Subcellular Application of Optiprep
OptiPrep™ Application Sheet S01
Preparation of gradient solutions (mammalian)
1. OptiPrep TM
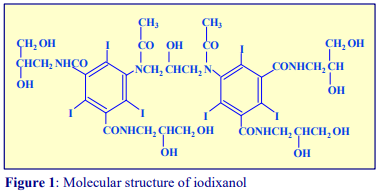
OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml. Iodixanol is a nonionic molecule with a molecular mass of 1550 (see Figure 1).
 2. Handling OptiPrep
2. Handling OptiPrep
Exposure (several months) of iodixanol solutions to direct sunlight will cause a slow release of iodine (solution turns yellow); OptiPrep™ should therefore be stored away from strong sunlight. On standing, iodixanol may „settle out“ of concentrated solutions, which should be well mixed before use.
3. Osmolality
The observed osmolality of OptiPrep™ depends on the mode of measurement (vapour pressure or freezing point); moreover the situation is complicated by the tendency of the iodixanol molecules to associate non-covalently in a concentrated aqueous solution. Measured values for its osmolality are thus lower than might be expected. Importantly however, when OptiPrep™ is diluted with a buffered isoosmotic solution, the iodixanol oligomers dissociate and all dilutions are isoosmotic. Under normal operating conditions therefore OptiPrep™ behaves as if it had an osmolality of approx 290 mOsm.
4. Preparation of density solutions for all organelles, except nuclei
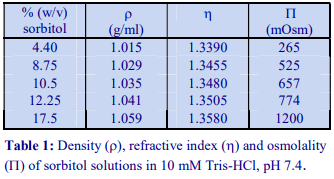
The recommended procedure for the production of density gradient solutions or for adjustment of the density of organelle suspensions is to use a working solution (WS) whose composition is compatible with the particles to be separated. The following methodology is based on the use of 0.25 M sucrose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.4 as homogenization medium (HM). To keep the concentrations of EDTA and buffer constant in the gradient constant first prepare a 50% (w/v) iodixanol working solution by mixing 5 vol. of OptiPrep™ with 1 vol. of 0.25 M sucrose, 6mM EDTA, 60 mM Tris-HCl, pH 7.4. The gradient solutions are then produced from the Working Solution (WS) by dilution with the HM according to Table 1.
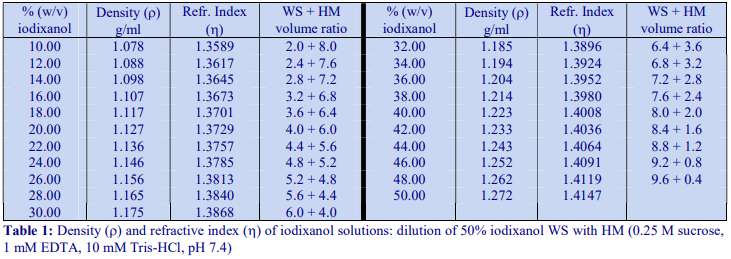
The osmolality of all the dilutions is in the range 295-310 mOsm. The use of alternative organic buffers at similar concentrations will have no significant effect on the density and osmolality of the WS. The concentration of buffer and EDTA in all of the gradient solutions will be the same as in the HM. If a low concentration (1-5 mM) of any other additive (e.g. DTT or a detergent) needs to be kept constant in the gradient, this can also be added to the OptiPrep™ diluent at the appropriate concentration.
- It may be permissible to produce density solutions simply by diluting OptiPrep™ with homogenization medium. The osmolality will be satisfactory but the concentration of buffer and additives in the gradient will decrease as the iodixanol concentration increases.
- The 50% (w/v) iodixanol WS is also suitable for adding to the homogenate or differential centrifugation fraction (suspended in buffered 0.25 M sucrose) in order to adjust its density; although it may be acceptable to add OptiPrep™ directly.
 5. Other non-ionic osmotic balancers
5. Other non-ionic osmotic balancers
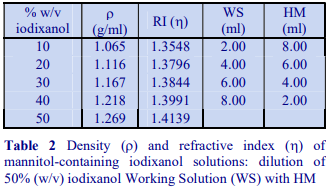
Occasionally mannitol (or sorbitol) may be preferred over sucrose as an osmotic balancer for mammalian systems. Mannitol in particular is widely used in media for the isolation of mitochondria and sometimes it is used for suspending cells when a nonionic medium is required. Isoosmotic density solutions based on an HM containing 4.4% (w/v) mannitol (or sorbitol), 10 mM Tris-HCl, pH 7.4 ( = 1.015 g/ml) are produced in the same manner as those based on 0.25 M sucrose. A 50% (w/v) iodixanol WS is produced by diluting 5 vol of OptiPrep™ with 1 vol of 4.4% (w/v) mannitol, 60 mM Tris-HCl, pH 7.4. This is then diluted further with HM. The properties of a few selected dilutions are given in Table 2. The osmolality of solutions is 290-310 mOsm.
6. Preparation of density solutions for nuclei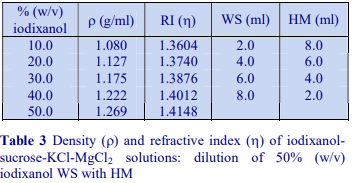
The majority of homogenization solutions for the isolation of nuclei contain KCl and MgCl2 as opposed to EDTA. An homogenization medium (HM) of 0.25 M sucrose, 25 mM KCl, 5 mM MgCl2, 20 mM Tris-HCl, pH 7.8 is often recommended. Mix 5 vol. of OptiPrep™ with 1 vol. of 150 mM KCl, 30 mM MgCl2, 120 mM Tris-HCl, pH 7.8, to produce a WS with a density of 1.269 g/ml and osmolality of 320 mOsm. Dilute the WS with HM (p=1.033 g/ml) to provide solutions of the appropriate density (see Table 3).
7. Homogenization media containing ionic osmotic balancers
Although the use of non-ionic osmotic balancers such as sucrose (or mannitol) is more or less a tradition in organelle isolation, there has been trend over the last ten years to move to the use of ionic osmotic balancers (KCl or NaCl) either on their own or in combination with sucrose, particularly for cultured cells. Solutions with a higher ionic strength may be particularly useful for cells in which the proteins of the cytoskeleton tend to form a gel during homogenization. Some examples are (the buffer is given as the final component in each example): 0.25 M sucrose, 130 mM KCl, 5 mM MgCl2, 25 mM Tris-HCl, pH 7.4; 130 mM KCl, 25 mM NaCl, 1 mM EGTA, 25 mM Tris-HCl, pH 7.4; 120 mM NaCl, 20 mM KCl, 1 mM EGTA, 1 mM EDTA, 10 mM Tris-HCl, pH 7.5 and 0.25 M sucrose, 78 mM KCl, 4 mM MgCl2, 8.32 mM CaCl2, 10 mM EGTA, 50 mM Hepes-KOH, pH 7.0
8. Density calculations
The density of any gradient solution can be calculated using Equation 1, so long as the densities of
the iodixanol-containing solution and of the diluent are known.
Equation 1:
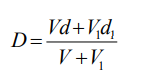
density of mixture; V = volume of iodixanol stock solution; d = density iodixanol stock solution;
V1 = volume of diluent; d1 = density of diluent
OptiPrepTM Application Sheet S01; 8th edition, January 2020
OptiPrep™ Application Sheet S02
Preparation of gradient solutions (non-mammalian)
1. OptiPrep
OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml. Iodixanol is a nonionic molecule with a molecular mass of 1550 (see Figure 1).
2. Handling OptiPrep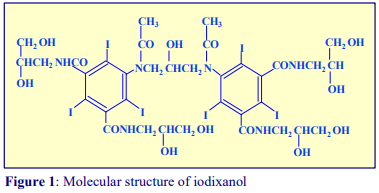
Exposure (several months) of iodixanol solutions to direct sunlight will cause a slow release of iodine (solution turns yellow); OptiPrep™ should therefore be stored away from strong sunlight. On standing, iodixanol may „settle out“ of concentrated solutions, which should be well mixed before use.
3. Osmolality
The observed osmolality of OptiPrep™ depends on the mode of measurement (vapour pressure or freezing point); moreover the situation is complicated by the tendency of the iodixanol molecules to associate non-covalently in a concentrated aqueous solution. Measured values for its osmolality are thus lower than might be expected. Importantly however, when OptiPrep™ is diluted with a buffered isoosmotic solution, the iodixanol oligomers dissociate and all dilutions are isoosmotic. Under normal operating conditions therefore OptiPrep™ behaves as if it had an osmolality of approx 290 mOsm. Homogenates of yeast and plants and the solutions used to isolate organelles from these sources frequently contain either mannitol or sorbitol at concentrations (and osmolarities) significantly higher than those used for mammalian systems. The isolation of protoplasts or spheroplasts (from plants and yeast respectively) is also carried out in such media in order to shrink the intact cell away from the cell coat. Media containing 400-600 mM mannitol or sorbitol are common but for yeast mitochondria concentrations as high as 0.8-1 M sorbitol are not unknown. Sucrose may also be used at concentrations up to 0.5 M, thus a special strategy has to be adopted to provide density solutions of the appropriate osmolality.
 4. Preparation of density solutions
4. Preparation of density solutions
The general strategy is to produce a high density working solution (WS) of the correct osmolality by diluting OptiPrep™ with a sorbitol (or mannitol) containing diluent and then diluting this solution with the normal homogenization medium (HM) or organelle suspension medium. Table 1 gives the properties of sorbitol solutions in 10 mM Tris-HCl, pH 7.4. Examples of use of these diluents to produce gradient solutions of a constant osmolality are given below. Prepare a WS of 40% (w/v) iodixanol by diluting 4 vol of OptiPrep™ with 2 vol of 12.25% (w/v) sorbitol, 30 mM Tris-HCl, pH 7.4. This has a density of 1.225 g/ml. Dilute the WS with 8.75% (w/v) sorbitol, 10 mM Tris-HCl, pH 7.4 to provide gradient solutions of a suitable density (see Table 2). All of the solutions have an osmolality of approx 545 mOsm.
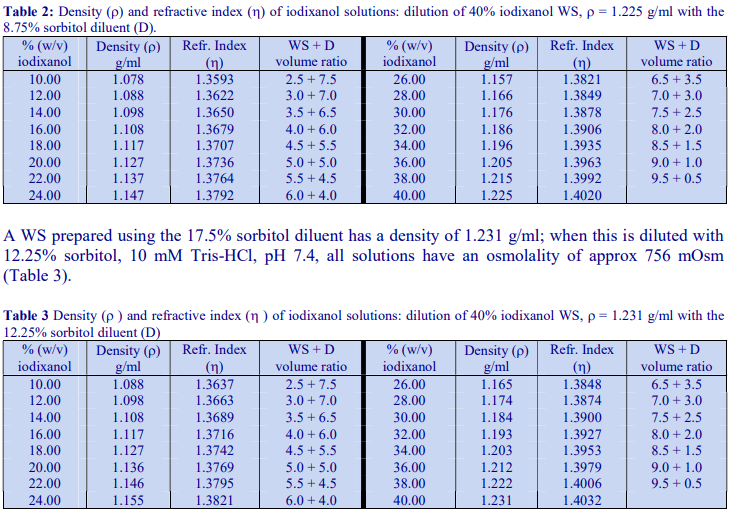
Diluents containing mannitol of the same concentration provide solutions of exactly the same density and osmolality. Sorbitol (or mannitol) solutions of 17.5%, 12.25% and 8.75% (w/v) are equivalent to 0.96 M, 0.67 M and 0.48 M respectively.
5. Concentration of buffer and other additives in the gradient
It may be important to maintain constant low concentrations (1-5 mM) of some additives such as EDTA or DTT in the gradient. In which case add them to the OptiPrep™ diluent at 3x the required gradient concentration when the WS is prepared. If the additives are also included in the WS diluent (at their required concentration) then their concentration in all density gradient solutions will be constant.
6. Other osmotic balancers
The density of any gradient solution can be calculated using Equation 1 (below), so long as the densities of the iodixanol-containing solution and of the diluent are known.
Equation 1:
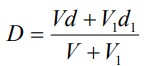
D = density of mixture; V = volume of iodixanol stock solution; d = density iodixanol stock solution;
V1 = volume of diluent; d1 = density of diluent
OptiPrepTM Application Sheet S02; 8th edition, January 2020
OptiPrep™ Application Sheet S03
Preparation of discontinuous and continuous gradients
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
1. Discontinuous gradients
1a Overlayering technique
The most widely used method for producing discontinuous gradients is to start with the densest solution and layer solutions of successively lower densities on top using some form of pipette or syringe. Tilt the centrifuge tube (approx. 45); place the tip of the pipette or syringe against the wall of the tube, about 1 cm above the meniscus of the denser solution, and gently deliver a slow and steady stream of liquid. This allows the liquid to spread over the tube surface and minimizes any mixing due to a sudden increase in liquid flow. Once a steady flow is established keep the tip of the pipette or syringe just above the meniscus of the liquid and against the wall of the tube.
From a pipette
Use a rubber two- or three-valve pipette filler to aliquot and dispense the gradient solutions. Check that the release valve when pressed gently, allows the delivery of a slow and steady flow of liquid. Do not use a pipette filler that uses positive pressure to deliver the liquid, as a slow even flow is often difficult to attain. Always take up more of the gradient medium than is required as it is easier and more accurate to empty the pipette to a graduation mark than to try to empty it completely.
From an automatic pipette
For small volume gradients an automatic pipette may be used. Always cut off the end of the plastic pipette tip to reduce the flow velocity of the liquid.
From a Pasteur pipette
Plastic Pasteur pipettes can be used conveniently for larger volume gradients, particularly those in calibrated centrifuge tubes. It requires some practise however to maintain a steady liquid flow by depressing the bulb of the pipette.
From a syringe
A syringe with a wide-bore metal filling cannula (i.d. approx 1 mm) is suitable for most gradient volumes, but make sure that the barrel can move easily and smoothly when a small pressure is applied. Placing the index finger around the bottom of the plunger, rather than around the barrel, restricts the movement of the plunger when it is depressed and thus achieves a more controlled liquid flow. Always take up more of the gradient medium than is required for the step as it is more accurate to empty the syringe to a graduation mark than to try to empty it completely.
- Metal filling cannulas can be purchased from most surgical instrument suppliers. Contact John Graham at jgrescon@outlook.com for information.
1b Underlayering technique
Although the overlayering technique is probably the most widely used, the easier method is to underlayer successively denser solutions beneath the lighter solutions. The only important requirement is that no air bubbles are introduced which may disturb the lower density layers above; for this reason a syringe with a metal filling cannula is the best tool for this procedure. Generally the existing steps are disturbed less as the outflowing liquid spreads upwards through the hemispherical section of the bottom of the tube.
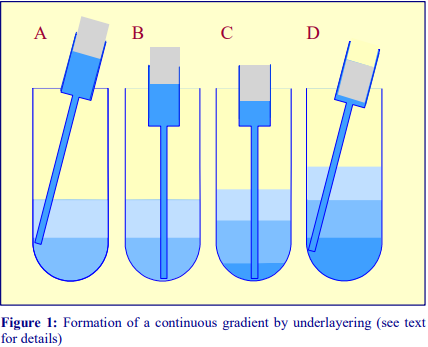 1. To underlayer 4 ml of liquid, take up 5 ml into the syringe and expel to the 4.5 ml mark to ensure that the cannula is full of liquid.
1. To underlayer 4 ml of liquid, take up 5 ml into the syringe and expel to the 4.5 ml mark to ensure that the cannula is full of liquid.
2. Dry the outside of the cannula.
3. Move the tip of the cannula to the bottom of the tube, sliding it slowly down the wall of the tube (Figure 1A-B)
4. Depress the plunger to the 0.5 ml mark (Figure 1C).
5. After a few seconds (to allow all of the liquid to be delivered into the tube) slowly withdraw the cannula, again against the wall of the tube (Figure 1D).
6. Dry the outside of the cannula and repeat the procedure with successively denser solutions.
2. Continuous gradients
Continuous gradients may be made by allowing discontinuous gradients to diffuse or by using a gradient maker specifically designed for this purpose.
 2a By diffusion of discontinuous gradients
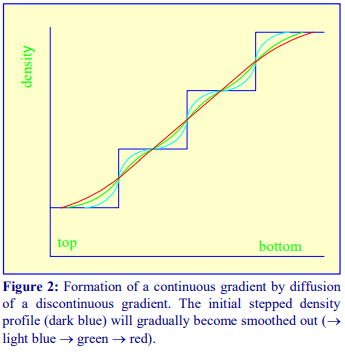
2a By diffusion of discontinuous gradients
Once a discontinuous gradient is formed, the sharp boundaries between the layers, which are observed as a sudden change in refractive index, start to disappear as the solute molecules diffuse down the concentration gradient from each denser layer to each lighter layer. Thus the density discontinuities between each layer will slowly even out and the gradient will eventually become linear
(Figure 2), and given sufficient time the density will become completely uniform.
For a particular medium, the rate of diffusion across an interface is dependent on temperature and the cross-sectional area of the interface. In addition the rate at which the gradient becomes linear will also be a function of the distance between the interfaces. Thus a linear gradient will form more rapidly at room temperature than at 4°C and if the distance between interfaces is reduced and the cross-sectional area increased. This can be achieved as follows (Figure 3).
1. Produce a discontinuous gradient by the underlayering (Section 1b) or overlayering (Section1a) method. Unless the gradient is to be very shallow use 3 or 4 layers that increase in steps of about 5-10% (w/v) iodixanol.
2. Seal the tube well with Parafilm and carefully rotate the tube to a horizontal position and leave for 45-60 min.
3. Return the tube to the vertical, cool to 4 °C if required and apply the sample to the gradient (either over- or underlayered).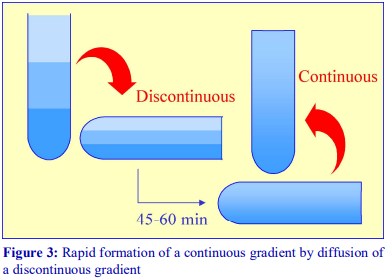
The precise timing for the formation of a continuous linear gradient will depend on the dimensions of the tube, the number of layers and the concentrations of iodixanol. A series of trial experiments should be carried out in which the time is varied and the density profile of the formed gradient checked by fractionation and refractive index measurement.
Because the continuous gradient is formed by a physical process, so long as the temperature and time are well controlled, the shape of the gradient is highly reproducible. If the diffusion is allowed to occur in a vertically maintained tube the process will take longer and at 4°C it may take more than 10 h. If however the gradients can be prepared the day before the experiment and left in the refrigerator overnight then this can be a convenient approach. Gradients prepared rapidly at room temperature need to be equilibrated at 4°C prior to loading of the sample. Incorporation of the sample into one or more of the layers eliminates interfaces and can improve resolution, but this useful strategy (used with cells) is unlikely to suit subcellular membranes that need maintaining at 4°C.
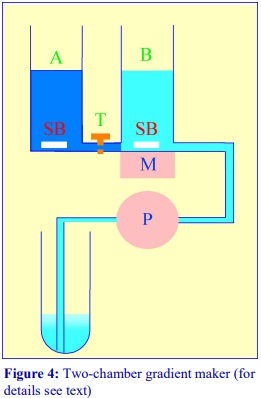 2b Using a two-chamber gradient maker
2b Using a two-chamber gradient maker
The traditional way of constructing a continuous gradient is to use a standard two-chamber gradient maker (Figure 4). It consists of two identical chambers connected close to their bases by a tapped channel (T). One of the chambers (the mixing chamber – B in Figure 4)) has an outlet directly opposite the inlet from the tapped channel.
1. Set up the device as shown in Figure 4 with the mixing chamber (B) resting on a magnetic stirrer (M) and the outlet tube leading via a peristaltic pump (P) to the bottom of the centrifuge tube.
2. Place the chosen high-density solution in the non-mixing chamber (A) and then momentarily open the tap (T) to allow dense liquid to fill the connecting tube.
3. Pour an equal volume of the low-density solution in the mixing chamber (B).
4. Place two identical stirring bars (SB) in the two chambers (this ensures that the height of the two solutions is the same.
5. In rapid sequence, switch on the pump (P) and the magnetic stirrer (M) and then open the connecting tap (T). As the levels in the two chambers fall synchronously, reduce the speed of the stirrer to avoid generating air bubbles that may enter the gradient and disturb it.
6. Make sure that the pump is turned off before any air bubbles reach the bottom of the delivery tube at the end of the operation.
- The larger the density difference between the two gradient solutions the more vigorous must be the stirring to ensure good mixing. If the stirring bar is too close to the inlet from the connecting tube, it is possible in the initial stages for the low-density medium to back flow into the highdensity medium.
- The correct pumping speed depends on the volume of the gradient and the quality of the pump (ideally the outflow from the pump should not pulsate), but for a standard 10-30% (w/v) or iodixanol gradient (of 12-15 ml total volume) a flow rate of approx 2 ml/min is satisfactory. Pumps that impart little or no pulsation to the liquid flow are commonly available from many sources.
- The gradient can alternatively be produced high density end-first, in which case the location of the two solutions needs to be reversed and the delivery tube to the centrifuge tube must be placed against the wall of the centrifuge tube near to its top, so the gradient flows down the tube smoothly. This is can pose some problems of mixing in the centrifuge tube if the flow down the tube wall is in the form of large drops rather than a continuous stream (this may be minimized by tilting the tube), on the other hand the tendency of the low density medium to float to the surface of the high density medium in the mixing chamber aids mixing. The Auto Densi-Flow gradient unloader can be used to deposit a gradient high-density end first with no disturbance. Although this device is no longer commercially available, it will be found in many laboratories. For details of this device see Section 4e of Application Sheet S08.
- To guard against air bubbles entering the delivery tube, a bubble trap could be included between mixer and pump. Although air bubbles are a major problem if they reach the bottom of the centrifuge tube (low density first delivery) they are no less a problem for high-density first delivery as they interfere with the smooth flow of liquid down the tube wall.
- It is possible to produce up to three gradients at a time; some gradient mixers have a three-outlet manifold. However such a device requires three tubes to pass through the peristaltic pump. It is the only reliable configuration of the delivery tube; simply splitting the liquid flow from a single tube through the pump cannot guarantee precisely equal delivery to all three tubes.
 2c Gradient Master
2c Gradient Master
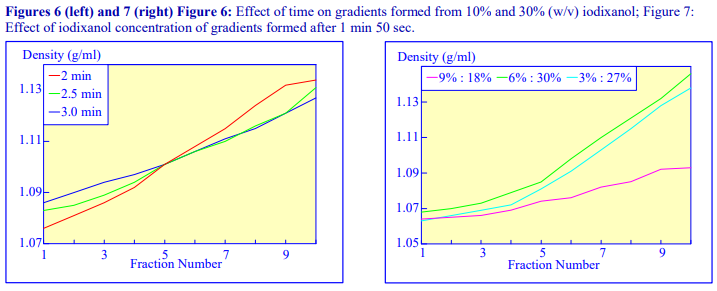
An alternative device for the generation of continuous density gradients – the Gradient Master – produces the gradient by controlled mixing of the low and high-density solutions layered in the centrifuge tube. The tubes are rotated at a pre-set angle – usually 80° – to increase the cross-sectional area of the interface – and speed (usually 20 rpm) for about 2 min (Figure 5). The density profile of the gradient generally becomes more shallow with time. The simplicity of the technique and the highly reproducible nature of the gradients make this a very attractive method; up to 6 gradients (17 ml tubes) can be formed at once. Some examples with iodixanol solutions are given in Figures 6 and 7.
- A very important advantage of this technique over the use of a two-chamber gradient mixer is that if it is necessary to make the sample part of the gradient, any potentially hazardous biological sample is contained within the centrifuge tube and does not contaminate the gradient forming device and ancillary tubing.
- For more information on the Gradient Master and other similar instruments contact the manufacturers at www.biocompinstruments.com
 2d Freeze-thawing
2d Freeze-thawing
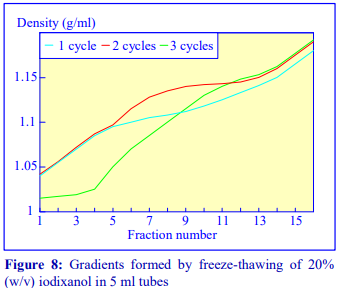
The final manner in which continuous gradients can be produced is by freezing a solution of uniform density for at least 30 min at -20°C and then thawing at room temperature for 30-60 min. These times are for tubes of approximately 5 ml volume. The freeze-thaw cycles can then be repeated; this modulates the density profile of the gradient. Generally as the number of freeze-thaw cycles increases, the gradient becomes markedly less dense at the top. The method can produce gradients that are more or less linear. Because the shape of the gradient depends on the rate of freezing and thawing, as well as the number of freeze-thaw cycles (and the volume of the tube), the precise conditions required need to be worked out for a particular laboratory. Under well-controlled conditions however, the profiles are highly reproducible. An example of the procedure with an iodixanol solution is given in Figure 8 (data kindly supplied by Dr C A Borneque, CNRS, Centre de Génétique Moléculaire, 91198 Gif sur Yvette,
France).
2e Non-linear gradients
It is not always desirable to use a linear gradient and either convex, concave, S-shaped or more complex gradient density profiles may be required to effect a particular resolution of particles. Convex gradients are sometimes particularly useful for the resolution of a sample containing a high concentration of particles of a wide range of densities. The steep density profile at the top of the gradient provides stable conditions for high capacity and the shallower high-density region provides high resolution.
From discontinuous gradients by diffusion
If each of the layers of the initially discontinuous gradient is of the same volume then diffusion will produce a linear gradient. The diffusion process however is also a very convenient way of producing a gradient that is not linear with volume. Convex or concave gradients or gradients containing a shallow median section can be produced by increasing the volume of the denser, lighter or median density layers respectively. The shape of the gradient may also be altered by changing the density interval between adjacent layers. Clearly reducing the density interval will make the gradient more shallow. It is important to test the density profile that is formed from such discontinuous gradients, but once satisfactory conditions are established the profile will be highly reproducible.
Using a gradient mixer or Gradient Master
Convex and concave gradients cannot be produced with the standard two-chamber gradient mixer (see Figure 4). However if the non mixing chamber is made twice the diameter of the mixing chamber, then with low-density solution in the mixing chamber a convex gradient is produced; if the locations of the low density and high-density solutions are reversed, a concave gradient is produced. In a Gradient Master non-equal volumes of the two density solutions will change gradient shape.
 3. Types of rotor used with preformed gradients
3. Types of rotor used with preformed gradients
Traditionally, preformed gradients of sucrose are run in a swinging-bucket rotor and today this remains the most popular choice of rotor for any density gradient centrifugation. Sedimentation path lengths tend to be long, but because of the relatively low viscosity of iodixanol solutions, centrifugation times need not be correspondingly long. In a standard 6×17 ml swinging-bucket rotor (path length approx 100 mm) for example, the major organelles (Golgi, lysosomes, mitochondria and peroxisomes) from mammalian liver can be resolved by flotation through a 10-30% (w/v) iodixanol gradient (p = 1.078-1.175 g/ml) at 50,000gav for 90 min. However, because iodixanol is able to form its own gradient by selfgeneration in the centrifugal field, it is not good practice to carry out buoyant density banding of smaller particles (such as membrane vesicles through pre-formed gradients at RCFs in excess of 250,000gav) for more than 3-4 h. Under these conditions iodixanol molecules towards the bottom of the tube may start to form a self-generated gradient and thus may deform the pre-formed density profile in the high-density region. At lower RCFs (e.g. 100,000 g) there will essentially be no density profile modulation.
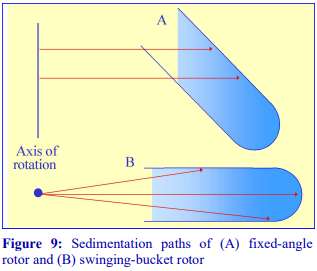
Fixed-angle rotors are generally less frequently used for pre-formed gradients. Because of the angle at which the tube is held, particles tend to sediment to the wall of the tube due to the radial centrifugal field (Figure 9A); this does not occur in a swinging-bucket rotor, although even in this type of rotor, only those particles in the middle of the sample move in a plane parallel to the walls of the tube (Figure 9B). Swinging-bucket rotors were also often perceived as having an advantage over fixed-angle rotors for gradient work since the gradient always maintains the same orientation with respect to the long axis of the tube. However, so long as the particles do not adhere to the wall of the tube, a fixed-angle rotor can provide a useful alternative and there are many successful examples, particularly now that slow acceleration and deceleration facilities are now widely available on centrifuges to permit smooth reorientations of the gradient.
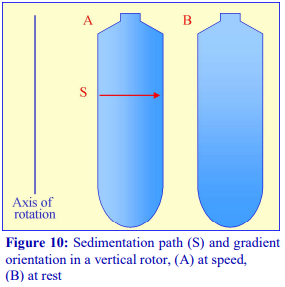 If a fixed-angle rotor is satisfactory for a particular gradient separation, then the shorter sedimentation path length of such a rotor compared to that of a swingingbucket rotor of the same capacity permits a shorter centrifugation time. This situation is taken to its logical conclusion with a vertical rotor which will have the shortest path length, i.e. the width of the tube and, like the swinging-bucket rotor, the sedimentation or flotation of particles is relatively unaffected by the tube wall (Figure 10). In these rotors therefore, centrifugation times are reduced to a minimum. In a rotor such as the Beckman VTi65.1 or VTi50 the long axis of the tubes is much larger than their diameter (Figure 10), so the steep gradient formed during centrifugation reorients to a relatively shallow one at rest. Therefore, so long as there is no mixing of the tube contents during deceleration, small volume fractionation of the gradient will provide very high resolution.
If a fixed-angle rotor is satisfactory for a particular gradient separation, then the shorter sedimentation path length of such a rotor compared to that of a swingingbucket rotor of the same capacity permits a shorter centrifugation time. This situation is taken to its logical conclusion with a vertical rotor which will have the shortest path length, i.e. the width of the tube and, like the swinging-bucket rotor, the sedimentation or flotation of particles is relatively unaffected by the tube wall (Figure 10). In these rotors therefore, centrifugation times are reduced to a minimum. In a rotor such as the Beckman VTi65.1 or VTi50 the long axis of the tubes is much larger than their diameter (Figure 10), so the steep gradient formed during centrifugation reorients to a relatively shallow one at rest. Therefore, so long as there is no mixing of the tube contents during deceleration, small volume fractionation of the gradient will provide very high resolution.
- It is important that the gradient is so designed to prevent particles from sedimenting to the wall of the tube, as these will tend to fall back into the medium during reorientation and unloading and thus contaminate the rest of the gradient.
- Near-vertical rotors, which hold the tube at approx. 8° to the vertical, overcome this pellet problem.
- Vertical and near-vertical rotors can provide the most efficient form of centrifugation in gradients. They can be particularly effective for rate-zonal separations, since any sample placed on top of a gradient achieves a very small radial thickness after reorientation.
- Because the surface area of any banded material is much higher in a vertical or near-vertical rotor than in a swinging-bucket rotor during centrifugation, particles that have a significant rate of diffusion (Mr<5×105) may exhibit band broadening due to this diffusion.
The use of large volume zonal rotors for gradient centrifugation is beyond the scope of this text; for information the reader is referred to relevant review articles [1,2].
4. Types of tube for gradient centrifugation
Choice of tube material (polyallomer, polycarbonate etc) is usually governed by considerations of optical transparency, resistance to chemicals or sterilizing (autoclaving) procedures (see manufacturers specifications for more information); generally speaking there is no specific advantage or disadvantage of using one particular type of tube material for gradient centrifugation from a fractionation point of view. The tube material may also restrict the maximum permitted RCF.
Choice of tube type (open-topped, screw-capped, sealed etc) is dictated by the selection of rotor type, the RCF that is required (many tube types cannot be run at the maximum speed of the rotor); the degree of containment that is required and a consideration of the type of gradient harvesting that is to be carried out. For details on gradient harvesting see Section 4i of Application Sheet S08.
Gradient centrifugation in low-speed and high-speed centrifuges is not generally carried out in special tubes, unless special containment is required; the standard thick walled polycarbonate, polyallomer, polypropylene or polystyrene tubes employed for all low- and high-speed centrifugation are satisfactory.
In ultracentrifugation a wide range of tube styles are available and the reader is directed to the appropriate technical manuals published by the centrifuge companies. Principally polyallomer and polycarbonate or Ultra-Clear (Beckman trade-name) are used. For simplicity and convenience only those tubes manufactured by Beckman Instruments will be described although other companies supply an essentially similar range of tubes although there may be some differences in the mode of sealing.
- Swinging-bucket rotors Tubes for swinging bucket rotors are traditionally open topped (the seal being provided the screw-cap on the bucket), thin-walled and made from polyallomer or UltraClear. Occasionally thick-walled polycarbonate is available. See also “Vertical rotors” below.
- Fixed-angle rotors The types of material used for tubes for fixed-angle rotors are broadly similar to those for swinging-bucket rotors. Some of the thick walled tubes are open-topped and do not require caps, others have a variety of capping devices. Thin walled tubes always require caps. The thick walled variety with a simple screw cap is not ideally suited to some forms of gradient harvesting. For details on gradient harvesting see Section 4i of Application Sheet S08. See also “Vertical rotors”, below.
- Vertical rotors The only types of tube recommended for vertical rotors are thin-walled sealed tubes made either from polyallomer or Ultraclear; these are also available for many swingingbucket and fixed-angle rotors. In swinging-bucket rotors however there is usually a variable reduction in tube volume compared to the standard thin-walled open-topped tubes. Beckman manufacture two types: Quick-Seal and Optiseal, the former are sealed by a heat and the latter by a central plastic plug.
- Through the use of adaptors and spacers most rotors accommodate a range of tubes of a volume considerably smaller than that
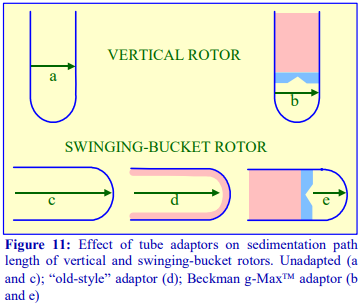 of the rotor tube pocket, many of which may have a restricted maximum RCF (compared to the standard thin walled tube). Traditionally the smaller volume tubes for swinging-bucket and fixed-angle had a much-reduced diameter but the length was only slightly less than that of the fullvolume tube (Figure 11).
of the rotor tube pocket, many of which may have a restricted maximum RCF (compared to the standard thin walled tube). Traditionally the smaller volume tubes for swinging-bucket and fixed-angle had a much-reduced diameter but the length was only slightly less than that of the fullvolume tube (Figure 11).
- g-Max tubes: Because of the advantage of a short sedimentation path length, some of the swinging-bucket and fixed-angle rotors have been adapted to take shorter sealed tubes so that the path length is reduced. They are also available for vertical rotors but in these rotors the sedimentation path length is unchanged, only the volume is altered (Figure 1)
5. References
1. Graham, J. M. (1978) In Centrifugal separations in molecular and cell biology (ed G. D. Birnie and D. Rickwood). Butterworths, London, pp 63-85.
2. Graham, J. M. (1992) In Preparative centrifugation – a practical approach (ed D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 315-350
OptiPrepTM Application Sheet S03; 9th edition, January 2020
OptiPrep Application Sheet S04
Preparation of self-generated gradients
1. Background
Iodixanol, like solutions of heavy metal salts (e.g. CsCl) can form a gradient from a solution of uniform density under the influence of the centrifugal field. Once the solute begins to sediment through the solvent a concentration gradient is formed which is opposed by back-diffusion of the solute. With a sufficiently high RCF, at equilibrium, the sedimentation of the solute is exactly balanced by the diffusion and the gradient is stable. It is possible to calculate the time for a selfgenerating gradient to reach equilibrium and it is described by the following equation:
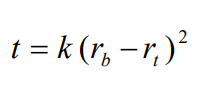
t is the time in hours; rb and rt the distance from the centre of rotation to the bottom and top of the gradient respectively and k is a constant, which depends on the diffusion coefficient and viscosity of the solute and on temperature [1]. The slope of the gradient is given by the equation:
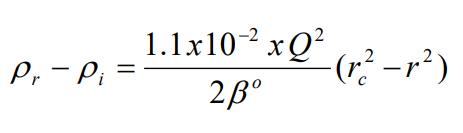
where p r is the density at a point r cm from the axis of rotation, p i is the starting density of the homogeneous solution, rc is the distance in cm from the axis of rotation where the density of the gradient = p i , Q is the rotor speed in rpm and ß°
o is a constant depending on the solute [1].
The shape of the gradient that is formed for a particular solute thus depends on the following factors:
- sedimentation path length of the rotor
- time of centrifugation
- speed of centrifugation
- temperature
The big advantages of the use of any self-generated gradient are the ease of sample handling (the sample is simply adjusted to the required starting concentration of iodixanol) and the great reproducibility of the gradient density profile under a particular set of centrifugation parameters.
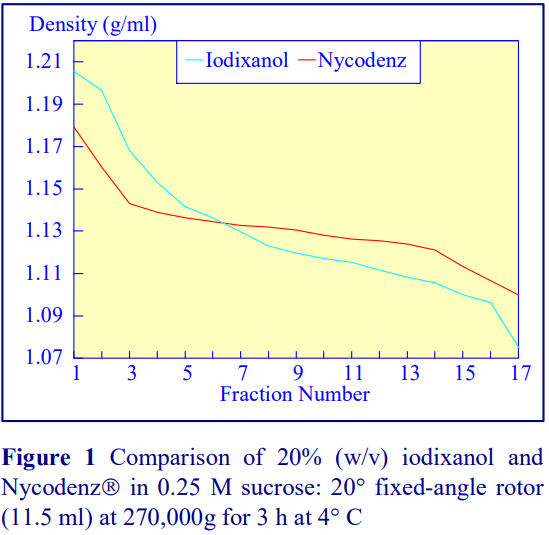 2. Self-generated gradient formation
2. Self-generated gradient formation
Iodixanol is able to form useful self-generating gradients in 1-4 h depending on the centrifugation speed and the rotor [2]. Figure 1 compares the gradient density profile generated from 20% (w/v) iodixanol and 20% (w/v) NycodenzⓇ in 0.25 M sucrose in a 20° fixed-angle rotor at 270,000gav for 3 h at 4 °C. Clearly a steeper gradient is formed from the iodixanol and this is a function of the higher molecular mass of iodixanol (approx. twice that of NycodenzⓇ): it therefore sediments rather more rapidly and diffuses more slowly.
2a. Types of rotor
Swinging-bucket rotors, which have rather long sedimentation path lengths, are little used for the formation of self-generating gradients. The shorter sedimentation path length rotors are much better suited to this task. Vertical and near-vertical rotors are particularly useful, although some fixed-angle rotors (preferably those with shallow angles of 20-24°) may be used. Gradients generated in the Beckman TLN100 near-vertical rotor (for the TLX120 table-top ultracentrifuge) which accommodates tubes of 3.5-4.0 ml, the Beckman VTi65.1 vertical rotor (for an appropriate floor-standing ultracentrifuge) which accommodates tubes of approx 11.0 ml (but which can be adapted down to smaller volumes) and the Beckman NVT65 (a near vertical rotor of similar tube capacity to that of the VTi65.1) are particularly useful for iodixanol self-generated gradients. The TLN100 and VTi65.1 rotors have approximately the same sedimentation path length (about 17 mm), that of the NVT65 is marginally longer (approx 25 mm); consequently under the same centrifugation conditions, they generate rather similar gradient profiles.
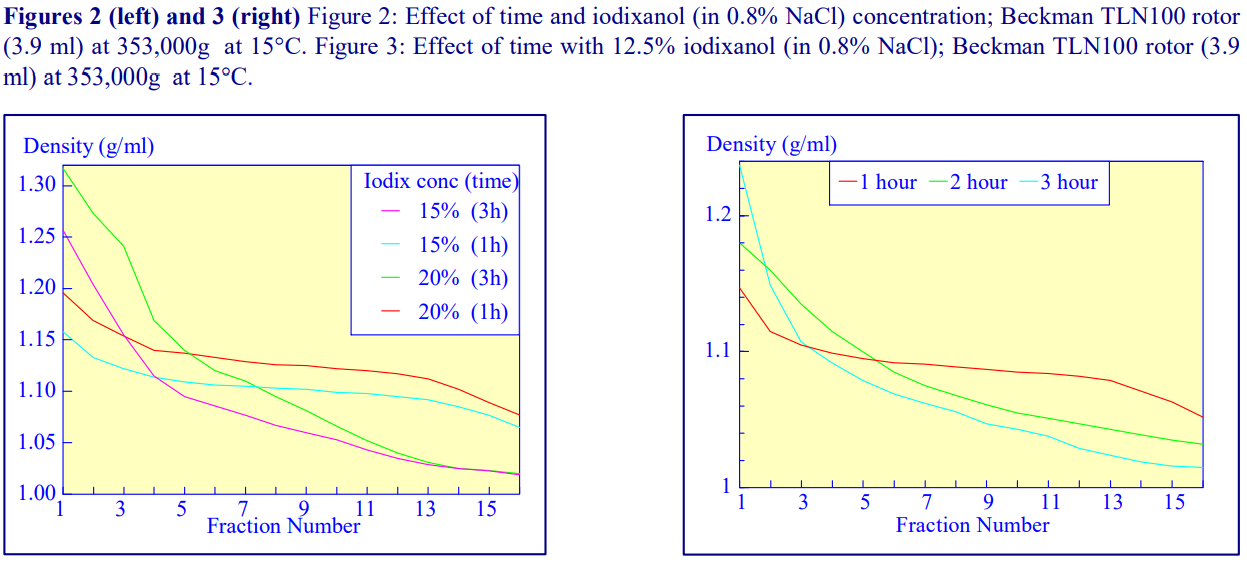
2b. Time of centrifugation
After 1 h at 15-18oC, centrifugation at approx 350,000gav, gradients generated in the TLN100 are Sshaped (i.e. they contain a relatively shallow region in the middle) and span a relatively narrow density range, while after 3 h, gradients are considerably steeper and cover a much wider density range. Figure 2 compares two starting concentrations of iodixanol at these two times, while Figure 3 compares three times (1, 2 and 3 h) using a 12.5% (w/v) iodixanol starting concentration with the same rotor. The exponential nature of the gradient becomes more apparent with time but times greater than 3 h result in little further change in the shape of the gradient at 350,000g, indicating that an equilibrium point has been reached.
2c. Temperature
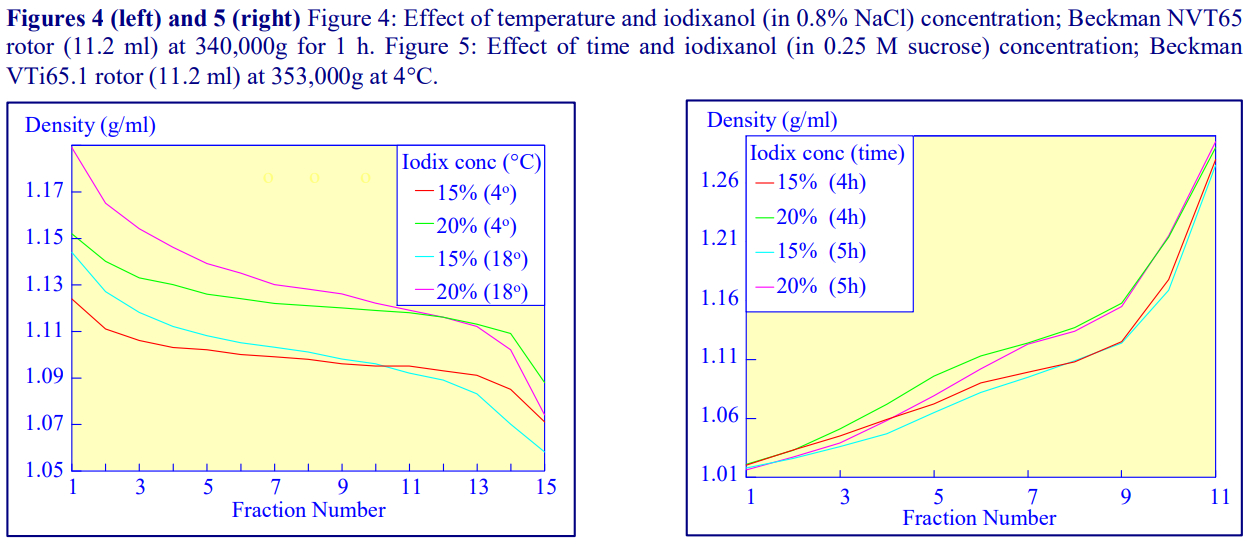
Higher temperatures tend to promote the formation of steeper gradients, although this effect is more apparent at shorter times of 1 h than at longer times of centrifugation. Figure 4 compares the formation of gradients at 4C and 18C in the NVT65 rotor at two iodixanol concentrations after centrifugation at approx 340,000gav for 1 h. At 4C, using 0.25 M sucrose as osmotic balancer, the gradients approach equilibrium more slowly: the excellent gradient profiles produced in the VTi65.1 with 15% or 20% (w/v) iodixanol at 4 h are very similar to those at 5 h (Figure 5), compare with Figure 2 (using NaCl as osmotic balancer at 15°C).
2d. Iodixanol concentration
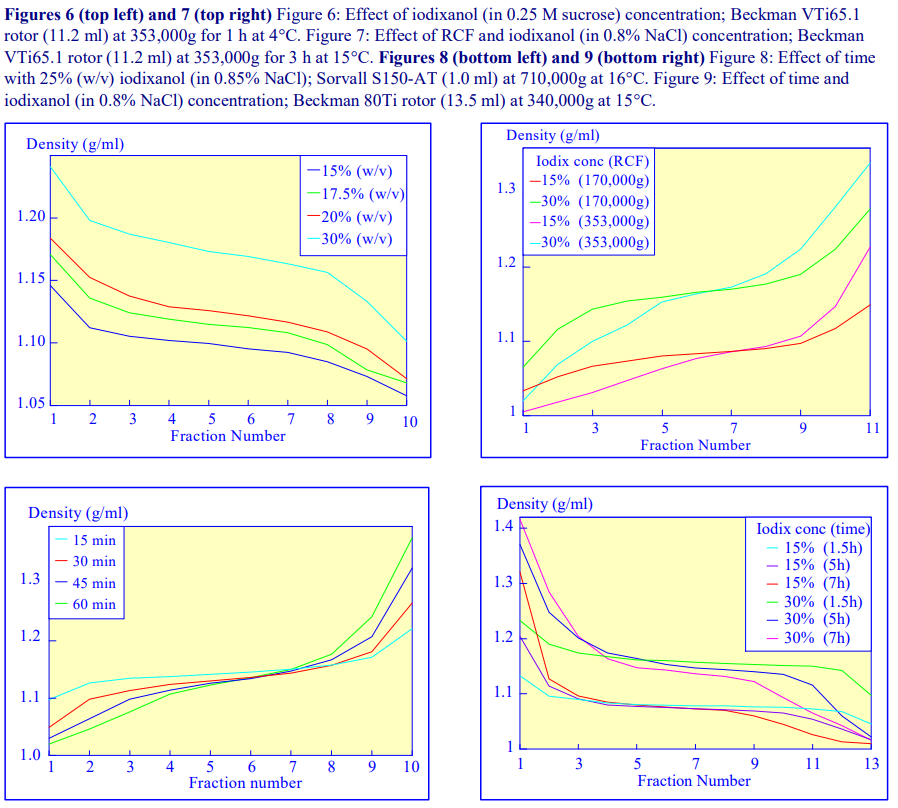
Other than changing the density range covered by the gradient (Figures 2 and 4-8) the starting concentration of iodixanol has rather little effect on the rate of gradient formation or shape of gradient profile. The shape of the gradient can be made more linear at lower RCFs by using two layers of iodixanol (e.g. 10% and 30%, w/v) rather than a single uniform concentration (20%, w/v).
2e. RCF
As the RCF decreases, the gradient becomes more shallow in the middle of the tube; the minimum RCF that produces a useful gradient will vary with the time and the rotor type. In the VTi65.1 vertical rotor, even at 170,000gav, a useful shallow gradient is produced within 3 h (Figure 7). In very high performance rotors that can run at up to 150,000 rpm (and also have very short sedimentation path lengths – see next section), self-generated gradients can form in as little as 15 min (Figure 8)
2f. Sedimentation path length
The longer the sedimentation path length of the rotor, the greater the tendency to form S-shaped gradients. Figure 9 compares the gradient formed from 15% or 30% (w/v) iodixanol using the 80Ti fixed-angle rotor with a sedimentation path length of 43 mm (13.5 ml tube volume) at a series of times. At 70,000 rpm, (equivalent to 345,000gav) approx 5 h is required to produce a useful gradient (compare with Figures 2-7).
3. References
1. Dobrota, M. and Hinton, R. (1992) Conditions for density gradient separations In Preparative centrifugation – a practical approach (ed D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 77-142.
2. Ford, T., Graham, J. and Rickwood, D. (1994) The preparation of subcellular organelles from mouse liver in selfgenerated gradients of iodixanol Anal. Biochem., 220, 360-366.
OptiPrepTM Application Sheet S04; 10th edition, January 2020
OptiPrep Application Sheet S05
Homogenization of mammalian tissues
1. Homogenization techniques
Mammalian tissues fall generally into two groups: soft tissues (e.g. rat liver) and hard tissues (e.g. bovine muscle) and routinely the types of homogenizer used to disrupt these tissues are liquid shear (Potter-Elvehjem or Dounce) or mechanical shear (e.g. Polytron) respectively. The situation is not clear-cut however since hard tissues may be rendered susceptible to liquid shear homogenization by treatment with hydrolytic enzymes. Whatever technique is used it is good practice to facilitate the homogenization by an initial coarse mincing of the tissue with scissors, scalpels or (for large masses of tissue) a mincer.
2. Removal of blood
Highly vascular tissues such as rat liver may require some form of perfusion to remove blood from the vasculature prior to homogenization. This is particularly true if the nuclear pellet is to be processed, for any erythrocytes in the homogenate will sediment at low g-forces. Erythrocytes may also interfere with the functional characterization of a particular organelle, for example the catalase in these cells may obscure any assessment of the fractionation of peroxisomes by measurements of this enzyme. Perfusion can be carried out after sacrificing the animal, simply by injection of buffered saline or homogenization medium through the portal vein after cutting the blood vessels above the liver. It is best carried out however under anaesthesia when the portal vein can be properly cannulated. This must be performed by a trained and licensed operative.
3. Homogenization media
Routinely, most soft tissues are homogenized in 0.25 M sucrose, buffered with low concentrations of an organic buffer such as Tris, Hepes or Tricine at a pH between 7 and 8. Often 1 mM EDTA is included to reduce aggregation, but if the organelle of interest is the nucleus, the EDTA is replaced with 25 mM KCl and 5 mM MgCl2, while for sheets of plasma membrane use 1 mM MgCl2. For mitochondria, the sucrose may be replaced by mannitol. Brain tissues are frequently disrupted in 0.32 M sucrose rather than 0.25 M. Hypoosmotic media (e.g. 10 mM Tris-HCl, pH 7.5 or 5 mM EDTA, pH 7.4) are often used with intestinal mucosa [1] and 1 mM NaHCO3 has been used for rat liver for the isolation of large sheets of plasma membrane although it is mow recognized that an isoosmotic medium can be just as effective [2].
Media for muscle homogenization are also quite variable and although compositions not unlike those for soft tissues have been used, KCl is often included (up to 180 mM) to solubilize some of the protein and prevent the formation of gels. The following media have been successfully used: 0.21 M mannitol, 70 mM sucrose, 0.1 mM EDTA, 0.5% bovine serum albumin (BSA), 10 mM Tris-HCl, pH 7.4 or 0.1 M sucrose, 10 mM EDTA, 46 mM KCl, 0.5% BSA, 100 mM Tris-HCl, pH 7.4 [3]. After coarse mincing of muscle tissue, it is commonly softened by incubating with Nagarse at 5-50 mg per 100ml at 4°C for about 5 min.
3. Homogenization of rat liver
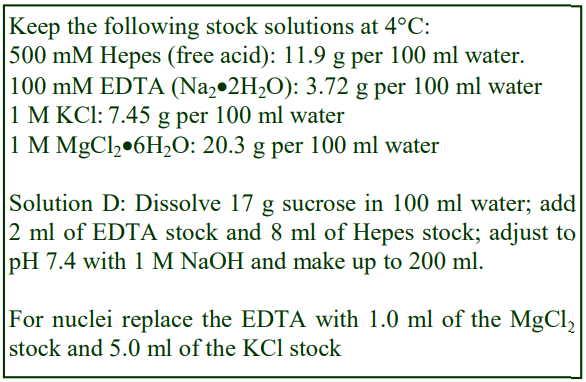
3a. Equipment and solutions required
A. Potter-Elvehjem homogenizer (30-40 ml), clearance approx 0.08 mm
B. Wall mounted, high-torque, thyristor controlled electric motor
C. Muslin or nylon mesh (75 µm pore size)
D. Homogenization medium (HM): 0.25 M sucrose, 1 mM EDTA, 20 mM HEPES-NaOH, pH 7.4; for
nuclei replace the EDTA with 5 mM MgCl2 and 25 mM KCl (see Box on p. 2)
 3b. Protocol
3b. Protocol
Keep all the equipment on ice.
1. Perfuse the liver if necessary, and then rapidly excise the tissue into ice-cold HM.
2. Transfer the liver into a 50 ml beaker (on ice) and mince with scissors, the pieces of tissue should be no more than 30 mm3.
3. For one liver (10-12 g) suspend the coarse mince in 40 ml of Solution D; stir and then decant the liquid after the mince has settled out. Repeat this process; finally suspend in 40 ml of Solution D and transfer half to the glass vessel of the homogenizer.
4. Secure the ice-cold pestle in the chuck of the electric motor and with the pestle rotating at 500-800 rpm homogenize the liver using 5-6 up-and-down strokes of the pestle. If the tissue becomes compacted at the bottom of the vessel; withdraw the pestle and allow the vortex action in the liquid to resuspend the tissue (see Notes 1-3).
5. Repeat the procedure with second half of the tissue suspension.
6. If required filter through nylon gauze or three layers of muslin to remove undisrupted cells and connective tissue. Do not force the suspension through the filter by squeezing.
4. Notes
1. This method can be used as a general-purpose homogenization procedure for the isolation of most organelles and membrane particles from most soft tissues or enzyme-digested hard tissues. See ref 4 for more information on homogenization techniques.
2. To isolate sheets of plasma membrane it may be preferable to replace the Potter-Elvehjem homogenizer with a loose-fitting Dounce homogenizer (clearance 0.1-0.3 mm) using about 10 strokes of the pestle and filter before processing further.
3. It is often advantageous to guard against possible protein hydrolysis in the homogenate by including a cocktail of protease inhibitors in HM: 1 mM phenylmethylsulphonyl fluoride (PMSF) and 2 µg/ml each of antipain, leupeptin and aprotinin.
5. References
1. Hopfer, U., Nelson, K., Perrotto, J. and Isselbacher, K. J. (1972) Glucose transport in isolated brush border membrane from rat small intestine J. Biol. Chem., 248, 25-32
2. Hubbard, A. L., Wall, D. A. and Ma, A. (1983) Isolation of rat hepatocyte plasma membranes. I. Presence of the three major domains J. Cell Biol., 96, 217-229
3. Bhattacharya, S. K., Thakar, J. H., Johnson, P. L. and Shanklin, D. R. (1991) Isolation of skeletal muscle mitochondria from hamsters using an lonic medium containing ethylenediaminetetraacetic acid and nagarse Anal. Biochem., 192, 344- 349
4. Graham, J. M. (1997) Homogenization of tissues and cells In Subcellular fractionation – a practical approach (ed Graham, J. M. and Rickwood, D,) Oxford University Press, Oxford, UK, pp 1-29
OptiPrepTM Application Sheet S05; 10th edition, January 2020
OptiPrep™ Application Sheet S06
Homogenization of mammalian cells
1. Introduction
Unlike an intact tissue such as rat liver, there are no definitive protocols for the homogenization of tissue culture cells that can be applied in all cases. The protocol depends crucially on whether the cells are grown as a monolayer or as a suspension culture. The former are much more easily disrupted than the latter. See ref 1 for a discussion of the methodology for homogenizing cultured cells. The aim of the homogenization procedure must be to produce at least 90% cell breakage, reproducibly, under the mildest conditions. Methods that employ hypoosmotic media and protracted use of homogenizers should be avoided, if at all possible. In all cases the homogenization procedure must be carried out at 4°C. For monolayer cells a very satisfactory method that uses an isoosmotic homogenization medium is based on the method of Marsh et al [2].
 2. Use of an isoosmotic medium
2. Use of an isoosmotic medium
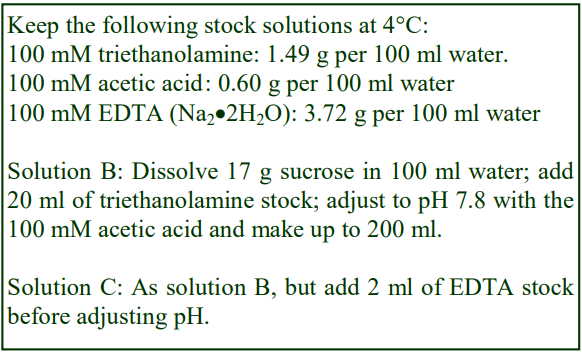
2a. Solutions required
A. Phosphate-buffered saline (PBS)
B. 0.25M sucrose, 10 mM triethanolamine-10 mM acetic acid, pH 7.8 (adjust to the correct pH with either triethanolamine or acetic acid, not HCl or NaOH)
C. Solution B containing 1 mM EDTA
2b. Protocol
1. Use a near confluent monolayer.
2. Remove the medium and rinse the monolayer at least three times with Solution A (at room temperature). Then wash the monolayer at least twice with Solution B (also at room temperature).
3. Add ice-cold Solution C to the dish (about 2 ml for a 9 cm dish) and scrape the cells into the medium with a rubber policeman. Do not try to produce a single cell suspension.
4. Transfer the crudely resuspended monolayer to a beaker on ice, washing the dish with a further 1 ml of Solution C to recover any remaining cells if necessary. Repeat the procedure for each dish.
5. If you end up with too large a volume, centrifuge the cells and resuspend the pellet in a smaller volume of Solution C. Again do not try to produce a single cell suspension.
6. Homogenize the cells using 10-25 strokes of the pestle of a tight-fitting Dounce homogenizer. Observe the suspension after 10 strokes under the phase contrast microscope. Continue homogenization until about 90% of cells have been broken.
7. The buffer is critical for the success of this method, no substitute is satisfactory.
2c. Problems
One of the major problems with cultured cells is the severity of the shearing forces required to effect efficient cell disruption. The greater the number of strokes of the pestle, the greater the possibility of causing nuclear rupture. Release of DNA, even from a few nuclei will cause severe aggregation of material: this will lead to the loss of large amounts of material into the nuclear pellet. It may therefore be advisable to add DNAase I to the homogenate to minimize this problem. Proteins from the cytoskeleton may also form a gel-like structure and cause aggregation of subcellular components. Inclusion of 10-15 mM KCl in the homogenization medium may alleviate this.
3. Alternative hypoosmotic homogenization media
Cells, which fail to homogenize in isoosmotic media, may require hypoosmotic swelling to render them susceptible to lysis by Dounce homogenization. Generally most suspension culture cells require osmotic stress. Osmotic stress involves exposing the cells to a hypoosmotic medium, normally at 4 °C for a few minutes prior to disruption by one of the liquid-shear techniques. There are many such media: 1 mM bicarbonate or any organic buffer at approx 10 mM concentration. Divalent cations Mg2+ or Ca2+ at 1-2 mM may be added to protect the nuclei against lysis but this may also have an unwanted stabilizing effect on the plasma membrane. Sometimes sufficient osmotic stress to produce lysis can be achieved by using a reduced sucrose concentration of 0.1 M. One of the most successful strategies, adapted from ref 3 is described below.
 3a. Solutions required
3a. Solutions required
A. 15mM KCl, 1.5 mM magnesium acetate, (MgOAc) 1 mM dithiothreitol (DTT), 10 mM Hepes-KOH, pH 7.5.
B. 375mM KCl, 22.5 mM MgOAc, 1mM DTT, 220 mM Hepes-KOH, pH 7.5
C. Hepes-buffered saline (HBS).
3b. Protocol
1. Wash the cells twice in Solution C to remove all traces of the culture medium.
2. Suspend the cells in 10 ml of Solution A and allow them to swell on ice for 10 min.
3. Centrifuge the cells and remove sufficient supernatant to leave a volume equivalent to 3.5x that of the cell pellet.
4. Homogenize in a tight-fitting Dounce homogenizer and then add 1/5th of the volume of Solution B.
- The ionic composition of the medium tends to avoid any „gel“ formation by cytoskeletal proteins and by homogenizing in a small volume, the organelles, which are released, are protected from hypoosmotic shock by the cytosolic proteins.
4. Other means of shear
The other principal liquid shear device, the Potter-Elvehjem homogenizer is generally less efficient than the Dounce type for cultured cells. However a third and very simple alternative for imposing a liquid shear force – repeated aspiration and ejection of a cell suspension through the narrow orifice of a syringe needle is a frequently used technique. Syringe needle gauges of 23-25G are common. Sometimes passage through a high gauge number needle is prefaced by using either a lower gauge number (larger i.d.) needle or by Dounce homogenization.
There are several commercially available devices, which can make the liquid shearing process more reproducible. In the Cell Cracker (ball-bearing homogenizer) the cell suspension is repeatedly passed (using two syringes) through the narrow annulus between a ball and a metal block. This is now regarded as one of the most reliable and gentle methods of homogenizing cultured cells (see ref 4). One source of the ball-bearing homogenizer is Isobiotec of Heidelberg, Germany. In the Stansted Cell Disruptor the cell suspension is forced, under high pressure from a piston or compressed nitrogen through a narrow orifice. The big advantage of this device is that the shear force is applied once to the entire cell suspension rather than repeatedly as in manually-operated versions.
Nitrogen cavitation involves the exposure of a stirred cell suspension to nitrogen gas at about 800 psi (5516 kPa) at 4oC for about 15 min within a stainless-steel pressure vessel. The suspension is then forced through a needle valve by the gas pressure, at which point cell rupture occurs by a combination of the sudden expansion of gas dissolved within the cytosol and the formation of bubbles of nitrogen gas in the medium. The method is successful with all types of cell. Gas equilibration parameters (time and pressure) and solution composition need to be tested to optimize the results.
5. References
1. Graham, J. M. (1997) Homogenization of tissues and cells In Subcellular fractionation – a practical approach (ed Graham, J. M. and Rickwood, D,) Oxford University Press, Oxford, UK, pp 1-29
2. Marsh, M., Schmid, S., Kern, H., Harms, E, Male, P., Mellman, I. and Helenius, A. (1987) Rapid analytical and preparative isolation of functional endosomes by free flow electrophoresis J. Cell Biol., 104, 875-886
3. Goldberg, D. E. and Kornfeld, S. (1983) Evidence for extensive subcellular organization of asparaginelinked oligosaccharide processing and lysosomal enzyme phosphorylation J. Biol. Chem., 258, 3159-3165
4. Balch, W. E. and Rothman, J. E. (1985) Characterization of protein transport between successive compartments of the Golgi apparatus: Asymmetric properties of donor and acceptor activities in a cell-free system Arch. Biochem. Biophys., 240, 413-425
OptiPrepTM Application Sheet S06; 9th edition, January 2020
OptiPrep™ Application Sheet S07
Differential centrifugation of homogenates
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
1. Background
The employment of differential centrifugation to prepare crude fractions of subcellular particles from homogenates is often a necessary first step to a subsequent purification of one or more particles on a density gradient. Buoyant density gradient purification of peroxisomes or lysosomes for example is almost invariably carried out on a light mitochondrial fraction so as to eliminate smaller particles that may have similar densities. Unless they are first removed, large rapidly sedimenting particles in homogenates may also disturb shallow gradients designed to fractionate small low density microsomes.
This Application Sheet describes the use differential centrifugation to fractionate a mammalian liver homogenate but similar methods should be applicable to all mammalian tissues and cultured cells. Refs 1-5 describe many of these procedures in more detail. Although the homogenization methods for other cells such as yeast are rather different to those for mammalian cells, the subsequent processing of the homogenate by differential centrifugation is probably rather similar. The processing of homogenates from plant tissues is rather more specialized and is not covered in this text.
 2. Homogenization medium
2. Homogenization medium
The solutions used for homogenization, washing and resuspension of the pellets, depend upon the organelle to be purified. They were developed for work with rat liver and other soft tissues and generally contain sucrose as the osmotic balancer.
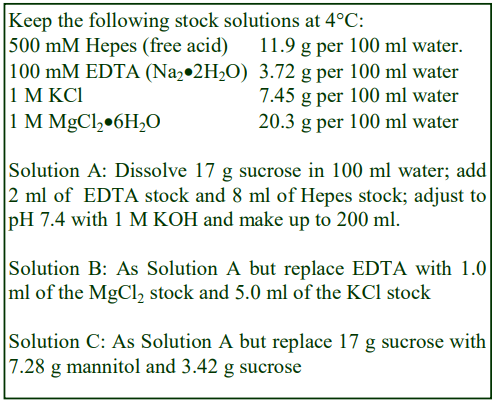
A. General Purpose: 0.25 M sucrose, 1 mM EDTA, 20 mM Hepes-KOH, pH 7.4
B. Nuclei: As General Purpose but replace 1 mM EDTA with 25 mM KCl, 5 mM MgCl2.
C. Peroxisomes: Add 0.1% (v/v) ethanol to Solution A.
D. Mitochondria: 0.2 M mannitol, 50 mM sucrose, 1 mM EDTA, 20 mM HEPES-KOH, pH 7.4.
Many cultured cells can also be homogenized in the General Purpose medium or some other similar isoosmotic medium, see OptiPrepTM Application Sheet S06.
- If the homogenization has been carried out in a hypoosmotic medium, then this should be adjusted to the recommended concentration of sucrose and other additives as soon as possible after homogenization is complete.
- It is very important to check by phase contrast microscopy that the homogenization process has been successful in breaking at least 90% of the cells before attempting to carry out any differential centrifugation.
3. Centrifugation Equipment
- To achieve the best resolution and recovery of a specific subcellular particle, a fixed-angle rotor should be used for all differential centrifugation. The shorter the sedimentation path length of the rotor, the better will be the resolution and recovery. For a full explanation of the choice of rotor see refs 6 and 7.
- After centrifugation in a fixed-angle rotor always decant the supernatant „away“ from the pellet or use a syringe and metal cannula to harvest each supernatant.
4. Protocol
Carry out all operations at 0-4 °C and all solutions should be pre-cooled on ice
1. Prepare the homogenate according to one of the methods described in OptiPrep Application Sheets S05 or S06.
2. If the nuclear pellet is to be processed, filter the homogenate through four layers of cheesecloth or fine nylon mesh (pore size 75 µm) to remove any unbroken cells and connective tissue. This filtration is not normally necessary for cultured cells.
3. Pellet the nuclear fraction by centrifugation at 1000gav for 10 min (see Notes 1-5).
4. Pellet the heavy mitochondrial fraction by centrifuging the post-nuclear supernatant at 3,000gav for 10min (see Notes 2-5).
5. Pellet the light mitochondrial fraction by centrifugation of the heavy mitochondrial supernatant at 15,000-17,000gav for 10 min (see Notes 2-5).
6. Pellet the microsomal fraction by centrifuging the light mitochondrial supernatant at 100,000gav for 45 min (see Note 5).
7. Resuspend all pellets in the appropriate medium by gentle homogenization with a loose-fitting Dounce homogenizer (approx. 0.5 mm clearance) to ensure complete dispersion of the pellets.
5. Notes
1. Centrifugation of the nuclear pellet is very often carried out in a swinging-bucket rotor rather than a fixed-angle rotor. In this case, the nuclei and debris are so large and rapidly sedimenting, compared to the other particles, that the long path length of such a rotor is not a real disadvantage.
2. To improve the recovery of more slowly-sedimenting particles and increase the purity of the differential centrifugation fractions it may be necessary to wash the pellets, in which case the resuspended pellets should be adjusted to about half of the volume of the homogenate and then recentrifuged at the same speed and time. The two supernatants are then combined prior to centrifugation at the next step.
3. Sometimes this washing is extended to three or more cycles of resuspension and recentrifugation; e.g. for the purification of mitochondria from the 3000g pellet.
4. Although the washing procedure can produce gains in recovery and/or purity of particles, it should always be a primary aim to minimize the amount of pelleting and resuspending as this causes progressive fragmentation of particles. It is also very time consuming.
5. The composition and analysis of the pellets are described in Sections 6 and 7
6. Composition of the pellets
The composition of the various fractions produced by differential centrifugation have been well defined for commonly used tissues such as mammalian liver, but for many cultured cells the distribution of the various membrane particles is rather less clear. The Nuclear Pellet contains, in addition to nuclei, mitochondria, sheets of plasma membrane (if present) and, if the homogenate has not been filtered, unbroken cells and debris (including connective tissue). Formation of this pellet is sometimes carried out at 500g rather than 1000g.
The Heavy Mitochondrial Pellet contains predominantly, mitochondria with rather few contaminants and is a common source of these organelles for respiratory studies. Minor components such as lysosomes, peroxisomes, Golgi membranes and various membrane vesicles are present largely because of entrapment during the pelleting process. Some plasma membrane fragments may also be present. These contaminants can be reduced by repeated washing.
The Light Mitochondrial Pellet contains mitochondria, lysosomes, peroxisomes, Golgi membranes and some endoplasmic reticulum. Of all differential centrifugation fractions it is the most variable in terms of the actual centrifugation parameters used: g-forces of 15-20,000g and times of 10- 20 min are the most common. Some methods are designed to maintain the Golgi membranes in their „stacked“ form so that they sediment at much lower g-forces (see ref 3 for more information)
The Microsomal Pellet is rather better defined and contains only membrane vesicles. Some of those vesicles will have been present in the cell before homogenization (e.g. endosomes, secretory vesicles and vesicles from the trans-Golgi network), others from the plasma membrane, Golgi and smooth and rough endoplasmic reticulum, will have been produced by the homogenization procedure.
7. Analysis of pellets
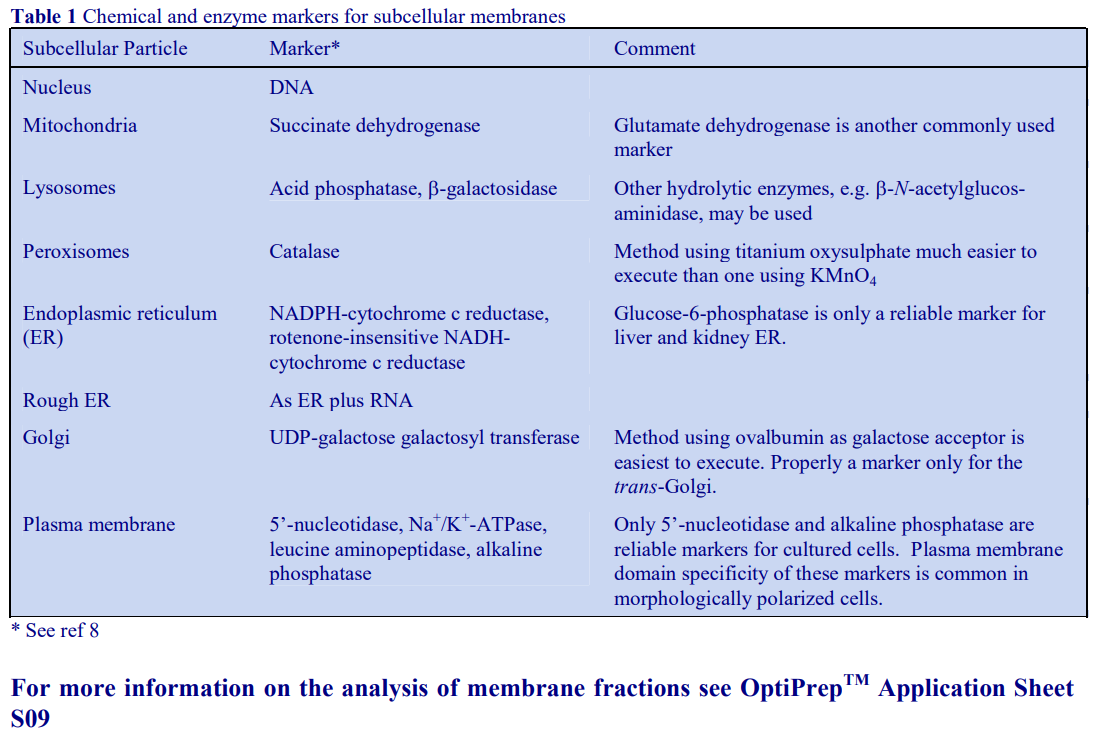
Although the operator may be interested only in processing one of the pellets, it is nevertheless important to analyze all of the pellets for chemical and enzyme markers (Table 1) and protein. This will allow determination of the recovery, not only of the particle of interest but also of contaminants, which may be difficult to remove. Analysis of the cytosolic fraction (100,000g supernatant) should always be included; this not only permits complete and valuable “book-keeping” of organelle markers, it can also give information on possible disruption to organelles and consequent release of organelle contents during the homogenization procedure.
8. References
1. Evans, W. H. (1992) Isolation and characterization of membranes and cell organelles In: Preparative Centrifugation – A Practical Approach (ed Rickwood, D.) Oxford University Press, Oxford, UK, 233-270
2. Graham, J.M. (1993) Isolation of mitochondria, mitochondrial membranes, lysosomes, peroxisomes and Golgi membranes from rat liver In: Methods in Molecular Biology 19, Biomembrane Protocols I (ed Graham, J. M. and Higgins, J. A.), Humana Press, Totowa, NJ, USA, pp 29-40
3. Graham, J. M. (1997) Homogenization of tissues and cells In: Subcellular Fractionation – a practical approach (ed Graham, J. M. and Rickwood, D.), Oxford University Press, Oxford, UK pp 1-29
4. Hinton, R. H. and Mullock, B. M. (1997) Isolation subcellular fractions In: Subcellular Fractionation – a practical approach (ed Graham, J. M. and Rickwood, D.), Oxford University Press, Oxford, UK pp 31-69
5. Graham, J.M. (2001) Fractionation of subcellular organelles In: Biological Centrifugation, Taylor and Francis Books Ltd, Oxford, UK, pp103-139
6. Graham, J.M. (2001) Principles and strategies of centrifugation In: Biological Centrifugation, Taylor and Francis Books Ltd, Oxford, UK, pp1-14
7. Graham, J.M. (2001) Centrifugation hardware In: Biological Centrifugation, Taylor and Francis Books Ltd, Oxford, UK, pp15-41
8. Graham J.M. (1993) The identification of subcellular fractions from mammalian cells In: Methods in Molecular Biology 19, Biomembrane Protocols I (ed Graham, J. M. and Higgins, J. A.), Humana Press, Totowa, NJ, USA, pp 1-18
OptiPrepTM Application Sheet S07; 10th edition January 2020
OptiPrep™ Application Sheet S08
Harvesting gradients
1. Introduction
The mode of harvesting depends very much on the type of tube used for the gradient, the distribution of particles in the gradient and the aim of the fractionation. Thick-walled tubes cannot be unloaded by any of the methods that involve piercing the tube wall with a needle and tubes with a narrow neck, such as some sealed tubes, make access with the tip of an automatic pipette impossible.
2. Tube handling prior to band or gradient recovery
The traditional open topped flexible-walled tubes for swinging-bucket rotors pose few, if any, problems for any mode of sample recovery. Heat-sealed or crimp-sealed tubes pose the biggest problems and for some modes of harvesting it may be necessary to slice off the top, to convert it to an open-topped tube.
- Do not use a scalpel blade
- Use a special tube cutter (Seton Scientific, Los Gatos, CA; sales@setonscientific.com) – the
Beckman tube slicer (Section 4f) is a possible alternative
3. Recovery of individual bands of material
If the position of the particles of interest has been clearly established and, if there is more than one band in the gradient, the linear separation of those bands is 1 cm, then the band(s) may be removed individually by aspiration.
3a. Using a Pasteur pipette or syringe (applicable to any open-topped tube)
If a syringe is used, attach it to a flat-tipped metal cannula (i.d. 0.8-1.0 mm) not to a syringe needle. Metal filling cannulas may be obtained from any surgical equipment supplies company.
- Place the tip of the pipette or cannula at the top of the band of interest and aspirate the liquid very slowly, moving it across the diameter of the tube.
- To minimise the aspiration of any liquid from below the band, the tip of a glass Pasteur pipette may be fashioned into an L-shape.
- If the band of interest is below other material in the gradient then remove the latter first.
3b. Using a syringe (flexible-walled tubes only)
It is also possible to collect a specific band within the gradient by puncturing the tube wall with a needle attached to a syringe.
- To allow easy piercing of the tube wall; the centrifuge tube is best restricted by some sort of tube clamp.
- Insert the needle just below the band and with the inlet to the needle (bevel uppermost); aspirate the band into the syringe (Figure 1).
- If a sealed tube is used, air must be allowed to displace the falling column of liquid in the tube (see Figure 1) by puncturing the tube close to its top with another syringe needle.
- Once the band has been aspirated, the syringe needle is withdrawn and the hole in the tube sealed with silicone grease.
- The procedure may be repeated to harvest a denser band.
4. Harvesting the entire gradient into a series of equal volume fractions
The volume of each fraction collected from a gradient is determined as much by the operator’s requirements as by the resolving power of the gradient. As a general rule however, the volume of each fraction should be approx 5% of the gradient volume, but this may be decreased or increased for higher or lower resolution respectively.
4a. Using a Pasteur pipette, automatic pipette or syringe (applicable to any open-topped tube)
Most Pasteur pipettes are calibrated on the stem so if the tip of the pipette or cannula (attached to a 1 or 2 ml syringe) is placed at the meniscus, the total gradient may be collected in suitably sized fractions. If an automatic pipette is used, trim the end of the tip to make the orifice diameter 0.8-1.0 mm. The method is however tedious, prone to error and difficult to obtain equal volume fractions because of the need to keep the tip of the cannula or pipette at the meniscus without occasionally aspirating some air or removing some of the gradient from below the meniscus. For a crude fractionation however into four or five gradient cuts it is quite satisfactory.
4b. Aspiration form the bottom using a peristaltic pump
Ideally the harvesting system should be devised so that the effluent from the tube should not have to pass through a pump, but as long as the dead space volume of the tubing is small compared to the volume of the gradient it is permissible to insert a narrow rigid tube to the bottom of the centrifuge tube and to aspirate the contents (dense-end first). Theoretically, mixing will occur in the vertical section of the collection tubing as the decreasingly dense medium enters the bottom of the tube. In practice however this seems not to be a serious problem, again as long as the enclosed volume of the collecting tube is small compared to that of the gradient.
- If there is a pellet, make sure that the tip of collecting tube is maintained above it.
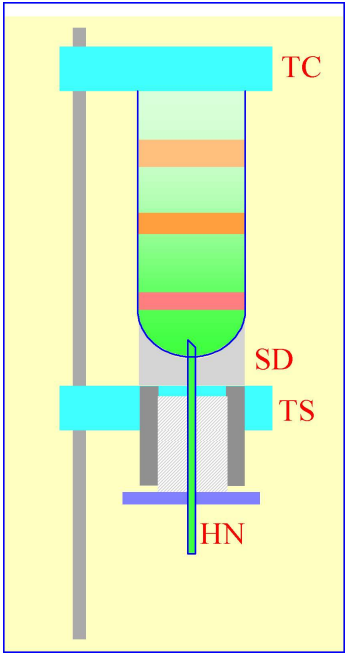 Figure 2: Gradient collection (dense-end first) by tube puncture. The tube is clamped between the sealing disc (SD) and the tube support (TS). A hollow needle (HN) is advanced through the bottom of the tube, sometimes by a screw-device (shown by the hatched area) or, more commonly by a pivoted lever.
Figure 2: Gradient collection (dense-end first) by tube puncture. The tube is clamped between the sealing disc (SD) and the tube support (TS). A hollow needle (HN) is advanced through the bottom of the tube, sometimes by a screw-device (shown by the hatched area) or, more commonly by a pivoted lever.
4c. Tube puncture
Practically this is best achieved by securing the tube vertically in some form of clamping device and to advance the needle through a rubber seal into the bottom of the tube by a screw or lever mechanism (Figure 2). The Beckman-Coulter Fraction Recovery System incorporates such a device. If sealed tubes are used, then either the central plug should be removed (Optiseal™) or the top punctured with a syringe needle (Quick-Seal™) to allow air to displace the liquid, which exits the tube under gravity. The system is simple and the dead space of the collecting tube is very small and the gradient is collected almost ideally, the hemispherical section of the bottom of the tube directing banded material into the collecting needle.
Because of the viscosity of the dense end of some gradients, gravitational flow will be slow at first and then speed up as the viscosity of the liquid decreases. To overcome this, the effluent from the hollow needle can be passed through a small volume peristaltic pump. So long as the dead space of the silicone tubing is small compared to the volume of the gradient, resolution is not seriously sacrificed.
- Thick-walled tubes cannot be used and it may not be a useful method if there is a large pellet, which may obstruct the hollow needle.
- Collecting equal volume fractions by this method or that described in Section 4b is not easy. The low-tech answer is to use calibrated collection tubes, although this requires continual attention from the operator to move on the delivery tube at the appropriate time. This problem is discussed further in Section 4h.
4d. Upwards displacement
A dense liquid introduced to the bottom of the tube can displace the entire gradient upwards and with a suitable device attached to the top of the tube, the gradient can be delivered into the collection tubes.
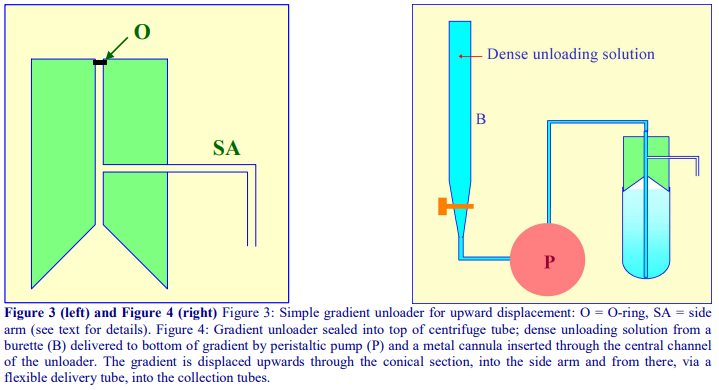
4d-1. Delivery of dense liquid through a central tube inserted into the gradient
A simple device fashioned from a cylindrical block of Perspex (Lucite or acrylic) shown in Figure 3 can be produced by any laboratory workshop. To fit flexible-walled tubes the cylinder should be slightly tapered towards the bottom (not shown in figure). The block contains a central channel, which leads to a hollowed-out cone, and a side-arm, which connects with the central channel. The dense unloading solution is introduced to the bottom via a long metal cannula inserted down the central channel and through the gradient (Figure 4). The gradient is displaced upwards by the incoming dense liquid into the cone and an O-ring around the cannula diverts the flow into the collection tubes via the side-arm.
- For rigid-walled open-topped tubes the collecting device requires sealing on to the tube with a gasket, under pressure. Such a device can indeed be used for any type of tube and one is incorporated as one of the options in the Beckman-Coulter Fraction Recovery System.
- By placing the dense unloading solution in a burette and delivering it to the bottom of the centrifuge tube via a peristaltic pump (Figure 4), the unloading process can be executed at a uniform flow rate
- By using the graduations on the burette to signal the manual advancement of the delivery tube to the next collection tube, it is the only method that guarantees equal volume fractions.
- The gradient could alternatively be collected using an automated fraction collector (see Sections 4g and 4h).
- The best unloading medium is a low viscosity, dense, non-water-miscible, fluorocarbon such as perfluorodecalin (p = 1.9 g/ml). This was previously commercially available from Axis-Shield and its distributors as Maxidens. Perfluorodecalin can currently be purchased from F2 Chemicals Ltd, Lea Lane, Lea Town, Preston PR4 0RZ, UK (tel: +44 (0)1772 775802, fax +44 (0)1772 775808); contact Helen McNamee (helen.mcnamee@f2chemicals.com). Also available from the same company is a similar fluorocarbon containing a blue dye (Flutec-blue), which makes visual assessment of the progress of gradient unloading very easy.
- The rate of gradient unloading should be 1-2 ml/min for 10-20 ml gradients and 0.5-1.0 ml/min
for smaller volume gradients.
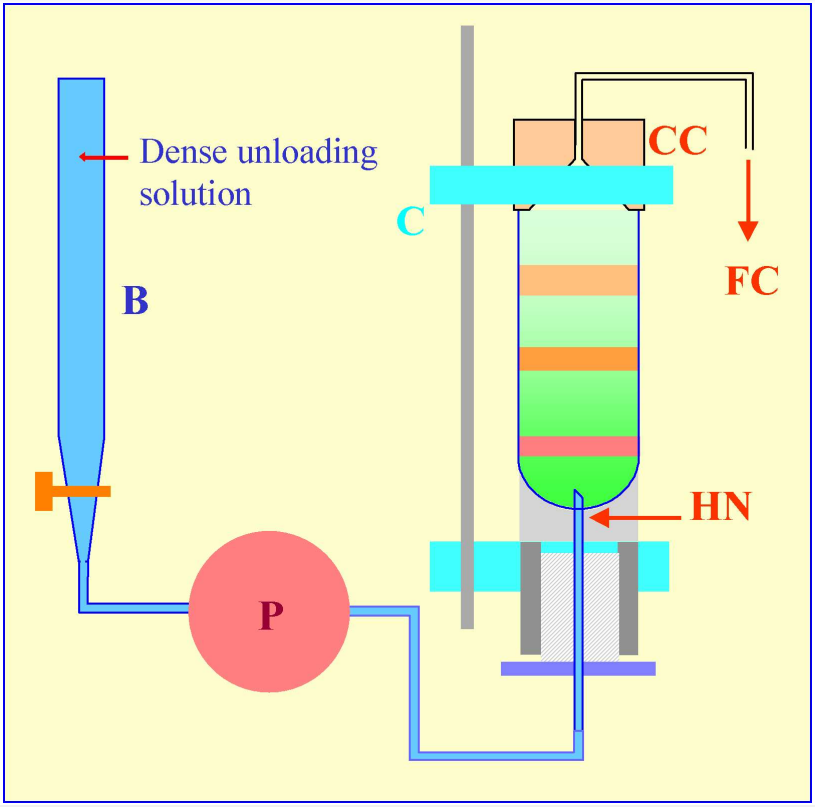 Figure 5: Gradient harvesting by upward displacement with a dense medium delivered by tube puncture. The hollow needle (HN) is completely filled with the dense unloading solution from the burette (B) using the peristaltic pump (P) before the tube is located within the clamping device of a Beckman-Coulter Fraction Recovery System. The conical collection head (CC) is located sealed on to the tube, and the tube held vertically, by the clamp (C). When the pump (P) is reactivated after puncturing the tube, the dense unloading solution displaces the gradient upwards through the conical collection head (CC) and into the fraction collection tubes (FC).
Figure 5: Gradient harvesting by upward displacement with a dense medium delivered by tube puncture. The hollow needle (HN) is completely filled with the dense unloading solution from the burette (B) using the peristaltic pump (P) before the tube is located within the clamping device of a Beckman-Coulter Fraction Recovery System. The conical collection head (CC) is located sealed on to the tube, and the tube held vertically, by the clamp (C). When the pump (P) is reactivated after puncturing the tube, the dense unloading solution displaces the gradient upwards through the conical collection head (CC) and into the fraction collection tubes (FC).
4d-2. Delivery of dense liquid by tube puncture
An alternative mode of delivering the dense unloading solution to the bottom of the tube is by tube puncture. In this case the burette is attached via the pump to the lower end of the hollow needle (Figure 5), which must be primed with the dense solution, prior to tube puncture. The hollow needle (HN) of the Beckman-Coulter Fraction Recovery System has an important design feature – the exit port is on the vertical side of the needle, thus its sharp point is solid. This not only facilitates tube puncture, fragments of tube material removed by the puncturing process or any pellet in the tube, are much less likely to impede the flow of the dense unloading solution than if the exit port was tip-located, as in a standard syringe needle.
4e. Automatic aspiration from the meniscus
The Auto Densi-Flow™, produced by the Labconco Corporation comprises a hollow metal tube that terminates in a small collection head (Figures 6 and 7); the upper end of the tube is connected to a peristaltic pump, which aspirates the gradient. The motor, which is activated when the electronic probe (mounted at the side of the collection head) is in a non-conductive medium (air), advances the collection head towards the gradient until the tip of the probe reaches the meniscus of the gradient (Figure 7, 1 and 2). Now the tip of the probe is in an aqueous conductive medium, the motor stops and the gradient starts to be aspirated by the pump and the meniscus falls (Figure 7, 3). The motor is consequently re-activated as the meniscus recedes from the probe and the collection head advances further downwards until again the probe reaches the meniscus (Figure 7,4) and so on. For clarity, the procedure has been described and shown in Figure 7 in an exaggerated step-like manner. In reality, the aspiration of the gradient and the steady advance of the collection head occur almost simultaneously. In this way the entire gradient is collected in a smooth and continuous fashion.
- Note that the collection head of this device also provides an excellent means of depositing a continuous gradient, dense end first, from a two-chamber gradient maker. In this mode the motor moves the collection head upwards; the sequential activation and deactivation of the motor by the rising meniscus being the reverse of the collection mode.
- IMPORTANT NOTE: although this device is no longer produced by Labconco, many remain available in laboratories and second hand machines are available from instrument “recycling” companies.
4f. Biocomp Instruments piston fractionator
Rather different to the other types of fractionator, the Biocomp piston fractionator comprises a piston containing a central channel, which at its lower end expands outwards in the form of a curved conical section. As the piston advances down the tube, the gradient is displaced upwards into the central channel. The progressively decreasing volume of the conical section experienced by the displaced liquid effectively increases the linear separation of particles in the gradient and so maximises resolution. The device is available in conjunction with a detection system (and the Biocomp Gradient Master™ gradient former. The device is only suitable for open-topped tubes and tubes of different diameters require their own piston.
4g. Integrated automatic gradient harvesting process
In the modern system illustrated in Figure 8, the Labconco Auto Densi-flow gradient unloader is being used to harvest the gradient (from the meniscus) from a standard tube for a swinging-bucket rotor. The effluent from the peristaltic pump on top of the Auto Densi-flow is directed to the collection head of a Gilson FC205 fraction collector for dispensing into a 96-well polypropylene “MasterBlock Deep-Well” plate (Greiner Bio-One Inc). These MasterBlocks can easily accommodate volumes of up to 2.0 ml. The multi-well plate format for gradient collection allows simple gradient analysis if the gradient fractions are subsequently sampled using a multiple channel automatic pipette (see below); it also provides an easy means of storage. A standard 96-well plate can replace the largevolume MasterBlock for the collection of smaller gradient volumes.
 Any gradient unloader that incorporates a peristaltic pump to maintain a reasonably consistent
Any gradient unloader that incorporates a peristaltic pump to maintain a reasonably consistent
flow rate can be linked up to fraction collector, but note that as the density of the liquid progressively changes
so do other physical parameters such as viscosity and surface tension. Drop size will thus vary during the collection process and fraction volumes will change progressively during a drop-counting collection process. So whether the fraction advance is signaled by drop number or time, there will be a progressive change in fraction volume whose severity depends on the densityrange of the gradient. Nevertheless if this change is acceptable, it will at least be reproducible from gradient to gradient. This fraction collection system has been used very successfully for analysis of lipoprotein banding in self-generated iodixanol gradients [1,2].
4h. Influence of tube type on harvesting strategy
- Open topped thin walled tubes (polyallomer, polycarbonate or Beckman Ultraclear™) for swinging-bucket or fixed-angle rotors can be unloaded by any of the above methods.
- Thick-walled open-top tubes can be unloaded by any of the methods except tube puncture (Sections 4c and 4d-1b) or slicing (Section 4f).
- Thick-walled tubes (screw-capped) with a wide shoulder are best unloaded from the meniscus (Section 4e) using the Labconco Auto Densi-flow™ or aspiration from the bottom (Section 4b).
- Upward displacement (Section 4d-1) may be satisfactory if the shoulder is narrow, sloped or rounded, otherwise material may get trapped at the shoulder.
- Heat-sealed or crimp-sealed tubes cannot be unloaded directly by any of the methods except tube puncture (Section 4c). Any other method of unloading requires the tube to be cut horizontally just below the shoulder (see Section 2). This is might cause disturbance to the gradient unless carried out very carefully.
- Sealed tubes that are sealed by a central plastic plug (e.g. Beckman Optiseal™ tubes) can be unloaded by any of the methods. Note however that upward displacement is best carried out using the Section 4d-2 option with a length of Teflon tubing secured to the neck of the tube by a silicone rubber collar to carry the gradient effluent to the collection tubes. Note also that the neck of some of the smaller volume sealed tubes may be too narrow to accept the collection head of the Labconco Auto Densi-flow machine (Section 4e).
OptiPrepTM Application Sheet S08; 6th edition, January 2020
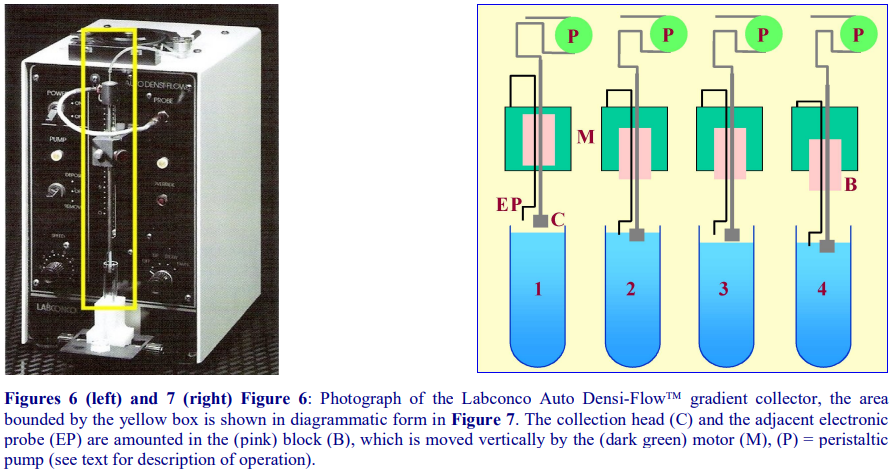
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
1. Density determination
Once gradients have been fractionated, it is often important that the density of each fraction is measured accurately. The most direct method is to weigh accurately known volumes of liquid using a pycnometer; however, this is very time consuming and it is more convenient to determine the density of a fraction by measuring the refractive index, which has the added advantage of requiring as little as 20-50 µl of sample. The simple linear relationship between refractive index (η) and the density (ρ) is ρ= Aη – B. The refractive index of gradient solutions is increased by the presence of other solutes (e.g. sucrose and NaCl), thus the values of the two constants A and B vary with the presence and concentration of the solute.
For tables relating % (w/v) concentration of iodixanol, density and refractive index of solutions used for the fractionation of subcellular membranes see Application Sheets S01and S02.
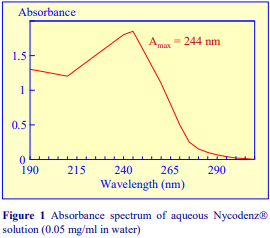 If a refractometer is not available then an alternative method of determining the density of gradient fractions is to measure the absorbance (optical density) of the fractions. All iodinated density gradient media absorb strongly in the UV (see Figure 1). If the absorbance is measured at approx 244 nm (the absorbance maximum for Nycodenz® and iodixanol) the gradient samples will need to be diluted 1:10,000 with water to get an absorbance value that can be measured accurately. Table 1 gives a few values for iodixanol solutions, measured in a standard 1 cm path length quartz cell in a single beam spectrophotometer. The need to dilute the solution also means that any other potentially interfering material will be diluted out at the same time.
If a refractometer is not available then an alternative method of determining the density of gradient fractions is to measure the absorbance (optical density) of the fractions. All iodinated density gradient media absorb strongly in the UV (see Figure 1). If the absorbance is measured at approx 244 nm (the absorbance maximum for Nycodenz® and iodixanol) the gradient samples will need to be diluted 1:10,000 with water to get an absorbance value that can be measured accurately. Table 1 gives a few values for iodixanol solutions, measured in a standard 1 cm path length quartz cell in a single beam spectrophotometer. The need to dilute the solution also means that any other potentially interfering material will be diluted out at the same time.
Alternatively, if the absorbance is measured at a higher wavelength, dilution is not required. Table 2 gives a few absorbance values for Nycodenz® solutions at 350 nm and 360 nm. Care must be taken to use the correct blank to ensure that other components in the gradient fractions that absorb at, or near these wavelengths do not interfere with the measurement of the gradient medium.

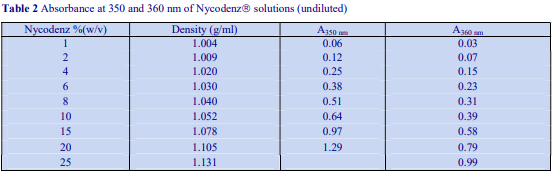
1a Absorbance measurements using a Multi-well Plate Reader
The wide availability of Multi-well Plate Readers which routinely have the facility for measurement of absorbance at 340 nm considerably simplify the measurement of absorbance on gradient fractions, particularly if the gradient has already been collected in a multi-well plate. Multiple-channel automatic pipettes also facilitate the transfer of liquid aliquots between plates.
1. Transfer 100 μl of each of the fractions into 100 μl of water in the wells of a second plate.
2. Complete the transfer and mixing by three repeated aspirations into and expulsions from the
pipette tips.
3. Measure the absorbance of the solutions in each well in a standard plate reader using a 340 nm
filter, against a suitable blank.
- For iodixanol concentrations above 35% (w/v), it may be necessary to make a second dilution of the solutions (again 100 μl into 100 μl of water) to avoid absorbance values above 1.2.
- Six different types of multi-well plate have been tested for their suitability. A flat-bottomed 96- well polystyrene plate, which has the lowest background absorbance of any plate tested (approx 0.130 at 340 nm), is available from Greiner BioOne Inc (Cat. # 655101). The inter-well variability of the absorbance was also one of the lowest of all those tested (± 0.007).
Absorbance values of a range of iodixanol solutions produce by dilution of OptiPrep™ with either saline or 0.25 M sucrose are given in Tables 3 and 4 respectively. The absorbance measurements were made against saline and 0.25 M sucrose blanks, which accounts for the slight distortion of the measured values of samples diluted with sucrose.
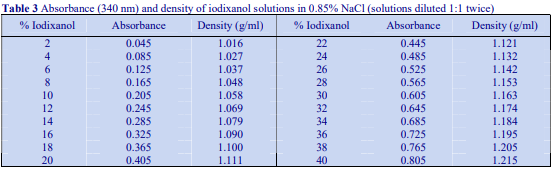
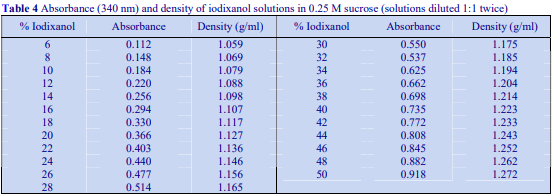
2. Particle detection
Although the quantitative distribution of cells through a gradient can be determined by using a haemocytometer or an electronic particle counter, turbidometric analysis is a more general method used for all types of light-scattering particles. Particulate matter can be detected and semi-quantified by light-scattering measurements at 500-600 nm, while particles containing macromolecules bearing porphyrin prosthetic groups (e.g. haem groups) can be monitored by Soret band absorbance at 400-420 nm.
3. Nucleic acids, proteins and polysaccharides
Although solutions of iodinated media absorb strongly in the ultraviolet region of the spectrum, as their absorbance maximum is different to that of proteins and nucleic acids, it may be possible in some cases, through use of the correct blank (i.e. from a blank gradient unloaded in exactly the same manner as the test gradient) to determine their distribution spectrophotometrically. Normally however, nucleic acids, proteins and polysaccharides are assayed spectrophotometrically by chemical methods (Table 5 and ref 2). Unlike metrizamide, neither Nycodenz® nor iodixanol contain a sugar residue, therefore they do not interfere with the orcinol or diphenylamine reactions for the estimation of the ribose and deoxyribose of RNA and DNA respectively [3]; polysaccharides and sugars can be determined using the phenol/H2SO4 assay [4]. Sensitive dye binding assays for protein [5,6] and DNA [7] are also unaffected by the presence of the gradient media. Protein assays based on Coomassie blue give the most reliable data. The Folin Ciocalteu reagent [8] however cannot be carried out unless the concentration of Nycodenz® or iodixanol is less than 5% (w/v): this situation however can often be attained if the final assay volume is 1-2 ml and the volume of gradient fraction used is 50 µl. Even at higher concentrations of gradient medium, an appropriate correction can be made to produce a linear relationship between protein concentration and absorbance (Table 6 gives an example). In addition to these spectrophotometric methods, fluorimetric assays of nucleic acids [9,10] and proteins [11] can also be carried out in the presence of Nycodenz® or iodixanol. Many of these protocols are listed in ref 12.
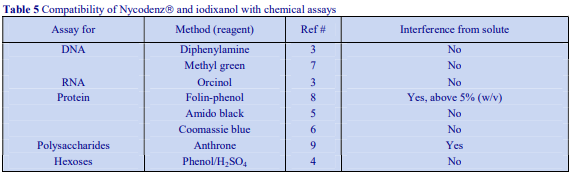

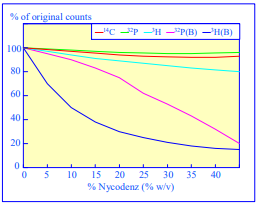

4. Enzymes
So long as the concentration of a subcellular membrane in a gradient fraction is sufficiently high, then many standard marker enzyme assays can be performed in the presence of either Nycodenz® or iodixanol [13]. Little or no inhibition is observed with these media (Table 7). This is in contrast to Percoll™, which because of its light scattering properties must be removed prior to spectrophoto-metric enzyme analysis. If the membrane does require concentration, then this can be done efficiently either by sedimentation or by ultrafiltration (see Section 7). pect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
5. Radioactivity assays
Analysis of gradients material may either involve the radiolabeling of the material prior to fractionation or the use of radiolabeled reagents in functional assays. Nycodenz® and iodixanol quench 3H, 32P and 14C to an extent that is dependent on the energy of the emission, although, as shown in Figure 2, the degree of quenching is also dependent upon the scintillant used. Toluene-based scintillant, containing 4.0 g 2,5-diphenyloxazole (PPO) and 0.05 g 1,4-bis-2(5-phenyloxazolyl) benzene, (POPOP) per litre and mixed with one half its volume of Triton X-100 is quite resistant to quenching, while Brays scintillant is much less suitable. The extent of quenching may be minimized by diluting the samples prior to counting, or it can be eliminated completely by acid precipitating the material in the gradient fractions and counting each precipitate after collection on filters and drying.
6. Electrophoresis
SDS-polyacrylamide and agarose gel electrophoresis can be carried out directly on gradient samples, as long as the concentration of protein or nucleic acid in the gradient fractions is sufficiently high for analysis. If the protein for example requires concentration, neither Nycodenz nor iodixanol interfere with TCA precipitation.
7. Removal of gradient medium and concentration of particles
It may be necessary to remove either Nycodenz® or iodixanol from the gradient fractions either to concentrate the particles or if the medium does interfere with some add-on process. Subcellular membranes and organelles can be pelleted from fractions after dilution with 1-2 volumes of a lowdensity buffer such as a buffered salt or sucrose solution. Particles should be sedimented at either a slightly higher RCF and/or longer centrifugation time than that used to pellet the particles from the low-density solution itself, to take account of the slightly raised density and viscosity caused by the presence of the gradient medium. RCFs in excess of 150,000g should be avoided for iodixanolcontaining solutions; otherwise there may be some sedimentation of the solute molecule itself. Removal of iodixanol and Nycodenz from gradient samples containing can also be achieved by filtration through microcentrifuge ultrafiltration cones such as those manufactured by Whatman (Vectaspin®) or Millipore (Amicon® Ultra 4). Successful use of two other membrane devices has been reported in the literature – Vivaspin membranes from Sartorius and Centricon Plus 70 centrifugal filters from Millipore. Alternatives are dialysis in large-pore size tubing or in a GeBAflex dialysis tube (Gene Bio Applications (GeBA) Ltd.). The latter are certainly more convenient than dialysis tubing for small volumes, the tubes are available with 0.25, 0.8 and 3.0 ml capacities and MWt cutoffs up to 14,000. Passage down a column of Sephadex G25 is another possibility.
8. References
1. Schroeder, M., Schafer, R. and Friedl, P. (1997) Spectrophotometric determination of iodixanol in subcellular fractions of mammalian cells Anal. Biochem., 244, 174-176
2. Rickwood, D., Ford, T. and Graham, J. (1982) Nycodenz: A new nonionic iodinated gradient medium Anal. Biochem., 123, 23-31
3. Schneider, W.C. (1957) Determination of nucleic acids in tissues by pentose analysis Meth. Enzymol., 3, 680-684
4. Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.E. and Smith, F. (1956) Colorimetric method for determination of sugars and related substances Anal. Chem., 28, 350-356
5. Schaffner, W. and Weissman, C. (1973) A rapid, sensitive, and specific method for the determination of protein in dilute solution Anal. Biochem., 56, 502-510
6. Bradford, M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding Anal. Biochem., 72, 248-254
7. Peters, D.L. and Dahmus, M.E. (1979) A method of DNA quantitation for localization of DNA in metrizamide gradients Anal. Biochem., 93, 306-311
8. Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with the Folin phenol reagent J. Biol. Chem., 193, 265-275
9. Fong, J., Schaffer, F.L. and Kirk, P.K. (1953) The ultramicrodetermination of glycogen in liver. A comparison of the anthrone and reducing-sugar methods Arch. Biochem. Biophys., 45, 319-326
10. Karsten, U. and Wollenberger, A. (1977) Improvements in the ethidium bromide method for direct fluorometric estimation of DNA and RNA in cell and tissue homogenates Anal. Biochem., 77, 464-469
11. Bohlen, P., Stein, S., Dairman, W. and Udenfriend, S. (1973) Fluorometric assay of proteins in the nanogram range Arch. Biochem. Biophys., 155, 213-220
12. Ford, T. and Graham, J.M. (1983) Enzymatic and chemical assays compatible with iodinated density gradient media In: Iodinated Density Gradient Media – a practical approach (ed D. Rickwood) IRL Press at Oxford University Press, Oxford, UK, pp 195 216
13. Ford, T., Graham, J. and Rickwood, D. (1994) Iodixanol: a non-ionic iso-osmotic centrifugation medium for the formation of self-generated gradients Anal. Biochem., 220, 360-366
OptiPrepTM Application Sheet S09; 6th edition, January 2020
OptiPrep™ Application Sheet S10a
Purification of nuclei from tissues and cells in iodixanol gradients – a methodological review
- Application Sheet S10a provides a summary of some of the methodological variations in the isolation of nuclei from the published literature: for full experimental details of the most commonly used method see Application Sheet S10.
1. Sucrose-barrier and detergent methods
The low-speed pellet produced by the centrifugation of tissues or cell homogenates at 600-1000 g for 10 min will contain 90-95% of the total nuclei, but it will be contaminated by significant amounts of mitochondria, large fragments of membrane, intact or partially broken cells and other organelles trapped by these rapidly sedimenting particles. Although the contamination may be partially reduced by repeated washing of the pellet, this procedure risks progressive damage to the nuclei by the shearing forces used to disperse the pellet. In the nineteen-sixties two influential papers [1,2] were published both of which reported the pelleting of nuclei through high-density sucrose solutions to remove the contamination effectively. In the method of Widnell and Tata [1] a crude nuclear pellet (homogenate centrifuged at 600 g for 10 min) was washed once in the homogenization medium; suspended in 2.2 M sucrose, 1 mM MgCl2, 10 mM Tris-HCl, pH 7.4 and then the nuclei pelleted at 60-80,000 g for 80 min at 4°C. The method of Blobel and Potter [2] omitted the low-speed pelleting of the nuclei directly from the homogenate: the sucrose concentration in the filtered homogenate was adjusted to 1.6 M by addition of 2.3 M sucrose, 50 mM Tris-HCl, pH 7.5, 25 mM KCl, 5 mM MgCl₂, and then layered over this 2.3 M sucrose solution. The nuclei were pelleted at 130,000 g for 30 min at 4°C.
The major disadvantages of these high-density sucrose barriers are:
- Difficulty in preparing and handling high concentration sucrose solutions that are close to saturation.
- The high viscosity of the solutions requires the use of an ultracentrifuge to pellet the nuclei.
- Solutions are grossly hyperosmotic (approx. 2,400 mOsm; the cytosol is approx. 290 mOsm). Nuclei lose
water and become highly condensed; loss of bound water from nucleoproteins may affect their stability.
2. Homogenization media
2a. Mammalian tissues
One aspect of the sucrose barrier methods that has been retained in many subsequent techniques is the nature of the homogenization medium; approximately isoosmotic solutions containing K+ and Mg2+ ions generally promote the retention of nuclear structure and function during their isolation. The marginally hyperosmotic Blobel and Potter medium, 0.25 M sucrose containing 25 mM KCl, 5 mM MgCl₂ buffered originally with 50 mM Tris-HCl, pH 7.4-7.8 [2], is widely used for rodent liver with some variation in the buffer, which has often been changed to the more organelle-friendly HEPES-KOH or Tricine-KOH and whose concentration is frequently reduced to 10 or 20 mM [3-9]. Provost et al [4] added 1 mM EDTA to the medium and Pyhtila et al [7] further added 1 mM DTT. The Blobel and Potter medium is also used for rat and sheep kidney [10-13], rodent brain [9,14,15], spleen [6], thymus [16] and testis [6,16]. In the case of frozen human brain tissue specimens the medium is supplemented with Brij [17,18]. Occasionally the medium is a generalpurpose buffered 0.25 M sucrose solution containing EDTA [19] or it may be supplemented with Ca2+, for example 0.25 M sucrose, 20 mM HEPES-KOH, pH 7.4, 200 μM CaCl₂ [20].
More rarely, the medium is significantly hyperosmotic: frozen hamster and squirrel livers have been homogenized in 25 mM KCl, 1 mM EDTA, 10 mM HEPES-KOH, pH 7.6 containing 40% glycerol [21,22]. Spermine and spermidine were also included to inhibit nitric oxide synthase. Hypoosmotic solutions are more often used for cultured cells, but they are occasionally used for tissues: rat brain tissue has been homogenized in 25 mM KCl, 2 mM MgCl₂, 10 mM HEPES-KOH, pH 7.5 [23] and spleen in 20 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1mM DTT, 1% NP40 [24].
2b. Cultured mammalian cells
Although there are examples of the use a standard, more or less isoosmotic, Blobel and Potter medium for carcinoma cells [25-27], hepatoma cells [28], epithelial cells [29], fibroblasts [30-32] and smooth muscle cells [33], efficient homogenization of cultured cells may require reductions in overall solution osmolality and/or divalent cation concentration and/or inclusion of chelating agent(s). Sometimes low concentrations of a nonionic detergent, to which nuclei are more resistant than the plasma membrane, are included in the medium. Some of the solutions are given in Table 1. Note that a diverse range of protease inhibitors is routinely included in all of these media
2c. Invertebrates and plants
Mussel tissue is homogenized in a HEPES-buffered Blobel and Potter medium supplemented with 1 mM EDTA [58-60]. A commonly used solution for Nicotiana tabacum BY2 protoplasts and Arabidopsis thaliana seedlings is 0.4 M sucrose, 10 mM MES, pH 5.3, 10 mM NaCl, 5 mM MgCl₂, 5 mM EDTA, 0.1 mM DTT [61-63], which for Arabidopsis was supplemented with 0.1% TX-100 [63].
3. OptiPrep methodology
3a. Discontinuous gradient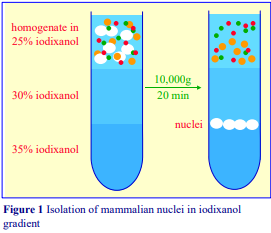 The original methodology devised by Graham et al [3] was specifically aimed at streamlining the purification procedure, thus, as with the Blobel and Potter method [2], it is not necessary to produce an initial crude nuclear fraction from the homogenate by lowspeed centrifugation. Instead the homogenate is simply adjusted to 25% (w/v) iodixanol by mixing with an equal volume 50% (w/v) iodixanol containing the same concentrations of buffer, KCl and MgCl₂ as the homogenate, and layered on top of a discontinuous iodixanol gradient as shown in Figure 1. The separation requires only a routine high-speed centrifuge, rather than an ultracentrifuge and a centrifugation time of only 20 min. A saving of at least 1 h in preparation time can be achieved over the standard sucrose gradient method.
The original methodology devised by Graham et al [3] was specifically aimed at streamlining the purification procedure, thus, as with the Blobel and Potter method [2], it is not necessary to produce an initial crude nuclear fraction from the homogenate by lowspeed centrifugation. Instead the homogenate is simply adjusted to 25% (w/v) iodixanol by mixing with an equal volume 50% (w/v) iodixanol containing the same concentrations of buffer, KCl and MgCl₂ as the homogenate, and layered on top of a discontinuous iodixanol gradient as shown in Figure 1. The separation requires only a routine high-speed centrifuge, rather than an ultracentrifuge and a centrifugation time of only 20 min. A saving of at least 1 h in preparation time can be achieved over the standard sucrose gradient method.
- Importantly the gradients are approx. isoosmotic throughout and have a low viscosity
A detailed methodology is described in the OptiPrep™ Application Sheet S10; this can be accessed via the following website: www.Optiprep.com (click on “Methodology”, then “Subcellular Membranes” and follow the links from the Index). The gradient solutions are best prepared as described in the Application Sheet, i.e. a 50% (w/v) iodixanol working solution is first prepared from 5 vol. of OptiPrep™ and 1 vol. of 150 mM KCl, 30 mM MgCl₂, 120 mM Tricine-KOH, pH 7.8 and any lower density solutions are made by dilution of the working solution with 0.25 M sucrose, 25 mM KCl, 5 mM MgCl₂, 20 mM Tricine-KOH, pH 7.8. Thus the concentrations of buffer, KCl and MgCl₂ and the osmotic pressure are approximately constant throughout the gradient.
3b. Discontinuous gradient – protocol variations
(1) If the cells have been homogenized in a hypoosmotic medium, very often the nuclei first are pelleted by low speed centrifugation and then resuspended in buffered 25% (w/v) iodixanol, 25 mM KCl, 5 mM MgCl₂. To avoid pelleting the nuclei prior to the gradient the homogenate has first been centrifuged at 300 g for 15 min over a 40% iodixanol cushion [42,63]; in the case of Drosophila and Caenorhabditis elegans the cushion was pure OptiPrep [64]
(2) Some gradient variations result in pelleting the nuclei rather than banding them at an interface by omission of the 35% (w/v) iodixanol layer; for example in refs 41,65, the homogenate was adjusted to 25% iodixanol and layered over 29% iodixanol.
(3) There are also some instances in which the gradient is altered, but the banding of the nuclei above a 35% (w/v) iodixanol layer is usually retained (but note #7), for example:
1. Homogenate layered over 12.5% and 35% (w/v) iodixanol [28]
2. 10%, 20% (nuclear fraction), 25%, 30% and 35% (w/v) iodixanol [10-13]
3. Homogenate layered over 30% and 35% (w/v) iodixanol [52,66,67]
4. Homogenate adjusted to 17.5% (w/v) iodixanol [20]
5. Nuclear pellet in buffered 0.32 M sucrose, layered over 25%, 30% and 35% iodixanol [55]
6. Nuclear pellet in buffered 0.25 M sucrose, layered over 15%, 20% and 35% iodixanol [31]
7. Plant nuclei were resolved by sedimenting at a 15%/45% iodixanol interface [68]
The centrifugation conditions generally deviate relatively little from the recommended g-force and time, with a few notable exceptions. Plant nuclei tend to be banded under more mild conditions, e.g. 3000 g for 30 min [61] and 1,500 g for 15 min [68]; lower g-forces (4,500 g for 30 min) have also been used for macrophage [66,67] and hepatoma [28] nuclei. Significantly higher g-forces are only rarely used; nuclei from mussel tissue and murine brain were banded at 100,000 g for 2 h [58-60] and 270,000 g for 3 h [20] respectively.
3c. Density barriers
A simpler variation is to layer either the homogenate or a crude nuclear fraction over a density barrier (usually 29-30% (w/v) iodixanol), through which the nuclei pellet when centrifuged at approx. 10,000 g for 10- 40 min; this approach has been used with brain tissue [69,70] and maize coleoptile [71]. An even more simple option is to adjust the sample to 20-30% (w/v) iodixanol, from which the nuclei sediment while the other organelles remain in suspension (12,000 g for 4 min and 10,000 g for 10 min have been used). This approach has been used for carcinoma cells [41,72,73], HEK [73] and ovarian cells [74] and the separations, described in detail by Guilluy et al [75], are often repeated. Palmowski et al [76] described a similar system for brain nuclei in 20% (w/v) iodixanol and centrifuged for a longer time of 40 min. A more unusual variant is to pellet endothelial cell nuclei through a 6% (w/v) iodixanol barrier at 20,000 g for just 30 sec [77].
- In a study of the uptake of plasmid DNA into the nucleus Cohen et al [78] compared the widely-used detergent method for solubilizing all non-nuclear membranes and the OptiPrep™ method and found that the latter “yielded nuclei with substantially less adhering plasmids on the outside of the nuclei”
4. References
1. Widnell, C.C. and Tata, J.R. (1964) A procedure for the isolation of enzymically active rat-liver nuclei Biochem. J. 92, 313-317
2. Blobel, G. and Potter, V.R. (1966) Nuclei from rat liver: Isolation method that combines purity with high yield Science 154: 1662–1665
3. Graham, J., Ford, T. and Rickwood, D (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
4. Provost, J.J., Fudge, J., Israelit, S., Siddiqi, A.R. and Exton, J.H. (1996) Tissue-specific distribution and subcellular distribution of phospholipase D in rat: evidence for distinct RhoA- and ADP-ribosylation factor (ARF)-regulated isoenzymes Biochem. J., 319, 285-291
5. Hofer, T. and Moller, L. (2002) Optimization of the workup procedure for the analysis of 8-oxo-7, 8-dihydro-2’-deoxyguanosine with electrochemical detection Chem. Res. Toxicol., 15, 426-432
6. Yamamoto, Y., Jones, K.A., Mak, B.C., Muehlenbachs, A. and Yeung, R.S. (2002) Multicompartmental distribution of tuberous sclerosis gene products, hamartin and tuberin Arch. Biochem. Biophys., 404, 210-
217
7. Pyhtila, B., Zheng, T., Lager, P.J., Keene, J.D., Reedy, M.C. and Nicchita, C.V. (2008) Signal sequenceand translation-independent mRNA localization to the endoplasmic reticulum RNA, 14, 445-453
8. Anderson, D.D. and Stover, P.J. (2009) SHMT1 and SHMT2 are functionally redundant in nuclear de novo thymidylate biosynthesis PLoS One, 4:e5839
9. Caro, P., Gómez, J., Arduini, A., González-Sánchez, M., González-García, M., Borrás, C., Viña, J., Puertas, M.J., Sastre, J. and Barja, G. (2010) Mitochondrial DNA sequences are present inside nuclear DNA in rat tissues and increase with age Mitochondrion 10, 479–486
10. Pendergrass, K.D., Averill, D.B., Ferrario, C.M., Diz, D.I. and Chappell, M.C. (2006) Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2.Lewis rat Am. J. Physiol. Renal Physiol., 290, F1497-F1506
11. Gwathmey, T.M., Pendergrass, K.D., Pirro, N.T., Shaltout, H.A., Reid, S.D., Rose, J.C. and Chappell, M.C. (2009) Nuclear AT2 receptors mediate angiotensin II-dependent generation of nitric oxide FASEB J., 23, Abstr. 606.9
12. Gwathmey, T.M., Shaltout, H.A., Pendergrass, K.D., Pirro, N.T., Figueroa, J.P., Rose, J.C., Diz, D.I. and Chappell, M.C. (2009) Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production Am. J. Physiol. Renal Physiol., 296, F1484–F1493
13. Gwathmey, T.M., Pendergrass, K.D., Reid, S.D., Rose, J.C., Diz, D.I. and Chappell, M.C. (2010) Angiotensin-(1-7)–angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus Hypertension, 55, 166-171
14. German, D.C., Ng, M.C., Liang, C.L., McMahon, A and Iacopino, A.M. (1997) Calbindin-D28k in nerve cell nuclei Neuroscience, 81, 735-743
15. Merritt, S.E., Mata, M., Nihalani, D., Zhu, C., Hu, X. and Holzman, L.B. (1999) The mixed lineage kinase DLK utilizes MKK7 and not MKK4 as substrate J. Biol. Chem., 274, 10195-10202
16. Ookawara, T., Kizaki, T., Takayama, E., Imazeki, N., Matsubara, O., Ikeda, Y., Suzuki, K., Ji, L.L., Tadakuma, T., Taniguchi, N. and Ohno, H. (2002) Nuclear translocation of extracellular superoxide dismutase Biochem. Biophys. Res. Commun., 296, 54-61
17. Iuchi, S., Hoffner, G., Verbeke, P., Djian, P. and Green, H. (2003) Oligomeric and polymeric aggregates formed by proteins containing expanded polyglutamine Proc. Natl. Acad. Sci. USA, 100, 2409-2414 (2003)
18. Hoffner, G., Island, M-L. and Djian, P. (2005) Purification of neuronal inclusions of patients with Huntington’s disease reveals a broad range of N-terminal fragments of expanded huntingtin and insoluble polymers J. Neurochem., 95, 125-136
19. Zhou, W., Zhang, Y., Hosch, M.S., Lang, A., Zwacka, R.M. and Engelhardt, J.F. (2001) Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion Hepatology, 33, 902-914
20. Clemen, C.S., Herr, C., Hövelmeyer, N. and Noegel, A.A. (2003) The lack of annexin A7 affects functions of primary astrocytes Exp. Cell Res., 291, 406-414
21. Van Breukelen, F. and Martin, S.L. (2002) Reversible depression of transcription during hibernation J. Comp. Physiol. B., 172, 355-361
22. Diaz, M.B., Lange, M., Heldmaier, G. and Klingenspor, M. (2004) Depression of transcription and translation during daily torpor in the Djungarian hamster (Phodopus sungorus) J. Comp. Physiol. B, 174, 495-502
23. Kumar, V., Jong, Y-J.I. and O’Malley, K.L. (2008) Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ release J. Biol. Chem., 283, 14072-14083
24. Thornburg, N.J., Kulwichit, W., Edwards, R.H., Shair, K.H.Y., Bendt, K.M. and Raab-Traub, N. (2006) LMP1 signaling and activation of NF-B in LMP1 transgenic mice Oncogene, 25, 288-297
25. He, D. and Falany, C.N. (2006) Characterization of proline-serine-rich carboxyl terminus in human sulfotransferase 2B1b: immunogenicity, subcellular localization, kinetic properties, and phosphorylation Drug. Metab. Dispos., 34, 1749-1755
26. Falany, C.N., He, D., Dumas, N., Frost, A.R. and Falany, J.L. (2006) Human cytosolic sulfotransferase 2B1: Isoform expression, tissue specificity and subcellular localization J. Steroid Biochem. Mol. Biol., 102, 214-221
27. Dumas, N.A., He, D., Frost, A.R. and Falany, C.N. (2008) Sulfotransferase 2B1b in human breast: Differences in subcellular localization in African American and Caucasian women J. Steroid Biochem Mol. Biol., 111, 171-177
28. Barthelson, R.A., Lambert, G.M., Vanier, C., Lynch, R.M. and Galbraith, D.W. (2007) Comparison of the contributions of the nuclear and cytoplasmic compartments to global gene expression in human cells BMC Genom., 8:340
29. Garcia-Mata, R., Dubash, A.D., Sharek, L., Carr, H.S., Frost, J.A. and Burridge, K. (2007) The nuclear RhoA exchange factor Net1 interacts with proteins of the Dlg family, affects their localization and influences their tumor suppressor activity Mol. Cell. Biol., 27, 8683-8697
30. Naranatt, P.P., Krishnan, H.H., Smith, M.S. and Chandran, B. (2005) Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus J. Virol., 79, 1191-1206
31. Krishnan, H.H., Sharma-Walia, N., Streblow, D.N., Naranatt, P.P. and Chandran, B. (2006) Focal adhesion kinase is critical for entry of Kaposi’s sarcoma-associated herpesvirus into target cells J. Virol., 80, 1167-1180
32. Gaboreanu, A-M., Hrstka, R., Xu, W., Shy, M., Kamholz, J., Lilien, J. and Balsamo, J. (2007) Myelin protein zero/P0 phosphorylation and function require an adaptor protein linking it to RACK1 and PKC J. Cell. Biol., 177, 707-716
33. Lucero, H.A., Kintsurashvili, E., Marketou, M.E. and Gavras, H. (2010) Cell signaling, internalization, and nuclear localization of the angiotensin converting enzyme in smooth muscle and endothelial cells J. Biol. Chem., 285, 5555-5568
34. Barta, C.A., Sachs-Barrable, K., Feng, F. and Wasan, K.M. (2008) Effects of monoglycerides on Pglycoprotein: modulation of the activity and expression in Caco-2 cell monolayers Mol. Pharmaceut., 5, 863-875
35. Thornburg, N.J., Pathmanathan, R. and Raab-Traub, N. (2003) Activation of nuclear factor-B p50 homodimer/Bcl-3 complexes in nasapharyngeal carcinoma Cancer. Res., 63, 8293-8301
36. Morrison, J.A., Gulley, M.L., Pathmanathan, R. and Raab-Traub, N. (2004) Differential signaling pathways are activated in the Epstein-Barr virus-activated malignancies nasopharyngeal carcinoma and Hodgkin lymphoma Cancer. Res.,64, 5251-5260
37. Morrison, T.E. and Kenney, S.C. (2004) BZLF1, an Epstein-Barr virus immediate-early protein, induces p65 nuclear translocation while inhibiting p65 transcriptional function Virology, 328, 219-232
38. Thornburg, N.J. and Raab-Traub, N. (2007) Induction of epidermal growth factor receptor expression by Epstein-Barr virus latent membrane protein 1 C-terminal-activating region 1 is mediated by NF-B p50 homodimer/Bcl-3 complexes J. Virol., 81, 12954-12961
39. Kung, C-P., and Raab-Traub, N. (2008) Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor through effects on Bcl-3 and STAT3 J. Virol., 82, 5486-5493
40. Kung, C-P. and Raab-Traub, N. (2010) Epstein-Barr virus latent membrane protein 1 modulates distinctive NF-B pathways through C-terminus-activating region 1 to regulate epidermal growth factor receptor expression J. Virol., 84, 6605-6614
41. Zippin, J.H., Farrell, J., Huron, D., Kamenetsky, M., Hess, K.C., Fischman, D.A., Levin, L.R. and Buck, J. (2004) Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear camp microdomain J. Cell Biol., 164, 527-534
42. Remillard-Labrosse, G., Guay, G. and Lippe, R. (2011) Reconstitution of Herpes Simplex virus type 1 nuclear capsid egress in vitro J. Virol., 80, 9741-9753
43. Iordanskiy, S.N. and Bukrinsky, M.I. (2009) Analysis of viral and cellular proteins in HIV-1 reverse transcription complexes by co-immunoprecipitation In: HIV Protocols 2nd edition, Methods Mol. Biol. 485 (eds. Prasad, V.R. and Kalpana, G.V.), Humana Press, Totowa, NJ pp 121-134 44. Duxin J.P., Dao, B., Martinsson, P., Rajala, N., Guittat, L., Campbell, J.L., Spelbrink, J.N. and Stewart, S.A. (2009) Human Dna2 is a nuclear and mitochondrial DNA maintenance protein Mol. Cell. Biol., 29, 4274-4282
45. Valenzuela, S.M., Martin, D.K., Por, S.B., Robbins, J.M., Warton, K., Bootcov, M.R., Schofield, P.R., Campbell, T.J. and Breit, S.N. (1997) Molecular cloning and expression of a chloride ion channel of cell nuclei J. Biol. Chem., 272, 12575-12582
46. Sinzger, C., Kahl, M., Laib, K., Klingel, K., Rieger, P., Plachter, B. and Jahn, G. (2000) Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus J. Gen. Virol., 81, 3021-3035
47. Qin, Z-H., Wang, Y., Kikly, K.K., Sapp, E., Kegel, K.B., Aronin, N. and DiFiglia, M. (2001) Pro-caspase8 is pre-dominantly localized in mitochondria and released into cytoplasm upon apoptotic stimulation J. Biol. Chem., 276, 8079-8086
48. Kegel, K., Meloni, A.R., Yi, Y., Kim, Y.J., Doyle, E., Cuiffo, B.G., Sapp, E., Wang, Y., Qin, Z-H. et al (2002) Huntingtin is present in the nucleus, interacts with the transcriptional corepressor C-terminal binding protein, and represses transcription J. Biol. Chem., 277, 7466-7476
49. Montgomery, S.A. and Johnston, R.E. (2007) Nuclear import and export of Venezuelan equine encephalitis virus nonstructural protein 2 J. Virol., 81, 10268-10279
50. Cooper, H.M. and. Spelbrink, J.N. (2008) The human SIRT3 protein deacetylase is exclusively mitochondrial Biochem. J., 411, 279-285
51. Morrison, J.A., Klingelhutz, A.J. and Raab-Traub, N. (2003) Epstein-Barr virus latent membrane protein 2A activates -catenin signaling in epithelial cells J. Virol., 77, 12276-12284
52. Morrison, J.A. and Raab-Traub, N. (2005) Roles of the ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of -catenin signaling J. Virol., 79, 2375-2382
53. Siler, C.A. and Raab-Traub, N. (2008) Rhesus lymphocryptovirus latent membrane protein 2A activates βcatenin signaling and inhibits differentiation in epithelial cells Virology, 377, 273-279
54. Tomlinson, C.C. and Damania, B. (2004) The K1 protein of Kaposi’s sarcoma-associated herpesvirus activates the Akt signaling pathway J. Virol., 78, 1918-1927
55. Thornburg, N.J., Kusano, S. and Raab-Traub, N. (2005) Identification of Epstein-Barr virus RK-BARF0- interacting proteins and characterization of expression pattern J. Virol., 78, 12848-12856 (2004)
56. Kegel, K.B., Kim, M., Sapp, E., McIntyre, C., Castano, J.G., Aronin, N. and DiFiglia, M. (2000) Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation and autophagy J. Neurosci., 20, 7268-7278
57. Kleene, R., Mzoughi, M., Joshi, G., Kalus, I., Bormann, U., Schulze, C., Xiao, M-F., Dityatev, A. and Schachner, M. (2010) NCAM-induced neurite outgrowth depends on binding of calmodulin to NCAM and on nuclear import of NCAM and fak fragments J. Neurosci., 30, 10784 –10798
58. Shaw, J.P., Large, A.T., Chipman, J.K., Livingstone, D.R. and Peters, L.D. (2000) Seasonal variation in mussel Mytilus edulis digestive gland cytochrome P4501A- and 2E-immunoidentified protein levels and DNA strand breaks (Comet assay) Marine Environ. Res., 50, 405-409
59. Shaw, J.P., Large, A.T., Livingstone, D.R., Doyotte, A., Renger, J., Chipman, J.K. and Peters, L.D. (2002) Elevation of cytochrome P450-immunopositive protein and DNA damage in mussels (Mytilus edulis) transplanted to a contaminated site Marine Environ. Res., 54, 505-509
60. Shaw, J.P., Large, A.T., Donkin, P., Evans, S.V., Staff, F.J., Livingstone, D.R., Chipman, J.K. and Peters, L.D. (2004) Seasonal varation in cytochrome P450 immunopositive protein levels, lipid peroxidation and genetic toxicity in digestive gland of the mussel Mytilus edulis Aquatic Tox., 67, 325-336
61. Xiong, T.C., Jauneau, A., Ranjeva, R. and Mazars, C. (2004) Isolated plant nuclei as mechanical and thermal sensors involved in calcium signaling Plant J., 40, 12-21
62. Lannoo, N., Peumans, W.J., Van Pamel, E., Alvarez, R., Xiong, T-C., Hause, G., Mazars, C. and Van Damme, E.J.M. (2006) Localization and in vitro binding studies suggest that the cytoplasmic/nuclear tobacco lectin can interact in situ with high-mannose and complex N-glyc FEBS Lett., 580, 6329-6337
63. Rémillard-Labrosse, G. and Lippé, R. (2011) In vitro nuclear egress of herpes simplex virus type 1 capsids Methods 55, 153–159
64. Steiner, F.A., Talbert, P.B., Kasinathan, S., Deal, R.B. and Henikoff, S. (2012) Cell-type-specific nuclei purification from whole animals for genome-wide expression and chromatin profiling Genome Res., 22:766–777
65. Jordi, E., Heiman, M., Marion-Poll, L., Guermonprez, P., Cheng, S.K., Nairn, A.C. Greengard, P. and Giraulta, J-A. (2013) Differential effects of cocaine on histone post-translational modifications in identified populations of striatal neurons Proc. Natl. Acad. Sci. USA, 110, 9511-9516
66. Bowick, G.C., Fennewald, S.M., Scott, E.P., Zhang, L-H., Elsom, B.L., Aronosn, J.F., Spratt, H.M., Luxon, B.A., Gorenstein, D.G. and Herzog, N.K. (2007) Identification of differentially activated cellsignaling networks associated with Pichinde virus pathogenesis by using systems kinomics J. Virol., 81, 1923-1933
67. Bowick, G.C., Spratt, H.M., Hogg, A.E., Endsley, J.J., Wiktorowicz, J.E., Kurosky, A., Luxon, B.A., Gorenstein, D.G. and Herzog, N.K. (2009) Analysis of the differential host cell nuclear proteome induced by attenuated and virulent hemorrhagic arenavirus infection J. Virol., 83, 687-700
68. Liu, Z., Zhu, Y., Gao, J., Yu, F., Dong, A. and Shen, W-H. (2009) Molecular and reverse genetic characterization of nucleosome assembly protein1 (NAP1) genes unravels their function in transcription and nucleotide excision repair in Arabidopsis thaliana Plant J., 59, 27–38
69. Grindberg, R.V., Yee-Greenbaum, J.L., McConnell, M.J., Novotny, M., O’Shaughnessy, A.L., Lambert, G.M., Araúzo-Bravo, M.J., Lee, J. et al (2013) RNA-sequencing from single nuclei Proc. Natl. Acad. Sci. USA, 110, 19802–19807
70. Mellén, M., Ayata, P., Dewell, S., Kriaucionis, S. and Heintz, N. (2012) MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system Cell 151, 1417–1430
71. Regulski, M., Lu, Z., Kendall, J., Donoghue, M.T.A., Reinders, J., Llaca, V., Deschamps, S., Smith, A., Levy, D. et al (2013) The maize methylome influences mRNA splice sites and reveals widespread paramutation-like switches guided by small RNA Genome Res., 23, 1651-1662
72. Huff, L.P., DeCristo, M.J., Trembath, D., Kuan, P.F., Yim, M., Liu, J., Cook, D.R., Miller, R., Der, C.J. and Cox, A.D. (2013) The role of Ect2 nuclear RhoGEF activity in ovarian cancer cell transformation Genes Cancer, 4, 460-475 Dubash, A.D., Guilluy, C., Srougi, M.C., Boulter, E., Burridge, K. and García-Mata, R. (2011) The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals PLoS One, 6: e17380
74. Chen, H., Guo, J.H., Lu, Y.C., Ding, G.L., Yu, M.K., Tsang, L.L., Fok, K.L., Liu, X.M., Zhang, X.H., Chung, Y.W., Huang, P., Huang, H. and Chan, H.C. (2012) Impaired CFTR-dependent amplification of FSH-stimulated estrogen production in cystic fibrosis and PCOS J. Clin. Endocrinol. Metab., 97, 923–932
75. Guilluy, C., Dubash, A.D. and García-Mata, R. (2011) Analysis of RhoA and Rho GEF activity in whole cells and the cell nucleus Nat. Protocols 6, 2050-2060
76. Palmowski, P., Rogowska-Wrzesinska, A., Williamson, J., Beck, H.C., Mikkelsen, J.D., Hansen, H.H. and Jensen, O.N. (2014) Acute phencyclidine treatment induces extensive and distinct protein phosphorylation in rat frontal cortex J. Proteome Res., 13,1578-1592
77. Hahn, A.S. and Desrosiers, R.C. (2013) Rhesus monkey rhadinovirus uses Eph family receptors for entry into B cells and endothelial cells but not fibroblasts PLoS Pathog., 9: e1003360
78. Cohen, R.N., van der Aa, M.A.E.M., Macaraeg, N., Lee, A.P. and Szoka, F.C. (2009) Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection J. Control. Release 135 (2009) 166–174
OptiPrepTM Application Sheet S10a 7th edition, January 2020
OptiPrep Application Sheet S10
Isolation of nuclei from animal and plant sources and cultured cells
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water: density = 1.32 g/ml
- OptiPrep™ Application Sheet S10a “Purification of nuclei from tissues and cells in isoosmotic iodixanol gradients – a methodological review” lists many of the protocol variations
- OptiPrep™ Reference List RS01 “Purification of nuclei from tissues and cells in iodixanol gradients” lists all the published papers. The methodology has been adapted to various plant and invertebrate sources
- To access RS01 return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- This Application Sheet describes a widely used discontinuous iodixanol gradient but simpler density barrier methods have also been used (see Section 4)
1. Background
Earlier methods for purifying nuclei involve pelleting through a 60% sucrose density barrier at 100,000 g for 1-2 h. Not only is the sedimentation of the particles very slow because of the high viscosity of the sucrose barrier, the nuclei become severely dehydrated because of loss of water from their internal space due to the osmotic gradient across the membrane. This process may disrupt the macromolecular structures, which are normally highly hydrated. In 1984 the introduction of Nycodenz®, whose solutions have a much lower viscosity and osmolality than those of sucrose, permitted for the first time, the banding of nuclei at an interface using only 13,000 g for 1 h. A commonly used method is to layer the crude nuclei in 35% Nycodenz® between layers of 20% and 40%, with a 50% cushion; the nuclei sediment to the 40%/50% Nycodenz® interface (the 40% and 50% Nycodenz® solutions are not however isoosmotic). The method, originally worked out for mouse liver [1-3] has been used for gut mucosa [3], HeLa cells [4], ovarian cells [5] and testicular cells [6,7]. Using iodixanol gradients nuclei can now be isolated by isopycnic banding in an iso-osmotic environment [8]. Because the nuclei retain their normal hydration their density is much lower than that in sucrose (1.20-1.22 against >1.32 g/ml) and slightly lower than in Nycodenz®. Buoyant density banding in iodixanol thus requires only 10,000 g for 20 min. The homogenate is adjusted to approx ρ = 1.14 g/ml (25% iodixanol); layered over two solutions of 30% and 35% iodixanol (ρ = 1.175 g/ml and 1.20 g/ml) and centrifuged at 10,000g to band the nuclei at the lower interface. This protocol, designed for mammalian liver, has been applied to many tissue types and to cultured cells; only in a few cases were small modifications required. It has also been applied to plant tissue (see Section 3).
