: Methods Mol. Biol., 1583 (ed. Gelissen, I.C. and Brown, A.J.), Springer Science+Business Media LLC, pp 141-161
Xiao, J., Luo, J., Hu, A., Xiao, T., Li, M., Kong, Z., Jiang, L., Zhou, Z., Liao, Y. et al (2019) Cholesterol transport through the peroxisome-ER membrane contacts tethered by PI(4,5)P2 and extended synapto-tagmins Sci. China Life. Sci., 62, 1117-1135
2c. Hep-G2 cells
Chen, X-F., Tian, M-X., Sun, R-Q., Zhang, M-L., Zhou, L-S., Jin, L., Chen, L-L., Zhou, W-J. et al (2018) SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is down-regulated in liver cancer EMBO Rep., 19: e45124
Wang, W., Xia, Z-J., Farré, J-C. and Subramani, S. (2017) TRIM37, a novel E3 ligase for PEX5-mediated peroxisomal matrix protein import J. Cell Biol., 216, 2843–2858
2d. Human fibroblasts
Beltran, P.M.J., Mathias, R.A. and Cristea, I.M. (2016) A portrait of the human organelle proteome in space and time during cytomegalovirus infection Cell Systems 3, 361–373
2e. Kidney (rodent)
Mi, J., Garcia-Arcos, I., Alvarez, R., and Cristobal, S. (2007) Age-related subproteomic analysis of mouse liver and kidney peroxisomes Proteome Sci., 5:19
Mi, J., Kirchner, E. and Cristobal, S. (2007) Quantitative proteomic comparison of mouse peroxisomes from liver and kidney Proteomics, 7, 1916-1928
2f. Liver (chick embryo)
Labitzke, E.M., Diani-Moore, S. and Rifkind, A.B. (2007) Mitochondrial P450-dependent arachidonic acid metabolism by TCDD-induced hepatic CYP1A5; conversion of EETs to DHETs by mitochondrial soluble epoxide hydrolase Arch. Biochem. Biophys., 468, 70-81
2g. Liver (rodent)
Amelina, H., Sjödin, M.O.D., Bergquist, J. and Cristobal, S. (2011) Quantitative subproteomic analysis of agerelated changes in mouse liver peroxisomes by iTRAQ LC -MS/MS J.Chromatogr. B, 879, 3393– 3400
Grant, P., Ahlemeyer, B., Karnati, S., Berg, T., Stelzig, I., Nenicu, A., Kuchelmeister, K., Crane, D.I. and Baumgart-Vogt, E. (2013) The biogenesis protein PEX14 is an optimal marker for the identification and localization of peroxisomes in different cell types, tissues, and species in morphological studies Histochem. Cell. Biol., 140, 423–442
Karnati, S., Lüers, G., Pfreimer, S. and Baumgart-Vogt, E. (2013) Mammalian SOD2 is exclusively located in mitochondria and not present in peroxisomes Histochem. Cell Biol., 140, 105–117
Lamhonwah, A-M., Skaug, J., Scherer, S. and Tein, I. (2003) A third human carnitine/organic cation transporter (OCTN3) as a candidate for the 5q31 Crohn’s disease locus (IBD5) Biochem. Biophys. Res. Commun., 301, 98-101
Mi, J., Garcia-Arcos, I., Alvarez, R., and Cristobal, S. (2007) Age-related subproteomic analysis of mouse liver and kidney peroxisomes Proteome Sci., 5:19
Mi, J., Kirchner, E. and Cristobal, S. (2007) Quantitative proteomic comparison of mouse peroxisomes from liver and kidney Proteomics, 7, 1916-1928
Salvi, M., Battaglia, V., Brunati, A.M., La Rocca, N., Tibaldi, E., Pietrangeli, P., Marcocci, L., Mondovi, B., Rossi, C.A. and Toninello, A. (2007) Catalase takes part in rat liver mitochondria oxidative stress defense J. Biol. Chem., 282, 24407-24415
Spinazzola, A., Viscomi, C., Fernandez-Vizarra, E., Carrara, F., D’Adamo, P., Calvo, S., Marsano, R.M., Donnini, C., Weiher, H., Strisciuglio, P., Parini, R., Sarzi, E., Chan, A., DiMauro, S., Rotig, A., Gasparini, P., Ferrero, I., Mootha, V.K., Tiranti, V. and Zeviani, M. (2006) MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion Nat. Genet., 38, 570-575
Weng, H., Ji, X., Naito, Y., Endo, K., Ma, X., Takahashi, R., Shen, C., Hirokawa, G., Fukushima, Y. and Iwai, N. (2013) Pex11α deficiency impairs peroxisome elongation and division and contributes to nonalcoholic fatty liver in mice Am. J. Physiol. Endocrinol. Metab., 304, E187–E196
2h. Mouse embryo fibroblasts
Zhang, J., Kim, J., Alexander, A., Cai, S., Tripathi, D.N., Dere, R., Tee, A.R., Tait-Mulder, J., Di Nardo, A., Han, J.M., Kwiatkowski, E., Dunlop, E.A., Dodd, K.M., Folkerth, R.D., Faust, P.L., Kastan, M.B., Sahin, M. and Walker, C.L. (2013) A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS Nat. Cell Biol., 15, 1186-1196
2i. Mussels
Apraiz, I., Mi, J. and Cristobal, S. (2006) Identification of proteomic signatures of exposure to marine pollutants in mussels (Mytilus edulis) Mol. Cell. Proteom., 5, 1274-1285
Apraiz, I., Cajaraville, M.P. and Cristobal, S. (2009) Peroxisomal proteomics: Biomonitoring in mussels after the Prestige’s oil spill Mar. Pollut. Bull., 58, 1815–1826
Cristobal, S. (2007) Proteomics-based method for risk assessment of peroxisome proliferating pollutants in the marine environment Methods Mol. Biol., 410, 123-135
Mi, J., Orbea, A., Syme, N., Ahmed, M., Cajaraville, M.P. and Cristobal, S. (2005) Peroxisomal proteomics, a new tool for risk assessment of peroxisome proliferating pollutants in the marine environment Proteomics, 5, 3954-2965
2j. Yeast
Nyathi, Y., De Marcos Lousa, C., van Roermund, C.W., Wanders, R.J.A., Johnson, B., Baldwin, S.A., Theodoulou, F.L. and Baker, A. (2010) The Arabidopsis peroxisomal ABC transporter, Comatose, complements the Saccharomyces cerevisiae pxa1 pxa2∆ mutant for metabolism of long-chain fatty acids and exhibits fatty
acyl-CoA-stimulated ATPase activity J. Biol., Chem., 285, 29892–29902
Nyathi, Y., Zhang, X., Baldwin, J.M., Bernhardt, K., Johnson, B., Baldwin, S.A., Theodoulou, F.L. and Baker, A. (2012) Pseudo half-molecules of the ABC transporter, COMATOSE, bind Pex19 and target to peroxisomes independently but are both required for activity FEBS Lett., 586, 2280–2286
3. Self-generated gradient
3a. CHO cells
Honsho, M., Yagita, Y., Kinoshita, N. and Fujuki, Y. (2008) Isolation and characterization of mutant animal cell line defective in alkyl-dihydroxyacetonephosphate synthase: Localization and transport of plasmalogens to post-Golgi compartments Biochim. Biophys. Acta, 1783, 1857-1865
Kobayashi, S., Tanaka, A. and Fujiki, Y. (2007) Fis1, DLP1 and Pex11p coordinately regulate peroxisome morphogenesis Exp. Cell Res., 313, 1675-1686
Matsuzaki, T. and Fujiki, Y. (2008) The peroxisomal membrane protein import receptor Pex3p is directly transported to peroxisomes by a novel Pex19p- and Pex16p-dependent pathway J. Cell Biol. 183, 1275–1286
3b. Hep-G2 cells
Morel, F., Rauch, C., Petit, E., Piton, A., Theret, N., Coles, B. and Guillouzo, A. (2004) Gene and protein characterization of the human glutathione S-transferase kappa and evidence for a peroxisomal localization J. Biol. Chem., 279, 16246-16253
3c. Liver (rodent)
Costello, J.L., Castro, I.G., Camões, F., Schrader, T.A., McNeall, D., Yang, J., Giannopoulou, E-A., Gomes, S., Pogenberg, V. et al (2017) Predicting the targeting of tail-anchored proteins to subcellular compartments in mammalian cells J. Cell Sci., 130, 1675-1687
He, D., Barnes, S. and Falany, C.N. (2003) Rat liver bile acid CoA:amino acid N-acyltransferase: expression, characterization, and peroxisomal localization J. Lipid Res., 44, 2242-2249
Graham, J., Ford, T. and Rickwood, D (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
Kurochkin, I.V., Mizuno, Y., Konagaya, A., Sakaki, Y., Schonbach, C. and Okazaki, Y. (2007) Novel peroxisomal protease Tysnd1 processes PTS1- and PTS2-containing enzymes involved in β-oxidation of fatty acids EMBO J., 26, 835-845
McClelland, G.B., Khanna, S., Gonzales, G.F., Butz, C.E. and Brooks, G.A. (2003) Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system Biochem. Biophys. Res. Commun., 304, 130-135
Miyata, N., Hosoi, K-i., Mukai, S. and Fujiki, Y. (2009) In vitro import of peroxisome-targeting signal type 2 (PTS2) receptor Pex7p into peroxisomes Biochim. Biophys. Acta, 1793, 860–870
Styles, N.A., Falany, J.L., Barnes, S. and Falany, C.N. (2007) Quantification and regulation of the subcellular distribution of bile acid coenzyme A:amino acid N-acyltransferase activity in rat liver J. Lipid Res., 48, 1305-1315
Yarmishyn, A.A., Kremenskoy, M., Batagov, A.O., Preuss, A., Wong, J.J. and Kurochkin, I.V. (2016) Genome-wide analysis of mRNAs associated with mouse peroxisomes BMC Genomics, 17 (Suppl 13): 1028
OptiPrepTM Reference List RS02; 4th edition, January 2020
OptiPrep Reference List RS03
Purification of mitochondria from mammalian sources
- This Reference List provides a complete list of publications reporting the use of OptiPrep™ for the isolation of mitochondria: the references are sorted into sections according cell or tissue type. Within each section references are listed alphabetically according to first author.
- Key words in the article titles are highlighted in light blue
- For yeast mitochondria see Reference List RS15 “Purification of subcelluar organelles and membrane compartments from Saccharomyces cerevisiae – a bibliography”
- Papers covering the purification of organelles from non-mammalian eukaryotes are listed in Reference List RS14
Reference List RS03 is divided into:
Section A papers: Analysis primarily of mitochondria, often with additional information on the banding of other organelles (e.g. lysosomes, peroxisomes and ER) from cultured cells (A1) and from tissues (A2)
Section B papers: analysis of mitochondrial interactions and association with the endoplasmic reticulum
SECTION A
A1 Mammalian cultured cells
Adenocarcinoma cells
Paterson, J.K. and Gottesman, M.M. (2007) P-Glycoprotein is not present in mitochondrial membranes Exp. Cell Res., 313, 3100-3105
Shen, S-M., Guo, M., Xiong, Z., Yu, Y., Zhao, X-Y., Zhang, F-F. and Chen, G-Q. (2015) AIF inhibits tumor
metastasis by protecting PTEN from oxidation EMBO Rep., 16, 1563–1580
Adipocytes
Den Hartigh, L.J., Han, C.Y., Wang, S., Omer, M. and Chait, A. (2013) 10E,12Z-conjugated linoleic acid impairs adipocyte triglyceride storage by enhancing fatty acid oxidation, lipolysis, and mitochondrial reactive oxygen species J. Lipid Res., 54, 2964–2978
Adrenoleukodystrophy fibroblasts
Wiesinger, C., Kunze, M., Regelsberger, G., Forss-Petter, S. and Berger, J. (2013) Impaired very long-chain Acyl-CoA β-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dyfunction J. Biol. Chem., 288, 19269-19279
C3HA mouse lymphoid cells
Maia, R.C., Culver, C.A. and Laster, S.M. (2006) Evidence against calcium as a mediator of mitochondrial dysfunction during apoptosis induced by arachidonic acid and other free fatty acids J. Immunol., 177, 6398-6404
Caco-2 cells
Bielaszewska, M., Ruter, C., Kunsmann, L., Greune, L., Bauwens, A., Zhang, W., Kuczius, T., Kim, K.S., Mellmann, A., Schmidt, M.A. and Karch, H. (2013) Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis PloS Pathog., 9: e1003797
Carcinoma cells (see also “Colon carcinoma/colorectal cells”)
Chattopadhyay, S., Mukherjee, A., Patra, U., Bhowmick, R., Basak, T., Sengupta, S., Chawla-Sarkar, M. (2017) Tyrosine phosphorylation modulates mitochondrial chaperonin Hsp60 and delays rotavirus NSP4-mediated apoptotic signaling in host cells Cell. Microbiol., 19: e12670
Maeda, H., Nagata, S., Wolfgang, C.D., Bratthauer, C.D., Bera, T.K. and Pastan, I. (2004) The T cell receptor γ chain alternate reading frame protein (TARP), a prostate-specific protein localized in mitochondria J. Biol. Chem., 279, 24561-24568
Sandin, M., Antberg, L., Levander, F. and James, P. (2015) A Breast Cell Atlas: Organelle analysis of the MDAMB-231 cell line by density-gradient fractionation using isotopic marking and label-free analysis EuPA Open Proteomics, 8, 68–77
Shen, S-M., Guo, M., Xiong, Z., Yu, Y., Zhao, X-Y., Zhang, F-F. and Chen, G-Q. (2015) AIF inhibits tumor metastasis by protecting PTEN from oxidation EMBO Rep., 16, 1563–1580
Weissleder, R., Tung, C-H., Mahmood, U. and Bogdanov, A.(1999) In vivo imaging of tumors with proteaseactivated near-infrared fluorescent probes Nature Biotech., 17, 375-378
Zhyvoloup, A., Nemazanyy, I., Panasyuk, G., Valovka, T., Fenton, T., Rebholz, H., Wang, M-L., Foxon, R., Lyzogubov, V. et al (2003) Subcellular localization and regulation of coenzyme A synthetase J. Biol. Chem., 278, 50316-50321
Cardiac myocytes
Nguyen, T., Ogbi, M. and Johnson, J.A. (2008) Delta protein kinase C Interacts with the d subunit of the F1F0 ATPase in neonatal cardiac myocytes exposed to hypoxia or phorbol ester: implications for F1F0 ATPase regulation J. Biol. Chem., 283, 29831-29840
Nguyen, T.T., Ogbi, M., Yu, Q., Fishman, J.B., Thomas, W., Harvey, B.J., Fulton, D. Johnson, J.A. (2010) Modulation of the protein kinase Cδ interaction with the “d” subunit of F1F0-ATP synthase in neonatal cardiac myocytes; development of cell-permeable, mitochondrially targeted inhibitor and facilitator peptides J. Biol. Chem., 285, 22164–22173
Nguyen, T.T., Ogbi, M., Yu, Q. and Johnson, J.A. (2010) Attenuation of the hypoxia-induced protein kinase Cδ interaction with the ‘d’ subunit of F1Fo-ATP synthase in neonatal cardiac myocytes: implications for energy preservation and survival Biochem. J., 429, 335–345
Ogbi, M. and Johnson, J.A. (2006) Protein kinase Cε interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning Biochem. J., 393, 191-199
Colon carcinoma/colorectal cells
Boohaker, R.J., Zhang, G., Carlson, A.L., Nemec, K.N. and Khaled, A.R. (2011) BAX supports the mitochondrial network, promoting bioenergetics in nonapoptotic cells Am. J. Physiol. Cell Physiol. 300, C1466–C1478
Dionne, S., Levy, E., Levesque D. and Seidman, E.G. (2010) PPARγ ligand 15-deoxy-delta 12,14-prostaglandin J2 sensitizes human colon carcinoma cells to TWEAK-induced apoptosis Anticancer Res., 30, 157-166
Margineantu, D.H., Emerson, C.B., Diaz, D. and Hockenberry, D.M. (2007) Hsp90 inhibition decreases mitochondrial protein turnover PLoS ONE, 10:e1066
Shen, S-M., Guo, M., Xiong, Z., Yu, Y., Zhao, X-Y., Zhang, F-F. and Chen, G-Q. (2015) AIF inhibits tumor metastasis by protecting PTEN from oxidation EMBO Rep., 16, 1563–1580
COS cells
Graf, S.A., Haigh, S.E., Corson, E.D. and Shirihai, O.S. (2004) Targeting, import, and dimerization of a mammalian mitochondrial ATP binding cassette (ABC) transporter, ABCB10 (ABC-me) J. Biol. Chem., 279, 42954-42963
Li, C-C., Wu, T-S., Huang, C-F., Jang, L-T., Liu, Y-T., You, S-T., Liou, G-G., Lee, F-J.S. (2012) GTP-bindingdefective ARL4D alters mitochondrial morphology and membrane potential PloS One, 7: e43552
Seyrantepe, V., Landry, K., Trudel, S., Hassan, J.A., Morales, C.R. and Pshezhetsky, A.V. (2004) Neu4, a novel human lysosomal lumen sialidase, confers normal phenotype to sialidosis and galactosialidosis cells J. Biol. Chem., 279, 37021-37029
Fibrosarcoma cells (human)
Zhao, H., Ruberu, K., Li, H. and Garner, B. (2013) Analysis of subcellular [57Co] cobalamin distribution in SHSY5Y neuronsand brain tissue J. Neurosci. Methods, 217, 67– 74
HEK cells
Bhowmick, R., Halder, U.C., Chattopadhyay, S., Chanda, S., Nandi, S., Bagchi, P., Nayak, M.K., Chakrabarti, O., Kobayashi, N. and Chawla-Sarkar, M. (2012) Rotaviral enterotoxin nonstructural protein 4 targets mitochondria for activation of apoptosis during infection J. Biol. Chem., 287, 35004–35020
Chen, Y., Feng, R., Luo, G., Guo, J., Wang, Y., Sun, Y., Zheng, L. and Wen, T. (2018) DCF1 subcellular localization and its function in mitochondria Biochimie, 144, 50-55
Choi, Y-S., Ryu, B-K., Min, H-K., Lee, S-W. and Pak, Y.K. (2005) Analysis of proteome bound to D-loop region of mitochondrial DNA by DNA-linked affinity chromatography and reverse-phase liquid chromatography/tandem mass spectrometry Ann. N.Y. Acad. Sci., 1042, 88-100
Choi, Y.B., Sandford, G. and Nicholas, J. (2012) Human herpesvirus 8 interferon regulatory factor-mediated BH3-only protein inhibition via Bid BH3-B mimicry PLoS Pathog., 8: e1002748
Chou, C-H., Lee, R-S., Yang-Yen, H-F. (2006) An internal EELD domain facilitates mitochondrial targeting of Mcl-1 via a Tom70-dependent pathway Mol. Biol. Cell, 17, 3952-3963
Gerhold, J.M., Cansiz-Arda, S., Lõhmus, M., Engberg, O., Reyes, A., van Rennes, H., Sanz, A., Holt, I.J., Cooper, H.M. and Spelbrink, J.N. (2015) Human mitochondrial DNA-protein complexes attach to a cholesterolrich membrane structure Sci. Rep., 5: 15292
Durigon, R., Mitchell, A.L., Jones, A.W.E., Manole, A., Mennuni, M., Hirst, E.M.A., Houlden, H., Maragni, G., Lattante, S. et al (2018) LETM1 couples mitochondrial DNA metabolism and nutrient preference EMBO Mol. Med., 10: e8550
Huang, J., Liu, P. and Wang, G. (2018) Regulation of mitochondrion-associated cytosolic ribosomes by mammalian mitochondrial ribonuclease T2 (RNASET2) J. Biol. Chem., 293, 19633–19644
Kobuchi, H., Moriya, K., Ogino, T., Fujita, H., Inoue, K., Shuin, T., Yasuda, T., Utsumi, K. and Utsumi, T. (2012) Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation PLoS One, 7: e50082
Landry, M-C., Champagne, C., Boulanger, M-C., Jetté, A., Fuchs, M., Dziengelewski, D. and Lavoie, J.N. (2014) A functional interplay between the small GTPase Rab11a and mitochondria-shaping proteins regulates mitochondrial positioning and polarization of the actin cytoskeleton downstream of Src family kinases J. Biol. Chem., 289, 2230–2249
Lu, M-Y. and Liao, F. (2011) Interferon-stimulated gene ISG12b2 is localized to the inner mitochondrial membrane and mediates virus-induced cell death Cell Death Differ., 18, 925–936
Luo, G., Sun, Y., Feng, R., Zhao, Q. and Wen, T. (2018) ARL3 subcellular localization and its suspected role in autophagy Biochimie, 154, 187-193
Ng, K-E., Schwarzer, S., Duchen, M.R. and Tinker, A. (2010) The intracellular localization and function of the ATP-sensitive K+ channel subunit Kir6.1 J. Membr. Biol., 234, 137–147
Rumlová, M., Křížová, I., Keprová, A., Hadravová, R., Doležal1, M., Strohalmová, K., Pichová, I., Hájek, M. and Ruml, T. (2014) HIV-1 protease-induced apoptosis Retrovirology, 11: 37
Shneyer, B.I., Ušaj, M. and Henn, A. (2016) Myo19 is an outer mitochondrial membrane motor and effector of starvation-induced filopodia J. Cell Sci., 129, 543-556
Witkowski, A., Thweatt, J. and Smith, S. (2011) Mammalian ACSF3 protein is a malonyl-CoA synthetase that supplies the chain extender units for mitochondrial fatty acid synthesis J. Biol. Chem., 286, 33729–33736
HeLa cells
Alonzo, J.R., Venkataraman, C., Field, M.S. and Stover, P.J. (2018) The mitochondrial inner membrane protein MPV17 prevents uracil accumulation in mitochondrial DNA J. Biol. Chem., 293, 20285–20294
Barth, S., Edlich, F., Berchner-Pfannschmidt, U., Gneuss, S., Jahreis, G., Hasgall, P.A., Fandrey, J., Wenger, R.H. and Camenisch, G. (2009) Hypoxia-inducible factor prolyl-4-hydroxylase PHD2 protein abundance depends on integral membrane anchoring of FKBP38 J. Biol. Chem., 284, 23046–23058
Costa, D., Costa, C., Caldeira, M., Cortes, L., Queiroz, J.A. and Cruz, C. (2017) Targeting of cellular organelles by fluorescent plasmid DNA nanoparticles Biomacromolecules, 18, 2928−2936
Huang, C-R. and Yang-Yen, H-F. (2010) The fast-mobility isoform of mouse Mcl-1 is a mitochondrial matrixlocalized protein with attenuated anti-apoptotic activity FEBS Lett., 584, 3323–3330
Liu, P., Huang, J., Zheng, Q., Xie, L., Lu, X., Jin, J. and Wang, G. (2017) Mammalian mitochondrial RNAs are degraded in the mitochondrial intermembrane space by RNASET2 Protein Cell, 8, 735–749
Tanaka, K., Sugiura, Y., Ichishita, R., Mihara, K. and Oka, T. (2011) KLP6: a newly identified kinesin that regulates the morphology and transport of mitochondria in neuronal cells J. Cell Sci., 124, 2457-2465
Hepatocytes, hepatoma cells and hepatocarcinoma cells
Beauchamp, E., Tekpli, X., Marteil, G., Lagadic-Gossmann, D., Legrand, P. and Rioux, V. (2009) NMyristoylation targets dihydroceramide D4-desaturase 1 to mitochondria: Partial involvement in the apoptotic effect of myristic acid Biochimie 91, 1411–1419
Bhattacharyya, S., Feferman, L. and Tobacman, J.K. (2016) Restriction of aerobic metabolism by acquired or innate arylsulfatase B deficiency: a new approach to the Warburg effect Sci. Rep., 6: 32885
Cheng, M-L., Chi, L-M., Wu, P-R. and Ho, H-Y. (2016) Dehydroepiandrosterone-induced changes in mitochondrial proteins contribute to phenotypic alterations in hepatoma cells Biochem.Pharmacol., 117, 20-34
Fantappi, O., Sassoli, C., Tani, A., Nosi, D., Marchetti, S., Formigli, L. and Mazzanti, R. (2015) Mitochondria of a human multidrug-resistant hepatocellular carcinoma cell line constitutively express inducible nitric oxide synthase in the inner membrane J. Cell. Mol. Med., 19, 1410-1417
Matsumoto, A., Comatas, K.E., Liu, L. and Stamler, J.S. (2003) Screening for nitric oxide-dependent proteinprotein interactions Science, 301, 657-661
Sultan, A.S., Miyoshi, E., Ihara, Y., Nishikawa, A., Tsukada, Y. and Taniguchi, N. (1997) Bisecting GlcNac structures act as negative sorting signals for cell surface glycoproteins in forskolin-treated rat hepatoma cells J. Biol. Chem., 272, 2866-2872
Tekpli, X., Rivedal, E., Gorria, M., Landvik, N.E., Rissel, M., Dimanche-Boitrel, M-T., Baffet, G., Holme, J.A. and Lagadic-Gossmann, D. (2010) The B[a]P-increased intercellular communication via translocation of connexin-43 into gap junctions reduces apoptosis Toxicol. Appl. Pharmacol., 242, 231–240
Hippocampal cells
Guan, D-F., Ren, P-Y., Hu, W. and Zhang, Y-L. (2015) The mGluR5 positive allosteric modulator CDPPB inhibits SO2-induced protein radical formation and mitochondrial dysfunction through activation of Akt in mouse hippocampal HT22 cells Cell. Mol. Neurobiol., 35, 573–583
Human breast cancer cells
Kim, H.M., Kim, C-S., Lee, J-H., Jang, S.J., Hwang, J.J., Ro, S. and Choi, J. (2013) CG0009, a novel glycogen synthase kinase 3 inhibitor, induces cell death through cyclin D1 depletion in breast cancer cells PLoS One, 8: e60383
Human fibroblasts
Beltran, P.M.J., Mathias, R.A. and Cristea, I.M. (2016) A portrait of the human organelle proteome in space and time during cytomegalovirus infection Cell Systems 3, 361–373
Mathias, R.A., Greco, T.M., Oberstein, A., Budayeva, H.G., Chakrabarti, R., Rowland, E.A., Kang, Y., Shenk, T. and Cristea, I.M. (2014) Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity Cell, 159, 1615–1625
Mathias, R.A., Greco, T.M. and Cristea, I.M. (2016) Identification of sirtuin4 (SIRT4) protein interactions: uncovering candidate acyl-modified mitochondrial substrates and enzymatic regulators In Histone Deacetylases: Methods and Protocols: Methods Mol. Biol., 1436 (ed. Sarkar, S.), Springer Science+Business Media, LLC, pp 213-239
Mathias, R.A., Greco, T.M. and Cristea, I.M. (2016) Identification of sirtuin4 (SIRT4) protein interactions: uncovering candidate acyl-modified mitochondrial substrates and enzymatic regulators In Histone Deacetylases: Methods and Protocols: Methods Mol. Biol., 1436 (ed. Sarkar, S.), Springer Science+Business Media, LLC, pp 213-239
Human glioblastoma cells
Paterson, J.K., Shukla, S., Black, C.M., Tachiwada, T., Garfield, S., Wincovitch, S., Ernst, D.N., Agadir, A., Li, X., Ambudkar, S.V., Szakacs, G., Akiyama, S-i. and Gottesman, M.M. (2007) Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane Biochemistry, 46, 9443-9452
Leukocytic/lymphoid cells/lymphocytes
Choi, Y.B. and Harhaj, E.W. (2014) HTLV-1 tax stabilizes MCL-1 via TRAF6-dependent K63-linked polyubiquitination to promote cell survival and transformation PLoS Pathog., 10: e1004458
Ebsen, H., Lettau, M., Kabelitz, D. and Janssen, O. (2015) Subcellular localization and activation of ADAM proteases in the context of FasL shedding in T lymphocytes Mol. Immunol., 65, 416–428
Hall, S.L., Hester, S., Griffin, J.L., Lilley, K.S. and Jackson, A.P. (2009) The organelle proteome of the DT40 lymphocyte cell line Mol.Cell. Proteom., 8, 1295–1305
Hwang, K.Y. and Choi, Y.B. (2016) Modulation of mitochondrial antiviral signaling by human herpesvirus 8 interferon regulatory factor 1 J. Virol., 90, 506-520
Kang, R., Tang, D., Yu, Y., Wang, Z., Hu, T., Wang, H. and Cao, L. (2010) WAVE1 regulates Bcl-2 localization and phosphorylation in leukemia cells Leukemia, 24, 177–18
Liang, P., Nair, J.R., Song, L., McGuire, J.J. and Dolnick, B.J. (2005) Comparative genomic analysis reveals a novel mitochondrial isoform of human rTS protein and unusual phylogenetic distribution of the rTS gene BMC Genomics, 6:125
Schmidt, H., Gelhaus, C., Nebendahl, M., Lettau, M., Wartzl, C., Kabelitz, D., Leippe, M. and Janssen, O. (2008) 2-D DIGE analyses of enriched secretory lysosomes reveal heterogeneous profiles of functionally relevant proteins in leukemic and activated human NK cells Proteomics, 8, 2911-2925
Schmidt, H., Gelhaus, C., Lucius, R., Nebendahl, M. Leippe, M. and Janssen, O. (2009) Enrichment and analysis of secretory lysosomes from lymphocyte populations BMC Immunol., 10:41
Schmidt, H., Gelhaus, C., Nebendahl, M., Lettau, M., Lucius, R., Leippe, M., Kabelitz, D. and Janssen, O. (2011) Effector granules in human T lymphocytes: proteomic evidence for two distinct species of cytotoxic effector vesicles J. Proteome Res., 10, 1603–1620
Smith, C.G., Kharkwal, H. and Wilson, D.W. (2017) Expression and subcellular localization of the Kaposi’s sarcoma-associated herpesvirus K15P protein during latency and lytic reactivation in primary effusion lymphoma Cells J. Virol., 91: e01370-17
Lung epithelial cells
Estrella, M.A., Du, J., Chen, L., Rath, S., Prangley, E., Chitrakar, A., Aoki, T., Schedl, P., Rabinowitz, J. and Korennykh, A. (2019) The metabolites NADP+
and NADPH are the targets of the circadian protein Nocturnin (Curled) Nat. Comm., 10: 2367
Macrophages
Andreyev, A.Y., Shen, Z., Guan, Z., Ryan, A., Fahy, E., Subramaniam, S., Raetz, C.R.H., Briggs, S. and Dennis, E.A. (2010) Application of proteomic marker ensembles to subcellular organelle identification Mol. Cell. Proteomics, 9, 388–402
Andreyev, A.Y., Fahy, E., Guan, Z., Kelly, S., Li, X., McDonald, J.G., Milne, S., Myers, D., Park, H., Ryan, A., Thompson, B.M. et al (2010) Subcellular organelle lipidomics in TLR-4-activated macrophages J. Lipid Res., 51, 2785–2797
DiMezzo, T.L., Ruthel, G., Brueggemann, E.E., Hines, H.B., Ribot, W.J., Chapman, C.E., Powell, B.S. and Welkos, S.L. (2009) In vitro intracellular trafficking of virulence antigen during infection by Yersinia pestis PLoS One, 4:e6281
Kassas, N., Tanguy, E., Thahouly, T., Fouillen, L., Heintz, D., Chasserot-Golaz, S., Bader, M-F., Grant, N.J. and Vitale, N. (2017) Comparative characterization of phosphatidic acid sensors and their localization during frustrated phagocytosis J. Biol. Chem., 292, 4266–4279
Mammary cells
Glunde, K., Guggino, S.E., Ichikawa, Y. and Bhujwalla, Z.M. (2003) A novel method of imaging lysosomes in living human mammary epithelial cells Mol. Imaging, 2, 24-36
Zhyvoloup, A., Nemazanyy, I., Panasyuk, G., Valovka, T., Fenton, T., Rebholz, H., Wang, M-L., Foxon, R., Lyzogubov, V. et al (2003) Subcellular localization and regulation of coenzyme A synthetase J. Biol. Chem., 278, 50316-50321
MDCK cells
Solazzo, M., Fantappiè, O., D’Amico, M., Sassoli, C., Tani, A., Cipriani, G., Bogani, C., Formigli, L, and Mazzanti, R. (2009) Mitochondrial expression and functional activity of breast cancer resistance protein in different multiple drug-resistant cell lines Cancer Res., 69, 7235-7242
Microvascular endothelial cells
Bielaszewska, M., Ruter, C., Kunsmann, L., Greune, L., Bauwens, A., Zhang, W., Kuczius, T., Kim, K.S., Mellmann, A., Schmidt, M.A. and Karch, H. (2013) Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis PloS Pathog., 9: e1003797
Monkey kidney cells
Bhowmick, R., Halder, U.C., Chattopadhyay, S., Chanda, S., Nandi, S., Bagchi, P., Nayak, M.K., Chakrabarti, O., Kobayashi, N. and Chawla-Sarkar, M. (2012) Rotaviral enterotoxin nonstructural protein 4 targets mitochondria for activation of apoptosis during infection J. Biol. Chem., 287, 35004–35020
Mukherjee, A., Patra, U., Bhowmick, R. and Chawla-Sarkar, M. (2018) Rotaviral nonstructural protein 4 triggers dynamin‐related protein 1‐dependent mitochondrial fragmentation during infection Cell. Microbiol., 20: e12831
Mouse/mouse embryo fibroblasts
Bär, S., Daeffler, L., Rommelaere, J. and Nüesch, J.P.F. (2008) Vesicular egress of non-enveloped lytic parvoviruses depends on gelsolin functioning PLoS Pathog., 4:e1000126
Choi, Y.B., Shembade, N., Parvatiyar, K., Balachandran, S. and Harhaja, E.W. (2017) TAX1BP1 restrains virusinduced apoptosis by facilitating itch-mediated degradation of the mitochondrial adaptor MAVS Mol. Cell. Biol., 37: e00422-16
Kim, Y., Kim, C., Kwon, O.Y., Nam, D., Kim, S.S., Park, J.H., Kim, S., Gallagher-Jones, M. et al (2017) Visualization of a mammalian mitochondrion by coherent X-ray diffractive imaging Sci. Rep., 7: 1850
Pandey, S., Talukdar, I., Jain, B.P. and Goswami, S.K. (2017) GSK3β and ERK regulate the expression of 78 kDa SG2NA and ectopic modulation of its level affects phases of cell cycle Sci. Rep., 7: 7555
Wang, H-Q., Nakaya, Y., Du, Z., Yamane, T., Shirane, M., Kudo, T., Takeda, M., Takebayashi, K., Noda, Y., Nakayama, K.I. and Nishimura, M. (2005) Interaction of presenilins with FKBP38 promotes apoptosis by reducing mitochondrial Bcl-2 Hum. Mol. Genet., 14, 1889-1902
Myeloid leukaemic cells
Yun, Y., Wang, L-S., Shen, S-M., Xia, L., Zhang, L., Zhu, Y-S. and Chen, G-Q. (2007) Subcellular proteome analysis of camptothecin analogue NSC606985-treated acute myeloid leukemic cells J. Proteome Res., 6, 3808-3818
Neuroblastoma/glioma cells
Greeve, I., Hermans-Borgmeyer, I., Brellinger, C., Kasper, D., Gomez-Isla, T., Behl, C., Levkau, B. and Nitsch, R.M. (2000) The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer’s diseaseassociated neurodegeneration and oxidative stress J. Neurosci., 20, 7345-7352
Sharer, J.D., Shern, J.S., Van Valkenburg, H., Wallace, D.C. and Kahn, R.A. (2002) ARL2 and BART enter mitochondria and bind the adenine nucleotide transporter Mol. Biol. Cell, 13, 71-83
Tibaldi, E., Brunati, A.M., Massimino, M.L., Stringaro, A., Colone, M., Agostinelli, E., Arancia, G. and Toninello, A. (2008) Src-Tyrosine kinases are major agents in mitochondrial tyrosine phosphorylation J. Cell. Biochem., 104, 840-849
Zhao, H., Ruberu, K., Li, H. and Garner, B. (2013) Analysis of subcellular [57Co] cobalamin distribution in SHSY5Y neurons and brain tissue J. Neurosci. Methods, 217, 67– 74
Neuronal cells
Zhao, H., Ruberu, K., Li, H. and Garner, B. (2013) Analysis of subcellular [57Co] cobalamin distribution in SHSY5Y neurons and brain tissue J. Neurosci. Methods, 217, 67– 74
NRK cells
Wang, C., Du, W., Su, Q.P., Zhu, M., Feng, P., Li, Y., Zhou, Y., Mi, N., Zhu, Y. et al (2015) Dynamic tubulation of mitochondria drives mitochondrial network formation Cell Res., 25, 108-1120
Osteosarcoma cells
Geladaki, A., Britovšek, N.K., Breckels, L.M., Smith, T.S., Vennard, O.L., Mulvey, C.M., Crook, O.M., Gatto, L. and Lilley, K.S. (2019) Combining LOPIT with differential ultracentrifugation for high-resolution spatial proteomics Nat. Comm., 10: 331
Jeon, J., Jeong J.H., Baek, J-H., Koo, H-J., Park, W-H., Yang, J-S., Yu, M-H., Kim, S. and Pak, Y.K. (2011) Network clustering revealed the systemic alterations of mitochondrial protein expression PLoS Comput Biol 7: e1002093
Pancreatic β cells
Nyblom, H.K., Thorn, K., Ahmed, M. and Bergsten, P. (2006) Mitochondrial protein patterns correlating with impaired insulin secretion from INS-1E cells exposed to elevated glucose concentrations Proteomics, 6, 5193- 5198
Pheochromocytoma cells
Pridgeon J.W., Olzmann, J.A., Chin, L-S. and Li, L. (2007) PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1 PLoS Biol 5, e172
Prostate cancer cells
Song, J-Y., Ryu, S-H., Cho, Y.M., Kim, Y.S., Lee, B-M., Lee, S-W. and Choi, J. (2013) Wip1 suppresses
apoptotic cell death through direct dephosphorylation of BAX in response to c-radiation Cell Death Dis., 4: e744
Trabecular meshwork cells
Sakai, H., Shen, X., Koga, T., Park, B-C., Noskina, Y., Tibudan, M. and Yue, B.Y.J.T. (2007) Mitochondrial association of myocilin, product of a glaucoma gene, in human trabecular meshwork cells J. Cell. Physiol., 213, 775-784
A2 Mammalian Tissues
Brain and spinal cord
Bergemalm, D., Jonsson, P.A., Graffmo, K.S., Andersen, P.M., Brännström, T., Rehnmark, A. and Marklund, S.L. (2006) Overloading of stable and exclusion of unstable human superoxide dismutase-1 variants in mitochondria of murine amyotrophic lateral sclerosis models J. Neurosci., 26, 4147-4154
Bergemalm, D., Forsberg, K., Srivastava, V., Graffmo, K.S., Andersen, P.M., Brännström, T., Wingsle, G. and Marklund, S.L. (2010) Supermoxide dismutase-1 and other proteins in inclusions from transgenic amyotrophic lateral sclerosis model mice J. Neurochem., 114, 408–418
Chronister, W.D., Burbulis, I.E., Wierman, M.B., Wolpert, M.J., Haakenson, M.F., Smith, A.C.B., Kleinman, J.E., Hyde, T.M. et al (2019) Neurons with complex karyotypes are rare in aged human neocortex Cell Reports 26, 825–835
El-Kadi, A.M., Bros-Facer, V., Deng, W., Philpott, A., Stoddart, E., Banks, G., Jackson, G.S., Fisher, E.M.C., Duchen, M.R et al (2010) The Legs at odd angles (Loa) mutation in cytoplasmic dynein ameliorates mitochondrial function in SOD1G93A mouse model for motor neuron disease J. Biol. Chem., 285, 18627-18639
Islinger, M., Kirsch, J., Angermüller, S., Rotaru, R., Abdolzade-Bavil, A. and Weber, G. (2011) Subcellular fractionation of brain tissue using free-flow electrophoresis In Neuroproteomics, Neuromethods, (ed. Li, K.W.) 57, Springer Science+Business Media, pp. 27-45
Israelson, A., Arbel, N., Da Cruz, S., Ilieva, H., Yamanaka, K., Shoshan-Barmatz, V. and Cleveland, D.W. (2010) Misfolded mutant SOD1 directly inhibits VDAC1 conductance in a mouse model of inherited ALS Neuron, 67, 575–587
Kwon, J., Han, E., Bui, C-B., Shin, W., Lee, J., Lee, S., Choi, Y-B., Lee, A-H., et al (2012) Assurance of mitochondrial integrity and mammalian longevity by the p62–Keap1–Nrf2–Nqo1 cascade EMBO Rep., 13, 150–156
Lee, M. and Shin, J. (2011) Triage of oxidation-prone proteins by Sqstm1/p62 within the mitochondria Biochem. Biophys. Res. Comm., 413, 122–127
Leyton-Jaimes, M.F., Benaim, C., Abu-Hamada, S., Kahn, J., Guetta, A., Bucala, R. and Israelson, A. (2016) Endogenous macrophage migration inhibitory factor reduces the accumulation and toxicity of misfolded SOD1 in a mouse model of ALS Proc. Natl. Acad. Sci. USA, 113, 10198–10203
Nawrotzki, R., Islinger, M., Vogel, I., Völkl, A. and Kirsch, J. (2012) Expression and subcellular distribution of gephyrin in non-neuronal tissues and cells Histochem. Cell. Biol., 137, 471–482
Park, E., Lee, G-J., Choi, S., Choi, S-K., Chae, S-J., Kang, S-W., Pak, Y.K. and Park, H-K. (2010) The role of glutamate release on voltage-dependent anion channels (VDAC)-mediated apoptosis in an eleven vessel occlusion model in rats PloS One 5: e15192
Parone, P.A., Da Cruz, S., Han, J.S., McAlonis-Downes, M., Vetto, A.P., Lee, S.K., Tseng, E., and Cleveland, D.W. (2013) Enhancing mitochondrial calcium buffering capacity reduces aggregation of misfolded SOD1 and motor neuron cell death without extending survival in mouse models of inherited amyotrophic lateral sclerosis J. Neurosci., 33, 4657-4671
Sarafian, T.A., Ryan, C.M., Souda, P., Masliah, E., Kar, U.K., Vinters, H.V., Mathern, G.W., Faull, K.F., Whitelegge, J.P. and Watson, J.B. (2013) Impairment of mitochondria in adult mouse brain overexpressing predominantly full-length, N-terminally acetylated human α-synuclein PLoS One, 8: e63557
Smith, S., Witkowski, A., Moghul, A., Yoshinaga, Y., Nefedov, M., de Jong, P., Feng, D., Fong, L., Tu, Y., Hu, Y., Young, S.G., Pham, T. et al (2012) Compromised mitochondrial fatty acid synthesis in transgenic mice results in defective protein lipoylation and energy disequilibrium PLoS One, 7: e47196
Vande Velde, C., Miller, T.M., Cashman, N.R. and Cleveland, D.W. (2008) Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria Proc. Natl. Acad. Sci., USA, 105, 4022-4027
Wang, H-Q., Nakaya, Y., Du, Z., Yamane, T., Shirane, M., Kudo, T., Takeda, M., Takebayashi, K., Noda, Y., Nakayama, K.I. and Nishimura, M. (2005) Interaction of presenilins with FKBP38 promotes apoptosis by reducing mitochondrial Bcl-2 Hum. Mol. Genet., 14, 1889-1902
Wang, X., Becker, K., Levine, N., Zhang, M., Lieberman, A.P., Moore, D.J. and Ma, J. (2019) Pathogenic alphasynuclein aggregates preferentially bind to mitochondria and affect cellular respiration Acta Neuropathol. Comm., 7: 41
Wood-Allum, C.A., Barber, S.C., Kirby, J., Heath, P., Holden, H., Mead, R., Higginbottom, A., Allen, S., Beaujeux, T., et al (2006) Impairment of mitochondrial anti-oxidant defence in SOD1-related motor neuron injury and amelioration by ebselen Brain, 129, 1693-1709
Zhao, H., Ruberu, K., Li, H. and Garner, B. (2013) Analysis of subcellular [57Co] cobalamin distribution in SHSY5Y neurons and brain tissue J. Neurosci. Methods, 217, 67– 74
Heart
Guo, D., Nguyen, T., Ogbi, M., Tawfik, H., Ma, G., Yu, Q., Caldwell, R.W. and Johnson, J.A. (2007) Protein kinase C-εoimmunoprecipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome-c oxidase activity and cardioprotection Am. J. Phsiol. Heart Circ. Physiol., 293, H2219-H2230
Jin, J-K., Whittaker, R., Glassy, M.S., Barlow, S.B., Gottlieb, R.A. and Glembotski, C.C. (2008) Localization of phosphorylated αB-crystallin to heart mitochondria during ischemia-reperfusion Am. J. Physiol. Heart Circ. Physiol., 294, H337-H344
Lee, K.H., Kwon, S.J., Woo, J-S., Lee, G-J., Lee, S-R., Jang, H-H. et al (2014) Effects of sildenafil on nanostructural and nanomechanical changes in mitochondria in an ischaemia-reperfusion rat model Clin. Exp. Pharmacol. Physiol., 41, 763–768
Lee, K.H., Ha, S.J., Woo, J-S., Lee, G-J., Lee, S-R., Kim, J.W., Park, H.K. and Kim, W. (2017) Exenatide prevents morphological and structural changes of mitochondria following ischaemia-reperfusion injury Heart Lung Circulat., 26, 519–523
Meng, C., Jin, X., Xia, L., Shen, S-M., Wang, X-L., Cai, J., Chen, G-Q., Wang, L-S. and Fang, N-Y. (2009) Alterations of mitochondrial enzymes contribute to cardiac hypertrophy before hypertension development in spontaneously hypertensive rats J. Proteome Res., 8, 2463-2475
Morrish, F., Buroker, N.E., Ge, M., Ning, X-H., Lopez-Guisa, J., Hockenbert, D., and Portman, M.A. (2006) Thyroid hormone receptor isoforms localize to cardiac mitochondrial matrix with potential for binding to receptor elements on mtDNA Mitochondrion, 6, 143-148
Ogbi, M., Obi, I. and Johnson, J.A. (2013) An inhibitor of the δPKC interaction with the d subunit of F1Fo ATP synthase reduces cardiac troponin I release from ischemic rat hearts: utility of a novel ammonium sulfate precipitation technique PLoS One, 8: e70580
Smith, S., Witkowski, A., Moghul, A., Yoshinaga, Y., Nefedov, M., de Jong, P., Feng, D., Fong, L., Tu, Y., Hu, Y., Young, S.G., Pham, T. et al (2012) Compromised mitochondrial fatty acid synthesis in transgenic mice results in defective protein lipoylation and energy disequilibrium PLoS One, 7: e47196
Witkowski, A., Joshi, A.K. and Smith, S. (2007) Coupling of the de novo fatty acid biosynthesis and lipoylation pathways in mammalian mitochondria J. Biol. Chem., 282, 14178-14185
Yu, Q., Nguyen, T., Ogbi, M., Caldwell, RW. and Johnson, J.A. (2008) Differential loss of cytochrome-c oxidase subunits in ischemia-reperfusion injury: exacerbation of COI subunit loss by PKC-ε inhibition Am. J. Physiol. Heart Circ. Physiol., 294, H2637–H2645
Kidney
Bergemalm, D., Jonsson, P.A., Graffmo, K.S., Andersen, P.M., Brännström, T., Rehnmark, A. and Marklund, S.L. (2006) Overloading of stable and exclusion of unstable human superoxide dismutase-1 variants in mitochondria of murine amyotrophic lateral sclerosis models J. Neurosci., 26, 4147-4154
Smith, S., Witkowski, A., Moghul, A., Yoshinaga, Y., Nefedov, M., de Jong, P., Feng, D., Fong, L. et al (2012) Compromised mitochondrial fatty acid synthesis in transgenic mice results in defective protein lipoylation and energy disequilibrium PLoS One, 7: e47196
Suna, S-H., Liu, S-Q., Cai, C-P., Cai, R., Chen, L. and Zhang, Q-B. (2012) Down-regulation of alpha-2u globulin in renal mitochondria of STZ-induced diabetic rats observed by a proteomic method Annales d’Endocrinologie 73, 530–541
Liver
Aboldzade, A., Islinger, M., Liebler, S., Eckerskorn, C., Völk, A. and Weber, G. (2007) Improved subcellular fractionation of the heavy mitochondrial pellet using free flow electrophoresis Mitochondrion 7, 419
Bhattacharyya, S., Feferman, L. and Tobacman, J.K. (2016) Restriction of aerobic metabolism by acquired or innate arylsulfatase B deficiency: a new approach to the Warburg effect Sci. Rep., 6: 32885
Fowler, S.L., Akins, M., Zhou, H., Figeys, D. and Bennett, S.A.L. (2013) The liver connexin32 interactome is a novel plasma membrane-mitochondrial signaling nexus J. Proteome Res., 12, 2597-2610
Fowler, S., Akins, M. and Bennett, S.A.L. (2016) Preparation of gap junctions in membrane microdomains for immunoprecipitation and mass spectrometry interactome analysis In Gap Junction Prototcols: Methods Mol. Biol., 1437 (ed. Vinken, M. and Johnstone, S.R), Springer Science+Business Media, LLC, pp 113-132
Gille, L. and Nohl, H. (2000) The existence of a lysosomal redox chain and the role of ubiquinone Arch. Biochem. Biophys., 375, 347-354
Graham, J., Ford, T. and Rickwood, D (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
Gringeri, E., Carraro, A., Tibaldi, E., d’Amico, F.E., Mancon, M., Toninello, A., Pagano, M.A., Vio, C., Cillo, U. and Brunati, A.M. (2010) Lyn-mediated mitochondrial tyrosine phosphorylation is required to preserve mitochondrial integrity in early liver regeneration Biochem. J., 425, 401–412
Hunt, M.C., Solaas, K., Kase, B.F and Alexon, E.H. (2002) Characterization of an acyl-CoA thioesterase that functions as a major regulator of peroxisomal lipid metabolism J. Biol. Chem., 277, 1128-1138
Kappler, L., Kollipara, L., Lehmann, R. and Sickmann, A. (2019) Investigating the role of mitochondria in type 2 diabetes – lessons from lipidomics and proteomics studies of skeletal muscle and liver In Mitochondria in Health and in Sickness, Advances in Experimental Medicine and Biology 1158 (ed. Urbani, A. and Babu, M.) Springer Nature Singapore Pte Ltd. pp. 143-182
Kerr, E.W., Shumar, S.A. and Leonardi, R. (2019) Nudt8 is a novel CoA diphosphohydrolase that resides in the mitochondria FEBS Lett., 593, 1133–1143
Kwon, J., Han, E., Bui, C-B., Shin, W., Lee, J., Lee, S., Choi, Y-B., Lee, A-H., et al (2012) Assurance of mitochondrial integrity and mammalian longevity by the p62–Keap1–Nrf2–Nqo1 cascade EMBO Rep., 13, 150–156
Islinger, M., Li, K.W., Seitz, J., Völkl, A. and Lüers, G.H. (2009) Hitchhiking of Cu/Zn superoxide dismutase to peroxisomes – evidence for a natural piggyback import mechanism in mammals Traffic, 10, 1711–1721
Israelson, A., Arbel, N., Da Cruz, S., Ilieva, H., Yamanaka, K., Shoshan-Barmatz, V. and Cleveland, D.W. (2010) Misfolded mutant SOD1 directly inhibits VDAC1 conductance in a mouse model of inherited ALS Neuron, 67, 575–587
Kwon, J., Han, E., Bui, C-B., Shin, W., Lee, J., Lee, S., Choi, Y-B., Lee, A-H., et al (2012) Assurance of mitochondrial integrity and mammalian longevity by the p62–Keap1–Nrf2–Nqo1 cascade EMBO Rep., 13, 150–156
Kushnareva, Y., Andreyev, A.Y., Kuwana, T. and Newmeyer, D.D. (2012) Bax activation initiates the assembly of a multimeric catalyst that facilitates bax pore formation in mitochondrial outer membranes PLoS Biol., 10: e1001394
Lewin T.M., Van Horn, C.G., Krisans, S.K. and Coelman, R.A. (2002) Rat liver acyl-CoA synthetase 4 is a peripheral-membrane protein located in two distinct subcellular organelles, peroxisomes and mitochondrialassociated membranes Arch. Biochem. Biophys., 404, 263-270
Matsumoto, A., Comatas, K.E., Liu, L. and Stamler, J.S. (2003) Screening for nitric oxide-dependent proteinprotein interactions Science, 301, 657-661
Mezzar, S., De Schryver, E., Asselberghs, S., Meyhi, E., Morvay, P.L., Baes, M. and Van Veldhoven, P.P. (2017) Phytol-induced pathology in 2-hydroxyacyl-CoA lyase (HACL1) deficient mice. Evidence for a second nonHACL1-related lyase BBA-Mol. Cell Biol. Lipids 1862, 972–990
Nakamura, Y., Ogura, M., Ogura, K., Tanaka, D. and Inagaki, N. (2012) SIRT5 deacetylates and activates urate oxidase in liver mitochondria of mice FEBS Lett., 586, 4076–4081
Nawrotzki, R., Islinger, M., Vogel, I., Völkl, A. and Kirsch, J. (2012) Expression and subcellular distribution of gephyrin in non-neuronal tissues and cells Histochem. Cell. Biol., 137, 471–482
Nohl, H. and Gille, L. (2002) The biofunctional activity of ubiquinone in lysosomal membranes Biogerontology, 3, 125-131
Noland, R.C., Woodlief, T.L., Whitfield, B.R., Manning, S.M., Evans, J.R., Dudek, R.W., Lust, R.M. and Cortright, R.N. (2007) Peroxisomal-mitochondrial oxidation in a rodent model of obesity-associated insulin resistance Am. J. Physiol. Endocrinol. Metab., 293, E986-E1001
Salvi, M., Battaglia, V., Brunati, A.M., La Rocca, N., Tibaldi, E., Pietrangeli, P., Marcocci, L., Mondovi, B., Rossi, C.A. and Toninello, A. (2007) Catalase takes part in rat liver mitochondria oxidative stress defense J. Biol. Chem., 282, 24407-24415
Smith, S., Witkowski, A., Moghul, A., Yoshinaga, Y., Nefedov, M., de Jong, P., Feng, D., Fong, L., Tu, Y., Hu, Y., Young, S.G., Pham, T. et al (2012) Compromised mitochondrial fatty acid synthesis in transgenic mice results in defective protein lipoylation and energy disequilibrium PLoS One, 7: e47196
Solaas, K., Sletta, R.J., Sρreide, O. and Kase, B.F. (2000) Presence of cholyl- and chenodexoy-cholylcoenzyme A thioesterase activity in human liver Scand. J. Clin. Lab. Invest., 60, 91-102
Solaas, K., Ulvestad, A., Soreide, O. and Kase, B.F. (2000) Subcellular organization of bile acid amidation in human liver: a key in regulating the biosynthesis of bile salts J. Lipid Res., 41, 1154-1162
Solaas, K., Kase, B.F., Pham, V., Bamberg, K., Hunt, M.C. and Alexson, S.E.H. (2004) Differential regulation of cytosolic and peroximal bile acid amidation by PPARα activation favors the formation of unconjugated bile acids J. Lipid. Res.,45, 1051-1060
Tschantz, W.R., Zhang, L. and Casey, P.J. (1999) Cloning, expression, and cellular localization of a human prenylcysteine lyase J. Biol. Chem., 274, 35802-35808
Yu, W., Liang, X., Ensenauer, R.E., Vockley, J., Sweetman, L. and Schultz, H. (2004) Leaky β-oxidation of a trans –fatty acid J. Biol. Chem., 279, 52160-52167
Zhou, W., Zhang, Y., Hosch, M.S., Lang, A., Zwacka, R.M. and Engelhardt, J.F. (2001) Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion Hepatology, 33, 902-914
Muscle (cardiac/skeletal)
Bensimon, M., Chang, A.I., Kuroski de Bold, M.L., Ponce, A., Carreras, D. and de Bold A.J. (2004) Participation of G protein in natriuretic peptide hormone secretion from heart atria Endocrinology, 145, 5313-5321
Fam, H.K., Chowdhury, M.K., Walton, C., Choi, K., Boerkoel, C.F. and Hendson, G. (2013) Expression profile and mitochondrial colocalization of Tdp1 in peripheral human tissues J. Mol. Hist., 44, 481–494
Flierl, A., Chen, Y., Coskun, P.E., Samulski, R.J. and Wallace, D.C. (2005) Adeno-associated virus mediated gene transfer of the heart/muscle adenine nucleotide translocator (ANT) in mouse Gene Ther., 12, 570-578
Goubaeva, F., Mikami, M., Giardina, S., Ding, B., Abe, J. and Yang, J. (2007) Cardiac mitochondrial connexin 43 regulates apoptosis Biochem. Biophys. Res. Commun., 352, 97-103
Kappler, L., Kollipara, L., Lehmann, R. and Sickmann, A. (2019) Investigating the role of mitochondria in type 2 diabetes – lessons from lipidomics and proteomics studies of skeletal muscle and liver In Mitochondria in Health and in Sickness, Advances in Experimental Medicine and Biology 1158 (ed. Urbani, A. and Babu, M.) Springer Nature Singapore Pte Ltd. pp. 143-182
Noland, R.C., Woodlief, T.L., Whitfield, B.R., Manning, S.M., Evans, J.R., Dudek, R.W., Lust, R.M. and Cortright, R.N. (2007) Peroxisomal-mitochondrial oxidation in a rodent model of obesity-associated insulin resistance Am. J. Physiol. Endocrinol. Metab., 293, E986-E1001
Ramos, E.S., Motori, E., Brüser, C., Kühl, I., Yeroslaviz, A., Ruzzenente, B., Kauppila, J.H.K., Busch, J.D., Hultenby, K. et al (2019) Mitochondrial fusion is required for regulation of mitochondrial DNA replication PLoS Genet., 15: e1008085
Smith, S., Witkowski, A., Moghul, A., Yoshinaga, Y., Nefedov, M., de Jong, P., Feng, D., Fong, L., Tu, Y., Hu, Y., Young, S.G., Pham, T. et al (2012) Compromised mitochondrial fatty acid synthesis in transgenic mice results in defective protein lipoylation and energy disequilibrium PLoS One, 7: e47196
SECTION B
Mitochondrial-associated membranes; interactions with the endoplasmic reticulum/Golgi
Bui, M., Gilady, S.Y., Fitzsimmons, R.E.B., Benson, M.D., Lynes, E.M., Gesson, K., Alto, N.M., Strack, S., Scott, J.D. and Simmen, T. (2010) Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties J. Biol. Chem., 285, 31590–31602
Giacomello, M. and Pellegrini, L. (2016) The coming of age of the mitochondria–ER contact: a matter of thickness Cell Death Differentiat., 23, 1417–1427
Gilady, S.Y., Bui, M., Lynes, E.M., Benson, M.D., Watts, R., Vance, J.E. and Simmen, T. (2010) Ero1α requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM) Cell Stress Chaperones, 15, 619–629
Huang, J., Liu, P. and Wang, G. (2018) Regulation of mitochondrion-associated cytosolic ribosomes by mammalian mitochondrial ribonuclease T2 (RNASET2) J. Biol. Chem., 293, 19633–19644
Ivanova, I.G. and Perkins, N.D. (2019) Hypoxia induces rapid, STAT3 and ROS dependent, mitochondrial translocation of RelA(p65) and IκBα Biosci. Rep., 39: BSR20192101
Kushnareva, Y., Andreyev, A.Y., Kuwana, T. and Newmeyer, D.D. (2012) Bax activation initiates the assembly of a multimeric catalyst that facilitates bax pore formation in mitochondrial outer membranes PLoS Biol., 10: e1001394
Lewin T.M., Van Horn, C.G., Krisans, S.K. and Coleman, R.A. (2002) Rat liver acyl-CoA synthetase 4 is a peripheral-membrane protein located in two distinct subcellular organelles, peroxisomes and mitochondrialassociated membranes Arch. Biochem. Biophys., 404, 263-270
Luo, G., Sun, Y., Feng, R., Zhao, Q. and Wen, T. (2018) ARL3 subcellular localization and its suspected role in autophagy Biochimie, 154, 187-193
Lynes, E.M., Bui, M., Yap, M.C., Benson, M.D., Schneider, B., Ellgaard, L., Berthiaume, L.G. and Simmen, T. (2012) Palmitoylated TMX and calnexin target to the mitochondria-associated membrane EMBO J., 31, 457–470
Lynes, E.M., Raturi, A., Shenkman, M., Sandoval, C.O., Yap, M.C., Wu, J., Janowicz, A., Myhill, N. et al (2013) Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling J. Cell Sci., 126, 3893–3903
Marchi, S., Patergnani, S. and Pinton, P. (2014) The endoplasmic reticulum–mitochondria connection: One touch, multiple functions Biochim. Biophys. Acta, 1837, 461–469
Myhill, N., Lynes, E.M., Nanji, J.A., Blagoveshchenskaya, A.D., Fei, H., Simmen, K.C., Cooper, T.J., Thomas, G. and Simmen, T. (2008) The subcellular distribution of calnexin is mediated by PACS-2 Mol. Biol. Cell, 19, 2777-2788
Raturi, A., Gutiérrez, T., Ortiz-Sandoval, C., Ruangkittisakul, A., Herrera-Cruz, M.S., Rockley, J.P., Gesson, K., Ourdev, D. et al (2016) TMX1 determines cancer cell metabolism as a thiolbased modulator of ER–mitochondria Ca2+ flux J. Cell Biol., 214, 433–444
Sane, S., Hafner, A., Srinivasan, R., Masood, D., Slunecka, J.I., Noldner, C.J., Hanson, A.D. et al (2018) UBXN2A enhances CHIP-mediated proteasomal degradation of oncoprotein mortalin-2 in cancer cells Mol. Oncol., 12, 1753–1777
Shapovalov, G., Ritaine, A., Bidaux, G., Slomianny, C., Borowiec, A-S., Gordienko, D., Bultynck, G., Skryma, R. and Prevarskaya, N. (2017) Organelle membrane derived patches: reshaping classical methods for new targets Sci. Rep., 7: 14082
Shintani-Ishida, K. and Yoshida, K-i. (2015) Mitochondrial m-calpain opens the mitochondrial permeability transition pore in ischemia–reperfusion Int. J. Cardiol., 197, 26–32
OptiPrepTM Reference List RS03; 5th edition, January 2020
OptiPrep Reference List RS04
Purification of and analysis of lysosomes
- References are divided alphabetically according to tissue or cell source and listed alphabetically according to first author
- Part(s) of the titles are highlighted in light blue to facilitate identification of particular research topic(s)
- IMPORTANT NOTE: PUBLICATIONS DEALING MORE SPECIFICALLY WITH THE INVOLVEMENT OF LYSOSOMES IN ENDOCYTIC PROCESSES ARE LISTED IN REFERENCE LIST RS12
Adipocytes
Villeneuve, J., Bassaganyas, L., Lepreux, S., Chiritoiu, M., Costet, P., Ripoche, J., Malhotra, V. Schekman, R. (2018) Unconventional secretion of FABP4 by endosomes and secretory lysosomes J. Cell Biol., 217 649-665
Bat kidney cells
Zheng, Y., Shang, J., Yang, Y., Liu, C., Wan, Y., Geng, Q., Wang, M., Baric, R. and Li, F. (2018) Lysosomal proteases are a determinant of coronavirus tropism J. Virol., 92, e01504-18
Blastocysts
Lee, J-H., Yu, W.H., Kumar, A., Lee, S., et al (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations Cell, 141, 1146–1158
Lee, J-H., McBrayer, M.K., Wolfe, D.M., Haslett, L.J., Kumar, A., Sato, Y., Lie, P.P.Y., Mohan, P. et al (2015) Presenilin 1 maintains lysosomal Ca2+ homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification Cell Rep., 12, 1430–1444
Bombyx mori silk gland
Shiba, H., Yabu, T., Sudayama, M., Mano, N., Arai, N., Nakanishi, T. and Hosono, K. (2016) Sequential steps of macroautophagy and chaperone-mediated autophagy are involved in the irreversible process of posterior silk gland histolysis during metamorphosis of Bombyx mori J. Exp. Biol., 219, 1146-1151
Brain
Andreyeva, A., Leshchyns’ka, I., Knepper, M., Betzel, C., et al (2010) CHL1 is a selective organizer of the presynaptic machinery chaperoning the SNARE complex PLoS One, 5: e12018
Annunziata, I., Patterson, A., Helton, D., Hu, H., et al (2013) Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid- secretion via deregulated lysosomal exocytosis Nat. Comm 4: 2734
Appu, A.P., Bagh, M.B., Sadhukhan, T., Mondal, A., Casey, S. and Mukherjee, A.B. (2019) Cln3-mutations underlying juvenile neuronal ceroid lipofuscinosis cause significantly reduced levels of Palmitoyl-protein thioesterases-1 (Ppt1)-protein and Ppt1-enzyme activity in the lysosome J. Inherit. Metab. Dis., 42, 944–954
Bagh, M.B., Pengm S., Chandra, G., Zhang, Z., Singh, S.P., Pattabiraman, N., Liu, A. and Mukherjee, A.B. (2017) Misrouting of v-ATPase subunit V0a1 dysregulates lysosomal acidification in a neurodegenerative lysosomal storage disease model Nat. Comm., 8: 14612
Dehay, B., Bové, J., Rodríguez-Muela, N., Perier, C., et al (2010) Pathogenic lysosomal depletion in Parkinson’s disease J. Neurosci., 30, 12535–12544
Gulbins, A., Schumacher, F., Becker, K.A., Wilker, B., Soddemann, M., Boldrin, F., Müller, C.P., Edwards, M.J., Goodman, M. et al (2018) Antidepressants act by inducing autophagy controlled by sphingomyelin– ceramide Mol. Psychiatr., 23, 2324–2346
Khundadze, M., Kollmann, K., Koch, N., Biskup, C., et al (2013) A hereditary spastic paraplegia mouse model supports a role of ZFYVE26/SPASTIZIN for the endolysosomal system PloS Genet., 9: e1003988
König, J., Besoke, F., Stuetz, W., Malarski, A., Jahreis, G., Grune, T. and Höhn, A. (2016) Quantification of age-related changes of a-tocopherol in lysosomal membranes in murine tissues and human fibroblasts Biofactors, 42, 307–315
McGlinchey, R.P. and Lee, J.C. (2015) Cysteine cathepsins are essential in lysosomal degradation of αsynuclein Proc. Natl. Acad. Sci. USA, 112, 9322–9327
McGlinchey, R.P., Lacy, S.M., Huffer, K.E., Tayebi, N., Sidransky, E. and Lee, J.C. (2019) C-terminal αsynuclein truncations are linked to cysteine cathepsin activity in Parkinson’s disease J. Biol. Chem., 294, 9973–9984
Meduri, G., Guillemeau, K., Dounane, O., Sazdovitch, V., Duyckaerts, C., Chambraud, B., Baulieu, E.E. and Giustiniani, J. (2016) Caspase-cleaved Tau-D421 is colocalized with the immunophilin FKBP52 in the autophagy-endolysosomal system of Alzheimer’s disease neurons Neurobiol. Aging, 46, 124-137
Murata, Y., Sun-Wada, G-H., Yoshimizu, T., Yamamoto, A., et al (2002) Differential localization of the vacuolar H+ pump with G subunit isoforms (G1 and G2) in mouse neurons J. Biol. Chem., 277, 36296-36303
Nawrotzki, R., Islinger, M., Vogel, I., Völkl, A., et al (2012) Expression and subcellular distribution of gephyrin in non-neuronal tissues and cells Histochem. Cell. Biol., 137, 471–482
Tian, X., Gala, U., Zhang, Y., Shang, W., Jaiswal, S.N., di Ronza, A., Jaiswa, M., Yamamoto, S., Sandoval, H. et al (2015) A voltage-gated calcium channel regulates lysosomal fusion with endosomes and autophagosomes and is required for neuronal homeostasis PLoS Biol 13: e1002103
Xiao, M-F., Xu, J-C., Tereshchenko, Y., Novak, D., et al (2009) Neural cell adhesion molecule modulates dopaminergic signaling and behavior by regulating dopamine D2 receptor internalization J. Neurosci., 29, 14752-14763
Zhao, H., Ruberu, K., Li, H. and Garner, B. (2013) Analysis of subcellular [57Co] cobalamin distribution in SHSY5Y neuronsand brain tissue J. Neurosci. Methods, 217, 67– 74
Breast cancer cells
Hao, Y., Kacal, M., Ouchida, A.T., Zhang, B., Norberg, E. and Vakifahmetoglu-Norberg, H. (2019) Targetome analysis of chaperone-mediated autophagy in cancer cells Autophagy, 15, 1558–1571
Caenorhabditis elegans
König, J., Besoke, F., Stuetz, W., Malarski, A., Jahreis, G., Grune, T. and Höhn, A. (2016) Quantification of age-related changes of a-tocopherol in lysosomal membranes in murine tissues and human fibroblasts Biofactors, 42, 307–315
Carcinoma cells: includes Caco2 cells, HeLa, lymphoma and hepatoma (Hep G2) cells
Al-Akhrass, H., Naves, T., Vincent, F., Magnaudeix, A., Durand, K., Bertin, F., Melloni, B., Jauberteau, M-O. and Lalloué, F. (2017) Sortilin limits EGFR signaling by promoting its internalization in lung cancer Nat. Comm., 8: 1182
Bertoli, F., Davies, G-L., Monopoli, M.P., Moloney, M., Gunko, Y.K., Salvati, A. and Dawson, K.A. (2014) Magnetic nanoparticles to recover cellular organelles and study the time resolved nanoparticle-cell interactome throughout uptake Small, 10, 3307–3315
Bielaszewska, M., Ruter, C., Kunsmann, L., Greune, L., et al (2013) Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis PloS Pathog., 9: e1003797
Cardoso, C.M.P., Groth-Pedersen, L., Høyer-Hansen, M., Kirkegaard, T., et al (2009) Depletion of kinesin 5B affects lysosomal distribution and stability and induces peri-nuclear accumulation of autophagosomes in cancer cells PLoS One, 4:e4424
Chekkat, N., Dahm, G., Chardon, E., Wantz, M., Sitz, J., Decossas, M., Lambert, O., Frisch, B., Rubbiani, R. et al (2106) N-Heterocyclic carbene−polyethylenimine platinum complexes with potent in vitro and in vivo antitumor efficacy Bioconjugate Chem., 27, 1942−1948
Costa, D., Costa, C., Caldeira, M., Cortes, L., Queiroz, J.A. and Cruz, C. (2017) Targeting of cellular organelles by fluorescent plasmid DNA nanoparticles Biomacromolecules, 18, 2928−2936
Edelmann, B., Bertsch, U., Tchikov, V., Winoto-Morbach, S., et al (2011) Caspase-8 and caspase-7 sequentially mediate proteolytic activation of acid sphingomyelinase in TNF-R1 receptosomes EMBO J., 30, 379–394
Grumet, L., Eichmann, T.O., Taschler, U., Zierler, K.A., Leopold, C., Moustafa, T., Radovic, B., Romauch, M. Yan, C. et al (2016) Lysosomal acid lipase hydrolyzes retinyl ester and affects retinoid turnover J. Biol., Chem., 291, 17977–17987
Kakar-Bhanot, R., Brahmbhatt, K., Chauhan, B., Katkam, R.R., Bashir, T., Gawde, H., Mayadeo, N., Chaudhari, U.K. and Sachdeva, G. (2019) Rab11a drives adhesion molecules to the surface of endometrial epithelial cells Human Reprod., 39, 519-529
Kidane, T.Z., Sauble, E. and Linder, M.C. (2006) Release of iron from ferritin requires lysosomal activity Am. J. Physiol. Cell Physiol., 291, C445-C455
Kinsey, C., Balakrishnan, V., O’Dell, M.R., Huang, J.L., et al (2014) Plac8 links oncogenic mutations to regulation of autophagy and is critical to pancreatic cancer progression Cell Rep., 7, 1143–1155
Lee, G-H., Lee, M-R., Lee, H-Y., Kim, S.H., et al (2014) Eucommia ulmoides cortex, geniposide and aucubin regulate lipotoxicity through the inhibition of lysosomal BAX PLoS One, 9: e88017
Le Guerroué, F., Eck, F., Jung, J., Starzetz, T., Mittelbronn, M. Kaulich, M. and Behrends, C. (2017) Autophagosomal content profiling reveals an LC3C-dependent piecemeal mitophagy pathway Mol. Cell, 68, 786–796
Li, F., Abuarab, N. and Sivaprasadarao, A. (2016) Reciprocal regulation of actin cytoskeleton remodelling and cell migration by Ca2+ and Zn2+: role of TRPM2 channels J. Cell Sci., 129, 2016-2029
Li, N., Zheng, Y., Chen, W., Wang, C., et al (2007) Adaptor protein LAPF recruits phosphorylated p53 to lysosomes and triggers lysosomal destabilization in apoptosis Cancer Res., 67, 11176-11185
Li, Y., Xu, M., Ding, X., Yan, C., Song, Z., Chen, L., Huang, X., Wang, X., Jian, Y., Tang, G. et al (2016) Protein kinase C controls lysosome biogenesis independently of mTORC1 Nat. Cell Biol., 18, 1065-1077
Liang, X-J., Shen, D-W., Garfield, S. and Gottesman, M.M. (2003) Mislocalization of membrane proteins associated with multidrug resistance in cisplastin-resistant cancer cell lines Cancer Res., 63, 5909-5916
Liu, L., Zhang, Z. and Xing, D. (2011) Cell death via mitochondrial apoptotic pathway due to activation of Bax by lysosomal photodamage Free Radic., Biol. Med., 51, 53–68
Luo, J., Liao, Y-C., Xiao, J. and Song, B-L. (2017) Measurement of cholesterol transfer from lysosome to peroxisome using an in vitro reconstitution assay In Cholesterol Homeostasis; Methods and Protocols: Methods Mol. Biol., 1583 (ed. Gelissen, I.C. and Brown, A.J.), Springer Science+Business Media LLC, pp 141-161
Matsuda, S., Okada, N., Kodama, T., Honda, T. and Iida, T. (2012) A Cytotoxic Type III Secretion Effector of Vibrio parahaemolyticus targets vacuolar H+-ATPase subunit c and ruptures host cell lysosomes PLoS Pathog., 8: e1002803
Matsui T., Jiang, P., Nakano, S., Sakamaki, Y., Yamamoto, H. and Mizushima, N. (2018) Autophagosomal YKT6 is required for fusion with lysosomes independently of syntaxin 17 J. Cell Biol. 217, 2633–2645
Meerovich, I., Koshkaryev, A., Thekkedath, R. and Torchilin, V.P. (2011) Screening and optimization of ligand conjugates for lysosomal targeting Bioconjugate Chem., 22, 2271–2282
Negoita, F., Blomdahl, J., Wasserstrom, S., Winberg, M.E., Osmark, P., Larsson, S., Stenkula, K.G., Ekstedt, M. et al (2019) PNPLA3 variant M148 causes resistance to starvation-mediated lipid droplet autophagy in human hepatocytes J. Cell Biochem., 120, 343-356
Prigozy, T.I., Naidenko, O., Qasba, P., Elewaut, D., et al (2001) Glycolipid antigen processing for presentation by CD1d molecules Science, 291, 664-667
Rock, B.M., Tometsko, M.E., Patel, S.K., Hamblett, K.J., Fansolow W.S. and Rock, D.A. (2015) Intracellular catabolism of an antibody drug conjugate with a noncleavable linker Drug Metab. Dispos., 43, 1341–1344
Seggewiß, N., Paulmann, D. and Dotzauer, A. (2016) Lysosomes serve as a platform for hepatitis A virus particle maturation and nonlytic release Arch. Virol., 161, 43–52
Shi, J., Chou, B., Choi, J.L., Ta, A.L., et al (2013) Investigation of polyethylenimine/DNA polyplex transfection to cultured cells using radiolabeling and subcellular fractionation methods Mol. Pharm., 10, 2145-2156
Sultan, A.S., Miyoshi, E., Ihara, Y., Nishikawa, A., et al (1997) Bisecting GlcNac structures act as negative sorting signals for cell surface glycoproteins in forskolin-treated rat hepatoma cells J. Biol. Chem., 272, 2866-2872
Takamura, A., Higaki, K., Ninomiya, H., Takai, T., Matsuda, J., Iida, M., Ohno, K., Suzuki, Y. and Nanba, E. (2011) Lysosomal accumulation of Trk protein in brain of GM1-gangliosidosis mouse and its restoration by chemical chaperone J. Neurochem., 118, 399–406
Udelnow, A., Kreyes, A., Ellinger, S., Landfester, K., et al (2011) Omeprazole inhibits proliferation and modulates autophagy in pancreatic cancer cells PLoS One, 6: e20143
Weissleder, R., Tung, C-H., Mahmood, U. and Bogdanov, A.(1999) In vivo imaging of tumors with proteaseactivated near-infrared fluorescent probes Nature Biotech., 17, 375-378
Xiao, J., Luo, J., Hu, A., Xiao, T., Li, M., Kong, Z., Jiang, L., Zhou, Z., Liao, Y. et al (2019) Cholesterol transport through the peroxisome-ER membrane contacts tethered by PI(4,5)P2 and extended synapto-tagmins Sci. China Life. Sci., 62, 1117-1135
Yin, J., Liu, X., He, Q., Zhou, L., Yuan, Z. and Zhao, S. (2016) Vps35-dependent recycling of Trem2 regulates microglial function Traffic, 17, 1286–1296
Zeng, J., Fan, Y., Hou, T., Wu, K., Chen, Y., He, D. and Li, L. (2019) Silibinin induced parallel activation of macroautophagy and inhibition of chaperone mediated autophagy in bladder cancer Eur. Urol. Suppl., 18, e1630
Zheng, Y., Shang, J., Yang, Y., Liu, C., Wan, Y., Geng, Q., Wang, M., Baric, R. and Li, F. (2018) Lysosomal proteases are a determinant of coronavirus tropism J. Virol., 92, e01504-18
Zhyvoloup, A., Nemazanyy, I., Panasyuk, G., Valovka, T., et al (2003) Subcellular localization and regulation of coenzyme A synthetase J. Biol. Chem., 278, 50316-50321
Caenorhabditis elegans
Li, Y., Chen, B., Zou, W., Wang, X., Wu, Y., Zhao, D., Sun, Y., Liu, Y., Chen, L., Miao, L, Yang, C. and Wang, X. (2016) The lysosomal membrane protein SCAV-3 maintains lysosome integrity and adult longevity J. Cell Biol., 215, 167–185
Cardiomyoblasts
Jaishy, B., Zhang, Q., Chung, H.S., Riehle, C., Soto, J., Jenkins, S., Abel, P., Cowart, L.A., Van Eyk, J.E. and Abel, E.D. (2015) Lipid-induced NOX2 activation inhibits autophagic flux by impairing lysosomal enzyme activity J. Lipid Res., 56, 546–561
Cladosporium
Goswami, P. and Cooney, J.J. (1999) Subcellular location of enzyme involved in oxidation on n-alkane by Cladosporium resinae Appl. Microbiol. Biotechnol., 51, 860-864
COS cells
Cao, Q., Zhao, K., Zhong, X.Z., Zou, Y., Yu, H., Huang, P., Xu, T-L. and Dong, X-P. (2014) SLC17A9 protein functions as a lysosomal ATP transporter and regulates cell viability 289, 23189–23199
Huang, P., Zou, Y., Zhong, X.Z., Cao, Q., et al (2014) P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH J. Biol. Chem., 289, 17658–17667
Seyrantepe, V., Landry, K., Trudel, S., Hassan, J.A., et al (2004) Neu4, a novel human lysosomal lumen sialidase, confers normal phenotype to sialidosis and galactosialidosis cells J. Biol. Chem., 279, 37021-37029
Wang, W., Zhang, X., Gao, Q., Lawas, M., Yu, L., Cheng, X., Gu, M., Sahoo, N. et al (2017) A voltagedependent K+ channel in the lysosome is required for refilling lysosomal Ca2+ stores J. Cell Biol., 216, 1715–1730
Zhong, X.Z., Cao, Q., Sun, X. and Dong, X-P., (2016) Activation of lysosomal P2X4 by ATP transported into lysosomes via VNUT/SLC17A9 using V-ATPase generated voltage gradient as the driving force J. Physiol., 594, 4253–42
Dictyostelium
Marchesini, N., Ruiz, F.A., Vieira, M. and Docampo, R. (2002) Acidocalcisomes are functionally linked to the contractile vacuole of Dictyostelium discoideum J. Biol. Chem., 277, 8146-8153 (2002)
Endothelial cells
Bielaszewska, M., Ruter, C., Kunsmann, L., Greune, L., et al (2013) Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis PloS Pathog., 9: e1003797
Justice, M.J., Bronova, I., Schweitzer, K.S., Poirier, C., Blum, J.S., Berdyshev, E.V. and Petrache, I. (2018) Inhibition of acid sphingomyelinase disrupts LYNUS signaling and triggers autophagy J. Lipid Res., 59, 596–606.
Liu, J., Weaver, J., Jin, X., Zhang, Y., Xu, J., Liu, K.J., Li, W. and Liu, W. (2016) Nitric oxide interacts with caveolin-1 to facilitate autophagy-lysosome-mediated claudin-5 degradation in oxygen-glucose deprivationtreated endothelial cells Mol. Neurobiol., 53, 5935–5947
Mu, R., Cutting, A.S., Del Rosario, Y., Villarino, N., Stewart, L., Weston, T.A., Patras, K.A. and Doran, K.S. (2016) Identification of CiaR regulated genes that promote group B streptococcal virulence and interaction with brain endothelial cells PLoS One, 11: e0153891
Epidermal cells
Raymond, A-A., de Peredo, A.G., Stella, A., Ishida-Yamamoto, A., et al (2008) Lamellar bodies of human epidermis: proteomics characterization by high throughput mass spectrometry and possible involvement of CLIP-170 in their trafficking/secretion Mol. Cell. Proteomics, 7, 2151-2175
Epithelial cells
Soni, L.E., Warren, C.M., Bucci, C., Orten, D.J., et al (2005) The unconventional myosin-VIIa associates with lysosomes Cell Motil. Cytoskeleton, 62, 13-26
Wang, W., Zhang, X., Gao, Q., Lawas, M., Yu, L., Cheng, X., Gu, M., Sahoo, N. et al (2017) A voltagedependent K+ channel in the lysosome is required for refilling lysosomal Ca2+ stores J. Cell Biol., 216, 1715–1730
Epithelial cells (boine mammary)
Luo, C., Zhao, S., Dai, W., Zheng, N. and Wang, J. (2018) Proteomic analysis of lysosomal membrane proteins in bovine mammary epithelial cells illuminates potential novel lysosome functions in lactation J. Agric. Food Chem., 66, 13041−13049
Fibroblasts (incl. human fibroblasts)
Beltran, P.M.J., Mathias, R.A. and Cristea, I.M. (2016) A portrait of the human organelle proteome in space and time during cytomegalovirus infection Cell Systems 3, 361–373
Benitez, B.A. and Sands, M.S. (2017) Primary fibroblasts from CSPα mutation carries recapitulate hallmarks of the adult onset neuronal ceroid lipofuscinosis Sci. Rep., 7: 6362
Dehay, B., Ramirez, A., Martinez-Vicente, M., Perier, C., et al (2012) Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration Proc. Natl. Acad. Sci. USA, 109, 9611–9616
Fehrenbacher, N., Bastholm, L., Kirkegaard-Sørenson, T., Rafn, B., et al (2008) Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-associated membrane proteins 1 and 2 Cancer Res., 68, 6623-6633
Higaki, K., Li, L., Bahrudin, U., Okuzawa, S., Takamuram, A., et al (2011) Chemical chaperone therapy: chaperone effect on mutant enzyme and cellular pathophysiology in β-galactosidase deficiency Hum. Mutat., 32, 843–852 920
Karaca, I., Tamboli, I.Y., Glebov, K., Richter, J., et al (2014) Deficiency of sphingosine-1-phosphate lyase impairs lysosomal metabolism of the amyloid precursor protein J. Biol. Chem., 289, 16761–16772
König, J., Besoke, F., Stuetz, W., Malarski, A., Jahreis, G., Grune, T. and Höhn, A. (2016) Quantification of age-related changes of a-tocopherol in lysosomal membranes in murine tissues and human fibroblasts Biofactors, 42, 307–315
Li, M., Zhang, C-S., Zong, Y., Feng, J-W., Ma, T., Hu, M., Lin, Z., Li, X., Xie, C. et al (2019) Transient receptor potential V channels are essential for glucose sensing by aldolase and AMPK Cell Metab., 30, 508–524
Marchesan, D., Cox, T.M. and Deegan, P.B. (2012) Lysosomal delivery of therapeutic enzymes in cell models of Fabry disease J. Inherit. Metab. Dis., 35, 1107–1117
Morrison, C., Sauble, E.N., Nguyen, A., La, A., Bach, G. and Linder, M.C. (2009) Potential abnormalities in iron metabolism in hyperlipidemia patient fibroblasts FASEB J., 23, Abstr. 105.4
Oberle, C., Huai, J., Reinheckel, T., Tacke, M., et al (2010) Lysosomal membrane permeabilization and cathepsin release is a Bax/Bak-dependent, amplifying event of apoptosis in fibroblasts and monocytes Cell Death Differ., 17, 1167–1178
Takai, T., Higaki, K., Aguilar-Moncayo, M., Mena-Barragan, T., et al (2013) A bicyclic 1- deoxygalactonojirimycin derivative as a novel pharmacological chaperone for GM1 gangliosidosis Mol. Ther., 21, 526–532
Wiesinger, C., Kunze, M., Regelsberger, G., Forss-Petter, S., et al (2013) Impaired very long-chain Acyl-CoA β-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dyfunction J. Biol. Chem., 288, 19269-19279
Zhao, H., Ruberu, K., Li, H. and Garner, B. (2013) Analysis of subcellular [57Co] cobalamin distribution in SHSY5Y neuronsand brain tissue J. Neurosci. Methods, 217, 67– 74
Zhong, X.Z., Zou, Y., Sun, X., Dong, G., Cao, Q., Pandey, A., Rainey, J.K., Zhu, X. and Dong, X-P. (2017) Inhibition of transient receptor potential channel mucolipin-1 (TRPML1) by lysosomal adenosine involved in severe combined immunodeficiency diseases J. Biol. Chem., 292, 3445–3455
Glioblastoma cells (see “Neuroblastoma)
HEK cells
Alexia, C., Poalas, K., Carvalho, G., Zemirli, N., et al (2013) The endoplasmic reticulum acts as a platform for ubiquitylated components of nuclear factor kB signaling Sci. Signal., 6(291), ra79
Chen, Y., Zheng, S., Tecedor, L. and Davidson, B.L. (2018) Overcoming limitations inherent in sulfamidase to improve mucopolysaccharidosis IIIA gene therapy Mol. Ther., 26, 1118-1126
Clark, N.E., Metcalf, M.C., Best, D., Fleet, G.W.J. and Garman, S.C. (2012) Pharmacological chaperones for human α-N-acetylgalactosaminidase Proc. Natl. Acad. Sci. USA, 109, 17400-17405
Kawaguchi, K., Okamoto, T., Morita, M. and Imanaka, T. (2016) Translocation of the ABC transporter ABCD4 from the endoplasmic reticulum to lysosomes requires the escort protein LMBD1 Sci. Rep., 6: 30183
Luo, G., Sun, Y., Feng, R., Zhao, Q. and Wen, T. (2018) ARL3 subcellular localization and its suspected role in autophagy Biochimie, 154, 187-193
Nguyen, A., Zhao, N., Morrison, C., Gonzalez, A., Sauble, E., La, A., Linder, M.C. and Knutson, M. (2009) Mechanisms of iron release from lysosomes FASEB J., 23, Abstr. 921.11
Sekiguchi, T., Furuno, N., Ishii, T., Hirose, E., Sekiguchi, F., Wang, Y. and Kobayashi, H. (2019) RagA, an mTORC1 activator, interacts with a hedgehog signaling protein, WDR35/IFT121 Genes Cells 24, 151–161
Wang, W., Zhang, X., Gao, Q., Lawas, M., Yu, L., Cheng, X., Gu, M., Sahoo, N. et al (2017) A voltagedependent K+ channel in the lysosome is required for refilling lysosomal Ca2+ stores J. Cell Biol., 216, 1715–1730
Wang, X., Zhang, X., Dong, X-p., Samie, M., et al (2012) TPC proteins are phosphoinositide-activated sodiumselective ion channels in endosomes and lysosomes Cell, 151, 372–383
Wu, X., Zhao, L., Chen, Z., Ji, X., Qiao, X., Jin, Y. and Liu, W. (2016) FLCN maintains the leucine level in lysosome to stimulate mTORC1 PLoS One, 11: e0157100
Yin, J., Liu, X., He, Q., Zhou, L., Yuan, Z. and Zhao, S. (2016) Vps35-dependent recycling of Trem2 regulates microglial function Traffic, 17, 1286–1296
Zheng, J., Chen, L., Skinner, O.S., Ysselstein, D., Remis, J., Lansbury, P., Skerlj, R., Mrosek, M., Heunisch, U. et al (2018) β‑Glucocerebrosidase modulators promote dimerization of β‑glucocerebrosidase and reveal an allosteric binding site J. Am. Chem. Soc., 140, 5914−5924
Zheng, Y., Shang, J., Yang, Y., Liu, C., Wan, Y., Geng, Q., Wang, M., Baric, R. and Li, F. (2018) Lysosomal proteases are a determinant of coronavirus tropism J. Virol., 92, e01504-18
Zong, Y., Zhang, C-S., Li, M., Wang, W., Wang, Z., Hawley, S.A., Ma, T., Feng, J-W., Tian, X. et al (2019) Hierarchical activation of compartmentalized pools of AMPK depends on severity of nutrient or energy stress Cell Res., 29, 460–473
Zhou, X., Paushter, D.H., Pagan, M.D., Kim, D., Santos, M.N., Lieberman, R.L., Overkleeft, H.S., Sun, Y., Smolka, M.B. and Hu, F. (2019) Progranulin deficiency leads to reduced glucocerebrosidase activity PLoS One 14: e0212382
HeLa cells/Hepatoma cells see “Carcinoma cells”
Human gastric epithelial cells
Hu, W., Zhang, L., Li, M.X., Shen, J., Liu, X.D., Xiao, Z.G., Wu, D.L., Ho, I.H.T., Wu, J.C.Y. et al (2019) Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells Autophagy 15, 707–725
Kidney/kidney cells
König, J., Besoke, F., Stuetz, W., Malarski, A., Jahreis, G., Grune, T. and Höhn, A. (2016) Quantification of age-related changes of a-tocopherol in lysosomal membranes in murine tissues and human fibroblasts Biofactors, 42, 307–315
Zeng, B., Chen, G-L., Garcia-Vaz, E., Bhandari, S., Daskoulidou, N., Berglund, L.M., Jiang, H., Hallett, T. Zhou, L-P. et al (2017) ORAI channels are critical for receptor-mediated endocytosis of albumin Nat. Comm., 8: 1920
Liver (bovine/human/rodent): for Hep G2 cells, see “Carcinoma cells”
Desai, M.M., Gong, B., Chan, T., Davey, R.A., Soong, L., Kolokoltsov, A.A. and Sun, J. (2011) Differential, type I interferon-mediated autophagic trafficking of hepatitis C virus proteins in mouse liver Gastroenterology, 141, 674–685
Ferreira, J.V., Soares, A.R., Ramalho, J.S., Pereira, P. and Girao, H. (2015) K63 linked ubiquitin chain formation is a signal for HIF1A degradation by Chaperone-Mediated Autophagy Sci. Rep., 5: 10210
Gille, L. and Nohl, H. (2000) The existence of a lysosomal redox chain and the role of ubiquinone Arch. Biochem. Biophys., 375, 347-354
Graham, J., Ford, T. and Rickwood, D (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
Islinger, M., Li, K.W., Seitz, J., Völkl, A, et al (2009) Hitchhiking of Cu/Zn superoxide dismutase to peroxisomes – evidence for a natural piggyback import mechanism in mammals Traffic, 10, 1711–1721
König, J., Besoke, F., Stuetz, W., Malarski, A., Jahreis, G., Grune, T. and Höhn, A. (2016) Quantification of age-related changes of a-tocopherol in lysosomal membranes in murine tissues and human fibroblasts Biofactors, 42, 307–315
Lu, W., Zhang, Y., McDonald, D.O., Jing, H., Carroll, B., Robertson, N., Zhang, Q., et al (2014) Dual proteolytic pathways govern glycolysis and immune competence Cell, 159, 1578–1590
Nawrotzki, R., Islinger, M., Vogel, I., Völkl, A., et al (2012) Expression and subcellular distribution of gephyrin in non-neuronal tissues and cells Histochem. Cell. Biol., 137, 471–482
Nohl, H. and Gille, L. (2002) The biofunctional activity of ubiquinone in lysosomal membranes Biogerontology, 3, 125-131
Nohl, H., Gille, L. and Stanick, K. (2006) OH radical formation from the lysosomal electron carriers Biochim. Biophys. Acta, 1757, Suppl. 1, 217
Solaas, K., Sletta, R.J., Søreide, O. and Kase, B.F. (2000) Presence of cholyl- and chenodexoycholyl- coenzyme A thioesterase activity in human liver Scand. J. Clin. Lab. Invest., 60, 91-102
Solaas, K., Ulvestad, A., Soreide, O. and Kase, B.F. (2000) Subcellular organization of bile acid amidation in human liver: a key in regulating the biosynthesis of bile salts J. Lipid Res., 41, 1154-1162
Tschantz, W.R., Zhang, L. and Casey, P.J. (1999) Cloning, expression, and cellular localization of a human prenylcysteine lyase J. Biol. Chem., 274, 35802-35808
Zhou, X., Paushter, D.H., Pagan, M.D., Kim, D., Santos, M.N., Lieberman, R.L., Overkleeft, H.S., Sun, Y., Smolka, M.B. and Hu, F. (2019) Progranulin deficiency leads to reduced glucocerebrosidase activity PLoS One 14: e0212382
Lung
Lo, S., Yuan, S-S.F. Hsu, C., Cheng, Y-J., et al (2013) Lc3 over-expression improves survival and attenuates lung injury through increasing autophagosomal clearance in septic mice Ann. Surg., 257, 352–363
Lymph node cells
Jin, R.U. and Mills, J.C. (2014) RAB26 coordinates lysosome traffic and mitochondrial localization J. Cell Sci., 127, 1018–1032
Lymphocytic/lymphoblastic/lymphoma cells
Alexia, C., Poalas, K., Carvalho, G., Zemirli, N., et al (2013) The endoplasmic reticulum acts as a platform for ubiquitylated components of nuclear factor kB signaling Sci. Signal., 6(291), ra79
Hall, S.L., Hester, S., Griffin, J.L., Lilley, K.S., et al (2009) The organelle proteome of the DT40 lymphocyte cell line Mol.Cell. Proteom., 8, 1295–1305
Kung Sutherland, M.S., Sanderson, R.J., Gordon, K.A., Andreyka, J., et al (2006) Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates J. Biol. Chem., 281, 10540-10547
Lettau, M., Kabelitz, D. and Janssen, O. (2015) Lysosome-related effector vesicles in T lymphocytes and NK cells Scand. J. Immunol., 82, 235–243
Lettau, M., Dietz, M., Dohmen, K., Leippe, M., Kabelitz, D. and Janssen, O. (2019) Granulysin species segregate to different lysosome-related effector vesicles (LREV) and get mobilized by either classical or nonclassical degranulation Mol. Immunol., 107, 44–53
Liang, P., Nair, J.R., Song, L., McGuire, J.J., et al (2005) Comparative genomic analysis reveals a novel mitochondrial isoform of human rTS protein and unusual phylogenetic distribution of the rTS gene BMC Genomics, 6:125
Ruppert, S.M., Li, W., Zhang, G., Carlson, A.L., Limaye, A., et al (2012) The major isoforms of Bim contribute to distinct biological activities that govern the processes of autophagy and apoptosis in interleukin-7 dependent lymphocytes Biochim. Biophys. Acta, 1823, 1877–1893
Schmidt, H., Gelhaus, C., Lucius, R., Nebendahl, M. et al (2009) Enrichment and analysis of secretory lysosomes from lymphocyte populations BMC Immunol., 10:41
Schmidt, H., Gelhaus, C., Nebendahl, M., Lettau, M., et al (2011) Effector granules in human T lymphocytes: proteomic evidence for two distinct species of cytotoxic effector vesicles J. Proteome Res., 10, 1603–1620
Smith, C.G., Kharkwal, H. and Wilson, D.W. (2017) Expression and subcellular localization of the Kaposi’s sarcoma-associated herpesvirus K15P protein during latency and lytic reactivation in primary effusion lymphoma Cells J. Virol., 91: e01370-17
Macrophages
DiMezzo, T.L., Ruthel, G., Brueggemann, E.E., Hines, H.B., et al (2009) In vitro intracellular trafficking of virulence antigen during infection by Yersinia pestis PLoS One, 4:e6281
Jena, P.V., Roxbury, D., Galassi, T.V., Akkari, L., Horoszko, C.P., Iaea, D.B., Budhathoki-Uprety, J., Pipalia, N. et al (2017) A carbon nanotube optical reporter maps endolysosomal lipid flux ACS Nano, 11, 10689−10703
La, A., Nguyen, T., Tran, K., Sauble, E., Tu, D., Gonzalez, T.Z.K., Soriano, C., Morgan, J., Doan, M. et al (2018) Mobilization of iron from ferritin: new steps and details Metallomics, 10, 154-168
Xu, X., Yuan, X., Li, N., Dewey, W.L., Li, P-L. and Zhang, F. (2016) Lysosomal cholesterol accumulation in macrophages leading to coronary atherosclerosis in CD38/
mice J. Cell. Mol. Med. 20, 1001-1013
Xu, X., Zhang, A., Halquist, M.S., Yuan, X., Henderson, S.C., Dewey, W.L., Li, P-L., Li, N. and Zhang, F. (2016) Simvastatin promotes NPC1-mediated free cholesterol efflux from lysosomes through YP7A1/LXRa signalling pathway in oxLDL-loaded macrophages J. Cell. Mol. Med., 20, 1-11
Mammary tissues and cells
Arnandis, T., Ferrer-Vicens, I., García-Trevijano, E.R., Miralles, V.J., et al (2012) Calpains mediate epithelialcell death during mammary gland involution: mitochondria and lysosomal destabilization Cell Death Differ., 19, 1536–1548
Glunde, K., Guggino, S.E., Ichikawa, Y. and Bhujwalla, Z.M. (2003) A novel method of imaging lysosomes in living human mammary epithelial cells Mol. Imaging, 2, 24-36
Nemeth, B.A., Tsang, S.W.Y., Geske, R.S. and Haney, P. (2000) Golgi targeting of the GLUT1 glucose transporter in lactating mouse mammary gland Pediatr Res., 47, 444-450
MDCK cells
Sabo, S.L., Lanier, L.M., Ikin, A.F., Khorkova, O., et al (1999) Regulation of -amyloid secretion by FE65, an amyloid protein precursor-binding protein J. Biol. Chem., 274, 7952-7957 (1999)
Van Itallie, C.M., Tietgens, A.J., LoGrande, K., Aponte, A., Gucek, M. and Anderson, J.M. (2012) Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes Journal of Cell Science 125, 4902–4912
Mesothelial (peritoneal) cells
Cao, S., Li, S., Li, H., Xiong, L., et al (2013) The potential role of HMGB1 release in peritoneal dialysisrelated peritonitis PLoS One, 8: e54647
Microglial cells
Yin, J., Liu, X., He, Q., Zhou, L., Yuan, Z. and Zhao, S. (2016) Vps35-dependent recycling of Trem2 regulates microglial function Traffic, 17, 1286–1296
Monocytes
Hazenbos, W.L.W., Kajihara, K.K., Vandlen, R., Morisaki, H., et al (2013) Novel staphylococcal glycosyltransferases SdgA and SdgB mediate immunogenicity and protection of virulence-associated cell wall proteins PLoS Pathog., 9: e1003653
Mouse lens epithelial cells
Cui, X., Feng, R., Wang, J., Du, C., Pi, X., Chen, D., Li, J., Li, H., Zhang, J., Zhang, J. et al (2020) Heat shock factor 4 regulates lysosome activity by modulating the αBcrystallin-ATP6V1A-mTOR complex in ocular lens BBA – Gen. Subjects, 1864: 129496
Mouse embryo fibroblasts
Zhang, C-S., Li, M., Zong, Y. and Lin, S-C. (2018) Determining AMPK activation via the lysosomal v-ATPaseRagulator-AXIN/LKB1 axis In AMPK: Methods and Protocols, Methods in Mol. Biol., 1732 (eds. Neumann, D. and Viollet, B.) Springer Science+Business Media, LLC, pp 393-411
Zhang, M. and Ge, L. (2019) Cell-free reconstitution of autophagic membrane formation In Autophagy: Methods and Protocols, Methods in Molecular Biology, 1880 (ed. Ktistakis, N. and Florey, O.), Springer Science+Business Media LLC New York, pp 135-148
Zheng, Y., Shang, J., Yang, Y., Liu, C., Wan, Y., Geng, Q., Wang, M., Baric, R. and Li, F. (2018) Lysosomal proteases are a determinant of coronavirus tropism J. Virol., 92, e01504-18
Myosarcoma
Elzi, D.J., Song, M., Hakala, K., Weintraub, S.T. and Shiio, Y. (2014) Proteomic analysis of the EWS-Fli‑1 interactome reveals the role of the lysosome in EWS-Fli‑1 turnover J. Proteome Res. 13, 3783-3791
Neuroblastoma/glioblastoma cells
Bär, S., Daeffler, L., Rommelaere, J. and Nüesch, J.P.F. (2008) Vesicular egress of non-enveloped lytic parvoviruses depends on gelsolin functioning PLoS Pathog., 4:e1000126
Di Piazza, M., Mader, C., Geletneky, K., Herrero y Calle, M., et al (2007) Cytosolic activation of cathepsins mediates parvovirus H-1-induced killing of cisplatin and TRIAL-resistant glioma cells J. Virol., 81, 4186-4198
Dong, H., Qin, Y., Huang, Y., Ji, D. and Wu, F. (2019) Poloxamer 188 rescues MPTP-induced lysosomal membrane integrity impairment in cellular and mouse models of Parkinson’s disease Neurochem. Int., 126, 178–186
Ishibashi, D., Homma, T., Nakagaki, T., Fuse, T., Sano, K., Takatsuki, H., Atarashi, R. and Nishida, N. (2015) Strain-dependent effect of macroautophagy on abnormally folded prion protein degradation in infected neuronal cells PLoS One, 10: e0137958
Mamada, N., Tanokashira, D., Ishii, K., Tamaoka, A. and Araki, W. (2017) Mitochondria are devoid of amyloid β-protein (Aβ)-producing secretases: Evidence for unlikely occurrence within mitochondria of Ab generation from amyloid precursor protein Biochem. Biophys. Res. Comm., 486, 321-328
Sevlever, D., Jiang, P. and Yen, S-H.C. (2008) Cathepsin D is the main lysosomal enzyme involved in the degradation of α-synuclein and generation of its carboxy-terminally truncated species Biochemistry, 47, 9678-9687
Sharer, J.D., Shern, J.S., Van Valkenburg, H., Wallace, D.C., et al (2002) ARL2 and BART enter mitochondria and bind the adenine nucleotide transporter Mol. Biol. Cell, 13, 71-83
Tringali, C., Cirillo, F., Lamorte, G., Papini, N., et al (2012) NEU4L sialidase overexpression promotes β-catenin signaling in neuroblastoma cells, enhancing stem-like malignant cell growth Int. J. Cancer, 131, 1768– 1778
Watanabe, S., Hayakawa, T., Wakasugi, K. and Yamanaka, K. (2014) Cystatin C protects neuronal cells against mutant copper-zinc superoxide dismutase-mediated toxicity Cell Death Dis., 5, e1497
Wei, J., Fujita, M., Nakai, M., Waragai, M., et al (2009) Protective role of endogenous gangliosides for lysosomal pathology in a cellular model of synucleinopathies Am. J. Pathol., 174, 1891–1909
Zhao, H., Ruberu, K., Li, H. and Garner, B. (2013) Analysis of subcellular [57Co] cobalamin distribution in SHSY5Y neurons and brain tissue J. Neurosci. Methods, 217, 67– 74
Neutrophils
Hazenbos, W.L.W., Kajihara, K.K., Vandlen, R., Morisaki, H., et al (2013) Novel staphylococcal glycosyltransferases SdgA and SdgB mediate immunogenicity and protection of virulence-associated cell wall proteins PLoS Pathog., 9: e1003653
NK cells
Iizuka, Y., Cichocki, F., Sieben, A., Sforza, F., Karim, R., Coughlin, K., Isaksson Vogel, R., Gavioli, R. and McCullar, V. (2015) UNC-45A is a nonmuscle myosin IIA chaperone required for NK cell cytotoxicity via control of lytic granule secretion J. Immunol., 195, 4760–4770
NRK cells
Chen, Y., Su, Q.P. and Yu, L. (2019) Studying autophagic lysosome reformation in cells and by an in vitro reconstitution system In Autophagy: Methods and Protocols, Methods in Molecular Biology, vol. 1880 (ed. Ktistakis, N. and Florey, O.), Springer Science+Business Media LLC New York, pp 163-172
Chen, Y., Su, Q.P., Sun, Y. and Yu, L. (2018) Visualizing autophagic lysosome reformation in cells using in vitro reconstitution systems Curr. Protocols Cell Biol., 78, 11.24.1–11.24.15
Du, W., Su, Q.P., Chen, Y., Zhu, Y. Jiang, D., Rong, Y., Zhang, S., Zhang, Y., Ren, H., Zhang, C. et al (2016) Kinesin 1 drives autolysosome tubulation Dev. Cell 37, 326–336
Yu, L., McPhee, C.K., Zheng, L., Mardones, G.A., Rong, Y., Peng, J., Mi, N., Zhao, Y. et al (2010) Termination of autophagy and reformation of lysosomes regulated by mTOR Nature 465, 942-946
Lee, W-K., Probst, S., Santoyo‑Sanchez, M.P., Al‑Hamdani, W., Diebels, I., von Sivers, J-K., Kerek, E., Prenner, E.J. and Thévenod, F. (2017) Initial autophagic protection switches to disruption of autophagic flux by lysosomal instability during cadmium stress accrual in renal NRK-52E cells Arch. Toxicol., 91, 3225–3245
Osteoblasts/Osteoclasts
Kariya, Y., Homma, M., Aoki, S., Chiba, A., et al (2009) Vps33a mediates RANKL storage in secretory lysosomes in osteoblastic cells J. Bone Mineral Res., 24, 1741-1752
Zhao, H., Ito, Y., Chappel, J., Andrews, N., Ross, F.P., et al (2010) How do bone cells secrete proteins? In Osteoimmunology, Adv. Exp. Med.Biol., 658 (ed. Choi, Y.), Springer Science+Business Media, pp 105-109
PC12 cells
Wu, F., Xu, H-D., Guan, J-J., Hou, Y-S., Gu, J-H., Zhen, X-C. and Qin, Z-H. (2015) Rotenone impairs autophagic flux and lysosomal functions in Parkinson’s disease Neuroscience, 284, 900–911
Placental tisseue (human)
Schröder, B.A., Wrocklage, C., Hasilik, A. and Saftig, P. (2010) The proteome of lysosomes Proteomics, 10, 4053–4076
Schröder, B., Wrocklage, C., Pan, C., Jäger, R., Kösters, B., Schäfer, H., Elsässer, H-P., Mann, M. and Hasilik, A. (2007) Integral and associated lysosomal membrane proteins Traffic, 8, 1676-1686
Promyeloid cells
Nathanson, C-M., Wasselius, J., Wallin, H. and Abrahamson, M. (2002) Regulated expression and intracellular localization of cystatin F in human U937 cells Eur. J. Biochem., 269, 5502-5511
Renal cortex
Dobrinskikh, E., Giral, H., Caldas, Y.A., Levi, M., et al (2010) Shank2 redistributes with NaPilla during regulated endocytosis Am. J. Physiol. Cell Physiol., 299, C1324–C1334
Skin fibroblasts
Rísquez-Cuadro, R., Matsumoto, R., Ortega-Caballero, F., Nanba, E., Higaki, K., García Fernández, J.M. and Mellet, C.O. (2019) Pharmacological chaperones for the treatment of α‑mannosidosis J. Med. Chem., 62, 5832−5843
Sáez, J., Diaz, J., Ibañez, J., Bozo, J.P., Reyes, F.C., Alamo, M., Gobert, F-X., Obino, D., et al (2019) The exocyst controls lysosome secretion and antigen extraction at the immune synapse of B cells J. Cell Biol., 218, 2247–2264
Substantia nigra (SN4741) cells
Li, W., Zhu, J., Dou, J., She, H., Tao, K., Xu, H., Yang, Q. and Mao, Z. (2017) Phosphorylation of LAMP2A by p38 MAPK couples ER stress to chaperone-mediated autophagy Nat. Comm., 8: 1763
Thymocytes (mouse)
Melum, E., Jiang, X., Baker, K.D., Macedo, M.F., Fritsch, J., Dowds, C.M., Wang, J., Pharo, A., Kaser, A. et al (2019) Control of CD1d-restricted antigen presentation and inflammation by sphingomyelin Nat. Immunol., 20, 1644–1655
Toxoplasma
Hakansson, S., Charron, A.J. and Sibley, L.D. (2001) Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole EMBO J., 20, 3132-3144
Trabecular meshwork cells
Porter, K.M., Jeyabalan, N., Skiba, N.P., Epstein, D.L. and Liton, P.B. (2012) Proteomic analysis of the lysosomal fraction in trabecular meshwork cells subjected to chronic oxidative stress Invest. Ophthalmol. Vis. Sci., 53, Abstr. 3248- A93
OptiPrepTM Reference List RS04, 8th edition, January 2020
OptiPrep Reference List RS05
Analysis of membrane trafficking in mammalian tissues and cells: fractionation of ER, Golgi, TGN, PM and endosomes
- This Reference List provides in Sections 1-5 a brief summary of some of the OptiPrep™ methodology and in Section 6 a comprehensive list of references
1. Continuous buoyant density gradients
One of the first published papers to report the use of iodixanol gradients for the analysis of endoplasmic reticulum (ER), Golgi and plasma membrane (PM) from COS-7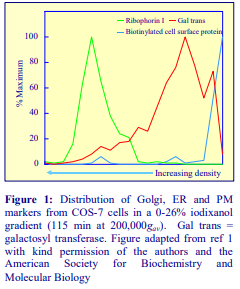 cells was by Yang et al [1] in 1997. A post-nuclear supernatant, loaded on to a 0-26% (w/v) iodixanol gradient was centrifuged at 200,000g for approx. 2 h. Analysis of the gradient established that the density of the three major membrane compartments increased in the order PM<Golgi<ER (see Fig. 1) and that banding sequence has been widely observed for the membranes from many other, but not all, mammalian cultured cells. This gradient and centrifugation format (spanning an approximate density range of 1.04-1.075 g/ml) has since been widely used at broadly similar (g x time) factors e.g. 280,000 g for 2 h and 100-200,000 g for 3h in a swinging-bucket rotor of approx. tube volume 13 ml (e.g. Beckman SW41Ti). Shorter times at a lower radial centrifugal force (RCF) (e.g. 150,000 g for 1.5 h) may be required for smaller volume rotors (e.g. 5 ml tubes) with a shorter sedimentation path length.
cells was by Yang et al [1] in 1997. A post-nuclear supernatant, loaded on to a 0-26% (w/v) iodixanol gradient was centrifuged at 200,000g for approx. 2 h. Analysis of the gradient established that the density of the three major membrane compartments increased in the order PM<Golgi<ER (see Fig. 1) and that banding sequence has been widely observed for the membranes from many other, but not all, mammalian cultured cells. This gradient and centrifugation format (spanning an approximate density range of 1.04-1.075 g/ml) has since been widely used at broadly similar (g x time) factors e.g. 280,000 g for 2 h and 100-200,000 g for 3h in a swinging-bucket rotor of approx. tube volume 13 ml (e.g. Beckman SW41Ti). Shorter times at a lower radial centrifugal force (RCF) (e.g. 150,000 g for 1.5 h) may be required for smaller volume rotors (e.g. 5 ml tubes) with a shorter sedimentation path length.