2. Mammalian tissues and cultured cells
2a. Solutions required (see Section 2c, Note 1)
A. OptiPrep™
B. Diluent: 150 mM KCl, 30 mM MgCl₂, 120 mM Tricine-KOH, pH 7.8
C. Working solution containing 50% (w/v) iodixanol: mix 5 vol. of solution A with 1 vol. of solution B
D. Homogenization Medium: 0.25M Sucrose, 25 mM KCl, 5 mM MgCl₂, 20 mM Tricine-KOH, pH 7.8
E. Gradient solutions: Prepare two gradient solutions of 30% and 35% (w/v) iodixanol by diluting solution C with solution D (6 vol. + 4 vol. and 7 vol. + 3 vol. respectively)
2b. Protocol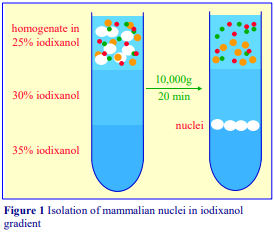
Carry out all operations at 0-4°C
1. Produce an homogenate of the tissue or cell using solution D and use this for Step 2. Alternatively produce a crude nuclear pellet by centrifugation at 1000 g for 10 min; resuspend the pellet in solution D and use this for Step 2.
2. Mix equal volumes of the sample (homogenate or resuspended nuclear pellet) and Solution C and transfer 10-15 ml to a suitable centrifuge tube (40-50 ml) for a swinging-bucket rotor of a high-speed centrifuge (see Section 2c, Note 2).
3. Underlayer the sample with 10 ml of the 30% iodixanol and 5-10 ml of the 35% iodixanol; for more information on creating discontinuous gradients see Application Sheet S03.
4. Centrifuge at 10,000 gav for 20 min (see Section 2c, Notes 3-5).
5. Collect the band of nuclei at the 30%-35% iodixanol interface (see Figure 1).
- The 30-35% interface material contains >90% of the total DNA. Phase contrast microscopy shows no discernible contaminants and 95% of the succinate dehydrogenase is recovered in the top layer.
2c. Notes
1. Protease inhibitors (PMSF, leupeptin, antipain, aprotinin etc) may be included in any or all of the media at the operator’s discretion. The preparation of a working solution of 40% or 50% (w/v) iodixanol as described ensures that the ionic concentration is constant through the gradient. If this is considered unnecessary, the gradient solutions may be prepared simply from OptiPrep™.
2. With a crude nuclear (1000 g) pellet, the protocol can be simplified by resuspending the pellet in homogenization medium and adjusting it to 30% iodixanol. After centrifugation the nuclei will form a pellet and contaminating membranes will float to the top. If this approach is used with an homogenate, the pellet of nuclei will be tend to be contaminated by peroxisomes.
3. The rapid sedimentation rate of nuclei, compared to that of other particles present, ensures that only the nuclei are able to sediment through the 30% iodixanol layer at the time and rotor speed used. Other particles remain in the sample or at the sample/ 30% iodixanol interface (see Figure 1)
4. With mammalian liver, the protocol can be carried out at g-forces as low as 5000 g (for 20 min) without any significant reduction in recovery of nuclei.
5. The density and/or the rate of sedimentation of nuclei from other tissues and from cultured mammalian cells may be different to those from mammalian liver. However, the described protocol seems to have a quite wide application; according to the published papers rather few modifications have been required for the satisfactory purification of nuclei from a range of cells and tissues. It may however be necessary to modulate either the centrifugation time or the density of the layers. Some variations are:
- CHO cell nuclei banded at a 25%/30% interface, centrifugation time 40 min [9].
- Crude nuclear pellet suspended in median 30% iodixanol layer, HeLa [10] and Caco2 [11]
cells - Invertebrates: 15-20% (w/v) iodixanol continuous gradient, 100,000g for 2h [12-14]
- Mouse liver: 12,000 g for 2 h [15]
- Squirrel/hamster liver: 0-35% (w/v) iodixanol continuous gradient [16,17]
3. Plant protoplasts
3a. Background
Xiong et al [18] and Lannoo et al [19] used a slightly modified iodixanol gradient to prepare nuclei from cultured BY2 tobacco cells, but the method almost certainly has a wider application to any lysed plant protoplast preparation. This Application Sheet is not primarily concerned with a detailed description of the method for protoplast preparation, which will probably vary from source to source. A method for the purification of protoplasts from green leaf tissue by flotation through a low-density iodixanol barrier is given in Application Sheet C19. The protocol for protoplast preparation from BY2 cells is clearly detailed by Xiong et al [18]. Once the plant cells had been digested in a mixture of pectolyase, cellulose and driselase and washed, they were isolated by flotation through a 20%, 10%, 0% (w/v) Ficoll 400 gradient.
3b. Solutions required (see Section 3d, Notes 1-2)
A. OptiPrep™
B. Lysis buffer: 0.4 M sucrose, 10 mM NaCl, 5 mM MgCl₂, 0.1 mM dithiothreitol (DTT), 5 mM EDTA, 10 mM MES-KOH, pH 5.3
C. OptiPrep™ diluent: 30 mM NaCl, 15 mM MgCl₂, 0.3 mM dithiothreitol (DTT), 15 mM EDTA, 30 mM MES-KOH, pH 5.3
D. Iodixanol (40% w/v) Working Solution (WS): mix 4 vol. of OptiPrep™ with 2 vol. of Solution C
3c. Protocol (adapted from ref 18)
1. Wash the protoplasts in Solution B using 160 g for 5 min to pellet the protoplasts; suspend in Solution B and disrupt by eight passages through a 26G syringe needle.
2. Prepare solutions of 10%, 25%, 30% and 36% (w/v) iodixanol by diluting Solution D with Solution B (1:3, 2.5:1.5, 3:1 and 3.6:0.4 v/v respectively).
3. Prepare a discontinuous iodixanol gradient from 2 ml of each gradient solution; for more information on preparing gradients see Application Sheet S03 (see Notes 3-6).
4. Layer the lysed protoplast suspension on top (2×106 protoplasts per 8 ml gradient) and centrifuge the gradients at 3000 g for 30 min. Harvest the nuclei from the 30%/36% iodixanol interface.
3d. Notes
1. Protease inhibitors may be included in any or all of the media at the operator’s discretion. The preparation of a working solution of 40% or 50% (w/v) iodixanol as described ensures that the concentration of ions, EDTA etc is constant through the gradient.
2. Because of the variable hydration of MES powder, use of a commercial MES stock is preferred.
3. Nuclei from wheat-germ have been isolated using 0.4 M sucrose, 25 mM KCl, 5 mM MgCl₂, 10 mM MES, pH 6.2 as a lysis medium [20]. Gradient solutions of 1.167 and 1.234 g/ml were used (OptiPrep™: lysis medium of 8.5:11.5 and 13.5:6.5 respectively). The osmolality of these solutions is 480-500 mOsm. A crude nuclear suspension (20 ml) was layered over 5 ml each of the gradient solutions. After centrifugation at 5,600 g for 30 min the nuclei band at the lower interface, other organelles at the top interface and the starch granules pellet.
4. As with animal tissues and cells, the density of the gradient layers (and the centrifugation conditions) may also require modulation to optimize the purification for specific types of plant tissue. Nuclei have been isolated from Sorghum bicolor leaves (in this study the authors preferred iodixanol to Percoll®) [21] and cow-pea leaves [22]. Preliminary indications suggest that for yeast nuclei it is necessary to increase the density of the bottom layer to at least 1.26 g/ml.
5. For Xanthi protoplasts a simplified gradient of 25% and 36% (w/v) iodixanol was used [23] under the same centrifugation conditions.
6. Liu et al [24] separated a cytoplasmic and nuclear fraction from Arabidopsis thaliana by loading an homogenate on top of a two layer gradient of 15% and 45% (w/v) iodixanol. Using 1,500 g for 15 min resolved a cytoplasmic fraction (sample zone) and purified nuclei at the interface of the two iodixanol solutions.
4. Density barrier methods
There are a few examples of the use of a simple density barrier, first reported for HEK cells [25] in which a crude pelleted nuclear fraction was suspended in 30% (w/v) iodixanol and centrifuged at 10,000 g for 10 min; subsequently the pellet was resuspended in the same medium and the centrifugation repeated. The method has been applied with minor variations) to neural progenitor cells [26] carcinoma cells [27] and Xenopus embryo [28]. In the latter case the g-force used was only 1000 g. Rat brain nuclei have been pelleted through a 20% iodixanol barrier [29] and those from endothelial cells and fibroblasts have been pelleted through 6% iodixanol at 20,000 g for 30 sec [30].
5. References
1. Graham, J., Ford, T. and Rickwood, D. (1990) Isolation of the major subcellular organelles from mouse liver using Nycodenz gradients without use of an ultracentrifuge Anal. Biochem., 187, 318-323
2. Quattrochi, L.C., Mills, A.S., Barwick, J.L., Yockey, C.B. and Guzelian, P.S. (1995) A novel cis-acting element in a liver cytochrome P450 3A gene confers synergistic induction by glucocoticoids plus antiglucocorticoids J. Biol. Chem., 270, 28917-28923
3. Carraway, R.E., Mitra, S.P. and Cochrane, D.E. (2000) Pro-xenopsin(s) in vesicles of mammalian brain, liver, stomach and intestine is apparently released into blood and cerebral spinal fluid Regul. Pept., 95, 115-124
4. Ladner, R.D., McNulty, D.E., Carr, S.A., Roberts, G.D. and Caradonna, S.J. (1996) Characterization of distinct nuclear and mitochondrial forms of human deoxyuridine triphosphate nucleotidohydrolase J. Biol. Chem., 271, 7745-7751
5. Hiscock, D.R., Yanagishita, M. and Hascall, V.C. (1994) Nuclear localization of glycosaminoglycans in rat ovarian granulose cells J. Biol. Chem., 269, 4539-4546
6. Bläuer, M., Husgafvel, S., Syvälä, H., Tuohimaa, P. And Ylikomi, T. (1999) Identification of a nuclear localization signal in activin/inhibin A subunit; intranuclear A in rat spermatogenic cells Biol. Reprod., 60, 588-593
7. Furland, N.E., Zanetti, S.R., Oresti, G.M., Maldonado, E.N. and Aveldano, M.L. (2007) Ceramides and sphingomyelins with high proportions of very long-chain polyunsaturated fatty acids in mammalian germ cells J. Biol. Chem., 282, 18141-18150
8. Graham, J., Ford, T. and Rickwood, D. (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
9. Valenzuela, S. M., Martin, D. K., Por, S.B., Robbins, J. M., Warton, K., Bootcov, M. R., Schofield, P. R., Campbell, T. J. and Breit, S. N. (1997) Molecular cloning and expression of a chloride ion channel of cell nuclei J. Biol. Chem., 272, 12575-12582
10. Zippin, J.H., Farrell, J., Huron, D., Kamenetsky, M., Hess, K.C., Fischman, D.A., Levin, L.R. and Buck, J. (2004) Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear camp microdomain J. Cell Biol., 164, 527-534
11. Barta, C.A., Sachs-Barrable, K., Feng, F. and Wasan, K.M. (2008) Effects of monoglycerides on Pglycoprotein: modulation of the activity and expression in Caco-2 cell monolayers Mol. Pharmaceut., 5, 863-875
12. Shaw, J.P., Large, A.T., Chipman, J.K., Livingstone, D.R. and Peters, L.D. (2000) Seasonal variation in mussel Mytilus edulis digestive gland cytochrome P4501A- and 2E-immunoidentified protein levels and DNA strand breaks (Comet assay) Marine Environ. Res., 50, 405-409
13. Shaw, J.P., Large, A.T., Livingstone, D.R., Doyotte, A., Renger, J., Chipman, J.K. and Peters, L.D. (2002) Elevation of cytochrome P450-immunopositive protein and DNA damage in mussels (Mytilus edulis) transplanted to a contaminated site Marine Environ. Res., 54, 505-509
14. Shaw, J.P., Large, A.T., Donkin, P., Evans, S.V., Staff, F.J., Livingstone, D.R., Chipman, J.K. and Peters, L.D. (2004) Seasonal varation in cytochrome P450 immunopositive protein levels, lipid peroxidation and genetic toxicity in digestive gland of the mussel Mytilus edulis Aquatic Tox., 67, 325-336
15. Zhou, W., Zhang, Y., Hosch, M.S., Lang, A., Zwacka, R.M. and Engelhardt, J.F. (2001) Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion Hepatology, 33, 902-914
16. Van Breukelen, F. and Martin, S.L. (2002) Reversible depression of transcription during hibernation J. Comp. Physiol. B., 172, 355-361
17. Diaz, M.B., Lange, M., Heldmaier, G. and Klingenspor, M. (2004) Depression of transcription and translation during daily torpor in the Djungarian hamster (Phodopus sungorus) J. Comp. Physiol. B, 174, 495-502
18. Xiong, T. C., Jauneau, A., Ranjeva, R. and Mazars, C. (2004) Isolated plant nuclei as mechanical and thermal sensors involved in calcium signaling The Plant J., 40, 12-21
19. Lannoo, N., Peumans, W.J., Van Pamel, E., Alvarez, R., Xiong, T-C., Hause, G., Mazars, C. and Van Damme, E.J.M. (2006) Localization and in vitro binding studies suggest that the cytoplasmic/nuclear tobacco lectin can interact in situ with high-mannose and complex N-glyc FEBS Lett., 580, 6329-6337
20. Ford, T. C., Baldwin, J. P. and Lambert, S. J. (1998) Rapid enzyme-free preparation of starch-free nuclei from plants facilitates studies of chromatin structure Plant Club Ann. Symp. York, UK., Abstr. 54
21. Bedell, J.A., Budiman, M.A., Nunberg, A., Citek, R.W., Robbins, D., Jones, J., Flick, E., Rohlfing, T., Fries, J., Bradford, K., McMenamy, J., Smith, M., Holeman, H., Roe, B.A., Wiley, G., Korf, I.F., Rabinowicz, P.D., Lakey, N., McCombie, W.R., Jeddeloh, J.A. and Martienssen, R.A. (2005) Sorghum genome sequencing by methylation filtration PLoS Biol 3:e13
22. Timko, M.P., Rushton, P.J., Laudeman, T.W., Bokowiec, M.T., Chipumuro, E., Cheung, F., Town, C.D. and Chen, X. (2008) Sequencing and analysis of the gene-rich space of cowpea BMC Genomics, 9:103
23. Schouppe, D., Ghesquière, B., Menschaert, G., De Vos, W.H., Bourque, S., Trooskens, G., Proost, P., Gevaert, K. and Van Damme, E.J.M. (2011) Interaction of the tobacco lectin with histone proteins Plant Physiol., 155, 1091–1102
24. Liu, Z., Zhu, Y., Gao, J., Yu, F., Dong, A. and Shen, W-H. (2009) Molecular and reverse genetic characterization of nucleosome assembly protein1 (NAP1) genes unravels their function in transcription and nucleotide excision repair in Arabidopsis thaliana Plant J., 59, 27–38
25. Guilluy, C., Dubash, A.D. and García-Mata, R. (2011) Analysis of RhoA and Rho GEF activity in whole cells and the cell nucleus Nat. Protocols 6, 2050-2060
26. Grindberg, R.V., Yee-Greenbaum, J.L., McConnell, M.J., Novotny, M., O’Shaughnessy, A.L., Lambert, G.M., Araúzo-Bravo, M.J., Lee, J., Fishman, M., Robbins, G.E., Lin, X., Venepally, P., Badger, J.H., Galbraith, D.W., Gage, F.H. and Lasken, R.S. (2013) RNA-sequencing from single nuclei Proc. Natl. Acad. Sci. USA, 110, 19802–19807
27. Huff, L.P., DeCristo, M.J., Trembath, D., Kuan, P.F., Yim, M., Liu, J., Cook, D.R., Miller, R., Der, C.J. and Cox, A.D. (2013) The role of Ect2 nuclear RhoGEF activity in ovarian cancer cell transformation Genes Cancer, 4, 460-475
28. Amin, N.M., Greco, T.M., Kuchenbrod, L.M., Rigney, M.M., Chung, M-I., Wallingford, J.B., Cristea, I.M. and Conlon, F.L. (2014) Proteomic profiling of cardiac tissue by isolation of nuclei tagged in specific cell types (INTACT) Development, 141, 962-973
29. Palmowski, P., Rogowska-Wrzesinska, A., Williamson, J., Beck, H.C., Mikkelsen, J.D., Hansen, H.H. and Jensen, O.N. (2014) Acute phencyclidine treatment induces extensive and distinct protein phosphorylation in rat frontal cortex J. Proteome Res., 13,1578-1592
30. Hahn, A.S. and Desrosiers, R.C. (2013) Rhesus monkey rhadinovirus uses Eph family receptors for entry into B cells and endothelial cells but not fibroblasts PLoS Pathog., 9: e1003360
OptiPrepTM Application Sheet S10; 11th edition, February, 2020
OptiPrep™ Application Sheet S11
Purification of mammalian peroxisomes in a continuous gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- An OptiPrep™ Reference List RS02 “Purification of peroxisomes – reference list” lists published papers reporting the use of OptiPrep™ according to tissue or cell type: to access return to the initial list of Folders and select “Reference Lists”
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Methods for the isolation of mitochondria (Application Sheets S14-S16) or lysosomes (Application Sheet S55) may also provide enriched peroxisomal fractions in the same gradient. All of these organelles may be purified from a “light” or “heavy+light” mitochondrial pellet or from a post-nuclear supernatant (see Application Sheet S07).
1. Background
Nycodenz® and Percoll® gradients have been widely used for the purification of peroxisomes; both offered a big improvement in resolution from mitochondria and lysosomes compared to sucrose gradients. Hiltunen et al [1] commented however that to reduce contamination from mitochondria to a minimum Nycodenz was the gradient medium of choice. Moreover in Percoll® gradients peroxisomes and endoplasmic reticulum (ER) have a very similar banding density; in Nycodenz® the ER has a much lower density. Since 1994 the use of iodixanol gradients has gained much popularity for peroxisome isolation. Iodixanol offers even more advantages than Nycodenz®. In both Nycodenz® and iodixanol the peroxisomes are the densest of the major subcellular organelles ( = 1.19-1.23 g/ml) and well separated from the other organelles in the light mitochondrial fraction. In iodixanol the density of these other organelles is lower than in Nycodenz®; mitochondria have a median density of approx 1.145 g/ml and lysosomes approx 1.12 g/ml; slightly lower than the figures in Nycodenz®; the separation from peroxisomes is thus potentially greater in iodixanol. The other big advantage of using iodixanol is that the gradient solutions are prepared simply by dilution of OptiPrep™. Section 2 describes the OptiPrep™ method as applied to rat liver in detail; it is adapted from the method of Van Veldhoven et al [2,3]. Section 3 describes some of the procedural variations.
2. Iodixanol gradient methodology
2a. Solutions Required (see Section 2d, Note 1)
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 1mM EDTA, 0.1% (v/v) ethanol, 5 mM Mops pH 7.2.
C. 6 mM EDTA, 0.6% ethanol, 30 mM Mops, pH 7.2.
D. 1 M sucrose.
E. Gradient solutions: Make up from solutions A, C,
D and water using respectively, these ratios by volume:
E1: 5 + 0.6 + 0.4 + 0.0 (50% iodixanol)
E2: 4 + 0.6 + 0.7 + 0.7 (40% iodixanol)
E3: 2 + 0.6 + 1.1 + 2.3 (20% iodixanol)
2b. Ultracentrifuge rotor requirements
Any 30-50 ml fixed-angle rotor for an ultracentrifuge, capable of approx 100,000 g (see Section 3)
2c. Protocol
Carry out all operations at 0-4°C.
1. Mince the liver very finely with scissors and transfer to a Potter-Elvehjem homogenizer with Solution B (use 10 ml medium for every 2.5 g tissue). Homogenize using approx 6 strokes of the pestle (500-700 rpm) (see Section 2d, Note 2).
2. Centrifuge the homogenate at 3000 gav in a fixed-angle rotor for 10 min to pellet the nuclei and heavy mitochondria. This pellet may be rehomogenized in solution B and the centrifugation repeated.
3. Centrifuge the supernatant(s) at 17,000 gav for 10-15 min.
4. Resuspend the 17,000 g pellet in solution B using a loose-fitting Dounce homogenizer (2-3 strokes of the pestle). Adjust to a volume of about 0.5 ml per g of tissue.
5. Use a two chamber gradient maker or a Gradient Master to prepare a linear gradient from 9 ml each of gradient solutions E2 and E3 in thick-walled polycarbonate tubes for a 36-40 ml fixed-angle rotor and underlayer each gradient with 2 ml of gradient solution E1 (see Section 2d, Note 3).
6. Layer 3 ml of the suspension over each gradient and centrifuge at 105,000 gav for 1 h.
7. Allow the rotor to decelerate from 1000 rpm without the brake, the collect the gradient in 1 ml fractions dense end first (see Section 2d, Note 4).
2d. Notes
1. The variable volume of 1 M sucrose maintains each solution isoosmotic. Keep these solutions, and carry out all subsequent operations, at 0-4°C. Protease inhibitors may be included in any or all of the media at the operator’s discretion.
2. For more information on homogenization of tissues and cells and differential centrifugation of an homogenate see respectively Application Sheets S05, S06 and S07.
3. Thin-walled tubes can be used but may require some capping or sealing device. For more details on the preparation of pre-formed iodixanol gradients see Application Sheet S03.
4. Gradients can be unloaded dense end first by carefully introducing a narrow metal cannula (connected to a peristaltic pump) to the bottom of the tube. Thin-walled tubes can be collected by tube puncture. For more information on unloading gradients see Application Sheet S08.
2e. Analysis
A typical result with rat liver is shown in Figure 1, which shows the distribution of marker enzymes across the gradient. The catalase (peroxisome) band is well separated from all of the mitochondria (glutamate dehydrogenase) and lysosomes (acid phosphatase). The endoplasmic reticulum (glucose-6-phosphatase) is the least dense membrane type. This is in contradistinction to Percoll gradients in which ER and peroxisomes always overlap. Similar separations of peroxisomes are obtained with mouse kidney [4] and hepatocytes [5]. The yield of peroxisomes is 80-90% with no detectable contamination from any other organelle. Yields of peroxisomes from Percoll® gradients are often low due to loss of material during the final centrifugation step to remove the Percoll®. This is normally obligatory as Percoll frequently interferes with subsequent analyses. If the concentration of peroxisomes in the gradient fraction(s) is sufficiently high for the analytical technique to provide reliable information, it is usually unnecessary to remove the gradient medium. Unlike Percoll®, iodixanol does not interfere with any spectrophotometric assays in the visible region of the spectrum nor does it affect the proper running of SDS-polyacrylamide gels. Standard spectrophotometric methods (carried out above 340 nm), for measuring organelle enzyme markers can be performed directly on gradient fractions [6]. Protein can also be measured directly by any Coomassie blue-based method [6]. If it is necessary to remove the gradient medium, dilute fractions with an equal volume of buffer; pellet at approx 30,000gav for 10 min and resuspend in a suitable buffer. For SDS-PAGE proteins can be precipitated directly in TCA, iodixanol is soluble in acid solutions.
3. Alternative centrifugation/gradient formats for iodixanol gradient separations
1. Joly et al [7] used exactly the same iodixanol gradient as described in this OptiPrep Application Sheet but carried out the centrifugation at 125,000 g for 1 h in a Beckman SW41 swinging-bucket rotor. An almost identical distribution of organelle markers was reported. The authors investigated the localization of malonyl-CoA decarboxylase.
2. Light and dense peroxisomes have been resolved in a non-linear continuous gradient [8-12]. It was generated from a discontinuous one of 1.12, 1.155, 1.19, 1.225 and 1.26 g/ml (equivalent to 18.5%, 26%, 32.5%, 40.5% and 47.5% iodixanol) by freezing in liquid nitrogen (and storage at – 20°C) followed by rapid thawing (approx. 30 min). The volumes of solution used were 10, 7, 6, 3 and 4 ml respectively and the crude fraction was layered on top. After centrifugation at 39,000 gmax for 30 min in a Beckman VTi50 rotor the light peroxisomes banded at approx 1.21 g/ml and the dense peroxisomes at approx. 1.24 g/ml. Islinger and Weber [10] showed that iodixanol gradients could even identify a very low density population. This gradient was also described in refs 11 and 12.
3. In a comprehensive methodological review paper Islinger et al [13] reserved the sigmoidal gradient for isolation of the peroxisomes from a light mitochondrial fraction; whilst the peroxisomes from a heavy mitochondrial fraction were isolated in a discontinuous gradient [13] – see Application
Sheet S14a.
4. Antonenkov et al [14] used an iodixanol gradient as a final step for the production of highly purified peroxisomes, virtually devoid of any contamination. An initially discontinuous gradient comprised 6 ml each of 20%, 25%, 30% and 35% (w/v) iodixanol, together with 4 ml each of 40% and 50% iodixanol, in tubes for the Beckman VTi50 rotor. After being allowed to diffuse overnight at 4°C, 8-9 ml of crude peroxisomes was layered on top and centrifuged at 65,000 gav for 1 h. The peroxisomes produced by this procedure were used in physical [14], membrane transport [15] and fatty acid binding protein studies [16]. In a more recent paper the centrifugation conditions were 100,000 g for 90 min [17].
5. Note that using a vertical rotor confers significant advantages for organelle isolation. The short sedimentation path lengths of such rotors, compared to either swinging-bucket or fixed-angle rotors permits both the use of lower g- forces and shorter times. This also means that the fragile organelles are exposed to much lower hydrostatic pressures.
5. References
1. Hiltunen, J.K., Karki, T., Hassinen, I.E. and Osmundsen, H. (1987) Beta-oxidation of polyunsaturated fatty acids by rat liver peroxisomes. A role for 2,4-dienoyl-coenzyne A reductase in peroxisomal -oxidation J. Biol. Chem., 261, 16484-16493
2. Van Veldhoven, P. P., Baumgart, E. and Mannaerts, G. P. (1996) Iodixanol (OptiPrep), an improved density gradient medium for the iso-osmotic isolation of rat liver peroxisomes Anal. Biochem., 237, 17-23
3. Gijsbers, S., Van der Hoeven, G. and Van Veldhoven, P.P. (2001) Subcellular study of sphingoid base phosphorylation in rat tissues: evidence for multiple sphingosine kinases Biochim. Biophys. Acta, 1532, 37-50
4. Westin, M.A.K., Hunt, M.C. and Alexson, S.E.H. (2007) Peroxisomes contain a specific phytanoylCoA/Pristanoyl-CoA thioesterase acting as a novel auxiliary enzyme in - and -oxidation of methyl-branched fatty acids in mouse J. Biol. Chem., 282, 26707-26716
5. Priore, P., Giudetti, A.M., Natali, F., Gnoni, G.V. and Geelen, M.J.H. (2007) Metabolism and short-term metabolic effects of conjugated linoleic acids in rat hepatocytes Biochim. Biophys. Acta, 1771, 1299-1307
6. Ford. T., Graham. J, and Rickwood, D. (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of self generated gradients Anal. Biochem., 220, 360-366
7. Joly, E., Bendyan, M., Roduit, R., Saha, A.K., Ruderman, N.B. and Prentki, M. (2005) Malonyl-CoA decarboxylase is present in the cytosolic, mitochondrial and peroxisomal compartments of rat hepatocytes FEBS Lett., 579, 6581-6586
8. Koch, A., Thiemann, M., Grabenbauer, M., Yoon, Y., McNiven, M.A. and Schrader, M. (2003) Dynamin-like protein 1 is involved in peroxisomal fission J. Biol. Chem., 278, 8597-8605
9. Koch, A., Yoon, Y., Bonekamp, N.A., McNiven, M.A. and Schrader, M. (2005) A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells Mol. Biol. Cell, 16, 5077-5086
10. Islinger, M. and Weber, G. (2008) Free flow isoelectric focusing: a method for the separation of both hydrophilic and hydrophobic proteins of rat liver peroxisomes In Methods in Molecular Biology, 432, Organelle Proteomics (ed. Pflieger, D, and Rossier, J.) Humana Press, Totowa, NJ, pp 199-215
11. Islinger, M., Li, K.W., Seitz, J., Völkl, A. and Lüers, G.H. (2009) Hitchhiking of Cu/Zn superoxide dismutase to peroxisomes – evidence for a natural piggyback import mechanism in mammals Traffic, 10, 1711–1721
12. Nawrotzki, R., Islinger, M., Vogel, I., Völkl, A. and Kirsch, J. (2012) Expression and subcellular distribution of gephyrin in non-neuronal tissues and cells Histochem. Cell. Biol., 137, 471–482
13. Islinger, M., Abdolzade-Bavil, A., Liebler, S., Weber, G. and Völkl, A. (2012) Assessing heterogeneity of peroxisomes: isolation of two subpopulations from rat liver In Liver Proteomics: Methods and Protocols, Methods Mol. Biol., 909 (eds Josic, D. and Hixson, D.C.) Springer Science+Business Media, New York 2012
14. Antonenkov, V.D., Sormunen, R.T. and Hiltunen, J.K. (2004) The behavior of peroxisomes in vitro: mammalian peroxisomes are osmotically sensitive particles Am. J. Physiol. Cell Physiol., 287, C1623-C1635
15. Antonenkov, V.D., Rokka, A., Sormunen, R.T., Benz, R. and Hiltunen, J.K. (2005) Solute traffic across mammalian peroxisomal membrane – single-channel conductance monitoring reveals pore-forming activities in peroxisomes Cell. Mol. Life Sci., 62, 2886-2895
16. Antonenkov, V.D., Sormunen, R.T., Ohlmeier, S., Amery, L., Fransen, M., Mannaerts, G.P. and Hiltunen, J.K. (2006) Localization of a portion of the liver isoform of fatty-acid-binding protein (L-FABP) to peroxisomes Biochem. J., 394, 475-484
17. Antonenkov, V.D., Ohlmeier, S., Sormunen, R.T. and Hiltunen, J.K. (2007) UK114, a YjgF/Yer057p/UK114 family protein highly conserved from bacteria to mammals, is localized in rat liver peroxisomes Biochem. Biophys. Res. Comm., 357, 252-257
OptiPrepTM Application Sheet S11; 10th edition, February 2020
OptiPrep™ Application Sheet S12
Purification of mammalian peroxisomes on a discontinuous gradient or density barrier
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Reference List RS02 “Purification of peroxisomes – reference list” covers all published papers reporting the use of OptiPrep™ for the isolation of these organelles from all mammalian cells and tissues and from non-mammalian sources. To access the Reference List return to the initial list of Folders and select “Reference Lists”
- OptiPrep™ Application Sheet S57 deals with the isolation of peroxisomes from yeast.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
1. Background
Peroxisomes can be purified in iodixanol gradients in high yield (80-90%) with no detectable contamination from any other organelle [1,2]. This is a property unique to iodixanol because the densities of other organelles, particularly that of mitochondria (approx ρ = 1.14 g/ml) and endoplasmic reticulum (approx ρ = 1.13 g/ml) are much lower than that of peroxisomes (approx ρ = 1.19-1.23g/ml). In Nycodenz® mitochondria have a significantly higher density (approx ρ = 1.165 g/ml) than in iodixanol because only the latter can provide an iso-osmotic medium at densities above ρ = 1.15-1.16 g/ml. The density of peroxisomes on the other hand is relatively little affected by the type of iodinated density gradient medium because of their lack of an osmotic space. In Percoll® both peroxisomes and endoplasmic reticulum have the same banding density and these two organelles cannot be resolved. Ghosh and Hajra [3] developed a rapid density barrier method for the isolation of peroxisomes using Nycodenz® in which approx. 2 ml of the light mitochondrial fraction (LMF) is layered over 15ml of 30% (w/v) Nycodenz® and centrifuged at 131,000 gav for 1 h in a fixed-angle rotor. The peroxisomes form a loose pellet. This has been adapted to the use of OptiPrep™ in the protocol described in Section 2. The density of the barrier has been increased slightly to improve the resolution from the other organelles. Some alternative one- or two-layer gradients are described in Section 3.
2. Use of a density barrier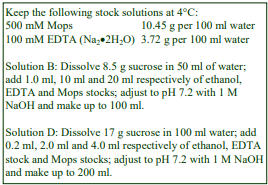
2a. Solutions Required (all iodixanol solutions are give as % w/v; see also Section 2e, Note 1)
A. OptiPrep™
B. OptiPrep™ diluent: 0.25 M sucrose, 10 mM EDTA, 1% (v/v) ethanol, 100 mM Mops-NaOH, pH 7.2
C. Working Solution of 54% (w/v) iodixanol (ρ = 1.291 g/ml): 9 vol. of OptiPrep™ + 1 vol. of Solution B.
D. Homogenization medium: 0.25 M sucrose, 1 mM EDTA, 0.1% (v/v) ethanol, 10 mM Mops-NaOH, pH 7.2.
E. Gradient solutions: Make up two dilutions of solution C, containing 47% iodixanol (ρ = 1.257 g/ml) and 32% iodixanol (ρ = 1.185 g/ml) by mixing solutions C and D, 8.7 + 1.3 and 6.0 + 4.0, v/v, respectively).
2b. Ultracentrifuge rotor requirements
Any swinging-bucket rotor for an ultra-centrifuge capable of approx 100,000g with a tube capacity of approx 17 ml tubes (see Section 2e, Note 2)
2c. Protocol
Keep all solutions and carry out all operations, at 0-4°C.
1. Mince the liver (wet weight approx 10 g) very finely with scissors and transfer to a PotterElvehjem (Teflon and glass) homogenizer.
2. Homogenize the mince in solution D (use 10 ml medium for every 2.5 g tissue), using approx 6 strokes of the pestle (500-700 rpm).
3. Centrifuge the homogenate at 3000gav in a fixed-angle rotor for 10 min to pellet the nuclei and heavy mitochondria (see Section 2e, Notes 3 and 4).
4. Centrifuge the supernatant(s) at 17,000gav for 10-15 min to produce a light mitochondrial pellet.
5. Resuspend this pellet in approx 4.0 ml of solution D using a loose-fitting Dounce homogenizer (2-3 strokes of the pestle) and add an equal volume of 47% iodixanol. The refractive index of this suspension should be 1.3782; adjust to this value with Solutions C or D if necessary. The density of this suspension is approx 1.145 g/ml.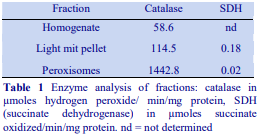
6. Transfer approx 14 ml of the suspension to a 17 ml tube for a suitable swinging-bucket rotor and underlayer with 2 ml of the 32% iodixanol. Top up the tube with suspension or solution D if necessary. Centrifuge at 110,000g for 2 h.
7. The peroxisomes band towards the bottom of the tube, partly within the 32% iodixanol layer and partly as a loose pellet. Aspirate and discard the liquid above the 32% layer, then harvest this layer and the pellet of peroxisomes.
2d. Analysis
Iodixanol does not significantly inhibit any enzyme so far tested. Standard spectrophotometric methods (carried out above 340 nm), for measuring organelle enzyme markers can be performed directly on gradient fractions [4]. Protein can also be measured directly by any Coomassie blue-based method [4]. A typical enzyme analysis is shown in Table 1. If it is necessary to remove the gradient medium, fractions can be diluted with an equal volume of buffer; pelleted at approx 30,000gav for 10 min and resuspended in a suitable buffer (recoveries are >90%). The specific activity of catalase in the peroxisome harvest represents an approximately 25-fold purification over the homogenate and approx 37% of the total catalase activity of the LMF was recovered. Barrier techniques, while simple, are inevitably a compromise between purity and yield. Because of the density heterogeneity of all organelles it is impossible to choose a density for the suspending medium which is greater than that all of the mitochondria and less than that of all of the peroxisomes.
2e. Notes
1. The density of Solution E can be adjusted to suit the operator’s requirements, if it is reduced, more of the peroxisomes will pellet; if it is increased, the peroxisomes will band predominantly at the interface between the sample and the barrier. If it is preferable to band the peroxisomes at an interface then it may be advisable to underlayer the sample with an additional 2 ml layer of approx. 25% iodixanol. Protease inhibitors may be included in any or all of the media at the operator’s discretion. Strategies for preparing gradient solutions for mammalian tissues are given in Application Sheet S01.
2. Choice of rotor is not particularly critical; Ghosh and Hajra [3] used a fixed-angle rotor. The separation may be adapted to larger or smaller volume rotors.
3. This pellet may be rehomogenized in solution D and the centrifugation repeated.
4. For more information on homogenization of tissues and cells and differential centrifugation of an homogenate see respectively Application Sheets S05, S06 and S07.
3. Alternative gradient formats
Lamhonwah et al [5] adjusted the light mitochondrial suspension to a slightly higher concentration of iodixanol (27.5%) and omitted the denser cushion; 5 ml of this was centrifuged at 250,000 g for 4 h in a swinging-bucket rotor to pellet the peroxisomes. By contrast Mi et al [6] adjusted the LMF to 28% iodixanol (15 ml) and underlayered it with 2 ml of 50% iodixanol, so that after 2 h at 131,000 g the peroxisomes banded at the interface. This format can probably be applied to LMFs from any source – an identical two-layer format was used for mussel peroxisomes [7]. A three-layer format [8] in which the LMF in 22.5% iodixanol (4.5 ml) is sandwiched between layers of 20% and 27.5% iodixanol (2 ml each) resulted in the peroxisomes from chicken embryo liver banding in the densest layer.
4. References
1. Van Veldhoven, P. P., Baumgart, E. and Mannaerts, G. P. (1996) Iodixanol (OptiPrep), an improved density gradient medium for the iso-osmotic isolation of rat liver peroxisomes Anal. Biochem., 237, 17-23
2. Graham, J., Ford, T. and Rickwood, D. (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
3. Ghosh, M. K. and Hajra, A. K. (1986) A rapid method for the isolation of peroxisomes from rat liver Anal. Biochem., 159, 169-174
4. Ford, T., Graham, J. and Rickwood, D. (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of self generated gradients Anal. Biochem., 220, 360-366
5. Lamhonwah, A-M., Skaug, J., Scherer, S. W. and Tein, I. (2003) A third human carnitine/organic cation transporter (OCTN3) as a candidate for the 5q31 Crohn’s disease locus (IBD5) Biochem. Biophys. Res. Comm., 301, 98-101
6. Mi, J., Garcia-Arcos, I., Alvarez, R., and Cristobal, S. (2008) Age-related subproteomic analysis of mouse liver and kidney peroxisomes Proteome Sci., 5:19
7. Apraiz, I., Mi, J. and Cristobal, S. (2006) Identification of proteomic signatures of exposure to marine pollutants in mussels (Mytilus edulis) Mol. Cell. Proteom., 5, 1274-1285
8. Labitzke, E.M., Diani-Moore, S. and Rifkind, A.B. (2007) Mitochondrial P450-dependent arachidonic acid metabolism by TCDD-induced hepatic CYP1A5; conversion of EETs to DHETs by mitochondrial soluble epoxide hydrolase Arch. Biochem. Biophys., 468, 70-81
OptiPrepTM Application Sheet S12 9th edition, January 2020
OptiPrep™ Application Sheet S13
Purification of mammalian peroxisomes in a self-generated gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Reference List RS02 “Purification of peroxisomes – reference list” covers all published papers reporting the use of OptiPrep™: to access return to the initial list of Folders and select “Reference Lists”
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Methods for the isolation of mitochondria (Application Sheets S14-S16) or lysosomes (Application Sheets S55) may also provide enriched peroxisomal fractions in the same gradient. All of these organelles may be purified from a “light” or “heavy+light” mitochondrial pellet or from a post-nuclear supernatant (see Application Sheet S07).
1. Background
Peroxisomes can be purified in self-generated iodixanol gradients in high yield (80-90%) with no detectable contamination from any other organelle. In iodixanol peroxisomes are the densest of the major subcellular organelles (ρ = 1.18-1.20 g/ml) present in the light mitochondrial fraction (LMF) from mammalian tissues and cells. Mitochondria have a median density of approx 1.145 g/ml and lysosomes approx 1.115 g/ml. Metrizamide or Nycodenz® gradients have been used previously to purify peroxisomes [1,2] but because mitochondria have a higher density in Nycodenz® or metrizamide than in iodixanol, the resolution of these two organelles is much easier in iodixanol gradients [3]. In Percoll® both peroxisomes and endoplasmic reticulum (ER) have the same banding density and these two organelles cannot be resolved; in iodixanol the ER has a much lower density. The protocol below is for mammalian liver, but similar techniques using other tissues or cells have been published. Variations in centrifugation conditions and a brief summary of some methodological variations is provided in Section 7. Formation of self-generated gradients requires ideally a vertical or a near-vertical rotor with a sedimentation path length of <25 mm. Some small volume fixed-angle rotors may be satisfactory, they tend to produce S-shaped gradients that are relatively shallow in the middle of the tube and steep at both ends in the centrifugation times which are valid for organelles (2-3 h). This particular protocol does require a slightly S shaped gradient for its efficacy however, thus some fixed-angle rotors (<10 ml tube volume) may be satisfactory.
2. Solutions Required (see Section 6, Note 1)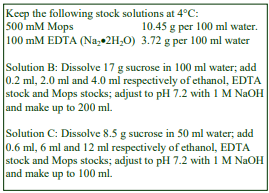
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 1 mM EDTA, 0.1% (v/v) ethanol, 10 mM Mops-NaOH, pH 7.4
C. Dilution medium: 0.25 M sucrose, 6 mM EDTA, 0.6% (v/v) ethanol, 60 mM Mops-NaOH, pH 7.4
D. Working Solution of 50% iodixanol (ρ = 1.272 g/ml): mix 5 vol. of solution A with 1 vol. solution C
3. Ultracentrifuge rotor requirements
A vertical or near vertical rotor with a tube capacity of 10-14 ml capable of >180,000g or a fixed-angle rotor with a tube capacity of <10 ml (see Section 6, Note 2)
4. Protocol (adapted from ref 3)
Carry out all operations at 0-4°C.
1. Mince the liver very finely with scissors and transfer to a Potter-Elvehjem (Teflon and glass)chomogenizer with solution B (use 10 ml medium for every 2.5 g tissue). Homogenize using approx 6 strokes of the pestle (see Section 6, Note 3).
2. Centrifuge at 3000 g for 10 min to pellet the nuclei and heavy mitochondria. This pellet may be rehomogenized in solution B and the centrifugation repeated (see Section 6, Note 3).
3. Centrifuge the supernatant(s) at 17,000 g for 10-15 min to produce a „light mitochondrial pellet“.
4. Resuspend this pellet in solution B using a loose-fitting Dounce homogenizer (2-3 strokes of the pestle). Adjust to a volume of about 15 ml per 10 g tissue; then mix with an equal volume of solution D (final iodixanol concentration = 25%: ρ = 1.150 g/ml).
5. Transfer to a suitable tube (10-14 ml) for a vertical, near-vertical or low-angle (less than 24°) fixed-angle rotor and centrifuge at a minimum of 180,000gav. The time required for formation of the gradient will depend on the rotor type; at 180,000g it will be about 3 h, at higher g-forces the time can be reduced (see Section 6, Notes 4-7).
6. Allow the rotor to decelerate from 3000 rpm without the brake and collect the gradient by upward displacement or by tube puncture, or with a syringe (see Section 6, Note 8). For more information on harvesting gradients see Application Sheet S08.
5. Analysis
Iodixanol does not significantly inhibit any enzyme so far tested. Spectrophotometric assays carried out above 340 nm, can be performed directly on gradient fractions: this includes the standard assays for catalase, succinate dehydrogenase and ß-galactosidase [4]. Protein can also be measured directly by any Coomassie blue-based method [4].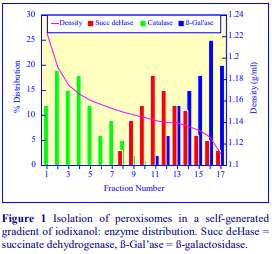
If it is necessary to remove the gradient medium, fractions can be diluted with an equal volume of buffer; pelleted at approx 30,000gav for 10 min and resuspended in a suitable buffer.
A typical result is shown in Figure 1, which shows the distribution of marker enzymes across the gradient. The activity in each fraction is expressed as a % of the total in the tube before centrifugation. Fractions 1-7 contain more than 90% of the total catalase with no detectable contamination from mitochondria or lysosomes. The Golgi membranes (not shown) band at the top of the gradient (far right of figure).
6. Notes
1. Protease inhibitors may be included in any or all of the media at the operator’s discretion. Strategies for preparing working solutions for mammalian tissues are given in Application Sheet S01.
2. The lower the angle of a fixed-angle rotor, the more suitable it is for the creation of self-generated gradients (ideally the angle should be <24°). Rather few modern fixed-angle rotors however conform to this specification and in such cases it may be necessary to use a smaller volume tube (preferably a Beckman g-Max adapted tube, which reduces the height of the tube rather than its diameter) in order to restrict the sedimentation path length.
3. For more information on homogenization of tissues and cells and differential centrifugation of an homogenate see respectively Application Sheets S05, S06 and S07.
4. Although much higher g-forces are required to generate the gradient than to band the organelles in a pre-formed gradient; the use of vertical rotors, which have very short sedimentation path lengths, means that the hydrostatic pressure on the organelles is no greater than in a swinging-bucket rotor at a lower g-force.
5. The precise conditions required for peroxisome purification in self-generated gradients, depends very much on the rotor type. Any vertical rotor, with a sedimentation path length of <25 mm would provide a very simple and efficient system; at approx 350,000gav separation would take place in 1-2 h. Vertical and near-vertical rotors can produce a range of density profile shapes; for more information see Application Sheet S04.
6. Optimal separation of the mitochondria and peroxisomes depends on the formation of a relatively shallow gradient in the middle of the tube (see Fig 1) and a sharp gradient at the bottom to prevent the peroxisomes from hitting the wall of the tube.
7. Always check the gradient density profile that is generated in a particular rotor using a blank gradient before using it for any fractionation and adjust the centrifugation conditions as appropriate. Refractive index (RI) is the most accurate method for determination of density and density tables giving RI values are given in Application Sheet S01. If a refractometer is not available then absorbance measurement is a possible alternative, see Application Sheet S09.
8. Once the banding position of the peroxisomes has been established, the reproducibility of selfgenerated gradients is so high, that a syringe can be used to harvest a standard volume from the bottom of the gradient.
7. Methodological variations
1. McClelland et al. [5] adjusted the LMF to 25% (w/v) in the manner described in the Protocol and centrifuged at 180,000g for 2.5 h in a Beckman NVT65 near-vertical rotor. Kurochkin et al [6] used the same rotor but centrifuged for 3.5 h and the LMF was mixed with an equal volume a working solution containing 5 vol. of OptiPrep™ and 1 vol. of 0.16 M sucrose, 12% (w/v) PEG1500, 60 mM Mops, pH 7.4, 6 mM EDTA, 6 mM DTT and 0.6% ethanol.
2. He et al [7] used a Hepes buffer rather than Mops and centrifuged at 180,000g for 3 h in a Beckman 50.2Ti fixed-angle rotor to study the expression and to characterize bile acid CoA:amino acid N-acyltransferase in peroxisomes.
3. Morel et al [8] isolated the peroxisomes from human hepatoblastoma (HepG2) cells. The homogenization buffer contained 0.25 M sucrose, 10 mM triethanolamine-acetic acid, pH 7.8 and the centrifugation was carried out at 180,000g for 4.5 h. Characterization of the human glutathione S-transferase kappa gene and protein was carried out.
4. A post-nuclear fraction rather than an LMF from CHO cells was processed according to the usual protocol [9].
5. In a large volume fixed-angle rotor with 39 ml tubes (Beckman 50.2Ti) fractionation was achieved at 150,000 g for 4 h [10].
8. References
1. Volkl, A. and Fahimi, H. D. (1985) Isolation and characterization of peroxisomes from the liver of normal untreated rats Eur. J. Biochem., 149, 257-265
2. Appelkvist, E-L., Reinhart, M., Fischer, R., Billheimer, J. and Dallner, G. (1990) Presence of individual enzymes of cholesterol biosynthesis in rat liver peroxisomes Arch. Biochem. Biophys., 282, 318-325.
3. Graham, J., Ford, T. and Rickwood, D. (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
4. Ford, T., Graham, J. and Rickwood, D. (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of self generated gradients Anal Biochem., 220, 360-366
5. McClelland, G. B., Khanna, S., Gonzalez, G. F., Butz, C. E. and Brooks, G. A. (2003) Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system Biochem. Biophys. Res. Comm., 304, 130-135
6. Kurochkin, I.V., Mizuno, Y., Konagaya, A., Sakaki, Y., Schonbach, C. and Okazaki, Y. (2007) Novel peroxisomal protease Tysnd1 processes PTS1- and PTS2-containing enzymes involved in -oxidation of fatty acids EMBO J., 26, 835-845
7. He, D., Barnes, S. and Falany, C. N. (2003) Rat liver bile acid CoA:amino acid N-acyltransferase: expression, characterization, and peroxisomal localization J. Lipid. Res., 44, 2242-2249
8. Morel, F., Rauch, C., Petit, E., Piton, A., Theret, N., Coles, B. and Guillouzo, A. (2004) Gene and protein characterization of the human glutathione S-transferase kappa and evidence for a peroxisomal localization J. Biol. Chem., 279, 16246-16253
9. Kobayashi, S., Tanaka, A. and Fujiki, Y. (2007) Fis1, DLP1 and Pex11p coordinately regulate peroxisome morphogenesis Exp. Cell Res., 313, 1675-1686
10. Styles, N.A., Falany, J.L., Barnes, S. and Falany, C.N. (2007) Quantification and regulation of the subcellular distribution of bile acid coenzyme A:amino acid N-acyltransferase activity in rat liver J. Lipid Res., 48, 1305-1315
OptiPrepTM Application Sheet S13; 9th edition, January 2020
OptiPrep™ Application Sheet S14a
Purification and analysis of mammalian mitochondrial fractions
- This Application Sheet presents the options available for resolution of mitochondria (and other organelles) from mammalian tissues and cultured cells. Reference List RS03 lists all the published papers, reporting the use of iodixanol gradients, according to tissue/cell type
1. Introduction
The choice of homogenization procedure, pre-gradient differential centrifugation and type of gradient can all contribute to the successful purification of mitochondria. Almost all the methodology was developed from work with rat or mouse liver, translation to other tissues and cells often requires changes to one or more protocol parameters.
2. Homogenization media
There are many examples of the use of a standard isoosmotic medium such as 0.25 M sucrose, 1 mM EDTA, buffered with 10-25 mM Tris to pH 7.0-7.8 for a variety of tissues and cells; the Tris often being replaced by the more organelle-friendly HEPES or Tricine. Functional studies with mitochondria may benefit from the inclusion of mannitol in the homogenization medium (HM) and there are many examples in which 0.25 M sucrose is replaced by 190-220 mM mannitol + 50-110 mM sucrose (e.g. refs 1-7). EGTA (at 1 mM) may replace the EDTA [1,4,7,8] or supplement the EDTA [9]. Virtually all media for tissues conform broadly to these recipes. An excellent description of the homogenization of bovine heart can be found in ref 10. Note that all media are routinely supplemented with a wide range of protease inhibitors. These isoosmotic media may also be used for cultured cells but there is a much more diverse range of medium composition, sometimes tailored to the cell type and/or to the subsequent analysis. For example the sucrose/mannitol medium for neonatal cardiac myocytes also included 2 mM taurine, 1 mM carnitine, 1 mM pyrophosphate and 1 mM calyculin [1,11-13]. Sometimes bovine serum albumin is included at 0.25-0.5% [8,12].
To achieve an effective and rapid homogenization some cultured cells must be swollen in a hypoosmotic medium, which, after cell lysis, is adjusted back to isoosmolality by the addition of a concentrated sucrose solution. Table 1 describes some of the variations. Sometimes MgCl₂ (2 mM final concentration) is added to protect the nuclei once homogenization is completed [17].

3. Differential centrifugation
A classical differential centrifugation scheme is shown in Figure 1. The g-force used to produce the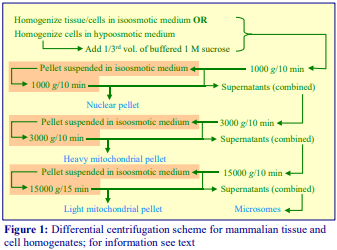 nuclear pellet varies from 600 to 1000 g with times of 5-10 min. Values outside these ranges are rare, though only 200 g for 10 min was used for macrophages [17]. Occasionally the “heavy mitochondrial pellet” (HMP) is used as the gradient input [5,14,15,17,18], in which case the g-force is usually increased to 5000 g. The use of this pellet has the advantage of eliminating many of the peroxisomes and lysosomes that normally sediment only at the higher g-forces used to produce the “light mitochondrial pellet” (LMP), but there is a significant loss of mitochondria, particularly those from cultured cells, which are more slowly sedimenting than those from a tissue
nuclear pellet varies from 600 to 1000 g with times of 5-10 min. Values outside these ranges are rare, though only 200 g for 10 min was used for macrophages [17]. Occasionally the “heavy mitochondrial pellet” (HMP) is used as the gradient input [5,14,15,17,18], in which case the g-force is usually increased to 5000 g. The use of this pellet has the advantage of eliminating many of the peroxisomes and lysosomes that normally sediment only at the higher g-forces used to produce the “light mitochondrial pellet” (LMP), but there is a significant loss of mitochondria, particularly those from cultured cells, which are more slowly sedimenting than those from a tissue
such as liver. Sometimes the g-force used to produce the LMP is increased to 17,000 g [3,19,20]. The LMP will contain all of the remaining mitochondria, lysosomes and peroxisomes plus some of the more rapidly sedimenting microsomal vesicles, principally the rough endoplasmic reticulum (RER). Most of the microsomal vesicle populations will remain in the LMP supernatant, although any large plasma membrane fragments and/or tubular Golgi elements may be in the HMP or LMP. In any differential centrifugation scheme such as that shown in Figure 1, the organelles that sediment at a particular g force often trap smaller particles in the process. This is often reduced by resuspending the pellet in the homogenization medium; repeating the centrifugation and combining the supernatants (see orange zones in Figure 1). The downside of this procedure is that the preparation time is increased and resuspension of the pellet may cause fragmentation of the organelles, unless the process is done very gently.
In gradient purification of mitochondria, the 3000 g sedimentation is frequently omitted and a total HMP+LMP fraction produced from the post-nuclear supernatant (PNS) [2,19-23]. To reduce contamination from microsomal vesicles the g-force used to pellet the total mitochondrial fraction from the PNS may be reduced to 10,000 g [8], 11,000 g [24] or 12,000 g [25-27]. There are also many examples in which the gradient input is the total PNS [1,9-13,28-33]. The advantages of this strategy are the considerable saving of time and the minimization of any loss of organelles; disadvantages are that the huge variety of particles in the sample severely tests the resolving power of the gradient and the possible inconvenient size of the sample. It may therefore be necessary to concentrate the particles by centrifugation at 100,000 g for 45-60 min [15,34,35]. If the study requires simultaneous analysis of mitochondria and other microsomal compartments such as the ER or Golgi, using a PNS may be the best option. Removal of the cytoplasmic proteins in the 100,000 g supernatant may also be an advantage. A problem with pelleting all the organelles at 100,000 g, aside from the considerable increase in preparation time and tendency of particles to aggregate together, might be the sensitivity of mitochondrial function to hydrostatic pressure.
- The differential centrifugation of brain homogenates has sometimes been omitted entirely and replaced by filtration of the homogenate through a 5 m nylon mesh at 20 g for 10 min [7,36]. This may be particularly effective for removing nuclei and myelin fragments.
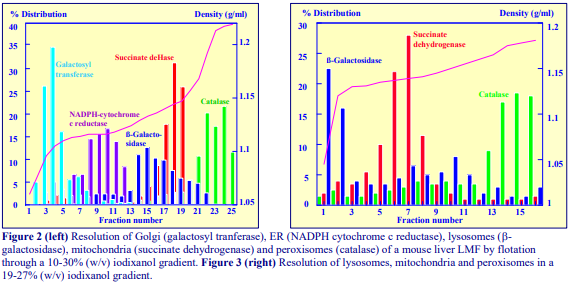
4. Gradient centrifugation
4a. Continuous gradients
The first report of the use of iodixanol [37] for the resolution of organelles from a crude mitochondrial fraction from mouse liver used a 10-30% (w/v) continuous gradient that was bottom loaded with the sample in 35% (w/v) iodixanol and centrifuged at 52,000 g for 1.5 h (Figure 2). The analysis showed the potential of iodixanol gradients since all of the membrane particles, Golgi (galactosyl transferase), ER (NADPH-cytochrome c reductase), -galactosidase (lysosomes), succinate dehydrogenase (mitochondria) and catalase (peroxisomes) displayed a characteristic median density. Subsequently, a more shallow gradient of 19-27% iodixanol was used to improve the resolution of the major organelles (Figure 3). Examples of the use of continuous gradients are given in Table 2.
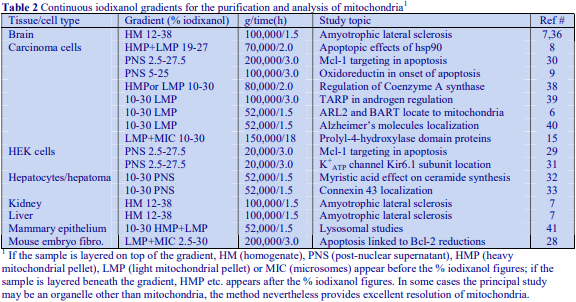
4b. Discontinuous gradients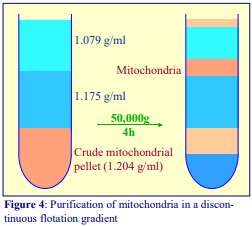
A three-layered discontinuous flotation gradient of 1.079 g/ml (10% iodixanol), 1.175 g/ml (30% w/v iodixanol) and approx 1.2 g/ml (35-36% iodixanol), the latter containing the crude mitochondria, was first described by Zhou et al [27] for the purification of mouse liver mitochondria from both lighter and denser organelles (see Figure 4). Choi et al [24] used a similar strategy for HEK293 mitochondria but based the method on one for the purification of yeast mitochondria by Meeusen et al [42] in which the density of the three layers was 1.10 (14.5 % iodixanol), 1.16 (27% iodixanol) and 1.27 g/ml (50% iodixanol).
Bottom-loaded flotation gradients can certainly provide excellent resolution of organelles; from a functional point of view however top-loading may be preferred because of the known sensitivity of mitochondria to hydrostatic pressure [43], which is highest at the bottom of the tube of a swinging bucket rotor. In contrast to continuous gradients, discontinuous gradients offer the easy compromise of median-loading and this was first reported by Graham et al [44] in which the LMP in 25% Nycodenz® , was underlayered by 40% Nycodenz® and overlayered by 23%, 20%, 15% and 10% Nycodenz®. The gentle centrifugation conditions of 52,000 g for 1.5 h might also be well suited to the isolation of functionally competent mitochondria. While there was good separation of mitochondria from lysosomes and lysosomes from Golgi membranes in the 10-23% region of the gradient, resolution of mitochondria from peroxisomes was relatively poor. This was subsequently remedied by the introduction of additional layers of 30% and 34% Nycodenz® [45], which allowed the peroxisomes to band at the 34%/40% Nycodenz® interface. Teoh et al [46] identified the material at each interface by Western blotting of SDS-PAGE analyzed fractions. Material at the 25%/34% interface was rich in cytochrome oxidase but completely lacking in catalase (peroxisomes) and GRP78 (endoplasmic reticulum). The median-loaded strategy has been very successfully adapted to the use of iodixanol [47] for HEK cells and for rat liver [48].
The vast majority of published papers have reported the use of discontinuous gradients with the crude fraction either top- or bottom-loaded and some of these are given in Table 3. Table 3 also includes examples of the use of a hybrid gradient incorporating 6% (v/v) Percoll® over 17% and 35% (w/v) iodixanol [1], which was introduced for neonatal cardiomyocytes. It may be beneficial for some cell types in which the peroxisomes and mitochondria have rather overlapping densities. Percoll® produces a pronounced shift in the density of peroxisomes to very low values.
- Note that in bottom-loaded formats any cytoplasmic proteins remain at in the sample zone.
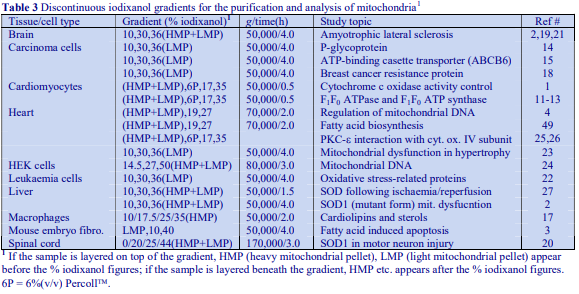
More discriminating multi-step discontinuous gradients may be able to give concomitant and significant enrichments of other membranes. For example a gradient of 10,15,20,25 and 30% (w/v) iodixanol on to which a concentrated liver PNS fraction was loaded and centrifuged at 100,000 g for 3 h provided an initial resolution of Golgi, ER and lysosomes; the mitochondria+peroxisome fraction was recovered from the bottom fractions of the gradient [34,35]. The mitochondria and peroxisomes were then completely resolved in a second gradient of 20, 25, 30 and 35% iodixanol run under the same centrifugation conditions.
4c. Self-generated gradients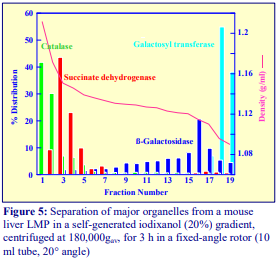
The formation of self-generated gradients requires higher g-forces than might be used with a pre-formed gradient. But, because the sedimentation path length of the rotors used for self-generated gradients (vertical, near-vertical or some fixed-angle rotors) is much lower than that of a swinging-bucket rotor, the hydrostatic pressure on the organelles is rather similar in the two systems. Compared to pre-formed gradients, there are three advantages to the use of self-generated gradients for organelle fractionation. (1) Multiple samples can be handled far more easily. (2) Resolution in any preformed gradient may be affected by the high particle concentration in the sample and the build-up of particles at interfaces during centrifugation. With preformed gradients this can be avoided only by incorporating the sample into the whole gradient (not always very convenient) – but this ideal is a natural consequence for self-generated gradients as the sample is simply mixed with a suitable solution of iodixanol. (3) Gradient density profiles and hence fractionations are far more reproducible than with pre-formed gradients.
Figure 5 shows a typical separation from ref 37. The methodology is probably more useful for studying the localization of a molecule or function amongst different membranes; i.e. the gradient is used analytically rather than preparatively for the isolation of one specific organelle. In the literature however self-generated gradients have largely been used for studying lysosomal rather than mitochondrial function.
5. Technical information
Detailed methods for the purification of mitochondria are described in three OptiPrep™ Application Sheets (S12-S14); these can be accessed via the following website www.Optiprep.com. Click on “Methodology”, then “Subcellular membranes” and follow the links from the index. Some ancillary OptiPrep™ Application Sheets on solution making, pre-gradient techniques, gradient preparation and gradient analysis are also listed below and may be accessed from the preface to the index.
- Application Sheet S14 describes the purification of mammalian mitochondria in a discontinuous iodixanol gradient; scroll down to “Mitochondria” in the index
- Application Sheet S15 describes the purification of mammalian mitochondria in a continuous iodixanol gradient; scroll down to “Light mitochondrial fraction, analysis” in the index
- Application Sheet S16 describes the purification of mammalian mitochondria in a self-generated iodixanol gradient; scroll down to “Light mitochondrial fraction, analysis” in the index
- Application Sheet S01 – Preparation of gradient solutions
- Application Sheet S03 – Preparation of continuous and discontinuous gradients
- Application Sheet S04 – Preparation of self-generated gradients
- Application Sheet S05 – Homogenization of mammalian tissues
- Application Sheet S06 – Homogenization of mammalian cells
- Application Sheet S07 – Differential centrifugation of homogenates
- Application Sheet S08 – Gradient harvesting
- Application Sheet S09 – Gradient analysis
6. References
1. Ogbi, M. and Johnson, J.A. (2006) Protein kinase Cε interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning Biochem. J., 393, 191-199
2. Israelson, A., Arbel, N., Da Cruz, S., Ilieva, H., Yamanaka, K., Shoshan-Barmatz, V. and Cleveland, D.W. (2010) Misfolded mutant SOD1 directly inhibits VDAC1 conductance in a mouse model of inherited ALS Neuron, 67, 575–587
3. Maia, R.C., Culver, C.A. and Laster, S.M. (2006) Evidence against calcium as a mediator of mitochondrial dysfunction during apoptosis induced by arachidonic acid and other free fatty acids J. Immunol., 177, 6398-6404
4. Morrish, F., Buroker, N.E., Ge, M., Ning, X-H., Lopez-Guisa, J., Hockenbert, D., and Portman, M.A. (2006) Thyroid hormone receptor isoforms localize to cardiac mitochondrial matrix with potential for binding to receptor elements on mtDNA Mitochondrion, 6, 143-148
5. Jin, J-K., Whittaker, R., Glassy, M.S., Barlow, S.B., Gottlieb, R.A. and Glembotski, C.C. (2008) Localization of phosphorylated αB-crystallin to heart mitochondria during ischemia-reperfusion Am. J. Physiol. Heart Circ. Physiol., 294, H337-H344
6. Sharer, J.D., Shern, J.S., Van Valkenburg, H., Wallace, D.C. and Kahn, R.A. (2002) ARL2 and BART enter mitochondria and bind the adenine nucleotide transporter Mol. Biol. Cell, 13, 71-83
7. Bergemalm, D., Jonsson, P.A., Graffmo, K.S., Andersen, P.M., Brännström, T., Rehnmark, A. and Marklund, S.L. (2006) Overloading of stable and exclusion of unstable human superoxide dismutase-1 variants in mitochondria of murine amyotrophic lateral sclerosis models J. Neurosci., 26, 4147-4154
8. Margineantu, D.H., Emerson, C.B., Diaz, D. and Hockenberry, D.M. (2007) Hsp90 inhibition decreases mitochondrial protein turnover PLoS One, 10:e1066
9. Gilady, S.Y., Bui, M., Lynes, E.M., Benson, M.D., Watts, R., Vance, J.E. and Simmen, T. (2010) Ero1α requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM) Cell Stress Chaperones, 15, 619–629
10. Smith, A.L. (1967) Preparation, properties and conditions for assay of mitochondria: slaughterhouse material, small scale Methods Enzymol., 10, 81-86
11. Nguyen, T., Ogbi, M. and Johnson, J.A. (2008) Delta protein kinase C interacts with the d subunit of the F1F0 ATPase in neonatal cardiac myocytes exposed to hypoxia or phorbol ester: implications for F1F0 ATPase regulation J. Biol. Chem., 283, 29831-29840
12. Nguyen, T.T., Ogbi, M., Yu, Q., Fishman, J.B., Thomas, W., Harvey, B.J., Fulton, D. Johnson, J.A. (2010) Modulation of the protein kinase C interaction with the “d” subunit of F1F0-ATP synthase in neonatal cardiac myocytes; development of cell-permeable, mitochondrially targeted inhibitor and facilitator peptides J. Biol. Chem., 285, 22164–22173
13. Nguyen, T.T., Ogbi, M., Yu, Q. and Johnson, J.A. (2010) Attenuation of the hypoxia-induced protein kinase Cδ interaction with the ‘d’ subunit of F1Fo-ATP synthase in neonatal cardiac myocytes: implications for energy preservation and survival Biochem. J., 429, 335–345
14. Paterson, J.K. and Gottesman, M.M. (2007) P-Glycoprotein is not present in mitochondrial membranes Exp. Cell Res., 313, 3100-3105
15. Paterson, J.K., Shukla, S., Black, C.M., Tachiwada, T., Garfield, S., Wincovitch, S., Ernst, D.N., Agadir, A. et al (2007) Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane Biochemistry, 46, 9443-9452
16. Barth, S., Edlich, F., Berchner-Pfannschmidt, U., Gneuss, S., Jahreis, G., Hasgall, P.A., Fandrey, J., Wenger, R.H. and Camenisch, G. (2009) Hypoxia-inducible factor prolyl-4-hydroxylase PHD2 protein abundance depends on integral membrane anchoring of FKBP38 J. Biol. Chem., 284, 23046–23058
17. Andreyev, A.Y., Fahy, E., Guan, Z., Kelly, S., Li, X., McDonald, J.G., Milne, S., Myers, D., Park, H., Ryan, A. et al (2010) Subcellular organelle lipidomics in TLR-4-activated macrophages J. Lipid Res., 51, 2785–2797
18. Solazzo, M., Fantappiè, O., D’Amico, M., Sassoli, C., Tani, A., Cipriani, G., Bogani, C., Formigli, L, and Mazzanti, R. (2009) Mitochondrial expression and functional activity of breast cancer resistance protein in different multiple drug-resistant cell lines Cancer Res., 69, 7235-7242
19. Van de Velde, C., Miller, T.M., Cashman, N.R. and Cleveland, D.W. (2008) Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria Proc. Natl. Acad. Sci., USA, 105, 4022-4027
20. Wood-Allum, C.A., Barber, S.C., Kirby, J., Heath, P., Holden, H., Mead, R., Higginbottom, A., Allen, S. et al (2006) Impairment of mitochondrial anti-oxidant defence in SOD1-related motor neuron injury and amelioration by ebselen Brain, 129, 1693-1709
21. El-Kadi, A.M., Bros-Facer, V., Deng, W., Philpott, A., Stoddart, E., Banks, G., Jackson, G.S., Fisher, E.M.C. et al (2010) The Legs at odd angles (Loa) mutation in cytoplasmic dynein ameliorates mitochondrial function in SOD1G93A mouse model for motor neuron disease J. Biol. Chem., 285, 18627-18639
22. Yun, Y., Wang, L-S., Shen, S-M., Xia, L., Zhang, L., Zhu, Y-S. and Chen, G-Q. (2007) Subcellular proteome analysis of camptothecin analogue NSC606985-treated acute myeloid leukemic cells J. Proteome Res., 6, 3808-3818
23. Meng, C., Jin, X., Xia, L., Shen, S-M., Wang, X-L., Cai, J., Chen, G-Q., Wang, L-S. and Fang, N-Y. (2009) Alterations of mitochondrial enzymes contribute to cardiac hypertrophy before hypertension development in spontaneously hypertensive rats J. Proteome Res., 8, 2463-2475
24. Choi, Y-S., Ryu, B-K., Min, H-K., Lee, S-W. and Pak, Y.K. (2005) Analysis of proteome bound to D loop region of mitochondrial DNA by DNA-linked affinity chromatography and reverse-phase liquid chromatography/tandem mass spectrometry Ann. N.Y. Acad. Sci., 1042, 88-100
25. Guo, D., Nguyen, T., Ogbi, M., Tawfik, H., Ma, G., Yu, Q., Caldwell, R.W. and Johnson, J.A. (2007) Protein kinase C-ε coimmunoprecipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome-c oxidase activity and cardioprotection Am. J. Phsiol. Heart Circ. Physiol., 293, H2219-H2230
26. Yu, Q., Nguyen, T., Ogbi, M., Caldwell, RW. and Johnson, J.A. (2008) Differential loss of cytochrome-c oxidase subunits in ischemia-reperfusion injury: exacerbation of COI subunit loss by PKC- inhibition Am. J. Physiol. Heart Circ. Physiol., 294, H2637–H2645
27. Zhou, W., Zhang, Y., Hosch, M.S., Lang, A., Zwacka, R.M. and Engelhardt, J.F. (2001) Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion Hepatology, 33, 902-914
28. Wang, H-Q., Nakaya, Y., Du, Z., Yamane, T., Shirane, M., Kudo, T., Takeda, M. et al (2005) Interaction of presenilins with FKBP38 promotes apoptosis by reducing mitochondrial Bcl-2 Hum. Mol. Genet., 14, 1889-1902
29. Chou, C-H., Lee, R-S., Yang-Yen, H-F. (2006) An internal EELD domain facilitates mitochondrial targeting of Mcl-1 via a Tom70-dependent pathway Mol. Biol. Cell, 17, 3952-3963 30.
30. Huang, C-R. and Yang-Yen, H-F. (2010) The fast-mobility isoform of mouse Mcl-1 is a mitochondrial matrix-localized protein with attenuated anti-apoptotic activity FEBS Lett., 584, 3323–3330
31. Ng, K-E., Schwarzer, S., Duchen, M.R. and Tinker, A. (2010) The intracellular localization and function of the ATP-sensitive K+ channel subunit Kir6.1 J. Membr. Biol., 234, 137–147
32. Beauchamp, E., Tekpli, X., Marteil, G., Lagadic-Gossmann, D., Legrand, P. and Rioux, V. (2009) Myristoylation targets dihydroceramide D4-desaturase 1 to mitochondria: Partial involvement in the apoptotic effect of myristic acid Biochimie 91, 1411–1419
33. Tekpli, X., Rivedal, E., Gorria, M., Landvik, N.E., Rissel, M., Dimanche-Boitrel, M-T., Baffet, G., Holme, J.A. and Lagadic-Gossmann, D. (2010) The B[a]P-increased intercellular communication via translocation of connexin-43 into gap junctions reduces apoptosis Toxicol. Appl. Pharmacol., 242, 231– 240
34. Salvi, M., Battaglia, V., Brunati, A.M., La Rocca, N., Tibaldi, E., Pietrangeli, P., Marcocci, L., Mondovi, B., Rossi, C.A. and Toninello, A. (2007) Catalase takes part in rat liver mitochondria oxidative stress defense J. Biol. Chem., 282, 24407-24415
35. Gringeri, E., Carraro, A., Tibaldi, E., d’Amico, F.E., Mancon, M., Toninello, A., Pagano, M.A., Vio, C., Cillo, U. and Brunati, A.M. (2010) Lyn-mediated mitochondrial tyrosine phosphorylation is required to preserve mitochondrial integrity in early liver regeneration Biochem. J., 425, 401–412
36. Bergemalm, D., Forsberg, K., Srivastava, V., Graffmo, K.S., Andersen, P.M., Brännström, T., Wingsle, G. and Marklund, S.L. (2010) Supermoxide dismutase-1 and other proteins in inclusions from transgenic amyotrophic lateral sclerosis model mice J. Neurochem., 114, 408–418
37. Graham, J., Ford, T. and Rickwood, D (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
38. Zhyvoloup, A., Nemazanyy, I., Panasyuk, G., Valovka, T., Fenton, T., Rebholz, H., Wang, M-L. et al (2003) Subcellular localization and regulation of coenzyme A synthetase J. Biol. Chem., 278, 50316 50321
39. Maeda, H., Nagata, S., Wolfgang, C.D., Bratthauer, C.D., Bera, T.K. and Pastan, I. (2004) The T cell receptor chain alternate reading frame protein (TARP), a prostate-specific protein localized in mitochondria J. Biol. Chem., 279, 24561-24568
40. Greeve, I., Hermans-Borgmeyer, I., Brellinger, C., Kasper, D., Gomez-Isla, T., Behl, C., Levkau, B. and Nitsch, R.M. (2000) The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer’s disease-associated neurodegeneration and oxidative stress J. Neurosci., 20, 7345-7352
41. Glunde, K., Guggino, S.E., Ichikawa, Y. and Bhujwalla, Z.M. (2003) A novel method of imaging lysosomes in living human mammary epithelial cells Mol. Imaging, 2, 24-36
42. Meeusen, S., Tieu, Q., Wong, E., Weiss, E., Schieltz, D., Yates, J.R. and Nunnari, J. (1999) Mgm101p is a novel component of the mitochondrial nucleoid that binds DNA and is required for the repair of oxidatively damaged mitochondrial DNA J. Cell Biol., 145, 291-304
43. Wattiaux, R. and Wattiaux-De Coninck, S. (1983) Isolation of cell organelles In Iodinated Density Gradient Media – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 119-137
44. Graham, J.M., Ford, T. and Rickwood, D. (1990) Isolation of the major subcellular organelles from mouse liver using Nycodenz gradients without use of an ultracentrifuge Anal. Biochem., 187, 318-323
45. Okado-Matsumoto, A. and Fridovich, I. (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver J. Biol. Chem., 276, 38388-38393
46. Teoh, M.L.T., Walasek, P.J. and Evans, D.H. (2003) Leporipoxvirus Cu, Zn-superoxide dismutase (SOD) homologs are catalytically inert decoy proteins that bind copper chaperone for SOD J. Biol. Chem., 278, 33175-33184
47. Matsumoto, A., Comatas, K.E., Liu, L. and Stamler, J.S. (2003) Screening for nitric oxide-dependent protein-protein interactions Science, 301, 657-661
48. Islinger, M., Li, K.W., Seitz, J., Völkl, A. and Lüers, G.H. (2009) Hitchhiking of Cu/Zn superoxide dismutase to peroxisomes – evidence for a natural piggyback import mechanism in mammals Traffic, 10, 1711–1721
49. Witkowski, A., Joshi, A.K. and Smith, S. (2007) Coupling of the de novo fatty acid biosynthesis and lipoylation pathways in mammalian mitochondria J. Biol. Chem., 282, 14178-14185
OptiPrepTM Application Sheet S14a; 6th edition, December 2019
OptiPrep™ Application Sheet S14
Purification of mammalian mitochondria in a discontinuous gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- OptiPrep™ Application Sheet 14a “Purification and analysis of mammalian mitochondria” provides a referenced protocol review.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- For a bibliography of all published papers return to the initial list of Folders and select “Reference Lists” to open Reference List RS03 which provides a complete list of published papers.
- In a Methodological Supplement (Section 8) a summary of methods from recent papers (2011- 2018) is given in this Application Sheet; it includes its own reference section.
- Methods for the isolation of peroxisomes (Application Sheets S11-S13) or lysosomes (Application Sheet S55) may also provide enriched mitochondrial fractions in the same gradient. All of these organelles may be purified from a “light” or “heavy+light” mitochondrial pellet or from a post-nuclear supernatant (see Application Sheet S07).
1. Background
Fractionation of particles is often improved by bottom-loading of a sample and this technique was used by Zhou et al [1] who described the flotation of liver mitochondria through a discontinuous iodixanol gradient of 1.175 and 1.079 g/ml, from a dense solution (approx 1.2 g/ml). The method provides very good resolution of the mitochondria from both lighter and denser organelles. Choi et al [2] used a similar flotation strategy for HEK293 mitochondria but based the method on one for the purification of yeast mitochondria by Meeusen et al [3].
Top-loading may however be preferred because of the known sensitivity of mitochondria to hydrostatic pressure [4]. Graham et al [5] first reported the use of a compromise median-loaded Nycodenz® gradient in which the LMF in 25% Nycodenz® , was underlayered by 40% Nycodenz® and overlayered by 23%, 20%, 15% and 10% Nycodenz® . The gentle centrifugation conditions of 52,000 g for 1.5 h might also be well suited to the isolation of organelles. While there was good separation of mitochondria from lysosomes and lysosomes from Golgi membranes, resolution of mitochondria from peroxisomes was relatively poor. This was remedied by the introduction of additional layers of 30% and 34% Nycodenz [6], which allowed the peroxisomes to band at the 34%/40% Nycodenz® interface, later confirmed by Teoh et al [7]. Material at the 25%/34% interface was rich in cytochrome oxidase but completely lacking in peroxisome and ER markers. The strategy is satisfactory for both tissues and cultured cells and it has been adapted to the use of iodixanol [8]. For convenience the methodology below is based on the use of Optiprep™.
2. Solutions required
A. OptiPrep™ (60% w/v iodixanol)
B. Homogenization medium: 0.25 M sucrose, 1 mM EDTA, 20 mM Hepes-NaOH, pH 7.4.
C. Diluent: 0.25 M sucrose, 6 mM EDTA, 120 mM Hepes-NaOH, pH 7.4.
D. Working Solution of 50% iodixanol (ρ = 1.272 g/ml): 5 vol of solution A + 1 vol of solution C
Protease inhibitors may be included at the operator’s discretion. For more information on gradient solution preparation see Application Sheet S01.
3. Ultracentrifuge rotor requirements
Any swinging-bucket rotor with 14 ml tubes (e.g. Beckman SW41TI or Sorvall TH641) or 38 ml tubes (e.g. Beckman SW28, Sorvall AH629 or equivalent) may also be used.
4. Preparation of a crude mitochondrial fraction
Keep all solutions, and carry out all operations, at 0-4°C.
1. Liver or other tissue: Mince the tissue very finely with scissors (or with a tissue chopper) and transfer to a Potter-Elvehjem (Teflon and glass) homogenizer with Solution B (use 10 ml medium for every 2.5 g tissue). Homogenize using approx 6 strokes of the pestle (500-700 rpm). For more information about homogenization of tissues see Application Sheet S05.
2. For cells: Wash 1-3×10⁸ cells in 5 ml of phosphate buffered saline and again with 5 ml of Solution B. Suspend the cells in 3 ml of Solution B and homogenize in a ball-bearing homogenizer using five passes or homogenize in a tight-fitting Dounce homogenizer (Wheaton Type A) using 20 strokes of the pestle. For more information about homogenization of cells see Application Sheet S06.
3. Centrifuge the homogenate at 1000 gav in a fixed-angle rotor for 10 min to pellet the nuclei.
4. Aspirate and retain the supernatant.
5. Re-homogenize the pellet gently in Solution B using 2-3 gentle strokes of the pestle of a loosefitting Dounce homogenizer and the centrifugation repeated.
6. Combine the supernatants and centrifuge the supernatant(s) at 10-17,000 gav for 10-15 min t produce a crude heavy + light mitochondrial fraction. For more information on differential centrifugation see Application Sheet S07 and also Section 8c.
7. Resuspend this pellet in 2.0-3.0 ml of Solution B using a loose-fitting (Wheaton Type B) Dounce homogenizer (2-3 strokes of the pestle).
5. Bottom or median loaded gradients
5a. Gradient solutions and centrifugation (adapted from ref. 1)
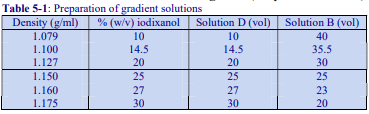 For bottom loading adjust the crude fraction from Step 7 (Section 4) to 36% (w/v) iodixanol (1.204 g/ml) by mixing with Solution D (1.4 + 3.6 vol. respectively). In some cases the sample may be adjusted to a higher density, for example 44% (w/v) iodixanol [9]. In approx. 14 ml tubes layer 4.0-4.5 ml of this suspension and overlay with 4.5 ml of each of the chosen gradient solutions (see Table 1): 1.079 and 1.175 g/ml [1]; 1.10 and 1.16 g/ml [2] or 1.127 and 1.150 g/ml [9]; the latter was used for mouse spinal cord. Mitochondria from KB-V1 and MCF7 cells have also been purified in the 1.079- 1.175 g/ml format [10].
For bottom loading adjust the crude fraction from Step 7 (Section 4) to 36% (w/v) iodixanol (1.204 g/ml) by mixing with Solution D (1.4 + 3.6 vol. respectively). In some cases the sample may be adjusted to a higher density, for example 44% (w/v) iodixanol [9]. In approx. 14 ml tubes layer 4.0-4.5 ml of this suspension and overlay with 4.5 ml of each of the chosen gradient solutions (see Table 1): 1.079 and 1.175 g/ml [1]; 1.10 and 1.16 g/ml [2] or 1.127 and 1.150 g/ml [9]; the latter was used for mouse spinal cord. Mitochondria from KB-V1 and MCF7 cells have also been purified in the 1.079- 1.175 g/ml format [10].
For median loading adjust the crude fraction from Step 7 (Section 4) to 25-30% (w/v) iodixanol and layer between 36% (w/v) iodixanol and one of the lower density solutions. Gradients may be completed with an optional 1-2 ml of the homogenization medium on top. This facilitates the collection of the lowest density material. Centrifuge at approx 50,000 gav for 3-4 h. Use a slow deceleration program or allow the rotor to decelerate from 2000 rpm without the brake.
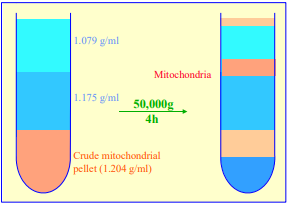 Figure 1 Purification of mammalian
Figure 1 Purification of mammalian
liver mitochondria in a discontinuous
iodixanol gradient, according to ref 1.
For larger volumes of crude mitochondrial fraction, in other rotors (36-38 ml tubes), the volumes should be scaled up proportionately. For more details on the preparation of pre-formed discontinuous iodixanol gradients see Application Sheet S03.
5b. Analysis
Harvest the mitochondria which band just above the upper interface (see Figure 1) or collect
the gradient in 1 ml fractions either low density end first by upward displacement or dense end first by carefully introducing a narrow metal cannula (connected to a peristaltic pump) to the bottom of the tube. For more information on gradient harvesting see Application Sheet S08.
Iodixanol does not significantly inhibit any enzyme so far tested. Standard spectrophotometric methods (carried out above 340 nm), for measuring organelle enzyme markers can be performed directly on gradient fractions [11]. Protein can also be measured directly by any Coomassie blue-based method [11]. If it is necessary to remove the gradient medium, fractions can be diluted with an equal volume of buffer; pelleted at approx 30,000gav for 10 min and resuspended in a suitable buffer.
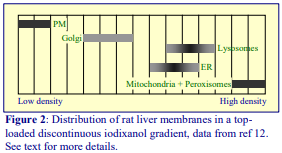 The 1.127 and 1.150 g/ml format [9] produced a particularly good separation of endoplasmic reticulum at the top of the 1.127 g/ml layer from the mitochondria at the next interface.
The 1.127 and 1.150 g/ml format [9] produced a particularly good separation of endoplasmic reticulum at the top of the 1.127 g/ml layer from the mitochondria at the next interface.
6. Top-loaded gradients
A multi-step iodixanol gradient of 10, 15, 20, 25 and 30% (w/v) iodixanol centrifuged at 100,000 g for 3 h [12] gave an excellent resolution of several membrane compartments (plasma membrane, Golgi,
endoplasmic reticulum + lysosomes and mitochondria + peroxisomes) from a rat liver post-nuclear supernatant (see Figure 2). The mitochondria + peroxisome fraction was re-run on a second gradient of 20%, 25%, 30% and 35% iodixanol, centrifuged under the same conditions to resolve these organelles completely.
Cardiac monocytes mitochondria are often purified on a composite discontinuous gradient of 6% (v/v) Percoll® and 17% and 35% (w/v) iodixanol, centrifuged at 50,000 g for 30 min [13-15]. The mitochondria band at the interface between the two iodixanol solutions. It is not known if the Percoll® layer could be replaced by an iodixanol solution of identical density (approx. 1.04 g/ml or 3% (w/v) iodixanol).
Brain mitochondria: More recently Islinger et al [16] reported the use of free-flow electrophoresis as the optimal method for removing synaptosomal contamination of brain mitochondria and the authors recommended the use of a top-loaded discontinuous gradient run in a vertical rotor for preparing the mitochondria. The gradient used was prepared by dilution of OptiPrep™ with a hypoosmotic medium of 5 mM HEPES-KOH, 1 mM EDTA, 2 mM PMSF, 1 mM DTT, 1 mM ε- aminocaproic acid, pH 7.4. We have adapted this to gradient solution preparation by dilution with a routine 0.25 M sucrose-containing buffer. Tubes for a Beckman VTi50 vertical rotor, are loaded with 3 ml 25%, 10 ml, 21%, 13 ml 14.5% and 4 ml 8.5% (w/v) iodixanol; the crude mitochondrial suspension is loaded on top, to fill the tube. The rotor is centrifuged at 33,000 g for approx 35 min. The mitochondria band close to the bottom of the tube.
7. References
1. Zhou, W., Zhang, Y., Hosch, M. S., Lang, A., Zwacka, R. M. and Engelhardt, J. F. (2001) Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion Hepatology, 33, 902-914
2. Choi, Y-S., Ryu, B-K., Min, H-K., Lee, S-W. and Pak, Y.K. (2005) Analysis of proteome bound to D loop region of mitochondrial DNA by DNA-linked affinity chromatography and reverse-phase liquid chromatography/tandem mass spectrometry Ann. N.Y. Acad. Sci., 1042, 88-100
3. Meeusen, S., Tieu, Q., Wong, E., Weiss, E., Schieltz, D., Yates, J. R. and Nunnari, J. (1999) Mgm101p is a novel component of the mitochondrial nucleoid that binds DNA and is required for the repair of oxidatively damaged mitochondrial DNA J. Cell Biol., 145, 291-304
4. Wattiaux, R. and Wattiaux-De Coninck, S. (1983) Isolation of cell organelles In Iodinated Density Gradient Media – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 119-137
5. Graham, J., M., Ford, T. and Rickwood, D. (1990) Isolation of the major subcellular organelles from mouse liver using Nycodenz gradients without use of an ultracentrifuge Anal. Biochem., 187, 318-323
6. Okado-Matsumoto, A. and Fridovich, I. (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver J. Biol. Chem., 276, 38388-38393
7. Teoh, M.L.T., Walasek, P.J. and Evans, D.H. (2003) Leporipoxvirus Cu, Zn-superoxide dismutase (SOD) homologs are catalytically inert decoy proteins that bind copper chaperone for SOD J. Biol. Chem., 278, 33175-33184
8. Matsumoto, A., Comatas, K.E., Liu, L. and Stamler, J.S. (2003) Screening for nitric oxide-dependent protein-protein interactions Science, 301, 657-661
9. Wood-Allum, C.A., Barber, S.C., Kirby, J., Heath, P., Holden, H., Mead, R., Higginbottom, A., Allen, S., Beaujeux, T., Alexson, S.E., Ince, P.G. and Shaw, P.J. (2006) Impairment of mitochondrial anti oxidant defence in SOD1-related motor neuron injury and amelioration by ebselen Brain, 129, 1693 1709
10. Paterson, J.K. and Gottesoman, M.M. (2007) P-Glycoprotein is not present in mitochondrial membranes Exp. Cell Res., 313, 3100-3105
11. Ford, T., Graham, J. and Rickwood, D. (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of self generated gradients Anal. Biochem., 220, 360-366
12. Salvi, M., Battaglia, V., Brunati, A.M., La Rocca, N., Tibaldi, E., Pietrangeli, P., Marcocci, L., Mondovi, B., Rossi, C.A. and Toninello, A. (2007) Catalase takes part in rat liver mitochondria oxidative stress defense J. Biol. Chem., 282, 24407-24415
13. Ogbi, M. and Johnson, J.A. (2006) Protein kinase C interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning Biochem. J., 393, 191-199
14. Yu, Q., Nguyen, T., Ogbi, M., Caldwell, RW. and Johnson, J.A. (2008) Differential loss of cytochrome-c oxidase subunits in ischemia-reperfusion injury: exacerbation of COI subunit loss by PKC- inhibition Am. J. Physiol. Heart Circ. Physiol., 294, H2637–H2645
15. Nguyen, T., Ogbi, M. and Johnson, J.A. (2008) Delta protein kinase C Interacts with the d subunit of the F1F0 ATPase in neonatal cardiac myocytes exposed to hypoxia or phorbol ester: implications for F1F0 ATPase regulation J. Biol. Chem., 283, 29831-29840
16. Islinger, M., Kirsch, J., Angermüller, S., Rotaru, R., Abdolzade-Bavil, A. and Weber, G. (2011) Subcellular fractionation of brain tissue using free-flow electrophoresis In Neuroproteomics, Neuromethods, (ed. Li, K.W.) 57, Springer Science+Business Media, pp. 27-45
8. Application Sheet S14 – Methodological Supplement
This supplement is divided into method type and includes its own reference section (8g).
8a Removal of myelin from spinal cord material
If contamination of mitochondria is a serious problem, a simple short spin density barrier centrifugation might be of use. Parone et al [1] suspended a 12,000 g – 10 min pellet from a spinal cord post-nuclear supernatant in 12% iodixanol in 210 mM mannitol, 70 mM sucrose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.2 and centrifuged it at 17,000 g for 10 min. The myelin layer at the top is separated from the organelle pellet.
8b Homogenization of cells
As the use of an EDTA containing homogenization medium may destabilize nuclei, HEK cells were first homogenized in 150 mM MgCl₂, 10 mM KCl, 20 mM HEPES pH 7.4; this was adjusted to 8.5% (w/v) sucrose and the nuclei removed at 1000 g for 10 min. A 5000 g – 10 min pellet was washed once in the same buffered sucrose/Mg/K medium and finally resuspended in a routine buffered sucrose/EDTA solution [2].
An interesting method was adopted by Bhowmick et al [3] for monkey kidney cells that avoided the use of any shearing forces that might be potentially damaging to mitochondria. The cells were suspended in 1-2% (w/v) Triton X100, 0.01-0.03 % Nonidet P40 and 0.4-0.6% CHAPS for 30 min on ice. A 7000 g – 10 min pellet, prepared from a post-nuclear supernatant, was then suspended in a routine buffered 0.25 M sucrose solution prior to the gradient purification. 8c Pre-gradient centrifugation A standard approach is to remove the nuclei from the homogenate and then to pellet a heavy + light mitochondrial fraction as described in Section 4. However many authors prefer to reduce the presence of more slowly sedimenting particles by using just a heavy mitochondrial fraction by using 5000 g for 10-20 min
8d Three-layer bottom-loaded gradients
A commonly used flotation format first used by Choi et al [4,5] in which the crude mitochondrial fraction is suspended in 36% (w/v) iodixanol; overlaid with solutions of 30% and 10% and centrifuged at approx 50,000 g for 4 h. The solutions are prepared by dilution of OptiPrep™ with the homogenization buffer and the mitochondria band at the 10%/30% interface. This has been used for brain/spinal cord [6,7], kidney [8], osteosarcoma cells [9] and HEK cells [2,10]. In a variant of this format, the sample layer was adjusted to 50% iodixanol with centrifugation at 80,000 g for 3 h [10]. Variants of this format were developed by Wood-Allum et al [11] of 44%, 25% and 20% iodixanol (also employed for HEK cells, monkey kidney cells and neuroblastoma cells [3]); 36%, 25%, 17.5% iodixanol gradient for liver [12] and a 36%, 25%, 20% iodixanol gradient centrifuged at 100,000 g for 4 h for HeLa cells [13]. More recently, mitochondria from colon cancer cells [14] and lymphoma cells [15] were purified by flotation from a 36% (w/v) iodixanol solution overlaid by 30% and 10% iodixanol, with centrifugation at 80,000 g [14] or 50,000 g for 3 h [15]. The method as shown in Figure 1 was used for both HEK and HeLa cells [16]
In all these flotation gradients the mitochondria band at an interface between the two lower density iodixanol solutions, a layer of homogenization medium on top is thus not strictly required, but should be included if top-banding organelles are also to be analyzed.
8e Top-loaded gradients
A crude mitochondrial fraction in 15% iodixanol (w/v) from neuroblastoma cells and fibroblasts [17], layered on a 30, 27, 23, 20 and 17% iodixanol gradient centrifuged at 145,000 g for 4 h resolves mitochondria (23-27% interface) from lysosomes (top of gradient). This discontinuous 17%-30% iodixanol gradient, (with the crude fraction in 10% or 15% iodixanol), centrifuged for 2 h, has also been used for mouse hepatic tissue [18] Caco-2 cells [19] and endothelial cells [19] . Similar toplayered gradients of 10, 15, 20, 25, 30% iodixanol, centrifuged at 100,000 g for 1 h, have been used for HEK cells [20], brain, kidney, liver, heart and skeletal muscle [21]. In this gradient the mitochondria banded around the mid-point of the gradient.
8f Median-loaded gradients
In an analysis of the organelles from PHA-blast cells generated from human peripheral blood mononuclear cells, Schmidt et al [22,23] used a discontinuous gradient of 27%, 22.5%, 19%, 16%, 12%, 8% (v/v) Optiprep™, with the sample median loaded in the 19% (v/v) layer and centrifuged at 150,000 g for 5 h. This gradient covers a much lower density range than that which is regularly used; whether this is a requirement of the specific cell type is not known. The lowest and highest density layers are equivalent to approx. 5% and 16% (w/v) iodixanol. The gradient was primarily used to purify secretory lysosomes.
8g References
1. Parone, P.A., Da Cruz, S., Han, J.S., McAlonis-Downes, M., Vetto, A.P., Lee, S.K., Tseng, E., and Cleveland, D.W. (2013) Enhancing mitochondrial calcium buffering capacity reduces aggregation of misfolded SOD1 and motor neuron cell death without extending survival in mouse models of inherited amyotrophic lateral sclerosis J. Neurosci., 33, 4657- 4671
2. Kobuchi, H., Moriya, K., Ogino, T., Fujita, H., Inoue, K., Shuin, T., Yasuda, T., Utsumi, K. and Utsumi, T. (2012) Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid mediated protoporphyrin IX accumulation PLoS One, 7: e50082
3. Bhowmick, R., Halder, U.C., Chattopadhyay, S., Chanda, S., Nandi, S., Bagchi, P., Nayak, M.K., Chakrabarti, O., Kobayashi, N. and Chawla-Sarkar, M. (2012) Rotaviral enterotoxin nonstructural protein 4 targets mitochondria for activation of apoptosis during infection J. Biol. Chem., 287, 35004–35020
4. Choi, Y-S., Ryu, B-K., Min, H-K., Lee, S-W. and Pak, Y.K. (2005) Analysis of proteome bound to D loop region of mitochondrial DNA by DNA-linked affinity chromatography and reverse-phase liquid chromatography/tandem mass spectrometry Ann. N.Y. Acad. Sci., 1042, 88-100
5. Lee, K.H., Kwon, S.J., Woo, J-S., Lee, G-J., Lee, S-R., Jang, H-H. et al (2014) Effects of sildenafil on nanostructural and nanomechanical changes inmitochondria in an ischaemia-reperfusion rat model Clin. Exp. Pharmacol. Physiol., 41, 763–768
6. Lee, M. and Shin, J. (2011) Triage of oxidation-prone proteins by Sqstm1/p62 within the mitochondria Biochem. Biophys. Res. Comm., 413, 122–127
7. Sarafian, T.A., Ryan, C.M., Souda, P., Masliah, E., Kar, U.K., Vinters, H.V., Mathern, G.W., Faull, K.F., Whitelegge, J.P. and Watson, J.B. (2013) Impairment of mitochondria in adult mouse brain overexpressing predominantly full-length, N-terminally acetylated human -synuclein PLoS One, 8: e63557
8. Suna, S-H., Liu, S-Q., Cai, C-P., Cai, R., Chen, L. and Zhang, Q-B. (2012) Down-regulation of alpha 2u globulin in renal mitochondria of STZ-induced diabetic rats observed by a proteomic method Annales d’Endocrinologie 73, 530–541
9. Jeon, J., Jeong J.H., Baek, J-H., Koo, H-J., Park, W-H., Yang, J-S., Yu, M-H., Kim, S. and Pak, Y.K. (2011) Network clustering revealed the systemic alterations of mitochondrial protein expression PLoS Comput Biol 7: e1002093
10. Kim, H.M., Kim, C-S., Lee, J-H., Jang, S.J., Hwang, J.J., Ro, S. and Choi, J. (2013) CG0009, a novel glycogen synthase kinase 3 inhibitor, induces cell death through cyclin D1 depletion in breast cancer cells PLoS One, 8: e60383
11. Wood-Allum, C.A., Barber, S.C., Kirby, J., Heath, P., Holden, H., Mead, R., Higginbottom, A., Allen, S., Beaujeux, T., Alexson, S.E., Ince, P.G. and Shaw, P.J. (2006) Impairment of mitochondrial anti oxidant defence in SOD1-related motor neuron injury and amelioration by ebselen Brain, 129, 1693-1709
12. Kushnareva, Y., Andreyev, A.Y., Kuwana, T. and Newmeyer, D.D. (2012) Bax activation initiates the assembly of a multimeric catalyst that facilitates bax pore formation in mitochondrial outer membranes PLoS Biol., 10: e1001394
13. Tanaka, K., Sugiura, Y., Ichishita, R., Mihara, K. and Oka, T. (2011) KLP6: a newly identified kinesin that regulates the morphology and transport of mitochondria in neuronal cells J. Cell Sci., 124, 2457-2465
14. Shen, S-M., Guo, M., Xiong, Z., Yu, Y., Zhao, X-Y., Zhang, F-F. and Chen, G-Q. (2015) AIF inhibits tumor metastasis by protecting PTEN from oxidation EMBO Rep., 16, 1563–1580
15. Hwang, K.Y. and Choi, Y.B. (2016) Modulation of mitochondrial antiviral signaling by human herpesvirus 8 interferon regulatory factor 1 J. Virol., 90, 506-520
16. Rumlová, M., Křížová, I., Keprová, A., Hadravová, R., Doležal1, M., Strohalmová, K., Pichová, I., Hájek, M. and Rum, T. (2014) HIV-1 protease-induced apoptosis Retrovirology, 11: 37
17. Zhao, H., Ruberu, K., Li, H. and Garner, B. (2013) Analysis of subcellular [57Co] cobalamin distribution in SH-SY5Y neuronsand brain tissue J. Neurosci. Methods, 217, 67– 74
18. Bhattacharyya, S., Feferman, L. and Tobacman, J.K. (2016) Restriction of aerobic metabolism by acquired or innate arylsulfatase B deficiency: a new approach to the Warburg effect Sci. Rep., 6: 32885
19. Bielaszewska, M., Ruter, C., Kunsmann, L., Greune, L., Bauwens, A., Zhang, W., Kuczius, T., Kim, K.S., Mellmann, A., Schmidt, M.A. and Karch, H. (2013) Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis PloS Pathog., 9: e1003797
20. Witkowski, A., Thweatt, J. and Smith, S. (2011) Mammalian ACSF3 protein is a malonyl-CoA synthetase that supplies the chain extender units for mitochondrial fatty acid synthesis J. Biol. Chem., 286, 33729–33736
21. Smith, S., Witkowski, A., Moghul, A., Yoshinaga, Y., Nefedov, M., de Jong, P., Feng, D., Fong, L., Tu, Y., Hu, Y., Young, S.G., Pham, T., Cheung, C., Katzman, S.M., Brand, M.D., Quinlan, C.L., Fens, M., Kuypers, F., Misquitta, S., Griffey, S.M., Tran, S., Gharib, A., Knudsen, J., Hannibal-Bach, H.K., Wang, G., Larkin, S., Thweatt, J. and Pasta, S. (2012) Compromised mitochondrial fatty acid synthesis in transgenic mice results in defective protein lipoylation and energy disequilibrium PLoS One, 7: e47196
22. Schmidt, H., Gelhaus, C., Lucius, R., Nebendahl, M. Leippe, M. and Janssen, O. (2009) Enrichment and analysis of secretory lysosomes from lymphocyte populations BMC Immunol., 10:41
23. Schmidt, H., Gelhaus, C., Nebendahl, M., Lettau, M., Lucius, R., Leippe, M., Kabelitz, D. and Janssen, O. (2011) Effector granules in human T lymphocytes: proteomic evidence for two distinct species of cytotoxic effector vesicles J. Proteome Res., 10, 1603–1620
OptiPrepTM Application Sheet S14 9th edition, January 2020
OptiPrep™ Application Sheet S15
Light mitochondrial fraction analysis (mitochondria, lysosomes and peroxisomes) in continuous gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Application Sheet S14a summarizes the range of methodologies for purifying and fractionating a total or light mitochondrial fraction. Reference List RS03 provides the titles of papers describing the isolation of mitochondria, RS04 – lysosomes and RS02 – peroxisomes. To access these Reference List files return to the initial list of Folders and select “Reference Lists”
- Centrifugation of a post-nuclear supernatant (PNS) at 3000 g for 10 min produces the heavy mitochondrial fraction (HMF) containing principally mitochondria plus some of the lysosomes and peroxisomes. The light mitochondrial fraction (LMF) is the material that sediments from the 3000 g supernatant at 12-20,000g for 10-20 min and contains mitochondria, lysosomes, peroxisomes and some of the microsomes. For a total mitochondrial fraction the 3,000g step is omitted.
1. Background
Both Nycodenz® and iodixanol have been widely used to analyze the organelles of the LMF or HMF+LMF. In iodixanol gradients the density of lysosomes is slightly lower than that in gradients of Nycodenz®; the density of mitochondria is much lower (ρ = 1.13-1.15 against 1.18-1.20 g/ml) while the density of peroxisomes is virtually the same [1]. The density of the endoplasmic reticulum (ER) present in the LMF is rather more variable and may overlap either the lysosomes or the mitochondria. Nevertheless each of the organelles has a distinctive banding pattern in both gradient media. In Percoll® gradients the ER co-bands with peroxisomes [2] and the two organelles cannot be resolved. Modulation of the density range of the gradient may be necessary to optimize a particular separation from a particular tissue or cell type.
- Section 2 of this Application Sheet describes the preparation of the LMF or HMF+LMF by differential centrifugation (for more information see Application Sheet S07.)
- Section 3 describes the use of iodixanol gradients (variants are given in Table 1 in Section 3e).
2. Preparation of LMF or HMF+LMF
2a. Solutions required (see Box 1)
Homogenization medium (HM): 0.25 M sucrose, 1 mM EDTA, 20 mM Hepes-NaOH, pH 7.4 (see Section 2c, Notes 1 and 2)
2b. Protocol
Carry out all operations at 0-4°C.
To prepare the HMF+LMF omit steps 6-8 and use the combined 1000 g supernatants (from step 5) instead of the 3000 g supernatant in step 9.
1. For soft tissues: Mince the tissue very finely with scissors (or with a tissue chopper) and transfer to a Potter-Elvehjem (Teflon and glass) homogenizer with the HM (use 10 ml for every 2.5 g tissue). Homogenize using approx 6 strokes of the pestle at 500-700 rpm (see Section 2c, Note 3).
2. For cells: Wash 1-3×10⁸ cells in 5 ml of phosphate buffered saline and again with 5 ml of HM. Suspend the cells in 3 ml of HM and homogenize in a ball-bearing homogenizer using five passes (see Section 2c, Note 4).
3. Centrifuge the homogenate at 1000 gav for 5 min to pellet the nuclei (do not use the brake to decelerate the rotor); then carefully decant the supernatant or aspirate it using a syringe and metal cannula and retain on ice.
4. Resuspend the pellet in 10 ml (5 ml for cells) of HM using 2-3 gentle strokes of the pestle of a loose-fitting Dounce homogenizer (see Section 2c, Note 5).
5. Repeat the centrifugation and combine the supernatants.
6. To pellet the HMF centrifuge the suspension at 3000 gav for 10 min, then aspirate or decant the supernatant and retain on ice.
7. Resuspend the 3000 g pellet (HMF) in about half the original volume of HM.
8. Gently homogenize the pellet using a loose-fitting Dounce homogenizer and repeat step 6.
9. Centrifuge the combined 3000 g supernatants at 17,000 gav for 10-15 min.
10. Resuspend the pellet (LMF) in a small volume (approx. 2 ml) of HM using a loose-fitting Dounce homogenizer (see Section 2c, Notes 6 and 7).
2c. Notes
1. Protease inhibitors may be included in the HM at the operator’s discretion.
2. Any suitable buffered isoosmotic solution may be used and there is considerable variation in the detailed composition of the HM in the literature. Media, which are most “mitochondria-friendly”, are based on 0.25 mM mannitol rather than sucrose and the EDTA is often replaced with 0.1 mM EGTA for rat liver. Alternatively peroxisome-specific media often contain 0.1% (v/v) ethanol. MOPS is another frequently used buffer.
3. The described methodology applies to tissues such as rodent liver and kidney. Other tissues such as skeletal and cardiac muscle, intestine and brain require special treatments and the operator should consult relevant texts. For more information see Application Sheet S05.
4. The ball-bearing homogenizer (cell cracker) is generally regarded as one of the best devices for cultured cells; delicate organelles are best preserved by this technique. If one is not available, shearing by several passages through a syringe needle may be a reliable alternative. For more information see Application Sheet S06.
5. The nuclei may be very fragile since any homogenization medium containing EDTA is not well suited to the preservation of these organelles. The pellet must be washed by very gently.
6. The LMF may be washed to remove trapped microsomes by suspension to the original volume with HM and repeating steps 9 and 10.
7. If the suspension is layered beneath a gradient, it may be resuspended in the appropriate solution or mixed with a dense solution. In the latter case the total volume may be doubled or trebled.
3. Fractionation of LMF (or HMF+LMF) in a continuous iodixanol gradient
3a. Solutions required (see Section 3d, Note 1)
OptiPrep™ Homogenization medium (HM): see Section 2a OptiPrep™ Diluent (OD): 0.25 M sucrose, 6 mM EDTA, 120 mM Hepes-NaOH, pH 7.4 (see Box 2) Working Solution (WS) of 50% iodixanol (ρ = 1.272 g/ml): 5 vol. of solution OptiPrep™ + 1 vol. of OD
Homogenization medium (HM): see Section 2a OptiPrep™ Diluent (OD): 0.25 M sucrose, 6 mM EDTA, 120 mM Hepes-NaOH, pH 7.4 (see Box 2) Working Solution (WS) of 50% iodixanol (ρ = 1.272 g/ml): 5 vol. of solution OptiPrep™ + 1 vol. of OD
3b. Ultracentrifuge rotor Requirements
Any swinging-bucket rotor for an ultracentrifuge capable of 100,000g with a tube capacity of approx
17 ml tubes, e.g. Beckman SW28.1 or Sorvall AH629 (see Section 3d, Note 2)
3c. Protocol
Bring gradients to 0-4°C and carry out all subsequent steps at this temperature.
1. Dilute WS with HM to produce gradient solutions with iodixanol concentrations of (1) 19% and 27% (w/v) iodixanol (see Section 3d, Notes 3 and 4).
2. Use a two chamber gradient maker or a Gradient Master™ to prepare a linear gradient from 6.0 ml each of the two gradient solutions in 17 ml tubes for the swinging-bucket rotor (see Section 3d, Note 5).
3. Adjust the LMF or HMF+LMF suspension to 30% iodixanol by mixing it with WS and layer 3-4 ml beneath the gradient.
4. Layer 1-2 ml HM on top of the gradient to fill the tube to 3-4 mm from the top of the tube and centrifuge in a suitable swinging-bucket rotor at approx 70,000 gav for 1.5-2 h. Use a slow deceleration program, or if one is not available allow the rotor to decelerate from 2000 rpm without the brake.
5. Collect the gradient in 1 ml fractions either low density end first by upward displacement or dense end first by carefully introducing a narrow metal cannula (connected to a peristaltic pump) to the bottom of the tube, by tube puncture or aspiration from the meniscus. For more information on harvesting gradients see Application Sheet S08.
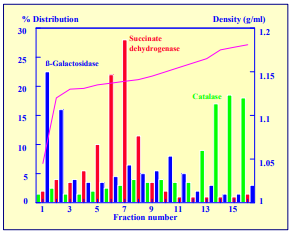 Figure 1 Fractionation of LMF on a pre-formed iodixanol gradient (19-27%): enzyme distribution; Succ deHase = succinate dehydrogenase; ß-Gal’ase = ß-galactosidase.
Figure 1 Fractionation of LMF on a pre-formed iodixanol gradient (19-27%): enzyme distribution; Succ deHase = succinate dehydrogenase; ß-Gal’ase = ß-galactosidase.
3d. Notes
1. Protease inhibitors may be included in OD at the operator’s discretion. Strategies for preparing gradient solutions for mammalian tissues and cells are given in Application Sheet S01.
2. Rotors of other tube capacity are permissible, e.g. Beckman SW41Ti (approx. 13 ml) or SW28 (approx. 39 ml). Scale up or down all the volumes given in the Protocol proportionately. A vertical rotor such as the Beckman VTi50 may also be used. The short path length of such a rotor reduces the hydrostatic pressure on the organelles and allows a reduction in either g-force or centrifugation time (see Table 1).
3. The density of the 19% and 27% (w/v) iodixanol solutions is 1.124 and 1.162 g/ml respectively. Typical results with the gradient are shown in Figure 1.
4. Modulation of the density range of the gradient may be required for other tissue or cell types and for customization to individual requirements (see Section 3e).
5. If one of these devices is not available a continuous gradient can be prepared by diffusion of a discontinuous gradient. The 19-27% iodixanol gradient could be produced from equal volumes of 19%, 22%, 25%, and 27% iodixanol. For more details on the preparation of gradients see Application Sheet S03.
3e. Summary of published gradient conditions
Published papers reporting the use of the method described in this Application Sheet (or a modification of the method) have been sorted alphabetically according to tissue or cell source in Table 1 (see next page). Each entry has a summary of the iodixanol gradient and centrifugation conditions used; whether the LMF or LMF+HMF was loaded on top or beneath the gradient and also an indication of the organelles that were identified in the gradient.
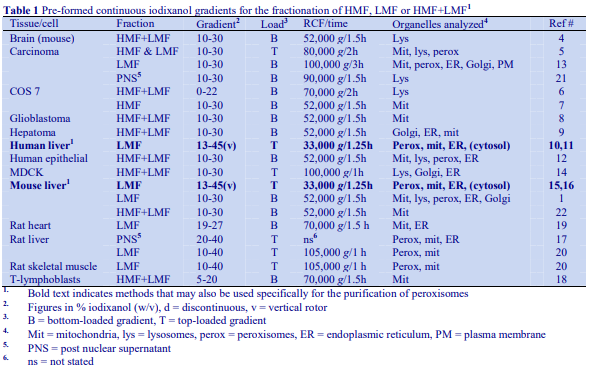
5. Gradient analysis
Iodixanol does not significantly inhibit any enzyme so far tested. Standard spectrophotometric methods (carried out above 340 nm), for measuring organelle enzyme markers can be performed directly on gradient fractions [3]. Protein can also be measured directly by any Coomassie blue-based method [3]. If it is necessary to remove the gradient medium, fractions can be diluted with an equal volume of buffer; pelleted at approx 30,000 gav for 10 min and resuspended in a suitable buffer. Schmidt et al [23] noted that the extensive washing of organelles that was required for organelles purified in Percoll® led to a serious loss of functionality. For more information on analyzing gradients see Application Sheet S09.
6. References
1. Graham, J., Ford, T. and Rickwood, D. (1994) The preparation of subcellular organelles from mouse liver in selfgenerated gradients of iodixanol Anal. Biochem., 220, 367-373
2. Mannaerts, G. P., Van Veldhoven, P., Van Broekhoven, A., Vandebroek, G. and Debeer, L. J. (1982) Evidence that peroxisomal acyl-CoA synthetase is located at the cytoplasmic side of the peroxisomal membrane Biochem. J., 204, 17-23
3. Ford, T., Graham, J. and Rickwood, D. (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of self generated gradients Anal. Biochem., 220, 360-366
4. Murata, Y., Sun-Wada, G-H., Yoshimizu, T., Yamamoto, A., Wada, Y. and Futai, M. (2002) Differential localization of the vacuolar H+ pump with G subunit isoforms (G1 and G2) in mouse neurons J. Biol. Chem., 277, 36296-36303
5. Graf, S.A., Haigh, S.E., Corson, E.D. and Shirhai, O.S. (2004) Targeting, import and dimerization of a mammalian mitochondrial ATP binding cassette (ABC) transporter, ABC10 (ABC-me) J. Biol. Chem., 279, 42954-4296
6. Zhyvoloup, A., Nemazanyy, I., Panasyuk, G., Valovka, T., Fenton, T., Rebholz, H., Wang, M-L., Foxon, R., Lyzogubov, V., Usenko, V., Kyyamova, R., Gorbenko, O., Matsuka, G., Filonenko, V. and Gout, I. T. (2003) Subcellular localization and regulation of coenzyme A synthetase J. Biol. Chem., 278, 50316-50321
7. Seyrantepe, V., Landry, K., Trudel, S., Hassan, J.A., Morales, C.R. and Pshezhetsky, A.V. (2004) Neu4, a novel human lysosomal lumen sialidase, confers normal phenotype to sialidosis and galactosialidosis cells J. Biol. Chem., 279, 37021-37029
8. Sharer, J.D., Shern, J.S., Van Valkenburg, H., Wallace, D.C., and Kahn, R.A. (2002) ARL2 and BART enter mitochondria and bind the adenine nucleotide transporter Mol. Biol. Cell, 13, 71-83
9. Greeve, I., Hermans-Borgmeyer, I., Brellinger, C., Kasper, D., Gomez-Isla, T., Behl, C., Levkau, B. and Nitsch, R.M. (2000) The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer’s disease-associated neurodegeneration and oxidative stress J. Neurosci., 20, 7345-7352
10. Solaas, K., Ulvestad, A., Soreide, O. and Kase, B.F. (2000) Subcellular organization of bile acid amidation in human liver: a key in regulating the biosynthesis of bile salts J. Lipid Res., 41, 1154-1162
11. Solaas, K., Sletta, R.J., Soreide, O. and Kase, B.F. (2000) Presence of cholyl- and chenodexoycholyl- coenzyme A thioesterase activity in human liver Scand. J. Clin. Lab. Invest., 60, 91-102
12. Glunde, K., Guggino, S.E., Ichikawa, Y. and Bhujwalla, Z.M. (2003) A novel method of imaging lysosomes in living human mammary epithelial cells Mol. Imaging, 2, 24-36
13. Maeda, H., Nagata, S., Wolfgang, C.D., Bratthauer, C.D., Bera, T.K. and Pastan, I. (2004) The T cell receptor chain alternate reading frame protein (TARP), a prostate-specific protein localized in mitochondria J. Biol. Chem., 279, 24561-24568
14. Sabo, S.L., Lanier, L.M., Ikin, A.F., Khorkova, O., Sahasrabudhe, S., Greengard, P. and Buxbaum, J.D. (1999) Regulation of -amyloid secretion by FE65, an amyloid protein precursor-binding protein J. Biol. Chem., 274, 7952- 7957
15. Hunt, M.C., Solaas, K., Kase, B.F and Alexon, E.H. (2002) Characterization of an acyl-CoA thioesterase that functions as a major regulator of peroxisomal lipid metabolism J. Biol. Chem., 277, 1128-1138
16. Solaas, K., Kase, B.F., Pham, V., Bamberg, K., Hunt, M.C. and Alexson, S.E.H. (2004) Differential regulation of cytosolic and peroximal bile acid amidation by PPAR activation favors the formation of unconjugated bile acids J. Lipid. Res., 45, 1051-1060
17. Lewin T.M., Van Horn, C.G., Krisans, S.K. and Coleman, R.A. (2002) Rat liver acyl-CoA synthetase 4 is a peripheralmembrane protein located in two distinct subcellular organelles, peroxisomes and mitochondrial-associated membranes Arch. Biochem. Biophys., 404, 263-270
18. Liang, P., Nair, J.R., Song, L., McGuire, J.J. and Dolnick, B.J. (2005) Comparative genomic analysis reveals a novel mitochondrial isoform of human rTS protein and unusual phylogenetic distribution of the rTS gene BMC Genomics, 6:125
19. Goubaeva, F., Mikami, M., Giardina, S., Ding, B., Abe, J. and Yang, J. (2007) Cardiac mitochondrial connexin 43 regulates apoptosis Biochem. Biophys. Res. Comm., 352, 97-103
20. Noland, R.C., Woodlief, T.L., Whitfield, B.R., Manning, S.M., Evans, J.R., Dudek, R.W., Lust, R.M. and Cortright, R.N. (2007) Peroxisomal-mitochondrial oxidation in a rodent model of obesity-associated insulin resistance Am. J. Physiol. Endocrinol. Metab., 293, E986-E1001
21. Weissleder, R., Tung, C-H., Mahmood, U. and Bogdanov, A.(1999) In vivo imaging of tumors with protease-activated near-infrared fluorescent probes Nat. Biotech., 17, 375-378
22. Kwon, J., Han, E., Bui, C-B., Shin, W., Lee, J., Lee, S., Choi, Y-B., Lee, A-H., Lee, K-H., Park, C., Obin, M.S., Park, S.K., Seo, Y.J., Oh, G.T., Lee, H-W. and Shin, J. (2012) Assurance of mitochondrial integrity and mammalian longevity by the p62–Keap1–Nrf2–Nqo1 cascade EMBO Rep., 13, 150–156
23. Schmidt, H., Gelhaus, C., Lucius, R., Nebendahl, M. Leippe, M. and Janssen, O. (2009) Enrichment and analysis of secretory lysosomes from lymphocyte populations BMC Immunol., 10:41
OptiPrepTM Application Sheet S15; 9th edition, January 2020
OptiPrep™ Application Sheet S16
Light mitochondrial fraction analysis (mitochondria, lysosomes and peroxisomes) in self-generated gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- OptiPrep™ Application Sheet S14a provides a protocol review of papers reporting the use of OptiPrep™ for the fractionation and analysis of a total, heavy or light mitochondrial fraction.
- Reference List RS03 focuses on the isolation of mitochondria, RS04 – lysosomes and RS02 – peroxisomes: to access return to the initial list of Folders and select “Reference Lists”
- Centrifugation of a post-nuclear supernatant (PNS) at 3000 g for 10 min produces the heavy mitochondrial fraction (HMF) containing principally mitochondria plus some of the lysosomes and peroxisomes. The light mitochondrial fraction (LMF) is the material that sediments from the 3000 g supernatant at 12-20,000g for 10-20 min and contains mitochondria, lysosomes, peroxisomes and some of the microsomes. For a total mitochondrial fraction the 3,000g step is omitted.
1. Background
This Application Sheet describes the fractionation principally of lysosomes, mitochondria, and peroxisomes from mammalian liver in self-generated gradients of iodixanol. Usually an LMF is prepared for the gradient, but sometimes the material includes the HMF (i.e. the 3000g step of the standard differential centrifugation protocol is omitted). The primary use of the gradients is an analytical one, but they may in certain circumstances also be used preparatively.
In iodixanol gradients the densities of lysosomes is slightly lower than those in gradients of Nycodenz® ; the density of mitochondria is much lower (ρ = 1.13-1.15 against 1.18-1.20 g/ml) while the density of peroxisomes is virtually the same [1]. Note in some published methods other membranes such as Golgi, ER and/or plasma membrane may be identified. The formation of self-generated gradients requires higher g-forces than would be used with a preformed gradient. But, because the sedimentation path length of the rotors used for self generated gradients (vertical, near-vertical or some fixed-angle rotors) is much lower than that of a swingingbucket rotor, the hydrostatic pressure on the organelles is rather similar in the two systems. Compared to pre-formed gradients, there are several major advantages to the use of self-generated gradients for organelle fractionation. (1) Multiple samples can be handled far more easily. (2) To maximize resolution, high concentrations of particles in the sample and the build-up of particles at interfaces during centrifugation should be avoided. With pre-formed gradients this can be achieved only by incorporating the sample into the gradient (not always very convenient) – but this aim is a natural consequence for self-generated gradients as the sample is simply mixed with a suitable solution of iodixanol. (3) Gradient density profiles and hence fractionations are far more reproducible than with pre-formed gradients.
- Section 2 of this Application Sheet describes the preparation of the LMF or HMF+LMF by differential centrifugation. For more information on differential centrifugation see Application Sheet S07.
- Section 3 describes the fractionation of LMF or HMF+LMF in self-generated iodixanol gradients.
- Section 4 describes how modulation of the centrifugation conditions affects the generated density profile and hence the separation of the organelles. For more information on self-generated gradient generation see Application Sheet S04. It also has important information on the analysis of membranes from iodixanol gradients.
- The self-generated gradients were developed for mouse liver but have been adapted to a variety of tissues and cells; some of the variations in protocol are summarized in Section 5.
2. Preparation of LMF or HMF+LMF
2a. Solutions required (see Box 1)
Homogenization medium (HM): 0.25 M sucrose, 1 mM EDTA, 20 mM Hepes-NaOH, pH 7.4 (see Section 2c, Notes 1 and 2)
2b. Protocol
Carry out all operations at 0-4°C.
To prepare the HMF+LMF omit steps 6-8 and use the combined 1000 g supernatants (from step 5) instead of the 3000 g supernatant in step 9.
1. For soft tissues: Mince the tissue very finely with scissors (or with a tissue chopper) and transfer to a Potter-Elvehjem (Teflon and glass) homogenizer with the HM (use 10 ml for every 2.5 g tissue). Homogenize using approx 6 strokes of the pestle at 500-700 rpm (see Section 2c, Note 3).
2. For cells: Wash 1-3×10⁸ cells in 5 ml of phosphate buffered saline and again with 5 ml of HM. Suspend the cells in 3 ml of HM and homogenize in a ball-bearing homogenizer using five passes (see Section 2c, Note 4).
3. Centrifuge the homogenate at 1000 gav for 5 min to pellet the nuclei (do not use the brake to decelerate the rotor); then carefully decant the supernatant or aspirate it using a syringe and metal cannula and retain on ice.
4. Resuspend the pellet in 10 ml (5 ml for cells) of HM using 2-3 gentle strokes of the pestle of a loose-fitting Dounce homogenizer (see Section 2c, Note 5).
5. Repeat the centrifugation and combine the supernatants.
6. To pellet the HMF centrifuge the suspension at 3000 gav for 10 min, then aspirate or decant the supernatant and retain on ice.
7. Resuspend the 3000 g pellet (HMF) in about half the original volume of HM.
8. Gently homogenize the pellet using a loose-fitting Dounce homogenizer and repeat step 6.
9. Centrifuge the combined 3000 g supernatants at 17,000 gav for 10-15 min.
10. Resuspend the pellet (LMF) in a small volume (approx. 2 ml) of HM using a loose-fitting Dounce homogenizer (see Section 2c, Notes 6 and 7).
2c. Notes
1. Protease inhibitors may be included in the HM at the operator’s discretion.
2. Any suitable buffered isoosmotic solution may be used and there is considerable variation in the detailed composition of the HM in the literature. Media, which are most “mitochondria-friendly”, are based on 0.25 mM mannitol rather than sucrose and the EDTA is often replaced with 0.1 mM EGTA for rat liver. Alternatively peroxisome-specific media often contain 0.1% (v/v) ethanol. MOPS is another frequently used buffer (see Section 4a).
3. The described methodology applies to tissues such as rodent liver and kidney. Other tissues such as skeletal and cardiac muscle, intestine and brain require special treatments and the operator should consult relevant texts. For more information see Application Sheet S05.
4. The ball-bearing homogenizer (cell cracker) is generally regarded as one of the best devices for cultured cells; delicate organelles are best preserved by this technique. If one is not available, shearing by several passages through a syringe needle may be a reliable alternative. For more information see Application Sheet S06.
5. The nuclei may be very fragile since any homogenization medium containing EDTA is not well suited to the preservation of these organelles. The pellet must be washed by very gently.
6. The LMF may be washed to remove trapped microsomes by suspension to the original volume with HM and repeating steps 9 and 10. 
3. Self-generated gradient fractionation
3a. Solutions required (see Section 3d, Note 1)
OptiPrep
OptiPrep diluent (OD): 0.25 M sucrose, 6 mM
EDTA, 120 mM Hepes-NaOH, pH 7.4 (see Box 2)
Working Solution (WS): 50% iodixanol ( = 1.272 g/ml): 5 vol. of OptiPrepsolution A + 1 vol. of OD
Homogenization medium (HM): see Section 2a
3b. Ultracentrifuge rotor requirements
A vertical or near vertical rotor with a tube capacity of 10-14 ml or a fixed-angle rotor (tube angle <24°) with a tube capacity <10 ml. The rotor should be able to achieve an RCF of ≥ 180,000g (see Section 3d, Note 2).
3c. Protocol
Carry out all operations at 0-4°C.
1. Mix the resuspended light mitochondrial pellet with WS to the chosen final concentration of iodixanol: 15%, 17.5% or 20% (w/v) (see Section 3d, Note 3).
2. Transfer to tubes for a vertical, near-vertical or low-angle fixed angle rotor (approx 20°). Fill about 90 95% of the tube volume with sample and then layer HM on top to fill the tube.
3. Centrifuge a vertical or near-vertical rotor at 353,000 gav for 1-2 h or a fixed-angle at 270,000 gav for 3 h; allow the rotor to decelerate from 2000 rpm without the brake or use a slow deceleration program (see Section 3d, Note 4).
4. Unload the gradients in 0.5-1.0 ml fractions by upward displacement or, if this is impractical, use tube puncture or aspiration from the bottom. For more information on harvesting gradients, see Application Sheet S08.
5. Analyze the gradients as required, see Application Sheet S09.
3d. Notes
1. Protease inhibitors may be included in OD at the operator’s discretion. Strategies for preparing gradient solutions for mammalian tissues are given in Application Sheet S01.
2. The efficacy of a rotor for self-generated gradient formation should always be assessed by determining the density profile of a blank gradient and comparing it with those described in the Figures in the Section 4 below. The density of the collected fractions is most accurately determined by refractive index (RI) measurement. RI values are given in Application Sheet S01. If a refractometer is not available, then absorbance measurement is another option, for information see Application Sheet S09.
3. The optimum final concentration of iodixanol will depend on the requirements of the operator (i.e. whether it is necessary to resolve all of the organelles or one organelle in particular), see Section 5.
4. The optimum centrifugation condition will depend on the rotor and the required density profile; see Section 5.
4. Gradient Analysis 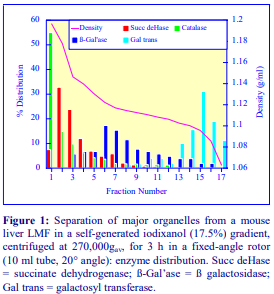
The gradient (see Figure 1) gives acceptable resolution of all the major organelles from mammalian liver and would be ideal for use in an analytical mode. Any ER (not shown) in the light mitochondrial fractions sometimes bands between the mitochondria and the lysosomes, sometimes between the lysosomes and the Golgi (depending on the tissue or cell type). It is also important that the specific activity profiles of the four enzyme markers (not shown) are very similar to the percentage distribution, i.e. yield and purity go together (see ref 1 for more information).
The relative separation of the organelles can be modulated by changing the starting concentration of the iodixanol. If, for example, it is required that the separation of the denser lysosomes from the mitochondria should be improved, at the expense of the resolution of the lighter lysosomes from the Golgi, this can be achieved by increasing the iodixanol starting concentration to 20% (w/v) – see Figure 2. Note the shallower central region of the gradient caused by the use of a lower g-force of 180,000gav rather than 270,000gav. The same lowangle fixed-angle rotor was used in all the separations described.
If the g-force is maintained at the lower value of 180,000gav and the starting iodixanol concentration is reduced to 15% (w/v) then the gradient may be used principally to purify the lighter Golgi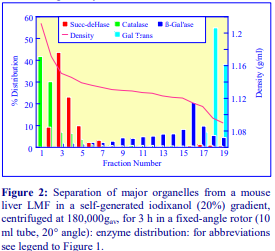 membranes, while the denser organelles are largely confined to the bottom third of the gradient (Figure 3). A fourth variation is raising the iodixanol concentration to 25%, see also Application Sheet S13.
membranes, while the denser organelles are largely confined to the bottom third of the gradient (Figure 3). A fourth variation is raising the iodixanol concentration to 25%, see also Application Sheet S13.
Enzyme activities can be measured directly on the gradient fractions as iodixanol neither inhibits enzymes nor does it interfere with spectrophotometric assays above 340 nm [2]. If however, it is necessary to remove the gradient medium, fractions can be diluted with an equal volume of buffer; pelleted at approx 30,000gav for 10 min and resuspended in a suitable buffer. For more information on analyzing gradients see Application Sheet S09.
Schmidt et al [19] noted that the extensive washing of organelles that was required for organelles purified in Percoll® led to a serious loss of functionality.
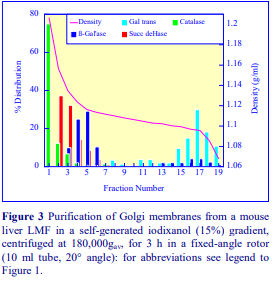
5. Summary of published gradient conditions
Published papers reporting the use of the method described in this Application Sheet (or a modification of the method) have been sorted alphabetically according to tissue or cell source in Table 1). Each entry has a summary of the iodixanol gradient and centrifugation conditions used, the type of rotor that was used, whether a LMF or LMF+HMF fraction was analyzed and also an indication of the organelles that were identified in the gradient.
A strategy first reported by Zhang et al [17,18], who used a large-volume low-angle fixed-angle rotor rather than a vertical or near-vertical rotor. The gradient was first generated from 21 ml of 30% Nycodenz® (layered on top of 1.5 ml of 60% sucrose) at 60,000 g for 24 h. The LMF was then layered on top of the gradient and re-centrifuged for 1 h at 76,000 g. Only a fixed-angle rotor with open-topped tubes allows this novel approach to be executed. Excellent resolution of mitochondria and peroxisomes (rat liver) was observed. In a way it combines the best of both worlds, easy and highly reproducible gradient formation and low g-forces for the organelle separation. It could certainly be adapted to the use of OptiPrep™ but this has not been reported. Occasionally a total post-nuclear supernatant is used, for example as described for retinal pigment epithelial cells [26] and rat hepatoma cells [27].