- This methodology is described in Application Sheet S21
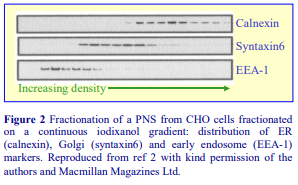 Some workers maintain that the highest resolution of membrane vesicles is only obtained if the centrifugation is carried out at relatives low RCFs for extended time periods; for example 50,000-100,000 g for 18 h. An example is given in Figure 2; an 8-34% (w/v) iodixanol gradient was used to study the processing of the amyloid β peptide From CHO cells stably transfected with PS1[2]. The gradient gave excellent resolution of early endosomes Golgi and ER. The method has also been able to resolve perinuclear ER from the bulk ER of mouse 3T3 fibroblasts [3]. This long-spin strategy however is not the only one that provides the ability to fractionate sub-domains of the same membrane compartment (see Section 2)
Some workers maintain that the highest resolution of membrane vesicles is only obtained if the centrifugation is carried out at relatives low RCFs for extended time periods; for example 50,000-100,000 g for 18 h. An example is given in Figure 2; an 8-34% (w/v) iodixanol gradient was used to study the processing of the amyloid β peptide From CHO cells stably transfected with PS1[2]. The gradient gave excellent resolution of early endosomes Golgi and ER. The method has also been able to resolve perinuclear ER from the bulk ER of mouse 3T3 fibroblasts [3]. This long-spin strategy however is not the only one that provides the ability to fractionate sub-domains of the same membrane compartment (see Section 2)
- This methodology is described in Application Sheet S22
2. Discontinuous gradients
Discontinuous iodixanol gradients in which the sample is bottom loaded in the densest solution are rather more variable in their detail; most run from 3 or 5% to 25% (w/v) iodixanol in 3.5% or 5% steps (e.g. refs 4 and 5), others are much simpler running from 20%-32% in three steps [6]. The centrifugation times are also variable: 88,000 g for 18 h [5] to 90,000 g for 2.5 h [6]. The banding position of the membranes is also rather variable, but some of this variability may arise from the variety of cell types. The 5-25% iodixanol gradient [5] resolved ER, Golgi and early endosomes from cortical neurons.
- This methodology is described in Application Sheet S23
There are many more examples in which the discontinuous gradients have been top-loaded. In many cases the gradients span the usual range from of 2.5-30% (w/v)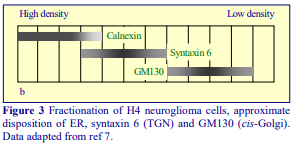 iodixanol, but often neither the increment between each step nor the volume of each step are constant. During the centrifugation, which is never more than 3h, diffusion of iodixanol will occur and a non-linear continuous gradient will form. Nonlinear gradients can provide an important feature that may potentially improve the resolution of certain membrane compartments. Figure 3 shows that this gradient effectively resolves ER, TGN and cis-Golgi from neuroglioma cells [7]. At the longer centrifugation time separations are certainly based on buoyant density but some of the shorter ones (1 h) are probably sedimentation velocity (see Section 3).
iodixanol, but often neither the increment between each step nor the volume of each step are constant. During the centrifugation, which is never more than 3h, diffusion of iodixanol will occur and a non-linear continuous gradient will form. Nonlinear gradients can provide an important feature that may potentially improve the resolution of certain membrane compartments. Figure 3 shows that this gradient effectively resolves ER, TGN and cis-Golgi from neuroglioma cells [7]. At the longer centrifugation time separations are certainly based on buoyant density but some of the shorter ones (1 h) are probably sedimentation velocity (see Section 3).
- This methodology is described in Application Sheet S24
- It is important to note that 5-25% (w/v) iodixanol discontinuous gradients (3h at 90,000 g) were able to resolve mitochondrial associated ER from the bulk of the ER [8 10] and that unlike Percoll®, OptiPrep™ was able to achieve this separation effectively and reliably [11].
3. Sedimentation velocity gradients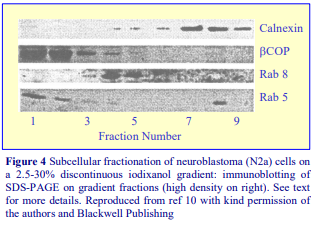
This strategy using either a multi-step discontinuous gradient (2.5%, 5%, 7.5%, 10%,12.5%, 15%, 17.5%, 20%, and 30% (w/v) iodixanol) or a continuous gradient over the same density range were introduced by Majoul et al [12] and Schroder et al [13] respectively. The former was widely used by the St. GeorgeHyslop group at the Centre for Research in Neurodegenerative Diseases in Toronto [14] for studying the processing of the β-amyloid precursor protein in neuroblastoma cells. The RCFs vary from 54,000-126,000 g and the centrifugation times from 25 to 90 min. An example of the fractionating power of this rapid centrifugation is shown in Figure 4.
- This methodology is described in Application Sheet S25
- There are also papers reporting the use of a self-generated iodixanol gradient, normally used for the separation of Golgi and smooth and rough ER – see Application Sheets S18 and S20
4. Pre-gradient strategies
A point worth noting is the widely adopted strategy of using a post-nuclear supernatant (PNS) as a gradient input. It has much to recommend it: notably time-saving, minimal sample manipulations and avoidance of any losses of membrane vesicle material. It should be borne in mind however that, with the exception of nuclei, every other major organelle and membrane vesicle derived from every membrane compartment, are going to band somewhere in the gradient. The use of a more substantial differential centrifugation scheme prior to the gradient was much more commonly used twenty or thirty years ago than is now the case. Removal of major organelle fractions prior to gradient fractionation may improve the purity of membrane vesicle fractions. A classic differential centrifugation scheme of 1000 g for 5-10 min, 3000 g for 10 min and 15,000 g for 15 min however is time-consuming and will lead to progressive loss of vesicles unless each pellet is carefully washed.
Other Application Sheets that are relevant to the fractionation of subcellular membranes are:
- Application Sheet S01 – Preparation of gradient solutions
- Application Sheet S03 – Preparation of continuous and discontinuous gradients
- Application Sheet S04 – Preparation of self-generated gradients
- Application Sheet S05 – Homogenization of mammalian tissues
- Application Sheet S06 – Homogenization of mammalian cells
- Application Sheet S07 – Differential centrifugation of homogenates
- Application Sheet S08 – Gradient harvesting
- Application Sheet S09 – Gradient analysis
5. References (for Sections 1-3)
1. Yang, M., Ellenberg, J., Bonifacino, J.S. and Weissman, A.M. (1997) The transmembrane domain of a carboxylterminal anchored protein determines localization to the endoplasmic reticulum J. Biol. Chem., 272, 1970-1975
2. Puglielli, L., Konopka, G., Pack-Chung, E., MacKenzie Ingano, L .A., Berezovska, O., Hyman, B.T., Chang, T. Y., Tanzi, R.E. and Kovacs, D.M. (2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide Nature Cell Biol., 3, 905-912
3. Woods, A.J., Roberts, M.S., Choudhary, J., Barry, S.T., Mazaki, Y., Sabe, H., Morley, S.J., Critchley, D.R. and Norman, J.C. (2002) Paxillin associates with poly(A)-binding protein 1 at the dense endoplasmic reticulum and the leading edge of migrating cells J. Biol. Chem., 277, 6428-6437
4. Decaffmeyer, M., Shugla, Y.V., Dicu, A.O., Thomas, A., Truant, R., Topham, M.K., Brasseur, R. and Epand, R.M. (2008) Determination of the topology of the hydrophobic segment of mammalian diacylglycerol kinase epsilon in a cell membrane and its relationship to predictions from modeling J. Mol. Biol., 383, 797-809
5. Yano, H. and Chao, M.V. (2004) Mechanisms of neurotrophin receptor vesicular transport J. Neurobiol., 58, 244-257
6. Li, Q., Zhang, Y., Marden, J.J., Banfi, B. and Engelhardt, J.F. (2008) Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury Biochem. J., 411, 531-541
7. Chang, Y., Tesco, G., Jeong, W.J., Lindsley, L., Eckman, E.A., Eckman, C.B., Tanzi, R.E. and Guenette, S.Y. (2003) Generation of the β-amyloid peptide and the amyloid percursor protein C-terminal fragment γ are potentiated by FE65L1 J. Biol. Chem., 278, 51100-51107
8. Myhill, N., Lynes, E.M., Nanji, J.A., Blagoveshchenskaya, A.D., Fei, H., Simmen, K.C., Cooper, T.J., Thomas, G. and Simmen, T. (2008) The subcellular distribution of calnexin is mediated by PACS-2 Mol. Biol. Cell, 19, 2777-2788
9. Gilady, S.Y., Bui, M., Lynes, E.M., Benson, M.D., Watts, R., Vance, J.E. and Simmen, T. (2010) Ero1α requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM) Cell Stress Chaperones, 15, 619–629
10. Bui, M., Gilady, S.Y., Fitzsimmons, R.E.B., Benson, M.D., Lynes, E.M., Gesson, K., Alto, N.M., Strack, S., Scott, J.D. and Simmen, T. (2010) Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties J. Biol. Chem., 285, 31590–31602
11. Lynes, E.M. and Simmen, T. (2011) Urban planning of the endoplasmic reticulum (ER): How diverse mechanisms segregate the many functions of the ER Biochim. Biophys. Acta, 1813, 1893–1905
12. Majoul, I.V., Bastiaens, P.I.H. and Soling H-D (1996) Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells J. Cell Biol., 133, 777-789
13. Schroder, M., Schafer, R. and Friedl, P. (2002) Induction of protein aggregation in an early secretory compartment by elevation of expression level Biotechnol. Bioeng., 78, 131-140
14. Chen, F., Yang, D-S., Petanceska, S., Yang, A., Tandon, A., Yu, G., Rozmahel, R., Ghiso, J., Nishimura, M., Zhang, D.M., Kawarai, T., Levesque, G., Mills, J., Levesque, L., Song, Y-Q., Rogaeva, E., Westaway, D., Mount, H., Gandy, S., St. George-Hyslop, P. and Fraser, P.E (2000) Carboxyl-terminal fragments of Alzheimer β-amyloid precursor protein accumulate in restricted and unpredicted intracellular compartments in presenilin 1 deficient cells J. Biol. Chem., 275, 36794-36802
6. Comprehensive bibliography
Papers have been divided into mammalian cells (pp 3-25) or tissue (pp 25-38) Within each group, papers are listed alphabetically according to first author. Multiple first author papers are sorted chronologically. Key words in the titles have been highlighted in light blue to facilitate visual searches for a particular research topic.
If the fractionation of more than one cell type is reported in a paper, the reference will appear in each relevant cell section.
IMPORTANT NOTE
Although many of the publications described below may include the analysis of endosomes OptiPrep Reference List RS12 deals more specifically with endocytosis
Mammalian cells
1. Adipocytes
Khalifeh-Soltani, A., McKleroy, W., Sakuma, S., Cheung, Y.Y., 1,3, Tharp, K., Qiu, Y., Turner, S.M., Chawla, A., Stah, A. and Atabai, K. (2014) Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids Nat. Med., 20, 175-183
2. Astrocytoma
Choi, K.S., Aizaki, H. and Lai, M.M. (2005) Murine coronavirus requires lipid rafts for virus entry and cell-cell fusion but not for virus release J. Virol., 79, 9862-9871
3. Blastocysts
Lee, J-H., Yu, W.H., Kumar, A., Lee, S., Mohan, P.S., Peterhoff, C.M., Wolfe, D.M., Martinez-Vicente, M., et al (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations Cell, 141, 1146–1158
4. Bronchial epithelial
Bomberger, J.M., Ely, K.H., Bangia, N., Ye, S., Green, K.A., Green, W.R., Enelow, R.I. and Stanton, B.A. (2014) Pseudomonas aeruginosa Cif protein enhances the ubiquitination and proteasomal degradation of the transporter associated with antigen processing (TAP) and reduces major histocompatibility complex (MHC) class I antigen presentation J. Biol. Chem., 289, 152-162
Kwon, S-H., Pollard, H. and Guggino, W.B. (2007) Knockdown of NHERF1 enhances degradation of temperature rescued F508 CFTR from the cell surface of human airway cells Cell Physiol. Biochem., 20, 763-772
Wang, J., Farris, A.B., Xu, K., Wang, P., Zhang, X., Duong, D.M., Yi, H., Shu, H-K., Sun, S-Y. and Wang, Y. (2106) GPRC5A suppresses protein synthesis at the endoplasmic reticulum to prevent radiation-induced lung tumorigenesis Nat. Comm., 7: 11795
5. Caco-2 cells
Jaschke, A., Chung, B., Hesse, D., Kluge, R., Zahn, C., Moser, M., Petzke, K-J. et al (2012) The GTPase ARFRP1 controls the lipidation of chylomicrons in the Golgi of the intestinal epithelium Hum. Mol. Genet., 21, 3128–3142
6. Carcinoma (incl. HeLa)
Agudo-Ibañez, L., Crespo, P. and Casar, B. (2017) Analysis of Ras/ERK compartmentalization by subcellular fractionation In ERK Signaling: Methods and Protocols, Methods Mol. Biol., 1487 (ed. Jiménez, G.) Springer Science+Business Media, New York, pp 151-162
Ahmed, I.S., Rohe, H.J., Twist, K.E. and Craven, R.J. (2010) Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates Erlotinib sensitivity J. Biol. Chem., 285, 24775–24782
Barth, S., Edlich, F., Berchner-Pfannschmidt, U., Gneuss, S., Jahreis, G., Hasgall, P.A., Fandrey, J., Wenger, R.H. and Camenisch, G. (2009) Hypoxia-inducible factor prolyl-4-hydroxylase PHD2 protein abundance depends on integral membrane anchoring of FKBP38 J. Biol. Chem., 284, 23046–23058
Boulter, E., Garcia-Mata, R., Guilluy, C., Dubash, A., Rossi, G., Brennwald, P.J. and Burridge, K. (2010) Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1 Nat. Cell Biol., 12, 477-483
Bui, M., Gilady, S.Y., Fitzsimmons, R.E.B., Benson, M.D., Lynes, E.M., Gesson, K., Alto, N.M., Strack, S., Scott, J.D. and Simmen, T. (2010) Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties J. Biol. Chem., 285, 31590–31602
Butler, E.C. and Bradbury, N.A. (2015) Signal dependent ER export of lemur tyrosine kinase 2 BMC Cell Biol., 16: 26
Chang, K., Wang, T. and Luo, G. (2009) Proteomics study of the hepatitis C virus replication complex In: Hepatitis C Methods and Protocols, Methods Mol. Biol., 510 (ed. Tang, H.), Humana Press, Totowa, NJ pp 185-193
Chansard, M., Hong, J-H., Park, Y-U., Park, S.K. and Nguyen, M.D. (2011) Ndel1, Nudel (Noodle): flexible in the cell? Cytoskeleton, 68, 540–554
Cuevas, E.P., Eraso, P., Mazón, M.J., Santos, V., Moreno-Bueno, G., Cano, A, and Portillo, F. (2017) LOXL2 drives epithelial mesenchymal-transition via activation of IRE1-XBP1 signalling pathway Sci. Rep., 7: 44988
Du, X., Kumar, J., Ferguson, C., Schulz, T.A., Ong, Y.S., Hong, W., Prinz, W.A., Parton, R.G., Brown, A.J. and Yang, H. (2011) A role for oxysterol-binding protein–related protein 5 in endosomal cholesterol trafficking J. Cell Biol., 192, 121–135
Earley, A.K., Chan, W.M. and Ward, B.M. (2008) The vaccinia virus B5 protein requires A34 for efficient intracellular trafficking from the endoplasmic reticulum to the site of wrapping and incorporation into progeny virions J. Virol., 82, 2161-2169
Eggert, S., Gonzalez, A.C., Thomas, C., Schilling, S., Schwarz, S.M., Tischer, C., Adam, V., Strecker, P. (2018) Dimerization leads to changes in APP (amyloid precursor protein) trafficking mediated by LRP1 and SorLA Cell. Mol. Life Sci., 75, 301–322
Fraldi, A., Zito, E., Annunziata, F., Lombardi, A., Cozzolino, M., Monti, M., Spampanato, C., Ballabio, A., Pucci, P., Sitia, R. and Cosma, M.P. (2008) Multistep, sequential control of the trafficking and function of the multiple sulfatase deficiency gene product, SUMF1 by PDI, ERGIC-53 and ERp44 Human Mol. Genet., 17, 2610-2621
Gachet, Y., Tournier, S., Lee, M., Lazaris-Karatzas, A., Poulton, T. and Bommer, U-A. (1999) The growthrelated, translationally controlled protein P23 has properties of a tubulin protein and associates transiently with microtubules during the cell cycle J. Cell Sci., 112, 1257-1271
Ghosh, S., Bose, M., Ray, A. and Bhattacharyya, S.N. (2015) Polysome arrest restricts miRNA turnover by preventing exosomal export of miRNA in growth-retarded mammalian cells Mol. Biol. Cell, 26, 1072-1083
Gilady, S.Y., Bui, M., Lynes, E.M., Benson, M.D., Watts, R., Vance, J.E. and Simmen, T. (2010) Ero1α requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM) Cell Stress Chaperones, 15, 619–629
Giles, D.K. and Wyrick, P.B. (2008) Trafficking of chlamydial antigens to the endoplasmic reticulum of infected epithelial cells Microbes Infect., 10, 1494-1503
Heaton, N.S., Moshkina, N., Fenouil, R., Gardner, T.J., Aguirre, S., Shah, P.S., Zhao, N., Manganaro, L., Hultquist, J.F. et al (2016) Targeting viral proteostasis limits influenza virus, HIV, and Dengue virus infection Immunity, 44, 46–58
Hieronimus, B., Pfohl, J., Busch, C. and Graeve, L. (2017) Expression and characterization of membrane-type 4 matrix metalloproteinase (MT4-MMP) and its different forms in melanoma Cell. Physiol. Biochem., 42, 198-210
Hirose, H., Arasaki, K., Dohmae, N., Takio, K., Hatsuzawa, K., Nagahama, M., Tani, K., Yamamoto, A., Tohyama, M. and Tagaya, M. (2004) Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi EMBO J., 23, 1267-1278
Hood, J.L., Brooks, W.H. and Roszman, T.L. (2004) Differential compartmentalization of the calpain/calpastatin network with the endoplasmic reticulum and Golgi apparatus J. Biol. Chem., 279, 43126-43135
Hu, T., Li, C., Cao, Z., Van Raay, T.J., Smith, J.G., Willert, K., Solnica-Krezel, L. and Coffey, R.J. (2010) Myristoylated Naked2 antagonizes Wnt-β-catenin activity by degrading Dishevelled-1 at the plasma membrane J. Biol. Chem., 285, 13561-13568
Jheng, J-R., Wang, S-C., Jheng, C-R. and Horng, J-T. (2016) Enterovirus 71 induces dsRNA/PKR-dependent cytoplasmic redistribution of GRP78/BiP to promote viral replication Emerg. Microbes Infect., 5: e23
Kanemoto, S., Kobayashi, Y., Yamashita, T., Miyamoto, T. Cui, M., Asada, R., Cui, X., Hino, K., Kaneko, M. et al (2015) Luman is involved in osteoclastogenesis through the regulation of DC-STAMP expression, stability and localization J. Cell Sci., 128, 4353-4365
Kiyohara, T., Miyano, K., Kamakura, S., Hayase, J., Chishiki, K., Kohda, A. and Sumimoto, H. (2018) Differential cell surface recruitment of the superoxide-producing NADPH oxidases Nox1, Nox2 and Nox5: The role of the small GTPase Sar1 Genes Cells, 23, 480–493
Landry, M-C., Sicotte, A., Champagne, C. and Lavoie, J.N. (2009) Regulation of cell death by recycling endosomes and Golgi membrane dynamics via a pathway involving Src-family kinases, Cdc42 and Rab11a Mol Biol. Cell, 20, 4091-4106
Li, C-C., Kuo, J-C., Waterman, C.M., Kiyama, R., Moss, J. and Vaughan, M. (2011) Effects of brefeldin Ainhibited guanine nucleotide exchange (BIG) 1 and KANK1 proteins on cell polarity and directed migration during wound healing Proc. Natl. Acad. Sci. USA, 108, 19228–19233
Li, C-C., Le, K.,. Kato, J., Moss, J. and Vaughan, M. (2016) Enhancement of β-catenin activity by BIG1 plus BIG2 via Arf activation and cAMP signals Proc.Natl. Acad. Sci. USA, 113, 5946–5951
Li, J-H., Huang, W., Lin, P., Wu, B., Fu, Z-G., Shen, H-M., Jing, L., Liu, Z-Y. et al (2016) N-linked glycosylation at Asn152 on CD147 affects protein folding and stability: promoting tumour metastasis in hepatocellular carcinoma Sci. Rep., 6: 35210
Li, Q., Harraz, M.M., Zhou, W., Zhang, L.N., Ding, W., Zhang, Y., Eggleston, T., Yeaman, C., Banfi, B. and Engelhardt, J.F. (2006) Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAFg to endosomal interleukin-1 receptor complexes Mol. Cell. Biol., 26, 140-154
Li, Q., Zhang, Y., Marden, J.J., Banfi, B. and Engelhardt, J.F. (2008) Endosomal NADPH oxidase regulates cSrc activation following hypoxia/reoxygenation injury Biochem. J., 411, 531-541
Li, Z., Zhuang, M., Zhang, L., Zheng, X., Yang, P. and Li, Z. (2016) Acetylation modification regulates GRP78 secretion in colon cancer cells Sci. Rep., 6: 30406
Liang, J., Yin, C., Doong, H., Fang, S., Peterhoff, C., Nixon, R.A. and Monteiro, M.J. (2006) Characterization of erasin (UBXD2): a new ER protein that promotes ER-associated protein degradation J. Cell Sci., 119, 4011-4024
Liang, X-J., Shen, D-W., Chen, K.G., Wincovitch, S.M., Garfield, S.H. and Gottesman, M.M. (2005) Trafficking and localization of platinum complexes in cisplatin-resistant cell lines monitored by fluorescencelabeled platinum J. Cell. Physiol., 202, 635-641
Maher, C.M., Thomas, J.D., Haas, D.A., Longen, C.G., Oyer, H.M., Tong, J.Y. and Kim, F.J. (2018) Smallmolecule sigma1 modulator induces autophagic degradation of PD-L1 Mol. Cancer Res., 16, 244-255 Matsuda, S., Matsuda, Y., Snapp, E.L. and D’Adamioa, L. (2011) Maturation of BRI2 generates a specific inhibitor that reduces APP processing at the plasma membrane and in endocytic vesicles Neurobiol. Aging, 32, 1400–1408
Meng, X., Embry, A., Rose, L., Yan, B., Xu, C. and Xiang, Y. (2012) Vaccinia virus A6 is essential for virion membrane biogenesis and localization of virion membrane proteins to sites of virion assembly J. Virol., 86, 5603-5613
Mookherjee, D., Majumder, P., Mukherjee, R., Chatterjee, D., Kaul, Z., Das, S., Sougrat, R., Chakrabarti, S. and Chakrabarti, O. (2019) Cytosolic aggregates in presence of non-translocated proteins perturb endoplasmic reticulum structure and dynamics Traffic, 20, 943–960
Mukherjee, K., Ghoshal, B., Ghosh, S., Chakrabarty, Y., Shwetha, S., Das, S. and Bhattacharyya, S.N. (2016) Reversible HuR-microRNA binding controls extracellular export of miR-122 and augments stress response EMBO Rep., 17, 1184-1203
Mumbengegwi, D.R., Li, Q., Li, C., Bear, C.E. and Engelhardt, J.F. (2008) Evidence for a superoxide permeability in endosomal membranes Mol. Cell. Biol., 28, 3700-3712
Myhill, N., Lynes, E.M., Nanji, J.A., Blagoveshchenskaya, A.D., Fei, H., Simmen, K.C., Cooper, T.J., Thomas, G. and Simmen, T. (2008) The subcellular distribution of calnexin is mediated by PACS-2 Mol. Biol. Cell, 19, 2777-2788
Niu, Y., Zhang, C., Sun, Z., Hong, Z., Li, K., Sun, D., Yang, Y., Tian, C., Gong, W. and Liu, J-J. (2013) PtdIns(4)P regulates retromer-motor interaction to facilitate dynein-cargo dissociation at the trans-Golgi network Nat. Cell Biol., 15, 417-429
Oh, Y., Jeon, Y-J., Hong, G-S., Kim, I., Woo, H-N. and Jung, Y-K. (2012) Regulation in the targeting of TRAIL receptor 1 to cell surface via GODZ for TRAIL sensitivity in tumor cells Cell Death Differ., 19, 1196–1207
Pourcelot, M., Zemirli, N., Da Costa, L.S., Loyant, R., Garcin, D., Vitour, D., Munitic, I., Vazquez, A. and Arnoult, D. (2016) The Golgi apparatus acts as a platform for TBK1 activation after viral RNA sensing BMC Biol., 14: 69
Raturi, A., Gutiérrez, T., Ortiz-Sandoval, C., Ruangkittisakul, A., Herrera-Cruz, M.S., Rockley, J.P., Gesson, K., Ourdev, D. et al (2016) TMX1 determines cancer cell metabolism as a thiolbased modulator of ER–mitochondria Ca2+ flux J. Cell Biol., 214, 433–444
Robb, V.A., Gerber, M.A., Hart-Mahon, E.K. and Gutmann, D.H. (2005) Membrane localization of the U2 domain of protein 4.1B is necessary and sufficient for meningioma growth suppression Oncogene, 24, 1946-1957
Roth, D., Lynes, E., Riemer, J., Hansen, H.G., Althaus, N., Simmen, T. and Ellgaard, L. (2010) A di-arginine motif contributes to the ER localization of the type I transmembrane ER oxidoreductase TMX4 Biochem. J., 425, 195–205
Saito, K., Yamashiro, K.,. Shimazu, N., Tanabe, T., Kontani, K. and Katada, T. (2014) Concentration of Sec12 at ER exit sites via interaction with cTAGE5 is required for collagen export J. Cell Biol. 206, 751–762
Saka, H.A., Thompson, J.W., Chen, Y-S., Kumar, Y., Dubois, L.G., Moseley, A. and Valdivia, R.H. (2011) Quantitative proteomics reveals metabolic and pathogenic properties of Chlamydia trachomatis developmental forms Mol. Microbiol., 82, 1185–1203
Sandin, M., Antberg, L., Levander, F. and James, P. (2015) A Breast Cell Atlas: Organelle analysis of the MDA-MB-231 cell line by density-gradient fractionation using isotopic marking and label-free analysis EuPA Open Proteomics, 8, 68–77
Sane, S., Abdullah, A., Boudreau, D.A., Autenried, R.K., Gupta, B.K., Wang, X., Wang, H. et al (2014) Ubiquitin-like (UBX)-domain-containing protein, UBXN2A, promotes cell death by interfering with the p53- Mortalin interactions in colon cancer cells Cell Death Dis., 5: e1118
Shi, J., Chou, B., Choi, J.L., Ta, A.L. and Pun, S.H. (2013) Investigation of polyethylenimine/DNA polyplex transfection to cultured cells using radiolabeling and subcellular fractionation methods Mol. Pharm., 10, 2145-2156
Shiwaku, H., Yoshimura, N., Tamura, T., Sone, M., Ogishima, S., Watase, K., Tagawa, K. and Okazawa, H. (2010) Suppression of the novel ER protein Maxer by mutant ataxin-1 in Bergman glia contributes to non-cellautonomous toxicity EMBO J., 29, 2446–2460
Tasetto, M., Maizel, A., Osorio, J. and Joliot. A. (2005) Plant and animal homeodomains use convergent mechanisms for intercellular transfer EMBO Rep., 6, 885-890
Thorne, R.F., Ralston, K.J., de Bock, C.E., Mhaidat, N.M., Zhang, X.D., Boyd, A.W. and Burns, G.F. (2010) Palmitoylation of CD36/FAT regulates the rate of its post-transcriptional processing in the endoplasmic reticulum Biochim. Biophys. Acta, 1803, 1298–1307
Wan, J., Zhu, F., Zasadil, L.M., Yu, J., Wang, L., Johnson, A., Berthier, E. et al (2014) A Golgi-localized pool of the mitotic checkpoint component Mad1 controls integrin secretion and cell migration Curr. Biol., 24, 2687-2692
Wanga, Y-N., Wang, H., Yamaguchi, H., Lee, H-J., Lee, H-H. and Hung, M-C. (2010) COPI-mediated retrograde trafficking from the Golgi to the ER regulates EGFR nuclear transport Biochem. Biophys. Res. Comm., 399, 498–504
Witze, E.S., Connacher, M.K., Houel, S., Schwartz, M.P., Morphew, M.K., Reid, L., Sacks, D.B., Anseth, K.S. and Ahn, N.G. (2013) Wnt5a directs polarized calcium gradients by recruiting cortical endoplasmic reticulum to the cell trailing edge Dev. Cell, 26, 645–657
Wolf, J., Reimer, T.A., Schuck, S., Rüder, C., Gerlach, F., Müller, E-C., Otto, A., Dörken, B. Rehm, A. (2010) Role of EBAG9 protein in coat protein complex I-dependent glycoprotein maturation and secretion processes in tumor cells FASEB J., 24, 4000–4019
Wu, X., Meng, X., Yan, B., Rose, L., Deng, J. and Xianga, Y. (2012) Vaccinia virus virion membrane biogenesis protein A11 associates with viral membranes in a manner that requires the expression of another membrane biogenesis protein, A6 J. Virol., 86, 11276–11286
Xiao, J., Luo, J., Hu, A., Xiao, T., Li, M., Kong, Z., Jiang, L., Zhou, Z., Liao, Y. et al (2019) Cholesterol transport through the peroxisome-ER membrane contacts tethered by PI(4,5)P2 and extended synapto-tagmins Sci. China Life. Sci., 62, 1117-1135
Yang, X., Yu, W., Shi, L., Sun, L., Liang, J., Yi, X., Li, Q., Zhang, Y., Yang, F., Han, X., Zhang, D., Yang, J., Yao, Z. and Shang, Y. (2011) HAT4, a Golgi apparatus-anchored B-type histone acetyltransferase, acetylates free histone H4 and facilitates chromatin assembly Mol. Cell, 44, 39–50
Yu, J., Chia, J., Canning, C.A., Jones, M., Bard, F.A. and Virshup, D.M. (2014) WLS retrograde transport to the endoplasmic reticulum during Wnt secretion Dev. Cell, 29, 277–291
7. Cardiomyocytes
Tseng, L.T-L., Lin, C-L., Tzen, K-Y., Chang, S.C. and Chang, M-F. (2013) LMBD1 Protein serves as a specific adaptor for insulin receptor internalization J. Biol. Chem., 288, 32424-32432
8. CHO cells
Andersen, O.M., Reiche, J., Schmidt, V., Gotthardt, M., Spoelgen, R., Behlke, J., von Arnim, C.A.F., Breiderhoff, T. et al (2005) Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein Proc. Natl. Acad. Sci. USA, 102, 13461-13466
Apostolou, A., Shen, Y., Linag, Y., Luo, J. and Fang, S. (2008) Armet, a UPR-upregulated protein, inhibits cell proliferation and ER stress-induced cell death Expt. Cell Res., 314, 2454,2467
Augustin, R., Riley, J. and Moley, K.H. (2005) GLUT8 contains a [DE]XXXL[LI] sorting motif and localizes to a late endosomal/lysosomal compartment Traffic, 6, 1196-1212
Banerji, S., Ngo, M., Lane, C.F., Robinson, C-A., Minogue, S. and Ridgway, N.D. (2010) Oxysterol binding protein-dependent activation of sphingomyelin synthesis in the Golgi apparatus requires phosphatidylinositol 4-kinase II Mol. Biol. Cell, 21, 4141-4150
Baulac, S., LaVoie, M.J., Kimberly, W.T., Strahle, J., Wolfe, M.S., Selkoe, D.J. and Xia, W. (2003) Functional γ-secretase complex assembly in Golgi/trans-Golgi network: interactions among presenilin, nicastrin, Aph1, Pen-2, and γ-secreatase substrates Neurobiol. Dis., 14, 194-204
Bhattacharyya, R., Barren, C. and Kovacs, D.M. (2013) Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts J. Neurosci., 33, 11169-11183
Campbell, C., Beug, S., Nickerson, P.E.B., Peng, J., Mazerolle, C., Bassett, E.A., Ringuette, R., Jama, F.A. et al (2016) Sortilin regulates sorting and secretion of Sonic hedgehog J. Cell Sci., 129, 3832-3844
Campbell, W.A., Iskandar, M-K., Reed, M.L.O. and Xia, W. (2002) Endoproteolysis of presenilin in vitro: inhibition by γ-secretase inhibitors Biochemistry, 41, 3372-3379
Campbell, W.A., Reed, M.L.O., Strahle, J., Wolfe, M.S. and Xia, W. (2003) Presenilin endoproteolysis mediated by an aspartyl protease activity pharmacologically distinct from γ-secretase J. Neurochem., 85, 1563-1574
Costantini, C., Ko, M.H., Jonas, M.C. and Puglielli, L. (2007) A reversible form of lysine acetylation in the ER and Golgi lumen controls the molecular stabilization of BACE1 Biochem. J., 407, 383-395
Esler, W.P., Das, C., Campbell, W.A., Kimberly, W.T., Kornilova, A.Y., Diehl, T.S., Ye, W. et al (2002) Amyloid-lowering isocoumarins are not direct inhibitors of -secretase Nat. Cell Biol., 4, E110
Giraudo, C.G. and Maccioni, J.F.(2003) Endoplasmic reticulum export of glycosyltransferases depends on interaction of a cytoplasmic dibasic motif with Sar1 Mol. Biol. Cell, 14, 3753-3766
Grimsby, J.L., Lucero, H.A., Trackman, P.C., Ravid, K. and Kagan, H.M. (2010) Role of lysyl oxidase propeptide in secretion and enzyme activity J. Cell. Biochem., 111, 1231–1243
Honsho, M., Yagita, Y., Kinoshita, N. and Fujuki, Y. (2008) Isolation and characterization of mutant animal cell line defective in alkyl-dihydroxyacetonephosphate synthase: Localization and transport of plasmalogens to post-Golgi compartments Biochim. Biophys. Acta, 1783, 1857-1865
Huttunen, H.J., Guénette, S.Y., Peach, C., Greco, C., Zia, W., Kim, D.Y., Barren, C., Tanzi, R.E. and Kovacs, D.M. (2007) HtrA2 regulates -amyloid precursor protein (APP) metabolism through endoplasmic reticulumassociated degradation J. Biol. Chem., 282, 28285-28295
Huttunen, H.J., Puglielli, L., Ellis, B.C., MacKenzie Ingano, L.A. and Kovacs, D.M. (2009) Novel N-terminal cleavage of APP precludes Aβ generation in ACAT-defective AC29 cells J. Mol. Neurosci., 37, 6-15
Huttunen, H.J., Peach, C., Bhattacharyya, R., Barren, C., Pettingell, W., Hutter-Paier, B., Windisch, M., Berezovska, O. and Kovacs, D.M. (2009) Inhibition of acyl-coenzyme A: cholesterol acyl transferase modulates amyloid precursor protein trafficking in the early secretory pathway role in the pathogenesis of Alzheimer’s disease (AD) FASEB J., 23, 3819–3828
Ikonomov, O.C., Fligger, J., Sbrissa, D., Dondapati, R., Mlak, K., Deeb, R. and Shisheva, A. (2009) Kinesin adapter JLP links PIKfyve to microtubule-based endosome-to-trans-Golgi network traffic of furin J. Biol. Chem., 284, 3750–3761
Kimberly, W.T., LaVoie, M.J., Ostaszewski, B.L., Ye, W., Wolfe, M.S. and Selkoe, D.J. (2002) Complex Nlinked glycosylated nicastrin associates with active γ-secretase and undergoes tight cellular regulation J. Biol. Chem., 277, 35113-35117
Matenia, D., Greisshaber, B., Li, X-y., Thiessen, A., Johne, C., Jiao, J., Mandelkow, E. and Mandelkow, E-M. (2005) PAK5 kinase is an inhibitor of MARK/Par-1, which leads to stable microtubules and dynamic actin Mol. Biol. Cell, 16, 4410-4422
Meli, G., Lecci, A. and Cattaneo, A. (2010) Targeting intracellular Aβb oligomers through conformational intrabodies Alzheimers Dementia 6, Suppl 1, S253-S254 Ohshiro, T., Kobayashi, K., Ohba, M., Matsuda, D., Rudel, L.L., Takahashi, T., Doi, T. and Tomoda, H. (2017) Selective inhibition of sterol O-acyltransferase 1 isozyme by beauveriolide III in intact cells Sci. Rep., 7: 4163
Patrana, D.V., Yun, C.H., McKee, M.L. and Fitzgerald, D.J. (2008) Mammalian cell expression of an active site mutant of Pseudomonas exotoxin disrupts LRP1 maturation J. Biomed. Sci., 15, 427-439
Posavec, M., Boskovic, D., Goate, A., Hecimovic, S. and Boskovic, R. (2008) Subcellular distribution of APP/C99 and amyloid-beta peptide is altered in CHO NPC1-null cells compared to CHOwt Alzheimers Dement., 4, Suppl. 1, T353
Puglielli, L., Konopka, G., Pack-Chung, E., MacKenzie Ingano, L .A., Berezovska, O., Hyman, B.T., Chang, T. Y., Tanzi, R.E. and Kovacs, D.M. (2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide Nat. Cell Biol., 3, 905-912
Schmidt, V., Sporbert, A., Rohe, M., Reimer, T., Rehm, A., Andersen, A.M. and Willnow, T.E. (2007) SorLA/LR11 regulates processing of amyloid precursor protein via interaction with adaptors GGA and PACS-1 J. Biol. Chem., 282, 32956-32964
Schroder, M., Schafer, R. and Friedl, P. (2002) Induction of protein aggregation in an early secretory compartment by elevation of expression level Biotechnol. Bioeng., 78, 131-140
Selkoe, D.J. (2001) Presenilin, notch, and the genesis and treatment of Alzheimer’s disease Proc. Natl. Acad, Sci. USA, 98, 111039-11041
Selkoe, D.J., Xia, W., Kimberly, W.T., Vekrellis, K., Walsh, D., Esler, W.P. and Wolfe, M.S. (2001) Mechanisms of Aβ production and Aβ degradation: routes to the treatment of Alzheimer’s disease In Alzheimer’s Disease: Advances in Ethiology, Pathogenesis and Therapeutics (Eds Iqbal, K. Sisodia, S.S. and Winblad, B.), John Wiley Ltd. pp. 421-432
Uliana, A.S., Giraudo, C.G. and Maccioni, H.J.F. (2006) Cytoplasmic tails of SialT2 and GalNacT impose their respective proximal and distal Golgi localization Traffic, 7, 604-612
Uliana, A.S., Crespo, P.M., Martina, J.A., Daniotti, J.L. and Maccioni, H.J.F. (2006) Modulation of GalT1 and SialT1 sub-Golgi localization by SialT2 expression reveals an organellar level of glycolipid synthesis control J. Biol. Chem., 281, 32852-32860
Urano, Y., Watanabe, H., Murphy, S.R., Shibuya, Y., Geng, Y., Peden, A.A., Chang, C.C.Y. and Chang, T.Y. (2008) Transport of LDL-derived cholesterol from the NPC1 compartment to the ER involves the trans-Golgi network and the SNARE protein complex Proc. Natl. Acad. Sci. USA, 105, 16513-16518
Walsh, D.M., Klyubin, I., Fadeeva, J.V., Cullen, W.K., Anwyl, R., Wolfe, M.S., Rowan, M.J. and Selkoe, D.J. (2002) Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo Nature, 416, 535-539
Wolfe, M.S., Xia, W., Ostraszewski, B.L., Diehl, T.S., Kimberly, W.T and Selkoe, D.J. (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity Nature 398, 513-517
Xia, W., Zhang, J., Ostraszewski, B.L., Kimberly, W.T., Seubert, P., Koo, E.H., Shen, J. and Selkoe, D.J. (1998) Presenilin 1 regulates the processing of β-amyloid precursor protein C-terminal fragments and the generation of amyloid β-protein in endoplasmic reticulum and Golgi Biochemistry, 37, 16465-71
Xia, W., Ray, W.J., Ostraszewski, B.L., Rahmati, T., Kimberly, W.T., Wolfe, M.S., Zhang, J., Goate, A.M. and Selkoe, D. (2000) Presenilin complexes with the C-terminal fragments of amyloid precursor protein at the sites of amyloid β-protein generation Proc. Natl. Acad. Sci. USA, 97, 9299-9304
Xu, D., Wang, Z., Zhang, Y., Jiang, W., Pan, Y., Song, B-L. and Chen, Y. (2015) PAQR3 modulates cholesterol homeostasis by anchoring Scap/SREBP complex to the Golgi apparatus Nat. Commun., 6: 8100
Yoneten, K.K., Kasap, M., Akpinar, G., Kanli, A. and Karaoz, E. (2019) Comparative proteomics analysis of four commonly used methods for identification of novel plasma membrane proteins J. Memb. Biol., 252, 587–608
Zhang, J., Kang, D.E., Xia, W., Okochi, M., Mori, H., Selkoe, D.J. and Koo, E.H. (1998) Subcellular distribution and turnover of presenilins in transfected cells J. Biol. Chem., 273, 12436-12442
9. CHO 7PA2/7WD4 cells
Meli, G., Lecci, A., Manca, A., Krako, N., Albertini, V., Benussi, L., Ghidoni, R. and Cattaneo, A. (2014) Conformational targeting of intracellular A oligomers demonstrates their pathological oligomerization inside the endoplasmic reticulum Nat. Comm., 5: 3867
10. COS cells
Chen, S.S-L., Lee, S-F. and Wang, C-T. (2001) Cellular membrane-binding ability of the C-terminal cytoplasmic domain of human immunodeficiency virus type 1 envelope transmembrane protein gp41 J. Virol., 75, 9925-9938
Cole, N.B., Ellenberg, J., Song, J., DiEuliis, D. and Lippincott-Schwarz, J. (1998) Retrograde transport of Golgi-localized proteins to the ER J. Cell Biol., 140, 1-15
Decaffmeyer, M., Shugla, Y.V., Dicu, A.O., Thomas, A., Truant, R., Topham, M.K., Brasseur, R. and Epand, R.M. (2008) Determination of the topology of the hydrophobic segment of mammalian diacylglycerol kinase epsilon in a cell membrane and its relationship to predictions from modeling J. Mol. Biol., 383, 797-809
Hirota, Y., Kurata, Y., Kato, M., Notsu, T., Koshida, S., Inoue, T., Kawata, Y., Miake, J. et al (2008) Functional stabilization of Kv1.5 protein by Hsp70 in mammalian cell lines Biochem. Biophys. Res. Commun., 372, 469-474
Kim, Y.J., Guzman-Hernandez, M.L. and Balla, T. (2011) A highly dynamic ER-derived phosphatidyl-inositolsynthesizing organelle supplies phosphoinositides to cellular membranes Dev. Cell, 21, 813–824
Koshida, S., Kurata, Y., Notsu, T., Hirota, Y., Kuang, T.Y., Li, P., Bahrudin, U., Harada, S. et al (2009) Stabilizing effects of eicosapentaenoic acid on Kv1.5 channel protein expressed in mammalian cells Eur. J. Pharmacol., 604, 93–102
Küch, E-M., Vellaramkalayil, V., Zhang, I., Lehnen, D., Brügger, B., Stremmel, W., Ehehalt, R., Poppelreuther, M. and Füllekrug, J. (2014) Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol Biochim. Biophys. Acta, 1841, 227–239
Ohsaki, Y., Sugimoto, Y., Suzuki, M., Hosokawa, H., Yoshimori, T., Davies, J.P. et al (2006) Cholesterol depletion facilitates ubiquitylation of NPC1 and its association with SKD1/Vps4 J. Cell Sci., 119, 2643-2653
Olmos, Y., Perdrix-Rosell, A. and Carlton, J.G. (2016) Membrane binding by CHMP7 coordinates ESCRT-IIIdependent nuclear envelope reformation Curr. Biol., 26, 2635–2641
Simonova, M., Shtanko, O., Serrgeyev, N., Weissleder, R. and Bogdanov Jr, A. (2003) Engineering of technetium-99m-binding artificial receptors for imaging gene expression J. Gene Med., 5, 1956-1066
Suzuki, S., Kurata, Y., Li, P., Notsu, T., Hasegawa, A., Ikeda, N., Kato, M., Miake, J., Sakata, S., Shiota, G., Yoshida, A., Ninomiya, H., Higaki, K., Yamamoto, K., Shirayoshi, Y. and Hisatome, I. (2012) Stabilization of Kv1.5 channel protein by bepridil through its action as a chemical chaperone Eur. J. Pharmacol., 696, 28–34
Yang, M., Ellenberg, J., Bonifacino, J.S. and Weissman, A.M. (1997) The transmembrane domain of a carboxyl-terminal anchored protein determines localization to the endoplasmic reticulum J. Biol.Chem., 272, 1970-1975
11. Dendritic cells
Imai, J., Hasegawa, H., Maruya, M., Koyasu, S. and Yahara, I. (2005) Exogenous antigens are processed through the endoplasmic reticulum-associated degradatin (ERAD) in cross-presentation by dendritic cells Int. Immunol., 17, 45-53
12. Embryo fibroblasts
Benyair, R., Ogen-Shtern, N., Mazkereth, N., Shai, B., Ehrlich, M. and Lederkremer, G.Z. (2015) Mammalian ER mannosidase I resides in quality control vesicles, where it encounters its glycoprotein substrates Mol. Biol. Cell, 26, 172-184
Curto, M., Cole, B.K., Lallemand, D., Liu, C-H. and McClatchey, A.I. (2007) Contact-dependent inhibition of EGFR signaling by Nf2/Merlin J. Cell Biol., 177, 893-903
Ge, L., Melville, D., Zhang, M. and Schekman, R. (2013) The ER–Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis eLife, 2: e00947
Ge, L., Zhang, M. and Schekman, R. (2014) Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment eLife, 3: e04135
Khalifeh-Soltani, A., McKleroy, W., Sakuma, S., Cheung, Y.Y., 1,3, Tharp, K., Qiu, Y., Turner, S.M., Chawla, A., Stah, A. and Atabai, K. (2014) Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids Nat. Med., 20, 175-183
Leitman, J., Shenkman, M., Gofman, Y., Shtern, N.O., Ben-Tal, N., Hendershot, L.M. and Lederkremer, G.Z. (2014) Herp coordinates compartmentalization and recruitment of HRD1 and misfolded proteins for ERAD Mol. Biol. Cell, 25, 1050-1060
Li, M., Zhang, C-S., Zong, Y., Feng, J-W., Ma, T., Hu, M., Lin, Z., Li, X., Xie, C. et al (2019) Transient receptor potential V channels are essential for glucose sensing by aldolase and AMPK Cell Metab., 30, 508–524
Marutani, T., Maeda, T., Tanabe, C., Zou, K., Araki, W., Kokame, K., Michikawa, M., Komano, H. (2011) ER-stress-inducible Herp, facilitates the degradation of immature nicastrin Biochim. Biophys. Acta, 1810, 790–798
Mathews, P.M., Guerra, C.B., Jiang, Y., Grbovic, O.M., Kao, B.H., Schmidt, S.D., Dinakar, R. et al (2002) Alzheimer’s disease-related overexpression of the cation dependent mannose-6-phosphate receptor increases Aβ secretion: Role for altered lysosomal hydrolase distribution in β-amyloidogenesis J. Biol. Chem., 277, 5299-5307
Molinari, M., Eriksson, K.K., Calanca, V., Galli, C., Cresswell, P., Michalak, M. and Helenius, A. (2004) Contrasting functions of calreticulin and calnexin in glycoprotein folding and ER quality control Mol. Cell, 13, 125-135
Pandey, S., Talukdar, I., Jain, B.P. and Goswami, S.K. (2017) GSK3β and ERK regulate the expression of 78 kDa SG2NA and ectopic modulation of its level affects phases of cell cycle Sci. Rep., 7: 7555
Pohl, J., Ring, A., Korkmaz, U., Ehehalt, R and Stremmel, W. (2005) FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts Mol. Biol. Cell, 16, 24-31
Poirier, S., Mayer, G., Murphy, S.R., Garver, W.S., Chang, T.Y., Schu, P. and Seidah, N.G. (2013) The cytosolic adaptor AP-1A is essential for the trafficking and function of Niemann-Pick type C proteins Traffic, 14, 458-469
Rai, S., Tanaka, H., Suzuki, M., Ogoh, H., Taniguchi, Y., Morita, Y., Shimada, T., Tanimura, A., Matsui, K., Yokota, T. et al (2014) Clathrin assembly protein CALM plays a critical role in KIT signaling by regulating its cellular transport from early to late endosomes in hematopoietic cells PLoS One, 9: e109441
Ring, A., Le Lay, S., Pohl, J., Verkade, P. and Stremmel, W. (2006) Caveolin-1 is required for fatty acid translocase (FAT/CD36) localization and function at the plasma membrane of mouse embryonic fibroblasts Biochim. Biophys. Acta, 1761, 416-423
Shulga, Y.V., Myers, D.S., Ivanova, P.T., Milne, S.B., Brown, H.A., Topham, M.K. and Epand, R.M. (2010) Molecular species of phosphatidylinositol-cycle intermediates in the endoplasmic reticulum and plasma membrane Biochemistry 2010, 49, 312–317
Van’t Hof, W. and Resh, M.D. (1997) Rapid plasma membrane anchoring of newly synthesized p59fyn Selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3 J. Cell Biol., 136, 1023-1035
Wang, H-Q., Nakaya, Y., Du, Z., Yamane, T., Shirane, M., Kudo, T., Takeda, M., Takebayashi, K. et al (2005) Interaction of presenilins with FKBP38 promotes apoptosis by reducing mitochondrial Bcl-2 Hum. Mol. Genet., 14, 1889-1902
Woods, A.J., Roberts, M.S., Choudhary, J., Barry, S.T., Mazaki, Y., Sabe, H., Morley, S.J., Critchley, D.R. and Norman, J.C. (2002) Paxillin associates with poly(A)-binding protein 1 at the dense endoplasmic reticulum and the leading edge of migrating cells J. Biol. Chem., 277, 6428-6437
Woods, A.J., White, D.P., Caswell, P.T. and Norman, J.C. (2004) PKD1/PKCμ promotes αvβ3 integrin recycling and delivery to nascent focal adhesions EMBO J., 23, 2531-2543
Zou, K., Hosono, T., Nakamura, T., Shiraishi, H., Maeda, T., Komano, H., Yanagisawa, K. and Michikawa, M. (2008) Novel role of presenilins in maturation and transport of integrin β1 Biochemistry, 47, 3370-3378
13. Embryo stem cells
Bergmann, A., Hansson, E.M., Pursglove, S.E., Farmery, M.R., Lannfelt, L., Lendahl, U., Lundkvist, J. and Naslund, J. (2004) Pen-2 is sequestered in the endoplamic reticulum and subjected to ubiquitylation and proteasome-mediated degradation in the absence of presenilin J. Biol. Chem., 279, 16744-16753
Mulvey, C.M., Breckels, L.M., Geladaki, A., Britovšek, N.K., Nightingale, D.J.H., Christoforou, A., Elzek, M., Deery, M.J., Gatto, L. and Lilley, K.S. (2017) Using hyperLOPIT to perform high-resolution mapping of the spatial proteome Nat. Protoc., 12, 1110-1135
Laudon, H., Karlstrom, H., Mathews, P.M., Farmery, M.R., Gandy, S.E., Lundqvist, J., Lendahl, U. and Naslund, J. (2004) Functional domains in presenilin: The TYR-288 residue controls γ-secretase activity and endoprotoeolysis J. Biol. Chem., 279, 23925-23932
Siman, R. and Velji, J. (2003) Localization of presenilin-nicastrin complexes and γ-secretase activity to the trans-Golgi network J. Neurochem., 84, 1143-1153
Sohaskey, M.L., Jiang, Y., Zhao, J.J., Mohr, A., Roemer, F. and Harland, R.M. (2010) Osteopotentia regulates osteoblast maturation, bone formation, and skeletal integrity in mice J. Cell Biol., 189, 511–525
14. Endothelial cells
Gómez-Escudero, J., Clemente, C., García-Weber, D., Acín-Pérez, R., Millán, J., Enríquez, J.A., Bentley, K., Carmeliet, P. and Arroyo, A.G. (2019) PKM2 regulates endothelial cell junction dynamics and angiogenesis via ATP production Sci. Rep., 9: 15022
Herbert, S.P., Odell, A.F., Ponnambalam, S. and Walker, J.H. (2007) The confluence-dependent interaction of cytosolic phospholipase A2- with annexin A1 regulates endothelial cell prostaglandin E2 generation J. Biol. Chem., 282, 34468-34478
Manickam, V., Tiwari, A., Jung, J-J., Bhattacharya, R., Goel, A., Mukhopadhyay, D. and Choudhury, A. (2011) Regulation of vascular endothelial growth factor receptor 2 trafficking and angiogenesis by Golgi localized t-SNARE syntaxin 6 Blood, 117, 1425-1435
Wu, R-F., Ma, Z., Liu, Z. and Terada, L.S. (2010) Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation Mol. Cell. Biol., 30, 3553-3568
Wu, R.F., Liao, C., Hatoum, H., Fu, G., Ochoa, C.D. and Terada, L.S. (2017) RasGRF couples Nox4-dependent endoplasmic reticulum signaling to Ras Arterioscler. Thromb. Vasc. Biol., 37: 98-107
Yamada, K.H., Nakajima, Y., Geyer, M., Wary, K.K., Ushio-Fukai, M., Komarova, Y. and Malik, A.B. (2014) KIF13B regulates angiogenesis through Golgi to plasma membrane trafficking of VEGFR2 J. Cell Sci., 127, 4518–4530
15. Eosinophils
Spencer, L.A., Melo, R.C.N., Perez, S.A.C., Bafford, S.P., Dvorak, A.M. and Weller, P.F. (2006) Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion Proc. Natl. Acad. Sci. USA, 103, 3333-3338
Glial/glioma cells (see Neuroglioma cells)
16. Glomerular epithelial
Liu, J., Takano, T., Papillon, J., Khadir, A. and Cybulsky, A.V. (2001) Cytosolic phospholipase A2-α associates with plasma membrane, endoplasmic reticulum and nuclear membrane in glomerular epithelial cells Biochem. J., 353, 79-90
17. HEK cells (see also Kidney cells)
Ai, L-S., Lee, Y-W., and Chen, S.S-L. (2009) Characterization of hepatitis C virus core protein multimerization and membrane envelopment: revelation of a cascade of core-membrane interactions J. Virol., 83, 9923-9939
Ballar, P., Zhong, Y., Nagahama, M., Tagaya, M., Shen, Y. and Fang, S. (2007) Identification of SVIP as an endogenous inhibitor of endoplasmic reticulum-associated degradation J. Biol. Chem., 282, 33908-33914
Brunkan, A.L., Martinez, M., Wang, J., Walker, E.S. and Goate, A.M. (2005) A domain at the C-terminus of PS1 is required for presenilinase and -secretase activities J. Neurochem., 92, 1158-1169
Brunkan, A.L., Martinez, M., Wang, J., Walker, E.S., Beher, D., Shearman, M.S. and Goate, A.M. (2005) Two domains within the first putative transmembrane domain of presenilin 1 differentially influence presenilinase and γ-secretase activity J. Neurochem., 94, 1315-1328
Cai, Y., Maeda, Y., Cedzich, A., Torres, V.E., Wu, G., Hayashi, T., Mochizuki, T., Park, J.H., Witzgall, R. and Somlo, S. (1999) Identification and characterization of polycystin-2, the PKD2 gene product J. Biol. Chem., 274, 28557-28565
Campodonico, E.M., Roy, C.R. and Ninio, S. (2016) Legionella pneumophila Type IV effectors YlfA and YlfB are SNARE-like proteins that form homo- and heteromeric complexes and enhance the efficiency of vacuole remodeling PLoS One, 11: e0159698
Chan, W.K.B., Chen, V.P., Luk, W.K.W., Choi, R.C.Y. and Tsim, K.W.K. (2012) N-linked glycosylation of proline-rich membrane anchor (PRiMA) is not required for assembly and trafficking of globular tetrameric acetylcholinesterase Neurosci., Lett., 523, 71– 75
Chen, D., Sandford, G. and Nicholas, J. (2009) Intracellular signaling mechanisms and activities of human herpes virus 8 interleukin-6 J. Virol., 83, 722-733
Chen, D., Choi, Y.B., Sandford, G. and Nicholas, J. (2009) Determinants of secretion and intracellular localization of human herpesvirus 8 interleukin-6 J. Virol., 83, 6874-6882
Chen, V.P., Choi, R.C.Y., Chan, W.K.B., Leung, K.W., Guo, A.J.Y., Chan, G.K.L., Luk, W.K.W. and Tsim, K.W.K. (2011) The assembly of proline-rich membrane anchor (PRiMA)-linked acetylcholine-esterase enzyme– glycosylation is required for enzymatic activity but not for oligomerization J. Biol. Chem., 286, 32948–32961
Chen, W-T., Hsieh, Y-F., Huang, Y-J., Lin, C-C., Lin, Y-T., Liu, Y-C., Lien, C-C. and Cheng, I.H-J. (2015) G206D mutation of presenilin-1 reduces Pen2 interaction, increases Aβ42/Aβ40 ratio and elevates ER Ca2+ accumulation Mol. Neurobiol., 52, 1835-849
Chou, C-H., Lee, R-S., Yang-Yen, H-F. (2006) An internal EELD domain facilitates mitochondrial targeting of Mcl-1 via a Tom70-dependent pathway Mol. Biol. Cell, 17, 3952-3963
Cottam, N.P., Wilson, K.M., Ng, B.G., Körner, C., Freeze, H.H. and Ungar, D. (2014) Dissecting functions of the conserved oligomeric Golgi tethering complex using a cell-free assay Traffic, 15, 12-21
Cunningham, M.R., McIntosh, K.A., Pediani, J.D., Robben, J., Cooke, A.E., Nilsson, M., Gould, G.W., Mundell, S., Milligan, G. and Plevin, R. (2012) Novel role for proteinase-activated receptor 2 (PAR2) in membrane trafficking of proteinase-activated receptor 4 (PAR4) J. Biol. Chem., 287, 16656–16669
Cunningham, M.R., Nisar, S.P., Cooke, A.E., Emery, E.D. and Mundell, S.J. (2013) Differential endosomal sorting of a novel P2Y12 purinoreceptor mutant Traffic, 14, 585–598
Ding, Q., Gros, R., Chorazyczewski, J., Ferguson, S.S.G. and Feldman, R.D. (2005) Isoform-specific regulation of adenylyl cylase function by disruption of membrane trafficking Mol. Pharmacol., 67, 564-571
Ding, W., Albrecht, B., Luo, R., Zhang, W., Stanley, J.R.L., Newbound, G.C. and Lairmore, M.D. (2001) Endoplasmic reticulum and cis-Golgi localization of human T lymphotropic virus type 1 p12I : association with calreticulin and calnexin J. Virol., 75, 7672-7682
Ding, W-G., Xie, Y., Toyoda, F. and Matsuura, H. (2014) Improved functional expression of human cardiac Kv1.5 channels and trafficking-defective mutants by low temperature treatment. PLoS One 9: e92923
Drummer, H.E., Maerz, A. and Poumbourios, P. (2003) Cell surface expression of functional hepatitis C virus E1 and E2 glycoproteins FEBS Lett., 546, 385-390
Duvernay, M.T., Dong, C., Zhang, X., Robitaille, M., Hébert, T.E. and Wu, G. (2009) A single conserved leucine residue on the first intracellular loop regulates ER export of G protein-coupled receptors Traffic, 10, 552–566
Eisses, J.F., Chi, Y. and Kaplan, J.H. (2005) Stable plasma membrane levels of hCTR1 mediate cellular copper uptake J. Biol. Chem., 280, 9635-9639
Finzi, A., Orthwein, A., Mercier, J. and Cohen, E.A. (2007) Productive human immunodeficiency virus type 1 assembly rakes place at the plasma membrane J. Virol., 81, 7476-7490
Finzi, A., Perlman, M., Bourgeois-Daigneault, M-C., Thibodeau, J. and Cohen, E.A. (2013) Major histocompatibility complex class-II molecules promote targeting of human immunodeficiency virus type 1 virions in late endosomes by enhancing internalization of nascent particles from the plasma membrane Cell. Microbiol., 15, 809–822
Fukumori, A., Okochi, M., Tagami, S., Jiang, J., Itoh, N., Nakayama, T., Yanagida, K., Ishizuka-Katsura, Y., Morihara, T. et al (2006) Presenilin-dependent -secretase on plasma membrane and endosomes is functionally distinct Biochemistry, 45, 4907-4914
Fumagalli, F., Noack, J., Bergmann, T.J., Cebollero, E., Brambilla Pisoni, G., Fasana, E., Fregno, I. Galli, C., Loi, M. et al (2016) Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery Nat. Cell Biol., 18, 1173-1184
Gallagher, A.R., Obermüller, N., Cedzich, A., Gretz, N. and Witzgall, R. (2000) An ever-expanding story of cyst formation Cell Tissue Res., 300, 361-371
Gilady, S.Y., Bui, M., Lynes, E.M., Benson, M.D., Watts, R., Vance, J.E. and Simmen, T. (2010) Ero1α requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM) Cell Stress Chaperones, 15, 619–629
Gu, Y., Chen, F., Sanjo, N., Kawarai, T., Hasegawa, H., Duthie, M., Li, W., Ruan, X., Luthra, A., Mount, H.T.J., Tandon, A., Fraser, P.E. and St George Hyslop, P. (2003) APH-1 Interacts with mature and immature forms of presenilins and nicastrin and may play a role in maturation of presenilin-nicastrin complexes J. Biol. Chem., 278, 7374-7380
Gustafsen, C., Glerup, S., Pallesen, L.T., Olsen, D., Andersen, O.M., Nykjær, A., Madsen, P. and Petersen, C.M. (2013) Sortilin and SorLA display distinct roles in processing and trafficking of amyloid precursor protein J. Neurosci., 33, 64 –71
Haffner, C., Dettmer, U., Weller, T. and Haass, C. (2007) The nicastrin-like protein nicalin regulates assembly and stability of the nicalin-nodal modulator (NOMO) membrane protein complex J. Biol. Chem., 282, 10632-10638
Hasegawa, H., Sanjo, N., Chen, F., Gu, Y-J., Shier, C., Petit, A., Kawarai, T., Katayama, T., Schmidt, S.D. et al (2004) Both the sequence and length of the C terminus of PEN-2 are critical for intermolecular interactions and function of presenilin complexes J. Biol. Chem., 279, 46455-46463
Iavarone, C., Ramsauer, K., Kubarenko, A.V., Debasitis, J.C., Leykin, I., Weber, A.N.R., Siggs, O.M., Beutler, B. et al (2011) A point mutation in the amino terminus of TLR7 abolishes signaling without affecting ligand binding J. Immunol., 186, 4213–4222
Joyal, J-S., Nim, S., Zhu, T., Sitaras, N. et al (2014) Subcellular localization of coagulation factor II receptorlike 1 in neurons governs angiogenesis Nature Med., 20, 1165-1173
Kim, J., Noh, S.H., Piao, H., Kim, D.H., Kim, K., Cha, J.S., Chung, W.Y., Cho, H-S., Kim, J.Y. and Lee, M.G. (2016) Monomerization and ER relocalization of GRASP is a requisite for unconventional secretion of CFTR Traffic, 17, 733–753
Kim, Y-S., Zhuang, H., Koehler, R.C. and Dore, S. (2005) Distinct protective mechanisms of HO-1 and HO-2 against hydroperoxide-induced cytotoxicity Free Radical Biol. Med., 38, 85-92
Kume, H., Murayama, K.S. and Araki,, W. (2009) The two-hydrophobic domain tertiary structure of reticulon proteins is critical for modulation of β-secretase BACE1 J. Neurosci. Res., 87, 2963-2972
Larsen, J.V., Hansen, M., Møller, B., Madsen, P., Scheller, J., Nielsen, M. and Petersen, C.M. (2010) Sortilin facilitates signaling of ciliary neurotrophic factor and related helical type 1 cytokines targeting the gp130/leukemia inhibitory factor receptor β heterodimer Mol. Cell. Biol., 17, 4175-4187
Larsen, J.V. and Petersen, C.M. (2017) SorLA in interleukin-6 signaling and turnover Mol. Cell. Biol., 37: e00641-16
Lee, S.M., Olzmann, J.A., Chin, L-S. and Li, L. (2011) Mutations associated with Charcot–Marie–Tooth disease cause SIMPLE protein mislocalization and degradation by the proteasome and aggresome–autophagy pathways J. Cell Sci., 124, 3319–3331
Lefterov, I.M., Koldamova, R.P. and Lazo, J.S. (2000) Human bleomycin hydrolase regulates the secretion of amyloid precursor protein FASEB J., 14, 1837-1847
Li, P., Ninomiya, H., Kurata, Y., Kato, M., Miake, J., Yamamoto, Y., Igawa, O., Nakai, A. et al (2011) Reciprocal control of hERG stability by Hsp70 and Hsc70 with implication for restoration of LQT2 mutant stability Circ. Res., 108, 458-468
Lim, P.J., Danner, R., Liang, J., Doong, H., Harman, C., Srinivasan, D., Rothenberg, C., Wang, H., Ye, Y., Fang, S. and Monteiro, M.J. (2009) Ubiquilin and p97/VCP bind erasin, forming a complex involved in ERAD J. Cell Biol., 187, 201–217
Liu, G., Coyne, A.N., Pei, F., Vaughan, S., Chaung, M., Zarnescu, D.C. and Buchan, J.R. (2017) Endocytosis regulates TDP-43 toxicity and turnover Nat. Comm., 8: 2092
Lu, S.H-J., Jeon, A.H.W., Schmitt-Ulms, G., Qamar, S., Dodd, R., McDonald, B., Li, Y., Meadows, W. et al (2012) Vigilin interacts with signal peptide peptidase Proteome Sci., 10:33
Lu, M-Y. and Liao, F. (2011) Interferon-stimulated gene ISG12b2 is localized to the inner mitochondrial membrane and mediates virus-induced cell death Cell Death Differ., 18, 925–936
Luk, W.K.W., Chen, V.P., Choi, R.C.Y. and Tsim, K.W.K. (2012) N-linked glycosylation of dimeric acetylcholinesterase in erythrocytes is essential for enzyme maturation and membrane targeting FEBS J., 279, 3229–3239
Majer, O., Liu, B., Kreuk, L.S.M., Krogan, N. and Barton, G.M. (2019) UNC93B1 recruits syntenin-1 to dampen TLR7 signalling and prevent autoimmunity Nature, 575, |366-370
Miyazaki, T., Neff, L., Tanaka, S., Horne, W.C. and Baron, R. (2003) Regulation of cytochrome c oxidase activity by c-Src in osteoclasts J. Cell Biol., 160, 709-718
Moonschi, F.H., Fox-Loe, A.M., Fu, X. and Richards, C.I. (2018) Mammalian cell-derived vesicles for the isolation of organelle specific transmembrane proteins to conduct single molecule studies Bio. Protoc., 8: 2825
Ng, K-E., Schwarzer, S., Duchen, M.R. and Tinker, A. (2010) The intracellular localization and function of the ATP-sensitive K+ channel subunit Kir6.1 J. Membr. Biol., 234, 137–147
Okamoto, Y., Bernstein, J.D. and Shikano, S. (2013) Role of C-terminal membrane-proximal basic residues in cell surface trafficking of HIV coreceptor GPR15 protein J. Biol. Chem., 288, 9189–9199
Pallesen, L.T., Gustafsen, C., Cramer, J.F., Petersen, S.V., Thirup, S.S., Madsen, P. and Petersen, C.M. (2020) PAK kinases target sortilin and modulate its sorting Mol. Cell. Biol., 40: e00411-19
Papadakis, M., Hawkins, L.M. and Stephenson, F.A. (2004) Appropriate NR1-NR1 disulfide-linked homodimer formation is requisite for efficient expression of functional, cell surface N-methyl-D-aspartate NR1/NR2 receptors J. Biol. Chem., 279, 14703-14712
Pourcelot, M., Zemirli, N., Da Costa, L.S., Loyant, R., Garcin, D., Vitour, D., Munitic, I., Vazquez, A. and Arnoult, D. (2016) The Golgi apparatus acts as a platform for TBK1 activation after viral RNA sensing BMC Biol., 14: 69
Reynard, O., Borowiak, M., Volchkova, V.A., Delpeut, S., Mateo, M. and Volchkov, V.E. (2009) Ebola virus glycoprotein GP masks both its own epitopes and the presence of cellular surface proteins J. Virol., 83, 9596-9601
Reynard, O., Nemirov, K., Page, A., Mateo, M., Raoul, H., Weissenhorn, W. and Volchkov, V.E. (2011) Conserved proline-rich region of Ebola virus matrix protein VP40 is essential for plasma membrane targeting and virus-like particle release J. Infect. Dis., 204, S884–S891
Sánchez-Sánchez, F., Martínez-Redondo, F., Aroca-Aguilar, J.D., Coca-Prados, M. and Escribano, J. (2007) Characterization of the intracellular proteolytic cleavage of myocilin and identification of calpain II as a myocilin-processing protease J. Biol. Chem., 282, 27810-27824
Santiago, F.W., Covaleda, L.M., Sanchez-Aparicio, M.T., Silvas, J.A., Diaz-Vizarreta, A.C., Patel, J.R., Popov, V., Yu, X-j., García-Sastre, A. and Aguilara, P.V. (2014) Hijacking of RIG-I signaling proteins into virusinduced cytoplasmic structures correlates with the inhibition of type I interferon responses J. Virol., 88, 4572–4585
Shi, Q., Prior, M., He, W., Tang, X., Hu, X. and Yan, R. (2009) Reduced amyloid deposition in mice overexpressing RTN3 is adversely affected by preformed dystrophic neurites J. Neurosci., 29, 9163–9173
Simonova, M., Wall, A., Weissleder, R. and Bogdanov, A. (2000) Tyrosine mutants are capable of prodrug activation in transfected nonmelanotic cells Cancer Res., 60, 6656-6662
Spoerri, L., Vella, L.J., Pham, C.L.L., Barnham, K.J. and Cappai, R. (2012) The amyloid precursor protein copper binding domain histidine residues 149 and 151 mediate APP stability and metabolism J. Biol. Chem., 287, 26840–26853
Stendel, C., Roos, A., Kleine, H., Arnaud, E., Özçelik, M., Sidiropoulos, P.N.M., Zenker, J., Schüpfer, F., Lehmann, U., Sobota, R.M., Litchfield, D.W., Lüscher, B., Chrast, R., Suter, U. and Senderek, J. (2010) SH3TC2, a protein mutant in Charcot–Marie–Tooth neuropathy, links peripheral nerve myelination to endosomal recycling Brain, 133, 2462–2474
Tanahashi, H. and Tabira, T. (2007) A novel beta-site amyloid precursor protein cleaving enzyme (BACE) isoform regulated by nonsense-mediated mRNA decay and proteasome-dependent degradation Neurosci. Lett., 428, 103-108
Torsvik, J., Johansson, B.B., Dalva, M., Marie, M., Fjeld, K., Johansson, S., Bjørkøy, G., Saraste, J., Njølstad, P.R. and Molven, A. (2014) Endocytosis of secreted carboxyl ester lipase in a syndrome of diabetes and pancreatic exocrine dysfunction J. Biol. Chem., 289, 29097–29111
Wagner, T., Dieckmann, M., Jaeger, S., eggen, S. and Pietrzika, C.U. (2013) Stx5 is a novel interactor of VLDL-R to affect its intracellular trafficking and processing Exp. Cell Res., 319, 1956-1972
Wang, J., Brunkan, A.L., Hecimovic, S., Walker, E. and Goate, A. (2004) Conserved “PAL” sequence in presenilins is essential for -secretase activity, but not required for formation or stabilization of γ-secretase complexes Neurobiol. Dis., 15, 654-666
Wilson, S.J., Cavanagh, C.C., Lesher, A.M., Frey, A.J., Russell, S.E. and Smyth, E.M. (2009) Activationdependent stabilization of the human thromboxane receptor: role of reactive oxygen species J. Lipid Res. 50, 1047–1056
Xie, Y., Ding, W-G., Liu, Y., Yu, M., Sun, X. and Matsuura, H. (2019) Long-term 4-AP treatment facilitates functional expression of human Kv1.5 channel Eur. J. Pharmacol., 844, 195–203
Yang, D-S., Tandon, A., Chen, F., Yu, G., Yu, H., Arawaki, S., Hasegawa, H., Duthie, M. et al (2002) Mature glycosylation and trafficking of nicastrin modulate its binding to presenilins J. Biol. Chem., 277, 28135-28142
Yin, J-A., Gao, G., Liu, X-J., Hao, Z-Q., Li, K., Kang, X-L., Li, H., Shan, Y-H. et al (2017) Genetic variation in glia–neuron signalling modulates ageing rate Nature 551, 198-203
Yu, G., Chen, F., Nishimura, M., Steiner, H., Tandon, A., Kawarai, T., Arawaka, S., Supala, A. et al (2000) Mutation of conserved aspartates affect maturation of presenilin 1 and presenilin 2 complexes Acta Neurol. Scand., Suppl. 176, 6-11
Yu, G., Chen, F., Nishimura, M., Steiner, H., Tandon, A., Kawarai, T., Arakawa, S., Supala, A. et al (2000) Mutation of conserved aspartates affects maturation of both aspartate mutant and endogenous presenilin 1 and presenilin 2 complexes J. Biol. Chem., 275, 27348-27353
Yu, G., Chen, F., Nishimura, M., Steiner, H., Tandon, A., Kawarai, T., Arawaka, S., Supala, A. et al (2009) UBXD4, a UBX-containing protein, regulates the cell surface number and stability of α3-containing nicotinic acetylcholine receptors J. Neurosci., 29, 6883– 6896
Zhang, J., Kang, D.E., Xia, W., Okochi, M., Mori, H., Selkoe, D.J. and Koo, E.H. (1998) Subcellular distribution and turnover of presenilins in transfected cells J. Biol. Chem., 273, 12436-12442
Zhu, L., Wang, L., Luo, X., Zhang, Y., Ding, Q., Jiang, X., Wang, X., Pan, Y.and Chen, Y. (2012) Tollip, an intracellular trafficking protein, is a novel modulator of the transforming growth factor-β signaling pathway J. Biol. Chem., 287, 39653–39663
18. Hepatocytes
Boson, B., Granio, O., Bartenschlager, R., Cosset, F-L. (2011) A concerted action of hepatitis C virus P7 and nonstructural protein 2 regulates core localization at the endoplasmic reticulum and virus assembly PloS Pathog., 7: e1002144
Buqué, X., Cano, A., Miquilena-Colina, M.E., García-Monzón, C., Ochoa, B. and Aspichueta, P. (2012) High insulin levels are required for FAT/CD36 plasma membrane translocation and enhanced fatty acid uptake in obese Zucker rat hepatocytes Am. J. Physiol. Endocrinol. Metab., 303, E504–E514
Hainan, L., Huilin, L., Khan, M., Xin, Z., YuJiang, Y., Hui, Z. and Naiquan, Y. (2018) The basic route of the nuclear translocation porcine growth hormone (GH)-growth hormone receptor (GHR) complex (pGH/GHR) in porcine hepatocytes Gen. Comp. Endocrinol., 266, 101–109
Majeau, N., Fromentin, R., Savard, C., Duval, M., Tremblay, M.J. and Leclerc, D. (2009) Palmitoylation of hepatitis C virus core protein is important for virion production J. Biol. Chem., 284, 33915–33925
Nagata, J., Guerra, M.T., Shugrue, C.A., Gomes, D.A., Nagata, N. and Nathanson, M.H. (2007) Lipid rafts establish calcium waves in hepatocytes Gastroenterology, 133, 256-267
19. Hepatoma/hepatocarcinoma cells
Barthel, S.R., Medvedev, R., Heinrich, T., Buchner, S.M., Kettern, N. and Hildt, E. (2016) Hepatitis B virus inhibits insulin receptor signaling and impairs liver regeneration via intracellular retention of the insulin receptor Cell. Mol. Life Sci., 73, 4121–4140
Camarota, L.M., Chapman, J.M., Hui, D.Y. and Howles, P.N. (2004) Carboxyl ester lipase cofractionates with scavenger receptor BI in hepatocyte lipid rafts and enhances selective uptake and hydrolysis of cholesteryl esters from HDL3 J. Biol. Chem., 279, 27599-27606
Christova, T. and Templeton, D.M. (2007) Effect of hypoxia on the binding and subcellular distribution of iron regulatory proteins Mol. Cell. Biochem., 301, 21-32
De Wispelaere, M. and Yang, P.L. (2012) Mutagenesis of the DI/DIII linker in Dengue virus envelope protein impairs viral particle assembly J. Virol., 86, 7072-7083
Garbarino, J., Pan, M., Chin, H.F., Lund, F.W., Maxfield, F.R. and Breslow, J.L. (2012) STARD4 knockdown in HepG2 cells disrupts cholesterol trafficking associated with the plasma membrane, ER, and ERC J. Lipid Res, 53, 2716–2725
Irías-Mata, A., Sus, N., Flory, S., Stock, D., Woerner, D., Podszun, M. and Frank, J. (2018) α-Tocopherol transfer protein does not regulate the cellular uptake and intracellular distribution of α- and γ-tocopherols and -tocotrienols in cultured liver cells Redox Biol., 19, 28–36
Küch, E-M., Vellaramkalayil, V., Zhang, I., Lehnen, D., Brügger, B., Stremmel, W., Ehehalt, R., Poppelreuther, M. and Füllekrug, J. (2014) Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol Biochim. Biophys. Acta, 1841, 227–239
Lin, C-C., Tsai, P., Sun, H-Y., Hsu, M-C., Lee, J-C., Wu, I-C., Tsao, C-W., Chang, T-T. and Young, K-C. (2014) Apolipoprotein J, a glucose-upregulated molecular chaperone, stabilizes core and NS5A to promote infectious hepatitis C virus virion production J. Hepatol., 61, 984–993
Manna, D., Aligo, J., Xu, C., Park, W.S., Koc, H., Heo, W.D. and Konan, K.V. (2010) Endocytic Rab proteins are required for hepatitis C virus replication complex formation Virology 398, 21–37
Masaki, T., Suzuki, R., Murakami, K., Aiziki, H., Ishii, K., Murayama, A., Date, T., Matsuura, Y., Miyamura, T., Wakita, T. and Suzuki, T. (2008) Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles J. Virol., 82, 7964-7976
Masaki, T., Matsunaga, S., Takahashi, H., Nakashima, K., Kimura, Y., Ito, M., Matsuda, M. et al (2014) Involvement of hepatitis C virus NS5A hyperphosphorylation mediated by casein kinase I-α in infectious virus production J. Virol., 88, 7541–7555
Massarweh, A., Bosco, M., Iatmanen-Harbi, S., Tessier, C., Amana, L., Busca, P., Chantret, I., GravierPelletier, C. and Moore, S.E.H. (2016) Brefeldin A promotes the appearance of oligosaccharyl phosphates derived from Glc3Man9GlcNAc2-PP-dolichol within the endomembrane system of HepG2 cells J. Lipid Res., 57, 1477–1491
Nonaka, M., Ma, B.Y., Ohtani, M., Yamamoto, A., Murata, M., Totani, K., Ito, Y. et al (2007) Subcellular localization and physiological significance of intracellular mannan binding protein J. Biol. Chem., 282, 17908-17920
Roth, J., Yam, G.H-F., Fan, J., Hirano, K., Gaplovska-Kysela, K., Le Fourn, V., Guhl, B., Santimaria, R., Torossi, T., Ziak, M. and Zuber, C. (2008) Protein quality control: the who’s who, the where’s and therapeutic escapes Histochem. Cell Biol., 129, 163-177
Schaub, B.E., Berger, B., Berger, E.G. and Rohrer, J. (2006) Transition of galactosyltransferase 1 from transGolgi cisterna to the trans-Golgi network is signal mediated Mol. Biol. Cell, 17, 5153-5162
Schaub, B.E., Berger, B., Berger, E. and Rohrer, J. (2006) Transition of galactosyltransferase 1 from transGolgi cisterna to the trans Golgi network (and back) is signal mediated Mol. Biol. Cell, 17 (Suppl), abstr. 1814
Smith, S.E., Granell, S., Salcedo-Sicilia, L., Baldini, G., Egea, G., Teckman, J.H. and Baldini, G. (2011) Activating transcription factor 6 limits intracellular accumulation of mutant α1-antitrypsin Z and mitochondrial damage in hepatoma cells J. Biol. Chem., 286, 41563–41577
Stone, M., Jia, S., Heo, W.D., Meyer, T. and Konan, K.V. (2007) Participation of Rab5, an early endosomes protein, in hepatitis C virus RNA replication machinery J. Virol., 81, 4551-4563
Tzatsos, A. (2009) Raptor binds the SAIN (Shc and IRS-1 NPXY binding) domain of insulin receptor substrate1 (IRS-1) and regulates the phosphorylation of IRS-1 at Ser-636/639 by mTOR J. Biol. Chem., 284, 22525–22534
Wang, Y., Lam, W., Chen, S-R., Guan, F-L., Dutchman, G.E., Francis, S., Baker, D.C. and Cheng, Y-C. (2016) Tylophorine analog DCB-3503 inhibited cyclin D1 translation through allosteric regulation of heat shock cognate protein 70 Sci. Rep., 6: 32832
Wang, Y., Lee, S., Ha, Y., Lam, W., Chen, S-R., Dutschman, G., Gullen, E.A., Grill, S.P., Cheng, Y. et al (2017) Tylophorine analogs allosterically regulates heat shock cognate protein 70 and inhibits hepatitis C virus replication Sci. Rep., 7: 10037
Wu, M-J., Ke, P-Y., and Horng, J-T. (2014) RacGTPase-activating protein 1 interacts with hepatitis C virus polymerase NS5B to regulate viral replication Biochem. Biophy. Res. Comm., 454, 19–24
Yanatori, I., Yasui, Y., Noguchi, Y. and Kishi, F. (2015) Inhibition of iron uptake by ferristatin II is exerted through internalization of DMT1 at the plasma membrane Cell. Biol. Int., 39, 427–434
Zuber, C., Cormier, J.H., Guhl, B., Santimaria, R., Hebert, D.N. and Roth, J. (2007) EDEM1 reveals a quality control vesicular transport pathway out of the endoplasmic reticulum not involving the COPII exit sites Proc. Natl. Acad. Sci. USA, 104, 4407-4412
20. Hep-2 epithelial cells
Olson, R.M. and Anderson, D.M. (2017) Fractionation techniques to examine effector translocation In Type 3 Secretion Systems: Methods and Protocols, Methods Mol. Biol., 1531, (ed. Nilles, M.L. and Jessen Condry) Springer Science+Business Media, New York, pp 101-109
Yanatori, I., Richardson, D.R., Toyokuni, S. and Kishi, F. (2017) The iron chaperone poly(rC)-binding protein 2 forms a metabolon with the heme oxygenase 1/cytochrome P450 reductase complex for heme catabolism and iron transfer J. Biol. Chem., 292 13205–13229
21. HL60 cells
Bach, J-P., Borta, H., Ackermann, W., Faust, F., Borchers, O. and Schrader, M. (2006) The secretory granule protein syncollin localizes to HL-60 cells and neutrophils J. Histochem. Cytochem., 54, 877-888
22. Human fibroblasts (incl. lung fibroblasts)
Beltran, P.M.J., Mathias, R.A. and Cristea, I.M. (2016) A portrait of the human organelle proteome in space and time during cytomegalovirus infection Cell Systems 3, 361–373
Gorur, A., Yuan, L., Kenny, S.J., Baba, S., Xu, K. and Schekman, R. (2017) COP II-coated membranes function as transport carriers of intracellular procollagen I J. Cell Biol., 216, 1745–1759
Liao, G., Ma, X. and Liu, G. (2011) An RNA-zipcode-independent mechanism that localizes Dia1 mRNA to the perinuclear ER through interactions between Dia1 nascent peptide and Rho–GTP J. Cell Sci., 124, 589-599
Lin, H., Sugimoto, Y., Ohsaki, Y., Ninomiya, H., Oka, A., Taniguchi, M., Ida, H., Eto, Y., Ogawa, S., Matsuzaki, Y. et al (2004) N-octyl-β-valienamine up-regulates activity of F213I mutant β-glucosidase in cultured cells: a potential chemical chaperone therapy for Gaucher disease Biochim. Biophys. Acta, 1689, 219-228
Ohsaki, Y., Sugimoto, Y., Suzuki, M., Hosokawa, H., Yoshimori, T., Davies, J.P., Ioannou, Y.A., Vanier, M.T., Ohno, K. and Ninomiya, H. (2006) Cholesterol depletion facilitates ubiquitylation of NPC1 and its association with SKD1/Vps4 J. Cell Sci., 119, 2643-2653
Seo, J-Y. and Britt, W.J. (2006) Sequence requirements for localization of human cytomegalovirus tegument protein pp28 to the virus assembly compartment and for assembly of infectious virus J. Virol., 80, 5611-5626
Seo, J-Y. and Britt, W.J. (2008) Multimerization of tegument protein pp28 within the assembly compartment is required for cytoplasmic envelopment of human cytomegalovirus J. Virol., 82, 6272-6287
Wang, H. and Stefanovic, B. (2014) Role of LARP6 and nonmuscle myosin in partitioning of collagen mRNAs to the ER membrane PLoS One, 9: e108870
Willett, M., Brocard, M., Davide, A. and Morley, S.J. (2011) Translation initiation factors and active sites of protein synthesis co-localize at the leading edge of migrating fibroblasts Biochem. J., 438, 217–227
23. Human mammary epithelial cells
Ip, C.K.M., 1, Ng, P.K.S., Jeong, K.J., Shao, S.H., 2, Ju, Z., Leonard, P.G., Hua, X., Vellano, C.P. (2018) Neomorphic PDGFRA extracellular domain driver mutations are resistant to PDGFRA targeted therapies Nat. Comm., 9: 4583
24. Human melanocytes
Elassiuty, Y.E., Klarquist, J., Speiser, J., Yousef, R.M., 3, El Refaee, A.A., Hunter, N.S., Shaker, O.G., Gundeti, M., Nieuweboer-Krobotova, L. and Le Poole, I.C. (2011) Heme oxygenase-1 expression protects melanocytes from stress-induced cell death: implications for vitiligo Exp. Dermatol., 20, 496–501
25. Human ocular cells (incl. trabecular meshwork cells)
Jia, L-Y., Gong, B., Pang, C-P., Huang, Y., Lam, D.S-C., Wang, N. Yam, G.H-F. (2009) Correction of the disease phenotype of myocilin-causing glaucoma by a natural osmolyte Invest. Ophthalmol. Vis. Sci., 50, 3743–3749
Park, B-C., Shen, X., Samaraweera, M. and Yue, B.Y.J.T. (2006) Studies of optineurin, a glaucoma gene: Golgi fragmentation and cell death from overexpression of wild-type and mutant optineurin in two ocular cell types Am. J: Pathol., 169, 1976-1989
Sakai, H., Shen, X., Koga, T., Park, B-C., Noskina, Y., Tibudan, M. and Yue, B.Y.J.T. (2007) Mitochondrial association of myocilin, product of a glaucoma gene, in human trabecular meshwork cells J. Cell. Physiol., 213,775-784
Wentz-Hunter, K., Ueda, J., Shimuzi, N. and Yue, B.Y.J.T. (2002) Myocilin is associated with mitochondria in human trabecular meshwork cells J. Cell. Physiol., 190, 46-53
Wentz-Hunter, K., Shen, X. and Yue, B.Y.J. (2003) Distribution of myocilin, a glaucoma gene product, in human corneal fibroblasts Mol. Vision., 9, 308-314
26. Jurkat cells
Finetti, F., Patrussi, L., Masi, G., Onnis, A., Galgano, D., Lucherini, O.M., Pazour, G.J. and Baldari, C.T. (2014) Specific recycling receptors are targeted to the immune synapse by the intraflagellar transport system J. Cell Sci., 127, 1924–1937
Matsuda-Lennikov, M., Biancalana, M., Zou, J., Ravell, J.C., Zheng, L., Kanellopoulou, C., Jiang, P., Notarangelo, G., Jing, H. et al (2019) Magnesium transporter 1 (MAGT1) deficiency causes selective defects in N-linked glycosylation and expression of immune-response genes J. Biol. Chem., 294, 13638–13656
27. Kidney cells (see also HEK cells and Kidney fibroblast cells)
Collecting duct cells
Procino, G., Barbieri, C., Carmosino, M., Rizzo, F., Valenti, G. and Svelto, M. (2010) Lovastatin-induced cholesterol depletion affects both apical sorting and endocytosis of aquaporin-2 in renal cells Am. J. Physiol. Renal Physiol., 298, F266–F278
28. MDCK cells
Bahloul, A., Simmler, M-C., Michel, V., Leibovici, M., Perfeettini, I., Roux, I., Weil, D., Nouaille, S. et al (2009) Vezatin, an integral membrane protein of adherens junctions, is required for the sound resilience of cochlear hair cells EMBO Mol. Med., 1, 125-138
Bakrač, B., Kladnik, A., Maček, P., McHaffie, G., Werner, A., Lakey, J.H. and Anderluh, G. (2010) A toxinbased probe reveals cytoplasmic exposure of Golgi sphingomyelin J. Biol. Chem., 285, 22186–22195
Bivona, T.G., Wiener, H.H., Ahearn, I.M., Siletti, J., Chiu, V.K. and Philips, M.R. (2004) Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion J. Cell Biol., 164, 461-470
Cao, Z., Li, C., Higginbotham, J.N., Franklin, J.L., Tabb, D.L., Graves-Deal, R., Hill, S., Cheek, K. et al (2008) Use of fluorescence-activated vesicle sorting for isolation of Naked2-associated, basolaterally targeted exocytic vesicles for proteomics analysis Mol. Cell. Proteomics, 7, 1651-1667
Guo, X., Mattera, R., Ren, X., Chen, Y., Retamal, C., González, A. and Bonifacino, J.S. (2013) The adaptor protein-1 μ1B subunit expands the repertoire of basolateral sorting signal recognition in epithelial cells Dev. Cell, 27, 353–366
Li, C., Hao, M., Cao, Z., Ding, W., Graves-Deal, R., Hu, J., Piston, D.W. and Coffey, R.J. (2007) Naked2 acts as a cargo recognition and targeting protein to ensure proper delivery and fusion of TGF-α-containing exocytic vesicles at the lower lateral membrane of polarized MDCK cells Mol. Biol. Cell 18, 3081-3093
Liu, F., Jia, L., Thompson-Baine, A-M., Puglise, J.M., ter Beest, M.B.A. and Zegers, M.M.P. (2010) Cadherins and Pak1 control contact inhibition of proliferation by Pak1-βPIX-GIT complex-dependent regulation of cellmatrix signaling Mol. Cell. Biol., 30, 1971-1983
Michaelson, D. and Philips, M. (2006) The use of GFP to localize Rho GTPases in living cells Methods Enzymol., 406, 296-315
Nguyen, S., Baker, K., Padman, B.S., Patwa, R., Dunstan, R.A., Weston, T.A., Schlosser, K., Bailey, B. et al (2017) Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers mBio 8: e01874-17
Sabo, S.L., Lanier, L.M., Ikin, A.F., Khorkova, O., Sahasrabudhe, S., Greengard, P. and Buxbaum, J.D. (1999) Regulation of β-amyloid secretion by FE65, an amyloid protein precursor-binding protein J. Biol. Chem., 274, 7952-7957 (1999)
Tokuo, H. and Coluccio, L.M. (2013) Myosin-1c regulates the dynamic stability of E-cadherin–based cell–cell contacts in polarized Madin–Darby canine kidney cells Mol. Biol. Cell, 24, 2820-2833
29. Monkey kidney cells
De Wilde, A.H., Li, Y., van der Meer, Y., Vuagniaux, G., Lysek, R., Fang, Y., Snijder, E.J. and van Hemert, M.J. (2013) Cyclophilin inhibitors block arterivirus replication by interfering with viral RNA synthesis J. Virol., 87, 1454-1464
Dunphy, J.T., Schroeder, H., Leventis, R., Greentree, W., Knudsen, J.K., Silvius, J.R. and Linder, M.E. (2000) Differential effects of acyl-CoA binding protein on enzymatic and non-enzymatic thioacylation of protein and peptide substrates Biochim. Biophys. Acta, 1485, 185-198
Majoul, I.V., Bastiaens, P.I.H. and Soling H-D (1996) Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells J. Cell Biol., 133, 777-789
Majoul, I., Ferrari, D. and Soling, H-D. (1997) Reduction of protein disulfide bonds in an oxidizing environment FEBS Lett., 401, 104-108
McKenzie, J., Johannes, L., Taguchi, T.and Sheff, D. (2009) Passage through the Golgi is necessary for Shiga toxin B subunit to reach the endoplasmic reticulum FEBS J., 276, 1581–1595
Unger, B., Mercer, J., Boyle, K.A. and Traktman, P. (2013) Biogenesis of the vaccinia virus membrane: genetic and ultrastructural analysis of the contributions of the A14 and A17 proteins J. Viorl., 87, 1083-1097
30. Mouse kidney cells
Bui-Xuan, E-F., Li, Q., Chen, X-Z., Boucher, C.A., Sandford, R., Zhou, J., Basora, N. (2006) More than colocalizing with polycystin-1, polycystin-L is in the centrosome Am. J. Physiol. Renal Physiol., 291, F395-F406
Newby, L.J., Streets, A.J., Zhao, Y., Harris, P.C., Ward, C.J. and Ong, A.C.M. (2002) Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex J. Biol. Chem., 277, 20763-20773
Wu, Y., Dai, X-Q., Li, Q., Chen, C.X., Mai, W., Hussain, Z., Long, W., Montalbetti, N. et al (2006) Kinesin-2 mediates physical and functional interactions between polycystin-2 and fibrocystin Hum. Mol. Genet., 15, 3280-3292
31. NRK cells
Arasaki, K., Uemura, T., Tani, K. and Tagaya, M. (2007) Correlation of Golgi localization of ZW10 and centrosomal accumulation of dynactin Biochem. Biophys. Res. Commun., 359, 811-816
32. Porcine kidney cells
Choukroun, G.J., Marshansky, V., Gustafson, C.E., McKee, M., Hajjar, R.J., Rosenzweig, A., Brown, D. and Bonventre, J.V. (2000) Cytosolic phospholipase A2 regulates Golgi structure and modulates intracellular trafficking of membrane proteins J. Clin. Invest., 106, 983-993
33. Proximal tubule cells
Merkulova, M., Hurtado-Lorenzo, A., Hosokawa, H., Zhuang, Z., Brown, D., Ausiello, D.A. and Marshansky, V. (2011) Aldolase directly interacts with ARNO and modulates cell morphology and acidic vesicle distribution Am. J. Physiol. Cell. Physiol., 300, C1442–C1455
34. Kidney fibroblast cells
34-1. BHK
Andrade, J., Zhao, H., Titus, B., Pearce, T. and Barroso, M.(2004) EF-hand Ca2+-binding protein p22 plays a role in microtubule and endoplasmic reticulum organization and dynamics with distinct Ca2+-binding requirements Mol. Biol. Cell, 15, 481-496
Fontana, J., Lopez-Montero, N., Elliott, R.M., Fernandez, J.J. and Risco, C. (2008) The unique architecture of Bunyamwera virus factories around the Golgi complex Cell. Microbiol., 10, 2112-2028
Frank, S., Upender, S., Hansen, S.H. and Casanova, J.E. (1998) ARNO is a guanine nucleotide exchange factor for ADP-ribosylation factor 6 J. Biol. Chem., 273, 23-27
Liu, H., Liu, Y., Wang, S., Zhang, Y., Zu, X., Zhou, Z., Zhang, B., Xiao, G. (2015) Structure-based mutational analysis of several sites in the E protein: implications for understanding the entry mechanism of Japanese encephalitis virus J. Virol., 89, 5668-5686
Moffat, K., Knox, C., Howell, G., Clark, S.J., Yang, H., Belsham, G.J., Ryan, M. and Wileman, T. (2007) Inhibition of the secretory pathway by foot-and mouth disease virus 2BC protein is reproduced by co-expression of 2B with 2C and the site of inhibition is determined by the subcellular location of 2C J. Virol., 81, 1129-1139
34-2. Human
Takeda, K., Inoue, H., Tanizawa, Y., Matsuzaki, Y., Oba, J., Watanabe, Y., Shinoda, K. and Oka, Y. (2001) WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain Hum. Mol. Genet., 10, 477-484
34-3. Monkey
Pedrazzini, E., Villa, A., Longhi, R., Bulbarelli, A. and Borgese, N. (2000) Mechanism of residence of cytochrome b(5), a tail-anchored protein, in the endoplasmic reticulum J. Cell Biol., 148, 899-913
Roy, M-O., Leventis, R. and Silvius, J.R. (2000) Mutational and biochemical analysis of plasma membrane targeting mediated by the farnesylated, polybasic carboxy terminus of K-ras4B Biochemistry, 39, 8298-8307
35. Lacrimal acinar cells
Gierow, J., Andersson, S.V. and Sjögren, E.C. (2002) Regulated secretion and subcellular distribution of rabbit lacrimal gland alpha-fucosidases Invest. Ophthamol. Vis. Sci., 43, E-Abstract 3125
36. Lung cells
Li, X. and Donowitz, M. (2008) Fractionation of subcellular membrane vesicles of epithelial and nonepithelial cells by OptiPrep™ density gradient ultracentrifugation In Methods Mol. Biol., 440, Exocytosis and Endocytosis (ed. Ivanov, A.I.) Humana Press, Totowa, NJ, pp 97-110
Nguyen, S., Baker, K., Padman, B.S., Patwa, R., Dunstan, R.A., Weston, T.A., Schlosser, K., Bailey, B. et al (2017) Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers mBio 8: e01874-17
37. Lymphocytic/lymphoma cells
Antberg, L., Cifani, P., Sandin, M., Levander, F. and James, P. (2012) Critical comparison of multidimensional separation methods for increasing protein expression coverage J. Proteome Res., 11, 2644−2652
Hall, S.L., Hester, S., Griffin, J.L., Lilley, K.S. and Jackson, A.P. (2009) The organelle proteome of the DT40 lymphocyte cell line Mol.Cell. Proteom., 8, 1295–1305
Hivroz, C., Larghi, P., Jouve, M. and Ardouin, L. (2017) Purification of LAT-containing membranes from resting and activated T lymphocytes In The Immune Synapse: Methods and Protocols, Methods Mol. Biol., 1584, (ed. Baldari, C.T. and Dustin, M.L.) Springer Science+Business Media, LLC, pp 355-368
Saveanu, L., Zucchetti, A.E., Evnouchidou, I., Ardouin, L. and Hivroz, C. (2019) Is there a place and role for endocytic TCR signaling? Immunolog. Rev. 291, 57–74
Schmidt, H., Gelhaus, C., Nebendahl, M., Lettau, M., Wartzl, C., Kabelitz, D., Leippe, M. and Janssen, O. (2008) 2-D DIGE analyses of enriched secretory lysosomes reveal heterogeneous profiles of functionally relevant proteins in leukemic and activated human NK cells Proteomics, 8, 2911-2925
Smith, C.G., Kharkwal, H. and Wilson, D.W. (2017) Expression and subcellular localization of the Kaposi’s sarcoma-associated herpesvirus K15P protein during latency and lytic reactivation in primary effusion lymphoma Cells J. Virol., 91: e01370-17
Xiong, Q. and Rikihisa, Y. (2012) Subversion of NPC1 pathway of cholesterol transport by Anaplasma phagocytophilum Cell. Microbiol., 14, 560–576
Zucchetti, A.E., Bataille, L., Carpier, J-M., Dogniaux, S., Roman-Jouve, M.S., Maurin. M., Stuck, M.W., Rios, R.M. et al (2019) Tethering of vesicles to the Golgi by GMAP210 controls LAT delivery to the immune synapse Nature Comm., 10:2864
38. Macrophages
Andreyev, A.Y., Shen, Z., Guan, Z., Ryan, A., Fahy, E., Subramaniam, S., Raetz, C.R.H., Briggs, S. and Dennis, E.A. (2010) Application of proteomic marker ensembles to subcellular organelle identification Mol. Cell. Proteomics, 9, 388–402
Andreyev, A.Y., Fahy, E., Guan, Z., Kelly, S., Li, X., McDonald, J.G., Milne, S. et al (2010) Subcellular organelle lipidomics in TLR-4-activated macrophages J. Lipid Res., 51, 2785–2797
Chakraborty, S., Srivastava, A., Jha, M.K., Nair, A., Pandey, S.P., Srivastava, N., Kumari, S., Singh, S., Krishnasastry, M.V. and Saha, B. (2015) Inhibition of CD40-induced N-Ras activation reduces Leishmania major infection J. Immunol., 194, 3852–3860
Kassas, N., Tanguy, E., Thahouly, T., Fouillen, L., Heintz, D., Chasserot-Golaz, S., Bader, M-F., Grant, N.J. and Vitale, N. (2017) Comparative characterization of phosphatidic acid sensors and their localization during frustrated phagocytosis J. Biol. Chem., 292, 4266–4279
Korthals, M., Langnaese, K., Smalla, K-H., Kähne, T., Herrera-Molina, R., Handschuh, J., Lehmann, A-C., Mamula, D. et al (2017) A complex of neuroplastin and plasma membrane Ca2+ ATPase controls T cell activation Sci. Rep. 7: 8358
Lipinski, S., Pfeuffer, S., Arnold, P., Treitz, C., Aden, K., Ebsen, H., Falk-Paulsen, M., Gisch, N. et al (2019) Prdx4 limits caspase-1 activation and restricts inflammasome-mediated signaling by extracellular vesicles EMBO J., 38: e101266
Sakashita, N., Chang, C.C.Y., Lei, X., Fujiwara, Y., Takeya, M. and Chang, T-Y. (2010) Cholesterol loading in macrophages stimulates formation of ER-derived vesicles with elevated ACAT1 activity J. Lipid Res., 51, 1263–1272
Schaheen, B., Dang, H. and Fares, H. (2009) Derlin-dependent accumulation of integral membrane proteins at cell surfaces J. Cell Sci., 122, 228-2239
Yan, W., Hwang, D. and Aebersold, R. (2008) Quantitative proteomic analysis to profile dynamic changes in the spatial distribution of cellular proteins In Methods Mol. Biol., 432, Organelle Proteomics (ed. Pflieger, D, and Rossier, J.) Humana Press, Totowa, NJ, pp 389-401
Xia, W-F., Tang, F-L., Xiong, L., Xiong, S., Jung, J-U., Lee, D-H., Li, X-S., Feng, X., Mei, L. and Xiong, W-C. (2013) Vps35 loss promotes hyperresorptive osteoclastogenesis and osteoporosis via sustained RANKL signaling J. Cell. Biol., 200, 821-837
39. Mammary epithelial cells
Herrero, A., Casar, B., Colón-Bolea, P., gudo-Ibáñez, L. and Crespo, P. (2016) Defined spatiotemporal features of RAS-ERK signals dictate cell fate in MCF-7 mammary epithelial cells Mol. Biol. Cell, 27, 1958-1968
40. Mast cells
Chahdi, A., Choi, W.S., Kim, Y. M. and Beaven, M.A. (2003) Mastoparan selectively activates phospholipase D2 in cell membranes J. Biol. Chem., 278, 12039-12045
41. Melanoma cells
Hieronimus, B., Pfohl, J., Busch, C. and Graeve, L. (2017) Expression and characterization of membrane-type 4 matrix metalloproteinase (MT4-MMP) and its different forms in melanoma Cell. Physiol. Biochem., 42, 198-210
McKenzie, A.J., Hoshino, D., Hong, N.H., Cha, D.J., Franklin, J.L., Coffey, R.J., Patton, J.G. and Weaver, A.M. (2016) KRAS-MEK signaling controls Ago2 sorting into exosomes Cell Rep., 15, 978–987
42. Mesenchymal stem cells
Makdissy, N., Haddad, K., D’arc AlBacha, J., Chaker, D., Ismail, B., Azar, A., Oreibi, G., Ayoub, D., Achkar, I., Quilliot, D. and Fajloun, Z. (2018) Essential role of ATP6AP2 enrichment in caveolae/lipid raft microdomains for the induction of neuronal differentiation of stem cells Stem Cell Res. Ther., 9: 132
43. Monocytic cells
Barnett, K.C., Coronas-Serna, J.M., Zhou, W., Ernandes, M.J., Cao, A., Kranzusch, P.J. and Kagan, J.C. (2019) Phosphoinositide interactions position cGAS at the plasma membrane to ensure efficient distinction between self- and viral DNA Cell 176, 1432–1446
Gibbings, D.J., Ciaudo, C., Erhardt, M. and Voinnet, O. (2009) Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity Nat. Cell Biol., 11, 1143-1149
Sakashita, N., Chang, C.C.Y., Lei, X., Fujiwara, Y., Takeya, M. and Chang, T-Y. (2010) Cholesterol loading in macrophages stimulates formation of ER-derived vesicles with elevated ACAT1 activity J. Lipid Res., 51, 1263–1272
44. Murine fibroblasts
Dudek, E., Millott, R., Liu, W-X., Beauchamp, E., Berthiaume, L.G. and Michalak, M. (2015) Nmyristoyltransferase 1 interacts with calnexin at the endoplasmic reticulum Biochem. Biophys. Res. Comm., 468, 889-893
Graham, J.F., Agarwal, S., Kurian, D., Kirby, L., Pinheiro, T.J.T. and Gill, A.C. (2010) Low density subcellular fractions enhance disease-specific prion protein misfolding J. Biol. Chem., 285, 9868–9880
Jain, B.P., Pandey, S., Saleem, N., Tanti, G.K., Mishra, S. and Goswami, S.K. (2017) SG2NA belongs to a three-member striatin subfamily SG2NA is a regulator of endoplasmic reticulum (ER) homeostasis as its depletion leads to ER stress Cell Stress Chaper., 22, 853–866
Jung, J., Wang, J., Groenendyk, J., Lee, D., Michalak, M. and Agellon, L.B. (2017) Fatty acid binding protein (Fabp) 5 interacts with the calnexin cytoplasmic domain at the endoplasmic reticulum Biochem. Biophys. Res, Comm., 493, 202-206
Khalifeh-Soltani, A., McKleroy, W., Sakuma, S., Cheung, Y.Y., 1,3, Tharp, K., Qiu, Y., Turner, S.M., Chawla, A., Stah, A. and Atabai, K. (2014) Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids Nat. Med., 20, 175-183
Lee, D., Kraus, A., Prins, D., Groenendyk, J., Aubry, I., Liu, W-X., Li, H-D., Julien, O. et al (2015) UBC9- dependent association between calnexin and protein tyrosine phosphatase 1B (PTP1B) at the endoplasmic reticulum J. Biol. Chem., 290, 5725-5738
Pourcelot, M., Zemirli, N., Da Costa, L.S., Loyant, R., Garcin, D., Vitour, D., Munitic, I., Vazquez, A. and Arnoult, D. (2016) The Golgi apparatus acts as a platform for TBK1 activation after viral RNA sensing BMC Biol., 14: 69
Takahashi, Y., Shinoda, A., Furuya, N., Harada, E., Arimura, N., Ichi, I., Fujiwara, Y., Inoue, J.and Sato, R. (2013) Perilipin-mediated lipid droplet formation in adipocytes promotes sterol regulatory element-binding protein-1 processing and triacylglyceride accumulation PLoS One, 8: e64605
Tanabe, C., Maeda, T., Zou, K., Liu, J., Liu, S., Nakajima, T. and Komano, H. (2012) The ubiquitin ligase synoviolin up-regulates amyloid β production by targeting a negative regulator of γ-secretase, Rer1, for degradation J. Biol. Chem., 287, 44203–44211
Zhang, M. and Ge, L. (2019) Cell-free reconstitution of autophagic membrane formation In Autophagy: Methods and Protocols, Methods in Molecular Biology, vol. 1880 (ed. Ktistakis, N. and Florey, O.), Springer Science+Business Media LLC New York, pp 135-148
45. Murine muscle cells
Gigel, M., Staffer, S., Ehehalt, F., Stremmel, W., Ehehalt, R. and Füllekrug, J. (2011) FATP4 contributes as an enzyme to the basal and insulin mediated fatty acid uptake of C2C12 muscle cells Am. J. Physiol. Endocrinol. Metab., 301, E785–E796, 2011
46. Myoblasts
Gracida-Jiménez, V., Mondragón-González, R., Vélez-Aguilera, G., Vásquez-Limeta, A., Laredo-Cisneros, M.S., Gómez-López, J. de-D., Vaca, L., Gourlay, S.C. et al (2017) Retrograde trafficking of β-dystroglycan from the plasma membrane to the nucleus Sci. Rep., 7: 9906
Gyobu, S., Miyata, H., Ikawa, M., Yamazaki, D., Takeshima, H., Suzuki, J., Nagata, S. (2016) A role of TMEM16E carrying a scrambling domain in sperm motility Mol. Cell. Biol., 36, 645-659
Mizuta, K., Tsutsumi, S., Inoue, H., Sakamoto, Y., Miyatake, K., Miyawaki, K., Noji, S., Kamata, N. and Itakura, M. (2007) Molecular characterization of GDD1/TMEM16E, the gene product responsible for autosomal dominant gnathodiaphyseal dysplasia Biochem. Biophys. Res. Commun., 357, 126-132
Valkova, C., Albrizio, M., Röder, I.V., Schwake, M., Betto, R., Rudolf, R. and Kaether, C. (2011) Sorting receptor Rer1 controls surface expression of muscle acetylcholine receptors by ER retention of unassembled αsubunits Proc. Natl. Acad. Sci. USA, 108, 621–625
47. Myotubes
Gavriilidis, C., Laredj, L., Solinhac, R., Messaddeq, N., Viaud, J., Laporte, J., Sumara, I. and Hnia, K. (2018) The MTM1–UBQLN2–HSP complex mediates degradation of misfolded intermediate filaments in skeletal muscle Nat. Cell Biol., 20, 198–210
48. Neuroblastoma cells
Colla, E., Coune, P., Liu, Y., Pletnikova, O., Troncoso, J.C., Iwatsubo, T., Schneider, B.L. and Lee, M.K. (2012) Endoplasmic reticulum stress is important for the manifestations of α-synucleinopathy in vivo J,.Neurosci., 32, 3306 –3320
Giles, L.M., Li, L. and Chin, L-S. (2009) Printor, a novel torsinA-interacting protein implicated in dystonia pathogenesis J. Biol. Chem., 284, 21765–21775