6. References
1. Graham, J., Ford, T. and Rickwood, D. (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
2. Ford, T., Graham, J. and Rickwood, D. (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of self generated gradients Anal. Biochem., 220, 360-366
3. Tschantz, W. R., Zhang, L. and Casey, P. (1999) Cloning, expression, and cellular localization of a human prenylcysteine lyase J. Biol. Chem., 274, 35802-35808
4. Liang, X-J., Shen, D-W., Garfield, S. and Gottesman, M. M. (2003) Mislocalization of membrane proteins associated with multidrug resistance in cisplastin-resistant cancer cell lines Cancer Res., 63, 5909-5916
5. Lukong, K. E., Seyrantepe, V., Landry, K., Trudel, S., Ahmad, A., Gahl, W. A., Lefrancois, S., Morales, C.R. and Pshezhetsky (2001) Intracellular distribution of lysosomal sialidase is controlled by the internalisation signal in its cytoplasmic tail J. Biol. Chem., 276, 46172-46181
6. Bär, S., Daeffler, L., Rommelaere, J. and Nüesch, J.P.F. (2008) Vesicular egress of non-enveloped lytic parvoviruses depends on gelsolin functioning PloS Pathog., 4:e1000126
7. Nathanson, C-M, Wasselius, J., Wallin, H. and Abrahamson, M. (2002) Regulated expression and intracellular localization of cystatin F in human U937 cells Eur. J. Biochem., 269, 5502-5511
8. Prigozy, T.I., Naidenko, O., Qasba, P., Elewaut, D., Brossay, L., Khurans, A., Natori, T., Koezuka, Y., Kulkarni, A. and Kronenberg, M. (2001) Glycolipid antigen processing for presentation by CD1d molecules Science, 291, 664-667
9. Kung Sutherland, M.S., Sanderson, R.J., Gordon, K.A., Andreyka, J., Cerveny, C.G., Yu, C., Lewis, T.S., Meyer, D.L., Zabinski, R.F., Doronina, S.D., Senter, P.D., Law, C-L., Wahl, A.F. (2006) Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti CD30-auristatin conjugates J. Biol. Chem., 281, 10540-10547
10. Nemeth, B.A., Tsang, S.W.Y., Geske, R.S. and Haney, P. (2000) Golgi targeting of the GLUT1 glucose transporter in lactating mouse mammary gland Pediatr Res., 47, 444-450
11. Flierl, A., Chen, Y., Coskun, P.E., Samulski, R.J. and Wallace, D.C. (2005) Adeno-associated virus mediated gene transfer of the heart/muscle adenine nucleotide translocator (ANT) in mouse Gene Ther., 12, 570-578
12. Bensimon, M., Chang, A.I., Kuroski de Bold, M.L., Ponce, A., Carreras, D. and de Bold A.J. (2004) Participation of G proteins in natriuretic peptide hormone secretion from heart atria Endocrinology, 145, 5313-5321
13. Sultan, A.S., Miyoshi, E., Ihara, Y., Nishikawa, A., Tsukada, Y. and Taniguchi, N. (1997) Bisecting GlcNac structures act as negative sorting signals for cell surface Glycoproteins in forskolin-treated rat hepatoma cells J. Biol. Chem., 272, 2866-2872
14. Gille, L. and Nohl, H. (2000) The existence of a lysosomal redox chain and the role of ubiquinone Arch. Biochem. Biophys., 375, 347-354
15. Nohl, H. and Gille, L. (2002) The biofunctional activity of ubiquinone in lysosomal membranes Biogerontology, 3, 125-131
16. Yu, W., Liang, X., Ensenauer, R.E., Vockley, J., Sweetman, L. and Schultz, H. (2004) Leaky oxidation of a trans –fatty acid J. Biol. Chem., 279, 52160-52167
17. Zhang, D., Liang, X., He, X-Y., Alipui, O. D., Yang, S-Y. and Schulz, H. (2001) 3,5 , 2,4-dienoyl-CoA isomerase is a multifunctional isomerase J. Biol. Chem., 276, 13622-13627
18. Zhang, D., Yu, W., Geisbrecht, B. V., Gould, S., Sprecher, H. and Schulz, H. (2002) Functional characterization of 3 , 2 -enoyl-CoA isomerases from rat liver J. Biol. Chem., 277, 9127-9132
19. Schmidt, H., Gelhaus, C., Lucius, R., Nebendahl, M. Leippe, M. and Janssen, O. (2009) Enrichment and analysis of secretory lysosomes from lymphocyte populations BMC Immunol., 10:41
20. Hall, S.L., Hester, S., Griffin, J.L., Lilley, K.S. and Jackson, A.P. (2009) The organelle proteome of the DT40 lymphocyte cell line Mol.Cell. Proteom., 8, 1295–1305
21. DiMezzo, T.L., Ruthel, G., Brueggemann, E.E., Hines, H.B., Ribot, W.J., Chapman, C.E., Powell, B.S. and Welkos, S.L. (2009) In vitro intracellular trafficking of virulence antigen during infection by Yersinia pestis PLoS One, 4:e6281
22. Nakamura, Y., Ogura, M., Ogura, K., Tanaka, D. and Inagaki, N. (2012) SIRT5 deacetylates and activates urate oxidase in liver mitochondria of mice FEBS Lett., 586, 4076–4081
23. Di Piazza, M., Mader, C., Geletneky, K., Herrero y Calle, M., Weber, E., Schlehofer, L. and Rommelaere, J. (2007) Cytosolic activation of cathepsins mediates parvovirus H-1-induced killing of cisplatin and TRIALresistant glioma cells J. Virol., 81, 4186-4198
24. Li, N., Zheng, Y., Chen, W., Wang, C., Liu, X., He, W., Xu, H. and Cao, X. (2007) Adaptor protein LAPF recruits phosphorylated p53 to lysosomes and triggers lysosomal destabilization in apoptosis Cancer Res., 67, 11176-11185
25. Tringali, C., Cirillo, F., Lamorte, G., Papini, N., Anastasia, L., Lupo, B., Silvestri, I., Tettamanti, G. and Venerando, B. (2012) NEU4L sialidase overexpression promotes -catenin signaling in neuroblastoma cells, enhancing stem-like malignant cell growth Int. J. Cancer, 131, 1768–1778
26. Soni, L.E., Warren, C.M., Bucci, C., Orten, D.J. and Hasson, T. (2005) The unconventional myosin VIIa associates with lysosomes Cell Motil. Cytoskeleton, 62, 13-26
27. Kidane, T.Z., Sauble, E. and Linder, M.C. (2006) Release of iron from ferritin requires lysosomal activity Am. J. Physiol. Cell Physiol., 291, C445-C455
28. Shneyer, B.I., Ušaj, M. and Henn, A. (2016) Myo19 is an outer mitochondrial membrane motor and effector of starvation-induced filopodia J. Cell Sci., 129, 543-556
29. Bertoli, F., Davies, G-L., Monopoli, M.P., Moloney, M., Gunko, Y.K., Salvati, A. and Dawson, K.A. (2014) Magnetic nanoparticles to recover cellular organelles and study the time resolved nanoparticle cell interactome throughout uptake Small, 10, 3307–3315
OptiPrepTM Application Sheet S16; 8th edition, January 2020
OptiPrep™ Application Sheet S17
Purification of yeast spheroplast mitochondria
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- See Section 5 for more information on iodixanol gradients.
1. Introduction
This text is primarily concerned with the application of iodixanol gradients to the purification of yeast spheroplast mitochondria. However because of the popularity and efficacy of the Nycodenz® – based method however, first published by Glick and Pon [1] in 1995, this methodology is summarized in Section 3. Section 4 is devoted to the use of iodixanol gradients. These sections are prefaced by Section 2, which is devoted to the preparation of spheroplasts from cultured yeast cells and the subsequent isolation of a crude mitochondrial fraction. 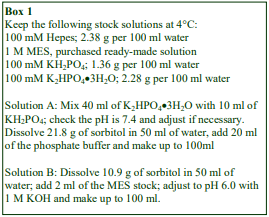
2 Isolation of a crude mitochondrial fraction from yeast spheroplasts
2a. Solutions required (see Section 2d, Notes 1and 2)
A. Spheroplast buffer: 1.2 M sorbitol, 20 mM phosphate buffer, pH 7.4 (see Box 1)
B. Spheroplast lysis medium: 0.6 M sorbitol, 20 mM MES-KOH, pH 6.0 (see Box 1)
2b. Spheroplast production (adapted from ref 1)
The protocol is designed for a 5 l culture of yeast cells, i.e. approx. 35-40 g of packed yeast cells.
1. Use 2.5 mg of Zymolase 20T per g of cells and dissolve in Solution A (2 ml per g of cells).
2. Wash the cells in 40 ml of Solution A and centrifuge at 2000 g for 5 min.
3. Decant the supernatant and suspend the cell pellets in the Zymolase solution and incubate at 30° for 30 min with gentle shaking.
2c. Production of crude mitochondria (adapted from ref 1)
Carry out all operations at 0-4°C. The protocol can be scaled down proportionately as required.
1. Centrifuge the spheroplast suspension at 4000 g for 5 min.
2. Suspend the spheroplasts in Solution A (Box 1) at 40 ml per spheroplast pellet from approx 35 g wet weight of yeast cells and centrifuge at 4000 g for 5 min.
3. Remove the supernatant and repeat steps 1 and 2.
4. Resuspend each spheroplast pellet in 50 ml of Solution B (Box 1) and homogenize in a tight-fitting Dounce homogenizer (Wheaton Type A) using 15 strokes of the pestle (see Section 2d, Note 3).
5. Dilute the homogenate with Solution B (1.5 vol.) and centrifuge at 1500 g for 5 min to pellet the nuclei and unbroken cells.
6. Decant and retain the supernatants; as the pellets may be loosely packed it may be better to
aspirate the supernatants using 20 ml syringe and metal cannula.
7. Resuspend each pellet in Solution B and repeat Steps 4-6.
8. Combine the supernatants and centrifuge at 12,000 g for 10 min.
9. Decant the supernatants and resuspend the crude mitochondrial pellets in Solution B using 3-4 gentle strokes of the pestle of a loose-fitting (Wheaton Type B) Dounce homogenizer (see Note 4).
10. Centrifuge the suspensions at 1500 g for 5 min to remove any aggregates and debris. 11. Using a syringe and metal cannula, aspirate the supernatants carefully; repeat step 8 and resuspend the pellets in one of the solutions described in Sections 3-5.
2d. Notes
1. It is normal practice to add protease inhibitors to all the solutions used in the lysis of the spheroplasts and in all subsequent operations. The chosen inhibitors should be added from routine stock solutions just prior to use. Because of the variable hydration of powdered MES, a commercially available 1 M solution is more convenient.
2. Spheroplasts may be prepared from yeast by zymolase digestion and disrupted in a Dounce homogenizer [1] as described in this Application Sheet; there are however a number of variations. In the method use by Ishihara et al [2] for example, Solution A contained 1.4 M sorbitol, 20 mM Tris-HCl, pH 7.5, in SD(-N) medium, while Solution B contained 1M sorbitol, 0.5% polysucrose (Ficoll) and 1 mM MgCl₂.
3. Alternative methods of homogenization of the spheroplasts are passage of the spheroplast suspension through a 3 μm pore size polycarbonate filter [2] or repeated passage through a fine syringe needle.
4. Resuspension of any crude mitochondrial pellets must be carried out as gently as possible.
3. Nycodenz® gradients
3a Introduction
For yeast grown in a semi-synthetic lactate medium, a top-loaded discontinuous gradient of 14.5% and 18% (w/v) Nycodenz® was devised by Glick and Pon [1]; the mitochondria that band at the interface of the two solutions are highly purified and metabolically active. Glick and Pon [1] however pointed out that the density of the mitochondria depends on the yeast strain and on the growth conditions. Thus if the 14.5%/18% Nycodenz® system was found to be unsatisfactory it was necessary to determine the true density of the mitochondria either in a shallow discontinuous gradient or a continuous gradient first. Once the banding density of the mitochondria, and of the contaminating organelles, has been established, it may be possible to devise a simpler discontinuous gradient. In some instances however, it may be desirable to use a more discriminating continuous gradient if other organelles in the crude mitochondrial fraction are to be studied at the same time. Section 3b will summarize the discontinuous Nycodenz® gradient methodology for wild-type yeast strains (D273-10B, MATα; ATCC 25657) grown in a semi-synthetic lactate medium.
3b. Gradient methodology (see ref 1)
1. Prepare the 14.5% and 18% (w/v) Nycodenz® solutions by diluting 14.5 ml and 18 ml of a 50% Nycodenz® stock solution with 25 ml of 1.2 M sorbitol, 40 mM MES-KOH, pH 6.0 and make up each to 50 ml with water.
2. Resuspend the crude mitochondria pellets in lysis medium (approx. 1 ml per 10 g of original yeast wet weight) using a small volume loose-fitting Dounce homogenizer.
3. In 13 ml tubes for a swinging-bucket rotor (approx 13 ml tubes, e.g. Beckman SW 41Ti, Sorvall TH641 or equivalent), underlayer 6 ml of the 14.5% Nycodenz® solution with the same volume of 18% Nycodenz® and layer 1 ml of the crude mitochondrial suspension on top.
4. Centrifuge at 200,000 gav for 30 min and collect the mitochondria from the interface of the two Nycodenz® solutions; dilute the suspension with approx 4-5 vol. of 0.6 M sorbitol, 20 mM HepesKOH, pH 7.4 Solution C. Pellet the mitochondria at 12,000 g for 10 min and resuspend in the same buffer.
- If the density of these Nycodenz solutions is unsuitable, Glick and Pon [1] recommended
investigating the following alternatives 15% and 21%, 14% and 20%, 13% and 19% or 12% and
18% (w/v).
4. Iodixanol gradients
Solutions of iodixanol of the same concentration as those of Nycodenz® (described above) can be substituted; indeed Lindahl et al [3] reported that this was successful. Although the solutions will have the same density, if prepared in the same way as described in Section 3b (step 1) they will have a lower osmotic pressure. Meeusen et al [4] was the first to describe the use of a customized discontinuous gradient of iodixanol in which the crude mitochondrial fraction is layered beneath the gradient, so that the organelles float to their banding density. Continuous gradients of iodixanol have also been developed; see section 4b.
gradient of iodixanol in which the crude mitochondrial fraction is layered beneath the gradient, so that the organelles float to their banding density. Continuous gradients of iodixanol have also been developed; see section 4b.
4a. Flotation in a discontinuous iodixanol gradient
4a-1. Solutions required (see Section 4a-4, Note 1)
A. OptiPrep™
B. OptiPrep™ dilution buffer: 0.8 M sorbitol, 60 mM Hepes-KOH, pH 7.4 (see Box 2)
C. Iodixanol (40% w/v) working solution: 2 vol. of Solution A + 1 vol. Solution B
D. Mitochondria suspension buffer: 0.6 M sorbitol, 20 mM Hepes-KOH, pH 7.4 (see Box 2)
4a-2. Ultracentrifuge rotor requirements
38 ml swinging-bucket rotor: e.g. Beckman SW28 OR 17 ml swinging-bucket rotor: e.g. Beckman SW28.1 OR 13 ml swinging-bucket rotor: e.g. Beckman SW 41Ti
4a-3. Protocol (adapted from ref 2)
Carry out all operations at 0-4°C. The protocol can be scaled down proportionately as required.
1. Suspend crude mitochondria in approx. 40 ml of Solution D using two or three gentle strokes of the pestle of a loose-fitting Dounce homogenizer and recentrifuge at 12,000 g for 10 min (see Section 4a-4, Note 2).
2. Suspend the washed crude mitochondrial pellet in 20 ml of Solution C (see Section 4a-4, Notes 3 and 4).
3. Prepare iodixanol solutions of ρ = 1.10 and 1.16 g/ml by diluting Solution C with Solution D (3 + 7 and 6.25 + 3.75 v/v respectively).
4. In tubes for the 38 ml swinging-bucket rotor layer 10 ml of the mitochondrial suspension and approx 14 ml each of the ρ = 1.10 and 1.16 g/ml solutions. Scale down all volumes proportionately if smaller volume rotors are used.
5. Centrifuge at 80,000 g for 3 h.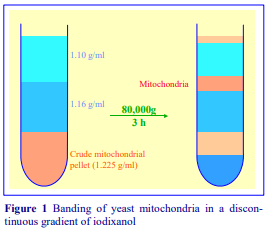
6. Collect the band of mitochondria (see Figure 1); dilute with four volumes of Solution D and harvest the purified organelles at 10,000 g for 10 min (see Section 4a-4, Note 5).
7. Remove any aggregated material by recentrifugation at 3,000 g for 5 min.
4a-4. Notes
1. Yeast spheroplast lysates are usually adjusted to an osmolality of 500-600 mOsm using sorbitol as the principal osmotic balancer. Preparation of solutions of approx 560 mOsm and 750 mOsm using a 40% iodixanol working solution are described in Application Sheet S02. If solutions of a higher iodixanol concentration are required, it is strongly advised that the osmolality of the solutions is checked by using an osmometer and Solution A adjusted as required so that Solutions B has the required osmolality.
2. Resuspension of any crude mitochondrial pellets must be carried out as gently as possible.
3. In the method of Meeusen et al [4], the crude mitochondrial pellet was resuspended in 50% iodixanol rather than 40% iodixanol. This should not affect the efficacy of the separation.
4. It is worth noting that the discontinuous gradient could be adapted to a median loading of the mitochondrial pellet in the 1.16 g/ml solution; a strategy that was found to give better preservation of function than bottom loading in the case of mammalian mitochondria in similar Nycodenz® gradients. It is believed that the lower hydrostatic pressure experienced by the median-loaded mitochondria is responsible. For more information see Application Sheet S14.
5. The method was also used by Tamura et al [5] and Chatterjee et al [6].
4b. Sedimentation in a continuous iodixanol gradient
4b-1. Solutions required
See Section 4a-1 for details.
4b-2. Rotor requirements
Although any of the rotors described in Section 4a-2 may be used, the procedure that is presented in Section 4b-3 uses only 12,000 g so many swinging-bucket rotors for high-speed centrifuges are adequate. Note however that continuous gradients may be used for a more comprehensive fractionation of spheroplast homogenates that will also involve analysis of membranes of the ER, endosomes and vacuole; in these instances much higher g-forces are used (see Section 4b-5)
4b-3. Protocol (adapted from ref 7)
Carry out all operations at 0-4°C. The protocol can be scaled down proportionately as required.
1. Prepare two iodixanol solutions of 2% and 25% (w/v) by mixing Solutions C and D (see Section 4a-1) at 2:38 and 25:15 v/v ratios respectively.
2. Construct a linear gradient from equal volumes of the two solutions using a two chamber gradient maker or a Gradient Master™. In a 13 ml tube, the total gradient volume should be approx 11 ml; in a 17 ml tube approx 14 ml and in a 38 ml tube approx 30 ml (see Section 4b-4, Notes 1-3).
3. Suspend the crude mitochondria in approx 40 ml of Solution D using two or three gentle strokes of the pestle of a loose-fitting Dounce homogenizer and recentrifuge at 12,000g for 10 min.
4. Suspend the washed crude mitochondrial pellet in 10-20 ml of Solution D and layer on top of the linear gradients, filling the tubes according to the manufacturer’s specifications.
5. Centrifuge at 12,000 g for 2 h.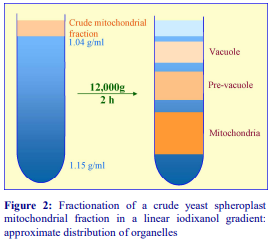
6. Harvest the gradient (see Figure 2) in 0.5-2.0 ml fractions (depending on gradient volume) by tube puncture, upward displacement with a dense medium or aspiration from the meniscus. For more information on the harvesting of gradients see Application Sheet S08
4b-4. Notes
1. If a gradient maker is not available, the gradient may be prepared by allowing a discontinuous gradient of equal volumes of 2%, 8%, 14%, 20% and 25% iodixanol to diffuse. For more information on making gradients see Application Sheet S03.
2. Chen and Kaplan [7] quoted a 0-25% iodixanol gradient, but the 2-25% gradient, which will not affect the subsequent separation, is easier to load with the sample.
3. This gradient has been used [7-12] in studies on the accumulation of Fe by yeast mitochondria.
4b-5. Other continuous gradients
The gradient used by Ishihara et al [13] comprised the following iodixanol (w/v) solutions: 10% (1.5 ml), 15% (2 ml), 20% (2 ml), 25% (1.5 ml), 30% (1 ml), 40% (1 ml) and 50% (0.5 ml) in Solution B (see Section 2a). It was centrifuged at 174,000g for 16 h and will become continuous during the centrifugation. In this gradient endosomes and vacuoles banded in the top third of the gradient and the mitochondria peaked quite sharply at approx 25%. The final continuous density profile of the gradient would reflect both diffusion of iodixanol across the interfaces and sedimentation of iodixanol molecules close to the bottom of the tube.
Narrower range continuous gradients were used by Kerssen et al [14]; the 15.5-36% (w/v) iodixanol gradient, which also contained 18% sucrose, was primarily used for a study of the peroxisome import receptor (Pex5p) but produced a very clear mitochondrial band as well. Gradients of 2.25-24% (w/v) iodixanol centrifuged in a vertical rotor for 90 min at 30,000 g [15] or 48,000 g [16] also gave very good separation of mitochondria from peroxisomes.
5. Summary of other information on iodixanol gradients
Nunnari et al [17] who used a four layer flotation gradient of 1.03, 1.10, 1.16 and 1.29 g/ml at 47,000 for 3 h emphasized the importance of the isoosmotic nature of the gradient. Similar gradients in which the sample was loaded beneath layers of 1.10 and 1.16 g/ml were used by He et al [18, 19] and Lahari et al [20]. Suzuki et al [21] used the gradient to resolve mitochondria and autophagosomes; contact points between ER and mitochondria have also been studied [22].
A study of all the major organelles of yeast cells employed both sucrose and iodixanol gradients; it also stressed the advantages of using nitrogen cavitation as a means of disrupting the cells [23]; the gradients are centrifuged at higher g-forces of approx 90,000 g for 18 h. Sakakibara et al [24] also used a linear iodixanol gradient centrifuged at 150,000 g for 16 h to obtain distinctive profiles of mitochondria ER, cis-Golgi, endosomes and vacuoles.
Gold et al [25-27] developed a small scale separation in a discontinuous iodixanol gradient of 0-27% (in 3% steps) in a Beckman TLS55 rotor (2.2 ml tube volume), to analyze quantum dot labeled mitochondria.
- Important note: Reference List RS15 lists papers reporting the isolation of yeast mitochondria (and other organelles) in iodixanol gradients: to access return to the initial list of Folders and select “Reference Lists” to open Reference List RS15.
6. References
1. Glick, B. J. and Pon, L. A. (1995) Isolation of highly purified mitochondria from Saccharomyces cerevisiae Meth. Enzymol., 260, 213-223
2. Ishihara, N., Hamasaki, M., Yokota, S., Suzuki, K., Kamada, Y., Kihara, A., Yoshimori, T., Noda, T. and Ohsumi, Y (2001) Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion Mol. Biol. Cell, 12, 3690-3702
3. Lindahl, P.A. Garber Morales, J., Miao, R. and Holmes-Hampton, G. (2009) Isolation of Saccharomyces cerevisiae mitochondria for Mössbauer, EPR, and electronic absorption spectroscopic analyses Methods Enzymol., 456, 267-285
4. Meeusen, S., Tieu, Q., Wong, E., Weiss, E., Schieltz, D., Yates, J. R. and Nunnari, J. (1999) Mgm101p is a novel component of the mitochondrial nucleoid that binds DNA and is required for the repair of oxidatively damaged mitochondrial DNA J. Cell Biol., 145, 291-304
5. Tamura, Y., Harada, Y., Nishikawa, S-I, Yamano, K., Kamiya, M., Shiota, T., Kuroda, T., Kuge, O., Sesaki,
H., Imai, K., Tomii, K. and Endo, T. (2013) Tam41 is a CDP-diacylglycerol synthase required for cardiolipin biosynthesis in mitochondria Cell Metab., 17, 709–718
6. Chatterjee, N., Pabla, R. and Siede, W. (2013) Role of polymerase in mitochondrial mutagenesis of Saccharomyces cerevisiae Biochem. Biophys. Res. Comm., 431, 270–273
7. Chen, O. S. and Kaplan, J. (2000) CCC1 suppresses mitochondrial damage in the yeast model of Friedreich’s ataxia by limiting mitochondrial iron accumulation J. Biol. Chem., 275, 7626-7632
8. Radisky, D. C., Babcock, M. C. and Kaplan, J. (1999) The yeast frataxin homologue mediates mitochondrial iron efflux J. Biol. Chem., 274, 4497-4499
9. Yun, C-W., Ferea, T., Rashford, J., Ardon, O., Brown, P. O., Botstein, D., Kaplan, J. and Philpott, C.C. (2000) Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake J. Biol. Chem., 275, 10709-10715
10. Chen, O. S. and Kaplan, J. (2001) YFH1-mediated iron homeostasis is independent of mitochondrial respiration FEBS Lett., 509, 131-134
11. Chen, O. S., Hemenway, S. and Kaplan, J. (2001) Genetic analysis of iron citrate toxicity in yeast: implications for mammalian iron homeostasis Proc. Natl. Acad. Sci. USA, 99, 16922-16927
12. Crisp, R. J., Pollington, A., Galea, C., Jaron, S., Yamaguchi-Iwai, Y. and Kaplan, J. (2003) Inhibition of heme biosynthesis prevents transcription of iron uptake genes in yeast J. Biol. Chem., 278, 45499 45506
13. Ishihara, N., Hamasaki, M., Yokota, S., Suzuki, K., Kamada, Y., Kihara, A., Yoshimori, T., Noda, T. and Ohsumi, Y (2001) Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion Mol. Biol. Cell, 12, 3690-3702
14. Kerssen, D., Hambruch, E., Klaas, W., Platta, H.W., de Kruijff, B., Erdmann, R., Kunau, W-H. and Schleibs, W. (2006) Membrane association of the cycling peroxisome import receptor Pex5p J. Biol. Chem., 281, 27003-27015
15. Oeljeklaus, S., Reinartz, B.S., Wolf, J., Wiese, S., Tonillo, J., Podwojski, K., Kuhlmann, K., Stephan, C., Meyer, H.E., Schliebs, W., Brocard, C., Erdmann, R. and Warscheid, B. (2012) Identification of core components and transient interactors of the peroxisomal importomer by dual-track stable isotope labeling with amino acids in cell culture analysis J. Proteome Res. 2012, 11, 2567−2580
16. Welker, S., Rudolph, B., Frenzel, E., Hagn, F., Liebisch, G., Schmitz, G., Scheuring, J., Kerth, A., Blume, A., Weinkauf, S., Haslbeck, M., Kessler, H. and Buchner, J. (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function Mol. Cell, 39, 507–520
17. Nunnari, J., Wong, E.D., Meeusen, S. and Wagner, J.A. (2002) Studying the behaviour of mitochondria Methods Enzymol., 351, 381-393
18. He, C.H., Xie, L.X., Allan, C.M., Tran, UP.C. and Clarke, C.F. (2014) Coenzyme Q supplementation or overexpression of the yeast Coq8 putative kinase stabilizes multi-subunit Coq polypeptide complexes in yeast coq null mutants Biochim. Biophys. Acta, 1841, 630–644
19. He, C.H., Black, D.S., Nguyen, T.P.T., Wang, C., Srinivasan, C. and Clarke, C.F. (2015) Yeast Coq9 controls deamination of coenzyme Q intermediates that derive from para-aminobenzoic acid Biochim. Biophys. Acta, 1851, 1227–1239
20. Lahiri, S., Chao, J.T., Tavassoli, S., Wong, A.K.O., Choudhary, V. et al (2014) A conserved endoplasmic reticulum membrane protein complex (EMC) facilitates phospholipid transfer from the ER to mitochondria PLoS Biol., 12: e1001969
21. Suzuki, K., Nakamura, S., Morimoto, M., Fujii, K., Noda, N.N., Inagaki, F. and Ohsumi, Y. (2014) Proteomic profiling of autophagosome cargo in Saccharomyces cerevisiae PloS One, 9: e91651
22. Kannan, M., Lahiri, S., Liu, L-K., Choudhary, V. and Prinz, W.A. (2017) Phosphatidylserine synthesis at membrane contact sites promotes its transport out of the ER J. Lipid Res., 58, 553–562
23. Wang, Y., Lilley, K.S. and Oliver, S.G. (2014) A protocol for the subcellular fractionation of Saccharomyces cerevisiae using nitrogen cavitation and density gradient centrifugation Yeast, 31, 127 135
24. Sakakibara, K., Eiyama, A., Suzuki, S.W., Sakoh-Nakatogawa, M., Okumura, N., Tani, M., Hashimoto, A., Nagumo, S., Kondo-Okamoto, N. et al (2015) Phospholipid methylation controls Atg32 mediated mitophagy and Atg8 recycling EMBO J., 134, 2703-2719
25. Gold, V.A.M., Ieva, R., Walter, A., Pfanner, N., van der Laan, M. and Kühlbrandt, W. (2014) Visualizing active membrane protein complexes by electron cryotomography Nat. Commun., 5: 4129
26. Gold, V.A.M., Brandt, T., Cavellini, L., Cohen, M.M., Ieva, R. and van der Laan, M. (2017) Analysis of mitochondrial membrane protein complexes by electron cryo-tomography In Mitochondria: Practical Protocols, Methods in Mol. Biol., 1567, (ed. Mokranjac, D. and Perocchi, F.) Springer Science+Business Media, New York, pp 315-336
27. Gold, V.A.M., Chroscicki, P., Bragoszewski, P. and Chacinska, A. (2017) Visualization of cytosolic ribosomes on the surface of mitochondria by electron cryo-tomography EMBO Rep., 18, 1786-1800
OptiPrepTM Application Sheet S17; 9th edition, February 2020
OptiPrep™ Application Sheet S18
Fractionation of rough and smooth endoplasmic reticulum in self-generated gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- An alternative protocol using a sedimentation velocity iodixanol gradient is described in Application Sheet S19
- An OptiPrep™ Reference List (RS05) “Analysis of membrane trafficking in mammalian tissues and cells: fractionation of ER, Golgi, TGN, PM and endosomes” provides a bibliography of all published papers reporting the use of OptiPrep™ for analysis of these membranes: to access return to the initial list of Folders and select “Reference Lists”.
1. Background
For the analysis of protein synthesis and translocation, the widely-used routine method for separation of the smooth and rough endoplasmic reticulum (SER and RER), which was devised by Walter and Blobel [1], involves a simple 1.3 M sucrose density barrier. In this system the SER bands at the interface while the RER and ribosomes pellet. Call et al [2] noted however that for some cell types (these workers were using plasmacytoma cells) the method did not yield high activity ER microsomes. These workers consequently chose [2,3] the more efficacious self-generated iodixanol gradient protocol that is described in this Application Sheet. Morand et al [4] also observed that this method provided the highly purified SER and RER that was necessary for proteomic analysis. The technique takes advantage of the ability of iodixanol to form reproducible self-generated gradients very rapidly in vertical or near vertical rotors. It simply involves mixing a total microsome fraction with an iodixanol working solution followed by a 2 h centrifugation in a vertical rotor and thus it is actually easier to set up, and takes no longer to execute, than the sucrose density barrier method. Although the banded material in the gradient is normally recovered by collection of the gradient in 10-20 equal volume fractions, the major SER and RER zones are so clearly defined that they may also be retrieved using a syringe. Moreover, as the RER forms a band in the gradient, rather than a gelatinous co-pellet with the ribosomes, its recovery is rather easier. The method was first described for analysis of the assembly of very low density lipoproteins in rabbit hepatocytes [5]. It is thus a very useful means of providing a „snapshot“ of the secretory process at a particular time following administration of appropriate radiolabelled precursors. The protocol is based on data from ref 5.
2. Solutions required (see Section 6, Note 1)
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 10 mM Tris-HCl, pH 7.4
C. OptiPrep™ Diluent: 0.25 M sucrose, 100 mM Tris-HCl, pH 7.4
D. 54% Iodixanol Working Solution (ρ = 1.291 g/ml): mix 9 vol. of Solution A with 1 vol. of Solution C
3. Ultracentrifuge rotor requirements
Vertical or near vertical rotor (10-14 ml tube capacity) capable of approx 350,000 gav, such as the Beckman VTi65.1 or NVT65 or Sorvall 65V13 (see Section 6, Notes 2 and 3)
4. Protocols
Carry out all operations at 0-4°C
4a. Microsome Preparation
1. Prepare an homogenate of the tissue or cells; in this protocol the tissue (liver) is homogenized in solution B using a Potter-Elevehjem homogenizer with 5-6 gentle strokes of the pestle (see Section 6, Note 4).
2. Remove the nuclei by centrifugation at 1000 g for 10 min (see Section 6, Note 5).
3. Carefully decant the 1000 g supernatant and centrifuge it at 15,000 gav for 20 min to pellet the mitochondria, lysosomes, peroxisomes and Golgi membranes.
4. Aspirate the 15,000 g supernatant carefully, using a syringe and metal cannula to avoid disturbing the sometimes loosely-packed upper zone of the pellet (see Section 6, Note 6).
5. Pellet the microsomes from the 15,000 g supernatant by centrifugation at 100,000 gav for 45 min (see Section 6, Notes 7 and 8) and resuspend the pellet in solution B (30 ml per 10g liver).
4b. Fractionation of microsomes
1. Mix 6.3 vol. of microsome suspension with 3.7 vol. of solution D (final iodixanol concentration 20%, w/v; ρ = 1.127 g/ml).
2. Transfer to tubes (11-14 ml) for a vertical rotor or near-vertical rotor.
3. Centrifuge at 350,000 gav for 2 h, turning off the brake during deceleration from approx. 3000 rpm (see Section 6, Note 9).
4. Harvest the gradients by upward displacement with a dense solution or by tube puncture in 0.5-1.0 ml fractions for analysis (see Section 6, Note 10)
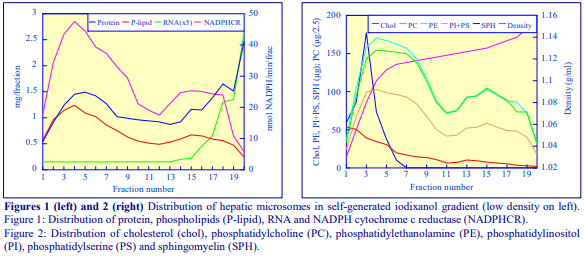
5. Analysis
Protein, lipid and NADPH cytochrome c reductase profiles of the gradient (Figure 1) reveal two broad bands of material. RNA increases gradually in the lower third of the gradient, the very sharp increase in the bottom fraction being accompanied by a rapid fall in phospholipid and NADPH cytochrome c reductase. The SER is thus distributed broadly in the top half of the gradient, the RER in the bottom third and ribosomes band in the last fraction (see Section 6, Note 11).
A phospholipid profile of the gradient (Figure 2) shows that most types follow the same biphasic profile as the protein and total phospholipid; the sphingomyelin on the other hand peaks very sharply in the third fraction. This plasma membrane marker indicates that the lightest vesicles are either derived from the plasma membrane or are destined for incorporation into this membrane. The cholesterol profile is similar to that of the sphingomyelin, in that it increases towards the low-density end of the gradient but it is nevertheless quite distinctive in detail.
This system has been used to show that although the major site of lipid assembly into VLDL occurs in a discrete fraction of the SER, other distinct intracellular pools of lipid may be involved in apolipoprotein B transit into the RER lumen. See ref. 5 for more details.
- The method has been used for analysis of mammalian liver [4, 8-11], rabbit hepatocytes [5], rabbit enterocytes [12,13], human hepatoma [6], T-cell [3], plasmacytoma cells [2], mouse hybridoma [14] and human carcinoma cells [7].
- In a variation of the self-generated gradient, Wang et al [15,16] adjusted the microsomal fraction from carcinoma cell lines to 20% (w/v) iodixanol and centrifuged small volume fractions (1 ml) at 200,000 g overnight. This may be a useful option for laboratories that do not have access to the rotor/ultracentrifuge required as described above.
6. Notes
1. Protease inhibitors (PMSF, leupeptin, antipain, aprotinin etc) may be included in any or all of the media at the operator’s discretion. Strategies for preparing working solutions for mammalian tissues are given in Application Sheet S01.
2. The sedimentation path length of the tube should be approx 17 mm. The preparation may be scaled down to approx 5 ml tubes with, for example, the Beckman VTi65.2 vertical rotor. It should be possible to reduce the centrifugation time to approx 1 h 45 min with this smaller volume rotor, although Morand et al [4] actually used just over 2 h. It may be feasible to use a small volume high-performance fixed-angle rotor but it will be necessary to modulate the centrifugation conditions in order to produce a gradient with the correct density profile. For more information on formation of self-generated gradients see Application Sheet S04.
3. The tubes of choice are Optiseal tubes, which are only available for Beckman rotors. Since they are sealed with a central plastic plus rather then heat- or crimp-sealed, they are easy to use and most importantly, gradients within them can be unloaded by any of the standard techniques available to open-topped tubes for swinging-bucket rotors.
4. Solution B may be any buffered isoosmotic sucrose solution. Alternatively, for some cultured cells, it may be necessary to swell them prior to homogenization. Call et al [2,3,] for example used 10 mM Hepes-KOH, pH 7.5 to swell plasmacytoma cells. Always return the homogenate to isoosmotic conditions as soon as possible. For more information about homogenization of tissues and cells see respectively Application Sheets S05 and S06.
5. The 1000 g step may be omitted, but retention of step 2 may improve yields. If the homogenate is treated directly as in step 4, large amounts of rapidly sedimenting nuclei and debris may trap a lot of other smaller particles.
6. The centrifugation conditions used to remove the light mitochondrial pellet organelles vary from 10,000 g to 17,000 g and from 10-20 min. For more information on differential centrifugation see Application Sheet S07.
7. Centrifugation conditions for pelleting microsomes vary; Higashi et al [6] used 138,000 g for 1 h
8. It is possible to omit the 100,000 g sedimentation of the microsomes and simply take the 12,000 g supernatant and adjust this to 20% iodixanol. Although this is quicker, it has the disadvantage that the cytosolic proteins contaminate the gradient fractions; these can however be removed subsequently by pelleting the membranes.
9. Higashi et al [6] used 100,000 g for 13 h, although rotor was not specified. Liao and Carpenter [7] also used an overnight centrifugation (at 200,000 g) in small volumes (1 ml/tube) but again the rotor was not given.
10. For more information on the harvesting of gradients Application Sheet S08.
11. The density profile (see Figure 2), which is steep at the top of the gradient, is not optimal for resolving vesicles in the low-density region. To study these vesicles more satisfactorily, or, for example, if it is required to study specifically either the SER or the RER, the density profile of the gradient can be altered, either by changing the starting concentration of the iodixanol or the centrifugation conditions, see Application Sheet S04.
7. References
1. Walter, P. and Blobel, G, (1983) Preparation of microsomal membranes for cotranslational protein translocation Meth. Enzymol., 96, 84-93
2. Call, M.E., Pyrdol, J., Wiedmann, M. and Wucherpfennig, K.W. (2002) The organizing principle in the formation of the T cell receptor-CD3 complex Cell, 111, 967-979
3. Call, M.E., Pyrdol, J. and Wucherpfennig, K.W. (2004) Stoichiometry of the T-cell receptor-CD3 complex and key intermediates assembled in the endoplasmic reticulum EMBO J., 22, 2348-2357
4. Morand, J-P. F., Macri, J. and Adeli, K. (2005) Proteomic profiling of hepatic endoplasmic reticulumassociated proteins in an animal model of insulin resistance and metabolic dyslipidemia J. Biol. Chem., 280, 17626-17633
5. Cartwright, I. J., Higgins, J. A., Wilkinson, J., Bellavia, S., Kendrick, J. S. and Graham, J. M. (1997) Investigation of the role of lipids in the assembly of very low density lipoproteins in rabbit hepatocytes J. Lipid Res., 38, 531-545
6. Higashi, Y., Itabe, H., Fukase, H., Mori, M., Fujimoto, Y., Sato, R., Imanaka, T. and Takano, T. (2002) Biochim. Biophys. Acta, 1581, 127-136
7. Liao, H-J. and Carpenter, G. (2007) Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression Mol. Biol. Cell, 18, 1064-1072
8. Iddon, R., Wilkinson, J., Bennett, A.J., Bennett, J., Salter, A.M. and Higgins, J.A. (2001) A role for smooth endoplasmic reticulum membrane cholesterol ester in determining the intracellular location and regulation of sterol-regulatory element-binding protein-2 Biochem. J., 358, 415-422
9. Wilkinson, J., Higgins, J.A., Fitzsimmons, C. and Bowyer, D.E. (1998) Dietary fish oils modify the assembly of VLDL and expression of the LDL receptor in rabbit liver Arterioscler. Thromb. Vasc. Biol., 18, 1490-1497
10. Oyadomari, S., Yun, C., Fisher, E.A., Kreglinger, N., Kreibich, G., Oyadomari, M., Harding, H.P., Goodman, A.G., Harant, H., Garrison, J.L., Taunton, J., Katze, M.G. and Ron, D. (2006) Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload Cell, 126, 727-739
11. Massarweh, A., Bosco, M., Iatmanen-Harbi, S., Tessier, C., Auberger, N., Busca, P., Chantret, I., GravierPelletier, C. and Moore, S.E.H. (2106) Demonstration of an oligosaccharide-diphosphodolichol diphosphatase activity whose subcellular localization is different than those of dolichyl-phosphate dependent enzymes of the dolichol cycle J. Lipid Res., 57, 1029–1042
12. Cartwright, I.J., Plonne, D. and Higgins, J.A. (2000) Intracellular events in the assembly of chylomicrons in rabbit enterocytes J. Lipid Res., 41, 1728-1739
13. Cartwright, I.J. and Higgins, J.A. (2001) Direct evidence for a two-step assembly of apoB48 containing lipoproteins in the lumen of the smooth endoplasmic reticulum of rabbit enterocytes J. Biol. Chem., 276, 48048-48057
14. Xu, C., Call, M.E. and Wucherpfennig, K.W. (2006) A membrane-proximal tetracysteine motif contributes to assembly of CD3 and CD3 dimers with the T cell receptor J. Biol. Chem., 281, 36977-36984
15. Wang, Y-N., Yamaguchi, H., Huo, L., Du, Y., Lee, H-J., Lee, H-H., Wang, H., Hsu, J-M. Hung, M-C. (2010) The translocon Sec61 localized in the inner nuclear membrane transports membrane-embedded EGF receptor to the nucleus J. Biol. Chem., 285, 38720–38729
16. Wang, Y-N., Lee, H-H., Lee, H-J., Du, Y., Yamaguchi, H. and Hung, M-C. (2012) Membrane-bound trafficking regulates nuclear transport of integral epidermal growth factor receptor (EGFR) and ErbB-2 J. Biol. Chem., 287, 16869-16879
OptiPrepTM Application Sheet S18; 9th edition, January 2020
OptiPrep™ Application Sheet S19
Fractionation of rough and smooth endoplasmic reticulum and subfractionation of ER from cultured mammalian cells
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- An alternative self-generated iodixanol gradient protocol is described in Application Sheet S18
- See Section 3 for a comment on the use of pancreatic tissue.
- Section 4 contains some comments about ER subfractionation.
- An OptiPrep™ Reference List (RS05) “Analysis of membrane trafficking in mammalian tissues and cells: fractionation of ER, Golgi, TGN, PM and endosomes” provides a bibliography of all published papers reporting the use of OptiPrep™ for analysis of these membranes: to access return to the initial list of Folders and select “Reference Lists”.
1. Sedimentation velocity gradients
1a. Background
For the analysis of protein synthesis and translocation, the widely-used routine method for separation of the smooth and rough endoplasmic reticulum (SER and RER), which was devised by Walter and Blobel [1], involves a simple 1.3 M sucrose density barrier. In this system the SER bands at the interface while the RER and ribosomes pellet. Call et al [2] noted however that for some cell types the method did not yield high activity ER microsomes.
The protocol in this Application Sheet describes an alternative discontinuous iodixanol gradient that separates the SER and RER by sedimentation velocity. Although the gradient set-up time is longer than that of the self-generated gradient or the sucrose density barrier, the centrifugation time is only 30 min and uses a routine swinging-bucket rotor. As with the self-generated gradient, the RER forms a band in the gradient, rather than the gelatinous co-pellet with the ribosomes of the sucrose barrier technique, so its recovery from the gradient is rather easier. The method may also permit some subfractionation within both the SER and RER bands.
Although the banded material in the gradient is normally recovered by collection of the gradient in 10-20 equal volume fractions, the major SER and RER zones are so clearly defined that they may also be retrieved using a syringe.
The method was first described by Majoul et al [3] for analyzing KDEL protein transport from the plasma membrane to the ER in Vero cells and much more recently by Sannerud et al [4] in the analysis of ER-Golgi trafficking. The protocol is adapted from refs 3 and 4.
membrane to the ER in Vero cells and much more recently by Sannerud et al [4] in the analysis of ER-Golgi trafficking. The protocol is adapted from refs 3 and 4.
1b. Solutions required (see Section 1e, Note 1)
A. OptiPrep™
B. Phosphate buffered saline
C. Cell wash medium: 140 mM NaCl, 30 mM KCl, 10 mM EDTA, 25 mM Tris-HCl, pH 7.4
D. Homogenization medium: 130 mM KCl, 25 mM NaCl, 1 mM EGTA, 25 mM Tris-HCl, pH 7.4
1c. Ultracentrifuge rotor requirements
Any swinging-bucket rotor, capable of approx 150,000g, with approx. 13-14 ml tubes (e.g. Beckman SW 41 or Sorvall TH641) or approx 17 ml tubes (e.g. Beckman SW28 or SW28.1 or Sorvall AH629)
1d. Protocol
Carry out all operations at 0-4°C
- Although the published method involved construction of a multi-step continuous gradient, the gradient becomes essentially continuous and linear during preparation and centrifugation. A more simple option may be to make a continuous gradient initially (see options in Steps 1 and 5)
1. For the discontinuous gradient: prepare solutions of 5%, 7.5%, 10%, 12.5%, 15%, 17.5%, 20%, 22.5% and 25% (w/v) iodixanol by diluting OptiPrep with Solution D OR for the continuous gradient prepare 5% and 25% (w/v) iodixanol only (see Section 1e, Note 2).
2. Wash the cells twice in Solution B and twice in Solution C.
3. Resuspend the cells in Solution D and prepare an homogenate using a ball-bearing homogenizer (cell cracker) or by repeated passage (approx. 10 passages) through a narrow gauge syringe needle (24-25G) or use a tight-fitting Dounce homogenizer (see Section 1e, Notes 3 and 4)
4. Remove the nuclei and heavy mitochondria fractions by centrifugation at 3000 g for 10 min (see Step 5 and Section 1e, Note 5).
5. During the centrifugation prepare a discontinuous gradient from 1.2 ml (13 ml tube) or 1.7 ml (17 ml tube) each of the nine iodixanol solutions OR using a two-chamber gradient maker or Gradient Master™ from equal volumes (5.5 ml or 7.5 ml) of the two iodixanol solutions for the 13 or 17 ml tubes respectively (see Section 1e, Note 6).
6. Layer 0.5-1.0 ml of the supernatant from step 4 on top of each gradient (see Section 1e, Note 7).
7. Centrifuge at 126,000 g for 30 min.
8. Harvest the gradients by upward displacement with a dense solution, by tube puncture or aspiration from the meniscus in 0.5-1.0 ml fractions for analysis (see Section 1e, Notes 8 and 9).
1e. Notes
1. Protease inhibitors (PMSF, leupeptin, antipain, aprotinin etc) may be included in any or all of the media at the operator’s discretion. Strategies for preparing working solutions for mammalian tissues are given in Application Sheet S01.
2. Majoul et al [3] used a slightly different discontinuous iodixanol gradient in which the two densest solutions were 25% and 30% (w/v) iodixanol rather than 22.5% and 25%.
3. Some cells may require osmotic swelling prior to homogenization. The hypoosmotic medium can be a simple low concentration buffer such as 10 mM Hepes-KOH, pH 7.5. Always return the homogenate to isoosmotic conditions as soon as possible. For more information about homogenization of cells see Application Sheet S06.
4. Note that the volume of the homogenate should be kept to a minimum; as the separation is based on sedimentation velocity only 0.5-1.0 ml of material may be placed atop each gradient.
5. An optional 1000 g/10 min step may be inserted prior to the 3000 g centrifugation. This may prevent large amounts of rapidly sedimenting nuclei and debris trapping a lot of other smaller particles. Note that in buoyant density fractionations (see Section 2a) it is common to use a high gforce (12,000 g) to remove the larger organelles.
6. For more information on construction of both discontinuous and continuous gradients see Application Sheet S03.
7. If the volume of the 3000g supernatant is inconveniently large, it may be necessary to pellet all of the particulate material at 100,000 g for 45 min and resuspending the pellet in a smaller volume. Centrifugation conditions for pelleting microsomes vary, Higashi et al [5] used 138,000 g for 1 h
8. For more information on the harvesting of gradients see Application Sheet S08.
9. Once the position of the SER and RER bands has been established it may be possible to remove them with a syringe. The SER peaks approx 1/3rd and the RER approx 2/3rd of the way down the tube.
2. Buoyant density fractionations
2a. Discontinuous gradients
In an alternative method devised by Kleene et al [6] for neuroblastoma cells, a microsomal fraction was sedimented from a 12,000 g/15 min supernatant; this was then resuspended in 0.32 M sucrose, 10 mM Tris-HCl, pH 7.4 and adjusted to 20% (w/v) iodixanol. It was layered between 30% and 15% iodixanol and centrifuged at 150,000 g for 3 h. The SER banded at the 15%/20% iodixanol interface and the RER 20%/30% iodixanol interface. This is a good example of using flotation of one membrane type and sedimentation of another to maximize resolution.
The strategy of layering the crude microsomes in 20% (w/v) iodixanol between 30% and 15% iodixanol is part of that the protocol recommended in the Sigma-Aldrich Endoplasmic Reticulum Isolation Kit. Its use has been reported in publications for neuroblastoma cells [7,8] and mouse pancreas [9]. Some methods omit the light mitochondrial centrifugation step and centrifuge a postnuclear supernatant (PNS) at 200,000 g [9]; the presence of all of the other major organelles (mitochondria, peroxisomes and lysosomes) in the gradient input will however severely test the gradient’s efficacy. The inclusion of a 12,000 g centrifugation of the PNS is strongly recommended.
More recently Ramming et al [10] used a discontinuous gradient of 20%, 16.25%, 12.5%, 8.75% and 5% (w/v) iodixanol, centrifuged at approx. 100,000 g for 3 h in a small volume swinging-bucket rotor (2.2 ml tubes).
2b. Continuous gradients
Geiger et al [11] sedimented (100,000 g for 1 h) all of the membranes from a post-nuclear supernatant from CHO cells; resuspended them in 0.25 M sucrose, 1 mM EDTA, 10 mM Hepes-NaOH Tris-HCl, pH 7.4 and layered 0.5 ml on top of an approx. 13 ml 0-15% (w/v) iodixanol gradient (prepared by dilution of OptiPrep™ with the same medium). After centrifugation at 75,000 g for 18 h, the RER banded at the bottom of the gradient and the SER→ERGIC banded at the top. A rather broader density range (0-26% iodixanol) was used by Uribe et al [12] for macrophages. A 2000 g supernatant from a HeLa cell homogenate was adjusted to 40% (w/v) iodixanol and loaded beneath a 0-35% (w/v) iodixanol gradient. Centrifugation at 94,000 g for 17 h separated a broadly banded ER from the cytoskeleton, which remained at the bottom of the gradient [13]. Bottom-loading is certainly the sample-loading method of choice in resolving potentially dense protein components from membranes.
3. Analysis of pancreatic RER
Hori et al [14] incubated RER (isolated by differential centrifugation) in either a low- or high-salt buffer and then layered them on to a 20-30% (w/v) iodixanol gradient and centrifuged in a small volume fixed-angle rotor (Beckman TLA100.4) and centrifuged at approx. 100,000 gav for 140 min.
4. Subfractionation of ER
There is evidence that iodixanol gradients are able to fractionate ER on the basis of its function and cytoplasmic localization; Woods et al [15] were able to use a continuous iodixanol gradient to separate perinuclear ER from 3T3 cells on the basis of its paxillin enrichment. For more details see Application Sheet S22. Separation of transitional and peripheral ER from mouse embryo fibroblasts was achieved on 10-40% (w/v) iodixanol gradients centrifuged at 48,000 g for 18 h [16]. See also Application Sheet S41.
More recently use of a 10-34% (w/v) iodixanol gradient (approx 100,000 g for 16 h) showed that α1,2 mannosidase I located to a distinct subset of the ER; its sharp-peaked distribution pattern contrasted markedly with a broad distribution of a general ER-marker (calnexin) and was quite distinct from the Golgi [17,18]. A high-density fraction of liver tissue ER harvested from a sucrose gradient has also been further fractionated in an iodixanol gradient in a study of non-alcoholic steatohepatitis [19]. Wang et al [20] showed that the ER distribution of unesterified cholesterol in a 6-27% iodixanol gradient was altered by reductions in intracellular Ca2+ of mouse embryonic fibroblasts.
5. References
1. Walter, P. and Blobel, G, (1983) Preparation of microsomal membranes for cotranslational protein translocation Meth. Enzymol., 96, 84-93
2. Call, M.E., Pyrdol, J., Wiedmann, M. and Wucherpfennig, K.W. (2002) The organizing principle in the formation of the T cell receptor-CD3 complex Cell, 111, 967-979
3. Majoul, I.V., Bastiaens, P.I.H. and Soling H-D (1996) Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells J. Cell Biol., 133, 777-789
4. Sannerud, R., Marie, M., Nizak, C., Dale, H.A., Pernet-Gallay, K., Perez, F., Goud, B. and Saraste, J. (2006) Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells Mol. Biol. Cell, 17, 1514-1526
5. Higashi, Y., Itabe, H., Fukase, H., Mori, M., Fujimoto, Y., Sato, R., Imanaka, T. and Takano, T. (2002) Distribution of microsomal triglyceride transfer protein within sub-endoplasmic reticulum in human hepatoma cells Biochim. Biophys. Acta, 1581, 127-136
6. Kleene, R., Mzoughi, M., Joshi, G., Kalus, I., Bormann, U., Schulze, C., Xiao, M-F., Dityatev, A. and Schachner, M. (2010) NCAM-induced neurite outgrowth depends on binding of calmodulin to NCAM and on nuclear import of NCAM and fak fragments J. Neurosci., 30, 10784 –10798
7. Lutz, D., Wolters-Eisfeld, G., Joshi, G., Djogo, N., Jakovcevski, I., Schachner, M. and Kleene, R. (2012) Generation and nuclear translocation of sumoylated transmembrane fragment of cell adhesion molecule L1 J. Biol. Chem., 287, 17161–17175
8. Tringali, C., Cirillo, F., Lamorte, G., Papini, N., Anastasia, L., Lupo, B., Silvestri, I., Tettamanti, G. and Venerando, B. (2012) NEU4L sialidase overexpression promotes -catenin signaling in neuroblastoma cells, enhancing stem-like malignant cell growth Int. J. Cancer, 131, 1768–1778
9. Chambers, J.E., Petrova, K., Tomba, G., Vendruscolo, M. and Ron, D. (2012) ADP ribosylation adapts an ER chaperone response to short-term fluctuations in unfolded protein load J. Cell Biol., 198, 371–385
10. Ramming, T., Hansen, H.G., Nagata, K., Ellgaard, L. and Appenzeller-Herzog, C. (2014) GPx8 peroxidase prevents leakage of H2O2 from the endoplasmic reticulum Free Radical Biol. Med., 70, 106–116
11. Geiger, R., Gautschi, M., Thor, F., Hayer, A. and Helenius, A. (2011) Folding, quality control, and secretion of pancreatic ribonuclease in live cells J. Biol. Chem., 286, 5813–5822
12. Uribe. K.B., Etxebarria., A., Martin. C. and Ostolaza, H. (2013) Calpain-mediated processing of adenylate cyclase toxin generates a cytosolic soluble catalytically active N-terminal domain PLoS One, 8: e67648
13. Maruri-Avidal, L., Weisberg, A.S. and Moss, B. (2013) Association of the vaccinia virus A11 protein with the endoplasmic reticulum and crescent precursors of immature virions J. Virol., 87, 10195–10206
14. Hori, O., Miyazaki, M., Tamatini, T., Ozawa, K., Takano, K., Okabe, M., Ikawa, M., Hartmann, E., Mai, D.M., Kitao, M. and Ogawa, S. (2006) Deletion of SERP1/RAMP4, a component of the endoplasmic reticulum (ER) translocation sites, leads to ER stress Mol. Cell. Biol., 26, 4257-4267
15. Woods, A.J., Roberts, M.S., Choudhary, J., Barry, S.T., Mazaki, Y., Sabe, H., Morley, S.J., Critchley, D.R. and Norman, J.C. (2002) Paxillin associates with poly(A)-binding protein 1 at the dense endoplasmic reticulum and the leading edge of migrating cells J. Biol. Chem., 277, 6428-6437
16. Cali, T., Galli, C., Olivari, S. and Molinari, M. (2008) Segregation and rapid turnover of EDEM1 by an autophagy-like mechanism modulates standard ERAD and folding activities Biochem. Biophys. res. Commun., 371, 405-410
17. Benyair, R., Ogen-Shtern, N., Mazkereth, N., Shai, B., Ehrlich, M. and Lederkremer, G.Z. (2015) Mammalian ER mannosidase I resides in quality control vesicles, where it encounters its glycoprotein substrates Mol. Biol. Cell, 26, 172-184
18. Ogen-Shtern, N., Avezov, E., Shenkman, M., Benyair, R. and Lederkremer, G.Z. (2016) Mannosidase IA is in quality control vesicles and participates in glycoprotein targeting to ERAD J. Mol. Biol., 428, 3194–3205
19. Bashiri, A., Nesan, D., Tavallaee, G., Sue-Chue-Lam, I., Chien, K., Maguire, G.F., Naples, M. et al (2016) Cellular cholesterol accumulation modulates high fat high sucrose (HFHS) diet-induced ER stress and hepatic inflammasome activation in the development of non-alcoholic steatohepatitis Biochim. Biophys. Acta, 1861, 594–605
20. Wang, W-A., Liu, W-X., Durnaoglu, S., Lee, S-K., Lian, J., Lehner, R., Ahnn, J., Agellon, L.B. and Michalak, M. (2017) Loss of calreticulin uncovers a critical role for calcium in regulating cellular lipid homeostasis Sci. Rep., 7: 5941
OptiPrepTM Application Sheet S19; 9th edition, January 2020
OptiPrep™ Application Sheet S20
Fractionation of smooth and rough endoplasmic reticulum and separation from Golgi (and other organelles)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- For a simplified system for separating ER and Golgi see Section 6
- An OptiPrep™ Reference List (RS05) “Analysis of membrane trafficking in mammalian tissues and cells: fractionation of ER, Golgi, TGN, PM and endosomes” provides a bibliography of all published papers reporting the use of OptiPrep™ for analysis of these membranes: to access return to the initial list of Folders and select “Reference Lists”
1. Background
The self-generated gradient system described in Application Sheet S18 provides excellent resolution of the smooth and rough endoplasmic reticulum (SER and RER), but there is no clear separation of Golgi membranes from the lighter SER vesicles. In this Application Sheet the gradient has been modified to take account of the requirement for a gradient that can achieve simultaneous resolution of Golgi, SER and RER.
Such a gradient needs to be reasonably shallow over its entire density range. Although it is possible to achieve such a gradient profile by increasing the centrifugation time (to approx 3 h) using the 20% iodixanol starting concentration described in Application Sheet S18, Plonné et al [1] preferred an alternative approach of using a biphasic iodixanol starting concentration (equal volumes of 15% and 20% iodixanol). Such a technique has been previously shown to provide shallow gradients over a quite wide density range while maintaining either relatively short centrifugation times or low RCFs [2]. It is particularly important in the use of density gradients to analyze secretion and endocytosis to keep the centrifugation time and any concomitant proteolysis to a minimum.
If the microsomal fraction is only included in the high density layer (20% iodixanol), the Golgi and smooth ER will float out of the load zone into the gradient formed within the 15% iodixanol layer. Any soluble proteins in the crude microsomes on the other hand will tend to sediment through the 20% layer. If it is important to resolve soluble and membrane-bound proteins, such a system might be preferable to one in which the crude microsomes are distributed throughout the starting solution. The protocol described in this Application Sheet was designed for rat liver or for isolated rat hepatocytes [1], but might be extended (with or without modification) to other tissue or cell types.
2. Solutions required (see Section 7, Notes 1 and 2)
A. OptiPrep™
B. Isolation medium: 0.25 M sucrose, 10 mM HepesNaOH, pH 7.8
C. Diluent: 0.25 M sucrose, 60 mM Hepes-NaOH, pH 7.4
D. Working Solution of 50% (w/v) iodixanol (ρ = 1.272 g/ml): 5 vol. of solution A + 1 vol. of solution C
3. Ultracentrifuge rotor requirements
A vertical or near-vertical rotor (tube size approx 11 ml) capable of producing >300,000gav, such as the Beckman VTi 65.1 or NVT65 or Sorvall 65V13 (see Section 7, Notes 3 and 4)
4. Protocol (for rat liver)
Carry out all operations at 0-4°C.
1. Mince the liver with scissors and then homogenize in Solution B (4 ml/g liver) using 30 strokes of the pestle of a loose-fitting Dounce homogenizer (see Section 7, Notes 5 and 6)
2. Centrifuge the homogenate at 10,000 g for 20 min to pellet most of the larger organelles (see Section 7, Note 7).
3. Centrifuge the 10,000 g supernatant at 100,000 g for 40 min and resuspend the microsomal pellet in Solution B (5.0 ml per 2 g liver), using 20 strokes of the pestle of the Dounce homogenizer.
4. Mix 3 vol of the microsome suspension with 2 vol of Solution D (see Section 7, Note 8).
5. Transfer 4.5 ml to a vertical rotor tube and underlayer with 1.8 ml of 30% (w/v) iodixanol, made from 3 vol. of Solution D + 2 vol. Solution B (see Section 7, Note 9).
6. Layer approx 4.5 ml of 15% (w/v) iodixanol (1.5 vol. of Solution D + 3.5 vol. of Solution B) on top to fill the tube.
7. Centrifuge at 350,000 gav for 2 h at speed (see Section 7, Note 10).
8. Collect the gradient in 20×0.5 ml fractions by upward displacement with a dense unloading solution, by tube puncture or aspiration from the meniscus (see Section 7, Note 11).
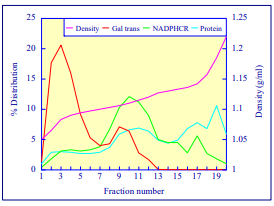 5. Analysis
5. Analysis
A typical distribution of Golgi and ER markers is shown Figure 1. The top six fractions contain almost exclusively Golgi membranes, while the smooth ER bands in the mid-region of the gradient. RNA (not shown) increases from tube 16, indicating that the rough ER is located towards the bottom of the gradient. See Section 7, Note 12 for more analytical information.
Figure 1 Distribution of Golgi and ER markers in gradient. Gal trans = galactosyl transferase, NADPHCR = NADPHcytochrome c reductase
6. Other separations
If separation of smooth and rough ER is not a requisite, then Golgi and ER may be separated in a simplified system. Yamaguchi et al [3] adjusted the total microsomes to 12.5% (w/v) iodixanol and centrifuged in a near-vertical rotor (Beckman NVT65.2) at 365,000g for 2.5 h. The ER banded close to the bottom of the gradient and the Golgi biphasically in the middle and at the top of the gradient. The single density starting format described in Application Sheet S18 has also been used for analysis of the ER and Golgi from intestinal cells and from brain [4, 5]. The method described in this Application Sheet has been used very successfully for a variety of cell types including astrocytes [6], neuroglioma cells [7] Chlamydia [8] and COS cells [9]. A recent review has compared some of the methods required for proteomic studies of ER [10].
7. Notes
1. It is important for the success of this protocol that EDTA is not included in isolation media.
2. Protease inhibitors (PMSF, leupeptin, antipain, aprotinin etc) may be included in any or all of the media at the operator’s discretion. Strategies for preparing working solutions for mammalian tissues are given in Application Sheet S01.
3. Smaller volume rotors such as the NVT65.2 (5 ml tubes) will require more or less the same centrifugation conditions. It is possible to use a fixed-angle rotor but it needs to be a low volume, high-performance rotor to be able to form the appropriate density gradient profile in 2 h. The gradient forming capacity of such a rotor will need confirming before use. For more information on the formation of self-generated gradients see Application Sheet S04.
4. The tubes of choice are Optiseal™ tubes, which are only available for Beckman rotors. Since they are sealed with a central plastic plus rather then heat- or crimp-sealed, they are easy to use and most importantly, gradients within them can be unloaded by any of the standard techniques available to open-topped tubes for swinging-bucket rotors.
5. If isolated hepatocytes are used, allow them to “recover” in a culture medium (e.g. DMEM) gassed with 95% O2/5% CO2 for 30 min at 37C before pelleting the cells at 800g for 2 min at 4°C. Wash the cells in ice-cold PBS and then allow them to swell in 10 mM Hepes-NaOH, pH 7.8 for 5-15 min on ice. Adjust the cell suspension to 0.25 M sucrose and then homogenize in the Dounce homogenizer using 30 strokes of the pestle [1].
6. For more information about homogenization of tissues and cells see, respectively, Application Sheets S05 and S06.
7. An optional 1000g/10 min step may be inserted prior to the 3000g centrifugation. This may prevent large amounts of rapidly sedimenting nuclei and debris trapping a lot of smaller particles. For more information on differential centrifugation see Application Sheet S07.
8. With smaller scale preparations it may be satisfactory to add Solution D directly to the 10,000g supernatant.
9. The small volume of 30% iodixanol cushion is included to prevent free ribosomes and protein reaching the wall of the tube.
10. Use a slow acceleration and deceleration program to and from 2000 rpm.
11. For more information on harvesting gradients see Application Sheet S08.
12. Ozawa et al [11] have used the method for rat C6 glioma cells; immunoblotting of gradient fractions with anti-TGN38 and anti-calnexin confirmed the enzyme distribution shown in Figure 1. Moreover, immunoblotting of rat hepatocyte fractions with anti-TGN38 and anti-GS28 (probes for the trans and cis Golgi domains) indicates that the trans domain is concentrated in fractions 1-2, while the cis domain is more evenly distributed across fractions 1-4 [12]. This suggests that there may be an opportunity for Golgi subfractionation using this system. If the top half of the gradient is made shallower by increasing the centrifugation time to 2.5-3 h higher resolution of these Golgi sub-domains may be obtained. This self-generated gradient technology was also used by Massarweh et al [13] in a study the involvement of the ER in the hydrolysis of oligosaccharide diphosphodolichol during protein N-glycosylation
8. References
1. Plonné, D., Cartwright, I., Lin, W., Dargel, R., Graham, J.M. and Higgins, J.A. (1999) Separation of the intracellular secretory compartment of rat liver and isolated rat hepatocytes in a single step using selfgenerating gradients of iodixanol Anal. Biochem., 276, 88-96
2. Ford, T., Graham, J. and Rickwood, D. (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of self generated gradients Anal. Biochem., 220, 360-366
3. Yamaguchi, J., Gamble, M.V., Conlon, D., Liang, J-S. and Ginsberg, H.N. (2003) The conversion of apoB100 low density lipoprotein/high density lipoprotein particles to apoB100 very low density lipoproteins in response to oleic acid occurs in the endoplasmic reticulum and not in the Golgi in Mca RH7777 cells J. Biol. Chem., 278, 42643-42651
4. Hui, D.Y. and Howles, P.N. (2005) Molecular mechanisms of cholesterol absorption and transport in the intestine Semin. Cell Develop. Biol., 16, 183-192
5. Silvestre, D.C., Maccioni, H.J.F. and Caputto, B.L. (2009) Content of endoplasmic reticulum and Golgi complex membranes positively correlates with the proliferative status of brain cells J. Neurosci. Res., 87, 857-865
6. Takano, K., Kitao, Y., Inagi, R., Momoi, T., Matsuyama, T., Miyata, T., Yoneda, Y., Iso, H., Stern, D.M., Hori, O. and Ogawa, S. (2006) A rat model of human FENIB (familial encephalopathy with neuroserpin inclusion bodies) Biochim. Biophys. Res. Comm, 346, 1040-1047
7. Huttunen, H.J., Greco, C. and Kovacs, D.M. (2007) Knockdown of ACAT-1 reduces amyloidogenic processing of APP FEBS Lett., 581, 1688-1692
8. Giles, D.K. and Wyrick, P.B. (2008) Trafficking of chlamydial antigens to the endoplasmic reticulum of infected epithelial cells Microbes Infect., 10, 1494-1503
9. Bonekamp, N.A., Vormund, K., Jacob, R. and Schrader, M. (2010) Dynamin-like protein 1 at the Golgi complex: A novel component of the sorting/targeting machinery en route to the plasma membrane Exp. Cell Res., 316, 3454-3467
10. Chen, X., Karnovsky, A., Dolors Sans, M., Andrews, P.C. and Williams, J.A., (2010) Molecular characterization of the endoplasmic reticulum: Insights from proteomic studies Proteomics, 10, 4040-4052
11. Ozawa, K., Tsukamoto, Y., Hori, H., Kitao, Y., Yanagi, H., Stern, D.M. and Ogawa, S. (2001) Regulation of tumor angiogenesis by oxygen-regulated protein 150, an inducible endoplasmic reticulum chaperone Cancer Res., 61, 4206-4213
12. Higgins, J.A. (2000) personal communication
13. Massarweh, A., Bosco, M., Iatmanen-Harbi, S., Tessier, C., Auberger, N., Busca, P., Chantret, I., GravierPelletier, C. and Moore, S.E.H. (2016) Demonstration of an oligosaccharide-diphosphodolichol diphosphatase activity whose subcellular localization is different than those of dolichyl-phosphate dependent enzymes of the dolichol cycle J. Lipid Res., 57, 1029–1042
OptiPrepTM Application Sheet S20; 8th edition, February 2020
OptiPrep™ Application Sheet S21
Analysis of ER, plasma membrane, endosomes, Golgi, ERGIC, TGN from mammalian cells and tissues in continuous iodixanol gradients (1.5-3 h spin)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RS05) “Analysis of membrane trafficking in mammalian tissues and cells: fractionation of ER, Golgi, TGN, PM and endosomes” provides a bibliography of all published papers reporting the use of OptiPrep™ for analysis of these membranes: to access return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Section 7 of this Application Sheet is a short review of some of the variations in the methodology according to cell or tissue type and indicates the type of membranes that were analyzed.
1. Background
The protocol described in this Application Sheet is based on methods first published by Yang et al [1] and Zhang et al [2]. Yang et al [1] used a linear 0-26% (w/v) iodixanol gradient to study the localization of UBC6 ubiquitin-containing protein in COS-7 cells. By using the gradient to separate endoplasmic reticulum (ER) and Golgi, they established that the transmembrane domain of a carboxylterminal anchored protein predisposes it to locate to the ER, while modulation of this domain resulted in re-targeting of the protein to the Golgi. Zhang et al [2] used a 1-20% (w/v) iodixanol gradient also to separate the ER and Golgi from transfected CHO and human embryonic kidney (HEK293) cells. The authors showed that the full-length presenilins (PS1 and PS2) were located in the ER while N- and Cterminal fragments were distributed to the Golgi membranes.
Although the ER and Golgi were the membranes of interest in these studies, the gradient may provide simultaneous purification of the plasma membrane (PM). The density of the three types of membrane generally increases in the order PM<Golgi<ER; some exceptions to this have however been observed. These gradients may also identify other membrane compartments such as early and late endosomes, cis-Golgi, trans-Golgi network (TGN) and occasionally lysosomes and mitochondria.
- The relative banding patterns of membranes in the gradient often depends on the type of cell, the homogenization procedure and the precise gradient and centrifugation conditions.
2. Solutions required (see Section 6, Notes 1 and 2)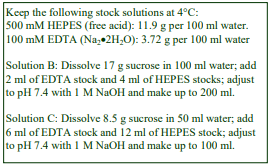
A. OptiPrep™
B. Homogenization medium (HM): 0.25 M sucrose, 1 mM EDTA 10 mM HEPES-NaOH, pH 7.4
C. Diluent: 0.25 M sucrose, 6 mM EDTA, 60 mM Hepes-NaOH, pH 7.4
D. Working Solution (WS) of 50% (w/v) iodixanol (ρ = 1.272 g/ml): 5 vol. of solution A + 1 vol. of solution C
3. Ultracentrifuge rotor requirements (see Section 6, Note 3)
Any swinging-bucket rotor capable of approx 200,000-300,000 g with tube volumes of 5 ml (e.g. Beckman SW 55Ti or Sorvall AH650) or 14 ml (e.g. Beckman SW 41 or Sorvall TH641).
4. Protocol
Carry out all operations at 0-4°C.
1. Wash the cells twice in phosphate-buffered saline to remove the culture medium, and then once in Solution B before resuspending in this solution (see Section 6, Note 4).
2. Suspend the cells in a small volume of Solution B (0.5-5.0 ml) and disrupt them by Dounce homogenization, repeated passages through a fine syringe needle or a ball-bearing homogenizer (see Section 6, Note 5).
3. Centrifuge the homogenate at 2000 g for 10 min and harvest the supernatant (see Section 6, Note 6).
4. Optional step Centrifuge the supernatant at 100,000 g for 40 min and then resuspend the pellet in 1-2 ml of Solution B (see Section 6, Note 7).
5. Prepare solutions of 2% and 25% (w/v) iodixanol solution by Solutions B and D 24:1 and 1:1 (v/v) respectively (see Section 6, Note 8).
6. Prepare 12-13 ml gradients in tubes for the swinging-bucket rotor from equal volumes of the 2% and 25% iodixanol solutions using a two-chamber gradient maker or a Gradient Master (see Section 6, Note 9).
7. Layer the vesicle suspension on top of the gradient and centrifuge at 200,000 g for 2-3 h (see Section 6, Notes 10 and 11).
8. Collect the gradient in 0.5 ml fractions (see Section 6, Note 12).
5. Analysis (see Section 7.5)
A typical distribution of plasma membrane (biotinylated cell surface proteins), Golgi (galactosyl transferase) and ER (ribophorin I) markers is shown Figure 1. Plasma membrane bands at the very top of the gradient, the Golgi is broadly banded in the top third and the ER in the bottom third of the gradient.
6. Notes
1. Protease inhibitors may be included in Solutions B and C at the operator’s discretion. Solutions B and C may contain alternative buffers (e.g. Tris, Tricine or triethanolamine. Strategies for preparing working solutions for mammalian tissues/cells are given in Application Sheet S01.
2. Although traditionally a buffered isoosmotic solution of sucrose containing EDTA has been used as the HM for organelle fractionation, there has been a trend to use more ionic media, containing NaCl or KCl (or both), particularly for cultured cells. See Section 7.1 for some of the media that have been used.
3. Other swinging-bucket rotors or even vertical rotors may be used. Larger volume swinging-bucket rotors may require longer centrifugation times but vertical rotors will need shorter times. Most of the gradients have been run in 5 or 14 ml tubes.
4. Washing the cells with saline may be carried out at room temperature rather than at 4°C if preferred. Yang et al [1] used a modified homogenization buffer containing 0.21 M sucrose, 0.75 mM KCl, 19 mM NaCl, 1 mM EDTA to wash the cells prior to homogenization. See Section 7.1 for some variations to this theme.
5. The homogenization protocol will need to be tailored to the cell type. Dounce homogenization or 12-15 passages through a 25-gauge syringe needle [1] or sometimes a combination of both [2] are common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices. See Section 7.2 for more information.
6. The pellet may be resuspended in Solution B; the centrifugation repeated and the two supernatants combined, if necessary. This strategy recovers membrane vesicles trapped in the first pellet.
7. The advantage of using a low-speed supernatant for the gradient input is that the procedure is quicker and that vesicles in the supernatant are not further exposed to the shearing forces that are required to resuspend a 100,000g pellet. On the other hand if it is important to remove any soluble cytosolic proteins, or if the volume of low-speed supernatant is inconveniently large, then preparing a 100,000g pellet may be a useful step. See Section 7.3 for more information.
8. A 2% iodixanol solution (rather than Solution B) has been chosen to allow easier layering of the sample. The concentrations of iodixanol used to form the gradient are variable; they may be customized to the operator’s requirements. If the main interest is the lower density fractions (e.g. Golgi, PM or endosomes) then a 2-18% iodixanol gradient may be more useful. If the main interest is in denser compartments then a 15%-40% gradient may be required.
9. If neither of these gradient-making devices is available then a continuous gradient can be prepared by diffusion of a discontinuous gradient. For more information on gradient construction see Application Sheet S03.
10. There is evidence that flotation of particles can provide better resolution than sedimentation. To use this approach the gradient should be modulated to 2-22% (w/v) iodixanol and the vesicle suspension adjusted to 25% (w/v) iodixanol before being layered beneath the gradient using a syringe and metal cannula (i.d. 0.9 mm). Some examples are given in Sections 7.3.
11. The centrifugation time and/or RCF (g-force) may also be modulated; smaller volume gradients may require shorter times (e.g. 1.5 h) and/or lower RCFs (100-150,000g). – see Section 7.4 for more information.
12. Collect the gradient by tube puncture, upward displacement or aspiration from the meniscus. For more information on harvesting gradients see Application Sheet S08.
7. Literature review
7.1. Homogenization media
Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility towards functional studies on subcellular membranes; moreover these low ionic strength HMs and gradients permit the direct use of fractions for SDS-PAGE. Although 0.25 M sucrose buffered with either Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and containing 1 mM EDTA is still a widely used HM, supplementation with inorganic salts, is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation. There is no obvious advantage of one organic buffer over another, but generally, Hepes or Tricine are considered to be more “biological particle-friendly” than is Tris.
Table 1 summarizes some of the other HMs that have been reported in the literature. Other examples are given in Application Sheets S05 (tissues) and S06 (cells). Usually a solution containing the same reagents is used to dilute the OptiPrep, there are however a few instances where this is not the case.
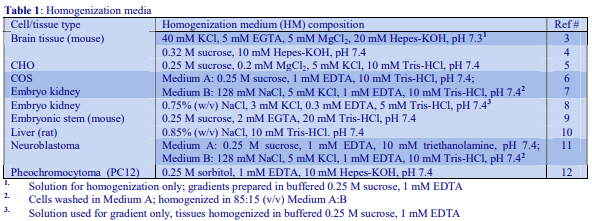
7.2. Cell or tissue homogenization
There are certainly no rigid guidelines regarding the homogenization procedure; ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some hints on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
7.3. Sample preparation for gradient
Interposing the pelleting of particles from the PNS risks some loss of the smallest vesicles; the subsequent resuspension may cause disruption of organelles such as lysosomes and the process adds considerably to the preparation time. Nuclear pelleting may be carried out at 500-3000g for 5-10 min; the higher RCFs (g-forces) resulting in removal of some of the mitochondria. To recover any vesicles trapped in the pellet (more serious at the higher RCFs), the pellet is sometimes resuspended in HM, recentrifuged and the two supernatants combined. A possible disadvantage of this practice is that unless the resuspension of the pellet is carried out very gently, the nuclei may be damaged, with consequent leakage of DNA, which may lead to almost irreversible aggregation of the subcellular membranes.
Suspension of a 100,000g pellet in a dense solution of iodixanol, or adjustment of the PNS to a high density allows the sample to be loaded beneath the gradient. Cytosolic proteins are retained in the sample zone and are less likely the membranes that float into the gradient. Some examples of these alternative sample handlings are given in Table 2.
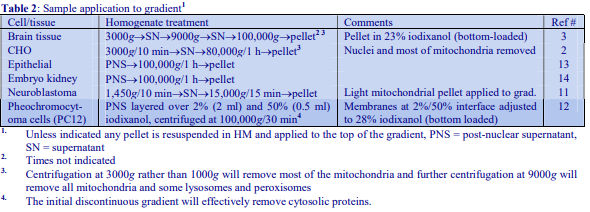
7.4. Centrifugation conditions
As far as is known the optimal centrifugation time and RCF have not been thoroughly investigated. Commonly used regimes are approx 280,000g for 2 h and 100-200,000g for 3 h. Short times at a relatively low RCF (e.g. 150,000g for 1.5 h) have generally only been used with 5 ml gradients. The separations are described as buoyant density therefore as long as the vesicles have had sufficient time to reach their banding density, the actual time and RCF are probably not critical. But if sedimentation velocity plays some part in the resolution, time and RCF will be more important and the sample volume must be no more than 10% of that of the gradient.
It should be pointed out that to get true equilibrium density banding centrifugation for at least 12 h at RCFs below 100,000g is required and there is evidence that such a practice can produce enhanced resolution – see Application Sheet S22.
7.5. Membrane analysis
Using the standard iodixanol gradient, resolution of the ER and cis-medial Golgi may be less than ideal; by adopting a 10-24% iodixanol gradient Drummer et al [8] were able to obtain a complete separation of calnexin and giantin (resident proteins for these two membranes respectively) from HEK cells. A 5-25% iodixanol gradient on the other hand provided more complete resolution of the lighter TGN from cis-Golgi but the latter banded very closely to the ER from embryo stem cells [9]. In two examples, from human primary fibroblasts [15] and from neuroblastoma cells [11], the Golgi banded at a higher density than the ER. In the case of the neuroblastoma cells, the gradient input was slightly unusual, inasmuch as it comprised a heavy+light mitochondrial fraction, i.e. it would be enriched in mitochondria, lysosomes, peroxisomes and maybe Golgi tubules and impoverished in vesicular microsomes. Whether this has any bearing on the relative densities of the Golgi and ER is not clear.
The plasma membrane from most cultured cells tends to be well defined in iodixanol gradients and almost invariably less dense than the Golgi and ER, but the PM from tissues is more heterogeneous. Thus when Newby et al [14] used the same procedure for both kidney and for mouse embryo kidney cells, in both cases the ER and Golgi were well separated but only in the case of the cultured cells was the PM also distinct and separate from the other two membranes. Aside from the unpredictable nature of the banding of plasma membrane from tissues however, there is evidence from liver [10,16], brain and spleen [16] that the density of early endosomes, Golgi (or TGN) and ER increases reproducibly in that order, as with most cultured cells. Late endosomes tend to overlap the ER.
Other organelles such as mitochondria tend to be denser than the ER. The density of lysosomes, is more variable, again it is usually denser than the ER but can, as in the case of pheochromocytoma (PC12) cells, band between the early endosomes and Golgi [12].
- Neural tissue poses additional requirements on the gradient; synaptic vesicles in particular may need to be identified. Araki et al [4] reported that, in mouse brain neurons, there was a clear distinction in the banding of ER, synaptic vesicles and Golgi (in order of decreasing density) in iodixanol gradients.
8. References
1. Yang, M., Ellenberg, J., Bonifacino, J.S. and Weissman, A.M. (1997) The transmembrane domain of a carboxyl-terminal anchored protein determines localization to the endoplasmic reticulum J. Biol. Chem., 272, 1970-1975
2. Zhang, J., Kang, D.E., Xia, W., Okochi, M., Mori, H., Selkoe, D.J. and Koo, E.H. (1998) Subcellular distribution and turnover of presenilins in transfected cells J. Biol. Chem., 273, 12436-12442
3. Kamal, A., Almenar-Queralt, A., LeBlanc, J.F., Roberts, E.A. and Goldstein, L.S.B. (2001) Kinesin mediated axonal transport of a membrane compartment containing -secretase and presenilin-1 requires APP Nature, 414, 643-648
4. Araki, Y., Tomita, S., Yamaguchi, H., Miyagi, N., Sumioka, A., Kirino, Y. and Suzuki, T. (2003) Novel cadherin-related membrane proteins, alcadeins, enhance the X11-like protein-mediated stabilization of amyloid -protein precursor metabolism J. Biol.Chem., 278, 49448-49458
5. Lefterov, I.M., Koldamova, R.P. and Lazo, J.S. (2000) Human bleomycin hydrolase regulates the secretion of amyloid precursor protein FASEB J., 14, 1837-1847
6. Eickmann, M., Kiermayer, S., Kraus, I., Gossl, M., Richt, J.A. and Garten, W. (2005) Maturation of Borma virus disease virus glycoprotein FEBS Lett., 579, 4751-4756
7. Cai, Y., Maeda, Y., Cedzich, A., Torres, V.E., Wu, G., Hayashi, T., Mochizuki, T., Park, J.H., Witzgall, R. and Somlo, S. (1999) Identification and characterization of polycystin-2, the PKD2 gene product J. Biol. Chem., 274, 28557-28565
8. Drummer, H.E., Maerz, A. and Poumbourios, P. (2003) Cell surface expression of functional hepatitis C virus E1 and E2 glycoproteins FEBS Lett., 546, 385-390
9. Siman, R. and Velji, J. (2003) Localization of presenilin-nicastrin complexes and -secretase activity to the trans-Golgi network J. Neurochem., 84, 1143-1153
10. Andrade, J., Zhao, H., Titus, B., Pearce, T. and Barroso, M. (2003) The EF-hand Ca2+-binding protein p22 plays a role in microtubule and endoplasmic reticulum organization and dynamics with distinct Ca2+-binding requirements Mol. Biol. Cell, 15, 481-496
11. Kim, S-H., Lah, J.J., Thinakaran, G., Levey, A. and Sisodia S.S (2000) Subcellular localization of presenilins: association with a unique membrane pool in cultured cells Neurobiol. Dis., 7, 99-117
12. Hasegawa, H., Zinsser, S., Rhee, Y., Vik-Mo, E.O., Davanger, S. and Hay, J.C. (2003) Mammalian Ykt6 is a neuronal SNARE targeted to a specialized compartment by its profilin-like amino terminal domain Mol. Biol. Cell, 14, 698-720
13. Choukroun, G.J., Marshansky, V., Gustafson, C.E., McKee, M., Hajjar, R.J., Rosenzweig, A., Brown, D. and Bonventre, J.V. (2000) Cytosolic phospholipase A2 regulates Golgi structure and modulates intracellular trafficking of membrane proteins J. Clin. Invest., 106, 983-993
14. Newby, L.J., Streets, A.J., Zhao, Y., Harris, P.C., Ward, C.J. and Ong, A.C.M. (2002) Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex J. Biol. Chem., 277, 20763-20773
15. Takeda, K., Inoue, H., Tanizawa, Y., Matsuzaki, Y., Oba, J., Watanabe, Y., Shinoda, K. and Oka, Y. (2001) WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain Hum. Mol. Genet., 10, 477-484
16. Yamamoto, Y., Jones, K.A., Mak, B.C., Muehlenbachs, A. and Yeung, R.S. (2002) Multicompartmental distribution of tuberous sclerosis gene products, hamartin and tuberin Arch. Biochem. Biophys., 404, 210-217
9. Acknowledgements
We wish to thank Dr Allan Weissman, Laboratory of Immune Cell Biology, National Cancer Institute, Bethesda, MD 20892, USA and Dr Edward Koo, Dept of Neurosciences 0691, University of California at San Diego, La Jolla, CA 92093, USA for their cooperation in the preparation of this text.
OptiPrepTM Application Sheet S21; 9th edition, February 2020
OptiPrep™ Application Sheet S22
Analysis of ER, plasma membrane, endosomes, Golgi, ERGIC and TGN from mammalian cells and tissues in continuous gradients (16-18 h spin)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml.
- An OptiPrep™ Reference List (RS05) “Analysis of membrane trafficking in mammalian tissues and cells: fractionation of ER, Golgi, TGN, PM and endosomes” provides a bibliography of all published papers reporting the use of OptiPrep™ for analysis of these membranes: to access return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Section 7 of this Application Sheet is a short review of some of the variations in the methodology according to cell or tissue type and indicates the type of membranes that were analyzed.
1. Background
Investigations into the processing of macromolecules, membrane trafficking and cell signaling often use density gradients, in association with confocal microscopy, to analyze these events. Such analyses usually involve detection of compartments such as endoplasmic reticulum (ER), Golgi, transGolgi network (TGN), ER-Golgi Intermediate Compartment (ERGIC), endosomes and the plasma membrane (PM). This Application Sheet describes “long-spin” protocols that have successfully resolved some of these compartments. There are many examples reported in the literature in which advantage of the low viscosity of iodixanol gradients (compared to those of sucrose) has been taken to carry out separations in 1.5-3 h, usually at 200,000-300,000g. Such short spin times can be important if the molecules and processes under study seriously degrade over time. The methodology is described in Application Sheet S21. There is evidence however that to obtain true equilibrium density banding it is preferable to carry out the centrifugation at lower RCFs (g-forces) for longer times and that under such conditions resolution is improved compared to the “short-spin” gradients.
- Some separations using intermediate centrifugation times of 5-6 h are also reported here
The following protocol is based on a method described by Woods et al [1] for the localization of the adaptor protein paxillin, in mouse 3T3 fibroblasts; it uses a continuous 10-40% iodixanol gradient and a long centrifugation time (18 h); a rather similar gradient and centrifugation conditions were used by Puglielli et al [2] for the fractionation of CHO cell membranes.
- The precise banding patterns of membranes in the gradient may depend on the type of cell, the homogenization medium, the homogenization procedure and the density range of the gradient
2. Solutions required (see Section 6, Notes 1-3)
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 140 mM NaCl, 1 mM EDTA, 20 mM Tris-HCl, pH 8.0
C. Diluent: 0.25 M sucrose, 140 mM NaCl, 3 mM EDTA, 60 mM Tris-HCl, pH 8.0
D. Working solution of 40% (w/v) iodixanol (ρ = 1.224 g/ml): 4 vol. of solution A + 2 vol. of solution C
3. Ultracentrifuge rotor requirements
Any swinging-bucket rotor for an ultracentrifuge capable of 100,000g with a tube capacity of approx 14 ml tubes, e.g. Beckman SW41Ti or Sorvall TH641 (see Section 6, Note 4)
4. Protocol
The protocol is described for a cultured cell monolayer. Step 1 may be carried out at room temperature, carry out all subsequent steps at 4°C.
1. Wash the cell monolayer twice with phosphate-buffered saline and once with Solution B (without the EDTA)
2. Scrape the cell monolayer into Solution B and homogenize the cells using no more than 20 strokes of the pestle of a Dounce homogenizer (see Section 6, Note 5).
3. Pellet debris and nuclei by centrifugation for 5 min at 700-800 g
4. Decant and retain the post-nuclear supernatant (PNS).
5. Prepare the two gradient solutions of 10% and 40% (w/v) iodixanol by diluting Solution D with Solution B.
6. Use either a standard two-chamber gradient maker or a Gradient Master™ to prepare 10-12 ml continuous 10-40% (w/v) iodixanol gradients from equal volumes of he two iodixanol solution in tubes for the suitable swinging bucket rotor (see Section 6, Notes 6 and 7).
7. Load the PNS (1-3 ml) on to each gradient (see Section 6, Note 8).
8. Centrifuge the gradients at 48,000 g for 18 h.
9. Unload the gradients in approx 0.5 ml fractions by upward displacement, aspiration from the meniscus or by tube puncture (see Section 6, Note 9) and analyze the fractions for appropriate membrane markers.
5. Analysis
The top half of the gradient resolves endosomes (approx 1.055- 1.070 g/ml) and clathrin-coated vesicles (approx 1.08-1.09 g/ml). These densities correlate very closely with those observed in self-generated iodixanol gradients for these two endocytic compartments from rat liver (described in Application Sheet S45) The bottom half of the gradient resolves two fractions of ER, a light one (approx 1.110 g/ml) and a dense one (approx 1.145 g/ml). Paxillin (not shown) co-purifies with the dense ER [1] and confocal microscopy studies indicate that this dense ER is perinuclear. Puglielli et al [2] used a similar iodixanol gradient (8-34%) and centrifugation at a slightly higher RCF (100,000g) for the same time to study the processing of the amyloid β peptide From CHO cells stably transfected with PS1 the authors obtained a very satisfactory resolution (see Figure 2) of early endosomes (EEA-1 as marker), Golgi (syntaxin6 as marker) and ER (calnexin as marker). For more information on analysis see Section 7.5.
6. Notes
1. Although traditionally a simple buffered isoosmotic solution of sucrose containing EDTA has been used as the HM for organelle fractionation, there has been a trend to use more ionic media, containing NaCl or KCl (or both), particularly for cultured cells. The solutions used for preparing density solutions may contain alternative buffers such as Tris, Tricine or triethanolamine. See Section 7.1 for some of the media that have been used.
2. Protease inhibitors may be included in Solutions B and C at the operator’s discretion.
3. The diluent (Solution C), used for the preparation of the 40% iodixanol working solution (Solution D) ensures that the concentrations of EDTA and buffer remain constant throughout the gradient. If it is considered the NaCl should also be constant throughout the gradient, then this should be present in Solution C at 3x the given concentration. Under these conditions however, the gradient solutions will be hyperosmotic. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
4. The method should be adaptable to larger or smaller volume swinging-bucket rotors; increase or decrease the volumes of sample and gradient proportionately.
5. The homogenization protocol will need to be tailored to the cell type. Dounce homogenization or passages through a 25-gauge syringe needle or sometimes a combination of both are common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices. See Section 7.2 for more information.
6. If neither of these gradient-making devices is available then a continuous gradient can be prepared by diffusion of a discontinuous gradient. For more information on gradient construction see Application Sheet S03.
7. An alternative approach is a discontinuous gradient prepared from multiple layers, the density increment between layers being very small. Such a gradient can be regarded essentially as continuous and linear. An example is provided by Lee et al [3] who used 1 ml each of 5.0, 6.5, 8.5, 10.5, 12.5, 14.5, 16.5, 18.5 and 20% (w/v) iodixanol.
8. There is evidence that flotation of particles can provide better resolution than sedimentation. To use this approach the gradient should be modulated to 10-35% (w/v) iodixanol and the vesicle suspension adjusted to 40% (w/v) iodixanol before being layered beneath the gradient using a syringe and metal cannula (i.d. 0.9 mm). Some examples are given in Section 7.3.
9. For more information on harvesting gradients see Application Sheet S08.
7. Technical review
7.1. Homogenization media
Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility towards functional studies on subcellular membranes; moreover these low ionic strength HMs and gradients permit the direct use of fractions for SDS-PAGE. Although 0.25 M sucrose buffered with either Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and containing 1 mM EDTA is still a widely used HM, supplementation with inorganic salts, such as the 140 mM NaCl used in this protocol, is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation. Table 1 summarizes some of the other HMs that have been used. Other examples are given in Application Sheets S05 (tissues) and S06 (cells). Usually a solution containing the same reagents is used to dilute the OptiPrep™, there are however a few instances where this is not the case (see Table 1).
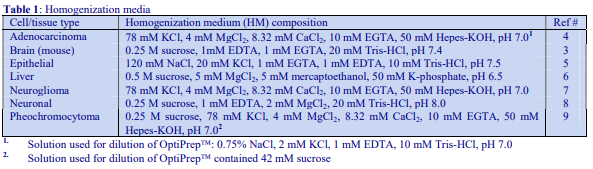
7.2. Cell or tissue homogenization
There are certainly no rigid guidelines regarding the homogenization procedure; ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some hints on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
7.3. Sample preparation for gradient
Probably the most common approach is the simplest one: to remove the nuclei from the homogenate and apply a total post-nuclear supernatant (PNS) to the top of the gradient. The big advantage of using a low-speed supernatant is that little of the total vesicle fraction or the smaller and less dense organelles will be lost; moreover the particles separated in the gradient will not have experienced the serious aggregation that can occur during pelleting and it saves a considerable amount of time. Nuclear pelleting is normally carried out at 500-1000g for 5-10 min. To recover any vesicles trapped in the pellet, the latter is sometimes resuspended in the HM, recentrifuged and the two supernatants combined. A possible disadvantage of this practice is that unless the resuspension of the pellet is carried out very gently, the nuclei may be damaged, with consequent leakage of DNA. If the RCF used for this preliminary “clarification” is raised to 3000g, a variable proportion of the mitochondria will also be removed.
If the PNS is inconveniently large for application to the gradient, then an alternative approach is to centrifuge this supernatant at approx. 80,000-150,000 g for 45-60 min and to resuspend the pellet in HM before applying to the gradient. This strategy also removes some of the cytosolic proteins; these can sediment and diffuse from a PNS into the gradient during the subsequent centrifugation. The extent of this movement into the gradient depends of course on the molecular mass of the proteins, centrifugation time and RCF. A variation in this strategy, which avoids contamination from cytosolic proteins, is to suspend the 80,000-150,000g pellet in a solution of iodixanol so that it can be loaded beneath the gradient rather than on top, so the membranes float rather than sediment to their banding positions. Some examples of these alternative sample handlings are given in Table 2.

7.4. Centrifugation conditions
The most commonly used regime is 100,000g for 18 h, but the advantage of using a lower RCF of 48,000g is that any tendency for the iodixanol molecules to sediment, and so make the linear gradient slightly steeper close to the bottom of the tube, is minimized. A compromise regime that uses a higher RCF (200,000g) for a shorter time (6 h) has also been reported [12]
7.5. Membrane analysis
As a general rule the density of membrane compartments separated on these long-spin iodixanol gradients increases in the order PM<early endosomes<TGN<cis-medial Golgi<ERGIC<ER<late endosomes<lysosomes. There are however exceptions to this, in pheochromocytoma (PC12) cells for example the early endosomes banded just below the middle of a 5-20% iodixanol gradient; they were however predictably less dense than the late endosomes [9]. Their position may simply reflect the lower density range of the gradient and/or the hyperosmotic nature of the homogenization medium. In shallow flotation gradient lysosomes from mouse brain banded between the early endosomes and Golgi [3].
The method described above has also been used more recently by Woods et al [13], who confirmed the banding of endosomes and ER shown in Figure 1 and showed in addition that the plasma membrane banded at the very top of the gradient, while the kinase PKD1 showed no correlation with any of these three major membrane compartments, banding instead between the endosomes and ER. The β3 integrin on the other hand co-purified with all three of the major compartments from a wild-type cell, but appeared additionally in the PKD1-containing zone from cells expressing catalytically inactive PKD1. Often the “tight” banding of membranes in iodixanol gradients permits the definitive identification of a particular compartment, even though its separation from a neighbouring compartment is minimal. For example [12] although the early endosomes from HeLa cells in a 0-28% iodixanol gradient were only slightly less dense than the Golgi, the definition was nevertheless very clear.
These gradients are also useful for at least partial resolution of sub-compartments of the Golgi and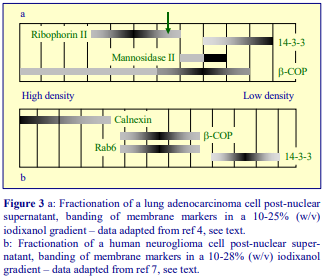 ERGIC. Panel a of Figure 3 indicates the banding of four protein markers within a 10-25% (w/v) iodixanol: ribophorin II (an ER resident protein), mannosidase II (an enzyme characteristic of the medial-trans Golgi) and 14-3-3 (a transGolgi marker) from an adenocarcinoma post-nuclear supernatant [4]. The fraction marked with a green arrow was identified as ERGIC. Panel b of Figure 3 similarly shows the banding of markers from a human neuroglioma cell post-nuclear supernatant; the median fractions containing -COP and Rab6 identified this region as being enriched in membranes associated with Golgi-to-ER retrograde transport and the presence of immature APP suggested that this was either ERGIC or early Golgi [7]. These two examples are chosen not only to display the fine resolution achievable with these gradients, but also to highlight that although the gradient and centrifugation conditions were almost identical and the relative densities of the various membrane compartments were very similar, significant differences in detailed banding can be observed, which is almost certainly related to the use of difference cell types.
ERGIC. Panel a of Figure 3 indicates the banding of four protein markers within a 10-25% (w/v) iodixanol: ribophorin II (an ER resident protein), mannosidase II (an enzyme characteristic of the medial-trans Golgi) and 14-3-3 (a transGolgi marker) from an adenocarcinoma post-nuclear supernatant [4]. The fraction marked with a green arrow was identified as ERGIC. Panel b of Figure 3 similarly shows the banding of markers from a human neuroglioma cell post-nuclear supernatant; the median fractions containing -COP and Rab6 identified this region as being enriched in membranes associated with Golgi-to-ER retrograde transport and the presence of immature APP suggested that this was either ERGIC or early Golgi [7]. These two examples are chosen not only to display the fine resolution achievable with these gradients, but also to highlight that although the gradient and centrifugation conditions were almost identical and the relative densities of the various membrane compartments were very similar, significant differences in detailed banding can be observed, which is almost certainly related to the use of difference cell types.
8. References
1. Woods, A.J., Roberts, M.S., Choudhary, J., Barry, S.T., Mazaki, Y., Sabe, H., Morley, S.J., Critchley, D.R. and Norman, J.C. (2002) Paxillin associates with poly(A)-binding protein 1 at the dense endoplasmic reticulum and the leading edge of migrating cells J. Biol. Chem., 277, 6428-6437
2. Puglielli, L., Konopka, G., Pack-Chung, E., MacKenzie Ingano, L .A., Berezovska, O., Hyman, B.T., Chang, T. Y., Tanzi, R.E. and Kovacs, D.M. (2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid -peptide Nature Cell Biol., 3, 905-912
3. Lee, M-S., Kao, S-C., Lemere, C.A., Xia, W., Tseng, H-C., Zhou, Y., Neve, R., Ahlijanian, M.K. and Tsai, LH. (2003) APP processing is regulated by cytoplasmic phosphorylation J. Cell Biol., 163, 83-95
4. Hood, J.L., Brooks, W.H. and Roszman, T.L. (2004) Differential compartmentalization of the calpain/calpastatin network with the endoplasmic reticulum and Golgi apparatus J. Biol. Chem., 279, 43126-43135
5. Koulen, P., Cai, Y., Geng, L., Maeda, Y., Nishimura, S., Witzgall, R., Ehrlich, B.E. and Somlo, S. (2002) Polycystin-2 is an intracellular calcium release channel Nature Cell Biol., 4, 191-197
6. Trombetta, E.S. and Helenius, A. (1999) Glycoprotein reglucosylation and nucleotide sugar utilization in the secretory pathway: identification of a nucleoside diphosphatase in the endoplasmic reticulum EMBO J., 18, 3282-3292
7. Tekirian, T.L., Merriam, D.E., Marshansky, V., Miller, J., Crowley, A.C., Chan, H., Ausiello, D., Brown, D., Buxbaum, J.D., Xia, W. and Wasco, W. (2001) Subcellular localization of presenilin 2 endoproteolytic Cterminal fragments Mol. Brain Res., 96, 14-20
8. Kim, A.H., Yano, H., Cho, H., Meyer, D., Monks, B., Margolis, B., Birnbaum, M.J. and Chao, M.V. (2002 Akt1 regulates a JNK scaffold during excitotoxic apoptosis Neuron, 35, 697-709
9. Li, Y., Chin, L-S., Levey, A.I. and Li, L. (2002) Huntingtin-associated protein 1 interacts with hepatocyte growth factor-regulated tyrosine kinase substrate and functions in endosomal trafficking J. Biol. Chem., 277, 28212-28221
10. Augustin, R., Riley, J. and Moley, K.H. (2005) GLUT8 contains a DEXXXLLI sorting motif and localizes to a late endosomal/lysosomal compartment Traffic, 6, 1196-1212
11. Molinari, M., Eriksson, K.K., Calanca, V., Galli, C., Cresswell, P., Michalak, M. and Helenius, A. (2004) Contrasting functions of calreticulin and calnexin in glycoprotein folding and ER quality control Mol. Cell, 13, 125-135
12. Hirose, H., Arasaki, K., Dohmae, N., Takio, K., Hatsuzawa, K., Nagahama, M., Tani, K., Yamamoto, A., Tohyama, M. and Tagaya, M. (2004) Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi EMBO J., 23, 1267-1278
13. Woods, A.J., White, D.P., Caswell, P.T. and Norman, J.C. (2004) PKD1/PKC promotes v3 integrin recycling and delivery to nascent focal adhesions EMBO J., 23, 2531-2543
OptiPrepTM Application Sheet S22; 8th edition, February 2020
OptiPrep™ Application Sheet S23
Analysis of ER, plasma membrane, endosomes, Golgi, ERGIC and TGN from mammalian cells and tissues by flotation in discontinuous gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- An OptiPrep™ Reference List (RS05) “Analysis of membrane trafficking in mammalian tissues and cells: fractionation of ER, Golgi, TGN, PM and endosomes” provides a bibliography of all published papers reporting the use of OptiPrep™ for analysis of these membranes: to access return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Section 7 of this Application Sheet is a short review of some of the variations in the methodology according to cell or tissue type and indicates the type of membranes that were analyzed.
1. Background
During the extended period of centrifugation, used in the protocol described in this Application Sheet, there will occur diffusion of iodixanol across the interfaces formed in the discontinuous gradient, but opposing this there will be some sedimentation of the iodixanol molecules. In tall narrow tubes (such as in a Beckman SW41Ti) there may be at least some retention of the discontinuous nature of the gradient, even after an overnight centrifugation. This will be particularly true of gradients of just three or four layers, but as far as is known however, the density profile of such gradients has not been checked after centrifugation. Flotation gradients such can be highly discriminating as they avoid the disturbance to the gradient and interfacial aggregation that may be created in the sedimentation format by rapidly sedimenting dense particles. It is also impossible to overload the gradient. This discontinuous gradient in which the membranes reach their banding density by flotation from a dense sample was first used for PC12 cells [1]. In this method the post-nuclear supernatant (PNS) produced by low-speed centrifugation of the homogenate was used as the gradient input, hence to avoid the use of multiple gradients, the homogenate was restricted to 1 ml (the PNS requires dilution with an equal volume of 50% iodixanol for bottom-loading). The gradient was used primarily to identify plasma membrane and early endosomes. The method was subsequently adapted: a 16,000g/30 min pellet, produced from a PNS, was used as the gradient input rather than the PNS itself, thus in this format the volume of homogenate is not critical. In this format the gradient was used to fractionate both PC12 and murine cortical neurons [2-4] and it is this format that is described in this OptiPrep Application Sheet.
2. Solutions required (see Section 5, Note 1)
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 2 mM MgCl₂, 1 mM EDTA, 20 mM Tricine-NaOH, pH 7.8 (see Section 5, Note 2)
C. Diluent: 0.25 M sucrose, 12 mM MgCl₂, 6 mM EDTA, 120 mM Tricine-NaOH, pH 7.8
D. Working Solution of 50% (w/v) iodixanol (ρ = 1.272 g/ml): 5 vol of solution A + 1 vol of solution C
3. Ultracentrifuge rotor requirements
Any swinging-bucket rotor capable of approx 200,000-300,000g with tube volumes of 13 ml, such as Beckman SW 41 or Sorvall TH641 (see Section 5, Note 3)
4. Protocol
Carry out all operations at 0-4°C.
1. Suspend the cells or tissues in Solution B and homogenize using a Dounce homogenizer. Cells may require repeated passage through a 25G or 27G needle instead of (or sometimes in addition to) Dounce homogenization, (see Section 5, Note 4).
2. Centrifuge the homogenate at 800-1000 g for 10 min. The pellet may be resuspended in homogenization medium; the centrifugation repeated and the two supernatants combined, if necessary (see Section 5, Note 5).
3. Centrifuge the supernatant(s) at 16,000 g for 30 min (see Section 5, Note 6).
4. Resuspend the pellet in 1.5 ml (total volume) of Solution B and mix with an equal volume of Solution D.
5. Prepare 10 ml each of 5%, 10%, 15%, and 20% (w/v) iodixanol solution by mixing the appropriate volumes of Solutions B and D.
6. In approx. 13 ml tubes for the swinging-bucket rotor form a discontinuous gradient from 2.5 ml each of the four gradient solutions and the sample, by the underlayering technique using a syringe and metal cannula. If necessary top up the tube with 5% iodixanol to the correct filling. For more information on making discontinuous gradients see Application Sheet S03.
7. Centrifuge at 88,000 g for 18 h (see Section 5, Note 7).
8. If the material within the gradient is visible as a series of well-defined bands collect them using a syringe and metal cannula. Otherwise unload the gradient in 0.5-1.0 ml fractions either by tube puncture, aspiration from the meniscus or upward displacement. For more information about harvesting gradients see Application Sheet S08.
5. Notes
1. Protease inhibitors may be included in any or all of the media at the operator’s discretion. The solutions used for preparing density solutions may contain alternative buffers such as Tris, Hepes or triethanolamine.
2. This diluent, used for the preparation of the 50% iodixanol working solution (Solution D) ensures that the concentrations of EDTA and buffer remain constant throughout the gradient, which will be more or less isoosmotic. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
3. Other swinging-bucket rotors of larger or smaller tube capacities may be used; scale all volumes up or down proportionately.
4. The homogenization protocol will need to be tailored to the cell or tissue type. For cells the ballbearing homogenizer (“cell cracker”) provides a very reliable alternative, see Application Sheet S06. For tissues see Application Sheet S05. It is not known what effect different homogenization media or protocols will have on the separation.
5. Washing the pellet in the manner described can release a significant amount of smaller material adventitiously trapped in the nuclear pellet, but the procedure does run the risk of causing nuclear disruption.
6. To apply the PNS to the gradient, omit this step and mix the PNS with an equal volume of Solution D.
7. Larger volume rotors (such as the Beckman SW28 or SW28.1) have similar tube lengths and the centrifugation conditions should not require modification. For smaller volume rotors (e.g. the Beckman SW55Ti) the centrifugation may be carried at 133,000g for 5 h [2].
6. Analysis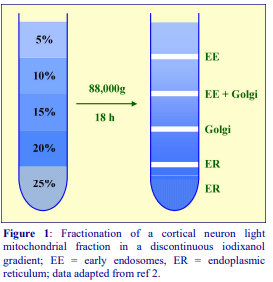
An example of the resolution that is achievable with the long-spin discontinuous iodixanol gradient is shown in Figure 1. Although the gradient was unloaded in a series of equal volume fractions, from the top of the gradient [2], major bands of material were found at the position of the original interfaces and the composition of these is shown in the figure. Although early endosomes were found across the position of the original 10% iodixanol layer, most of these subcellular membranes were found at the top interface. Golgi membranes (monitored by a cis-Golgi marker) were more or less evenly distributed in the two fractions shown in the figure, while all the ER was concentrated below the 20% iodixanol layer. As an example of the variation from tissue to tissue and cell to cell, Wu et al [1] found that major plasma membrane band from PC12 cells (identified by the EGFR) resided predominantly at the position of the top interface with a minor band at the second interface, while early endosomes banded at the bottom interface. Aside from the use of a PNS fraction rather than a 16,000g pellet, the only difference was the use of PC12 cells rather than cortical neurons.
7. Technical review
The strategy described in this OptiPrep™ Application Sheet has been used for studying nerve growth factor signaling in PC12 cells [1, 5 and 6] and neurotophin receptor signaling and transport in both cortical neurons [2-4] and PC12 cells [3]. Other gradient formats are summarized in Table 1.
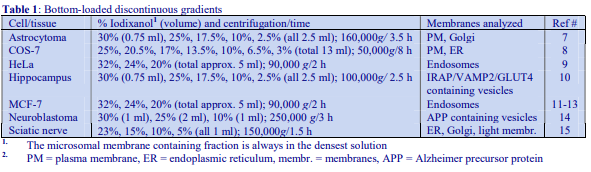
In a less commonly used format the sample was median-loaded [16]. A 4000g/10 min supernatant from a myocardial homogenate (in 0.1 M sucrose, 10 mM EDTA, 46 mM KCl, 5 mM NaN3, 100 mM Tris-HCl, pH 7.4); after adjusting to 5% (w/v) iodixanol; 3.0 ml was layered between 0.5 ml each of 15% iodixanol and the homogenization medium and centrifuged at 80,000 g for 16 h. In the five visible layers, the plasma membrane was restricted to the lowest density (Figure 2), while the ER, as determined by SERCA Ca2+ pump, banded in the two densest fractions, only one of which also contained the glucose transporter GLUT4 [5]. The method was used in studies on the translocation of GLUT4 to the plasma membrane during the raised status of glucose oxidation in heart failure [16] and nitric oxide inhibition of myocardial glucose transport [17].
pH 7.4); after adjusting to 5% (w/v) iodixanol; 3.0 ml was layered between 0.5 ml each of 15% iodixanol and the homogenization medium and centrifuged at 80,000 g for 16 h. In the five visible layers, the plasma membrane was restricted to the lowest density (Figure 2), while the ER, as determined by SERCA Ca2+ pump, banded in the two densest fractions, only one of which also contained the glucose transporter GLUT4 [5]. The method was used in studies on the translocation of GLUT4 to the plasma membrane during the raised status of glucose oxidation in heart failure [16] and nitric oxide inhibition of myocardial glucose transport [17].
8. References
1. Wu, C., Lai, C-F. and Mobley, W.C. (2001) Nerve growth factor activates persistent Rap1 signaling in endosomes J. Neurosci., 21, 5406-5416
2. Yano, H. and Chao, M.V. (2004) Mechanisms of neurotrophin receptor vesicular transport J. Neurobiol., 58, 244-257
3. Yano, H. and Chao, M.V. (2005) Biochemical characterization of intracellular membranes bearing Trk neurotrophin receptors Neurochem. Res., 30, 767-777
4. Arevalo, J.C., Pereira, D.B., Yano, H., Teng, K.K. and Chao, M.V. (2006) Identification of a switch in neurotrophin signaling by selective tyrosine phosphorylation J. Biol. Chem., 281, 1001-1007
5. Wu, C., Ramirez, A., Cui, B., Ding, J., Delcroix, J-D.M., Valletta, J.S., Yang, Y., Chu, S. and Mobley, W.C. (2007) A functional dynein-microtubule network is required for NGF signaling through the Rap1/MAPK pathway Traffic, 8, 1503-1520
6. Wan, J., Cheung, A.Y., Fu, W-Y., Wu, C., Zhang, M., Mobley, W.C., Cheung, Z.H. and Ip, N.Y. (2008) Endophilin B1 as a novel regulator of nerve growth factor/ TrkA trafficking and neurite outgrowth J. Neurosci., 28, 9002-9012
7. Choi, K.S., Aizaki, H. and Lai, M.M. (2005) Murine coronavirus requires lipid rafts for virus entry and cellcell fusion but not for virus release J. Virol., 79, 9862-9871
8. Decaffmeyer, M., Shugla, Y.V., Dicu, A.O., Thomas, A., Truant, R., Topham, M.K., Brasseur, R. and Epand, R.M. (2008) Determination of the topology of the hydrophobic segment of mammalian diacylglycerol kinase epsilon in a cell membrane and its relationship to predictions from modeling J. Mol. Biol., 383, 797-809
9. Li, Q., Zhang, Y., Marden, J.J., Banfi, B. and Engelhardt, J.F. (2008) Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury Biochem. J., 411, 531-541
10. Fernando, R.N., Albiston, A.L. and Chai, S.Y. (2008) The insulin-regulated aminopeptidase IRAP is colocalised with GLUT4 in the mouse hippocampus – potential role in modulation of glucose uptake in neurones? Eur. J. Neurosci., 28, 588-598
11. Li, Q., Harraz, M.M., Zhou, W., Zhang, L.N., Ding, W., Zhang, Y., Eggleston, T., Yeaman, C., Banfi, B. and Engelhardt, J.F. (2006) Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAFg to endosomal interleukin-1 receptor complexes Mol. Cell. Biol., 26, 140-154
12. Li, Q., Zhang, Y., Marden, J.J., Banfi, B. and Engelhardt, J.F. (2008) Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury Biochem. J., 411, 531-541
13. Mumbengegwi, D.R., Li, Q., Li, C., Bear, C.E. and Engelhardt, J.F. (2008) Evidence for a superoxide permeability in endosomal membranes Mol. Cell. Biol., 28, 3700-3712
14. Shrivastava-Ranjan, P., Faundez, V., Fang, G., Rees, H., Lah, J.J., Levey, A.I. and Kahn, R.A. (2008) Mint3/X11 is an ADP-ribosylation factor-dependent adaptor that regulates the traffic of the Alzheimer’s precursor protein from the trans-Golgi network Mo. Biol. Cell, 19, 51-64
15. Kamal, A., Almenar-Queralt, A., LeBlanc, J.F., Roberts, E.A. and Goldstein, L.S.B. (2001) Kinesin mediated axonal transport of a membrane compartment containing -secretase and presenilin-1 requires APP Nature, 414, 643-648
16. Lei, B., Lionetti, V., Young, M.E., Chandler, M.P., d’Agostino, C., Kang, E., Altarejos, M., Matsuo, K., Hintze, T.H., Stanley, W.C. and Recchia, F.A. (2004) Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure J. Mol. Cell. Cardiol., 36, 567-576
17. Lei, B., Matsuo, K., Labinsky, V., Sharma, N., Chandler, M.P., Ahn, A., Hintze, T.H., Stanley, W.C. and Recchia, F.A. (2005) Exogenous nitric oxide reduces glucose transporters translocation and lactate production in ischemic myocardium in vivo Proc. Natl. Acad. Sci. USA, 102, 6966-6971
OptiPrepTM Application Sheet S23; 8th edition, February 2020
OptiPrep™ Application Sheet S24
Analysis of ER, plasma membrane, endosomes, Golgi, ERGIC and TGN from mammalian cells and tissues by sedimentation in discontinuous gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- An OptiPrep™ Reference List (RS05) “Analysis of membrane trafficking in mammalian tissues and cells: fractionation of ER, Golgi, TGN, PM and endosomes” provides a bibliography of all published papers reporting the use of OptiPrep™ for analysis of these membranes: to access return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Section 5 of this Application Sheet is a short review of some of the variations in the methodology according to cell or tissue type and indicates the type of membranes that were analyzed.
1. Background
The strategy of using a multi-step discontinuous iodixanol gradient was first published by Xia et al [1] in 1998; subsequently many similar methods have been published and the number of layers varies from five to nine. Diffusion of iodixanol down the concentration gradient will occur across the interfaces during the centrifugation, but for relatively short time periods such as 1.5-3 h the gradients will certainly not become continuous. Moreover, the diffusion will be partly counterbalanced by sedimentation of the iodixanol molecules in the opposite direction; the higher the RCF (g-force) the more likely this is to occur. The retention of the discontinuous nature of the gradient also depends on the interfacial surface area and the linear distance between the interfaces. So to reproduce a particular published fractionation pattern it is probably good practice to use the recommended rotor, rather than adapt the method to a larger or smaller volume gradient.
- The separations are probably based on buoyant density but sedimentation velocity may make some contribution. Compare the shorter times and generally lower RCFs (g-forces) used in the protocol described in Application Sheet S25; in which the resolution is likely to be principally by sedimentation velocity.
Although layering each iodixanol solution can be rather tedious, by changing the increment in volume and/or density between adjacent layers, the gradients can reproducibly be made concave or convex or to contain irregular shallow or steep regions. In this manner, the gradients may provide better linear separation of some particles. Nevertheless there are many examples where excellent resolution is achieved with discontinuous gradients of uniform density increment and volume.
These discontinuous iodixanol gradients have been used to separate not only the major membrane compartments, plasma membrane (PM), Golgi and endoplasmic reticulum (ER), but also some of the important sub-compartments such as cis-Golgi, trans-Golgi network (TGN) and the endoplasmic reticulum-Golgi intermediate compartment (ERGIC). The density of these compartments generally increases in the order PM<Golgi<ER with the trans-Golgi network (TGN) being less dense than the cis-Golgi and ERGIC less dense than the ER. In some instances early and late endosomes, lysosomes and mitochondria have also been identified.
- The precise banding patterns of membranes in the gradient may depend on the type of cell, the homogenization medium, the homogenization procedure, the type of gradient and the centrifugation conditions (see Section 5.7).
- The system described by Xia et al [1] has been used by a number of workers and it forms the basis of the protocol described in this Application Sheet.
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 1 mM EDTA 10 mM Hepes-NaOH, pH 7.4
C. Diluent: 0.25 M sucrose, 6 mM EDTA, 60 mM Hepes-NaOH, pH 7.4
D. Working Solution of 50% (w/v) iodixanol (ρ = 1.272 g/ml): 5 vol of solution A + 1 vol of solution C
3. Ultracentrifuge rotor requirements (see Section 5.2)
Any swinging-bucket rotor capable of approx 200,000-300,000 g with tube volumes of approx. 13 ml (e.g. Beckman SW 41 or Sorvall TH641)
4. Protocol
Carry out all operations (except the phosphate-buffered saline washes in step 1) at 0-4°C.
1. Wash the cells twice in phosphate-buffered saline to remove the culture medium, and then once in the homogenization medium before resuspending in this medium.
2. Suspend the cells in a small volume of homogenization medium (0.5-5.0 ml) and disrupt them by Dounce homogenization, repeated passages through a fine syringe needle or a ball-bearing homogenizer (see Section 5.3)
3. Centrifuge the homogenate at 1500 g for 10 min. The pellet may be resuspended in homogenization medium; the centrifugation repeated and the two supernatants combined, if necessary (see Section 5.4.1)
4. Centrifuge the supernatant(s) at 65,000 g for 1 h and then resuspend the pellet in 1-2 ml of Solution B (see Section 5.4.2).
5. Prepare 10 ml each of 2.5%, 5%, 7.5%, 10%, 12.5%, 15%, 17.5%, 20%, and 30% (w/v) iodixanol solution by mixing the appropriate volumes of Solutions B and D (see Section 5.5.1).
6. In tubes for the swinging-bucket rotor: layer 1 ml of 2.5%, 2 ml each of 5%, 7.5% and 10%, 0.5 ml of 12.5%, 2 ml of 15%, and 0.5 ml each of 17.5%, 20% and 30% (see Sections 5.5.1 and 5.5.2).
7. Layer the vesicle suspension (0.8-1.0 ml) on top of the gradient and centrifuge at 200,000 gav for 2.5 h; allow the rotor to decelerate from 2000 rpm without the brake (see Sections 5.4.2 and 5.6).
8. Collect the gradient in 0.5 ml fractions either by tube puncture or upward displacement. For more information on harvesting gradients see Application Sheet S08.
9. Analyze the fractions as appropriate (for examples of analyses from the published literature see 5.7)
5. Technical review
5.1. Homogenization media and gradient solutions
The homogenization medium often has to be tailored to the tissue or cell type and it is not known if the composition of the HM is relevant to the separation. Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility in functional studies on subcellular membranes; moreover these low ionic strength HMs and gradient solutions permit the direct use of fractions for SDS-PAGE. Although 0.25 M sucrose buffered with either Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and containing 1 mM EDTA is still a widely used HM, supplementation with inorganic salts is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation. Table 1 summarizes some of the other HMs that have been used. Other examples are given in Application Sheets S05 (tissues) and S06 (cells).
Protease inhibitors may be included in Solutions B and C at the operator’s discretion. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
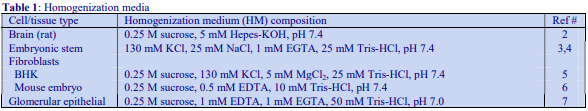
5.2. Ultracentrifuge rotors
Many of these separations have been performed in 13 ml tubes for a Beckman SW41 type rotor at 200,000g for 2-3 h. Other swinging-bucket rotors or even vertical rotors may be used. Larger volume swinging-bucket rotors may require longer centrifugation times but smaller volume rotors and vertical rotors will need shorter times. Gradients and sample volume should be scaled up or down proportionately as required. Note however that the progressive change in gradient density profile (due to diffusion and sedimentation of the iodixanol molecules) may also be modulated in other rotors and affect the final resolution.
5.3. Homogenization
The homogenization protocol should be tailored to the cell (or tissue) type. Potter-Elevhjem homogenization for tissues and Dounce homogenization for cells used to be the standard procedures. For cells use of 12-15 passages through a 27- or 25-gauge syringe needle, sometimes preceded by Dounce homogenization, is more common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices. Although the Polytron homogenizer is normally restricted to tissues, Iwata et al [8] successfully used this device for neuroblastoma cells. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some hints on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
5.4. Differential centrifugation
5.4.1. Removal of nuclei
Nuclear pelleting may be carried out at 500-3000g for 5-10 min; the higher RCFs (g-forces) resulting in removal of some of the mitochondria, which can facilitate subsequent layering of the sample on the gradient. To recover any vesicles trapped in the pellet (more serious at the higher RCFs), the pellet is sometimes resuspended in HM, recentrifuged and the two supernatants combined. A possible disadvantage of this practice is that unless the resuspension of the pellet is carried out very gently, the nuclei may be damaged, with consequent leakage of DNA, which may lead to almost irreversible aggregation of the subcellular membranes.
5.4.2. Preparation of sample for gradient loading
Almost without exception, papers reporting the use of the method of Xia et al [1] describe the preparation of 65,000g (or sometimes 100,000g) pellet from the PNS and application of this to the gradient rather than the PNS itself. However if the volume of the 1500g supernatant (step 3) is sufficiently small it might be applied directly to the top of the gradient (in Step 7). Interposing the pelleting of particles from the PNS risks loss of the smallest vesicles and the subsequent resuspension may cause disruption of organelles such as lysosomes. On the other hand if it is important to remove the soluble cytosolic proteins, or if the volume of low-speed supernatant is inconveniently large, then preparing a 65,000g pellet is an essential step, although it does add significantly to the preparation time. Note that direct application of a PNS to a discontinuous gradient was used for hamster embryo kidney cells [9] and embryonic stem cells [3,4] Suspension of a high-speed pellet in a dense solution of iodixanol, or adjustment of the PNS to a high density allows the sample to be loaded beneath the gradient. Cytosolic proteins are retained in the sample zone and are less likely to contaminate the membranes that float into the gradient. There is also some evidence that improved resolution can be obtained with this strategy, as the gradient is not disturbed by rapidly sedimenting dense and/or large particles. This approach was used for mouse embryo fibroblasts [6] and glomerular epithelial cells [7].
5.5. Density gradients
5.5.1. Alternative formats
Some variations in gradient format are given in Table 2. Those gradients comprising larger numbers of layers with smaller incremental densities are potentially more discriminating. Gradients of, for example 10-30% (w/v) iodixanol are more suited to fractionation of denser components such as ER and mitochondria than those of for example 1-25% (w/v) iodixanol. The latter are more suited to resolving lighter components such as Golgi and PM.
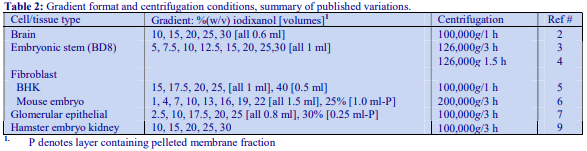
5.5.2. Construction
Discontinuous gradients are most easily prepared by underlayering (i.e. low density first) using a syringe (1-2 ml) and a long metal cannula); overlayering small volumes is more difficult using either a syringe or Pasteur pipette. An alternative for overlayering is to use a small volume (low-pulsating) peristaltic pump; first to take up the required volume of solution into the attached tubing and second to expel it slowly on to a denser layer in the centrifuge tube. For more information on gradient construction see Application Sheet S03 If necessary, adjust all volumes proportionately so that (after sample application) tubes are properly filled according to the manufacturer’s instructions.
5.6. Centrifugation conditions
As far as is known the optimal centrifugation time and RCF have not been thoroughly investigated. Commonly used regimes are in the range 100-200,000g for 2-3 h. Some variations are given in Table 2. Short times at a relatively low RCF (e.g. 100,000g for 1 h) have generally only been used with smaller volume (<5 ml) gradients. It should be pointed out that to get true equilibrium density banding centrifugation for at least 12 h at RCFs below 100,000g is required and there is evidence that such a practice can produce enhanced resolution – see Application Sheet S22.
5.7. Analysis
In one of the few reports of a direct comparison between the use of iodixanol and sucrose gradients [7], the former were found to give markedly better resolution of PM, ER and mitochondria in a much reduced time (3 h against 16 h). There may also be analytical reasons for the choice of iodixanol over sucrose. Iodixanol gradients were chosen by Campbell et al [10] because their isoosmotic nature (in contradistinction to the hyperosmotic nature of sucrose gradients) preserved vesicles structure and function and allowed the study of the de novo synthesis of the amyloid protein in in vitro incubations of isolated membrane fractions.
These 2.5-30% iodixanol gradients are ideal for resolving ER and Golgi (see Figure 1) and are probably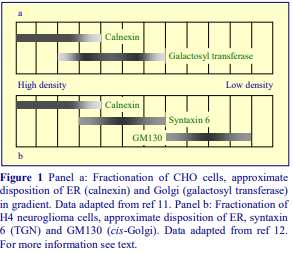 applicable to most cultured cells; although the density of PM<Golgi<ER generally increases in that order, the fine detail of the fractionation varies from cell type. The Golgi (as
applicable to most cultured cells; although the density of PM<Golgi<ER generally increases in that order, the fine detail of the fractionation varies from cell type. The Golgi (as
measured by galactosyl transferase from CHO cells appears biphasic [11], while the gradient
seems to be able to resolve the TGN and cisGolgi from neuroglioma cells, with the TGN being the denser of the two compartments [12]. On a 5-30% iodixanol gradient (see Table 2) the cis-Golgi from BD8 blastocyst-derived embryonic stem cells was more centrally located; in cells expressing PS1, this enabled some PS2 to be localized to a less dense non-ER, non-Golgi compartment.
Bottom-loaded 1 25% iodixanol gradients (see Table 2) were able to resolve ER, Golgi and PM from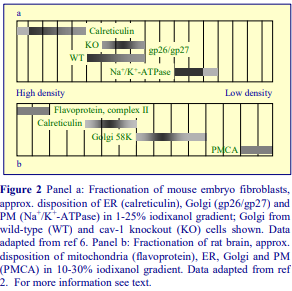 mouse embryo fibroblasts (Figure 2, Panel a) with essentially no overlap of markers at all [6]. The possible variation in detailed banding position between different types of cell is emphasized in the observation that the Golgi from wild-type cells was skewed to a slightly higher density than that from cells of a cav-1 knockout mouse. Gradients covering the range 10-30% iodixanol (see Table 2) are also able to resolve
mouse embryo fibroblasts (Figure 2, Panel a) with essentially no overlap of markers at all [6]. The possible variation in detailed banding position between different types of cell is emphasized in the observation that the Golgi from wild-type cells was skewed to a slightly higher density than that from cells of a cav-1 knockout mouse. Gradients covering the range 10-30% iodixanol (see Table 2) are also able to resolve
denser organelles such as mitochondria (Figure 2, Panel b) [2] or both mitochondria and lysosomes [9]. Although the plasma membrane from cultured cells is invariably the lightest membrane compartment, that from organized tissues may be denser if it is associated with an extensive cytoskeleton. None of these gradients are particularly successful in resolving recycling [9] or early [13] endosomes from Golgi/TGN or ER membranes respectively. Generally endosomes are better resolved in gradients centrifuged for longer times see Application Sheet S22.
6. References
1. Xia, W., Zhang, J., Ostraszewski, B.L., Kimberly, W.T., Seubert, P., Koo, E.H., Shen, J. and Selkoe, D.J. (1998) Presenilin 1 regulates the processing of -amyloid precursor protein C-terminal fragments and the generation of amyloid -protein in endoplasmic reticulum and Golgi Biochemistry, 37, 16465 16471
2. Salvi, M., Stringaro, A., Brunati, A.M., Agostinelli, E., Arancia, G., Clari, G. and Toninello, A. (2004) Tyrosine phosphatase activity in mitochondria: presence of Shp-2 phosphatase in mitochondria Cell. Mol. Life Sci., 61, 2393-2404
3. Bergmann, A., Hansson, E.M., Pursglove, S.E., Farmery, M.R., Lannfelt, L., Lendahl, U., Lundkvist, J. and Naslund, J. (2004) Pen-2 is sequestered in the endoplamic reticulum and subjected to ubiquitylation and proteasome-mediated degradation in the absence of presenilin J. Biol. Chem., 279, 16744-16753
4. Laudon, H., Karlstrom, H., Mathews, P.M., Farmery, M.R., Gandy, S.E., Lundqvist, J., Lendahl, U. and Naslund, J. (2004) Functional domains in presenilin. The TYR-288 residue controls -secretase activity and endoprotoeolysis J. Biol. Chem., 279, 23925-23932
5. Andrade, J, Zhao, H., Titus, B., Pearce, T. and Barroso, M. (2003) The EF-hand Ca2+-binding protein p22 plays a role in microtubule and endoplasmic reticulum organization and dynamics with distinct Ca2+-binding requirements Mol. Biol. Cell, 15, 481-496
6. Ring, A., Le Lay, S., Pohl, J., Verkade, P. and Stremmel, W. (2006) Caveolin-1 is required for fatty acid translocase (FAT/CD36) localization and function at the plasma membrane of mouse embryonic fibroblasts Biochim. Biophys. Acta, 1761, 416-423
7. Liu, J., Takano, T., Papillon, J., Khadir, A. and Cybulsky, A.V. (2001) Cytosolic phospholipase A2-
associates with plasma membrane, endoplasmic reticulum and nuclear membrane in glomerular epithelial cells Biochem. J., 353, 79-90
8. Iwata, H., Tomita, T., Maruyama, H. and Iwatsubo, T. (2001) Subcellular compartment and molecular subdomain of -amyloid precursor protein relevant to the A42-promoting effects of Alzheimer mutant presenilin 2 J. Biol. Chem., 276, 21678-21685
9. Miyazaki, T., Neff, L., Tanaka, S., Horne, W.C. and Baron, R. (2003) Regulation of cytochrome c oxidase activity by c-Src in osteoclasts J. Cell Biol., 160, 709-718
10. Campbell, W.A., Iskandar, M-K., Reed, M.L.O. and Xia, W. (2002) Endoproteolysis of presenilin in vitro: inhibition by -secretase inhibitors Biochemistry, 41, 3372-3379
11. Xia, W., Ray, W.J., Ostraszewski, B.L., Rahmati, T., Kimberly, W.T., Wolfe, M.S., Zhang, J., Goate, A.M. and Selkoe, D. (2000) Presenilin complexes with the C-terminal fragments of amyloid precursor protein at the sites of amyloid -protein generation Proc. Natl. Acad. Sci. USA, 97, 9299-9304
12. Chang, Y., Tesco, G., Jeong, W.J., Lindsley, L., Eckman, E.A., Eckman, C.B., Tanzi, R.E. and Guenette, S.Y. (2003) Generation of the -amyloid peptide and the amyloid percursor protein C-terminal fragment are potentiated by FE65L1 J. Biol. Chem., 278, 51100-51107
13. Walsh, D.M., Klyubin, I., Fadeeva, J.V., Cullen, W.K., Anwyl, R., Wolfe, M.S., Rowan, M.J. and Selkoe, D.J. (2002) Naturally secreted oligomers of amyloid protein potently inhibit hippocampal long-term potentiation in vivo Nature, 416, 535-539
OptiPrepTM Application Sheet S24; 8th edition, February 2020
OptiPrep™ Application Sheet S25
Analysis of ER, plasma membrane, endosomes, Golgi, ERGIC and TGN from cells and tissues in sedimentation velocity gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- An OptiPrep™ Reference List (RS05) “Analysis of membrane trafficking in mammalian tissues and cells: fractionation of ER, Golgi, TGN, PM and endosomes” provides a bibliography of all published papers reporting the use of OptiPrep™ for analysis of these membranes: to access return to the initial list of Folders and select “Reference Lists”.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Section 5 of this Application Sheet is a short review of some of the variations in the methodology according to cell or tissue type and indicates the type of membranes that were analyzed.
1. Background
Using modern OptiPrep™ technology, fractionation and subfractionation of membrane compartments have generally been carried out by buoyant density banding in either pre-formed or selfgenerated gradients. Fractionation on the basis of sedimentation velocity however, may provide increased resolving power, particularly of the multiple compartments of relatively low density membranes (ERGIC, Golgi, TGN etc). Both sucrose and glycerol gradients have been used for sedimentation velocity analysis of membrane vesicles but their usefulness is compromised by the increasing osmolality of the gradients, as a result of which, osmotically-active vesicles progressively decrease in size during their progress through the gradient. Since the size of the vesicle is the predominant factor in determining its sedimentation velocity, hyperosmotic gradients are less than ideal for these separations. Iodixanol gradients on the other hand can be made isoosmotic throughout their length. Sedimentation velocity separations are traditionally carried out in continuous linear gradients, such gradients are essential, for example, for the sedimentation analysis of proteins. For the membrane analysis described herein however such strictures have been relaxed and both discontinuous and continuous iodixanol gradients have been used. Moreover, by manipulation of the density of each layer of a discontinuous gradient, gradients can easily and reproducibly adjusted to optimize the separation of both slowly and rapidly sedimenting particles. Furthermore by making the difference in density between adjacent layers and the volume of these layers relatively small, a discontinuous gradient will, more or less, approach a continuous format. The protocol described in this Application Sheet provides discontinuous (adapted from ref 1) and continuous (adapted from ref 2) gradient alternatives.