Grimm, M.O.W., Kuchenbecker, J., Grösgen, S., Burg, V.K., Hundsdörfer, B., Rothhaar, T.L., Friess, P., de Wilde, M.C. et al (2011) Docosahexaenoic acid reduces amyloid β production via multiple pleiotropic mechanisms J. Biol., Chem., 286, 14028–14039
Ito, D., Fujisawa, T., Iida, H. and Suzuki, N. (2008) Characterization of seipin/BSCL2, a protein associated with spastic paraplegia 17 Neurobiol. Dis., 31 266-277
Iwata, H., Tomita, T., Maruyama, H. and Iwatsubo, T. (2001) Subcellular compartment and molecular subdomain of β-amyloid precursor protein relevant to the Aβ42-promoting effects of Alzheimer mutant presenilin 2 J. Biol. Chem., 276, 21678-21685
Jung, S. (2010) ELGA stimulates gamma-secretase activity to increase Aβ generation Alzheimers Dementia 6, Suppl 1, S260
Kim, S.H., Lah, J.J., Thinakaran, G., Levey, A. and Sisodia S.S. (2000) Subcellular localization of presenilins: association with a unique membrane pool in cultured cells Neurobiol. Dis., 7, 99-117
Leem, J.Y., Vijayan, S., Han, P., Cai, D., Machura, M., Lopes, K.O., Veselits, M.L., Xu, H. and Thinakaran, G. (2002) Presenilin 1 is required for maturation and cell surface accumulation of nicastrin J. Biol. Chem., 277, 19236-19240
Omi, T., Tanimukai, H., Kanayama, D., Sakagami, Y., Tagami, S., Okochi, M., Morihara, T., Sato, M., Yanagida, K., et al (2014) Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor Cell Death Dis., 5: e1332
Petanceska, S., Seeger, M., Checler, F. and Gandy, S. (2000) Mutant presenilin 1 increases the levels of Alzheimer amyloid β-peptide Aβ42 in late compartments of the constitutive secretory pathway J. Neurochem., 74, 1878-1884
Qin, W. and Jia, J. (2008) Down-regulation of insulin-degrading anzume by presenilin 1 V97L mutant potentially underlies increased levels of amyloid beta 42 Eur. J. Neurosci., 27, 2425-2432
Shrivastava-Ranjan, P., Faundez, V., Fang, G., Rees, H., Lah, J.J., Levey, A.I. and Kahn, R.A. (2008) Mint3/X11γ is an ADP-ribosylation factor-dependent adaptor that regulates the traffic of the Alzheimer’s precursor protein from the trans-Golgi network Mo. Biol. Cell, 19, 51-64
Takahashi, M., Dore, S., Ferris, C.D., Tomita, T., Sawa, A., Wolosker, H., Borchelt, D. R. et al (2000) Amyloid precursor proteins inhibit heme oxygenase activity and augment neurotoxicity in Alzheimer’s disease Neuron, 28, 461-473
Takasugi, N., Takahashi, Y., Morohashi, Y. and Tomita, T. (2002) The mechanism of γ-secretase activities through high molecular weight complex formation of presenilins is conserved in Drosophila melanogaster and mammals J. Biol. Chem., 277, 50198-50205
Tibaldi, E., Brunati, A.M., Massimino, M.L., Stringaro, A., Colone, M., Agostinelli, E., Arancia, G. and Toninello, A. (2008) Src-Tyrosine kinases are major agents in mitochondrial tyrosine phosphorylation J. Cell. Biochem., 104, 840-849
Tomita, T., Watabiki, T., Takikawa, R., Morohashi, Y., Takasugi, N., Kopan, R., de Strooper, B. and Iwatsubo, T. (2001) The first proline of PALP motif at the C terminus of presenilins is obligatory for stabilization, complex formation, and γ-secretase activities of presenilins J. Biol. Chem., 276, 33273-33281
Urano, Y., Ochiai, S. and Noguchi, N. (2013) Suppression of amyloid-β production by 24S-hydroxy-cholesterol via inhibition of intracellular amyloid precursor protein trafficking FASEB J., 27, 4305–4315
Wang, Y.P., Biernat, J., Pcikhardt, M., Mandelkow, E. and Mandelkow, E-M. (2007) Stepwise proteolysis liberates tau fragments that nucleate the Alzheimer-like aggregation of full-length tau in a neuronal cell model Proc. Natl. Acad. Sci. USA, 104, 10252-10257
49. Neuroglioma cells
Brockway, S.M., Clay, C.T., Lu, X.T. and Denison, M.R. (2003) Characterization of the expression, intracellular localization, and replication complex association of the putative mouse hepatitis virus RNAdependent RNA polymerase J. Virol., 77, 10515-10527
Chang, Y., Tesco, G., Jeong, W.J., Lindsley, L., Eckman, E.A., Eckman, C.B., Tanzi, R.E. and Guenette, S.Y. (2003) Generation of the β-amyloid peptide and the amyloid percursor protein C-terminal fragment γ are potentiated by FE65L1 J. Biol. Chem., 278, 511000-51107
Lane, R.F., Steele, J.W., Cai, D., Ehrlich, M.E., Attie, A.D. and Gandy, S. (2013) Protein sorting motifs in the cytoplasmic tail of SorCS1 control generation of Alzheimer’s amyloid-β peptide J. Neurosci., 33, 7099 -7107
Patton, S.M., Pinero, D.J., Surguladze, N., Beard, J. and Connor, J.R. (2005) Subcellular localization of iron regulatory proteins to Golgi and ER membranes J. Cell Sci., 118, 4365-4373
Podyma-Inoue, K.A., Moriwaki, T, Rajapakshe, A,R., Terasawa, K. and Hara-Yokoyama, M. (2016) Characterization of heparan sulfate proteoglycan-positive recycling endosomes isolated from glioma cells Canc. Genom. Proteom., 13, 443-452
Richt, J.A. and Garten, W. (2005) Maturation of Borma virus disease virus glycoprotein FEBS Lett., 579, 4751-4756
Sanjo, N., Katayama, T., Hasegawa, H., Jin, H., Duthie, M., Mount, H.T.J., Mizusawa, H., St George-Hyslop, P. and Fraser, P.E. (2010) Localization and trafficking of endogenous anterior pharynx-defective 1, a component of Alzheimer’s disease related -secretase Neurosci. Lett., 483, 53–56
Sims, A.C., Ostermann, J. and Denison, M.R. (2000) Mouse hepatitis virus replicase proteins associate with two distinct populations of intracellular membranes J. Virol., 74, 5647-5654
Tekirian, T.L., Merriam, D.E., Marshansky, V., Miller, J., Crowley, A.C., Chan, H., Ausiello, D. et al (2001) Subcellular localization of presenilin 2 endoproteolytic C-terminal fragments Mol. Brain Res., 96, 14-20
Tien, N.T., Karaca, I., Tamboli, I.Y. and Walter, J. (2016) Trehalose alters subcellular trafficking and the metabolism of the Alzheimer-associated amyloid precursor protein J. Biol. Chem., 291, 10528–10540
50. Neuronal cells
Akassoglou, K., Malester, B., Xu, J., Tessarollo, L., Rosenbluth, J. and Chao, M.V. (2004) Brain-specific deletion of neuropathy target esterase/swisscheese results in neurodegeneration Proc. Natl. Acad. Sci. USA, 101, 5075-5080
Antoniou, A., Khudayberdiev, S., Idziak, A., Bicker, S., Jacob, R. and Schratt, G. (2018) The dynamic recruitment of TRBP to neuronal membranes mediates dendritogenesis during development EMBO Rep., 19: e44853
Arévalo, J., Pereira, D.B., Yano, H., Teng, K.K. and Chao, M.V. (2006) Identification of a switch in neurotrophin signaling by selective tyrosine phosphorylation J. Biol. Chem., 281, 1001-1007
Arévalo, J.C., Wu, S.H., Takahashi, T., Zhang, H., Yu, T., Yano, H., Milner, T.A., Tessarollo, L., Ninan, I., Arancio, O. and Chao, M.V. (2010) The ARMS/Kidins220 scaffold protein modulates synaptic transmission Mol. Cell. Neurosci., 45, 92–100
Barthet, G., Jordà-Siquier, T., Rumi-Masante, J., Bernadou, F., Müller, U. and Mulle, C. (2018) Presenilinmediated cleavage of APP regulates synaptotagmin-7 and presynaptic plasticity Nat. Comm., 9: 4780
Delcroix, J-D., Valletta, J.S., Wu, C., Hunt, S.J., Kowal, A.S. and Mobley, W.C. (2003) NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals Neuron, 39, 69-84
Kamal, A., Almenar-Queralt, A., LeBlanc, J.F., Roberts, E.A. and Goldstein, L.S.B. (2001) Kinesin-mediated axonal transport of a membrane compartment containing β-secretase and presenilin-1 requires APP Nature, 414, 643-648
Kao, S-C., Krichevsky, A.M., Kosik, K.S. and Tsai, L-H. (2004) BACE1 suppression by RNA interferences in primary cortical neurons J. Biol. Chem., 279, 1942-1949
Kim, A.H., Yano, H., Cho, H., Meyer, D., Monks, B., Margolis, B., Birnbaum, M.J. and Chao, M.V. (2002) Akt1 regulates a JNK scaffold during excitotoxic apoptosis Neuron, 35, 697-709
Kim, Y-S., Zhuang, H., Koehler, R.C. and Dore, S. (2005) Distinct protective mechanisms of HO-1 and HO-2 against hydroperoxide-induced cytotoxicity Free Radical Biol. Med., 38, 85-92
Lee, S.M., Sha, D., Mohammed, A.A., Asress, S., Glass, J.D., Chin, L-S. and Li, L. (2013) Motor and sensory neuropathy due to myelin infolding and paranodal damage in a transgenic mouse model of Charcot–Marie–Tooth disease type 1C Hum. Mol. Genet., 22, 1755–1770
Lei, P., Ayton, S., Finkelstein, D.I., Spoerri, L., Ciccotosto, G.D., Wright, D.K., Wong, B.X.W., Adlard, P.A., Cherny, R.A., et al (2012) Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export Nat. Med., 18, 291-295
López-Menéndez, C., Simón-García, A., Gamir-Morralla, A., Pose-Utrilla, J., Luján, R., Mochizuki, N., DíazGuerra, M. and Iglesias, T. (2019) Excitotoxic targeting of Kidins220 to the Golgi apparatus precedes calpain cleavage of Rap1-activation complexes Cell Death Dis., 10: 535
Rezvani, K., Baalman, K., Teng, Y., Mee, M.P., Dawson, S.P., Wang, H., De Biasi. M. and Mayer, R.J. (2012) Proteasomal degreadation of the metabotropic glutamate receptor 1α is mediated by Homer-3 via the proteasomal S8 ATPase J. Neurochem. (2012) 122, 24–37
Schäfer, M.K.E., Nam, Y-C., Moumen, A., Keglowich, L., Bouché, E., Küffner, M., Bock, H.H., Rathjen, F.G., Raoul, C. and Frotscher, M. (2010) L1 syndrome mutations impair neuronal L1 function at different levels by divergent mechanisms Neurobiol. Dis., 40, 222–237
Won, J-S., Im, Y-B., Khan, M., Contreras, M., Singh, A.K. and Singh, I. (2008) Lovastatin inhibits amyloid precursor protein (APP) β-cleavage through reduction of APP distribution in Lubrol WX extractable low density lipid rafts J. Neurochem., 105, 1536-1549
Yano, H. and Chao, M.V. (2004) Mechanisms of neurotrophin receptor vesicular transport J. Neurobiol., 58, 244-257
51. Osteoblasts/osteosarcoma cells
Delorme-Axford, E., Morosky, S., Bomberger, J., Stolz, D.B., Jackson, W.T. and Coyne, C.B. (2014) BPIFB3 regulates autophagy and coxsackievirus B replication through a noncanonical pathway independent of the core initiation machinery mBio, 5, e02147-14
Ribe, D. and Stenbeck, G. (2010) Modulation of intracellular trafficking by TGFB and rhoa Bone 47, S130-S131
Yuan, L., Kenny, S.J., Hemmati, J., Xu, K. Schekman, R. (2018) TANGO1 and SEC12 are copackaged with procollagen I to facilitate the generation of large COPII carriers Proc. Natl. Acad. Sci. USA 115, E12255–E12264
52. Pancreatic cells
Bearrows, S.C., Bauchle, C.J., Becker, M., Haldeman, J.M., Swaminathan, S. and Stephens, S.B. (2019) Chromogranin B regulates early-stage insulin granule trafficking from the Golgi in pancreatic islet β-cells J. Cell Sci., 132, jcs231373
Gupta, S., McGrath, B. and Cavener, D.R. (2010) PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway Diabetes, 59, 1937–1947
Raffaniello, R., Fedorova, D., Ip, D. and Rafiq, S. (2009) Hsp90 co-localizes with Rab-GDI-1 and regulates agonist-induced amylase release in AR42J cells Cell. Physiol. Biochem., 24, 369-378
53. Pheochromocytoma (PC12) cells
Hasegawa, H., Zinsser, S., Rhee, Y., Vik-Mo, E.O., Davanger, S. and Hay, J.C. (2003) Mammalian Ykt6 is a neuronal SNARE targeted to a specialized compartment by its profilin-like amino terminal domain Mol. Biol. Cell, 14, 698-720
Kametani, F., Usami, M., Tanaka, K., Kume, H. And Mori, H. (2004) Mutant presenilin (A260V) affects Rab8 in PC12D cell Neurochem. Int., 44, 313-320
Kim, C-H., Leung, A., Huh, Y.H., Yang, E., Kim, D-J., Leblanc, P., Ryu, H. et al (2011) Norepinephrine deficiency is caused by combined abnormal mRNA processing and defective protein trafficking of dopamine β-hydroxylase J. Biol. Chem., 286, 9196–9204
Li, Y., Chin, L-S., Levey, A.L. and Li, L. (2002) Huntingtin-associated protein 1 interacts with hepatocyte growth factor-regulated tyrosine kinase substrate and functions in endosomal trafficking J. Biol. Chem., 277, 28212-28221
Patranabis, S. and Bhattacharyya, S.N. (2016) Phosphorylation of Ago2 and subsequent inactivation of let-7a RNP specific microRNAs control differentiation of mammalian sympathetic neurons Mol. Cell. Biol., 36, 1260-1271
Pryor, S., McCaffrey, G., Young. L.R. and Grimes, M.L. (2012) NGF causes TrkA to specifically attract microtubules to lipid rafts PLoS One 7: e35163
Rezvani, K., Teng, Y. and De Biasi, M. (2010) The ubiquitin–proteasome system regulates the stability of neuronal nicotinic acetylcholine receptors J. Mol. Neurosci., 40, 177–184
Sannerud, R., Marie, M., Nizak, C., Dale, H.A., Pernet-Gallay, K., Perez, F., Goud, B. and Saraste, J. (2006) Rab1 defines a novel pathway connecting the pre-Golgi intermediate compartment with the cell periphery Mol. Biol. Cell, 17, 1514-1526
Sannerud, R., Marie, M., Hansen, B.B. and Saraste, J. (2008) Use of polarized PC12 cells to monitor protein localization in the early biosynthetic pathway In Methods Mol. Biol., 457, Membrane Trafficking (ed. Vancura, A.) Humana Press, Totowa, NJ, pp 253-265
Thayanidhi, N., Liang, Y., Hasegawa, H., Nycz, D.C., Oorschot, V., Klumperman, J. and Hay, J.C. (2012) RSNARE ykt6 resides in membrane-associated protease-resistant protein particles and modulates cell cycle progression when over-expressed Biol. Cell, 104, 397–417
Van Niel, G., Bergam, P., Di Cicco, A., Hurbain, I., Lo Cicero, A., Dingli, F., Palmulli, R., Fort, C. et al (2015) Apolipoprotein E regulates amyloid formation within endosomes of pigment cells Cell Rep., 13, 43–51
Wan, J., Cheung, A.Y., Fu, W-Y., Wu, C., Zhang, M., Mobley, W.C., Cheung, Z.H. and Ip, N.Y. (2008) Endophilin B1 as a novel regulator of nerve growth factor/ TrkA trafficking and neurite outgrowth J. Neurosci., 28, 9002-9012
Wu, C., Lai, C-F. and Mobley, W.C. (2001) Nerve growth factor activates persistent Rap1 signaling in endosomes J. Neurosci., 21, 5406-5416
Wu, C., Ramirez, A., Cui, B., Ding, J., Delcroix, J-D.M., Valletta, J.S., Yang, Y., Chu, S. and Mobley, W.C. (2007) A functional dynein-microtubule network is required for NGF signaling through the Rap1/MAPK pathway Traffic, 8, 1503-1520
Yano, H. and Chao, M.V. (2005) Biochemical characterization of intracellular membranes bearing Trk neurotrophin receptors Neurochem. Res., 30, 767-777
54. Platelets
Albarran, L., Berna-Erro, A., Dionisio, N., Redondo, P.C., Lopez, E., Lopez, J.J., Salido, G.M., Brull Sabate, J.M. and Rosado, J.A. (2014) TRPC6 participates in the regulation of cytosolic basal calcium concentration in murine resting platelets Biochim. Biophys. Acta, 1843, 789–796
Flaumenhaft, R., Dilks, J.R., Rozenvayn, N., Monahan-Earley, R., Feng, D. and Dvorak, A.M. (2005) The actin cytoskeleton differentially regulates platelet α-granule and dense-granule secretion Blood, 105, 3879-3887
55. Polyoma virus producing JCI cells
Suzuki, T., Orba. Y., Okada, Y., Sunden, Y., Kimura, T., Tanaka, S., Nagashima, K., Hall, W.W. and Sawa, H. (2010) The human polyoma JC virus agnoprotein acts as a viroporin PLoS Pathogens, 6: e1000801
56. Prostate carcinoma cells
Thomas, J.D., Longen, C.G., Oyer, H.M., Chen, N., Maher, C.M., Salvino, J.M., Kania, B., Anderson, K.N. et al (2017) Sigma1 targeting to suppress aberrant androgen receptor signaling in prostate cancer Cancer Res., 77, 2439-2452
57. Retinal ganglion cells
Joyal, J-S., Nim, S., Zhu, T., Sitaras, N. et al (2014) Subcellular localization of coagulation factor II receptorlike 1 in neurons governs angiogenesis Nature Med., 20, 1165-1173
58. Skin fibroblasts
Li, Q., Zhang, Y., Marden, J.J., Banfi, B. and Engelhardt, J.F. (2008) Endosomal NADPH oxidase regulates cSrc activation following hypoxia/reoxygenation injury Biochem. J., 411, 531-541
59. Stellate cells (hepatic)
Brunati, A.M., Tibaldi, E., Carraro, A., Gringeri, E., D’Amico, F., Toninello, A., Massimino, M.L., Pagano, M.A., Nalesso, G. and Cillo, U. (2008) Cross-talk between PDGF and S1P signalling elucidates the inhibitory effect and potential antifibrotic action of the immunomodulator FTY720 in activated HSC-cultures Biochim. Biophys. Acta 1783 347-359
Tibaldi, E., Brocca, A., Sticca, A., Gola, E., Pizzi, M., Bordin, L., Pagano, M.A., Mazzorana, M., Donà, G. et al (2020) Fam20C-mediated phosphorylation of osteopontin is critical for its secretion but dispensable for its action as a cytokine in the activation of hepatic stellate cells in liver fibrogenesis FASEB J., 34, 1122–1135
60. Stem cells
Christoforou, A., Mulvey, C.M., Breckels, L.M., Geladaki, A., Hurrell, T., Hayward, P.C., Naake, T., Gatto, L., Viner, R., Arias, A.M. and Lilley, K.S. (2016) A draft map of the mouse pluripotent stem cell spatial proteome Nat. Comm., 7: 8992
61. Vascular smooth muscle cells
Cavet, M.E., Pang, J., Yin, G. And Berk, B.C. (2008) An epidermal growth factor (EGF) –dependent interaction between GIT1 and sorting nexin 6 promotes degradation of the EGF receptor FASEB J., 22, 3607-3616
Lucero, H.A., Ravid, K., Grimsby, J.L., Rich, C.B., DiCamillo, S.J., Mäki, J.M., Myllyharju, J. and Kagan, H.M. (2008) Lysyl oxidase oxidizes cell membrane proteins and enhances the chemotactic response of vascular smooth muscle cells J. Biol. Chem., 283, 24103-24117
Masori, M., Hamamoto, A., Mawatari, K., Harada, N., Takahasi, A. and Nakaya, Y. (2007) Angiotensin II decreases glucose uptake by downregulation of GLUT1 in the cell membrane of the vascular smooth muscle cell line A10 J. Cardiovasc. Pharmacol., 50, 267-273
Mammalian tissues
1. Brain
Araki, Y., Tomita, S., Yamaguchi, H., Miyagi, N., Sumioka, A., Kirino, Y. and Suzuki, T. (2003) Novel cadherin-related membrane proteins, alcadeins, enhance the X11-like protein-mediated stabilization of amyloid β-protein precursor metabolism J. Biol.Chem., 278, 49448-49458
Araki, Y., Kawano, T., Taru, H., Saito, Y., Wada, S., Miyamoto, K., Kobayashi, H. et al (2007) The novel cargo Alcadein induces vesicle association of kinesin-1 motor components and activates axonal transport EMBO J., 26, 1475-1486
Ballar, P., Shen, Y., Zhong, Y., Nagahama, M., Tagaya, M., Apostolou, A. and Fang, S. (2007) SVIP interacts with Derlin1 and regulates ER-associated degradation FASEB J., 21, 803.3
Chakraborty, G., Saito, M., Shah, R., Mao, R-F., Vadasz,C. and Saito, M. (2012) Ethanol triggers sphingosine 1-phosphate elevation along with neuroapoptosis in the developing mouse brain J. Neurochem., 121, 806–817
Chen, F., Yang, D-S., Petanceska, S., Yang, A., Tandon, A., Yu, G., Rozmahel, R., Ghiso, J., Nishimura, M., Zhang, D.M., Kawarai, T. et al P.E (2000) Carboxyl-terminal fragments of Alzheimer β-amyloid precursor protein accumulate in restricted and unpredicted intracellular compartments in presenilin 1 deficient cells J. Biol. Chem., 275, 36794-36802
Chen, F., Tandon, A., Sanjo, N., Gu, Y-J., Hasegawa, H., Arawaka, S., Lee, F.J.S., Ruan, X., Mastrangelo, P. et al (2003) Presenilin 1 and Presenilin 2 have differential effects on the stability and maturation of Nicastrin in mammalian brain J. Biol. Chem., 278, 19974-19979
D’Acunzo, P., Hargash, T., Pawlik, M., Goulbourne, C.N., Perez‐Gonzalez, R. and Levy, E. (2019) Enhanced generation of intraluminal vesicles in neuronal late endosomes in the brain of a Down syndrome mouse model with endosomal dysfunction Devel. Neurobiol., 79, 656–663
Fernando, R.N., Albiston, A.L. and Chai, S.Y. (2008) The insulin-regulated aminopeptidase IRAP is colocalised with GLUT4 in the mouse hippocampus – potential role in modulation of glucose uptake in neurones? Eur. J. Neurosci., 28, 588-598
Frykman, S., Hur, J-Y., Franberg, J., Aoki, M., Winblad, B., Nahalkova, J., Behbahani, H. and Tjernberg, L.O. (2010) Synaptic and endosomal localization of active -secretase in rat brain PLoS One, 5: e8948
Galliciotti, G., Glatzel, M., Kinter, J., Kozlov, S.V., Cinelli, P., Rülicke, T. and Sonderegger, P. (2007) Accumulation of mutant neuroserpin precedes development of clinical symptoms in familial encephalopathy with neuroserpin inclusion bodies Am. J. Pathol., 170, 1305-1313
Gandy, S., Zhang, Y-w., Ikin, A., Schmidt, S.D., Levy, E., Sheffield, R., Nixon, R.A., Liao, F-F., Mathews, P.M., Xu, H. and Ehrlich, M.E. (2007) Alzheimer’s presenilin 1 modulates sorting of APP and its carboxyterminal fragments in cerebral neurons in vivo J. Neurochem., 102, 619-626
Green, K.N., Khashwji, H., Estrada, T. and LaFerla, F.M. (2011) ST101 induces a novel 17kDa APP cleavage that precludes Aβ generation in vivo Ann. Neurol., 69, 831-844
Hryciw, T., MacDondald, J.I.S., Phillips, R., Seah, C., Pasternak, S. and Meakin, S. (2010) The fibroblast growth factor receptor substrate 3 adapter is a developmentally regulated microtubule-associated protein expressed in migrating and differentiated neurons J. Neurochem., 112, 924-939
Jacobs, S.B.R., Basak, S., Murray, J.I. and Attardi, L.D. (2007) Siva is an apoptosis-selective p53 target gene important for neuronal cell death Cell Death Differ., 14, 1374-1385
Kumada, T., Yamanaka, Y., Kitano, A., Shibata, M., Awaya, T., Kato, T., Okawa, K., Abe, T., Oshima, N., Nakahata, T. and Heike, T. (2010) Ttyh1, a Ca2+-binding protein localized to the endoplasmic reticulum, is required for early embryonic development Dev. Dyn., 239, 2233–2245
Kume, H., Konishi, Y., Murayama, K.S., Kametani, F. and Araki, W. (2009) Expression of reticulon 3 in Alzheimer’s disease brain Neuropathol. Appl. Neurobiol., 35, 178–188
Lee, M-S., Kao, S-C., Lemere, C.A., Xia, W., Tseng, H-C., Zhou, Y., Neve, R., Ahlijanian, M.K. and Tsai, L-H. (2003) APP processing is regulated by cytoplasmic phosphorylation J. Cell Biol., 163, 83-95
Li, Q., Sullivan, N.R., McAllister, C.E., Van de Kar, L.D. and Muma, N. A. (2013) Estradiol accelerates the effects of fluoxetine on serotonin 1A receptor signaling Psychoneuroendocrinology, 38, 1145—1157
Liu, L., Watanabe, N., Akatsu, H. and Nishimura, M. (2016) Neuronal expression of ILEI/FAM3C and its reduction in Alzheimer’s disease Neuroscience, 330, 236–246
Lochhead, J.J., McCaffrey, G., Quigley, C.E., Finch, J., DeMarco, K.M., Nametz, N. and Davis, T.P. (2010) Oxidative stress increases blood–brain barrier permeability and induces alterations in occluding during hypoxia–reoxygenation J. Cereb. Blood Flow Metab., 30, 1625–1636
MacDonald, J.I.S., Dietrich, A., Gamble, S., Hryciw, T., Grant, R.I. and Meakin, S. (2012) Nesca, a novel neuronal adapter protein, links the molecular motor kinesin with the pre-synaptic membrane protein, syntaxin1, in hippocampal neurons J. Neurochem., 121, 861–880
Motodate, R., Saito, H., Sobu, Y., Hata, S., Yuhki, Y., Nakaya, T. and Suzuki, T. (2019) X11 and X11-like proteins regulate the level of synaptic glutamate receptors J. Neurochem., 148, 480-498
Nahalkova, J., Volkmann, I., Aoki, M., Winblad, B., Bogdanovic, N., Tjernberg, L.O. and Behbahani, H. (2010) CD147, a γ-secretase associated protein is up-regulated in Alzheimer’s disease brain and its cellular trafficking is affected by presenilin-2 Neurochem. Int., 56, 67–76
Polyak, M.J., Li, H., Shariat, N. and Deans, J.P. (2008) CD20 Homo-oligomers physically associate with the B cell antigen receptor: dissociation upon receptor engagement and recruitment of phosphoproteins and calmodulin-binding proteins J. Biol. Chem., 283, 18545-18552
Rezvani, K., Baalman, K., Teng, Y., Mee, M.P., Dawson, S.P., Wang, H., De Biasi. M. and Mayer, R.J. (2012) Proteasomal degreadation of the metabotropic glutamate receptor 1α is mediated by Homer-3 via the proteasomal S8 ATPase J. Neurochem. (2012) 122, 24–37
Salvi, M., Stringaro, A., Brunati, A.M., Agostinelli, E., Arancia, G., Clari, G. and Toninello, A. (2004) Tyrosine phosphatase activity in mitochondria: presence of Shp-2 phosphatase in mitochondria Cell. Mol. Life Sci., 61, 2393-2404
Sano, Y., Syuzo-Takabatake, A., Nakaya, T., Saito, Y., Tomita, S., Itohara, S. and Suzuki, T. (2006) Enhanced amyloidogenic metabolism of the amyloid β-protein precursor in the X11L-deficient mouse brain J. Biol. Chem., 281, 37853-37860
Sano, Y., Nakaya, T., Pedrini, S., Takeda, S., Iijima-Ando, K., Iijima, K., Mathews, P.M., Itohara, S., Gandy, S. and Suzuki, T. (2006) Physiological mouse brain Aβ levels are not related to the phsophorylation state of threonine-668 of Alzheimer’s APP PLoS ONE, 1:e51
Schwenk, B.M., Lang, C.M., Hogl, S., Tahirovic, S., Orozco, D., Rentzsch, K., Lichtenthaler, S.F., Hoogenraad, C.C., Capell, A., Haass, C. and Edbauer, D. (2014) The FTLD risk factor TMEM106B and MAP6 control dendritic trafficking of lysosomes EMBO J., 33, 450-467
Song, W-J., Son, M-Y., Lee, H-W., Seo, H., Kim, J.H. and Chung, S-H. (2015) Enhancement of BACE1 activity by p25/Cdk5-mediated phosphorylation in Alzheimer’s disease PLoS One, 10: e0136950
Srinivasan, K., Roosa, J., Olsen, O., Lee, S-H., Bredt, D.S. and McConnell, S.K. (2008) MALS-3 regulates polarity and early neurogenesis in the developing cerebral cortex Development, 135, 1781-1790
Szodorai, A., Kuan, Y-H., Hunzelmann, S., Engel, U., Sakane, A., Sasaki, T., Takai, Y., Kirsch, J., U., Müller, Beyreuther, K., Brady, S., Morfini, G. and Kins, S. (2009) APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle J. Neurosci., 29, 14534-14544
Tenga, Y., Rezvanib, K. and De Biasia, M. (2015) UBXN2A regulates nicotinic receptor degradation by modulating the E3 ligase activity of CHIP Biochem. Pharmacol., 97, 518–530
Tindi, J.O., Cha´vez, A.E., Cvejic, S., Calvo-Ochoa, E., Castillo, P.E. Jordan, B.A. (2015) ANKS1B gene product AIDA-1 controls hippocampal synaptic transmission by regulating GluN2B subunit localization J. Neurosci., 35, 8986–8996
Wang, J., Lu, R., Yang, J., Li, H., He, Z., Jing, N., Wang, X. and Wang, Y. (2015) TRPC6 specifically interacts with APP to inhibit its cleavage by γ-secretase and reduce Aβ production Nat. Comm., 6: 8876
Wang, T., Liu, Y., Xu, X-H., Deng, C-Y., Wu, K-Y., Zhu, J., Fu, X-Q., He, M. and Luo, Z-G. (2011) Lgl1 activation of Rab10 promotes axonal membrane trafficking underlying neuronal polarization Devel. Cell 21, 431–444
Watanabe, T., Hikichi, Y., Willuweit, A., Shintani, Y. and Horiguchi, T. (2012) FBL2 regulates amyloid precursor protein (APP) metabolism by promoting ubiquitination-dependent APP degradation and inhibition of APP endocytosis J. Neurosci., 32, 3352–3365
Yamamoto, Y. and Yeung, R.S. (1999) Subcellular distribution of tuberin in cells derived from brain and liver Proc. Amer. Assoc. Cancer Res., 40, #4515
Yamamoto, Y., Jones, K.A., Mak, B.C., Muehlenbachs, A. and Yeung, R.S. (2002) Multicompartmental distribution of tuberous sclerosis gene products, hamartin and tuberin Arch. Biochem. Biophys., 404, 210-217
Zhang, Z., Hao, C-J., Li, C-G., Zang, D-J., Zhao, J., Li, X-N., Wei, A-H., Wei, Z-B. et al (2014) Mutation of SLC35D3 causes metabolic syndrome by impairing dopamine signaling in striatal D1 neurons PLoS Genet., 10: e1004124
2. Heart
Hong, M., Kefaloyianni, E., Bao, L., Malester, B., Delaroche, D., Neubert, T.A. and Coetzee, W.A. (2011) Cardiac ATP-sensitive K+
channel associates with the glycolytic enzyme complex FASEB J., 25, 2456–2467
Hund, T.J. and Mohler, P.J. (2011) Differential roles for SUR subunits in KATP channel membrane targeting and regulation Am. J. Physiol. Heart Circ. Physiol., 300, H33–H35
Lei, B., Lionetti, V., Young, M.E., Chandler, M.P., d’Agostino, C., Kang, E., Altarejos, M., Matsuo, K., Hintze, T.H., Stanley, W.C. and Recchia, F.A. (2004) Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure J. Mol. Cell. Cardiol., 36, 567-576
Lei, B., Matsuo, K., Labinsky, V., Sharma, N., Chandler, M.P., Ahn, A., Hintze, T.H., Stanley, W.C. and Recchia, F.A. (2005) Exogenous nitric oxide reduces glucose transporters translocation and lactate production in ischemic myocardium in vivo Proc. Natl. Acad. Sci. USA, 102, 6966-6971
3. Intestine
Mansbach, C.M., Siddiqi, S. and Mahan, J. (2016) Vesicle-associated membrane protein 7 is crucial for lipid absorption J. Investig. Med.,54, S288
Siddiqi, S., Mathan, J., Siddiqi, S., Gorelick, F.S. and Mansbach, C.M. (2005) Vesicle-associated membrane protein 7 is expressed in intestinal ER J. Cell Sci., 119, 943-950
4. Kidney
Cai, Y., Maeda, Y., Cedzich, A., Torres, V.E., Wu, G., Hayashi, T., Mochizuki, T., Park, J.H., Witzgall, R. and Somlo, S. (1999) Identification and characterization of polycystin-2, the PKD2 gene product J. Biol. Chem., 274, 28557-28565
Inoue, Y., Sohara, E., Kobayashi, K., Chiga, M., Rai, T., Ishibashi, K., Horie, S., Su, X., Zhou, J., Sasaki, S. and Uchida, S. (2014) Aberrant glycosylation and localization of polycystin-1 cause polycystic kidney in an AQP11 knockout model J. Am. Soc. Nephrol., 25, 2789–2799
Kawaguchi, K., Hatano, R., Matsubara, M. and Asano, S. (2018) Internalization of NKCC2 is impaired in thick ascending limb of Henle in moesin knockout mice Pflügers Archiv Eur. J. Physiol., 470, 1055–1068
Koulen, P., Cai, Y., Geng, L., Maeda, Y., Nishimura, S., Witzgall, R., Ehrlich, B.E. and Somlo, S. (2002) Polycystin-2 is an intracellular calcium release channel Nat. Cell Biol., 4, 191-197
Lemale, J., Bloch-Faure, M., Grimont, A., El Abida, B., Imbert-Teboul, M. and Crambert, G. (2008) Membrane progestin receptors α and γ in renal epithelium Biochim. Biophys. Acta, 1783, 2234-2240
Mansbach, C.M., Siddiqi, S. and Mahan, J. (2016) Vesicle-associated membrane protein 7 is crucial for lipid absorption J. Investig. Med.,54, S288
Newby, L.J., Streets, A.J., Zhao, Y., Harris, P.C., Ward, C.J. and Ong, A.C.M. (2002) Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex J. Biol. Chem., 277, 20763-20773
Siddiqi, S., Mathan, J., Siddiqi, S., Gorelick, F.S. and Mansbach, C.M. (2005) Vesicle-associated membrane protein 7 is expressed in intestinal ER J. Cell Sci., 119, 943-950
5. Liver
Andrade, J., Zhao, H., Titus, B., Pearce, T. and Barroso, M.(2004) EF-hand Ca2+-binding protein p22 plays a role in microtubule and endoplasmic reticulum organization and dynamics with distinct Ca2+-binding requirements Mol. Biol. Cell, 15, 481-496
Desai, M.M., Gong, B., Chan, T., Davey, R.A., Soong, L., Kolokoltsov, A.A. and Sun, J. (2011) Differential, type I interferon-mediated autophagic trafficking of hepatitis C virus proteins in mouse liver Gastroenterology, 141, 674–685
Fowler, S., Akins, M. and Bennett, S.A.L. (2106) Preparation of gap junctions in membrane microdomains for immunoprecipitation and mass spectrometry interactome analysis In Gap Junction Prototcols: Methods Mol. Biol., 1437 (ed. Vinken, M. and Johnstone, S.R), Springer Science+Business Media, LLC, pp 113-132
Gringeri, E., Carraro, A., Tibaldi, E., d’Amico, F.E., Mancon, M., Toninello, A., Pagano, M.A., Vio, C., Cillo, U. and Brunati, A.M. (2010) Lyn-mediated mitochondrial tyrosine phosphorylation is required to preserve mitochondrial integrity in early liver regeneration Biochem. J., 425, 401–412
Mansbach, C.M., Siddiqi, S. and Mahan, J. (2016) Vesicle-associated membrane protein 7 is crucial for lipid absorption J. Investig. Med.,54, S288
Massarweh, A., Bosco, M., Iatmanen-Harbi, S., Tessier, C., Auberger, N., Busca, P., Chantret, I., GravierPelletier, C. and Moore, S.E.H. (2106) Demonstration of an oligosaccharide-diphosphodolichol diphosphatase activity whose subcellular localization is different than those of dolichyl-phosphate-dependent enzymes of the dolichol cycle J. Lipid Res., 57, 1029–1042
Mhamdi, M., Funk, A., Hohenberg, H., Will, H. and Sirma, H. (2007) Assembly and budding of a hepatitis B virus is mediated by a novel type of intracellular vesicles Hepatology, 46, 95-106
Patton, S.M., Pinero, D.J., Surguladze, N., Beard, J. and Connor, J.R. (2005) Subcellular localization of iron regulatory proteins to Golgi and ER membranes J. Cell Sci., 118, 4365-4373
Salvi, M., Battaglia, V., Brunati, A.M., La Rocca, N., Tibaldi, E., Pietrangeli, P., Marcocci, L., Mondovi, B., Rossi, C.A. and Toninello, A. (2007) Catalase takes part in rat liver mitochondria oxidative stress defense J. Biol. Chem., 282, 24407-24415
Siddiqi, S., Mathan, J., Siddiqi, S., Gorelick, F.S. and Mansbach, C.M. (2005) Vesicle-associated membrane protein 7 is expressed in intestinal ER J. Cell Sci., 119, 943-950
Skarpen, E., Oksvold, M.P., Grøsvik, H., Widnes, C. and Huitfeldt, H.S. (2005) Altered regulation of EGF receptor signaling following a partial hepatectomy J. Cell. Physiol., 202, 707-716
Smith, S.E., Granell, S., Salcedo-Sicilia, L., Baldini, G., Egea, G., Teckman, J.H. and Baldini, G. (2011) Activating transcription factor 6 limits intracellular accumulation of mutant α1-antitrypsin Z and mitochondrial damage in hepatoma cells J. Biol. Chem., 286, 41563–41577
Tibaldi, E., Brocca, A., Sticca, A., Gola, E., Pizzi, M., Bordin, L., Pagano, M.A., Mazzorana, M., Donà, G. et al (2020) Fam20C-mediated phosphorylation of osteopontin is critical for its secretion but dispensable for its action as a cytokine in the activation of hepatic stellate cells in liver fibrogenesis FASEB J., 34, 1122–1135
Trombetta, E.S. and Helenius, A. (1999) Glycoprotein reglucosylation and nucleotide sugar utilization in the secretory pathway: identification of a nucleoside diphosphatase in the endoplasmic reticulum EMBO J., 18, 3282-3292
Yamamoto, Y. and Yeung, R.S. (1999) Subcellular distribution of tuberin in cells derived from brain and liver Proc. Amer. Assoc. Cancer Res., 40, #4515
Yamamoto, Y., Jones, K.A., Mak, B.C., Muehlenbachs, A. and Yeung, R.S. (2002) Multicompartmental distribution of tuberous sclerosis gene products, hamartin and tuberin Arch. Biochem. Biophys., 404, 210-217
6. Melanoma tumours
Lee, E-Y., Park, K-S., Yoon, Y.J., Lee, J., Moon, H-G., Jang, S.C., Choi, K-H., Kim, Y-K. and Gho, Y.S. (2012) Therapeutic effects of autologous tumor-derived nanovesicles on melanoma growth and metastasis PLoS One 7: e33330
7. Murine embryo
Wang, X., Pralhada Rao, R., Kosakowska-Cholody, T., Masood, M.A., Southon, E., Zhang. H., Berthet, C. et al (2009) Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice J. Cell Biol., 184, 143-158
8. Salivary gland
Reddy, R.N., Pena, J.A., Roberts, B.R., Williams, S.R., Price, S.R. and Gooch, J. L. (2011) Rescue of calcineurin Aα -/-
mice reveals a novel role for the α isoform in the salivary gland Am. J. Pathol., 78, 1605–1613
9. Skeletal muscle
Cacciottolo, M., Belcastro, V., Laval, S., Bushby, K., di Bernardo, D. and Nigro, V. (2011) Reverse engineering gene network identifies new dysferlin-interacting proteins J. Biol. Chem., 286, 5404–5413
Gyobu, S., Miyata, H., Ikawa, M., Yamazaki, D., Takeshima, H., Suzuki, J., Nagata, S. (2016) A role of TMEM16E carrying a scrambling domain in sperm motility Mol. Cell. Biol., 36, 645-659
10. Spleen
Yamamoto, Y., Jones, K.A., Mak, B.C., Muehlenbachs, A. and Yeung, R.S. (2002) Multicompartmental distribution of tuberous sclerosis gene products, hamartin and tuberin Arch. Biochem. Biophys., 404, 210-217
11. Testis
Yamamoto, Y., Jones, K.A., Mak, B.C., Muehlenbachs, A. and Yeung, R.S. (2002) Multicompartmental distribution of tuberous sclerosis gene products, hamartin and tuberin Arch. Biochem. Biophys., 404, 210-217
OptiPrepTM Reference List RS05; 7th edition, January 2020
OptiPrep Reference List RS06
Lipid rich detergent-resistant domains (mammalian sources)
- The main aim of this OptiPrep™ Reference List is to present a bibliography of all of the current papers reporting the use of an iodixanol gradient to purify and analyze lipid rafts from vertebrate cells/tissues as detergent-resistant domains (see Section 2). Section 1 contains a brief survey of the technique; it has its own short reference list (Section 1f) distinct from the comprehensive reference list in Section 2.
1. Methodological survey
1a. Introduction
Lipid-rich plasma membrane domains have commonly been isolated from tissues and cultured cells as detergent-resistant membranes (DRMs), principally by flotation from a dense solution through a discontinuous or continuous density gradient containing a non-ionic detergent, usually Triton X-100 (TX100), but sometimes CHAPS. The resolution of DRMs is based on their low buoyant density and a flotation strategy is regarded as the best method for resolving any minor low-density fraction from predominantly denser material. Sucrose gradients were originally used in the separations but the much lower viscosity of Nycodenz® solutions, compared to those of sucrose, allowed the centrifugation time at 200,000 gav to be reduced from 18 h to 4 h [1]. Although iodixanol gradients are slightly more viscous than the corresponding Nycodenz® ones, the ease of preparation of gradient solutions from a commercial 60% (w/v) solution (OptiPrep™) has made use of this medium very popular.
1b. Cell lysis and iodixanol discontinuous gradients
Figure 1 summarizes the most commonly used basic strategy and includes the three types of pre-gradient procedure. Using the blue pathway sometimes the membranes maybe concentrated by a 100,000 g centrifugation prior to addition of detergent. The lysis buffer composition varies; an isotonic medium containing 150 mM NaCl is probably the most commonly used, buffered with 15-50 mM Tris, HEPES or MES (pH 7.0-7.6), usually containing 1-5 mM EDTA and sometimes including 1-5 mM DTT. The osmotic balancer sometimes is 0.25 M sucrose with or without 50 mM NaCl. Other lysis media are hypotonic, for example 25 mM MES, 2 mM EDTA, 5 mM DTT. The detergent is usually TX100 at concentrations from 0.1-1.0% (w/v); occasionally CHAPS is used at approx 20 mM.
The gradient is routinely comprises three layers: the bottom sample layer, which may be 35-45% (w/v) iodixanol, the middle layer of 25-25% iodixanol and the top layer of 5-10% iodixanol or the lysis buffer. The volume of the middle layer is always larger than that of the sample layer (2-8x) to maximize the linear separation of the DRM and detergent soluble fractions. The volume of the top layer is usually no more than about 10% of the total volume. Sometimes an additional layer is inserted into the gradient, e.g. 50% (sample layer), 30%, 25% and 3% iodixanol or 35% (sample layer), 30%, 20% iodixanol and buffer. There are also some examples of six-layer gradients, e.g. 40% (sample layer), 35%, 30%, 25%, 20% iodixanol and buffer. The simple three-layer gradients are the most widely used for the separation of DRM and detergent-soluble fractions but they do not generally allow much scope for possible resolution of raft sub-domains. The more the number of layers the greater the opportunity for the identification of DRMs of different density.
1c.Centrifugation
Centrifugation is normally in the range 200,000-300,000 g, usually for 2-6 h, but may be for as long as 16h. Small volume gradients are commonly carried out in the Beckman TLS55, large volume gradients in the Beckman SW41Ti (or similar). During extended centrifugations significant diffusion of the iodixanol will occur and the gradient will become continuous (but not necessarily linear) and this too may permit some finer resolution of subdomains of lipid-rich complexes.
1d. Double gradient strategies
There are a few published examples of the use of a primary discontinuous gradient not containing detergent to obtain a low-density membrane fraction, which is then further fractionated in a detergent-containing gradient. The first gradient will eliminate all of the major organelles such as mitochondria, lysosomes and peroxisomes and also most of the endoplasmic reticulum, Golgi and denser endosomes. Since the plasma membrane is one of the lightest of the cellular membranes in iodixanol, it is highly likely that the DRMs obtained in the second gradient are indeed derived from (or at least preferentially enriched in) this membrane rather than from internal membranes. This strategy was first reported for adult mouse brain [2]; the tissue was homogenized in 0.25 M sucrose, 50 mM NaCl, 1 mM DTT, 20 mM Tris-HCl, pH 7.4 and a post-nuclear supernatant adjusted to 35% (w/v) iodixanol, upon which were layered 30%, 20% and 5% iodixanol (total vol. approx. 13 ml). After centrifugation at approx. 200,000 gav for 3 h the low-density membrane from the 20%/5% iodixanol interface was harvested; adjusted to 35% iodixanol and 0.25% TX100 and overlaid with 30% iodixanol and buffer (containing 0.1% TX100). The gradient (total vol. 2.2 ml) was centrifuged at approx 170,000 gav for 1 h. In other examples there are small variations in the precise make-up of the two gradients; the method has been used for cultured cortical neurons [3], rat brain [4,5] and cultured chicken ciliary ganglion neurons [6]
1e. Other gradient strategies
Before OptiPrep™ became the medium of choice Naslavsky et al [7] described the use of a very effective Nycodenz® gradient in the analysis of prion proteins in neuroblastoma cells. The lysate was adjusted to 35% (w/v) Nycodenz (total volume 0.8 ml) and overlaid by 0.2 ml each of 25, 22.5, 20, 18, 15, 12 and 8% Nycodenz®. The small volume of the gradient and the small difference in density between each layer probably allows the gradient to become more or less continuous by the end of the centrifugation (200,000 g for 4 h). This gradient was used widely in studies on neuroblastoma cells [e.g. refs 8-10]. The gradients cover predominantly a range of lower densities than the iodixanol ones and maybe enhance the potential for DRM subfractionation. The importance of using lower density gradients was confirmed by Hering et al [11] who, in their studies on rat brain, were able to show a clear density separation of two DRM markers (Thy-1 and caveolin) in 30%, 25%, 15% and 5% Nycodenz® gradients; this was less definitive in 30%, 25% and 5% gradients.
Rouvinski et al [12] used the same 8-25% Nycodenz® flotation gradient (in 2.2 ml tubes) as in ref 7 but introduced a novel two-gradient concept in which the first gradient was centrifuged for only 45 min (separation by rate of flotation), after which the low density fractions were collected; adjusted to a 35% Nycodenz® and
analyzed in a second identical gradient centrifuged for 150 min (density separation). In this manner it was possible to identify large, intermediate and small DRMs [12] from neuroblastoma cells.
- It is likely that these higher resolution strategies can be directly transcribed to the use of OptiPrep™
A detailed description of the OptiPrep™ methodology (see Application Sheet S32) can be found via the releveant OptiPrep™ Application Sheets Index on the following website: www.Optiprep.com (click on “Methodology”, then “Organelles and Subcellular Membranes”) and scroll down the Index.
1f. References to Section 1
1. Naslavsky, N., Stein, R., Yanai, A., Friedlander, G. and Taraboulos, A. (1997) Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform J. Biol. Chem., 272, 6324-6331
2. Bruckner, K., Labrador, J.P., Schieffele, P., Herb, A., Seeburg, P.H. and Klein, R. (1999) EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains Neuron, 22, 511-524
3. Palmer, A., Zimmer, M., Erdmann, K.S., Eulenburg, V., Porthin, A., Heumann, R., Deutsch, U. and Klein, R. (2002) EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase Mol. Cell, 9, 725-737
4. Ma, L., Huang, Y.Z., Pitcher, G.M., Valtschanoff, J. ., Ma, Y.H., Feng, L.Y., Lu, B., Xiong, W. ., Salter, M.W., Weinberg, R.J. and Mei, L. (2003) Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts J. Neurosci., 23, 3164-3175
5. Yu, W., Guo, W. and Feng, L. (2004) Segregation of Nogo66 receptors into lipid rafts in rat brain and inhibition of Nogo66 signaling by chosesterol depletion FEBS Lett., 577, 87-92
6. Bruses, J.L., Chauvet, N. and Rutishauser, U. (2001) Membrane lipid rafts are necessary for the maintenance of the α7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons J. Neurosci., 21, 504-512
7. Naslavsky, N., Stein, R., Yanai, A., Friedlander, G. and Taraboulos, A. (1997) Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform J. Biol. Chem., 272, 6324-6331
8. Naslavsky, N., Shmeeda, H., Friedlander, G., Yanai, A., Futerman, A.H., Barenholz. Y. and Taraboulos, A. (1999) Sphingolipid depletion increases formation of the scrapie prion protein in neuroblastoma cells infected with prions J. Biol. Chem., 274, 20763-20771
9. Ben-Zaken, O., Tzaban, S., Tal, Y., Horonchik, L., Esko, J.D., Vlodavsky, I. and Taraboulos, A. (2003) Cellular heparan sulfate participates in the metabolism of prions J. Biol. Chem., 278, 40041-40049
10. Gilch, S., Kehler, C. and Schätzl, H.M. (2006) The prion protein requires cholesterol for cell surface localization Mol. Cell. Neurosci.,31, 346-353
11. Hering, H., Lin, C-C- and Sheng, M. (2003) Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability J. Neurosci., 23, 3262-3271
12. Rouvinski, A., Gahali-Sass, I., Stav, I,m Metzer, E., Atlan, H. and Taraboulos, A. (2003) Both raft- and non-raft proteins associate with CHAPS-insoluble complexes: some APP in large complexes Biochem. Biophys. Res. Comm., 308, 750-758
2. Comprehensive bibliography of OptiPrep™ papers
Papers have been divided into:
(1) Mammalian cells and tissues
(2) Mammalian subcellular organelles
(3) Review articles
Within each major group papers are listed alphabetically according to cell or tissue type When required, papers have also been sorted according to research topic. Within each group, papers are listed alphabetically according to first author. To facilitate identification of references of interest key words in titles are highlighted in light blue. When a paper reports the study of more than one cell type, reference to that paper will appear under all relevant headings.
1. Mammalian cells and tissues
1-1. Adipocytes
Jacobs, C., Onnockx, S., Vandenbroere, I. and Pirson, I. (2004) Endogenous SHIP2 does not localize in lipid rafts in 3T3-L1 adipocytes FEBS Lett., 565, 70-74
Jansen, M., Pietiäinen, V.M., Pölönen, H., Rasilainen, L. et al (2008) Cholesterol substitution increases the structural heterogeneity of caveolae J. Biol. Chem., 283, 14610-14618
Mansbach, C.M. and Siddiqi, S. (2016) Control of chylomicron export from the intestine Am. J. Physiol. Gastrointest. Liver Physiol., 310, G659–G668
Nakamichi, Y., Wada, E., Aoki, K., Ohara-Imaizumi, M. et al (2004) Functions of pancreatic β cells and adipocytes in bombesin receptor subtype-3-deficient mice Biochem. Biophys. Res. Commun., 318, 698-703
Pohl, J., Ring, A., Korkmaz, U., Ehehalt, R. et al (2005) FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts Mol. Biol. Cell, 16, 24-31
1-2. Amyloidogenesis
Cacciottolo, M., Morgan, T.E., Saffari, A.A., Shirmohammadi, F., Forman, H.J., Sioutas, C. and Finch, C.E. (2020) Traffic-related air pollutants (TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts Free Radical Biol. Med., 147, 242–251
1-3. BHK cells
Abrami, L., Leppla, S.H. and Gisou van der Gaat, F. (2006) Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis J. Cell Biol., 171, 309-320
Bhattacharya, B. and Roy, P. (2008) Bluetongue virus outer capsid protein VP5 interacts with membrane lipid rafts via a SNARE domain J. Virol., 82, 10600-10612
Fivaz, M., Vilbois, F., Thurnheer, S., Pasquali, C., Abrami, L., et al (2002) Differential sorting and fate of endocytosedGPI-anchored proteins EMBO J., 21, 3989-4000
Glende, J., Schwegmann-Wessels, C., Al-Falah, M., Pferfferle, S., et al (2008) Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2 Virology, 381, 215-221
Harder, T., Scheiffele, P., Verkade, P. and Simons K (1998) Lipid domain structure of the plasma membrane revealed by patching of membrane components J. Cell Biol., 141, 929-942
Heino, S., Lusa, S., Somerharju, P., Ehnholm, C., Olkkonen, V.M., et al (2000) Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface Proc. Natl. Acad. Sci., USA, 97, 8375-8380
Simons, M., Kramer, E-M., Macchi, P., Rathke-Hartlieb, S., et al (2002) Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: implications for Pelizaeus-Merzbacher disease J. Cell Biol., 157, 327-336
Zhao, Y., Ishigami, M., Nagao, K., Hanada, K., Kono, N., Arai, H., Matsuo, M., Kioka, N. and Ueda. K. (2015) ABCB4 exports phosphatidylcholine in a sphingomyelin-dependent manner J. Lipid Res., 56, 644–652
Brain (see “1-32. Neural tissue, neural cells and related cells” )
1-4. Caco-2 cells
Broquet, A.H., Thomas, G., Masliah, J., Trugnan, G., et al (2003) Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release J. Biol. Chem., 278, 21601-21606
Cuadras, M.A. and Greenberg, H.B. (2003) Rotavirus infectious particles use lipid rafts during replication for transport to the cell surface in vitro and in vivo Virology, 313, 308-321
Ehehalt, R., Krautter, M., Zorn, M., Sparla, R., et al (2008) Increased basolateral sorting of carcinoembryonic antigen in a polarized colon carcinoma cell line after cholesterol depletion-Implications for treatment of inflammatory bowel disease World J. Gastroenterol., 14, 1528-1533
Li, X. and Donowitz, M. (2008) Fractionation of subcellular membrane vesicles of epithelial and nonepithelial cells by OptiPrep™ density gradient ultracentrifugation In Methods Mol. Biol., 440, Exocytosis and Endocytosis (ed. Ivanov, A.I.) Humana Press, Totowa, NJ, pp 97-110
Sakseena, S., Tyagi, S., Goyal, S., Gill, R.K., et al (2010) Stimulation of apical Cl-/HCO3- (OH-) exchanger, SLC26A3 by neuropeptide Y is lipid raft dependent Am. J. Physiol. Gastrointest. Liver Physiol., 299, G1334–G1343, 2010
Sapin, C., Colard, O., Delmas, O., Tessier, C., et al (2002) Rafts promote assembly and atypical targeting of a nonenveloped virus, rotavirus, in Caco-2 cells J. Virol., 76, 4591-4602
Treede, I., Braun, A., Jeliaskova, P., Giese, T., et al (2009) TNF-α-induced up-regulation of pro-inflammatory cytokines is reduced by phosphatidylcholine in intestinal epithelial cells BMC Gastroenterol., 9:53
Tyska, M.J. and Mooseker, M.S. (2004) A role for myosin-1A in the localization of a brush border disaccharidase J. Cell Biol., 165, 395-405
1-5. Cancer-related
Angiogenesis/carcinogenesis (see also “Cannabinoid receptor”)
Bourguignon, L.Y.W., Singleton, P.A., Diedrich, F., Stern, R. et al (2004) CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironment leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion J. Biol. Chem., 279, 26691-27007
Grass, G.D., Bratoeva, M. and Toole, B.P. (2012) Regulation of invadopodia formation and activity by CD147 J. Cell Sci., 125, 777–788
Hitosugi, T., Sato, M., Sasaki, K. and Umezawa, Y. (2007) Lipid raft-specific knockdown of Src family kinase activity inhibits cell adhesion and cell cycle progression of breast cancer cells Cancer Res., 67, 8139-8148
Mazzone, M., Baldassarre, M., Beznoussenko, G., Giacchetti, G., et al (2004) Intracellular processing and activation of membrane type 1 matrix metalloprotease depends on its partitioning into lipid domains J. Cell Sci., 117, 6275-6287
Mira, E., Lacalle, R.A., Buesa, J.M., Gonzalez de Buitrago, G., et al (2004) Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface J. Cell Sci., 117, 1847-1856
Remacle-Bonnet, M., Garrouste, F., Baillat, G., Andre, F., et al (2005) Membrane rafts segregate pro- from anti-apoptotic insulin-like growth factor-I receptor signaling in colon carcinoma cells stimulated by members of the tumor necrosis factor superfamily Am. J. Pathol., 167, 761-773
Yang, J., Qian, J., Wezeman, M., Wang, S., et al (2006) Targeting β2-microglobulin for induction of tumor apoptosis in human hematological malignancies Cancer Cell, 10, 295-307
Apoptosis
Elyassaki, W. and Wu, S. (2006) Lipid rafts mediate ultraviolet light-induced Fas aggregation in M624 melanoma cells Photochem. Photobiol., 82, 787-792
Katsogiannou, M., El Boustany, C., Gackiere, F., Delcourt, P., et al (2009) Caveolae contribute to the apoptosis resistance induced by the a1A-adrenoceptor in androgen-independent prostate cancer cells Plos One, 4: e7068
Xu, L., Zhang, Y., Liu, J., Qu, J., et al (2012) TRAIL-activated EGFR by Cbl-b-regulated EGFR redistribution in lipid rafts antagonises TRAIL-induced apoptosis in gastric cancer cells Eur. J. Cancer, 48, 3288–3299
Xu, L., Hu, X., Qu, X., Hou, K., et al (2013) Cetuximab enhances TRAIL-induced gastric cancer cell apoptosis by promoting DISC formation in lipid rafts Biochem. Biophys. Res. Comm., 439, 285–290
Xu, L., Qu, X., Hu, X., Zhu, Z., et al (2103) Lipid raft-regulated IGF-1R activation antagonizes TRAIL-induced apoptosis in gastric cancer cells FEBS Lett., 587, 3815–3823
Yang, J., Zhang, X., Wang, J., Qian, J., et al (2007) Anti-β2-microglobulin monoclonal antibodies induce apoptosis in myeloma cells by recruiting MHC class I to and excluding growth and survival cytokine receptors from lipid rafts Blood, 110, 3028-3035
Bacterial toxins
Abrami, L., Kunz, B., Deuquet, J., Bafico, A., et al (2008) Functional interactions between anthrax toxin receptors and the WNT signalling protein LRP6 Cell. Microbiol., 10, 2509-2519
Abrami, L., Bischofberger, M., Kunz, B., Groux, R. et al (2010) Endocytosis of the anthrax toxin is mediated by clathrin, actin and unconventional adaptors PLoS Pathogens 6: e1000792
Backert, S., Tegtmeyer, N. and Selbach, M. (2010) The versatility of Helicobacter pylori CagA effector protein functions: the master key hypothesis Helicobacter, 15, 163–176
Deng, Q., Zhang, Y. and Barbieri, J.T. (2007) Intracellular trafficking of Pseudomonas ExoS, type III cytotoxin Traffic, 8, 1331-1345
Falguieres, T., Mallard, F., Baron, C., Hanau, D., et al (2001) Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes Mol. Biol. Cell, 12, 2453-2468
Gauthier, N.C., Monzo, P., Kaddai, V., Doye, A., et al (2005) Helicobacter pylori VacA cytotoxin: a probe for a clathrin-independent and Cdc42-dependent pinocytic pathway routed to late endosomes Mol. Biol. Cell, 16, 4852-4866
Gauthier, N.C., Ricci, V., Gounon, P., Doye, A., et al (2004) Glycosylphosphatidylinositol-anchored proteins and actin cytoskeleton modulate chloride transport by channels formed by the Helicobacter pylori vacuolating cytotoxin VacA in HeLa cells J. Biol. Chem., 279, 9481-9489
Gupta, V.R., Patel, H.K., Kostolansky, S.S., Ballivian, R.A., et al (2008) Sphingomyelin functions as a novel
receptor for Helicobacter pylori VacA PloS Pathog., 4: e1000073
Gupta, V.R., Wilson, B.A. and Blanke, S.R. (2010) Sphingomyelin is important for the cellular entry and intracellular localization of Helicobacter pylori Cell. Microbiol., 12, 1517–1533
Hutton, M.L., Kaparakis-Liaskos, M., Turner, L., Cardona, A., et al (2010) Helicobacter pylori exploits cholesterol-rich microdomains for induction of NF-B-dependent responses and peptidoglycan delivery in epithelial cells Infect. Immun., 78, 4523–4531
Lafont, F., Van Nhieu, G.T., Hanada, K., Sansonetti, P., et al (2002) Initial steps of Shigella infection depend on the cholesterol/shingolipid raft-mediated CD44-IpaB interaction EMBO J., 21, 4449-4457
Lai, C-H., Chang, Y-C., Du, S-Y., Wang, H-J., et al (2008) Cholesterol depletion reduces Helicobacter pylori CagA translocation and CagA-induced responses in AGS cells Infect. Immun., 76, 3293-3303
Smith, D.C., Sillence, D.J., Falguières, T., Jarvis, R.M., et al (2006) The association of Shiga-like toxin with detergent-resistant membranes is modulated by glucosylceramide and is an essential requirement in the endoplasmic reticulum for a cytotoxic effect Mol. Biol. Cell, 17, 1375-1387
Smith, D.C., Spooner, R.A., Watson, P., Murray, J.L., Hodge, T.W. (2006) Internalized Pseudomonas exotoxin A can exploit multiple pathways to reach the endoplasmic reticulum Traffic, 7, 379-393
Van der Goot, F.G., Tran van Nhieu, G., Allaoui, A., Sansonetti, P., et al (2004) Rafts can trigger contactmediated secretion of bacterial effectors via a lipid-based mechanism J. Biol. Chem., 279, 47792-47798
Breast cancer cells
Badana, A., Chintala, M., Varikuti, G., Pudi, N., Kumari, S., Kappala, V.R. and Malla, R.R. (2016) Lipid raft integrity is required for survival of triple negative breast cancer cells J. Breast Canc., 19, 372-384
Elia, J., Carbonnelle, D., Logé, C., Ory, L., Huvelin, J-M., Tannoury, M., Diab-Assaf, M., Petit, K. and Nazih, H. (2019) 4-cholesten-3-one decreases breast cancer cell viability and alters membrane raft-localized EGFR expression by reducing lipogenesis and enhancing LXR-dependent cholesterol transporters Lipids Health Dis., 18: 168
Ca2+-signalling
Pulli, I., Blom, T., Löf, C., Magnusson, M., Rimessi, A., Pinton, P. and Törnquist, K. (2015) A novel chimeric aequorin fused with caveolin-1 reveals a sphingosine kinase 1-regulated Ca2+ microdomain in the caveolar compartment Biochim. Biophys. Acta, 1853, 2173–2182
Cannabinoid receptor
DeMorrow, S., Glaser, S., Francis, H., Venter, J., et al (2007) Opposing actions of endocannabinoids on cholangiocarcinoma growth J. Biol. Chem., 282, 13098-13113
Sarnataro, D., Grimaldi, C., Pisanti, S., Gazzerro, P., et al (2005) Plasma membrane and lysosomal localization of CB1 cannabinoid receptor are dependent on lipid rafts and regulated by anandamide in human breast cancer cells FEBS Lett., 579, 6343-6349
Sarnataro, D., Pisanti, S., Santoro, A., Gazzero, P., et al (2006) The cannabinoid CB1 receptor antagonist Rimonabant (SR141716) inhibits human breast cancer cell proliferation through a lipid taft-mediated mechanism Mol. Pharmacol., 70, 1298-1306
Chemotaxis
Manes, S., Mira, E., Gomez-Mouton, C., Lacalle, R.A., et al (1999) Membrane raft microdomains mediate front-rear polarity in migrating cells EMBO J., 18, 6211-6220
Prag, S., Parsons, M., Keppler, M.D., Ameer-Beg, S.M., et al (2007) Activated ezrin promotes cell migration through recruitment of the GEF Db1 to lipid rafts and preferential downstream activation of Cdc42 Mol. Biol. Cell, 18, 2935-2948
Van Rheenen, J., Song, X., van Roosmalen, W., Cammer, M., et al (2007) EGF-induced PIP2 hydrolysis releases and activates cofilin locally in carcinoma cells J. Cell Biol., 179, 1247-1259
Chronic obstructive pulmonary disease Singh, D.P., Kaur, G., Bagam, P., Pinkston, R. and Batra, S. (2018) Membrane microdomains regulate NLRP10- and NLRP12-dependent signalling in A549 cells challenged with cigarette smoke extract Arch. Toxicol., 92, 1767–1783
Colon cancer
Ali, M. and Mocarski, E.S. (2018) Proteasome inhibition blocks necroptosis by attenuating death complex aggregation Cell Death and Disease 9: 346
Endothelial cell adhesion
Chanvorachote, P. and Chunhacha, P. (2013) Caveolin-1 regulates endothelial adhesion of lung cancer cells via reactive oxygen species-dependent mechanism PLoS One, 8: e57466
Glycolipids (see also “Lipid composition”)
Bonardi, D., Papini, N., Pasini, M., Dileo, L., et al (2014) Sialidase NEU3 dynamically associates to different membrane domains specifically modifying their ganglioside pattern and triggering Akt phosphorylation PLoS One, 9, e99405
Marques, L., Auriac, A., Willemetz, A., Banha, J., et al (2012) Immune cells and hepatocytes express glycosylphosphatidylinositol-anchored ceruloplasmin at their cell surface Blood Cells Mol. Dis., 48, 110–120
Simonis, D., Schlesinger, M., Seelandt, C., Borsig, L., et al (2010) Analysis of SM4 sulfatide as a P-selectin ligand using model membranes Biophys. Chem., 150, 98–104
Green tea phenolics
Sánchez-Tena, S., Vizán, P., Dudeja, P.K., Centelles, J.J. et al, (2013) Green tea phenolics inhibit butyrateinduced differentiation of colon cancer cells by interacting with monocarboxylate transporter Biochim. Biophys. Acta, 1832, 2264–2270
Growth factor receptors
Grass, G.D., Tolliver, L.B., Bratoeva, M. and Toole, B.P. (2013) CD147, CD44, and the epidermal growth factor receptor (EGFR) signaling pathway cooperate to regulate breast epithelial cell invasiveness J. Biol. Chem., 288, 26089–26104
Kyriakakis, E., Maslova, K., Philippova, M., Pfaff, D., et al, (2012) T-Cadherin is an auxiliary negative regulator of EGFR pathway activity in cutaneous squamous cell carcinoma: impact on cell motility J. Invest. Dermatol., 132, 2275–2285
Xu, L., Zhang, Y., Liu, J., Qu, J., et al (2012) TRAIL-activated EGFR by Cbl-b-regulated EGFR redistribution in lipid rafts antagonises TRAIL-induced apoptosis in gastric cancer cells Eur. J. Cancer, 48, 3288–3299
Hepatitis C virus
Wang, H. and Tai, A.W. (2019) Nir2 is an effector of VAPs necessary for efficient hepatitis C virus replication and phosphatidylinositol 4-phosphate enrichment at the viral replication organelle J. Virol., 93: e00742
Wu, M-J., Shanmugam, S., Welsch, C. and Yia, MK. (2020) Palmitoylation of hepatitis C virus NS2 regulates its subcellular localization and NS2-NS3 autocleavage J. Virol., 94: e00906-19
Human colon cancer cells
Ali, M., Roback, L. and Mocarski, E.S. (2019) Herpes simplex virus 1 ICP6 impedes TNF receptor 1–induced necrosome assembly during compartmentalization to detergent-resistant membrane vesicles J. Biol. Chem., 294, 991–1004
Ion transport
Chen, Y-F., Chou, C-Y., Wilkins, R.J., Ellory, J.C., et al (2009) Motor protein–dependent membrane trafficking of KCl cotransporter-4 is important for cancer cell invasion Cancer Res., 69, 8585–8593
Kinases/signaling
Baillat, G., Siret, C., Delamarre, E. and Luis, J. (2008) Early adhesion induces interaction of FAK and Fyn in lipid domains and activates raft-dependent Akt signaling in SW480 colon cancer cells Biochim. Biophys. Acta, 1783, 2323-2331
Cinar, B., Mukhopadhyay, N.K., Meng, G. and Freeman, M.R. (2007) Phosphoinositide 3-kinase-independent non-genomic signals transit from androgen receptor to Akt1 in membrane raft microdomains J. Biol. Chem., 282, 29584-29593
Hitosugi, T., Sato, M., Sasaki, K. and Umezawa, Y. (2007) Lipid raft-specific knockdown of Src family kinase activity inhibits cell adhesion and cell cycle progression of breast cancer cells Cancer Res., 67, 8139-8148
Piazza, T.M., Lu, J-C., Carver, K.C. and Schuler, L.A. (2009) Src family kinases accelerate prolactin receptor internalization, modulating trafficking and signaling in breast cancer cells Mol. Endocrinol., 23, 202-212
Lipid composition
Hynynen, R., Laitinen, S., Kakela, R., Tanhuanpaa, K., et al (2005) Overexpression of OSBP-related protein 2 (ORP2) induces changes in cellular cholesterol metabolism and enhances endocytosis Biochem. J., 390, 273-283
Neumann-Giesen, C., Falkenbach, B., Beicht, P., Claasen, S., et al (2004) Membrane and raft association of reggie-1/flotillin-2: role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression Biochem. J., 378, 509-518
Pike, L.J., Han, X., Chung, K-N and Gross, R.W. (2002) Lipid rafts are enriched in arachidonic acid and plasmenyl-ethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis Biochemistry, 41, 2075-2088
Yamauchi, Y., Furukawa, K., Hamamura, K. and Furukawa, K. (2011) Positive feedback loop between PI3KAkt-mTORC1 signaling and the lipogenic pathway boosts Akt signaling: induction of the lipogenic pathway by a melanoma antigen Cancer Res., 71, 4989–4997
Melanoma
Yang, J., Price, M.A., Wanshura, L.E.C., He, J., Yi, M., Welch, D.R., Li, G., Conner, S., Sachs, J., Turley, E.A. and McCarthy, J.B. (2019) Chondroitin sulfate proteoglycan 4 enhanced melanoma motility and growth requires a cysteine in the core protein transmembrane domain Melanoma Res., 29, 365–375
Plasmalogen biosynthesis
Honsho, M., Abe, Y. and Fujiki, Y. (2017) Plasmalogen biosynthesis is spatiotemporally regulated by sensing plasmalogens in the inner leaflet of plasma membranes Sci. Rep., 7: 43936
Prostate cancer cells
Grolez, G.P., Gordiendko, D.V., Clarisse, M., Hammadi, M., Desruelles, E., Fromont, G., Prevarskaya, N., Slomianny, C. and Gkika, D. (2019) TRPM8-androgen receptor association within lipid rafts promotes prostate cancer cell migration Cell Death Dis., 10: 652
Reactive oxygen species
Chanvorachote, P. and Chunhacha, P. (2013) Caveolin-1 regulates endothelial adhesion of lung cancer cells via reactive oxygen species-dependent mechanism PLoS One, 8: e57466
Shiga toxin
Falguierres, T., Römer, W., Amessou, M., Afonso, C., Wolf, C., Tabet, J-C., Lamaze, C. and Johannes, L. (2006) Functionally different pools of Shiga toxin receptor, globotriaosyl ceramide, in HeLa cells FEBS J., 273, 5205-5218
Sphingosine kinase
Pulli, I., Blom, T., Löf, C., Magnusson, M., Rimessi, A., Pinton, P. and Törnquist, K. (2015) A novel chimeric aequorin fused with caveolin-1 reveals a sphingosine kinase 1-regulated Ca2+ microdomain in the caveolar compartment Biochim. Biophys. Acta, 1853, 2173–2182
Vero cells
Ali, M., Roback, L. and Mocarski, E.S. (2019) Herpes simplex virus 1 ICP6 impedes TNF receptor 1–induced necrosome assembly during compartmentalization to detergent-resistant membrane vesicles J. Biol. Chem., 294, 991–1004
Viral interactions
Gosselin-Grenet, A-S., Mottet-Osman, G. and Roux, L. (2006) From assembly to virus particle budding: pertinence of the detergent resisitant membranes Virology, 344, 296-303
Fleming, E.H., Kolokoltsov, A.A., Davey, R.A., Nichols, J.E. et al, (2006) Respiratory syncytial virus F envelope protein associates with lipid rafts without a requirement for other virus proteins J. Virol., 80, 12160-12170
Kataoka, C., Kaname, Y., Taguwa, S., Abe, T., Fukuhara, T., Tani, H., Moriishi, K. and Matsuura, Y. (2012) Baculovirus GP64-mediated entry into mammalian cells J. Virol., 86, 2610-2620
Martín, J.J., Holguera, J., Sánchez-Felipe, L., Villar, E. and Muñoz-Barroso, I. (2012) Cholesterol dependence of Newcastle disease virus entry Biochim. Biophys. Acta, 1818, 753–761
Ono, A., Waheed, A.A., Joshi, A. and Freed, E.O. (2005) Association of human immunodeficiency virus type 1 Gag with membrane does not require highly basic sequences in the nucleocapsid: use of a novel Gag multimerization assay J. Virol., 79, 14131-14140
Wu, X., Meng, X., Yan, B., Rose, L., Deng, J. and Xianga, Y. (2012) Vaccinia virus virion membrane biogenesis protein A11 associates with viral membranes in a manner that requires the expression of another membrane biogenesis protein, A6 J. Virol., 86, 11276–11286
Zhao, W-L., Zhang, F., Feng, D., Wu, J., et al (2009) A novel sorting strategy of trichosanthin for hijacking human immunodeficiency virus type 1 Biochem. Biophys. Res. Commun., 384, 347–351
1-6. CHO cells
Bacterial toxins
Abrami, L., Liu, S., Cosson, P., Leppla, S.H., et al (2003) Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process J. Cell Biol., 160, 321-328
Abrami, L., Leppla, S.H., and Gisou van der Gaat, F. (2006) Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis J. Cell Biol., 171, 309-320
Lafont, F., Van Nhieu, G.T., Hanada, K., Sansonetti, P., et al (2002) Initial steps of Shigella infection depend on the cholesterol/shingolipid raft-mediated CD44-IpaB interaction EMBO J., 21, 4449-4457
Glycolipids/Phospholipids
Abrami, L., Fivaz, M., Kobayashi, T., Kinoshita, T., et al (2001) Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains J. Biol. Chem., 276, 30729-30736
Haberkant, P., Schmitt, O., Contreras, E-X., Thiele, C., et al (2008) Protein-sphingolipid interactions within cellular membranes J. Lipid. Res., 49, 251-262
Honsho, M., Yagita, Y., Kinoshita, N. and Fujuki, Y. (2008) Isolation and characterization of mutant animal cell line defective in alkyl-dihydroxyacetonephosphate synthase: Localization and transport of plasmalogens to post-Golgi compartments Biochim. Biophys. Acta, 1783, 1857-1865
Sprong, H., Degroote, S., Nilsson, T., Kawakita, M., et al (2003) Association of the Golgi UDP-galactose transporter with UDP-galactose: ceramide galactosyltransferase allows UDP-galactose import in the endoplasmic reticulum Mol. Biol. Cell, 14, 3482-3493
GPI-anchored proteins
Abe, Y., Inoue, H., Ashida, H., Maeda, Y., Kinoshita, T. and Kitada, S. (2017) Glycan region of GPI anchoredprotein is required for cytocidal oligomerization of an anticancer parasporin-2, Cry46Aa1 protein, from Bacillus thuringiensis strain A1547 J. Invertebr. Pathol., 142, 71–81
Jaensch, N., Corrêa Jr, I.R. and Watanabe, R. (2014) Stable cell surface expression of GPI-anchored proteins, but not intracellular transport, depends on their fatty acid structure Traffic, 15, 1305–1329
Nucleic acids
Lucero, H., Gae, D. and Taccioli, G.E. (2003) Novel localization of the DNA-PK complex in lipid rafts: a putative role in the signal transduction pathway of the ionizing radiation response J. Biol. Chem., 278, 22136-22143
O-glycans
Shao, B., Yago, T., Setiadi, H., Wang, Y., Mehta-D’souza, P., Fu, J., Crocker, P.R., Rodgers, W., Xia, L. and McEver, R.P.l (2015) O-glycans direct selectin ligands to lipid rafts on leukocytes Proc. Natl. Acad. Sci. USA, 112, 8661–8666
Plasmalogen biosynthesis
Honsho, M., Abe, Y. and Fujiki, Y. (2017) Plasmalogen biosynthesis is spatiotemporally regulated by sensing plasmalogens in the inner leaflet of plasma membranes Sci. Rep., 7: 43936
Protein/receptor translocation
Bournazos, S., Hart, S.P., Chamberlain, L.H., Glennie, M.J., et al. (2009) Association of FcRIIa (CD32a) with lipid rafts regulates ligand binding activity J. Immunol., 182, 8026-8036
Chiang, N-Y., Hsiao, C-C., Huang, Y-S., Chen, H-Y., et al (2011) Disease-associated GPR56 mutations cause bilateral frontoparietal polymicrogyria via multiple mechanisms J. Biol. Chem., 286, 14215–14225
Frenzel, K.E. and Falls, D.L. (2001) Neuregulin-1 proteins in rat brain and transfected cells are localized to lipid rafts J. Neurochem., 77, 1-12
Renner, U., Glebov, K., Lang, T., Papusheva, E., et al (2007) Localization of the mouse 5-hydroxytryptamine1A receptor in lipid microdomains depends on its palmitoylation and is involved in receptor-mediated signaling Mol. Pharmacol., 72, 502-513
Schilling, K., Opitz, N., Wisenthal, A., Oess, S., et al (2006) Translocation of endothelial nitric-oxide synthase involves a ternary complex with caveolin-1 and NOSTRIN Mol. Biol. Cell, 17, 3870-3880
Setiadi, H. and McEver, R.P. (2008) Clustering endothelial E-selectin in clathrin-coated pits and lipid rafts enhances leukocyte adhesion under flow Blood, 111, 1989-1998
Tournaviti, S., San Pietro, E., Terjung, S., Schafmeier, T., et al (2009) Reversible phosphorylation as a molecular switch to regulate plasma membrane targeting of acylated SH4 domain proteins Traffic 2009; 10, 1047–1060
Sterols and sterol binding proteins
Laitinen, S., Lehto, M., Lehtonen, S., Hyvarinen, K., et al (2002) ORP2, a homolog of oxysterol binding protein, regulates cellular cholesterol metabolism J. Lipid Res., 43, 245-255
Lusa, S., Blom, T.S., Eskelinen, E-L., Kuismanen, E., et al (2001) Depletion of rafts in late endocytic membranes is controlled by NPC1-dependent recycling of cholesterol to the plasma membrane J. Cell Sci., 114, 1893-1900
Maiwald, A., Bauer, O. and Gimpl, G. (2017) Synthesis and characterization of a novel rhodamine labeled cholesterol reporter Biochim.Biophys. Acta, 1859, 1099–1113
Vainio, S., Jansen, M., Koivusalo, M., Rog, T., et al (2006) Significance of sterol structural specificity J. Biol. Chem., 281, 348-355
Wiegand, V., Chang, T-Y., Strauss, J.F., Fahrenholz, F., et al (2003) Transport of plasma membrane cholesterol and the function of Niemann-Pick C1 protein FASEB J., 17, 782-784
Yamauchi, Y., Yokoyama, S. and Chang, T-Y. (2016) ABCA1-dependent sterol release: sterol molecule specifi city and potential membrane domain for HDL biogenesis J. Lipid Res., 57, 77–88
1-7. Chondrocytes
Zhou, Z., Xie, J., Lee, D., Liu, Y., et al (2010) Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation Dev. Cell, 19, 90–102
1-8. Cochlear spiral ligament pericytes
Ghelfi, E., Grondin, Y., Millet, E.J., Bartos, A., Bortoni, M., Gomes dos Santos, C.O., Trevino-Villarreal, H.J., Sepulveda, R. and Rogers, R. (2018) In vitro gentamicin exposure alters caveolae protein profile in cochlear spiral ligament pericytes Proteome Sci., 16: 7
1-9. COS cells
G-protein signalling
Hiol, A., Davey, P.C., Osterhout, J.L., Waheed, A.A., et al (2003) Palmitoylation regulates regulators of Gprotein signaling (RGS) 16 function: I Mutation of amino terminalcysteine residues on RGS16 prevents its targeting to lipid rafts and palmitoylation of an internal cysteine residue J. Biol. Chem., 278, 19302-19308
Osterhout, J.L., Waheed, A.A., Hiol, A., Ward, R.J., et al (2003) Palmitoylation regulates regulators of Gprotein signaling (RGS) 16 function: II Palmitoylation of a cysteine residue in the RGS box is critical for RGS16 GTPase accelerating activity and regulation of Gi-coupled signaling J. Biol. Chem., 278, 19309-19316
Waheed, A.A. and Jones, T.L.Z. (2002) Hsp90 interactions and acylation target the G protein Gα12 but not Gα13 to lipid rafts J. Biol. Chem., 277, 32409-32412
Lipids/fatty acids
Kai, M., Sakane, F., Jia, Y-J., Imai, S-i., et al (2006) Lipid phosphate phosphatases 1 and 3 are localized in distinct lipid rafts J. Biochem., 140, 677-686
Liang, X., Nazarian, A., Erdjument-Bromage, H., Bornmann, W., et al (2001) Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction J. Biol. Chem., 276, 30987-30994
Matsutomo, D., Isozaki, T., Sakai, H. and Sakane, F. (2013) Osmotic shock-dependent redistribution of diacylglycerol kinase η1 to non-ionic detergent-resistant membrane via pleckstrin homology and C1 domains J. Biochem., 153, 179–190
Thankamony, S.P. and Knudson, W. (2006) Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis J. Biol. Chem., 281, 34601-34609
Neuron-derived factors
Cabedo, H., Luna, C., Fernandez, A.M., Gallar, J., et al (2002) Molecular determinants of the sensory and motor neuron-derived factor insertion into plasma membrane J. Biol. Chem., 277, 19905-19912
Cabedo, H., Carteron, C. and Ferrer-Montiel, A. (2004) Oligomerization of the sensory and motor neuronderived factor prevents protein O-glycosylation J. Biol. Chem., 279, 33623-33629
Protein kinases
Chen, X. and Resh, M.D. (2001) Activation of mitogen-activated protein kinase by membrane targeted Raf chimeras is independent of raft localization J. Biol. Chem., 276, 34617-34623
Trafficking
Blancet, M-H., Le Good, J.A., Mesnard, D., Oorschot, V., et al (2008) Cripto recruits Furin and PACE4 and controls Nodal trafficking during proteolytic maturation EMBO J., 27, 2580-2591
Conklin, M.W., Ada-Nguema, A., Parsons, M., Riching, K.M., et al (2010) R-Ras regulates β1-integrin trafficking via effects on membrane ruffling and endocytosis BMC Cell Biol., 11:14
Lupo, V., Galindo, M.I., Martínez-Rubio, D., Sevilla, T., et al (2009) Missense mutations in the SH3TC2 protein causing Charcot-Marie-Tooth disease type 4C affect its localization in the plasma membrane and endocytic pathway Hum. Mol. Genet., 18, 4603–4614
Viruses/virus components
Anastasia, L., Holguera, J., Bianchi, A., D’Avila, F., et al (2008) Over-expression of mammalian sialidase NEU3 reduces Newcastle disease virus entry and propagation in COS7 cells Biochim. Biophys. Acta, 1780, 504-512
Halwani, R., Khorchid, A., Cen, S. and Kleiman, L. (2003) Rapid localization of Gag/GagPol complexes to detergent-resistant membrane during the assembly of human immunodeficiency virus type 1 J. Virol., 77, 3973-3984
Kroupa, T., Langerová, H., Doležal, M., Prchal, J., Spiwok, V., Hunter, E., Rumlová, M., Hrabal, R. and Ruml, T. (2016) Membrane Interactions of the Mason-Pfizer monkey virus matrix protein and its budding deficient mutants J. Mol. Biol., 428, 4708-4722
Lindwasser, O.W. and Resh, M.D. (2001) Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains J. Virol., 75, 7913-7924
Lindwasser, O.W. and Resh, M.D. (2002) Myristoylation as a target for inhibiting HIV assembly: Unsaturated fatty acids block viral budding Proc. Natl. Acad. Sci. USA, 99, 13037-13042
Martinez, N., Xue, X., Berro, R.G., Kreitzer, G., et al (2008) Kinesin KIF4 regulates intracellular trafficking and stability of the human immunodeficiency virus type 1 Gag polyprotein J. Virol., 82, 9937-9950
1-10. Dendritic cells
Ocaña-Morgner, C., Reichardt, P., Chopin, M., Braungart, S., et al (2011) Sphingosine 1-phosphate–induced motility and endocytosis of dendritic cells is regulated by SWAP-70 through RhoA J. Immunol., 186, 5345–5355
Peng, W., Martaresche, C., Escande-Beillard, N., Cedile, O., Reynier-Vigouroux, A. and Boucraut, J. (2007) Influence of lipid rafts on CD1d presentation by dendritic cells Mol. Membr. Biol., 24, 475-484
Wang, S-h., Yuan, S-g., Peng, D-q, and Zhao, S-p. (2012) HDL and ApoA-I inhibit antigen presentationmediated T cell activation by disrupting lipid rafts in antigen presenting cells Atherosclerosis, 225, 105-114
1-11. Embryonic limb bud explants
Long, J., Tokhunts, R., Old, W.M., Houel, S., Rodgriguez-Blanco, J., Singh, S., Schilling, N., Capobianco, A.J., Ahn, N.G. and Robbins, D.J. (2015) Identification of a family of fatty-acid-speciated sonic hedgehog proteins, whose members display differential biological properties Cell Rep., 10, 1280–1287
1-12. Endometrial cells
Banadakoppa, M., Goluszko, P., Liebenthal, D. and Yallampalli, C. (2012) Nitric oxide induces segregation of decay accelerating factor (DAF or CD55) from the membrane lipid-rafts and its internalization in human endometrial cells Cell Biol. Int., 36, 901–907
1-13. Endothelial cells
Angiogenesis/angiotensin
Amiya, E., Watanabe, M., Takeda, N., Saito, T., et al. (2013) Angiotensin II impairs endothelial nitric-oxide synthase bioavailability under free cholesterol-enriched conditions via intracellular free cholesterol-rich membrane microdomains 288, 14497–14509
Chen, P-K., Chang, B-I., Kuo, C-H., Chen, P-S., et al (2013) Thrombomodulin functions as a plasminogen receptor to modulate angiogenesis FASEB J., 27, 4520–4531
Han, W-Q., Chen, W-D., Zhang, K., Liu, J-J., Wu, Y-J. and Gao, P-J. (2016) Ca2+-regulated lysosome fusion mediates angiotensin II-induced lipid raft clustering in mesenteric endothelial cells Hypertens. Res., 39, 227–236
Liu, P., Li, X., Song, F., Li, P., Wei, J., Yan, Q., Xu, X., Yang, J., Li, C. and Fu, X (2017) Testosterone promotes tube formation of endothelial cells isolated from veins via activation of Smad1 protein Mol. Cell. Endocrinol., 446, 21-31
Tiwari, A., Jung, J.J., Inamdar, S.M., Nihalani, D., et al., (2013) The myosin motor Myo1c is required for VEGFR2 delivery to the cell surface and for angiogenic signaling Am. J. Physiol. Heart. Circ. Physiol., 304, H687–H696
Urbinati, C., Ravelli, C., Tanghetti, E., Belleri, M., et al (2012) Substrate-immobilized HIV-1 Tat drives VEGFR2/αvβb3–integrin complex formation and polarization in endothelial cells Arterioscler. Thromb. Vasc. Biol., 32, e25-e34
Wang, X., Zou, Z., Deng, Z., Liang, D., Zhou, X., Sun, R. and Lan, K. (2017) Male hormones activate EphA2 to facilitate Kaposi’s sarcoma-associated herpesvirus infection: Implications for gender disparity in Kaposi’s sarcoma PLoS Pathog., 13: e100658
Bacterial/fungal infections
He, X., Shi, X., Puthiyakunnon, S., Zhang, L., Zeng, Q., Li, Y., Boddu, S., Qiu, J., Lai, Z. et al (2016) CD44- mediated monocyte transmigration across Cryptococcus neoformans-infected brain microvascular endothelial cells is enhanced by HIV-1 gp41-I90 ectodomain J. Biomed. Sci., 23: 28
Huang, S-H., Wu, C-H., Jiang, S., Bahner, I., et al (2011) HIV-1 gp41 ectodomain enhances Cryptococcus neoformans binding to human brain microvascular endothelial cells via gp41 core-induced membrane activities Biochem. J., 438, 457–466
Huang, S-H., Long, M., Wu, C-H., Kwon-Chung, K.J., et al (2011) Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells is mediated through the lipid rafts-endocytic pathway via the dual specificity tyrosine phosphorylation-regulated kinase 3 (DYRK3) J. Biol. Chem., 286, 34761–34769
Huang, S-H., Wu, C-H., Chang, Y.C., Kwon-Chung, K.J., et al (2012) Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection PLoS One, 7, e48570
Jong, A., Wu, C-H., Shackleford, G.M., Kwon-Chung, K.J., et al (2008) Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells Cell. Microbiol., 10, 1313-1326
Maruvada, R., Argon, Y. and Prasadaroa, N. (2008) Escherichia coli interaction with human brain microvascular endothelial cells induces signal transducer and activator of transcription 3 association with the C-terminal domain of Ec-gp96, the outer membrane protein A receptor for invasion Cell. Microbiol., 10, 2326-2338
Warnier, M., Römer, W., Geelen, J., Lesieur, J., et al (2006) Trafficking of Shiga toxin/Shiga-like toxin-1 in human glomerular microvascular endothelial cells and human mesangial cells Kidney Int., 70, 2085-2091
Cell adhesion
Hara, T., Kondo, N., Nakamura, H., Okuyama, H., et al (2007) Cell-surface thioredoxin-1: possible involvement in thiol-mediated leukocyte-endothelial cell interaction through lipid rafts Antioxid. Redox Signal., 9, 1427-1437
Setiadi, H. and McEver, R.P. (2008) Clustering endothelial E-selectin in clathrin-coated pits and lipid rafts enhances leukocyte adhesion under flow Blood, 111, 1989-1998
Urbinati, C., Ravelli, C., Tanghetti, E., Belleri, M., et al (2012) Substrate-immobilized HIV-1 Tat drives VEGFR2/αvβb3–integrin complex formation and polarization in endothelial cells Arterioscler. Thromb. Vasc. Biol., 32, e25-e34
Endothelial barrier function/cytoskeleton
Armstrong, S.M., Khajoee, V., Wang, C., Wang, T., et al (2012) Co-regulation of transcellular and paracellular leak cross microvascular endothelium by dynamin and Rac Am. J. Pathol., 180, 1308–1323
Dudek, S.M., Chiang, E.T., Camp, S.M., Guo, Y., et al (2010) Abl tyrosine kinase phosphorylates nonmuscle myosin light chain kinase to regulate endothelial barrier function Mol. Biol. Cell, 21, 4042–4056
Ephstein, Y., Singleton, P.A., Chen, W., Wang, L., et al (2013) Critical role of S1PR1 and integrin β4 in HGF/c-Met-mediated increases in vascular integrity J. Biol. Chem., 288, 2191–2200
Mammoto, A., Huang, S. and Ingber, D.E. (2007) Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts J. Cell Sci., 120, 456-467
Ni, X., Epshtein, J., Chen, W., Zhou, T., et al (2014) Interaction of integrin β4 with S1P receptors in S1P- and HGF-induced endothelial barrier enhancement J. Cell. Biochem., 115, 1187–1195
Singleton, P.A., Dudek, S.M., Chiang, E.T. and Garcia, J.G.N. (2003) Regulation of sphingosine 1-phosphateinduced endothelial cytoskeletal rearrangement and barrier enhancement by SIP1 receptor, PI3 kinase, Tiam1/Rac1, and α-actinin FASEB J., 19, 1646-1656
Singleton, P.A., Salgia, R., Moreno-Vinasco, L., Moitra, J., et al., (2007) CD44 regulates hepatocyte growth factor-mediated vacular integrity: role of c-Met, Tiam 1/Rac1, dynamin 2 and cortactin J. Biol. Chem., 282, 30643-30657
Singleton, P.A., Mirzapoiazova, T., Guo, Y., Sammani, S., et al (2010) High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness Am. J. Physiol. Lung Cell Mol. Physiol., 299, L639–L651, 2010
Urbinati, C., Ravelli, C., Tanghetti, E., Belleri, M., et al (2012) Substrate-immobilized HIV-1 Tat drives VEGFR2/αvβb3–integrin complex formation and polarization in endothelial cells Arterioscler. Thromb. Vasc. Biol., 32, e25-e34
Usatyuk, P.V., Singleton, P.A., Pendyala, S., Kalari, S.K., et al (2012) Novel role for non-muscle myosin light chain kinase (MLCK) in hyperoxia-induced recruitment of cytoskeletal proteins, NADPH oxidase activation, and reactive oxygen species generation in lung endothelium J. Biol. Chem., 287, 9360–9375
Wickström, S.A., Alitalo, K. and Keski-Oja, J. (2003) Endostatin associates with lipid rafts and induces reorganisation of the actin cytoskeleton via down-regulation of RhoA activity J. Biol. Chem., 278, 37895-37901
Zhao, J., Singleton, P.A., Brown, M.E., Dudek, S.M., et al (2009) Phosphotyrosine protein dynamics in cell membrane rafts of sphingosine-1-phosphate-stimulated human endothelium: Role in barrier enhancement Cell. Signal., 21, 1945–1960
Glycolipids, see “Lipids, lipoproteins and lipid precursors”
Ion Transport
Sones, W.R., Davis, A.J., Leblanc, N. and Greenwood, I.A. (2010) Cholesterol depletion alters amplitude and pharmacology of vascular calcium-activated chloride channels Cardiovasc. Res., 87, 476–484
Lipids, lipoproteins and lipid precursors
Chen, J., Chen, L., Wang, G. and Tang, H. (2007) Cholesterol-dependent and –independent CD40 internalization and signaling activation in cardiovascular endothelial cells Arterioscl. Thromb. Vasc. Biol., 27, 2005-2013
Chen, Y., Wang, S., Lu, X., Zhang, H., et al (2011) Cholesterol sequestration by nystatin enhances the uptake and activity of endostatin in endothelium via regulating distinct endocytic pathways Blood, 117, 6392-6403
Cheng, A.M., Handa, P., Tateya, S., ,Schwartz, J., et al (2012) Apolipoprotein A-I attenuates palmitatemediated NF-kB astivation by reducing toll-like receptor-4 recruitment into lipid rafts PLoS One, 7: e33917
Kondo, Y., Ikeda, K., Tokuda, N., Nishitani, C., Ohto, U., Akashi-Takamura, S., Ito, Y., Uchikawa, M., Kuroki, Y., Taguchi, R., Miyake, K., Zhang, Q., Furukawa, K. and Furukawa, K. (2013) TLR4–MD-2 complex is negatively regulated by an endogenous ligand, globotetraosylceramide Proc. Natl. Acad. Sci. USA, 110, 4714-4719
Predescu, S.A., Predescu,, D.N., Shimizu, K., Klein, I.K., et al (2005) Cholesterol-dependent syntaxin-4 and SNAP-23 clustering regulates caveolar fusion with the endothelial plasma membrane J. Biol. Chem., 280, 37130-37138
Singleton, P.A., Dudek, S.M., Ma, S-F. and Garcia, J.G.N. (2006) Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation: novel role for hyaluronan and CD44 receptor family J. Biol. Chem., 281, 34381-34393
Singleton, P.A., Chatchavalvanich. S., Fu, P., Xing, J., et al (2009) Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids Circ. Res. 104, 978-986
Wang, L., Sapuri-Butti, A.R., Aung, H.H., Parikh, A.N., et al (2008) Triglyceride-rich lipoprotein lipolysis increases aggregation of endothelial cell membrane microdomains and produces reactive oxygen species Am. J. Physiol., Heart Circ. Physiol., 295, H237-H244
Wilkerson, B.A., Grass, G.D., Wing, S.B., Argraves, W.S. and Argraves, K.M. (2012) Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier – High density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compares with albumin-S1P via effects on levels, trafficking, and signaling of S1P1 J. Biol. Chem., 287, 44645–44653
Lysosome-related functions
Han, W-Q., Xia, M., Xu, M., Boini, K.M., et al (2012) Lysosome fusion to the cell membrane is mediated by the dysferlin C2A domain in coronary arterial endothelial cells J. Cell Sci., 125, 1225–1234
Xu, M., Xia, M., Li, X-X., Han, W-Q., et al (2012) Requirement of translocated lysosomal V1 H+-ATPase for activation of membrane acid sphingomyelinase and raft clustering in coronary endothelial cells Mol. Biol. Cell, 23, 1546-1557
Protein/membrane trafficking
Cailleteau, L., Estrach, S., Thyss, R., Boyer, L., et al (2010) α2β1 Integrin controls association of Rac with the membrane and triggers quiescence of endothelial cells J. Cell Sci., 123, 2491-2501
Huttunen, H.J., Guénette, S.Y., Peach, C., Greco, C., et al (2007) HtrA2 regulates β-amyloid precursor protein (APP) metabolism through endoplasmic reticulum-associated degradation J. Biol. Chem., 282, 28285-28295
Popescu, N.I., Lupu, C. and Lupu, F. (2010) Calcium ionophore-induced tissue factor (TF) decryption induces TF immobilization into lipid rafts and negative regulation of TF procoagulant activity Blood (ASH Ann. Meeting Abstr.) 116, Abstr. 1131
Song, N., Ding, Y., Zhuo, W., He, T., Fu, Z., Chen, Y., Song, X., Fu, Y. and Luo, Y. (2012) The nuclear translocation of endostatin is mediated by its receptor nucleolin in endothelial cells Angiogenesis, 15, 697–711
Zhan, R., Leng, X., Liu, X., Wang, X., et al. (2009) Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes Biochem. Biophys. Res. Comm., 387, 229–233
Signalling
Impagnatiello, M-A., Weitzer, S., Gannon, G., Compagni, A., et al (2001) Mammalian sprouty-1 and –2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells J. Cell Biol., 152, 1087-1098
Katoh, S-Y., Kamimoto, T., Yamakawa, D. and Takakura, N. (2009) Lipid rafts serve as signaling platforms for Tie2 receptor tyrosine kinase in vascular endothelial cells Exp. Cell Res., 315, 2818-2823
Singleton, P.A. and Bourguignon, L.Y.W. (2004) CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling to nitric oxide production and endothelial cell adhesion and proliferation Exp. Cell Res., 295, 102-118
Xu, M., Xia, M., Li, X-X., Han, W-Q., et al (2012) Requirement of translocated lysosomal V1 H+-ATPase for activation of membrane acid sphingomyelinase and raft clustering in coronary endothelial cells Mol. Biol. Cell, 23, 1546-1557
Yu, J., Akishita, M., Eto, M., Koizumi, H., et al (2012) Src kinase-mediates androgen receptor-dependent nongenomic activation of signaling cascade leading to endothelial nitric oxide synthase Biochem. Biophys. Res. Comm., 424, 538–543
Zhung, A.Y., Yi, F., Zhang, G., Gulbins, E., et al (2006) Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells Hypertension, 47, 74-80
Enterocytes: see “1-13 Epithelial cells”
1-14. Epithelial cells (see also MDCK cells and Retinal Cells)
Bomberger, J.M., MacEachran, D.P., Coutermarsh, B.A., Ye, S., et al (2009) Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles PLoS Pathog., 5:e1000382
Borot, F., Vieu, D-L., Faure, G., Fritsch, J., et al (2009) Eicosanoid release is increased by membrane destabilization and CFTR inhibition in Calu-3 cells PLoS One 4: e7116
Caputo, A., Sarnataro, D., Campana, V., Costanzo, M., et al (2010) Doppel and PrPC co-immunoprecipitate in detergent-resistant membrane domains of epithelial FRT cells Biochem. J., 425, 341–351
Jandu, N., Ceponis, P.J.M., Kato, S., Riff, J.D., et al (2006) Conditioned medium from enterohemorrhagic Escherichia coli-infected T84 cells inhibits signal transducer and activator of transcription 1 activation by gamma interferon Infect. Immun., 74, 1809-1818
Oliferenko, S., Paiha, K., Harder, T., Gerke, V., et al (1999) Analysis of CD44-containing lipid rafts: Recruitment of Annexin II and stabilization by the actin cytoskeleton J. Cell Biol., 146, 843-854
Peng, W., Martaresche, C., Escande-Beillard, N., Cedile, O., et al (2007) Influence of lipid rafts on CD1d presentation by dendritic cells Mol. Membr. Biol., 24, 475-484
Sarnataro, D., Caputo, A., Casanova, P., Puri, C., et al (2009) Lipid rafts and clathrin cooperate in the internalization of PrPC in epithelial FRT cells PLoS One, 4:e5829
Sharpe, S.W., Kuehn, M.J. and Mason, K.M. (2011) Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of non-typeable haemophilus influenzae Infect. Immun., 79, 4361-4369
Shen-Tu, G., Schauer, D.B., Jones, N.L., and Sherman, P.M. (2010) Detergent-resistant microdomains mediate activation of host cell signaling in response to attaching–effacing bacteria Lab. Invest., 90, 266–281
Shen-Tu, G., Kim, H., Liu, M., Johnson-Henry, K.C., et al (2014) Protein kinase C mediates enterohemorrhagic Escherichia coli O157:H7-induced attaching and effacing lesions Infect. Immun., 82, 1648–1656
Siddiqi, S. and Mansbach, C.M. (2011) Dietary fatty acid in rat intestinal cytosol is associated with caveolae Gastroenterology, 140, Suppl. 1 S542
Siddiqi, S., Polly, S., Siddiqi, T. and Mansbach, C.M. (2015) Apolipoprotein-Av resists proteolysis in the intestinal tract and is absorbed intact, controlled by dietary phosphatidylcholine Gastroenterology, 148, Suppl. 1, S880-881
Smith, J.L., Campos, S.K. and Ozbun, M.A. (2007) Human papillomavirus type 31 uses a caveolin 1- and dynamin 2-mediated entry pathway for infection of human keratinocytes J. Virol., 81, 9922-9931
Valuckaite, V., Zaborina, O., Long, J., Hauer-Jensen, M., et al (2009) Oral PEG 15–20 protects the intestine against radiation: role of lipid rafts Am. J. Physiol. Gastrointest. Liver Physiol., 297, G1041–G1052
1-15. Erythrocytes (human)
D’Avila, F., Tringali, C., Papini, N., Anastasia, L., et al (2013) Identification of lysosomal sialidase NEU1 and plasma membrane sialidase NEU3 in human erythrocytes J. Cell. Biochem., 114, 204–211
Mandal, S., Mukherjee, S., Chowdhury, K.D., Sarkar, A., et al (2012) S-allyl cysteine in combination with clotrimazole downregulates Fas induced apoptotic events in erythrocytes of mice exposed to lead Biochim. Biophys. Acta, 1820, 9–23
Kathuria, R., Mondal, A.K., Sharma, R., Bhattacharyya, S. and Chattopadhyay, K. (2018) Revisiting the role of cholesterol in regulating the pore-formation mechanism of Vibrio cholerae cytolysin, a membrane-damaging βbarrel pore-forming toxin Biochem. J., 475, 3039–3055
1-16. Fibroblasts
Asano, S., Kitatani, K., Taniguchi, M., Hashimoto, M., et al, (2012) Regulation of cell migration by sphingomyelin synthases: sphingomyelin in lipid rafts decreases responsiveness to signaling by the CXCL12/CXCR4 pathway Mol. Cell. Biol., 32, 3242-3252
Baker, T.L., Zheng, H., Walker, J., Coloff, J.L.,et al (2003) Distinct rates of palmitate turnover on membranebound cellular and oncogenic H-Ras J. Biol. Chem., 278, 19292-19300
Hathaway, H.J., Evans, S.C., Dubois, D.H., Foote, C.I.,et al (2003) Mutational analysis of the cytoplasmic domain of β1,4-galactosyltransferase I: influence of phosphorylation on cell surface expression J. Cell Sci., 116, 4319-4330
Hayward, R.D., Hume, P.J., Humphreys, D., Phillips, N., et al, (2009) Clustering transfers the translocated Escherichia coli receptor into lipid rafts to stimulate reversible activation of c-Fyn Cell. Microbiol., 11, 433-441
Holzer, R.G., Park, E-J., Li, N., Tran, H., et al., (2011) Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation Cell 147, 173–184
Jacobs, C., Onnockx, S., Vandenbroere, I. and Pirson, I. (2004) Endogenous SHIP2 does not localize in lipid rafts in 3T3-L1 adipocytes FEBS Lett., 565, 70-74
Kai, M., Sakane, F., Jia, Y-J., Imai, S-i., et al, (2006) Lipid phosphate phosphatases 1 and 3 are localized in distinct lipid rafts J. Biochem., 140, 677-686
Kant, S., Standen, C.L., Morel, C., Jung, D.Y., Kim, J.K., Swat, W., Flavell, R.A. and Davis, R.J. (2017) A protein scaffold coordinates SRC-mediated JNK activation in response to metabolic stress Cell Rep., 20, 2775-2783
Koivusalo, M., Jansen, M., Somerharju, P. and Ikonen, E. (2007) Endocytic trafficking of sphingomyelin depends on its acyl chain length Mol. Biol. Cell, 18, 5113-5123
Lallemand-Breitenbach, V., Quesnoit, M., Braun, V., El Marjou, A., et al (2004) CLIPR-59 is a lipid raftassociated protein containing a cytoskeleton-associated protein glycine-rich domain (CAP-Gly) that perturbs microtubule dynamics J. Biol. Chem., 279, 41168-41178
Le Lay, S., Li, Q., Proschogo, N., Rodriguez, M., et al (2009) Caveolin-1-dependent and -independent membrane domains J. Lipid Res. 50, 1609–1620
Lusa, S., Blom, T.S., Eskelinen, E-L., Kuismanen, E., et al (2001) Depletion of rafts in late endocytic membranes is controlled by NPC1-dependent recycling of cholesterol to the plasma membrane J. Cell Sci., 114,1893-1900
Mani, T., Hennigan, R.F., Foster, L.A., Conrady, D.G., et al, (2011) FERM domain phosphoinositide binding targets merlin to the membrane and is essential for its growth-suppressive function Mol. Cell. Biol., 31, 1983–1996
Martín, J.J., Holguera, J., Sánchez-Felipe, L., Villar, E., et al, (2012) Cholesterol dependence of Newcastle disease virus entry Biochim. Biophys. Acta, 1818, 753–761
Midgley, A.C., Rogers, M., Hallett, M.B., Clayton, A., et al, (2013) Transforming growth factor-β1 (TGF-β1)- stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts J. Biol. Chem., 288, 14824–14838
Okada, S., Yamada, E., Saito, T., Ohshima, K., Hashimoto, K., et al (2008) CDK5-dependent phosphorylation of the Rho family GTPase TC10α regulates insulin-stimulated GLUT4 translocation J. Biol. Chem., 283, 35455-35463
Peng, W., Martaresche, C., Escande-Beillard, N., Cedile, O., et al, (2007) Influence of lipid rafts on CD1d presentation by dendritic cells Mol. Membr. Biol., 24, 475-484
Renner, U., Glebov, K., Lang, T., Papusheva, E., et al (2007) Localization of the mouse 5-hydroxytryptamine1A receptor in lipid microdomains depends on its palmitoylation and is involved in receptor-mediated signaling Mol. Pharmacol., 72, 502-513
Rylaarsdam, L.E., Johnecheck, G.N., Looyenga, B.D. and Louters, L.L. (2019) GLUT1 is associated with sphingolipid-organized, cholesterol independent domains in L929 mouse fibroblast cells Biochimie 162, 88-96
Sharon-Friling, R. and Shenk, T. (2014) Human cytomegalovirus pUL37x1-induced calcium flux activates PKCα, inducing altered cell shape and accumulation of cytoplasmic vesicles Proc. Natl. Acad. Sci. USA, 111, E1140–E1148
Sordella, R., Jiang, W., Chen, G-C., Curto, M. et al (2003) Modulation of Rho GTPase signaling regulates a switch between adigenesis and myogenesis Cell, 113, 147-158
Smith, D.C., Spooner, R.A., Watson, P., Murray, J.L., et al (2006) Internalized Pseudomonas exotoxin A can exploit multiple pathways to reach the endoplasmic reticulum Traffic, 7, 379-393
Stickney, J.T., Bacon, W.C., Rojas, M., Ratner, N., et al (2004) Activation of the tumor suppresor merlin modulates its interaction with lipid rafts Cancer Res., 64, 2717-2724
Wang, Y., Buggia-Prévot, V., Zavorka, M.E., Bleackley, R.C., MacDonald, R.G., Thinakaran, G. and Kara, S. (2015) Overexpression of the insulin-like growth factor II receptor increases β-amyloid production and affects cell viability Mol. Cell Biol., 35, 2368-2384
Woeste, M.A., Stern, S., Raju, D.N., Grahn, E., Dittmann, D., Gutbrod, K., Dörmann, P., Hansen, J.N.,Schonauer, S. et al (2019) Species-specific differences in nonlysosomal glucosylceramidase GBA2 function underlie locomotor dysfunction arising from loss-of-function mutations J. Biol. Chem., 294, 3853–3871
Yamauchi, Y., Yokoyama, S. and Chang, T-Y. (2016) ABCA1-dependent sterol release: sterol molecule specifi city and potential membrane domain for HDL biogenesis J. Lipid Res., 57, 77–88
1-17. Gastric cancer cells
Xu, L., Guo, T., Qu, X., Hu, X., Zhang, Y., Che, X., Song, H., Gong, J. et al (2018) β-elemene increases the sensitivity of gastric cancer cells to TRAIL by promoting the formation of DISC in lipid rafts Cell Biol Int 42 (2018) 1377–1385
1-18. Heart tissue
Lin, L., Kim, S.C., Wang, Y., Gupta, S., et al (2007) HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis Am. J. Physiol. Heart Circ. Physiol., 293 H2238-H2247
1-19. Hepatocytes and hepatoma cells
Baron, W., Ozgen, H., Klunder, B., de Jonge, J.C., Nomden, A., Plat, A., Trifilieff, E., de Vries, H. and Hoekstra, D. (2015) The major myelin-resident protein PLP is transported to myelin membranes via a transcytotic mechanism: involvement of sulfatide Mol. Cell. Biol., 35, 288-302
Chao, T-C., Su, W-C., Huang, J-Y., Chen, Y-C., et al, (2012) Proline-serine-threonine phosphatase-interacting protein 2 (PSTPIP2), a host membrane-deforming protein, is critical for membranous web formation in hepatitis C virus replication J. Virol., 86, 1739-1749
Elazar, M., Cheong, K.H., Liu, P., Greenberg, H.B., et al (2003) Amphipathic helix-dependent localization of NS5A mediates hepatitis C virus RNA replication J. Virol., 77, 6055-6061
Kataoka, C., Kaname, Y., Taguwa, S., Abe, T., et al, (2012) Baculovirus GP64-mediated entry into mammalian cells J. Virol., 86, 2610-2620
Khan, I., Katikaneni, D.S., Han, Q., Sanchez-Felipe, L., Hanada, K., Ambrose, R.L., Mackenzie, J.M. and Konana, K.V. (2014) Modulation of hepatitis C virus genome replication by glycosphingolipids and fourphosphate adaptor protein 2 J. Virol., 88, 12276–12295
Li, Y., Masaki, H., Shimakami, T., and Lemon, S.M. (2104) hnRNP L and NF90 interact with hepatitis C virus 5’terminal untranslated RNA and promote efficient replication J. Virol., 88, 7199–7209
Lusa, S., Heino, S. and Ikonen, E. (2003) Differential mobilization of newly synthesized cholesterol and biosynthetic sterol precursors from cells J. Biol. Chem., 278, 19844-19851
Majeau, N., Fromentin, R., Savard, C., Duval, M., et al, (2009) Palmitoylation of hepatitis C virus core protein is important for virion production J. Biol. Chem., 284, 33915–33925
Marques, L., Auriac, A., Willemetz, A., Banha, J., et al, (2012) Immune cells and hepatocytes express glycosylphosphatidylinositol-anchored ceruloplasmin at their cell surface Blood Cells Mol. Dis., 48, 110–120
Matto, M., Rice, C.M., Aroeti, B. and Glenn, J.S. (2006) Hepatitis C virus core protein associates with detergent-resistant membranes distinct from classical plasma membrane rafts J. Virol., 78, 12047-12053
Nagata, J., Guerra, M.T., Shugrue, C.A., Gomes, D.A., et al (2007) Lipid rafts establish calcium waves in hepatocytes Gastroenterology, 133, 256-267
Nguyen, L.N., Lim, Y-S., Pham, L.V., Shin, H-Y., Kim, Y-S. and Hwang, S.B. (2014) Stearoyl coenzyme A desaturase 1 is associated with hepatitis C virus replication complex and regulates viral replication J. Virol., 88, 12311–12325
Park, J-W., Park, W-J., Kuperman, Y., Boura-Halfon, S., et al (2013) Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes Hepatology, 57, 525-532
Park, W-J., Park, J-W., Erez-Roman, R., Kogot-Levin, A., et al (2013) Protection of a ceramide synthase 2 null mouse from drug-induced liver injury: role of gap junction dysfunction and connexin 32 mislocalization J. Biol. Chem., 288, 30904-30916
Park, W-J., Park, J-W., Merrill Jr., A.H., Storch, J., Pewzner-Jung, Y. and Futerman, A.H. (2014) Hepatic fatty acid uptake is regulated by the sphingolipid acyl chain length Biochim. Biophys. Acta, 1841, 1754–1766
Saxena, V., Lai, C-K., Chao, T-C., Jeng, K-S., et al, (2012) Annexin A2 is involved in the formation of hepatitis C virus replication complex on the lipid raft J. Virol., 86, 4139-4150
Shanmugam, S., Saravanabalaji, D. and Yi, M. (2015) Detergent-resistant membrane association of NS2 and E2 during hepatitis C virus replication J. Virol., 89, 4562-4574
Vainio, S., Heino, S., Mansson, J-E., Fredman, P., Kuismanen, E., et al (2002) Dynamic association of human insulin receptor with lipid rafts in cells lacking caveolae EMBO Rep., 3, 1-6
Wang, H., Perry, J.W., Lauring, A.S., Neddermann, P., et al (2014) Oxysterol-binding protein is a phosphatidylinositol 4-kinase effector required for HCV replication membrane integrity and cholesterol trafficking Gastroenterology, 146, 1373–1385
1-20. Human embryonic kidney cells
Amyloid precursor protein
Brandimarti, R., Hill, G.S., Geiger, J.D. and Meucci, O. (2017) The lipid raft-dwelling protein US9 can be manipulated to target APP compartmentalization, APP processing, and neurodegenerative disease pathogenesis Sci. Rep., 7: 15103
Kojro, E. Gimpl, G., Lammich, S., Marz, W., et al, (2001) Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10 Proc. Natl. Acad. Sci. USA, 98, 5815-5820
Nagarathinam, A., Hölinger, P., Bühler, A., Schäfer, C., et al (2013) Membrane-anchored Aβ accelerates amyloid formation and exacerbates amyloid-associated toxicity in mice J. Neurosci., 33, 19284 –19294
Sano, O., Tsujita, M., Shimizu, Y., Kato, R., Kobayashi, A., Kioka, N., Remaley, A.T., Michikawa, M.. Ueda, K. and Matsuo, M. (2016) ABCG1 and ABCG4 suppress γ-secretase activity and amyloid β production PLoS One, 11: e0155400
Apoptosis
Cruz, A.C., Ramaswamy, M., Ouyang, C., Klebanoff, C.A., Sengupta, P., Yamamoto, T.N., Meylan, F., Thomas, S.K. et al (2016) Fas/CD95 prevents autoimmunity independently of lipid raft localization and efficient apoptosis induction Nat, Comm., 7, 13895
ATP binding casettes
Sano, O., Ito, S., Kato, R., Shimizu, Y., Kobayashi, A., Kimura, Y., Kioka, N., Hanada, K., Ueda, K. and Matsuo, M. (2014) ABCA1, ABCG1, and ABCG4 are distributed to distinct membrane meso-domains and disturb detergent-resistant domains on the plasma membrane PLoS One, 9: e109886
Bile acid transporter
Annaba, F., Kumar, P., Dudeja, A.K., Saksena, S., et al, (2010) Green tea catechin EGCG inhibits ileal apical sodium bile acid transporter ASBT Am . J. Physiol. Gastrointest. Liver Physiol., 298, G467–G473
Brij/Triton comparison: see “ATP binding cassettes”
G-protein, see “Signaling/G-protein”
Ion channels
Carnally, S.M., Johannessen, M., Henderson, R.M., Jackson, M.B., et al, (2010) Demonstration of a direct interaction between s-1 receptors and acid-sensing ion channels Biophys. J. 98, 1182–1191
Hinzpeter, A., Fritsch, J., Borot, F., Trudel, S., et al (2007) Membrane cholesterol content modulates ClC-2 gating and sensitivity to oxidative stress J. Biol. Chem., 282, 2423-2432
Morenilla-Palao, C. Pertusa, M., Meseguer, V., Cabedo, H., et al (2009) Lipid raft segregation modulates TRPM8 channel activity J. Biol. Chem., 284, 9215–9224
Schwarzer, S., Nobles, M. and Tinker, A. (2010) Do caveolae have a role in the fidelity and dynamics of receptor activation of G-protein-gated inwardly rectifying potassium channels? J. Biol. Chem., 285, 27817–27826
Lipid/lipoprotein metabolism and organization
Hatch, G.M., Smith, A.J., Xu, F.Y., Hall, A.M., et al (2002) FATS1 channels exogenous FA into 1,2,3-triacylsn-glycerol and down-regulates sphingomyelin and cholesterol metabolism in growing 293 cells J. Lipid. Res., 43, 1380-1389
Vieira, C.R., Munoz-Olaya, J.M., Sot, J., Jiménez-Baranda, S., et al (2010) Dihydrosphingomyelin impairs HIV-1 infection by rigidifying liquid-ordered membrane domains Chem. Biol., 17, 766–775
Yamauchi, Y., Yokoyama, S. and Chang, T-Y. (2016) ABCA1-dependent sterol release: sterol molecule specificity and potential membrane domain for HDL biogenesis J. Lipid Res., 57, 77–88
Neuronal development and signalling
Cabrera, J.R., Sanchez-Pulido, L., Rojas, A.M., Valencia, A., et al (2006) Gas1 is related to the glial cellderived neurotrophic factor family receptors α and regulates ret signaling J. Biol. Chem., 281, 14330-14339
Caprini, M., Gomis, A., Cabedo, H., Planells-Cases, R.,et al (2003) GAP43 stimulates inositol trisphosphatemediated calcium release in response to hypotonicity EMBO J., 22, 3004-3014
Cezanne, L., Lecat, S., Lagane, B., Millot, C., et al (2004) Dynamic confinement of NK2 receptors in the plasma membrane J. Biol. Chem., 279, 45057-45067
Fournier, A.E., Gould, G.C., Liu, B.P. and Strittmatter, S.M. (2002) Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin J. Neurosci., 22, 8876-8883
Yang, G., Liu, Y., Yang, K., Liu, R., et al (2012) Isoform-specific palmitoylation of JNK regulates axonal development Cell Death Differ., 19, 553–561
Prion protein
Christensen, H.M. and Harris, D.A. (2009) A deleted prion protein that is neurotoxic in vivo is localized normally in cultured cells J. Neurochem., 108, 44-56
Solomon, I.H., Khatri, N., Biasini, E., Massignan, T., et al (2011) An N-terminal polybasic domain and cell surface localization are required for mutant prion protein toxicity J. Biol. Chem., 286, 14724–14736
Turnbaugh, J.A., Unterberger, U., Saá, P., Massignan, T., et al (2012) The N-terminal, polybasic region of PrPC dictates the efficiency of prion propagation by binding to PrPSc J. Neurosci., 32, 8817– 8830
Westergard, L., Turnbaugh, J.A. and Harris, D.A. (2011) A nine amino acid domain is essential for mutant prion protein toxicity J. Neurosci., 31, 14005–14017
Signaling/G-protein
Chiang, N-Y., Chang, G-W.l, Huang, Y-S., Peng, Y-M., Hsiao, C-C., Kuo, M-L. and Lin, H-H. (2016) Heparin interacts with the adhesion GPCR GPR56, reduces receptor shedding, and promotes cell adhesion and motility J. Cell Sci., 129, 2156-2169
Hiol, A., Davey, P.C., Osterhout, J.L., Waheed, A.A., et al (2003) Palmitoylation regulates regulators of Gprotein signaling (RGS) 16 function: I Mutation of amino terminalcysteine residues on RGS16 prevents its targeting to lipid rafts and palmitoylation of an internal cysteine residue J. Biol. Chem., 278, 19302-19308
Huang, Y-S., Chiang, N-Y., Hu, C-H., Hsiao, C-C., et al (2012) Activation of myeloid cell-specific adhesion class G protein-coupled receptor EMR2 via ligation-induced translocation and interaction of receptor subunits in lipid raft microdomains Mol. Cell. Biol., 32, 1408-1420
Johnson, E.N., Seasholtz, T.M., Waheed, A.A., Kreutz, B., et al (2003) RGS16 inhibits signaling through the Gα13-Rho axis Nat. Cell Biol., 5, 1095-1103
Osterhout, J.L., Waheed, A.A., Hiol, A., Ward, R.J., et al (2003) Palmitoylation regulates regulators of Gprotein signaling (RGS) 16 function: II Palmitoylation of a cysteine residue in the RGS box is critical for RGS16 GTPase accelerating activity and regulation of Gi-coupled signaling J. Biol. Chem., 278, 19309-19316
Waheed, A.A. and Jones, T.L.Z. (2002) Hsp90 interactions and acylation target the G protein Gα12 but not Gα13 to lipid rafts J. Biol. Chem., 277, 32409-32412
Signaling/kinases
Bruckner, K., Labrador, J.P., Schieffele, P., Herb, A., et al (1999) EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains Neuron, 22, 511-524
Cinar, B., Mukhopadhyay, N.K., Meng, G. and Freeman, M.R. (2007) Phosphoinositide 3-kinase-independent non-genomic signals transit from androgen receptor to Akt1 in membrane raft microdomains J. Biol. Chem., 282, 29584-29593
Huai, J.and Drescher, U. (2001) An ephrinA-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120 kDa protein J. Biol. Chem., 276, 6689-6694
Lacalle, R.A., Mira, E., Gomez-Mouton, C., Jimenez-Baranda, S., et al (2002) Specific SHP-2 partitioning in raft domains triggers integrin-mediated signaling via Rho activation J. Cell Biol., 157, 277-289
Signaling/receptors
Crowley, J.T., Toledo, A.M., LaRocca, T.J., Coleman, J.L., et al (2013) Lipid exchange between Borrelia burgdorferi and host cells PLoS Pathog., 9: e1003109
Gimpl, G. and Fahrenholz, F. (2002) Human oxytocin receptors in cholesterol-rich vs. cholesterol-poor microdomains of the plasma membrane Eur. J. Biochem., 267, 2483-2497
Hang, Q., Isaji, T., Hou, S., Im, S., Fukuda, T. and Gu, J. (2015) Integrin α5 suppresses the phosphorylation of epidermal growth factor receptor and its cellular signaling of cell proliferation via N-glycosylation J. Biol. Chem., 290, 29345–29360
Hartung, A., Bitton-Worms, K., Rechtman. M.M., Wenzel, V., et al (2006) Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling Mol. Cell. Biol., 26, 7791-7805
Huang, Y-S., Chiang, N-Y., Hu, C-H., Hsiao, C-C., et al (2012) Activation of myeloid cell-specific adhesion class G protein-coupled receptor EMR2 via ligation-induced translocation and interaction of receptor subunits in lipid raft microdomains Mol. Cell. Biol., 32, 1408-1420
Lamb, M.E., Zhang, C., Shea, T., Kyle, D.J., et al (2002) Human B1 and B2 bradykinin receptors and their agonists target caveolae-related lipid rafts to different degrees in HEK293 cells Biochemistry, 41, 14340-14347
Özhan, G., Sezgin, E., Wehner, D., Pfister, A.S., et al (2013) Lypd6 enhances Wnt/β-catenin signaling by promoting Lrp6 phosphorylation in raft plasma membrane domains Dev. Cell, 26, 331–345
Sezgin, E., Azbazdar, Y., Ng, X.W., The, C., Simons, K., Weidinger, G., Wohland, T., Eggeling, C. and Ozhan, G. (2017) Binding of canonical Wnt ligands to their receptor complexes occurs in ordered plasma membrane environments FEBS J., 284, 2513–2526
Thankamony, S.P. and Knudson, W. (2006) Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis J. Biol. Chem., 281, 34601-34609
Urea transporter
Feng, X., Huang, H., Yang, Y., Fröhlich, O., et al (2009) Caveolin-1 directly interacts with UT-A1 urea transporter: the role of caveolae/lipid rafts in UT-A1 regulation at the cell membrane Am. J. Physiol. Renal Physiol., 296, F1514–F1520
Virus proteins
Aguilar, H., Matreyek, K.A., Choi, D.Y., Filone, C.M., et al (2007) Polybasic KKR motif in the cytoplasmic tail of Nipah virus fusion protein modulates membrane fusion by inside-out signaling J. Virol., 81, 4520-4532
Beer, C., Pedersen, L. and Wirth, M. (2005) Amphotropic murine leukaemia virus envelope protein is associated with cholesterol-rich microdomains Virology J., 2:36
Del Real, G., Jimenez-Baranda, S., Lacalle, R.A., Mira, E., et al (2002) Blocking of HIV-1 infection by targeting CD4 to nonraft membrane domains J. Exp. Med., 196, 293-301
De Rocquigny, H., El Meshri, S.E., Richert, L., Didier, P., Darlix, J-L. and Mély, Y. (2014) Role of the nucleocapsid region in HIV-1 Gag assembly as investigated by quantitative fluorescence-based microscopy Virus Res., 193, 78–88
Ding, L., Derdowsky, A., Wang, J-J- and Spearman, P. (2003) Independent segregation of human immunodeficiency virus type I Gag protein complexes and lipid rafts J. Virol., 77, 1916-1926
Dou, J., Wang, J-J., Chen, X., Li, H., et al (2009) Characterization of a myristoylated, monomeric HIV Gag protein Virology 387, 341–352
Fenard, D., Yonemoto, W., de Noronha, C., Cavrois, M., et al (2005) Nef is physically recruited into the immunological synapse and potentiates T cell activation early after TCR engagement J. Immunol., 175, 6050-6057
Fritz, J.V., Tibroni, N., Keppler, O.T. and Fackler, O.T. (2012) HIV-1 Vpu’s lipid raft association is dispensable for counteraction of the particle release restriction imposed by CD317/Tetherin Virology, 424, 33–44
Giese, S.I., Woerz, I., Homann, S., Tibroni, N., et al (2006) Specific and distinct determinants mediate membrane binding and lipid raft incorporation of HIV-1SF2 Nef Virology, 355, 175-191
Kaname, Y., Tani, H., Kataoka, C., Shiokawa, M., et al (2010) Acquisition of complement resistance through incorporation of CD55/decay-accelerating factor into viral particles bearing baculovirus GP64 J. Virol., 84, 3210–3219
Krautkrämer, E., Giese, S.I., Gasteier, J.E., Muuranyi, W., et al (2004) Human immunodeficiency virus type 1 Nef activates p21-activated kinase via recruitment into lipid rafts J. Virol., 78, 4085-4097
Kroupa, T., Langerová, H., Doležal, M., Prchal, J., Spiwok, V., Hunter, E., Rumlová, M., Hrabal, R. and Ruml, T. (2016) Membrane interactions of the Mason-Pfizer monkey virus matrix protein and its budding deficient mutants J. Mol. Biol., 428, 4708-4722
Manes, S., del Real, G., Lacalle, R.A., Lucas, P., et al (2000) Membrane raft microdomains mediate lateral assemblies for HIV-1 infection EMBO Rep., 1, 190-196
Matskova, L., Ernberg, I., Pawson, T. and Winberg, G. (2001) C-terminal domain of the Epstein-Barr virus LMP2A membrane protein contains a clustering signal J. Virol., 75, 10941-10949
Okamoto, K., Mori, Y., Komoda, Y., Okamoto, T., et al (2008) Intramembrane processing by signal peptide peptidase regulates the membrane localization of hepatitis C virus core protein and viral propagation J. Virol., 82, 8349-8361
Pulkkinen, K., Renkema, G.H., Kirchhoff, F. and Saksela, K. et al (2004) Nef associates with p21-activated kinase 2 in a p21-GTPase-dependent dynamic activation complex within lipid rafts J. Virol., 78, 12773-12780
Rasbach, A., Abel, T., Münch, R.C., Boller, K., et al (2013) The receptor attachment function of measles virus hemagglutinin can be replaced with an autonomous protein that binds Her2/neu while maintaining its fusionhelper function J. Virol., 87, 6246–6256
Schneider, F., Neugebauer, J., Griese, J., Liefold, N., et al (2008) The viral oncoprotein LMP1 exploits TRADD for signaling by masking its apoptotic activity PloS Biology, 6, 86-98
Tanaka, T., Kasai, H., Yamashita, A., Okuyama-Dobashi, K., Yasumoto, J., Maekawa, S., Enomoto, N., Okamoto, T. et al (2014) Hallmarks of hepatitis C virus in equine hepacivirus J. Virol., 88, 13352–13366
Vieira, C.R., Munoz-Olaya, J.M., Sot, J., Jiménez-Baranda, S., et al (2010) Dihydrosphingomyelin impairs HIV-1 infection by rigidifying liquid-ordered membrane domains Chem. Biol., 17, 766–775
Wang, S-W. and Aldovini, A. (2002) RNA incorporation is critical for retroviral particle integrity after cell membrane assembly of Gag complexes J. Virol., 76, 11853-11865
Intestinal cells see “1-13 Epithelial cells”
1-21. Jurkat cells (see also “1-23 Lymphoid/leukaemia cells)
Apoptosis
Muppidi, J. and Siegel, R.M. (2004) Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death Nat. Immunol., 5, 182-189
Cell-cell interactions
Hara, T., Kondo, N., Nakamura, H., Okuyama, H., et al (2007) Cell-surface thioredoxin-1: possible involvement in thiol-mediated leukocyte-endothelial cell interaction through lipid rafts Antioxid. Redox Signal., 9, 1427-1437
Trucy, M., Barbat, C., Sorice, M., Fischer, A., et al (2004) CD4-induced down-regulation of T cell adhesion to B cells is associated with localization of phosphatidyl inositol 3-kinase and LFA-1 in distinct membrane domains Eur. J. Immunol., 34, 2168-2178
Lipids and fatty acids
Ishii, H., Mori, T., Shriatsuchi, A., Nakai, Y., et al (2004) Distinct localization of lipid rafts and externalized phosphatidylserine at the surface of apoptotic cells Biochem. Biophys. Res. Commun., 327, 94-99
Liang, X., Nazarian, A., Erdjument-Bromage, H., Bornmann, W., et al (2001) Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction J. Biol. Chem., 276, 30987-30994
HIV1 Nef
Chase, A.J., Wombacher, R. and Fackler, O.T. (2018) Intrinsic properties and plasma membrane trafficking route of Src family kinase SH4 domains sensitive to retargeting by HIV-1 Nef J. Biol. Chem., 293, 7824–7840
Methodology
Korzeniowski, M., Kwiatkowska, K., and Sobota, A. (2003) Insights into the association of FcçRII and TCR with detergent-resistant membrane domains: isolation of the domains in detergent-free density gradients facilitates membrane fragment reconstitution Biochemistry, 42, 5358-5367
Schatzlmaier, P., Supper, V., Göschl, L., Zwirzitz, A., Eckerstorfer, P., Ellmeier, W., Huppa, J.B. and Stockinger, H. (2015) Rapid multiplex analysis of lipid raft components with single-cell resolution Sci Signal., 8 (395) rs11
Signalling
Dienz, O., Moller, A., Strecker, A., Stephan, N., et al (2002) Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDA and phospholipase Cγ1 are required for NF-κB activation and lipid raft recruitment of protein kinase Cθ induced by T cell co-stimulation J. Immunol., 169, 365-372
Gomez-Mouton, C., Abad, J.L., Mira, E., Lacalle, R.A., et al (2001) Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization Proc. Natl. Acad, Sci., USA, 98, 9642-9647
Harder, T. and Kuhn, M. (2000) Selective accumulation of raft-associated membrane protein LAT in T cell receptor signaling assemblies J. Cell Biol., 151, 199-207
Hartgrove, L., Lin, J., Langen, H., Zech, T., et al (2003) Synergistic assembly of linker for activation of T cells signaling protein complexes in T cell plasma membrane domains J. Biol. Chem., 278, 20389-20394
Huang, J., Ren, T., Guan, H., Jiang, Y. et al (2009) HTLV-1 tax is a critical lipid raft modulator that hijacks IκB kinases to the microdomains for persistent activation of NF-κB J. Biol. Chem., 284, 6208–6217
Sakamoto, T., Kobayashi, M., Tada, K., Shinohara, M., et al (2014) CKIP-1 is an intrinsic negative regulator of T-cell activation through an interaction with CARMA1 PLoS One, 9: e85762
Sebald, A., Mattioli, I. and Schmitz, M.L. (2005) T cell receptor-induced lipid raft recruitment of the IκB kinase complex is necessary and sufficient for NF-κB activation occurring in the cytosol Eur. J. Immunol., 35, 318-325
Szymańska, E., Korzeniowski, M., Raynal, P., Sobota, A. et al (2009) Contribution of PIP-5 kinase Iα to raftbased FcγRIIA signaling Exp. Cell Res., 315, 981-995
Yan, G., Huang, J., Jarbadan, N.R., Jiang, Y. et al (2008) Sequestration of NF-B signaling complexes in lipid rafts contributes to repression of NF-B in T lymphocytes under hyperthermia stress J. Biol. Chem., 283, 12489-12500
Viruses
Brügger, B., Krautmrämer, E., Tibroni, N., Munte, C.E., et al (2007) Human immunodeficiency virus type 1 Nef protein modulates the lipid composition of virions and host cell membrane microdomains Retrovirology, 4:70
Fenard, D., Yonemoto, W., de Noronha, C., Cavrois, M., et al (2005) Nef is physically recruited into the immunological synapse and potentiates T cell activation early after TCR engagement J. Immunol., 175, 6050-6057
Holm, K., Weclewicz,K., Hewson, R. and Suomalainen, M. (2003) Human immunodeficiency virus type 1 assembly and lipid rafts: Pr55gag associates with membrane domains J. Virol.,77, 4805-4817
Krautkrämer, E., Giese, S.I., Gasteier, J.E., Muuranyi, W., et al (2004) Human immunodeficiency virus type 1 Nef activates p21-activated kinase via recruitment into lipid rafts J. Virol., 78, 4085-4097
Pan, X., Geist, M.M., Rudolph, J.M., Nickel, W., et al (2013) HIV-1 Nef disrupts membrane-microdomainassociated anterograde transport for plasma membrane delivery of selected Src family kinases Cell. Microbiol., 15, 1605–1621
Rauch, S., Pulkkinen, K., Saksela, K. and Fackler, O. (2008) Human immunodeficiency virus type 1 Nef recruits the guanine exchange factor Vav1 via an unexpected interface into plasma membrane microdomains for association with p21-activated kinase 2 activity J. Virol., 82 2918-2929
Witte, V., Laffert, B., Gintschel, P., Krautkrämer, E., et al (2008) Induction of HIV transcription by Nef involves Lck activation and protein kinase Cθ raft recruitment leading to activation of ERK1/2 but not NFκB J. Immunol., 181, 8425-8432
1-22. Keratinocytes
Gagnoux-Palacios, L., Gagnoux-Palacios, L., Dans, M., van’t Hoff, W., Mariotti, A., et al (2003) Compartmentalization of integrin α6β4 signaling in lipid rafts J. Cell Biol., 162, 1189-1196
George, K.S., Elyassaki, W., Wu, Q. and Wu, S. (2012) The role of cholesterol in UV light B-induced apoptosis Photochem. Photobiol., 88, 1191–1197
Grether-Beck, S., Salahshour-Fard, M., Timmer, A., Brenden, H., et al. (2008) Ceramide and raft signaling are linked with each other in UVA radiation-induced gene expression Oncogene, 27, 4768-4778
Wu, Q. and Wu, S. (2017) The role of lipid raft translocation of prohibitin in regulation of Akt and Rafprotected apoptosis of HaCaT cells upon ultraviolet B irradiation Mol. Carcinog., 56, 1789–1797
1-23. Kidney cells (proximal tubules and glomeruli)
N.B. This section is primarily concerned with kidney function; cultured kidney cells are also used for the study of other processes – see for example “1-25 MDCK cells” or “1-27 Monkey kidney cells”
Methodology reviews
Salant, D.J. and Topham, P.S. (2003) Role of nephrin in proteinuric renal diseases Immunopathol., 24, 423-439
Yu, P., Villar, V.A. and Jose, P.A. (2013) Methods for the study of dopamine receptors within lipid rafts of kidney cells In, Dopamine: Methods and Protocols, Methods Mol, Biol., 964 (ed. Kabbani, N.)Springer Science+Business Media, pp 14-24
Nephrin
Barletta, G-M., Kovari, I.A., Verma, R.K., Kerjaschki, D., et al (2003) Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers J. Biol. Chem., 278, 19266-19271
Simons, M., Schwarz, K., Kriz, W., Miettinen, A., et al (2001) Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm Am. J. Pathol., 159, 1069-1077
Verma, R., Wharram, B., Kovar, I., Kunkel, R., et al (2003) Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin J. Biol. Chem., 278, 20716-20723
Verma, R., Kocvari, I., Soofi, A., Nihalani, D., et al (2006) Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization J. Clin. Invest., 116, 1346-1359
Verma, R., Venkatareddy, M., Kalinowski, A., Patel S.R., Salant, D.J. and Garga, P. (2016) Shp2 associates with and enhances nephrin tyrosine phosphorylation and is necessary for foot process spreading in mouse models of podocyte injury Mol. Cell. Biol., 36, 596-614
Yuan, H.Y., Takeuchi, E., and Salant, D.J. (2002) Podocyte slit-diaphragm protein nephrin is linked to the actin cytoskeleton Am. J. Physiol., Renal Physiol., 282, F585-F591
Renal fibrosis
Han, W-Q., Xu, L., Tang, X-F., Chen, W-D., Wu, Y-J. and Gao, P-J. (2018) Membrane rafts–redox signalling pathway contributes to renal fibrosis via modulation of the renal tubular epithelial–mesenchymal transition J. Physiol., 596.16, 3603–3616
Renal medulla
Kawaguchi, K., Hatano, R., Matsubara, M. and Asano, S. (2018) Internalization of NKCC2 is impaired in thick ascending limb of Henle in moesin knockout mice Pflügers Archiv Eur. J. Physiol., 470, 1055–1068
Signalling and trafficking
Arif, E., Wagner, M.C., Johnstone, D.B., Wong, H.N., et al (2011) Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane Mol. Cell. Biol., 31, 2134-2150
Arif, E., Rathore, Y.S., Kumari, B., Ashish, F., et al (2014) Slit diaphragm protein Neph1 and its signaling – a novel therapeutic target for protection of podocytes against glomerular injury J. Biol. Chem., 289, 9502–9518
Song, J.H., Kim, M., Park, S.W., Chen, S.W., et al (2010) Isoflurane via TGF-β1 release increases caveolae formation and organizes sphingosine kinase signaling in renal proximal tubules Am. J. Physiol. Renal Physiol., 298, F1041–F1050
Villar, V.A.M., Cuevas, S., Zheng, X. and Jose, P.A. (2016) Localization and signaling of GPCRs in lipid rafts Meth. Cell Biol., 132, 3-23
Zhang, C., Hu, J-J., Xia, M., Boini, K.M., et al (2010) Redox signaling via lipid raft clustering in homocysteine-induced injury of podocytes Biochim. Biophys. Acta, 1803, 482–491
Transporters and ion exchangers
Carmosino, M., Rizzo, F., Procino, G., Basco, D. (2010) MAL/VIP17, a new player in the regulation of NKCC2 in the kidney Mol. Biol. Cell, 21, 3985–3997
Feng, X., Huang, H., Yang, Y., Fröhlich, O. et al (2009) Caveolin-1 directly interacts with UT-A1 urea transporter: the role of caveolae/lipid rafts in UT-A1 regulation at the cell membrane Am. J. Physiol. Renal Physiol., 296, F1514–F1520
McDonough, A.A. (2010) Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure Am. J. Physiol. Regul. Integr. Comp. Physiol., 298, R851–R861
Yang, J., Singh, V., Cha, B., Chen, T-E., et al, (2013) NHERF2 protein mobility rate is determined by a unique C-terminal domain that is also necessary for its regulation of NHE3 protein in OK cells J. Biol. Chem., 288, 16960–16974
Virus interactions
Favoreel, H.W., Mettenleiter, T.C. and Nauwynck, H.J. (2004) Copatching and lipid raft association of different viral glycoproteins expressed on the surfaces of Pseudorabies virus-infected cells J. Virol., 78, 5279-5287
Desplanques, A.S., Nauwynck, H.J., Tilleman, K., Deforce, D., et al (2007) Tyrosine phosphorylation and lipid raft association of pseudorabies virus glycoprotein E during antibody-mediated capping Virology, 362, 60-66
1-24 Leydig cells
Prasad, M., Pawlak, K.J., Burak, W.E., Perry, E.E., Marshall, B., Whittal, R.M. and Bose, H.S. (2017) Mitochondrial metabolic regulation by GRP78 Sci. Adv. 3: e1602038
Liver: see “1-19 Hepatocytes and hepatoma cells”
1-25. Lymphoid/leukemia cells
ADAM proteases
Ebsen, H., Lettau, M., Kabelitz, D. and Janssen, O. (2015) Subcellular localization and activation of ADAM proteases in thecontext of FasL shedding in T lymphocytes Mol. Immunol., 65, 416–428
Apoptosis
Ayllon, V., Fleischer, A., Cayla, X., Garcia, A., et al (2002) Segregation of Bad from lipid rafts is implicated in the induction of apoptosis J. Immunol., 168, 3387-3393
Griner, L.N., McGraw, K.L., Johnson, J.O., List, A.F., et al (2013) JAK2-V617F-mediated signalling is dependent on lipid rafts and statins inhibit JAK2-V617F-dependent cell growth Br. J. Haematol., 160, 177-187
Kondo, N., Ishii, Y., Kwon, Y-W., Tanito, M., et al (2007) Lipid raft-mediated uptake of cysteine-modified thioredoxin-1: apoptosis enhancement by inhibiting the endogenous thioredoxin-1 Antioxid. Redox Signal., 9, 1439-1448
Muppidi, J. and Siegel, R.M. (2004) Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death Nat. Immunol., 5, 182-189
Ramaswamy, M., Cruz, A.C., Cleland, S.Y., Deng, M., et al (2011) Specific elimination of effector memory CD4+ T cells due to enhanced Fas signaling complex formation and association with lipid raft microdomains Cell Death Differ., 18, 712–720
Rusyn, E., Mousallem, T., Persaud-Sawin, D-A., Miller, S., et al (2008) CLN3p impacts galactosylceramide transport, raft morphology and lipid content Pediatr. Res., 63, 625-63
Yan, G., Huang, J., Jarbadan, N.R., Jiang, Y., et al (2008) Sequestration of NF-κB signaling complexes in lipid rafts contributes to repression of NF-κB in T lymphocytes under hyperthermia stress J. Biol. Chem., 283, 12489-12500
Yang, J., Qian, J., Wezeman, M., Wang, S., et al (2006) Targeting β2-microglobulin for induction of tumor apoptosis in human hematological malignancies Cancer Cell, 10, 295-307
Cholesterol
Griner, L.N., McGraw, K.L., Johnson, J.O., List, A.F., et al (2013) JAK2-V617F-mediated signalling is dependent on lipid rafts and statins inhibit JAK2-V617F-dependent cell growth Br. J. Haematol., 160, 177-187
Murai, T., Sato, C., Sato, M., Nishiyama, H., et al (2103) Membrane cholesterol modulates the hyaluronan binding ability of CD44 in T lymphocytes and controls rolling under shear flow J. Cell Sci., 126, 3284–3294
Erythropoietin receptor
McGraw, K.L., Basiorka, A.A., Johnson, J.O., Clark, J., Caceres, G., Padron, E. et al (2014) Lenalidomide induces lipid raft assembly to enhance erythropoietin receptor signaling in myelodysplastic syndrome progenitors PloS One, 9: e114249
Leukaemia-related effects
Allsup, D.J., Kamiguti, A.S., Lin, K., Sherrington, P.D., et al (2005) B-cell receptor translocation to lipid rafts and associated signaling differ between prognostically important subgroups of chronic lymphocytic leukemia Cancer Res., 65, 7328-7337
Huang, J., Ren, T., Guan, H., Jiang, Y., et al (2009) HTLV-1 tax is a critical lipid raft modulator that hijacks IκB kinases to the microdomains for persistent activation of NF-κB J. Biol. Chem., 284, 6208–6217
Kamiguti, A.S., Serrander, L., Lin, K., Harris, R.J., Cawley, J.C., et al (2005) Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia J. Immunol., 175, 8424-8430
Zhang, H., Chen, L., Cai, S-H. and Cheng, H. (2016) Identification of TBK1 and IKKε, the non-canonical IκB kinases, as crucial pro-survival factors in HTLV-1-transformed T lymphocytes Leukemia Res.,46, 37–44
Myelodysplastic syndrome
McGraw, K.L., Basiorka, A.A., Johnson, J.O., Clark, J., Caceres, G., Padron, E. et al (2014) Lenalidomide induces lipid raft assembly to enhance erythropoietin receptor signaling in myelodysplastic syndrome progenitors PloS One, 9: e114249
Raft components
Schatzlmaier, P., Supper, V., Göschl, L., Zwirzitz, A., Eckerstorfer, P., Ellmeier, W., Huppa, J.B. and Stockinger, H. (2015) Rapid multiplex analysis of lipid raft components with single-cell resolution Sci Signal., 8 (395) rs11
Reactive oxygen species
Kamiguti, A.S., Serrander, L., Lin, K., Harris, R.J., Cawley, J.C., et al (2005) Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia J. Immunol., 175, 8424-8430
Kondo, N., Ishii, Y., Kwon, Y-W., Tanito, M., et al (2007) Lipid raft-mediated uptake of cysteine-modified thioredoxin-1: apoptosis enhancement by inhibiting the endogenous thioredoxin-1 Antioxid. Redox Signal., 9, 1439-1448
Receptors and signaling
Allsup, D.J., Kamiguti, A.S., Lin, K., Sherrington, P.D., et al (2005) B-cell receptor translocation to lipid rafts and associated signaling differ between prognostically important subgroups of chronic lymphocytic leukemia Cancer Res., 65, 7328-7337
Barbat, C., Trucy, M., Sorice, M., Garofalo, T., et al (2007) p56lck, LFA-1 and PI3K but not SHP-2 interact with GM1- or GM3-enriched microdomains in a CD4-p56lck association-dependent manner Biochem. J. 402, 471-481
Beekman, J.M., van der Linden, J.A., van de Winkel, J.G.J. and Leusen, J.H.W. (2008) FcRI (CD64) resides constitutively in lipid rafts Immunol. Lett., 116, 149-155
Bournazos, S., Hart, S.P., Chamberlain, L.H., Glennie, M.J., et al (2009) Association of FcRIIa (CD32a) with lipid rafts regulates ligand binding activity J. Immunol., 182, 8026-8036
Griner, L.N., McGraw, K.L., Johnson, J.O., List, A.F., et al (2013) JAK2-V617F-mediated signalling is dependent on lipid rafts and statins inhibit JAK2-V617F-dependent cell growth Br. J. Haematol., 160, 177-187
Hao, S. and August, A. (2005) Actin depolymerization transduces the strength of B-cell receptor stimulation Mol. Biol. Cell, 16, 2275-2294
Kheirallah, S., Caron, P., Gross, E., Quillet-Mary, A., et al., (2010) Rituximab inhibits B-cell receptor signaling Blood, 115, 985-994
Kondo, N., Ishii, Y., Kwon, Y-W., Tanito, M., et al (2007) Lipid raft-mediated uptake of cysteine-modified thioredoxin-1: apoptosis enhancement by inhibiting the endogenous thioredoxin-1 Antioxid. Redox Signal., 9, 1439-1448
Malapati, S. and Pierce, S.K. (2001) The influence of CD40 on the association of the B cell antigen receptor with lipid rafts in mature and immature cells Eur. J. Immunol., 31, 3789-3797
Martinez, E., Brzostowski, J.A., Long, E.O. and Gross, C.C. (2011) NKG2D-dependent cytotoxicity is controlled by ligand distribution in the target cell membrane J. Immunol., 186, 5538–5542
McGraw, K.L., Fuhler, G.M., Johnson, J.O., Clark, J.A., Caceres, G.C., Sokol, L. and List, A.F. (2012) Erythropoietin receptor signaling is membrane raft dependent PLoS One, 7: e34477
McGraw, K.L., Basiorka, A.A., Johnson, J.O., Clark, J., Caceres, G., Padron, E. et al (2014) Lenalidomide induces lipid raft assembly to enhance erythropoietin receptor signaling in myelodysplastic syndrome progenitors PloS One, 9: e114249
Misra, R.S., Russell, J.Q., Koenig, A., Hinshaw-Makepeace, J.A., et al (2007) Caspase-8 and c-FLIP in lipid rafts with NF-κB adaptors during T cell activation J. Biol. Chem., 282, 19365-19374
Shakor, A.B.A., Kwiatkowska, K. and Sobota, A. (2004) Cell surface ceramide generation precedes and controls FcγRII clustering and phosphorylation in rafts J. Biol. Chem., 279, 36778-36787
Sproul, T.W., Malapati, S., Kim, J. and Pierce, S. (2000) B cell antigen receptor signaling occurs outside lipid rafts in immature B cells J. Immunol., 165, 6020-6023
Szymańska, E., Korzeniowski, M., Raynal, P., Sobota, A., et al (2009) Contribution of PIP-5 kinase Iα to raftbased FcγRIIA signaling Exp. Cell Res., 315, 981-995
Yan, G., Huang, J., Jarbadan, N.R., Jiang, Y., et al (2008) Sequestration of NF-B signaling complexes in lipid rafts contributes to repression of NF-κB in T lymphocytes under hyperthermia stress J. Biol. Chem., 283, 12489-12500
Structure and assembly
Hao, S. and August, A. (2005) Actin depolymerization transduces the strength of B-cell receptor stimulation Mol. Biol. Cell, 16, 2275-2294
McQuade, K.J. and Repraeger, A.C. (2003) Syndecan-1 transmembrane and extracellular domains have unique and distinct roles in cell spreading J. Biol. Chem., 278, 46607-46615
Rusyn, E., Mousallem, T., Persaud-Sawin, D-A., Miller, S., et al (2008) CLN3p impacts galactosylceramide transport, raft morphology and lipid content Pediatr. Res., 63, 625-631
Thomas, S., Preda-Pais, A., Casares, S. and Brumeanu, T-D. (2004) Analysis of lipid rafts in T cell Mol. Immunol., 41, 399-409
T-cell interactions (see also “Leukaemia-related effects”)
Arenas-Del Angel, M., Legorreta-Herrera, M., Mendoza-Hernandez, G., Garfias, Y., Chavez, R., Zenteno. E. and Lascurain, R. (2015) Amaranthus leucocarpus lectin recognizes a moesin-like O-glycoprotein and costimulates murine CD3-activated CD4+ T cells Immun. Inflamm. Dis., 3, 182–195
Dobenecker, M-W., Schmedt, C., Okada, M. and Tarakhovsky, A. (2005) The ubiquitously expressed Csk adaptor protein Cbp is dispensable for embryogenesis and T-cell development and function Mol. Cell. Biol., 25, 10533-10542
Misra, R.S., Russell, J.Q., Koenig, A., Hinshaw-Makepeace, J.A., et al (2007) Caspase-8 and c-FLIP in lipid rafts with NF-κB adaptors during T cell activation J. Biol. Chem., 282, 19365-19374
Murai, T., Sato, C., Sato, M., Nishiyama, H., et al (2103) Membrane cholesterol modulates the hyaluronan binding ability of CD44 in T lymphocytes and controls rolling under shear flow J. Cell Sci., 126, 3284–3294
Secinaro, M.A., Fortner, K.A., Dienz, O., Logan, A., Murphy, M.P., Anathy, V., Boyson, J.E. and Budd, R.C. (2018) Glycolysis promotes caspase-3 activation in lipid rafts in T cells Cell Death Dis., 9: 62
Thioredoxin
Kondo, N., Ishii, Y., Kwon, Y-W., Tanito, M., et al (2007) Lipid raft-mediated uptake of cysteine-modified thioredoxin-1: apoptosis enhancement by inhibiting the endogenous thioredoxin-1 Antioxid. Redox Signal., 9, 1439-1448
Toxins
Atapattu, D.N. and Czuprynski, C.J. (2007) Mannheimia haemolytica leukotoxin binds to lipid rafts in bovine lymphoblastoid cells and is internalized in a dynamin-2- and clathrin-dependent manner Infect. Immun., 75, 4719-4727
Smith, D.C., Sillence, D.J., Falguières, T., Jarvis, R.M., Johannes, L., et al (2006) The association of Shiga-like toxin with detergent-resistant membranes is modulated by glucosylceramide and is an essential requirement in the endoplasmic reticulum for a cytotoxic effect Mol. Biol. Cell, 17, 1375-1387
Virus-related effects (see also “Leukaemia-related effects”)
Brügger, B., Glass, B., Haberkant, P., Leibrecht, I., Wieland, F.T. et al (2006) The HIV lipodome: a raft with an unusual composition Proc. Natl. Acad. Sci. USA, 103, 2641-2646
Brügger, B., Krautmrämer, E., Tibroni, N., Munte, C.E., et al (2007) Human immunodeficiency virus type 1 Nef protein modulates the lipid composition of virions and host cell membrane microdomains Retrovirology, 4:70
Hunte, R., Alonso, P., Thomas, R., Bazile, C.A., Ramos, J.C., van der Weyden, L., Dominguez-Bendala, J., Khan, W.N. and Shembade, N. (2018) CADM1 is essential for KSHV-encoded vGPCR and vFLIP-mediated chronic NF-κB activation PLoS Pathog. 14: e1006968
Pujari, R., Hunte, R., Thomas, R., van der Weyden, L., Rauch, D., Ratner, L., Nyborg, J.K., Ramos, J.C., Takai, Y. and Shembade, N. (2015) Human T-Cell leukemia virus type 1 (HTLV-1) Tax requires CADM1/TSLC1 for inactivation of the NF-κB inhibitor A20 and constitutive NF-κB signaling PLoS Pathog., 11: e1004721
Yasui, T., Luftig, M., Soni, V. and Kieff, E. (2004) Latent infection membrane protein transmembrane FWLY is critical for intermolecular interaction, raft localization, and signaling Proc. Natl.Acad. Sci. USA, 101, 278-283
1-26. Macrophages
Activation
Kim, G.T., Hahn, K.W., Sohn, K-Y., Yoon, S.Y. and Kim, J.W. (2019) PLAG enhances macrophage mobility for efferocytosis of apoptotic neutrophils via membrane redistribution of P2Y2 FEBS J., 286, 5016–5029
Adhesion
Vadali, S. and Post, S.R. (2014) Lipid rafts couple class A scavenger receptors to phospholipase A2 activation during macrophage adhesion J. Leukoc. Biol., 96, 873–881
Bacterial and parasitic infections
Houde, M., Gottschalk, M., Gagnon, F., Van Calsteren, M-R., et al (2012) Streptococcus suis capsular polysaccharide inhibits phagocytosis through destabilization of lipid microdomains and prevents lactosylceramide-dependent recognition Infect. Immun., 80, 506-517
Krishna Prasad, G.V.R., Dhar, V. and Mukhopadhaya, A. (2019) Vibrio cholerae OmpU mediates CD36- dependent reactive oxygen species generation triggering an additional pathway of MAPK activation in macrophages J. Immunol., 202, 2431-2450
Mukherjee, M., Ball, W.B. and Das, P.K. (2014) Leishmania donovani activates SREBP2 to modulate macrophage membrane cholesterol and mitochondrial oxidants for establishmentof infection Int. J. Biochem. Cell Biol., 55, 196–208
Sen, S., Roy, K., Mukherjee, S., Mukhopadhyay, R., et al (2011) Restoration of IFNγR subunit assembly, IFNγ signaling and parasite clearance in Leishmania donovani infected macrophages: role of membrane cholesterol PloS Pathog., 7: e1002229
Cystic fibrosis
Lévêque, M., Penna, A., Le Trionnaire, S., Belleguic, C., Desrues, B., Brinchault, G., Jouneau, S., LagadicGossmann, D. and Martin-Chouly, C. (2018) Phagocytosis depends on TRPV2-mediated calcium influx and requires TRPV2 in lipids rafts: alteration in macrophages from patients with cystic fibrosis Sci. Rep., 8: 4310
Endocytosis
Auriac, A., Willemetz, A. and Canonne-Hergaux, F. (2010) Lipid raft-dependent endocytosis: a new route for hepcidin-mediated regulation of ferroportin in macrophages Haematologica, 95, 1269-1277
Delputte, P.L., Van Gorp, H., Favoreel, H.W., Hoebeke, I., et al (2011) Porcine sialoadhesin (CD169/Siglec-1) is an endocytic receptor that allows targeted delivery of toxins and antigens to macrophages PloS One 6: 16827
Immune/inflammatory reactions
Murshid, A., Gong, J., Prince, T., Borges, T.J. and Calderwood, S.K. (2015) Scavenger receptor SREC-I mediated entry of TLR4 into lipid microdomains and triggered inflammatory cytokine release in RAW 264.7 cells upon LPS activation PLoS One, 10: e0122529
Zitzler, S., Hellwig, A., Hartl, F-U., Wieland, F., et al (2008) Distinct binding sites for the ATPase and substrate-binding domain of human Hsp70 on the cell surface of antigen presenting cells Mol. Immunol., 45, 3974-3983
Receptors and signalling
Fernandez-Lizarbe, S., Pascual, M., Gascon, M.S., Blanco, A., et al (2008) Lipid rafts regulate ethanolinduced activation of TLR4 signaling in murine macrophages Mol. Immunol., 45 2007-2016
Sen, S., Roy, K., Mukherjee, S., Mukhopadhyay, R., et al (2011) Restoration of IFNγR subunit assembly, IFNγ signaling and parasite clearance in Leishmania donovani infected macrophages: role of membrane cholesterol PloS Pathog., 7: e1002229
Villacorta, L., Chang, L., Salvatore, S.R., Ichikawa, T., et al (2103) Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts Cardiovasc. Res., 98, 116–124
Virus interactions
Carter, G.C., Bernstone, L., Sangani, D., Wynter Bee, J., et al (2009) HIV entry in macrophages is dependent on intact lipid rafts Virology 386, 192–202
Cui, H.L., Grant, A., Mukhamedova, N., Pushkarsky, T., Jennelle, L., Dubrovsky, L., Gaus, K., Fitzgerald, M.L., Sviridov, D. and Bukrinsky, M. (2012) HIV-1 Nef mobilizes lipid rafts in macrophages through a pathway that competes with ABCA1-dependent cholesterol efflux J. Lipid Res., 53, 696–708 1-27. MDCK cells
Apical (versus basolateral) trafficking
Benting, J.H., Rietveld, A.G. and Simons, K. (1999) N-glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells J. Cell Biol., 146, 313-320
Chmelar, R.S. and Nathanson, N.M. (2006) Identification of a novel apical sorting motif and mechanism of targeting of the M2 muscarinic acetylcholine receptor J. Biol. Chem., 281, 35381-35396
Francesconi, A. and Duvoisin, R.M. (2002) Alternative splicing unmasks dendritic and axonal targeting signals in metabotropic glutamate receptor 1 J. Neurosci., 22, 2196-2205
Fullekrug, J., Scheiffele, P. and Simons, K (1999) VIP36 localisation to the early secretory pathway J. Cell Sci., 112, 2813-2821
Hanwell, D., Ishikawa, T., Saleki, R., and Rotin, D. (2002) Trafficking and cell surface stability of the epithelial Na+ channel expressed in epithelial Madin-Darby canine kidney cells J. Biol. Chem., 277, 9772-9779
Lafont, F., Lecat, S., Verkade, P. and Simons K. (1998) Annexin XIIIb associates with lipid microdomains to function in apical delivery J. Cell Biol., 142, 1413-1427
Lafont, F., Verkade, P., Galli, T., Wimmer, C., et al (1999) Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells Proc. Natl. Acad. Sci. USA, 96, 3734-3738
Lecat, S., Verkade, P., Thiele, C., Fiedler, K., et al (2000) Different properties of two isoforms of annexin XIII in MDCK cells J. Cell Sci., 113, 2607-2618
Plant P.J., Lafont, F., Lecat, S., Verkade, P., et al (2000) Apical membrane targeting of Nedd4 is mediated by an association of its C2 domain with annexin XIIIb J. Cell Biol., 149, 1473-1483
Verkade, P., Harder, T., Lafont, F. and Simons, K. (1999) Induction of caveolae in the apical plasma membrane of Madin-Darby canine kidney cells J. Cell Biol., 148, 727-739
Cholesterol
Ogawa, Y. and Tanaka, M. (2016) A fluorescent cholesterol analogue for observation of free cholesterol in the plasma membrane of live cells Anal. Biochem., 492, 49-55
Glycolipids
Benting, J., Rietveld, A., Ansorge, I. and Simons, K (1999) Acyl and alkyl chain length of GPI-anchors is critical for raft association in vitro FEBS Lett., 462, 47-50
Castillon, G.A., Michon, L. and Watanabe, R. (2013) Apical sorting of lysoGPI-anchored proteins occurs independent of association with detergent-resistant membranes but dependent on their N-glycosylation Mol. Biol. Cell, 24, 2021-2033
Puig, B., Altmeppen, H.C., Thurm, D., Geissen, M., et al (2011) N-Glycans and glycosylphosphatidylinositolanchor act on polarized sorting of mouse PrPC in Madin-Darby canine kidney cells PLoS One, 6: e24624
Junctional permeability
Dudez, T., Borot, F., Huang, S., Kwak, B.R., et al (2008) CFTR in a lipid raft-TNFR1 complex modulates gap junctional intercellular communication and IL-8 secretion Eur. J. Clin. Invest., 38 (Suppl. 1), 20
Dudez, T., Borot, F., Huang, S., Kwak, B.R., et al (2008) CFTR in a lipid raft-TNFR1 complex modulates gap junctional intercellular communication and IL-8 secretion Biochim. Biophys. Acta, 1783, 779-788
Huang, S., Jornot, L., Wiszniewski, L., Rochat, T., et al (2003) Src signaling links mediators of inflammation to Cx43 gap junction channels in primary and transformed CFTR-expressing airway cells Cell Commun. Adhesion, 10, 279-285
Leung, L.W., Contreras, R.G., Flores-Maldomado, C., Cereijido, M., et al (2003) Inhibitors of glycosphingolipid biosynthesis reduce transepithelial electrical resistance in MDCK I and FRT cells Am. J. Physiol. Cell Physiol., 284, C1021-C1030
Lynch., R.D., Francis, S.A., McCarthy, K.M., Casas, E., et al (2007) Cholesterol depletion alters detergentspecific solubility profiles of selected tight junction proteins and the phosphorylation of occludin Exp. Cell Res., 313, 2597-2610
Prion proteins
Puig, B., Altmeppen, H.C., Thurm, D., Geissen, M., et al (2011) N-Glycans and glycosylphosphatidylinositolanchor act on polarized sorting of mouse PrPC in Madin-Darby canine kidney cells PLoS One, 6: e24624
Surface architecture, composition and interactions
Benaud, C.B., Gentil, B.J., Assard, N., Court, M., et al (2004) AHNAK interaction with the annexin 2/S100A10 complex regulates cell membrane cytoarchitecture J. Cell Biol., 164, 133-144
Fernández-Mũnoza, B., Yurrita, M.M., Martín-Villar, E., Carrasco-Ramírez, P., et al (2011) The transmembrane domain of podoplanin is required for its association with lipid rafts and the induction of epithelial-mesenchymal transition Int. J. Biochem. Cell Biol., 43, 886–896
Jansen, M., Pietiäinen, V.M., Pölönen, H., Rasilainen, L., et al. (2008) Cholesterol substitution increases the structural heterogeneity of caveolae J. Biol. Chem., 283, 14610-14618
Paterson, R.G., Takeda, M., Ohigashi, Y., Pinto, L.H. et al (2003) Influenza B virus BM2 protein is an oligomeric integral membrane protein expressed at the cell surface Virology, 306, 7-17
Pauchet, Y., Luton, F., Castella, C., Charles, J-F., et al (2005) Effects of a mosquito-cidal toxin on a mammalian epithelial cell line expressing its target receptor Cell. Microbiol., 7, 1335-1344
Schuck, S., Honsho, M., Ekroos, K., Shevchenko, A. et al (2002) Resistance of cell membranes to different antigens Proc. Natl. Acad. Sci. USA, 100, 5795-5800
Wei, W-C., Lin, H-H., Shen, M-R. and Tang, M-J. (2008) Mechanosensing machinery for cells under low substratum rigidity Am. J. Physiol.Cell Physiol., 295, 1579-1589
1-28. Melanoma cells
Yamauchi, Y., Furukawa, K., Hamamura, K. and Furukawa, K. (2011) Positive feedback loop between PI3KAkt-mTORC1 signaling and the lipogenic pathway boosts Akt signaling: induction of the lipogenic pathway by a melanoma antigen Cancer Res., 71, 4989–4997
1-29. Monkey kidney cells
Bacterial toxins
Smith, D.C., Spooner, R.A., Watson, P., Murray, J.L., et al (2006) Internalized Pseudomonas exotoxin A can exploit multiple pathways to reach the endoplasmic reticulum Traffic, 7, 379-393
Virus interactions
Cordo, S.M., Valko, A., Martinez, G.M. and Candurra, N.A. (2013) Membrane localization of Junín virus glycoproteins requires cholesterol and cholesterol rich membranes Biochem. Biophys. Res. Comm., 430, 912–917
Cuadras, M.A. and Greenberg, H.B. (2003) Rotavirus infectious particles use lipid rafts during replication for transport to the cell surface in vitro and in vivo Virology, 313, 308-321
Cuadras, M.A., Bordier, B.B., Zambrano, J.L., Ludert, J.E., et al (2006) Dissecting rotavirus particle-raft interaction with small interfering RNAs: Insights into rotavirus transit through the secretory pathway J. Virol., 80, 3935-3946
Isa, P., Realpe, M., Romero, P., Lopez, S., et al (2004) Rotavirus RRV associates with lipid membrane microdomains during cell entry Virology, 322, 370-381
Kukkonen, S.K.J., Vaheri, A. and Plyusin, A. (2004) Tula hantavirus L protein is a 250 kDa perinuclear membrane-associated protein J. Gen. Virol., 85, 1181-1189
Gosselin-Grenet, A-S., Mottet-Osman, G. and Roux, L. (2006) From assembly to virus particle budding: pertinence of the detergent resisitant membranes Virology, 344, 296-303
Martínez, M.A., López, S., Arias, C.F. and Isa, P. (2013) Gangliosides have a functional role during rotavirus cell entry J. Virol., 87, 1115-1122
1-30. Monocytic cells
Favoreel, H.W., Mettenleiter, T.C. and Nauwynck, H.J. (2004) Copatching and lipid raft association of different viral glycoproteins expressed on the surfaces of Pseudorabies virus-infected cells J. Virol., 78, 5279-5287
Horvatova, A., Utaipan, T., Otto, A-C., Zhang, Y., Gan-Schreier, H., Pavek, P., Pathil, A., Stremmel, W. and Chamulitrat, W. (2018) Ursodeoxycholyl lysophosphatidylethanolamide negatively regulates TLRmediated lipopolysaccharide response in human THP-1-derived macrophages Eur. J. Pharmacol., 825, 63–74
Kulma, M., Hereć, M., Grudziński, W., Anderluh, G., et al (2010) Sphingomyelin-rich domains are sites of lysenin oligomerization: Implications for raft studies Biochim. Biophys. Acta, 1798, 471–481
Kulma, M., Kwiatkowska, K. and Sobota, A. (2012) Raft coalescence and FcγRIIA activation upon sphingomyelin clustering induced by lysenin Cell. Signal., 24, 1641–1647
Kwiatkowska, K. and Sobota, A. (2001) The clustered Fcγ receptor II is recruited to Lyn-containing membrane domains and undergoes phosphorylation in a cholesterol-dependent manner Eur. J. Immunol., 31, 989-998(2001)
Kwiatkowska, K., Frey, J. and Sobota, A. (2003) Phosphorylation of FcRIIA is required for the receptorinduced actin rearrangement and capping: the role of membrane rafts J. Cell Sci., 116, 537-550
Richardson, D.D., Tol1, S., Valle-Encinas, E., Pleguezuelos, C., Bierings, R., Geerts, D. and Fernandez-Borja1, M. (2015) The prion protein inhibits monocytic cell migration by stimulating β1 integrin adhesion and uropod formation J. Cell Sci., 128, 3018-3029
Schaefer, M.B., Schaefer, C.A., Schifferings, S., Kuhlmann, C.R.W., Urban, A., Benscheid, U., Fischer, T., Hecker, M., Morty, R.E., Vadasz, I. et al (2016) N-3 vs. n-6 fatty acids differentially influence calcium signalling and adhesion of inflammatory activated monocytes: impact of lipid rafts Inflamm. Res., 65, 881–894
Schatzlmaier, P., Supper, V., Göschl, L., Zwirzitz, A., Eckerstorfer, P., Ellmeier, W., Huppa, J.B. and Stockinger, H. (2015) Rapid multiplex analysis of lipid raft components with single-cell resolution Sci Signal., 8 (395) rs11
Shakor, A.B.A., Kwiatkowska, K. and Sobota, A. (2004) Cell surface ceramide generation precedes and controls FcγRII clustering and phosphorylation in rafts J. Biol. Chem., 279, 36778-36787
Szymańska, E., Korzeniowski, M., Raynal, P., Sobota, A., et al (2009) Contribution of PIP-5 kinase Iα to raftbased FcγRIIA signaling Exp. Cell Res., 315, 981-995
1-31. Muscle cells (incl. myoblasts, myogenic and satellite cells, and myometrium)
Baron, C.B. and Coburn, R.F. (2004) Smooth muscle raft-like membranes J. Lipid Res.,45, 41-53
Berrou, E. and Bryckaert, M. (2009) Recruitment of protein phosphatase 2A to dorsal ruffles by plateletderived growth factor in smooth muscle cells: dephosphorylation of Hsp27 Exp. Cell Res., 315, 826-848
Buxton, I.L.O., Milton, D., Barnett, S.D. and Tichenor, S.D. (2010) Agonist-specific compartmentation of cGMP action in myometrium J. Pharmacol. Exp. Ther., 335, 256-263
Chu, C.L., Buczek-Thomas, J.A. and Nugent, M.A. (2004) Heparan sulphate proteoglycans modulate fibroblast growth factor-2 binding through a lipid-raft-mediated mechanism Biochem. J., 379, 331-341
Guzman, A., Zelman-Femiak, M., Boergermann, J.H., Paschkowsky, S., et al (2012) SMAD versus non-SMAD signaling is determined by lateral mobility of bone morphogenetic protein (BMP) receptors J. Biol. Chem., 287, 39492–39504
Hartung, A., Bitton-Worms, K., Rechtman. M.M., Wenzel, V., et al (2006) Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling Mol. Cell. Biol., 26, 7791-7805
Jia, S.J., Jin, S., Zhang, F., Yi, F., et al (2008) Formation and function of ceramide-enriched membrane platforms with CD38 during M1-receptor stimulation in bovine coronary arterial myocytes Am. J. Physiol. Heart Circ. Physiol., 295, H1743-H1752
Long, J., Tokhunts, R., Old, W.M., Houel, S., Rodgriguez-Blanco, J., Singh, S., Schilling, N., Capobianco, A.J., Ahn, N.G. and Robbins, D.J. (2015) Identification of a family of fatty-acid-speciated sonic hedgehog proteins, whose members display differential biological properties Cell Rep., 10, 1280–1287
Mukai, A., Kurisaki, T., Sato, S.B., Kobayashi, T., et al (2009) Dynamic clustering and dispersion of lipid rafts contribute to fusion competence of myogenic cells Exp. Cell Res., 315, 3052-3063
Mukai, A.M. and Hashimoto, N. (2013) Regulation of pre-fusion events: recruitment of M-cadherin to microrafts organized at fusion-competent sites of myogenic cells BMC Cell Biology 14: 37
Sammar, M., Sieber, C. and Knaus, P. (2009) Biochemical and functional characterization of the Ror2/BRIb receptor complex Biochem. Biophys. Res, Commun., 381, 1–6
1-32. Neural tissue, neural cells, neuroblastoma and related cells
Brain tissue
Amyloid -β(AB) peptide
Nagarathinam, A., Hölinger, P., Bühler, A., Schäfer, C., et al (2013) Membrane-anchored Aβ accelerates amyloid formation and exacerbates amyloid-associated toxicity in mice J. Neurosci., 33, 19284 –19294
Scheider, A., Schulz-Schaeffer, W., Hartmann, T., Schulz, J.B., et al (2006) Cholesterol depletion reduces aggregation of amyloid-beta peptide in hippocampal neurons Neurobiol. Dis., 23, 573-577
Cocaine effects
Huang, C-C., Liang, Y-C., Lee, C-C., Wu, M-Y. et al (2009) Repeated cocaine administration decreases 5- HT2A receptor-mediated serotonergic enhancement of synaptic activity in rat medial prefrontal cortex Neuropsychopharmacology, 34, 1979–1992
Ephrin B
Bruckner, K. Labrador, J.P., Schieffele, P., Herb, A., et al (1999) EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains Neuron, 22, 511-524
Multiple sclerosis
Maier, O., Baron, W. and Hoekstra, D. (2007) Reduced raft-association of NF155 in active MS-lesions is accompanied by the disruption of the paranodal junction Glia, 55, 885-895
Neurotrophic factor
Kao, H-T., Ryoo, K., Lin, A., Janoschka, S.R., Augustine, G.J. and Porton, B. (2017) Synapsins regulate brainderived neurotrophic factor-mediated synaptic potentiation and axon elongation by acting on membrane rafts Eur. J. Neurosci., 45, 1085–1101
Neuregulins
Frenzel, K.E. and Falls, D.L. (2001) Neuregulin-1 proteins in rat brain and transfected cells are localized to lipid rafts J. Neurochem., 77, 1-12
Prion proteins
Abid, K., Morales, R. and Soto, C. (2010) Cellular factors implicated in prion replication FEBS Lett., 584, 2409–2414
Baron, G.S., Hughson, A.G., Raymond, G.J., Offerdahl, D.K., et al (2011) Effect of glycans and the glycophosphatidylinositol anchor on strain dependent conformations of scrapie prion protein: improved purifications and infrared spectra Biochemistry, 50, 4479–4490
Baumann, F., Tolnay, M., Brabeck, C., Pahnke, J., et al (2007) Lethal recessive myelin toxicity of prion protein lacking its central domain EMBO J., 26, 538-547
Baumann, F., Pahnke, J., Radovanovic, I., Rülicke, T., et al (2009) Functionally relevant domains of the prion protein identified in vivo Plos One 4: e6707
Kobayashi, A., Qi, Z., Shimazaki, T., Munesue, Y., Miyamoto, T., Isoda, N., Sawa, H., Aoshima, K., Kimura, T. et al (2019) Ganglioside synthase knockout reduces prion disease incubation time in mouse models Am. J. Pathol., 189, 677-686
Castilla, J., Saa, P., Morales, R., Abid, K., et al (2006) Protein misfolding cyclic amplification for diagnosis and prion propagation studies Methods Enzymol., 412, 3-21
Lemaire-Vieille, C., Bailly, Y., Erlich, P., Loeuillet, C., et al (2013) Ataxia with cerebellar lesions in mice expressing chimeric PrP-Dpl protein J. Neurosci., 33, 1391–1399
Radovanovic, I., Braun, N., Giger, O.T., Mertz, K., et al (2005) Truncated prion protein and doppel are myelinotoxic in the absence of oligodendrocytic PrPC J. Neurosci., 25, 4879-4888
Rutishauser, D., Mertz, K.D., Moos, R., Brunner, E., et al (2009) The comprehensive native interactome of a fully functional tagged prion protein PLoS One, 4:e4446
Sigurdson, C.J., Nilsson, K.P.R., Hornemann, S., Heikenwalder, M., et al (2009) De novo generation of a transmissible spongiform encephalopathy by mouse transgenesis Proc. Natl. Acad. Sci. USA, 106, 304-309
Recptor proteins and signalling
Amritraj, A., Posse de Chaves, E.I., Hawkes, C., MacDonald, R.G., et al (2012) Single-trans-membrane domain IGF-II/M6P receptor: potential interaction with G protein and its association with cholesterol-rich membrane domains Endocrinology, 153, 4784–4798
Huo, J., Cortez, M.A. and Snead III, O.C. (2009) GABA receptor proteins within lipid rafts in the AY-9944 model of atypical absence seizures Epilepsia, 50, 776-788
Ma, L., Huang, Y.Z., Pitcher, G.M., Valtschanoff, J., et al (2003) Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts J. Neurosci., 23, 3164-3175
Scorticati, C., Formoso, K. and Frasch, A.C. (2011) Neuronal glycoprotein M6a induces filopodia formation via association with cholesterol-rich lipid ratfs J. Neurochem., 119, 521–531
Yu, W., Guo, W. and Feng, L. (2004) Segregation of Nogo66 receptors into lipid rafts in rat brain and inhibition of Nogo66 signaling by chosesterol depletion FEBS Lett., 577, 87-92
Sphingomyelinase
Stoffel, W., Jenke, B., Blöck, B., Zumbansen, M., et al (2005) Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development Proc. Natl. Acad. Sci. USA, 102, 4554-4559
Synapsins
Kao, H-T., Ryoo, K., Lin, A., Janoschka, S.R., Augustine, G.J. and Porton, B. (2017) Synapsins regulate brainderived neurotrophic factor-mediated synaptic potentiation and axon elongation by acting on membrane rafts Eur. J. Neurosci., 45, 1085–1101
Ciliary ganglia neurons
Bruses, J.L., Chauvet, N. and Rutishauser, U. (2001) Membrane lipid rafts are necessary for the maintenance of the α7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons J. Neurosci., 21, 504-512
Cortical neurons
Arevalo, J., Pereira, D.B., Yano, H., Teng, K.K., et al (2006) Identification of a switch in neurotrophin signaling by selective tyrosine phosphorylation J. Biol. Chem., 281, 1001-1007
Assaife-Lopes, N., Sousa, V.C., Pereira, D.B., Ribeiro, J.A., et al (2010) Activation of adenosine A2A receptors induces TrkB translocation and increases BDNF-mediated phospho-TrkB localization in lipid rafts: implications for neuromodulation J. Neurosci., 30, 8468–8480
Assaife-Lopes, N., Sousa, V., Pereira, D.B., Ribeiro, J.A., et al (2014) Regulation of TrkB receptor translocation to lipid rafts by adenosine A2A receptors and its functional implications for BDNF-induced regulation of synaptic plasticity Purinergic Signal., 10, 251–267
Ma, L., Huang, Y.Z., Pitcher, G.M., Valtschanoff, J., et al (2003) Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts J. Neurosci., 23, 3164-3175
Palmer, A., Zimmer, M., Erdmann, K.S., Eulenburg, V., et al (2002) EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase Mol. Cell, 9, 725-737
Dorsal root ganglia neurons
Pristerá, A. and Okuse, K. (2012) Building excitable membranes: lipid rafts and multiple controls on trafficking of electrogenic molecules Neuroscientist, 18, 70–81
Pristerà, A., Baker, M.D. and Okuse, K. (2012) Association between tetrodotoxin resistant channels and lipid rafts regulates sensory neuron excitability PloS One, 7: e40079
Glial cells
Querbes, W., O’Hara, B.A., Williams, G. and Atwood, W.J. (2006) Invasion of host cells by JC virus identifies a novel role for caveolae in endosomal sorting of noncaveolar ligands J. Virol., 80, 9402-9413
Weerth, S.H., Holtzclaw, L.A. and Russell, J.T. (2007) Signaling proteins in raft-like microdomains are essential for Ca2+ wave propagation in glial cells Cell Calcium, 41, 155-167
Glioma/glioblastoma cells
Bomben, V.C., Turner, K.L., Barclay, T-T.C. and Sontheimer, H. (2011) Transient receptor potential canonical channels are essential for chemotactic migration of human malignant gliomas J. Cell. Physiol., 226, 1879–1888
Chetty, C., Lakka, S.S., Bhoopathi, P., Gondi, C.S., et al (2010) Urokinase plasminogen activator receptor and/or matrix metalloproteinase-9 inhibition induces apoptosis signaling through lipid rafts in glioblastoma xenograft cells Mol Cancer Ther., 9, 2605–17
Ghildiya, R., Dixit, D. and Sen, E. (2013) EGFR inhibitor BIBU induces apoptosis and defective autophagy in glioma cells Mol. Carcinog., 52, 970–982
McFerrin, M.B. and Sontheimer, H. (2006) A role for ion channels in glioma cell invasion Neuron Glia Biol., 2, 39–49
Strale, P-O., Clarhaut, J., Lamiche, C., Cronier, L., et al (2012) Down-regulation of connexin43 expression reveals the involvement of caveolin-1 containing lipid rafts in human U251 glioblastoma cell invasion Mol. Carcinog., 51, 845–860
Weaver, A.K., Olsen, M.L., McFerrin, M.B. and Sontheimer, H. (2007) BK channels are linked to inositol 1,4,5-triphosphate receptors via lipid rafts J. Biol. Chem., 282, 31558-31568
Wu, H., Jiang, H., Lu, D., Xiong, Y., et al (2009) Effect of Simvastatin on glioma cell proliferation, migration, and apoptosis Neurosurgery, 65, 1087-1097
Hippocampal neurons
Ledesma, M.D., Da Silva, J.S., Crassaerts, K., Delacourte, A., et al (2000) Brain plasmin enhances APP α- cleavage and Aβ degradation and is reduced in Alzheimer’s disease brains EMBO Rep., 11, 530-535
Ledesma, M.D., Abad-Rodruigez, J., Galvan, C., Biondi, E., et al (2003) Raft disorganization leads to reduced plasmin activity in Alzheimer’s disease brains EMBO Rep., 4, 1190-1196
Ledesma, M.D., Da Silva, J.S., Schevchenko, A., Wilm, M., et al (2003) Proteomic characterisation of neuronal sphingolipid-cholesterol microdomains: role in plasminogen activation Brain Res., 987, 107-116
Simons, M., Keller, P., De Strooper, B., Beyreuther, K., Dotti, C.G. and Simons, K. (1998) Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons Proc. Natl. Acad. Sci. USA, 95, 6460-6464
Xu, H., Bae, M., Tovar-y-Romo, L.B., Patel, N., et al (2011) The human immunodeficiency virus coat protein gp120 promotes forward trafficking and surface clustering of NMDA receptors in membrane microdomains J. Neurosci., 31, 17074 –17090
Hypothalamic neurons
Toni, M., Spisni, E., Griffoni, C., Santi, S., et al (2006) Cellular prion protein and caveolin-1 interaction in a neuronal cell line precedes Fyn/Erk 1/2 signal transduction J. Biomed. Biotech., 2006: 69469
Motor neurons
Muraro, L., Tosatto, S., Motterlini, L., Rossetto, O., et al (2009) The N-terminal half of the receptor domain of botulinum neurotoxin A binds to microdomains of the plasma membrane Biochem. Biophys. Res. Commun., 380, 76–80
Myelin
Karim, S.A., Barrie, J.A., McCulloch, M.C., Montague, P., et al (2010) PLP/DM20 expression and turnover in a transgenic mouse model of Pelizaeus-Merzbacher disease Glia, 58, 1727–1738
Meixner, M., Jungnickel, J., Grothe, C., Gieselmann, V., et al (2011) Myelination in the absentce of UDPgalactose: ceramide galactosyl-transferase and fatty acid 2-hydroxylase BMC Neuroscience, 12: 22
Saher, G., Quintes, S., Möbius, W., Wehr, M.C., et al (2009) Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction J. Neurosci., 29, 6094–6104
Zöller, I., Meixner, M., Hartmann, D., Büssow, H., et al (2008) Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration J. Neurosci., 28, 9741-9754
Neural stem cells (Neurospheres)
Campos, L., Decker, L., Taylor, V. and Skarnes, W. (2006) Notch, epidermal growth factor receptor, and β1-integrin pathways are coordinated in neural stem cells J. Biol. Chem., 281, 5300-5309
Das, S., Chakraborty, S. and Basu, A. (2010) Critical role of lipid rafts in virus entry and active ation of phosphoinositide 3’ kinase/Akt signaling during early stages of Japanese encephalitis virus infection in neural stem/progenitor cells J. Neurochem., 115, 537–549
Neuroblastoma cells
Badawy, S.M.M., Okada, T., Kajimoto, T., Hirase, M., Matovelo, S.A., Nakamura, S., Yoshida, D., Ijuin, T. and Nakamura, S-i., (2018) Extracellular α-synuclein drives sphingosine 1-phosphate receptor subtype 1 out of lipid rafts, leading to impaired inhibitory G-protein signalling J. Biol. Chem., 293, 8208–8216
Baron, G.S., Wehrly, K., Dorward, D.W., Chesebro, B., et al (2002) Conversion of raft associated prion protein to the protease-resistant state requires insertion of PrP-res (PrPSc) into contiguous membranes EMBO J., 21, 1031-1040
Cabrera, J.R., Sanchez-Pulido, L., Rojas, A.M., Valencia, A., et al (2006) Gas1 is related to the glial cellderived neurotrophic factor family receptors α and regulates ret signaling J. Biol. Chem., 281, 14330-14339
Castilla, J., Saa, P., Morales, R., Abid, K., Maundrelli, K. and Soto, C. (2006) Protein misfolding cyclic amplification for diagnosis and prion propagation studies Methods Enzymol., 412, 3-21
Chung, J., Phukan, G., Vergote, D., Mohamed, A., Maulik, M., Stahn, M., Andrew, R.J., Thinakaran, G., Posse de Chaves, E. and Kara, S. (2018) Endosomal-lysosomal cholesterol sequestration by U18666A differentially regulates amyloid precursor protein (APP) metabolism in normal and APP-overexpressing cells Mol. Cell. Biol., 38: e00529-17
Ehehalt, R.E., Keller, P., Haass, C., Thiele, C., et al (2003) Amyloidgenic processing of the Alzheimer β-amyloid precursor protein depends on lipid rafts J. Cell Biol., 160, 113-123
Ehehalt, R., Sparla, R., Kulaksiz, H., Herrmann, T., et al (2008) Uptake of long chain fatty acids is regulated by dynamic interaction of FAT/CD36 with cholesterol/sphingolipid enriched microdomains (lipid rafts) BMC Cell Biol., 9:45
Fernández, M., Segura, M.F., Solé, C., Comella, J.X. et al (2007) Lifeguard/neuronal membrane protein 35 regulates Fas ligand-mediated apoptosis in neurons via microdomain recruitment J. Neurochem., 103, 190-203
Hudry, E., Van Dam, D., Kulik, W., De Deyn, P.P., et al (2010) Adeno-associated virus gene therapy with cholesterol 24-hydroxylase reduces the amyloid pathology before or after the onset of amyloid plaques in mouse models of Alzheimer’s disease Mol. Ther., 18, 44–53
Li, L., Chen, H., Wang, M., Chen, F., Gao, J., Sun, S., Li, Y. and Gao, D. (2017) NCAM-140 translocation into lipid rafts mediates the neuroprotective effects of GDNF Mol. Neurobiol., 54, 2739–2751
Lucero, H., Gae, D. and Taccioli, G.E. (2003) Novel localization of the DNA-PK complex in lipid rafts: a putative role in the signal transduction pathway of the ionizing radiation response J. Biol. Chem., 278, 22136-22143
Lundgren, T.K., Luebke, M., Stenqvist, A. and Ernfors, P. (2008) Differential membrane compartmentalization of Ret by PTB-adaptor engagement FEBS. J., 275, 2055-2066
Massimino, M.L., Ballarin, C., Bertoli, A., Casonato, S., et al (2004) Human Doppel and prion protein share common membrane microdomains and internalization pathways Int. J. Biochem. Cell Biol., 36, 2016-2031
Nakagawa, T., Morotomi, A., Tani, M., Sueyoshi, N., et al (2005) C18:3-GM1a induces apoptosis in Neuro2 cells: enzymatic remodeling of fatty acyl chains of glycosphingolipids J. Lipid Res., 46, 1103-1112
Port, M.D., Gibson, R.M. and Nathanson, N.M. (2007) Differential stimulation-induced receptor localization in lipid rafts for interleukin-6 family cytokines signaling through the gp130/leukemia inhibitory factor receptor complex J. Neurochem., 101, 782-793
Tansey, M.G., Baloh, R.H., Milbrandt, J. and Johnson, Jr., E.M. (2000) GFRα –mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival Neuron, 25, 611-623
Tewari, R., Sharma, V., Koul, N. and Sen, E. (2008) Involvement of miltefosine-mediated ERK activation in glioma cell apoptosis through Fas regulation J. Neurochem., 107, 616-627
Yang, J., Lindahl, M., Lindholm, P., Virtanen, H., et al (2004) PSPN/GFRα4 has a significantly weaker capacity than GDNF/GFR1 to recruit RET to rafts, but promotes neuronal survival and neurite outgrowth FEBS Lett., 569, 267-271
Yamagushi, H., Shiraishi, M., Fukami, K., Tanabe, A., et al (2009) MARCKS regulates lamellipodia formation induced by IGF-I via association with PIP2 and β-actin at membrane microdomains J. Cell. Physiol., 220, 748-755
Olfactory neurons
Kobayakawa, K., Hayashi, R., Morita, K., Miyamichi, K., et al (2002) Stomatin-related olfactory protein, SRO, spedifically expressed in the murine olfactory sensory neurons J. Neurosci., 22, 5931-5937
Oligodendrocytes
Baron, W., Decker, L., Colognato, H. and ffrench-Constant, C. (2003) Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains Curr. Biol., 13, 151-155
Baron, W., Bijlard, M., Nomden, A., de Jonge, J.C., et al (2014) Sulfatide-mediated control of extracellular matrix-dependent oligodendrocyte maturation Glia, 62, 927–942
Baron, W., Ozgen, H., Klunder, B., de Jonge, J.C., Nomden, A., Plat, A., Trifilieff, E., de Vries, H. and Hoekstra, D. (2015) The major myelin-resident protein PLP is transported to myelin membranes via a transcytotic mechanism: involvement of sulfatide Mol. Cell. Biol., 35, 288-302
Bijlard, M., de Jonge, J.C., Klunder, B., Nomden, A., Hoekstra, D. and Baron, W. (2016) MAL is a regulator of the recruitment of myelin protein PLP to membrane microdomains PLoS One, 11: e0155317
Decker, L. and ffrench-Constant, C. (2004) Lipid rafts and integrin activation regulate oligodendrocyte survival J. Neurosci., 24, 3816-3825
Fitzner, D., Schneider, A., Kippert, A., Möbius, W., et al (2006) Myelin basic protein-dependent plasma membrane reorganization in the formation of myelin EMBO J., 25, 5037-5048
Krämer-Alber, E-M., Gehrig-Burger, K., Thiele, C., Trotter, J., et al (2006) Perturbed interactions of mutant proteolipid protein/DM20 with cholesterol and lipid rafts in oligodendroglia: implications for dysmyelination in spastic paraplegia J. Neurosci., 26, 11743-11752
Maier, O., van der Heide, T., van Dam, A-M., Baron, W., et al (2005) Alteration of the extracellular matrix interferes with raft association of neurofascin in oligodendrocytes. Potential significance for multiple sclerosis Mol. Cell. Neurosci., 28, 390-401
Ozgen, H., Schrimpf, W., Hendrix, J., de Jonge, J.C., et al (2014) The lateral membrane organization and dynamics of myelin proteins PLP and MBP are dictated by distinct galactolipids and the extracellular matrix PLoS One, 9: e101834
Simons, M., Keller, P., De Strooper, B., Beyreuther, K., et al (1998) Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons Proc. Natl. Acad. Sci. USA, 95, 6460-6464
Simons, M., Kramer, E-M., Thiele, C., Stoffel, W., et al (2000) Assembly of myelin by association of proteolipid protein with cholesterol and galactosylceramide-rich membrane domains J. Cell Biol., 151, 143-153
Simons, M., Kramer, E-M., Macchi, P., Rathke-Hartlieb, S., et al (2002) Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: implications for Pelizaeus-Merzbacher disease J. Cell Biol., 157, 327-336
PC12 cells (see “1-34 Pheochromocytoma cells”)
Spinal cord neurons (spinal cord ganglia)
Maier, O., van der Heide, T., van Dam, A-M., Baron, W., et al (2005) Alteration of the extracellular matrix interferes with raft association of neurofascin in oligodendrocytes. Potential significance for multiple sclerosis Mol. Cell. Neurosci., 28, 390-401
Pierchala, B.A., Milbrandt, J. and Johnson Jr., E.M. (2006) Glial cell line-derived neurotrophic factordependent recruitment of ret into lipid rafts enhances signaling by partitioning ret from proteasome-dependent degradation J. Neurosci., 26, 2777-2787
Roth, A.L. and Berg, D.K. (2003) Large clusters of α7-containing nicotinic acetylcholine receptors on chick spinal cord neurons J. Comp. Neurol., 465, 195-204
Striatum
Vanderwerf, S.M., Buck, D.C., Wilmarth, P.A., Sears, L.M., David, L.L., Morton, D.B. and Neve, K.A. (2015) Role for Rab10 in methamphetamine-induced behavior PloS One, 10: e0136167
1-33. Neutrophils
Fernandes, M.J.G., Rollet-Labelle, E., Pare, G., Marios, S., et al (2006) CD16b associates with high-density, detergent-resistant membranes in human neutrophils Biochem. J., 393, 351-359
Fuhler, G.M., Blom, N.R., Coffer, P.J., Drayer, A.L. et al (2007) The reduced GM-CSF priming of ROS production in granulocytes from patients with myelodysplasia is associated with an impaired lipid raft formation J. Leuk. Biol., 81, 449-457
Lafont, F. and Simons, K. (2001) Raft-partitioning of the ubiquitin ligases CbI and Nedd4 upon IgE-triggered cell signaling Proc. Natl. Acad. Sci., USA., 98; 3180-3184
Marois, L., Vaillancourt, M., Marois, S., Proulx, S., et al (2009) The ubiquitin ligase c-Cbl down-regulates FcRIIa activation in human neutrophils J. Immunol., 182, 2374 –2384
Marois, L., Paré, G., Vaillancourt, M., Rollet-Labelle, E., et al (2011) FcγRIIIb triggers raft-dependent calcium influx in IgG-mediated responses in human neutrophils J. Biol. Chem., 286, 3509–3519
Marois, L., Vaillancourt, M., Paré, G., Gagné, V., et al (2011) CIN85 modulates the down-regulation of FcγRIIa expression and function by c-Cbl in a PKC-dependent manner in human neutrophils J. Biol. Chem., 286, 15073–15084
Miner, J.J., Xia, L., Yago, T., Kappelmayer, J., et al (2008) Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow Blood, 112, 2035-2045
Nebl, T., Pestonjamasp, K.N., Leszyk, J.D., Crowley, J.L., et al (2002) Proteomic analysis of a detergentresistant membrane skeleton from neutrophil plasma membranes J. Biol. Chem., 277, 43999-43409
Nuzzi, P.A., Senetar, M.A. and Huttenlocher, A. (2007) Asymmetric localization of calpain 2 during neutrophil chemotaxis Mol. Biol. Cell, 18, 795-805
Pereira, D.B. and Chao, M.V. (2007) The tyrosine kinase Fyn determines the localization of TrkB receptors in lipid rafts J. Neurosci., 27, 4859-4869
Rollet-Labelle, E., Marois, S., Barbeau, K., Malawista, S.E. et al (2004) Recruitment of the cross-linked opsonic receptor CD32A (FcRIIA) to high density detergent-resistant membrane domains in human neutrophils Biochem. J., 381, 919-928
Shao, B., Yago, T., Setiadi, H., Wang, Y., Mehta-D’souza, P., Fu, J., Crocker, P.R., Rodgers, W., Xia, L. and McEver, R.P.l (2015) O-glycans direct selectin ligands to lipid rafts on leukocytes Proc. Natl. Acad. Sci. USA, 112, 8661–8666
1-34. Osteosarcoma cells
Rao-Bindal, K., Zhou, Z. and Kleinerman, E.S. (2012) MS-275 sensitizes osteosarcoma cells to Fas ligand induced cell death by increasing the localization of Fas in membrane lipid rafts Cell Death Dis., 3: e369
1-35. Pancreatic cells
β-cells
Somanath, S., Barg, S., Marshall, C., Silwood, C.J., et al (2009) High extracellular glucose inhibits exocytosis through disruption of syntaxin 1A-containing lipid rafts Biochem. Biophys. Res. Commun., 389, 241–246
Cancer cells
Gupta, V.K. and Banerjee, S. (2017) Isolation of lipid raft proteins from CD133+ cancer stem cells In Lipidomics: Methods and Protocols: Methods Mol. Biol., 1609 (ed. Bhattacharya, S.K.) Springer Science+Business Media, LLC, pp 25-31
1-36. Pheochromocytoma cells
Kratchmarov, R., Taylor, M.O. and Enquist, L.W. (2013) Role of Us9 phosphorylation in axonal sorting and anterograde transport of pseudorabies virus PloS One, 8: e58776
Lyman, M.G., Curanovic, D. and Enquist, L.W. (2008) Targeting of pseudorabies virus structural proteins to axons requires association of the viral Us9 protein with lipid rafts PLoS Pathog., 4:e1000065
Lyman, M.G:, Curanovic, D., Brideau, A.D. and Enquist, L.W. (2008) Fusion of enhanced green fluorescent protein to the Pseudorabies virus axonal sorting protein Us9 blocks anterograde spread of infection in mammalian neurons J. Virol., 82, 10308-10311
Pryor, S., McCaffrey, G., Young. L.R. and Grimes, M.L. (2012) NGF causes TrkA to specifically attract microtubules to lipid rafts PLoS One 7: e35163
1-37 Placenta
Szilagyi, J.T., Vetrano, A.M., Laskin, J.D. and Aleksunes, L.M. (2017) Localization of the placental BCRP/ABCG2 transporter to lipid rafts: Role for cholesterol in mediating efflux activity Placenta 55, 29-36
1-38. Platelets
Liu, L., Zhang, K., Tan, L., Chen, Y-H. and Cao, Y-P., (2015) Alterations in cholesterol and ganglioside GM1 content of lipid rafts in platelets from patients with Alzheimer disease Alzheimer. Dis. Assoc. Disord., 29, 63–69
1-39 Pulmonary mesenchymal cells
McGowan, S.E. and McCoy, D.M. (2018) Neuropilin-1 and platelet-derived growth factor receptors cooperatively regulate intermediate filaments and mesenchymal cell migration during alveolar septation Am. J. Physiol. Lung Cell. Mol. Physiol., 315, L102–L115
1-40. Retinal cells (incl. trabecular meshwork cells and other optical structures)
Beverley, R.M., Nolan, M.J., McCarty, R.D., Yue, B.Y.J.T., et al (2009) Omega-3 protects trabecular meshwork from metabolic stress Invest. Ophthalmol. Vis. Sci., 50, E-Abstract 4866
Bhat, S.P., Gangalum, R.K. and Jing, Z. (2009) The small heat shock protein αB-crystallin is secreted from retinal pigment epithelial cells ARPE in culture Invest. Ophthalmol. Vis. Sci., 50, E-Abstract 4183
Gangalum, R.K., Atanasov, I.C., Zhou, Z.H. and Bhat, S.P. (2011) B-Crystallin is found in detergentresistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells J. Biol. Chem., 286, 3261–3269
Giovingo, M.C., McCarty, R.D., Beverley, R.M., Nolan, M.J., et al (2010) Soluble CD44 increases outflow resistance in porcine organ cultures Invest. Ophthalmol. Vis. Sci., 51, E-Abst. 5838
Giovingo, M., Nolan, M., McCarty, R., Pang, I-H., et al (2013) sCD44 overexpression increases intraocular pressure and aqueous outflow resistance Mol. Vis., 19, 2151-2164
Härtinger, T., Walczak, Y., Weber, B.H.F. and Langmann, T. (2011) Partial colocalization of retinoschisin (RS1) and the Na+/K+-ATPase subunit in membrane rafts and their role in signaling Medizinische Genetik, 23, 156
Hsu, K-S., Otsu, W., Li, Y., Wang, H-C., Chen, S., Tsang, S.H., Chuang, J-Z. and Sung, C-H. (2019) CLIC4 late endosomal trafficking and matrix degradation activity of MMP14 at focal adhesions in RPE cells Sci. Rep., 9: 12247
Knepper, P.A., Beverley, R.M., McCarty, R.D., Nolan, M.J., et al (2009) Wnt signaling and trabecular meshwork lipid rafts Invest. Ophthalmol. Vis. Sci., 50, E-Abstract 4875
LaVail, J.H., Huang, G., Cortez, D.A., Sucher, A., et al (2010) Differential targeting of HSV structural proteins to axons requires association of viral Us9 protein to lipid rafts Invest. Ophthalmol. Vis. Sci., 51, E-Abstr. 3812
Lyman, M.G., Curanovic, D., Brideau, A.D. and Enquist, L.W. (2008) Fusion of enhanced green fluorescent protein to the Pseudorabies virus axonal sorting protein Us9 blocks anterograde spread of infection in mammalian neurons J. Virol., 82, 10308-10311
McCarty, R.D., Nolan, M.J., Beverley, R.M., Quinn, K., et al (2009) Gamma secretase and trabecular meshwork stress Invest. Ophthalmol. Vis. Sci., 50, E-Abstract 4870
McCarty, R.D., Beverley, R.M., Giovingo, M.C., Nolan, M.J., et al (2010) Differential pattern of junctional complex lipid raft proteins in human conventional outflow Invest. Ophthalmol. Vis. Sci., 51: E-Abst. 3204
Nolan, M.J., Koga, T., Yue, B.Y.J.T., Giovingo, M.C., et al (2008) Caveolin-1 expression intrabecular meshwork cells Invest. Ophthalmol. Vis. Sci., 49, E-Abstr. 1627
Ramseger, R., White, R. and Kröger, S. (2009) Trans-membrane form agrin-induced process formation requires lipid rafts and the activation of Fyn and MAPK J. Biol. Chem., 284, 7697–7705
Sergeeva, A. and van der Goot, F.G. (2019) Anthrax toxin requires ZDHHC5-mediated palmitoylation of its surface-processing host enzymes Proc. Natl. Acad. Sci. USA, 116, 1279-1288
Vijayasarathy, C., Takada, Y., Zeng, Y., Bush, R.A. et al (2007) Retinoschisin is a peripheral membrane protein with affinity for anionic phospholipids and affected by divalent cations Invest. Ophthalmol. Vis. Sci., 48, 991-1000
1-41. Secretory cells
Briand, O., Lestavel, S., Pilon, A., Torpier, G., et al (2003) SR-BI does not require raft/caveola localization for cholesteryl ester selective uptake in the human adrenal cell line NCI-H295R Biochim. Biophys. Acta, 163, 42-50
Chasserto-Golaz, S., Vitale, N., Umbrecht-Jenck, E., Knight, D., et al (2005) Annexin 2 promotes the formation of lipid microdomains required for calcium-regulated exocytosis of dense-core vesicles Mol. Biol. Cell, 16, 1108-1119
Costa, M.J., Song, Y., Macours, P., Massaart, C., et al (2004) Sphingolipid-cholesterol domains (lipid rafts) in normal human and dog thyroid follicular cells are not involved in thyrotropin receptor signaling Endocrinology, 145, 1464-1472
Ishikawa, Y., Yuan, Z., Inoue, N., Skowronski, M.T., et al (2005) Identification of AQP5 in lipid rafts and its translocation to apical membranes by activation of M3 mAChRs in interlobular ducts of rat parotid gland Am. J. Physiol Cell Physiol., 289, C1303-C1311
Lang, T., Bruns, D., Wenzel, D., Riedel, D., et al (2001) SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis The EMBO J., 20, 2202-2213
Leung, L.W., Contreras, R.G., Flores-Maldomado, C., Cereijido, M. et al (2003) Inhibitors of glycosphingolipid biosynthesis reduce transepithelial electrical resistance in MDCK I and FRT cells Am. J. Physiol. Cell Physiol., 284, C1021-C1030
Lockwich, T.P., Liu, X., Singh, B.B., Jadlowiec, J., et al (2000) Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains J. Biol. Chem., 275, 11934-11942
Ohara-Imaizumi, M., Nishiwaki, C., Kikuta, T., Konosuke, K., et al (2004) Site of docking and fusion of insulin secretory granules in live MIN6 cells analyzed by TAT-conjugated anti-syntaxin 1 antibody and total internal reflection fluorescence microscopy J. Biol. Chem., 279, 8403-8408
1-42. Sperm cells
Cross, N.L. (2004) Reorganization of lipid rafts during capacitation of human sperm Biol. Reprod., 71, 1367-1373
Nishimura, H., Myles, D.G. and Primakoff, P. (2007) Identification of an ADAM2-ADAM3 complex on the surface of mouse testicular germ cells and cauda epididymal sperm J. Biol. Chem., 282, 17900-17907
1-43. Splenocytes
Michel, B., Ferguson, A., Johnson, T., Bender, H., et al (2012) Genetic depletion of complement receptors CD21/35 prevents terminal prion disease in a mouse model of chronic wasting disease J. Immunol., 189, 4520–4527
Vascular endothelial cells (see “1-13 Endothelial cells”)
1-44. Vero cells
Agnihothram, S.S., Dancho, B., Grant, K.W., Grimes, M.L., et al (2009) Assembly of arenavirus envelope glycoprotein GPC in detergent-soluble membrane microdomains J. Virol., 83, 9890-9900
Glende, J., Schwegmann-Wessels, C., Al-Falah, M., Pferfferle, S., et al (2008) Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2 Virology, 381, 215-221
Kang, M-H., Roy, B.B., Finnen, R., Le Sage, V., et al (2013) The Us2 gene product of herpes simplex virus 2 is a membrane-associated ubiquitin-interacting protein J. Virol., 87, 9590–9603
1-45. Virus processing/interactions
Chao, T-C., Su, W-C., Huang, J-Y., Chen, Y-C., Jeng, K-S., Wang, H-D. and Laia, M.M.C. (2012) Prolineserine-threonine phosphatase-interacting protein 2 (PSTPIP2), a host membrane-deforming protein, is critical for membranous web formation in hepatitis C virus replication J. Virol., 86, 1739-1749
Martín, J.J., Holguera, J., Sánchez-Felipe, L., Villar, E. and Muñoz-Barroso, I. (2012) Cholesterol dependence of Newcastle disease virus entry Biochim. Biophys. Acta, 1818, 753–761
Sharon-Friling, R. and Shenk, T. (2014) Human cytomegalovirus pUL37x1-induced calcium flux activates PKCα, inducing altered cell shape and accumulation of cytoplasmic vesicles Proc. Natl. Acad. Sci. USA, 111, E1140–E1148
2. Mammalian subcellular organelles
2-1. Endocytic/lysosomal/phagocytic systems
Goyette, G., Dermine, J-F., Brunet, S., Boulais, J., et al (2004) Phagosome microdomains define foci of specialized functions in innate immunity ICI/FOCIS 2004 meeting Abstr. 2246
Goyette, G., Boulais, J., Carruthers, N.J., Landry, et al (2012) Proteomic characterization of phagosomal membrane microdomains during phagolysosome biogenesis and evolution Mol. Cell. Proteom., 11, 1365–1377
Houde, M., Bertholet, S., Gagnon, E., Brunet, S., et al (2003) Phagosomes are competent organelles for antigen cross-presentation Nature, 425, 402-406
Jutras, I., Laplante, A., Boulais, J., Brunet, S., et al (2005) -Secretase is a functional component of phagosomes J. Biol. Chem., 280, 36310-36317
Lafourcade, C., Sobo, K., Kieffer-Jaquinod, S., Garin, J., et al (2008) Regulation of the V-ATPase along the endocytic pathway occurs through reversible subunit association and membrane localization PloS One, 3:e2758
Siddiqi, S., Sheth, A., Patel, F., Barnes, M., et al (2013) Intestinal caveolin-1 is important for dietary fatty acid absorption Biochim. Biophys. Acta, 1831, 1311–1321
Sobo, K., Chevallier, J., Parton, R.G., Gruenberg, J., et al (2007) Diversity of raft-like domains in late endosomes PloS ONE, 4: e391
Sobo, K., Le Blanc, I., Luyet, P-P., Fivaz, M., et al (2007) Late endosomal cholesterol accumulation leads to impaired intra-endosomal trafficking PloS ONE, 9: e851
Taute, A., Watzig, K., Simons, B., Lohaus, C., et al (2002) Presence of detergent-resistant microdomains in lysosomal membranes Biochem. Biophys. Res. Commun., 298, 5-9
2-2. Golgi
Stoffel, W., Jenke, B., Blöck, B., Zumbansen, M., et al (2005) Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development Proc. Natl. Acad. Sci. USA, 102, 4554-4559
2-3. Intestinal brush border
Annaba, F., Sarwar, Z., Kumar, P., Saksena, S., et al (2008) Modulation of ileal bile acid transporter (ASBT) activity by depletion of plasma membrane cholesterol: association with lipid rafts Am J Physiol Gastrointest Liver Physiol 294, G489-G497 Li, X., Galli, T., Leu, S., Wade, J.B., et al (2001) Na+-H+ exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3 J. Physiol., 537, 537-552
Li, X. and Donowitz, M. (2008) Fractionation of subcellular membrane vesicles of epithelial and nonepithelial cells by OptiPrep™ density gradient ultracentrifugation In Methods Mol. Biol., 440, Exocytosis and Endocytosis (ed. Ivanov, A.I.) Humana Press, Totowa, NJ, pp 97-110
Luo, M., Liu, Y., Riederer, B., Patrucco, E., et al (2014) CGMP-dependent kinase 2, Na+/H+ regulatory factor 2, and Na+/ H+ exchanger isoform 3 dynamically assemble within lipid rafts in murine small intestinal brush border membrane Gastroenterology, 146, Suppl. 1, S651-S652
McConnell, R.E., Higginbotham, J.N., Shifrin, D.A., Tabb, D.L., et al (2009) The enterocyte microvillus is a vesicle-generating organelle J. Cell Biol., 185, 1285–1298
Sakseena, S., Tyagi, S., Goyal, S., Gill, R.K., et al (2010) Stimulation of apical Cl- /HCO3- (OH-) exchanger, SLC26A3 by neuropeptide Y is lipid raft dependent Am. J. Physiol. Gastrointest. Liver Physiol., 299, G1334–G1343, 2010
Siddiqi, S., Sheth, A., Patel, F., Barnes, M., et al (2013) Intestinal caveolin-1 is important for dietary fatty acid absorption Biochim. Biophys. Acta, 1831, 1311–1321
Sultan, A., Riederer, B., Xia, W., Lamprecht, G., et al (2012) The NHE3 interacting PDZ protein NHERF2 is strongly lipid raft-associated and determines the raft association of the apical Na+/H+ exchanger NHE3 in murine small intestinal brush border membrane Gastroenterology, 142 (Suppl. 1), S691-S692
Sultan, A., Luo, M., Yu, Q., Riederer, B., et al (2013) Differential association of the Na+/H+ exchanger regulatory factor (NHERF) family of adaptor proteins with the raft-and the non-raft brush border membrane fractions of NHE3 Cell. Physiol. Biochem., 32, 1386-1402
Tyska, M.J. and Mooseker, M.S. (2004) A role for myosin-1A in the localization of a brush border disaccharidase J. Cell Biol., 165, 395-405
Tyska, M.J., Mackey, A.T., Huang, J-D., Copeland, N.G., et al (2005) Myosin-1a is critical for normal brush border structure and composition Mol. Biol. Cell, 16, 2443-2457
Wani, N.A. and Kaur, J. (2011) Reduced levels of folate transporters (PCFT and RFC) in membrane lipid rafts result in colonic folate malabsorption in chronic alcoholism J. Cell. Physiol., 226, 579–587
Wani, N.A., Thakur, S., Najar, R.A., Nada, R., et al (2013) Mechanistic insights of intestinal absorption and renal conservation of folate in chronic alcoholism Alcohol, 47, 121-130
2-4. Kidney brush border
Blaine, J.T., Takahashi, H., Barry, N., Digman, M., et al (2006) The heterogeneity of NaPi protein dynamics and NaPi cotransport activity in renal brush border membranes FASEB J., 20, A59
Cheng, M., Ruan, Q., Barry, N., Breusegem, S., et al (2004) Membrane dynamics in giant unilaminar vesicles (GUVS) of raft and non-raft fractions of brush border membranes Biophys., Meeting 2004 Abstr.