- Two types of solution preparation are provided:
- Type I is a standard general-purpose sucrose-based homogenization medium containing a buffer and EDTA
- Type II contains KCl as the major osmotic component instead of sucrose. KCl-containing solutions may be useful for any homogenate that contains a lot of microfilament-derived proteins, which tend to form a gel in the absence of salt.
- In some instances it may be necessary to use a hypoosmotic solution in order to achieve a satisfactory homogenization of a particular cell type. Some of these variations are described in Section 5.1
2. Solutions required (see Section 5.1)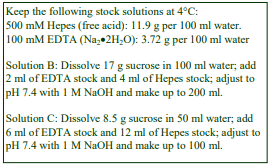
Type I
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 1 mM EDTA 10 mM Hepes-NaOH, pH 7.4
C. Diluent: 0.25 M sucrose, 6 mM EDTA, 60 mM Hepes-NaOH, pH 7.4
D. Working Solution of 50% (w/v) iodixanol (ρ = 1.272 g/ml): 5 vol of solution A + 1 vol of solution C
Type II
A. OptiPrep™
B. Homogenization medium: 130 mM KCl, 25 mM NaCl, 1 mM EGTA, 25 mM Tris-Cl, pH 7.4
C. Diluent: 130 mM KCl, 25 mM NaCl, 6 mM EGTA, 25 mM Tris-Cl, pH 7.4
D. Working Solution of 50% (w/v) iodixanol: 5 vol. of solution A + 1 vol. of solution C.
3. Ultracentrifuge rotor requirements
Any swinging-bucket rotor capable of approx 200,000g with a tube volume of 13 ml, e.g. Beckman SW 41 or Sorvall TH641 (see Section 5.2)
4. Protocol
Carry out all operations at 0-4°C.
1. Produce an homogenate from the chosen tissue or cells using Solution B (Type I or II). See Section 5.3 for more information.
2. Centrifuge the homogenate at 1000 g for 10 min (see Section 5.4).
3. Centrifuge the supernatant at 3,000 g for 10 min and keep the supernatant for subsequent gradient separation (see Section 5.4).
4. For a discontinuous gradient: Prepare 10 ml each of 2.5%, 5%, 7.5%, 10%, 12.5%, 15%, 17.5%, 20%, and 30% (w/v) iodixanol solution by mixing the appropriate volumes of Solutions B and D (Type I or II). For a continuous gradient: Prepare 20 ml of the 2.5% and 30% solutions (see Section 5.5.1).
5. In 13 ml tubes for the swinging-bucket rotor (discontinuous gradient): layer 1.2 ml each of the nine gradient solutions (see Notes 10 and 11); (continuous gradient): use a two-chamber gradient maker or Gradient Master™ to make an approx. 12 ml gradient from equal volumes of the 10% and 30% iodixanol solutions (see Section 5.5.2).
6. Layer the vesicle suspension on top of the gradient and centrifuge at 55,000 g for 90 min or 126,000 g for 25 min (see Section 5.6).
7. Collect the gradient in 0.5 fractions by tube puncture, aspiration form the meniscus or upward displacement. For more information on harvesting gradients see Application Sheet S08.
8. Analyze the fractions as appropriate (for examples of analyses from the published literature see Section 5.7)
5. Technical Notes and Review
5.1. Homogenization media and gradient solutions
The homogenization medium often has to be tailored to the tissue or cell type and it is not known if the composition of the HM is relevant to the separation. Although 0.25 M sucrose buffered with either Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and containing 1 mM EDTA is still a widely used HM, supplementation with inorganic salts is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation. Rather more unusually hamster embryo fibroblasts were homogenized in phosphate-buffered saline [3]. If a hypoosmotic medium is required to swell the cells in order to achieve an adequate degree of homogenization it is important to return the homogenate to isoosmotic conditions as soon as possible. Schroder et al [2] for example washed CHO cells first in an isoosmotic sucrose buffer (0.25 M sucrose, 1 mM EDTA, 10 mM triethanolamine-HOAc, pH 7.4); suspended 3×106 cells in 0.5 ml of 83 mM sucrose (in the same EDTA-buffer) and allowed them to swell for 3 min at room temperature. This step is often carried out on ice, but more efficient swelling may be obtained at the higher temperature. Homogenization (see 5.3) was performed by two passages through a 28G needle. Isoosmolality was reestablished by adding an equal volume of 415 mM sucrose before three additional passages through the needle was carried out. Human meningioma cells were swollen in 10 mM KCl, 1.5 mM MgCl₂, 10 mM Tris-HCl, pH 7.4 for 15 min on ice before being disrupted in a in a Dounce homogenizer [4]. Other examples of homogenization media are given in Application Sheets S05 (tissues) and S06 (cells). The composition of Solution C is designed so that when Solution D is diluted with Solution B the concentrations of important additives such as EDTA or EGTA are constant. Protease inhibitors may be included in Solutions B and C at the operator’s discretion. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
5.2. Ultracentrifuge rotors
Many of these separations have been performed in 13 ml tubes for a Beckman SW41 type rotor. Other swinging-bucket rotors or even vertical rotors may be used. Larger volume swinging-bucket rotors may not require longer centrifugation times (they normally have similar sedimentation path lengths to the SW41) but smaller volume rotors and vertical rotors will certainly need shorter times. Gradients and sample volume should be scaled up or down proportionately as required. Note however that the progressive change in gradient density profile (due to diffusion and sedimentation of the iodixanol molecules) may also be modulated in other rotors and affect the final resolution.
5.3. Homogenization
The homogenization protocol should be tailored to the cell (or tissue) type. Potter-Elevhjem homogenization for tissues and Dounce homogenization for cells used to be the standard procedures. For cells use of 5-15 passages through a 27- or 25-gauge syringe needle, sometimes preceded by Dounce homogenization, is more common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some hints on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
5.4. Differential centrifugation
Nuclear pelleting may be carried out at 500-3000g for 5-10 min; sometimes the homogenate is centrifuged sequentially at 1000g/10 min and 3000g/10 min [1]. Exposure of the homogenate to 3000g will remove some of the mitochondria, which can facilitate subsequent layering of the sample on the gradient. In one instance a higher RCF (g-force) was used (17,000g for 15 min) prior to gradient loading [5]. To recover any vesicles trapped in the pellet (more serious at the higher RCFs), the pellet is sometimes resuspended in HM, recentrifuged and the two supernatants combined. A possible disadvantage of this practice is that unless the resuspension of the pellet is carried out very gently to avoid damage to the organelles. If the nuclei are damaged consequent leakage of DNA may lead to almost irreversible aggregation of the subcellular membranes. As the separation is based on sedimentation velocity, it is important that the volume of sample applied to the top of the gradient should be <10% of the gradient volume. This is why there is a tendency to keep the homogenate volume to an absolute minimum. If the homogenate volume is inconveniently large then the membrane vesicles might be sedimented at approx 100,000g for 30 min prior to resuspension in a smaller volume of buffer, but this has rarely been reported for this method. The necessary re-homogenization of the pellet may lead to further reductions in vesicle size – with unpredictable consequences for the subsequent gradient separation.
5.5. Density gradients
5.5.1. Alternative formats
The discontinuous gradients that have been reported in published papers all conform to the format described in this OptiPrep Application Sheet. Continuous gradients are slightly more variable; for example, for CHO cells a 10-22.5% (w/v) iodixanol gradient has been used [2] and a 0-40% gradient for MDCK cells [5]. A summary is given in Table 1.
5.5.2. Construction
Discontinuous gradients are normally most easily prepared by underlayering (i.e. low density first) using a syringe (1-2 ml) and a long metal cannula; overlayering small volumes is more difficult using either a syringe or Pasteur pipette. One alternative for overlayering is to use a small volume (lowpulsating) peristaltic pump; first to take up the required volume of solution into the attached tubing and second, to reverse the flow, in order to expel it slowly on to a denser layer in the centrifuge tube. If neither a two chamber gradient maker nor a Gradient Master is available for making continuous gradients then these may be formed from diffusion of discontinuous gradients. For more information on gradient construction see Application Sheet S03. If necessary, adjust all volumes proportionately so that tubes are properly filled according to the manufacturer’s instructions.
5.6. Centrifugation conditions
As far as is known the optimal centrifugation time and RCF have not been thoroughly investigated. Commonly used regimes are summarized in Table 1.
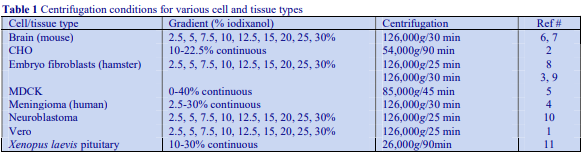
5.7. Analysis
In most cases these gradients have been used as a simple, rapid means of separating ER and Golgi, which band predominantly in the bottom third and between one third and halfway down the gradient respectively, although these positions may vary with the cell type and/or the gradient. When the iodixanol concentration top of the gradient was 10% (w/v) the Golgi and ER banding from CHO cells was predictably shifter upwards [2]. MDCK microsomes run on a 0-40% (w/v) iodixanol continuous gradient (85,000g for 45 min) display in addition a small vesicle fraction at the top of the gradient that contained early endosomes and TGN [5]. Interestingly when a light mitochondrial fraction was substituted for the microsomes, the Golgi banded at the top, while the ER was approx. half way down the gradient.
These gradients can also achieve further resolution. An example of this is given in Figure 1, taken from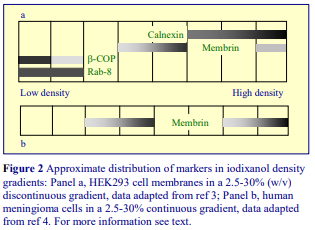 ref 10. Blots of SDSPAGE gels of gradient fractions were probed with calnexin (ER marker), βCOP (cis- /medial-Golgi marker), rab8 (TGN vesicle marker). Early endosomes, as identified by rab5 show a unique biphasic distribution, locating to both low- and high- density fractions. There is evidence however that fine detail of distribution in sedimentation velocity gradients may vary with the cell type or with the homogenization protocol (or both). With HEK293 cells for example both βCOP and rab8 overlapped at the top of the gradient (Figure 2a), although as with neuroblastoma cells, the βCOP tended to be associated with the least dense compartment [3]. Membrin was used as a Golgi marker in this study and although the vast majority was (as expected) in a compartment less dense than the ER, a minor fraction partly co-banded with the ER. This may reflect the function of membrin as an ER-Golgi SNARE. The biphasic distribution of membrin was even more pronounced in fractions from meningioma cells (Figure 2b). In this study Protein 4.1B (ΔU2) was identified at the top of the gradient (plasma membrane) while an E465-S851 mutant of this protein was confined to the denser part of the gradient [4].
ref 10. Blots of SDSPAGE gels of gradient fractions were probed with calnexin (ER marker), βCOP (cis- /medial-Golgi marker), rab8 (TGN vesicle marker). Early endosomes, as identified by rab5 show a unique biphasic distribution, locating to both low- and high- density fractions. There is evidence however that fine detail of distribution in sedimentation velocity gradients may vary with the cell type or with the homogenization protocol (or both). With HEK293 cells for example both βCOP and rab8 overlapped at the top of the gradient (Figure 2a), although as with neuroblastoma cells, the βCOP tended to be associated with the least dense compartment [3]. Membrin was used as a Golgi marker in this study and although the vast majority was (as expected) in a compartment less dense than the ER, a minor fraction partly co-banded with the ER. This may reflect the function of membrin as an ER-Golgi SNARE. The biphasic distribution of membrin was even more pronounced in fractions from meningioma cells (Figure 2b). In this study Protein 4.1B (ΔU2) was identified at the top of the gradient (plasma membrane) while an E465-S851 mutant of this protein was confined to the denser part of the gradient [4].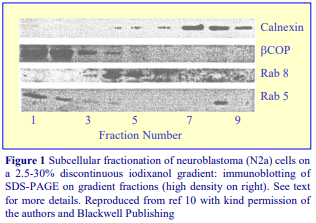
- The fractionation pattern from Xenopus laevi [11] was broadly similar to the mammalian examples, in a 10-30% iodixanol gradient, the Golgi banded close to the top and the ER approx. in the middle of the gradient. Interestingly distinctive banding patterns (in the Golgi region) were displayed for p24 putative cargo receptors and for the pro-opiomelanocortin prohormone from white-adapted and black-adapted animals.
6. References
1. Majoul, I.V., Bastiaens, P.I.H. and Soling H-D (1996) Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells J. Cell Biol., 133, 777-789
2. Schroder, M., Schafer, R. and Friedl, P. (2002) Induction of protein aggregation in an early secretory compartment by elevation of expression level Biotechnol. Bioeng., 78, 131-140
3. Brunkan, A.L., Martinez, M., Wang, J., Walker, E.S. and Goate, A.M. (2005) A domain at the C terminus of PS1 is required for presenilinase and -secretase activities J. Neurochem., 92, 1158-1169
4. Robb, V.A., Gerber, M.A., Hart-Mahon, E.K. and Gutmann, D.H. (2005) Membrane localization of the U2 domain of protein 4.1B is necessary and sufficient for meningioma growth suppression Oncogene, 24, 1946-1957
5. Sabo, S.L., Lanier, L.M., Ikin, A.F., Khorkova, O., Sahasrabudhe, S., Greengard, P. and Buxbaum, J.D. (1999) Regulation of -amyloid secretion by FE65, an amyloid protein precursor-binding protein J. Biol. Chem., 274, 7952-7957
6. Chen, F., Yang, D-S., Petanceska, S., Yang, A., Tandon, A., Yu, G., Rozmahel, R., Ghiso, J., Nishimura, M., Zhang, D.M., Kawarai, T., Levesque, G., Mills, J., Levesque, L., Song, Y-Q., Rogaeva, E., Westaway, D., Mount, H., Gandy, S., St. George-Hyslop, P. and Fraser, P.E (2000) Carboxyl-terminal fragments of Alzheimer -amyloid precursor protein accumulate in restricted and unpredicted intracellular compartments in presenilin 1 deficient cells J. Biol. Chem., 275, 36794-36802
7. Chen, F., Tandon, A., Sanjo, N., Gu, Y-J., Hasegawa, H., Arawaka, S., Lee, F.J.S., Ruan, X., Mastrangelo, P., Erdebil, S., Wang, L., Westaway, D., Mount, H.T.J., Yankner, B., Fraser, P.E. and St George Hyslop, P. (2003) Presenilin 1 and Presenilin 2 have differential effects on the stability and maturation of Nicastrin in mammalian brain J. Biol. Chem., 278, 19974-19979
8. Yang, D-S., Tandon, A., Chen, F., Yu, G., Yu, H., Arawaki, S., Hasegawa, H., Duthie, M., Schmidt, S.D., Ramabhadran, T.V., Nixon, R.A., Mathews, P.M., Gandy, S.E., Mount, H.T.J., St George-Hyslop, P. and Fraser, P.E. (2002) Mature glycosylation and trafficking of nicastrin modulate its binding to presenilins J. Biol. Chem., 277, 28135-28142
9. Brunkan, A.L., Martinez, M., Wang, J., Walker, E.S., Beher, D., Shearman, M.S. and Goate, A.M. (2005) Two domains within the first putative transmembrane domain of presenilin 1 differentially influence presenilinase and -secretase activity J. Neurochem., 94, 1315-1328
10. Petanceska, S., Seeger, M., Checler, F. and Gandy, S. (2000) Mutant presenilin 1 increases the levels of Alzheimer amyloid -peptide A42 in late compartments of the constitutive secretory pathway J. Neurochem., 74, 1878-1884
11. Kuiper, R.P., Bouw, G., Janssen, K.P.C., Rotter, J., van Herp, F. and Martens, G.J.M. (2001) Localization of p24 putative cargo receptors in the early secretory pathway depends on the biosynthetic activity of the cell Biochem. J., 360, 421-429
OptiPrepTM Application Sheet S25; 8th edition, January 2020
OptiPrep™ Application Sheet S26
Isolation of plasma membrane from cardiac muscle
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5)
1. Background
Using iodixanol gradients, isolation of a plasma membrane (PM) fraction is often achieved simultaneously with the purification of other subcellular membranes. Most of these methods are suitable for work with cultured cells and soft tissues such as liver but tissues such as muscle often require a customized method. Nevertheless some of the other iodixanol gradients may be adaptable to muscle tissue; for other plasma membrane methods return to the Index. Research into cardiac muscle function often involves the complex intracellular processes by which the GLUT4 glucose transport protein is translocated to and from the plasma membrane (sarcolemma). Thus in fractionations of cardiac muscle, a prime requirement is to be able resolve the plasma membrane from GLUT-4 transport vesicles and other membrane compartments. The overall strategy of this protocol is thus similar to that using a cultured cell line or other tissue but the details of the technique are rather different. The PM is usually the least dense of all the subcellular membranes and this Application Sheet highlights a novel exploitation of this property [1-3]. In this protocol, a crude plasma membrane suspension is adjusted to a density (1.049 g/ml, equivalent to 5% w/v iodixanol) just greater than that of the plasma membrane of cardiac muscle, thus during the centrifugation the plasma membrane will float to the top the liquid. By layering the sample upon a small cushion of 15% (w/v) iodixanol (1.103 g/ml) and centrifuging for 16 h, a shallow gradient will form due to diffusion of iodixanol and also to some sedimentation of iodixanol molecules close to the bottom of the tube. This will therefore allow not only isolation of the plasma membrane, but also partial fractionation of some of the slightly denser membrane compartments. The particular format described in this protocol also allows the sample to be contained within a relatively large volume; this also minimizes interactions between particles in the gradient.
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Homogenization medium (HM): 0.1 M sucrose, 10 mM EDTA, 46 mM KCl, 5 mM NaN3, 100 mM Tris-HCl, pH 7.4
C. OptiPrep™ Diluent: 0.25 M sucrose, 6 mM EDTA, 60 mM Tris-HCl, pH 7.4
D. Working Solution (WS) of 50% (w/v) iodixanol (ρ = 1.272 g/ml): 5 vol. of solution A + 1 vol. of solution C
E. WS diluent: 0.25 M sucrose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.4
3. Ultracentrifuge rotor requirements (see Section 5.2)
Any swinging-bucket rotor capable of 100,000-200,000g with tube volumes of approx. 14 ml (e.g. Beckman SW 41 or Sorvall TH641)
4. Protocol (adapted from refs 1 and 2]
Carry out all operations at 0-4°C.
1. Place 5 g of frozen tissue in saline and strip off epicardium and endocardium.
2. Place the tissue in Solution B (0.2 g/ml) and mince finely to produce 2 mm3 fragments.
3. Homogenize the tissue using 30 strokes of the pestle of a loose-fitting Dounce homogenizer (Wheaton Type B) followed by the same number of strokes in a tight-fitting Dounce homogenizer (Wheaton Type A).
4. Centrifuge the homogenate at 4000 g for 10 min and harvest the supernatant (see Section 5.3).
5. Adjust the 4000 g supernatant to 5% (w/v) iodixanol by mixing 9 vol. with 1 vol. of Solution D (see Section 5.1).
6. Prepare a solution 15% (w/v) iodixanol solution by mixing 1.5 vol. and 3.5 vol., respectively, of Solutions D and E (see Section 5.1).
7. To tubes for the swinging-bucket rotor transfer 10 ml of 4000 g supernatant in 5% iodixanol; using a syringe and metal cannula underlay with 1.5 ml of the 15% iodixanol and then overlay with approx 1 ml of Solution E (see Section 5.4).
8. Centrifuge at 80,000 g for 16 h (see Section 5.5).
9. Collect the plasma membrane fraction from the top of the gradient or harvest the gradient in 0.5 ml fractions either by tube puncture, aspiration from the meniscus or upward displacement. For more information on harvesting gradients see Section 5.6 and Application Sheet S08.
- See Section 5.7 for other method strategies
5. Technical Notes and Review
5.1. Homogenization media and gradient solutions
The homogenization medium has been tailored to the tissue type but whether other media can be been used without any effect on the fractionation is not known. An alternative medium containing 0.1 M sucrose, 5 mM MgCl₂, 100 mM KCl, 10 mM EDTA, 10 mM azide and 50 mM Tris-HCl, pH 7.4 had no discernible influence on the subsequent behaviour of the plasma membrane [4]. The inclusion of divalent cations can guard against nuclear breakage and stabilize membranes generally, but may lead to aggregation. Azide was added as a protease inhibitor [1] but alternative or additional inhibitors may be included in Solution B at the operator’s discretion. Protease inhibitors may also be included in Solutions C and E. If it is considered desirable that the concentrations of EDTA and KCl in the gradient should be identical with those of the homogenization medium then Solution C should contain 0.1 M sucrose, 60 mM EDTA and 276 mM KCl and the WS diluent (Solution E) should be replaced with Solution B. Concomitantly increasing the sucrose concentration to 0.6 M however should not be adopted as this will considerably raise the osmolality. The validity of these solutions will however require testing. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
5.2. Ultracentrifuge rotors
The method was originally developed for the 4 ml tubes of a Beckman SW60 swinging-bucket rotor using 3 ml of sample. Larger volume swinging-bucket rotors may require longer centrifugation times but smaller volume rotors may need shorter times. All volumes should be scaled up or down proportionately as required. Note however that the progressive change in gradient density profile (due to diffusion and sedimentation of the iodixanol molecules) may also be modulated in other rotors and affect the final resolution.
5.3. Low speed centrifugation of the homogenate
Nuclear pelleting is routinely carried out at 500-4000 g for 5-10 min; the higher RCFs (g-forces) resulting in removal of some of the mitochondria. To recover any vesicles trapped in the pellet (more serious at the higher RCFs), the pellet is sometimes resuspended in HM, recentrifuged and the two supernatants combined. A possible disadvantage of this practice is that unless the resuspension of the pellet is carried out very gently, the nuclei may be damaged, with consequent leakage of DNA, which may lead to almost irreversible aggregation of the subcellular membranes.
5.4. Density gradients
For more information on gradient construction see Application Sheet S03. If necessary, adjust all volumes proportionately so that tubes (after layering Solution D) are properly filled according to the manufacturer’s instructions.
5.5. Centrifugation conditions
The 80,000 g recommended in ref 1, has subsequently been reduced to 65,000 g in ref 2.
5.6. Analysis
Five visible layers were observed in the gradient after centrifugation, the plasma membrane (Na+ -K+ -ATPase) was restricted to the lowest density (Figure 1), while the ER, as determined by SERCA2 Ca2+ pump, banded in the two densest fractions, only one of which (the lighter one) also contained the glucose-transporter GLUT4 [1]. The method was used in studies on the translocation of GLUT4 to the plasma membrane during the raised status of glucose oxidation in heart failure [1], nitric oxide regulation of the myocardium in hyperhomocysteinemia [2]; nitric oxide inhibition of myocardial glucose transport [3] aquaporin expression [5] and for studying the roles for SUR subunits in KATP channel membrane targeting and regulation [6].
5.7 Other methodological modifications
Hong et al [7] increased the g-force and time centrifugation time to 200,000 g for 2.5 h and observed seven distinct zones of material. Subsequent modifications have included an increase in the density of the lower layer to 18% (w/v) iodixanol and a reduction in the centrifugation time to 1 h [8].
- A review of the technology for studying cardiac muscle biochemistry can be found in ref 9.
6. References
1. Lei, B., Lionetti, V., Young, M.E., Chandler, M.P., d’Agostino, C., Kang, E., Altarejos, M., Matsuo, K.,
Hintze, T.H., Stanley, W.C. and Recchia, F.A. (2004) Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure J. Mol. Cell. Cardiol., 36, 567-576
2. Lei, B., Matsuo, K., Labinsky, V., Sharma, N., Chandler, M.P., Ahn, A., Hintze, T.H., Stanley, W.C. and Recchia, F.A. (2005) Hyperhomocysteinemia, a cardiac metabolic disease role of nitric oxide and the p22phox subunit of NADPH oxidase Proc. Natl. Acad. Sci. USA, 102, 6966-6971
3. Becker, J.S., Adler, A., Schneeberger, A., Huang, H., Wang, Z., Walsh, E., Koller, A. and Hintze, T.H. (2005) Exogenous nitric oxide reduces glucose transporters translocation and lactate production in ischemic myocardium in vivo Circulation, 111, 2112-2118
4. Lei, B. (2006) Personal communication
5. Butler, T.L., Au, C.G., Yang, B., Egan, J.R., Tan, Y.M., Hardeman, E.C., North, K.N., Verkman, A.S. and Winlaw, D.S. (2006) Cardiac aquaporin expression in humans, rats, and mice Am. J. Physiol. Heart Circ. Physiol., 291, H705-H713
6. Hund, T.J. and Mohler, P.J. (2011) Differential roles for SUR subunits in KATP channel membrane targeting and regulation Am. J. Physiol. Heart Circ. Physiol., 300, H33–H35
7. Hong, M., Kefaloyianni, E., Bao, L., Malester, B., Delaroche, D., Neubert, T.A. and Coetzee, W.A. (2011) Cardiac ATP-sensitive K+ channel associates with the glycolytic enzyme complex FASEB J., 25, 2456–2467
8. Bao, L., Kefaloyianni, E., Lader, J., Hong, M., Morley, G., Fishman, G.I., Sobie, E.A. and Coetzee, W.A. (2011) Unique properties of the ATP-sensitive K+ channel in the mouse ventricular cardiac conduction system Circ. Arrhythm. Electrophysiol., 4, 926-935
9. Kefaloyianni, E., Bao, L., Rindler, M.J., Hong, M., Patel, T., Taskin, E. and Coetzee, W.A. (2012) Measuring and evaluating the role of ATP-sensitive K+ channels in cardiac muscle J. Mol. Cell. Cardiol., 52, 596–607
7. Acknowledgements
We would like to thank Dr Biao Lei and Dr Thomas H Hintze (Department of Physiology, New York Medical College, Valhalla, NY 10595) for their kind and invaluable help in the preparation of this OptiPrep™ Application Sheet.
OptiPrepTM Application Sheet S26; 7th edition, January 2020
OptiPrep™ Application Sheet S27
Fractionation of plasma membrane and plasma membrane domains using cationic colloidal silica
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 4)
1. Background
1a. Colloidal silica strategy
There have been many attempts to stabilize the plasma membrane (PM) of cultured cells against fragmentation during homogenization; thus retain it as a large sheet and so facilitate its separation from all the other subcellular membranes. In 1968 the use of chemical “hardening” agents such as Zn²+ and fluoroscein mercuric acetate [1] was introduced. While undoubtedly effective, the toxic nature of these agents meant that functional studies were compromised and the method never gained a wide popularity. Then in 1977, the adsorption of the PM on to a positively charged polylysine (or polyethylenimine) coated bead, followed by shearing away of the rest of the cell, was developed by Jacobson and Branton [2]. The remainder of (unbound) PM was however lost in the shearing process. More recently this problem of selective isolation of only part of the PM was avoided by coating the cells (in suspension) sequentially with cationic silica (approx 500Å diameter) and an anionic polymer (polyacrylic acid), thus forming a dense coat (pellicle) around the cell [3]. The polyacrylic acid cross-links and stabilizes the silica colloid and also blocks the residual positive charge on the surface of the colloidal particles. Repeating the binding of the cationic silica and anionic polymer can increase the thickness and the density of the pellicle [3]. The procedure has also been adapted to the isolation of basolateral and apical domains of the PM from polarized cells by coating the exposed (apical) surface of a bovine aortic endothelial cell monolayer, then lysing the cells by liquid shear in a jet of hypotonic solution, to leave the basolateral domain remaining on the substratum (e.g. ref 4). 1b. Separation of colloidal silica-bound membranes using Nycodenz® The colloidal silica membranes are usually separated from the native membranes by a high-density solution of Nycodenz® (usually 70%, w/v) upon which the total lysate (adjusted to approx 50% Nycodenz®) is layered [5-8]. The Si-bound membranes are then allowed to pellet at approx 20,000 g for 20 min [5-7] or at 60,000 g for 40 min [8]. Sometimes the lysate is not density-adjusted [9,10]. Occasionally the density-adjusted lysate is layered over a continuous gradient of 55-70% (w/v) Nycodenz® [11-13] or discontinuous gradient of 55%, 60%, 65% and 70% (w/v) Nycodenz® [14]. The relative efficacy of a continuous or discontinuous gradient is not clear. 1c. Separation of colloidal silica-bound membranes using Optiprep™ The rapidly-sedimenting PM pellicle complexes can be separated from other organelles either on a simple density barrier of OptiPrep™ (60%, w/v iodixanol) [15-17] or on a discontinuous iodixanol gradient [18]. The lower concentrations of iodixanol (compared to Nycodenz®) may reflect the lower density of native organelles in this medium and the simplicity of use of OptiPrep™ certainly makes this the medium of choice. Mansour et al [17] used the technology to separate silica-coated plasma membranes containing streptavidin-Dynabead-bound EphA3 clusters, which sediment through an OptiPrep™ cushion, while nuclei, unbound streptavidin beads, other sub-cellular particles and cytosol remain above the cushion.
- The following protocols describe the techniques as applied to monolayers of cultured cells.
- Section 2 describes the binding of colloidal silica to the plasma membrane and homogenization.
- In Section 2b monolayer cells are detached from the culture dish and coated with silica to provide a total PM fraction.
- In Section 2c the cell monolayer is coated with silica before detachment to provide the apical and basolateral domains of the PM.
- Section 3 describes the gradient purification using either OptiPrep™ or Nycodenz®.
2. Treatment of cell monolayers with colloidal silica (adapted from refs 3 and 4)
2a. Solutions required (see Section 4.1)
A. Hank’s Balanced Salt Solution (HBSS)
B. Coating buffer: 135 mM NaCl, 1 mM MgCl₂, 0.5 mM CaCl₂, 20 mM MES-NaOH, pH 5.5
C. Cationic silica colloid diluted to 1% (w/v) with Solution B
D. Polyacrylic acid: 1 mg/ml in Solution B, adjusted to pH 5.0 with NaOH
E. Lysis buffer: (LB): 1 mM MgCl₂, 0.5 mM CaCl₂, 2.5 mM imidazole-HCl, pH 7.0
2b. Coating and homogenization of detached cells
Carry out all operations, except Step 1, at 0-4°C.
1. Detach the cells from the substratum using the usual regime of EDTA and/or trypsin or collagenase and wash the cells twice in Solution A.
2. Wash the cell three times in Solution B.
3. Suspend the cells (e.g. 50 μl packed cell volume) in 0.2 ml of Solution B (see Section 4.2).
4. Rapidly mix 1.25 ml of the diluted cationic silica colloid (Solution C) is with the cell suspension (see Section 4.2).
5. Immediately after mixing dilute with approx. 8 vol. of Solution B and centrifuge the suspension at 700 g for 5 min to pellet the cells.
6. Aspirate and discard the colloidal silica supernatant.
7. Suspend the cells in the original volume of Solution B and mix with 5 vol. of Solution D (see Section 4.3).
8. Immediately after mixing dilute with approx. 8 vol. of Solution B and centrifuge the suspension at 700 g for 5 min. to pellet the cells.
9. Suspend the cell pellet in Solution E and leave for 30 min.
10. Homogenize the cells using a Dounce homogenizer (see Section 4.4).
11. Centrifuge the homogenate at 900 g for 10 min.
12. Resuspend the pellet in 2 ml of E, by sonication for 1 sec at a low setting (see Section 4.5).
2c. Coating and lysis of cell monolayers (see Section 4.6)
Carry out all operations, except Step 1 at 0-4°C.
1. Wash the monolayer twice in Solution A.
2. Wash the monolayer twice in Solution B.
3. Coat the cells with Solution C and then wash the monolayer with Solution B.
4. Coat the cells with Solution D and wash the monolayer twice with Solution B.
5. Wash the monolayer very quickly with a small volume of Solution E and aspirate to make sure that all residual amounts of Solution B are removed.
6. Pour 2-3 ml of Solution E on to the monolayer and allow to stand on ice for approx 30 min to lyse the cells and monitor lysis using light microscopy.
7. Assist lysis if necessary by repeatedly expelling Solution E from a syringe attached to a short narrow metal cannula (approx 18G), but be careful not to cause detachment of the cells.
8. When approx 90% of the cells have been lysed aspirate and retain the suspension, which contains the apical PM domain plus organelles released from the lysed cells.
9. Carefully wash the residual material (basolateral PM) with Solution E and add the washes to the original aspirate. Residual material may be solubilized for analysis by SDS-PAGE and Western blotting.
10. Centrifuge the aspirate (plus washes) at 900 g for 10 min.
11. Resuspend the pellet in 2 ml of E, by sonication for 1 sec at a low setting (see Section 4.5).
3. Gradient purification of the total PM or apical PM domain
3a. Ultracentrifuge rotor requirements
Any swinging-bucket rotor capable of approx 100,000 g with a tube volume of approx. 5 ml (e.g. Beckman SW55Ti)
3b. Using OptiPrep™
Separate the total PM or the apical PM domain from the sonicate by layering upon 3 ml of OptiPrep™ OR 1 ml each of 5%, 43% (w/v) iodixanol and undiluted OptiPrep™ in tubes for the chosen rotor. Prepare the two lower density solutions by diluting OptiPrep™ with Solution E (see Section 2a) 1:11 and 43:17 v/v, respectively. Centrifuge at 30,000 g for 30 min at 4°C (see Section 4.7) and resuspend the pellets of PM or apical PM in a suitable solution for analysis.
3c. Using Nycodenz®
To make a 100% (w/v) Nycodenz® stock solution: place 55 ml of Solution E (see Section 2a) in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 100 g of Nycodenz® in small amounts until dissolved. Allow the solution to cool to room temperature and if necessary make up to 100 ml with Solution E. Dilute this stock solution with Solution E to make up any lower density solutions. It is easier to make up a stock solution of a lower Nycodenz® concentration of say 70% or 75% (w/v), but larger volumes will be required for the adjustment of the sample to 50% (w/v). See Section 1b for gradient and centrifugation conditions and see Section 4.7. For more information on the preparation of both continuous and discontinuous gradients see Application Sheet S03.
4. Technical Notes and Review
4.1 Coating and Homogenization Solutions
The solutions used for coating the cells with the pellicle of cationic silica and polyacrylic acid are all buffered with MES and are marginally acidic (pH 5-5.5) but otherwise quite variable in composition. The same solution is normally used for initially suspending the cells and the vehicle for the two coating reagents. Chaney and Jacobson [3] used 140 mM sorbitol, 20 mM MES, pH 5.0 for Dictyostelium, while for mouse embryo fibroblasts Cain et al [15] used a hyperosmotic buffer containing 280 mM sorbitol, 150 mM NaCl, 20 mM MES. The buffer used for CHO cells [16] and for bovine aortic endothelial cells [4] however was more similar to a routine buffered saline solution (supplemented with low levels of divalent cations) and approx. isoosmotic and is the one that is described in this OptiPrep™ Application Sheet. What effect the osmolality and composition of the coating solution has on the coating process is not clear. It is necessary to have the silica colloid in excess and in the original method published by Chaney and Jacobson [3], the concentration of cationic silica colloid used in the coating process was higher (3- 4% w/v, final concentration) than the approx 1% (w/v) more widely used in later studies. In the earlier study however much larger numbers of non-mammalian (Dictyostelium) cells were used (see Section 4.3) Cationic silica colloid can either be prepared according to the method of Chaney and Jacobson [3] or purchased commercially from Sigma-Aldrich or EKA Chemicals. Lower concentrations of polyacrylic acid (approx 0.2 mg/ml) have also been used [15]; and while this is normally in the same coating solution as is the silica colloid, for Dictyostelium, the polyacrylic acid was dissolved in 70 mM NaCl, 20 mM MES-NaOH, pH 6.5 [3]. The lysis buffer may be any suitable buffer that will permit efficient disruption of the silica pellicle coated cells. Although Application Sheet S06 describes a variety of buffers that are routinely used for the homogenization of cultured cells, they may be unsuitable for coated cells. Protease inhibitors may be included in Solution E at the operator’s discretion.
4.2 Preparation of cells for coating
For larger numbers of cells, Chaney and Jacobson [3] used a different approach. Dictyostelium (2 ml packed cell volume) in 140 mM sorbitol, 20 mM MES, pH 5.0 (4 ml final volume) was added slowly to 4 ml of the cationic silica colloid (diluted to 6-8%, w/v with buffered sorbitol) while vortexing very gently. It may be necessary to optimize the coating conditions for different cell types and different numbers of cells.
4.3 Reaction with polyacrylic acid
Chaney and Jacobson [3] used the same method of slow addition of the cell suspension to polyacrylic acid on a vortex mixer as described for the silica colloid in Section 4.2.
4.4 Homogenization of coated cells
The homogenization protocol should be tailored to the cell (or tissue) type. The coat may render the cells less susceptible to disruption by routine liquid shear techniques, although the use of hypoosmotic lysis buffer appears to obviate any such problems and Cain et al. [15] were then subsequently able to homogenize mouse embryo fibroblasts in a Dounce homogenizer. Other standard procedures such as 12-20 passages through a syringe needle (Gauge Number (G) varies from 21 to 25), sometimes preceded by Dounce homogenization, or the ball-bearing homogenizer (“cell cracker”) may be applicable, but which is the most effective for coated cells can only be determined experimentally. Pellicle-coated Dictyostelium was most effectively homogenized by nitrogen cavitation [3]. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Some hints on homogenization of “native” cells are given in Application Sheet S06.
4.5 Resuspension of coated PM fractions
Using a Branson Sonifier 185, fitted with a microprobe, setting 2 was used by Stolz and Jacobsen [4]. It is important to that any coated material is applied to gradients as an homogeneous suspension. Sonication is the recommended procedure but since the coated PM pellet will also contain nuclei, this needs to be carried out as gently as possible otherwise any DNA released from the nuclei may cause serious aggregation.
4.6 Alternative cell handling
Note that the cells may also be grown on micro-carrier beads [4].
4.7 Gradient centrifugation of coated PM fractions
For the isolation of the coated fraction it is not clear if continuous gradients [11-13] or discontinuous gradients [e.g. refs 14 and 16] have any practical advantages over the simple density barrier format [5-8, 15, 17]. The g-forces range from 20,000-60,000g and centrifugation times from 20- 45 min.
5. References
1. Warren, L. and Glick, M.C. (1968) Membranes of animal cells II: The metabolism and turnover of the surface membrane J. Cell Biol. 37, 729-746
2. Jacobson, B.S. and Branton, D. (1977) Plasma membrane: rapid isolation and exposure of the cytoplasmic surface by the use of positively charged beads Science, 195, 302-304
3. Chaney, L.K. and Jacobson, B.S. (1983) Coating cells with colloidal silica for high yield isolation of plasma membrane sheets and identification of transmembrane proteins J. Biol. Chem., 258, 10062 10072
4. Stolz, D.B. and Jacobson, B.S. (1992) Examination of transcellular membrane protein polarity of bovine aortic endothelial cells in vitro using the cationic colloidal silica micro-bead membrane isolation method J. Cell Sci., 103, 39-51
5. Stolz, D.B., Ross, M.A., Salem, H.M., Mars, W.M., Michalopoulos, G.K. and Enomoto, K. (1999) Cationic colloidal silica membrane perturbation as a means of examining changes at the sinusoidal surface during liver regeneration Am. J. Pathol., 155, 1478-1498
6. Mora, M.P., Tourne-Peteilh, C., Charveron, M., Fabre, B., Milon, A. and Muller. I. (1999) Optimisation of plant sterols incorporation in human keratinocyte plasma membrane and modulation of membrane fluidity Chem. Phys. Lipids, 101, 255-265
7. Marchetti, M., Monier, M.N., Fradagrada, A., Mitchell, K., Baychelier, F., Eid, P., Johannes, L. and Lamaze, C. (2006) Stat-mediated signaling induced by type I and type II interferons (IFNs) is differentially controlled through lipid microdomain association and clathrin-dependent endocytosis of IFN receptors Mol. Biol. Cell, 17, 2896-2909
8. Li, L., Hisamoto, K. Kim, K.H., Haynes, M.P., Bauer, P.M., Sanjay, A., Collinge, M., Baron, M., Sessa, W.C. and Bender, J.R. (2007) Variant estrogen receptor–c-Src molecular interdependence and c-Src structural requirements for endothelial NO synthase activation Proc. Natl. Acad. Sci. USA, 104, 16468-16473
9. Rathmell, J.C., Fox, C.J., Plas, D.R., Hammerman, P.S., Cinalli, R.M. and Thompson, C.B. (2003) Aktdirected glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival Mol. Cell. Biol., 23, 7315-7328
10. Hynynen, R., Laitinen, S., Käkelä, R., Tanhuanpää, K., Lusa, S., Ehnholm, C., Somerharju, P., Ikonen, E. and Olkkonen, V.M. (2005) Overexpression of OSBP-related protein 2 (ORP2) induces changes in cellular cholesterol metabolism and enhances endocytosis Biochem. J., 390, 273-283
11. Garver, W.S., Krishnan, K., Gallagos, J.R., Michikawa, M., Francis, G.A. and Heidenreich, R.A. (2002) Niemann-Pick C1 protein regulates cholesterol transport to the trans-Golgi network and plasma membrane caveolae J. Lipid Res., 43, 579-589
12. Kincer, J.F., Uittenbogaard, A., Dressman, J., Guerin, T.M., Febbraio, M., Guo, L. and Smart, E.J. (2002) Hypercholesterolemia promotes a CD36-dependent and endothelial nitric-oxide synthase-mediated vascular dysfunction J. Biol. Chem., 277, 23525-23533
13. Rizzo, V., Morton, C., DePaola, N., Schnitzer, J.E. and Davies, P.F. (2003) Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro Am. J. Physiol. Heart Circ. Physiol., 285, H1720-H1729
14. Ramos, M., Lame, M.W., Segall, H.J. and Wilson, D.W. (2006) The BMP type II receptor is located in lipid rafts, including caveolae, of pulmonary endothelium in vivo and in vitro Vasc. Pharmacol., 44, 50-59
15. Cain, R.J., Hayward, R.D. and Koronakis, V. (2004) The target cell plasma membrane is a critical interface for Salmonella cell entry effector-host interplay Mol. Microbiol., 54, 887-904
16. Mathias, R.A., Chen, Y-S., Goode, R.J.A., Kapp, E.A., Mathivanan, S., Moritz, R.L., Zhu, H-J. and Simpson, R.J. (2011) Tandem application of cationic colloidal silica and Triton X-114 for plasma membrane protein isolation and purification: Towards developing an MDCK protein database Proteomics, 11, 1238–1253
17. Mansour, M., Nievergall, E., Gegenbauer, K., Llerena, C., Atapattu, L., Halle, M., Tremblay, M.L., Janes, P.W. and Lackmann, M. (2016) PTP-PEST controls EphA3 activation and ephrin-induced cytoskeletal remodelling J. Cell Sci., 129, 277-289
18. Pastrana, D.V., Hanson, A.J., Knisely, J., Bu, G. and Fitzgerald, D.J. (2005) LRP1B functions as a receptor for Pseudomonas endotoxin Biochem. Biophys. Acta, 1741, 224-239
OptiPrepTM Application Sheet S27; 8th edition, January 2020
OptiPrep™ Application Sheet S28
Fractionation of brush border and basolateral plasma membrane domains from intestinal (ileal) mucosa
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5)
1. Background
The resolution of plasma membrane domains, primarily the basolateral and apical domains from polarized tissues such as intestine, liver and kidney and also from polarized cells such as human colon adenocarcinoma (Caco-2) cells and Madin-Darby canine kidney (MDCK) cells, is an important preliminary requirement for studies on how functional dichotomy at the cell surface is achieved. Methods often involve the use of divalent cations. Brush border preparations, from for example intestinal mucosa, treated with 10 mM MgSO4, allow residual basolateral membrane and intracellular membranes to be removed by low-speed centrifugation [1]. The isolation of the basolateral domain from intestinal mucosa cells, originally carried out in a variety of sucrose gradients, was transferred to Percoll® gradients by Scalera et al [2] and used subsequently by other workers, e.g. Cohen et al [1]. Distribution of the basolateral membrane within the Percoll® gradient is however inconveniently broad, the band extending over more than 80% of the gradient volume and, as with all Percoll® gradients, it is necessary to remove the colloidal silica before any analysis can be carried out. Moreover isolation of the brush border domain had to be carried out separately using the divalent cation precipitation technique. Although this is less of a problem for preparative work, for analytical work it is much less suitable. Iodixanol gradients are however now being increasingly used to provide the high resolution necessary to purify both the apical and basolateral membranes away from other intracellular membranes, using a total membrane fraction sedimented from an homogenized ileal cell post-nuclear supernatant; thus permitting the use of a single separation technique [3].
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Homogenization buffer: 2 mM DTT, 1 mM EGTA/1.006 mM CaCl₂, 20 mM Tris-HCl, pH 7.5
C. OptiPrep™ dilution buffer: 150 mM NaCl, 50 mM NaF, 20 mM Hepes-NaOH, pH 7.2
3. Ultracentrifuge rotor requirements (See Section 5.2)
Swinging-bucket rotor with 13-14 ml tubes (e.g. Beckman SW41Ti, Sorvall TH641 or similar)
4. Protocol (adapted from refs 1 and 3)
Carry out all operations except Step 1 at 0-4°C.
1. Incubate the ileal segments in gassed Ringer’s-bicarbonate buffer at 37°C as required.
2. Place the segments on a chilled glass plate and scrape off the mucosa gently with a glass slide.
3. Transfer the mucosa to a small beaker and pour on 10 vol. of Solution B and place in ice.
4. Homogenize using a Polytron™ homogenizer using 10 x 10 sec bursts, each burst being separated by a 20 sec rest. During the rest periods swirl the contents of the beaker in the ice to ensure efficient cooling (see Section 5.3).
5. Centrifuge the homogenate 3000 g for 5 min to pellet unbroken cells, nuclei and debris.
6. Carefully decant the supernatant repeat the centrifugation.
7. Centrifuge the supernatant from step 6 at 160,000 g for 1 h. Carry out Steps 8 and 9 during the centrifugation.
8. Prepare solutions of 10%, 12.5%, 15%, 17.5%, 20%, 22.5%, 25%, 27.5% and 30% (w/v) iodixanol by diluting OptiPrep™ with Solution C (see Section 5.1).
9. Using a syringe and metal cannula layer 1.25 ml of each solution in tubes for the swinging-bucket rotor (see Section 5.4.1).
10. Resuspend the pellet from Step 7 in Solution C and layer approx. 1.3 ml on top of each gradient, to fill the tube (see Section 5.4.2).
11. Centrifuge at approx 64,000 gav for 90 min; allow the rotor to decelerate without the brake or use a slow deceleration program.
12. Unload the gradient in approx. 0.6 ml fractions using upward displacement, tube puncture or aspiration from the meniscus. For more information on unloading gradients see Application Sheet S08.
- For details of the expected separation of the apical and basolateral domains Section 5.5.
5. Technical Notes and Review
5.1 Homogenization media and gradient solutions
Additional protease inhibitors may be included in Solutions B and C at the operator’s discretion. Preparation of the density gradient solutions by diluting OptiPrep™ with Solution C will mean that the concentration of NaCl, NaF and Hepes will fall as the iodixanol concentration increases. If this is deemed undesirable then first dilute 5 vol. of OptiPrep™ with 1 vol. of a solution containing 150 mM NaCl, 300 mM NaF, 120 mM Hepes-NaOH, pH 7.2 and then dilute this 50% (w/v) iodixanol Working Solution with Solution C to prepare the density gradient solutions. The NaF and Hepes concentrations will then be constant throughout the gradient. If the OptiPrep™ diluent also contains 6×150 mM NaCl, all the density solutions will be grossly hyperosmotic, which would probably be undesirable. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01. Other homogenization media have been used: Scalera et al [2] used a simple one of 0.25 M sucrose, 10 mM triethanolamine-HCl, pH 7.6
5.2 Ultracentrifuge rotors
These separations have been performed in 13 ml tubes. Other swinging-bucket rotors or even vertical rotors may be used. Larger volume swinging-bucket rotors may require longer centrifugation times but smaller volume rotors and vertical rotors will need shorter times. All volumes should be scaled up or down proportionately. Note however that the progressive change in gradient density profile (due to diffusion of the iodixanol molecules) may also be modulated in other rotors and affect the final resolution.
5.3 Homogenization
Although the Polytron is the homogenizer of choice, less sophisticated rotating blades homogenizers such as the Waring blender have also been used [2].
5.4 Density gradients
5.4.1 Construction
Discontinuous gradients are normally most easily prepared by underlayering (i.e. low density first) using a syringe (1-2 ml) and a long metal cannula); overlayering small volumes is more difficult using either a syringe or Pasteur pipette. One alternative for overlayering the small volumes used in this protocol is to use a small volume (low-pulsating) peristaltic pump; first to take up the required volume of solution into the attached tubing and second, to reverse the flow, in order to expel it slowly on to a denser layer in the centrifuge tube. For more information on gradient construction see Application Sheet S03.
5.4.2 Tube loading
In swinging-bucket rotors of different tube volumes scale up or down the volumes proportionately. The separation achieved in this protocol is probably based at least partly on sedimentation velocity, so the sample volume should never be more than 10% of the gradient volume. If necessary, adjust all volumes proportionately so that tubes are properly filled according to the manufacturer’s instructions.
5.5 Gradient analysis
If it is necessary to concentrate a fraction or to remove the iodixanol before analysis, see Application Sheet S09. In this gradient system, the banding of the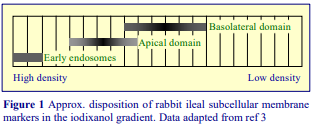 apical and basolateral domains of the ileal mucosal cell plasma membrane is shown in Figure 1. The apical domain was identified using sucrase as a marker and the basolateral marker was Na+/K+ ATPase; EEA1 was used as an early endosomes marker. Li et al [3,4] were able to demonstrate that the Na+-H+ exchanger (NH3) was localized to the apical domain and to the early endosomes. More recently the Ser/Thr kinase Akt has also been localized principally to the apical domain [5]. The gradient has also been used for the fractionation of Caco-2 cell membranes [5]; for more information see Application Sheet S29. The gradient may be applicable to the resolution of basolateral and apical plasma membrane domains from other tissues and cells but this can only be determined experimentally.
apical and basolateral domains of the ileal mucosal cell plasma membrane is shown in Figure 1. The apical domain was identified using sucrase as a marker and the basolateral marker was Na+/K+ ATPase; EEA1 was used as an early endosomes marker. Li et al [3,4] were able to demonstrate that the Na+-H+ exchanger (NH3) was localized to the apical domain and to the early endosomes. More recently the Ser/Thr kinase Akt has also been localized principally to the apical domain [5]. The gradient has also been used for the fractionation of Caco-2 cell membranes [5]; for more information see Application Sheet S29. The gradient may be applicable to the resolution of basolateral and apical plasma membrane domains from other tissues and cells but this can only be determined experimentally.
- The OptiPrep™ method has been applied to ileal cells from rabbit [3-6], mouse jejunal mucosal
cells [7], mouse ileal cells [8] and frozen human jejunal specimens [9]. - Excellent methodological reviews are provided in refs 6 and 10
- Refs 11 and 12 review the function of the Na/H exchanger NH3 from data obtained in OptiPrep™
fractionations - Ref 13 reviews proteomic analysis associated with inflammatory bowel disease
6. References
1. Cohen, M.E., Wesolek, J., McCullen, J., Rys-Sikora, K., Pandol, S., Rood, R.P., Sharp, G.W.G. and Donowitz, M. (1991) Carbachol- and elevated Ca2+-induced translocation of functionally active protein kinase C to brush borders of rabbit ileal Na+ absorbing cells J. Clin. Invest., 88, 855-863
2. Scalera, V., Storelli, C., Storelli-Joss, C., Haase, W. and Muer, H. (1980) A simple and fast method for the isolation of basolateral plasma membranes from rat small intestinal epithelial cells Biochem. J., 186, 177-181
3. Li, X., Galli, T., Leu, S., Wade, J.B., Weinman, E.J., Leung, G., Cheong, A., Louvard, D. and Donowitz, M. (2001) Na+ -H+ exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3 J. Physiol., 537, 537-552
4. Li, X., Zhang, H., Cheong, A., Leu, S., Chen, Y., Elowsky, C.G. and Donowitz, M. (2004) Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent J. Physiol., 556.3, 791-804
5. Li, X., Leu, S., Cheong, A., Zhang, H., Baibakov, B., Shih, C., Birnbaum, M.J. and Donowitz, M. (2004) Akt2, phosphatidylinositol 3-kinase, and PTEN are in lipid rafts of intestinal cells: role in absorption and differentiation Gastroenterol., 126, 122-135
6. Li, X. and Donowitz, M. (2008) Fractionation of subcellular membrane vesicles of epithelial and nonepithelial cells by OptiPrep™ density gradient ultracentrifugation In Methods Mol. Biol., 440, Exocytosis and Endocytosis (ed. Ivanov, A.I.) Humana Press, Totowa, NJ, pp 97-110
7. Tobin, V., Le Gall, M., Fioramonti, X., Stolarczyk, E., Blazquez, A.G., Klein, C., Prigent, M., Serradas, P., Cuif, M.H., Magnan, C., Leturque, A., and Brot-Laroche, E. (2008) Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice Diabetes, 57, 555-562
8. Sullivan, S., Alex, P., Dassopoulos, T., Zachos, N.C., Iacobuzio-Donahue, C., Donowitz, M., Brant, S.R., Cuffari, C., Harris, M.L., Datta, L.W., Conklin, L., Chen, Y. and Li, X. (2009) Down-regulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: potential contributors to IBDassociated diarrhea Inflamm. Bowel Dis., 15, 261–274
9. Ait-Omar, A., Monteiro-Sepulveda, M., Poitou, C., Le Gall, M., Cotillard, A., Gilet, J., Garbin, K., Houllier, A., Château, D., Lacombe, A., Veyrie, N., Hugol, D., Tordjman, J., Magnan, C., Serradas, P., Clément, K., Leturque, A. and Brot-Laroche, E. (2011) GLUT2 accumulation in enterocyte apical and intracellular membranes a study in morbidly obese human subjects and ob/ob and high fat–fed mice Diabetes 60, 2598–2607
10. Li, X. and Donowitz, M. (2014) Fractionation of subcellular membrane vesicles of epithelial and nonepithelial cells by OptiPrep™ density gradient ultracentrifugation In Exocytosis and Endocytosis, Methods in Molecular Biology, 1174 (ed. Ivanov, A,I.) Springer Science+Business Media New York 2014, pp 85-99
11. Donowitz, M. and Li, X. (2007) Regulatory binding partners and complexes of NHE3 Physiol. Rev., 87, 825-872
12. Donowitz, M., Mohan, S., Xinjun Zhu, C., Chen, T-E., Lin, R., Cha, B., Zachos, N.C., Murtazina, R., Sarker, R. and Li, X. (2009) NHE3 regulatory complexes J. Exp. Biol., 212, 1638-1646
13. Alex, P., Gucek, M. and Li, X. (2009) Applications of proteomics in the study of inflammatory bowel
diseases: current status and future directions with available technologies Inflamm. Bowel Dis., 15, 616 629
OptiPrepTM Application Sheet S28; 7th edition, January 2020
OptiPrep™ Application Sheet S29
Fractionation of apical and basolateral plasma membrane domains from Caco-2 cells
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5)
1. Background
The resolution of plasma membrane (PM) domains, primarily the basolateral and apical domains from polarized tissues such as intestine, liver and kidney and also from polarized cells such as human colon adenocarcinoma (Caco-2) cells and Madin-Darby canine kidney (MDCK) cells, is an important preliminary requirement for studies on how functional dichotomy at the cell surface is achieved. Methods often involve the use of divalent cations. Brush border preparations, from for example intestinal mucosa, treated with 10 mM MgSO4, allow residual basolateral membrane and intracellular membranes to be removed by low-speed centrifugation [1]. Ellis et al [2] used a modification of this procedure; basolateral and apical membranes from Caco-2 cells were separated in a sucrose gradient and 10 mM MgCl₂ was used to remove contaminating intracellular membranes from the basolateral domain band. Iodixanol gradients are however now being increasingly used to provide the high resolution necessary to purify both the apical and basolateral membranes away from other intracellular membranes. This Application Sheet describes the use of CaCl₂ to separate the apical domain from the basolateral domain + endomembranes from a post-mitochondrial supernatant of Caco-2 cells and then subsequently an iodixanol gradient to resolve the basolateral domain from endomembranes [3]. Methods for the resolution of some other plasma membrane domains may be accessed via the Index.
2. Solutions required (see Section 5.1)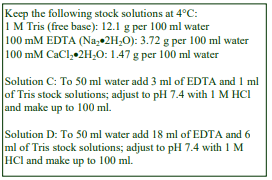
A. OptiPrep™
B. Phosphate-buffered saline (PBS)
C. Homogenization buffer: 3 mM EDTA, 10 mM Tris-HCl, pH 7.4
D. OptiPrep™ dilution buffer: 18 mM EDTA, 60 mM Tris-HCl, pH 7.4
E. Working Solution (50% w/v iodixanol): mix 5 vol. of Optiprep™ with 1 vol. of Solution D
F. Ca₂+ solution: 100 mM CaCl2
3. Ultracentrifuge rotor requirements (see Section 5.2)
Swinging-bucket rotor with 13-14 ml tubes (e.g. Beckman SW41Ti, Sorvall TH641 or similar)
4. Protocol (adapted from ref 3)
Carry out all operations except Step 1 at 0-4°C.
1. Grow Caco-2 cells on permeable supports as required.
2. Scrape off the cells from the filter using a policeman into ice-cold PBS.
3. Pellet the cells at 600 g for 10 min (or 20 sec in a microfuge) and wash the cells once with PBS.
4. Suspend the cells in 5 ml of Solution C and homogenize in a tight-fitting Dounce (glass-glass) or Potter-Elvehjem (Teflon-glass) homogenizer (see Section 5.3).
5. Centrifuge the homogenate 1000 g for 5 min to pellet unbroken cells, nuclei and debris (see Section 5.4).
6. Carefully aspirate the supernatant and centrifuge it at 10,000 g to pellet the mitochondria and most of the lysosomes and peroxisomes.
7. Carefully aspirate the supernatant; add 1/10th of the volume of Solution F and leave on ice for 30 min.
8. During the incubation on ice prepare solutions of 40%, 30% and 5% (w/v) iodixanol by diluting Solution E with Solution C at volume ratios of 4:1, 3:2 and 1:9 respectively (see Section 5.1).
9. Centrifuge the suspension (Step 7) at 10,000 g for 15 min.
10. Aspirate and retain the supernatant and resuspend the pellet containing the basolateral PM domain in 1-2 ml of 40% iodixanol.
11. Pellet the apical PM domain from the supernatant at 100,000 g for 30 min.
12. During this centrifugation prepare 11-12 ml continuous gradients from equal volumes of the 30% and 5% (w/v) iodixanol solutions using either a two chamber gradient maker or a Gradient Master™ in tubes for the swinging-bucket rotor (see Section 5.5.1).
13. Using a syringe and metal cannula underlay the gradient with the basolateral PM domain suspension from Step 10 (see Section 5.5.2).
14. Centrifuge the gradients at approx. 165,000 gav for 3 h and allow the rotor to decelerate without the brake below 2000 rpm or use a controlled deceleration program.
15. Unload the gradient in 0.5-1.0 ml fractions using upward displacement, tube puncture or aspiration from the meniscus. For more information on unloading gradients see Application Sheet S08; for more information on gradient analysis see Section 5.6.
5. Technical Notes and Review
5.1 Homogenization media and gradient solutions
The homogenization medium often has to be tailored to the tissue or cell type and it is not known if the composition of the HM is relevant to the separation described in this Application Sheet. Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility in functional studies on subcellular membranes; moreover these low ionic strength HMs and gradient solutions permit the direct use of fractions for SDS-PAGE. The most commonly used isoosmotic HMs contain 0.25 M sucrose buffered either with Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and often, but not always, containing 1 mM EDTA. Supplementation of the HM with inorganic salts is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). Some other examples of homogenization media for cultured cells are given in Application Sheet S06. Often however, as in this protocol, a frankly hypoosmotic medium is used to swell the cells and so facilitate homogenization. This may be important to the plasma membrane domain isolation but it may also cause some organelles such mitochondria and lysosomes to fragment. Only experimentation can determine if other homogenization media are permissible with Caco-2 cells or with other polarized cells. By using the strategy of first preparing a 50% (w/v) iodixanol Working Solution (Solution E) containing 3 mM EDTA, 10 mM Tris-HCl, pH 7.4, the concentration of EDTA and Tris will be
constant throughout the gradient. This will not be the case if the gradient solutions are simply prepared by diluting OptiPrep™ with Solution C. Strategies for preparing working solutions (WSs) for mammalian tissues and cells are given in Application Sheet S01.
- Protease inhibitors may be added to Solutions C and D at the operator’s discretion.
5.2 Ultracentrifuge rotors
These separations have been performed in 13 ml tubes. Other swinging-bucket rotors or even vertical rotors may be used. Larger volume swinging-bucket rotors may require longer centrifugation times but smaller volume rotors and vertical rotors will need shorter times. All volumes should be scaled up or down proportionately. Note however that the progressive change in gradient density profile (due to diffusion of the iodixanol molecules) may also be modulated in other rotors and affect the final resolution.
5.3 Homogenization
Although Musch et al [3] used 20 strokes of the pestle of a tight fitting Potter-Elvehjem homogenizer this may not be the only homogenization protocol that is valid. Moreover the method may need customizing to other polarized cells. Dounce and Potter-Elvehjem homogenization were the most widely used procedures at one time but the ball-bearing homogenizer (“cell cracker”) is now regarded as one of the most effective and reproducible of devices. If this is not available however 10-20 passages through a syringe needle (the Gauge Number (G) varies from 21 to 25) is usually an efficient alternative. Occasionally use of a syringe needle is prefaced by Dounce homogenization. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some hints on homogenization are given in Application Sheet S06.
5.4 Differential centrifugation
Musch et al [3] omitted this step and centrifuged the homogenate directly at 10,000g. Nuclei in particular will sediment very rapidly under these conditions and this may lead to entrapment and loss of smaller particles.
5.5 Density gradients
5.5.1 Construction
If neither of these gradient-making devices is available then a continuous gradient can be prepared by diffusion of a discontinuous gradient (use equal volumes of 5%, 10%, 15%, 20%, 25% and 30% (w/v) iodixanol). For more information on gradient construction see Application Sheet S03.
5.5.2 Tube loading
In swinging-bucket rotors of different tube volumes scale up or down the volumes proportionately. If necessary, adjust all volumes (also proportionately) so that tubes are properly filled according to the manufacturer’s instructions
5.6 Gradient analysis
If it is necessary to concentrate a fraction or to remove the iodixanol before analysis, see Application Sheet S09. The basolateral membrane bands in the 10-15% (w/v) iodixanol region [4], this is at slightly higher density than that reported for in other plasma membrane fractionation gradients (5-10% iodixanol) but it may reflect the binding of the CaCl2, unique to this type of isolation.
- An excellent review of the iodixanol methodology as applied to epithelial cells is given in ref 5.
6. References
1. Cohen, M.E., Wesolek, J., McCullen, J., Rys-Sikora, K., Pandol, S., Rood, R.P., Sharp, G.W.G. and Donowitz, M. (1991) Carbachol- and elevated Ca2+-induced translocation of functionally active protein kinase C to brush borders of rabbit ileal Na+ absorbing cells J. Clin. Invest., 88, 855-863
2. Ellis, J.A., Jackman, M.R. and Luzio, J.P. (1992) The post-synthetic sorting of ensogenous membrane proteins examined by the simultaneous purification of apical and basolateral plasma membrane fractions from Caco-2 cells Biochem. J., 283, 553-560
3. Musch, M.W., Walsh-Reitz, M.M. and Chang, E.B. (2006) Roles of ZO-1, occludin, and actin in oxidantinduced barrier disruption Am. J. Physiol. Gastrointest. Liver Physiol., 290, 222-231
4. Musch, M.W. (2006) Personal communication
5. Li, X. and Donowitz, M. (2014) Fractionation of subcellular membrane vesicles of epithelial and nonepithelial cells by OptiPrep™ density gradient ultracentrifugation In Exocytosis and Endocytosis, Methods in Molecular Biology, 1174 (ed. Ivanov, A,I.) Springer Science+Business Media New York 2014, pp 85-99
OptiPrepTM Application Sheet S29; 7th edition, January 2020
OptiPrep™ Application Sheet S30
Fractionation of plasma membrane microdomains of the brush border from renal cortex tissue and from glomeruli (slit diaphragms)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and
select the appropriate S-number.
1. Renal cortex
1a. Background
The resolution of plasma membrane domains, primarily the basolateral and apical domains from polarized tissues such as intestine, liver and kidney and also from polarized cells such as human colon adenocarcinoma (Caco-2) cells and Madin-Darby canine kidney (MDCK) cells, is an important preliminary requirement for studies on how functional dichotomy at the cell surface is achieved. Methods often involve the use of divalent cations. Brush border preparations from, for example intestinal mucosa, treated with 10 mM MgSO4, allow residual basolateral membrane and intracellular membranes to be removed by low-speed centrifugation [1]. The basolateral membranes are then prepared in a separate density gradient protocol. Ellis et al [2] used a modification of this procedure; basolateral and apical membranes from Caco-2 cells were separated in a sucrose gradient and 10 mM MgCl₂ was used to remove contaminating intracellular membranes from the basolateral domain band. Iodixanol gradients are however now being increasingly used to provide the high resolution necessary to purify both the apical and basolateral membranes away from other intracellular membranes, as part of single procedure, sometimes without the use of divalent cations. Methods for the resolution of some other plasma membrane domains may be accessed via the Index. The high resolving power of iodixanol gradients can however achieve a separation of other plasma membrane microdomains that have hitherto been difficult to study with sucrose gradients. Biemesderfer et al [3] reported that shallow continuous iodixanol gradients are capable of resolving the microvillar and intermicrovillar domains from the renal brush border and it is this technology is presented in this Application Sheet.
- Important technical notes and a summary of the analyses that have been carried out on the membrane fractions are given in Section 1e
1b. Solutions required (see Section 5.1)
A. OptiPrep™
B. OptiPrep™ dilution buffer: 0.25 M sucrose, 120 mM Tricine-NaOH, pH 7.8
C. Working Solution of 50% (w/v) iodixanol: mix 5 vol. of OptiPrep™ with 1 vol. of Solution B.
D. Homogenization buffer: 0.25 M sucrose, 20 mM Tricine-NaOH, pH 7.8
E. Gradient solutions: Dilute Solution C with Solution D to produce solutions containing 5%, 15% and 25% (w/v) iodixanol
1c. Ultracentrifuge rotor requirements (see Section 5.2)
Swinging-bucket rotor with 13-14 ml tubes (e.g. Beckman SW41Ti, Sorvall TH641 or similar)
1d. Protocol (adapted from ref 1)
Carry out all operations at 0-4°C.
1. Separate the renal cortex from the excised kidney.
2. Homogenize the tissue in 35 ml of Solution D using a loose-fitting Potter-Elvehjem homogenizer and centrifuge the homogenate at 1900 g for 15 min.
3. Aspirate the supernatant and centrifuge at 21,000 g for 20 min.
4. The lower well-packed layer of the bipartite pellet contains the major organelles while the upper loosely-packed layer contains the denser microsomes.
5. Aspirate the supernatant plus the loosely packed microsomes using a syringe and metal cannula and centrifuge at 48,000 g for 30 min.
6. Resuspend the pellet in the 5% iodixanol solution (10-20 mg protein/ml).
7. Using a two-chamber gradient maker or Gradient Master prepare linear 15-25% iodixanol gradients (12-13 ml), then layer approx 0.5 ml of the microsome suspension on top to fill the tube (see Section 5.3).
8. Centrifuge at 100,000 g for 3 h and collect the gradient either by tube puncture, aspiration from the meniscus or upward displacement in 0.5-1.0 ml fractions (see Section 5.4).
1e. Technical Notes and Review
Homogenization media and gradient solutions
Protease inhibitors may be included in Solutions B and D at the operator’s discretion. The preparation of a Working Solution as described, ensures that the concentration of buffer is constant throughout the gradient. If this is deemed unimportant the 5%, 15% and 25% iodixanol solutions may be prepared by diluting OptiPrep™ with Solution D. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
Ultracentrifuge rotors
These separations have been performed in 13 ml tubes. Other swinging-bucket rotors or even vertical rotors may be used. Larger volume swinging-bucket rotors may require longer centrifugation times but smaller volume rotors and vertical rotors will need shorter times. All volumes should be scaled up or down proportionately. Note however that the progressive change in gradient density profile (due to diffusion and sedimentation of the iodixanol molecules) may also be modulated in other rotors and affect the final resolution.
Density gradients
If neither of these devices is available, form a continuous iodixanol gradient by allowing a discontinuous one to diffuse. For more information on gradient construction see Application Sheet S03. If necessary, adjust all volumes proportionately so that tubes are properly filled according to the manufacturer’s instructions.
Harvesting the gradient
Methods for the efficient harvesting of density gradients are described in Application Sheet S08. If it is necessary to concentrate a fraction or to remove the iodixanol before analysis, see Application Sheet S09.
Gradient Analysis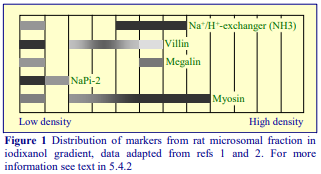
Two major areas of microdomain markers have been identified; one narrow band close to the top of the gradient contains typical microvillar markers such as villin and the Na-Pi co-transporter NaPi-2. Towards the middle of the gradient is broader band that was highly enriched in the Na+/H+ exchanger isoform NH3, which was only a very minor component of the villin-containing band [3]. Megalin (and clathrin) were present in both regions [3,4]. The median NH3 containing band was identified as the intermicrovillae microdomain, which was later shown to be highly enriched in myosin VI [5]. Interestingly the distribution of villin in the median region of the gradient was distinctive from that of the myosin. This data suggests that a shallow 15%-25% (w/v) iodixanol gradient (covering the approx. density range 1.10-1.14 g/ml is capable of very fine discrimination and may be applicable to plasma domain resolution from other tissues. More recently megalin processing in the brush border [6], processing of the Na+/H+ exchanger [7]; its down-regulation by dipeptidyl peptidase IV inhibition [8] and its reduction in spontaneously-hypertensive rate [9] have been reported. Studies on the Type IIc Na-Pi exchanger have used an identical method [10].
2. Glomeruli (slit diaphragms)
The self-generated gradients, produced from a three-iodixanol layer discontinuous gradient, developed by Yeaman [11] and Vogelmann and Nelson [12] (see Application Sheet S31) have also been used in the analysis of junctional proteins from glomeruli [13]. A PNS fraction from the glomerular homogenate is mixed with OptiPrep™ is adjusted to 30% (w/v) iodixanol and overlaid with equal volumes of 20% and 10% iodixanol. Self-generated gradients are normally run in either vertical or near-vertical rotors, since for efficient formation a rotor with a short sedimentation path length is required, but Fukasawa et al [13] used a Beckman SW60 Ti (4 ml tubes) at 350,000 g for 3 h. It is however known that in the absence of a vertical or near vertical rotor, if a two layer starting format is used (rather than a uniform concentration of iodixanol), effective gradients can be generated in, for example, a 10 ml fixed-angle rotor [14]. The gradient displayed remarkable resolving power: for example, occludin, cadherins, claudin-5 and crumbs-3 all had very distinctive distributions through the gradient. For more information see ref 13.
3. References
1. Cohen, M.E., Wesolek, J., McCullen, J., Rys-Sikora, K., Pandol, S., Rood, R.P., Sharp, G.W.G. and Donowitz, M. (1991) Carbachol- and elevated Ca2+-induced translocation of functionally active protein kinase C to brush borders of rabbit ileal Na+ absorbing cells J. Clin. Invest., 88, 855-863
2. Ellis, J.A., Jackman, M.R. and Luzio, J.P. (1992) The post-synthetic sorting of ensogenous membrane proteins examined by the simultaneous purification of apical and basolateral plasma membrane fractions from Caco-2 cells Biochem. J., 283, 553-560
3. Biemesderfer, D., DeGray, B. and Aronson, P. S. (2001) Active (9.6S) and inactive (21S) oligomers of NHE3 in distinct microdomains of the renal brush border J. Biol. Chem., 276, 10161-10167
4. Girardi, A. C. C., Degray, B. C., Nagy, T., Biemesderfer, D. and Aronson, P. S. (2001) Association of Na+-H+ exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule J. Biol. Chem., 276, 46671-46677
5. Biemesderfer, D., Mentone, S. A., Mooseker, M. and Hasson, T. (2002) Expression of myosin VI within the early endocytic pathway in adult and developing proximal tubules Am. J. Physiol. Renal Physiol. 282, F785-794
6. Zou, Z., Chung, B., Nguyen, T., Mentone, S., Thomson, B. and Biemesderfer, D. (2004) Linking receptormediated endocytosis and cell signaling, evidence for regulated intramembrane proteolysis of megalin in proximal tubule J. Biol. Chem., 278, 34302-343310
7. Kocinsky, H.S., Girardi, A.C.C., Biemsderfer, D., Nguyen, T., Mentone, SA., Orlowski, J. and Aronson, P.S. (2005) Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+ /H+ exchanger NHE3 at PKA consensus sites Am. J. Physiol. Renal Physiol., 289, F249-F258
8. Castello, A., Girardi, C., Fukuda, L.E., Rossoni, L.V., Malnic, G. and Reboucas¸ N.A. (2008) Dipeptidyl peptidase IV inhibition down-regulates Na+-H+ exchanger NHE3 in rat renal proximal tubule Am. J. Physiol. Renal Physiol., 294, F414-F422
9. Crajoinas, R.O., Lessa, L.M.A., Carraro-Lacroix, L.R., Davel, A.P.C., Pacheco, B.P.M., Rossoni, L.V., Malnic, G. and Girardi, A.C.C. (2010) Post-translational mechanisms associated with reduced NHE3 activity in adult vs. young prehypertensive SHR Am. J. Physiol. Renal Physiol., 299, F872–F881
10. Segawa, H., Yamanaka, S., Mikiko, I., Kuwahata, M., Shono, M., Yamamoto, T. and Miyamoto, K-i. (2005) Internalization of renal type Iic Na-Pi cotransporter in response to a high-phosphate diet Am. J. Renal Physiol., 288, F587-F596
11. Yeaman, C. (2003) Ultracentrifugation-based approaches to study regulation of Sec6/8 (exocyst) complex function during development of epithelial cell polarity Methods, 30, 198-206
12. Vogelmann, R. and Nelson, W.J. (2007) Separation of cell-cell adhesion complexes by differential centrifugation Meth. Mol. Biol., 370, 11-22
13. Fukasawa, H., Bornheimer, S., Kudlicka, K. and Farquhar, M.G. (2009) Slit diaphragms contain tight junction proteins J. Am. Soc. Nephrol., 20, 1491–1503
14. Ford, T., Graham, J. and Rickwood, D. (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of self generated gradients Anal. Biochem., 220, 360-366 (1994)
OptiPrepTM Application Sheet S30; 7th edition, January 2020
OptiPrep™ Application Sheet S31
Analysis of plasma membrane domains and apical junctional complex from polarized epithelial cells in a self-generated gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5)
1. Background
The resolution of plasma membrane domains, primarily the basolateral and apical domains from polarized tissues such as intestine, liver and kidney and also from polarized cells such as human colon adenocarcinoma (Caco-2) cells and Madin-Darby canine kidney (MDCK) cells, is an important preliminary requirement for studies on how functional dichotomy at the cell surface is achieved. Methods often involve the use of divalent cations. Brush border preparations, from for example intestinal mucosa, treated with 10 mM MgSO4, allow residual basolateral membrane and intracellular membranes to be removed by low-speed centrifugation [1]. The basolateral membranes are then prepared in a separate density gradient protocol. Ellis et al [2] used a modification of this procedure; basolateral and apical membranes from Caco-2 cells were separated in a sucrose gradient and 10 mM MgCl₂ was used to remove contaminating intracellular membranes from the basolateral domain band. Musch et al [3] used the established divalent cation procedure to resolve the apical and basolateral domains of Caco-2 cells, but then used an iodixanol gradient to improve the subsequent purification of the basolateral domain: see Application Sheet S29. However ileal brush border and basolateral membranes from a total microsomal fraction can be well resolved in a single iodixanol gradient: see Application Sheet S28. Clearly there are important analytical advantages to the ability to separate the two domains in the same gradient. Moreover, the high resolving power of iodixanol gradients can achieve a separation of other plasma membrane microdomains that have hitherto been difficult to study with sucrose gradients. Biemesderfer et al [4] reported that shallow continuous iodixanol gradients are capable of resolving the microvillar and intermicrovillar domains from the renal brush border: see Application Sheet S30. This Application Sheet describes the use of a self-generated gradient to analyze proteins in the apical and basolateral domains and the apical junctional complex from MDCK cells. Such gradients can be prepared simply by adjusting the sample (e.g. a post-nuclear supernatant) to a uniform concentration of iodixanol; transferring the suspension to a tube for a vertical or near-vertical rotor and centrifuging for 1-3 h at approx 350,000g. These gradients are highly reproducible, very simple to prepare and the absence of any interfaces contributes to a minimization of particle aggregation. However if it is necessary to establish whether a protein might have a cytosolic or membrane location, the standard approach is to load the PNS in a dense solution beneath a discontinuous gradient so that the membranes float to their banding position while all of the soluble proteins and protein complexes (their density in iodixanol is >1.26 g/ml) remain in the load zone or sediment. For this methodology see Application Sheet S35. This strategy has now been combined with that of a selfgenerated gradient. The post-nuclear supernatant is adjusted to 30% (w/v) iodixanol and overlaid with equal volumes of 20% and 10% iodixanol and centrifuged in a vertical or near-vertical rotor [5-7]. Some variations on this format are described in Section 5. This discontinuous gradient format has one other interesting property; the density profile that is generated by a combination of diffusion and selfgeneration is more closely linear in the top 90% of the gradient volume than with the single concentration format. For information on the latter see Application Sheet S04.
2. Solutions required (see Section 5.1)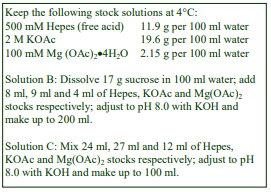
A. OptiPrep™
B. Homogenization medium (HM): 0.25 M sucrose, 90 mM KOAc, 2 mM Mg(OAc)₂, 20 mM HepesKOH, pH 8.0
C. Diluent: 540 mM KOAc, 12 mM Mg(OAc)₂, 120 mM Hepes-KOH, pH 8.0
D. Working Solution (50% iodixanol): mix 5 vol. of Solution A with 1 vol. of Solution C.
3. Ultracentrifuge rotor requirements (see Section 5.2)
Vertical rotor with 11-13 ml tubes (e.g. Beckman VTi65.1 or Sorvall 65V13, or near-vertical rotor (e.g. Beckman NVT65)
4. Protocol (adapted from refs 5-7)
Carry out all operations at 0-°C. All iodixanol concentrations are given as % (w/v).
1. Wash the cell monolayer twice with Solution B and scrape the cells into the same solution.
2. Pellet the cells at 600 g for 10 min and resuspend them in 1-3 ml of Solution B.
3. Homogenize the cells in a cell cracker (ball-bearing homogenizer) using 4-10 passages or pass the suspension through a 22G needle followed by a 26G needle (six times through each). Monitor the efficacy of the homogenization by phase contrast microscopy (see Section 5.3).
4. Centrifuge the homogenate at 3000 g for 10 min to pellet the denser mitochondria, nuclei and cell debris (see Section 5.4.1).
5. Aspirate the 3000 g supernatant and adjust it to 30% (w/v) iodixanol by mixing with Solution D, volume ratio of 2:3 (see Section 5.4.2).
6. Make up solutions of 10%, 20% (w/v) iodixanol by mixing Solution D with Solution B, volume ratios of 1:4 and 2:3 respectively (see Section 5.4.2).
7. Transfer 3.75 ml of the three iodixanol solutions to an Optiseal tube (11.2 ml) for the vertical or near-vertical rotor by under- or over-layering (see Section 5.5).
8. Centrifuge at 353,000 gav for 3 h using a slow acceleration program.
9. Allow the centrifuge to decelerate to rest from 2000 rpm without the brake or use a slow deceleration program.
10. Collect the gradient in 0.5 ml fractions by tube puncture, upward displacement or aspiration from the meniscus. For more information on harvesting gradients from sealed tubes see Application Sheet S08.
- For information on analysis of the gradient fractions see Section 5.6
5. Technical Notes and Review
5.1 Homogenization media
The homogenization medium (HM) often has to be tailored to the tissue or cell type and it is not known if the composition of the HM is relevant to the separation. Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility in functional studies on subcellular membranes; moreover these low ionic strength HMs and gradient solutions permit the direct use of fractions for SDS-PAGE. Supplementation of the HM with inorganic salts (containing K+ or Na+ ions) is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation. The 0.25 M sucrose, 90 mM KOAc, 2 mM Mg(OAc)2, 20 mM Hepes-KOH, pH 8.0 described in this protocol was also used by Yeaman et al [8] for NRK-49F and NRK-52E rat kidney cells. Solutions are buffered with Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and it is unlikely if the type of buffer significantly influences the fractionation, although triethanolamine does seem to offer some advantages in homogenization efficiency. Other examples of homogenization media are given in Application Sheet S06. The preparation of a Working Solution as described, ensures that the concentrations of KOAc, Mg(OAc)₂ and buffer are constant throughout the gradient, while the sucrose and iodixanol act as osmotic balancers to maintain an approx. constant osmolality. If this is deemed unimportant the iodixanol solutions may be prepared simply by diluting OptiPrep™ with Solution B. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01. In some cases the surface proteins are cross linked by dithiobis(succinimidylproprionate) prior to homogenization [5-7]. Protease inhibitors may be included in Solutions B and C at the operator’s discretion.
5.2 Ultracentrifuge rotors
Other rotors with different sedimentation path lengths may be suitable but the optimal centrifugation conditions will require investigation; only vertical, near-vertical or low-angle small volume fixed-angle rotors can normally be used for self-generated gradients. For more information see Application Sheet S04.
5.3 Homogenization
Dounce (or sometimes Potter-Elvehjem) homogenization was the most widely used procedure at one time but the ball-bearing homogenizer or “cell cracker”, with the standard 0.3747 in (9.52 mm) ball bearing, is now regarded as one of the most effective and reproducible of devices. If this is not available however 10-20 passages through a syringe needle (the Gauge Number (G) varies from 21 to 26) is usually an efficient alternative. Sometimes, as in this protocol, the efficacy of this method is improved by switching to a second finer syringe needle for half the passes. Occasionally use of a syringe needle is prefaced by Dounce homogenization. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some other hints on homogenization are given in Application Sheet S06.
5.4 Differential centrifugation
5.4.1 Removal of nuclei
Nuclear pelleting may be carried out at 500-3000g for 5-10 min; the higher RCFs (g-forces) resulting in removal of some of the mitochondria. To recover any vesicles trapped in the pellet (more serious at the higher RCFs), the pellet is sometimes resuspended in HM, recentrifuged and the two supernatants combined. A possible disadvantage of this practice is that unless the resuspension of the pellet is carried out very gently, the nuclei may be damaged, with consequent leakage of DNA, which may lead to almost irreversible aggregation of the subcellular membranes.
5.4.2 Preparation of sample for gradient loading
If the size of the 3000g supernatant is inconveniently large, then the microsomes may need pelleting at 100,000g for 45 min and resuspension in a total volume of 1.5 ml of Solution B before adjusting to 30% (w/v) iodixanol. Note that most of the cytosolic proteins will be removed in the 100,000g supernatant. Yeaman [6] also suggested an alternative gradient made up from 10%, 15%, 20%, 25% and 30% (w/v) iodixanol, in which case the 3000g supernatant or the resuspended microsomes need to be adjusted to 35% (w/v) iodixanol. This format was better at resolving very slowly sedimenting low MWt proteins from the floating denser membranes. The 10% and 20% iodixanol layers may also be prepared by mixing the 3000g supernatant with Solution D; this permits a greater sample loading per gradient but resolution of the membrane compartments from the smaller soluble proteins is probably less clear [6,8].
5.5 Setting up the gradient
Although underlayering with a syringe and metal cannula is the recommended method for making discontinuous gradients, overlayering maybe more convenient since the tubes need to be filled exactly to the bottom of the neck. For more information on gradient construction see Application Sheet S03. If necessary, adjust all volumes proportionately so that tubes are properly filled according to the manufacturer’s instructions.
5.6 Analysis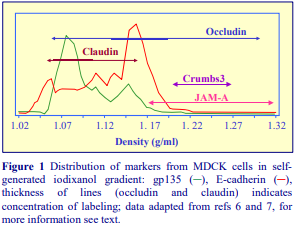
Figure 1 describes the resolution of the apical and basolateral domains of MDCK cells. Using E-cadherin as a basolateral marker and gp135 as an apical marker the gradient was clearly very effective at resolving these domains [6]. The major bands of E-cadherin and gp135 from MDCK cells were separated by as much as 0.1 g/ml. Na+/K+ ATPase co-banded with the Ecadherin [7]. Note however that the major Ecadherin band from the epithelial-like NRK-52E cells was found at a much lower density than that from MDCK cells [9] emphasizing the difference in plasma membrane density that may be found when these gradients are used to fractionate the membrane compartments from different cell types. Each cell type may have its own distinctive membrane banding patterns. Vogelmann and Nelson [7] found that occludin was widely distributed through the gradient but it was most heavily concentrated in the basolateral domain region of the gradient (Figure 1), while claudin was predominantly in the apical domain region. JAM-A and crumbs3 were confined to denser regions of the gradient. If however the densest region of the gradient is harvested; diluted to 10% (w/v) iodixanol; made part of a second 10%, 20%, 30% iodixanol gradient and re-centrifuged under the same conditions, the JAM-A now bands in the middle of the gradient and can be completely resolved from the much more densely-banding crumbs3 [7]. This may indicate that both JAM-A and crumbs3 reside in particles that are denser than those of the basolateral or apical domains, but the JAM-A containing particles are much smaller. This gradient system displays much finer resolving powers than is suggested by Figure 1. For example although the figure suggests occludin was widely distributed but mainly confined to the basolateral domain, its distribution actually revealed three distinct peaks [7]. Vogelmann and Nelson [7] used the gradient, in association with immunofluorescence microscopy, to reassess the distribution of a variety of proteins at the surface of MDCK cells and to study the temporal role of the epithelial apical junctional complex in development of surface polarity. Yeaman [6] compared the translocation and localization of the Sec6/8 complex in contact-naïve and polarized MDCK cells, while Amieva et al [5] used the gradient system to separate fractions containing intercellular junctions, apical and basolateral domains and cytosol from MDCK cells, in their study of the disruption of the epithelial cell apical-junctional complex by the CagA protein from Helicobacter pylori. In the study by Gromley et al [10] of centriolin anchoring of exocyst and SNARE complexes in MDCK cells, centriolin was mostly associated with membranes and furthermore co-fractionated with a fraction of Sec8 that was slightly less dense than the junction associated peak. A clear separation of basolateral and apical domains from Golgi in these gradients showed the importance of the coupling of microtubules to post-Golgi trafficking in polarized cells [11]. Wang et al [12] increased the centrifugation time to 4h in their studies on E-cadherin trafficking. Using the alternative sample loading format in which the 3000 g supernatant is mixed into all three of the iodixanol layers, Yeaman et al [8] studied the redistribution of occludin and Sec8 during the induction of Ca₂+ dependent cell adhesion of MDCK cells. Self-generated iodixanol gradients have also been used to study the association of Par complex proteins with the tight junction of MDCK cells [13] and in the identification of occludin-containing vesicles from the medium surrounding the basolateral membrane [14]
- A methodological review by Vogelmann and Nelson [15] provides an excellent description of the use of these iodixanol gradients in combination with a variety of other techniques in the study of membrane trafficking and exocytosis.
5.7 Matrix adherence
Gerl et al [16] used a novel method for capturing the free-floating apical membranes from MDCK cells grown on permeable supports. They were overlaid with semidry filter paper (Whatman 3-MM) for 10 min and then rehydrated for 30 min in 150 mM ammonium bicarbonate and the surface scraped with a cell scraper. Supernatants, which contained membranes and filter paper remnants were underlaid with pure OptiPrep™ and centrifuged for 3 h at 160,000 g, in 13 ml tubes (e.g. Beckman SW 41Ti). The bulk of the supernatant was removed, leaving 1 ml and the membranes , which banded at the interface were harvested in the remaining 1 ml plus 2 ml of the OptiPrep (final iodixanol concentration of 40% (w/v) iodixanol) and overlaid with 30% iodixanol in ammonium bicarbonate and then with ammonium bicarbonate. After centrifugation for 90 min at 280,000 g, the membranes were harvested from the 30% iodixanol/ammonium bicarbonate interface.
5.8 Other plasma membrane domains
Bandyopadhyay et al [17] were able to resolve, from HepG2 cells homogenized in a Brijcontaining solution, light and dense plasma membrane domains in a 2.5%, 10%, 20%, 30% iodixanol gradient (125,000 g for 12h). The light domain was enriched in Thy-1 (a GPI-anchored protein), while Lyn and glyceraldehyde phosphate dehydrogenase were in the denser domain.
6. References
1. Cohen, M.E., Wesolek, J., McCullen, J., Rys-Sikora, K., Pandol, S., Rood, R.P., Sharp, G.W.G. and Donowitz, M. (1991) Carbachol- and elevated Ca2+-induced translocation of functionally active protein kinase C to brush borders of rabbit ileal Na+ absorbing cells J. Clin. Invest., 88, 855-863
2. Ellis, J.A., Jackman, M.R. and Luzio, J.P. (1992) The post-synthetic sorting of endogenous membrane proteins examined by the simultaneous purification of apical and basolateral plasma membrane fractions from Caco-2 cells Biochem. J., 283, 553-560
3. Musch, M.W., Walsh-Reitz, M.M. and Chang, E.B. (2006) Roles of ZO-1, occludin, and actin in oxidantinduced barrier disruption Am. J. Physiol. Gastrointest. Liver Physiol., 290, 222-231
4. Biemesderfer, D., DeGray, B. and Aronson, P. S. (2001) Active (9.6S) and inactive (21S) oligomers of NHE3 in distinct microdomains of the renal brush border J. Biol. Chem., 276, 10161-10167
5. Amieva, M. R., Vogelman, R., Covacci, A., Tompkins, L. S., Nelson, W. J. and Falkow, S. (2003) Disruption of the epithelial apical-junction complex by Helicobacter pylori CagA Science, 300, 1430-1434
6. Yeaman, C. (2003) Ultracentrifugation-based approaches to study regulation of Sec6/8 (exocyst) complex function during development of epithelial cell polarity Methods, 30, 198-206
7. Vogelmann, R. and Nelson, W.J. (2005) Fractionation of the epithelial apical junctional complex:
reassessment of protein distributions in different substructures Mol. Biol. Cell, 16, 701-716
8. Yeaman, C., Grindstaff, K. K. and Nelson, W. J. (2003) Mechanism of recruiting Sec6/8 (exocyst) complex to the apical juntional complex during polarization of epithelial cells J. Cell Sci., 117, 559-570
9. Yeaman, C., Grindstaff, K. K., Wright, J. R. and Nelson, W. J. (2001) Ultracentrifugation-based approaches to study regulation of Sec6/8 (exocyst) complex function during the development of epithelial cell polarity J. Cell Biol., 155, 593-604
10. Gromley, A., Yeaman, C., Rosa, J., Redick, S., Chen, C-T., Mirabelle, S., Guha, M., Sillibourne, J. and Doxsey, S.J. (2005) Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission Cell, 123, 75-87
11. Spiliotis, E.T., Hunt, S.J., Hu, Q., Kinoshita, M. and Nelson, W.J. (2008) Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules J. Cell. Biol., 180, 295-303
12. Wang, Q., Chen, X-W. and Margolis, B. (2007) PALS1 regulates E-cadherin trafficking in mammalian epithelial cells Mol. Biol. Cell, 18, 874-885
13. Cline, E.G. and Nelson, W.J. (2007) Characterization of mammalian Par 6 as a dual-location protein Mol. Cell. Biol., 27, 4431-4443
14. Casas, E., Barron, C., Francis, S.A., McCormack, J.M., McCarthy, K.M., Schneeberger, E.E. and Lynch, R.D. (2010) Cholesterol efflux stimulates metalloproteinase-mediated cleavage of occludin and release of extracellular membrane particles containing its C-terminal fragments Exp. Cell Res., 316, 353-365
15. Vogelmann, R. and Nelson, W.J. (2007) Separation of cell-cell adhesion complexes by differential centrifugation Meth. Mol. Biol., 370, 11-22
16. Gerl, M.J., Sampaio, J.L., Urban, S., Kalvodova, L., Verbavatz, J-M., Binnington, B., Lindemann, D., Lingwood, C.A., Shevchenko, A., Schroeder, C. and Simons, K. (2012) Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane J. Cell Biol., 196, 213-221
17. Bandyopadhyay, D., Sanchez, J.L., Guerrero, A.M., Chang, F-M., Granados, J.C., Short, J.D. and Banik, B.K. (2015) Design, synthesis and biological evaluation of novel pyrenyl derivatives as anticancer agents Eur. J. Medicinal Chem., 89, 851-862
OptiPrepTM Application Sheet S31; 8th edition, January 2020
OptiPrep™ Application Sheet S32
Purification of lipid rafts from cells and tissues (detergent method)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List RS06 “Analysis of lipid rich detergent-resistant domains” provides a protocol review and list of all published papers reporting the use of OptiPrep: to access return to the initial list of Folders and select “Reference Lists”. The references are divided into cell or tissue type and also highlight the analytical content.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes and information regarding methodological variations are contained in the “Technical Notes and Review” section (Section 5)
1. Background
The importance of lipid-rich microdomains of the plasma membrane in signal-transduction events, in lipid transport, in various internalization processes and in the regulation of plasma membranecytoskeleton interactions have become well established. A number of important cholesterol and sphingolipid-rich structures have been identified and studied, notably caveolae and lipid rafts. The isolation of caveolae using OptiPrep™ is the subject of Application Sheet S34. A widely used method for the isolation of lipid rafts is based on the insolubility of these structures in a non-ionic detergent (usually TritonX-100). Either the intact cells are treated with a detergentcontaining solution or a post-nuclear supernatant is prepared from a cell homogenate and then detergent is added to this supernatant. The former approach was adopted by Oliferenko et al [1] for EpH4 cells; the latter by Lafont et al [2] for MDCK cells. The detergent-treated material is then adjusted to a high density and layered under a discontinuous iodixanol gradient (also usually containing the detergent). The lipid rafts, which have a relatively low density, float away from soluble proteins and detergent-soluble cytoskeleton-associated proteins, which remain in the load zone. An alternative detergent-free method is provided in Application Sheet S33. This protocol is based on refs 1 and 2. Lafont et al [2] adjusted the post-nuclear fraction to either 35% iodixanol (overlayered by 30% and 0%) or 40% iodixanol (overlayered by 30%, 20% and 5%). Oliferenko et al [1] used more steps 35%, 30%, 25%, 20% and 0% (with a total cell extract in 40%) iodixanol. In all cases however the lipid rafts band close to the top of the gradient. In a more recent modification to the gradient format Lindwasser and Resh [3] adjusted the sample to 50% iodixanol and overlayered with 40%, 30%, 20% and 10% iodixanol. By harvesting the modified gradient in smaller volume fractions the authors were able to identify subfractions of these lipid-rich domains, which displayed a heterogeneous cholesterol, GM1 glycolipid and caveolin-1 content. The gradient might therefore be usefully modified in the low-density region to be able to discriminate different low-density sub-domains.
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Isolation medium: 150 mM NaCl, 5 mM dithiothreitol (DTT), 5 mM EDTA, 25 mM TrisHCl, pH 7.4 supplemented with a cocktail of protease inhibitors
C. Triton X-100
D. Solution B + 1% (w/v) Triton X100
E. Phosphate-buffered saline (PBS)
3. Ultracentrifuge rotor requirements (see Section 5.2)
Any small volume (approx 4 ml) swinging bucket rotor for an ultracentrifuge (e.g. Beckman SW60Ti or Sorvall TH660)
4. Protocol
Carry out all operations at 0-4°C
4a. Isolation from a total cell lysate
1. Wash the cell monolayer twice with PBS and scrape into this medium.
2. Pellet the cells and resuspend in 0.2 ml of Solution D; then leave on ice for 30 min (see Section 5.3).
4b. Isolation from a post-nuclear supernatant
1. Homogenize the cells in Solution B (see Section 5.3) and centrifuge the homogenate at 1000 g for 10 min.
2. Adjust the supernatant to 1% Triton X-100 and leave on ice for 30 min.
4c. Gradient separation
1. Add 2 vol. of OptiPrep™ to 1 vol. of either the homogenate or 1000 g supernatant.
2. Dilute OptiPrep™ with Solution D to give 35%, 30%, 25% and 20% (w/v) iodixanol (see Sections 5.4 and 5.5)
3. In tubes for the swinging-bucket rotor layer 0.6 ml each of the sample, the four gradient solutions and Solution D to fill the tube.
4. Centrifuge at 160,000 gav for 4 h (see Section 5.5).
5. Collect the lipid rafts from the top interface (see Figure 1) or harvest the gradient in a number of equal volume fractions and analyze as required (see Section 5.6).
5. Technical Notes and Review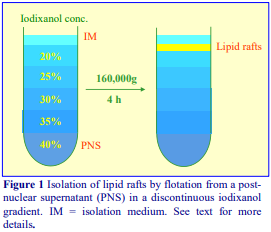
5.1 Homogenization media and gradient solutions
If the cells are homogenized in the absence of detergent (Protocol 4B), then the homogenization medium may be tailored to the tissue or cell type. A medium containing NaCl, EDTA and a buffer is perhaps the most popular for the isolation of rafts, but there are variations. For example the level of DTT used by Oliferenko et al [1] was 1 mM rather than 5 mM and EDTA was omitted. Some proteins, which associate with lipid rafts, exhibit a Ca₂+
– dependence, so inclusion of a chelating agent may be detrimental to the study. DTT may be omitted and the concentration of Triton X-100 may be as low as 0.1% and as high as 2%. Sometimes the level of detergent in the discontinuous gradient is lower than in the sample layer. CHAPS or Brij may replace Triton X100 as the detergent. Just a few of the variations in isolation media are given in Table 1. Protease inhibitors such as PMSF, leupeptin, antipain, aprotinin etc should be included in Solutions B and D as
5.2 Ultracentrifuge rotors
Many of these separations have been performed in relatively small volume swinging-bucket rotors (4-5 ml) but the gradients and sample volume may be scaled up or down proportionately as required.
5.3 Homogenization
To achieve complete cell lysis in the presence of detergent (Protocol 4a) it may be necessary to supplement this with some mechanical means, such as repeated passage through a syringe needle. If the cells are to be homogenized prior to addition of detergent (Protocol 4b) then use an homogenization protocol tailored to the cell (or tissue) type. Potter-Elevhjem homogenization for tissues and Dounce homogenization for cells used to be the standard procedures. For cells however use of 5-15 passages through a 27- or 25-gauge syringe needle, sometimes preceded by Dounce homogenization, is more common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei since these are to be removed from the homogenate before detergent is added. Some hints on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
- Note that for yeast cells it is common to break them by vortexing with glass beads using the same NaCl, EDTA, Tris buffer
- Note that sometimes, particularly with a tissue, a plasma membrane or other organelle is isolated using standard techniques prior to detergent extraction. For isolation of specific membranes see the Index
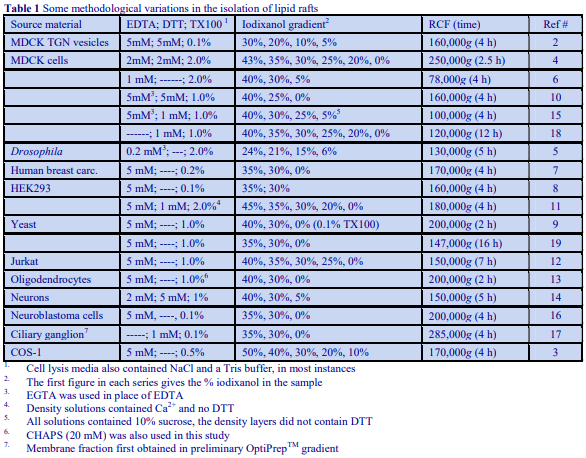
5.4 Forming the discontinuous gradient
Discontinuous gradients are normally most easily prepared by underlayering (i.e. low density first) using a 1 ml syringe and a long metal cannula; overlayering small volumes is more difficult using either a syringe or Pasteur pipette. One alternative for overlayering is to use a small volume (lowpulsating) peristaltic pump; first to take up the required volume of solution into the attached tubing and second, to reverse the flow, in order to expel it slowly on to a denser layer in the centrifuge tube. For more information on gradient construction see Application Sheet S03. If necessary, adjust all volumes proportionately so that tubes are properly filled according to the manufacturer’s instructions.
5.5 Gradient and centrifugation conditions
There are wide variations in the details of the discontinuous gradient; sometimes it is a relatively simple three-layer gradient (including the dense sample), sometimes there may be up to 6 layers. The latter are more likely to provided resolution of different detergent-resistant domains. Some commonly used formats are summarized in Table 1. As far as is known the optimal centrifugation time and RCF have not been thoroughly investigated. Oliferenko et al [1] used a longer centrifugation time of 12 h at a slightly lower RCF (120,000gav). Because of the relatively short sediment path length of the rotor, 4 h at the higher RCF is probably satisfactory, but the centrifugation conditions may vary with the mode of preparation and there is evidence from other work that for optimal resolution long centrifugation times (>12 h) at relatively low g-forces (<100,000g) are recommended. Some commonly used regimes are summarized in Table 1.
- Adapting a protocol developed by Yeaman et al [20], Lynch et al [21] set up a discontinuous gradient of 10%, 20% and 30% (w/v) iodixanol (each layer containing the sample) in tubes for Beckman VTi90 vertical rotor and centrifuged it at 350,000 g for 3 h. During that time a linear gradient will form by a self generation and diffusion. It provided a means of distinguishing multiple membrane domains.
5.6 Gradient analysis
Depending on the resolution that is required it may be sufficient to use an automatic pipette to collect the gradient in four or five broad zones. Alternatively for higher resolution the gradient should be unloaded either by tube puncture, upward displacement or automatic aspiration from the meniscus. For more information see Application Sheet S08. Always check on the distribution of raft and non-raft markers in the gradient to confirm that the centrifugation has achieved a satisfactory resolution and recovery of rafts.
6. References
1. Oliferenko, S., Paiha, K., Harder, T., Gerke, V., Schwarzler, C., Schwarz, H., Beug, H., Gnthert, U. and Huber, L.A. (1999) Analysis of CD44-containing lipid rafts: Recruitment of Annexin II and stabilization by the actin cytoskeleton J. Cell Biol., 146, 843-854
2. Lafont, F., Verkade, P., Galli, T., Wimmer, C., Louvard, D. and Simons, K. (1999) Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells Proc. Natl. Acad. Sci., USA, 96, 3734-3738
3. Lindwasser, O.W. and Resh, M.D. (2001) Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains J. Virol., 75, 7913-7924
4. Lafont, F., Lecat, S., Verkade, P. and Simons, K. (1998) Annexin XIIIb associates with lipid microdomains to function in apical delivery J. Cell Biol., 142, 1413-1427
5. Rietveld, A., Neutz, S., Simons, K. and Eaton, S. (1999) Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophilia raft lipid microdomains J. Biol. Chem., 274, 12049-12054
6. Benting, J.H., Rietveld, A.G. and Simons, K. (1999) N-glycans mediate the apical sorting of a GPI anchored, raft-associated protein in Madin-Darby canine kidney cells J. Cell Biol., 146, 313-320
7. Manes, S., Mira, E., Gomez-Mouton, C., Lacalle, R.A., Keller, P., Labrador, J.P. and Martinez-A, C. (1999) Membrane raft microdomains mediate front-rear polarity in migrating cells EMBO J., 18, 6211-6220
8. Bruckner, K., Labrador, J.P., Scheiffele, P., Herb, A., Seeburg, P.H. and Klein, R. (1999) EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains Neuron, 22, 511-524
9. Bagnat, M., Keranen, S., Shevchenko, A., Shevchenko, A. and Simons, K. (2000) Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast Proc. Nat. Acad. Sci. USA, 97, 3254-3259
10. Plant, P.J., Lafont, F., Lecat, S., Verkade, P., Simons, K. and Rotin, D. (2000) Apical membrane targeting of Nedd4 is mediated by an association of its C2 domain with annexin XIIIb J. Cell Biol., 149, 1473-1483
11. Gimpl, G. and Fahrenholz, F. (2000) Human oxytocin receptors in cholesterol-rich vs. cholesterol-poor microdomains of the plasma membrane Eur. J. Biochem., 267, 2483-2497
12. Harder, T. and Kuhn, M. (2000) Selective accumulation of raft-associated membrane protein LAT in T cell receptor signaling assemblies J. Cell Biol., 151, 199-207
13. Simons, M., Kramer, E-M., Thiele, C., Stoffel, W. and Trotter, J. (2000) Assembly of myelin by association of proteolipid protein with cholesterol and galactosylceramide-rich membrane domains J. Cell Biol., 151, 143-153
14. Ledesma, M.D., Da Silva, J.S., Crassaerts, K., Delacourte, A., De Stropper, B. and Dotti, C.G. (2000) Brain plasmin enhances APP -cleavage and A degradation and is reduced in Alzheimer’s disease brains EMBO Rep., 1, 530-535
15. Lecat, S., Verkade, P., Thiele, C., Fiedler, K., Simons, K. and Lafont, F. (2000) Different properties of two isoforms of annexin XIII in MDCK cells J. Cell Sci., 113, 2607-2618
16. Tansey, M.G., Baloh, R.H., Milbrandt, J. and Johnson, E.M. (2000) GFR –mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival Neuron, 25, 611-623
17. Bruses, J.L., Chauvet, N. and Rutishauser, U. (2001) Membrane lipid rafts are necessary for the maintenance of the 7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons J. Neurosci., 21, 504-512
18. Hanwell, D., Ishikawa, T., Saleki, R. and Rotin, D. (2002) Trafficking and cell surface stability of the
epithelial Na+ channel expressed in epithelial Madin-Darby canine kidney cells J. Biol. Chem., 277, 9772-9779
19. Lee, M.C., Hamamoto, S. and Schekman, R. (2002) Ceramide biosynthesis is required for the formation of the oligomeric H+ -ATPase Pma1p in the yeast endoplasmic reticulum J. Biol. Chem., 277, 22395-22401
20. Yeaman, C., Grindstaff, K. K. and Nelson, W. J. (2003) Mechanism of recruiting Sec6/8 (exocyst) complex to the apical juntional complex during polarization of epithelial cells J. Cell Sci., 117, 559-570
21. Lynch., R.D., Francis, S.A., McCarthy, K.M., Casas, E., Thiele, C. and Schneeberger, E.E. (2007) Cholesterol depletion alters detergent-specific solubility profiles of selected tight junction proteins and the phosphorylation of occluding Exp. Cell Res., 313, 2597-2610
7. Acknowledgements
We wish to thank Dr Lukas Huber, IMP, Research Institute of Molecular Pathology, A-1030, Vienna, Austria and Dr Kai Simons EMBL, Cell Biology and Biophysics Program, 69117 Heidelberg, Germany for their kind cooperation in the preparation of this text.
OptiPrepTM Application Sheet S32; 9th edition, January 2020
OptiPrep™ Application Sheet S33
Purification of lipid rafts from cells and tissues (detergent-free method)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RS07) “Detergent-free strategy for lipid raft isolation from mammalian cells, tissues and organelles” lists all published papers reporting the use of OptiPrep™: to access return to the initial list of Folders and select “Reference Lists”. The references are divided into cell or tissue type and also highlight the analytical content.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes and information regarding methodological variations and analysis are contained in the “Technical Notes” section (Section 5)
1. Background
The importance of lipid-rich microdomains of the plasma membrane in signal-transduction events, in lipid transport, in various internalization processes and in the regulation of plasma membranecytoskeleton interactions have become well established over the last six years. A number of important cholesterol and sphingolipid-rich structures have been identified and studied, notably caveolae and lipid rafts. The isolation of caveolae using OptiPrep is the subject of Application Sheet S34. Isolation of the two types of lipid-rich particle developed along quite distinct lines. Caveolae were purified from a plasma membrane fraction after sonication by sequential flotation in continuous and discontinuous iodixanol gradients (e.g. refs 1 and 2). Lipid rafts on the other hand were isolated, also by flotation through a discontinuous iodixanol gradient, as detergent resistant membranes (e.g. refs 3 and 4), generally from a post-nuclear supernatant (PNS). Usually the PNS and gradient contained Triton X100 as the detergent, but sometimes other detergents such as CHAPS or Brij were used. Lipid rafts, which like caveolae have a relatively low density, float away from detergent soluble proteins and detergent-insoluble cytoskeleton-associated proteins, which remain in the load zone. The method is described in the companion Application Sheet S32. Both methodologies have been widely applied and both appear in a huge number of publications. The sonication method is rather lengthy and yields are rather poor. The use of detergents may however introduce artifacts, notably the coalescence of smaller lipid domains. In spite of this, the detergentcontaining iodixanol gradients have been able to resolve lipid-rich domains of different densities (e.g. refs 5 and 6). In 2005 Macdonald and Pike [7] introduced a detergent-free flotation method, which incorporated a continuous iodixanol gradient very similar to that used by Smart et al [1], but used a PNS as the source rather than a purified plasma membrane fraction. The following protocol for monolayer-grown cells is adapted from ref 7.
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Isolation medium: 0.25 M sucrose, 1 mM CaCl₂, 1 mM MgCl₂, 20 mM Tris-HCl, pH 7.8
C. OptiPrep™ diluent: 0.25 M sucrose, 120 mM Tris-HCl, pH 7.8
D. Working Solution (50% iodixanol): mix 5 vol. of OptiPrep™ with 1 vol. of Solution C
E. Working Solution diluent: 0.25 M sucrose, 20 mM Tris-HCl, pH 7.8
F. Phosphate-buffered saline (PBS)
3. Ultracentrifuge rotor requirements (see Section 5.2)
Swinging bucket rotor for an ultracentrifuge with a tube volume of approx 13 ml (e.g. Beckman SW41Ti or Sorvall TH641)
4. Protocol
Carry out all operations, except the PBS washes in Step 3, at 0-4°C
1. Prepare a 20% (w/v) solution of iodixanol from 2 vol. of Solution D and 3 vol. of Solution E.
2. In tubes for the swinging-bucket rotor prepare 8-9 ml gradients from equal volumes of the 20% iodixanol and Solution B using a two-chamber gradient maker or Gradient Master™ (see Section 5.3) and keep at 4°C.
3. Wash the cell monolayer once in PBS and twice Solution B; then scrape the cells into Solution B.
4. Pellet the cells at 250 g for 2 min and then resuspend the cell pellet in 1 ml of Solution B.
5. Homogenize the cells by 20 passages through a 22G syringe needle (see Section 5.4)
6. Centrifuge the homogenate at 1000 g for 10 min.
7. Carefully aspirate and retain the supernatant.
8. Resuspend the pellet in 1 ml of Solution B and repeat the homogenization by repeated passage through the 22G syringe needle.
9. Centrifuge the suspension at 1000 g for 10 min.
10. Aspirate the supernatant and mix the combined supernatants with an equal volume of Solution D.
11. Using a syringe and metal cannula underlayer the gradient with the dense sample to fill the tube (see Section 5.3).
12. Centrifuge at 52,000 g for 90 min (see Section 5.5).
13. Collect the gradient in approx 0.5-0.75 ml fractions by tube puncture, upward displacement with a dense medium or aspiration from the meniscus and analyze the fractions as required (see Sections 5.6 and 5.7).
5. Technical Notes
5.1 Homogenization media and gradient solutions
The solutions, as described, were used by Macdonald and Pike [7] and Pike et al [8] for CHO cells. The same strategy was also used for HeLa cells [7]. Detergent-free lipid raft fractions have also been prepared from renal brush border membranes [9] and from neuroblastoma cells [10] homogenized in Tris-buffered 150 mM NaCl, 5 mM EDTA. The relevance of the type of homogenization medium to the efficacy of the technique is not known. The preparation of a Working Solution as described, ensures that the concentration of buffer is constant throughout the gradient, while the sucrose and iodixanol act as osmotic balancers to maintain an approx. constant osmolality. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
Protease inhibitors such as PMSF, leupeptin, antipain, aprotinin etc should be included in all of the media.
5.2 Ultracentrifuge rotors
The method can probably be scaled down to smaller volume swinging-bucket rotors, but this will need experimental verification. Since the g-force and time are both quite modest, the resolution may in part be based on rate of flotation, if this is so then shorter tubes may require shorter times.
5.3 Forming the gradient and loading the sample
If neither of these devices is available then a continuous gradient may be prepared from diffusion of a discontinuous gradient. Equal volumes of Solution B and 5%, 10%, 15% and 20% iodixanol would be suitable. For more information on gradient construction see Application Sheet S03. In the nondetergent preparation of rafts from brush border membranes [9] and neuroblastoma cells [10] the gradients were discontinuous rather than continuous. Formation of discontinuous gradients and underlayering the gradient with the sample are best achieved using a syringe with metal cannula (i.d. approx 0.8 mm). Metal filling cannulas can be obtained from most surgical instrument supply companies. Make sure that the tubes are filled in accordance with the manufacturer’s instructions.
5.4 Homogenization
For some suspension culture cells it may be necessary to use a smaller gauge needle (27G or 25G). The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices. Choice of a suitable device will require experimentation. Brush border membranes were fragmented using a Dounce homogenizer [9]. Some hints on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
5.5 Centrifugation conditions
Centrifugation times and g-forces used by other workers have generally been more severe: 170,000 g for 4 h [9] or 200,000 g for 18 h [10].
5.6 Gradient analysis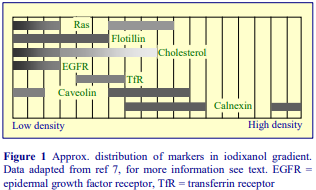
For more information on harvesting gradients see Application Sheet S08. Always check on the distribution of raft and non-raft markers in the gradient to confirm that the centrifugation has achieved a satisfactory resolution and recovery of rafts. Once the pattern of marker banding in the gradient has bee confirmed as being reproducible, then it may be possible to retrieve the raft and other fractions using a syringe and metal cannula or an automatic pipette.
5.7 Technical review
Macdonald and Pike [7] reported that 70% of the EGFR and 40-50% of the ras and flotillin were found in the major raft fraction at the top of the gradient (Figure 1). Caveolin distribution was not dissimilar to that found by Smart et al [1] in the first continuous iodixanol gradient, i.e. it was present in the low-density raft fractions but most was detected in denser fractions that also contained calnexin. Because the gradient is continuous rather than discontinuous as in the detergent-containing strategy (see Application Sheet S32) the marker distribution displays not unexpectedly a considerably greater complexity. For example, flotillin, an established raft marker protein is not confined to the topmost three fractions but extends to higher densities rather like the cholesterol and although they both overlap non raft markers such as the transferrin receptor, they have a distribution that suggests that the gradient may be able to resolve partially a slightly denser raft fraction.
A study of the effect of glycosphingolipids, an α-linolenic acid-containing GM1a (C18:3-GM1a), a stearic acid-containing molecule (C18:0-GM1a) and a lyso-GM1a, on the distribution of neural cell adhesion molecule (NCAM) in neuroblastoma cells [10], compared detergent-containing sucrose gradients and detergent-free iodixanol gradients. Whilst the lyso-GM1a totally removed NCAM from the low-density raft fraction in both gradients, C18:3-GM1 only shifted the NCAM to a high density in the detergent-free iodixanol gradients. Although these observations emphasize the need for more studies in comparisons between the various lipid-rich domain isolation strategies, there are some clear indications that detergent and non-detergent methods can provide similar data. Inoue et al [9] investigated the type IIa sodium/phosphate co-transporter protein (NaPi) in brush border membranes and reported simple detergent extraction of the membranes and detergent-free iodixanol gradients both showed the protein to have a raft location.
6. References
1. Smart E. J., Ying, Y-S., Mineo, C. and Anderson, R. G. W. (1995) A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane Proc. Natl. Acad. Sci., USA, 92, 10104 10108
2. Uittenbogaard, A., Ying, Y. and Smart, E. J. (1998) Characterization of a cytosolic heat-shock proteincaveolin chaperone complex J. Biol. Chem., 273, 6525-6532
3. Oliferenko, S., Paiha, K., Harder, T., Gerke, V., Schwarzler, C., Schwarz, H., Beug, H., Gnthert, U. and Huber, L.A. (1999) Analysis of CD44-containing lipid rafts: Recruitment of Annexin II and stabilization by the actin cytoskeleton J. Cell Biol., 146, 843-854
4. Lafont, F., Verkade, P., Galli, T., Wimmer, C., Louvard, D. and Simons, K. (1999) Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells Proc. Natl. Acad. Sci., USA, 96, 3734-3738
5. Lindwasser, O.W. and Resh, M.D. (2001) Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains J. Virol., 75, 7913-7924
6. Opekarova, M., Malinska, K., Novakova, L. and Tanner, W. (2005) Differential effect of phosphatidylethanolamine depletion on raft proteins. Further evidence for diversity of rafts in Saccharomyces cerevisiae Biochim. Biophys. Acta, 1711, 87-95
7. Macdonald, J.L. and Pike, L.J. (2005) A simplified method for the preparation of detergent-free lipid rafts J. Lipid Res., 46, 1061-1067
8. Pike, L.J., Han, X. and Gross, R.W. (2005) Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids J. Biol. Chem., 280, 26796-26804
9. Inoue, M., Digman, M.A., Cheng, M., Breusegem, S.Y., Halahiel, N., Sorribas, V., Mantulin, W.W., Gratton, E., Barry, N.P. and Levi, M. (2004) Partitioning of NAPi cotransporter in cholesterol-, sphingonyelin-, and glycophingolipid-enriched membrane domains modulates NAPi protein diffusion, clustering, and activity J. Biol. Chem., 279, 49160-49171
10. Nakagawa, T., Morotomi, A., Tani, M., Sueyoshi, N., Komori, H. and Ito, M. (2005) C18:3-GM1a induces apoptosis in Neuro2 cells: enzymatic remodeling of fatty acyl chains of glycosphingolipids J. Lipid Res., 46, 1103-1112
OptiPrepTM Application Sheet S33; 7th edition, February 2020
OptiPrep™ Application Sheet S34
Purification of caveolae from cells and tissues by sonication of a plasma membrane fraction (detergent-free)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List (RS08) “Purification of caveolae in gradients prepared from OptiPrep™” lists all published papers reporting the use of OptiPrep™: to access return to the initial list of Folders and select “Reference Lists”. The references are divided into cell or tissue type and also highlight the analytical content.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes and information regarding methodological variations are contained in the “Technical Notes and Review” section (Section 5)
1. Background
Although the existence of caveolae has been recognized in the electron microscope since the early fifties, it is only relatively recently that their importance in a number of internalization processes has been established in a variety of epithelial and endothelial cells (e.g. see ref 1 for more information). This in turn has led to the development of isolation processes in order to study their composition and to define their functions at the molecular level and now it is widely recognized that lipid-rich domains, such as caveolae and lipid rafts, in the plasma membrane are critical in cell signaling and virus processing.
Early methods to purify these surface membrane domains relied on the relative detergent-resistance of caveolae compared to that of rest of the plasma membrane. Although exposure of membranes to Triton X-100 does lead to the selective solubilization of the bulk of the plasma membrane, loss of some proteins from the caveolae themselves is also liable to occur (see ref 1). Smart et al [1] therefore developed a method that avoids the use of Triton X-100. After isolation of a plasma membrane fraction from either human skin fibroblasts or MA104 cells, the caveolae are released by sonication in a standard cell homogenization medium. The first part of the isolation procedure is a flotation through a continuous iodixanol gradient; this gradient is essentially a resolving gradient in which the caveolin-rich vesicles are concentrated in the top third of the gradient, while the predominantly caveolin-poor vesicles band in denser regions. A second discontinuous gradient is essentially a concentration gradient to band the caveolin-rich vesicles sharply at an interface. Figure 1 summarizes this procedure. The protocol in this Application Sheet has been adapted from refs 1 and 2.
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Diluent: 0.25 M sucrose, 6 mM EDTA, 120 mM Tricine-NaOH, pH 7.6
C. Working solution of 50% iodixanol (ρ = 1.282 g/ml): mix 5 vol. of solution A with 1 vol. of solution B
D. Suspension medium: 0.25 M sucrose, 1 mM EDTA, 20 mM Tricine-NaOH, pH 7.6
3. Ultracentrifuge rotor requirements (see Section 5.2)
Any 13-17 ml swinging bucket (e.g. Beckman SW41 or SW28.1, Sorvall TH641 or AH629)
4. Protocol
Carry out all operations at 0-4°C.
1. Prepare a plasma membrane fraction by a suitable technique (see Section 5.3).
2. Suspend the plasma membrane in 2 ml of solution D in a suitable tube (approx 1.5 cm diameter).
3. Place the tube in ice and introduce the tip of a sonicator probe (approx diameter 3 mm) to a point equidistant from the top and bottom of the suspension (see Section 5.3).
4. Sonicate twice for 6 sec at a total power of 50 J/W per sec, then allow to rest for 2 min before repeating the sonication procedure twice more, i.e. a total of six sonication bursts.
5. Add 1.84 ml of solution C and 0.16 ml of solution D. The final iodixanol concentration is 23% (w/v).
6. Produce two gradient solutions of 10% (w/v) iodixanol (ρ = 1.076 g/ml) and 20% (w/v) iodixanol ( = 1.125 g/ml) by diluting solution C with solution D (1 + 4 and 2 + 3 v/v respectively).
7. Using a standard two chamber gradient former or a Gradient Master produce a 9 ml linear 10-20% iodixanol gradient in an approx 13 ml tube for the swinging-bucket rotor (see Section 5.4).
8. Underlayer the gradient with 4 ml of the sample and centrifuge at 53,000 gmax for 90 min.
9. Collect the top 5 ml of the gradient (see Figure 1);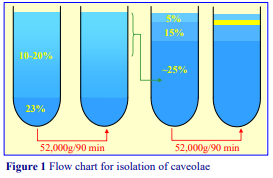 transfer to a new centrifuge tube and mix with 4 ml of solution C (see Section 5.5).
transfer to a new centrifuge tube and mix with 4 ml of solution C (see Section 5.5).
10. Produce two new gradient solutions of 5% and 15% (w/v iodixanol by diluting solution C with solution D (1 + 9 and 3 + 7 v/v respectively)
11. Layer 1.0 ml of 15% and 0.5 ml of 5% iodixanol over the sample and centrifuge at 52,000 g for 90 min (see Section 5.6).
12. Collect the caveolae-rich opaque layer that forms above the 15% iodixanol layer (see Figure 1).
5. Technical Notes and Review
5.1 Homogenization media and gradient solutions
Protease inhibitors (PMSF, leupeptin, antipain, aprotinin etc) may be included in Solutions B and D at the operator’s discretion. Strategies for preparing gradient solutions for mammalian tissues are given in Application Sheet S01.
5.2 Ultracentrifuge rotors
Most of the published papers quote the use of swinging-bucket rotor of the tube volume specified, but there are examples in which the method has been scaled up larger volume rotors (such as the Beckman SW28) but rather rarely scaled down to smaller volume rotors.
5.3 Plasma membrane preparation and release of caveolae
It is beyond the scope of this Application Sheet to cover the precise methodology for preparing the plasma membrane from cultured cells. The vast majority of published papers reporting the use of the protocol described in this OptiPrep Application Sheet for the isolation of caveolae have also cited a Percoll® gradient method for initial isolation of the plasma membrane, as described by Smart et al [1].
However, unless the colloidal silica particles of the Percoll contribute to the success of the sonication and the subsequent gradient separation, there is no obvious reason why other methods for the isolation of the plasma membrane should not be used. See “Plasma membrane” in the Index for some examples of OptiPrep™ based methods. There is moreover an interesting alternative approach for the isolation of the plasma membrane that uses the binding of cationic silica to the surface of intact cells, first described by Chaney and Jacobson [3], the silica raises the density of the plasma membrane and thus permits its simple isolation. Kincer et al [4] perfused the cationic silica through the mouse vasculature to isolate the plasma membrane of the endothelial cells lining the femoral arteries. The method may however be used less elaborately for the density perturbation of the plasma membrane from any cultured cells. The methodology is described in Application Sheet S27.
Schnitzer et al [5] also used the colloidal silica method for rat lung endothelial cells. The size and structural disposition in the membrane of the caveolae abrogates their ability to bind the colloidal silica, thus they can be recovered as a detergent-resistant membrane [5]. Subsequently the colloidal silica may be detached by raising the ionic strength of the medium and non-caveolae lipid-rich domains isolated, also as detergent-resistant membranes [5].
Song et al [6] introduced another option to the use of detergent; MDCK cells were homogenized in 500 mM sodium carbonate sequentially in a Dounce homogenizer, a Polytron homogenizer and by sonication before harvesting the caveolin-rich membranes by flotation through a sucrose gradient. In the case of vascular smooth muscle cells Ishizaka et al [7] used the same strategy but omitted the Polytron homogenization; HEK cells were disrupted by the same Dounce homogenization/sonication strategy but the caveolae were isolated by the double iodixanol gradient described in this OptiPrep Application Sheet [8].
Caveolin-rich low-density membranes have also been isolated from hepatoma cells without sonication or detergent [9]. After an initial homogenization in a routine homogenization medium (137 mM NaCl, 50 mM Hepes, pH 7.4, containing vanadate) by 20 passages through a 27G syringe needle, the post-nuclear supernatant was loaded on to a 2%, 10%, 15% and 18% (w/v) iodixanol gradient and centrifuged at 235,000g for 90 min. The caveolin-rich domain banded at the 2%/10% iodixanol interface. Yanase and Madaio [10] used a similar strategy, also for hepatoma cells, but omitted the 2% iodixanol from the gradient.
5.4 Iodixanol gradient preparation
A gradient volume needs to be used so that the tube is properly filled (according to the manufacturers instructions) when the 4 ml sample is underlayered. An approx 9 ml gradient was used by Smart et al [1]. The method can be scaled up or down as required, adjusting the volumes of sample and gradient proportionately. If the volume of the gradient is increased, the volume occupied by the caveolae-containing fractions may also increase. As an approximation the top third-to-half of the gradient should be removed. If the relative volumes of gradient and sample are significantly different it may be advisable to check the distribution of the caveolae by assaying fractions for caveolin by electroblotting. For more information on gradient preparation see Application Sheet S03.
5.5 Collecting the continuous gradient
It is probably sufficient simply to collect the top 5 ml of the gradient with a syringe attached to a flat-tipped metal cannula (i.d. approx 0.8 mm) or an automatic pipette, whose tip has been cut to enlarge the orifice. Either way the gradient must be withdrawn very slowly to avoid aspiration denser regions. The gradient may alternatively be collected by tube puncture, upward displacement with a dense medium or aspiration from the meniscus for a more complete analysis. More information about harvesting material from gradients may be found in Application Sheet S08.
5.6 The discontinuous gradient
The volumes used in the second gradient may also have to be modified for larger volume tubes; it is probably good practice to increase the volumes of the sample and the two iodixanol layers, proportionally. For more information on gradient preparation see Application Sheet S03. Collect interfacial band with a syringe attached to a flat-tipped metal cannula (i.d. approx 0.8 mm) or an automatic pipette, whose tip has been cut to enlarge the orifice.
6. References
1. Smart E. J., Ying, Y-S., Mineo, C. and Anderson, R. G. W. (1995) A detergent-free method for purifying caveolae membrane from tissue culture cells Proc. Natl. Acad. Sci., USA, 92, 10104-10108
2. Uittenbogaard, A., Ying, Y. and Smart, E. J. (1998) Characterization of a cytosolic heat-shock proteincaveolin chaperone complex J. Biol. Chem., 273, 6525-6532
3. Chaney, L.K. and Jacobson, B.S. (1983) Coating cells with colloidal silica for high yield isolation of plasma membrane sheets and identification of transmembrane proteins J. Biol. Chem., 258, 10062-10072
4. Kincer, J.F., Uittenbogaard, A., Dressman, J., Guerin, T. M., Febbraio, M., Guo, L. and Smart, E.J. (2002) Hypercholesterolemia promotes a CD36-dependent and endothelial nitric oxide synthase mediated vascular dysfunction J. Biol. Chem., 277, 23525-23533
5. Schnitzer, J.E., McIntosh, D.P., Dvorak, A.M., Liu, J. and Oh, P. (1995) Separation of caveolae from associated microdomains of GPI-anchored proteins Science, 269¸1435-1439
6. Song, K.S., Li, S., Okamoto, T., Quilliam, L.A., Sargiacomo, M. and Lisanti, M.P. (1996) Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains J. Biol. Chem., 271, 9690-9697
7. Ishizaka, N,, Griendling, K.K., Lassègue, B. and Alexander, R.W. (1998) Angiotensin II type 1 receptor: relationship with caveolae and caveolin after initial agonist stimulation Hypertension, 32, 459-466
8. Sabourin, T., Bastien, L., Bachvarov, D.R., and Marceau, F. (2002) Agonist-induced translocation of the kinin B1 receptor to caveolae-related rafts Mol. Pharmacol., 61, 546-553
9. Smith, R.M., Harada, S., Smith, J.A., Zhang, S. and Jarett, L. (1998) Insulin-induced protein tyrosine phosphorylation cascade and signalling molecules are localized in a caveolin-enriched cell membrane domain Cell Signal., 10, 355-362
10. Yanase, K. and Madaio, M.P. (2005) Nuclear localization anti-DNA antibodies enter cells via caveoli and modulate expression of caveolin and p53 J. Autoimmun., 24, 145-151
7. Acknowledgements
We wish to thank Dr Eric Smart, Dept of Physiology, University of Kentucky, Lexington, KY and Dr Richard G W Anderson, Dept of Cell Biology and Neuroscience, University of Texas Southwestern Medical school, Dallas, TX for their kind cooperation in the preparation of the text.
OptiPrepTM Application Sheet S34; 9th edition, January 2020
OptiPrep™ Application Sheet S35
Separation of membrane-bound and cytosolic proteins (flotation through a discontinuous gradient)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List RS13 “The resolution of soluble cytosolic proteins from membrane vesicles and organelles: a bibliography” provides a list of all published papers reporting the use of OptiPrep™: to access return to the initial list of Folders and select “Reference Lists”. The references are divided into cell or particle type.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Sections 5.1-5.5 contain some important technical notes for processing mammalian cells
- Section 5.6.1 lists some of the gradient variations used with different mammalian cells; 5.6.2. and 5.6.3 deal with bacteria and yeast cells respectively. For plant cells see Application Sheet S61.
1. Background
There are many situations where it is necessary to provide an efficient separation of membrane vesicles from the cytosol in order to distinguish the location of a protein to one or other compartment. Permeabilization of cultured cells to release cytoplasmic vesicles of the exocytic pathway and the isolation of vesicles budded from the plasma membrane by selective hypoosmotic cell disruption also require subsequent resolution of these vesicles from the cytosol which is also released. A number of protocols have been published which all rely on the same strategy; i.e. flotation of the vesicles through a gradient (usually discontinuous, but sometimes continuous) of either Nycodenz® or iodixanol from a dense load zone. An early example of this flotation strategy was elaborated and widely used for isolation of budded vesicles from yeast. The crude vesicle containing fraction was adjusted to approx 37% (w/v) Nycodenz®; 7 ml of this overlaid with 3 ml each of 35%, 25% and 15% Nycodenz® and centrifuged at 100,000 g for 12 h [1-3]. Later the Nycodenz® flotation strategy was applied more widely to the separation of vesicles and cytosol [4-7]. More recently the method has been adapted to 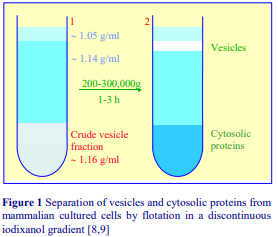 the use of OptiPrep™ and because of ease of use of this medium compared to Nycodenz®, it has become the method of choice and it is the one that is described in this Application Sheet. Another advantage of iodixanol gradients is that because of the lower osmolality of iodixanol solutions, compared to those of Nycodenz®, the difference in density between proteins and vesicles is enhanced. In iodixanol proteins have a density of approx. 1.26 g/ml, while that of membrane vesicles is generally < 1.13 g/ml, thus if the crude vesicle containing fraction is adjusted to 30% (w/v) iodixanol (approx 1.16 g/ml) and overlayered with 25% iodixanol (approx 1.14 g/ml) then the vesicles will float through the lower density layer and the proteins will tend to sediment from the load zone. This is an ideal way of separating the two compartments, which are separated by the median-density layer. A typical fractionation is described diagrammatically in Figure 1. Generally higher g-forces and shorter centrifugation times are used.
the use of OptiPrep™ and because of ease of use of this medium compared to Nycodenz®, it has become the method of choice and it is the one that is described in this Application Sheet. Another advantage of iodixanol gradients is that because of the lower osmolality of iodixanol solutions, compared to those of Nycodenz®, the difference in density between proteins and vesicles is enhanced. In iodixanol proteins have a density of approx. 1.26 g/ml, while that of membrane vesicles is generally < 1.13 g/ml, thus if the crude vesicle containing fraction is adjusted to 30% (w/v) iodixanol (approx 1.16 g/ml) and overlayered with 25% iodixanol (approx 1.14 g/ml) then the vesicles will float through the lower density layer and the proteins will tend to sediment from the load zone. This is an ideal way of separating the two compartments, which are separated by the median-density layer. A typical fractionation is described diagrammatically in Figure 1. Generally higher g-forces and shorter centrifugation times are used.
2. Solutions required 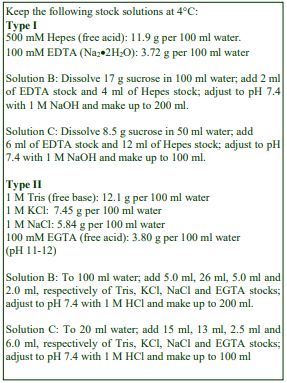
Choose Type I or Type II (see Section 5.1)
2a. Type I
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 1 mM EDTA 10 mM Hepes-NaOH, pH 7.4
C. OptiPrep™ Diluent: 0.25 M sucrose, 6 mM EDTA, 60 mM Hepes-NaOH, pH 7.4
D. Working Solution of 50% (w/v) iodixanol (ρ = 1.272 g/ml): 5 vol. of solution A + 1 vol. of solution C
2b. Type II
A. OptiPrep™
B. Homogenization medium: 130 mM KCl, 25 mM NaCl, 1 mM EGTA, 25 mM Tris-HCl, pH 7.4
C. OptiPrep™ Diluent: 130 mM KCl, 25 mM NaCl, 6 mM EGTA, 150 mM Tris-HCl, pH 7.4
D. Working Solution of 50% (w/v) iodixanol: 5 vol. of solution A + 1 vol. of solution C
3. Ultracentrifuge rotor requirements
Swinging-bucket rotor (e.g. Beckman SW55 or Sorvall TH660; the procedure can be scaled down or up as a required. For smaller volumes use the Beckman TLS55; for larger volumes use the Beckman SW41 or Sorvall TH641.
4. Protocol
Carry out all operations at 0-4°C.
If the gradient is being used to isolate budded vesicles or cytoplasmic vesicles from permeabilized cells then remove the cells from the vesicle-containing suspension either in a microcentrifuge or by centrifugation at 1000 g for 5 min in a low-speed centrifuge and use the supernatant for Step 3.
1. Suspend the washed cells in Solution B and disrupt them by Dounce homogenization, repeated passages through a fine syringe needle or a ball-bearing homogenizer (see Section 5.2).