Inoue, M., Digman, M.A., Cheng, M., Breusegem, S.Y., et al (2004) Partitioning of NAPi cotransporter in cholesterol-, sphingomyelin-, and glycophingolipid-enriched membrane domains modulates NAPi protein diffusion, clustering, and activity J. Biol. Chem., 279, 49160-49171
Lee, D.H., Riquier, A.D., Yang, L.E., Leong, P.K., et al (2009) Acute hypertension provokes acute trafficking of distal tubule Na-Cl cotransporter (NCC) to subapical cytoplasmic vesicles Am. J. Physiol. Renal Physiol., 296, F810–F818
Riquier, A.D.M., Lee, D.H. and McDonough, A.A. (2009) Renal NHE3 and NaPi2 partition into distinct membrane domains Am. J. Physiol. Cell. Physiol., 296, C900–C910
Wani, N.A., Thakur, S., Najar, R.A., Nada, R., et al (2013) Mechanistic insights of intestinal absorption and renal conservation of folate in chronic alcoholism Alcohol, 47, 121-130
2-5. Plasma membrane
Hiol, A., Davey, P.C., Osterhout, J.L., Waheed, A.A., et al (2003) Palmitoylation regulates regulators of Gprotein signaling (RGS) 16 function: I Mutation of amino terminalcysteine residues on RGS16 prevents its targeting to lipid rafts and palmitoylation of an internal cysteine residue J. Biol. Chem., 278, 19302-19308
Marois, L., Vaillancourt, M., Marois, S., Proulx, S., et al (2009) The ubiquitin ligase c-Cbl down-regulates FcRIIa activation in human neutrophils J. Immunol., 182, 2374 –2384
Marois, L., Paré, G., Vaillancourt, M., Rollet-Labelle, E., et al (2011) FcγRIIIb triggers raft-dependent calcium influx in IgG-mediated responses in human neutrophils J. Biol. Chem., 286, 3509–3519
Marois, L., Vaillancourt, M., Paré, G., Gagné, V., et al (2011) CIN85 modulates the down-regulation of FcγRIIa expression and function by c-Cbl in a PKC-dependent manner in human neutrophils J. Biol. Chem., 286, 15073–15084
Vainio, S., Bykov, I., Hermansson, M., Jokitalo, E., et al (2005) Defective insulin receptor activation and altered lipid rafts in Niemann-Pick type C diseases hepatocytes Biochem. J., 391, 465-472
Wani, N.A., Nada, R. and Kaur, J. (2011) Biochemical and molecular mechanisms of folate transport in rat pancreas; interference with ethanol ingestion PloS One 6: e28599
Wani, N.A., Nada, R., Khanduja, K.L. and Kaur, J. (2013) Decreased activity of folate transporters in lipid rafts resulted in reduced hepatic folate uptake in chronic alcoholism in rats Genes Nutr., 8, 209–219
2-6. Synapses/Synaptosomes
Brodde, A., Teigler, A., Brugger, B., Lehmann, W.D., et al (2012) Impaired neurotransmission in ether lipiddeficient nerve terminals Hum. Mol. Genet., 21, 2713–2724
Jia, J-y., Lamer, S., Schümann, M., Schmidt, M.R., et al (2006) Quantitative proteomics analysis of detergentresistant membranes from chemical synapses: Evidence for cholesterol as spatial organizer of synaptic vesicle cycling Mol. Cell. Proteom., 5, 2060-2071
3. Review articles
Aureli, M., Grassi, S., Sonnino, S. and Prinetti, A. (2016) Isolation and analysis of detergent-resistant membrane fractions In Lipid Signaling Protocols, Methods in Molecular Biology, 1376 (ed. Waugh, M.G.) Springer Science+Business Media, LLC, pp 107-131
Balbis, A. and Posner, B.I. (2010) Compartmentalization of EGFR in cellular membranes: role of membrane rafts J. Cell. Biochem., 109, 1103–1108
Bittmann, R. (2004) The 2003 ASBMB-Avanti Award in Lipids Address: applications of novel synthetic lipids to biological problems Chem. Lipids, 129, 111-131
Briggs, J.A., Wilk, T. and Fuller, S.D. (2003) Do lipid raft mediate virus assembly and pseudotyping? J. Gen. Virol., 84, 757-768
Brown, D.A. and London, E. (1998) Functions of lipid rafts in biological membranes Annu. Rev. Cell Dev. Biol., 14, 111-136
Callera, G.E., Bruder-Nascimento, T. and Touyz, R.M. (2017) Assessment of caveolae/lipid rafts in isolated cells In Hpertension Methods and Protocols: Methods Mol. Biol., 1527 (ed. Touyz, R.M. and Schiffrin, E.L.), Springer Science+Business Media, LLC, pp 251-269
Casem, M.L. (2016) Cytoskeleton and intracellular motility In “Case studies in cell biology” Elsevier Inc, pp 127-156
Chazal, N. and Gerlier, D. (2003) Virus entry, assembly, budding and membrane rafts (Review article) Microbiol. Mol. Biol. Rev., 67, 226-237
Dotti, C.G., Galvan, C. and Ledesma, M.D. (2004) Plasmin deficiency in Alzheimer’s disease brains: causal or casual? Neurodegener. Dis., 1, 205-212
Fölsch, H., Mattila, P.E. and Weisz, O.A. (2009) Taking the scenic route: biosynthetic traffic to the plasma membrane in polarized epithelial cells Traffic, 10, 972–981
Foster, L.J. and Chan, Q.W.T. (2007) Lipid raft proteomics: more than just detergent-ersistant membranes In Subcellular Proteomics (ed. Bertrand, E. and Faupel, M.) Springer Science + Business Media, Berlin, pp 35-47
Gajate, C. and Mollinedo, F. (2015) Lipid rafts and raft-mediated supramolecular entities in the regulation of CD95 death receptor apoptotic signaling Apoptosis, 20, 584–606
Gimpl, G. (2010) Cholesterol–protein interaction: methods and cholesterol reporter molecules In Subcellular Biochemistry vol. 51 Cholesterol Binding and Cholesterol Transport Proteins (ed. Harris, J.R.), Springer Science
Gimpl, G. and Gehrig-Burger, K. (2012) Specific and non specific regulation of GPCR function by cholesterol In Regulation of ion channels and receptors (Ed. Levitan, I. and Barrantes, F.J.) John Wiley & Sons, Inc., pp 205-230
Ishikawa, Y., Cho, G., Yuan, Z., Inoue, N. and Nakae, Y. (2006) Aquaporin-5 water channel in lipid rafts of rat parotid glands Biochim. Biophys. Acta, 1758, 1053-1060
Klappe, K., Hummel, I., Hoekstra, D. and Kok, J.W. (2009) Lipid dependence of ABC transporter localization and function Chem. Phys. Lipids, 161, 57–64
Landry, A. and Xavier, R. (2006) Isolation and analysis of lipid rafts in cell-cell interactions Methods Mol. Biol., 341, 251-282
Lasley, R.D. (2011) Adenosine receptors and membrane microdomains Biochim. Biophys. Acta, 1808, 1284–1289
Lingwood, D. and Simons, K. (2007) Detergent resistance as a tool in membrane research Nat. Protoc., 2, 2159-2165
Maguy, A., Hebert, T.E. and Nattel, S. (2006) Involvement of lipid rafts and caveolae in cardiac ion channel function Cardiovasc. Res., 69, 798-807
Minogue, S. and Waugh, M.G. (2012) Lipid rafts, microdomain heterogeneity and inter-organelle contacts: Impacts on membrane preparation for proteomic studies Biol. Cell, 104, 618–627
Nothdurfter, C., Rammes, G., Rein, T. and Rupprecht, R. (2007) Pitfalls in isolating lipid rafts Nat. Rev. Neurosci., 8, 567-568
Pike, L.J. (2003) Lipid rafts: bringing order to chaos J. Lipid Res., 44, 655-667
Resh, M.D. (2006) Use of analogs and inhibitors to study the functional significance of protein palmitoylation Methods, 40, 191-197
Stillwell, W. (2016) Membrane isolation methods In “An Introduction to Biological Membranes” Elsevier Inc, pp 247-271
Stillwell, W. (2016) Membrane reconstitution In “An Introduction to Biological Membranes” Elsevier Inc, pp 273-312
Villar, V.A.M., Cuevas, S., Zheng, X. and Jose, P.A. (2016) Localization and signaling of GPCRs in lipid rafts Meth. Cell Biol., 132, 3-23
Yi, F., Jin, S. and Li, P-L. (2009) Lipid raft-redox signaling platforms in plasma membrane In Methods Mol. Biol., 579, Lipidomics (ed. Armstrong, D) Humana Press, Totowa, NJ, pp 93-107
Zheng, Y.Z. and Foster, L.J. (2009) Contributions of quantitative proteomics to understanding membrane microdomains J. Lipid Res., 50, 1976–1985
OptiPrepTM Reference List RS06; 8th edition, January 2020
OptiPrep Reference List RS07
Detergent-free strategy for lipid raft isolation from mammalian cells, tissues and organelles
The aim of this OptiPrepTM Reference List is to present a bibliography of all of the current papers
reporting the use of an iodixanol gradient to purify and analyze lipid rafts from vertebrate cells/tissues
prepared in the absence of detergent (see Section 2). Section 1 contains a brief survey of the technique.
1. Background
Five years before McDonald and Pike [1] published their method for the isolation of lipid rafts in the
absence of detergent, Kasahara et al [2] used a broadly similar approach in which cerebellar granule cells were
disrupted in a Potter-Elvehjem homogenizer in buffered 0.25 M sucrose, 1 mM EDTA and then sonicated before
adjusting to 25% iodixanol and fractionated by flotation through a 10-20% iodixanol linear gradient at approx
180,000 g for 18 h. McDonald and Pike [1] performed two rounds of homogenization of CHO cells using
multiple passages through a syringe needle. A post-nuclear supernatant was similarly adjusted to 25% (w/v)
iodixanol and loaded under a 0-20% iodixanol gradient. The centrifugation was considerably different however
– 52,000 g for 90 min; although subsequent papers using the technology have reported the use of much more
severe conditions – e.g. 170,000 g for 4 h [3] or 200,000 g for 18 h [4]. The milder centrifugation conditions
recall those used by Smart et al [5] in the isolation of caveolae.
A detailed description of the OptiPrep methodology (Application Sheet S33) can be found via the
releveant OptiPrep Application Sheets Index on the following website: www.Optiprep.com (click on
“Methodology”, then “Organelles and Subcellular Membranes”) and scroll down the Index.
1. Macdonald, J.L. and Pike, L.J. (2005) A simplified method for the preparation of detergent-free lipid rafts
J. Lipid Res., 46, 1061-1067
2. Kasahara, K., Watanabe, K., Takeuchi, K., Kaneko, H., Oohira, A., Yamamoto, T. and Sanai, Y. (2000)
Involvement of gangliosides in GPI-anchored neuronal cell adhesion molecule TAG-1 signaling in lipid
rafts J. Biol. Chem., 275, 34701-34709
3. Inoue, M., Digman, M.A., Cheng, M., Breusegem, S.Y., Halahiel, N., Sorribas, V., Mantulin, W.W.,
Gratton, E., Barry, N.P. and Levi, M. (2004) Partitioning of NAPi cotransporter in cholesterol-,
sphingonyelin-, and glycophingolipid-enriched membrane domains modulates NAPi protein diffusion,
clustering, and activity J. Biol. Chem., 279, 49160-49171
4. Nakagawa, T., Morotomi, A., Tani, M., Sueyoshi, N., Komori, H. and Ito, M. (2005) C18:3-GM1a induces
apoptosis in Neuro2 cells: enzymatic remodeling of fatty acyl chains of glycosphingolipids J. Lipid Res.,
46, 1103-1112
5. Smart E. J., Ying, Y-S., Mineo, C. and Anderson, R. G. W. (1995) A detergent-free method for purifying
caveolae membrane from tissue culture cells Proc. Natl. Acad. Sci., USA, 92, 10104-10108
2. Comprehensive bibliography of papers
Papers reporting the use of the non-detergent method have been divided into cell type (or occasionally tissue).
Within each group, papers are listed alphabetically according to first author. To facilitate identification of
references of interest key words in titles are highlighted in light blue. There is also a Section 2-18. “Subcellular
membranes” listing papers that report the isolation of rafts from an isolated subcellular membrane fraction
rather than whole cells and at the end, a list of Review articles (Section 2-19).
2-1. Adipocytes
Han, C.Y., Umemoto, T., Omer, M., Den Hartigh, L.J., et al (2012) NADPH oxidase-derived reactive oxygen
species increases expression of monocyte chemotactic factor genes in cultured adipocytes J. Biol. Chem., 287,
10379–10393
Umemoto, T., Han, C.Y., Mitra, P., Averill, M.M., et al (2013) Apolipoprotein AI and high-density lipoprotein
have anti-inflammatory effects on adipocytes via cholesterol transporters ATP-binding cassette A-1, ATPbinding cassette G-1, and scavenger receptor B-1 Circ. Res., 112, 1345-1354
Yamada, H., Umemoto, T., Kawano, M., Kawakami, M., Kakei, M., Momomura, S-i., Ishikawa, S-e and Hara,
K. (2017) High-density lipoprotein and apolipoprotein A-I inhibit palmitate induced translocation of toll-like receptor 4 into lipid rafts and inflammatory cytokines in 3T3-L1 adipocytes Biochem. Biophys. Res. Comm.,
484, 403-408
BHK cells (see “2-9. Kidney cells”)
Brain (see “2-13. Neural and related cells” )
2-2. Carcinoma cells (see also “HeLa cells” and “Hepatoma cells”)
Basiouni, S., Stöckel, K., Fuhrmann, H. and Schumann, J. (2012) Polyunsaturated fatty acid supplements
modulate mast cell membrane microdomain composition Cell. Immunol., 275, 42–46
Danza, G., Di Serio, C., Ambrosio, M.R., Sturli, N., et al (2013) Notch3 is activated by chronic hypoxia and
contributes to the progression of human prostate cancer Int. J. Cancer, 133, 2577–2586
Dutta, S., Bandyopadhyay, C., Bottero, V., Veettil, M.V., et al (2014) Angiogenin interacts with the
plasminogen activation system at the cell surface of breast cancer cells to regulate plasmin formation and cell
migration Mol. Oncol., 8, 483-507
Hashimoto, A., Oikawa, T., Hashimoto, S., Sugino, H., Yoshikawa, A., Otsuka, Y., Handa, H., Onodera, Y.,
Nam, J-M. et al (2016) P53- and mevalonate pathway–driven malignancies require Arf6 for metastasis and
drug resistance J. Cell Biol., 213, 81–95
Irwin, M.E., Mueller, K.L., Bohin, N., Ge, Y., et al (2011) Lipid raft localization of EGFR alters the response
of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib J. Cell. Physiol., 226, 2316–2328
Jiang, S., Wang, X., Song, D., Liu, KJ., Gu, Y., Xu, Z., Wang, X., Zhang, X., Ye, Q. et al (2019) Cholesterol
induces epithelial-to-mesenchymal transition of prostate cancer cells by suppressing degradation of EGFR
through APMAP Cancer Res., 79, 3063–75
Kozyulina, P.Y., Loskutov, Y.V., Kozyreva, V.K., Rajulapati, A., Ice, R.J., Jones, B.C. and Pugacheva, E.N.
(2015) Prometastatic NEDD9 regulates individual cell migration via caveolin-1–dependent trafficking of
integrins Mol. Cancer Res., 13, 423-438
Kyriakakis, E., Maslova, K., Frachet, A., Ferri, N., et al (2013) Cross-talk between EGFR and T-cadherin:
EGFR activation promotes T-cadherin localization to intercellular contacts Cell. Signal., 25, 1044–1053
Lee, C-Y., Lai, T-Y., Tsai, M-K., Ou-Yang, P., Tsai, C-Y., Wu, S-W., Hsu, L-C. and Chen, J-S. (2016) The
influence of a caveolin-1 mutant on the function of P-glycoprotein Sci. Rep., 6: 20486
Lin, H-C., Lai, P-Y., Lin, Y-p., Huang, J-Y. et al (2012) Fas ligand enhances malignant behavior of tumor cells
through interaction with Met, hepatocyte growth factor receptor, in lipid rafts J. Biol. Chem., 287, 20664–
20673
Rogers, K.R., Kikawa, K.D., Mouradian, M., Hernandez, K., et al (2010) Docosahexaenoic acid alters
epidermal growth factor receptor-related signaling by disrupting its lipid raft association Carcinogenesis, 31,
1523–1530
Rutkowski, R., Mertens-Walker I., Lisle, J.E., Herington, A.C., et al (2012) Evidence for a dual function of
EphB4 as tumor promoter and suppressor regulated by the absence or presence of the ephrin-B2 ligand Int. J.
Cancer, 131, E614–E624
Soung, Y.H. and Chung, J. (2011) Curcumin inhibition of the functional interaction between integrin a6b4 and
the epidermal growth factor receptor Mol. Cancer Ther., 10, 883–91
Tawadros, T., Brown, M.D., Hart, C.A. and Clarke, N.W. (2012) Ligand-independent activation of EphA2 by
arachidonic acid induces metastasis-like behaviour in prostate cancer cells Br. J. Cancer, 107, 1737–1744
2-3. Breast cancer cells
Hu, J., Wang, W., Liu, C., Li, M., Nice, E. and Xu, H. (2019) Receptor tyrosine kinase inhibitor Sunitinib and
integrin antagonist peptide HM-3 show similar lipid raft dependent biphasic regulation of tumor angiogenesis
and metastasis J. Exp. Clin. Canc. Res., 38: 381
2-4. CHO cells
Adak, S., DeAndrade, D. and Pike, L.J. (2011) The tethering arm of the EGF receptor is required for negative
cooperativity and signal transduction J. Biol. Chem., 286, 1545-1555
Latif, R., Ando, T. and Davies, T.F. (2007) Lipid rafts are triage centers for multimeric and monomeric
thyrotropin receptor regulation Endocrinology 148, 3164-3175
Macdonald, J.L. and Pike, L.J. (2005) A simplified method for the preparation of detergent-free lipid rafts J.
Lipid Res., 46, 1061-1067
Macdonald, J., Li, Z., Su, W. and Pike, L.J. (2006) The membrane proximal disulfides of the EGF receptor
extracellular domain are required for high affinity binding and signal transduction but do not play a role in the
localization of the receptor to lipid rafts Biochim. Biophys. Acta, 1763, 870-878
Pike, L.J., Han, X. and Gross, R.W. (2005) Epidermal growth factor receptors are localized to lipid rafts that
contain a balance of inner and outer leaflet lipids J. Biol. Chem., 280, 26796-26804
Zhang, X., Brovkovycha, V., Zhanga, Y., Tan, F. and Skidgel, R.A. (2015) Downregulation of kinin B1
receptor function by B2 receptor heterodimerization and signaling Cell. Signal., 27, 90–103
2-5 COS cells
Yu, X., Noll, R.R., Romero Dueñas, B.P., Allgood, S.C., Barker, K., Caplan, J.L., Machner, M.P., LaBaer, J.,
Qiu, J. and Neunuebel, M.R. (2018) Legionella effector AnkX interacts with host nuclear protein PLEKHN1
BMC Microbiol., 18: 5
2-6. Endothelial cells (incl. microvascular and progenitor): see also Section 16
Bandyopadhyay, C., Valiya-Veettil, M., Dutta, D., Chakraborty, S., et al (2014) CIB1 synergizes with ephrinA2
to regulate Kaposi’s sarcoma-associated herpesvirus macropinocytic entry in human microvascular dermal
endothelial cells PLoS Pathog., 10: e1003941
Bandyopadhyay, C., Veettil, M.V., Dutta, S. and Chandran, B. (2014) p130Cas scaffolds the signalosome to
direct adaptor-effector cross talk during Kaposi’s sarcoma-associated herpesvirus trafficking in human
microvascular dermal endothelial cells J. Virol., 13858–13878
Chakraborty, S., Veettil, M.V., Sadagopan, S., Paudel, N., et al (2011) c-Cbl-mediated selective virus-receptor
translocations into lipid rafts regulate productive Kaposi’s sarcoma-associated herpesvirus infection in
endothelial cells J. Virol., 85, 12410–12430
Chakraborty, S., Veetti, M.V., Bottero, V. and Chandran, B. (2012) Kaposi’s sarcoma-associated herpesvirus
interacts with EphrinA2 receptor to amplify signaling essential for productive infection Proc. Natl. Acad. Sci.
USA, 109, E1163-E1172
Chi, F., Jong, T.D., Wang, L., Ouyang, Y., et al (2010) Vimentin-mediated signalling is required for IbeA+ E.
coli K1 invasion of human brain microvascular endothelial cells Biochem. J., 427, 79–90
Han, W-Q., Chen, W-D., Zhang, K., Liu, J-J., Wu, Y-J. and Gao, P-J. (2016) Ca2+-regulated lysosome fusion
mediates angiotensin II-induced lipid raft clustering in mesenteric endothelial cells Hypertens. Res., 39, 227–
236
Huang, S-H., Chi, F., Peng, L., Bo, T., Zhang, B., Liu, L.Q., Wu, X., Mor-Vaknin, N., Markovitz, D.M., Cao,
H. and Zhou, Y-H. (2016) Vimentin, a novel NF-KB regulator, is required for meningitic Escherichia coli K1-
induced pathogen invasion and PMN transmigration across the blood-brain barrier PloS One, 11: e0162641
Margheri, F., Chilla, A., Laurenzana, A., Serratì, S., et al (2011) Endothelial progenitor cell–dependent
angiogenesis requires localization of the full-length form of uPAR in caveolae Blood, 118, 3743-3755
Sverdlov, M., Shinin, V., Place, A.T., Castellon, M., et al (2009) Filamin A regulates caveolae internalization
and trafficking in endothelial cells Mol. Biol. Cell, 20, 4531-4540
2-7. Epithelial cells
Wang, Y., Maciejewski, B.S., Drouillard, D., Santos, M., et al (2010) A role for caveolin-1 in
mechanotransduction of fetal type II epithelial cells Am. J. Physiol. Lung Cell. Mol. Physiol., 298, L775–L783
2-8. Fibroblasts
Dutta, D., Chakraborty, S., Bandyopadhyay, C., Veettil, M.V., et al (2013) EphrinA2 regulates clathrin
mediated KSHV endocytosis in fibroblast cells by coordinating integrin-associated signaling and c-Cbl directed
polyubiquitination PLoS Pathog., 9: e1003510
Klappe, K., Hummel, I. and Kok, J.W. (2013) Separation of actin-dependent and actin-independent lipid rafts
Anal. Biochem., 438, 133–135
Hofman, E.G., Ruonala, M.O., Bader, A.N., van den Heuvel, D., et al (2008) EGF induces coalescence of
different lipid rafts J. Cell Sci., 121, 2519-2528
Meszaros, P., Hummel, I., Klappe, K., Draghiciu, O., et al (2013) The function of the ATP-binding cassette
(ABC) transporter ABCB1 is not susceptible to actin disruption Biochim. Biophys. Acta, 1828, 340–351
Morris, D.P., , B., Lei, Wu, Y-X., Michelotti, G.A., et al (2008) The 1a-adrenergic receptor occupies
membrane rafts with its G protein effectors but internalizes via clathrin-coated pits J. Biol. Chem., 283, 2973-
2985
HEK cells: see “2-10 Kidney cells”
2-9. HeLa cells
Kim, H.Y., Kim, S., Pyun, H.J., Maeng, J. and Lee, K. (2015) Cellular uptake mechanism of TCTP-PTD in
human lung carcinoma cells Mol. Pharmaceutics, 12, 194−203
Lee, J.J., Kim, D.G., Kim, D.H., Simborio, H.L., Min, W., Lee, H.J., Her, M., Jung, S.C., Watarai, M. and Kim,
S. (2013) Interplay between clathrin and Rab5 controls the early phagocytic trafficking and intracellular
survival of Brucella abortus within HeLa cells J. Biol. Chem., 288, 28049–28057
Macdonald, J.L. and Pike, L.J. (2005) A simplified method for the preparation of detergent-free lipid rafts J.
Lipid Res., 46, 1061-1067
Milev, M.P., Brown, C.M. and Mouland, A.J. (2010) Live cell visualization of the interactions between HIV-1
Gag and the cellular RNA-binding protein Staufen1 Retrovirology, 7: 41
White, A.B., Givogri, M.I., Lopez-Rosas, A., Cao, H., et al (2009) Psychosine accumulates in membrane
microdomains in the brain of Krabbe patients, disrupting the raft architecture J. Neurosci. 29, 6068–6077
Zarubica, A., Plazzo, A.P., Stöckl, M., Trombik, T., et al (2009) Functional implications of the influence of
ABCA1 on lipid microenvironment at the plasma membrane: a biophysical study FASEB J. 23, 1775–1785
2-10. Hepatoma cells
Bandyopadhyay, D., Sanchez, J.L., Guerrero, A.M., Chang, F-M., Granados, J.C., Short, J.D. and Banik, B.K.
(2015) Design, synthesis and biological evaluation of novel pyrenyl derivatives as anticancer agents Eur. J.
Medicinal Chem., 89, 851-862
Carrasco, M.P., Jiménez-López, J.M., Ríos-Marco, P., Segovia, J.L., et al (2010) Disruption of cellular
cholesterol transport and homeostasis as a novel mechanism of action of membrane-targeted alkylphospholipid
analogues Br. J. Pharmacol., 160, 355–366
Chakraborty, S., Lakshmanan, M., Swa, H.L.F., Chen, J., Zhang, X., Ong, Y.S. et al (2015) An oncogenic role
of Agrin in regulating focal adhesion integrity in hepatocellular carcinoma Nat. Commun. 6: 6184
Kim, H.Y., Kim, S., Pyun, H.J., Maeng, J. and Lee, K. (2015) Cellular uptake mechanism of TCTP-PTD in
human lung carcinoma cells Mol. Pharmaceutics, 12, 194−203
Li, Y., Masaki, H., Shimakami, T., and Lemon, S.M. (2104) hnRNP L and NF90 interact with hepatitis C virus
5’terminal untranslated RNA and promote efficient replication J. Virol., 88, 7199–7209
Lin, H-C., Lai, P-Y., Lin, Y-p., Huang, J-Y., et al (2012) Fas ligand enhances malignant behavior of tumor
cells through interaction with Met, hepatocyte growth factor receptor, in lipid rafts J. Biol. Chem., 287, 20664–
20673
Jurkat cells: see “2-14 Lymphocytic cells”
2-11. Kidney cells
2-11-1. BHK cells
Hummel, I., Klappe, K., Ercan, C. and Kok, J.W. (2011) Multidrug resistance-related protein 1 (MRP1)
function and localization depend on cortical actin Mol. Pharmacol., 79, 229-240
Lund-Katz, S., Lyssenko, N.N., Nickel, M., Nguyen, D., et al (2103) Mechanisms responsible for the
compositional heterogeneity of nascent high density lipoprotein J. Biol. Chem., 288, 23150–23160
2-11-2. Foetal monkey cells
Ohmine, S., Singh, R.D., Marks, D.L., Meyer, M.A., Pagano, R.E. and Ikeda, Y. (2013) Viral attachment
induces rapid recruitment of an innate immune sensor (TRIM5α) to the plasma membrane J. Innate Immun., 5,
414–424
2-11-3. HEK cells
Barceló, C., Paco, N., Beckett, A.J., Alvarez-Moya, B., et al (2013) Oncogenic K-ras segregates at spatially
distinct plasma membrane signaling platforms according to its phosphorylation status J. Cell Sci., 126, 4553–
4559
Fenstermaker, R.A., Figel, S.A., Qiu, J., Barone, T.A., Dharma, S.S., Winograd, E.K., Galbo, P.M., Wiltsie,
L.M. and Ciesielski, M.J. (2018) Survivin monoclonal antibodies detect survivin cell surface expression and
inhibit tumor growth in vivo Clin. Cancer Res., 24, 2642-2652
Fornoni, A., Sageshima, J., Wei, C., Merscher-Gomez, S., et al (2011) Rituximab targets podocytes in recurrent
focal segmental glomerulosclerosis Sci. Translat Med., 3: 85ra46
Tao, B., Bu, S., Yang, Z., Siroky, B., et al (2009) Cystin localizes to primary cilia via membrane microdomains
and a targeting motif J. Am. Soc. Nephrol., 20, 2570–2580
Zhang, X., Tan, F., Zhang, Y. and Skidgel, R.A. (2008) Carboxypeptidase M and kinin B1 receptors interact to
facilitate efficient B1 signaling from B2 agonists J. Biol. Chem., 283, 7994-8004
Zhang, X., Brovkovycha, V., Zhanga, Y., Tan, F. and Skidgel, R.A. (2015) Downregulation of kinin B1
receptor function by B2 receptor heterodimerization and signaling Cell. Signal., 27, 90–103
2-11-4. Human podocytes
Fornoni, A., Sageshima, J., Wei, C., Merscher-Gomez, S., et al (2011) Rituximab targets podocytes in recurrent
focal segmental glomerulosclerosis Sci. Translat Med., 3: 85ra46
2-11-5. LLC-PK1
Foster, J.D., Adkins, S.D., Lever, J.R. and Vaughan, R.A. (2008) Phorbol ester induced traffickingindependent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane
rafts and cholesterol J. Neurochem., 105, 1683-1699
2-11-6. MDCK cells
Polymenidou, M., Trusheim, H,., Stallmach, L., Moos, R., et al (2008) Canine MDCK cell lines are refractory
to infection with human and mouse prions Vaccine, 26, 2601-2614
2-11-7. Mouse kidney cells
Follit, J.A., Li, L., Vucica, Y. and Pazour, G.J. (2010) The cytoplasmic tail of fibrocystin contains a ciliary
targeting sequence J. Cell Biol., 188, 21–28
2-11-8. Opossum kidney
Breusegem, S.Y., Halaihel, N., Inoue, M., Zajicek, H., et al (2005) Acute and chronic changes in cholesterol
modulate Na-Pi
cotransport activity in OK cells Am. J. Physiol., 289, FF154-F165
2-11-9. Rat glomerular
Boini, K.M., Zhang, C., Xia, M., Han, W-Q., et al (2010) Visfatin-induced lipid raft redox signaling platforms
and dysfunction in glomerular endothelial cells Biochim. Biophys. Acta, 1801, 1294–1304
2-12. Liver cells
Hahn-Obercyger, M., Graeve, L. and Madar, Z. (2009) A high-cholesterol diet increases the association
between caveolae and insulin receptors in rat liver J. Lipid Res., 50, 98–107
Morita, S-y., Tsuda, T., Horikami, M., Teraoka, R., Kitagawa, S. and Terada, T. (2013) Bile salt-stimulated
phospholipid efflux mediated by ABCB4 localized in non-raft membranes J. Lipid Res., 54, 1221–1230
2-13. Lung cancer cells
Chen, Q., Pan, Z., Zhao, M., Wang, Q., Qiao, C., Miao, L. and Ding, X. (2018) High cholesterol in lipid rafts
reduces the sensitivity to EGFR-TKI therapy in non-small cell lung cancer J. Cell Physiol., 233, 6722–6732
2-14. Lymphocytic cells (incl. Jurkat cells)
Kennedy, C., Nelson, M.D. and Bamezai, A.K. (2011) Analysis of detergent-free lipid rafts isolated from a
CD4+ T cell line: interaction with antigen presenting cells promotes coalescing of lipid rafts Cell Commun.
Signal., 9: 31
Morales-Garzia, M.G., Fournie, J-J., Morena-Altamirano, M.M.B., Rodriguez-Luna, G., et al (2008) A flowcytometry method for analyzing the composition of membrane rafts Cytometry Part A, 73A, 918-925
Mutch, C.M., Sanyal, R., Unruh, T.L., Grigoriou. L., et al (2007) Activation-induced endocytosis of the raftassociated transmembrane adaptor protein LAB/NTAL in B lymphocytes: evidence for a role in internalization
of the B cell receptor Int. Immunol., 19, 19-30
Polyak, M.J., Li, H., Shariat, N. and Deans, J.P. (2008) CD20 Homo-oligomers physically associate with the B
cell antigen receptor: dissociation upon receptor engagement and recruitment of phosphoproteins and
calmodulin-binding proteins J. Biol. Chem., 283, 18545-18552
Quattrocchi, S., Ruprecht, N., Bönsch, C., Bieli, S., et al (2012) Characterization of the early steps of human
parvovirus B19 infection J. Virol., 86, 0274-9284
Talaty, P., Emery, A., Holthusen, K. and Everly, Jr, D.N. (2012) Identification of transmembrane protein 134
as a novel LMP1-binding protein by using bimolecular fluorescence complementation and an enhanced
retroviral mutagen J. Virol., 86, 11345–11355
2-15. Macrophages/Monocytes
Hellwing, C., Tigistu-Sahle, F., Fuhrmann, H., Käkelä, R. and Schumann, J. (2018) Lipid composition of
membrane microdomains isolated detergent-free from PUFA supplemented RAW264.7 macrophages J. Cell.
Physiol., 233, 2602–2612
Morales-Garzia, M.G., Fournie, J-J., Morena-Altamirano, M.M.B., Rodriguez-Luna, G., et al (2008) A flowcytometry method for analyzing the composition of membrane rafts Cytometry Part A, 73A, 918-925
Schumann, J., Leichtle, A., Thiery, J. and Fuhrmann, H. (2011) Fatty acid and peptide profiles in plasma
membrane and membrane rafts of PUFA supplemented RAW264.7 macrophages PLoS One 6: e24066
Serezani, C.H., Aronoff, D.M., Sitrin, R.G. and Peters-Golden, M. (2009) FcRI ligation leads to a complex
with BLT1 in lipid rafts that enhances rat lung macrophage antimicrobial functions Blood, 114, 3316-3324
Zhu, X., Owen, J.S., Wilson, M.D., Li, H., et al (2010) Macrophage ABCA1 reduces MyD88-dependent Tolllike receptor traffi cking to lipid rafts by reduction of lipid raft cholesterol J. Lipid Res., 51, 3196–3206
2-16. Neural and related cells
2-16-1. Brain cells, tissue and microvessels
Lochhead, J.J., McCaffrey, G., Sanchez-Covarrubias, L., Finch, J.D., et al. (2012) Tempol modulates changes
in xenobiotic permeability and occludin oligomeric assemblies at the blood-brain barrier during inflammatory
pain Am. J. Physiol. Heart Circ. Physiol., 302, H582–H593
McCaffrey, G., Staatz, W.D., Quigley, C.A., Nametz, N., et al (2007) Tight junctions contain oligomeric
protein assembly critical for maintaining blood-brain barrier integrity in vivo J. Neurochem., 103, 2540-2555
McCaffrey, G., Seelbach, M.J., Staatz, W.D., Nametz, N., et al (2008) Occludin oligomeric assembly at tight
junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia J. Neurochem., 106,
2395-2409
McCaffrey, G., Willis, C.L., Staatz, W.D., Nametz, N., et al (2009) Occludin oligomeric assemblies at tight
junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress J. Neurochem., 110, 58-71
McCaffrey, G., Staatz, W.D., Sanchez-Corravubias, L., Finch, J.D., et al (2012) P-glycoprotein trafficking at
the blood-brain barrier altered by peripheral inflammatory hyperalgesia J. Neurochem., 122, 962–975
Persaud-Sawin, D.A., Lightcap, S. Harry, G.J. (2009) Isolation of rafts from mouse brain tissue by a detergentfree method J. Lipid Res., 50, 759–767
Tome, M.E., Jarvis, C.K., Schaefer, C.P., Jacobs, L.M., Herndon, J.M., Hunn, K.C., Arkwright, N.B., Kellohen,
K.E., Mierau, P.C. and Davis, T.P. (2018) Acute pain alters P-glycoprotein-containing protein complexes in rat
cerebral microvessels: Implications for P-glycoprotein trafficking J. Cereb. Blood Flow Metab., 38, 2209–2222
2-16-2.Glial/ Microglial cells
Lundgren, T.K., Luebke, M., Stenqvist, A. and Ernfors, P. (2008) Differential membrane compartmentlization
of Ret by PTB-adaptor engagement FEBS. J., 275, 2055-2066
Rimmerman, N., Bradshaw, H.B., Kozela, E., Levy, R., et al (2012) Compartmentalization of endocannabinoids into lipid rafts in a microglial cell line devoid of caveolin-1 Br. J. Pharmacol., 165, 2436–2449
Rimmerman, N., Ben-Hail, D., Porat, Z., Juknat, A., et al (2013) Direct modulation of the outer mitochondrial
membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: a novel mechanism for
cannabinoid-induced cell death Cell Death Dis., 4: e949
2-16-3. Hypothalamic cells
Cui, H.L., Guo, B., Scicluna, B., Coleman, B.M., et al (2014) Prion infection impairs cholesterol metabolism in
neuronal cells J. Biol. Chem., 289, 789-802
2-16-4. Neuroblastoma cells
Hummel, I., Klappe, K., Ercan, C. and Kok, J.W. (2011) Multidrug resistance-related protein 1 (MRP1)
function and localization depend on cortical actin Mol. Pharmacol., 79, 229-240
Klappe, K., Dijkhuis, A-J., Hummel, I., van Dam, A., et al (2010) Extensive sphingolipid depletion does not
affect lipid raft integrity or lipid raft localization and efflux function of the ABC transporter MRP1 Biochem. J.,
430, 519–529
Klappe, K., Hummel, I. and Kok, J.W. (2013) Separation of actin-dependent and actin-independent lipid rafts
Anal. Biochem., 438, 133–135
Meszaros, P., Klappe, K., Hummel, I., Hoekstra, D. et al (2011) Function of MRP1/ABCC1 is not dependent on
cholesterol or cholesterol-stabilized lipid rafts Biochem. J., 437, 483–491
Nakagawa, T., Morotomi, A., Tani, M., Sueyoshi, N., et al (2005) C18:3-GM1a induces apoptosis in Neuro2
cells: enzymatic remodeling of fatty acyl chains of glycosphingolipids J. Lipid Res., 46, 1103-1112
Qiu, Y., Wang, Y., Law, P-Y., Chen, H-Z. et al (2011) Cholesterol regulates -opioid receptor-induced -
arrestin 2 translocation to membrane lipid rafts Mol. Pharmacol., 80, 210–218
Sontag, J-M., Nunbhakdi-Craig, V. and Sontag, E. (2013) Leucine carboxyl methyltransferase 1 (LCMT1)-
dependent methylation regulates the association of protein phosphatase 2A and tau protein with plasma
membrane microdomains in neuroblastoma cells J. Biol. Chem., 288, 27396–27405
2-16-5. Neuronal cells
Asimaki, O., Leondaritis, G., Lois, G., Sakellaridis, N. et al (2011) Cannabinoid 1 receptor-dependent
transactivation of fibroblast growth factor receptor 1 emanates from lipid rafts and amplifies extracellular
signal-regulated kinase 1/2 activation in embryonic cortical neurons J. Neurochem., 116, 866–873
Kang, S.S., Zhang, Z., Liu, X., Manfredsson, F.P., Benskey, M.J., Cao, X., Xu, J., Sun. Y.E. and Ye, K. (2017)
TrkB neurotrophic activities are blocked by α-synuclein, triggering dopaminergic cell death in Parkinson’s
disease Proc. Natl. Acad. Sci. USA, 114, 10773–10778
Kasahara, K., Watanabe, K., Takeuchi, K., Kaneko, H., et al (2000) Involvement of gangliosides in GPIanchored neuronal cell adhesion molecule TAG-1 signaling in lipid rafts J. Biol. Chem., 275, 34701-34709
Salinas, S., Zussy, C., Loustalo, F., Henaff, D., et al (2014) Disruption of the coxsackievirus and adenovirus
receptor-homodimeric interaction triggers lipid microdomain- and dynamin-dependent endocytosis and
lysosomal targeting J. Biol. Chem., 289, 680-695
Schindler, M., Fabre, C., de Weille, J., Carreau, S., et al (2012) Disruption of nongenomic testosterone
signaling in a model of spinal and bulbar muscular atrophy Mol. Endocrinol., 26, 1102–1116
Wang, J. and Yua, R.K. (2013) Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal
ability of mouse neural stem cells in vitro Proc. Natl. Acad. Sci. USA, 110, 19137–19142
2-17. Polymorphonuclear leukocytes
Kannan, K.B., Barlos, D. and Hauser, C.J. (2007) Free cholesterol alters lipid raft structure and function
regulating neutrophil Ca2+ entry and respiratory burst: correlations with calcium channel raft trafficking J.
Immunol., 178, 5253-5261
2-18. Retinal pigment epithelial cells
Opreanu, M., Tikhonenko, M., Bozack, S., Lydic, T.A., Reid, et al (2011) The unconventional role of acid
sphingomyelinase in regulation of retinal microangiopathy in diabetic human and animal models Diabetes 60,
2370–2378
2-19. Smooth muscle vascular cells
Dey, K., Roy, S., Ghosh, B. and Chakraborti, S, (2012) Role of protein kinase C in phospholemman mediated
regulation of 21 isozyme of Na+
/K+
-ATPase in caveolae of pulmonary artery smooth muscle cells Biochimie,
94, 991-1000
Kiyan, J., Haller, H. and Dumler, I. (2009) The tyrosine phosphatase SHP-2 controls urokinase-dependent
signaling and functions in human vascular smooth muscle cells Exp. Cell Res., 315, 1029-1039
Kiyan, J., Smith, G., Haller, H. and Dumler, I. (2009) Urokinase-receptor-mediated phenotypic changes in
vascular smooth muscle cells require the involvement of membrane rafts Biochem. J., 423, 343–351
Norambuena, A., Poblete, M.I., Donoso, M.V., Espinoza, C.S., et al (2008) P2Y1 receptor activation elicits its
partition out of membrane rafts and its rapid internalization from human blood vessels: implications for
receptor signaling Mol. Pharmacol., 74, 1666-1677
Norambuena, A., Palma, F., Poblete, I., Donoso, V., et al (2010) UTP controls cell surface distribution and
vasomotor activity of the human P2Y2 receptor through an epidermal growth factor receptor-transregulated
mechanism J. Biol. Chem., 285, 2940-2950
2-20 Thyroid
Campana, V., Caputo, A., Sarnataro, D., Paladino, S., et al (2007) Characterization of the properties and
trafficking of an anchorless form of the prion protein J. Biol. Chem., 282, 22747-22756
2-21. Subcelluar membranes
Inoue, M., Digman, M.A., Cheng, M., Breusegem, S.Y., et al (2004) Partitioning of NAPi cotransporter in
cholesterol-, sphingomyelin-, and glycophingolipid-enriched membrane domains modulates NAPi protein
diffusion, clustering, and activity J. Biol. Chem., 279, 49160-49171
Morita, S-y., Tsuda, T., Horikami, M., Teraoka, R., et al (2013) Bile salt-stimulated phospholipid efflux
mediated by ABCB4 localized in non-raft membranes J. Lipid Res., 54, 1221–1230
2-22. Reviews
Balbis, A. and Posner, B.I. (2010) Compartmentalization of EGFR in cellular membranes: role of membrane
rafts J. Cell. Biochem., 109, 1103–1108
Busija, A.R., Patel, H.H. and Insel, P.A. (2017) Caveolins and cavins in the trafficking, maturation, and
degradation of caveolae: implications for cell physiology Am. J. Physiol. Cell Physiol., 312, C459–C477
Callera, G.E., Bruder-Nascimento, T. and Touyz, R.M. (2017) Assessment of caveolae/lipid rafts in isolated
cells In Hpertension Methods and Protocols: Methods Mol. Biol., 1527 (ed. Touyz, R.M. and Schiffrin, E.L.),
Springer Science+Business Media, LLC, pp 251-269
Dodelet-Devillers, A., Cayrol, R., van Horssen, J., Haqqani, A.S., et al (2009) Functions of lipid raft membrane
microdomains at the blood–brain barrier J. Mol. Med., 87, 765–774
Gimpl, G. and Gehrig-Burger, K. (2012) Specific and non specific regulation of GPCR function by cholesterol
In Regulation of ion channels and receptors (Ed. Levitan, I. and Barrantes, F.J.) John Wiley & Sons, Inc., pp
205-230
Landry, A. and Xavier, R. (2006) Isolation and analysis of lipid rafts in cell-cell interactions Methods Mol.
Biol., 341, 251-282
Płóciennikowska, A., Hromada-Judycka, A., Borzęcka, K. and Kwiatkowska, K. (2015) Co-operation of TLR4
and raft proteins in LPS-induced pro-inflammatory signaling Cell. Mol. Life Sci., 72, 557–581
Shah, M.B. and Sehgal, P.B. (2007) Non-detergent isolation of rafts Methods Mol. Biol., 398, 21-28
Zheng, Y.Z. and Foster, L.J. (2009) Biochemical and proteomic approaches for the study of membrane
microdomains J. Proteom., 72, 12-22
OptiPrepTM Reference List RS07 6th edition, January 2020
OptiPrep Reference List RS08
Purification of caveolae in gradients prepared from OptiPrep
The principal aim of this OptiPrep Reference List is to present a bibliography of all of the current
papers reporting the use of an iodixanol gradient to purify and analyse caveolae from vertebrate and nonvertebrate cells/tissues (see Section 2). Section 1 contains a brief survey of the technique; it has its own
short reference list distinct from the comprehensive reference list in Section 2.
1. Background
Early methods for the purification of lipid-rich plasma membrane domains largely relied on their
insolubility in Triton X-110 (or some other non-ionic detergent) relative to that of the bulk plasma membrane or
that of all the other subcellular membranes. Sometimes detergent was added to the whole homogenate or more
frequently a partially-purified plasma membrane fraction was first isolated before treating with detergent. Smart
et al [1] however pointed out that, while use of a non-ionic detergent did permit the isolation of a lipid-rich
membrane domain, that some characteristic caveolar proteins can be lost in the procedure. These workers
therefore developed a method that avoids the use of Triton X-100. After isolation of a plasma membrane
fraction from either human skin fibroblasts or MA104 cells, the caveolae are released by sonication in a
standard cell homogenization medium. The first part of the isolation procedure is a flotation through a
continuous iodixanol gradient (0-20%); this gradient is essentially a resolving gradient in which the caveolinrich vesicles are concentrated in the top third of the gradient, while the predominantly caveolin-poor vesicles
band in denser regions. A second discontinuous gradient is essentially a concentration gradient to band the
caveolin-rich vesicles sharply at an interface.
Smart et al [1] used a Percoll-based method for the initial purification of the plasma membrane, but there
is no obvious requirement that such a method must be used. Kumanogoh et al [2] for example used a sucrose
gradient to purify a synaptic plasma membrane before using the method devised by Smart et al [1]. There are
many examples in the literature of iodixanol gradients being used to purify plasma membrane from a
homogenate. The McDonald and Pike [3] method for the isolation of lipid rafts incorporates elements of both
the detergent and the sonicated plasma membrane approaches. It involves performing two rounds of homogenization of CHO cells using multiple passages through a syringe needle. A post-nuclear supernatant is then adjusted
to 25% (w/v) iodixanol and loaded under a 0-20% iodixanol gradient for floating the lipid-rich plasma
membrane fragments. In effect it resembles a Smart et al [3] method without a plasma membrane purification
step. It also omits the final iodixanol caveolae concentration gradient. Occasionally the Percoll gradient is
omitted, see for example ref 4.
1. Smart, E.J., Mineo, C. and Anderson, R.G.W. (1996) Clustered folate receptors deliver 5-methyltetrahydrofolate to cytoplasm of MA104 cells J. Cell Biol., 134, 1169-1177
2. Kumanogoh, H., Miyata, S., Sokawa, Y. and Maekawa, S. (2001) Biochemical and morphological analysis
on the localization of Rac1 in neurons Neurosci. Res., 39, 189-196
3. Macdonald, J.L. and Pike, L.J. (2005) A simplified method for the preparation of detergent-free lipid rafts
J. Lipid Res., 46, 1061-1067
4. Tome, M.E., Schaefer, C.P., Jacobs, L.M., Zhang, Y., Hemdon, J.M., Matty, F.O. and Davis, T.P. (2015)
Identification of P- glycoprotein co-fractionating proteins and specific binding partners in rat brain
microvessels J. Neurochem., 134, 200-210
Detailed descriptions of the OptiPrep-based techniques for isolation of caveolae and lipid rafts can be
found in the following OptiPrep Application Sheets:
OptiPrepTM Application Sheet S34: Isolation of caveolae
OptiPrepTM Application Sheet S32: Isolation of lipid rafts (detergent strategy)
OptiPrepTM Application Sheet S33: Isolation of lipid rafts (detergent-free strategy)
These can be found via the releveant OptiPrep Application Sheets Index on the following website:
www.Optiprep.com (click on “Methodology”, then “Organelles and Subcellular Membranes”). Scroll down the
Index to “Plasma membrane domains”. There is also a large literature on the fractionation of plasma membrane,
endoplasmic reticulum, Golgi and endosomes and several Application Sheets, based on this literature, may be
accessed from the Index entry for “Endoplasmic reticulum”.
2. Comprehensive bibliographies
Papers have been divided into cell or tissue type; and additionally, when required, into research topic.
Within each group papers are listed alphabetically according to first author. To facilitate identification of
references of interest key words in titles are highlighted in light blue. When a paper reports the study of more
than one cell type, reference to that paper will appear under all relevant cell headings.
All publications reporting brain-derived caveolae are listed under “22. Neural tissue and cells”. Papers
reporting on heart-derived caveolae are listed under “3. Cardiac muscle” but see also “27. Smooth muscle
and smooth muscle cells” for related cells. Cultured cell and tissue-derived caveolae will generally have been
prepared from a partially purified plasma membrane fraction. An exception to this is a paper on “signalosomes”,
cytoplasmic organelles, that resemble caveolae and which have been purified from cardiac muscle (see Quinlan
et al in Section 3). Review articles are listed in Section 30. Note also the following:
OptiPrepTM Reference List RS06: Lipid rich detergent-resistant membranes from mammalian cells, tissues and
organelles
OptiPrepTM Reference List RS07: Detergent-free strategy for lipid raft isolation from mammalian cells and
tissues
1. Caco-2 cells
Delmas, O., Breton, M., Sapin, C., Le Bivic, A., Colard, O. and Trugnan, G. (2007) Heterogeneity of raft-type
membrane micrdomains associated with VP4, the rotavirus spike protein, in Caco-2 and MA 104 cells J. Virol.,
81, 1610-1618
2. Carcinoma cells
Cai, C., Zhu, H. and Chen, J. (2004) Overexpression of caveolin-1 increases plasma membrane fluidity and
reduces P-glycoprotein function in Hs578T/Dox Biochem. Biophys Res. Commun., 320, 868-874
Chaterjee, S., Cao, S., Peterson, T.E., Simari, et al (2003) Inhibition of GTP-dependent vesicle trafficking
impairs internalization of plasmalemmal eNOS and cellular nitric oxide production J. Cell Sci., 116, 3645-3655
Grądzka I., Sochanowicz, B., Brzóska, K., Wójciuk, G., et al (2013) Cis-9,trans-11-conjugated linoleic acid
affects lipid raft composition and sensitizes human colorectal adenocarcinoma HT-29 cells to X-radiation
Biochim. Biophys. Acta, 1830, 2233–2242
Huhtakangas, J.A., Olivera, C.J., Bishop, J.E., Zanello, L.P. et al (2004) The vitamin D receptor is present in
caveolae-enriched plasma membranes and binds 1,25(OH)2-vitamin D3 in vivo and in vitro Mol. Endocrinol.,
18, 2660-2671
McDonald, J.F., Zheleznyak, A. and Frazier, W.A. (2004) Cholesterol-independent interactions with CD47
enhance v3
activity J. Biol. Chem., 279, 17301-17311
Nion, S., Briand, O., Lestavel, S., Torpier, G., et al (1997) High-density-lipoprotein subfraction 3 interaction
with glycosylphosphatidyl-inositol-anchored proteins Biochem. J., 328, 415-423
Piazza, T.M., Lu, J-C., Carver, K.C. and Schuler, L.A. (2009) Src family kinases accelerate prolactin receptor
internalization, modulating trafficking and signaling in breast cancer cells Mol. Endocrinol., 23, 202-212
Pitto, M., Parenti, M., Guzzi, F., Magni, F., et al (2002) Palmitic is the main fatty acid carried by lipids of
detergent-resistant membrane fractions from neural and non-neural cells Neurochem. Res., 27, 729-734
Sitaraman, S.V., Wang, L., Wong, M., Bruewer, M., et al (2002) The adenosine 2b receptor is required to the
plasma membrane and associates with E3KARP and ezrin upon agonist stimulation J. Biol. Chem., 277, 33188-
33195
Sun, J., Nanjundan, M., Pike, L.J., Wiedmer, T., et al (2002) Plasma membrane phospholipid scramblase 1 is
enriched in lipid rafts and interacts with the epidermal growth factor receptor Biochemistry, 41, 6338-6345
Thiel, K.W. and Carpenter, G. (2006) ErbB-4 and TNF-α converting enzyme localization to membrane
microdomains Biochem. Biophys. Res. Commun., 350, 629-633
Turk, H.F., Barhoumi, R. and Chapkin, R.S. (2012) Alteration of EGFR spatiotemporal dynamics suppresses
signal transduction PLoS One, 7: e39682
Waugh, M.G., Lawson, D., Tan, S.K. and Hsuan, J.J. (1998) Phosphatidylinositol 4-phosphate synthesis in
immunoisolated caveolae-like vesicles and low buoyant non-caveolar membranes J. Biol. Chem., 273, 17115-
17121
Widatalla, S.E., Korolkova, O.Y., Whalen, D.S., Goodwin, J.S., Williams, K.P., Ochieng, J. and Sakwe, A.M.
(2019) Lapatinib-induced annexin A6 upregulation as an adaptive response of triple-negative breast cancer
cells to EGFR tyrosine kinase inhibitors Carcinogenesis, 40, 998–1009
3. Cardiac muscle (see also “Smooth muscle”)
Huhtakangas, J.A., Olivera, C.J., Bishop, J.E., Zanello, L.P., et al (2004) The vitamin D receptor is present in
caveolae-enriched plasma membranes and binds 1,25(OH)2-vitamin D3 in vivo and in vitro Mol. Endocrinol.,
18, 2660-2671
Gabrielová, E., Bartošíková, L., Nečas, J. and Modrianský, M. (2019) Cardioprotective effect of 2,3-
dehydrosilybin preconditioning in isolated rat heart Fitoterapia 132, 12–21
Quinlan, C.L., Costa, A.D.T., Costa, C.L., Pierre, S.V., et al Conditioning the heart induces formation of
signalosomes that interact with mitochondria to open mitoKATP channels Am. J. Physiol. Heart Circ. Physiol.,
295, H953-H961
4. CHO cells
Babitt, J., Trigatti, B., Rigotti, A., Smart, E.J., et al (1997) Murine SR-BI, a high density lipoprotein receptor
that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane
caveolae J. Biol. Chem., 272, 13242-13249
Graf, G.G., Connell, P.M., van der Westhuyzen, D.R. and Smart, E.J. (1999) The class B, type I scavenger
receptor promotes the selective uptake of high density lipoprotein cholesterol ethers into caveolae J. Biol.
Chem., 274, 12043-12048
Guo, L., Chen, M., Song, Z., Daugherty, A., et al (2011) C323 of SR-BI is required for SR-BI-mediated HDL
binding and cholesteryl ester uptake J. Lipid Res., 52, 2272–2278
Uittenbogaard, A., Everson, W.V., Matveev, S.V. and Smart, E.J. (2002) Cholesteryl ester is transported from
caveolae to internal membranes as part of a caveolin-annexin II lipid-protein J. Biol. Chem., 277, 4925-4931
Webb, N.R., Connell, P.M., Graf, G.A., Smart, E.J., et al (1998) SR-BII, an isoform of the scavenger receptor
BI containing an alternate cytoplasmic tail, mediates lipid transfer between high density lipoprotein and cells J.
Biol. Chem., 273, 15241-15248
Zhang, J., Chu, W. and Crandall, I. (2008) Lipoprotein binding preference of CD36 is altered by filipin
treatment Lipids Health Dis., 7, 23
5. Chondrocytes
Elbaradie, K.B.Y., Wang, Y., Boyan, B.D. and Schwartz, Z. (2013) Sex-specific response of rat costochondral
cartilage growth plate chondrocytes to 17β-estradiol involves differential regulation of plasma membrane
associated estrogen receptors Biochim. Biophys. Acta, 1833, 1165–1172
6. COS cells
Grossmann, S., Higashiyama, S., Oksche, A., Schaefer, M., et al (2009) Localisation of endothelin B receptor
variants to plasma membrane microdomains and its effects on downstream signaling Mol. Memb. Biol., 26,
279-292
Heberden, C., Reine, F., Grosse, B., Henry, C., et al (2006) Detection of a raft-located estrogen receptor-like
protein distinct from ER Int. J. Biochem. Cell Biol., 38, 376-391
Hinkovska-Galcheva, V., Boxer, L.A., Kindzelski, A., Hiraoka, M., Abe, A., Goparju, S., Spiegel, S., Petty,
H.R. and Shayman, J.A. (2005) Ceramide 1-phosphate, a mediator of phagocytosis J. Biol. Chem., 280, 26612-
26621
Hinkovska-Galcheva, V., Clark, A., VanWay, S., Huang, J-B., et al (2008) Ceramide kinase promotes Ca21
signaling near IgG-opsonized targets and enhances phagolysosomal fusion in COS-1 cells J. Lipid Res., 49,
531-542
Ikezu, T., Trapp, B.D., Song, K.S., Schlegel, A., et al (1998) Caveolae, plasma membrane microdomains for -
secretase-mediated processing of the amyloid precursor protein J. Biol. Chem., 273, 10485-10495
Mansfield, P.J., Hinkovska-Galcheva, V., Borofsky, M.S., Shayman, J.A. et al (2005) Phagocytic signaling
molecules in lipid rafts of COS-1 cells transfected with FcRIIA Biochem. Biophys. Res. Commun., 331, 132-
138
Nishiyama, K., Trapp, B.D., Ikezu, T., Ransohoff, et al (1999) Caveolin-3 upregulation activates -secretasemediated cleavage of the amyloid precursor protein in Alzheimer’s disease J. Neurosci., 19, 6538-6548
Oh, P. and Schnitzer, J.E. (1999) Immunoisolation of caveolae with high affinity antibody binding to the
oligomeric caveolin cage J. Biol. Chem, 274, 23144-23154
7. Embryonic stem cells
Hernandez, V.J., Weng, J., Ly, P., Pompey, S., et al (2013) Cavin-3 dictates the balance between ERK and Akt
signaling eLife, 2: e00905
8. Endothelial (vascular) cells
8-1. Alzheimer’s disease
David, M.A., Jones, D.R. and Tayebi, M. (2014) Potential candidate camelid antibodies for the treatment of
protein-misfolding diseases J. Neuroimmunol., 272, 76–85
8-2. ATP synthase
Yamamoto, K., Shimizu, N., Obi, S., Kumagaya, S., Taketani, Y., Kamiya, A. and Ando, J. (2007) Involvement
of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells Am. J. Physiol. Heart
Circ. Physiol., 293, H1646-H1653
8-3. Caspase-3
Oxhorn, B.C. and Buxton, I.L.O. (2003) Caveolar compartmentation of caspase-3 in cardiac endothelial cells
Cell. Signal., 15, 489-496
8-4. Caveolin-2
Boyd, N.L., Park, H., Sun, W-P., Coleman, S.E., et al (2004) Bovine caveolin-2 cloning and effects of shear
stress on its localization in bovine aortic endothelial cells Endothelium, 11, 189-198
8-5. FC5
Abulrop, A., Sprong, H., Van Bergen en Henegouwen P. and Stanimirovic, D. (2005) The blood-brain barrier
transmigrating single domain antibody: mechanism of transport and antigenic epitopes in human brain
endothelial cells. J. Neurochem., 95, 1201-1214
8-6. Glycolipids
Czarny, M., Liu, J., Oh, P. and Schnitzer, J.E. (2003) Transient mechanoactivation of neutral sphingomyelinase
in caveolae to generate ceramide J. Biol. Chem., 278, 4424-4430
Shu, L. and Shayman, J.A. (2007) Caveolin-associated accumulation of globotriaosylceramide in the vascular
endothelium of -glactosidase A null mice J. Biol. Chem., 282, 20960-20967
8.-7. HDL uptake
Balazs, Z., Panzenboeck, U., Hammer, A., Sovic, A., et al. (2004) Uptake and transsport of high-density
lipoprotein (HDL) and HDL-associated -tocopherol by an in vitro blood-brain barrier model J. Neurochem.,
89, 939-950
8-8. Ion transport
Wang, X-L., Ye, D., Peterson, T.E., Cao, S., et al (2005) Caveolae targeting and regulation of large
conductance Ca2+ -activated K+
channels in vascular endothelial cells J. Biol. Chem., 280, 11656-11664
8-9. Lung
Jiang, Y., Sverdlov, M.S., Toth, P.T., Huang, L.S., Du, G., Liu, Y., Natarajan, V. Minshall, R,D, (2016)
Phosphatidic acid produced by RalA-activated PLD2 stimulates caveolae-mediated endocytosis and trafficking
in endothelial cells J. Biol. Chem., 291, 20729–20738
8-10. Nitric oxide synthase
Blair, A., Shaul, P.W., Yuhanna, I.S., Conrad, P.A., et al (1999) Oxidized low density lipoprotein displaces
endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation J. Biol
Chem., 274, 32512-32519 (1999)
Joshi, M.S., Mineo, C., Shaul, P.W. and Bauer, J.A. (2007) Biochemical consequences of the NOS3 Glu298Asp
variation in human endothelium: altered caveolar localization and impaired response to shear FASEB J., 21,
2655-2663
Kincer, J.F., Uittenbogaard, A., Dressman, J., Guerin, T. M., et al (2002) Hypercholesterolemia promotes a
CD36-dependent and endothelial nitric oxide synthase mediated vascular dysfunction J. Biol. Chem., 277,
23525-23533
Peterson, T.E., Poppa, V., Ueba, H., Wu, A., et al (1999) Opposing effects of reactive oxygen species and
cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. Circ. Res., 85, 29-37
Peterson, T.E., d’Uscio, L.V., Cao, S., Wang, X-L., et al (2009) Guanosine triphosphate cyclohydrolase I
expression and enzymatic activity are present in caveolae of endothelial cells Hypertension, 53, 189-195
Shaul, P.W., Smart, E.J., Robinson, L.J., German, Z., et al (1996) Acylation targets endothelial nitric-oxide
synthase to plasmalemmal caveolae J. Biol. Chem., 271, 6518-6522 (1996)
Uittenbogaard, A., Shaul, P.W., Yuhanna, I.S., Blair, A. et al (2000) High density lipoprotein prevents
oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and
activation in caveolae J. Biol. Chem., 275, 11278-11283
8-11. Vascular barrier/integrity
Birukova, A.A., Singleton, P.A., Gawlak, G., Tian, X., et al (2014) GRP78 is a novel receptor initiating a
vascular barrier protective response to oxidized phospholipids Mol. Biol. Cell, 25, 2006-2016
David, M.A., Jones, D.R. and Tayebi, M. (2014) Potential candidate camelid antibodies for the treatment of
protein-misfolding diseases J. Neuroimmunol., 272, 76–85
Heemskerk, N., Asimuddin, M., Oort, C., van Rijssel, J. and van Buul, J.D. (2016) Annexin A2lLimits
neutrophil transendothelial migration by organizing the spatial distribution of ICAM-1 J. Immunol., 196, 2767–
2778
8-12. VEGF Receptors
Galvagni, F., Anselmi, F., Salameh, A., Orlandini, M., et al (2007) Vascular endothelial growth factor
receptor-3 activity is modulated by its association with caveolin-1 on endothelial membrane Biochemistry, 46,
3998-4005
Ikeda, S., Ushio-Fukai, M., Zuo, L., Tojo, T., et al (2005) Novel role of ARF6 in vascular endothelial growth
factor-induced signaling and angiogenesis Circ. Res., 96, 467-475
Labrecque, L., Royal, I., Surprenant, D.S., Patterson, C., et al (2003) Regulation of vascular endothelial growth
factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol Mol. Biol. Cell, 14, 334-347
9. Endothelial progenitor cells
Chilla, A., Magherini, F., Margheri, F., Laurenzana, A., et al (2013) Proteomic identification of VEGFdependent protein enrichment to membrane caveolar-raft microdomains in endothelial progenitor cells. Mol.
Cell. Proteom., 12, 1926-1938
Margheri, F., Chilla, A., Laurenzana, A., Serratì, S., et al (2011) Endothelial progenitor cell–dependent
angiogenesis requires localization of the full-length form of uPAR in caveolae Blood, 118, 3743-3755
10. Epithelial cells
Bolander Jr., F.F. (2005) The compartmentalization of prolactin signaling in the mouse mammary gland Mol.
Cell. Endocrinol., 245, 105-110
Bradbury, N.A., Clark, J.A., Watkins, S.C., Widnell, C.C., et al (1999) Characterization of the internalization
pathways for the cystic fibrosis transmembrane conductance regulator Am. J. Physiol. Lung Cell. Mol.
Physiol., 276, L659-L668
Briand, O., Lestavel, S., Pilon, A., Torpier, G., et al (2003) SR-BI does not require raft/caveola localization for
cholesteryl ester selective uptake in the human adrenal cell line NCI-H295R Biochim. Biophys. Acta, 163, 42-
50
Chen, J., Chen, J-K. and Harris, R.C. (2012) Angiotensin II induces epithelial-to-mesenchymal transition in
renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth
factor receptor–extracellular signal-regulated kinase signaling pathway Mol. Cell. Biol., 32, 981–991
Delmas, O., Breton, M., Sapin, C., Le Bivic, A., et al (2007) Heterogeneity of raft-type membrane micrdomains
associated with VP4, the rotavirus spike protein, in Caco-2 and MA 104 cells J. Virol., 81, 1610-1618
Heberden, C., Reine, F., Grosse, B., Henry, C., et al (2006) Detection of a raft-located estrogen receptor-like
protein distinct from ER Int. J. Biochem. Cell Biol., 38, 376-391
Huang, C., Hepler, J.R., Chen, L.T., Gilman, A.G., et al (1997) Organization of G proteins and adenylyl
cyclase at the plasma membrane Mol. Biol. Cell, 8, 2365-2378
Kifor, O., Diaz, R., Butters, R., Kifor, I., et al (1998) The calcium-sensing receptor is localized in caveolin-rich
plasma membrane domains of bovine parathyroid cells J. Biol. Chem., 273, 1708-21713
Lalor, D., Liu, P. and Hayashi, J. (2004) Fas ligand is enriched in the caveolae membrane domains of thymic
epithelial cells Cell. Immunol., 230, 10-16
McMahon, K-A., Zhu, M., Kwon, S.W., Liu, P., et al (2006) Detergent-free caveolae proteome suggests an
interaction with ER and mitochondria Proteomics, 6, 143-152
Norman, A.W., Olivera, C.J., Silva, F.R.M.B. and Bishop, J.E. (2002) A specific binding protein/receptor for
1,25-dihydroxyvitamin D3 is present in an intestinal caveolae membrane fraction Biochem. Biophys. Res.
Commun., 298, 414-419
Pike, L.J., Han, X., Chung, K-N and Gross, R.W. (2002) Lipid rafts are enriched in arachidonic acid and
plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative
electrospray ionization/mass spectrometric analysis Biochemistry, 41, 2075-2088
Smart, E.J., Mineo, C. and Anderson, R.G.W. (1996) Clustered folate receptors deliver 5-methyltetrahydrofolate to cytoplasm of MA104 cells J. Cell Biol., 134, 1169-1177
Subramanian, P.S. and Johnson, H.M. (2002) Lipid microdomains are required sites for the selective
endocytosis and nuclear translocation of IFN-, its receptor chain IFN- receptor-1, and phosphorylation and
nuclear translocation of STAT1 J. Immunol, 169, 1959-1969
11. Fibroblasts
11-1. Cholesterol
Dufour, D., Zhao, W-Q., Ravindranath, L. and Alkon, D.L. (2003) Abnormal cholesterol processing in
Alzheimer’s disease patient’s fibroblasts Neurobiol. Lipids., 1, 34-44
Furuchi, T. and Anderson, R.G.W. (1998) Cholesterol depletion of caveolae causes hyperactivation of
extracellular signal-related kinase (ERK) J. Biol. Chem., 273, 21009-21104
Gallegos, A.M., McIntosh, A.L., Atshaves, B.P. and Schroeder, F. (2004) Structure and cholesterol domain
dynamics of an enriched caveolae/raft isolate Biochem. J., 382, 451-461
Laitinen, S., Lehto, M., Lehtonen, S., Hyvarinen, K., et al (2002) ORP2, a homolog of oxysterol binding
protein, regulates cellular cholesterol metabolism J. Lipid Res., 43, 245-255
Landry, Y.D., Denis, M., Nandi, S., Bell, S., et al (2006) ATP-binding cassette transporter A1 expression
disrupts raft membrane microdomains through its ATPase-related functions J. Biol. Chem., 281, 36091-36101
Lu, X., Kambe, F., Cao, X., Yoshida, T., et al (2006) DHCR24-Knockout embryonic fibroblasts are susceptible
to serum withdrawal-induced apoptosis because of dysfunction of caveolae and insulin-Akt-Bad signaling
Endocrinology, 147, 3123-3132
Matthews, L.C., Taggart, M.J. and Westwood, M. (2005) Effect of cholesterol depletion on mitogenesis and
survival: the role of caveolar and noncaveolar domains in insulin-like growth factor-mediated cellular function
Endocrinology, 146, 5463-5473
Smart, E.J., Ying, Ys., Donzell, W.C. and Anderson, R.G.W. (1996) A role for caveolin in transport of
cholesterol from endoplasmic reticulum to plasma membrane J. Biol. Chem., 271, 29427-29435
Uittenbogaard, A., Ying, Y.S. and Smart, E.J. (1998) Characterization of a cytosolic heat-shock proteincaveolin chaperone complex J. Biol. Chem., 273, 6525-6532
Yin, Y., Liu, P., Anderson, R.G.W. and Sampson, N.S. (2002) Construction of a catalytically inactive
cholesterol oxidase mutant: inverstigation of the interplay between active site-residues glutamate 361 and
histidine 447 Arch. Biochem. Biophys., 402, 235-242
11-2. Composition and structure
Landry, Y.D., Denis, M., Nandi, S., Bell, S., et al (2006) ATP-binding cassette transporter A1 expression
disrupts raft membrane microdomains through its ATPase-related functions J. Biol. Chem., 281, 36091-36101
Marks, D.L., Bittman, R. and Pagano, R.E. (2008) Use of Bodipy-labeled sphingolipid and cholesterol analogs
to examine membrane microdomains in cells Histochem. Cell. Biol., 130, 819-832
McMahon, K-A., Zhu, M., Kwon, S.W., Liu, P., et al (2006) Detergent-free caveolae proteome suggests an
interaction with ER and mitochondria Proteomics, 6, 143-152
Smart, E.J., Ying, Y-S, Mineo, C. and Anderson, R.G.W. (1995) A detergent-free method for purifying
caveolae membrane from tissue culture cells Proc. Natl. Acad. Sci. USA, 92, 10104-10108
Westermann, M., Leutbecher, H. and Meyer, H.W. (1999) Membrane structure of caveolae and isolated
caveolin-rich vesicles Histochem. Cell Biol., 111, 71-81
11-3. Glycosphingolipids
Kim, S-Y., Wang, T-k., Singh, R.D., Wheatley, C.L., Marks, D.L. and Pagano, R.E. (2009) Proteomic
identification of proteins translocated to membrane microdomains upon treatment of fibroblasts with the
glycosphingolipid, C8-β-D-lactosylceramide Proteomics, 9, 4321-4328
11-4. Growth factor receptors (see also “Protein targeting and activation” and “Signal transdiction”)
Boucher, P., P., Liu, P., Gotthardt, M., Hiesberger, T., et al (2002) Platelet-derived growth factor mediates
tyrosine phosphorylation of the cytoplasmic domain of the low density lipoprotein receptor-related protein in
caveolae J. Biol. Chem., 277, 15507-15513
Liu, P., Ying, Y., Ko, Y.G and Anderson, R.G.W. (1996) Localization of platelet-derived growth factorstimulated phosphorylation cascade to caveolae J. Biol. Chem., 271, 10299-10303
Liu, P., Ying, Y-S., and Anderson. R.G.W. (1997) Platelet-derived growth factor activates mitogen-activated
protein kinase in isolated caveolae Proc. Natl. Acad. Sci., USA, 94, 13666-13670
Liu, P. and Anderson, R.G.W. (1999) Spatial organization of EGF receptor transmodulation by PDGF
Biochem. Biophys. Res. Commun., 261, 695-700
Matthews, L.C., Taggart, M.J. and Westwood, M. (2005) Effect of cholesterol depletion on mitogenesis and
survival: the role of caveolar and noncaveolar domains in insulin-like growth factor-mediated cellular function
Endocrinology, 146, 5463-5473
Matveev, S.V. and Smart, E.J. (2002) Heterologous desensitization of EGF receptors and PDGF receptors by
sequestration in caveolae Am. J. Physiol Cell Physiol, 282, C935-C946
Mineo, C., James, G.L., Smart, E.J. and Anderson, R.G.W. (1996) Localization of epidermal growth
factor-stimulated Ras/Raf-1 interaction to caveolae membrane J. Biol. Chem., 271, 11930-11935
Mineo, C., Gill, G.N. and Anderson, R.G.W. (1999) Regulated migration of epidermal growth factor receptor
from caveolae J. Biol. Chem., 274, 30636-30643
Yamabhai, M. and Anderson, R.G.W. (2002) Second cysteine-rich region of epidermal growth factor receptor
contains targeting information for caveolae/rafts J. Biol. Chem., 277, 24843-24846
Yang, N., Huang, Y., Jiang, J. and Frank, S.J. (2004) Caveolar and lipid raft localization of the growth
hormone receptor ans its signaling elements J. Biol. Chem., 279 20898-20905
11-5. Lipoproteins
Boucher, P., P., Liu, P., Gotthardt, M., Hiesberger, T., et al (2002) Platelet-derived growth factor mediates
tyrosine phosphorylation of the cytoplasmic domain of the low density lipoprotein receptor-related protein in
caveolae J. Biol. Chem., 277, 15507-15513
Nion, S., Briand, O., Lestavel, S., Torpier, G., et al (1997) High-density-lipoprotein subfraction 3 interaction
with glycosylphosphatidyl-inositol-anchored proteins Biochem. J., 328, 415-423
11-6. Protein targeting and activation
Li, W-P., Liu, P., Pilcher, B.K. and Anderson, R.G.W. (2001) Cell-specific targeting of caveolin-1 to caveolae,
secretory vesicles, cytoplasm or mitochondria J. Cell Sci., 114, 1397-1408
Michaely, P.A., Mineo, C. Ying, Y-S. and Anderson, R.G.W. (1999) Polarized distribution of endogenous Racl
and RhoA at the cell surface J. Biol. Chem., 274, 21430-21436
Mineo, C., James, G.L., Smart, E.J. and Anderson, R.G.W. (1996) Localization of epidermal growth
factor-stimulated Ras/Raf-1 interaction to caveolae membrane J. Biol. Chem., 271, 11930-11935
Mineo, C., Anderson, R.G.W. and White, M.A. (1997) Physical association with Ras enhances activation of
membrane-bound Raf (RafCAAX) J. Biol. Chem., 272, 10345-10348
Shu, L., Lee, L., Chang, Y., Holzman, L.B., et al (2000) Caveolar structure and protein sorting are maintained
in NIH cells independent of glycosphingolipid depletion Arch. Biochem. Biophys., 373, 83-90
Uittenbogaard, A., Ying, Y.S. and Smart, E.J. (1998) Characterization of a cytosolic heat-shock proteincaveolin chaperone complex J. Biol. Chem., 273, 6525-6532
11-7. Signal transduction
Bilderback, T.R., Gazula, V-R., Lisanti, M.P. and Dobrowsky, R.T. (1999) Caveolin interacts with Trk A and
p75NTR and regulates neurotrophin signaling pathways J. Biol. Chem., 274, 257-263
Chen, J., Doroudi, M., Cheung, J., Grozier, A.L., et al (2013) Plasma membrane Pdia3 and VDR interact to
elicit rapid responses to 1α,25(OH)2D3 Cell. Signal., 25, 2362–2373
Huang, C., Hepler, J.R., Chen, L.T., Gilman, et al (1997) Organization of G proteins and adenylyl cyclase at
the plasma membrane Mol. Biol. Cell, 8, 2365-2378
Liu, P., Wang, P-y., Michaely, P., Zhu, M., et al (2000) Presence of oxidized cholesterol in caveolae uncouples
active platelet-derived growth factor receptors from tyrosine kinase substrates J. Biol. Chem., 275, 31648-
31654
Lu, X., Kambe, F., Cao, X., Yoshida, T., et al (2006) DHCR24-Knockout embryonic fibroblasts are susceptible
to serum withdrawal-induced apoptosis because of dysfunction of caveolae and insulin-Akt-Bad signaling
Endocrinology, 147, 3123-3132
Mineo, C., Ying, Y-S, Chapline, C., Jaken, S., et al (1998) Targeting of protein kinase C to caveolae J. Cell
Biol., 141, 601-610
Yin, Y., Liu, P., Anderson, R.G.W. and Sampson, N.S. (2002) Construction of a catalytically inactive
cholesterol oxidase mutant: inverstigation of the interplay between active site-residues glutamate 361 and
histidine 447 Arch. Biochem. Biophys., 402, 235-242
12. HEK cells
Balijepalli, R.C., Delisle, B.P., Balijepalli, S.Y., Foell, J.D., et al (2007) Kv11.1 (ERH1) K+
channels localize in
cholesterol and sphingolipid enriched membranes and are modulated by cholesterol Channels, 1, 263-272
Fortin, J-P., Rivard, G.E., Adam, A. and Marceau, F. (2005) Studies on rabbit natural and recombinant tissue
factors: intracellular retention and regulation of surface expression in cultured cells Am. J. Physiol., 288,
H2192-H2202
Ikezu, T., Trapp, B.D., Song, K.S., Schlegel, A., et al (1998) Caveolae, plasma membrane microdomains for -
secretase-mediated processing of the amyloid precursor protein J. Biol. Chem., 273, 10485-10495
Lamb, M.E., de Weerd, W.F.C. and Leeb-Lundberg, L.M.F. (2001) Agonist-promoted trafficking of human
bradykinin receptors: arrestin- and dynamin-independent sequestration of the B2 receptor and bradykinin in
HEK293 cells Biochem. J., 355, 741-750
Lamb, M.E., Zhang, C., Shea, T., Kyle, D.J., et al (2002) Human B1 and B2 bradykinin receptors and their
agonists target caveolae-related lipid rafts to different degrees in HEK293 cells Biochemistry, 41, 14340-14347
Sabourin, T., Bastien, L., Bachvarov, D.R., and Marceau, F. (2002) Agonist-induced translocation of the kinin
B1 receptor to caveolae-related rafts Mol. Pharmacol., 61, 546-553
Sorci-Thomas, M.G., Owen, J.S., Fulp, B., Bhat, S., Zhu, X., Parks, J.S., Shah, D., Jerome, W.G., Gerelus, M.,
Zabalawi, M. and Thomas, M.J. (2012) Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts
and are structurally organized by three apoA-I monomers J. Lipid Res., 53, 1890–1909
13. Hepatoma cells
Burger, H-M., Abela, S. and Gelderblom, W.C.A. (2018) Modulation of key lipid raft constituents in primary
rat hepatocytes by fumonisin B1 – Implications for cancer promotion in the liver Food Chem. Toxicol., 115, 34–
41
Smith, R.M., Harada, S., Smith, J.A., Zhang, S., et al (1998) Insulin-induced protein tyrosine phosphorylation
cascade and signalling molecules are localized in a caveolin-enriched cell membrane domain Cell. Signalling,
10, 355-362
Truong, T.Q., Aubin, D., Bourgeois, P., Falstrault, L., et al (2006) Opposite effect of caveolin-1 in the
metabolism of high-density and low-density lipoproteins Biochim. Biophys. Acta, 1761, 24-36
Yanase, K. and Madaio, M.P. (2005) Nuclear localization anti-DNA antibodies enter cells via caveoli and
modulate expression of caveolin and p53 J. Autoimmun., 24, 145-151
13a. Human mammary epithelial cells
Zhuo, D. and Guan, F. (2019) Ganglioside GM1 promotes contact inhibition of growth by regulating the
localization of epidermal growth factor receptor from glycosphingolipid‐enriched microdomain to caveolae
Cell Prolif. 52: e12639
14. Intestine
Huhtakangas, J.A., Olivera, C.J., Bishop, J.E., Zanello, L.P., et al (2004) The vitamin D receptor is present in
caveolae-enriched plasma membranes and binds 1,25(OH)2-vitamin D3
in vivo and in vitro Mol. Endocrinol.,
18, 2660-2671
15. Kidney
Chen, J., Chen, J-K. and Harris, R.C. (2012) Angiotensin II induces epithelial-to-mesenchymal transition in
renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth
factor receptor–extracellular signal-regulated kinase signaling pathway Mol. Cell. Biol., 32, 981–991
Hill, W.G., Butterworth, M.B., Wang, H. Edinger, R.S., et al (2007) The epithelial sodium channel (ENaC)
traffics to apical membrane in lipid rafts in mouse cortical collecting duct cells J. Biol. Chem., 282, 37402-
37411
Huhtakangas, J.A., Olivera, C.J., Bishop, J.E., Zanello, L.P et al (2004) The vitamin D receptor is present in
caveolae-enriched plasma membranes and binds 1,25(OH)2-vitamin D3 in vivo and in vitro Mol. Endocrinol.,
18, 2660-2671
Yosef, E., Katz, A., Peleg, Y., Mehlman, T. and Karlish, S.J.D. (2016) Do Src kinase and caveolin interact
directly with Na,K-ATPase? J. Biol. Chem., 291, 11736–11750
16. Liver (fish)
Zehmer, J.K. and Hazel, J.R. (2004) Membrane order conservation in raft and non-raft regions of hepatocyte
plasma membranes from thermally acclimated rainbow trout Biochim, Biophys. Acta., 1664, 108-116
Zehmer, J.K. and Hazel, J.R. (2005) Thermally induced changes in lipid composition of raft and non-raft
regions of hepatocyte plasma membranes of rainbow trout J. Exp. Biol., 208, 4283-4290
17. Liver (rodent)
Huhtakangas, J.A., Olivera, C.J., Bishop, J.E., Zanello, L.P. et al (2004) The vitamin D receptor is present in
caveolae-enriched plasma membranes and binds 1,25(OH)2-vitamin D3 in vivo and in vitro Mol. Endocrinol.,
18, 2660-2671
Wang, Y., Posner, B.I. and Balbis, A. (2009) Compartmentalization of epidermal growth factor receptor in
liver plasma membrane J. Cell. Biochem., 107, 96-103
18. Lung
Hill, W.G., Almasri, E., Ruiz, W.G., Apodaca, G., et al (2005) Water and solute permeability of rat lung
caveolae: high permeabilities explained by acyl chain unsaturation Am. J. Physiol., 289, C33-C41
Huhtakangas, J.A., Olivera, C.J., Bishop, J.E., Zanello, L.P., et al (2004) The vitamin D receptor is present in
caveolae-enriched plasma membranes and binds 1,25(OH)2-vitamin D3
in vivo and in vitro Mol. Endocrinol.,
18, 2660-2671
Oh, P. and Schnitzer, J.E. (1999) Immunoisolation of caveolae with high affinity antibody binding to the
oligomeric caveolin cage J. Biol. Chem, 274, 23144-23154
Oh, P. and Schnitzer, J.E. (2001) Segregation of heterotrimeric G proteins in cell surface microdomains Mol.