2. Centrifuge the homogenate at 1000 g for 15 min and aspirate the supernatant (see Section 5.3).
3. Adjust the supernatant to 30% (w/v) iodixanol by thorough mixing with Solution D (volume ratio of 2:3 respectively).
4. Prepare solutions containing 25% and 5% (w/v) iodixanol by diluting Solution D with Solution B (volume ratios of 1:1 and 1:9).
5. In tubes for a swinging-bucket rotor layer 2 ml each of the crude vesicle fraction in 30% iodixanol and the 25% iodixanol and fill the tube by overlayering with 5% iodixanol (see Section 5.4).
6. Centrifuge at approx 250,000 g for 3 h and collect the vesicles which band at the top interface and the bottom layer containing cytosolic proteins (see Section 5.5). Alternatively collect the gradient in 0.5 ml fractions by tube puncture, upward displacement or aspiration from the meniscus. For more information on harvesting gradients see Application Sheet S08.
- Section 5.6 lists some of the cell types and gradient formats that have been used
5. Technical Notes and Technological Review
5.1 Solution strategies for mammalian cells
The solutions that are recommended are general purpose ones for the homogenization of cultured cells and tissues. The homogenization medium often has to be tailored to the tissue or cell type but it is unlikely that the composition of the HM is relevant to the separation described in this OptiPrep™ Application Sheet. Organic osmotic balancers such as sucrose (Type I) were introduced for their compatibility in functional studies on subcellular membranes; moreover these low ionic strength HMs and gradient solutions permit the direct use of fractions for SDS-PAGE. The most commonly used isoosmotic HMs contain 0.25 M sucrose buffered either with Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and often, but not always, containing 1 mM EDTA or EGTA. Supplementation of the HM with inorganic salts is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media (Type II) that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). Often a frankly hypoosmotic medium is needed used to swell the cells and so facilitate homogenization. Some other examples of homogenization media for cultured cells are given in Application Sheet S06.
In cases where the gradient is being used to isolate either budded vesicles or cytoplasmic vesicles released by the permeabilization of cultured cells special solutions may be used. A 140 mM KCl, 10 mM Hepes-KOH, pH 7.2 solution containing either 2 mM EGTA and 1 mM DTT or 2.5 mM Mg(OAC)₂ was used for budded vesicles [1] or vesicles from permeabilised cells [2] respectively. The preparation of a Working Solution (Solution D) using an OptiPrep™ diluent as described, ensures that the concentrations of reagents such as EDTA, EGTA and the buffer are constant throughout the gradient.
The concentrations of the osmotic balancers such as sucrose, KCl or NaCl are not similarly raised in the OptiPrep™ diluent; if they were, the solutions would be grossly hyperosmotic. If the maintenance of constant EDTA, EGTA and buffer concentrations is deemed unimportant, the gradient solutions may be prepared directly from OptiPrep™ . For more information about preparing gradient solutions see Application Sheet S01. Protease inhibitors may be included in Solutions B and C at the operator’s discretion.
5.2 Homogenization
Dounce (or sometimes Potter-Elvehjem) homogenization was the most widely used procedure at one time but the ball-bearing homogenizer or “cell cracker”, with the standard 0.3747 in (9.52 mm) ball bearing, is now regarded as one of the most effective and reproducible of devices. If this is not available however 10-20 passages through a syringe needle (the Gauge Number (G) varies from 21 to 26) is usually an efficient alternative. Sometimes the efficacy of this method is improved by switching to a second finer syringe needle for half the passes. Occasionally use of a syringe needle is prefaced by Dounce homogenization.
Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some other hints on homogenization are given in Application Sheet S06.
5.3 Differential centrifugation
Although centrifugation at 1000g will remove the nuclei, there is no obvious reason why this step should not be carried out at 3000g to remove some of the mitochondria as well.
5.4 Preparing the discontinuous gradient
As with all flotation methods a small volume of buffer or 5-10% iodixanol is always layered on top of the density barrier to prevent banding of the vesicles at an air/liquid interface. The latter often leads to adherence of particles to the wall of the tube and aggregation. Sometimes the layer of 5% iodixanol is replaced by the lysis buffer. For information on preparing gradients see Application Sheet S03.
5.5 Collection of fractions from the gradient
Small volume gradients may be divided into three of four zones simply by very careful aspiration using a syringe and metal cannula (i.d. approx 0.8 mm); an automatic pipette may be used but the end of the pipette tip should be cut off to enlarge the orifice.
5.6 Review of methodology 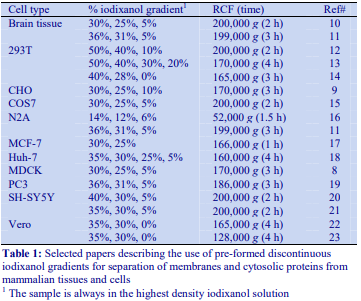
5.6.1 Mammalian cells
Some of the cells and tissues that have been analyzed by this flotation strategy, the gradient format and centrifugation conditions are listed in Table 1. There is no obvious reason why more than three layers are required for a simple separation of the total membrane vesicle fraction from the cytosol, unless it is important to discriminate between vesicles of different density. In an analysis of vesicles budded from NRK cells Joglekar et al (2003) top-loaded a 7%, 50% discontinuous gradient (100,000 g for 40 min), the top layer containing cytosolic proteins was then removed; the remainder mixed and layered under a 5-25% (w/v) continuous iodixanol gradient centrifuged at 100,000 g for 90 min to separate the ER and Golgi [24]. A top-loaded discontinuous gradient of 2% and 50% (w/v) iodixanol (100,000 g for 1 h) was used to establish that IL-32 was almost exclusively membrane-bound [25]
5.6.2 Bacteria
De Leeuw et al [26] devised a basically similar strategy for separating the membranes and soluble protein fractions from E. coli. A membrane pellet in 500 mM KOAc, 5 mM Mg(OAc)₂, 50 mM HepesKOH, pH 7.6 was mixed with 0.25 M sucrose, 50% iodixanol in the same buffer (volume ratio of 0.15:1.05) and overlaid with 5.8 vol. of 125 mM sucrose, 30% iodixanol in the same buffer and 3 vol. of buffer. The gradient was centrifuged at 166,000 g for 3 h. This system has been frequently used [e.g. 26-28].
5.6.3 Yeast
Although in most cases yeast cells are homogenized via spheroplast formation, they are occasionally homogenized with glass beads with a lysis buffer of 150 mM NaCl, 5 mM EDTA, 50 mM Tris-HCl, pH 7.4 [29]. The clarified lysate, adjusted to 40% (w/v) iodixanol, overlaid by 30% iodixanol and the lysis buffer was centrifuged at 55,000 g for 2 h. Medkova et al [30] prepared spheroplast lysates in a 20 mM triethanolamine-acetate buffer, pH 7.2, containing 0.8 M sorbitol and 1 mM EDTA, which were clarified at 10,000g for 10 min. The lysates were adjusted to 40% (w/v) iodixanol and overlaid by 35% iodixanol. The iodixanol solutions contained 0.4 M sorbitol and 1 mM EDTA in the same buffer and the gradients were centrifuged in a small volume fixed-angle rotor (Beckman TLA120.2) at 100,000 g for 3 h. Medkova et al [30] included 50 mM MES, pH 6.5 in the lysate and 20 mM MES in the 35% iodixanol solution to stabilize the membrane-protein association. Although sorbitol is a common component of yeast lysates, it is by no means universally included. Wang et al [31] used the same buffered NaCl/EDTA solution as Ge et al [29]. The lysate was adjusted to 40% (w/v) iodixanol by simple addition of half the volume of Optiprep™ , overlaid with 30% iodixanol and lysis buffer and centrifuged at 200,000 g for 5 h in a small volume Beckman TLS55 rotor. More complex discontinuous iodixanol gradients have also been used [32]; the spheroplast lysate was adjusted to 37% (w/v) iodixanol and layered beneath, 30%, 25%, 19%, 0% iodixanol and centrifuged at 75,000 gav for 4 h to separate cytosolic and vacuolar fractions. A continuous gradient was generated in a Gradient Master™ from equal volumes of lysis medium (400mM Sucrose, 100mM NaCl, 20mM HEPES pH7.2) and a lysate (adjusted to 27% w/v iodixanol) was centrifuged for 16 h in a Beckman SW41 rotor at 190,000 g. It resolved low density lysed vacuole membranes from denser membranes and soluble cytosolic proteins at the bottom of the gradient [33].
6. References
1. Yeung, T., Barlowe, C. and Schekman, R. (1995) Uncoupled packaging of targeting and cargo molecules during transport vesicle budding from the endoplasmic reticulum J. Biol. Chem., 270, 30567-30570
2. Belden, W.J. and Barlowe, C. (1996) Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport J. Biol. Chem., 271, 26939-26946
3. Lupashin, V.V., Hamamoto, S. and Schekman, R.W. (1996) Biochemical requirements for the targeting and fusion of ER-derived transport vesicles with purified yeast Golgi membranes J. Cell Biol., 132, 277-289
4. Miller, D.J. and Ahlquist, P. (2002) Flock house virus RNA polymerase is a transmembrane protein with amino-terminal sequences sufficient for mitochondrial localization and membrane insertion J. Virol., 76, 9856-9867
5. Penin, F., Brass, V., Appel, N., Ramboarina, S., Montserret, R., Ficheux, D., Blum, H.E., Bartenschlager, R. and Moradpour, D. (2004) Structure and function of the membrane anchor domain of hepatitis C virus nonstructural protein 5A J. Biol. Chem., 279, 40835-40843
6. Guo, Y.X., Chan, S-W. and Kwang, J. (2004) Membrane association of greasy grouper nervous necrosis virus protein A and characterization of its mitochondrial localization targeting signal J. Virol., 78, 6498-6508
7. Lisman, Q., Pomorski, T., Vogelzangs, C., Urli-Stam, D., de Cocq van Delwijnen, W. and Holthuis, J.C.M. (2004) Protein sorting in the late Golgi of Saccharomyces cerevisiae does not require mannosylated sphingolipids J. Biol. Chem., 279, 1020-1029
8. Scheiffele, P., Verkade, P., Fra, A. M., Virta, H., Simons, K. and Ikonen, E. (1998) Caveolin-1 and –2 in the exocytic pathway of MDCK cells J. Cell. Biol., 140, 795-806
9. Love, H. D., Lin, C-C., Short, C.S., and Ostermann, J. (1998) Isolation of functional Golgi-derived vesicles with a possible role in retrograde transport J. Cell Biol., 140, 541-551
10. Ding, T. T., Lee, S-J., Rochet, J-C. and Lansbury, Jr., P. T. (2002) Annular -synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes Biochemistry, 41, 10209-10217
11. Wang, X., Wang, F., Arterburn, L., Wollmann, R. and Ma, J. (2006) The interaction between cytoplasmic Prion protein and the hydrophobic lipid core of membrane correlates with neurotoxicity J. Biol. Chem., 281, 13559-13565
12. Derdowski, A., Ding, L. and Spearman, P. (2004) A novel fluorescence resonance energy transfer assay demonstrates that the human immunodeficiency virus type I Pr55Gag I domain mediates Gag-Gag interactions J. Virol., 78, 1230-1242
13. Halwani, R., Cen, S., Javanbakht, H., Saadatmand, J., Kim, S., Shiba, K. Kleiman, L. (2004) Cellular distribution of lysyl-tRNA synthetase and its interaction with Gag during human immunodeficiency virus type 2 assembly J. Virol., 78, 7553-7564
14. Gottwein, E. and Krausslich, H-G. (2005) Analysis of human immunodeficiency virus type 1 Gag ubiquitination J. Virol., 79, 9134-9144
15. Lee, H-J., Choi, C. and Lee, S-J. (2002) Membrane-bound -synuclein has a high aggregation propensity and the ability to seed the aggregation of cytosolic form J. Biol. Chem., 277, 671-678
16. Yu, C., Kim, S-H., Ikeuchi, T., Xu, H., Gasparini, L., Wang, R. and Sisodia, S. S. (2001) Characterization of a presenilin-mediated APP carboxyl terminal fragment : Evidence for distinct mechanisms involved in “gamma-secretase processing of the APP and notch 1 transmembrane domains J. Biol. Chem., 276, 43756-43760
17. Mira, E., Lacalle, R. A., Buesa, J. M., Gonzalez de Buitrago, G., Jimenez-Baranda, S., Gomez-Mouton, C., Martinez-A, C and Manes, S. (2004) Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface J. Cell Sci., 117, 1847-1856
18. Elazar, M., Liu, P., Rice, C. M. and Glenn, J. S. (2004) An N-terminal amphipathic helix in hepatitis C virus (HCV) NS4B mediates membrane association, correct localization of replication complex proteins, and RNA replication J. Virol., 78, 11393-11400
19. Wang, X., Wang, F., Sy, M-S. and Ma, J. (2005) Calpain and other cytosolic proteases can contribute to the degradation of retro-translocated prion protein in the cytosol J. Biol. Chem., 280, 317-325
20. Kim, Y.S., Laurine, E., Woods, W. and Lee, S-J. (2006) A novel mechanism of interaction between α-synuclein and biological membranes J. Mol. Biol., 360, 386-397
21. Lee, H-J., Patel, S. and Lee, S-J. (2005) Intravesicular localization and exocytosis of -synuclein and its aggregates J. Neurosci., 25, 6016-6024
22. Kraus, I., Eickmann, M., Kiermayer, S., Scheffczik, H., Fluess, M., Richt, J. A. and Garten, W. (2001) Open reading frame III of Borna disease virus encodes a nonglycosylated matrix protein J. Virol., 75, 12098-12104
23. Eichler, R., Strecker, T., Kolesnikova, L., ter Meulen, J., Weissenhorn, W., Becker, S., Klenk, H. D., Garten, W. and Lenz, O. (2004) Characterization of the Lassa virus matrix protein Z: electron microscopic study of virus-like particles and interaction with the nucleoprotein (NP) Virus Res., 100, 249-255
24. Joglekar, A. P., Xu, D., Rigotti, D. J., Fairman, R. and Hay, J. C. (2003) The SNARE motif contributes to rbet1 intracellular targeting and dynamics independently of SNARE interactions J. Biol. Chem., 278, 14121-14133
25. Hasegawa, H., Thomas, H.J., Schooley, K. and Born, T.L. (2011) Native IL-32 is released from intestinal epithelial cells via a non-classical secretory pathway as a membrane-associated protein Cytokine, 53, 74–83
26. De Leeuw, E., Poland, D., Mol, O., Sinning, I., ten Hagen-Jongman, C. M., Oudega, B. and Luirink, J. (1997) Membrane association of FtsY, the E. coli SRP receptor FEBS Lett., 416, 225-229
27. Hersovits, A. A., Seluanov, A., Rajsbaum, R., ten Hagen-Jongman, C. M., Henrichs, T., Bochkareva, E. S., Phillips, G. J., Probst, F. J., Nakae, T., Ehrmann, M., Luirink, J. and Bibi, E. (2001) Evidence for coupling of membrane targeting and function of the signal recognition particle (SRP) receptor FtsY EMBO Rep., 2, 1040-1046
28. Valent, Q. A., Scotti, P. A., High, S., de Gier, J-W. L., von Heijne, G., Lentzen, G., Wintermeyer, W., Oudega, B. and Luirink, J. (1998) The Escherichia coli SRP and SecB targeting pathways converge at the translocon EMBO J., 17, 2504-2512
29. Ge, W., Chew, T.G., Wachtler, V., Naqvi, S.N. and Balasubramanian, M.K. (2005) The novel fission yeast protein Pal1p interacts with Hip1-related Sla2p/End4p and is involved in cellular morphogenesis Mol. Biol. Cell, 16, 4124-4136
30. Medkova, M., France, Y.E., Coleman, J. and Novick, P. (2006) The rab exchange factor Sec2p reversibly associates with the exocyst Mol. Biol. Cell, 17, 2757-2769
31. Wang, X., Lee, W-M., Watanabe, T., Schwartz, M., Janda, M. and Ahlquist, P. (2005) Brome mosaic virus 1a nucleoside triphosphatase/helicase domain plays crucial roles in recruiting RNA replication templates J. Virol., 79, 13747-13758
32. Satyanarayana, C., Schroder-Kohne, S., Craig, E. A., Schu, P. V. and Horst, M. (2000) Cytosolic Hsp70s are involved in the transport of aminopeptidase 1 from the cytoplasm into the vacuole FEBS Lett., 470, 232-238
33. Urbanowski, J.L. and Piper, R.C. (2001) Ubiquitin sorts proteins into the intralumenal degradative compartment of the late-endosome/vacuole Traffic, 2, 622-630
OptiPrepTM Application Sheet S35; 9th edition, January 2020
OptiPrep™ Application Sheet S36
Separation of membrane vesicles and cytosol in a self-generated gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The OptiPrep™ Reference List RS13 “The resolution of soluble cytosolic proteins from membrane vesicles and organelles: a bibliography” provides a list of all published papers reporting the use of OptiPrep™: to access return to the initial list of Folders and select “Reference Lists”. The references are divided into cell or particle type.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and
select the appropriate S-number. - Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5)
1. Background
There are many situations where it is necessary to provide an effective separation of membrane vesicles from cytosolic proteins. The discontinuous gradient flotation strategy, see Application Sheet S35, uses a swinging-bucket rotor and allows the dense cytosolic proteins to remain in a dense load zone, while lower density membranes float to the top of a lower density solutions layered on top of the sample. This application sheet describes a similar strategy, i.e. the sample is adjusted to a high density and a lower density solution is layered on top, but in this case the use of a small volume high performance fixed-angle rotor permits the formation of an almost linear gradient by self-generation [1]. Since a linear gradient is formed, the method may allow a more detailed analysis of the membrane other than its simple resolution from cytosolic proteins. The method was developed by Du and Novick [1] for determining whether the GTPase activating protein Gyp1p was membrane-bound in yeast, but the gradient is more widely applicable to any protein in any cell type. The method used by Du and Novick [1] is ideally suited to multiple samples because of simple tube filling using open-topped (1 ml) polycarbonate tubes for a fixed-angle rotor. The method employs the usual sorbitol based solutions characteristic of yeast, but this can be adapted to mammalian cells (an example is given in Section 2.2). Note that the protocol below, adapted from ref 1 does not contain details of spheroplast or cell lysis.
2. Solutions required
2.1 Yeast (see Section 5.1)
A. OptiPrep™
B. OptiPrep™ diluent: 2.4 M sorbitol, 6 mM EDTA, 120 mM tetraethylammonium acetate, pH 7.2.
C. Working Solution (50% iodixanol): mix 5 vol. of OptiPrep™ with 1 vol. of Solution B.
D. Lysis buffer: 0.4 M sorbitol, 1 mM EDTA, 20 mM tetraethyl-ammonium acetate, pH 7.2.
2.2 Mammalian cells (see Sections 5.1, 5.2 and 5.3)
E. OptiPrep™
F. OptiPrep™ diluent: 0.25 M sucrose, 6 mM EDTA, 120 mM Hepes-NaOH, pH 7.4
G. Working Solution (50% iodixanol): mix 5 vol. of OptiPrep™ with 1 vol. of Solution B.
H. Homogenization buffer: 0.25 M sucrose, 1 mM EDTA, 10 mM Hepes-NaOH, pH 7.4
3. Ultracentrifuge rotor requirements (see Section 5.4)
Small-volume fixed angle rotor: Beckman TLA120.2, TLA100.2, Sorvall S120-AT2, S150-AT or equivalent
4. Protocol
Carry out all operations at 0-4°C.
1. Centrifuge the spheroplast lysate or cell homogenate at 1000 g for 5 min to remove nuclei and unbroken cells.
2. Remove the supernatant and adjust to 40% (w/v) iodixanol by mixing with Solution C or G (1 + 4 vol. respectively).
3. Prepare a solution of 35% (w/v) iodixanol by diluting Solution C (or G) with Solution D (or H); place 0.9 ml in a tube for the ultracentrifuge fixed-angle rotor and underlayer it with the postnuclear supernatant in 40% iodixanol.
4. Transfer to the ultracentrifuge fixed-angle rotor and centrifuge at 100-120,000 rpm for 3 h (see Section 5.5).
5. Unload the gradient using an automatic pipette or a Labconco Auto Densi-flow fractionator in approx. 0.1 ml fraction (see Section 5.6).
5. Technical Notes and Review
5.1 Preparing solutions
If Solution D (or H) contains low concentrations of other reagents such as DTT or MgOAc then these can be included in the OptiPrep™ diluent (Solution B or F) at 6x the normal concentration. Thus the 50% (w/v) iodixanol working solution (Solution C or G) and all the gradient solutions produced from it by dilution with Solution D or H, will contain these reagents at the same concentration as in Solutions D or H. Strategies for preparing working solutions for mammalian cells and for yeast are given respectively in Application Sheets S01 and S02. Protease inhibitors may be included in Solutions B, D, F and H at the operator’s discretion.
5.2 Homogenization media and gradient solutions for mammalian cells
The homogenization medium often has to be tailored to the tissue or cell type and it is not known if the composition of the HM is relevant to the separation. Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility in functional studies on subcellular membranes; moreover these low ionic strength HMs and gradient solutions permit the direct use of fractions for SDS-PAGE. Although 0.25 M sucrose buffered with either Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and containing 1 mM EDTA is still a widely used HM, supplementation with inorganic salts is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation. If a hypoosmotic medium is required to swell the cells in order to achieve an adequate degree of homogenization it is important to return the homogenate to isoosmotic conditions as soon as possible. Some examples of homogenization media for mammalian cells are given in Application Sheet S06.
5.3 Homogenization
The homogenization protocol should be tailored to the cell (or tissue) type. Potter-Elevhjem homogenization for tissues and Dounce homogenization for cells used to be the standard procedures. For cells use of 5-15 passages through a 27- or 25-gauge syringe needle, sometimes preceded by Dounce homogenization, is more common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles. Some hints on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
5.4 Ultracentrifuge rotor
Almost any high-performance fixed-angle rotor can be used, as long as the sedimentation path length of the tube is less than 20 mm. Information on the formation of self-generated gradients is given in Application Sheet S04.
5.5 Centrifugation
With such small sedimentation path length rotors it is very likely that the centrifugation time could be reduced to 2 h without seriously affecting the resolution of the gradient. Rotors with a maximum speed of 100,000 rpm may need a slightly longer centrifugation time but the gradient formation and particle banding should be satisfactory after 3 h at the lower RCF with these short path length rotors.
5.6 Analysis
Cytosolic proteins will remain in the load zone, while membrane vesicles will band in the top half of the gradient. For more information on harvesting gradients see Application Sheet S08. Compare the protocol in this Application Sheet with that in Application Sheet S37 that describes specifically the separation of membranes from large protein complexes.
6. References
1. Du, L-L. and Novick, P. (2001) Yeast Rab GTPase-activating protein Gyp1p localizes to the Golgi apparatus and is a negative regulator of Ypt1p Mol. Biol. Cell, 12, 1215-1226
OptiPrepTM Application Sheet S36; 8th edition, January 2020
OptiPrep™ Application Sheet S37
Determination of the cytosolic or membrane location of a large protein
complex
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5)
1. Background
Differential centrifugation or sedimentation through a sucrose gradient does not easily provide an unambiguous answer to the question of whether a particular protein shifts from a cytosolic location to a membrane location during subcellular processing or during some functional modulation. Differential centrifugation requires several washes of the membrane pellet to remove all traces of cytosolic proteins. In differential centrifugation and sedimentation through a sucrose gradient, the proteins are also moving in the same direction as the membrane vesicles by sedimentation and diffusion. Application Sheet S35 describes a system in which the membranes are allowed to float through a density gradient from a dense load zone. This is an ideal format because:
- Proteins sediment, particularly as they are exposed to the gmax, while membranes migrate in the opposite direction
- The difference in density between the membranes and the proteins is much greater in iodixanol
than in sucrose - The lower viscosity of iodixanol gradients means that the particles move more quickly.
If the protein is part of a rapidly sedimenting large complex however, it may be possible to simplify the protocol considerably by avoiding bottom-loading of the sample. Instead, the sample is merely adjusted to a uniform concentration of iodixanol and centrifuged in a vertical or near-vertical rotor for approx 1 h at approx 350,000 g [1]. Plasma membrane vesicles (from any source) have a low density (<1.1 g/ml) in iodixanol, while proteins have a density of approx 1.26 g/ml and at 350,000 g rapidly sedimenting protein complexes will sediment through the gradient within 1 h in a shortsedimentation path length rotor. The gradient that is formed under these centrifugation conditions will have a wide density range with a shallow median section. Such a gradient is ideal for the separation of two particles with dissimilar densities, which will band at opposite ends of the gradient.
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 90 mM KOAc, 2 mM Mg(OAc)2, 20 mM Hepes-KOH, pH 8.0 (see Note 3)
C. Diluent: 540 mM KOAc, 12 mM Mg(OAc)2, 120 mM Hepes-KOH, pH 8.0.
D. Working Solution (50% iodixanol): mix 5 vol. of Solution A with 1 vol. of Solution C.
3. Rotor requirements (see Section 5.2)
Vertical rotor with 11-13 ml tubes (e.g. Beckman VTi65.1 or Sorvall 65V13, or near-vertical rotor (e.g. Beckman NVT65).
4. Protocol (adapted from ref 1)
Carry out all operations at 0-4°C.
1. Homogenize the cells in Solution B in a cell cracker (ball-bearing homogenizer) using 4-6 passages. Monitor the efficacy of the homogenization by phase contrast microscopy (see Section 5.3).
2. Centrifuge the homogenate at 1000 g for 5 min to pellet the nuclei.
3. Mix 2 vol. of post-nuclear supernatant (PNS) with 3 vol. of Solution D.
4. Centrifuge at approx 350,000 gav for 1 h.
5. Unload the gradient by tube puncture, upward displacement with a dense medium or aspiration from the meniscus in 0.5-1 ml fractions. For more information on harvesting gradients see Application Sheet S08.
- A brief review of some of the publications that have used this methodology is given Section 5.4.
5. Technical Notes and Review
5.1 Homogenization media and gradient solutions
The homogenization medium often has to be tailored to the tissue or cell type and it is not known if the composition of the HM is relevant to the separation. Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility in functional studies on subcellular membranes; moreover these low ionic strength HMs and gradient solutions permit the direct use of fractions for SDS-PAGE. Although 0.25 M sucrose buffered with either Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and containing 1 mM EDTA is still a widely used HM, supplementation with inorganic salts is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due
to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl (for example at 140 mM) or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation. Some other examples are given in Application Sheets S05 (tissues) and S06 (cells).
Strategies for preparing working solutions (WSs) for mammalian tissues and cells are given in Application Sheet S01. These strategies were originally devised for simple HMs such as 0.25 M sucrose, 1 mM EDTA, 10 mM Hepes-KOH, pH.7.4. Diluting 5 vol. of OptiPrep™ with 1 vol. of 0.25 M sucrose, 6 mM EDTA, 60 mM Hepes-KOH, pH.7.4 produces a 50% iodixanol solution containing the same concentration of EDTA and buffer as the HM and more or less isoosmotic, thus when diluted with the HM all solutions will also contain these same concentrations and also be isoosmotic. Because OptiPrep™ itself behaves as an isoosomotic solution, it is unnecessary to raise the concentration of the osmotic balancer in the OptiPrep™ diluent. However inclusion of extra components at significantly higher concentrations (e.g. the 90 mM in Solution B) compromises the strategy slightly. The preparation of a 50% (w/v) iodixanol working solution (WS) from OptiPrep™ and Solution C ensures that when this is added to the PNS, the KOAc, Mg(OAc)₂ and buffer concentrations will not change but the osmolality will be raised slightly. Only experimentation will determine which is more important – keeping the KOAc concentration or the osmolality constant. Nürnberger et al [2] first prepared a 54% (w/v) iodixanol solution by diluting OptiPrep™ with 1/10th vol. of 900 mM KOAc, 20 mM Mg(OAc)₂, 200 mM Hepes-KOH, pH 8.0
- Protease inhibitors may be included in any of the homogenization media and diluents at the operator’s discretion.
5.2 Ultracentrifuge rotors and tubes
The manner in which iodixanol forms self-generated gradients and the rotor requirements for rapid formation of such gradients is described in ref 3 and also in Application Sheet S04. Other rotors that have been used for the analysis of membranes and cytosolic proteins are the small volume (3.3-3.9 ml tube volume) TLN100 near-vertical rotor [2] and also the MLN-80 (up to 8 ml) near-vertical rotor [4], both of which are accommodated into the Beckman Tabletop Ultracentrifuge. With Beckman rotors it is strongly recommended that Optiseal™ tubes be used for their ease of use, both when setting up and unloading the gradients.
5.3 Homogenization
The homogenization protocol should be tailored to the cell (or tissue) type. Potter-Elevhjem homogenization for tissues and Dounce homogenization for cells used to be the standard procedures. For cells use of 5-15 passages through a 27- or 25-gauge syringe needle, sometimes preceded by Dounce homogenization, is more common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles. Some hints on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
5.4 Analysis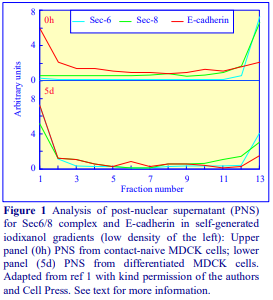
Grindstaff et al [1] were able to demonstrate that the plasma membrane protein, E-cadherin banded almost exclusively in fractions 1-2 in the membranes at the top of the gradient in both contact-naive and differentiated MDCK cells. On the other hand the Sec6/8 complex changed its location from an exclusively cytosolic one in the contact-naive cells to a predominantly membrane-bound one in the differentiated cells (Figure 1). This protocol has also been used in the detection of membrane located Rac1:effector complexes [5,6] and cytosol located GFP tagged Sec3 fusion proteins [7] in MDCK cells. In a study of inversin complex formation with catenins and N-cadherin in polarized epithelial cells [2], immunoblotting of the gradient fractions with an antibody to inversin detected a 140 kDa protein distributed broadly in the gradient at a low level, while a 125 kDa protein was only detected in the low density membrane fraction, along with pancadherin and β-catenin. Clathrin coat components in Triton X-100 treated post-mitochondrial supernatants from stably transfected LLC-PK1 cells were virtually restricted to the cytoplasmic fraction, while from cells expressing transfected μ1B, clathrin components along with Sec8, Exo70 and the AP-1B cargo molecule TfnR were located in the low-density membrane fraction while the AP-1A cargo molecule furin remained in the high-density cytosol region [4].
- For other examples of the use of this methodology see refs 8-10.
- Note that a self-generated gradient strategy can be used to determine if an uncomplexed protein has a membrane or cytosolic location (see Application Sheet S36).
6. References
1. Grindstaff, K. K., Yeaman, C., Anandasabapathy, N., Hsu, S-C., Rodriguez-Boulan, E., Scheller, R. H. and Nelson, W. J. (1998) Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells Cell, 93, 731-740
2. Nürnberger, J., Bacallao, R. L. and Phillips, C. L. (2002) Inversin forms a complex with catenins and Ncadherin in polarized epithelial cells Mol. Biol. Cell, 13, 3096-3106
3. Ford, T., Graham, J. and Rickwood, D. (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of self generated gradients Anal. Biochem., 220, 360-366
4. Fölsch, H., Pypaert, M., Maday, S., Pelletier, L. and Mellman, I. (2003) The AP-1A and AP1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains J. Cell Biol., 163, 351-362
5. Hansen, M. D. H. and Nelson, W. J. (2001) Serum-activated assembly and membrane translocation of an endogenous Rac1: effector complex Curr. Biol., 11, 356-360
6. Hansen, M. D. H., Ehrlich, J. S. and Nelson, W. J. (2002) Molecular mechanism for orienting membrane and actin dynamics to nascent cell-cell contacts in epithelial cells J. Biol. Chem., 277, 45371-45376
7. Matern, H.T., Yeaman, C., Nelson, W.J. and Scheller, R.H. (2001) The Sec6/8 complex in mammalian cells: characterization of mammalian Sec3, subunit interactions, and expression of subunits in polarized cells Proc. Natl. Acad. Sci. USA, 98, 9648-9653
8. Yeaman, C., Grindstaff, K.K., Wright, J.R. and Nelson, W.J. (2001) Sec6/8 complexes on trans-Golgi network and plasma membrane regulate stages of exocytosis in mammalian cells J. Cell Biol., 155, 593-604
9. Gromley, A., Yeaman, C., Rosa, J., Redick, S., Chen, C-T., Mirabelle, S., Guha, M., Sillibourne, J. and Doxsey, S.J. (2005) Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission Cell, 123, 75-87
10. Zhao, H., Ito, Y., Chappel, J., Andrews, N.W., Teitelbaum, S.L. and Ross, F.P. (2008) Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion Dev. Cell, 14, 914-925
7. Acknowledgements
We wish to thank Dr Kent Grindstaff, Dr Charles Yeaman and Dr W James Nelson, Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA 94305- 5345, for their kind cooperation in the preparation of this text.
OptiPrepTM Application Sheet S37; 8th edition, January 2020
OptiPrep™ Application Sheet S38
Isolation of secretory granules from pancreatic cells
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and
select the appropriate S-number. - Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (see Section 4)
- Cultured murine βTC6-F7 cells, mouse pancreatic β cells and insulinoma cells have been used in the study of insulin secretion; all three are presented in this Application Sheet.
- See Section 4.9 for information on exocrine (acinar) cell granules
1. Background
A crude mitochondrial/secretory granule fraction from pancreatic islet β cells can be obtained as a pellet by a simple centrifugation of a post-nuclear supernatant over a 0.3 M sucrose barrier at 15,000 rpm for 12 min [1]. Sucrose gradients are however not particularly successful for preparing secretory granules at relatively high purity and yields, principally because the high osmolality of the gradients causes all osmotically active organelles to approach a limiting density. Protocols using Percoll® gradients are able to resolve intact granules from mitochondria, peroxisomes, endoplasmic reticulum and plasma membrane [2], all of which are significantly less dense than the granules in isoosmotic Percoll® gradients. In these gradients however, lysosomes have a significantly higher density than the other major organelles and consequently tend to overlap the granule fraction significantly. Hutton et al [2] ameliorated this problem by carrying out two Percoll® gradients, the first low-density gradient to obtain granule/lysosome fractions and then a second denser gradient to remove the lysosome contamination. Even so, the final granule fraction contained 12% of the total lysosomes and was enriched over the homogenate in the lysosome marker enzyme, aryl sulphatase, some nine-fold. Another disadvantage of using Percoll® is that it is necessary to wash the pelleted granule fraction from the final gradient three times to remove the colloidal silica particles.
2. Gradient selection
Continuous iodixanol gradients appear to be the most successful in resolving the insulin containing granule fraction from both a cultured murine cell line, βTC6-F7 [3] and also from mouse pancreatic β (islet) cells [4,5]. Buchanan et al [3] found that the Percoll® based technology was unsatisfactory for cultured cells and developed a single-step iodixanol gradient purification, which provides both a purer product and also avoids repeated centrifugations to remove the gradient medium.
3. Solutions required (see Box and Section 4.1)
A. OptiPrep™
B. Homogenization medium: 0.3 M sucrose, 1 mM K EDTA, 1 mM MgSO4, 10 mM MES-NaOH, pH 6.5
C. OptiPrep™Diluent: 0.3 M sucrose, 6 mM KEDTA, 6 mM MgSO4, 60 mM MES-NaOH, pH 6.5
D. Working Solution of 50% (w/v) iodixanol: 5 vol. of solution A + 1 vol. of solution C
4. Ultracentrifuge rotor requirements
Any swinging-bucket rotor capable of approx 200,000 g with tube volumes of 13 ml, e.g. Beckman SW 41 or Sorvall TH641 (see Section 4.2)
5. Protocol (adapted from refs 3-5)
Carry out all operations at 0-4°C.
1. Homogenize the cells in Solution B using a ball-bearing homogenizer (see Section 4.3).
2. Centrifuge the homogenate at 500 g for 10 min and retain the post-nuclear supernatant (PNS).
3. Prepare 20 ml each of 8% and 19% (w/v) iodixanol by mixing Solutions D and B using volume ratios of 8:42 and 19:31 (see Section 4.4 for alternative gradients).
4. Use a two-chamber gradient maker or Gradient Master to make an approx. 12 ml gradient from equal volumes of the 8 and 19% iodixanol solutions tubes for the swinging-bucket rotor (see Section 4.5).
5. Layer the PNS on top of the gradient and centrifuge at 160,000 gav for 16 h (see Section 4.6).
6. Collect the gradient in 0.5-1.0 ml fractions either by tube puncture, upward displacement or aspiration from the meniscus. For more information on harvesting gradients see Application Sheet S08.
- For information on analysis of the gradient fractions see Section 4.7.
4. Technical Notes and Review
4.1 Homogenization media
The homogenization medium (HM) often has to be tailored to the tissue or cell type. The most common osmotic balancer is 0.25 M sucrose, although slightly higher concentrations, as in this protocol, are quite common. The use of 0.3 M sucrose in the homogenization medium and in the OptiPrep™ diluent (Solution C), slightly increases the density and osmolality of the gradient solutions, compared to those given in Application Sheet S01, which are based on 0.25 M sucrose. Supplementation of the HM with inorganic salts (containing K+ or Na+ ions) is becoming increasingly common in the analysis of endoplasmic reticulum, plasma membrane and Golgi in iodixanol gradients; it can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Whether the fractionations reported in this OptiPrep™ Application Sheet would benefit from such modifications can only be assessed by experimentation. The inclusion of divalent cations guards against nuclear breakage and stabilizes membranes generally, but in some cases can lead to aggregation. The preparation of a Working Solution as described, ensures that the concentrations of EDTA, MgSO₄ and buffer are constant throughout the gradient. If this is deemed unimportant the iodixanol solutions may be prepared simply by diluting OptiPrep with Solution B. The sucrose concentration in Solution C should not be increased 6x, in line with the other components. If it were, the Working Solution would be grossly hyperosmotic. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01. Protease inhibitors may be included in Solutions B and C at the operator’s discretion.
4.2 Ultracentrifuge rotors
Smaller or larger volume swinging-bucket rotors may be used for smaller or larger amounts of material. It may also be possible to use a vertical or near-vertical rotor; use of these rotors would speed up the separation considerably at the same g-force, because of the much shorter sedimentation path length.
4.3 Homogenization
Dounce (or sometimes Potter-Elvehjem) homogenization was the most widely used procedure at
one time but the ball-bearing homogenizer or “cell cracker”, with the standard 0.3747 in (9.52 mm) ball
bearing, is now regarded as one of the most effective and reproducible of devices. If this is not
available however 10-20 passages through a syringe needle (the Gauge Number (G) varies from 21 to
26) is usually an efficient alternative. Sometimes, as in this protocol, the efficacy of this method is
improved by switching to a second finer syringe needle for half the passes. Occasionally use of a syringe needle is prefaced by Dounce homogenization. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. Some other hints on homogenization are given in Application Sheet S06.
4.4 Density gradient selection
In a later publication the use of a 12-24% (w/v) iodixanol gradient was described [6]. Most gradient separations are based on buoyant density, but see Section 4.9 for a sedimentation rate option.
4.5 Density gradient preparation
If neither of these gradient-making devices is available then a continuous gradient can be prepared by diffusion of a discontinuous gradient (use equal volumes of 8%, 12%, 16% and 20% (w/v) iodixanol). For more information on gradient construction see Application Sheet S03. Application of this technique to the isolation of granule fractions from other cells may need modification to the density range of the gradient.
4.6 Tube loading
In swinging-bucket rotors of different tube volumes scale up or down the volumes proportionately. If necessary, adjust all volumes (also proportionately) so that tubes are properly filled according to the manufacturer’s instructions.
4.7 Gradient analysis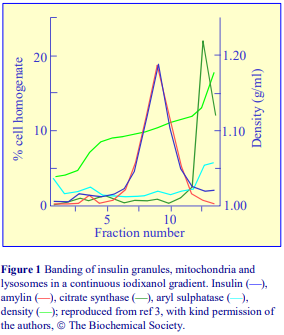
If it is necessary to concentrate a fraction or to remove the iodixanol before analysis, see Application Sheet S09. Figure 1 shows the distribution of granule markers (insulin and amylin), a mitochondrial marker (citrate synthase) and a lysosomal marker (aryl sulphatase) from βTC6-F7 cells in the iodixanol gradient. Both the mitochondria and lysosomes are well resolved from the granule fraction in the steep gradient that is formed towards the bottom of the tube. Only 2% of the lysosomes and 0.9% of the mitochondria were recovered in the granule fraction. Although the continuous gradient originally formed is essentially linear, some sharpening of the gradient at the top and bottom of the tube will always occur at the RCF and time used in this purification, due to sedimentation of the iodixanol molecules
themselves (i.e. some degree of self-generation will occur). Note that mitochondria are denser in iodixanol than they are in Percoll®; this accounts for the differences in banding patterns of lysosomes and mitochondria in the two media.
Using an iodixanol gradient, Buchanan et al [3] investigated the functions and properties of these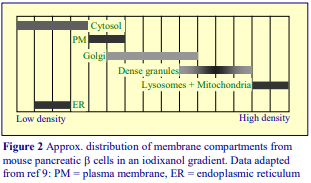 granules more fully and to identify a new peptide involved in regulating glucosemediated insulin secretion. The large dense-core insulin containing vesicles of mouse pancreatic β cells banded in a similar position to the insulin granules of βTC6-F7 cells (Figure 2), close to, but wellresolved from the lysosomes and mitochondria [4]. Varadi et al [4] also identified the cytosolic, plasma membrane (PM) and Golgi containing regions that banded in the lower density regions of the gradient. The only significant overlap of the insulin containing vesicles was observed with the denser Golgi membranes. Endosomes banded close to the top of the gradient. The banding of the various non-granule membrane compartments was more or less in line with that observed by other workers, i.e. the density increased in the order endosomes<PM<Golgi<lysosomes<mitochondria; the ER however was less dense than the PM and Golgi; the reverse is normally the case. Varadi et al [4] identified the SUR1 and Kir6.2 subunits of the ATP-sensitive K+ channel in the large dense-core insulin containing vesicles and in ref 10 the involvement of myosin Va in secretory vesicle transport. The original iodixanol gradient described by Buchanan et al [3] was also used by Jurczyk et al [7] in a study on the secretion of insulin granules from pancreatic β cells. A review by Cooper [8] includes observations made on granules isolated by different methodologies. More recently Chen et al [9] used a discontinuous gradient of 8.8%, 13.2%, 17.6%, 23.4% and 30% (w/v) iodixanol, the top-loaded sample was centrifuged at 100,000 g for 75 min. Granules banded on top of and below the 17.6% layer; the denser granules were more mature [9].
granules more fully and to identify a new peptide involved in regulating glucosemediated insulin secretion. The large dense-core insulin containing vesicles of mouse pancreatic β cells banded in a similar position to the insulin granules of βTC6-F7 cells (Figure 2), close to, but wellresolved from the lysosomes and mitochondria [4]. Varadi et al [4] also identified the cytosolic, plasma membrane (PM) and Golgi containing regions that banded in the lower density regions of the gradient. The only significant overlap of the insulin containing vesicles was observed with the denser Golgi membranes. Endosomes banded close to the top of the gradient. The banding of the various non-granule membrane compartments was more or less in line with that observed by other workers, i.e. the density increased in the order endosomes<PM<Golgi<lysosomes<mitochondria; the ER however was less dense than the PM and Golgi; the reverse is normally the case. Varadi et al [4] identified the SUR1 and Kir6.2 subunits of the ATP-sensitive K+ channel in the large dense-core insulin containing vesicles and in ref 10 the involvement of myosin Va in secretory vesicle transport. The original iodixanol gradient described by Buchanan et al [3] was also used by Jurczyk et al [7] in a study on the secretion of insulin granules from pancreatic β cells. A review by Cooper [8] includes observations made on granules isolated by different methodologies. More recently Chen et al [9] used a discontinuous gradient of 8.8%, 13.2%, 17.6%, 23.4% and 30% (w/v) iodixanol, the top-loaded sample was centrifuged at 100,000 g for 75 min. Granules banded on top of and below the 17.6% layer; the denser granules were more mature [9].
4.8 Sedimentation velocity separation
Although the majority of fractionations are achieved on the basis of particle buoyant density (and there is evidence such separations are more effective when carried out for more than 6 h), the alternative sedimentation velocity strategy, which requires very short centrifugation times, may have advantages if the functions being studied are rather labile. Cao et al [10] homogenized INS-1E insulinoma cells in 0.3 M sucrose, 1 mM EDTA, 1 mM MgSO₄, 10 mM MES-KOH, pH 6.5 and layered a PNS on a discontinuous gradient of 30%, 23.4%, 17.6%, 13.2% and 8.8% (w/v) iodixanol (10 ml total volume) and centrifuged it at 100,000 g for 75 min. The authors used the gradient to show that PICK1 co-localizes with insulin granules that band approx. in the middle of the gradient.
4.9 Pancreatic exocrine cells
Secretory granules from pancreatic exocrine tumour cells have also been isolated and separated from the lighter ER and from the cytosol in 10-30% (w/v) iodixanol gradients (137,000 g for 2h) in a small volume rotor (0.2 ml of PNS on top of a 2 ml gradient) [11,12]. Recently granules have been isolated from cultured pancreatic exocrine cells in a 0-30% (w/v) iodixanol gradient centrifuged at 20,000 g for 3 h [13]. Granules banded in the bottom half of the gradient, with a sharp peak at the high density end; treatment of the cells with β-D xyloside broadened the band towards lower densities.
4.10 Pancreatic islet cells
An 8-20% (w/v) iodixanol gradient, centrifuged at 160,000 g for approx. 16 h was used to show that insulin granule production in the Golgi of pancreatic islet cells is regulated by the prohormone VGF [14].
Note: for recent publications on insulin granules see refs 15 and 16.
5. References
1. Michael, J., Carroll, R., Swift, H. and Steiner, D. (1987) Studies on the molecular organization of rat insulin secretory granules J. Biol. Chem., 262, 16531-16535
2. Hutton, J. C., Penn, E. J. and Peshavaria, M. (1982) Isolation and characterization of insulin secretory granules from a rat islet cell tumour Diabetologia, 23, 365-373
3. Buchanan, C. M., Phillips, A. R. J. and Cooper, G. J. S. (2001) Preptin derived from proinsulin-like growth factor II (proIGF-II) is secreted from pancreatic islet -cells and enhances insulin secretion Biochem. J., 360, 431-439
4. Varadi, A., Grant, A., McCormack, M., Nicolson, T., Magistri, M., Mitchell, K.J., Halestrap, A.P., Yuan, H., Schwappach, B. and Rutter, G.A. (2006) Intracellular ATP-sensitive K+ channels in mouse pancreata beta cells: against a role in organelle cation homeostasis Diabetologia, 49, 1567-1577
5. Varadi, A., Tsuboi, T. and Rutter, G.A. (2005) Myosin Va transports dense core secretory vesicles in pancreatic MIN6 -cells Mol. Biol. Cell, 16, 2670-2680
6. Hickey, A.J.R., Bradley, J.W.I., Skea, G.L., Middleditch, M.J., Buchanan, C.M., Phillips, A.R.J.and Cooper, J.G.S. (2009) Proteins associated with immunopurified granules from a model pancreatic islet -cell system: proteomic snapshot of an endocrine secretory granule J. Proteome Res., 8, 178-186
7. Jurczyk, A., Pino, S.C., O’Sullivan-Murphy, B., Addorio, M., Lidstone, E.A., di Iorio, P., Lipson, K.L., Standley, C., Fogarty, K., Lifshitz, L., Urano, F., Mordes, J.P., Greiner, D.L., Rossini, A.A. and Bortell, R. (2010) A novel role for the centrosomal protein, pericentrin, in regulation of insulin secretory vesicle docking in mouse pancreatic -cells PloS One, 5: 11812
8. Cooper, G.J.S. (2011) Proteomic analysis of the pancreatic islet β-cell secretory granule: current understanding and future opportunities In BetaSys, Systems Biology, 2 (ed. B. Booß-Bavnbek et al.), Springer Science+Business Media, pp 327-362
9. Chen, Y., Xia, Z., Wang, L., Yu, Y., Liu, P., Song, E. and Xu, T. (2015) An efficient two-step subcellular fractionation method for the enrichment of insulin granules from INS-1 cells Biophys. Rep., 1, 34–40
10. Cao, M., Mao, Z., Kam, C., Xiao, N., Cao, X., Shen, C., Cheng, K.K.Y., Xu, A., Lee, K-M., Jiang, L. and Xia, J. (2013) PICK1 and ICA69 control insulin granule trafficking and their deficiencies lead to impaired glucose tolerance PLoS Biol., 11: e1001541
11. Raffaniello, R., Fedorova, D., Ip, D. and Rafiq, S. (2009) Hsp90 co-localizes with Rab-GDI-1 and regulates agonist-induced amylase release in AR42J cells Cell. Physiol. Biochem., 24, 369-378
12. Limi, S., Ojakian, G. and Raffaniello, R. (2012) Rab3D regulated amylase levels, not agonist induced amylase release, in AR42J cells Cell. Mol. Biol. Lett., 17, 258-273
13. Aroso, M., Agricola, B., Hacker, C. and Schrader, M. (2015) Proteoglycans support proper granule formation in pancreatic acinar cells Histochem. Cell. Biol., 144, 331–346
14. Stephens, S.B., Edwards, R.J., Sadahiro, M., Lin, W-J., Jiang, C., Salton, S.R. and Newgard, C.B. (2017) The prohormone VGF regulates β cell function via insulin secretory granule biogenesis Cell Rep., 20, 2480-2489
15. Hussain, S.S., Harris, M.T., Kreutzberger, A.J.B., Inouye, C.M., Doyle, C.A., Castle, A.M., Arvan, P. and Castle, J.D. (2018) Control of insulin granule formation and function by the ABC transporters ABCG1 and ABCA1 and by oxysterol binding protein OSBP Mol. Biol. Cell, 29, 1238-1257
16. Bearrows, S.C., Bauchle, C.J., Becker, M., Haldeman, J.M., Swaminathan, S. and Stephens, S.B. (2019) Chromogranin B regulates early-stage insulin granule trafficking from the Golgi in pancreatic islet β-cells J. Cell Sci., 132, jcs231373
6. Acknowledgements
We wish to thank Dr C.M. Buchanan, Dr A.R.J. Phillips and Dr G.J.S. Cooper, School of Biological Sciences, University of Auckland, N.Z., and the Biochemical Society, for their kind cooperation in the preparation of this text.
OptiPrepTM Application Sheet S38; 10th edition, January 2020
OptiPrep™ Application Sheet S39
Isolation of a granule fraction from eosinophils
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” sections (Section 5)
1. Background
A number of published protocols for the isolation of secretory or storage granules from eosinophils use a Nycodenz® gradient. Some of these were prepared from Nycoprep® 1.15, an isoosmotic solution of density 1.15 g/ml containing 27.6% Nycodenz®. The latter is no longer commercially available, thus all Nycodenz® solutions must be made from the powder. The Nycodenz® method for the isolation of crystalloid granules from human peripheral blood eosinophils was first described by Levi-Schaffer et al [1] in the study of their association with granulocyte-macrophage colony-stimulating factor. Subsequently this methodology was also applied to study their release of interleukin-2 [2] and interleukin-6 [3] in inflammatory reactions, the mobilization of the chemokine RANTES in response to γ-interferon [4], the release of interleukin-13 [5] and the mechanism of the eosinophil respiratory burst in asthma [6]. More recently the method has been adapted to an iodixanol gradient covering approximately the same density range [7-14]. It is this method that is described below and it is adapted from ref 12.
2. Solutions required (see Section 5.1)
A. Homogenization medium: 0.25 M sucrose, 1 mM EGTA, 2 mM MgCl₂, 10 mM Hepes-NaOH, pH 7.4
B. OptiPrep™ diluent: 0.25 M sucrose, 6 mM EGTA, 60 mM Hepes-NaOH 7.4
C. Working solution of 50% (w/v) iodixanol: Mix 5 vol. of OptiPrep™ with 1 vol. of Solution B
D. Gradient solution: 45% (w/v) iodixanol; mix 4.5 vol. of Solution C with 0.5 vol. of Solution A
- Note that Solution A may also adjusted to 1 mM ATP before use [11]. Protease inhibitors may be included in Solutions A-C at the operator’s discretion [11].
3. Ultracentrifuge rotor requirements
Any swinging-bucket rotor with tube volumes of approx. 13 ml (e.g. Beckman SW 41 or Sorvall TH641)
4. Protocol
4a. Cell preparation
Prepare polymorphonuclear leukocytes (PMNs) from fresh human blood (anticoagulant EDTA) as described in Application Sheet C12, but carry out the centrifugation at 4°C. In Application sheet C12 the method is described as being carried out at room temperature; it may be necessary to increase the centrifugation time to allow for the increased liquid viscosity at the lower temperature. Wash and resuspend the PMNs harvested from the gradient in a buffered saline and then purify the eosinophils using immunomagnetic beads by negative selection. Anti-CD16 will remove neutrophils;
anti-CD14 and anti-CD3 will remove any residual mononuclear cells. The use of immunomagnetic beads to negatively select the eosinophils has become the method of choice and it should be carried out in accordance with the manufacturer’s recommendations and ref 15. Simple discontinuous gradients are however still in use for the subsequent separation of normodense and hypodense eosinophils. For more information see ref. 15.
4b. Isolation of granules
Carry out all steps at 4°C
1. Wash the cells twice in Solution A and then suspend the eosinophils in this solution; typically, 5×107 eosinophils are used at approx. 1×107 cells/ml.
2. Homogenize the cells by nitrogen cavitation at 600 psi for 10 min (see Section 5.2).
3. Centrifuge the homogenate at 200 g for 10 min (see Section 5.2).
4. Prepare gradients in tubes for the SW41 rotor from 4 ml of Solution A and 5 ml of Solution D using either a two-chamber gradient maker or Gradient Master™. Finally underlayer with 0.5 ml of Solution D. (see Section 5.3).
5. Layer the 200 g supernatant on top of the gradient (approx 2 ml per gradient) and centrifuge at 100,000 g for 1 h. Allow the rotor to decelerate using a slow deceleration program or turn off the brake below 2000 rpm.
6. Collect the gradient in 0.5-1.0 ml fractions either by tube puncture, upward displacement or aspiration from the meniscus. For more information on harvesting gradients see Application Sheet S09. For information on analysis of the gradient fractions see Section 5.4.
5. Technical Notes and Review
5.1. Solutions
Supplementation of the homogenization medium with inorganic salts (containing K+ or Na+
ions) is becoming increasingly common in the analysis of endoplasmic reticulum, plasma membrane and Golgi in iodixanol gradients; it can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Whether the fractionations reported in this Application Sheet would benefit from such modifications can only be assessed by experimentation. Levi-Schaffer et al [1] adjusted Solution A to 2mM MgCl2 and 1 mM ATP for the final suspension of the eosinophils prior to homogenization, to enhance the retention of granule functional activity.
The preparation of a Working Solution as described, ensures that the concentrations of EGTA and buffer are constant throughout the gradient. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
5.2. Homogenization and production of a post-nuclear supernatant
The ball-bearing homogenizer or “cell cracker”, with the standard 0.3747 in (9.52 mm) ball bearing, is now regarded as one of the most effective and reproducible of devices; it was used by LeviSchaffer et al [1], while nitrogen cavitation was used by Spencer et al [2]. If neither of these devices is available however 10-20 passages through a syringe needle (the Gauge Number (G) varies from 21 to 26) can be an efficient alternative, but its efficacy must be tested before using it as an alternative for granule preparation. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. Neves et al [11] used 1 ml of cell suspension. Some other hints on homogenization are given in Application Sheet S06.
Neves et al [11] resuspended the pellet from step 3 in 1ml of Solution A; repeated the cavitation and 200 g centrifugation (step 3) and combined the two supernatants. In earlier methods the first lowspeed centrifugation was carried out at 400 g for 10 min.
5.3. Density gradients
Note that the earlier Nycodenz® gradients the low-density gradient solution was 2% (w/v) and a permissible alternative is to use 2% (w/v) iodixanol in this method (prepare this from a mixture of Solution D (2 vol.) and Solution A (43 vol.). If the top of the gradient is 0% (w/v) iodixanol, layering of the post-nuclear supernatant might be difficult, hence a 2% low-density solution may be preferable. If neither of the gradient-making devices is available then a continuous gradient may be generated from a discontinuous one by diffusion: use 1 ml of 2% (or 0%) and equal volumes of 5%, 15%, 25%, 35%, 40% and 45% (w/v) iodixanol. For more information on gradient construction see Application Sheet S03. It is also worth noting the use of a very simple discontinuous gradient in which a post-nuclear supernatant from a HL60 cells is mixed with an equal volume of a dense solution (ρ = 1.12 g/ml) and 14 ml layered between 14 ml of 1.05 g/ml and 5 ml of 1.12 g/ml solutions [16]. The two densities are approx equivalent to 19% and 4% (w/v) iodixanol. The gradient was centrifuged at 37,000 g for only 35 min to purify azurophilic granules.
5.4. Analysis of the gradients
The granules band close to the bottom of the gradient at approx 1.20 g/ml, while cytosolic proteins remain at the top of the gradient. Under the short centrifugation time condition soluble proteins will sediment and diffuse only a short distance from the sample layer. The plasma membrane will also band close to the top of the gradient. Spencer et al [7] confirmed the identity and purity of the granule fraction by light and electron microscopy and also by Major Basic Protein and CD63 staining in flow cytometry analysis. The authors were able to demonstrate the existence of preformed cytokine receptors within the granules. Iodixanol gradients described in this Application Sheet are isoosmotic, it is not possible to make Nycodenz® with similar osmotic properties and this is thought to be responsible for the iodixanol gradient’s unique ability to resolve eosinophil vesicles from the granules [11]. See Application Sheet S09 for more general information on gradient analysis.
- For a more detailed account of the methodology see ref 17
6. References
1. Levi-Schaffer, F., Lacy, P., Severs, N.J., Newman, T.M., North, J., Gomperts, B., Kay, A.B. and Moqbel, R. (1995) Association of granulocyte-macrophage colony-stimulating factor with the crystalloid granules of human eosinophils Blood 85, 2579-2586
2. Levi-Schaffer, F., Barkans, J., Newman, T. M., Ying, S., Wakelin, M., Hohenstein, R., Barak, V., Lacy, P., Kay, A. B. and Moqbel, R. (1996) Identification of interleukin-2 in peripheral blood eosinophils Immunology, 87, 155-161.
3. Lacy, P., Levi-Schaffer, F., Mahmudi-Azer, S., Bablitz, B., Hagen, S. C., Velazquez, J. R., Kay, A. B. and Moqbel, R. (1998) Intracellular localization of interleukin-6 in eosinophils from atopic asthmatics and effects of interferon Blood, 91, 2508-2516.
4. Lacy, P., Mahmudi-Azer, S., Bablitz, B., Hagen, S. C., Velazquez, J. R., Man, S. F. P. and Moqbel, R. (1999) Rapid mobilization of intracellularly stored RANTES in response to interferon- in human eosinophils Blood, 94, 23-32.
5. Woerly, G., Lacy, P., Younes, A. B., Roger, N., Loiseau, S., Moqbel, R., Capron, M. (2002) Human eosinophils express and release IL-13 following CD28-dependent activation J. Leukocyte Biol., 72, 769 779.
6. Lacy, P., Latif, D. A., Steward, M., Musat-Marcu, S., Man, S. F. P. and Moqbal, R. (2003) Divergence of mechanisms regulating respiratory burst in blood and sputum eosinophils and neutrophils from atopic subjects J. Immunol., 170, 2670-2679.
7. Spencer, L.A., Melo, R.C.N., Perez, S.A.C., Bafford, S.P., Dvorak, A.M. and Weller, P.F. (2006) Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion Proc. Natl. Acad. Sci. USA, 103, 3333-3338
8. Neves, J.S., Perez, S.A.C., Spencer, L.A., Melo, R.C.N., Reynolds, L., Ghiran, I., Mahmudi-Azer, S., Odemuyiwa, S.O., Dvorak, A.M., Moqbel, R. and Weller, P.F. (2008) Eosinophil granules function extracellularly as receptor-mediated secretory organelles Proc. Natl. Acad. Sci. USA, 105, 18478-18483
9. Melo, R.C.N., Perez, S.A.C., Spencer, L.A., Dvorak, A.M. and Weller, P.F. (2005) Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils Traffic, 6, 866-879
10. Spencer, L.A., Szela, C.T., Perez, S.A.C., Kirchhoffer, C.L., Neves, J.S., Radke, A.L. and Weller, P.F. (2009) Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially J. Leukoc. Biol., 85, 117-123
11. Neves, J.S., Perez, S.A.C., Spencer, L.A., Melo, R.C.N. and Weller, P.F. (2009) Subcellular fractionation of human eosinophils: Isolation of functional specific granules on isoosmotic density gradients J. Immunol. Methods, 344, 64-72
12. Melo, R.C.N., Spencer, L.A., Perez, S.A.C., Neves, J.S., Bafford, S.P., Morgan, E.S., Dvorak, A.M. and Weller, P.F. (2009) Vesicle-mediated secretion of human eosinophil granule-derived major basic protein Lab. Invest., 89, 769–781
13. Neves, J.S., Radke, A.L., Weller, P.F. (2010) Cysteinyl leukotrienes acting via granule membrane expressed receptors elicit secretion from within cell-free human eosinophil granules J. Allergy Clin. Immunol., 125, 477-82
14. Shamri, R., Melo, R.C.N., Young, K.M., B-B., M., Xenakis, J.J., Spencer, L.A., Weller, P.F. (2012) CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules FASEB J., 26, 2084 –2093
15. Miyasato, M., Tsuda, S., Kitamura, N., Shirouzu, K., Nakama, T. and Sasai, Y. (1995) Purification of human blood eosinophils by a combination method using anti-CD16 monoclonal antibody, immunobeads, and Nycodenz density gradient J. Dermatol. Sci., 10, 118-129.
16. Bach, J-P., Borta, H., Ackermann, W., Faust, F., Borchers, O. and Schrader, M. (2006) The secretory granule protein syncollin localizes to HL-60 cells and neutrophils J. Histochem. Cytochem., 54, 877-888
17. Baptista-dos-Reis, R., Muniz, V.S. and Neves, J.S. (2014) Isolation and functional assessment of eosinophil crystalloid granules In Eosinophils: Methods and Protocols, Methods in Molecular Biology, 1178 (ed. Walsh, G.M), Springer Science+Business Media, LLC, pp 1-24
OptiPrepTM Application Sheet S39; 6th edition, January 2020
OptiPrep™ Application Sheet S40a
Preparation of synaptosomes, synaptoneurosomes, neuromelanin granules and synaptic vesicles – a methodological survey
- This short Application Sheet summarizes the presently available published papers that describe the use of iodixanol gradients for the purification of a small range of membranous particles that are unique to neural tissues.
1. Synaptosomes
Kiebler et al [1] was the first group to describe the use of a discontinuous iodixanol gradient in the isolation of dendritic spines from mouse hippocampus. The gradient comprised four layers of 9%, 12.5%, 15% and 25% (w/v) iodixanol (equivalent to densities of 1.076, 1.095, 1.105 and 1.152 g/ml); the densest solution contained the material pelleted from the hippocampal homogenate at 900 g for 10 min. The gradient was centrifuged at 18,000 g for 20 min and it was described as a velocity flotation separation. The primary aim of the gradient was to isolate dendritic spines and the material that banded at the 9%/12.5% interface was further fractionated in a secondary Percoll-sucrose gradient.
The basic methodology has subsequently been adapted to the isolation of synaptosomes. Bagni et al [2] were the first group to report this approach and they reversed the order of the Percoll and the iodixanol flotation gradients. Material at the 15-23% Percoll interface has generally been used for subsequent purification in the iodixanol gradient, which is generally modified to include a denser layer of 35% (w/v) iodixanol and the centrifugation conditions changed to 10,000 g for 20 min. Synaptosomes band at the 15%/25% iodixanol interface. Essentially the same methodology has been used by other workers [3-10].
Rather fewer papers have reported the use of the original gradient sequence of iodixanol then Percoll [11-14]. Troca-Marín et al [14] underlayered the gradient with the crude fraction in 40% iodixanol to purify synaptoneurosomes and this was also used more recently by Heintz et al [15].
Yuan et al [16] used only a 0-30% (w/v) iodixanol gradient in their analysis of hippocampal tissue, which showed that neurofilament proteins banded with synaptosomes. Tenga et al [17] using a similar 2.5-30% (w/v) iodixanol gradient (approx 120,000 g for 18 h) fractionated murine pre-frontal cortex material to establish that UBXN2A co-fractionated with an ER/cis-Golgi compartment rather than in synaptosomes.
2. Neuromelanin granules
A 1000 g/20 min pellet was resuspended in 20% (w/v) iodixanol and layered over discontinuous gradient of 26%, 31%, 36% and 50% iodixanol and centrifuged for 3 h at 81,000 g [18,19]. The neuromelanin granules banded at the 26%-31% iodixanol interface.
3. Synaptic vesicles
3a. Discontinuous flotation gradients
Hu et al [20] was the first group to describe the use of iodixanol gradients for the purification of synaptic vesicles prepared from synaptosomes, which had been isolated by differential centrifugation. The synaptosomes were osmotically lysed and the more rapidly sedimenting vesicles removed by centrifugation at 34,000 g for 20 min. The light membrane suspension was then mixed with an equal volume of OptiPrep™ (final iodixanol concentration of 30% w/v) and overlaid by 24% iodixanol in 140 mM potassium gluconate, 4 mM MgCl₂, 20 mM HEPES, pH 7.3. The SVs were recovered from the top of the 24% iodixanol layer after centrifugation at 500,000 g for 1 h in a small volume (5 ml) fixed-angle rotor. This method was also used by Ferraci et al [21]. It was scaled up by Richards et al [22] to a Beckman SW28 swinging-bucket rotor centrifuged at 95,000 g for 17 h; the 24% iodixanol was prepared in HEPES-buffered saline (HBS) and a layer of HBS was included on top. In a final concentration step the SV suspension was mixed with an equal volume of 80% Nycodenz®; overlaid with HBS and centrifuged for 8 h at 200,000 g [22]. Importantly Holt et al [23] compared the simple discontinuous iodixanol flotation gradient (as in ref 16) with the earlier sucrose gradients + sizeexclusion controlled pore glass bead method and found the two preparation gave very similar data in fusion experiments between synaptic vesicles and proteoliposomes. Discontinuous flotation methods similar to that introduced by Hu et al [20] have been used to monitor the synaptobrevin content of the synaptic vesicles [24] and the role of synaptotagmin in their exocytosis and endocytosis [25].
- A detailed protocol of the flotation method is provided in ref 26.
3b. Discontinuous sedimentation gradients
Low density membrane vesicles fraction from lysed synaptosomes have also been purified on similar iodixanol gradients top-loaded with the sample rather than bottom loaded [27]. The lowdensity membrane fraction in 145 mM NaCl, 1 mM EDTA, 25 mM HEPES-KOH, pH 7.4 was layered over 5%, 10%, 15% and 20% iodixanol in the same buffer. After centrifugation at 140,000 g for 16 h (Beckman SW28 rotor) the SVs banded at the 5%/10% iodixanol interface.
3c. Continuous sedimentation gradients
Weible et al [28] used a sciatic nerve homogenate as a source of synaptic vesicles and after an initial centrifugation at 1000 g for 10 min, cytosolic proteins were removed from the supernatant by a second centrifugation over a cushion of 10% (w/v) sucrose at 100,000 g for 1 h. The pellets were resuspended in buffer. Continuous iodixanol gradients were generated by three cycles of freezethawing of either 15% or 30% (w/v) iodixanol. So long as the freeze-thawing conditions are well controlled this can be an effective and simple means of creating a continuous gradient (see OptiPrep™ Application Sheet S03 for details). The resuspended membranes were layered over the gradients and centrifuged at 200,000 g for 3 h. The gradients were very effective in discriminating between lowdensity neurotrophin-containing vesicles and denser synaptic vesicles. In a study to determine the physical parameters of synaptic vesicles Takamori et al [29] loaded the vesicles on to a 5-35% (w/v) iodixanol gradient; centrifugation at 180,000 g for 5 h demonstrated a median density of 1.09 g/ml.
3d Self-generated gradients
The big advantages of the use of any self-generated gradient are the ease of sample handling (the sample is simply adjusted to the required starting concentration of iodixanol) and the great reproducibility of the gradient density profile under a particular set of centrifugation parameters. Iodixanol is able to form useful self-generating gradients in 1-4 h depending on the centrifugation speed and the rotor. The most widely used rotors are vertical or near-vertical rotors with tube sizes of approx. 3, 5 or 13 ml, which are capable of generating forces of at least 265,000 gav. Near-vertical rotors have an advantage over vertical ones inasmuch as any material that pellets is confined to the bottom of the tube in a near-vertical rotor while in a vertical rotor such a pellet is distributed over the entire length of the tube at its outermost point. The shape of the gradient is determined principally by the time of centrifugation and the g-force. A couple of examples are given in Figures 1and 2 As a general rule, the gradients after a short period of centrifugation (1 h) are S-shaped and are very shallow in the middle part of the tube; as the centrifugation time is increased the gradients become more linear, although the gradients always become steeper in the high-density region (Figure 1). After 4-5 h (Figure 2) the gradients reach in equilibrium position in which the tendency of the iodixanol molecules to sediment is counterbalanced by their diffusion down the gradient. A self-generated gradient was first used by Hashiramoto and James [30] in their studies on glucose transport vesicles in adipocytes; they were able to isolate two distinctive populations of GLUT-4 containing vesicles from a low-density membrane vesicle preparation. Ferguson et al [31]
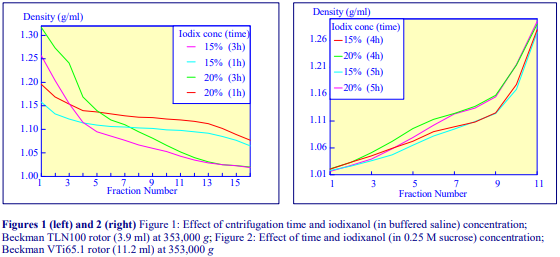
used the same strategy in which a synaptic vesicle fraction was prepared from synaptosomes. The fraction was adjusted to 14% (w/v) iodixanol and centrifuged at 265,000 g in a vertical rotor for 4 h and a low-density fraction rich in choline transporter was identified. Similar self-generated iodixanol gradients have also been used in the analysis of vesicles from Canton-S Drosophila heads. In the gradient Syt-1 synaptic vesicles were distinctly banded from the remaining synaptotagmins [32]. Although similar information was given by sedimentation velocity gradients, the ease of executing self-generated gradients makes their use very attractive alternative. Darios et al also reported the use of a self-generated gradient [33]. Iodixanol gradients for the purification of synaptic vesicles have also been reported by Herrera et al [34]
- For more information on the formation of self-generated gradients see Application Sheet S04, which can be found on the OptiPrep Subcellular Membranes Index, via the following website: www.OptiPrep.com.
4. References
1. Kiebler, M.A., Lopez-Garcia, J.C. and Leopold, P.L. (1999) Purification and characterization of rat hippocamal CA3-dendritic spines associated with mossy fiber terminals FEBS Lett., 445, 80-86
2. Bagni, C., Mannucci, L., Dotti, C.G. and Amaldi, F. (2000) Chemical stimulation of synaptosomes modulates -Ca2+/calmodulin-dependent protein kinase II mRNA association to polysomes J. Neurosci., 20 RC76, 1-6
3. Bence, M., Arbuckle, M.I., Dickson, K.S. and Grant, S.G.N. (2005) Analyses of murine Postsynaptic density-95 identify novel isoforms and potential translational control elements Mol. Brain Res., 133, 143-152
4. Wang, Z., Ruan, Q. and Wang, D. (2005) Different effects of intracochlear sensory and neuronal injury stimulation on expression of synaptic N-methyl-D-aspartate receptors in the auditory cortex of rats in vivo Acta Oto-Laryngol., 125, 1145-1151
5. Davidovic, L., Bechara, E., Gravel, M., Jaglin, X.H., Tremblay, S., Sik, A., Bardoni, B. and Khandjian, E.W. (2006) The nuclear microspherule protein 58 is a novel RNA-binding protein that interacts with fragile X mental retardation in polyribosomal mRNPs from neurons Hum. Mol. Genet., 15, 1525-1538
6. Matsumoto, M., Setou, M. and Inokuchi, K. (2007) Transcriptome analysis reveals the population of dendritic RNAs and their redistribution by neural acvitity Neurosci. Res., 57, 411-423
7. Cougot, N., Bhattacharyya, S.N., Tapia-Arancibia, L., Bordonné, R., Filipowicz, W., Bertrand, E. and Rage, F. (2008) Dendrites of mammalian neurons contain specialized P-body-like structures that respond to neuronal activation J. Neurosci., 28, 13793-13804
8. Alves-Sampaio, A., Troca-Marín, J.A. and Montesinos, M.L. (2010) NMDA-mediated regulation of DSCAM dendritic local translation is lost in a mouse model of Down’s syndrome J. Neurosci., 30, 13537–13548
9. Néant-Fery, M., Pérès, E., Nasrallah, C., Kessner, M., Gribaudo, S., Greer, C., Didier, A., Trembleau, A. and Isabelle Caillé, I. (2012) A role for dendritic translation of CaMKIIa mRNA in olfactory plasticity PloS One, 7: e40133
10. La Via, L., Bonini, D., Russo, I., Orlandi, C., Barlati, S. and Barbon, A. (2013) Modulation of dendritic AMPA receptor mRNA trafficking by RNA splicing and editing Nucleic Acids Res., 41, 617–631
11. Havik, B., Rokke, H., Bardsen, K., Davanger, S. and Bramham, C.R. (2003) Bursts of high frequency stimulation trigger rapid delivery of pre-existing -CaMKII mRNA to synapses: a mechanism in dendritic protein synthesis during long-term potentiation in adult awake rats Eur. J. Neurosci., 17, 2679-2689
12. Gilman, C.P., Chan, S.L., Guo, Z., Zhu, X., Greig, N. and Mattson, M.P. (2003) p53 is present in synapses where it mediates mitochondrial dysfunction and synaptic degeneration in response to DNA damage, and oxidative and excitotoxic insults Neuromol. Med., 3, 159-172
13. Kanhema, T., Dagestad, G., Panja, D., Tiron, A., Messaoudi, E., Havik, B., Ying, S-W., Nairn, A.C., Sonenberg, N. and Bramham, C.R. (2006) Dual regulation of translation initiation and peptide chain elongation during BDNF-induced LTP in vivo: evidence for compartment-specific translation control J. Neurochem., 99, 1328-1337
14. Troca-Marín, J.A., Alves-Sampaio, A., Tejedor, F.J. and Montesinos, M.L. (2010) Local translation of
dendritic RhoA revealed by an improved synaptoneurosome preparation Mol. Cell. Neurosci., 43, 308-314
15. Heintz, T.G., Eva, R. and Fawcett, J.W. (2016) Regional regulation of Purkinje cell dendritic spines by integrins and Eph/ephrins PLoS One, 11: e0158558
16. Yuan, A., Sershen, H., Veeranna, Basavarajappa, B.S., Kumar, A., Hashim, A., Berg, M., Lee, J-H., Sato, Y. et al (2015) Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo Mol. Psychiatry, 20, 986–994
17. Tenga, Y., Rezvanib, K. and De Biasia, M. (2015) UBXN2A regulates nicotinic receptor degradation by modulating the E3 ligase activity of CHIP Biochem. Pharmacol., 97, 518–530
18. Plum, S., Hellinga, S., Theiss, C., Leite, R.E.P., May, C., Jacob-Filho, W., Eisenacher, M., Kuhlmann, K., Meyer, H.E., Riederer, P., Grinberg, L.T., Gerlach, M. and Marcus, K. (2013) Combined enrichment of neuromelanin granules and synaptosomes from human substantia nigra pars compacta tissue for proteomic analysis J. Proteomics, 94, 202-206
19. Plum, S., Steinbach, S., Abel, L., Marcus, K., Helling, S. and May, C. (2015) Proteomics in neurodegenerative diseases: Methods for obtaining a closer look at the neuronal proteome Proteomics Clin. Appl., 9, 848–871
20. Hu, K., Carroll, J., Fedorovich, S., Rickman, C., Sukhodub, A. and Davletov, B. (2002) Vesicular restriction of synaptobrevin suggests a role for calcium in membrane fusion Nature, 415, 646-650
21. Ferraci, G., Seagar, M., Joël, C., Miquelis, R. and Leveque, C. (2004) Real time analysis of intact organelle using surface plasmon resonance Anal. Biochem., 334, 367-375
22. Richards, D.A., Bai, J. and Chapman, E.R. (2005) Two modes of exocytosis at hippocampal synapses revealed by rate of FM1-43 efflux from individual vesicles J. Cell Biol., 168, 929-939
23. Holt, M., Riedel, D., Stein, A., Schuette, C. and Jahn, R. (2008) Synaptic vesicles are constitutively active fusion machines that function independently of Ca2+ Curr. Biol., 18, 715-722
24. Darios, F., Wasser, C., Shakirzyanova, A., Giniatullin, A., Goodman, K., Munoz-Bravo, J.L., Raingo, J., Jorgačevski, J. et al (2009) Sphingosine facilitates SNARE complex assembly and activates synaptic vesicle exocytosis Neuron 62, 683–694
25. Yao, J., Kwon, S.E., Gaffaney, J.D., Dunning, F.M. and Chapman, E.R. (2012) Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles Nat. Neurosci., 15, 243-249
26. DiGiovanni, J., Sun, T. and Sheng, Z-H. (2012) Characterizing synaptic vesicle proteins using synaptosomal fractions and cultured hippocampal neurons Curr. Protoc. Neurosci., 59, 2.7.1-2.7.22, John Wiley & Sons, Inc
27. Baldwin, M.R. and Barbieri, J.T. (2007) Association of botulinum neurotoxin serotypes A and B with synaptic vesicle protein complexes Biochemistry, 46, 3200-3210
28. Weible II, M.W., Ozsarac, N., Grimes, M.L. and Hendry, I.A. (2004) Comparison of nerve terminal events in vivo effecting retrograde transport of vesicles containing neurotrophins or synaptic vesicle components J. Neurosci. Res., 75, 771-781
29. Takamori, S., Holt, M., Stenius, K., Lemke, E.A., Grønborg, M., Riedel, D., Urlaub, H., Schenck, S., Brügger, B. et al (2006) Molecular anatomy of a trafficking organelle Cell, 127, 831-846
30. Hashiramoto, M. and James, D.E. (2000) Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3-L1 adipocytes Mol. Cell Biol., 20, 416-427
31. Ferguson, S.M., Savchenko, V., Apparsundaram, S., Zwick, M., Wright, J., Heilman, C.J., Yi, H., Levey, A.I. and Balkely, R.D. (2003) Vesicular localization and activity-dependent trafficking of presynaptic choline transporters J. Neurosci., 23, 9697-9709
32. Adolfsen, B., Sarawati, S., Yoshihara, M. and Littleton, J.T. (2004) Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment J. Cell Biol., 166, 249-260
33. Darios, F.D., Jorgacevski, J., Flašker, A., Zorec, R., García-Martinez, V., Villanueva, J., Gutiérrez, L.M., Leese, C., Bal, M., Nosyreva, E., Kavalali, E.T. and Davletov, B. (2017) Sphingomimetic multiple sclerosis drug FTY720 activates vesicular synaptobrevin and augments neuroendocrine secretion Sci. Rep., 7: 5958
34. Herrera, A., Muñoz, P., Paris, I., Díaz-Veliz, G., Mora, S., Inzunza, J., Hultenby, K., Cardenas, C., Jaña, F. et al (2016) Aminochrome induces dopaminergic neuronal dysfunction: a new animal model for Parkinson’s disease Cell. Mol. Life Sci., 73, 3583–3597
OptiPrepTM Mini-Review S40a: 7th edition, January 2020
OptiPrep™ Application Sheet S40
Isolation of vesicular and granular fractions from various sources
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- This Application Sheet briefly summarizes the current information on the isolation of granules from the following cell/tissue types (1-6) (see Note below regarding neural tissue fractions);
- (1) Adrenal chromaffin cells
- (2) Lymphocytic cells
- (3) Neutrophils and HL-60 cells
- (4) Neuromelanin granules
- (5) Parotid acinar cells
- (6) Platelets
- (7) References
Important Note: Because of the diversity of studies on the neural system and the variety of methodologies, Section 8 contains an additional list of references sorted according to the nature or source of particle(s) under investigation. Papers are listed alphabetically according to first author.
1. Adrenal chromaffin cells
The gradient methodology which was originally published in 2008 [1] described an homogenization medium of 0.3 M sucrose, 1 mM MgSO₄, 1mM EDTA and 10 mM HEPES (pH 7.0). The gradient solutions were prepared from a stock solution containing 50% (w/v) iodixanol: OptiPrep™ was diluted 5:6 v/v with 0.3 M sucrose, 6 mM MgSO₄, 6mM EDTA and 60 mM HEPES (pH 7.0) [1-3]. Later [4,5] 10 mM KCl was included in the homogenization medium; consequently the OptiPrep™ diluent contained 60 mM KCl and the sucrose concentration reduced to 0.25 M (to maintain isoosmolality). Preceding the gradient a standard differential centrifugation of 1000 g for 10 min to remove nuclei, followed by 10,000 g for 20 min to pellet the granules (large dense core vesicles) is common to most methods. The major contaminant of the granules is therefore mitochondria; most (but not all) of the lysosomes and other smaller organelles will remain in the supernatant. Early gradients [1-3] were discontinuous and comprised iodixanol solutions of 8% and 16% [1,2] or 8% and 18% (w/v) iodixanol [3]; centrifugation conditions were 100,000 g for 1 h or [1,2] or 10,000 g for 10h [3]. More recently continuous gradients of 8-26% (w/v) iodixanol [4,5] centrifuged at 100,000 g for 1 h (sample was applied in 5% iodixanol), allowed the granule fraction to reach the bottom of the tube. Similar gradients are described in ref 6 with centrifugation at 110,000 g for 15-16 h [6].
2. Lymphocytic cells
Separations of lytic particles containing Granzyme B and/or granulysin have generally been carried out in gradients containing 8-27% (w/v) iodixanol [7-9], centrifuged at 150,000 g for 5h. After homogenization, the postnuclear supernatant from a lymphocytic cell suspension was centrifuged at 18,000 g for 20 min, to produce a light+heavy mitochondrial fraction, which was analyzed in the iodixanol gradient. In ref 8, which provides a detailed account of the methodology, the gradient was constructed from 8, 12, 16, 19, 22.5 and 27% (w/v) iodixanol and the sample was median loaded in the 19% (w/v) iodixanol layer. During the centrifugation the gradient will have become more or less linear. Granulysin was strongly enriched in a median and the densest band in the gradient. Median-loading can have a distinct advantage over top-loading: in the latter all the particles are sedimenting while in the former some will be moving up the gradient, others down, thus improving particle resolution. A slightly different continuous gradient of 6-30% (w/v) iodixanol at a lower g-force (100,000 g for 2h) was used by Kozlowski et al [10].
3. Neutrophils and HL60 cells (see ref 11)
HL60 cells were homogenized in a buffered HEPES-buffered 0.25 M sucrose medium containing 0.3 mM EDTA and mixed with an equal volume of iodixanol (1.12 g/ml density); layered between an equal volume of a 1.05 g/ml solution and 5 ml of the 1.12 g/ml solution. After centrifugation at 37,000 g for 35 min azurophilic granules (identified by syncollin) banded predominantly around the lower interface.
4. Neuromelanin granules (see ref 12)
Neuromelanin granules released from human brain synaptosomes were adjusted to 20% iodixanol and layered over a discontinuous 26%, 31%, 36% and 50% iodixanol gradient and centrifuged at 81,000 g for 3h, the granules banded on the 20-26% interface.
5. Parotid acinar cells (see ref 13)
Amylase-containing granules released from rat paraotid gland, homogenized in a routine isoosmotic Trisbuffered 0.25 M sucrose solution. The post-nuclear supernatant was layered on top of a continuous 10-30% (w/v) iodixanol gradient (solutions prepared by dilution of OptiPrepTM with homogenization medium) and centrifuged at 137,000 g for 2h: the granules banded around 20% (w/v) iodixanol.
6. Platelets
Platelets are most effectively homogenized by nitrogen cavitation [14] and the iodixanol gradients used for granule isolation, described in refs 14 and 15, are based on the use of this method . Homogenization of platelets is carried out in 145 mM NaCl, 5 mM KCl, 1 mM MgSO₄, 10 mM glucose, 0.5 mM EGTA, 25 mM Hepes-NaOH, pH 7.4. A 50% (w/v) iodixanol working solution is prepared from 5 vol. of OptiPrep TM and 1 vol. of 145 mM NaCl, 30 mM KCl, 6 mM MgSO₄, 60 mM glucose, 3 mM EGTA, 25 mM Hepes-NaOH, pH 7.4. Further dilutions are made with the homogenization medium. The cavitate is adjusted to 11% (w/v) iodixanol, transferred to a centrifuge tube for a swinging-bucket rotor and underlaid with a 30% (w/v) iodixanol solution. After centrifugation at 38,000 g for 3 h interfacial band is collected and dialyzed to remove the iodxanol. A second gradient is prepared from 2 ml each of 1%, 15%, 20%, 25% and 30% (w/v) iodixanol; the dialyzed sample (approx 2.5 ml containing approx 2 mg protein/ml) layered on top and centrifuged at 38,000 g for 3 h. The rotor is allowed to decelerate from 2000 rpm without the brake (or a controlled deceleration program is used). It is not known if the band from the first gradient might be collected in the 30% iodixanol solution and used as the second gradient bottom layer. If the separation is based on density alone then this is a valid alternative; if on the other hand the separation is based on sedimentation rate, then such an approach is invalid. The α-granules band at the 25%/30% interface
7. References
1. Camacho, M., Machado, J.D., Alvarez, J. and Borges, R. (2008) Intravesicular calcium release mediates the motion and exocytosis of secretory organelles: a study with adrenal chromaffin cells J. Biol. Chem., 283, 22383-22389
2. Díaz-Vera, J., Morales, Y.G., Hernández-Fernaud, J.R., Camacho, M., Montesinos, M.S., Calegari, F., Huttner, W.B., Borges, R. and Machado, J.D. (2010) Chromogranin B gene ablation reduces the catecholamine cargo and decelerates exocytosis in chromaffin secretory vesicles J. Neurosci., 30, 950–957
3. Díaz-Vera, J., Camacho, M., Machado, J.D., Domínguez, N., Montesinos, M.S., Hernández-Fernaud, J.R., Luján, R. and Borges, R. (2012) Chromogranins A and B are key proteins in amine accumulation, but the catecholamine secretory pathway is conserved without them FASEB J. 26, 430–438
4. Estévez-Herrera, J., Domínguez, N., Pardo, M.R., González-Santana, A., Westhead, E.W., Borges, R. and Machado, J.D. (2016) ATP: The crucial component of secretory vesicles Proc. Natl. Acad. Sci. USA, 113, E4098–E4106
5. Pardo, M.R., Estévez-Herrera, J., Castañeyra, L., Borges, R. and Machado, J.D. (2017) Isolation of mouse chromaffin secretory vesicles and their division into 12 fractions Anal. Biochem., 536, 1-7
6. Hao, Z., Wei, L., Feng, Y., Chen, X., Du, W., Ma, J., Zhou, Z., Chen, L. and Li, W. (2015) Impaired maturation of large dense-core vesicles in muted-deficient adrenal chromaffin cells J. Cell Sci., 128, 1365–1374
7. Sanborn, K.B., Rak, G.D., Maru, S.Y., Demers, K., Difeo, A., Martignetti, J.A., Betts, M.R., Favier, R., Banerjee, P.P. and Orange, J.S. (2009) Myosin IIA associates with NK cell lytic granules to enable their interaction with F-actin and function at the immunological synapse J. Immunol., 182, 6969–6984
8. Schmidt, H., Gelhaus, C., Lucius, R., Nebendahl, M. Leippe, M. and Janssen, O. (2009) Enrichment and analysis of secretory lysosomes from lymphocyte populations BMC Immunol., 10:41
9. Tuli, A., Thiery, J., James, A.M., Michelet, X., Sharma, M., Garg, S., Sanborn, K.B., Orange, J.S., Lieberman, J. and Brenner, M.B. (2013) Arf-like GTPase Arl8b regulates lytic granule polarization and natural killer cell–mediated cytotoxicity Mol. Biol. Cell, 24, 3721-3735
10. Kozlowski, M., Schorey, J., Portis, T., Grigoriev, V. and Kornbluth, J. (1999) NK lytic-associated molecule: A novel gene selectively expressed in cells with cytolytic function J. Immunol., 163, 1775-1785
11. Bach, J-P., Borta, H., Ackermann, W., Faust, F., Borchers, O. and Schrader, M. (2006) The secretory granule protein syncollin localizes to HL-60 cells and neutrophils J. Histochem. Cytochem., 54, 877-888
12. Plum, S., Helling, S., Theiss, C., Leite, R.E.P., May, C., Jacob-Filho, W., Eisenacher, M., Kuhlmann, K., Meyer, H.E., Riederer, P., Grinberg, L.T., Gerlach, M. and Marcus, K. (2013) Combined enrichment of neuromelanin granules and synaptosomes from human substantia nigra pars compacta tissue for proteomic analysis J. Proteomics, 94, 202-206
13. Chan, D., Lin, J. and Raffaniello, R.D. (2000) Expression and localization of rab escort protein isoforms in parotid acinar cells from rat Cell. Physiol., 185, 339-34
14. Flaumenhaft, R., Dilks, J.R., Rozenvayn, N., Monahan-Earley, R., Feng, D. and Dvorak, A.M. (2005) The actin cytoskeleton differentially regulates platelet -granule and dense-granule secretion Blood, 105, 3879-3887
15. Woronowicz, K., Dilks, J.R., Rozenvayn, N., Dowal, L. Blair, P.S., Peters, C.G., Woronowicz, L. and Flaumenhaft, R. (2010) The platelet actin cytoskeleton associates with SNAREs and participates in R granule secretion Biochemistry, 49, 4533–4542
8. Additional references
Adrenal medulla
Estévez-Herrera, J., Domínguez, N., Pardo, M.R., González-Santana, A., Westhead, E.W., Borges, R. and Machado, J.D. (2016) ATP: The crucial component of secretory vesicles Proc. Natl. Acad. Sci. USA, 113, E4098–E4106
Choline transporter membranes
Ferguson, S.M., Savchenko, V., Apparsundaram, S., Zwick, M., Wright, J., Heilman, C.J., Yi, H., Levey, A.I. and Balkely, R.D. (2003) Vesicular localization and activity-dependent trafficking of presynaptic choline transporters J. Neurosci., 23, 9697-9709
Dendritic spines
Kiebler, M.A., Lopez-Garcia, J.C. and Leopold, P.L. (1999) Purification and characterization of rat hippocamal CA3-dendritic spines associated with mossy fiber terminals FEBS Lett., 445, 80-86
HL60 cells
Davis, J.M., Carvalho, H.M., Rasmussen, S.B. and O’Brien, A.D. (2006) Cytotoxic necrotizing factor type 1 delivered by outer membrane vesicles of uropathogenic Escherichia coli attenuates polymorphonuclear leukocyte antimicrobial activity and chemotaxis Infect. Immun., 74, 4401-4408
Neuromelanin granules
Pluma, S., Hellinga, S., Theiss, C., Leite, R.E.P., May, C., Jacob-Filho, W., Eisenacher, M., Kuhlmann, K., Meyer, H.E., Riederer, P., Grinberg, L.T., Gerlach, M. and Marcus, K. (2013) Combined enrichment of neuromelanin granules and synaptosomes from human substantia nigra pars compacta tissue for proteomic analysis J. Proteomics, 94, 202-206
Neurotrophin-containing vesicles
Weible II, M.W., Ozsarac, N., Grimes, M.L. and Hendry, I.A. (2004) Comparison of nerve terminal events in vivo effecting retrograde transport of vesicles containing neurotrophins or synaptic vesicle components J. Neurosci. Res., 75, 771-781
NK cells
Gil-Krzewska, A., Saeed, M.B., A. Oszmiana, A., Fischer, E.R., Lagrue, K., Gahl, W.A., Introne, W.J. et al (2018) An actin cytoskeletal barrier inhibits lytic granule release from natural killer cells in patients with ChediakHigashi syndrome J. Allergy Clin. Immunol., 142, 914-927
Post-synaptic vesicles
Bence, M., Arbuckle, M.I., Dickson, K.S. and Grant, S.G.N. (2005) Analyses of murine Postsynaptic density-95 identify novel isoforms and potential translational control elements Mol. Brain Res., 133, 143-152
Pre-frontal cortex (endoplasmic reticulum/Golgi)
Tenga, Y., Rezvanib, K. and De Biasia, M. (2015) UBXN2A regulates nicotinic receptor degradation by modulating the E3 ligase activity of CHIP Biochem. Pharmacol., 97, 518–530
Synaptic vesicles
Adolfsen, B., Sarawati, S., Yoshihara, M. and Littleton, J.T. (2004) Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment J. Cell Biol., 166, 249-260
Baldwin, M.R. and Barbieri, J.T. (2007) Association of botulinum neurotoxin serotypes A and B with synaptic vesicle protein complexes Biochemistry, 46, 3200-3210
Darios, F., Wasser, C., Shakirzyanova, A., Giniatullin, A., Goodman, K., Munoz-Bravo, J.L., Raingo, J., Jorgačevski, J., Kreft, M. et al (2009) Sphingosine facilitates SNARE complex assembly and activates synaptic vesicle exocytosis Neuron 62, 683–694
Darios, F.D., Jorgacevski, J., Flašker, A., Zorec, R., García-Martinez, V., Villanueva, J., Gutiérrez, L.M., Leese, C., Bal, M., Nosyreva, E., Kavalali, E.T. and Davletov, B. (2017) Sphingomimetic multiple sclerosis drug FTY720 activates vesicular synaptobrevin and augments neuroendocrine secretion Sci. Rep., 7: 5958
DiGiovanni, J., Sun, T. and Sheng, Z-H. (2012) Characterizing synaptic vesicle proteins using synapto-somal fractions and cultured hippocampal neurons Curr. Protoc. Neurosci., 59, 2.7.1-2.7.22, John Wiley & Sons, Inc.
Ferraci, G., Seagar, M., Joël, C., Miquelis, R. and Leveque, C. (2004) Real time analysis of intact organelle using surface plasmon resonance Anal. Biochem., 334, 367-375
Herrera, A., Muñoz, P., Paris, I., Díaz-Veliz, G., Mora, S., Inzunza, J., Hultenby, K., Cardenas, C., Jaña, F. et al (2016) Aminochrome induces dopaminergic neuronal dysfunction: a new animal model for Parkinson’s disease Cell. Mol. Life Sci., 73, 3583–3597
Holt, M., Riedel, D., Stein, A., Schuette, C. and Jahn, R. (2008) Synaptic vesicles are constitutively active fusion machines that function independently of Ca2+ Curr. Biol., 18, 715-722
Hu, K., Carroll, J., Fedorovich, S., Rickman, C., Sukhodub, A. and Davletov, B. (2002) Vesicular restriction of synaptobrevin suggests a role for calcium in membrane fusion Nature, 415, 646-650
Richards, D.A., Bai, J. and Chapman, E.R. (2005) Two modes of exocytosis at hippocampal synapses revealed by rate of FM1-43 efflux from individual vesicles J. Cell Biol., 168, 929-939
Takamori, S., Holt, M., Stenius, K., Lemke, E.A., Grønborg, M., Riedel, D., Urlaub, H., Schenck, S. et al (2006) Molecular anatomy of a trafficking organelle Cell, 127, 831-846
Yao, J., Kwon, S.E., Gaffaney, J.D., Dunning, F.M. and Chapman, E.R. (2012) Uncoupling the roles of
synaptotagmin I during endo- and exocytosis of synaptic vesicles Nat. Neurosci., 15, 243-249
Synaptodendrosomes
Havik, B., Rokke, H., Bardsen, K., Davanger, S. and Bramham, C.R. (2003) Bursts of high-frequency stimulation trigger rapid delivery of pre-existing -CaMKII mRNA to synapses: a mechanism in dendritic protein synthesis during long-term potentiation in adult awake rats Eur. J. Neurosci., 17, 2679-2689
Kanhema, T., Dagestad, G., Panja, D., Tiron, A., Messaoudi, E., Havik, B., Ying, S-W., Nairn, A.C., Sonenberg, N. and Bramham, C.R. (2006) Dual regulation of translation initiation and peptide chain elongation during BDNF-induced LTP in vivo: evidence for compartment-specific translation control J. Neurochem., 99, 1328-1337
Synaptoneurosomes
Alves-Sampaio, A., Troca-Marín, J.A. and Montesinos, M.L. (2010) NMDA-mediated regulation of DSCAM dendritic local translation is lost in a mouse model of Down’s syndrome J. Neurosci., 30, 13537–13548
Cougot, N., Bhattacharyya, S.N., Tapia-Arancibia, L., Bordonné, R., Filipowicz, W., Bertrand, E. and Rage, F. (2008) Dendrites of mammalian neurons contain specialized P-body-like structures that respond to neuronal activation J. Neurosci., 28, 13793-13804
Heintz, T.G., Eva, R. and Fawcett, J.W. (2016) Regional regulation of Purkinje cell dendritic spines by integrins and Eph/ephrins PLoS One, 11: e0158558
La Via, L., Bonini, D., Russo, I., Orlandi, C., Barlati, S. and Barbon, A. (2013) Modulation of dendritic AMPA receptor mRNA trafficking by RNA splicing and editing Nucleic Acids Res., 41, 617–631
Troca-Marín, J.A., Alves-Sampaio, A., Tejedor, F.J. and Montesinos, M.L. (2010) Local translation of dendritic RhoA revealed by an improved synaptoneurosome preparation Mol. Cell. Neurosci., 43, 308-314
Synaptosomes
Bagni, C., Mannucci, L., Dotti, C.G. and Amaldi, F. (2000) Chemical stimulation of synaptosomes modulates - Ca2+/calmodulin-dependent protein kinase II mRNA association to polysomes J. Neurosci., 20 RC76, 1-6
Castora, F.J. and Trevino, M.B. (2012) An improved procedure for isolation of functional synaptosomes for the transient generation of cybrids from frozen human brain FASEB J., 26: 586.4
Davidovic, L., Bechara, E., Gravel, M., Jaglin, X.H., Tremblay, S., Sik, A., Bardoni, B. and Khandjian, E.W. (2006) The nuclear microspherule protein 58 is a novel RNA-binding protein that interacts with fragile X mental retardation in polyribosomal mRNPs from neurons Hum. Mol. Genet., 15, 1525-1538
Gilman, C.P., Chan, S.L., Guo, Z., Zhu, X., Greig, N. and Mattson, M.P. (2003) p53 is present in synapses where it mediates mitochondrial dysfunction and synaptic degeneration in response to DNA damage, and oxidative and excitotoxic insults Neuromol. Med., 3, 159-172
Matsumoto, M., Setou, M. and Inokuchi, K. (2007) Transcriptome analysis reveals the population of dendritic RNAs and their redistribution by neural acvitity Neurosci. Res., 57, 411-423
Néant-Fery, M., Pérès, E., Nasrallah, C., Kessner, M., Gribaudo, S., Greer, C., Didier, A., Trembleau, A. and Isabelle Caillé, I. (2012) A role for dendritic translation of CaMKIIa mRNA in olfactory plasticity PloS One, 7: e40133
Yuan, A., Sershen, H., Veeranna, Basavarajappa, B.S., Kumar, A., Hashim, A., Berg, M., Lee, J-H., Sato, Y. et al (2015) Neurofilament subunits are integral components of synapses and modulate neuro-transmission and behavior in vivo Mol. Psychiatry, 20, 986–994
Synaptosomes (auditory cortex)
Wang, Z., Ruan, Q. and Wang, D. (2005) Different effects of intracochlear sensory and neuronal injury
stimulation on expression of synaptic N-methyl-D-aspartate receptors in the auditory cortex of rats in vivo Acta Oto-Laryngol., 125, 1145-1151
OptiPrepTM Application Sheet S40; 8th edition, January, 2020
OptiPrep™ Application Sheet S41
Resolution of smooth endoplasmic reticulum (SER), SER domains, study of SER communication with other organelles and lipid droplets
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and
select the appropriate S-number.
1. Background
It is widely recognized that the smooth endoplasmic reticulum contains specialized domains that are structurally and functionally associated with other membrane compartments. Application Sheet S22 describes the fractionation of endoplasmic reticulum (ER), Golgi, plasma membrane and endosomes in continuous gradients of iodixanol, using relatively low g-forces for at least 12 h. The method is widely used; it highlights a paper by Woods et al [1], which describes this strategy as applied to 3T3 cells. Calreticulin shows a distinctive biphasic distribution in a 10-40% (w/v) iodixanol gradient but only the denser fraction also contains paxillin, which identifies this ER subfraction as perinuclear [1]. This functional and structural specialization of the ER is now widely recognized as more and more functional specializations have been discovered. Lynes and Simmen [2], amongst others, have reviewed some of these domain-specific functions; for example the peripheral ER that is closely associated with the plasma membrane, the mitochondria-associated membranes (MAM) and domains of the smooth ER that are associated with peroxisome biogenesis and the formation of lipid droplets.
2. MAM analysis
Lewin et al [3] prepared a standard rat liver homogenate (see Application Sheet S05) and loaded a post-nuclear supernatant (PNS) on to 20-40% iodixanol gradient. No other details about the gradient were given. The principal aim of their studies was to determine the localization of acyl-CoA synthetase 4. The gradient fractions were analyzed for wide range of markers including: acyl-CoA synthetase 1 (ER located); mitochondria were identified by acyl-CoA synthetase 5, glutamate dehydrogenase and glycerol-3-phosphate acyltransferase. MAM was identified by phosphatidylethanolamine methyltransferase. The gradient was able to resolve peroxisomes, mitochondria, MAM and endoplasmic reticulum. An approximate indication of the distribution of the major membrane compartments that were analyzed is given in Figure 1.
In studies by Myhill et al [4], HeLa cells were homogenized in a routine HEPES- buffered 0.25 M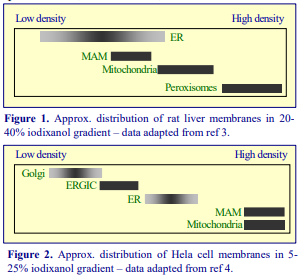 sucrose solution containing 1 mM EDTA, using a ball-bearing homogenizer. A PNS was loaded on to a continuous 5-25% (w/v) iodixanol gradient (produced by diffusion from a 5%-interval step gradient) at approx. 120,000 g for 3 h. An approximate indication of the distribution of the major membrane compartments that were analyzed is given in Figure 2. A very noticeable difference between the two patterns is the relative banding positions of MAM to the ER and mitochondria. In the case of rat liver the MAM was well resolved from the mitochondria but overlapped the ER, while in the case of HeLa cells the MAM was well resolved from the ER but co-banded with the mitochondria. It is not clear if this is a distinction between the two sources or the difference in the density range of the iodixanol gradient, or both. The 20-40% (w/v) iodixanol gradient used by Lewin et al [3] covers the range 1.127-1.223 g/ml while that used by Myhill et al [4] was 1.054 1.151 g/ml. The latter gradient has also been used to study (a) the localization of the ER oxidoreductin (Ero1) to MAM in HEK cells, which was found the be dependent on the oxidizing conditions in the ER [5] and (b) that in HeLa cells Rab32 regulates the properties of MAM, notably Ca2+ and the enrichment of calnexin [6]. Discontinuous gradients of 10-30% (w/v) iodixanol, similar to those used by Myhill et al [4] and Gilady et al [5] also resolved Golgi, ER and MAM [7] and revealed that palmitoylation of the trans-membrane thioredoxin family protein (TMX) and calnexin influence their enrichment in MAM. Moreover the palmitoylation of calnexin influenced its functional properties [8].
sucrose solution containing 1 mM EDTA, using a ball-bearing homogenizer. A PNS was loaded on to a continuous 5-25% (w/v) iodixanol gradient (produced by diffusion from a 5%-interval step gradient) at approx. 120,000 g for 3 h. An approximate indication of the distribution of the major membrane compartments that were analyzed is given in Figure 2. A very noticeable difference between the two patterns is the relative banding positions of MAM to the ER and mitochondria. In the case of rat liver the MAM was well resolved from the mitochondria but overlapped the ER, while in the case of HeLa cells the MAM was well resolved from the ER but co-banded with the mitochondria. It is not clear if this is a distinction between the two sources or the difference in the density range of the iodixanol gradient, or both. The 20-40% (w/v) iodixanol gradient used by Lewin et al [3] covers the range 1.127-1.223 g/ml while that used by Myhill et al [4] was 1.054 1.151 g/ml. The latter gradient has also been used to study (a) the localization of the ER oxidoreductin (Ero1) to MAM in HEK cells, which was found the be dependent on the oxidizing conditions in the ER [5] and (b) that in HeLa cells Rab32 regulates the properties of MAM, notably Ca2+ and the enrichment of calnexin [6]. Discontinuous gradients of 10-30% (w/v) iodixanol, similar to those used by Myhill et al [4] and Gilady et al [5] also resolved Golgi, ER and MAM [7] and revealed that palmitoylation of the trans-membrane thioredoxin family protein (TMX) and calnexin influence their enrichment in MAM. Moreover the palmitoylation of calnexin influenced its functional properties [8].
- Reviews of some of the methodology for the study of MAM is provided in refs 9 and 10.
- Iodixanol gradients have also been used for the clear resolution of SER, principally from lysosomes, but also other organelles such as peroxisomes and mitochondria, subsequent to an initial sucrose gradient fractionation. The methodology, developed by Radhakrishnan et al for CHO-K1 cells [11,12], has been extended to HEK cells [13-15], Niemann-Pick type C cells [16], HepG2 cells [17], HeLa cells [18] and mouse liver [19]
- For more recent publications on MAM see refs 20 and 21.
3. ER-Golgi transport (COPII containing vesicles)
Iodixanol gradients are able to isolate the donor membrane vesicles that bud from the endoplasmic reticulum: Gorur et al [22] removed the intact ER membranes by sedimentation at 7000 g and subsequently concentrated the vesicles by flotation from the supernatant (adjusted to 22% iodixanol) layer through an upper 18% iodixanol layer (250,000 g for 90 min). The total gradient volume was <0.25 ml. On a larger scale Ding et al [23] layered 3 ml of a post-nuclear supernatant over a 9 ml 5-30% iodixanol gradient (200,000 g for 4 h) separating the dense calnexin-containing ER from the much lighter COPII vesicles. Recent publications on these ER-Golgi interactions can be found in refs 24 and 25.
4. Lipid droplets
Lipid droplets have been observed to be associated with the ER for over thirty years but it is only relatively recently that they have been shown to be involved in viral infection and a number of lipidassociated diseases. Presently, rather few papers have been published in which an OptiPrep-based method has been used in their isolation. Because of the growing interest in these particles however a short summary of the methodology is included here. The most complete information comes from a paper by Heid et al [26]. Human hepatocellular carcinoma cells were homogenized by nitrogen cavitation and the PNS was adjusted to 30% (w/v) iodixanol. It was overlaid with layers of 20% and 10% iodixanol and centrifuged either at 190,000 g for 3 h or 220,000g for 2 h. The lipid droplets were concentrated close to the top of the gradient. The same three layer flotation gradient was adopted by Suzuki et al [27] for HeLa cells and by Akil et al [28] for Huh7 cells; the centrifugation conditions were however rather different: 166,000 g for 5 h and 200,000 g for 16 h respectively. A similar flotation approach was used by Buers et al [29] for studies on macrophages, the gradient however spanned a higher density range, it comprised 40%, 30% and 20% (w/v) iodixanol, but a much reduced g-force of 10,000 g for only 1h. In all cases the lipid droplets were recovered from the top of the gradient. Flotation through 10%, 20%, 30% (w/v) iodixanol discontinuous gradients has also been reported in refs 30-33. More recently Jayson et al [34], Hashani et al [35] and Schott et al [36], using iodixanol gradients, have investigated the lipid droplets from human mammary carcinoma cells, skeletal muscle tissue and mouse hepatocytes respectively.
5. References
1. Woods, A.J., Roberts, M.S., Choudhary, J., Barry, S.T., Mazaki, Y., Sabe, H., Morley, S.J., Critchley, D.R. and Norman, J.C. (2002) Paxillin associates with poly(A)-binding protein 1 at the dense endoplasmic reticulum and the leading edge of migrating cells J. Biol. Chem., 277, 6428-6437
2. Lynes, E.M. and Simmen, T. (2011) Urban planning of the endoplasmic reticulum (ER): How diverse mechanisms segregate the many functions of the ER Biochim. Biophys. Acta, 1813, 1893–1905
3. Lewin T.M., Van Horn, C.G., Krisans, S.K. and Coleman, R.A. (2002) Rat liver acyl-CoA synthetase 4 is a peripheral-membrane protein located in two distinct subcellular organelles, peroxisomes and mitochondrialassociated membranes Arch. Biochem. Biophys., 404, 263-270
4. Myhill, N., Lynes, E.M., Nanji, J.A., Blagoveshchenskaya, A.D., Fei, H., Simmen, K.C., Cooper, T.J., Thomas, G. and Simmen, T. (2008) The subcellular distribution of calnexin is mediated by PACS-2 Mol. Biol. Cell, 19, 2777-2788
5. Gilady, S.Y., Bui, M., Lynes, E.M., Benson, M.D., Watts, R., Vance, J.E. and Simmen, T. (2010) Ero1α requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM) Cell Stress Chaperones, 15, 619–629
6. Bui, M., Gilady, S.Y., Fitzsimmons, R.E.B., Benson, M.D., Lynes, E.M., Gesson, K., Alto, N.M., Strack, S., Scott, J.D. and Simmen, T. (2010) Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties J. Biol. Chem., 285, 31590–31602
7. Lynes, E.M., Bui, M., Yap, M.C., Benson, M.D., Schneider, B., Ellgaard, L., Berthiaume, L.G. and Simmen, T. (2012) Palmitoylated TMX and calnexin target to the mitochondria-associated membrane EMBO J., 31, 457–470
8. Lynes, E.M., Raturi, A., Shenkman, M., Sandoval, C.O., Yap, M.C., Wu, J., Janowicz, A., Myhill, N., Benson, M.D., Campbell, R.E., Berthiaume, L.G., Lederkremer, G.Z. and Simmen, T. (2013) Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling J. Cell Sci., 126, 3893–3903
9. Marchi, S., Patergnani, S. and Pinton, P. (2014) The endoplasmic reticulum–mitochondria connection: One touch, multiple functions Biochim. Biophys. Acta, 1837, 461–469
10. Giacomello, M. and Pellegrini, L. (2016) The coming of age of the mitochondria–ER contact: a matter of thickness Cell Death Differentiat., 23, 1417–1427
11. Radhakrishnan, A., Goldstein, J.L., McDonald, J.G. and Brown, M.S. (2008) Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance Cell Metab., 8, 512-521
12. Sokolov, A. and Radhakrishnan, A. (2010) Accessibility of cholesterol in endoplasmic reticulum membranes and activation of SREBP-2 switch abruptly at a common cholesterol threshold J. Biol. Chem., 285, 29480-29490
13. Harrison, K.D., Miao, R.Q., Fernandez-Hernándo, C., Suárez, Y., Dávalos, A. and Sessa, W.C. (2009) NogoB receptor stabilizes Niemann-Pick type C2 protein and regulates intracellular cholesterol trafficking Cell Metab., 10, 208–218
14. Alexia, C., Poalas, K., Carvalho, G., Zemirli, N., Dwyer, J., Dubois, S.M., Hatchi, E.M., Cordeiro, N., Smith, S.S., Castanier, C., Le Guelte, A., Wan, L., Kang, Y., Vazquez, A., Gavard, J., Arnoult, D. and Bidère, N. (2013) The endoplasmic reticulum acts as a platform for ubiquitylated components of nuclear factor kB signaling Sci. Signal., 6(291), ra79
15. Kawaguchi, K., Okamoto, T., Morita, M. and Imanaka, T. (2016) Translocation of the ABC transporter ABCD4 from the endoplasmic reticulum to lysosomes requires the escort protein LMBD1 Sci. Rep., 6: 30183
16. Abi-Mosleh, L., Infante, R.E., Radhakrishnan, A., Goldstein, J.L. and Brown, M.S. (2009) Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells Proc. Natl. Acad. Sci., 106, 19316–19321
17. Wang, Y., Lam, W., Chen, S-R., Guan, F-L., Dutchman, G.E., Francis, S., Baker, D.C. and Cheng, Y-C. (2016) Tylophorine analog DCB-3503 inhibited cyclin D1 translation through allosteric regulation of heat shock cognate protein 70 Sci. Rep., 6: 32832
18. Ferencz, C-M., Guigas, G., Veres, A.,Neumann, B., Stemmann, O. and Weiss, M. (2016) Shaping the endoplasmic reticulum in vitro Biochim. Biophys. Acta, 1858, 2035–2040
19. Hager, L., Li, L., Pun, H., Liu, L., Hossain, M.A., Maguire, G.F., Naples, M., Baker, C., Magomedova, L., Tam, J., Adeli, K., Cummins, C.L., Connelly, P.W. and Ng, D.S. (2012) Lecithin:cholesterol acyltransferase deficiency protects against cholesterol-induced hepatic endoplasmic reticulum stress in mice J. Biol. Chem., 287, 20755–20768
20. Shapovalov, G., Ritaine, A., Bidaux, G., Slomianny, C., Borowiec, A-S., Gordienko, D., Bultynck, G.,
Skryma, R. and Prevarskaya, N. (2017) Organelle membrane derived patches: reshaping classical methods for new targets Sci. Rep., 7: 14082
21. Ivanova, I.G. and Perkins, N.D. (2019) Hypoxia induces rapid, STAT3 and ROS dependent, mitochondrial translocation of RelA(p65) and IκBα Biosci. Rep., 39: BSR20192101
22. Gorur, A., Yuan, L., Kenny, S.J., Baba, S., Xu, K. and Schekman, R. (2017) COP II-coated membranes function as transport carriers of intracellular procollagen I J. Cell Biol., 216, 1745–1759
23. Ding, J., Shao, L., Yao, Y., Tong, X., Liu, H., Yue, S., Xie, J. and Cheng, S.Y. (2017) DGKδ triggers
endoplasmic reticulum release of IFT88-containing vesicles destined for the assembly of primary cilia Sci. Rep., 7: 5296
24. Melville, D., Gorur, A. and Schekman, R. (2019) Fatty-acid binding protein 5 modulates the SAR1 GTPase cycle and enhances budding of large COPII cargoes Mol. Biol. Cell 30, 387-399
25. Matsuda-Lennikov, M., Biancalana, M., Zou, J., Ravell, J.C., Zheng, L., Kanellopoulou, C., Jiang, P.,
Notarangelo, G., Jing, H. et al (2019) Magnesium transporter 1 (MAGT1) deficiency causes selective defects in N-linked glycosylation and expression of immune-response genes J. Biol. Chem., 294, 13638–13656
26. Heid, H., Rickelt, S., Zimbelmann, R., Winter, S., Schumacher, H. and Dorflinger, Y. (2013) Lipid droplets, perilipins and cytokeratins – unravelled liaisons in epithelium-derived cells PLoS One, 8: e63061
27. Suzuki, M., Murakami, T., Cheng, J., Kano, H., Fukata, M. and Fujimoto, T. (2015) ELMOD2 is anchored to lipid droplets by palmitoylation and regulates adipocyte triglyceride lipase recruitment Mol. Biol. Cell, 26, 2333-2342
28. Akil, A., Peng, J., Omrane, M., Gondeau, C., Desterke, C., Marin, M., Tronchère, H., Taveneau, C., Sar, S. et al (2016) Septin 9 induces lipid droplets growth by a phosphatidylinositol-5-phosphate and microtubuledependent mechanism hijacked by HCV Nat. Comm., 7: 12203
29. Buers, I., Robenek, H., Lorkowski, S., Nitschke, Y., Severs, N.J. and Hofnagel, O. (2009) TIP47, a lipid cargo protein involved in macrophage triglyceride metabolism Arterioscler. Thromb. Vasc. Biol., 29, 767-773
30. Vogt D.A., Camus, G., Herker, E., Webster, B.R., Tsou, C.L., Greene, W.C., Yen, T.S., and Ott, M. (2013). Lipid droplet-binding protein TIP47 regulates hepatitis C virus RNA replication through interaction with the viral NS5A protein PLoS Pathog, 9: e1003302.
31. Wong, M-T. and Chen, S.S. (2016) Human choline kinase- promotes hepatitis C virus RNA replication through modulation of membranous viral replication complex formation J. Virol., 90, 9075-9095
32. Boson, B., Denolly, S., Turlure, F., Chamot, C., Dreux, M. and Cosset, F-L. (2017) Daclatasvir prevents hepatitis C virus infectivity by blocking transfer of the viral Genome to assembly sites Gastroenterology, 152, 895-907
33. Gainullin, M.R., Zhukov, I.Y., Zhou, X., Mo, Y., Astakhova, L., Ernberg, I. and Matskova, L. (2017)
Degradation of cofilin is regulated by Cbl, AIP4 and Syk resulting in increased migration of LMP2A positive nasopharyngeal carcinoma cells Sci. Rep., 7: 9012
34. Jayson, C.B.K., Arlt, H., Fischer, A.W., Laia, Z.W., Farese, Jr. R.V.and Walther, T.C. (2018) Rab18 is not necessary for lipid droplet biogenesis or turnover in human mammary carcinoma cells Mol. Biol. Cell, 29, 2045-2054
35. Hashani, M., Witzel, H.R., Pawella, L.M., Lehmann-Koch, J., Schumacher, J., Mechtersheimer, G.,
Schnölzer, M., Schirmacher, P., Roth, W. and Straub, B.K. (2018) Widespread expression of perilipin 5 in normal human tissues and in diseases is restricted to distinct lipid droplet subpopulations Cell Tissue Res., 374: 121–136
36. Schott, M.B., Weller, S.G., Schulze, R.J., Krueger, E.W., Drizyte-Miller, K., Casey, C.A. and McNiven, M.A. (2019) Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes J. Cell Biol., 218, 3320–3335
OptiPrepTM Application Sheet S41; 4th edition, January 2020
OptiPrep™ Application Sheet S42
Endocytosis analysis – a review of density gradient methods
1. Nycodenz® gradients
1a. Long-spin Nycodenz® continuous gradients
Some of the first reports on the use of Nycodenz® for the fractionation of endosomes were published in the mid-nineteen eighties by Howard Evans and his co-workers, working at the National Institute for Medical Research in London. They worked primarily on the endocytosis of a variety of 125I-labelled asialo-glycoproteins by the perfused rat liver. After differential centrifugation of the homogenate, a supernatant from a light mitochondrial pellet was first applied to a continuous 15-43% (w/v) sucrose gradient (over layers of 43% and 70% sucrose) and centrifuged at 140,000 g for 3.5 h. The lightest fraction (1.095-1.117 g/ml) was re-centrifuged on a 13.8-27.6% (w/v) Nycodenz gradient at 110,000 g for 18 h. Two very distinct, well-separated peaks of radioactivity were obtained at 1.090 and 1.115 g/ml. The denser material was identified as early endosomes and the lighter as late endosomes [1].
1b. Short-spin Nycodenz® velocity gradients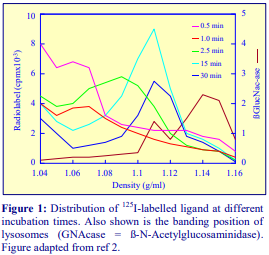
At about the same time, Trond Berg’s group at the University of Oslo, working primarily with isolated hepatocytes, developed a variety of Nycodenz® gradients for the analysis of ligand internalization. The gradients were continuous 0-45% (w/v) Nycodenz®, centrifuged at 85,000 g for a variety of times, with the sample either top- or bottom-loaded. Two principal times were chosen, either 5 h for buoyant density analysis or 45 min for ratezonal (sedimentation or flotation velocity) analysis. The top-loaded 45 min gradients showed that, with increasing internalization time (30 sec to 30 min), asialofetuin was
associated with membrane compartments of increasing sedimentation velocity [2], which were all distinct from the bulk of the lysosomes (see Figure 1). If the centrifugation was carried out for 3 h, so that the membrane vesicles reached their banding density, then there was only a relatively small difference between the peak density at 1 and 15 min incubation times (1.10 and 1.11 g/ml respectively). Using the 45 min gradient format, it was later shown, using a polyethylene glycolmodified asialofetuin, that the peak around 1.05-1.06 g/ml was early endosomes and that at 1.09 g/ml comprised multi-vesicular bodies; the latter were quite distinct from lysosomes at 1.14 g/ml [3].
1c. Hybrid Nycodenz®/polysucrose gradients
Branch et al [4] compared the efficacy of short-spin (1 h) continuous polysucrose and Nycodenz® gradients in the analysis of the membrane compartments in rat liver during the transcytosis of polymeric IgA and endocytosis of asialofetuin. In both instances a Beckman VTi50, vertical rotor was used at 206,000 g. The authors concluded that while polysucrose gradients were superior for resolving light (early) and dense (late) endosomes, Nycodenz® gradients provided far greater discrimination between late endosomes and lysosomes; moreover discrimination was achieved between lysosomes and very dense endosomes [5]. This led to the use of hybrid polysucrose-Nycodenz® gradients for the simultaneous isolation of early and late endosomes and lysosomes [6-9]; also, a simplified discontinuous gradient for the separation of lysosomes, very dense endosomes and other less dense endosomes was developed.
2. OptiPrep™
2a. Flotation velocity gradients
In 1997 the first paper reporting the use of an iodixanol gradient in endocytic studies was published by Orlandi [10]; the bottom-loaded 9-30% (w/v) iodixanol gradient centrifuged at 52,000 g for 90 min was similar to the Nycodenz® flotation velocity gradient (see Section 1b) of Kindberg et al [2]. It was used to analyze the internalization of cholera toxin by Caco-2 cells and over a period of 1 h the toxin peak showed a very clear shift from approx. 1.11 (0 min) to 1.12 (30 min) to 1.13 (60 min) g/ml.
2b. Overnight buoyant density gradients
A gradient system that has been widely used is that of Sheff et al [11]. In a study of the internalization of transferrin by MDCK cells transfected with the human Tfn receptor a shallow 5-20% (w/v) iodixanol gradient centrifuged at 100,000 g for 18 h was used to analyze a post-nuclear supernatant. Although many workers take advantage of the availability of high-performance rotors and the much lower viscosity of iodixanol gradients (compared to those of sucrose) to carry out buoyant density separations in 2-3 h, there is a widespread view that for the highest resolution of particles relatively low g-forces for long times are to be preferred. Sheff et al [11] were able to identify clearly a separation of early (EE) from recycling endosomes (RE), which were both well separated from the plasma membrane (PM) and lysosomes, which banded at lower densities (Figure 2). It is important to note however that there may be significantly different patterns of banding in the case of other studies with other cell types. For example, in both HeLa [12] and COS-7 cells [13] EE banded at a lower density than RE, while the HeLa cell PM [12] banded close to the bottom of the gradient. LAMP-2 positive particles (late endosomes (LE) and lysosomes) from PC12 cells [14] and HeLa cells [15] banded at a higher density than the Rab5 positive EE. The banding patterns may also be influenced by practical variations in the handling of the cell homogenization. In a study on the effect of moesin on receptor recycling in HeLa cells [16], this gradient system revealed two well separated bands of Rab7, the lighter one of which overlapped the lower density Rab5-containing vesicles (Figure 3). Barroso-González et al [16] compared control “scrambled” cells (nucleofected with scrambled oligonucleotides) and test cells nucleofected with siRNA moesin oligonucleotides. The TfR, which was located predominantly between the two Rab7 bands from control cells, shifted significantly into the lowdensity region in the siRNA moesin oligonucleotide-treated cells (Figure 3). A very detailed analysis of the membranes from monkey kidney cells on an 8-25% (w/v) iodixanol gradient [17] identified (in increasing banding density) PM, lysosomes, endosomes + Golgi and two distinct bands of endoplasmic reticulum (ER). McKenzie et al [17] used the gradient to study the internalization of Shiga toxin (and its B subunit) and detected a single toxin-rich band in the Golgi + endosomes region; AlF4 – treatment caused a pronounced dichotomy of the band. In an unrelated study Woods et al [18] also reported the resolution of two distinct fractions of ER from 3T3 cells, the denser of which was identified as perinuclear. Other uses of the long-spin continuous gradient are given in Table 1.

Idkowiak-Baldys et al [23] used a similar long-spin, low g-force strategy but started with a discontinuous 5%, 10%, 15% and 20% iodixanol gradient; although this will have become continuous during the centrifugation there may be small important differences in the final density profile when compared with a preformed continuous gradient. In HEK cells the PM and ER were concentrated at the light and dense ends respectively, while LAMP1-1 positive late endosomes banded about 2/3rds down the gradient and three distinct Rab11 areas were observed (Figure 4). In phorbol-ester treated cells, protein kinase C, which was broadly distributed in control cells, became markedly and distinctively concentrated in the lightest of the Rab11 bands.
- In contrast to iodixanol gradients, Percoll® gradients cannot be centrifuged for long time periods
 at the g-forces required for effective membrane fractionation without most of colloidal silica pelleting; thus its use in endocytosis studies is very restrictive. In studies on the trafficking of LDL cholesterol Percoll® gradients were unable to resolve PM and EE from CHO cells [24]; long-spin iodixanol gradients were able to exhibit distinctive banding patterns not only for PM and EE, but also LE and Golgi.
at the g-forces required for effective membrane fractionation without most of colloidal silica pelleting; thus its use in endocytosis studies is very restrictive. In studies on the trafficking of LDL cholesterol Percoll® gradients were unable to resolve PM and EE from CHO cells [24]; long-spin iodixanol gradients were able to exhibit distinctive banding patterns not only for PM and EE, but also LE and Golgi.
2c. Short-spin continuous density gradients
Gradients covering more or less the same density as those described in 2b, when centrifuged for only 3 h at 100-130,000 g can provide excellent separation of EE from PM and Golgi from HeLa and HEK cells [25,26]. In the case of human HT1080 (human sarcoma) cells two LE fractions were identified; moreover chloroquine treatment of the cells induced a pronounced shift of Rab9 to the denser LE [27].
2d. Double-gradient strategy
An initial sedimentation velocity gradient followed by a second buoyant density gradient enabled Lin et al [28] to achieve very fine fractionation of light endosomes fractions from PC12 cells, in their studies on the internalization of nerve growth factor. To obtain a membrane vesicle fraction the cells were initially permeabilized by a single passage through a ball-bearing homogenizer. Semi-intact cells and larger membrane particles were removed by low-speed centrifugation. The supernatant was first layered on top of a 0-30% (w/v) iodixanol gradient and centrifuged at 133,000 g for 1.5 h. Fractions from this gradient were adjusted to 32.5% iodixanol by mixing with OptiPrep™; layered under a second 0-30% iodixanol gradient and centrifuged at the same speed for 18 h. The second fraction from the velocity gradient was resolved into two completely distinct non-overlapping sub-fractions; the lighter contained the neurotrophin receptor pTrkA plus its associated proteins APPL1 and GIPC1; the denser only contained APPL1. More recently [29] the same double-gradient strategy was able to resolve three distinct subclasses of endosomes: (1) those bearing activated receptor tyrosine kinases, (2) those containing p75NTR (tumor necrosis receptor superfamily) and (3) those exhibiting PAC1 (a G proteincoupled receptor).
 2e. Self-generated gradients
2e. Self-generated gradients
The use of self-generated gradients was first reported by Billington et al [30] for analysis of the internalization of asialoglycoproteins by the perfused rat liver. One of its attractions is its simplicity and reproducibility of gradient formation. After homogenization of the liver, a 3000 g supernatant was simply adjusted to 12.5% (w/v) iodixanol, underlaid with 1 ml of 20% iodixanol and centrifuged in a vertical or near-vertical rotor at 350,000 gav for 1.5 h. The results of a 1 min pulse of labelled ligand after various chase times are shown in Figure 5. The data is interpreted as follows: (1 min chase) the ligand is found initially in a dense clathrin-coated vesicle; (2 min) uncoating of the vesicle reduces its density; (10 min) the ligand has been transferred to a low-density endosome and (20 min) the ligand appears in a lysosomal or pre-lysosomal compartment. Sometimes the gradient is generated not from an iodixanol solution of uniform density but from a discontinuous iodixanol gradient in of 10%, 20% and 30% (w/v) iodixanol (with the sample in the latter). Centrifugation for 3 h will produce a more or less linear gradient (that produced from a single concentration will tend to be shallower towards the top and steeper towards the bottom). This tri-layered gradient has an additional feature; all of the cytosolic proteins remain at the bottom of the gradient. It was used to investigate the distribution of Ra1A (a GTPase which interacts with the exocyst complex) that resides in recycling endosomes. The Ra1A in COS cells overlapped only the denser parts of the TfR and Rab11 region, but not with EEA1, syntaxin 6 or the cytosolic Akt. Indeed the best coincidence was observed with another recycling endosome marker – Rab4, which was clearly associated with a denser particle than Rab11 [31]. This gradient format has also been used for studying the changes induced by the adenovirus early region 4 open reading frame 4 protein in 293T cells [32].
- See Reference List RS-12 for a complete bibliography of all the published papers that have reported gradients prepared from OptiPrep™ for the analysis of all aspects of endocytosis and trafficking within the endosomal system. To access return to the initial list of Folders and select “Reference Lists”.
- There are several relevant OptiPrep Application Sheets that provide detailed protocols:
- Cultured cells – buoyant density: Application Sheet S46
- Rat liver/hepatocytes – lysosome/late-endosome events: Application Sheet S54
- Rat liver/hepatocytes – sedimentation velocity gradients: Application Sheet S44
- Clathrin-coated vesicles/endosomes/lysosomes (self-generated gradient): Application Sheet S45
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- It is not known if all or any of the Nycodenz®-based methods can be transposed directly to iodixanol. Nycodenz® and iodixanol solutions of the same % (w/v) have approximately the same density but the former have a higher osmolality (see Application Sheets for more information).
3. References
1. Evans, W.H. and Flint, N. (1985) Subfractions of hepatic endosomes in Nycodenz gradients and by freeflow electrophoresis Biochem. J., 232, 25-32
2. Kindberg, G.M., Ford, T., Blomhoff, R., Rickwood, D. and Berg, T. (1984) Separation of endocytic vesicles in Nycodenz gradients Anal. Biochem., 142, 455-462
3. Roseng, I., Tolleshaug, H. and Berg, T. (1992) Uptake, intracellular transport, and degradation of polyethylene glycol-modified asialofetuin in hepatocytes J. Biol. Chem., 257, 22987-22993
4. Branch, W.J., Mullock, B.M. and Luzio, J.P. (1987) Rapid subcellular fractionation of the rat liver endocytic compartments involved in transcytosis of polymeric immunoglobulin A and endocytosis of asialofetuin Biochem. J., 244, 311-315
5. Perez, J. H., Branch, W. J., Smith, L., Mullock, B. M. and Luzio, J.P. (1988) Investigation of endosomal compartments involved in endocytosis and transcytosis of polymeric immunoglobulin A by subcellular fractionation of perfused isolated rat liver Biochem. J., 251, 763-770
6. Mullock, B. M., Perez, J. H., Kuwana, T., Gray, S. R. and Luzio, J. P. (1994) Lysosomes can fuse with a late endosomal compartment in a cell-free system from rat liver J. Cell Biol., 126, 1173-1182
7. Mullock, B. M., Bright, N. A., Fearon, C. W., Gray, S. R. and Luzio, J. P. (1998) Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent J. Cell. Biol., 140, 591-601
8. Mullock, B. M., Smith, C. W., Ihrke, G., Bright, N. A., Lindsay, M., Parkinson, E. J., Brooks, D. A. Parton, R. G., James, D. E., Luzio, J. P. and Piper, R. C. (2000) Syntaxin 7 is localized to late endosome compartments, associates with Vamp 8, and is required for late endosome-lysosome fusion Mol. Biol. Cell 11, 3137-3153
9. Pryor, P. R., Mullock, B. M., Bright, N. A., Gray, SD. R. and Luzio, J. P. (2000) The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles J. Cell Biol., 149, 1053-1062
10. Orlandi, P.A.(1997) Protein-disulfide isomerase-mediated reduction of the A subunit of cholera toxin in a human intestinal cell line J. Biol. Chem., 272, 4591-4599
11. Sheff, D.R., Daro, E.A., Hull, M. and Mellmann, I. (1999) The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions J. Cell Biol., 145, 123-139
12. Meyers, J.M. and Prekeris, R. (2002) Formation of mutually exclusive Rab11 complexes with members of the family of Rab11-interacting proteins regulates Rab11 endocytic targeting and function J. Biol. Chem., 277, 49003-49010
13. Shen, X., Xu, K-F., Fan, Q., Pacheco-Rodriguez, G., Mos, J. and Vaughan, M. (2006) Association of
brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) with recycling endosomes during transferring uptake Proc. Natl. Acad. Sci. USA, 103, 2635-2640
14. Li, Y., Chin, L-S., Levey, A.L. and Li, L. (2002) Huntingtin-associated protein 1 interacts with hepatocyte growth factor-regulated tyrosine kinase substrate and functions in endosomal trafficking J. Biol. Chem., 277, 28212-28221
15. Chin, L-S., Raynor, M.C., Wei, X., Chen, H-Q. and Li, L. (2001) Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor J. Biol. Chem., 276, 7069-7078
16. Barroso-González, J., Machado, J-D., García-Expósito, L. and Valenzuela-Fernández, A. (2009) Moesin regulates the trafficking of nascent clathrin-coated vesicles J. Biol. Chem., 284, 2419–2434
17. McKenzie, J., Johannes, L., Taguchi, T.and Sheff, D. (2009) Passage through the Golgi is necessary for Shiga toxin B subunit to reach the endoplasmic reticulum FEBS J., 276, 1581–1595
18. Woods, A.J., Roberts, M.S., Choudhary, J., Barry, S.T., Mazaki, Y., Sabe, H., Morley, S.J., Critchley, D.R. and Norman, J.C. (2002) Paxillin associates with poly(A)-binding protein 1 at the dense endoplasmic reticulum and the leading edge of migrating cells J. Biol. Chem., 277, 6428-6437
19. Wiesinger, J.A., Buwen, J.P., Cifelli, C.J., Unger, E.L., Jones, B.C. and Beard, J.L. (2007) Downregulation of dopamine transporter by iron chelation in vitro is mediated by altered trafficking, not synthesis J. Neurochem., 100, 167-179
20. Manunta, M., Izzo, L., Duncan, R. and Jones, A.T. (2007) Establishment of subcellular fractionation techniques to monitor the intracellular fate of polymer therapeutics II: Identification of endosomal and lysosomal compartments in HepG2 cells combining single-step subcellular fractionation and fluorescent imaging J. Drug Target., 15, 37-50
21. Proikas-Cezanne, T., Gaugel, A., Frickey, T. and Nordheim, A. (2006) Rab14 is part of the early endosomal clathrin-coated TGN microdomain FEBS Lett., 580, 5241-5246
22. Mairhofer, M., Steiner, M., Salzer, U. and Prohaska, R. (2009) Stomatin-like protein-1 interacts with stomatin and is targeted to late endosomes J. Biol. Chem., 284, 29218-29229
23. Idkowiak-Baldys, J., Becker, K.P., Kitatani, K. and Hannum, Y.A. (2006) Dynamic sequestration of the recycling compartment by classical protein kinase C J. Biol. Chem., 281, 22321-22331
24. Sugii, S., Reid, P.C., Ohgami, N., Du, H. and Chang, T-Y. (2003) Distinct endosomal compartments in early trafficking of low density lipoprotein-derived cholesterol J. Biol. Chem., 278, 27180-27189
25. Tagami, S., Okochi, M., Yanagida, K., Ikuta, A., Fukumori, A., Matsumoto, N., Ishizuka-Katsura, Y., Nakayama, T., Itoh, N., Jiang, J., Nishitomi, K., Kamino, K., Morihara, T., Hashimoto, R., Tanaka, T., Kudo, T., Chiba, S. and Takeda, M. (2008) Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1 Mol. Cell. Biol., 28, 165-76
26. Fukumori, A., Okochi, M., Tagami, S., Jiang, J., Itoh, N., Nakayama, T., Yanagida, K., Ishizuka Katsura, Y., Morihara, T., Kamino, K., Tanaka, T., Kudo, T., Tanii, H., Ikuta, A., Haass, C. and Takeda, M. (2006) Presenilin-dependent -secretase on plasma membrane and endosomes is functionally distinct Biochemistry, 45, 4907-4914
27. Molle, D., Segura-Morales, C., Camus, G., Berlioz-Torrent, C., Kjems, J., Basyuk, E. and Bertrand, E. (2009) Endosomal trafficking of HIV-1 Gag and genomic RNAs regulates viral egress J. Biol. Chem., 284, 19727-19743
28. Lin, D.C., Quevedo, C., Brewer, N.E., Bell, A., Testa, J.R., Grimes, M.L., Miller, F.D. and Kaplan, D.R. (2006) APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction Mol. Cell. Biol., 26, 8928-8941
29. McCaffrey, G., Welker, J., Scott, J., van der Salm, L. and Grimes, M.L. (2009) High-resolution fractionation of signaling endosomes containing different receptors Traffic, 10, 938–950
30. Billington, D., Maltby, P.J. Jackson, A.P. and Graham, J.M. (1998) Dissection of hepatic receptormediated endocytic pathways using self-generated gradients of iodixanol (OptiPrep) Anal. Biochem., 258, 251-258
31. Chen, X-W., Inoue, M., Hsu, S. and Saltiel, A.R. (2006) RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis J. Biol. Chem., 281, 38609-38616
32. Landry, M-C., Sicotte, A., Champagne, C. and Lavoie, J.N. (2009) Regulation of cell death by recycling endosomes and Golgi membrane dynamics via a pathway involving Src-family kinases, Cdc42 and Rab11a Mol Biol. Cell, 20, 4091-4106
OptiPrepTM Application Sheet S42 6th edition, January 2020
OptiPrep™ Application Sheet S43
Lysosomes – a methodological review
- THIS APPLICATION SHEET SUMMARIZES MANY OF THE POSSIBLE METHOD OPTIONS FOR DIFFERENTIAL AND GRADIENT CENTRIFUGATION
- APPLICATION SHEET S55 PROVIDES A DETAILED METHODOLOGY
- SEE REFERENCE LIST RS04 FOR A COMPLETE LYSOSOME BIBLIOGRAPHY
METHODOLOGICAL REVIEW
1. Homogenization
Tissues are generally homogenized (in a Potter-Elvehjem apparatus) in buffered 0.25 M sucrose usually, but not invariably, containing 1 mM EDTA. The buffer (Figure 1) is usually 10-20 mM Tris-HCl or Hepes-NaOH. For cultured cells the homogenization medium (HM) is rather more variable; with lymphoid cells for example the buffered sucrose medium contained 1 mM MgCl2 [1] and the homogenizer may be a ball-bearing device [1], nitrogen cavitation vessel [2] or Dounce homogenizer [2]. It is generally accepted that for cultured cells the ball-bearing device provides the necessary gentle conditions suited to intact lysosome recovery.
2. Differential centrifugation
The production of a light mitochondrial pellet (L) by differential centrifugation that is described in Figure 1 is based on the scheme originally published by de Duve et al [3], but there are many variations on this theme. The first phase (green in Figure 1) or second phase (blue in Figure 1) is sometimes omitted; sometimes both blue and magenta phases are omitted and the gradient is thus loaded with a post-nuclear supernatant. Additionally the g-forces and/or times for one or more of the phases may vary from those given in Figure 1. For example the L pellet from lymphoid cells has been recovered at 22,000 g for 60 min [1] or 17,000 g for 20 min [4]. Occasionally the differential centrifugation follows a rather different format: for example Klein et al [5-7] first centrifuged a liver homogenate at 10,700 g for 20 min; the pellet was resuspended in HM and centrifuged at 120 g for 10 min. This nuclear pellet was washed twice and the combined supernatants were centrifuged at 23,000 g for 10 min. This L pellet was washed twice before being resolved on a Nycodenz® gradient. The authors used the supernatant from the first centrifugation as a source of microsomes and cytosol and for comparative analytical purposes this approach has much to recommend it. In the more widely-used sequence of centrifugations shown in Figure 1 soluble and fragmented material derived from partial disruption of organelles during manipulation of the N, M and L pellets will eventually end up in the microsomal supernatant.
- The L pellet is further resolved by a variety of gradient strategies and these are reviewed in Section 3.
3. Discontinuous – bottom-loaded
In sucrose gradients the buoyant density banding positions of lysosomes and mitochondria are too close to permit a useful separation, consequently it became common to use a strategy first described by Leighton et al [8] in 1968 in which the density of rat liver lysosomes was artificially reduced by prior administration of Triton WR1339 to the animals. However a number of functional changes in the lysosomes are caused by the detergent. In the late nineteen-seventies Wattiaux’s group at the University of Namur in Belgium investigated the alternative use of the first non-ionic iodinated density gradient medium – metrizamide – for the fractionation of subcellular organelles from the light mitochondrial fraction from rat liver [9,10]. These workers showed that the resolution of lysosomes from the denser mitochondria was much improved if the light mitochondrial fraction was layered in a dense solution beneath the gradient rather than layered on top of the gradient. For routine preparation of lysosomes from rat liver a bottom-loaded discontinuous gradient was recommended. More recently metrizamide, which is now commercially unavailable, was replaced by Nycodenz® [10-13] and it is this method that is summarized in Figure 1. In a typical experiment using rat liver the enrichment of a lysosomal marker such as N-acetyl-β-glucosaminidase in the 20%/25% Nycodenz® band is approx. 100-fold over the homogenate [10,11]. The method as has also been applied to HepG2 cells [14]. Organelles, particularly mitochondria, are very sensitive to hydrostatic pressure and there are many examples of mitochondrial purification methods using discontinuous gradients in which the crude organelle fraction is loaded in a median layer rather than bottom-loaded in order to reduce the hydrostatic pressure on the sample. This strategy was first used by Okado-Matsumoto and Fridovich [15]. It may be good practice to reduce the hydrostatic pressure in all flotation fractionations of the light mitochondrial pellet. Cabrita et al [16] used 40%, 30%, 25%, 23%, 20%, 15% and 10% (w/v) Nycodenz® (0.5, 1.0, 3.5, 2.0, 2.0, 2.0 and 1.0 ml respectively) with the light mitochondrial pellet in the 25% layer; the lysosomes from rat liver banded at the 15%-20% Nycodenz® interface. The centrifugation conditions were also very mild – 52,000 g for 90 min. Another means of reducing the hydrostatic pressure on the sample is to use a vertical rotor rather than a swinging-bucket rotor.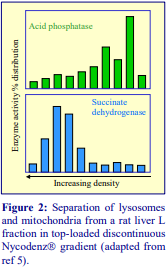
- Although no published papers have reported the use of OptiPrep™ in this flotation mode, there is no obvious reason why this would not be effective.
4. Discontinuous gradients – top-loaded
An L fraction from rat liver (in 2 ml of HM) layered over 24%, 27%, 28%, 33% and 40% (w/v) Nycodenz® (2 ml, 2 ml, 3 ml, 1 ml and 1 ml respectively) and centrifuged at 74,000 g for 3 h provided a very useful separation of mitochondria and lysosomes [5-7] as s described in Figure 2. Marshall et al [2] employed a novel discontinuous gradient in which the low-density layer contained 6% Percoll and the two denser layers comprised 17% and 35% Nycodenz. Moreover these workers highlighted two problems that are often overlooked; in particular, with cultured cells the homogenization strategy often needs to be tailored to the cell type and the organelles from different cell types may behave distinctively in gradients. Human breast carcinoma cells were homogenized using nitrogen cavitation, while human T-cell leukaemia cells were lysed in a Dounce homogenizer. Moreover while the lysosomes from breast carcinoma cells banded at the 17%-33% Nycodenz interface, those from the leukaemia cells banded at the 6% Percoll-17% Nycodenz interface [2]. Optimization of the centrifugation conditions may also be needed; at 20,000 g breast carcinoma cell lysates required 20 min, leukaemia cells – 30 min. Whether this organelle density difference is a consequence of the cell type or the mode of homogenization, or both, is not known. Iodixanol gradients have been widely used in the top-loaded discontinuous gradient mode, usually covering a slightly lower density range. Layers of 17%, 20%, 23%, 27% and 30% (w/v) or 8, 12, 16, 19, 22.5 and 27% (w/v) iodixanol are quite common. Sometimes the crude fraction is a total cell lysate, sometimes a post-nuclear supernatant (PNS) and sometimes a light mitochondrial fraction (see Section 2). Table 1 lists the cell types that have been analyzed in these gradients and summarizes the gradient format.

In the 15%, 17%, 20%, 23%, 27%, 30% (w/v) iodixanol gradient format the sample (normally a light mitochondrial pellet) is usually in the 15% iodixanol layer. The centrifugation conditions vary rather widely: 50,000 g for 17 h [21]; 145,000 g for 2 h [27,28,30]; 150,000 g for 4 h [26,29]; 150,000 g for 5 h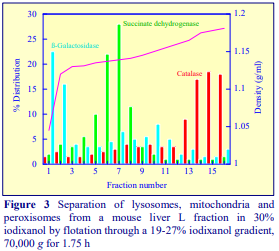 [22,25] and 100,000 g for 16 h [17]. The lysosomes are normally located in the top quarter of the gradient and the recoveries are very good; the lysosome fraction, recovered from the top of the gradient, contained over 80% of the total cathepsin D activity [27]. Lysosomes from osteoclasts have also been fractionated in a discontinuous gradient [31]. Over the longer periods of centrifugation the gradient will become essentially continuous.
[22,25] and 100,000 g for 16 h [17]. The lysosomes are normally located in the top quarter of the gradient and the recoveries are very good; the lysosome fraction, recovered from the top of the gradient, contained over 80% of the total cathepsin D activity [27]. Lysosomes from osteoclasts have also been fractionated in a discontinuous gradient [31]. Over the longer periods of centrifugation the gradient will become essentially continuous.
5. Continuous gradients
This methodology has been widely used with iodixanol. Graham et al [32] were the first to report this technology with mouse liver. A variety of density profiles were studied; an efficacious system comprises a 19-27% (w/v) iodixanol gradient, overlaid with HM and underlaid by the L fraction adjusted to 30% iodixanol. After 70,000 gav for 1.5-2 h the distribution of markers is as shown in Figure 3, with the lysosomes at the top of the gradient. Separations similar to those shown in Figure 3 have been obtained with an L-fraction from human breast carcinoma cells [33] loaded in 35% (w/v) under a 10-30% iodixanol gradient (52,000 g for 1.5 h) or top-loaded in 5% iodixanol [34]. A light mitochondrial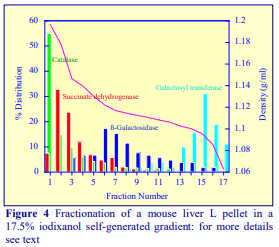 fraction from carcinoma cells has been fractionated on a 4-24% (w/v) iodixanol gradient; the g-force was only 20,000 g but the time was extended to 17 h [35], the lysosomes banded around 1.12 g/ml and the gradient was used in the localization of the KIF5B kinesin heavy chain protein. Higaki et al [36] fractionated a human skin fibroblast PNS on a continuous 5-20% (w/v) iodixanol graident (90,000 g for 20 h) that was first described by Sugii et al [37] in endocytosis studies.
fraction from carcinoma cells has been fractionated on a 4-24% (w/v) iodixanol gradient; the g-force was only 20,000 g but the time was extended to 17 h [35], the lysosomes banded around 1.12 g/ml and the gradient was used in the localization of the KIF5B kinesin heavy chain protein. Higaki et al [36] fractionated a human skin fibroblast PNS on a continuous 5-20% (w/v) iodixanol graident (90,000 g for 20 h) that was first described by Sugii et al [37] in endocytosis studies.
6. Self-generated gradients
Graham et al [32] were the first to demonstrate the usefulness of self-generated gradients of iodixanol to fractionate the light mitochondrial fraction from mouse liver. Self-generated gradients are simple to set up and the lack of any interfaces between the sample and the gradient reduces particulate aggregation. The L fraction is adjusted to, for example 17.5% (w/v) iodixanol, and centrifuged in a suitable rotor, either vertical, nearvertical or low-angle fixed-angle rotor. In the example in Figure 4 a fixed-angle rotor (10 ml tube, 20 angle) was used at 270,000 gav, for 3 h. A small tube volume (2 ml) Beckman TLV-100 vertical rotor allowed the centrifugation time to be reduced to 1.5 h for a light mitochondrial fraction from glioma cells [4]. The strategy also appears very successful with promyeloid [38] and lymphoma cells [39]; in both these cases the crude organelle fraction was adjusted to 20% iodixanol and the lysosomes recovered from close to the top of the gradient. This 20% (w/v) iodixanol gradient may be less successful in resolving the lysosomes from any Golgi membranes in the fraction but this will also depend on the density profile of the gradient, and that depends not only on the rotor type but also on the g-force and the centrifugation time. Beckman VTi65.2 vertical rotor (350,000 g for 3 h) and a Beckman NVT90 near-vertical rotor (320,000 g for 3 h) have also been used for these gradient separations for retinal epithelial cells [40] and Caco-2 cells [41] respectively. In the latter case the starting concentration of iodixanol was 30% and consequently the lysosomes banded close to the top of the gradient. A detailed description of the OptiPrep methodologies can be found from the relevant OptiPrep™ Applications Sheets index on the following website: www.Optiprep.com (click on “Methodology”, then “Organelles and Subcellular Membranes”) and scroll down the Index.
- Application Sheet S55 describes the use of discontinuous gradients
- Application Sheet S15 describes the use of continuous gradients
- Application Sheet S16 describes the use of self-generated gradients
- Application Sheet S04 describes the construction of self-generated gradients
7. References
1. Levade, T., Leruth, M., Graber, D., Moisand, A., Vermeersch, S., Salvayre, R. and Courtoy, P.J. (1996) In situ assay of acid sphingomyelinase and ceramidase based on LDL-mediated lysosomal targeting of ceramide-labeled sphingomyelin J. Lipid Res., 37, 2525-2538
2. Marshall, L.A., Rhee, M.S., Hofmann, L., Khodjakov, A. and Schneider, E. (2005) Increased lysosomal uptake of methotrexate-polyglutamates in two methotrexate-resistant cell lines with distinct mechanisms of resistance Biochem. Pharmacol., 71, 203-213
3. de Duve, C., Pressman, B.C., Gianetto, R., Wattiaux, R. and Appelmans, F. (1955) Tissue fractionation studies 6: intracellular distribution patterns of enzymes in rat liver tissues Biochem. J., 60, 604-617
4. Di Piazza, M., Mader, C., Geletneky, K., Herrero y Calle, M., Weber, E., Schlehofer, L. and Rommelaere, J. (2007) Cytosolic activation of cathepsins mediates parvovirus H-1-induced killing of cisplatin and TRIAL-resistant glioma cells J. Virol., 81, 4186-4198
5. Klein, D., Lichtmannegger, J., Heinzmann, U., Müller-Höcker, J., Michaelsen, S. and Summer, K.H.
(1998) Association of copper to metallothionein in hepatic lysosomes of Long-Evans cinnamon (LEC) rats during the development of hepatitis Eur. J. Clin. Invest., 28, 302-310
6. Klein, D., Lichtmannegger, J., Heinzmann, U. and Summer, K.H. (2000) Dissolution of copper-rich granules in hepatic lysosomes by D-penicillamine prevents the development of fulminant hepatitis in LongEvans cinnamon rats J. Hepatol., 32, 193-201
7. Klein, D., Lichtmannegger, J., Heinzmann, U. and Summer, K.H. (2000) Dissolution of copper-rich granules in hepatic lysosomes by D-penicillamine prevents the development of fulminant hepatitis in LongEvans cinnamon rats J. Hepatol., 32, 193-201
8. Leighton, F., Poole, B., Beaufay, H., Baudhuin, P., Coffey, J.W., Fowler, S. and de Duve, C. (1968) The large scale separation of peroxisomes, mitochondria and lysosomes from the livers of rats injected with Triton-WR1339 J. Cell Biol., 37, 482-513
9. Wattiaux, R., Wattiaux-De Coninck, S., Ronveaux-Dupal, M.F. and Dubois, F. (1978) Isolation of rat liver lysosomes by isopycnic centrifugation in metrizamide gradients J. Cell Biol., 78, 349-368
10. Wattiaux, R., Wattiaux-De Coninck, S. (1983) Separation of cell organelles In Iodinated density gradient media – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 119-137
11. Olsson, G.M., Svensson, I., Zdolsek, J.M. and Brunk, U.T. (1989) Lysosomal enzyme leakage during the hypoxanthine/xanthine oxidase reaction Virchows Arch. B Cell Pathol., 56, 385-391
12. Decharneux, T., Dubois, F., Beauloye, C., Wattiaux-De Coninck, S. and Wattiaux, R. (1992) Effect of various flavonoids on lysosomes subjected to an oxidative or an osmotic stress Biochem. Pharmacol., 44, 1243-1248
13. Enrich, C., Verges, M. and Evans, W.H. (1995) Functional identification of three major phosphoproteins in endocytic functions from rat liver Eur. J. Biochem., 231, 802-808
14. Jadot, M., Andrianaivo, F., Dubois, F. and Wattiaux, R. (2001) Effects of methylcyclodextrin on lysosomes Eur. J. Biochem., 268, 1392-1399
15. Okado-Matsumoto, A. and Fridovich, I. (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver J. Biol. Chem., 276, 38388-38393
16. Cabrita, M.A., Hobman, T.C., Hogue, D.L., King, K.M. and Cass, C.E. (1999) Mouse transporter protein, a membrane protein that regulates cellular multidrug resistance, is localized to lysosomes Cancer Res., 59, 4890-4897
17. Lee, J-H., Yu, W.H., Kumar, A., Lee, S., Mohan, P.S., Peterhoff, C.M., Wolfe, D.M., Martinez Vicente, M., Massey, A.C., Sovak, G., Uchiyama, Y., Westaway, D., Cuervo, A.M. and Nixon, R.A. (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations Cell, 141, 1146–1158
18. Xiao, M-F., Xu, J-C., Tereshchenko, Y., Novak, D., Schachner, M. Kleene, R. (2009) Neural cell adhesion molecule modulates dopaminergic signaling and behavior by regulating dopamine D2 receptor internalization J. Neurosci., 29, 14752-14763
19. Andreyeva, A., Leshchyns’ka, I., Knepper, M., Betzel, C., Redecke, L., Sytnyk, V. and Schachner, M. (2010) CHL1 is a selective organizer of the presynaptic machinery chaperoning the SNARE complex PLoS One, 5: e12018
20. Dehay, B., Bové, J., Rodríguez-Muela, N., Perier, C., Recasens, A., Boya, P. and Vila, M. (2010) Pathogenic lysosomal depletion in Parkinson’s disease J. Neurosci., 30, 12535–12544
21. Fehrenbacher, N., Bastholm, L., Kirkegaard-Sørenson, T., Rafn, B., Bøttzauw, T., Nielsen, C., Weber, E., Shirasawa, S., Kallunki, T. and Jäättelä (2008) Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-Associated membrane proteins 1 and 2 Cancer Res., 68, 6623-6633
22. Edelmann, B., Bertsch, U., Tchikov, V., Winoto-Morbach, S., Perrotta, C., Jakob, M., Adam-Klages, S., Kabelitz, D. and Schütze, S. (2011) Caspase-8 and caspase-7 sequentially mediate proteolytic activation of acid sphingomyelinase in TNF-R1 receptosomes EMBO J., 30, 379–394
23. Udelnow, A., Kreyes, A., Ellinger, S., Landfester, K., Walther, P., Klapperstueck, T., Wohlrab, J., HenneBruns, D., Knippschild, U. and Würl, P. (2011) Omeprazole inhibits proliferation and modulates autophagy in pancreatic cancer cells PLoS One, 6: e20143
24. Liu, L., Zhang, Z. and Xing, D. (2011) Cell death via mitochondrial apoptotic pathway due to activation of Bax by lysosomal photodamage Free Radic., Biol. Med., 51, 53–68
25. Schmidt, H., Gelhaus, C., Nebendahl, M., Lettau, M., Lucius, R., Leippe, M., Kabelitz, D. and Janssen, O. (2011) Effector granules in human T lymphocytes: proteomic evidence for two distinct species of cytotoxic effector vesicles J. Proteome Res., 10, 1603–1620
26. Oberle, C., Huai, J., Reinheckel, T., Tacke, M., Rassner, M., Ekert, P.G., Buellesbach, J. and Borner, C. (2010) Lysosomal membrane permeabilization and Cathepsin release is a Bax/Bak-dependent, amplifying event of apoptosis in fibroblasts and monocytes Cell Death Differ., 17, 1167–1178
27. Sevlever, D., Jiang, P. and Yen, S-H.C. (2008) Cathepsin D is the main lysosomal enzyme involved in the degradation of α-synuclein and generation of its carboxy-terminally truncated species Biochemistry, 47, 9678-9687
28. Wei, J., Fujita, M., Nakai, M., Waragai, M., Sekigawa, A., Sugama, S., Takenouchi, T., Masliah, E. and Hashimoto, M. (2009) Protective role of endogenous gangliosides for lysosomal pathology in a cellular model of synucleinopathies Am. J. Pathol., 174, 1891–1909
29. Sanborn, K.B., Rak, G.D., Maru, S.Y., Demers, K., Difeo, A., Martignetti, J.A., Betts, M.R., Favier, R., Banerjee, P.P. and Orange, J.S. (2009) Myosin IIA associates with NK cell lytic granules to enable their interaction with F-actin and function at the immunological synapse J. Immunol., 182, 6969–6984
30. Dobrinskikh, E., Giral, H., Caldas, Y.A., Levi, M. and Doctor, R.B. (2010) Shank2 redistributes with NaPilla during regulated endocytosis Am. J. Physiol. Cell Physiol., 299, C1324–C1334
31. Zhao, H., Ito, Y., Chappel, J., Andrews, N., Ross, F.P. and Teitelbaum, S.L. (2010) How do bone cells secrete proteins? In Osteoimmunology, Adv. Exp. Med.Biol., 658 (ed. Choi, Y.), Springer Science+Business Media, pp 105-109
32. Graham, J., Ford, T. and Rickwood, D (1994) The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol Anal. Biochem., 220, 367-373
33. Glunde, K., Guggino, S.E., Ichikawa, Y. and Bhujwalla, Z.M. (2003) A novel method of imaging lysosomes in living human mammary epithelial cells Mol. Imaging, 2, 24-36
34. Zhyvoloup, A., Nemazanyy, I., Panasyuk, G., Valovka, T., Fenton, T., Rebholz, H., Wang, M-L., Foxon, R., Lyzogubov, V., Usenko, V., Kyyamova, R., Gorbenko, O., Matsuka, G., Filonenko, V. and Gout, I. T. (2003) Subcellular localization and regulation of coenzyme A synthetase J. Biol. Chem., 278, 50316-50321
35. Cardoso, C.M.P., Groth-Pedersen, L., Høyer-Hansen, M., Kirkegaard, T., Corcelle, E., Andersen, J.S., Jäättelä, M. and Nylandsted, J. (2009) Depletion of kinesin 5B affects lysosomal distribution and stability and induces peri-nuclear accumulation of autophagosomes in cancer cells PloS One, 4:e4424
36. Higaki, K., Li, L., Bahrudin, U., Okuzawa, S., Takamuram, A., Yamamoto, K., Adachi, K., Paraguison, R.C., Takai, T., Ikehata, H., Tominaga, L., Hisatome, I., Iida, M., Ogawa, S., Matsuda, J., Ninomiya, H., Sakakibara, Y., Ohno, K., Suzuki, Y. and Nanba, E. (2011) Chemical chaperone therapy: chaperone effect on mutant enzyme and cellular pathophysiology in -galactosidase deficiency Hum. Mutat., 32, 843–852
37. Sugii, S., Reid, P.C., Ohgami, N., Du, H. and Chang, T-Y. (2003) Distinct endosomal compartments in early trafficking of low density lipoprotein-derived cholesterol J. Biol. Chem., 278, 27180-27189
38. Nathanson, C-M., Wasselius, J., Wallin, H. and Abrahamson, M. (2002) Regulated expression and intracellular localization of cystatin F in human U937 cells Eur. J. Biochem., 269, 5502-5511
39. Prigozy, T.I., Naidenko, O., Qasba, P., Elewaut, D., Brossay, L., Khurans, A., Natori, T., Koezuka, Y., Kulkarni, A. and Kronenberg, M. (2001) Glycolipid antigen processing for presentation by CD1d molecules Science, 291, 664-667
40. Soni, L.E., Warren, C.M., Bucci, C., Orten, D.J. and Hasson, T. (2005) The unconventional myosin VIIa associates with lysosomes Cell Motil. Cytoskeleton, 62, 13-26
41. Kidane, T.Z., Sauble, E. and Linder, M.C. (2006) Release of iron from ferritin requires lysosomal activity Am. J. Physiol. Cell Physiol., 291, C445-C455
OptiPrepTM Application Sheet S43; 4th edition, January 2020
OptiPrep™ Application Sheet S44
Endocytosis of ligands: analysis in sedimentation velocity gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5)
- See Section 5.7 for a brief review of methodology which includes a buoyant density separation of fractions from an initial velocity gradient; this technology maximizes resolution of compartments
- For a more detailed methods review see OptiPrep™ Application Sheet S42 “Endocytosis analysis – a review of density gradient methods”.
- See also RS12 “Endocytosis – a bibliographical review” which lists all the relevant papers reporting the use of OptiPrep™: to access this file return to the initial list of Folders and select “Reference Lists”.
1. Background
There are many examples of the use of both Nycodenz® and iodixanol gradients for the analysis of the endocytic process. The procedure described in this Application Sheet fractionates endocytic compartments in 0-45% Nycodenz® gradients at 85,000 g for 45 min i.e. the compartments are resolved by sedimentation rate (rather than density). It demonstrates that as the endocytic process progresses, ligands are associated with endosomal compartments of increasing sedimentation velocity [1-7]. This strategy is also able to identify a “pre-lysosomal compartment” which bands at a slightly lower density than the lysosomes. It has been used for the analysis of the internalization of asialoglycoproteins [1,2,4-7] and LDL [3]. The protocol has been developed primarily for rat hepatocytes but it can be used for any cell type or perfused tissue, although only the use of cultured monolayers of cells is ideally suited to some of the shorter ligand internalization times. The denser parts of the Nycodenz® gradient are hyperosmotic. When iodixanol is substituted for Nycodenz®, the gradient can be isoosmotic throughout its entire density range. In all other respects however, the properties of the two gradient media are essentially identical (solutions of the same % w/v concentration give the same density). What effect the osmolality has on the resolution in the densest part of the gradient is not known. However, because of the ease of use of iodixanol (OptiPrep™ can be simply diluted with the homogenization medium), the Nycodenz® methodology described in refs 2 and 4 has been adapted to iodixanol in this Application Sheet. he banding of endocytic compartments might vary with the type of cell or tissue and the type of ligand under investigation.
iodixanol in this Application Sheet. he banding of endocytic compartments might vary with the type of cell or tissue and the type of ligand under investigation.
2. Solutions required (see Section 5.1)
2a. Homogenization medium (HM): 0.25 M sucrose, 1 mM EGTA, 10 mM Hepes-NaOH, pH 7.2 (see Box)
2b. Iodixanol stock solution (see Box –>)
Mix 4.5 vol. with 1.5 vol. of OptiPrep diluent Add protease inhibitors as required to any of the solutions as required.
3. Ultracentrifuge rotor requirements (see section 5.2)
Any swinging-bucket rotor for an ultracentrifuge capable of 100,00 g with a tube capacity of approx 38 ml tubes (e.g. Beckman SW28 or Sorvall AH629).
4. Protocol
Suspensions of hepatocytes are allowed to bind 125I-labelled asialofetuin at 4°C for 60 min in a suitable incubation buffer. The ligand is then internalized during subsequent incubation at 37°C for periods of 30 sec, 60 sec, 2.5 min, 15 min or 30 min (see Note 1). At the end of each time period, the internalization is stopped by addition of ice-cold incubation buffer containing EGTA. Perform all of the following at 4°C
1. Wash the cells in solution HM to remove any surface-bound ligand and then homogenize them in this solution using 20 strokes of the pestle of a Dounce homogenizer (see Section 5.3).
2. Pellet debris and nuclei by centrifugation for 5 min at 2000 gav; then decant and retain the supernatant.
3. Resuspend the pellet in HM; re-centrifuge at 2000 g and combine the two supernatants.
4. Use either a standard two-chamber gradient maker or a Gradient Master to prepare 34 ml continuous 0-45% (w/v) iodixanol gradients from equal volumes of the stock solution and the HM in 36-40 ml tubes for a suitable swinging bucket rotor (see Section 5.4).
5. Load approx 4 ml aliquots of each sample on to each gradient (see Section 5.5).
6. Centrifuge the gradients at 85,000 gav for 45 min at 4C; allow the rotor to decelerate from 2000 rpm without the brake.
7. Unload the gradients in 2 ml fractions by upward displacement and analyze the fractions (see Section 5.6). For more information on harvesting gradients see Application Sheet S08.
5. Technical Notes and Review
5.1 Homogenization media and gradient solutions
The homogenization medium often has to be tailored to the tissue or cell type and it is not known if the composition of the HM is relevant to the separation. Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility in functional studies on subcellular membranes; moreover these low ionic strength HMs and gradient solutions permit the direct use of fractions for SDS-PAGE. Although 0.25 M sucrose buffered with either Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) is still a widely used HM, supplementation with inorganic salts is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation. If a hypoosmotic medium is used to swell the cells to achieve an adequate degree of homogenization, it is important to return the homogenate to isoosmotic conditions as soon as possible. Other examples of homogenization media are given in Application Sheets S05 (tissues) and S06 (cells). The use of the OptiPrep™ Diluent keeps the concentration of EGTA and buffer constant through the gradient. If this is not regarded as critical the OptiPrep™ may be diluted with the HM. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
5.2 Ultracentrifuge rotors
Smaller volume rotors or vertical (or near-vertical) rotors may be used but since this method relies on sedimentation velocity for its efficacy, the separations will certainly need shorter times in rotors with smaller sedimentation path lengths. In vertical or near-vertical rotors, the sample occupies a very narrow zone after reorientation in the tube and thus should provide the ideal format.
5.3 Homogenization
The homogenization protocol should be tailored to the cell (or tissue) type. Potter-Elvehjem homogenization for tissues and Dounce homogenization for cells used to be the standard procedures. For cells use of 5-15 passages through a 27- or 25-gauge syringe needle, sometimes preceded by Dounce homogenization, is more common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some hints on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
5.4 Gradient construction
If neither a two-chamber gradient maker nor a Gradient Master™ is available for making continuous gradients then these may be formed from diffusion of discontinuous gradients. In this case layer equal volumes of 0%, 10%, 20%, 30% and 40% (w/v) iodixanol. For more information on gradient construction see Application Sheets S03. If necessary, adjust all volumes proportionately and make sure that, after loading of the sample, tubes are properly filled according to the manufacturer’s instructions.
5.5 Sample layering
As with all sedimentation velocity density gradients the volume of sample should not exceed approx 10% of the total gradient volume. Scale down all sample and gradient volumes proportionately.
5.6 Analysis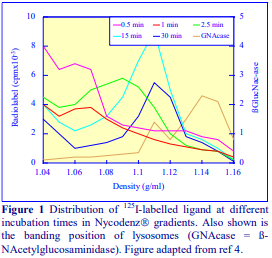
Figure 1 shows that (in Nycodenz® gradients) with increasing times of incubation at 37°C (internalization) after binding of the ligand to the cell surface, the peak of radiolabel shifts progressively to higher densities. After 30 sec of internalization, the main peak of activity is found near the top of the gradient, at a density of about 1.06 g/ml. After 60 sec, the total amount of internalized ligand increases, with the main peak at a slightly increased density, but with more of the label distributed further into the gradient. After 2.5 min incubation, total internalized ligand increases by about 5 times, with the main peak at 1.09 g/ml, but widely spread to a density of 1.12 g/ml. At 15 min incubation the density of the main peak shifts to 1.11 g/ml with approx the same amount of internalized ligand as after 2.5 min, while after 30 min, the amount of ligand had decreased by half, with the peak still at 1.11 g/ml. The 1.11 g/ml material is clearly resolved from the main lysosome band, which peaks around 1.15 g/ml. The pattern of labelling is interpreted in the following manner. Upon uptake of the ligand from the cell surface, it is contained in small, slowly sedimenting particles, which, with time, increase in size as shown by their increased sedimentation rate. After 15 min, the particles increase to a size sufficient to allow them to reach their buoyant density within the 45 min centrifugation. Under the recommended centrifugation conditions only the largest endosomes reach their buoyant density. If the centrifugation time is increased to 3 h, the radiolabel at the early time points also peaks close to 1.11 g/ml. The particles, which band at this density after a 45 min centrifugation are probably a pre-lysosomal compartment from which the ligand exits and is degraded between 2.5 and 30 min.
The use of tyramine-cellobiose attached to the ligand is recommended for the study of the degradation steps. Tyramine-cellobiose promotes the retention of degradation products in the cell. In this way the degradation process from a pre-lysosomal compartment through primary lysosomes to secondary lysosomes can be studied by comparing acid soluble and acid precipitable radiolabel [8].
5.7 Combined sedimentation velocity and buoyant density analysis
A study of nerve growth factor-mediated signal transduction that investigated the role of the TrkA receptor tyrosine kinase and associated proteins (APPL1 and GIPC1) implicated a special population of endosomes [9,10]. A post-nuclear fraction of PC12 cells was first layered over a 0-30% (w/v) iodixanol gradient and centrifuged at 133,000 g for 90 min. The gradient was collected in five sequential fractions and to facilitate the subsequent processing they were adjusted to an iodixanol concentration of at least 32.5% (w/v) iodixanol and overlaid with a continuous 0-30% (w/v) iodixanol gradient (or the fractions might be underlaid previously formed gradients) and recentrifuged for approx.17 h. From the second fraction of the first gradient two completely distinct populations of membrane vesicles were resolved: the lighter population (approx 1.12 g/ml) contained predominantly pTrk and smaller amounts of APPL1 and GIPC1; the denser population (approx. 1.20 g/ml) contained almost exclusively APPL1. The methodology was later applied to human neuroblastoma cells [11]. In a detailed account of the methodology and the analytical procedures McCaffrey et al [12] showed that Rab5 and Rab4 and Rab7, respectively markers for primary endocytic vesicles, recycling endosomes and multivesicular carrier vesicles, showed distinctive distributions through the gradients.
6. References
1. Berg, T., Ford, T., Kindberg, G. M., Blomhoff, R. and Drevon, C. (1985) Intracellular transport of asialoglycoproteins in rat hepatocytes Exp. Cell Res., 156, 570-574
2. Berg, T., Kindberg, G. M., Ford, T. and Blomhoff, R. (1985) Intracellular transport of asialoglycoproteins in rat hepatocytes. Evidence for two subpopulations of lysosomes Exp. Cell Res., 161, 285-296.
3. Gudmundsen, O., Nenseter, M. S. and Berg, T. (1993) Endocytosed LDL and -VLDL follow different intracellular pathways in rat liver Biochim. Biophys. Acta, 1210, 63-72.
4. Kindberg, G. M., Ford, T., Blomhoff, R., Rickwood, D. and Berg, T. (1984) Separation of endocytic vesicles in Nycodenz gradients Anal. Biochem., 142, 455-462.
5. Kindberg, G. M., Refsnes, M., Christofferson, T., Norum, K. R. and Berg, T. (1987) The relationship between autophagy and the intracellular degradation of asialoglycoproteins in cultured rat hepatocytes J. Biol. Chem., 262, 7066-7071.
6. Kindberg, G. M., Tolleshaug, H., Gjoen, T. and Berg, T. (1991) Lysosomal and endosomal heterogeneity in the liver: a comparison of the intracellular pathways of endocytosis in rat liver cells Hepatology, 13, 254-259.
7. Malaba, L., Smeland, S., Senoo, H., Norum, K. R., Berg, T., Blomhoff, R. and Kindberg, G. M. (1995) Retinol –binding protein and asialo-orosomucoid are taken up by different pathways in liver-cells J. Biol. Chem., 270, 15686-15692.
8. Pittman, R. C., Green, S. R., Attie, A. D. and Steinberg, D. (1979) Radiolabeled sucrose covalently linked to protein. A device for quantifying degradation of plasma proteins catabolized by lysosomal mechanisms J. Biol. Chem., 254, 6876-6879
9. Lin, D.C., Quevedo, C., Brewer, N.E., Bell, A., Testa, J.R., Grimes, M.L., Miller, F.D. and Kaplan, D.R. (2006) APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction Mol. Cell. Biol., 26, 8928-8941
10. Pryor, S., McCaffrey, G., Young. L.R. and Grimes, M.L. (2012) NGF causes TrkA to specifically attract microtubules to lipid rafts PLoS One 7: e35163
11. Xin, X., Gfeller, D., Cheng, J., Tonikian, R., Sun, L., Guo, A., Lopez, L., Pavlenco, A., Akintobi, A., Zhang, Y., Rual, JF., Currell, B., Seshagiri, S., Hao, T., Yang, X., Shen, Y.A., Salehi-Ashtiani, K., Li, J., Cheng, A.T., Bouamalay, D., Lugari, A., Hill, D.E., Grimes, M.L., Drubin, D.G., Grant, B.D., Vidal, M., Boone, C., Sidhu, S.V. and Bader, G.D. (2013) SH3 interactome conserves general function over specific form Mol. Systems Biol., 9: 652
12. McCaffrey, G., Welker, J., Scott, J., van der Salm, L. and Grimes, M.L. (2009) High-resolution fractionation of signaling endosomes containing different receptors Traffic, 10, 938–950
OptiPrepTM Application Sheet S44; 8th edition, January 2020
OptiPrep™ Application Sheet S45
Endocytosis of ligands: fractionation of clathrin-coated vesicles, endosomes and lysosomes by buoyant density in a self-generated gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5)
- Note the following: OptiPrep™ Application Sheet S42 – “Endocytosis analysis – a review of density gradient methods”.
- Reference List RS12 “Endocytosis – a bibliographical review” lists all papers reporting the use of OptiPrep™: to access return to the initial list of Folders and select “Reference Lists”.
1. Background
By using the ability of iodixanol to form self-generating gradients, the resolution of endosomes can be accomplished simply by mixing a heavy-mitochondrial supernatant (or any other suitable fraction containing endosomal vesicles) with iodixanol to an appropriate starting concentration and centrifuging in a vertical rotor for 1-2 hours. From homogenization to collection of fractions takes less than 3 hours. Self-generated gradients offer the potential for high resolution since the migration of particles within the centrifugation tube is not impeded by any interface, thus the possibilities for particle aggregation are minimized.
2. Solutions required (see Box–> and Section 5.1)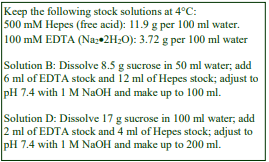
A. OptiPrep™
B. Diluent: 0.25 M sucrose, 6 mM EDTA, 60 mM Tris-HCl, pH 7.4
C. Working solution of 50% iodixanol (ρ = 1.272 g/ml): 5 volumes of Solution A + 1 volume of Solution B.
D. Homogenization medium: 0.25 M sucrose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.4
3. Ultracentrifuge rotor requirements (see Section 5.2)
A vertical or near vertical rotor capable of approx 350,000g (e.g. Beckman VTi65.1, VTi65.2, NVT65, NVT90 or Sorvall TV1665, 65V13 or 70V6), or a high-performance fixed-angle rotor with a tube capacity of <6ml (e.g. Beckman 80Ti with 4.2 ml g-Max tubes)
4. Protocol
Carry out any ligand binding, uptake and processing, as required using the tissue or cell system of choice. Subsequently all operations must be carried out at 4°C.
1. Homogenize the tissue (or cells) in Solution D: for mammalian liver use 6-8 strokes of the pestle of a Potter-Elvehjem homogenizer (500 rpm). Use about 4 ml per gram of tissue (see Section 5.3).
2. Centrifuge the homogenate in a swinging-bucket rotor at 3000 gav for 10 min. The pellet may be washed with Solution D if necessary and the two supernatants combined.
3. Make a 20% iodixanol solution (ρ = 1.127 g/ml) by diluting Solution C with Solution D.
4. Dilute the 3000 g supernatant with Solution C, 3:1 (final concentration = 12.5% iodixanol).
5. Transfer approx 9 ml of the suspension to a suitable tube (10-12 ml) for a vertical or near-vertical rotor; underlay with 1.5 ml 20% iodixanol and overlay with Solution D to fill the tube (see Section 5.4).
6. Centrifuge at approx 350,000 gav for 1.5 h (slow acceleration program to 800 rpm). Use a slow deceleration program (or no brake) from 800 rpm.
7. Collect the gradient by tube puncture, upward displacement with a dense medium or aspiration from the meniscus in approx 0.5 ml fractions and analyze as required (see Section 5.5).
8. If it is necessary to remove cytosolic proteins from the fractions and/or to concentrate them, dilute with an equal volume of buffer and sediment the membranes at approx 350,000g for 15 min (see Section 5.6).
- Information regarding the expected resolution of endocytic compartments can be found in Sections 5.7 and 5.8
5. Technical Notes and Review
5.1 Homogenization media and gradient solutions
The homogenization medium often has to be tailored to the tissue or cell type and it is not known if the composition of the HM is relevant to the separation. Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility in functional studies on subcellular membranes; moreover these low ionic strength HMs and gradient solutions permit the direct use of fractions for SDS-PAGE. Although 0.25 M sucrose buffered with either Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and containing 1 mM EDTA is still a widely used HM for both tissues and cultured cells, for the latter in particular, supplementation with inorganic salts is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation.
If a hypoosmotic medium is required to swell the cells in order to achieve adequate homogenization it is important to return the homogenate to isoosmotic conditions as soon as possible. Other examples of homogenization media are given in Application Sheets S05 (tissues) and S06 (cells). Protease inhibitors may be included in Solutions B and C at the operator’s discretion. Methods for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
5.2 Ultracentrifuge rotors
The sedimentation path length of the tube should be <24 mm. The protocol provides centrifugation times and g-forces for 11 ml Optiseal tubes for the Beckman VTi65.1, they may need to be optimized to produce the required iodixanol density gradient in other rotors. Smaller volume rotors can be used with little or no modification to the protocol, but larger volumes may require significantly longer centrifugation times. For more information on the rotor requirements for self-generated gradients see Application Sheet S04.
5.3 Homogenization
The homogenization protocol should be tailored to the cell (or tissue) type. Potter-Elevhjem homogenization for tissues and Dounce homogenization for cells used to be the standard procedures. For cells use of 5-15 passages through a 27- or 25-gauge syringe needle, sometimes preceded by Dounce homogenization, is more common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some hints on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
5.4 Sealed tube preparation for vertical and near-vertical rotors
Beckman Optiseal™ tubes are certainly the tubes of choice for these rotors. Note that all sealed tubes must be filled in accordance with the manufacturer’s recommendations and volumes should be scaled up or down proportionately for larger or smaller volume tubes. If a near-vertical rotor is used, the 20% iodixanol cushion may be omitted; it is present in tubes for vertical rotors to prevent any dense particle reaching the wall of the centrifuge tube. Use of an overlay is a convenient way of filling the tube.
5.5 Unloading sealed tubes
Heat sealed tubes may only be conveniently unloaded by tube puncture, unless the top of the tube is cut off. The latter procedure may however cause considerable disturbance to the gradient and cannot be recommended. Beckman Optiseal tubes however may be unloaded by any of the routine methods. For more information on harvesting gradients see Application Sheet S08.
5.6 Cytosolic proteins
By removing cytosolic proteins after fractionation, endosomes need not be pelleted and resuspended prior to separation. Small volume open-topped thick-walled tubes for a microultracentrifuge are a convenient way of recovering sedimented membrane fractions. Do not use more than 15 min at 350,000 g or 1-1.5 h at 100,000 g otherwise sedimentation of the iodixanol molecules themselves may interfere with pellet formation.
5.7 Gradient analysis
99mTc labeled neogalactosylalbumin, a ligand that is taken into hepatocytes by the asialoglycoprotein receptor, was injected into a perfused rat liver system for 1 min and then chased with cold medium, for periods up to 20 min. After homogenization of the blanched liver, a 3000 g supernatant was processed according to the protocol described in this Application Sheet [1].
The gradients were analyzed for radiolabel (see Figure 1). After a 1 min chase the radiolabel peaked at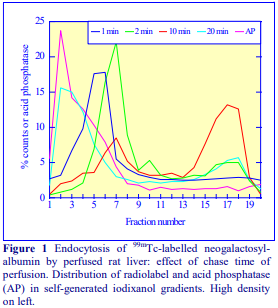 a density of approx 1.105 g/ml and after a further 1 min chase moved to a marginally lower density (approx 1.10 g/ml). The fractions in this region contained clathrin (not shown). After a 10 min chase there was a pronounced shift in the main radiolabelcontaining material to a much lower density (approx 1.075 g/ml) region, which was devoid of clathrin. After a 20 min chase the radiolabel had moved into a denser compartment, which was coincident with the acid phosphatase. The data is interpreted as follows: (1 min) the ligand is found initially in a dense clathrin-coated vesicle; (2min) uncoating of the vesicle reduces its density; (10 min) the ligand has been transferred to a low density endosome and (20 min) the ligand appears in a lysosome or pre-lysosomal compartment. The data is discussed in more detail in ref 1. Under the recommended conditions, the gradient contains a central shallow region that separates light and dense endosomes. Lysosomes band in the sharp gradient formed at the bottom of the tube, while mitochondria and peroxisomes band below the lysosomes. Plasma membrane (not shown) bands between the lysosomes and the densest endosomes (Figure 1). For a more linear gradient use longer centrifugation times. The minimum g-force required for efficient self-generation of iodixanol gradients is 180,000 gav but linear gradients are difficult to achieve at this low g-force. To subfractionate the early clathrin-coated vesicles and the plasma membrane, which tend to have a high density, the starting concentration of iodixanol should be increased to 15% or 17.5% (w/v). The plasma membrane, which has been identified in this region, (see ref 1) also contains clathrin and accounts for its relatively high density. To analyze more effectively the low-density endosomes, the starting concentration of iodixanol might be reduced to 10% (w/v).
a density of approx 1.105 g/ml and after a further 1 min chase moved to a marginally lower density (approx 1.10 g/ml). The fractions in this region contained clathrin (not shown). After a 10 min chase there was a pronounced shift in the main radiolabelcontaining material to a much lower density (approx 1.075 g/ml) region, which was devoid of clathrin. After a 20 min chase the radiolabel had moved into a denser compartment, which was coincident with the acid phosphatase. The data is interpreted as follows: (1 min) the ligand is found initially in a dense clathrin-coated vesicle; (2min) uncoating of the vesicle reduces its density; (10 min) the ligand has been transferred to a low density endosome and (20 min) the ligand appears in a lysosome or pre-lysosomal compartment. The data is discussed in more detail in ref 1. Under the recommended conditions, the gradient contains a central shallow region that separates light and dense endosomes. Lysosomes band in the sharp gradient formed at the bottom of the tube, while mitochondria and peroxisomes band below the lysosomes. Plasma membrane (not shown) bands between the lysosomes and the densest endosomes (Figure 1). For a more linear gradient use longer centrifugation times. The minimum g-force required for efficient self-generation of iodixanol gradients is 180,000 gav but linear gradients are difficult to achieve at this low g-force. To subfractionate the early clathrin-coated vesicles and the plasma membrane, which tend to have a high density, the starting concentration of iodixanol should be increased to 15% or 17.5% (w/v). The plasma membrane, which has been identified in this region, (see ref 1) also contains clathrin and accounts for its relatively high density. To analyze more effectively the low-density endosomes, the starting concentration of iodixanol might be reduced to 10% (w/v).
A second example of the use of this gradient system is taken from Molinari et al [2], who separated late endosomes, vacuoles and lysosomes in their studies on the vacuolation induced in HBK cells by Helicobacter pylori vacuolating toxin. In Figure 2 the β-glucosaminidase and Rab7 profiles identify the lysosomes in the dense region of the gradient (principally fractions 1-3) while the late endosomes peaked at a much lower density around fraction 9. CI-M6PR is a late-endosome and transGolgi network marker, and shows that the latter bands at a slightly lower density (peak fraction 4) than the lysosomes. This separation was carried out in a Beckman NVT90 near-vertical rotor.
5.8 Other self-generated gradient methods
Gradients comprising three layers rather than two have also been reported: 1.2 ml of 10%, 1.3 ml of 20% and 2.4 ml of 30% (w/v) iodixanol, the latter containing a 3000 g–10 min supernatant from CHO cells was used to create a gradient that was very close to linear in a Beckman NVT90 nearvertical rotor centrifuged at 350,000 g for 3 h. The gradient was used to analyze early and recycling endosomes [3]. A more standard strategy of starting with a solution of uniform density (30% iodixanol) centrifuged for 4 h at 365,000 g separated the ER and TGN from endosomes in a study of progression of endosomal transport [4].
Landry et al [5] used a similar gradient system to that described by Chen et al [3]; 2.5 ml of a HeLa cells post-nuclear supernatant (PNS) was adjusted to 30% iodixanol (2.5 ml total); layered under approx. 1.2 ml each of 20% and 10% iodixanol and centrifuged at 360,000 g for 3 h. The system was used to demonstrate that cell death signaling is associated with a diversion of recycling endosomes trafficking to the Golgi. In a simpler gradient system the PNS from a population of brain cells was simply adjusted to 13% iodixanol; the self-generated iodixanol gradient was used to separate early and late endosomes in a study of norepinephrine transporter trafficking [6]. Self-generated gradients have also been used in a study of the uptake by macrophages of the virulence antigen during infection by Yersinia pestis [7].
Lampugnani et al [8] used a strategy first described by Yeaman et al [9] to create a virtually linear gradient by using equal volumes of 10%, 20% and 30% (w/v) iodixanol, in tubes for the Beckman Vti65.1 vertical rotor, centrifuged at 350,000 g for 3 h. The gradients analyzed the internalization of vascular endothelial cadherin by endothelial cells and were able to provide distinctive banding patterns for plasma membranes, clathrin-coated vesicles and early endosomes.
A low-density membrane fraction from 3T3-L1 adipocytes, adjusted to 14% (w/v) iodixanol, was fractionated in a gradient, self-generated at 295,000 g for 1 h in a Beckman TLN100 rotor [10]. The gradient was able to resolve the constitutive recycling pool (endosomal recycling compartments) and the insulin-sensitive GLUT4 storage vesicles.
6. References
1. Billington, D., Maltby, P. J., Jackson, A. P. and Graham, J. M. (1998) Dissection of hepatic receptormediated endocytic pathways using self-generated gradients of iodixanol (OptiPrep) Anal. Biochem., 258, 251-258
2. Molinari, M., Galli, C., Norais, N., Telford, J. L., Rappuoli, R., Luzio, J. P. and Montecucco, C. (1997)
Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers J. Biol. Chem., 272, 25339-25344
3. Chen, X-W., Inoue, M., Hsu, S. and Saltiel, A.R. (2006) RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis J. Biol. Chem., 281, 38609-38616
4. Sbrissa, D., Ikonomov, O.C., Fu, Z., Ijuin, T., Gruenberg, J., Takenawa, T. and Shisheva, A. (2007) Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport J. Biol. Chem., 282, 23878-23891
5. Landry, M-C., Sicotte, A., Champagne, C. and Lavoie, J.N. (2009) Regulation of cell death by recycling endosomes and Golgi membrane dynamics via a pathway involving Src-family kinases, Cdc42 and Rab11a Mol Biol. Cell, 20, 4091-4106
6. Matthies, H.J.G., Moore, J.L., Saunders, C., Matthies, D.S., Lapierre, L.A., Goldenring, J.R., Blakely, R.D. and Galli, A. (2010) Rab11 supports amphetamine-stimulated norepinephrine transporter trafficking J. Neurosci., 30, 7863–7877
7. DiMezzo, T.L., Ruthel, G., Brueggemann, E.E., Hines, H.B., Ribot, W.J., Chapman, C.E., Powell, B.S. and Welkos, S.L. (2009) In vitro intracellular trafficking of virulence antigen during infection by Yersinia pestis PLoS One, 4:e6281
8. Lampugnani, M.G., Orsenigo, F., Gagliani, M.C., Tacchetti, C. and Dejana, E. (2006) Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments J. Cell Biol., 174, 593-604
9. Yeaman, C., Grindstaff, K.K., Wright, J.R. and Nelson, W.J. (2001) Sec6/8 complexes on trans-Golgi
network and plasma membrane regulate stages of exocytosis in mammalian cells J. Cell Biol., 155, 593-604
10. Sadler, J.B.A., Lamb, C.A., Gould, G.W. and Bryant, N.J. (2016) Iodixanol gradient centrifugation to separate components of the low-density membrane fraction from 3T3-L1 adipocytes Cold Spring Harb. Protoc., doi:10.1101/pdb.prot083709
OptiPrepTM Application Sheet S45; 8th edition, January 2020
OptiPrep™ Application Sheet S46
Endocytosis in cultured cells: analysis of endosomes, lysosomes and plasma membrane by buoyant density
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5)
- A summary of some of the more recent papers is given in Section 5.7
- Note OptiPrep™ Application Sheet: S42 – “Endocytosis analysis – a review of density gradient methods”. Reference List RS12 “Endocytosis – a bibliographical review” lists all the relevant papers reporting the use of OptiPrep™: to access the latter return to the initial list of Folders and select “Reference Lists”.
1. Background
The protocol in this Application Sheet was devised by Sheff et al [1] for the separation of two types of endosomes from transfected MDCK cells: peripheral early endosomes and perinuclear recycling endosomes in a study of transferrin internalization. These endosomes are involved in the sorting of proteins for delivery to the apical and basolateral domains of polarized epithelial cells and are differentially associated with rab4, rab11 and the transferrin receptor [1]. The method uses a conventional approach of a pre-formed gradient, centrifuged over night to equilibrium density in a swinging-bucket rotor.
Although there are many published methods in which buoyant density gradient centrifugation is carried out in 1-3 h at 150,000-200,000g, there is evidence that longer centrifugation times at lower RCFs, as used in this protocol, are required for true equilibrium density banding of membrane vesicles and that this strategy achieves their optimal separation. If times of 12-18 h are used, then the RCFs should be <100,000g to prevent significant gradient density profile distortion by the sedimentation of iodixanol molecules.
The following protocol is adapted from ref 1.
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Optiprep™ diluent: 235 mM KCl, 12 mM MgCl2, 25 mM CaCl₂, 30 mM EGTA, 150 mM HepesNaOH pH 7.0
C. 40% iodixanol working solution (WS): 2 vol. of Solution A + 1 vol. of Solution B
D. WS diluent: 78 mM KCl, 4 mM MgCl2, 8.4 mM CaCl₂, 10 mM EGTA, 50 mM Hepes-NaOH pH 7.0
E. Homogenization medium: 0.25 M sucrose, 78 mM KCl, 4 mM MgCl₂, 8.4 mM CaCl2, 10 mM EGTA, 50 mM Hepes-NaOH pH 7.0
3. Ultracentrifuge rotor requirements (see Section 5.2)
Swinging-bucket rotor with tube size approx 14 ml, e.g. Beckman SW41Ti or Sorvall TH641
4. Protocol
Following any experimental procedures to allow binding, uptake and processing of a ligand or other functional manipulations, all operations must be carried out at 4°C.
1. Remove any unbound ligand or other components from the cell surface by washing the cell monolayer twice in any solution compatible with the study and then scrape the cells into 1 ml of Solution E.
2. Homogenize the cells using a ball-bearing homogenizer (cell cracker); four passes of the cell suspension should be sufficient. Check for adequate homogenization by phase contrast microscopy. If such a device is not available then use several passages through a fine gauge syringe needle or a Dounce homogenizer (see Section 5.3).
3. Centrifuge the homogenate in a swinging-bucket rotor at 1000 g for 5 min to pellet the nuclei and any cell debris. The pellet may be washed with Solution E if necessary and the two supernatants combined.
4. Prepare the low and high density gradient solutions of 5% and 20% (w/v) iodixanol by diluting Solution C with Solution D.
5. In tubes for the swinging-bucket rotor prepare 12-13 ml 5-20% (w/v) iodixanol gradients using either a two-chamber gradient maker or a Gradient Master (see Section 5.4).
6. Layer the 1000g supernatant(s) on top of the gradient and centrifuge at 90,000 gav for 18-20 h (see Section 5.5).
7. Collect the gradient in approx 0.25 ml fractions either by upward displacement with a dense liquid, tube puncture or aspiration from the meniscus. For more information on harvesting gradients see Application Sheet S08.
8. If it is necessary to remove the iodixanol, fractions can be pelleted at 200,000 g for 20 min after dilution with 2 vol of Solution E. For more information see Section 5.6.
- Information regarding the analysis of endocytic compartments can be found in Section 5.7.
5. Technical Notes and Review
5.1 Homogenization media and gradient solutions
The homogenization medium often has to be tailored to the tissue or cell type. Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility in functional studies on subcellular membranes; moreover these low ionic strength HMs and gradient solutions permit the direct use of fractions for SDS-PAGE. Although 0.25 M sucrose buffered with either Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and containing EDTA or EGTA is still a widely used HM for both tissues and cultured cells, for the latter in particular, supplementation with inorganic salts, as in this protocol, is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation.
The use of an Optiprep™ diluent (solution B) containing 235 mM KCl, 12 mM MgCl₂, 25 mM
CaCl₂, 30 mM EGTA, 150 mM Hepes-NaOH pH 7.0 to produce a 40% (w/v) iodixanol working solution ensures that the concentrations of KCl, MgCl2, CaCl2, EGTA and buffer remain constant in the gradient when this solution is diluted with Solution D. Indeed the osmolality of the gradient will also be approximately the same as in the HM (Solution E) the iodixanol and the sucrose providing almost identical osmotic contributions to the solutions. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
The same homogenization medium and gradient solutions as those described in Section 2 have been used for PC12 cells [2], HeLa cells [3-6], CHO cells [7], COS-7 cells [9] HEK293 cells [10], renal epithelial cells [11], a fibrosarcoma cell line [12], polarized human airway epithelial cells [13] and cultured monocytes [14,15]. Other homogenization media are given in Table 1.
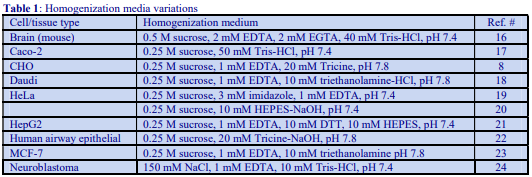
If a hypoosmotic medium has to be used to swell the cells in order to achieve an adequate degree of homogenization it is important to return the homogenate to isoosmotic conditions as soon as possible. Other examples of homogenization media are given in Application Sheets S05 (tissues) and S06 (cells).
- Protease inhibitors may be included in Solutions B, D and E at the operator’s discretion.
7
5.2 Ultracentrifuge rotors
The method may scaled up or down to the use of larger or smaller volume swinging-bucket rotors to accommodate other samples sizes.
5.3 Homogenization
The homogenization protocol should be tailored to the cell (or tissue) type. Potter-Elevhjem homogenization for tissues and Dounce homogenization for cells used to be the standard procedures. For cells use of 5-15 passages through a 27- or 25-gauge syringe needle, sometimes preceded by Dounce homogenization, is more common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices. Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some hints on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
- Ref 25 describes the use of a complex treatment of PC12 cells for internalization of nerve growth factor, followed by mechanical permeabilization of the cells (one pass through a ball-bearing homogenizer) to release the internal membrane vesicles.
5.4 Gradients and centrifugation conditions
If neither of these gradient-making devices is available then a continuous gradient can be prepared by diffusion of a discontinuous gradient. For more information on gradient construction see Application Sheet S03. If necessary, adjust all volumes proportionately so that tubes (after sample application) are properly filled according to the manufacturer’s instructions.
The long-spin 5-20% (w/v) iodixanol gradients described in the above protocol have now been used for a variety of cell types with small variations in g-force and centrifugation time (90,000-125,000 g for 15-20h). The cell types include including CHO [7,8], COS-7 [9], HeLa [3-6,19], HepG2 [21] human airway epithelial cells [13] and PC-12 [2]. Gradients covering other density ranges and/or centrifuged for different times have also been used (see Table 2).
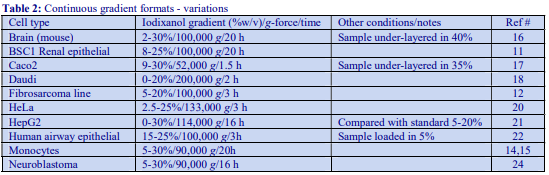
Other strategies
Idkowiak-Baldys et al [10] used a discontinuous gradient of 5%, 10%, 15% and 20% (w/v) iodixanol for HEK cells, rather than the recommended 5-20% continuous gradient, but since the gradient is centrifuged at a relatively low g-force for at least 16 h the gradient will become continuous and more or less linear by diffusion.
Lin et al [25] used PC12 cells, permeabilized by a single passage through a ball-bearing homogenizer to isolate a vesicle-containing fraction. This was first separated on a 0-30% (w/v) iodixanol sedimentation velocity gradient and fractions from this gradient adjusted to 32% iodixanol and further fractionated by flotation through a long-spin 0-30% iodixanol gradient at 133,000 g for 18 h. The authors were investigating the trafficking of the TrkA neurotrophin receptor and were able to completely resolve one population of vesicles (ρ = 1.12 g/ml) which contained both TrkA and the TrkA-associated protein APPL1 from a denser one (ρ = 1.2 g/ml) which contained only APPL1. See Application Sheet S42 (Section 5.7) for other examples of this double gradient strategy. Li et al [23] used a discontinuous gradient of 2%, 24% and 32% (w/v) iodixanol (with the sample in the latter), centrifuged at 83,000 g for 2 h to separate (in order of increasing density) the plasma membrane, early/recycling endosomes, mitochondria and peroxisomes from MCF7 cells.
5.5 Removal of iodixanol from gradient samples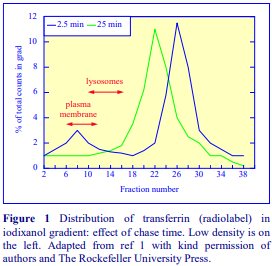
Large pore size dialysis tubing, Maxi GeBAflex (www.geba.org) dialysis tubes (highest MWt cut off), centrifugal ultrafiltration cones or a G25 Sephadex column may be used. More information about the removal of iodixanol can be found in Section 7 of Application Sheet S09.
5.6 Analytical review
Sheff et al [1] carried out a number of elegant experiments to investigate the endosomal pathways of transfected MDCK cells. In one of these, the uptake of 125I-transferrin into MDCK cells transfected with human transferrin receptor was
studied by a classical pulse-chase technique. The data is summarized in Figure 1. The early endosomes (2.5 min) band at a significantly higher density than the recycling endosomes (25 min) and the equilibrium density of both of these are distinctively higher than either plasma membrane or lysosomes. The position of the latter was determined by ß-hexosaminidase activity [1]. The gradients are generally able to resolve plasma membrane from early and late endosomes, although the relative position of the plasma membrane is variable; it is usually lighter than the endosomes, but there may be instances where it is denser, see for example refs 4 and 8. Sugii et al [8] also demonstrated that there was also a distinctive banding of the TGN (denser than the late endosomes) from CHO cells. In nearly all instances early endosomes are reported as being less dense than the late endosomes and there may be some distinctive banding of recycling endosomes, although there is usually significant overlap between the latter and early endosomes. Sheff et al [1] reported a slightly lower density for recycling endosomes (see Figure 1).
5.7 Summary of other papers reporting use of this continuous gradient technology
- Urbanska et al [26] compared the resolving power of sucrose and iodixanol gradients in the characterization of APPL endosomes from HeLa cells. The distinctive banding patterns in iodixanol gradients of EEA-1, APPL1, AP50 and Rab5 were not seen in sucrose gradients.
- The 5-20% (w/v) iodixanol gradient format described above achieved a complete separation of plasma membrane and late endosomes/lysosomes from brain tissue [27] and early endosomes and late endosomes/lysosomes from HeLa cells [28].
- Keith et al [29] did not use a post-nuclear supernatant (PNS) from HEK cells as the gradient input; instead the PNS was centrifuged at 12,000 g for 20 min; the pellet resuspended in lysis buffer and layered on a 2.5%-17% (w/v) iodixanol gradient, which was centrifuged at 90,000 g for 7 h. The gradient achieved effective resolution of early endosomes (EEA1), recycling endosomes (Rab11) and late endosomes (Rab7).
- Niu et al [30] studied the infection of HL60 cells by the obligate intracellular bacterium Anaplasma phagocytophilum (Ap) The continuous iodixanol gradient was able to resolve the characteristic Ap inclusions, from early endosomes (EEA1), late endosomes+lysosomes (LAMP2), ER (calnexin) and autophagosomes (LC3). Interestingly in the infected cells the ER was shifted to slightly higher densities compared to that in uninfected cells.
6. References
1. Sheff, D. R., Daro, E. A., Hull, M. and Mellman, I. (1999) The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions J. Cell Biol., 145, 123-139
2. Li, Y., Chin, L-S., Levey, A.I. and Li, L. (2002) Huntingtin-associated protein 1 interacts with hepatocyte growth factorregulated tyrosine kinase substrate and functions in endosomal trafficking J. Biol. Chem., 277, 28212-28221
3. Chin, L-S., Raynor, M. C., Wei, X., Chen, H-Q. and Li, L. (2001) Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor J. Biol. Chem., 276, 7069-7078
4. Meyers, J. M. and Prekeris, R. (2002) Formation of mutually exclusive Rab11 complexes with members of the family of Rab11-interacting proteins regulates Rab11 endocytic targeting and function J. Biol. Chem., 277, 49003-49010
5. Proikas-Cezanne, T., Gaugel, A., Frickey, T. and Nordheim, A. (2006) Rab14 is part of the early endosomal clathrincoated TGN microdomain FEBS Lett., 580, 5241-5246
6. Barroso-González, J., Machado, J-D., García-Expósito, L. and Valenzuela-Fernández, A. (2009) Moesin regulates the trafficking of nascent clathrin-coated vesicles J. Biol. Chem., 284, 2419–2434
7. Daro, E., Sheff, D., Gomez, M., Kreis, T. and Mellman, I. (1997) Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component -COP J. Cell Biol., 139, 1747-1759
8. Sugii, S., Reid, P.C., Ohgami, N., Du, H. and Chang, T-Y. (2003) Distinct endosomal compartments in early trafficking of low density lipoprotein-derived cholesterol J. Biol. Chem., 278, 27180-27189
9. Shen, X., Xu, K-F., Fan, Q., Pacheco-Rodriguez, G., Mos, J. and Vaughan, M. (2006) Association of brefeldin Ainhibited guanine nucleotide-exchange protein 2 (BIG2) with recycling endosomes during transferring uptake Proc. Natl. Acad. Sci. USA, 103, 2635-2640
10. Idkowiak-Baldys, J., Becker, K.P., Kitatani, K. and Hannun, Y.A. (2006) Dynamic sequestration of the recycling compartment by classical protein kinase C J. Biol. Chem., 281, 22321-22331
11. McKenzie, J., Johannes, L., Taguchi, T.and Sheff, D. (2009) Passage through the Golgi is necessary for Shiga toxin B subunit to reach the endoplasmic reticulum FEBS J., 276, 1581–1595
12. Molle, D., Segura-Morales, C., Camus, G., Berlioz-Torrent, C., Kjems, J., Basyuk, E. and Bertrand, E. (2009) Endosomal trafficking of HIV-1 Gag and genomic RNAs regulates viral egress J. Biol. Chem., 284, 19727-19743
13. Bomberger, J.M., Guggino, W.B. and Stanton, B.A. (2011) Methods to monitor cell surface expression and endocytic trafficking of CFTR in polarized epithelial cells In Cystic Fibrosis, Methods Mol. Biol. (eds. Amaral, M.D. and Kunzelmann, K.) Springer Science+Business Media, pp 271-283
14. Gibbings, D.J., Ciaudo, C., Erhardt, M. and Voinnet, O. (2009) Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity Nat. Cell Biol., 11, 1143-1149
15. Gibbings, D. (2011) Continuous density gradients to study argonaute and GW182 complexes associated with the endocytic pathway In Argonaute Proteins: Methods and Protocols, Methods Mol. Biol., 725, (ed. Hobman. T.C. and Duchaine, T.F.) Springer Science+Business Media, pp 63-76
16. Shinohara, M., Sato, N., Kurinami, H., Takeuchi, D., Takeda, S., Shimamura, M., Yamashita, T., Uchiyama, Y., Rakugi, H. and Morishita, R. (2010) Reduction of brain -amyloid (A) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and A clearance J. Biol. Chem., 285, 22091–22102
17. Orlandi, P.A (1997) Protein-disulfide isomerase-mediated reduction of the A subunit of cholera toxin in a human intestinal cell line J. Biol. Chem., 272, 4591-4599 (1997)
18. Payelle-Brogard, B. and Pellegrini, S. (2010) Biochemical monitoring of the early endocytic traffic of the type I interferon receptor J. Interferon Cytokine Res., 30, 89-98
19. Mairhofer, M., Steiner, M., Salzer, U. and Prohaska, R. (2009) Stomatin-like protein-1 interacts with stomatin and is targeted to late endosomes J. Biol. Chem., 284, 29218-29229
20. Tagami, S., Okochi, M., Yanagida, K., Ikuta, A., Fukumori, A., Matsumoto, N., Ishizuka-Katsura, Y., Nakayama, T., Itoh, N., Jiang, J., Nishitomi, K., Kamino, K., Morihara, T., Hashimoto, R., Tanaka, T., Kudo, T., Chiba, S. and Takeda, M. (2008) Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1 Mol. Cell. Biol., 28, 165-76
21. Manunta, M., Izzo, L., Duncan, R. and Jones, A.T. (2007) Establishment of subcellular fractionation techniques to monitor the intracellular fate of polymer therapeutics II: Identification of endosomal and lysosomal compartments in HepG2 cells combining single-step subcellular fractionation and fluorescent imaging J. Drug Target., 15, 37-50
22. Bomberger, J.M., Ye, S., MacEachran, D.P., Koeppen, K., Barnaby, R.L., O’Toole, G.A. and Stanton, B.A. (2011) A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system PLoS Pathog., 7: e1001325
23. Li, Q., Harraz, M.M., Zhou, W., Zhang, L.N., Ding, W., Zhang, Y., Eggleston, T., Yeaman, C., Banfi, B. and Engelhardt, J.F. (2006) Nox2 and Rac1 regulate H2O2 -dependent recruitment of TRAFg to endosomal interleukin-1 receptor complexes Mol. Cell. Biol., 26, 140-154
24. Wiesinger, J.A., Buwen, J.P., Cifelli, C.J., Unger, E.L., Jones, B.C. and Beard, J.L. (2007) Down-regulation of dopamine transporter by iron chelation in vitro is mediated by altered trafficking, not synthesis J. Neurochem., 100, 167-179
25. Lin, D.C., Quevedo, C., Brewer, N.E., Bell, A., Testa, J.R., Grimes, M.L., Miller, F.D. and Kaplan, D.R. (2006) APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction Mol. Cell. Biol., 26, 8928-8941
26. Urbanska, A., Sadowski, L., Kalaidzidis, Y. and Miaczynska, M. (2011) Biochemical characterization of APPL endosomes: the role of annexin A2 in APPL membrane recruitment Traffic, 12, 1227–1241
27. Takamura, A., Higaki, K., Ninomiya, H., Takai, T., Matsuda, J., Iida, M., Ohno, K., Suzuki, Y. and Nanba, E. (2011) Lysosomal accumulation of Trk protein in brain of GM1-gangliosidosis mouse and its restoration by chemical chaperone J. Neurochem., 118, 399–406
28. Huotari, J., Meyer-Schaller, N., Hubner, M., Stauffer, S., Katheder, N., Horvath, P., Mancini, R., Helenius, A. and Peter, M. (2012) Cullin-3 regulates late endosome maturation Proc. Natl. Acad. Sci. USA, 109, 823–828
29. Keith, D.J., Wolfrum, K., Eshleman, A.J. and Janowsky, A. (2012) Melittin initiates dopamine transporter internalization and recycling in transfected HEK-293 cells Eur. J. Pharmacol., 690, 13–21
30. Niu, H., Xiong, Q., Yamamoto, A., Hayashi-Nishino, M. and Rikihisa, Y. (2012) Autophagosomes induced by a bacterial Beclin 1 binding protein facilitate obligatory intracellular infection Proc. Natl. Acad. Sci. USA, 109, 20800–20807
7. Acknowledgements
We wish to thank Dr David Sheff, Department of Cell Biology, Yale University School of Medicine, New Haven, CT 06520, USA, for his kind cooperation in the preparation of this Application Sheet.
OptiPrepTM Application Sheet S46; 9th edition, December 2019
OptiPrep™ Application Sheet S47a
Intracellular exocytic vesicle trafficking and exocyst complex – a short methodological summary
1. Introduction
There are two areas of investigation where iodixanol gradients have been widely used in studies of exocytosis: (a) the control and organization of membrane trafficking within the cells that permits the movement of vesicles to, and ultimately their fusion with, the plasma membrane or a specific plasma membrane domain and (b) analysis of the process of cytokinesis and in particular the role of the evolutionary-conserved exocyst complex in controlling the final abscission process.
2. Membrane trafficking within the cytoplasm
2a. Cell homogenization
There is no particular consensus regarding the nature of the homogenization medium or the method of homogenization and a selection of examples is provided in Table 1.
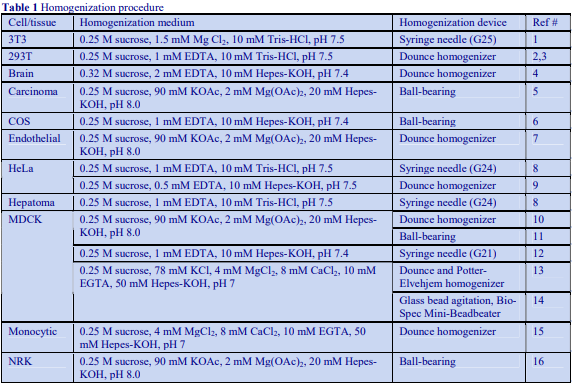
- Any cocktail of protease inhibitors may be added to the homogenization medium
2b Pre-gradient processing
In most cases the fraction that is applied to the gradient, or incorporated into the gradient, is a post-nuclear supernatant (PNS). The single centrifugation may be carried out at any g-force from 500- 1000 g for 5-10 min, although occasionally a post-heavy mitochondrial supernatant (3000 g) is used (see e.g. ref 6). Sometimes a more extensive differential centrifugation is carried out, for example an MDCK cell homogenate was centrifuged sequentially at 1000 g for 10 min, 5,000 g for 40 min and then 100,000 g for 2 h; the pellet from the last centrifugation was applied to the gradient [14]. Rather more rarely the differential centrifugation may be more extensive: e.g. 1000 g for 10 min, 5000 g for 10 min, 10,000 g for 20 min, 15,000 g for 20 min and then the final pellet at 100,000 g for 1 h was resuspended and incorporated into a continuous gradient [13]. The advantage of using a PNS is the reduction in time between homogenization and gradient and losses of vesicles will be minimal. The disadvantage is the presence of all the other large organelles (mitochondria, lysosomes, peroxisomes) that will band in the denser regions of the gradient. Maybe a single 5000 g centrifugation, which will eliminate most of the mitochondria and some of the peroxisomes and lysosomes, might be a useful compromise.
2c. Self-generated gradients
The 10%-20%-30% (w/v) iodixanol starting format for the preparation of gradients that are close to linear was first introduced by Yeaman et al [16] in 2001. These gradients are best prepared in a vertical or near-vertical rotor with a tube volume of no more than 13 ml; for example the Beckman VTi65.1 or NVT65 or the Sorvall 65V13. Smaller volume rotors (5-6 ml tubes) such as the Beckman VTi65.2 or NVT65.2 or the Sorvall 70V6 are equally acceptable. The method has often been by Yeaman and his colleagues in studies on the influence of the Sec6/8 exocyst complex in controlling membrane vesicle delivery to the plasma membrane of polarized cells [17,18]. In more recent experiments the gradient was modified: an MDCK cell PNS was adjusted to 30% iodixanol and layers of 25%, 20%, 15% and 10% (w/v) iodixanol layered on top, otherwise the centrifugation conditions were the same [5]. The gradient clearly resolved the Sec8 complex in the denser from the lighter Na+/K+-ATPase, while the paxillin marker was biphasic with only the lighter peak co-migrating with the Sec8 (see Figure 1). The standard 10%-20%-30% (w/v) iodixanol starting format was used in a study of the relationship between the Sec3 containing exocyst complex and desmosome assembly [11].
Kolesnikova et al [8] used the 10-20-30% iodixanol gradient to monitor the translocation of the VP40 matrix protein of Marburg virus in infected cells. At 7 h post-infection most of the VP40 was associated with the small vesicle fraction but as the infection progressed (up to 24 h) the gradient permitted the demonstration of a shift through the endosomal/ER zone to the plasma membrane. A HeLa cell PNS was fractionated on the 10%-20%-30% (w/v) iodixanol gradient, with the PNS only in 30% layer and centrifuged at 330,000 g for 3 h: M-Sec and RalA formed a clear biphasic distribution, only the denser material co-fractionating with the Sec6/8 exocyst complex [9]. Using the same gradient and centrifugation format Chen et al [6] studied exocyst regulation of vesicle delivery to the centrosome prior to cytokinesis in COS cells: they observed a co-banding of RalA with TfR and Rab11 but not with early endosomes, Golgi or cytosol markers.
An additional layer of 15% iodixanol was inserted by Wang et al [12] who found that in PALS1 knockdown cells there was a significant shift in the banding of Sec8 and E-cadherin compared to wild-type cells.
- Similar self-generated gradients have also been used for the analysis of secretory proteins and the exocytic process for Drosophila [19] and in the study of the secretion of bone matrix proteins by osteoclasts [20].
2d. Gradients in swinging bucket rotors
Leblanc et al [1] working with 3T3 cells used a three-layer 10-30% (w/v) iodixanol gradient, again with the PNS in the densest layer in small volume (4 ml) swinging-bucket rotor at 260,000g for 3 h. A continuous gradient will form during the centrifugation mainly by diffusion, although some self generation may also occur. The separation of dense small vesicles from lighter endosomes and plasma membrane was similar to that in ref 8. Other gradients have conformed to the traditional format of top-loading of pre-formed gradients centrifuged at lower g-forces. A microsomal fraction placed on top of a continuous 10-40% (w/v) iodixanol gradient, centrifuged at 90,000 g for 18 h to study the delivery of TGF-α to the basolateral surface [13] and the targeting of exosomes to the same surface domain [14] of MDCK cells. The method in Ref 14 provides a particularly impressive purification of vesicles containing Naked-2- enhanced green fluorescent protein). A 5-30% (w/v) iodixanol gradient centrifuged under the same conditions was used in a study of miRNA effector proteins in exosomes derived from multivesicular bodies in monocytic cells [15].
A simple flotation discontinuous density gradient can separate soluble cytosolic proteins from a total vesicle fraction [21]
For some recent publications of studies of exocytic vesicles in polarised cells see refs 22-24.
3. References
1. Leblanc, P., Alais, S., Porto-Carriero, I., Lehmann, S., Grassi, J., Raposo, G. and Darlix, J.L. (2006) Retrovirus infection strongly enhances scrapie infectivity release in cell culture EMBO J., 25, 2674-2685
2. Grigorov, B., Arcanger, F., Roingeard, P., Darlix, J-L. and Muriaux, D. (2006) Assembly of infectious HIV1 in human epithelial and T-lymphoblastic cell lines J. Mol. Biol., 359, 848-862
3. Grigorov, B., Décimo, D., Smagulova, F., Péchoux, C., Mougel., M., Muriaux, D. and Darlix, J-L. (2007) Intracellular HIV-1 Gag localization is impaired by mutations in the nucleocapsid zinc fingers Retrovirology, 4:54
4. Jacobs, S.B.R., Basak, S., Murray, J.I. and Attardi, L.D. (2007) Siva is an apoptosis-selective p53 target gene important for neuronal cell death Cell Death Differ., 14, 1374-1385
5. Spiczka, K.S. and Yeaman, C. (2008) Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration J. Cell Sci., 121, 2880-2891
6. Chen, X-W., Inoue, M., Hsu, S. and Saltiel, A.R. (2006) RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis J. Biol. Chem., 281, 38609-38616
7. Lampugnani, M.G., Orsenigo, F., Gagliani, M.C., Tacchetti, C. and Dejana, E. (2006) Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments J. Cell Biol., 174, 593-604
8. Kolesnikova, L., Bamberg, S., Berghöfer, B. and Becker, S. (2004) The matrix protein of Marburg virus is transported to the plasma membrane along cellular membranes: exploiting the retrograde late endosomal pathway J. Virol., 78, 2382-2393
9. Hase, K., Kimura, S., Takatsu, H., Ohmae, M., Kawano, S., Kitamura, H., Ito, M., Watarai, H., Hazelett, C.C., Yeaman, C. and Ohno, H. (2009) M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex Nat. Cell Biol., 11, 1427-1432
10. Yeh, T-Y.J., Meyer, T.N., Schwesinger, C., Tsun, Z-Y., Lee, R.M. and Chi, N-W. (2006) Tankyrase recruitment to the lateral plasma membrane in polarized epithelial cells: regulation by cell-cell contact and protein poly(ADP-ribosyl)ation Biochem J., 399, 415-425
11. Andersen, N.J. and Yeaman, C. (2010) Sec3-containing exocyst complex is required for desmosome assembly in mammalian epithelial cells Mol. Biol. Cell, 21, 152-164
12. Wang, Q., Chen, X-W. and Margolis, B. (2007) PALS1 regulates E-cadherin trafficking in mammalian epithelial cells Mol. Biol. Cell, 18, 874-885
13. Li, C., Hao, M., Cao, Z., Ding, W., Graves-Deal, R., Hu, J., Piston, D.W. and Coffey, R.J. (2007) Naked2 acts as a cargo recognition and targeting protein to ensure proper delivery and fusion of TGF-- containing exocytic vesicles at the lower lateral membrane of polarized MDCK cells Mol. Biol. Cell 18, 3081-3093
14. Cao, Z., Li, C., Higginbotham, J.N., Franklin, J.L., Tabb, D.L., Graves-Deal, R., Hill, S., Cheek, K., Jerome, W.G., Lapierre, L.A., Goldenring, J.R., Ham, A-J.L. and Coffey, R.J. (2008) Use of fluorescenceactivated vesicle sorting for isolation of Naked2-associated, basolaterally targeted exocytic vesicles for proteomics analysis Mol. Cell. Proteomics, 7, 1651-1667
15. Gibbings, D.J., Ciaudo, C., Erhardt, M. and Voinnet, O. (2009) Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity Nat. Cell Biol., 11, 1143-1149
16. Yeaman, C., Grindstaff, K.K., Wright, J.R. and Nelson, W.J. (2001) Sec6/8 complexes on trans-Golgi network and plasma membrane regulate stages of exocytosis in mammalian cells J. Cell Biol., 155, 593-604
17. Yeaman, C., Grindstaff, K.K. and Nelson, W.J. (2004) Mechanism of recruiting Sec6/8 (exocyst) complex to the apical juntional complex during polarization of epithelial cells J. Cell Sci., 117, 559-570
18. Gromley, A., Yeaman, C., Rosa, J., Redick, S., Chen, C-T., Mirabelle, S., Guha, M., Sillibourne, J. and Doxsey, S.J. (2005) Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission Cell, 123, 75-87
19. Beronja, S., Laprise, P., Papoulas, O., Pellikka, M., Sisson, J. and Tepass, U. (2005) Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells J. Cell Biol., 169, 635-646
20. Zhao, H., Ito, Y., Chappel, J., Andrews, N., Ross, F.P. and Teitelbaum, S.L. (2010) How do bone cells secrete proteins? In Osteoimmunology, Adv. Exp. Med.Biol., 658 (ed. Choi, Y.), Springer Science+Business Media, pp 105-109
21. Jang, A., Lee, H-J., Suk, J-E., Jung, J-W., Kim, K-P. and Lee, S-J. (2010) Non-classical exocytosis of - synuclein is sensitive to folding states and promoted under stress conditions J. Neurochem., 113, 1263–1274
22. Caballero-Lima, D., Hautbergue, G.M., Wilson, S.A. and Sudbery, P.E. (2014) In Candida albicans hyphae, Sec2p is physically associated with SEC2 mRNA on secretory vesicles Mol. Microbiol., 94, 828–842
23. Majumdar, R., Tavakoli Tameh, A. and Parent, C.A. (2016) Exosomes mediate LTB4 release during neutrophil chemotaxis PLoS Biol., 14: e1002336
24. Kreutzberger, A.J.B., Kiessling, V., Liang, B., Seelheim, P., Jakhanwal, S., Jahn, R., Castle, D. and Tamm, L.K. (2017) Reconstitution of calcium-mediated exocytosis of dense-core vesicles Sci. Adv., 3: e1603208
OptiPrepTM Application Sheet S47a 7th edition, January 2020
OptiPrep™ Application Sheet S47
Analysis of exocytosis, exocyst function and plasma membrane domain targeting in (A) self-generated and (B) pre-formed gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Application Sheet S47a “Intracellular exocytic vesicle trafficking and exocyst complex – a short methodological summary” provides a brief protocol review of papers reporting the use of OptiPrep™ which complements this Application Sheet.
- Important technical notes and information regarding alternative methodologies are contained in the “Technical Notes and Review” section (Section 5); for non-mammalian cells see Section 5.8
A. Self-generated gradients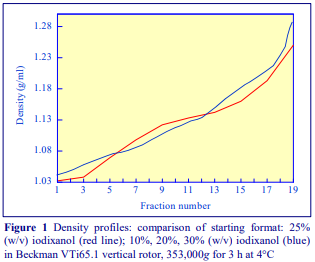
1. Background
Self-generated gradients offer an important advantage for the fractionation of membrane compartments involved with trafficking and signaling; they are highly reproducible and very simple to prepare. In a vertical or near-vertical rotor with a sedimentation path length of <25 mm, gradient formation takes place in 1-3 h at approx 350,000 g. They can be prepared simply by adjusting the sample (usually a post-nuclear supernatant) to a uniform concentration of iodixanol and then transferring the suspension to the centrifuge tube. Some of the density profiles that can be obtained in this manner are described in Application Sheet S04.
In the example in Figure 1, centrifugation of a 25% (w/v) iodixanol solution for 3 h centrifugation at 353,000 gav (at 4°C) produces a gradient that is more or less linear from 1.02 g/ml to 1.15 g/ml but is steeper on the densest region and clearly retains the slight sigmoidal form more prominent in shorter spin gradients. To overcome the latter, Yeaman et al [1] used a starting format of a discontinuous gradient, comprising equal volumes of 10%, 20% and 30% (w/v) iodixanol. The gradient formed under these conditions retains its steeper densest portion and the sigmoidal form is lost (Figure 1).
This gradient is very effective for the study of the transfer of proteins or protein complexes to the surface or the secretion of virus and virus-like particles (see Section A5.6).
2. Solutions required (see Section 5.1 for important information)
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 90 mM KOAc, 2 mM Mg(OAc)₂, 20 mM Hepes-KOH, pH 8.0
3. Ultracentrifuge rotor requirements (see Section 5.2)
Vertical rotor with 11-13 ml tubes (e.g. Beckman VTi65.1 or Sorvall 65V13, or near-vertical rotor (e.g. Beckman NVT65)
4. Protocol (adapted from ref 1)
Carry out all operations at 0-4°C. All iodixanol concentrations are given as % (w/v).
1. Homogenize the cells in Solution B in a cell cracker (ball-bearing homogenizer) using 4-6 passages. Monitor the efficacy of the homogenization by phase contrast microscopy (see Section 5.3).
2. Centrifuge the homogenate at 800 g for 5 min to pellet the nuclei.
3. Mix the post-nuclear supernatant (PNS), OptiPrep™ and solution B in the following volume ratios (3:3:0, 3:2:1 and 3:1:2) to produce three suspensions containing 30%, 20% and 10% iodixanol respectively (see Sections 5.4 and 5.7).
4. Layer equal volumes the three suspensions in tubes for the vertical or near-vertical rotor; 11.2 ml Optiseal™ tubes for the Beckman VTi65.1 or NVT65 rotor are the recommended ones (see Sections 5.5 and 5.7).
5. Centrifuge at 353,000 gav for 3 h using a slow acceleration program.
6. Allow the centrifuge to decelerate to rest from 2000 rpm without the brake or use a slow deceleration program.
7. Collect the gradient in 0.5 ml fractions either by tube puncture, aspiration from the meniscus or upward displacement; the latter two options are only permissible with Beckman Optiseal™ tubes. For more information on harvesting gradients see Application Sheet S08.
- A brief summary of the analytical capabilities of this gradient system is given in Section 5.6.
5. Technical Notes and Review
5.1 Homogenization media
The homogenization medium (HM) often has to be tailored to the tissue or cell type and it is not known if the composition of the HM is relevant to the separation. Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility in functional studies on subcellular membranes; moreover these low ionic strength HMs and gradient solutions permit the direct use of fractions for SDS-PAGE.
Supplementation of the HM with inorganic salts (containing K+ or Na+ ions) is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation. Solutions are buffered with Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and it is unlikely if the type of buffer significantly influences the fractionation, although triethanolamine does seem to offer some advantages in homogenization efficiency [2]
The 0.25 M sucrose, 90 mM KOAc, 2 mM Mg(OAc)₂, 20 mM Hepes-KOH, pH 8.0 described in this protocol was used by Yeaman et al [1] for NRK-49F and NRK-52E rat kidney cells. Kolesnikova et al [3] who used the same gradient homogenized human hepatoma (HUHT-7) and HeLa cells in a standard 0.25 M sucrose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.5, while Leblanc et al [4] replaced the 1 mM EDTA with 1.5 mM MgCl₂ for mouse embryo fibroblasts (3T3 cells). Other examples of homogenization media are given in Application Sheet S06. Protease inhibitors may be included in Solution B at the operator’s discretion.
5.2 Ultracentrifuge rotors
Other rotors with different sedimentation path lengths may be suitable but the optimal centrifugation conditions will require investigation; only vertical, near-vertical or low-angle small volume fixed-angle rotors can normally be used for self-generated gradients. For more information see Application Sheet S04.
Interestingly Leblanc et al [4] used a small volume (4 ml maximum) swinging-bucket rotor at a lower g-force (260,000g). This rotor type is not normally used for self-generated gradients; diffusion may be sufficiently rapid to form the correct continuous gradient with this small volume rotor. Moreover, sedimentation of iodixanol molecules will occur at g-forces >180,000g, thus gradient selfgeneration is likely even in this rotor.
5.3 Homogenization
The homogenization protocol should be tailored to the cell type. Dounce homogenization was the most widely used procedure at one time but the ball-bearing homogenizer (“cell cracker”) is now regarded as one of the most effective and reproducible of devices. If this is not available however 10-20 passages through a syringe needle (the Gauge Number (G) varies from 21 to 25) is usually an efficient alternative [3,4]. Occasionally use of a syringe needle is prefaced by Dounce homogenization.
Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some hints on homogenization are given in Application Sheet S06.
5.4 Gradient solutions
If it is considered that it is important to maintain one or more of the reagents present in the homogenization medium at the same concentration throughout the gradient, then make up a Working Solution of 50% iodixanol first. For example, if the homogenization buffer is 0.25 M sucrose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.5, then dilute 5 vol. of OptiPrep™ with 1 vol. of 0.25 M sucrose, 6 mM EDTA, 60 mM Tris-HCl, pH 7.5. If this is used in place of the OptiPrep™ in Step 3, then the concentrations of Tris and EDTA will be the same in all three suspensions. The concentration of the osmotic balancer (0.25 M sucrose) is normally not changed in the Working Solution otherwise the solutions would become significantly hyperosmotic. The volume ratios for the three suspensions using a 50% iodixanol working solution instead of OptiPrep would need to be changed to 2:3:0; 2:2:1 and 2:1:2, respectively. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
5.5 Gradient set-up and formation
Sealed tubes require complete filling; a small volume of Solution B may be added to fill the tube to the required level if necessary.
The ability of a two or three-layer starting format to modulate the density profile obtained by selfgeneration is often a useful means of achieving a more linear gradient [5]. Although it may be considered that one of the important advantages of using a single uniform iodixanol concentration format (i.e. the lack of any interfaces) is lost in the layered format, this is ameliorated by the fact that the particles in the post-nuclear supernatant are diffusely and uniformly spread through the gradient. Diffusion of iodixanol across the interfaces will, moreover, rapidly “soften” the original density discontinuities. This happens more effectively in a vertical rotor than in a swinging-bucket rotor because of the large interfacial surface area. Although Yeaman et al [1] developed this methodology using a vertical rotor, a near-vertical rotor is probably the rotor of choice since any soluble proteins sediment towards the bottom of the tube. In a vertical rotor the proteins will sediment towards the entire length of the wall of that part of the tube furthest from the rotor axis. Subsequently Andersen and Yeaman [6] used a near-vertical rotor.
- It is important to note that although a three-layer discontinuous gradient was often constructed, the selection of vertical, near-vertical or small volume high-performance fixed-angle rotors, centrifuged at approx. 300,000 g will permit the formation of a self-generated continuous (but not necessarily linear) gradient.
5.6 Gradient analysis
The density of the fractions from a blank gradient can be checked by refractometry. Absorbance measurements are an alternative method. For more information see Application Sheet S09.
Figure 2 summarizes the distribution of plasma membrane, endosomes (and TGN) and cytosolic proteins in the gradient. The endosome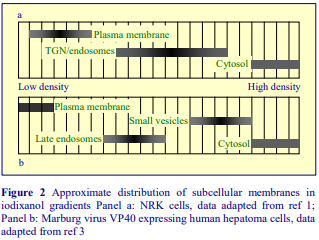 marker (panel a) used by Yeaman et al [1] was syntaxin13, present in early and recycling endosomes. Late endosomes, ER and lysosomes were detected in denser fractions overlapping the lighter cytosolic protein region. On the other hand, late endosomes from human hepatoma cell (panel b) were clearly less dense than their NRK counterparts. Although these gradients can be used for a variety of cell types, the fine detail of the membrane fractionation patterns in these gradients is clearly cell-dependent.
marker (panel a) used by Yeaman et al [1] was syntaxin13, present in early and recycling endosomes. Late endosomes, ER and lysosomes were detected in denser fractions overlapping the lighter cytosolic protein region. On the other hand, late endosomes from human hepatoma cell (panel b) were clearly less dense than their NRK counterparts. Although these gradients can be used for a variety of cell types, the fine detail of the membrane fractionation patterns in these gradients is clearly cell-dependent.
Yeaman et al [1] compared the distribution of the Sec6/8 complex, which is involved with the functioning of the exocytic pathway, in two forms of NRK cells: NRK-49F, which formed fibroblastlike junctions and NRK-52E, which formed epithelial-like junctions. The authors were able to show that in NRK-52E cells the Sec6/8 complex co-fractionated principally with the plasma membrane, while relatively little was detected in the TGN/endosomes or in cytosolic protein regions. In NRK-49F cells on the other hand significant amounts of the complex were detected in the cytosolic proteins and in two distinctive fractions in the TGN/endosome region. The pattern in the latter was quite different to that of the syntaxin13 or VAMP4 profiles.
Kolesnikova et al [3] were able to use the gradient to monitor the translocation of the VP40 matrix protein of Marburg virus in infected cells. At 7 h post-infection most of the VP40 was associated with the small vesicle fraction but as the infection progressed (up to 24 h) the gradient permitted the demonstration of a shift through the endosomal/ER zone to the plasma membrane (Figure 2, panel b).
HEK cells [7,8] and endothelial cells [9] also show the distinctive patterns of plasma membrane, endosomes, small vesicles and soluble proteins shown in Figure 2 (panel b); while if the primary objective is a simple and convenient preparation of plasma membrane, the crude fraction can simply be adjusted to 30% iodixanol; this has been executed with such diverse material as mouse brain [10] and MDCK cells [11].
- Ref 12 reviews the use of self-generated iodixanol gradients in the analysis of the way that exocytic trafficking is involved with the establishment of distinctive plasma membrane domains in epithelial cells.
5.7 Alternative gradient formats
Sometimes the PNS is only present in the densest of the gradient layers; this was the case in the analysis of HeLa cells [13] of recycling and early endosomes from COS cells [14]; a detailed study of MDCK cells, which also included a 15% (w/v) iodixanol layer [15] and an investigation into the membrane association of paxillin in prostate cancer cells in which the PNS in 30% (w/v) iodixanol underlaid 25%, 20%, 15% and 10% (w/v) iodixanol [16]; a near-vertical rotor was used, thus the gradient formed remains a self-generated one.
5.8 Non-mammalian cells
Drospophila exocyst function has been analyzed using the method as described in Sections 2-4 [17] and polarized secretory vesicle delivery in yeast cells has been down-scaled to a TLA120.2 fixedangle rotor; the spheroplast lysate was adjusted to 40% (w/v); underlaid beneath a 35% (w/v) layer and centrifuged at 100,000 g for 3 h [18]. The low g-force used to create this gradient is a consequence of the very small volumes used – a sample volume of 0.1 ml was overlaid by 1 ml of the 35% iodixanol solution in a small volume TLA120.2 fixed-angle rotor. Chang et al [19] and Caballero-Lima et al [20] used the same small volume rotor to generate the gradient in studies of yeast secretory vesicles.
B. Pre-formed gradients
The following table summarizes the cell types and gradient formats that have been used; a short description of the analysis is also included.
C. References
1. Yeaman, C., Grindstaff, K. K., Wright, J. R. and Nelson, W. J. (2001) Sec6/8 complexes on trans-Golgi network and plasma membrane regulate stages of exocytosis in mammalian cells J. Cell Biol., 155, 593-604
2. Marsh, M., Schmid, S., Kern, H., Harms, E., Male, P., Mellman, I. and Helenius, A. (1987) Rapid analytical and preparative isolation of functional endosomes by free flow electrophoresis J. Cell Biol., 104, 875-886
3. Kolesnikova, L., Bamberg, S., Berghöfer, B. and Becker, S. (2004) The matrix protein of Marburg virus is transported to the plasma membrane along cellular membranes: exploiting the retrograde late endosomal pathway J. Virol., 78, 2382-2393
4. Leblanc, P., Alais, S., Porto-Carriero, I., Lehmann, S., Grassi, J., Raposo, G. and Darlix, J.L. (2006) Retrovirus infection strongly enhances scrapie infectivity release in cell culture The EMBO J., 25, 2674-2685
5. Graham, J., Ford, T. and Rickwood, D. (1994) Iodixanol: A nonionic iso-osmotic centrifugation medium for the formation of self generated gradients Anal. Biochem. 220, 360-366
6. Andersen, N.J. and Yeaman, C. (2010) Sec3-containing exocyst complex is required for desmosome assembly in mammalian epithelial cells Mol. Biol. Cell, 21, 152-164
7. Grigorov, B., Arcanger, F., Roingeard, P., Darlix, J-L. and Muriaux, D. (2006) Assembly of infectious HIV-1 in human epithelial and T-lymphoblastic cell lines J. Mol. Biol., 359, 848-862
8. Grigorov, B., Décimo, D., Smagulova, F., Péchoux, C., Mougel., M., Muriaux, D. and Darlix, J-L. (2007) Intracellular HIV-1 Gag localization is impaired by mutations in the nucleocapsid zinc fingers Retrovirology, 4:54
9. Lampugnani, M.G., Orsenigo, F., Gagliani, M.C., Tacchetti, C. and Dejana, E. (2006) Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments J. Cell Biol., 174, 593-604
10. Jacobs, S.B.R., Basak, S., Murray, J.I. and Attardi, L.D. (2007) Siva is an apoptosis-selective p53 target gene important for neuronal cell death Cell Death Differ., 14, 1374-1385
11. Yeh, T-Y.J., Meyer, T.N., Schwesinger, C., Tsun, Z-Y., Lee, R.M. and Chi, N-W. (2006) Tankyrase recruitment to the lateral plasma membrane in polarized epithelial cells: regulation by cell-cell contact and protein poly(ADP-ribosyl)ation Biochem J., 399, 415-425
12. Yeaman, C. (2003) Ultracentrifugation-based approaches to study regulation of Sec6/8 (exocyst) complex function during development of epithelial cell polarity Methods, 30, 198-206
13. Hase, K., Kimura, S., Takatsu, H., Ohmae, M., Kawano, S., Kitamura, H., Ito, M., Watarai, H., Hazelett, C.C., Yeaman, C. and Ohno, H. (2009) M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex Nat. Cell Biol., 11, 1427-1432
14. Chen, X-W., Inoue, M., Hsu, S. and Saltiel, A.R. (2006) RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis J. Biol. Chem., 281, 38609-38616
15. Wang, Q., Chen, X-W. and Margolis, B. (2007) PALS1 regulates E-cadherin trafficking in mammalian epithelial cells Mol. Biol. Cell, 18, 874-885
16. Spiczka, K.S. and Yeaman, C. (2008) Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration J. Cell Sci., 121, 2880-2891
17. Beronja, S., Laprise, P., Papoulas, O., Pellikka, M., Sisson, J. and Tepass, U. (2005) Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells J. Cell Biol., 169, 635-646
18. Medkova, M., France, Y.E., Coleman, J. and Novick, P. (2006) The rab exchange factor Sec2p reversibly associates with the exocyst Mol. Biol. Cell, 17, 2757-2769
19. Chang, W., Zaarour, R.F., Reck-Peterson, S., Rinn, J., Singer, R.H., Snyder, M., Novick, P. and Mooseker, M.S. (2008) Myo2p, a class V myosin in budding yeast, associates with a large ribonucleic acid–protein complex that contains mRNAs and subunits of the RNA-processing body RNA, 14, 491-502
20. Caballero-Lima, D., Hautbergue, G.M., Wilson, S.A. and Sudbery, P.E. (2014) In Candida albicans hyphae, Sec2p is physically associated with SEC2 mRNA on secretory vesicles Mol. Microbiol., 94, 828–842
21. Cantin, R., Diou, J., Belanger, D., Tremblay, A.M. and Gilbert, C. (2008) Discrimination between exosomes and HIV-1: Purification of both vesicles from cell-free supernatants J. Immunol. Methods, 338, 21-30
22. Lenassi, M., Cagney, G., Liao, M., Vaupotǐc, T., Bartholomeeusen, K., Cheng, Y., Krogan, N.J., Plemenitǎ, A. and Peterlin, B.M. (2010) HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells Traffic, 11, 110–122
23. Neto, H., Kaupisch, A., Collins, L.L. and Gould, G.W, (2013) Syntaxin 16 is a master recruitment factor for cytokinesis Mol. Biol. Cell, 24, 3663-3674
24. Cao, Z., Li, C., Higginbotham, J.N., Franklin, J.L., Tabb, D.L., Graves-Deal, R., Hill, S., Cheek, K., Jerome, W.G., Lapierre, L.A., Goldenring, J.R., Ham, A-J.L. and Coffey, R.J. (2008) Use of fluorescence-activated vesicle sorting for isolation of Naked2-associated, basolaterally targeted exocytic vesicles for proteomics analysis Mol. Cell. Proteomics, 7, 1651-1667
25. Li, C., Hao, M., Cao, Z., Ding, W., Graves-Deal, R., Hu, J., Piston, D.W. and Coffey, R.J. (2007) Naked2 acts as a cargo recognition and targeting protein to ensure proper delivery and fusion of TGF--containing exocytic vesicles at the lower lateral membrane of polarized MDCK cells Mol. Biol. Cell 18, 3081-3093
26. Majumdar, R., Tavakoli Tameh, A. and Parent, C.A. (2016) Exosomes mediate LTB4 release during neutrophil chemotaxis PLoS Biol., 14: e1002336
27. Kreutzberger, A.J.B., Kiessling, V., Liang, B., Seelheim, P., Jakhanwal, S., Jahn, R., Castle, D. and Tamm, L.K. (2017) Reconstitution of calcium-mediated exocytosis of dense-core vesicles Sci. Adv., 3: e1603208
OptiPrepTM Application Sheet S47; 8th edition, January 2020
OptiPrep™ Application Sheet S48
Analysis of membrane trafficking and intracellular signaling in selfgenerated gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- The protocols in this Application Sheet were developed principally as a simple and reproducible means of fractionation low-density microsomes for analysis of the complex endosome-TGN transport cycle and trafficking of the GLUT4 glucose transporter
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5)
1. Background
It is often difficult to devise suitable and reliable gradients which are able to resolve the multiple smooth membrane compartments which are involved in (a) the trafficking of macromolecules between the trans-Golgi network (TGN), plasma membrane and parts of the endocytic system and (b) the complex patterns of intracellular signaling which control many cellular processes. Sucrose gradients have been partially successful in dissecting out these smooth membranes but their high osmolality tends to compromise the resolving power of both equilibrium density and sedimentation velocity gradients.
Self-generated isoosmotic iodixanol gradients offer a superior strategy for the study of the interrelationships of these complex smooth membrane compartments. A major advantage of the use of these gradients is that centrifugation conditions can be chosen which produce suitably shallow gradients reproducibly. Moreover the density range of the shallowest part of the gradient can be adjusted by varying either the starting concentration of iodixanol or the centrifugation time or both. Similar preformed continuous (or discontinuous) gradients can be difficult to prepare. Adjusting the sample to a uniform concentration of iodixanol and centrifuging it in a vertical or near-vertical rotor is not only a simple way of achieving the right sort of gradient, the lack of any interfaces which may cause accumulation and aggregation of particles is avoided. Some of the density profiles that can be obtained in this manner are described in Application Sheet S04.
This protocol, developed for analyzing the translocation of GLUT4 containing vesicles between the TGN and the plasma membrane by Hashiramoto and James [1], has been taken up by a number of research groups working in similar areas [2-4] and adapted to studies of other membranes [2]. It incorporates the strategy of using a low-density microsome fraction as the gradient input, commonly used in GLUT 4 studies that may have a wider application to other investigations. This avoids the inclusion of any of the denser organelles such as lysosomes, peroxisomes and rough endoplasmic reticulum in the gradient; an advantage denied the vast preponderance of methods that use a postnuclear supernatant.
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 1 mM EDTA, 20 mM Hepes-NaOH, pH 7.4
C. Diluent: 0.25 M sucrose, 6 mM EDTA, 120 mM Hepes-NaOH, pH 7.4 (optional)
D. Working Solution (50%, w/v iodixanol): Mix 5 vol. of Solution A with 1 vol. of Solution C (optional)
3. Ultracentrifuge rotor requirements
Fixed angle rotors (e.g. Beckman 80Ti or 70.1Ti or Sorvall T865.1 or T-1270) for sedimentation of LDM fraction
Near vertical rotor with a tube capacity 4-5 ml (Beckman NVT65.2) or a vertical rotor (e.g. Beckman VTi65.2 or VTi90) with Optiseal™ tubes for the self-generated gradient (see Section 5.2)
4. Protocol (adapted from refs 1 and 4)
Following any metabolic labeling steps performed at 37C; carry out all subsequent operations at 0-4°C.
1. Wash the cells two or three times in Solution B to remove any culture medium (it is important to remove all traces of any culture/incubation medium prior to homogenization).
2. Suspend the cells in Solution B and homogenize either in a Dounce homogenizer, by repeated passages through a fine syringe needle (22-gauge) or in a “cell cracker” (see Section 5.3).
3. Centrifuge the homogenate at 1000 g for 10 min to pellet the nuclei and cell debris (see Section 5.4).
4. Centrifuge the 1000g supernatant at 27,000 gmax in a fixed-angle rotor for 15 min to pellet all of the major organelles and most of the high-density microsomes (see Section 5.4).
5. Centrifuge the 27,000 g supernatant at 235,000 gmax to pellet the low-density microsomes (LDM).
6. Resuspend the LDM in Solution B and mix well with Solution D or OptiPrep™ to give the appropriate iodixanol concentration, e.g. 14% or 30 w/v (see Sections 5.1 and 5.5).
7. Transfer 4-5 ml to a sealed tube for a near-vertical or vertical rotor and centrifuge at 265,000 gav for 4 h (see Section 5.5).
8. Allow the rotor to decelerate using a controlled deceleration program or turn off the brake at 2000 rpm.
9. Collect the gradients from Beckman Optiseal tubes by tube puncture, upward displacement with a dense liquid or aspiration from the meniscus (see Section 5.2). For more information on harvesting gradients see Application Sheet S08.
- A brief summary of the analytical capabilities of this gradient system is given in Section 5.6
5. Technical Notes and Review
5.1 Homogenization media and gradient solutions
The homogenization medium often has to be tailored to the tissue or cell type and it is not known if the composition of the HM is relevant to the separation. Organic osmotic balancers such as sucrose, mannitol and sorbitol were introduced for their compatibility in functional studies on subcellular membranes; moreover these low ionic strength HMs and gradient solutions permit the direct use of fractions for SDS-PAGE. All of the published methods using the type of protocol described in this OptiPrep™ Application Sheet have employed a 0.25 M sucrose buffered with either Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and often, but not always, containing 1 mM EDTA.
Supplementation of the HM with inorganic salts is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation. Other examples are given in Application Sheets S05 (tissues) and S06 (cells).
The optional production of a working solution of iodixanol containing the same concentrations of EDTA and buffer as the homogenization medium allows these concentrations to be maintained in the LDM suspension when its density is raised (see Step 6). If this is not regarded as important the density can be raised by mixing with OptiPrep™ (as used in the original method).
Protease inhibitors may be included in Solutions B and C at the operator’s discretion. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
5.2 Ultracentrifuge rotors and tubes
A near-vertical rotor of slightly smaller tube volume such as the TLN100 (3 ml) will also be very suitable. Larger volume vertical and near-vertical rotors can also be used for self-generated gradients but the optimal centrifugation conditions of those with longer sedimentation path lengths may require investigation. The use of Beckman Optiseal™ tubes is recommended because of the ease of use and the ability to use a variety of options for gradient unloading (see Step 9); for other tubes such as heatsealed tubes, tube puncture is the only safe and reliable option.
5.3 Homogenization
The homogenization protocol should be tailored to the cell (or tissue) type. Potter-Elevhjem or Dounce homogenization for tissues and Dounce homogenization for cells used to be the standard procedures. For cells however use of 12-20 passages through a syringe needle (the Gauge Number (G) varies from 21 to 25) sometimes preceded by Dounce homogenization, has become very common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices.
Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. Some tips on homogenization are given in Application Sheets S05 (tissues) and S06 (cells).
5.4 Preparation of the LDM fraction
The 1000g centrifugation may be omitted but removal of nuclei and cell debris at a low speed may enhance the recovery of smaller less dense particles later on. If the source material is a tissue (e.g. skeletal muscle) or primary cells from a tissue, the LDM is normally prepared by centrifugation of a 10,000g supernatant through a 0.4/1.5M sucrose gradient (see ref 1 for more details).
5.5 Self-generated gradient formation
By modulating the starting concentration of the iodixanol and the time of centrifugation it is possible to enhance the resolution of vesicles of different densities; 14% or 30% (w/v) iodixanol and 1 or 4 h are the two commonly used starting concentrations and centrifugation times respectively, but these should be modified in the light of experience. For more information on the influence of these parameters on the density profile see Application Sheet S04.
If a vertical rotor is used, it may be necessary to include a small cushion of 20% iodixanol to prevent any dense contaminants from reaching the wall of the tube. In a near-vertical rotor this poses no problem.
5.6 Analysis
Immunoblotting of the gradient fractions with antibodies to GLUT4, TfR, sortilin, VAMP2 and Rab4 showed that the gradient formed from 14% iodixanol was capable of fine discrimination of the complex endosomal-TGN system [1]. In 4 h gradients it was possible to detect at least two populations of GLUT4 containing vesicles, coincident with TGN or endosomal markers.
The steep nature of the denser regions of this gradient however does not allow simultaneous resolution of denser vesicles. From this 14% iodixanol gradient, for example (top 3 panels of Figure 1), two populations of GLUT4 containing vesicles are evident but it is not entirely clear if the phosphoinositide kinases PIKfyve and P85 PI3K are entirely confined to a denser membrane compartment [2]. The gradient formed from 30% iodixanol on the other hand not only completely resolves the kinases from endosome-TGN markers (bottom five panels of Figure 1), it is also able to partially resolve vesicles containing the two types of kinase [2]. PhosphoSer-p40 was found to co-fractionate with PIKfyve, while the recycling protein IRAP was detected principally in fractions of lower density [6].
Using the 14% iodixanol starting concentration, Maier and Gould [3] reduced the centrifugation time to 1 h in the Beckman TLN100 rotor and were able to identify the denser fraction as the GLUT4 storage vesicle (GSV), whose GLUT4 responded hugely to short term insulin stimulation. The lower density fraction was coincident with TGN and endosome markers, which was also rich in the cysteinestring protein Csp-1 [4]. Moreover Syntaxin STX16 was targeted to the GSV, while STX6 targeted the TNG/endosomes [5]. Yeh et al [7] and Liu et al [8] also used the standard 14% iodixanol starting concentration and centrifugation times of 4 h and 2.5 h respectively in GLUT4 transport studies. Ma et al [9], used a broadly similar methodology to that of Liu et al [8], to demonstrate that insulin-generated oxidative stress directs GLUT4 to lysosomes.
Ikonomov al [10] employed the same self-generated gradient system [2] to show that the kinesin adapter, JLP, interacts with PIKfyve and that both proteins and their association are required in microtubule-based, but not in microtubule independent, endosome-to-TGN cargo transport. A selfgenerated gradient system used by Chen et al [11] to study exocyst regulation of vesicle delivery to the centrosome prior to cytokinesis in COS cells, in which they observed a co-banding of RalA with TfR and Rab11 but not with early endosomes, Golgi or cytosol markers has been adapted by Landry et al [12] to 293T cells to identify TGN, cis-Golgi and recycling endosomes (RE) in the gradient and to study RE trafficking to the Golgi. Xie et al [13] also used a self-generated gradient for investigating the insertion of GLUT4 into the plasma membrane.
Karunanithi et al [14] used the established 14% (w/v) iodixanol starting concentration to discover a G protein cascade that regulated GLUT4 trafficking. The same gradient formula also demonstrated that VAMP2 also has a major influence on GLUT4 trafficking [15].
- Sadler et al [16] have recently produced a detailed methodology for the use of iodixanol gradients in studies on GLUT4.
6. References
1. Hashiramoto, M. and James, D. E. (2000) Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3-L1 adipocytes Mol. Cell. Biol., 20, 416-427.
2. Shisheva, A., Rusin, B., Ikonomov, O. C., DeMarco, C. and Sbrissa, D. (2001) Localization and insulinregulated relocation of phosphoinositide 5-kinase PIKfyve in 3T3-L1 adipocytes J. Biol. Chem., 276, 11859-11869.
3. Maier, V. H. and Gould, G.W. (2000) Long-term insulin treatment of 3T3-L1 adipocytes results in mistargeting of GLUT4: implications for insulin-stimulated glucose transport Diabetologia, 43, 1273-1281.
4. Chamberlain, L. H., Graham, M. E., Kane, S., Jackson, J. L., Maier, V. H., Burgoyne, R. D. and Gould, G. W. (2001) The synaptic vesicle protein, cysteine-string, is associated with the plasma membrane in 3T3-L1 adipocytes and interacts with syntaxin 4 J. Cell Sci., 114, 445-455.
5. Perera, H. K. I., Clarke, M., Morris, N. J., Hong, W., Chamberlain, L. H. and Gould, G. W. (2003) Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes Mol. Biol. Cell, 14, 2946-2958
6. Ikonomov, O.C., Sbrissa, D., Mlak, K., Deeb, R., Fligger, J., Soans, A., Finley, R.L. and Shisheva, A. (2003) Active PIKfyve associates with and promotes the membrane attachment of the late endosome-to-trans-Golgi network transport factor Rab9 effector p40 J. Biol. Chem., 278, 50863-50871
7. Yeh, T-Y.J., Sbodio, J.I., Tsun, Z-Y., Luo, B. and Chi, N-W. (2007) Insulin-stimulated exocytosis of GLUT4 is enhanced by IRAP and its partner tankyrase Biochem. J. 402, 279-290
8. Liu, L-B., Omata, W., Kojima, I. and Shibata, H. (2007) The SUMO conjugating enzyme Ubc9 is a regulator of GLUT4 turnover and targeting to the insulin-responsive storage compartment in 3T3-L1 adipocytes Diabetes, 56, 11977-1985
9. Ma, J., Nakagawa, Y., Kojima, I. and Shibata, H. (2014) Prolonged insulin stimulation down-regulates GLUT4 through oxidative stress-mediated retromer inhibition by a protein kinase CK2-dependent mechanism in 3T3-L1 J. Biol. Chem., 289, 133-142
10. Ikonomov, O.C., Fligger, J., Sbrissa, D., Dondapati, R., Mlak, K., Deeb, R. and Shisheva, A. (2009) Kinesin adapter JLP links PIKfyve to microtubule-based endosome-to-trans-Golgi network traffic of furin J. Biol. Chem., 284, 3750–3761
11. Chen, X-W., Inoue, M., Hsu, S. and Saltiel, A.R. (2006) RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis J. Biol. Chem., 281, 38609-38616
12. Landry, M-C., Sicotte, A., Champagne, C. and Lavoie, J.N. (2009) Regulation of cell death by recycling endosomes and Golgi membrane dynamics via a pathway involving Src-family kinases, Cdc42 and Rab11a Mol Biol. Cell, 20, 4091-4106
13. Xie, X., Gong, Z., Mansuy-Aubert, V., Zhou, Q.L., Tatulian, S.A., Sehrt, D., Gnad, F., Brill, L.M. Motamedchaboki, K., Chen, Y., Czech, M.P., Mann, M., Krüger, M. and Jiang, Z.Y. (2011) C2 domaincontaining phosphoprotein CDP138 regulates GLUT4 insertion into the plasma membrane Cell Metab., 14, 378–389
14. Karunanithi, S., Xiong, T., Uhm, M., Leto, D., Sun, J., Chen, X-W., and Saltiel, A.R. (2014) A Rab10:RalA G protein cascade regulates insulin-stimulated glucose uptake in adipocytes Mol. Biol. Cell, 25, 3059-3069
15. Sadler, J.B.A., Bryant, N.J. and Gould, G.W. (2015) Characterization of VAMP isoforms in 3T3-L1 adipocytes: implications for GLUT4 trafficking Mol. Biol. Cell, 26, 530-536
16. Sadler, J.B.A., Lamb, C.A., Gould, G.W. and Bryant, N.J. (2016) Iodixanol gradient centrifugation to separate components of the low-density membrane fraction from 3T3-L1 adipocytes Cold Spring Harb. Protoc., doi:10.1101/pdb.prot083709
OptiPrepTM Application Sheet S48; 8th edition, January 2020
OptiPrep™ Application Sheet S49
Analysis of membranes from Drosophila
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- This Application Sheet contains the following: Section 1 – Fractionation of ER, Golgi and PM; Section 2 – Lipid rafts; Section 3 – Other analyses.
- At the end of Sections 1 and 2 there are important technical reviews.
- Section 4 summarizes some of the more recent papers.
1. Fractionation of endoplasmic reticulum (ER), Golgi and plasma membrane (PM)
1a. Background
Although iodixanol gradients have been used widely since 1996 for the fractionation of these subcellular membrane compartments from mammalian cells, their application to the membranes from Drosophila has only been realized relatively recently (mainly since 2004). The methodology described in this OptiPrep™ Application Sheet employs a pre-formed 10%, 20%, 30% (w/v) iodixanol discontinuous gradient in a near-vertical rotor [1]. A crude membrane fraction was incorporated into each iodixanol solution and in this respect the method is similar to that devised by Yeaman et al [2] for MDCK cells. A more or less linear continuous gradient will from, partly by self-generation in the centrifugal field and partly by diffusion. Membranes are separated on the basis of buoyant density. It is quite likely that other iodixanol gradients, developed for mammalian cells would also be applicable, but this can only be verified by experimentation.
1b. Solutions required (see Section 1e-1)
A. OptiPrep™
B. Wash Solution 1: 0.7% (w/v) NaCl, 0.03% (w/v) Triton X-100
C. Homogenization Medium (HM): 0.25 M sucrose, 5 mM EDTA, 10 mM Tris-HCl, pH 7.5
D. KCl: 2M KCl
E. Sucrose Cushion: 0.5 M sucrose, 0.5 mM EDTA, 50 mM KCl, 15 mM Tris-HCl, pH 7.0
F. Wash Solution 2: 5 mM EDTA, 50 mM KCl, 10 mM Tris-HCl, pH 7.5
G. Membrane Suspension Buffer: 0.25 M sucrose, 50 mM KCl, 5 mM EDTA, 10 mM Tris-HCl, pH 7.5
H. Optiprep™ Dilution Buffer: 300 mM KCl, 30 mM EDTA, 60 mM Tris-HCl, pH 7.5
I. Optiprep™ Working Solution (50% iodixanol): Dilute 5 vol. OptiPrep™ with 1 vol. of Solution H Protease inhibitors may be included in Solutions B, C and E-H at the operator’s discretion.
1c. Ultracentrifuge rotor requirements (see Section 1e-2)
Swinging-bucket rotor (e.g. Beckman SW55Ti or SW41Ti) and a near-vertical rotor (e.g. Beckman NVT90 or NVT65.2)
1d. Protocol (adapted from refs 1 and 3)
Carry out all operations at 0-4°C.
Option A: Membranes in the 3000 g supernatant (from Step 4) may simply be pelleted and resuspended before iodixanol gradient centrifugation (Step 5)
Option B: Membranes may be separated from cytosolic proteins and small contaminants on a sucrose density barrier (Steps 6-8) before iodixanol gradient centrifugation.
1. Wash dechorionated 1-3 h embryos twice in Solution B and once in Solution C.
2. Suspend embryos in 10 vol. of Solution C and homogenize firstly in a loose-fitting Dounce (glassglass) homogenizer (Wheaton type B) and then a tight-fitting one (Wheaton type A). Monitor the homogenization by light microscopy.
3. Add Solution D to the homogenate (0.1 vol. + 3.9 vol. respectively) to adjust the KCl concentration to 50 mM (see Section 1e-1).
4. Centrifuge the homogenate at 3000 g for 10 min to remove debris and larger organelles.
5. Option A: Pellet the membranes from the 3000 g supernatant at 100,000 g for 1 h (total membrane fraction) OR at 20,000 g for 30 min to isolate a Golgi-rich fraction (see Section 1e-3).
6. Option B: Layer the 3000 g supernatant on a cushion of Solution E and centrifuge in a swingingbucket rotor at 100,000 g for 1 h (see Section 1e-3).
7. Option B: Using a syringe and metal cannula (i.d. 0.8-1.0 mm) aspirate as much of the supernatant as possible without disturbing the pellet.
8. Option B: Resuspend the pellet in the residual solution; mix with 5 vol. of Solution F and recentrifuge at 100,000 g for 1 h.
9. Using a syringe and metal cannula aspirate the supernatant from Step 5 or Step 8 and resuspend the pellet in approx. 1 ml of Solution G.
10. Prepare three gradient solutions (approx. 2 ml each) from the following volume ratios of Solution I, the membrane suspension and Solution G: 3:2:0, 2:2:1 and 1:2:2 (see Section 1e-4).
11. Layer 1.8 ml of each gradient solution in Optiseal™ tubes for the near-vertical rotor by, under- or over-layering (see Section 1e-5).
12. Centrifuge at 340,000 gav for 3 h using a slow acceleration program.
13. Allow the centrifuge to decelerate to rest from 2000 rpm without the brake or use a slow deceleration program.
14. Collect the gradient in 0.25 ml fractions by tube puncture, upward displacement or aspiration from the meniscus. For more information on harvesting gradients from sealed tubes see Application Sheet S08.
- For information on analysis of the gradient fractions and a short review of some of the other papers see Section 1e-6.
1e. Technical Notes and Review
1e-1. Homogenization media
The homogenization medium (HM) often has been specifically tailored to fractionation of Drosophila membranes. In the example given solutions are buffered with Tris but Hepes, Tricine or triethanolamine (at the same concentration) may be used if preferred. It is unlikely that the type of buffer significantly influences the homogenization or fractionation, although with mammalian cells triethanolamine does seem to offer some particular advantages in homogenization efficiency.
The preparation of a Working Solution (Solution I) as described, ensures that the concentrations of KCl, EDTA and Tris buffer are constant throughout the gradient, while the sucrose and iodixanol act as osmotic balancers to maintain an approx. constant osmolality. If this is deemed unimportant the gradient solutions may be prepared directly from OptiPrep™, but if this option is chosen then the concentrations of KCl, EDTA and buffer will decrease with increasing solution density.
Beronja et al [1] adjusted the KCl concentration in the homogenate (50 mM) as described in the protocol, but Papoulas et al [3] adjusted it to 100 mM. If the KCl concentration is adjusted to 100 mM KCl, then all subsequent solutions used (Solutions E-H) should be similarly adjusted.
Tan et al [4] used 0.25 M sucrose, 1 mM EDTA, 1 mM DTT, 10 mM HEPES, pH 7.4 as an homogenization medium in their proteomic studies and in some cases the osmotic balancer is NaCl and not sucrose, e.g. 150 mM NaCl, 0.2 mM EGTA, 100 mM Tris, pH 7.4 [5]
1e-2. Ultracentrifuge rotors
Choose whichever swinging-bucket rotor is most suitable for the amount of material available (see Step 6). The iodixanol gradient centrifugation is carried out in a near-vertical rotor. The gradient is formed partly by self-generation, partly by diffusion. Other rotors with different sedimentation path lengths may be suitable but the optimal centrifugation conditions will require investigation. Nearvertical rotors are preferred over vertical ones because any very dense particles will form a well defined pellet close to the bottom of the tube (as in a fixed-angle rotor), while in a vertical rotor any dense material will pellet along the entire length of the wall of the tube and may contaminate fractions during unloading. The use of Beckman Optiseal™ tubes is recommended because of the ease of use and the ability to use a variety of options for gradient unloading (see Step 14); for other tubes such as heat-sealed tubes, tube puncture is the only safe and reliable option. For more information selfgenerated gradients see Application Sheet S04.
1e-3. Preliminary purification
For the analysis of the major membrane fractions (plasma membrane, Golgi and ER) the 3000 g supernatant may be simply centrifuged at 100,000 g to pellet the microsomes [3] or partially purified by sedimentation through a sucrose cushion [1]. The latter will more effectively remove soluble proteins, which will remain principally in the sample zone. The 100,000 g step was replaced by Papoulas et al [3] by a 20,000 g step if the aim was to isolate primarily the Golgi for use in vitro incubations.
1e-4. Gradient variations
Adolfson et al [6] adopted a simpler iodixanol gradient format; the post-nuclear extract was adjusted to 26% (w/v) iodixanol to form a self-generated gradient at 300,000 gav in a near-vertical rotor, while Niimura et al [7] used a discontinuous 2.5-30% (w/v) iodixanol gradient.
1e-5 Gradient layering
Although underlayering with a syringe and metal cannula is the recommended method for making discontinuous gradients, overlayering maybe more convenient since the tubes need to be filled exactly to the bottom of the neck. For more information on gradient construction see Application Sheet S03. If necessary, adjust all volumes proportionately so that tubes (after sample application) are properly filled according to the manufacturer’s instructions.
1e-6. Method review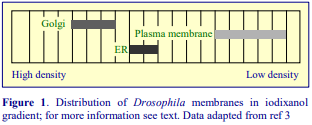
In the gradient system described in this OptiPrep™ Application Sheet, the Golgi, ER and PM banded approximately as shown in Figure 1. The Golgi was identified by the Lava lamp protein, the ER by BiP and the PM by α-spectrin, which was also present in the Golgi region [3]. Unlike most mammalian cells the Golgi was denser than the ER; but like mammalian cells, the PM banded close to the top of the gradient. Dynein also co-banded with the Golgi but was also present in some denser fractions. These gradients were used in characterization of the Sec6 component of the exocyst complex [1], the dynein based motility of Golgi membranes [3] and to confirm the association of dLgl and dFmr1 with the Golgi [8]. The method has also been used in proteomic studies [9] and to identify an ER location for the Seele protein [10].
In the 2.5-30% discontinuous iodixanol gradients used by Niimura et al [7] the ER banded at a higher density than the Golgi. In the self generated gradients reported by Adolfsen et al [6], synaptic vesicles banded close to the top of the gradient and clearly discriminated low-density vesicles containing Synaptotagmin 1 from denser ones containing Synaptotagmins 4 and 7. Paneels et al [11] used a 5%, 30%, 40% iodixanol flotation gradient to band the plasma membrane at the 5%/30% interface (see Section B).
A 10-40% (w/v) iodixanol gradient (250,000 g, for 3 h) separates the plasma membrane from the cytoskeleton [12]. A simple three-layer gradient in which a 1000 g supernatant in 40% iodixanol is layered beneath layers of 5% and 30% iodixanol [5,13] centrifuged at 100,000 g for 3 h results in the plasma membrane banding below the 5% layer. Tan et al [4] underlaid a 2000 g/5 min supernatant with 6% and 8% (w/v) iodixanol and centrifuged at 100,000 g for 90 min to concentrate the membranes at the interface of the two iodixanol solutions. The recovered membranes were adjusted to 12.5% iodixanol and centrifuged in a Beckman VTi65.1 vertical rotor for 1 h. The method resolved PM, Golgi, ER and mitochondria.
- For some more recent publications see Section 4
2. Isolation of lipid rafts (as detergent-resistant membranes)
2a. Background
Rietveld et al [14] were the first to report isolation of lipid rafts from Drosophila using flotation in iodixanol gradients. A crude PM fraction was first produced from a post-nuclear supernatant of the homogenate (adjusted to 1.4 M sucrose) by flotation through a layer of 1.22 M sucrose. Hoehne et al [15] used a similar approach. Paneels et al [11] and Eroglu et al [16] adapted this plasma membrane isolation method to iodixanol. The crude low-density membrane fraction was then extracted with Triton X-100 and the lipid rafts isolated by flotation through a discontinuous iodixanol gradient. Zhai et al [17] prepared lipid rafts from both Drosophila and from the Drosophila S2 cell line directly from a 5000g supernatant of the homogenate, without a preliminary preparation of a crude PM fraction.
- The method below is adapted from refs 11, 14 and 16. Some of the variants are described in the Technical Notes and Review Section (Section 2e).
2b. Solutions required (see Box on next page and Section 2e-1)
A. OptiPrep™
B. Wash Solution 1: 0.9% (w/v) NaCl, 0.1% (w/v) Triton X-100
C. Wash Solution 2: 0.9% (w/v) NaCl
D. Homogenization Medium (HM): 0.3 M sucrose, 150 mM NaCl, 0.2 mM EGTA, 100 mM Tris-HCl, pH 7.5
E. TNE: 150 mM NaCl, 0.2 mM EGTA, 100 mM Tris-HCl, pH 7.5
F. Optiprep™ Dilution Buffer: 150 mM NaCl, 1.2 mM EGTA, 100 mM Tris-HCl, pH 7.5
G. Optiprep™ Working Solution (50% iodixanol): Dilute 5 vol. OptiPrep™ with 1 vol. of Solution F
2c. Ultracentrifuge rotor requirements (see Section 2e-2)
Swinging-bucket rotors: approx. 27 ml tubes (e.g. Beckman SW28) and approx. 5 ml tubes (e.g. Beckman SW55)
2d. Protocol
Carry out all operations, except step 1, at 0-4°C.
1. Wash the dechorionated embryos twice in Solution B, three times in Solution C.
2. Wash the embryos twice in Solution D.
3. Suspend washed embryos in 10 vol. of Solution D and homogenize firstly in a loose-fitting Dounce (glass-glass) homogenizer (Wheaton type B) and then a tight-fitting one (Wheaton type A). Monitor the homogenization by light microscopy (see Section 2e-3).
4. Centrifuge the homogenate at approx. 3000 g for 10 min to remove nuclei and debris.
5. Adjust the iodixanol concentration of the 3000 g supernatant to 40% (w/v) iodixanol by mixing 1 vol. with 4 vol. of Solution G (see Section 2e-4).
6. Prepare solutions of 30% and 5% (w/v) iodixanol from Solution G and Solution D (volume ratios of 3:2 and 1:9 respectively).
7. Distribute the 3000 g supernatant (in 40% iodixanol) equally amongst tubes for the 27 ml swinging-bucket rotor and layer 10 ml and 5 ml respectively of the 30% and 5% iodixanol solutions on top to fill the tube (see Section 2e-5).
8. Centrifuge at 100,000 g for 3 h.
9. Collect the plasma membrane enriched fraction from the 5%/30% iodixanol interface and dilute with 3 vol. of Solution E.
10. Pellet the membranes at 50,000 g for 30 min.
11. Aspirate the supernatant; resuspend the pellet in Solution E and repeat Step 10.
12. Resuspend the pellet in 0.5 ml of Solution E and mix with an equal volume of Solution E containing 2% (w/v) Triton X-100 (or other chosen detergent at twice the required concentration).
13. Keep at 4°C for 30 min to solubilize the detergent-sensitive membranes.
14. During the solubilization prepare solutions of 21%, 15% and 6%(w/v) iodixanol by diluting Solution G with Solution E at volume ratios of 2.1:2.9, 1.5:3.5 and 0.6:4.4 respectively. Note that there are important published variations in the density of the gradient solutions (see Section 2e-6).
15. Mix the suspension from Step 13 with an equal volume of Solution G.
16. In tubes for the approx. 5 ml swinging-bucket rotor, over layer 2 ml of the sample with 1 ml each of the 21%, 15% and 5% (w/v) iodixanol solutions (see Section 2e-5).
17. Centrifuge at approx 150,000 gav for 5-6 h (see Section 2e-7).
18. Allow the rotor to decelerate without the brake below 2000 rpm or use a controlled deceleration program.
19. The lipid rafts band as a visible layer at the top interface. Harvest this layer or collect the gradient in 0.25-0.5 ml fractions by tube puncture, upward displacement or aspiration from the meniscus. For more information on harvesting gradients see Application Sheet S08.
2e. Technical Notes and Review
2e-1. Homogenization media
The homogenization medium (HM) often has been specifically tailored to fractionation of Drosophila membranes. In the example given solutions are buffered with Tris but Hepes, Tricine or triethanolamine (at the same concentration) may be used if preferred. It is unlikely that the type of buffer significantly influences the homogenization or fractionation, although with mammalian cells triethanolamine does seem to offer some particular advantages in homogenization efficiency.
HM variations include 30 mM NaCl, 5 mM EDTA, 20 mM HEPES, pH 7.5, 1% TX-100 for Drosophila photoreceptors [18], 150 mM NaCl, 20 mM EGTA, 100 mM Tris-HCl pH 7.5, 1% TX-100 for a Drosophila neuronal cell line [19] and Drosophila heads [20].
The preparation of a Working Solution (Solution G) as described, ensures that the concentration of EGTA is constant throughout the gradients. If this is deemed unimportant the gradient solutions may be prepared directly from OptiPrep™. An advantage of this approach is that the final volume of dense membrane suspension (Steps 5 and 15 of the protocol) is smaller.
Protease inhibitors may be included in Solutions D-F at the operator’s discretion.
2e-2. Ultracentrifuge rotors
For smaller amounts of starting material a rotor such as a 14 ml rotor (e.g. Beckman SW41) may be substituted for the SW28.
2e-3. Homogenization
Other means of homogenization have been reported; Rietveld et al [14] used a Potter-Elvehjem (glass-Teflon) homogenizer before the double Dounce homogenization. Zhai et al [17] homogenized the embryos directly in a detergent-containing buffer by passing the suspension 20x through the fine needle (27G) of a syringe, thus obviating the first plasma membrane gradient.
2e-4. Adjustment of density of 3000g supernatant
If OptiPrep™ is used to adjust the density then mix 2 vol. of OptiPrep™ with 1 vol. of supernatant. Although reducing the volume, the EGTA concentration will also be reduced.