Biol. Cell, 12, 685-698
19. Lymphoid/monocytic cells
Huhtakangas, J.A., Olivera, C.J., Bishop, J.E., Zanello, L.P., et al (2004) The vitamin D receptor is present in
caveolae-enriched plasma membranes and binds 1,25(OH)2-vitamin D3
in vivo and in vitro Mol. Endocrinol.,
18, 2660-2671
Matveev, S., van der Westhuyzen, D.R. and Smart, E.J. (1999) Co-expression of scavenger receptor-BI and
caveolin-1 is associated with enhanced selective cholesterol ester uptake in THP-1 macrophages J. Lipid Res.,
40, 1647-1654
McMahon, K-A., Zhu, M., Kwon, S.W., Liu, P., et al (2006) Detergent-free caveolae proteome suggests an
interaction with ER and mitochondria Proteomics, 6, 143-152
Poussin, C., Foti, M., Carpentier, J.L. and Pugin, J. (1998) CD14-dependent endotoxin internalization via a
macropinocytic pathway J. Biol. Chem., 273, 20285-20291
Uittenbogaard, A. and Smart, E.J. (2000) Palmitoylation of caveolin-1 is required for cholesterol binding,
chaperone complex formation, and rapid transport of cholesterol to caveolae J. Biol. Chem., 275, 25595-25599
20. Macrophages
Sorci-Thomas, M.G., Owen, J.S., Fulp, B., Bhat, S., et al (2012) Nascent high density lipoproteins formed by
ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers J. Lipid Res., 53, 1890–
1909
Zhu, X., Owen, J.S., Wilson, M.D., Li, H., et al (2010) Macrophage ABCA1 reduces MyD88-dependent Tolllike receptor trafficking to lipid rafts by reduction of lipid raft cholesterol J. Lipid Res., 51, 3196–3206
21. MDCK (and other kidney cells)
Gallegos, A.M., Storey, S.M., Kier, A.B., Schroeder, F., et al (2006) Structure and cholesterol dynamics of
caveolae/raft and nonraft plasma membrane domains Biochemistry, 45, 12100-12116
Heller, B., Adu-Gyamfi, E., Smith-Kinnaman, W., Babbey, C., et al (2010) Amot recognizes a juxtanuclear
endocytic recycling compartment via a novel lipid binding domain J. Biol. Chem., 285, 12308-12320
Huang, C., Hepler, J.R., Chen, L.T., Gilman, A.G., et al (1997) Organization of G proteins and adenylyl
cyclase at the plasma membrane Mol. Biol. Cell, 8, 2365-2378
Huang, C., Duncan, J.A., Gilman, A.G. and Mumby, S.M (1999) Persistent membrane association of activated
and depalmitoylated G protein subunits Proc. Natl. Acad. Sci. USA, 96, 412-417
Ikezu, T., Trapp, B.D., Song, K.S., Schlegel, A., Lisanti, M.P. and Okamoto, T. (1998) Caveolae, plasma
membrane microdomains for -secretase-mediated processing of the amyloid precursor protein J. Biol. Chem.,
273, 10485-10495
McMahon, K-A., Zhu, M., Kwon, S.W., Liu, P., et al (2006) Detergent-free caveolae proteome suggests an
interaction with ER and mitochondria Proteomics, 6, 143-152
Nashiki, K., Taketani, Y., Takeichi, T., Sawada, N., et al (2005) Role of membrane microdomains in PTHmediated down-regulation of NaPi-Iia in opossum kidney cells Kidney Int., 68, 1137-1147
22. Neural tissue and cells
Abulrob, A., Giuseppin, S., Andrade, M.F., McDermid, A., et al (2004) Interactions of EGFR and caveolin-1 in
human glioblastoma cells: evidence that tyrosine phosphorylation regulates EGFR association with caveolae
Oncogene, 23, 6967-6979
Brouillett, E., Trembleau, A., Galanaud, D., Volovitch, M., et al (1999) The amyloid precursor protein
interacts with Go heterotrimeric protein within a cell compartment specialized in signal transduction J.
Neurosci., 19, 1717-1727
Cameron, P.L., Ruffin, J.W., Bollag, R., Rasmussen, H., et al (1997) Identification of caveolin and caveolinrelated proteins in the brain J. Neurosci., 17, 9520-9535
Karouzaki, S., Peta, C., Tsirimonaki, E. and Mangoura, D. (2019) PKCε-dependent H-Ras activation
encompasses the recruitment of the RasGEF SOS1 and of the RasGAP neurofibromin in the lipid rafts of
embryonic neurons Neurochem. Int., 131: 104582
Kelly, J.F., Storie, K., Skamra, C., Bienias, J., et al (2005) Relationship between Alzheimer’s disease clinical
stage and Gq/11 in subcellular fractions of frontal cortex J. Neural Transm., 112, 1049-1056
Kumanogoh, H., Miyata, S., Sokawa, Y. and Maekawa, S. (2001) Biochemical and morphological analysis on
the localization of Rac1 in neurons Neurosci. Res., 39, 189-196
Langelier, B., Linard, A., Bordat, C., Lavialle, M., et al (2010) Long chain-polyunsaturated fatty acids
modulate membrane phospholipid composition and protein localization in lipid rafts of neural stem cell cultures
J. Cell. Biochem., 110, 1356–1364
Nishiyama, K., Trapp, B.D., Ikezu, T., Ransohoff, R.M., et al (1999) Caveolin-3 upregulation activates -
secretase-mediated cleavage of the amyloid precursor protein in Alzheimer’s disease J. Neurosci., 19, 6538-
6548
Ren, Q. and Bennett, V. (1998) Palmitoylation of neurofascin at a site in the membrane-spanning domain
highly conserved among the L1 family of cell adhesion molecules J. Neurochem., 70, 1839-1849
Rimmerman, N., Hughes, H.V., Bradshaw, H.B., Pazos, M.X., et al (2008) Compartmentalization of
endocannabinoids into lipid rafts in a dorsal root ganglion cell line Br. J. Pharmacol., 153, 380–389
Tome, M.E., Schaefer, C.P., Jacobs, L.M., Zhang, Y., Hemdon, J.M., Matty, F.O. and Davis, T.P. (2015)
Identification of P- glycoprotein co-fractionating proteins and specific binding partners in rat brain
microvessels J. Neurochem., 134, 200-210
Toran-Allerand, C.D., Guan, X., MacLusky, N.J., Horvath, T.L., et al (2002) ER-X: A novel, plasma
membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain
injury J. Neurosci., 22, 8391-8401
Vey, M., Pilkuhn, S., Wille, H., Nixon, R., et al (1996) Subcellular colocalization of the cellular and scrapie
prion proteins in caveolae-like membranous domains Proc. Natl. Acad. Sci. USA, 93, 14945-14949
Wu, C., Butz, S., Ying, Y-S and Anderson, R.G.W. (1997) Tyrosine kinase receptors concentrated in caveolaelike domains from neuronal plasma membrane J. Biol. Chem., 272, 3554-3559
Zhai, J., Ström, A.L., Kilty, R., Venkatakrishnan, P., et al (2009) Proteomic characterization of lipid raft
proteins in amyotrophic lateral sclerosis mouse spinal cord FEBS J., 276, 3308-3323
23. Ocular photoreceptor membrane
McClellan, M.E. and Elliott, M.H. (2017) Analysis of fatty acid and cholesterol content from detergentresistant and detergent-free membrane microdomains In Lipidomics: Methods and Protocols: Methods Mol.
Biol., 1609 (ed. Bhattacharya, S.K.) Springer Science+Business Media, LLC, pp 185-194
24. Osteoblasts/osteoclasts
Chen, J., Olivares-Navarrete, R., Wang, Y., Herman, T.R., et al (2010) Protein-disulfide isomerase-associated
3 (Pdia3) mediates the membrane response to 1,25-dihydroxyvitamin D3 in osteoblasts J. Biol. Chem., 285,
37041–37050
Chen, J., Doroudi, M., Cheung, J., Grozier, A.L., et al (2013) Plasma membrane Pdia3 and VDR interact to
elicit rapid responses to 1α,25(OH)2D3 Cell. Signal., 25, 2362–2373
Doroudi, M., Schwartz, Z., and Boyan, B.D. (2012) Phospholipase A2 activating protein is required for 1,25-
dihydroxyvitamin D3 dependent rapid activation of protein kinase C via Pdia3 J. Steroid Biochem. Mol. Biol.,
132, 48– 56
Doroudi, M., Olivares-Navarrete, R., Hyzy, S.L., Boyan, B.D. amd Schwartz, Z. (2014) Signaling components
of the 1α,25(OH)2D3-dependent Pdia3 receptor complex are required for Wnt5a calcium-dependent signaling
Biochim. Biophys. Acta, 1843, 2365–2375
Doroudi, M., Plaisance, M.C., Boyan, B.D. and Schwartz, Z. (2015) Membrane actions of 1,25(OH)2D3 are
mediated by Ca2+/calmodulin-dependent protein kinase II in bone and cartilage cells J. Steroid Biochem. Mol.
Biol., 145, 65–74
25. Platelets
Silvagno, F., De Vivo, E., Attanasio, A., Gallo, V., et al (2010) Mitochondrial localization of vitamin D
receptor in human platelets and differentiated megakaryocytes PLoS One 5: e8760
26. Pre-adipocytes
Wang, X., Yang, N., Deng, L., Li, X., et al (2009) Interruption of growth hormone signaling via SHC and ERK
in 3T3-F442A preadipocytes upon knockdown of insulin receptor substrate-1 Mol. Endocrinol., 23, 486–496
Renal cells/tissue see “15. Kidney”
27. Smooth muscle and smooth muscle cells
27-1. Airway
Grim, K.J., Abcejo, A.J., Barnes, A., Sathish, V., Smelter, D.F., Ford, G.C., Thompson, M.A., Prakash, Y.S.
and Pabelick, C.M. (2012) Caveolae and propofol effects on airway smooth muscle Br. J. Anaesth., 109, 444–53
Prakash, Y.S., Thompson, M.A., Vaa, B., Matabdin, I., et al (2007) Caveolins and intracellular calcium
regulation in human airway smooth muscle Am. J. Physiol. Lung Cell. Mol. Physiol., 293, L1118-L1126
Sathish, V., Yang, B., Meuchel, L.W., Van Oosten, S.K., et al (2011) Caveolin-1 and force regulation in
porcine airway smooth muscle Am. J. Physiol. Lung Cell. Mol. Physiol., 300, L920–L929
Sathish, V., Abcejo, A.J., VanOosten, S.K., Thompson, M.A., et al (2011) Caveolin-1 in cytokine-induced
enhancement of intracellular Ca2+ in human airway smooth muscle Am. J. Physiol. Lung Cell. Mol. Physiol.,
301, L607–L614
Sathish, V., Thompson, M.A., Sinha, S., Sieck, G.C., et al (2014) Inflammation, caveolae and CD38-mediated
calcium regulation in human airway smooth muscle Biochim. Biophys. Acta, 1843, 346–351
27-2. Cardiac myocytes
Doyle, D.D., Goings, G., Upshaw-Earley, J., Ambler, S.K., et al (2000) Dystrophin associates with a caveolae
of rat cardiac myocytes Circ. Res., 87, 480-488
Lasley, R.D., Narayan, P., Uittenbogaard, A. and Smart, E.J. (2000) Activated cardiac adenosine A1 receptors
translocate out of caveolae J. Biol. Chem., 275, 4417-4421
Liu, L., Mohammadi, K., Aynafshar, B., Wang, H., et al (2003) Role of caveolae in signal-tranducing function
of cardiac Na+
/K+
-ATPase Am. J. Physiol. Cell Physiol., 284, C1550-C1560
Rybin, V.O., Xu, X., Lisanti, M.P. and Steinberg, S.F. (2000) Differential targeting of -adrenergic receptor
subtypes and adenyl cyclase to cardiomyocyte caveolae: A mechanism to functionally regulate the cAMP
signaling pathway J. Biol. Chem., 275, 41447-41457
27-3. Gall bladder
Cong, P., Pricolo, V., Biancani, P. and Behar, J. (2010) Effects of cholesterol on CCK-1 receptors and caveolin3 proteins recycling in human gallbladder muscle Am. J. Physiol. Gastrointest. Liver Physiol., 299, G742–G750
Xiao, Z., Schmitz, F., Pricolo, V.E., Biancani, P., et al (2007) Role of caveolae in the pathogenesis of
cholesterol-induced gall bladder muscle hypomotility Am. J. Physiol. Gastrointest. Liver Physiol., 292, G1641-
G1649
27-4. MF-2 cells
De Weerd, W.F.C. and Leeb-Lundberg, L.M.F. (1997) Bradykinin sequesters B2 bradykinin receptors and the
receptor-coupled G subunits Gq and Gi in caveolae in DDT1 MF-2 smooth muscle cells J. Biol. Chem., 272,
17858-17866
27-5. Vascular (primarily pulmonary artery)
Angiotensin receptors
Zuo, L., Ushio-Fukai, M., Hilenski, L.L. and Alexander, R.W. (2004) Microtubules regulate angiotensin II type
1 receptor and Rac1 localization in caveolae/lipid rafts Arterioscler. Thromb. Vasc. Biol., 24, 1223-1228
Zuo, L., Ushio-Fukai, M., Ikeda, S., Hilenski, L., et al (2005) Caveolin-1 is essential for activation of Rac1 and
NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells Arterioscler.
Thromb. Vasc. Biol., 25, 1824-1830
27-6. Growth factor receptors
Ashino, T., Sudhahar, V., Urao, N., Oshikawa, J., et al (2010) Unexpected role of the copper transporter
ATP7A in PDGF-induced vascular smooth muscle cell migration Circ. Res., 107, 787-799
Bousserouel, S., Raymondjean, M., Brouillet, A., Bereziat, G., et al (2004) Modulation of cyclin D1 and early
growth response factor-1 gene expression in interleukin-1-treated rat smooth muscle cells by n-6 and n-3
polyunsaturated fatty acids Eur. J. Biochem., 271, 4462-4473
27-7. Lipoprotein receptor
Von Arnim, C.A.F., Kinoshita, A., Peltan, I.D., Tangredi, M.M., et al (2005) The low density lipoprotein
receptor-related protein (LRP) is a novel -secretase (BACE1) substrate J. Biol. Chem., 280, 17777-17785
Na+
/K+
-ATPase and Ca2+ regulation
Ghosh, B., Kar, P., Mandal, A., Dey, K., et al (2009) Ca2+ influx mechanisms in caveolae vesicles of pulmonary
smooth muscle plasma membrane under inhibition of α2β1 isozyme of Na+
/K+
-ATPase by ouabain Life Sci., 84,
39–148
Ghosh, B., Chakraborti, T., Kar, P., Dey, K., et al (2009) Solubilization, purification, and reconstitution of a2b1
isozyme of Na+
/K+
-ATPase from caveolae of pulmonary smooth muscle plasma membrane: comparative studies
with DHPC, C12E8, and Triton X-100 Mol. Cell. Biochem., 323, 169–184
Shaikh, S., Samanta, K., Kar, P., Roy, S., et al (2010) m-Calpain-mediated cleavage of Na+
/Ca2+ exchanger-1
in caveolae vesicles isolated from pulmonary artery smooth muscle Mol. Cell. Biochem., 341, 167–180
27-8. Signaling pathways (signalosomes)
Gabrielová, E., Bartošíková, L., Nečas, J. and Modrianský, M. (2019) Cardioprotective effect of 2,3-
dehydrosilybin preconditioning in isolated rat heart Fitoterapia 132, (2019) 12–21
Hilenski, L.L., Clempus, R.E., Quinn, M.T., Lambeth, J.D. and Griendling, K.K. (2004) Distinct subcellular
localizations of Nox1 and Nox4 in vascular smooth muscle cells Arterioscler. Thromb. Vasc. Biol., 24, 1-8
27-9. Tissue factors
Fortin, J-P., Rivard, G.E., Adam, A. and Marceau, F. (2005) Studies on rabbit natural and recombinant tissue
factors: intracellular retention and regulation of surface expression in cultured cells Am. J. Physiol., 288,
H2192-H2202
27-10. Uterine
Kiss, A.L., Turi, A., Mullner, N., Kovacs, E., et al (2005) Oestrogen-mediated tyrosine phosphorylation of
caveolin-1 and its effect on the oestrogen receptor localization: an in vivo study Mol. Cell. Endocrinol., 245,
128-137
28. Thyroid
Lin, C-I., Barletta, J.A., Nehs, M.A., Morris, Z.S., et al (2011) Thyroid-specific knockout of the tumor
suppressor mitogen-inducible gene 6 activates epidermal growth factor receptor signaling pathways and
suppresses nuclear factor-B activity Surgery, 150, 1295-302
29. Trypanosomes
Sharma, A.I., Olson, C.L., Mamede, J.I., Gazos-Lopes, F., Epting, C.L., Almeida, I.C. and Engman, D.M.
(2017) Sterol targeting drugs reveal life cycle stage-specific differences in trypanosome lipid rafts Sci. Rep., 7:
9105
30. Review articles
Balbis, A. and Posner, B.I. (2010) Compartmentalization of EGFR in cellular membranes: role of membrane
rafts J. Cell. Biochem., 109, 1103–1108
Brown, D.A. and London, E. (1998) Functions of lipid rafts in biological membranes Annu. Rev. Cell Dev.
Biol., 14, 111-136
Casem, M.L. (2016) Cytoskeleton and intracellular motility In “Case studies in cell biology” Elsevier Inc, pp
127-156
Gimpl, G. and Gehrig-Burger, K. (2012) Specific and non specific regulation of GPCR function by cholesterol
In Regulation of ion channels and receptors (Ed. Levitan, I. and Barrantes, F.J.) John Wiley & Sons, Inc., pp
205-230
Head, B.P., Patel, H.H. and Insel, P.A. (2014) Interaction of membrane/lipid rafts with the cytoskeleton: Impact
on signaling and function. Membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling
Biochim. Biophys. Acta, 1838, 532–545
Kim, W., Chapkin, R.S., Barhoumi, R. and Ma, D.W.L. (2009) A novel role for nutrition in the alteration of
functional microdomains on the cell surface In Methods Mol. Biol., 579, Lipidomics (ed. Armstrong, D)
Humana Press, Totowa, NJ, pp 261-270
Landry, A. and Xavier, R. (2006) Isolation and analysis of lipid rafts in cell-cell interactions Methods Mol.
Biol., 341, 251-282
Lasley, R.D. (2011) Adenosine receptors and membrane microdomains Biochim. Biophys. Acta, 1808, 1284–
1289
Matveev, S., Li, X., Everson, W. and Smart, E.J. (2001) The role of caveolae and caveolin in vesicle-dependent
and vesicle-independent trafficking Adv. Drug. Deliver. Rev., 49, 237-240
Mingpeng, S. and Zongli, W. (1999) The protective role of high-density lipoproteins in atherosclerosis Exp.
Gerontol., 34, 539-548
Minogue, S. and Waugh, M.G. (2012) Lipid rafts, microdomain heterogeneity and inter-organelle contacts:
Impacts on membrane preparation for proteomic studies Biol. Cell, 104, 618–627
Pilch, P.F., Souto, R.P., Liu, L., Jedrycjowski, M.P., et al (2007) Cellular spelunking: exploring adipocyte
caveolae J. Lipid Res., 48, 2103-2111
Shaul, P.W. and Anderson, R.G.W. (1998) Role of plasmalemmal caveolae in signal transduction Am. J.
Physiol., 275, 843-851
Stillwell, W. (2016) Membrane isolation methods In “An Introduction to Biological Membranes” Elsevier Inc,
pp 247-271
Svobada, P. and Novotny, J. (2002) Hormone-induced subcellular redistribution of trimeric G proteins Cell.
Mol. Life Sci., 59, 501-512
Thomas, C.M. and Smart, E.J. (2008) Caveolae structure and function J. Cell Mol. Med., 12, 796-809
OptiPrepTM Reference List RS08; 7th edition, January 2020
OptiPrep Reference List RS09
Lipid-rich membranes from non-mammalian sources
The companion OptiPrep Reference List RS06 “Lipid-rich detergent-resistant domains
(mammalian sources) ” contains a brief summary of the methodology for the isolation of these plasma
membrane domains in addition to the complete reference list of the published papers. Strategies used for
invertebrate cells, plant cells, algae, fungi and protozoa are broadly similar. This Reference List is thus confined
to the provision of a bibliography of published papers concerned with this diverse group of organisms.
Detailed description of the OptiPrep methodology (see Application Sheets S32 and S33) can be found
via the releveant OptiPrep Application Sheets Index on the following website: www.Optiprep.com (click on
“Methodology”, then “Organelles and Subcellular Membranes”) and scroll down the Index.
Papers have been divided into organism or cell type (listed alphabetically) and additionally, when
required, into research topic. Within each group papers are listed alphabetically according to first author.
When a paper reports the study of more than one cell type, reference to that paper will appear under multiple
cell headings. A paper may also appear under two or more research topic headings.
Part(s) of the titles are highlighted in blue to facilitate identification of particular research topic(s)
For “Yeast” see “Fungi”.
1. Amphibia
Bates, R.C., Fees, C.P., Holland, W.L., Winger, C.C., Batbayar, K., Ancar, R., Bergren, T., Petcoff, D. and
Stith, B.J. (2014) Activation of Src and release of intracellular calcium by phosphatidic acid during Xenopus
laevis fertilization Dev. Biol., 386, 165-180
2. Bacteria
Borrelia burgdorferi
Coleman, J.L., Toledo, A. and Benach, J.L. (2016) Borrelia burgdorferi HtrA: evidence for twofold proteolysis
of outer membrane protein p66 Mol. Microbiol., 99, 135–150
LaRocca, T.J., Crowley, J.T., Cusack, B.J., Pathak, P., et al (2010) Cholesterol lipids of Borrelia burgdorferi
form lipid rafts and are required for the bactericidal activity of a complement-independent antibody Cell Host
Microbe 8, 331–342
Toledo, A., Crowley, J.T., Coleman, J.L., LaRocca, T.J., et al (2014) Selective association of outer surface
lipoproteins with the lipid rafts of Borrelia burgdorferi mBio, 5: e00899-14
Toledo, A., Pérez, A., Coleman, J.L. and Benach, J. L. (2015) The lipid raft proteome of Borrelia burgdorferi
Proteomics, 15, 3662–3675
Escherichia coli
Guzman-Flores, J.E., Alvarez, A.F., Poggio, S., Gavilanes-Ruiz, M. and Georgellis, D. (2017) Isolation of
detergent-resistant membranes (DRMs) from Escherichia coli Anal. Biochem., 518, 1-8
Guzmán-Flores, J.E., Steinemann-Hernández, L., González de la Vara, L.E., Gavilanes-Ruiz, M., Romeo, T.,
Alvarez, A.F. and Georgellis, D. (2019) Proteomic analysis of Escherichia coli detergent-resistant membranes
(DRM) PLoS One, 14: e0223794
3. Chicken embryo
Long, J., Tokhunts, R., Old, W.M., Houel, S., Rodgriguez-Blanco, J., Singh, S., Schilling, N., Capobianco,
A.J., Ahn, N.G. and Robbins, D.J. (2015) Identification of a family of fatty-acid-speciated sonic hedgehog
proteins, whose members display differential biological properties Cell Rep., 10, 1280–1287
4. Coccolithophores
Rose, S.L., Fulton, J.M., Brown, C.M., Natale, F., Van Mooy, B.A.S. and Bidle, K.D. (2014) Isolation and
characterization of lipid rafts in Emiliania huxleyi: a role for membrane microdomains in host–virus
interactions Environ. Microbiol., 16, 1150–1166
5. Drosophila melanogaster
Eroglu, C., Brügger, B., Wieland, F. and Sinning, I. (2003) Glutamate-binding affinity of Drosophila
metabotropic glutamate receptor is modulated by association with lipid rafts Proc. Natl. Acad. Sci. USA, 100,
10219-10224
Fernandez-Funez, P., Casas-Tinto, S., Zhang, Y., Gómez-Velazquez, M., et al (2009) In vivo generation of
neurotoxic prion protein: role for Hsp70 in accumulation of misfolded isoforms PLoS One, 5:e1000507
Goyal, G., Zheng, J., Adam, E., Steffes, G., Jain, M., Klavins, K. and Hummel, T. (2019) Sphingolipiddependent Dscam sorting regulates axon segregation Nat. Comm., 10: 813
Hebbar, S., Lee, E., Manna, M., Steinert, S., et al (2008) A fluorescent sphingolipid binding domain peptide
probe interacts with sphingolipids and cholesterol-dependent raft domains J. Lipid Res. 49, 1077-1089
Hoehne, M., de Couet, H.G., Stuermer, C.A.O. and Fischbach, K-F. (2005) Loss- and gain-of-function analysis
of the lipid raft proteins reggie/flotillin in Drosphilia: they are posttranslationally regulated, and misexpression
interferes with wing and eye development Mol. Cell. Neurosci., 30, 326-338
Rietveld, A., Neutz, S., Simons, K. and Eaton, S. (1999) Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophilia raft lipid microdomains J. Biol. Chem., 274, 12049-12054
Sanxaradis, P.D., Cronin, M.A., Rawat, S.S., Waro, G., et al (2007) Light-induced recruitment of INADsignaling complexes to detergent-resistant lipid rafts in Drosophila receptors Mol Cell. Neurosci., 36, 36-46
West, R.J.H., Briggs, L., Fjeldstad, M.P., Ribchester, R.R. and Sweeney, S.T. (2018) Sphingolipids regulate
neuromuscular synapse structure and function in Drosophila J. Comp. Neurol., 526, 1995–2009
Zhai, L., Chaturvedi, D. and Cumberledge, S. (2004) Drosophila Wnt-1 undergoes a hydrophobic modification
and is targeted to lipid rafts, a process that requires porcupine J. Biol. Chem., 279, 33220-33227
6. Echinoderms
Loza-Huerta, A., Vera-Estrella, R., Darszon, A. and Beltrána, C. (2013) Certain Strongylocentrotus purpuratus
sperm mitochondrial proteins co-purify with low density detergent-insoluble membranes and are PKA or PKCsubstrates possibly involved in sperm motility regulation Biochim. Biophys. Acta, 1830, 5305–5315
Vacquier, V.D., Loza-Huerta, A., García-Rincón, J., Darszon, A. and Beltrán, C. (2014) Soluble adenylyl
cyclase of sea urchin spermatozoa Biochim. Biophys. Acta, 1842, 2621–2628
7. Fish and fish embryo
Adachi, T., Sato, C. and Kitajima, K. (2007) Membrane microdomain formation in crucial in epiboly during
gastrulation of medaka Biochem. Biophys. Res. Commun., 358, 848-853
Adachi, T., Sato, C., Kishi, Y., Totani, K., et al, (2009) Membrane microdomains from early gastrula embryos
of medaka, Oryzias latipes, are a platform of E-cadherin- and carbohydrate-mediated cell–cell interactions
during epiboly Glycoconj. J. 26, 285–299
Sezgin, E., Azbazdar, Y., Ng, X.W., The, C., Simons, K., Weidinger, G., Wohland, T., Eggeling, C. and Ozhan,
G. (2017) Binding of canonical Wnt ligands to their receptor complexes occurs in ordered plasma membrane
environments FEBS J., 284, 2513–2526
Zehmer, J.K. and Hazel, J.R. (2003) Plasma membrane rafts of rainbow trout are subject to thermal
acclimation J. Exp. Biol., 206, 1657-1667
Zehmer, J.K. and Hazel, J.R. (2005) Thermally induced changes in lipid composition of raft and non-raft
regions of hepatocyte plasma membranes of rainbow trout J. Exp. Biol., 208, 4283-4290
8. Fungi
8-1. Candida albicans
Aeed, P.A., Sperry, A.E., Young, C.L., Nagiec, M.M., et al (2004) Effect of membrane perturbants on the
activity and phase distribution of inositol phosphorylceramide synthase; development of a novel assay
Biochemistry, 43, 8483-8493
Insenser, M., Nombela, C., Molero, G. and Gil, C. (2006) Proteomic analysis of detergent-resistant membranes
from Candida albicans Proteomics, 6, Suppl. 1., S74-S81
Martin, S.W. and Konopka, J.B. (2004) Lipid raft polarization contributes to hyphal growth in Candida
albicans Eukary. Cell, 3, 675-684
Ragni, E., Calderon, J., Fascio, U., Sipiczki, M., et al (2011) Phr1p, a glycosylphosphatidylinsitol-anchored
(1,3)-glucanosyltransferase critical for hyphal wall formation, localizes to the apical growth sites and septa in
Candida albicans Fungal Genet. Biol., 48, 793–805
Wang, L., Jia, Y., Tang, R-J., Xu, Z., Cao, Y-B., Jia, X-M. and Jiang, Y-Y. (2012) Proteomic analysis of
Rta2p-dependent raft-association of detergent-resistant membranes in Candida albicans PLoS One, 7: e37768
8-2. Saccharomyces cerevisiae
ABC transporters
Rockwell, N.C., Wolfger, H., Kuchler, K. and Thorner, J. (2009) ABC transporter Pdr10 regulates the
membrane microenvironment of Pdr12 in Saccharomyces cerevisiae J Membr. Biol., 229, 27–52
Acyl chains
Gaigg, B., Toulmay, A. and Scheiter, R. (2006) Very long-chain fatty acid-containing lipids rather than
sphingolipids per se are required for raft association and stable surface transport of newly synthesized plasma
membrane ATPase in yeast J. Biol. Chem., 281, 34135-34145
Peng, Y., Tang, F. and Weisman, L.S. (2006) Palmitoylation plays a role in targeting Vac8p to specific
membrane subdomains Traffic, 7, 1378-1387
Tatzer, V., Zellnig, G., Kohlwein, S.D. and Schneiter, R. (2002) Lipid-dependent subcellular relocalization of
the acyl chain desaturase in yeast Mol. Biol. Cell, 13, 4429-4442
Actin organization
Aronova, S., Wedaman, K., Anderson, S., Yates, J., et al (2007) Probing the membrane environment of the
TOR kinases reveals functional interactions between TORC1, actin and membrane trafficking in Saccharomyces
cerevisiae Mol. Biol. Cell, 18, 2779-2794
Balguerie, A., Bagnat, M., Bonneu, M., Aigle, M., et al (2002) RVS161p and sphingolipids are required for
actin repolarization following salt stress Eukaryot. Cell, 1, 1021-1031
Germann, M., Swain, E., Bergman, L. and Nickels, Jr. J.T. (2005) Characterizing the sphingolipid signaling
pathway that remediates defects associated with loss of the yeast amphiphysin-like orthologs, Rvs161p and
Rvs167p J. Biol. Chem., 280, 4270-4278
Kishimoto, T., Yamamoto, T. and Tanaka, K. (2005) Defects in structural integrity of ergosterol and the
Cdc50p-Drs2p putative phospholipid translocase cause accumulation of endocytic membranes, onto which actin
patches are assembled in yeast Mol. Biol. Cell, 16, 5592-5609
Proszynski, T.J., Klemm, R.W., Gravert, M., Hsu, P.P., et al (2005) A genome-wide visual screen reveals a
role for sphingolipids and ergosterol in cell surface delivery in yeast Proc. Natl. Acad. Sci. USA, 102, 17981-
17986
Amino acid transport
Lauwers, E., Grossmann, G. and André, B. (2007) Evidence for coupled biogenesis of yeast Gap1 permease
and sphingolipids: essential role in transport activity and normal control by ubiqutination Mol. Biol. Cell 18,
3068-3080
Apoptosis
Büttner, S., Delay, C., Franssens, V., Bammens, T., et al (2010) Synphilin-1 enhances -synuclein aggregation
in yeast and contributes to cellular stress and cell death in a Sir2-dependent manner PloS One 5: e13700
Ca2+- CaM
Ana, B., Chen, Y., Li, B., Qin, G. and Tian, S. (2014) Ca2+–CaM regulating viability of Candida guilliermondii
under oxidative stress by acting on detergent resistant membrane proteins J. Proteom., 109, 38–49
Cholesterol
Souza, C.M., Schwabe, T.M.E., Pichler, H., Ploier, B., et al (2011) A stable yeast strain efficiently producing
cholesterol instead of ergosterol is functional for tryptophan uptake, but not weak organic acid resistance
Metab. Eng., 13, 555–569
Coenzyme Q uptake
Padilla-López, S., Jiménez-Hidalgo, M., Martín-Montalvo, A., Clarke, C.F., et al (2009) Genetic evidence for
the requirement of the endocytic pathway in the uptake of coenzyme Q6 in Saccharomyces cerevisiae Biochim.
Biophys. Acta 1788, 1238–1248
Endocytosis
Kashiwazaki, J., Yamasaki, Y., Itadani, A., Teraguchi, E., et al (2011) Endocytosis is essential for dynamic
translocation of a syntaxin 1 orthologue during fission yeast meiosis Mol. Biol. Cell, 22, 3658-3670
Ergosterol
Bagnat, M. and Simons, K. (2002) Cell surface polarization during yeast mating Proc. Natl. Acad. Sci. USA,
99, 14283-14188
Eisenkolb, M., Zenzmaier, C., Leitner, E., and Schneiter, R. (2002) A specific structural requirement for
ergosterol in long-chain fatty acid synthesis mutants important for maintaining raft domains in yeast Mol. Biol.
Cell, 13, 4414-4428
Grossmann, G., Opekarova, M., Novakova, L., Stolz, J., et al (2006) Lipid raft-based membrane
compartmentation of a plant transport protein expressed in Saccharomyces cerevisiae Eukary. Cell, 5, 945-953
Iwaki, T., Iefuji, H., Hiraga, Y., Hosomi, A., et al (2008) Multiple functions of ergosterol in the fission yeast
Schizosaccharomyces pombe Microbiology 154, 830-841
Kishimoto, T., Yamamoto, T. and Tanaka, K. (2005) Defects in structural integrity of ergosterol and the
Cdc50p-Drs2p putative phospholipid translocase cause accumulation of endocytic membranes, onto which actin
patches are assembled in yeast Mol. Biol. Cell, 16, 5592-5609
Li, Y. and Priz, W.A. (2004) ATP-binding cassette (ABC) transporters mediate nonvesicular, raft-modulatd
sterol movement from the plasma membrane to the endoplasmic reticulum J. Biol. Chem., 279, 45226-45234
Pasrija, R., Panwar, S.L. and Prasad, R. (2008) Multidrug transporters CaCdr1p and CaMdr1p of Candida
albicans display different lipid specificities: both ergosterol and sphingolipids are essential for targeting of
CaCdr1p to membrane rafts Antimicrob. Agents Chemother., 52, 694-704
Pineau, L., Bonifait, L., Berjeaud, J-M., Alimardani-Theuil, P., et al (2008) A lipid-mediated quality control
process in the Golgi apparatus in yeast Mol. Biol. Cell, 19, 807-821
Proszynski, T.J., Klemm, R.W., Gravert, M., Hsu, P.P., et al (2005) A genome-wide visual screen reveals a
role for sphingolipids and ergosterol in cell surface delivery in yeast Proc. Natl. Acad. Sci. USA, 102, 17981-
17986
Souza, C.M., Schwabe, T.M.E., Pichler, H., Ploier, B., et al (2011) A stable yeast strain efficiently producing
cholesterol instead of ergosterol is functional for tryptophan uptake, but not weak organic acid resistance
Metab. Eng., 13, 555–569
Takeda, T. and Chang, F. (2005) Role of fission yeast myosin I in organization of sterol-rich membrane
domains Curr. Biol., 15, 1331-1336
Umebayashi, K. and Nakano, A. (2003) Ergosterol is required for targeting of tryptophan permease to the
yeast plasma membrane J. Cell Biol., 161, 1117-1131
Valachovic, M., Bareither, B.M., Alam Bhuiyan, M.S., Eckstein, J., et al (2006) Cumulative mutations affecting
sterol biosynthesis in the yeast Saccharomyces cerevisiae result in synthetic lethality that is suppressed by
alterations in sphingolipid profiles Genetics, 173, 1893-1908
Glycophospholipids
Yoko-o, T., Ichikawa, D., Miyagishi, Y., Kato, A., Umemura, M., Takase, K., Ra, M., Ikeda, K., Taguchi, R.
and Jigami Y. (2013) Determination and physiological roles of the glycosylphosphatidylinositol lipid
remodelling pathway in yeast Mol. Microbiol., 88, 140–155
GPI-proteins
Dupre, S. and Haguenauer-Tsapis, R. (2003) Raft partitioning of the yeast uracil permease during trafficking
along the endocytic pathway Traffic, 4, 83-9
Eisenkolb, M., Zenzmaier, C., Leitner, E., and Schneiter, R. (2002) A specific structural requirement for
ergosterol in long-chain fatty acid synthesis mutants important for maintaining raft domains in yeast Mol. Biol.
Cell, 13, 4414-4428
Fujita, M., Umemura, M., Yoko-o, T. and Jigami, Y. (2006) PER1 is required for GPI-Phospholipase A2
activity and involved in lipid remodeling of GPI-anchored proteins Mol. Biol. Cell, 17, 5253-5264
Okamoto, M., Yoko-o, T., Umemura, M., Nakayama, K-i., et al (2006) Glycosylphosphatidyl-inositol-anchored
proteins are required for the transport of detergent-resistant microdomain-associated membrane proteins Tat2p
and Fur4p J. Biol. Chem., 281, 4013-4023
Sikorska, N., Lemus, L., Aguilera-Romero, A., Manzano-Lopez, J., Riezman, H., Muñiz, M. and Goder, V.
(2016) Limited ER quality control for GPI-anchored proteins J. Cell Biol., 213, 693–704
Umemura, M., Fujita, M., Yoko-o, T., Fukamizu, A, et al (2007) Saccharomyces cerevisiae CWH43 is involved
in the remodeling of the lipid moiety of GPI anchors to ceramides Mol. Biol. Cell, 18, 4304-4316
Growth properties
Bagnat, M. and Simons, K. (2002) Cell surface polarization during yeast mating Proc. Natl. Acad. Sci. USA,
99, 14283-14188
Claret, S., Roumanie, O., Prouzet-Mauleon, V., Lefebvre, F., et al (2011) Evidence for functional links between
theRgd1-Rho3RhoGAPGTPasemodule and Tos2, a protein involved in polarized growth in Saccharomyces
cerevisiae FEMS Yeast Res., 11, 179–191
Tani, M., Khara, A. and Igarashi, Y. (2006) Rescue of cell growth by sphingosine with disruption of lipid
microdomain formation in Saccharomyces cerevisiae deficient in sphingolipid biosynthesis Biochem. J., 394,
237-242
H
+
-ATPase Pma1P
Bagnat, M., Chang, A. and Simons, K. (2001) Plasma membrane proton ATPase Pma1p requires raft
association for surface delivery in yeast Mol. Biol. Cell, 12, 4129-4138
Czyz, O., Bitew, T., Cuesta-Marban, A., McMaster, C.R., et al (2013) Alteration of plasma membrane
organization by an anticancer lysophosphatidylcholine analogue induces intracellular acidification and
internalization of plasma membrane transporters in yeast J. Biol. Chem., 288, 8419–8432
Gaigg, B., Toulmay, A. and Scheiter, R. (2006) Very long-chain fatty acid-containing lipids rather than
sphingolipids per se are required for raft association and stable surface transport of newly synthesized plasma
membrane ATPase in yeast J. Biol. Chem., 281, 34135-34145
Lee, M.C., Hamamoto, S. and Schekman, R. (2002) Ceramide biosynthesis is required for the formation of the
oligomeric H+
-ATPase Pma1p in the yeast endoplasmic reticulum J. Biol. Chem., 277, 23395-23401
Liu, Y. and Chang, A. (2006) Quality control of a mutant plasma membrane ATPase: ubiquitylation prevents
cell-surface stability J. Cell Sci., 119, 360-369
Malinska, K., Malinsky, J., Opekarova, M and Tanner, W. (2003) Visualization of protein compartmentation
within the plasma membrane of living yeast cells Mol. Biol. Cell., 14, 4427-4436
Malinska, K., Malinsky, J., Opekarova, M. and Tanner, W. (2004) Distribution of Can1p into stable domains
reflects lateral protein segregation within the plasma membrane of living S. cerevisiae cells J. Cell Sci., 117,
6031-6041
Pizzirusso, M. and Chang, A. (2004) Ubiquitin-mediated targeting of a mutant plasma membrane ATPase,
Pma1-7, to the endosomal/vacuolar system in yeast Mol. Biol. Cell, 15, 2401-2409
Iron regulated transport
Tan, S., Zhang, P., Xiao, W., Feng, B., Chen, L-Y., Li, S., Li, P., Zhao, W-Z., Qi, X-T. and Yin, L-P. (2018)
TMD1 domain and CRAC motif determine the association and disassociation of MxIRT1 with detergentresistant membranes Traffic, 19, 122–137
Membrane trafficking
Aronova, S., Wedaman, K., Anderson, S., Yates, J. et al (2007) Probing the membrane environment of the TOR
kinases reveals functional interactions between TORC1, actin and membrane trafficking in Saccharomyces
cerevisiae Mol. Biol. Cell, 18, 2779-2794
Cuesta-Marbán, A., Botet, J., Czyz, O., Cacharro, L.M., et al (2013) Drug uptake, lipid rafts, and vesicle
trafficking modulate resistance to an anticancer lysophosphatidyl-choline analogue in yeast J. Biol. Chem., 288,
8405–8418
Czyz, O., Bitew, T., Cuesta-Marban, A., McMaster, C.R., et al (2013) Alteration of plasma membrane
organization by an anticancer lysophosphatidylcholine analogue induces intracellular acidification and
internalization of plasma membrane transporters in yeast J. Biol. Chem., 288, 8419–8432
Watanabe, R., Castillon, G.A., Meury, A. and Riezman, H. (2008) The presence of an ER exit signal
determines the protein sorting upon ER exit in yeast Biochem. J., 414, 237-245
Zabrocki, P., Bastiaens, I., Delay, C., Bammens, T., Ghillebert, R., Pellens, K., De Virgilio, C., Van Leuven, F.
and Winderickx, J. (2008) Phosphorylation, lipid raft interaction and traffic of α-synuclein in a yeast model for
Parkinson Biochim. Biophys. Acta., 1783, 1767-1780
Morphogenesis
Rolli, E., Ragni, E., Calderon, J., Porello, S., et al (2009) Immobilization of the glycosylphosphatidylinositolanchored Gas1 protein into the chitin ring and septum is required for proper morphogenesis in yeast Mol. Biol.
Cell, 20, 4856–4870
Na+
/H+
antiport
Mitsui, K., Hatakeyama, K., Matsushita, M. and Kanazawa, H. (2009) Saccharomyces cerevisiae Na+
/H+
antiporter Nha1p associates with lipid rafts and requires sphingolipid for stable localization to the plasma
membrane J. Biochem., 145, 709–720
Oxidative stress
Ana, B., Chen, Y., Li, B., Qin, G., et al (2014) Ca2+–CaM regulating viability of Candida guilliermondii under
oxidative stress by acting on detergent resistant membrane proteins J. Proteom., 109, 38–49
Phospholipids
Cuesta-Marbán, A., Botet, J., Czyz, O., Cacharro, L.M., et al (2013) Drug uptake, lipid rafts, and vesicle
trafficking modulate resistance to an anticancer lysophosphatidyl-choline analogue in yeast J. Biol. Chem., 288,
8405–8418
Czyz, O., Bitew, T., Cuesta-Marban, A., McMaster, C.R., et al (2013) Alteration of plasma membrane
organization by an anticancer lysophosphatidylcholine analogue induces intracellular acidification and
internalization of plasma membrane transporters in yeast J. Biol. Chem., 288, 8419–8432
Kato, U., Emoto, K., Fredriksson, C., Nakamura, H., et al (2002) A novel membrane protein, Ros3p, is required
for phospholipid translocation across the plasma membrane in Saccharomyces cerevisiae J. Biol. Chem., 277,
37855-37862
Kishimoto, T., Yamamoto, T. and Tanaka, K. (2005) Defects in structural integrity of ergosterol and the
Cdc50p-Drs2p putative phospholipid translocase cause accumulation of endocytic membranes, onto which actin
patches are assembled in yeast Mol. Biol. Cell, 16, 5592-5609
Kobayashi, T., Takematsu, H., Yamaji, T., Hiramoto, S., et al (2005) Disturbance of sphingolipid biosynthesis
abrogates the signaling of Mss4, phosphatidylinositol-4-phosphate 5-kinase, in yeast J. Biol. Chem., 289,
18087-18094
Noji, T., Yamamoto, T., Saito, K., Fujimura-Kamada, K., et al (2006) Mutational analysis of the Lem3p-Dnf1p
putative phospholipid-translocating P-type ATPase reveals novel regulatory roles for Lem3p and a carboxylterminal region of Dnf1p independent of the phospholipid-translocating activity of Dnf1p in yeast Biochem.
Biophys. Res. Commun., 344, 323-331
Okamoto, M., Yoko-o, T., Umemura, M., Nakayama, K-i., et al (2006) Glycosylphosphatidyl-inositol-anchored
proteins are required for the transport of detergent-resistant microdomain-associated membrane proteins Tat2p
and Fur4p J. Biol. Chem., 281, 4013-4023
Opekarova, M., Malinska, K., Novakova, L. and Tanner, W. (2005) Differential effect of phosphatidylethanolamine depletion on raft proteins. Further evidence for diversity of rafts in Saccharomyces cerevisiae
Biochim. Biophys. Acta, 1711, 87-95
Pineau, L., Bonifait, L., Berjeaud, J-M., Alimardani-Theuil, P., et al (2008) A lipid-mediated quality control
process in the Golgi apparatus in yeast Mol. Biol. Cell, 19, 807-821
Takeda, T. and Chang, F. (2005) Role of fission yeast myosin I in organization of sterol-rich membrane domains
Curr. Biol., 15, 1331-1336
Takeda, T. and Chang, F. (2005) Role of fission yeast myosin I in organization of sterol-rich membrane
domains Curr. Biol., 15, 1331-1336
Zaremberg, V., Gajate, C., Cacharro, L.M., Mollinedo, F., et al (2005) Cytotoxicity of an anti-cancer
lysophospholipid through selective modification of lipid raft composition J. Biol. Chem., 280, 38047-38058
Protein localization
Bagnat, M., Keranen, S., Shevchenko, A., Shevchenko, A. and Simons, K (2000) Lipid rafts function in
biosynthetic delivery of proteins to the cell surface in yeast Proc. Natl. Acad. Sci., USA, 97, 3254-3259
Fujita, M., Umemura, M., Yoko-o, T. and Jigami, Y. (2006) PER1 is required for GPI-Phospholipase A2
activity and involved in lipid remodeling of GPI-anchored proteins Mol. Biol. Cell, 17, 5253-5264
Grossmann, G., Opekarova, M., Novakova, L., Stolz, J., et al (2006) Lipid raft-based membrane
compartmentation of a plant transport protein expressed in Saccharomyces cerevisiae Eukary. Cell, 5, 945-953
Lauwers, E. and Andre, B. (2006) Association of yeast transporters with detergent-resistant membranes
correlates with their cell-surface location Traffic, 7, 1045-1059
Malinska, K., Malinsky, J., Opekarova, M and Tanner, W. (2003) Visualization of protein compartmentation
within the plasma membrane of living yeast cells Mol. Biol. Cell., 14, 4427-4436
Malinska, K., Malinsky, J., Opekarova, M. and Tanner, W. (2004) Distribution of Can1p into stable domains
reflects lateral protein segregation within the plasma membrane of living S. cerevisiae cells J. Cell Sci., 117,
6031-6041
Okamoto, M., Yoko-o, T., Umemura, M., Nakayama, K-i., et al (2006) Glycosylphosphatidyl-inositol-anchored
proteins are required for the transport of detergent-resistant microdomain-associated membrane proteins Tat2p
and Fur4p J. Biol. Chem., 281, 4013-4023
Opekarova, M., Malinska, K., Novakova, L. and Tanner, W. (2005) Differential effect of phosphatidylethanolamine depletion on raft proteins. Further evidence for diversity of rafts in Saccharomyces cerevisiae
Biochim. Biophys. Acta, 1711, 87-95
Peng, Y., Tang, F. and Weisman, L.S. (2006) Palmitoylation plays a role in targeting Vac8p to specific
membrane subdomains Traffic, 7, 1378-1387
Řičicová, M., Kučerova, H., Váchová, L. and Palková, Z. (2007) Association of putative ammonium exporters
Ato with depergent-resistant compartments of plasma membrane during yeast colony development: pH affects
Ato1p localization in patches Biochim. Biophys. Acta, 1768, 1170-1178
Takeda, T. and Chang, F. (2005) Role of fission yeast myosin I in organization of sterol-rich membrane
domains Curr. Biol., 15, 1331-1336
Sphingolipids
Bagnat, M. and Simons, K. (2002) Cell surface polarization during yeast mating Proc. Natl. Acad. Sci. USA,
99, 14283-14188
Balguerie, A., Bagnat, M., Bonneu, M., Aigle, M., et al (2002) RVS161p and sphingolipids are required for
actin repolarization following salt stress Eukaryot. Cell, 1, 1021-1031
Dupre, S. and Haguenauer-Tsapis, R. (2003) Raft partitioning of the yeast uracil permease during trafficking
along the endocytic pathway Traffic, 4, 83-96
Eisenkolb, M., Zenzmaier, C., Leitner, E., and Schneiter, R. (2002) A specific structural requirement for
ergosterol in long-chain fatty acid synthesis mutants important for maintaining raft domains in yeast Mol. Biol.
Cell, 13, 4414-4428
Ferreira, C. and Lucas, C. (2008) The yeast O-acyltransferase Gup1p interferes in lipid metabolism with direct
consequences on the sphingolipid-sterol-ordered domains integrity/assembly Biochim. Biophys. Acta, 1778,
2648-2653
Fröhlich, F., Moreira, K., Aguilar, P.S., Hubner, N.C., et al (2009) A genome-wide screen for genes affecting
eisosomes reveals Nce102 function in sphingolipid signaling J. Cell Biol., 185, 1227–1242
Gaigg, B., Toulmay, A. and Scheiter, R. (2006) Very long-chain fatty acid-containing lipids rather than
sphingolipids per se are required for raft association and stable surface transport of newly synthesized plasma
membrane ATPase in yeast J. Biol. Chem., 281, 34135-34145
García-Marqués, S., Randez-Gil, F., Dupont, S., Garre, E. and Prieto, J.A. (2016) Sng1 associates with Nce102
to regulate the yeast Pkh–Ypk signaling module in response to sphingolipid status Biochim. Biophys. Acta,
1863, 1319–1333
Germann, M., Swain, E., Bergman, L. and Nickels, Jr. J.T. (2005) Characterizing the sphingolipid signaling
pathway that remediates defects associated with loss of the yeast amphiphysin-like orthologs, Rvs161p and
Rvs167p J. Biol. Chem., 280, 4270-4278
Grossmann, G., Opekarova, M., Novakova, L., Stolz, J., et al (2006) Lipid raft-based membrane
compartmentation of a plant transport protein expressed in Saccharomyces cerevisiae Eukary. Cell, 5, 945-953
Idkowiak-Baldys, J., Grilley, M.M. and Takemoto, J.Y. (2004) Sphingolipid C4 hydroxylation influences
properties of yeast detergent-insoluble glycolipid-enriched membranes FEBS Lett., 569, 272-276
Kobayashi, T., Takematsu, H., Yamaji, T., Hiramoto, S., et al (2005) Disturbance of sphingolipid biosynthesis
abrogates the signaling of Mss4, phosphatidylinositol-4-phosphate 5-kinase, in yeast J. Biol. Chem., 289,
18087-18094
Lauwers, E., Grossmann, G. and André, B. (2007) Evidence for coupled biogenesis of yeast Gap1 permease
and sphingolipids: essential role in transport activity and normal control by ubiqutination Mol. Biol. Cell 18,
3068-3080
Lee, M.C., Hamamoto, S. and Schekman, R. (2002) Ceramide biosynthesis is required for the formation of the
oligomeric H+
-ATPase Pma1p in the yeast endoplasmic reticulum J. Biol. Chem., 277, 23395-23401
Mitsui, K., Hatakeyama, K., Matsushita, M. and Kanazawa, H. (2009) Saccharomyces cerevisiae Na+
/H+
antiporter Nha1p associates with lipid rafts and requires sphingolipid for stable localization to the plasma
membrane J. Biochem., 145, 709–720
Pasrija, R., Panwar, S.L. and Prasad, R. (2008) Multidrug transporters CaCdr1p and CaMdr1p of Candida
albicans display different lipid specificities: both ergosterol and sphingolipids are essential for targeting of
CaCdr1p to membrane rafts Antimicrob. Agents Chemother., 52, 694-704
Proszynski, T.J., Klemm, R.W., Gravert, M., Hsu, P.P., et al (2005) A genome-wide visual screen reveals a
role for sphingolipids and ergosterol in cell surface delivery in yeast Proc. Natl. Acad. Sci. USA, 102, 17981-
17986
Tani, M., Khara, A. and Igarashi, Y. (2006) Rescue of cell growth by sphingosine with disruption of lipid
microdomain formation in Saccharomyces cerevisiae deficient in sphingolipid biosynthesis Biochem. J., 394,
237-242
Tanigawa, M., Kihara, A., Terashima, M., Takahara, T. et al (2012) Sphingolipids regulate the yeast highosmolarity glycerol response pathway Mol. Cell. Biol., 32, 2861-2870
Valachovic, M., Bareither, B.M., Alam Bhuiyan, M.S., Eckstein, J., et al (2006) Cumulative mutations affecting
sterol biosynthesis in the yeast Saccharomyces cerevisiae result in synthetic lethality that is suppressed by
alterations in sphingolipid profiles Genetics, 173, 1893-1908
-Synuclein
Büttner, S., Delay, C., Franssens, V., Bammens, T., et al (2010) Synphilin-1 enhances -synuclein aggregation
in yeast and contributes to cellular stress and cell death in a Sir2-dependent manner PloS One 5: e13700
Thioredoxin
Takeuchi, Y., Nomura, W., Ohdate, T., Tamasu, S., et al (2007) Release of thioredoxin from Saccharomyces
cerevisiae with environmental stimuli: solubilization of thioredoxin with ethanol Appl. Microbiol. Biotechnol.,
75, 1393-1399
Tryptophan transport
Abe, F. and Iida, H. (2003) Pressure-induced differential regulation of the two tryptophan permeases Tat1 and
Tat2 by ubiquitin ligase Rsp5 and its binding proteins, Bu11 and Bu12 Mol. Cell. Biol., 23, 7566-7584
Daicho, K., Makino, N., Hiraki, T., Ueno, M., Uritani, M., Abe, F. and Ushimaru, T. (2009) Sorting defects of
the tryptophan permease Tat2 in an erg2 yeast mutant FEMS Microbiol. Lett., 298, 218-227
Hiraki, T. and Abe, F. (2010) Overexpression of Sna3 stabilizes tryptophan permease Tat2, potentially
competing for the WW domain of Rsp5 ubiquitin ligase with its binding protein Bul1 FEBS Lett., 584, 55–60
Souza, C.M., Schwabe, T.M.E., Pichler, H., Ploier, B., Leitner, E., Guan, X.L., Wenke, M.R., Riezman, I. and
Riezman, H. (2011) A stable yeast strain efficiently producing cholesterol instead of ergosterol is functional for
tryptophan uptake, but not weak organic acid resistance Metab. Eng., 13, 555–569
Umebayashi, K. and Nakano, A. (2003) Ergosterol is required for targeting of tryptophan permease to the
yeast plasma membrane J. Cell Biol., 161, 1117-1131
Ubiquitin
Abe, F. and Iida, H. (2003) Pressure-induced differential regulation of the two tryptophan permeases Tat1 and
Tat2 by ubiquitin ligase Rsp5 and its binding proteins, Bu11 and Bu12 Mol. Cell. Biol., 23, 7566-7584
Kinner, A. and Kölling, R. (2003) The yeast deubiquitinating enzyme Ubp16 is anchored to the outer
mitochondrial membrane FEBS Lett., 549, 135-140
Lauwers, E. and Andre, B. (2006) Association of yeast transporters with detergent-resistant membranes
correlates with their cell-surface location Traffic, 7, 1045-1059
Lauwers, E., Grossmann, G. and André, B. (2007) Evidence for coupled biogenesis of yeast Gap1 permease
and sphingolipids: essential role in transport activity and normal control by ubiqutination Mol. Biol. Cell 18,
3068-3080
Pizzirusso, M. and Chang, A. (2004) Ubiquitin-mediated targeting of a mutant plasma membrane ATPase,
Pma1-7, to the endosomal/vacuolar system in yeast Mol. Biol. Cell, 15, 2401-2409
Uracil transport
Dupre, S. and Haguenauer-Tsapis, R. (2003) Raft partitioning of the yeast uracil permease during trafficking
along the endocytic pathway Traffic, 4, 83-96
Hearn, J.D., Lester, R.L. and Dickson, R.C. (2003) The uracil transporter Fur4p associates with lipid rafts J.
Biol. Chem., 278, 3679-3686
Okamoto, M., Yoko-o, T., Umemura, M., Nakayama, K-i. and Jigami, Y. (2006) Glycosylphosphatidylinositol-anchored proteins are required for the transport pg detergent-resistant microdomain-associated
membrane proteins Tat2p and Fur4p J. Biol. Chem., 281, 4013-4023
Viral replication studies
Zhang, J., Zhang, Z., Chukkapalli, V., Nchoutmboube, J.A., Li, J., Randall, G., Belov, G.A. and Wang, X.
(2016) Positive-strand RNA viruses stimulate host phosphatidylcholine synthesis at viral replication sites Proc.
Natl. Acad. Sci. USA, 113, E1064–E1073
9. Insect larva brush border
Bayyareddy, K., Zhu, X., Orlando, R. and Adang, M.J. (2012) Proteome analysis of Cry4Ba toxin-interacting
Aedes aegypti lipid rafts using geLC-MS/MS J. Proteome Res., 11, 5843-5855
Bravo, A., Gomez, I., Conde, J., Munoz-Garay, C., Sanchez, J., Miranda, R., Zhuang, M., Gill, S.S. and
Soberon, M. (2004) Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to
aminopeptidase N receptor leading to insertion into membrane microdomains Biochim. Biophys. Acta, 1667,
38-46
Ito, T., Bando, H. and Asano, S-i. (2006) Activation process of the mosquitocidal δ-endotoxin Cry39A produced
by Bacillus thuringiensis subsp. aizawai BUN1-14 and binding property to Anopheles stephensi BBMV J. Invert.
Pathol., 93, 29-35
Liu, J-G., Yang, A-Z., Shen, X-H., Hua, B-G., Shi, G-L. (2011) Specific binding of activated Vip3Aa10 to
Helicoverpa armigera brush border membrane vesicles results in pore formation J. Invertebr. Pathol., 108, 92–
97
10. Plant tissue
Belugin, B.V., Zhestkova, I.M. and Trofimova, M.S. (2011) Affinity of PIP-aquaporins to sterol-enriched
domains in plasma membrane of the cells of etiolated pea seedlings Biochemistry (Moscow) Suppl. Series A:
Membr. Cell Biol., 5, 56–63
Carmona-Salazar, L., El Hafidi, M., Enríquez-Arredondo, C., Vázquez-Vázquez, C., González de la Vara,
L.E. and Gavilanes-Ruíz, M. (2011) Isolation of detergent-resistant membranes from plant photosynthetic and
non-photosynthetic tissues Anal. Biochem., 417, 220–227
Carmona-Salazar, L., El Hafidi, M., Gutierrez-Najera, N., Noyola-Martinez, L., Gonzalez-Solis, A. and
Gavilanes-Ruiz, M. (2015) Fatty acid profiles from the plasma membrane and detergent resistant membranes of
two plant species Phytochemistry, 109, 25–35
Han, B., Yang, N., Pu, H. and Wang, T. (2018) Quantitative proteomics and cytology of rice pollen sterol-rich
membrane domains reveals pre-established cell polarity cues in mature pollen J. Proteome Res., 17, 1532−1546
Liu, P., Li, R-L., Zhang, L., Wang, Q-L., Niehaus, K., Baluška, F., Šamaj, J. and Lin, J-X. (2009) Lipid
microdomain polarization is required for NADPH oxidase-dependent ROS signaling in Picea meyeri pollen
tube tip growth Plant J., 60, 303–313
Moscatelli, A., Gagliardi, A., Maneta-Peyret, L., Bini, L., Stroppa, N., Onelli, E., Landi, C., Scali, M., Idilli,
A.I. and Moreau, P. (2015) Characterisation of detergent-insoluble membranes in pollen tubes of Nicotiana
tabacum Biol. Open 4, 378–399
Nagano, M., Ishikawa, T., Fujiwara, M./, Fukao, Y., Kawano, Y. Kawai-Yamada, M. and Shimamoto, K.
(2016) Plasma membrane microdomains are essential for Rac1-RbohB/H-mediated immunity in rice Plant Cell,
28, 1966–1983
Ohta, D. and Mizutani, M. (2012) Sterol C22-desaturase and its biological roles In Isoprenoid Synthesis in
Plants and Microorganisms: New Concepts and Experimental Approaches (eds. Bach, T.J. and Rohmer, M.)
Springer Science+Business Media New York, pp 381-391
Šamajová, O., Takác, T., von Wangenheim, D., Stelzer, E. and Šamaj, J. (2012) Update on methods and
techniques to study endocytosis in plants In Endocytosis in Plants (ed. Šamaj, J.) Springer-Verlag Berlin
Heidelberg, pp 1-36
11. Protozoa
11-1. Giardia
De Chatterjee, A., Mendez, T.L., Roychowdhury, S. and Dasa, S. (2015) The assembly of GM1 glycolipid- and
cholesterol-enriched raft-like membrane microdomains is important for Giardial encystation Infect. Immun. 83,
2030-2042
11-2. Leishmania
Denny, P.W., Field, M.C. and Smith, D.F. (2001) GPI-anchored proteins and glycoconjugates segregate into
lipid rafts in Kinetoplastida FEBS Lett., 491, 148-153
Dermine. J-F., Goyette, G., Houde, M., Turco, S.J., et al (2005) Leishmania donovani lipophosphoglycan
disrupts phagosome microdomains in J774 macrophages Cell. Microbiol., 7, 1263-1270
Fridberg, A., Buchanan, K.T. and Engman, D.M. (2007) Flagellar membrane trafficking in kinetoplastids
Parasitol. Res., 100, 205-212
Sen, S., Roy, K., Mukherjee, S., Mukhopadhyay, R. and Roy, S. (2011) Restoration of IFNR subunit assembly,
IFN signaling and parasite clearance in Leishmania donovani infected macrophages: role of membrane
cholesterol PloS Pathog., 7: e1002229
Yao, C., Donelson, J.E. and Wilson, M.E. (2003) The major surface protease (MSP or GP63) of Leishmania sp.
Biosynthesis, regulation of expression and function Mol. Biol. Parasitol., 132, 1-16
11-3. Trypanosoma
De Paulo Martins, V., Okura, M., Maric, D., Engman, D.M., Vieira, M., Docampo, R. and Moreno, S.N.J.
(2010) Acylation-dependent export of Trypanosoma cruzi phosphoinositide-specific phospholipase C to the
outer surface of amastigotes J. Biol. Chem., 285, 30906-30917
Emmer, B.T., Souther, C., Toriello, K.M., Olson, C.L., Epting, C.L. and Engman, D.M. (2009) Identification of
a palmitoyl acyltransferase required for protein sorting to the flagellar membrane J. Cell Sci., 122, 867-874
Fridberg, A., Buchanan, K.T. and Engman, D.M. (2007) Flagellar membrane trafficking in kinetoplastids
Parasitol. Res., 100, 205-212
Fridberg, A., Olson, C.L., Nakayasu, E.S., Tyler, K.M., Almeida, I.C. and Engman, D.M. (2008) Sphingolipid
synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei J. Cell Sci., 121, 522-
535
Lantos, A.B., Carlevaro, G., Araoz, B., Diaz, P.R., de los Milagros Camara, M., Buscaglia, C.A., Bossi, M., Yu,
H., Chen, X. et al (2016) Sialic acid glycobiology unveils Trypanosoma cruzi trypomastigote membrane
physiology PloS Pathog., 12, e1005559
Maric, D., McGwire, B.S., Buchanan, K.T., Olson, C.L., Emmer, B.T., Epting, C.L. and Engman, D.M. (2011)
Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding
protein J. Biol. Chem., 286, 33109–33117
Maric, D., Olson, C.L., Xu, X., Ames, J.B. and Engman, D.M. (2015) Calcium-dependent membrane
association of a flagellar calcium sensor does not require calcium binding Mol. Biochem. Parasitol., 201, 72–75
Mucci, J., Lantos, A.B., Buscaglia, C,A,m Leguizamón, M.S. and Campetella, O. (2017) The Trypanosoma
cruzi surface, a nanoscale patchwork quilt Trends Parasitol., 33, 102-112
Niyogi, S., Mucci, J., Campetella, O. and Docampo, R. (2014) Rab11 regulates trafficking of trans-sialidase to
the plasma membrane through the contractile vacuole complex of Trypanosoma cruzi PLOS Pathog., 10:
e1004224
Sharma, A.I., Olson, C.L., Mamede, J.I., Gazos-Lopes, F., Epting, C.L., Almeida, I.C. and Engman, D.M.
(2017) Sterol targeting drugs reveal life cycle stage-specific differences in trypanosome lipid rafts Sci. Rep., 7:
9105
Tyler, K.M., Fridberg, A., Toriello, K.M., Olson, C.L., Cieslak, J.A., Hazlett, T.L. and Engman, D.M. (2009)
Flagellar membrane localization via association with lipid rafts J. Cell Sci., 122, 859-866
12. Rice pollen grains
Han, B., Yang, N., Pu, H. and Wang, T. (2018) Quantitative proteomics and cytology of rice pollen sterol-rich
membrane domains reveals pre-established cell polarity cues in mature pollen J. Proteome Res., 17, 1532−1546
13. Xenopus sperm
Bates, R.C., Fees, C.P., Holland, W.L., Winger, C.C., Batbayar, K., Ancar, R., Bergren, T., Petcoff, D. and
Stith, B.J. (2014) Activation of Src and release of intracellular calcium by phosphatidic acid during Xenopus
laevis fertilization Dev. Biol., 386, 165-180
OptiPrepTM Reference List RS09; 8th edition, January 2020
OptiPrep™ Reference List RS11
Extracellular vesicles from non-mammalian sources
1. Introduction
It is widely recognized that mammalian cells, bacteria, algae and fungi release extracellular vesicles into the surrounding medium; these vesicles are involved in communication between cells and the delivery of biologically and clinically important molecules to other cells. With regard to bacteria the term “extracellular vesicles” (EVs) covers the outer membrane vesicles (OMVs) produced by Gramnegative bacteria and the membrane vesicles (MVs) produced by Gram-positive bacteria and other organisms such as algae and fungi. In all cases; EVs are distinct from the intracellular vesicles present in the cytoplasm. The OMVs from Gram-negative bacteria in particular are widely researched and have been shown to be important in the transfer of virulence factors and the initiation of immune and inflammatory responses in host cells.
- Section 2 briefly reviews the current OptiPrep™-based methodology; this is described in detail in Application Sheet S60, which has its own short reference list.
- The principal aim of this Mini-Review however is to provide a bibliography of all the published papers that have reported the use of an iodixanol gradient. Published papers have been divided into the flowing sections:
- Section 3a: Gram –ve bacteria
- Section 3b: Gram +ve bacteria and mycobacteria
- Section 3c: Bacterial exosomes derived from human fluids and waste water
- Section 3d: Haloarchaea (halobacteria)
- Section 3e: Biofilm structures
- Section 3f: Mycoplasmas
- Section 3g: Protozoa, algae, fungi and archaea
- Section 3h: Invertebrates
- Section 3i: Trematodes
- Section 3j: Nematodes
- Section 3k: Arthropoda
- Section 3l: Plants
- Section 4: References to methodological and functional reviews
- All references are listed alphabetically according to first author
Related research areas that have reported the use of gradients prepared from OptiPrep™ are:
- The analysis of the microvesicles that are expressed from the surface of mammalian cells is covered in OptiPrep™ Reference List RS10 and OptiPrep™ Application Sheet S63
- The control and organization of membrane trafficking within mammalian cells that permits the movement of vesicles to, and ultimately their fusion with, the plasma membrane or a specific plasma membrane domain is covered in OptiPrep™ Application Sheet S47
- These and other OptiPrepTM Reference Lists or OptiPrep™ Application Sheets can be accessed via the relevant OptiPrep Index from the following website: www.Optiprep.com, click on “Reference Lists” or “Methodology” respectively.
2. Methodological summary
Various forms of pre-gradient processing are employed, during which intact bacteria and aggregated material in the culture medium are mostly removed and the EVs concentrated. This is covered in much greater detail in Application Sheet S62
The first step is clarification of the bacterial broth to remove intact cells by centrifugation; the time and g-force used varies widely and reflects the size of the organism. Generally rcfs of approx. 10,000 g are used for 10-20 min, but there are examples both of lower and higher g-forces so that the range spans 4,000-12,000 g. Occasionally this first step is carried out in two stages in which the broth is centrifuged for 30 min at 5,000 g and then the supernatant at 7,500 g. This strategy may minimize the entrapment (and consequent loss) of vesicles into the pellet by the rapidly sedimenting much larger bacteria. Fungal cells appear to sediment satisfactorily at lower speeds such as 2,500 g for 10 min.
Commonly the second step is volume reduction since ultimately the vesicles will be sedimented in a fixed-angle ultracentrifuge rotor, prior to gradient purification. Large volumes of clarified medium can pose a problem for this final pre-gradient step. The Beckman 45Ti for example which accommodates 6×94 ml tubes has a maximum g-force of 235,000 g, but the vesicles at the top of the sample experience initially only 80,000 g. Tangential filtration devices (e.g. 70-100 kDa cut-off) are popular or centrifugal filters are popular for this volume reduction step.
Residual bacteria in the broth may then be removed by vacuum filtration through either a 0.45 or 0.22 μm filter or both pore size filters sequentially, before the EVs are sedimented in a fixed-angle rotor; the conditions vary quite widely, from approx. 40,000 g for 1 h to 140-150,000 g for 2-3 h..
Ammonium sulphate precipitation of OMVs from the clarified broth of Gram-ve bacteria has been used a few cases. The precipitation process may only require about half an hour at 4°C but can be as long as overnight. Moreover the resuspended pellet requires dialysis overnight prior to further processing.
Iodixanol gradient purification of the EVs, regardless of the pre-gradient technology, involves adjustment of the suspension to a density of approx. 1.241 g/ml (sometimes higher 1.267 g/ml or lower 1.215 g/ml).for subsequent flotation through a discontinuous iodixanol gradient; During centrifugation at 100-200,000 g for 12-16 h the gradient will become more or less continuous; gradients run for shorter times (e.g. 2 h) will retain some of their discontinuous nature.
The density of MVs in iodixanol gradients is generally >1.11 g/ml, many banding between 1.13 and 1.15 g/ml; although occasionally densities of as a high as 1.20 g/ml have been observed. There is also evidence of heterogeneity amongst EV populations from a single bacterial type.
The first published paper to describe the use of OptiPrep™, of which we have a record, was by Horstman and Kuehn, published in 2000. It documented the purification of OMVs from enterotoxincontaining Escherichia coli; the OMVs were shown to contain the pathogenic enterotoxin, lipids and specific proteins characteristic of the outer membrane and proteins from the periplasmic space, but no markers of the cytosol or inner membrane (see Section 3a – Escherichia coli ).
3. Bibliography of publications reporting analytical studies on iodixanol-purified OMVs and MVs
References are sorted alphabetically according to first author. Research topics are highlighted in blue in the titles.
3a. Gram-negative bacteria
Acetobacter pasteurianus
Hashimoto, M., Matsumoto, T., Tamura-Nakano, M., Ozono, M., Hashiguchi, S. and Suda, Y. (2018) Characterization of outer membrane vesicles of Acetobacter pasteurianus NBRC3283 J.Biosci. Bioeng., 125, 425-431
Acinetobacter baumannii
Chatterjee, S., Mondal, A., Mitra, S. and Basu, S. (2017) Acinetobacter baumannii transfers the blaNDM-1 gene via outer membrane vesicles J. Antimicrob. Chemother., 72, 2201–2207
Marion, C.R., Lee, J., Sharma, L., Park, K-S., Lee, C., Liu, W., Liu, P., Feng, J., Gho, Y.S. and Dela Cruz, C.S. (2019) Toll-like receptors 2 and 4 modulate pulmonary inflammation and host factors mediated by outer membrane vesicles derived from Acinetobacter baumannii Infect. Immun. 87: e00243-19
Wachino, J-i., Jin, W., Kimura, K. and Arakawa, Y. (2019) Intercellular transfer of chromosomal antimicrobial resistance genes between Acinetobacter baumannii strains mediated by prophages Antimicrob. Agents Chemother., 63: e00334-19
Aggregatibacter actinomycetemcomitans
Kieselbach, T. and Oscarsson, J. (2017) Dataset of the proteome of purified outer membrane vesicles from the human pathogen Aggregatibacter actinomycetemcomintans Data in Brief, 10, 426-431
Rompikuntal, P.K., Thay, B., Khan, M.K., Alanko, J., Penttinen, A-M., Asikainen, S., Wai, S.N. and Oscarsson, J. (2012) Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans Infect. Immun., 80, 31-42
Alteromonas
Biller, S.J., McDaniel, L.D., Breitbart, M., Rogers, E., Paul, J.H. and Chisholm, S.W. (2017) Membrane vesicles in sea water: heterogeneous DNA content and implications for viral abundance estimates ISME J., 11, 394–404
Bacteroides vulgaris
Maerz, J.K., Steimle, A., Lange, A., Bender, A., Fehrenbacher, B. and Frick, J-S. (2018) Outer membrane vesicles blebbing contributes to B. vulgatus mpk-mediated immune response silencing Gut Microbes., 9(1), 1–12
Borrelia burgdorferi
Coleman, J.L., Crowley, J.T., Toledo, A.M. and Benach, G.L. (2013) The HtrA protease of Borrelia burgdorferi degrades outer membrane protein BmpD and chemotaxis phosphatase CheX Mol. Microbiol., 88, 619–633
Crowley, J.T., Toledo, A.M., LaRocca, T.J., Coleman, J.L., London, E. and Benach, J.L. (2013) Lipid exchange between Borrelia burgdorferi and host cells PLoS Pathog., 9: e1003109
Toledo, A., Coleman, J.L., Kuhlow, C.J., Crowley, J.T. and Benach, J.L. (2012) The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles Infect. Immun., 80, 359-368
Burkholderia glumae
Kang. Y., Goo, E., Kim, J. and Hwang, I. (2017) Critical role of quorum sensing-dependent glutamate metabolism in homeostatic osmolality and outer membrane vesiculation in Burkholderia glumae Sci. Rep., 7: 44195
Burkholderia pseudomallei
Nieves, W., Heang, J., Asakrah, S., Höner zu Bentrup, K., Roy, C.J. and Morici, L.A. (2010) Immuno-specific responses to bacterial elongation factor Tu during Burkholderia infection and immunization PloS One 5: e14361
Campylobacter jejuni
Jang, K-S., Sweredoski, M.J., Graham, R.L.J., Hess, S. and Clemons Jr., W.M. (2014) Comprehensive proteomic profiling of outer membrane vesicles from Campylobacter jejuni J. Proteom., 98, 90-98
Edwardsiella tarda
Chen, S., Yang, D., Wen, Y., Jiang, Z., Zhang, L., Jiang, J., Chen, Y., Hu, T., Wang, Q., Zhang, Y. and Liu, Q. (2018) Dysregulated hemolysin liberates bacterial outer membrane vesicles for cytosolic lipopolysaccharide sensing PLoS Pathog., 14: e1007240
Edwardsiella piscicida
Wen, Y., Chen, S., Jiang, Z., Wang, Z., Tan, J., Hu, T., Wang, Q., Zhou, X., Zhang, Y., Liu, Q. and Yang, D. (2019) Dysregulated haemolysin promotes bacterial outer membrane vesicles‐induced pyroptotic‐like cell death in zebrafish Cell. Microbiol., 21: e13010
Escherichia coli
Balsalobre, C., Silvan, J.M., Berglund, S., Mizunoe, Y., Uhlin, B.E. and Wai, S.N. (2006) Release of the type I secreted α-haemolysin via outer membrane vesicles from Escherichia coli Mol. Microbiol., 59, 99-112
Bielaszewska, M., Ruter, C., Kunsmann, L., Greune, L., Bauwens, A., Zhang, W., Kuczius, T., Kim, K.S., Mellmann, A., Schmidt, M.A. and Karch, H. (2013) Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis PloS Pathog., 9: e1003797
Bielaszewska, M., Ruter, C., Kunsmann, L., Greune, L., Bauwens, A., Zhang, W., Kuczius, T., Kim, K.S., Mellmann, A., Schmidt, M.A. and Karch, H. (2013) Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis PloS Pathog., 9: e1003797
Bielaszewska, M., Rüter, C., Bauwens, A., Greune, L., Jarosch, K-A., Steil, D., Zhang, W., He, X. et al (2017) Host cell interactions of outer membrane vesicle-associated virulence factors of enterohemorrhagic Escherichia coli O157: Intracellular delivery, trafficking and mechanisms of cell injury PloS Pathog., 13: e1006159
Bielaszewska, M., Marejková, M., Bauwens, A., Kunsmann-Prokscha, L., Mellmann, A. and Karch, H. (2018) Enterohemorrhagic Escherichia coli O157 outer membrane vesicles induce interleukin 8 production in human intestinal epithelial cells by signaling via Toll-like receptors TLR4 and TLR5 and activation of the nuclear factor NF-κB Int. J, Med. Microbiol., 308, 882–889
Blenkiron, C., Simonov, D., Muthukaruppan, A., Tsai, P., Dauros, P., Green, S., Hong, J., Print, C.G., Swift, S. and Phillips, A.R. (2016) Uropathogenic Escherichia coli releases extracellular vesicles that are associated with RNA PLoS One, 11, e0160440
Chen, L., Valentine, J.L., Huang, C-Jr., Endicott, C.E., Moeller, T.D., Rasmussen, J.A., Fletcher, J.R., Boll, J.M. et al (2016) Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies Proc. Natl. Acad. Sci. USA, 113, E3609–E3618
Daleke-Schermerhorn, M.H., Felix, T., Soprova, Z., ten Hagen-Jongman, C.M., Vikström, D., Majlessi, L., Beskers, J., Follmann, F., de Punder, K., van der Wel, N.N. et al (2014) Decoration of outer membrane vesicles with multiple antigens by using an autotransporter approach Appl, Environ. Microbiol., 80, 5854–5865
Davis, J.M., Carvalho, H.M., Rasmussen, S.B. and O’Brien, A.D. (2006) Cytotoxic necrotizing factor type 1 delivered by outer membrane vesicles of uropathogenic Escherichia coli attenuates polymorphonuclear leukocyte antimicrobial activity and chemotaxis Infect. Immun., 74, 4401-4408
Ficurilli, M., Liu, C., Riviello, C., Pozo, M.J. and Meers, P.R. (2015) Delivery of liposomal contents to outer membrane vesicles from Gram negative bacteria Biophys. J. 108 (Suppl.1), 408a
Ghosal, A., Upadhyaya, B.B., Fritz, J.V., Heintz-Buschart, A., Desai, M.S., Yusuf, D., Huang, D. et al (2015) The extracellular RNA complement of Escherichia coli Microbiol.Open, 4, 252–266
Hong, J., Dauros-Singorenko, P., Whitcombe, A., Payne, L., Blenkiron, C., Phillips, A. and Swift, S. (2019) Analysis of the Escherichia coli extracellular vesicle proteome identifies markers of purity and culture conditions J. Extracell. Ves., 8: 1632099
Horstman, A.L. and Kuehn, M.J. (2000) Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles J. Biol. Chem., 275, 12489-12496
Kesty, N.C. and Kuehn, M.J. (2004) Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles J. Biol. Chem., 279, 2069-2076
Kim, J-Y., Doody, A.M., Chen, D.J., Cremona, G.H., Shuler, M.L., Putnam, D. and DeLisa, M.P. (2008) Engineered bacterial outer membrane vesicles with enhances functionality J. Mol. Biol., 380, 51-66
Kim, O.Y., Choi, S.J., Jang, S.C., Park, K-S., Kim, S.R., Choi, J.P., Lim, J.H., Lee, S-W. et al (2015) Bacterial protoplast-derived nanovesicles as vaccine delivery system against bacterial infection Nano Lett. 15, 266−274
Kim, O.Y., Dinh, N.T.H., Park, H.T., Choi, S.J., Hong, K. and Gho, Y.S. (2017) Bacterial protoplast-derived nanovesicles for tumor targeted delivery of chemotherapeutics Biomaterials, 113, 68-79
Kim, O.Y., Park, H.T., Dinh, N.T.H., Choi, S.J., Lee, J., Kim, J.H., Lee, S-W. and Gho, Y.S. (2017) Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response Nat. Comm., 8: 626
Kunsmann, L., Rüter, C., Bauwens, A., Greune, L., Glüder, M., Kemper, B., Fruth, A., Wai, S.N., He, X., Lloubes, R. et al (2015) Virulence from vesicles: Novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain Sci. Rep., 5: 13252
McBroom, A.J., Johnson, A.P., Vemulapalli, S. and Kuehn, M.J. (2006) Outer membrane vesicle production by Escherichia coli is independent of membrane instability J. Bacteriol., 188, 5385-5392
McBroom, A.J. and Kuehn, M.J. (2007) Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response Mol. Microbiol., 63, 545-558
Park, M., Sun, Q., Liu, F., DeLisa, M.P. and Chen, W. (2014) Positional assembly of enzymes on bacterial outer membrane vesicles for cascade reactions PloS One, 9: e97103
Roier, S., Zingl, F.G., Cakar, F., Durakovic, S., Kohl, P., Eichmann, T.O., Kiug, L., Gadermaier, B. et al (2016) A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria Nat. Comm., 7: 10515
Roy, K., Hamilton, D.J., Munson, G.P. and Fleckenstein, J.M. (2011) Outer membrane vesicles induce immune responses to virulence proteins and protect against colonization by enterotoxigenic Escherichia coli Clin. Vaccine Immunol., 18, 1803–1808
Tiana, Y., Chen, C., Niu, Q., Zhu, S. and Yan, X. (2019) Analysis of fluorescent labelling efficiency of extracellular vesicles derived from different kingdoms of life with lipid-binding dyes via nano-flow cytometry J. Extracell. Ves., 8 (Suppl.1) PF06.06
Valentine, J.L., Chen, L., Perregaux, E.C., Weyant, K.B., Rosenthal, J.A., Heiss, C., Azadi, P., Fisher, A.C., Putnam, D. et al (2016) Immunization with outer membrane vesicles displaying designer glycotopes yields classswitched, glycan-specific antibodies Cell Chem. Biol., 23, 655–665
Zhao, H. and Martinis, S.A. (2017) Isolation of bacterial compartments to track movement of protein synthesis factors Methods, 113, 120–126
Flavobacterium columnare
Laanto, E., Penttinen, R.K., Bamford, J.K.H. and Sundberg, L-R. (2014) Comparing the different morphotypes of a fish pathogen – implications for key virulence factors in Flavobacterium columnare BMC Microbiol., 14:170
Francisella novicida
McCaig, W.D., Koller, A. and Thanassi, D.G. (2013) Production of outer membrane vesicles and outer membrane tubes by Francisella novicida J. Bacteriol., 195, 1120-1132
Francisella tularensis
Chen, L., Valentine, J.L., Huang, C-Jr., Endicott, C.E., Moeller, T.D., Rasmussen, J.A., Fletcher, J.R., Boll, J.M. et al (2016) Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies Proc. Natl. Acad. Sci. USA, 113, E3609–E3618
Chen, F., Cui, G., Wang, S., Nair, M.K.M., He, L., Qi, X., Han, X., Zhang, H., Zhang, J-R. and Su, J (2017) Outer membrane vesicle-associated lipase FtlA enhances cellular invasion and virulence in Francisella tularensis LVS Emerg. Microbes Infect., 6: e66
Sampath, V., McCaig, W.D. and Thanassi, D.G. (2018) Amino acid deprivation and central carbon metabolism regulate the production of outer membrane vesicles and tubes by Francisella Mol.Microbiol., 107, 523–541
Fusobacterium nucleatum
Liu, J., Hsieh, C-L., Gelincik, O., Devolder, B., Sei, S., Zhang, S., Lipkin, S.M. and Chang, Y.F. (2019) Proteomic characterization of outer membrane vesicles from gut mucosa derived Fusobacterium nucleatum J. Proteom., 195, 125–137
Haemophilus influenzi
Roier, S., Blume, T., Klug, L., Wagner, G.E., Elhenawy, W., Zangger, K., Prassl, R., Reidl, J., Daum, G., Feldman, M.F. and Schild, S. (2015) A basis for vaccine development: comparative characterization of Haemophilus influenzae outer membrane vesicles Int. J. Med. Microbiol., 305, 298–309
Roier, S., Zingl, F.G., Cakar, F., Durakovic, S., Kohl, P., Eichmann, T.O., Kiug, L., Gadermaier, B. et al (2016) A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria Nat. Comm., 7: 10515
Sharpe, S.W., Kuehn, M.J. and Mason, K.M. (2011) Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of non-typeable haemophilus influenzae Infect. Immun., 79, 4361-4369
Haemophilus parasuis
McCaig, W.D., Loving, C.L., Hughes, H.R. and Brockmeier, S.L. (2016) Characterization and vaccine potential of outer membrane vesicles produced by Haemophilus parasuis PLoS One 11: e0149132
Helicobacter pylori
Choi, H-I., Choi, J-P., Seo, J., Kim, B.J., Rho, M., Han, J.K. and Kim, J.G. (2017) Helicobacter pylori-derived extracellular vesicles increased in the gastric juices of gastric adenocarcinoma patients and induced inflammation mainly via specific targeting of gastric epithelial cells Exp. Mol. Med., 49, e330
Liu, Q., Li, X., Zhang, Y., Song, Z., Li, R., Ruan, H. and Huang, X. (2019) Orally-administered outermembrane vesicles from Helicobacter pylori reduce H. pylori infection via Th2-biased immune responses in mice Pathog. Dis., 77: ftz050
Zavan, L., Bitto, N.J., Johnston, E.L., Greening, D.W. and Kaparakis-Liaskos, M. (2019) Helicobacter pylori growth stage determines the size, protein composition, and preferential cargo packaging of outer membrane vesicles Proteomics, 19: 1800209
Klebsiella pneumoniae
Cahill, B.K., Seeley, K.W., Gutel, D. and Ellis, T.N. (2015) Klebsiella pneumoniae O antigen loss alters the outer membraneprotein composition and the selective packaging of proteins into secreted outer membrane vesicles Microbiol. Res., 180, 1–10
Legionella pneumophila
Fernandez-Moreira, E., Helbig, J.H. and Swanson, M.S. (2006) Membrane vesicles shed by Legionella pneumophila inhibit fusion of phagosomes with lysosomes Infect. Immun., 74, 3285-3295
Lysobacter enzymogenes
Meers, P.R., Liu, C., Chen, R., Bartos, W. Davis, J. Dziedzic, N. Orciuolo, J., Kutyla, S., Jose, M. et al (2018) Vesicular delivery of the antifungal antibiotics of Lysobacter enzymogenes C3 Appl. Environ. Microbiol., 84, e01353-18
Marinobacter guineae
See “Shewanella livingstonensis”
Mycobacteria
See Section 3b
Neisseria gonorrhoeae
Pérez-Cruz, C., Delgado, L., López-Iglesias, C. and Mercade, E. (2015) Outer-inner membrane vesicles naturally secreted by Gram-negative pathogenic bacteria PLoS One, 10: e0116896
Deo, P., Chow, S.H., Hay, I.D., Kleifeld, O., Costin, A., Elgass, K.D., Jiang, J-H., Ramm, G. et al (2018) Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis PLoS Pathog., 14: e1006945
Neisseria meningitides
Matthias, K.A., Strader, M.B., Nawar, H.F., Gao, Y.S., Lee, J., Patel, D.S., Im, W. and Bash, M.C. (2017) Heterogeneity in non-epitope loop sequence and outer membrane protein complexes alters antibody binding to the major porin protein PorB in serogroup B Neisseria meningitides Mol. Microbiol., 105, 934–953
Porphyromonas gingivalis
Cecil, J.D., O’Brien-Simpson, N.M., Lenzo, J.C., Holden, J.A., Chen, Y-Y., Singleton, W., Gause. K.T., Yan, Y., Caruso, F. and Reynolds, E.C. (2016) Differential responses of pattern recognition receptors to outer membrane vesicles of three periodontal pathogens PLoS One 11: e0151967
Prochlorococcus
Biller, S.J., McDaniel, L.D., Breitbart, M., Rogers, E., Paul, J.H. and Chisholm, S.W. (2017) Membrane vesicles in sea water: heterogeneous DNA content and implications for viral abundance estimates ISME J., 11, 394–404
Pseudoalteromona
See “Shewanella livingstonensis”
Pseudomonas aeruginosa
Ballok, A.E., Filkins, L.M., Bomberger, J.M., Stanton, B.A. and O’Toole, G.A. (2014) Epoxide-mediated differential packaging of Cif and other virulence factors into outer membrane vesicles J. Bacteriol., 196, 3633–3642
Barnaby, R., Koeppen, K. and Stanton B.A. (2018) Cyclodextrins reduce the ability of Pseudomonas aeruginosa outer-membrane vesicles to reduce CFTR Cl–secretion Am. J. Physiol. Lung Cell Mol. Physiol., 316, L206–L215
Bauman, S.J. and Kuehn, M.J. (2006) Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response Microbes Infect., 8, 2400-2408
Bauman, S.J. and Kuehn, M.J. (2009) Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells BMC Microbiol., 9:26
Bomberger, J.M., MacEachran, D.P., Coutermarsh, B.A., Ye, S., O’Toole, G.A. and Stanton, B.A. (2009) Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles PLoS Pathog., 5:e1000382
Couto, N., Schooling, S.R., Dutcher, J.R. and Barber, J. (2015) Proteome profiles of outer membrane vesicles and extracellular matrix of Pseudomonas aeruginosa biofilms J. Proteome Res., 14, 4207−4222
Ellis, T.N., Leiman, S.A. and Kuehn, M.J. (2010) Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components Infect. Immun., 78, 3822-3831
Esoda, C.N., Kuehn, M.J. (2019) Pseudomonas aeruginosa leucine aminopeptidase influences early biofilm composition and structure via vesicle-associated antibiofilm activity Host-Microbe Biol., 10: e02548-19
Koeppen, K., Hampton, T.H., Jarek, M., Scharfe, M., Gerber, S.A., Mielcarz, D.W., Demers, E.G., Dolben, E.L. et al (2016) A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles PloS Pathog., 12: e1005672
Koeppen, K., Barnaby, R., Jackson, A.A., Gerber, S.A., Hogan, D.A. and Stanton, B.A. (2019) Tobramycin reduces key virulence determinants in the proteome of Pseudomonas aeruginosa outer membrane vesicles PLoS One, 14: e0211290
MacDonald, I.A. and Kuehn, M.J. (2013) Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa J. Bacteriol., 195, 2971–2981
MacEachran, D.P., Ye, S., Bomberger, J.M., Hogan, D.A., Swiatecka-Urban, A., Stanton, B.A. and O’Toole, G.A. (2007) The Pseudomonas aeruginosa secreted protein PA2934 decreases apical membrane expression of the cystic fibrosis transmembrane conductance regulator Infect. Immun., 75, 3902-3912
Schooling, S.R., Hubley, A. and Beveridge, T.J. (2009) Interactions of DNA with biofilm-derived membrane vesicles J. Bacteriol., 191, 4097-4102
Tashiro, Y., Sakai, R., Toyofuku, M., Sawada, I., Nakajima-Kambe, T., Uchiyama, H. and Nomura, N. (2009) Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa J. Bacteriol., 191, 7509-7519
Tashiro, Y., Ichikawa, S., Shimizu, M., Toyofuku, M., Takaya, N., Nakajima-Kambe, T., Uchiyama, H. and Nomura, N. (2010) Variation of physiochemical properties and cell association activity of membrane vesicles with growth phase in Pseudomonas aeruginosa Appl. Environ. Microbiol., 76, 3732-3239
Toyofuku, M., Roschitzki, B., Riedel, K. and Eberl, L. (2012) Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix J. Proteome Res., 11, 4906−4915
Toyofuku, M., Zhou, S., Sawada, I., Takaya, N., Uchiyama, H. and Nomura, N. (2014) Membrane vesicle formation is associated with pyocin production under denitrifying conditions in Pseudomonas aeruginosa PAO1 Environ. Microbiol., 16, 2927–2938
Turnbull, L., Toyofuku, M., Hynen, A.L., Kurosawa, M., Pessi, G., Petty, N.K., Osvath, S.R., CárcamoOyarce, G. et al (2016) Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms Nat. Comm., 7: 11220
Zhao, K., Deng, X., He, C., Yue, B. and Wu, M. (2013) Pseudomonas aeruginosa outer membrane vesicles modulate host immune responses by targeting the Toll-like receptor 4 signaling pathway Infect. Immun., 81, 4509-4518
Pseudomonas panacis (from faeces)
Choi, Y., Kwon, Y., Kim, D-K., Jeon, J., Jang, S.C., Wang, T., Ban, M., Kim, M-H., Jeon, S.G. et al (2015) Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle Sci. Rep., 5: 15878
Psychrobacter fozii
See “Shewanella livingstonensis”
Salinicola
Biller, S.J., McDaniel, L.D., Breitbart, M., Rogers, E., Paul, J.H. and Chisholm, S.W. (2017) Membrane vesicles in sea water: heterogeneous DNA content and implications for viral abundance estimates ISME J., 11, 394–404
Salmonella enterica
Bai, J., Kim, S.I., Ryu, S. and Yoon, H. (2014) Identification and characterization of outer membrane vesicleassociated proteins in Salmonella enterica Serovar Typhimurium Infect. Immun., 82, 4001–4010
Bonnington, K.E. and Kuehn, M.J. (2016) Outer membrane vesicle production facilitates LPS remodeling and outer membrane maintenance in Salmonella during environmental transitions mBio, 7: e01532-16
Daleke-Schermerhorn, M.H., Felix, T., Soprova, Z., ten Hagen-Jongman, C.M., Vikström, D., Majlessi, L., Beskers, J., Follmann, F., de Punder, K., van der Wel, N.N. et al (2014) Decoration of outer membrane vesicles with multiple antigens by using an autotransporter approach Appl, Environ. Microbiol., 80, 5854–5865
Habier, J., May, P., Heintz-Buschart, A., Ghosal, A., Wienecke-Baldacchino, A.K., Nolte-‘t Hoen, E.N.M., Wilmes, P. and Fritz, J.V. (2018) Extraction and analysis of RNA isolated from pure bacteria-derived outer membrane vesicles In Bacterial Regulatory RNA: Methods and Protocols, Methods in Mol. Biol., 1737 (eds. Arluison, V. and Valverde, C.) Springer Science+Business Media, LLC, pp 213-230
Kim, O.Y., Park, H.T., Dinh, N.T.H., Choi, S.J., Lee, J., Kim, J.H., Lee, S-W. and Gho, Y.S. (2017) Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response Nat. Comm., 8: 626
Kitagawa, R., Takaya, A., Ohya, M., Mizunoe, Y., Takade, A., Yoshida, S-i., Isogai, E. and Yamamoto, T. (2010) Biogenesis of Salmonella enterica serovar Typhimurium membrane vesicles provoked by induction of PagC J. Bacteriol., 192, 5645–5656
Liu, Q., Liu, Q., Yi, J., Liang, K., Liu, T., Roland, K.L., Jiang, Y. and Kong, Q. (2016) Outer membrane vesicles derived from Salmonella Typhimurium mutants with truncated LPS induce cross-protective immune responses against infection of Salmonella enterica serovars in the mouse model Int. J. Med. Microbiol., 306, 697–706
Muralinath, M., Kuehn, M.J., Roland, K.L. and Curtiss III, R. (2011) Immunization with Salmonella enterica serovar Typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae Infect. Immun., 79, 887–894
Shewanella livingstonensis
Frias, A., Manresa, A., de Oliveira, E., López-Iglesias, C. and Mercade, E. (2010) Membrane vesicles: a common feature in the extracellular matter of cold-adapted Antarctic bacteria Microb. Ecol., 59, 476–486
Yokoyama, F., Kawamoto, J., Imai, T. and Kurihara, T. (2017) Characterization of extracellular membrane vesicles of an Antarctic bacterium, Shewanella livingstonensis Ac10, and their enhanced production by alteration of phospholipid composition Extremophiles 21: 723–731
Shewanella veisiculosa
Frias, A., Manresa, A., de Oliveira, E., López-Iglesias, C. and Mercade, E. (2010) Membrane vesicles: a common feature in the extracellular matter of cold-adapted Antarctic bacteria Microb. Ecol., 59, 476–486
Pérez-Cruz, C., Carrión, O., Delgado, L., Martinez, G., López-Iglesias, C. and Mercade, E. (2013) New type of outer membrane vesicle produced by the Gram-negative bacterium Shewanella vesiculosa M7T: implications for DNA content Appl. Environ. Microbiol., 79, 1874-1881
Tannerella forsythia
Cecil, J.D., O’Brien-Simpson, N.M., Lenzo, J.C., Holden, J.A., Chen, Y-Y., Singleton, W., Gause. K.T., Yan, Y., Caruso, F. and Reynolds, E.C. (2016) Differential responses of pattern recognition receptors to outer membrane vesicles of three periodontal pathogens PLoS One 11: e0151967
Veith, P.D., Chen, Y-Y., Chen, D., O’Brien-Simpson, N.M., Cecil, J.D., Holden, J.A., Lenzo, J.C. and Reynolds, E.C. (2015) Tannerella forsythia outer membrane vesicles are enriched with substrates of the type IX secretion system and TonB-dependent receptors J. Proteome Res., 14, 5355−5366
Thalassospria
Biller, S.J., McDaniel, L.D., Breitbart, M., Rogers, E., Paul, J.H. and Chisholm, S.W. (2017) Membrane vesicles in sea water: heterogeneous DNA content and implications for viral abundance estimates ISME J., 11, 394–404
Treponema denticola
Cecil, J.D., O’Brien-Simpson, N.M., Lenzo, J.C., Holden, J.A., Chen, Y-Y., Singleton, W., Gause. K.T., Yan, Y., Caruso, F. and Reynolds, E.C. (2016) Differential responses of pattern recognition receptors to outer membrane vesicles of three periodontal pathogens PLoS One 11: e0151967
Veith, P.D., Glew, M.D., Gorasia, D.G., Chen, D., O’Brien-Simpson, N.M. and Reynolds, E.C. (2019) Localization of outer membrane proteins in Treponema denticola by quantitative proteome analyses of outer membrane vesicles and cellular fractions J. Proteome Res., 18, 1567−1581
Vibrio cholerae
Bitar, A., Aung, K.M., Wai, S.N., and Hammarström, M-L. (2019) Vibrio cholerae derived outer membrane vesicles modulate the inflammatory response of human intestinal epithelial cells by inducing microRNA-146a Sci. Rep., 9: 7212
Elluri, S., Enow, C., Vdovikova, S., Rompikuntal, P.K., Dongre, M., Carlsson, S., Pal, A., Uhlin, B.E., Wai, S.N. (2014) Outer membrane vesicles mediate transport of biologically active Vibrio cholerae cytolysin (VCC) from V. cholerae strains PLoS One, 9: e106731
Kohl, P., Zingl, F.G., Eichmann, T.O. and Schild, S. (2018) Isolation of outer membrane vesicles including their quantitative and qualitative analyses In Vibrio Cholerae: Methods and Protocols, Methods in Mol. Biol., 1839, (ed. Sikora, A.E.), Springer Science+Business Media, LLC, pp 117-134
Mondal, A., Tapader, R., Chatterjee, N.S., Ghosh, A., Sinha, R., Koley, H., Saha, D.R., Chakrabarti, M.K., Wai, S.N. and Pala, A. (2016) Cytotoxic and inflammatory responses induced by outer membrane vesicleassociated biologically active proteases from Vibrio cholerae Infect. Immun., 84, 1478-1490
Roier, S., Zingl, F.G., Cakar, F., Durakovic, S., Kohl, P., Eichmann, T.O., Kiug, L., Gadermaier, B. et al (2016) A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria Nat. Comm., 7: 10515
Sjöström, A.E., Sandblad, L., Uhlin, B.E. and Wai, S.N. (2015) Membrane vesicle-mediated release of bacterial RNA Sci. Rep., 5: 15329
Vibrio shilonii
Li, J., Azam, F. and Zhang, S. (2016) Outer membrane vesicles containing signalling molecules and active hydrolytic enzymes released by a coral pathogen Vibrio shilonii AK1 Environ. Microbiol., 18, 3850–3866
Vibrio tasmaniensis
Vanhove, A.S., Duperthuy, M., Charrière, G.M., Le Roux, F., Goudenège, D., Gourbal, B., Kieffer-Jaquinod, S., Couté, Y., Wai, S.N. and Destoumieux-Garzón, D. (2015) Outer membrane vesicles are vehicles for the delivery of Vibrio tasmaniensis virulence factors to oyster immune cells Environ. Microbiol., 17, 1152–1165
Yersinia pestis
Eddy, J.L., Gielda, L.M., Caulfield, A.J., Rangel, S.M. and Lathem, W.W. (2014) Production of outer membrane vesicles by the plague pathogen Yersinia pestis PloS One, 9: e107002
Yersinia pseudotuberculosis
Monnappa, A.K., Bari, W., Seo, J.K. and Mitchell, R.J. (2018) The cytotoxic necrotizing factor of Yersinia pseudotuberculosis (CNFy) is carried on extracellular membrane vesicles to host cells Sci. Rep., 8:14186
3b. Gram-positive bacteria and mycobacteria
Acholeplasma
Medvedeva, E.S., Mouzykantov, A.A.,Baranova, N.B., Dramchini, M.A., Chernova, O.A. and Chernov, V.M. (2019) Data on proteomic profiling of cells and extracellular vesicles of the melittin-resistant Acholeplasma laidlawii strain Data in brief, 25, 104169
Bacillus anthracis
Wolf, J.M., Rivera, J. and Casadevall, A. (2012) Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles Cellular Microbiology (2012) 14(5), 762–773
Bacillus subtilis
Brown, L., Kessler, A., Cabezas-Sanchez, P., Luque-Garcia, J.L. and Casadevall, A. (2014) Extracellular vesicles produced by the Gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin Mol. Microbiol., 93, 183–198
Prados-Rosales, R., Brown, L., Casadevall, A., Montalvo-Quiros, S. and Luque-Garcia, J.L. (2014) Isolation and identification of membrane vesicle-associated proteins in Gram-positive bacteria and mycobacteria MethodsX, 1, 124–129
Clostridium perfringens
Jiang. Y., Kong, Q., Roland, K.L. and Curtiss III, R. (2014) Membrane vesicles of Clostridium perfringens type A strains induce innate and adaptive immunity Int. J, Med. Microbiol., 304, 431–443
Obana, N., Nakao, R., Nagayama, K., Nakamura, K., Senpuku, H. and Nomuraa, N. (2017) Immunoactive clostridial membrane vesicle production is regulated by a sporulation factor Infect. Immun., 85: e00096-17
Enterococcus faecium
Wagner, T., Joshi, B., Janice, J., Askarian, F., Škalko-Basnet, N., Hagestad, O.C., Mekhlif, A., Wai, S.N., Hegstad, K. and Johannessen, M. (2018) Enterococcus faecium produces membrane vesicles containing virulence factors and antimicrobial resistance related proteins J.Proteom., 187, 28–38
Lactobacillus acidophilus
Kim, O.Y., Park, H.T., Dinh, N.T.H., Choi, S.J., Lee, J., Kim, J.H., Lee, S-W. and Gho, Y.S. (2017) Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response Nat. Comm., 8: 626
Lactobacillus sakei
Yamasaki-Yashiki, S., Yuki Miyoshi, Y., Nakayama, T., Kunisawa, J. and Katakura, Y. (2019) IgA-enhancing effects of membrane vesicles derived from Lactobacillus sakei subsp. sakei NBRC15893 Biosci. Microbiota Food Health, 38, 23–29
Listeria monocytogenes
Coelho, C., Brown, L., Maryam, M., Vij, R., Smith, D.F.Q., Burnet, M.C., Kyle, J.E., Heyman, H.M., Ramirez, J. et al (2019) Listeria monocytogenes virulence factors, including listeriolysin O, are secreted in biologically active extracellular vesicles J. Biol. Chem., 294, 1202–1217
Mycobacterium smegmatis
Dauros Singorenko, P., Chang, V., Whitcombe, A., Simonov, D., Hong, J., Phillips, A., Swift, S. and Blenkiron, C. (2017) Isolation of membrane vesicles from prokaryotes: a technical and biological comparison reveals heterogeneity J. Extracell. Ves., 6: 1324731
Mycobacterium tuberculosis
D’Lima, N.G. and Teschke, C.M. (2015) A method to investigate protein association with intact sealed mycobacterial membrane vesicles Anal. Biochem. 485, 109–111
Lee, J., Kim, S-H., Choi, D-S., Lee, J.S., Kim, D-K., Go, G., Park, S-M., Kim, S.H., Shin, J.H., Chang, C.L. and Gho, Y.S. (2015) Proteomic analysis of extracellular vesicles derived from Mycobacterium tuberculosis Proteomics, 15, 3331–3337
Palacios, A., Sampedro, L., Sevilla, I.A., Molina, E., Gil, D., Azkargorta, M., Elortza, F., Garrido, J.M., Anguita, J. and Prados-Rosales, R. (2019) Mycobacterium tuberculosis extracellular vesicle-associated lipoprotein LpqH as a potential biomarker to distinguish paratuberculosis infection or vaccination from tuberculosis infection BMC Vet. Res., 15: 188
Prados-Rosales, R., Weinrick, B.C., Piqué, D.G., Jacobs, Jr., W.R., Casadevall,A. and Rodriguez, G.M. (2014) Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition J. Bacteriol., 196, 1250–1256
Prados-Rosales, R., Brown, L., Casadevall, A., Montalvo-Quiros, S. and Luque-Garcia, J.L. (2014) Isolation and identification of membrane vesicle-associated proteins in Gram-positive bacteria and mycobacteria MethodsX, 1, 124–129
Ratha, P., Huang, C., Wang, T., Wang, T., Li, H., Prados-Rosales, R., Elemento, O., Casadevall, A. and Nathan, C.F. (2013) Genetic regulation of vesiculogenesis and immunomodulation in Mycobacterium tuberculosis Proc. Natl. Acad. Sci. USA, 110, E4790–E4797
Proprionibacterium acnes
Jeon, J., Park, S.C., Her, J., Lee, J.W., Han, J-K., Kim, Y-K., Kim, K.P. and Ban, C, (2018) Comparative lipidomic profiling of the human commensal bacterium Propionibacterium acnes and its extracellular vesicles RSC Adv., 8, 15241–15247
Staphylococcus aureus
Askarian, F., Lapek Jr., J.D., Dongre, M., Tsai, C-M., Kumaraswamy, M., Kousha, A., Valderrama, J.A., Ludviksen, J.A. et al (2018) Staphylococcus aureus membrane-derived vesicles promote bacterial virulence and confer protective immunity in murine infection models Front. Physiol., 9: 262
Askarian, F., Lapek Jr., J.D., Dongre, M., Tsai, C-M., Kumaraswamy, M., Kousha, A., Valderrama, J.A., Ludviksen, J.A. et al (2018) Staphylococcus aureus membrane-derived vesicles promote bacterial virulence and confer protective immunity in murine infection models Front. Physiol., 9: 262
Streptococcus haemolyticus
Cavanagh, J.P., Askarian, F., Pain, M., Bruun, J-A., Urbarova, I., Wai, S.N., Schmidt, F. and Johannessen, M. (2019) Proteome profiling of secreted and membrane vesicle associated proteins of an invasive and a commensal Staphylococcus haemolyticus isolate Data in Brief, 22, 914–919
Cavanagh, J.P., Pain, M., Askarian, F., Bruune, J-A., Urbarova, I., Waif, S.N., Schmidt, F. and Johannessen, M. (2019) Comparative exoproteome profiling of an invasive and a commensal Staphylococcus haemolyticus isolate J. Proteom., 197, 106–114
Cavanagh, J.P., Askarian, F., Pain, M., Bruun, J-A., Urbarova, I., Wai, S.N., Schmidt, F. and Johannessen, M. (2019) Proteome profiling of secreted and membrane vesicle associated proteins of an invasive and a commensal Staphylococcus haemolyticus isolate Data in Brief, 22, 914–919
Cavanagh, J.P., Pain, M., Askarian, F., Bruune, J-A., Urbarova, I., Waif, S.N., Schmidt, F. and Johannessen, M. (2019) Comparative exoproteome profiling of an invasive and a commensal Staphylococcus haemolyticus isolate J. Proteom., 197, 106–114
Askarian, F., Lapek Jr., J.D., Dongre, M., Tsai, C-M., Kumaraswamy, M., Kousha, A., Valderrama, J.A., Ludviksen, J.A. et al (2018) Staphylococcus aureus membrane-derived vesicles promote bacterial virulence and confer protective immunity in murine infection models Front. Physiol., 9: 262
Streptococcus haemolyticus
Cavanagh, J.P., Askarian, F., Pain, M., Bruun, J-A., Urbarova, I., Wai, S.N., Schmidt, F. and Johannessen, M. (2019) Proteome profiling of secreted and membrane vesicle associated proteins of an invasive and a commensal Staphylococcus haemolyticus isolate Data in Brief, 22, 914–919
Cavanagh, J.P., Pain, M., Askarian, F., Bruune, J-A., Urbarova, I., Waif, S.N., Schmidt, F. and Johannessen, M. (2019) Comparative exoproteome profiling of an invasive and a commensal Staphylococcus haemolyticus isolate J. Proteom., 197, 106–114
Lee, E-Y., Choi, D-Y., Kim, D-K., Kim, J-W., Park, J O., Kim, S., Kim, S-H., Desiderio, D.M., Kim, Y-K., Kim, K-P- and Gho, Y.S. (2009) Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles Proteomics, 9, 5425-5436
Kim, O.Y., Park, H.T., Dinh, N.T.H., Choi, S.J., Lee, J., Kim, J.H., Lee, S-W. and Gho, Y.S. (2017) Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response Nat. Comm., 8: 626
Schlatterer, K., Beck, C., Hanzelmann, D., Lebtig, M., Fehrenbacher, B., Schaller, M., Ebner, P., Nega, M. et al (2018) The mechanism behind bacterial lipoprotein release: phenol-soluble modulins mediate toll-like receptor 2 activation via extracellular vesicle release from Staphylococcus aureus mBio, 9, e01851-18
Thay, B., Wai, S.N. and Oscarsson, J. (2013) Staphylococcus aureus α-toxin-dependent induction of host cell death by membrane-derived vesicles PloS One, 8: e54661
Tiana, Y., Chen, C., Niu, Q., Zhu, S. and Yan, X. (2019) Analysis of fluorescent labelling efficiency of extracellular vesicles derived from different kingdoms of life with lipid-binding dyes via nano-flow cytometry J. Extracell. Ves., 8 (Suppl.1) PF06.06
Wang, X., Thompson, C.D., Weidenmaier, C. and Lee, J.C. (2018) Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform Nat. Comm., 9: 1379
Streptococcus pneumoniae
Codemo, M., Muschiol, S., Iovino, F., Nannapaneni, P., Plant, L., Wai, S.N. and Henriques-Normark, B. (2018) Immunomodulatory effects of pneumococcal extracellular vesicles on cellular and humoral host defenses mBio 9: e00559-18
Jhelum, H., Sori, H. and Sehgal, D. (2018) A novel extracellular vesicle associated endodeoxyribonuclease helps Streptococcus pneumoniae evade neutrophil extracellular traps and is required for full virulence Sci. Rep., 8: 7985
Olaya-Abrila, A., Prados-Rosales, R., McConnell, M.J., Martín-Peña, R., González-Reyes, J.A., JiménezMunguía, I., Gómez-Gascón, L.., Fernández, J., et al (2014) Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae J. Proteomics, 106, 46-60
Streptomyces
Hoefler, B.C., Stubbendieck, R.M., Josyula, N.K., Moisan, S.M., Schulze, E.M. and Straight, P.D. (2017) A link between linearmycin biosynthesis and extracellular vesicle genesis connects specialized metabolism and bacterial membrane physiology Cell Chem. Biol., 24, 1238–1249
3c Bacterial exosomes derived from human fluids and waste water
Choi, Y., Kwon, Y., Kim, D-K., Jeon, J., Jang, S.C., Wang, T., Ban, M., Kim, M-H., Jeon, S.G. et al (2015) Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle Sci. Rep., 5: 15878
Choi, Y.J., Lee, D.H., Kim, H.S. and Kim, Y-K. (2018) An exploratory study on the effect of daily fruits and vegetable juice on human gut microbiota Food Sci. Biotechnol., 27, 1377–1386
Maestre-Carballa, L., Gomez, M.L., Navarro, A.A., Garcia-Heredia, I., Martinez-Hernandez, F. and MartinezGarcia, M. (2019) Insights into the antibiotic resistance dissemination in a wastewater effluent microbiome: bacteria, viruses and vesicles matter Environ. Microbiol., 21, 4582–4596
Tulkens, J., De Wever, O. and Hendrix, A. (2020) Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization Nat. Protoc., 15, 40–67
3d. Halobacteria
Erdmann, S., Tschitschko, B., Zhong, L., Raftery, M.J. and Cavicchioli, R. (2017) A plasmid from an Antarctic haloarchaeon uses specialized membrane vesicles to disseminate and infect plasmid-free cells Nat. Microbiol., 1446, 1446–1455
3e. Biofilms
Ficurilli, M., Liu, C., Riviello, C., Pozo, M.J. and Meers, P.R. (2015) Delivery of liposomal contents to outer membrane vesicles from Gram negative bacteria Biophys. J. 108 (Suppl.1), 408a
Laanto, E., Penttinen, R.K., Bamford, J.K.H. and Sundberg, L-R. (2014) Comparing the different morphotypes of a fish pathogen – implications for key virulence factors in Flavobacterium columnare BMC Microbiol., 14:170
Turnbull, L., Toyofuku, M., Hynen, A.L., Kurosawa, M., Pessi, G., Petty, N.K., Osvath, S.R., CárcamoOyarce, G. et al (2016) Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms Nat. Comm., 7: 11220
3f. Mycoplasmas
Acholeplasma laidlawii
Chernov,, V.M., Mouzykantov, A.A., Baranova, N.B., Medvedeva, E.S., Yu Grygorieva, T., Trushina, M.V., Vishnyakov, I.E., Sabantsev, A.V., Borchsenius, S.N. and Chernova, O.A. (2014) Extracellular membrane vesicles secreted by mycoplasma Acholeplasma laidlawii PG8 are enriched in virulence proteins J. Proteom., 110, 117–128
3g. Protozoa, algae, fungi and archaea
Caeiro, L.D., Alba-Soto, C.D., Rizzi, M., Solana, M.E., Rodriguez, G., Chidichimo, A.M., Rodriguez, M.E. et al (2018) The protein family TcTASV-C is a novel Trypanosoma cruzi virulence factor secreted in extracellular vesicles by trypomastigotes and highly expressed in bloodstream forms PLoS Negl. Trop. Dis., 12: e0006475
Camacho, E., Vij, R., Chrissian, C., Prados-Rosales, R., Gil, D., O’Meally, R.N., Cordero, R.J.B., Cole, R.N., McCaffery, J.M., Stark, R.E. and Casadevall, A. (2019) The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans J. Biol. Chem., 294, 10471–10489
Johnson, T.B., Mach, C., Grove, R., Kelly, R., Van Cott, K. and Blum, P. (2018) Secretion and fusion of biogeochemically active archaeal membrane vesicles Geobiology, 16, 659–673
Sampaio, N.G., Cheng, L. and Eriksson, E.M. (2017) The role of extracellular vesicles in malaria biology and pathogenesis Malar. J., 16 245
Long, H., Zhang, F., Xu, N., Liu, G., Diener, D.R., Rosenbaum, J.L. and Huang, K. (2017) Comparative analysis of ciliary membranes and ectosomes Curr. Biol., 26, 3327–3335
Oliveira, D.L., Nimrichter, L., Miranda, K., Frases, S., Faull, K.F., Casadevall, A. and Rodrigues, M.L. (2009) Cryptococcus neoformans cryoultramicrotomy and vesicle fractionation reveals an intimate association between membrane lipids and glucuronoxylomannan Fungal Genet. Biol., 46, 956–963
Regev-Rudzki, N., Wilson, D.W., Carvalho, T.G., Sisquella, X., Coleman, B.M., Rug, M., et al (2013) Cell– cell communication between malaria-infected red blood cells via exosome-like vesicles Cell, 153, 1120–1133
Rodrigues, M.L., Oliveira, D.L., Vargas, G., Girard-Dias, W., Franzen, A.J., Frasés, S., Miranda, K. and Nimrichter, L. (2016) Analysis of yeast extracellular vesicles In Unconventional Protein Secretion: Methods and Protocols, Methods Mol. Biol., 1459 (ed. Pompa, A. and De Marchis, F.), Springer Science+Business Media New York, pp 175-190
Schatz, D., Rosenwasser, S., Malitsky, S., Wolf, S.G., Feldmesser, E. and Vardi, A. (2017) Communication via extracellular vesicles enhances viral infection of a cosmopolitan alga Nat. Microbiol., 2, 1485–1492
Wolf, J.M., Rivera, J. and Casadevall, A. (2012) Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles Cellular Microbiology (2012) 14(5), 762–773
3h. Invertebrates
Matusek, T., Wendler, F., Polès, S., Pizette, S., D’Angelo, G., Fürthauer, M. and Thérond, P.P. (2014) The ESCRT machinery regulates the secretion and long-range activity of Hedgehog Nature, 516, 99-103
Shibata, T., Hadano, J., Kawasaki, D., Dong, X. and Kawabata, S-i. (2017) Drosophila TG-A transglutaminase is secreted via an unconventional Golgi-independent mechanism involving exosomes and two types of fatty acylations J. Biol. Chem., 292, 10723–10734
Thoene, J., Goss, T., Witcher, M., Mullet, J., N’Kuli, F., Van Der Smissen, P., Courtoy, P. and Hahn, S.H. (2013) In vitro correction of disorders of lysosomal transport by microvesicles derived from baculovirusinfected Spodoptera cells Mol. Genet. Metab., 109, 77–85
Vora, A., Zhou, W., Londono-Renteria, B., Woodson, M., Sherman, M.B., Colpitts, T.M., Neelakanta, G. and Sultana, H. (2018) Arthropod EVs mediate dengue virus transmission through interaction with a tetraspanin domain containing glycoprotein Tsp29Fb Proc. Natl. Acad. Sci. USA, 115, E6604–E6613
Zhou, W., Woodson, M., Neupane, B., Bai, F., Sherman, M.B., Choi, K.H., Neelakanta, G., and Sultana, H. (2018) Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells PLoS Pathog., 14: e1006764
3i. Trematodes
Schistosoma mansonii
Meningher, T., Lerman, G., Regev-Rudzki, N., Gold, D., Ben-Dov, I.Z., Sidi, Y., Avni, D. and Schwartz, E. (2017) Schistosomal microRNAs isolated from extracellular vesicles in sera of infected patients: a new tool for diagnosis and follow-up of human schistosomiasis J. Infect. Dis., 215, 378–86
Sotillo, J., Pearson, M., Potriquet, J., Becker, L., Pickering, D., Mulvenna, J. and Loukas, A. (2016) Extracellular vesicles secreted by Schistosoma mansoni contain proteinvaccine candidates Int. J. Parasitol., 46, 1–5
3j Nematodes
Eichenberger, R.M., Talukder, H., Field, M.A., Wangchuk, P., Giacomina, P., Loukas, A. and Sotillo, J. (2018) Characterization of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host–parasite communication J. Extracell. Ves., 7: 1428004
Eichenberger, R.M., Talukder, H. Field, M.A., Wangchuk, P., Giacomin, P.R., Loukas, A. and Sotillo, J. (2018) Extracellular vesicles from the parasitic nematode Trichuris muris: new insights into host–parasite communications J. Extracell. Ves., 7, Suppl. 1, Abstr. # PT01.09
3k. Arthropoda
Zaborowski, M.P., Cheah, P.S., Zhang, X., Bushko, I., Lee, K., Sammarco, A., Zappulli, V., Maas, S.L.N., Allen, R.M. et al (2019) Membrane-bound Gaussia luciferase as a tool to track shedding of membrane proteins from the surface of extracellular vesicles Sci. Rep., 9: 17387
3l. Arabidopsis
Rutter, B.D. and Innes, R.W. (2017) Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins Plant Physiol., 173, 728–741
4. Methodology and functional reviews
Chutkan, H., MacDonald, I., Manning, A. and Kuehn, M.J. (2013) Quantitative and qualitative preparations of bacterial outer membrane vesicles In, Bacterial cell surfaces: Methods and Protocols, Methods Mol, Biol., 966 (ed. Delcour, A.H.) Springer Science+Business Media, pp 259-272
Coelho, C. and Casadevall, A. (2019) Answers to naysayers regarding microbial extracellular vesicles Biochem. Soc. Trans., 47, 1005–1012
Dauros Singorenko, P., Chang, V., Whitcombe, A., Simonov, D., Hong, J., Phillips, A., Swift, S. and Blenkiron, C. (2017) Isolation of membrane vesicles from prokaryotes: a technical and biological comparison reveals heterogeneity J. Extracell. Ves., 6: 1324731
Gnopo, Y.M.D., Watkins, H.C., Stevenson, T.C., DeLisa, M.P. and Putnama, D. (2017) Designer outer membrane vesicles as immunomodulatory systems – Reprogramming bacteria for vaccine delivery Advanced Drug Delivery Rev., 114, 132–142
Kim, O.Y., Lee, J. and Gho, Y.S. (2017) Extracellular vesicle mimetics: Novel alternatives to extracellular vesicle-based theranostics, drug delivery, and vaccines Semin.Cell Dev. Biol., 67, 74–82
Klimentová, J. and Stulík, J. (2015) Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria Microbiol., Res.,170, 1–9
D’Lima, N.G. and Teschke, C.M. (2015) A method to investigate protein association with intact sealed mycobacterial membrane vesicles Anal. Biochem. 485, 109–111
Qing, G., Gong, N., Chen, X., Chen, J., Zhang, H., Wang, Y., Wang, R., Zhang, S., Zhang, Z. (2019) Natural and engineered bacterial outer membrane vesicles Biophys. Rep., 5, 184–198
Toyofuku, M., Tashiro, Y., Hasegawa, Y., Kurosawa, M. and Nomura, N. (2015) Bacterial membrane vesicles, an overlooked environmental colloid: Biology, environmental perspectives and applications Adv. Colloid Interface Sci., 226, 65–77
Tulkens, J., De Wever, O. and Hendrix, A. (2020) Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization Nat. Protoc., 15, 40–67
OptiPrepTM Reference List RS11; 8th edition, January 2020
OptiPrep™ Reference List RS12
Endocytosis – studies on various tissue and cell types
The following references are concerned with the pathways of the endocytic process and describe the use of iodixanol gradients for the purification of a variety of membrane compartments.