2e-5. Layering the gradient
Although underlayering with a syringe and metal cannula is the recommended method for making discontinuous gradients, overlayering should be acceptable in view of the large difference in density between the solutions. For more information on gradient construction see Application Sheet S03. If necessary, adjust all volumes proportionately so that tubes (after sample application) are properly filled according to the manufacturer’s instructions.
2e-6. Lipid raft gradient
There is considerable scope for variation in the exact format of the iodixanol gradient: (1) sample in 40% iodixanol, overlaid with 1.2 ml of 30% iodixanol and 0.2 ml of 0% iodixanol [11,18]; (2) sample in 40% iodixanol, overlaid with 1ml each of 30% iodixanol, 20%, 5% and 0% iodixanol [15] and (3) sample in 40% iodixanol, overlaid with 0.9 ml of 30% iodixanol and 0.3 ml of 5% iodixanol [16,19,20]. The latter was carried out in the small volume Beckman TLS55 rotor. Zhai et al [17] also used this format for Drosophila S2 cells in the much larger volume Beckman SW60 rotor.
It is worth noting that usually [11,14,15] Triton X-100 (or other detergent) was only included in the dense sample layer, but Zhai et al [17] included 1% Triton X-100 in all the gradient solutions.
2e-7. Centrifugation
The centrifugation conditions vary from laboratory to laboratory; shorter times at higher g-forces (e.g. 2 h at 280,000 g) may be used or lower g-forces for longer times.
3. Other analyses
3a. Cytosolic and membrane proteins
A very simple method for resolving the cytosolic from membrane proteins involves homogenization of the Drosophila heads in 0.25 M sucrose, 10 mM KOAc, 2 mM Mg(OAc)2, 5 mM DTT, 30 mM HEPES, pH 7.4; clarifying the lysate three times at 1000 g for 5 min, then adjusting the supernatant to 30% (w/v) iodixanol and centrifuging at 350,000 g for 1 h. All of the membranes float to the top and the soluble proteins sediment [21].
3b. Early endosomes
A 3000 g supernatant of an embryo lysate in 100 mM KCl, 0.25 M sucrose, 5 mM EDTA, 10 mM Tris, pH 7.5 was layered over a 2.5-30% (w/v) iodixanol gradient, centrifuged at 37,000 g, for 1 h. Early endosomes were clearly identified by Rab5 antibodies [22].
3c. Rhabdomere membranes
The resolution of rhabdomere membranes from Drosophila eyes is dependent on the severity of the homogenization (reciprocating shaker in the presence of silica beads or an Ultra-Turrax macerator). The smaller membrane fragments produced by the latter permitted the resolution of Rh-1 and HsSERT containing subpopulations. For more information please see ref 23.
3d. Nuclei
A simple cushion of Optiprep™ (1000 g for 10 min) was used to band nuclei (repeated twice), principally to remove cytoplasmic components but the method would also remove smaller particles without the damage that may be caused by repeated pelleting [24]. Ye et al [25] also used just a single round of this cushion method. Alternatively the crude nuclear fraction may be suspended in 25% (w/v) (OptiPrep™ diluted as usual, with 0.25 M sucrose, 25 mM KCl, 5 mM MgCl₂, 20 mM Tris-Cl, pH 7.8) and the nuclei pelleted by centrifugation at 10,000 × g for 10 min. After removal of the supernatant the process was repeated [26].
4. Short review of other recent publications
Endoplasmic reticulum. Iodixanol gradients were able to monitor a banding density shift of Rab7 +ve vesicles during photoreceptor cell degeneration [27]. The method described in ref 9 was used by Sekine et al [28] to establish the ER location of the nucleotide sugar transporter Meigo. Kruppa et al [29] used a discontinuous gradient of 0-30% (v/v) OptiPrep™ underlayered by a 3000 g supernatant (adjusted to 35% v/v OptiPrep™) in studies on the -amyloid peptide. A microsomal fraction from Drosophila brain tissue, loaded on to a discontinuous gradient of 40%, 35%, 30%, 25%, 20%, 10%, 5% and 2.5 % (w/v) iodixanol, centrifuged 340,000 g for 3 hr showed considerable functional diversity: in particular HSC3 distribution in HTorAΔE-expressing brains was different from HTorAWT-expressing brains [30].
Endoplasmic reticulum and Golgi. Wan et al [31] used a median loaded discontinuous gradient in which the microsomal sample was adjusted to 20% (w/v) iodixanol and sandwiched between 20% and 15% (w/v) iodixanol solutions. After centrifugation for 3h at 150,000 g, Golgi and ER were separated across the original sample layer. This is an ideal way of separating the two membranes,
Endosomes and endoplasmic reticulum were well separated on a 5-20% (w/v) iodixanol gradient centrifuged at 90,000 g for 18 h [32] in a study of miRNA, in particular the association of a particular type of miRNAinduced silencing complex with these membranes.
Mitochondria were isolated in a discontinuous iodixanol gradient covering a similar density range to that used for mammalian cells [33]
Exovesicles banded in a 10%, 25%, 35%, 45% (w/v) iodixanol gradient centrifuged for 16–18 h at 120,000 g, were shown to contain Hedgehog proteins; these membranes banded around 35% (w/v) iodixanol [34].
5. References
1. Beronja, S., Laprise, P., Papoulas, O., Pellikka, M., Sisson, J. and Tepass, U. (2005) Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells J. Cell Biol., 169, 635-646
2. Yeaman, C., Grindstaff, K.K., Wright, J.R. and Nelson, W.J. (2001) Sec6/8 complexes on trans-Golgi network and plasma membrane regulate stages of exocytosis in mammalian cells J. Cell Biol., 155, 593-604
3. Papoulas, O., Hays, T.S. and Sisson, J.C. (2005) The golgin lava lamp mediates dynein-based Golgi movements during Drosophila cellularization Nature Cell Biol., 7, 612-618
4. Tan, D.J.L., Dvinge, H., Christoforou, A., Bertone, P., Arias, A.M. and Lilley, K.S. (2009) Mapping organelle proteins and protein complexes in Drosophila melanogaster J. Proteome Res., 8, 2667–2678
5. Dasgupta, U., Bamba, T., Chiantia, S., Karim, P., Abou Tayoun, A.N., Yonamine, I., Rawat, S.S. et al (2009) Ceramide kinase regulates phospholipase C and phosphatidylinositol 4, 5, bisphosphate in phototransduction Proc. Natl. Acad. Sci. USA, 106, 20063-20068
6. Adolfsen, B., Sarawati, S., Yoshihara, M. and Littleton, J.T. (2004) Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment J. Cell Biol., 166, 249-260
7. Niimura, M., Isoo, N., Takasugi, N., Tsuruoka, M., Ui-Tei, K., Saigo, K., Morohashi, Y., Tomita, T. and Iwatsubo, T. (2005) Aph-1 contributes to the stabilization and trafficking of the -secretase complex through mechanisms involving intermolecular and intramolecular interactions J. Biol.Chem., 280, 12967-12975
8. Zarnescu, D.C., Jin, P., Betschinger, J., Nakamoto, M., Wang, Y., Dockendorff, T.C., Feng, Y., Jongens, T.A., Sisson, J.C., Knoblich, J.A., Warren, S.T. and Moses, K. (2005) Fragile X protein functions with LgI and the PAR complex in flies and mice Develop. Cell, 8, 43-52
9. Khanna, M.R., Stanley, B.A. and Thomas, G.H. (2010) Towardsta membrane proteome in Drosophila: a method for the isolation of plasma membrane BMC Genomics 2010, 11: 302
10. Stein, D., Charatsi, I., Cho, Y.S., Zhang, Z., Nguyen, J., DeLotto, R., Luschnig, S. and Moussian, B. (2010) Localization and activation of the Drosophila protease Easter require the ER-resident saposin-like protein Seele Curr. Biol., 20, 1953–1958
11. Panneels, V., Eroglu, C., Cronet, P. and Sinning, I. (2003) Pharmacological characterization and immunoaffinity purification of metabotropic glutamate receptor from Drosophila overexpressed in Sf9 cells Protein Expr. Purif., 20, 275-282
12. Betschinger, J., Eisenhaber, F. and Knoblich, J.A. (2005) Phosphorylation-induced autoinhibition regulates the cytoskeletal protein lethal (2) giant larvae Curr. Biol., 15, 276-282
13. Rao, R.P., Yuan, C., Allegood, J.C., Rawat, S.S., Edwards, M.B., Wang, X., Merrill, A.H., Acharya, U. and Acharya, J.K. (2007) Ceramide transfer protein function is essential for normal oxidative stress response and lifespan Proc. Natl. Acad. Sci. USA, 104, 11364-11369
14. Rietveld, A., Neutz, S., Simons, K. and Eaton, S. (1999) Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophilia raft lipid microdomains J. Biol. Chem., 274, 12049-12054
15. Hoehne, M., de Couet, H.G., Stuermer, C.A.O. and Fischbach, K-F. (2005) Loss- and gain-of-function analysis of the lipid raft proteins reggie/flotillin in Drosphilia: they are post-translationally regulated, and misexpression interferes with wing and eye development Mol. Cell. Neurosci., 30, 326-338
16. Eroglu, C., Brügger, B., Wieland, F. and Sinning, I. (2003) Glutamate-binding affinity of Drosophila metabotropic glutamate receptor is modulated by association with lipid rafts Proc. Natl. Acad. Sci. USA, 100, 10219-10224
17. Zhai, L., Chaturvedi, D. and Cumberledge, S. (2004) Drosophila Wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine J. Biol. Chem., 279, 33220-33227
18. Sanxaradis, P.D., Cronin, M.A., Rawat, S.S., Waro, G., Acharya, U. and Tsunoda, S. (2007) Light-induced recruitment of INAD-signaling complexes to detergent-resistant lipid rafts in Drosophila receptors Mol Cell. Neurosci., 36, 36-46
19. Hebbar, S., Lee, E., Manna, M., Steinert, S., Kumar, G.S., Wenk, M., Wohland, T., and Kraut, R. (2008) A fluorescent sphingolipid binding domain peptide probe interacts with sphingolipids and cholesteroldependent raft domains J. Lipid Res. 49, 1077-1089
20. Fernandez-Funez, P., Casas-Tinto, S., Zhang, Y., Gómez-Velazquez, M., Morales-Garza, M.A., CepedaNieto, A.C., Castilla, J., Soto, C. and Rincon-Limas, D.E. (2009) In vivo generation of neurotoxic prion protein: role for Hsp70 in accumulation of misfolded isoforms PLoS One, 5:e1000507
21. Lee, Y.S., Pressman, S., Andress, A.P., Kim, K., White, J.L., Cassidy, J.J., Li, X., Lubell, K., Lim, D.H., Cho, I.S., Nakahara, K., Preall, J.B., Bellare, P., Sontheimer, E.J. and Carthew, R.W. (2009) Silencing by small RNAs is linked to endosomal trafficking Nat. Cell Biol., 11, 1150-1157
22. Tiklová, K., Senti, K-A., Wang, S., Gräslund, A. and Samakovlis, C. (2010) Epithelial septate junction assembly relies on melanotransferrin iron binding and endocytosis in Drosophila Nature Cell. Biol., 12, 1071-1078
23. Panneels, V., Kock, I., Krijnse-Locker, J., Rezgaoui, M., Sinning, I. (2011) Drosophila photoreceptor cells exploited for the production of eukaryotic membrane proteins: receptors, transporters and channels PLoS One 6: e18478
24. Steiner, F.A., Talbert, P.B., Kasinathan, S., Deal, R.B. and Henikoff, S. (2012) Cell-type-specific nuclei purification from whole animals for genome-wide expression and chromatin profiling Genome Res., 22:766–777
25. Ye, Y., Gu, L., Chen, X., Shi, J., Zhang, X. and Jiang, C. (2016) Chromatin remodeling during the in vivo glial differentiation in early Drosophila embryos Sci. Rep., 6: 33422
26. Groen, C.M., Jayo, A., Parsons, M. and Tootle, T.L. (2015) Prostaglandins regulate nuclear localization of Fascin and its function in nucleolar architecture Mol. Biol. Cell, 26, 1901-1917
27. Lee, J., Song, M. and Hong, S. (2013) Negative regulation of the novel norpAP24 suppressor, diehard4, in the endo-lysosomal trafficking underlies photoreceptor cell degeneration PLoS Genet., 9: e1003559
28. Sekine, S.U., Haraguchi, S., Chao, K., Kato, T., Luo, L., Miura, M. and Chihara, T. (2013) Meigo governs dendrite targeting specificity by modulating Ephrin level and N-glycosylation Nat. Neurosci., 16, 683-691
29. Kruppa, A.J., Ott, S., Chandraratna, D.S., Irving, J.A., Page, R.M., Speretta, E., Seto, T., Camargo, L.M., Marciniak, S.J., Lomas, D.A. and Crowther, D.C. (2013) Suppression of Aβ toxicity by puromycin-sensitive aminopeptidase is independent of its proteolytic activity Biochim. Biophys. Acta, 1832, 2115–2126
30. Kim, A-Y., Seo, J.B., Kim, W-t., Choi, H.J., Kim, S-Y., Morrow, G., Tanguay, R.M., Steller, H. and Koh, Y.H. (2015) The pathogenic human Torsin A in Drosophila activates the unfolded protein response and increases susceptibility to oxidative stress BMC Genom., 16: 338
31. Wan, D., Zhang, Z.C., Zhang, X., Li, Q. and Han, J. (2015) X chromosome-linked intellectual disability protein PQBP1 associates with and regulates the translation of specific mRNAs Hum. Mol. Genet., 24, 4599–4614
32. Wu, P-H., Isaji, M. and Carthew, R.W. (2013) Functionally diverse microRNA effector complexes are regulated by extracellular signaling Mol. Cell. 52, 113–123
33. Sing, A., Tsatskis, Y., Fabian, L., Hester, I., Rosenfeld, R., Serricchio, M., Yau, N., Bietenhader, M., Shanbhag, R., Jurisicova, A. et al (2014) The atypical cadherin fat directly regulates mitochondrial function and metabolic state Cell, 158, 1293–1308
34. Matusek, T., Wendler, F., Polès, S., Pizette, S., D’Angelo, G., Fürthauer, M. and Thérond, P.P. (2014) The ESCRT machinery regulates the secretion and long-range activity of Hedgehog Nature, 516, 99-103
OptiPrepTM Application Sheet S49; 7th edition, January 2020
OptiPrep™ Application Sheet S50
Fractionation of acidocalcisomes from trypanosomes (and other protozoa) and Gram-negative bacteria
- In Gram-negative bacteria these particles are often termed “volutin granules”
- The methodology can also resolve contractile vacuoles from protozoa (see Section 2c-2)
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding the extension of the technology to a variety of organisms and membrane analysis are contained in the “Technical Notes and Review” (Section 5).
- See also Application Sheet S58 for other organelle isolation methods from protozoa
- IMPORTANT NOTE: OptiPrep™ Reference List RS14 gives an up-to-date bibliography of all the published papers reporting the use of iodixanol gradient methodology for the purification of organelles from non-mammalian eukaryotes. For yeast see Reference List RS15. To access these two files return to the initial list of Folders and select “Reference Lists”.
1. Background
Acidocalcisomes, the electron-dense, acidic, calcium-storing organelles, which are rich in calcium and polyphosphate, were originally identified in Trypanosoma cruzi by Docampo et al [1] in 1995. They have now been detected in a range of prokaryotic and eukaryotic cells but there are significant differences in their characteristics from organism to organism. Although strict parallels in mammalian and non-mammalian cells may not exist, some dense granules in human platelets [2] and sea urchin eggs [3] share the same high levels of calcium and polyphosphate and may have similar functions.
Scott and Docampo [4] developed a discontinuous iodixanol gradient for purifying acidocalcisomes that effectively replaces the previous Percoll® method and overcomes many of the disadvantages associated with the use of Percoll®. The more general problem, observed with the recovery of mammalian organelles (e.g. lysosomes and peroxisomes) free from the colloidal silica particles with which they tend to co-sediment, is more serious in the case of acidocalcisomes because of their higher density and more rapid rate of sedimentation. Iodixanol, being a true solute, does not pose this problem at all. Moreover, since it is non-light-scattering, most spectrophotometric assays and electrophoresis can be carried out directly on gradient fractions without the need to remove the medium, so long as the concentration of the organelle in the gradient fraction is sufficiently high for accurate analysis. In some cases the gradient is able to provide simultaneous resolution of some other organelles.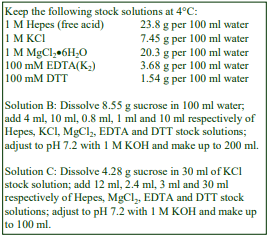
2. Trypanosomes and other protozoa
2a. Solutions required (see Section 2d-1)
A. OptiPrep™ (60% w/v, iodixanol)
B. Lysis buffer: 0.125 M sucrose, 50 mM KCl, 4 mM MgCl₂, 0.5 mM EDTA, 5 mM dithiothreitol (DTT), 20 mM Hepes-KOH, pH 7.2
C. OptiPrep™ diluent: 0.125 M sucrose, 0.3 M KCl, 24 mM MgCl₂, 3.0 mM EDTA, 30 mM DTT, 120 mM Hepes-KOH, pH 7.2
D. Iodixanol (50% w/v) working solution: mix 5 vol. of OptiPrep™ + 1 vol. of Solution C
2b. Ultracentrifuge rotor requirements
Ultracentrifuge with swinging-bucket rotors to accommodate approx 30 ml thick-walled tubes, e.g. Beckman SW28, Sorvall AH629 or similar (see Section 2d-2)
2c. Protocols
Carry out all operations at 0-4°C.
2c-1. Isolation of acidocalcisomes (adapted from ref 4)
1. Lyse the washed epimastigotes by grinding with silicon carbide using standard procedures (see ref 3 for further details).
2. Centrifuge the lysed cells in Solution B at 144 g for 5 min (see Section 2d-3).
3. Decant the supernatant and centrifuge this at 325 g for 10 min (see Section 2d-3).
4. Decant and retain the supernatant.
5. Resuspend the pellet in Solution B and repeat the centrifugation at 325 g for 10 min.
6. Combine the two supernatants and centrifuge at 10,500 g for 30 min (see Section 2d-4).
7. Resuspend the pellet in Solution B (4 ml) by repeated passages through the 22-gauge needle of a syringe (see Section 2d-4).
8. Prepare density gradient solutions containing 24%, 28%, 34%, 37% and 40% (w/v) iodixanol by diluting Solution D with Solution B (see Section 2d-5).
9. In tubes for the chosen rotor, layer 4 ml of each of the density gradient solutions and layer the resuspended 10,500 g pellet on top of the discontinuous gradient, to fill the tube (see Section 2d6).
10. Centrifuge at 50,000 g for 60 min.
11. The acidocalcisomes form a pellet at the bottom of the gradient. If the primary interest is isolation of the acidocalcisomes, aspirate the gradient and resuspend the pellet in a suitable medium. If the aim is to analyze other organelles as well, then collect the gradient in a series of 15-20 equal volume fractions (see Sections 2d-7 and 2d-8).
2c-2. Isolation of acidocalcisomes and contractile vacuoles (adapted from ref 5)
1. Clarify the lysate sequentially using two centrifugations at 36 g for 5 min and one at 144 g for 10 min (see Section 2d-3).
2. Centrifuge the final supernatant at 100,000 g for 1 h.
3. Resuspend the pellet in Solution B (2 ml) by repeated passages through the 22-gauge needle of a syringe (see Section 2d-4).
4. Mix the suspension with an equal volume of Solution D and 0.15 ml of Solution B to adjust it to 24% (w/v) iodixanol.
5. Prepare density gradient solutions containing 15%, 20%, 28%, 34%, 37% and 40% (w/v) iodixanol by diluting Solution D with Solution B.
6. In tubes for the chosen rotor, layer 4 ml of each of the density gradient solutions, including the sample in 24% iodixanol (see Section 2d-6).
7. Centrifuge at 50,000 g for 60 min.
8. Collect the gradient in a series of 15-20 equal volume fractions (see Section 2d-7 and 2d-8).
2d. Technical Notes and Review
2d-1. Homogenization media
Solutions are commonly buffered with Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and it is unlikely if the type of buffer significantly influences the fractionation.
The preparation of a Working Solution as described, ensures that the concentrations of KCl, MgCl₂, EDTA, DTT and the buffer (Hepes-KOH, pH 7.2) are constant throughout the gradient. If this is deemed unimportant the iodixanol solutions may be prepared simply by diluting OptiPrep™ with Solution B. Strategies for preparing working solutions are given in Application Sheet S01. Protease inhibitors may be included in Solutions B and C at the operator’s discretion.
2d-2 Ultracentrifuge rotors
The gradient + sample volume is 20-24 ml, thus thick-walled tubes which may be partially filled are the recommended ones for the rotor (total tube volume = approx. 30 ml). There is no obvious reason however why the gradient cannot be scaled up to allow the use of thin-walled tubes, which may be more convenient if complete unloading of the gradient into multiple fractions is envisaged. Similarly the procedure may be scaled down for use in rotors with a smaller tube volume.
2d-3 Clarification of the lysate
In many cases of acidocalcisome preparation, for example from Toxoplasma gondii [6-8], the 144 g and 325 g steps have been replaced by 36 g and 144 g.
2f-4 Preparation of a crude acidocalcisome fraction
In the case of Toxoplasma gondii [6,7] the crude acidocalcisomes were sedimented at 15,000 g rather than 10,000 g. Suspension of the crude fraction should be carried out as gently as possible to avoid damage not only to the organelles of interest but also to any other organelles present – particularly those which may release degradative enzymes. If median loading of the sample in the gradient is chosen, rather than top-loading, suspend the pellet in no more than approx 2 ml of Solution B so the volume after adjustment of the density with Solution D remains manageable.
2d-5 Gradient format (all iodixanol concentrations are % (w/v)
In some instances, for example in the isolation of acidocalcisomes from Dictylostelium discoideum the 40% iodixanol layer has been omitted [9]. To avoid any possible loss of material due to the rapid accumulation of particles at the sample/24% iodixanol interface in Protocol 2c-1, it may be preferable to suspend the crude pellet in one of the gradient layers, e.g. 24% or 28% iodixanol as in Protocol 4b. In Protocol 2c-2 the lower density layers (15% and 20% iodixanol) are included to improve the resolution of the contractile vacuole from the denser mitochondria, glycosomes and lysosomes; such a format may generally be beneficial in any studies in which a more complete fractionation is required.
For Toxoplasma gondii the gradient comprised 10%, 15%, 20% (sample), 25% and 30% iodixanol [6,7], while for isolation of acidocalcisomes from the plant trypanosomatid Phytomonas françai Protocol 2c-1 was used [10]. In proteomic analytical studies of acidocalcisomes, Ferella et al [11] used a discontinuous 20-50% (w/v) iodixanol gradient and in a similar study of the contractile vacuole the discontinuous gradient comprised 15, 20, 25, 30, 34, 37 and 40% iodixanol, with the sample in the 25% layer [12].
2d-6 Forming the discontinuous gradient
Although overlayering (i.e. starting with the densest layer) is the most common means of creating
a discontinuous gradient, underlayering (i.e. starting with the least dense layer) with a syringe and
metal cannula is more reliable and the recommended method for making discontinuous gradients. For
more information on gradient construction see Application Sheet S03. If necessary, adjust all volumes
proportionately so that tubes (after sample application) are properly filled according to the
manufacturer’s instructions.
2d-7 Fractionating the gradient
If thick-walled tubes are used then aspiration from the bottom of the tube or from the meniscus
(with a Labconco Auto Densi-flow device) are acceptable methods for harvesting gradients. Unloading
by upward displacement with a dense liquid may be less acceptable since the pellet may become
dispersed. So long as the acidocalcisomes do not form a too firmly-packed pellet, tube puncture may be
satisfactory for a thin-walled tube. For more information on harvesting gradients see Application Sheet
S52.
2d-8 Analysis
Protocol 2c-1 has been used preparatively for the isolation of acidocalcisomes from Trypanosoma cruzi [4,13-15], Trypanosoma brucei [15] and Leishmania major [15] in studies on Ca2+ and phosphate metabolism. This protocol also permits the partial resolution of some other organelles [16], although glycosomes, lysosomes and the vacuolar compartment tend to overlap close to the top interface between the sample and the top layer of the gradient.
There are however quite clear differences in the manner in which organelles from different organisms behave using Protocol 2c1. Figure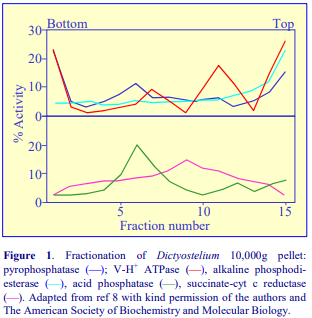 1 shows the distribution of organelles from a 10,500g pellet prepared from a post-nuclear supernatant of a Dictyostelium discoideum homogenate, which was layered on top of the gradient, as in Protocol 2c-1, but omitting the 40% iodixanol layer [9]. Alkaline phosphodiesterase shows a clear concentration at the top of the gradient (Figure 1); this is an established marker for contractile vacuoles from a number of microorganisms. The vacuole invariably bands at a lower density than any other organelle. On the other hand both pyrophosphatase and the vacuolar H+-ATPase (V-H+ ATPase) are present in both the vacuole and the dense acidocalcisome, confirming a functional link between these two particles [9]. Acid phosphatase (lysosomes) and succinate cytochrome c reductase (mitochondria) each show distinctive profiles. The distribution of the mitochondria is broad (compared to the usual pattern from mammalian cells); nevertheless fractions 8-10 which demonstrate the highest succinate-cytochrome c reductase are also significantly impoverished in markers for other organelles. It is also notable that the lysosomes from Dictyostelium are denser than are the bulk of the mitochondria (the reverse is true in all mammalian cells so far studied).
1 shows the distribution of organelles from a 10,500g pellet prepared from a post-nuclear supernatant of a Dictyostelium discoideum homogenate, which was layered on top of the gradient, as in Protocol 2c-1, but omitting the 40% iodixanol layer [9]. Alkaline phosphodiesterase shows a clear concentration at the top of the gradient (Figure 1); this is an established marker for contractile vacuoles from a number of microorganisms. The vacuole invariably bands at a lower density than any other organelle. On the other hand both pyrophosphatase and the vacuolar H+-ATPase (V-H+ ATPase) are present in both the vacuole and the dense acidocalcisome, confirming a functional link between these two particles [9]. Acid phosphatase (lysosomes) and succinate cytochrome c reductase (mitochondria) each show distinctive profiles. The distribution of the mitochondria is broad (compared to the usual pattern from mammalian cells); nevertheless fractions 8-10 which demonstrate the highest succinate-cytochrome c reductase are also significantly impoverished in markers for other organelles. It is also notable that the lysosomes from Dictyostelium are denser than are the bulk of the mitochondria (the reverse is true in all mammalian cells so far studied).
- In the case of Chlamydomonas reinhardtii Protocol 2c-1 was also able to resolve very clearly an acidocalcisome fraction and in this case the mitochondria were also well resolved about a third of the way down the gradient, but the chloroplasts were rather broadly distributed [17].
Protocol 2c-2 provides a much more clear resolution in the case of Toxoplasma gondii [6,7] and Trypanosoma cruzi [5] of the vacuole, lysosomes and glycosomes and the acidocalcisomes which band towards the top, middle and bottom of the gradient respectively.
- Other papers using this methodology for Trypanosoma brucei report that modulation of polyphosphate alters acidocalcisome biogenesis and function [18]; acidocalcisome lipids and glycolipids [19] and that the inositol 1,4,5-trisphosphate receptor present on acidocalcisomes has an important role in growth and infectivity [20].
- A discontinuous iodixanol gradient has also been used to purify contractile vacuoles from Dictyostelium [21].
3. Bacteria
3a Clarification of the lysate
The lysates from Rhodospirillum rubrum and Agrobacterium tumerfaciens) were clarified at 1000g for 5 min [22,23].
3b Preparation of a crude acidocalcisome fraction
For bacteria 14,500g was used to produce a crude organelle fraction [22,23]. Thus to maximize yields and purity of the acidocalcisomes it may be necessary to optimize the differential centrifugation of the homogenate.
3c Gradient format (all iodixanol concentrations are % (w/v)
Bacteria suspensions (Rhodospirillum rubrum and Agrobacterium tumerfaciens) were adjusted to 30% iodixanol and made part of a 24%, 28%, 30%, 35%, 40% iodixanol gradient [22,23] Note that in some bacteria the acidocalcisomes may be termed volutin granules [23]. A 14,500 g (10 min) fraction from a Corynebacterium matruchotii lysate was also median loaded as the 30% (w/v) step in a 24%, 28%, 30%, 35%, 40% (w/v) iodixanol gradient and centrifuged at 235,000 gav for 1 h. The particles banded at the 30%-35% interface [24].
- A review of the functional significance of acidocalcisomes is to be found in ref 25
- A very thorough proteomic analysis of acidocalcisomes from a variety of sources, using variants of the above technology, see ref 26.
4. References
1. Docampo, R., Scott, D. A., Vercesi, A. E. and Moreno, S. N. J. (1995) Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi Biochem J., 310, 1005-1012
2. Docampo, R., de Souza, W., Miranda, K., Rohloff, P. and Moreno, S.N.J. (2005) Acidocalcisomes – conserved from bacteria to man Nature Rev. Microbiol., 3, 251-261
3. Ramos, I.B., Miranda, K., Pace, D.A., Verbist, K.C., Lin, F-Y., Zhang, Y., Oldfield, E., Machado, E.A., de Souza, W. and Docampo, R. (2010) Calcium- and polyphosphate-containing acidic granules of sea urchin eggs are similar to acidocalcisomes, but are not the targets for NAADP Biochem. J., 429, 485–495
4. Scott, D. A. and Docampo, R. (2000) Characterization of isolated acidocalcisomes of Trypanosoma cruzi J. Biol. Chem., 275, 24215-24221
5. Rohloff, P., Montalvetti, A. and Docampo, R. (2003) Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi J. Biol. Chem., 279, 52270-52281
6. Rodrigues, C.O., Ruiz, F.A., Rohloff, P., Scott, D.A. and Moreno, S.N.J. (2002) Characterization of isolated acidocalcisomes from Toxoplasma gondii Tachyzoites reveals a novel pool of hydrolysable polyphosphate J. Biol. Chem., 277, 48650-48656
7. Rohloff, P., Miranda, K., Rodrigues, J.C.F., Fang, J., Galizzi, M., Plattner, H., Hentschel, J. and Moreno, S.N.J. (2011) Calcium uptake and proton transport by acidocalcisomes of Toxoplasma gondii PloS One 6: e18390
8. Ferreira, D. da S., Menezes Resende, I.T. and Lopez, J.A. (2014) Proteome investigation of an organellar fraction of Toxoplasma gondii: a preliminary study BMC Proc., 8 (Suppl 4): P74
9. Marchesini, N., Ruiz, F.A. Vieira, M. and Docampo, R. (2002) Acidocalcisomes are functionally linked to the contractile vacuole of Dictyostelium discoideum J. Biol. Chem., 277, 8146-8153
10. Miranda, K., Rodrigues, C.O., Hentchel, J., Vercesi, A., Plattner, H., de Souza, W. and Docampo, R. (2004) Acidocalcisomes of Phytomonas francai possess distinct morphological characteristics and contain iron Microsc. Microanal., 10, 647-655
11. Ferella, M., Nilsson, D., Darban, H., Rodrigues, C., Bontempi, E.J., Docampo, R. and Andersson, B. (2008) Proteomics in Trypanosoma cruzi – localization of novel proteins to various organelles Proteomics, 8, 2735-2749
12. Ulrich, P.N., Jimenez, V., Park, M., Martins, V.P., Atwood III, J., Moles, K., Collins, D., Rohloff, P., Tarleton, R., Moreno, S.N.J., Orlando, R. and Docampo, R. (2011) Identification of contractile vacuole proteins in Trypanosoma cruzi PLoS One 6: e18013
13. Martinez, R., Wang, Y., Benaim, G., Benchimol, M., de Souza, W., Scott, D. A. and Docampo, R. (2002) A proton pumping pyrophosphatase in the Golgi apparatus and plasma membrane vesicles of Trypanosoma cruzi Mol. Biochem. Parasitol., 120, 205-213
14. Rohloff, P., Rodrigues, C. O. and Docampo, R., (2003) Regulatory volume decrease in Trypanosoma cruzi involves amoni acid efflux and changes in intracellular calcium Mol. Biochem. Parasitol., 126, 219-230
15. Moreno, B., Urbina, J.A., Oldfield, E., Bailey, B.N. Rodrigues, C.O. and Docampo, R. (2000) 31P NMR spectroscopy of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major J. Biol. Chem., 275, 28356-28362
16. Ruiz, F.A., Rodrigues, C.O. and Docampo, R. (2001) Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation and environmental stress in Trypanosoma cruzi J. Biol. Chem., 276, 26114-26121
17. Ruiz, F.A., Marchesini, N., Seufferheld, M., Govindjee and Docampo, R. (2001) The polyphosphate bodies of Chlamydomas reinhardtii possess a proton pumping pyrophosphatase and are similar to acidocalcisomes J. Biol. Chem., 276, 46196-46203
18. Fang, J., Rohloff, P., Miranda, K. and Docampo, R. (2007) Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect Biochem. J., 407, 161-170
19. Salto, M.L., Kuhlenschmidt, T., Kuhlenschmidt, M., de Lederkremer, R.M. and Docampo, R. (2008) Phospholipid and glycolipid composition of acidocalcisomes of Trypanosoma cruzi Mol. Biochem. Parasitol., 158 120-130
20. Huang, G., Bartlett, P.J., Thomas, A.P., Moreno, S.N.J. and Docampo, R. (2013) Acidocalcisomes of Trypanosoma brucei have an inositol 1,4,5-trisphosphate receptor that is required for growth and infectivity Proc. Natl. Acad. Sci. USA, 110, 1887–1892
21. Sivaramakrishnan, V. and Fountain, S.J. (2012) A mechanism of intracellular P2X receptor activation J. Biol. Chem., 287, 28315–28326
22. Suefferheld, M., Lea, C.R., Vieira, M., Oldfield, E. and Docampo, R. (2004) The H+ -pyrophosphatase of Rhodospirillum rubrum is predominantly located in polyphosphate-rich acidocalcisomes J. Biol. Chem., 279, 51193-51202
23. Seufferheld, M., Vieira, M. C. F., Ruiz, F.A., Rodrigues, C.O., Moreno, S.N.J. and Docampo, R. (2003) Identification of organelles in bacteria similar to acidocalsisomes of unicellular eukaryotes J. Biol. Chem., 278, 29971-29978
24. Linton, K.M., Tapping, C.R., Adams, D.G., Carter, D.H., Shore, R.C. and Aaron, J.E. (2013) A silicon cell cycle in a bacterial model of calcium phosphate mineralogenesis Micron, 44, 419–432
25. Patel, S. and Docampo, R. (2010) Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling Trends Cell Biol., 20, 277-286
26. Huang, G., Ulrich, P.N., Storey, M., Johnson, D., Tischer, J.. Tovar, J.A., Moreno, S.N.J., Orlando, R. and Docampo, R. (2014) Proteomic analysis of the acidocalcisome, an organelle conserved from bacteria to human cells PLoS Pathog., 10: e1004555
OptiPrepTM Application Sheet S50: 10th edition, January 2020
OptiPrep™ Application Sheet S51
Isolation of optical system membranes and structures
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
This Application Sheet covers the purification of the following particles:
Section 1: Retinal rod outer segments (ROS) and ROS disks
Section 2: Photoreceptor outer segments
Section 3a: References
Section 3b: Recent references (after 2018)
1. Retinal rod outer segments (ROS) and ROS disks
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 1.5).
- Section 1.5 includes information on some variations to the given format and some of the more recent references
1.1 Background
The use of OptiPrep™ for the isolation of mouse retinal rod outer segments (ROS) was first published in 1998 [1]. After removal of the retinas, they are repeatedly vortexed in a Ringer’s solution containing iodixanol (usually, but not always, 8% v/v/ OptiPrep™) and centrifuged at low speed. During this procedure the ROS are released into the supernatants and subsequently banded in a twostep 10%, 18% (v/v) OptiPrep™ gradient. The method was extended without modification to the isolation of ROSs from human tissue [2]. Howes et al [3] and Calvert et al [4] replaced the two-layer iodixanol gradient with a three layer one for the purification of the ROS, although the densities of the three layers were slightly different and Ringer’s solution was replaced by Locke’s solution.
More recently Liang et al [5] introduced a continuous iodixanol gradient for the purification of the ROSs and subsequently, after osmotic lysis of the ROSs, the optical disks were recovered on a second continuous iodixanol gradient. For convenience, the methodology described in this Application Sheet is adapted from ref 5 but the extraction and gradient variations for ROS purification are presented in Section 5.3.
Section 5.3.
1.2 Solutions required (see Section 5.1)
A. OptiPrep™
B. Ringers Solution: 130 mM NaCl, 3.6 mM KCl, 2.4 mM MgCl2, 1.2 mM CaCl2, 0.02 mM EDTA, 10 mM Hepes-NaOH, pH 7.4
C. Lysis buffer: 2 mM Tris-HCl, pH 7.4
1.3 Centrifuge requirements
Swinging-bucket rotor for a high-speed centrifuge with a tube volume of approx 15 ml
1.4 Protocol
1. Make an 8% (v/v) OptiPrep™ solution by diluting 8 ml of Solution A to 100 ml with Solution B.
2. Suspend 12 mouse retinas in 0.12 ml of this solution. Carry out this and the following steps at room temperature.
3. Vortex for 1 min and then centrifuge at 200 g for 1 min.
4. Carefully recover the supernatant (without disturbing the pellet) and retain.
5. Resuspend the pellet in 0.12 ml of the 8% OptiPrep™ solution and repeat steps 3-4.
6. Repeat this procedure five or six times.
7. Prepare two gradient solutions of 10% and 30% (v/v) OptiPrep™ by diluting 1 ml and 3 ml of Solution A to 10 ml with Solution B respectively.
8. Using a two-chamber gradient maker or a Gradient Master™ prepare a 10-12 ml continuous gradient from these two solutions in tubes for the swinging-bucket rotor (see Section 5.2).
9. Layer the combined supernatants (approx. 1.5 ml) on top and centrifuge at 26,500 g for 30 min.
10. Collect the band of ROSs (about two thirds of the way from the top of the gradient).
11. Dilute with 3 vol. of Solution B and centrifuge at 500 g for 3 min to pellet the nuclei.
12. Harvest the ROSs by centrifuging at 26,500 g for 30 min.
13. To prepare the optical disks, lyse the ROSs by suspending them in 2 ml of Solution C and maintain them at 0°C for 15 h.
14. Prepare two gradient solutions of 15% and 40% (v/v) OptiPrep™ from 1.5 ml and 4 ml of Solution A, each diluted to 10 ml with Solution B respectively.
15. Using a two-chamber gradient maker or a Gradient Master™ prepare a 10-12 ml continuous gradient from these two solutions in tubes for the swinging-bucket rotor (see Section 5.2).
16. Layer the lysed ROSs on top of the gradient and centrifuge at 26,500 g for 30 min.
17. Harvest the disks that band about two thirds of the way from the top of the gradient
1.5 Technical Notes and Review
1.5.1 Homogenization media and gradient solutions
All iodixanol solutions in this Optiprep™ Application Sheet are prepared simply by diluting Optiprep™ with Ringers Solution, which will mean that the levels of KCl, MgCl₂, CaCl₂, EDTA and buffer will be reduced. If it is considered important that these levels should be maintained in all density solutions then a working solution of 50% (w/v) iodixanol should be first prepared by diluting 5 vol. of Optiprep™ with 1 vol. of 130 mM NaCl, 21.6 mM KCl, 14.4 mM MgCl₂, 7.2 mM CaCl₂, 0.12 mM EDTA, 60 mM Hepes-NaOH, pH 7.4. The working solution would thus contain the same concentrations of KCl, MgCl₂, CaCl₂, EDTA and Hepes as the Ringers buffer. Further dilutions with Ringers buffer should then need to be adjusted appropriately from those given in the protocol to give gradient solutions of the correct density. Note that if the concentration of NaCl in the diluent were also raised then the osmolality of the 50% (w/v) iodixanol will be unacceptably high. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01.
1.5.2 Construction of gradients
If neither of these gradient-making devices is available then a continuous gradient can be prepared by diffusion of a discontinuous gradient. For example, the 10-30% (v/v) Optiprep™ gradient (step 7) might be generated from a discontinuous gradient of equal volumes of 10%, 18%, 24% and 30% Optiprep™. Likewise the 15-40% (v/v) Optiprep™ gradient (step 14) might be generated from a discontinuous gradient of equal volumes of 15%, 21%, 27%, 33% and 40% Optiprep™. For more information on gradient construction see Application Sheet S03.
1.5.3 Variations in gradient and centrifugation format
Continuous gradients Continuous iodixanol gradients normally conform to the above protocol [5-13], although Peshenko et al [14,15] used a 20-30% (v/v) OptiPrep™gradient and a higher g-force of 75,000 g, for 40 min.
Discontinuous gradients
The commonly used discontinuous gradient format involves layering the extracted ROSs in 8% (v/v) Optiprep™ over a two layers of 10% and 18% (v/v) Optiprep; the centrifugation conditions are however somewhat diverse: 3,300 g for 10 min [1,2], 50,000 g for 1 h [16] 70,000 g for 1 h [17]. Variants in the density of the gradient layers include 8.3% and 20% with the sample in 6.7%, centrifuged at only 1,425 g, for 15 min [4]. Gradients comprising three layers: 10, 20 and 30% (sample in 8%), after centrifugation at 17,000 g for 50 min the ROSs banded at the 20%/30% interface [3] and four layers: 8%, 12%, 16% and 20% (sample in 2%) [18,19].
- Occasionally after repeated extractions with 8% (v/v) Optiprep™ no gradient is used [20].
- Refs 21-23 are meetings abstracts, reporting the use of Optiprep™ but no details are provided.
- Refs 24-35 all report the use of iodixanol gradients prepared from OptiPrep™ for ROS isolation using methods identical or very similar to those described above
2. Photoreceptor outer segments (POS)
Jiang et al [36] and Hazim et al [37] described homogenizing retinas in 130 mM NaCl, 3.6 mM KCl, 2.4 mM MgCl₂, 1.2 mM CaCl₂, 0.02 mM EDTA, and 10 mM HEPES, pH 7.4; then after centrifugation at 100 g for 1 min, the supernatant was layered over 8, 10 and 15% (w/v) iodixanol and centrifuged at 12,000 g for 20 min. The outer segments banded at the 10-15% interface. In an interesting variation Pelkonen et al [38] described homogenizing the tissue in 8% iodixanol (in a bicarbonate buffer). After an initial centrifugation at 720 g for 3 min (repeated five times); the suspension was diluted to 2% iodixanol and layered over 10% and 20% iodixanol, banding the POS at 14,000 g for 30 min. Rao et al [39] used a similar approach to that described in ref 38 but the gradient was a continuous one (10-30% iodixanol).
3a. References
1. Tsang, S.H., Burns, M.E., Calvert, P.D., Gouras, P., Baylor, D.A., Goff, S.P. and Arshavsky, V.Y. (1998) Role for the target enzyme in deactivation of photoreceptor G protein in vivo Science, 282, 117-121
2. Mata, N.L., Weng, J. and Travis, G.H. (2000) Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR–mediated retinal and macular degeneration Proc. Natl. Acad. Sci. USA, 97, 7154-7159
3. Howes, K.A., Pennesi, M.E., Sokal, I., Church-Kopish, J., Schmidt, B., Margolis, D., Frederick, J.M., Rieke, F., Palczewski, K., Wu, S.M., Detwiler, P.B. and Baehr, P. (2002) GCAP1 and rescues rod photoreceptor response in GCAP1/GCAP2 knockout mice The EMBO J., 21, 1545-1554
4. Calvert, P.D., Govardovskii, V.I., Krasnoperova, N., Anderson, R.E., Lem, J. and Makino, C.L. (2001) Membrane protein diffusion sets the speed of rod phototransduction Nature, 411, 90-94
5. Liang, Y., Fotiadis, D., Filipek, S., Saperstein, D. A., Palczewski, K. and Engel, A. (2003) Organization of the G protein-coupled receptors rhodopsion and opsin in native membranes J. Biol. Chem., 278, 21655-21662
6. Fotiadis, D., Liang, Y., Filipek, S., Saperstein, D. A., Engel, A. and Palczewski, K. (2004) The G proteincoupled receptor rhodosin in the native membrane FEBS Lett., 564, 281-288
7. Liang, Y., Fotiadis, D., Filipek, S., Saperstein, D.A., Engel, A. and Palczewski, K. (2004) Rhodopsin signaling and organization in heterozygote rhodosin knockout mice J. Biol. Chem., 279, 48189-48196
8. Maeda, A., Maeda, T., Imanishi, Y., Kuksa, V., Alekseev, A., Bronson, J.D., Zhang, H., Zhu, L., Sun, W., Saperstein, D.A., Ricke, F., Baehr, W. and Palczewski, K. (2005) Role of photoreceptor-specific retinal dehydrogenase in the retinoid cycle in vivo J. Biol. Chem., 280, 18822-18832
9. Saperstein, D.A., Fotiadis, D., Liang, Y., Filipek, S., Palczewski, K. and Engel, A. (2003) The structure of murine outer segment disk membranes using atomic force microscopy Invest. Ophthalmol Vis. Sci., 44, Eabstract, 3175
10. Nickell, S., Park, P.S-H., Baumeister, W. and Palczewski, K. (2007) Three-dimensional architecture of murine outer rod segments determined by cryoelectron tomography J. Cell Biol., 177, 917-925
11. Mustafi, D., Kevany, B.M., Genoud, C., Okano, K., Cideciyan, A.V., Sumaroka, A., Roman, A.J., Jacobson, S.G., Engel, A., Adams, M.D. and Palczewski, K. (2011) Defective photoreceptor phagocytosis in a mouse model of enhanced S-cone syndrome causes progressive retinal degeneration FASEB J. 25, 3157–3176
12. Ziccardi, L., Vijayasarathy, C., Bush, R.A. and Sieving, P.A. (2012) Loss of retinoschisin (RS1) cell surface protein in maturing mouse rod photoreceptors elevates the luminance threshold for light-driven translocation of transducin but not arrestin J. Neurosci., 32, 13010 –13021
13. Gilliam, J.C., Chang, J.T., Sandoval, I.M., Zhang, Y., Li, T., Pittler, S.J., Chiu, W. and Wensel, T.G. (2012) Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration Cell. 151, 1029–1041
14. Peshenko, I.V., Olshevskaya, E.V., Savchenko, A.B., Karan, S., Palczewski, K., Baehr, W. and Dizhoor, A.M. (2011) Enzymatic properties and regulation of the native isozymes of retinal membrane guanylyl cyclase (RetGC) from mouse photoreceptors Biochemistry, 50, 5590–5600
15. Peshenko, I.V., Olshevskaya, E.V., Azadi, S., Molday, L.L., Molday, R.S. and Dizhoor, A.M. (2011) Retinal degeneration 3 (RD3) protein inhibits catalytic activity of retinal membrane guanylyl cyclase (RetGC) and its stimulation by activating proteins Biochemistry, 50, 9511− 9519
16. Dizhoor, A.M. Woodruff, M.L., Olshevskaya, E.V., Cilluffo, M.C., Cornwall, M.C., Sieving, P.A. and Fain, G.L. (2008) Night blindness and the mechanism of constitutive signaling of mutant G90D rhodopsin J. Neurosci., 28, 11662-11672
17. Burns, M.E., Mendez, A., Chen, C-K., Almuete, A., Quillinan, N., Simon, M.I., Baylor, D.A. and Chen, J. (2006) Deactivation of phosporylated and nonphosphorylated rhodopsin by arrestin splice variants J. Neurosci., 26, 1036-1044
18. Krispel, C.M., Chen, D., Melling, N., Chen, Y-J., Martemyanov, K.A., Quillinan, N., Arshavsky, V.Y., Wensel, T.G., Chen, C-K. and Burns, M.E. (2006) RGS expression rate-limits recovery of rod photoresponses Neuron, 51, 409-416
19. Gross, O.P. and Burns, M.E. (2010) Control of rhodopsin’s active lifetime by arrestin-1 expression in mammalian rods J. Neurosci., 30, 3450 –3457
20. Nair, K.S., Hanson, S.M., Mendez, A., Gurevich, E.V., Kennedy, M.J., Shestopalov, V.I., Vishnivetskiy, S.A., Chen, J., Hurley, J.B., Gurevich, V.V. and Slepak, V.Z. (2005) Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions Neuron, 46, 555-567
21. Wang, Q., Hu, G., Leitges, M. and Wensel, T.G. (2006) Phosphorylation of phototransduction GAP RGS9-1 depends on the isoform of protein kinase C Invest. Ophthalmol. Vis. Sci., 47, E-Abstr 822
22. Finnemann, S.C. and Chang, Y. (2006) Regulation of RPE phagocytosis by integrin receptor-tetraspanin surface membrane domains Invest. Ophthalmol. Vis. Sci., 47, E-Abstr 822
23. Coleman, J.A., Djajadi, H.R., Molday, L.L. and Molday, R.S. (2012) Disruption of the P4-ATPase aminophospholipid flippase Atp8a2 gene suggests a role for phosphatidylserine in photoreceptor outer segments Invest. Ophthalmol. Vis. Sci., 53, Abstr. 743- 620
24. Moaven, H., Koike, Y., Jao, C.C., Gurevich, V.V., Langen, R. and Chen, J. (2013) Visual arrestin interaction with clathrin adaptor AP-2 regulates photoreceptor survival in the vertebrate retina Proc. Natl. Acad. Sci. USA, 110, 9463–9468
25. Skiba, N.P., Spencer, W.J., Salinas, R.Y., Lieu, E.C., Thompson, J.W. and Arshavsky, V.Y. (2013) Proteomic identification of unique photoreceptor disc components reveals the presence of PRCD, a protein linked to retinal degeneration J. Proteome Res., 12, 3010-3018
26. Coleman, J.A., Zhu, X., Djajadi, H.R., Molday, L.L., Smith, R.S., Libby, R.T., John, S.W.M. and Molday, R.S. (2014) Phospholipid flippase ATP8A2 is required for normal visual and auditory function and photoreceptor and spiral ganglion cell survival J. Cell Sci., 127, 1138–1149
27. Wensel, T.G. and Gilliam, J.C. (2015) Three-dimensional architecture of murine rod cilium revealed by cryo-EM In Methods Mol. Biol., 1271, Rhodopsin: Methods and Protocols (ed. Jastrzebska, B.), Springer Science+Business Media, New York, pp 267-292
28. Rakshit, T., Senapati, S., Sinha, S., Whited, A.M. amd Park, P.S-H. (2015) Rhodopsin forms nanodomains in rod outer segment disc membranes of the cold-blooded Xenopus laevis PLoS One, 10: e0141114
29. Calzia, D., Panfoli, I., Heinig, N., Schumann, U., Ader, M., Traverso, C.E., Funk, R.H.W. and Roehlecke, C. (2016) Impairment of extramitochondrial oxidative phosphorylation in mouse rod outer segments by blue light irradiation Biochimie, 125, 171-178
30. He, F., Agosto, M.A., Anastassov, I.A., Tse, D.Y., Wu, S.M. and Wensel, T.G. (2016) Phosphatidylinositol3-phosphate is light regulated and essential for survival in retinal rods Sci. Rep., 6: 26978
31. Berry, J., Frederiksen, R., Yao, Y., Nymark, S., Chen, J. and Cornwall, C. (2016) Effect of rhodopsin phosphorylation on dark adaptation in mouse rods J. Neurosci., 36, 6973– 6987
32. Peshenko, I.V., Olshevskaya, E.V. and Dizhoor, A.M. (2016) Functional study and mapping sites for interaction with the target enzyme in retinal degeneration 3 (RD3) protein J. Biol. Chem., 291, 19713–19723
33. Rajala, R.V.S., Rajala, A., Kooker, C., Wang, Y. and Anderson, R.E. (2016) The Warburg effect mediator pyruvate kinase M2 expression and regulation in the retina Sci. Rep., 6: 37727
34. Rakshit, T., Senapati, S., Parmar, V.M., Sahu, B., Maeda, A. and Park, P.S-H. (2017) Adaptations in rod outer segment disc membranes in response to environmental lighting conditions BBA – Mol. Cell Res., 1864, 1691-1702
35. Maity, S., Ilieva, N., Laio, A, Torre, V. and Mazzolini, M. (2017) New views on phototransduction from atomic force microscopy and single molecule force spectroscopy on native rods Sci. Rep., 7: 12000
36. Jiang, M., Esteve-Rudd, J., Lopes, V.S., Diemer, T., Lillo, C., Rump, A. and Williams, D.S. (2015) Microtubule motors transport phagosomes in the RPE, and lack of KLC1 leads to AMD-like pathogenesis J.
Cell Biol., 210, 595–611
37. Hazim, R., Jiang, M., Esteve-Rudd, J., Diemer, T., Lopes, V.S. and Williams, D.S. (2015) Live-cell imaging of phagosome motility in primary mouse RPE cells In Retinal Degenerative Diseases, Adv. Exp. Med. Biol., 854, (ed. C. Bowes Rickman et al.) Springer International Publishing Switzerland, pp 751-755
38. Pelkonen, L., Sato, K., Reinisalo, M., Kidron, H., Tachikawa, M., Watanabe, M., Uchida, Y., Urtti, A and Terasaki, T. (2017) LC−MS/MS based quantitation of ABC and SLC transporter proteins in plasma membranes of cultured primary human retinal pigment epithelium cells and immortalized ARPE19 cell line Mol. Pharmaceutics, 14, 605-613
3b. Recent references
1. Makino, C.L., Duda, T., Pertzev, A. and Sharma, R.K. (2018) Experimental approaches for defining the role of the Ca2+-modulated ROS-GC system in retinal rods of mouse In Mouse Retinal Phenotyping: Methods and Protocols, Methods in Mol. Biol., 1753 (ed. Tanimoto, N.), Springer Science+Business Media, LLC, pp 129-158
2. Senapati, S. and Park, P.S-H. (2019) Investigating the nanodomain organization of rhodopsin in native membranes by atomic force microscopy In Atomic Force Microscopy: Methods and Protocols, Methods in Molecular Biology, vol. 1886 (ed Santos, N.C. and Carvalho, F.A. Springer Science+Business Media LLC New York, pp 61-74
3. Dilan, T.L., Moye, A.R., Salido, E.M., Saravanan, T., Kolandaivelu, S., Goldberg, A.F.X. and Ramamurthy, V. (2019) ARL13B, a Joubert syndrome-associated protein, is critical for retinogenesis and elaboration of mouse photoreceptor outer segments J. Neurosci., 39, 1347–1364
4. Spencer, W.J., Ding, J-D., Lewis, T.R., Yu, C., Phan, S., Pearring, J.N., Kim, K-Y., Thor, A., Mathew, R. et al (2019) PRCD is essential for high-fidelity photoreceptor disc formation Proc. Natl. Acad. Sci., 116, 13087–13096
OptiPrepTM Application Sheet S51; 8th edition, January 2020
OptiPrep™ Application Sheet S52
Fractionation of vacuoles, pre-vacuoles, vacuolar, subvacuolar vesicles, secretory vesicles and Cvt vesicles from yeast spheroplasts
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5).
- See also the related Application Sheet S53
1. Background
Spheroplasts are prepared from yeast by a standard zymolase digestion. They are then lysed (or permeabilized) in a low concentration sorbitol buffer [1,2] from which a high-speed particulate and a soluble fraction are obtained. The vacuoles are first isolated from the particulate fraction in a discontinuous polysucrose (Ficoll) gradient and subsequently exposed to a hypoosmotic medium. A discontinuous iodixanol gradient is used to resolve vacuolar, subvacuolar and other subfractions [2]. This is described in Parts A-C of the Application Sheet.
Satyanarayana et al [3] also used a discontinuous iodixanol gradient to resolve the vacuole and Cvt vesicles from a yeast spheroplast lysate and also used the same gradient to analyze the vacuole fraction isolated on a polysucrose gradient, in a similar manner to that described by Harding et al [1] and by Scott et al [2]. This is described in Section 4d.
Section 4 of this Application Sheet describes the following procedures
a. Formation of yeast spheroplasts (adapted from ref 1)
b. Isolation and vesiculation of the vacuoles (adapted from ref 2)
c. Separation of the vacuolar and subvacuolar vesicles (adapted from ref 2)
d. Separation of vacuoles and Cvt vesicles from a yeast spheroplast lysate (adapted from ref 3)
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Wash buffer: 10 mM DTT, 10 mM Tris-SO4, pH 9.4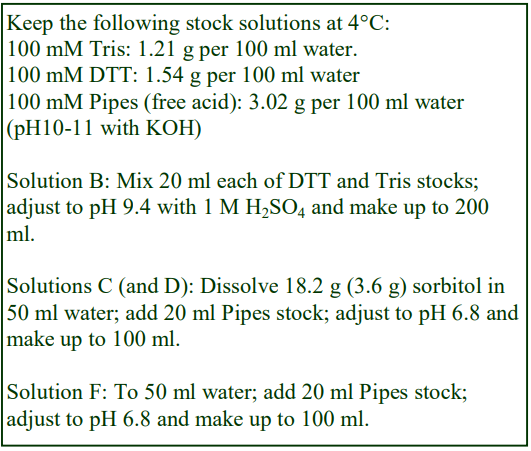
C. Spheroplast buffer: 1 M sorbitol, 20 mM PipesKOH, pH 6.8.
D. Spheroplast lysis medium: 200 mM sorbitol, 20 mM Pipes-KOH, pH 6.8
E. Polysucrose solutions: 4%, 10% and 12% (w/v) polysucrose in Solution D.
F. Vesiculation medium: 20 mM Pipes-KOH, pH 6.8
3. Ultracentrifuge rotor requirements (see Section 5.2)
For Protocol 4c: Swinging-bucket rotor to accommodate approx 2 ml tubes (e.g. Beckman TLS55)
For Protocol 4d: Swinging-bucket rotor to accommodate 13 ml tubes (e.g. Beckman SW41Ti, Sorvall TH641 or similar)
4. Protocol
4a. Spheroplast isolation
1. Harvest cells (20 OD600 units) at OD600 of 0.8-1.2 (scale up or down as required) and wash once in Solution B.
2. Resuspend in solution B (OD600 = 2.0) and incubate at 30C for 15 min with shaking.
3. Harvest the cells and resuspend in Solution C (OD600 = 1.0).
4. Dissolve Zymolase 20T (0.2 mg) by gentle inversion and incubate at 30°C for 15 min with occasional gentle shaking.
5. Harvest the spheroplasts at 3000 g for 3 min.
6. Resuspend the pellet in Solution C and transfer to microcentrifuge tubes.
7. Centrifuge the spheroplast suspension at 3000 g for 3 min.
4b. Isolation of vacuoles
For 700-800 OD600 units of spheroplasts
1. Resuspend the spheroplast pellet in 1 ml of Solution D.
2. Incubate at 23°C for 5 min (inverting once at 2.5 min).
3. Sediment the vacuole-containing fraction by centrifugation at 5000 g for 5 min.
4. Suspend the vacuolar pellet in 4 ml of the 12% polysucrose solution.
5. In 13 ml tubes for the swinging-bucket rotor layer 4 ml each of the sample in 12% polysucrose, 10% and 4% polysucrose and 1 ml of Solution D.
6. Centrifuge at 100,000 g for 90 min at 8C.
7. Collect the vacuoles from the 4% polysucrose/Solution D interface (see Section 5.3).
8. Dilute the vacuole fraction with 2 vol. of Solution D and harvest by centrifugation at 60,000 g for 15 min.
4c. Separation of vacuolar and subvacuolar vesicles
1. Gradient solutions: dilute Solution A with Solution F to give 11% and 22% (w/v) iodixanol (1.06 and 1.12 g/ml respectively).
2. Suspend the vacuole fraction in 0.2 ml of Solution F.
3. In approx 2 ml tubes for the TLS55 swinging-bucket rotor layer 0.5 ml of 22% (w/v) iodixanol, 1.3 ml of 11% (w/v) iodixanol and 0.3 ml of the sample (see Section 5.2).
4. Centrifuge at 160,000 g for 60 min at 12°C.
5. Vacuolar vesicles band at the Solution F/11% iodixanol interface and subvacuolar vesicles at the 11%/22% iodixanol interface (see Sections 5.3 and 5.4).
4d. Separation of vacuoles and Cvt vesicles from a yeast spheroplast lysate
1. Make up a 50% (w/v) iodixanol working solution (WS) containing 10 mM K-Pipes, pH 6.8 and dilute with the same buffer to give 30%, 25% and 19% (w/v) iodixanol gradient solutions.
2. Lyse spheroplasts (from Protocol 4a) in water; adjust lysate to 10 mM K-Pipes, pH 6.8 and mix with WS so that the final iodixanol concentration is 37% (w/v).
3. In tubes for a Beckman SW41Ti, Sorvall TH641 (or similar) overlayer 3 ml of this lysate with 2 ml of each gradient solution and K-Pipes buffer to fill the tube.
4. Centrifuge at approx 80,000 g for 4 h at 4₂C.
5. The vacuoles band at the buffer/19% interface and the Cvt vesicles at the 37%/30% interface (see Section 5.3).
5. Technical Notes and Review
5.1 Lysis media and gradient solutions
Protease inhibitors should be added to solutions used in the lysis of the spheroplasts and in solutions for subsequent operations as required.
5.2 Ultracentrifuge rotors
The Beckman TLS55 is accommodated in the Beckman Table-top ultracentrifuge and is used in Protocol 4c. A 5 ml rotor (e.g. Beckman SW50.1 or similar) with 3.0-3.5 ml adapted tubes is an alternative. In this case it will be necessary either to increase the volume of the sample in order to fill the tube or a cushion of 30% iodixanol could be included. Alternatively it may be acceptable simply to scale up all the volumes proportionately for larger volume tubes. The use of alternative rotors needs to be validated.
5.3 Harvesting the banded material from the gradients
In separations as well-resolved as these, the material of interest can simply be aspirated into a syringe fitted with a flat-tipped metal cannula (i.d approx 0.8 mm). If it is considered useful to unload the gradients in a series of equal volume fractions then for more information see Application Sheet S08. Note that the small volume tubes are probably best unloaded by tube puncture or aspiration from the meniscus.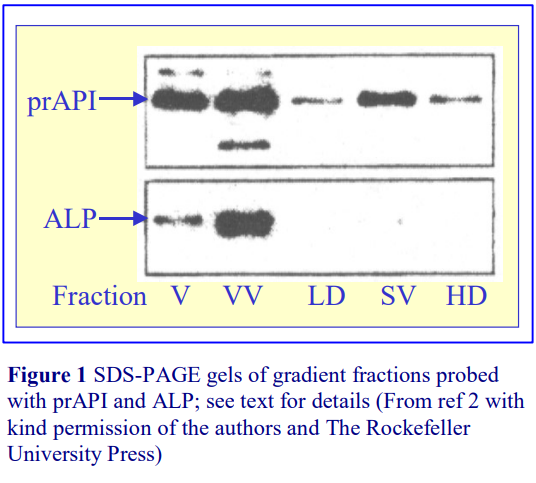
5.4 Analysis of results
The fractions from the small volume discontinuous iodixanol gradients (Protocol 4c) were run on SDS gels and probed for Precursor API (prAPI) and ALP (subvacuolar and vacuolar markers respectively). See ref 2 for more information on the analysis of this material. Figure 1 shows an analysis of the crude vacuole fraction (V) and the banded material at the sample/11% iodixanol interface which is enriched in vacuolar vesicles (VV), the 11/22% iodixanol interface, enriched in subvacuolar vesicles (SV) and also the two 11% and 22% iodixanol layers (LD and HD respectively).
5.5 Vacuole and pre-vacuole separations
The continuous iodixanol gradient system devised by Chen and Kaplan [4] and subsequently reported in a number of other papers [5-9] and used primarily for the study of the uptake of iron into yeast mitochondria, also separates vacuole and pre-vacuole fractions. It is described in Application Sheet S17.
In a study of yeast Myo2p, Chang et al [10] used a small volume self-generated iodixanol gradient. Differential centrifugation of a 2000 g/10 min supernatant of a yeast lysate in 150 mM KCl, 2 mM MgCl₂, 1 mM EGTA, 0.2% TX100, 2 mM DTT, 20 mM HEPES-NaOH, pH 7.2 was carried out at 2000 g for 10 min, 30,000 g for 30 min and 100,000 g for 1h. The final centrifugation included a 0.1 ml 60% (w/v) sucrose cushion. The 100,000 g pellet was resuspended in 0.75 ml of the lysis medium (plus the cushion) and 0.85 ml of OptiPrep™. This was centrifuged at 287,000 g for 2 h in a small volume rotor (Beckman TLA 120.2). Although vertical (or near-vertical) rotors are usually used for the creation of self-generated gradients, this small volume fixed-angle rotor has an ideal low sedimentation path length (approx 15 mm). The vacuole banded close to the top of the gradient, in secretory vesicles and ribosomes also showed distinctive banding patterns,
6. References
1. Harding, T.M., Morano, K.A., Scott, S.V. and Klionsky, D.J. (1995) Isolation and Characterization of Yeast Mutants in the Cytoplasm to Vacuole Protein Targeting Pathway J. Cell Biol., 131, 591-602
2. Scott, S.V., Baba, M., Ohsumi, Y. and Klionsky, D.J. (1997) Aminopeptidase I is targeted to the vacuole by a nonclassical vesicular mechanism J. Cell Biol., 138, 37-44
3. Satyanarayana, C., Schroder-Kohne, S., Craig, E.A., Schu, P.V. and Horst, M. (2000) Cytosolic Hsp70s are involved in the transport of aminopeptidase 1 from the cytoplasm into the vacuole FEBS Lett., 470, 232-238
4. Chen, O. S. and Kaplan, J. (2000) CCC1 suppresses mitochondrial damage in the yeast model of Friedreich’s ataxia by limiting mitochondrial iron accumulation J. Biol. Chem., 275, 7626-7632
5. Radisky, D. C., Babcock, M. C. and Kaplan, J. (1999) The yeast frataxin homologue mediates mitochondrial iron efflux J. Biol. Chem., 274, 4497-4499
6. Yun, C-W., Ferea, T., Rashford, J., Ardon, O., Brown, P. O., Botstein, D., Kaplan, J. and Philpott, C.C. (2000) Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake J. Biol. Chem., 275, 10709-10715
7. Chen, O. S. and Kaplan, J. (2001) YFH1-mediated iron homeostasis is independent of mitochondrial respiration FEBS Lett., 509, 131-134
8. Chen, O. S., Hemenway, S. and Kaplan, J. (2001) Genetic analysis of iron citrate toxicity in yeast: implications for mammalian iron homeostasis Proc. Natl. Acad. Sci. USA, 99, 16922-16927
9. Crisp, R. J., Pollington, A., Galea, C., Jaron, S., Yamaguchi-Iwai, Y. and Kaplan, J. (2003) Inhibition of heme biosynthesis prevents transcription of iron uptake genes in yeast J. Biol. Chem., 278, 45499-45506
10. Chang, W., Zaarour, R.F., Reck-Peterson, S., Rinn, J., Singer, R.H., Snyder, M., Novick, P. and Mooseker, M.S. (2008) Myo2p, a class V myosin in budding yeast, associates with a large ribonucleic acid–protein complex that contains mRNAs and subunits of the RNA-processing body RNA, 14, 491-502
OptiPrepTM Application Sheet S52; 10th edition, January 2020
OptiPrep™ Application Sheet S53
Fractionation of ER, Golgi, TGN, endosomes and vacuoles from yeast spheroplasts
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water; density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Important technical notes, information regarding alternative methodologies and membrane analysis are contained in the “Technical Notes and Review” section (Section 5).
1. Background
This application sheet is concerned with the use of iodixanol gradients in an analytical mode to study the membrane localization of a particular protein or function. Continuous gradients are best suited to this task. One of the protocols described in this application sheet starts with a discontinuous gradient [1], but since the gradient is centrifuged at 174,000 g for 16 h it will become continuous by diffusion. Some sedimentation of the iodixanol (self-generated gradient formation) will also occur and contribute further to the creation of a more or less linear density profile in the top three quarters of the gradient. Kim et al [2] used a pre-formed continuous gradient, also for 16 h, but at a lower RCF (100,000 g). Iodixanol will sediment rather less at this RCF and so the shape of density profile will change relatively little. Note that the discontinuous iodixanol gradients described in Application Sheet S50 were centrifuged for much shorter times.
Shintani et al [1] used a gradient between 10 and 50% (w/v) iodixanol, while that used by Kim et al [2] had a lower density range (0-40%). The two groups also used different spheroplast lysis buffers and protocols. Choice of buffer will depend on the subsequent analysis of the gradient fractions; in the following description only a basic buffer is described (see Section 5.1).
- A method for producing spheroplasts from a yeast culture is provided in Application Sheet S50.
- Two options are provided for the iodixanol gradient, a discontinuous or a continuous one, although the former will becomes continuous during the centrifugation.
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Spheroplast lysis buffer: 0.2 M sorbitol, 1 mM EDTA, 20 mM Pipes-KOH, pH 6.8
C. OptiPrep™ diluent: 0.6 M sorbitol, 6 mM EDTA, 120 mM Pipes-KOH, pH 6.8
D. Iodixanol (50% w/v) working solution: mix 5 vol. of OptiPrep™ + 1 vol. of Solution B
E. Discontinuous gradient solutions: 40%, 30%, 25%, 20%, 15% and 10% (w/v) iodixanol (dilute Solution D with Solution B)
F. Continuous gradient: 40% (w/v) iodixanol (dilute Solution D with Solution B)
3. Ultracentrifuge rotor requirements (see Section 5.2)
Ultracentrifuge with swinging-bucket rotors to accommodate 13 ml tubes (e.g. Beckman SW41Ti, Sorvall TH641 or similar
4. Protocol (adapted from refs 1 and 2).
Carry out all operations at 0-4C.
1. Prepare a spheroplast lysate using standard procedures (see Section 5.3).
2. Sediment cell debris and nuclei by centrifugation at 500 g for 5 min.
3. Remove the supernatant by aspiration and centrifuge this at 100,000 g for 20-30 min to prepare a total membrane fraction (see Section 5.4)
4. Resuspend the pellet in 1-2 ml of Solution B.
5. In tubes for the swinging-bucket rotor EITHER form a 12 ml linear gradient (using a two-chamber gradient maker or a Gradient Master™) from Solution B and 40% (w/v) iodixanol OR form a discontinuous gradient from 0.5 ml of 50%, 1.5 ml of 40% and 30%, 2.0 ml of 25%, 3 ml of 20% and 15%, and 2 ml of 10% (w/v) iodixanol (see Section 5.5)
6. Layer 1 ml of the 100,000 g pellet suspension over the gradient to fill the tube (see Section 5.5).
7. Centrifuge at 100-180,000 g for 16 h and allow the rotor to decelerate from 2000 rpm without the brake or use a controlled slow deceleration program.
8. Collect the gradient in 0.5-1.0 ml fractions by tube puncture, upward displacement with a dense medium or aspiration from the meniscus. For more information on gradient harvesting see Application Sheet S08.
- Some examples of the resolution that can be achieved with these gradients are given in Section 5.6. This section also contains in Table 1 a summary of the gradient and centrifugation conditions reported in other papers and the analysis that was carried out.
5. Technical Notes and Review
5.1 Lysis media and gradient solutions
The lysis buffer used by Shintani et al [1] contained in addition to the listed reagents in Solution B, 50 mM sodium acetate. On the other hand that used by Kim et al [2] contained in addition 1 mM DTT and 1 mM MgCl₂ (see Note 2). Protease inhibitors should also be added as required.
The preparation of gradient solutions is carried out by dilution of a 50% (w/v) iodixanol working solution (Solution D) that contains the same concentrations of EDTA (1 mM) and buffer (20 mM PIPES) as the lysis buffer and 0.1 M sorbitol. The concentration of sorbitol is lower than in the lysis buffer because the iodixanol is also contributing to the osmolality of the solution. To account for the additional components in the lysis buffer, Solution C might also contain 6x their normal concentration as well (e.g. 300 mM sodium acetate or 6 mM DTT + 6 mM MgCl₂). In this manner the concentration of EDTA, buffer and either acetate or DTT + MgCl₂ remain constant in the gradient. If however this is not considered important (or even desirable) then the gradient solutions may simply be prepared by diluting OptiPrep™ with the lysis buffer. A description of the preparation of gradient solutions for yeast spheroplasts is given in Application Sheet S02. Protease inhibitors should be added to Solutions B and C as required.
5.2 Ultracentrifuge rotors
The method may be scaled up or down as required to the use of larger or smaller volume rotor. It might be adaptable to a vertical or near-vertical rotor, in which case centrifugation times can be considerably reduced.
5.3 Spheroplast lysing
To disrupt the spheroplasts Shintani et al [1] extruded the spheroplast suspension through a filter (3 μm pore size); other workers use the standard liquid shear homogenization devices such as PotterElvehjem or tight-fitting Dounce homogenizer or differential lysis [3]. Whatever method is used the aim must be to disrupt the spheroplasts as gently as possible to avoid damage to delicate organelles but at the same time achieve at least 90% breakage.
5.4 Differential centrifugation
A centrifugation of the 500 g supernatant at 10,000 g for 10 min to pellet most of the larger organelles might be interposed if required. Alternatively, if the volume of the lysate is small, the 100,000 g step could be omitted and the entire 500 g supernatant applied to the gradient. Cytosolic proteins in this fraction however will both diffuse and sediment into the gradient during the overnight centrifugation.
5.5 Gradient construction and sample layering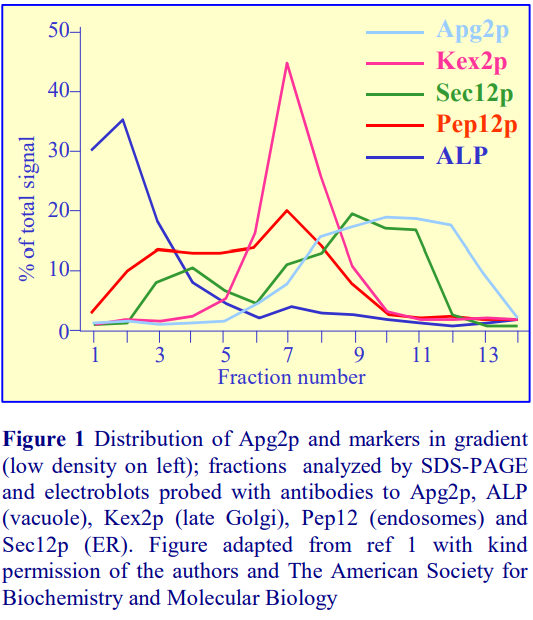
Some of the options for layering discontinuous gradients and for preparing continuous gradients are described in Application Sheet S03. An alternative to layering the sample on top of the gradient might be to layer the sample in a dense medium beneath the gradient – strategy often used with mammalian cell membrane fractionation that often produces improved resolution (see Section 5.7)
5.6 Gradient resolution
Examples of the resolving power of the iodixanol gradients are given in Figures 1 and 2. Shintani et al [1] used the 10-50% gradient (formed by diffusion of a discontinuous gradient) to study the distribution of a protein (Apg2p), which is essential for autophagosome formation. From Figure 1, it is clear that the Apg2p did not precisely co-localize with any of the other recognized markers, although it overlapped the ER marker, some denser fractions containing Apg2p were devoid of the ER marker (for more information see ref 1). For information on autophagosomes see Section 5.9
Kim et al [2], studying the transport of cytoplasmic material to the vacuole, identified a cytoplasmto-vacuole targeting (Cvt) pathway. In particular the gradient (continuous 0-40% iodixanol) was used to determine the localization of Cvt9, a protein required for the selective delivery of prAPI to the vacuole. The Cvt9 did not co-localize to the vacuole, endosomes, TGN or ER; indeed it had its own very distinctive distribution pattern (for more information see ref 2).
See Table 1 for a summary of the types of density gradient reported in some other publications, all using overnight centrifugation at a minimum of 100,000 g. See Section 5.8 re centrifugation time.
By making the gradient span a smaller range of density Sakakibara et al [16] were able to improve the resolution of some of the membrane compartments; the gradient was constructed from 16-60% (v/v) Optiprep™, which is approx. equivalent to 9-36% (w/v) iodixanol, which was centrifuged at 150,000 g for 16h after top-loading the 500 g supernatant. The gradient was able to provide very distinctive banding of the vacuole, endosomes, endoplasmic reticulum, cis-Golgi and mitochondria. Interestingly the banding from the wild-type yeast was different to that of the opi3∆ variant.
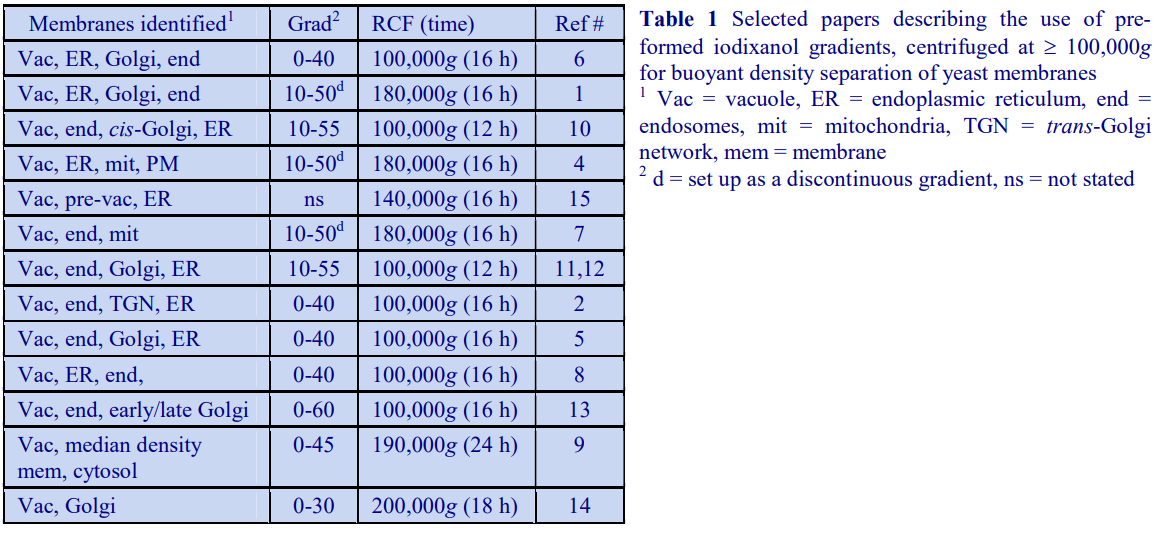
More recently Sakakibara et al [26] using 0.2 M sorbitol, 5 mM EDTA, 20 mM HEPES-KOH buffer (pH 7.2) to produce a 15-60% (v/v) OptiPrep™ gradient (150,000 g for 16 h) obtained distinctive profiles for vacuoles, endosomes, ER, mitochondria and cis-Golgi.
5.7 Flotation separation
Mitsui et al [17] suspended spheroplast lysate membranes in (0.8% sorbitol, 10 mM triethanolamine, 1 mM EDTA, pH 7.4. The suspension was adjusted to 35% (w/v) iodixanol and overlaid with a continuous 12–30% (w/v) iodixanol density gradient and centrifuged 100,000 g for 16 h. An easier alternative is to prepare the gradient first and then underlay with the sample. Late endosome and vacuole fractions were recovered at the top of the gradient and these were very well resolved from plasma membrane, which peaked about a third the way down the gradient. Cytoplasmic proteins remained in the original sample zone.
5.8 Shorter centrifugation time methods
There are several examples of the use of both shorter times and lower g-forces for the analysis of yeast membranes. Some examples of these separations are outlined below. The gradients often, but not exclusively, span a smaller and lower density range.
1. A 16,000g pellet from a spheroplast homogenate was resolved on a 0-25% (w/v) iodixanol gradient centrifuged at only 14,000g for 2 h. The gradient system was able to resolve ER, vacuole and late endosomes fractions and was used to locate Bph1p to late endosomes and vacuolar structures in a study on cell wall formation and protein sorting [18].
2. Welker et al [19] used a 2.25-24% (w/v) iodixanol gradient in a vertical rotor, centrifuged at 48,000 g for 1.5 h. Distinctive, but overlapping profiles of vacuole, plasma membrane, ER, endosomes, mitochondria and peroxisome markers. A stress protein (Hsp12) localized mainly to the PM and endosomes.
3. A similar gradient demonstrated that Yke4p (a zinc transporter) associated with the ER rather than the Golgi [20].
4. Diaz et al [21] tracked the bromovirus replication protein 1a using a flotation gradient. The spheroplasts were lysed in 150 mM NaCl, 5 mM EDTA, 30 mM Tris-HCl, pH 7.5. After clarification at 500 g for 5 min, the lysate was adjusted to 40% (w/v) iodixanol and 0.6 ml overlaid with 1.4 ml of 30% (w/v) iodixanol (and topped up with lysis buffer). After centrifugation at approx. 200,000 gav for 2 h, the ER had floated to the top of the gradient. Lack of expression of either the CAP or HEL fragments of 1a considerably reduced the ER flotation efficiency.
5. Work on PM-ER contact sites by Toulmay and Prinz [22], identified (using more or less the same methodology) the low density banding of the plasma membrane marker Pma1p and observed that the synaptogamin-like-mitochondrial-lipid binding proteins (SMP) domains shifted the GFP from a high- to a low-density
5.9 Autophagosomes
Autophagosomes were resolved on a very shallow gradient by Yamamoto et al [23]. A clarified lysate was layered on to an 4.5-18% (w/v) iodixanol gradient and centrifuged at 200,000 g for 1 hr. Golgi membranes banded sharply in the top two fractions; endosomes banded in fractions 1-7 while autophagosomes were recovered principally from fractions 8-10. More recently these particles have
also been identified in the long-spin gradients described in Section 4 above: a 0-30% (w/v) iodixanol gradient was centrifuged at 100,000 g for 20 h [24]
5.10 Self-generated gradients
In their study of vesiculogenic membranes, a 10,000 g pellet was suspended in 1 ml of 35% (w/v) iodixanol and layered under an equal volume of 30% (w/v) iodixanol, with 1 ml of lysis buffer on top, in tubes for a Beckman TLA 100.3 rotor. The samples were centrifuged at approx. 150,000g for 18 h [25]. The gradients that were formed occurred partly by diffusion and partly by self-generation.
6. References
1. Shintani, T., Suzuki, K., Kamada, Y., Noda, T. and Ohsumi, Y. (2001) Apg2p functions in autophagosome formation on the perivacuolar structure J. Biol. Chem., 276, 30452-30460
2. Kim, J., Kamada, Y., Stromhaug, P.E., Guan J., Hefner-Gravink, A., Baba, M., Scott, S.V., Ohsumi, Y., Dunn, W.A. and Klionsky, D.J. (2001) Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole J. Cell Biol., 153, 381-396
3. Kim, J., Dalton, V.M.., Eggerton, K.P., Scott, S.V. and Klionsky, D.J. (1999) Apg7p/Cvt2p is required for the cytoplasm-to-vacuole targeting, macroautophagy and peroxisome degradation pathways Mol. Biol. Cell, 10, 1337-1351
4. Kametaka, S., Okano, T., Ohsumi, M. and Ohsumi, Y. (1998) Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast Saccharomyces cerevisiae J. Biol. Chem., 273, 22284-22291
5. Teter, S.A., Eggerton, K.P., Scott, S.V., Kim, J., Fischer, A.M. and Klionsky, D.J. (2001) Degradation of lipid vesicles in the yeast vacuole requires function of Cvt17, a putative lipase J. Biol. Chem., 276, 2083-2087
6. Wang, C-W., Kim, J., Huang, W-P., Abeliovich, H., Stromhaug, P.E., Dunn, W. A. and Klionsky, D.J. (2001) Apg2 is a novel protein required for the cytoplasm to vacuole targeting, autophagy, and pexophagy pathways J. Biol. Chem., 276, 30442-30451
7. Ishihara, N., Hamasaki, M., Yokota, S., Suzuki, K., Kamada, Y., Kihara, A., Yoshimori, T., Noda, T. and Ohsumi, Y. (2001) Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion Mol. Biol. Cell, 12, 3690-3702
8. Guan, J., Stromhaug, P.E., George, M.D., Habiibzadegh-Tari, P., Bevan, A., Dunn, W.A. and Klionsky, D.J. (2001) Cvt18/Gsa12 is required for cytoplasm-to-vacuole transport, pexophagy, and autophagy in Saccharomyces cerevisiae and Pichia pastoris Mol. Biol. Cell, 12, 3821-3838
9. Urbanowski, J. L. and Piper, R.C. (2001) Ubiquitin sorts proteins into the intralumenal degradative compartment of the late-endosome/vacuole Traffic, 2, 622-630
10. Kim, J., Huang, W-P., Stromhaug, P.E. and Klionsky, D.J. (2002) Convergence of multiple autophagy and cytoplasm to vacuole components to a perivacuolar membrane compartment prior to de novo vesicle formation J. Biol. Chem., 277, 763-773
11. Wang, C-W., Stromhaug, P.E., Shima, J. and Klionsky, D.J. (2002) The Ccz1-Mon1 protein complex is required for the late step of multiple vacuole delivery pathways J. Biol. Chem., 277, 47917-47927
12. Wang, C-W., Stromhaug, P.E., Kauffman, E. J., Weisman, L.S. and Klionsky, D.J. (2003) Yeast homotypic vacuole fusion requires the Ccz1-Mon1 complex during the tethering/docking stage J. Cell Biol., 163, 973-985
13. Chantalat, S., Park, S-K., Hua, Z., Liu, K., Gobin, R., Peyroche, A., Rambourg, A., Graham, T.R. and Jackson, C. L. (2004) The Arf activator Gea2p and P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae J. Cell Sci., 117, 711-722
14. Dove, S.K., Piper, R.C., McEwen, R.K., Yu, J.W., King, M.C., Hughes, D.C., Thuring, J., Holmes, A. B., Cooke, F. T., Michell, R.H., Parker, P.J. and Lemmon, M.A. (2004) Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors The EMBO J., 23, 1922-1933
15. Meiling-Wesse, K., Barth, H., Voss, C., Eskelinen, E-L., Epple, U.D. and Thumm, M. (2004) Atg21 is required for effective recruitment of Atg8 to the preautophagosomal structure during the Cvt pathway J. Biol. Chem., 279, 37741-37759
16. Sakakibara, K., Eiyama, A., Suzuki, S.W., Sakoh-Nakatogawa, M., Okumura, N., Tani, M., Hashimoto, A., Nagumo, S., Kondo-Okamoto, N. et al (2015) Phospholipid methylation controls Atg32-mediated mitophagy and Atg8 recycling EMBO J., 134, 2703-2719
17. Mitsui, K., Koshimura, Y., Yoshikawa, Y., Matsushita, M. and Kanazawa, H. (2011) The endosomal Na+ /H+ exchanger contributes to multivesicular body formation by regulating the recruitment of ESCRT-0 Vps27p to the endosomal membrane J. Biol. Chem., 286, 37625–37638
18. Shiflett, S.L., Vaughn, M.B., Huynh, D., Kaplan, J. and McVey Ward, D. (2004) Bph1p, the Saccharomyces cerevisiae homologue of CHS1/beige, functions in cell wall formation and protein sorting Traffic, 5, 700-710
19. Welker, S., Rudolph, B., Frenzel, E., Hagn, F., Liebisch, G., Schmitz, G., Scheuring, J., Kerth, A., Blume, A., Weinkauf, S., Haslbeck, M., Kessler, H. and Buchner, J. (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function Mol. Cell, 39, 507–520
20. Kumanovics, A., Poruk, K.E., Osborn, K.A., Ward, D.M. and Kaplan, J. (2006) YKE4 (YIL023C) Encodes a bidirectional zinc transporter in the endoplasmic reticulum of Saccharomyces cerevisiae J. Biol. Chem., 281, 22566-22574
21. Diaz, A., Gallei, A. and Ahlquist, P. (2012) Bromovirus RNA replication compartment formation requires concerted action of 1a’s self-interacting RNA capping and helicase domains J. Virol., 86, 821–834
22. Toulmay, A. and Prinz, W.A. (2012) A conserved membrane-binding domain targets proteins to organelle contact sites J. Cell Sci., 125, 49–58
23. Yamamoto, H., Kakuta, S., Watanabe, T.M., Kitamura, A., Sekito, T., Kondo-Kakuta, C., Ichikawa, R., Kinjo, M. and Ohsumi, Y. (2012) Atg9 vesicles are an important membrane source during early steps of autophagosome formation J. Cell Biol., 198, 219–233
24. Suzuki, K., Nakamura, S., Morimoto, M., Fujii, K., Noda, N.N., Inagaki, F. and Ohsumi, Y. (2014) Proteomic profiling of autophagosome cargo in Saccharomyces cerevisiae PloS One, 9: e91651
25. Chen, J., Korostyshevsky, D., Lee, S. and Perlstein, E.O. (2012) Accumulation of an antidepressant in vesiculogenic membranes of yeast cells triggers autophagy PLoS One, 7: e34024
26. Sakakibara, K., Eiyama, A., Suzuki, S.W., Sakoh-Nakatogawa, M., Okumura, N., Tani, M., Hashimoto, A., Nagumo, S., Kondo-Okamoto, N. et al (2015) Phospholipid methylation controls Atg32-mediated mitophagy and Atg8 recycling EMBO J., 134, 2703-2719
OptiPrepTM Application Sheet 53; 10th edition, January 2020
Application Sheet S54
Endocytosis in rat liver: analysis of lysosome and late endosome events using a two-phase Nycodenz®-polysucrose gradient
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
1. Background
Branch et al [1] compared the efficacy of short-spin (1 h) continuous polysucrose and Nycodenz® gradients in the analysis of the membrane compartments in rat liver during the transcytosis of polymeric IgA and endocytosis of asialofetuin. In both instances a Beckman VTi50, vertical rotor was used at 206,000 g. The authors concluded that while polysucrose gradients were superior for resolving light (early) and dense (late) endosomes, Nycodenz® gradients provided far greater discrimination between late endosomes and lysosomes; moreover discrimination was achieved between lysosomes and very dense endosomes [2]. This led to the use of hybrid polysucrose-Nycodenz® gradients for the simultaneous isolation of early and late endosomes and lysosomes [3-6]; also, a simplified discontinuous gradient for the separation of lysosomes, very dense endosomes and other less dense endosomes was developed. The latter is described in Section 2 of this Application Sheet.
A more recent development has been the use of in vitro systems to study the transfer of molecules between endosomes and lysosomes that occurs during fusion between these two compartments. The use of such a system allows detailed study of this process in isolation from other events. Hybrid polysucrose-Nycodenz® gradients were also used to analyze the result and have identified and permitted the isolation of endosome-lysosome hybrids subsequent to incubation of previously purified late endosomes and lysosomes [3-6].
2. Discontinuous polysucrose-Nycodenzgr® adients (adapted from refs 3-7)
This methodology applies to rat liver and although in principle it may be applied to other tissues and cultured cells, but in view of the functional uniqueness of liver, it is likely that some optimization of the gradient and centrifugation conditions may be necessary.
2a. Solutions required (see Section 2d, Note 1)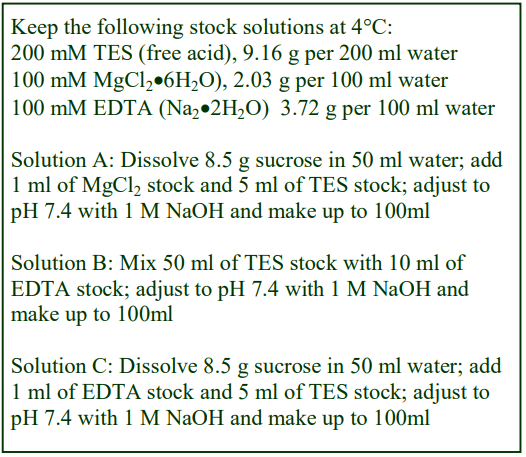
A. Homogenization medium: 0.25 M sucrose, 1 mM MgCl₂, 10 mM TES-NaOH, pH 7.4
B. Nycodenz® buffer: 10 mM EDTA, 100 mM TESNaOH, pH 7.4
C. Gradient diluent: 0.25 M sucrose, 1 mM EDTA, 10 mM TES-NaOH, pH 7.4
Add protease inhibitors as required to Solutions A-C.
For polysucrose solution see Section 2c, Step 3.
2b. Ultracentrifuge rotor requirements
Beckman VTi50 rotor (or equivalent) with a tube volume of approx 36 ml (see Section 2d, Note 2)
2c. Protocol
1. Prepare a Nycodenz® stock solution (45%, w/v): into approx. 50 ml of water at approx. 50°C in a 150 ml beaker on a heated magnetic stirrer add slowly 45 g of Nycodenz®. When all the Nycodenz® has dissolved allow the solution to cool to room temperature; add 10 ml of Solution B and make up to 100 ml with water. This may be filter sterilized and stored at 4°C.
2. Make up a 20% (w/v) Nycodenz®: mix 2 vol. of the 45% Nycodenz® solution prepared in Step 1 with 2.5 vol. of Solution C.
3. Make up a 20% (w/v) polysucrose in Solution C (see Section 2d, Notes 3 and 4).
4. Keep the solutions prepared in Steps 1-3 on ice and carry out the following at 4°C.
5. After perfusing the liver with an appropriate ligand, perfuse with Solution A until the lobes are well blanched (see Section 2d, Note 5)
6. Excise the liver into a beaker on ice and chop finely with scissors.
7. Stir the liver mince in approx. 10 ml of Solution A, then decant after allowing the mince to settle.
8. Transfer about half the mince to a Potter-Elvehjem homogenizer in approx 20 ml of Solution A and homogenize using 3-5 strokes of the pestle rotating at approx. 2000 rpm (see Section 2d, Note 6).
9. Repeat the procedure with the other half of the mince.
10. Centrifuge the homogenate at 2000 g for 10 min to sediment cell debris, nuclei and most of the heavy mitochondria.
11. Transfer 12.5 ml of the 20% polysucrose to 36 ml Optiseal tubes for the VTi50 rotor, then using a syringe and metal cannula underlayer with 12.5 ml of 20% Nycodenz® and 4 ml of 45% Nycodenz® solution (see Notes Section 2d, 7 and 8).
12. Layer the 2000 g supernatant on top, to fill the tube, as specified by the manufacturer.
13. Centrifuge at 200,000 g for 1 h, using a slow acceleration and deceleration programs up to and below 2000 rpm (alternatively turn off the brake below 2000 rpm).
14. Harvest the gradient in a series of equal volume fractions prior to analysis (see Notes Section 2d, 9-11).
2d. Notes
1. It is not known if iodixanol can be substituted for Nycodenz® in this application. Certainly the availability of iodixanol as a 60% (w/v) solution (OptiPrep™) makes gradient solution preparation much easier than is the case with Nycodenz®. Iodixanol and Nycodenz® solutions of the same % (w/v) concentration have almost identical densities, but solutions of Nycodenz® are hyperosmotic above 1.15 g/ml, in contrast to those of iodixanol which can be made isoosmotic at all densities. Whether the osmolality of Nycodenz® solutions plays an important role in achieving the separations described in this Application Sheet is not known. Comparisons can only be made empirically. For the preparation of iodixanol gradient solutions see Application Sheet S01.
2. If a vertical rotor is unavailable, either a fixed-angle or a swinging-bucket rotor may be used, but the longer sedimentation path length of these rotors will require longer centrifugation times.
3. Although Ficoll was used in the original method, the Axis-Shield product polysucrose, which has an almost identical molecular weight, may be substituted. When making up solutions of these high molecular weight sucrose polymers, it is better to add small aliquots (2-3 ml) of the solvent to the weighed-out powder, using a glass rod to mix well after each addition.
4. Ellis et al [7] made up a much more concentrated Ficoll solution in water (1 ml per g) before dialyzing it for 2 h against a large volume of water and adjusting it to the appropriate concentration in 0.25 M sucrose, 1 mM EDTA, 10 mM TES-NaOH, pH 7.4. It is probably easier to make up a 25% (w/v) polysucrose in 0.25 M sucrose, 1 mM EDTA, 10 mM TES-NaOH, pH 7.4 and to dialyze it against the same medium, before checking and adjusting the volume to make it 20% with respect to polysucrose.
5. See ref 7 for more information about liver perfusion.
6. See Application Sheets S05 and S06 respectively for more information on homogenization of tissues and cells.
7. A simplified method for separating lysosomes and endosomes in which the 20% polysucrose layer was omitted has also been used [4].
8. More detailed analysis of the light and dense endosomes may be carried out on continuous 1-22% polysucrose gradients (with a 45% Nycodenz® cushion) using approx. the same gradient volume, rotor and centrifugation conditions. The material containing lysosomes and dense endosomes at the cushion interface may be reanalyzed in a 0-35% or 0-45% Nycodenz® gradient, see refs 3-7 for
more details.
9. Collect the gradient in approx 1 ml fractions either by tube puncture, upward displacement with Maxidens®, or aspiration from the meniscus. For more information on gradient harvesting see Application Sheet S08.
10. Lysosomes band at the 45%/20% Nycodenz® interface, very dense endosomes at the 20% polysucrose/20% Nycodenz®; all other endosomes band at the sample/20% polysucrose interface.
11. The methodology has been widely used [8-10] to study the subcellular distributions of sterols and oxysterols in lipid-loaded macrophages (foam cells); their ability to oxidise LDL [11] and the mobilization of free and esterified cholesterol in response to cyclodextrins [12]. It has also been used to study the role of lysosomal membrane proteins in intracellular lactosylceramide traffic [13].
3. References
1. Branch, W. J., Mullock, B. M. and Luzio, J. P. (1987) Rapid subcellular fractionation of the rat liver endocytic compartments involved in transcytosis of polymeric immunoglobulin A and endocytosis of asialofetuin Biochem. J., 244, 311-315
2. Perez, J. H., Branch, W. J., Smith, L., Mullock, B. M. and Luzio, J.P. (1988) Investigation of endosomal compartments involved in endocytosis and transcytosis of polymeric immunoglobulin A by subcellular fractionation of perfused isolated rat liver Biochem. J., 251, 763-770
3. Mullock, B. M., Perez, J. H., Kuwana, T., Gray, S. R. and Luzio, J. P. (1994) Lysosomes can fuse with a late endosomal compartment in a cell-free system from rat liver J. Cell Biol., 126, 1173-1182
4. Mullock, B. M., Bright, N. A., Fearon, C. W., Gray, S. R. and Luzio, J. P. (1998) Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent J. Cell. Biol., 140, 591-601
5. Mullock, B. M., Smith, C. W., Ihrke, G., Bright, N. A., Lindsay, M., Parkinson, E. J., Brooks, D. A. Parton, R. G., James, D. E., Luzio, J. P. and Piper, R. C. (2000) Syntaxin 7 is localized to late endosome compartments, associates with Vamp
8, and is required for late endosome-lysosome fusion Mol. Biol. Cell 11, 3137-3153 6. Pryor, P. R., Mullock, B. M., Bright, N. A., Gray, SD. R. and Luzio, J. P. (2000) The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles J. Cell Biol., 149, 1053-1062
7. Ellis, J. A., Jackman, M. R., Perez, J. H., Mullock, B. M. and Luzio, J. P. (1992) Membrane traffic pathways in polarized epithelial cells In Protein Targeting: a practical approach (ed. Magee, A. I. and Wileman, T.) IRL Press at Oxford University Press, Oxford, UK, pp 25-57
8. Kritharides, L., Jessup, W., Mander, E.L. and Dean, R.T. (1995) Apolipoprotein A-I-mediated efflux of sterols from oxidized LDL-loaded macrophages Arterioscler. Thromb. Vasc. Biol., 15, 276-289
9. Gelissen, I.C. et al (1996) Sterol efflux is impaired from macrophage foam cells selectively enriched with 7- ketocholesterol J. Biol. Chem., 271, 17852-17860
10. Brown, A.J. et al (2000) Cholesterol and oxysterol metabolism and subcellular distribution in macrophage foam cells: accumulation of oxidized esters in lysosomes J. Lipid Res., 41, 226-237
11. Baoutina, A., Dean, R.T. ands Jessup, W. (1998) -Tocopherol supplementation of macrophages does not influence their ability to oxidize LDL J. Lipid Res., 39, 114-130
12. Liu, S.M. et al (2003) Cyclodextrins differentially mobilize free and esterified cholesterol from primary human foam cell macrophages J. Lipid Res., 44, 1156-1166
13. Pryor, P.R., Reichmann, F., Gribble, F.M. and Luzio, J.P. (2006) Mucolipin-1 is a lysosomal membrane protein required for intracellular lactosylceramide traffic Traffic, 7, 1388-1398
OptiPrepTM Application Sheet S54; 5th edition, January 2020
OptiPrep™ Application Sheet S55
Isolation of mammalian lysosomes in discontinuous gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- An OptiPrep™ Reference List (RS04) “Lysosomes – a bibliographical review” provides a bibliography of all published papers reporting the use of OptiPrep™ for analysis of these organelles: to access return to the initial list of Folders and select “Reference Lists”. The references are divided into cell or tissue type and highlight the analytical content.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- See Application Sheet S15 for information on the use of pre-formed continuous gradients
- See Application Sheet S16 for information on the use of self-generated gradients
1. Background
Wattiaux et al [1] were the first to describe the use of metrizamide for the purification of mammalian liver lysosomes. They were also the first to point out the huge advantage of using a gradient of much lower osmolality than the traditional sucrose gradient, which can only provide a reasonable separation of lysosomes and mitochondria if the density of the former is artificially reduced by Triton WR1339 [2]. It was also established that, by bottom loading of the density gradient, advantage could be taken of the greater sensitivity of mitochondria to hydrostatic pressure, thereby causing an increase in their density relative to that of the lysosomes [3].
In a discontinuous gradient of 30%, 26%, 24% and 19% (w/v) metrizamide (covering the density range of 1.16-1.105 g/ml), rat liver lysosomes float up from a light mitochondrial fraction, loaded in approx 35% metrizamide, to band at the 24%/19% interface after centrifugation at 95,000 g for 2 h [1,3]. The relative specific activity of β-galactosidase (over the homogenate) was reported as 80, while for a mitochondrial marker (cytochrome oxidase) the value was only 0.17 [3]. Metrizamide is no longer commercially available and Olsson et al [4] translated the flotation method to Nycodenz® and reported an even higher relative specific activity for N-acetyl-β-glucosaminidase of 108. The method has also been extended to HepG2 cells [5].
The procedure has now been adapted to the use of OptiPrep™ and a large number of published papers have predominantly reported the use of a discontinuous iodixanol gradient. In some cases the centrifugation is carried out for approx. 5 h, in others for 20 h and under these conditions the continuous gradient will become continuous, but not necessarily linear. Note that the density of lysosomes in iodixanol gradients is lower than that in either metrizamide or Nycodenz® gradients because of the reduced osmolality of iodixanol gradients.
2. Solutions required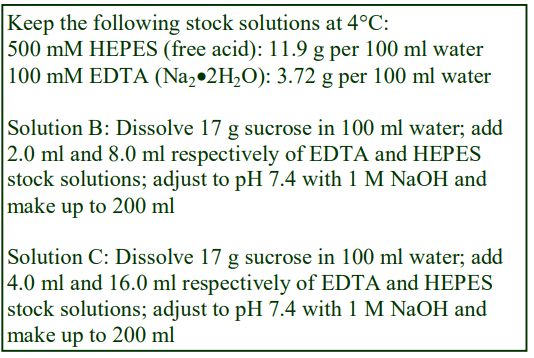
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 1 mM EDTA, 20 mM HEPES-NaOH, pH 7.4 (see Section 4)
C. OptiPrep™ diluent: 0.25 M sucrose, 2 mM EDTA, 40 mM HEPES-NaOH, pH 7.4
D. 30% (w/v) Iodixanol working solution: Mix equal volumes of Solutions A and C Add protease inhibitors as required to Solutions B and D.
3. Ultracentrifuge rotor requirements
In this protocol, a swinging-bucket rotor with a tube capacity of approx 13 ml (e.g. Beckman SW 41Ti, Sorvall TH641 or equivalent) or 4-5 ml (e.g. Beckman SW60Ti, TH-660 or equivalent) is suitable. The use of a vertical rotor is a valid alternative; the short sedimentation path length not only reduces the centrifugation time, the reduced hydrostatic pressure also favours retention of organelle integrity. This alternative has not been used with iodixanol gradients as far as is known, but it is quite a common practice with Nycodenz gradient purification of mitochondria.
4. Homogenization
For tissues: Mince the tissue very finely with scissors (or with a tissue chopper) and transfer to a Potter-Elvehjem (Teflon and glass) homogenizer with solution A (use 10 ml medium for every 2.5 g tissue). Homogenize using approx 6 strokes of the pestle (500-700 rpm).
For cells: Wash 1-3×108 cells in 5 ml of phosphate buffered saline and again with 5 ml of Solution A. Suspend the cells in 3 ml of Solution B and homogenize in a ball-bearing homogenizer using five passes.
Any suitable buffered isoosmotic solution may be used. The recommended version is a common one (see e.g. refs 6 and 7). But there are some significant variations (see Table 1). For more information about homogenization media for tissues and cells see Application Sheets S05 and S06 respectively.
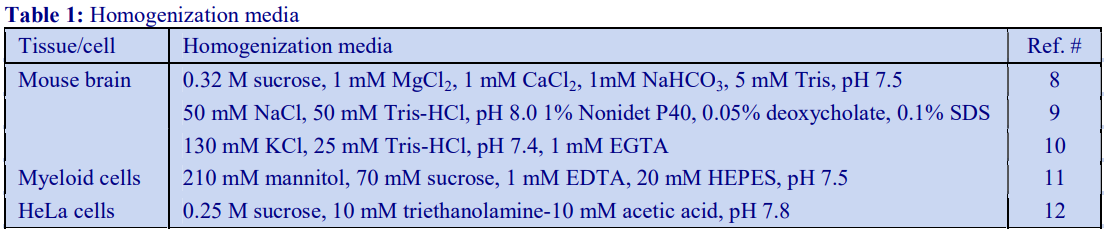
For the isolation of lysosomes from cultured cells, the ball bearing homogenizer is considered to offer the gentlest means of disruption. Most soft tissues can be homogenized in a Potter-Elvehjem homogenizer, but alternatives such as the Polytron homogenizer have been used. Some procedures for tissues and cells are described in Application Sheets S05 and S06 respectively. Always monitor the efficacy of the homogenization by phase contrast microscopy.
5. Preparation of the light mitochondrial fraction
1. Pellet the nuclei, cell debris and any unbroken cells by centrifuging at 800-1000 g for 10 min.
2. Decant or aspirate the supernatant and retain on ice.
3. Resuspend the pellet in Solution B using 2-3 gentle strokes of the pestle of a loose-fitting Dounce homogenizer. Resuspension of the pellet must be carried out under the mildest of conditions, to avoid damage to the delicate organelles.
4. Repeat the centrifugation and combine the supernatants.
5. Centrifuge the combined supernatants at 3,000 g for 10 min to pellet the heavy mitochondria. Aspirate the supernatant and keep on ice.
6. Resuspend the pellet in Solution B (see Step 3) and repeat the 3000 g centrifugation
7. Combine the two 3000 g supernatants and centrifuge at 17-20,000 g for 10 min to produce a light mitochondrial pellet.
8. Resuspend the light mitochondrial pellet in Solution B and repeat the 17-20,000 g centrifugation. This pellet is used for the gradient input.
A variety of centrifugation conditions have been used for this part of the procedure. In some cases a light mitochondrial fraction is used for the gradient input, which may be prepared in the mode described. There are wide variations in the protocol used to produce the gradient input. The first 800 g step [6] or the 3000 g step [8,9] may be omitted. Sometimes the entire 3000 g supernatant is applied to the gradient [7] or the entire 800-1000 g (post-nuclear) supernatant (PNS) is used [12-14] and in more rare examples the whole homogenate is used [15].
The advantage of using a PNS is that the pre-gradient procedure is accelerated and organelles are less likely to be lost due to the repeated centrifugation and re-suspension steps, on the other hand the presence of all the membrane-bound organelles and vesicles (except the nuclei) will severely test the resolving power of the gradient.
- See Application Sheet S07 for more information on differential centrifugation
6. Discontinuous gradient centrifugation
- Note that in some published methods the gradient is described in terms of % (w/v) iodixanol and sometimes as % (v/v) OptiPrep™; the two conventions give quite different densities: 20% (w/v) iodixanol is 1.127 g/ml (using a 0.25 M sucrose diluent), 20% (v/v) OptiPrep™ is equivalent to 12% (w/v) iodixanol and a density of 1.088 g/ml. For clarity all of the gradients are expressed as % (w/v) iodixanol in this Application Sheet.
The most commonly used discontinuous gradient format is 10%, 12%, 14%, 16%, 18% (w/v) iodixanol (equal volumes of each) prepared by diluting Solution D with Solution B. The crude lysosomal fraction is adjusted to 9% (w/v) and layered on top of the gradient. Tubes for the SW41 can accommodate 2 ml of each of the density gradient solutions and sample. For smaller volume rotors, all volumes should be scaled down proportionately. Gradients are normally centrifuged at 145,000 g for 2 h. Lysosomes band close to the top of the gradient. The method has been used for the following cell types: myeloid cells [11] neuroblastoma [12,13], renal cortex cells [14], pancreatic cancer cells [16], human lung carcinoma cells [17].
Other discontinuous gradients have been used to fractionate a tissue or cell PNS.
1. A gradient of 4,10,16 and 24% (w/v) iodixanol, with the sample loaded on top in the homogenization medium was used for HeLa cells [6] and 3T3 fibroblasts [7]. In this example, the gradients were centrifuged for 17 h at 20,000 g, during which time they will certainly become continuous.
2. A mouse blastocyst homogenate was fractionated on a 6, 9, 12,15, 18% (w/v) iodixanol gradient, centrifuged at 100,000 g for 16 h [15].
3. HeLa cell lysosomes were isolated on a 12, 14, 16, 18, 20% (w/v) iodixanol gradient, centrifuged at 150,000 g for 5 h [18]
4. In a 12.8, 16, 19, 22.5, 27% (w/v) iodixanol gradient, a lymphocyte light mitochondrial fraction was median loaded in the 19% iodixanol layer with centrifugation was at 150,000 g for 5 h [19,20]. Median loading has the advantage that the lysosomes will float to their banding density while most of the denser organelles will sediment. Very good resolution of lysosomes, mitochondria and dense membrane-bound granules was observed.
5. Renal cortex lysosomes have been purified from a PNS adjusted to 15% (w/v) iodixanol, loaded on to a 17, 20, 23, 27 and 30% (w/v) iodixanol (145,000 g for 2 h); the organelles banded at the 15- 17% iodixanol interface [21]. The same gradient and centrifugation conditions have been used for HeLa cells [22]. Purification of lymphocyte lysosomes [23] used a slightly modified gradient of 17, 23, 25, 27, 29 and 30% (w/v) iodixanol. Broadly similar discontinuous gradients were used for a mammary gland PNS [24], microvascular endothelial cells [25], Caco-2 cells [25] and brain [9]. Note that several commercial companies including Sigma-Aldrich, Pierce Biotechnology and Thermo-Scientific produce kits for the purification of lysosomes using OptiPrep™, which recommend iodixanol gradients of the type described in this section. In all these gradients the lysosomes will band towards the top of the gradient.
6. A rather more elaborate system was devised by Khundadze et al [26] for brain material. Essentially a heavy mitochondrial supernatant was first fractionated into a heavy and light membrane fraction on a discontinuous sucrose gradient; lysosomes were then enriched from the heavy fraction by flotation through a 25, 23, 21 and 19% (w/v) iodixanol gradient (centrifuged at 110,000 g for 2 h).
- For more information on the preparation of discontinuous gradients see Application Sheet S03.
- Lysosomes from approximately 30 different types of cell or tissue have been purified in discontinuous iodixanol gradients; for further information see OptiPrep™ Reference List RS04.
7. References
1. Wattiaux, R., Wattiaux-De Coninck, S., Ronveaux-Dupal, M. P. and Dubois, F. (1978) Isolation of rat liver lysosomes by isopycnic centrifugation in a metrizamide gradient J. Cell Biol., 78, 349-368
2. Leighton, F., Poole, B., Beaufay, H., Baudhuin, P., Coffey, J. W., Fowler, S. de Duve, C. (1968) The large scale separation of peroxisomes, mitochondria and lysosomes from livers of rats injected with Triton WR1339 J. Cell Biol., 37, 482-516.
3. Wattiaux, R. and Wattiaux-De Coninck, S. (1983) Separation of cell organelles In: Iodinated Density Gradient Media – a practical approach (ed. Rickwood, D.) IRL Press at Oxford University Press, Oxford, UK, pp 119-137
4. Olsson, G. M., Svensson, I., Zdoisek, J. M. and Brunk, U. T. (1989) Lysosomal enzyme leakage during the hypoxanthine/xanthine oxidase reaction Virchows Arch. B Cell Pathol., 56, 385-391
5. Jadot, M., Andrianaivo. F., Dubois, F. and Wattiaux, R. (2001) Effects of methylcyclodextrin on lysosomes Eur. J. Biochem., 268, 1392-1399
6. Cardoso, C.M.P., Groth-Pedersen, L., Høyer-Hansen, M., Kirkegaard, T., Corcelle, E., Andersen, J.S., Jäättelä, M. and Nylandsted, J. (2009) Depletion of kinesin 5B affects lysosomal distribution and stability and induces peri-nuclear accumulation of autophagosomes in cancer cells PLoS One, 4:e4424
7. Fehrenbacher, N., Bastholm, L., Kirkegaard-Sørenson, T., Rafn, B., Bøttzauw, T., Nielsen, C., Weber, E., Shirasawa, S., Kallunki, T. and Jäättelä (2008) Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-Associated membrane proteins 1 and 2 Cancer Res., 68, 6623-6633
8. Xiao, M-F., Xu, J-C., Tereshchenko, Y., Novak, D., Schachner, M. Kleene, R. (2009) Neural cell adhesion molecule modulates dopaminergic signaling and behavior by regulating dopamine D2 receptor internalization J. Neurosci., 29, 14752-14763
9. Annunziata, I., Patterson, A., Helton, D., Hu, H., Moshiach, S., Gomero, E., Nixon, R. and d’Azzo, A. (2013) Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid- secretion via deregulated lysosomal exocytosis Nat. Comm 4: 2734
10. Khundadze, M., Kollmann, K., Koch, N., Biskup, C., Nietzsche, S., Zimmer, G., Hennings, J.C., Huebner, A.K., Symmank, J., Jahic, A., Illina, E.I., Karle, K., Schöls, L., Kessels, M.., Braulke, T., Qualmann, B., Kurth, I., Beetz, C. and Hübner, C.A. (2013) A hereditary spastic paraplegia mouse model supports a role of ZFYVE26/SPASTIZIN for the endolysosomal system PloS Genet., 9: e1003988
11. Oberle, C., Huai, J., Reinheckel, T., Tacke, M., Rassner, M., Ekert, P.G., Buellesbach, J. and Borner, C. (2010) Lysosomal membrane permeabilization and Cathepsin release is a Bax/Bak-dependent, amplifying event of apoptosis in fibroblasts and monocytes Cell Death Differ., 17, 1167–1178
12. Sevlever, D., Jiang, P. and Yen, S-H.C. (2008) Cathepsin D is the main lysosomal enzyme involved in the degradation of α-synuclein and generation of its carboxy-terminally truncated species Biochemistry, 47, 9678-9687
13. Wei, J., Fujita, M., Nakai, M., Waragai, M., Sekigawa, A., Sugama, S., Takenouchi, T., Masliah, E. and Hashimoto, M. (2009) Protective role of endogenous gangliosides for lysosomal pathology in a cellular model of synucleinopathies Am. J. Pathol., 174, 1891–1909
14. 1556
15. Lee, J-H., Yu, W.H., Kumar, A., Lee, S., Mohan, P.S., Peterhoff, C.M., Wolfe, D.M., Martinez-Vicente, M., Massey, A.C., Sovak, G., Uchiyama, Y., Westaway, D., Cuervo, A.M. and Nixon, R.A. (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations Cell, 141, 1146–1158
16. Udelnow, A., Kreyes, A., Ellinger, S., Landfester, K., Walther, P., Klapperstueck, T., Wohlrab, J., HenneBruns, D., Knippschild, U. and Würl, P. (2011) Omeprazole inhibits proliferation and modulates autophagy in pancreatic cancer cells PLoS One, 6: e20143
17. Liu, L., Zhang, Z. and Xing, D. (2011) Cell death via mitochondrial apoptotic pathway due to activation of Bax by lysosomal photodamage Free Radic., Biol. Med., 51, 53–68
18. Edelmann, B., Bertsch, U., Tchikov, V., Winoto-Morbach, S., Perrotta, C., Jakob, M., Adam-Klages, S., Kabelitz, D. and Schütze, S. (2011) Caspase-8 and caspase-7 sequentially mediate roteolytic activation of acid sphingomyelinase in TNF-R1 receptosomes EMBO J., 30, 379–394
19. Schmidt, H., Gelhaus, C., Lucius, R., Nebendahl, M. Leippe, M. and Janssen, O. (2009) Enrichment and analysis of secretory lysosomes from lymphocyte populations BMC Immunol., 10:41
20. Schmidt, H., Gelhaus, C., Nebendahl, M., Lettau, M., Lucius, R., Leippe, M., Kabelitz, D. and Janssen, O. (2011) Effector granules in human T lymphocytes: proteomic evidence for two distinct species of cytotoxic effector vesicles J. Proteome Res., 10, 1603–1620
21. Dobrinskikh, E., Giral, H., Caldas, Y.A., Levi, M. and Doctor, R.B. (2010) Shank2 redistributes with NaPilla during regulated endocytosis Am. J. Physiol. Cell Physiol., 299, C1324–C1334
22. Meerovich, I., Koshkaryev, A., Thekkedath, R. and Torchilin, V.P. (2011) Screening and optimization of ligand conjugates for lysosomal targeting Bioconjugate Chem., 22, 2271–2282
23. Ruppert, S.M., Li, W., Zhang, G., Carlson, A.L., Limaye, A., Durum, S.K. and Khaled, A.R. (2012) The major isoforms of Bim contribute to distinct biological activities that govern the processes of autophagy and apoptosis in interleukin-7 dependent lymphocytes Biochim. Biophys. Acta, 1823, 1877–1893
24. Arnandis, T., Ferrer-Vicens, I., García-Trevijano, E.R., Miralles, V.J., García, C., Torres, L., Viña, J.R. and Zaragozá, R. (2012) Calpains mediate epithelial-cell death during mammary gland involution: mitochondria and lysosomal destabilization Cell Death Differ., 19, 1536–1548
25. Bielaszewska, M., Ruter, C., Kunsmann, L., Greune, L., Bauwens, A., Zhang, W., Kuczius, T., Kim, K.S., Mellmann, A., Schmidt, M.A. and Karch, H. (2013) Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis PloS Pathog., 9: e1003797
26. Khundadze, M., Kollmann, K., Koch, N., Biskup, C., Nietzsche, S., Zimmer, G., Hennings, J.C., Huebner, A.K., Symmank, J., Jahic, A., Illina, E.I., Karle, K., Schöls, L., Kessels, M.., Braulke, T., Qualmann, B., Kurth, I., Beetz, C. and Hübner, C.A. (2013) A hereditary spastic paraplegia mouse model supports a role of ZFYVE26/SPASTIZIN for the endolysosomal system PloS Genet., 9: e1003988
OptiPrepTM Application Sheet S55; 3rd edition, January 2020
OptiPrep™ Application Sheet S56
Analysis of mammalian lysosomes (ER, endosomes and plasma membrane) in continuous gradients
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- An OptiPrep™ Reference List (RS04) “Lysosomes – a bibliographical review” provides a bibliography of all published papers reporting the use of OptiPrep™ for analysis of these organelles: to access return to the initial list of Folders and select “Reference Lists”. The references are divided into cell or tissue type and highlight the analytical content.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- See Application Sheet S55 for information on the use of discontinuous gradients
- See Application Sheet S55 also for cell and tissue homogenization.
- See Application Sheet S16 for information on the use of self-generated gradients
1. Introduction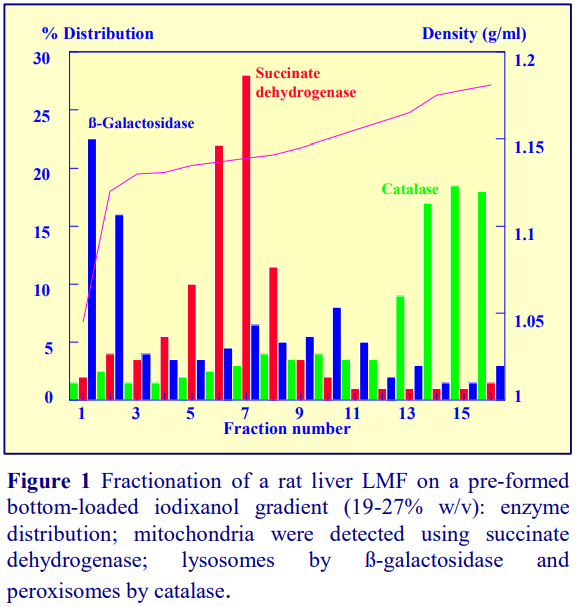
Continuous gradients may be prepared by allowing discontinuous gradients to diffuse; by using one of the standard two-chamber devices or a Gradient Master™. These methods are fully described in Application Sheet S03.
As an illustration of the effectiveness of continuous iodixanol gradients in resolving lysosomes, Figure 1 shows the separation of the major organelles from a rat liver light mitochondrial fraction (LMF). The LMF was adjusted to approx. 35% (w/v) iodixanol, layered beneath a 19-27% (w/v) iodixanol gradient and centrifuged 70,000 gav for 2 h. The sharp reduction in density in the top fraction occurs because 1-2 ml of 0.25 M sucrose was layered on top of the gradient (after underlayering of the LMF) to fill the tube. The distribution of enzyme markers shows the efficacy of such gradients in resolving all of the principal organelles.
2. Solutions required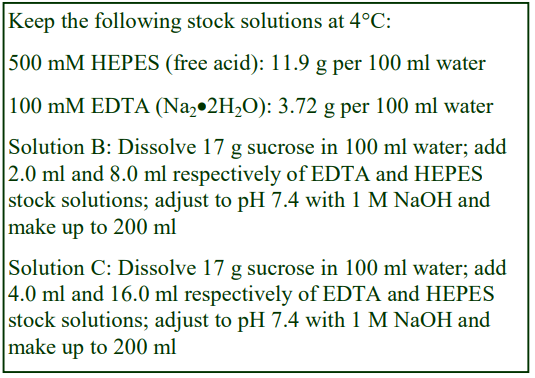
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 1 mM EDTA, 20 mM HEPES-NaOH, pH 7.4 (see Section 4)
C. OptiPrep™ diluent: 0.25 M sucrose, 2 mM EDTA, 40 mM HEPES-NaOH, pH 7.4
D. 30% (w/v) Iodixanol working solution: Mix equal volumes of Solutions A and C
Add protease inhibitors as required.
The solutions described here may be unsuitable to the cell or tissue type under study; they are what may be regarded as the “traditional ones” devised originally for rat liver but may be unsuitable for a particular cell type or study. For brief summaries of some of the other solutions used in lysosome studies see Application Sheets S55, S05 and S06; see also the publications described in Section 3.
3. A review of the published methodology
Other tissues or cultured cells not listed below may require modification of the density gradient configuration.
3a. HeLa cells. An LMF was loaded on top a continuous gradient formed from layers of 4, 10, 16 and 24% (w/v) iodixanol and centrifuged at 20,000 g for 17 h [1]. There was clear distinction in the distribution of mitochondrial and lysosomal markers, although the separation of these two organelles might have been enhanced in a more shallow gradient spanning a higher density range. The optimal density range may also be cell or tissue specific. A 15-30% (w/v) iodixanol has also been used for HeLa cells [2]. See also Section 3c.
3b. Osteoclasts. The chosen density range may also be a function of the aim of the study and the type of cell. A post-nuclear supernatant (PNS) from osteoblast cells was loaded on to a 0-17% (w/v) iodixanol gradient and centrifuged at approx. 150,000 gav for 100 min [3]. More unusually, this was carried out in a fixed-angle rotor, but so long as the acceleration to and from 2000 rpm is carried slowly and smoothly during the reorientation of the gradient in the centrifuge tube, there is no obvious reason why such a rotor cannot be used. The gradient completely separated the lighter plasma membrane from the denser lysosomes.
3c. Human skin fibroblsts. A 5-20% (w/v) iodixanol gradient, centrifuged for 20 h at 90,000 g, which was used for separation of ER and lysosomes from human skin fibroblasts [4]. These gradient and centrifugation conditions were originally described by Sugii et al [5] for studying endocytosis in CHO cell lines. Sugii et al [4] commented that Percoll gradients were unable to resolve plasma membrane from endosomal compartments, while in the iodixanol gradient the Na+/K+-ATPase was completely separated from EEA1, Rab9 and syntaxin 6 regions, each of which showed distinctive gradient banding patterns. This gradient has also been used for HeLa cells [6]
3d. Mouse brain. The homogenization medium contained 150 mM NaCl as the main osmotic balancer, in 1mm EDTA, 1 mM EGTA, 10 mM Tris-HCl, pH 7.4, containing 1% Triton X-100. Inclusion of detergent in the medium is a brain-specific strategy to overcome the myelin problem. The same gradient and centrifugation conditions as in Section 3c. The gradient completely resolves the lysosomes+late endosomes from the plasma membrane [7].
3e. HEK cells. This informative paper displayed how important it is to adapt methods to a particular cell or analytical requirement. An original 7.5-25% (w/v) iodixanol gradient was modified to an 11-22% gradient to maximize the separation of lysosomes and ER for demonstrating the localization of mature and immature forms of α-N-acetylgalactosaminidase to lysosomes and ER respectively [8].
3f. Primary human fibroblasts. A 10-30% (w/v) iodixanol gradient with a 40% iodixanol cushion, top-loaded with an LMF and centrifuged in a vertical rotor at 55,000 gav for 90 min completely separated the lysosomes, mitochondria and peroxisomes but the lighter ER overlapped the lysosomes [9]. It is difficult to assess whether a longer centrifugation time may have resolved the ER and mitochondria more effectively.
- Lysosomes from other cells that have isolated using similar continuous iodixanol gradients are MDCK cells [10], HepG2 cells [11] and also from he Bombyx mori silk gland [12].
4. References
1. Cardoso, C.M.P., Groth-Pedersen, L., Høyer-Hansen, M., Kirkegaard, T., Corcelle, E., Andersen, J.S., Jäättelä, M. and Nylandsted, J. (2009) Depletion of kinesin 5B affects lysosomal distribution and stability and induces peri-nuclear accumulation of autophagosomes in cancer cells PLoS One, 4:e4424
2. Matsuda, S., Okada, N., Kodama, T., Honda, T. and Iida, T. (2012) A Cytotoxic Type III Secretion Effector of Vibrio parahaemolyticus targets vacuolar H+ -ATPase subunit c and ruptures host cell lysosomes PLoS Pathog., 8: e1002803
3. Kariya, Y., Homma, M., Aoki, S., Chiba, A. and Suzuki, H. (2009) Vps33a mediates RANKL storage in secretory lysosomes in osteoblastic cells J. Bone Mineral Res., 24, 1741-1752
4. Higaki, K., Li, L., Bahrudin, U., Okuzawa, S., Takamuram, A., Yamamoto, K., Adachi, K., Paraguison, R.C., Takai, T., Ikehata, H., Tominaga, L., Hisatome, I., Iida, M., Ogawa, S., Matsuda, J., Ninomiya, H., Sakakibara, Y., Ohno, K., Suzuki, Y. and Nanba, E. (2011) Chemical chaperone therapy: chaperone effect on mutant enzyme and cellular pathophysiology in -galactosidase deficiency Hum. Mutat., 32, 843–852
5. Sugii, S., Reid, P.C., Ohgami, N., Du, H. and Chang, T-Y. (2003) Distinct endosomal compartments in early trafficking of low density lipoprotein-derived cholesterol J. Biol. Chem., 278, 27180-27189
6. Takamura, A., Higaki, K., Ninomiya, H., Takai, T., Matsuda, J., Iida, M., Ohno, K., Suzuki, Y. and Nanba, E. (2011) Lysosomal accumulation of Trk protein in brain of GM1-gangliosidosis mouse and its restoration by chemical chaperone J. Neurochem., 118, 399–406
7. Shi, J., Chou, B., Choi, J.L., Ta, A.L. and Pun, S.H. (2013) Investigation of polyethylenimine/DNA polyplex transfection to cultured cells using radiolabeling and subcellular fractionation methods Mol. Pharm., 10, 2145-2156
8. Clark, N.E., Metcalf, M.C., Best, D., Fleet, G.W.J. and Garman, S.C. (2012) Pharmacological chaperones for human α-N-acetylgalactosaminidase Proc. Natl. Acad. Sci. USA, 109, 17400-17405
9. Wiesinger, C., Kunze, M., Regelsberger, G., Forss-Petter, S. and Berger, J. (2013) Impaired very long-chain Acyl-CoA -oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dyfunction J. Biol. Chem., 288, 19269-19279
10. Van Itallie, C.M., Tietgens, A.J., LoGrande, K., Aponte, A., Gucek, M. and Anderson, J.M. (2012) Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes Journal of Cell Science 125, 4902–4912
11. Seggewiß, N., Paulmann, D. and Dotzauer, A. (2016) Lysosomes serve as a platform for hepatitis A virus particle maturation and nonlytic release Arch. Virol., 161, 43–52
12. Shiba, H., Yabu, T., Sudayama, M., Mano, N., Arai, N., Nakanishi, T. and Hosono, K. (2016) Sequential steps of macroautophagy and chaperone-mediated autophagy are involved in the irreversible process of posterior silk gland histolysis during metamorphosis of Bombyx mori J. Exp. Biol., 219, 1146-1151
OptiPrepTM Application Sheet S56; 4th edition, January 2020
OptiPrep™ Application Sheet S57
Isolation of peroxisomes from yeast spheroplasts
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Recent published papers reporting the use of iodixanol gradients are given in Section 7.
1. Background
Wild-type yeast strains contain rather few peroxisomes, however they can be induced to proliferate and there are a number of peroxisome gene-deletion strains of the organism [1,2]. Yeast systems are therefore a popular means of investigating peroxisome development.
Although Percoll® has been used for peroxisome isolation from mammalian cells and yeast spheroplasts, the organelles are contaminated by both Golgi and smooth ER membranes, which have similar low densities in this medium. In both iodixanol and Nycodenz® however peroxisomes are denser than any other organelle in the light mitochondrial fraction and contamination by ER and Golgi is never a problem. Nycodenz® gradients have been widely used for the isolation of peroxisomes, but in the last eight years there has been increasing use of OptiPrep™ for both mammalian and yeast peroxisomes. Although Nycodenz® has remained a popular choice for yeast peroxisomes, the availability of iodixanol as a 60% (w/v) solution (OptiPrep™) makes gradient solution preparation much easier than with Nycodenz®. Iodixanol and Nycodenz® solutions of the same % (w/v) concentration have almost identical densities, but only iodixanol permits the banding of peroxisomes under more or less isoosmotic conditions and it is only the OptiPrep™ option that is presented in this Application Sheet.
Spheroplasts are homogenized in a sorbitol-containing medium using a Dounce homogenizer. Some variation exists in the sorbitol concentration in this medium; Watkins et al [2] used 0.6 M, while Crane et al [3] used 1.0 M. The iodixanol methodology is adapted from refs 4-5, 7-10.
2. Solutions required (see Box)
A. Spheroplast wash solution: 1.2 M sorbitol, 20 mM phosphate buffer, pH 7.4
B. Spheroplast lysis buffer: 0.6 M sorbitol, 1 mM EDTA, 1 mM KCl, 0.1% (v/v) ethanol, 5 mM MES-NaOH pH 6.0
C. OptiPrep™
D. OptiPrep™ diluent: 18% (w/v) sucrose, 3 mM EDTA, 3 mM KCl, 0.3% ethanol, 15 mM MESNaOH, pH 6.0
E. Gradient diluent: 18% (w/v) sucrose, 1 mM EDTA, 1 mM KCl, 0.1% ethanol, 5 mM MESNaOH, pH 6.0
Add protease inhibitors to solutions as required.
3 Preparation of gradient solutions
Mix 2 vol. of OptiPrep™ with 1 vol. of Solution D to produce a 40% (w/v) iodixanol stock solutions and then dilute further with Solution E to produce solutions of 2.25% and 24% (w/v) iodixanol (see Section 6, Notes 1 and 2)
4 Ultracentrifuge rotor requirements
Vertical rotor with a tube capacity of approx 36 ml tubes (e.g. Beckman VTi50) with appropriate sealed tubes (see Section 6, Note 3).
5. Protocol
Carry out all operations at 0-4°C
1. Prepare spheroplasts from 1 litre of yeast culture, grown in YPD medium (OD600 = 0.5-1.0).
2. Suspend the spheroplasts in Solution A (10-15 ml).
3. Centrifuge in an 8×50 ml fixed-angle rotor (high-speed centrifuge) at 4000 g for 5 min.
4. Remove the supernatant and repeat steps 1 and 2.
5. Suspend the spheroplasts in 35 ml of Solution B and homogenize in a tight-fitting Dounce homogenizer (Wheaton Type A), using 10 up-and-down strokes of the pestle.
6. Centrifuge the homogenate at 1,500 g for 10 min.
7. Using a syringe and metal cannula aspirate and retain the supernatant on ice (see Section 6, Note 4).
8. Resuspend the pellet in 35 ml of Solution B and repeat steps 5-7.
9. Combine the two supernatants and centrifuge at 25,000 g for 30 min.
10. Resuspend the light mitochondrial pellet in 6 ml of Solution B using 10 gentle strokes of the pestle of the small volume loose-fitting (Wheaton Type B) Dounce homogenizer (see Section 6, Notes 5 and 6).
11. Using a two chamber gradient maker or Gradient Master™ prepare 30 ml linear gradients from equal volumes of the 2.25 and 24% (w/v) iodixanol in tubes for the vertical rotor (see Section 6, Notes 7 and 8).
12. Underlayer the gradient with 1.0 ml of 0.5 ml of OptiPrep™.
13. Layer the sample on top of the gradient to fill the tube and seal it (see Section 6, Note 9).
14. Centrifuge at 40,000 g for 90 min. Use a controlled acceleration and deceleration programs to ensure a smooth reorientation of the gradient. If these are not available, turn off the brake below 2000 rpm.
15. Collect the gradient dense end first in 0.5 ml fractions; the peroxisomes band close to the bottom of the gradient. For more information on gradient harvesting and analysis see respectively Application Sheets S08 and S09.
6. Notes
1. In the gradient EDTA was omitted in the method of ref 7.
2. Originally the gradient covered a wider density range 15-36% (w/v) iodixanol [4,5], but more recently the 2.25-24% or 2.25-22.5% [7-14] iodixanol gradients have become more popular. Discontinuous iodixanol gradients are less often [15,16]; the light mitochondrial fraction was adjusted to 23.5% (w/v) iodixanol and layered over 35% (w/v) iodixanol and centrifuged at 110,000 g for 2 h. The peroxisomes band at the interface.
3. The use of a vertical rotor is very common in many organelle purifications. The short sedimentation path length of the rotor means that the particles reach their banding density very quickly and the low hydrostatic pressure in the gradient preserves organelle integrity. A smaller rotor such as the VTi65.1 is permissible. If a vertical rotor is not available, fixed-angle rotor or swinging-bucket rotors may be substituted, but the centrifugation times will need to be increased.
4. Metal cannulas (i.d. approx 0.8 mm) can be obtained from most surgical instrument supply companies.
5. To avoid damage to the delicate organelles only use very gentle strokes of the pestle.
6. A post-nuclear supernatant was used instead of a light mitochondrial fraction according to ref 5.
7. If neither of these devices is available then first construct a discontinuous gradient from equal volumes of 15%, 25%, 30% and 36% (w/v) iodixanol and allow them to diffuse. For more information on preparing gradients see Application Sheet S03.