- References are primarily sorted into cell/tissue type, or occasionally a cell process: e.g. virus processing
- Each cell/tissue type may be sorted according to the principal analytical study.
- In each section or subsection references are listed alphabetically according to first author; a particular reference may appear in more than one subsection
- Note that the Application Sheet S42 summarizes the available methods for fractionation of components of the endocytic system
There are also several Application Sheets, accessible from the “Subcellular Membranes Index”, devoted to the use of cultured cells or mammalian liver:
- Cultured cells – buoyant density: Application Sheet S46
- Rat liver/hepatocytes – lysosome/late-endosome events: Application Sheet S54
- Rat liver/hepatocytes – sedimentation velocity gradients: Application Sheet S44
- Clathrin-coated vesicles/endosomes/lysosomes (self-generated gradient): Application Sheet S45
There are three other Reference Lists that provide bibliographies of papers reporting the analysis of lipid-rich plasma membrane domains, which may also be relevant in the endocytic process
- RS06 Lipid rich detergent-resistant domains from mammalian cells, tissues and organelles
- RS07 Detergent-free strategy for lipid raft isolation from mammalian cells and tissues
- RS08 Purification of caveolae in gradients prepared from OptiPrep™
- All of the Application Sheets and Reference Lists referred to above can be found via the following website: www.Optiprep.com. One the website click on the “Methodology” tab for the Application Sheets or the “Reference Lists” tab.
- To assist the identification of a relevant reference in the following index key words are highlighted in blue.
Adipocytes
Methodology
Sadler, J.B.A., Lamb, C.A., Gould, G.W. and Bryant, N.J. (2016) Iodixanol gradient centrifugation to separate components of the low-density membrane fraction from 3T3-L1 adipocytes Cold Spring Harb. Protoc., doi:10.1101/pdb.prot083709
Sadler, J.B.A., Lamb, C.A., Welburn, C.R., Adamson, I.S., D., Kioumourtzoglou1, Chi, N-W., Gould, G.W. and Bryant, N.J. (2019) The deubiquitinating enzyme USP25 binds tankyrase and regulates trafficking of the facilitative glucose transporter GLUT4 in adipocytes Sci. Rep., 9: 4710
Airway epithelial cells
Cystic fibrosis membrane conductance regulator
Bomberger, J.M., MacEachran, D., Ye, S., Swiatecka-Urban, A., et al (2007) CFTR inhibitory factor (CIF) reduces the plasma membrane expression of CFTR by altering intracellular trafficking of CFTR to the lysosomal pathway FASEB J., 21, 944.4
Bomberger, J.M., Ye, S., MacEachran, D.P., Koeppen, K., et al (2011) A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system PLoS Pathog., 7: e1001325
Bomberger, J.M., Guggino, W.B. and Stanton, B.A. (2011) Methods to monitor cell surface expression and endocytic trafficking of CFTR in polarized epithelial cells In Cystic Fibrosis, Methods Mol. Biol. (eds. Amaral, M.D. and Kunzelmann, K.) Springer Science+Business Media, pp 271-283
Astrocytes
Autophagy
Luo, G., Sun, Y., Feng, R., Zhao, Q. and Wen, T. (2018) ARL3 subcellular localization and its suspected role in autophagy Biochimie, 154, 187-193
Notch signalling
Valapala, M., Hose, S., Gongora, C., Dong, L., et al (2013) Impaired endolysosomal function disrupts Notch signalling in optic nerve astrocytes Nat. Commun., 4: 1629
Persistent fetal vasculature
Zigler Jr. J.S., Valapala, M., Shang, P., Hose, S., Goldberg, M.F. and Sinha, D. (2016) βA3/A1-crystallin and persistent fetal vasculature (PFV) disease of the eye Biochim. Biophys. Acta, 1860, 287–298
Bacterial phagosomes
Lee, B-Y., Jethwaney, D., Schilling, B., Clemens, D.L., Gibson, B.W. and Horwitz, M.A. (2010) The Mycobacterium bovis bacilli Calmette-Guérin phagosome proteome Mol. Cell. Proteom., 9, 32–53
Li, Q., Jagannath, C., Rao, P.K., Singh, C.R. and Lostumbo, G. (2010) Analysis of phagosomal proteomes: From latex-bead to bacterial phagosomes Proteomics, 10, 4098–4116
BHK cells
Helicobacter pylori toxin
Molinari, M., Galli, C., Norais, N., Telford, J.L., et al (1997) Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers J. Biol. Chem., 272, 25339-25344
Brain tissue/neural cells (see also “Astrocytes” and “Glial cells”)
Adaptins
Zizioli, D., Geumann, C., Kratzke, M., Mishra, R., Borsani, G., Finazzi, D., Candiello, E. and Schua, P. (2017) ɣ2 and ɣ1AP-1 complexes: Different essential functions and regulatory mechanisms in clathrin-dependent protein sorting Eur. J. Cell Biol., 96, 356–368
β-Amyloid protein
Barbero-Camps, E., Roca-Agujetas, V., Bartolessis, I., de Dios, C., Fernández-Checa, J.C., Marí, M., Morales, A, Hartmann, T. and Colell, A. (2018) Cholesterol impairs autophagy-mediated clearance of amyloid beta while promoting its secretion. Autophagy, 14, 1129-1154
Sato, N., Shinohara, M., Rakugi, H. and Morishita, R. (2012) Dual effects of statins on A metabolism: upregulation of the degradation of APP-CTF and A clearance Neurodegener. Dis., 10, 305–308
Shinohara, M., Sato, N., Kurinami, H., Takeuchi, D., et al (2010) Reduction of brain -amyloid (A) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and A clearance J. Biol. Chem., 285, 22091–22102
Tamboli, I.Y., Hampel, H., Sandhoff, K. and Walter, J. (2006) Accumulation of sphingolipids increases secretion of the amyloid β-peptide by stabilization of the β-amyloid precursor protein Alzheimers Dement., 2, Suppl. 1, S528-S529
Dendritic trafficking
Schwenk, B.M., Lang, C.M., Hogl, S., Tahirovic, S., et al (2014) The FTLD risk factor TMEM106B and MAP6 control dendritic trafficking of lysosomes EMBO J., 33, 450-467
Down syndrome mouse model
D’Acunzo, P., Hargash, T., Pawlik, M., Goulbourne, C.N., Perez‐Gonzalez, R. and Levy, E. (2019) Enhanced generation of intraluminal vesicles in neuronal late endosomes in the brain of a Down syndrome mouse model with endosomal dysfunction Devel. Neurobiol., 79, 656–663
Early endosome maturation
Candiello, E., Kratzke, M., Wenzel, D., Cassel, D. and Schu, P. (2016) AP-1/σ1A and AP-1/σ1B adaptorproteins differentially regulate neuronal early endosome maturation via the Rab5/Vps34-pathway Sci. Rep., 6: 29950
Glycolipids
Takamura, A., Higaki, K., Ninomiya, H., Takai, T., et al (2011) Lysosomal accumulation of Trk protein in brain of GM1-gangliosidosis mouse and its restoration by chemical chaperone J. Neurochem., 118, 399–406
Hereditary spastic paraplegia
Khundadze, M., Kollmann, K., Koch, N., Biskup, C., et al (2013) A hereditary spastic paraplegia mouse model supports a role of ZFYVE26/SPASTIZIN for the endolysosomal system PloS Genet., 9: e1003988
Neurite outgrowth
Tao, T., Sun, J., Peng, Y., Li, Y., Wang, P., Chen, X., Zhao, W., Zheng, Y-Y., Wei, L. et al (2019) Golgiresident TRIO regulates membrane trafficking during neurite outgrowth J. Biol. Chem., 294, 10954–10968
Trk protein
Fu, X., Yang, Y., Xu, C., Niu, Y., et al (2011) Retrolinkin cooperates with endophilin A1 to mediate BDNF–TrkB early endocytic trafficking and signaling from early endosomes Mol. Biol. Cell, 22, 3684-3698
Takamura, A., Higaki, K., Ninomiya, H., Takai, T., et al (2011) Lysosomal accumulation of Trk protein in brain of GM1-gangliosidosis mouse and its restoration by chemical chaperone J. Neurochem., 118, 399–406
Caco-2 cells
Cholera toxin
Orlandi, P.A. (1997) Protein-disulfide isomerase-mediated reduction of the A subunit of cholera toxin in a human intestinal cell line J. Biol. Chem., 272, 4591-4599
Van den Broeck, D., Lagrou, A.R. and De Wolf, M.J.S. (2007) Distinct role of clathrin-mediated endocytosis in the functional uptake of cholera toxin Acta Biochim. Polonica, 54, 757-767
Methodology
Li, X. and Donowitz, M. (2008) Fractionation of subcellular membrane vesicles of epithelial and nonepithelial cells by OptiPrep™ density gradient ultracentrifugation In Methods Mol. Biol., 440, Exocytosis and Endocytosis (ed. Ivanov, A.I.) Humana Press, Totowa, NJ, pp 97-110
Li, X. and Donowitz, M. (2014) Fractionation of subcellular membrane vesicles of epithelial and non-epithelial cells by OptiPrep™ density gradient ultracentrifugation In Exocytosis and Endocytosis, Methods in Molecular Biology, 1174 (ed. Ivanov, A,I.) Springer Science+Business Media New York 2014, pp 85-99
Carcinoma cells (incl. HeLa)
Adaptor proteins
Urbanska, A., Sadowski, L., Kalaidzidis, Y. and Miaczynska, M. (2011) Biochemical characterization of APPL endosomes: the role of annexin A2 in APPL membrane recruitment Traffic, 12, 1227–1241
Autophagy
Cohen-Kaplan, V., Livneh, I., Kwon, Y.T. and Ciechanover, A. (2019) Monitoring stress-induced autophagic engulfment and degradation of the 26S proteasome in mammalian cells Meth. Enzymol., 619, 337-366
Gui, X., Yang, H., Li, T., Tan, X., Shi, P., Li, M., Du, F., Chen, Z.J. (2019) Autophagy induction via STING trafficking is a primordial function of the cGAS pathway Nature 567, 262-285
β-Amyloid precursor protein
Matsuda, S., Matsuda, Y., Snapp, E.L. and D’Adamioa, L. (2011) Maturation of BRI2 generates a specific inhibitor that reduces APP processing at the plasma membrane and in endocytic vesicles Neurobiol. Aging, 32, 1400–1408
Vorobyeva, A.G., Lee, R., Miller, S., Longen, C., Sharoni, M. et al (2014) Cyclopamine modulates -secretasemediated cleavage of amyloid precursor protein by altering its subcellular trafficking and lysosomal degradation J. Biol. Chem., 289, 33258–33274
Biogenesis and cargo selection
Dengje, J., Høyer-Hansen, M., Nielsen, M.O., Eisenberg, T., et al (2012) Identification of autophagosomeassociated proteins and regulators by quantitative proteomic analysis and genetic screens Mol. Cell. Proteom., 11: M111.014035
Clathrin-mediated
Barroso-González, J., Machado, J-D., García-Expósito, L. and Valenzuela-Fernández, A. (2009) Moesin regulates the trafficking of nascent clathrin-coated vesicles J. Biol. Chem., 284, 2419–2434
Colon cancer
Duong, H.Q., Nemazanyy, I., Rambow, F., Tang, S.C., Delaunay, S., Tharun, L., Florin, A., Büttner, R., et al (2018) The endosomal protein CEMIP links WNT signaling to MEK1–ERK1/2 activation in selumetinibresistant intestinal organoids Cancer Res. 78, 4533–4548
Ohata, H., Shiokawa, D., Obata, Y., Sato, A., Sakai, H., Fukami, M., Hara, W., Taniguchi, H. et al (2019) NOX1-Dependent mTORC1 activation via S100A9 oxidation in cancer stem-like cells leads to colon cancer progression Cell Rep., 28, 1282–1295
COPI COPII vesicles
Adolf, F., Rhiel, M., Hessling, B., Gao, Q., Hellwig, A., Béthune, J. and Wieland, F.T. (2019) Proteomic profiling of mammalian COPII and COPI vesicles Cell Rep., 26, 250–265
Cytokinesis
Neto, H., Kaupisch, A., Collins, L.L. and Gould, G.W, (2013) Syntaxin 16 is a master recruitment factor for cytokinesis Mol. Biol. Cell, 24, 3663-3674
Endosome maturation and processing
Gireud-Goss, M., Reyes, S., Wilson, M., Farley, M., Memarzadeh, K., Srinivasan, S., Sirisaengtaksin, N., Yamashita, S., Tsunoda, S. et al (2018) Distinct mechanisms enable inward or outward budding from late endosomes/multivesicular bodies Exp. Cell Res., 372,1–15
Huotari, J., Meyer-Schaller, N., Hubner, M., Stauffer, S., et al (2012) Cullin-3 regulates late endosome maturation Proc. Natl. Acad. Sci. USA, 109, 823–828
Li, Q., Spencer, N.Y., Oakley, F.D., Buettner, G.R. and Engelhardt, J.F. (2009) Endosomal Nox2 facilitates redox-dependent induction of NF-κB by TNF-α Antioxid. Redox Signal., 11, 1249–1263
Perini, E.D., Schaefer, R., Stöter, M., Kalaidzidis, Y. and Zerial, M. (2014) Mammalian CORVET Is required for fusion and conversion of distinct early endosome subpopulations Traffic, 15, 1366–1389
Growth factors
Chin, L-S., Raynor, M.C., Wei, X., Chen, H-Q., et al (2001) Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor J. Biol. Chem., 276, 7069-7078
Yakymovych, I., Yakymovych, M., Zang, G., Mu, Y., Bergh, A., Landström, M. and Heldin, K.H. (2015) CIN85 modulates TGFβ signaling by promoting the presentation of TGFβ receptors on the cell surface J. Cell Biol., 210, 319–332
Interleukin-1 receptor complex
Li, Q., Harraz, M.M., Zhou, W., Zhang, L.N., et al (2006) Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAFg to endosomal interleukin-1 receptor complexes Mol. Cell. Biol., 26, 140-154
Lipid droplets
Velikkakath, A.K.G., Nishimura, T., Oita, E., Ishihara, N., et al (2012) Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets Mol. Biol. Cell, 23, 896-909
Multivesicular bodies
Gireud-Goss, M., Reyes, S., Wilson, M., Farley, M., Memarzadeh, K., Srinivasan, S., Sirisaengtaksin, N., Yamashita, S., Tsunoda, S. et al (2018) Distinct mechanisms enable inward or outward budding from late endosomes/multivesicular bodies Exp. Cell Res., 372,1–15
Notch signalling
Tagami, S., Okochi, M., Yanagida, K., Ikuta, A., et al (2008) Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1 Mol. Cell. Biol., 28, 165-76
Rab GTPase
Meyers, J.M. and Prekeris, R. (2002) Formation of mutually exclusive Rab11 complexes with members of the family of Rab11-interacting proteins regulates Rab11 endocytic targeting and function J. Biol. Chem., 277, 49003-49010
Proikas-Cezanne, T., Gaugel, A., Frickey, T. and Nordheim, A. (2006) Rab14 is part of the early endosomal clathrin-coated TGN microdomain FEBS Lett., 580, 5241-5246
Urbanska, A., Sadowski, L., Kalaidzidis, Y. and Miaczynska, M. (2011) Biochemical characterization of APPL endosomes: the role of annexin A2 in APPL membrane recruitment Traffic, 12, 1227–1241
ROS
Mumbengegwi, D.R., Li, Q., Li, C., Bear, C.E., et al (2008) Evidence for a superoxide permeability in endosomal membranes Mol. Cell. Biol., 28, 3700-3712
Salmonella-containing vacuole
Santos, J.C., Duchateau, M., Fredlund, J., Weiner, A., Mallet, A., Schmitt, C., Matondo, M., Hourdel, V., Chamot-Rooke, J. and Enninga, J. (2015) The COPII complex and lysosomal VAMP7 determine intracellular Salmonella localization and growth Cell. Microbiol., 17, 1699–1720
Syntaxins
Neto, H., Kaupisch, A., Collins, L.L. and Gould, G.W, (2013) Syntaxin 16 is a master recruitment factor for cytokinesis Mol. Biol. Cell, 24, 3663-3674
Virus internalization and interactions
Ding, W., Zhang, L.N., Yeaman, C. and Engelhardt, J.F. (2006) rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion Mol. Ther., 13, 671-682
Ganti, K., Massimi, P., Manzo-Merino, J., Tomaić, V., Pim, D., Playford, M.P., Lizano, M., Roberts, S., Kranjec, C., Doorbar, J. and Banks, L. (2016) Interaction of the human papillomavirus E6 oncoprotein with sorting nexin 27 modulates endocytic cargo transport pathways PLoS Pathog., 12: e1005854
Su, W-C., Chen, Y-C., Tseng, C-H., Hsu, P.W-C., et al (2013) Pooled RNAi screen identifies ubiquitin ligase Itch as crucial for influenza A virus release from the endosome during virus entry Proc. Natl. Acad. Sci. USA, 110, 17516–17521
Cardiac tissue
KATP channel
Hund, T.J. and Mohler, P.J. (2011) Differential roles for SUR subunits in KATP channel membrane targeting and regulation Am. J. Physiol. Heart Circ. Physiol., 300, H33–H35
Yang, H.Q., Foster, M.N., Jana, K., Ho, J., Rindler, M.J. and Coetzee, W.A. (2016) Plasticity of sarcolemmal KATP channel surface expression: relevance during ischemia and ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol., 310, H1558–H1566
CHO cells
β-amyloid precursor protein
Huttunen, H.J., Puglielli, L., Ellis, B.C., MacKenzie Ingano, L.A. and Kovacs, D.M. (2009) Novel N-terminal cleavage of APP precludes Aβ generation in ACAT-defective AC29 cells J. Mol. Neurosci., 37, 6-15
Coatamer COPI protein
Daro, E., Sheff, D., Gomez, M., Kreis, T., et al (1997) Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component ε-COP J. Cell Biol., 139, 1747-1759
GLUT8 transporter
Augustin, R., Riley, J. and Moley, K.H. (2005) GLUT8 contains a [DE]XXXL[LI] sorting motif and localizes to a late endosomal/lysosomal compartment Traffic, 6, 1196-1212
LDL receptor
Sugii, S., Reid, P.C., Ohgami, N., Du, H. et al (2003) Distinct endosomal compartments in early trafficking of low density lipoprotein-derived cholesterol J. Biol. Chem., 278, 27180-27189
NCAM
Westphal, N., Loers, G., Lutz, D., Theis, T., Kleene, R. and Schachner, M. (2017) Generation and intracellular trafficking of a polysialic acid carrying fragment of the neural cell adhesion molecule NCAM to the cell nucleus Sci. Rep., 7: 8622
Corneal epithelial cells
Clathrin-mediated
Argueso, P., Guzman-Aranguez, A., Woodward, A. and Pintor, J.J. (2011) Inhibition of mucin O-glycosylation promotes endocytosis and nanopartical uptake in corneal epithelial cells Invest. Ophthalmol. Vis. Sci., 52, EAbstr. 4394
COS-7 cells
Amyloid β-precursor protein
Takasugi, N., Araya, R., Kamikubo, Y., Kaneshiro, N., Imaoka, R., Jin, H., Kashiyama, T., Hashimoto, Y. Kurosawa, M. et al (2018) TMEM30A is a candidate interacting partner for the β-carboxyl-terminal fragment of amyloid-β precursor protein in endosomes PLoS One, 13: e0200988
Calmodulin
Cao, Q., Zhong, X.Z., Zou, Y., Murrell-Lagnado, R., Zhu. M.X. and Dong, X-P. (2015) Calcium release through P2X4 activates calmodulin to promote endolysosomal membrane fusion J. Cell Biol., 209, 879–894
Parkinson’s disease
Yoshida, S., Hasegawa, T., Suzuki, M., Sugeno, N., Kobayashi, J., Ueyama, M., Fukuda, M., Ido-Fujibayashi, A., Sekiguchi, K. et al (2018) Parkinson’s disease-linked DNAJC13 mutation aggravates alpha-synucleininduced neurotoxicity through perturbation of endosomal trafficking Hum. Mol. Genet., 27, 823–836
Sialidase
Lukong, K.E., Seyrantepe, V., Landry, K., Trudel, S., et al (2001) Intracellular distribution of lysosomal sialidase is controlled by the internalisation signal in its cytoplasmic tail J. Biol. Chem., 276, 46172-46181
Transferrin receptor
Shen, X., Xu, K-F., Fan, Q., Pacheco-Rodriguez, G., et al (2006) Association of brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) with recycling endosomes during transferrin uptake Proc. Natl. Acad. Sci. USA, 103, 2635-2640
Cytokinesis
Chen, X-W., Inoue, M., Hsu, S. and Saltiel, A.R. (2006) RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis J. Biol. Chem., 281, 38609-38616
Dendritic cells
Phagosomes
Romao, S., Gasser, N., Becker, A.C., Guhl, B., Bajagic, M., Vanoaica, D., Ziegler, U., Roesler, J., Dengjel, J., Reichenbach, J. and Münz, C. (2013) Autophagy proteins stabilize pathogen-containing phagosomes for prolonged MHC II antigen processing J. Cell Biol., 203, 757–766
Endothelial cells
Growth factor receptors
Lampugnani, M.G., Orsenigo, F., Gagliani, M.C., Tacchetti, C. et al (2006) Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments J. Cell Biol., 174, 593-604
Enterocytes
Intestinal chylomicron output
Siddiqi, S. and Mansbach II, C.M. (2015) Dietary and biliary phosphatidylcholine activates PKCξ in rat intestine J. Lipid Res., 56, 859–870
Glial/glioma cells
Proteoglycans
Podyma-Inoue, K.A., Moriwaki, T, Rajapakshe, A.R., Terasawa, K. and Hara-Yokoyama, M. (2016) Characterization of heparan sulfate proteoglycan-positive recycling endosomes isolated from glioma cells Canc. Genom. Proteom., 13, 443-452
Virus processing
Querbes, W., O’Hara, B.A., Williams, G. and Atwood, W.J. (2006) Invasion of host cells by JC virus identifies a novel role for caveolae in endosomal sorting of noncaveolar ligands J. Virol., 80, 9402-9413
Green monkey kidney (Vero) cells
Toxins
McKenzie, J., Johannes, L., Taguchi, T.and Sheff, D. (2009) Passage through the Golgi is necessary for Shiga toxin B subunit to reach the endoplasmic reticulum FEBS J., 276, 1581–1595
Majoul, I.V., Bastiaens, P.I.H. and Soling H-D (1996) Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells J. Cell Biol., 133, 777-789
HEK cells
Adhesion/growth regulatory galectins
Kutzner. T.J., Higuero. A.M., Susmair, M., Kopitz, J., Hingar, M., Diez-Revuelta, N., Caballero, G.G.C., Kaltner, H., Lindner, I. et al (2020) How presence of a signal peptide affects human galectins-1 and -4: Clues to explain common absence of a leader sequence among adhesion/growth regulatory galectins Biochem. Biophys. Acta – Gen. Subjects, 1864: 129449
Amyloid β protein
Liu, L., Lauro, B.M., Ding, L., Rovere, M., Wolfe, M.S. and Selkoe, D.J. (2019) Multiple BACE1 inhibitors abnormally increase the BACE1 protein level in neurons by prolonging its half-life Alzheimers Dement., 15, 1183-1194
Autophagy
Gui, X., Yang, H., Li, T., Tan, X., Shi, P., Li, M., Du, F., Chen, Z.J. (2019) Autophagy induction via STING trafficking is a primordial function of the cGAS pathway Nature 567, 262-285
Kumar, S., Gu, Y., Abudu, Y.P., Bruun, J-A., Jain, A., Farzam, F., Mudd, M., Anonsen, J.H. et al (2019) Phosphorylation of syntaxin 17 by TBK1 controls autophagy initiation Dev. Cell, 49, 130–144
Luo, G., Sun, Y., Feng, R., Zhao, Q. and Wen, T. (2018) ARL3 subcellular localization and its suspected role in autophagy Biochimie, 154, 187-193
Nnah,I.C., Wang, B., Saqcena, C., Weber, G.F., Bonder, E.M., Bagley, D., De Cegli, R., Napolitano, G. et al (2019) TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy Autophagy, 15, 151-164
β-Catenin
Layton, M.J., Faux, M.C., Church, N.L., Catimel, B., et al (2012) Identification of a Wnt-induced protein complex by affinity proteomics using an antibody that recognizes a sub-population of β-catenin Biochim. Biophys. Acta, 1824, 925–937
Clathrin-mediated
Idkowiak-Baldys, J., Becker, K.P., Kitatani, K. and Hannum, Y.A. (2006) Dynamic sequestration of the recycling compartment by classical protein kinase C J. Biol. Chem., 281, 22321-22331
Neel, N.F., Lapierre, L.A., Goldenring, J.R. and Richmond, A. (2007) RhoB plays an essential role in CXCR2 sorting decisions J. Cell Sci., 120, 1559-1571
Dendritic cell factor
Chen, Y., Feng, R., Luo, G., Guo, J., Wang, Y., Sun, Y., Zheng, L. and Wen, T. (2018) DCF1 subcellular localization and its function in mitochondria Biochimie, 144, 50-55
Dopamine transporter
Keith, D.J., Wolfrum, K., Eshleman, A.J. and Janowsky, A. (2012) Melittin initiates dopamine transporter internalization and recycling in transfected HEK-293 cells Eur. J. Pharmacol., 690, 13–21
GLUT8 transporter
Augustin, R., Riley, J. and Moley, K.H. (2005) GLUT8 contains a DEXXXLLI sorting motif and localizes to a late endosomal/lysosomal compartment Traffic, 6, 1196-1212
Immune receptors
Qi, R., Singh, D. and Kao, C.C. (2012) Proteolytic processing regulates toll-like receptor 3 stability and endosomal localization J. Biol. Chem., 287, 32617–32629
Neimann-Pick disease
Kim, H., Chun, Y., Che, L. Kim, J., Lee, S. and Lee, S. (2017) The new obesity-associated protein, neuronal growth regulator 1 (NEGR1), is implicated in Niemann-Pick disease Type C (NPC2)-mediated cholesterol trafficking Biochem. Biophys. Res. Comm., 482, 1367-1374
Notch signalling
Tagami, S., Okochi, M., Yanagida, K., Ikuta, A., et al (2008) Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1 Mol. Cell. Biol., 28, 165-76
Pericentrion
El-Osta, M.A., Idkowiak-Baldys, J. and Hannun, Y.A. (2011) Delayed phosphorylation of classical protein kinase C (PKC) substrates requires PKC internalization and formation of the pericentrion in a phospholipase D (PLD)-dependent manner J. Biol. Chem., 286, 19340–19353
Phosphatidylinositol
Sbrissa, D., Ikonomov, O.C., Fu, Z., Ijuin, T., et al (2007) Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport J. Biol. Chem., 282, 23878-23891
mRNA, mi-RNA targetting
Barman, B. and Bhattacharyya, S.N. (2015) mRNA targeting to endoplasmic reticulum precedes Ago protein interaction and microRNA (miRNA)-mediated translation repression in mammalian cells J. Biol. Chem., 290, 24650–24656
Bose, M., Barman, B., Goswami, A., Bhattacharyya, S.N., (2017) Spatiotemporal uncoupling of microRNAmediated translational repression and target RNA degradation controls microRNP recycling in mammalian cells Mol. Cell. Biol., 37: e00464-16
Rab GTPase
Urbanska, A., Sadowski, L., Kalaidzidis, Y. and Miaczynska, M. (2011) Biochemical characterization of APPL endosomes: the role of annexin A2 in APPL membrane recruitment Traffic, 12, 1227–1241
Somatostatin receptor 2
Olsen, C., Memarzadeh, K., Ulu, A., Carr, H.S., Bean, A.J. and Frost, J.A. (2019) Regulation of somatostatin receptor 2 trafficking by C-tail motifs and the retromer Endocrinology, 160, 1031–1043
Stotmatin
Mairhofer, M., Steiner, M., Salzer, U. and Prohaska, R. (2009) Stomatin-like protein-1 interacts with stomatin and is targeted to late endosomes J. Biol. Chem., 284, 29218-29229
Tau protein
Simón, D., García-García, E., Royo, F., Falcón-Pérez, J.M. et al (2012) Proteostasis of tau. Tau overexpression results in its secretion via membrane vesicles FEBS Lett., 586, 47–54
Virus internalization
Su, W-C., Chen, Y-C., Tseng, C-H., Hsu, P.W-C., et al (2013) Pooled RNAi screen identifies ubiquitin ligase Itch as crucial for influenza A virus release from the endosome during virus entry Proc. Natl. Acad. Sci. USA, 110, 17516–17521
HeLa cells (see “Carcinoma cells”)
Hepatocytes
Autophagosomes/phagosomes
Berg, T.O., Fengsrud, M., Stromhaug, P.E., Berg, T., et al (1998) Isolation and characterization of rat liver amphisomes J. Biol. Chem., 273, 21883-21892
Fengsrud, M., Erichsen, E.S., Berg, T.O., Raiborg, C. et al (2000) Ultrastructural characterization of the delimiting membranes of isolated autophagosomes and amphisomes by freeze-fracture electron microscopy Eur. J. Cell Biol., 79, 871-882
Strømhaug, P.E., Berg, T.O. and Seglen, P.O. (1998) Purification and characterization of autophagosomes from rat hepatocytes Biochem. J., 335, 217-224
Sætre, F., Hagen, L.K., Engedal, N. and Seglen, P.O. (2015) Novel steps in the autophagic-lysosomal pathway FEBS J., 282, 2202–2214
Szalaia, P., Korseberg Hagen, L., Sætre, F., Luhr, M., Sponheim, M., Øverbye, A., Mills, I.G., Seglen, P.O. and Engedal, N. (2015) Autophagic bulk sequestration of cytosolic cargo is independent of LC3, but requires GABARAPs Exp. Cell Res., 333, 21-38
Lipid droplets
Schulze, R.J., Weller, S.G., Schroeder, B., Krueger, E.W., et al (2013) Lipid droplet breakdown requires Dynamin 2 for vesiculation of autolysosomal tubules in hepatocytes J. Cell Biol., 203, 315–326
miRNA / siRNA
Ghosh, S., Bose, M., Ray, A. and Bhattacharyya, S.N. (2015) Polysome arrest restricts miRNA turnover by preventing exosomal export of miRNA in growth-retarded mammalian cells Mol. Biol. Cell, 26, 1072-1083
Xu, Y., Ou, M., Keough, E., Roberts, J., et al (2014) Quantitation of physiological and biochemical barriers to siRNA liver delivery via lipid nanoparticle platform Mol. Pharmaceutics, 11, 1424-434
Reactive oxygen species
Oakley, F.D., Abbott, D., Li, Q. and Engelhardt, J.F. (2009) Signaling components of redox active endosomes: the redoxosomes Antioxid. Redox Signal., 11, 1313–1333
Hepatoma/hepatocarcinoma cells
Alpha-1-antitrypsin deficiency
Khodayari, N., Marek, G., Lu, Y., Krotova, K., Wang, R.L. and Brantly, M. (2017) Erdj3 has an essential role for Z variant alpha-1-antitrypsin degradation J. Cell. Biochem., 118, 3090–3101
Lipid droplets
Li, Z., Schulze, R.J., Weller, S.G., Krueger, E.W., Schott, M.B., Zhang, X., Casey, C.A., Liu, J., Stöckli, J., James, D.E. and McNiven, M.A. (2016) A novel Rab10-EHBP1-EHD2 complex essential for the autophagic engulfment of lipid droplets Sci. Adv., 2: e1601470
Schott, M.B., Rasineni, K., Weller, S.G., Schulze, R.J., Sletten, A.C., Casey, C.A. and McNiven, M.A. (2017) β-Adrenergic induction of lipolysis in hepatocytes is inhibited by ethanol exposure J. Biol. Chem., 292, 11815–11828
Lysosomal acid lipase
Grumet, L., Eichmann, T.O., Taschler, U., Zierler, K.A., Leopold, C., Moustafa, T., Radovic, B., Romauch, M. Yan, C. et al (2016) Lysosomal acid lipase hydrolyzes retinyl ester and affects retinoid turnover J. Biol., Chem., 291, 17977–17987
mRNA, mi-RNA
Mukherjee, K., Ghoshal, B., Ghosh, S., Chakrabarty, Y., Shwetha, S., Das, S. and Bhattacharyya, S.N. (2016) Reversible HuR-microRNA binding controls extracellular export of miR-122 and augments stress response EMBO Rep., 17, 1184-1203
Wang, Y., Lam, W., Chen, S-R., Guan, F-L., Dutchman, G.E., Francis, S., Baker, D.C. and Cheng, Y-C. (2016) Tylophorine analog DCB-3503 inhibited cyclin D1 translation through allosteric regulation of heat shock cognate protein 70 Sci. Rep., 6: 32832
Transferrin receptor
Manunta, M., Izzo, L., Duncan, R. and Jones, A.T. (2007) Establishment of subcellular fractionation techniques to monitor the intracellular fate of polymer therapeutics II: Identification of endosomal and lysosomal compartments in HepG2 cells combining single-step subcellular fractionation and fluorescent imaging J. Drug Target., 15, 37-50
Virus interactions
Abdul, F., Ndeboko, B., Buronfosse, T., Zoulim, F., et al (2012) Potent inhibition of late stages of hepadnavirus replication by a modified cell penetrating peptide PLoS One, 7: e48721
Shaikh F.Y., Utley, T.J., Craven, R.E., Rogers, M.C., et al (2012) Respiratory syncytial virus assembles into structured filamentous virion particles independently of host cytoskeleton and related proteins PLoS One, 7: e40826
HepG2 cells
Hepatitis B virus production
Inoue, J., Ninomiya, M., Umetsu, T., Nakamura, T., Kogure, T., Kakazu, E., Iwata, T., Takai, S., Sano, A. et al (2019) Small interfering RNA screening for the small GTPase Rab proteins identifies Rab5B as a major regulator of hepatitis B virus production J. Virol., 93: e00621-19
Human lung adenocarcinoma epithelial cells
Su, W-C. and Lai, M.M.C. (2018) Quantitative RT-PCR analysis of influenza virus endocytic escape In Influenza Virus Methods and Protocols, Meth. Mol. Biol., vol. 1836 (ed. Yamauchi, Y.) Springer Science+Business Media LLC, New York 2018, pp 185-194
Human osteosarcoma cells
Bryant, D., Liu, Y., Datta, S., Hariri, H., Seda, M., Anderson, G., Peskett, E., Demetriou, C., Sousa, S. et al (2018) SNX14 mutations affect endoplasmic reticulum associated neutral lipid metabolism in autosomal recessive spinocerebellar ataxia 20 Hum. Mol. Genet., 27, 1927-1940
Human promyelocyte leukaemia cells
Xiong, Q., Lin, M., Huang, W., Rikihisa, Y. (2019) Infection by Anaplasma phagocytophilum requires recruitment of low-density lipoprotein cholesterol by flotillins mBIO 10: e02783-18
Human skin fibroblasts
Nakasone, N., Nakamura, Y.S., Higaki, K., Oumi, N., Ohno, K. and Ninomiya, H. (2014) Endoplasmic reticulum-associated degradation of Niemann-Pick C1: evidence for the role of heat shock proteins and identification of lysine residues that accept ubiquitin J. Biol. Chem., 289, 9714–19725
Hypothalamic neural cells
Yamasaki, T., Suzuki, A., Hasebe, R. and Horiuchi, M. (2018) Retrograde transport by clathrin-coated vesicles is involved in intracellular transport of PrPSc in persistently prion-infected cells Sci. Rep., 8: 1224
Ileal brush border
Na+-H+ exchanger
Li, X. and Donowitz, M. (2008) Fractionation of subcellular membrane vesicles of epithelial and nonepithelial cells by OptiPrep™ density gradient ultracentrifugation In Methods Mol. Biol., 440, Exocytosis and Endocytosis (ed. Ivanov, A.I.) Humana Press, Totowa, NJ, pp 97-110
Li, X., Zhang, H., Cheong, A., Leu, S., et al (2004) Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent J. Physiol., 3, 791-804
Li, X. and Donowitz, M. (2014) Fractionation of subcellular membrane vesicles of epithelial and non-epithelial cells by OptiPrep™ density gradient ultracentrifugation In Exocytosis and Endocytosis, Methods in Molecular Biology, 1174 (ed. Ivanov, A,I.) Springer Science+Business Media New York 2014, pp 85-99
Jurkat cells
Transferrin receptor
Shakor, A.B.A., Atia, M.M., Kwiatkowska, K. and Sobota, A. (2012) Cell surface ceramide controls translocation of transferrin receptor to clathrin-coated pits Cell. Signal., 24, 677–684
Keratinocytes
Clathrin-mediated
Guzman-Aranguez, A., Woodward, A.M., Pintor, J. and Argüeso, P. (2012) Targeted disruption of core 1 1,3-galactosyltransferase (C1galt1) induces apical endocytic trafficking in human corneal keratinocytes PLoS One, 7: e36628
Kidney
Aquaporin
Procino, G., Barbieri, C., Carmosino, M., Rizzo, F., et al M. (2010) Lovastatin-induced cholesterol depletion affects both apical sorting and endocytosis of aquaporin-2 in renal cells Am. J. Physiol. Renal Physiol., 298, F266–F278
Megalin (LDL receptor gene family)
Zou, Z., Chung, B., Nguyen, T., Mentone, S., Thomson, B. and Biemesderfer, D. (2004) Linking receptormediated endocytosis and cell signaling J. Biol. Chem., 279, 34302-34310
Myosin VI
Biemesderfer, D., Mentone, S.A., Mooseker, M. and Hasson, T. (2002) Expression of myosin VI within the early endocytic pathway in adult and developing proximal tubules Am. J. Physiol., Ren. Physiol., 282, F785-F794
LD9 cells
Prion protein
Graham, J.F., Agarwal, S., Kurian, D., Kirby, L., et al (2010) Low density subcellular fractions enhance disease-specific prion protein misfolding J. Biol. Chem., 285, 9868–9880
Liver (rodent)
Late endosomal/lysosomal/mitochondrial sorting
Lim, J.M., Lim, J.C., Kim, G. and Levine, R.L. (2018) Myristoylated methionine sulfoxide reductase A is a late endosomal protein J. Biol. Chem., 7355–7366
Pribasnig, M.A., Mrak I., Grabner, G.F., Taschler, U., Knittelfelder, O., Scherz, B., Eichmann, T.O., Heier, C., Grumet, L. et al (2015) / Hydrolase domain-containing 6 (ABHD6) degrades the late endosomal/ lysosomal lipid bis(monoacylglycero)phosphate J. Biol. Chem., 290, 29869–29881
Neogalactosylalbumin uptake
Billington, D., Maltby, P.J. Jackson, A.P. and Graham, J.M. (1998) Dissection of hepatic receptor-mediated endocytic pathways using self-generated gradients of iodixanol (OptiPrep) Anal. Biochem., 258, 251-258
Sialidase
Lukong, K.E., Seyrantepe, V., Landry, K., Trudel, S., et al (2001) Intracellular distribution of lysosomal sialidase is controlled by the internalisation signal in its cytoplasmic tail J. Biol. Chem., 276, 46172-46181
Lung cells
Su, W-C. and Lai, M.M.C. (2018) Quantitative RT-PCR analysis of influenza virus endocytic escape In Influenza Virus Methods and Protocols, Meth. Mol. Biol., vol. 1836 (ed. Yamauchi, Y.) Springer Science+Business Media LLC, New York 2018, pp 185-194
Yuan, L., Kenny, S.J., Hemmati, J., Xu, K. Schekman, R. (2018) TANGO1 and SEC12 are copackaged with procollagen I to facilitate the generation of large COPII carriers Proc. Natl. Acad. Sci. USA 115, E12255–E12264
Lymphocytes, leukeamia and lymphoma cells
Anaplasma infection/Beclin-1
Niu, H., Xiong, Q., Yamamoto, A., Hayashi-Nishino, M.et al (2012) Autophagosomes induced by a bacterial Beclin 1 binding protein facilitate obligatory intracellular infection Proc. Natl. Acad. Sci. USA, 109, 20800–20807
Antigen processing
Vaithilingam, A., Lai, N.Y., Duong, E., Boucau, J., et al (2013) A simple methodology to assess endolysosomal protease activity involved in antigen processing in human primary cells BMC Cell Biol., 14: 35
Cysteine proteases
Kung Sutherland, M.S., Sanderson, R.J., Gordon, K.A., Andreyka, J., Cerveny, C.G., Yu, C., Lewis, T.S., Meyer, D.L., Zabinski, R.F., Doronina, S.D., Senter, P.D., Law, C-L., Wahl, A.F. (2006) Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates J. Biol. Chem., 281, 10540-10547
Granzyme B
Baginska, J., Viry, E., Berchem, G., Poli, A., et al (2013) Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia Proc. Natl. Acad. Sci. USA, 110,17450–17455
Interferon receptor (Type-1)
Payelle-Brogard, B. and Pellegrini, S. (2010) Biochemical monitoring of the early endocytic traffic of the type I interferon receptor J. Interferon Cytokine Res., 30, 89-98
Lymphoma-targetting antibody-polymer conjugates
Berguig, G.Y., Convertine, A.J., Shi, J., Palanca-Wessels, M.C., et al (2012) Intracellular delivery and trafficking dynamics of a lymphoma-targeting antibody-polymer conjugate Mol. Pharm., 9, 3506−3514
Lytic granules
Tuli, A., Thiery, J., James, A.M., Michelet, X., et al (2013) Arf-like GTPase Arl8b regulates lytic granule polarization and natural killer cell–mediated cytotoxicity Mol. Biol. Cell, 24, 3721-3735
Macrophages
Derlin-dependent proteins
Schaheen, B., Dang, H. and Fares, H. (2009) Derlin-dependent accumulation of integral membrane proteins at cell surfaces J. Cell Sci., 122, 228-2239
Leishmania-infected
Chakrabarty, T. and Bhattacharyya, S.N. (2017) Leishmania donovani restricts mitochondrial dynamics to enhance miRNP stability and target RNA repression in host macrophages Mol. Biol. Cell, 28, 2091-2105
Leucine-rich repeat kinase2
Schapansky, J., Nardozzi. J.D., Felizia, F. and LaVoie, M.J. (2014) Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy Hum. Mol. Genet., 23, 4201–4214
Methodology
Gibbings, D.J. (2011) Continuous density gradients to study argonaute and GW182 complexes associated with the endocytic pathway In Argonaute Proteins: Methods and Protocols, Methods Mol. Biol., 725, (ed. Hobman. T.C. and Duchaine, T.F.) Springer Science+Business Media, pp 63-76
miRNA
Chakrabarty, T. and Bhattacharyya, S.N. (2017) Leishmania donovani restricts mitochondrial dynamics to enhance miRNP stability and target RNA repression in host macrophages Mol. Biol. Cell, 28, 2091-2105
Gibbings, D.J., Ciaudo, C., Erhardt, M. and Voinnet, O. (2009) Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity Nat. Cell Biol., 11, 1143-1149
Phagosomes
Romao, S., Gasser, N., Becker, A.C., Guhl, B., Bajagic, M., Vanoaica, D., Ziegler, U., Roesler, J., Dengjel, J., Reichenbach, J. and Münz, C. (2013) Autophagy proteins stabilize pathogen-containing phagosomes for prolonged MHC II antigen processing J. Cell Biol., 203, 757–766
Toll-like receptors
Schapansky, J., Nardozzi. J.D., Felizia, F. and LaVoie, M.J. (2014) Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy Hum. Mol. Genet., 23, 4201–4214
Yersinia pestis V antigen
DiMezzo, T.L., Ruthel, G., Brueggemann, E.E., Hines, et al (2009) In vitro intracellular trafficking of virulence antigen during infection by Yersinia pestis PLoS One, 4:e6281
MCF-7 cells (human mammary epithelial tumor)
Redox-active endosomes
Shahin, W.S. and Engelhardt, J.F. (2019) Isolation of redox-active endosomes (Redoxosomes) and assessment of NOX activity In NADPH Oxidases: Methods and Protocols, Methods in Molecular Biology, vol. 1982 (ed. Knaus, U.G. and Thomas L.), Springer Science+Business Media LLC New York, pp 461-472
MDCK cells
Transferrin receptor
Sheff, D.R., Daro, E.A., Hull, M. and Mellmann, I. (1999) The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions J. Cell Biol., 145, 123-139
Virus internalization
Su, W-C., Chen, Y-C., Tseng, C-H., Hsu, P.W-C., et al (2013) Pooled RNAi screen identifies ubiquitin ligase Itch as crucial for influenza A virus release from the endosome during virus entry Proc. Natl. Acad. Sci. USA, 110, 17516–17521
Monocytic cells
Autophagosomes
Kimura, T., Jia, J., Kumar, S., Choi, S.W., Gu, Y., Mudd, M., Dupont, N., Jiang, S., et al (2017) Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy EMBO J., 36, 42-60
Mouse embryo fibroblasts
Autophagy
Ganley, I.G., Wong, P-M., Gammoh, N. and Jiang, X. (2011) Distinct autophagosomal-lysosomal fusion mechanism revealed by thapsigargin-induced autophagy arrest Mol. Cell, 42, 731–743
Gui, X., Yang, H., Li, T., Tan, X., Shi, P., Li, M., Du, F., Chen, Z.J. (2019) Autophagy induction via STING trafficking is a primordial function of the cGAS pathway Nature 567, 262-285
Young, M.M., Takahashi, Y., Fox, T.E., Yun, J.K., Kester, M. and Wang, H-G. (2016) Sphingosine kinase 1 cooperates with autophagy to maintain endocytic membrane trafficking Cell Rep., 17, 1532–1545
Zhang, M. and Ge, L. (2019) Cell-free reconstitution of autophagic membrane formation In Autophagy: Methods and Protocols, Methods in Molecular Biology, vol. 1880 (ed. Ktistakis, N. and Florey, O.), Springer Science+Business Media LLC New York, pp 135-148
Insulin receptor
Pedersen, D.J., Diakanastasis, B., Stöckli, J., Schmitz-Peiffer, C. (2013) Protein kinase C modulates insulin receptor localization and trafficking in mouse embryonic fibroblasts PLoS One, 8: e58046
Metal (copper) transporter
Öhrvik, H., Nose, Y., Wood, L.K., Kim, B-E., et al (2013) Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain Proc. Natl. Acad. Sci. USA, 110, E4279-E4288
Öhrvik, H., Logeman, B., Turk, B., Reinhecke, T. l and Thiele, D.J. (2016) Cathepsin protease controls copper and cisplatin accumulation via cleavage of the Ctr1 metal-binding ectodomain J. Biol. Chem., 291, 13905–13916
Sphinghosine kinase
Young, M.M., Takahashi, Y., Fox, T.E., Yun, J.K., Kester, M. and Wang, H-G. (2016) Sphingosine kinase 1 cooperates with autophagy to maintain endocytic membrane trafficking Cell Rep., 17, 1532–1545
Nerve tissue/neurons (see also SH-SY5Y cells)
Growth factor receptors
Weible II, M.W., Ozsarac, N., Grimes, M.L. and Hendry, I.A. (2004) Comparison of nerve terminal events in vivo effecting retrograde transport of vesicles containing neurotrophins or synaptic vesicle components J. Neurosci. Res., 75, 771-781
Neuronal signalling
Ammar, M.R., Thahouly, T., Hanauer, A., Stegner, D., Nieswandt, B. and Vitale, N. (2015) PLD1 participates in BDNF-induced signalling in cortical neurons Sci. Rep., 5: 14778
Neuroblastoma cells
Alzheimer’s disease
Burg, V.K., Grimm, H.S., Rothhaar, T.L., Grösgen, S., et al (2013) Plant sterols the better cholesterol in Alzheimer’s disease? A mechanistical study J. Neruosci., 33, 16072-16087
Grimm, M.O.W., Stahlmann, C.P., Mett, J., Haupenthal, V.J., Zimmer, V.C., Lehmann, J., Hundsdorfer, B., Endres, K., Grimm, H.S. and Hartmann, T. (2015) Vitamin E: curse or benefit in Alzheimer´s disease? A systematic investigation of the impact of α-, γ- and δ-tocopherol on Aß generation and degradation in
neuroblastoma cells J. Nutr. Health Aging, 19, 646-654
Kim, N-Y., Cho, M-H., Won, S-H., Kang, H-J., Yoon, S-Y. and Kim, D-H. (2017) Sorting nexin-4 regulates βamyloid production by modulating β-site-activating cleavage enzyme-1 Alzheimer’s Res. Ther., 9: 4
vATPase
Kratzke, M., Candiello, E., Schmidt, B., Jahn, O. and Schu, P. (2015) AP-1/σ1B-dependent SV protein recycling is regulated in early endosomes and is coupled to AP-2 endocytosis Mol. Neurobiol., 52, 142–161
Autophagosomes
Osaka, M., Ito, D. and Suzuki, N. (2016) Disturbance of proteasomal and autophagic protein degradation pathways by amyotrophic lateral sclerosis-linked mutations in ubiquilin 2 Biochem. Biophys. Res. Comm., 472, 324-331
Dopamine receptor
Wiesinger, J.A., Buwen, J.P., Cifelli, C.J., Unger, E.L., et al (2007) Down-regulation of dopamine transporter by iron chelation in vitro is mediated by altered trafficking, not synthesis J. Neurochem., 100, 167-179
PrPSc transport in prion infected cells
Yamasaki, T., Suzuki, A., Hasebe, R. and Horiuchi, M. (2018) Retrograde transport by clathrin-coated vesicles is involved in intracellular transport of PrPSc in persistently prion-infected cells Sci. Rep., 8: 1224
Src homology 3
Xin, X., Gfeller, D., Cheng, J., Tonikian, R., et al (2013) SH3 interactome conserves general function over specific form Mol. Systems Biol., 9: 652
α-Synuclein
Dettmer, U., Ramalingam, N., von Saucken, V.E., Kim, T-E., Newman, A.J., Terry-Kantor, E., Nuber, S., Ericsson, M. et al (2017) Loss of native α-synuclein multimerization by strategically mutating its amphipathic helix causes abnormal vesicle interactions in neuronal cells Hum. Mol. Genetics, 26, 3466–3481
Transferrin receptor
Wiesinger, J.A., Buwen, J.P., Cifelli, C.J., Unger, E.L., et al (2007) Down-regulation of dopamine transporter by iron chelation in vitro is mediated by altered trafficking, not synthesis J. Neurochem., 100, 167-179
NRK cells
Caveolin
Pol, A., Lu, A., Pons, M., Peiro, S., et al (2000) Epidermal growth factor-mediated caveolin recruitment to early endosomes and MAPK activation J. Biol. Chem., 275, 30566-30572
Nanotube formation
Su, Q.P., Du, W., Ji, Q., Xue, B., Jiang, D., Zhu, Y., Lou, J., Yu, L. and Sun, Y. (2016) Vesicle size regulates nanotube formation in the cell Sci. Rep., 6: 24002
Osteosarcoma cells
Autophagy
Merrill, N.M., Schipper, J.L., Kames, J.B., Kauffman, A.L., Martin, K.R. and MacKeigan, J.P. (2017) PI3KC2a knockdown decreases autophagy and maturation of endocytic vesicles PLoS One, 12: e0184909
Proteomics
Geladaki, A., Britovšek, N.K., Breckels, L.M., Smith, T.S., Vennard, O.L., Mulvey, C.M., Crook, O.M., Gatto, L. and Lilley, K.S. (2019) Combining LOPIT with differential ultracentrifugation for high-resolution spatial proteomics Nat. Comm., 10: 331
Pancreas
Pancreatitis
Mareninova, O.A., Yakubov, I., Gukovsky, I and Gukovskaya, A.S. (2018) Disordering of endo-lysosomal system in pancreatitis Circulation, 138, Suppl.1, abstr.
PC12 cells
Neurotrophin receptor
Lin, D.C., Quevedo, C., Brewer, N.E., Bell, A., et al (2006) APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction Mol. Cell. Biol., 26, 8928-8941
Receptors (various)
McCaffrey, G., Welker, J., Scott, J., van der Salm, L., et al (2009) High-resolution fractionation of signaling endosomes containing different receptors Traffic, 10, 938–950
Growth factor receptors
Li, Y., Chin, L-S., Levey, A.L. and Li, L. (2002) Huntingtin-associated protein 1 interacts with hepatocyte growth factor-regulated tyrosine kinase substrate and functions in endosomal trafficking J. Biol. Chem., 277, 28212-28221
Pryor, S., McCaffrey, G., Young. L.R. and Grimes, M.L. (2012) NGF causes TrkA to specifically attract microtubules to lipid rafts PLoS One 7: e35163
Neurotrophin receptor
Fu, X., Zang, K., Zhou, Z., Reichardt, L.F., et al (2010) Retrograde neurotrophic signaling requires a protein interacting with receptor tyrosine kinases via C2H2 zinc fingers Mol. Biol. Cell, 21, 36-49
Peritoneal mesothelial cells
Vacuolar trafficking
Oba-Yabana, I., Mori, T., Takahashi, C., Hirose, T., Ohsaki, Y., Kinugasa, S., Muroya, Y., Sato, E. et al (2018) Acidic organelles mediate TGF-β1-induced cellular fibrosis via (pro)renin receptor and vacuolar ATPase trafficking in human peritoneal mesothelial cells Sci. Rep., 8: 2648
PS120 cells
Gradient methodology
Li, X. and Donowitz, M. (2008) Fractionation of subcellular membrane vesicles of epithelial and non-epithelial cells by OptiPrep™ density gradient ultracentrifugation In Methods Mol. Biol., 440, Exocytosis and Endocytosis (ed. Ivanov, A.I.) Humana Press, Totowa, NJ, pp 97-110
SH-SY5Y cells
Parkinson’s disease
Yoshida, S., Hasegawa, T., Suzuki, M., Sugeno, N., Kobayashi, J., Ueyama, M., Fukuda, M., Ido-Fujibayashi, A., Sekiguchi, K. et al (2018) Parkinson’s disease-linked DNAJC13 mutation aggravates alpha-synucleininduced neurotoxicity through perturbation of endosomal trafficking Hum. Mol. Genet., 27, 823–836
T cells
TCR signaling
Saveanu, L., Zucchetti, A.E., Evnouchidou, I., Ardouin, L. and Hivroz, C. (2019) Is there a place and role for endocytic TCR signaling? Immunolog. Rev. 291, 57–74
Extracelluar vesicles
Chiou, N-T., Kageyama, R. and Ansel, K.M. (2018) Selective export into extracellular vesicles and function of tRNA fragments during T cell activation Cell Rep., 25, 3356–3370
U2OS cells
Spinocerebellar ataxia (ER/endosomal/lysosomal system)
Bryant, D., Liu, Y., Datta, S., Hariri, H., Seda, M., Anderson, G., Peskett, E., Demetriou, C., Sousa, S. et al (2018) SNX14 mutations affect endoplasmic reticulum associated neutral lipid metabolism in autosomal recessive spinocerebellar ataxia 20 Hum. Mol. Genet., 27, 1927-1940
U251 cells
Autophagy
Luo, G., Sun, Y., Feng, R., Zhao, Q. and Wen, T. (2018) ARL3 subcellular localization and its suspected role in autophagy Biochimie, 154, 187-193
Yeast
Multivesicular body
Mitsui, K., Koshimura, Y., Yoshikawa, Y., Matsushita, M., et al (2011) The endosomal Na+/H+ exchanger contributes to multivesicular body formation by regulating the recruitment of ESCRT-0 Vps27p to the endosomal membrane J. Biol. Chem., 286, 37625–37638
OptiPrepTM Reference List RS12; 7th edition, January 2020
OptiPrep™ Reference List RS13
Resolution of soluble cytosolic proteins from membrane vesicles and organelles
There are three Application Sheets listed in the Application Sheet Index under “Protein localization (membrane versus cytosol)” which describe different gradient strategies. These Application Sheets (described below) can be accessed via the following website www.OptiPrep.com (click on “Methodology”, then “Organelles and subcellular membranes” and follow the links from the Index):
- Discontinuous gradient: OptiPrepTM Application Sheet S35
- Self-generated gradient: OptiPrepTM Application Sheet S36
- A special strategy for rapid resolution of protein complexes and cytosol: OptiPrepTM Application Sheet S37
- Note that Reference Lists of papers addressing the resolution of mammalian cell exosomes and other microvesicles from soluble proteins are covered in OptiPrepTM Reference List RS10 and the similar resolution of bacterial and fungal microvesicles in RS11.
The reference list, which follows, includes principally papers describing the separation of membranes and soluble (cytosolic) proteins (Section 1); it is divided alphabetically into source material (cell or tissue type). It includes both mammalian and non-mammalian sources and in each of the 26 sections, references are listed alphabetically according to first author. References in Section 1a describe the use of the gradients to isolate in addition, lipid droplets. Section 2 lists a few papers that report the study of previously prepared subcellular membranes to determine the distribution of a particular protein between the soluble fraction and the organelle(s). Others describe the separation of vesicles either budded from the cells or obtained from permeabilized cells.
- Key words in titles are highlighted in light blue.
1. Cells or tissues
1.1. Algae
Baquero, E., Fedry, J., Legrand, P., Krey, T. and Rey, F.A. (2019) Species-specific functional regions of the green alga gamete fusion protein HAP2 revealed by structural studies Structure 27, 113–124
Wood, C.R. and Rosenbaum, J.L. (2014) Proteins of the ciliary axoneme are found on cytoplasmic membrane vesicles during growth of cilia Curr. Biol., 24, 1114-1120
1.2. Bacteria
De Leeuw, E., Poland, D., Mol, O., Sinning, I., et al (1997) Membrane association of FtsY, the E. coli SRP receptor FEBS Lett., 416, 225-229
Herskovits, A.A., Seluanov, A., Rajsbaum, R., ten Hagen-Jongman, C.M., et al (2001) Evidence for coupling of membrane targeting and function of the signal recognition particle (SRP) receptor FtsY EMBO Rep., 2, 1040-1046
Valent, Q.A., Scotti, P.A., High, S., de Gier, J-W.L., et al (1998) The Escherichia coli SRP and SecB targeting pathways converge at the translocon EMBO J., 17, 2504-2512
1.3. Brain
Ding, T.T., Lee, S-J., Rochet, J-C. and Lansbury, Jr., P.T. (2002) Annular -synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes Biochemistry, 41, 10209-10217
Wang, X., Bowers, S.L., Wang, F., Pu, X-a., et al (2009) Cytoplasmic prion protein induces forebrain neurotoxicity Biochim. Biophys. Acta 1792, 555–563
1.4. Carcinoma (incl HeLa)cells
Adolf, F., Rhiel, M., Hessling, B., Gao, Q., Hellwig, A., Béthune, J. and Wieland, F.T. (2019) Proteomic profiling of mammalian COPII and COPI vesicles Cell Rep., 26, 250–265
Blake Richardson, R., Ohlson, M.B., Eitson, J.L., Kumar, A., McDougal, M.B., Boys, I.N., Mar, K.B., De La Cruz-Rivera, P.C. et al (2018) A CRISPR screen identifies IFI6 as an ER-resident interferon effector that blocks flavivirus replication Nat. Microbiol. 3, 1214–1223
Collins, L.L., Simon, G., Matheson, J., Wu, C., et al (2012) Rab11-FIP3 is a cell cycle-regulated phosphoprotein BMC Cell Biol., 13: 4
Guan, J-J., Zhang, X-D., Sun, W., Qi, L., Wu, J-C. and Qin, Z-H. (2015) DRAM1 regulates apoptosis through increasing protein levels and lysosomal localization of BAX Cell Death Dis., 6: e1624
Hasegawa, H., Thomas, H.J., Schooley, K. and Born, T.L. (2011) Native IL-32 is released from intestinal epithelial cells via a non-classical secretory pathway as a membrane-associated protein Cytokine, 53, 74–83
Jorgensen, I., Bednar, M.M., Amin, V., Davis, B.K., et al (2011) The Chlamydia protease CPAF regulates host and bacterial proteins to maintain pathogen vacuole integrity and promote virulence Cell Host Microbe, 10, 21–32
Kaeser-Pebernard, S., Diedrich, B. and Dengjel, J (2019) Identification and regulation of multimeric protein complexes in autophagy via SILAC-based mass spectrometry approaches In Autophagy: Methods and Protocols, Methods in Molecular Biology, vol. 1880 (ed. Ktistakis, N. and Florey, O.), Springer Science+Business Media LLC New York, pp 341-357
Lee, E-Y., Park, K-S., Yoon, Y.J., Lee, J., et al (2012) Therapeutic effects of autologous tumor-derived nanovesicles on melanoma growth and metastasis PLoS One 7: e33330
Lee, S.M., Olzmann, J.A., Chin, L-S. and Li, L. (2011) Mutations associated with Charcot–Marie–Tooth disease cause SIMPLE protein mislocalization and degradation by the proteasome and aggresome–autophagy pathways J. Cell Sci., 124, 3319–3331
Méndez, E., Aguirre-Crespo, G., Zavala, G. and Arias, C.F. (2007) Association of the astrovirus structural protein VP90 with membranes plays a role in virus morphogenesis J. Virol., 81, 10649-10658
Mira, E., Lacalle, R.A., Buesa, J.M., Gonzalez de Buitrago, G., et al (2004) Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface J. Cell Sci., 117, 1847-1856
Murillo, A., Vera-Estrella, R., Barkla, B.J., Méndez, E. and Arias, C.F. (2015) Identification of host cell factors associated with astrovirus replication in Caco-2 cells J. Virol., 89, 10359 –10370
Salman, E.D., He, D., Runge-Morris, M., Kocarek, T.A., et al (2011) Site-directed mutagenesis of human cytosolic sulfotransferase (SULT) 2B1b to phospho-mimetic Ser348Asp results in an isoform with increased catalytic activity J. Steroid Biochem. Mol. Biol., 127, 315– 323
Wang, X., Wang, F., Sy, M-S- and Ma, J. (2005) Calpain and other cytosolic proteases can contribute to the degradation of retro-translocated prion protein in the cytosol J. Biol. Chem., 280, 317-325
Welsch, S., Habermann, A., Jäger, S., Müller, B., et al (2006) Ultrastructural analysis of ESCRT proteins suggests a role for endosome-associated tubular–vesicular membranes in ESCRT function Traffic, 7, 1551-1566
1.5. CHO cells
Lin, C-C., Love, H.D., Gushue, J.N., Bergeron, J.J.M., et al (1999) ER/Golgi intermediates acquire Golgi enzymes by Bredelfin A – sensitive retrograde transport in vitro J. Cell. Biol., 147, 1457-1472
Love, H.D., Lin, C.C., Short, C.S. and Ostermann, J. (1998) Isolation of functional Golgi-derived vesicles with a possible role in retrograde transport J. Cell Biol., 140, 541-551
1.6. COS cells
Dicu, A.O., Topham, M.K., Ottaway, L. Epand, R.M. (2007) Role of the hydrophobic segment of diacylglycerol kinase Biochemistry, 46, 6109-6117
Kim, Y.S., Laurine, E., Woods, W. and Lee, S-J. (2006) A novel mechanism of interaction between α-synuclein and biological membranes J. Mol. Biol., 360, 386-397
Lee, H-J., Choi, C. and Lee, S-J. (2002) Membrane-bound -synuclein has a high aggregation propensity and the ability to seed the aggregation of cytosolic form J. Biol. Chem., 277, 671-678
1.7. Glioblastoma cells
Bruntz, R.C., Taylor, H.E., Lindsley, C.W. and Brown, H.A. (2014) Phospholipase D2 mediates survival signaling through direct regulation of Akt in glioblastoma cells J. Biol. Chem., 289, 600-616
1.8. HEK cells
Burnett, A. and Spearman, P. (2007) APOBEC3G multimers are recruited to the plasma membrane for packaging into human immunodeficiency virus type 1 virus-like particles in an RNA-dependent process requiring the NC basic linker J. Virol., 81, 5000-5013
Chatel-Cjaix, L., Abrahamyan, L., Fréchina, C., Mouland, A.J., et al (2007) The host protein Staufen1 participates in human immunodeficiency virus type 1 assembly in live cells by influencing pr55Gag multimerization J. Virol., 81, 6216-6230
Derdowski, A., Ding, L. and Spearman, P. (2004) A novel fluorescence resonance energy transfer assay demonstrates that the human immunodeficiency virus type I Pr55Gag I domain mediates Gag-Gag interactions J.Virol., 78, 1230-1242
Dou, J., Wang, J-J., Chen, X., Li, H., et al (2009) Characterization of a myristoylated, monomeric HIV Gag protein Virology 387, 341–352
Gottwein, E. and Kräusslich, H-G. (2005) Analysis of human immunodeficiency virus type 1 Gag ubiquitination J. Virol., 79, 9134-9144
Halwani, R., Cen, S., Javanbakht, H., Saadatmand, J., et al (2004) Cellular distribution of lysyl-tRNA synthetase and its interaction with Gag during human immunodeficiency virus type 2 assembly J. Virol., 78, 7553-7564
Jäger, S., Gottwein, Kräusslich, H-G. (2007) Ubiqutination of human immunodeficiency virus type 1 Gag is highly dependent on Gag membrane association J. Virol., 81, 9193-9201
Li, H., Dou, J., Ding, L and Spearman, P. (2007) Myristoylation is required for human immunodeficiency virus type 1 Gag-Gag multimerization in mammalian cells J. Virol., 81, 12899-12910
Lee, S.M., Olzmann, J.A., Chin, L-S. and Li, L. (2011) Mutations associated with Charcot–Marie–Tooth disease cause SIMPLE protein mislocalization and degradation by the proteasome and aggresome–autophagy pathways J. Cell Sci., 124, 3319–3331
Runkler, N., Pohl, C., Schneider-Schaulles, S., Klenk, H-D., et al (2007) Measles virus nucleocapsid transport to the plasma membrane requires stable expression and surface accumulation of the viral matrix protein Cell. Microbiol., 9, 1203-1214
Schlehe, J.S., Lutz, A.K., Pilsl, A., Lämmermann, K., et al (2008) Aberrant folding of pathogenic Parkin mutants: Aggregation versus degradation J. Biol. Chem., 283, 13771-13779
Shtanko, O., Imai, M., Goto, H., Lukashevich, I.S., et al (2010) A role for the C terminus of Mopeia virus nucleoprotein in its incorporation into Z protein-induced virus-like particles J. Virol., 84, 5415-5422
1.9. Hepatocytes
Aligo, J., Jia, S., Manna, D. and Konan, K.V. (2009) Formation and function of hepatitis C virus replication complexes require residues in the carboxy-terminal domain of NS4B protein Virology 393, 68–83
1.10. Hepatoma cells
Cho, N-J., Cheong, K.H., Lee, C.H., Frank, C.W. et al (2007) Binding dynamics of hepatitis C virus NS5A amphipathic peptide to cell and model membranes J. Virol., 81, 6682-6689
Döring, T., Zeyen, L., Bartusch, C. and Prange, R. (2018) Hepatitis B virus subverts the autophagy elongation complex Atg5-12/16L1 and does not require Atg8/LC3 lipidation for viral maturation J. Virol., 92: e01513-17
Elazar, M., Liu, P., Rice, C.M. and Glenn, J.S. (2004) An N-terminal amphipathic helix in hepatitis C virus (HCV) NS4B mediates membrane association, correct localization of replication complex proteins, and RNA replication J. Virol., 78, 11393-11400
Han, Q., Aligo, J., Manna, D., Belton, K., et al (2011) Conserved GXXXG- and S/T-like motifs in the transmembrane domains of NS4B protein are required for hepatitis C virus replication J. Virol., 85, 6464–6479
Strecker, T., Maisa, A., Daffis, S., Eichler, R., et al (2006) The role of myristoylation in the membrane association of the Lassa virus matrix protein Z Virology, J., 3, 93
Vogt, D.A., Camus, G., Herker, E., Webster, B.R., Tsou, C-L., Greene, W.C., Yen, T-S.B. and Ott, M. (2013) Lipid droplet-binding protein TIP47 regulates hepatitis C virus RNA replication through interaction with the viral NS5A protein PLoS Pathog., 9: e1003302
1.11 Insect cells
Hatanaka, R., Hagiwara-Komoda, Y., Furuki, T., Kanamori, Y., et al (2013) An abundant LEA protein in the anhydrobiotic midge, PvLEA4, acts as a molecular shield by limiting growth of aggregating protein particles Insect Biochem. Mol. Biol., 43, 1055-1067
Kruppa, A.J., Ott, S., Chandraratna, D.S., Irving, J.A., et al (2013) Suppression of Aβ toxicity by puromycinsensitive aminopeptidase is independent of its proteolytic activity Biochim. Biophys. Acta, 1832, 2115–2126
1.12. Jurkat cells
Frankel, A.D., Alber, T., Zhou, Q. and Krogan, N.J. (2011) Purification and characterization of HIV–human protein complexes Methods, 53, 13–19
Geist, M.M., Pan, X., Bender, S., Bartenschlager, R., et al (2014) Heterologous Src homology 4 domains support membrane anchoring and biological activity of HIV-1 Nef J. Biol. Chem., 289, 14030–14044
1.13. Kidney proximal tubule cells (incl. LLC-PK1)
Fölsch, H., Pypaert, M., Maday, S., Pelletier, L., et al (2003) The AP-1A and AP1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains J. Cell Biol., 163, 351-362
Nürnberger, J., Bacallao, R.L. and Phillips, C.P. (2002) Inversin forms a complex with catenins and Ncadherin in polarized epithelial cells Mol. Biol. Cell, 13, 3096-3106
1.14. Liver
Kanayama, M., Xu, S., Danzaki, K., Gibson, J.R., Inoue, M., Gregory, S.G. and Shinohara, M.L. (2017) Skewing of the population balance of lymphoid and myeloid cells by secreted and intracellular osteopontin Nat. Immunol., 18, 973-984
Nakatsuka, A., Wada, J., Iseda, I., Teshigawara, S., et al (2012) Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex Diabetes, 61, 2823–2832
1.15 Lung cells
Kanayama, M., Xu, S., Danzaki, K., Gibson, J.R., Inoue, M., Gregory, S.G. and Shinohara, M.L. (2017) Skewing of the population balance of lymphoid and myeloid cells by secreted and intracellular osteopontin Nat. Immunol., 18, 973-984
1.16 Lymphocytic cells
Onnis, A., Cianfanelli, V., Cassioli, C., Samardzic, D., Pelicci, P.G., Cecconi, F. and Baldari, C.T. (2018) The pro-oxidant adaptor p66SHC promotes B cell mitophagy by disrupting mitochondrial integrity and recruiting LC3-II Autophagy 14, 2117–2138
1.17 MDCK cells
Grindstaff, K.K., Yeaman, C., Anandasabapathy, N., Hsu, S.C., et al (1998) Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells Cell, 93, 731-740
Gromley, A., Yeaman, C., Rosa, J., Redick, S., et al (2005) Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission Cell, 123, 75-87
Hansen, M.D.H. and Nelson, W.J. (2001) Serum-activated assembly and membrane translocation of an endogenous Rac1: effector complex Curr. Biol., 11, 356-360
Hansen, M.D., Ehrlich, J.S. and Nelson, W.J. (2002) Molecular mechanism for orienting membrane and actin dynamics to nascent cell-cell contacts in epithelial cells J. Biol. Chem., 277, 45371-45376
Imai, M., Kawasaki, K. and Odagiri, T. (2008) Cytoplasmic domain of influenza virus BM2 protein plays critical roles in production of infectious virus J. Virol., 82, 728-739
Matern, H.T., Yeaman, C., Nelson, W.J. and Scheller, R.H. (2001) The Sec6/8 complex in mammalian cells: characterization of mammalian Sec3, subunit interactions, and expression of subunits in polarized cells Proc. Natl. Acad. Sci, USA, 98, 9648-9653
Scheiffele, P., Verkade, P., Fra, A.M., Simons, K., et al (1998) Caveolin-1 and –2 in the exocytic pathway of MDCK cells J. Cell Biol., 140, 795-806
Weiskircher, E., Aligo, J., Ning, G. and Konan, K.V. (2009) Bovine viral diarrhea virus NS4B protein is an integral membrane protein associated with Golgi markers and rearranged host membranes Virol. J. 6: 185
1.18. Monkey kidney cells
McKenzie, J., Johannes, L., Taguchi, T.and Sheff, D. (2009) Passage through the Golgi is necessary for Shiga toxin B subunit to reach the endoplasmic reticulum FEBS J., 276, 1581–1595
1.19. Mouse embryo fibroblasts
Lee, H-J., Cho, E-D., Lee, K.W., Kim, J-H., et al (2013) Autophagic failure promotes the exocytosis and intercellular transfer of -synuclein Exp. Mol. Medi., 45, e22
1.20. Neuroblastoma cells
Bae, E-J., Ho, D-H., Park, E., Jung, J.W., et al (2013) Lipid peroxidation product 4-hydroxy-2-nonenal promotes seeding-capable oligomer formation and cell-to-cell transfer of α-synuclein Antioxid. Redox Signal., 18, 770–783
Kim, Y.S., Laurine, E., Woods, W. and Lee, S-J. (2006) A novel mechanism of interaction between α-synuclein and biological membranes J. Mol. Biol., 360, 386-397
Jang, A., Lee, H-J., Suk, J-E., Jung, J-W., et al (2010) Non-classical exocytosis of α-synuclein is sensitive to folding states and promoted under stress conditions J. Neurochem., 113, 1263–1274
Lee, H.J., Patel, S. and Lee, S-J. (2005) Intravesicular localization and exocytosis of α-synuclein and its aggregates J. Neurosci., 25, 6016-6024
Lee, H-J., Cho, E-D., Lee, K.W., Kim, J-H., et al (2013) Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein Exp. Mol. Medi., 45, e22
Lee, S.M., Olzmann, J.A., Chin, L-S. and Li, L. (2011) Mutations associated with Charcot–Marie–Tooth disease cause SIMPLE protein mislocalization and degradation by the proteasome and aggresome–autophagy pathways J. Cell Sci., 124, 3319–3331
Mamada, N., Tanokashira, D., Ishii, K., Tamaoka, A. and Araki, W. (2017) Mitochondria are devoid of amyloid β-protein (Aβ)-producing secretases: Evidence for unlikely occurrence within mitochondria of Ab generation from amyloid precursor protein Biochem. Biophys. Res. Comm., 486, 321-328
Schlehe, J.S., Lutz, A.K., Pilsl, A., Lämmermann, K., et al (2008) Aberrant folding of pathogenic Parkin mutants: Aggregation versus degradation J. Biol. Chem., 283, 13771-13779
Wang, X., Wang, F., Arterburn, L., Wollmann, R., et al (2006) The interaction between cytoplasmic prion protein and the hydrophobic lipid core of membrane correlates with neurotoxicity J. Biol. Chem., 281, 13559-13565
Yu, C., Kim, S-H., Ikeuchi, T., Xu, H., et al (2001) Characterization of a presenilin-mediated APP carboxyl terminal fragment γ: Evidence for distinct mechanisms involved in gamma-secretase processing of the APP and notch 1 transmembrane domains J. Biol. Chem., 276, 43756-43760
1.21. NRK cells
Joglekar, A.P., Xu, D., Rigotti, D.J., Fairman, R., et al (2003) The SNARE motif contributes to rbet1 intracellular targeting and dynamics independently of SNARE interactions J. Biol. Chem., 278, 14121-14133
Yeaman, C., Grindstaff, K.K., Wright, J.R. and Nelson, W.J. (2001) Sec6/8 complexes on trans-Golgi network and plasma membrane regulate stages of exocytosis in mammalian cells J. Cell Biol., 155, 593-604
1.22 Pheochromocytoma (PC12) cells
Thayanidhi, N., Liang, Y., Hasegawa, H., Nycz, D.C., et al (2012) R-SNARE ykt6 resides in membraneassociated protease-resistant protein particles and modulates cell cycle progression when over-expressed Biol. Cell, 104, 397–417
1.23 Plant cells
Liu, Z., Zhu, Y., Gao, J., Yu, F., et al (2009) Molecular and reverse genetic characterization of nucleosome assembly protein1 (NAP1) genes unravels their function in transcription and nucleotide excision repair in Arabidopsis thaliana Plant J., 59, 27–38
Mahon, P. and Dupree, P. (2001) Quantitative and reproducible two-dimensional gel analysis using Phoretix 2D Full Electrophoresis, 22, 2075-2085
1.24 Squid axons
LaPointe, N.E., Morfini, G., Pigino, G., Gaisina, I.N., et al (2009) The amino terminus of tau inhibits kinesindependent axonal transport: Implications for filament toxicity J. Neurosci. Res., 87, 440-451
Zamponi, E., Buratti, F., Cataldi, G., Caicedo, H.H., Song, Y., Jungbauer, L.M., LaDu, M.J., Bisbal, M. et al (2017) Prion protein inhibits fast axonal transport through a mechanism involving casein kinase 2 PLoS One, 12: e0188340
1.25 Vero cells
Eichler, R., Strecker, T., Kolesnikova, L., ter Meulen, J., et al (2004) Characterization of the Lassa virus matrix protein Z: electron microscopic study of virus-like particles and interaction with the nucleoprotein (NP) Virus Res., 100, 249-255
Kim, M., Mackenzie, J.M. and Westaway, E.G. (2004) Comparisons of physical separation methods of Kunjin virus-induced membranes J. Virol. Methods, 120, 179-187
Kraus, I., Eickmann, M., Kiermeyer, S., Scheffczik, H., et al (2001) Open reading frame III of Borna disease virus encodes a nonglycosylated matrix protein J. Virol., 75, 12098-12104
1.26 Xenopus
Hülsmann, B.B., Labokha, A.A. and Görlich, D. (2012) The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model Cell, 150, 738–751
1.27 Yeast
Chen, S.H., Chen, S., Tokarev, A.A., Liu, F., et al (2005) Ypt3132 GTPases and their novel F-Box effector protein Rcy1 regulate protein recycling Mol. Biol. Cell, 16, 178-192
Cox, R., Chen, S.H., Yoo, E. and Segev, N. (2007) Conservation of the TRAPPII-specific subunits of a Ypt/Rab exchanger complex BMC Evol. Biol., 7:12
Diaz, A., Zhang, J., Ollwerther, A., Wang, X. and Ahlquist, P. (2015) Host ESCRT proteins are required for bromovirus RNA replication compartment assembly and function PLoS Pathog., 11: e1004742
Du, L-L. and Novick, P. (2001) Yeast Rab GTPase-activating protein Gyp1p localizes to the Golgi apparatus and is a negative regulator of Ypt1p Mol. Biol. Cell, 12, 1215-1226
Elkind., N.B., Walch-Solimena, C. and Novick, P.J. (2002) The role of the COOH terminus of Sec2p in the transport of post-Golgi vesicles J. Cell Biol., 149, 95-110
Ge, W., Chew, T.G., Wachtler, V., Naqvi, S.N., et al (2005) The novel fission yeast protein Pal1p interacts with Hip1-related Sla2p/End4p and is involved in cellular morphogenesis Mol. Biol. Cell, 16, 4124-4138
Huch, S., Gommlich, J., Muppavarapu, M., Beckham, C. and Nissan, T. (2016) Membrane-association of mRNA decapping factors is independent of stress in budding yeast Sci. Rep., 6: 25477
Liu, L., Westler, W.M., den Boon, J.A., Wang, X., et al (2009) An amphipathic α-helix controls multiple roles of Brome mosaic virus protein 1a in RNA replication complex assembly and function PLoS Pathog., 5:e1000351
Medkova, M., France, Y.E., Coleman, J. and Novick, P. (2006) The rab exchange factor Sec2p reversibly associates with the exocyst Mol. Biol. Cell, 17, 2757-2769
Pawelec, A., Arsić, J. and Kölling, R. (2010) Mapping of Vps21 and HOPS binding sites in Vps8 and effect of binding site mutants on endocytic trafficking Eukaryot. Cell, 9, 602-610
Roberts-Galbraith, R.H., Ohi, M.D., Ballif, B.A., Chen, J-S., et al (2010) Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site Mol. Cell, 39, 86–99
Ruggiano, A., Mora, G., Buxó, L. and Carvalho, P. (2016) Spatial control of lipid droplet proteins by the ERAD ubiquitin ligase Doa10 EMBO Rep., 17, 1644-1655
Satyanarayana, C., Schroder-Kohne, S., Craig, E.A., Schu, P.V., et al (2000) Cytosolic Hsp70s are involved in the transport of aminopeptidase 1 from the cytoplasm into the vacuole FEBS Lett., 470, 232-238
Schmitz, C., Kinner, A. and Kölling, R. (2005) The deubiquitinating enzyme Ubp1 affects sorting of the ATPbinding cassette-transporter Ste6 in the endocytic pathway Mol. Biol. Cell, 16, 1319-1329
Schuldiner, M., Metz, J., Schmid, V., Denic, V., Rakwalska, M., et al (2008) The GET complex mediates insertion of tail-anchored proteins into the ER membrane Cell, 134, 634-645
Sciskala, B. and Kölling, R. (2013) Interaction maps of the Saccharomyces cerevisiae ESCRT-III protein Snf7 Eukaryot. Cell, 12, 1538–1546
Urbanowski, J.L. and Piper, R.C. (2001) Ubiquitin sorts proteins into the intralumenal degradative compartment of the late-endosome/vacuole Traffic, 2, 622-630
Wang, F., Whynot, A., Tung, M. and Denic, V. (2011) The mechanism of tail-anchored protein insertion into the ER membrane Mol. Cell, 43, 738–750
Wang, X., Lee, W-M., Watanabe, T., Schwartz, M., et al (2005) Brome mosaic virus 1a nucleoside triphosphatase/helicase domain plays crucial roles in recruiting RNA replication templates J. Virol., 79, 13747-13758
Wang, F., Chan, C., Weir, N.R. and Denic, V. (2014) The Get1/2 transmembrane complex is an endoplasmicreticulummembrane protein insertase Nature, 512, 441-444
Weiss, P., Huppert, S. and Kölling, R. (2009) Analysis of the dual function of the ESCRT-III protein Snf7 in endocytic trafficking and in gene expression Biochem. J., 424, 89–97
Zhang, J., Diaz, A., Mao, L., Ahlquist, P. et al (2012) Host acyl coenzyme A binding protein regulates replication complex assembly and activity of a positive-strand RNA vrus J. Virol., 86, 5110-5121
1a. Additional separation of lipid droplets
Schott, M.B., Rasineni, K., Weller, S.G., Schulze, R.J., Sletten, A.C., Casey, C.A. and McNiven, M.A. (2017) β-Adrenergic induction of lipolysis in hepatocytes is inhibited by ethanol exposure J. Biol. Chem., 292, 11815–11828
Vogt, D.A., Camus, G., Herker, E., Webster, B.R., Tsou, C-L., Greene, W.C., Yen, T-S.B. and Ott, M. (2013) Lipid droplet-binding protein TIP47 regulates hepatitis C virus RNA replication through interaction with the viral NS5A protein PLoS Pathog., 9: e1003302
Ysselstein, D., Dehay, B., Costantino, I.M., McCabe, G.P., Frosch, M.P., George, J.M., Bezard, E. and Rochet, J-C. (2017) Endosulfine-alpha inhibits membrane induced α-synuclein aggregation and protects against αsynuclein neurotoxicity Acta Neuropathol. Comm., 5: 3
2. Subcellular membranes
2.1 Golgi membranes
Fath, K.R. (2005) Characterization of myosin-II binding to Golgi stacks in vitro Cell Motil. Cytoskeleton, 60, 222-235
2.2 Lysosomes/endosomes
Morrison, C., Sauble, E.N., Nguyen, A., La, A., et al (2009) Potential abnormalities in iron metabolism in hyperlipidemia patient fibroblasts FASEB J., 23, Abstr. 105.4
Nguyen, A., Zhao, N., Morrison, C., Gonzalez, A., et al (2009) Mechanisms of iron release from lysosomes FASEB J., 23, Abstr. 921.11
2.3 Vesicles (budded and from permeablized cells)
Joglekar, A.P., Xu, D., Rigotti, D.J., Fairman, R., et al (2003) The SNARE motif contributes to rbet1 intracellular targeting and dynamics independently of SNARE interactions J. Biol. Chem., 278, 14121-14133
Lin, C-C., Love, H.D., Gushue, J.N., Bergeron, J.J.M., et al (1999) ER/Golgi intermediates acquire Golgi enzymes by Bredelfin A – sensitive retrograde transport in vitro J. Cell. Biol., 147, 1457-1472
Love, H.D., Lin, C.C., Short, C.S. and Ostermann, J. (1998) Isolation of functional Golgi-derived vesicles with a possible role in retrograde transport J. Cell Biol., 140, 541-551
Scheiffele, P., Verkade, P., Fra, A.M., Simons, K., et al (1998) Caveolin-1 and –2 in the exocytic pathway of MDCK cells J. Cell Biol., 140, 795-806
OptiPrepTM Reference List RS13; 6th edition, January 2020
OptiPrep™ Reference List RS14
Purification of organelles and membranes from non-mammalian eukaryotes
- This is a Reference List of publications reporting the use of OptiPrep™ for the purification and analysis of organelles from a variety of non-mammalian eukaryotic cells and tissues.
- This Reference List does not contain information on organelles and membranes from Saccharomyces cerevisiae: for this source see RS15
- Important Note: RS12 “Endocytosis – a bibliographical review”, although containing principally references on mammalian cells also contains sections on non-mammalian cells.
- For extracellular vesicle references see RS11
- For centrifugation strategies see Application Sheet S50
- The reference list is divided into eleven principal eukaryotic groups; within each of these groups papers are further sorted according to species and/or organelle type.
- References are listed alphabetically according to first author and then, if required, chronologically. To aid identification of research topics, these are highlighted in blue.
- For completeness Section 11 lists a few papers describing Gram-negative bacteria containing acidocalcisome-like particles
- Review articles (Section 12) are also listed.
Published papers have been assigned to one of the following principal sections:
1. Algae
2. Amphibia
3. Fish
4. Fungi (other than Saccharomyces cerevisiae). For Saccharomyces cerevisiae
organelles – see OptiprepTM Reference List RS15)
5. Insects
6. Marine invertebrates
7. Nematodes, trematodes, annelids
8. Phytoplankton
9. Plants and plant cells
10. Protozoa
11. Gram-negative bacteria
12. Review articles, including a sub-section on proteomics
1. Algae
1-1. Chlamydomonas reinhardtii
Acidocalcisomes-like organelles, chloroplasts and mitochondria
Ruiz, F.A., Marchesini, N., Seufferheld, M., Govindjee and Docampo, R. (2001) The polyphosphate bodies of Chlamydomas reinhardtii possess a proton pumping pyrophosphatase and are similar to acidocalcisomes J. Biol. Chem., 276, 46196-46203
Ciliary structure and function
Diener, D.R., Lupetti, P. and Rosenbaum, J.L. (2015) Proteomic analysis of isolated ciliary transition zones reveals the presence of ESCRT proteins Curr. Biol., 25, 379–384
Long, H., Zhang, F., Xu, N., Liu, G., Diener, D.R., Rosenbaum, J.L. and Huang, K. (2017) Comparative analysis of ciliary membranes and ectosomes Curr. Biol., 26, 3327–3335
Cytoplasmic vesicles
Casem, M.L. (2016) Cytoskeleton and intracellular motility In “Case studies in cell biology” Elsevier Inc, pp 127-156
Wood, C.R. and Rosenbaum, J.L. (2014) Proteins of the ciliary axoneme are found on cytoplasmic membrane vesicles during growth of cilia Curr. Biol., 24, 1114-1120
Flagella membrane vesicles
Huang, K., Diener, D.R., Mitchell, A., Pazour, G.J., Witman, G.B. and Rosenbaum, J.L. (2007) Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella J. Cell Biol., 179, 501-514
HAP2 fusion protein analysis
Baquero, E., Fedry, J., Legrand, P., Krey, T. and Rey, F.A. (2019) Species-specific functional regions of the green alga gamete fusion protein HAP2 revealed by structural studies Structure 27, 113–124
1-2. Cyanidioschyzon merolae
Chloroplasts/mitochondria
Nishida, K., Yagisawa, F., Kuroiwa, H., Nagata, T. and Kuroiwa, T. (2005) Cell cycle-regulated microtubuleindependent organelle division in Cyanidioschyzon merolae Mol. Biol. Cell, 16, 2493-2502
Mitochondria
Nishida, K., Yagisawa, F., Kuroiwa, H., Yoshida, Y. and Kuroiwa, T. (2007) WD40 protein Mda1 is purified with Dnm1 and forms a dividing ring for mitochondria before Dnm1 in Cyanidioschyzon merolae Proc. Natl. Acad. Sci. USA, 104, 4736-4741
Peroxisomes
Imoto, Y., Abe, Y., Okumoto, K., Honsho, M., Kuroiwa, H., Kuroiwa, T. and Fujiki, Y. (2017) Defining the dynamin-based ring organizing center on the peroxisome-dividing machinery isolated from Cyanidioschyzon merolae J. Cell Sci., 130, 853-867
Polyphosphate vacuoles
Yagisawa, F., Nishida, K., Yoshida, M., Ohnuma, M., Shimada, T., Fujiwara, T., Yoshida, Y., Misumi, O., Kuroiwa, H. and Kuroiwa, T. (2009) Identification of novel proteins in isolated polyphosphate vacuoles in the primitive red alga Cyanidioschyzon merolae Plant J., 60, 882–893
1-3. Emiliania huxleyi
Extracellular vesicles
Schatz, D., Rosenwasser, S., Malitsky, S., Wolf, S.G., Feldmesser, E. and Vardi, A. (2017) Communication via extracellular vesicles enhances viral infection of a cosmopolitan alga Nat. Microbiol., 2, 1485–1492
2. Amphibia (Xenopus)
ER/Golgi/plasma membrane
Carattino, M.D., Liu, W., Hill, W.G., Satlin, L.M. and Kleyman, T.R. (2007) Lack of a role of membraneprotein interactions in flow-dependent activation of ENaC Am. J. Physiol. Renal Physiol., 293, F316-F324
Kuiper, R.P., Bouw, G., Janssen, K.P.C., Rotter, J., van Herp, F. and Martens, G.J.M. (2001) Localization of p24 putative cargo receptors in the early secretory pathway depends on the biosynthetic activity of the cell Biochem. J., 360, 421-429
Lipid rafts
Bates, R.C., Fees, C.P., Holland, W.L., Winger, C.C., Batbayar, K., Ancar, R., Bergren, T., Petcoff, D. and Stith, B.J. (2014) Activation of Src and release of intracellular calcium by phosphatidic acid during Xenopus laevis fertilization Dev. Biol., 386, 165-180
Membrane/cytoplasm
Hülsmann, B.B., Labokha, A.A. and Görlich, D. (2012) The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model Cell, 150, 738–751
Nuclei
Amin, N.M., Greco, T.M., Kuchenbrod, L.M., Rigney, M.M., Chung, M-I., Wallingford, J.B., Cristea, I.M. and Conlon, F.L. (2014) Proteomic profiling of cardiac tissue by isolation of nuclei tagged in specific cell types (INTACT) Development, 141, 962-973
3. Fish
Endosomes/lysosomes
Yue, Y., Behra, R., Sigg, L., Suter, M, J-F., Pillai, S and Schirmer, K. (2016) Silver nanoparticle–protein interactions in intact rainbow trout gill cells Environ. Sci. Nano, 3, 1174
Exosomes
Hyenne, V., Ghoroghi, S, Collot, M., Bons, J., Follain, G., Harlepp, S., Mary, B., Bauer, J., Mercier, L., Busnelli, I et al (2019) Studying the fate of tumor extracellular vesicles at high spatiotemporal resolution using the zebrafish embryo Devel. Cell, 48, 554–572
Lipid rafts and caveolae
Adachi, T., Sato, C. and Kitajima, K. (2007) Membrane microdomain formation in crucial in epiboly during gastrulation of medaka Biochem. Biophys. Res. Commun., 358, 848-853
Adachi, T., Sato, C., Kishi, Y., Totani, K., Murata, T. Usui, T. and Kitajima, K. (2009) Membrane microdomains from early gastrula embryos of medaka, Oryzias latipes, are a platform of E-cadherin- and carbohydrate-mediated cell–cell interactions during epiboly Glycoconj. J. 26, 285–299
Sezgin, E., Azbazdar, Y., Ng, X.W., The, C., Simons, K., Weidinger, G., Wohland, T., Eggeling, C. and Ozhan, G. (2017) Binding of canonical Wnt ligands to their receptor complexes occurs in ordered plasma membrane environments FEBS J., 284, 2513–2526
Zehmer, J.K. and Hazel, J.R. (2003) Plasma membrane rafts of rainbow trout are subject to thermal acclimation J. Exp. Biol., 206, 1657-1667
Zehmer, J.K. and Hazel, J.R. (2005) Thermally induced changes in lipid composition of raft and non-raft regions of hepatocyte plasma membranes of rainbow trout J. Exp. Biol., 208, 4283-4290
Migrasomes
Jiang, D., Jiang, Z., Lu, D., Wang, X., Liang, H., Zhang, J., Meng, Y., Li, Y., Wu, D., Huang, Y. et al (2019) Migrasomes provide regional cues for organ morphogenesis during zebrafish gastrulation Nat. Cell Biol., 21, 966–977
Mitochondria
Yue, Y., Behra, R., Sigg, L., Suter, M, J-F., Pillai, S and Schirmer, K. (2016) Silver nanoparticle–protein interactions in intact rainbow trout gill cells Environ. Sci. Nano, 3, 1174 Signalling organelles: see “Migrasomes” above
4. Fungi
4-1. Candida albicans
Plasma membane (lipid rafts)
Aeed, P.A., Sperry, A.E., Young, C.L., Nagiec, M.M. and Elhammer, A.P. (2004) Effect of membrane perturbants on the activity and phase distribution of inositol phosphorylceramide synthase; development of a novel assay Biochemistry, 43, 8483-8493
Insenser, M., Nombela, C., Molero, G. and Gil, C. (2006) Proteomic analysis of detergent-resistant membranes from Candida albicans Proteomics, 6, Suppl. 1., S74-S81
Ragni, E., Calderon, J., Fascio, U., Sipiczki, M., Fonzi, W.A. and Popolo, L. (2011) Phr1p, a glycosylphosphatidylinsitol-anchored (1,3)-glucanosyltransferase critical for hyphal wall formation, localizes to the apical growth sites and septa in Candida albicans Fungal Genet. Biol., 48, 793–805
Wang, L., Jia, Y., Tang, R-J., Xu, Z., Cao, Y-B., Jia, X-M. and Jiang, Y-Y. (2012) Proteomic analysis of Rta2p-dependent raft-association of detergent-resistant membranes in Candida albicans PLoS One, 7: e37768
Secretory vesicles
Caballero-Lima, D., Hautbergue, G.M., Wilson, S.A. and Sudbery, P.E. (2014) In Candida albicans hyphae, Sec2p is physically associated with SEC2 mRNA on secretory vesicles Mol. Microbiol., 94, 828–842
4-2. Cladosporium resinae
Mitochondria, vacuoles
Goswami, P. and Cooney, J.J. (1999) Subcellular location of enzyme involved in oxidation on n-alkane by Cladosporium resinae Appl. Microbiol. Biotechnol., 51, 860-864
4-3. Cryptococcus neoformans
Exocytosis and extracellular vesicles
Oliveira, D.L., Nimrichter, L., Miranda, K., Frases, S., Faull, K.F., Casadevall, A. and Rodrigues, M.L. (2009) Cryptococcus neoformans cryoultramicrotomy and vesicle fractionation reveals an intimate association between membrane lipids and glucuronoxylomannan Fungal Genet. Biol., 46, 956–963
Wolf, J.M., Rivera, J. and Casadevall, A. (2012) Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles Cellular Microbiology (2012) 14, 762–773 Lipid rafts
He, X., Shi, X., Puthiyakunnon, S., Zhang, L., Zeng, Q., Li, Y., Boddu, S., Qiu, J., Lai, Z. et al (2016) CD44- mediated monocyte transmigration across Cryptococcus neoformans-infected brain microvascular endothelial cells is enhanced by HIV-1 gp41-I90 ectodomain J. Biomed. Sci., 23: 28
Huang, S-H., Wu, C-H., Chang, Y.C., Kwon-Chung, K.J., Brown, R.J. and Jong, A. (2012) Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection PLoS One, 7, e48570
4-4. Neurospora crassa
Glyoxysomes
Managadze, D., Würtz, C., Wiese, S., Meyer, H.E., Niehaus, G., Erdmann, R., Warscheid, B. and Rottensteiner, H. (2010) A proteomic approach towards the identification of the matrix protein content of the two types of microbodies in Neurospora crassa Proteomics, 10, 3222–3234
4-5. Paracoccidioides brasiliensis
Mitochondria and peroxisomes
Brito, W. deA., Rezende, T.C.V., Parente, A.F., Ricart, C.A.O., de Sousa, M.V., Báo, N. and Soares, C.M.deA. (2011) Identification, characterization and regulation studies of the aconitase of Paracoccidioides brasiliensis Fungal Biol., 115, 697-707
5. Insects (arthropoda)
5-1 Aedes cell lines
Vora, A., Zhou, W., Londono-Renteria, B., Woodson, M., Sherman, M.B., Colpitts, T.M., Neelakanta, G. and Sultana, H. (2018) Arthropod EVs mediate dengue virus transmission through interaction with a tetraspanin domain containing glycoprotein Tsp29Fb Proc. Natl. Acad. Sci. USA, 115, E6604–E6613
5-2. Bombyx mori
Lysosomes
Shiba, H., Yabu, T., Sudayama, M., Mano, N., Arai, N., Nakanishi, T. and Hosono, K. (2016) Sequential steps of macroautophagy and chaperone-mediated autophagy are involved in the irreversible process of posterior silk gland histolysis during metamorphosis of Bombyx mori J. Exp. Biol., 219, 1146-1151
5-3. Chironomids
Membrane vesicles, separation from proteins
Hatanaka, R., Hagiwara-Komoda, Y., Furuki, T., Kanamori, Y., Fujita, M., Cornette, R., Sakurai, M., Okuda, T. and Kikawada, T. (2013) An abundant LEA protein in the anhydrobiotic midge, PvLEA4, acts as a molecular shield by limiting growth of aggregating protein particles Insect Biochem. Mol. Biol., 43, 1055-1067
5-4. Drosophila/Flies
Endosomes/endocytosis
Lee, J., Song, M. and Hong, S. (2013) Negative regulation of the novel norpAP24 suppressor, diehard4, in the endo-lysosomal trafficking underlies photoreceptor cell degeneration PLoS Genet., 9: e1003559
Lee, Y.S., Pressman, S., Andress, A.P., Kim, K., White, J.L., Cassidy, J.J., Li, X., Lubell, K. et al (2009) Silencing by small RNAs is linked to endosomal trafficking Nat. Cell Biol., 11, 1150-1157
Tiklová, K., Senti, K-A., Wang, S., Gräslund, A. and Samakovlis, C. (2010) Epithelial septate junction assembly relies on melanotransferrin iron binding and endocytosis in Drosophila Nature Cell. Biol., 12, 1071- 1078
ER/Golgi/plasma membrane
Adolfsen, B., Sarawati, S., Yoshihara, M. and Littleton, J.T. (2004) Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment J. Cell Biol., 166, 249-260
Beronja, S., Laprise, P., Papoulas, O., Pellikka, M., Sisson, J. and Tepass, U. (2005) Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells J. Cell Biol., 169, 635-646
Betschinger, J., Eisenhaber, F. and Knoblich, J.A. (2005) Phosphorylation-induced autoinhibition regulates the cytoskeletal protein lethal (2) giant larvae Curr. Biol., 15, 276-282
Gatto, L., Breckels, L.M., Burger, T., Nightingale, D.J.H., Groen, A.J., Campbell, C., Nikolovski, N., Mulvey, C.M. et al (2014) A foundation for reliable spatial proteomics data analysis Mol. Cell. Proteom., 13, 1937-1952
Khanna, M.R., Stanley, B.A. and Thomas, G.H. (2010) Towardsta membrane proteome in Drosophila: a method for the isolation of plasma membrane BMC Genomics 2010, 11: 302
Kim, A-Y., Seo, J.B., Kim, W-t., Choi, H.J., Kim, S-Y., Morrow, G., Tanguay, R.M., Steller, H. and Koh, Y.H. (2015) The pathogenic human Torsin A in Drosophila activates the unfolded protein response and increases susceptibility to oxidative stress BMC Genom., 16: 338
Niimura, M., Isoo, N., Takasugi, N., Tsuruoka, M., Ui-Tei, K., Saigo, K., Morohashi, Y., Tomita, T. and Iwatsubo, T. (2005) Aph-1 contributes to the stabilization and trafficking of the γ-secretase complex through mechanisms involving intermolecular and intramolecular interactions J. Biol. Chem., 280, 12967-12975
Panneels, V., Eroglu, C., Cronet, P. and Sinning, I. (2003) Pharmacological characterization and immunoaffinity purification of metabotropic glutamate receptor from Drosophila overexpressed in Sf9 cells Prot. Expr. Purif., 20, 275-282
Papoulas, O., Hays, T.S. and Sisson, J.C. (2005) The golgin lava lamp mediates dynein-based Golgi movements during Drosophila cellularization Nat. Cell Biol., 7, 612-618
Satori, C.P., Henderson, M.M., Krautkramer, E.A., Kostal, V., Distefano, M.M. and Arriaga, E.A. (2013) Bioanalysis of eukaryotic organelles Chem. Rev., 113, 2733−2811
Stein, D., Charatsi, I., Cho, Y.S., Zhang, Z., Nguyen, J., DeLotto, R., Luschnig, S. and Moussian, B. (2010) Localization and activation of the Drosophila protease Easter require the ER-resident saposin-like protein Seele Curr. Biol., 20, 1953–1958
Tan, D.J.L., Dvinge, H., Christoforou, A., Bertone, P., Arias, A.M. and Lilley, K.S. (2009) Mapping organelle proteins and protein complexes in Drosophila melanogaster J. Proteome Res., 8, 2667–2678
Wan, D., Zhang, Z.C., Zhang, X., Li, Q. and Han, J. (2015) X chromosome-linked intellectual disability protein PQBP1 associates with and regulates the translation of specific mRNAs Hum. Mol. Genet., 24, 4599–4614
Zarnescu, D.C., Jin, P., Betschinger, J., Nakamoto, M., Wang, Y., Dockendorff, T.C., Feng, Y., Jongens, T.A., Sisson, J.C., Knoblich, J.A., Warren, S.T. and Moses, K. (2005) Fragile X protein functions with LgI and the PAR complex in flies and mice Dev. Cell, 8, 43-52
Exocytosis and exosomes
Beronja, S., Laprise, P., Papoulas, O., Pellikka, M., Sisson, J. and Tepass, U. (2005) Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells J. Cell Biol., 169, 635-646
Kerr, C,H., Dalwadi, U., Scott, N.E., Yip, C.K., Foster, L.J. and Jan, E. (2018) Transmission of Cricket paralysis virus via exosome-like vesicles during infection of Drosophila cells Sci. Rep., 8: 17353
Matusek, T., Wendler, F., Polès, S., Pizette, S., D’Angelo, G., Fürthauer, M. and Thérond, P.P. (2014) The ESCRT machinery regulates the secretion and long-range activity of Hedgehog Nature, 516, 99-103
Shibata, T., Hadano, J., Kawasaki, D., Dong, X. and Kawabata, S-i. (2017) Drosophila TG-A transglutaminase is secreted via an unconventional Golgi-independent mechanism involving exosomes and two types of fatty acylations J. Biol. Chem., 292, 0723–10734
Lipid rafts
Eroglu, C., Brügger, B., Wieland, F. and Sinning, I. (2003) Glutamate-binding affinity of Drosophila metabotropic glutamate receptor is modulated by association with lipid rafts Proc. Natl. Acad. Sci. USA, 100, 10219-10224
Goyal, G., Zheng, J., Adam, E., Steffes, G., Jain, M., Klavins, K. and Hummel, T. (2019) Sphingolipiddependent Dscam sorting regulates axon segregation Nat. Comm., 10: 813
Hebbar, S., Lee, E., Manna, M., Steinert, S., Kumar, G.S., Wenk, M., Wohland, T., and Kraut, R. (2008) A fluorescent sphingolipid binding domain peptide probe interacts with sphingolipids and cholesterol-dependent raft domains J. Lipid Res. 49, 1077-1089
Hoehne, M., de Couet, H.G., Stuermer, C.A.O. and Fischbach, K-F. (2005) Loss- and gain-of-function analysis of the lipid raft proteins reggie/flotillin in Drosphilia: they are posttranslationally regulated, and misexpression interferes with wing and eye development Mol. Cell. Neurosci., 30, 326-338
Rietveld, A., Neutz, S., Simons, K. and Eaton, S. (1999) Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophilia raft lipid microdomains J. Biol. Chem., 274, 12049-12054
Sanxaradis, P.D., Cronin, M.A., Rawat, S.S., Waro, G., Acharya, U. and Tsunoda, S. (2007) Light-induced
recruitment of INAD-signaling complexes to detergent-resistant lipid rafts in Drosophila receptors Mol Cell. Neurosci., 36, 36-46
West, R.J.H., Briggs, L., Fjeldstad, M.P., Ribchester, R.R. and Sweeney, S.T. (2018) Sphingolipids regulate neuromuscular synapse structure and function in Drosophila J. Comp. Neurol., 526, 1995–2009
Zhai, L., Chaturvedi, D. and Cumberledge, S. (2004) Drosophila Wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine J. Biol. Chem., 279, 33220-33227
Membrane vesicles, separation from proteins
Kruppa, A.J., Ott, S., Chandraratna, D.S., Irving, J.A., Page, R.M., Speretta, E., Seto, T., Camargo, L.M., Marciniak, S.J., Lomas, D.A. and Crowther, D.C. (2013) Suppression of Aβ toxicity by puromycin-sensitive aminopeptidase is independent of its proteolytic activity Biochim. Biophys. Acta, 1832, 2115–2126
Sing, A., Tsatskis, Y., Fabian, L., Hester, I., Rosenfeld, R., Serricchio, M., Yau, N., Bietenhader, M., Shanbhag, R., Jurisicova, A. et al (2014) The atypical cadherin fat directly regulates mitochondrial function and metabolic state Cell, 158, 1293–1308
Mitochondria
Odnokoz, O., Nakatsuka, K., Klichko, V.I., Nguyen, J., Solis, L.C., Ostling, K., Badinloo, M., Orr, W.C. and Radyuk, S.N. (2017) Mitochondrial peroxiredoxins are essential in regulating the relationship between Drosophila immunity and aging Biochim. Biophy. Acta, 1863, 68–80
Satori, C.P., Henderson, M.M., Krautkramer, E.A., Kostal, V., Distefano, M.M. and Arriaga, E.A. (2013) Bioanalysis of eukaryotic organelles Chem. Rev., 113, 2733−2811
Tan, D.J.L., Dvinge, H., Christoforou, A., Bertone, P., Arias, A.M. and Lilley, K.S. (2009) Mapping organelle proteins and protein complexes in Drosophila melanogaster J. Proteome Res., 8, 2667–2678
Neuroligin 3
Wua, J., Tao, N., a, Tian, Y., Xing, G., Lv, H., Han, J., Lin, C. and Xie, W. (2018) Proteolytic maturation of Drosophila Neuroligin 3 by tumor necrosis factor α-converting enzyme in the nervous system BBA – Gen. Subjects, 1862, 440–450
Nuclei
Groen, C.M., Jayo, A., Parsons, M. and Tootle, T.L. (2015) Prostaglandins regulate nuclear localization of Fascin and its function in nucleolar architecture Mol. Biol. Cell, 26, 1901-1917
Steiner, F.A., Talbert, P.B., Kasinathan, S., Deal, R.B. and Henikoff, S. (2012) Cell-type-specific nuclei purification from whole animals for genome-wide expression and chromatin profiling Genome Res., 22, 766– 777
Steiner, F.A. and Henikoff, S. (2015) Cell type-specific affinity purification of nuclei for chromatin profiling in whole animals In The Nucleus, Methods in Mol. Biol. 1228 (ed. Hancock, R.) Springer Science+Business Media New York, pp 3-14
Ye, Y., Gu, L., Chen, X., Shi, J., Zhang, X. and Jiang, C. (2016) Chromatin remodeling during the in vivo glial differentiation in early Drosophila embryos Sci. Rep., 6: 33422
Ye, Y., Li, M., Gu, L., Chen, X., Shi, J., Zhang, X. and Jiang, C. (2017) Chromatin remodeling during in vivo neural stem cells differentiating to neurons in early Drosophila embryos Cell Death Different., 24, 409–420
Plasma membrane
Dasgupta, U., Bamba, T., Chiantia, S., Karim, P., Abou Tayoun, A.N., Yonamine, I., Rawat, S.S., Rao, R.P. et al (2009) Ceramide kinase regulates phospholipase C and phosphatidylinositol 4, 5, bisphosphate in phototransduction Proc. Natl. Acad. Sci. USA, 106, 20063-20068
Rao, R.P., Yuan, C., Allegood, J.C., Rawat, S.S., Edwards, M.B., Wang, X., Merrill, A.H., Acharya, U. and Acharya, J.K. (2007) Ceramide transfer protein function is essential for normal oxidative stress response and lifespan Proc. Natl. Acad. Sci. USA, 104, 11364-11369
Stowers, R.S., Megeath, L.J., Gorska-Andrzejak, J., Meinertzhagen, I.A. and Schwartz, T.L. (2002) Axonal transport of mitochondria to synapses depends on Milton, a novel Drosophilia protein Neuron, 36, 1063-1077
Rhabdomere membranes
Panneels, V., Kock, I., Krijnse-Locker, J., Rezgaoui, M., Sinning, I. (2011) Drosophila photoreceptor cells exploited for the production of eukaryotic membrane proteins: receptors, transporters and channels PLoS One 6: e18478
Toll pathway
Shmueli, A., Shalit, T., Okun, E. and Shohat‑Ophir, G, (2018) The Toll pathway in the central nervous system of flies and mammals Neuromol. Med., 20, 419–436
5-5 Insect larvae lipid rafts
Bayyareddy, K., Zhu, X., Orlando, R. and Adang, M.J. (2012) Proteome analysis of Cry4Ba toxin-interacting Aedes aegypti lipid rafts using geLC-MS/MS J. Proteome Res., 11, 5843-5855
Ito, T., Bando, H. and Asano, S-i. (2006) Activation process of the mosquitocidal δ-endotoxin Cry39A produced by Bacillus thuringiensis subsp. aizawai BUN1-14 and binding property to Anopheles stephensi BBMV J. Invert. Pathol., 93, 29-35
Liu, J-G., Yang, A-Z., Shen, X-H., Hua, B-G., Shi, G-L. (2011) Specific binding of activated Vip3Aa10 to Helicoverpa armigera brush border membrane vesicles results in pore formation J. Invertebr. Pathol., 108, 92– 97
5-6. Leech microglia
Arab, T., Raffo-Romero, A., Van Camp, C., Lemaire, Q., Le Marrec-Croq, F., Drago, F., Aboulouard, S., Slomianny, C., Lacoste, A-S. et al (2019) Proteomic characterisation of leech microglia extracellular vesicles (EVs): comparison between differential ultracentrifugation and Optiprep™ density gradient isolation J. Extracell. Ves., 8: 1603048
5-7. Rhodnius prolixus
Yolk granules
Gomes, F.M., Oliveira, D.M.P., Motta, L.S., Ramos, I.B., Miranda, K.M. and Machado, E.A. (2010) Inorganic polyphosphate inhibits an aspartic protease-like activity in the eggs of Rhodnius prolixus (Stahl) and impairs yolk mobilization in vitro J. Cell. Physiol., 222, 606–611
5-8. sf9 cells
Plasma membrane
Eisses, J.F., Chi, Y. and Kaplan, J.H. (2005) Stable plasma membrane levels of hCTR1 mediate cellular copper uptake J. Biol. Chem., 280, 9635-9639
5-9. Spodoptera
Extracellular vesicles
Thoene, J., Goss, T., Witcher, M., Mullet, J., N’Kuli, F., Van Der Smissen, P., Courtoy, P. and Hahn, S.H. (2013) In vitro correction of disorders of lysosomal transport by microvesicles derived from baculovirusinfected Spodoptera cells Mol. Genet. Metab., 109, 77–85
6. Marine invertebrates
6.1 Haloarcheons
Erdmann, S., Tschitschko, B., Zhong, L., Raftery, M.J. and Cavicchioli, R. (2017) A plasmid from an Antarctic haloarchaeon uses specialized membrane vesicles to disseminate and infect plasmid-free cells Nat. Microbiol., 1446, 1446–1455
6-2 Molluscs
Mannosomes
Knigge, T., Mann, N., Parveen, Z., Perry, C., Gernhofer, M., Triebskorn, R., Kohler, H-R. and Connock, M. (2002) Mannosomes: a molluscan intracellular tubular membrane system related to heavy metal stress Comp. Biochem. Physiol. Part C, 131, 259-269
Mitochondria, peroxisomes, lysosomes, microsomes
Apraiz, I., Mi, J. and Cristobal, S. (2006) Identification of proteomic signatures of exposure to marine pollutants in mussels (Mytilus edulis) Mol. Cell. Proteom., 5, 1274-1285
Apraiz, I., Cajaraville, M.P. and Cristobal, S. (2009) Peroxisomal proteomics: Biomonitoring in mussels after the Prestige’s oil spill Mar. Pollut. Bull., 58, 1815–1826
Cristobal, S. (2007) Proteomics-based method for risk assessment of peroxisome proliferating pollutants in the marine environment Methods Mol. Biol., 410, 123-135
Grewal, N., Parveen, Z., Large, A., Perry, C. and Connock, M. (2000) Gastropod mollusc aliphatic alcohol oxidase: subcellular localisation and properties Comp. Biochem. Biophys., 125, 543-554
Mi, J., Orbea, A., Syme, N., Ahmed, M., Cajaraville, M.P. and Cristobal, S. (2005) Peroxisomal proteomics, a new tool for risk assessment of peroxisome proliferating pollutants in the marine environment Proteomics, 5, 3954-2965
Nuclei
Shaw, J.P., Large, A.T., Chipman, J.K., Livingstone, D.R. and Peters, L.D. (2000) Seasonal variation in mussel Mytilus edulis digestive gland cytochrome P4501A- and 2E-immunoidentified protein levels and DNA strand breaks (Comet assay) Marine Environ. Res., 50, 405-409
Shaw, J.P., Large, A.T., Livingstone, D.R., Doyotte, A., Renger, J., Chipman, J.K. and Peters, L.D. (2002) Elevation of cytochrome P450-immunopositive protein and DNA damage in mussels (Mytilus edulis) transplanted to a contaminated site Marine Environ. Res., 54, 505-509
Shaw, J.P., Large, A.T., Donkin, P., Evans, S.V., Staff, F.J., Livingstone, D.R., Chipman, J.K. and Peters, L.D. (2004) Seasonal varation in cytochrome P450 immunopositive protein levels, lipid peroxidation and genetic toxicity in digestive gland of the mussel Mytilus edulis Aquatic Tox., 67, 325-336
6-3 Sea urchin eggs/sperm
Acidocalcisomes
Ramos, I.B., Miranda, K., Pace, D.A., Verbist, K.C., Lin, F-Y., Zhang, Y., Oldfield, E., Machado, E.A., de Souza, W. and Docampo, R. (2010) Calcium- and polyphosphate-containing acidic granules of sea urchin eggs are similar to acidocalcisomes, but are not the targets for NAADP Biochem. J., 429, 485–495
Lipid rafts
Loza-Huerta, A., Vera-Estrella, R., Darszon, A. and Beltrána, C. (2013) Certain Strongylocentrotus purpuratus sperm mitochondrial proteins co-purify with low density detergent-insoluble membranes and are PKA or PKCsubstrates possibly involved in sperm motility regulation Biochim. Biophys. Acta, 1830, 5305–5315
Vacquier, V.D., Loza-Huerta, A., García-Rincón, J., Darszon, A. and Beltrán, C. (2014) Soluble adenylyl cyclase of sea urchin spermatozoa Biochim. Biophys. Acta, 1842, 2621–2628
6-4 Squid
Axoplasmic vesicles
LaPointe, N.E., Morfini, G., Pigino, G., Gaisina, I.N., Kozikowski, A.P., Binder, L.I. and Brady, S.T. (2009) The amino terminus of tau inhibits kinesin-dependent axonal transport: Implications for filament toxicity J. Neurosci. Res., 87, 440-451
Zamponi, E., Buratti, F., Cataldi, G., Caicedo, H.H., Song, Y., Jungbauer, L.M., LaDu, M.J., Bisbal, M. et al (2017) Prion protein inhibits fast axonal transport through a mechanism involving casein kinase 2 PLoS One, 12: e0188340
7. Nematodes, trematodes, flatworms, annelids
Extracellular vesicles
Eichenberger, R.M., Talukder, H., Field, M.A., Wangchuk, P., Giacomina, P., Loukas, A. and Sotillo, J. (2018) Characterization of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host–parasite communication J. Extracell. Ves., 7: 1428004
Meningher, T., Lerman, G., Regev-Rudzki, N., Gold, D., Ben-Dov, I.Z., Sidi, Y., Avni, D. and Schwartz, E. (2017) Schistosomal microRNAs isolated from extracellular vesicles in sera of infected patients: a new tool for diagnosis and follow-up of human schistosomiasis J. Infect. Dis., 215, 378–86
Sotillo, J., Pearson, M., Potriquet, J., Becker, L., Pickering, D., Mulvenna, J. and Loukas, A. (2016) Extracellular vesicles secreted by Schistosoma mansoni contain proteinvaccine candidates Int. J. Parasitol., 46, 1–5
Lysosomes
Li, Y., Chen, B., Zou, W., Wang, X., Wu, Y., Zhao, D., Sun, Y., Liu, Y., Chen, L., Miao, L, Yang, C. and Wang, X. (2016) The lysosomal membrane protein SCAV-3 maintains lysosome integrity and adult longevity J. Cell Biol., 215, 167–185
Mitochondria
Haynes, C.M., Yang, Y., Blais, S.P., Neubert, T.A. and Ron, D. (2010) The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans Mol. Cell, 37, 529– 540
Nuclei
Steiner, F.A., Talbert, P.B., Kasinathan, S., Deal, R.B. and Henikoff, S. (2012) Cell-type-specific nuclei purification from whole animals for genome-wide expression and chromatin profiling Genome Res., 22, 766– 777
Steiner, F.A. and Henikoff, S. (2015) Cell type-specific afinity purification of nuclei for chromatin profiling in whole animals In The Nucleus, Methods in Mol. Biol. 1228 (ed. Hancock, R.) Springer Science+Business Media New York, pp 3-14
Tweetena, K.A. and Morris, S.J. (2016) Flow cytometry analysis of DNA ploidy levels and protein profiles distinguish between populations of Lumbriculus (Annelida: Clitellata) Invert. Biol., 135, 385–399
Multivesicular bodies
Kobuna, H., Inoue, T., Shibata, M., Gengyo-Ando, K., Yamamoto, A., Mitani, S. and Arai, H. (2010) Multivesicular body formation requires OSBP–related proteins and cholesterol PloS Genet., 6: e1001055
8. Phytoplankton (Emiliania huxleyi)
Lipid rafts
Rose, S.L., Fulton, J.M., Brown, C.M., Natale, F., Van Mooy, B.A.S. and Bidle, K.D. (2014) Isolation and characterization of lipid rafts in Emiliania huxleyi: a role for membrane microdomains in host–virus interactions Environ. Microbiol., 16, 1150–1166
9. Plants, plant cells, trees
9-1. Arabidopsis
Chloroplasts
Laganowsky, A., Gómez, S.M., Whitelegge, J.P., Nishio, J.N. (2009) Hydroponics on a chip: Analysis of the Fe deficient Arabidopsis thylakoid membrane proteome J. Proteom., 72, 397-415
Zheng, Y., Liao, C., Zhao, S., Wang, C. and Guo, Y. (2017) The glycosyltransferase QUA1 regulates chloroplast-associated calcium signaling during salt and drought stress in Arabidopsis Plant Cell Physiol., 58, 329–341
Cytoplasm
Liu, Z., Zhu, Y., Gao, J., Yu, F., Dong, A. and Shen, W-H. (2009) Molecular and reverse genetic characterization of nucleosome assembly protein1 (NAP1) genes unravels their function in transcription and nucleotide excision repair in Arabidopsis thaliana Plant J., 59, 27–38
ER and Golgi
Busse-Wicher, M., Gomes, T.C.F., Tryfona, T., Nikolovski, N., Stott, K., Grantham, N.J., Bolam, D.N., Skaf, M.S. and Dupree, P. (2014) The pattern of xylan acetylation suggests xylan may interact with cellulose microfibrils as a twofold helical screw in the secondary plant cell wall of Arabidopsis thaliana Plant J., 79, 492–506
Dunkley, T.P.J., Watson, R., Griffin, J.L., Dupree, P. and Liley, K.S. (2004) Localization of organelle proteins by isotope tagging (LOPIT) Mol. Cell. Proteom., 3, 1128-1134
Zheng, Y., Liao, C., Zhao, S., Wang, C. and Guo, Y. (2017) The glycosyltransferase QUA1 regulates chloroplast-associated calcium signaling during salt and drought stress in Arabidopsis Plant Cell Physiol., 58, 329–341
ER, Golgi, plasma membrane, mitochondria, vacuolar membrane
Dunkley, T.P.J., Hester, S., Shadforth, I.P., Runions, J., Weimer, T., Hanton, S.L., Griffin, J.L., Besssant, C., Brandizzi, F. et al (2006) Mapping the Arabidopsis organelle proteome Proc. Natl. Acad. Sci. USA, 103, 6518- 6523
Gatto, L., Breckels, L.M., Burger, T., Nightingale, D.J.H., Groen, A.J., Campbell, C., Nikolovski, N., Mulvey, C.M., Christoforou, A., Ferro, M. and Lilley, K.S. (2014) A foundation for reliable spatial proteomics data analysis Mol. Cell. Proteom., 13, 1937-1952
Groen, A.J., de Vries, S.C. and Lilley, K.S. (2008) A proteomics approach to membrane trafficking Plant Physiol., 147, 1584-1589
Lilley, K.S. and Dunkley, T.P.J. (2008) Determination of genuine residents of plant endomembrane organelles using isotope tagging and multivariate statistics In Methods Mol. Biol., 432, Organelle Proteomics (ed. Pflieger, D, and Rossier, J.) Humana Press, Totowa, NJ, pp 373-387
Nikolovski, N., Shliaha, P.V., Gatto, L., Dupree, P. and Lilley, K.S. (2014) Label-free protein quantification for plant Golgi protein localization and abundance Plant Physiol., 166, 1033–1043
Sadowski, P.G., Dunkley, T.P.J., Shadforth, I.P., Dupree, P., Bessant, J.L. and Lilley, K.S. (2006) Quantitative proteomic approach to study subcellular localization of membrane proteins Nat. Protoc., 1, 1778-1789
Zhang, Y., Nikolovski, N., Sorieul, M., Vellosillo, T., McFarlane, H.E., Dupree, R., Kesten, C., Schneider, R. et
al (2016) Golgi-localized STELLO proteins regulate the assembly and trafficking of cellulose synthase complexes in Arabidopsis Nat. Comm., 7: 11656
Extracellular vesicles
Hou, Y., Zhai, Y., Feng, L., Karimi, H.Z., Rutter, B.D., Zeng, L., Choi, D.S., Zhang, B., Gu, W. et al (2019) A Phytophthora effector suppresses trans-kingdom RNAi to promote disease susceptibility Cell Host Microbe 25, 153–165
Rutter, B.D. and Innes, R.W. (2017) Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins Plant Physiol., 173, 728–741
Membrane vesicles, separation from proteins
Mahon, P. and Dupree, P. (2001) Quantitative and reproducible two-dimensional gel analysis using Phoretix 2D Full Electrophoresis, 22, 2075-2085
Mitochondria
Breckels, L.M., Gatto, L., Christoforou, A., Groen, A.J., Lilley, K.S. and Trotter, M.W.B. (2013) The effect of organelle discovery upon sub-cellular protein localization J. Proteom., 88, 129-140
Hartman, N.T., Sicilia, F., Lilley, K.S. and Dupree, P. (2007) Proteomic complex detection using sedimentation Anal. Chem., 79, 2078-2083
Mitochondria, rough ER, plastid membranes
Berg, M., Parbel, A., Pettersen, H., Fenyo, D. and Björkesten, L. (2006) Reproducibility of LC-MS-based protein identification J. Exp. Botany, 57, 1509-1514
Nuclei
Liu, Z., Zhu, Y., Gao, J., Yu, F., Dong, A. and Shen, W-H. (2009) Molecular and reverse genetic characterization of nucleosome assembly protein1 (NAP1) genes unravels their function in transcription and nucleotide excision repair in Arabidopsis thaliana Plant J., 59, 27–38
Liu, Z., Zhu, Y., Gao, J., Yu, F., Dong, A. and Shen, W-H. (2009) Molecular and reverse genetic characterization of nucleosome assembly protein1 (NAP1) genes unravels their function in transcription and nucleotide excision repair in Arabidopsis thaliana Plant J., 59, 27–38
Oil bodies
Deruyffelaere, C., Bouchez, I., Morin, H., Guillot, A., Miquel, M., Froissard, M., Chardot, T., and D’Andrea, S. (2015) Ubiquitin-mediated proteasomal degradation of oleosins is involved in oil body mobilization during post-germinative seedling growth in Arabidopsis Plant Cell Physiol., 56, 1374–1387
Peroxisomes
Palma, J.M., Corpas, F.J. and del Rio, L.A. (2009) Proteome of plant peroxisomes: new perspectives on the role of these organelles in cell biology Proteomics, 9, 2301-2312
Reumann, S. (2011) Toward a definition of the complete proteome of plant peroxisomes: Where experimental proteomics must be complemented by bioinformatics Proteomics 11, 1764–1779
Plasma membrane
Alexandersson, E., Gustavsson, N., Bernfur, K., Kjellbom, P. and Larsson, C. (2007) Plasma membrane proteomics In Plant Proteomics (ed. Samaj, J. and Thelen, J.) Springer Science + Business Media, Berlin, pp 186-206
Subcellular membrane markers
Hooper, C.M., Stevens, T.J., Saukkonen, A., Castleden, I.R., Singh, P., Mann, G.W., Fabre, B., Jun Ito, J. et al (2017) Multiple marker abundance profiling: combining selected reaction monitoring and data-dependent acquisition for rapid estimation of organelle abundance in subcellular samples Plant J., 92, 1202–1217
9-2. Ferns
Tonoplast
Shen, H., He, Z., Yan, H., Xing, Z., Chen, Y., Xu, W., Xu, W. and Ma, M. (2014) The fronds tonoplast quantitative proteomic analysis in arsenic hyperaccumulator Pteris vittata L. J. Proteom., 105, 46–57
9-3. Fruit
Tomato ripening
Pontiggia, D., Spinelli, F., Fabbri, C., Licursi, V., Negri, R., De Lorenzo, G. and Mattei, B. (2019) Changes in the microsomal proteome of tomato fruit during ripening Sci. Rep., 9: 14350
Tonoplast
Liu, R., Wang, Y., Qin, G. and Tian, S. (2016) iTRAQ-based quantitative proteomic analysis reveals the role of the tonoplast in fruit senescence J. Proteom., 146, 80–89
9-4. Ginger
Li, Z., Wang, H., Yin, H., Bennett, C., Zhang, H-g. and Guo, P. (2018) Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression Sci. Rep., 8: 14644
9-5. Grasses, grains and related crops
Golgi/microsomes
Chateigner-Boutin, A-L., Suliman, M., Bouchet, B., Alvarado, C., Lollier, V., Rogniaux, H., Guillon, F. and Larré, C. (2015) Endomembrane proteomics reveals putative enzymes involved in cell wall metabolism in wheat grain outer layers J. Exp. Botany, 66, 2649–2658
Suliman, M., Chateigner-Boutin, A-L., Francin-Allami, M., Partier, A., Bouchet, B., Salse, J., Pont, C., Marion, J., Rogniaux, H., Tessier, D., Guillon, F. and Larréa, C. (2013) Identification of glycosyltransferases involved in cell wall synthesis of wheat endosperm J. Proteom., 78, 508–521
Lipid rafts
Carmona-Salazar, L., El Hafidi, M., Gutierrez-Najera, N., Noyola-Martinez, L., Gonzalez-Solis, A. and Gavilanes-Ruiz, M. (2015) Fatty acid profiles from the plasma membrane and detergent resistant membranes of two plant species Phytochemistry, 109, 25–35
Han, B., Yang, N., Pu, H. and Wang, T. (2018) Quantitative proteomics and cytology of rice pollen sterol-rich membrane domains reveals pre-established cell polarity cues in mature pollen J. Proteome Res., 17, 1532−1546
Nagano, M., Ishikawa, T., Fujiwara, M./, Fukao, Y., Kawano, Y. Kawai-Yamada, M. and Shimamoto, K. (2016) Plasma membrane microdomains are essential for Rac1-RbohB/H-mediated immunity in rice Plant Cell, 28, 1966–1983
Nuclei
Bedell, J.A., Budiman, M.A., Nunberg, A., Citek, R.W., Robbins, D., Jones, J., Flick, E., Rohlfing, T., Fries, J. et al (2005) Sorghum genome sequencing by methylation filtration PLoS Biol 3: e13
Ford, T.C., Baldwin, J.P. and Lambert, S.J. (1998) Rapid enzyme-free preparation of starch-free nuclei from plants facilitates studies of chromatin structure. Plant proteins in abiotic stress responses Plant Protein Club, 1998 Annual Symposium, University of York, p24
Plasma membrane
Nagano, M., Ishikawa, T., Fujiwara, M./, Fukao, Y., Kawano, Y. Kawai-Yamada, M. and Shimamoto, K. (2016) Plasma membrane microdomains are essential for Rac1-RbohB/H-mediated immunity in rice Plant Cell, 28, 1966–1983
Protein bodies
Llop-Tous, I., Madurga, S., Giralt, E., Marzabal, P., Torrent, M. and Ludevid, M.D. (2010) Relevant elements of
a maize γ-zein domain involved in protein body biogenesis J. Biol. Chem., 285, 35633–35644
9-6. Legumes
Nuclei
Timko, M.P., Rushton, P.J., Laudeman, T.W., Bokowiec, M.T., Chipumuro, E., Cheung, F., Town, C.D. and Chen, X. (2008) Sequencing and analysis of the gene-rich space of cowpea BMC Genomics, 9: 103
Lipid rafts
Belugin, B.V., Zhestkova, I.M. and Trofimova, M.S. (2011) Affinity of PIP-aquaporins to sterol-enriched domains in plasma membrane of the cells of etiolated pea seedlings Biochemistry (Moscow) Suppl. Series A: Membr. Cell Biol., 5, 56–63
Carmona-Salazar, L., El Hafidi, M., Enríquez-Arredondo, C., Vázquez-Vázquez, C., González de la Vara, L.E. and Gavilanes-Ruíz, M. (2011) Isolation of detergent-resistant membranes from plant photosynthetic and non-photosynthetic tissues Anal. Biochem., 417, 220–227
Carmona-Salazar, L., El Hafidi, M., Gutierrez-Najera, N., Noyola-Martinez, L., Gonzalez-Solis, A. and Gavilanes-Ruiz, M. (2015) Fatty acid profiles from the plasma membrane and detergent resistant membranes of two plant species Phytochemistry, 109, 25–35
Microsomal/plasma membranes
Belugin, B.V., Zhestkova, I.M., Piotrovskii, M.S., Lapshin, N.K. and Trofimova, M.S. (2017) PIP1 aquaporins, sterols, and osmotic water permeability of plasma membranes from etiolated pea seedlings Biochemistry (Moscow), Suppl. Ser. A: Membrane and Cell Biol., 11, 168–176
Peroxisomes
Arai, Y., Hayashi, M. and Nishimura, M. (2008) Proteomic analysis of highly purified peroxisomes from etiolated Soybean cotyledons Plant Cell Physiol., 49, 526-539
Hossain, Z. and Komatsu, S. (2014) Soybean proteomics In Plant Proteomics: Methods Mol. Biol., 1072 (ed. Jorrin-Novo, J.V. et al), Springer Science+Business Media, LLC, pp 315-331
Komatsu, S. and Ahsan, N. (2009) Soybean proteomics and its application to functional analysis J. Proteom., 72, 325-336
9-7. Nicotiana benthamiana
Endoplasmic reticulum
Joseph, M., Ludevid, D., Torrent, M., Rofidal, V., Tauzin, M., Rossignol, M. and Peltier, J-B. (2012) Proteomic characterisation of endoplasmic reticulum-derived protein bodies in tobacco leaves BMC Plant Biol., 12: 36
9-8. Nicotiana tabacum
ER/Golgi/plasma membrane/tonoplast
Hagiwara, Y., Komoda, K., Yamanaka, T., Tamai, A., Meshi, T., Funada, R., Tsuchiya, T., Naito, S and Ishikawa, M. (2003) Subcellular localization of host and viral proteins associated with tobamovirus RNA replication EMBO J., 22, 344-353
Potocký, M., Pejchar, P., Gutkowska, M., Jiménez-Quesada, M.J., Potocká, A., de Dios Alchéc, J., Kost, B. and Žárský, V. (2012) NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipids and Rac/Rop GTPases J. Plant Physiol., 169, 1654– 1663
Lipid rafts
Carmona-Salazar, L., El Hafidi, M., Enríquez-Arredondo, C., Vázquez-Vázquez, C., González de la Vara, L.E. and Gavilanes-Ruíz, M. (2011) Isolation of detergent-resistant membranes from plant photosynthetic and non-photosynthetic tissues Anal. Biochem., 417, 220–227
Moscatelli, A., Gagliardi, A., Maneta-Peyret, L., Bini, L., Stroppa, N., Onelli, E., Landi, C., Scali, M., Idilli, A.I. and Moreau, P. (2015) Characterisation of detergent-insoluble membranes in pollen tubes of Nicotiana tabacum (L.) Biol. Open 4, 378–399
Membrane/cytosol fractionation
Hagiwara-Komoda, Y., Hirai, K., Mochizuki, A., Nishiguchi, M., Meshi, T. and Ishikawa, M. (2008) Overexpression of a host factor TOM1 inhibits tomato mosaic virus propagation and suppression of RNA silencing Virology, 376, 132-139
Nuclei
Dahan, J., Pichereaux, C., Rossignol, M., Blanc, S., Wendehenne, D., Pugin, A. and Bourque, S. (2009) Activation of a nuclear-localized SIPK in tobacco cells challenged by cryptogein, an elicitor of plant defence reactions Biochem. J., 418, 191–200
Lannoo, N., Peumans, W.J., Van Pamel, E., Alvarez, R., Xiong, T-C., Hause, G., Mazars, C. and Van Damme, E.J.M. (2006) Localization and in vitro binding studies suggest that the cytoplasmic/nuclear tobacco lectin can interact in situ with high-mannose and complex N-glyc FEBS Lett., 580, 6329-6337
Mazars, C., Bourque, S., Mithöfer, A., Pugin, A. and Ranjeva, R. (2009) Calcium homeostasis in plant cell nuclei New Phytologist, 181, 261-274
Schouppe, D., Ghesquière, B., Menschaert, G., De Vos, W.H., Bourque, S., Trooskens, G., Proost, P., Gevaert, K. and Van Damme, E.J.M. (2011) Interaction of the tobacco lectin with histone proteins Plant Physiol., 155, 1091–1102
Testard, A., Da Silva, D., Ormancey, M., Pichereaux, C., Pouzet, C., Jauneau, A., Grat, S., Robe, E., Brière, C., Cotelle, V., Mazars, C. and Thuleau, P. (2016) Calcium- and nitric oxide-dependent nuclear accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase in response to long chain bases in tobacco BY-2 cells Plant Cell. Physiol., 57, 2221–2231
Xiong, T.C., Jauneau, A., Ranjeva, R. and Mazars, C. (2004) Isolated plant nuclei as mechanical and thermal sensors involved in calcium signaling Plant J., 40, 12-21
Protein bodies
Llop-Tous, I., Madurga, S., Giralt, E., Marzabal, P., Torrent, M. and Ludevid, M.D. (2010) Relevant elements of a maize γ-zein domain involved in protein body biogenesis J. Biol. Chem., 285, 35633–35644
9-9. Picea meyeri (pollen tubes)
Lipid rafts
Liu, P., Li, R-L., Zhang, L., Wang, Q-L., Niehaus, K., Baluška, F., Šamaj, J. and Lin, J-X. (2009) Lipid microdomain polarization is required for NADPH oxidase-dependent ROS signaling in Picea meyeri pollen tube tip growth Plant J., 60, 303–313
9-10. Suaeda altissima
Golgi
Shuvalov, A.V., Orlova, J.V., Khalilova, L.A., Myasoedov, N.A., Andreev, I.M., Belyaev, D.V. and Balnokin, Y.V. (2015) Evidence for the functioning of a Cl– /H+
antiporter in the membranes isolated from root cells of the halophyte Suaeda altissima and enriched with Golgi membranes Russ. J. Plant Physiol., 62, 45–56
10. Protozoa
10-1. Apicomplexia protozoa (Eimeria tenella)
Refractile body
De Venevelles, P., Chich, J.F., Faigle, W., Lombard, B., Loew, D., Péry, P. and M. Labbé (2006) Study of proteins associated with the Eimeria tenella refractile body by a proteomic approach Int. J. Parasitol., 36, 1399- 1407
10-2. Dictyostelium
Acidocalcisomes, contractile vacuoles and mitochondria
Marchesini, N., Ruiz, F.A., Vieira, M. and Docampo, R. (2002) Acidocalcisomes are functionally linked to the contractile vacuole of Dictyostelium discoideum J. Biol. Chem., 277, 8146-8153
G-proteins
Alamer, S., Kageyama, Y. and Gundersen, R.E. (2018) Localization of palmitoylated and activated G protein α-subunit in Dictyostelium discoideum J Cell Biochem., 119, 4975–4989
Phagosomes
Shevchuk, O., Batzilla, C., Hägele, S., Kusch, H., Engelmann, S., Hecker, M., Haas, A., Heuner, K., Glöckner, G., Steinert, M. (2009) Proteomic analysis of Legionella-containing phagosomes isolated from Dictyostelium Int. J. Med. Microbiol., 299, 489–508
Secretory vesicles (CoA binding protein)
Cabral, M., Anjard, C., Malhotra, V., Loomis, W.F. and Kuspa, A. (2010) Unconventional secretion of AcbA in Dictyostelium discoideum through a vesicular intermediate Eukaryot. Cell, 9, 1009-1017
Vacuoles (pathogen-containing)
Shevchuk, O. and Steinert, M. (2013) Isolation of pathogen-containing vacuoles In Dictyostelium discoideum Protocols, Methods Mol. Biol., 983, (eds Eichinger, L. and Rivero, F.) Springer Science+Business Media, pp 419-429
10-3. Giardia
Lipid rafts
De Chatterjee, A., Mendez, T.L., Roychowdhury, S. and Dasa, S. (2015) The assembly of GM1 glycolipid- and cholesterol-enriched raft-like membrane microdomains is important for Giardial encystation Infect. Immun. 83, 2030-2042
Mitochondria
Jedelský, P.L., Doležal, P., Rada, P., Pyrih, J., Šmíd, O., Hrdý, I., Šedinová, M., Marcinčiková, M. et al (2011) The minimal proteome in the reduced mitochondrion of the parasitic protist Giardia intestinalis PLoS One, 6: e17285
10-4. Leishmania
Acidocalcisomes
Adhikari, A., Biswas, S., Mukherjee, A., Das, S. and Adak, S. (2019) PAS domain-containing phosphoglycerate kinase deficiency in Leishmania major results in increased autophagosome formation and cell death Biochem. J., 476, 1303–1321
Moreno, B., Urbina, J.A., Oldfield, J.A., Bailey, B.N., Rodrigues, C.O. and Docampo, R. (2000) 31P NMR spectroscopy of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major J. Biol. Chem., 275, 28356- 28362
Lipid rafts
Denny, P.W., Field, M.C. and Smith, D.F. (2001) GPI-anchored proteins and glycoconjugates segregate into lipid rafts in Kinetoplastida FEBS Lett., 491, 148-153
Fridberg, A., Buchanan, K.T. and Engman, D.M. (2007) Flagellar membrane trafficking in kinetoplastids Parasitol. Res., 100, 205-212
Sen, S., Roy, K., Mukherjee, S., Mukhopadhyay, R. and Roy, S. (2011) Restoration of IFNγR subunit assembly, IFNγ signaling and parasite clearance in Leishmania donovani infected macrophages: role of membrane cholesterol PloS Pathog., 7: e1002229
Yao, C., Donelson, J.E. and Wilson, M.E. (2003) The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression and function Mol. Biol. Parasitol., 132, 1-16
Micro-RNAs
Bose, M., Barman, B., Goswami, A., Bhattacharyya, S.N., (2017) Spatiotemporal uncoupling of microRNAmediated translational repression and target RNA degradation controls microRNP recycling in mammalian cells Mol. Cell. Biol., 37: e00464-16
Chakrabarty, T. and Bhattacharyya, S.N. (2017) Leishmania donovani restricts mitochondrial dynamics to enhance miRNP stability and target RNA repression in host macrophages Mol. Biol. Cell, 28, 2091-2105
Nuclei
Jardim, A., Hardie, D.B., Boitz, J. and Borchers, C.H. (2018) Proteomic profiling of Leishmania donovani promastigote subcellular organelles J. Proteome Res., 17, 1194−1215
Transport vesicles
Legare, D., Richard, D., Mukhopadyay, R., Stierhof, Y-D., Rosen, B.P., Haimeur, A., Papadopoulou, B. and Ouellette, M. (2001) The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase J. Biol. Chem., 276, 26301-26307
10-5. Mastigamoeba balamuthi
Hydrogenosomes
Nývltová, E., Stairs, C.W., Hrdý, I., Rídl, J., Mach, J., Pačes, J., Roger, A.J. and Tachezy, J. (2015) Lateral Gene transfer and gene duplication played a key role in the evolution of Mastigamoeba balamuthi hydrogenosomes Mol. Biol. Evol., 32, 1039–1055
10-6. Paramecium organelles
Schilde, C., Lutter, K., Kissmehl, R. and Plattner, H. (2008) Molecular identification of a SNAP-25-like SNARE protein in Paramecium Eukaryot. Cell, 7, 1387-1402
10-7. Phytomonas françai
Acidocalcisomes
Miranda, K., Rodrigues, C.O., Hentchel, J., Vercesi, A., Plattner, H., de Souza, W. and Docampo, R. (2004) Acidocalsisomes of Phytomonas francai possess distinct morphological characteristics and contain iron Microsc. Microanal., 10, 647-655
10-8. Toxoplasma
Acidocalcisomes
Ferreira, D. da S., Menezes Resende, I.T. and Lopez, J.A. (2014) Proteome investigation of an organellar fraction of Toxoplasma gondii: a preliminary study BMC Proc., 8 (Suppl 4): P74
Rodrigues, C.O., Ruiz, F.A., Rohloff, P., Dcott, D.A. and Moreno, S.N.J. (2002) Characterization of isolated acidocalcisomes from Toxoplasma gondii Tachyzoites reveals a novel pool of hydrolysable polyphosphate J. Biol. Chem., 277, 48650-48656
Rohloff, P., Miranda, K., Rodrigues, J.C.F., Fang, J., Galizzi, M., Plattner, H., Hentschel, J. and Moreno, S.N.J. (2011) Calcium uptake and proton transport by acidocalcisomes of Toxoplasma gondii PloS One 6: e18390
Mitochondria
Hakansson, S., Charron, A.J. and Sibley, L.D. (2001) Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole EMBO J., 20, 3132-3144
Vacuole-like organelle
Miranda, K., Pace, D.A., Cintron, R., Rodrigues, J.C.F., Fang, J., Smith, A., Rohloff, P., Coelho, E., de Haas, F. et al (2010) Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole Mol. Microbiol., 76, 1358–1375
10-9. Trichomonas vaginalis
Endomembrane trafficking
Hsu, H-M., Huang, Y-H., Aryal, S., Liu, H-W., Chen, C., Chen, S-H., Chu, C-H. and Tai, J-H. (2020) Endomembrane protein trafficking regulated by a TvCyP2 cyclophilin in the protozoan parasite, Trichomonas vaginalis Sci. Rep., 10: 1275
Hydrogenosomes
Beltrán, N.C., Horváthová, L., Jedelský, P.L., Šedinová, M., Rada, P., Marcinčiková, M., Hrdý, I. and Tachezy, J. (2013) Iron-induced changes in the proteome of Trichomonas vaginalis hydrogenosomes PLoS One, 5: e65148
Hsu, H-M., Chu, C-H., Wang, Y-T., Lee, K., Wei, S-Y., Liu, H-W., Ong, S-J., Chen, C. and Tai, J-H. (2014) Regulation of nuclear translocation of the Myb1 transcription factor by TvCyclophilin 1 in the protozoan parasite Trichomonas vaginalis 289, 19120–19136
Hsu, H-M., Huang, Y-H., Aryal, S., Liu, H-W., Chen, C., Chen, S-H., Chu, C-H. and Tai, J-H. (2020) Endomembrane protein trafficking regulated by a TvCyP2 cyclophilin in the protozoan parasite, Trichomonas vaginalis Sci. Rep., 10: 1275
Kay, C., Lawler, K., Self, T.J., Dyall, S.D. and Kerr, I.D. (2012) Localisation of a family of complex-forming - barrels in the T. vaginalis hydrogenosomal membrane FEBS Lett., 586, 4038–4045
Low and high density membranes
Nievas, Y.R., Vashisht, A.., Corvi, M.M., Metz, S., Johnson, P.J., Wohlschlegel, J.A. and de Miguel, N. (2018) Protein palmitoylation plays an important role in Trichomonas vaginalis adherence Mol. Cell. Proteomics 17, 2229–2241
10-10. Trypanosomes
Acidocalcisomes
Docampo, R. (2000) New and re-emerging diseases: A dedication to Norman D. Levine Parasitology Today, 16, 316
Fang, J., Rohloff, P., Miranda, K. and Docampo, R. (2007) Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect Biochem. J., 407, 161-170
Ferella, M., Nilsson, D., darban, H., Rodrigues, C., Bontempi, E.J., Docampo, R. and Andersson, B. (2008) Proteomics in Trypanosoma cruzi – localization of novel proteins to various organelles Proteomics, 8, 2735- 2749
Huang, G., Bartlett, P.J., Thomas, A.P., Moreno, S.N.J. and Docampo, R. (2013) Acidocalcisomes of Trypanosoma brucei have an inositol 1,4,5-trisphosphate receptor that is required for growth and infectivity Proc. Natl. Acad. Sci. USA, 110, 1887–1892
Huang, G., Ulrich, P.N., Storey, M., Johnson, D., Tischer, J.. Tovar, J.A., Moreno, S.N.J., Orlando, R. and Docampo, R. (2014) Proteomic analysis of the acidocalcisome, an organelle conserved from bacteria to human cells PLoS Pathog., 10: e1004555
Martinez, R., Wang, Y., Benaim, G., Benchimol, M., de Souza, W., Scott, D.A. and Docampo, R.A (2002) Proton pumping pyrophosphatase in the Golgi apparatus and plasma membrane vesicles of Trypanosoma cruzi Mol. Biochem. Parasitol., 120, 205-213
Moreno, B., Urbina, J.A., Oldfield, J.A., Bailey, B.N., Rodrigues, C.O. and Docampo, R. (2000) 31P NMR spectroscopy of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major J. Biol. Chem., 275, 28356- 28362
Negreiros, R.S., Lander, N., Huang, G., Cordeiro, C.D., Smith, S.A., Morrissey, J.H. and Docampo, R. (2018) Inorganic polyphosphate interacts with nucleolar and glycosomal proteins in trypanosomatids Mol. Microbiol., 110, 973–994
Rohloff, P., Rodrigues, C.O. and Docampo, R. (2003) Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium Mol. Biochem. Parasitol., 126, 219-230
Rohloff, P., Montalvetti, A. and Docompo, R. (2004) Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi J. Biol. Chem., 279, 52270-52281
Ruiz, F.A., Rodrigues, C.O. and Docampo, R. (2001) Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation and environmental stress in Trypanosoma cruzi J. Biol. Chem., 276, 26114-26121
Salto, M.L., Kuhlenschmidt, T., Kuhlenschmidt, M., de Lederkremer, R.M. and Docampo, R. (2008) Phospholipid and glycolipid composition of acidocalcisomes of Trypanosoma cruzi Mol. Biochem. Parasitol., 158, 120-130
Scott, D.A. and Docampo, R. (2000) Characterization of isolated acidocalcisomes of Trypanosoma cruzi J. Biol. Chem., 275, 24215-24221
Contractile vacuoles
Rohloff, P., Montalvetti, A. and Docompo, R. (2004) Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi J. Biol. Chem., 279, 52270-52281
Ulrich, P.N., Jimenez, V., Park, M., Martins, V.P., Atwood III, J., Moles, K., Collins, D., Rohloff, P., Tarleton, R., Moreno, S.N.J., Orlando, R. and Docampo, R. (2011) Identification of contractile vacuole proteins in Trypanosoma cruzi PLoS One 6: e18013
Exosomes
Caeiro, L.D., Alba-Soto, C.D., Rizzi, M., Solana, M.E., Rodriguez, G., Chidichimo, A.M., Rodriguez, M.E. et al (2018) The protein family TcTASV-C is a novel Trypanosoma cruzi virulence factor secreted in extracellular vesicles by trypomastigotes and highly expressed in bloodstream forms PLoS Negl. Trop. Dis., 12: e0006475
Glycosomes
Colasante, C., Ellis, M., Ruppert, T. and Voncken, F. (2006) Comparative proteomics from bloodstream form and procyclic form Trypanosoma brucei brucei Proteomics, 6, 3275-3293
Gualdron-López, M., Vapola, M.H., Miinalainen, I.J., Hiltunen, J.K., Michels, P.A.M. and Antonenkov, V.D. (2012) Channel-forming activities in the glycosomal fraction from the bloodstream form of Trypanosoma brucei PLoS One, 7: e34530
Gualdrón-López, M., Chevalier, N., Van Der Smissen, P., Courtoy, P.J., Rigden, D.J. and Michels, P.A.M. (2013) Ubiquitination of the glycosomal matrix protein receptor PEX5 in Trypanosoma brucei by PEX4 displays novel features Biochim. Biophys. Acta, 1833, 3076–3092
Negreiros, R.S., Lander, N., Huang, G., Cordeiro, C.D., Smith, S.A., Morrissey, J.H. and Docampo, R. (2018) Inorganic polyphosphate interacts with nucleolar and glycosomal proteins in trypanosomatids Mol. Microbiol., 110, 973–994
Lipid rafts and other lipid domains
De Paulo Martins, V., Okura, M., Maric, D., Engman, D.M., Vieira, M., Docampo, R. and Moreno, S.N.J. (2010) Acylation-dependent export of Trypanosoma cruzi phosphoinositide-specific phospholipase C to the outer surface of amastigotes J. Biol. Chem., 285, 30906-30917
Emmer, B.T., Souther, C., Toriello, K.M., Olson, C.L., Epting, C.L. and Engman, D.M. (2009) Identification of
a palmitoyl acyltransferase required for protein sorting to the flagellar membrane J. Cell Sci., 122, 867-874 Fridberg, A., Buchanan, K.T. and Engman, D.M. (2007) Flagellar membrane trafficking in kinetoplastids Parasitol. Res., 100, 205-212
Fridberg, A., Olson, C.L., Nakayasu, E.S., Tyler, K.M., Almeida, I.C. and Engman, D.M. (2008) Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei J. Cell Sci., 121, 522- 535
Lantos, A.B., Carlevaro, G., Araoz, B., Diaz, P.R., de los Milagros Camara, M., Buscaglia, C.A., Bossi, M., Yu, H., Chen, X. et al (2016) Sialic acid glycobiology unveils Trypanosoma cruzi trypomastigote membrane physiology PloS Pathog., 12: e1005559
Maric, D., McGwire, B.S., Buchanan, K.T., Olson, C.L., Emmer, B.T., Epting, C.L. and Engman, D.M. (2011) Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein J. Biol. Chem., 286, 33109–33117
Maric, D., Olson, C.L., Xu, X., Ames, J.B. and Engman, D.M. (2015) Calcium-dependent membrane association of a flagellar calcium sensor does not require calcium binding Mol. Biochem. Parasitol., 201, 72–75
Mucci, J., Lantos, A.B., Buscaglia, C.A.M., Leguizamón, M.S. and Campetella, O. (2017) The Trypanosoma cruzi surface, a nanoscale patchwork quilt Trends Parasitol., 33, 102-112
Niyogi, S., Mucci, J., Campetella, O. and Docampo, R. (2014) Rab11 regulates trafficking of trans-sialidase to the plasma membrane through the contractile vacuole complex of Trypanosoma cruzi PLOS Pathog., 10: e1004224
Tyler, K.M., Fridberg, A., Toriello, K.M., Olson, C.L., Cieslak, J.A., Hazlett, T.L. and Engman, D.M. (2009) Flagellar membrane localization via association with lipid rafts J. Cell Sci., 122, 859-866
Mitochondria
Colasante, C., Zheng, F., Kemp, C. and Voncken, F. (2018) A plant-like mitochondrial carrier family protein facilitates mitochondrial transport of di- and tricarboxylates in Trypanosoma brucei Mol. Biochem. Parasitol., 221, 36–51
11. Gram-negative bacteria
Linton, K.M., Tapping, C.R., Adams, D.G., Carter, D.H., Shore, R.C. and Aaron, J.E. (2013) A silicon cell cycle in a bacterial model of calcium phosphate mineralogenesis Micron, 44, 419–432
Seufferheld, M., Vieira, M. C. F., Ruiz, F.A., Rodrigues, C.O., Moreno, S.N.J. and Docampo, R. (2003) Identification of organelles in bacteria similar to acidocalcisomes of unicellular eukaryotes J. Biol. Chem., 278, 29971-29978
Seufferheld, M., Lea, C.R., Vieira, M., Oldfield, E. and Docampo, R. (2004) The H + -pyrophosphatase of Rhodospirillum rubrum is predominantly located in polyphosphate-rich acidocalcisomes J. Biol. Chem., 279, 51193-51202
12. Review articles
12-1. Methodology
Ohta, D. and Mizutani, M. (2012) Sterol C22-desaturase and its biological roles In Isoprenoid Synthesis in Plants and Microorganisms: New Concepts and Experimental Approaches (eds. Bach, T.J. and Rohmer, M.) Springer Science+Business Media New York, pp 381-391
Šamajová, O., Takác, T., von Wangenheim, D., Stelzer, E. and Šamaj, J. (2012) Update on methods and techniques to study endocytosis in plants In Endocytosis in Plants (ed. Šamaj, J.) Springer-Verlag Berlin Heidelberg, pp 1-36
12-2. Proteomic review articles
Agrawal, G.K., Bourguignon, J., Rolland, N., Ephritikhine, G., Ferro, M., Jaquinod, M., Alexiou, K.G., Chardot, T., Chakraborty, N., Jolivet, P., Doonan, J.H. and Rakwal1, R. (2011) Plant organelle proteomics: collaborating for optimal cell function Mass Spectrom. Rev., 30, 772– 853
Au, C.E., Bell, A.W., Gilchrist, A., Hiding, J., Nilsson, T. and Bergeron, J.J.M. (2007) Organellar proteomics to create the cell map Curr. Opin. Cell Biol., 19, 376-385
Chen, X., Karnovsky, A., Dolors Sans, M., Andrews, P.C. and Williams, J.A., (2010) Molecular characterization of the endoplasmic reticulum: Insights from proteomic studies Proteomics, 10, 4040–4052
Dengjel, J., Jakobsen, L. and Andersen, J.S. (2010) Organelle proteomics by label-free and SILAC-based protein correlation profiling In LC-MS/MS in Proteomics, Methods Mol. Biol., 658, (ed. Cutillas, P.R. and Timms, J.F.) Springer Science+Business Media, pp 255-265
Kota, U. and Goshe, M.B. (2011) Advances in qualitative and quantitative plant membrane proteomics Phytochemistry, 72, 1040–1060
Lee, Y.H., Tan, H.T. and Chung, M.C.M. (2010) Subcellular fractionation methods and strategies for proteomics Proteomics 10, 3935–3956
Lilley, K.S. and Dupree, P. (2007) Plant organelle proteomics Curr. Opin. Plant Biol., 10, 594-599
Oeljeklaus, S., Meyer, H.E. and Warscheid, B. (2009) Advancements in plant proteomics using quantitative mass spectrometry J. Proteom., 72, 545-554
Sadowski, P.G., Groen, A.J., Dupree, P. and Lilley, K.S. (2008) Sub-cellular localization of membrane proteins Proteomics, 8, 3991-4011
Schröder, B.A., Wrocklage, C., Hasilik, A. and Saftig, P. (2010) The proteome of lysosomes Proteomics, 10, 4053–4076
Trotter, M.W.B., Sadowski, P.G., Dunkley, T.P.J., Groen, A.J. and Lilley, K.S. (2010) Improved sub-cellular resolution via simultaneous analysis of organelle proteomics data across varied experimental conditions Proteomics, 10, 4213–4219
Vertommena, A., Panisa, B., Swennena, R. and Carpentiera, S.C. (2011) Challenges and solutions for the identification of membrane proteins in non-model plants J. Proteom., 74, 1165-1181
OptiPrep™ Reference List RS14 6th edition, January 2020
OptiPrep™ Reference List RS15
Purification of subcelluar organelles and membrane compartments from Saccharomyces cerevisiae – a bibliography
This Reference List covers all published papers that have reported the use of iodixanol gradients for the purification of organelles and membrane compartments from yeast (Saccharomyces cerevisiae) spheroplasts. For detailed protocols please refer to the following OptiPrepTM Application Sheets, which can be accessed from the following section: “Subcellular Membranes (Non-mammalian)”.
Endosomes, endoplasmic reticulum, Golgi, TGN and vacuoles see Application Sheet S53
Membrane trafficking (vacuole, Cvt vesicles etc) see Application Sheet S52
Mitochondria see Application Sheet S17
Peroxisomes see Application Sheet S57
See also Reference List RS09 “Lipid rich detergent-resistant membranes from non-mammalian sources – a bibliography”, this contains a section on yeast analysis.
See also Reference List RS13 “Resolution of soluble cytosolic proteins from membrane vesicles and organelles – a bibliography”; this contains a section on yeast analysis.
Both RS09 and RS13 have sections on yeast.
In the following bibliography the published papers have been sorted according to the principal membrane compartments under study or research topic and listed alphabetically according to first author. Key words are highlighted in blue.
1. Autophagosomes/autophagic vacuoles
Cohen-Kaplan, V., Livneh, I., Kwon, Y.T. and Ciechanover, A. (2019) Monitoring stress-induced autophagic engulfment and degradation of the 26S proteasome in mammalian cells Meth. Enzymol., 619, 337-366
Gao, J., Reggiori, F. and Ungermann, C. (2018) A novel in vitro assay reveals SNARE topology and the role of Ykt6 in autophagosome fusion with vacuoles J. Cell Biol., 217, 3670–3682
Ishihara, N., Hamasaki, M., Yokota, S., Suzuki, K., Kamada, Y., Kihara, A., Yoshimori, T., Noda, T. and Ohsumi, Y. (2001) Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion Mol. Biol. Cell, 12, 3690-3702
Kametaka, S., Okano, T., Ohsumi, M. and Ohsumi, Y. (1998) Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast Saccharomyces cerevisiae J. Biol. Chem., 273, 22284-22291
Kim, J., Huang, W-P., Stromhaug, P.E. and Klionsky, D.J. (2002) Convergence of multiple autophagy and cytoplasm to vacuole components to a perivacuolar membrane compartment prior to de novo vesicle formation J. Biol. Chem., 277, 763-773
Meiling-Wesse, K., Barth, H., Voss, C., Eskelinen, E-L., Epple, U.D. and Thumm, M. (2004) Atg21 is required for effective recruitment of Atg8 to the preautophagosomal structure during the Cvt pathway J. Biol. Chem., 279, 37741-37759
Shintani, T., Suzuki, K., Kamada, Y., Noda, T. and Ohsumi, Y. (2001) Apg2p functions in autophagosome formation on the perivacuolar structure J. Biol. Chem., 276, 30452-30460
Suzuki, K., Nakamura, S., Morimoto, M., Fujii, K., Noda, N.N., Inagaki, F. and Ohsumi, Y. (2014) Proteomic profiling of autophagosome cargo in Saccharomyces cerevisiae PloS One, 9: e91651
Wang, C-W., Kim, Huang, W-P., Abeliovich, H., Stromhaug, P.E., Dunn, W.A. and Klionsky, D.J. (2001) Apg2 is a novel protein required for the cytoplasm to vacuole targeting, autophagy, and pexophagy pathways J. Biol. Chem, 276, 30442-30451
Yamamoto, H., Kakuta, S., Watanabe, T.M., Kitamura, A., Sekito, T., Kondo-Kakuta, C., Ichikawa, R., Kinjo, M. and Ohsumi, Y. (2012) Atg9 vesicles are an important membrane source during early steps of autophagosome formation J. Cell Biol., 198, 219–233
2. Cvt vesicles plus endosomes/Golgi/ER/TGN/vacuole (see also Section 12)
Chantalat, S., Park, S-K., Hua, Z., Liu, K., Gobin, R., Peyroche, A., Rambourg, A., Graham, T. and Jackson, C.L. (2004) The Arf activator Gea2p and P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae J. Cell Sci., 117, 711-722
Dove, S.K., Piper, R.C., McEwen, R.K., Yu, J.W., King, M.C., Hughes, D.C., Thuring, J., Holmes, A.B., Cooke, F.T., Michell, R.H., Parker, P.J. and Lemmon, M.A. (2004) Svp1p defines a family of phosphatidylinositol 3,5- bisphosphate effectors EMBO J., 23, 1922-1933
Guan, J., Stromhaug, P.E., George, M.D., Habibzadegh-Tari, P., Bevan, A., Dunn, W.A. and Klionsky, D.J. (2001) Cvt18/Gsa12 is required for cytoplasm-to-vacuole transport, pexophagy, and autophagy in Saccharomyces cerevisiae and Pichia pastoris Mol. Biol. Cell, 12, 3821-3838
Kim, J., Kamada, Y., Stromhaug, P.E., Guan J., Hefner-Gravink, A., Baba, M., Scott, S.V., Ohsumi, Y., Dunn, W.A. and Klionsky, D.J. (2001) Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole J. Cell Biol., 153, 381-396
Mitsui, K., Koshimura, Y., Yoshikawa, Y., Matsushita, M. and Kanazawa, H. (2011) The endosomal Na+/H+ exchanger contributes to multivesicular body formation by regulating the recruitment of ESCRT-0 Vps27p to the endosomal membrane J. Biol. Chem., 286, 37625–37638
Sakakibara, K., Eiyama, A., Suzuki, S.W., Sakoh-Nakatogawa, M., Okumura, N., Tani, M., Hashimoto, A., Nagumo, S., Kondo-Okamoto, N. et al (2015) Phospholipid methylation controls Atg32-mediated mitophagy and Atg8 recycling EMBO J., 134, 2703-2719
Shiffert, S.L., Vaughn, M.B., Huynh, D., Kaplan, J. and McVey Ward, D. (2004) Bph1p, the Saccharomyces cerevisiae homologue of CHS1/beige, functions in cell wall formation and protein sorting Traffic, 5, 700-710
Teter, S.A., Eggerton, K.P., Scott, S.V., Kim, J., Fischer, A.M. and Klionsky, D.J. (2001) Degradation of lipid vesicles in the yeast vacuole requires function of Cvt17, a putative lipase J. Biol. Chem., 276, 2083-2087
Urbanowski, J.L. and Piper, R.C. (2001) Ubiquitin sorts proteins into the intralumenal degradative compartment of the late-endosome/vacuole Traffic, 2, 622-630
Wang, C-W., Stromhaug, P.E., Shima, J. and Klionsky, J. (2002) The Ccz1-Mon1 protein complex is required for the late step of multiple vacuole delivery pathways J. Biol. Chem., 277, 47917-47927
Wang, C-W., Stromhaug, P.E., Kauffman, E.J., Weisman, L.S. and Klionsky, D.J.(2003) Yeast homotypic vacuole fusion requires the Ccz1-Mon1 complex during the tethering/docking stage J. Cell Biol., 163, 973-985
3. Cvt vesicles plus vacuole/vacuolar vesicles
Satyanarayana, C., Schroder-Kohne, S., Craig, E.A., Schu, P.V. and Horst, M. (2000) Cytosolic Hsp70s are involved in the transport of aminopeptidase 1 from the cytoplasm into the vacuole FEBS Lett., 470, 232-238
Scott, S.V., Baba, M., Ohsumi, Y. and Klionsky, D.J. (1997) Aminopeptidase I is targeted to the vacuole by a nonclassical vesicular mechanism J. Cell Biol., 138, 37-44
4. Cytosolic proteins: resolution from membrane vesicles and organelles: see MS-17
5. Endoplasmic reticulum
Diaz, A., Gallei, A. and Ahlquist, P. (2012) Bromovirus RNA replication compartment formation requires concerted action of 1a’s self-interacting RNA capping and helicase domains J. Virol., 86, 821–834
Welker, S., Rudolph, B., Frenzel, E., Hagn, F., Liebisch, G., Schmitz, G., Scheuring, J., Kerth, A., Blume, A., Weinkauf, S., Haslbeck, M., Kessler, H. and Buchner, J. (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function Mol. Cell, 39, 507–520
6. Endoplasmic reticulum/Golgi
Chen, J., Korostyshevsky, D., Lee, S. and Perlstein, E.O. (2012) Accumulation of an antidepressant in vesiculogenic membranes of yeast cells triggers autophagy PLoS One, 7: e34024
Kumanovics, A., Poruk, K.E., Osborn, K.A., Ward, D.M. and Kaplan, J. (2006) YKE4 (YIL023C) encodes a bidirectional zinc transporter in the endoplasmic reticulum of Saccharomyces cerevisiae J. Biol. Chem., 281, 22566-22574
Sakakibara, K., Eiyama, A., Suzuki, S.W., Sakoh-Nakatogawa, M., Okumura, N., Tani, M., Hashimoto, A., Nagumo, S., Kondo-Okamoto, N. et al (2015) Phospholipid methylation controls Atg32-mediated mitophagy and Atg8 recycling EMBO J., 134, 2703-2719
Suzuki, K., Nakamura, S., Morimoto, M., Fujii, K., Noda, N.N., Inagaki, F. and Ohsumi, Y. (2014) Proteomic profiling of autophagosome cargo in Saccharomyces cerevisiae PloS One, 9: e91651
Wang, Y., Lilley, K.S. and Oliver, S.G. (2014) A protocol for the subcellular fractionation of Saccharomyces cerevisiae using nitrogen cavitation and density gradient centrifugation Yeast, 31, 127–135
7. Endosomes
Chen, J., Korostyshevsky, D., Lee, S. and Perlstein, E.O. (2012) Accumulation of an antidepressant in vesiculogenic membranes of yeast cells triggers autophagy PLoS One, 7: e34024
Suzuki, K., Nakamura, S., Morimoto, M., Fujii, K., Noda, N.N., Inagaki, F. and Ohsumi, Y. (2014) Proteomic profiling of autophagosome cargo in Saccharomyces cerevisiae PloS One, 9: e91651
Welker, S., Rudolph, B., Frenzel, E., Hagn, F., Liebisch, G., Schmitz, G., Scheuring, J., Kerth, A., Blume, A., Weinkauf, S., Haslbeck, M., Kessler, H. and Buchner, J. (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function Mol. Cell, 39, 507–520
8. Exosomes
Rodrigues, M.L., Oliveira, D.L., Vargas, G., Girard-Dias, W., Franzen, A.J., Frasés, S., Miranda, K. and Nimrichter, L. (2016) Analysis of yeast extracellular vesicles In Unconventional Protein Secretion: Methods and Protocols, Methods Mol. Biol., 1459 (ed. Pompa, A. and De Marchis, F.), Springer Science+Business Media New York, pp 175-190
9. Fractionation technology
Wang, Y., Lilley, K.S. and Oliver, S.G. (2014) A protocol for the subcellular fractionation of Saccharomyces cerevisiae using nitrogen cavitation and density gradient centrifugation Yeast, 31, 127–135
10. Mitochondria (see also 11. Nucleic acids and ribosomes)
10-1 Co-enzyme Q
He, C.H., Xie, L.X., Allan, C.M., Tran, UP.C. and Clarke, C.F. (2014) Coenzyme Q supplementation or overexpression of the yeast Coq8 putative kinase stabilizes multi subunit Coq polypeptide complexes in yeast coq null mutants Biochim. Biophys. Acta, 1841, 630–644
He, C.H., Black, D.S., Nguyen, T.P.T., Wang, C., Srinivasan, C. and Clarke, C.F. (2015) Yeast Coq9 controls deamination of coenzyme Q intermediates that derive from para-aminobenzoic acid Biochim. Biophys. Acta, 1851, 1227–1239
Xie, L.X., Ozeir, M., Tang, J.Y., Chen, J.Y., Jaquinod, S-K., Fontecave, M., Clarke, C.F. Pierrel, F. (2012) Overexpression of the Coq8 kinase in Saccharomyces cerevisiae coq null mutants allows for accumulation of diagnostic intermediates of the coenzyme Q6 biosynthetic pathway J. Biol. Chem., 287, 23571–23581
10-2 Energy metabolism
Nishimura, A., Nasuno, R., Yoshikawa, Y., Jung, M., Ida, T., Matsunaga, T., Morita, M., Takagi, H., Motohashi, H. and Akaike, T. (2019) Mitochondrial cysteinyl-tRNA synthetase is expressed via alternative transcriptional initiation regulated by energy metabolism in yeast cells J. Biol. Chem., 294, 13781–13788
10-3 Iron analysis/transport
Chen, O.S. and Kaplan J. (2000) CCC1 suppresses mitochondrial damage in the yeast model of Friedreich’s ataxia by limiting mitochondrial iron accumulation J. Biol. Chem., 275, 7626-7632
Chen, O.S. and Kaplan, J. (2001) YFH1-mediated iron homeostasis is independent of mitochondrial respiration FEBS Lett., 509, 131-134
Chen, O.S., Hemenway, S. and Kaplan, J. (2002) Genetic analysis of iron citrate toxicity in yeast: implications for mammalian iron homeostasis Proc. Natl. Acad. Sci., USA, 99, 16922-16927
Crisp, R.J., Pollington, A., Galea, C., Jaron, S., Yamaguchi-Iwai, Y. and Kaplan, J. (2003) Inhibition of heme biosynthesis prevents transcription of iron uptake genes in yeast J. Biol. Chem., 278, 45499-45506
Lindahl, P.A. Garber Morales, J., Miao, R. and Holmes-Hampton, G. (2009) Isolation of Saccharomyces cerevisiae mitochondria for Mössbauer, EPR, and electronic absorption spectroscopic analyses Methods Enzymol., 456, 267-285
Radisky, D.C., Babcock, M.C. and Kaplan, J. (1999) The yeast frataxin homologue mediates mitochondrial iron efflux J. Biol. Chem., 274, 4497-4499
Yun, C-W., Ferea, T., Rashford, J., Ardon, O., Brown, P.O., Botstein, D., Kaplan, J. and Philpott, C.C. (2000) Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake J. Biol. Chem., 275, 10709-10715
10-4 Lipid metabolism/transport/
Kannan, M., Lahiri, S., Liu, L-K., Choudhary, V. and Prinz, W.A. (2017) Phosphatidylserine synthesis at membrane contact sites promotes its transport out of the ER J. Lipid Res., 58, 553–562
Lahiri, S., Chao, J.T., Tavassoli, S., Wong, A.K.O., Choudhary, V. et al (2014) A conserved endoplasmic reticulum membrane protein complex (EMC) facilitates phospholipid transfer from the ER to mitochondria PLoS Biol., 12: e1001969
Sakakibara, K., Eiyama, A., Suzuki, S.W., Sakoh-Nakatogawa, M., Okumura, N., Tani, M., Hashimoto, A., Nagumo, S., Kondo-Okamoto, N. et al (2015) Phospholipid methylation controls Atg32-mediated mitophagy and Atg8 recycling EMBO J., 134, 2703-2719
Tamura, Y., Harada, Y., Nishikawa, S-I, Yamano, K., Kamiya, M., Shiota, T., Kuroda, T., Kuge, O., Sesaki, H., Imai, K., Tomii, K. and Endo, T. (2013) Tam41 is a CDP-diacylglycerol synthase required for cardiolipin biosynthesis in mitochondria Cell Metab., 17, 709–718
10-5 Mitophagy
Sakakibara, K., Eiyama, A., Suzuki, S.W., Sakoh-Nakatogawa, M., Okumura, N., Tani, M., Hashimoto, A., Nagumo, S., Kondo-Okamoto, N. et al (2015) Phospholipid methylation controls Atg32-mediated mitophagy and Atg8 recycling EMBO J., 134, 2703-2719
Vigié, P., Cougouilles, E., Bhatia-Kissová, I., Salin, B., Blancard, C. and Camougrand, N. (2019) The mitochondrial phosphatidylserine decarboxylase Psd1 is involved in nitrogen starvation-induced mitophagy in yeast J, Cell Sci., 132: jcs221655
10-6 Protein complexes
Chatterjee, N., Pabla, R. and Siede, W. (2013) Role of polymerase η in mitochondrial mutagenesis of Saccharomyces cerevisiae Biochem. Biophys. Res. Comm., 431, 270–273
Gold, V.A.M., Brandt, T., Cavellini, L., Cohen, M.M., Ieva, R. and van der Laan, M. (2017) Analysis of mitochondrial membrane protein complexes by electron cryo-tomography In Mitochondria: Practical Protocols, Methods in Mol. Biol., 1567, (ed. Mokranjac, D. and Perocchi, F.) Springer Science+Business Media, New York, pp 315-336
Gold, V.A.M., Ieva, R., Walter, A., Pfanner, N., van der Laan, M. and Kühlbrandt, W. (2014) Visualizing active membrane protein complexes by electron cryotomography Nat. Commun., 5: 4129
10-7 Proteome
Nightingale, D.J.H., Oliver, S.G. and Lilley, K.S. (2019) Mapping the Saccharomyces cerevisiae spatial proteome with high resolution using hyperLOPIT In Yeast Systems Biology: Methods and Protocols, Methods in Molecular Biology, vol. 2049 (ed. Oliver, S.G. and Castrillo, J.I.), Springer Science+Business Media LLC New York, pp 165-190
10-8 Stress proteins
Welker, S., Rudolph, B., Frenzel, E., Hagn, F., Liebisch, G., Schmitz, G., Scheuring, J., Kerth, A., Blume, A., Weinkauf, S., Haslbeck, M., Kessler, H. and Buchner, J. (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function Mol. Cell, 39, 507–520
11. Nucleic acids and ribosomes
Chang, W., Zaarour, R.F., Reck-Peterson, S., Rinn, J., Singer, R.H., Snyder, M., Novick, P. and Mooseker, M.S. (2008) Myo2p, a class V myosin in budding yeast, associates with a large ribonucleic acid–protein complex that contains mRNAs and subunits of the RNA-processing body RNA, 14, 491-502
Gold, V.A.M., Chroscicki, P., Bragoszewski, P. and Chacinska, A. (2017) Visualization of cytosolic ribosomes on the surface of mitochondria by electron cryo-tomography EMBO Rep., 18, 1786-1800
Meeusen, S., Tieu, Q., Wong, E., Weiss, E., Schieltz, D., Yates, J.R. and Nunnari, J. (1999) Mgm101p is a novel component of the mitochondrial nucleoid that binds DNA and is required for the repair of oxidatively damaged mitochondrial DNA J. Cell Biol., 145, 291-304
12. Organelle contact sites
Toulmay, A. and Prinz, W.A. (2012) A conserved membrane-binding domain targets proteins to organelle contact sites J. Cell Sci., 125, 49–58
13. Peroxisomes
Antonenkov, V.D., Mindthoff, S., Grunau, S., Erdmann, R. and Hiltunen, J.K. (2009) An involvement of yeast peroxisomal channels in transmembrane transfer of glyoxylate cycle intermediates Int., J. Biochem. Cell Biol., 41, 2546–2554
Cramer, J., Effelsberg, D., Girzalsky, W. and Erdmann, R. (2015) Isolation of peroxisomes from yeast Cold Spring Harb. Protoc; doi:10.1101/pdb.top074500
Cramer, J., Effelsberg, D., Girzalsky, W. and Erdmann, R. (2015) Small-scale purification of peroxisomes for analytical applications Cold Spring Harb. Protoc; doi:10.1101/pdb.prot083717
Debelyy, M.O., Platta, H.W., Saffian, D., Hensel, A., Thoms, S., Meyer, H.E., Warscheid, B., Girzalsky, W. and Erdmann, R. (2011) Ubp15p, a ubiquitin hydrolase associated with the peroxisomal export machinery J. Biol. Chem., 286, 28223–28234
Effelsberg, D., Cruz-Zaragoza, L.D., Schliebs, W. and Erdmann, R. (2016) Pex9p is a new yeast peroxisomal import receptor for PTS1-containing proteins J. Cell Sci., 129, 4057-4066
Effelsberg, D., Cruz-Zaragoza, L.D, Tonillo, J., Schliebs, W. and Erdmann, R. (2015) Role of Pex21p for piggyback import of Gpd1p and Pnc1p into peroxisomes of Saccharomyces cerevisiae J. Biol. Chem., 290, 25333–25342
Effelsberg, D., Cruz-Zaragoza, L.D., Schliebs, W. and Erdmann, R. (2016) Pex9p is a new yeast peroxisomal import receptor for PTS1-containing proteins J. Cell Sci., 129, 4057-4066
Einwachter, H., Sowinski, S., Kunau, W-H. and Schliebs, W. (2001) Yarrowia lipolytica Pex20p, Saccharomyces cerevisiae Pex18p/Pex 21p and mammalian Pex5pL fulfil a common function in the early steps of the peroxisomal PTS2 import pathway EMBO Rep., 2, 1035-1039
Grunau, S., Mindthoff, S., Rottensteiner, H., Sormunen, R.T., Hiltunen, J.K., Erdmann, R. and Antonenkov, V.D. (2009) Channel-forming activities of peroxisomal membrane proteins from the yeast Saccharomyces cerevisiae FEBS J., 276, 1698–1708
Grunau, S., Lay, D., Mindthoff, S., Platta, H.W., Girzalsky, W., Just, W.W. and Erdmann, R. (2011) The phosphoinositide 3-kinase Vps34p is required for pexophagy in Saccharomyces cerevisiae Biochem. J. 434, 161–170
Kerssen, D., Hambruch, E., Klaas, W., Platta, H.W., de Kruijff, B., Erdmann, R., Kunau, W-H. and Schleibs, W. (2006) Membrane association of the cycling peroxisome import receptor Pex5p J. Biol. Chem., 281, 27003-27015
Mindthoff, S., Grunau, S., Steinfort, L.L., Girzalsky, W., Hiltunen, J.K., Erdmann, R. and Antonenkov, V.D. (2016) Peroxisomal Pex11 is a pore-forming protein homologous to TRPM channels Biochim. Biophys. Acta, 1863, 271–283
Oeljeklaus, S., Reinartz, B.S., Wolf, J., Wiese, S., Tonillo, J., Podwojski, K., Kuhlmann, K., Stephan, C. et al (2012) Identification of core components and transient interactors of the peroxisomal importomer by dual-track stable isotope labeling with amino acids in cell culture analysis J. Proteome Res. 2012, 11, 2567−2580
Platta, H.W., Grunau, S., Rosenkrantz, K., Girzalsky, W. and Erdmann, R. (2005) Functional role of the AAA peroxins in dislocation of the cycling PTS1 receptor back to the cytosol Nat. Cell Biol., 7, 817-822
Schäfer, A., Kerssen, D., Veenhuis, M., Kunau, W-H. and Schliebs, W. (2004) Functional similarity between the peroxisomal PTS2 receptor binding protein Pex18p and the N-terminal half of the PTS1 receptor Pex5p Mol. Cell Biol., 24, 8895-8906
Thoms, S., Debelyy, M.O., Nau, K., Meyer, H.E. and Erdmann, R. (2008) Lpx1p is a peroxisomal lipase required for normal peroxisome morphology FEBS J., 275, 504-514
Welker, S., Rudolph, B., Frenzel, E., Hagn, F., Liebisch, G., Schmitz, G., Scheuring, J., Kerth, A., Blume, A., Weinkauf, S., Haslbeck, M., Kessler, H. and Buchner, J. (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function Mol. Cell, 39, 507–520
Wróblewska, J.P., Cruz-Zaragoza, L.D., Yuan, W., Schummer, A., Chuartzman, S.G., de Boer, R., Oeljeklaus, S., Schuldiner, M. et al (2017) Saccharomyces cerevisiae cells lacking Pex3 contain membrane vesicles that harbor a subset of peroxisomal membrane proteins BBA Mol. Cell Res., 1864, 656–1667
14. Plasma membrane
Wang, Y., Lilley, K.S. and Oliver, S.G. (2014) A protocol for the subcellular fractionation of Saccharomyces cerevisiae using nitrogen cavitation and density gradient centrifugation Yeast, 31, 127–135
Welker, S., Rudolph, B., Frenzel, E., Hagn, F., Liebisch, G., Schmitz, G., Scheuring, J., Kerth, A., Blume, A., Weinkauf, S., Haslbeck, M., Kessler, H. and Buchner, J. (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function Mol. Cell, 39, 507–520
15. Vacuole/pre-vacuole
Chang, W., Zaarour, R.F., Reck-Peterson, S., Rinn, J., Singer, R.H., Snyder, M., Novick, P. and Mooseker, M.S. (2008) Myo2p, a class V myosin in budding yeast, associates with a large ribonucleic acid–protein complex that contains mRNAs and subunits of the RNA-processing body RNA, 14, 491-502
Chen, O.S. and Kaplan J. (2000) CCC1 suppresses mitochondrial damage in the yeast model of Friedreich’s ataxia by limiting mitochondrial iron accumulation J. Biol. Chem., 275, 7626-7632
Toulmay, A. and Prinz, W.A. (2012) A conserved membrane-binding domain targets proteins to organelle contact sites J. Cell Sci., 125, 49–58
Welker, S., Rudolph, B., Frenzel, E., Hagn, F., Liebisch, G., Schmitz, G., Scheuring, J., Kerth, A., Blume, A., Weinkauf, S., Haslbeck, M., Kessler, H. and Buchner, J. (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function Mol. Cell, 39, 507–520
Yun, C-W., Ferea, T., Rashford, J., Ardon, O., Brown, P.O., Botstein, D., Kaplan, J. and Philpott, C.C. (2000) Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake J. Biol. Chem., 275, 10709-10715
OptiPrepTM Reference List RS15: 4th edition, January 2020