8. For smaller rotors scale down all volumes proportionately.
9. There are a variety of sealed tubes that are commercially available but the easiest sealed tubes to use are Beckman Optiseal tubes.
7. Recent publications
In a Cold Spring Harbor Protocols publication Cramer et al [17] provided a detailed and interesting comparison of sucrose gradients, iodixanol gradients in which the OptiPrep™ was diluted with a simple buffer and iodixanol gradients in which the OptiPrep™ was diluted with a standard buffered sucrose homogenization medium and found that the latter clearly gave superior results. Wróblewska et al [18] using an iodixanol gradient containing 18% (w/v) sucrose, based on the methods described by Cramer [17] were able to resolve not only very pure peroxisomes, but also a population of vesicles that contained a subset of peroxisomal membrane proteins. Effelsberg et al [19] used a 15.5-36% (w/v) iodixanol gradient, also containing 18% (w/v) sucrose. Mindthoff et al [20] and Effelsberg et al [21], used the 2.25-25% (w/v) iodixanol gradient previously described by Grunau et al [9].
8. References
1. Thieringer, R., Shio, H., Han, Y., Cohen, G. and Lazarow, P. B. (1991) Peroxisomes in Saccharomyces cerevisiae: immunofluorescence analysis and import of catalase A into isolated peroxisomes Mol. Cell. Biol., 11, 510-522
2. Watkins, P. A., Lu, J-F., Steinberg, S. J., Gould, S. J., Smith, K. D. and Braiterman, L. T. (1998) Disruption of the Saccharomyces cerevisiae FAT1 gene decreases very long-chain fatty acyl-CoA synthetase activity and elevates intracellular very long-chain fatty acid concentrations J. Biol. Chem., 273, 18210-18219
3. Crane, D. I., Kalish, J. E. and Gould, S. J. (1994) The Pichia pastoris PAS4 gene encodes a ubiquitinconjugating enzyme required for peroxisome assembly J. Biol. Chem., 269, 21835-21844
4. Einwachter, H., Sowinski, S., Kunau, W-H. and Schliebs, W. (2001) Yarrowia lipolytica Pex20p, Saccharomyces cerevisiae Pex18p/Pex 21p and mammalian Pex5pL fulfil a common function in the early steps of the peroxisomal PTS2 import pathway EMBO Rep., 2, 1035-1039
5. Schäfer, A., Kerssen, D., Veenhuis, M., Kunau, W-H. and Schliebs, W. (2004) Functional similarity between the peroxisomal PTS2 receptor binding protein Pex18p and the N-terminal half of the PTS1 receptor Pex5p Mol. Cell Biol., 24, 8895-8906
6. Kerssen, D., Hambruch, E., Klaas, W., Platta, H.W., de Kruijff, B., Erdmann, R., Kunau, W-H. and Schleibs, W. Membrane association of the cycling peroxisome import receptor Pex5p J. Biol. Chem., 281, 27003- 27015
7. Platta, H.W., Grunau, S., Rosenkrantz, K., Girzalsky, W. and Erdmann, R. (2005) Functional role of the AAA peroxins in dislocation of the cycling PTS1 receptor back to the cytosol Nat. Cell Biol., 7, 817-822
8. Thoms, S., Debelyy, M.O., Nau, K., Meyer, H.E. and Erdmann, R. (2008) Lpx1p is a peroxisomal lipase required for normal peroxisome morphology FEBS J., 275, 504-514
9. Grunau, S., Mindthoff, S., Rottensteiner, H., Sormunen, R.T., Hiltunen, J.K., Erdmann, R. and Antonenkov, V.D. (2009) Channel-forming activities of peroxisomal membrane proteins from the yeast Saccharomyces cerevisiae FEBS J., 276, 1698–1708
10. Antonenkov, V.D., Mindthoff, S., Grunau, S., Erdmann, R. and Hiltunen, J.K. (2009) An involvement of yeast peroxisomal channels in transmembrane transfer of glyoxylate cycle intermediates Int., J. Biochem. Cell Biol., 41, 2546–2554
11. Welker, S., Rudolph, B., Frenzel, E., Hagn, F., Liebisch, G., Schmitz, G., Scheuring, J., Kerth, A., Blume, A., Weinkauf, S., Haslbeck, M., Kessler, H. and Buchner, J. (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function Mol. Cell, 39, 507–520
12. Grunau, S., Lay, D., Mindthoff, S., Platta, H.W., Girzalsky, W., Just, W.W. and Erdmann, R. (2011) The phosphoinositide 3-kinase Vps34p is required for pexophagy in Saccharomyces cerevisiae Biochem. J. 434, 161–170
13. Debelyy, M.O., Platta, H.W., Saffian, D., Hensel, A., Thoms, S., Meyer, H.E., Warscheid, B., Girzalsky, W. and Erdmann, R. (2011) Ubp15p, a ubiquitin hydrolase associated with the peroxisomal export machinery J. Biol. Chem., 286, 28223–28234
14. Oeljeklaus, S., Reinartz, B.S., Wolf, J., Wiese, S., Tonillo, J., Podwojski, K., Kuhlmann, K., Stephan, C., Meyer, H.E., Schliebs, W., Brocard, C., Erdmann, R. and Warscheid, B. (2012) Identification of core components and transient interactors of the peroxisomal importomer by dual-track stable isotope labeling with amino acids in cell culture analysis J. Proteome Res. 2012, 11, 2567−2580
15. Nyathi, Y., De Marcos Lousa, C., van Roermund, C.W., Wanders, R.J.A., Johnson, B., Baldwin, S.A., Theodoulou, F.L. and Baker, A. (2010) The Arabidopsis peroxisomal ABC transporter, Comatose, complements the Saccharomyces cerevisiae pxa1 pxa2 mutant for metabolism of long-chain fatty acids and exhibits fatty acyl-CoA-stimulated ATPase activity J. Biol., Chem., 285, 29892–29902
16. Nyathi, Y., Zhang, X., Baldwin, J.M., Bernhardt, K., Johnson, B., Baldwin, S.A., Theodoulou, F.L. and Baker, A. (2012) Pseudo half-molecules of the ABC transporter, COMATOSE, bind Pex19 and target to peroxisomes independently but are both required for activity FEBS Lett., 586, 2280–2286
17. Cramer, J., Effelsberg, D., Girzalsky, W. and Erdmann, R. (2015) Isolation of peroxisomes from yeast Cold Spring Harb. Protoc; doi:10.1101/pdb.top074500
18. Wróblewska, J.P., Cruz-Zaragoza, L.D., Yuan, W., Schummer, A., Chuartzman, S.G., de Boer, R., Oeljeklaus, S., Schuldiner, M. et al (2017) Saccharomyces cerevisiae cells lacking Pex3 contain membrane vesicles that harbor a subset of peroxisomal membrane proteins BBA – Mol. Cell Res., 1864, 656–1667
19. Effelsberg, D., Cruz-Zaragoza, L.D, Tonillo, J., Schliebs, W. and Erdmann, R. (2015) Role of Pex21p for piggyback import of Gpd1p and Pnc1p into peroxisomes of Saccharomyces cerevisiae J. Biol. Chem., 290, 25333–25342
20. Mindthoff, S., Grunau, S., Steinfort, L.L., Girzalsky, W., Hiltunen, J.K., Erdmann, R. and Antonenkov, V.D. (2016) Peroxisomal Pex11 is a pore-forming protein homologous to TRPM channels Biochim. Biophys. Acta, 1863, 271–283
21. Effelsberg, D., Cruz-Zaragoza, L.D., Schliebs, W. and Erdmann, R. (2016) Pex9p is a new yeast peroxisomal import receptor for PTS1-containing proteins J. Cell Sci., 129, 4057-4066
OptiPrepTM Application Sheet S57; 6th edition January 2020
OptiPrep™ Application Sheet S58
Isolation of organelles from protozoa and amoeba
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- For the isolation of acidocalcisomes see Application Sheet S50
- Organelles from following organisms are covered: Trypanosoma (Section 1), Dictyostelium (Section 2), Leishmania (Section 3), Mastigamoeba and Trichomonas (Section 4), Giardia (Section 5), Paramecium (Section 6) and Toxoplasma (Section 7).
1. Trypanosoma brucei
1a Glycosomes
1a-1. Background
Isolation of glycosomes from Trypansoma brucei in double sucrose gradients, in Nycodenz® gradients and in Percoll® gradients was compared by Aman and Wang [1]. Those isolated in Percoll® produced the least enriched fraction and while those in Nycodenz® were marginally less homogenous than those from the double sucrose gradient, the convenience of using a single gradient of a more particle-friendly medium may be more important [1-3]. More recently however a more convenient OptiPrep™-based method has been developed by Colasante et al [4].
1a-2. Solutions required
A. OptiPrep™
B. Homogenization medium: 0.25 M sucrose, 1mM EDTA, 0.1% (v/v) ethanol, 5 mM Mops pH 7.2.
C. 6 mM EDTA, 0.6% ethanol, 30 mM Mops, pH 7.2.
D. 1 M sucrose.
E. Gradient solutions: Make up from solutions A, C, D and water using respectively, these ratios by volume:
E1: 5 + 0.6 + 0.4 + 0.0 (50% iodixanol)
E2: 4 + 0.6 + 0.7 + 0.7 (40% iodixanol)
E3: 2 + 0.6 + 1.1 + 2.3 (20% iodixanol)
Add protease inhibitors as required to solutions as required. Solution A can also be used as a wash medium (see Section 2d) for the trypanosomes; protease inhibitors may be omitted from the solution when used for this purpose.
1a-3. Ultracentrifuge rotor requirements
Vertical rotor with a tube capacity of approx 38 ml tubes (e.g. Beckman VTi50 or Sorvall TV860) with appropriate sealed tubes (see Section 1a-6, Notes 1 and 2).
1a-4. Pre-gradient protocols
See Colasante et al [4] for details of harvesting, washing and homogenization of the trypanosomes by grinding with silicon carbide.
1a-5. Centrifugation protocol
Carry out all operations at 0-4°C
1. Remove the silicone carbide by centrifugation at 100 g for 15min.
2. Decant the supernatant and centrifuge at 1000 g for 15 min to pellet the nuclei.
3. Decant the supernatant and centrifuge at 17,000 g for 15 min to give a pellet of crude glycosomes.
4. Decant and discard the supernatant and resuspend the pellet in approx 3 ml of Solution B using a few gentle strokes of the pestle of a loose-fitting Dounce homogenizer (Wheaton Type B).
5. During the differential centrifugation steps use a two chamber gradient maker or a Gradient Master to prepare linear gradients from 16 ml each of gradient solutions E2 and E3 in tubes for the vertical rotor and underlayer each gradient with 3.5 ml of gradient solution E1. Transfer the suspension from step 4 to the top of the gradient and centrifuge at 170,000 g for 1 h. Use a slow acceleration and deceleration programme if available; alternatively turn off the brake during deceleration from 2000 rpm (see Section 1a-6, Notes 3 and 4).
6. Harvest the purified glycosomes, which form the densest band in the gradient or unload the gradient in a number of equal volume fractions. See also Section 1a-6, Notes 5 and 6. For more information on gradient collection see Application Sheet S08.
1a-6. Notes
1. If the preparation is scaled down to smaller tubes (13 ml) use the Beckman VTi65.1 or Sorvall 65V13 rotor. If no vertical rotor is available then a swinging-bucket rotor may be used but the centrifugation time will need to be increased to take account of the longer path length.
2. The sealed tubes that are the easiest to load and unload are Optiseal™ tubes for Beckman rotors.
3. For smaller volume rotors scale all gradient and sample volumes down proportionately. For methods describing the construction of continuous gradients see Application Sheet S03.
4. A continuous gradient of 20%–35%, v/v OptiPrep (with a 50%, v/v OptiPrep™ cushion) was used by Gualdron-López et al [5], centrifuged at 100,000 g for 2 h in a vertical rotor. Later the centrifugation time was extended to 15 h [6].
5. See also Section 3d below.
6. The same method has been used by Bauer et al [7].
1b. Contractile vacuole
The supernatant from a series of low-speed centrifugations was centrifuged at 100,000 g for 1 h and the resuspended pellet median-loaded (25% iodixanol) in a discontinuous gradient of 15%, 20%, 25%, 30%, 34%, 37% and 40% (w/v). It was centrifuged at 50,000 g 65 min; the contractile vacuole banded near the top of the gradient [8,9].
1c. Lipid rafts
For the isolation of these structures see Application Sheets S32 and S33 and Reference List RS09 (to access return to the initial list of Folders and select “Reference Lists”).
1d. Acidocalcisomes
For the isolation of these structures see Application Sheet S50
2. Dictyostelium
2a. Acidocalcisomes
For the isolation of these structures see Application Sheet S50 The iodixanol gradient also provides mitochondria- and contractile vacuole-rich fractions from Dictyostelium. Sivaramakrishnan and Fountain [10] reported that iodixanol gradients also resolve the ER from contractile vacuoles.
2b. Phagosomes (Legionella-containing)
A post-nuclear supernatant from which the lysosomes had been removed by antibody binding, was centrifuged through a 5-30% (w/v) iodixanol at 100,000g for 2h [11]. The phagosomes banded approx. a third of the way down the gradient. Denser Legionella-containing vacuoles partially overlap the mitochondria; this was interestingly resolved by increasing the density of the mitochondria by addition of iodophenylnitrophenyltetrazolium (INT) to the homogenate [12]. Iodixanol gradients have also been used in the analysis of acyl CoA binding protein (AcbA) which is unusually sequestered into a dense vesicle population in a mutant form of the protozoan [13]
3. Lieshmania
3a. Acidocalcisomes
For the isolation of these structures see Application Sheet S50
3b. Lipid rafts
For the isolation of these structures see Application Sheets S32 and S33 and Reference List RS09 (to access return to the initial list of Folders and select “Reference Lists).
3c. Subcellular membranes
A low-speed supernatant was loaded on to discontinuous 18.5%, 28% and 40% (w/v) iodixanol gradients, centrifuged in a Beckman SW41 rotor at approx 190,000 g for 90 min [14]. The gradient was used primarily to analyze the distribution of ATPase; it was detected in a number of fractions with a distinct pattern. This pattern of enzyme distribution was however changed in those cells that were PGPHA transfected.
3d. Glycosomes
A method, alternative to that described for Leishmania glycosomes (see Section 1) is to use a density barrier [15,16]. The crude glycosome containing fraction was produced at 26,000 g for 50 min (rather than the conditions described in Step 7); 2 ml of this was layered over 10 ml of 30% (w/v) Nycodenz® and centrifuged at 105,000 g for 50 min to pellet the glycosomes.
4 Hydrogenosomes
4.1 Mastigamoeba balamuthi
A discontinuous iodixanol covering the range 15–40% was centrifuged at 100,000 g overnight to resolve a hydrogenosome fraction [17].
4.2 Trichomonas
Crude hydrogenosomes from Trichomonas vaginalis (homogenized by sonication in 0.25 M sucrose, 0.5 mM KCl, 10 mM Tris-HCl, pH 7.2 and the usual protease inhibitors), were prepared from a post-nuclear supernatant by centrifugation at 17,000 g for 20 min [18]. The gradient solutions were prepared (containing sucrose, EDTA and Tris buffer) in the same manner as described in OptiPrep Application Sheet S01. Either continuous (18-36% w/v iodixanol) or a discontinuous over the same concentration range (in 2% steps. The sample was underlayered in 50% (w/v) iodixanol and centrifuged at 200,000 g for 2 h. Hydrogenosomes banded at approx. 30% (w/v) iodixanol. In an alternative method published by Kay et al [19] the crude fraction was suspended in 0.25 M sucrose, 20 mM HEPES, 5 mM DTT containing 6% (w/v) iodixanol and the usual protease inhibitors and layered over a 12-24% (w/v) iodixanol gradient centrifuged at 70,000 g for 2 h. The banding position of the hydrogenosomes is identified by their characteristic light brown colour. More recently a 15,000 g pellet was fractionated in a discontinuous 12-30% iodixanol gradient at 200,000 g for 12 h [20]
5. Giardia
Giardia contains a lysosome-like organelle [21], which like the mammalian lysosomes has relatively low density. When a 100,000 g particulate fraction of a post-nuclear supernatant (prepared from a Giardia sonicate) is fractionated in a 1.05-1.25 g/ml Nycodenz® gradient, the organelle bands at approx 1.07 g/ml [21] after centrifugation at approx. 150,000 g for 2.5 h. Jedelský et al [22] used a discontinuous OptiPrepTM gradient (15%, 20%, 25%, 30% and 50%) centrifuged at 120,000 g for 24 h to fractionate a Giardia homogenate in a study which demonstrated the reduced proteome in the mitosomes of Giardia intestinalis (compared to that of mammalian mitochondria). The authors concluded that this was a result of the low oxygen content of its environment, which required a reduced set of metabolic processes.
6. Paramecium
Parmecium tetraurelia homogenates have been fractionated on a 10 to 30% (w/v) iodixanol gradient at 46,000 g for 18 h in SNARE protein distribution studies [23]
7. Toxoplasma
Host cells were fractionated to investigate the organelle association of evacuoles: a light mitochondrial fraction of the cells was adjusted to 20% (w/v) iodixanol and a self-generated gradient formed by centrifugation at 180,000 g for 4 h in a small volume near-vertical rotor [24]. More recently “plant-like vacuoles” were isolated from toxoplasma tachyzoites in a discontinuous gradient of 15%, 20% (containing a “microsomal fraction”), 25%, 30%, 34% and 38% iodixanol and centrifuged at 50,000 g for 1 h. The vacuoles banded close to the top of the gradient [25].
8. References
1. Aman, R. A. and Wang, C. C. (1986) An improved purification of glycosomes from the procyclic trypomastigotes of Trypanosoma-brucei Mol. Biochem. Parasitol., 21, 211-220
2. Opperdoes, F. R., Baudhuin, P., Coppens, I., de Roe, C., Edwards, S. W., Weijers, P. J. and Misset, O. (1984) Purification, morphometric analysis, and characterization of the glycosomes (microbodies) of the protozoan hemoflagellate Trypanosoma brucei J. Cell Biol., 98, 1178-1184
3. Aman, R. A., Kenyon, G. L. and Wang, C. C. (1985) Cross-linking of the enzymes in the glycosome of Trypanosoma brucei J. Biol. Chem., 260, 6966-6973
4. Colasante, C., Ellis, M., Ruppert, T. and Voncken, F. (2006) Comparative proteomics from bloodstream form and procyclic form Trypanosoma brucei brucei Proteomics, 6, 3275-3293
5. Gualdron-López, M., Vapola, M.H., Miinalainen, I.J., Hiltunen, J.K., Michels, P.A.M. and Antonenkov, V.D. (2012) Channel-forming activities in the glycosomal fraction from the bloodstream form of Trypanosoma brucei PLoS One, 7: e34530
6. Gualdrón-López, M., Chevalier, N., Van Der Smissen, P., Courtoy, P.J., Rigden, D.J. and Michels, P.A.M. (2013) Ubiquitination of the glycosomal matrix protein receptor PEX5 in Trypanosoma brucei by PEX4 displays novel features Biochim. Biophys. Acta, 1833, 3076–3092
7. Bauer, S.T., McQueeney, K.E., Patel, T. and Morris, M.T. (2017) Localization of a trypanosome peroxin to the endoplasmic reticulum J. Eukaryot. Microbiol., 64, 97–105
8. Rohloff, P., Montalvetti, A. and Docompo, R. (2004) Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi J. Biol. Chem., 279, 52270-52281
9. Ulrich, P.N., Jimenez, V., Park, M., Martins, V.P., Atwood III, J., Moles, K., Collins, D., Rohloff, P. et al (2011) Identification of contractile vacuole proteins in Trypanosoma cruzi PLoS One 6: e18013
10. Sivaramakrishnan, V. and Fountain, S.J. (2012) A mechanism of intracellular P2X receptor activation J. Biol. Chem., 287, 28315–28326
11. Shevchuk, O. and Steinert, M. (2013) Isolation of pathogen-containing vacuoles In Dictyostelium discoideum Protocols, Methods Mol. Biol., 983, (eds Eichinger, L. and Rivero, F.) Springer Science+Business Media, pp 419-429
12. Shevchuk, O., Batzilla, C., Hägele, S., Kusch, H., Engelmann, S., Hecker, M., Haas, A., Heuner, K., Glöckner, G., Steinert, M. (2009) Proteomic analysis of Legionella-containing phagosomes isolated from Dictyostelium Int. J. Med. Microbiol., 299, 489–508
13. Cabral, M., Anjard, C., Malhotra, V., Loomis, W.F. and Kuspa, A. (2010) Unconventional secretion of AcbA in Dictyostelium discoideum through a vesicular intermediate Eukaryot. Cell, 9, 1009-1017
14. Légaré, D., Richard, D., Mukhopadyay, R., Stierhof, Y-D., Rosen, B.P., Haimeur, A., Papadopoulou, B. and Ouellette, M. (2001) The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase J. Biol. Chem., 276, 26301-26307
15. Raychaudhury, B., Gupta, S., Banerjee, S., Das, B. and Datta, S.C. (2004) Isolation of Leishmania glycosomes by a rapid method Anal. Biochem., 332, 404-408
16. Gupta, S., Raychaudhury, B., Banerjee, S., Das, B. and Datta, S.C. (2006) An intracellular calcium store is present in Leishmania donovani glycosomes Exp. Parasitol., 113, 161-167
17. Nývltová, E., Stairs, C.W., Hrdý, I., Rídl, J., Mach, J., Pačes, J., Roger, A.J. and Tachezy, J. (2015) Lateral Gene transfer and gene duplication played a key role in the evolution of Mastigamoeba balamuthi hydrogenosomes Mol. Biol. Evol., 32, 1039–1055
18. Beltrán, N.C., Horváthová, L., Jedelský, P.L., Šedinová, M., Rada, P., Marcinčiková, M., Hrdý, I. and Tachezy, J. (2013) Iron-induced changes in the proteome of Trichomonas vaginalis hydrogenosomes PLoS One, 5: e65148
19. Kay, C., Lawler, K., Self, T.J., Dyall, S.D. and Kerr, I.D. (2012) Localisation of a family of complex-forming -barrels in the T. vaginalis hydrogenosomal membrane FEBS Lett., 586, 4038–4045
20. Hsu, H-M., Chu, C-H., Wang, Y-T., Lee, K., Wei, S-Y., Liu, H-W., Ong, S-J., Chen, C. and Tai, J-H. (2014) Regulation of nuclear translocation of the Myb1 transcription factor by TvCyclophilin 1 in the protozoan parasite Trichomonas vaginalis 289, 19120–19136
21. Thirion, J., Wattiaux, R. and Jadot, M. (2003) The acid phosphatase positive organelles of the Giardia lamblia trophozoite contain a membrane bound cathepsin C activity Biol. Cell, 95, 99-105
22. Jedelský, P.L., Doležal, P., Rada, P., Pyrih, J., Šmíd, O., Hrdý, I., Šedinová, M., Marcinčiková, M., Voleman, L., Perry, A.J., Beltrán, N.C., Lithgow, T. and Tachezy, J. (2011) The minimal proteome in the reduced mitochondrion of the parasitic protist Giardia intestinalis PLoS One, 6: e17285
23. Schilde, C., Lutter, K., Kissmehl, R. and Plattner, H. (2008) Molecular identification of a SNAP-25-like SNARE protein in Paramecium Eukaryot. Cell, 7, 1387-1402
24. Hakansson, S., Charron, A.J. and Sibley, L.D. (2001) Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole EMBO J., 20, 3132-3144
25. Miranda, K., Pace, D.A., Cintron, R., Rodrigues, J.C.F., Fang, J., Smith, A., Rohloff, P., Coelho, E., de Haas, F., et al (2010) Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole Mol. Microbiol., 76, 1358–1375
OptiPrepTM Application Sheet S58; 6th edition, January 2020
OptiPrep™ Application Sheet S59
Isolation of organelles from algae and fungi
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- Yeast organelles are covered in other Application Sheets; see Subcellular Membrane Index
- This Application Sheet summarizes all of the known OptiPrep™ applications for algae and fungi; a detailed methodology is only provided in a few instances.
- Some of the more recent papers are summarized in Section 7.
1. Chlamydomonas reinhardtii
Gradients that are used primarily for the isolation of acidocalcisomes also resolve mitochondria; see Application Sheet S50. Membrane vesicles can be shed from flagella by shaking in 25 mM KCl, 5 mM MgSO4, 1 mM DTT, 0.5 mM EDTA, 10 mM Hepes, pH 7.2 containing 0.1% NP40 (plus protease inhibitors). Axonemes are then removed by repeated centrifugation at 16,000 g for 10 min and the vesicles harvested from the supernatant at 228,000 g for 30 min. The crude vesicles in 1 ml of the medium are adjusted to 15% (w/v) iodixanol; underlaid with an equal column of 30% iodixanol and centrifuged at 431,000 g for approx 1 h. The vesicles band in the middle of the gradient [1].
In a study of the development of cilia in Wood and Rosenbaum in Chlamydomonas reinhardtii [2] sCytoplasmic extract was adjusted to 30% w/v iodixanol and overlaid by layers of 25%, 20%, 15%, 12%, 10%, and 5% and centrifuged for 3 hr at 200,000 g. The gradients were used to study the attachment of proteins to cytoplasmic membranes during flagellar development.
2. Cladopsorium resinae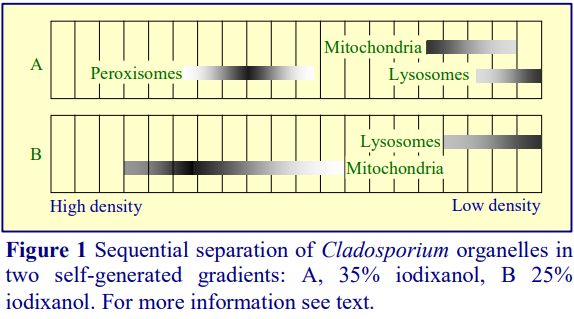
The LMF in a routine buffered 0.25 M sucrose solution containing 1 mM EDTA was adjusted to 35% (w/v) iodixanol and a gradient
formed by self-generation in near vertical NVT65 rotor centrifuged at 202,000 g for 4 h [3]. The organelle distribution is shown diagrammatically in Figure 1A; if the overlapping fractions of mitochondria and lysosomes are collected and adjusted to 25% iodixanol and re-centrifuged then a complete separation of these two organelles is obtained (Figure 1B).
3. Cyanidioschyzon merolae
3.1 Microtubule-linked organelles
Cells were lysed in a microtubule stabilizing medium: 1.2 M sorbitol, 2 mM MgSO₄, 2 mM KCl, 1 mM DTT, 4 mM EGTA, 10 mM Tris-HCl, pH 7.2, containing, 50μM paclitaxel and 0.1% BSA, plus protease inhibitors [4,5]. The homogenate was mixed with an equal volume of 10% (w/v) iodixanol in 0.6 M sorbitol, 2 mM MgSO₄, 2 mM KCl, 1 mM DTT, 4 mM EGTA, 10 mM Tris-HCl, pH 7.2, containing, 10μM paclitaxel (plus protease inhibitors). A discontinuous gradient of 15%, 25% and 40% (w/v) iodixanol (8 ml, 8 ml and 4 ml respectively), in the same buffer, were overlaid with 15 ml of 300 g supernatant and centrifuged at 141,000 g for 30 min. The microtubule-linked organelles banded at the lowest interface.
3.2 Polyphosphate vacuoles
Yagisawa et al [6] isolated these polyphosphate-rich vacuoles from Cyanidioschyzon merolae and identified a number of important proteins. The discontinuous gradient used by the authors was an unusual one, comprising three layer one of 27%, 62% and 80% (w/v) iodixanol. To achieve the latter two solutions, the authors first produced a very high density stock by evaporating the water from OptiPrep™. For safety reasons this is not a procedure that the manufacturers of OptiPrep™ can recommend. Since however the vacuoles banded at the 27%/62% iodixanol interface we suggest that the densest layer is omitted and the gradient formed from OptiPrep™ and 27% (w/v) iodixanol. To maintain the other solution properties used by the authors first prepare a diluent of 62% (w/w) sucrose solution containing 40 mM MgCl₂, 100 mM KCl, 400 mM Tris-HCl, pH 7.6. For the dense gradient solution add 1 ml of the diluent to 20 ml of OptiPrep™. From this prepare a 27% (w/v) iodixanol solution by diluting 2.7 vol. with 3.3 ml of 0.25 M sucrose, 2 mM MgCl₂, 5 mM KCl, 20 mM TrisHCl, pH 7.6. Use the latter to resuspend the total membrane pellet. Layer 0.6 ml of this on to 2 ml of the dense solution and overlay with 1.4 ml of the lysis solution and centrifuge at 50,000 g for 1 h at 8°C. The vacuoles band at the lower interface.
- More recently, iodixanol gradients have been used to study peroxisomes from this algae [7].
4. Cryptococcus neoformans extracellular vesicles (EVs)
The glucuronoxylomannan of this fungus appears to be transported into the extracellular space in a membrane vesicle. This exocytic process is akin to that which occurs in mammalian cells and the shallow 6-18% (w/v) iodixanol gradients used to purify the EVs released from such cells have also been used for similar studies with Cryptococcus neoformans. The gradients were centrifuged at 250,000 g for 75 min [8]. A complex banding pattern of the glucuronoxylomannan and sterol suggest a heterogeneity of the budded vesicles. Discontinuous iodixanol gradients have been used by Wolf et al [9] comprising 3 ml of 30% and 2 ml each of 25%, 20%, 15% and 10% (w/v) iodixanol. The crude membrane vesicles in 5% (w/v) iodixanol were layered on top and centrifuged at 140 000 g, at 4°C, for 15 h. The peak of EVs occurred just below the mid-point of the gradient and interestingly the lightest ones were deficient in glucuronoxylomannan.
5. Neurospora crassa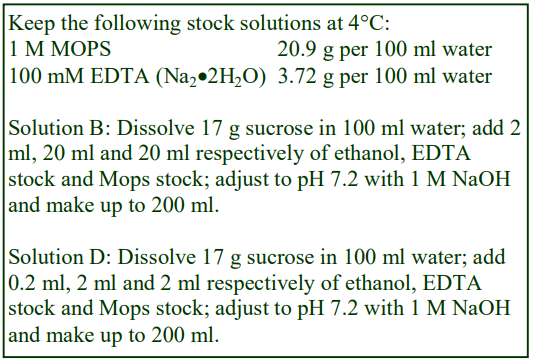
Glyoxysomes have been prepared from an N. crassa post-nuclear supernatant (PNS) using a gradient similar [10] to that developed for mammalian peroxisomes see Application Sheet S12. The following method is adapted from ref 10.
5.1 Solutions required
A. OptiPrep™
B. OptiPrep™ diluent: 0.25 M sucrose, 10 mM EDTA, 100 mM MOPS-NaOH pH 7.2, 1% (v/v) ethanol (see Box)
C. 54% (w/v) Iodixanol stock solution: 9 vol. of Solution A + 1 vol. Solution B.
D. Homogenization medium: 0.25 M sucrose, 1 mM EDTA, 10 mM MOPS-NaOH, pH 7.2, containing 0.1% (v/v) ethanol.
E. Gradient solutions of 47% and 35% (w/v) iodixanol: dilute Solution C with Solution D.
5.2 Ultracentrifuge rotor
Sorvall TH641 or Beckman SW41 or equivalent rotor with approx. 30 ml tubes
5.3 Protocol
1. Prepare an homogenate of N crassa in Solution D.
2. Centrifuge the homogenate at 500 g for 5 min and repeat this three times to remove all of the abrasive powder and nuclei.
3. Prepare a crude organelle pellet was by centrifugation of the PNS at 25,000 g for 30 min and resuspend the pellet in Solution D.
4. Mix the resuspended pellet with an equal volume of the 47% (w/v) iodixanol solution.
5. Transfer 9-10 ml of the crude organelle suspension in 23.5% iodixanol and underlayer with 1-2 ml of the 35% iodixanol solution. If necessary top up the tubes to the required filling volume by layering Solution D on top.
6. Centrifuge at 110,000 g for 2 h. The glyoxysomes band at the interface between the two iodixanol solutions.
6. Paracoccidioides brasiliensis
The mitochondria and peroxisomes from the yeast phase of Paracoccidioides brasiliensis have been isolated using a gradient identical to that used for preparing mitochondria from S. cerevisiae. Section 4 of Application Sheet S17 describes the discontinuous gradient that is used [11]; the densest band contains the peroxisomes. See also Application Sheet S57 for more information of the isolation of peroxisomes from S. cerevisiae.
7. Recent publications
A CsCl gradient was used primarily to separate flagella, transition zones, and empty flagellar collars from Chlamydomonas elegans . To improve the ourity of the transition zone fraction, it was diluted with buffer and harvested by centrifugation at approx 190,000 gav for 20 min. The pellet was made was resuspended in buffer; adjusted to 30% iodixanol and made part of a discontinuous 60%, 30%, 20% (w/v) iodixanol step gradient After centrifugation for 3 hr at approx 165,000 gav. The transitional zones banded at the 60%/30% iodixanol interface, diluted. For more details see ref 12. Secretory vesicles from Candida albicans [13] were fractionated according to a method first described by Chang et al (see Application Sheet S52).
- Note the other Application Sheets devoted to algae and fungi:
- Algae: Acidocalcisomes (S50)
- Fungi: Extracellular vesicles (S62)
8. References
1. Huang, K., Diener, D.R., Mitchell, A., Pazour, G.J., Witman, G.B. and Rosenbaum, J.L. (2007) Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella J. Cell Biol., 179, 501-514
2. Wood, C.R. and Rosenbaum, J.L. (2014) Proteins of the ciliary axoneme are found on cytoplasmic membrane vesicles during growth of cilia Curr. Biol., 24, 1114-1120
3. Goswami, P. and Cooney, J.J. (1999) Subcellular location of enzyme involved in oxidation on n-alkane by Cladosporium resinae Appl. Microbiol. Biotechnol., 51, 860-864
4. Nishida, K., Yagisawa, F., Kuroiwa, H., Nagata, T. and Kuroiwa, T. (2005) Cell cycle-regulated microtubeli-independent organelle division in Cyanidioschyzon merolae Mol. Biol. Cell, 16, 2493-2502
5. Nishida, K., Yagisawa, F., Kuroiwa, H., Yoshida, Y. and Kuroiwa, T. (2007) WD40 protein Mda1 is purified with Dnm1 and forms a dividing ring for mitochondria before Dnm1 in Cyanidioschyzon merolae Proc. Natl. Acad. Sci. USA, 104, 4736-4741
6. Yagisawa, F., Nishida, K., Yoshida, M., Ohnuma, M., Shimada, T., Fujiwara, T., Yoshida, Y., Misumi, O., Kuroiwa, H. and Kuroiwa, T. (2009) Identification of novel proteins in isolated polyphosphate vacuoles in the primitive red alga Cyanidioschyzon merolae Plant J., 60, 882–893
7. Imoto, Y., Abe, Y., Okumoto, K., Honsho, M., Kuroiwa, H., Kuroiwa, T. and Fujiki, Y. (2017) Defining the dynamin-based ring organizing center on the peroxisome-dividing machinery isolated from Cyanidioschyzon merolae J. Cell Sci., 130, 853-867
8. Oliveira, D.L., Nimrichter, L., Miranda, K., Frases, S., Faull, K.F., Casadevall, A. and Rodrigues, M.L. (2009) Cryptococcus neoformans cryoultramicrotomy and vesicle fractionation reveals an intimate association between membrane lipids and glucuronoxylomannan Fungal Genet. Biol., 46, 956–963
9. Wolf, J.M., Rivera, J. and Casadevall, A. (2012) Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles Cellular Microbiology (2012) 14(5), 762–773
10. Managadze, D., Würtz, C., Wiese, S., Meyer, H.E., Niehaus, G., Erdmann, R., Warscheid, B. and Rottensteiner, H. (2010) A proteomic approach towards the identification of the matrix protein content of the two types of microbodies in Neurospora crassa Proteomics, 10, 3222–3234
11. Brito, W.deA., Rezende, T.C.V., Parente, A.F., Ricart, C.A.O., de Sousa, M.V., Báo, N. and Soares, C.M.deA. (2011) Identification, characterization and regulation studies of the aconitase of Paracoccidioides brasiliensis Fungal Biol., 115, 697-707
12. Diener, D.R., Lupetti, P. and Rosenbaum, J.L. (2015) Proteomic analysis of isolated ciliary transition zones reveals the presence of ESCRT proteins Curr. Biol., 25, 379–384
13. Caballero-Lima, D., Hautbergue, G.M., Wilson, S.A. and Sudbery, P.E. (2014) In Candida albicans hyphae, Sec2p is physically associated with SEC2 mRNA on secretory vesicles Mol. Microbiol., 94, 828–842
OptiPrepTM Application Sheet S59; 6th edition, January 2020
OptiPrep™ Application Sheet S60
Isolation of organelles from plants and plant cells
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- The purification of plant protoplasts that is often a prelude to subcellular membrane fractionation is covered in Application C19 (see Mammalian and Non-mammalian Cell Index)
- This Application Sheet covers the purification of the following organelles: chloroplasts, peroxisomes and glyoxysomes, mitochondria, amyloplasts, leucoplasts and vacuoles. For nuclei see Application Sheet S10
- Reference List RS01 “Purification of nuclei from tissues and cells in iodixanol gradients” lists all the published papers. The methodology has been adapted to various plant sources. To access RS01 return to the initial list of Folders and select “Reference Lists”.
- Application Sheet S61 describes methods for analysis of membrane trafficking, protein processing and proteomic analysis; it covers plasma membrane, ER, Golgi and mitochondria.
1. Introduction
Until recently Nycodenz® was used for most plant organelle separations. It is not known if iodixanol can be substituted directly for Nycodenz® in any of the Nycodenz® applications. Certainly the availability of iodixanol as a 60% (w/v) solution (OptiPrep™) makes gradient solution preparation much easier than is the case with Nycodenz®. Iodixanol and Nycodenz® solutions of the same % (w/v) concentration have almost identical densities, but solutions of Nycodenz® are hyperosmotic above 1.15g/ml, in contrast to those of iodixanol which can be made isoosmotic at all densities. Whether the osmolality of Nycodenz® solutions plays an important role in achieving the separations described in this Application Sheet is not known. Comparisons can only be made empirically. For the preparation of iodixanol gradient solutions see Application Sheet S01.
2. Chloroplasts
Laganowsky et al [1] purified and pelleted chloroplasts from a filtered homogenate of Arabidopsis thaliana through a 36% (w/v) iodixanol cushion, containing 0.27 M sucrose, 2 mM EDTA-Na₂, 1 mM MgCl₂, 0.2% bovine serum albumin, 50 mM HEPES-NaOH, pH 7.6, centrifuged at 1200 g for 10 min at 4°. The authors commented that there was no difference between iodixanol-purified chloroplasts and the more traditional Percoll®-purified organelles in terms of electron transport properties. It is important to point out however that add-on procedures such as SDS-PAGE require the removal of residual Percoll; however in the case of iodixanol, this is only true for electron microscopy..
3. Peroxisomes/glyoxysomes
Although peroxisomes and glyoxysomes are essentially very similar in structure, the latter being often referred to as a type of the former, it is not clear if all the methods published are equally applicable to both organelles.
3a. Nycodenz® gradients
Tobacco leaf peroxisomes can be purified from a leaf homogenate in 0.5 M sucrose, 10 mM KCl, 1 mM EDTA, 25 mM MES-KOH, pH 6.0 in a discontinuous gradient of 1 ml each of 17.5% and 25% and 1.55 ml of 35% (w/v) Nycodenz® [2]. Make up the 35% Nycodenz® solution as follows: place 50 ml of the homogenization medium (HM) in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 35 g of Nycodenz® in small amounts until dissolved. Allow the solution to cool to room temperature and make up to 100 ml with HM. It may be filter-sterilized if required. Dilute this further with HM to give the lower density solutions. In a swinging-bucket rotor such as the Beckman SW50.1, a 1000 g (10 min) supernatant is layered on top and centrifuged at 190,000 g for 2.5 h. The peroxisomes band sharply in the bottom half of the gradient and are well separated from mitochondria and other membranes (see comments in Section 1).
Peroxisomes from etiolated Soybean cotyledons have now been isolated in 15.5-36% (w/v) iodixanol gradients (approx 13 ml) + a 2 ml 50% iodixanol cushion; 2 ml of the crude fraction were layered on top and centrifuged for 2.5 h at 100,000 g in a swinging-bucket rotor [3].
Sunflower seeds have been used widely as the plant source for glyoxysomes [4-7], but there is no obvious reason why the gradient might not have a wider applicability. The homogenization medium and Nycodenz® solutions are made up exactly as described in Section 3, but the 17.5% (w/v) Nycodenz® is omitted. The 1000 g supernatant is layered (to fill the tube) over 10 ml of 35% and 8 ml of 25% (w/v) Nycodenz® in tubes for a Beckman SW 28 rotor gradients; the tubes are centrifuged at approx. 120,000 g for 2.5 h. Unload the gradients dense-end first in 1 ml fractions (for more information on gradient collection see Application Sheet S08). The glyoxysomes are recovered close to the top of the 25% Nycodenz®.
3b. OptiPrep
The first paper to report the use of OptiPrep™ for the isolation of peroxisomes from etiolated soybean cotyledons [8] used an initial Percoll® gradient followed by a layering of the partially purified organelles on to a 15.5-36% (w/v) iodixanol gradient in 0.3M sucrose, 1mM EDTA, 0.1% ethanol and 5mM MOPS-HCl pH 7.2; underlayered with a cushion of 50% (w/v) iodixanol containing 25mM sucrose, 0.5mM EDTA, 0.05% ethanol, 2.5mM MOPS-HCl pH 7.2. After centrifugation at 100,000 g for 2.5 h the peroxisomes banded very sharply close to the cushion. Whether the Percoll® gradient can be omitted is not clear. Several reviews have pointed out the advantages of the Arai et al [8] method [9- 11]. Hossain and Komatsu [12] stressed the advantages of using iodixanol.
4. Mitochondria
Hartman et al [13] analysed the mitochondria from cultures of Arabidopsis thaliana by homogenizing the cells in a routine organelle medium of 0.25 M sucrose, 1 mM EDTA, HEPES-NaOH, pH 7.4. After removing unbroken cells, cell wall fragments and nuclei at 2,200 g for 5 min (the supernatant was centrifuged under the same conditions), the remaining organelles were concentrated on to a 16% (w/v) iodixanol cushion at 100,000 g for 2h. Whether the g-force can be reduced should be tested experimentally. The membrane band was collected; readjusted to 16% (w/v) iodixanol and the membranes banded in a self-generated gradient (approx. 11 ml tubes in a vertical rotor at 350,000 g for 3 h. The mitochondria banded approx. three-quarters of the way down the gradient.
- Note that mitochondria are also often identified in iodixanol gradients that are used in proteomic analysis; see Application Sheet S61
5. Amyloplasts
Amyloplasts are rapidly sedimenting organelles that can be isolated without a centrifuge; a wide variety of gradient systems using Nycodenz® have however been used. Most use a discontinuous gradient, although one of the earliest published methods used a continuous gradient.
5a. Continuous gradients (adapted from refs 14-16)
5a-1. Gradient solutions
Make up a 60% (w/v) Nycodenz® stock solution as follows: Place 50 ml of 50 mM Hepes-NaOH, pH 7.5 in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 60 g of Nycodenz® in small amounts until dissolved. Allow the solution to cool to room temperature and make up to 100 ml with buffer. It may be filter-sterilized if required. Make dilutions of this 60% Nycodenz® with 0.5 M sucrose, 50 mM Hepes-NaOH, pH 7.5 to give solutions of 40%, 20% and 10% (w/v) Nycodenz®.
5a-2. Gradient separation
In a glass tube layer 6 ml each of the four Nycodenz® solutions and allow a continuous gradient to form by diffusion at room temperature. For methods describing the construction of continuous gradients see Application Sheet S03. Bring the gradients to 4°C and layer 8 ml of a plant protoplast lysate on top and leave for 4-6 h at 4°C. The amyloplasts band a third to half way down the gradient.
5b. Discontinuous gradients
The amyloplasts are allowed to sediment through a 2% (w/v) Nycodenz® in 0.8 M sorbitol, 1 mM KCl, 2 mM MgCl₂, 1 mM EDTA, 0.1% BSA, 50 mM Hepes-NaOH, pH 7.5 solution on to a 1% agar cushion at 30 g for 10 min [17-19]. Note however that although this same medium was used for Hordeum spontaneum, for Hordeum murinum the Nycodenz® concentration was reduced to 0.5% [20]. This is a widely used methodology [21-25].
In a variant of this method, the agar cushion is replaced by 60% (w/v) Nycodenz® and the sedimentation is carried at 1 g. The Nycodenz® solutions were prepared in 1 M sucrose, 1 mM KCl, 1 mM MgCl₂, 1 mM EDTA, 0.2% BSA, 5 mM DTT, 50 mM Hepes-NaOH, pH 7.6 [26,27].
6. Leucoplasts
6.1 Gradient solutions
Protoplasts are homogenized in 21% (w/v) sucrose, 10 mM KCl, 1mM MgCl₂, 1 mM EDTA, 50 mM Tricine-KOH, pH 7.4 (Solution A). This has an osmolality of approx. 800 mOsm and the 0-40% (w/v) Nycodenz® gradient is constructed in such a way as maintain the same osmolality [28,29]. Make up the 40% Nycodenz® solution as follows: place 50 ml of the 0.3 M sucrose, 10 mM KCl, 1mM MgCl₂, 1 mM EDTA, 50 mM Tricine-KOH, pH 7.4 in a 150 ml beaker on a heated magnetic stirrer set at approx. 50°C and add 40 g of Nycodenz® in small amounts until dissolved. Allow the solution to cool to room temperature and make up to 100 ml with the same medium. It may be filter-sterilized if required.
6.2 Centrifugation
In tubes for a Beckman SW28 swinging-bucket rotor (or equivalent) construct a continuous gradient (approx 30 ml) from equal volumes of the 40% Nycodenz® and Solution A using a twochamber gradient maker or a Gradient Master™. For methods describing the construction of continuous gradients see Application Sheet S03. Layer 7-8 ml of the protoplast lysate on top and centrifuge at 90,000 g for 90 min. Unload the gradients low-density end first in 1 ml fractions (for more information on gradient collection see Application Sheet S08). The leucoplasts are recovered close to the bottom of the gradient.
7. Vacuoles
These particles can be isolated by flotation through low-density solutions under very low g-forces. Protoplasts in 550 mM sorbitol, 10 mM Hepes-KOH, pH 7.4 [29] are osmotically shocked by rapid addition of 1 ml to 1.75 ml of a low osmolality solution of 7.9 mM EDTA, 10 mM Hepes-KOH, pH 7.3 [30] or 5 mM EDTA, 25 mM MES-Tris, pH 7.3 [31]. Sometimes 0.5 % BSA is included in the
solutions. After maintenance at 4°C for 10-30 min, with occasional shaking, the lysate is mixed with 10 ml of 350 mM sorbitol, 8.5% (w/v) Nycodenz® in the same low osmolality medium. After layering 1 ml of the protoplast medium on top, the tubes are centrifuged at 160 g for 3 [30] or 10 [31] min. The vacuoles are recovered from the top half of the protoplast medium.
Sometimes crude vacuoles from plant tissues (rather than protoplasts) are pelleted at 2000 g for 10 min from a homogenate before suspension in 5ml of 15% (w/v) Nycodenz®, 1.2 M sorbitol, 1 mM EDTA, 25 mM MES-Tris, pH 8.0 [32]. This is overlaid with 5 ml of 10% Nycodenz® in the same medium and 2 ml of medium and after centrifugation at 650 g for 5 min the vacuoles collect at the top interface.
In a few published methods very different centrifugation conditions are reported. A filtered suspension (16 ml) of an 8,000 g –15 min pellet (from a cauliflower bud homogenate) in 15% (w/v) Nycodenz, 1.5 M sorbitol, 1mM EDTA, 4 mM DTT, 10 mM Tris-HCl, pH 7.6 is overlaid with 5 ml of 8% (w/v) Nycodenz® in the same medium and 4 ml of medium [32]. After centrifugation at 100,000 g for 45 min (very slow acceleration and deceleration) the vacuoles band at the top interface [33].
8. Tonoplasts
The micrsomal pellet from a Pteris vittata homogenate was adjusted to 15% (w/v) iodixanol; ayered beneath an 8% (w/v) iodixanol solution and the tube filled with an upper layer of isolation medium. After centrifugation at ed (100,000 g, for 45 min, the tonoplasts banded at the 0% and 8% iodixanol interface [34]. In a modified method, a pellet from an 8000 g supernatant was suspended in 15% (w/v) iodixanol (in suspension medium), overlaid with suspension medium and centrifuged at 100,000 g for 1 h. The interfacial material (in 15% iodixanol) was overlaid with 8%, 4% (w/v) iodixanol and the suspension medium. After repeating the centrifugation, the tonoplast vesicles banded at the top interface [35].
9. References
1. Laganowsky, A., Gómez, S.M., Whitelegge, J.P., Nishio, J.N. (2009) Hydroponics on a chip: Analysis of the Fe deficient Arabidopsis thylakoid membrane proteome J. Proteom., 72, 397-415
2. Onyeocha, I., Behari, R., Hill, D. and Baker, A. (1993) Targeting of castor bean glyoxysomal isocitrate lyase to taco leaf peroxisomes Plant Mol. Biol., 22, 385-396
3. Arai, Y., Hayashi, M. and Nishimura, M. (2008) Proteomic analysis of highly purified peroxisomes from etiolated Soybean cotyledons Plant Cell Physiol., 49, 526-539
4. Behari, R. and Baker, A. (1993) The carboxyl terminus of isocitrate lyase is not essential for import into glyoxysomes in an in vitro system J. Biol. Chem., 268, 7315-7322
5. Horng, J-T., Behari, R., Bruke, L.E.C-A. and Baker, A. (1995) Investigation of the energy requirement and targeting signal for the import of glycolate oxidase into glyoxysomes Eur. J. Biochem., 230, 157-163
6. Pool, M.R., Lopez-Huertas, E., Homg, J-T- and Baker, A. (1998) NADPH is a specific inhibitor of protein import into glyoxysomes Plant J., 15, 1-14
7. Tugal, H.B., Pool, M. and Baker, A. (1999) Arabidopsis 22-kilodalton peroxisomal membrane protein. Nucleotide sequence analysis and biochemical characterization Plant Physiol., 120, 309-320
8. Arai, Y., Hayashi, M. and Nishimura, M. (2008) Proteomic analysis of highly purified peroxisomes from etiolated Soybean cotyledons Plant Cell Physiol., 49, 526-539
9. Komatsu, S. and Ahsan, N. (2009) Soybean proteomics and its application to functional analysis J. Proteom., 72, 325-336
10. Palma, J.M., Corpas, F.J. and del Rio, L.A. (2009) Proteome of plant peroxisomes: new perspectives on the role of these organelles in cell biology Proteomics, 9, 2301-2312
11. Reumann, S. (2011) Toward a definition of the complete proteome of plant peroxisomes: Where experimental proteomics must be complemented by bioinformatics Proteomics 11, 1764–1779
12. Hossain, Z. and Komatsu, S. (2014) Soybean proteomics In Plant Proteomics: Methods Mol. Biol., 1072 (ed. Jorrin-Novo, J.V. et al), Springer Science+Business Media, LLC, pp 315-331
13. Hartman, N.T., Sicilia, F., Lilley, K.S. and Dupree, P. (2007) Proteomic complex detection using sedimentation Anal. Chem., 79, 2078-2083
14. Entwistle, G., Tyson, R.H. and ap Rees, T (1988) Isolation of amyloplasts from wheat endosperm Phytochemistry, 27, 993-996
15. Entwistle, G. and Rees, T.A. (1988) Enzymic capacities of amyloplasts from wheat (Triticum aestivum) endosperm Biochem. J., 255, 391-396
16. Coates, S. and ap Rees, T. (1994) Metabolism of glucose monophosphates by leucoplasts and amyloplasts from soybean suspension cultures Phytochemistry, 35, 881-883
17. Tetlow, I.J., Blissett, K.J. and Emes, M.J. (1993) A rapid method for the isolation of purified amyloplasts from wheat endosperm Planta, 189, 597-600
18. Tetlow, I.J., Bowsher, C.G. and Emes, M.J. (1996) Reconstitution of the hexose phosphate translocation from the envelope membranes of wheat endosperm amyloplasts Biochem. J., 319, 717-723
19. Wischmann, B., Nielsen, T.H. and Møller, B.L. (1999) In vitro biosynthesis of phosphorylated starch in intact potato amyloplasts Plant Physiol., 119, 455-462
20. Beckles, D.M., Smith, A.M. and ap Rees, T. (2001) A cytosolic ADP-glucose pyrophosphorylase is a feature of graminaceous endosperms, but not of other starch-storing organs Plant Physiol., 125, 818-827
21. Davies, E.J., Tetlow, I.J., Bowsher, C.G. and Emes, M.J. (2003) Molecular and biochemical characterization of cytosolic phosphoglucomutase in wheat endosperm (Triticum aestivum L. cv. Axona) J. Exp. Bot., 54, 1351-1360
22. Tetlow, I.J., Davies, E.J., Vardy, K.A., Bowsher, C.G., Burrell, M.M. and Emes, M.J. (2003) Subcellular localization of ADPglucose pyrophosphorylatase in developing wheat endosperm and analysis of the properties of a plastidial isoform J. Exp. Bot., 54, 715-725
23. Balmer, Y., Vensel, W.H., Cai, N., Manieri, W., Schürmann, P., Hurkman, W.J. and Buchanan, B.B. (2006) A complete ferrodoxin/thioredoxin system regulates fundamental processes in amyloplasts Proc. Natl. Acad. Sci, USA, 103, 2988-2993
24. Balmer, Y., Vensel, W.H., DuPont, F.M., Buchanan, B.B. and Hurkman, W.J. (2006) Proteome of amyloplasts isolated from developing wheat endosperm presents evidence of broad metabolic capability J. Exp. Bot., 57, 1591-1602
25. Hurkmann, W.J. and Tanaka, C.K. (2007) Extraction of wheat endosperm proteins for proteome analysis J. Chromatogr. B, 849, 344-350
26. Sweetlove, L.J:, Burrell, M.M. and ap Rees, T. (1996) Characterization of transgenic popato (Solanum tuberosum) tubers with increased ADPglucose pyrophosphorylase Biochem. J., 320, 487-492
27. Naeem, M., Tetlow, I.J. and Emes, M.J. (1997) Starch synthesis in amyloplasts purified from developing potato tubers Plant J., 11, 1095-1103
28. Coates, S.A. and ap Rees, T. (1993) Carbohydrate oxidation by leucoplasts from suspension cultures of soybean (Glycine max L.) Planta, 189, 516-521
29. Coates, S. and ap Rees, T. (1994) Metabolism of glucose monophosphates by leucoplasts and amyloplasts from soybean suspension cultures Phytochemistry, 35, 881-883
30. Renaudin, J.P., Brown, S.C., Barbier-Brygoo, H. and Guern, J. (1986) Quantitative characterization of protoplasts and vacuoles from suspension-cultured cells of Catharanthus roseus Physiol. Plantarium, 68, 695-703
31. Sottomayor, M., de Pinto, M.C., Salema, R., DiCosmo, F., Pedreno, M.A. and Barcelo, A.R. (1996) The vacuolar localization of a basic peroxidase isoenzyme responsible for the synthesis of α-31, 41-anhydrovinblastine in Catharanthus roseus (L.) G. Don leaves Plant Cell Envir., 19, 761-767
32. Orsomando, G., de la Garza, R.D., Green, B.J., Peng, M., Rea, P.A., Ryan, T.J., Gregory, J.F. and Hanson, A.D. (2005) Plant -glutamyl hydrolyses and folate polyglutamates J. Biol. Chem., 280, 28877-28884
33. Schmidt, U.G., Endler, A., Schlebert, S., Brunner, A., Schnell, M., Neuhaus, H.E., Marty-Mazars, D., Marty, F., Baginsky, S. and Martinoia,E.(2007) Novel tonoplast transporters identified using a proteomic approach with vacuoles isolated from cauliflower buds Plant Physiol., 145, 216-229
34. Shen, H., He, Z., Yan, H., Xing, Z., Chen, Y., Xu, W., Xu, W. and Ma, M. (2014) The fronds tonoplast quantitative proteomic analysis in arsenic hyperaccumulator Pteris vittata L. J. Proteom., 105, 46–57
35. Liu, R., Wang, Y., Qin, G. and Tian, S. (2016) iTRAQ-based quantitative proteomic analysis reveals the role of the tonoplast in fruit senescence J. Proteom., 146, 80–89
OptiPrepTM Application Sheet S60; 5th edition, January 2020
OptiPrep™ Application Sheet S61
Membrane trafficking and proteomic analysis of plants and plant cells
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- The purification of plant protoplasts that is often a prelude to subcellular membrane fractionation is covered in Application Sheet C19 (see Mammalian and Non-mammalian Cell Index)
- This Application Sheet describes methods for analysis of membrane trafficking, protein processing and proteomic analysis; it covers, plasma membrane, ER and Golgi in particular.
- Application Sheet S60 covers the purification of the following organelles: chloroplasts, peroxisomes and glyoxysomes, mitochondria, amyloplasts, leucoplasts, tonoplasts and vacuoles. For nuclei see Application Sheets S10 and S10a.
- For information on lipid rafts see Reference List RS09 “Lipid-rich membranes from nonmammalian sources – a bibliography” lists all the published papers. The methodology has been adapted to various plant sources.
- To access RS09, return to the initial list of Folders and select “Reference Lists”.
1. Flotation gradients
1a. Two-layer discontinuous format
Background
There are many situations where it is necessary to provide an efficient separation of either a total membrane fraction and the cytosol or a specific membrane type and the cytosol. In both cases the aim is to be able to allocate one or more proteins to one or other compartment or both compartments. A simple discontinuous gradient flotation strategy, first used with Nycodenz®, has been adapted to the use of OptiPrep™. Such gradients are widely used in studies with cultured animal cell studies. An advantage of iodixanol gradients is that because of the lower osmolality of iodixanol solutions, compared to those of Nycodenz®, the difference in density between cytoplasmic proteins (approx. 1.26 g/ml) and all membrane vesicles and most membrane organelles (<1.18 g/ml) is enhanced. With animal cells a post nuclear supernatant is usually adjusted to approx. 30% (w/v) iodixanol and overlayered with 25% iodixanol (approx 1.14 g/ml); membrane-bound particles float through the lower density layer and the proteins will tend to sediment from the load zone. While this has not been extrapolated exactly to plant cells and tissues, some similar procedures have been adopted.
Methodology
In order to carry out a 2D electrophoresis analysis of Arabidopsis membranes Mahon and Dupree [1] overlaid a crude fraction with a discontinuous gradient of 25% and 9% (w/v) iodixanol and centrifuged was at 90,000 g for 1.5 h; to band the membranes at the upper interface. This flotation strategy would work less well for the densest organelles (nuclei), thus Liu et al [2] layered the crude Arabidopsis fraction (after clarification through a 100 m filter) over 15% and 45% (w/v) iodixanol and centrifuged at 1,500 g for 15 min; the nuclei banded at the interface 15%-45% interface. Routinely the OptiPrep™ is diluted with extraction buffer to provide the gradient solutions; in this case 400 mM sucrose, 10 mM NaCl, 5 mM MgCl₂, 5 mM EDTA, 0.1% Triton X100, 0.1 mM DTT, 10 mM MESNaOH, pH 5.3 (plus protease inhibitors).
1b. Flotation through continuous iodixanol gradients
Analysis of tonoplast, ER, Golgi and protein bodies
BY-2 cells and tobacco (Nicotiana benthamiana) leaf protoplasts have been homogenized in a standard medium (0.25 M sucrose, 1 mM EDTA, 10 mM HEPES-NaOH, pH 7.4 supplemented with 10 mM DTT). Unbroken cells and nuclei were removed at 400 g for 10 min and the supernatant adjusted to 35% (w/v) iodixanol. This was layered beneath a 0-30% (w/v) iodixanol gradient and centrifuged at 114,000 g for 16 h [3] in 13 ml tubes for the Beckman SW41 rotor. The tonoplast banded at the top of the gradient and ER/Golgi in the middle. In this gradient tobacco mosaic virus 130K and 180K banded in the tonoplast and ER/Golgi regions but also in the bottom fractions, which probably contained predominantly soluble proteins. By reducing the volume of the gradient to 2.2 ml the centrifugation time can be reduced to 2h at 106,000 g [4].
Tonoplasts from Pteris vittata [5] and apple tissue [6] may be purified by flotation through discontinuous iodixanol gradients (see Application Sheet S60)
2. Sedimentation gradients
Protein bodies
Endoplasmic reticulum-sourced Protein bodies have been analyzed in a discontinuous gradient of 1.11, 1.17, 1.19, 1.21, 1.23 and 1.25 g/ml; this is equivalent to the following % (w/v) solutions prepared by diluting OptiPrep™ with the homogenization medium (0.25 M sucrose, 10 mM Tris-HCl, pH 8.0): 17%, 29%, 33%, 37%, 41.4% and 45.5%. The gradients (approx. 13 ml), which were centrifuged at 80,000 g, for 2 h, were used to analyze the protein bodies from tobacco (Nicotiana benthamiana) leaves. This method was first reported by Llop-Tous et al [7] in studies on the N-terminal proline-rich domain of the maize storage protein γ-zein (Zera) fused to other proteins. A dense fraction at the 1.19/1.21 g/ml interface contained Zera linked to enhanced cyan fluorescent protein, while other leaf proteins remained in the supernatant and lower density fractions (microsomes). Iodixanol solutions were prepared from OptiPrep™ as described in Application Sheet S01. More recently protein body-targeting of Zera linked xylanase [8] and linked to DsRed [9] were studied using the same gradient system.
3. Self-generated gradients
- For more information on the creation of self-generated gradients of iodixanol see Application Sheet S04.
3a. Organelles and proteomic analysis of Arabidopsis thaliana
The first example of the use of a self-generated iodixanol for the analysis of Arabidopsis membranes was by Dunkley et al [10]. After homogenization of the plant tissue in 0.25 M sucrose, 1 mM EDTA, 1 mM DTT, 10 mM HEPES-NaOH, pH 7.4, a post-nuclear supernatant was obtained by centrifugation at 2200 g for 5 min (carried out twice). A working solution of 50% (w/v) iodixanol containing 1 mM EDTA, 1 mM DTT, 10 mM HEPES-NaOH, pH 7.4 was diluted with the homogenization medium to produce solutions of 16% and 18% (w/v) iodxanol. The 2200 g supernatant was layered over 18% (w/v) iodixanol and the total membranes concentrated at the interface by centrifugation at 100,000 g for 2 h [10,11]. Most of the supernatant was removed and the residual material mixed to provide a crude membrane suspension in 16% iodixanol. This was transferred to a 13ml tube for a Beckman VTi65.1 and a gradient created by self-generation at 350,000 g for 3 h [10].
The gradient successfully resolves the Golgi and smooth ER membranes from the denser mitochondria membranes and this method has been used by and been reported by several groups [12-17]. The method has also been the subject of many review articles [18-23] and described in detail in refs 21-23.
Oil bodies from Arabidopsis thaliana have also been successfully purified on discontinuous iodixanol gradients [27]
3b. Wheat-grain endosperm
The source material was homogenized in 0.25 M sucrose, 1 mM EDTA, 1 mM DTT, 10 mM HEPES-NaOH, pH 7.4 and the suspension centrifuged twice at 2200 g for 5 min to remove rapidly sedimenting material and debris. A microsome fraction was obtained by sedimentation on to an 18% (w/v) iodixanol barrier (OptiPrep™ diluted with the homogenization medium) at 100,000 g for 2 h in a swinging-bucket rotor. Golgi fractions were then isolated in a self generated iodixanol gradient (starting concentration 14% (w/v)). It was formed by centrifugation ay 200,000 g for 12 h in a Beckman 70Ti rotor. Although self-generated gradients are normally formed at higher g-forces for much shorter times, it is known that some fixed angle rotors, particularly those with low angles such as the 70Ti (23°) can be a suitable substitute. Golgi and ER markers banded distinctively under these conditions [28].
- Iodixanol gradient analysis of plant tissue has played an important part in identifying the subcellular localization of many plant proteins [29].
4. References
1. Mahon, P. and Dupree, P. (2001) Quantitative and reproducible two-dimensional gel analysis using Phoretix 2D Full Electrophoresis, 22, 2075-2085
2. Liu, Z., Zhu, Y., Gao, J., Yu, F., Dong, A. and Shen, W-H. (2009) Molecular and reverse genetic characterization of nucleosome assembly protein1 (NAP1) genes unravels their function in transcription and nucleotide excision repair in Arabidopsis thaliana Plant J., 59, 27–38
3. Hagiwara, Y., Komoda, K., Yamanaka, T., Tamai, A., Meshi, T., Funada, R., Tsuchiya, T., Naito, S and Ishikawa, M. (2003) Subcellular localization of host and viral proteins associated with tobamovirus RNA replication EMBO J., 22, 344-353
4. Hagiwara-Komoda, Y., Hirai, K., Mochizuki, A., Nishiguchi, M., Meshi, T. and Ishikawa, M. (2008) Overexpression of a host factor TOM1 inhibits tomato mosaic virus propagation and suppression of RNA silencing Virology, 376, 132-139
5. Shen, H., He, Z., Yan, H., Xing, Z., Chen, Y., Xu, W., Xu, W. and Ma, M. (2014) The fronds tonoplast quantitative proteomic analysis in arsenic hyperaccumulator Pteris vittata L. J. Proteom., 105, 46–57
6. Liu, R., Wang, Y., Qin, G. and Tian, S. (2016) iTRAQ-based quantitative proteomic analysis reveals the role of the tonoplast in fruit senescence J. Proteom., 146, 80–89
7. Llop-Tous, I., Madurga, S., Giralt, E., Marzabal, P., Torrent, M. and Ludevid, M.D. (2010) Relevant elements of a maize -zein domain involved in protein body biogenesis J. Biol. Chem., 285, 35633–35644
8. Llop-Tous, I., Ortiz, M., Torrent, M. and Ludevid, M.D. (2011) The expression of a xylanase targeted to ERprotein bodies provides a simple strategy to produce active insoluble enzyme polymers in tobacco plants PLoS One 6: e19474
9. Joseph, M., Ludevid, D., Torrent, M., Rofidal, V., Tauzin, M., Rossignol, M. and Peltier, J-B. (2012) Proteomic characterisation of endoplasmic reticulum-derived protein bodies in tobacco leaves BMC Plant Biol., 12: 36
10. Dunkley, T.P.J., Watson, R., Griffin, J.L., Dupree, P. and Liley, K.S. (2004) Localization of organelle proteins by isotope tagging (LOPIT) Mol. Cell. Proteom., 3, 1128-1134
11. Zhang, Y., Nikolovski, N., Sorieul, M., Vellosillo, T., McFarlane, H.E., Dupree, R., Kesten, C., Schneider, R. et al (2016) Golgi-localized STELLO proteins regulate the assembly and trafficking of cellulose synthase complexes in Arabidopsis Nat. Comm., 7: 11656
12. Berg, M., Parbel, A., Pettersen, H., Fenyo, D. and Björkesten, L. (2006) Reproducibility of LC-MS-based protein identification J. Exp. Botany, 57, 1509-1514
13. Dunkley, T.P.J., Hester, S., Shadforth, I.P., Runions, J., Weimer, T., Hanton, S.L., Griffin, J.L., Besssant, C., Brandizzi, F., Hawes, C., Watson, R.B., Duprese, P. and Lilley, K.S. (2006) Mapping the Arabidopsis organelle proteome Proc. Natl. Acad. Sci. USA, 103, 6518-6523
14. Sadowski, P.G., Dunkley, T.P.J., Shadforth, I.P., Dupree, P., Bessant, J.L. and Lilley, K.S. (2006) Quantitative proteomic approach to study subcellular localization of membrane proteins Nat. Protoc., 1, 1778-1789
15. Au, C.E., Bell, A.W., Gilchrist, A., Hiding, J., Nilsson, T. and Bergeron, J.J.M. (2007) Organellar proteomics to create the cell map Curr. Opin. Cell Biol., 19, 376-385
16. Alexandersson, E., Gustavsson, N., Bernfur, K., Kjellbom, P. and Larsson, C. (2007) Plasma membrane proteomics In Plant Proteomics (ed. Samaj, J. and Thelen, J.) Springer Science + Business Media, Berlin, pp 186-206
17. Lilley, K.S. and Dupree, P. (2007) Plant organelle proteomics Curr. Opin. Plant Biol., 10, 594-599
18. Trotter, M.W.B., 1, Sadowski, P.G., Dunkley, T.P.J., Groen, A.J. and Lilley, K.S. (2010) Improved subcellular resolution via simultaneous analysis of organelle proteomics data across varied experimental conditions Proteomics, 10, 4213–4219
19. Kota, U. and Goshe, M.B. (2011) Advances in qualitative and quantitative plant membrane proteomics Phytochemistry, 72, 1040–1060
20. Groen, A.J., de Vries, S.C. and Lilley, K.S. (2008) A proteomics approach to membrane trafficking Plant Physiol., 147, 1584-1589
21. Oeljeklaus, S., Meyer, H.E. and Warscheid, B. (2009) Advancements in plant proteomics using quantitative mass spectrometry J. Proteom., 72, 545-554
22. Agrawal, G.K., Bourguignon, J., Rolland, N., Ephritikhine, G., Ferro, M., Jaquinod, M., Alexiou, K.G., Chardot, T., Chakraborty, N., Jolivet, P., Doonan, J.H. and Rakwal1, R. (2011) Plant organelle proteomics: collaborating for optimal cell function Mass Spectrom. Rev., 30, 772– 853
23. Vertommena, A., Panisa, B., Swennena, R. and Carpentiera, S.C. (2011) Challenges and solutions for the identification of membrane proteins in non-model plants J. Proteom., 74, 1165-1181
24. Lilley, K.S. and Dunkley, T.P.J. (2008) Determination of genuine residents of plant endomembrane organelles using isotope tagging and multivariate statistics In Methods Mol. Biol., 432, Organelle Proteomics (ed. Pflieger, D, and Rossier, J.) Humana Press, Totowa, NJ, pp 373-387
25. Gatto, L., Breckels, L.M., Burger, T., Nightingale, D.J.H., Groen, A.J., Campbell, C., Nikolovski, N., Mulvey, C.M., Christoforou, A., Ferro, M. and Lilley, K.S. (2014) A foundation for reliable spatial proteomics data analysis Mol. Cell. Proteom., 13, 1937-1952
26. Nikolovski, N, Shliaha, P.V., Gatto, L., Dupree, P. and Lilley, K.S. (2014) Label-free protein quantification for plant Golgi protein localization and abundance Plant Physiol., 166, 1033–1043
27. Deruyffelaere, C., Bouchez, I., Morin, H., Guillot, A., Miquel, M., Froissard, M., Chardot, T., and D’Andrea, S. (2015) Ubiquitin-mediated proteasomal degradation of oleosins is involved in oil body mobilization during post-germinative seedling growth in Arabidopsis Plant Cell Physiol., 56, 1374–1387
28. M. Suliman, 1, A.-L. Chateigner-Boutin, 1, M. Francin-Allami, A. Partier, B. Bouchet, J. Salse, C. Pont, J. Marion, H. Rogniaux, D. Tessier, F. Guillon, C. Larré (2013) Identification of glycosyltransferases involved in cell wall synthesis of wheat endosperm J. Proteom., 78, 508–521
29. Breckels, L.M., Gatto, L., Christoforou, A., Groen, A.J., Lilley, K.S. and Trotter, M.W.B. (2013) The effect of organelle discovery upon sub-cellular protein localization J. Proteom., 88, 129-140
OptiPrepTM Application Sheet S61; 5th edition, January 2020
OptiPrep™ Application Sheet S62
Extracellular vesicles from non-mammalian sources
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- OptiPrep™ Reference List (RS11) “Extracellular vesicles from non-mammalian sources” provides a full reference list: return to the initial list of Folders and select “Reference Lists”.
- See a companion Application Sheet (S63) on mammalian cell exosomes and other microvesicles from conditioned medium and a corresponding OptiPrep Reference List (RS10).
- Samples often require pre-gradient treatment for the removal of larger particles and reduction in sample volume; these are considered in Section 2.
1. Introduction
It is widely recognized that mammalian cells, bacteria and fungi release extracellular vesicles into the surrounding medium; these vesicles are involved in communication between cells and the delivery of biologically and clinically important molecules to other cells. With regard to bacteria and fungi, the term “extracellular vesicles” (EVs) covers the outer membrane vesicles (OMVs) produced by Gram-negative bacteria and the membrane vesicles (MVs) produced by Gram-positive bacteria and other organisms such as fungi. In all cases; EVs are distinct from the intracellular vesicles present in the cytoplasm. The OMVs from Gram-negative bacteria in particular are widely researched and have been shown to be important in the transfer of virulence factors and the initiation of immune and inflammatory responses in host cells.
Gradients prepared from OptiPrep™ represent a simple and effective means for separating the EVs and MVs from any soluble proteins and the strategies are not unlike those used for the separation of mammalian cell membrane vesicles from cytosolic proteins. They are described in Application Sheets S35 and S36. The principle is to adjust the sample to a high density by addition of OptiPrep™ or a dense- buffered solution of 40-50% (w/v) iodixanol, prepared from OptiPrep™. This is then laid under either a continuous or discontinuous iodixanol gradient. During the centrifugation the proteins, which have a density of approx.1.26 g/ml, either remain in the load zone or sediment very slowly, while the vesicles float up to their banding position. It provides the ideal separating strategy and since the gradients are more less isoosmotic the vesicles remain fully hydrated and their density is not significantly altered by the gradient. This is unlike sucrose gradients, whose high osmolality causes any vesicles to lose water and hence raises their density.
The gradient methodology is described in Section 3. It is common to preface the iodixanol gradient purification with one or more treatments that remove larger contaminants and/or concentrate the vesicles from the often rather large volumes of starting material.
2. Pre-gradient methodology
Various forms of pre-gradient processing (including filtration, ultrafiltration, centrifugation and ultracentrifugation techniques) are employed, during which intact bacteria, aggregated material and soluble components of the culture medium are mostly removed and the EVs concentrated.
2a. Removal of cells by medium-speed centrifugation
All methods incorporate an initial clarification of the bacterial broth to remove intact cells; 10,000 g is the most commonly used speed, usually for 10-20 min, although there are examples both of higher g-forces, 13,000 g for 15 min [1] and 12,000 g for 30 min [2] and of lower g-forces, 8,000 g for 30 min [3], 6,000 g for 10-15 min [4-7], 5,000 g for 10-15 min [8,9] and 4,000 g for 12 min [10,11]. Francisella novicida bacteria [12] were removed in two steps of 5,000 g and 7,500 g (both for 30 min); this may improve yields of EVs by reducing their entrapment by large numbers of rapidly-sedimenting intact bacteria. The choice of centrifugation conditions does not appear to be bacteria type-specific. Fungal cells (e.g. Cryptococcus neoformans) however appear to sediment satisfactorily at lower speeds – 2,500 g for 10 min [13].
2b. Concentration by volume reduction
Large volumes of clarified medium can be awkward to deal with in the final ultracentrifugation step used to pellet the EVs prior to density gradient analysis. For the purification of OMVs from Escherichia coli Horstman and Keuhn [14] reduced the volume to 1/25th of the original by using a 70 kDa cut-off tangential filtration device (Pall-Gelman) after the first medium-speed centrifugation. The same device with a 100 kDa cut-off has also been used with Pseudomonas aeruginosa [15-17], Francisella novicida [12] and Haemophilus influenzae [18]. S. aureus and B. subtilis MVs have been concentrated in the QuixStand Benchtop System with a 100 kDa hollow-fibre membrane (Amersham Biosciences) [19]. Cryptococcus neoformans MVs were concentrated in an Amicon-Ultra centrifugal filter (also with a 100 kDa cut-off) [13]. In most cases the volume reduction is applied to the supernatant from the first medium-speed centrifugation step (see Section 2a) prior to any filtration steps to remove residual bacteria (see Section 2c). Sometimes ultrafiltration is performed after an initial passage through a 0.45 μm filter [19]. Occasionally a subsequent repeat of the initial mediumspeed centrifugation is carried out [17].
2c. Removal of residual bacteria by filtration
Following the first centrifugation step, residual bacteria and other larger contaminants are usually removed by vacuum filtration through either a 0.45 (most common) or a 0.22 μm filter; sometimes the filtration through a 0.22 μm filter is immediately repeated [10,11]; sometimes the 0.45 and 0.22 μm filters are used sequentially [5,6]. It is also quite common to repeat a filtration after the membrane vesicles have been pelleted (see Section 2d) and resuspended in a suitable medium prior to density gradient loading [14,15,20-23] or after an ultrafiltration concentration step [19].
2d.Sedimentation of membrane vesicles
Prior to gradient fractionation MVs are routinely pelleted by ultracentrifugation (in a fixed-angle rotor); the centrifugation conditions are surprisingly variable. Most papers report the use of 34-40,000 g for 1 h [14-16, 18,20-26] but there are examples of higher g-forces: 85,000 g for 1-2 h [2,3], 100,000 g for 1 h [9-13] and 140-150,000 g for 2-3 h [4-6,10,19].
2e. Precipitation with ammonium sulphate
In a few cases the MVs were harvested from the clarified fluid by precipitation with ammonium sulphate. This was first reported by Ferndandez-Moreira et al [27] for Legionella pneumophila OMVs. After removing the bacteria by medium-speed centrifugation and filtration of the culture fluid through a 0.45 μm filter, the OMVs were precipitated in 70% (NH4)2SO4 at 4°C for 30 min and centrifugation at 10,000 g for 15 min. The pellet was resuspended in PBS and dialyzed against this medium overnight. Concentration was carried out by centrifugal ultrafiltration (>100 kDa cut-off) – see Section 2b. Bauman and Kuehn [15] subsequently increased the (NH4)2SO4 concentration (71 or 75%) and the precipitation time to >3h; the resuspended pellet was dialyzed against HEPES buffer and concentrated by >50kDa cut-off ultrafiltration. The (NH4)2SO4 precipitation time was increased to overnight by Ellis et al [17] and Nieves et al [7].
3. Gradient methodology
3a. Solution preparation
A. OptiPrep™
B. 0.85% (w/v) NaCl, 60 mM HEPES (or Tricine) -NaOH, pH 7.4
C. 0.85% (w/v) NaCl, 10 mM HEPES (or Tricine) -NaOH, pH 7.4
Prepare a 50% (w/v) iodixanol stock solution (approx. ρ = 1.268 g/ml) by mixing 5 vol. of OptiPrep™ with 1 vol. of Solution B and then to make lower density solutions dilute this stock with Solution C. This ensures that the buffer concentration and osmolality is more or less constant throughout the gradient. If this is deemed unnecessary then simply dilute the OptiPrep™ with Solution C. For more information on the preparation of gradient solutions using NaCl as an osmotic balancer see Application Sheet C01 (Cell index); for use of sucrose as an osmotic balancer see Application Sheet S01.
Horstman and Kuehn [14] who published the first paper on the use of this strategy for OMVs from Escherichia coli, suspended the OMVs in 50 mM HEPES-NaOH pH 6.8 buffer (occasionally 20 mM HEPES is used, see ref 12). This simple buffer is still widely used at pHs from 6.8 to 7.5. It is however hypoosmotic. In a later paper Kesty and Kuehn [24] included NaCl in the same buffer at a lower concentration (0.85% w/v NaCl, 10 mM HEPES-NaOH, pH 6.8). Sometimes a routine phosphate-buffered saline has been used [27,28].
- Returning the vesicle suspension to an isoosmotic NaCl-containing medium before adjusting the suspension to a high density is probably the most convenient method for preparing the sample for the density gradient.
3b. Gradient preparation (general comments)
The most widely-used iodixanol concentration for the sample is 45% (w/v). This was first used by Horstman and Kuehn [14] and has been widely adopted. Occasionally higher 50% (w/v) [5,19,24] or lower 40% (w/v) [10,12] or 35% (w/v) [13] concentrations have been used.
Without exception all of the gradients are discontinuous, the layers being deposited on the dense sample. However, those gradients that are centrifuged overnight (16-18 h) will become more or less continuous (but not necessarily linear) owing to diffusion of the solute from high to low concentration solutions. If the gradient solutions are prepared by diluting OptiPrep™ with the same buffered 0.85% (w/v) NaCl solution used for the EV suspension, then the entire gradient will be approximately isoosmotic.
- Although layering of discontinuous gradients is most often executed by overlayering (i.e. starting with the most dense layer first), underlayering starting with the least dense solution first is often more easily carried out, using a syringe and wide-bore (approx. 0.8 mm internal diameter) metal filling cannula. For more information see OptiPrep™ Application Sheet S03.
3c. Gradient format
Horstman and Kuehn [14] developed a multiple-layered gradient in which the sample (in 45% w/v iodixanol) was overlaid by 35%, 30%, 25%, 20%, 15% and 10% (w/v) iodixanol, centrifuged at 180,000 g for 3 h. It has been adapted to a wide range of tube sizes, but the volume ratio of the different layers remained more or less constant (0.4:3:3:2:2:1:1) [4,8,9]; the volume of the two least dense layers is sometimes increased [2,3]. The same solutions have been used by other workers, but at significantly different volumes Davis et al [28] used 1 ml for all layers and included 3 ml of buffer as the topmost layer. Tashiro et al [5] used 0.5 ml of each (and added a layer of 40% w/v iodixanol). Later the same group [6] used 1.0 ml of each, except for the sample and 10% (w/v) iodixanol layers (0.5 ml).
Since the density of MVs in iodixanol gradients is generally >1.11 g/ml (many banding between 1.13 and 1.15 g/ml); it might be considered unnecessary to include layers of 15% and 10% (w/v) iodixanol which have densities of 1.085 and 1.058 g/ml respectively. Thus Kesty and Kuehn [24] introduced a discontinuous gradient of 50%, 45%, 40%, 35%, 30% and 25% (w/v) iodixanol (2 ml of each). Similar gradients (excluding the 50% layer) have been used by other groups [27], sometimes including a 20% (w/v) iodixanol layer [9,17,18,26], which may replace the 25% layer [25].
The density range of some gradients has been shifted by deleting the top 25% (w/v) iodixanol layer; in these cases the MVs band very sharply at, or towards, the top of the gradient [20,21]; the type of separation is similar to that for purifying any membrane vesicles away from denser particles and soluble proteins (see OptiPrep Application Sheet S35).
Some publications report the use of much simplified systems. MVs from the Gram +ve organisms Staphylococcus aureus and Bacillus subtilis [19] have been purified in a discontinuous gradient of 50%, 40% and 10% (w/v) iodixanol. This relatively simple gradient separated soluble proteins, protein aggregates and denatured MVs from the MVs, which had a density of 1.16-1.20 g/ml.
3d Centrifugation conditions
Although the centrifugation conditions reported in the earliest publications, which continue to be widely used for OMVs and MVs are 180,000 g for 3 h, they have become quite diverse; for example 100,000 g for 3 h [5,6,27], 111,000 g for 2 h [7]; 200,000 g for 2 h [19] and 100,000 g for 16-20 h [12, 16-18,20,22,23,25,26]. Long centrifugation times at reduced g-forces are often regarded as producing superior resolution of mammalian intracellular membranes (e.g. plasma membrane, Golgi and endoplasmic reticulum); it is not known if this has been studied rigorously for MVs.
The gradients used for the isolation of Pseudomonas aeruginosa OMVs have been tailored to the use of different sources [15,16] Isolation from culture and from soil: 40%, 35%, 30%, 25% and 20% (w/v) iodixanol; for cystic fibrosis isolates the 25% solution was omitted; furthermore the volumes of each layer were also optimised to the separation from soluble proteins and flagella (see refs 15 and 16 for more information.) OMVs banded very close to the top of the gradient after centrifugation at 100,000 g for 16 h.
- Tashiro et al [5] noted that the OMVs from Pseudomonas aeruginosa showed a biphasic distribution, with a major peak at 1.15 g/ml density and a minor one a 1.20 g/ml. Moreover in a timed study these workers observed a steady shift in the density of the OMVs between 4.5 h (approx. 1.12 g/ml) and 12 h (approx. 1.20 g/ml) in culture. They proposed that there was significant heterogeneity amongst the OMV population.
- Although the majority of the published papers are concerned with the analysis of OMVs from Escherichia coli and Pseudomonas aeruginosa, other sources have been investigated using similar gradients. Those from Borrelia burgdorferi also band at approx. 1.12 g/ml [10,11]. OMVs from Aggregatibacter actinomycetemcomitans [3], Burkholderia pseudomallei [7], Haemophilus influenzae [18], Legionella pneumophila [27], Marinobacter guineae [26], Pseudoalteromonas [26], Psychrobacter fozii [26], Salmonella enterica [1,22], Shewanella livingstonensis [26], Shewanella vesiculosa [26].
- It is also worth noting that there may be considerable heterogeneity in the physical characteristics of exosomes from different bacteria; this was emphasized by Singorenko et al [29] in their studies of Mycobacterium smegmatis and Escherichia coli and the nature of the analytical methodology.
3e Gradient harvesting
The banded vesicles may be harvested simply by aspiration using a syringe attached to a flattipped metal filling cannula or the entire gradient maybe unloaded either low-density or high-density end first. For more information on harvesting gradients see Application Sheet S08
4. Fungi
MVs from cultures of the fungus Cryptococcus neoformans [13] were suspended in 35% (w/v) iodixanol (3 ml) and overlaid by 3 ml of 30% and 2 ml each of 25, 20,15 and 10% iodixanol. After centrifugation at 140,000 g for only 15 min the MVs banded broadly approx. in the middle of the gradient.
5. References
1. Kitagawa, R., Takaya, A., Ohya, M., Mizunoe, Y., Takade, A., Yoshida, S-i., Isogai, E. and Yamamoto, T. (2010) Biogenesis of Salmonella enterica serovar Typhimurium membrane vesicles provoked by induction of PagC J. Bacteriol., 192, 5645–5656
2. Thay, B., Wai, S.N. and Oscarsson, J. (2013) Staphylococcus aureus a-toxin-dependent induction of host cell death by membrane-derived vesicles PloS One, 8: e54661
3. Rompikuntal, P.K., Thay, B., Khan, M.K., Alanko, J., Penttinen, A-M., Asikainen, S., Wai, S.N. and Oscarsson, J. (2012) Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans Infect. Immun., 80, 31-42
4. Balsalobre, C., Silvan, J.M., Berglund, S., Mizunoe, Y., Uhlin, B.E. and Wai, S.N. (2006) Release of the type I secreted -haemolysin via outer membrane vesicles from Escherichia coli Mol. Microbiol., 59, 99-112
5. Tashiro, Y., Sakai, R., Toyofuku, M., Sawada, I., Nakajima-Kambe, T., Uchiyama, H. and Nomura, N. (2009) Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa J. Bacteriol., 191, 7509-7519
6. Tashiro, Y., Ichikawa, S., Shimizu, M., Toyofuku, M., Takaya, N., Nakajima-Kambe, T., Uchiyama, H. and Nomura, N. (2010) Variation of physiochemical properties and cell association activity of membrane vesicles with growth phase in Pseudomonas aeruginosa Appl. Environ. Microbiol., 76, 3732-3239
7. Nieves, W., Heang, J., Asakrah, S., Höner zu Bentrup, K., Roy, C.J. and Morici, L.A. (2010) Immunospecific responses to bacterial elongation factor Tu during Burkholderia infection and immunization PloS One 5: e14361
8. Kim, J-Y., Doody, A.M., Chen, D.J., Cremona, G.H., Shuler, M.L., Putnam, D. and DeLisa, M.P. (2008) Engineered bacterial outer membrane vesicles with enhances functionality J. Mol. Biol., 380, 51-66
9. Roy, K., Hamilton, D.J., Munson, G.P. and Fleckenstein, J.M. (2011) Outer membrane vesicles induce Immune responses to virulence proteins and protect against colonization by enterotoxigenic Escherichia coli Clin. Vaccine Immunol., 18, 1803–1808
10. Toledo, A., Coleman, J.L., Kuhlow, C.J., Crowley, J.T. and Benach, J.L. (2012) The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles Infect. Immun., 80, 359-368
11. Crowley, J.T., Toledo, A.M., LaRocca, T.J., Coleman, J.L., London, E. and Benach, J.L. (2013) Lipid exchange between Borrelia burgdorferi and host cells PLoS Pathog., 9: e1003109
12. McCaig, W.D., Koller, A. and Thanassi, D.G. (2013) Production of outer membrane vesicles and outer membrane tubes by Francisella novicida J. Bacteriol., 195, 1120-1132
13. Wolf, J.M., Rivera, J. and Casadevall, A. (2012) Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles Cellular Microbiology (2012) 14(5), 762–773
14. Horstman, A.L. and Kuehn, M.J. (2000) Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles J. Biol. Chem., 275, 12489-12496
15. Bauman, S.J. and Kuehn, M.J. (2006) Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response Microbes Infect., 8, 2400-2408
16. Bauman, S.J. and Kuehn, M.J. (2009) Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells BMC Microbiol., 9:26
17. Ellis, T.N., Leiman, S.A. and Kuehn, M.J. (2010) Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components Infect. Immun., 78, 3822-3831
18. Sharpe, S.W., Kuehn, M.J. and Mason, K.M. (2011) Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of non-typeable haemophilus influenzae Infect. Immun., 79, 4361-4369
19. Lee, E-Y., Choi, D-Y., Kim, D-K., Kim, J-W., Park, J O., Kim, S., Kim, S-H., Desiderio, D.M., Kim, Y-K., Kim, K-P- and Gho, Y.S. (2009) Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles Proteomics, 9, 5425-5436
20. McBroom, A.J., Johnson, A.P., Vemulapalli, S. and Kuehn, M.J. (2006) Outer membrane vesicle production by Escherichia coli is independent of membrane instability J. Bacteriol., 188, 5385-5392
21. McBroom, A.J. and Kuehn, M.J. (2007) Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response Mol. Microbiol., 63, 545-558
22. Muralinath, M., Kuehn, M.J., Roland, K.L. and Curtiss III, R. (2011) Immunization with Salmonella enterica serovar Typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae Infect. Immun., 79, 887–894
23. Pérez-Cruz, C., Carrión, O., Delgado, L., Martinez, G., López-Iglesias, C. and Mercade, E. (2013) New type of outer membrane vesicle produced by the Gram-negative bacterium Shewanella vesiculosa M7T: implications for DNA Appl. Environ. Microbiol., 79, 1874-1881
24. Kesty, N.C. and Kuehn, M.J. (2004) Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles J. Biol. Chem., 279, 2069-2076
25. MacEachran, D.P., Ye, S., Bomberger, J.M., Hogan, D.A., Swiatecka-Urban, A., Stanton, B.A. and O’Toole, G.A. (2007) The Pseudomonas aeruginosa secreted protein PA2934 decreases apical membrane expression of the cystic fibrosis transmembrane conductance regulator Infect. Immun., 75, 3902-3912
26. Frias, A., Manresa, A., de Oliveira, E., López-Iglesias, C. and Mercade, E. (2010) Membrane vesicles: a common feature in the extracellular matter of cold-adapted Antarctic bacteria Microb. Ecol., 59, 476–486
27. Fernandez-Moreira, E., Helbig, J.H. and Swanson, M.S. (2006) Membrane vesicles shed by Legionella pneumophila inhibit fusion of phagosomes with lysosomes Infect. Immun., 74, 3285-3295
28. Davis, J.M., Carvalho, H.M., Rasmussen, S.B. and O’Brien, A.D. (2006) Cytotoxic necrotizing factor type 1 delivered by outer membrane vesicles of uropathogenic Escherichia coli attenuates polymorphonuclear leukocyte antimicrobial activity and chemotaxis Infect. Immun., 74, 4401-4408
29. Dauros Singorenko, P., Chang, V., Whitcombe, A., Simonov, D., Hong, J., Phillips, A., Swift, S. and Blenkiron, C. (2017) Isolation of membrane vesicles from prokaryotes: a technical and biological comparison reveals heterogeneity J. Extracell. Ves., 6: 1324731
OptiPrepTM Application Sheet S62; 5th edition, January 2020
OptiPrep™ Application Sheet S63
Mammalian cell exosomes and other microvesicles from conditioned medium
1. Introduction
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
- OptiPrep™ Reference List (RS10) “Mammalian (including all chordata) cell exosomes and other microvesicles from tissues, cells and conditioned medium” provides a full reference list: to access return to the initial list of Folders and select “Reference List”.
- There is a companion to Application Sheet (S62) on purification of bacterial and fungal extracellular vesicles from culture medium and a corresponding OptiPrep Reference List (RS11).
- The culture medium is often purified before contact with the cells under study (see Section 2).
- After the period of culture, the medium usually undergoes pre-gradient treatment for the removal of larger particles and reduction in sample volume; these are considered in Sections 3a-3d.
2. Preparation of the culture medium
To minimize contamination of the exosomes expressed into the culture medium, the latter is often, but not always, centrifuged at approx 100,000 g for 1-16 h and/or filtered (usually a 0.2-0.22 μm filter) to remove any particulate material, before it is put in contact with the cell monolayer. Sometimes it is just the foetal bovine serum, used as a supplement to the culture medium, that is clarified using the same ultracentrifugation and/or filtration. Occasionally serum-free medium is used.
Once the culture medium has been in contact with the cell monolayer for the requisite length of time, the conditioned medium (CM) is harvested and then variously clarified, partially purified and/or concentrated prior to iodixanol gradient purification.
3 Pre-iodixanol gradient treatment
3a. Differential centrifugation (low and high-speed)
Sometimes a single low-speed centrifugation is used to remove cells and other large particles such as apoptotic bodies, e.g. 1000-2000 g for 10 min [1]; more often a more extensive differential centrifugation scheme is used prior to the final recovery of exosomes (and other microparticles) by ultracentrifugation. Although it might be tempting to delete the first low-speed centrifugation, failure to include this step will probably lead to the entrapment and loss of small vesicles into aggregates of rapidly-sedimenting larger particles at a higher g-force. Some examples are given in Table 1.
- Microvesicles have also been isolated from human plasma [12] and have been identified as agents in the transport of miRNAs between cells. The plasma was treated to a similar sequence of differential centrifugation steps of 300 g, 1200 g, and 10,000 g
- See Section 3c for details of the final centrifugation steps to concentrate the exosomes prior to iodixanol gradient purification.
3b. Filtration
Removal of larger contaminants is commonly performed using a 0.20 or 0.22 μm syringe filter, occasionally with a 0.1μm [2,6] or 0.45μm [13] filter. The filtration may be carried out before [3], or more frequently after the first low-speed centrifugation [1,2,11,14]; or occasionally after a second higher speed centrifugation [6]. Occasionally filtration is the only pre-gradient treatment [13,15,16,17].
3c. Concentration of exosomes and other vesicles by pelleting
Virtually all of the published methods involve a final pelleting of the exosomes and other microvesicles at 100-150,000 g for 1-2 h before resuspending in a suitable buffered medium for application to the iodixanol gradient. The exceptions to the use of these g-forces are the methods that use 20,000 g for muscle cell microparticles [8] and vexosomes [9] – see Table 1.
- Duelli et al [14] described a method in which the CM was initially centrifuged at 500 g and filtered, before being centrifuged sequentially at 12,000 g, 70,000 g and 110,000 g. In the differential centrifugation fractions, exosomal fusogenic activity was heavily concentrated in the 70,000 g pellet, with rather little in the 110,000 g pellet and it was the former that was further purified in the iodixanol density gradient. The lower g-force of 70,000 g was also reported in [11].
Some protocols, particularly those that are applied to large volumes of CM, include a preliminary concentration using centrifugal ultrafiltration, to reduce the total volume prior to this final pelleting at 100-150,000 g. This conveniently avoids the use of large volume ultracentrifuge rotors. These ultrafilters have cut-offs from 5 kDa [2,6] to 100 kDa [4,17].
3d. Sucrose gradients
As part of the pre-iodixanol gradient purification and concentration procedure a discontinuous sucrose gradient is sometimes included. The combination of multiple two-layer sucrose density gradients and iodixanol gradient separations was investigated by Choi et al in their studies on exosomes from colon carcinoma cells [18]. The exosomes were concentrated at the interface of 0.8 and 2.7 M sucrose layers after centrifugation at 100,000 g for 4 h. Microvesicles from mesenchymal stem cells were interface-concentrated in the same two-layer gradient at 100,000 g for 2 h [4].
4. Iodixanol gradient methodology
4a. Gradient solution preparation
A. OptiPrep™
B. 0.85% (w/v) NaCl, 60 mM HEPES (or Tricine) -NaOH, pH 7.4
C. 0.85% (w/v) NaCl, 10 mM HEPES (or Tricine) -NaOH, pH 7.4
When OptiPrep™ is diluted with an isoosmotic solution such as a buffered saline the gradients produced from such a solution will be positive with respect to iodixanol and negative with respect to all the diluent components. If it is considered advantageous to maintain the buffer composition constant then make up the solutions described in Box 1 and produce solutions B and C. Dilute 5 vol. of OptiPrep™ with 1 vol. of Solution B to produce a working stock of 50% (w/v) iodixanol containing 10 mM buffer (ρ = 1.268 g/m l). When this is subsequently diluted with Solution C all solutions will contain 10 mM buffer.
The same strategy can be applied to other isoosmotic media such as a typical homogenisation medium such as 0.25 M sucrose, 1 mM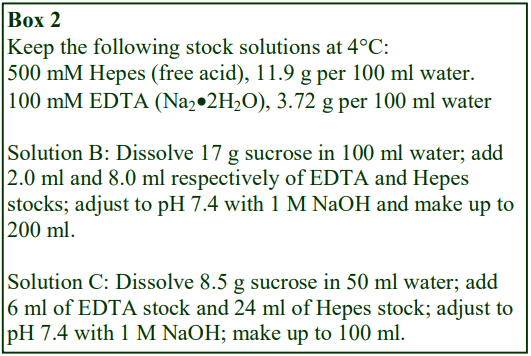 EDTA, 20 mM HEPES-NaOH, pH 7.4. Solutions B and C are prepared as in Box 2 and handled in the same manner as described for the NaCl based media; the concentrations of EDTA and buffer will be constant in all solutions. An additional sophistication was described in ref 13. A 6% (w/v) iodixanol solution was made up in 215 mM sucrose and a 65.4% (w/v) iodixanol solution in 5 mM sucrose, both solutions containing 2 mM EDTA, 10 mM Tris-HCl (pH 8.0).
EDTA, 20 mM HEPES-NaOH, pH 7.4. Solutions B and C are prepared as in Box 2 and handled in the same manner as described for the NaCl based media; the concentrations of EDTA and buffer will be constant in all solutions. An additional sophistication was described in ref 13. A 6% (w/v) iodixanol solution was made up in 215 mM sucrose and a 65.4% (w/v) iodixanol solution in 5 mM sucrose, both solutions containing 2 mM EDTA, 10 mM Tris-HCl (pH 8.0).
The crude vesicle fraction and the diluent for the OptiPrep™ are quite frequently a cell buffer such as phosphate-buffered saline [e.g. refs 1,3,11,14 and15], occasionally supplemented with 2.5 mM MgCl₂ [9]. Rather than a phosphate buffer the saline may contain organic buffer such as 20 mM HEPES-NaOH, pH 7.2 [4,5,18]. Dilutions with 0.25 M sucrose, 10 mM Tris-HCl pH 7.5 are also common [2,6,8]. In some instances the exosomes are suspended in, and the gradient solutions prepared in, a simple buffer such as 20 mM HEPES-NaOH, pH 7.4 [10,17]. A 20 mM HEPESbuffered solution of 100 mM KCl and 2 mM MgCl₂ was used in ref 12 and in the case of the selfgenerated gradient method [16], the layers were simply produced by adjusting the density of the crude exosome suspension with OptiPrep™.
4b. Purification and analysis in a top-loaded sedimentation velocity (short spin) gradient
Dettenhoffer and Yu [19] developed a sedimentation velocity iodixanol gradient to purify HIV-1 virions without affecting the infectivity of the virus. The authors noted that in buoyant density sucrose gradients the extracellular Vif gene always co-purifies with the virus and the latter is also contaminated with cell-derived microvesicles. In rate-zonal iodixanol gradients on the other hand the HIV-1 was effectively separated both from Vif and from the microvesicles. Another important point about sucrose gradients is that although sucrose is generally less deleterious to viral infectivity than CsCl, it can nevertheless have serious effects on viral structure; in particular the loss of surface glycoproteins from retroviruses has been noted [20]. This may be related to its viscosity, which is much higher than that of iodixanol. The loss of surface glycoproteins may not be restricted to the virus; it is feasible that similar losses may occur with membrane-bound vesicles.
The sedimentation velocity gradient originally developed by Dettenhoffer and Yu [19], for the purification of HIV particles, comprises a 6 18% (w/v) iodixanol gradient and is usually run in a 12- 13 ml swinging-bucket rotor, such as a Beckman SW41. It was constructed as a discontinuous gradient from multiple solutions; the concentration interval from step to step was only 1.2% (w/v) iodixanol. This method was also described in Table 2, lines 6, 7 and 11. It was adapted to a nearvertical (Beckman NVT65) rotor, with tubes of approx. the same volume by Cantin et al [15] and Park and He [21] see Table 2 lines 10 and 12. Because of the much reduced radial distance occupied by the sample, vertical or near-vertical rotors are regarded as the ideal ones for sedimentation velocity. Taking into account the much reduced path length of the near-vertical rotor, compared to the swinging-bucket rotor, it is perhaps surprising that the g-forces and centrifugation times used with these two types of rotor were so similar.
- Because of the small iodixanol concentration interval between steps of the Dettenhoffer and Yu gradient [19] and the small volume of each step, it is highly likely that the gradient will rapidly become more or less continuous during the preparation and run times. An easier gradient preparation alternative therefore may be to create a continuous 6-18% (w/v) iodixanol at the outset.
- See OptiPrep™ Application Sheet V34 (virus index) for practical details on this method.
- See OptiPrep™ Application Sheet S03 for more information on the construction of gradients
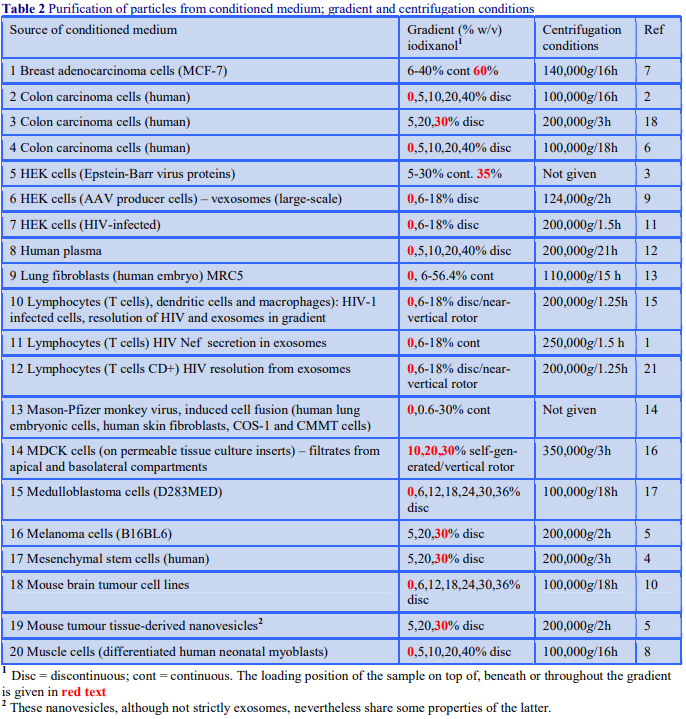
4c. Purification and analysis in a short-spin, bottom-loaded gradients
Although the gradients described in Section 4b were all continuous (or close to continuous) toploaded gradients, those in this section are all discontinuous with centrifugation times of 2-3 h. Because they were all bottom-loaded it is likely that the separation is based on density but some contribution from the rate of flotation cannot be ruled out. A two-layered gradient of 5 and 20% (w/v) iodixanol under-layered by the crude sample adjusted to 30% (w/v) iodixanol and centrifugation at 200,000 g is a common format (see lines 3, 16, 17 and 19 in Table 2).
4d. Purification and analysis in buoyant density gradients
Some of these long-spin (16-21 h) gradients are set up as pre-formed continuous gradients (for example see lines 1 and 9 in Table 2). In line 1, the gradient is bottom loaded, in line 9 it is toploaded; the advantage of bottom loading is that any residual soluble proteins will remain at the bottom of the gradient. If the gradient is top loaded soluble proteins will sediment through the gradient at a rate proportional to their molecular mass. All other examples (lines 2, 4, 8, 15, 18 and 20 in Table 2) feature discontinuous gradients, but these will become more or less continuous by diffusion during the centrifugation. The g-forces are generally 100-150,000 g but may be as high as 200,000 g.
4e. Purification and analysis in self-generated density gradients
The final variation (line 14 in Table 2) relies on the ability of iodixanol to form a self-generated gradient in a vertical (or near-vertical rotor); self-generated gradient formation is discussed in OptiPrep™ Application Sheet S04. An advantage of the use of self-generated gradients is that the sample can be simply adjusted to a single iodixanol concentration (for example 20% w/v iodixanol) and then centrifuged at approx 350,000 g for 2-3 h. The gradients produced by this method are often not completely linear (see OptiPrep™ Application Sheet S04), but there are three big advantages to the use of such gradients:
- The gradient profiles are very reproducible
- The tube set-up is very simple
- The particles in the starting solution are very dilute and do not encounter any interfaces during their sedimentation or flotation (compared to bottom- or top-loading): thus aggregation between particles is minimal.
In the example in Table 2 the set-up of the three-layer format (sample contained in each layer) is a little more time-consuming; it’s big advantage however is that an almost completely linear gradient is achieved in a very short time.
4f. Density barrier
Occasionally it may be sufficient to use a simple density barrier to concentrate the exosomes, rather than use a more sophisticated gradient to provide the additional resolution from other membrane bound particles is no required. Hasegawa et al [22] layered conditioned medium from an epithelial cell line over layers of 2% and 50% (w/v) iodixanol and centrifuged at 100,000 g for 1 h. The soluble proteins remained in the sample layer and the exosomes banded at the 2%-50% iodixanol interface.
5. Banding density of exosomes
The observed banding density of the exosomes in the iodixanol gradient is going to depend on a number of operational factors. In the long-spin fractionations (15-21h) the exosomes should reach their buoyant banding density, which will probably not depend on whether the gradient was originally top- or bottom-loaded (see Table 2). Differences in the observed banding density are likely to be the result of the use of different cell and tissue sources. In the case of shorter centrifugation times (1.25- 3h), the exosomes may not reach their true banding density. This is particularly true of those toploaded gradients of 6-18% (w/v) iodixanol (see Section 4b and Table 2) in which the exosomes are separated from other particles mainly on the basis of their sedimentation velocity. Other gradient systems fall in between these two extremes (see Section 4c and Table 2) and it is difficult to determine whether the exosomes have or have not reached their buoyant density. The path length of the swinging-bucket rotor tube and the distance the exosomes have either to sediment or to float to their banding density is also a consideration in deciding whether the particles have or have not reached that position in the gradient. Although the self-generated iodixanol gradient (see Section 4e and Table 2) also requires a relatively short spin-time, the much higher g-force and the short sedimentation path length of the vertical rotor mean that the exosomes will certainly have reached their buoyant density banding position.
- The banding position and broadness of the observed exosome band will depend on the inherent heterogeneity of the vesicles (both in terms of size and content) and all of the operational variables described above.
- Larger vesicles may have a more rapid sedimentation (or flotation) rate than smaller ones, but the latter may have a higher buoyant density
- Some of the reported banding densities of exosomes are given in Table 3.
In Type 4b gradients (see Section 4)
In separations on the 6-18% (w/v) iodixanol sediment-velocity gradient the separation of exosomes and HIV is much better defined than on a sucrose gradient.
- Cantin et al [15] noted that the exosomes banded at 8.4–12% (w/v) iodixanol, while HIV-1 banded at 15.6%; these concentrations are equivalent to 1.050-1.069 and 1.087 g/ml respectively. Separation from denser apoptotic vesicles was also noted. Sucrose gradients are unable to provide such a satisfactory resolving power, with HIV banding at 1.16-1.18 g/ml, while the exosomes at 1.13-1.21 g/ml completely overlapped the virus.
- Lenassi et al [1] reported a density of 1.04-1.055 g/ml and 1.065-1.10 g/ml for the exosomes and HIV respectively. The virus protein p24 was absent from the exosome peak, which was rich in Nef.
- Although no specific density data was provided in ref 11, a large linear separation of acetylcholinesterase (exosome marker) in the top 1/3rd of the gradient and viral vesicles in the bottom 1/3rd was observed. In Type 4c/4d gradients (see Section 4)
- The expected much lower density banding in iodixanol compared to sucrose gradients (1.03-1.08 and 1.13-1.18 g/ml respectively) is also observed [3].
- Duelli et al [14] isolated exosomes from conditioned culture media from a variety of cells lines; the authors commented that an iodixanol gradient was far more effective for the purpose than a sucrose gradient.
6. References
1. Lenassi, M., Cagney, G., Liao, M., Vaupotǐc, T., Bartholomeeusen, K., Cheng, Y., Krogan, N.J., Plemenitǎ, A. and Peterlin, B.M. (2010) HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells Traffic, 11, 110–122
2. Mathivanan, S., Lim, J.W.E., Tauro, B.J., Ji, H., Moritz, R.L. and Simpson, R.J. (2010) Proteomics analysis of A33 immunoaffinity purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature Mol. Cell. Proteomics, 9, 197–208
3. Ruiss, R., Jochum, S., Mocikat, R., Hammerschmidt, W. and Zeidler, R. (2011) EBV-gp350 confers B-cell tropism to tailored exosomes and is a neo-antigen in normal and malignant B cells – new option for the treatment of B-CLL PLoS One, 6: e25294
4. Kim, H-S., Choi, D-Y., Yun, S.J., Choi, S-M., Kang, J.W., Jung, J.W., Hwang, D., Kim, K.P. and Kim, DW. (2012) Proteomic analysis of microvesicles derived from human mesenchymal stem cells J. Proteome Res., 11, 839−849
5. Lee, E-Y., Park, K-S., Yoon, Y.J., Lee, J., Moon, H-G., Jang, S.C., Choi, K-H., Kim, Y-K. and Gho, Y.S. (2012) Therapeutic effects of autologous tumor-derived nanovesicles on melanoma growth and metastasis PLoS One 7: e33330
6. Tauro, B.J., Greening, D.W., Mathias, R.A., Ji, H., Mathivanan, S., Scott, A.M. and Simpson, R.J. (2012) Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863 derived exosomes Methods, 56, 293–304
7. Baietti, M.F., Zhang, Z., Mortier, E., Melchior, A., Degeest, G., Geeraerts, A., Ivarsson, Y., Depoortere, F., Coomans, C., Vermeiren, E., Zimmermann, P. and David, G. (2012) Syndecan-syntenin-ALIX regulates the biogenesis of exosomes Nat. Cell Biol., 14, 677-685
8. Le Bihan, M-C., Bigot, A., Jensen, S.S., Dennis, J.L., Rogowska-Wrzesinska, A., Lainé, J., Gache, V., Furling, D., Jensen, O.N., Voita, T., Mouly, V., Coulton, G.R. and Butler-Browne, G. (2012) In-depth analysis of the secretome identifies three major independent secretory pathways in differentiating human myoblasts J. Proteom., 77, 344-356
9. Maguire, C.A., Balaj, L., Sivaraman, S., Crommentuijn, M.H.W., Ericsson, M., Mincheva-Nilsson, L., Baranov, V., Gianni, D., Tannous, B.A., Sena-Esteves, M., Breakefield, X.O. and Skog, J. (2012) Microvesicle-associated AAV vector as a novel gene delivery system Mol. Ther., 20, 960–971
10. Graner, M.W., Alzate, O., Dechkovskaia, A.M., Keene, J.D., Sampson, J.H., Mitchell, D.A. and Bigner, D.D. (2009) Proteomic and immunologic analyses of brain tumor exosomes FASEB J., 23, 1541–1557
11. Columba Cabezas, S. and Federico, M. (2013) Sequences within RNA coding for HIV-1 Gag p17 are efficiently targeted to exosomes Cell. Microbiol., 15, 412–429
12. Zhang, Y., Liu, D., Chen, X., Li, J., Li, L., Bian, Z., Sun, F., Lu, J., Yin, Y., Cai, X., Sun, Q., Wang, K., Ba, Y., Wang, Q., Wang, D., Yang, J., Liu, P., Xu, T., Yan, Q., Zhang, J., Zen, K. and Zhang, C-Y. (2010) Secreted monocytic miR-150 enhances targeted endothelial cell migration Mol. Cell, 39, 133–144
13. Mangeot, P-E., Dollet, S., Girard, M., Ciancia, C., Joly, S., Peschanski, M. and Lotteau, V. (2011) Protein transfer into human cells by VSV-G-induced nanovesicles Mol. Ther., 19, 1656–1666
14. Duelli, D.M., Hearn, S., Myers, M.P. and Lazebnik, Y. (2005) A primate virus generates transformed human cells by fusion J. Cell Biol., 171, 493-503
15. Cantin, R., Diou, J., Belanger, D., Tremblay, A.M. and Gilbert, C. (2008) Discrimination between exosomes and HIV-1: Purification of both vesicles from cell-free supernatants J. Immunol. Methods, 338, 21-30
16. Casas, E., Barron, C., Francis, S.A., McCormack, J.M., McCarthy, K.M., Schneeberger, E.E. and Lynch, R.D. (2010) Cholesterol efflux stimulates metalloproteinase-mediated cleavage of occludin and release of extracellular membrane particles containing its C-terminal fragments Exp. Cell Res., 316, 353-365
17. Epple, L.M., Griffiths, S.G., Dechkovskaia, A.M., Dusto, N.L., White, J., Ouellette, R.J., Anchordoquy, T.J., Bemis, L.T. and Graner, M.W. (2012) Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles PLoS One, 7: e42064
18. Choi, D-S., Park, J.O., Jang, C.S., Yoon, Y.J., Jung, J.W., Choi, D-Y., Kim, J-W., Kang, J.S., Park, J., Hwang, D., Lee, K-H., Park, S-H., Kim, Y-K., Desiderio, D.M., Kim, K.P. and Gho, Y.S. (2011) Proteomic analysis of microvesicles derived from human colorectal cancer ascites Proteomics, 11, 2745–2751
19. Dettenhoffer, M. and Yu, X-F. (1999) Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif virions J. Virol., 73, 1460-1467
20. Palker, T.J. (1990) Mapping of epitopes on human T-cell leukemia virus type 1 envelope glycoprotein In: Human Retrovirology: HTLV (ed. Blattner, W.A.) Raven Press, NY, pp 435-445
21. Park, I-W. and He, J.J. (2010) HIV-1 is budded from CD4+ T lymphocytes independently of exosomes Virol. J., 7: 234
22. Hasegawa, H., Thomas, H.J., Schooley, K. and Born, T.L. (2011) Native IL-32 is released from intestinal epithelial cells via a non-classical secretory pathway as a membrane-associated protein Cytokine, 53, 74–83
OptiPrepTM Application Sheet S63; 5th edition, January 2020
OptiPrep™ Application Sheet S64
Isolation of plasma membrane from cultured cells by flotation through a discontinuous gradient
- OptiPrep™ is a 60% (w/v) solution of iodixanol in water, density = 1.32 g/ml
- An OptiPrep™ Reference List (RS05) “Analysis of membrane trafficking in mammalian cells…..” provides a bibliography of published papers reporting the use of OptiPrep for analysis of these membranes; return to the initial list of folders and select “Reference List”. The references are listed according to cell type.
- To access other Application Sheets referred to in the text: return to the 2020SMemapp file and select the appropriate S-number.
1. Background
Using iodixanol gradients, isolation of a plasma membrane (PM) fraction is often achieved simultaneously with the purification of other subcellular membranes in either continuous (linear) or discontinuous iodixanol gradients, centrifuged for 1-3 h or overnight (see Application Sheets S21-25). This Application Sheet highlights examples in which the PM is particularly well separated from intracellular compartments.
The PM is usually the least dense of all the subcellular membranes. In such situations, flotation through one or more low-density solutions from a high-density suspension often provides improved recovery and/or resolution than does sedimentation from the top of the gradient. If the density at the top of the gradient is in the range 1.04-1.08 g/ml (equivalent to approx 2.5-10% w/v iodixanol), the PM is usually well resolved from slightly denser components such as Golgi and early endosomes. Additionally, overloading the gradient (instability of the sample/gradient interface) cannot occur in a flotation format and resolution of the PM from cytoplasmic proteins, which remain in the dense sample zone, is much improved. In top-layering cytoplasmic proteins move down the gradient by both sedimentation and diffusion and are likely to contaminate the least dense material.
This flotation technique has been used for mouse astrocytoma cells [1], PC12 pheochromocytoma cells [2] and MCF7 human breast adenocarcinoma cells [3]. All used a routine buffered solution of 0.25 M sucrose and 1 mM EDTA, although 2 mM MgCl₂ was included for the PC12 cells. The latter is included as an option in Section 2 (below). Whether this a specific requirement for PC12 cells or whether it would of more general benefit can only be determined experimentally. The protocol below is adapted from ref 1, while Sections 5.5.1 and 5.6 describe alternative gradients and centrifugation conditions from refs 2 and 3.
2. Solutions required (see Section 5.1)
A. OptiPrep™
B. Homogenization buffer (HB): 0.25 M sucrose, 1 mM EDTA, 2 mM MgCl₂ (optional), 20 mM Hepes-NaOH, pH 7.4
C. Diluent: 0.25 M sucrose, 6 mM EDTA, 12 mM MgCl₂ (optional), 120 mM Hepes-NaOH, pH 7.4
D. Working Solution (WS) of 50% (w/v) iodixanol ( = 1.272 g/ml): 5 vol. of solution A + 1 vol. of solution C
3. Ultracentrifuge rotor requirements (see Section 5.2)
Any swinging-bucket rotor capable of 100,000-200,000g with tube volumes of approx. 14 ml (e.g. Beckman SW41 or Sorvall TH641) or 5 ml (e.g. Beckman SW55 or Sorvall AH650)
4. Protocol
Carry out all operations, except the washed with phosphate-buffered saline (Step 1) at 0-4°C.
1. Wash the cells twice in phosphate-buffered saline to remove the culture medium, and then once in Solution B.
2. Suspend the cells in a small volume of Solution B (2.5×107 cells in 0.5-1.0 ml)
3. Disrupt the cells by repeated passages through a fine syringe needle (e.g. up to 20x using a 25G needle), in a ball-bearing homogenizer (3-10 passes) or with 20 strokes of the pestle of a tightfitting Dounce homogenizer (see Section 5.3).
4. Centrifuge the homogenate at 2000 g for 10 min and harvest the supernatant (see Section 5.4.1).
5. Centrifuge the supernatant at 100,000 g for 40-60 min (see Section 5.4.2).
6. Prepare solutions of 2.5%, 10%, 17.5, 25% and 30% (w/v) iodixanol solution by mixing Solutions D and B 2.5:47.5, 1:5, 17.5:32.5, 1:1 and 3:2 (v/v) respectively (see Section 5.5.1).
7. Suspend the pellet from Step 5 in 0.75 ml of 30% iodixanol (see Section 5.5.1).
8. In 13 ml tubes for the swinging-bucket rotor prepare a discontinuous gradient from 3 ml each of the 2.5%, 10%, 17.5% and 25% iodixanol by the underlayering technique, using a syringe and metal cannula and finally underlayer the sample in 30% iodixanol (see Section 5.5.2).
9. Centrifuge at 165,000 gav for 3.5 h (see Section 5.6).
10. Collect the gradient in 0.5 ml fractions by tube puncture, upward displacement or aspiration from the meniscus. For more information on harvesting gradients see Application Sheet S08.
5. Technical Notes and Review
5.1. Homogenization media and gradient solutions
The homogenization medium often has to be tailored to the tissue or cell type and it is not known if the composition of the HM is relevant to the separation. All of the published methods using the type of protocol used in this OptiPrep™ Application Sheet have used a 0.25 M sucrose buffered with either Tris, Hepes, Tricine or triethanolamine (at 10-20 mM concentration) and often, but not always, containing 1 mM EDTA. Supplementation of the HM with inorganic salts is becoming increasingly common and can reduce ionic interactions, aggregation between membranes and combat any raised viscosity of the homogenate due to cytoskeletal proteins. Some media that omit sucrose entirely use either NaCl or KCl or both as the principal osmotic balancer(s). The composition of the HM should also be compatible with any subsequent analytical process. The inclusion of divalent cations can guard against nuclear breakage; stabilize membranes generally, but may lead to aggregation. Table 1 summarizes some of the other HMs that have been used. Other examples are given in Application Sheets S05 (tissues) and S06 (cells).
The preparation of a 50% iodixanol working solution (Solution D) ensures that the concentrations of EDTA (and MgCl₂, if included) and buffer are constant in all gradient solutions. Strategies for preparing working solutions for mammalian tissues and cells are given in Application Sheet S01. Protease inhibitors may be included in Solutions B and C at the operator’s discretion.
5.2. Ultracentrifuge rotors
These separations have been performed either in 13 ml tubes or 5 ml tubes. Other swinging-bucket
rotors or even vertical rotors may be used. Larger volume swinging-bucket rotors may require longer
centrifugation times but smaller volume rotors and vertical rotors will need shorter times. Note however that the progressive change in gradient density profile (due to diffusion and sedimentation of the iodixanol molecules) may also be modulated in other rotors and affect the final resolution.
5.3. Homogenization
The homogenization protocol should be tailored to the cell (or tissue) type. Potter-Elevhjem or Dounce homogenization for tissues and Dounce homogenization for cells used to be the standard procedures. For cells however use of 12-20 passages through a syringe needle (the Gauge Number (G) varies from 21 to 25) sometimes preceded by Dounce homogenization, has become very common. The ball-bearing homogenizer (“cell cracker”) is now widely regarded as one of the most effective and reproducible of devices.
Ideally the procedure should be as gentle and reproducible as possible, the aim being to cause at least 95% cell disruption without damage to the major organelles, particularly the nuclei and lysosomes. The type and severity of the homogenization process will have consequences for the integrity of the organelles and the size of the vesicles produced from tubular structures in the cytoplasm. Therefore the pattern of membrane banding in any subsequent gradient may not be easily predicted. For hints on homogenization see Application Sheets S05 (tissues) and S06 (cells).
5.4. Differential centrifugation
5.4.1. Removal of nuclei
Nuclear pelleting may be carried out at 500-3000g for 5-10 min; the higher RCFs (g-forces) resulting in removal of some of the mitochondria, which can facilitate subsequent layering of the sample on the gradient. To recover any vesicles trapped in the pellet (more serious at the higher RCFs), the pellet is sometimes resuspended in HM, recentrifuged and the two supernatants combined. A possible disadvantage of this practice is that unless the resuspension of the pellet is carried out very gently, the nuclei may be damaged, with consequent leakage of DNA, which may lead to almost irreversible aggregation of the subcellular membranes.
5.4.2. Preparation of sample for gradient loading
If the size of the sample is sufficiently small this step may be omitted and the 2000g supernatant itself adjusted to a high density for loading under the gradient. Since the cytosolic proteins remain in at the bottom of the gradient during the subsequent centrifugation, this practice should not be detrimental to the purity of the recovered plasma membrane.
5.5. Density gradients
5.5.1. Alternative formats
Other gradients that have been used for plasma membrane flotation are:
For PC12 cells [2], 5%, 10%, 15% and 20% (w/v) iodixanol, sample underlaid in 25% (w/v) iodixanol
For MCF-7 cells [3], 20% and 24% (w/v) iodixanol, sample underlaid in 32% (w/v) iodixanol
A three layer discontinuous gradient, which was originally designed for the isolation of PM from Drosophila [4], was also used for its isolation from mouse embryos [6]. The embryos were homogenized in 150 mM NaCl, 0.2 mM EGTA, 100 mM Tris-HCl, pH 7.4 and a post-nuclear supernatant was mixed with OptiPrep™ to 40% (w/v) iodixanol solution and overlaid by 30% and 5% iodixanol. After centrifugation at 100,000 g for 3 h the plasma membrane was harvested from the top interface [5].
5.5.2. Construction
Although underlayering is the recommended method for making discontinuous gradients, overlayering is also an option. For more information on gradient construction see Application Sheet S03. If necessary, adjust all volumes proportionately so that tubes (after sample application) are properly filled according to the manufacturer’s instructions. Gradients and sample volume should be scaled up or down proportionately as required for larger or smaller rotors.
5.6. Centrifugation conditions
Other centrifugation conditions that have been used are: for PC12 cells [2], 88,000g for 17 h; for MCF-7 cells [3], and 83,000g for 2 h
5.7. Analysis
In a 5%, 10%, 15%, 25%, 30% iodixanol flotation gradient from PC12 cells [2] the plasma membrane, as identified by the EGF receptor banded at the 5/10% and 10/15% interface. The PM from MCF-7 cells also banded at the top of the 20% iodixanol layer (Figure 1, Panel a), while that from mouse astrocytoma cells (Figure 1, Panel b) was also the least dense membrane (banding mainly at approx. 10% iodixanol). In the latter case the PM marker (transferrin receptor) showed a distinct biphasic trait and interestingly the murine coronavirus spike protein (S-protein) was confined to the Golgi and the denser plasma membrane component. The implication is that this gradient may be able to resolve subfractions of the PM.
The plasma membrane overlapped with neither endosomes [2,3] nor Golgi markers [3]. Endosome markers EEA1 and Rab5B from PC12 cells were both recovered almost entirely from the 25/30% interface region (1.15-1.175 g/ml) and in the case of MCF-7 cells both Rab5 and Rab 11 (Figure 1, Panel a) banded at >20% iodixanol. The Golgi marker (Syntaxin 6) from mouse astrocytoma cells
(Figure 1, Panel b) banded towards the bottom of the gradient in the 17.5-25% iodixanol zone. Thus compared to top-loaded gradients (see for example Application Sheet S21) the banding density of both endosomes and Golgi is unexpectedly high in these flotation gradients. On the other hand the position of mitochondria (mHSP70 marker) from MCF-7 cells (Figure 1, Panel a) was more or less as expected.
6. References
1. Choi, K.S., Aizaki, H. and Lai, M.M.C. (2005) Murine coronavirus requires lipid rafts for virus entry and cell-cell fusion but not for virus release J. Virol., 79, 9862-9871
2. Wu, C., Lai, C-F. and Mobley, W.C. (2001) Nerve growth factor activates persistent Rap1 signaling in endosomes J. Neurosci., 21, 5406-5416
3. Li, Q., Harraz, M.M., Zhou, W., Zhang, L.N., Ding, W., Zhang, Y., Eggleston, T., Yeaman, C., Banfi, B. and Engelhardt, J.F. (2006) Nox2 and Rac1 regulate H2O2 -dependent recruitment of TRAFg to endosomal interleukin-1 receptor complexes Mol. Cell. Biol., 26, 140-154
4. Eroglu, C., Brügger, B., Wieland, F. and Sinning, I. (2003) Glutamate-binding affinity of Drosophila metabotropic glutamate receptor is modulated by association with lipid rafts Proc. Natl. Acad. Sci. USA, 100, 10219-10224
5. Wang, X., Pralhada Rao, R., Kosakowska-Cholody, T., Masood, M.A., Southon, E., Zhang. H., Berthet, C., Nagashim, K., Veenstra, T.K., Tessarollo, L., Acharya, U. and Acharya, J.K. (2009) Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice J. Cell Biol., 184, 143-158
OptiPrepTM Application Sheet S64; 9th edition, January 2020
OPTIPREP™ APPLICATION SHEET INDEX SUBCELLULAR MEMBRANES
- The Index is divided into three sections:
A. General methods for preparing gradients; preparing crude organelle or membrane fractions and analysing the gradient separation
B. An alphabetical subcellular membrane index. Membranes and organelles from tissues and cells from mammals and most higher eukaryotes are listed according to the membrane or organelle or subcellular process (e.g. endocytosis) .
C. Membranes and organelles from protozoa, fungi, algae, plants etc are listed alphabetically
according to the source.
- In some cases more than one Application Sheet for a specific membrane type may be provided if different practical strategies are available.
- To open an Application Sheet click on the relevant [Application Sheet S–]
A. GENERAL METHODS
Gradient solution preparation (mammalian)
Gradient solution preparation (non-mammalian)
Gradients (discontinuous and continuous)
Gradients (self-generated gradients)
Homogenization of mammalian tissues
Homogenization of mammalian cells
Homogenates, differential centrifugation of
Gradient harvesting
Gradient analysis
B. SUBCELLULAR MEMBRANES (MAMMALIAN)
Caveolae
See “Plasma membrane domains”
Cytoplasmic vesicles
See “Protein localization (membrane versus cytosol)”
Cytosol/membrane vesicle separation
See “Protein localization (membrane versus cytosol)”
Endocytosis analysis
Cultured cells (endosomes/lysosomes/plasma membrane)
Lysosome/late endosome events (rat liver)
Methodological review
Self-generated gradients; buoyant density separation
Lysosome/late endosome analysis using Nycodenz®
Endoplasmic reticulum
See “Membrane trafficking” for resolution of endoplasmic reticulum from other membrane
compartments, e.g. plasma membrane, Golgi, trans-Golgi network, endosomes, ERGIC
Endoplasmic reticulum domains
Lipid droplets
Mitochondria-associated
Perinuclear
Endoplasmic reticulum – rough/smooth fractionation
Cultured cells (continuous gradients)
Self-generated gradients
Separation from Golgi
Endosomes
See “Endocytosis analysis”
See “Membrane trafficking” for resolution of endosomes from other membrane compartments, e.g. endoplasmic reticulum, plasma membrane, Golgi, trans-Golgi network, ERGIC
Exocyst function
Plasma membrane domain targeting
Exocyst vesicle trafficking – methodological summary
Exosomes and other microvesicles (mammalian)
Extracellular vesicles (non-mammalian sources)
GLUT 4 trafficking (self-generated gradient)
Golgi and trans-Golgi network
See “Endoplasmic reticulum – rough/smooth fractionation – separation from Golgi”
See also “Membrane trafficking” for resolution of Golgi (or trans-Golgi network) from other membrane compartments – e.g. endoplasmic reticulum, plasma membrane, endosomes, ERGIC
Intracellular signalling
Light mitochondrial fraction, analysis of
Continuous gradient
Self-generated gradient
See also “Lysosomes”
See also “Mitochondria”
See also “Peroxisomes”
Lipid rafts
See “Plasma membrane domains”
Lysosomes
Discontinuous gradient
Methodological review
See also “Light mitochondrial fraction, analysis of”
See also “Endocytosis analysis/lysosome/late endosome events
Lysosomes (ER/endosomes/plasma membrane)
Continuous gradient
Membrane trafficking (endoplasmic reticulum, plasma
membrane, Golgi, trans-Golgi network, endosomes, ERGIC)
Continuous gradient (short spin)
Continuous gradient (long spin)
Discontinuous gradient (flotation)
Discontinuous gradient (sedimentation)
Sedimentation velocity
Self-generated gradient
Membrane vesicle/cytosol separation
See “Protein localization”
Mitochondria:
Mammalian (discontinuous gradient)
Purification and analysis
See also “Light mitochondrial fraction, analysis of”
Neural tissue organelles
Synaptosomes, synaptoneurosomes, neuromelanin granules
and synaptic vesicles – a methodological survey
Nuclei
Discontinuous gradients and density barrier
Methodological review
Peroxisomes (mammalian)
Continuous gradient
Discontinuous gradient or density barrier
Self-generated gradient
See also “Light mitochondrial fraction, analysis”
Plasma membrane
See “Membrane trafficking” for resolution of plasma membrane from other membrane compartments, e.g. endoplasmic reticulum, Golgi, trans-Golgi network, endosomes, ERGIC
Cardiac muscle, from
Cationic colloidal silica
Flotation in a discontinuous gradient
Plasma membrane domains
Caco-2 cells (apical/basolateral)
Cationic colloidal silica
Caveolae
Epithelial cells (apical junction complex)
Intestinal mucosa (brush border/basolateral)
Lipid rafts
Detergent strategy
Detergent-free strategy
Renal cortex (brush border/glomeruli/slit diaphragm)
MDCK cells
See “Epithelial cells (apical junction complex)”
NRK cells
“See Epithelial cells (apical junction complex) ”
Plasma membrane domain targeting (exocyst)
Protein localization (large protein complexes)
Protein localization (membrane versus cytosol)
Discontinuous flotation gradient
Self-generated gradient
Retinal rod outer segments
Optical disks
Secretory granules
Eosinophils
Pancreatic cells
Adrenal chromaffin, lymphocytic, neutrophil-derived,
synaptosomal, parotid and platelets
Storage granules (vesicles)
See “Secretory granules”
Trans-Golgi Network (TGN)
See “Membrane trafficking” for resolution of trans-Golgi network from other membrane compartments, e.g. endoplasmic reticulum, endosomes, plasma membrane, Golgi, , ERGIC
C. SUBCELLULAR MEMBRANES (NON-MAMMALIAN)
Algae
Organelles (various)
Amoeba
Organelles (various)
Arabidopsis thaliana
See “Plants”
Bacteria
Extracellular vesicles
Rhodospirillum rubrum:
Organelles, including acidocalcisomes
Chlamydomonas reinhardtii
See “Algae”
Dictyostelium discoideum
See “Protozoa”
Drosophila membranes
Fungi
Extracellular vesicles
Organelles
Yeast spheroplasts
Endosomes, ER, Golgi, TGN, vacuole
Membrane trafficking (vacuole, Cvt vesicles etc.)
Mitochondria
Peroxisomes
Phytomonas francai
See “Protozoa”
Plant tissues
Organelles
Proteomic analysis of Arabidopsis
Protein processing
Protozoa
Acidocalcisomes
Contractile vacuoles
Other organelles
[Application Sheet S01]
[Application Sheet S02]
[Application Sheet S03]
[Application Sheet S04]
[Application Sheet S05]
[Application Sheet S06]
[Application Sheet S07]
[Application Sheet S08]
[Application Sheet S46]
[Application Sheet S44]
[Application Sheet S42]
[Application Sheet S45]
[Application Sheet S54]
[Application Sheet S41]
[Application Sheet S41]
[Application Sheet S41]
[Application Sheet S19]
[Application Sheet S18]
[Application Sheet S20]
[Application Sheet S47]
[Application Sheet S47a]
[Application Sheet S63]
[Application Sheet S62]
[Application Sheet S48]
[Application Sheet S48]
[Application Sheet S15]
[Application Sheet S16]
[Application Sheet S55]
[Application Sheet S43]
[Application Sheet S56]
[Application Sheet S21]
[Application Sheet S22]
[Application Sheet S23]
[Application Sheet S24]
[Application Sheet S25]
[Application Sheet S48]
[Application Sheet S14]
[Application Sheet S14a]
[Application Sheet S40a]
[Application Sheet S10]
[Application Sheet S10a]
[Application Sheet S11]
[Application Sheet S12]
[Application Sheet S13]
[Application Sheet S26]
[Application Sheet S27]
[Application Sheet S64]
[Application Sheet S29]
[Application Sheet S27]
[Application Sheet S34]
[Application Sheet S31]
[Application Sheet S28]
[Application Sheet S32]
[Application Sheet S33]
[Application Sheet S30]
[Application Sheet S47a]
[Application Sheet S37]
[Application Sheet S35]
[Application Sheet S36]
[Application Sheet S51]
[Application Sheet S39]
[Application Sheet S38]
[Application Sheet S40]
[Application Sheet S59]
[Application Sheet S58]
[Application Sheet S62]
[Application Sheet S50]
[Application Sheet S49]
[Application Sheet S62]
[Application Sheet S59]
[Application Sheet S53]
[Application Sheet S52]
[Application Sheet S17]
[Application Sheet S57]
[Application Sheet S60]
[Application Sheet S61]
[Application Sheet S58]
[Application Sheet S48]
[Application Sheet S48]
[Application Sheet S58]
OptiPrepTM Application Sheet Index: February 2020
